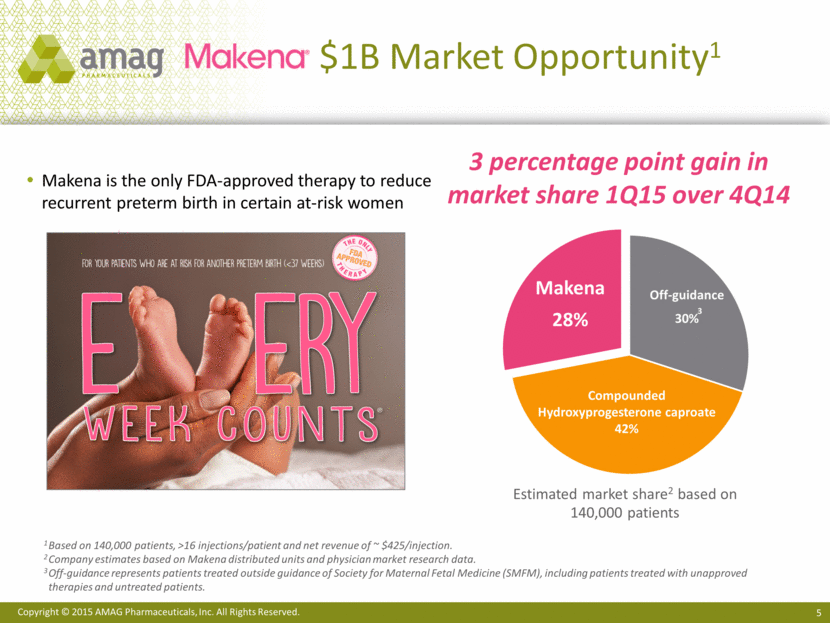Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a15-10781_1ex99d1.htm |
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a15-10781_18k.htm |
Exhibit 99.2
|
|
AMAG Pharmaceuticals 1Q 2015 Financial Results May 5, 2015 |
|
|
This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including among others, statements regarding AMAG’s updated 2015 financial guidance, including net product sales, adjusted EBITDA and cash earnings; the market opportunity for Makena, including opportunities to increase market share; Makena’s growth strategies and drivers for 2015; potential regulatory approval and launch of the single-dose, preservative-free Makena formulation and expectations for other lifecycle management initiatives; Feraheme growth opportunities, including in the chronic kidney disease (CKD) market and if approval of the broader iron deficiency anemia (IDA) label is pursued and received; AMAG’s future expansion opportunities, business development targeting strategy, ability to fund future acquisitions and positioning; expectations regarding significant cash generation in connection with planned deleveraging and portfolio expansion opportunities; AMAG’s 2015 goals, including product growth across the portfolio, increase in adjusted EBITDA margin, execution on Makena’s line extension/lifecycle management program, the path forward (if any) for the broad IDA indication in the U.S. and plans to further expand AMAG’s product portfolio are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others, those risks identified in Forward-Looking Statements AMAG’s filings with the U.S. Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K for the year ended December 31, 2014 and subsequent filings with the SEC. Any of the above risks and uncertainties could materially and adversely affect AMAG’s results of operations, its profitability and its cash flows, which would, in turn, have a significant and adverse impact on AMAG’s stock price. Use of the term “including” in the two paragraphs above shall mean in each case “including, but not limited to.” AMAG cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals® and Feraheme® are registered trademarks of AMAG Pharmaceuticals, Inc. Lumara Health™ is a trademark of Lumara Health Inc. Makena® is a registered trademark of Lumara Health Inc. MuGard® is a registered trademark of PlasmaTech Biopharmaceuticals, Inc. |
|
|
1Q15 Earnings Call Speakers: Bill Heiden, Chief Executive Officer Frank Thomas, President & Chief Operating Officer Scott Holmes, Senior Vice President, Finance & Investor Relations Linda Lennox, Vice President, Investor Relations & Corporate Communications Agenda Company Highlights 1Q15 Product Performance Makena Feraheme 1Q15 Results and Financial Update Portfolio Expansion Update 2015 Goals |
|
|
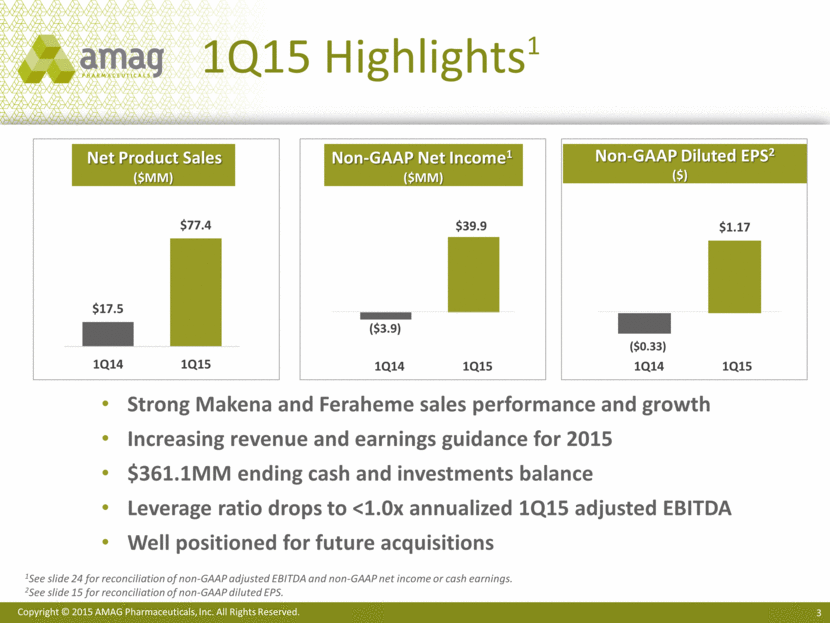
1Q15 Highlights1 Net Product Sales ($MM) Non-GAAP Net Income1 ($MM) Non-GAAP Diluted EPS2 ($) 1Q14 1Q15 Strong Makena and Feraheme sales performance and growth Increasing revenue and earnings guidance for 2015 $361.1MM ending cash and investments balance Leverage ratio drops to <1.0x annualized 1Q15 adjusted EBITDA Well positioned for future acquisitions 1Q14 1Q15 1See slide 24 for reconciliation of non-GAAP adjusted EBITDA and non-GAAP net income or cash earnings. 2See slide 15 for reconciliation of non-GAAP diluted EPS. $39.9 ($3.9) ($0.33) $1.17 |
|
|
Maternal Health |
|
|
$1B Market Opportunity1 Estimated market share2 based on 140,000 patients 1 Based on 140,000 patients, >16 injections/patient and net revenue of ~ $425/injection. 2 Company estimates based on Makena distributed units and physician market research data. 3 Off-guidance represents patients treated outside guidance of Society for Maternal Fetal Medicine (SMFM), including patients treated with unapproved therapies and untreated patients. Makena is the only FDA-approved therapy to reduce recurrent preterm birth in certain at-risk women 3 percentage point gain in market share 1Q15 over 4Q14 Makena 28% Compounded Hydroxyprogesterone caproate 42% Off-guidance 30% 3 |
|
|
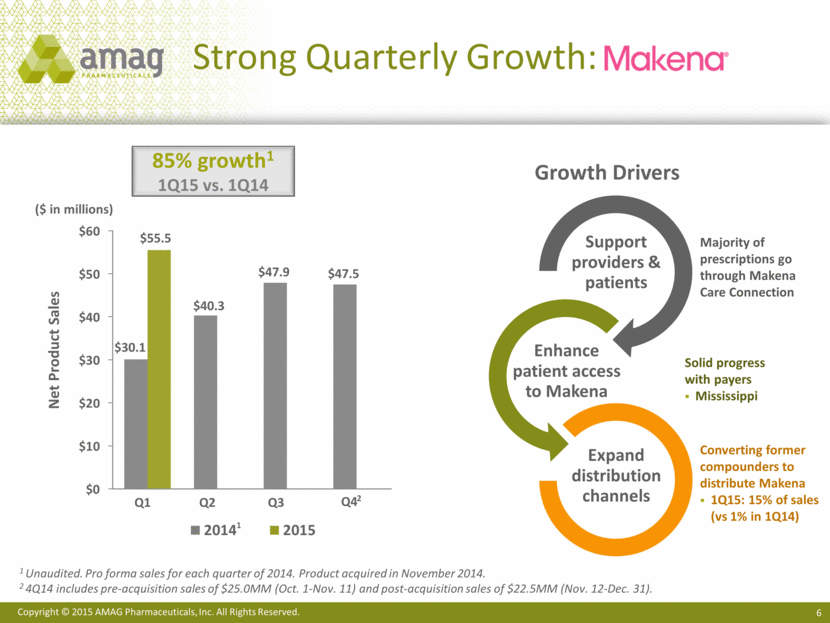
Strong Quarterly Growth: ($ in millions) 1 Unaudited. Pro forma sales for each quarter of 2014. Product acquired in November 2014. 2 4Q14 includes pre-acquisition sales of $25.0MM (Oct. 1-Nov. 11) and post-acquisition sales of $22.5MM (Nov. 12-Dec. 31). Net Product Sales 85% growth1 1Q15 vs. 1Q14 Majority of prescriptions go through Makena Care Connection Solid progress with payers Mississippi Converting former compounders to distribute Makena 1Q15: 15% of sales (vs 1% in 1Q14) Growth Drivers $0 $10 $20 $30 $40 $50 $60 $30.1 $40.3 $47.9 $47.5 $55.5 2014 2015 1 Q1 Q3 Q2 Q4 2 Support providers & patients Enhance patient access to Makena Expand distribution channels |
|
|
Significant Opportunity to Drive Growth in 2015 Educate physicians and patients on treatment guidelines Physician and nurse education through field sales force Support for independent educational programs with physician societies New Makena field-based medical affairs team Facilitate timely, unencumbered access to Makena Continue work with payers to remove barriers to patient access Makena Care Connection FDA enforcement of DQSA Expand compounding pharmacy distribution channel Enhance Makena compliance Drive enrollment in new adherence program designed to facilitate improved patient compliance (increase avg. # injections per patient) Execute on multi-pronged lifecycle management plan Continued focus on the physician and patient experience with Makena 5-dose vial |
|
|
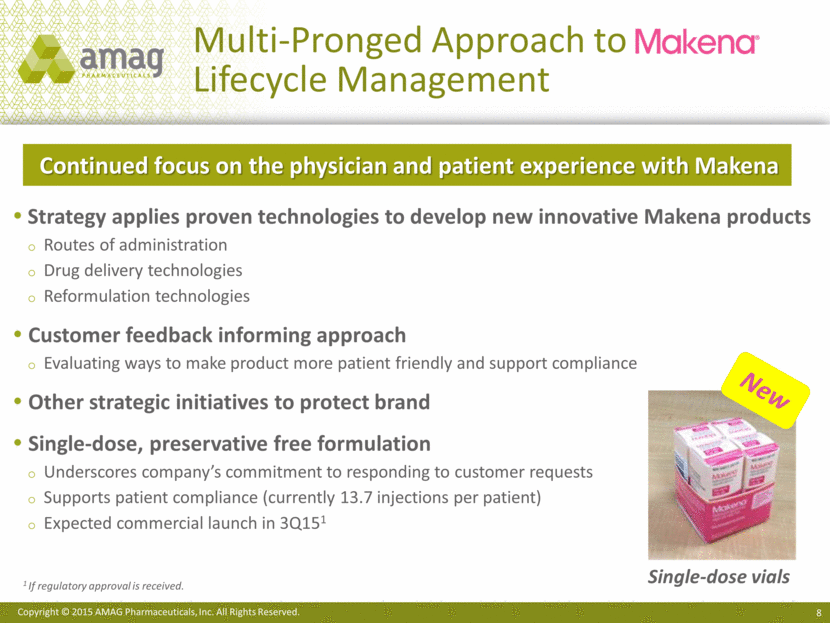
Multi-Pronged Approach to Lifecycle Management Strategy applies proven technologies to develop new innovative Makena products Routes of administration Drug delivery technologies Reformulation technologies Customer feedback informing approach Evaluating ways to make product more patient friendly and support compliance Other strategic initiatives to protect brand Single-dose, preservative free formulation Underscores company’s commitment to responding to customer requests Supports patient compliance (currently 13.7 injections per patient) Expected commercial launch in 3Q151 Single-dose vials 1 If regulatory approval is received. New Continued focus on the physician and patient experience with Makena |
|
|
Hematology / Oncology and Hospital |
|
|
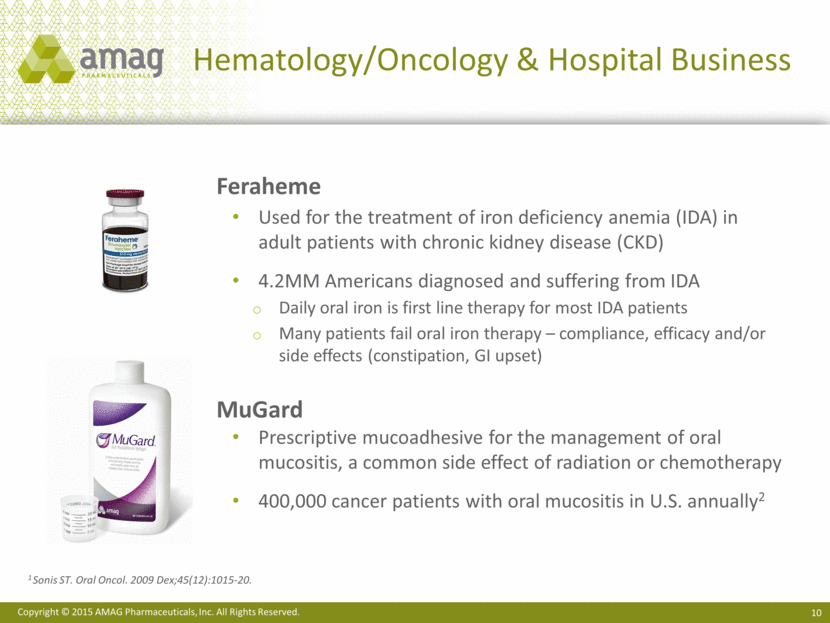
Hematology/Oncology & Hospital Business Feraheme Used for the treatment of iron deficiency anemia (IDA) in adult patients with chronic kidney disease (CKD) 4.2MM Americans diagnosed and suffering from IDA Daily oral iron is first line therapy for most IDA patients Many patients fail oral iron therapy – compliance, efficacy and/or side effects (constipation, GI upset) MuGard Prescriptive mucoadhesive for the management of oral mucositis, a common side effect of radiation or chemotherapy 400,000 cancer patients with oral mucositis in U.S. annually2 1 Sonis ST. Oral Oncol. 2009 Dex;45(12):1015-20. |
|
|
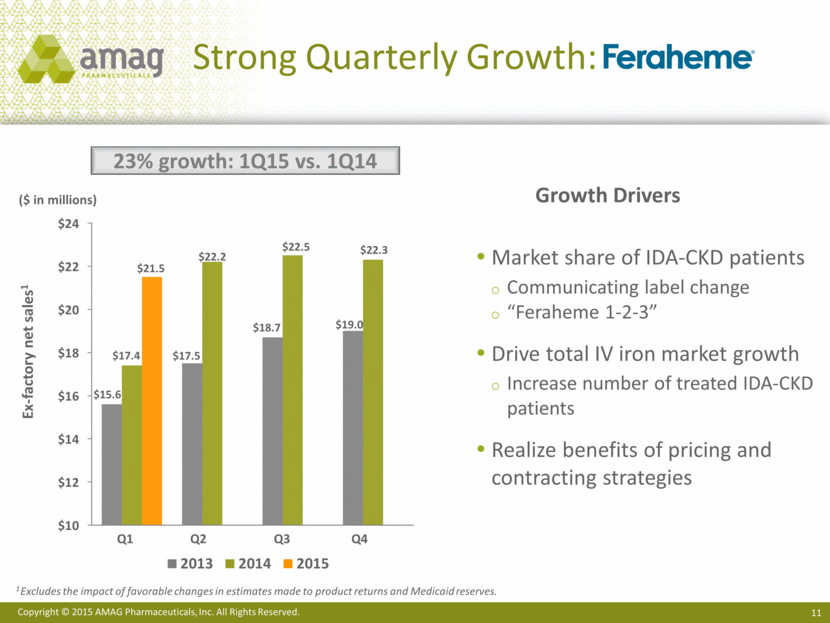
Strong Quarterly Growth: ($ in millions) Ex-factory net sales1 23% growth: 1Q15 vs. 1Q14 Market share of IDA-CKD patients Communicating label change “Feraheme 1-2-3” Drive total IV iron market growth Increase number of treated IDA-CKD patients Realize benefits of pricing and contracting strategies 1 Excludes the impact of favorable changes in estimates made to product returns and Medicaid reserves. Growth Drivers $10 $12 $14 $16 $18 $20 $22 $24 $15.6 $17.5 $18.7 $19.0 $17.4 $22.2 $22.5 $22.3 $21.5 2013 2014 2015 Q1 Q3 Q2 Q4 |
|
|
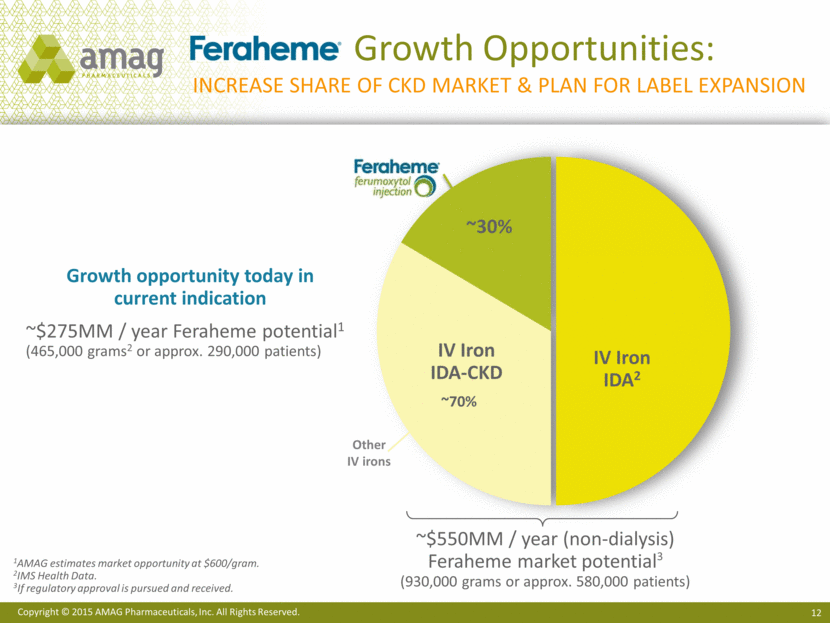
Growth Opportunities: Increase share of ckd market & plan for label expansion ~30% 1AMAG estimates market opportunity at $600/gram. 2IMS Health Data. 3 If regulatory approval is pursued and received. ~$550MM / year (non-dialysis) Feraheme market potential3 (930,000 grams or approx. 580,000 patients) IV Iron IDA2 ~70% IV Iron IDA-CKD Growth opportunity today in current indication ~$275MM / year Feraheme potential1 (465,000 grams2 or approx. 290,000 patients) Other IV irons |
|
|
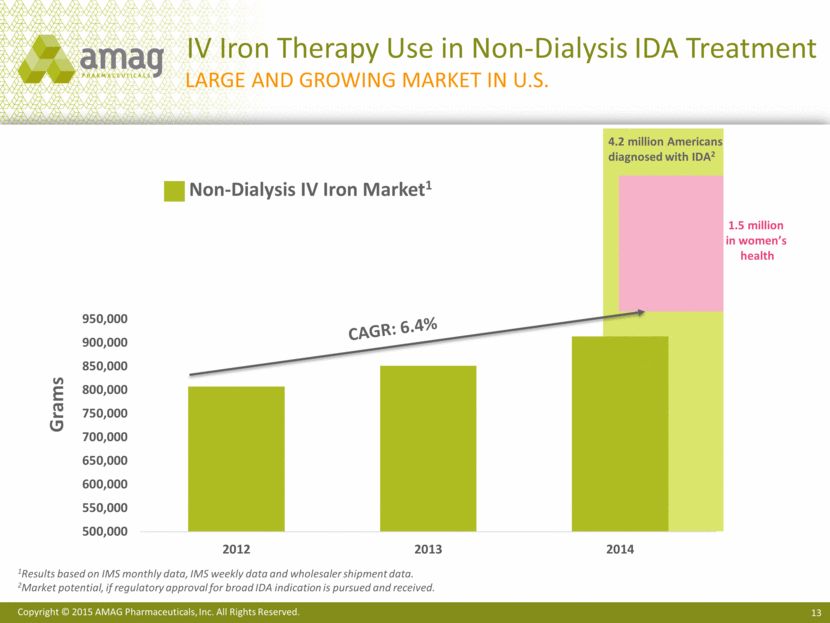
IV Iron Therapy Use in Non-Dialysis IDA Treatment 13 Large and growing market in U.S. CAGR: 6.4% 1Results based on IMS monthly data, IMS weekly data and wholesaler shipment data. 2Market potential, if regulatory approval for broad IDA indication is pursued and received. 4.2 million Americans diagnosed with IDA2 1.5 million in women’s health Non-Dialysis IV Iron Market1 Grams |
|
|
Financial Update |
|
|
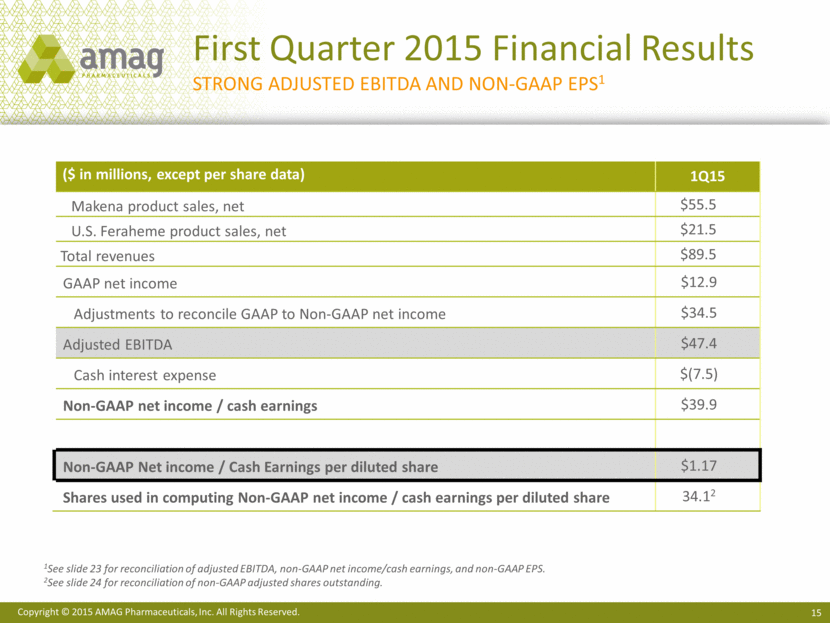
First Quarter 2015 Financial Results Strong Adjusted EBITDA and non-gaap eps1 ($ in millions, except per share data) 1Q15 Makena product sales, net $55.5 U.S. Feraheme product sales, net $21.5 Total revenues $89.5 GAAP net income $12.9 Adjustments to reconcile GAAP to Non-GAAP net income $34.5 Adjusted EBITDA $47.4 Cash interest expense $(7.5) Non-GAAP net income / cash earnings $39.9 Non-GAAP Net income / Cash Earnings per diluted share $1.17 Shares used in computing Non-GAAP net income / cash earnings per diluted share 34.12 1See slide 23 for reconciliation of adjusted EBITDA, non-GAAP net income/cash earnings, and non-GAAP EPS. 2See slide 24 for reconciliation of non-GAAP adjusted shares outstanding. |
|
|
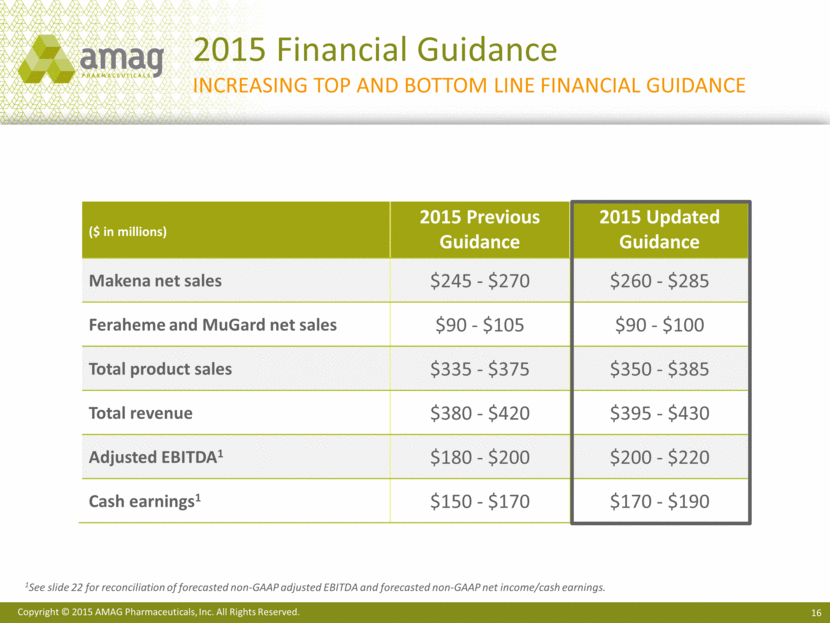
2015 Financial Guidance Increasing Top and Bottom line financial guidance 1See slide 22 for reconciliation of forecasted non-GAAP adjusted EBITDA and forecasted non-GAAP net income/cash earnings. ($ in millions) 2015 Previous Guidance 2015 Updated Guidance Makena net sales $245 - $270 $260 - $285 Feraheme and MuGard net sales $90 - $105 $90 - $100 Total product sales $335 - $375 $350 - $385 Total revenue $380 - $420 $395 - $430 Adjusted EBITDA1 $180 - $200 $200 - $220 Cash earnings1 $150 - $170 $170 - $190 |
|
|
Future Expansion Opportunities |
|
|
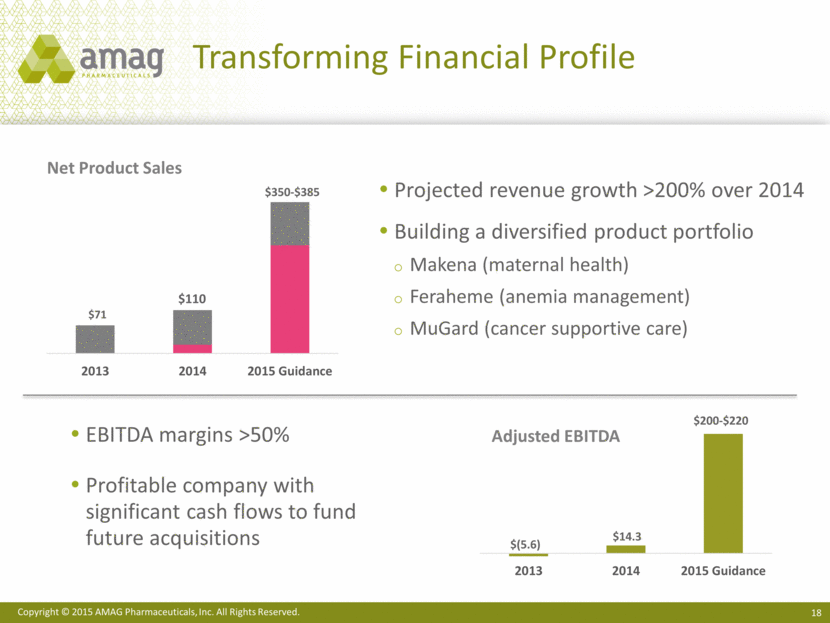
Projected revenue growth >200% over 2014 Building a diversified product portfolio Makena (maternal health) Feraheme (anemia management) MuGard (cancer supportive care) Transforming Financial Profile Net Product Sales Adjusted EBITDA EBITDA margins >50% Profitable company with significant cash flows to fund future acquisitions $350-$385 $200-$220 $14.3 2013 2014 2015 Guidance $(5.6) |
|
|
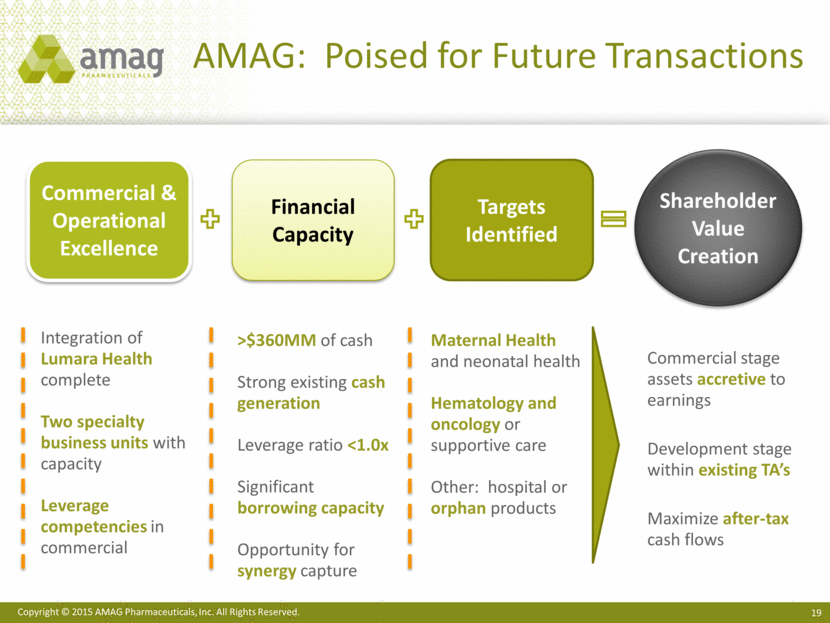
AMAG: Poised for Future Transactions Commercial & Operational Excellence Financial Capacity Targets Identified Shareholder Value Creation Integration of Lumara Health complete Two specialty business units with capacity Leverage competencies in commercial >$360MM of cash Strong existing cash generation Leverage ratio <1.0x Significant borrowing capacity Opportunity for synergy capture Maternal Health and neonatal health Hematology and oncology or supportive care Other: hospital or orphan products Commercial stage assets accretive to earnings Development stage within existing TA’s Maximize after-tax cash flows |
|
|
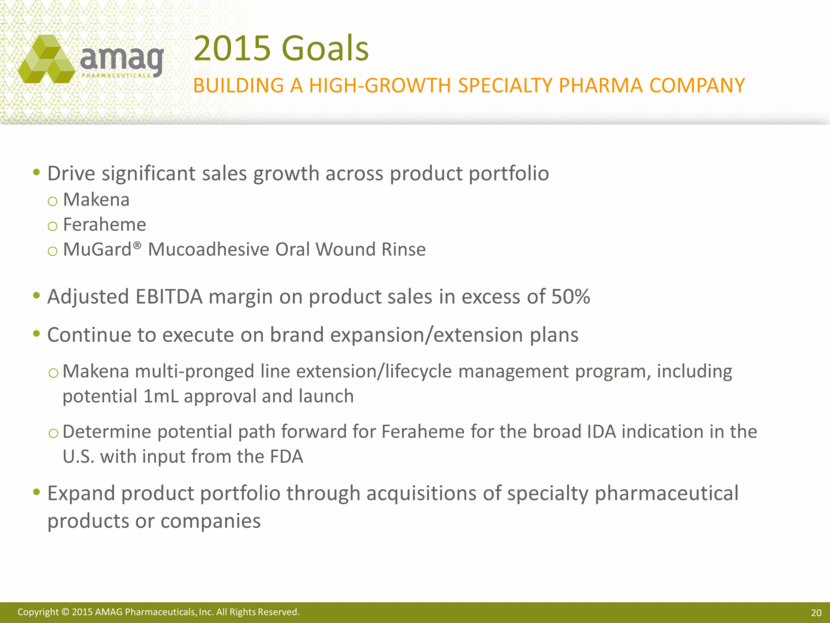
2015 Goals building a high-growth specialty pharma company Drive significant sales growth across product portfolio Makena Feraheme MuGard® Mucoadhesive Oral Wound Rinse Adjusted EBITDA margin on product sales in excess of 50% Continue to execute on brand expansion/extension plans Makena multi-pronged line extension/lifecycle management program, including potential 1mL approval and launch Determine potential path forward for Feraheme for the broad IDA indication in the U.S. with input from the FDA Expand product portfolio through acquisitions of specialty pharmaceutical products or companies |
|
|
AMAG Pharmaceuticals 1Q15 Financial Results May 5, 2015 |
|
|
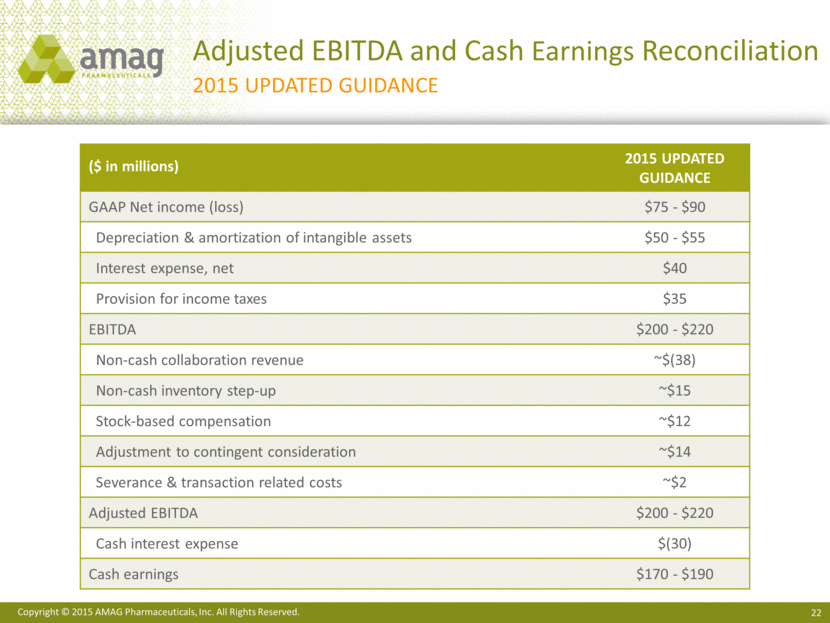
Adjusted EBITDA and Cash Earnings Reconciliation 2015 Updated Guidance ($ in millions) 2015 Updated Guidance GAAP Net income (loss) $75 - $90 Depreciation & amortization of intangible assets $50 - $55 Interest expense, net $40 Provision for income taxes $35 EBITDA $200 - $220 Non-cash collaboration revenue ~$(38) Non-cash inventory step-up ~$15 Stock-based compensation ~$12 Adjustment to contingent consideration ~$14 Severance & transaction related costs ~$2 Adjusted EBITDA $200 - $220 Cash interest expense $(30) Cash earnings $170 - $190 |
|
|
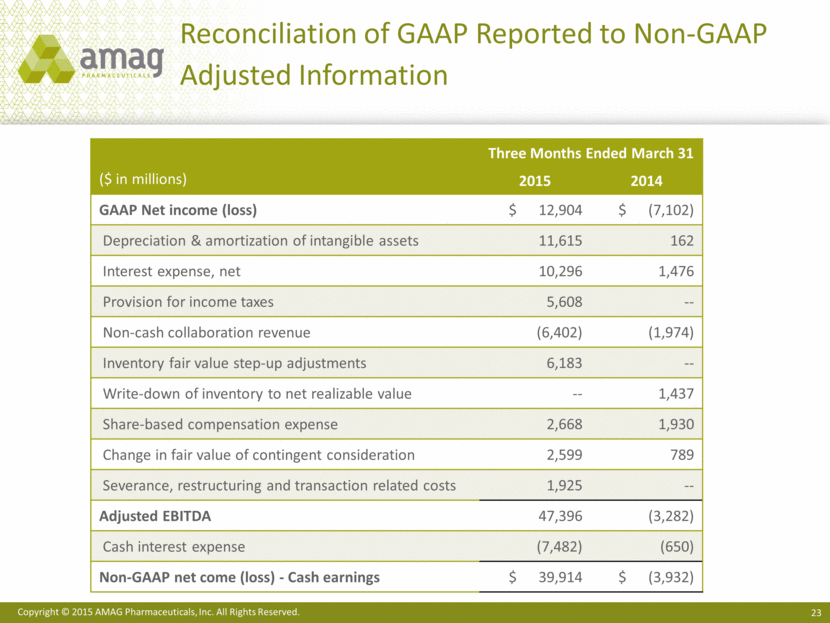
Reconciliation of GAAP Reported to Non-GAAP Adjusted Information Three Months Ended March 31 ($ in millions) 2015 2014 GAAP Net income (loss) $ 12,904 $ (7,102) Depreciation & amortization of intangible assets 11,615 162 Interest expense, net 10,296 1,476 Provision for income taxes 5,608 -- Non-cash collaboration revenue (6,402) (1,974) Inventory fair value step-up adjustments 6,183 -- Write-down of inventory to net realizable value -- 1,437 Share-based compensation expense 2,668 1,930 Change in fair value of contingent consideration 2,599 789 Severance, restructuring and transaction related costs 1,925 -- Adjusted EBITDA 47,396 (3,282) Cash interest expense (7,482) (650) Non-GAAP net come (loss) - Cash earnings $ 39,914 $ (3,932) |
|
|
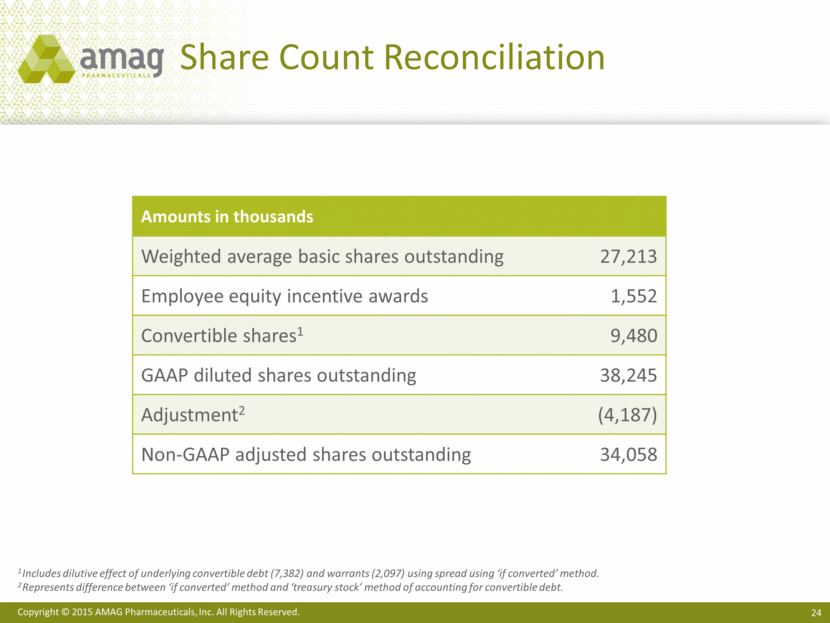
Share Count Reconciliation Amounts in thousands Weighted average basic shares outstanding 27,213 Employee equity incentive awards 1,552 Convertible shares1 9,480 GAAP diluted shares outstanding 38,245 Adjustment2 (4,187) Non-GAAP adjusted shares outstanding 34,058 1 Includes dilutive effect of underlying convertible debt (7,382) and warrants (2,097) using spread using ‘if converted’ method. 2 Represents difference between ‘if converted’ method and ‘treasury stock’ method of accounting for convertible debt. |