Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - SurePure, Inc. | Financial_Report.xls |
| EX-32.2 - EXHIBIT 32.2 - SurePure, Inc. | v400934_ex32-2.htm |
| EX-31.1 - EXHIBIT 31.1 - SurePure, Inc. | v400934_ex31-1.htm |
| EX-32.1 - EXHIBIT 32.1 - SurePure, Inc. | v400934_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - SurePure, Inc. | v400934_ex31-2.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT
(Mark One)
þAnnual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2014
¨Transition Report under Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from to
SurePure, Inc.
(Exact name of registrant as specified in its charter)
Nevada
(State or other jurisdiction of
Incorporation |
3556
(Primary Standard Industrial |
26-3550286
(I.R.S. Employer Identification No.) |
405 Lexington Avenue, 25th Floor
New York, New York 10174
Registrant’s telephone number, including area code:
(917) 368-8480
Securities registered pursuant to section 12(g) of the Act:
Title of Class: Common stock, par value $0.001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data file required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| ¨ Large Accelerated Filer | ¨ Accelerated Filer | ¨ Non-Accelerated Filer | þ Smaller Reporting Company |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes¨ No þ
The aggregate market value of voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter: $16,754,928
The number of shares of the registrant’s Common Stock, par value $0.001 per share, outstanding as of March 30, 2015 was 47,345,816.
DOCUMENTS INCORPORATED BY REFERENCE
None.
SUREPURE, INC.
| 2 |
Cautionary Note Regarding Forward-Looking Statements
Certain statements included in this annual report on Form 10-K, other than statements of historical fact, are forward-looking statements (as such term is defined in Section 27A of the Securities Act and Section 21E of the Exchange Act, and the regulations promulgated thereunder), which are intended to be covered by the safe harbors created thereby. Forward-looking statements contained in this annual report on Form 10-K can be identified by the use of terms and phrases such as “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect,” and the like, and/or future-tense or conditional constructions “may,” “could,” “should,” etc. In addition, statements that contemplate or make assumptions about actual or potential future sales, market size, collaborations, and trends or operating results also constitute forward-looking statements. These forward-looking statements involve risks and uncertainties and are based on the beliefs and assumptions of our management and on information available at the time these statements and disclosures were prepared. Forward-looking statements include, but are not limited to:
| · | the availability, if any, of equity or debt financing to us, our access to operating capital and the period of time after the date of this annual report on Form 10-K for which the proceeds from private financings will enable us to fund our operations and other costs and expenses; and |
| · | the rate at which the Company’s proprietary technology is commercialized and generates significant revenues. |
In addition to these risks and those identified under “Item 1A. Risk Factors,” many important factors affect our ability to achieve our plans and objectives and to successfully develop and commercialize our technology and the products that utilize it, including our ability:
| · | to obtain funds to operate our business and pay other expenses; |
| · | to enforce our patents against infringers; |
| · | to demonstrate the safety and effectiveness of our technology in the purification of liquids; |
| · | to meet applicable regulatory standards and receive required regulatory approvals; |
| · | to manufacture and distribute Turbulator systems in commercial quantities at reasonable costs; and |
| · | to compete successfully against other products and to market products in a profitable manner. |
Therefore, current and prospective stockholders are cautioned that there can be no assurance that the forward-looking statements included in this annual report on Form 10-K will prove to be accurate. In light of the significant uncertainties inherent to the forward-looking statements included in this annual report on Form 10-K, the inclusion of such information should not be regarded as a representation or warranty by the Company or any other person that the objectives and plans of the Company will be achieved in any specified time frame, if at all. Except to the extent required by applicable laws or rules, the Company does not undertake any obligation to update any forward-looking statements or to announce revisions to any of the forward-looking statements.
| 3 |
CERTAIN TERMS USED IN THIS REPORT
As used in this annual report on Form 10-K, unless the context otherwise requires, “SurePure,” the “Company,” “we,” “us” and “our” refer to SurePure, Inc., a Nevada corporation, as well as its subsidiaries, SurePure Investment Holding AG, a Swiss corporation, SurePure Operations AG, a Swiss corporation, SurePure Participations, AG, a Swiss Corporation, and SurePure Latin America—Maquinas de Purificação U.V.C. LTDA, a Brazilian private company, and also refer to SurePure Holdings South Africa (Pty) Ltd. and SurePure Marketing South Africa (Pty) Ltd., both of which are private South African companies.
The following names refer to the following entities:
| “SPI” | means SurePure Investment Holding AG, a Swiss corporation that serves as the holding company and contains our international administrative and financial functions; | |
| “SPO” | means SurePure Operations AG, a Swiss corporation that is our principal operating business and holds all of our patents other than the patent issued in South Africa; | |
| “SPP” | means SurePure Participations AG, a Swiss corporation, a company formed by XOptics to hold certain shares of SPI; and | |
| “SPHSA” | means SurePure Holdings South Africa (Pty) Ltd., a South African private company that serves as the holding company for SPMSA and owns the patent issued in South Africa; | |
| “SPMSA” | means SurePure Marketing South Africa (Pty) Ltd., a South African private company that commercializes our technology in South Africa and contains the administrative and financial functions of our business in South Africa; | |
| “SPLAM” | means SurePure Latin America—Maquinas de Purificação U.V.C. LTDA, a Brazilian private company that commercializes our technology in Latin America; |
| 4 |
We use other defined terms throughout this annual report on Form 10-K, including the following:
| · | “Commission” means the U.S. Securities and Exchange Commission; |
| · | “Exchange Act” means the U.S. Securities Exchange Act of 1934, as amended; |
| · | “Nonvoting Convertible Preferred Stock” means our Nonvoting Convertible Preferred Stock, par value $.01 per share; |
| · | “Regency” means Regency Capital Corporation, a company formed under the laws of the Turks and Caicos Islands; |
| · | “Securities Act” means the U.S. Securities Act of 1933, as amended; |
| · | “Share Exchange” means the exchange of shares of SPI for shares of our common stock, which occurred on December 12, 2012, under the terms and conditions of the Share Exchange Agreement; |
| · | “Share Exchange Agreement” means the Amended and Restated Share Exchange Agreement that we entered into on December 12, 2012, under which we are obligated to issue shares of our common stock to the shareholders of SPI; |
| · | “SurePure Photopurification Technology” means our proprietary Turbulator systems for liquid photopurification technology; |
| · | Our “Technology” has the same meaning as “SurePure Photopurification Technology”; |
| · | “Trinity” means Trinity Asset Management Ltd. of Cape Town, South Africa; and |
| · | “XOptics” means XOptics (PTC), Ltd., a private company formed under the laws of the British Virgin Islands. |
All references in this annual report on Form 10-K to “$” shall be to U.S. Dollars. All references in this annual report on Form 10-K to “CHF” shall be to Swiss Francs. All references in this annual report on Form 10-K to “ZAR” shall be to South African Rands.
Rounding
Certain figures included in this annual report on Form 10-K have been rounded for ease of presentation. Percentage figures included in this annual report on Form 10-K have not in all cases been calculated on the basis of such rounded figures but on the basis of such amounts prior to rounding. For this reason, certain percentage amounts in this annual report on Form 10-K may vary from those obtained by performing the same calculations using the figures in our financial statements. Certain other amounts that appear in this annual report on Form 10-K may not sum due to rounding.
| 5 |
Item 1. Description of the Business
Overview
The Company designs, manufactures, markets, sells or licenses and maintains its proprietary Turbulator systems for innovative liquid photopurification technology in the global marketplace (“SurePure Photopurification Technology” or our “Technology”). We have been engaged in these activities since 2005. Currently, our Technology uses Ultraviolet (“UV”) light in the C band (“UVC”) to process, preserve and sustain the natural quality of food ingredients, such as liquid egg and animal feed constituents, and beverage products, such as juices and concentrates, sugar syrup bases, alcoholic beverages and farm milk. In addition to the foregoing applications now in use, our SurePure Photopurification Technology is being tested for its capacity to reduce the microbial loads in turbid liquids intended for human consumption, such as dairy products, flavored water and coconut water, liquids with industrial applications, such as diesel and bio-ethanol, and liquids with pharmaceutical applications, such as eye preparations, saline drips and personal care products.
The use of UVC light energy with a specific wavelength to disinfect food surfaces, water and other beverages is well accepted. In 2001, the U.S. Food and Drug Administration (“FDA”) amended its regulations to permit the use of UVC light as a means of purification for water and fruit juices under certain conditions. There are multiple systems that effectively use UVC to purify or disinfect water and transparent liquids. Beverages and other liquids, such as milk and fruit juice, and water and other liquids used in industrial processes, are cloudy, opaque and contain suspended matter, and are often referred to as “turbid.” The ability to use UVC to purify turbid liquids requires technology that passes limited amounts of liquid at high flow rates very close to the UVC light source so that microbes and other pathogens in the liquid are exposed most directly and intensely to UVC waves.
It is our goal to secure approval for our Technology in 2015 and 2016 in those markets where approval is a precondition for the commercial implementation, as well as to market and distribute our SurePure Photopurification Technology on a worldwide basis. After concluding research and successfully testing at various research institutions during the past several years, we are seeking approval from the FDA and EU Novel Food Clearance for use of the SurePure Photopurification Technology as an adjunct to and in certain cases as an alternative for, pasteurization in dairy applications and from the International Organisation of Vine and Wine for the purification of wine. We are simultaneously increasing the general awareness of our Technology through demonstrations at trade shows and exhibitions of our Technology to selected customers who are successfully running internal tests.
Based on research that we have sponsored, we believe that our SurePure Photopurification Technology improves beverage and liquid food quality and safety by safely disinfecting these products beyond existing technology, such as conventional pasteurization. Because our Technology does not require heat to be generated for purification to occur and therefore requires less energy to operate, we also believe that it enables processors of food, beverages and industrial liquids that use our Technology to reduce their operating expenditures. Our research also leads us to believe that our SurePure Photopurification Technology can be applied to virtually every consumable beverage, many industrial and processing liquids and any liquid pharmaceutical products that may suffer from microbiological contamination. Consequently, the markets that we serve can encompass all industries that manufacture or distribute consumable beverages, industries that use water or other liquids in industrial processes in the manufacturing or processing of foods and beverages and industries that produce liquids that must meet standards for high levels of purification.
| 6 |
Our Industry
Currently, conventional means for purifying dairy products, juices, wine, beer and other beverages are pasteurization and chemical. Pasteurization was developed in the nineteenth century and received widespread adoption in the late nineteenth and twentieth centuries. Pasteurization operates by heating milk or other liquid to a specific temperature level (generally, 73° C or 164° F, although the exact temperature will vary according to need) and holding the temperature constant for a specified period of time, during which period pathogen cells are ruptured as a result of the heat transferred by the liquid. As a result of the heating process, pasteurization transforms components of the liquid, including taste and other sensory attributes, and does not remove the ruptured microbes from the fluid. In order to create sufficient heat to raise the temperature of the liquid to meet pasteurization standards, large amounts of energy must be consumed and CO2 emissions are created.
Another principal means for purifying liquids has been the addition of certain chemicals, such as sulfites and other preservatives. The addition of these elements often affects the taste and consistency of the liquid or food to be treated. Published research concludes that the additives used to achieve purification or preservation may lead to unhealthy or otherwise undesirable side effects among certain consumers.
Notwithstanding the general effectiveness of pasteurization and other means of purification, certain spore-forming bacteria, such as Bacillus, remain a serious concern in the food industry, since they can survive heat, chemicals, desiccation and radiation and can cause food poisoning and spoilage. Certain species of Bacillus, such as B. sporothermodurans, are heat resistant and can survive the process of pasteurization. We believe that the food and dairy industries are seeking alternative methods to render their products microbiologically safe for consumption against the widest possible range of microorganisms.
Our Technology
UVC occupies the light spectrum with wavelengths of 10 nanometers to 290 nanometers. UVC has been successfully used as a process for the disinfection of air, water and surfaces. In recent years, scientists have studied UVC irradiation technology as an alternative to heat (pasteurization) or chemically-based processes (preservatives) that currently are being used in the purification and/or sterilization or preservation of foods and liquids. Our Technology uses UVC waves to purify microbiologically sensitive turbid (cloudy or hazy fluids caused by individual particles (or suspended solids)) that are generally invisible to the naked eye by inactivating contaminating microorganisms, such as bacteria, yeasts, moulds and viruses in treated liquids. The UV wavelength most effective for killing microorganisms is between 254 and 260 nanometers. Based on this, our Technology employs UVC radiation at the germicidal wavelength of 254 nanometers of sufficient energy and duration to inactivate contaminating pathogens, including viruses. On the basis of research that we have sponsored, we believe that since our Technology transfers energy directly to microbial DNA, it also limits secondary effects on liquid proteins, enzymes and other components and concomitantly minimizes effects on nutritional, sensory and marketing attributes of the product being purified.
| 7 |
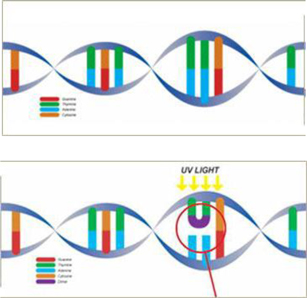
UVC light can penetrate the outer cell membranes of microorganisms, such as viruses, bacteria, mold and mildew. Exposure to UVC causes the DNA molecules of the microorganisms to become fused, or mutated. The mutations further result in the death of the organisms as their metabolic functions such as replication cannot be completed after the mutation results. In addition to being safe, our process is non-thermal, chemical-free, fast and replicable.
Our Product
We provide our Technology, the Turbulator, to commercial clients for use in their plants. The Turbulator is a mechanical tube system within a double-walled stainless steel cylinder that creates turbulation in the fluid, moves the liquid to be purified in spiral flows over the source of the UVC at a specified distance from the source which maximizes the efficacy of the UVC waves, and ensures that all the liquid is exposed to the UVC source. Existing laminar flow systems, in which the liquid passes across a light source in sheets, do not allow for the sustained, controlled exposure that is necessary for effective purification, particularly for turbid liquids. The Turbulator system can be installed in parallel or series, resulting in a system that uses multiple lamps, with a modular approach enabling the treatment of a number of liquids requiring varying levels of exposure and flow rates. The proprietary design of the Turbulator is based on an internal lamp which is inserted into a quartz sleeve which in turn is placed inside a metal housing. Because of the proximity of the light source to the target liquid within the housing, there is a very high level of energy transfer to individual micro-organisms within the target liquid. The Turbulator system includes a data recording component which records quality assurance and other critical data relating to its operation.
| 8 |
We have designed the Turbulator system to maximize the range of liquids that our Technology can purify. The color and turbidity of the liquid being treated plays a role as UVC can only penetrate roughly 1 mm into juice and even less so into milk. The efficacy of the UVC is therefore dependent on the properties of the liquids being treated, including the color, viscosity, absorbance and presence of suspended solids. The Turbulator has been designed so that the necessary adjustments can be made to optimize its effectiveness in relation to the properties of the liquids being treated. Moreover, because of the design of the Turbulator and its components, our system can treat liquids where the use of heat as a purifying agent would be detrimental to the product. The Turbulator also allows the processor to set the precise exposure of a treated liquid to the UVC source, so that the exposure can match the exact purification requirements of the liquid.
The principal benefit of our SurePure Photopurification Technology is that it purifies liquids by inactivating pathogens without the use of heat energy. Because of the proprietary characteristics of the Turbulator system that control the flow of liquid over the UVC source, the Technology has been effective on all pathogens for which we have performed tests, including those that we believe are resistant to heat. In addition, we believe that our product has other benefits as well:
| · | Because our Turbulator systems do not rely on the generation of heat or other intense energy to perform purification, they consume substantially less power and therefore lead to less power-related expense, both in terms of power inputs and cooling needs; |
| · | Because our Turbulator systems require substantially less power than conventional means of heat-based purification, they substantially reduce the carbon footprint of the process; |
| · | Because the liquids that have been purified have not been subject to significant change in temperature, the quality of the end product remains intact; |
| · | For most liquids treated by our Technology, shelf life improves because we have inactivated the microbes and spores that result in spoilage; |
| · | Because our Turbulator systems are effective in destroying pathogens, the need to use preservatives in the liquid product that has been treated is substantially reduced; |
| · | Because our Turbulator systems effectively purify liquids used in industrial processes for washing and rinsing, among other purposes, those liquids can then be reused, which substantially reduces water consumption and the need to dispose of waste products; and |
| · | Because our Technology does not rely on heat, our Turbulator systems can possibly create new products in situations in which the application of heat has been a limiting factor in their purification. |
| 9 |
More than 400 Turbulator systems, including test systems, have been installed since our inception in 2005 and either have been or are in operation in the following locations: the United States, Europe, South America and South Africa. Each installed Turbulator system is comprised of one to 40 separate Turbulators. We continue to evaluate the performance of our Turbulator systems. As we gain customer feedback, we incorporate it into the design of new Turbulator installations.
In certain geographic markets and for applications to certain liquids, we have not obtained or are not required to obtain governmental approvals for the sale or distribution of our Turbulator products. Accordingly, we or our distributors can market our Technology at the present time. In those markets and for those applications where governmental or similar approvals are required, our timelines for market entry are estimated to be between one and three years. See “—Regulation.” Rather, our principal task is to fund research by credible and competent third parties that will generate the scientific data that underpin our requests for clearance and that will convincingly demonstrate the efficacy, as well as what we believe to be the advantages of, our Technology. We believe that the aggregate cost of these reports will be approximately $100,000 during 2015 and 2016.
We do not, and do not expect in the near term, to manufacture Turbulator systems through a facility of our own. Rather, we rely on third-party manufacturers to build our Turbulator components to our specifications and for our final assembly. Our limited financial resources impair our ability to contract with manufacturers, since, among other things, our manufacturers generally require full payment before product delivery and installation. Our current manufacturers are located in France and South Africa. Our products are generally delivered to our customers at their particular industrial sites where our own personnel install and test the systems, and then provide training to the customer. The materials used to manufacture and assemble Turbulator systems are commodities and, at the current time, are not in short supply. If any of our suppliers were unable to provide components, there are sufficient backup sources for components that would permit us to maintain our pricing intact.
Our Competitive Strengths
Based on our proprietary patented Turbid Fluid Photopurification Technology, we are attempting to become an industry leader in the liquids purification and treatment industry. We believe our competitive strengths include the following:
| · | Although UV light has long been used as a surface sterilizer and has been successfully applied to clear water, we have developed the first product that processes opaque or turbid liquids using UV light: |
| 10 |
| § | Our patented Turbulator design increases the liquid’s exposure to UVC, enabling greater efficacy and consistency in purification; |
| § | The turbulent flow of the liquid over the UVC source for an extended period at controlled distances from the source ensures a non-fouling, self-cleaning system; and |
| § | The multiple-lamp system used in the Turbulator system provides greater flexibility; |
| · | The Technology is an alternative to comparable heat - or chemical-based processes and has a greater microbiological efficacy than conventional, laminar-flow UV systems in turbid liquid applications; and |
| · | The Turbulator uses a low maintenance, non-fouling technology that offers significant process and energy savings. |
The principal commercial benefits of our Technology and the systems that incorporate it include:
| · | an effective alternative to pasteurization; |
| · | replacement of chemical and other preservatives; |
| · | significant savings of energy and water; |
| · | extended shelf life of treated products; |
| · | preservation of the taste, smell and look of the raw materials or finished products; and |
| · | delivery of replicable, predictable germicidal efficacy. |
Notwithstanding the above advantages of our product, we nonetheless encounter and therefore must overcome serious challenges to further adoption and implementation of our Technology. As is the case with any business that seeks to replace an existing conventional modality, such as purification heat or preservatives, we have the burden of persuading customers and others of the superiority of the results that we produce and the value to our customers of the return on their investment in our technology. In applications, such as industrial application, where there is no regulatory involvement, we rely on various “cost/benefit” analyses to substantiate the case for acquiring and installing our system. In applications, such as dairy or foods, where there is regulatory involvement, we must demonstrate to the regulators that our Technology and its use of UVC either fits within the scope and application of existing rules and regulations, or, more often, we must persuade the regulators, by evidence they deem to be satisfactory, that they can amend or vary their regulations so that customers can employ our Technology. In many circumstances in which our technology is regulated, we prefer to present initially our Technology, as an adjunct to, and not as a replacement of, existing purification technologies. Although we believe that adjunct use will eventually lead to replacement, we do not yet have an established track record in this respect.
| 11 |
Sales
We have focused our sales efforts on “early adopter” global clients with multiple processing plants who have a substantial market share in their industries. Most of our sales activity takes place within our subsidiary SPO, as that company owns and administers all of our patents other than the South African patent. Most of our current sales arrangements are through SPO. During 2014, we generated approximately $500,000 of revenues under a few existing customer agreements.
For example, we have focused on the brewing industry and its need to purify liquid inputs into its final products, such as simple sugar syrup, as well as producers of fruit juice and wine. For example, we have focused on the brewing industry in emerging economies because we believe that these economies exhibit favorable opportunities for growth in demand for our perspective clients’ products. In 2009, we sold a Turbulator system to a facility a major international brewer located in South Africa. During 2014, we delivered two additional units to the same brewer. We also have engaged in trials with a craft brewer. Depending on regulatory developments (see “—Regulation”), we will target businesses that both produce and use liquids in their production processes and require the highest levels of purification of those liquids. The customers that we serve in one particular geographic market may not be the same type of customers that we serve in another geographic market, as we expect to continue to be opportunistic with respect to sales and market penetration.
During 2014, we also sold our patented Turbulator systems to a supplier of natural ingredients, a supplier of aromatherapy oils, a producer of liquid egg products and a producer of animal feeds. To date in 2015, we have received sales orders from a producer of natural vegetable juices and new orders from an existing customer which supplies equipment to customers who produce milk for consumption by calves and other farm animals.
We rely predominately on direct sales, and also have distributors in certain limited international markets. To date, we have selected distributors by type of liquid and by geographic region. Currently, we are represented by approximately eight distributors, two of which are based in Africa. Putting into place a distribution network requires that we identify the appropriate players to serve the specific industries in which our end users operate, that we provide them with that equipment and training that enables them to both demonstrate and explain the advantages of our Technology and that we design our Turbulator systems so that they are a compelling offering that permits our distributors to effectively utilize their resources in placing our products. The customer sales cycle is long for any new technology and our distributors may face resistance as a result of the historical investment that customers have made in existing facilities. See “Item 1A. Risk Factors— Risks Relating to our Business, Financial Condition and Corporate Structure—We do not have an expansive or well developed distribution network.”
We must raise sufficient working capital to support any distribution network that we establish. In addition, our distribution network will require sales support. Optimally, we would provide the support of a microbiologist/biochemist and an expert in the design and construction of engineered processes to our distributors to assist them in their sales efforts. In addition, we will require working capital to pay the general and administrative costs relating to in-house staff additions such as sales trainers and regulatory experts.
| 12 |
As of March 15, 2015, we have one existing multi-year customer agreement which generates a non-material amount of revenue. Under that agreement we have licensed a Turbulator system to a U.S.-based manufacturer of equipment used to sterilize milk and milk components used in the feeding of calves with farm milk. The license expires in April 2018. The manufacturer has an order pending with us for the delivery of 100 additional Turbulators during the second quarter of 2015. We have a contract with a customer for the delivery of a unit containing 360 Turbulators during the second quarter of 2015. In February 2015, we entered into a sales agreement for a unit with 240 Turbulators, also for delivery during the second half of 2015. Our ability to deliver and install equipment is dependent on financing the manufacturing and delivery of the equipment, either from the customer, internal working capital or a combination of both.
We also have entered into a patent license agreement with a major dairy operator in the United Kingdom under which the licensee is evaluating our technology in connection with the extension of the shelf life of milk. The license was entered into in October 2010, at which time the licensee paid us a one-time fee of approximately $40,000. The territory of the license includes both the United Kingdom and the Republic of Ireland. The license permits the licensee to evaluate our Technology and to use that evaluation to pursue a Novel Food Application with the European Union Commission. The license provides that if the European Union Commission approves the Novel Foods Application, then the licensee may, at its option, extend the license so that it is able to produce liquid milk commercially for a period of 24 months after the approval, contingent on the licensee’s agreeing to purchase from us one purification system with at least 200 Turbulators. If the licensee elects to produce milk commercially using our Technology, the parties have agreed to negotiate the terms under which equipment may be purchased from us, subject to a limit on equipment prices that the parties have agreed to. If not terminated earlier by mutual agreement, the license remains in force until nine months after a final decision of the European Union Commission on the Novel Foods Application for the Technology or, if the licensee has exercised its right to option to extend the license, for 24 months after the final decision of the European Union Commission.
We are currently in discussions with a number of prospective customers, but cannot predict whether we will be able to enter into revenue-generating contracts with customers during 2015. Our sales cycle is long and often requires negotiation with multiple levels and groups within a customer. Therefore, we cannot be certain of the timing of these contracts and the amount of revenues to be generated either on a period-by-period basis or over the life of the contract. See “Item 1A. Risk Factors— Risks Relating to our Business, Financial Condition and Corporate Structure—We face significant obstacles to the commercialization of SurePure Photopurification Technology and our Turbulator systems.”
| 13 |
Marketing
We have primarily utilized public relations, trade shows and customer trials to convert awareness into interest and purchase. Because our financial resources are limited, however, we will not be able to utilize any of these methods unless we receive increased funding.
Our current working strategy is to locate markets and market participants who require effective means for purifying liquids. Since inception, we have focused on producers of milk and other dairy products, fruit juices, wine and sugar syrups in the United States, the United Kingdom, the European Union, South Africa and India. In each case, we seek to approach the strongest market participants and explain the advantages of our Technology as an adjunct to or as a replacement of existing conventional processes. To the extent that permitted uses of our Technology is subject to regulation that limits, restricts or prohibits the use of UVC as a means of purification of a specific liquid or food, we work with the customer in various tests to support an application to amend or replace the applicable regulation.
Our technology was developed in South Africa. Since our management team is familiar with the dairy, fruit juice and wine industries in South Africa, we initially focused on the South African market. During the last five years, however, we have been working with customers in the United States, the United Kingdom and the European Union, as well as Australia, New Zealand, South America and Canada. We also continue to explore emerging economies, such as India, and during 2012 entered into an agreement with a distributor for our equipment in India. Our Indian distributor is conducting trials of our equipment at various small scale farmer dairies and is planning broader trials during the balance of 2015.
Regulatory Matters
The application of our SurePure Photopurification Technology to purify or disinfect milk, other dairy products, wine and juice generally requires that we take various steps to obtain regulatory clearances in each of the jurisdictions in which we plan to market our Technology. The compliance measures that we are required to take differ based on whether we are seeking to have the Technology sold as an adjunct to pasteurization or as an alternative to pasteurization, the latter having a higher standard for clearance. Other uses of our Technology—industrial uses, for example—do not require compliance with regulations governing food or dairy products. We are pursuing compliance efforts principally in the United States of America, the European Union and the Republic of South Africa, but our efforts in this respect may be impaired by our inability to pay our consultants who are providing some or all of these efforts.
United States of America
In 2001, the FDA amended its regulations to permit the use of UVC to purify water and certain fruit juices, as long as certain performance characteristics are complied with. With regard to milk and other dairy products in the USA, we plan to market our Technology first as an adjunct to pasteurization. Specifically, we are applying to treat raw milk, which is milk prior to any pasteurization or any other similar process. Although we have not committed to doing so, we plan to pursue clearance from the FDA as an alternative to thermal pasteurization in the future. The timeline for this process may be as long as four to six years and will likely require that we devote additional resources to fund the necessary testing.
| 14 |
The FDA classifies the use of UVC to treat milk or other food as a food additive, the use of which must be expressly permitted. The current FDA regulation that permits the use of ultraviolet radiation for the processing and treatment of food does not include the use of UV light as a means of purification of dairy products, although it does permit the use of UV light in treating potable water and juice products, as well as high fat-content food. In June 2012, in compliance with the FDA’s procedures, we submitted a draft application to amend the FDA’s food radiation regulation to permit use of UV light. We filed a draft of our petition to amend the regulation to the Office of Food Additive Safety (“OFAS”) of the FDA’s Center for Food Safety and Applied Nutrition (“CFSAN”). In August 2012, CFSAN confirmed our view that an amendment of the FDA’s food radiation regulation would be required and noted certain deficiencies in our draft petition for the use of our Technology in the pasteurization of milk. When we have successfully completed tests that generate the necessary data, we intend to submit those data and studies to address directly CFSAN’s comments in an effort to establish the safety of our treatment process. Once OFAS receives our formal petition, it must act within 15 days to determine whether it is suitable for filing, in which case we are so notified and OFAS publishes a notice to that effect in the Federal Register. At that time, OFAS also determines whether there is any need for review by agencies in addition to CFSAN, and formal review by OFAS commences. If all required safety information has been included in our petition, the scientific review should be completed within approximately six months. The wording of our suggested amendment to the food radiation regulation also undergoes a review for approximately six months, at which point OFAS drafts a final rule, which we will then review. When OFAS has completed its full consideration of our petition, and if that review is positive, the FDA will publish a final rule in the Federal Register. We understand that the timeframe for publication is two to three months and that the average length of time between submission of a final petition and publication of the rule is 24 months. Accordingly, full approval is possible in late 2015 or 2016.
In addition, the U.S. Food Drug and Cosmetic Act, which prohibits the manufacture or sale of misbranded foods, provides that any food that purports to be or is represented as pasteurized must have been subjected to a safe process or treatment that meets certain standards. We must satisfy these standards to the satisfaction of the FDA if our purification process does not satisfy the applicable FDA regulations. The submissions that we have made to date to the FDA have not produced a determination that our Technology satisfies its standards.
We also are required to obtain FDA approval for the use of our Technology for wines or other alcoholic beverages before the Alcohol & Tobacco Tax & Trade Bureau will make any determinations with respect to the application of our Technology to purify those products.
| 15 |
European Union
In the European Union, before our Technology can be used commercially with respect to foods, food ingredients and beverages, it must receive clearance under Regulation (EC) No. 258/97 of the European Parliament and of the Council of 27 January 1997 Concerning Novel Foods and Novel Food Ingredients. The application pertaining to our Technology has been submitted by a member company of the EU dairy industry that is a customer of ours. The matter is being reviewed by the Novel Foods, Additives and Supplements branch of the EU’s Food Standards Agency. In the application regarding our Technology, our customer has claimed that its use significantly increases the levels of Vitamin D in milk and extends the shelf life of dairy foods. The purpose of the EU Novel Foods review is to determine whether the use of our Technology presents a danger to the consumer, misleads the consumer or results in dairy products that are nutritionally disadvantageous to the consumer. The EU process requires the party who will be distributing the product to submit a request to an individual member state of the EU (the “Member State”), which, in our case, is the United Kingdom. The Member State designates an official committee to review the application, which determines whether additional assessment is required. At the same time, the request to distribute the novel food is forwarded to the European Commission. We understand that the initial assessment of the application should be completed within three months of the initial request to the Member State, not counting any time required to complete the submission if it is deemed incomplete in some respect. If the official committee requests additional assessment or if there are objections from other Member States, the Commission then asks the European Food Safety Authority to render an opinion. When the opinion is rendered, the Commission and the Standing Committee of the Food Chain and Animal Health make their decision. One of our customers has submitted a request to the Food Safety Authority in the United Kingdom. Our customer received the initial assessment of our SurePure Photopurification Technology in April 2013 and its application was resubmitted in November 2014. In February 2015 our customer received responses in the form of additional comments, to which it currently is responding. Our client has in turn responded to the responses, and we expect a determination by the end of 2015.
Republic of South Africa
In South Africa, we are engaged in a process to draft or amend a series of regulations of the Ministry of Health that apply to various foods and foodstuffs. In May 2012 we filed an application to amend certain regulations relating to Milk and Dairy Products GN R1555 of 21 November 1998 applicable to milk and dairy products, wine, fruit juice and other microbiologically sensitive liquids to permit our Product to be used as an alternative to pasteurization. In the case of certain regulations, draft regulations have been published for comment in the government’s official publication. In other cases, publication of the regulations may not occur until we have responded to requests for further information from the government’s Directorate: Food Control, including information that relates to the disposition of applications that we have submitted in the US and the EU. The Directorate of Food Control, Department of Health is not prepared to make an application to the Minister of Health to amend the regulation until such time that we have received either FDA or Novel Food Approval. In addition, the Directorate has advised us that the Agricultural Products Standards Act will have to be amended to conform with any amendments to South Africa’s regulations relating to milk and dairy products. The Directorate has further advised that, due to constraints in its human resource capacity, it has requested the Dairy Standards Agency of South Africa to assist. The Dairy Standards Agency undertook a scientific review of our Technology and other similar technologies during late 2014. We understand that if the results of the scientific review are favorable, the process for amending the regulations will be progressed. This may not however, be complete until late 2015 at the earliest.
| 16 |
Competition
Generally, we compete with a number of engineered services businesses who design and, in some cases, install purification systems for beverages, dairies, wineries and other manufacturers or processors of beverages. We also compete with those businesses that manufacture the systems that are used to pasteurize or otherwise purify beverages and other liquids. Both the service providers and manufacturers with which we compete generally are private companies, although a number are associated with large international conglomerates.
We believe that the principal competitive factors in our industry that create barriers to entry include, but are not limited to, reliability, effectiveness of technology to achieve requisite levels of purification, reputation, availability of resources and regulatory compliance for certain applications. Our limited financial resources and lack of a clear financial future may also be factors that make it difficult for us to compete in this marketplace.
We believe that any current thermal or chemical preservative process for liquids is a direct competitor to our business. Other developing processes, such as ultrasound, high-pressure or sterile filtration, are also a source of competitive threat.
Our strategy for dealing with our competition is to gain commercial acceptance of our Technology by pursuing those applications, such as dairy, that have rigorous regulatory standards that must be met, and at the same time work with large international businesses to receive their backing for our Technology. We also focus our efforts to gain acceptance in emerging growth economies where we believe that our Technology has competitive advantages, such as requiring the use of less power than other means of purification and where there are a large number of “Greenfield” installations for our Technology.
Intellectual Property
Our patented Turbid Fluid Photopurification Technology is protected by patents which have been granted in 66 countries, including South Africa, United States, China, Japan, Australia and the countries of the European Union. A predecessor entity to SPHSA applied for our patents in October 2000. Each of the patents is based on the same international patent application and relates to the same invention. The patents will expire beginning on October 12, 2020. Our United States patent was granted on July 12, 2005 and will expire on October 12, 2020. Patent applications for our invention remain outstanding in Brazil. If we do not have adequate funds, we may not be able to make the required payments to maintain our patents in all countries in which they have been issued.
| 17 |
The applications for these patents were examined independently by each of the patent offices where patent applications were filed, and each of the examiners in the various countries conducted an independent international novelty search for related published technology and then assessed whether or not the invention was new and inventive and thus qualified for a patent. Each examiner decided independently at the end of his examination that a patent should be granted. Accordingly, we believe that it is unlikely that a third party could successfully challenge the validity of any of our patents, and we further believe that our patents are enforceable with respect to the claims that they describe. The costs of enforcing a patent against an alleged infringer vary significantly from the relatively minor cost of sending a simple cease-and-desist letter which could result in cessation of the infringement, to the significant cost of bringing and maintaining a full patent infringement action. We believe that a third party may be infringing our Brazilian patent right and using our trade secrets in Brazil and that another third party may be infringing our patent rights in the United Kingdom. We have engaged counsel to advise us of our rights in this matter and whether we have an effective remedy available to us. See “Item 1A. Risk Factors—We rely heavily upon our patents and other intellectual property.”
We plan to continue to develop our Technology with a view to applying for further patents.
We also own the registered trademark “SurePure” in various formats in South Africa. We hold trademark registrations for “Turbulator” in South Africa and the European Community, and for “SurePure Turbulator” in the USA and Switzerland.
Research
We have contracted for and funded research efforts regarding the use of UVC as employed by our SurePure Photopurification Technology in the purification of liquids generally, and foods and beverages in particular, at academic institutions throughout the world. Generally, we have commissioned research in areas such as flow dynamics, biochemistry and microbiology. We, and in one or two cases, our customers, have provided funding for the studies, which generally covered both direct costs as well as overhead, and we also have provided our equipment to the institutions. In certain cases, we have provided annual grants to academic institutions to study the effects of UVC energy on foods and beverages. The academic institutions that we contracted to perform studies of our Technology or that received grants from us include:
| Ø | University of Wisconsin – Madison, Wisconsin, United States of America |
| Ø | Cornell University – Ithaca, New York, United States of America |
| Ø | Queens College – Belfast, Northern Ireland |
| Ø | Illinois Institute of Technology, Illinois, United States of America |
| Ø | Campdens BRI – Gloucestershire, United Kingdom |
| 18 |
| Ø | University of Birmingham – Birmingham, United Kingdom |
| Ø | University of Stellenbosch – Stellenbosch , South Africa |
| Ø | University of the Western Cape – South Africa |
| Ø | Cape Peninsula University of Technology – Cape Town, South Africa |
| Ø | IFV – Bordeaux, France |
By way of example, results of studies conducted at these institutions include the following:
| ○ | A 2008 published study that concluded that UVC radiation was successful in reducing bacterial counts in raw bovine milk, as well as apple juice, guava juice, pineapple juice and orange juice, while preserving the nutrients of the beverages being treated. |
| ○ | A 2010 unpublished study of the use of our Technology to produce grape juice and wine that is free of microorganisms that found that using the UVC treatment on grape juice and wine samples in five different wineries could provide wine significantly free of microorganisms (i.e., to the extent of less than 1 colony-forming unit per milliliter). |
| ○ | A 2011 published study that explains the effects of UVC radiation on microorganisms found in grape juice and wine products which found that our Technology “clearly indicated significant germicidal effect against the wine specific microorganisms; therefore, UVC radiation may stabilize grape juice and wine microbiologically in conjunction with reduced sodium dioxide levels” and that UVC radiation effectively inactivates wine-associated microorganisms such as Brettanomyces, Saccharomyces, Acetobacter, Lactrobacillus, Pediococcus and Oenococcus. |
| ○ | A 2011 published study that investigated the efficacy of UVC radiation technology to inactivate microorganisms in milk and concluded that UVC treatments produced microbial reductions similar to traditional heat pasteurization. The study also noted that UVC has the advantage of not producing chemical residues, by-products or radiation; is a cold process requiring very low maintenance at low cost; and does not require energy to produce heat. |
| ○ | A 2011 unpublished study currently under peer review that investigated whether UVC treatment could reduce the number of Alicyclobacillus acidoterrestris cells in water, fruit concentrate and grape concentrate and found that our Technology is a viable way to control contamination of juice concentrate by species of that bacterium. |
| ○ | A 2012 published study that examined the use of UVC light to inactivate microorganisms that found a 100% improvement in predicted microbial elimination due to the novel swirl design of our Turbulator. |
| 19 |
We believe that these studies were undertaken by qualified scientists who were employed by academic institutions with experience in the areas of scientific endeavor that relates to our Technology. We also believe that these scientists performed their research in accordance with the ethical and scientific guidelines of their respective universities. Prior to being published, the results were reviewed by scientific peers and presented by journals with international following. Accordingly, we believe that we and others can rely on these studies as proof of the efficacy of our Technology as presented in the studies. We have presented these studies to regulators as evidence of the efficacy of our Technology.
In addition to evaluating the results of our Technology as a means of purification, these studies also provided information with respect to certain critical process parameters, such as optimal dosage and flow rates. We plan to commission additional studies to gather additional information on these topics as well as certain toxicological and chemical safety studies. The methods to be used for our studies must comply with various regulatory standards. Any academic institutions with whom we might deal are likely to require that we fund these studies, and, accordingly, we will require additional financing so that we are able to pay all of the expenses and overhead costs that we are required to pay.
Employees
As of December 31, 2014, we employed 11 people directly, 9 of whom are based at our operational headquarters near Cape Town, South Africa. We continue to utilize the services of key academic and professional consultants in the areas of microbiology, biochemistry process engineering and manufacturing to enable us to grow the business and service our customer base on a global basis.
| 20 |
Our operations and securities are subject to a number of risks. Below we have identified and discussed the material risks that we are likely to face. Should any of the following risks occur, they may adversely affect our business, financial condition, and/or results of operations as well as the future trading price and/or the value of our securities.
Risks Relating to our Business, Financial Condition and Corporate Structure
We have a history of significant operating losses for the past eight years and these losses may continue in the future.
We have incurred net losses of approximately $34,325,000 for the period from inception through December 31 2014. We have been without significant revenue since inception, and our current agreements provide relatively limited amounts of revenue. Our historical losses are expected to continue into the future unless we are able to generate significant amounts of revenue in our business. Our history of significant operating losses has also made it difficult for us to raise additional working capital.
We require immediate capital to fund our operations, and we may not be able to obtain sufficient capital and may be forced to limit the scope of our operations or to liquidate our business.
We have significant working capital needs, including approximately $110,000 of U.S. and foreign taxes. Our management team has worked without receiving cash compensation for as much as 27 months and the total accrued compensation and related expense reimbursements owed to them is approximately $1,148,000. We have other current liabilities, net of customer deposits, of approximately $1,521,000. If we are unable to secure adequate financing on reasonable terms, or on any terms, we may not be able to pay these or other operating expenses and to continue to operate our business. There is no assurance that financing will be available to us, in which case we may be compelled to wind down or liquidate our business.
Even if we do find a source of capital, we may not be able to negotiate terms and conditions for receiving the capital that are acceptable to us or our shareholders. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to the units. We cannot provide any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us.
| 21 |
Even if we obtain capital to fund our operations, the capital may not be sufficient to fund our strategies on which the potential growth of our business is premised.
Even if we raise capital to fund our day-to-day operations, that capital may not be sufficient to enable us to execute the strategies that we have formulated for our growth. In connection with our growth strategies, we will likely experience increased capital needs and accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the release of competitive products by our competitors; (iii) the level of our investment in research development; and (iv) our need to obtain certain regulatory approvals. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
Our efforts to raise capital, if successful, will subject us to ongoing costs and expenses.
Each of our efforts to raise working capital, when successful, has required that we enter into obligations to register for resale with the Commission the securities purchased by our investors. Because our trading price is low, the aggregate market value of the shares of our Common Stock held by shareholders other than our affiliates is less than $75,000,000 and, accordingly, we are not able to take advantage of those forms and procedures of the Commission that allow us to incorporate by reference filings that we have made. As a result, our registration process is more expensive and time-consuming than it may be for other companies that can register their shares more efficiently.
Since March 2013 we have not been able to raise equity capital from persons other than existing shareholders. We cannot predict whether existing shareholders will continue to fund our business in the absence of commercial results or otherwise.
As of the date of this annual report on Form10-K, no investors are obligated to purchase any of our shares. Between September 2012 and March 2013, two institutional investors, Trinity and RD Active Limited of London, United Kingdom (“RD Active”) have purchased our shares. As of December 31, 2013, an affiliate of Trinity had the obligation to purchase 900,000 shares of our Common Stock by March 25, 2014. Prior to March 25, 2014, that affiliate purchased only 184,825 shares of our Common Stock.
During the first three quarters of 2014, we were able to raise equity capital only from persons who are, or are related to, significant shareholders of the Company. During that 9-month period ended September 31, 2014, Regency purchased 1,006,250 shares and Trinity purchase 184,000, for total gross proceeds of $1,190,250. Regency is an affiliate of XOptics, which holds 14,800,000 shares of our Non-Voting Convertible Preferred Stock as of March 15, 2015. We believe that Regency funded its purchases with the proceeds of sales of our shares to Trinity and others. Trinity has not purchased shares from the Company since March 2014. See “Item 13. Certain Relationships and Related Transactions—Transactions with Regency.” The ability of Regency to provide additional financing to us by purchasing additional shares may be limited to the extent that it is able to resell shares of our Common Stock to Trinity and receives available funds from those sale transactions.
| 22 |
In direct transactions with Trinity and Regency, we priced our shares at not less than $1.00 per share, which is greater than the prevailing prices at which our shares generally trade in the OTCQB. See “Item 5. Market for Common Equity, Related Stockholder Matters—Market for Common Stock”.
We continue to discuss and negotiate possible financing transactions with our shareholders, but cannot be certain that we can conclude financing transactions with them or, if completed, that our shareholders will perform their obligations to purchase shares from us.
We do not currently have any independent directors on our Board.
As of March 27, 2013, we ceased to have any independent directors. As of the date of this annual report on Form 10-K, we have two members of our board and they both are members of management. Until an independent director becomes a member of our board, all board decisions will be made by these two members. Because we do not have an independent director, our board of directors has no nominating or compensation committees and our audit committee has no members. In addition, without independent directors, the Company does not have any independent oversight or administration of its management and operations. The Company’s decision-making may suffer from the lack of independent directors, since only officers and employees of the Company will make all important decisions regarding the Company. For example, our executive officers, who are also stockholders and directors, could establish policies and enter into transactions without independent review and approval thereof. This could present the potential for a conflict of interest between the Company and its shareholders generally and the controlling officers or directors.
Our success is dependent on our ability to commercialize our SurePure Photopurification Technology and our Turbulator photopurification systems to generate sufficient revenues to sustain and expand our operations.
The execution of our business plan in the near-term is wholly dependent on our ability to commercialize our Technology through the use of our Turbulator line of photopurification systems to produce sufficient revenues to cover our operating expenses. The success of these endeavors will require that sufficient funding is available to us to finance the manufacture, marketing and sale of our current Turbulator systems and the development of new photopurification products and applications for those systems. Should we be unable to improve our financial condition through debt or equity offerings, our ability to successfully advance our business plan will be severely limited and we could be forced to discontinue or liquidate our business. Were this to occur, our shareholders would not be likely to receive any significant return, or any return at all, on their investment.
| 23 |
We face significant obstacles to the commercialization of SurePure Photopurification Technology and our Turbulator systems.
The industries in which we intend to commercialize our Technology are characterized by existing processes and technologies that have been used for very long periods, such as pasteurization, filtration and chemical and other preservatives. It is difficult for us as a business with limited resources to displace these existing processes and technologies and to gain acceptance even as an adjunct to the existing processes. In the case of businesses with existing facilities, a change to our Technology may require the abandonment in whole or in part of existing facilities which have been in place for an extended period of time. In the case of businesses about to build or invest in new facilities, we face the challenge of marketing a newer and often lesser-known technology.
In addition, the principal industries that we have targeted—dairy products, juice products, beer, wine and food—are regulated by government agencies or subject to standards promulgated by industry bodies. These agencies and bodies operate under and enforce regulations which often do not permit UVC purification without change in applicable legislation or regulations or both or classify UVC purification as a food additive. As a result, we are not permitted to address the dairy industry in major markets, such as the United States of America, the European Union or South Africa, until the restrictive regulations have been changed to permit the use of our Technology or until our Technology has been approved for use in the dairy industry. The processes that we have undertaken in our attempts to change legislation and other regulation are often prolonged and difficult. See “Item 1. Business—Regulatory Matters.”
In order to address these challenges, we seek to create awareness of the advantages of our Turbulator systems among businesses that have purification requirements in their processes. We also plan to continue our efforts to obtain regulatory and similar approvals where required. At the same time, we plan to keep pace with outside technological developments, to respond to technological developments and to seek out additional industries whose liquid purification needs are not being met adequately or at all. In order to pursue these strategies, we believe we will be required to expend significant amounts to hire or engage and manage those persons with the networks, skills, experience and resources to execute these plans. We believe we must also continue to fund independent research at qualified academic and similar institutions to investigate fully the scientific basis of our Technology. The financial and other resources that we currently have available are not adequate for all of these purposes, and we will require significant additions to enable us to execute these plans. If we are not able to secure additional funding, we may not be able to address these challenges adequately or at all and therefore could be forced to discontinue our business. Were this to occur, our shareholders would not likely receive any significant return, or any return at all, on their investment.
| 24 |
Our SurePure Photopurification Technology may become obsolete.
The ability of UVC to destroy microbes and other pathogens has been known for several years. We believe the principal advantage of our Turbulator systems is that they purify turbid liquids in a more efficient and effective manner as compared to conventional processes. There is no assurance, however, that another business will not discover and commercialize a system that would use UVC or some other energy source in a manner more efficient and/or effective than our Turbulator systems, in which case our Technology might be rendered obsolete. Were this obsolescence to occur, we could encounter significant difficulty in selling or otherwise deploying our systems and could be forced to write off the value of our investment in our Technology. In addition, if we were not able to deploy technology or systems that met the challenge of these developments, we could be forced to discontinue our business. Were this to occur, our shareholders would not likely receive any significant return, or any return at all, on their investment.
We compete with established purification processes with wide market acceptance and installed bases.
Since we acquired the SurePure Photopurification Technology in 2005, we have faced considerable difficulty entering the marketplace. Although we believe that our Technology has considerable advantages over conventional modalities, businesses in the market have made considerable fixed cost investments in the installation of the conventional processes, and the replacement of their installed technology with our Technology would entail added costs in the short-term. The success of our business is likely to depend principally upon our ability to convincingly demonstrate the longer-term technical and cost advantages of our Turbulator systems and secure a reliable base of customers who are willing to install our Technology together with or in replacement of the existing purification technologies. It is possible that, despite our ability to prove our effectiveness and our cost efficiencies across a range of industries, our customers will, because of their reluctance to adopt a new technology, prefer to fully amortize their investment in existing purification systems or, because of simple inertia, remain unwilling to purchase our Turbulator systems or otherwise acquire our Technology.
At the same time, many of the businesses that sell and install the existing processes with which our Technology competes have considerable resources and sophisticated marketing plans and operating distribution networks. These factors have made and may continue to make it difficult for us to compete with these businesses. In addition, many of our potential customers engage and rely on industrial engineering firms to recommend and select purification systems for their facilities. The fact that we are a relatively new business with a newly developed technology and with very limited resources may make it difficult for these engineering firms to recommend our Technology over the existing processes.
In addition, competitors could develop technologies or systems similar to or better than our own, finish development of new technologies in advance of us, or be more successful at marketing new products, any of which factors may hurt our prospects for success.
| 25 |
If we are not able to deal effectively with these competitive factors either directly or by focusing on geographic or industrial markets where these competitive factors are less intense, we may not be able to generate revenues for our business. As a result, without revenues, we may be forced to discontinue or sell our business. Were this to occur, our shareholders would not likely receive any significant return, or any return at all, on their investment.
General economic conditions will affect our operations.
Changes in general domestic and international economic conditions may adversely affect the financial performance of the Company. Factors that may contribute to a change in economic conditions include slow or non-existent GDP growth or recession in developed and/or emerging market economies, volatility, uncertainty or actual or potential downturns in banking and/or financial markets, fluctuations in interest rates, availability of credit, inflation rates or currency exchange rates, labor disputes and political and social unrest or reform. Further, the delayed recovery of the global economy is not conducive to growth, particularly of technology companies with newly commercialized products. Any adverse development in economic conditions, whether in banking or financial markets, or in levels of economic activity generally, could increase our expenses and make it more difficult for us to market and commercialize our Technology.
To date, we have chosen to direct our marketing efforts to markets in emerging economies, which economies may exhibit less stability and predictability than developed economies.
Since we acquired our Technology, we have chosen to devote a considerable amount of our resources to marketing it in South Africa and other emerging economies. We believe that emerging economies tend to offer certain opportunities, such as growing consumer populations, which may lead to the construction of new breweries, dairies and other food and beverage processing facilities. We also believe that it may be easier for the owner or operator of a facility that is about to be built to adopt our Technology than for the owner or operator of an existing facility which already has a purification system installed. Doing business in emerging economies, however, is subject to substantial risks. Among other things, economic conditions in those countries may be volatile. The role of governmental agencies and other regulators may be subject to change without advance notice or without any notice. The plans of our current and potential customers to construct or complete the planned construction of their facilities therefore may change. In addition, perception of risk in any one particular emerging market country or region can heighten the perception of risk in emerging market countries in general, whether among banking or financial markets or otherwise. As a result, the investment that we have made with respect to the projects may not have the return that we anticipated or any return at all.
| 26 |
We do not have an expansive or well-developed distribution network.
To date, we have relied on distributors to sell our Technology on a country-by-country or region-by-region basis without putting into place a coordinated international distribution network. We believe that the establishment and maintenance of an effective worldwide distribution network will require a substantial amount of financing which has not yet been available to us. The lack of an effective worldwide distribution network may place us at a competitive disadvantage to other providers of liquid purification technologies. If we are not able to secure adequate equity or debt financing in the future, we may not be able to create channels of distribution for our systems, and, as a result, our revenues may not increase.
We rely on third parties to manufacture our Turbulator systems and do not have our own manufacturing facilities.
Although our own employees design, assemble and install our Turbulator systems in conjunction with our distributors and customers, we have contracted the manufacturing of the components of our Turbulator systems to third parties. As a result, we do not directly control these components and are subject to risks, including late or non-delivery, defective manufacture by third parties, breakage in transit and insufficient supply. Any of these problems, as well as other problems deriving from outsourced manufacture of components, could lead us to default on our obligations to our customers, which could have adverse financial consequences for us and as well as adverse consequences for our business reputation.
We rely heavily upon our patent and other intellectual property.
We rely on a combination of the counterparts of our issued patent, trade secrets and licenses, and, to a lesser extent, trademarks, together with non-disclosure and confidentiality agreements, to establish and protect proprietary rights to our Technology. See “Item 1. Business—Intellectual Property.” Our success depends, in part, on our ability to obtain additional United States and foreign patent protection for our products, their uses and our processes to preserve our trade secrets and to maintain the patents that we hold. We do not know whether any of the claims in our issued patents or pending applications will provide us with any significant protection against competitive products or otherwise be commercially valuable. Legal standards regarding the validity of patents and the proper scope of their claims are still evolving, and there is no consistent law or policy regarding the valid breadth of claims. Additionally, there may be third-party patents, patent applications and other intellectual property relevant to our products and technology which are not known to us and that block or compete with our products. Since we do business in emerging economies where legal means for effectively addressing infringement of our patent may not be available, we may not be able to prevent infringement or to obtain judicial relief when infringement occurs.
Our limited financial resources impede our ability to assert our patents against infringers and to obtain additional patents. Accordingly, we may not be able to exercise our rights to their full extent. As a result, infringement may have a material and adverse affect on our ability of our SurePure Technology to compete.
| 27 |
We may not be able to effectively manage our growth.
Over the longer term we will seek to achieve considerable future growth in our business. This growth may come from the market acceptance of our Technology, increased licensing and commercialization of our Turbulator systems and expanding applications and markets for our Technology. To achieve growth in an efficient and timely manner, we will have to maintain strict controls over our internal management, technical, accounting, marketing, and research and development functions in all of our companies, both in the United States and elsewhere. We believe that we will continue to employ sufficient quality personnel to manage our anticipated future growth. Should we be unable to successfully manage our anticipated future growth by employing or otherwise engaging personnel and professionals who will maintain these standards, our costs may increase, our growth could be impaired and our ability to operate in existing and new marketplaces may be impaired.
Failure to receive regulatory approval and other governmental action may adversely affect our business.
We have developed our SurePure Photopurification Technology such that it can be used by industries without being subject to prior regulatory approval as well as in those industries where regulatory approval is required. Should our Technology not receive regulatory approval, the applications of our Technology will become limited to those industries where regulatory approval is not required, and our business and financial results would thereby be seriously harmed.
In those markets and industries in which regulatory approval is required, the approval process is frequently long, unpredictable and costly. See “Item 1. Business—Regulatory Matters.” Although we believe that we have accumulated sufficient scientific data to prove that our Technology is both safe and effective, governmental agencies frequently request additional or updated data. To satisfy these requests, we may be required to expend significant amounts and delay our entry into the market place, both of which adversely affect our business. The regulatory standards that we seek to satisfy are in large part subjective, and therefore we cannot be certain that our evidence will be satisfactory in any given case until the regulatory body so determines.
Although regulatory bodies in different jurisdictions may consider approval in one jurisdiction to be persuasive on certain issues of concern, in general, we must address each regulatory body separately and attempt to satisfy its own set of standards in each case. This process is extremely time-consuming and costly. Frequently delays arise because of, among other reasons, changes in the staffing of regulatory agencies or because of changes in priorities within the regulatory agencies. Although we have employees dedicated to regulatory issues, in almost all cases we must rely on outside consultants for each jurisdiction in which we are seeking regulatory approval. Although these consultants have frequent contact and wide experience with the government agency in question, these consultants are expensive and cannot assure us of a favorable outcome or that delays will be avoided.
| 28 |
In certain jurisdictions, regulatory approval requires that an existing law or regulation be modified. Typically, the existing laws or regulations limit the means by which foods and beverages can be purified to the existing modalities. The modification process requires publication or debate of the intended change. Publication and debate give our competitors and those businesses that have vested interests in maintaining the exclusivity of the existing modalities the opportunity to oppose our efforts to modify the existing limitations. As a result, we may be forced to spend significant resources to counter these efforts, and we may not be successful in doing so. If we are not successful, then we could be prohibited from selling or distributing our Technology in that market. Even if we are successful, we would likely undergo significant delays and expend significant resources, which may impair or prevent us from operating in such markets.
Moreover, because we are regulated in certain markets as a food additive, we are subject to ongoing scrutiny and subject to changes in regulatory actions even after approval has been obtained. Furthermore, changes in legislation and governmental policy could also negatively impact us.
Because our Technology is applied to food and beverage products to be consumed by the public, we may face liability claims.
Although we have conducted testing programs to identify potential material defects in our Technology and have relied on published, peer-reviewed studies of UVC by independent investigators, these tests and studies may not have detected all potential defects. Any undetected defects could harm our business reputation, diminish our customer base, shrink revenues and expose us to product liability claims. We carry product liability insurance in amounts that we consider to be adequate for the size of our business. However, we cannot assure you that we will have adequate insurance to cover all claims that we may face, and any determination of liability that is not covered by insurance or is in excess of insurance coverage could have a material adverse effect on our business, results of operations and financial condition.
Consumer advocates and others may question the safety of the effects of our Technology.
We are aware that certain organizations that purport to be consumer advocates and others may question the safety of irradiation for purposes of preservation and avoiding spoilage. Often, these persons question the safety testing that has been done with respect to the consumption of foods and beverages that have been irradiated and attempt to establish a link between irradiated food and cancer, despite the FDA’s efforts to educate the public on questions relating to irradiation. These persons may attempt to use the Internet or other media to bring pressure on governmental agencies and generate publicity against the use of irradiation. As a result, and because UVC may be characterized as a form of radiation, their concerns may adversely affect the reputation of our Technology and our ability to increase our revenues. Were their concerns to become pervasive, our business could be damaged. In that case, we might have to restrict our marketing and sales efforts to markets and industries that were not affected by governmental actions or publicity.
| 29 |
We may be unable to attract and retain qualified employees and management personnel necessary for the operation of our business.
Our future success also depends upon our continuing ability to attract and retain highly qualified personnel. Expansion of our business and the management and operation will require additional managers and employees with industry experience, and our success will be highly dependent on our ability to attract and retain skilled management personnel and other employees. There can be no assurance that we will be able to attract or retain highly qualified personnel. Competition for skilled personnel in our industry and in the locations in which we operate is significant. This competition may make it more difficult and expensive to attract, hire and retain qualified managers and employees.
The elimination of monetary liability against our directors, officers and employees under Nevada law and the existence of indemnification rights to our directors, officers and employees may result in substantial expenditures by us and may discourage lawsuits against our directors, officers and employees.
Our Articles of Incorporation contains a specific provision that eliminates the liability of directors for monetary damages that may be awarded to us and our shareholders; further, we provide indemnification to our directors and officers to the extent provided by Nevada law. We may also have contractual indemnification obligations under our employment agreements with our executive officers. The foregoing indemnification obligations could result in our incurring substantial expenditures to cover the cost of settlement or damage awards against our directors and officers, which we may be unable to recover. These provisions and resultant costs may also discourage us from bringing lawsuits against our directors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation by our shareholders against our directors and officers, even though such actions, if successful, might otherwise benefit us and our shareholders.
We may not have obtained shareholder approval for the transfer of certain of our assets.
As required by the Share Exchange Agreement, on December 12, 2012 we redeemed and then cancelled 23,180,000 outstanding shares of our Common Stock then held by Ratree Yabamrung and Kotchaporn Bousing, two of our officers, directors and shareholders prior to the Share Exchange. We redeemed Ms. Yabamrung’s shares by assigning to her all of our assets and liabilities immediately prior to the closing. We did not obtain the approval of our shareholders for the transaction that we executed with Ms. Yabamrung. Although we believe that shareholder approval may not have been required under Nevada law for the transfer of those assets that were transferred to her, it is possible that persons who were shareholders at the time of the Share Exchange may claim that their approval was required, in which case litigation might follow. Although we believe that there would be meritorious defenses to the claim and that few shareholders would have an incentive to bring such a claim, nevertheless defense of the claim may be difficult, costly and time consuming.
| 30 |
We are controlled by our current officers, directors and principal shareholders.
Our directors and executive officers and their affiliates beneficially own approximately 4.1% of the outstanding shares of our Common Stock. See “Item 12. Security Ownership Of Certain Beneficial Owners and Management and Related Stockholder Matters.” Accordingly, our directors, executive officers and principal shareholders will have substantial influence over, and may have the ability to control, the election of our board of directors and the outcome of issues submitted to a vote of our shareholders. Although our board members and officers owe a fiduciary duty to our shareholders and must act in good faith in a manner they reasonably believe to be in the best interests of our shareholders, as a shareholder such persons are entitled to vote their shares in their own interests, which may not always be in the interests of shareholders generally.
Risks Relating to our Securities
Shares of our Common Stock are very thinly traded, and the price may not reflect our value and there can be no assurance that there will be an active market for shares of our Common Stock either now or in the future.
Although our Common Stock is quoted on the OTCQB, shares of our Common Stock are very thinly traded, and the price of our Common Stock, if traded, may not reflect our value. OTCQB securities are not listed and traded on the floor of an organized national or regional stock exchange. Instead, OTCQB securities transactions are conducted through a computer network connecting dealers in stocks. OTCQB stocks are traditionally smaller companies that do not meet the financial and other listing requirements of a regional or national stock exchange. As a result, there can be no assurance that there will be an active market for shares of our Common Stock either now or in the future. Market liquidity will depend on the perception of our operating business and any steps that our management might take to bring us to the awareness of investors. There can be no assurance given that there will be any awareness generated. Consequently, shareholders may not be able to liquidate their investment in us or liquidate it at a price that reflects the value of our business. As a result holders of our securities may not find purchasers for our securities should they desire to sell the securities that they hold. Consequently, only investors having no need for liquidity in their investment and who can hold our securities for an indefinite period of time should purchase our securities.
If a more active market for our shares should develop, the price of shares of our Common Stock may be highly volatile. Because there may be a low price for shares of our Common Stock, many brokerage firms may not be willing to effect transactions in our securities. Even if an investor finds a broker willing to effect a transaction in the shares of our Common Stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price.
| 31 |
If we are not able to satisfy the eligibility standards of the OTCQB market, the trading of our shares will become more difficult.
During 2014 the OTCQB market underwent changes that require us to comply with its new eligibility standards. If we become unable to satisfy the eligibility standards of the OTCQB market, OTCQB has advised us that it would move the trading of our shares to OTC Pink, a market in which it could be substantially more difficult for volume in our shares to increase and for buyers and sellers to transact with each other. This development, in turn, may make it more difficult for us to sell shares directly to investors because our shares will not have the liquidity of other shares of other companies. To date, we have been able to comply with the standards.
Our shares may lose their status as “DTC-Eligible.”
Shares of our Common Stock became “DTC-eligible” during the first quarter of 2013. This means that our shares can be electronically transferred between brokerage accounts. It is, however, possible that DTC could in the future change the status of our shares to other than “DTC-eligible” or otherwise subject our shares to a limited status which DTC references as a “chill.” Were either or both of these events to occur, our shares would not be able to be electronically transferred between brokerage accounts and the transfer process would therefore become manual. Manual processing may take several days and is not a favored option for companies such as us that rely on broker dealers for stock transactions. If we were to lose DTC-eligibility, our shares may not be able to trade with any volume.
We cannot assure our shareholders that an active market for shares of our Common Stock can be sustained.
We cannot assure our shareholders that an active trading market for our Common Stock can be sustained. A shareholder may find it difficult to dispose of shares or obtain accurate quotations as to the market value of our shares on the OTCQB. Securities quoted on the OTCQB may be subject to a Commission rule that imposes various practice requirements on broker-dealers who sell securities governed by the rule to persons other than established customers and accredited investors. Consequently, this rule may deter broker-dealers from recommending or selling such securities, which may further limit the liquidity of our shares. If applicable, this rule could also make it more difficult for us to raise additional capital.
Sales by our major shareholders under Rule 144, Regulation S or another exemption from the Securities Act, or pursuant to a registration of our shares, may depress the price of our Common Stock.
As of the date of this annual report on Form 10-K, our stockholder XOptics holds 14,800,000 shares of our Nonvoting Convertible Preferred Stock. Those shares are convertible into 14,800,000 shares of our Common Stock on a 1-to-1 basis under certain terms and conditions, including upon any sale to a third party purchaser. Any shares of Common Stock that are issued to XOptics upon conversion of its shares will be restricted securities unless the shares are first registered prior to their resale.
| 32 |
XOptics is able to sell, or convert and then sell, the 14,800,000 shares of our Nonvoting Convertible Preferred Shares that it holds under Rule 144 under the Securities Act, which permits XOptics to resell, subject to various terms and conditions, its restricted securities. In addition, because XOptics is not a “U.S. person,” as defined in Regulation S under the Securities Act, it may be able to sell shares of our Nonvoting Convertible Preferred, or convert those shares and then sell the conversion shares, in offshore transactions.
Similarly, our other major shareholders—Trinity, Regency and RD Active—are not “U.S. persons” and have sold and may continue to sell our restricted securities outside of the United States at prices that are below the prices quoted on the OTCQB.
Any sale of a substantial number of shares at one time or from time to time may depress or limit the market price of our shares. Moreover, because these sales could occur in the future, the anticipated effect of the shares becoming available in the market through future sales, also known as overhang, could reduce the trading price of shares of our Common Stock.
Our efforts to raise capital, if successful, will subject our shareholders to dilution.
As described under “—We require immediate capital to fund our operations, and we may not be able to obtain sufficient capital and may be forced to limit the scope of our operations or to liquidate our business,” we require immediate capital to fund our operations. Our efforts to raise capital have resulted in multiple subscription and share purchase agreements. Since the date of the Share Exchange and through March 30, 2015, we have issued 26,478,457 shares of our Common Stock, or an increase of approximately 114% of our issued and outstanding shares. Of the shares issued, 16,840,010 have been issued to shareholders in exchange for consideration and 9,865,447 have been issued upon conversion of shares of our Nonvoting Convertible Preferred Stock. These issuances have diluted the interests of our shareholders, any future issuances will continue to dilute their interests. Therefore, any investment in our shares of our Common Stock carries that risk that such investment will be diluted by a subsequent share issuance by us as a result of, among other things, our need for external sources of capital.
| 33 |
We are incurring increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could harm our operating results.
As a public company, we are incurring significant legal, accounting and other expenses, including costs associated with public company reporting requirements. We also are incurring substantial expenses in connection with the preparation and filing of this registration statement and responding to the Commission’s comments in connection with its review of the registration statement. We may be required to incur costs associated with current corporate governance requirements, including requirements under provisions of the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley”), as well as rules implemented by the Commission or any stock exchange or quotation system on which shares of our Common Stock may be listed in the future. The expenses incurred by public companies for reporting and corporate governance purposes have increased dramatically in recent years. We expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. We are unable to currently estimate these costs with any degree of certainty. We also expect these rules and regulations may make it difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage available to privately-held companies. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as our executive officers.
If we fail to maintain proper and effective internal controls, our ability to produce accurate and timely financial statements could be impaired, which could harm our operating results, our ability to operate our business and investors’ views of us.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Our failure to maintain the effectiveness of our internal controls in accordance with the requirements of Sarbanes-Oxley could have a material adverse effect on our business. We could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on the price of our Common Stock. In addition, if our efforts to comply with new or changed laws, regulations, and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Because we became public by means of a reverse acquisition, we have not been able to attract the attention of major brokerage firms.
Additional risks may exist as a result of our having become a public reporting company through a “reverse acquisition.” Security analysts of major brokerage firms do not cover us or our stock. Because we became public through a reverse acquisition, there may be less incentive for brokerage firms to recommend the purchase of our Common Stock. No assurance can be given that brokerage firms will want to provide analyst coverage of us or our stock in the future, which may result in less liquidity and lower trading prices for our shareholders.
| 34 |
We are subject to Sarbanes-Oxley and Dodd Frank and the reporting requirements of federal securities laws, which can be expensive and time-consuming.
We are subject to Sarbanes-Oxley and the Dodd Frank Wall Street Reform and Consumer Protection Act (“Dodd Frank”), as well as related rules implemented by the Commission and reporting requirements of the Exchange Act and other federal securities laws. The costs of compliance with Sarbanes-Oxley and of preparing and filing annual and quarterly reports, proxy statements and other information with the Commission, and furnishing audited reports to shareholders, are significant and require us to expend significant amount on our costs and expenses of complying with these rules, principally the fees and expenses of our professional advisors.
Our executive officers have had limited experience managing a public company subject to U.S. securities laws, which could adversely impact our ability to comply with the reporting requirements of those laws.
Prior to December 12, 2012, none of our management team had managed a public company subject to U.S. securities laws. Our management’s lack of experience could adversely impact our ability to comply with legal, regulatory, and reporting requirements of those laws. Our management may not be able to implement programs and policies in an effective and timely manner to adequately respond to such legal, regulatory and reporting requirements, including the establishment and maintenance of internal control over financial reporting. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our ability to comply with the reporting requirements of the Exchange Act, which are necessary to maintain public company status. If we were to fail to fulfill those obligations, our ability to operate as a public company would be in jeopardy, in which event the shareholders could lose their investment. Our ability to operate successfully may depend on our ability to attract and retain qualified personnel with appropriate experience in the management of a public company. Our ability to find and retain qualified personnel on our terms and budget may be very limited.
We have never paid dividends on our Common Stock, and we do not intend to do so for the foreseeable future.
We have never paid dividends on our Common Stock, and we do not anticipate that we will pay any dividends on our Common Stock for the foreseeable future. Accordingly, any return on an investment in our Common Stock will be realized, if at all, only when a shareholder sells shares of our Common Stock. In addition, our failure to pay dividends may make our stock less attractive to investors, adversely impacting trading volume and price.
| 35 |
Commission rules prohibit a reverse acquisition company from listing on a national securities exchange until the company has been in the U.S. over-the-counter market or on another regulated U.S. or foreign exchange for at least one complete fiscal year.
Commission rules seek to improve the reliability of the reported financial results of reverse acquisition companies by requiring a pre-listing “seasoning period” during which the post- reverse acquisition public company must produce financial and other information in connection with its required Commission filings. The company also must maintain a requisite minimum share price for at least 30 of the most recent 60 trading days prior to the date of the initial listing application and the date of listing on any national securities exchange. As a result of these rules, it is unlikely that we will be eligible to list on a national securities exchange until 2015 at the earliest, and then only if our stock trades above the requisite minimum price in accordance with the listing requirements of the applicable national securities exchange.
A significant portion of the total outstanding shares of our Common Stock may be sold into the public market in the near future, which could cause the market price to drop significantly, even if our business is doing well.
During 2013 and 2014 we registered for resale by our shareholders a total of 38,982,753 shares, or approximately 82.3% of the issued and outstanding shares of our Common Stock. We believe that, despite these registrations, our shareholders have not sold most of those shares of our Common Stock but would likely do if there were broader demand for our shares in the market or if the bid price increased. Sales of a substantial number of shares in the public market, or the perception in the market that the holders of a large number of shares intend to sell shares, could depress the trading price of our Common Stock or limit increases in the trading price.
Because it may be considered a “penny stock,” our shareholders may have difficulty selling shares of our Common Stock.
Our Common Stock may be considered a “penny stock” if the price for our Common Stock that does not trade on an exchange drops below $5.00 per share and we do not meet certain net asset or revenue thresholds. These thresholds include the possession of net tangible assets (i.e., total assets less intangible assets and liabilities) in excess of $2,000,000 if we have been operating for at least three years or $5,000,000 if we have been operating for fewer than three years, and the recognition of average revenues equal to at least $6,000,000 for each of the last three years. In that case, our shares will be subject to the requirements of Rule 15g-9 under the Exchange Act. Under this rule, broker-dealers who recommend penny stocks to persons other than established customers and accredited investors must satisfy special sales practice requirements. The broker-dealer must make an individualized written suitability determination for the purchaser, considering such purchaser’s financial situation; investment experience and investment objectives, with respect to penny stock transactions, and receive the purchaser’s written consent prior to the transaction.
The penny stock rules severely limit the liquidity of securities in the secondary market, and many brokers choose not to participate in penny stock transactions. As a result, there is generally less trading in penny stocks. If you become a holder of our Common Stock, you may not always be able to resell shares of our Common Stock publicly at the time and price that a shareholder believes are fair or appropriate.
| 36 |
Item 1B. UNRESOLVED STAFF COMMENTS
As a smaller reporting company, as defined in Rule 12b-2 of the Exchange Act, and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information requested by this Item.
SPHSA leases two premises, each having approximately 2,000 square feet, in South Africa under separate operating leases. Both leases terminate at the end of May 2015. If we are not able to raise additional working capital, we may not be able to extend these leases or lease any space whatsoever, in which case we might not be able to maintain a principal office.
We are not involved in any material legal proceedings that are pending as of the date of this annual report on Form 10-K. From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and adverse results in contested matters that may arise from time to time may harm our business.
Item 4. MINE SAFETY DISCLOSURES
Not Applicable.
| 37 |
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market for Common Stock
Our Common Stock has been approved for quotation on the OTCQB under the symbol “SURP.” The Company stock began trading on OTCQB on September 20, 2010. The table below sets forth the high and low bid prices for our Common Stock for the eight quarters ended December 31, 2014 as reported on the OTCQB and OTCBB websites for the relevant quarters. The information reflects inter-dealer prices, without retail mark-ups, mark-downs or commissions and may not necessarily represent actual transactions. See “Item 1A. Risk Factors—Shares of our Common Stock are very thinly traded, and the price may not reflect our value and there can be no assurance that there will be an active market for shares of our Common Stock either now or in the future” and “Item 1A. Risk Factors —Because it may be considered a “penny stock,” our shareholders may have difficulty selling shares of our Common Stock.” Also, see “Item 1A. Risk Factors— If we are not able to satisfy the eligibility standards of the OTCQB market, the trading of our shares will become more difficult”
| Common Stock | Market Price | |||||||
| Quarter Ended | Bid High ($) | Bid Low ($) | ||||||
| December 31, 2014 | 0.19 | 0.04 | ||||||
| September 30, 2014 | 0.44 | 0.13 | ||||||
| June 30, 2014 | 0.70 | 0.47 | ||||||
| March 31, 2014 | 0.91 | 0.40 | ||||||
| December 31, 2013 | 0.91 | 0.75 | ||||||
| September 30, 2013 | 1.17 | 0.85 | ||||||
| June 30, 2013 | 1.34 | 0.92 | ||||||
| March 31, 2013 | 1.32 | 1.00 | ||||||
As of December 31, 2014, 47,345,816 shares of our Common Stock were issued and outstanding, an additional 14,800,000 shares of our Common Stock were issuable upon the conversion of the issued and outstanding shares of our Nonvoting Convertible Preferred Stock., We have reserved 3,000,000 shares of our Common Stock for future issuance under the 2012 Stock Plan, under which we issued options to purchase 475,000 shares of our Common Stock in 2013.
Record Holders
As of December 31, 2014, there were approximately 90 holders of record of our Common Stock.
| 38 |
Equity Compensation Plan Information
As of December 31, 2013, options to purchase 475,000 shares of our Common Stock had been issued under our 2012 Nonqualified Stock Option Plan (the “2012 Stock Plan”). Under the terms of the grants of those options, options to purchase 190,000 of those shares vested on the date of grant, options to purchase 142,500 shares vested on November 12, 2014 and options to purchase the remaining 142,500 shares vest on November 12, 2015, all subject to early vesting upon a change in control of the Company.
DTC Eligibility
DTC, a financial industry service provider that immobilizes and makes "book-entry" changes to ownership of securities, has advised us that shares of our Common Stock became “DTC eligible”. This means that shares of our Common Stock can be electronically transferred between brokerage accounts. It is, however, possible that in the future DTC could change the status of our shares to other than “DTC eligible” or otherwise subject them to a limited status that DTC refers to as a “chill.” Were either or both of these events to occur, the process for transferring our shares between brokerage accounts would become manual. Manual processing may take several days and is not a favored option for companies such as us that rely on broker dealers for stock transactions. Unless and until we again became DTC eligible, our shares might not be able to trade with any volume. See “Item 1A. Risk Factors—Our shares may lose their status as “DTC-eligible.””
Dividend Policy
We have never paid or declared any dividends on our shares of Common Stock. Any future decisions regarding dividends will be made by our board of directors. We currently intend to retain and use any future earnings for the development and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Our board of directors has complete discretion on whether to pay dividends. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant.
Performance Graph
As a smaller reporting company, as defined in Rule 12b-2 promulgated under the Exchange Act, and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide this the information requested by this Item.
| 39 |
Item 6. SELECTED FINANCIAL DATA
As a smaller reporting company, as defined in Rule 12b-2 promulgated under the Exchange Act, and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information requested by this Item.
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward Looking Statements
This “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other parts of this Annual Report on Form 10-Q contains “forward-looking statements” that involve risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially from those expressed or implied by such forward-looking statements. The statements contained in this Annual Report on Form 10-Q that are not purely historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Forward-looking statements are often identified by the use of words such as, but not limited to, “anticipate,” “believe,” “can,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “will,” “plan,” “project,” “seek,” “should,” “target,” “will,” “would,” and similar expressions or variations intended to identify forward-looking statements. These statements are based on the beliefs and assumptions of our management based on information currently available to them. Such forward-looking statements are subject to risks, uncertainties and other important factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2013 filed with the Securities and Exchange Commission (the “Commission”), as well as in other documents that we have filed with the Commission. Furthermore, such forward-looking statements speak only as of the date of this Annual Report on Form 10-K. Except as required by law, we undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date of such statements.
Rounding
Certain figures included in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other parts of this Annual Report on Form 10-K have been rounded for ease of presentation. Percentage figures included in this Annual Report on Form 10-K have not in all cases been calculated on the basis of such rounded figures but on the basis of such amounts prior to rounding. For this reason, certain percentage amounts in this Annual Report on Form 10-K may vary from those obtained by performing the same calculations using the figures in our financial statements. Certain other amounts that appear in this Annual Report on Form 10-K may not sum due to rounding.
Overview
The results presented in this “Management’s Discussion and Analysis of Financial Conditions and Results of Operations” are those of SurePure, Inc. and its subsidiaries (all of which are collectively referred to in this Management’s Discussion and Analysis of Financial Condition and Results of Operations as “we”) as reflected in our unaudited consolidated financial statements included in this Annual Report on Form 10-K. The following discussion should be read in conjunction with such consolidated financial statements and the notes thereto. The results presented below pertain to our audited consolidated financial statements for the years ended December 30, 2014 and 2013.
| 40 |
Insufficient Funding
For the period from our inception on August 24, 2005 to December 31, 2014, we had net losses from operations of approximately $34,325,000. During the year ended December 31, 2014, we continued to experience both insufficient operating revenues and a significant shortfall in funding from investors. As a result, we require significant additional funding to maintain our operations and execute our business plan. Funding provided during the first half of the year ended December 31, 2014 was provided by certain of our current shareholders. If we are unable to obtain financing from our current shareholders or other sources, we will be unable to meet our business objectives and may scale back or even discontinue our operations until such time, if ever, that we are able to obtain financing. Notwithstanding our efforts to obtain financing, there is no assurance that any financing will be available on acceptable terms or at all. See “—Liquidity and Capital Resources.”
Business Overview
SurePure Inc. (SURP) is quoted on the USA OTC QB market and owns the international patent for a novel liquid purification technology that uses UV, rather than heat, to sterilise liquids. Using ultraviolet C light, the photopurification process can deliver the same or superior microbiological efficacy to pasteurisation on both clear and turbid liquids without the concomitant energy consumption or degradation of the liquid, either biochemically or organoleptically
Whilst UV light has long been used as a surface steriliser and has been applied to clear water successfully, this is the first technology of its kind that can process opaque or turbid liquids. Its scope of application is therefore virtually unlimited. Food-grade liquid application in beverages abound; technical application to process water and liquid raw materials, as well as liquids such as diesel are possible; even the medical application of this technology from blood to breast milk is being explored. We continue to do research work either by ourselves or in conjucntion with clients to establish the the efficacy on other liquids including various consumer beverages and home care products.
.
The heart of the SurePure photopurification technology is the turbulator technology for which SurePure holds 1 United States and 16 foreign patents. The unique and self-cleaning design of the turbulator enables the purification of turbid liquids on an industrial processing scale.
We have experienced negative cash flows from operations with respect to our business since our inception. As of December 31, 2014, we did not have adequate working capital resources to satisfy our current liabilities and as a result we have substantial doubt about our ability to continue as a going concern. Based on our current projections, including equity financing subsequent to December 31, 2014, we are not assured that we will have the cash resources to enable us to continue to fund normal operations.
We reported a number of accomplishments during period from January 1, 2014 to the date of this report:
| ● | On February 26th, we announced developments in the non-thermal processing of potato rinse water; |
| ● | On March 6th, we announced a further flavor preservation application and technology sale to a South African producer of fruit juice, carbomated soft drinks, water and non-alcoholic malt drinks; |
| ● | On March 12th, we announced additional brewing breakthrough for craft brewing; |
| ● | On March 28th, we announced our commercial deal with Helpac, a major essential oils and floral water bio-producer; |
| ● | On April 4th, we announced the publication in the World of Food Ingredients demonstrating value of SurePure’s photopurification technology in protein based liquid ingredients; |
| 41 |
| ● | On April 9th, we announced results of dairy study underlining efficacy of patented photopurification technology; |
| ● | On April 23rd, we announced a breakthrough in comtaminated diesel purification without biocides; |
| ● | On July 21st, we announced the production of cider for craft brewing market; |
| ● | On July 24th, we announced preliminary results for additional commercial application of our patented photopurification technology in the dairy industry. |
| ● | On August 6th, we announced that our technology demonstrates energy savings to the brewing industry through our colaboration with SAB Miller ; |
| ● | On August 7th, we announced that we concluded an agreement for the sale of photopurification units to a major US producer of high quality protein product, delivery of which is expected to be take place during the first quarter of 2015; and |
| ● | During the period to date in 2015 we have received orders to the value of US$940 000, delivery of which is expected to be take place during the second quarter of 2015. |
Additionally we concluded the final step in the acquisition of the South Africa entities with the registration for sale of those stockholders shares in a Form S1.
Plan of Operation
We seek to expand the commercial acceptance of our SurePure Turbulator purification systems. To pursue this goal, contingent on our obtaining sufficient capital to maintain our operations, we plan to execute the following steps:
| · | Attend and promote our product at international trade shows in 2015 and 2016 for the brewing, dairy industries, liquid egg and industrial applications. At these venues we will seek to establish contact with industry participants, including distributors of engineered systems, and to make them aware of our product’s advantages and the expanded applications of the product that we have developed over the past 36 months; | |
| · | Continue to pursue regulatory clearances and approvals for applications, such as dairy products, that are required for our technology to be sold in those industries. In particular, we expect to take the following actions with regulatory agencies: |
| · | To request review of the SurePure technology by the US Food and Drug Administration (“FDA”) for a number of applications so that the technology may be categorized as “generally recognized as safe,” or “GRAS”; |
| · | To receive approval of the material that we and a UK customer submitted to the UK Food Standard Authority in June 2011 to obtain EU Novel Food approval; and |
| · | To receive approval from the International Organization of Wine and Vine (“OIV”) for the use of the SurePure technology for wine industry applications. |
| · | Seek to market and sell our product and our technology for industrial applications; | |
| · | Seek to expand the level of commercialization of our technology in the liquid egg, wine, dairy, fruit juice and other beverage industries; |
| 42 |
| · | Expand our distribution networks for our products by identifying additional distributors according to geographic market, expertise with specific liquids and existing client relationships and negotiating agreements with the identified distributors on terms acceptable to both the distributors and to us; and | |
| · | Obtain additional equity financing to support our growth from current shareholders, institutional investors or other investors to provide capital to support and augment our operations. |
Our goal is to grow significantly over the next five years through the use of working capital and equity financing, if capital and financing are available to us. If we are able to obtain sufficient financing, we believe that it will be feasible for us to grow our operations based on our strategy of targeting strategic global regions with multiple potential clients for the commercialization of our technology. However, we will also seek to carefully monitor the risks associated with achieving our goal to increase commercialization and meet client expectations while also becoming financially solvent.
During the year ended December 31, 2014, we focused on the following activities:
| · | continuing with commercial trials with key customers; | |
| · | concluding agreements with customers; | |
| · | working with regulatory authorities such that our technology can be employed in key areas; | |
| · | manufacturing, delivering and commissioning equipment ordered by customers; and | |
| · | negotiating financing arrangements. |
Subsequent to December 31, 2014, we have focused on the following activities:
| · | continuing with commercial trials with key customers; | |
| · | concluding agreements and accepting orders from new customers; | |
| · | pursuing the expansion of our customer base and distributor network internationally; | |
| · | manufacturing, delivering and commissioning equipment ordered by customers; and | |
| · | negotiating possible equity financing agreements. |
Results of Operations
The following table summarizes our results of operations for the year ended December 31, 2014 compared with our results of operations for the year ended December 31, 2013:
| 43 |
| Years Ended: | Variance | |||||||||||||||
| December 31, 2014 | December 31, 2013 | $ | % | |||||||||||||
| Revenues | $ | 557,000 | $ | 171,000 | 386,000 | 226 | % | |||||||||
| General and Admin Expenses | 2,960,000 | 4,021,000 | (1,061,000 | ) | -26 | % | ||||||||||
| Research and Development | 193,000 | 227,000 | (34,000 | ) | -15 | % | ||||||||||
| Interest Expense | 179,000 | 152,000 | 27,000 | 18 | % | |||||||||||
| Net Loss | 3,099,000 | 4,419,000 | (1,320,000 | ) | -30 | % | ||||||||||
Revenues
We had revenues of approximately $557,000 during the year ended December 31, 2014, representing a 226% increase as compared to approximately $171,000 for the year ended December 31, 2013. This increase as compared to the 2013 year was primarily due to the increase in new customer installations during the period.
Although we are negotiating agreements for the commercialization of our SurePure technology, it is unlikely that more than one or two of those agreements as currently structured will generate significant amounts of revenue during the next six months. As a general matter, we expect that revenues will increase to the extent that our commercialization efforts are successful. These commercialization efforts seek to introduce our technology to a broader range of clients and geographic areas. Our business model is largely based on sales of equipment, usage royalties or usage fees. We do not expect, however, an increased level of commercialization to occur before the third quarter of 2015, at the earliest.
General and Administrative Expenses
We had general and administrative expenses of approximately $2,960,000 during the year ended December 31, 2014, representing a 26% decrease as compared with approximately $4,021,000 during the year ended December 31, 2013. This decrease as compared to 2013 was primarily due to lower professional fees resulting from the conclusion of the incorporation of the SA entities during 2013 as well as lower travel expenditures due to timing of customer visits.
General and administrative expenses are largely attributable to employment costs, professional and consulting fees and business travel expenses.
| 44 |
We expect that our general and administrative expenses will increase in future periods if we are able to obtain funding for our operations, as the increased level of commercialization of our technology will require increased levels of staffing and associated expenditures.
Research and Development Expenses
We had research and development expenses of approximately $193,000 for the year ended December 31, 2014, representing a 15% decrease as compared with approximately 227,000 for the year ended December 31, 2013.
We expect that our research and development expenses will increase in future periods as we pursue new applications and clients for our technology.
Interest Expense
We had interest expense of approximately $179,000 for the year ended December 31, 2014, representing a 18% increase, as compared to approximately $152,000 of interest expense for the year ended December 31, 2013. This increase was primarily due to the interest cost relating to the convertible note, partly offset by the lower interest costs resulting from the restructuring of the shareholder loans into equity as part of the acquisition of the SA entities during 2013.
Net Loss
For the reasons discussed above, we had a net loss of approximately $3,099,000 for the year ended December 31, 2014, representing a 30% decrease in net losses as compared to a net loss of approximately $4,419,000 for the year ended December 31, 2013.
We have not as yet generated sufficient revenue to fund our operations, and we expect our net losses to continue until such time as our commercialization efforts related to our technologies produce an increase in revenues sufficient to meet the cost of our operations. Accordingly, we expect to continue to operate at a loss through at least Q3, 2015.
Income Tax Expense (Benefit)
We have realized net operating losses in Switzerland, South Africa and the United States. In addition to net operating losses in prior periods, our net operating losses during the 2014 year may result in a potential future income tax benefit, to the extent that any unexpired net operating loss carry-forwards can offset future operating profits.
Impact of Inflation
We believe that inflation has not had a material effect on our operations for the period from August 24, 2005 (when our operations began) to December 31, 2014.
Capital Expenditures
We have spent approximately $182,000 dollars on property and equipment for the period from August 24, 2005 (when our operations began) to December 31, 2014.
| 45 |
Liquidity and Capital Resources
Our principal sources of liquidity have historically been through sales of our Common Stock to our investors. During the year ended December 31, 2013, we sold shares of our Common Stock to two institutional investors. During 2013, Regency Capital Corporation (“Regency”) purchased 2,349,500 of the 3,237,318 shares of our Common Stock that we sold to investors during the year, all at a cash purchase price of $1.00 per share. During 2014, Regency purchased an additional 1,006,250 shares of our Common Stock at a cash purchase price of $1.00 per share, all of which purchases occurred during the first __ months of the year. Regency is an affiliate of XOptics (PTC) Ltd., a British Virgin Islands corporation, which holds 14,800,000 shares of our Non-Voting Convertible Preferred Stock. We believe that the ability of Regency to provide financing to us by purchasing additional shares is limited, and we do not know whether Regency will be able provide significant amounts of financing to us during 2014 or thereafter.
An affiliate of Trinity purchased 184,825 shares from us for $184,825 during the first quarter of 2014. We cannot be certain that Trinity or its affiliates will purchase additional shares from us at the price that we have offered, or at any price.
We continue to discuss potential financing transactions with our shareholders and others, but we cannot provide any assurance that we will be able to conclude financing transactions with these or any other investors.
Our current assets are insufficient to meet our current obligations or to satisfy our cash needs over the next twelve months and, as such, we will require debt or equity financing. We are pursuing prospective sources for equity financing in an amount that would allow us to meet our commercialization targets. We face significant obstacles to attracting new financing due to our historical and current record of net losses and working capital deficits. Therefore, despite our efforts, we can provide no assurances that we will be able to obtain the financing required to meet our stated objectives or even to continue our business as a going concern.
The following table summarizes our financial position at December 31, 2014 compared with our financial position at December 31, 2013:
:
| As of: | Variance | |||||||||||||||
| December 31, 2014 | December 31, 2013 | $ | % | |||||||||||||
| Cash | $ | 1,000 | $ | 21,000 | (20,000 | ) | -95 | % | ||||||||
| Other Current Assets | 423,000 | 175,000 | 248,000 | 142 | % | |||||||||||
| Total Assets | 519,000 | 319,000 | 207,000 | 63 | % | |||||||||||
| Current Liabilities | 3,079,000 | 1,671,000 | 1,408,000 | 84 | % | |||||||||||
| 46 |
As noted above under “Insufficient Funding” and as also noted below under this heading, our current cash resources are extremely limited.
We have been in the development stage since we began operations in August 2005. As of December 31, 2014, we had cash of approximately $1,000 and other current assets of approximately $423,000, consisting of accounts receivable and prepaid expenses, and total assets of approximately $519,000. As of December 31, 2014, we had current liabilities of approximately $3,079,000, consisting of accounts payable, accounts payable to related parties and accrued liabilities. Of this amount, approximately $1,148,000 represented liabilities that were due to officers and stockholders, an increase in liabilities to officers and stockholders of approximately $422,000 as compared to such amount as of December 31, 2013. Our shareholders’ deficit was approximately $2,560,000 as of December 31, 2014, compared with deficit of approximately $1,353,000 as of December 31, 2013.
Cash Flow Analysis
For the period from August 24, 2005 (when we began operations) to December 31, 2014, our cash used in operating activities was approximately $27,676,000. We expect to continue to have negative cash flows from operating activities until such time as we fully commercialize our technology.
For the year ended December 31, 2014 our cash used in operating activities was $1,315,000, which amount corresponded mainly to net losses from our operations partly offset by an increase in accounts payable and amounts due to officers and stockholders.
We do not expect to pay cash dividends in the foreseeable future.
We have a defined stock option plan and contractual commitments with all of our officers and directors. See “Market for Registrants Common Equity, Related Equity Stockholder Matters and Issuer Purchases of Equity Securities—Equity Compensation Plan Information” below in this Annual Report on Form 10-K.
We plan to increase the number of employees to meet the anticipated demand if we are able to achieve wider acceptance of our technology.
Off Balance Sheet Arrangements
As of December 31, 2014, we had no off-balance sheet arrangements.
| 47 |
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
As a smaller reporting company, as defined in Rule 12b-2 promulgated under the Exchange Act, and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information requested by this Item.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The consolidated financial statements and supplementary data required by this item are included at page F-1 herein.
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
Item 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We designed our disclosure controls and procedures, as such term is defined in Rule 13a-15(e) and 15d-15(e) under the Exchange Act, to provide reasonable assurance that information required to be disclosed by us in reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosures.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures as of the end of the period covered by this report. Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective to provide such reasonable assurance.
In designing and evaluating the disclosure controls and procedures, management recognized that such controls and procedures, as any controls and procedures, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
| 48 |
Management’s Annual Report on Internal Control Over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2014. In making this assessment, the Company’s management used the criteria set forth by the Committee of Sponsoring Organization of the Treadway Commission (“COSO”) in “Internal Control-Integrated Framework.” Based on this assessment, management concluded that as of December 31, 2014, the Company’s internal control over financial reporting is effective.
As a smaller reporting company, the Company is not required to include in this annual report a report on Form 10-K the effectiveness of internal control over financial reporting by the Company’s independent registered public accounting firm.
Changes in Internal Control Over Financial Reporting
During management’s assessment of the effectiveness of the Company’s internal control over financial reporting, management did not identify any change that occurred during the year ended December 31, 2014 that has materially affected or is reasonably likely to materially affect the Company’s internal control over financial reporting.
None.
| 49 |
Item 10. DIRECTORS AND EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table sets forth the names and ages of the Company’s directors and executive officers as of December 31, 2014. At this time, our board of directors has no nominating or compensation committees and our audit committee has no members.
| Name | Age | Position with the Company | Officer/Director Since | |||
| Guy Kebble | 48 | Chief Executive Officer | December 12, 2012 | |||
| President | December 12, 2012 | |||||
| Director | January 6, 2013 | |||||
| Stephen Robinson | 51 | Chief Financial Officer | December 12, 2012 | |||
| Chief Accounting Officer | December 12, 2012 | |||||
| Treasurer | December 12, 2012 | |||||
| Secretary | December 12, 2012 | |||||
| Director | December 12, 2012 |
Term of Office
Our directors and executive officers hold office until the earlier of their death, resignation, removal or until their successors have been duly elected and qualified. Our executive officers are employed by us on full time basis and are appointed by the board of directors and serve at the discretion of the board, subject to the provisions of the employment and consulting agreements referred to under “Item 11. Executive Compensation—Employment and Consulting Agreements.” There are no family relationships among our directors and executive officers.
Agreements and Understandings
Our executive officers have entered into employment or consulting contracts with SPO and SPMSA. The terms and conditions of those agreements are described under “Item 11. Executive Compensation—Employment and Consulting Agreements.” Except as disclosed under “Item 11. Executive Compensation—Employment and Consulting Agreements,” there are no arrangements or understandings between any of our directors and officers, and any other person, pursuant to which he serves as our officer or director.
The business experience during the past five years of our directors and executive officers is as follows:
Stephen Robinson —Prior to the Share Exchange, Mr. Robinson served as the chief financial officer of the SurePure group of companies since January 2008. Mr. Robinson also is a member of the board of directors of each of the companies in the SurePure group. Mr. Robinson’s role incorporates the following key areas of responsibility: finance and accounting support, legal and compliance; governance and operational support.
| 50 |
Mr. Robinson is a Chartered Accountant and attained his Bachelor of Accounting degree from the University of the Witwatersrand in South Africa in 1989. He has been registered with the South African Institute of Chartered Accountants since 1990. Mr. Robinson has over 20 years of financial executive experience in the mining and technology sectors during which he acquired wide ranging financial and general management skills honed in international corporate and medium sized companies in the Technology, Mining, Manufacturing, Logistics and Shared Service sectors. From September 2004 through July 2007, Mr. Robinson was employed as group finance manager for the Trans Hex Group Ltd., a South African diamond mining company.
Mr. Robinson was appointed as a member of the board of directors due to his strong experience in management, structuring and finance planning of international businesses. In addition to his extensive familiarity with SurePure group of companies, he had been employed by Samancor (then part of the BHP Billiton Group) for ten years where he became group finance manager and was involved with international joint ventures, corporate treasury and international reporting, as well as the corporate governance and risk management portfolios. For two years, he was employed by the largest mining section in the Anglo Platinum Group to develop and manage a multidisciplinary services unit, which deployed logistical, engineering, financial and human resources support to four key operating units accounting for a substantial portion of Anglo Platinum’s outputs. Mr. Robinson is a citizen of the United Kingdom and a resident of Switzerland.
Guy Kebble —Prior to the Share Exchange, Mr. Kebble served as the chief executive officer and a director of SPHSA and SPMSA since the inception of the SurePure business in 2005, both of which companies are variable interest entities of SurePure US. Mr. Kebble’s role at SurePure incorporates the following key areas of responsibility: shareholder interaction, strategic development, brand and market assessment, key stakeholder relationships and team building.
Mr. Kebble began his career in 1990 working for Natal Rugby Union as a professional rugby player. From 1996 to 2004 he managed private property portfolio managed investments. His involvement as a financier in the South African mining business led to the SurePure technology being brought forward as a project. Mr. Kebble is a citizen and resident of South Africa. His enthusiasm, passion and drive have been instrumental in bringing the technology to the current stage of technical development and market awareness. These qualifications, among others, led to the conclusion that Mr. Kebble should serve as a member of our board of directors.
Family Relationships
None of our officers or directors is related to any other officer or director.
| 51 |
Involvement in Certain Legal Proceedings
During the past ten years no director, executive officer, promoter or control person of SurePure US, SPI or any of their subsidiaries, and no person who became a director of SurePure US in January 2013, has:
| · | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| · | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| · | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| · | been found by a court of competent jurisdiction in a civil action or by the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| · | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| · | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Procedures for Security Holders to Nominate Candidates to the Board of Directors
During 2014, there were no changes made to the procedures by which shareholders may nominate candidates to our board of directors. The full board of directors will consider all director candidates nominated by shareholders during such times as the Company is actively considering appointment of new directors. Candidates recommended by shareholders will be evaluated based on the same criteria described above. Shareholders desiring to suggest a candidate for consideration should send a letter to the Company’s Corporate Secretary and include:
| 52 |
| · | a statement that the writer is a shareholder (providing evidence if the person’s shares are held in street name) and is proposing a candidate for consideration; |
| · | the name and contact information for the candidate; |
| · | a statement of the candidate’s business and educational experience; |
| · | information regarding the candidate’s qualifications to be director, including but not limited to an evaluation of the factors discussed above which the board would consider in evaluating a candidate; |
| · | information regarding any relationship or understanding between the proposing shareholder and the candidate; |
| · | information regarding potential conflicts of interest; and |
| · | a statement that the candidate is willing to be considered and willing to serve as director if nominated and elected. |
There is no assurance that all shareholder proposed candidates will be fully considered, that all candidates will be considered equally, or that the proponent of any candidate or the proposed candidate will be contacted by the Company or the board, and no undertaking to do so is implied by the willingness to consider candidates proposed by shareholders.
Our bylaws do not set forth procedures by which our shareholders may recommend nominees to our board of directors.
Director Independence
We are not currently a “listed company” under the rules of the Commission and are therefore not required to have a board comprised of a majority of independent directors or separate committees comprised of independent directors. We do not have any independent directors. We use the definition of “independence” under Rule 5605 of the Nasdaq Stock Market Rules, as applicable and as may be modified or supplemented from time to time and the interpretations thereunder, to determine if the members of our board are independent. In making this determination, our board considers, among other things, transactions and relationships between each director and his immediate family and the Company, including those described under “Item 11. Executive Compensation.” The purpose of this review is to determine whether any such relationships or transactions are material and, therefore, inconsistent with a determination that the directors are independent.
Board Committees
As of the date of this annual report on Form 10-K, we do not have any board committees. We may in the future adopt certain board committees, although we cannot assure you when or if this will occur. Accordingly, the descriptions below are for informational purposes only.
| 53 |
Audit Committee
We do not have an Audit Committee.
Executive Compensation, and Director Nomination and Corporate Governance Function
We do not have a compensation committee, nominating or corporate governance committee.
Section 16(a) Beneficial Ownership Reporting Compliance
Executive officers, directors and certain persons who own more than 10% of our outstanding Common Stock are required by Section 16(a) of the Exchange Act to file with the Commission an initial report of ownership of our Common Stock on Form 3 and reports of change s in ownership on Form 4 or Form 5, and such persons are required to furnish us with copies of all such forms that they file. We believe that all such Section 16(a) reports were filed on a timely basis in 2014.
Code of Ethics
We have a Code of Ethics and Business Conduct (the “Code”) that applies to all directors, officers and employees, which will be posted on its website or can be obtained by writing to us at 405 Lexington Avenue, 25th/26th Floor, New York, New York 10174 c/o Corporate Secretary. All of our directors, officers and employees are expected to be familiar with the Code and to adhere to those principles and procedures set forth in the Code that apply to them. The Company will post any amendments to the Code, as well as any waivers that are required to be disclosed by the rules of the Commission, on the Company’s web site.
Item 11. EXECUTIVE COMPENSATION
In determining executive compensation, we consider the skills and added value of the individuals concerned, as well as relative cost of living and the statutory requirements in the jurisdiction in which the individual provides his services. Since our executives provide their services to various of our subsidiaries, in some cases we use separate employment contracts.
| 54 |
Summary Compensation Table
The following table sets forth, for the periods indicated, all of the compensation awarded to, earned by or paid to (i) each individual serving as a principal executive officer of SurePure or any of the subsidiaries or related operating entities of SurePure during our last completed fiscal year; and (ii) each other individual that served as an executive officer or significant employee of SurePure or any of the subsidiaries or related operating entities of SurePure at the conclusion of the fiscal year ended December 31, 2014 and who received in excess of $100,000 in compensation during such fiscal year (collectively, the “named executive officers”).
| Name and Principal | Option | |||||||||||||||||||
| Position | Year | Salary | Bonus | Awards | Total | |||||||||||||||
| Guy R. Kebble, | 2014 | $ | 274,512 | $ | - | $ | - | $ | 274,512 | |||||||||||
| Director, President and Chief Executive Officer (1) | 2013 | $ | 285,840 | $ | - | $ | 69,700 | $ | 355,540 | |||||||||||
| Stephen M. Robinson, | 2014 | $ | 439,708 | $ | - | - | $ | 439,708 | ||||||||||||
| Director and Chief Financial Officer (2) | 2013 | $ | 433,758 | $ | - | $ | 69,700 | $ | 503,458 | |||||||||||
| Stephen Miller, Vice | 2014 | $ | - | $ | - | - | $ | - | ||||||||||||
| President-Sales and Marketing Manager (3) | 2013 | $ | 249,840 | $ | - | $ | 52,275 | $ | 302,115 | |||||||||||
| (1) | Has served as chief executive officer of the companies controlled by SPI and as Chairman of SPMSA since January 1, 2010. |
| (2) | Has served as chief financial officer of the companies controlled by SPI and as the sole director of SPI, SPO and SPP and as a director of SPMSA and SPHSA since January 1, 2008. |
| (3) | Mr. Miller’s responsibilities were modified on December 20, 2013, and he left our employ on August 31, 2014. |
In 2015, Messrs. Kebble and Robinson agreed to reduce their compensation effective January 1, 2015 to the amounts described below under “Employment and Consultancy Agreements.”
| 55 |
Employment and Consulting Agreements
Guy Kebble and SPMSA entered into an updated Service Agreement dated July 1, 2012, under which SPMSA appointed Mr. Kebble as a permanent employee in the position of chief executive officer. The appointment is of indefinite duration, but may be terminated either by the employer or by Mr. Kebble on not less than three months’ prior written notice to the other and may also be summarily terminated by the employer if the executive commits a material breach of his contractual obligations under the agreement or in any circumstances justifying such termination at law. As chief executive officer, Mr. Kebble reports to the board of directors. His compensation under the agreement is ZAR 960,000 per annum (approximately $96,000) and is to be reviewed annually by the board of directors with a view to granting inflation-related or other increases. Mr. Kebble is also to be reimbursed for expenses incurred on behalf of SPMSA. The agreement provides for 20 days of vacation per year.
Mr. Kebble also has entered into an updated Consulting Agreement with SPO, dated January 1, 2012, under which he provides his services as an independent contractor to develop markets globally for those products and services marketed by SPO and to provide such other assistance to SPO as agreed with the directors of SPO from time to time. Under this agreement, Mr. Kebble has agreed to serve as the chief executive officer of SurePure US and its subsidiaries. Effective January 1, 2015, Mr. Kebble’s remuneration from SPO is $150,000 per annum payable in equal monthly installments. Either party to the consulting agreement may terminate it for convenience on three months’ prior written notice. SPO has the right to terminate the agreement for cause with immediate notice. The agreement further provides that during the term of the agreement and for one year after the completion of his services to SPO, Mr. Kebble will not provide any services relating to the products or services of SPO to its clients. The agreement further provides that Mr. Kebble may not disclose any of SPO’s confidential information or other trade secrets and may not solicit or contact any employee, advisor or other individual working on behalf of clients in connection with SPO’s products or services for the purpose of obtaining or executing new work for the same 12-month period. The agreement is governed by Swiss law.
Stephen Robinson entered into an Employment Agreement with SPO, dated July 1, 2012, under which he is employed as the chief financial officer of SPO. In performing his duties, Mr. Robinson reports to the chief executive officer and board of directors of SPO. His duties are to be determined by the board of directors and include those normally associated with the position of chief financial officer. Subject to provisions of applicable law which permit termination for cause, Mr. Robinson’s appointment may be terminated either by SPO or by Mr. Robinson on not less than three months’ prior written notice to the other. Effective January 1, 2015 Mr. Robinson’s remuneration from SPO is CHF 318,000 (approximately $348,000) per annum payable in equal monthly installments and is to be reviewed annually by the board of directors with a view to granting inflation-related or other increases. In addition, the employer agrees to make contributions for social security and other compulsory expenses, plus the cost of certain airfare, and other local travel expenses in Switzerland. Mr. Robinson also is entitled to receive an allowance of CHF 500 (approximately $600) per month. The agreement further provides that Mr. Robinson may not disclose any of SPO’s confidential information or other trade secrets.
| 56 |
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information regarding each unexercised option held by each of the named executive officers as of December 31, 2014:
Option Awards
| Number of Securities Underlying Unexercised Options | Number of Securities Underlying Unexercised Options | Stock Option | Stock Option | |||||||||||
| Name | Exercisable | Unexercisable | Exercise Price | Expiration Date | ||||||||||
| Guy R. Kebble, Director, President and Chief Executive Officer | 70,000 | 30,000 | 0.89 | 11/11/2023 | ||||||||||
| Stephen M. Robinson, Director and Chief Financial Officer | 70,000 | 30,000 | 0.89 | 11/11/2023 | ||||||||||
Each grant of options vests and becomes exercisable as follows: 40% on the date of grant; the next 30% on the first anniversary of the date of grant; and the remaining 30% on the second anniversary of the date of grant. The exercise price of each option is $0.89 per share which was the fair market value of our Common Stock, as ascertained by our Board of Directors.
2012 Stock Plan
In December of 2012, our directors and a majority of our shareholders, acting by written consent, approved the 2012 Stock Plan. We have reserved 3,000,000 shares of our Common Stock for future issuance under the 2012 Stock Plan, of which 475,000 shares are subject to outstanding grants of options.
| 57 |
The 2012 Stock Plan authorizes the granting of non-qualified stock options to our directors and to any independent consultants. The Plan will be administered by our board, or a committee appointed by our board (in either case, the “Committee”). The Committee may determine persons eligible for grants and the timing, type, amount, fair market value and other provisions of such grants. The Committee will have authority, subject to the express provisions of the Plan, to construe the Plan and the option agreements granted pursuant to the Plan, to prescribe, amend and rescind rules and regulations relating to the Plan, and to make all other determinations in the judgment of the Committee necessary or desirable for the administration of the Plan. The Committee may correct any defect or supply any omission or reconcile any inconsistency in the Plan or in any option agreement in the manner and to the extent it shall deem expedient to promote the best interests of the Company. Our board may suspend or terminate the Plan at any time.
The Plan provides that the determination of the option price per share for any option rests in the sole and unfettered discretion of the Committee. Options granted under the Plan must be granted within ten years from the date on which the Plan has been adopted. No options granted under the Plan may be exercisable after ten years from the date of grant. The Committee may establish installment exercise terms for an option such that the option becomes fully exercisable in a series of accumulating portions. The Committee may also accelerate the exercise of any option. Options may be exercised by delivery of a written notice of exercise and payment of the full price of the Common Stock underlying the option. The exercise price of an option may be paid in cash, or, at the discretion of the Committee, through the delivery of fully paid and nonassessable shares of Common Stock having an aggregate fair market value as of the date of exercise equal to the option price, or by a combination of both. The Committee has the right to determine acceptable methods for tendering shares of Common Stock as payment upon exercise and may impose such limitations and prohibitions on the use of Common Stock to exercise an option as it deems appropriate. With the consent of the optionee, the Committee may cancel any options issued under the Plan and issue a new option to the optionee. Except by will or the laws of descent and distribution, or with the written consent of the Committee, no optionee may assign his interest in any option and no interest of any optionee in a stock option may become subject to any lien or obligation of the optionee. Options shall be exercisable during the optionee's lifetime only by the optionee or his assignees or by the duly appointed legal representative of an incompetent optionee or after the optionee's lifetime only by the duly appointed legal representative of the deceased optionee.
If the Company becomes a party to any merger, consolidation, reorganization or sale of assets, each outstanding option applies to the securities or property which a holder of the Company’s Common Stock be entitled to receive pursuant to such transaction. Whenever a change in control occurs, certain optionees are entitled to receive, in lieu of the exercise of their options, a cash payment equal to the difference between the exercise price of the option and the final offer price per share paid for common stock or such lower price as the Committee may determine to conform an option to preserve its status or the aggregate fair market value of the Common Stock underlying the stock options.
At any time and from time to time the Company’s board of directors may suspend or terminate the Plan in whole or in part or amend the Plan in such respects as the board may deem appropriate and in the best interest of the company. No amendment, suspension or termination of the Plan shall, however, without the optionee's consent, alter or impair any of the rights or obligations granted under the Plan.
| 58 |
Each option granted under the plan is to be embodied in a written stock option agreement and shall be signed by the optionee and an officer of the Company. Option agreements are to be subject to applicable provisions of the Plan and such other provisions as the Committee may adopt.
The Plan terminates on December 9, 2022.
Director Compensation
We have not yet determined any policy for the payment of compensation to our directors in their capacities as directors. Our directors received no compensation in their capacity as such during 2013 or 2014. We may make grants of options from time to time under the 2012 Stock Plan to our directors in addition to those grants made in November 2013.
Our directors have been, and will continue to be, reimbursed for the reasonable out-of-pocket costs incurred by them in connection with travel to and from board meetings.
| 59 |
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table summarizes certain information regarding the beneficial ownership (as such term is defined in Rule 13d-3 under the Exchange Act) of our outstanding Common Stock as of December 31, 2014 by (i) each person known by us to be the beneficial owner of more than 5% of our outstanding Common Stock, (ii) each of our directors, (iii) each of our named executive officers (as defined in Item 402(m) of Regulation S-K under the Securities Act), and (iv) all executive officers and directors as a group. Shares of our common stock subject to options, warrants, or other rights currently exercisable or exercisable within 60 days of the date of the Share Exchange are deemed to be beneficially owned and outstanding for purposes of computing the share ownership and percentage of the person holding such options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other person. An asterisk (*) denotes less than 1%.
| Name of Beneficial Owner | Number of Shares Beneficially Owned (1) | Percent Beneficially Owned (1) | ||||||
| Guy Kebble P.O. Box 71 Milnerton Cape Town South Africa, 7435 | 1,070,000 | (2) | 2.3 | % | ||||
| Stephen Robinson Dammstrasse 19, CH-6301, Zug, Switzerland | 853,637 | (3) | 1.8 | % | ||||
| All Officers and Directors as a Group | 1,923,637 | 4.1 | % | |||||
| (1) | Based on the sum of (a) 47,345,816 shares issued and outstanding as of December 31, 2013, and (b) as to each listed beneficial owner, any additional rights to acquire shares of our Common Stock that are exercisable within the 60 days after December 31, 2013. | |
| (2) | Includes options to purchase 70,000 shares of our Common Stock granted to Mr Kebble under our 2012 Stock Plan, which are currently exercisable. | |
| (3) | Includes options to purchase 70,000 shares of our Common Stock granted to Mr Robinson under our 2012 Stock Plan, which are currently exercisable. |
| 60 |
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Transactions with Related Persons
Transactions with XOptics
On December 12, 2012, we entered into an Amended and Restated Share Exchange Agreement (the “Amended and Restated Share Exchange Agreement”) with all of the shareholders of SPI, including XOptics, that provided for the exchange of all shares of capital stock of SPI, par value CHF 0.01, for shares of our common stock, par value $.001 per share (our “Common Stock”), and for shares of our Nonvoting Convertible Preferred Stock, par value $.01 per share (the “Nonvoting Convertible Preferred Stock”), each share of which will be convertible, subject to certain terms and conditions, into one share of our Common Stock. The Amended and Restated Share Exchange Agreement amended and restated the Share Exchange Agreement (the “Original Share Exchange Agreement”), dated October 28, 2011, which we had entered into with the shareholders of SPI at that time. The Original Share Exchange Agreement was entered into to replace the Agreement and Plan of Merger that we had entered into with SPI on July 8, 2011, and which the parties thereafter terminated and replaced due to unanticipated regulatory and tax complications that made a merger transaction between them impractical. The Amended and Restated Share Exchange Agreement contained customary representations, warranties and conditions to closing. The Amended and Restated Share Exchange Agreement required that, as a condition to closing, we first have redeemed and then cancelled 23,180,000 outstanding shares of our common stock held by Ratree Yabamrung and Kotchaporn Bousing, two of our officers, directors and shareholders prior to the Share Exchange. By its approval of the Amended and Restated Share Exchange Agreement, our board of directors authorized and directed us to redeem Ms. Yabamrung’s shares immediately prior to the closing in exchange for her being assigned all of our assets (of which there were none on the date of the Share Exchange) and her assumption of all our liabilities. Ms. Bousing tendered all of her shares to us for no consideration, and we then took the initial steps to cancel all of the shares owned by each Ms. Bousing and Ms. Yabamrung. The principal effect of the cancellations of these shares is that the existing shareholders of SPI acquired greater percentage ownership interests in SurePure US and may have acquired more influence or control and greater ability to delay, defer or prevent any potential changes in control. We did not obtain the approval of our shareholders for the transaction that we executed with Ms. Yabamrung. Although we believe that shareholder approval was not required under Nevada law for the transaction with her, it is possible that persons who were shareholders at the time of the Share Exchange may claim that their approval was required, in which case litigation might follow. See “Item 1A. Risk Factors—We Did Not Obtain Shareholder Approval for a Transaction with a Former Shareholder.”
Transactions between XOptics and Certain of our Other Shareholders Prior to the Share Exchange
On or about March 3, 2011, XOptics purchased 2,906,937 shares of our Common Stock (after giving effect to the 15.2:1 stock split that we effected on June 14, 2011) from our shareholders in a resale by those shareholders under our registration statement which was initially filed with the Commission on March 23, 2009. The aggregate purchase price for the shares was $145,347, and XOptics paid the purchase price to the selling shareholders in cash from its working capital.
| 61 |
In the second and third quarters of 2011, XOptics sold 1,857,000 of our shares in approximately 19 transactions at prices ranging from $1.00 to $1.25 per share for aggregate proceeds of approximately $1,970,500. None of the buyers of the shares were U.S. Persons (as defined in Regulation S promulgated by the Commission) or were resident in the United States. Upon completing a sale of shares on June 15, 2011, XOptics held less than 5% of our outstanding shares of common stock.
During the fourth quarter of 2011 and the first four months of 2012, XOptics sold a total of 376,167 shares of our Common Stock in approximately 11 transactions at prices ranging from $1.10 to $1.26 per share for aggregate proceeds of approximately $425,400. None of the buyers of the shares were persons who were U.S. Persons or were resident in the United States.
As of December 31, 2013, XOptics beneficially owned no shares of our Common Stock.
Transactions between XOptics and SPI and Subsidiaries of SPI
In October 2007, XOptics and its 100%-owned subsidiary SPP purchased 18,360,000 shares of the common shares of SPI for approximately $174,000, or approximately $0.01 per share, as part of the initial capitalization of SPI.
In September 2008, XOptics acquired an additional 163,347 shares from SPI at a price of $2.45 per share.
In December 2010, as part of a sale of 2,781,818 shares to its then existing shareholders at $0.01 per share in proportion to their shareholdings at that time, SPI sold 1,298,182 of its common shares to XOptics and 370,909 of its common shares to SPP.
From time to time between January 1, 2011 and July 31, 2012, XOptics advanced funds to SPI. The advances were not documented and did not state either a rate of interest or maturity date. There was, however, a written subordination agreement that provided that the advances would not be classified as current liabilities of SPI or be payable within one year if doing so would have caused SPI to be considered insolvent. The funds that XOptics advanced to SPI were principally derived from the sale of SPI shares by XOptics in a series of private transactions, all of which occurred outside of the United States. The advances made were as follows:
| Quarter and Year | Amount of Advance | |||
| 2nd Quarter 2011 | $ | 1,686,815 | ||
| 3rd Quarter 2011 | $ | 370,000 | ||
| 4th Quarter 2011 | $ | 1,084,523 | ||
| 1st Quarter 2012 | $ | 894,500 | ||
| 2nd Quarter 2012 | $ | 1,043,500 | ||
| 3rd Quarter 2012 | $ | - | ||
| 4th Quarter 2012 | $ | - | ||
| Total | $ | 5,079,338 | ||
| 62 |
The loan balance was reduced by a loan conversion of $1,390,130 for 1,390,130 shares of SPI in November 2011. The loan balance also included loans outstanding to SPI in the aggregate principal amount of $300,000 that XOptics purchased from three other shareholders of SPI. None of the shareholders that sold the loans to XOptics was an affiliate of XOptics. The acquired loans were included in the loan balance that was converted in July 2012, as discussed in the following paragraph.
The loan balance was paid in full by the conversion of the remaining balance of $3,689,208 into 7,378,416 common shares of SPI at the rate of $0.50 per share in July 2012. In order to satisfy a condition to the investment by the investor referred to under “—Financing Transactions with Other Related Persons—RD Active Subscription Agreement,” SPI convened an annual general meeting on July 18, 2012. All of the shareholders of SPI attended by proxy and at the meeting there was unanimous approval for an increase in the authorized share capital of SPI for the purpose of, among other matters, satisfying the proposed conversion of outstanding shareholder loans into common shares of SPI. The shareholders of SPI further authorized the board of directors to determine the issue price of the new shares to be issued. Based on this authorization, the board of directors of SPI resolved to issue 7,378,416 common shares to XOptics to convert the existing shareholder loans due to XOptics in the amount of $3,689,208 at the rate of $0.50 per share. As part of the Share Exchange, XOptics exchanged the common shares of SPI issued in the conversion of the shareholder loans, together with other common shares of SPI owned by it at the time, for shares of our Nonvoting Convertible Preferred Stock at the rate of one share of our Nonvoting Convertible Preferred Stock for each common share of SPI owned by it.
Ownership of Our Shares by XOptics Immediately Post-Share Exchange
As of the Share Exchange, XOptics beneficially owned 22,665,447 common shares of SPI. Upon the closing of the Share Exchange, these shares were exchanged for an equivalent number of our shares of Nonvoting Convertible Preferred Stock. XOptics owned 19,800,000 shares of our Nonvoting Convertible Preferred Stock as of December 31, 2013.
The terms of our Nonvoting Convertible Preferred Stock provide that no holder of shares of our Nonvoting Convertible Preferred Stock which, on a fully converted basis, and when combined with shares of our Common Stock, would constitute the holder thereof the beneficial holder of more than 5.0% of shares of our Common Stock, may enter into any transaction with us or with any of our subsidiaries without the prior approval of a majority of our independent directors or, if we have no independent directors, the prior approval of a majority of the shares of our Common Stock not owned by the beneficial holder in question and its affiliates.
| 63 |
Certain Transactions between Officers and Directors of SPI and XOptics Prior to the Share Exchange
Prior to the Share Exchange and while SPI was a privately-owned company, XOptics made certain transfers of shares to the key executives of the business, and in one case to a family member of a key executive, for no or for nominal consideration. Stephen Robinson, a member of our board of directors and our chief financial officer, acquired 463,637 common shares of SPI from XOptics on December 20, 2010. Stephen Miller, formerly our vice president sales and marketing, also acquired 463,637 shares of SPI from XOptics on December 20, 2010. In February 2011, Mr. Robinson acquired 210,000 additional common shares of SPI and Mr. Miller acquired 110,000 additional common shares of SPI from XOptics. As a result of these transfers, after giving effect to the Share Exchange, Mr. Robinson and Mr. Miller owned 673,637 and 573,637 shares respectively of our Common Stock in addition to the 140,000 and 109,033 shares of our Common Stock that they respectively owned prior to the Share Exchange, having acquired these shares directly from us in March 2011.
On November 1, 2012 XOptics transferred 1,000,000 common shares of SPI to Guy Kebble, a member of our board of directors and our chief executive officer, and 70,000 shares of SPI to Oliver Kebble, who is the adult son of Guy Kebble.
Transactions between XOptics and Trinity
During 2013 XOptics sold 410,631 restricted shares of our Common Stock to Trinity in a private transaction. On the basis of information provided to us by XOptics and Trinity, we believe that the sales were conducted outside of the United States and that neither XOptics nor Trinity is a “U.S. person” as defined by Regulation S under the Securities Act. In September 2013, XOptics sold 900,000 registered shares of our Common Stock to Trinity in a private transaction.
Transactions with Regency
Regency is a corporation formed under the laws of the Turks and Caicos Islands which is controlled by Richard G. Wilson. Mr. Wilson also controls XOptics. During the first half of 2013, Regency purchased 538,000 shares of our Common Stock from us as an assignee of RD Active under the Additional Subscription Agreement. For information regarding that Agreement, see “—Financing Transactions with Other Related Persons—RD Active Subscription Agreement.”
| 64 |
During the second half of 2013, we entered into three separate agreements under which Regency acquired an aggregate of 1,811,500 of our shares during 2013. On September 2, 2013, we entered into the Share Purchase Agreement, dated July 25, 2013, with Regency, for the purchase of 661,500 shares of our Common Stock at an aggregate price of $661,500. Regency purchased 220,500 shares of our Common Stock upon executing the agreement and purchased the remaining 441,000 shares of our Common Stock in August and September as required by the agreement. We also entered into a Registration Rights Agreement, dated the same date, with Regency, under which we are obligated to file with the Commission a registration statement for the resale of the shares of our Common Stock that we issued to Regency and to use our best efforts to cause that registration to become effective as promptly as possible after its filing. We filed the registration statement on Form S-1 on February 3, 2014, which registration statement has not yet been declared effective. We also are obligated to use our reasonable best efforts to keep the registration statement that we have filed continuously effective until all of the shares covered by the registration statement have been sold or may be sold under Rule 144 without volume or manner of sale restrictions, including the filing of such post-effective amendments as may be necessary. The Registration Rights Agreement provides that we are to bear all fees and expenses incident to the performance of or compliance with the agreement whether or not any shares are sold pursuant to the registration statement. These costs and expenses include all registration and filing fees (including the fees and expenses of our counsel and independent certified public accountants), as well as all internal expenses incurred in connection with the registration. In addition, if prior to filing the required registration statement, we file a registration statement for the resale by other shareholders of shares of our Common Stock, including that which we are required to file for the former shareholders of SPHSA under the terms of the Acquisition Agreement that is referred to under “—Acquisition of SPHSA,” we are required to notify Regency of our determination to file a registration statement and include in the registration statement any shares purchased by Regency that Regency requests.
Regency also purchased 600,000 shares of our Common Stock under the Share Purchase Agreement, dated October 25, 2013, for an aggregate purchase price of $600,000. The Share Purchase Agreement also obligates us to include the shares that Regency has purchased in any registration statement that we file with Commission to register the resale by other shareholders of their shares, including that which we are required to file for the former shareholders of SPHSA under the terms of the Acquisition Agreement that is referred to below under “—Acquisition of SPHSA.” In that event, we are required to notify Regency of our determination to file a registration statement and include in the registration statement any shares purchased by Regency under the Share Purchase that Regency requests. We also are obligated to use our reasonable best efforts to keep the registration statement that we have filed continuously effective until all of the shares covered by the registration statement have been sold or may be sold under Rule 144 without volume or manner of sale restrictions, including the filing of such post-effective amendments as may be necessary. Our agreement with Regency provides that we are to bear all fees and expenses incident to the performance of or compliance with the agreement whether or not any shares are sold pursuant to the registration statement. These costs and expenses include all registration and filing fees (including the fees and expenses of our counsel and independent certified public accountants), as well as all internal expenses incurred in connection with the registration.
Regency also agreed to purchase 600,000 shares of our Common Stock under the Share Purchase Agreement, dated November 22, 2013. On November 22, 2013, Regency purchased 170,000 shares of our Common Stock for an aggregate price of $170,000. On each of December 6 and 18, 2013. Regency purchased an additional 90,000 shares of our Common Stock under this agreement for an aggregate purchase price of $180,000, and on December 30, 2013, Regency purchased an additional 200,000 shares of our Common Stock for an aggregate purchase price of $200,000. Under the November 22, 2013 agreement, Regency had the right to purchase 50,000 shares of our Common Stock through January 31, 2014 at a purchase price which is the greater of $1.00 per share and 92% of the volume weighted average price for the twenty trading days ending on the third trading day prior to the date of the purchase. The November 22, 2013 Share Purchase Agreement included registration provisions that are substantially identical with those in the Share Purchase Agreement, dated as of October 25, 2013, with Regency.
| 65 |
During 2014 we amended the November 22, 2013 Share Purchase Agreement three times. On February 13, 2014, we amended the November 22, 2013 Share Purchase Agreement to grant Regency the option to purchase an additional 500,000 shares of our Common Stock. On March 19, 2014, we amended the November 22, 2013 Share Purchase Agreement to grant Regency the option to purchase an additional 1,000,000 shares of our Common Stock prior to March 31, 2014. On June 24, 2014 we amended the November 22, 2013 Share Purchase Agreement to extend the expiration date of Regency’s option to June 30, 2014. During the first quarter of 2014, Regency purchased 615,000 shares of our Common Stock. During the second quarter of 2014, Regency purchased 250,000 shares. During the third quarter of 2014, Regency purchased 141,250 shares. All purchases were priced at $1.00 per share.
During 2013 Regency resold 1,339,369 shares of our Common Stock to Trinity in a private transaction. On the basis of information provided to us by Regency and Trinity, we believe that Regency applied a substantial portion of the proceeds of its resales to Trinity to purchase shares of our Common Stock under the share purchase agreements described above. See “Item 1A. Risk Factors—Since March 2013 we have not been able to raise equity capital from persons other than existing shareholders. We cannot predict whether existing shareholders will continue to fund our business in the absence of commercial results or otherwise” and “Item 13. Certain Relationships and Related Transactions—Transactions with Trinity and Affiliates—Trinity Purchases from XOptics and Regency.” We also believe that the resales were conducted outside of the United States and that neither Regency nor Trinity is a “U.S. person” as defined by Regulation S under the Securities Act.
Transactions with Trinity and Affiliates
We believe that during 2013 and parts of 2014 Trinity was the beneficial owner of 7,246,000, or 17.5%, of the shares of our Common Stock as of December 31, 2013, after including as beneficially owned 900,000 shares of our Common Stock referred to under “Item 13. Certain Relationships and Related Transactions—Transactions with Trinity Asset Management Limited and Affiliates—TAMIL Share Purchase Agreement.” See “Item 12. Security Ownership Of Certain Beneficial Owners and Management and Related Stockholder Matters.” We now believe that because of changes to its authority to vote and dispose of shares of our Common Stock, it no longer beneficially owns 5% or more of shares of our Common Stock.
| 66 |
Subscription Agreement for Shares
On July 23, 2012, SPI entered into a subscription agreement (the "Subscription Agreement") with Trinity Asset Management (PTE) Ltd., an institutional investor located in Cape Town, South Africa under which Trinity agreed, subject to the terms and conditions of the Subscription Agreement, to purchase 5,000,000 common shares of SPI from SPI and 1,000,000 common shares of SPI from XOptics in a series of six share installments, all at an issue price of $1.00 per share. The first two installments were for 500,000 shares each; the other four installments were for 1,000,000 shares each. The Subscription Agreement provided that not less than 75% of each installment was to be paid for shares issued by SPI or by us, as SPI’s successor under the Subscription Agreement. Consequently, not more than 25% of each installment could be for the purchase of shares from XOptics. Also, XOptics was not permitted to sell more than 1,000,000 of its shares to Trinity under the Subscription Agreement.
Prior to the Share Exchange, Trinity had purchased 3,094,737 of the 6,000,000 shares covered by the Subscription Agreement all at the price of $1.00 per share, as follows:
| Date of Purchase | Total Shares Purchased | Shares Purchased from SPI | Shares Purchased from XOptics | |||||||||
| July 30, 2012 | 500,000 | 375,000 | 125,000 | |||||||||
| August 17, 2012 | 500,000 | 375,000 | 125,000 | |||||||||
| September 28, 2012 | 1,767,737 | 1,517,737 | 250,000 | |||||||||
| October 9, 2012 | 327,000 | 232,263 | 94,737 | |||||||||
| Total | 3,094,737 | 2,500,000 | 594,737 | |||||||||
As a result, 2,500,000 additional common shares of SPI were issued after June 30, 2012. In accordance with the terms and conditions of the Share Exchange Agreement, shares of SPI that are owned by Trinity, by persons on whose behalf Trinity purchased shares from SPI or XOptics or by persons to whom Trinity assigned its rights under the Subscription Agreement were to be exchanged for shares of our Common Stock on the basis of the Exchange Ratio. Trinity and SPI agreed that the installments due on August 31, 2012 and September 30, 2012 could be paid together and SPI waived any penalty resulting from the investor’s late performance.
Under the terms of the Subscription Agreement, three installments of shares remained to be purchased on each of October 31, November 30 and December 31, 2012, all at the price of $1.00 per share. Of the 2,905,263 shares remaining to be purchased by Trinity, 2,500,000 were to have been purchased from SPI and 405,263 were to have been purchased from XOptics. Trinity failed to purchase shares as agreed on October 31, 2012. Exercising its rights under the Subscription Agreement, SPI terminated the Subscription Agreement and all rights of the investor to purchase additional shares, including both the shares referenced in the table above as well as additional shares as to which SPI had granted Trinity three purchase options, together with Trinity’s right to appoint directors. The termination of the Subscription Agreement also applied to the obligation of Trinity to purchase shares from XOptics.
| 67 |
Trinity Share Purchase Agreement dated May 24, 2013
On May 24, 2013, we and Trinity entered into the Share Purchase Agreement (the “May 24 2013 Share Purchase Agreement”), dated that date, under which Trinity agreed to purchase 1,000,000 shares of our Common Stock, par value $0.001 per share, in three installments during the period from May 24 through June 28, 2013 each at a cash purchase price of $1.00 per share. At the time of the execution of the Share Purchase Agreement by both parties, Trinity purchased 250,000 shares of our Common Stock for $250,000 and thereafter purchased 151,318 shares of our Common Stock.
The shares purchased by Trinity under the May 24 2013 Share Purchase Agreement are restricted shares and bear a restrictive legend. The May 24 2013 Share Purchase Agreement provided that, if Trinity fully performed its obligations to purchase 1,000,000 shares, which it did not, we would be obligated to use our best efforts to include the shares so purchased in any registration statement that we file with the Commission on or after July 31, 2013 for the resale of shares of our Common Stock by the shareholders. On or after June 1, 2014, Trinity may demand that we use our best efforts to register the shares and any additional shares purchased for resale by Trinity. We have elected to register the shares that Trinity purchased under the May 24 2013 Share Purchase Agreement to avoid becoming subject to the registration obligation later in 2014.
Tamil Share Purchase Agreement dated September 19, 2013, as Amended
On September 20, 2013, we and Trinity Asset Management International Limited (“TAMIL”), a company formed under the laws of Mauritius, entered into the Share Purchase Agreement (the “TAMIL Share Purchase Agreement”), dated as of September 19, 2013, under which TAMIL agreed to purchase 900,000 shares of our Common Stock, par value $0.001 per share, during the period ending March 25, 2014 in a series of nine installments. On November 7, 2013, we and TAMIL entered into an amendment of the TAMIL Share Purchase Agreement Under the terms of the TAMIL Share Purchase Agreement, as amended, TAMIL may purchase the 900,000 shares of our Common Stock from us from time to time during the period ending March 25, 2014 at a cash purchase price equal to the greater of $1.00 per share and 92% of the volume-weighted average trading price of shares of our Common Stock during the 20 trading day period ending on the day that is 3 days prior to the closing of the purchase and sale of our shares.
During the first quarter of 2014, TAMIL purchased 184,825 shares of our Common Stock.
The shares purchased and to be purchased by TAMIL under the TAMIL Share Purchase Agreement are restricted shares and bear a restrictive legend. In conjunction with the TAMIL Share Purchase Agreement, we and TAMIL entered into a separate registration rights agreement which provides that, within 60 days after the date when TAMIL has purchased all of the shares required to be purchased under the TAMIL Share Purchase Agreement, we will file a registration statement for the resale of the shares sold under that agreement.
| 68 |
In the TAMIL Share Purchase Agreement TAMIL represented to us that it was an “accredited investor” as defined in Regulation D promulgated under the Securities Act, and was also not a “U.S. Person” within the meaning of Regulation S promulgated under the Securities Act. TAMIL also represented that it was not acquiring the shares under the TAMIL Share Purchase Agreement for the benefit of any U.S. Person, that it will be the sole beneficial owner of the shares and that it has not prearranged any sale to any persons in the United States. TAMIL was outside of the United States when it entered into the TAMIL Share Purchase Agreement and is expected to be outside of the United States at such time as it purchases the shares under that agreement. On the basis of the representations and warranties that TAMIL has made to us in the TAMIL Share Purchase Agreement, we believe that the sales of shares of our Common Stock under the TAMIL Share Purchase Agreements will be exempt from the registration requirements of the Securities Act.
TAMIL has advised us that it is related to Trinity.
Agreement for Subscription of Shares dated May 20, 2014
On May 20, 2014 we and Trinity entered into the Agreement for the Subscription of Shares (the “May 2014 Trinity Subscription Agreement”). Under the May 2014 Trinity Subscription Agreement, Trinity agreed to arrange, from time to time until July 30, 2014, for persons that desired to subscribe for shares of our Common Stock to tender and deliver to us separate written offers to purchase up to 850,000 shares of our Common Stock. Upon our receipt of an offer from a subscriber, if we accepted the offer we were obligated to notify Trinity of our acceptance and to issue to the subscriber named in the offer the shares of our Common Stock that were subscribed for against payment of the purchase price for the shares. On July 24, 2014, we and Trinity extended the date by which it could arrange for subscriptions to September 30, 2014.
Trinity entered into the May 2014 Trinity Subscription Agreement in part to obtain our release of TAMIL from its obligations under the TAMIL Share Purchase Agreement. As noted above, under the TAMIL Share Purchase Agreement, TAMIL was obligated to purchase 900,000 shares of our Common Stock and had purchased only 184,825 shares prior to the termination of the Share Purchase Agreement on March 25, 2014. As part of the May 2014 Trinity Subscription Agreement, upon the issue, sale and delivery of all of the 850,000 shares available for subscription under the May 2014 Trinity Subscription Agreement, TAMIL was to have been released from its obligations under the TAMIL Share Purchase Agreement. During 2014 Trinity did not arrange any offers to subscribe for shares of our Common Stock under the May 2014 Trinity Subscription Agreement or otherwise.
Trinity Purchases from XOptics and Regency
We believe that, during 2013, Trinity acquired 1,750,000 shares of our Common Stock from XOptics and Regency. The shares acquired from Regency have the benefit of the registration rights that we granted to Trinity in the applicable purchase agreements and related agreements. We further believe that Regency applied a substantial amount of the proceeds from resales it made to Trinity to Regency’s purchases of shares of our Common Stock from us. We are not aware of any purchases by Trinity from Regency during 2014.
| 69 |
Acquisition of SPHSA
On June 12, 2013, we completed the acquisition of SPHSA and SPHSA’s wholly-owned subsidiary, SPMSA. The terms and conditions of the acquisition were set forth in the Acquisition Agreement, dated August 16, 2012 (the “Acquisition Agreement”), between our subsidiary, SPI, and SPHSA. On December 12, 2012, as part of the Share Exchange, we assumed the obligations of SPI under the Acquisition Agreement. As a result of acquisition, both SPHSA and SPMSA have become our wholly-owned subsidiaries. The financial statements of SPHSA and SPMSA will be fully consolidated with our financial statements, beginning with our financial statements for the quarter ended June 30, 2013. In addition, we have been able to directly control the intellectual property of these entities.
The purchase price for the shares of SPHSA was ZAR 4,000 (approximately $500.00 as of the date of the Acquisition Agreement) per share. The Acquisition Agreement provides that we will settle the purchase price in our shares in the ratios of (i) 1,000 shares of our Common Stock and (ii) 1,000 common shares of our Common Stock for each ZAR 4,000 in principal amount of loans owed by SPHSA to its shareholders.
As a result of the acquisition of SPHSA, we issued 9,864,811 shares of our Common Stock to the former shareholders of SPHSA, after giving effect to conversions of shareholder loans. Of those shares 8,389,836 were issued to Roger Kebble, a shareholder of SPHSA, after giving effect to the conversion of his loans to SPHSA under the terms of the acquisition, and the remaining 1,474,976 shares of its Common Stock were issued to the other 35 shareholders of SPHSA. Transferability of the shares was restricted under the provisions of the Securities Act of 1933, as amended.
Our acquisition of the shares of SPHSA was subject to several conditions imposed by the Reserve Bank of South Africa (“SARB”), the governmental agency which is responsible for administration of that country’s exchange control laws and regulations. With respect to this transaction, SARB required that all shares of our Common Stock that were issued in exchange for the shares of SPHSA be held in custody by a South African bank so that the proceeds of all sales of shares are remitted to South Africa. The custodian bank agreed that it would place orders for the sales of the shares, remit the proceeds of sales only to shareholder banks accounts in South African banks and ensure that the sale process conforms to the sale process as approved by SARB. SARB also required that we place restrictive legends on the share certificates representing the shares of our Common Stock issued to the shareholders of SPHSA, that we complete the registration of the resale of those shares of our Common Stock with the Commission within five months after their issuance and that the former shareholders of SPHSA complete the resales of their shares of our Common Stock within 180 days after our registration statement is declared effective.
Consulting and Employment Agreements
For a more complete discussion of those agreements under which our directors and officers provide their services, see “Item 11. Executive Compensation—Employment and Consulting Agreements.”
| 70 |
Transactions with Other Parties
RD Active Subscription Agreement
On November 26, 2012, SPI entered into a Subscription Agreement (the “Additional Subscription Agreement”) with RD Active Capital Limited, a United Kingdom-based investment manager (“RD Active”), in which RD Active agreed to purchase up to 300,000 new common shares over the period ending January 31, 2013 and under which RD Active had the right to purchase up to 2,700,000 new common shares from the Company during the period ending March 29, 2013 as long as RD Active has purchased the initial 300,000 common shares which it agreed to purchase. All common shares are to be purchased and sold at an issue price of US $1.00 per share. RD Active agreed to purchase the 300,000 new common shares on an installment basis, with 100,000 shares to be purchased on or before each of November 28, 2012, December 21, 2012 and January 31, 2013. On March 28, 2013, we and RD Active agreed to extend the termination date for the Additional Subscription Agreement to April 12, 2013, and on April 12, 2013 we further agreed to extend the ending date to May 31, 2013. As of May 31, 2013, when the agreement had terminated, RD Active and its other purchasers, including Regency, had purchased an aggregate of 1,159,500 shares.
As required by the Share Exchange Agreement, we assumed the obligations of SPI under the Additional Subscription Agreement. Specifically, we agreed that the following terms and conditions will govern the exchange of shares acquired by RD Active and its other purchasers: (i) we will not have any issued and outstanding securities other than our Common Stock and our Nonvoting Convertible Preferred Stock; (ii) shares of our Common Stock will continue to be quoted on the OTCBB or another comparable interdealer quotation system as long as we file all reports with the Commission that are required to be filed; and (iii) the remainder of the shares to be acquired under the Additional Subscription Agreement will be shares of our Common Stock and SPI will no longer have any obligations to issue its common shares.
As part of the Additional Subscription Agreement, we agreed that we would use our best efforts to register for resale to the public all of our Common Shares that RD Active and any other purchasers under the Additional Subscription Agreement have purchased. Each purchase of shares under the Additional Subscription Agreement was subject to the accuracy of certain representations and warranties made by the purchasers of the shares. Among the representations and warranties made, RD Active represented that it has no offices or presence in the United States of America and none of the accounts that it manages are beneficially owned by citizens or residents of the United States of America.
The Additional Subscription Agreement provided that for purposes of the Securities Act, the shares sold under the Additional Subscription Agreement are restricted securities and may not be resold to any person who is a “U.S. person” as defined by Regulation S under the Securities Act. Moreover, RD Active agreed not to sell or transfer the shares without our prior written consent or in a transaction which has been notified to us and does not involve any U.S. person or any means of commerce in the United States until one year and four days after the Share Exchange. Similarly, any shares which were not included in the registration statement which we were obligated to file may not be sold without our prior written consent or in a transaction of which the Company has been notified and does not involve any U.S. Person or any means of commerce in the United States.
| 71 |
The shares of our Common Stock that we issued to RD Active and the other purchasers under the Additional Subscription Agreement bear legends restricting their transferability until such shares have been sold as part of a registered public offering.
Minaurum Share Purchase Agreement
On September 2, 2013, we entered into the Share Purchase Agreement, dated that July 25, 2013, with Minaurum Limited (“Minaurum”), an existing shareholder, for the purchase of 100,000 shares of our Common Stock at an aggregate price of $100,000. Minaurum performed its obligations under the agreement by purchasing the shares upon full execution of the agreement. We also entered into a Registration Rights Agreement, dated the same date, with Minaurum, under which we are obligated to file with the Commission a registration statement for the resale of the shares of our Common Stock that we issued to Minaurum and to use our best efforts to cause that registration to become effective as promptly as possible after its filing. We filed such registration statement on Form S-1 on February 3, 2014, which registration statement has not yet been declared effective. We also are obligated to use our reasonable best efforts to keep the registration statement that we have filed continuously effective until all of the shares covered by the registration statement have been sold or may be sold under Rule 144 without volume or manner of sale restrictions, including the filing of such post-effective amendments as may be necessary. The Registration Rights Agreement provides that we are to bear all fees and expenses incident to the performance of or compliance with the agreement whether or not any shares are sold pursuant to the registration statement. These costs and expenses include all registration and filing fees (including the fees and expenses of our counsel and independent certified public accountants), as well as all internal expenses incurred in connection with the registration. In addition, if prior to filing the required registration statement, we file a registration statement for the resale by other shareholders of shares of our Common Stock, including that which we are required to file for the former shareholders of SPHSA under the terms of the Acquisition Agreement referred to below under “Item 13. Certain Relationships and Related Transactions—Financing Agreements with Other Persons—Acquisition of SPHSA,” we are required to notify Minaurum Limited of our determination to file a registration statement and include in the registration statement any shares purchased by Minaurum that Minaurum requests.
| 72 |
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
RSSM CPA LLP (“RSSM”), formerly known as Rosen Seymour Shapss Martin & Company LLP, served as the our independent registered public accounting firm for the fiscal years ended December 31, 2012, 2013 and 2014 and is expected to serve in that capacity for the 2015 fiscal year. RSSM served as the independent certified public accounting firm for SPI for 2011 and 2012. Total principal accounting fees for professional services rendered by RSSM for the twelve months ended December 31, 2014 and December 31, 2013, are summarized as follows:
| Fiscal 2014 | Fiscal 2013 | |||||||
| Audit | $ | 117,500 | $ | 253,224 | ||||
| Tax | — | — | ||||||
| Total | $ | 117,500 | $ | 253,224 | ||||
Audit Fees. RSSM’s 2014 audit fees were for professional services rendered in connection with the audit of SurePure’s 2014 consolidated financial statements included in this annual report on Form 10-K and RSSM’s review of our quarterly financial statements during 2014. RSSM’s 2013 audit fees were for professional services rendered in connection with the audit of SurePure’s 2013 consolidated financial statements included in this annual report on Form 10-K and RSSM’s review of our quarterly financial statements during 2013.
Tax Fees. There were no tax fees for professional services related to tax compliance, tax advice and tax planning within the United States that were paid to RSSM.
Board of Directors Pre-Approval Policies and Procedures. At its regularly scheduled meetings, the board of directors, in lieu of an established audit committee, considers and pre-approves any audit and non-audit services to be performed by our independent registered public accounting firm. The board of directors has the authority to grant pre-approvals of non-audit services.
| 73 |
The board of directors has not, as of the time of filing this annual report on Form 10-K with the Commission, adopted policies and procedures for pre-approving audit or permissible non-audit services performed by our independent auditors. Instead, the board of directors as a whole has pre-approved all such services. In the future, our board of directors may approve the services of our independent registered public accounting firm pursuant to pre-approval policies and procedures adopted by the board of directors, provided the policies and procedures are detailed as to the particular service, the board of directors is informed of each service, and such policies and procedures do not include delegation of the board of director’s responsibilities to our management.
The board of directors has determined that the provision of services by RSSM described above is compatible with maintaining RSSM’s independence at our independent registered public accounting firm.
| 74 |
Item 15. Exhibits and Financial Statement Schedules.
Financial Statement Schedules
Required information is included in the footnotes to the Consolidated Financial Statements.
| 75 |
EXHIBIT INDEX
| Exhibit No. |
Description | |
| 3.1 | Articles of Incorporation of SurePure, Inc., as amended. (1) | |
| 3.2 | Bylaws of SurePure, Inc. (2) | |
| 10.2 | Amended and Restated Share Exchange Agreement, dated December 12, 2012, by and among SurePure, Inc. (formerly SOEFL Inc.), XOptics (PTY) Limited and the holders of all shares in SurePure Investment Holdings AG (3) | |
| 10.3 | Acquisition Agreement, dated August 16, 2012, between SurePure Investment Holding AG and SurePure Holdings South Africa (Pty) Ltd. (3) | |
| 10.4 | Amendment to Acquisition Agreement, dated November 26, 2012 (3) | |
| 10.5 | 2012 Nonqualified Stock Option Plan. (3) | |
| 10.6 | Subscription Agreement, dated July 23, 2012, between SurePure Investment Holding AG and Trinity Investment Management (Pte) Limited. (3) | |
| 10.7 | Termination Letter, dated November 22, 2012, from SurePure Investment Holding AG to Trinity Investment Management (Pte) Limited. (3) | |
| 10.8 | Employment Agreement dated July 1, 2012, between SurePure Marketing South Africa Ltd. and Guy Kebble. (3) | |
| 10.9 | Consulting Agreement, dated July 1, 2012, between SurePure Operations AG and Guy Kebble. (3) | |
| 10.10 | Employment Agreement, dated July 1, 2012, between SurePure Operations AG and Stephen Robinson. (3) | |
| 10.11 | Employment Agreement dated July 1, 2012, between SurePure Marketing South Africa Ltd. and Stephen Miller. (3) | |
| 10.12 | Consulting Agreement, dated July 1, 2012, between SurePure Operations AG and Stephen Miller. (3) | |
| 10.13 | Agreement of Lease dated February 8, 2012, between Anglo African Development (Pty) Ltd. and SurePure Marketing South Africa (Pty) Ltd., effective March 1, 2012 until February 28, 2014, for the premises located at One Lagoon Beach, Unit 204, Lagoon Beach Milnerton, South Africa. (3) |
| 76 |
| 10.14 | Memorandum of Agreement of Lease, dated April 25, 2012, between HY Jack Property Holdings CC and SurePure Marketing South Africa Ltd., effective May 1, 2008, for the premises located at 4 Acteon Street, Unit 2, Paarden Eiland, South Africa. (3) | |
| 10.15 | Agreement of Extension of Lease, dated April 19, 2012, between HY Jack Property Holdings CC and SurePure Marketing South Africa Ltd, effective January 5, 2012 until November 11, 2012, for the premises located at 4 Acteon Street, Unit 2, Paarden Eiland, South Africa. (3) | |
| 10.17 | Form of Indemnification Agreement. (3) | |
| 10.18 | Subscription Agreement, dated November 26, 2012, between SurePure Investment Holding AG and RD Active Capital Limited. (4) | |
| 10.19 | Share Purchase Agreement, dated May 24, 2013, between SurePure, Inc. and Trinity Asset Management (Proprietary) Ltd. (5) | |
| 10.20 | Waiver under the Share Purchase Agreement, dated May 24, 2013, between SurePure, Inc. and Trinity Asset Management (Proprietary) Ltd. (6) | |
| 10.21 | Share Purchase Agreement, dated as of July 25, 2013, between SurePure, Inc. and MinAurum Limited. (7) | |
| 10.22 | Registration Rights Agreement, dated as of July 25, 2013, between SurePure, Inc. and MinAurum Limited. (7) | |
| 10.23 | Share Purchase Agreement, dated as of July 25, 2013, between SurePure, Inc. and Regency Capital Corporation. (7) | |
| 10.24 | Registration Rights Agreement, dated as of July 25, 2013, between SurePure, Inc. and Regency Capital Corporation. (7) | |
| 10.25 | Share Purchase Agreement, dated as of September 19. 2013, between SurePure, Inc. and Trinity Asset Management International Limited (8) | |
| 10.26 | Registration Rights Agreement, dated as of September 19. 2013, between SurePure, Inc. and Trinity Asset Management International Limited. (8) | |
| 10.27 | Share Purchase Agreement, dated as of October 25, 2013, between SurePure, Inc. and Regency Capital Corporation. (9) | |
| 10.28 | Amendment, dated as of November 7, 2013, to the Share Purchase Agreement, dated as of September 19. 2013, between SurePure, Inc. and Trinity Asset Management International Limited. (10) |
| 77 |
| 10.29 | Share Purchase Agreement, dated as of November 22, 2013, between SurePure, Inc. and Regency Capital Corporation (11) | |
| 10.30 | Amendment, dated February 13, 2014, to the Share Purchase Agreement, dated as of November 22, 2013, between SurePure, Inc. and Regency Capital Corporation (13) | |
| 10.31 | Second Amendment, dated March 19, 2014, to the Share Purchase Agreement, dated as of November 22, 2013, between SurePure, Inc. and Regency Capital Corporation (14) | |
| 10.32 | Agreement for the Subscription of Shares, dated as of May 20, 2014, between SurePure, Inc. and Trinity Asset Management (Proprietary) Limited (15) | |
| 10.33 | Lease Agreement, dated May 20, 2014, between Southern African Landmark Properties CC and SurePure Marketing South Africa (Pty) Ltd. (15) | |
| 10.34 | Third Amendment, dated June 26, 2014, to the Share Purchase Agreement, dated as of November 22, 2013, between SurePure, Inc. and Regency Capital Corporation (16) | |
| 10.35 | Securities Purchase Agreement, dated June 23, 2014, between the Company and Peak One (17) | |
| 10.36 | Debenture, issued June 23, 2014 by the Company to Peak One (17) | |
| 10.37 | Registration Rights Agreement, dated June 23, 2014, between the Company and Peak One (17) | |
| 10.38 | Guaranty Agreement, dated June 23, 2014, among SurePure Investment Holdings, AG, SurePure Operations, AG, SurePure Participations, AG, SurePure Holdings South Africa (Pty) Limited, SurePure Marketing South Africa (Pty) Limited and Peak One (17) | |
| 10.39 | Amendment, dated July 24, 2014, to the Agreement for the Subscription of Shares, dated as of May 20, 2014, between SurePure, Inc. and Trinity Asset Management (Proprietary) Limited (18) | |
| 16.2 | Letter from Seale & Beers, CPAs, dated December 21, 2012, to the Securities and Exchange Commission (12) | |
| 31.1* | Certification of the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2* | Certification of the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 78 |
| 32.1** | Certification of the Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2** | Certification of the Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101* | The following materials from SurePure, Inc. Form 10-K for the year ended December 31, 2014, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets at December 31, 2014 and 2013, (ii) Consolidated Statements of Operations for the years ended December 31, 2014 and 2013 and August 24, 2005 (inception) to December 31, 2013, (iii) Consolidated Statements of Other Comprehensive Income (Loss) for the years ended December 31, 2014 and 2013 and August 24, 2005 (inception) to December 31, 2014, (iv) Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2013 and 2012, and (v) Consolidated Statements of Cash Flows for the years ended December 31, 2014 and 2013 and August 24, 2005 (inception) to December 31, 2014 and (v) Notes to Consolidated Financial Statements. |
| (1) Incorporated by reference from Exhibit 3 to Form S-1 filed on March 23, 2009, Exhibit 3.1 to Form 8-K filed on July 29, 2011 and Exhibit 3.3 to Form 8-K filed on December 16, 2011. | ||
| (2) Incorporated by reference to Exhibit 3.2 to the registration statement of SOEFL Inc. on Form S-1, as filed with the Securities and Exchange Commission on March 23, 2009. | ||
| (3) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on December 12, 2012. | ||
| (4) Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the Commission on April 1, 2013. | ||
| (5) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on May 29, 2013 | ||
| (6) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on June 26, 2013. | ||
| (7) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on September 5, 2013. | ||
| (8) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on September 26, 2013 |
| 79 |
| (9) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on October 30, 2013 | ||
| (10) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on November 12, 2013 | ||
| (11) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on November 25, 2013 | ||
| (12) Incorporated herein by reference to the Company’s Current Report on Form 8-K/A filed with the Commission on December 21, 2012. | ||
| (13) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on February 18, 2014. | ||
| (14) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on March 25, 2014. | ||
| (15) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on May 22, 2014. | ||
| (16) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on June 26, 2014. | ||
| (17) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on June 27, 2014. | ||
| (18) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on July 28, 2014. | ||
| * Filed herewith | ||
| ** Furnished herewith |
| 80 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Guy R. Kebble | Chief Executive Officer; Director | March 30, 2015 | ||
| Guy R. Kebble | (Principal Executive Officer) | |||
| /s/ Stephen M. Robinson | Chief Financial Officer; Director | March 30, 2015 | ||
| Stephen M. Robinson |
(Principal Financial Officer and Principal Accounting Officer) |
|||
| 81 |
EXHIBIT INDEX
The following exhibits are filed as part of, or are incorporated by reference into, this annual report on Form 10-K:
| Exhibit No. |
Description | |
| 3.1 | Articles of Incorporation of SurePure, Inc., as amended. (1) | |
| 3.2 | Bylaws of SurePure, Inc. (2) | |
| 10.2 | Amended and Restated Share Exchange Agreement, dated December 12, 2012, by and among SurePure, Inc. (formerly SOEFL Inc.), XOptics (PTY) Limited and the holders of all shares in SurePure Investment Holdings AG (3) | |
| 10.3 | Acquisition Agreement, dated August 16, 2012, between SurePure Investment Holding AG and SurePure Holdings South Africa (Pty) Ltd. (3) | |
| 10.4 | Amendment to Acquisition Agreement, dated November 26, 2012 (3) | |
| 10.5 | 2012 Nonqualified Stock Option Plan. (3) | |
| 10.6 | Subscription Agreement, dated July 23, 2012, between SurePure Investment Holding AG and Trinity Investment Management (Pte) Limited. (3) | |
| 10.7 | Termination Letter, dated November 22, 2012, from SurePure Investment Holding AG to Trinity Investment Management (Pte) Limited. (3) | |
| 10.8 | Employment Agreement dated July 1, 2012, between SurePure Marketing South Africa Ltd. and Guy Kebble. (3) | |
| 10.9 | Consulting Agreement, dated July 1, 2012, between SurePure Operations AG and Guy Kebble. (3) | |
| 10.10 | Employment Agreement, dated July 1, 2012, between SurePure Operations AG and Stephen Robinson. (3) | |
| 10.11 | Employment Agreement dated July 1, 2012, between SurePure Marketing South Africa Ltd. and Stephen Miller. (3) | |
| 10.12 | Consulting Agreement, dated July 1, 2012, between SurePure Operations AG and Stephen Miller. (3) |
| 82 |
| 10.13 | Agreement of Lease dated February 8, 2012, between Anglo African Development (Pty) Ltd. and SurePure Marketing South Africa (Pty) Ltd., effective March 1, 2012 until February 28, 2014, for the premises located at One Lagoon Beach, Unit 204, Lagoon Beach Milnerton, South Africa. (3) | |
| 10.14 | Memorandum of Agreement of Lease, dated April 25, 2012, between HY Jack Property Holdings CC and SurePure Marketing South Africa Ltd., effective May 1, 2008, for the premises located at 4 Acteon Street, Unit 2, Paarden Eiland, South Africa. (3) | |
| 10.15 | Agreement of Extension of Lease, dated April 19, 2012, between HY Jack Property Holdings CC and SurePure Marketing South Africa Ltd, effective January 5, 2012 until November 11, 2012, for the premises located at 4 Acteon Street, Unit 2, Paarden Eiland, South Africa. (3) | |
| 10.17 | Form of Indemnification Agreement. (3) | |
| 10.18 | Subscription Agreement, dated November 26, 2012, between SurePure Investment Holding AG and RD Active Capital Limited. (4) | |
| 10.19 | Share Purchase Agreement, dated May 24, 2013, between SurePure, Inc. and Trinity Asset Management (Proprietary) Ltd. (5) | |
| 10.20 | Waiver under the Share Purchase Agreement, dated May 24, 2013, between SurePure, Inc. and Trinity Asset Management (Proprietary) Ltd. (6) | |
| 10.21 | Share Purchase Agreement, dated as of July 25, 2013, between SurePure, Inc. and MinAurum Limited. (7) | |
| 10.22 | Registration Rights Agreement, dated as of July 25, 2013, between SurePure, Inc. and MinAurum Limited. (7) | |
| 10.23 | Share Purchase Agreement, dated as of July 25, 2013, between SurePure, Inc. and Regency Capital Corporation. (7) | |
| 10.24 | Registration Rights Agreement, dated as of July 25, 2013, between SurePure, Inc. and Regency Capital Corporation. (7) | |
| 10.25 | Share Purchase Agreement, dated as of September 19. 2013, between SurePure, Inc. and Trinity Asset Management International Limited (8) | |
| 10.26 | Registration Rights Agreement, dated as of September 19. 2013, between SurePure, Inc. and Trinity Asset Management International Limited. (8) | |
| 10.27 | Share Purchase Agreement, dated as of October 25, 2013, between SurePure, Inc. and Regency Capital Corporation. (9) |
| 83 |
| 10.28 | Amendment, dated as of November 7, 2013, to the Share Purchase Agreement, dated as of September 19. 2013, between SurePure, Inc. and Trinity Asset Management International Limited. (10) | |
| 10.29 | Share Purchase Agreement, dated as of November 22, 2013, between SurePure, Inc. and Regency Capital Corporation (11) | |
| 10.30 | Amendment, dated February 13, 2014, to the Share Purchase Agreement, dated as of November 22, 2013, between SurePure, Inc. and Regency Capital Corporation (13) | |
| 10.31 | Second Amendment, dated March 19, 2014, to the Share Purchase Agreement, dated as of November 22, 2013, between SurePure, Inc. and Regency Capital Corporation (14) | |
| 10.32 | Agreement for the Subscription of Shares, dated as of May 20, 2014, between SurePure, Inc. and Trinity Asset Management (Proprietary) Limited (15) | |
| 10.33 | Lease Agreement, dated May 20, 2014, between Southern African Landmark Properties CC and SurePure Marketing South Africa (Pty) Ltd. (15) | |
| 10.34 | Third Amendment, dated June 26, 2014, to the Share Purchase Agreement, dated as of November 22, 2013, between SurePure, Inc. and Regency Capital Corporation (16) | |
| 10.35 | Securities Purchase Agreement, dated June 23, 2014, between the Company and Peak One (17) | |
| 10.36 | Debenture, issued June 23, 2014 by the Company to Peak One (17) | |
| 10.37 | Registration Rights Agreement, dated June 23, 2014, between the Company and Peak One (17) | |
| 10.38 | Guaranty Agreement, dated June 23, 2014, among SurePure Investment Holdings, AG, SurePure Operations, AG, SurePure Participations, AG, SurePure Holdings South Africa (Pty) Limited, SurePure Marketing South Africa (Pty) Limited and Peak One (17) | |
| 10.39 | Amendment, dated July 24, 2014, to the Agreement for the Subscription of Shares, dated as of May 20, 2014, between SurePure, Inc. and Trinity Asset Management (Proprietary) Limited (18) | |
| 16.2 | Letter from Seale & Beers, CPAs, dated December 21, 2012, to the Securities and Exchange Commission. (12) | |
| 31.1* | Certification of the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 84 |
| 31.2* | Certification of the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1** | Certification of the Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2** | Certification of the Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101* | The following materials from SurePure, Inc. Form 10-K for the year ended December 31, 2014, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets at December 31, 2014 and 2013, (ii) Consolidated Statements of Operations for the years ended December 31, 2014 and 2013 and August 24, 2005 (inception) to December 31, 2014, (iii) Consolidated Statements of Other Comprehensive Income (Loss) for the years ended December 31, 2014 and 2013 and August 24, 2005 (inception) to December 31, 2014, (iv) Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2013 and 2012, and (v) Consolidated Statements of Cash Flows for the years ended December 31, 2014 and 2013 and August 24, 2005 (inception) to December 31, 2014 and (v) Notes to Consolidated Financial Statements. |
| (1) Incorporated by reference from Exhibit 3 to Form S-1 filed on March 23, 2009, Exhibit 3.1 to Form 8-K filed on July 29, 2011 and Exhibit 3.3 to Form 8-K filed on December 16, 2011. | ||
| (2) Incorporated by reference to Exhibit 3.2 to the registration statement of SOEFL Inc. on Form S-1, as filed with the Securities and Exchange Commission on March 23, 2009. | ||
| (3) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on December 12, 2012. | ||
| (4) Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the Commission on April 1, 2013. | ||
| (5) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on May 29, 2013. | ||
| (6) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on June 26, 2013. | ||
| (7) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on September 5, 2013. |
| 85 |
| (8) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on September 26, 2013. | ||
| (9) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on October 30, 2013. | ||
| (10) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on November 12, 2013. | ||
| (11) Incorporated by reference to the Company’s Current Report on Form8-K filed with the Commission on November 25, 2013. | ||
| (12) Incorporated herein by reference to the Company’s Current Report on Form 8-K/A filed with the Commission on December 21, 2012. | ||
| (13) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on February 18, 2014 | ||
| (14) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on March 25, 2014 | ||
| (15) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on May 22, 2014 | ||
| (16) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on June 26, 2014 | ||
| (17) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on June 27, 2014. | ||
| (18) Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Commission on July 28, 2014. | ||
| * Filed herewith | ||
| ** Furnished herewith |
| 86 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
Consolidated Financial Statements
Years Ended December 31, 2014 and 2013 and
From August 24, 2005 (inception) to December 31, 2014
SUREPURE, INC. AND SUBSIDIARIES
(A Development Stage Company)
CONTENTS
| Page | |
| Consolidated Financial Statements | |
| Report of Independent Registered Public Accountants | F-2 |
| Consolidated Balance Sheets as of December 31, 2014 and 2013 | F-3 |
| Consolidated Statements of Operations for the years ended December 31, 2014 and 2013 and for the period from August 24, 2005 (inception) to December 31, 2014 | F-4 |
| Consolidated Statements of Other Comprehensive Income (Loss) for the years ended December 31, 2014 and 2013 and for the period from August 24, 2005 (inception) to December 31, 2014 | F-5 |
| Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2014 and 2013 | F-6 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2014 and 2013 and for the period from August 24, 2005 (inception) to December 31, 2014 | F-8 |
| Notes to Consolidated Financial Statements | F-9 to F-28 |
| F-1 |
Report of Independent Registered Public Accounting Firm
Board of Directors
SurePure Inc. and Subsidiaries
New York, New York
We have audited the accompanying consolidated balance sheets of SurePure, Inc. and Subsidiaries (“SurePure” or the “Company”) (a development stage company) as of December 31, 2014 and 2013 and the related consolidated statements of operations, stockholders’ deficit, other comprehensive income (loss) and cash flows for each of the years in the two-year period ended December 31, 2014, and for the period from August 24, 2005 (inception) to December 31, 2014. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of SurePure, Inc. and Subsidiaries at December 31, 2014 and 2013, and the results of its consolidated operations and its cash flows for each of the years in the two-year period ended December 31, 2014, and for the period from August 24, 2005 (inception) to December 31, 2014 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As shown in the consolidated financial statements, the Company has experienced significant losses and negative cash flows, resulting in increased accumulated deficits during its development stage. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are described in Note 15. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/RSSM CPA LLP
CERTIFIED PUBLIC ACCOUNTANTS
New York, New York
March 30, 2015
| F-2 |
SUREPURE, INC. AND SUBSIDIARIES
(A Development Stage Company)
DECEMBER 31, 2014 AND DECEMBER 31, 2013
| December 31, 2014 | December 31, 2013 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash | $ | 526 | $ | 20,870 | ||||
| Accounts receivable | 16,800 | 29,029 | ||||||
| Prepaid expenses and other current assets | 406,280 | 156,382 | ||||||
| Total current assets | 423,606 | 206,281 | ||||||
| Property and equipment, net | - | - | ||||||
| Other assets: | ||||||||
| Intangible assets, net | 95,622 | 112,290 | ||||||
| Total other assets | 95,622 | 112,290 | ||||||
| Total assets | $ | 519,228 | $ | 318,571 | ||||
| Liabilities and Stockholders' Equity (Deficit) | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and other current liabilities | $ | 1,252,203 | $ | 703,421 | ||||
| Customer deposits | 412,703 | 10,000 | ||||||
| Due to officers/stockholders | 1,148,085 | 725,699 | ||||||
| Income taxes payable | 480 | 533 | ||||||
| Loan payable | 265,522 | 231,490 | ||||||
| Total current liabilities | 3,078,993 | 1,671,143 | ||||||
| Total liabilities | 3,078,993 | 1,671,143 | ||||||
| Stockholders' equity (deficit): | ||||||||
| Common stock, $.001 par value, 200,000,000 shares authorized, 47,345,816 shares issued and outstanding (39,937,432 shares issued and outstanding at December 31, 2013) | 47,346 | 39,937 | ||||||
| Preferred stock, $.01 par values, 31,155,282 shares authorized, 14,800,000 shares issued and outstanding (19,800,000 shares issued and outstanding at December 31, 2013) | 148,000 | 198,000 | ||||||
| Additional paid-in capital | 30,570,760 | 28,728,466 | ||||||
| Other comprehensive income | 999,456 | 907,756 | ||||||
| Deficit accumulated | (34,325,327 | ) | (31,226,731 | ) | ||||
| Total stockholders' equity (deficit) | (2,559,765 | ) | (1,352,572 | ) | ||||
| Total liabilities and stockholders' equity (deficit) | $ | 519,228 | $ | 318,571 | ||||
See notes to Consolidated Financial Statements.
| F-3 |
SUREPURE, INC. AND SUBSIDIARIES
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF OPERATIONS
YEARS ENDED DECEMBER 31, 2014 AND 2013
AND CUMULATIVE FROM AUGUST 24, 2005 (INCEPTION) TO DECEMBER 31, 2014
| Cumulative | |||||||||||||
| From | |||||||||||||
| August 24, | |||||||||||||
| Year Ended | 2005 | ||||||||||||
| December 31, | (inception) to | ||||||||||||
| 2014 | 2013 | December 31, 2014 | |||||||||||
| Revenue | $ | 557,341 | $ | 170,838 | $ | 2,992,589 | |||||||
| Cost of revenue | 249,523 | 8,602 | 1,795,621 | ||||||||||
| Gross profit | 307,818 | 162,236 | 1,196,968 | ||||||||||
| Expenses: | |||||||||||||
| General and administrative expenses (includes equity-based compensation of $136,568, $151,053, and $287,621, respectively) | 2,959,571 | 4,020,530 | 27,372,608 | ||||||||||
| Promotion and marketing | 58,467 | 165,355 | 815,208 | ||||||||||
| Research and development | 192,870 | 227,011 | 4,049,181 | ||||||||||
| Depreciation and amortization | 16,668 | 21,283 | 214,050 | ||||||||||
| Impairment of patent | - | - | 537,631 | ||||||||||
| Total expenses | 3,227,576 | 4,434,179 | 32,988,678 | ||||||||||
| Loss from operations | (2,919,758 | ) | (4,271,943 | ) | (31,791,710 | ) | |||||||
| Other income (expense): | |||||||||||||
| Interest income | 11 | 2,033 | 373,902 | ||||||||||
| Interest expense | (178,849 | ) | (152,097 | ) | (2,788,172 | ) | |||||||
| Exchange rate gains and losses | - | 2,885 | (22,502 | ) | |||||||||
| Loss on disposition of fixed assets | - | - | (64,172 | ) | |||||||||
| Total other (expense) income | (178,838 | ) | (147,179 | ) | (2,500,944 | ) | |||||||
| Loss before provision for taxes | (3,098,596 | ) | (4,419,122 | ) | (34,292,654 | ) | |||||||
| Provision for income taxes | - | - | 32,673 | ||||||||||
| Net loss | (3,098,596 | ) | (4,419,122 | ) | (34,325,327 | ) | |||||||
| Net loss attributable to non-controlling interest | - | (17,653 | ) | (1,633,120 | ) | ||||||||
| Net loss attributable to SurePure | $ | (3,098,596 | ) | $ | (4,401,469 | ) | $ | (32,692,207 | ) | ||||
| Loss per share – basic and diluted attributable | |||||||||||||
| to SurePure common stockholders | $ | (0.07 | ) | $ | (0.14 | ) | $ | (1.10 | ) | ||||
| Weighted average shares outstanding - | |||||||||||||
| basic and diluted | 44,931,481 | 31,780,016 | 29,832,060 | ||||||||||
See notes to Consolidated Financial Statements.
| F-4 |
SUREPURE INC. AND SUBSIDIARIES
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF OTHER COMPREHENSIVE INCOME (LOSS)
YEARS ENDED SEPTEMBER 31, 2014 AND 2013
AND CUMULATIVE FROM AUGUST 24, 2005 (INCEPTION) TO DECEMBER 31, 2014
| Cumulative | ||||||||||||
| From | ||||||||||||
| August 24, | ||||||||||||
| Years Ended | 2005 | |||||||||||
| December 31, | (inception) to | |||||||||||
| 2014 | 2013 | December 30, 2014 | ||||||||||
| Net loss | $ | (3,098,596 | ) | $ | (4,419,122 | ) | $ | (34,325,327 | ) | |||
| Other comprehensive income, net of tax | ||||||||||||
| Unrealized gain on foreign currency translation | 91,700 | 461,073 | 999,456 | |||||||||
| Comprehensive loss | (3,006,896 | ) | (3,958,049 | ) | (33,325,871 | ) | ||||||
| Less: Comprehensive (loss) attributable to noncontrolling interest | - | 122,083 | (1,407,165 | ) | ||||||||
| Comprehensive loss attributable to SurePure, net of tax | $ | (3,006,896 | ) | $ | (4,080,132 | ) | $ | (31,918,706 | ) | |||
See notes to Consolidated Financial Statements.
| F-5 |
SUREPURE INC. AND SUBSIDIARIES
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' DEFICIT
CUMULATIVE FROM AUGUST 24, 2005 (INCEPTION) TO DECEMBER 31, 2014
| Equity in | Accumulated | |||||||||||||||||||||||||||||||||||||||||||
| Common | Range of | Additional | Variable | Retained | Other | Stock | ||||||||||||||||||||||||||||||||||||||
| Shares | Price Per | Common | Preferred | Paid-in | Interest | Earnings | Noncontrolling | Comprehensive | Subscription | |||||||||||||||||||||||||||||||||||
| Issued | Share | Stock | Stock | Capital | Entities | (Deficit) | Interest | Income | Receivable | Total | ||||||||||||||||||||||||||||||||||
| August 24, 2005 – inception | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||||||||||||
| Issuances of common stock: | ||||||||||||||||||||||||||||||||||||||||||||
| 2007 | 18,360,000 | $ | 0.01 | 173,832 | 173,832 | |||||||||||||||||||||||||||||||||||||||
| 2007 | 2,040,000 | $ | 2.45 | 19,314 | 4,980,686 | 5,000,000 | ||||||||||||||||||||||||||||||||||||||
| 2008 | 2,781,965 | $ | 0.01 | 26,340 | 26,340 | |||||||||||||||||||||||||||||||||||||||
| 2008 | 367,200 | $ | 2.45 | 3,477 | 896,523 | 900,000 | ||||||||||||||||||||||||||||||||||||||
| 2009 | 323,050 | $.01 to $2.60 | 3,113 | 836,887 | 840,000 | |||||||||||||||||||||||||||||||||||||||
| Total from issuance of common stock | 23,872,215 | 226,076 | 6,714,096 | 6,940,172 | ||||||||||||||||||||||||||||||||||||||||
| Imputed interest on stockholder loans | 1,187,154 | 1,187,154 | ||||||||||||||||||||||||||||||||||||||||||
| Net income (loss) for the period from August 24, 2005 (inception) to December 31, 2009 | (12,815,358 | ) | 74,127 | (12,741,231 | ) | |||||||||||||||||||||||||||||||||||||||
| Equity of VIE's prior to January 1, 2010 | - | - | - | 1,004,150 | - | (1,202,762 | ) | - | (42,141 | ) | (240,753 | ) | ||||||||||||||||||||||||||||||||
| Balances-December 31, 2009 | 23,872,215 | 226,076 | 7,901,250 | 1,004,150 | (12,815,358 | ) | (1,202,762 | ) | 74,127 | (42,141 | ) | (4,854,658 | ) | |||||||||||||||||||||||||||||||
| 2010 | 1,390,130 | $ | 1.00 | 14,775 | - | 14,775 | ||||||||||||||||||||||||||||||||||||||
| 2010 | 225,000 | $ | 2.00 | 2,392 | 447,608 | 450,000 | ||||||||||||||||||||||||||||||||||||||
| 2010 | 974,870 | $ | 2.60 | 10,362 | 2,524,301 | 2,534,663 | ||||||||||||||||||||||||||||||||||||||
| 2010 | 360,000 | $ | 2.85 | 3,826 | 1,022,174 | 1,026,000 | ||||||||||||||||||||||||||||||||||||||
| Imputed interest on stockholder loans | 380,664 | 380,664 | ||||||||||||||||||||||||||||||||||||||||||
| Net (loss) for the year | (4,066,384 | ) | (70,123 | ) | (4,136,507 | ) | ||||||||||||||||||||||||||||||||||||||
| Unrealized (loss) on foreign currency translation adjustment | - | - | - | - | - | (152,250 | ) | (403,381 | ) | - | (555,631 | ) | ||||||||||||||||||||||||||||||||
| Balances-December 31, 2010 (audited) | 26,822,215 | 257,431 | 12,275,997 | 1,004,150 | (16,881,742 | ) | (1,425,135 | ) | (329,254 | ) | (42,141 | ) | (5,140,694 | ) | ||||||||||||||||||||||||||||||
| Conversion of stockholders loans to additional paid-in capital | 1,376,229 | 1,376,229 | ||||||||||||||||||||||||||||||||||||||||||
| Imputed interest on stockholder loans | 415,705 | 415,705 | ||||||||||||||||||||||||||||||||||||||||||
| Net (loss) income for the year | (4,009,727 | ) | (41,235 | ) | (4,050,962 | ) | ||||||||||||||||||||||||||||||||||||||
| Unrealized gain on foreign currency translation adjustment | - | - | - | - | - | - | 210,058 | 593,256 | - | 803,314 | ||||||||||||||||||||||||||||||||||
| Balances-December 31, 2011 (audited) | 26,822,215 | 257,431 | 14,067,931 | 1,004,150 | (20,891,469 | ) | (1,256,312 | ) | 264,002 | (42,141 | ) | (6,596,408 | ) | |||||||||||||||||||||||||||||||
| Issuance of common stock, pre-merger | 2,500,000 | $ | 1.00 | 25,501 | 2,474,499 | 2,500,000 | ||||||||||||||||||||||||||||||||||||||
| Conversion of stockholder loans | 7,378,416 | $ | .50 | 75,206 | 3,614,002 | 3,689,208 | ||||||||||||||||||||||||||||||||||||||
| Imputed interest on stockholder loans | 380,414 | 380,414 | ||||||||||||||||||||||||||||||||||||||||||
| Adjustment for reverse merger | (13,393,447 | ) | (334,831 | ) | 226,654 | 149,206 | (83,170 | ) | 42,141 | - | ||||||||||||||||||||||||||||||||||
| Issuance of common stock, post-merger | 235,000 | $ | 1.00 | 235 | 234,765 | 235,000 | ||||||||||||||||||||||||||||||||||||||
| Net (loss) for the year | (4,300,673 | ) | (117,786 | ) | (4,418,459 | ) | ||||||||||||||||||||||||||||||||||||||
| Unrealized gain on foreign currency translation adjustment | - | - | - | - | - | - | 38,409 | 96,462 | - | 134,871 | ||||||||||||||||||||||||||||||||||
| Balances-December 31, 2012 (audited) | 23,542,184 | 23,542 | 226,654 | 20,920,817 | 920,980 | (25,192,142 | ) | (1,335,689 | ) | 360,464 | - | (4,075,374 | ) | |||||||||||||||||||||||||||||||
| Issuance of common stock for cash | 3,237,318 | $ | 1.00 | 3,237 | 3,234,081 | 3,237,318 | ||||||||||||||||||||||||||||||||||||||
| Imputed interest on stockholder loans | 105,925 | 105,925 | ||||||||||||||||||||||||||||||||||||||||||
| Conversion of preferred stock to common stock | 2,865,447 | 2,865 | (28,654 | ) | 25,789 | - | ||||||||||||||||||||||||||||||||||||||
| Conversion of other loans payable to common stock | 222,672 | $ | 1.00 | 223 | 222,449 | 222,672 | ||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for consulting services | 205,000 | 205 | 190,295 | 190,500 | ||||||||||||||||||||||||||||||||||||||||
| Conversion of stockholders loans to former VIE to common stock | 6,864,811 | 6,865 | 2,766,519 | 2,773,384 | ||||||||||||||||||||||||||||||||||||||||
| F-6 |
SUREPURE INC. AND SUBSIDIARIES
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' DEFICIT
CUMULATIVE FROM AUGUST 24, 2005 (INCEPTION) TO DECEMBER 31, 2014
| Equity in | Accumulated | |||||||||||||||||||||||||||||||||||||||||||
| Common | Range of | Additional | Variable | Retained | Other | Stock | ||||||||||||||||||||||||||||||||||||||
| Shares | Price Per | Common | Preferred | Paid-in | Interest | Earnings | Noncontrolling | Comprehensive | Subscription | |||||||||||||||||||||||||||||||||||
| Issued | Share | Stock | Stock | Capital | Entities | (Deficit) | Interest | Income | Receivable | Total | ||||||||||||||||||||||||||||||||||
| Adjustment to reflect acquisition of former VIE's | 3,000,000 | 3,000 | 1,111,538 | (920,980 | ) | (1,633,120 | ) | 1,213,606 | 225,956 | - | - | |||||||||||||||||||||||||||||||||
| Equity-based compensation | 151,053 | 151,053 | ||||||||||||||||||||||||||||||||||||||||||
| Net (loss) for the year | (4,401,469 | ) | (17,653 | ) | (4,419,122 | ) | ||||||||||||||||||||||||||||||||||||||
| Unrealized gain on foreign currency translation adjustment | - | - | - | - | - | - | 139,736 | 321,336 | - | 461,072 | ||||||||||||||||||||||||||||||||||
| Balances- December 31, 2013 (audited) | 39,937,432 | 39,937 | 198,000 | 28,728,466 | - | (31,226,731 | ) | - | 907,756 | - | (1,352,572 | ) | ||||||||||||||||||||||||||||||||
| Issuance of common stock for cash | 1,191,075 | $ | 1.00 | 1,192 | 1,189,884 | 1,191,076 | ||||||||||||||||||||||||||||||||||||||
| Conversion of preferred stock to common stock | 5,000,000 | 5,000 | (50,000 | ) | 45,000 | - | ||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for consulting services | 180,000 | 180 | 77,821 | 78,001 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock to settle liabilities | 575,000 | 575 | 229,425 | 230,000 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock in connection with sale of convertible debentures | 462,309 | 462 | 96,288 | 96,750 | ||||||||||||||||||||||||||||||||||||||||
| Beneficial conversion feature of convertible debentures | 67,308 | 67,308 | ||||||||||||||||||||||||||||||||||||||||||
| Equity-based compensation | 136,568 | 136,568 | ||||||||||||||||||||||||||||||||||||||||||
| Net (loss) for the year | (3,098,596 | ) | (3,098,596 | ) | ||||||||||||||||||||||||||||||||||||||||
| Unrealized gain on foreign currency translation adjustment | - | - | - | - | - | - | - | 91,700 | 91,700 | |||||||||||||||||||||||||||||||||||
| Balances- December 31, 2014 (audited) | 47,345,816 | $ | 47,346 | $ | 148,000 | $ | 30,570,760 | $ | - | $ | (34,325,327 | ) | $ | - | $ | 999,456 | $ | - | $ | (2,559,765 | ) | |||||||||||||||||||||||
See notes to Consolidated Financial Statements.
| F-7 |
SUREPURE INC. AND SUBSIDIARIES
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
YEARS ENDED DECEMBER 31, 2014 AND 2013
AND CUMULATIVE FROM AUGUST 24, 2005 (INCEPTION) TO DECEMBER 31, 2014
| Cumulative From | ||||||||||||
| Years Ended | August 24, 2005 | |||||||||||
| December 31, | (inception) to | |||||||||||
| 2014 | 2013 | December 31, 2014 | ||||||||||
| Cash from operating activities: | ||||||||||||
| Net loss | $ | (3,098,596 | ) | $ | (4,419,122 | ) | $ | (34,325,327 | ) | |||
| Adjustments to reconcile net loss to cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 16,668 | 21,283 | 214,050 | |||||||||
| Non-cash interest expense and loan costs | 191,344 | - | 191,344 | |||||||||
| Impairment of patent | - | - | 537,631 | |||||||||
| Loss on sale of property and equipment | - | - | 64,172 | |||||||||
| Imputed interest on stockholders loans | - | 105,925 | 2,469,862 | |||||||||
| Issuance of common stock for consulting services | 78,000 | 190,500 | 268,500 | |||||||||
| Equity-based compensation | 136,568 | 151,053 | 287,621 | |||||||||
| Changes in assets and liabilities: | - | |||||||||||
| Accounts receivable | 12,229 | (9,081 | ) | (16,800 | ) | |||||||
| Prepaid expenses and other current assets | (249,898 | ) | (17,736 | ) | (406,280 | ) | ||||||
| Accounts payable and other current liabilities | 624,137 | (87,509 | ) | 1,327,558 | ||||||||
| Customer deposits payable | 402,703 | 412,703 | ||||||||||
| Due to officers/stockholders | 572,386 | 478,440 | 1,298,085 | |||||||||
| Income taxes payable | (53 | ) | 124 | 480 | ||||||||
| Total cash used in operating activities | (1,314,512 | ) | (3,586,123 | ) | (27,676,401 | ) | ||||||
| Cash from investing activities: | ||||||||||||
| Purchase of property and equipment | - | - | (181,760 | ) | ||||||||
| Proceeds from sales of property and equipment | - | - | 16,860 | |||||||||
| Acquisition of patents | - | - | (746,576 | ) | ||||||||
| Total cash used in investing activities | - | - | (911,476 | ) | ||||||||
| Cash from financing activities: | ||||||||||||
| Proceeds from issuance of equity | 1,191,077 | 3,237,318 | 18,086,864 | |||||||||
| Proceeds from issuance of convertible debt | 37,500 | - | 37,500 | |||||||||
| Proceeds from equity of former variable interest entities | - | - | 83,309 | |||||||||
| Proceeds from loans from stockholders | - | - | 9,421,605 | |||||||||
| Proceeds from other loans payable | - | 234,612 | 454,161 | |||||||||
| Total cash provided by financing activities | 1,228,577 | 3,471,930 | 28,083,439 | |||||||||
| Effect of exchange rate changes on cash and cash equivalents | 65,591 | (7,310 | ) | 504,964 | ||||||||
| Net (decrease) increase in cash | (20,344 | ) | (121,503 | ) | 526 | |||||||
| Cash, beginning of period | 20,870 | 142,373 | - | |||||||||
| Cash, end of period | $ | 526 | $ | 20,870 | $ | 526 | ||||||
| Supplemental disclosures: | ||||||||||||
| Interest paid | $ | - | $ | 1,309 | $ | 70,005 | ||||||
| Income taxes paid | $ | - | $ | - | $ | 32,673 | ||||||
| Conversion of stockholders' and other loans to equity | $ | - | $ | 2,996,056 | $ | 8,061,493 | ||||||
| Conversion of other loans payable to stockholders' loans | $ | - | $ | - | $ | 300,000 | ||||||
| Conversion of stockholders' loans to equity of former variable interest entities | $ | - | $ | - | $ | 1,114,400 | ||||||
| - | ||||||||||||
| Imputed interest on stockholders' loans reported as an increase to additional paid-in capital | $ | - | $ | 105,925 | $ | 2,469,862 | ||||||
| Conversion of equity of former variable interest entities to equity of company | $ | - | $ | 1,114,539 | $ | 1,114,539 | ||||||
| Conversion of preferred stock to common stock and additional paid-in capital | $ | 50,000 | $ | 28,654 | $ | 78,654 | ||||||
| Issuance of common stock in connection with issuance of convertible debentures | $ | 36,750 | $ | - | $ | 36,750 | ||||||
See notes to Consolidated Financial Statements.
| F-8 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
1. Organization and Significant Accounting Policies
Description of Business
SurePure Investment Holding AG (“SPI”) was incorporated in Switzerland in 2007. From 2007 to December 12, 2012 SPI was the holding company of the SurePure Group (the “Group”), which included subsidiaries and other entities whose activities primarily benefit the Group. On December 12, 2012, SPI entered into an Amended and Restated Share Exchange Agreement with SurePure, Inc. (“SurePure US” or the “Company”) pursuant to which SurePure US acquired SPI in a share exchange (the “Share Exchange”) and became the holding company for the Group, including SPI. Although SurePure US is the legal acquirer of SPI, SPI is treated as the acquirer for accounting and financial reporting purposes and under this method, SurePure US retains SPI’s financial reporting history.
On June 12, 2013, we completed the acquisition of SPHSA and SPHSA’s wholly-owned subsidiary, SPMSA both incorporated in 2005. The terms and conditions of the acquisition were set forth in the Acquisition Agreement, dated August 16, 2012 (the “Acquisition Agreement”), between our subsidiary, SPI, and SPHSA. On December 12, 2012, as part of the Share Exchange, we assumed the obligations of SPI under the Acquisition Agreement. As a result of acquisition, both SPHSA and SPMSA have become our wholly-owned subsidiaries.
Under the Share Exchange, each share of the capital stock of SPI was exchanged for one share of SurePure US common stock, par value $.001 per share (“Common Stock”), and, in the case of one shareholder of SPI, one share of Nonvoting Convertible Preferred Stock, par value $.01 per share (the “Nonvoting Convertible Preferred Stock”).
The Group has developed technology for using shortwave ultraviolet light (“UV-C”) to purify turbid liquids such as wine, fruit juice and milk. Although initially designed to treat food-grade applications, it has successfully been applied to liquids such as dairy products for animal consumption, protein products, bovine blood plasma, water, brines and sugar syrup solutions. The Group holds patents in 66 countries for this technology. The Group has been engaged in raising capital, continuing research and development of its technologies and developing markets for its products.
Basis of Presentation and Consolidation
The accompanying audited consolidated financial statements of the Company and subsidiaries have been prepared in accordance accounting principles generally accepted in the United States of America (“GAAP”).
The Company’s wholly-owned subsidiaries are as follows:
· SurePure Investment Holding AG (“SPI”), which holds the investment in SPO;
· SurePure Operations AG (“SPO”), which markets the products of the Group and earns its revenue by selling or otherwise distributing equipment utilizing the Group’s technology globally. SPO owns a patent for the Group’s technology in various countries;
· SurePure Participations AG (“SPP”), which was a minority stockholder of SPI and is part of the common holding structure of the Group. SPP has no operations and all of its expenses have been and will continue to be paid by SPI. Formerly a VIE, SPP became a subsidiary as a result of the Share Exchange on December 12, 2012;
| F-9 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
· SurePure Holdings South Africa (Pty) Ltd. and its wholly-owned subsidiary, SurePure Marketing South Africa (Pty) Ltd. (“SPMSA”), which holds the South African patent, manufacture the products of the Group and earn revenue from selling or otherwise distributing equipment utilizing the Group’s technology. Formerly VIE’s, these entities became subsidiaries as a result of their acquisition on June 12, 2013; and
· SurePure Latin America Maqinas de Purificasao UVC Ltda. (“SPLAM”), which conducts no operations currently.
The Group’s reporting currency is the United States Dollar (“USD”) and these consolidated financial statements are presented in USD or “$.”
Use of Estimates
GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates.
Reclassification
Certain previously reported amounts have been reclassified to conform to the presentation used in the December 31, 2014 financial statements.
Income Taxes
The Group accounts for income taxes using the liability method. Under this method, deferred income taxes are recognized based on the tax effects of temporary differences between the financial statement and tax bases of assets and liabilities, as measured by current enacted tax rates. Valuation allowances are recorded to reduce the deferred tax assets to an amount that will more likely than not provide a future tax benefit.
GAAP requires that, in applying the liability method, the consolidated financial statement effects of an uncertain tax position be recognized based on the outcome that is more likely than not to occur. Under this criterion, the most likely resolution of an uncertain tax position should be analyzed based on technical merits and one that will likely be sustained under examination. There are no uncertain tax positions requiring adjustment to or disclosure in these consolidated financial statements.
| F-10 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
Accounts Receivable
Accounts receivable represent amounts due from customers and are carried at original invoice amount less an estimate made for doubtful receivables. The Group establishes a provision for estimated doubtful accounts, if necessary. No allowance for doubtful accounts was deemed to be necessary at December 31, 2014 or at December 31, 2013.
Property, Equipment and Related Depreciation
Property and equipment are recorded at cost, less accumulated depreciation. Cost includes the price paid to acquire the asset and any expenditures that substantially increase the asset’s value or extend the useful life of an existing asset. Depreciation is computed using the straight-line method over the estimated useful lives of the property and equipment. Major repairs and betterments that significantly extend original useful lives or improve productivity are capitalized and depreciated over the period benefited. Expenditures for routine repairs and maintenance are expensed as incurred. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts and any gain or loss is recognized in operations.
Depreciation is provided over the following estimated useful lives:
| Plant machinery | 3 to 5 years | |
| Furniture and fixtures | 3 to 5 years | |
| Motor vehicles | 5 years | |
| Office and computer equipment | 3 to 5 years |
Intangible Assets
Intangible assets consist of patents in various countries around the world for the Company’s UV-C purification technology. The patents were initially recognized at their cost and are being amortized on a straight-line basis over their estimated useful lives of twelve years beginning with the acquisition of the patents in 2008. The patents expire in October 2020.
The Group evaluates the carrying value of its intangible assets for impairment at least annually or when events or changes in circumstances are identified by management that indicate that such carrying values may not be fully recoverable. The evaluation involves estimating the future undiscounted cash flows expected to be derived from the assets to assess whether or not a potential impairment exists. As a result of its evaluations, management determined that it was not necessary to recognize a loss on impairment of its intangible assets for the years ended December 31, 2014 and December 31, 2013. During the period from August 24, 2005 (inception) to December 31, 2014, impairment losses on intangible assets of $537,631 were recognized.
| F-11 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
Revenue
Revenue is earned from sales or use of equipment that uses the Company’s patented technology and is recognized, net of returns and discounts, when persuasive evidence of an arrangement exists, delivery has occurred, the sales price is fixed or determinable and collectability is reasonably assured. These criteria are usually met upon delivery of the product to the customer, which is also when the risk of ownership and title passes to the customer.
Research and Development
Research and development costs are charged to expense as incurred.
Equity-Based Compensation
The Company measures compensation cost for all stock options granted based on fair value on the measurement date, which is typically the grant date. The fair value of each stock option granted is estimated on the grant date using the Black-Scholes-Merton option valuation model. The fair value of each share is based on the fair market value of the Company’s common stock on the date of the grant. Equity-based compensation expense is recognized on a straight-line basis over the requisite service period for each stock option or stock grant expected to vest with forfeitures estimated at the date of grant based on the Company’s historical experience and future expectations.
Foreign Currency Translations
These consolidated financial statements are presented in USD, which is the Group’s reporting currency. The consolidated financial statements of the Group members have been translated into USD in accordance with GAAP. All assets and liability accounts on the consolidated balance sheets have been translated using the spot exchange rate in effect at the consolidated balance sheet date. Equity accounts have been translated at their historical rates when the capital transaction occurred. Income and expenses have been translated at the average exchange rates for the periods presented. Adjustments resulting from the translation of the Group’s consolidated financial statements are included in the consolidated statement of other comprehensive income (loss). Actual transaction gains and losses are included in the consolidated statements of operations as incurred.
| F-12 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
The functional currencies of the companies included in the Group are their respective local currencies. Accordingly, the Group is exposed to transaction gains and losses that result from changes in various foreign currency exchange rates.
Applicable functional currencies are:
| SPI, SPO, and SPP | Swiss Francs – CHF | |
| SPLAM | Brazilian Real – BRL | |
| SPHSA and SPMSA | South African Rand – ZAR |
Exchange rates used for conversion of foreign items to USD at the end of each period and the average for each period were:
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| CHF: | ||||||||
| Reporting date | 1.0105 | 1.1230 | ||||||
| Average for period | 1.0938 | 1.0790 | ||||||
| BRL: | ||||||||
| Reporting date | 0.3722 | 0.4232 | ||||||
| Average for period | 0.4257 | 0.4645 | ||||||
| ZAR: | ||||||||
| Reporting date | 0.0861 | 0.0952 | ||||||
| Average for period | 0.0922 | 0.1040 | ||||||
| F-13 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
Earnings (Loss) per Share
Basic and diluted earnings (loss) per share are computed by dividing net income or loss by the weighted average number of shares of Common Stock outstanding during the period. Diluted earnings (loss) per share reflects the potential dilution that could occur if securities or other contracts to issue shares of Common Stock, such as options, convertible notes and convertible preferred stock, were exercised or converted into shares of Common Stock or could otherwise cause the issuance of shares of Common Stock that then would share in earnings (losses). Such potential issuances of additional shares of Common Stock are included in the computation of diluted earnings per share. Except as disclosed in Note 7 of these Notes to Consolidated Financial Statements, the Company has no securities or other contracts to issue shares of Common Stock that could cause any dilution of earnings. In addition, when there is a loss, diluted loss per share is not computed because any potential additional common shares of Common Stock would reduce the reported loss per share and therefore have an anti-dilutive effect.
2. Property and Equipment
Property and equipment consists of the following:
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Machinery and equipment | $ | 5,010 | $ | 5,010 | ||||
| Furniture and fixtures | 12,753 | 12,753 | ||||||
| Motor vehicles | 14,400 | 14,400 | ||||||
| Office and computer equipment | 12,647 | 12,647 | ||||||
| 44,810 | 44,810 | |||||||
| Less: Accumulated depreciation | 44,810 | 44,810 | ||||||
| Property and equipment, net | $ | - | $ | - | ||||
Depreciation expense was $0 and approximately $4,600 for the years ended December 31, 2014 and 2013, respectively, and approximately $97,300 for the period from inception to December 31, 2014.
3. Intangible Assets
Intangible assets consist of the following:
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Patents | $ | 208,943 | $ | 208,943 | ||||
| Less: Accumulated amortization | 113,321 | 96,653 | ||||||
| Intangible assets, net | $ | 95,622 | $ | 112,290 | ||||
Amortization expense was approximately $16,700 and $16,700 for the years ended December 31, 2014 and 2013, and approximately $116,800 for the period from inception to December 31, 2014.
4. Due to Officers/Stockholders
Due to officers/stockholders consists of unpaid salaries, accrued leave and advances from two executives and one employee/stockholder totaling $1,148,085 and $725,699 at December 31, 2014 and December 31, 2013, respectively. During the period from May to December 2014, the Company’s Chief Executive Officer advanced $58,720 to a subsidiary of the Company. The Company’s chief executive officer has not been paid a portion of his salary since September 30, 2013 and as of December 31, 2014 $303,116 was due. In accordance to an agreement entered into on September 10, 2014 the amount due to the Company’s chief executive officer was ceded to the Company’s chief financial officer in part settlement of an obligation to the Company’s chief financial officer. The Company’s chief financial officer has not been paid any of his salary or and a portion of his business expenses since October 2012. The amount owed to the chief financial officer as of December 31, 2014 includes unpaid salaries of $621,065 and unreimbursed expenses paid on behalf of the Company of $84,103.
| F-14 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
5. Other Loans Payable
In October 2012, the Company and the three lenders whose balances were reflected in Other Loans Payable at December 31, 2012 revised their agreements orally and these agreements were later formalized in writing effective January 2013. The Company agreed with each of these lenders that it would pay interest at 5% per annum on the outstanding balances retroactive to the time that the loans were made in 2011 and it would repay each of these lenders $105,000 in October 2012. On April 4, 2013, these lenders and the Company further agreed that the lenders would be granted the right to convert each $1.00 of principal and interest that was unpaid under their agreements into one share of Common Stock upon the effective date of the Company’s registration statement which the Company had filed to register the resale of certain shares of the Common Stock. Pursuant to these agreements, these loans were converted into shares of Common Stock at the time that the registration statement was declared effective on May 16, 2013.
On April 4, 2013, SPMSA borrowed ZAR 2 million (approximately $201,000) from a lender pursuant to a note. The terms of the loan provide for interest of 2% per month on the outstanding balance which is payable together with the principal balance no later than December 31, 2013. An officer and stockholder of the Company has deposited 1,000,000 shares of Common Stock with the lender as security for the repayment of the loan to SPMSA. On September 26, 2013, the repayment date was extended to October 31, 2013. On October 31, 2013, the repayment date was extended to November 30, 2013. On November 21, 2013, the repayment date was further extended to March 31, 2014. On March 19, 2014, the repayment date was further extended to June 30, 2014. On June 24, 2014, the repayment date was further extended to September 30, 2014. On September, 30, 2014, the repayment date was further extended to December 31, 2014. On November 26, 2014, the repayment date was further extended to June 30, 2015. Interest on this loan was approximately $60,100 and $41,700 for the years ended December 31, 2014 and 2013 and approximately $101,800 for the period from inception to December 31, 2014, respectively. As of December 31, 2014, the unpaid balance on this loan was $265,522 and is included in loans payable in the accompanying Consolidated Balance Sheet.
| F-15 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
6. Convertible debentures payable
On June 23, 2014, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with Peak One Opportunity Fund, L.P., a Delaware limited partnership (“Peak One”), under which the Company has the right, subject to the terms and conditions of the Securities Purchase Agreement, to issue up to three convertible debentures each in the face amount of $125,000. . Each debenture is payable in full on the third anniversary of the date of issue (the “maturity date”). Each debenture provides for a discount of $12,500 upon issuance and sale and a zero percent stated interest rate. The Company issued the first debenture (the “Debenture”) to Peak One on June 23, 2014. The Company paid Peak One $10,000 for legal and other costs, that were deducted from the proceeds of issuance. The Securities Purchase Agreement also provided for the issuance of 75,000 shares of the Company’s Common Stock to an affiliate of Peak One upon the issuance of the Debenture. These shares were issued and valued at $.49 per share, which was the closing price of the Company’s Common Stock on the date of issuance. The Debenture provides Peak One with the option to convert any outstanding balance under the Debenture into shares of Common Stock of the Company at 65% of the lowest closing price of the Company’s Common Stock during the twenty trading days prior to conversion. This conversion price constitutes a beneficial conversion feature (“BCF”) that was valued at $67,308 on the date of issuance. The Company accounted for the BCF as a reduction of the balance of the related debt at the date of issuance and as an additional cost that is to be treated as noncash interest. The total costs of issuance were $126,558, including the value of 75,000 shares of the Company’s Common Stock issued, the discount and the BCF, which are treated as interest, and the legal and other costs that are treated as loan costs. The Company has expensed these costs currently due to the fact that the option to convert into shares rests solely with Peak One. Of the total costs associated with the Securities Purchase Agreement, $116,558 was charged to interest expense and $10,000 of loan costs was included in general and administrative expenses. Following the issuance of the Debenture, the Company registered 1,227,416 shares of its Common Stock in connection with Peak One’s conversion rights.
At June 30, 2014, the unpaid balance of the Debenture was $125,000 and was included in long-term debt. On July 18, 2014, Peak One converted $30,000 of the Debenture into 177,514 shares of the Company’s Common Stock. On August 5, 2014, Peak One converted an additional $30,000 of the Debenture into 209,795 shares of the Company’s Common Stock. On September 2, 2014, the Company repaid the outstanding amount of the debenture that it had issued to Peak One Opportunity Fund, L.P. on June 23, 2014. On September 3, 2014 the Company and Peak One Opportunity Fund, L.P. terminated the Securities Purchase Agreement, dated June 23, 2014.
7. Equity
Common Stock through the date of the Share Exchange consisted of the common stock of SPI. The amounts presented for periods prior to the Share Exchange were denominated in CHF and have been translated from CHF to USD using the exchange rates in effect on the date of each issuance. As of December 31, 2011, SPI had 26,822,215 common shares issued and outstanding. During 2012 prior to the Share Exchange, SPI issued 2,500,000 shares in connection with the subscription agreement referenced in Note 7 of these Notes to Consolidated Financial Statements and 7,378,416 shares in connection with the conversion of SPI stockholder loans to common shares, resulting in 36,700,631 common shares of SPI being outstanding immediately prior to the Share Exchange.
| F-16 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
On December 12, 2012, SurePure US designated 31,155,282 of its authorized shares of preferred stock as Nonvoting Convertible Preferred Stock, par value $0.01 per share. Under the terms of the Certificate of Designation (the “Certificate”), each share of Nonvoting Convertible Preferred Stock is convertible into one share of Common Stock, subject to certain limitations and restrictions as defined in the Certificate.
The issued shares of Nonvoting Convertible Preferred Stock automatically convert, at the applicable conversion ratio as defined in the Certificate, into shares of the Company’s Common Stock upon the assignment, sale or other transfer of shares to any person other than an affiliate of the holder of the seller. Any assignee, purchaser or other transferee may surrender certificates representing the assigned shares to the Company and will receive shares of the Company’s Common Stock in return.
During the year ended December 31, 2014, the preferred stockholder converted 5,000,000 shares of the Company’s preferred stock into 5,000,000 shares of the Company’s Common Stock. At December 31, 2014 and December 31, 2013, there were 14,800,000 and 19,800,000 issued and outstanding shares of the Company’s preferred stock.
Effective with the acquisition of SPHSA on June 12, 2013, 3,000 common shares of SPHSA were then exchanged for 3,000,000 shares of the Company’s Common Stock, which shares are treated as being outstanding as of June 12, 2013. The shareholder loans of SPHSA were converted to 6,865 common shares of SPHSA which were then exchanged for 6,864,811 shares of the Company’s Common Stock, which shares are treated as being outstanding as of June 12, 2013.
On September 1, 2013, the Company issued 60,000 common shares to a consultant in exchange for services. The shares issued and the related consulting services were valued at $60,000 based upon a value of $1.00 per share, which was the closing price of the shares of the Company’s Common Stock on the last trading day prior to September 1, 2013. On December 19, 2013, the Company issued 145,000 common shares to two consultants in exchange for services. The shares issued and the related consulting services were valued at $130,500 based upon a value of $.90 per share, which was the closing price of the shares of the Company’s Common Stock on the last trading day prior to December 19, 2013.
On April 1, 2014, the Company entered into an agreement with a consultant to issue 30,000 common shares on the first business day of each month through September, 2014 in exchange for services. A total of 90,000 common shares were issued during the year ended December 31, 2014. The shares issued and the related consulting services were valued at $51,300 based upon the closing price on each date of issuance.
On June 23, 2014, the Company issued 75,000 common shares to Peak One as part of the cost of the financing in connection with the sale of the convertible debentures. The shares issued were valued at $.49, the closing share price on that day. On July 18, 2014, Peak One converted $30,000 of the Debenture into 177,514 shares of the Company’s Common Stock. On August 5, 2014, Peak One converted an additional $30,000 of the Debenture into 209,795 shares of the Company’s Common Stock.
At December 31, 2014 and December 31, 2013, after giving effect to the acquisition of SPHSA, there were 47,345,816 and 39,937,432 shares, respectively, of the Company’s Common Stock issued and outstanding.
| F-17 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
8. Stock Subscription Agreements; Share Purchase Agreements
On November 26, 2012, the Company entered into a Subscription Agreement with RD Active Capital Limited, a United Kingdom-based investment manager (“RD Active”), in which RD Active agreed to purchase up to 300,000 new shares of Common Stock over the period ending January 31, 2013 and acquired the right to purchase up to 2,700,000 new shares of Common Stock through March 31, 2013 as long as RD Active purchased the 300,000 new shares of Common Stock. The right to purchase shares of Common Stock was extended on March 28, 2013 to April 12, 2013, and on April 12, 2013 the right to purchase shares of Common Stock was further extended to May 31, 2013. All shares of Common Stock were purchased and sold at an issue price of $1.00 per share. Under the terms of the Subscription Agreement, RD Active had the right to exercise the additional purchase right on its own behalf and resell to other purchasers or had the right to place the additional shares directly with other purchasers. Pursuant to the terms and conditions of the Share Exchange, the Company had assumed the obligations of SPI under the Subscription Agreement. Under this Subscription Agreement, RD Active had purchased 1,159,000 shares of Common Stock, including 924,500 shares of Common Stock during the year ended December 31, 2013. RD Active’s right to purchase additional shares of Common Stock under the waiver terminated on May 31, 2013.
On May 24, 2013, the Company entered into a Share Purchase Agreement with Trinity Asset Management (Proprietary) Limited, a South Africa-based investment manager (“Trinity”), in which Trinity agreed to purchase 1,000,000 new shares of the Company’s Common Stock at $1.00 per share in three installments: 250,000 shares on May 24, 2013, 250,000 shares May 31, 2013 and 500,000 shares on June 28, 2013. If Trinity complied with its purchase commitment, it would also have had the right to purchase up to 1,000,000 additional shares of Common Stock at $1.35 per share before July 31, 2013 by providing written notice three days prior to the purchase of the additional shares. As of June 12, 2013, Trinity had purchased 50,000 of the second installment of 250,000 shares of Common Stock, and demand was made for the unpaid portion of the second installment. On June 26, 2013, the Company granted a waiver of time until July 31, 2013 for Trinity to purchase the remaining shares of Common Stock that were to have been purchased by June 28, 2013. During June and July 2013, Trinity purchased 101,318 additional shares of the second installment. Trinity’s right to purchase additional shares under its share purchase agreement terminated on July 31, 2013.
Effective July 25, 2013, Regency Capital Corporation, a Turks and Caicos Islands corporation (“Regency”), agreed to subscribe for and purchase 661,500 shares of the Company’s Common Stock in three equal installments of 220,500 shares each over the period ending September 13, 2013. Regency completed the purchase of all 661,500 shares as agreed. All shares of Common Stock under this share purchase agreement were purchased and sold at an issue price of $1.00 per share.
Effective July 25, 2013, the Company entered into a Share Purchase Agreement with Minaurum Limited, a Malta-based investment manager (“Minaurum”), in which Minaurum agreed to purchase 100,000 new shares of the Company’s Common Stock on that day. Minaurum completed the purchase as agreed. These shares of Common Stock were purchased and sold at an issue price of $1.00 per share.
| F-18 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
On September 20, 2013, the Company and Trinity Asset Management International Limited (“TAMIL”), a company formed under the laws of Mauritius, entered into the Share Purchase Agreement (the “TAMIL Share Purchase Agreement”), dated as of September 19, 2013, under which TAMIL agreed to purchase 900,000 shares of the Company’s Common Stock in monthly installments during the period ending March 25, 2014. The agreement required that TAMIL purchase the first 360,000 shares on September 30, 2013 or on such later date, not later than October 25, 2013 as the Company and TAMIL may agree. TAMIL agreed to purchase the remaining 540,000 shares in six installments of 90,000 shares each during the months beginning October 2013 and ending March 2014. TAMIL did not purchase any shares during the third quarter or fourth quarter of 2013. On November 7, 2013, the Company and TAMIL amended the Share Purchase Agreement to permit TAMIL to purchase the 900,000 shares of Common Stock at any time until March 25, 2014 and to modify the purchase price to be paid for the shares to be the greater of $1.00 per share or 92% of the volume weighted average price per share for the 20 trading days prior to the third business day before the purchase and sale of the shares. On March 5, 2014, TAMIL purchased 184,825 shares of the Company’s Common Stock under the agreement for a purchase price of $184,825. The agreement included a liquidated damages provision in the event that TAMIL failed to purchase the agreed upon number of shares. The liquidated damages provision required TAMIL to pay an amount equal to the difference between $900,000 and the amount paid for the shares under the agreement. TAMIL did not purchase any of the remaining 715,175 shares of the Company’s Common Stock under this agreement as amended. The Company did not enforce its rights under the liquidated damages provision.
On October 25, 2013, the Company entered into an additional Share Purchase Agreement with Regency, an existing stockholder, under which Regency agreed to purchase 600,000 shares of Common Stock during the period ended October 31, 2013. All shares of Common Stock under this additional share purchase agreement with Regency were purchased at an issue price of $1.00 per share during October 2013.
On November 22, 2013, the Company entered into an additional Share Purchase Agreement with Regency, under which Regency agreed to purchase a total of 170,000 shares of Common Stock during the period ended November 30, 2013. In addition, Regency was given an option to purchase an additional 430,000 shares of Common Stock before January 31, 2014. Regency purchased the 170,000 shares of Common Stock as agreed and purchased an additional 380,000 shares of Common Stock through December 31, 2013. The remaining 50,000 shares of the Company’s Common Stock were purchased on January 24, 2014. All shares of Common Stock under this additional share purchase agreement with Regency were purchased at an issue price of $1.00 per share.
On February 13, 2014, effective as of January 31, 2014, this agreement was amended to grant Regency an option to purchase an additional 500,000 common shares not later than March 31, 2014 at the greater of $1.00 and 92% of the volume-weighted average price of for the 20 trading days ending on the third trading day before each additional share closing (the “Amendment Option Price”). Under this agreement as amended and through June 30, 2014, Regency has purchased 500,000 shares of the Company’s Common Stock at an issue price of $1.00 per share for an aggregate purchase price of $500,000.
| F-19 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
On March 19, 2014, the Company and Regency entered into a second amendment of the Share Purchase Agreement that granted Regency the right to purchase 1,000,000 additional shares of Common Stock during the period ending June 30, 2014 at a cash purchase price equal to the Amendment Option Price. Under the second amendment and through June 30, 2014, Regency has purchased 315,000 shares of the Company’s Common Stock for an aggregate purchase price of $315,000.
On May 20, 2014, the Company and Trinity Asset Management (Proprietary) Limited (“Trinity”) entered into an Agreement for the Subscription of Shares that permitted Trinity arrange for the purchase of 850,000 shares of the Company’s Common Stock by offers to purchase by other subscribers at the price of $1.00 per share before July 30, 2014. In addition, Trinity agreed to assume the obligations of TAMIL with respect to the liquidated damages in connection with the unpurchased shares under the TAMIL agreement dated as of September 19, 2013 as amended. As of July 24, Trinity had not provided any offers to purchase to the Company and requested an extension of time to September 30, 2014 to complete it obligations under the agreement. Subsequent to June 30, 2014, no such offers to purchase have been made to the Company and no sales of Common Stock have taken place under the agreement.
On June 24, 2014, the Company and Regency entered into a third amendment of the Share Purchase Agreement that extended to September 30, 2014 Regency’s right to purchase the remaining 685,000 shares of the Company’s Common Stock under the second amendment. Through September 30, 2014, 141,250 shares were purchased under the third amendment.
During the year ended December 31, 2014, the Company issued an aggregate of 1,191,075 shares of its Common Stock for aggregate gross proceeds of $1,190,075, 575,000 shares of its Common Stock in settlement of liabilities to executives and consultants, 180,000 shares of its Common Stock in connection with a consulting agreement, 75,000 shares of its Common Stock in connection with the issuance of the Peak One convertible debentures and 387,309 shares issued in connection with the conversion rights under the Peak One convertible debentures.
During the year ended December 31, 2014, the holder of the Company’s convertible preferred shares converted 5,000,000 shares of Preferred Stock into 5,000,000 shares of Common Stock.
9. Stockholders’ Deficit
The consolidated stockholders’ equity of the Company consists of common stock, additional paid-in capital, accumulated deficit and accumulated other comprehensive income. The amounts for equity in variable interest entities and noncontrolling interest have been reclassified to additional paid-in capital, retained earnings and accumulated other comprehensive income effective with the acquisition of SPHSA on June 12, 2013.
| F-20 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
10. Income Taxes
The Company and its subsidiaries file income tax returns in Switzerland, South Africa, the United States and Brazil. The components of income (loss) from operations before income taxes, by jurisdiction, are as follows:
| From August 24, 2005 | ||||||||||||
| Years Ended December 31, | (inception) to | |||||||||||
| 2014 | 2013 | December 31, 2014 | ||||||||||
| Switzerland | $ | (1,561,194 | ) | $ | (2,883,099 | ) | $ | (20,960,178 | ) | |||
| South Africa | (616,550 | ) | (234,099 | ) | (10,152,768 | ) | ||||||
| United States | (920,852 | ) | (1,301,924 | ) | (2,238,210 | ) | ||||||
| Brazil | - | - | (941,498 | ) | ||||||||
| Total Net Loss | $ | (3,098,596 | ) | $ | (4,419,122 | ) | $ | (34,292,654 | ) | |||
The provision for income taxes shown in the accompanying consolidated statements of operations consists of the following for the years ended December 31, 2014 and 2013 and for the period from inception to December 31, 2014:
| From August 24, 2005 | ||||||||||||
| Years Ended December 31, | (inception) to | |||||||||||
| 2014 | 2013 | December 31, 2014 | ||||||||||
| Current tax provision: | ||||||||||||
| Switzerland | $ | - | $ | - | $ | 32,673 | ||||||
| South Africa | - | - | - | |||||||||
| United States | - | - | - | |||||||||
| Brazil | - | - | - | |||||||||
| Total current tax provision | - | $ | - | 32,673 | ||||||||
| Deferred tax provision: | ||||||||||||
| Switzerland | (278,301 | ) | $ | (513,023 | ) | (3,621,534 | ) | |||||
| South Africa | (171,594 | ) | (19,435 | ) | (2,012,578 | ) | ||||||
| United States | (322,299 | ) | (455,673 | ) | (783,374 | ) | ||||||
| Brazil | - | - | (235,375 | ) | ||||||||
| Change in valuation allowance | 772,194 | 988,131 | 6,652,861 | |||||||||
| Total deferred provision | - | $ | - | - | ||||||||
| Total | $ | - | $ | - | $ | 32,673 | ||||||
The Company has determined that the future tax benefits from net operating losses are not likely to be realized in future periods and a full valuation allowance has been provided for all periods.
The income tax effect of each type of temporary difference giving rise to the net deferred tax asset as follows:
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | 6,652,861 | $ | 5,880,667 | ||||
| Less: valuation allowance | (6,652,861 | ) | (5,880,667 | ) | ||||
| Total | $ | - | $ | - | ||||
| F-21 |
The following reconciles the effective income tax rates with the statutory rates for the year ended December 31, 2014 and 2013, and for the period from inception to December 31, 2014:
| Switzerland | South Africa | United States | Brazil | Total | ||||||
| Statutory rate of tax | 8.5%/18% | 28.0% | 35.0% | 25.0% |
| Year ended December 31, 2014: | ||||||||||||||||||||
| Net (loss) from operations before taxes | $ | (1,561,194 | ) | $ | (616,550 | ) | $ | (920,852 | ) | $ | - | $ | (3,098,596 | ) | ||||||
| As calculated at the statutory rate | (278,301 | ) | (172,634 | ) | (322,299 | ) | - | (773,234 | ) | |||||||||||
| Permanent differences | - | 1,040 | - | - | 1,040 | |||||||||||||||
| Change in valuation reserves | 278,301 | 171,594 | 322,299 | - | 772,194 | |||||||||||||||
| Provision for income taxes | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
| Year ended December 31, 2013: | ||||||||||||||||||||
| Net (loss) from operations before taxes | $ | (2,883,099 | ) | $ | (234,099 | ) | $ | (1,301,924 | ) | $ | - | $ | (4,419,122 | ) | ||||||
| As calculated at the statutory rate | (513,023 | ) | (65,548 | ) | (455,673 | ) | - | (1,034,244 | ) | |||||||||||
| Permanent differences | - | 46,113 | - | - | 46,113 | |||||||||||||||
| Change in valuation reserves | 513,023 | 19,435 | 455,673 | - | 988,131 | |||||||||||||||
| Provision for income taxes | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
| For the period from August 24, 2005 (inception) to December 31, 2014: | ||||||||||||||||||||
| Net (loss) from operations before taxes | $ | (20,960,178 | ) | $ | (10,152,768 | ) | $ | (2,238,210 | ) | $ | (941,498 | ) | $ | (34,292,654 | ) | |||||
| As calculated at the statutory rate | (3,601,186 | ) | (2,842,775 | ) | (783,374 | ) | (235,375 | ) | (7,462,710 | ) | ||||||||||
| Permanent differences | 12,325 | 830,197 | - | - | 842,522 | |||||||||||||||
| Change in valuation reserves | 3,621,534 | 2,012,578 | 783,374 | 235,375 | 6,652,861 | |||||||||||||||
| Provision for income taxes | $ | 32,673 | $ | - | $ | - | $ | - | $ | 32,673 |
Permanent differences are principally related to loss on disposal of property and equipment, interest and penalties and unallowable expenses.
The Company and Group members remain subject to tax examinations for the year ended December 31, 2014 in Switzerland and South Africa, for the six years ended December 31, 2014 in the U.S and for the four years ended December 31, 2014 in Brazil.
The Company was notified by the U.S. Internal Revenue Service during December 2013 of assessments totaling $40,000 for the failure by the management of the Company prior to the December 12, 2012 share exchange to file certain information returns for the years 2008-2011. The Company requested but did not receive administrative relief from the assessments. The Company has entered into an agreement with the Internal Revenue Service to pay the outstanding balance over four months in installments of $10,000 each commencing August 15, 2014. The Company made the payment of the first installment of $10,000 on August 7, 2014. The Company obtained an updated settlement amount from the U.S. Internal Revenue Service during February 2015 and paid the amount in full during March 2015.
| F-22 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
11. Acquisition of SPHSA
On August 16, 2012, SPI entered into an agreement (the "Acquisition Agreement") with SPHSA providing for the acquisition of 100% of the outstanding shares of SPHSA from its shareholders. The Company has assumed the executory obligations of SPI under the Acquisition Agreement.
Prior to its acquisition, SPHSA was accounted for as a subsidiary of SPI under variable interest accounting rules. The shareholdings in SPHSA had remained largely unchanged since the formation of that company in 2005. Since October 2007, SPHSA has also provided various management services for SPI and SPO. In addition, our subsidiary SPO had loaned approximately $2,000,000 to SPHSA, all of which was due on demand. There had been and continue to be various intercompany transactions relating to the sale and purchase of goods and services between SPI and SPHSA. Accordingly, the boards of directors of both SPI and SPHSA believed it to be in the best interest of their respective companies to combine such that SPHSA would become a wholly-owned subsidiary of SPI and SPO would convert its loans and advances to SPHSA into equity, thereby improving the financial condition of SPHSA.
Subject to the terms and conditions of the Acquisition Agreement, on August 21, 2012, SPI made its offer, which initially was irrevocable for a period of 20 business days after the date of the offer to purchase from each of the shareholders of SPHSA all of their shares of SPHSA at a closing to be held September 19, 2012, which was the 21st business day after the date of the offer. The shares of SPHSA subject to SPI’s offer to purchase included shares that reflected the value of shareholder loans outstanding at the time of the offer. On September 21, 2012, SPI extended the period during which its offer remained open until November 26, 2012 to permit the satisfaction of conditions to the closing of the offer. On November 30, 2012, SPI and SPHSA agreed to amend the Acquisition Agreement to provide that the offer would remain open until February 28, 2013, so as to permit the fulfillment of certain closing conditions. On March 8, 2013, SPI extended the date that the offer would remain open to June 28, 2013. The Acquisition Agreement provided that SPI would settle the purchase price it is paying for the shares of SPHSA in shares of SPI in the ratios of (i) 1,000 common shares of SPI for each share of SPHSA and (ii) 1,000 common shares of SPI for each ZAR 4,000 in principal amount of loans owed by SPHSA to its shareholders. The purchase price for the shares of SPHSA under the offer was ZAR 4,000 (approximately $500.00) per share of SPHSA. The Acquisition Agreement provided that SPI would settle the purchase price it paid for the shares of SPHSA in shares of SPI in the ratio of (i) 1,000 common shares of SPI for each share of SPHSA and (ii) 1,000 common shares of SPI for each ZAR 4,000 in principal amount of loans owed by SPHSA to its shareholders.
| F-23 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
Acting under the terms of the Share Exchange, at the time of the Share Exchange SPI assigned its rights under the Acquisition Agreement to SurePure US, and SurePure US assumed the obligations of SPI to issue shares to the shareholders of SPHSA at such time, if any, that the pending acquisition of SPHSA is completed.
On May 23, 2013, SPHSA received a final determination from the South African Reserve Bank that the transaction could proceed with certain conditions and controls designed to repatriate the funds of any sale of Company common stock by the South African shareholders. On June 12, 2013, the acquisition was completed. In the acquisition, SPHSA issued one common share in exchange for each ZAR 4,000 of SPHSA shareholder loans in the aggregate amount of ZAR 27,459,245 (approximately $2,775,000). These newly issued 6,865 common shares of SPHSA and the previously issued 3,000 common shares of SPHSA were then exchanged at the rate of 1,000 shares of Common Stock for each SPHSA common share and a total of 9,864,811 shares of Common Stock were issued.
12. 2012 Stock Option Plan
On December 11, 2012, the Company adopted the 2012 Stock Plan (“the Plan”). Under the terms of the Plan, 3,000,000 shares of our Common Stock have been reserved for future issuance. The Plan authorizes the granting of non-qualified stock options to our directors and to any independent consultants. The Plan is administered by the Company’s board of directors, and may also be administered by a committee appointed by the board of directors (the “Committee”). The Committee may determine persons eligible for grants and the timing, type, amount, fair market value and other provisions of such grants. The Committee will have authority, subject to the express provisions of the Plan, to construe the Plan and the option agreements granted pursuant to the Plan, to prescribe, amend and rescind rules and regulations relating to the Plan, and to make all other determinations in the judgment of the Committee necessary or desirable for the administration of the Plan. The Committee may correct any defect or supply any omission or reconcile any inconsistency in the Plan or in any option agreement in the manner and to the extent it shall deem expedient to promote the best interests of the Company. The Plan terminates on December 9, 2022.
The Plan provides that the determination of the option price per share for any option rests in discretion of the Committee. Options granted under the Plan must be granted within ten years from the date on which the Plan has been adopted. No options granted under the Plan may be exercisable after ten years from the date of grant. The Company’s board of directors may suspend or terminate the Plan in whole or in part or amend the Plan in such respects as the board may deem appropriate and in the best interest of the company. No amendment, suspension or termination of the Plan shall, however, without the optionee's consent, alter or impair any of the rights or obligations granted under the Plan.
On November 12, 2013, the Company granted non-qualified stock options to purchase an aggregate of 475,000 shares of the Company’s Common Stock under the plan, each at an exercise price of $0.89 per share. The options vest over three years as follows:
| F-24 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
| Options vested on November 12, 2013 | 190,000 | |||
| Options vesting on November 12, 2014 | 142,500 | |||
| Options vesting on November 12, 2015 | 142,500 | |||
| 475,000 |
The Company uses the Black-Scholes-Merton option pricing model to estimate the fair value of stock options. The principal assumptions utilized in valuing stock options include the expected stock price volatility (based on the most recent historical period beginning December 12, 2012); the expected option life; the expected dividend yield; and the risk-free rate (an estimate based upon the yields of Treasury constant maturities) equal to the expected life of the option. The fair values for the options, all of which were granted in 2013, were determined using the Black-Scholes-Merton option pricing model with the following assumptions:
| Dividend yield | 0.00 | % | ||
| Risk-free interest rates | 2.80 | % | ||
| Expected volatility | 72.68 | % | ||
| Expected term (in years) | 10 |
There was no reduction for estimated forfeitures. Under this model and the foregoing assumptions, the options granted in 2013 were valued at $151,053. Of this amount, $87,452, $15,900 and $47,701 is attributable to executives, employees and consultants, respectively. Equity-based compensation for the years ended December 31, 2014 and December 31, 2013 was $136,568 and $151,053, respectively and $287,621 for the period from inception to December 31, 2014. These amounts are included in general and administrative expenses in the accompanying Consolidated Statements of Operations.
13. Commitments, Contingencies and Tax Obligation
Lease Commitments
The Group leases two premises in South Africa under operating leases. One location is an office facility and the other is a workshop. The office facility lease was originally scheduled to expire on April 30, 2012 and has been extended in a short-term basis several times. The lease term terminated on February 28, 2014 and the Company leased this office for an additional month in March 2014. The Company executed a one-year lease for a new office facility effective April 1, 2014 and was able to terminate this lease in May 2014. The lease for the Group’s workshop facility originally expired on April 30, 2012 and was also extended on a short-term basis. The workshop is being leased on a month-to-month basis. On June 1, 2014, the Company lease new office and workshop facilities for a one-year term. The monthly rent for the office lease is approximately $1,800 and the monthly rent for the workshop is $900. In addition to the payments required under the preceding leases, payments are made for space rentals on an as-needed basis. The total rent expense was approximately $52,000 and $71,000 for the years ended December 31, 2014 and 2013, respectively, and approximately $902,000 for the period from inception to December 31, 2014. Rent expense is included in general and administrative expenses in the accompanying Consolidated Statements of Operations.
The future rent commitments under the above leases for the twelve months ending December 31, 2015 is approximately $17,000.
| F-25 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
Payroll Commitments
The Group’s employees in South Africa have employment contracts that provide for one month of notice before the employee can be terminated. As of December 31, 2014, the total monthly salary commitment applicable to these employees was approximately $21,000.
Payroll Tax Obligation
SPMSA has received notification from the South African Revenue Service regarding unpaid payroll taxes of approximately $185,000. SPMSA requested additional time to arrange a payment plan that is suitable to both parties and has commenced making monthly payments against this balance. Due to insufficient funds the SPMSA has been unable to continue making additional payments in this regard and additionally SPMSA has been unable to meet current payroll tax obligations. As at December 31, 2014 and December 31, 2013, the unpaid balance of this liability was approximately $80,000 and $81,000, respectively and is included in accounts payable and other current liabilities in the accompanying consolidated balance sheets. Effective October 31, 2014, the South African Revenue Service placed a collection order on the bank account of SPMSA for the outstanding amounts due.
Employment and Consulting Agreements
The Company has entered into agreements to secure the services of two executives. These agreements provide for annual compensation and require a termination notice period by the Group of three months. The executives all are stockholders of the Company.
The total compensation to these executives and one former executive was approximately $814,000 and $990,000 for the years ended December 31, 2014 and 2013, respectively, and approximately $6,896,000 for the period from inception to December 31, 2014.
The Company entered into agreements with unrelated third party consultants and institutions for consulting, research and professional services. With the exception of one agreement that has a remaining term of three months as of December 31, 2013, these agreements can be terminated by either party with between two weeks and three months written notice or immediately if for cause. The amounts due vary according to the nature of the service arrangement and the length of notice required for termination. The minimum amount due under these agreements is approximately $75,000 for the year ending December 31, 2015.
The Company entered into a six-month consulting agreement effective April 1, 2014 under which the consultant was compensated by the issuance of 30,000 shares of Company Common Stock on a monthly basis for each month covered by the agreement. This contract ended on September 20, 2014 and has not been renewed.
Other Commitments
The Company is obligated to make payments of approximately $415,000 subsequent to December 31, 2014 in connection with purchase orders open as of December 31, 2014 for the purchase of machines to be delivered in 2015. Payments to vendors relating to these purchases are scheduled to be made prior to June 30, 2015 from the proceeds of the sales to which they relate.
| F-26 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
14. Subsequent Events
The Company has evaluated its subsequent events since December 31, 2014. Except as disclosed in Note 10 with respect to income taxes payable and Note 13 with respect to open purchase orders and payroll tax obligations, the Company has determined that there were no material subsequent events requiring adjustment to or disclosure in the accompanying Consolidated Financial Statements.
15. Going Concern
The accompanying Consolidated Financial Statements have been prepared on a going concern basis, which contemplates the continuation of the Group as a going concern. Due to the start-up nature of its business, the Group has generated recurring losses and expects to incur additional losses as it expands its photopurification technology and develops marketing, sales and financial plans. As reflected in the accompanying Consolidated Financial Statements, the Company’s total consolidated liabilities exceed total consolidated assets at December 31, 2014 and December 31, 2013 by $2,259,765 and $1,352,572, respectively, and the Company has incurred cumulative consolidated operating losses since the date of inception.
To date, the Company’s cash flow has been funded with cash investments by its shareholders, certain third parties and to a considerably lesser extent, sales and interest income. These inflows have been insufficient to enable the company to cover its costs. For the year ended December 31, 2014 there has been a substantial increase in current liabilities. Additionally as disclosed in Note 10 with respect to income taxes payable and Note 14 with respect to payroll tax obligations, the Company has been unable to meet its obligations in terms of these taxes.
The Company requires additional capital to continue its development and to achieve sufficient revenues to support its operations. The extent of the Company’s future capital requirements depend on many factors, including its ability to maintain revenues and to manage expenses. The Company requires additional financing either through borrowings or the sale of additional equity to support its operations.
The Company’s access to additional financing, if any, will depend on a variety of factors over which the Company has little or no control. These factors include market conditions, the general unavailability of equity financing to the Company in light of its limited revenues, the actual financial and operational results of the Company on a consolidated basis, as well as investors’ perception of the Company’s short-term and long-term financial prospects. If future financing is not available on acceptable terms, or on any other terms, the Company will not be able to continue as a going concern.
Management is currently pursuing plans to address the cash flow requirements of the Company for the twelve months ending December 31, 2015 and beyond. The Company is engaged in discussions with current and other potential investors, and the Company is also considering additional offerings of Common Stock. The Company was not, however, able to secure any additional equity financing during the second half of 2014 from existing shareholders or otherwise, despite its efforts to do so. In addition, the Company projects that increase in the commercialization of its technology may provide additional cash flow for operations. However, the Company did not enter into any commercial agreements during 2013 or in the year ended December 31, 2014 that provided materials amounts of revenue.
| F-27 |
SurePure, Inc. and Subsidiaries
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years ended December 31, 2014 and 2013 |
The Consolidated Financial Statements do not include any adjustments that might be necessary should the Company be unable to continue as a going concern.
| F-28 |
