Attached files
| file | filename |
|---|---|
| 8-K - BIOTIME INC 8-K 4-29-2015 - Lineage Cell Therapeutics, Inc. | form8k.htm |
Exhibit 99.1

Novel Strategies for the Scalable Manufacture of Cellular Therapeutics from Pluripotent Stem Cells: Commercial Implications GTCbio 4th Stem CellProduct Development &Commercialization April 29 2015

Safe Harbor Statement 2 The matters discussed in this presentation include forward looking statements which are subject to various risks, uncertainties, and other factors that could cause actual results to differ materially from the results anticipated. Such risks and uncertainties include but are not limited to the success of BioTime in developing new stem cell products and technologies; results of clinical trials of BioTime products; the ability of BioTime and its licensees to obtain additional FDA and foreign regulatory approval to market BioTime products; competition from products manufactured and sold or being developed by other companies; the price of and demand for BioTime products; and the ability of BioTime to raise the capital needed to finance its current and planned operations. Any statements that are not historical fact (including, but not limited to statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. As actual results may differ materially from the results anticipated in these forward-looking statements they should be evaluated together with the many uncertainties that affect the business of BioTime and its subsidiaries, particularly those mentioned in the cautionary statements found in BioTime's Securities and Exchange Commission filings. BioTime disclaims any intent or obligation to update these forward-looking statements.

The Opportunity in Pluripotency 3 Scalable source of all human cell typesImmortal substrate allowing complex genetic modifications

Manufacturing Technology 1.0 ES Cells Purification ofdesired cell type Problem of impurities Differentiation Traditional Manufacture The Challenge

OPC1: Previous Phase 1 Trial 5 Feasible and Safe Five subjects received 2 mil OPC1 cells, followed for >4 years Clean safety profile observed to date:No serious adverse events related to surgery, OPC1, or immunosuppressionNo unexpected neurological changesNo adverse changes on MRIMonitoring through one year shows no evidence of immune responses to OPC1Potential evidence of biological activity:MRI results in 4 of 5 subjects are consistent with prevention of lesion cavity formation
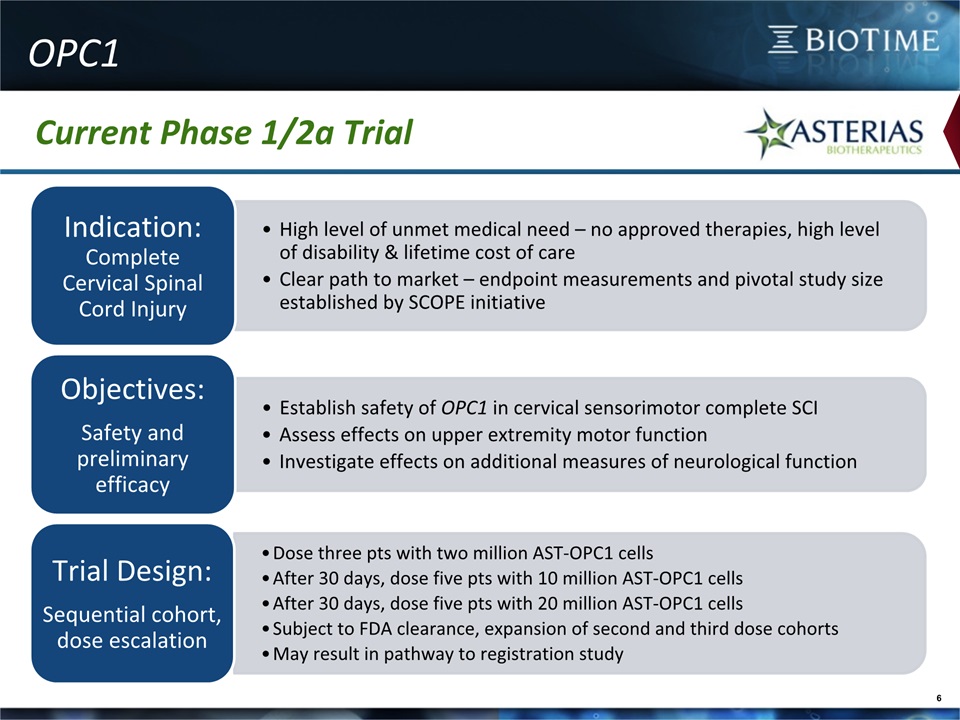
6 OPC1 Current Phase 1/2a Trial
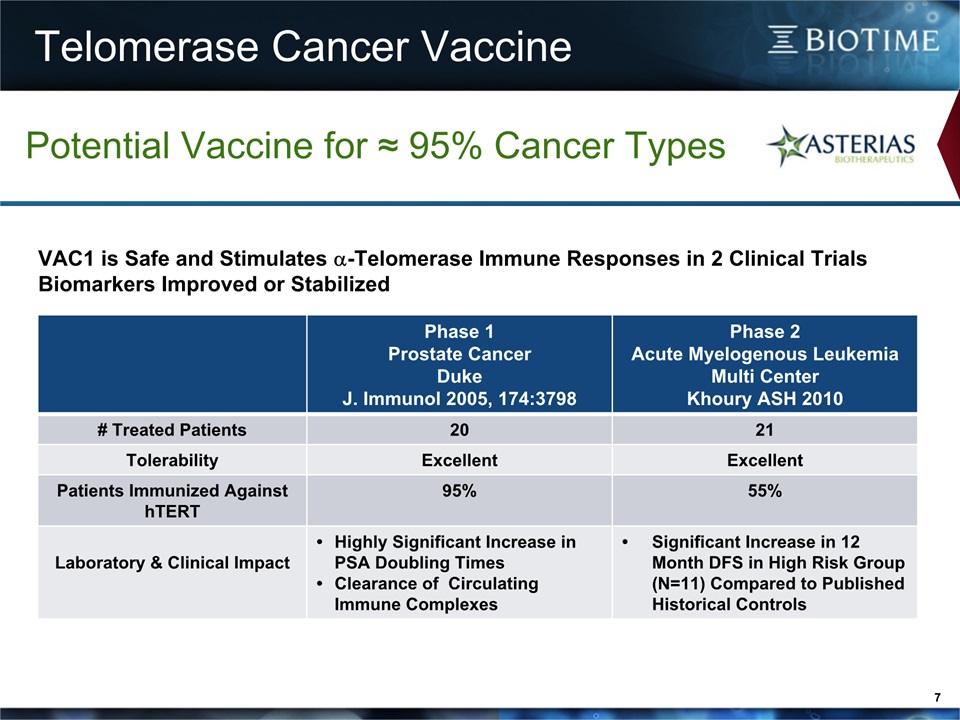
Telomerase Cancer Vaccine 7 Potential Vaccine for ≈ 95% Cancer Types Phase 1 Prostate CancerDukeJ. Immunol 2005, 174:3798 Phase 2Acute Myelogenous Leukemia Multi CenterKhoury ASH 2010 # Treated Patients 20 21 Tolerability Excellent Excellent Patients Immunized Against hTERT 95% 55% Laboratory & Clinical Impact Highly Significant Increase in PSA Doubling Times Clearance of Circulating Immune Complexes Significant Increase in 12 Month DFS in High Risk Group (N=11) Compared to Published Historical Controls VAC1 is Safe and Stimulates a-Telomerase Immune Responses in 2 Clinical Trials Biomarkers Improved or Stabilized

Telomerase Cancer Vaccine 8 The VAC2 Platform

9 1 Source: Cancer Research Technologies, 2 Source: National Institutes of Health. 3 Source: Decision Resources VAC2 Trial Design

Age-Related Macular Degeneration (AMD) OpRegen Photoreceptor function and angiogenesisinhibition depend on RPE cellsLoss of RPE cells can cause either thedry or wet forms of AMD Retinal PigmentEpithelium (RPE) Choroid Photoreceptors Drusen 10
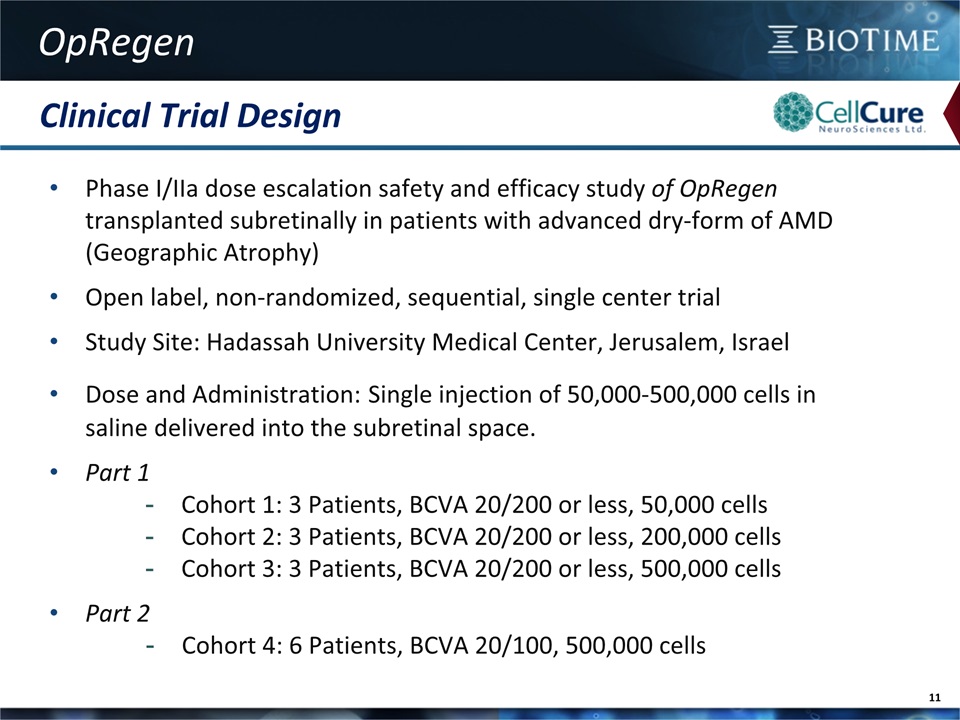
Clinical Trial Design OpRegen 11 Phase I/IIa dose escalation safety and efficacy study of OpRegen transplanted subretinally in patients with advanced dry-form of AMD (Geographic Atrophy)Open label, non-randomized, sequential, single center trialStudy Site: Hadassah University Medical Center, Jerusalem, IsraelDose and Administration: Single injection of 50,000-500,000 cells in saline delivered into the subretinal space. Part 1Cohort 1: 3 Patients, BCVA 20/200 or less, 50,000 cellsCohort 2: 3 Patients, BCVA 20/200 or less, 200,000 cellsCohort 3: 3 Patients, BCVA 20/200 or less, 500,000 cellsPart 2Cohort 4: 6 Patients, BCVA 20/100, 500,000 cells

Manufacturing Technology 1.0 12 In addition to the challenge of the >1000-fold complexity of cell types coming from ES cells, and the challenge of manufacturing pure and identified product, the highly complex fate decisions lead to a challenge of lot-to-lot variability. Embryoid Body Activin A FGF2 TGFb3 Desired Cell Type The Challenge

Challenges of Pluripotency 13 ScalabilityReproducibilityPurityIdentity
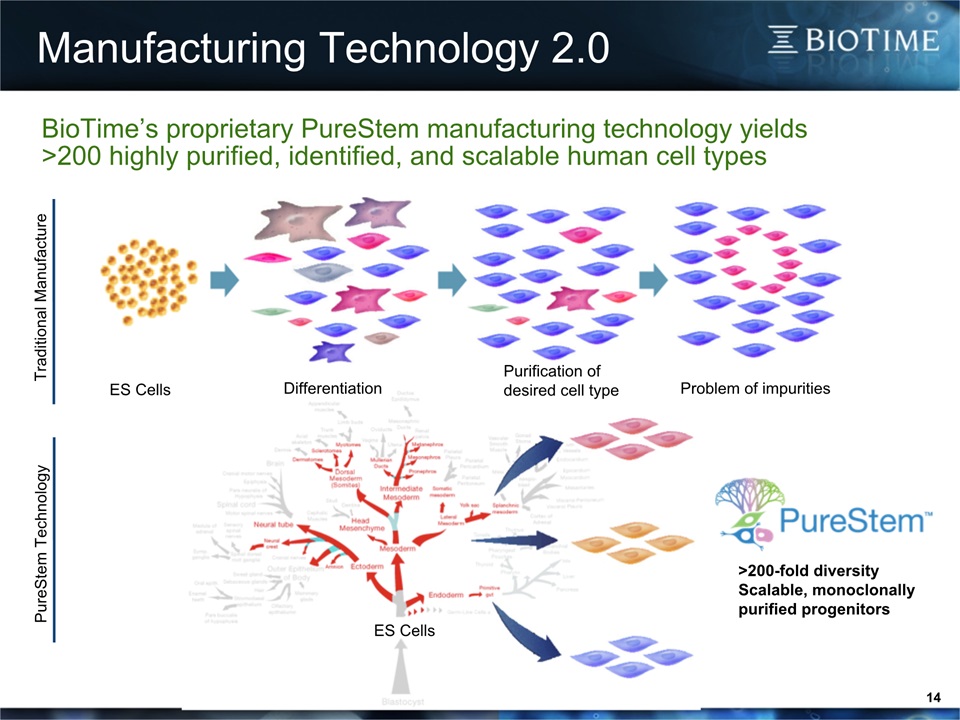
Manufacturing Technology 2.0 14 ES Cells Purification ofdesired cell type Problem of impurities Differentiation >200-fold diversityScalable, monoclonallypurified progenitors BioTime’s proprietary PureStem manufacturing technology yields >200 highly purified, identified, and scalable human cell types Traditional Manufacture PureStem Technology ES Cells

15 Manufacturing Technology 2.0

16 Manufacturing Technology 2.0
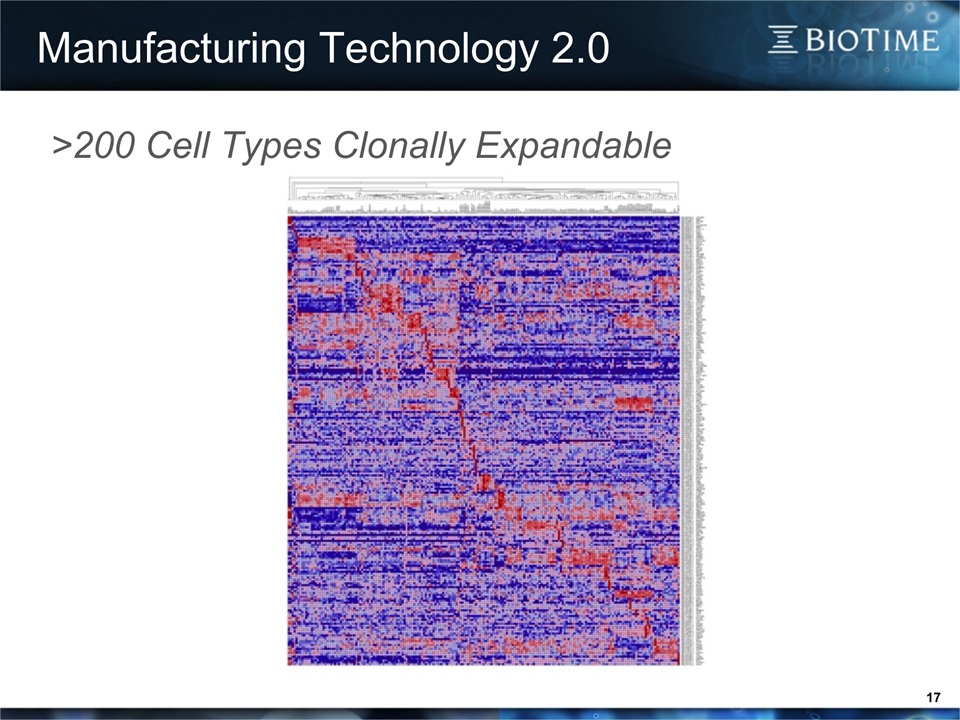
17 Manufacturing Technology 2.0 >200 Cell Types Clonally Expandable

HyStem – A Critical Combination 18 Polymerizes safely in vivo Stays as liquid for ~ 20 minutes Supports cells including adipocytes in 3-D Cast Hydrogel Cells in Sponge Injectable Multiple Formulations Durable Films 3-D Lattices Heparin-mediated Slow Release
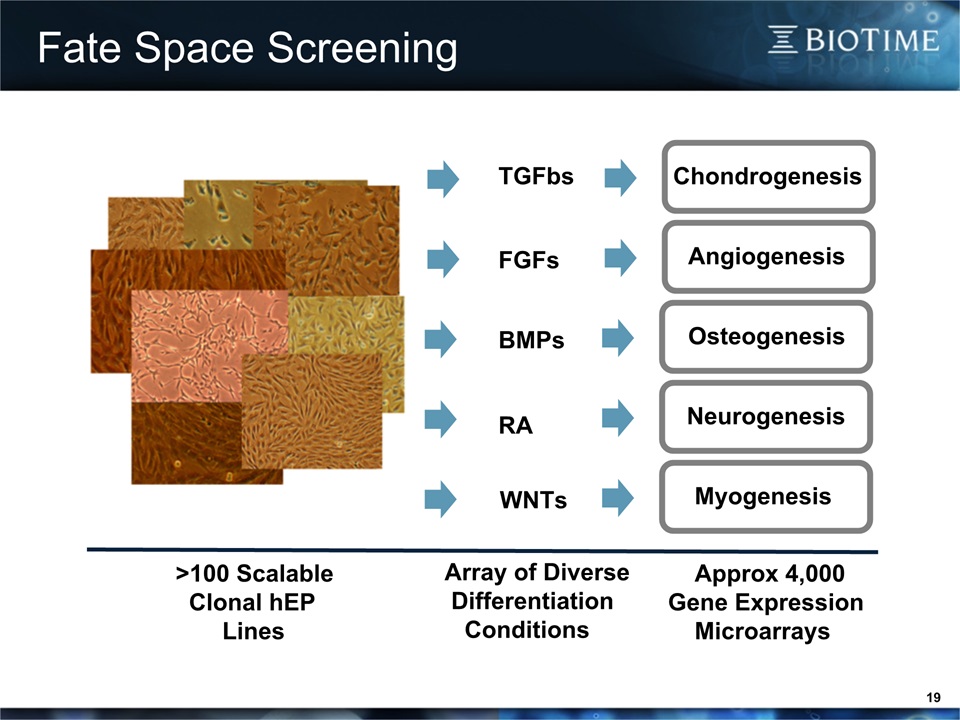
Fate Space Screening 19 Chondrogenesis Angiogenesis Osteogenesis Neurogenesis Myogenesis TGFbs FGFs BMPs RA WNTs >100 Scalable Clonal hEP Lines Array of Diverse Differentiation Conditions Approx 4,000Gene Expression Microarrays

Fate Space Screening 20 T42 in MM Culture T42 in HyStem Culture

Purified Endothelium Purity Monoclonal Endothelium GFP Endothelium (168 hrs) Monoclonal Heterogeneous
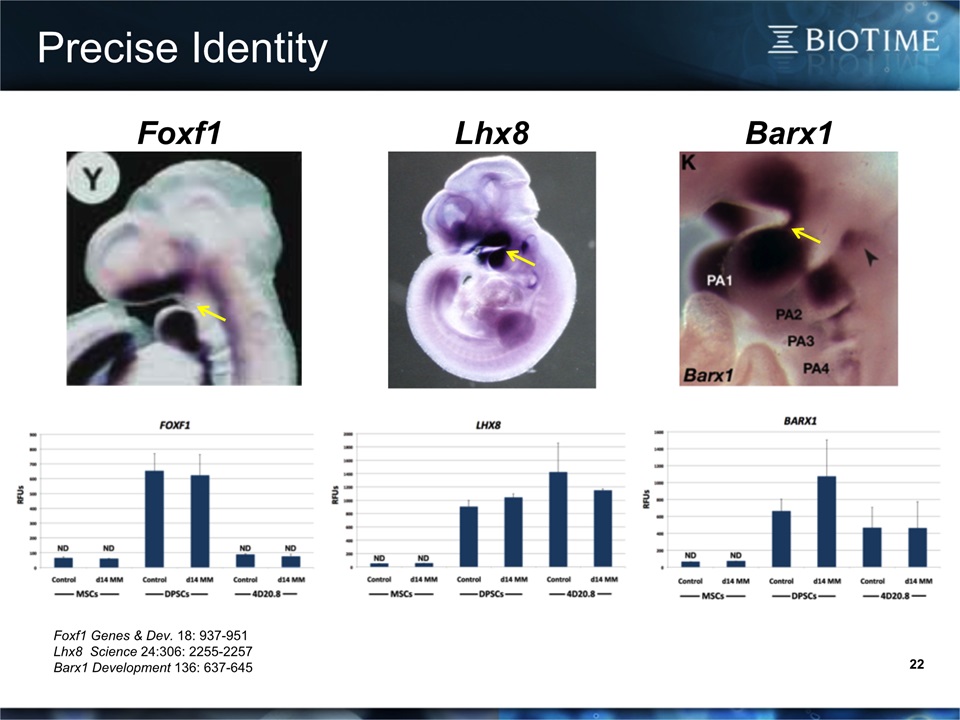
22 Precise Identity Foxf1 Genes & Dev. 18: 937-951Lhx8 Science 24:306: 2255-2257Barx1 Development 136: 637-645 Lhx8 Foxf1 Barx1
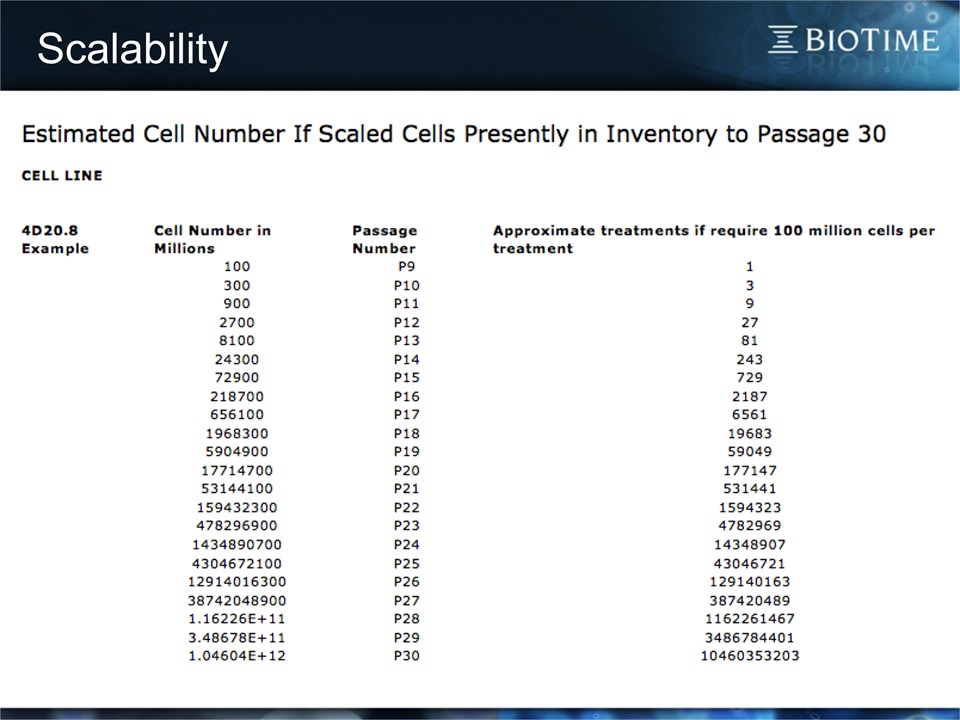
Scalability

Osteochondral Differentiation 24 NHAC MSCs 4D20.8 SM30 E15 MEL2 7SMOO32 7PEND24 SK11 COL2A1 qPCR Micromass with TGFb3 Condition

Osteochondral Differentiation 25 PureStem progenitor lines targeting orthopedics OTX-CP03 Experimentally-induced trauma 4 weeks

Reproducibility 26

Limb Bud Markers 27 Distal LPM displays unique molecular markers Taher L, Collette NM, Murugesh D, Maxwell E, Ovcharenko I, et al. (2011) Global Gene Expression Analysis of Murine Limb Development. PLoS ONE 6(12): e28358. doi:10.1371/journal.pone.0028358 MandibularMesenchyme Forelimb Hindlimb

Limb Bud Markers 28 B16 E44 RAD20.5 MSCs Xgene NHAC RASMO19 RAPEND18 C4ELSR10 SK11 SM30

29 Precise Neural Crest Cell Types E69 T42

30 Precise Neural Crest Cell Types Choroid PlexusOf 4th Ventricle MeningesOf Upper Medulla Basio-Occipital Bone CYP26B1 TTR In situ images from Genepaint.org
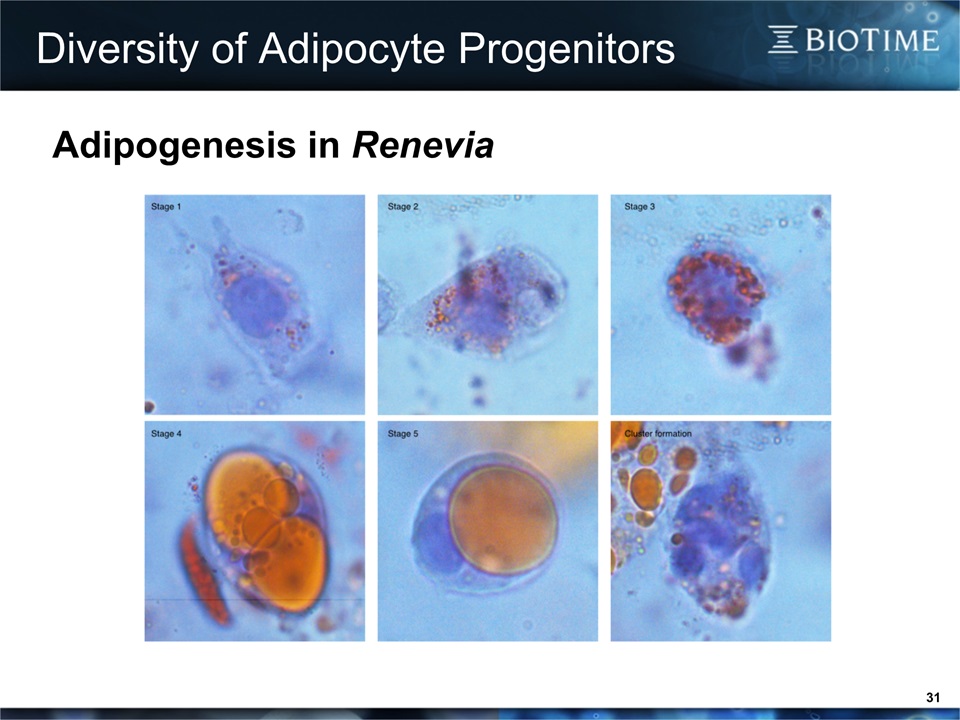
Diversity of Adipocyte Progenitors 31 Adipogenesis in Renevia

HyStem Trial - Renevia™ 32 Renevia™ is an injectable matrix designed to safely produce 3-D tissue in vivo, keeping cells where the surgeon places them. It is expected to have numerous applications in multiple tissue typesPivotal trial for CE mark for use in HIV-associated lipoatrophy in combination with autologous lipotransfer now underwayEstimated 3.5M people worldwide have HIV-related lipoatrophy In addition, a greater number of people have lipoatrophy due to trauma or agingMany other potential applications in combination with adult and ESC therapies Age-Related Lipoatrophy
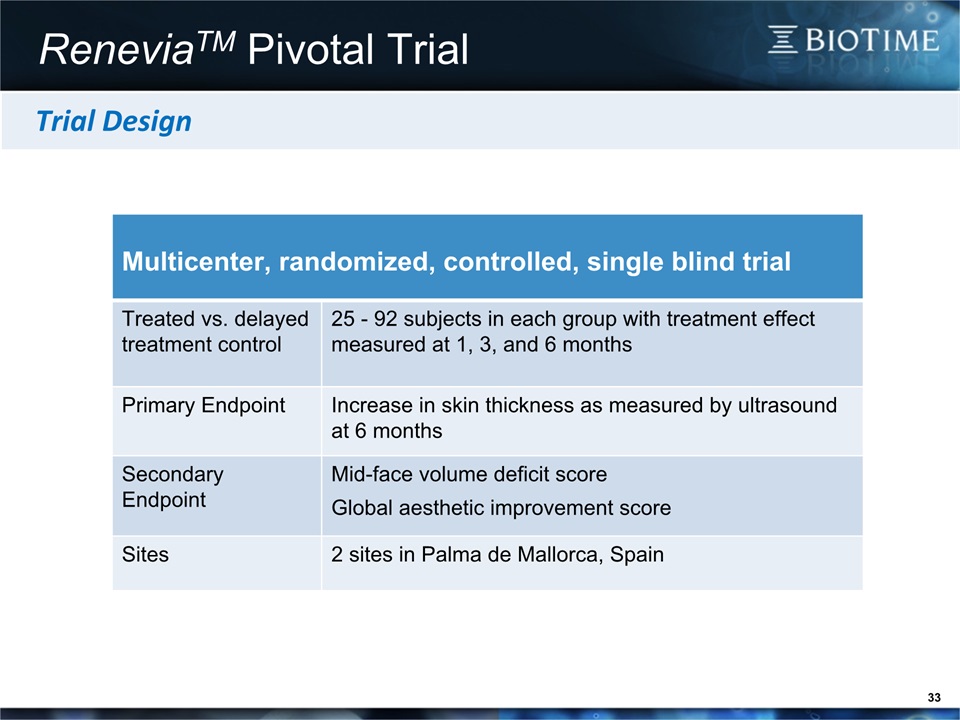
33 ReneviaTM Pivotal Trial Multicenter, randomized, controlled, single blind trial Treated vs. delayed treatment control 25 - 92 subjects in each group with treatment effect measured at 1, 3, and 6 months Primary Endpoint Increase in skin thickness as measured by ultrasound at 6 months Secondary Endpoint Mid-face volume deficit score Global aesthetic improvement score Sites 2 sites in Palma de Mallorca, Spain Trial Design
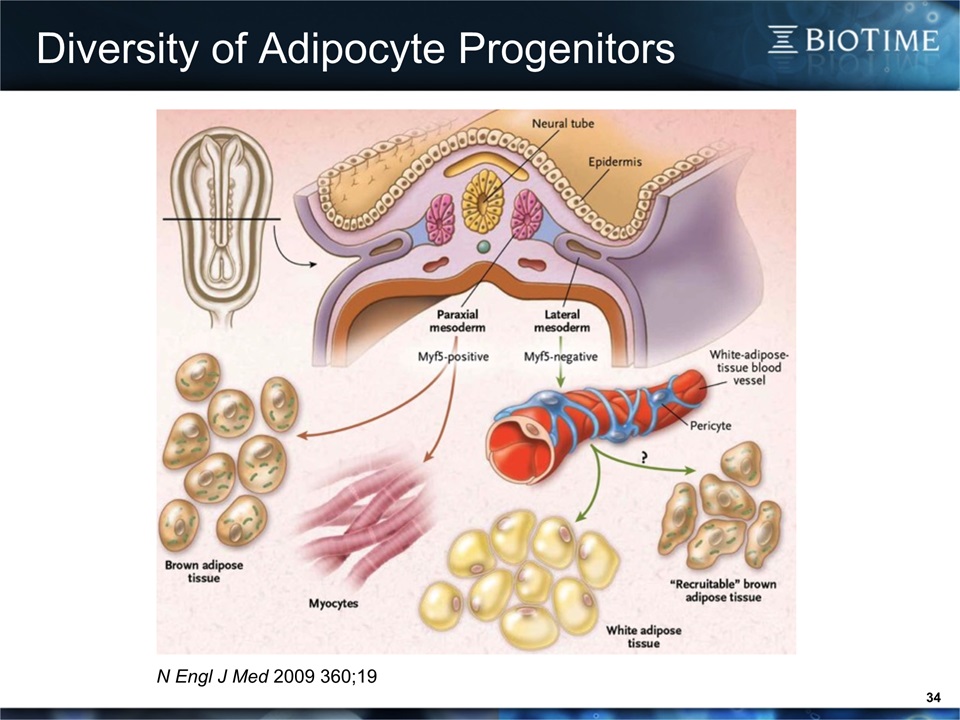
Diversity of Adipocyte Progenitors 34 N Engl J Med 2009 360;19

Brown Adipocyte Progenitors

Advantages of HyStem & Clonal EPs 36 Rare and potentially valuable cell typesScalable & reproducible productPurity and identity of cellsA formulation optimizing viability & immobilization of engraftment
