Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zosano Pharma Corp | d909210d8k.htm |
| Exhibit 99.1
|
Corporate Presentation
April 2015
|
|
Forward-Looking Statements
This presentation contains forward looking statements. All statements other than statements of historical fact contained in this presentation, including statements regarding our commercialization, our research and other development programs, our ability to undertake certain activities and accomplish certain goals, projected timelines for our research and development activities (including any clinical trials), our ability to secure and further possible regulatory approvals, the enforceability of our intellectual property rights, our capital requirements, the prospects for third-party reimbursement for our products, the expected pricing of our products, our expectations regarding the relative benefits of our product candidates versus competitive therapies, our expectations regarding the possibility of licensing or collaborating with third parties regarding our product candidates or research, our business strategy, our expectations regarding potential markets or market sizes, and our expectations regarding the therapeutic and commercial potential of our product candidates, research, technologies and intellectual property, are forward looking statements. In some cases, you can identify these statements by forward-looking words, such as the words “believe,” “may,” “estimate,” “continue,” “anticipate,” “design,” “intend,” “expect,” “potential” and similar expressions, as well as the negative version of these words and similar expressions. The forward looking statements in this presentation do not constitute guarantees of future performance. Statements in this presentation that are not strictly historical statements are subject to a number of known and unknown risks and uncertainties that could cause actual results to differ materially and adversely from those anticipated or implied in the forward looking statements, including, without limitation, those described under the heading “Risk Factors” in our Form 10-K filed with the SEC on March 26 , 2015, and new risks emerge from time to time. These forward looking statements are based upon our current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward looking statements as a result of various risks and uncertainties which include, without limitation, risks associated with the process of discovering, developing and commercializing products that are safe and effective for use as human therapeutics and risks inherent in the effort to build a business around such products. Although we believe that the expectations reflected in these forward looking statements are reasonable, we cannot in any way guarantee that the future results, level of activity, performance or events and circumstances reflected in forward-looking statements will be achieved or occur. Any forward looking statement made by us in this presentation speaks only as of the date this presentation is actually delivered by us in person. We assume no obligation or undertaking to update or revise any forward looking statements contained herein to reflect any changes in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based, except as required by law.
| 1 |
|
|
|
Zosano Pharma (ZSAN)
Investment Highlights
Differentiated transdermal microneedle ZP-Patch delivery platform
Capable of delivering small molecules, peptides/proteins and vaccines Fast onset with short Tmax: injection-comparable or better
Convenient and easy-to-use: room temperature stable, portable
Well validated pipeline with multiple near term catalysts
ZP-PTH (teriparatide) entering Phase 3 for osteoporosis – partnered
ZP-Glucagon for severe hypoglycemia emergency rescue – Phase 2 data Q3 2015 ZP-Triptan (zolmitriptan) for migraine – Phase 1 data by year-end 2015
GLP-1 analogues for type 2 diabetes – partnered
Robust IP and life cycle management options across entire portfolio
January 2015 IPO and concurrent private placement with net proceeds of $60m
Includes net proceeds of $15.0 million investment by Eli Lilly at the IPO price Sufficient to fund operations through mid-2016
2
|
|
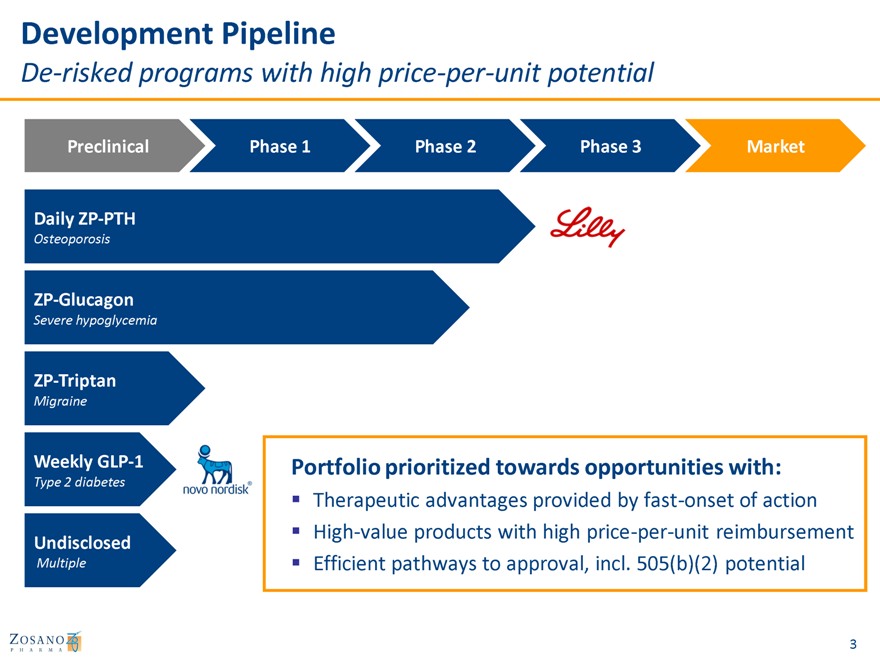
Development Pipeline
De-risked programs with high price-per-unit potential
Preclinical Phase 1 Phase 2 Phase 3 Market
Daily ZP-PTH
Osteoporosis
ZP-Glucagon
Severe hypoglycemia
ZP-Triptan
Migraine
Weekly GLP-1 Portfolio prioritized towards opportunities with:
Type 2 diabetes
Therapeutic advantages provided by fast-onset of action High-value products with high price-per-unit reimbursement
Undisclosed
Multiple Efficient pathways to approval, incl. 505(b)(2) potential
3
|
|
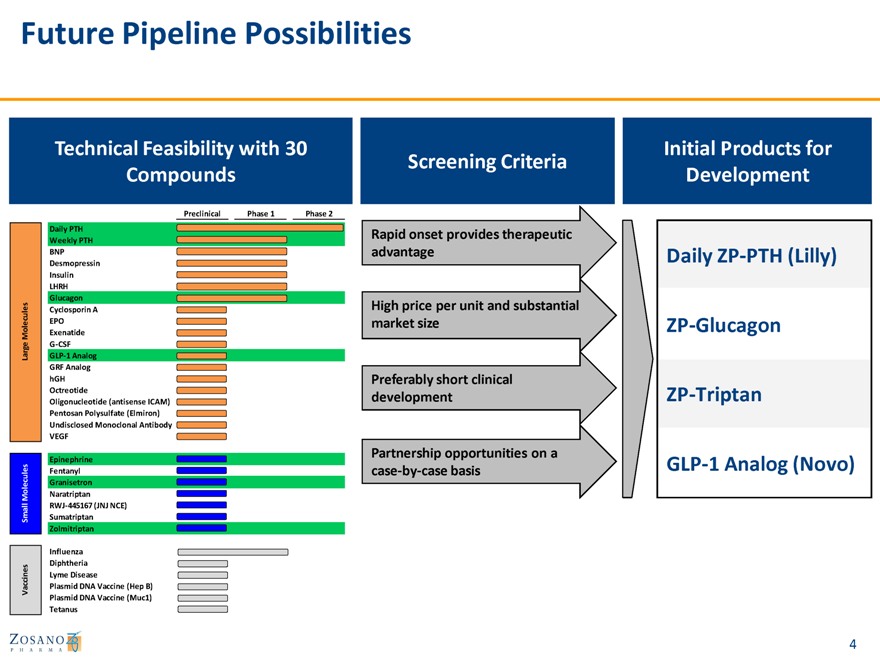
Future Pipeline Possibilities
Technical Feasibility with 30 Initial Products for
Screening Criteria
Compounds Development
Preclinical Phase 1 Phase 2
Daily PTH Rapid onset provides therapeutic
Weekly PTH
BNP advantage Daily ZP-PTH (Lilly)
Desmopressin Insulin LHRH
Glucagon
es Cyclosporin A High price per unit and substantial
u l
e c EPO market size ZP-Glucagon
M ol Exenatide ge G-CSF Lar GLP-1 Analog GRF Analog
hGH Preferably short clinical
Octreotide ZP-Triptan Oligonucleotide (antisense ICAM) development Pentosan Polysulfate (Elmiron) Undisclosed Monoclonal Antibody VEGF
Partnership opportunities on a
Epinephrine GLP-1 Analog (Novo)
Fentanyl case-by-case basis
ecules Granisetron Mol Naratriptan
RWJ-445167 (JNJ NCE) Small Sumatriptan Zolmitriptan
Influenza Diphtheria Lyme Disease
Vaccines Plasmid DNA Vaccine (Hep B) Plasmid DNA Vaccine (Muc1) Tetanus
4
|
|
2014 in Review
A Successful Foundational Year
ZP-Patch validation through business development
Signed Novo Nordisk partnership in Q1 2014 for their weekly GLP-1 analog
Signed Eli Lilly partnership in Q4 2014 for Zosano’s ZP-PTH
Critical advancement of internal programs
Completed ZP-Glucagon Phase 1 in H1 2014
Discussion with FDA mid-2014 regarding ZP-Glucagon path forward
Preclinical and formulation progress for ZP-Triptan
Solidified funding and capital base
Embarked on a successful IPO process generating total proceeds of $60m
IPO included concurrent $15.0 million private placement by Eli Lilly
5
|
|
2015 Outlook
Expected to Be a Transformational Year for Zosano
Prepare for Phase 3 of ZP-PTH for severe osteoporosis
Confirm Phase 3 clinical trial design with regulatory authorities by YE 2015
Establish significant clinical study inventory by Q4 2015
Prepare for Phase 3 of ZP-Glucagon for severe hypoglycemia
Complete Phase 2 by Q3 2015
File IND in H2 2015
Initiate Phase 3 by YE 2015
Prepare for Phase 2 of ZP-Triptan for migraine
Commence Phase 1 and complete by Q4 2015
Discuss Phase 2 & Phase 3 development with FDA by Q1 2016
6
|
|
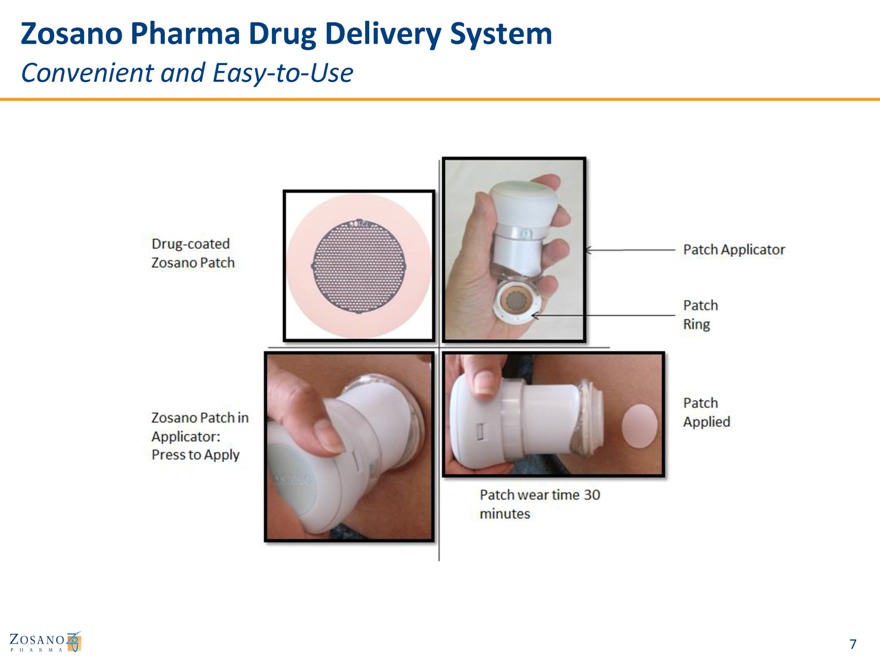
Zosano Pharma Drug Delivery System
Convenient and Easy-to-Use
| 7 |
|
|
|
Zosano Pharma Drug Delivery System
Rapid Onset and High Bioavailability
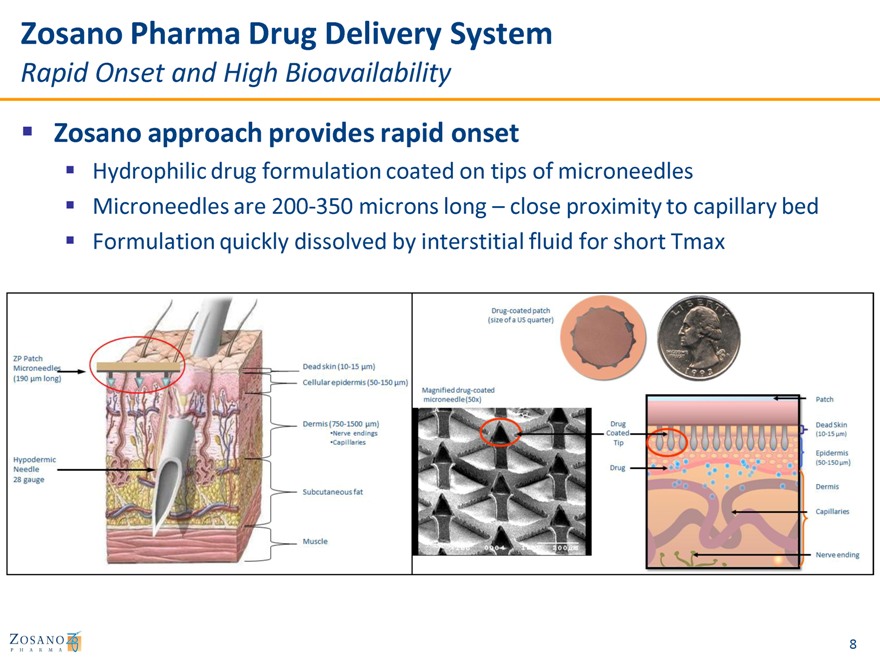
Zosano approach provides rapid onset
Hydrophilic drug formulation coated on tips of microneedles
Microneedles are 200-350 microns long – close proximity to capillary bed
Formulation quickly dissolved by interstitial fluid for short Tmax
8
|
|
Zosano Pharma Drug Delivery System
Formulations Stable at Room Temperature
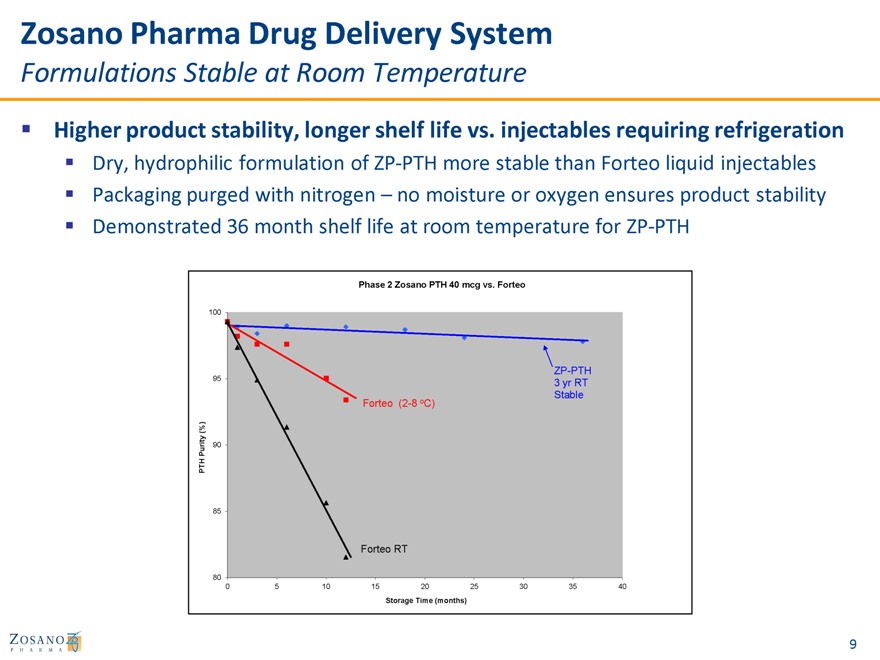
Higher product stability, longer shelf life vs. injectables requiring refrigeration
Dry, hydrophilic formulation of ZP-PTH more stable than Forteo liquid injectables Packaging purged with nitrogen – no moisture or oxygen ensures product stability
Demonstrated 36 month shelf life at room temperature for ZP-PTH
Phase 2 Zosano PTH 40 mcg vs. Forteo
100
95 ZP-PTH 3 yr RT Stable Forteo (2-8 oC)
(%) u rity 90 P PT H
85
Forteo RT
80
0 5 10 15 20 25 30 35 40
Storage Time (months)
9
|
|
Overview of ZP-PTH Collaboration with Eli Lilly
Zosano grants exclusive global licensing for PTH to Lilly
Zosano responsible for financing and achieving critical success factors leading to regulatory approvals and commercial readiness Lilly responsible for commercialization of product
Deal terms with significant pre-launch value
$15 million equity investment in private placement concurrent with IPO $300 million at regulatory approvals
$125 million upon sales milestones Double-digit royalty
Zosano to be exclusive supplier of commercial material
Confidential
10
|
|
Daily ZP-PTH Patch for Osteoporosis
Phase 2 Trial Design
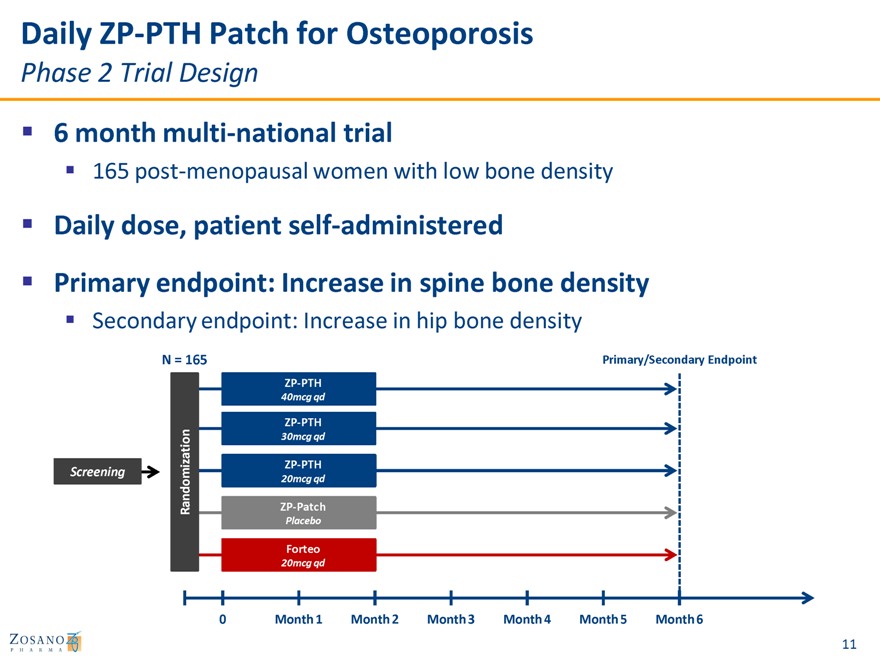
6 month multi-national trial
165 post-menopausal women with low bone density
Daily dose, patient self-administered
Primary endpoint: Increase in spine bone density
Secondary endpoint: Increase in hip bone density
N = 165 Primary/Secondary Endpoint
ZP-PTH
40mcg qd
ZP-PTH
30mcg qd
ZP-PTH
Screening
20mcg qd
Randomization ZP-Patch
Placebo
Forteo
20mcg qd
0 Month 1 Month 2 Month 3 Month 4 Month 5 Month 6
11
|
|
Daily ZP-PTH Patch Program—PK Profile
Pulsatile Delivery Critical for Building Bone
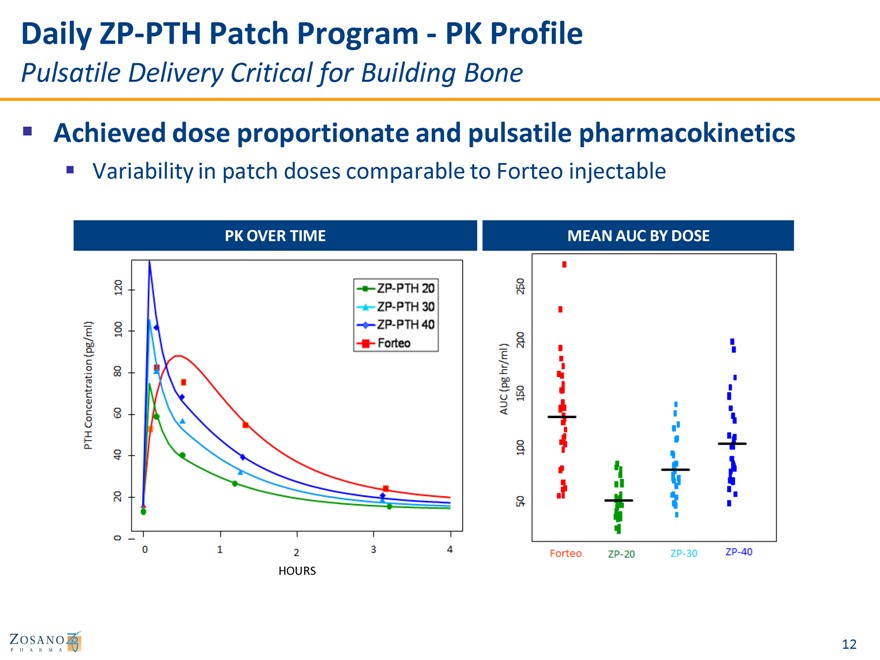
Achieved dose proportionate and pulsatile pharmacokinetics
Variability in patch doses comparable to Forteo injectable
PK OVER TIME MEAN AUC BY DOSE
HOURS
12
|
|
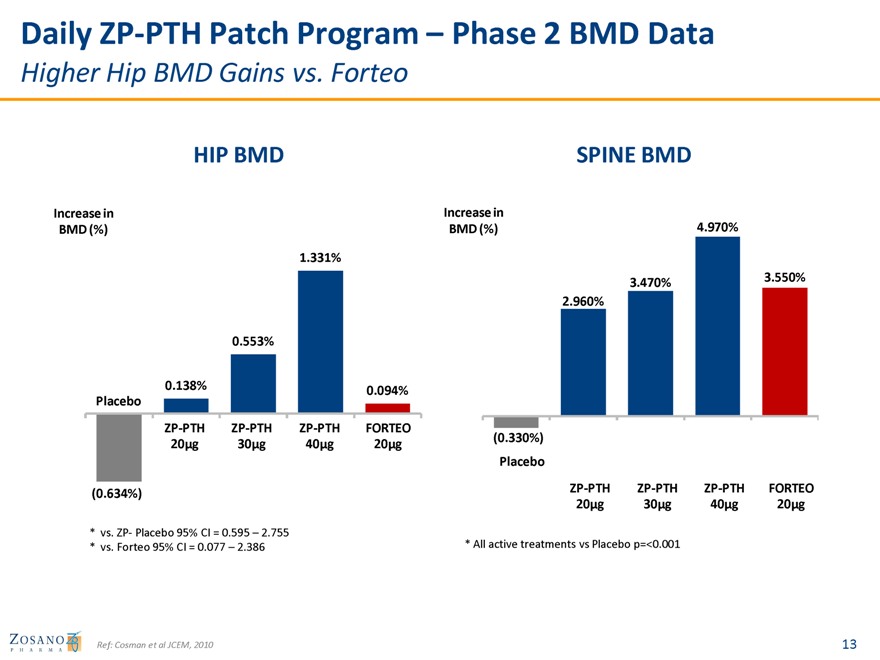
Daily ZP-PTH Patch Program – Phase 2 BMD Data
Higher Hip BMD Gains vs. Forteo
HIP BMD SPINE BMD
Increase in Increase in
BMD (%) BMD (%) 4.970%
1.331%
3.470% 3.550%
2.960%
0.553%
0.138% 0.094% Placebo
ZP-PTH ZP-PTH ZP-PTH FORTEO
(0.330%) 20µg 30µg 40µg 20µg Placebo
ZP-PTH ZP-PTH ZP-PTH FORTEO (0.634%) 20µg 30µg 40µg 20µg
* vs. ZP- Placebo 95% CI = 0.595 – 2.755
* vs. Forteo 95% CI = 0.077 – 2.386 * All active treatments vs Placebo p=<0.001
Ref: Cosman et al JCEM, 2010
13
|
|
Daily ZP-PTH for Osteoporosis
Next Steps
Agreement for Phase 3 design with FDA, EMA
Phase 3 clinical trial design confirmation by regulatory agencies in 2015
Manufacturing infrastructure scale-up and commercial readiness
Establish significant clinical study inventory by Q4 2015
Single, active-comparator Phase 3 trial for approval
Larger and longer version of successful Phase 2 study
Trial size: 400 postmenopausal women per arm
Primary endpoint: non-inferior change in BMD vs. Forteo at 12 months
Six month safety extension
No long-term fracture study needed
14
|
|
ZP-Glucagon for Severe Hypoglycemia
Potential to Expand Highly Underserved Market
Severe hypoglycemia is life-threatening, requiring emergency rescue
Ease-of-use critical for third-party caregivers who may lack medical training Rapid onset important for fast recovery
Underpenetrated market generates ~$160 million in US sales
Only two glucagon products currently marketed in U.S. High current unit selling price in U.S. (>$150 per injection) Concentrated prescriber base
Current glucagon injections are cumbersome with poor stability
Dry powder needs to be reconstituted at time of injection Route of administration limited to injection/infusion
Efficient clinical development pathway
Clinical development expected to be complete by H1 2016 with estimated remaining spend of $7 million
15
|
|
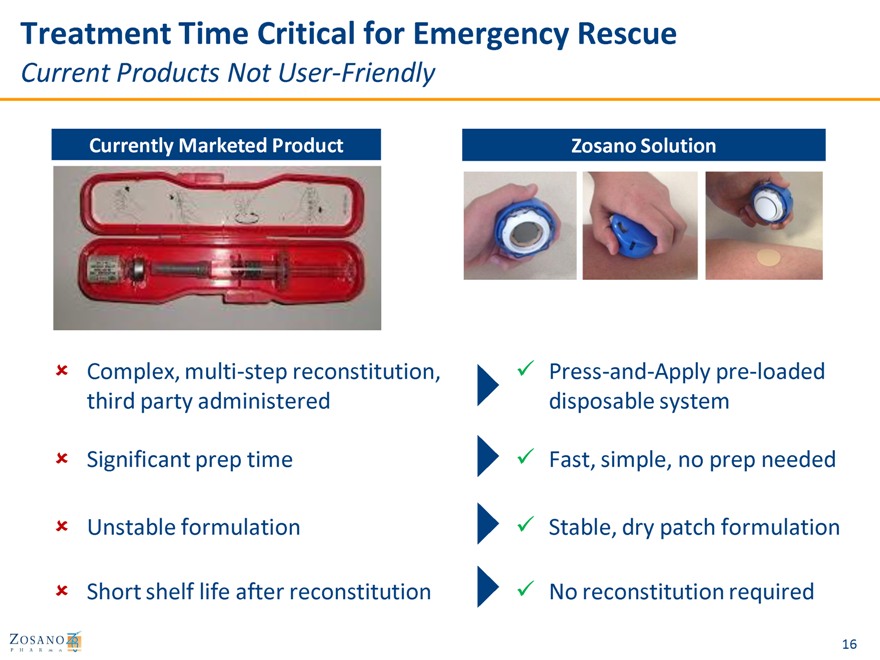
Treatment Time Critical for Emergency Rescue
Current Products Not User-Friendly
Currently Marketed Product Zosano Solution
Complex, multi-step reconstitution, Press-and-Apply pre-loaded third party administered disposable system
Significant prep time Fast, simple, no prep needed
Unstable formulation Stable, dry patch formulation
Short shelf life after reconstitution No reconstitution required
16
|
|
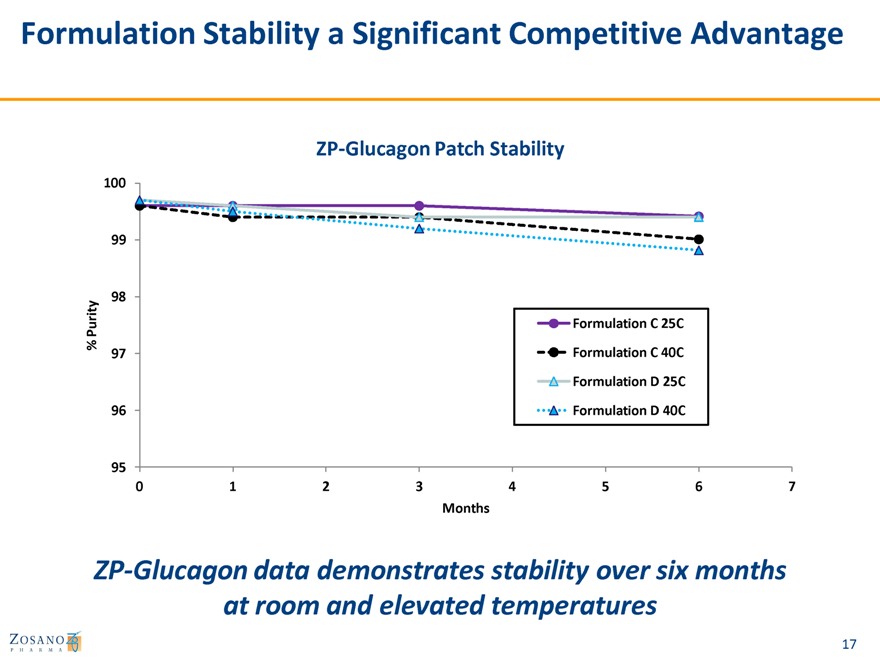
Formulation Stability a Significant Competitive Advantage
ZP-Glucagon Patch Stability
100
99
98
Purity Formulation C 25C %
97 Formulation C 40C Formulation D 25C
96 Formulation D 40C
95
0 1 2 3 4 5 6 7 Months
ZP-Glucagon data demonstrates stability over six months at room and elevated temperatures
17
|
|
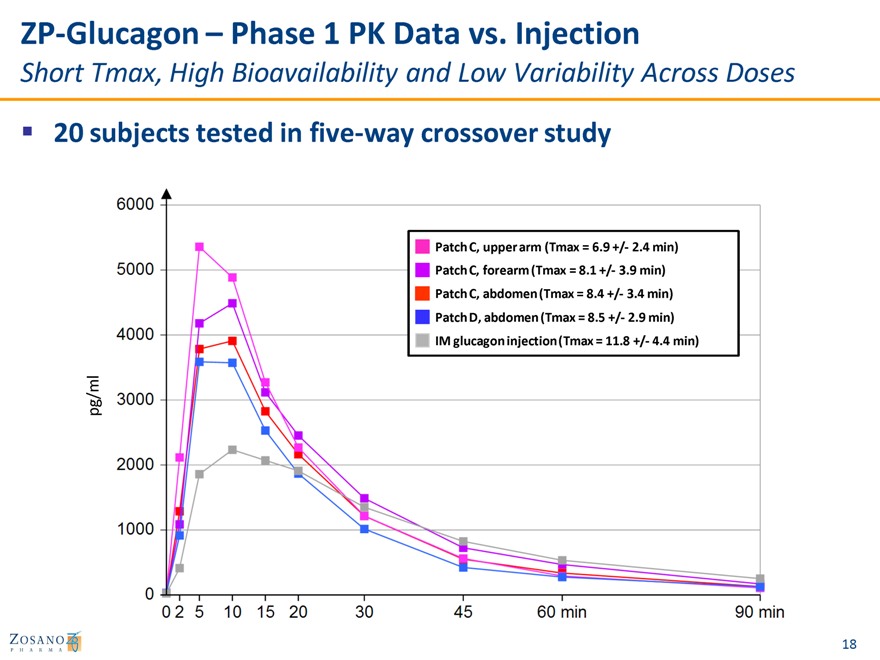
ZP-Glucagon – Phase 1 PK Data vs. Injection
Short Tmax, High Bioavailability and Low Variability Across Doses
20 subjects tested in five-way crossover study
Patch C, upper arm (Tmax = 6.9 +/- 2.4 min) Patch C, forearm (Tmax = 8.1 +/- 3.9 min) Patch C, abdomen (Tmax = 8.4 +/- 3.4 min) Patch D, abdomen (Tmax = 8.5 +/- 2.9 min) IM glucagon injection (Tmax = 11.8 +/- 4.4 min)
pg/ml
18
|
|
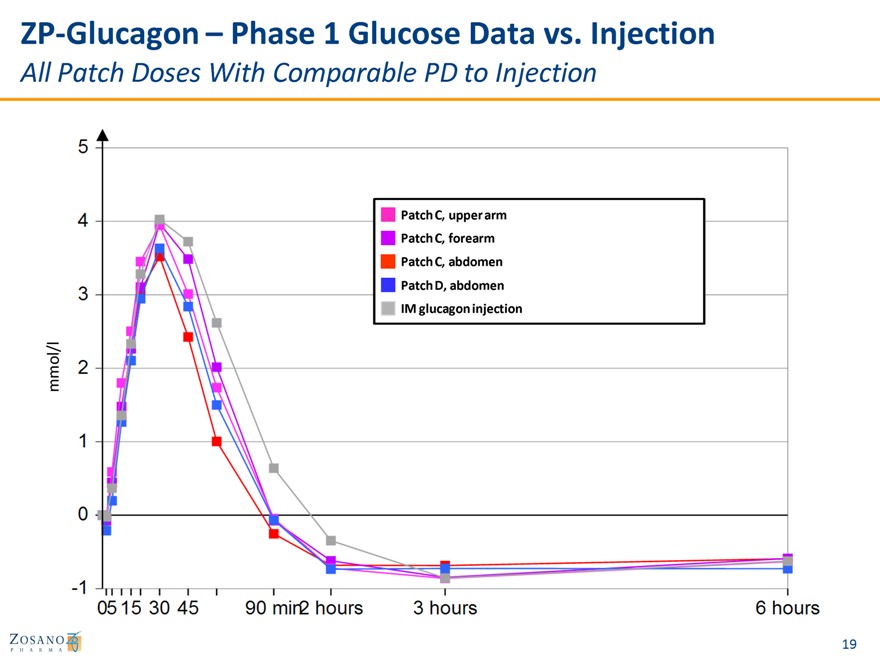
ZP-Glucagon – Phase 1 Glucose Data vs. Injection
All Patch Doses With Comparable PD to Injection
Patch C, upper arm Patch C, forearm Patch C, abdomen Patch D, abdomen IM glucagon injection
mmol/l
19
|
|
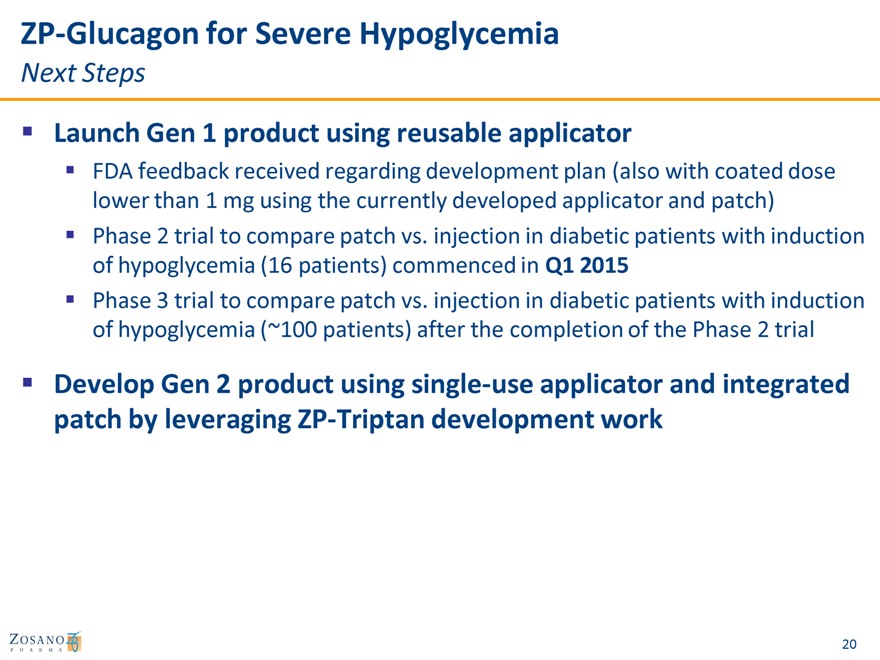
ZP-Glucagon for Severe Hypoglycemia
Next Steps
Launch Gen 1 product using reusable applicator
FDA feedback received regarding development plan (also with coated dose lower than 1 mg using the currently developed applicator and patch) Phase 2 trial to compare patch vs. injection in diabetic patients with induction of hypoglycemia (16 patients) commenced in Q1 2015 Phase 3 trial to compare patch vs. injection in diabetic patients with induction of hypoglycemia (~100 patients) after the completion of the Phase 2 trial
Develop Gen 2 product using single-use applicator and integrated patch by leveraging ZP-Triptan development work
20
|
|
ZP-Triptan for Migraine
Highly Suited for Acute Condition Requiring Immediate Relief
Potential best-in-class rapid onset: Tmax = 9 minutes
Comparable or better onset of action than existing triptans/injectables
Self-administration provides significant advantage vs. injectables
Large, growing market with attractive unit pricing for injectables
US $1.9 billion total migraine market ($1.1 billion for triptans) Multiple products in a large and growing market but no clear effective solution
21
|
|
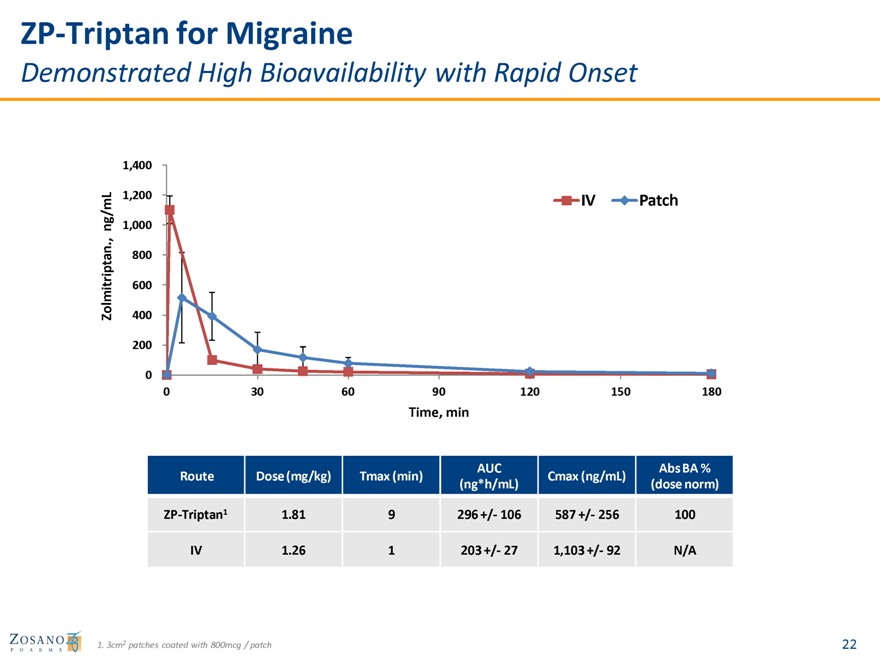
ZP-Triptan for Migraine
Demonstrated High Bioavailability with Rapid Onset
1,400
1,200
IV Patch
ng/mL 1,000
,
.
800
600
Zolmitriptan 400
200
0
0 30 60 90 120 150 180
Time, min
AUC Abs BA % Route Dose (mg/kg) Tmax (min) Cmax (ng/mL) (ng*h/mL) (dose norm)
ZP-Triptan1 1.81 9 296 +/- 106 587 +/- 256 100
IV 1.26 1 203 +/- 27 1,103 +/- 92 N/A
1. 3cm2 patches coated with 800mcg / patch
22
|
|
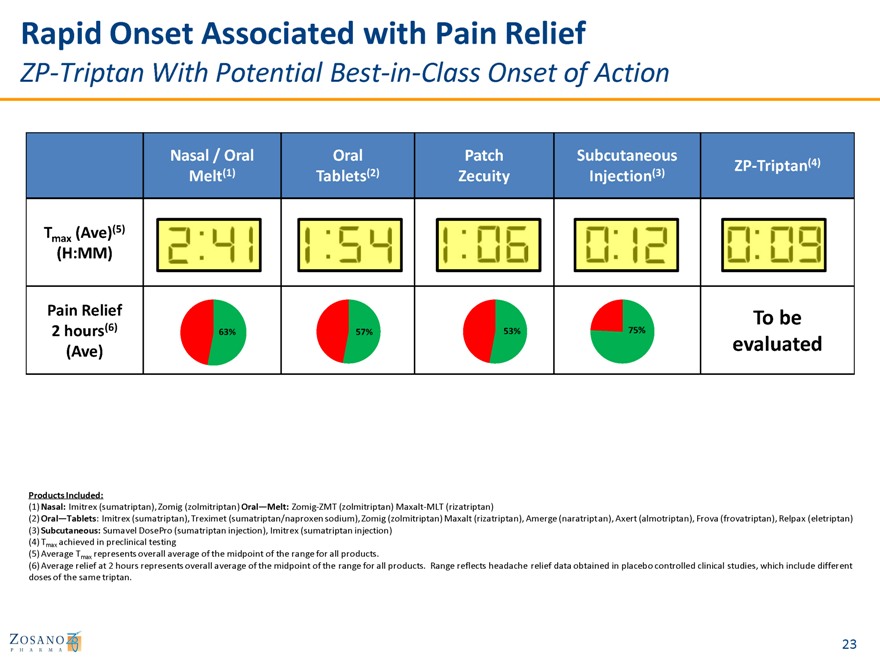
Rapid Onset Associated with Pain Relief
ZP-Triptan With Potential Best-in-Class Onset of Action
Nasal / Oral Oral Patch Subcutaneous
ZP-Triptan(4) Melt(1) Tablets(2) Zecuity Injection(3)
T (Ave)(5)
max
(H:MM)
Pain Relief To be
(6) 53% 75%
2 hours 63% 57% evaluated
(Ave)
Products Included:
(1) Nasal: Imitrex (sumatriptan), Zomig (zolmitriptan) Oral—Melt: Zomig-ZMT (zolmitriptan) Maxa
(2) Oral—Tablets: Imitrex (su (sumatriptan omig (zolmitr tan), Amerge almotriptan), F ax (eletriptan) (3) Subcutaneous: Sumavel D injection), Im ection) (4) Tmax achieved in preclinica (5) Average Tmax represents overall average of the midpoint of the range for all products.
(6) Average relief at 2 hours represents overall average of the midpoint of the range for all products. Range reflects headache relief data obtained in placebo controlled clinical studies, which include different doses of the same triptan.
23
|
|
ZP-Triptan for Migraine
Next Steps
Launch product using current applicator and patch
Phase 1 development to commence Q2 2015
Single/multi dose crossover study versus SC/IM injection
Multiple patch doses with zolmitriptan compared to one subcutaneous injection of sumatriptan in healthy volunteers
Phase 2 trial with 200 patients planned
Expected to compare three ZP-Triptan doses vs. placebo
Primary endpoint: reduction in headache severity in 2 hours or less Subcutaneous sumatriptan injection as reference arm
24
|
|
Once-Weekly GLP-1 Analog Patch for Type 2 Diabetes
Novo Nordisk partnered program with potential to replace injectable Victoza (liraglutide) in the growing diabetes market
2014 Victoza sales = approximately $2 billion USD
Worldwide collaboration on multiple Novo GLP-1 analogues
Novo Nordisk responsible for all commercialization Preclinical, clinical, regulatory and sales milestones
Up to $60 million total for first product and $55 million for each additional
Reimbursement of all Zosano development and manufacturing costs Zosano eligible for royalties on sales
25
|
|
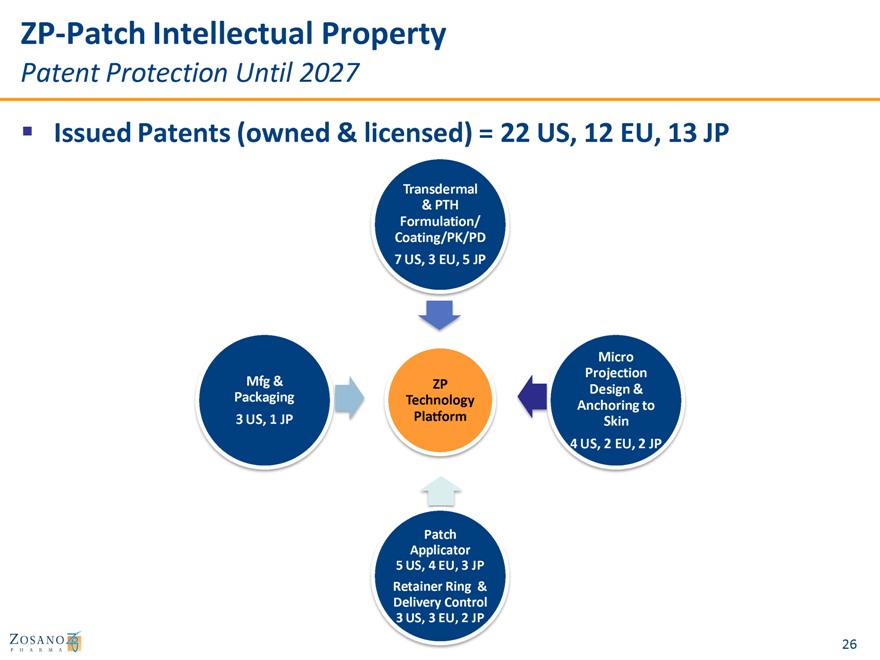
ZP-Patch Intellectual Property
Patent Protection Until 2027
Issued Patents (owned & licensed) = 22 US, 12 EU, 13 JP
Transdermal
& PTH
Formulation/ Coating/PK/PD
7 US, 3 EU, 5 JP
Micro Projection Mfg & ZP
Design & Packaging Technology Platform Anchoring to
3 US, 1 JP Skin
4 US, 2 EU, 2 JP
Patch Applicator
5 US, 4 EU, 3 JP
Retainer Ring & Delivery Control
3 US, 3 EU, 2 JP
26
|
|
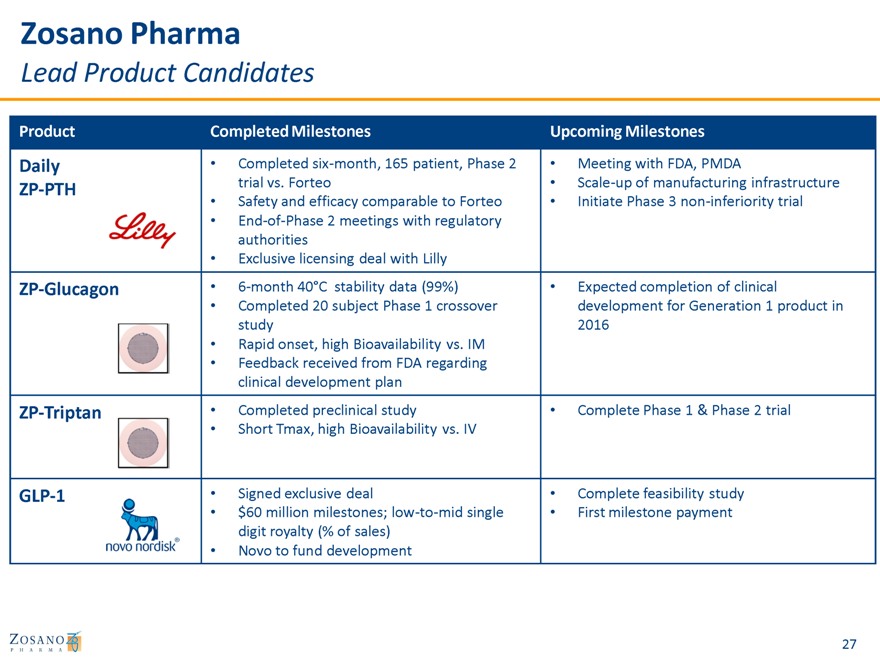
Zosano Pharma
Lead Product Candidates
Product Completed Milestones Upcoming Milestones
Daily • Completed six-month, 165 patient, Phase 2 • Meeting with FDA, PMDA
ZP-PTH trial vs. Forteo • Scale-up of manufacturing infrastructure
Safety and efficacy comparable to Forteo Initiate Phase 3 non-inferiority trial
End-of-Phase 2 meetings with regulatory authorities
Exclusive licensing deal with Lilly
ZP-Glucagon • 6-month 40°C stability data (99%) • Expected completion of clinical
Completed 20 subject Phase 1 crossover development for Generation 1 product in study 2016
Rapid onset, high Bioavailability vs. IM
Feedback received from FDA regarding clinical development plan
ZP-Triptan • Completed preclinical study • Complete Phase 1 & Phase 2 trial
Short Tmax, high Bioavailability vs. IV
GLP-1 • Signed exclusive deal • Complete feasibility study
$60 million milestones; low-to-mid single First milestone payment digit royalty (% of sales)
Novo to fund development
27
|
|
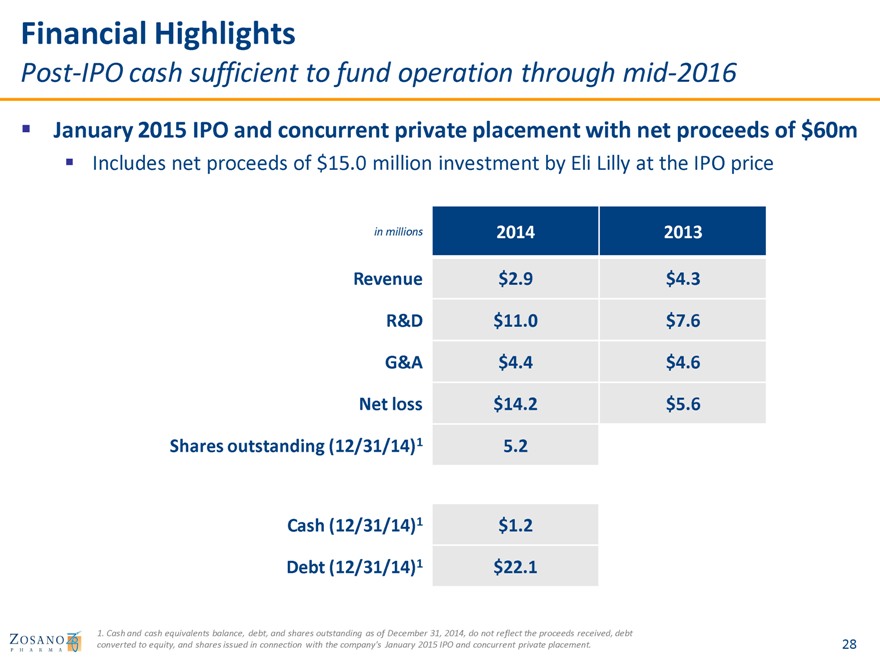
Financial Highlights
Post-IPO cash sufficient to fund operation through mid-2016
January 2015 IPO and concurrent private placement with net proceeds of $60m
Includes net proceeds of $15.0 million investment by Eli Lilly at the IPO price
in millions 2014 2013
Revenue $2.9 $4.3 R&D $11.0 $7.6 G&A $4.4 $4.6 Net loss $14.2 $5.6 Shares outstanding (12/31/14)1 5.2
Cash (12/31/14)1 $1.2
Debt (12/31/14)1 $22.1
1. Cash and cash equivalents balance, debt, and shares outstanding as of December 31, 2014, do not reflect the proceeds received, debt converted to equity, and shares issued in connection with the company’s January 2015 IPO and concurrent private placement.
28
|
|
Zosano Pharma (ZSAN)
Investment Highlights
Differentiated transdermal microneedle ZP-Patch delivery platform
Capable of delivering small molecules, peptides/proteins and vaccines Fast onset with short Tmax: injection-comparable or better
Convenient and easy-to-use: room temperature stable, portable
Well validated pipeline with multiple near term catalysts
ZP-PTH (teriparatide) entering Phase 3 for osteoporosis – partnered
ZP-Glucagon for severe hypoglycemia emergency rescue – Phase 2 data Q3 2015 ZP-Triptan (zolmitriptan) for migraine – Phase 1 data by year-end 2015
GLP-1 analogues for type 2 diabetes– partnered
Robust IP and life cycle management options across entire portfolio
January 2015 IPO and concurrent private placement with net proceeds of $60m
Includes net proceeds of $15.0 million investment by Eli Lilly at the IPO price Sufficient to fund operations through mid-2016
29
|
|
Corporate Presentation
April 2015