Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REPROS THERAPEUTICS INC. | v406790_8-k.htm |
Exhibit 99.1

Enclomiphene ( fka Androxal®) A Mechanistically Consistent Approach to the Treatment of Secondary Hypogonadism

Repros Disclaimer Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to various risks, uncertainties and other factors that could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by such forward - looking statements . These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “believe,” “plan,” “seek,” “could,” “can,” “should” or similar expressions . These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current conditions, expected future developments and other factors the Company believes are appropriate in these circumstances . Forward - looking statements include, but are not limited to, those relating to anticipated milestones for Androxal® and Proellex®, the conduct of planned clinical studies and the timing and nature of the results thereof, the markets for the Company’s products and the potential success of the Company in penetrating those markets and that the Company’s need for and use of financial resources . Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factors that may cause actual events to be materially different from those expressed or implied by such forward - looking statements, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of its Androxal® and Proellex® programs, the ability to have success in the clinical development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks as are identified in the Company's most recent Annual Report on Form 10 - K and the subsequent quarterly report on Form 10 - Q and in the prospectus supplement and the accompanying prospectus included in the registration statement mentioned below . These documents are available on request from Repros or at www . sec . gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise . In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry . We obtained this information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates . Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information . While we believe that each of these studies and publications is reliable, we have not independently verified such data, and we make no any representation as to the accuracy of such information . Similarly, we believe our internal research is reliable, but it has not been verified by any independent sources . 2


Androxal (Patented) Trans Isomer (Pure Estrogen Antagonist) of Clomiphene Commercial Clomid (60% Trans, 40% Cis) 0 20 40 60 80 100 0.01 1 100 10000 % Concentration ( nM ) Estrogen Receptor Binding Affinity zu cis Trough Concentration. of Androxal In Serum at Steady State Achieved within 10 Days Of Administration Cis Isomer Exhibits Agonist/Antagonist Activity Mikkelson et al, Fertility & Sterility, Vol. 46, No. 3, Sept. 1986 In animal studies the zu isomer achieves > 10 fold concentrations in serum and prolonged residence in all tissues including the eye and male reproductive tissues

Clomiphene Isomers Exhibit Different Impact on Animal Urogenital Tract Tissues 0 5 10 15 20 25 Blood Testis Epididymis Seminal vesicles Kidneys ng Equivalent [ 14 C] clomiphene isomers /Kg Zuc 4 Hr Zuc 24 Hr Enc 4 Hr Enc 24 Hr

Enclomiphene Regulatory Status • NDA accepted by FDA – Treatment of secondary hypogonadism in overweight men – NDA filing requests NCE (New Chemical Entity) status • EMA (European Medicines Agency) notes enclomiphene is eligible for submission for a centralized marketing authorisation application (MAA) as a New Active Substance – MAA anticipated filing in 2016 – If approved potential for extended patent life

How Androxal Works A ndroxal blocks estrogen at the level of the H - P axis increasing LH levels which in turn increases endogenous production of T 0 1 2 3 4 5 6 7 Endroxal LH Androgel LH LH ( mIU /mL) Impact on LH in 16 Wk Study ZA - 304 Baseline End of Study 0 50 100 150 200 250 300 350 400 450 500 Endroxal T Androgel T Morning T (ng/ dL ) Impact on T in 16 Wk Study ZA - 304 Baseline End of Study p<0.0001 p=0.0007

ZA - 304 & ZA - 305 Results Androxal Yields Meaningful Reproducible Outcomes Study ZA - 304 (p - values Treatment vs A ndroxal ) ZA - 305 (p - values Treatment vs A ndroxal ) Treatment Androxal Androgel Placebo Androxal Androgel Placebo N 41 43 45 44 42 41 Age (SD) 49.1 (7.4) 47.4 (7.2) 47.2 (9.0) 47.3 (8.8) 45.0 (8.2) 47.5 (8.9) BMI 33.1 (4.4) 34.0 (4.4) 32.6 (4.3) 33.8 (4.6) 33.1 (4.6) 33.5 (4.4) Baseline T (ng/dL) 203.3 (52.4) 208.6 (54.0) 200.3 (43.1) 212.9 (48.0) 229.8 (44.0) 206.0 (48.2) Baseline Sperm (10 6 /ml) 98.3 (87.2) 78.9 (68.5) 95.3 (90.8) 79.0 (55.2) 75.1 (45.8) 80.5 (62.3) 16 Wk T 445.8 (186.4) 350.6 (338.1)) p = 0.0007 236.4 (144.7) p < 0.0001 412.9 (130.3) 387.4 (244.8) p = 0.0368 214.4 (58.7) p < 0.0001 % Change Sperm @ 16 Wk 11.7 (80.3) - 56.6 (48.2) p < 0.0001 4.1 (57.2) p = 0.5482 15.2 (55.8) - 32.8 (63.2) p = 0.0003 - 7.6 (89.6) p = 0.2007 % Sperm < 15 MM/mL 4.9 48.8 p < 0.0001 4.4 p = 1.0000 2.3 23.8 p = 0.0031 2.4 p = 1.000

Androxal Exhibits No Negative Effects on Spermatogenesis Unlike Exogenous T -70 -60 -50 -40 -30 -20 -10 0 10 20 % Change from Baseline Impact on Sperm Concentration After 16 Wks Treatment ZA - 304 Endroxal Androgel Placebo 0 10 20 30 40 50 60 % of Oligospermic Subjects % of Subjects Becoming Oligospermic After 16 Wks Treatment ZA - 304 Endroxal Androgel Placebo Subjects on Androgel exhibited shrinkage of testes after 16 wks compared to Androxal (p=0.014) p<0.0001 p<0.0001

Androxal Averages > 75 % of Men Studied in Normal Range • 10 studies conducted with durations up to 1 year • Table determined as LOCF for morning T Study Subjects Receiving Androxal Study Duration % Morning T in Normal Range (LOCF) ZN - 018 22 2 Wks. 77.3 ZA - 003 97 3 Mo. 63.9 ZA - 203 57 3 Mo. 68.4 ZA - 204 31 6 Wks. 93.5 ZA - 300 499 6 Mo. 81.3 ZA - 301 112 18 Wks. 83.9 ZA - 302 134 18 Wks. 80.6 ZA - 303 213 1 Yr. 69.9 ZA - 304 41 16 Wks. 80.0 ZA - 305 44 16 Wks. 70.7 Summary 1250 77.2

What Predicts Response? • How long has subject been diagnosed as secondary hypogonadal? – Obesity suppresses responsiveness of the HPT axis • Previous TRT lessens response to Androxal – Drastically suppresses HPT axis • Should intervention in the obese male start sooner – Age related morning T – T < 300 ng/dL, “Is that an appropriate starting point for men < 50 years of age?”

Initial Screening Findings for ZA - 304 & 305 Age ≤ 60, BMI > 25 Category Number of Men Age Mean ( StDev ) T ng/ dL ** Mean ( StDev ) BMI Mean ( StDev ) Total Screened 642 47.5 (8.6) 267 (83.6) 33.3 (4.6) Randomized 256 47.3 (8.3) 237 (43.8) 33.4 (4.4) Screen Failed 386 BMI>42 2 48 (5.7) - - Eye Exam (cataracts, etc.) 10 49.2 (5.4) 228 (31.2) 35.2 (5.8) LH High (primary failure?) 13 51.1 (7.6) 214 (44.4) 36.8 (3.9) PSA 7 52.7 (9.4) 251 (36.5) 31.6 (1.4) Sperm Conc. <15x10 6 /mL* 86 49.0 (8.3) 223 (56.5) 34.4 (4.7) Testosterone >300 ng/ dL 156 (24%) 46.4 (9.2) 372 (79.7) 32.0 (4.9) Other 112 47.5 (8.8) 228 (55.6) 33.6 (5.1) * 9 subjects with LH below normal suggesting previous T use ** Maximum T during screening assessments

Overweight Men Exhibit Lower Than Normal T Compared to Aged Matched Controls 0 20 40 60 80 100 N= Distribution of Morning T for Men Screen Failing Due to High T Mean Age: 46.3 100 300 500 700 900 Framingham Heart Study 40- 49 Framingham Heart Study 50- 59 European Male Aging Study 40-49 European Male Aging Study 50-59 ZA-304 & 305 Morning T ng/dL Comparative T Mean ± StDev ZA - 304 & ZA - 305 Vs Two Published Studies

Influence of Prior Testosterone on Response ZA - 300, 6 month study
Androxal Exhibits Unique Profile with Numerous Advantages vs Approved Hormone Replacement • The Androxal Advantages – Oral – Not controlled substance, cannot be abused – No supernormal levels of T achieved – No transference risk – Restores normal function (no loss of testicular function) – Does not develop dependency – Avoids withdrawal symptoms – With lifestyle change can reverse disorder and result in no need for therapy

$ Sales of Testosterone and Clomiphene 0 100 200 300 400 500 600 700 0 50000 100000 150000 200000 250000 Clomiphene Retail Sales $(000) Testosterone Retail Sales $(000) Monthly Sales (January 2013 – November 2014) Testosterone clomiphene

Androxal US Commercial Plan/Schedule • Q2 - 15 – Orders placed for API and Drug Product – KOL Board in place and active – Publications and Scientific Mtgs – Hire Chief Commercial Officer • Q3 - 15 – Finish preparation for Adcom – Adcom ? – Continue Education – Commence pre - launch activities • Q4 - 15 – Build launch inventory – Award sales contract • Q1 - 16 PDUFA date • Q2 - 16 – Launch into Endo & Uro Spec space (~ 25% of all prescriptions)
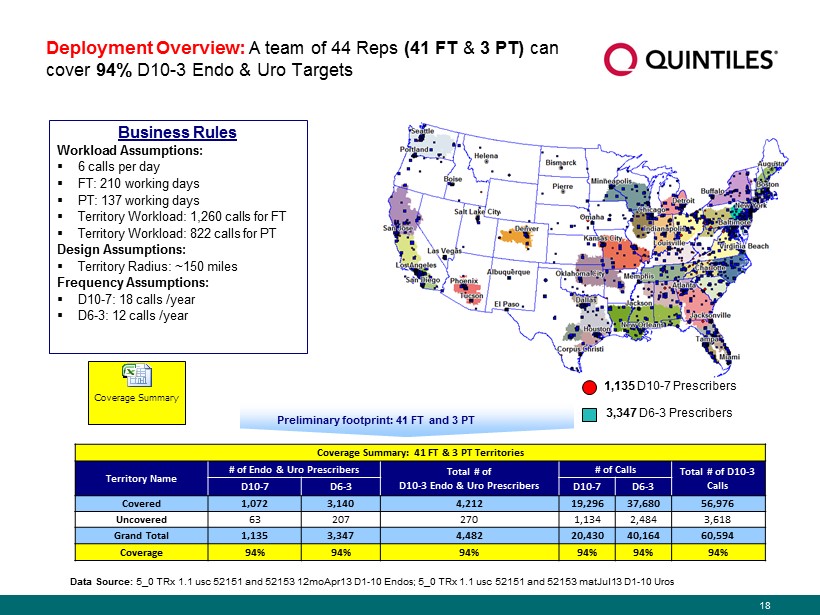
18 Preliminary footprint : 41 FT and 3 PT Business Rules Workload Assumptions : ▪ 6 calls per day ▪ FT: 210 working days ▪ PT: 137 working days ▪ Territory Workload: 1,260 calls for FT ▪ Territory Workload: 822 calls for PT Design Assumptions : ▪ Territory Radius: ~150 miles Frequency Assumptions: ▪ D10 - 7: 18 calls /year ▪ D6 - 3: 12 calls /year Deployment Overview : A team of 44 Reps ( 41 FT & 3 PT) can cover 94% D10 - 3 Endo & Uro Targets 1,135 D10 - 7 Prescribers 3,347 D6 - 3 Prescribers Data Source: 5_0 TRx 1.1 usc 52151 and 52153 12moApr13 D1 - 10 Endos; 5_0 TRx 1.1 usc 52151 and 52153 matJul13 D1 - 10 Uros Coverage Summary Coverage Summary: 41 FT & 3 PT Territories Territory Name # of Endo & Uro Prescribers Total # of D10 - 3 Endo & Uro Prescribers # of Calls Total # of D10 - 3 Calls D10 - 7 D6 - 3 D10 - 7 D6 - 3 Covered 1,072 3,140 4,212 19,296 37,680 56,976 Uncovered 63 207 270 1,134 2,484 3,618 Grand Total 1,135 3,347 4,482 20,430 40,164 60,594 Coverage 94% 94% 94% 94% 94% 94%

NOTES : 1. The prior assumptions and caveats under the original scenario remain. 2. We believe that the only material change to these expenses are related to the size of the Sales Team. We have kept other expenses the same. Commercial Function Pre - Launch (Launch Minus 18 Months) Launch Year Launch Year + 1 Total Executive & Mktg Team $1,700,000 $1,300,000 $1,350,000 $4,350,000 MSL Team (including P/T) $1,190,000 $1,730,000 $1,890,000 $4,810,000 Sales Team (including P/T) $9,700,000 $9,400,000 $19,100,000 Trade / Managed Markets $875,000 $1,350,000 $1,350,000 $3,575,000 Med Info / Pharmacovigilance $100,000 $200,000 $200,000 $500,000 TOTAL $3,865,000 $ 14,280,000 $ 14,190,000 $32,335,000 Marketing Budget Estimate $1,000,000 $10,000,000 $10,000,000 $21,000,000 Total Commercial Budget Necessary $4,865,000 $24,280,000 $24,190,000 $53,335,000 New Endo & Uro Scenario Expense Budget 19

Proellex Status and Plans Going Forward • Ongoing Phase 2b Proellex studies – Uterine fibroids (vaginal and oral administration studies) • Rapid and maintained reduction in bleeding • Reduction of fibroid bulk • Reduction of bulk associated symptoms – Endometriosis • Rapid reduction in menstrual and non - menstrual pain • Significant reduction in narcotic and non - narcotic analgesic use • Proellex safety profile very encouraging at lower doses – General adverse events are mild to moderate and transient • Request Type C meeting with FDA to discuss overall exposure for safety late 2015 pending enrollment
Financial Summary • Cash and equivalents: (audited year - end 2014) $46.6 M • Estimated Burn in 2015 : $22.0 M • Cash runway: Through 2016 – Anticipate Androxal approved • Current shares outstanding: 24.3 M shares – Warrants Outstanding – Series A – 39,595 (purchased in unit deal @ $2.45); Series B – 429,704 @ $2.49 exercise price.
