Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - WHITE FOX VENTURES, INC. | Financial_Report.xls |
| EX-32.1 - EXHIBIT 32.1 - WHITE FOX VENTURES, INC. | ex32_1.htm |
| EX-31.1 - EXHIBIT 31.1 - WHITE FOX VENTURES, INC. | ex31_1.htm |
| EX-32.2 - EXHIBIT 32.2 - WHITE FOX VENTURES, INC. | ex32_2.htm |
| EX-23.1 - EXHIBIT 23.1 - WHITE FOX VENTURES, INC. | ex23_1.htm |
| EX-31.2 - EXHIBIT 31.2 - WHITE FOX VENTURES, INC. | ex31_2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C.
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2014
or
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES
EXCHANGE ACT OF 1934
For the transition period from: _____________ to _____________
Commission file number 333-178624
|
BREATHE ECIG CORP.
|
|
(Exact Name of Registrant as Specified in its Charter)
|
|
322 Nancy Lynn Lane, Suite 7, Knoxville, TN 37919
|
|
(ADDRESS OF PRINCIPAL EXECUTIVE OFFICES)
|
|
(865) 337-7549
|
|
REGISTRANT’S TELEPHONE NUMBER
|
|
DNA Precious Metals, Inc.
|
|
9125 Pascal Gagnon, Suite 204, Saint Leonard, Quebec, Canada H1P 1Z4
|
|
|
|
(FORMER NAME OR FORMER ADDRESS, IF CHANGES SINCE LAST REPORT)
|
All correspondence to:
Joshua Kimmel, CEO
Breath eCig Corp.
322 Nancy Lynn Lane, Suite 7, Knoxville, TN 37919
Office: (800) 905-4013
Email: joshbreathe@outlook.com
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, $0.001 par value | None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Acto Yesx No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act from their obligations under those Sections.o Yesx No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). x Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o Yes x No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer o
|
Non-accelerated Filer o
|
|
|
Accelerated Filer o
|
Smaller Reporting Company x
|
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant computed by reference to the price at which the common equity was last sold, or the average bid and ask price for such common equity was approximately$12,605,460 (based on a quoted price of $0.21 per common stock share at the close of business on June 30, 2014.
APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY
PROCEEDINGS DURING THE PRECEDING FIVE YEARS:
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court.
o Yes o No
APPLICABLE ONLY TO CORPPORATE ISSUERS
Indicate the number of shares outstanding of each of the issuer’s classes of common stock as of the last practical date: 276,352,667 shares of common stock, $0.001 par value as of April 6, 2015.
DOCUMENTS INCORPORATED BY REFERENCE
None.
Table of Contents
|
PART 1
|
|||
| Item 1. | 1 | ||
| Item 1A. | 17 | ||
| Item 1B. | 45 | ||
| Item 2. | 46 | ||
| Item 3. | 46 | ||
| Item 4. | 46 | ||
|
PART II
|
|||
| Item 5. | 46 | ||
| Item 6. | 50 | ||
| Item 7. | 51 | ||
| Item 8. | 53 | ||
| Item 9. | 54 | ||
| Item 9A. | 54 | ||
| Item 9B. | 55 | ||
|
PART III
|
|||
| Item 10. | 56 | ||
| Item 11. | 61 | ||
| Item 12. | 64 | ||
| Item 13. | 66 | ||
| Item 14. | 66 | ||
|
PART IV
|
|||
| Item 15. | 67 | ||
Corporate History
Breathe eCig Corp. is a Nevada corporation organized June 2, 2006. Our original name was Celtic Capital, Inc. On October 20, 2008, we changed our name to Entertainment Education Arts Inc. On May 12, 2010, we changed our name to DNA Precious Metals, Inc. On March 5, 2015 pursuant to an agreement and plan of merger, the Company changed its name to Breathe eCig Corp. (Breathe eCig Corp. may also be referred to as “Breathe”, “we”, “us” or the “Company”)
From May 2010 through December 31, 2014, we were an exploration stage mining company. The Company’s focus was the development of the the Montauban Mining Project, located in the Montauban and Chavigny townships near Grondines-West in Portneauf County, Quebec, Canada (“Montauban Mine Property” or “Property”). Recognizing the need to secure significant additional capital to put the Property into production, management began to focus its attention on other business opportunities which would not require the significant capital required to expand the Property. In furtherance thereof, on January 16, 2015 the Company entered into a share exchange agreement with Breathe LLC, a Tennessee limited liability company, whereby the Company acquired all of the issued and outstanding membership interests in Breathe LLC in consideration for the issuance of 150,000,000 shares of the Company’s common stock to the members of Breathe, LLC
In connection with the acquisition of Breath LLC, on March 5, 2015 we entered into an Agreement and Plan of Merger (the “Merger Agreement”) pursuant to which the Company merged with its wholly owned subsidiary, Breathe eCig Corp., the sole purpose of the Merger was to effect a change of the Company's name from DNA Precious Metals, Inc. to Breathe eCig Corp. This name change more accurately reflects the Company’s current operations.
With the acquisition of Breathe LLC, it was contemplated that the Company would spin-off the operations of its mining operations which were being conducted through the Company’s wholly owned subsidiary, DNA Canada, Inc (“DNAC”). Effective February 3, 2015, we declared a stock dividend whereby each of the Company’s shareholders on the record date (February 3. 2015) would receive one share of DNAC for every two shares of the Company’s common stock owned on the record date.
On February 3, 2015, the Company declared a stock dividend of its wholly owned subsidiary, DNA Canada, Inc. (“DNAC”) to the shareholders of record on February 3, 2015 whereby each shareholder of record received one share of DNAC for every two shares of DNA owned as of the record date. The former members of Breathe LLC tendered their shares of DNAC for redemption by the Company.
With the completion of the spin-off, the Company is no longer in the mining field and its sole and exclusive business operations will be marketing its electronic cigarettes and related vapor devices.
Our Operations during the Fiscal Year ended December 31, 2014.
The following discussion does not include a material change in our business which occurred with the acquisition of Breathe LLC following our year end. We are no longer an exploration stage mining company nor do we have any interest in any mining operations. The following discussion relates to our operations during 2014. We are no longer in the mining field. To better understand our current operations you are urged to review the business description disclosed under the heading “Subsequent Events”.
1
During 2014 we were an exploration stage mining company whose business objective was to identify proven reserves of gold and silver, construct a mill, build out the Property’s infrastructure and place the mine into production. The Montauban Mining Project is located in the Montauban and Chavigny townships near Grondines-West in Portneauf County, Quebec, Canada (“Montauban Mine Property” or “Property”). The Montauban Mine Property does not contain any known ore reserves according to the definition of ore reserves under Industry Guide 7 promulgated by the Securities and Exchange Commission (“SEC”). Further work is required on the Property before a final determination as to the economic and legal feasibility of a mining venture can be made. There is no assurance that a commercially viable deposit will be proven through our exploration efforts. The funds expended on our properties may not be successful in leading to the delineation of ore reserves that meet the criteria established under SEC guidelines.
THE PROPERTY
The Property is composed of 122 mining claims, a mining lease and mining concession totaling 4183 hectares located in the Montauban-les-Mines sector of the Notre-Dame-de-Montauban municipality, in the Montauban Township, Portneuf County, Province of Quebec, Canada. The Property is located 120 km east of Quebec City and 80 km north of Trois-Rivières. The Property is located one kilometer west of Montauban-les-Mines with multiple land accesses. Manpower, water and electric power are easily available within the very same distance.
Mining Claims Previously Acquired
The Company acquired ten (10) mining claims, which became fifteen (15) mining claims under new government regulations, on June 9, 2011 through the issuance of 5,000,000 common shares with a valuation of $15,000. The mining claims are located in the Montauban and Chavigny townships near Grondines-West in the Portneuf County of Quebec, Canada (“Montauban Mine Property” or “Property”).
On October 30, 2013 and November 27, 2013, the Company entered into binding agreements for the asset acquisitions of an undivided one hundred percent (100%) interest in certain mineral claims and mining assets located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf, specifically Mining Lease BM 748 and Mining Concession Miniere CM 410. The purchase price was CDN$75,000 together with the issuance of 1,050,000 common shares of the Company. The common shares for the acquisition were valued at their fair market value on the day they were issued which totaled $496,860. In connection with the asset purchase, the Company also issued 40,000 shares of common stock to a former supplier of the vendor for mining related information of the assets purchased valued at $20,000 along with cash consideration of CDN$20,000. The total cost of the acquisition amounted to $612,431. The Company had been awaiting confirmation of the contemplated transaction from a bankruptcy court in Montreal, Quebec over viewing the financial restructuring of the vendor. The bankruptcy court approved the transaction on April 17, 2014 and has been included in mining rights on the consolidated balance sheet as at June 30, 2014.
On January 10, 2014, the Company entered into an asset purchase agreement for an undivided one hundred percent (100%) interest in certain mineral claims located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf, including claims, rights, concessions and leases.
2
The purchase price was CDN$70,000, 1,000,000 common shares of the Company and a one percent (1%) net smelter return (“NSR”). The Company paid CDN$10,000 upon the signing of the asset purchase agreement with the cash balance due, along with the common shares, upon the closing of the asset purchase agreement and transfer of the mineral claims in the name of the Company. The transfer of the mineral claims was completed in February 2014 whereby the remaining cash balance due and the common shares were released to the vendor. The common shares for the acquisition will be valued at their fair market value on the day they were issued which totaled $340,000. The total cost of the acquisition amounts to $403,840.
On April 14, 2014, the Company entered into an asset purchase agreement for an undivided one hundred percent (100%) interest in fifty seven (57) mining claims located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf. The purchase price was CDN$5,000 (U.S. $4,547). The transfer of the mining claims has been completed by the Province of Quebec in the name of the Company.
There are mining residues, or tailings, (“Montauban Tailings”) located on the Montauban Property which are considered by the Quebec Provincial Government as toxic wastes. There are no environmental liabilities as such to our Company. However, the Company will have to obtain the necessary permits from the Quebec Government Authorities to realize any further fieldwork having an impact on the environment, especially if re-mobilization of mining residues is contemplated.
DRILLING SUMMARY
A systematical sampling program was developed to provide an accurate and homogeneous grid of data to estimate the Montauban Tailings potential. A 24-hole percussion drilling campaign was performed totaling 143.1 meters. This percussion drilling campaign was considered part of completing a previous 25-hole drilling campaign performed earlier. A total of 49 holes totaling 302.3 meters of drilling were completed. No proven or indicated reserves were identified.
PROPERTY DESCRIPTION AND LOCATION
The Property is composed of 122 mining claims, a mining lease and mining concession totaling 4183 hectares located in the Montauban-les-Mines sector of the Notre-Dame-de-Montauban municipality, in the Montauban Township, Portneuf County, Province of Quebec, Canada. The Property is located 120 km east of Quebec City and 80 km north of Trois-Rivières. The Property is located one kilometer west of Montauban-les-Mines with multiple land accesses. Manpower, water and electric power are easily available within the very same distance
ACCESS, CLIMATE, LOCAL INFRASTRUCTURES AND PHYSIOGRAPHY
The Montauban municipality is accessible by route 363 from highway 40 linking Quebec City (120 km to the east) and Trois-Rivières (80 km to the southeast). Access to railway is also available less than 10 km to the northeast in Notre-Dame-des-Anges. The Montauban Mine Property is located one kilometer west of Montauban-les-Mines with multiple land accesses.
Manpower, water and electric power are easily available within one-kilometer distance from the Montauban-les-Mines village. The region is rural, most of the farmers growing potatoes and corn. The equipment and personnel specialized in quarries are available within a 30 km radius from the Montauban Tailings in the surrounding municipalities (Notre-Dame-de-Montauban, St-Ubalde, Lac-aux-Sables, St-Casimir, St-Marc-des-Carrières and Ste-Thècle).
3
The area’s physiography is characterized by argilitic and sandy plateaus forming the foothills of the Laurentides. The Montauban Mine Property is limited to the North West by the Batiscan River, which is the main effluent in the area draining most of the Property towards the south to the St-Lawrence River. The topography consists of numerous small hills reaching an altitude of up to 220 m above the sea level from the valleys standing in average at 160 m elevation.
GEOLOGY
Regional geology consists of three main rock groups: the basement crust, the supracrustal rocks and the intrusive rocks which were respectively identified as the Mekinac Group, the Montauban Group and the La Bostonnais Complex.
The Montauban Group is composed of Helikian supracrustal rocks. Those are various gneiss, quartzites, amphibolites, metabasalts and calcosilicated rocks reaching less than 2 kilometers in thickness. The Montauban deposit is located in the upper part of this unit.
The Montauban Group is bordered to the East by the La Bostonnais Complex, an intrusive rocks complex formed of basic, tonalitic and felsic igneous rocks. To the West, the Montauban Group is in contact with the Mekinac Group mostly composed of charnockitic migmatites.
The Montauban Deposit is a three-kilometer long mineralized formation with a geology that is fairly complex being located within an extensively folded sequence of amphibolite facies rocks that are sandwiched between intrusions of granodioritic to gabbroic composition. In the mine area, these metamorphic rocks strike roughly North-South and dip ±60° to the East and consist of migmatitic biotite gneiss, amphibolite, quartzofeldspathic biotite gneiss and quartzite.
MINERALIZATION
The base metal mineralization found in Montauban is massive to semi-massive sulphides, coarsely grained and mostly composed of sphalerite, galena, pyrrhotite, pyrite and chalcopyrite with minor quantities of cubanite, tetrahedrite and molybdenite.
The gold bearing mineralization is marginal and consists of disseminated pyrrhotite, galena, sphalerite and chalcopyrite with a large range of minor sulphides, sulphosalts and native minerals.
MONTAUBAN TAILINGS
The Montauban Mine Property covers a total area of 53,093 m² and amounts to a total volume of 250,750 m³. Since this volume is composed of tailings and that the water table is located within most of the blocks derived from each hole, the specific gravity of the material had to be evaluated to estimate the tonnage that is present on site.
4

Montauban Mine Property View Looking South
DRILLING RESULTS
The distribution of metals within the tailings is not homogeneous. It was demonstrated with the 49 holes drilled on the Montauban Mine Property that recoveries dropped from 81 to less than 64 % below the 4,6 m (15 ft.) horizon, which is more or less the location of the water table within the Tailings. The impact is seen on metal content when gold is 67 % richer over this horizon, silver is up 73 %, Copper also up 63 %, and the winner being lead with a jump of 149 %. The only one being evenly distributed is zinc.
MILL CONSTRUCTION
We are constructing a mill to process mining residues. Our focus was to produce gold and silver concentrate in addition to the mica product. By extracting mica and producing the gold and silver concentrate, we will reduce the sulphide content of the tailings and thus lowering the environmental impact and cost for the project closure at the end of the operation.
The on-site mill production equipment to be constructed and installed will incorporate cyanization circuit and separation equipment consisting of spiral classifiers and Nelson concentrators in addition to other equipment. Test work to date has indicated that this configuration will effectively segregate the mica and produce a gold/silver concentrate.
To keep expenditures as low as possible, we use refurbished milling equipment when possible. Our larger expenses include the mill building, electrical distribution, pumps, pipe valves, spiral classifiers, Nelson concentrators, table separator, trommel, loader and a conveyor.
PROPERTY DEVELOPMENT
The Company has completed construction of all access roads to and from the new milling facility. The hydropower source to the milling facility totaling 1.3 kilometers has been completed. The main power line consists of 2,500 amperes total output power and has been brought inside the newly erected 16,000 sq/ft. steel structure building.
5
We have also secured the necessary mining permits. The two (2) Certificates of Authorization issued to us will allow for the construction and installation of equipment facilities to recuperate mica (phlogorite) and precious metals (gold and silver) from the mining residues (tailings) located on the Montauban Mine Tailings.
Total mining rights as of December 31, 2014 and 2013 were $1,035,818 and $15,000, respectively.
DNA CRYTO CORP.
On June 20, 2014, DNA Crypto Corp. (“DNAC”), a wholly-owned subsidiary of DNA Precious Metals, Inc., signed a definitive Asset Purchase Agreement with Lynx Mining LLC, a Texas limited liability company (“Lynx”), whereby DNAC acquired the assets of Lynx, being its intellectual property rights. On September 15, 2014 we terminated for cause the Asset Purchase Agreement.
SUBSEQUENT EVENTS
Breathe Acquisition and Spin-off of DNA Canada, Inc.
On January 16, 2015 the Company entered into a share exchange agreement with Breathe LLC, a Tennessee limited liability company, whereby the Company acquired all of the issued and outstanding membership interests in Breathe LLC in consideration for the issuance of 150,000,000 shares of the Company’s common stock to the members of Breathe, LLC
As a result of the transaction effected by the Exchange Agreement, at closing Breathe LLC. became a wholly owned subsidiary of the Company, with the former Breathe LLC members owning approximately 56% of the then issued and outstanding common stock of the Company.
The operations of Breathe:
ABOUT BREATHE SMART CIGARETTE
Breathe, LLC was formed in October 2013 and Breathe eCigs. Corp. was formed on December 31, 2014. On December 31, 2014, Breathe, LLC entered into a Bill of Sale to transfer 100% of the assets to Breathe eCigsCorp.
Since formation, Breathe has operated as a development stage company, with the intentions of designing marketing and distributing electronic cigarettes (“E-cigarettes”), vaporizers, e-liquids (i.e., liquid nicotine) and related accessories. As of December 31, 2014 Breathe had no revenues and limited assets.
E-cigarettes and vaporizers are replacements for traditional cigarettes allowing smokers to reproduce the smoking experience. Although they do contain nicotine, E-cigarettes and vaporizers do not burn tobacco and are not smoking cessation devices.
6
Breathe’s initial line of products will focus on E-cigarettes. The present day E-Cigarette is a smokeless, battery-powered device that vaporizes liquid nicotine for delivery via inhalation by the user. The E-Cigarette does not contain tobacco, only nicotine derived from the tobacco plant and trace amounts of secondary chemical ingredients. The component parts of an E-Cigarette are the nicotine cartridge; the atomizer (which vaporizes the liquid nicotine); the rechargeable battery that powers it; and a light-emitting diode (LED) indicator at the end that is activated when the user draws in air (collectively referred to as the “Component Parts”). Breathe will partner with manufacturers in the United States who will be responsible for producing the liquid nicotine filling the nicotine cartridge with liquid nicotine; thereby ensuring a safe and high standard process for producing a consumer product.
Market Opportunity For E-Cigarettes
Breathe operates within the rapidly growing and global e-cigarette industry, an emerging product category that is taking market share from the $783 billion global tobacco industry. The American Cancer Society estimates that there are 1.3 billion tobacco smokers in the world, consuming approximately 6 trillion cigarettes per year, or 190 thousand cigarettes per second. Tobacco use is the leading cause of preventable illness and death, causing more than 5 million annual deaths across the globe according to the CDC. We believe e-cigarettes offer an alternative for current smokers of traditional cigarettes.
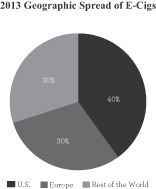
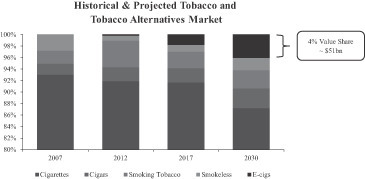
Still in the early stages of its market penetration, the e-cigarette industry is highly fragmented with approximately 250 brands worldwide according to the CDC. Primarily propelled by the cannibalization of the traditional tobacco industry, the global e-cigarette industry has recently experienced dramatic growth. According to Euromonitor, e-cigarettes accounted for approximately $3.5 billion in 2013 global retail sales, with approximately 40% of sales generated in the U.S., 30% of sales generated in Europe, and 30% of sales generated in the rest of the world. Euromonitor estimated that significant market growth was achieved from 2012 to 2013 with the U.S., Europe and the rest of the world generating growth rates of 180%, 160%, and 150%, respectively. Euromonitor also projects e-cigarette sales to represent approximately $51 billion, or 4% of the global tobacco and tobacco alternatives market by 2030. We believe that we are well positioned to benefit from, and take advantage of, these attractive market trends in the coming years.
 |
 |
Source: Euromonitor International 2013.
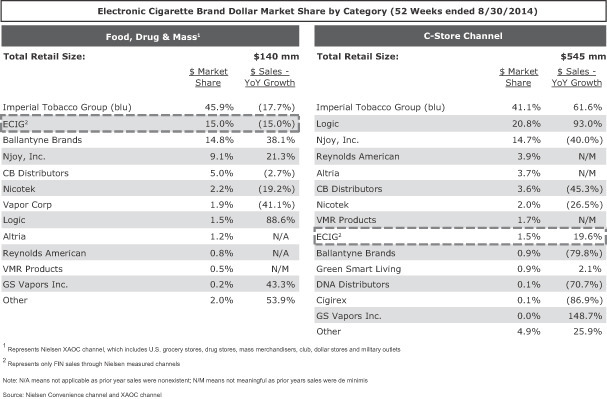
Below is a table presenting the market shares in the United States of various e-cigarette brands for the 52 weeks ended August 30, 2014:
7
BUSINESS STRATEGY
Breathe’s strategic goal is to profitably expand its operations. The business strategies employed by Breathe to achieve this goal are defined succinctly through the Company’s mission statement of creating Socially Responsible Innovation in the E-Cigarette and Vaporizer industries and by fulfilling the following objectives:
|
●
|
Building a strong brand through a concentration of operational focus on the design, market and
distribution of exceptional quality electronic cigarettes and vaporizers.
|
|
●
|
Specializing in the development of great tasting proprietary organic and naturally flavored
e-liquids with nicotine from Tennessee-sourced Tobacco plants.
|
|
|
●
|
Exceptional Packaging – The Company’s high-end products will comprise high quality packaging,
unique and customizable labeling for specific customers and retailers.
|
|
●
|
Age Verification A commitment to verifying and ensuring that all Breathe customers are
at least 21 years old, through specific product labeling and marketing efforts focused on the adult
population age 21 and older.
|
|
●
|
Environmentally Conscious Production and Disposal Process A commitment to
establishing an environmentally aware production and disposal process, which shall include a special
recycling program for eligible retailers where (a) said retailers will be provided with a self-mailer option
to ship expended lithium batteries and other recyclables to a designated facility and (b) where proceeds
from these eligible recyclables will then be shared with the respective retailers.
|
|
●
|
Developing Our Organizational Capability Continuing to develop our organizational capability
through recruiting, retaining and rewarding highly capable people and through performance
management.
|
8
|
●
|
Pursuing Growth Opportunities Focusing on pursuing growth opportunities after launching our
current product offerings and seeking brands, other products, and partnerships to complement our high-end quality products.
|
|
●
|
Maximizing our financial performance Continuing to drive our business activities to deliver improved
financial performance.
|
|
●
|
Developing a global distribution platform with the emphasis of serving customers throughout the entire
world.
|
License to Intellectual Property and Brand Portfolio
Breathe has the exclusive licensing rights to sell the following product lines:
|
●
|
Mini e-cigarette Pack – a standard e-cigarette pack designed for vending machines and convenience
stores.
|
|
●
|
Original non CP – a standard rechargeable single unit without the child protective device.
|
|
●
|
Original with CP - a standard rechargeable single unit with the child protective device.
|
|
●
|
Smart e-cigarette PCC – Smart e-cigarette carrying case, 2 rechargeable mini-e-cigarettes with 5
cartridges and iPhone chargeable connections.
|
|
●
|
5 Pack mini Ref – 5 mini cartridges for the mini size e-cigarette.
|
|
●
|
5 pack Standard Ref – 5 refillable cartridges for the mini-size e-cigarettes.
|
Pricing, Sales Model; e-Commerce and Retail
Breathe plans to offer its Products at prices, determined based on pricing strategies that are developed by the Company from time to time and which management believes to be best suited to achieve the Company’s goals at such time. These pricing strategies are expected to be developed based on a number of factors, including the needs and behaviors of customers, purchase volumes, market specific criteria, and the Company’s costs of goods.
The price of the brand portfolio of products are broken down as follows, with prices varying based on product type and distribution channel (e-commerce vs. retail):
|
Product
|
Mini e-Cig
Pack
|
Original non
CP
|
Original with
CP
|
Smart E-Cig
PCC
|
5 Pack mini
Ref.
|
5 pack
Standard
Ref.
|
|
E-Commerce
Price
|
19.95
|
19.95
|
19.95
|
38.00
|
19.95
|
19.95
|
|
Retail Price
|
10.00
|
10.00
|
10.50
|
25.00
|
10.50
|
10.50
|
9
Management believes that the elegant design of the packaging, along with high quality products which feature excellent tasting, proprietary and handcrafted flavors justifies the costs and increases the margins.
Production and Supply for e-cigarette Lines
The launch of a new E-Cigarette line involves input from many different sources, from the manufacturer to the customer.
The stages of the development, manufacturing, production and distribution process of the E-cigarette can be summarized as follows:
|
●
|
Discussions with designers and creators (includes analysis and factory trends, target clientele and
market communication);
|
|
●
|
Concept choice;
|
|
●
|
Produce mock-ups for final acceptance of unit device, packaging and flavoring;
|
|
●
|
Receive bids from component suppliers;
|
|
●
|
Choose suppliers;
|
|
●
|
Schedule production and packaging;
|
|
●
|
Issue component part purchase orders;
|
|
●
|
Follow quality control procedures for incoming components;
|
|
●
|
Follow packaging and inventory control procedures;
|
|
●
|
Engage U.S. based FDA certified e-liquid manufacturer to produce and fill nicotine cartridges after
receiving Component Parts; and
|
|
●
|
Production specialists who carry out packaging or logistics for storage, order preparation and shipment.
|
Procurement and Distribution
In launching E-Cigarette lines, the Company must be able to coordinate procuring the Component Parts, manufacturing the product, packaging the product, storage, distribution and order processing. The Company has been in discussions with a Canadian-based and Chinese based manufacturer who will produce the pen devices. The Company has been in discussions with a U.S. based manufacturer who will produce the e-liquids and who will also fill the cartridges with the e-liquids, which in turn will allow all of the Company’s consumables to be U.S. oriented. Therefore, after the pen devices are manufactured overseas, the e-liquid filled cartridges will be inserted in the U.S. and ready for distribution.
The Company has been in discussions with a distribution center and warehouse located in Knoxville, TN who will procure the component goods from the manufacturers and other suppliers, package the Company’s products for distribution, manage purchase orders and the electronic data interchange.
10
Additionally, the distribution partner, under the supervision of the Company’s leadership, will be responsible for negotiate pricing and payment terms with suppliers, manufacture and package the products and coordinate payment to the suppliers.
Finally, the Company’s experienced leadership team will be responsible for all component costs, transportation, assembly costs and a management fee paid to the Distribution and Manufacturer.
Market Opportunity
The e-cigarette industry is booming – approximately 3.5 million Americans regularly use e-cigarettes, according to a 2013 study done by Mary Diduch. The Centers for Disease Control show that e-cigarette use quadrupled in a single year from 2009 to 2010. Based on 2011 numbers alone, 21% of adult smokers in the United States have used e-cigarettes, 6% of all adults have tried e-cigarettes, and general awareness of e-cigarettes rose to 60% of all adults, up from 40% from 2010 according to a 2013 study published by the CDC. The co-founder of the Tobacco Vapor Electronic Cigarette Association stated in March 2012 that nearly 20 million e-cigarette cartridges are sold in the United States, per week.
Moreover, there is currently a favorable regulatory environment with certain federal, local and state regulation focused at advertising, age verification and use bans in public areas.
Marketing and Growth Strategies
In order to increase brand awareness, the Company began to focus its marketing initiatives and efforts through the development of a proprietary system that has accumulated over 20 million individuals that have the potential to see very advertisement and social media post produced by the Company. In addition to hosting a secure web portal, www.breathecig.com, that promotes the Company’s products and will handle orders, the Company has also been marketed on major social media platforms: LinkedIn, Facebook, Twitter, Instagram, Google and Pinterest. Because of this successful initial marketing effort:
|
●
|
The Company has already received hundreds of requests for more information on its products.
|
|
●
|
These initial efforts have been cost effective and have not involved a substantial drive to promote sales.
|
|
●
|
The Company’s website has received over 600,000 visitors during the last 18 months.
|
|
●
|
The Company has received numerous requests from customers interested in purchasing Breathe’s products including but not limited to major retail groups, Hotel Chains, Restaurants and Club Owners.
|
Retail and Wholesale Distribution:
The Company is developing unique and distinct brands for its E-cigarette Products for purposes of marketing and selling such branded E-cigarette Products, initially in North America, China, Africa, and Europe, through retail and wholesale distribution channels, including convenience stores, retail chains, wholesale trade, pharmacies, gas stations, hotels, industrial customers, clubs, casinos and duty free stores.
11
In addition, the Company intends to enter into exclusive agreements with various distributors providing them with exclusivity on certain brands of Product in defined territories and markets worldwide.
E-Commerce:
The Company intends to distribute its branded Products through its website, www.breathecig.com, and other online sales platforms. Through its e-commerce sales initiatives, the Company hopes to generate recurring purchases of its exclusive brands of E-cigarettes from customers who are legally allowed to purchase cigarettes in the United States and other regions. Management expects that its marketing strategy will include various forms of social media as a key element in its marketing strategies and in further establishing and growing the Company’s business.
White Label/Private Brand Distribution:
The Company is actively pursuing opportunities and relationships to develop and offer its Products on a “white label”, private branded basis. Management of the Company believes that there is an opportunity to supply Products on a custom branded basis to a variety of customers for purposes of resale. These potential customers may include wholesale and retail customers that have or wish to develop a private customizable label.
Government Regulations
A recent United States Federal Court decision permits the United States Food and Drug Administration to regulate electronic cigarettes as “tobacco products” under the Family Smoking Prevention and Tobacco Control Act of 2009 and the United States Food and Drug Administration has indicated that it intends to do so.
Based on the December 2010 U.S. Court of Appeals for the D.C. Circuit’s decision in Sottera, Inc. v. Food & Drug Administration, 627 F.3d 891 (D.C. Cir. 2010), the United States Food and Drug Administration (the “FDA”) is permitted to regulate electronic cigarettes as “tobacco products” under the Family Smoking Prevention and Tobacco Control Act of 2009 (the “Tobacco Control Act”).
Under this Court decision, the FDA is not permitted to regulate electronic cigarettes as “drugs” or “devices” or a “combination product” under the Federal Food, Drug and Cosmetic Act unless they are marketed for therapeutic purposes.
Because we do not market our electronic cigarettes for therapeutic purposes, our electronic cigarettes are subject to being classified as “tobacco products” under the Tobacco Control Act. The Tobacco Control Act grants the FDA broad authority over the manufacture, sale, marketing and packaging of tobacco products, although the FDA is prohibited from issuing regulations banning all cigarettes or all smokeless tobacco products, or requiring the reduction of nicotine yields of a tobacco product to zero. Among other measures, the Tobacco Control Act (under various deadlines):
12
|
●
|
Increases the number of health warnings required on cigarette and smokeless tobacco products,
increases the size of warnings on packaging and in advertising, requires the FDA to develop graphic
warnings for cigarette packages and grants the FDA authority to require new warnings;
|
|
|
●
|
Requires practically all tobacco product advertising to eliminate color and imagery and instead
consist solely of black text on white background;
|
|
|
●
|
Imposes new restrictions on the sale and distribution of tobacco products, including significant new
restrictions on tobacco product advertising and promotion as well as the use of brand and trade names;
|
|
|
●
|
Bans the use of “light,” “mild,” “low” or similar descriptors on tobacco products;
|
|
|
●
|
Gives the FDA the authority to impose tobacco product standards that are appropriate for the
protection of the public health (by, for example, requiring reduction or elimination of the use of
particular constituents or components, requiring product testing, or addressing other aspects of tobacco
product construction, constituents, properties or labeling);
|
|
|
●
|
Requires manufacturers to obtain FDA review and authorization for the marketing of certain new or
modified tobacco products;
|
|
|
●
|
Requires pre-market approval by the FDA for tobacco products represented (through
labels, labeling, advertising, or other means) as presenting a lower risk of harm or tobacco-related
disease;
|
|
|
●
|
Requires manufacturers to report ingredients and harmful constituents and requires the FDA to
disclose certain constituent information to the public;
|
|
|
●
|
Mandates that manufacturers test and report on ingredients and constituents identified by the FDA as
requiring such testing to protect the public health, and allows the FDA to require the disclosure of testing
results to the public;
|
|
|
●
|
Requires manufacturers to submit to the FDA certain information regarding the health, toxicological,
behavioral or physiologic effects of tobacco products;
|
|
|
●
|
Prohibits use of tobacco containing a pesticide chemical residue at a level greater than allowed under
federal law;
|
|
|
●
|
Requires the FDA to establish “good manufacturing practices” to be followed at tobacco
manufacturing facilities;
|
|
|
●
|
Requires tobacco product manufacturers (and certain other entities) to register with the FDA; and
|
|
|
●
|
Grants the FDA the regulatory authority to impose broad additional restrictions.
|
The Tobacco Control Act also requires establishment, within the FDA’s new Center for Tobacco Products, of a Tobacco Products Scientific Advisory Committee to provide advice, information and recommendations with respect to the safety, dependence or health issues related to tobacco products. As indicated above, the Tobacco Control Act imposes significant new restrictions on the advertising and promotion of tobacco products. For example, the law requires the FDA to finalize certain portions of regulations previously adopted by the FDA in 1996 (which were struck down by the Supreme Court in 2000 as beyond the FDA’s authority). As written, these regulations would significantly limit the ability of manufacturers, distributors and retailers to advertise and promote tobacco products, by, for example, restricting the use of color, graphics and sound effects in advertising, limiting the use of outdoor advertising, restricting the sale and distribution of non-tobacco items and services, gifts, and sponsorship of events and imposing restrictions on the use for cigarette or smokeless tobacco products of trade or brand names that are used for non-tobacco products. The law also requires the FDA to issue future regulations regarding the promotion and marketing of tobacco products sold or distributed over the internet, by mail order or through other non-face-to-face transactions in order to prevent the sale of tobacco products to minors.
It is likely that the Tobacco Control Act could result in a decrease in tobacco product sales in the United States, including sales of our electronic cigarettes.
13
While the FDA has not yet mandated electronic cigarettes be regulated as tobacco products, the FDA issued proposed regulations on April 25, 2014 seeking to regulate electronic cigarettes as tobacco products. Under these proposed rules, products meeting the statutory definition of “tobacco products,” except accessories of a proposed deemed tobacco product, would be subject to the Federal Food, Drug, and Cosmetic Act (the FD&C Act), as amended by the Family Smoking Prevention and Tobacco Control Act (Tobacco Control Act). The Tobacco Control Act provides FDA authority to regulate cigarettes, cigarette tobacco, roll-your-own tobacco, smokeless tobacco, and any other tobacco products that the Agency by regulation deems to be subject to the law. Option 1 of the proposed rule would extend the Agency's “tobacco product” authorities in the FD&C Act to all other categories of products, except accessories of a proposed deemed tobacco product, that meet the statutory definition of “tobacco product” in the FD&C Act. Option 2 of the proposed rule would extend the Agency's “tobacco product” authorities to all other categories of products, except premium cigars and the accessories of a proposed deemed tobacco product, that meet the statutory definition of “tobacco product” in the FD&C Act. FDA also is proposing to prohibit the sale of “covered tobacco products” to individuals under the age of 18 and to require the display of health warnings on cigarette tobacco, roll-your own tobacco, and covered tobacco product packages and in advertisements. Further, the FDA proposed to extend its authority to cover additional products that meet the definition of a tobacco product under the proposed rule: Tobacco Products Deemed To Be Subject to the Food, Drug & Cosmetic Act (Deeming). Currently FDA regulates cigarettes, cigarette tobacco, roll-your-own tobacco and smokeless tobacco. Proposed newly “deemed” products would include: electronic cigarettes, cigars, pipe tobacco, certain dissolvables that are not “smokeless tobacco,” gels and water pipe tobacco.
If electronic cigarettes are regulated as proposed, the FDA will implement new rules to govern the labeling of electronic cigarettes, including smokeless tobacco product warning labels and “light,” “low,” “mild” or similar descriptors for tobacco products. These new laws are expected to have a significant public health impact by decreasing the number of people using tobacco products, resulting in lives saved, increased life expectancy, and lower medical costs. In addition, the FDA will also restrict the way electronic cigarette manufacturers, retailers, and distributers can advertise and promote cigarettes and smokeless tobacco products, especially marketing efforts designed to appeal to youth. Such restrictions relating to marketing, advertising, and promotion will include: prohibiting tobacco brand name sponsorship of any athletic, musical, or other social or cultural event, or any team or entry in those events, requiring that audio ads use only words with no music or sound effects, prohibiting the sale or distribution of items, such as hats and tee shirts, with cigarette and smokeless tobacco brands or logos and requiring that manufacturers receive a written order from FDA permitting the legal marketing of a new tobacco product. The FDA’s traditional “safe and effective” standard for evaluating medical products does not currently apply to tobacco. FDA evaluates new tobacco products based on a public health standard that considers the risks and benefits of the tobacco product on the population as a whole, including users and non-users. To legally market new tobacco products, a written order from FDA must be received permitting its marketing in the United States.
The application of the Tobacco Control Act to electronic cigarettes could impose, among other things, restrictions on the content of nicotine in electronic cigarettes, the advertising, marketing and sale of electronic cigarettes, the use of certain flavorings and the introduction of new products. We cannot predict the scope of such regulations or the impact they may have on our company specifically or the electronic cigarette industry generally, though if enacted, they could have a material adverse effect on our business, results of operations and financial condition.
14
In this regard, total compliance and related costs are not possible to predict and depend substantially on the future requirements imposed by the FDA under the Tobacco Control Act. Costs, however, could be substantial and could have a material adverse effect on our business, results of operations and financial condition. In addition, failure to comply with the Tobacco Control Act and with FDA regulatory requirements could result in significant financial penalties and could have a material adverse effect on our business, financial condition and results of operations and ability to market and sell our products. At present, we are not able to predict whether the Tobacco Control Act will impact us to a greater degree than competitors in the industry, thus affecting our competitive position.
Competition
Competition in the electronic cigarette industry is intense. We compete with other sellers of electronic cigarettes, most notably Lorillard, Inc., Altria Group, Inc. and Reynolds American Inc., big tobacco companies, through their electronic cigarettes business segments; the nature of our competitors is varied as the market is highly fragmented and the barriers to entry into the business are low.
We compete primarily on the basis of product quality, brand recognition, brand loyalty, service, marketing, advertising and price. We are subject to highly competitive conditions in all aspects of our business. The competitive environment and our competitive position can be significantly influenced by weak economic conditions, erosion of consumer confidence, competitors’ introduction of low-priced products or innovative products, cigarette excise taxes, higher absolute prices and larger gaps between price categories, and product regulation that diminishes the ability to differentiate tobacco products.
Our principal competitors are “big tobacco”, U.S. cigarette manufacturers of both conventional tobacco cigarettes and electronic cigarettes like Altria Group, Inc., Lorillard, Inc. and Reynolds American Inc. We compete against “big tobacco” who offers not only conventional tobacco cigarettes and electronic cigarettes but also smokeless tobacco products such as “snus” (a form of moist ground smokeless tobacco that is usually sold in sachet form that resembles small tea bags), chewing tobacco and snuff. Furthermore, we believe that “big tobacco” will devote more attention and resources to developing and offering electronic cigarettes as the market for electronic cigarettes grows. Because of their well-established sales and distribution channels, marketing expertise and significant resources, “big tobacco” is better positioned than small competitors like us to capture a larger share of the electronic cigarette market. We also compete against numerous other smaller manufacturers or importers of cigarettes. There can be no assurance that we will be able to compete successfully against any of our competitors, some of whom have far greater resources, capital, experience, market penetration, sales and distribution channels than us. If our major competitors were, for example, to significantly increase the level of price discounts offered to consumers, we could respond by offering price discounts, which could have a materially adverse effect on our business, results of operations and financial condition.
15
With the acquisition of Breathe, management determined that it would be in the best interest of the Company and its shareholders to operate each company separate and independently of each other. The operation of DNA Canada, Inc. and Breathe were inconsistent. Breathe is a manufacturer and distributor of e-cigarette and related products while DNA Canada, Inc. is an exploration stage mining company. The spin-off of DNA Canada, Inc. will allow each company to focus on its principal business activity and facilitate capital formation.
On March 5, 2015, the Company entered into an Agreement and Plan of Merger pursuant to which the Company merged with its wholly owned subsidiary, Breathe eCig Corp., a Nevada corporation with no material operations and created for the sole purpose of affecting a name change pursuant to Chapter 92A.180 of the Nevada Revised Statutes. Upon the consummation of the Merger on March 11, 2015, the separate existence of Breathe eCig Corp. ceased, and the shareholders of the Company became shareholders of the surviving company, named Breathe eCig Corp. As permitted under Nevada law, the sole purpose of the Merger was to effect a change to the Company’s name from DNA Precious Metals, Inc. to Breathe eCig Corp. This change to the Amended Articles of Incorporation and name change took effect on March 11, 2015.
Spinoff of Mining operations:
The Company declared a stock dividend to its shareholders of record as of February 3, 2015 of its wholly owned subsidiary, DNA Canada, Inc. Each shareholder of record on this date received one share of DNA Canada, Inc. for every two shares of DNA Precious Metals Inc. owned by the shareholder on this date. All stock dividends will be rounded up to the next whole number. With the completion of the stock dividend, the Company, no longer has an equity interest in DNA Canada, Inc.
The former shareholders of Breathe participating in the stock dividend were required to tender for redemption any shares of DNA Canada, Inc. common stock received pursuant to the stock dividend in accordance with the Exchange Agreement.
The following table represents the approximate value of the assets transferred and liabilities assumed by DNAC in connection with the spinoff:
|
Net Assets Spun-off
|
||
|
Cash
|
$25,514
|
|
|
Prepaid expenses
|
137,919
|
|
|
Sales tax receivable
|
17,097
|
|
|
Fixed assets
|
138,124
|
|
|
Mining rights
|
1,035,818
|
|
|
Accounts payable
|
(127,640)
|
|
|
Asset retirement obligation
|
(107,749)
|
|
|
Note payable
|
(127,523)
|
|
|
Accumulated comprehensive income
|
108,337
|
|
|
Due to DNAP
|
(5,288,703)
|
|
|
Adjustment to Additional Paid in Capital
|
$(4,188,806)
|
|
16
The $5,288,703 represents the receivable that the Company would be due from DNAC following the stock dividend. This amount is not expected to be received as DNA Canada, Inc. does not have sufficient cash to repay the balance due. The Company will write off this receivable balance against the additional paid in capital of $4,188,806, and the remaining $1,099,897 will be reflected as a dividend.
Risks related to our mining operations in 2014. The following does not represent our current operations. You should read the risk factors following this discussion to identify those risks associated with Breathe.
We are an exploration stage company and our business plan is unproven. We have generated no revenues from our operations and incurred operating losses since our inception. The Company as a going concern is in doubt.
We are an exploration stage company, our business plan is unproven, and we cannot assure you that we will ever achieve profitability or, if we achieve profitability, that it will be sustainable. We are subject to all of the risks inherent in a new business. We have not generated any revenues to date. At December 31, 2014 we have a working deficit of $165,472 and an accumulated deficit of $7,770,337. We require additional capital to finance our mining activities. We currently have no commitment for additional funding and there can be no assurance that we will be able to secure additional funding, or if available, available on terms acceptable to us.
We have no proven reserves.
The Property does not have known reserves of commercial gold or silver. Our long-term success will be related to the cost and success our exploration and mining programs. Mining for gold and silver and base metals is a highly speculative business, involving a high degree of risk. Few properties that are explored are ultimately developed into producing mines. There is no assurance that our exploration program will result in any discoveries of commercial quantities of gold or silver. There is also no assurance that, even if commercial quantities of gold or silver are discovered, a mine can be brought into commercial production. Production/discovery of gold and silver is dependent upon a number of factors, not the least of which is the technical skill of the exploration personnel involved. The commercial viability of a mine is also dependent upon a number of factors, many of which are beyond our control, such as the worldwide economy, the price of gold and silver, government regulations, including regulations relating to royalties, allowable production and environmental protection.
17
During our operations unexpected events may occur, including labor unrest, changes in government regulations, fires, floods, or earthquakes. It is not always possible to fully insure against such risks and we may decide not to take out insurance against such risks as a result of high premiums or for other reasons. Should such liabilities arise, they may impede our exploration activities, raise costs and otherwise reduce the commercial viability of the Property.
We may not identify proven reserves and our estimates may be inaccurate.
There is no certainty that any expenditures made in our exploration program will result in discoveries of commercially recoverable quantities of gold, silver or any base metal. Most exploration projects do not result in the discovery of commercially extractable deposits of gold or silver and no assurance can be given that any particular level of recovery will in fact be realized or that any identified leasehold interest will ever qualify as a commercially developed. Estimates of mineralization, reserves, deposits and production costs can also be affected by such factors as environmental regulations and requirements, weather, environmental factors, unforeseen technical difficulties, unusual or unexpected geological formations and work interruptions. Material changes in estimated reserves, exploration and mining costs may affect the economic viability of any project.
We will be required to locate mineral reserves for our long-term success.
Mines have limited lives based on proven and probable mineral reserves that are depleted in the course of production. To ensure continued viability we must offset depleted reserves by replacing and expanding our mineral reserves, through further exploration at the Property and/or the acquisition of new properties. Even if additional reserves are discovered, the process from exploration to production can take many years, during which the economic feasibility of production may change. Therefore, our ability to maintain or increase annual production of gold and other base or precious metals once mining activities commence, if at all, will be dependent almost entirely on our ability to bring new mines into production.
Mining is inherently dangerous and subject to conditions or events beyond our control, which could have a material adverse effect on our business.
Mining involves various types of risks and hazards, including:
|
•
|
environmental hazards;
|
|
|
•
|
power outages;
|
|
|
•
|
metallurgical and other processing problems;
|
|
|
•
|
unusual or unexpected geological formations;
|
|
|
•
|
flooding, fire, explosions, cave-ins, landslides and rock-bursts;
|
|
|
•
|
inability to obtain suitable or adequate machinery, equipment, or labor;
|
|
|
•
|
metals losses; and
|
|
|
•
|
periodic interruptions due to inclement or hazardous weather conditions.
|
These risks could result in damage to, or destruction of, mineral properties, production facilities or other properties, personal injury, environmental damage, delays in mining, increased production costs, monetary losses and possible legal liability.
18
Exploration for economic deposits of gold and silver is speculative.
Our business is very speculative since there is generally no way to recover any of the funds expended on exploration unless the existence of commercially exploitable reserves are established and the Company can exploit those reserves by either commencing mining operations, selling or leasing its interest in the property, or entering into a joint venture with a larger company that can further develop the property. Unless we can establish and exploit reserves before our funds are exhausted, we will have to discontinue operations.
Changes in the market price of gold, silver and other metals, which in the past has fluctuated widely, will affect the profitability of our operations and financial condition.
Our profitability and long-term viability depend, in large part, upon the market price of gold and other metals and minerals produced from our mineral properties. The market price of gold and other metals is volatile and is impacted by numerous factors beyond our control, including:
|
•
|
expectations with respect to the rate of inflation;
|
|
|
•
|
the relative strength of the U.S. dollar and certain other currencies;
|
|
|
•
|
interest rates;
|
|
•
|
global or regional political, financial, or economic conditions;
|
|
|
•
|
supply and demand for jewelry and industrial products containing metals; and
|
|
|
•
|
sales by central banks and other holders, speculators and producers of gold and
other metals in response to any of the above factors.
|
A decrease in the market price of gold and other metals could affect the commercial viability of our Montauban Mine Property and our anticipated development and production assumptions. Lower gold prices could also adversely affect our ability to finance future development at the Montauban Mine Property, all of which would have a material adverse effect on our financial condition and results of operations. There can be no assurance that the market price of gold and other metals will remain at current levels or that such prices will improve.
Our estimates of resources are subject to uncertainty.
Estimates of resources are subject to considerable uncertainty. Such estimates are arrived at using standard acceptable geological techniques, and are based on the interpretations of geological data obtained from drill holes and other sampling techniques. Engineers use drilling results to derive estimates of cash operating costs based on anticipated tonnage and grades of ore to be mined and processed, the predicted configuration of the ore bodies, expected recovery rates of metal from ore, comparable facility and operating costs and other factors. Actual cash operating costs and economic returns on projects may differ significantly from the original estimates, primarily due to fluctuations in the current prices of metal commodities extracted from the deposits, changes in fuel costs, labor rates, changes in permit requirements, and unforeseen variations in the characteristics of the ore body. Due to the presence of these factors, there is no assurance that any geological reports will accurately reflect actual quantities of gold or silver that can be economically processed and mined by us.
19
The mineralization estimates are based on interpretation and assumptions and may yield less mineral production, if any, under actual conditions than is currently estimated.
We have relied on independent geologists to conduct drilling samples on the Property. When making determinations whether to continue any project, we must rely upon such estimated calculations as to the mineral reserves and grades of mineralization on the Property. Until ore is actually mined and processed, mineral reserves and grades of mineralization must be considered as estimates only.
These estimates are imprecise and depend upon geological interpretation and statistical inferences drawn from drilling and sampling analysis, which may prove to be unreliable. We cannot assure you that:
|
•
|
these estimates will be accurate;
|
|
|
•
|
reserve or other mineralization estimates will be accurate; or
|
|
|
•
|
this mineralization can be mined or processed profitably.
|
Any material changes in mineral reserve estimates and grades of mineralization may affect the economic viability of placing a property into production and a property’s return on capital. Because we have not started mining operations at the Property and have not commenced actual production, mineralization estimates may require adjustments or downward revisions based upon further drilling and/or actual production experience.
In addition, the grade of ore ultimately mined, if any, may differ from that indicated by our testing results to date. There can be no assurance that minerals recovered in small-scale tests will be duplicated in large-scale tests under on-site conditions or in production scale. Declines in market prices for gold and silver may render portions of our mineralization, reserve estimates uneconomic and result in reduced reported mineralization or adversely affect the commercial viability of the Property. Any material reductions in estimates of mineralization, or of our ability to extract this mineralization, could have a material adverse effect on our results of operations or financial condition.
Our exploration activities at the Montauban Mine Property may not be successful, which could lead us to abandon our plans to develop the property and our investments in exploration.
Our long-term success depends on our ability to identify proven reserves and mine the Property and any other properties we may acquire, if any. Exploration activities are highly speculative in nature, which involves many risks and is frequently non-productive. These risks include unusual or unexpected geologic formations, and the inability to obtain suitable or adequate machinery, equipment or labor. The success of our exploration program is determined in part by the following factors:
|
•
|
the identification of potential gold mineralization based on surficial analysis;
|
|
|
•
|
availability of government-granted exploration permits;
|
|
|
•
|
the quality of our management and our geological and technical expertise;
and
|
|
|
•
|
the capital available for exploration.
|
20
Substantial expenditures are required to establish proven and probable reserves through drilling and analysis, to develop metallurgical processes to extract metal, and to develop the mining and processing facilities and infrastructure at the Property. Whether a mineral deposit will be commercially viable depends on a number of factors, which include, without limitation, the particular attributes of the deposit, such as size, grade and proximity to infrastructure; metal prices, which fluctuate widely; and government regulations, including, without limitation, regulations relating to prices, taxes, royalties, land tenure, land use, importing and exporting of minerals and environmental protection. We may invest significant capital and resources in exploration activities and abandon such investments if we are unable to identify commercially exploitable mineral reserves. We cannot assure you that we will discover mineralized resources in sufficient quantities on the Property to commence commercial development.
Actual capital costs, operating costs, production and economic returns may differ significantly from those we have anticipated and there is no assurance that our development activities will result in profitable mining operations.
We plan to estimate operating and capital costs for the Property based on information available to us and that we believe to be accurate. However, costs for labor, regulatory compliance, energy, mine and plant equipment and materials needed for mine development and construction may fluctuate significantly. In light of these factors, actual costs related to our proposed mine development and construction may exceed any estimates we may make. We do not have an operating history upon which we can base estimates of future operating costs related to the Property. We intend to rely upon our analysis of the future economic feasibility of the project and any estimates that may be contained therein. Studies derive estimates of cash operating costs based upon, among other things:
|
·
|
anticipated tonnage, grades and metallurgical characteristics of the ore to
be mined and processed;
|
|
|
·
|
anticipated recovery rates of gold and other metals from the ore;
|
|
·
|
cash operating costs of comparable facilities and equipment; and
|
|
·
|
anticipated climatic conditions.
|
Capital and operating costs, production and economic returns, and other estimates may differ significantly from actual costs, and there can be no assurance that our actual capital and operating costs will not be higher than anticipated or disclosed.
In addition, any calculations of cash costs and cash cost per ounce may differ from similarly titled measures of other companies and are not intended to be an indicator of projected operating profit.
There can be no assurance that we will be successful in establishing mining operations or profitably exploiting mineral deposits.
We are subject to all of the risks associated with establishing new mining operations and business enterprises including:
|
•
|
the timing and cost, which can be considerable, of the construction of mining
and processing facilities;
|
|
|
•
|
the ability to find sufficient gold reserves to support a mining operation;
|
|
|
•
|
the availability and costs of skilled labor and mining equipment;
|
21
|
•
|
the availability and cost of appropriate smelting and/or refining arrangements;
|
|
|
•
|
compliance with environmental and other governmental approval and permit
requirements;
|
|
|
•
|
the availability of funds to finance construction and development activities;
|
|
|
•
|
potential opposition from non-governmental organizations, environmental
groups, local groups or local inhabitants which may delay or prevent
development activities; and
|
|
|
•
|
potential increases in construction and operating costs due to changes in the
cost of fuel, power, materials, supplies, and other costs.
|
It is common in new mining operations to experience unexpected problems and delays during construction, development and mine start-up; delays in the commencement of mineral production often occur. Accordingly, we cannot assure you that our activities will result in profitable mining operations or that we will successfully establish mining operations or profitably extract gold or silver at the Property.
Historical production at the Property may not be indicative of the potential for future development.
We currently have no commercial production at the Property and have never recorded any revenues from gold or silver production. You should not rely on the fact that there were historical mining operations at the Property as an indication that we will ever have future successful commercial operations at the Property. We expect to continue to incur losses unless and until such time, if ever, as the Property enters into commercial production and generates sufficient revenues to fund our continuing operations. The development of new mining operations requires the commitment of substantial resources for operating expenses and capital expenditures, which may increase in subsequent years as needed consultants, personnel and equipment associated with advancing exploration, development and commercial production are added. The amount and timing of expenditures will depend on the progress of ongoing exploration and development, the results of consultants’ analysis and recommendations, the rate at which operating losses are incurred, the execution of any joint venture agreements with strategic partners and other factors, many of which are beyond our control.
We have no history as a Company engaged in the mining business.
We have no history of earnings or cash flow from mining activities. If we identify proven reserves and are able to proceed to production, commercial viability will be affected by factors that are beyond our control such as the particular attributes of the deposit, the fluctuation in the prices of gold and silver, the cost of construction and operating a mining operation, the availability of economic sources for energy, government regulations including regulations relating to prices, royalties, restrictions on production, quotas on exploration as well as the costs of protection of the environment.
We face many operating hazards.
The development and operation of a mining property involves many risks, which even a combination of experience, knowledge and careful evaluation may not be able to overcome. These risks include, among other things, ground fall, flooding, environmental hazards and the discharge of toxic chemicals, explosions and other accidents. Such occurrences may result in work stoppages, delays in production, increased production costs, damage to or destruction of mines and other producing facilities, injury or loss of life, damage to property, environmental damage and possible legal liability for such damages.
22
A shortage of critical equipment, supplies and resources could adversely affect our operations.
We are dependent on equipment, supplies and resources to carry out our mining operations, including input commodities, drilling equipment and skilled labor. A shortage in the market for any of these factors could cause unanticipated cost increases and delays in delivery times, which could in turn adversely impact production schedules and costs.
Operations at the Property will require a significant amount of water. Successful mining and processing will require careful control of project water usage and efficient reclamation of project solutions in the process.
Current global financial conditions have made access to financing more difficult.
Since the fall of 2008 there has been severe deterioration in global credit and equity markets. This has resulted in the need for government intervention in major banks, financial institutions and insurers, and has also led to greater volatility, increased credit losses and tighter credit conditions. These unprecedented disruptions in the credit and financial markets have had a significant adverse impact on a number of financial institutions and have limited access to capital and credit for many companies. These disruptions could, among other things, make it more difficult for us to obtain, or increase our cost of obtaining, capital and financing for our operations.
We do not insure against all risks to which we may be subject in our planned operations.
Any insurance that we secure will in all likelihood not cover all of the potential risks associated with a mining company’s operations, and we may be unable to maintain insurance to cover these risks at economically feasible premiums. Insurance coverage may not be available or may not be adequate to cover any resulting liability. Moreover, we expect that insurance against certain hazards as a result of exploration and production may be prohibitively expensive to obtain for a company of our size and financial means.
We might also become subject to liability for pollution or other hazards which may not be insured against or which we may elect not to insure against because of premium costs or other reasons. Insurance against certain environmental risks, including potential liability for pollution or other hazards as a result of the disposal of waste products occurring from production, is not generally available to us or to other companies within the mining industry.
Losses from events that are not covered by our insurance policies may cause us to incur significant costs that could negatively affect our financial condition and ability to fund our activities on the Property. A significant loss could force us to terminate our operations.
Drilling operations are hazardous, raise environmental concerns and raise insurance risks.
We intend to conduct our business in a way that safeguards public health and the environment and in compliance with applicable laws and regulations. Environmental hazards may exist on properties in which we hold an interest which are unknown to us and may have been caused by prior owners. Changes to drilling and mining laws and regulations could require additional capital expenditures and increase operating and/or reclamation costs. Although we are unable to predict what additional legislation, if any, might be proposed or enacted, additional regulatory requirements could render certain operations uneconomic.
23
Local infrastructure may impact our exploration activities and results of operations.
Our activities depend, to one degree or another, on adequate infrastructure. Reliable roads, bridges and power and water supplies are important determinants that affect capital and operating costs. Unusual or infrequent weather phenomenon, sabotage or government or other interference in the maintenance of such infrastructure could adversely affect our activities.
We are subject to significant governmental regulations.
The Montauban Mine Property is located in Quebec, Canada and is subject to extensive federal, provincial, and local laws and regulations governing various matters, including:
|
|
•
|
environmental protection;
|
|
•
|
management and use of toxic substances and explosives;
|
|
|
•
|
management of natural resources;
|
|
|
•
|
labor standards and occupational health and safety, including mine safety; and
|
|
|
•
|
historic and cultural preservation.
|
Noncompliance may result in civil or criminal fines or penalties or enforcement actions, including orders issued by regulatory or judicial authorities enjoining or curtailing operations or requiring corrective measures, installation of additional equipment or remedial actions, any of which could result in us incurring significant expenditures. We may also be required to compensate private parties suffering loss or damage by reason of a breach of such laws, regulations or permitting requirements. It is also possible that future laws and regulations will be more stringent which could cause additional expense, capital expenditures, restrictions on our operations and delays in the development of the Property.
Our activities are subject to environmental laws and regulations that may increase our costs of doing business and restrict our operations.
All of our exploration and potential development and production activities are in the province of Quebec, Canada and are subject to regulation by governmental agencies under various environmental laws. These laws address, among other things, emissions into the air, discharges into water, management of waste, management of hazardous substances, protection of natural resources, antiquities and endangered species and reclamation of lands disturbed by mining operations.
Additionally, our operations will result in emissions of greenhouse gases, which may be subject to increased regulation in the future. In general, environmental legislation is evolving and the trend has been towards stricter standards and enforcement, increased fines and penalties for non-compliance, more stringent environmental assessments of proposed projects and increasing responsibility for companies and their officers, directors and employees. Compliance with environmental laws and regulations requires significant capital outlays, and future changes in these laws and regulations may cause material changes or delays in our financial position, operations and future activities. More stringent regulation may cause us to re-evaluate our activities.
24
Land reclamation requirements for the Property may be burdensome.
Land reclamation requirements are generally imposed on mineral exploration companies (as well as companies with mining operations) in order to minimize long-term effects of land disturbance.
Reclamation may include requirements to:
|
•
|
control dispersion of potentially deleterious effluents; and
|
|
|
•
|
reasonably re-establish pre-disturbance landforms and vegetation.
|
In order to carry out reclamation obligations we will have to allocate a portion of our financial resources that might otherwise be spent on further exploration and development programs. Unanticipated reclamation work will adversely impact our operations.
We may not be able to comply with permitting requirements.
We have obtained required permitting to commence production activities. Maintaining the permits may require us to comply with more stringent government regulation or new regulatory controls may be instituted which will require us to implement more stringent controls and procedures over our production activities. There can be no assurance that we will be able to comply with more stringent government regulations or that additional costs will be required to remain compliant. This may result in production delays and impact our budgeted resources.
We may experience difficulty attracting and retaining qualified management.
We are dependent on the services of our executive officers. We will have to hire other highly skilled and experienced consultants. Due to our relatively small size, the loss of these persons or our inability to attract and retain highly skilled employees may have a material adverse effect on our business or future operations. We do not maintain key-man life insurance on any of our officers or directors.
We compete with larger, better-capitalized competitors in the mining industry.
The mining industry is intensely competitive in all of its phases, including financing, technical resources, personnel and property acquisition. It requires significant capital, technical resources, personnel and operational experience to effectively compete in the mining industry. Larger companies with significant resources have an advantage over us. Competition for resources at all levels is very intense, particularly affecting the availability of manpower, drill rigs, mining equipment and production equipment. As a result, we may be unable to maintain or acquire financing, personnel or technical resources.
There are differences in U.S. and Canadian practices for reporting reserves and resources.
Since our operations are in Canada, resource estimates disseminated outside the United States are not directly comparable to those made in filings subject to SEC reporting and disclosure requirements. These practices are different from the practices used to report reserve and resource estimates in reports and other materials filed with the SEC. It is Canadian practice to report measured, indicated and inferred resources, which are generally not permitted in filings with the SEC. In the United States, mineralization may not be classified as a “reserve” unless the determination has been made that the mineralization could be economically and legally produced or extracted at the time the reserve determination is made. United States investors are cautioned not to assume that all or any part of measured or indicated resources will ever be converted into reserves. Further, “inferred resources” have a great amount of uncertainty as to their existence and as to whether they can be mined legally or economically.
25
Our directors and officers may have conflicts of interest as a result of their relationships with other companies.
Our directors and officers may serve as officers or directors for other companies engaged in natural resource exploration and development. The directors and officers owe us a fiduciary obligation. We have not yet established a policy to deal with potential conflicts of interest.
Compliance with SEC reporting requirements can be costly.
We do not have any employees to segregate responsibilities and may be unable to afford increasing our staff or engaging outside consultants or professionals to overcome our lack of employees. During the course of our operations, we may identify other deficiencies that we may not be able to remedy in time to satisfy the requirements imposed by the Sarbanes-Oxley Act for compliance with that Section 404. If we fail to achieve and maintain the adequacy of our internal controls, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act. Moreover, effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information.
Legislation, including the Sarbanes-Oxley Act of 2002, may make it difficult for us to retain or attract officers and directors.
We may be unable to attract and retain qualified officers, directors and members of board committees required to provide for our effective management as a result of rules and regulations that govern publicly held companies. The Sarbanes-Oxley Act has resulted in a series of rules and regulations that increase responsibilities and liabilities of directors and executive officers. We are a small company with a limited operating history and no revenues. This may influence the decisions of potential candidates we may recruit as directors or officers. The perceived increased personal risk associated with these recent changes may deter qualified individuals from accepting these roles.
Risks related to our operations as an eCigarette manufacturer.
The following risk factors reflect our current operations as a result of our acquisition of Breathe LLC. And Breathe eCigs Corp. (“Breathe” or “Breathe OldCo.”)
Breathe has a limited operating history, and may not be successful in developing profitable business operations.
26
Breathe is a development stage company focused on the e-cigarette market. Breathe was organized in October 2013. Breathe’s business operations must be considered in light of the risks, expenses and difficulties frequently encountered by a developmental stage company. As of December 31, 2014, Breathe has not generated any revenues and has limited operations. Breathe will require additional capital to finance its ongoing operations. There is nothing at this time on which to base an assumption that our business operations will be successful in the long-term. Breathe’s future operating results will depend on many factors, including:
|
●
|
Breathe’s ability to raise adequate working capital;
|
|
●
|
success of in developing and marketing our e-cigarettes;
|
|
●
|
demand for e-cigarettes;
|
|
●
|
increased regulations of e-cigarettes
|
|
●
|
the level of our competition; and
|
|
●
|
Breathe’s ability to attract and maintain key management and employees.
|
While our officers and directors have significant business experience, there can be no assurance that this experience will help us fully implement its business plan. Prospects for success must be considered in the context of a new company in a highly competitive industry with few barriers to entry.
We have limited capital and will need to raise additional capital in the future.
Breathe does not currently have sufficient capital to fund both our continuing operations and its planned growth. Breathe will require additional capital to continue to expand its operations. Breathe may be unable to obtain additional capital when required. Future business development activities, as well as our administrative requirements (such as salaries, insurance expenses and general overhead expenses, as well as legal compliance costs and accounting expenses) will require a substantial amount of additional capital and cash flow.
Breathe may pursue sources of additional capital through various financing transactions or arrangements, including joint venturing of projects, debt financing, equity financing or other means. Breathe may not be successful in identifying suitable financing transactions in the time period required or at all, and we may not obtain the capital we require by other means. If Breathe does not succeed in raising additional capital, Breathe’s operations will terminate and there is a risk of loss of your entire investment.
Any additional capital raised through the sale of equity may dilute the ownership percentage of our stockholders. Raising any such capital could also result in a decrease in the fair market value of your equity securities because our assets would be owned by a larger pool of outstanding equity. The terms of securities we issue in future capital transactions may be more favorable to our new investors, and may include preferences, superior voting rights and the issuance of other derivative securities, and issuances of incentive awards under equity employee incentive plans, which may have a further dilutive effect.
27
The Company’s ability to obtain financing may be impaired by such factors as the capital markets (both generally and in our industry in particular), our limited operating history, national unemployment rates and the departure of key employees. Further, economic downturns will likely decrease our revenues may increase our requirements for capital. If the amount of capital we are able to raise from financing activities, together with our revenues from operations, is not sufficient to satisfy our capital needs (even to the extent that we reduce our operations), we may be required to cease our operations, divest our assets at unattractive prices or obtain financing on unattractive terms.
We may not be able to successfully manage our growth, which could lead to our inability to implement our business plan.
Our growth is expected to place a significant strain on our managerial, operational and financial resources, especially considering that we currently only have a small number of executive officers, employees and advisors. Further, as we enter into various contracts or other transactions, we will be required to manage multiple relationships with various consultants, businesses and other third parties. There can be no assurance that our systems, procedures and/or controls will be adequate to support our operations or that our management will be able to achieve the rapid execution necessary to successfully implement our business plan. If we are unable to manage our growth effectively, our business, results of operations and financial condition will be adversely affected, which could lead to us being forced to abandon or curtail our business plan and operations.
Our executive officers and key employees will be crucial to our business, and we may not be able to recruit, integrate and retain the personnel we need to succeed.
Our future success is dependent, in a large part, on retaining the services of our current officers and directors. The knowledge, leadership and technical expertise of management would be difficult to replace. While no director has plans to leave or retire in the near future, the loss of any of our directors could have a material adverse effect on our operating and financial performance, including our ability to develop and execute our long The loss of the services of any key personnel, or our inability to attract, integrate and retain highly skilled technical, management, sales and marketing personnel could result in significant disruption to our operations, including our inability or limited success in developing our job verticals, completion of our initiatives, including growth plans and the results of our operations. Any failure by us to find suitable replacements for our key management may be disruptive to our operations. Competition for such personnel can be intense, and we may be unable to attract, integrate and retain such personnel successfully.
Breathe does not have any independent directors and have not implemented various corporate governance measures, in the absence of which, stockholders may have more limited protections against interested director transactions, conflicts of interest and similar matters.
Breathe does not have any independent directors to evaluate its decisions nor have we adopted corporate governance measures. Although not required by rules or regulations applicable to us, corporate governance measures such as the presence of independent directors, or the establishment of an audit and other independent committees of our Board of Directors, would be beneficial to our stockholders. We do not presently maintain any of these protections for our stockholders. It is possible that if our Board of Directors included independent directors and if we were to adopt corporate governance measures, stockholders would benefit from greater assurance that decisions were being made with impartiality by directors and that policies had been implemented to define conduct of our management and board members. For example, in the absence of audit, nominating and compensation committees comprised of at least a majority of independent directors, decisions concerning matters such as compensation packages to our officers and recommendations for director nominees may be made by existing members of the Board of Directors, who may have a direct interest in the outcome. Although we anticipate expanding the Board of Directors to include independent directors at some point in the future, when and if this will occur is uncertain.
28
Our management controls a significant percentage of our current outstanding common stock.
As of the date of this report, our officers and directors collectively and beneficially own a more than 50% of our outstanding common stock. This concentration of voting control gives management substantial influence over any matters which require a stockholder vote, including without limitation the election of directors and approval of merger and/or acquisition transactions, even if their interests may conflict with those of other stockholders. It could have the effect of delaying or preventing a change in control of, or otherwise discouraging, a potential acquirer from attempting to obtain control of the company. This could have a material adverse effect on the market price of our common stock or prevent our stockholders from realizing a premium over the then prevailing market prices for their shares of common stock.
Our business will suffer if we cannot adequately protect our patent and proprietary rights
We have a patent pending. The patent application may be challenged. In addition, there can be no assurance that the patent will provide us with meaningful protection from competition, or that we will possess the financial resources necessary to enforce any of our patents. Also, we cannot be certain that any products that we (or a licensee) develop will not infringe upon any patent or other intellectual property right of a third party. We also rely upon trade secrets, know-how, and continuing technological advances to develop and maintain our competitive position.
We are vulnerable to intellectual property infringement claims brought against us by others.
Successful intellectual property infringement claims against us could result in monetary liability or a material disruption in the conduct of our business. We cannot be certain that our products, content and brand names do not or will not infringe valid patents, trademarks, copyrights or other intellectual property rights held by third parties. We expect that infringement claims in our markets will increase in number. We may be subject to legal proceedings and claims from time to time relating to the intellectual property of others in the ordinary course of our business. If we were found to have infringed the intellectual property rights of a third party, we could be liable to that party for license fees, royalty payments, lost profits or other damages, and the owner of the intellectual property might be able to obtain injunctive relief to prevent us from using the technology or software in the future. If the amounts of these payments were significant or we were prevented from incorporating certain technology into our products, our business could be significantly harmed. We may incur substantial expenses in defending against these third party infringement claims, regardless of their merit. As a result, due to the diversion of management time, the expense required to defend against any claim and the potential liability associated with any lawsuit, any significant litigation could significantly harm our business, financial condition and results of operations.
29
If we are unable to protect our, proprietary rights or maintain our rights to use key technologies of third parties, our business may be harmed.
We may be issued patents. Our patent applications or patents may be later challenged, invalidated or circumvented, and the rights granted under patents may not provide us with a competitive advantage. We may also face risks associated with any trademarks to which we own the rights. Policing unauthorized use of our patented, proprietary technology and other intellectual property rights could involve significant expense and could be difficult or impossible, particularly given the global nature of the Internet and the fact that the laws of certain other countries may afford us little or no effective protection of our intellectual property.
We may incur significant increased costs as a result of operating as a public company, and our management may be required to devote substantial time to new compliance initiatives.
In the future, we may incur significant legal, accounting and other expenses as a result of operating as a public company. The Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), as well as new rules subsequently implemented by the SEC, have imposed various new requirements on public companies, including requiring changes in corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these new compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these new rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage.
In addition, the Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. In particular, we are required to perform system and process evaluation and testing on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline, and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
Risks Related to e-cigarettes:
E-cigarettes may be subject to extensive government regulations.
E-cigarettes are likely to become subject to increasing government regulation Compliance with these government regulations may result in increased costs. If E-cigarettes become subject to taxes similar to regular cigarettes, or restriction on where individuals can smoke e-cigarettes, demand may decrease.
30
The FDA has concluded that electronic cigarettes may contain ingredients that are known to be toxic to humans and may contain other ingredients that may not be safe.
The FDA conducted a preliminary analysis on some samples of electronic cigarettes and components from two leading brands. These samples included 18 of the various flavored, nicotine, and no-nicotine cartridges offered for use with these products. These cartridges were obtained in order to test some of the ingredients contained in them and inhaled by users of electronic cigarettes. The FDA's Center for Drug Evaluation, Division of Pharmaceutical Analysis (DPA) analyzed the cartridges from these electronic cigarettes for nicotine content and for the presence of other tobacco constituents, some of which are known to be harmful to humans, including those that are potentially carcinogenic or mutagenic. The DPA's analysis of the electronic cigarette samples showed that the product contained detectable levels of known carcinogens and toxic chemicals to which users could potentially be exposed. DPA's testing also suggested that quality control processes used to manufacture these products are inconsistent or non-existent. Specifically, the DPA's analysis of the electronic cigarette cartridges from the two leading brands revealed the following:
|
|
·
|
Diethylene glycol was detected in one cartridge at approximately 1%. Diethylene glycol, an
ingredient used in antifreeze, is toxic to humans.
|
|
·
|
Certain tobacco-specific nitrosamines which are human carcinogens were detected in half of the
samples tested.
|
|
|
·
|
Tobacco-specific impurities suspected of being harmful to humans-anabasine, myosmine, and-
nicotyrine-were detected in a majority of the samples tested.
|
|
|
·
|
The electronic cigarette cartridges that were labeled as containing no nicotine had low levels of nicotine
present in all cartridges tested, except one.
|
|
|
·
|
Three different electronic cigarette cartridges with the same label were tested and each cartridge
emitted a markedly different amount of nicotine with each puff. The nicotine levels per puff ranged
from 26.8 to 43.2 mcg nicotine/100 mL puff.
|
|
|
·
|
One high-nicotine cartridge delivered twice as much nicotine to users when the vapor from that
electronic cigarette brand was inhaled than was delivered by a sample of the nicotine inhalation product
(used as a control) approved by FDA for use as a smoking cessation aid.
|
Because our products may contain ingredients that are known to be toxic to humans, any decreases in tobacco product sales in the United States, including sales of our electronic cigarettes, could have a material adverse effect on our business, results of operations and financial condition.
The FDA has received voluntary reports of adverse events involving e-cigarettes from consumers, health professionals and concerned members of the public.
The FDA regularly receives voluntary reports of adverse events involving e-cigarettes from consumers, health professionals and concerned members of the public. The adverse events described in these reports have included hospitalization for illnesses such as:
|
|
·
|
Pneumonia
|
|
·
|
Congestive heart failure,
|
|
|
·
|
Disorientation,
|
|
|
·
|
Seizure,
|
|
|
·
|
Hypotension, and
|
|
|
·
|
Other health problems.
|
31
Whether e-cigarettes caused these reported adverse events is unknown. Some of the adverse events could be related to a pre-existing medical condition or to other causes that were not reported to the FDA. Because our products may contribute to adverse events requiring hospitalization, any decreases in tobacco product sales in the United States as a result, including sales of our electronic cigarettes could have a material adverse effect on our business, results of operations and financial condition.
There have been publicized incidents of electronic cigarettes exploding if improperly used.
There have been several news reports publicizing incidents in which electronic cigarettes have exploded. According to such news reports, the cause of such electronic cigarettes exploding was due to improper use by users of such electronic cigarettes that charged the devices with equipment that was not intended for such devices. Although we notify users of our products with regard to how to properly use our products, we may be unable to prevent improper use that may result in explosions of our products similar to that publicized in recent news reports, which could have a material adverse effect on our business, results of operations and financial condition.
The recent development of electronic cigarettes has not allowed the medical profession to study the long-term health effects of electronic cigarette use.
Because electronic cigarettes were recently developed the medical profession has not had a sufficient period of time to study the long-term health effects of electronic cigarette use. Currently, therefore, there is no way of knowing whether or not electronic cigarettes are safe for their intended use. If the medical profession were to determine conclusively that electronic cigarette usage poses long-term health risks, electronic cigarette usage could decline, which could have a material adverse effect on our business, results of operations and financial condition.
Our business, results of operations and financial condition could be adversely affected if we are taxed like other tobacco products or if we are required to collect and remit sales tax on certain of our internet sales.
Presently the sale of electronic cigarettes is not subject to federal excise taxes, and most state and local excise taxes, like the sale of conventional cigarettes or other tobacco products, all of which have faced significant increases in the amount of taxes collected on their sales. At present, the sale of electronic cigarettes is subject to state excise taxes in only a small number of states, Minnesota and North Carolina. However, should federal, state and local governments and or other taxing authorities impose excise taxes similar to those levied by Minnesota and North Carolina, it may have a material adverse effect on the demand for our products, as consumers may be unwilling to pay the increased costs for our products.
We may be unable to establish the systems and processes needed to track and submit the excise and sales taxes we collect through Internet sales, which would limit our ability to market our products through our websites which would have a material adverse effect on our business, results of operations and financial condition. States such as New York, Hawaii, Rhode Island and North Carolina have begun collecting sales taxes on Internet sales where companies have used independent contractors in those states to solicit sales from residents of that state. The requirement to collect, track and remit sales taxes based on independent affiliate sales may require us to increase our prices, which may affect demand for our products or conversely reduce our net profit margin, either of which would have a material adverse effect on our business, results of operations and financial condition.
32
The market for electronic cigarettes is a niche market, subject to a great deal of uncertainty and is still evolving.
Electronic cigarettes, having recently been introduced to market, are at an early stage of development, represent a niche market and are evolving rapidly and are characterized by an increasing number of market entrants. Our future sales and any future profits are substantially dependent upon the widespread acceptance and use of electronic cigarettes. Rapid growth in the use of, and interest in, electronic cigarettes is recent, and may not continue on a lasting basis. The demand and market acceptance for these products is subject to a high level of uncertainty. Therefore, we are subject to all of the business risks associated with a new enterprise in a niche market, including risks of unforeseen capital requirements, failure of widespread market acceptance of electronic cigarettes, in general or, specifically our products, failure to establish business relationships and competitive disadvantages as against larger and more established competitors.
We face intense competition and our failure to compete effectively could have a material adverse effect on our business, results of operations and financial condition.
We are a developmental stage company with limited capital. We will face intense competition from larger, well established e-cigarette manufacturers including big tobacco companies which are increasingly entering into the e-cigarette market. There can be no assurance that we will be able to compete based on the superior quality of our product and niche marketing opportunities.
Sales of conventional tobacco cigarettes have been declining, which could have a material adverse effect on our business.
The overall U.S. market for conventional tobacco cigarettes has generally been declining in terms of volume of sales, as a result of restrictions on advertising and promotions, funding of smoking prevention campaigns, increases in regulation and excise taxes, a decline in the social acceptability of smoking, and other factors, and such sales are expected to continue to decline. Recently, a national drug store chain announced that it would cease selling tobacco products. If other national drug store chains also decide to cease selling tobacco products, cigarette sales could decline further. While the sales of electronic cigarettes have been increasing over the last several years, the electronic cigarette market is only developing and is a fraction of the size of the conventional tobacco cigarette market. A continual decline in cigarette sales may adversely affect the growth of the electronic cigarette market, which could have a material adverse effect on our business, results of operations and financial condition.
Electronic cigarettes face intense media attention and public pressure.
Electronic cigarettes are new to the marketplace and since their introduction certain members of the media, politicians, government regulators and advocate groups, including independent medical physicians have called for an outright ban of all electronic cigarettes, pending regulatory review and a demonstration of safety. A partial or outright ban would have a material adverse effect on our business, results of operations and financial condition.
33
We may experience product liability claims in our business, which could adversely affect our business.
The tobacco industry in general has historically been subject to frequent product liability claims. As a result, we may experience product liability claims from the marketing and sale of electronic cigarettes. Any product liability claim brought against us, with or without merit, could result in:
|
●
|
Liabilities that substantially exceed our product liability insurance, which we would then be
required to pay from other sources, if available;
|
|
|
●
|
An increase of our product liability insurance rates or the inability to maintain insurance coverage
in the future on acceptable terms, or at all;
|
|
|
●
|
Damage to our reputation and the reputation of our products, resulting in lower sales;
|
|
|
●
|
Regulatory investigations that could require costly recalls or product modifications;
|
|
|
●
|
Litigation costs; and
|
|
|
●
|
The diversion of management’s attention from managing our business.
|
Any one or more of the foregoing could have a material adverse effect on our business, results of operations and financial condition.
If we experience product recalls, we may incur significant and unexpected costs and our business reputation could be adversely affected.
We may be exposed to product recalls and adverse public relations if our products are alleged to cause illness or injury, or if we are alleged to have violated governmental regulations. A product recall could result in substantial and unexpected expenditures that could exceed our product recall insurance coverage limits and harm to our reputation, which could have a material adverse effect on our business, results of operations and financial condition. In addition, a product recall may require significant management time and attention and may adversely impact on the value of our brands. Product recalls may lead to greater scrutiny by federal or state regulatory agencies and increased litigation, which could have a material adverse effect on our business, results of operations and financial condition.
Product exchanges, returns and warranty claims may adversely affect our business.
If we are unable to maintain an acceptable degree of quality control of our products we will incur costs associated with the exchange and return of our products as well as servicing our customers for warranty claims. Any of the foregoing on a significant scale may have a material adverse effect on our business, results of operations and financial condition.
Adverse economic conditions may adversely affect the demand for our products.
Electronic cigarettes are new to market and may be regarded by users as a novelty item and expendable as such demand for our products may be extra sensitive to economic conditions. When economic conditions are prosperous, discretionary spending typically increases; conversely, when economic conditions are unfavorable, discretionary spending often declines. Any significant decline in economic conditions that affects consumer spending could have a material adverse effect on our business, results of operations and financial condition.
34
Our success is dependent upon our marketing efforts.
We intend to undertake extensive marketing activities to promote brand awareness and our portfolio of products. If we are unable to generate significant market awareness for our products and our brands at the consumer level or unable to capitalize on significant marketing, advertising or promotional campaigns we undertake, our business, financial condition and results of operations could be adversely affected.
We depend on third party manufacturers for our products.
We depend on third party manufacturers for our electronic cigarettes. Our customers associate certain characteristics of our products including the weight, feel, draw, unique flavour, packaging and other attributes of our products to the brands we market, distribute and sell. Any interruption in supply and/or consistency of our products may adversely impact our ability to deliver our products to our wholesalers, distributors and customers and otherwise harm our relationships and reputation with customers, and have a materially adverse effect on our business, results of operations and financial condition.
Although we believe that several alternative sources for the components, chemical constituents and manufacturing services necessary for the production of our products are available, any failure to obtain any of the foregoing would have a material adverse effect on our business, results of operations and financial condition.
We may be unable to promote and maintain our brands.
We believe that establishing and maintaining the brand identities of our products is a critical aspect of attracting and expanding a large customer base. Promotion and enhancement of our brands will depend largely on our success in continuing to provide high quality products. If our customers and end users do not perceive our products to be of high quality, or if we introduce new products or enter into new business ventures that are not favorably received by our customers and end users, we will risk diluting our brand identities and decreasing their attractiveness to existing and potential customers.
Moreover, in order to attract and retain customers and to promote and maintain our brand equity in response to competitive pressures, we may have to increase substantially our financial commitment to creating and maintaining a distinct brand loyalty among our customers. If we incur significant expenses in an attempt to promote and maintain our brands, our business, results of operations and financial condition could be adversely affected.
We expect that new products and/or brands we develop will expose us to risks that may be difficult to identify until such products and/or brands are commercially available.
We are currently developing, and in the future will continue to develop, new products and brands, the risks of which will be difficult to ascertain until these products and/or brands are commercially available. For example, we are developing new formulations, packaging and distribution channels. Any negative events or results that may arise as we develop new products or brands may adversely affect our business, financial condition and results of operations.
35
The market for e-cigarettes is a niche market, subject to a great deal of uncertainty, and is still evolving.
E-cigarettes, having recently been introduced to market, are at an early stage of development, represent a niche market and are evolving rapidly and are characterized by an increasing number of market entrants. Our future sales and any future profits are substantially dependent upon the widespread acceptance and use of e-cigarettes. Rapid growth in the use of, and interest in, e-cigarettes is recent, and may not continue on a lasting basis. The demand and market acceptance for these products is subject to a high level of uncertainty. Therefore, we are subject to all of the business risks associated with a new enterprise in a niche market, including risks of unforeseen capital requirements, failure of widespread market acceptance of e-cigarettes, in general or, specifically our products, failure to establish business relationships and competitive disadvantages as against larger and more established competitors.
Our business is in a relatively new consumer product segment, which is difficult to forecast.
Our industry segment is relatively new, and is constantly evolving. As a result, there is a dearth of available information with which to forecast industry trends or patterns. For instance, after several quarters of rapid growth within the segment, there was a decrease in industry growth in the second quarter of 2014 as retailers grappled with high inventory levels from 2013, coupled with changes in preferences and attitudes among users of e-cigarettes and related products. There is no assurance that sustainable industry trends or preferences will develop that will lead to predictable growth or earnings forecasts for individual companies or the industry segment as a whole. We are also unable to determine what impact future governmental regulation may have on trends and preferences or patterns within our industry segment. See “Risks Related to Government Regulation” for a discussion of the risks associated with governmental regulation.
The recent development of e-cigarettes has not allowed the medical profession to study the long-term health effects of e-cigarette use.
Because e-cigarettes were recently developed the medical profession has not had a sufficient period of time to study the long-term health effects of e-cigarette use. Currently, therefore, there is no way of knowing whether or not e-cigarettes are safe for their intended use. If the medical profession were to determine conclusively that e-cigarette usage poses long-term health risks, e-cigarette usage could decline, which could have a material adverse effect on our business, results of operations and financial condition.
The use of e-cigarettes may pose health risks as great as, or greater than, regular tobacco products.
According to the FDA, e-cigarettes may contain ingredients that are known to be toxic to humans and may contain other ingredients that may not be safe. In addition, other publicized recent studies contain assertions that additional carcinogens, including formaldehyde, may be produced through the use of tank systems. Additionally, e-cigarettes may be attractive to young people and may lead them to try other tobacco products, including conventional cigarettes that are known to cause disease. Because clinical studies about the safety and efficacy of e-cigarettes have not been submitted to the FDA, consumers currently have no way of knowing whether e-cigarettes are safe before their intended use; what types or concentrations of potentially harmful chemicals are found in these products; or how much nicotine is being inhaled.
36
Our products contain nicotine, which is considered to be a highly addictive substance.
Certain of our products contain nicotine, a chemical found in cigarettes and other tobacco products which is considered to be highly addictive. The Family Smoking Prevention and Tobacco Control Act, empowers the FDA to regulate the amount of nicotine found in tobacco products, but may not require the reduction of nicotine yields of a tobacco product to zero. Any FDA regulation may require us to reformulate, recall and or discontinue certain of the products we may sell from time to time, which may have a material adverse effect on us.
We need to maintain state of the art software and websites.
Website and Internet technologies are constantly changing. In order for us to remain competitive we must continue to develop and or utilize state of the art software. We must also continue to upgrade our websites to make visitors to our websites an educational and rewarding experience. If the software and technologies used in our websites should fall behind, we success of our business could be materially adversely affected.
If Internet search engines’ methodologies are modified or our search result page rankings decline for other reasons, our user engagement could decline.
We will depend in part on various Internet search engines, such as Google, Bing and Yahoo!, to direct a significant amount of traffic to our website. Our ability to maintain the number of visitors directed to our website is not entirely within our control. Our competitors’ search engine optimization, or “SEO,” efforts may result in their websites receiving a higher search result page ranking than ours, or Internet search engines could revise their methodologies in an attempt to improve their search results, which could adversely affect the placement of our search result page ranking. If search engine companies modify their search algorithms in ways that are detrimental to our new user growth or in ways that make it harder for our users to use our website, or if our competitors’ SEO efforts are more successful than ours, overall growth in our user base could slow, user engagement could decrease, and we could lose existing users. These modifications may be prompted by search engine companies entering the online professional networking market or aligning with competitors. Any reduction in the number of users directed to our website would harm our business and operating results.
Risks Related to Government Regulation
Changes in laws, regulations and other requirements could adversely affect our business, results of operations or financial condition.
In addition to the anticipated regulation of our business by the FDA, our business, results of operations or financial condition could be adversely affected by new or future legal requirements imposed by legislative or regulatory initiatives, including, but not limited to, those relating to health care, public health and welfare and environmental matters. For example, in recent years, states and many local and municipal governments and agencies, as well as private businesses, have adopted legislation, regulations or policies which prohibit, restrict, or discourage smoking; smoking in public buildings and facilities, stores, restaurants and bars; and smoking on airline flights and in the workplace. Furthermore, some states prohibit and others are considering prohibiting the sales of electronic cigarettes to minors. Other similar laws and regulations are currently under consideration and may be enacted by state and local governments in the future. At present, it is not clear if electronic cigarettes, which omit no smoke or noxious odors, are subject to such restrictions. If electronic cigarettes are subject to restrictions on smoking in public and other places, our business, operating results and financial condition could be materially and adversely affected. New legislation or regulations may result in increased costs directly for our compliance or indirectly to the extent such requirements increase the prices of goods and services because of increased costs or reduced availability. We cannot predict whether such legislative or regulatory initiatives will result in significant changes to existing laws and regulations and/or whether any changes in such laws or regulations will have a material adverse effect on our business, results of operations or financial condition.
37
Restrictions on the public use of electronic cigarettes may reduce the attractiveness and demand for our electronic cigarettes.
Certain states, such as North Dakota, New Jersey, Utah, Arkansas, Colorado, Delaware, Hawaii, Kansas, Maryland, New Hampshire, Oklahoma, Oregon, South Dakota and Vermont, and certain cities, such as New York City, Boston, Chicago and San Francisco, have adopted limitations on the use of electronic cigarettes in certain public areas, while others are considering banning the use of electronic cigarettes. If the use of electronic cigarettes is banned anywhere the use of traditional tobacco burning cigarettes is banned, electronic cigarettes may lose their appeal as an alternative to traditional tobacco burning cigarettes, which may reduce the demand for our products and, thus, have a material adverse effect on our business, results of operations and financial condition.
Limitation by states on sales of electronic cigarettes may have a material adverse effect on our ability to sell our products.
If one or more states from which we generate or anticipate generating significant sales bring actions to prevent us from selling our products unless we obtain certain licenses, approvals or permits and if we are not able to obtain the necessary licenses, approvals or permits for financial reasons or otherwise and/or any such license, approval or permit is determined to be overly burdensome to us then we may be required to cease sales and distribution of our products to those states, which would have a material adverse effect on our business, results of operations and financial condition.
We may face the same governmental actions aimed at conventional cigarettes and other tobacco products.
Tobacco industry expects significant regulatory developments to take place over the next few years, driven principally by the World Health Organization’s Framework Convention on Tobacco Control (“FCTC”). The FCTC is the first international public health treaty on tobacco, and its objective is to establish a global agenda for tobacco regulation with the purpose of reducing initiation of tobacco use and encouraging cessation. Regulatory initiatives that have been proposed, introduced or enacted include:
|
|
●
|
The levying of substantial and increasing tax and duty charges;
|
|
●
|
Restrictions or bans on advertising, marketing and sponsorship;
|
|
|
●
|
The display of larger health warnings, graphic health warnings and other labeling requirements;
|
|
|
●
|
Restrictions or bans on the display of tobacco product packaging at the point of sale, and restrictions
or bans on cigarette vending machines;
|
38
|
●
|
Requirements regarding testing, disclosure and performance standards for tar, nicotine, carbon monoxide and other smoke constituents levels;
|
|
|
●
|
Requirements regarding testing, disclosure and use of tobacco product ingredients;
|
|
|
●
|
Increased restrictions on smoking in public and work places and, in some instances, in private places and outdoors;
|
|
|
●
|
Elimination of duty free allowances for travelers; and
|
|
|
●
|
Encouraging litigation against tobacco companies.
|
If electronic cigarettes are subject to one or more significant regulatory initiates enacted under the FCTC, our business, results of operations and financial condition could be materially and adversely affected.
Limitation by states and cities on sales of e-cigarettes may have a material adverse effect on our ability to sell our products in the United States.
Certain states and cities have enacted laws which preclude the use of e-cigarettes where traditional tobacco-burning cigarettes cannot be used and others have proposed legislation that would categorize e-cigarettes as tobacco products, which if enacted, would be regulated in a manner equivalent to their tobacco burning counterparts. For example, San Francisco, California has passed legislation that includes e-cigarettes under its anti-smoking laws, including only allowing the use of e-cigarettes in areas where traditional cigarettes may be smoked and requiring a tobacco permit to sell e-cigarettes. Chicago, Illinois has passed legislation prohibiting the use of e-cigarettes in most public indoor places and requires that e-cigarettes may only be sold from “behind the counter.” New York City has amended its Smoke Free Air Act to ban the use of e-cigarettes anywhere that traditional cigarettes may not be used, such as bars, parks, restaurants and beaches. Similarly, Boston, Massachusetts has banned the use of e-cigarettes in the workplace and restricted the use of e-cigarettes to adults. Additionally, New Jersey, North Dakota and Utah have included bans on the use of e-cigarettes in designated smoke-free areas such as restaurants and bars, and New York has proposed law that will prohibit the sale or provision of any quantity of e-liquid used to fill e-cigarettes or cartridges. Several states and cities are currently considering similar initiatives and if such states and cities pass or further legislate to ban the use of e-cigarettes anywhere the use of traditional tobacco burning cigarettes are banned, e-cigarettes may lose their appeal as an alternative to traditional cigarettes; which may have the effect of reducing the demand for our products and as a result have a material adverse effect on our business, results of operations and financial condition.
Restrictions on the public use of e-cigarettes may reduce the attractiveness and demand for our e-cigarettes.
Certain states, cities, businesses, providers of transportation and public venues in the U.S. have already banned the use of e-cigarettes, while others are considering banning the use of e-cigarettes. If the use of e-cigarettes is banned anywhere the use of traditional tobacco burning cigarettes is banned, e-cigarettes may lose their appeal as an alternative to traditional tobacco burning cigarettes, which may reduce the demand for our products and, thus, have a material adverse effect on our business, results of operations and financial condition
39
We may not be able to establish sustainable relationships with large retailers or national chains.
We believe the best way to develop brand and product recognition and increase sales volume is to establish relationships with large retailers and national chains. We currently do not have established relationships with any retailers or national chains Any agreement that we reach, we may have to pay “slotting fees”, to carry and offer our products for sale based on the number of stores our products will be carried in.
We face a risk of product liability claims and may not be able to obtain adequate insurance, and any such claims could materially and adversely affect our reputation and brand image.
Our business exposes us to potential liability risks that may arise from the clinical testing, manufacture, and sale of our products. Substantial damage awards have been issued in certain jurisdictions against pharmaceutical and tobacco companies based on claims for injuries allegedly caused by the use of pharmaceutical and tobacco products, and similar claims may be brought against manufacturers and distributors of e-cigarette products. Liability claims may be expensive to defend and result in large judgments against us. We currently carry liability insurance, however there is no assurance that it will continue to be available to us at an affordable price if at all. Our insurance may not reimburse us, or the coverage may not be sufficient to cover claims made against us. We cannot predict any or all of the possible harms or side effects that may result from the use of our current products or any future products and, therefore, the amount of insurance coverage we currently hold may not be adequate to cover all liabilities we might incur. If we are sued for any injury allegedly caused by our products, our liability could exceed our ability to pay the liability. Whether or not we are ultimately successful in any adverse litigation, such litigation could consume substantial amounts of our financial and managerial resources, all of which could have a material adverse effect on our business, financial condition, results of operations, prospects and stock price. In addition, even if a product liability claim is found to be without merit or is otherwise unsuccessful, the negative publicity surrounding such assertions regarding our products or processes could materially and adversely affect our reputation and brand image. Any loss of consumer confidence in the safety and quality of our products would be difficult and costly to overcome.
If we have improperly marketed and distributed certain of our products in violation of governmental regulations we may be subject to fines, sanctions, administrative actions, penalties and other liability, either civil and or criminal.
We may be subject to disciplinary, administrative, regulatory and or legal actions if the FDA, or any regulatory agencies in either the United Sates or any foreign jurisdiction in which our products are sold determines that our products or the means by which we marketed and sold our products was effected without the proper regulatory approvals. As a distributor and marketer of a product that a government or regulatory agency may assert is a smoking cessation device and or a tobacco product, the Company faces potential fines, sanctions, administrative actions, penalties, and other liability for: improper sales, labeling, making improper claims, referencing or publishing to its websites, marketing materials, advertisements, testimonials or representations that certain of our products have the ability or potential to treat, cure or otherwise improve a medical condition, and or provide a healthier alternative to other more traditional tobacco products.
40
Moreover, in light of the FDA’s recently issued proposed rule seeking to regulate various deemed tobacco products such as e-cigarettes, we may be required to follow federal and state tobacco labeling laws, and could face potential fines, sanctions, administrative actions, penalties and other liability, either civil and or criminal, for any violations thereof.
Any violation of law with respect to the Company’s marketing materials and or labeling could expose our company to liability including but not limited to fines, sanctions, administrative actions, penalties, civil actions and or criminal prosecution. And although our company maintains general liability insurance, our company’s insurance may not cover potential claims of this type or may not be adequate to indemnify our company for all liability that may be imposed. Any imposition of liability that is not covered by insurance or is in excess of insurance coverage could have a material adverse effect on our company’s business, results of operations and financial condition.
Risks Related To Our Securities
As an “emerging growth company” under the JOBS Act, we are permitted to rely on exemptions from certain disclosure requirements.
We qualify as an “emerging growth company” under the JOBS Act. As a result, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. For so long as we are an emerging growth company, we will not be required to:
Report pursuant to Section 404(b) of the Sarbanes-Oxley Act;
|
|
·
|
Comply with all requirements that may be adopted by the Public Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and financial statements;
|
|
|
·
|
Submit certain execute compensation matters to shareholder advisory votes, such as “say-on-pay” and “say on frequency;”
|
|
|
·
|
Disclose certain executive compensation related items such as the correlation between executive compensation and performance.
|
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
We will remain an “emerging growth company” for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our total annual gross revenues exceed $1 billion, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, which would occur if the market value of our ordinary shares that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.
41
Our financial statements may not be comparable to those of companies that comply with new or revised accounting standards.
We have elected to take advantage of the benefits of the extended transition period that Section 107 of the JOBS Act provides an emerging growth company, as provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
Our status as an “emerging growth company” under the JOBS Act OF 2012 may make it more difficult to raise capital when we need to do it.
Because of the exemptions from various reporting requirements provided to us as an “emerging growth company” and because we will have an extended transition period for complying with new or revised financial accounting standards, we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
We will incur increased costs and demands upon management as a result of complying with the laws and regulations that affect public companies, which could materially adversely affect our results of operations, financial condition, business and prospects.
As a public company and particularly after we cease to be an “emerging growth company,” we will incur significant legal, accounting and other expenses that we did not incur as a private company, including costs associated with public company reporting and corporate governance requirements. These requirements include compliance with Section 404 and other provisions of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as rules implemented by the SEC and NASDAQ. In addition, our management team will also have to adapt to the requirements of being a public company. We expect that compliance with these rules and regulations will substantially increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
The increased costs associated with operating as a public company will decrease our net income or increase our net loss, and may require us to reduce costs in other areas of our business or increase the prices of our products or services. Additionally, if these requirements divert our management’s attention from other business concerns, they could have a material adverse effect on our results of operations, financial condition, business and prospects.
However, for as long as we remain an “emerging growth company” as defined in the JOBS Act, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.”
42
We will not be required to comply with certain provisions of the Sarbanes-Oxley Act for as long as we remain an “emerging growth company.”
Our independent registered public accounting firm is not required to formally attest to the effectiveness of our internal control over financial reporting until the later of the year following our first annual report required to be filed with the SEC, or the date we are no longer an “emerging growth company.” At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating.
Reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
As an “emerging growth company,” we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including not being required to comply with the auditor attestation requirements of section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In the event that your investment in our shares is for the purpose of deriving dividend income or in expectation of an increase in market price of our shares from the declaration and payment of dividends, your investment will be compromised because we do not intend to pay dividends.
We have never paid a dividend to our shareholders. We intend to retain cash for the continued development of our business. As a result, your return on investment will be solely determined by your ability to sell your shares in a secondary market.
The market valuation of our business may fluctuate due to factors beyond our control and the value of your investment may fluctuate correspondingly.
The market valuation of developmental stage companies, such as us, frequently fluctuate due to factors unrelated to the past or present operating performance of such companies. Our market valuation may fluctuate significantly in response to a number of factors, many of which are beyond our control, including:
|
changes in securities analysts’ estimates of our financial performance, although there are
currently no analysts covering our stock;
|
|
|
·
|
fluctuations in stock market prices and volumes, particularly among securities of emerging
growth companies;
|
|
|
·
|
changes in market valuations of similar companies;
|
43
|
|
·
|
announcements by us or our competitors of significant contracts, new technologies,
acquisitions, commercial relationships, joint ventures or capital commitments;
|
|
|
·
|
variations in our quarterly operating results;
|
|
|
·
|
fluctuations in related commodities prices; and
|
|
|
·
|
additions or departures of key personnel.
|
Currently there is no established public market for our common stock, and there can be no assurances that any established public market will ever develop or that our common stock will be quoted for trading.
There is currently no established public market whatsoever for our securities. A market maker has submitted an application with FINRA on our behalf so as to be able to quote the shares of our common stock on the OTCBB maintained by FINRA. The Company has been issued a symbol “DNAP”. There can be no assurances as to whether;
|
|
·
|
any market will develop;
|
|
|
·
|
the prices at which our common stock will trade; or
|
|
|
·
|
the extent to which investor interest in us will lead to the development of an active, liquid trading markets generally result in lower price volatility and more efficient execution of buy and sell orders for investors.
|
In addition, our common stock is unlikely to be followed by any market analysts, and there may be few institutions acting as market makers for our common stock. Either of these factors could adversely affect the liquidity and trading price of our common stock. Until our common stock is fully distributed and an orderly market develops in our common stock, if ever, the price at which it trades is likely to fluctuate significantly. Prices for our common stock will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity of the market for shares of our common stock, developments affecting our business, including the impact of the factors referred to elsewhere in these Risk Factors, investor perception of exploratory stage mining companies and general economic and market conditions.
Because the SEC imposes additional sales practice requirements on brokers who deal in penny stocks, some brokers may be unwilling to trade them. This means that you may have difficulty reselling your shares.
Our common stock is classified as a penny stock and will be covered by Section 15(g) of the Securities Exchange Act of 1934. These rules impose additional sales practice requirements on brokers/dealers who sell our securities in this offering or in the aftermarket. For sales of our securities, the broker/dealer must make a special suitability determination and receive from you a written agreement prior to making a sale for you. Because of the imposition of the foregoing additional sales practices, it is possible that brokers will not want to make a market in our shares. This could prevent you from reselling your shares and may cause the price of the shares to decline.
44
FINRA sales practice requirements may limit a stockholder’s ability to buy and sell our stock.
FINRA has adopted rules that require broker-dealer to have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may have the effect of reducing the level of trading activity and liquidity of our common stock. Further, many brokers charge higher transactional fees for penny stock transactions. As a result, fewer broker-dealers may be willing to make a market in our common stock, which may limit your ability to buy and sell our stock.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report contains “forward-looking statements”. Such forward-looking statements concern our anticipated results and developments in our operations in future periods, planned exploration and development of its properties, plans related to its business and other matters that may occur in the future. These statements relate to analyses and other information that are based on forecasts of future results, estimates of amounts not yet determinable and assumptions of our management.
Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions or future events or performance (often, but not always, using words or phrases such as “expects” or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “estimates” or “intends”, or stating that certain actions, events or results “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved) are not statements of historical fact and may be forward-looking statements. Forward-looking statements are subject to a variety of known and unknown risks, uncertainties and other factors which could cause actual events or results to differ from those expressed or implied by the forward-looking statements
Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those anticipated, believed, estimated or expected. We caution readers not to place undue reliance on any such forward-looking statements, which speak only as of the date made. We disclaim any obligation subsequently to revise any forward-looking statements to reflect events or circumstances after the date of such statements or to reflect the occurrence of anticipated or unanticipated events, except as required by law.
None.
45
During 2014, our corporate headquarters are located at 9125 rue Pascal Gagnon, Suite 204 Saint Leonard, Quebec Canada HIP IZ4 where we rent approximately 1100 square feet of office space at a cost of $1,447cdn per month.
In March 2015 we signed a 38 month lease for approximately 1,289 square feet of space at 322 Nancy Lynn Lane, Suite 7, Knoxville, TN 37919. The monthly rent is approximately $1,200. We believe that the space is adequate for our current needs.
None.
Under the Dodd-Frank Wall Street Reform and Consumer Protection Act (“Dodd-Frank Act”) each operator of a mine is required to include certain mine safety results in its periodic reports filed with the Securities and Exchange Commission. Since our mining operations are located in Quebec, Canada, we are not subject to the Federal Mine Safety and Health Administration under the Federal Mine Safety and Health Act of 1977.
Nonetheless, it should be noted that during 2014, we were subject to certain mining rules and regulations as promulgated by the Quebec government and other municipalities and we have never been cited for any mining violations.
As to our current operations, this disclosure is not applicable.
PART II
A. Market Information
Our common stock does not trade on any Exchange or electronic quotation system and there can be no assurance that our common stock will trade on any Exchange or electronic quotation system. Moreover, even if a market in our common stock develops there can be no assurance that an active trading market will be sustained.
Our common stock is quoted on the OTC Bulletin Board under the symbol “BVAP.” Trading in our common stock in the over-the-counter market has been very limited and the quotations set forth below are not necessarily indicative of actual market conditions. The high and low sales prices for our common stock for each quarter of the fiscal years ended December 31, 2014 and 2013, according to OTC Markets Group Inc., were as follows:
46
|
Quarter Ended
March 31, 2015
|
High
0.10
|
Low
0.04
|
||||||
|
December 31, 2014
|
$
|
0.08
|
$
|
0.02
|
||||
|
September 30, 2014
|
$
|
0.21
|
$
|
0.10
|
||||
|
June 30, 2014
|
$
|
0.31
|
$
|
0.28
|
||||
|
March 31, 2014
|
$
|
0.37
|
$
|
0.32
|
||||
|
December 31, 2013
|
$
|
0.59
|
$
|
0.38
|
||||
|
September 30, 2013
|
$
|
0.59
|
$
|
0.38
|
||||
|
June 30, 2013*
|
$
|
N/A
|
$
|
|||||
*The Company’s Common Stock did not begin trading until August 2013
B. Holders.
As of December 31, 2014 there were 106 shareholders of record of our Common Stock.
Our transfer agent is Olde Monmouth Stock Transfer Co., Inc. Their telephone number is (732) 872-2727 and there mailing address is 200 Memorial Parkway, Atlantic Highlands, NJ 07716.
C. Dividends.
Holders of our common stock are entitled to receive such dividends as our board of directors may declare from time to time from any surplus that we may have. We have not paid dividends on our common stock since the date of our incorporation and we do not anticipate paying any common stock dividends in the foreseeable future. We anticipate that any earnings will be retained for development and expansion of our businesses and we do not anticipate paying any cash dividends in the foreseeable future. Future dividend policy will depend upon our earnings, financial condition, contractual restrictions and other factors considered relevant by our Board of Directors and will be subject to limitations imposed under Nevada law.
D. Equity Compensation Plan.
None.
47
E. Sale of Unregistered Securities
We have issued shares of our common stock for services rendered, capital formation and corporate acquisitions. We relied on the exemptive provisions of Section 4(2) of the Securities Act. We have also offered shares pursuant to the exemptive provisions of Regulation S.
2010
In May 2010, we issued to 28 purchasers residing in Canada 18,000,000 shares of our common stock at a purchase price of $.003 per share for a total of $54,000. Those shares were issued in pursuant to Regulation S of the Securities Act of 1933, as amended. Accordingly, none of those purchasers are U.S. persons as that term is defined in Regulation S. No underwriters were used, and no commissions or other remuneration was paid except to us. The securities were sold in an offshore transaction relying on Rule 903 of Regulation S of the Securities Act. No directed selling efforts were made in the United States by us, any distributor, any of their respective affiliates or any person acting on behalf of any of the foregoing. We are subject to Category 3 of Rule 903 of Regulation S and accordingly we implemented the offering restrictions required by Category 3 of Rule 903 of Regulations S by including a legend on all offering materials and documents which stated that the shares have not been registered under the Securities Act of 1933 and may not be offered or sold in the United States or to US persons unless the shares are registered under the Securities Act, or an exemption from the registration requirements of the Securities Act is available.
In June 2010, we offered and sold to 5 purchasers residing in the United States 2,000,000 shares of our common stock at a purchase price of $.003 per share for a total of $6,000. The transactions pursuant to which those shares were issued to those purchasers did not involve a public offering of our securities and, therefore, were exempt from the registration and prospectus delivery requirements of the Securities Act pursuant to the provisions of Section 4(2) of that Act. In connection with the offer and sale of those 2,000,000 shares, no general solicitation or advertising was used. Those 5 purchasers had pre-existing relationships with us on the dates we sold those 2,000,000 shares to them. No commission was paid in connection with the offer and sale of those 2,000,000 shares.
2011
In 2011, the Company issued 5,000,000 shares of common stock to acquire mining rights at a value of $15,000; 5,000,000 shares of common stock to board members for services at a value of $15,000; 1,000,000 shares of common stock for payment of interest on the promissory note of $3,000; 3,350,000 shares of common stock pursuant to a private placement of our securities totaling $670,000, 1,500,000 issued pursuant to employment agreements valued at $1,500,000 and 250,000 shares valued at $50,000 for engineering services. We relied on the exemption provisions of Securities Act Section 4(2) in issuing these securities.
2012
None.
48
2013
On or about September 18, 2013, we issued 200,000 restricted common stock shares to Small Cap Voice in return for investor relations services. The shares were valued at $0.38.
On or about September 18, 2013, we sold an aggregate of 400,000 restricted common stock shares to 3 persons for $0.25 per share for an aggregate purchase price of $100,000.
On or about October 16, 2013, we sold an aggregate of 1,808,000 restricted common stock shares to 7 persons and 2 entities for $0.25 per share for an aggregate purchase price of $457,000.
On or about October 30, 2013, we issued 40,000 restricted common stock shares to MRB & Associates in return for geological data. We valued the shares at $0.50.
On or about November 12, 2013, we issued 350,000 restricted common stock shares for the acquisition of the Excel Property Mortgage. We valued the shares at $0.40 per share.
On or about November 25, 2013, we issued 700,000 restricted common stock shares to Excel for purchaser of the Excel Property. We valued the shares at $0.40.
On or about November 26, 2013, we issued 500,000 restricted common stock shares to First Level Capital LLC in return for investor relations services. We valued the shares at $0.45 per share.
On or about December 29, 2013, Marquest Mining Quebec 2013-II Super Flow – Through LP, purchased 1,000,000 restricted common stock shares through its purchase of 1,000,000 restricted common stock shares through its purchase of 1,000,000 units valued at CDN$500,000. We valued the shares at $0.50 per share.
2014
On or about January 14, 2014, we issued a total of 1,000,000 restricted common stock shares valued at US$340,000 to Fayz Yacoub and Ramy Yacoub for the purchase of the St. Anne Claims. We valued the shares at $0.34 per share.
On or about April 3, 2014 we issued 50,000 restricted common stock shares valued at $0.25 per share to G.E(Ted) Creber for the purchase of warrants.
On or about June 03, 2014, we issued a total of 400,000 restricted common stock shares to Momentum Public Relations Inc. and Agoracom Investor Relations Co. in return for investor relations’ services. We valued the shares at $0.31 per share.
On or about November 04, 2014, we issued 250,000 restricted common stock shares valued at $ 0.08 per share to Tony Giuliano in relation to his termination package.
On or about November 10th we sold an aggregate of 4,810,000 restricted common stock shares to 4 persons and 4 corporate entities for $0.05 per share for an aggregate purchase price of $240,500. In addition we issued 500 000 common stock shares to Michel Lebeuf for consulting services in connection with an agreement in which he agreed to serve as V.P Legal and Corporate Development. In 2015 these shares were cancelled.
49
On or about December 18th we sold an aggregate of 4,800,000 restricted common stock shares to 5 persons and 4 corporate entities for $0.05 per share for an aggregate purchase price of $240,000.
On or about December 26th we sold an aggregate of 4,700,000 restricted common stock shares to 6 persons and 2 corporate entities for $0.05 per share for an aggregate purchase price of $235,000
With respect to the sale of the securities identified above, we relied on the exemptions of Section 4(2), Regulation S or Section 3(a) 10 of the Securities Act.
|
o
|
At all times relevant the securities were offered subject to the following terms and conditions:
|
|
o
|
The sale was made to a sophisticated or accredited investor, as defined in Rule 502 or were issued pursuant to a specific exemption;
|
|
o
|
we gave the purchaser the opportunity to ask questions and receive answers concerning the terms and conditions of the offering and to obtain any additional information which we possessed or could acquire without unreasonable effort or expense that is necessary to verify the accuracy of information furnished;
|
|
o
|
at a reasonable time prior to the sale of securities, we advised the purchaser of the limitations on resale in the manner contained in Rule 502(d)2; and
|
|
o
|
neither we nor any person acting on our behalf sold the securities by any form of general solicitation or general advertising.
|
We used these proceeds of these securities sales for working capital purposes including but not limited to infrastructure build-out, purchase of machines and equipment, mill construction and professional fees.
Sale of Registered Stock:
During the fiscal year ended December 31, 2014, we issued 15,760,000 shares of our registered common stock for a total of $1,177,000. We used the proceeds for general working capital expenses, payments of outstanding notes, exploration expenses including infrastructure build-out.
The following consolidated financial data has been derived from and should be read in conjunction with our audited interim financial statements for the years ended December 31, 2014 and 2013.
50
|
Year Ended
December 31, 2014
|
Year Ended
December 31, 2013
|
|||||||
|
Revenue
|
$
|
-0-
|
$
|
-0-
|
||||
|
Operating Expenses
|
$
|
2,772,549
|
$
|
2,992,167
|
||||
|
Cash
|
$
|
53,813
|
$
|
535,934
|
||||
|
Total Assets
|
$
|
1,457,592
|
$
|
2,690,691
|
||||
|
Current Liabilities
|
$
|
436,756
|
$
|
186,729
|
||||
|
Total Liabilities
|
$
|
649,819
|
$
|
186,729
|
||||
|
Working Capital
|
$
|
(298,632)
|
$
|
1,187,577
|
||||
|
Accumulated Deficit
|
$
|
7,770,337
|
$
|
4,710,522
|
||||
The following discussion should be read in conjunction with, and is qualified in its entirety by, our financial statement, and certain other financial information included elsewhere in this prospectus.
It should be noted that during the year ended December 31, 2014 we were an exploratory stage-mining corporation. We did not commence mining operations nor did we generate revenues.
We are no longer an exploratory stage mining company. With the acquisition of Breathe LLC in January 2015, our sole business objective is the eCigarette industry. We divested our mining operations pursuant to a stock dividend to our shareholders of our wholly owned subsidiary, DNA Precious Metals Canada, which conducted all of our mining operations.
As such, you should not view our operating results for 2014 as indicative of, or as a basis for, our operations in 2015 as our operations in 2015 will be solely related to our acquisition of Breathe LLC and the eCigarette industry.
We are an Emerging Growth Company:
We qualify as an “emerging growth company” under the JOBS Act. As a result, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. For so long as we are an emerging growth company, we will not be required to:
|
●
|
have an auditor report on our internal controls over financial reporting pursuant to
Section 404(b) of the Sarbanes-Oxley Act;
|
|
●
|
comply with any requirement that may be adopted by the Public Company Accounting
Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s
report providing additional information about the audit and the financial statements
(i.e., an auditor discussion and analysis);
|
|
●
|
submit certain executive compensation matters to shareholder advisory votes, such as
“say-on-pay” and “say-on-frequency;” and
|
51
|
●
|
disclose certain executive compensation related items such as the correlation between
executive compensation and performance and comparisons of the CEO’s compensation
to median employee compensation.
|
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
Management’s Discussion and Analysis
There is no historical mining information about us upon which you may rely to base an evaluation of our performance.
Results of Operations for Fiscal Years Ended December 31, 2014 and 2013
Since our inception, we have not generated any revenues. Operating expenses for the years ended December 31, 2014 and 2013 totaled $2,772,549 and $2,922,167 respectively. Our Net Loss for 2014 and 2013 was $3,059,815 and $2,937,475 respectively. Our Accumulated Deficit since Inception totaled $7,770,337.
Our single largest expenses during 2014 were the impairment of our assets and shares issued for services rendered and common stock options issued as compensation, was for salaries and related expenses totaling $427,568 as compared to $1,674,752 in 2013. Exploration costs in 2014 totaled $349,597 and $88,247 in 2013. Professional fees, primarily for legal and accounting services totaled $303,229 in 2014 as compared to $388,810 in 2013. General and administrative expenses totaled $389,521 in 2014 as compared to $721,454 in 2013. Rental costs in 2014 were $40,005 as compared to $40,385 in 2013.
Liquidity and Capital Resources
Assets and Liabilities.
To date, our operations have been primarily funded through debt and equity financing. We have secured approximately $4,894,874 in equity financing and $619,173 in debt financing. We will continue to rely on debt and equity financing for our ongoing operations.
At December 31, 2014 we had cash of $53,813, prepaid expenses of $189,528 and sales tax receivable of $27,943. Total current assets were $271,284. Our fixed assets totaled $138,124, deferred financing fees were $12,366 and our mining rights totaled $1,035,818. Total assets were $1,457,592. At December 31, 2013 we had cash totaling $535,934, prepaid expenses and deposits of $802,945, and sales tax receivable of $35,427. Fixed assets totaled $1,276,304, deferred financing fees totaled $25,081 and our mining rights totaled $15,000. Total assets were $2,690,691. The primary reason for the significant decrease in our assets is attributable to the write off of the land, building and mill equipment to satisfy accounting requirements since the assets were not being used and have been impaired we utilized the funds secured from the sale of our securities to invest in our mining project and the acquisition of mining assets.
52
Current liabilities at December 31, 2014 totaled $436,756 as compared to $186,729 at December 31, 2013. We had long term liabilities at December 31, 2014 totaling $213,063. We had no long term liabilities at December 31, 2013.
We had a working capital deficit at December 31, 2014 of $165,472 as compared to a working capital surplus on December 31, 2013 of $1,187,577. Most of our funds have been invested to acquire mining properties. Unless we secure additional financing, or generate sufficient cash flow, we will not have sufficient liquidity to continue operations for the next twelve months.
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements. We do not anticipate entering into any off-balance sheet arrangements during the next 12 months.
Foreign Currency Exchange Rate Risk
The Company holds cash balances in both U.S. and Canadian dollars. We transact most of our business in US and Canadian dollars. Some of our expenses, including labor and operating supplies are denominated in Canadian dollars. As a result, currency exchange fluctuations may impact our operating costs. We do not manage our foreign currency exchange rate risk through the use of financial or derivative instruments, forward contracts or hedging activities.
In general, the strengthening of the U.S. dollar will positively impact our expenses transacted in Canadian dollars. Conversely, any weakening of the U.S dollar will increase our expenses transacted in Canadian Dollars. We do not believe that any weakening of the U.S. dollar as compared to the Canadian dollar will have an adverse material effect on our operations.
Interest Rate Risk
The Company’s investment policy for its cash and cash equivalents is focused on the preservation of capital and supporting the liquidity requirements of the Company. The Company’s interest earned on its cash balances is impacted on the fluctuations of U.S. and Canadian interest rates. We do not use interest rate derivative instruments to manage exposure to interest rate changes. We do not believe that interest rate fluctuations will have any effect on our operations
Our financial statements have been examined to the extent indicated in their reports by KBL, LLP. and have been prepared in accordance with generally accepted accounting principles and pursuant to Regulation S-K as promulgated by the Securities and Exchange Commission and are included herein, on Page F-1 hereof.
53
None.
Evaluation of Disclosure Controls and Procedures
Our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) are designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission. Our disclosure controls and procedures are also designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officer, to allow timely decisions regarding required disclosure. Our chief executive officer and chief financial officer, with assistance from other members of our management, have reviewed the effectiveness of our disclosure controls and procedures as of December 31, 2014 based on their evaluation, have concluded that the disclosure controls and procedures were effective.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) during the year ended December 31, 2014 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as that term is defined in Rule 13a-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our principal executive and principal financial officers, we assessed, as of December 31, 2014, the effectiveness of our internal control over financial reporting. This assessment was based on criteria established in the framework in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our assessment using those criteria, management concluded that our internal control over financial reporting as of December 31, 2014 were effective.
Internal control over financial reporting is defined as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
54
|
|
·
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
|
|
·
|
provide reasonable assurance that transactions are recorded as necessary to permit the preparation of financial statements in accordance with U.S. generally accepted accounting principles and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and
|
|
|
·
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
|
|
|
·
|
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the internal control system are met. Because of the inherent limitations of any internal control system, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected.
|
This Annual Report on Form 10-K does not include an attestation report of the Company's independent registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by the Company's independent registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit the Company to provide only management's report in this Annual Report on Form 10-K.
Evaluation of Changes in Internal Controls over Financial Reporting
There was no change in the internal control over financial reporting that occurred during the fiscal year ended December 31, 2014 that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
None.
55
PART III
The following information sets forth the names of our Officers and Directors during 2014 and our current. Officers and Directors, their present positions, and some brief information about their background.
| Name | Age | Position(s) |
|
Tony Giuliano
630-44th Avenue
Lachine, Quebec H8T 2K8
|
56
|
Former: Chief Executive Officer,
President, Chief Financial Officer and Director
|
|
James Chandik
229 Bergerac
Repentigny, Quebec J6A 7V9
|
52
|
Former Executive-Vice President, Chief Operating Officer and Current Director
|
|
Yves Gagnon
401 Route 132 Est
L’Isle Verte, Quebec G0L 1K0
|
58
|
Former
Vice-President, Operations and Director
|
|
Joshua Kimmel
9921 Lanni Lane
Knoxville, TN, 37932
|
40
|
Chief Executive Officer,
President and Director
|
|
Chris Clark
P.O. Box 8204
Atlanta, GA,31106
|
32
|
Vice-President Legal Affairs and Director
|
Background of Officers and Directors
Former officers and directors serving during the year ended December 31, 2014:
Tony J. Giuliano
Tony J. Giuliano is our former President/ Chief Executive Officer and a director. Mr. Giuliano is an experienced financial executive having worked for several public companies listed in the United States and Canada. Recently, from October 2010-June 2012, Mr. Giuliano was the Chief Financial Officer for Sand Technology Inc., a U.S. public company involved in the development of specialized software. From October 2008-March 2010, Mr. Giuliano was Director of Finance for Unisource Canada Inc., the largest distributor of commercial printer paper in Canada, and from July 2006-April 2008, Mr. Giuliano was Chief Financial Officer of Avensys Corporation, a U.S. public company involved in the manufacture high-tech components for the telecommunications industry. Mr. Giuliano has been involved in all aspects of accounting, finance, taxation, mergers and acquisitions, international operations and ensuring SEC and Canadian regulatory compliance. Mr. Giuliano spent the first nine years of his career with Deloitte Canada, a major international accounting firm, in Montreal where he gained expertise with Canadian public companies operating in both the manufacturing and financial services sectors. Mr. Giuliano is a Certified Professional Accountant/Chartered Accountant and a member of the Quebec Order of Chartered Accountants since December 1982. He received a Diploma in Public Accountancy from McGill University in 1982 and a Bachelor of Commerce from Concordia University in 1979.
56
Yves Gagnon
Yves Gagnon is a former Vice-President of Operations and a Director. He is a geological engineer with a master degree in geochemistry from the Polytechnic School of the Montréal University (1983). He has been actively working for the last 35 years within the mining industry, recently acting as professional consultant developing junior companies in the process of becoming public through the startup of small mining/milling operations (Sep. 2009 to Feb. 2013). Prior to that, he was President/Chief Executive Officer of C2C Inc., a public mining company involved in the startup of a gold mine in Ecuador (Jul. 2007 to Aug. 2009). Mr. Gagnon has expertise in exploration, in mining management and in environmental mine closure planning. He acted as president/director/manager or consultant for other public and private companies: Yorbeau Resources (1984-89), Géospex Sciences Inc. (1989-96), Espalau Corp. (1996-99), Abcourt Mines (2000-07) and Métanor Resources (2001-07). He managed multi-disciplinary teams of up to 400 peoples and multi-million dollar projects, both nationally and internationally. He was instrumental in the discovery of the Bell Allard South copper-zinc deposit (for Noranda), in the return to life of the Bachelor Mining Complex (with Espalau and then with Métanor), and in the closure and reclaim of the Goldfields Mining Complex (for Barrick).
Background of Officers and Directors
Current officers and directors:
Joshua Kimmel
Mr. Kimmel was appointed our president, chief executive officer and a director in 2015. Has an extensive experience at the management level with companies primarily focused in the hospitality arena.
Since October of 2013, as the President and Founder of Breathe, Mr. Kimmel has developed an innovative entry into the E-cigarette market, possessing unique features not found in other market entries. He has patents pending that focus on consumer safety, including child lock features. Having worked as an advanced level sommelier and chef for almost two decades, Mr. Kimmel created proprietary handcrafted flavors manufactured in his hometown of Knoxville, Tennessee.
From September 2013 through present, Mr. Kimmel has also served on the Board of Directors of Electronic Magnetic Power Solutions, which implements disruptive patented technology licensed from Virginia Tech University for the express purpose of alternative energy use in the consumer space.
57
From November 1999 through March of 2009, Mr. Kimmel worked as the Vice President of Operations for The Addison Restaurant Group on the creation and opening of multiple restaurants. From October 2006 through December 2008, Mr. Kimmel took a hiatus from the Addison Restaurant Group to work with the founding team of Seasons 52, one of Darden Restaurants test concepts at the time.
James Chandik
James Chandik is our former Executive Vice-President/Chief Operating Officer having resigned these positions in January 2015. Since June 2011 through the present, he has served as a director of the Company and currently retains his position as a director. Mr. Chandik received an Economic Degree from McGill University in 1977. Since our inception until March 14, 2013, Mr. Chandik was our President/Chief Executive Officer. Mr. Chandik has served as a General Manager for Disaster Kleenup Canada from February 2009 to April 2010 and prior to that position, he was the General Manager of Datacom Wireless Corporation from September 2004 to October 2008. Mr. Chandik also served as a Manager from 1997 to 2001, with Navigata Communications, Metaphor Communications, Axxent Communication.
Chris Clark
Mr. Clark has since January 2015 served as the chief legal counsel and head of business affairs for companies in the television, telecommunications, media, new technology and global security consultancy sectors. Since August 2014, Chris has served the role as General Counsel of a global security consultancy firm, Red Alert Group, Inc., based in Atlanta Georgia. Mr. Clark provides legal and business advisory counsel to the Chief Executive Officer on matters concerning the firm’s operational, financial and media content sources that it has developed over the past ten years. Additionally, through his work as General Counsel to a private equity affiliate charged with media acquisitions, Mr. Clark has been responsible for structuring special financing to launch new media properties, establish joint ventures with existing television networks and affiliates, and creating a multi-video programming distribution platform.
From January 2012 to July 2014, Mr. Clark served as the Executive Vice President of Business and Legal Affairs of a startup media and technology company in Little Rock, Arkansas, Soul of the South Television where he coordinated approximately $20 million in public and private financing to launch its television network operations. Mr. Clark was also involved in the day to day operations of the business, from overseeing contracts, human resources, managing outside counsel and vendor relationships, and ensuring that the company reached both short and long term management objectives.
From September 2008 to December 2011, Mr. Clark served as an associate in the general litigation practice of Dewey & LeBoeuf, LLP. Mr. Clark’s work focused on sports, antitrust, and labor and employment law, with rewarding experience representing multinational technology companies, players unions, and international figures.
Mr. Clark is a graduate of Columbia Law School, Class of 2008, and the University of Miami, Class of 2005.
Family Relationships
There are no family relationships among our Directors and/or Officers.
58
Committees of the Board of Directors
We presently do not have an Audit Committee, compensation committee, nominating committee, corporate governance committee or any other committee of our Board of Directors. Our entire Board of Directors meets to undertake the responsibilities which would otherwise be delegated to a committee of our Board of Directors.
Compensation of Directors
Our Directors do not receive cash compensation for their services as Directors but are reimbursed for their reasonable expenses incurred in attending board or committee meetings.
Terms of Office
There are no family relationships among our Directors and/or Officers. Our Directors are appointed for one-year terms to hold office until the next annual general meeting of shareholders or until removed from office in accordance with our by-laws. Our Officers are appointed by our Board of Directors and hold office until removed by our Board of Directors or terminated pursuant to their employment agreements. However, pursuant to the Share Exchange Agreement between DNA Precious Metals, Inc. and Breathe LLC James Chandick was to tender his resignation upon the spin-off of DNA Canada, Inc. It is anticipated that Mr. Chandick will tender his resignation in February 2015 and the Board of Directors will appoint a new Director.
Involvement in Certain Legal Proceedings
During the past ten years:
1) No petition pursuant to the federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of any of our officers or directors, or any partnership in which any such officer or director was a general partner at or within 2 years before the time of such filing, or any corporation or business association of which any such officer or director was an executive officer at or within 2 years before the time of such filing;
2) None of our Officers or Directors has been convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
3) None of our Officers or Directors has been the subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining any such officer or director from, or otherwise limiting, the following activities:
(i) Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
59
(ii) Engaging in any type of business practice; or
(iii) Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of federal or state securities laws or federal commodities laws;
4) None of our Officers or Directors has been the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any federal or state authority barring, suspending or otherwise limiting for more than 60 days the right of any such officer or director to engage in any activity described in paragraph (f) (3) (i) of Item 401(f) of Regulation S-K, or to be associated with persons engaged in any such activity;
5) None of our Officers or Directors has been found by a court of competent jurisdiction in a civil action or by the Securities and Exchange Commission to have violated any federal or state securities law, and the judgment in such civil action or finding by the Securities and Exchange Commission has not been subsequently reversed, suspended, or vacated;
6) None of our Officers or Directors has been found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
7) None of our Officers or Directors has been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
(i) Any federal or state securities or commodities law or regulation; or
(ii) Any law or regulation respecting financial institutions or insurance companies, including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
(iii) Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
8) None of our Officers or Directors has been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3 (a) (26) of the Exchange Act (15 U.S.C. 78c (a) (26)), any registered entity (as defined in Section 1 (a) (29) of the Commodity Exchange Act (7 U.S.C. 1 (a) (29)), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
Code of Ethics
Code of Ethics for Senior Executive Officers and Senior Financial Officers
We have adopted a Code of Ethics for our Officers. The code provides as follows:
60
|
▪
|
Each Officer is responsible for full, fair, accurate, timely and understandable disclosure in all periodic reports and financial disclosures required to be filed by us with the Securities and Exchange Commission or disclosed to our stockholders and/or the public.
|
|
▪
|
Each Officer shall immediately bring to the attention of the Audit Committee, or disclosure compliance officer, any material information of which the officer becomes aware that affects the disclosures made by us in our public filings and assist the audit committee or disclosure compliance officer in fulfilling its responsibilities for full, fair, accurate, timely and understandable disclosure in all periodic reports required to be filed with the Securities and Exchange Commission.
|
|
▪
|
Each Officer shall promptly notify our general counsel, if any, or the president or chief executive officer as well as the audit committee of any information he may have concerning any violation of our Code of Business Conduct or our Code of Ethics, including any actual or apparent conflicts of interest between personal and professional relationships, involving any management or other employees who have a significant role in our financial reporting, disclosures or internal controls.
|
|
▪
|
Each Officer shall immediately bring to the attention of our general counsel, if any, the president or the chief executive officer and the audit committee any information he may have concerning evidence of a material violation of the securities or other laws, rules or regulations applicable to us and the operation of our business, by us or any of our agents.
|
|
▪
|
Any waiver of this Code of Ethics for any officer must be approved, if at all, in advance by a majority of the independent directors serving on our board of directors. Any such waivers granted will be publicly disclosed in accordance with applicable rules, regulations and listing standards.
|
Section 16(a) Beneficial Ownership Reporting Compliance
Compliance with Section 16(a) of the Securities Exchange Act of 1934
For companies registered pursuant to section 12(g) of the Exchange Act, Section 16(a) of the Exchange Act requires our executive officers and directors, and persons who beneficially own more than ten percent of our equity securities, to file reports of ownership and changes in ownership with the Securities and Exchange Commission. Officers, directors and greater than ten percent shareholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file. When the Company becomes subject to section 12(g) of the Securities Act, it is the intent of all officers, directors and 5% shareholders to comply with this requirement.
Overview of Compensation Program
Our compensation philosophy is based on our belief that our compensation programs should: be aligned with stockholders’ interests and business objectives; reward performance; and be externally competitive and internally equitable. We seek to achieve three objectives, which serve as guidelines in making compensation decisions:
61
|
Providing a total compensation package which is competitive and therefore enables us
to attract and retain, high-caliber executive personnel;
|
|
|
Integrating compensation programs with our short-term and long-term strategic plan
and business objectives; and
|
|
|
Encouraging achievement of business objectives and enhancement of stockholder
Value by providing executive management long-term incentive through equity ownership.
|
We may compensate our Officers with cash compensation, common stock and common stock options. We have not established any quantifiable criteria with respect to the level of compensation, stock grants or options. Rather, the Board of Directors will evaluate cash, stock grants and stock options paid by similar mining companies. We do not have a Compensation Committee of the Board of Directors.
With respect to stock grants and options which may be issued to the Company’s Officers and Directors, the Board will consider an overall compensation package that includes both cash and stock based compensation which would be in line with the Company’s overall operations and compensation levels paid to similar mining companies. On August 12, 2013, the Company approved and enacted the 2013 Stock Incentive Plan (the “Plan”). Under the 2013 Stock Incentive Plan, the Company may grant options or share awards to its full-time employees, executive officers, directors and consultants up to a maximum of 8,000,000 common shares. Under the Plan, the exercise price of each option has been established at $0.25. Stock options vest as stipulated in the stock option agreement and their maximum term is 8 years.
The following table sets forth the compensation paid by us to our Officers for the fiscal years ended December 31, 2014, 2013, 2012 and 2011. This information includes the dollar value of base salaries, bonus awards and number of stock options granted, and certain other compensation, if any. The compensation discussed addresses all compensation awarded to, earned by, or paid or named executive officers.
62
Executive Officer Compensation Table
|
Name and
|
|||||||||||||||||||
|
Position
|
Year
|
Salary
|
Stock Awards
|
Option Awards(7)
|
Total*
|
||||||||||||||
|
Tony Giuliano (1)
|
2014
|
$ | 42,462 | 0 | 0 | $ | 42,462 | ||||||||||||
|
Former
|
2013
|
$ | 66,667 | $ | 250,000 | $ | 77,937 | $ | 394,604 | ||||||||||
|
CEO/CFO/Director
|
|||||||||||||||||||
|
Ronald K. Mann (2)
|
2014
|
$ | 18,462 | 0 | 0 | $ | 18,462 | ||||||||||||
|
Former
|
2013
|
$ | 95,000 | $ | 125,000 | $ | 0 | $ | 220,000 | ||||||||||
|
CEO/Director
|
2012
|
0 | 0 | 0 | 0 | ||||||||||||||
|
James Chandik (3)
|
2014
|
$ | 88,615 | $ | 0 | 0 | $ | 88,615 | |||||||||||
|
Director
|
2013
|
$ | 120,000 | $ | 0 | $ | 18,000 | $ | 138,000 | ||||||||||
|
Former CEO/COO
|
|||||||||||||||||||
|
Yves Gagnon (4)
|
2014
|
$ | 60,923 | 0 | 0 | $ | 60,923 | ||||||||||||
|
Former
|
2013
|
$ | 120,000 | $ | 500,000 | $ | 18,000 | $ | 638,000 | ||||||||||
|
VP/Director
|
|||||||||||||||||||
|
Claud Girard (5)
|
2014
|
$ | 60,923 | 0 | 0 | $ | 60,923 | ||||||||||||
|
Former
|
2013
|
$ | 120,000 | $ | 500,000 | $ | 18,000 | $ | 638,000 | ||||||||||
|
VP/Director
|
|||||||||||||||||||
|
Jeffrey Bercovitch (6)
|
2014
|
$ | 0 | $ | 0 | 0 | $ | 0 | |||||||||||
|
Former
|
2013
|
$ | 48,000 | $ | 0 | 0 | $ | 48,000 | |||||||||||
|
CFO/Director
|
|||||||||||||||||||
* In accordance with the rules promulgated by the Securities and Exchange Commission, certain
columns relating to information that is not applicable have been omitted from this table.
(1) Chief Financial Officer from March 15, 2013 to October 20, 2014. Chief Executive Officer from February 21, 2014 to March 27 2014. The value of the stock awards includes 1,000,000 common shares of stock at a value of $0.25 per share of which 750,000 common shares were subsequently cancelled.
(2) Chief Executive Officer from March 15, 2013 to February 19, 2014. The value of the stock awards includes 2,000,000 common shares of stock at a value of $0.25 per share of which 1,500,000 common shares were subsequently cancelled. .
(3) Chief Executive Officer from June 1, 2011 until March 15, 2013 and from March 27 2014 to January 16, 2015. COO from March 15, 2013 to March 27, 2014.The value of the stock awards includes 2,000,000 shares of stock at a value of $0.003 per share.
(4) Vice-President from November 10, 2012 to January 16, 2015. The value of the stock awards includes 2,000,000 common shares of stock at a value of $0.25 per share. These shares were subsequently cancelled.
(5) Vice-President from June 1, 2011 to October 31, 2011. The value of the stock awards includes 300,000 common shares of stock at a value of $0.003 per share.
(6) Chief Financial Officer from June 1, 2011 to March 15, 2013. The value of the stock awards includes 1,000,000 common shares of stock at a value of $0.003 per share.
(7) The valuation of the stock options is based on the Black-Scholes pricing formula calculated at the August 12, 2013 grant date.
The compensation discussed herein addresses all compensation awarded to, earned by, or paid to our named executive officers through December 31, 2014.
63
Employment Agreements-
James Chandik - Mr. Chandik signed an employment agreement with the Company commencing June 1, 2011. The agreement was terminated when he stepped down as a corporate officer in January 2015. Effective June 1, 2011 he received an annual salary of $120,000. He was issued 2,000,000 shares of the Company’s common stock in consideration for agreeing to serve as our Chief Executive Officer at that time. His annual salary commencing June 1, 2012 and June 1, 2013 remained at $120,000 per year and no additional shares of common stock were issued.
There are currently no employment agreements with either of our new officers, Josh Kimmel or Chris Clark.
Compensation of Directors
Our Directors are not compensated for their services as Directors. The Board of Directors has not awarded any options to our Directors. There are no contractual arrangements with any member of the Board of Directors. We have no Director’s service contracts.
Long-Term Incentive Plan Awards
We do not have any long-term incentive plans that provide compensation intended to serve as incentive for performance.
Indemnification
Under our Articles of Incorporation and Bylaws of the Company, we may indemnify an Officer or Director who is made a party to any proceeding, including a lawsuit, because of his position, if he acted in good faith and in a manner he reasonably believed to be in our best interest. We may advance expenses incurred in defending a proceeding. To the extent that the officer or director is successful on the merits in a proceeding as to which he is to be indemnified, we must indemnify him against all expenses incurred, including attorney’s fees. With respect to a derivative action, indemnity may be made only for expenses actually and reasonably incurred in defending the proceeding, and if the officer or director is judged liable, only by a court order. The indemnification is intended to be to the fullest extent permitted by the laws of the State of Nevada.
Regarding indemnification for liabilities arising under the Securities Act of 1933, which may be permitted to directors or officers under Nevada law, we are informed that, in the opinion of the Securities and Exchange Commission, indemnification is against public policy, as expressed in the Act and is, therefore, unenforceable.
The following table sets forth certain information as of April 1, 2015 with respect to the beneficial ownership of the Company's Common Stock by: (i) all persons known by the Company to be beneficial owners of more than 5% of the Company's Common Stock, (ii) each director and Named Executive Officer, and (iii) by all executive officers and directors as a group.
64
|
Name
|
No. of Shares of
Common Stock
|
No. of Options
|
Percent of
Class(1)(2)
|
|||||||||
|
Josh Kimmel
9921 Lanni Lane
Knoxville, TN, 37932
|
127,500,000 |
none
|
47.2 | % | ||||||||
|
James Chandik
229 Bergerac
Repentigny, Quebec
J6A 7V9
|
2,000,000 | 100,000 | .8 | % | ||||||||
|
Chris Clark
P.O. Box 8204
Atlanta, GA,31106
|
3,000,000 |
none
|
1.1 | % | ||||||||
|
54 Western Ore Place Inc.
30 French Cay Close
Providenciales TCI
British West Indies
|
15,000,000 | 0 | 5.4 | % | ||||||||
|
55 Strathmore Capital Inc.
34 Hawksbill Lane
Providenciales, TCI
British West Indies
|
15,000,000 | 0 | 5.4 | % | ||||||||
|
All Officers and Directors (3)
|
132,500,000 | 100,000 | 49.1 | % | ||||||||
(1) Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares
(2) Based on 276,352,667 issued and outstanding shares of common stock on April 6, 2015.
65
Except as described below, none of the following persons has any direct or indirect material interest in any transaction to which we are a party during the past two years, or in any proposed transaction to which the Company is proposed to be a party:
|
|
o
|
any director or officer;
|
|
|
o
|
any proposed nominee for election as a director;
|
|
|
o
|
any person who beneficially owns, directly or indirectly, shares carrying more than 5% of the voting rights attached to our common stock; or
|
|
|
o
|
any relative or spouse of any of the foregoing persons, or any relative of such spouse, who has the same house as such person or who is a director or officer of any parent or subsidiary.
|
We issued shares of our common stock to our officers and directors for services rendered.
Related Party Transactions
None.
Director Independence.
We do not have an independent Board of Directors. Each of our Directors also serves as an Officer of the Company.
AUDIT FEES. The aggregate fees billed for professional services rendered was $18,000 and $22,500 for the audit of our annual financial statements for the fiscal years ended December 31, 2014 and 201 3, respectively, and $12,000 and $10,500 for the reviews of the financial statements included in our Forms 10-Q for the fiscal years ended 2014 and 2013 respectively.
AUDIT-RELATED FEES. No fees were billed in each of the last two fiscal years for assurance and related services by the principal accountant that are reasonably related to the performance of the audit or review of our financial statements and not reported under the caption "Audit Fee."
TAX FEES. No fees were billed in each of the last two fiscal years for professional services rendered by the principal accountant for tax compliance, tax advice and tax planning services.
ALL OTHER FEES. Other than the services described above, there were no other services provided by our principal accountants for the fiscal years ended December 31, 2014 and 2013.
We do not have an Audit Committee. Therefore, our entire Board of Directors (the "Board") serves in the capacity of the Audit Committee. In discharging its oversight responsibility as to the audit process, our Board obtained from the independent auditors a formal written statement describing all relationships between the auditors and us that might bear on the auditors' independence as required by Independence Standards Board Standard No. 1, "Independence Discussions with Audit Committees."
Our Board discussed with the auditors any relationships that may impact their objectivity and independence, including fees for non-audit services, and satisfied itself as to the auditors' independence. The Board also discussed with management and the independent auditors the quality and adequacy of its internal controls. The Board reviewed with the independent auditors their management letter on internal controls.
66
Our Board discussed and reviewed with the independent auditors all matters required to be discussed by auditing standards generally accepted in the United States of America, including those described in Statement on Auditing Standards No. 61, as amended, "Communication with Audit Committees." Our entire Board, acting in the capacity of the Audit Committee reviewed the audited consolidated financial statements of the Company as of and for the year ended December 31, 2014 with the independent auditors. Management has the responsibility for the preparation of the Company's financial statements and the independent auditors have the responsibility for the examination of those statements. Based on the above-mentioned review and discussions with the independent auditors our Board of Directors approved the Company's audited consolidated financial statements and recommended that they be included in its Annual Report on Form 10-K for the year ended December 31, 2014, for filing with the Securities and Exchange Commission.
PART IV
|
a)
|
The following report and consolidated financial statements are filed together with this Annual Report.
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
CONSOLIDATED BALANCE SHEETS AS OF DECEMBER 31, 2014 and 2013
|
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS FOR THE YEARS ENDED DECEMBER 31, 2014 AND DECEMBER 31, 2013
|
|
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
|
|
CONSOLIDATED STATEMENTS OF CASH FLOWS FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
|
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
b)
|
Index to Exhibits
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm
|
|
|
|
|
31.1
|
Certificate of the Chief Executive Officer and Chief Financial Officer pursuant Section 302 of the Sarbanes-Oxley Act of 2002
|
|
31.2
|
Certificate of Executive Vice-President and Chief Operating Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
32 .1
|
Certificate of the Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
32 .2
|
Certificate of the Executive Vice-President and Chief Operating Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 .
|
67
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
BREATHE ECIG CORP.
|
By: /s/ Joshua Kimmel
|
Date: April 7, 2015 | |
|
Joshua Kimmel
Chief Executive Officer/Chief Financial Officer and Director
|
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| By: /s/ Joshua Kimmel | Date: April 7, 2015 | |
|
|
||
|
Chief Executive Officer/Chief Financial Officer/Director
|
| By; /s/ James Chandik | Date: April 7, 2015 | |
|
|
||
|
James Chandik, Director
|
| By; /s/ Christopher R. Clark | Date: April 7, 2015 | |
|
Christopher R. Clark, Director
|
68
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Consolidated Financial Statements:
|
|
|
F-2
|
|
|
F-3
|
|
|
F-4
|
|
|
F-5
|
|
|
F-6
|
|
|
F-7
|
F-1

To the Directors of
Breathe eCig Corp.
(formerly DNA Precious Metals, Inc.)
We have audited the accompanying consolidated balance sheets of Breathe eCig Corp. (formerly DNA Precious Metals, Inc.) (the "Company") as of December 31, 2014 and 2013, and the related consolidated statements of operations and comprehensive loss, changes in stockholders' equity and cash flows for the years then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Breathe eCig Corp. (formerly DNA Precious Metals, Inc.) as of December 31, 2014 and 2013, and the results of its consolidated statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows for the years ended December 31, 2014 and 2013 in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company recently acquired a company in a different industry and spun out their existing mining operations. The lack of profitable operations to date raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in this regard are described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/KBL, LLP
New York, NY
April 7, 2015
F-2
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
DECEMBER 31, 2014 AND 2013
(in United States dollars)
|
DECEMBER 31,
|
DECEMBER 31,
|
|||||||
|
2014
|
2013
|
|||||||
| ASSETS | ||||||||
|
CURRENT ASSETS
|
||||||||
|
Cash
|
$ | 53,813 | $ | 535,934 | ||||
|
Prepaid deposit
|
- | 612,431 | ||||||
|
Prepaid expenses
|
189,528 | 190,514 | ||||||
|
Sales tax receivable
|
27,943 | 35,427 | ||||||
| Total current assets | 271,284 | 1,374,306 | ||||||
|
Fixed assets, net
|
138,124 | 1,276,304 | ||||||
|
Other Asset
|
||||||||
|
Deferred financing fees, net
|
12,366 | 25,081 | ||||||
|
Mining rights
|
1,035,818 | 15,000 | ||||||
| 1,048,184 | 40,081 | |||||||
|
|
||||||||
|
TOTAL ASSETS
|
$ | 1,457,592 | $ | 2,690,691 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Accounts payable and accrued expenses
|
$ | 217,507 | $ | 186,729 | ||||
|
Derivative liability - warrants
|
197,040 | - | ||||||
|
Loan payable - current portion
|
22,209 | - | ||||||
|
Total current liabilities
|
436,756 | 186,729 | ||||||
|
OTHER LIABILITIES
|
||||||||
|
Asset retirement obligation
|
107,749 | - | ||||||
|
Loan payable - net of current portion
|
105,314 | - | ||||||
|
Total other liabilities
|
213,063 | - | ||||||
|
TOTAL LIABILITIES
|
649,819 | 186,729 | ||||||
|
STOCKHOLDERS' EQUITY
|
||||||||
|
Preferred stock, $0.001 par value, 10,000,000 shares authorized
|
||||||||
|
Nil shares issued and outstanding
|
- | - | ||||||
|
Common stock, $0.001 par value, 500,000,000 shares authorized
|
||||||||
|
106,586,000 and 95,076,000 shares issued and outstanding, respectively
|
106,586 | 95,076 | ||||||
|
Additional paid in capital
|
8,579,861 | 7,313,888 | ||||||
|
Deferred compensation
|
- | (187,500 | ) | |||||
|
Accumulated deficit
|
(7,770,337 | ) | (4,710,522 | ) | ||||
|
Accumulated other comprehensive income (loss)
|
(108,337 | ) | (6,980 | ) | ||||
|
Total stockholders' equity
|
807,773 | 2,503,962 | ||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY
|
$ | 1,457,592 | $ | 2,690,691 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
|
BREATHE ECIG CORP.
|
|||||
|
(FORMERLY DNA PRECIOUS METALS, INC.)
|
|||||
|
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
|
|||||
|
(in United States dollars)
|
|
YEARS ENDED
|
||||||||
|
DECEMBER 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
REVENUE
|
$ | - | $ | - | ||||
|
COST OF REVENUES
|
- | - | ||||||
|
GROSS PROFIT
|
- | - | ||||||
|
OPERATING EXPENSES
|
||||||||
|
Exploration costs
|
349,597 | 88,247 | ||||||
|
Salaries and related expenses
|
427,568 | 1,674,152 | ||||||
|
Professional fees
|
303,229 | 388,810 | ||||||
|
Rent
|
40,005 | 40,385 | ||||||
|
Depreciation, amortization and impairment
|
1,262,629 | 9,120 | ||||||
|
General and administrative
|
389,521 | 721,453 | ||||||
|
Total operating expenses
|
2,772,549 | 2,922,167 | ||||||
|
OTHER (INCOME) EXPENSE
|
||||||||
|
Interest expense, net
|
5,452 | 1,403 | ||||||
|
Interest expense, convertible notes
|
140,651 | - | ||||||
|
Fair value adjustment in derivative liabilities
|
76,073 | - | ||||||
|
Loss on conversion of promissory note
|
- | 125,000 | ||||||
|
Exploration tax credits
|
- | (111,095 | ) | |||||
|
Loss on extinguishment of debt
|
65,090 | - | ||||||
|
Total other (income) expense
|
287,266 | 15,308 | ||||||
|
Net loss before provision for income taxes
|
(3,059,815 | ) | (2,937,475 | ) | ||||
|
Provision for income taxes
|
- | - | ||||||
|
NET LOSS
|
$ | (3,059,815 | ) | $ | (2,937,475 | ) | ||
|
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING
|
96,010,164 | 90,057,890 | ||||||
|
NET LOSS PER SHARE
|
$ | (0.03 | ) | $ | (0.03 | ) | ||
|
COMPREHENSIVE LOSS
|
||||||||
|
Net loss
|
$ | (3,059,815 | ) | $ | (2,937,475 | ) | ||
|
Currency translation adjustment
|
(101,357 | ) | (19,396 | ) | ||||
|
Total comprehensive loss
|
$ | (3,161,172 | ) | $ | (2,956,871 | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
|
BREATHE ECIG CORP.
|
|||||||||||||||||||||
|
(FORMERLY DNA PRECIOUS METALS, INC.)
|
|||||||||||||||||||||
|
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
|
|||||||||||||||||||||
|
(in United States dollars)
|
|
Accumulated
|
||||||||||||||||||||||||||||||||||||
|
Additional
|
Other
|
|||||||||||||||||||||||||||||||||||
|
Preferred Stock
|
Common Stock
|
Paid-In
|
Deferred
|
Accumulated
|
Comprehensive
|
|||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Compensation
|
Deficit
|
Income (Loss)
|
Total
|
||||||||||||||||||||||||||||
|
Balance - December 31, 2012
|
- | $ | - | 83,024,000 | $ | 83,024 | $ | 2,800,976 | $ | - | $ | (1,773,047 | ) | $ | 12,416 | $ | 1,123,369 | |||||||||||||||||||
|
Shares issued through S-1 registration statement
|
1,576,000 | 1,576 | 392,424 | - | - | - | 394,000 | |||||||||||||||||||||||||||||
|
Shares issued through S-1 registration statement
|
||||||||||||||||||||||||||||||||||||
|
in settlement of liability for stock to be issued
|
928,000 | 928 | 231,072 | - | - | - | 232,000 | |||||||||||||||||||||||||||||
|
Shares issued on conversion of promissory note
|
- | - | 2,500,000 | 2,500 | 625,050 | - | - | - | 627,550 | |||||||||||||||||||||||||||
|
Shares issued pursuant to employment contracts
|
- | - | 5,000,000 | 5,000 | 1,245,000 | (187,500 | ) | - | - | 1,062,500 | ||||||||||||||||||||||||||
|
Cancellation of shares previously issued
|
- | - | (1,500,000 | ) | (1,500 | ) | 1,500 | - | - | - | - | |||||||||||||||||||||||||
|
Shares issued pursuant to services
|
- | - | 900,000 | 900 | 350,000 | - | - | - | 350,900 | |||||||||||||||||||||||||||
|
Cancellation of shares previously issued
|
- | - | (1,700,000 | ) | (1,700 | ) | 1,700 | - | - | - | - | |||||||||||||||||||||||||
|
Shares issued pursuant to an asset purchase
|
- | - | 1,090,000 | 1,090 | 515,770 | - | - | - | 516,860 | |||||||||||||||||||||||||||
|
Shares issued in private placement
|
- | - | 3,208,000 | 3,208 | 992,573 | - | - | - | 995,781 | |||||||||||||||||||||||||||
|
Stock options exercised
|
- | - | 50,000 | 50 | 12,450 | - | - | - | 12,500 | |||||||||||||||||||||||||||
|
Warrants issued for deferred financing fees
|
- | - | - | - | 25,431 | - | - | - | 25,431 | |||||||||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 119,942 | - | - | - | 119,942 | |||||||||||||||||||||||||||
|
Net loss for the period
|
- | - | - | - | - | - | (2,937,475 | ) | (19,396 | ) | (2,956,871 | ) | ||||||||||||||||||||||||
|
Balance - December 31, 2013
|
- | - | 95,076,000 | 95,076 | 7,313,888 | (187,500 | ) | (4,710,522 | ) | (6,980 | ) | 2,503,962 | ||||||||||||||||||||||||
|
Shares issued pursuant to an asset purchase
|
- | - | 1,000,000 | 1,000 | 339,000 | - | - | - | 340,000 | |||||||||||||||||||||||||||
|
Cancellation of shares previously issued
|
- | - | (4,250,000 | ) | (4,250 | ) | 4,250 | - | - | - | - | |||||||||||||||||||||||||
|
Common shares issued for services
|
- | - | 400,000 | 400 | 121,100 | - | - | - | 121,500 | |||||||||||||||||||||||||||
|
Warrants issued for services
|
- | - | - | - | 28,743 | - | - | - | 28,743 | |||||||||||||||||||||||||||
|
Amortization of deferred compensation
|
- | - | - | - | - | 187,500 | - | - | 187,500 | |||||||||||||||||||||||||||
|
Shares issued in private placement
|
- | - | 14,310,000 | 14,310 | 701,190 | - | - | - | 715,500 | |||||||||||||||||||||||||||
|
Stock options exercised
|
- | - | 50,000 | 50 | 12,450 | - | - | - | 12,500 | |||||||||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 59,240 | - | - | - | 59,240 | |||||||||||||||||||||||||||
|
Net loss for the period
|
- | - | - | - | - | - | (3,059,815 | ) | (101,357 | ) | (3,161,172 | ) | ||||||||||||||||||||||||
|
Balance - December 31, 2014
|
- | $ | - | 106,586,000 | $ | 106,586 | $ | 8,579,861 | $ | - | $ | (7,770,337 | ) | $ | (108,337 | ) | $ | 807,773 | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
|
BREATHE ECIG CORP.
|
|||||
|
(FORMERLY DNA PRECIOUS METALS, INC.)
|
|||||
|
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
|
|||||
|
(in United States dollars)
|
|
YEARS ENDED
|
||||||||
|
DECEMBER 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net loss
|
$ | (3,059,815 | ) | $ | (2,937,475 | ) | ||
|
Adjustments to reconcile net (loss)
|
||||||||
|
to net cash (used in) operating activities:
|
||||||||
|
Depreciation, amortization and impairment
|
1,262,629 | 9,120 | ||||||
|
Non-cash interest charges
|
55,877 | - | ||||||
|
Fair value adjustment in derivative liabilities
|
76,073 | - | ||||||
|
Shares and options issued for compensation
|
246,740 | 1,182,442 | ||||||
|
Loss on conversion of promissory note
|
- | 125,000 | ||||||
|
Shares and warrants issued for services rendered
|
83,948 | 350,900 | ||||||
|
Loss on extinguishment of debt
|
65,090 | - | ||||||
|
Change in assets and liabilities
|
||||||||
|
(Increase) decrease in prepaid expenses and deposits
|
986 | (196,586 | ) | |||||
|
Decrease in sales tax receivable
|
7,483 | 23,724 | ||||||
|
Increase in accounts payable and accrued expenses
|
97,229 | 96,505 | ||||||
|
Total adjustments
|
1,896,055 | 1,591,105 | ||||||
|
Net cash (used in) operating activities
|
(1,163,760 | ) | (1,346,370 | ) | ||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Acquisition of fixed assets
|
(2,613 | ) | (68,684 | ) | ||||
|
Acquisition of mining rights
|
(68,387 | ) | - | |||||
|
Net cash (used in) investing activities
|
(71,000 | ) | (68,684 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Proceeds received from loan payable, net of repayments
|
127,523 | - | ||||||
|
Cash received for common stock and liability for stock to be issued
|
728,000 | 1,345,874 | ||||||
|
Net cash provided by financing activities
|
855,523 | 1,345,874 | ||||||
|
Effect of foreign currency
|
(102,884 | ) | 6,176 | |||||
|
NET (DECREASE) IN CASH AND CASH EQUIVALENTS
|
(482,121 | ) | (63,004 | ) | ||||
|
|
||||||||
|
CASH AND CASH EQUIVALENTS - BEGINNING OF YEAR
|
535,934 | 598,938 | ||||||
|
|
||||||||
|
CASH AND CASH EQUIVALENTS - END OF YEAR
|
$ | 53,813 | $ | 535,934 | ||||
|
SUPPLEMENTAL CASH FLOW INFORMATION:
|
||||||||
|
Cash paid during the period for:
|
||||||||
|
Interest
|
$ | 64,492 | $ | 1,403 | ||||
|
Income taxes
|
$ | - | $ | - | ||||
|
SUPPLEMENTAL NON-CASH ACTIVITY:
|
||||||||
|
Common stock issued for mining rights
|
$ | 340,000 | $ | - | ||||
|
Common stock issued for prepaid deposit
|
$ | - | $ | 516,860 | ||||
|
Conversion of promissory note to common stock
|
$ | - | $ | 627,550 | ||||
|
Deferred compensation for common stock
|
$ | 187,500 | $ | 1,062,500 | ||||
|
Deferred financing fees through the issuance of warrants
|
$ | - | $ | 25,431 | ||||
|
Issuance of common stock and warrants for prepaid expenses
|
$ | 103,743 | $ | - | ||||
|
Asset retirement obligation
|
$ | 107,749 | $ | - | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
DECEMBER 31, 2014 AND 2013
|
NOTE 1-
|
ORGANIZATION AND BASIS OF PRESENTATION
|
On June 2, 2006, Celtic Capital, Inc. was incorporated in the State of Nevada. On October 20, 2008, Celtic Capital, Inc. changed its name to Entertainment Educational Arts Inc. On May 12, 2010, the Company changed its name to DNA Precious Metals, Inc. (the “Company”). On October 29, 2010, the Company formed DNA Canada Inc., a Canadian (Province of Quebec) incorporated company, as a wholly-owned subsidiary. The Company will operate all of its exploration operations through this Canadian entity.
The Company is an exploration stage company that is in the business of identifying mineral claim rights in Canada and the United States. The Company has conducted minimal business to date.
The Company’s primary goal is to identify and acquire premium gold (Au) and silver (Ag) properties to create an international mining company. The mineralized properties that the Company will focus on acquiring, will have easy accessibility, transportation infrastructures in place on the property and most importantly, will have the potential to be brought into production quickly.
The Company acquired certain mining claims on June 9, 2011 located in the Montauban and Chavigny townships near Grondines-West in the Portneuf County of Quebec, Canada (“Montauban Mine Property” or “Property”). The transaction is shown as an asset, Mining rights, on the consolidated balance sheets at December 31, 2014 and 2013.
On October 30, 2013 and November 27, 2013, the Company entered into binding agreements for the asset acquisitions of an undivided one hundred percent (100%) interest in certain mineral claims and mining assets located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf, specifically Mining Lease BM 748 and Mining Concession Miniere CM 410. The purchase price was CDN$75,000 together with the issuance of 1,050,000 common shares of the Company. The common shares for the acquisition were valued at their fair market value on the day they were issued which totaled $496,860. In connection with the asset purchase, the Company also issued 40,000 shares of common stock to a former supplier of the vendor for mining related information of the assets purchased valued at $20,000 along with cash consideration of CDN$20,000. The Company had been awaiting confirmation of the contemplated transaction from a bankruptcy court in Montreal, Quebec overviewing the financial restructuring of the vendor. The bankruptcy court approved the transaction on April 17, 2014.
On January 10, 2014, the Company entered into an asset purchase agreement for an undivided one hundred percent (100%) interest in certain mineral claims located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf, including claims, rights, concessions and leases. The purchase price was CDN$70,000, 1,000,000 common shares of the Company and a one percent (1%) net smelter return (“NSR”). The Company paid CDN$10,000 upon the signing of the asset purchase agreement with the cash balance due, along with the common shares, upon the closing of the asset purchase agreement and transfer of the mineral claims in the name of the Company. The transfer of the mineral claims was completed in February 2014 whereby the remaining cash balance due and the common shares were released to the vendor.
F-7
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 1-
|
ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED)
|
The common shares for the acquisition will be valued at their fair market value on the day they were issued which totaled $340,000. The total cost of the acquisition amounted to $403,840.
On April 14, 2014, the Company entered into an asset purchase agreement for an undivided one hundred percent (100%) interest in fifty seven (57) mining claims located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf. The purchase price was CDN$5,000 (US $4,547). The transfer of the mining claims has been completed by the Province of Quebec in the name of the Company.
On December 4, 2014, the Company presented a renewal request with the Government of the Province of Quebec to renew all 122 claims and this has been granted through a decision dated February 23, 2015.
On January 16, 2015, Breathe eCigs Corp., a Tennessee corporation (“Breathe”), entered into a Share Exchange Agreement (the “Exchange Agreement”) with the Company, whereby the Company acquired all of the issued and outstanding shares of common stock of Breathe in consideration for the issuance of 150,000,000 shares of common stock.
As a result of the transaction effected by the Exchange Agreement, at closing Breathe became a wholly owned subsidiary of the Company, with the former Breathe shareholders owning approximately 56% of the then issued and outstanding common stock of the Company.
The Company declared a stock dividend to its shareholders of record as of February 3, 2015 of its wholly owned subsidiary, DNA Canada, Inc. Each shareholder of record on this date will receive one share of DNA Canada, Inc. for every two shares of DNA Precious Metals Inc. owned by the shareholder on this date. All stock dividends will be rounded down to the next whole number. With the completion of the stock dividend, the Company, no longer has an equity interest in DNA Canada, Inc.
The former shareholders of Breathe participating in the stock dividend were required to tender for redemption any shares of DNA Canada, Inc. common stock received pursuant to the stock dividend in accordance with the Exchange Agreement.
With the acquisition of Breathe, management determined that it would be in the best interest of the Company and its shareholders to operate each company separate and independently of each other. The operation of DNA Canada, Inc. and Breathe were inconsistent. Breathe is a manufacturer and distributor of e-cigarette and related products while DNA Canada, Inc. is an exploration stage mining company. The spin-off of DNA Canada, Inc. will allow each company to focus on its principal business activity and facilitate capital formation.
F-8
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 1-
|
ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED)
|
On March 5, 2015, the Company and Breathe entered into an Agreement and Plan of Merger pursuant to which the Company merged with its wholly owned subsidiary, Breathe. Upon the consummation of the Merger on March 11, 2015, the separate existence of Breathe ceased, and the shareholders of the Company became shareholders of the surviving company, named Breathe eCig Corp. As permitted under Nevada law, the sole purpose of the Merger was to effect a change to the Company’s name from DNA Precious Metals, Inc. to Breathe eCig Corp. This change to the Amended Articles of Incorporation and name change took effect on March 11, 2015.
Going Concern
The consolidated financial statements have been prepared on a going concern basis. The going concern basis of presentation assumes that the Company will continue in operation for the foreseeable future and be able to realize its assets and discharge its liabilities and commitments in the normal course of business. The Company has not generated revenues since inception and has generated losses totaling $3,059,815 and $2,937,475 for the years ended December 31, 2014 and 2013, respectively.
The Company’s continuation as a going concern is dependent upon, amongst other things, continued financial support from its shareholders, attaining a satisfactory revenue level, attainment of profitable operations and the generation of cash from operations and the ability to secure new financing arrangements and new capital to carry out its business plan. These matters are dependent on a number of items outside of the Company’s control and there exists material uncertainties that may cast significant doubt about the Company’s ability to continue as a going concern.
The Company can give no assurance that it will achieve profitability or be capable of sustaining profitable operations in its new business of Breathe. These consolidated financial statements do not include any adjustments relating to the recoverability and classification of the carrying amounts of assets or the amount and classification of liabilities that might result if the Company is unable to continue as a going concern. These factors raise substantial doubt regarding the ability of the Company to continue as a going concern.
The Company recently raised $715,500 in November and December 2014, to increase the exploration of the mining claims. Additionally, after the acquisition of Breathe, was able to raise capital in the form of convertible notes to assist Breathe in completing this transaction and acquire product for their initial production of the e-cigarettes.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for Securities and Exchange Commission (“SEC”) registrants.
F-9
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 1-
|
ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED)
|
All guidance contained in the Codification carries an equal level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Positions or Emerging Issue Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
Basis of Accounting
These consolidated financial statements are prepared in conformity with United States generally accepted accounting principles (“U.S. GAAP”) and are presented in U.S. dollars.
Exploration Stage Company
The Company is an exploration stage company as defined in ASC 915. The Company has made significant capital investments on a processing mill and related infrastructure pertaining to a mining site described earlier. The construction of the processing mill structure was commenced in the fourth quarter of fiscal 2012 and completed in the first quarter of fiscal 2013. Also, significant infrastructure work related to the processing mill has been completed.
Presently, the infrastructure construction includes the foundation, a 16,000 sq./ft. steel structure building and water and power supply installations. The Company has completed all the access infrastructural work to the future site where the milling facilities will be located. Due to SEC requirements, the land, building and mill equipment have been charged to impairment expense since these assets were not put into production since their acquisition.
On September 14, 2012, the Company received a Certificate of Authorization, from the Quebec Provincial Government, with respect to operating a gravimetric circuit to process the mining residues, or tailings, located on the Montauban Mine Property. On March 13, 2014, the Company received another Certificate of Authorization, also from the Quebec Provincial Government, with respect to operating a cyanization circuit to process the mining residues located on the Montauban Mine Property. Previously, on February 28, 2014, the Company received approval, from the Quebec Provincial Government, for the Restoration Plan on the Montauban Mine Property which will be implemented subsequent to the Company’s processing of the mining residues (tailings) on the site (see Note 11, Subsequent Events).
F-10
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
The two (2) Certificates of Authorization issued to the Company will allow for the construction and installation of equipment facilities to recuperate mica (muscovite) and precious metals (gold and silver) from the mining residues (tailings) located on the Montauban Mine Property.
Consequently, the primary objective will be to recuperate the mica and precious metals (gold and silver) from the mining residues. The recuperation of the precious metals from the mining residues will be less expensive than traditional mining operations primarily because the mining residues have already been crushed and grinded by prior mining companies.
In accordance with ASU 2014-10 (see Recent Accounting Pronouncements), the Company has elected early adoption whereby, amounts and disclosures of the Company’s exploration stage activities commencing with the period June 30, 2014 are no longer required to be presented. As a result, the Company removed this information.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. Estimates include but are not limited to stock-based compensation and tax valuation allowances.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, DNA Canada Inc. All intercompany transactions and accounts have been eliminated on consolidation.
Currency Translation
The Company’s functional is the Canadian dollar and its reporting currency is the United States dollar. Transactions denominated in the functional currency are converted into United States dollars using the exchange rate in effect at the date of the transaction or the average rate for the period in the case of revenue and expense transactions. Monetary assets and liabilities are re-valued into the reporting currency at each balance sheet date using the exchange rate in effect at the balance sheet date, with any resulting exchange gains or losses being credited or charged to accumulated other comprehensive income (loss). Non-monetary assets and liabilities are recorded in the reporting currency using the exchange rate in effect at the date of the transaction and are not revalued for subsequent changes in exchange rates.
F-11
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
Stock Options-Based Compensation
The Company estimates the fair value of stock options-based payment awards made to officers and directors related to the Company’s stock incentive plan, on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense rateably over the requisite service periods. The Company uses the Black-Scholes option pricing model to determine the fair value of the stock-based compensation that it grants to officers and directors. The Company is required to make certain assumptions in connection with this determination, the most important of which involves the calculation of volatility with respect to the price of its common stock. The computation of volatility is intended to produce a volatility value that is representative of the Company’s expectations about the future volatility of the price of its common stock over an expected term. The Company used an estimate of its future share price to determine volatility and cannot predict how the price of its common shares of common stock will react on the open market in the future. Shares of the Company commenced trading on August 22, 2013. As a result, the volatility value that the Company calculated may differ from the future volatility of the price of its shares of common stock.
Upon the exercise of stock options, any consideration received and the amounts previously recorded under stock-based compensation are credited to share capital. Upon the issuance of shares resulting from share awards, amounts previously recorded under stock options-based compensation are credited to share capital.
Comprehensive Income (Loss)
The Company adopted ASC 220-10, “Reporting Comprehensive Income,” (formerly SFAS No. 130). ASC 220-10 requires the reporting of comprehensive income in addition to net income from operations.
Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of information that historically has not been recognized in the calculation of net income.
Cash and Cash Equivalents
The Company considers all highly liquid debt instruments and other short-term investments with maturity of three months or less, when purchased, to be cash equivalents.
The Company maintains cash and cash equivalent balances at one major Canadian bank.
Exploration Tax Credits
The Company is entitled to certain exploration tax credits for the exploration expenditures they have incurred from the Canadian federal government and the government of the Province of Quebec. Some of the tax credits available from the Province of Quebec are in the form of cash. Qualifying expenditures include exploration costs and salaries to conduct the activities of the Company.
F-12
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
During the year ended December 31, 2014, the Company did not received any amount as tax credits for qualifying expenditures (2013 - $111,095). The Company’s policy is to record the tax credits when received rather than when applied for. Research tax credits must be reviewed and approved by the appropriate tax authorities when applied and it is possible that the amounts granted may differ from the amounts applied for.
Fixed Assets
Fixed assets are stated at cost, less accumulated depreciation. Depreciation will be provided using the straight-line method over the estimated useful lives of the related assets when those assets are placed into service. Costs of maintenance and repairs will be charged to expense as incurred.
Trade and Other Payables
Trade and other payables and accrued liabilities are obligations to pay for goods or services that have been acquired in the normal course of business. Trade and other payables and accrued liabilities are classified as current liabilities if payment is due within one year or less. If not, they are presented as non-current liabilities.
Recoverability of Long-Lived Assets
Although the Company does not have any long-lived assets at this point, for any long-lived assets acquired in the future, the Company will review their recoverability on a periodic basis whenever events and changes in circumstances have occurred which may indicate a possible impairment. The assessment for potential impairment will be based primarily on the Company’s ability to recover the carrying value of its long-lived assets from expected future cash flows from its operations on an undiscounted basis. If such assets are determined to be impaired, the impairment recognized is the amount by which the carrying value of the assets exceeds the fair value of the assets. Fixed assets to be disposed of by sale will be carried at the lower of the then current carrying value or fair value less estimated costs to sell.
Asset Retirement Obligations
The Company is subject to environmental laws and regulations enacted by Canadian federal and provincial authorities. As of the reporting date, management believes that the Company’s operations are in compliance with current laws and regulations. To take account of estimated cash flows required to settle the obligations arising from environmentally acceptable closure plans (such as dismantling and demolition of infrastructures, removal of residual matter and site restoration), provisions are recognized in the year that the harm to the environment occurs, that is when the Company has an actual obligation resulting from harm to the environment, it is likely that an outflow will be required in settlement of the obligation and the obligation is reasonably determinable. These provisions are determined on the basis of the best estimates of future costs, based on information available on the reporting date. Best estimates of future costs are the amount the Company would reasonably pay to settle its obligation on the closing date or to transfer it to a third party on the same date.
F-13
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
Future costs are discounted using pre-tax rates that reflect current market assessments of the time value of money and the risks specific to the liability. A corresponding asset is recognized in property, plant and equipment when establishing the provision.
The asset retirement obligation is reviewed quarterly to reflect changes in the estimated outflow of resources as a result of changes in obligations or legislation, changes in the current market-based discount rate or an increase that reflects the passage of time. The accretion expense is recognized in net earnings as a finance expense as incurred. The cost of the related asset is adjusted to reflect changes (other than accretion) in the reporting period.
Fair Value of Financial Instruments
The carrying amount reported in the consolidated balance sheets for cash and cash equivalents, accounts payable, and accrued expenses approximate fair value because of the immediate or short-term maturity of these financial instruments. The Company does not utilize derivative instruments.
Income Taxes
The Company uses the liability method of accounting for income taxes under which deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted or substantially enacted tax rates in effect for the year in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized as part of the provision for income taxes in the period that includes the enactment date. Deferred tax liabilities are always provided for in full. Deferred tax assets are recognized to the extent that it is probable that they will be able to be utilized against future taxable income. Deferred tax assets and liabilities are offset only when the Company has a right and intention to set off current tax assets and liabilities from the same taxation authority. Valuation allowances are established, when necessary, to reduce deferred tax assets to amounts that are expected to be realized.
The Company accounts for income taxes pursuant to the provision of ASC 740-10, “Accounting for Income Taxes” (“ASC 740-10”) which requires, among other things, an asset and liability approach to calculating deferred income taxes. The asset and liability approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities. A valuation allowance is provided to offset any net deferred tax assets for which management believes it is more likely than not that the net deferred asset will not be realized.
The Company follows the provision of ASC 740-10 related to Accounting for Uncertain Income Tax Position. When tax returns are filed, there may be uncertainty about the merits of positions taken or the amount of the position that would be ultimately sustained.
F-14
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
In accordance with the guidance of ASC 740-10, the benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions.
Tax positions that meet the more likely than not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above should be reflected as a liability for uncertain tax benefits in the accompanying balance sheet along with any associated interest and penalties that would be payable to the taxing authorities upon examination. The Company believes its tax positions are all highly certain of being upheld upon examination. As such, the Company has not recorded a liability for uncertain tax benefits.
The Company has adopted ASC 740-10-25, “Definition of Settlement”, which provides guidance on how an entity should determine whether a tax position is effectively settled for the purposes of recognizing previously unrecognized tax benefits and provides that a tax position can be effectively settled upon the completion and examination by a taxing authority without being legally extinguished. For tax positions considered effectively settled, an entity would recognize the full amount of tax benefit, even if the tax position is not considered more likely than not to be sustained based solely on the basis of its technical merits and the statute of limitations remains open. The federal and state income tax returns of the Company are subject to examination by the IRS and state taxing authorities generally for three years after they were filed.
Revenue Recognition
The Company will generate revenues from the sale of precious metals mined from its Property. Revenue from the sale of precious metals, namely gold, silver and mica, will be recognized upon delivery of the precious metals, collection is probable, the fee is fixed or determinable and the Company has transferred to the buyer the significant risks and rewards of ownership of the precious metals supplied. Significant risks and rewards are generally considered to be transferred to the buyer when the customer has taken undisputed delivery of the precious metals.
Loss Per Share of Common Stock
Basic net loss per share (“Basic EPS”) is computed by dividing net loss available to common shareholders by the weighted average number of common shares outstanding for the period.
Diluted earnings per share is computed by dividing adjusted net income available to common shareholders by the weighted average number of common shares outstanding adjusted for the effects of all dilutive common share issuances.
F-15
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
Dilutive common share issuances shall be deemed to have been converted into ordinary shares at the beginning of the period.
For the purpose of calculating diluted earnings per share, the Company shall assume the exercise of dilutive stock options and warrants. The assumed proceeds from these instruments shall be regarded as having been received from the issue of common shares at the average market price of common shares during the period. Dilutive common share issuances are not included in the computation of diluted earnings per share when the Company reports a loss because to do so would be anti-dilutive for the periods presented.
The following is a reconciliation of the computation for basic and diluted EPS:
|
Year Ended
|
||||||||
|
December 31,
|
December 31,
|
|||||||
|
2014
|
2013
|
|||||||
|
Net loss
|
$ | (3,059,815 | ) | $ | (2,937,475 | ) | ||
|
Weighted-average common shares
|
||||||||
|
outstanding (Basic)
|
96,010,164 | 90,057,890 | ||||||
|
Weighted-average common shares
|
||||||||
|
Equivalent
|
||||||||
|
Stock options
|
200,000 | 1,333,000 | ||||||
|
Warrants
|
1,540,625 | - | ||||||
|
Weighted-average common shares
|
||||||||
|
outstanding (Diluted)
|
97,750,789 | 91,390,890 | ||||||
Derivative Financial Instruments
The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. The Company evaluates all of its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period.
The accounting treatment of derivative financial instruments requires that the Company record the conversion option and related warrants at their fair values as of the inception date of the agreements, and at fair value as of each subsequent balance sheet date.
F-16
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
As a result of entering into the convertible notes, the Company is required to classify certain non-employee warrants as derivative liabilities and record them at their fair values at each balance sheet date. Any change in fair value was recorded as a change in the fair value of derivative liabilities for each reporting period at each balance sheet date. The Company reassesses the classification at each balance sheet date. If the classification changes as a result of events during the period, the contract is reclassified as of the date of the event that caused the reclassification.
The fair value of conversion options at a fixed number of shares are recorded using the intrinsic value method. Conversion options at variable rates and any options and warrants with ratchet provisions are deemed to contain a “down-round protection”. Accordingly, they do not meet the scope exception for treatment as a derivative under ASC 815 since “down-round protection” is not an input into the calculation of the fair value of the equity instruments and cannot be considered “indexed to the Company’s own stock”, which is a requirement for the scope exception as outlined under ASC 815.
The Company signed convertible notes and warrants and has determined that a conversion option is embedded in the note and it is required to bifurcate the conversion option from the host contract under ASC 815 and account for the derivatives at fair value. The estimated fair value of the conversion option was determined using the binomial model. The fair value of the conversion option will be classified as a liability until the debt is converted by the note holders or paid back by the Company. The fair value will be affected by changes in inputs to that model including our stock price, expected stock price volatility, the contractual term, and the risk-free interest rate. The Company will continue to classify the fair value of the conversion option as a liability until the conversion option is exercised, expires or is amended in a way that would no longer require these conversion options to be classified as a liability, whichever comes first. The Company has adopted a sequencing policy that reclassifies contracts (from equity to assets or liabilities) with the most recent inception date first. Thus any available shares are allocated first to contracts with the most recent inception date.
For the binomial lattice options pricing model, the Company used the following assumptions and weighted average fair value ranges as at the transaction date, April 28, 2014, and for the period ended December 31, 2014:
|
Convertible Notes:
|
April 28, 2014
|
December 31, 2014
|
|
|
Risk free interest rate
|
.0577%
|
N/A
|
|
|
Dividend yield
|
N/A
|
N/A
|
|
|
Volatility
|
86.31%
|
N/A
|
F-17
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
|
Warrants
|
April 28, 2014
|
December 31, 2014
|
|
|
Risk free interest rate
|
.1442%
|
..0400%
|
|
|
Dividend yield
|
N/A
|
N/A
|
|
|
Volatility
|
97.33%
|
175.00%
|
Recent Issued Accounting Standards
During August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements—Going Concern.” The provisions of ASU No. 2014-15 require management to assess an entity’s ability to continue as a going concern by incorporating and expanding upon certain principles that are currently in U.S. auditing standards. Specifically, the amendments (1) provide a definition of the term substantial doubt, (2) require an evaluation every reporting period including interim periods, (3) provide principles for considering the mitigating effect of management’s plans, (4) require certain disclosures when substantial doubt is alleviated as a result of consideration of management’s plans, (5) require an express statement and other disclosures when substantial doubt is not alleviated, and (6) require an assessment for a period of one year after the date that the financial statements are issued (or available to be issued). The amendments in this ASU are effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. The Company is currently assessing the impact of this ASU on the Company’s consolidated financial statements.
During June 2014, the FASB issued an Accounting Standards Update No. 2014-10, "Development Stage Entities (Topic 915) - Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation ("ASU 2014-10")". The objective of ASU 2014-10 is to improve financial reporting by reducing the cost and complexity associated with the incremental reporting requirements for development stage entities. ASU 2014-10 is effective for annual reporting periods beginning after December 15, 2014, and interim periods therein. The Company has elected early implementation, as permitted by the standard, for the interim period ending June 30, 2014. All exploration stage language disclosures and amounts have been removed as a result of the adoption of ASU 2014-10.
During July 2013, the FASB issued an Accounting Standards Update No. 2013-11, “Income Taxes (Topic 740) - Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carry forward, a Similar Tax Loss, or a Tax Credit Carry forward Exists (“ASU 2013-11”)”. The objective of ASU 2013-11 is to clarify the financial presentation of an unrecognized tax benefit when a net operating loss carry forward, a similar tax loss, or a tax credit carry forward exists. The assessment of whether a deferred tax asset is available is based on the unrecognized tax benefit and deferred tax asset that exist at the reporting date and should be made presuming disallowance of the tax position at the reporting date. ASU 2013-11 is effective for fiscal years, and interim periods within those years beginning after December 15, 2013.
F-18
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 2-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
The Company does not expect that the adoption of ASU 2013-11 will have a significant impact on the presentation of its financial statements.
In July 2012, the FASB issued ASU 2012-02, Intangibles – Goodwill and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment, on testing for indefinite-lived intangible assets for impairment. The new guidance provides an entity to simplify the testing for a drop in value of intangible assets such as trademarks, patents, and distribution rights. The amended standard reduces the cost of accounting for indefinite-lived intangible assets, especially in cases where the likelihood of impairment is low.
The changes permit businesses and other organizations to first use subjective criteria to determine if an intangible asset has lost value. The amendments to U.S. GAAP will be effective for fiscal years starting after September 15, 2012. The Company’s adoption of this accounting guidance does not have a material impact on the consolidated financial statements and related disclosures.
There were other updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries and are not expected to have a material impact on the Company’s financial position, results of operations or cash flows.
|
NOTE 3-
|
MINING RIGHTS
|
The Company acquired ten (10) mining claims, which became fifteen (15) mining claims under new government regulations, on June 9, 2011 through the issuance of 5,000,000 common shares with a valuation of $15,000. The mining claims are located in the Montauban and Chavigny townships near Grondines-West in the Portneuf County of Quebec, Canada (“Montauban Mine Property” or “Property”).
On October 30, 2013 and November 27, 2013, the Company entered into binding agreements for the asset acquisitions of an undivided one hundred percent (100%) interest in certain mineral claims and mining assets located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf, specifically Mining Lease BM 748 and Mining Concession Miniere CM 410. The purchase price was CDN$75,000 together with the issuance of 1,050,000 common shares of the Company. The common shares for the acquisition were valued at their fair market value on the day they were issued which totaled $496,860. In connection with the asset purchase, the Company also issued 40,000 shares of common stock to a former supplier of the vendor for mining related information of the assets purchased valued at $20,000 along with cash consideration of CDN$20,000. The total cost of the acquisition amounts $612,431. The Company had been awaiting confirmation of the contemplated transaction from a bankruptcy court in Montreal, Quebec overviewing the financial restructuring of the vendor. The bankruptcy court approved the transaction on April 17, 2014.
On January 10, 2014, the Company entered into an asset purchase agreement for an undivided one hundred percent (100%) interest in certain mineral claims located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf, including claims, rights, concessions and leases.
F-19
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 3-
|
MINING RIGHTS (CONTINUED)
|
The purchase price was CDN$70,000, 1,000,000 common shares of the Company and a one percent (1%) net smelter return (“NSR”). The Company paid CDN$10,000 upon the signing of the asset purchase agreement with the cash balance due, along with the common shares, upon the closing of the asset purchase agreement and transfer of the mineral claims in the name of the Company. The transfer of the mineral claims was completed in February 2014 whereby the remaining cash balance due and the common shares were released to the vendor. The common shares for the acquisition will be valued at their fair market value on the day they were issued which totaled $340,000. The total cost of the acquisition amounts $403,840.
On April 14, 2014, the Company entered into an asset purchase agreement for an undivided one hundred percent (100%) interest in fifty seven (57) mining claims located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf. The purchase price was CDN$5,000 (U.S.$4,547). The transfer of the mining claims has been completed by the Province of Quebec in the name of the Company.
Total mining rights as of December 31, 2014 and 2013 were $1,035,818 and $15,000, respectively.
|
NOTE 4-
|
ACQUISITION
|
On June 20, 2014, DNA Crypto Corp. (“DNAC”), a wholly-owned subsidiary of DNA Precious Metals, Inc., signed a definitive asset purchase agreement with Lynx Mining LLC, a Texas limited liability company (“Lynx”), whereby DNAC acquired the assets of Lynx, being its intellectual property rights. As part of the asset purchase agreement, DNAC issued 4.9 million shares of its common stock to Lynx Mining LLC. Following the issuance of the DNAC common stock, DNA Precious Metals, Inc. owned 5.1 million shares (51%) of the outstanding shares of common stock of DNAC and Lynx owns 4.9 million shares (49%) of DNAC common stock. The issuance of the 10 million shares represents 100% of DNAC’s authorized common stock. Lynx’s contribution of all of its intellectual property rights is in connection with the design of proprietary software to mine bitcoins. Lynx has developed formulas for how much hashing power must be added to negate the decreased Bitcoin generation.
The intellectual property rights acquired from Lynx did not have significant value as Lynx itself was a start-up entity and there had not been any significant amounts expensed by Lynx to design the proprietary software. The 4.9 million shares of DNAC issued to Lynx was for the proprietary software and had been valued at $10,000 as of the acquisition date which represents the approximate cost the individual owners of Lynx expended to develop the software.
From June 3, 2014 to September 14, 2014, aside from the asset purchase agreement, there had been limited transactions in DNAC as operations had not yet fully commenced. As a result, there was a nominal non-controlling interest shown on the consolidated statement of operations.
F-20
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 4-
|
ACQUISITION (CONTINUED)
|
On August 1, 2014, DNAC entered into an updated Asset Purchase Agreement (“Agreement”) with Lynx Mining LLC. The Agreement rescinds the prior Asset Purchase Agreement entered into between DNAC and Lynx dated June 20, 2014. The updated Agreement takes into account funding obligations by DNA Precious Metals, Inc. into DNAC. The accounting for DNAC as at and for the period ended June 30, 2014 remains the same as stipulated in the original Asset Purchase Agreement dated June 20, 2014. As part of the new Agreement and subsequent to its execution, the Company has remitted $11,000 to DNAC.
On September 15, 2014 DNA Precious Metals, Inc. terminated for cause the Asset Purchase Agreement entered into July 14, 2014 with Lynx Mining LLC, a Texas limited liability company (“Lynx”). The intellectual property rights transferred to DNA Crypto, Inc. and the services provided by Lynx to implement the Company’s business plan were deficient. DNA Precious Metals, Inc. wrote all of the costs associated with this transaction off, and will not pursue any action against Lynx. As a result, no assets or non-controlling equity interest are reflected herein.
|
NOTE 5-
|
CONVERTIBLE NOTES AND DERIVATIVE LIABILITIES
|
On August 6, 2014, the Company entered into an agreement with a U.S.-based private equity fund (“Investor”) under which the Company issued an unsecured Convertible Note (“Convertible Note”) in the principal amount of $250,000. The funds to be issued under the Convertible Note is $225,000 (“Consideration”). The Convertible Note includes an original issue discount of $25,000 (“OID”), calculated at 10% of the principal amount ($250,000). The initial Consideration paid to the Company on August 6, 2014 was $66,000. The Investor may pay additional Consideration to the Company in such amounts and at such dates as the Investor may choose in its sole discretion. The principal sum due to the Investor shall be prorated based on the Consideration actually paid by the Investor plus a 10% OID, as well as any other interest or fees, such that the Company is only required to repay the amounts funded and the Company is not required to repay any unfunded portions of the Convertible Note. The Company may repay the Convertible Note at any time on or before 90 days from the transaction date after which the Company may not make further payments on the Convertible Note prior to the Maturity Date without written approval from the Investor. If the Company makes a payment of Consideration on or before 90 days from the transaction date, the interest rate on that payment of Consideration shall be zero percent (0%). If the Company does not repay a payment of Consideration on or before 90 days from the transaction date, a one-time interest charge of 12% shall be applied to the principal amount. Any interest payable is in addition to the OID and that OID (or prorated OID, if applicable) remains payable regardless of time and manner of payment by the Company. The maturity date is two years from the transaction date of each payment ("Maturity Date") and is the date upon which the principal amount of the Convertible Note, as well as any unpaid interest and other fees, shall be due and payable. The Investor has the right, at any time after the transaction date, at its election, to convert all or part of the outstanding and unpaid principal amount and accrued interest (and any other fees) into fully paid and non-assessable shares of common stock of the Company.
F-21
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 5-
|
CONVERTIBLE NOTES AND DERIVATIVE LIABILITIES (CONTINUED)
|
The conversion price is the lesser of $0.16 or 60% of the lowest trade price in the 25 trading days prior to the conversion. Unless otherwise agreed in writing by both parties, at no time will the Investor convert any amount of the Convertible Note into common stock that would result in the Investor owning more than 4.99% of the common stock outstanding of the Company. At all times that this Convertible Note is outstanding, the Company agrees to reserve at least 10,000,000 shares of common stock for conversion. On October 30, 2014, the Company and the Investor entered into an amendment (“Amendment #1”), whereby the Company paid a partial note repayment in the amount of $33,333 on November 5, 2014 which equals 50% of the note balance which includes $3,333 in interest, with the remaining balance due by January 6, 2015, which was repaid on December 29, 2014.
On April 28, 2014, the Company entered into a Securities Purchase Agreement (“SPA”), with a U.S.-based private equity fund, under which the Company issued a Secured Convertible Promissory Note (the “Convertible Note”) in the amount of $552,500. The Convertible Note includes an original issue discount of $50,000 (“OID”), calculated at 10% of the principal amount ($500,000), plus an additional $2,500 (“Transaction Expense Amount”) to cover the investor’s due diligence and legal fees in connection therewith. The principal amount will be paid to the investor in six (6) tranches of an initial amount under the Convertible Note of $250,000 and five (5) additional amounts of $50,000, with each of the additional amounts represented by Investor Notes (the Convertible Note and the Investor Notes are collectively referred to herein as the “Notes”). The initial $250,000 in cash was paid to the Company on April 29, 2014. Payment of the Notes will be made on a monthly basis, beginning six months after the issue date when the Company received the initial $250,000, in the amount of $34,531 per month plus all accrued but unpaid interest and other costs, fees or charges payable, for sixteen (16) months until the balance is paid in full. The Notes are convertible into common stock, at the option of the investor, at a price of $0.40 per share subject to adjustment in the case of a default, reorganization or recapitalization. In the event the Company elects to prepay all or any portion of the Notes, the Company is required to pay to the investor an amount in cash equal to 125% of the outstanding balance of the Notes, plus accrued interest and any other amounts owing. Interest accrues at the rate of 10% per annum. If the Company failed to repay the Notes when due, or if other events of default thereunder apply, a default interest rate of 22% per annum would apply. In addition, if the Company failed to issue stock to the investor within three trading days of receipt of a notice of conversion, the Company must pay a penalty equal to the greater of greater of $500 per day and 2% of the applicable conversion amount or installment amount, as applicable (but, in any event, the cumulative amount of such late fees shall not exceed the applicable conversion amount or installment amount). The Notes were secured by an interest in all right, title, interest, claims and demands of the Company in and to the property described in the Security Agreement, and all replacements, proceeds, products, and accessions thereof.
F-22
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 5-
|
CONVERTIBLE NOTES AND DERIVATIVE LIABILITIES (CONTINUED)
|
The Notes were convertible into shares of our common stock in six tranches, consisting of (i) an initial tranche in an amount equal to $277,500 and any interest, costs, fees or charges accrued thereon or added thereto under the terms of this Note and the other Transaction Documents (as defined in the Securities Purchase Agreement), and (ii) five (5) additional tranches, each in the amount of $55,000, plus any interest, costs, fees or charges accrued thereon or added thereto under the terms of this Note and the other Transaction Documents. Except in the case of a Company default, the Notes are convertible by the investor at a price of $.40 per share. Concurrently with the Securities Purchase Agreement, the Company also issued to the investor warrants (the "Warrants") to purchase 690,625 shares of the Company’s common stock at an exercise price of $.75 per share subject to adjustment as more fully set forth in the warrant agreement. The Warrants also contains a cashless exercise provision. The Warrants are for a term of two (2) years.
In accordance with Financial Accounting Standards Board (“FASB”) ASC 815, Derivatives and Hedging, the embedded conversion option in the Convertible Note, as well as the Warrants issued by the Company, are required to be accounted for as derivative instrument liabilities. Such liabilities are initially and continuously carried at fair value with changes in their fair value reported in income in each reporting period. Accounting for the conversion option in the Convertible Note and for the Warrants as derivative instruments is required because both the Convertible Note and the Warrants have down-round anti-dilution protection, or ratchet exercise prices, whereby the conversion or exercise price is reduced if the Company subsequently issues common stock, convertible securities or stock options or stock warrants at a lower price or with a lower exercise or conversion price. Such a provision is inconsistent with the “fixed for fixed” nature of an equity option and therefore the instruments do not meet one of the required tests for equity classification. In addition, because the Convertible Note and the Warrants are denominated in a currency (U.S. dollars) that is different from the Company’s functional currency (Canadian dollars), they do not meet the test of being indexed only to the Company’s common stock. When one or more instruments are accounted for as derivative liabilities at fair value, the proceeds received are first allocated to the initial fair value of those derivative instruments, with any remaining proceeds allocated to the initial carrying value of the Convertible Note, which is accounted for at amortized cost. Interest is accrued on the initial carrying value of the Convertible Note at whatever effective interest rate is required in order to equate the present value of the expected future cash flows associated with the Convertible Note with their initial carrying value. Stated interest on the Note (10% per annum) is not accrued separately but is included in the effective interest rate on the Convertible Note.
The fair value of the embedded conversion option in the Note and the fair value of the Warrants have been calculated using the call option value output from a binomial Lattice model. A binomial Lattice model assumes that the price of the stock that underlies an option follows a probability distribution in which the underlying event only has one of two possible outcomes - the market price of the stock can either go up or go down in the future.
F-23
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 5-
|
CONVERTIBLE NOTES AND DERIVATIVE LIABILITIES (CONTINUED)
|
The Lattice valuation model takes into account all of the assumptions that market participants would likely consider in negotiating the transfer of the embedded conversion option and the Warrants, namely, stock price, exercise price, time to expiration, volatility, risk-free rate and dividends.
On April 28, 2014, the Convertible Note was issued as follows:
|
Total Convertible Note
|
$ | 552,500 | ||
|
Legal fees
|
( 2,500 | ) | ||
|
Original Issue Discount
|
( 50,000 | ) | ||
|
Net Convertible Note
|
$ | 500,000 |
Of the $500,000, the Company received net proceeds of $222,500 (after payment of legal fees of $2,500 and consideration of the original issue discount of $25,000).
As at April 28, 2014, the allocation of the debt facility was as follows:
|
Cash
|
$ | 222,500 | ||
|
Investor notes receivable
|
250,000 | |||
|
Assets recorded
|
472,500 | |||
|
Derivative Liabilities –
|
||||
|
Convertible notes – Investors
|
76,835 | |||
|
Warrants – Investors
|
200,715 | |||
| 277,550 | ||||
|
Convertible notes – Carrying value
|
58,541 | |||
|
Non-convertible notes – Carrying value
|
136,409 | |||
| 194,950 | ||||
|
Liabilities recorded
|
$ | 472,500 |
The Company between November 26, 2014 and December 31, 2014, repaid the entire convertible note balance of $277,500 thus extinguishing the note. Upon the intitail recording of the convertible note and warrants associated with the convertible note, a derivative liability was recorded as the convertible note and warrant each contained embedded derivatives as determined under ASC 815. Since the warrants associated with the convertible note remain outstanding as of December 31, 2014, the derivative liability associated with the warrants remain, however, the extinguishment of the debt resulted in a loss on extinguishment of $65,090 under ASC 470. The Company recognized a loss on the fair value of the derivative liabilities of $76,073 during the year ended December 31, 2014.
The effective interest expense for the Convertible Note for the year ended December 31, 2014 was $140,651.
F-24
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 6-
|
STOCKHOLDERS’ EQUITY
|
Preferred Stock
The Company was established on June 2, 2006 with 10,000,000 shares of preferred stock authorized with a par value of $0.001. The Company has not issued any preferred stock.
Common Stock
The Company was established on June 2, 2006 with 100,000,000 shares of common stock authorized with a par value of $0.001. On December 8, 2011, the Company amended the authorized stock to 150,000,000 shares. On March 17, 2014, the Company amended the authorized stock to 500,000,000 shares.
At incorporation, the Company issued 40,000,000 shares of common stock to the Company’s founders at par value of $40,000 for services rendered by the founder.
In November 2010, the Company issued 20,000,000 shares of common stock for $60,000 to investors ($0.003 per share – 33 investors).
In 2011, the Company issued:
|
|
·
|
5,000,000 shares of common stock on June 9, 2011 to acquire mining rights at a value of $15,000 which represented a per share value of $0.003 which was the price used in the November 2010 private placement. There was no change in the valuation of the Company from November 2010 to June 2011, therefore the same price was used;
|
|
|
·
|
5,000,000 shares of common stock on June 13, 2011 to board members for services at a value of $15,000 which represented a per share value of $0.003 which was the price used in the November 2010 private placement. There was no change in the valuation of the Company from November 2010 to June 2011, therefore the same price was used;
|
|
|
·
|
1,000,000 shares of common stock on June 13, 2011 for payment of interest on the promissory note of $3,000 which represented a per share value of $0.003 which was the price used in the November 2010 private placement. There was no change in the valuation of the Company from November 2010 to June 2011, therefore the same price was used;
|
|
|
·
|
3,350,000 shares of common stock from June 27, 2011 through October 20, 2011 for cash under a private placement. The Company issued the private placement at $0.20 per share to reflect the recent activity. There was no independent valuation report. The Company raised $670,000 for the shares of common stock.
|
|
|
·
|
1,500,000 shares of common stock on September 19, 2011 under employment agreements for a value of $300,000. The $0.20 value is the same value the Company used in raising funds under their private placement, and there were no changes to that value; and
|
F-25
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 6-
|
STOCKHOLDERS’ EQUITY (CONTINUED)
|
|
|
·
|
250,000 shares of common stock on October 20, 2011 to a consultant who assisted on the engineering of the building for a value of $0.20 per share amounting to $50,000. The $0.20 value is the same value the Company used in raising funds under their private placement, and there were no changes to that value
|
The methodologies, approaches and assumptions that the Company used are consistent with the American Institute of Certified Public Accountants, “Practice Guide on Valuation of Privately-Held Company Equity Securities Issued as Compensation”, considering numerous objective and subjective factors to determine common stock fair market value at each issuance date, including but not limited to the following factors: (a) arm’s length private transactions; (b) shares issued for cash as a basis to determine the value for shares issued for services to non-related third parties; and (c) fair value of service provided to non-related third parties as a basis to determine value per share. With respect to the sale of the securities identified above, the Company has relied on the exemption provisions of Section 4(2), Regulation S or Section 3(a) 10 of the Securities Act of 1933, as amended. The sale was made to a sophisticated or accredited investor, as defined in Rule 502, or were issued pursuant to a specific exemption.
In 2012, the Company issued:
|
|
·
|
6,924,000 shares of common stock from September 1, 2012 to December 31, 2012 for $1,731,000 through an S-1 registration statement at $0.25 per share.
|
In 2013, the Company issued:
|
|
·
|
1,576,000 shares of common stock from January 1, 2013 to February 28, 2013 for $394,000 through an S-1 registration statement at $0.25 per share. The shares were issued to thirteen different shareholders.
|
|
|
·
|
928,000 shares of common stock to nine different shareholders who purchased $232,000 through an S-1 registration statement at $0.25 per share at the end of December 2012. These shares were reflected as a liability for stock to be issued at December 31, 2012. The balance as of March 31, 2013 is $0.
|
|
|
·
|
2,500,000 shares of common stock on March 4, 2013 pursuant to an executed agreement with 754 2542 Canada Inc. to convert all amounts owing them, CDN$500,000 face value of the promissory note at $0.20 per share. The original promissory note executed between the parties was dated May 13, 2011. This exchange resulted in a loss on conversion of $125,000 and this is reflected in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2013.
|
F-26
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 6-
|
STOCKHOLDERS’ EQUITY (CONTINUED)
|
|
|
·
|
5,000,000 shares of common stock on March 15, 2013 to new Officers of the Company, pursuant to employment agreements, for value at $0.25 per share representing a total compensation expense of $1,250,000. 2,000,000 shares, valued at $500,000, vest immediately and 3,000,000 shares, valued at $750,000, vest quarterly over one year and are reflected as deferred compensation. As of December 31, 2013, $187,500 is reflected as deferred compensation on the consolidated balance sheet.
|
|
|
·
|
1,500,000 shares of common stock were cancelled on March 28, 2013.
|
|
|
·
|
200,000 shares of common stock pursuant to legal services rendered and 200,000 shares of common stock for investor relations valued at $126,000.
|
|
|
·
|
1,700,000 shares of common stock were cancelled on June 11, 2013.
|
|
|
·
|
2,208,000 shares of common stock from August 30, 2013 through October 16, 2013 for cash consideration of $523,564 (CDN$552,000) under a private placement. The Company issued the private placement at $0.25 per share.
|
|
|
·
|
On October 30, 2013 and November 27, 2013, the Company entered into binding agreements for the asset acquisitions of an undivided one hundred percent (100%) interest in certain mineral claims and mining assets located in the Province of Quebec’s Montauban and Chavigny townships near Grondines West, in the county of Portneuf, specifically Mining Lease BM 748 and Mining Concession CM 410.
|
|
|
|
The purchase price was CDN$75,000 together with the issuance of 1,050,000 common shares of the Company. The common shares for the acquisition were valued at their fair market value on the day they were issued which totaled $496,860. The transaction has not closed as of the date of these consolidated financial statements (see Note 11, Subsequent Events).
|
|
|
|
In connection with the asset purchase, the Company also issued 40,000 shares of common stock to a former supplier of the vendor for mining related information of the assets purchased valued at $20,000 along with cash consideration of CDN$20,000.
|
|
|
·
|
On November 26, 2013, the Company issued 500,000 common stock shares for investor relations. The common shares were valued at $224,900 representing their fair value on November 26, 2013.
|
|
|
·
|
50,000 shares of common stock on December 9, 2013 upon the exercise of 50,000 stock options by a Director of the Company, at an exercise price of $0.25, for total proceeds of $12,500.
|
F-27
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 6-
|
STOCKHOLDERS’ EQUITY (CONTINUED)
|
|
|
·
|
On December 23, 2013, the Company closed a flow-through financing transaction for $472,217 (CDN$500,000) from investors in exchange for 1,000,000 common stock shares and 500,000 warrants. The warrants are exercisable at $0.75 and expire in 2 years. Under Canadian law, the Company also paid finders’ fees of $50,000 in cash and 100,000 warrants that are exercisable at $0.50 and expire in 2 years. The flow-through financing requires the Company to spend substantially all of the funds raised on specific mining exploration activities.
|
|
|
In 2014, the Company issued:
|
|
|
·
|
During November and December 2014, 14,310,000 private placement shares were issued to investors for an amount totaling $715,500 ($0.05 per share).
|
|
|
·
|
On January 10, 2014, the Company issued 1,000,000 common shares in connection with an asset purchase valued at $340,000.
|
|
|
·
|
50,000 shares of common stock upon the exercise of 50,000 stock options by a Director of the Company, at an exercise price of $0.25, for total proceeds of $12,500.
|
|
|
·
|
400,000 shares were issued for services rendered evaluated at an amount of $121,500.
|
Also, 4,250,000 shares previously issued to directors or administrators were cancelled.
As of December 31, 2014, the Company has 106,586,000 shares of common stock issued and outstanding.
Stock Options
On August 12, 2013, the Company approved and enacted the 2013 Stock Incentive Plan (the “Plan”). Under the 2013 Stock Incentive Plan, the Company may grant options or share awards to its full-time employees, executive officers, directors and consultants up to a maximum of 8,000,000 common shares. Under the Plan, the exercise price of each option has been established at $0.25. Stock options vest as stipulated in the stock option agreement and their maximum term is 8 years.
F-28
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 6-
|
STOCKHOLDERS’ EQUITY (CONTINUED)
|
The following table summarizes information about the Company’s stock options:
|
December 31, 2014
|
December 31, 2013
|
|||||||||||||||
|
Weigthed
|
Weigthed
|
|||||||||||||||
|
average
|
average
|
|||||||||||||||
|
Number of
|
exercise
|
Number of
|
exercise
|
|||||||||||||
|
options
|
price
|
options
|
price
|
|||||||||||||
|
Options outstanding, beginning of period
|
1,283,000 | $ | 0.25 | - | $ | - | ||||||||||
|
Granted
|
- | - | 1,333,000 | 0.25 | ||||||||||||
|
Exercised
|
(50,000 | ) | (0.25 | ) | (50,000 | ) | (0.25 | ) | ||||||||
|
Forfeited
|
(1,033,000 | ) | (0.25 | ) | - | - | ||||||||||
|
Expired
|
- | - | - | - | ||||||||||||
| 200,000 | 0.25 | 1,283,000 | 0.25 | |||||||||||||
The following table summarizes the ranges of exercise prices of outstanding and exercisable options held by officers and directors as of December 31, 2014:
|
December 31, 2014
|
December 31, 2014
|
|||||||||||
|
Options Outstanding
|
Options Exercisable
|
|||||||||||
|
Weigthed
|
Weigthed |
Weigthed
|
Weigthed
|
|||||||||
|
average
|
average
|
average
|
average
|
|||||||||
|
Number of
|
remaining
|
exercise
|
Number of
|
remaining
|
exercise
|
|||||||
|
options
|
life (years)
|
price
|
options
|
life (years)
|
price
|
|||||||
|
200,000
|
6.70
|
$ |
0.25
|
200,000
|
6.70
|
$ |
0.25
|
|||||
The fair value of the stock options granted during the year ended December 31, 2014 amounted to $ - (2013-$239,930) and was determined using the Black-Scholes option–pricing model using the following weighted-average assumptions:
|
Risk-free interest rate
|
.32 | % | ||
|
Dividend yield
|
- | |||
|
Volatility
|
152.5 | % | ||
|
Expected life in years
|
2 years
|
|||
|
Exercise price
|
$ | 0.25 | ||
F-29
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 6-
|
STOCKHOLDERS’ EQUITY (CONTINUED)
|
Stock options-based compensation expense included in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2014 and 2013 was $59,240 and $119,942, respectively.
Warrants
The following table summarizes the Company’s share warrants outstanding as of December 31, 2014 and 2013:
|
December 31, 2014
|
December 31, 2013
|
|||||||||||||||||||||||
|
Weighted
|
Weigthed
|
Weighted
|
Weigthed
|
|||||||||||||||||||||
|
average
|
average
|
average
|
average
|
|||||||||||||||||||||
|
Number of
|
remaining
|
exercise
|
Number of
|
remaining
|
exercise
|
|||||||||||||||||||
|
warrants
|
life (years)
|
price
|
warrants
|
life (years)
|
price
|
|||||||||||||||||||
|
Warrants outstanding, beginning of year
|
600,000 | 2.0 | $ | 0.71 | - | - | $ | - | ||||||||||||||||
|
Granted
|
940,625 | 1.6 | 0.63 | 600,000 | 2.0 | 0.71 | ||||||||||||||||||
|
Exercised
|
- | - | - | - | ||||||||||||||||||||
|
Expired
|
- | - | - | - | - | - | ||||||||||||||||||
|
Warrants outstanding, end of year
|
1,540,625 | 1.5 | $ | 0.66 | 600,000 | 2.0 | $ | 0.71 | ||||||||||||||||
The following table summarizes the ranges of exercise prices of outstanding warrants as of December 31, 2014
|
December 31, 2014
|
||||||||||
|
Warrants Outstanding
|
||||||||||
|
Weighted
|
Weigthed
|
|||||||||
|
average
|
average
|
|||||||||
|
Number of
|
remaining
|
exercise
|
||||||||
|
options
|
life (years)
|
price
|
||||||||
| 250,000 | 0.7 | $ | 0.30 | |||||||
| 100,000 | 1.3 | 0.50 | ||||||||
| 1,190,625 | 1.6 | 0.75 | ||||||||
| 1,540,625 | 1.5 | $ | 0.66 | |||||||
F-30
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 7-
|
FIXED ASSETS
|
Fixed assets consist of the following as of December 31, 2014 and 2013:
|
Estimated
|
||||||||||||
|
Useful Lives
|
December 31,
|
December 31,
|
||||||||||
|
(Years)
|
2014
|
2013
|
||||||||||
|
Building
|
- | $ | - | $ | 1,089,415 | |||||||
|
Land
|
- | 108,974 | ||||||||||
|
Computers
|
5 | 5,868 | 4,424 | |||||||||
|
Office Equipment
|
5 | 14,711 | 14,711 | |||||||||
|
Mill Equipment
|
5 | - | 43,115 | |||||||||
|
Vehicle
|
5 | 25,127 | 25,127 | |||||||||
|
Assets retirement
|
107,749 | - | ||||||||||
|
Subtotal
|
153,455 | 1,285,766 | ||||||||||
|
Less: accumulated depreciation
|
(15,331 | ) | (9,462 | ) | ||||||||
|
Fixed assets, net
|
$ | 138,124 | $ | 1,276,304 | ||||||||
During the year ended December 31, 2014, the Company in accordance with Industry Guide 7, impaired $1,244,117 in land, building and the mill equipment as the Company is in the exploration stage and these assets should not be capitalized. Depreciation for the years ended December 31, 2014 and 2013 was $5,869 and $8,770, respectively.
|
NOTE 8-
|
DEFERRED FINANCING FEES
|
Deferred financing fees result from the issuance of share warrants as finders’ fees in connection with flow-through financing completed on December 23, 2013 and described in Note 3. The fair value of the warrants amounted to $25,431 and was determined using the Black-Scholes option–pricing model. The deferred financing fees are being amortized over the life of the warrants which is 2 years. Amortization of deferred financing fees for the year ended December 31, 2014 was $13,065.
|
NOTE 9-
|
PROVISION FOR INCOME TAXES
|
As of December 31, 2014, there is no provision for income taxes, current or deferred.
|
Net operating losses
|
$ | 2,164,429 | ||
|
Temporary and permanent tax differences
|
(378,000 | ) | ||
|
Valuation allowance
|
(1,786,429 | ) | ||
| $ | - |
F-31
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 9-
|
PROVISION FOR INCOME TAXES (CONTINUED)
|
At December 31, 2014, the Company had a net operating loss carry forward in the amount of $5,069,000, available to offset future taxable income through 2034. The Company has established a valuation allowance equal to the full amount of the deferred tax assets due to the uncertainty of the utilization of the operating losses in future periods.
A reconciliation of the Company’s effective tax rate as a percentage of income before taxes and the statutory rate for year ended December 31, 2014 is summarized below:
|
Canada
|
United States
|
||
|
Federal rate
|
12.6%
|
34.0%
|
|
|
State rate
|
-
|
0.0
|
|
|
Provincial rate
|
10.0
|
-
|
|
|
Mining duties
|
16.0
|
-
|
|
|
38.6
|
34.0
|
||
|
Valuation allowance
|
(38.6)%
|
(34.0)
|
|
|
0.0%
|
0.0%
|
|
NOTE 10-
|
LOAN PAYABLE / PROMISSORY NOTE |
The Company entered into a loan agreement with the Centre local de Développement (CLD) Mékinac on December 3, 2014 in the amount of CDN$150,000 that matures on November 22, 2019. The note had a default interest rate of 7.67% per annum, repayable in monthly instalments of $3,018 including interest. The loan is secured by a mortgage on the building. The balance at December 31, 2014 outstanding on this note was $127,523.
The note has the following maturities for the period ended December 31:
|
2015
|
$ | 22,209 | ||
|
2016
|
23,969 | |||
|
2017
|
25,872 | |||
|
2018
|
27,927 | |||
|
2019
|
27,546 | |||
|
Total
|
$ | 127,523 |
The Company entered into a promissory note with an investor on May 13, 2011 in the amount of CDN$500,000 that matures on May 31, 2014. The note had a default interest rate of 5% per annum should repayment not occur by the maturity date and the Company be in default of the promissory note agreement. In connection with the promissory note, the Company issued 1,000,000 shares of stock valued at CDN$3,000 in June 2011 for prepaid interest.
F-32
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
| NOTE 10- | LOAN PAYABLE / PROMISSORY NOTE (CONTINUED) |
On March 4, 2013, 754 2542 Canada Inc. executed an agreement with the Company whereby 754 2542 Canada Inc. agreed to accept 2,500,000 shares of common stock in satisfaction of all amounts due and owing 754 2542 Canada Inc. pursuant to the promissory note executed between the parties on May 13, 2011. As a result, the promissory note has been converted, and the Company recorded a loss on conversion of this note of $125,000 in the consolidated statement of operations.
|
NOTE 11-
|
COMMITMENTS |
The Company had the following financial commitments, represented by rental lease agreements, as of December 31, 2014:
|
Year ending December 31,
|
||||
|
2015
|
$ | 33,157 | ||
|
2016
|
20,688 | |||
|
2017
|
20,688 | |||
|
2018
|
20,688 | |||
|
2019
|
20,688 | |||
| $ | 115,909 | |||
Rent expense under the lease agreements for the years ended December 31, 2014 and 2013 were $40,005 and $40,385, respectively.
|
NOTE 12-
|
FAIR VALUE MEASUREMENTS
|
The Company adopted certain provisions of ASC Topic 820. ASC 820 defines fair value, provides a consistent framework for measuring fair value under generally accepted accounting principles and expands fair value financial statement disclosure requirements. ASC 820’s valuation techniques are based on observable and unobservable inputs. Observable inputs reflect readily obtainable data from independent sources, while unobservable inputs reflect our market assumptions. ASC 820 classifies these inputs into the following hierarchy:
Level 1 inputs: Quoted prices for identical instruments in active markets.
Level 2 inputs: Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable.
Level 3 inputs: Instruments with primarily unobservable value drivers.
The following table represents the fair value hierarchy for those financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2014:
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||
|
Cash and cash equivalents
|
$ | 53,813 | - | - | $ | 53,813 | ||||||||||
F-33
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
NOTE 12-
|
FAIR VALUE MEASUREMENTS (CONTINUED)
|
The derivative liabilities associated with the convertible notes that were repaid were measured at fair value using the binomial lattice options pricing model, and were classified within Level 3 of the valuation hierarchy. The Company recognized a loss of $64,949 on the fair value of the derivative liabilities for the year ended December 31, 2014.
|
NOTE 13-
|
SUBSEQUENT EVENTS
|
Breathe Acquisition and Spin-off of DNA Canada, Inc.
On January 16, 2015, Breathe eCigs Corp., a Tennessee corporation (“Breathe”), entered into a Share Exchange Agreement (the “Exchange Agreement”) with the Company, whereby the Company acquired all of the issued and outstanding shares of common stock of Breathe in consideration for the issuance of 150,000,000 shares of common stock.
As a result of the transaction effected by the Exchange Agreement, at closing Breathe became a wholly owned subsidiary of the Company, with the former Breathe shareholders owning approximately 56% of the then issued and outstanding common stock of the Company.
The Company declared a stock dividend to its shareholders of record as of February 3, 2015 of its wholly owned subsidiary, DNA Canada, Inc. Each shareholder of record on this date will receive one share of DNA Canada, Inc. for every two shares of DNA Precious Metals Inc. owned by the shareholder on this date. All stock dividends will be rounded down to the next whole number. With the completion of the stock dividend, the Company, no longer has an equity interest in DNA Canada, Inc.
The former shareholders of Breathe participating in the stock dividend were required to tender for redemption any shares of DNA Canada, Inc. common stock received pursuant to the stock dividend in accordance with the Exchange Agreement.
With the acquisition of Breathe, management determined that it would be in the best interest of the Company and its shareholders to operate each company separate and independently of each other. The operation of DNA Canada, Inc. and Breathe were inconsistent. Breathe is a manufacturer and distributor of e-cigarette and related products while DNA Canada, Inc. is an exploration stage mining company. The spin-off of DNA Canada, Inc. will allow each company to focus on its principal business activity and facilitate capital formation.
F-34
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 13-
|
SUBSEQUENT EVENTS (CONTINUED)
|
On March 5, 2015, the Company and Breathe entered into an Agreement and Plan of Merger pursuant to which the Company merged with its wholly owned subsidiary, Breathe. Upon the consummation of the Merger on March 11, 2015, the separate existence of Breathe ceased, and the shareholders of the Company became shareholders of the surviving company, named Breathe eCig Corp. As permitted under Nevada law, the sole purpose of the Merger was to effect a change to the Company’s name from DNA Precious Metals, Inc. to Breathe eCig Corp. This change to the Amended Articles of Incorporation and name change took effect on March 11, 2015.
The Company acquired the assets as noted below in consideration of the 150,000,000 shares in accordance with ASC 805. Based on the fair values at the effective date of acquisition the purchase price in was allocated as follows:
|
Net Assets Purchased
|
||||
|
Cash
|
$ | 7,301 | ||
|
Accounts payable and other current liabilities
|
(11,811 | ) | ||
|
Goodwill
|
9,004,510 | |||
|
Purchase Price
|
$ | 9,000,000 | ||
The goodwill will not be amortized but it will be tested annually for impairment.
With the spinoff of DNA Canada, Inc., in accordance with ASC 505-60-55-1, the following assets and liabilities were transferred to the new company in the form of a dividend:
|
Net Assets Spun-off
|
||||
|
Cash
|
$ | 25,514 | ||
|
Prepaid expenses
|
137,919 | |||
|
Sales tax receivable
|
17,097 | |||
|
Fixed assets
|
138,124 | |||
|
Mining rights
|
1,035,818 | |||
|
Accounts payable
|
(127,640 | ) | ||
|
Asset retirement
obligation
|
(107,749 | ) | ||
|
Note payable
|
(127,523 | ) | ||
|
Accumulated
comprehensive income
|
108,337 | |||
|
Due to DNAP
|
(5,288,703 | ) | ||
|
Adjustment to Additional Paid in Capital
|
$ | (4,188,806 | ) | |
F-35
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 13-
|
SUBSEQUENT EVENTS (CONTINUED)
|
The $5,288,703 represents the receivable that the Company would be due from DNA Canada, Inc. upon spinoff. This amount is not expected to be received as DNA Canada, Inc. does not have sufficient cash to repay the balance due. The Company has written off this receivable balance against the additional paid in capital of $4,188,806, and the remaining $1,099,897 will be reflected as a dividend.
The following table shows pro-forma results for the years ended December 31, 2014 and 2013 as if the acquisition had occurred on January 1, 2013. These unaudited pro forma results of operations are based on the historical financial statements and related notes of each of Breathe and the Company without DNA Canada, Inc. included.
|
For the years ended December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Revenues
|
$ | - | $ | - | ||||
|
Net loss
|
$ | (1,343,661 | ) | $ | (2,219,299 | ) | ||
|
Net loss per share
|
$ | (0.01 | ) | $ | (0.02 | ) | ||
Share Issuances
In January 2015, the Company issued 400,000 shares for $20,000, which represented the last of the issuance of shares related to the equity raise that occurred in November and December 2014.
The Company issued 9,150,000 shares for $457,500 in consulting services in January 2015.
The Company issued 2,500,000 shares for $125,000 in consulting services in February 2015.
The Company issued 5,050,000 shares for $189,375 in consulting services in March 2015.
The Company issued 2,666,667 shares for $100,000 in an investment in Tauriga Sciences, Inc (“TAUG”). The Company has entered into a license agreement with TUAG to jointly develop a new line of business involving CBD oil cartridges in March 2015. The Company received from TAUG 10,869,565 shares of TAUG common stock (with a value of $100,000) in exchange for their shares.
The Company received $50,000 at $0.03 per share (1,666,667 shares) in March 2015 under a subscription agreement with a company. The common shares have not been issued as of the date of this report.
Debt Issuances
On February 4, 2015, the Company entered into a convertible note in an amount up to $250,000 with an investor. The initial funding was in the gross amount of $27,500, with net proceeds received of $25,000. The $2,500 represents Original Issue Discount. The maturity date of this note is two years from the date that each tranche is paid. The note is convertible at the lesser of $0.065 or 60% of the lowest trade price in the 25 trading days previous to the conversion.
F-36
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 13-
|
SUBSEQUENT EVENTS (CONTINUED)
|
The Company may repay this note at any time on or before 90 days from the effective date (the date the Company receives the cash) of the note at no additional interest charge. If the note remains outstanding beyond the 90 days, there will be a one-time 12% interest charge applied in addition to the Original Issue Discount recognized at the onset of the note. The investor has the right to convert at any time after the effective date of this note.
On February 9, 2015, the Company entered into a convertible note in an amount of $110,000 with an investor. The gross amount of the note is $110,000, with net proceeds received of $100,000. The $10,000 represents Original Issue Discount. The note will also bear interest at 8%, compounded annually. The maturity date of this note is one year from execution. The note may be converted, in whole or in part, into shares of the Company’s common stock. The conversion price will be 60%, equivalent to a 40% discount, of the lowest trading price of the Company’s common stock during the 25 trading days prior to conversion. For defaults, the note is immediately due and payable and subject to a penalty interest rate of 20%.
The note may be prepaid according to the following schedule: Between 1 and 45 days from the date of execution, the note may be prepaid for 105% of face value plus accrued interest. Between 46 and 90 days from the date of execution, the note may be prepaid for 110% of face value plus accrued interest. Between 91 and 135 days from the date of execution, the note may be prepaid for 125% of face value plus accrued interest. Between 136 and 180 days from the date of execution, the note may be prepaid for 135% of face value plus accrued interest. After 180 days from the date of execution, the note may not be prepaid without written consent from the investor.
On March 6, 2015, the Company entered into an 8% convertible redeemable note with an investor in the amount of $31,500. The gross amount of the note is $31,500, with net proceeds received of $30,000. The $1,500 represents legal fees. This note matures on March 6, 2016. The investor is entitled, at its option, at any time, to convert all or any amount of the principal face amount of the note, then outstanding into shares of the Company’s common stock at a price for each share of common stock equal to 58% of the lowest trading price of the Company’s common stock as reported on the OTCQB for the fifteen prior trading days including the day upon which a notice of conversion is delivered.
The note may be prepaid according to the following schedule: Between 1 and 30 days from the date of execution, the note may be prepaid for 110% of face value plus accrued interest. Between 31 and 60 days from the date of execution, the note may be prepaid for 116% of face value plus accrued interest. Between 61 and 90 days from the date of execution, the note may be prepaid for 122% of face value plus accrued interest. Between 91 and 120 days from the date of execution, the note may be prepaid for 128% of face value plus accrued interest. Between 121 and 150 days from the date of execution, the note may be prepaid for 134% of face value plus accrued interest. Between 151 and 180 days from the date of execution, the note may be prepaid for 135% of face value plus accrued interest. After 180 days from the date of execution, the note may not be prepaid.
F-37
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 13-
|
SUBSEQUENT EVENTS (CONTINUED)
|
On March 11, 2015, the Company entered into an 8% convertible redeemable note with an investor in the amount of $52,500. The gross amount of the note is $52,500, with net proceeds received of $50,000. The $2,500 represents legal fees. This note matures on March 11, 2016. The investor is entitled, at its option, at any time, to convert all or any amount of the principal face amount of the note, then outstanding into shares of the Company’s common stock at a price for each share of common stock equal to 55% of the lowest trading price of the Company’s common stock as reported on the OTCQB for the fifteen prior trading days including the day upon which a notice of conversion is delivered.
The note may be prepaid according to the following schedule: Between 1 and 30 days from the date of execution, the note may be prepaid for 105% of face value plus accrued interest. Between 31 and 60 days from the date of execution, the note may be prepaid for 115% of face value plus accrued interest. Between 61 and 90 days from the date of execution, the note may be prepaid for 120% of face value plus accrued interest. Between 91 and 120 days from the date of execution, the note may be prepaid for 130% of face value plus accrued interest. Between 121 and 150 days from the date of execution, the note may be prepaid for 135% of face value plus accrued interest. Between 151 and 180 days from the date of execution, the note may be prepaid for 140% of face value plus accrued interest. After 180 days from the date of execution, the note may not be prepaid.
On March 13, 2015, the Company entered into an 8% convertible redeemable note with an investor in the amount of $52,500. The gross amount of the note is $52,500, with net proceeds received of $50,000. The $2,500 represents legal fees. This note matures on March 13, 2016.
The investor is entitled, at its option, at any time, to convert all or any amount of the principal face amount of the note, then outstanding into shares of the Company’s common stock at a price for each share of common stock equal to 55% of the lowest trading price of the Company’s common stock as reported on the OTCQB for the fifteen prior trading days including the day upon which a notice of conversion is delivered.
The note may be prepaid according to the following schedule: Between 1 and 30 days from the date of execution, the note may be prepaid for 105% of face value plus accrued interest. Between 31 and 60 days from the date of execution, the note may be prepaid for 115% of face value plus accrued interest. Between 61 and 90 days from the date of execution, the note may be prepaid for 120% of face value plus accrued interest. Between 91 and 120 days from the date of execution, the note may be prepaid for 130% of face value plus accrued interest. Between 121 and 150 days from the date of execution, the note may be prepaid for 135% of face value plus accrued interest. Between 151 and 180 days from the date of execution, the note may be prepaid for 140% of face value plus accrued interest. After 180 days from the date of execution, the note may not be prepaid.
On March 31, 2015, the Company entered into an 8% convertible redeemable note with an investor in the amount of $105,000. The gross amount of the note is $105,000, with net proceeds received of $100,000. The $5,000 represents legal fees. This note matures on March 31, 2016.
F-38
BREATHE ECIG CORP.
(FORMERLY DNA PRECIOUS METALS, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014 AND 2013
|
NOTE 13-
|
SUBSEQUENT EVENTS (CONTINUED)
|
The investor is entitled, at its option, at any time, to convert all or any amount of the principal face amount of the note, then outstanding into shares of the Company’s common stock at a price for each share of common stock equal to 55% of the lowest trading price of the Company’s common stock as reported on the OTCQB for the fifteen prior trading days including the day upon which a notice of conversion is delivered.
The note may be prepaid according to the following schedule: Between 1 and 30 days from the date of execution, the note may be prepaid for 105% of face value plus accrued interest. Between 31 and 60 days from the date of execution, the note may be prepaid for 115% of face value plus accrued interest. Between 61 and 90 days from the date of execution, the note may be prepaid for 120% of face value plus accrued interest. Between 91 and 120 days from the date of execution, the note may be prepaid for 130% of face value plus accrued interest. Between 121 and 150 days from the date of execution, the note may be prepaid for 135% of face value plus accrued interest. Between 151 and 180 days from the date of execution, the note may be prepaid for 140% of face value plus accrued interest. After 180 days from the date of execution, the note may not be prepaid.
In accordance with the warrant agreement as described in Note 5, the warrant price will be reset to equal the conversion price associated with these new debt agreements from the stated strike price of $0.75.
Distribution Agreements
The Company in March 2015 entered into Distribution Agreements with various distributors for the distribution of the Company’s products. The Company placed an intial order for merchandise in the amount of $327,200 on March 27, 2015. The Company prepaid $98,160 (30%) of this order on March 27, 2015.
F-39
