Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Lipocine Inc. | v407529_8-k.htm |

Efficacy & Pharmacokinetics of LPCN 1021, Novel Oral TRT ─ Obese & Non - Obese Hypogonadal Men Exhibit 99.1

Adrian Dobs • Research funding (Clinical researcher): Clarus, Endo; Honoraria (Advisory group): AbbVie , Endo, Lipocine , Inc. Anthony DelConte • Consulting fees: Lipocine, Inc. Martin Miner • Research funding (Clinical researcher): Forest, NERI; Consulting fees: AbbVie, Lipocine , Inc., Repros Jed Kaminetsky • Research funding (Principal investigator): AbbVie , Antares, Endo, Clarus, Ferring , Lipocine, Inc.; Consulting fees, Antares , Endo; Contracted fee (Speaker ): Endo Christina Wang • Research funding (Clinical researcher): Antares, Clarus, Lipocine , Inc., Prolor ; Consulting fees: Clarus, Lipocine; Honoraria (Advisory group): TesoRx Rest of the authors nothing to disclose Disclosures 2
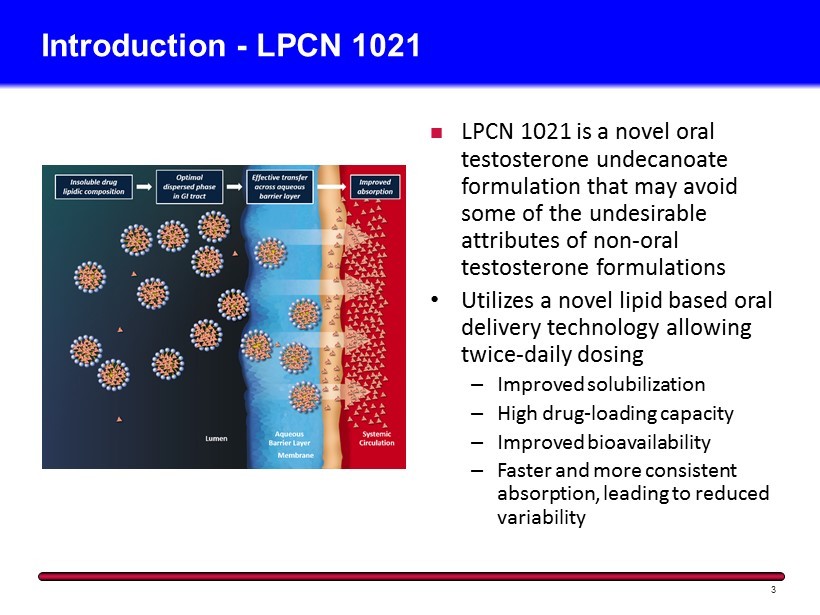
Introduction - LPCN 1021 3 LPCN 1021 is a novel oral testosterone undecanoate formulation that may avoid some of the undesirable attributes of non - oral testosterone formulations • Utilizes a novel lipid based oral delivery technology allowing twice - daily dosing – Improved solubilization – High drug - loading capacity – Improved bioavailability – Faster and more consistent absorption, leading to reduced variability
Males 18 - 80 years of age with documented onset of hypogonadism prior to age 65 Documented diagnosis of primary hypogonadism (congenital or acquired) or hypogonadotropic hypogonadism (congenital or acquired ) Serum T <300 ng/dL based on 2 blood samples, taken approximately same time of day on 2 different days (between 6 a.m. and 10 a.m.) Naïve to androgen replacement or discontinued current treatment and completed a washout of prior androgen therapy • Washout: 12 weeks following intramuscular androgens, 4 weeks following topical/buccal, 3 weeks following oral; or investigator discretion Key Inclusion Criteria 4
Abnormal prostate exam (I - PSS score >19 points) Body mass index (BMI) ≥38 kg/m 2 Clinically significant abnormal laboratory value Concurrent medications that could affect PK measurements or patient health Partner who is concurrently pregnant or planning to become pregnant Key Exclusion Criteria 5
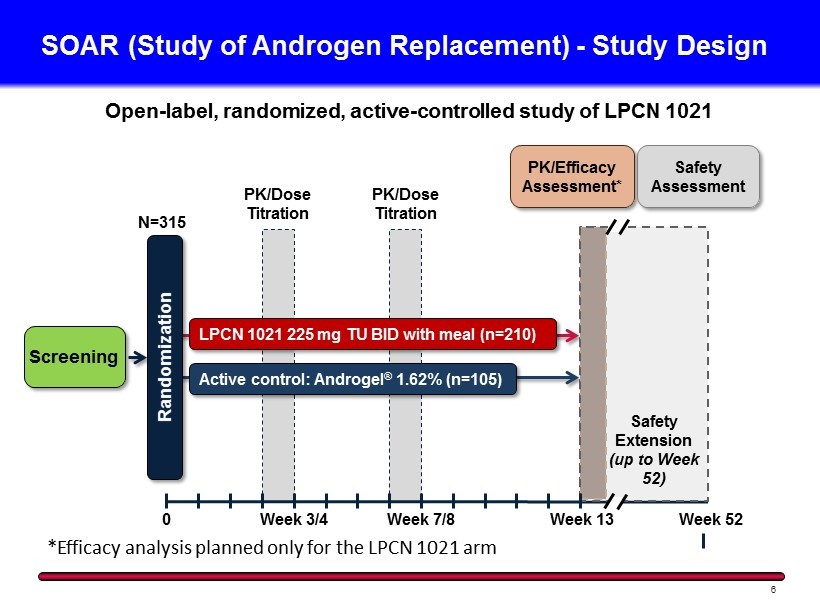
SOAR (Study of Androgen Replacement) - Study Design 6 Screening N=315 0 Week 3/4 Week 7/8 Randomization LPCN 1021 225 mg TU BID with meal (n=210 ) Active control: Androgel ® 1.62% (n=105 ) PK/Dose Titration PK/Dose Titration PK/Efficacy Assessment* Safety Assessment Week 13 Week 52 Safety Extension (up to Week 52) Open - label, randomized, active - controlled study of LPCN 1021 *Efficacy analysis planned only for the LPCN 1021 arm
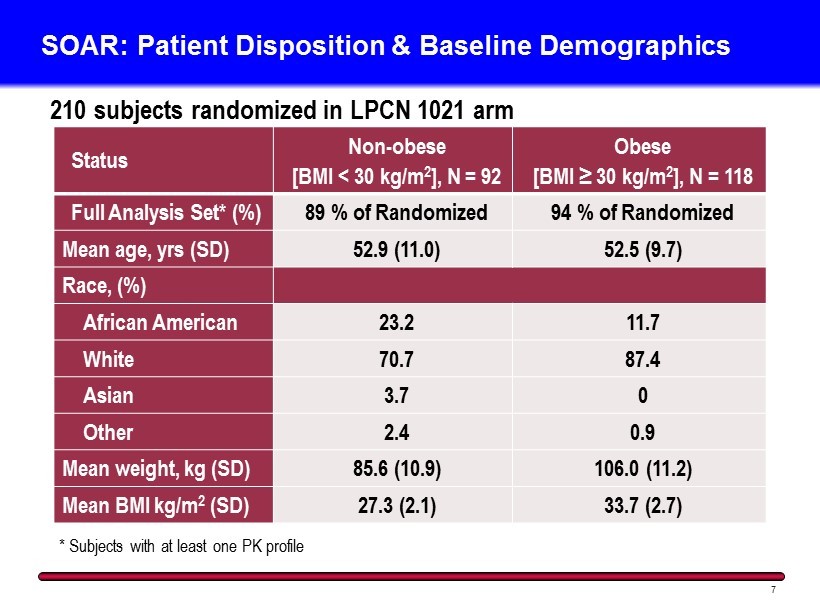
SOAR: Patient Disposition & Baseline Demographics 7 210 subjects randomized in LPCN 1021 arm Status Non - obese [BMI < 30 kg/m 2 ], N = 92 Obese [BMI ≥ 30 kg/m 2 ], N = 118 Full Analysis Set* (%) 89 % of Randomized 94 % of Randomized Mean age, yrs (SD) 52.9 (11.0) 52.5 (9.7) Race, (%) African American 23.2 11.7 White 70.7 87.4 Asian 3.7 0 Other 2.4 0.9 Mean weight, kg (SD) 85.6 (10.9) 106.0 (11.2) Mean BMI kg/m 2 (SD) 27.3 (2.1) 33.7 (2.7) * Subjects with at least one PK profile
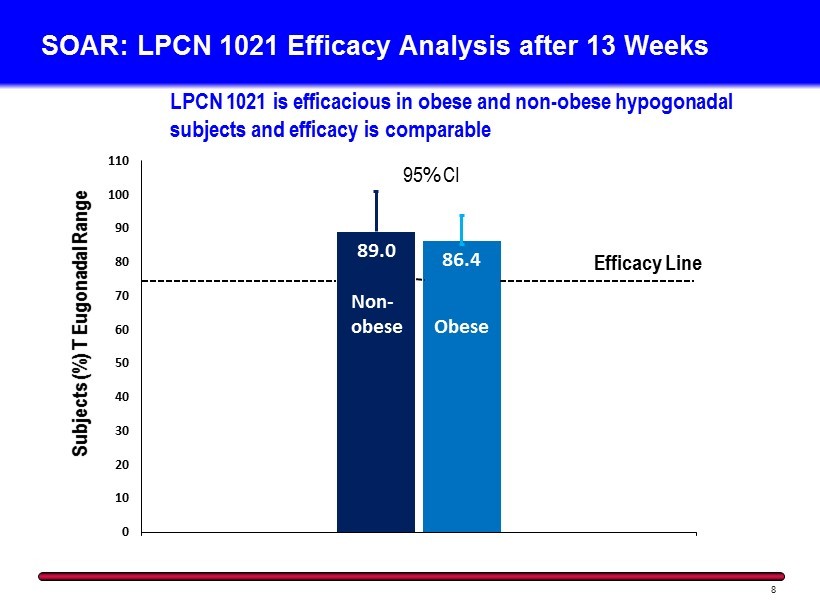
SOAR: LPCN 1021 Efficacy Analysis after 13 Weeks 8 89.0 86.4 0 10 20 30 40 50 60 70 80 90 100 110 Subjects (%) T Eugonadal Range Obese Efficacy Line LPCN 1021 is efficacious in obese and non - obese hypogonadal subjects and efficacy is comparable Non - obese 95% CI
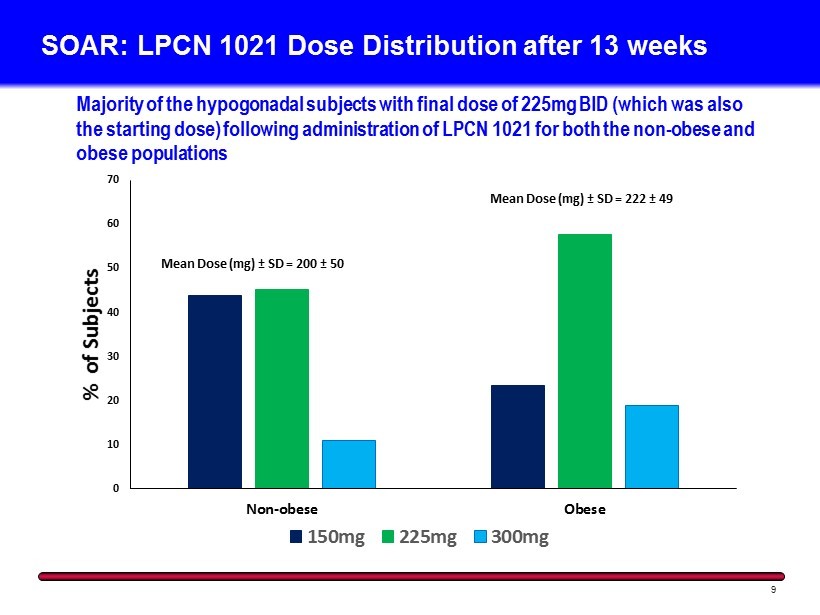
0 10 20 30 40 50 60 70 Non-obese Obese % of Subjects 150mg 225mg 300mg SOAR: LPCN 1021 Dose Distribution after 13 weeks 9 Majority of the hypogonadal subjects with final dose of 225mg BID (which was also the starting dose) following administration of LPCN 1021 for both the non - obese and obese populations Mean Dose (mg) ± SD = 200 ± 50 Mean Dose (mg) ± SD = 222 ± 49
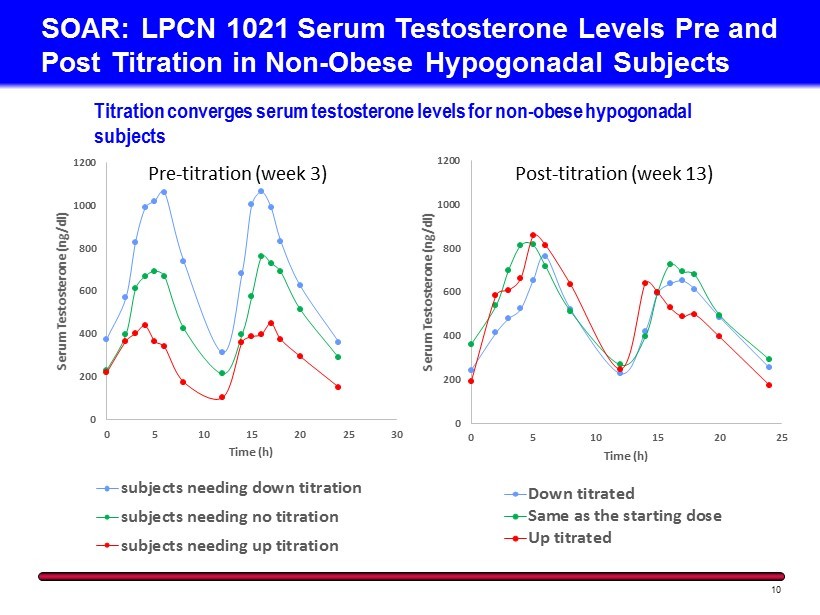
0 200 400 600 800 1000 1200 0 5 10 15 20 25 30 Serum Testosterone ( ng /dl) Time (h) subjects needing down titration subjects needing no titration subjects needing up titration SOAR: LPCN 1021 Serum Testosterone Levels P re and Post Titration in Non - Obese Hypogonadal Subjects 10 Titration converges serum testosterone levels for non - obese hypogonadal subjects 0 200 400 600 800 1000 1200 0 5 10 15 20 25 Serum Testosterone ( ng /dl) Time (h) Down titrated Same as the starting dose Up titrated Post - titration (week 13) Pre - titration (week 3)
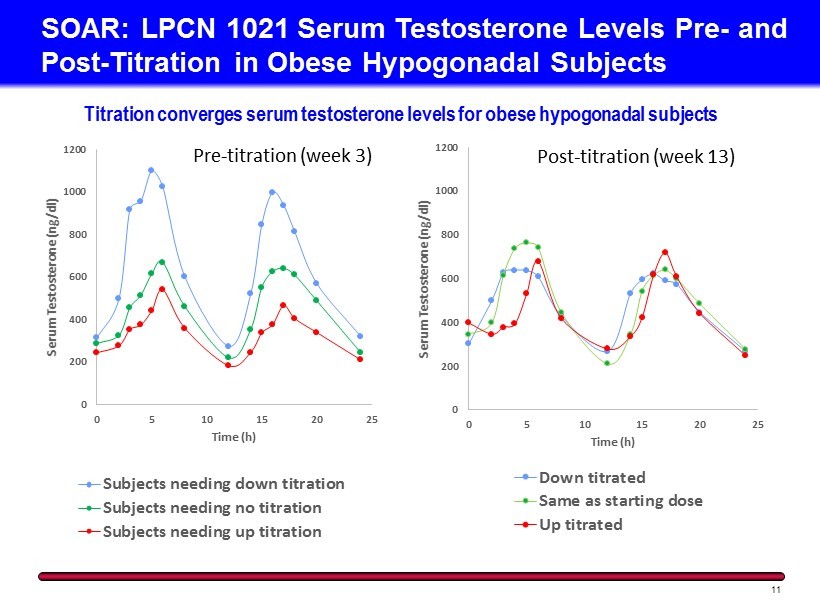
0 200 400 600 800 1000 1200 0 5 10 15 20 25 Serum Testosterone ( ng /dl) Time (h) Subjects needing down titration Subjects needing no titration Subjects needing up titration 0 200 400 600 800 1000 1200 0 5 10 15 20 25 Serum Testosterone ( ng /dl) Time (h) Down titrated Same as starting dose Up titrated SOAR: LPCN 1021 Serum Testosterone Levels Pre - and Post - Titration in Obese Hypogonadal Subjects 11 Titration converges serum testosterone levels for obese hypogonadal subjects Pre - titration (week 3) Post - titration (week 13)
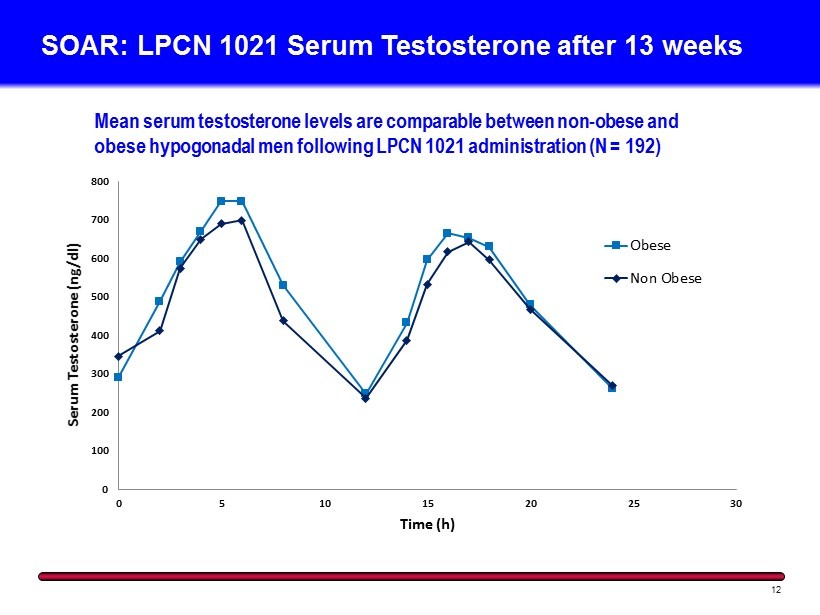
0 100 200 300 400 500 600 700 800 0 5 10 15 20 25 30 Serum Testosterone ( ng /dl) Time (h) Obese Non Obese Mean serum t estosterone l evels are comparable between non - obese and obese hypogonadal men following LPCN 1021 administration (N = 192) SOAR: LPCN 1021 Serum Testosterone after 13 weeks 12
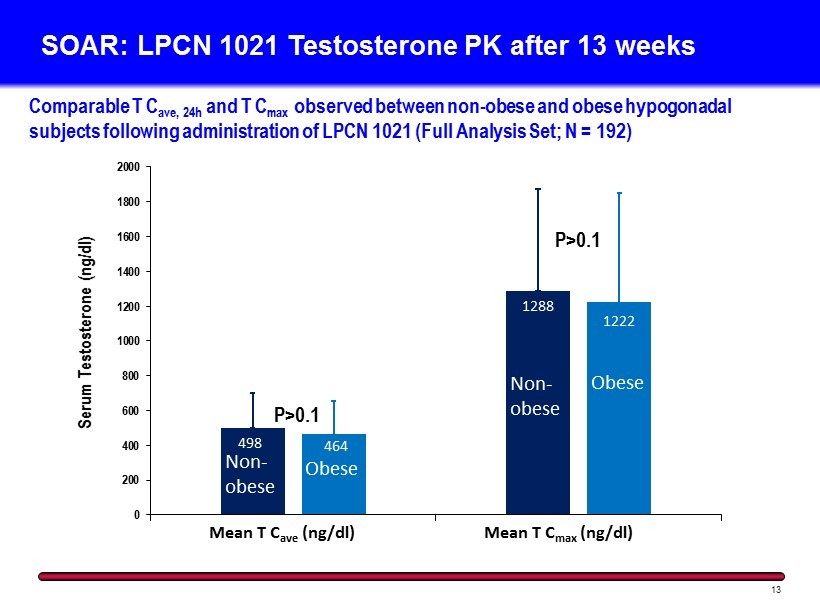
SOAR: LPCN 1021 Testosterone PK after 13 weeks 13 Comparable T C ave, 24h and T C max observed between non - obese and obese hypogonadal subjects following administration of LPCN 1021 (Full Analysis Set; N = 192) 498 1288 464 1222 0 200 400 600 800 1000 1200 1400 1600 1800 2000 Mean T Cave, 24h (ng/dl) Mean T Cmax (ng/dl) Serum Testosterone (ng/dl) Non - obese Obese Obese Mean T C ave (ng/dl) Mean T C max (ng/dl) Non - obese P>0.1 P>0.1
As of March 31, 2015 • No drug - related SAEs • No cardiac - related SAEs Serious Adverse Events 14
LPCN 1021 is an orally administered TRT product with acceptable serum T levels for both non - obese and obese hypogonadal men. Obese hypogonadal men may require a marginally higher mean dose (about 10%) compared to non - obese men LPCN 1021 is an option for men requiring testosterone therapy and may result in improved patient compliance and effectiveness Conclusions 15

Forward Looking Statements 16 This presentation contains and our discussions during this conference may include forward - looking statements about Lipocine Inc. (the “Company”). These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward - looking statements relate to the Company’s product candidates, clinical and regulatory processes and objectives, potential benefits of the Company’s product candidates, intellectual property and related matters, all of which involve known and unknown risks and uncertainties. Actual results may differ materially from the forward - looking statements discussed in this presentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials, the potential advantages of the Company’s product candidates and the Company’s capital needs. Among other things, the projected commencement and completion of the Company’s clinical trials may be affected by difficulties or delays. In addition, the Company’s results may be affected by its ability to manage its financial resources, difficulties or delays in developing manufacturing processes for its product candidates, preclinical and toxicology testing and regulatory developments. Delays in clinical programs, whether caused by competitive developments, adverse events, patient enrollment rates, regulatory issues or other factors, could adversely affect the Company’s financial position and prospects. Prior clinical trial program designs and results are not necessarily predictive of future clinical trial designs or results. If the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. The Company may not be able to enter into any strategic partnership agreements. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corporate priorities, the timing and outcomes of clinical trials, competitive developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale back or discontinue one or more of its drug development or discovery research programs. The Company is at an early stage of development and may not ever have any products that generate significant revenue. The forward - looking statements contained in this presentation are further qualified by the detailed discussion of risks and uncertainties set forth in the documents filed by the Company with the Securities and Exchange Commission, all of which can be obtained on the Company’s website at www.lipocine.com or on the SEC website at www.sec.gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations. This is not an offer of securities or the solicitation of an offer to buy securities of Lipocine Inc.
