Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Adhera Therapeutics, Inc. | t1500958_8k.htm |
Exhibit 99.1

Nucleic Acid Therapeutics and Rare Diseases World Orphan Drug Congress – 2015 23 April 2015

Forward Looking Statement This document contains forward - looking statements about Marina Biotech, Inc . (the “Company”) that are based on current management expectations . These statements reflect the Company’s current views with respect to uncertain future events and are based on imprecise estimates and assumptions and are subject to risk and uncertainties . Given these uncertainties, investors should not place undue reliance on the forward - looking statements contained in or made in connection with this document . The Company’s actual results, performance or achievements could differ materially from those contemplated, expressed or implied by these forward - looking statements for a variety of reasons . The Company undertakes no duty or obligation to update or revise forward - looking statements as a result of new information, future events or changes in the Company’s expectations . Factors that could cause actual results to differ materially from those contained in the forward - looking statements in this document include, but are not limited to : ( i ) the Company’s ability to obtain additional and substantial funding when required and on acceptable terms ; (ii) the Company’s ability to attract and/or maintain research, development, commercialization and manufacturing partners ; (iii) the ability of the Company and/or a partner to successfully complete product research and development, including pre - clinical and clinical studies and commercialization ; (iv) the ability of the Company and/or a partner to obtain required governmental approvals ; (v) the ability of the Company and/or a partner to develop and commercialize products that can compete favorably with those of its competitors ; (vi) the timing of costs and expenses related to the R&D programs of the Company and/or its partners ; (vii) the timing and recognition of revenue from milestone payments and other sources not related to product sales ; (viii) the Company’s ability to obtain suitable facilities in which to conduct its planned business operations on acceptable terms and on a timely basis ; (ix) the Company’s ability to attract and retain qualified officers, employees and consultants on a timely basis ; and (x) costs associated with any product liability claims, patent prosecution, patent infringement lawsuits and other lawsuits . Additional factors that could cause actual results to differ materially from those projected or suggested in any forward - looking statements are contained in the Company’s most recent filings with the Securities and Exchange Commission (the “SEC”), including the sections entitled “Risk Factors” and “Forward - Looking Statements” contained in the Company’s Annual Report on Form 10 - K for the fiscal year ended December 31 , 2014 . You may access the Company’s filings with the SEC for free by visiting the SEC’s website at http : //www . sec . gov . 2

Marina has technology capability equal to the three largest companies in the sector, combined, . . . Marina Biotech – A Leading RNA Therapeutics Company 3 Marina Antisense siRNA microRNA mimics & antagomirs . . . and a Rare Disease Pipeline with one Phase I program and two preclinical programs!
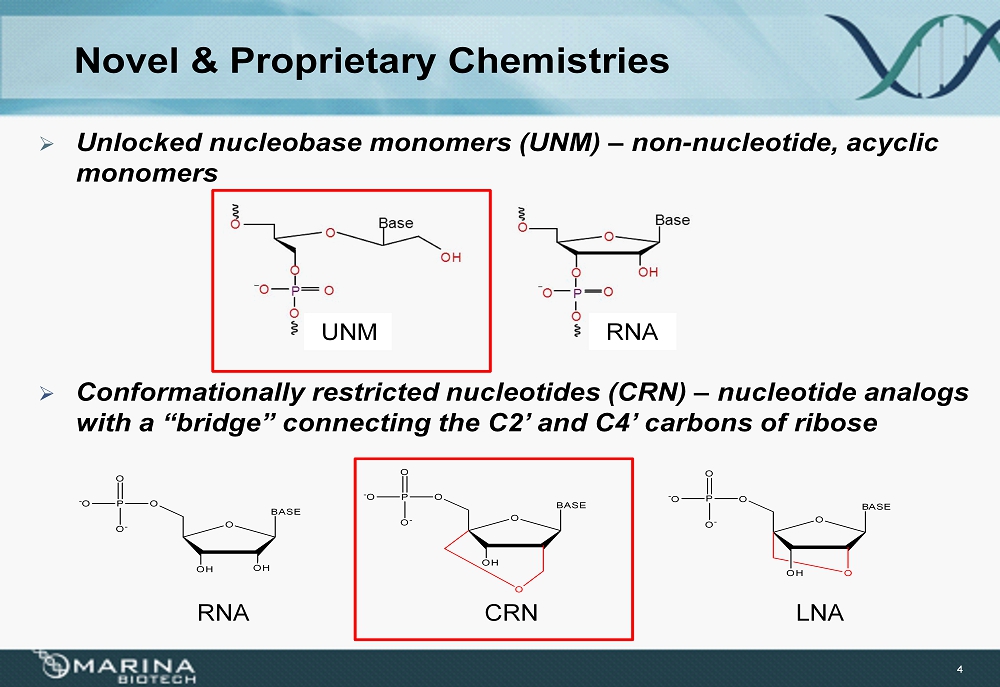
Novel & Proprietary Chemistries » Unlocked nucleobase monomers (UNM) – non - nucleotide, acyclic monomers 4 » Conformationally restricted nucleotides (CRN) – nucleotide analogs with a “bridge” connecting the C2’ and C4’ carbons of ribose RNA CRN LNA O OH BASE OH OP - O O - O OP - O O - O O OH O BASE O P - O O - O O O BASE OH RNA UNM

» SMARTICLES – Lipid - based system comprised of unique combinations of anionic and cationic lipids » DiLA2 – Lipid - based system comprised of unique combinations of naturally occurring or man - made amino acid head groups, linkers and alkyl chains » tkRNAi – Bacterial - based system utilizing n on - pathogenic bacteria engineered to produce, deliver and release nucleic acids to targeted tissue » Trp Cage Phage Display Library – Peptide - based system for identification of cell - targeting peptides for conjugation directly to nucleic acid compounds (e.g. ASOs) or other delivery systems (e.g. SMARTICLES ) 5 Novel & Proprietary Delivery Systems Potential Range of Administration: I.V ., subQ , Local, Topical and Oral

» Two separate and distinct delivery technologies in clinical development » SMARTICLES (lipid nanoparticle) » tk RNAi (engineered, non - pathogenic bacteria) » Only orally administered RNAi - based therapeutic ( tk RNAi ) in clinical development » Most versatile delivery system (SMARTICLES) in clinical development – Phase I & II » Effective d elivery of single - stranded and double - stranded nucleic acids » Delivery to cell nucleus and cell cytoplasm » Delivery outside the liver, i.e. tumors » Most widely adopted clinical stage delivery technology in the RNA therapeutic sector – second only to Tekmira in number of patients dosed (~100) » Third behind Tekmira (8 programs with LNP) and Alnylam (3 programs with GalNac ) in number of therapeutic compounds currently in clinical development » Self - assembly of lipids and oligonucleotides into liposomes » Negatively charged under physiological conditions and absence of PEG is a distinct approach from positively charged cationic liposomes 6 Marina’s Delivery Capability is Unparalleled Within the Sector

7 Clinical Program Pipeline Development Stage Program Myotonic Dystrophy Pre - IND Studies Phase I CEQ508 - FAP Duchenne Muscular Dystrophy IND 2017 2016 2017 2017 2018 2017 2017 2018 2019 Phase II Lead Selection

Familial Adenomatous Polyposis (FAP) » Rare hereditary disease » Mutation in Adenomatous Polyposis Coli (APC) gene » Causes dysregulation and accumulation of β - catenin » Results in numerous colon polyps appearing in early adolescence with potential for rapid disease progression » Clinical drug product, CEQ508, targets β - catenin oncogene » Unmet medical need » ~100,000 worldwide (orphan status) » Near 100% risk of colon cancer if untreated » Treatment options: » Surgical intervention (colectomy) is the only available treatment to prevent colon cancer progression » No generally accepted pharmaceutical approach is available » Currently, no other significant pharmaceutical advancements in clinical development » Opportunities to expand into sporadic CRC, other polyposis syndromes and other GI cancers 8 Potential for commercial launch in 2018

TransKingdom RNAi ( tk RNAi ™) 9 to Dicer for processing Bacterial lysis in endosome Receptor - mediated cell entry Endosome lysis and shRNA release into cytoplasm Invasin receptor Invasin shRNA Compound is taken orally by the patient Only orally a vailable RNAi clinical c andidate

Nucleic Acid - based Strategies for Therapeutic Intervention in DM1 10 Accumulation of altered DMPK mRNA in nucleus sequesters Muscleblind (MBNL1) protein resulting in loss - of - function » Steric Blocking » Displace/disrupt the MNBL - CUG n+x interaction » mRNA Translational Inhibition » RNAi or RNase - dependent degradation of DMPK mRNA » Splice Junction Inhibition » Modulate CUG n+x via altered splicing of DMPK gene to reduce/skip CUG n+x

Ability to Move Multiple Compounds into Preclinical Development for a Single Indication 11 Targeting DMPK mRNA to increase degradation and elimination Targeting the CUG - repeat for degradation/elimination or compete with proteins for binding sites Targeting intron - exon s plice to bias for less - toxic or non - toxic variants RNase H - dependent Translational Blocker Steric Blocker RNase H - dependent Translational Blocker RNAi - dependent Translational Blocker Splice Junction Inhibitor = CRN = UNM Creating a more robust clinical program with leads and back - ups Compounds may include delivery formulation or targeting moiety Multiple approaches for DM1

Partner of Choice for Rare Diseases » Marina Biotech has multiple novel and proprietary b iochemistries and delivery technologies » Development and delivery of single - and double - stranded constructs » Marina Biotech has the broadest, nucleic acid drug discovery platform » Validated drug discovery platform and most widely licensed technologies, licensees include: Mirna , MiNA , Monsanto, Novartis, ProNAi , Roche, Tekmira » Broad and young IP estate with over 140 issued/allowed patents and over 90 pending U.S. and foreign applications » Marina Biotech has the most versatile delivery technology in clinical development » Only technology delivering single - and double - stranded oligonucleotides » Only platform delivering RNA therapeutics systemically and orally » Marina Biotech has the ability to pursue robust clinical development programs 12

Thank You 13
