Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Aevi Genomic Medicine, Inc. | v407560_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Aevi Genomic Medicine, Inc. | v407560_ex99-1.htm |
Exhibit 99.2

Q1 2015 Results April 17, 2015

Forward Looking Statement This presentation includes certain estimates and other forward - looking statements within the meaning of Section 21 E of the Securities Exchange Act of 1934 , as amended, including statements with respect to anticipated operating and financial performance, clinical results, potential partnerships, licensing opportunities and other statements of expectation . Words such as “ expects, ” “ anticipates, ” “ intends, ” “ plans, ” “ believes, ” “ assumes, ” “ seeks, ” “ estimates, ” “ should ” and variations of these words and similar expressions, are intended to identify these forward - looking statements . While we believe these statements are accurate, forward - looking statements are inherently uncertain and we cannot assure you that these expectations will occur and our actual results may be significantly different . These statements by the Company and its management are based on estimates, projections, beliefs and assumptions of management and are not guarantees of future performance . Important factors that could cause actual results to differ from those in the forward - looking statements include the factors described in the Company ’ s filings with the U . S . Securities and Exchange Commission . The Company disclaims any obligation to update or revise any forward - looking statement based on the occurrence of future events, the receipt of new information, or otherwise . 2

Agenda 3 • Q1 Operational Update • Q1 Financial Update • CHOP Collaboration • 2015 Milestones

Q1 2015 Operational Update • Low - dose cohort fully enrolled – Positive response to therapy at ~100x lower c max and 10x lower overall exposure vs rHuEPO – Low - dose well tolerated; no treatment related SAE’s • Demonstrated ability to maintain Hb in target range for up to 10 months to date • Initiated enrollment in mid - dose cohort • Initiated TARGT EPO Study in Peritoneal Dialysis – Enrolled first patient in Israel – Filed IND with FDA for TARGT EPO renal failure indications – Anticipate initiating US Peritoneal Dialysis trial by mid year • Advanced CHOP and Harvard Collaborations – Anticipate initial program from CHOP to be announced mid - year – Preclinical data expected from Harvard CNS program H2 2015 4

MDGN - 201 (TARGT EPO ) – Open Label Ascending Dose 5 Clinical endpoints: (1) Serum eEPO levels (2) Hb levels (3) Duration (4) Safety Study Summary Group Treatment N A Low dose eEPO 18 - 25 IU/kg/day 3 + 3 * B Mid dose eEPO 35 - 45 IU/kg/day 3 + 3 * C High dose eEPO 55 - 65 IU/kg/day 3 + 3 * MDGN - 201 Phase 1/2 Trial Design Enrollment and dose selection Screening Harvest procedure Ex vivo viral transduction 9 days Treatment and follow - up 12 months Safety follow - up 6 months Implantation procedure 4 weeks run - in * Initial cohort of 3 , expandable to 6 based on response Objective : determine dose of TARGT EPO sufficient to maintain hemoglobin in target range [9 - 11,<12 g/ dL ] for > 6 months in ESRD patients
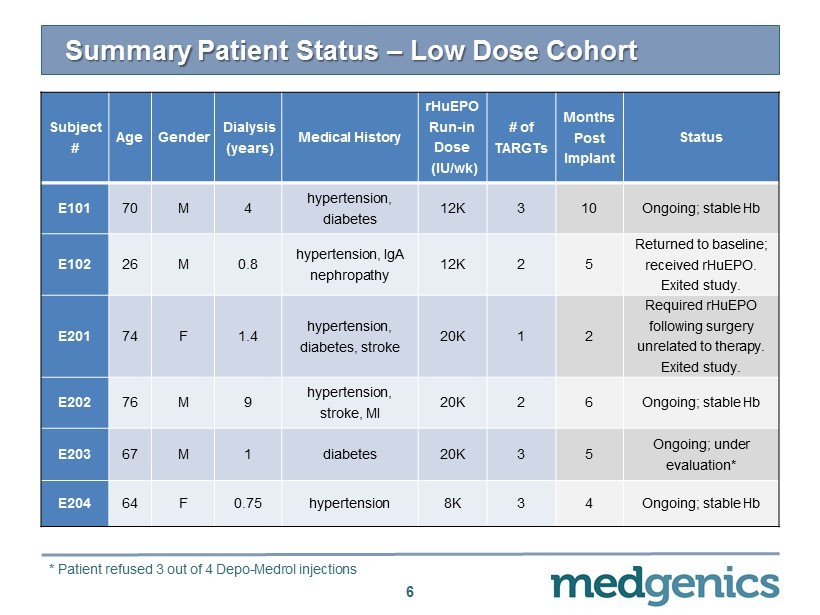
Summary Patient Status – Low Dose Cohort 6 Subject # Age Gender Dialysis (years) Medical History rHuEPO Run - in Dose (IU/ wk ) # of TARGT s Months Post Implant Status E101 70 M 4 hypertension, diabetes 12K 3 10 Ongoing ; s table Hb E102 26 M 0.8 hypertension, IgA nephropathy 12K 2 5 Returned to baseline; received rHuEPO . Exited study. E201 74 F 1.4 hypertension, diabetes, stroke 20K 1 2 Required rHuEPO following surgery unrelated to therapy. Exited study. E202 76 M 9 hypertension, stroke, MI 20K 2 6 Ongoing; stable Hb E203 67 M 1 diabetes 20K 3 5 Ongoing; under evaluation* E204 64 F 0.75 hypertension 8K 3 4 Ongoing; stable Hb * Patient refused 3 out of 4 Depo - Medrol injections

Potential Orphan Indications for TARGT EPO 7 INDICATION PATIENT / PAYOR NEED CLINICAL RATIONALE ESTIMATED PREVALENCE PERITONEAL DIALYSIS (PD) PD provides 20% cost reduction vs hemodialysis Improved compliance will boost outcomes and reduce costs for PD patients 20,000 (US) 6 ANEMIC CKD TRANSPLANT CANDIDATES Heightened immunogenic profile due to repeat transfusions Reduced transfusions will prevent increased immunogenicity, shorten wait times and improve transplant outcomes 10,000 (US) 6 HYPO - RESPONDERS TO rHuEPO Unable to manage anemia on high doses of rHuEPO ; often leading to hypertension Continuous, physiologic levels of eEPO will stimulate bone marrow more effectively and safely than intermittent IV dosing 5 40,000 – 70,000 (US) 6 MYELODYSPLATIC SYNDROMES (MDS) 20 - 55% of MDS patients respond to rHuEPO but use limited due to safety concerns 3 Reduced need for transfusions would limit iron overload 60,000 (US) 4 BETA THALASSEMIA INTERMEDIA Requires frequent transfusions (up to 12 per annum) rHuEPO shown ability to raise Hb in beta thalassemia 1 ; safety concerns limit use 15,000 (US/EU) 2 1. Oliveri NF et al. Blood 1992 , 80:3258 - 60 2. Weatherall , D.J. and Clegg, J.B. Bulletin of the World Health Organization , 2001 , 79 : 704 – 712 . 3. Giraldo , P. et al Cancer : 107 - 2807 - 14 4. Am Journal Med. 2012 , Jul: 125 ( 7 Suppl ):S 2 - 5 5 . Source : Besarab , A. Semin Nephrol . 2000 ; 20 ( 4 ): 364 6 . U.S . Renal Data System, USRDS 2013 Annual Data Report and primary company research
CHOP Collaboration 8
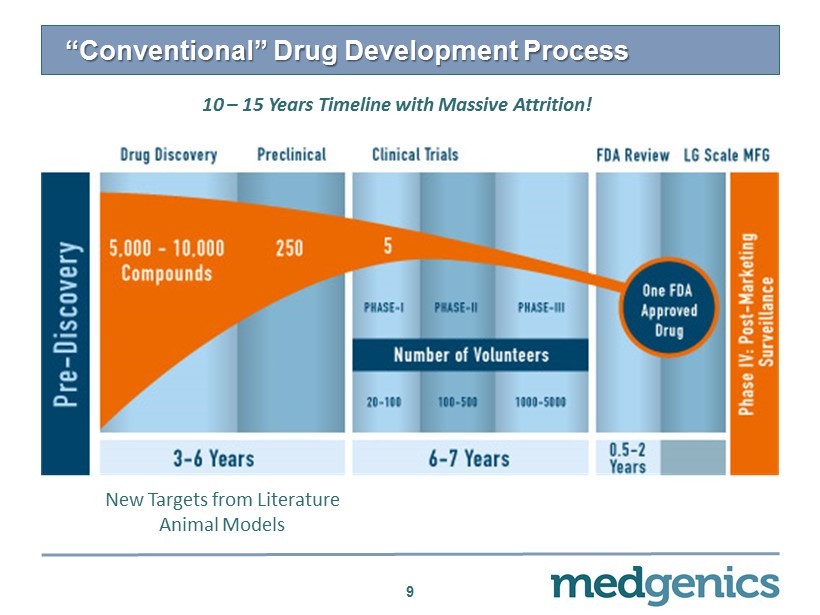
“Conventional” Drug Development Process 9 10 – 15 Years Timeline with Massive Attrition! New Targets from Literature Animal Models

Genomic Guided Precision Medicine Precision medicine is an emerging approach to disease treatment and prevention based on individual genetic and lifestyle/environmental variation Why now? Cheap and fast genome sequencing Availability of new tools for bioanalytics and large clinical datasets Government/payer support 10

Precision Development A pproach 11 Phenotype (Inherited/Extreme) Tissue Banking Screening/Genomics Hypothesis/Mechanism New “Validated” Target(s)

Precision Drug D evelopment A pproach Phenotype Validated Target & Mechanism Native Peptide/Protein Preclinical POC Enabling Tox Exploratory IND PD Markers Dosing POC Phase IB/II Dose Confirmation Pivotal Trials Submission & Approval 12 12 mos 12 mos 12 mos 12 - 24 mos 12 mos 5 - 6 Yrs

CHOP Medgenics Collaboration Prioritization Criteria • Well characterized and genetically defined rare/orphan disease with clear diagnostic and phenotypic criteria • Serious disease with significant impact on health and/or lifespan of affected individuals • Sufficient characterization of natural history of the disease to allow design of clinical intervention trials • Potential to substantially improve health outcomes for patients by intervention in the identified pathway(s) beyond outcomes achievable by existing therapies • At least 1,000 affected individuals in developed markets – or potential to expand intervention to related populations of at least 1,000 individuals 13

Collaboration Update 14 PARTNER SCOPE KEY DELIVERABLES Harvard University / Massachusetts General Hospital Funding of research program to a ssess the viability of TARGT micro - organs in the CNS Initiated collaboration and anticipate results from initial preclinical experiments mid - year. Stanford University License to novel AAV vector, pLK19, for ex vivo use in the TARGT platform Provide vector flexibility for TARGT platform . GMP manufacturing in H2 2015.

Financial Update 15
Q1 2015 Financial Update • Net R&D expenses for the 1 st Quarter were $3.9MM increasing from $2.1MM for the same period in 2014 due mainly to increased materials and sub - contractor costs and increased stock - based compensation expenses related to options granted to R&D personnel . • G&A expenses for the 1 st Quarter were $3.9MM increasing from $3.1MM for the same period in 2014 primarily due to increased stock - based compensation expenses related to options granted to directors and general and administrative personnel, offset in part by a decrease in professional fees . • Cash balance as of March 31 , 2015 was $25.2MM 16
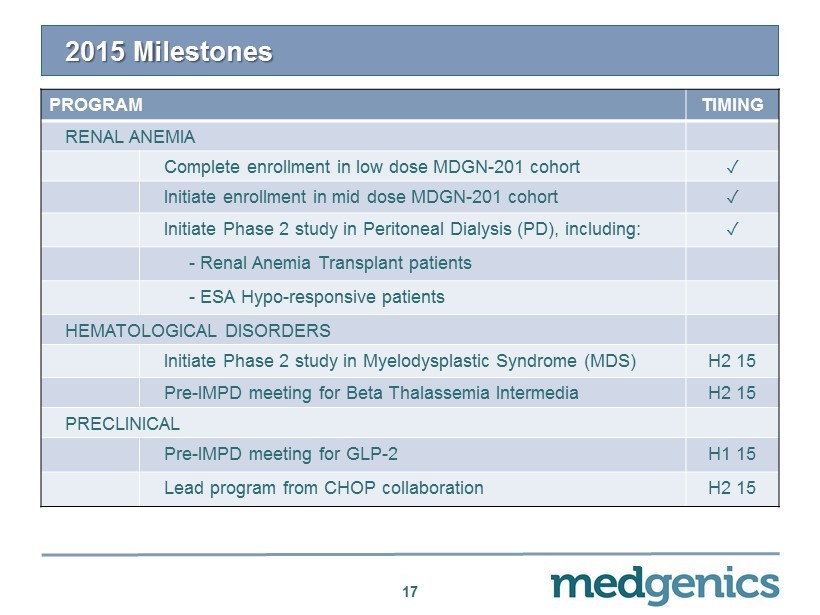
2015 Milestones 17 PROGRAM TIMING RENAL ANEMIA Complete enrollment in low dose MDGN - 201 cohort ✓ Initiate enrollment in mid dose MDGN - 201 cohort ✓ Initiate Phase 2 study in Peritoneal Dialysis (PD), including: ✓ - Renal Anemia Transplant patients - ESA Hypo - responsive patients HEMATOLOGICAL DISORDERS Initiate Phase 2 study in Myelodysplastic Syndrome (MDS) H2 15 Pre - IMPD meeting for Beta Thalassemia Intermedia H2 15 PRECLINICAL Pre - IMPD meeting for GLP - 2 H1 15 Lead program from CHOP collaboration H2 15
