Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT
REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
April 30, 2015
Date of Report (date of earliest event reported)
MyDx, Inc.
(Exact name of Registrant as specified in its charter)
| Nevada | 333-191721 | 99-0384160 | ||
| (State or other
jurisdiction of incorporation or organization) |
(Commission File Number) | (I.R.S. Employer Identification Number) |
4225
Executive Square, Suite 600
La Jolla, California 92037
(Address of principal executive offices)
(800) 814-4550
(Registrant’s telephone number, including area code)
Brista Corp.
302 San Anselmo Avenue, Suite 220
San Anselmo, California 94960
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Current Report on Form 8-K contains certain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which statements involve substantial risks and uncertainties. In some cases, it is possible to identify forward-looking statements because they contain words such as “anticipates,” believes,” “contemplates,” “continue,” “could,” “estimates,” “expects,” “future,” “intends,” “likely,” “may,” “plans,” “potential,” “predicts,” “projects,” “seek,” “should,” “target” or “will,” or the negative of these words or other similar terms or expressions that concern our expectations, strategy, plans or intentions. Many factors could cause our actual operations or results to differ materially from the operations and results anticipated in forward-looking statements. These factors include, but are not limited to:
| ● | our financial performance, including our history of operating losses; |
| ● | our ability to obtain additional funding to continue our operations; |
| ● | our ability to successfully develop and commercialize our products; |
| ● | changes in the regulatory environments of the United States and other countries in which we intend to operate; |
| ● | our ability to attract and retain key management and marketing personnel; |
| ● | competition from new market entrants; |
| ● | our ability to successfully transition from a research and development company to a marketing, sales and distribution concern; |
| ● | our ability to identify and pursue development of additional products; and |
| ● | the other factors contained in the section entitled “Risk Factors” contained in this Current Report on Form 8-K. |
We have based the forward-looking statements contained in this Current Report on Form 8-K primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition, results of operations and prospects. The outcome of the events described in these forward-looking statements are subject to risks, uncertainties, assumptions, and other factors including those described in the section of this Current Report on Form 8-K entitled “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements used herein.
You should not rely on forward-looking statements as predictions of future events. Except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements, and we undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date of such statements.
EXPLANATORY NOTE
As used in this Current Report on Form 8-K, (1) the terms the “Company,” “we,” “us,” and “our” refer to the combined enterprises of MyDx., Inc., a Nevada corporation, formerly named Brista Corp. (“Brista”), and CDx, Inc., a Delaware corporation (“CDx”), after giving effect to the Merger (defined below) and the related transactions described herein, (2) the term Brista refers to the business of Brista Corp., prior to the Merger, and (3) the term “CDx” refers to the business of CDx, Inc., prior to the Merger, in each case unless otherwise specifically indicated or as is otherwise contextually required. Although Brista Corp. changed its name to MyDx, Inc. on April 24, 2015, to avoid confusion and for purposes of clarity, the historical pre-merger operations of the Company are referred to in this Current Report as “Brista”.
| -1- |
This Current Report on Form 8-K is being filed in connection with a series of transactions consummated by us that relate to the Merger (as defined below) between us and CDx, Inc., which transactions are described herein, together with certain related actions taken by us.
The information contained in this Current Report on Form 8-K responds to the following items of Form 8-K:
| Item 1.01 | Entry into a Material Definitive Agreement. | |
| Item 2.01 | Completion of Acquisition or Disposition of Assets. | |
| Form 10 Information | ||
| Description of Business | ||
| Risk Factors | ||
| Management’s Discussion and Analysis | ||
| Description of Properties | ||
| Security Ownership of Certain Beneficial Owners and Management | ||
| Directors, Executive Officers and Corporate Governance | ||
| Executive Compensation | ||
| Certain Relationships and Related Transactions, and Director Independence | ||
| Legal Proceedings | ||
| Recent Sales of Unregistered Securities | ||
| Controls and Procedures | ||
| Market for Registrants Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | ||
| Description of Capital Stock | ||
| Indemnification of Officers and Directors | ||
| Financial Statements and Supplementary Data | ||
| Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | ||
| Exhibits, Financial Statement Schedules | ||
| Item 3.02 | Unregistered Sales of Equity Securities. | |
| Item 4.01 | Changes in Registrant’s Certifying Accountant. | |
| Item 5.01 | Changes in Control of Registrant. | |
| Item 5.02 | Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers. | |
| Item 5.03 | Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year. | |
| Item 5.06 | Change in Shell Company Status. | |
| Item 7.01 | Regulation FD Disclosure. | |
| Item 9.01 | Financial Statements and Exhibits. |
| Item 1.01. | Entry into a Material Definitive Agreement. |
On April 9, 2015, Brista entered into an Agreement and Plan of Merger (the “Merger Agreement”) with CDx Merger Inc., a Nevada corporation and wholly owned subsidiary of Brista (“Merger Sub”), and CDx. Pursuant to the Merger Agreement, Merger Sub merged with and into CDx with CDx surviving the merger as Brista’s wholly owned subsidiary (the “Merger”). On April 24, 2015, in anticipation of closing the Merger, Brista changed its name to MyDx, Inc.
For a description of the Merger and the material agreements entered into in connection with the Merger, please see the disclosures set forth in Item 2.01 of this Current Report on Form 8-K, which disclosures are incorporated into this Item 1.01 by reference. Item 2.01 of this Current Report on Form 8-K contain only a brief description of the material terms of the Merger Agreement, and does not purport to be a complete description of the rights and obligations of the parties thereunder, and such description is qualified in their entirety by reference to the Merger Agreement which is filed as an exhibit to this Current Report on Form 8-K.
| -2- |
| Item 2.01. | Completion of Acquisition or Disposition of Assets. |
The Merger and Related Transactions
On April 30, 2015, pursuant to the Merger Agreement, Merger Sub and CDx consummated the Merger, and CDx became a wholly owned subsidiary of Brista.
Pursuant to the Merger Agreement, upon consummation of the Merger, each share of CDx’s capital stock issued and outstanding immediately prior to the Merger was converted into the right to receive one (1) share of Brista’s common stock, par value $0.001 per share (the “Common Stock”). Additionally, pursuant to the Merger Agreement, upon consummation of the Merger, Brista assumed all of CDx’s options and warrants issued and outstanding immediately prior to the Merger, which are now exercisable for approximately 1,902,173 and 7,571,000 shares of Common Stock, respectively, as of the date of the Merger. Prior to and as a condition to the closing of the Merger, each then-current Brista stockholder agreed to sell certain shares of common stock held by such holder to Brista and the then-current Brista stockholders retained an aggregate of 1,990,637 shares of common stock. Therefore, following the Merger, CDx’s former stockholders now hold 19,484,615 shares of Brista common stock which is approximately 92% of the Company Common Stock outstanding.
Upon consummation of the Merger, Brista expanded its board of directors (the “Board”) from one to seven directors, each of whom will be directors designated by CDx.
Pursuant to the Merger Agreement, each party has made certain customary representations and warranties to the other parties thereto. The Merger was conditioned upon approval by CDx’s stockholders and certain other customary closing conditions.
The foregoing description of the Merger Agreement is only a summary and is qualified in its entirety by reference to the complete text of the Original Merger Agreement and the Amendment, which are filed as Exhibit 2.1 and Exhibit 2.2, respectively, to this Current Report on Form 8-K, and each of which is incorporated by reference herein.
Accounting Treatment
The Merger is being treated as a reverse acquisition of Brista, a public shell company, for financial accounting and reporting purposes. As such, CDx is treated as the acquirer for accounting and financial reporting purposes while Brista is treated as the acquired entity for accounting and financial reporting purposes. Further, as a result, the historical financial statements that will be reflected in the Company’s future financial statements filed with the United States Securities and Exchange Commission (“SEC”) will be those of CDx, and the Company’s assets, liabilities and results of operations will be consolidated with the assets, liabilities and results of operations of CDx.
Smaller Reporting Company
Following the consummation of the Merger, the Company will continue to be a “smaller reporting company,” as defined in Regulation S-K promulgated under the Exchange Act.
Stock Repurchase Agreement
In connection with the Merger, Brista entered into a Stock Repurchase Agreement (the "Repurchase Agreement") with each of its stockholders pursuant to which Brista repurchased 17,533,363 shares of its common stock (the "Repurchased Shares") from such shareholders. Upon the repurchase, Brista cancelled all of the Repurchased Shares.
| -3- |
FORM 10 INFORMATION
For purposes of this Current Report on Form 8-K, the Company is providing certain information that it would be required to disclose if it were a registrant filing a general form for registration of securities on Form 10 under the Exchange Act. As such, the terms the “Company,” “we,” “us,” and “our” refer to the combined enterprises of Brista and CDx, after giving effect to the Merger and the related transactions described below, except with respect to information for periods before the consummation of the Merger which refer expressly to CDx or Brista, as specifically indicated.
BUSINESS
Company Overview
Immediately following the Merger, the business of CDx became our business.
CDx, Inc. was incorporated under the laws of the State of Delaware on September 16, 2013. We are an early-stage science and technology company. We have developed and are commercializing technology and devices to accurately measure chemicals of interest in solid, liquid, or gas samples. Our mission is to enable people to live a healthier life by revealing the purity of certain compounds they eat, drink and inhale in real time through a device they can hold in the palm of their hand. We provide a quick, easy and affordable way for consumers to test the safety and composition of what they consume. We believe that the broad application and ease of use of our technology puts us in an ideal position to provide consumers with a practical and affordable way to trust and verify what they are putting into their bodies without leaving the comfort of their homes.
Our foundational proprietary technology derives from research developed at the California Institute of Technology, Pasadena, California, for the Jet Propulsion Laboratory, used by NASA as well as an additional project funded by the Bill & Melinda Gates Foundation for other exploratory research and medical applications. We have a portfolio of intellectual property rights covering principles and enabling instrumentation of chemical sensing technology across solid, liquid, and gas samples, including certain patented and patent pending technologies from a third party pursuant to a joint development agreement (the rights in this paragraph are sometimes referred to herein as the “Intellectual Property Rights”). See “Intellectual Property.”
We believe that our portfolio of Intellectual Property Rights provides us with a strong and defensible market position from which to commercialize our portable electronic nose and analyzing technology and to build our business by expanding our core technology across a variety of applications. Our current business strategy includes collaborations with a variety of industry partners for the development of products to support our portable sensing and analyzing technology.
Product Overview: MyDx
How MyDx Works
Our core technology is centered on a portable chemical sensing and analyzing method, represented by our first product, MyDx. MyDx is a portable chemical sensor, combined with a hand-held analyzer and associated mobile app, which together, acts as an electronic nose by detecting and analyzing molecules present in a given sample. MyDx aims to take chemical analysis out of the lab and to put it into the palm of the user’s hand. MyDx permits analysis of a wide array of substances using one device with interchangeable sensors which will be rolled out over coming years.
Each MyDx sensor has sensitivity in parts per billion, which the Company believes is unique for a handheld chemical analyzer at our anticipated consumer price-point for MyDx. The MyDx device has a user-friendly interface and is designed to easily communicate via Bluetooth with our mobile app, which can be downloaded on any iOS, Android, or Windows smartphone. Given the sensitivity of the MyDx device, once the app is downloaded and the device is synced, a small sample can be placed in the sample chamber, which is then stimulated, releasing the chemicals of interest into a vapor for identification by the MyDx Analyzer. The process of analysis takes about three minutes, after which the user will be able to view the parts per billion chemical composition of the sample on his or her mobile smart phone via our mobile app. In addition, the app will track and save the results of each analysis for future reference and comparison, and will aggregate reports on and analyses of various chemical compounds to help educate the consumer about the results. MyDx sensors can be switched by the user based on the type of the sample being tested, and the user will simply launch the associated app from their smartphone. The Company believes that, based on its portability, MyDx is the first portable chemical sensing and analyzing technology available for use by a broad range of consumers. This technology could help consumers to test for, among other things, pesticides in food, chemicals in water, toxins in the air in their own homes, and cannabis for its chemical content. Over the course of the next two years, the MyDx team plans to roll out four different sensors including Organa, Canna, Aero, and Aqua. These four sensor types are described in more detail below.
| -4- |
The Company currently has working prototypes of the Canna Sensor combined with the MyDx Analyzer.
About the MyDx Solution
The Company believes that MyDx is the first portable chemical analyzer available for use by consumers. Designed to work in combination with the Company’s mobile app, the MyDx solution provides seamless integration with iOS, Android, and Windows mobile smart phones and will make the analysis of chemical data meaningful to users. The Company’s first four planned interchangeable sensors include:
| ● | Organa – The Organa Sensor will be used to identify pesticides present in fruits and vegetables. MyDx will be able to analyze samples of food and detect levels of certain chemicals that might be harmful, such as Bisphenol-A, Phthalates, pesticides, herbicides, and countless others that could lead to hormone imbalances, sickness, cancer, and various other diseases. This product would allow parents to be more informed about the food they are feeding to their children, a population segment vulnerable to these chemicals. Many of the harmful chemicals detected by MyDx are legal in the United States. The MyDx device, once available, will be one of the first options consumers will have to educate themselves, in real-time about whether their foods contain such chemicals by actually testing the food themselves. In addition, because the mobile app paired with the MyDx Analyzer quickly and easily indicates the presence of various chemicals, consumers do not need to be fully educated about the various chemicals in order to use the device effectively. |
| ● | Aero – The Aero Sensor will be used for testing air quality. Air quality can vary greatly depending on the time of day and location. The Aero Sensor will allow the user to measure the local air quality in any location, and the information included in the mobile app will aid in their understanding of the air quality measurement. The Company expects, although it cannot assure, that there will be global interest in its air quality sensor, especially in urban and industrial areas where air quality is often poor. |
| ● | Aqua – The Aqua Sensor will be used for testing water quality. Water often contains harmful chemicals and minerals such as hexavalent chromium, fluoride, mercury, chlorine, lead, and many others. The use of the Aqua Sensor with the MyDx Analyzer will enable people to test water for these harmful chemicals. Because the MyDx device is easily transported, consumers will be able to test the local tap water while traveling, which the Company believes will be a popular use for the device. |
| ● | Canna – The Canna Sensor is used to identify the chemical composition of cannabis, the use of which is increasingly being permitted for medicinal and other purposes across North America. The cannabis plant is versatile, and certain strains could aid with the treatment of different ailments and symptoms. When presented with a sample of cannabis, the MyDx test and mobile app will identify the chemical compounds of the unique strain being tested, inform the user about its potency,and enable the consumer to associate feelings and ailments with the test results. With the introduction of our Canna Sensor, the Company’s goal is to promote patient and consumer safety by giving them the ability to correlate the chemical composition of their cannabis with feelings and ailments. This will allow consumers to use MyDx to educate themselves on responsible use and dosage by giving them an understanding of their body’s reaction to the sample being tested. Note that consumers cannot inhale or use cannabis with the MyDx device. It is simply an analyzer. We believe the legalization of cannabis both for medical and other uses will continue, and that the success of our Canna Sensor will benefit from this trend. |
The Company currently has working prototypes of the Canna Sensor combined with the MyDx Analyzer. We shipped the first 231 beta devices of our Canna Sensor in February 2015, and plan to ship commercial units with Canna Sensors over the remainder of 2015. However, unexpected circumstances could delay or completely alter our anticipated product rollout.
| -5- |
Product Features
MyDx Service

The MyDx Analyzer comes with a variety of product features that provide consumers with a user-friendly experience. The Company has developed a sleek and durable unit that, paired with our four sensors, has capabilities to analyze a variety of chemical compounds in the palm of the user’s hand.
The MyDx Service connects the MyDx Analyzer to the MyDx App using BLE (Bluetooth Low Energy) connection. The MyDx App communicates with the secure MyDx Cloud via the Internet.
MyDx Analyzer
The MyDx Analyzer comes with Bluetooth connectivity in order to engage the MyDx App, which provides the user with additional information to help them understand the chemicals found by the MyDx Analyzer. The MyDx Cloud compares what was sensed by the MyDx Analyzer with scientific data about that sample on the back-end, revealing Tetrahydrocannabinol (“THC”) and Cannabidiol (“CBD”) levels found in the sample.
Using the MyDx Analyzer is simple and easy. Below are the steps to power on/charge the MyDx Analyzer, open/close the Sample Chamber, and insert/remove the Sample Insert.
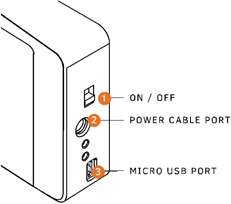 |
1) Power: Turn on and off MyDx 2) Power Cable Port: Charge MyDx Analyzer and power the device 3) Micro USB Port: Connect to computer to charge and power MyDx Analyzer 4) Press on the Chamber Release Button to open the Sliding Sample Chamber 5) Open the Sliding Sample Chamber to insert Disposable Sample Insert 6) After testing, remove the Disposable Sample Insert |
| -6- |
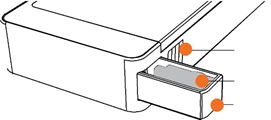 |
CHAMBER RELEASE BUTTON
DISPOSABLE SAMPLE INSERT
SLIDING SAMPLE CHAMBER |
 |
The MyDx App
All of the software necessary to run the MyDx Analyzer is built-in so the consumer will have no need to manage any software other than the MyDx App. The MyDx App is available for the iPhone, iPad, and IOS, Android and Windows platforms.
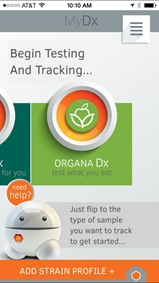
Once the MyDx App is installed on the consumer’s mobile device, the consumer merely turns on the MyDx Analyzer using the small button on the side next to the power cable slot. The green power light will illuminate. MyDx Analyzer and the mobile device must be within 5 feet of each other when testing, or they must be connected with the USB cable provided.
From the MyDx App home screen, the consumer can either click the center OrganaDx logo and click “Test with MyDx” or click the orange “Add Profile +” button at the bottom of the page and “Use MyDx”.
|
| -7- |
 |
Connect:
Next, the MyDx App will attempt to find and sync with the MyDx Analyzer via a wireless Bluetooth connection.
|
 |
Sample:
The consumer will then load their sample into the Sample Chamber. They will take an empty disposable Sample Insert and place their sample into the Sample Insert. This is done by gently grinding the sample between their fingers and letting the contents fall into the chamber. They fill the sample up to just below the rim of the insert. Once the sample is loaded and the chamber is closed, they follow the onscreen instructions and click “Next”.
|
 |
Analyze:
The MyDx Analyzer is placed on a flat surface, and the consumer presses the “Start” button. Three minutes later, they have their results. |
| -8- |
Target Audiences
The initial target audience for MyDx is consumers who are concerned with health and are interested in understanding what they are putting into their bodies. As described above, the MyDx Analyzer is intended to have a variety of sensors, and consumers will be able to insert the appropriate sensor to match the type of material to be analyzed. We believe that, as consumers become more educated about the risks of pesticides and other harmful chemicals that can be present in their food, water and in the air they breathe, the demand for a product like MyDx will increase.
Globally, the Company expects, but cannot guarantee, far-reaching adoption inasmuch as our MyDx product has the potential to address some of the world’s most important human health problems. Air quality and water quality, internationally, are of concern to many, and regulations attempting to limit air and water pollution exist in countries across the globe. The Aero and Aqua Sensors could be used to alert consumers that certain activities are emitting too many pollutants in to the air or water. MyDx’s capability of analyzing local air and water quality could be useful in other ways to locals and travelers alike, from parents measuring particulate matter levels in the air in their homes, to a traveler or foreign aid worker testing tap water in a foreign country before taking or offering a drink. Along similar lines, the Organa Sensor can be used to detect pesticides and other harmful chemicals in food. As the organic food movement grows and consumers become increasingly interested in identifying pesticide content in the fruits and vegetables they are eating, MyDx’s target audience will expand. We believe that MyDx’s interchangeable sensor design allows it to address a broad array of problems faced in the modern world.
The Canna Sensor may have broad appeal to individuals who use medicinal cannabis for their health. In many cases, the chemical compounds consumers need to know about are not correctly identified at the point-of-sale, and the consumers may not be aware of whether a particular strain will treat the symptoms they are experiencing. Using the Canna Sensor, consumers will be able to identify certain chemical compounds in different strains of cannabis that are associated with the desired response to its use. This makes MyDx an important tool for both cannabis patients and recreational users who want to understand the product they are consuming, how it affects their body and treats any symptoms they may have.
In addition to the consumer audience for our MyDx Canna Sensor, we expect businesses in the cannabis industry, including cultivators, processors, dispensaries, and retail distributors to use MyDx as an additional test for their own products or the products that they are buying for resale to consumers. However MyDx is not a replacement for commercial lab testing using standard gas chromatography and other lab equipment.
We believe the target audience for sales of our Organa, Aqua and Aero Sensors will be the individual consumer. We plan to offer those sensors for sale to consumers through our website shopping cart and at traditional brick and mortar retail stores.
Distribution and Marketing Strategy
The Company expects to distribute its products through various channels, including distributors, e-commerce via the Company’s website, direct to retail, and sales through affiliate partners. As part of our marketing strategy, the Company intends to partner with certain bloggers, websites and social media channels with large audiences, to ensure that the potential market knows and understands the promise of the MyDx Analyzer. The Company plans to use industry conferences, media outreach, and social media channels such as Facebook, LinkedIn, Twitter, Instagram, Google+ and more to push its message to the right audiences.
Despite having no marketing budget since its inception, to date, the Company has received hundreds of requests to purchase the device and has presold nearly 300 devices. Some of the Company’s channel partners have expressed interest in purchasing large unit quantities. The Company expects that when the first product is launched and the Company has the financial resources to implement its marketing strategy, the Company will be able to stimulate further interest and quickly generate product sales.
| -9- |
Market Overview
Organic Food Demand
The global organic food and beverage market is expected to grow from $80 billion in 2013 to $162 billion by 2018, which is a 15% compounded annual growth rate. Sales of organic products in the United States grew 12% in 2013 to $35 Billion. Over 10% of fruits and vegetables sold in the United States are organic (See Reportlinker.com; June 2014; Organic Trade Association - OTA’s 2014 Organic Industry Survey). The Company believes that the consumption of organic foods will continue to become more mainstream. The popularity of farmers’ markets in the United States has grown in conjunction with the rise in organic production and consumer interest in locally and organically produced foods See: http://www.ers.usda.gov/publications/vgs-vegetables-and-pulses-outlook; and http://www.ers.usda.gov; and http://www.organicconsumers.org/Organic/marketincrease73001.cfm). Recent surveys of parents across the country indicate that 81% of parents reported purchasing organic foods at least sometimes. (See http://www.prnewswire.com/news-releases/eight-in-ten-us-parents-report-they-purchase-organic-products-201477661.html) This trend toward “organics” is in part a function of consumers attempting to avoid harmful pesticides and foods created with genetically modified organisms (“GMOs”), as well as concerns regarding animal welfare and the environment.
We believe that this trend toward organic foods puts MyDx in a unique position to meet demand. The market for the Organa Sensor could grow alongside consumers becoming more concerned with the source and quality of their foods, and as farmer’s markets and organics become increasingly popular, MyDx could be used to test foods before they are purchased or sold. MyDx could, in this way, be a useful tool for consumers at farmer’s markets, and even at non-organic food stores, because MyDx can be used to test both organic and non-organic foods to ensure that consumers are given real-time and potentially comparative information about the foods they are buying.
Air Quality
As air pollution levels increase, air quality is rapidly deteriorating in countries across the globe. Air pollution consists of both gaseous and fine particulate contaminants present in the atmosphere. Pollutants can be released directly into the air, or can be formed when pollutants in the air react with one another. Based on a recent article by the World Health Organization (“WHO”), half of the urban populations of over 1,600 cities worldwide are exposed to air pollution every day. The WHO warns that air pollution for an average urban citizen is 2.5 times higher than what is considered a healthy environment. (See: http://www.who.int/phe/health_topics/outdoorair/databases/cities/en.) Air pollution has been linked to numerous health problems, including aggravation of respiratory and cardiovascular disease, decreased lung function, increased susceptibility to respiratory infection, and cancer (See http://www.epa.gov/airtrends/2011/report/airpollution.pdf; and http://www.epa.gov/airtrends/2011/). Certain “sensitive” populations, such as asthmatics, diabetics, children, and the elderly, experience greater impact from pollutants in the air.
In order to stem the tide of increasing air pollution, the United States Environmental Protection Agency has set National Ambient Air Quality Standards (“NAAQS”) for pollutants considered harmful to the environment and to the public health. Based on the NAAQS, all states in the United States are required to develop emission reduction strategies in order to lower levels of these harmful pollutants. Along the same lines, local governments are undertaking air quality studies to assess potential public health impacts pollutants in the air, and are promulgating rules to govern pollution-causing activities on the local level. State, federal, and local laws also exist to govern things like demolition and remodeling activities, emissions of asbestos fibers in the air, and the maintenance and removal of lead paint. In addition to regulations on air pollution, there are a variety of voluntary measures, including pollution prevention and control methods that can be implemented by willing individuals and businesses.
MyDx, paired with our Aero Sensor, is intended to present a solution for air quality monitoring for individuals within populations that are sensitive to air pollution, such as parents of asthmatic children who want to monitor the quality of the air in their homes. As both air pollution and public awareness of contaminants in the air increase, so will the market for a relatively low-cost air quality monitor. Furthermore, we believe that MyDx could be used both to help ensure compliance with air quality regulations and to help achieve voluntary control measures for pollution prevention. Individuals and small businesses conducting activities such as demolition or remodeling could use MyDx to ensure that their chosen means of conducting the activity is in compliance with the law. One step further, MyDx could be used to voluntarily ensure that, for instance, one’s car or lawn mower is not emitting harmful chemicals into the air. In any case, we believe that MyDx’s portability and low cost make it an ideal candidate to allow for convenient and accurate air quality monitoring from small sample, without the need for any governmental overhead or a trip to a laboratory. The Company does not intend to commercialize and offer for the sale the Aero Sensor during 2015.
| -10- |
Water Quality
Global water quality is also rapidly declining. Every day two million tons of sewage is discharged into our water. Recent United Nation estimates indicate that about 1,500 km3 of wastewater is produced annually, which is six times more water than is present in all the rivers of the world (See http://www.pacinst.org/wp-content/uploads/sites/21/2013/02/water_quality_facts_and_stats3.pdf). To put this issue in perspective, here are some facts about how water pollution can and is affecting everyone on a global scale:
| ● | Lack of adequate sanitation contaminates water courses worldwide and is one of the most significant forms of water pollution. Worldwide, 2.5 billion people live without improved sanitation. |
| ● | 18% of the world’s population, or 1.2 billion people (1 out of 3 in rural areas), defecate in the open. Open defecation significantly compromises quality in nearby water bodies and poses an extreme human health risk. |
| ● | Worldwide, infectious diseases such as waterborne diseases are the number one killer of children under five years old and more people die from unsafe water annually than from all forms of violence, including war. |
| ● | Unsafe or inadequate water, sanitation, and hygiene cause approximately 3.1% of all deaths worldwide, and 3.7 percent of disability adjusted life years worldwide. |
| ● | Unsafe water causes four billion cases of diarrhea each year, and results in 2.2 million deaths, mostly of children under five. This means that 15% of child deaths each year are attributable to diarrhea – a child dying every 15 seconds. In India alone, the single largest cause of ill health and death among children is diarrhea, which kills nearly half a million children each year. (See: http://www.pacinst.org/wpcontent/uploads/sites/21/2013/02/water_quality_facts_and_stats3.pdf; and http://www.un.org/waterforlifedecade/quality.shtml http://www.gemstat.org/ |
We believe that MyDx and our Aqua Sensor could be an indispensable tool for individuals and businesses trying to solve water contamination problems. Similar to air quality, water contamination has both local and far-reaching impacts. We believe that MyDx could be used by anyone from a parent testing the water in their own home, to a traveler testing the tap water in a foreign country, to a foreign aid worker trying to ensure that individuals in countries without adequate sanitation are not exposed to drinking contaminated water. As part of our long-term business plan, we believe that the Aqua Sensor could be used on the ground in water filtration and treatment facilities in developing countries that may not have access to a local testing laboratory. As a relatively low-cost and portable water testing device, MyDx paired with our Aqua Sensor, could therefore have a market that stretches around the globe. The Company does not intend to commercialize and offer for the sale the Aqua Sensor during 2015.
Cannabis
In 1996, Oregon and California passed legislation legalizing the possession and use of cannabis for medical purposes. As of 2014, both Colorado and Washington have passed laws allowing for recreational cultivation and use of cannabis among adults aged 21 and over, and over 20 states and the District of Columbia have passed regulations permitting cannabis use in one form or another. There are currently approximately 2.4 million medicinal cannabis users in the United States (See: www.ProCon.org). According to a recent study by Archview Market Research, the United States national legal cannabis market was valued at $1.53 billion in 2013, including all states that had active sale of cannabis to consumers legally allowed to possess cannabis. According to this report, this market is expected to grow to $10.2 billion in five years.
Given the relative youth of the industry, and the lack of federal legalization of either medical or recreational cannabis use, few practical cannabis quality control solutions exist today. Medicinal cannabis patients therefore often have a very difficult time understanding what psychotropic effects they should expect before consuming a particular strain of cannabis, even when the strain is labelled with the percentage concentration of THC or CBD, two common cannabinoids present in the cannabis plant that impact its potency and impact on the user. Both medical cannabis patients and recreational consumers deserve a practical and affordable solution to gain control over what to expect from a given strain before choosing to put it into their body. MyDx, used in conjunction with the app, is designed to enable those individuals to gain control over what they are consuming and to achieve some consistency in how they are feeling or how effectively certain symptoms are being treated. This is accomplished by having the user enter “feeling data” (e.g. anxious, tired, pain relief) into the app and comparing that feeling data to the specific chemical composition of the cannabis ingested. In such manner, a database can be developed enabling MyDx users to be more deliberate as to the strain and chemical features THC, TCB-2, etc.) of the cannabis they choose to procure in the future. MyDx users may also elect to develop and maintain their own personal database within the app.
| -11- |
Competition
Currently, the Company believes there are no portable, consumer-focused, reasonably-priced sensors on the market that can measure chemicals at parts per billion levels, as MyDx’s interchangeable sensors can. The Company is aware that there are companies developing devices that can measure at the parts per hundred or parts per thousand level although, to the Company’s knowledge, none of these products have yet been delivered. The Company is also aware of two hand-held analyzer devices in development which are being marketed to consumers. One is Scio, which plans to use spectrometer technology to externally scan food, medicine and plants. The other one is FOOD sniffer, which is being marketed as the world’s first electronic nose that helps consumers determine the quality of meat, poultry and fish before they eat it, thereby reducing the risk of food poisoning by testing food to confirm that it is safe to consume. In addition, larger gas chromatography units that range from $25,000 to well over $100,000, are available, but we believe these larger units ultimately will not compete for the consumer base to which that the Company plans to market MyDx.
In contrast, the Company intends to bring to market a consumer-focused, parts per billion device for under $700. The Company believes that the combination of a relatively low price point, parts per billion sensitivity, and patentable intellectual property provide barriers to competition. However, companies with far greater resources than the Company could develop competing technologies and products that could impact the market for the Company’s products.
The Company does not plan to commercialize its Aqua and Aero Sensors in 2015 and has not performed a competitive analysis with respect to those planned uses of MyDx.
Research and Development
MyDx is built on technology that has been available for at least a decade, but appears to never have been used at the consumer level. We have filed four United States provisional patent applications and an international patent application citing priority to those United States provisional patent applications is pending. We are developing our intellectual property rights in order to create and protect the MyDx Analyzer with respect to competitors. Our intellectual property is intended to aid us in bringing this technology to consumers in a portable, handheld unit. Moving forward, we expect many other applications of the technology may arise for both consumers and small businesses. For instance, we have had inquiries from the wine industry about analyzing chemicals in wine. We have also had inquiries from various consumer appliance manufacturers for detecting both spoiling foods and when food preparation is complete.
The Company incurred research and development expenses of approximately $1,500,000 in 2014 related to the development of the MyDx Analyzer and the sensors.
Current Sensor Commercialization R&D Status
Currently the Company has operational sensors in two different laboratories, and at its corporate headquarters in San Diego. The other units are newer but are testing with similarly favorable results. The Company is continuing to test its various sensors to ensure that the sensors and overall units are working appropriately.
Analyzing the results from these combined testing and development efforts, the Company is convinced that the MyDx technology is working and will soon be ready for commercial launch. However, until the Company begins to manufacture the products in commercial volumes, there can be no assurance that the development efforts will result in a marketable product that will achieve acceptance and profitable results of operations.
| -12- |
Manufacturing
CDx is using a contract manufacturing model. It is currently evaluating a variety of contract manufacturers who are capable of delivering over 20,000 units per month. The Company intends to sign an agreement with at least one contract manufacturer, and possibly more, if there is sufficient demand for the MyDx product. The Company’s contract manufacturer will not only deliver units, but will also help with design, manufacturing, and testing and review of the MyDx unit and sensor. In addition, the contract manufacturer will be responsible for customer logistics management, packaging, fulfillment and more. Our aim is to initially use contract manufacturers based in California, so that we can have hands-on control of product manufacturing to ensure the MyDx units produced are ready for sale.
Intellectual Property
We strive to protect the proprietary technologies that we believe are important to our business, including seeking and maintaining patent protection intended to cover the device products, their methods of use, related technology and other inventions that are important to our business. We also rely on trade secrets and careful monitoring of our proprietary information to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection.
We believe our success will depend significantly on our ability to (i) obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to our business; (ii) defend and enforce our patents;(iii) maintain our licenses to use intellectual property owned by third parties; (iv) preserve the confidentiality of our trade secrets; and (v) operate without infringing valid and enforceable patents and other proprietary rights of third parties. We also rely on know-how, and continuing technological innovation to develop, strengthen and maintain our proprietary position in the field of handheld sensor technology.
We plan to continue to expand our intellectual property portfolio by filing patent applications directed to handheld sensor technology. We anticipate seeking patent protection in the United States and internationally for compositions of matter, apparatuses, and methods related to handheld sensor technology.
On July 2, 2014, the Company filed assignments of all right, title and interest for four United States provisional patent applications that were recorded with the United States Patent and Trademark Office on July 3, 2014. The United States provisional patent applications assigned to the Company were acquired for consideration that included cash and stock. The Company filed an international patent application with an international filing date of July 14, 2014 that cites priority to the four United States provisional patent applications. The international patent application is currently pending. The Company also has an exclusive option to acquire specific patents with a third party development partner.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a Patent Cooperation Treaty (PCT) application or a non-provisional patent application, subject to any disclaimers or extensions. The term of a patent in the United States can be adjusted and extended due to the failure of the United States Patent and Trademark Office following certain statutory and regulation deadlines for issuing a patent.
The following is a list of the Company’s Trademarks.
AeroDx™
AquaDx™
CannaDx™
MyDx™
Trust & Verify™
| -13- |
Below is a current list of patent applications owned by the Company:
| ● | A PORTABLE CHEMICAL ANALYSIS METHOD BY VOLATILIZATION AND SUBLIMATION. US Patent Application Number 61/846,996; |
| ● | A PORTABLE GAS CHROMATOGRAPHIC ANALYSIS AND DISTRIBUTION SYSTEM. US Patent Application Number 61/864,515; |
| ● | A PORTABLE GAS CHROMATOGRAPHIC ANALYSIS AND DISTRIBUTION SYSTEM FOR THE EXTRACTION AND DELIVERY OF COMPOUNDS IN CANNABIS. US Patent Application Number 61/864,517; |
| ● | Method for Therapeutic Development, Preparation and Administration US Patent application Number 61/927,992; and |
| ● | Apparatus for Detection and Delivery of Volatilized Compounds and Related Methods International Patent application Number PCT/US 14/46500. |
In addition to the applications for patent owned by the Company, the Company has a joint development agreement with Next Dimension Technologies, Inc. (“NDT”) to develop and provide the sensor used in our MyDx devices. NDT was founded in May 2004 and is based in Pasadena, California. The company develops detection solutions for chemical sensing applications using novel sensor and sensor array technologies. NDT’s sensor-array technology allows a single, leveraged platform to address a wide variety of vapor detection markers and applications. NDT holds rights to a broad portfolio of patents covering many aspects of its unique sensor array technology. NDT’s intellectual property gives it significant advantages over traditional sensor technologies and provides significant competitive advantages.
Government Regulation
As of the date of this Current Report, our products require CE Marking (European Conformity), Federal Communications Commission and other government safety approvals. We are working with various partners to secure all approvals. Inasmuch as we are a sensor technology company, except with respect to government regulations related to the cannabis industry described in more detail below, we do not foresee any other probable government regulations on our business outside of normal business practices.
With respect to our Canna Sensor, there is a substantial amount of change occurring in the United States regarding both the medical and recreational use of cannabis, and a number of individual states have enacted state laws to enable distribution, possession, and use of cannabis for medical, and in some cases, recreational purposes. Despite the development of a legal medical or recreational cannabis industry under certain state laws, cannabis use and possession remains illegal under federal law, and such state laws are in conflict with the Federal Controlled Substances Act. The Obama Administration has effectively stated that it is not an efficient use of resources to direct federal law enforcement agencies to prosecute individuals lawfully abiding by state-designated laws allowing for use and distribution of medical and recreational cannabis. However, there is no guarantee that the Obama Administration will not change its stated policy regarding enforcement of federal laws in states where cannabis has been legalized. Further, the change in administration after the 2016 presidential election cycle could introduce a less favorable policy with respect to enforcement of federal laws concerning cannabis.
Also, the possession, use, cultivation, or transfer of cannabis remains illegal under the Federal Controlled Substances Act. Our Canna Sensor may be sold to customers that are engaged in the business of possession, use, cultivation, or transfer of cannabis. As a result, law enforcement authorities regulating the illegal use of cannabis may seek to bring an action or actions against us, including, but not limited, to a claim of aiding and abetting another’s criminal activities. The federal aiding and abetting statute provides that anyone who “commits an offense against the United States or aids, abets, counsels, commands, induces or procures its commission, is punishable as a principal.” 18 U.S.C. §2(a).
| -14- |
Employees
We presently have 15 full and part-time employees, and make extensive use of third-party contractors, consultants, and advisors to perform many of our present activities. We have not entered into any collective bargaining agreements with any of our employees, and we believe our relationships with our employees and any third-party contractors, consultants, and advisors are strong.
Legal Proceedings
We are not aware of any legal proceedings contemplated by any governmental authority or any other party involving us or our properties. As of the date of this Current Report, no director, officer or affiliate is (i) a party adverse to us in any legal proceeding, or (ii) has an adverse interest to us in any legal proceedings. Management is not aware of any other legal proceedings pending or that have been threatened against us or our properties.
Litigation
We are not presently a party to any litigation nor to the knowledge of management is any litigation threatened against us which may materially affect our business or its assets. However, we may become involved with various legal matters arising in the ordinary course of our business that are complex in nature and have outcomes that are difficult to predict.
Reports to Security Holders
Our Internet address is http://www.cdxlife.com. The content on our website is available for information purposes only. It should not be relied upon for investment purposes, nor is it incorporated by reference into this Current Report. The public may read and copy any materials we file with the SEC, including our annual reports, quarterly reports, current reports, proxy statements, information statements and other information, at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549, on official business days during the hours of 10 a.m. to 3 p.m. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at http://www.sec.gov.
| -15- |
RISK FACTORS
An investment in our Company involves a significant level of risk. Investors should carefully consider the risk factors described below together with the other information included in this Current Report on Form 8-K. If any of the risks described below occurs, or if other risks not identified below occur, our business, financial condition, and results of operations could be materially and adversely affected.
Risks Related to our Business
We have a limited operating history and a history of operating losses, and we may not be able to achieve or sustain profitability. In addition, we may be unable to continue as a going concern.
We were incorporated in September 2013 and have only a limited operating history. We are not profitable and have incurred losses since our inception. We continue to incur research and development and general and administrative expenses related to our operations.
We incurred a net loss of approximately $3,528,000 for the year ended December 31, 2014 and a cumulative net loss of approximately $3,530,000 from September 16, 2013 (date of inception) to December 31, 2014.
We expect to continue to incur losses for the foreseeable future, and these losses will likely increase as we continue to commercialize our products. The amount of future losses and when, if ever, we will achieve profitability are uncertain. If our products do not achieve market acceptance, we may never become profitable. The initial cost of completing development of our products and penetrating our anticipated markets will be substantial, and there is no assurance that we will be successful in doing so. We have no revenues to date and have not proven that we have been able to commercialize the product. Additionally, if we are not successful in growing revenues and controlling costs, we will not achieve profitable operations or positive cash flow, and even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Absent a significant increase in revenue or additional equity or debt financing, we may not be able to sustain our ability to continue as a going concern.
We are an early-stage company, and as such, we have no meaningful operating or financial history, we have no products in the marketplace, and we are pre-revenue at this time.
We commenced operations in January 2014. Therefore, there is limited historical financial information upon which to base an evaluation of our performance and future prospects. Due to our lack of operating history, our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in the early-stage of operations, including, without limitation, the following:
| ● | absence of an operating history; |
| ● | absence of any revenues; |
| ● | absence of any products in the marketplace; |
| ● | insufficient capital; |
| ● | expected continual losses for the foreseeable future; |
| ● | no history on which to evaluate our ability to anticipate and adapt to a developing market; |
| ● | uncertainty as to market acceptance of our initial and future products; |
| ● | limited marketing experience and lack of sales organization; and |
| ● | competitive and highly regulated environment. |
| -16- |
Because we are subject to these risks, potential investors may have a difficult time evaluating our business and their investment in our Company. We may be unable to successfully overcome these risks, any of which could irreparably harm our business.
The likelihood of our success must be considered in light of the problems, expenses, difficulties, complications, and delays frequently encountered in connection with a new enterprise, the commercial launch of a new product which still requires testing, and the operation in a competitive industry. We expect to sustain losses in the future as we implement our business plan. There can be no assurance that we will ever generate revenues or operate profitably.
We will require substantial additional funding, which may not be available to us on acceptable terms, or at all.
Our cash balance at December 31, 2014 was approximately $745,000. We may not have adequate funds to fully develop our business, and we may need other capital investment to fully implement our business plans. We also expect that we will need substantial additional capital following this Merger. We do not have any contracts or commitments for additional funding, and there can be no assurance that financing will be available in amounts or on terms acceptable to us, if at all. The inability to obtain additional capital will restrict our ability to grow and may reduce our ability to continue to conduct business operations. If we are unable to obtain additional financing, we will likely be required to curtail our development plans. In that event, current stockholders would likely experience a loss of most or all of their investment. Additional funding that we do obtain may be dilutive to the interests of existing stockholders.
We are dependent on our current management team. If we fail to attract and retain key management personnel, we may be unable to successfully develop or commercialize our products.
In the early stages of development, our business will be significantly dependent on our management team. Our success will be particularly dependent upon Daniel Yazbeck, Thomas Gruber, and Skip Sanzeri. The loss of any of their services could have a material adverse effect on us. We have not obtained key-man insurance on the lives of any members of our management team. Moreover, in order to successfully implement and manage our business plan, we will be dependent upon, among other things, successfully recruiting qualified sales and marketing personnel. Competition for qualified individuals is intense. There can be no assurance that we will be able to find, attract and retain existing employees or that we will be able to find, attract and retain qualified personnel to join the Company on acceptable terms.
We may not be able to attract or retain qualified management and research personnel in the future due to the intense competition for qualified personnel in our industry. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will impede significantly the achievement of our research and development objectives, our ability to raise additional capital and our ability to implement our business strategy. In particular, if we lose any members of our senior management team, we may not be able to find suitable replacements in a timely fashion or at all, and our business may be harmed as a result.
Initially we will be dependent on two products.
Our Aero and Aqua Sensors will not be commercialized in the near future. Therefore, when commercialized, the Canna and Organa Sensors will account for a substantial portion of our revenues for the foreseeable future. As a result, our future operating results are dependent upon market acceptance of those products, neither of which has been commercially launched as of the date hereof. Factors adversely affecting the pricing of, demand for, or market acceptance of the sensors, such as regulatory complications, competition, or technological change, could have a material adverse effect on our business, operating results, and financial condition.
| -17- |
One of our initial product candidates, the Canna Sensor, relates to cannabis, which is a controlled substance under Federal law.
Despite the development of a legal medical or recreational cannabis industry under certain state laws, cannabis use and possession remains illegal under Federal law, and such state laws are in conflict with the Federal Controlled Substances Act. Since one of our initial product candidates, the Canna Sensor, relates to the use of cannabis, it may generate public controversy and be the subject of federal regulation or other action. Political and social pressures and negative publicity could limit or restrict the introduction and marketing of our initial or future product candidates. Adverse publicity from cannabis misuse, adverse side effects from cannabis or other cannabinoid products may harm the commercial success or market penetration achievable by our Canna Sensor. The nature of our Canna Sensor product attracts a high level of public and media interest, and in the event of any resultant negative publicity, our reputation may be harmed.
Laws and regulations affecting the cannabis industry are constantly changing, which could detrimentally affect our business, and we cannot predict the impact that future legislation or changes in enforcement practices may have on our company.
There is a substantial amount of change occurring in the United States regarding both the medical and recreational use of cannabis, and a number of individual states have enacted state laws to enable distribution, possession, and use of cannabis for medical, and in some cases, recreational purposes. The Obama Administration has effectively stated that it is not an efficient use of resources to direct federal law enforcement agencies to prosecute individuals lawfully abiding by state-designated laws allowing for use and distribution of medical and recreational cannabis. However, there is no guarantee that the Obama Administration will not change its stated policy regarding enforcement of federal laws in states where cannabis has been legalized. Further, the change in administration after the 2016 presidential election cycle could introduce a less favorable policy with respect to enforcement of federal laws concerning cannabis.
Future active enforcement of the current federal regulatory position on cannabis on a regional or national basis may directly and adversely affect the willingness of customers to invest in or buy our Canna Sensor, which is used in connection with cannabis. In addition, federal or state legislation could be enacted in the future that could prohibit customers of our Canna Sensor from distributing, possessing, or using cannabis. If such legislation were enacted, customers may discontinue use of our Canna Sensor, our potential source of customers would be reduced, and our revenues may decline. Violation of any federal or state law, or allegations of such violations, could disrupt our business and result in a material adverse effect on our revenues, profitability, and financial condition.
As the possession and use of cannabis is illegal under the Federal Controlled Substances Act, we may be deemed to be aiding and abetting illegal activities through the services that we provide to users, and as such may be subject to enforcement actions which could materially and adversely affect our business
The possession, use, cultivation, or transfer of cannabis remains illegal under the Federal Controlled Substances Act. Our Canna Sensor may be sold to customers that are engaged in the business of possession, use, cultivation, or transfer of cannabis. As a result, law enforcement authorities regulating the illegal use of cannabis may seek to bring an action or actions against us, including, but not limited, to a claim of aiding and abetting another’s criminal activities. The Federal aiding and abetting statute provides that anyone who “commits an offense against the United States or aids, abets, counsels, commands, induces or procures its commission, is punishable as a principal.” 18 U.S.C. §2(a). As a result of such an action, we may be forced to cease operations and our investors could lose their entire investment. Such an action would have a material negative effect on our business and operations.
Due to the use of one of our products, the Canna Sensor, in the cannabis industry, we may have a difficult time obtaining the various insurances that are desired to operate our business, which may expose us to additional risk and financial liabilities.
Insurance that is generally readily available, such as workers compensation, general liability, and directors and officers insurance, may be more difficult for us to find, and more expensive, because our Canna Sensor provides a service to companies and customers in the cannabis industry. There are no guarantees that we will be able to secure such insurances in the future, or that the cost will be affordable to us. If we are forced to go without such insurances, we may be prevented from entering into certain business sectors, our growth may be inhibited, and we may be exposed to additional risks and financial liabilities.
| -18- |
We may not be able to compete effectively against products introduced into our market space.
The industry surrounding handheld consumer analyzers is rapidly evolving, and the market landscape is currently uncertain. However, we expect that as consumers begin to learn and adapt to having handheld analyzer capability, products that will compete with our offerings will rapidly proliferate. These competitive products could have similar applications, perhaps using superior technology, and may provide additional benefits that our sensors do not. We expect that the market could be occupied by larger competitors with greater financial and other resources, which could hinder our market share. We may be forced to modify or alter our business and regulatory strategy, as well as our sales and marketing plans, in response to, among other things, changes in the market, competition, and technological limitations. Such modifications may pose additional delays in achieving our goals.
Because our manufacturing capabilities are limited, we will rely on third parties to manufacture and supply all of our initial products.
Our initial products will manufactured by third parties. If these manufacturing partners are unable to produce our products or component parts in the amounts or on the timeline that we require, the development and initial commercialization of our products may be delayed, depriving us of potential product revenue and resulting in other losses. Our ability to replace any then-existing manufacturer may be difficult because the number of potential manufacturers is limited. It may be difficult or impossible for us to identify and engage a replacement manufacturer on acceptable terms in a timely manner, or at all. If we need to engage a replacement manufacturer but are unable to do so, our business and results of operations could be severely impacted.
Even after our initial product launch, we will depend on third-party suppliers for materials and components for our products.
We will depend on a limited number of third-party suppliers for the materials and components required to manufacture our products. A delay or interruption by our suppliers may harm our business, results of operations, and financial condition, and could also adversely affect our future profit margins. In addition, the lead time needed to establish a relationship with a new supplier can be lengthy, and we may experience delays in meeting demand in the event we must change or add new suppliers. Our dependence on our suppliers exposes us to numerous risks, including but not limited to the following: our suppliers may cease or reduce production or deliveries, raise prices, or renegotiate terms; we may be unable to locate a suitable replacement supplier on acceptable terms or on a timely basis, or at all; and delays caused by supply issues may harm our reputation, frustrate our customers, and cause them to turn to our competitors for future needs.
Our failure to effectively manage growth could impair our business.
Our business strategy envisions a period of rapid growth after our initial product launches, which may put a strain on our administrative and operational resources, and our funding requirements. Our ability to effectively manage growth will require us to successfully expand the capabilities of our operational and management systems, and to attract, train, manage, and retain qualified personnel during our initial product launch and future launches of our other products. There can be no assurance that we will be able to do so, particularly if losses continue and we are unable to obtain sufficient financing. If we are unable to appropriately manage growth, our business, prospects, financial condition, and results of operations could be adversely affected.
We may be subject to product liability claims, and may not have sufficient product liability insurance to cover any such claims, which may expose us to substantial liabilities.
We may be exposed to product liability claims from consumers of our products. It is possible that any product liability insurance coverage we obtain will be insufficient to protect us from future claims. Further, we may not be able to obtain or maintain insurance on acceptable terms or such insurance may be insufficient to cover any potential product liability claim or recall. Failure to obtain or maintain sufficient insurance coverage could have a material adverse effect on our business, prospects, and results of operations if claims are made that exceed our coverage.
| -19- |
We have incurred costs and expect to incur additional costs related to the Merger.
We have incurred, and expect to continue to incur, various non-recurring costs associated with the Merger, including, but not limited to, legal, accounting, and financial advisory fees. The substantial majority of our non-recurring expenses have been composed of these costs and expenses related to the execution of the acquisition
From time to time we may need to license patents, intellectual property, and proprietary technologies from third parties, which may be difficult or expensive to obtain.
We may need to obtain licenses to patents and other proprietary rights held by third parties to successfully develop, manufacture and market our products. As an example, it may be necessary to use a third party’s proprietary technology to reformulate a product in order to create a new type of sensor in response to market demand, or to improve the abilities of our current sensors. If we are unable to timely obtain these licenses on reasonable terms, our ability to commercially exploit our products may be inhibited or prevented.
If we are unable to adequately protect our technology or enforce our intellectual property rights, our business could suffer.
Our success with the products we will develop will depend, in part, on our ability to obtain and maintain patent protection for these products. The coverage claimed in a patent application can be significantly reduced before the patent is issued, and the patent’s scope can be modified after issuance. Furthermore, if patent applications that we file or license are not approved or, if approved, are not upheld in a court of law, our ability to competitively exploit our products would be substantially harmed. Additionally, such patents may or may not provide competitive advantages for their respective products or they may be challenged or circumvented by our competitors, in which case our ability to commercially exploit any related products may be diminished.
We also will rely on trade secret and contractual protections for our unpatented, confidential, and proprietary technology. Trade secrets are difficult to protect. While we will enter into proprietary information agreements with certain of our employees, consultants, and others, these agreements may not successfully protect our trade secrets or other confidential and proprietary information. It is possible that these agreements will be breached, or that they will not be enforceable in every instance, and that we will not have adequate remedies in the case of any such breach. It is also possible that our trade secrets will become known or independently developed by our competitors. If we are unable to adequately protect our technology, trade secrets, or proprietary know-how, or enforce our patents, our business, financial condition, and prospects could suffer.
Intellectual property litigation is increasingly common and increasingly expensive, and may result in restrictions on our business and substantial costs, even if we prevail.
Patent and other intellectual property litigation is becoming more common, and such litigation may be necessary to defend against or assert claims of infringement, to protect trade secrets, to determine the scope and validity of proprietary rights of third parties, or to enforce our patent rights, including those we may license from others. Currently, no third party is asserting that we are infringing upon their patent or other intellectual property rights, nor are we aware or believe that we are infringing or will infringe upon any third party’s patent or other intellectual property rights. We may, however, currently be infringing or infringe in the future upon a third party’s patent or other intellectual property rights. In that event, litigation asserting such claims might be initiated in which we may not prevail, or may not be able to obtain the necessary licenses on reasonable terms, if at all. All such litigation, whether meritorious or not, as well as litigation initiated by us against third parties, is time-consuming and very expensive to defend or prosecute and to resolve. In addition, if we infringe the intellectual property rights of others, we could lose our right to develop, manufacture, or sell our products or could be required to pay monetary damages or royalties to license proprietary rights from third parties. An adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing or selling our products, which could harm our business, financial condition, and prospects.
| -20- |
If our competitors prepare and file patent applications in the United States that claim technology we also claim, we may have to participate in interference or derivation proceedings required by the United States Patent and Trademark Office to determine priority of invention, which could result in substantial costs, even if we ultimately prevail. Results of these proceedings are highly unpredictable and may result in us having to try to obtain licenses in order to continue to develop or market our product candidate.
If our competitors file administrative challenges of our patent applications after grant, we may have to participate in post-grant challenge proceedings, such as oppositions, inter-partes review, post grant review or a derivation proceeding, that challenge our entitlement to an invention or the patentability of one or more claims in our patent applications or issued patents. Such proceedings could result in substantial costs, even if we ultimately prevail. Results of these proceedings are highly unpredictable and may result in us losing proprietary intellectual property rights as claimed in the challenged patents.
We may encounter unanticipated obstacles to execution of our business plan which may cause us to change or abandon our current business plan.
Our business plan may change significantly based on our encountering unanticipated obstacles. Many of our potential business endeavors are capital intensive, and may be subject to statutory or regulatory requirements and other factors that we cannot control, and which could be detrimental to our business plan. We believe that our chosen undertakings and strategies make our plans achievable in light of current economic and legal conditions and with the skills, background, and knowledge of our management team and advisors. We reserve the right to make significant modifications to our stated strategies depending on future events.
Risks Related to Our Common Stock
There is currently a limited public trading market for our common stock and one may never develop.
There currently is a limited public trading market for our securities, and it is not assured that any such public market will develop in the foreseeable future. While this is true of any small cap company, the fact that one of our initial products is a device that will be associated with the use of cannabis, the legal status of which has not been completely resolved at the state level in many states or on the federal level, may make the path to a listing on an exchange or actively traded in the over-the-counter market more problematic. Moreover, there can be no assurance that even if our common stock is approved for listing on an exchange or is quoted in the over-the-counter market in the future, that an active trading market will develop or be sustained. Therefore, we cannot predict the prices at which our common stock will trade in the future, if at all. As a result, our investors may have limited or no ability to liquidate their investments.
Trading in our common stock is conducted on the OTC Markets and on the Over The Counter Bulletin Board (“OTCBB”), as we currently do not meet the initial listing criteria for any registered securities exchange. The OTCBB and OTC Markets are less recognized markets than the registered securities exchanges and is often characterized by low trading volume and significant price fluctuations. These and other factors may further impair our stockholders’ ability to sell their shares when they want to and/or could depress our stock price. As a result, stockholders could find it difficult to dispose of, or obtain accurate quotations of the price of our securities because smaller quantities of shares could be bought and sold, transactions could be delayed and security analyst and news coverage of our Company may be limited. If a public market for our common stock does develop, these factors could result in lower prices and larger spreads in the bid and ask prices for our shares of common stock.
The market price of our common stock may be highly volatile and such volatility could cause you to lose some or all of your investment.
The market price of our common stock may fluctuate significantly in response to numerous factors, some of which are beyond our control, such as:
| ● | the announcement of new products or product enhancements by us or our competitors; |
| ● | developments concerning intellectual property rights; |
| -21- |
| ● | changes in legal, regulatory, and enforcement frameworks impacting our products; |
| ● | variations in our and our competitors’ results of operations; |
| ● | fluctuations in earnings estimates or recommendations by securities analysts, if our common stock is covered by analysts; |
| ● | the results of product liability or intellectual property lawsuits; |
| ● | future issuances of common stock or other securities; |
| ● | the addition or departure of key personnel; |
| ● | announcements by us or our competitors of acquisitions, investments or strategic alliances; and |
| ● | general market conditions and other factors, including factors unrelated to our operating performance. |
Further, the stock market has recently experienced extreme price and volume fluctuations. The volatility of our common stock could be further exacerbated due to low trading volume. Continued market fluctuations could result in extreme volatility in the price of our common stock, which could cause a decline in the value of our common stock and the loss of some or all of our investors’ investment.
Some or all of the “restricted” shares of our common stock held by our stockholders, including, but not limited to, shares issued in connection with: (i) our incorporation in 2013 and (ii) our 2014 private placements may be offered from time to time in the open market pursuant to an effective registration statement under the Securities Act, or without registration pursuant to Rule 144 promulgated thereunder, and these sales may have a depressive effect on the market price of our common stock.
Because our common stock may be a “penny stock,” it may be more difficult for investors to sell shares of our common stock, and the market price of our common stock may be adversely affected.
Our common stock may be a “penny stock” if, among other things, the stock price is below $5.00 per share, it is not listed on a national securities exchange, or it has not met certain net tangible asset or average revenue requirements. Broker-dealers who sell penny stocks must provide purchasers of these stocks with a standardized risk-disclosure document prepared by the SEC. This risk-disclosure document provides information about penny stocks and the nature and level of risks involved in investing in the penny-stock market. A broker must also give a purchaser, orally or in writing, bid and offer quotations and information regarding broker and salesperson compensation, make a written determination that the penny stock is a suitable investment for the purchaser and obtain the purchaser’s written agreement to the purchase. Broker-dealers must also provide customers that hold penny stock in their accounts with such broker-dealer a monthly statement containing price and market information relating to the penny stock. If a penny stock is sold to an investor in violation of the penny stock rules, the investor may be able to cancel its purchase and get their money back.
If applicable, the penny stock rules may make it difficult for stockholders to sell their shares of our common stock. Because of the rules and restrictions applicable to a penny stock, there is less trading in penny stocks and the market price of our common stock may be adversely affected. Also, many brokers choose not to participate in penny stock transactions. Accordingly, stockholders may not always be able to resell their shares of our common stock publicly at times and prices that they feel are appropriate.
Because we became public by means of a reverse merger, we may not be able to attract the attention of brokerage firms.
Additional risks may exist because we became public through a “reverse merger.” Securities analysts of brokerage firms may not provide coverage of our Company since there is little incentive for brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct secondary offerings on our behalf in the future.
| -22- |
Compliance with the reporting requirements of federal securities laws can be expensive.
We are a public reporting company in the United States, and accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, and the compliance obligations of the Sarbanes-Oxley Act of 2002. The costs of preparing and filing annual and quarterly reports and other information with the SEC and furnishing audited reports to stockholders are substantial. If we do not provide current information about our Company to market makers, they will not be able to trade our stock. Failure to comply with the applicable securities laws could result in private or governmental legal action against us or our officers and directors, which could have a detrimental impact on our business and financials, the value of our stock, and the ability of stockholders to resell their stock.
Our investors’ ownership in the Company may be diluted in the future.
In the future, we may issue additional authorized but previously unissued equity securities, resulting in the dilution of ownership interests of our present stockholders. We expect to need to issue a substantial number of shares of common stock or other securities convertible into or exercisable for common stock in connection with hiring or retaining employees, future acquisitions, raising additional capital in the future to fund our operations, and other business purposes. We expect to authorize an additional three million shares of common stock for issuance under the 2014 Equity Incentive Plan in connection with the Merger, and plan to issue equity awards to management and employees under the 2014 Equity Incentive Plan. Additional shares of common stock issued by us in the future will dilute an investor’s investment in the Company.
Directors, executive officers, principal stockholders and affiliated entities own a significant percentage of our capital stock, and they may make decisions that our stockholders do not consider to be in their best interests.
As of the date of this Current Report, our directors, executive officers, principal stockholders and affiliated entities beneficially own, in the aggregate, approximately 48% of our outstanding voting securities as of the date hereof. Daniel Yazbeck, the Chief Executive Officer and a director of the Company, is the beneficial owner of approximately 45% of our outstanding voting securities as of the date hereof. As a result, if some or all of them acted together, they would have the ability to exert substantial influence over the election of our board of directors and the outcome of issues requiring approval by our stockholders. This concentration of ownership may also have the effect of delaying or preventing a change in control of our Company that may be favored by other stockholders. This could prevent transactions in which stockholders might otherwise recover a premium for their shares over current market prices. This concentration of ownership and influence in management and board decision-making could also harm the price of our capital stock by, among other things, discouraging a potential acquirer from seeking to acquire shares of our capital stock (whether by making a tender offer or otherwise) or otherwise attempting to obtain control of our Company.
Our board of directors has historically had significant control over us and we have yet to establish committees comprised of independent directors.
Until March 26, 2015, CDx only had three directors. Because of such limited number of directors, each of our board members had significant control over all corporate issues. In addition, two of our three directors also held officer positions in CDx. We could not establish board committees comprised of independent members, and we did not have an audit or compensation committee comprised of independent directors. Our three directors performed these functions, despite not all being independent directors. Thus, there was potential conflict in that two of our directors were also engaged in management and participated in decisions concerning management compensation and audit issues that may affect management and CDx performance.
On March 27, 2015, CDx elected four additional independent directors including an Audit Committee Chairman. (see following section on Directors and Executive Officer for additional information on all officers and directors).
| -23- |
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or detect fraud. Consequently, investors could lose confidence in our financial reporting and this may decrease the trading price of our stock.
We must maintain effective internal controls to provide reliable financial reports and to detect and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as would be possible with an effective control system in place. We have not performed an in-depth analysis to determine if historical undiscovered failures of internal controls exist, and may in the future discover areas of our internal control that need improvement.
We have been assessing our internal controls to identify areas that need improvement. We are in the process of implementing changes to internal controls, but have not yet completed implementing these changes. Failure to implement these changes to our internal controls or any others that it identifies as necessary to maintain an effective system of internal controls could harm our operating results and cause investors to lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the trading price of our common stock.
For the year ended December 31, 2014, our auditors identified a material weakness in our internal controls over financial reporting due to the Company not maintaining a sufficient complement of personnel with an appropriate level of accounting knowledge and experience in the application of accounting for warrants to purchase common and preferred stock issued in connection with convertible notes payable and convertible preferred stock and accounting for non-employee stock options. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company's annual or interim financial statements will not be prevented or detected on a timely basis. If the Company does not address the material weaknesses, we may not be able to manage our business as effectively as would be possible with an effective control system in place.
| -24- |
MANAGEMENT DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with, and is qualified in its entirety by, CDx’s audited annual financial statements and the related notes thereto and CDx’s unaudited interim financial statements and the related notes thereto, each of which appear elsewhere in this Current Report on Form 8-K. This discussion contains certain forward-looking statements that involve risks and uncertainties, as described under the heading “Forward-Looking Statements” in this Current Report on Form 8-K. Actual results could differ materially from those projected in the forward-looking statements. For additional information regarding these risks and uncertainties, please see the disclosure under the heading “Risk Factors” elsewhere in this Current Report on Form 8-K. The Management Discussion and Analysis of Financial Condition and Results of Operations below is based upon only the financial performance of CDx.
For information regarding the financial results of Brista, you should refer to Brista’s Annual Report on Form 10-K for the year ended July 31, 2014, filed with the SEC on September 29, 2014, and its Quarterly Report on Form 10-Q for the quarter ended January 31, 2015, filed with the SEC on March 13, 2015, and the financial statements of Brista which are filed as Exhibit 99.2 of this Current Report.
Overview
CDx (referred to in this discussion and analysis set forth below as “CDx,” “we,” us,” or “our”) is a Delaware Corporation formed on September 16, 2013. CDx is an early-stage science and technology company. We have developed and are commercializing technology and devices to accurately measure chemicals of interest in any solid, liquid, or gas sample. Our mission is to enable people to live a healthier life by revealing the purity of certain compounds they eat, drink and inhale in real time through a device they can hold in the palm of their hand. We provide a quick, easy and affordable way for consumers to test the safety and composition of what they consume. We believe that the broad application and ease of use of our technology put us in an ideal position to provide consumers with a practical and affordable way to trust and verify what they are putting into their bodies without leaving the comfort of their homes.
Our foundational proprietary technology derives from research developed at the California Institute of Technology, Pasadena, California, for the Jet Propulsion Laboratory, used by NASA as well as an additional project funded by the Bill & Melinda Gates Foundation for other exploratory research and medical applications. We have a portfolio of intellectual property rights covering principles and enabling instrumentation of chemical sensing technology across solid, liquid, and gas samples. We believe that our portfolio of intellectual property rights provides us with a strong and defensible market position from which to commercialize our portable chemical sensing and analyzing technology and to build our business by expanding our core technology across a variety of applications.
Recent Events
CDx Bridge Loan
In August, September and October 2014 CDx issued convertible subordinated promissory notes (the “Notes”) bearing interest at 8% per annum in an aggregate amount of $2,000,000 (the “2014 Bridge Financing”). For every $4.00 in face value of the Notes issued in the 2014 Bridge Financing, CDx issued corresponding non-callable, five year warrants to purchase one share of its Common Stock, at an exercise price of $1.10 per share (the “Warrants”). CDx issued an aggregate of $2,000,000 of such Notes and corresponding Warrants. The outstanding Notes and accrued unpaid interest automatically converted into 1,882,282 shares of CDx Series B convertible preferred stock on February 12, 2015. The Warrants were assumed by the Company pursuant to the terms of the Merger.
Series B Preferred Stock Financing
Prior to the completion of the Merger, CDx completed the sale of Series B convertible preferred stock and corresponding, non-callable five year Series B preferred stock Purchase Warrants at an exercise price of $1.10 per share (together with the Series B convertible preferred stock, the “Series B Units”) in an aggregate amount of up to $5,000,000 (the “Series B Convertible Preferred Stock Financing”). CDx sold an aggregate of $4,812,518 of Series B Units. Each one share of CDx Series B convertible preferred stock was converted into one share of CDx common stock immediately prior to the Merger. Pursuant to the terms of the Merger, each one share of CDx common stock will be exchanged for one share of Company common stock. Pursuant to the terms of the Merger, the Warrants have been assumed by the Company.
| -25- |
Registration Rights
All of the securities issued in connection with the Series B Convertible Preferred Stock Financing are “restricted securities” and, as such, are subject to all applicable restrictions specified by federal and state securities laws.
On the closing dates of the 2014 Bridge Financing and Series B Convertible Preferred Stock Financing, we entered into a registration rights agreement with the investors in the respective offering. Under the terms of the registration rights agreements, we have committed to file a registration statement covering the resale of the common stock underlying the 2014 Bridge Financing Notes and Series B Units within 60 days after completion of the Merger, and shall use commercially reasonable efforts to cause the registration statement to become effective no later than 60 days after it is filed.
We have agreed to use commercially reasonable efforts to maintain the effectiveness of the registration statement until there are no longer registerable securities outstanding, but in no case longer than 36 months after the effectiveness of the registration statement, provided that if Rule 144(i)(1) applies to the Company, the requirement to maintain an effective registration statement will be extended to 60 months after effectiveness.
The above description of the registration rights agreement does not purport to be a complete description of the rights and obligations of the parties thereunder, and such description is qualified in their entirety by reference to the form of registration rights agreement which is filed as an exhibit to this Current Report on Form 8-K.
Merger
On April 30, 2015, pursuant to the Merger Agreement, Merger Sub and CDx consummated the Merger, and CDx became a wholly owned subsidiary of Brista.
Pursuant to the Merger Agreement, upon consummation of the Merger, each share of CDx’s capital stock issued and outstanding immediately prior to the Merger was converted into the right to receive one (1) share of Brista’s common stock, par value $0.001 per share (the “Common Stock”). Additionally, pursuant to the Merger Agreement, upon consummation of the Merger, Brista assumed all of CDx’s options and warrants issued and outstanding immediately prior to the Merger, which are now exercisable for approximately 1,902,173 and 7,571,000 shares of common stock, respectively as of the date of the Merger. Prior to and as a condition to the closing of the Merger, each then-current Brista stockholder agreed to sell certain shares of common stock held by such holder to Brista and the then-current Brista stockholders retained an aggregate of 1,990,637 shares of common stock. Therefore, following the Merger, CDx’s former stockholders now hold 19,484,615 shares of Brista common stock which is approximately 91% of the Company Common Stock outstanding.
The foregoing description of the Merger Agreement is only a summary and is qualified in its entirety by reference to the complete text of the Merger Agreement which is filed as Exhibit 2.1 to this Current Report on Form 8-K, and which is incorporated by reference herein.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations set forth below under the headings “Results of Operations” and “Liquidity and Capital Resources” have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and should be read in conjunction with our financial statements and notes thereto appearing elsewhere in this Current Report Form 8-K. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our critical accounting policies and estimates, including stock-based compensation and long-lived assets. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. A more detailed discussion on the application of these and other accounting policies can be found in Note 3 of the Notes to Financial Statements set forth in our financial statements as of and for the year ended December 31, 2014, which is attached as Exhibit 99.1 to this Current Report on Form 8-K. Actual results may differ from these estimates under different assumptions and conditions.
| -26- |
While all accounting policies impact the financial statements, certain policies may be viewed as critical. Critical accounting policies are those that are both most important to the portrayal of financial condition and results of operations and that require management’s most subjective or complex judgments and estimates. Our management believes the policies that fall within this category are the policies on accounting for stock-based compensation, intellectual property and long-lived assets.
Accounting for Stock-Based Compensation
We recognize as expense, the grant-date fair value of stock options and other stock-based compensation issued to our employees and non-employee directors over the requisite service periods, which are typically the vesting periods. CDx uses the Black-Scholes-Merton option pricing model to estimate the fair value of its stock-based payments. The volatility assumption used in the Black-Scholes-Merton option pricing model is based on the calculated historical volatility derived from an analysis of reported data for a peer group of companies. The expected term of options granted by us has been determined based upon the simplified method, because we do not have sufficient historical information regarding our options to derive the expected term. Under this approach, the expected term is the mid-point between the weighted-average of each vesting period and the contractual term. The risk-free interest rate is based on U.S. Treasury rates whose term is consistent with the expected life of the stock options. CDx has not paid cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero.
Long-Lived Assets
We review our long-lived assets including property and equipment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine the recoverability of our long-lived assets, we evaluate the probability that future estimated undiscounted net cash flows will be less than the carrying amount of the assets. If such estimated cash flows are less than the carrying amount of the long-lived assets, then such assets are written down to their fair value. Our estimates of anticipated cash flows and the remaining estimated useful lives of long-lived assets could be reduced in the future, resulting in a reduction to the carrying amount of long-lived assets.
Results of Operations
Results of Operations - For the Year Ended December 31, 2014
Revenue
From September 16, 2013 (date of inception) through December 31, 2014, the Company has not had any revenues. The Company is in the research and development stage, but projects its MyDx product will be released in 2015.
Operating Expenses
Research and development (“R&D”) expenses primarily consist of engineering and product development, incurred in the design, development, testing and enhancement of our products. For the year ended December 31, 2014, the Company expended approximately $1,539,000 for various research and development projects for hardware, database, software and sensor development.
| -27- |
Sales and marketing expenses include costs for sales and marketing personnel, travel, demonstration product, market development, tradeshows, marketing and consulting expenses. For the year ended December 31, 2014, the Company expended approximately $750,000 for sales and marketing activities.
General and administrative expenses (“G&A”) include costs for administrative personnel, facility-related costs, insurance, legal and accounting services, amortization of intellectual property, and depreciation expense. G&A costs and expenses were approximately $1,010,000 for the year ended December 31, 2014.
Non-Operating Expenses
Interest expense was approximately $227,500 for the year ended December 31, 2014. Interest expense was primarily due to the amortization of debt issuance costs and the accrual of interest on the convertible notes payable issued in 2014.
Net Loss
We incurred a loss of approximately $3,528,000 for the year ended December 31, 2014, and had an accumulated deficit of approximately $3,530,000 as of December 31, 2014 for the reasons cited above.
Liquidity
As of December 31, 2014, we had cash and cash equivalents of approximately $745,000. Our cash and cash equivalents increased by approximately $745,000 during the year ended December 31, 2014. As of December 31, 2013, we had cash of $150.
Operating Activities
For the year ended December 31, 2014, our operating activities used cash in the amount of $1,824,000 which is was caused primarily by our net loss of $3,528,000. The effect of the net loss was offset by increases in accounts payable, accrued liabilities and customer deposits as well as non-cash adjustments for services rendered in exchange for convertible notes and common stock and stock-based compensation.
Investing Activities
For the year ended December 31, 2014 cash used in investing activities totaled $121,000 which resulted from purchases of equipment and website development costs.
Financing Activities
For the year ended December 31, 2014, financing activities provided $2,690,000 in cash primarily from proceeds received from the issuance of $1,612,000 in convertible notes and $1,077,000 in preferred stock.
Sources of Liquidity and Capital
During 2014, CDx issued convertible notes for gross proceeds of $2,000,000 and issued 1,620,000 shares Series A convertible preferred for gross proceeds of $810,000. In addition, in November and December 2014, the Company issued 547,725 shares of Series B convertible preferred stock for gross proceeds of $657,500. The capital raised will be primarily used for continuation of the Company’s R&D efforts and to support its operations. As of December 31, 2014, the Company had cash of approximately $745,000 with $3,093,000 in current liabilities. Based upon the Company’s cash position as of December 31, 2014 and the proceeds received from the issuance of Series B convertible preferred stock in February 2015, we anticipate that the Company will be able to fund its liquidity needs for the next year after which the Company will need to commercialize its products and generate revenue and positive cash flow in order to continue its operations. The foregoing assumptions may prove to be wrong, and we may be required to use our available cash resources and seek additional financing sooner than anticipated. As a result of CDx’s significant operating expenditures and the lack of any significant product sales revenue, we expect to incur losses from operations for the near future.
| -28- |
To date, CDx has funded its operations primarily with proceeds from the issuance of common and convertible preferred stock and the incurrence of convertible debt. To the extent we raise additional capital by issuing equity securities or obtaining borrowings convertible into equity, ownership dilution to existing stockholders will result and future investors may be granted rights superior to those of existing stockholders. The incurrence of indebtedness or debt financing would result in increased fixed obligations and could also result in covenants that would restrict our operations. Our ability to obtain additional capital may depend on prevailing economic conditions and financial, business and other factors beyond our control. Economic crisis and disruptions in the U.S. and global financial markets may adversely impact the availability and cost of credit, as well as our ability to raise money in the capital markets. Instability in these market conditions may limit our ability to access the capital necessary to fund and grow our business.
Sources of Liquidity and Capital
The following paragraphs address our liquidity as a combined company.
As reported in this Current Report on Form 8-K, on April 30, 2015, Brista Corp. acquired CDx pursuant to the Merger. Prior to the Merger, CDx sold notes with an aggregate principal amount of $2,000,000 and associated warrants to purchase common stock at $1.10 per share. In addition, in November, 2014 thru February, 2015, investors purchased 4,402,272 Units, each consisting of one share of Series B convertible preferred stock and a warrant to purchase one share of Series B preferred stock at $1.10 per share, in a private placement at a price of $1.10 per unit, with net proceeds to CDx of approximately $4,213,000. The capital raised will be primarily used for the continuation of the combined company’s R&D efforts and to support its operations. As of March 31, 2015, the combined company had remaining cash of approximately $2,700,000 with $1,100,000 in current liabilities. Based upon the combined company’s current cash position and by monitoring its discretionary expenditures, we anticipate that the combined company will be able to fund its liquidity needs for the next year after which the combined company will need to raise additional funds in order to continue its operations. The foregoing on assumptions may prove to be wrong, and we may be required to use our available cash resources and seek additional financing sooner than anticipated. As a result of CDx’s and Brista Corp.’s combined significant operating expenditures and the lack of any significant product sales revenue, we expect to incur losses from operations for the foreseeable future.
To date, CDx and Brista Corp. have funded their operations primarily with proceeds from the private placement of common and preferred stock and the incurrence of debt. To the extent we raise additional capital by issuing equity securities or obtaining borrowings convertible into equity, ownership dilution to existing stockholders will result and future investors may be granted rights superior to those of existing stockholders. The incurrence of indebtedness or debt financing would result in increased fixed obligations and could also result in covenants that would restrict our operations. Our ability to obtain additional capital may depend on prevailing economic conditions and financial, business and other factors beyond our control. Economic crisis and disruptions in the U.S. and global financial markets may adversely impact the availability and cost of credit, as well as our ability to raise money in the capital markets. Instability in these market conditions may limit our ability to access the capital necessary to fund and grow our business.
Results of Operations - For the Year Ended December 31, 2013
Revenue
From September 16, 2013 through December 31, 2013, the Company had no revenues. The Company is in the research and development stage, but projects its MyDx product will be released in 2015.
Operating Expenses
G&A costs and expenses were $1,879 for the year ended December 31, 2013.
| -29- |
Net Loss
The Company incurred a net loss of $1,879 for the year ended December 31, 2013 for the reasons cited above.
Liquidity and Capital Resources
Our principal sources of cash have been proceeds from private placements of common and convertible preferred stock, incurrence of convertible debt.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as defined in Item 303(a)(4) of Regulation S-K.
Contractual Obligations
Below is a table of CDx’s contractual obligations as of December 31, 2014:
| Contractual obligations | Payments due by period | |||||||||||||||||||
| Total | Less than 1 year | 1 - 3 years | 3 - 5 years | More than 5 years | ||||||||||||||||
| Notes payable | $ | 1,974,058 | $ | 1,974,058 | $ | - | $ | - | $ | - | ||||||||||
| Total | $ | 1,974,058 | $ | 1,974,058 | $ | - | $ | - | $ | - | ||||||||||
| -30- |
DESCRIPTION OF PROPERTIES
The Company owns no properties and leases several properties. The Company leases its principal corporate offices which are located at 4225 Executive Square Suite 600, La Jolla, California. These offices consist of approximately 400 square feet, on a month-to-month basis, that commenced on May 2014. The monthly rent for these offices is $6,800.
On April 1, 2015, the Company signed a 31 month lease for approximately 6,200 square feet of office and laboratory space at 6335 Ferris Square, Suite B, San Diego, California. The facility includes approximately 1,500 square feet of laboratory space. Commencement date of the lease is May 1, 2015. Total net rent under this lease is $247,000 and expires on November 30, 2017.
The Company also leases three sales offices located in San Mateo, California. The offices are approximately 150 square feet; 200 square feet and 350 square feet. The leases are all on a month-to-month basis. The aggregate monthly rent for these offices is approximately $2,650.
The Company believes its leased office and warehouse facilities are adequate for its current needs.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security ownership of certain beneficial owners and management
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of April 30, 2015 for:
| ● | each of our directors and nominees for director; |
| ● | each of our named executive officers; |
| ● | all of our current directors and executive officers as a group; and |
| ● | each person, entity or group, who beneficially owned more than 5% of each of our classes of securities. |
We have based our calculations of the percentage of beneficial ownership on 21,475,252 shares of our common stock. We have deemed shares of our common stock subject to stock options that are currently exercisable within 60 days of April 30, 2015 to be outstanding and to be beneficially owned by the person holding the stock option or restricted stock unit for the purpose of computing the percentage ownership of that person. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
Unless otherwise indicated, the mailing address of each beneficial owner is c/o CDx, Inc., 4225 Executive Square, Suite 600, La Jolla, California. The information provided in the table is based on our records, information filed with the SEC, and information provided to us, except where otherwise noted.
| -31- |
| Name | Position | Number of Shares Beneficially Owned | Percentage of Common Stock Shares Beneficially Owned | |||||||||
| Daniel Yazbeck (1) | Chief Executive Officer | 10,351,042 | 44.28 | % | ||||||||
| Samuel Sanzeri (2) | President | 438,434 | 1.88 | % | ||||||||
| Thomas Gruber (3) | Chief Financial Officer and Chief Operating Officer | 265,000 | 1.13 | % | ||||||||
| Steven Katz (4) | Director | 53,542 | * | |||||||||
| George Jackoboice (5) | Director | 120,833 | * | |||||||||
| Edward Roffman (6) | Director | 20,833 | * | |||||||||
| Federico Pier (7) | Director | 20,833 | * | |||||||||
| Michael Harris (8) | Director | 20,833 | * | |||||||||
| All Executives as a Group (8 persons) (9) | 11,291,350 | 48.30 | % | |||||||||
| 5% Stockholders | ||||||||||||
| Daniel Yazbeck | 44.28 | % | ||||||||||
| * | Represents beneficial ownership of less than 1% of the outstanding shares of our common stock. |
| (1) | Consists of 10,000,000 shares held by Mr. Yazbeck and YCIG, Inc. and 351,042 shares issuable upon the exercise of outstanding equity awards exercisable within 60 days of April 30, 2015. |
| (2) | Consists of 14,100 shares of common stock and 424,334 shares issuable upon the exercise of outstanding equity awards exercisable within 60 days of April 30, 2015. |
| (3) | Consists of 10,000 shares of common stock and 255,000 shares issuable upon the exercise of outstanding equity awards exercisable within 60 days of April 30, 2015. |
| (4) | Consists of 53,542 shares issuable upon the exercise of outstanding equity awards exercisable within 60 days April 30, 2015. |
| (5) | Consists of 100,000 shares of common stock and 20,833 shares issuable upon the exercise of outstanding equity awards exercisable within 60 days April 30, 2015. |
| (6) | Consists of 20,833 shares issuable upon the exercise of outstanding equity awards exercisable within 60 days April 30, 2015. |
| (7) | Consists of 20,833 shares issuable upon the exercise of outstanding equity awards exercisable within 60 days April 30, 2015. |
| (8) | Consists of 20,833 shares issuable upon the exercise of outstanding equity awards exercisable within 60 days April 30, 2015. |
| (9) | Consists of (i) 10,124,100 shares beneficially owned by the Company’s directors and officers; and (ii) 1,167,250 shares issuable upon the exercise of outstanding options exercisable within 60 days of April 30, 2015. |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The table below sets certain information concerning our executive officers and directors as of the closing of the Merger, including their names, ages, anticipated positions with us. Our executive officers are chosen by our Board and hold their respective offices until their resignation or earlier removal by the Board.
In accordance with our certificate of incorporation, incumbent directors are elected to serve until our next annual meeting and until each director’s successor is duly elected and qualified.
| Name | Age | Position | ||
| Daniel Yazbeck | 37 | Chief Executive Officer, Director (Chairman of the Board) | ||
| Thomas Gruber | 70 | Chief Financial Officer, Chief Operating Officer, Director, Secretary | ||
| Stephen M. Katz | 61 | Director | ||
| Edward Roffman | 65 | Director and Audit Committee Chairman | ||
| Federico Pier | 47 | Director | ||
| George A. Jackoboice, Jr | 47 | Director | ||
| Michael J. Harris | 61 | Director | ||
| Samuel Sanzeri | 53 | President |
| -32- |
Directors
The following information pertains to the members of our Board effective as of the closing of the Merger, their principal occupations and other public company directorships for at least the last five years and information regarding their specific experiences, qualifications, attributes and skills:
Daniel Yazbeck – Chief Executive Officer, Director (Chairman of the Board)
Mr. Yazbeck became the Chief Executive Officer and a director of CDx in September 2013. Mr. Yazbeck has substantial experience in new market and business development, strategic partnering and negotiations from his tenure in top 50 Fortune 500 companies. Mr. Yazbeck also has an extensive scientific and technical engineering background, having invented, patented, secured resources for, managed, developed, and commercialized several successful pharmaceutical and healthcare related market products from conception to implementation.
Mr. Yazbeck joined Pfizer, Inc. in January 2002 as a scientist in their pharmaceuticals group where he specialized in chemical research and development technologies, including analytics. While at Pfizer, Mr. Yazbeck participated in creating a global center of technology for Pfizer in the field of biocatalysis, developing multiple patents issued in his name and authored a variety of research papers. After leaving Pfizer, Mr. Yazbeck joined Panasonic Corporation of North America in March 2005, spearheading their new market, business and strategic product development activities in the consumer electronics healthcare field. Mr. Yazbeck worked on many advanced Panasonic projects in the biotechnology and healthcare space, again creating multiple patents in his name.
Mr. Yazbeck founded the Yazbeck Consulting & Investment Group (YCIG, Inc.) in October of 2008. YCIG, Inc. seeded the capital, R&D, market development, legal and human resource investments required to create CDx, Inc in September 2013.
Mr. Yazbeck graduated with honors from McGill University in Canada in 2001, holds a Master’s Degree in Medicinal Chemistry, with a minor in Marketing Management, and served as a research/teaching assistant for 4 years prior to graduating and joining Pfizer.
Thomas Gruber – Chief Financial Officer, Chief Operations Officer, Director and Secretary
Mr. Gruber joined CDx as Chief Financial Officer in June 2014, appointed a director in July 2014 and Chief Operating Officer in February, 2015. He brings more than 20 years of financial and senior executive management experience to the company from publicly traded high-technology companies. Before joining the Company, Mr. Gruber served as US Corporate Controller for ATEN Technologies, Inc. from August 2013 to June 2014; Managing Partner for TLG Investments from April 2013 to August 2013; Chief Financial Officer and Chief Operating Officer for I/OMagic Corporation from November 2006 until March 2013; President and Chief Financial Officer for nStor Technologies, Inc. from January 2001 until October 2006; and, prior to that, as Chief Financial Officer for Western Digital Corporation.
Mr. Gruber received a Bachelor’s of Business Administration degree from Ohio University and a Master of Business Administration degree from Pepperdine University. He also earned a CPA certification.
Stephen M. Katz – Director
Dr. Stephen M. Katz became a director of CDx in July 2014. Dr. Katz is the founder and owner of the Bronx Veterinary Center, the largest full service veterinary hospital in the Bronx, NY, which he founded in 1994. Dr. Katz is currently serving in his second term as New York State Assemblyman, 94th District. Dr. Katz founded Enceladus Enterprises, LLC in 2012. As an elected official, Dr. Katz has been instrumental in writing and passing the New York State medical cannabis bill into law.
| -33- |
Dr. Katz obtained his Bachelors of Science degree from Cornell University and his Doctor of Veterinary Medicine from the University of Pennsylvania.
Edward Roffman - Director and Audit Committee Chairman
Mr. Roffman has over 40 years’ experience in accounting and finance. He has been a management consultant since April 2006 and is a principal of Creekside, LLC. In April 2014, Ed co-founded LERNA, LLC and serves as the company’s Chief Financial Officer. In January 2014, Ed co-founded AdSource, LLC and serves as their Chief Financial Officer. From January 2012, Mr. Roffman has served as Chief Financial Officer of Public Media Works, Inc. From January 2012 to June 2014, Ed Served as Chief Financial Officer of Emerge Digital, Inc. From January, 2008 to December 2009, Mr. Roffman served as Chief Financial Officer for Cryptic Studios. Mr. Roffman is also on the board of directors of XRpro Sciences, Inc. and Andalay Solar.
Mr. Roffman is a Certified Public Accountant and earned a Bachelor’s of Business Administration from Temple University. Mr. Roffman has served on the Board of Directors and Audit Committees of Andalay Solar, Inc. and XRPro Sciences, Inc.
Federico Pier - Director
Mr. Pier has more than 20 years of experience in the commercial and private banking sectors.
In May 2014, Mr. Pier has Abacus Asset Management, Inc. and serves as Managing Director. Abacus provides investment management advice to financial institutions. From November 2007 to May 2014, Mr. Pier was a senior director at Oppenheimer and Company Inc., providing institutional fixed income sales and coverage of middle-market fixed income accounts. Prior to joining Oppenheimer and Company, Fred held positions with Bear Stearns, Prudential Securities, and Sharp Capital.
Mr. Pier received a Bachelor’s degree from the University of North Texas and a Master’s of Business Administration from the Graduate School of Management at the University of Dallas.
George A. Jackoboice, Jr. - Director
Mr. Jackoboice has more than 20 years of senior management and portfolio company investment experience. Since 1998, Mr. Jackoboice has managed a portfolio of manufacturing, medical device, real estate and technology investments. From 1998 to 2007 Mr. Jackoboice was Chief Financial officer for Monarch Holdings, Inc. and President of Monarch Hydraulics business operations in China, Canada and the United Kingdom. Since 1992, Mr. Jackoboice worked for the Wing Group, a private power development company and established the Wing Group’s Beijing office in 1994. In 2004, Mr. Jackoboice cofounded and serves on the investment committee of Bridge Street Capital Partners, a private equity fund.
Mr. Jackoboice serves a Board Member for Cardiatis SA (Belgium), Artic Equipment Manufacturing (Canada), Courtyard Investments, Ltd. (China) Monarch Holdings, Inc. (USA) Toad Medical Corporation (USA) Clikthrough, Inc. (USA) and Health Leads (USA). George earned a Bachelor’s of Art degree from Dennison University and a Master’s of Business Administration from Harvard business school.
Michael J. Harris - Director
Mr. Harris has more than thirty-five years’ experience in various management positions with both domestic and international companies. Since 2007, Mr. Harris has served as President of The Dyson-Kissner-Moran Corporation. From 2001 to 2007, Mr. Harris was President and Chief Executive Officer of Thetford Corporation. Mr. Harris began his career in 1982 with Crystal Communications.
Mr. Harris has served on the Board of Directors for the Recreational Vehicle Industry Association, Lighthouse for the Blind, the Mid-Hudson Civic Center and the Dutchess County Arts Council. He is currently Secretary/Treasurer of the Millman Harris Romano Foundation, Poughkeepsie, New York.
| -34- |
Mr. Harris attended New York State University at New Paltz, New York.
In addition to the information above regarding each director’s business experience and service on the board of directors of other companies, our board of directors considered the following experience, qualifications or skills of each director in concluding that each director is qualified to service as a director. The information below is not intended to be an exhaustive list of the qualifications that the board of directors considered with respect to the directors:
| ● | Mr. Yazbeck is the founder of CDx has substantial experience in new market and business development, strategic partnering and negotiations from his tenure in top 50 Fortune 500 companies; |
| ● | Mr. Gruber has more than 20 years of financial and senior executive management experience in publicly traded high-technology companies; |
| ● | Dr. Katz is currently serving in his second term as New York State Assemblyman, 94th District, and as an elected official, Dr. Katz has been instrumental in writing and passing the New York State medical cannabis bill into law; |
| ● | Mr. Roffman has over 40 years’ experience in accounting and finance; |
| ● | Mr. Pier has more than 20 years of experience in the commercial and private banking sectors. |
| ● | Mr. Jackoboice has more than 20 years of senior management and portfolio company investment experience and serves on several boards of directors; and |
| ● | Mr. Harris has more than thirty-five years’ experience in various management positions with both domestic and international companies. |
Executive Officers
The following information pertains to our executive officers effective as of the Closing Date:
Daniel Yazbeck – Chief Executive Officer, Director
Mr. Yazbeck has served as the CDx Chief Executive Officer since September, 2013. See the disclosure under the heading “Directors” in this Current Report on Form 8-K for additional background information on Mr. Yazbeck.
Thomas Gruber – Chief Financial Officer, Chief Operations Officer, Director
Mr. Gruber has served as the CDx Chief Financial Officer since June, 2014. See the disclosure under the heading “Directors” in this Current Report on Form 8-K for additional background information on Mr. Gruber.
Skip Sanzeri – President
Samuel “Skip” Sanzeri joined the Company in January 2014 as Chief Operating Officer and was appointed President in February 2015. Mr. Sanzeri has 30 years of business experience with both private and public companies. From December 2001 to December 2013 Mr. Sanzeri was an advisor and management consultant working to help finance and operate many different early-stage companies and in November 2007 founded Social G2, a mobile apps company where he served as CEO. In February 1998 Mr. Sanzeri founded Open Source Group (later merged with Olliance) and served as CEO until November 2001. From February 1996 to January 1998 Skip worked at Quote.com as Vice President of Business Development. From January 1986 to January 1996 Mr. Sanzeri worked at I.A.C., a division of Ziff-Davis Publishing, progressing to become Director of Sales and Marketing.
Mr. Sanzeri received his Bachelor’s Degree in Economics from Claremont McKenna College and a Master’s Degree in Public Administration from College of Notre Dame.
| -35- |
Non-Executive Officers
David Bortolin – Chief Revenue Officer
Dave Bortolin joined the Company in April, 2014 as Chief Revenue Officer. Mr. Bortolin has over 22 years of enterprise software, SaaS direct sales, channel partner and marketing experience with companies ranging from start-ups to established organizations.
Mr. Bortolin served as Vice President of Worldwide Sales, Marketing, and Strategic Partnerships for AppCentral from March 2010 to August 2013. From May 2008 to January 2010, Mr. Bortolin served as Vice President Sales, Marketing and Strategic Partners for Exalead. From December 2005 to April 2008, Mr. Bortolin served as Vice President Sales and Marketing at Remedy Interactive. From January 2004 to January 2006, Mr. .Bortolin served as Vice President Sales and Marketing for the Milestone Group, and prior to that, he served in various management capacities at Ziff Davis Publishing.
Mr. Bortolin received a Bachelor’s degree in Business Administration from Saint Mary’s College.
Jean-Pierre LeBlanc – Chief Technology Officer
Jean-Pierre (JP) joined CDx, Inc. as its Chief Technology Officer in February, 2015. Mr. LeBlanc has more than 30 years senior management level experience in software architecture development and hardware design. From January 2014 to August 2014, Mr. LeBlanc was Chief Technology Officer and Vice President of Search Initiatives, Inc. From March 2012 to April 2013, Mr. LeBlanc was Vice President Software Development for Skinit, Inc. From January, 2011 to March, 2012, Mr. LeBlanc was General Manager of Qiwi USA, LLC. From February 2010 to December 2010 Mr. LeBlanc was Head of Architecture and Solution Management for Alcatel-Lucent, Inc. From July, 2008 to February, 2010, Mr. LeBlanc was Vice President Mobile Solutions/Vice President Marketing/General Manager America for Virtuallogix, Inc. Mr. LeBlanc also held senior management technology positions at Nokia Networks, Cellon, Inc., Borland Software and Santa Cruz Operations, Inc.
Mr. LeBlanc earned a Bachelor of Engineering Degree and a Masters of Engineering from Royal Military College of Canada and completed a PhD (Doctor of Philosophy) curriculum in Computer Science at Queens University, Canada. Mr. LeBlanc was an assistant professor in Computer Engineering at Royal Military College.
Rob Lewis – Chief Marketing Officer
Rob Lewis joined the Company as Chief Marketing Officer in January 2015. He brings 27 years of Technology Marketing and Product Management experience to the company from both public and private companies. Before joining the Company, Mr. Lewis served as the President of Local Value Marketing from January 2012 to January 2015, Vice President of Product Management from May 2010 to January 2012 for Telcentris, Director of Product Management for Semtek (Verifone), Product Manager at Qualcomm from March 2007 to September 2008, Vice President of Business Development for CommerceTel from May 2006 to December 2006, Vice President of Ameranth from September 2003 to May 2006, Vice President of Business Development at Vivenid/Universal from April 2002 to April 2003, and Vice President of Marketing and Business Development at Cloakworks Inc. from November 2001 to April 2002.
Mr. Lewis received a Bachelor’s of Arts degree from the University of California at San Diego and a Master of Business Administration from the University Of Virginia Darden Graduate School Of Business Administration.
Involvement in Legal Proceedings
To the Company’s knowledge, none of our officers or our directors has, during the last ten years:
| ● | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| -36- |
| ● | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| ● | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| ● | been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| ● | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| ● | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
To the Company’s knowledge, there are no material proceedings to which any director, officer or affiliate of the Company, any owner of record or beneficially of more than 5% of any class of voting securities of the Company, or any associate of any such director, officer, affiliate of the Company, or security holder is a party adverse to the Company or any of its subsidiaries or has a material interest adverse to the Company or any of its subsidiaries.
Arrangements for Appointment of Directors and Officers
Pursuant to the Merger Agreement, as of the Closing Date, CDx has the right to appoint each of the seven (7) directors of the Company and has the right to designate all of the executive officers of the Company.
The disclosures set forth in Item 5.01 of this Current Report on Form 8-K are incorporated by reference into this item.
Family Relationships
There are no family relationships among the members of our Board or our executive officers.
Composition of the Board
In accordance with our certificate of incorporation, our Board is elected annually as a single class.
Communications with our Board of Directors
Our stockholders may send correspondence to our board of directors c/o the Corporate Secretary at MyDx, Inc., 4225 Executive Square, Suite 600, La Jolla, California 92037. Our corporate secretary will forward stockholder communications to our board of directors prior to the board’s next regularly scheduled meeting following the receipt of the communication.
| -37- |
Code of Business Conduct and Ethics.
Effective as of April 30, 2015, the Company adopted a Code of Business Conduct and Ethics that applies to, among other persons, our president or chief executive officer as well as the individuals performing the functions of our chief financial officer, corporate secretary and controller. As adopted, our Code of Business Conduct and Ethics sets forth written standards that are designed to deter wrongdoing and to promote:
| ● | honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; |
| ● | full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with, or submit to regulatory agencies, including the Securities and Exchange Commission; |
| ● | the prompt internal reporting of violations of the Code of Business Conduct and Ethics to an appropriate person or persons identified in the Code of Business Conduct and Ethics; and |
| ● | accountability for adherence to the Code of Business Conduct and Ethics. |
Our Code of Business Conduct and Ethics requires, among other things, that all of our personnel be afforded full access to our president or chief executive officer with respect to any matter which may arise relating to the Code of Business Conduct and Ethics. Further, all of our personnel are to be afforded full access to our board of directors if any such matter involves an alleged breach of the Code of Business Conduct and Ethics by our president or chief executive officer.
In addition, our Code of Business Conduct and Ethics emphasizes that all employees, and particularly managers and/or supervisors, have a responsibility for maintaining financial integrity within our company, consistent with generally accepted accounting principles, and federal, provincial and state securities laws. Any employee who becomes aware of any incidents involving financial or accounting manipulation or other irregularities, whether by witnessing the incident or being told of it, must report it to his or her immediate supervisor or to our president or chief executive officer. If the incident involves an alleged breach of the Code of Business Conduct and Ethics by our president or chief executive officer, the incident must be reported to any member of our board of directors or use of a confidential and anonymous hotline phone number. Any failure to report such inappropriate or irregular conduct of others is to be treated as a severe disciplinary matter. It is against our company policy to retaliate against any individual who reports in good faith the violation or potential violation of our Code of Business Conduct and Ethics by another. Our Code of Business Conduct and Ethics is available, free of charge, to any stockholder upon written request to our Corporate Secretary at MyDx, Inc., 4225 Executive Square, Suite 600, La Jolla, California 92037. A copy of our Code of Business Conduct and Ethics is also attached as an exhibit to this Current Report on Form 8-K.
CORPORATE GOVERNANCE
Board Committees
Our board of directors intends to establish an audit committee, a nominating and governance committee and a compensation committee. The audit committee will review the results and scope of the audit and other services provided by the independent auditors and review and evaluate the system of internal controls. The compensation committee will manage any stock option plan we may establish and review and recommend compensation arrangements for the officers. The nominating and governance committee will assist our board of directors in fulfilling its oversight responsibilities and identify, select and evaluate our board of directors and committees. Mr. Roffman has been selected to be the Chairman of the Audit Committee. No final determination has yet been made as to the memberships of the other committees.
| -38- |
We will reimburse all directors for any expenses incurred in attending directors’ meetings provided that we have the resources to pay these fees. We will provide officers and directors liability insurance.
Leadership Structure
The chairman of our board of directors and chief executive officer positions are currently the same person, Mr. Daniel Yazbeck. Our bylaws do not require our board of directors to separate the roles of chairman and chief executive officer but provides our board of directors with the flexibility to determine whether the two roles should be combined or separated based upon the Company’s needs. Our board of directors believes the combination of the chairman and the chief executive officer roles is the appropriate structure for the company at this time. Our board of directors believes the current leadership structure serves as an aid in the board of directors’ oversight of management and it provides the Company with sound corporate governance practices in the management of its business.
Risk Management
The board of directors discharges its responsibilities, and assesses the information provided by the Company's management and the independent auditor, in accordance with its business judgment. Management is responsible for the preparation, presentation, and integrity of the Company's financial statements, and management is responsible for conducting business in an ethical and risk mitigating manner where decisions are undertaken with a culture of ownership. Our board of directors oversees management in their duty to manage the risk of our company and each of our subsidiaries. Our board of directors regularly reviews information provided by management as management works to manage risks in the business. Our board of directors intends to establish board committees to assist the full board of directors’ oversight by focusing on risks related to the particular area of concentration of the relevant committee. For example, the compensation committee will oversee risks related to our executive compensation plans and arrangements, the audit committee will oversee the financial reporting and control risks and the nominating and governance committee will oversee risks associated with the independence of our board of directors and potential conflicts of interest. If a risk is of sufficient magnitude, a committee will report on the discussions of the applicable relevant risk to the full board of directors during the committee reports portion of the board of directors meetings. The full board of directors will incorporate the insight provided by these reports into its overall risk management analysis.
Meetings
No director who served as a director during the past year of CDx attended fewer than 75% of the aggregate of the total number of meetings of the CDx board of directors.
EXECUTIVE COMPENSATION
CDx became our wholly owned subsidiary as a result of the consummation of the Merger on April 30, 2015. The following table summarizes all compensation earned in each of CDx’s last two fiscal years ended December 31, 2013 and 2014 by: (i) its principal executive officer; and (ii) its most highly compensated executive officer other than the principal executive officer who was serving as an executive officer of CDx as of the end of the last completed fiscal year. The tables below reflect the compensation for the CDx executive officers who are also named executive officers of the combined company.
| -39- |
Summary Compensation Table
The following table lists the summary compensation of CDx’s named executive officers for the prior two fiscal years (CDx was incorporated in 2013):
| Name and principal position | Year | Salary | Bonus | Stock awards | Option awards | All other comp. | Total | |||||||||||||||||||||
| Daniel R. Yazbeck (1)(2) | 2014 | $ | 97,500 | $ | - | $ | - | $ | 40,000 | $ | - | $ | 137,500 | |||||||||||||||
| Chief Executive Officer | 2013 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||
| Samuel Sanzeri (5) | 2014 | $ | 101,250 | $ | - | $ | - | $ | 40,000 | $ | 2,250 | $ | 143,500 | |||||||||||||||
| President | 2013 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||
| Thomas L. Gruber (3)(4) | 2014 | $ | 70,000 | $ | - | $ | - | $ | 16,000 | $ | 1,875 | $ | 87,875 | |||||||||||||||
| Chief Financial Officer and Chief Operating Officer | 2013 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||
| (1) | In 2014, CDx established its 2014 Equity Incentive Plan pursuant to which it is authorized to grant up to an aggregate of 6,200,000 options to purchase shares of common stock. The Company assumed CDx’s 2014 Equity Incentive Plan upon consummation of the Merger. |
| (2) | In July 2014, Mr. Yazbeck was granted an option to purchase 250,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of December 1, 2013. 1/12th of the underlying shares vest each month thereafter. |
| (3) | In July 2014, Mr. Yazbeck was granted an option to purchase 250,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of July 1, 2014. 1/24th of the underlying shares vest each month thereafter. |
| (4) | In July 2014, Mr. Gruber was granted an option to purchase 200,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of June 21, 2014. 1/24th of the underlying shares vest each month thereafter. |
| (5) | In July 2014, Mr. Sanzeri was granted an option to purchase 500,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of December 1, 2013. 1/24th of the underlying shares vest each month thereafter. |
| (6) | The current salaries for the named executive officers are $180,000; $180,000; and $150,000, respectively. |
Agreements with Named Executive Officers
Daniel R. Yazbeck, Chief Executive Officer
On October 15, 2014, CDx, Inc. entered into a five year employment agreement with Daniel R. Yazbeck. The employment agreement provides that Mr. Yazbeck shall serve as the CDx Chief Executive Officer or such other title or position as may be designated from time to time by the CDx board of directors. The employment agreement can be terminated pursuant to written notification by either CDx or Mr. Yazbeck, which notification may occur at any time for any reason. Mr. Yazbeck’s initial base salary is $180,000 per year, subject to periodic review by the CDx board of directors and may be increased in the discretion of CDx.
Under the terms of the employment agreement, Mr. Yazbeck is also eligible to participate in all group term life insurance plans, group health plans, accidental death and dismemberment plans and short-term disability programs and other perquisites which are made available to the CDx executives and for which Mr. Yazbeck qualifies. Additionally, Mr. Yazbeck is entitled to be reimbursed for supplemental insurance products including life insurance at a cost of up to an additional $15,000 per year. CDx also provides a leased vehicle with operating expenses, not to exceed $800 per month, which CDx commenced paying in March 2015.
Under the terms of the employment agreement, should CDx terminate Mr. Yazbeck’s employment other than for cause during the first 5 years of the employment agreement, or should Mr. Yazbeck resign for good reason, CDx shall have no further obligation under the employment agreement, except that CDx will continue to pay Mr. Yazbeck’s base salary for a two year period, (less, if applicable, any long-term disability payments) and the Target Bonus (as defined below) for a one year period following termination of Mr. Yazbeck’s employment on the normal payroll dates, and in addition one hundred percent (100%) of Mr. Yazbeck’s then-outstanding unvested stock options shall immediately vest.
| -40- |
The employment agreement provides that for each fiscal year during the employment agreement, Mr. Yazbeck shall be eligible for an incentive bonus. For each full fiscal year of employment, Mr. Yazbeck shall be eligible for an incentive bonus of up to one hundred percent (100%) of his annual base salary and his performance objectives shall be set such that one hundred percent (100%) completion of his objectives shall entitle him to at least seventy-five percent (75%) of the bonus (the “Target Bonus”). During the first year of employment Mr. Yazbeck shall be eligible for the bonus plan as outlined in the employment agreement. After the first year, the bonus amount will be based on the following factors: (1) the financial performance of CDx as determined and measured by CDx’s board of directors, and (2) Mr. Yazbeck’s achievement of management targets and goals as set by CDx. The bonus amount is intended to reward contribution to CDx’s performance over an entire fiscal year, and on the basis of continuing, cumulative contribution, and consequently will be paid only if Mr. Yazbeck is employed and in good standing at the time of bonus payments, which will occur each quarter. CDx accrued a $45,000 bonus for Mr. Yazbeck in compliance with the terms of his target bonus milestones for completion of a working prototype. The bonus was paid in March 2015.
The foregoing is only a brief description of the material terms of the employment agreement, and does not purport to be a complete description of the rights and obligations of the parties thereunder, and such description is qualified in their entirety by reference to the employment agreement which is filed as an exhibit to this Current Report on Form 8-K.
Outstanding Equity Awards at Fiscal Year End
The following table lists the outstanding equity awards held by CDx’s named executive officers at December 31, 2014:
| OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | ||||||||||||||||||||||||||||||||||
| OPTION AWARDS (1) | STOCK AWARDS | |||||||||||||||||||||||||||||||||
| Equity
Incentive Plan | Equity
Incentive Plan | Equity Incentive Plan Awards: | ||||||||||||||||||||||||||||||||
| Awards: | Market | Awards: | Market | |||||||||||||||||||||||||||||||
| Number of | Number of | Number of | Number of | Value of | Number of | or Payout | ||||||||||||||||||||||||||||
| Securities | Secutrities | Securities | Shares or | Shares or | Unearned | Value of | ||||||||||||||||||||||||||||
| Underlying | Underlying | Underlying | Units of | Units of | Shares, Units or | Unearned Shares, | ||||||||||||||||||||||||||||
| Unexercised | Unexercised | Unexercised | Option | Option | Stock that | Stock that | Other Rights | Units or Other | ||||||||||||||||||||||||||
| Options | Options | Unearned | Exerisable | Expiration | have Not | have not | that have Not | Rights that have | ||||||||||||||||||||||||||
| Name | Exercisable | Unexercisable | Options | Price | Date | Vested | Vested | Vested | Not Vested | |||||||||||||||||||||||||
| Daniel Yazbeck (2)(3) | 333,333 | 166,667 | 166,667 | $ | 0.08 | 7/11/2024 | - | - | - | - | ||||||||||||||||||||||||
| Thomas Gruber (4) | 50,000 | 150,000 | 150,000 | $ | 0.08 | 7/11/2024 | - | - | - | - | ||||||||||||||||||||||||
| Samuel Sanzeri (5) | 261,833 | 229,167 | 229,167 | $ | 0.08 | 7/11/2024 | - | - | - | - | ||||||||||||||||||||||||
| (1) | In 2014, CDx established its 2014 Equity Incentive Plan pursuant to which it is authorized to grant up to an aggregate of 6,200,000 options to purchase shares of common stock. The Company assumed CDx’s 2014 Equity Incentive Plan upon consummation of the Merger. |
| (2) | In July 2014, Mr. Yazbeck was granted an option to purchase 250,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of December 1, 2013. 1/12th of the underlying shares vest each month thereafter. |
| (3) | In July 2014, Mr. Yazbeck was granted an option to purchase 250,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of July 1, 2014. 1/24th of the underlying shares vest each month thereafter. |
| (4) | In July 2014, Mr. Gruber was granted an option to purchase 200,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of June 21, 2014. 1/24th of the underlying shares vest each month thereafter. In March 2015, Mr. Gruber was granted an option to purchase 550,000 shares of CDx’s common stock at $0.55 per share. The options subject to grant began vesting as of January 1, 2015. 1/18th of the underlying shares vest each month thereafter. |
| (5) | In July 2014, Mr. Sanzeri was granted an option to purchase 500,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of December 1, 2013. 1/24th of the underlying shares vest each month thereafter. In March 2015, Mr. Sanzeri was granted an option to purchase 500,000 shares of CDx’s common stock at $0.55 per share. The options subject to grant began vesting on January 1, 2015. 1/24th of the underlying shares vest each month thereafter. |
| -41- |
Director Compensation
The following table lists the compensation of CDx’s directors, during the last fiscal year ended December 31, 2014, that will be continuing as directors of the combined company following the Merger:
| DIRECTOR COMPENSATION | ||||||||||||||||||||||||||||
| Name | Fees Earned or Paid in Cash | Stock Awards | Option Awards (1) | Non-Equity Incentive Plan Compensation | Nonqualified Deferred Compensation Earnings | All Other Compensation | Total | |||||||||||||||||||||
| Daniel Yazbeck | — | — | — | (2)(3) | — | — | — | — | ||||||||||||||||||||
| Thomas Gruber | — | — | — | (4) | — | — | — | — | ||||||||||||||||||||
| Stephen M. Katz | — | — | — | (5) | — | — | — | — | ||||||||||||||||||||
| (1) | In 2014, CDx established its 2014 Equity Incentive Plan pursuant to which it is authorized to grant up to an aggregate of 6,200,000 options to purchase shares of common stock. The Company assumed CDx’s 2014 Equity Incentive Plan upon consummation of the Merger. |
| (2) | In July 2014, Mr. Yazbeck was granted an option to purchase 250,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of December 1, 2013. 1/12th of the underlying shares vest each month thereafter. |
| (3) | In July 2014, Mr. Yazbeck was granted an option to purchase 250,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of July 1, 2014. 1/24th of the underlying shares vest each month thereafter. |
| (4) | In July 2014, Mr. Gruber was granted an option to purchase 200,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of June 21, 2014. 1/24th of the underlying shares vest each month thereafter. In March 2015, Mr. Gruber was granted an option to purchase 550,000 shares of CDx’s common stock at $0.55 per share. The options subject to grant began vesting as of January 1, 2015. 1/18th of the underlying shares vest each month thereafter. |
| (5) | In July 2014, Mr. Katz was granted an option to purchase 50,000 shares of CDx’s common stock at $0.08 per share. The options subject to the grant began vesting as of July 1, 2014. 1/24th of the underlying shares vest each month thereafter. In March 2015, Mr. Katz was granted an option to purchase 250,000 shares of CDx’s common stock. The options subject to the grant began vesting on March 15, 2015. 1/24th of the underlying shares vest each month thereafter. |
Director Compensation Arrangements
Director Steve Katz was awarded options to purchase 300,000 shares in CDx’s common stock.
Director Edward Roffman, Audit Committee Chairman, receives an annual stipend of $25,000 as chairman of the audit committee. Mr. Roffman was also awarded options to purchase 250,000 shares in CDx’s common stock.
Director Federico Pier was awarded options to purchase 250,000 shares in CDx’s common stock.
Director George Jackoboice, Jr. was awarded options to purchase 250,000 shares in CDx’s common stock.
| -42- |
Director Michael Harris was awarded options to purchase 250,000 shares in CDx’s common stock.
It is anticipated that reasonable compensation would be awarded for new directors.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Related Persons
The following includes a summary of any transaction occurring since September 16, 2013 (inception) for CDx and since August 1, 2013 for Brista, or any proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds $120,000, and in which any related person had or will have a direct or indirect material interest (other than compensation described under "Executive Compensation" above). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm's-length transactions:
CDx
None.
Brista
On May 14, 2013, we offered and sold 3,000,000 shares of common stock to Andrejs Levaskovics, our President, Treasurer, sole Director and Secretary at a purchase price of $0.001 per share, for aggregate proceeds of $3,000.
As of July 31, 2014, Andrejs Levaskovics, the Company’s President, Treasurer, sole Director and Secretary had advanced $5,217 to the Company. This amount is payable upon demand, unsecured, non-interest bearing, has no term.
Review and Approval of Transactions with Related Persons
In reviewing and approving transactions with related persons, CDx’s board of directors considered all material factors in relation to such related person’s role in a proposed transaction, including, without limitation, the related person’s indirect or direct financial interest in the proposed transaction, other interests such related person may have in the proposed transaction, the terms and conditions of the proposed transaction, and whether such transaction is on an equivalent to arms-length basis. After reviewing and factoring all these considerations, CDx’s board of directors, and the disinterested directors, if applicable, determined whether to approve the proposed transaction with the respective related person. While CDx did not have any written polices with respect to review and approval of any such transactions with related persons, CDx’s believes the processes its board of directors has followed ensure the appropriateness of its entry into such transactions with related persons and that they were entered into on terms on an equivalent basis to an arms-length transaction.
Director Independence
Board of Directors
The Board, in the exercise of its reasonable business judgment, has determined that Edward Roffman, Frederico Pier, Stephen M. Katz, George Jackoboice and Michael Harris qualify as an independent directors pursuant to Nasdaq Stock Market Rule 5605(a)(2) and applicable SEC rules and regulations. Mr. Yazbeck and Mr. Gruber, are currently employed as our Chief Executive Officer and Chief Financial Officer, respectively, and therefore would not be considered independent directors.
| -43- |
Potential Conflicts of Interest
Since we did not have an audit or compensation committee comprised of independent directors, the functions that would have been performed by such committees were performed by our directors. Thus, there was an inherent conflict of interest.
LEGAL PROCEEDINGS
From time to time we may be involved in claims arising in the ordinary course of business. No legal proceedings, governmental actions investigations or claims are currently pending against us or involve us.
RECENT SALES OF UNREGISTERED SECURITIES
The description of the 2014 Bridge Note Financing and Series B Convertible Preferred Stock Financing in Item 2.01 is incorporated herein by reference. The issuance of the Notes, Warrants and Series B shares were made in reliance on the exemption provided by Section 4(2) of the Securities Act for the offer and sale of securities not involving a public offering, and Regulation D promulgated under the Securities Act of 1933, as amended (the “Securities Act”).
CONTROLS AND PROCEDURES
This report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of the company’s registered public accounting firm due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
MARKET
PRICE OF AND DIVIDENDS ON THE REGISTRANT’S
COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our common stock is currently quoted on the OTC Markets under the symbol “MYDX.” To date, there has been limited trading in our common stock.
As of March 31, 2015 there were approximately 30 record holders of our common stock.
We paid no dividends or made any other distributions in respect of our common stock since inception and we have no plans to pay any dividends or make any other distributions in the future.
DESCRIPTION OF CAPITAL STOCK
Our authorized capital stock consists of 375,000,000 shares of common stock, par value $0.001 per share.
Common Stock
Of the authorized common stock, 21,475,252 shares are outstanding as of immediately after the closing of the Merger and after giving effect to the shares to be issued to the former CDx shareholders as a result of the Merger. The holders of our common stock are entitled to receive dividends from our funds legally available therefor only when, as and if declared by our Board, and are entitled to share ratably in all of our assets available for distribution to holders of our common stock upon the liquidation, dissolution or winding-up of our affairs. Holders of our common stock do not have any preemptive, subscription, redemption or conversion rights. Holders of our common stock are entitled to one vote per share on all matters which they are entitled to vote upon at meetings of stockholders or upon actions taken by written consent pursuant to Nevada corporate law. The holders of our common stock do not have cumulative voting rights, which mean that the holders of a plurality of the outstanding shares can elect all of our directors. All of the shares of our common stock currently issued and outstanding are fully-paid and nonassessable. No dividends have been paid to holders of our common stock since our incorporation, and no cash dividends are anticipated to be declared or paid in the reasonably foreseeable future.
| -44- |
Warrants
Pursuant to the terms of the Merger, the Company has assumed the warrants previously issued by CDx which total 7,571,395. These warrants have a term of 5 years and have an exercise price of $1.10 per share. This description of the warrants does not purport to be a complete description of the rights and obligations of the parties thereunder, and such description is qualified in its entirety by reference to the form of warrant which is filed as an exhibit to this Current Report on Form 8-K.
Equity Compensation Plan Information
The following table summarizes our equity compensation plan information as of April 9, 2015. Information is included for equity compensation plans approved by our stockholders and equity compensation plans not approved by our stockholders.
| Plan Category | (a) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | (b) Weighted Average Exercise Price of Outstanding Options, Warrants and Rights | (c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) | |||||||||
| Equity compensation plans approved by stockholders | 6,191,000 | $ | 0.39 | 235,838 | ||||||||
| Equity compensation plans not approved by stockholders | None | N/A | N/A | |||||||||
In connection with the Merger, on April 30, 2015, the Company adopted the MyDx, Inc. 2015 Equity Incentive Plan (the “Plan”). The Plan is intended to attract and retain the best available personnel for positions of substantial responsibility; to provide additional incentive to employees, directors and consultants, and; to promote the success of the Company’s business. The Plan permits the grant of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock and restricted stock units. The maximum number of shares available to be issued under the Plan is currently 6,200,000 shares, subject to adjustments for any stock splits, stock dividends or other specified adjustments which may take place in the future. The Plan is administered by our Company’s Board of Directors. Persons eligible to participate in the Plan are: 1) employees; 2) non-employee members of our Company’s Board of Directors; and 3) consultants and other independent advisors who provide services to our Company. Options granted under the Plan are evidenced by agreement between the recipient and our Company, subject to the terms of the Plan.
The foregoing is only a brief description of the material terms of the Plan, and does not purport to be a complete description of the Plan, and such description is qualified in its entirety by reference to the Plan which is filed as an exhibit to this Current Report on Form 8-K.
| -45- |
Anti-Takeover Effects of Certain Provisions of our Certificate of Incorporation, our By-Laws and Delaware Law
Anti-takeover Effects of Nevada Law
Business Combination
The “business combination” provisions of Sections 78.411 to 78.444, inclusive, of the Nevada Revised Statutes (“NRS”), generally prohibit a Nevada corporation with at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved by the board of directors prior to the date the interested stockholder obtained such status; and extends beyond the expiration of the three-year period, unless:
| ● | the transaction was approved by the board of directors prior to the person becoming an interested stockholder or is later approved by a majority of the voting power held by disinterested stockholders, or |
| ● | if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
A “combination” is generally defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer or other disposition, in one transaction or a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, (c) 10% or more of the earning power or net income of the corporation, and (d) certain other transactions with an interested stockholder or an affiliate or associate of an interested stockholder.
In general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within three years, did own) 10% or more of a corporation’s voting stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire our Company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Currently, we have no Nevada shareholders and since this offering will not be made in the State of Nevada, no shares will be sold to its residents. Further, we do not do business in Nevada directly or through an affiliate corporation and we do not intend to do so. Accordingly, there are no anti-takeover provisions that have the effect of delaying or preventing a change in our control.
Control Share Acquisition
The “control share” provisions of Sections 78.378 to 78.3793, inclusive, of the NRS apply to “issuing corporations,” which are Nevada corporations with at least 200 stockholders, including at least 100 stockholders of record who are Nevada residents, and which conduct business directly or indirectly in Nevada. The control share statute prohibits an acquirer, under certain circumstances, from voting its shares of a target corporation’s stock after crossing certain ownership threshold percentages, unless the acquirer obtains approval of the target corporation’s disinterested stockholders. The statute specifies three thresholds: one-fifth or more but less than one-third, one-third but less than a majority, and a majority or more, of the outstanding voting power. Generally, once an acquirer crosses one of the above thresholds, those shares in an offer or acquisition and acquired within 90 days thereof become “control shares” and such control shares are deprived of the right to vote until disinterested stockholders restore the right. These provisions also provide that if control shares are accorded full voting rights and the acquiring person has acquired a majority or more of all voting power, all other stockholders who do not vote in favor of authorizing voting rights to the control shares are entitled to demand payment for the fair value of their shares in accordance with statutory procedures established for dissenters’ rights.
A corporation may elect to not be governed by, or “opt out” of, the control share provisions by making an election in its articles of incorporation or bylaws, provided that the opt-out election must be in place on the tenth day following the date an acquiring person has acquired a controlling interest, that is, crossing any of the three thresholds described above. We have not opted out of the control share statutes, and will be subject to these statutes if we are an “issuing corporation” as defined in such statutes.
| -46- |
At this time, we do not have 100 stockholders of record resident of Nevada. Therefore, the provisions of the control share acquisition act do not apply to acquisitions of our shares and will not until such time as these requirements have been met. At such time as they may apply to us, the provisions of the control share acquisition act may discourage companies or persons interested in acquiring a significant interest in or control of the Company, regardless of whether such acquisition may be in the interest of our stockholders.
Shares Eligible for Future Sale
As of the date hereof, there were 21,475,252 shares of our common stock outstanding. We are authorized to issue by our Articles of Incorporation, an aggregate of 375,000,000 shares of common stock, par value $0.001 per share.
Rule 144
| ● | Pursuant to Rule 144 of the Securities Act, a person who has beneficially owned restricted shares of our common stock or warrants for at least six months (or longer in the case of former shell companies as described below) would be entitled to sell their securities provided that (1) such person is not deemed to have been one of our affiliates at the time of, or at any time during the three months preceding, a sale, (2) we are subject to the Exchange Act reporting requirements for at least 90 days before the sale and (3) if the sale occurs prior to satisfaction of a one-year holding period, we provide current information at the time of sale. |
| ● | Persons who have beneficially owned restricted shares of our common stock or warrants for at least six months but who are our affiliates at the time of, or at any time during the three months preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of: 1% of total shares outstanding and the average weekly trading volume of such securities during the four calendar weeks preceding the filing of a 144 notice with respect to such sale (which average volume criteria only applies if the company’s securities become listed on NASDAQ or an exchange). |
Provided, in each case that we are subject to the Exchange Act periodic reporting requirements for at least three months before the sale.
However, since our shares are quoted on the OTC Markets, which is not an “automated quotation system,” our stockholders will not be able to rely on the market-based volume limitation described in the second bullet above. If, in the future, our securities are listed on an exchange or quoted on NASDAQ, then our stockholders would be able to rely on the market-based volume limitation. Unless and until our stock is so listed or quoted, our stockholders can only rely on the percentage based volume limitation described in the first bullet above.
Such sales by affiliates must also comply with the manner of sale, current public information and notice provisions of Rule 144. The selling stockholders will not be governed by the foregoing restrictions when selling their shares pursuant to this prospectus.
Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies
Brista was a shell company prior to the filing of this Current Report on Form 8-K. Historically, the SEC staff has taken the position that Rule 144 is not available for the resale of securities initially issued by companies that are, or previously were, blank check companies, like us. The SEC has codified and expanded this position in the amendments discussed above by prohibiting the use of Rule 144 for resale of securities issued by any shell companies (other than business combination related shell companies) or any issuer that has been at any time previously a shell company. The SEC has provided an important exception to this prohibition, however, if the following conditions are met:
| ● | the issuer of the securities that was formerly a shell company has ceased to be a shell company; |
| ● | the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; |
| -47- |
| ● | the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials), other than Current Reports on Form 8-K; and |
| ● | at least one year has elapsed from the time that the issuer filed current comprehensive disclosure with the SEC reflecting its status as an entity that is not a shell company. |
As a result, it is likely that pursuant to Rule 144 our stockholders will be able to sell their shares of our common stock from and after the one year anniversary of our filing of current comprehensive disclosure following in this Current Report on Form 8-K without registration.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Nevada law and certain provisions of our bylaws under certain circumstances provide for indemnification of our officers, directors and controlling persons against liabilities which they may incur in such capacities. A summary of the circumstances in which such indemnification is provided for is contained herein, but this description is qualified in its entirety by reference to our bylaws and to the statutory provisions.
In general, any officer, director, employee or agent may be indemnified against expenses, fines, settlements or judgments arising in connection with a legal proceeding to which such person is a party, if that person’s actions were in good faith, were believed to be in our best interest, and were not unlawful. Unless such person is successful upon the merits in such an action, indemnification may be awarded only after a determination by independent decision of our Board, by legal counsel, or by a vote of the stockholders, that the applicable standard of conduct was met by the person to be indemnified.
The circumstances under which indemnification is granted in connection with an action brought on our behalf is generally the same as those set forth above; however, with respect to such actions, indemnification is granted only with respect to expenses actually incurred in connection with the defense or settlement of the action. In such actions, the person to be indemnified must have acted in good faith and in a manner believed to have been in our best interest, and have not been adjudged liable for negligence or misconduct.
Indemnification may also be granted pursuant to the terms of agreements which may be entered into in the future or pursuant to a vote of stockholders or directors. The statutory provision cited above also grants the power to us to purchase and maintain insurance which protects our officers and directors against any liabilities incurred in connection with their service in such a position, and such a policy may be obtained by us.
A stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees regarding which indemnification is sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, this indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements set forth in Item 9.01(a) and (b) of this Current Report on Form 8-K are incorporated by reference into this item.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
The disclosures set forth in Item 4.01 of this Current Report on Form 8-K are incorporated by reference into this item.
| -48- |
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
| -49- |
The exhibits described in Item 9.01 of this Current Report on Form 8-K are incorporated by reference into this item.
| Item 4.01. | Change in Registrant’s Certifying Accountant. |
The financial statements as of and for the year ended December 31, 2014, and as of December 31, 2013 and for the period from September 16, 2013 (date of inception) through December 31, 2013 of our newly acquired Delaware formed subsidiary, CDx, Inc., were previously audited by Burr Pilger Mayer, Inc., which were the independent registered public accounting firm that audited the financial statements of CDx. Accordingly, financial statements prepared by Brista Corp for the period commencing its inception December 20, 2012 and ended July 31, 2014, were audited by Cutler & Co. LLC, which statements were relied upon, in part in preparing the unaudited proforma condensed combined Financial Statements annexed as an exhibit to this Report.
As the result of the Merger, CDx has become our subsidiary and, therefore, as of such date (e.g. review of the quarterly report for the period ended March 31, 2015 and thereafter) the independent registered public accounting firm of the Company, Burr Pilger Mayer Inc., will continue to act as the auditor for the Company and CDx until their resignation or removal.
Pursuant to Item 304 of Regulation S-K under the Securities Act of 1933, as amended, and under the Securities Exchange Act of 1934, as amended, the Company reports as follows:
| ● |
The dismissal of Cutler & Co. LLC, which took effect on April 30, 2015, and appointment of the new independent registered public accounting firm, Burr Pilger Mayer, Inc., which took effect on May 5, 2015, were related solely to the change of control of Brista Corp resulting from our acquisition of CDx, and was not related in any way to any disagreement with Cutler & Co. LLC. |
| ● | Cutler & Co. LLC’s report on the financial statements of Brista Corp since its inception in 2012, has not contained any adverse opinion or disclaimer of opinion and was not qualified or modified as to uncertainty, audit scope, or accounting principle, except for Cutler & Co. LLC’s audit report dated September 25, 2014, which contains an explanatory paragraph that cited certain conditions that raised substantial doubt about the Company’s ability to continue as a going concern. |
| ● | The decision to utilize the Company’s current accountants was approved by the Company’s board of directors, as the Company does not have an audit committee. |
| ● | Since inception of Brista Corp., neither the Company, nor, based on information, CDx, Inc., has had any disagreements with Burr Pilger Mayer, Inc. on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreement(s), if not resolved to the satisfaction of the former accountant, would have caused it to make reference to the subject matter of the disagreement(s) in connection with its report. |
A copy of the disclosure made herein by the Company has been provided to Cutter & Co. LLC prior to the date of filing of this Report with the Securities and Exchange Commission, and the independent registered public accounting firm have been requested to furnish the Company with a copy of a letter addressed to the Commission stating that it agrees with the statements made by the Company in this Item 4.01 of this Report. A copy of this letter is filed as an exhibit to this Report. A copy of this letter will be filed by an amendment to this Report once it is received.
As the result of the Merger, CDx has become our subsidiary and, therefore, as of such date (e.g. review of the quarterly report for the period ended March 31, 2015 and thereafter) the independent registered public accounting firm of CDx, Burr Pilger Mayer, Inc. will continue to act as the auditor for CDx and Brista Corp. until their resignation or removal. Since its inception in 2013, and through the acquisition date, and through the date of the Brista Corp.’s appointment of Burr Pilger Mayer, Inc., neither the Brista Corp. nor CDx, Inc. consulted Burr Pilger Mayer, Inc. with respect to the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s consolidated financial statements, or any other matter or reportable events listed in Items 304(a)(2)(i) and (ii) of Regulation S-K.
| -50- |
| Item 5.01. | Changes in Control of Registrant. |
The disclosures set forth in Item 2.01 of this Current Report on Form 8-K are incorporated by reference into this Item.
Upon consummation of the Merger, each share of CDx’s capital stock issued and outstanding immediately preceding the Merger was converted into the right to receive one share of common stock. Additionally, upon consummation of the Merger, Brista Corp. assumed all of CDx’s options and warrants issued and outstanding immediately prior to the Merger, which are now exercisable for shares of common stock, respectively. Following the Merger, CDx’s former stockholders now hold approximately 92% of the Company Common Stock outstanding.
Upon consummation of the Merger, we expanded our Board from one to seven (7) directors, each of whom was a director designated by CDx.
| Item 5.02. | Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers. |
Effective as of the Closing Date, Joseph Abrams resigned from the Board and from any committee of the Board of which he was a member. In addition, Joseph Abrams resigned as our President and Chief Executive Officer, also effective as of the Closing Date.
Also in connection with the closing of the Merger, Daniel Yazbeck, Thomas Gruber, Stephen Katz, Michael Harris, Edward Roffman, George Jackoboice and Federico Pier became members of our Board, with Mr. Yazbeck serving as the Chairman of the Board. Daniel Yazbeck, the Chief Executive Officer of CDx, became our Chief Executive Officer, Thomas Gruber became our Chief Financial and Chief Operating Officer and Samuel Sanzeri became our President.
Accordingly, after consummation of the Merger, our Board consists of Daniel Yazbeck, Thomas Gruber, Stephen Katz, Michael Harris, Edward Roffman, George Jackoboice and Federico Pier. Our executive officers are Daniel Yazbeck, Chief Executive Officer; Thomas Gruber, Chief Financial Officer and Chief Operating Officer; and Samuel Sanzeri, President.
The disclosures set forth under the headings “Form 10 Information—Directors and Officers,” “Form 10 Information—Executive Compensation” and “Form 10 Information—Certain Relationships and Related Transactions and Director Independence” of this Current Report on Form 8-K are incorporated by reference into this Item 5.02.
| Item 5.03. | Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year. |
On April 24, 2015, the Company amended its Articles of Incorporation to change its corporate name from “Brista Corp” to “MyDx, Inc.”
On April 30, 2015, the Company amended its Bylaws to (i) changed its fiscal year end from July 31st to December 31st; and (ii) set the number of directors of the Company to be established by the Board from time to time at a number between one (1) and nine (9).
| Item 5.06. | Change in Shell Company Status. |
As the result of the transactions effected by the closing of the Merger, as described above under Item 2.01 of this Current Report, we are no longer a shell company as that term is defined in Rule 12b-2 of the Securities Exchange Act of 1934. The disclosures under the heading “The Merger” set forth in Item 2.01 of this Current Report on Form 8-K are incorporated by reference into this Item 5.06.
| -51- |
| Item 7.01. | Regulation FD Disclosure. |
On April30, 2015, the Company issued a press release announcing consummation of the Merger with CDx pursuant to the Merger Agreement. A copy of the press release is furnished as Exhibit 99.2 to this Current Report on Form 8-K and incorporated by reference in this Item 7.01.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.2 furnished herewith, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing by the Company under the Securities Act.
| Item 9.01. | Financial Statements and Exhibits. |
| (a) | Financial Statements of the Businesses Acquired and of Brista |
In accordance with Item 9.01(a): (i) CDx’s audited financial statements for the years ended December 31, 2014 and December 31, 2013 are filed as Exhibit 99.1 to this Current Report on Form 8-K; and (ii) Brista’s audited financial statements for the years ended July 31, 2014 and July 31, 2013 and unaudited financial statements for the period ending January 31, 2015 are filed in this Current Report on Form 8-K as Exhibit 99.2
| (b) | Pro Forma Financial Information is filed in this Current Report on Form 8-K as Exhibit 99.3 |
| (d) | Exhibits |
The exhibits listed in the following Exhibit Index are filed as part of this Current Report on Form 8-K.
| Exhibit No. | Description | |
| 2.1 | Agreement and Plan of Merger, dated as of April 9, 2015, by and among Brista Corp., CDX Merge, Inc. and CDx, Inc.(1) | |
| 3.2 | Amendment to Articles of Incorporation (2) | |
| 10.1 | Joint Development Agreement, dated as of November 1, 2013, by and between CDx, Inc. and Next Dimension Technologies, Inc. (3) | |
| 10.2 | Patent Assignments, dated as of July 2, 2014, by and between CDx, Inc. and Richard Rouse. | |
| 10.3 | Employment Agreement, dated as of October 15, 2014, between CDx and Daniel Yazbeck | |
| 10.4 | Form of Series A Preferred Investor Rights Agreement, dated as of March 2014, by and among CDx and the investors party thereto. | |
| 10.5 | Form of Series B Preferred Stock and Warrant Purchase Agreement, dated as of October 2014, by and between CDx and the investors party thereto. | |
| 10.6 | Form of Registration Rights Agreement, dated as of October2014, by and among CDx and the investors party thereto. | |
| 10.7 | Form of Series B Preferred Warrant | |
| 10.8 | 2015 Equity Incentive Plan | |
| 10.9 | Office Lease dated April 1, 2015 | |
| 14.1 | Code of Ethics | |
| 23.1 | Consent of Cutler & Co., LLC | |
| 99.1 | CDx, Inc. audited financial statements for the fiscal years ended December 31, 2014 and 2013 | |
| 99.2 | Brista Corp. audited financial statements for the years ended July 31, 2014 and July 31, 2013 and unaudited financial statements for the period ending January 31, 2015 | |
| 99.3 | Pro Forma Financial Information | |
| 99.4 | Press Release, Dated April 30, 2015. |
| (1) | Incorporated by reference to the Company’s Form 8-K filed with the SEC on April 14, 2015. |
| (2) | Incorporated by reference to the Company’s Form 8-K filed with the SEC on April 29, 2015. |
| (3) | The Company has requested confidential treatment for portions of this agreement. Accordingly, certain portions of this agreement have been omitted in the version filed with this report and such confidential portions have been filed with the Securities and Exchange Commission. |
| -52- |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| MyDx, Inc. | |
| Date: May 6, 2015 | |
| Thomas Gruber | |
| Chief Financial Officer |
| -53- |
INDEX TO FINANCIAL STATEMENTS
CDx, Inc. Audited Financial Statements
| Report of Independent Registered Public Accounting Firm. | F-1 |
| Balance Sheets as of December 31, 2014 and 2013. | F-2 |
| Statements of Operations for the year ended December 31, 2014 and the period from September 16, 2013 (inception) to December 31, 2013. | F-3 |
| Statements of Changes in Stockholders’ Deficit for the year ended December 31, 2014 and the period from September 16, 2013 (inception) to December 31, 2013. | F-4 |
| Statements of Cash Flows for the year ended December 31, 2014 and the period from September 16, 2013 (inception) to December 31, 2013. | F-5 |
| Notes to Financial Statements. | F-6 |
Brista Corp. Financial Statements
| Condensed Balance Sheets as of January 31, 2015 (Unaudited) and July 31, 2014. | F-2 |
| Condensed Statements of Operations for the three months and six months ended January 31, 2015 and 2014 (Unaudited), and the period since inception (December 20, 2012) to January 31, 2015 (Unaudited). | F-3 |
| Condensed Statement of Changes in Stockholders’ Equity (Deficit) for the period since inception (December 20, 2012) to January 31, 2015. (Unaudited). | F-4 |
| Condensed Statements of Cash Flows for the six months ended January 31, 2015 and 2014 (Unaudited) and for the period since inception (December 20, 2012) to January 31, 2015 (Unaudited). | F-5 |
| Notes to Unaudited Condensed Financial Statements. | F-6 |
| Report of Independent Registered Public Accounting Firm. | F-12 |
| Balance Sheets at July 31, 2014 and July 31, 2013. | F-13 |
| Statements of Operations for the year ended July 31, 2014 and the period from December 20, 2012 (inception) to July 31, 2013 and from December 20, 2013 (inception) to July 31, 2014. | F-14 |
| Statements of Changes in Stockholders’ Deficit for the period from December 20, 2012 (inception) to July 31, 2014. | F-15 |
| Statements of Cash Flows for the year ended July 31, 2014 and the period from December 20, 2012 (inception) to July 31, 2013 and from December 20, 2013 (inception) to July 31, 2014. | F-16 |
| Notes to Financial Statements. | F-17 |
Unaudited Pro Forma Condensed Combined Financial Statements
| Proforma Condensed Combined Balance Sheets As of December 31, 2014. | 1 |
| Proforma Condensed Combined Statements of Operations For the year ended December 31, 2014. | 2 |
| Notes to Unaudited Pro Forma Condensed Combined Financial Statements. | 3 |
-54-
