Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - NORTHWEST BIOTHERAPEUTICS INC | Financial_Report.xls |
| EX-23.1 - EXHIBIT 23.1 - NORTHWEST BIOTHERAPEUTICS INC | v403266_ex23-1.htm |
| EX-31.1 - EXHIBIT 31.1 - NORTHWEST BIOTHERAPEUTICS INC | v403266_ex31-1.htm |
| EX-32.1 - EXHIBIT 32.1 - NORTHWEST BIOTHERAPEUTICS INC | v403266_ex32-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
| ¨ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______to _______
Commission File Number: 001-35737
NORTHWEST BIOTHERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware (State or Other Jurisdiction of Incorporation or Organization) |
94-3306718 (I.R.S. Employer
|
4800 Montgomery Lane, Suite 800, Bethesda, MD 20814
(Address of principal executive offices) (Zip Code)
(240) 497-9024
(Registrant's telephone number)
N/A
(Former Name, Former Address and Former Fiscal Year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, $0.001 par value | The NASDAQ Capital Market | |
| Warrants to purchase Common Stock | The NASDAQ Capital Market |
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 (the "Exchange Act") during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer x |
Non- accelerated filer ¨ (Do not check if a smaller reporting company) |
Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was $235,623,851 on June 30, 2014.
As of March 13, 2015 the registrant had 70,310,724 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
| 1 |
NORTHWEST BIOTHERAPEUTICS, INC.
FORM 10-K
TABLE OF CONTENTS
| 2 |
PART I
This Report on Form 10-K for Northwest Biotherapeutics, Inc. may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such forward-looking statements are characterized by future or conditional verbs such as "may," "will," "expect," "intend," "anticipate," believe," "estimate" and "continue" or similar words. You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward-looking information. Such statements are only predictions and our actual results may differ materially from those anticipated in these forward-looking statements. We believe that it is important to communicate future expectations to investors. However, there may be events in the future that we are not able to accurately predict or control. Factors that may cause such differences include, but are not limited to, those discussed under Item 1A. Risk Factors and elsewhere in this Form 10-K for the year ended December 31, 2014, as filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger-scale clinical trials, the risk that we will not obtain approval to market our products, the risks associated with dependence upon key personnel and the need for additional financing. We do not assume any obligation to update forward-looking statements as circumstances change.
Unless the context otherwise requires, “Northwest Biotherapeutics,” the “Company,” “we,” “us,” “our” and similar names refer to Northwest Biotherapeutics, Inc. DCVax® is a registered trademark of the Company.
| ITEM 1. | BUSINESS. |
Overview
We are a biotechnology company focused on developing immunotherapy products to treat cancers more effectively than current treatments, without toxicities of the kind associated with chemotherapies, and, through a proprietary batch manufacturing process, on a cost-effective basis, initially in both the United States and Europe (the two largest medical markets in the world).
We have developed a platform technology, DCVax, which uses activated dendritic cells to mobilize a patient's own immune system to attack their cancer. The DCVax technology is expected to be applicable to all solid tumor cancers, and is embodied in several distinct product lines. One of the product lines (DCVax-L) is designed to cover all solid tumor cancers in which the tumors can be surgically removed. Another product line (DCVax-Direct) is designed for all solid tumor cancers which are considered inoperable and cannot be surgically removed. We believe the broad applicability of DCVax to many cancers provides multiple opportunities for commercialization and partnering.
Our DCVax platform technology involves dendritic cells, the master cells of the immune system, and is designed to reinvigorate and educate the immune system to attack cancers. The dendritic cells are able to mobilize the overall immune system, including T cells, B cells and antibodies, natural killer cells and many others. Such mobilization of the overall immune system provides a broader attack on the cancer than mobilizing just a particular component, such as T cells alone, or a particular antibody alone. Likewise, our DCVax technology is designed to attack the full set of biomarkers, or antigens, on a patient’s cancer, rather than just a particular selected target or several targets. Clinical experience indicates that when just one or a few biomarkers on a cancer are targeted by a drug or other treatment, sooner or later the cancer usually develops a way around that drug, and the drug stops working. We believe that mobilizing all agents of the immune system, and targeting all biomarkers on the patient’s cancer, contributes to the effectiveness of DCVax.
Product Information
Immune therapies for cancer
Development of effective immune therapies for cancer has long been a goal of the medical and scientific communities. The human immune system is very powerful, and also very complex: an “army” with many divisions and many different kinds of weapons. A diagram of some key agents and weapons of the immune system is set forth below:
| 3 |
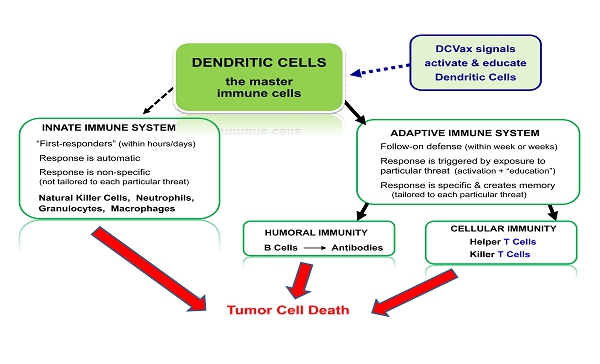
INNATE IMMUNE SYSTEM ADAPTIVE IMMUNE SYSTEM “First - responders” (within hours/days) Response is automatic Response is non - specific (not tailored to each particular threat ) Follow - on defense (within week or weeks) Response is triggered by exposure to particular threat (activation + “education”) Response is specific & creates memory (tailored to each particular threat) Natural Killer Cells, Neutrophils, Granulocytes, Macrophages DENDRITIC CELLS t he master immune cells DCVax signals activate & educate Dendritic Cells B Cells Antibodies Helper T Cells Killer T Cells CELLULAR IMMUNITY HUMORAL IMMUNITY Tumor Cell Death
Diagram 1: The immune system includes many diverse agents. Dendritic cells are the master cells of the immune system.
It has taken decades of research to identify the many different types of immune system agents, to determine the relationships among them, and to determine how they work together to attack and defeat invaders such as bacteria, viruses and cancers. While the research was in process, early versions of immune therapies against cancers were tried, with mixed results and a number of failures. Over the course of the 1990s and 2000s, the first commercially successful category of immune agents to treat cancers emerged: drugs that consisted of individual antibodies, such as Avastin, Herceptin and Erbitux.
Antibodies are just one category of weapon in the overall immune “army,” and there are many kinds of individual antibodies within this category. Each antibody drug, such as Avastin, consists of just a single one of the many kinds of antibodies within this one category of immune weapon. These drugs do not involve the numerous other important agents in the immune system, such as T cells, NK cells, and so on.
Antibody drugs have been moderate medical successes and huge commercial successes. These drugs have delivered moderate extensions of patient survival over traditional chemotherapy drugs, with somewhat lesser (though still significant) toxicity. On this basis, these antibody drugs are achieving multi-billion dollars per year in sales.
Now, other types of immune therapies are also being developed, including checkpoint inhibitor drugs that are designed to “take the brakes off” immune responses, and T cell based therapies that are designed to deliver targeted immune agents to attack cancers. In addition, more broad based immune therapies are starting to come of age: “therapeutic vaccines” designed to mobilize the entire immune “system,” rather than just a single agent or single category of agents. Therapeutic vaccines are similar to preventive vaccines in that they work by mobilizing the immune system. However, therapeutic vaccines are administered to patients who already have a given disease, for the purpose of preventing or delaying recurrence or progression of the existing disease.
Several of the therapeutic vaccines that are now coming of age are focusing on dendritic cells in various ways. These vaccines offer a broader potential immune response because dendritic cells are the master cells of the immune system. When dendritic cells are activated against a particular pathogen (or cancer) they, in turn, mobilize all of the other agents (including T cells as well as B cells, NK cells and others) to attack that pathogen (or cancer). The process by which dendritic cells mobilize other agents takes place to a large extent in the lymph nodes.
A major challenge faced by immune therapies for cancer has been that, unlike in a healthy patient with an infectious disease, in cancer patients the dendritic cells fail to do their job, and the other immune agents also fail to do their job. Pathologists analyzing tumor tissue removed from cancer patients have long observed that there are often substantial numbers of immune cells in the surrounding tissue, but they are not infiltrating and attacking the tumor - as though the immune cells have made it to the doorstep of the tumor and then stopped.
The mechanisms by which cancer cells selectively suppress or block the immune system are still the subject of much research. It is known that cancer cells have many such mechanisms, including secretion of biochemical signals that block normal immune signaling, that make tumor cells invisible to immune detection and/or that convey false messages to the immune system. Different therapeutic vaccines are taking different approaches to trying to overcome these cancer mechanisms and put the immune system back in action.
Many of the therapeutic vaccines for cancer have targeted existing dendritic cells in situ in a patient’s body, by administering various compounds or factors that are designed to attract dendritic cells to the tumor or enhance the tumor signals to the dendritic cells (in essence, making the tumor signals “louder”).
We and a few others are taking a different approach, based on the belief that existing dendritic cells in situ in a patient’s body are impaired and their ability to receive and process the necessary signals is blocked. Under this view, if the signaling is blocked, then no matter how “loud” the signal may be, it will not get through and will not achieve the activation needed.
| 4 |
The DCVax Technology
As described above, our platform technology, DCVax, is a personalized immune therapy which consists of a therapeutic vaccine that uses a patient's own dendritic cells, or DCs, the master cells of the immune system, as the therapeutic agent. The patient’s DCs are obtained through a blood draw, or leukapheresis. The DCs are then activated and loaded with biomarkers (“antigens”) from the patient’s own tumor. The loading of biomarkers into the DCs “educates” the DCs about what to attack. The activated, educated DCs are then isolated with very high purity and constitute the DCVax personalized vaccine.
We believe that injection of DCVax-L (the activated, educated, fresh, uncompromised dendritic cells of the patient) into the patient, through a simple intra-dermal injection, similar to a flu shot, in the upper arm, can initiate a potent immune response against cancer cells, mobilizing the overall immune system and doing so in the natural way, with the numerous immune agents acting in their normal roles and in combination with each other. We also believe that DCVax-Direct, which is directly injected into inoperable tumors, functions in a similar way. In short, DCVax is designed to restore the potent natural functioning of the immune system which has otherwise been impaired or blocked by the cancer.
Importantly, each activated, educated dendritic cell has a large multiplier effect, mobilizing hundreds of T cells and other immune cells. As a result, small doses of such dendritic cells can mobilize large and sustained immune responses. Also very importantly, dendritic cells activate diverse populations of T cells (i.e., T cells targeted at a variety of different biomarker targets on the patient’s cancer). In contrast, T cell based therapies employ T cells aimed at just one biomarker target on the cancer, similar to targeted drugs.
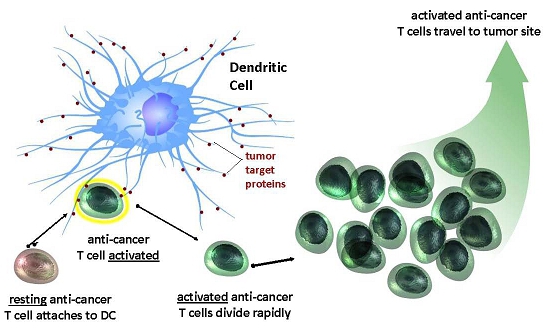
Dendritic Cell resting anti - cancer T cell attaches to DC anti - cancer T cell activated Dendritic Cell tumor target proteins activated anti - cancer T cells divide rapidly activated anti - cancer T cells travel to tumor site
Diagram 2: One Educated Dendritic Cell Activates Hundreds of Anti-Cancer T Cells
We believe that at least three key aspects of the DCVax technology contribute to the positive results (described more fully below) seen in clinical trials to date:
| (1) | DCVax is personalized, and targets the particular biomarkers expressed on that patient’s tumor. Extensive scientific evidence has shown that there is substantial variation in tumor profiles and characteristics among patients with the “same” cancer. The degree of variation is particularly large in some of the most aggressive cancers, such as GBM brain cancer and pancreatic cancer. Cancer drugs are typically keyed to a single target which is believed to be found on the cancer cells’ surface or in one of the cancer cells’ signaling pathways in a substantial percentage of patients with a given type of cancer. Such drugs are of no use in patients whose cancers do not happen to express that particular target, or cease expressing that target as the disease progresses. Most cancer drugs only achieve clinical benefits in a limited percentage of the patients with the type of cancer being targeted (e.g., 25 - 30% of the patients). In contrast, DCVax has achieved clinical benefits (i.e., longer delay in disease progression and longer extension of survival than with standard of care treatment) in over 80% of the patients who have received DCVax in clinical trials to date. Since DCVax is made with biomarkers from the patient’s own tumor, it is automatically tailored to targets that are present on that patient’s cancer. |
| (2) | DCVax is designed to target not just one but the full set of biomarkers on the patient’s tumor. As mentioned above, cancer drugs are typically rifle shots aimed at just one target on a patient’s cancer. However, cancer is a complex and variable disease. Tumor profiles vary among patients with the “same” cancer and also vary as the disease progresses. Further, when rifle shot drugs hit individual targets on cancers, the cancers find ways around them (called “escape variants”) - and the rifle shot treatments then usually stop working. DCVax takes the opposite approach: instead of aiming at a single target, DCVax is aimed at the full set of biomarkers on a patient’s cancer. Such treatment approach is expected to make it more difficult for tumors to develop escape variants. |
| (3) | DCVax is designed to mobilize the overall immune system, not just one among the many different categories of immune agents in that overall system. As described above, DCVax is comprised of activated, educated dendritic cells, and dendritic cells are the master cells of the immune system; they mobilize the rest of the immune system. Some of the prominent cancer drugs today are composed of just one type of antibody - and antibodies themselves are just one type of agent in the overall immune system (see Diagram 1 above). In contrast, the full immune system involves many types of antibodies, and also many other kinds of agents besides antibodies. There have been a variety of early immune therapies that failed in the past. These typically involved single agents, such as a single one among the many types of immune signaling molecules (e.g., a particular interferon or interleukin), or a single type of agent such as T cells alone, etc. In contrast, dendritic cells can mobilize all of these different categories of agents, comprising the overall immune system, in combination with each other and in their natural relationships to each other. |
DCVax Product Lines
We have developed several different product lines based on the DCVax technology, to address multiple different cancers and different patient situations. There are two main components to each DCVax product: the immune cells (dendritic cells) and the cancer biomarker targets (antigens).
All of our DCVax product lines are made from the patient’s own dendritic cells. The dendritic cells are freshly isolated, and newly matured and activated. We believe that the existing dendritic cells in a cancer patient have already been compromised by the cancer, and we believe that is the reason other vaccines aimed at the existing dendritic cells in patients have largely failed. However, the patient’s body continues to produce new precursors of dendritic cells, and these precursors (monocytes) circulate in the patient’s blood stream. For all DCVax products, these precursors are obtained through a blood draw, and then (through our proprietary manufacturing processes), the precursors are matured into a fresh, uncompromised batch of new dendritic cells.
| 5 |
The antigen (biomarker target) component, which is combined with the fresh, personalized dendritic cells, varies among the DCVax product lines.
Importantly, each of our product lines has an excellent safety profile. Patients may develop some flu-like symptoms, but there have been no toxicities such as are seen with chemotherapies and with some other types of immune therapies. Patients do not have to take a second set of drugs to manage side effects, there are no in-patient hospital stays to deal with side effects, and patients do not have to go home and stay in bed for several days to deal with side effects. Our DCVax patients typically go on with their work and their daily activities.
DCVax-L - is designed for operable solid tumors. It is made with cancer antigens from tumor lysate (a protein extract from processed tumor cells) from the patient’s own tumor tissue. As such, DCVax-L incorporates the full set of tumor antigens, making it difficult for tumors to find ways around it (“escape variants”), as described above. This is the DCVax product that has been used in our brain cancer and ovarian cancer clinical trials, and is currently in our 348-patient Phase III brain cancer trial. DCVax-L is expected to be applicable for any type of solid tumor cancers in situations in which the patient has their tumor surgically removed as part of standard of care.
DCVax-Direct - is designed for inoperable solid tumors – situations in which it is not feasible or not desirable for patients to have their tumors surgically removed, either due to multiple metastases or for other reasons. Like DCVax-L, DCVax-Direct also incorporates the full set of tumor antigens - but it does so in situ in the patient’s body rather than at the manufacturing facility. With DCVax Direct, the fresh, new dendritic cells are partially matured in a special proprietary way so as to be ready to pick up antigens directly from tumor tissue in the patient’s body, and also communicate the information about those antigens to other agents of the immune system, such as T cells. The partially matured dendritic cells are then injected directly into the patient’s tumor(s). There, the dendritic cells pick up the antigens in situ rather than picking up the antigens from lysate in a lab dish at the manufacturing facility, as is done with DCVax-L. DCVax-Direct is anticipated to be applicable to any type of inoperable solid tumors.
DCVax-Prostate - is designed specifically for late stage, hormone independent prostate cancer. Such cancer involves the spread of micro-metastases beyond the prostate tissue. In most patients, there is no focal tumor which can be surgically removed and used to make lysate, or into which dendritic cells can be directly injected. Instead, the cancer cells are diffuse. We have developed a DCVax product line using a particular antigen - PSMA (Prostate Specific Membrane Antigen) - which is found on essentially all late stage (hormone independent) prostate cancer. The PSMA is produced through recombinant manufacturing methods, and is then combined with the fresh, personalized dendritic cells to make DCVax-Prostate.
| 6 |
Clinical Programs, Clinical Trial Results and Early Access Programs
Overall Clinical Pipeline
Over the last ten years, we have built a robust clinical pipeline with DCVax products for multiple cancers, which we believe provides us with multiple opportunities for success. Our lead product, DCVax-L for GBM brain cancer has previously gone through early stage trials, and is in now the late stages of a 348-patient international Phase III trial. Our second product, DCVax-Direct for all types of inoperable solid tumors, has completed the 40-patient Phase I stage of a Phase I/II trial and is progressing into multiple Phase II trials. In addition to these trials, our DCVax-L product has also been administered in a small early stage trial for metastatic ovarian cancer, and DCVax-Prostate successfully completed a 35-patient Phase I/II trial and was cleared by the FDA some years ago for a 612-patient Phase III trial.
DCVax-L for Operable Solid Tumors: GBM Brain Cancer
The first cancer for which we are developing DCVax-L is Glioblastoma multiforme (GBM): the most aggressive and lethal type of brain cancer. With full standard of care treatment for GBM today, including surgery, radiation and chemotherapy, the cancer recurs in a median of just 6.9 months and kills the patient in a median of just 14.6 months. There has been very little improvement in clinical outcomes for GBM patients in the last 30 years. The incidence of GBM appears to be on the rise, for unknown reasons, and there is an urgent need for new and better treatments.
Prior Phase I/II Clinical Trials
We and our collaborator, Dr. Linda Liau, conducted two prior Phase I/II clinical trials at UCLA with DCVax-L for GBM brain cancer. These trials consisted of 39 patients with newly diagnosed GBM and recurrent GBM, and a couple of other gliomas. The newly diagnosed patients who received DCVax in addition to standard of care treatment typically did not have tumor recurrence for a median of approximately 2 years, which is more than triple the usual time with standard of care (6.9 months), and patients survived for a median of approximately 3 years, which is approximately 2½ times the usual period attained with standard of care treatment (14.6 months).
Furthermore, a substantial percentage of patients who received DCVax in the prior clinical trials have continued in a “long tail” far beyond even the 3 year median survival. As of the latest long-term data update several years ago, approximately 33% of the patients treated with DCVax in the Phase I/II trials had reached or exceeded 4 years’ survival, and approximately 27% had reached or exceeded 6 years’ survival, compared with 14.6 months median survival with full standard of care treatment today. Two of the DCVax-treated patients have exceeded 10 years’ survival to date.
| 7 |
Our Current Phase III Clinical Trial
Our DCVax-L product is currently in a 348-patient Phase III trial. As of February 28, 2015, there are more than 60 clinical sites open and operating for the trial across the U.S. and in the U.K., Germany and Canada, with more sites expected to become operational during 2015, particularly in Europe. The trial was originally getting under way when the financial crisis began in 2008, enrolled a limited number of patients at that time, and then suspended new enrollment into the ongoing trial until the summer of 2011. The trial initially resumed enrollment in 2011 at about 10 sites, all in the US, then gradually expanded to a growing number of sites over the course of 2012 through 2014. In 2012, the trial was approved by the U.K. regulatory authority to proceed in the U.K. In the fall of 2013, the trial was approved by the German regulatory authority to proceed there. In 2014, the trial was approved by the Canadian regulatory authority to proceed there as well. We plan to continue adding sites to the trial, because the same institutional approvals, contract negotiations, personnel training and logistics arrangements that are needed for the trial also serve to prepare the sites for commercialization.
The trial is a double-blind, randomized, placebo controlled trial with two treatment arms. Patients in one arm receive standard of care plus DCVax-L; patients in the other arm receive standard of care plus a placebo. Patients are assigned randomly between the two arms: two-thirds of the patients into the DCVax-L arm and one-third into the placebo arm. Standard of care includes surgical removal of the brain tumor, followed by 6 weeks of daily radiotherapy and chemotherapy, followed by monthly chemotherapy. The standard of care chemotherapy is Temodar (temozolamide). The primary endpoint of the trial is median Progression Free Survival. Secondary endpoints include median Overall Survival. The trial includes a crossover arm, in which patients who originally receive the standard of care plus a placebo have an opportunity, when their disease progresses, to cross over and start receiving DCVax-L. However, there is still no un-blinding at the time of crossover.
We anticipate that the Phase III trial will reach its first interim analysis for efficacy during 2015. Various factors may affect the timing of completion of the trial, including the pace of adding more sites in Europe during 2015, and the pace of enrollment in Europe. We anticipate that the Phase III trial will reach its primary endpoint next year, potentially in the spring or summer.
In the meantime, while the Phase III trial is moving toward completion, during 2015 we plan to be developing and growing our early access programs for providing DCVax-L to brain cancer patients, such as the Hospital Exemption program in Germany, as described below.
“Information Arm” Outside the Phase III Trial
In parallel with the Phase III trial of DCVax-L for GBM, we accepted a total of 55 patients into an “Information Arm” outside of the trial, who failed to meet the eligibility requirements for the trial. Most of these patients (51 of the 55) were actual or potential “rapid progressors” (patients in whom the brain cancer is already appearing to re-grow by the time the patient finishes the 6 weeks of daily radiotherapy and daily chemotherapy following surgical removal of the tumor). At least 19 of the 51 patients were confirmed as being clear rapid progressors, with such aggressive cancer that the brain tumors were already re-growing within weeks after the original surgery, and during the daily radiotherapy and chemotherapy. The rest of the 51 patients could not be classified as clearly, with today’s imaging and other technology. All of the 51 patients were treated with the same DCVax-L product as in the Phase III trial, on the same treatment schedule as in the trial, at the same medical centers as in the trial, in the same time period as the trial, and the data were collected and maintained by the same Contract Research Organization (CRO) as is managing the trial.
As we have reported, a significant extension of survival compared with expected survival times has been seen to date in these Information Arm patients, including the 19 confirmed rapid progressors. Such patients usually do not respond much to any treatments. We plan to continue following these patients during this year, and plan to report on further results.
| 8 |
DCVax-L Early Access Programs
In March 2014, we received approval from the German regulatory authority of a “Hospital Exemption” for DCVax-L for glioma brain cancers under Section 4b of the German Drug Law. This approval for DCVax-L was the first of its kind in a number of key ways, although the law had been in place for several years. Under this Hospital Exemption, we may provide DCVax-L to patients for the treatment of any glioma brain cancers (both Glioblastoma multiforme, the most severe grade, and lower grade, less-malignant gliomas), and both newly diagnosed and recurrent stages of disease, outside of our Phase III clinical trial, and charge full price for the product. The patients may be from Germany or elsewhere. This approval has a term of five years, and can be re-applied for and re-issued at the end of that period.
During 2014, we undertook preparations for this Hospital Exemption early access program (for which the parties would not engage until we had received regulatory approval) including numerous contract negotiations with medical centers, separate arrangements for international patients at the medical centers, development of a registry and system for data collection, obtaining local licenses, development of patient contracts and consent and release forms, logistics arrangements, and other steps. During 2015, we plan to continue these program development activities, undertake outreach activities, and gradually grow the program.
Also in early 2014, we received a determination from the German central reimbursement authority that DCVax-L is eligible for reimbursement on an extraordinary basis, even though it is still in clinical trials. The reimbursement must be negotiated with the German Sickness Funds (health insurance companies). One aspect of the process involves negotiations with hospitals to seek inclusion in overall budgets which the hospitals negotiate with the Sickness Funds. Another aspect of the process involves negotiation of individual patient cases, one by one.
During 2014, we undertook considerable work on pricing models, and began the process of hospital budget discussions and case by case discussions. We expect to continue these processes in 2015. Although these are labor intensive and lengthy processes, we believe it is highly valuable to have an opportunity to undertake these processes now, while DCVax-L is still finishing its clinical trials and prior to commercialization, as normally these processes can only be begun after full product approval has been received and commercialization has begun.
In the U.K., we also undertook early access program activities in 2014. In April, 2014, the U.K. government launched a new program for early access to innovative new treatments for serious unmet medical needs: the Early Access to Medicines Scheme (EAMS). The EAMS involves a 2-step process. First is a scientific evaluation by the U.K. regulatory authority of the new treatment and whether it is likely to offer a major advantage over existing treatments for a serious disease with high unmet medical need. If the evaluation is positive, it results in a “PIM” (Promising Innovative Medicine) designation. Our DCVax-L for brain cancer went through this evaluation and, as we reported in September, 2014, DCVax-L became the first product to receive a PIM designation under the new EAMS program. The second (and final) stage of the EAMS involves a further Scientific Opinion and an evaluation of the manufacturing. Our activities for 2015 include pursuit of the second (and final) stage of EAMS. Recently, the first EAMS approval was granted to a big pharma’s checkpoint inhibitor drug, which is designed to “take the brakes off” a patient’s immune response to cancer. We consider this a helpful precedent.
As we have consistently said in our public presentations, we believe the most important value of early access programs such as the above lies in the regulatory validation involved, and the invaluable opportunity to practice for commercialization outside of clinical trials and before actual commercialization. The opportunity for some early revenues is also encouraging, but in our view is secondary.
DCVax-Direct for Inoperable Solid Tumor Cancers
Our DCVax-Direct product offers a potential new treatment option for the wide range of clinical situations in which patients' tumors are considered inoperable because the patient has multiple tumors, or their tumor cannot be completely removed, or the surgery would cause undue damage to the patient and impair their quality of life.
| 9 |
A large number of patients with a variety of cancer types (including lung, colon, pancreatic, liver, ovarian, head and neck, and others) are faced with this situation, because their tumors are already locally advanced or have begun to metastasize by the time symptoms develop and the patients seek diagnosis and treatment. For these patients, the outlook today is bleak and survival remains quite limited.
DCVax-Direct is administered by direct injection into a patient's tumors. It can potentially be injected into any number of tumors, enabling patients with locally advanced disease or with metastases to be treated. DCVax-Direct can also be injected into tumors in virtually any location in the body: not only tissues at or near the surface of the body but also into interior tissues, with any form of image guidance (ultra-sound, MRI or CT).
In the fall of 2012, we initiated the processes for manufacturing DCVax-Direct for clinical trials. During the first half of 2013, we (through Cognate BioServices) expanded and accelerated the manufacturing preparations, including assay development, test runs and other qualification and optimization work, on both the product and the automated system for key stages of the manufacturing. In the second half of 2013 we launched the Phase I stage of our 60-patient Phase I/II clinical trial with DCVax-Direct, for all types of inoperable solid tumor cancers. The trial took place at two sites: MD Anderson Cancer Center in Houston and Orland Health (formerly MD Anderson, Orlando).
As a first-in-man study of a new type of treatment, the DCVax-Direct trial had to proceed slowly for an extended period, treating only about one patient per month to check for any safety or toxicity issues until about March, 2014. There was strong interest in the trial, and once the pacing limitations were lifted, the trial proceeded quickly. Most of the total recruitment was completed between March and June, 2014, and a few excess patients were accepted in July and early August. For each patient, the manufacturing, quality control and product release took several weeks and then the treatment regimen lasted up to 8 months.
The patients who were enrolled in the Phase I trial had failed other treatments, had multiple tumors and actively progressing disease – these were effectively no-option patients. In spite of this heavy disease burden, though, the treatment regimen in this first clinical trial was very conservative: only one tumor was injected in each patient, and treatments were spaced as much as a month or two apart. Despite these challenging circumstances, a number of case studies and interim data to date have been quite encouraging, as we have previously reported. Clinical effects seen in various patients include examples of tumor necrosis (i.e., cell death) in the injected tumors, shrinkage or stabilization in some non-injected tumors, and/or stabilization of disease. We are continuing to collect the data, and anticipate reporting on it after the data set is complete.
This Phase I trial is designed to be very informative: we are treating numerous diverse types of cancers (sarcoma, pancreatic, colorectal, lung, melanoma and others); we are testing three different dose levels and two different formulations of the DCVax-Direct product; we are testing different methods of image guidance for the intra-tumoral injections (all of which have worked); we are collecting both imaging and biopsy data, and correlating them with clinical effects in patients; we are evaluating both local effects in the injected tumors and systemic effects in the non-injected tumors; we are evaluating potential endpoints for future trials; and most importantly, we are evaluating safety.
Our experience to date with the Phase I stage of the DCVax-Direct Phase I/II trial is that (as has also been the case over the years with DCVax-L) the safety profile is excellent. The typical effects are that patients develop a fever after the injections, but only a couple of degrees and only for a day or two, and they do not experience any significant toxicities.
Based upon the data and experience over the course of 2014 and the first months of this year, we are planning to proceed with at least two Phase II trials of DCVax-Direct in different cancers, in parallel during this year. In these Phase II trials, we plan to inject multiple tumors, rather than just one tumor, and we plan to administer treatments weeks apart rather than months apart. We continue to receive strong interest for trials of DCVax-Direct in a variety of cancers.
| 10 |
Prostate Cancer
Prostate cancer is the most common cancer in men in the U.S., accounting for more than 25% of all cancers in men, and nearly twice as many cases per year as lung cancer in men, according to the American Cancer Society. For late stage prostate cancer, there is a pressing unmet need for new treatments. This late stage cancer includes two subsets of patients, comprising two distinct markets: (A) about 80 - 85% of patients do not yet show metastases, have a last good period of life, and typically live for about 36 months; and (B) about 15 - 20% of patients have more aggressive disease, show metastases right away, and only live for about 18 months. Nearly 100,000 men reach these late stages of prostate cancer every year in the United States alone (with similar numbers in Europe). Yet, there is no FDA approved standard of care drug specifically for the patients in group A, who comprise the vast majority of late stage prostate cancer patients.
For the patients in group B, there are a growing number of FDA approved drugs, including taxotere (docetaxel), Provenge, Zytiga, Xtandi and others. These drugs generally add about 4-5 months of survival.
We believe that DCVax-Prostate can potentially offer a much needed treatment option for late stage prostate cancer patients, and can potentially offer a materially longer extension of survival.
Our Prior Clinical Trials
Clinical experience with DCVax-Prostate dates back more than a decade. More than one hundred patients were treated with DCVax-Prostate in an academic clinical setting in the mid and late 1990s. Based on encouraging results from those treatments, we undertook a Phase I/II clinical trial with 35 patients at two leading clinical centers: MD Anderson and UCLA. Based upon positive results from that trial, we designed a large 612-patient, Phase III clinical trial, and previously obtained FDA clearance to proceed with this trial. The details of these clinical programs are described below.
Our Phase I/II clinical trial, conducted at MD Anderson and UCLA, included both subsets of hormone independent prostate cancer patients: group A, without visible metastases, and group B, with metastases. As is standard for Phase I/II trials, in our Phase I/II trial all patients in the trial received the DCVax treatment (there was no placebo control arm). For Group A patients, the information below shows a comparison of our clinical results with DCVax-Prostate vs. the natural course of the disease in group A (for whom there is no established standard of care treatment). For group B patients, the information below shows a comparison of our clinical results with the results reported in clinical trials with Provenge, the dendritic cell immunotherapy that was approved by the FDA several years ago for late stage prostate cancer and was recently acquired by Valeant.
| 11 |
Group A: Hormone Independent Prostate Cancer Patients without Metastases
| Natural Course of Disease | With DCVax-Prostate | |||
| Median time to disease progression (appearance of bone metastases) | 28 - 34 weeks | 59 weeks | ||
| Median survival | 36 months | >54 months and continuing** | ||
|
**(more than half of these patients still alive as of last data follow-up) |
Group B: Hormone Independent Prostate Cancer Patients with Metastases
| With Provenge | With DCVax-Prostate | |||
| Median survival | 25.9 months | 38.7 months | ||
| Overall survival at 3 years | 33% | 64% |
Thus, in the prior Phase I/II clinical trial, patients without metastases (group A) who were treated with DCVax-Prostate typically lived at least 1½ years longer than patients going through the natural course of the disease.
Patients with metastases (group B) who were treated with DCVax-Prostate lived more than a year longer than patients treated with Provenge immune therapy in the clinical trials upon which FDA approval of Provenge was based.
Following these positive results in both group A and group B patients, we decided to focus our Phase III clinical trial on the patients in group A, because 80-85% of late stage prostate cancer patients fall into this group, while only 15 - 20% fall into group B. In contrast, the Provenge clinical trials were focused on the group B patients, and obtained FDA approval only for that group of patients.
We are currently focusing on development of our DCVax-L and DCVax-Direct product lines. We plan to proceed with the previously planned Phase III trial of DCVax-Prostate only in the context of partnering arrangements.
Target Markets for DCVax Products
Since our DCVax-L product is potentially applicable to all types of operable solid tumors, and our DCVax-Direct product is potentially applicable to all types of inoperable solid tumors, the potential markets for DCVax products are particularly large. According to the American Cancer Society, 1 in 2 men, and 1 in 3 women in the U.S. will develop some form of cancer in their lifetime. There are nearly 1.5 million new cases of cancer per year in the U.S., and nearly 600,000 deaths from cancer. The statistics are similar in Europe and in much of the rest of the world.
Brain cancer
Brain cancers fall into two broad categories: primary (meaning the cancer first originates in the brain) and metastatic (meaning the cancer first appears elsewhere in the body, but subsequently metastasizes to the brain). In the U.S. alone, on an annual basis, there are some 40,000 new cases of primary brain cancer, and 160,000 new cases of metastatic brain cancer. The numbers are similar in Europe and the rest of the world.
Within the category of primary brain cancer, Grade 4 GBM is the most aggressive and lethal type. Among the approximately 40,000 new cases of primary brain cancer per year in the U.S., at least 12,000 cases are GBM (with some estimates as high as 17,000) and the incidence is increasing.
In addition, brain cancer is a serious medical problem in children 18 years and under. It is the second most frequent type of childhood cancers (after leukemias) and, following progress in reducing death rates from leukemias, it is now a leading cause of childhood cancer deaths.
Very little has changed in the last 30 years in the treatment and clinical outcomes for GBM. With all standard of care treatment today - surgery, radiation and chemotherapy - patients still die within a median of about 14.6 months from diagnosis.
| 12 |
Although many experimental treatments are in various stages of clinical development, the one drug which has been the standard of care chemotherapy treatment for GBM to date, Temodar, achieved market saturation extremely rapidly, within two years of product launch. Temodar added 10 weeks of survival (extending survival from its historical 12 months to the 14.6 months typical today), and did so in a limited percentage of patients. Other drugs approved by FDA for GBM, such as Avastin, did not extend survival at all.
Against this backdrop, we believe DCVax is well positioned for this target market. Further, after seeking regulatory approval for DCVax for the GBM subset of primary brain cancers, in the future we plan to conduct clinical trials and seek approval for other (lower grade) primary brain cancers and for metastatic brain cancers, as well as, ultimately, other types of solid tumor cancers.
Manufacturing of DCVax
We believe that our proprietary manufacturing process for DCVax products is a key to the practicality of our product and to favorable product economics. We have spent more than a decade honing this manufacturing process.
We have pioneered a DCVax-L manufacturing model under which at least 3 years of treatments are normally produced in a single personalized batch for each patient. Similarly, for DCVax-Direct, we produce a full course of treatment in a single personalized batch for a patient.
In addition, we have implemented special cryopreservation methods which enable this multi-year or multi-dose quantity of personalized product to be frozen, and kept frozen for years, while maintaining its viability and potency.
Both of these technologies, the personalized batch manufacturing for each patient and the cryopreservation, are essential elements of our manufacturing model and product economics. Together, they enable us to incur the high costs of manufacturing just one time for each patient, and then store the multi-year quantity of product, frozen, in single doses. This makes DCVax effectively an “off the shelf” product for the patient after the initial manufacturing, even though it is personalized, and we anticipate that this will enable the pricing of DCVax to be in line with other new cancer drugs. We also believe that both automation and economies of scale will further enhance the product economics. The manufacturing process today is also rapid: about 8 days for DCVax-L, and 7 days for DCVax-Direct, followed by quality control and release testing.
| 13 |
We contract out the manufacturing of our DCVax products to Cognate BioServices. Although there are many contract manufacturers for small molecule drugs and for biologics, there are only a few contract manufacturers in the U.S. and even fewer in Europe that specialize in producing living cell products and that have a track record of success with regulatory authorities. The manufacturing of living cell products is highly specialized and entirely different than production of biologics: the physical facilities and equipment are different, the types of personnel and skill sets are different, and the processes are different. The regulatory requirements relating to manufacturing of cellular products (especially personalized cellular products) are exceptionally difficult to meet and are one of the most frequent reasons for a company’s clinical trials and product development to be put on clinical hold (i.e., stopped by regulatory authorities).
Cognate BioServices is one of the few companies that specializes in the production of cellular products, and has been doing so for 13 years (far longer than others). Very importantly, Cognate also has a leading track record of regulatory success specifically with cellular products.
In addition to the rigorous regulatory requirements, our DCVax programs involve a particularly challenging operational and business requirement: our programs require a large amount of capacity in these specialized manufacturing facilities, and require that the large capacity be dedicated exclusively to our programs. Most medical products, including cellular products, are made in batches on a campaign basis. The same manufacturing suites are used for a number of companies’ products, at designated times scheduled in advance. In contrast, our products are fully personalized and can only be made in individual personalized batches, not large-scale batches of standardized products, and our products are made on demand, on an ongoing basis. So, the manufacturing suites must be dedicated entirely to NW Bio’s products. Among the few specialized contract manufacturers for cellular products, even fewer have the necessary capacity that can be dedicated exclusively to NW Bio.
Cognate BioServices’ manufacturing facility for clinical-grade cell products is located in Memphis, Tennessee, a major air shipping hub for both Federal Express and UPS. Cognate BioServices' facility is approximately 80,000 square feet and contains substantial expansion space in addition to the portions currently built out and in use. The current manufacturing facilities are sufficient to produce DCVax for at least several thousand patients per year - an amount well in excess of what is needed for the Phase III clinical trial under way. The expansion space will also allow us to procure significantly increasing capacity as we scale-up towards many more patients for commercialization. The facility planned for the U.K. will similarly allow for scale-up there.
Intellectual Property and Orphan Drug Designation
We have an integrated strategy for protection of our technology through both patents and other mechanisms, such as Orphan Drug status. As of December 31, 2014, we have over 136 issued patents and 47 pending patent applications worldwide, grouped into 12 patent families. Of these, 100 issued patents and 47 pending patent applications relate to our DCVax products. In the United States and Europe, some of our patents and applications relate to the composition and use of products, while other patents and applications related to other aspects such as manufacturing and quality control. For example, in the United States, we have three issued and five pending patent applications that relate to the composition and/or use of our DCVax products. We also have other U.S patents and applications that cover, among other things, quality control for DCVax, as well as an automated system which we believe will play a major role in the scale-up of production for large numbers of patients on a cost-effective basis. Similarly, in Europe, we have four patents issued by and six pending patent applications with the European Patent Office ("EPO") that cover our DCVax products, and other patents and applications that cover aspects such as manufacturing and quality control, and the automated system. In Japan, we have three issued patents and five pending patent applications relating to our DCVax products, as well as manufacturing related patents. Patents have been granted and are pending in other foreign jurisdictions we consider important potential future markets for our DCVax products.
During 2012, a dozen new patents, including one European and twelve other foreign patents, were issued to us as part of our worldwide patent portfolio. The newly issued patents covered a variety of subject matter, such as the proprietary partial maturation for DCVax-Direct, the machines and systems to manufacture DCVax-Direct, certain processes for enhancing the potency of dendritic cells in general, certain measures of product quality, and other matters.
During 2013, eight new patents, including three U.S. and five other foreign patents, were issued to us as part of our worldwide patent portfolio. The newly issued patents cover a variety of subject matter, such as the proprietary partial maturation for DC-Vax-Direct, the machine and systems to manufacture DCVax-Direct, and certain processes for enhancing the potency of dendritic cells in general, and other matters.
In September 2013, we announced that we had been issued U.S. patent #8,518,636, covering a next-generation process for manufacturing lower cost human dendritic cells of both a higher quality and higher reliability. This next generation system has already been cleared by the FDA for use in the manufacturing of dendritic cells for our clinical trials. These systems are now in use producing the vaccines which have already been injected into the tumors of DCVax-Direct patients.
During 2014, seven new patents (including one European patent validated into 26 patents in various European countries and seven other foreign patents) were issued to us as part of our worldwide patent portfolio. The newly issued patents cover a variety of subject matter, such as the machine and system to manufacture DCVax-Direct and certain processes for enhancing the potency of dendritic cells.
| 14 |
The expiration dates of the issued U.S. patents involved in our current business range from 2022 to 2028. The expiration dates of the issued European patents involved in our current business range from 2022 to 2028. For some of the earlier dates, we plan to seek extensions of the patent life, and believe we have reasonable grounds for doing so.
In addition to our patent portfolio, we have obtained Orphan Drug designation for our lead product, DCVax-L for glioma brain cancers. Such designation brings with it a variety of benefits, including potential market exclusivity for seven years in the U.S. and ten years in Europe if our product is the first of its type to reach the market.
This market exclusivity applies regardless of patents, (i.e., even if the company that developed it has no patent coverage on the product). In addition, the time period for such market exclusivity does not begin to run until product sales begin. In contrast, the time period of a patent begins when the patent is filed and runs down during the years while the product is going through development and clinical trials.
Competition
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. A large and growing number of companies (such as Celldex, Oxford Biomedica, Argos Therapeutics, Agenus, Prima Biomed, Avax Technologies, Lion Biotechnologies, Immunocellular Therapeutics, Bavarian Nordic, Bellicum Pharmaceuticals, KITE Pharma, Juno Therapeutics and many others) are actively involved in the research and development of immune therapies or cell-based therapies for cancer. In addition, many big pharma companies (including BMS, Merck, Pfizer, Astra Zeneca, Roche and others) are rapidly developing, and have begun obtaining accelerated approvals for, a new class of checkpoint inhibitor drugs to “take the brakes off” patients’ immune responses to cancer. Other novel technologies for cancer are also under development, such as the electro-therapy device of NovoCure. Additionally, many companies are actively involved in the research and development of monoclonal antibody-based and bi-specific antibody based cancer therapies. Currently, a substantial number of antibody-based drugs are approved for commercial sale for cancer therapy, and a large number of additional ones are under development. Many other third parties compete with us in developing alternative therapies to treat cancer, including: biopharmaceutical companies; biotechnology companies; pharmaceutical companies; academic institutions; and other research organizations, as well as some medical device companies (e.g., NovoCure).
We face extensive competition from companies developing new treatments for brain cancer. These include a variety of immune therapies, as mentioned above, as well as a variety of small molecule drugs and biologics. There are also a number of existing drugs used for the treatment of brain cancer that may compete with our product, including, Avastin® (Roche Holding AG), Gliadel® (Eisai Co. Ltd.), and Temodar® (Merck & Co., Inc.). Both checkpoint inhibitor drugs and T cell based therapies are rapidly pursuing clinical trials for brain cancer, as well.
Most of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing and sales than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly if they enter into collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel and collaborators, as well as in acquiring technologies complementary to our programs, and in obtaining sites for our clinical trials and enrolling patients.
Corporate Information
We were formed in 1996 and incorporated in Delaware in July 1998. Our principal executive offices are located in Bethesda, Maryland, and our telephone number is (240) 497-9024. Our website address is www.nwbio.com. The information on our website is not part of this report. We have included our website address as a factual reference and do not intend it to be an active link to our website.
Available Information
Our website address is www.nwbio.com. We make available, free of charge through our website, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports as soon as is reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission (the “SEC”), but other information on our website is not incorporated into this report. The SEC maintains an Internet site that contains these reports at www.sec.gov.
Employees and Contractors
As of February 28, 2015, we had 12 full-time, as well as manufacturing related employees in Germany. We believe our employee relations are positive.
In addition to our full-time employees, a substantial number of contractors provide various services for our corporate operations. We have contract management of our clinical trials and contract manufacturing of our products. In addition, since 2012 a third party firm has been performing the functions of a Chief Financial Officer, including financial systems and financial reporting. This third party firm specializes in providing such services to bio-pharma companies, both small and large, and the owners are highly experienced former partners of “Big Four” accounting firms.
This contract services approach has enabled us to have important financial functions performed by experienced specialists, and also to have large support teams for our work on an as-needed basis, without the ongoing expense. However, we plan to develop more internal systems and structures, and expand our internal personnel for these functions, over the coming year as our programs continue to grow and we move toward completion of certain clinical trials.
| 15 |
| ITEM 1A. | RISK FACTORS |
Our business, financial condition, operating results and prospects are subject to the following material risks. Additional risks and uncertainties not presently foreseeable to us may also impair our business operations. If any of the following risks actually occurs, our business, financial condition or operating results could be materially adversely affected. In such case, the trading price of our common stock could decline, and our stockholders may lose all or part of their investment in the shares of our common stock.
Risks Related to our Operations
We will need to raise substantial funds, on an ongoing basis, for general corporate purposes and operations, including our clinical trials. Such funding may not be available or may not be available on acceptable terms.
We will need substantial additional funding, on an ongoing basis, in order to continue execution of our clinical trials, to move our product candidates towards commercialization, to continue prosecution and maintenance of our large patent portfolio, to continue development and optimization of our manufacturing and distribution arrangements, and for other corporate purposes. Any financing, if available, may include restrictive covenants and provisions that could limit our ability to take certain actions, preference provisions for the investors, and/or discounts, warrants, anti-dilution rights, the provision of collateral, or other incentives. Any financing will involve issuance of equity and/or debt, and such issuances will be dilutive to existing shareholders. There can be no assurance that we will be able to complete any of the financings, or that the terms for such financings will be acceptable. If we are unable to obtain additional funds on a timely basis or on acceptable terms, we may be required to curtail or cease some or all of our operations at any time.
We are likely to continue to incur substantial losses, and may never achieve profitability.
As of December 31, 2014, we had a net cash outflows flows from operations, since inception. We may never achieve or sustain profitability.
Our auditors have issued a “going concern” audit opinion.
Our independent auditors have indicated in their report on our December 31, 2014 financial statements that there is substantial doubt about our ability to continue as a going concern. A “going concern” opinion indicates that the financial statements have been prepared assuming we will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities that may result if we do not continue as a going concern. Therefore, you should not rely on our consolidated balance sheet as an indication of the amount of proceeds that would be available to satisfy claims of creditors, and potentially be available for distribution to stockholders, in the event of liquidation.
Our management and our independent auditors have identified internal control deficiencies, which our management and our independent auditor believe constitute material weaknesses
In connection with the preparation of our financial statements for the year ended December 31, 2014, and prior years, our management and our independent auditor identified certain internal control deficiencies that, in the aggregate, represent material weaknesses, including the following. However, these weaknesses did not result in any material unadjusted differences when preparing the December 31, 2014, financial statements.
| · | Insufficient segregation of duties, oversight of work performed and lack of compensating controls in our finance and accounting function due to limited personnel. |
| · | Lack of controls in place, including those surrounding related party transactions, to ensure that all material transactions and developments impacting the financial statements are reflected and properly recorded. |
| · | Lack of documentation to support occurrences of review and approval procedures. |
| · | Design deficiencies that do not meet stated control objectives that elevate the level of risk of a material misstatement to our financial statements. |
| · | Policies and procedures with respect to the review, supervision and monitoring of our accounting operations throughout the organization were either not designed and in place or not operating effectively. |
| · | We did not maintain an adequate risk oversight function to evaluate and report on risks to financial reporting throughout the organization, including completion of a comprehensive risk assessment to identify all potential risk areas and evaluate the adequacy of controls to mitigate identified risk. |
| · | We did not maintain an effective anti-fraud program designed to detect and prevent fraud relating to (i) an effective whistle- blower program or other comparable mechanism and (ii) an ongoing program to manage identified fraud risks. |
| 16 |
As part of our independent auditors’ communications with our audit committee with respect to audit procedures for the year ended December 31, 2014, our independent auditors informed the audit committee that these deficiencies constituted material weaknesses, as defined by Auditing Standard No. 5, “An Audit of Internal Control Over Financial Reporting that is Integrated with an Audit of Financial Statements and Related Independence Rule and Conforming Amendments,” established by the Public Company Accounting Oversight Board, or PCAOB. Accordingly, the report of Marcum LLP on the Company’s internal control over financial reporting as of December 31, 2014, as well as management’s report as of the same date, which were included in the Annual Report, contained an adverse opinion thereon. Since 2012, we have retained a third party firm to perform our financial reporting function on a contract services basis. This third party firm specializes in technical accounting and SEC reporting services, and performs this function for many other bio-pharma companies, both small and large. This third party firm is owned and managed by individuals with significant “Big 4” accounting firm experience. In addition, we hired a “Big 4” accounting firm in early 2015 to review the significant estimates surrounding our derivative financial instruments. Management intends to take additional steps in due course to make the necessary improvements to address these deficiencies, but the timing of such steps is uncertain and the availability of funding and resources for such steps are also uncertain. Our ability to retain or attract qualified individuals to serve on our Board and to take on key management or other roles within our Company is also uncertain. Our failure to successfully complete the remediation of the existing weaknesses could lead to heightened risk for financial reporting mistakes and irregularities, and/or lead to a loss of public confidence in our internal controls that could have a negative effect on the market price of our common stock.
As a Company with a novel technology and unproven business strategy, an evaluation of our business and prospects is difficult
We are still in the process of developing our product candidates through clinical trials. Our technology is novel and involves mobilizing the immune system to fight a patient’s cancer. Immune therapies have been pursued by many parties for decades, and have experienced many failures. In addition, our technology involves personalized treatment products, a new approach to medical products that involves new product economics and business strategies, which have not yet been shown to be commercially feasible or successful. We have not yet gone through scale-up of our operations to commercial scale. The novelty of our technology, product economics, and business strategy, and the limited scale of our operations to date, makes it difficult to assess our prospects for generating revenues commercially in the future.
We will need to expand our management and technical personnel as our operations progress, and we may not be able to recruit such additional personnel and/or retain existing personnel.
As of February 28, 2015, we employed 12 full-time employees, plus manufacturing related employees in Germany. The rest of our personnel are retained on a consulting or contractor basis. Many biotech companies would typically have a larger number of employees by the time they reach late stage clinical trials. Such trials and other programs require extensive management capabilities, activities and skill sets, including scientific, medical, regulatory (for FDA and foreign regulatory counterparts), manufacturing, distribution and logistics, site management, reimbursement, business, financial, legal, public relations outreach to both the patient community and physician community, intellectual property, administrative, regulatory (SEC), investor relations and other.
In order to fully perform all these diverse functions, with late stage trials and other programs under way at many sites across the U.S. and in Europe, we will need to expand our management, technical and other personnel. However, with respect to management and technical personal, the pool of such personnel with expertise and experience with living cell products, such as our DCVax immune cell product, is very limited. In addition, we are a small company with limited resources, our business prospects are uncertain and our stock price is volatile. For some or all of such reasons, we may not be able to recruit all the management, technical and other personnel we need, and/or we may not be able to retain all of our existing personnel. In such event, we may have to continue our operations with a small team of personnel, and our business and financial results may suffer.
We rely at present on third-party contract manufacturers. As a result, we may be at risk for capacity limitations and/or supply disruptions.
We currently rely upon Cognate BioServices, Inc., or Cognate, to produce all of our DCVax product candidates in the U.S., and to supervise the production of our DCVax product candidates outside the U.S. The shareholders of Cognate BioServices include Toucan Capital Fund III, L.P., one of our stockholders, and its affiliates, including Linda Powers. We have an agreement in place with Cognate BioServices pursuant to which Cognate BioServices has agreed to provide manufacturing and other services for five years, in connection with our Phase III clinical trial of DCVax-L in brain cancer, and other programs. The agreement requires us to make certain minimum monthly payments to Cognate BioServices in order to have dedicated manufacturing capacity available for our products, irrespective of whether we actually order any DCVax products. The agreement also specifies the amounts we must pay for Cognate BioServices’ actual manufacturing of DCVax for patients.
Due to the large expansion of our Phase III trial with DCVax-L for brain cancer, and initiation of the trial in Europe, as well as initiation of our DCVax-Direct program, and certain advanced product development work, additional services that are required for logistics, distribution and tracking, and other pending programs, and the need for expanded manufacturing capacity, we entered into four new agreements with Cognate BioServices in January, 2014, for our DCVax-L and DCVax-Direct programs, Ancillary Services and Manufacturing Expansion Services. However, there can be no assurance that these expanded agreements will be sufficient. The agreements involved substantial upfront payments and provide for payment of at least half of all invoices to be paid in common stock and warrants of the Company, and the remainder in cash, at an initial price of $4.00 per share for an initial period in parallel with the lock-up period under the conversion transaction, subject to most favored nation treatment with respect to terms provided to other investors or creditors (including with respect to any warrants). The agreements may cover commercial as well as clinical activities, and will only be terminable early by either party for uncured material breach by the other party.
| 17 |
We have entered into an agreement with King’s College London to manufacture DCVax for our clinical trial and our compassionate use cases. Cognate BioServices will manage and supervise the processing in London. In addition, in Germany our partner, Fraunhofer IZI Institute in Germany, has received approval and certification from the regional and national regulatory agencies in Germany for the manufacture of DCVax for GBM. Fraunhofer IZI also received the necessary regulatory approvals to supply DCVax-L products to the U.K. for our clinical trial there. We anticipate that the manufacturing facilities in the U.K. will eventually obtain the necessary approvals, and that the German and U.K. facilities’ will be able to supply DCVax products for anywhere in Europe; however, this may not turn out to be feasible, for regulatory, operational and/or logistical reasons.
Problems with the manufacturing facilities, processes or operations of Cognate BioServices, or of our partners in the U.K. and/or Germany, could result in a failure to produce, or a delay in producing adequate supplies of our DCVax product candidates. A number of factors could cause interruptions or delays, including the inability of a supplier to provide raw materials, equipment malfunctions or failures, damage to a facility due to natural disasters or otherwise, changes in FDA or European regulatory requirements or standards that require modifications to our manufacturing processes, action by the FDA or European regulators, or by us that results in the halting or slowdown of production of components or finished products due to regulatory issues, our manufacturers going out of business or failing to produce product as contractually required, and/or other similar factors. Because manufacturing processes for our DCVax product candidates are highly complex, require specialized facilities (dedicated exclusively to DCVax production) and personnel that are not widely available in the industry, involve equipment and training with long lead times, and are subject to lengthy regulatory approval processes, alternative qualified production capacity may not be available on a timely basis or at all. Also, Cognate could choose to terminate its agreements with us if we are in breach. Difficulties, delays or interruptions in the manufacturing and supply of our DCVax product candidates could require us to stop enrolling new patients into our trials, and/or require us to stop the trial or other program, increase our costs, damage our reputation and, if our product candidates are approved for sale, cause us to lose revenue or market share if our manufacturers are unable to timely meet market demands.
The manufacturing of our product candidates will have to be greatly scaled up for commercialization, and neither we nor other parties in the industry have experience with such scale-up.
As is the case with any clinical trial, our Phase III clinical trial of DCVax-L for GBM involves a number of patients that is a small fraction of the number of potential patients for whom DCVax-L may be applicable in the commercial market. The same will be true of our other clinical programs with our other DCVax product candidates. If our DCVax-L, and/or other DCVax product candidates, are approved for commercial sale, it will be necessary to greatly scale up the volume of manufacturing, far above the level needed for the trials. Neither we nor our contract manufacturers have experience with such scale-up. In addition, there are very few consultants or advisors in the industry who have such experience and can provide guidance or assistance, because active immune therapies such as DCVax are a fundamentally new category of product in two major ways: these active immune therapy products consist of living cells, not chemical or biologic compounds, and the products are personalized. To our knowledge, no such products have successfully completed the necessary scale-up for commercialization without material difficulties. For example, Dendreon Corporation has encountered substantial difficulties trying to scale up the manufacturing of its Provenge® product for commercialization.
The necessary specialized facilities, equipment and personnel may not be available or obtainable for the scale-up of manufacturing of our product candidates.
The manufacture of living cells requires specialized facilities, equipment and personnel which are entirely different than what is required for the manufacturing of chemical or biologic compounds. Scaling up the manufacturing of living cell products to volume levels required for commercialization will require enormous amounts of these specialized facilities, equipment and personnel - especially where, as in the case of our DCVax product candidates, the product is personalized and must be made for each patient individually. Since living cell products are so new, and have barely begun to reach commercialization, the supply of the specialized facilities, equipment and personnel needed for them has not yet developed. It may not be possible for us or our manufacturers to obtain all of the specialized facilities, equipment and personnel needed for commercialization of our DCVax product candidates. This could delay or halt our commercialization.
Our technology is novel, involves complex immune system elements, and may not prove to be effective.
Data already obtained, or in the future obtained, from pre-clinical studies and clinical trials do not necessarily predict the results that will be obtained from later pre-clinical studies and clinical trials. Over the course of several decades, there have been many different immune therapy product designs - and many product failures and company failures. To our knowledge, to date, only one active immune therapy, Provenge, has been approved by the FDA. The human immune system is complex, with many diverse elements, and the state of scientific understanding of the immune system is still limited. Some immune therapies previously developed by other parties showed surprising and unexpected toxicity in clinical trials. Other immune therapies developed by other parties delivered promising results in early clinical trials, but failed in later stage clinical trials.
| 18 |
To date, we have only completed early stage trials with our first product (DCVax-L) in limited numbers of patients. Although the results of those trials were quite positive, those results may not be achieved in our later stage clinical trials, such as the 348-patient Phase III trial we are now conducting for GBM, and our product candidates may not ultimately be found to be effective. Further, although we have not seen toxicity with our DCVax-L product in the early stage clinical trials, toxicity may be seen as we treat larger numbers of patients in late stage clinical trials. If such toxicity occurs, it could limit, delay or stop further clinical development or commercialization of our DCVax-L product.
We have only conducted the Phase I portion of our first-in-man Phase I/II clinical trial with our third product - DCVax Direct - after prior early stage trials with DCVax-L and DCVax-Prostate. Although the early results have not indicated any significant toxicity, we do not yet know what efficacy or toxicity DCVax-Direct may show in a larger sample of human patients. This product may not ultimately be found to be effective, and/or it may be found to be toxic, which could limit, delay or stop clinical development or commercialization of DCVax-Direct.
Clinical trials for our product candidates are expensive and time consuming, and their outcome is uncertain.
The process of obtaining and maintaining regulatory approvals for new therapeutic products is expensive, lengthy and uncertain. Costs and timing of clinical trials may vary significantly over the life of a project owing to any or all of the following non-exclusive reasons:
| • | the duration of the clinical trial; |
| • | the number of sites included in the trials; |
| • | the countries in which the trial is conducted; |
| • | the length of time required and ability to enroll eligible patients; |
| • | the number of patients that participate in the trials; |
| • | the number of doses that patients receive; |
| • | the drop-out or discontinuation rates of patients; |
| • | per patient trial costs; |
| • | third party contractors failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner; |
| • | our final product candidates having different properties in humans than in laboratory testing; |
| • | the need to suspend or terminate our clinical trials; |
| • | insufficient or inadequate supply or quality of necessary materials to conduct our trials; |
| • | potential additional safety monitoring, or other conditions required by the FDA or comparable foreign regulatory authorities regarding the scope or design of our clinical trials, or other studies requested by regulatory agencies; |
| • | problems engaging independent review Boards, or IRBs, to oversee trials or in obtaining and maintaining IRB approval of studies; |
| • | the duration of patient follow-up; |
| • | the efficacy and safety profile of a product candidate; |
| • | the costs and timing of obtaining regulatory approvals; and |
| • | the costs involved in enforcing or defending patent claims or other intellectual property rights. |
| 19 |
Late stage clinical trials, such as our Phase III clinical trial for GBM patients, are especially expensive, typically requiring tens of millions of dollars, and take years to reach their outcomes. Such outcomes often fail to reproduce the results of earlier trials. It is often necessary to conduct multiple late stage trials (including multiple Phase III trials) in order to obtain sufficient results to support product approval, which further increases the expense. Sometimes trials are further complicated by changes in requirements while the trials are under way (for example, when the standard of care changes for the disease that is being studied in the trial). For example, while the Company’s lead program, the Phase III clinical trial of DCVax-L for brain cancer, has been under way, there has been a very large proliferation of new treatments in various stages of development, as well as some new product approvals, for brain cancer. Any of our current or future product candidates could take a significantly longer time to gain regulatory approval than we expect, or may never gain approval, either of which could delay or stop the commercialization of our DCVax product candidates.
We may be required to suspend or discontinue clinical trials due to unexpected side effects or other safety risks that could preclude approval of our product candidates.
Our clinical trials may be suspended at any time for a number of reasons. For example, we may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to the clinical trial patients. In addition, the FDA or other regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the clinical trial patients.
Administering any product candidate to humans may produce undesirable side effects. These side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities denying further development or approval of our product candidates for any or all targeted indications. Ultimately, some or all of our product candidates may prove to be unsafe for human use. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects as a result of participating in our clinical trials.
We have limited experience in conducting and managing clinical trials.
We rely on third parties to assist us, on a contract services basis, in managing and monitoring all of our clinical trials. We do not have experience conducting late stage clinical trials by ourselves without third party service firms, nor do we have experience in supervising such third parties in managing late stage, multi-hundred patient clinical trials, other than our current Phase III trial for GBM. Our lack of experience and/or our reliance on these third party service firms may result in delays or failure to complete these trials successfully and on time. If the third parties fail to perform, we may not be able to find sufficient alternative suppliers of those services in a reasonable time period, or on commercially reasonable terms, if at all. If we were unable to obtain alternative suppliers of such services, we might be forced to delay, suspend or stop our Phase III trial for GBM.
We may fail to comply with regulatory requirements.
Our success will be dependent upon our ability, and our collaborative partners’ abilities, to maintain compliance with regulatory requirements in multiple countries, including current good manufacturing practices, or cGMP, and safety reporting obligations. The failure to comply with applicable regulatory requirements can result in, among other things, fines, injunctions, civil penalties, total or partial suspension of regulatory approvals, refusal to approve pending applications, recalls or seizures of products, operating and production restrictions and criminal prosecutions.
Regulatory approval of our product candidates may be withdrawn at any time.
After any regulatory approval has been obtained for medicinal products (including any early approval such as our Hospital Exemption approval in Germany and/or our reimbursement eligibility determination in Germany), the product and the manufacturer are subject to continual review, including the review of adverse experiences and clinical results that are reported after our products are made available to patients, and there can be no assurance that such approval will not be withdrawn or restricted. Regulators may also subject approvals to restrictions or conditions, or impose post-approval obligations on the holders of these approvals, and the regulatory status of such products may be jeopardized if such obligations are not fulfilled. If post-approval studies are required, such studies may involve significant time and expense.
The manufacturer and manufacturing facilities we use to make any of our products will also be subject to periodic review and inspection by the FDA or EMA, as applicable. The discovery of any new or previously unknown problems with the product, manufacturer or facility may result in restrictions on the product or manufacturer or facility, including withdrawal of the product from the market. We will continue to be subject to the FDA or the European Medicines Agency, or EMA, requirements, as applicable, governing the labeling, packaging, storage, advertising, promotion, recordkeeping, and submission of safety and other post-market information for all of our product candidates, even those that the FDA or EMA, as applicable, had approved. If we fail to comply with applicable continuing regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approval, product recalls and seizures, operating restrictions and other adverse consequences.
Our Operations under Early Access Programs Such As the Hospital Exemption in Germany May Not Be Successful
There is not much accumulated or available experience, information or precedents in regard to early access programs such hospital exemption programs and/or similar programs, especially for new types of treatments such as immune therapies. Our DCVax-L product for glioma brain cancers is one of the first products to receive a Hospital Exemption approval in Germany. Establishing operations under this Hospital Exemption will require us to establish and implement new operational, contractual, financial and other arrangements with physicians, hospitals, patients and others. We may not be successful in establishing and implementing such arrangements, and/or such arrangements may not be financially satisfactory or viable.
| 20 |
We May Not Be Successful In Negotiating Acceptable or Viable Reimbursement
The reimbursement eligibility determination that we have received in Germany for our DCVax-L product for brain cancer allows us to negotiate reimbursement arrangements, but does not ensure a positive outcome. We must negotiate with hospitals and multiple Sickness Funds in Germany. Reimbursement at the current stage of our DCVax-L programs is extraordinary, and we do not have substantial precedents to refer to. Our DCVax-L product involves a different cost structure than traditional drugs and may require different reimbursement arrangements. The reimbursement arrangements also may be applied on a patient by patient basis. We may not be successful in negotiating or obtaining reimbursement, or obtaining it on acceptable or viable terms.
Our product candidates will require a different distribution model than conventional therapeutic products, and this may impede commercialization of our product candidates.
Our DCVax product candidates consist of living human immune cells. Such products are entirely different from chemical or biologic drugs, and require different handling, distribution and delivery than chemical or biologic drugs. One crucial difference is that the biomaterial ingredients (immune cells and tumor tissue) from which we make DCVax products and the finished DCVax products themselves are subject to time constraints in the shipping and handling. The biomaterial ingredients come from the medical centers to the manufacturing facility fresh and unfrozen, and must arrive within a certain time and in usable condition. Performance failures by the medical center or the courier company can result in biomaterials that are not usable, in which case it may not be possible to make DCVax product for the patient involved. The finished DCVax products are frozen, and must remain frozen throughout the process of distribution and delivery to the medical center or physician’s office, until the time of administration to the patient, and cannot be handled at room temperature until then. In addition, our DCVax product candidates are personalized and they involve ongoing treatment cycles over several years for each patient. Each product shipment for each patient must be tracked and managed individually. For all of these reasons, among others, we will not be able to simply use the distribution networks and processes that already exist for conventional drugs. It may take time for shipping companies, hospitals, pharmacies and physicians to adapt to the requirements for handling, distribution and delivery of these products, which may adversely affect our commercialization.
Our product candidates will require different marketing and sales methods and personnel than conventional therapeutic products. Also, we lack sales and marketing experience. These factors may result in significant difficulties in commercializing our product candidates.
The commercial success of any of our product candidates will depend upon the strength of our sales and marketing efforts. We do not have a marketing or sales force and have no experience in marketing or sales of products like our lead product, DCVax-L for GBM. To fully commercialize our product candidates, we will need to recruit and train marketing staff and a sales force with technical expertise and ability to manage the distribution of our DCVax-L for GBM. As an alternative, we could seek assistance from a corporate partner or a third party services firm with a large distribution system and a large direct sales force. However, since our DCVax products are living cell, immune therapy products, and these are a fundamentally new and different type of product than are on the market today, we would still have to train such partner’s or such services firm’s personnel about our products, and would have to make changes in their distribution processes and systems to handle our products. We may be unable to recruit and train effective sales and marketing forces or our own, or of a partner or a services firm, and/or doing so may be more costly and difficult than anticipated. Such factors may result in significant difficulties in commercializing our product candidates, and we may be unable to generate significant revenues.
The availability and amount of potential reimbursement for our product candidates by government and private payers is uncertain and may be delayed and/or inadequate.
The availability and extent of reimbursement by governmental and/or private payers is essential for most patients to be able to afford expensive treatments, such as cancer treatments. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payers tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for fundamentally novel products such as ours, as there is no body of established practices and precedents for these new products. To date, we are aware of only one active immune therapy that has reached the stage of a reimbursement decision (Provenge). Although CMS approved coverage and reimbursement for Provenge, and private payers followed suit, there remain substantial questions and concerns about reimbursement for Provenge, and such questions and concerns appear to be impeding sales.
Reimbursement agencies in Europe can be even more conservative than CMS in the U.S. A number of cancer drugs which have been approved for reimbursement in the U.S. have not been approved for reimbursement in certain European countries, and/or the level of reimbursement approved in Europe is lower than in the U.S.
| 21 |
Various factors could increase the difficulties for our DCVax products to obtain reimbursement. Costs and/or difficulties associated with the reimbursement of Provenge could create an adverse environment for reimbursement of other immune therapies, such as our DCVax products. Approval of other competing products (drugs and/or devices) for the same disease indications could make the need for our products and the cost-benefit balance seem less compelling. The cost structure of our product is not a typical cost structure for medical products, as the majority of our costs are incurred up front, when the manufacturing of the personalized product is done. Our atypical cost structure may not be accommodated in any reimbursement for our products. If we are unable to obtain adequate levels of reimbursement, our ability to successfully market and sell our product candidates will be adversely affected.
The manner and level at which reimbursement is provided for services related to our product candidates (e.g., for administration of our product to patients) are also important. If the reimbursement for such services is inadequate, that may lead to physician resistance and adversely affect our ability to market or sell our products.
The methodology under which CMS makes coverage and reimbursement determinations is subject to change, particularly because of budgetary pressures facing the Medicare program. For example, the Medicare Prescription Drug, Improvement, and Modernization Act, or Medicare Modernization Act, enacted in 2003, provided for a change in reimbursement methodology that has reduced the Medicare reimbursement rates for many drugs, including oncology therapeutics. The Affordable Care Act may also result in changes in reimbursement arrangements that adversely affect the prospects for reimbursement of our products.
In markets outside the U.S., where we plan to operate in the future, the prices of medical products are subject to direct price controls and/or to reimbursement with varying price control mechanisms, as part of national health systems. In general, the prices of medicines under such systems are substantially lower than in the U.S. Some jurisdictions operate positive and/or negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other countries allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. Accordingly, in markets outside the U.S., the reimbursement for our products may be reduced compared with the U.S. and may be insufficient to generate commercially reasonable revenues and profits.
Competition in the biotechnology and biopharmaceutical industry is intense, rapidly expanding and most of our competitors have substantially greater resources than we do.
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Several companies, such as Novartis, Amgen and Bluebird Bio, Dendreon, Celldex Therapeutics, Inc., Activartis, Oxford Biomedica plc, Argos Therapeutics, Inc., Agenus, Inc., Prima Biomed, Ltd., Avax Technologies, Inc., Immunocellular Therapeutics, Ltd., Bavarian Nordic, Bellicum Pharmaceuticals, Juno Therapeutics, KITE Pharma and a rapidly expanding number of other companies are actively involved in the research and development of immune therapies or cell-based therapies for cancer. In addition, other novel technologies for cancer are under development or commercialization, such as checkpoint inhibitor drugs (which are being rapidly developed by numerous big pharma companies including BMS, Merck, Pfizer, Astra Zeneca, Roche and others) and various T cell based therapies (which are also being rapidly developed by numerous companies with extraordinary resource backing), as well as the electro-therapy device of NovoCure. Additionally, many companies are actively involved in the research and development of monoclonal antibody-based cancer therapies. Currently, a substantial number of antibody-based products are approved for commercial sale for cancer therapy, and a large number of additional ones are under development, including late stage trials. Many other third parties compete with us in developing alternative therapies to treat cancer, including: biopharmaceutical companies; biotechnology companies; pharmaceutical companies; academic institutions; and other research organizations, as well as some medical device companies (e.g., NovoCure and MagForce Nano Technologies AG).
We face extensive competition from companies developing new treatments for brain cancer. These include a variety of immune therapies, as mentioned above (including T cell based therapies and checkpoint inhibitor drugs), as well as a variety of small molecule drugs and biologics drugs. There are also a number of existing drugs used for the treatment of brain cancer that may compete with our product, including, Avastin® (Roche Holding AG), Gliadel® (Eisai Co. Ltd.), and Temodar® (Merck& Co., Inc.), as well as NovoCure’s electrotherapy device.
Most of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing and sales than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly if they enter into collaborative arrangements with large and established companies.
These third parties compete with us in recruiting and retaining qualified scientific and management personnel and collaborators, as well as in acquiring technologies complementary to our programs, and in obtaining sites for our clinical trials and enrolling patients.
Our competitors may complete their clinical development more rapidly than we and our products do, may develop more effective or affordable products, or may achieve earlier or longer patent protection or earlier product marketing and sales. Any products developed by us may be rendered obsolete and non-competitive.
| 22 |
Competing generic medicinal products may be approved.
In the E.U., there exists a process for approval of generic biological medicinal products once patent protection and other forms of data and market exclusivity have expired. Arrangements for approval of generic biologics products exist in the U.S. as well, and the FDA recently approved the first bio-similar product. Other jurisdictions are considering adopting legislation that would allow the approval of generic biologic medicinal products. If generic biologic medicinal products are approved, competition from such products may substantially reduce sales of our products.
We may be exposed to potential product liability claims, and our existing insurance may not cover these claims, in whole or in part. In addition, insurance against such claims may not be available to us on reasonable terms in the future, if at all.
Our business exposes us to potential product liability risks that are inherent in the testing, manufacturing, marketing, sale and use of therapeutic products. We insurance coverage but this insurance may not cover any claims made. In the future, insurance coverage may not be available to us on commercially reasonable terms (including acceptable cost), if at all. Insurance that we obtain may not be adequate to cover claims against us. Regardless of whether they have any merit or not, and regardless of their eventual outcome, product liability claims may result in substantially decreased demand for our product candidates, injury to our reputation, withdrawal of clinical trial participants or physicians, and/or loss of revenues. Thus, whether or not we are insured, a product liability claim or product recall may result in losses that could be material.
We may need to store, handle, use and dispose of controlled hazardous, radioactive and biological materials in our business. Our development activities may result in our becoming subject to regulatory requirements, and if we fail to comply with applicable requirements we could be subject to substantial fines and other sanctions, delays in research and production, and increased operating costs. In addition, if regulated materials were improperly released at our current or former facilities or at locations to which we send materials for disposal, we could be liable for substantial damages and costs, including cleanup costs and personal injury or property damages, and we could incur delays in research and production and increased operating costs.
Insurance covering certain types of claims of environmental damage or injury resulting from the use of these materials is available but can be expensive and is limited in its coverage. We have no insurance specifically covering environmental risks or personal injury from the use of these materials and if such use results in liability, our business may be seriously harmed.
Collaborations play an important role in our business, and could be vulnerable to competition or termination.
We work with scientists and medical professionals at academic and other institutions, including UCLA, MD Anderson Cancer Center, Kings College Hospital and the Fraunhofer Institute, among others, some of whom have conducted research for us or have assisted in developing our research and development strategy. These scientists and medical professionals are collaborators, not our employees. They may have commitments to, or contracts with, other institutions or businesses (including competitors) that limit the amount of time they have available to work with us. We have little control over these individuals. We can only expect that they devote time to us and our programs as required by any license, consulting or sponsored research agreements we may have with them. In addition, these individuals may have arrangements with other companies to assist in developing technologies that may compete with our products. If these individuals do not devote sufficient time and resources to our programs, or if they provide substantial assistance to our competitors, our business could be seriously harmed.
The success of our business strategy may partially depend upon our ability to develop and maintain our collaborations and to manage them effectively. Due to concerns regarding our ability to continue our operations or the commercial feasibility of our personalized DCVax product candidates, these third parties may decide not to conduct business with us or may conduct business with us on terms that are less favorable than those customarily extended by them. If either of these events occurs, our business could suffer significantly.
We may have disputes with our collaborators, which could be costly and time consuming. Failure to successfully defend our rights could seriously harm our business, financial condition and operating results. We intend to continue to enter into collaborations in the future. However, we may be unable to successfully negotiate any additional collaboration and any of these relationships, if established, may not be scientifically or commercially successful.
Our business could be adversely affected by new legislation and/or product related issues.
Changes in applicable legislation and/or regulatory policies or discovery of problems with the product, production process, site or manufacturer may result in delays in bringing products to market, the imposition of restrictions on the product’s sale or manufacture, including the possible withdrawal of the product from the market, or may otherwise have an adverse effect on our business.
| 23 |
Our business could be adversely affected by animal rights activists.
Our business activities have involved animal testing, as such testing is required before new medical products can be tested in clinical trials in human patients. Animal testing has been the subject of controversy and adverse publicity. Some organizations and individuals have attempted to stop animal testing by pressing for legislation and regulation in these areas. To the extent that the activities of such groups are successful, our business could be adversely affected. Negative publicity about us, our pre-clinical trials and our product candidates could also adversely affect our business.
Multiple late stage clinical trials of DCVax-L for GBM, our lead product, may be required before we can obtain regulatory approval.
Typically, companies conduct multiple late stage clinical trials of their product candidates before seeking product approval. Our current Phase III 348-patient clinical trial of DCVax-L for GBM is our first late stage trial. We may be required to conduct additional late stage trials with DCVax-L for GBM before we can obtain product approval. This would substantially delay our commercialization. There is also some possibility that changes requested by the FDA could complicate the application process for product approval. In addition, a rapidly growing number of products are under development for brain cancer, including immunotherapies such as checkpoint inhibitor drugs and T cell based therapies, and some (e.g., NovoCure’s device) has been approved in the U.S. It is possible that the standard of care for brain cancer could change while our Phase III trial is still under way. This could necessitate further clinical trials with our DCVax-L product candidate for brain cancer.
Changes in manufacturing methods for DCVax-L could require us to conduct equivalency studies and/or additional clinical trials.
With biologics products, “the process is the product”: i.e., the manufacturing process is considered to be as integral to the product as is the composition of the product itself. If any changes are made in the manufacturing process, and such changes are considered material by the regulatory authorities, the company sponsor may be required to conduct equivalency studies to show that the product is equivalent under the changed manufacturing processes as under the original manufacturing processes, and/or the company sponsor may be required to conduct additional clinical trials. In addition, if there are multiple manufacturing locations, equivalency studies may be required to show that the products produced in the respective facilities are substantially the same. Our manufacturing processes have undergone some changes during the early clinical trials, and we have multiple manufacturing locations. Accordingly, we may be required to conduct equivalency studies, and/or additional clinical trials, before we can obtain product approval, unless the regulatory authorities are satisfied that the changes in processes do not affect the quality, efficacy or safety of the product, and satisfied that the products made in each manufacturing location are substantially the same.
We may not receive regulatory approvals for our product candidates or there may be a delay in obtaining such approvals.
Our products and our ongoing development activities are subject to regulation by regulatory authorities in the countries in which we and our collaborators and distributors wish to test, manufacture or market our products. For instance, the FDA will regulate the product in the U.S. and equivalent authorities, such as the EMA will regulate in Europe. Regulatory approval by these authorities will be subject to the evaluation of data relating to the quality, efficacy and safety of the product for its proposed use, and there can be no assurance that the regulatory authorities will find our data sufficient to support product approval of DCVax-L or DCVax-Direct. In addition, the endpoint against which the data is measured must be acceptable to the regulatory authorities. The primary endpoint of our Phase III trial of DCVax-L is progression free survival. Sometimes regulators have accepted this endpoint, and sometimes not. There can be no assurance that the regulatory authorities will find this to be an approvable endpoint for Glioblastoma multiforme cancer.
The time period required to obtain regulatory approval varies between countries. In the U.S., for products without “Fast Track” status, it can take up to 18 months after submission of an application for product approval to receive the FDA's decision. Even with Fast Track status, FDA review and decision can take up 12 months. At present, we do not have Fast Track status for our lead product, DCVax-L for GBM. We plan to apply for Fast Track status, but there can be no assurance that FDA will grant us such status for DCVax-L.
Different regulators may impose their own requirements and may refuse to grant, or may require additional data before granting, an approval, notwithstanding that regulatory approval may have been granted by other regulators. Regulatory approval may be delayed, limited or denied for a number of reasons, including insufficient clinical data, the product not meeting safety or efficacy requirements or any relevant manufacturing processes or facilities not meeting applicable requirements as well as case load at the regulatory agency at the time.
| 24 |
We may not obtain or maintain the benefits associated with orphan drug status, including market exclusivity.
Although our lead product, DCVax-L for GBM, has been granted orphan drug status in both the U.S. and the E.U., we may not receive the benefits associated with orphan drug designation (including the benefit providing for market exclusivity for a number of years). This may result from a failure to maintain orphan drug status, or result from a competing product reaching the market that has an orphan designation for the same disease indication. Under U.S. and E.U. rules for orphan drugs, if such a competing product reaches the market before ours does, the competing product could potentially obtain a scope of market exclusivity that limits or precludes our product from being sold in the U.S. for seven years or from being sold in the E.U. for ten years. Also, in the E.U., even after orphan status has been granted, that status is re-examined shortly prior to the product receiving any regulatory approval. The EMA must be satisfied that there is evidence that the product offers a significant benefit relative to existing therapies, in order for the therapeutic product to maintain its orphan drug status. Accordingly, our product candidates will have to re-qualify for orphan drug status prior to any potential product approval in the E.U.
Our intellectual property rights may be overturned, narrowed or blocked, and may not provide sufficient commercial protection for our product candidates, or third parties may infringe upon our intellectual property.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Patent laws afford only limited protection and may not protect our rights to the extent necessary to sustain any competitive advantage we may have. In addition, the laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in those countries. Moreover patents and patent applications relating to living cell products are relatively new, involve complex factual and legal issues, and are largely untested in litigation - and as a result, are uncertain. Our pending and future patent applications may not result in patents being issued which adequately protect our technology or products or which effectively prevent others from commercializing the same or competitive technologies and products. As a result, we may not be able to obtain meaningful patent protection for our commercial products, and our business may suffer as a result. Third parties may challenge our existing patents, and such challenges could result in overturning or narrowing some of our patents. Even if our patents are not challenged, third parties could assert that their patents block our use of technology covered by some or all of our patents
As of December 31, 2014, we had 60 pending patent applications and 136 issued patents worldwide relating to our product candidates and related matters such as manufacturing processes. The issued patents expire at various dates from 2022 to 2028. Our issued patents may be challenged, and such challenges may result in reductions in scope, cancellations or invalidations. Our pending patent applications may not result in issued patents. Moreover, our patents and patent applications may not be sufficiently broad to prevent others from using substantially similar technologies or from developing competing products. We also face the risk that others may independently develop similar or alternative technologies, or design around our patented technologies. As a result, no assurance can be given that any of our pending or future patent applications will be granted, that the scope of any patent protection currently granted or that may be granted in the future will exclude competitors or provide us with competitive advantages, that any of the patents that have been or may be issued to us will be held valid if subsequently challenged, or that other parties will not claim rights to or ownership of our patents or other proprietary rights that we hold.
We have taken security measures (including execution of confidentiality agreements) to protect our proprietary information, especially proprietary information that is not covered by patents or patent applications. These measures, however, may not provide adequate protection for our trade secrets or other proprietary information. In addition, others may independently develop substantially equivalent proprietary information or techniques or otherwise gain access to our trade secrets.
We may be exposed to claims or lawsuits that our products infringe patents or other proprietary rights of other parties.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. We have not conducted a comprehensive freedom-to-operate review to determine whether our proposed business activities or use of certain of the technology covered by patent rights owned by us would infringe patents issued to third parties.
| 25 |
There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and biopharmaceutical industries generally. The patent landscape is especially uncertain in regard to cell therapy products, as it involves complex legal and factual questions for which important legal principles remain unresolved. We may become party to, or be threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference proceedings before the U.S. Patent and Trademark Office. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. If the infringement is found to be willful, we could be liable for treble damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We have already been exposed to one patent lawsuit by a large company, which we vigorously defended. Our defense resulted in the plaintiff withdrawing nearly all of the claims it filed, and in settlement of the last claims without our paying the plaintiff anything. However, the litigation was expensive and time consuming. We have recently also been exposed to claims (without a lawsuit) by a competitor asserting or implying (and commentaries by third parties based on the claims by our competitor) that a patent issued to our competitor covers our products. We believe these claims to be without merit. However, if a lawsuit for infringement were brought against us, there can be no assurance that a judge or jury would agree with our position, and in any event such litigation would be expensive and time consuming. In the future, we may again be exposed to claims by third parties - with or without merit - that our products infringe their intellectual property rights.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
DCVax is our only technology in clinical development.
Unlike many pharmaceutical companies that have a number of products in development and which utilize many different technologies, we are dependent on the success of our DCVax platform technology. While the DCVax technology has a wide scope of potential use, and is embodied in several different product lines for different clinical situations, if the core DCVax technology is not effective or is toxic or is not commercially viable, our business could fail. We do not currently have other technologies that could provide alternative support for us.
Risks Related to our Common Stock
The market price of our common stock is volatile and can be adversely affected by several factors.
The share prices of publicly traded biotechnology and emerging pharmaceutical companies, particularly companies without consistent product revenues and earnings, can be highly volatile and are likely to remain highly volatile in the future. The price which investors may realize in sales of their shares of our common stock may be materially different than the price at which our common stock is quoted, and will be influenced by a large number of factors, some specific to us and our operations, and some unrelated to our operations. Such factors may cause the price of our stock to fluctuate frequently and substantially. Such factors may include large purchases or sales of our common stock, shorting of our stock, positive or negative events, commentaries or publicity relating to our company, management or products, or other companies, management or products, including other immune therapies for cancer or immune therapies or cancer therapies generally, positive or negative events relating to healthcare and the overall pharmaceutical and biotech sector, the publication of research by securities analysts and changes in recommendations of securities analysts, legislative or regulatory changes, and/or general economic conditions. In the past, shareholder litigation, including class action litigation, has been brought against other companies that experienced volatility in the market price of their shares and/or unexpected or adverse developments in their business. Whether or not meritorious, litigation brought against a company following such developments can result in substantial costs, divert management’s attention and resources, and harm the company’s financial condition and results of operations.
| 26 |
Our Common Stock may be considered a “penny stock” and may be difficult to sell.
The Commission has adopted regulations which generally define “penny stock” to be an equity security that has a market price of less than $5.00 per share or an exercise price of less than $5.00 per share, subject to specific exemptions. Historically, the price of our Common Stock has fluctuated greatly. If the market price of our common stock is less than $5.00 per share it therefore may be designated as a “penny stock” according to Commission rules. The “penny stock” rules impose additional sales practice requirements on broker-dealers who sell securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together with their spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of securities and have received the purchaser’s written consent to the transaction before the purchase. Additionally, for any transaction involving a penny stock, unless exempt, the broker-dealer must deliver, before the transaction, a disclosure schedule prescribed by the Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements must be sent disclosing recent price information on the limited market in penny stocks. These additional burdens imposed on broker-dealers may restrict the ability or decrease the willingness of broker-dealers to sell our common stock, and may result in decreased liquidity for our common stock and increased transaction costs for sales and purchases of our common stock as compared to other securities.
Toucan Capital and its affiliates, including Linda Powers and Cognate BioServices, are the principal holders of our shares of common stock, and this concentration of ownership may have a negative effect on the market price of our common stock.
As of February 28, 2015, Toucan Capital and its affiliates (including Cognate BioServices, Toucan Partners and Linda Powers, who also serves as our Chief Executive Officer and Chairperson of the Board of Directors), collectively, beneficially owned a significant percentage of our outstanding common stock on that date. This concentration of ownership could involve conflicts of interest, and may adversely affect the trading price of our common stock because investors may perceive disadvantages in owning stock of companies with controlling stockholders, including controlling stockholders who could have conflicts of interest. Toucan Capital and its affiliates, including Linda Powers and Cognate BioServices, have the ability to exert substantial influence over all matters requiring approval by our stockholders, including the election and removal of directors and any proposed merger, consolidation or sale of all or substantially all of our assets, as well as over our business plans, strategies or operations. This influence could have the effect of delaying, deferring or preventing a change in control, or impeding a merger or consolidation, takeover or other business combination or action that could be favorable to investors.
The requirements of the Sarbanes-Oxley Act of 2002 and other U.S. securities laws impose substantial costs, and may drain our resources and distract our management.
We are subject to certain of the requirements of the Sarbanes-Oxley Act of 2002 in the U.S., as well as the reporting requirements under the Exchange Act. The Exchange Act requires, among other things, filing of annual reports on Form 10-K, quarterly reports on Form 10-Q and periodic reports on Form 8-K following the happening of certain material events, with respect to our business and financial condition. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal controls over financial reporting. We have identified a number of material weaknesses in our internal controls, as described above under “- Our management and our independent auditors have identified internal control deficiencies, which our management and our independent auditor believe constitute material weaknesses.” Meeting the requirements of the Exchange Act and the Sarbanes-Oxley Act may strain our resources and may divert management's attention from other business concerns, both of which may have a material adverse effect on our business.
We do not intend to pay any cash dividends in the foreseeable future and, therefore, any return on your investment in our common stock must come from increases in the market price of our common stock.
We have not paid any cash dividends on our common stock to date in our history, and we do not intend to pay cash dividends on our common stock in the foreseeable future. We intend to retain future earnings, if any, for reinvestment in the development and expansion of our business. Also, any credit agreements which we may enter into with institutional lenders may restrict our ability to pay dividends. Therefore, any return on your investment in our capital stock must come from increases in the fair market value and trading price of our common stock. Such increases in the trading price of our stock may not occur.
| 27 |
Our certificate of incorporation and bylaws and Delaware law, have provisions that could discourage, delay or prevent a change in control.
Our certificate of incorporation and bylaws and Delaware law contain provisions which could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. We are authorized to issue up to 40,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our Board of Directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. No preferred stock is currently outstanding. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Provisions of our certificate of incorporation and bylaws and Delaware law also could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change in control, including changes a stockholder might consider favorable. Such provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, the certificate of incorporation and bylaws and Delaware law, as applicable, among other things:
| • | provide the Board of Directors with the ability to alter the bylaws without stockholder approval; |
| • | establish staggered terms for board members; |
| • | place limitations on the removal of directors; and |
| • | provide that vacancies on the Board of Directors may be filled by a majority of directors in office, although less than a quorum. |
We are also subject to Section 203 of the Delaware General Corporation Law which, subject to certain exceptions, prohibits “business combinations” between a publicly-held Delaware corporation and an “interested stockholder,” which is generally defined as a stockholder who becomes a beneficial owner of 15% or more of a Delaware corporation’s voting stock for a three-year period following the date that such stockholder became an interested stockholder.
We might not be able to maintain the listing of our common stock on The NASDAQ Capital Market.
Our common stock became listed on The NASDAQ Capital Market on December 12, 2012, under the symbol “NWBO.” We might not be able to maintain the listing standards of that exchange. If we fail to maintain the listing requirements, our common stock might move to the Over the Counter Bulletin Board or in the “pink sheets” maintained by Pink OTC Markets, Inc. The OTC Bulletin Board and the “pink sheets” are generally considered to be markets that are less efficient and less broad than The NASDAQ Capital Market. Our common stock was previously quoted on the OTC Bulletin Board from December 23, 2002 to July 23, 2012. From July 23, 2012 to December 12, 2012, our stock was quoted on the OTCQB.
A substantial number of shares of common stock may be sold in the market, which may depress the market price for our common stock.
Sales of a substantial number of shares of our common stock in the public market could cause the market price of our common stock to decline. A substantial majority of the outstanding shares of our common stock are freely tradable without restriction or further registration under the Securities Act. In addition, as of December 31, 2014, 29,385,000 shares of our common stock are issuable upon exercise of outstanding warrants and 1,551,000 shares of our common stock are issuable upon exercise of outstanding options.
We may have claims and lawsuits against us which may result in adverse outcomes.
From time to time we may be subject to a variety of claims and lawsuits. As described more fully in “Item 3. Legal Proceedings,” of Part I. of this Form 10-K, we are engaged in several potential claims seeking access to certain corporate books and records, and two potential claims relating to short swing profit matters which the Company itself identified and believes have been resolved. We believe these claims are without merit. However, litigation and claims are subject to inherent uncertainties, and adverse rulings or outcomes could occur, and/or could lead to further claims or litigation. Adverse outcomes or further litigation could result in significant monetary damages or injunctive relief that could adversely affect our business. A material adverse impact on our financial statements also could occur for the period in which the effect of an unfavorable final outcome becomes probable and reasonably estimable. In addition, litigation and claims may divert material amounts of management time and attention from our business, and/or involve significant legal costs and expenses.
| 28 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
On July 31, 2012, we entered into a non-cancelable operating lease for 7,097 square feet of office space in Bethesda, Maryland, which expires in March 2018. On October 28, 2013, we entered into a non-cancelable operating lease for 4,251 square feet of office space in Germany, which expires in December 2017. As of December 31, 2014, obligations for future minimum lease payments under these leases, in aggregate over the rest of the lease term, total approximately $1.3 million.
| ITEM 3. | LEGAL PROCEEDINGS |
Shareholder Books and Records Demands
We have received demand letters from three purported individual shareholders seeking to inspect our corporate books and records pursuant to Section 220 of the Delaware General Corporation Law. The demand letters are all substantially the same, and claim that their purpose is to investigate possible mismanagement and breaches of fiduciary duty by our directors and officers. They request a range of documents, including books and records relating to agreements and interactions with related parties, stock ownership, and the independence of our Board members.
One of the individual shareholders filed a complaint in the Delaware Court of Chancery seeking to enforce her books and records demand. The parties reached a negotiated settlement about limited records to be provided, under a confidentiality agreement. Discussions with counsel for the other two individual demanding shareholders are ongoing.
Section 16 Demand Letters
We have received demand letters from two purported individual shareholders seeking disgorgement of possible “short swing” profits within the meaning of Section 16(b) of the Exchange Act from Cognate Bioservices, Inc. and affiliated persons by reasons of certain sales of our common stock. However, prior to either of these demand letters, we had already filed a Form 8-K on December 19, 2014 in which we already disclosed the potential Section 16(b) short swing profits (which had been found in the course of a joint review by Cognate and us), and we disclosed that the disgorgement of those profits ($448,681) had already been agreed by Cognate and us, and resolved. We believe that this review and the payment from Cognate fully resolve the matters raised by the demand letters.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not Applicable.
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS, AND ISSUERS PURCHASES OF EQUITY SECURITIES |
Market for Common Equity and Related Stockholder Matters
Our common stock and certain warrants trade on The NASDAQ Capital Market under the trading symbols “NWBO” and “NWBOW” effective December 12, 2012. Prior to listing on The NASDAQ Capital Market, our common stock was quoted on the OTCQB beginning on July 23, 2012. Previously our common stock was quoted on the Over The Counter Bulletin Board from December 23, 2002 to July 23, 2012. The table below sets forth the high and low prices for our common stock for the last two fiscal years. Quotations reflect inter-dealer prices, without retail mark-up, mark-down commission, and may not represent actual transactions. No assurance can be given that an active market will exist for our common stock.
| High | Low | |||||||
| Year end December 31, 2014 | ||||||||
| First Quarter | $ | 10.64 | $ | 3.84 | ||||
| Second Quarter | 9.35 | 4.87 | ||||||
| Third Quarter | 7.52 | 4.98 | ||||||
| Fourth Quarter | 6.15 | 3.79 | ||||||
| Year end December 31, 2013 | ||||||||
| First Quarter | $ | 4.30 | $ | 3.00 | ||||
| Second Quarter | 4.64 | 3.12 | ||||||
| Third Quarter | 3.90 | 3.14 | ||||||
| Fourth Quarter | 6.89 | 3.10 | ||||||
As of February 28, 2015, there were approximately 136 holders of record of our common stock. Such holders may include any broker or clearing agencies as holders of record, and in such cases exclude the individual stockholders whose shares are held by such brokers or clearing agencies.
Effective September 25, 2012, all shares of our common stock issued and outstanding were combined and reclassified on a one-for-sixteen basis. All shares and per share amounts, except as noted, have been retroactively adjusted to give effect to the combination and reclassification.
| 29 |
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all future earnings, if any, to fund the ongoing development and growth of our business. We do not currently anticipate paying any cash dividends in the foreseeable future.
Securities Authorized for Issuance under Equity Compensation Plan
We have an employee stock option plan (“2007 Stock Option Plan”) under which an aggregate of 13,792,000 shares had been reserved for issuance as of December 31, 2014, including an aggregate of 12,241,000 shares that are issuable pursuant to outstanding options. The following table shows information with respect this plan as of December 31, 2014 (options or shares in thousands).
Equity Compensation Plan Information
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans | |||||||||
| Equity compensation plans approved by security holders | 1,551 | $ | 10.56 | 7,871 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 1,551 | $ | 10.56 | 7,871 | ||||||||
Amended and Restated 2007 Stock Option Plan
Our Board of Directors and the holders of a majority of the voting power of our stockholders have adopted the Amended and Restated 2007 Stock Option Plan or the Plan, under which an original aggregate of 2.25 million shares was reserved. The plan provides that, on the effective date of any increase in our capitalization, the aggregate numbers of shares of common stock that are available for issuance shall automatically be increased by such number of shares as is equal to the number of shares sufficient to cause the option pool to equal 20% of our issued and outstanding stock at such time. Pursuant to the Plan, if on the date of any increase in our capitalization 20% of our total issued and outstanding shares of stock is less than the number of shares of common stock available for issuance under the Plan, no change will be made to the aggregate number of shares of common stock issuable under the Plan for that year (such that the aggregate number of shares available for issuance under the Plan will never decrease).
Recent Sales of Unregistered Securities
On August 27, 2014 and September 16, 2014, we sold to an individual investor 435,202 shares of common stock at $5.17 per share and 448,207 shares of common stock at $5.02 per share, respectively. The total proceeds received were $4.5 million.
During the quarter ended September 30, 2014, we converted accounts payable owed to Cognate BioServices in the amount of approximately $7.9 million into 1,986,205 shares of common stock and warrants to purchase 1,121,404 shares of common stock. The shares and warrants are subject to most favored nation treatment with respect to the terms (including in regards to warrants) provided to any other investors or creditors, including share issuances upon the exercise of previously issued derivative securities. The shares are also subject to a 36-month lock-up as described above.
During the quarter ended September 30, 2014, we issued 14,519 shares of common stock in exchange for consulting services valued at $94,999.
On November 17, 2014, we sold $25 million of unregistered shares of common stock of the Company (4,317,789 shares), at a price of $5.79 per share (the closing price of the stock on November 14, 2014, the trading day prior to the sale of shares).
The securities sold in these transactions were exempt from registration under the Securities Act of 1933, as amended, in reliance on Section 4(a) (2) thereof.
The sales of the securities identified above were made pursuant to privately negotiated transactions that did not involve a public offering of securities and, accordingly, these transactions were exempt from the registration requirements of the Securities Act pursuant to Section 4(2) of the Securities Act.
| 30 |
| ITEM 6. | SELECTED FINANCIAL DATA |
Not Applicable
| ITEM 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read this discussion together with the Financial Statements, related Notes and other financial information included elsewhere in this Form 10-K. The following discussion contains assumptions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under “Risk Factors,” and elsewhere in this Form 10-K. These risks could cause our actual results to differ materially from those anticipated in these forward-looking statements
Overview
We are a biotechnology company focused on developing immunotherapy products to treat cancers more effectively than current treatments, without toxicities of the kind associated with chemotherapies, and, through a proprietary batch manufacturing process, on a cost-effective basis, initially in both the United States and Europe.
We have developed a platform technology, DCVax, which uses activated dendritic cells to mobilize a patient's own immune system to attack their cancer. The DCVax technology is expected to be applicable to all solid tumor cancers, and is embodied in several distinct product lines. One of the product lines (DCVax-L) is designed to cover all solid tumor cancers in which the tumors can be surgically removed. Another product line (DCVax-Direct) is designed for all solid tumor cancers which are considered inoperable and cannot be surgically removed. We believe the broad applicability of DCVax to many cancers provides multiple opportunities for commercialization and partnering.
Our DCVax platform technology involves dendritic cells, the master cells of the immune system, and is designed to reinvigorate and educate the immune system to attack cancers. The dendritic cells are able to mobilize the overall immune system, including T cells, B cells and antibodies, natural killer cells and many others. Such mobilization of the overall immune system provides a broader attack on the cancer than mobilizing just a particular component, such as T cells alone, or a particular antibody alone. Likewise, our DCVax technology is designed to attack the full set of biomarkers, or antigens, on a patient’s cancer, rather than just a particular selected target or several targets. Clinical experience indicates that when just one or a few biomarkers on a cancer are targeted by a drug or other treatment, sooner or later the cancer usually develops a way around that drug, and the drug stops working. We believe that mobilizing all agents of the immune system, and targeting all biomarkers on the patient’s cancer, contributes to the effectiveness of DCVax.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect our reported amounts of assets, liabilities, revenues and expenses.
On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses and stock-based compensation. We based our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the reported amounts of revenues and expenses that are not readily apparent from other sources. Actual results may differ from these estimates.
Warrant Liability
The Company accounts for the 12,555,198 common stock warrants issued in accordance with the guidance contained in ASC 815-40-15-7D, "Contracts in Entity's Own Equity" whereby under that provision they do not meet the criteria for equity treatment and must be recorded as a liability. Accordingly, the Company classifies the warrant instrument as a liability at its fair value and adjusts the instrument to fair value at each reporting period. This liability is subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in the Company's statements of operations. The fair value of the warrants issued by the Company in connection the Conversion Transaction has been estimated using a Monte Carlo simulation.
| 31 |
Fair Value Measurements
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| · | Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets; | |
| · | Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable | |
| · | Level 3 — inputs that are unobservable (for example, cash flow modeling inputs based on assumptions) |
Environmental Remediation Liabilities
We record environmental remediation liabilities for properties acquired. The environmental remediation liabilities are initially recorded at fair value. The liability is reduced for actual costs incurred in connection with the clean-up activities for each property. Upon completion of the clean-up, the environmental remediation liability is adjusted to equal the fair value of the remaining operation, maintenance and monitoring activities to be performed for the property. The reduction in the liability resulting from the completion of the clean-up is included in other income. As of December 31, 2014, we estimate that the total environmental remediation costs associated with the purchase of the UK Facility will be approximately $6.2 million. Contamination clean-up costs that improve the property from its original acquisition state are capitalized as part of the property’s overall development costs. We engaged a third party specialist to conduct certain surveys of the condition of the property which included, among other things, a preliminary analysis of potential environmental remediation exposures. We determined, based on information contained in the specialist’s report, that we would be required to estimate the fair value of an unconditional obligation to remediate specific ground contamination at an estimated fair of approximately $6.2 million. We computed its preliminary estimate of the fair value of this obligation using a probability weighted approach that measures the likelihood of the following two potential outcomes: (i) a higher probability requirement of erecting a protective barrier around the affected area at an estimated cost of approximately $4.5 million, and (ii) a lower probability requirement of having to excavate the affected area at an estimated cost of approximately $32 million. Our estimate is preliminary and therefore subject to change as further studies are conducted, and as additional facts come to our attention. Environmental remediation efforts are complex, technical and subject to various uncertainties. Accordingly, it is at least reasonably possible that any changes in our estimate could materially differ from the management’s preliminary discussed herein.
Stock-based Compensation
Compensation expense for all stock-based awards is measured at the grant date based on the fair value of the award and is recognized as an expense, on a straight-line basis, over the employee's requisite service period (generally the vesting period of the equity award). The fair value of each option award is estimated on the date of grant using a Black-Scholes option valuation model. Stock-based compensation expense is recognized only for those awards that are expected to vest using an estimated forfeiture rate. Estimates of pre-vesting forfeiture are periodically revised in subsequent periods if actual forfeitures differ from those estimates. To the extent that actual results differ from our estimates, such amounts will be recorded as cumulative adjustments in the period the estimates are revised.
Recent Accounting Pronouncements
In June 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No. 2014-10, Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation. The amendments in this update remove the definition of a development stage entity from the Master Glossary of the Accounting Standards Codification, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, the amendments eliminate the requirements for development stage entities to (1) present inception-to-date information in the statements of income, cash flows and shareholder equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. A public entity is required to apply the amendments for annual reporting periods beginning after December 15, 2014, and interim periods therein. An entity should apply the amendments retrospectively for all comparative periods presented. Early adoption is permitted. We adopted the guidance during the second quarter of 2014. Adoption of this standard did not have a material impact on our financial position, results of operations, or cash flows.
| 32 |
The FASB has issued Accounting Standards Update ASU No. 2014-12, Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. This ASU requires that a performance target that affects vesting, and that could be achieved after the requisite service period, be treated as a performance condition. As such, the performance target should not be reflected in estimating the grant date fair value of the award. This update further clarifies that compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. The amendments in this ASU are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Earlier adoption is permitted. The adoption of this standard is not expected to have a material impact on our consolidated financial position results of operation or cash flows.
The FASB has issued ASU No. 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The guidance, which is effective for annual reporting periods ending after December 15, 2016, extends the responsibility for performing the going-concern assessment to management and contains guidance on how to perform a going-concern assessment and when going-concern disclosures would be required under U.S. GAAP. We has elected to early adopt the provisions of ASU 2014-15 in connection with the issuance of these unaudited condensed consolidated financial statements. Management’s evaluation regarding the events and conditions that raise substantial doubt regarding our ability to continue as a going concern have been disclosed in Note 2.
Results of Operations
Operating costs:
Operating costs and expenses consist primarily of research and development expenses, including clinical trial expenses which increase when we are actively participating in clinical trials and are especially high when we are in a large ongoing international phase III trial (as we now are). Such costs have increased and will continue to increase as we bring on an additional clinical trial programs which are under way in parallel (as we have with our 60-patient Phase I/II trial with DCVax-Direct for all types of inoperable solid tumors), and general and administrative expenses. Such expenses also increase in later stage trials (such as our ongoing Phase III trial) as we begin to prepare for commercialization, which involves process optimization, validation and scale-up. The associated administrative expenses increase as such operating activities grow.
Our operating costs include ongoing development work relating to the DCVax-Direct product and its manufacturing, such as the design, engineering, sourcing, production, testing, modification and validation of the manufacturing automation systems, disposable sets to be used with the manufacturing automation systems, and manufacturing processes, product ingredients, product release assays, and other matters, as well as development of standard operating procedures (SOPs), batch production records, and other necessary materials.
Our operating costs also include the costs of preparations for the launch of new or expanded clinical trial programs including the Phase III trial in the UK and Germany (with DCVax-L for brain cancer), early access programs in Europe, and the Phase I/II trial (with DCVax-Direct for all inoperable solid tumor cancers). The preparation costs include upfront payments to the clinical trial sites and the CROs managing the trials and other service providers, and expenses related to institutional approvals, training of medical and other site personnel, trial supplies and other. Additional substantial costs relate to the expansion of manufacturing facilities and capacity, in both the US and Europe.
Research and development:
Discovery and preclinical research and development expenses include costs for substantial external scientific personnel, technical and regulatory advisers, and others, costs of laboratory supplies used in our internal research and development projects, travel, regulatory compliance, and expenditures for preclinical and clinical trial operation and management when we are actively engaged in clinical trials.
Because we are pre-revenue company, we do not allocate research and development costs on a project basis. We adopted this policy, in part, due to the unreasonable cost burden associated with accounting at such a level of detail and our limited number of financial and personnel resources.
| 33 |
General and administrative:
General and administrative expenses include administrative personnel related salary and benefit expenses, cost of facilities, insurance, travel, legal support, property and equipment and amortization of stock options and warrants.
For the Years Ended December 31, 2014 and 2013
We recognized a (combined cash and non-cash) net loss of $135.6 million for the year ended December 31, 2014 compared to a (combined cash and non-cash) net loss of $65.8 million for the year ended December 31, 2013.
Research and development expense
Research and development expense (combined cash and non-cash) was $85.6 million for year ended December 31, 2014 compared to $43.9 million for the year ended December 31, 2013. The increase was primarily attributable to an increased number of clinical trial sites open and recruiting across the US in our ongoing Phase III clinical trial of DCVax®-L immune therapy for Glioblastoma multiforme (GBM) brain cancer and initiation of this trial in Europe. Included in research and development expense was $10.0 million and $3.8 million, respectively, related to clinical site expenses such as clinical research organizations (“CRO”) fees and site related fees.
For the years ended December 31, 2014 and 2013, the Company made cash payments of approximately $18.7 million, and $12.4 million, respectively, to Cognate. A portion of the $18.7 million paid in 2014 was payment for unpaid invoices from 2013. At December 31, 2014 and 2013, the Company owed Cognate $5.7 million and $3.6 million, respectively, for unpaid invoices for services performed by Cognate (including manufacturing for both the Phase III and Phase I/II clinical trials, ongoing product and process development, expansion of several company programs and services related to expansion of manufacturing capacity).
During the years ended December 31, 2014 and 2013, the Company incurred non-cash equity based compensation (restricted common stock and warrants) expense related to Cognate BioServices of $21.3 million and $0, respectively. This equity compensation primarily involved one-time initiation payments of shares and warrants relating to the four new agreements the Company entered into with Cognate in January, 2014. The shares are vesting over a period of three years from the date of the agreements. The fair value calculation of these shares was determined using the market price for tradable shares; however the shares issued to Cognate were unregistered restricted shares. The equity compensation also included lock-up warrants (for the lock-up of Cognate shares) and most favored nation warrants.
For the years ended December 31, 2014 and 2013, Cognate’s unpaid payables and accrued expenses increased by $19.3 million (increase from 2013 to 2014) and $13.8 million (increase from 2012 to 2013), respectively, as the Company launched and carried out its DCVax-Direct program, and expanded its DCVax-L program.
General and administrative expense
General and administrative expense was $16.9 million for the year ended December 31, 2014 compared to $12.4 million for the year ended December 31, 2013. The increase was primarily due to an increase of $2.4 million in professional services.
Change in fair value of derivatives
During the years ended December 31, 2014 and December 31, 2013 we recognized a loss and gain on derivative liabilities of $15.1 million and $1.2 million, respectively, primarily due to the change in fair value of warrants issued to Cognate BioServices for research and development and warrants issued in connection with our Cognate BioServices service agreement entered in July 2013 and January 2014.
Inducement expense
During the years ended December 31, 2014 and December 31, 2013 we recognized an inducement expense of $18.9 million and $10.6 million, respectively. The increase in the inducement expense for the year ended December 31, 2014 was related to conversion expenses related to financing from an unrelated institutional investor, and to an increase of the conversion of accounts payable to common stock and warrants to Cognate BioServices in connection with the extinguishment of accounts payable.
Interest expense
Interest expense increased to $1.0 million for the year ended December 31, 2014 from $0.8 million for the year ended December 31, 2013. Interest expense increased for the year ended December 31, 2014 primarily related to the $17.5 million long term convertible note that we entered in August 2014.
Other income
During the year ended December 31, 2014 and December 31, 2013, we recorded $0.448 million and $0 income from settlement from Cognate BioServices under Section 16(b) (see Note 9).
Liquidity and Capital Resources
We have experienced recurring losses from operations. During the year ended December 31, 2014, net cash outflows from operations were $54.6 million for all of the Company’s ongoing programs (348-patient Phase III trial of DCVax-L at over 60 sites in the US and Europe, 41-patient Phase I stage of the Phase I/II DCVax-Direct trial, early access programs in both Germany and the UK) as well as substantial one-time expenditures, including for initiation payments related to the clinical trials and to development of large new manufacturing capacity in Europe.
At December 31, 2014, current assets totaled $14.6 million, compared to $18.6 million at December 31, 2013. Current assets less current liabilities produces a working capital deficit in the amount of $54.2 million at December 31, 2014. Non-cash derivative adjustments, primarily attributable to warrants, accounted for $44.7 million of this $54.2 million deficit. On December 31, 2013 the deficit was $1.0 million (excluding redeemable common stock amounting to $8.9 million). This non-cash accounting deficit increase in 2014 related to the conversion of financing from an unrelated institutional investor, conversion of $16.8 million of accounts payable to Cognate BioServices to common stock and warrants, estimation of environmental liability on the UK property of $6.2 million, and a decrease of $5.1 million in cash and cash equivalents as a result of substantial expansion of the DCVax-L and DCVax-Direct clinical trials during 2014.
| 34 |
On a going forward basis, commencing with August 2013, and continuing throughout the lock-up period, we and Cognate BioServices agreed to establish a vendor financing arrangement for regular ongoing payment of at least half of all invoices in unregistered, restricted common stock and warrants of our company at an initial price of $4.00 per share (the closing market price was $3.55 at that time), and the remainder in cash, subject to a most favored nation treatment with respect to terms provided to other investors or creditors (including with respect to any warrants), including share issuances upon exercise of previously issued derivative securities. Under the Cognate BioServices Agreements executed in January 2014, this arrangement will continue for 18 months from the execution of those agreements or until terminated by mutual agreement.
Since 2004, Toucan Capital Fund II, L.P. or Fund III (“Toucan Capital”), Toucan Partners, LLC (“Toucan Partners”), entities controlled by Ms. Linda Powers, our CEO and the managing director of Toucan Capital and managing member of Toucan Partners, and Ms. Linda Powers (collectively, “Toucan”) also have provided substantial funding to us. From 2004 to date, Toucan has provided ongoing financings to us through the purchase of common stock, preferred stock (which was all converted to common stock), loans and debt securities. As of December 31, 2014, Toucan (other than Cognate BioServices) held approximately 4% of common stock outstanding.
On August 19, 2014, we completed the acquisition of a facility and property in the U.K (“UK Facility”). The purchase price was £13 million ($20.8 million, excluding professional fees of $1.5 million associated with the purchase of the U.K Facility). The facility is an existing building of approximately 65,000 square feet. We plan to re-purpose the facility and have it built out as part of the expansion of manufacturing capacity, potentially doubling the building’s square footage. Such re-purposing requires approval of the applicable Planning Commission. If re-purposing is approved, then the specific design and engineering of the proposed build out will also have to be approved. In addition to the facility, the acquisition included about 25 acres of potentially developable land (as well as non-developable land). Any future development for business use will require removal of certain existing structures, permission from the Planning Commission for the intended purpose, and then permission from the Planning Commission for the specific designs and engineering.
On November 17, 2014, we entered into a private offering of $25 million of unregistered shares of common stock, at a price of $5.79 per share (the closing price of the stock on November 14, 2014, the trading day prior to the sale of shares). The shares were purchased by C.F. Woodford Equity Income Fund of the U.K.
We engaged a third party specialist to conduct certain surveys of the condition of the property which included, among other things, a preliminary analysis of potential environmental remediation exposures. We determined, based on information contained in the specialists report, that we would be required to estimate the fair value of an unconditional obligation to remediate specific ground contamination at an estimated fair of approximately $6.2 million. We computed our preliminary estimate of the fair value of this obligation using a probability approach that measures likelihood of the following two potential outcomes: (i) a higher probability (>95%) requirement of erecting a protective barrier around the affected area at an estimated cost of approximately $4.5 million, or (iii) lower probability (<5%) requirement of having to excavate the affected area at an estimated cost of approximately $32 million. Our estimate is preliminary and therefore subject to change as further studies are conducted, and as additional facts come to our attention. Environmental remediation obligations are complex and technical. Accordingly, it is at least reasonably possible that any changes in our estimates could materially differ from management’s preliminary estimates.
Operating Activities
We used $54.6 million in cash for operating activities during the year ended December 31, 2014. We used $37.8 million in cash for operating activities during the year ended December 31, 2013. The increase in cash used in operating activities was primarily attributable to the DCVax-Direct manufacturing and product development work described above, the launch and execution of the 41-patient Phase I stage of the Phase I/II clinical trial with DCVax-Direct for inoperable solid tumor cancers, the increased manufacturing of DCVax®-L for the ongoing Phase III brain cancer trial at a growing number of sites across the US and in Europe, the preparations for launch of the Phase III trial in Canada, ,extensive negotiations of dozens of program and hospital related contracts in Germany, as well as major regulatory submissions.
As of December 31, 2014, we had more than 60 clinical trial sites in operation in the US and Europe in our Phase III trial with DCVax-L, compared to 50 clinical trial sites at December 31, 2013, almost entirely in the US. The year before, in 2013, we also had substantially expanded our clinical trial related operations compared with 2012 including, for example, the launch of the Phase III DCVax-L clinical trial in the UK and approval for the trial in Germany, as described above, and launch of the Phase I/II DCVax-Direct trial in the U.S. as described above, in addition to costs related to develop fully operational and approved manufacturing in Germany, an established wholly owned German subsidiary, and clinical activity in the U.K.
Financing Activities
For the year ended December 31, 2014, our financing activities primarily consisted of net proceeds from the issuance of common stock and warrants amounting to $63.3 million and net proceeds from issuance of notes for $22.6 million; offset by the repayment of $0.03 million of interest on notes payable.
| 35 |
In April 2013, we entered into an agreement with one healthcare-dedicated institutional investor for a registered direct placement of $10.0 million of common stock at the closing market price of $3.90 per share. We issued to the investor warrants exercisable for 1,025,641 shares of common stock. The warrants have an exercise price of $4.29 per share and are exercisable beginning six months after closing, with an exercise period of five years.
On August 8, 2013, we entered into a securities purchase agreement with institutional investors for the sale of an aggregate of $15.0 million of units in a registered direct offering. Each unit consisted of one share of common stock, one long-term warrant to purchase 0.25 of a share of common stock and one over-allotment warrant to purchase 0.25 of a share of common stock. We issued to the investors 4,477,612 shares of common stock and long-term warrants exercisable for 1,119,403 shares of common stock, with an exercise price of $4.00 per share, exercisable six months after closing with a term of five years after they are first exercisable. We also issued to the investors over-allotment warrants exercisable for 1,119,403 shares of common stock, with an exercise price of $3.35 per share and exercisable immediately with a term of one year.
On November 25, 2013, we completed a public offering with institutional investors for the sale of an aggregate of 5,630,208 units at a public offering price of $4.80 per unit, resulting in gross proceeds of $27.0 million.
On April 9, 2014, the Company entered into a Securities Purchase Agreement with a single institutional investor for the sale of 2,272,727 shares of common stock at a purchase price of $6.60 per share, for a total purchase price of $15.0 million. Additionally, from the date of the closing until one year after the closing date, the investor has a non-transferable Over-allotment Right to purchase up to 2,272,727 additional shares of common stock at a price per share of $7.50, for an additional subscription amount of up to $17.05 million.
On August 13, 2014, the Company entered into a purchase agreement with Oppenheimer & Co. Inc., with respect to the Company’s issuance and sale of $17.5 million aggregate principal amount of the Senior Notes due on August 15, 2017 (the “Notes”). The offering of the Notes was completed on August 19, 2014.
On November 17, 2014, we entered into a private offering of $25 million of unregistered shares of common stock, at a price of $5.79 per share (the closing price of the stock on November 14, 2014, the trading day prior to the sale of shares). The shares were purchased by C.F. Woodford Equity Income Fund of the U.K.
Our financial statements indicate there is substantial doubt about our ability to continue as a going concern as we are dependent on our ability to obtain short term financing and ultimately to generate sufficient cash flow to meet our obligations on a timely basis, as well as successfully obtain financing on favorable terms to fund our long term plans. We can give no assurance that our plans and efforts to achieve the above steps will be successful.
In order to continue with our current activities under our DCVax®-L program, we will have to obtain substantial amounts of further funding, as described in the Risk Factors. Our on-going funding requirements will depend on many factors, including the extent to which we realize and draw upon various sources of non-dilutive funding. One such source of non-dilutive funding that we drew upon in 2014 is a $5.5 million German grant awarded on May 1, 2012, by the German government through its Saxony Development Bank. The grant provided funding on a matching basis for up to 50% of costs incurred by us for the DCVax-L clinical trial and manufacturing in Germany.
Other factors affecting our ongoing funding requirements include the number of staff we employ, the number of sites and pace of patient enrollment in our Phase III brain cancer trial and our Phase I/II clinical trial with DCVax-Direct, the possible launch of additional Phase II trials with DCVax-Direct, the costs of further development work relating to DCVax-Direct, the costs of expansion of manufacturing of both DCVax-L and DCVax-Direct, the cost of developing our Hospital Exemption program in Germany, and unanticipated developments. The extent of resources available to us will determine the pace at which we can move forward with both our DCVax-L program and our DCVax-Direct program.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK. |
Not applicable.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The full text of our audited consolidated financial statements as of December 31, 2014 and 2013 and for the fiscal years ended December 31, 2014 and 2013, begins on page F-1 of this Annual Report on Form 10-K.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
On July 25, 2013, we changed our independent registered public accounting firm from Peterson Sullivan to Marcum LLP for the year ended December 31, 2013. The change was approved by the Audit Committee and was made to provide us with an auditing firm with a broader national presence. Peterson Sullivan is located in Seattle, Washington, and had served as our independent registered public accounting firm since February 16, 2005.
| 36 |
Peterson Sullivan’s reports on our consolidated financial statements as of December 31, 2011 and 2012, and for the two years then ended and for the period from March 18, 1996 (inception) to December 31, 2012 did not contain an adverse opinion or a disclaimer of opinion, although the report contained an explanatory paragraph relating to our ability to continue as a going concern, and was not qualified or modified as to uncertainty, audit scope or accounting principles.
During the two years ended December 31, 2012 and through July 25, 2013, there were no: (a) disagreements with Peterson Sullivan on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to Peterson Sullivan’s satisfaction, would have caused Peterson Sullivan to make reference to the subject matter thereof in connection with its reports on our financial statements as of December 31, 2011 and 2012, and for the two years then ended and for the period from March 18, 1996 (inception) to December 31, 2012; or (b) “reportable events”, as defined under Item 304(a)(1)(v) of Regulation S-K. However, Peterson Sullivan identified material weaknesses in our financial reporting process with respect to segregation of duties and lack of controls over reporting of material transactions and developments in the financial statements.
Peterson Sullivan has indicated to us that it concurs with the foregoing statements contained in the paragraphs above as they relate to Peterson Sullivan and furnished a letter dated July 30, 2013 to the SEC to this effect. A copy of the letter from Peterson Sullivan is attached as Exhibit 16.1 to our current report on Form 8-K filed with the SEC on July 31, 2013.
During the two years ended December 31, 2012 and through July 25, 2013, neither us nor anyone acting on our behalf consulted with Marcum regarding any of the matters or events set forth in Item 304(a)(2)(i) or (ii) of Regulation S-K.
|
ITEM 9A. |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive, financial and accounting officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended. Based on this evaluation, our principal executive, financial and accounting officer concluded that, as of December 31, 2014, in light of the material weaknesses described below, our disclosure controls and procedures were not effective to ensure that the information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to management, including our chief executive officer, financial and accounting officer, to allow timely decisions regarding required disclosure, and that such information is recorded, processed, summarized and reported within the time periods prescribed by the SEC.
Management's Report on Internal Controls Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal controls over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our principal executive, financial and accounting officer, we conducted an evaluation of the effectiveness of our internal controls over financial reporting as of December 31, 2014. This evaluation was based on the framework in Internal Control-Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO").
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company's annual or interim financial statements will not be prevented or detected on a timely basis.
Based on management's evaluation as of December 31, 2014, our management identified the material weaknesses set forth below in our internal control over financial reporting:
| (i) | The Company's process for internally reporting material information in a systematic manner to allow for timely filing of material information is ineffective, due to its inherent limitations from being a small company, and there exist material weaknesses in internal control over financial reporting that contribute to the weaknesses in our disclosure controls and procedures. These weaknesses include: |
| · | Insufficient segregation of duties, oversight of work performed and lack of compensating controls in our finance and accounting function due to limited personnel. |
| · | Lack of controls in place, including those surrounding related party transactions, to ensure that all material transactions and developments impacting the financial statements are reflected and properly recorded. |
| · | Lack of documentation to support occurrences of review and approval procedures. |
| · | Design deficiencies that do not meet stated control objectives that elevate the level of risk of a material misstatement to our financial statements. |
| 37 |
| · | Policies and procedures with respect to the review, supervision and monitoring of our accounting operations throughout the organization were either not designed and in place or not operating effectively. |
| · | We did not maintain an adequate risk oversight function to evaluate and report on risks to financial reporting throughout the organization, including completion of a comprehensive risk assessment to identify all potential risk areas and evaluate the adequacy of controls to mitigate identified risk. |
| · | We did not maintain an effective anti-fraud program designed to detect and prevent fraud relating to (i) an effective whistle- blower program or other comparable mechanism and (ii) an ongoing program to manage identified fraud risks. |
Our company's management concluded that in light of the material weaknesses described above, our company did not maintain effective internal control over financial reporting as of December 31, 2014 based on the criteria set forth in Internal Control-Integrated Framework (1992) issued by the COSO.
Changes in Internal Control over Financial Reporting
There were no changes, in the Company's internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of the Exchange Act Rules 13a-15 or 15d-15 that occurred during the quarter ended December 31, 2014 that materially affected, or were reasonably likely to materially affect, the Company's internal control over financial reporting.
| 38 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
ON INTERNAL CONTROL OVER FINANCIAL REPORTING
To the Audit Committee of the
Board of Directors and Stockholders of
Northwest Biotherapeutics, Inc.
We have audited Northwest Biotherapeutics, Inc. and Subsidiaries (the “Company”) internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 1992. The Company's management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Management’s Annual Report on Internal Control over Financial Reporting”. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a control deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company's annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in “Management's Report on Internal Control Over financial Reporting”.
| 1. | Insufficient segregation of duties, oversight of work performed and lack of compensating controls in our finance and accounting function due to limited personnel. |
| 2. | Lack of controls in place, including those surrounding related party transactions, to ensure that all material transactions and developments impacting the financial statements are reflected and properly recorded. |
| 3. | Lack of documentation to support occurrences of review and approval procedures. |
| 4. | Design deficiencies that do not meet stated control objectives that elevate the level of risk of a material misstatement to the financial statements. |
| 5. | Policies and procedures with respect to the review, supervision and monitoring of the accounting operations throughout the organization were either not designed and in place or not operating effectively. |
| 6. | The Company did not maintain an adequate risk oversight function to evaluate and report on risks to financial reporting throughout the organization, including completion of a comprehensive risk assessment to identify all potential risk areas and evaluate the adequacy of controls to mitigate identified risk. |
| 7. | The Company did not maintain an effective anti-fraud program designed to detect and prevent fraud relating to (i) an effective whistle- blower program or other comparable mechanism and (ii) an ongoing program to manage identified fraud risks. |
These material weaknesses were considered in determining the nature, timing and extent of audit tests applied in our audit of the Company's consolidated financial statements for the year ended December 31, 2014, and this report does not affect our report dated March 17, 2014.
In our opinion, because of the effect of the material weaknesses described above on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control-Integrated Framework issued in 1992 by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets as of December 31, 2014 and 2013 and the related consolidated statements of operations, stockholders' deficit, and cash flows for the years ended December 31, 2014 and 2013 of the Company and our report dated March 16, 2015 expressed an unqualified opinion on those financial statements, which includes an explanatory paragraph as to the company’s ability to continue as a going concern.
/s/ Marcum llp
Marcum llp
New York, NY
March 17, 2015
| 39 |
| ITEM 9B. | OTHER INFORMATION |
None.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information regarding executive officers is included in Part I of this Annual Report on Form 10-K as permitted by General Instruction G (3).
Northwest Biotherapeutics, Inc. has adopted a formal “Code of Business Conduct and Ethics” applicable to all Board members, executive officers and employees. A copy of our Code of Business Conduct and Ethics will be provided free of charge upon request to: Secretary, Northwest Biotherapeutics, Inc., 4800 Montgomery Lane, Suite 800, Bethesda, Maryland, 20814.
Other information required by Item 10, including information regarding directors, membership and function of the audit committee, including the financial expertise of its members, and Section 16(a) compliance, appearing under the captions “Election of Directors,” “Information Regarding Board of Directors and Committees” and “Other Matters” of the Company’s Proxy Statement for the 2014 Annual Meeting of Stockholders is incorporated herein by reference. The Company intends to file its Proxy Statement with the Securities and Exchange Commission (the “SEC”) not later than 120 days after December 31, 2014.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by Item 11 appearing under the captions “Information Regarding Board of Directors and Committees—Compensation of Directors” and “Executive Compensation” of the Company’s Proxy Statement for the 2014 Annual Meeting of Stockholders is incorporated herein by reference. The Company intends to file its Proxy Statement with the SEC not later than 120 days after December 31, 2014.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by Item 12 appearing under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Executive Compensation—Equity Compensation Plan Information” of the Company’s Proxy Statement for the 2014 Annual Meeting of Stockholders is incorporated herein by reference. The Company intends to file its Proxy Statement with the SEC not later than 120 days after December 31, 2014.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by Item 13 appearing under the captions “Information Regarding Board of Directors and Committees” and “Certain Relationships and Related Transactions” of the Company’s Proxy Statement for the 2014 Annual Meeting of Stockholders is incorporated herein by reference. The Company intends to file its Proxy Statement with the SEC not later than 120 days after December 31, 2014.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by Item 14 appearing under the caption “Independent Registered Public Accounting Firm” of the Company’s Proxy Statement for the 2014 Annual Meeting of Stockholders is incorporated by reference. The Company intends to file its Proxy Statement with the SEC not later than 120 days after December 31, 2014.
| 40 |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
The Exhibits listed below are identified by numbers corresponding to the Exhibit Table of Item 601 of Regulation S-K. The Exhibits designated by an asterisk (*) are management contracts or compensatory plans or arrangements required to be filed pursuant to Item 15.
EXHIBIT INDEX
|
Exhibit |
Description | |
| 3.1 | Seventh Amended and Restated Certificate of Incorporation (incorporated by reference to exhibit 3.1 filed with the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1(File No. 333-134320) on July 17, 2006) | |
| 3.2 | Third Amended and Restated Bylaws of the Company (incorporated by reference to exhibit 3.1 filed with the Registrant’s Current Report on Form 8-K on June 22, 2007) | |
| 3.3 | Amendment to Seventh Amended and Restated Certificate of Incorporation (incorporated by reference to exhibit 3.2 filed with the Registrant’s Current Report on Form 8-K on June 22, 2007) | |
| 3.5 | Amendment to Seventh Amended and Restated Certificate of Incorporation (incorporated by reference to exhibit 3.1 filed with the Registrant’s Quarterly Report on Form 10-Q on May 21, 2012) | |
| 3.6 | Amendment to Seventh Amended and Restated Certificate of Incorporation (incorporated by reference to exhibit 3.1 filed with the Registrant’s Current Report on Form 8-K on September 26, 2012). | |
| 3.7 | Amendment to Third Amended and Restated Bylaws of the Company (incorporated by reference to exhibit 3.1 filed with the Registrant’s Current Report on Form 8-K on December 11, 2012) | |
| 4.1 | Form of common stock certificate (incorporated by reference to exhibit 4.1 filed with the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-67350) on November 14, 2001) | |
| 4.2 | Form of Warrant Agency Agreement by and between Northwest Biopharmaceuticals, Inc. and Computershare Trust Company, N.A. and Form of Warrant Certificate (incorporated by reference to Exhibit 4.2 filed with the Registrant’s Form S-1 on December 4, 2012). | |
| 10.4 | Form of Loan Agreement and 10% Convertible, Promissory Note between the Company and Toucan Partners, LLC (incorporated by reference to exhibit 10.4 filed with the Registrant’s Form 10-K on April 17, 2007) | |
| 10.5 | Second Amended and Restated Investor Rights Agreement dated June 22, 2007 between the Company and Toucan Capital Fund II, LLP (incorporated by reference to exhibit 10.3 filed with the Registrant’s Current Report on Form 8-K on June 22, 2007) | |
| 10.6 | Warrant to purchase securities of the Company dated July 26, 2005 issued to Toucan Capital Fund II, L.P (incorporated by reference to exhibit 10.3 filed with the Registrant’s Current Report on Form 8-K on August 1, 2005) | |
| 10.7 | Warrant to purchase securities of the Company dated September 7, 2005 issued to Toucan Capital Fund II, L.P (incorporated by reference to exhibit 10.3 filed with the Registrant’s Current Report on Form 8-K on September 9, 2005) | |
| 10.8 | Amended Form of Warrant to purchase securities of the Company dated November 14, 2005 and April 17, 2006, as amended April 14, 2007, issued to Toucan Partners, LLC (incorporated by reference to exhibit 10.21 filed with the Registrant’s Form 10-K on April 17, 2007) | |
| 10.9 | Form of Warrant to purchase securities of the Company dated April 14, 2007 issued to Toucan Partners, LLC (incorporated by reference to exhibit 10.22 filed with the Registrant’s Form 10-K on April 17, 2007) |
| 41 |
|
Exhibit |
Description |
| 10.10 | Loan Agreement and 10% Convertible Promissory Note in the principal amount of $100,000 between the Company and Toucan Partners, LLC, dated April 27, 2007 (incorporated by reference to exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K on May 3, 2007) | |
| 10.11 | Warrant to purchase securities of the Company issued to Toucan Partners, LLC, dated April 27, 2007 (incorporated by reference to exhibit 10.2 filed with the Registrant’s Current Report on Form 8-K on May 3, 2007) | |
| 10.12 | Form of Toucan Partners Loan Agreement and 10% Convertible Note, dated as of June 1, 2007 (incorporated by reference to exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K on June 7, 2007) | |
| 10.13 | Form of Toucan Partners Warrant, dated as of June 1, 2007 (incorporated by reference to exhibit 10.2 filed with the Registrant’s Current Report on Form 8-K on June 7, 2007) | |
| 10.14 | Amended and Restated Warrant to purchase Series A Preferred Stock issued to Toucan Capital Fund II, L.P., dated as of June 1, 2007 (incorporated by reference to exhibit 10.3 filed with the Registrant’s Current Report on Form 8-K on June 7, 2007) | |
| 10.15 | Warrant to purchase Series A-1 Preferred Stock issued to Toucan Capital Fund II, L.P., dated as of June 1, 2007 (incorporated by reference to exhibit 10.4 filed with the Registrant’s Current Report on Form 8-K on June 7, 2007) | |
| 10.16 | Warrant to purchase Series A-1 Preferred Stock issued to Toucan Capital Fund II, L.P., dated as of June 1, 2007 (incorporated by reference to exhibit 10.5 filed with the Registrant’s Current Report on Form 8-K on June 7, 2007) | |
| 10.17 | Northwest Biotherapeutics, Inc. $225,000 Demand Note dated June 13, 2007 (incorporated by reference to exhibit 10.1 filed with the Registrant’s Current Report on Form 8-K on June 18, 2007) | |
| 10.18 | Conversion Agreement dated June 15, 2007 and effective June 22, 2007 between the Company and Toucan Capital Fund II, LLP (incorporated by reference to exhibit 10.1filed with the Registrant’s Current Report on Form 8-K on June 22, 2007) | |
| 10.19* | Services Agreement between Cognate BioServices, Inc. and Northwest Biotherapeutics dated April 1, 2011 (incorporated by reference to exhibit 10.19 filed with the Registrant’s Registration Statement on Form S-1 (Registration No. 333-182470 on June 29, 2012) | |
| 10.20 | 1998 Stock Option Plan (incorporated by reference to exhibit 10.15 filed with the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-67350) on November 14, 2001) | |
| 10.21 | 1999 Executive Stock Option Plan (incorporated by reference to exhibit 10.16 filed with the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-67350) on November 14, 2001) | |
| 10.22 | 2001 Stock Option Plan (incorporated by reference to exhibit 10.17 filed with the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-67350) on November 14, 2001) | |
| 10.23 | 2001 Nonemployee Director Stock Incentive Plan (incorporated by reference to exhibit 10.18 filed with the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-67350) on November 14, 2001) | |
| 10.24 | Employee Stock Purchase Plan (incorporated by reference to exhibit 10.19 filed with the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-67350) on November 14, 2001) | |
| 10.25 | Form of Stock Option Agreement under the 2007 Stock Option Plan (incorporated by reference to exhibit 10.2 filed with the Registrant’s Registration Statement on Form S-8 on November 21, 2007) | |
| 10.26 | Loan Agreement and Promissory Note, dated May 6, 2008 between the Company and Al Rajhi Holdings WLL (incorporated by reference to exhibit 4.5 filed with the Registrant’s Current Report on Form 8-K on May 15, 2008) | |
| 10.27 | Loan Agreement and Promissory Note, dated August 19, 2008 between the Company and Toucan Partners LLC (incorporated by reference to exhibit 10.1 filed with the Registrant’s Quarterly Report on Form 10-Q on August 19, 2008) | |
| 42 |
|
Exhibit |
Description |
| 10.28 | Loan Agreement and Promissory Note, dated October 1, 2008 between the Company and SDS Capital Group SPC, Ltd (incorporated by reference to exhibit 10.2 filed with the Registrant’s Quarterly Report on Form 10-Q on November 19, 2008) | |
| 10.29 | Warrant, dated October 1, 2008, between the Company and SDS Capital Group SPC, Ltd (incorporated by reference to exhibit 10.3 filed with the Registrant’s Quarterly Report on Form 10-Q on November 19, 2008) | |
| 10.30 | Loan Agreement and Promissory Note, dated October 21, 2008, between the Company and SDS Capital Group SPC, Ltd (incorporated by reference to exhibit 10.4 filed with the Registrant’s Quarterly Report on Form 10-Q on November 19, 2008) | |
| 10.31 | Form of Loan Agreement and Promissory Note, between the Company and a Group of Private Investors (incorporated by reference to exhibit 10.5 filed with the Registrant’s Quarterly Report on Form 10-Q on November 19, 2008) | |
| 10.32 | Form of Warrant, between the Company and SDS Capital Group SPC. Ltd and a Group of Private Investors (incorporated by reference to exhibit 10.6 filed with the Registrant’s Quarterly Report on Form 10-Q on November 19, 2008) | |
| 10.33 | Loan Agreement and Promissory Note, dated December 22, 2008, between the Company and Toucan Partners LLC (incorporated by reference to exhibit 10.62 filed with the Registrant’s Form 10-K on April 15, 2009) | |
| 10.34 | Form of Warrant, dated December 22, 2008, between the Company and Toucan Partners LLC (incorporated by reference to exhibit 10.63 filed with the Registrant’s Form 10-K on April 15, 2009) | |
| 10.35 | Form of Securities Purchase Agreement, by and among the Company and Al Rajhi Holdings (incorporated by reference to exhibit 10.64 filed with the Registrant’s Form 10-K on April 15, 2009) | |
| 10.36 | Securities Purchase Agreement, by and among the Company and a Group of Equity Investors (incorporated by reference to exhibit 10.65 filed with the Registrant’s Form 10-K on April 15, 2009) | |
| 10.37 | Form of Warrant, between the Company and a Group of Equity Investors (incorporated by reference to exhibit 10.66 filed with the Registrant’s Form 10-K on April 15, 2009) | |
| 10.38 | Form of Loan Agreement and Promissory Note, dated March 27 2009, between the Company and a Group of Private Lenders (incorporated by reference to exhibit 10.67 filed with the Registrant’s Form 10-K on April 15, 2009) | |
| 10.39 | Amended and Restated Northwest Biotherapeutics, Inc. 2007 Stock Option Plan (incorporated by reference to Schedule 14A filed on December 3, 2013) | |
| 10.40 | DC Vax ®-L Manufacturing and Services Agreement between the Company and Cognate BioServices, Inc. dated January 17, 2014 (incorporated by reference to Exhibit 10.40 filed with the Company’s Quarterly Report on Form 10-Q on May 15, 2014. | |
| 10.41 | DC Vax ®-L Direct Manufacturing and Services Agreement between the Company and Cognate BioServices, Inc. dated January 17, 2014 (incorporated by reference to Exhibit 10.41 filed with the Company’s Quarterly Report on Form 10-Q on May 15, 2014. | |
| 10.42 | Ancillary Services Agreement between the Company and Cognate BioServices, Inc. dated January 17, 2014 (incorporated by reference to Exhibit 10.42 filed with the Company’s Quarterly Report on Form 10-Q on May 15, 2014. | |
| 10.43 | Manufacturing Expansion Service Agreement between the Company and Cognate BioServices, Inc. dated January 17, 2014 (incorporated by reference to Exhibit 10.43 filed with the Company’s Quarterly Report on Form 10-Q on May 15, 2014. | |
| 10.44 | Form of Warrant between the Company and H.C. Wainwright & Co., LLC dated April 9, 2014 (incorporated by reference to Exhibit 4.1 filed with the Company’s Quarterly Report on Form 10-Q on May 15, 2014. | |
| 10.45 | Securities Purchase Agreement between the Company and certain investors dated April 9, 2014 (incorporated by reference to Exhibit 10.1 filed with the Company’s Current Report on Form 8-K on April 14, 2014) | |
| 10.46 | Placement Agreement between the Company and H.C. Wainwright & Co., LLC (incorporated by reference as Exhibit 10.2 filed with the Company’s Current Report on Form 8-K on April 14, 2014) |
| 43 |
|
Exhibit |
Description | |
| 10.47 | Form of Purchase Agreement for 5.00% Convertible Senior Notes between the Company and certain investors dated August 13, 2014 (incorporated by reference as Exhibit 1.1 filed with the Company’s Current Report on Form 8-K on August 19, 2014.) | |
| 10.48 | Form of Indenture between the Company and The Bank of New York Mellon, as Trustee, dated August 19, 2014 (incorporated by reference as Exhibit 4.1 filed with the Company’s Current Report on Form 8-K on August 25, 2014.) | |
| 10.49 | Pledge and Escrow between the Company and The Bank of New York Mellon, as Trustee, dated August 19, 2014 (incorporated by reference as Exhibit 10.1 filed with the Company’s Current Report on Form 8-K on August 25, 2014.) | |
| 10.50 | Form of Warrant between the Company and certain investors (incorporated by reference as Exhibit 4.1 filed with the Company’s Current Report on Form 8-K on October 10, 2014.) | |
| 10.51 | Stock Purchase, Amendment and Issuance Agreement dated October 6, 2014 (incorporated by reference as Exhibit 10.1 filed with the Company’s Current Report on Form 8-K on October 10, 2014.) | |
| 10.52 | Amendment to Engagement Letter between the Company H.C. Wainwright & Co., LLC dated October 6, 2014 incorporated by reference as Exhibit 10.2 filed with the Company’s Current Report on Form 8-K on October 10, 2014.) | |
| 23.1 | Consent of Marcum LLP, Independent Registered Public Accounting Firm | |
| 31.1 | Certification of the Principal Executive and Principal Financial and Accounting Officer pursuant to Section 302 of the Sarbanes Oxley Act of 2002 | |
| 32.1 | Certification of the Principal Executive and Principal Financial and Accounting Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101 | The following materials from Northwest Biotherapeutics, Inc. Form 10-K for the year ended December 31, 2013, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets at December 31, 2013 and December 31, 2012, (ii) Consolidated Statements of Operations for the years ended December 31, 2013 and 2012 and for the Cumulative Period from March 18, 1996 (inception) through December 31, 2013, (iii) Consolidated Statements of Comprehensive Loss for the years ended December 31, 2013 and 2012 and for the Cumulative Period from March 18, 1996 (inception) through December 31, 2013, (iv) Consolidated Statements of Changes in Stockholders’ Equity for the year ended December 31, 2013, (v) Consolidated Statements of Cash Flows for the years ended December 31, 2013 and 2012 and for the Cumulative Period from March 18, 1996 (inception) through December 31, 2013 and (vi) Notes to the Consolidated Financial Statements. |
| * | Confidential information in this exhibit has been omitted and filed separately with the SEC pursuant to a confidential treatment request. |
| 44 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
|
NORTHWEST BIOTHERAPEUTICS, INC. (Registrant) | |||
| Date: March 17, 2015 | By: | /s/ Linda F. Powers | |
| Linda F. Powers, | |||
| Chief Executive Officer (Principal Executive Officer | |||
| and Principal Financial and Accounting Officer) | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Linda F. Powers | Chief Executive Officer (Principal | March 17, 2015 | ||
| Linda F. Powers | Executive Officer and Principal | |||
| Financial and Accounting Officer)) | ||||
| /s/ Alton L. Boynton | Director | March 17, 2015 | ||
| Alton L. Boynton | ||||
| /s/ Robert A. Farmer | Director | March 17, 2015 | ||
| Robert A. Farmer | ||||
| /s/ Navid Malik | Director | March 17, 2015 | ||
| Dr. Navid Malik | ||||
| /s/ Jerry Jasinowski | Director | March 17, 2015 | ||
| Jerry Jasinowski |
| S-1 |
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
NORTHWEST BIOTHERAPEUTICS, INC.
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of the
Board of Directors and stockholders of
Northwest Biotherapeutics, Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheets of Northwest Biotherapeutics, Inc. and Subsidiaries (the “Company”) as of December 31, 2014 and 2013, and the related consolidated statements of operations, changes in stockholders’ deficit and cash flows for the years then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Northwest Biotherapeutics, Inc. and Subsidiaries as of December 31, 2014 and 2013, and the consolidated results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Northwest Biotherapeutics, Inc. and Subsidiaries’ internal control over financial reporting as of December 31, 2014, based on the criteria established in Internal Control-Integrated Framework in issued by the Committee of Sponsoring Organizations of the Treadway Commission in 1992 and our report dated March 17, 2015 expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting because of the existence of material weaknesses.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company incurred operating losses of approximately $101.1 million and $55.5 million for the years ended December 31, 2014 and 2013, respectively. The Company also used approximately $54.6 million of cash in its operating activities, has an accumulated deficit of $520.5 million and limited capital resources to funds its business development plans. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might become necessary should the Company be unable to continue as a going concern. Management’s plans with regard to these matters are also discussed in Note 2 to the financial statements.
/s/ Marcum llp
New York, New York
March 17, 2015
| F-2 |
NORTHWEST BIOTHERAPEUTICS, INC.
(in thousands, except per share data)
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 13,390 | $ | 18,499 | ||||
| Restricted cash - interest payments held in escrow | 865 | - | ||||||
| Prepaid expenses and other current assets | 387 | 147 | ||||||
| Total current assets | 14,642 | 18,646 | ||||||
| Non-current assets: | ||||||||
| Property, plant and equipment, net | 39,999 | 83 | ||||||
| Deferred financing cost, net | 1,985 | - | ||||||
| Restricted cash - interest payments held in escrow, net of current portion | 1,760 | - | ||||||
| Other assets | 55 | 55 | ||||||
| Total non-current assets | 43,799 | 138 | ||||||
| Total assets | $ | 58,441 | $ | 18,784 | ||||
| LIABILITIES, REDEEMABLE COMMON STOCK AND STOCKHOLDERS' DEFICIT | ||||||||
| Current liabilities: | ||||||||
| Accounts payable (includes related party of $5,729 and $3,619 as of December 31, 2014 and December 31, 2013, respectively) | $ | 15,555 | $ | 8,937 | ||||
| Accrued expenses (includes related party of $8 and $5 as of December 31, 2014 and December 31, 2013, respectively) | 1,211 | 842 | ||||||
| Convertible notes, net (includes related party note of $50 and $75 as of December 31, 2014 and December 31, 2013, respectively) | 238 | 288 | ||||||
| Note payable - in dispute | 934 | 934 | ||||||
| Environmental remediation liability | 6,200 | - | ||||||
| Derivative liability | 44,742 | 8,688 | ||||||
| Total current liabilities | 68,880 | 19,689 | ||||||
| Non-current liabilities: | ||||||||
| Convertible note | 17,500 | - | ||||||
| Mortgage loan | 6,990 | - | ||||||
| Other accrued expenses | 98 | - | ||||||
| Total non-current liabilities | 24,588 | - | ||||||
| Total liabilities | 93,468 | 19,689 | ||||||
| Redeemable common stock ($0.001 par value); 0 and 1,444,788 shares issued and outstanding as of December 31, 2014 and December 31, 2013, respectively | - | 8,913 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders' deficit: | ||||||||
| Preferred stock ($0.001 par value); 40,000,000 shares authorized; 0 shares issued and outstanding as of December 31, 2014 and December 31, 2013, respectively | - | - | ||||||
| Common stock ($0.001 par value); 450,000,000 shares authorized; 68,957,469 and 45,666,315 shares issued and outstanding as of December 31, 2014 and December 31, 2013, respectively | 69 | 46 | ||||||
| Additional paid-in capital | 485,615 | 375,213 | ||||||
| Accumulated deficit | (520,521 | ) | (384,887 | ) | ||||
| Cumulative translation adjustment | (190 | ) | (190 | ) | ||||
| Total stockholders' deficit | (35,027 | ) | (9,818 | ) | ||||
| Total liabilities, redeemable common stock and stockholders' deficit | $ | 58,441 | $ | 18,784 | ||||
See accompanying notes to the consolidated financial statements
| F-3 |
NORTHWEST BIOTHERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
| For the years ended | ||||||||
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Revenues: | ||||||||
| Research grant and other | $ | 1,454 | $ | 809 | ||||
| Total revenues | 1,454 | 809 | ||||||
| Operating costs and expenses: | ||||||||
| Research and development | 85,637 | 43,906 | ||||||
| General and administrative | 16,949 | 12,365 | ||||||
| Depreciation and amortization | 11 | 12 | ||||||
| Total operating costs and expenses | 102,597 | 56,283 | ||||||
| Loss from operations | (101,143 | ) | (55,474 | ) | ||||
| Other income (expense): | ||||||||
| Inducement expense | (18,905 | ) | (10,599 | ) | ||||
| Accretion of redeemable securities | - | (31 | ) | |||||
| Change in fair value of derivative liability | (15,062 | ) | 1,157 | |||||
| Interest expense | (996 | ) | (842 | ) | ||||
| Section 16 settlement | 449 | - | ||||||
| Gain on foreign currency exchange | 23 | - | ||||||
| Net loss | $ | (135,634 | ) | $ | (65,789 | ) | ||
| Net loss per share applicable to common stockholders - basic and diluted | $ | (2.29 | ) | $ | (2.00 | ) | ||
| Weighted average shares used in computing basic and diluted loss per share | 59,248 | 32,865 | ||||||
See accompanying notes to the consolidated financial statements
| F-4 |
NORTHWEST BIOTHERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
(in thousands)
| Common Stock | Additional | Accumulated Deficit | Cumulative Translation | Total Stockholders' | ||||||||||||||||||||
| Shares | Par value | Paid-in Capital | Deficit | Adjustment | Equity (Deficit) | |||||||||||||||||||
| Balance at January 1, 2013 | 24,677 | $ | 25 | $ | 303,190 | $ | (319,098 | ) | $ | (190 | ) | $ | (16,073 | ) | ||||||||||
| Exercise of warrants | 347 | 1 | 594 | - | - | 595 | ||||||||||||||||||
| Issuance of common stock in connection with conversion of notes payable | 1,211 | 2 | 2,867 | - | - | 2,869 | ||||||||||||||||||
| Stock and warrants issued to induce an extension of redeemable securities | 763 | 1 | 2,803 | - | - | 2,804 | ||||||||||||||||||
| Reclassification of redeemable shares to stockholders' equity | 424 | 1 | 1,863 | - | - | 1,864 | ||||||||||||||||||
| Reclass of warrants to warrant liability | - | - | (217 | ) | - | - | (217 | ) | ||||||||||||||||
| Conversion of accounts payable to common stock - Cognate BioServices | 4,720 | 5 | 16,964 | - | - | 16,969 | ||||||||||||||||||
| Stock and warrants issued for cash | 12,954 | 13 | 53,013 | - | - | 53,026 | ||||||||||||||||||
| Reclassification between common par and additional paid-in-capital | - | (3 | ) | 3 | - | - | - | |||||||||||||||||
| Issuance of common stock in exchange for services - non-employees | 570 | 1 | 2,340 | - | - | 2,341 | ||||||||||||||||||
| Warrant liability recorded in connection with issuance of common stock | - | - | (5,602 | ) | - | - | (5,602 | ) | ||||||||||||||||
| Offering Cost | - | - | (3,955 | ) | - | - | (3,955 | ) | ||||||||||||||||
| Stock compensation expense | - | - | 1,350 | - | - | 1,350 | ||||||||||||||||||
| Net loss | - | - | - | (65,789 | ) | - | (65,789 | ) | ||||||||||||||||
| Balance December 31, 2013 | 45,666 | 46 | 375,213 | (384,887 | ) | (190 | ) | (9,818 | ) | |||||||||||||||
| Issuance of common stock for cash in a private placement | 291 | - | 2,059 | - | - | 2,059 | ||||||||||||||||||
| Issuance of common stock for cash | 2,273 | 2 | 14,998 | - | - | 15,000 | ||||||||||||||||||
| Offering costs | - | - | (1,105 | ) | - | - | (1,105 | ) | ||||||||||||||||
| Conversion of accounts payable to common stock - Cognate BioServices | 4,195 | 5 | 16,776 | - | - | 16,781 | ||||||||||||||||||
| Inducement expenses associated with Conversion of accounts payable to common stock and warrants - Cognate BioServices | - | - | 8,654 | - | - | 8,654 | ||||||||||||||||||
| Conversion of note payable and accrued interest to common stock | 70 | - | 217 | - | - | 217 | ||||||||||||||||||
| Proceeds from the issuance of common stock - Cognate BioServices | 563 | 1 | 2,249 | - | - | 2,250 | ||||||||||||||||||
| Inducement expenses associated with issuance of common stock and warrants for cash - Cognate BioServices | - | - | 1,525 | - | - | 1,525 | ||||||||||||||||||
| Issuance of common stock and warrants for cash | 7,490 | 8 | 40,227 | 40,235 | ||||||||||||||||||||
| Proceeds from warrants exercises | 1,312 | 1 | 4,816 | - | - | 4,817 | ||||||||||||||||||
| Issuance of common stock in exchange for services - non-employees | 279 | - | 1,817 | - | - | 1,817 | ||||||||||||||||||
| Cashless warrants exercises | 54 | - | - | - | - | - | ||||||||||||||||||
| Reclassification of redeemable securities | 1,445 | 1 | 8,912 | - | - | 8,913 | ||||||||||||||||||
| Issuance of common stock as deferred financing cost - mortgage loan | 38 | - | 197 | 197 | ||||||||||||||||||||
| Redeemable securities settlement | 110 | - | 398 | - | - | 398 | ||||||||||||||||||
| Adjustment for issuance of common stock | 72 | - | 2 | - | - | 2 | ||||||||||||||||||
| Stock based compensation - Cognate BioServices | 5,101 | 5 | 8,660 | - | - | 8,665 | ||||||||||||||||||
| Net loss | - | - | - | (135,634 | ) | - | (135,634 | ) | ||||||||||||||||
| Balance December 31, 2014 | 68,958 | $ | 69 | $ | 485,615 | $ | (520,521 | ) | $ | (190 | ) | $ | (35,027 | ) | ||||||||||
See accompanying notes to the consolidated financial statements
| F-5 |
NORTHWEST BIOTHERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| For the years ended | ||||||||
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net Loss | $ | (135,634 | ) | $ | (65,789 | ) | ||
| Reconciliation of net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 11 | 11 | ||||||
| Amortization of debt discount and accretion on redeemable securities | - | 531 | ||||||
| Amortization of deferred financing cost | 212 | - | ||||||
| Change in fair value of derivatives | 15,062 | (1,157 | ) | |||||
| Gain from section 16 settlement | (449 | ) | - | |||||
| Accrued interest converted to common stock | 77 | - | ||||||
| Stock-based compensation costs | - | 1,350 | ||||||
| Stock and warrants issued to Cognate BioServices as compensation under Cognate Agreements | 21,329 | - | ||||||
| Stock and warrants issued for services | 1,819 | 2,341 | ||||||
| Inducement expense | 18,905 | 10,465 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (240 | ) | (35 | ) | ||||
| Accounts payable and accrued expenses | 4,989 | 766 | ||||||
| Related party accounts payable and accrued expenses | 19,343 | 13,754 | ||||||
| Deposits and other non-current assets | - | (37 | ) | |||||
| Net cash used in operating activities | (54,576 | ) | (37,800 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Purchase of property and equipment, net | (33,727 | ) | 1 | |||||
| Funding of escrow - convertible notes | (2,625 | ) | - | |||||
| Net cash used in investing activities | (36,352 | ) | 1 | |||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from issuance of notes payable to related parties | - | 1,630 | ||||||
| Proceeds from mortgage loan | 6,990 | - | ||||||
| Deferred offering cost related to mortgage loan | (622 | ) | - | |||||
| Repayment of note payable to related parties | (25 | ) | (1,605 | ) | ||||
| Repayment of convertible promissory notes | - | (499 | ) | |||||
| Proceeds from issuance of convertible note, net of deferred financing cost | 17,500 | - | ||||||
| Deferred offering cost related to convertible note | (1,280 | ) | - | |||||
| Redemption of on redeemable common shares | - | (240 | ) | |||||
| Proceeds from issuance of common stock in a private placement | 2,059 | - | ||||||
| Proceeds from exercise of warrants | 4,817 | 595 | ||||||
| Proceeds from the issuance of common stock and warrants - Cognate BioServices | 2,250 | - | ||||||
| Proceeds from issuance common stock and warrants, net of offering cost | 40,235 | 53,026 | ||||||
| Proceeds from issuance common stock and overallotment rights, net of offering cost | 13,895 | - | ||||||
| Offering costs | - | (3,955 | ) | |||||
| Net cash provided by financing activities | 85,819 | 48,952 | ||||||
| Net decrease in cash and cash equivalents | (5,109 | ) | 11,153 | |||||
| Cash and cash equivalents at beginning of period | 18,499 | 7,346 | ||||||
| Cash and cash equivalents at end of period | $ | 13,390 | $ | 18,499 | ||||
See accompanying notes to the consolidated financial statements
| F-6 |
NORTHWEST BIOTHERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| For the years ended | ||||||||
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Supplemental schedule of non-cash financing activities: | ||||||||
| Issuance of common stock in connection with conversion of notes payable and accrued expenses | $ | 140 | $ | 2,869 | ||||
| Issuance of common stock and warrants in connection with conversion of accounts payable - Cognate BioServices | $ | 16,781 | $ | 16,968 | ||||
| Reclass of redeemable security to equity | $ | 8,913 | $ | 217 | ||||
| Adjustment for common stock no longer subject to redemption | $ | - | $ | 1,864 | ||||
| Deferred offering cost related to mortgage loan | $ | 98 | $ | - | ||||
| Issuance of common stock as deferred financing cost - mortgage loan | $ | 197 | $ | - | ||||
| Environmental remediation liability | $ | 6,200 | $ | - | ||||
See accompanying notes to the consolidated financial statements
| F-7 |
NORTHWEST BIOTHERAPEUTICS, INC.
Notes to the Consolidated Financial Statements
| 1. | Organization and Description of Business |
Northwest Biotherapeutics, Inc. and its wholly owned subsidiaries NW Bio Europe S.A.R.L, NW Bio Gmbh, and Aracaris Capital, Ltd (collectively, the “Company”, “we”, “us” and “our”) were organized to discover and develop innovative immunotherapies for cancer.
The Company is developing an experimental dendritic cell vaccine using its platform technology known as DCVax. DCVax is currently being tested for use in the treatment of certain types of cancers. The Company is currently conducting clinical trials in more than 60 trial sites in the United States and Europe.
Cognate BioServices, Inc. (“Cognate BioServices”), which is a company related by common ownership (Note 9), provides the Company with mission critical contract manufacturing services, research and development services, distribution and logistics, and related services, in compliance with the Company’s specifications and the applicable regulatory requirements for clinical grade cellular products. The Company and Cognate BioServices are currently parties to a series of contracts providing for these services as more fully described below. The Company is dependent on Cognate BioServices to provide the manufacturing services, and any interruption of such services could potentially have a material adverse effect on the Company’s ability to proceed with its clinical trials. Cognate BioServices’ manufacturing facility for clinical-grade cellular products is located in Memphis, Tennessee, a major air cargo hub for both Federal Express and UPS.
Although there are many contract manufacturers for small molecule drugs and for biologics, there are only a few contract manufacturers in the U.S. that specialize in producing living cell products and that have a track record of success with regulatory authorities. The manufacturing of living cell products is highly specialized and entirely different than production of biologics: the physical facilities and equipment are different, the types of personnel and skill sets are different, and the processes are different. The regulatory requirements relating to manufacturing and cellular products are especially challenging and are one of the most frequent reasons for the development of a company’s cellular products to be put on clinical hold (i.e., stopped by regulatory authorities).
In addition, NW Bio’s programs require a large amount of capacity in these specialized manufacturing facilities, and require that the large capacity be dedicated exclusively to NW Bio’s programs. Most medical products, including cellular products, are made in batches on a campaign basis. The same manufacturing suites are used for a number of companies’ products, at designated times scheduled in advance. In contrast, NW Bio’s products are fully personalized and not made in batches, and NW Bio’s products are made on demand, on an ongoing basis. So, the manufacturing suites must be dedicated entirely to NW Bio’s products. Among the few specialized contract manufacturers for cellular products, even fewer have the necessary capacity that can be dedicated exclusively to NW Bio.
Important Developments
On January 17, 2014, the Company entered into the following series of contract manufacturing and services agreements with Cognate BioServices (collectively, the “Cognate Agreements” or the “Agreements”) providing for Cognate BioServices to manufacture DCVax products, undertake ongoing DCVax development work, develop arrangements for and manage product storage, distribution and tracking, and conduct search, evaluation, acquisition and development activities for expansion of manufacturing capacity for NW Bio products:
| · | DCVax®-L Manufacturing and Services Agreement; |
| · | DCVax®-Direct Manufacturing and Services Agreement; |
| · | Ancillary Services Agreement; and |
| · | Manufacturing Expansion Services Agreement |
| 2. | Liquidity, Financial Condition and Management Plans |
The Company used approximately $54.6 million of cash in its operating activities for the year ended December 31, 2014. The Company incurred a $135.6 million aggregate combined cash and non-cash loss for the year ended December 31, 2014, including $57.0 million of net aggregate non-cash charges for the non-cash interest associated with the accretion of its convertible notes discount, stock based compensation, a mark to market charge for the change in the fair value of our derivative financial instruments, and the fair value of warrants issued to certain holders of the Company’s redeemable common shares in exchange for an extension of their optional redemption dates. The Company expects to incur substantial capital expenditures to develop its UK Property. Management believes that the Company has access to capital resources through the sale of equity and debt financing arrangements and may seek to obtain funding from Cognate BioServices, should Cognate BioServices have available capital. Notwithstanding, the Company has not secured any commitments for new financing for this specific purpose at this time.
By entering into the Cognate Agreements, cash outflows related to research and development were greatly reduced. In addition, because the payments were made in unregistered, restricted shares, and were also subject to a lock-up, the shares did not come into the marketplace. During 2014, $16.8 million of accounts payable related to the Cognate Agreements were settled for common stock and warrants.
On April 9, 2014, the Company entered into a Securities Purchase Agreement (the “April Offering”) with a single institutional investor for the sale of 2,272,727 shares of common stock at a purchase price of $6.60 per share, for total gross proceeds of $15.0 million, less offering costs of $1.1 million. Additionally, the investor had a one-year non-transferable contractual Over-allotment Right to purchase up to 2,272,727 additional shares of common stock at a price per share of $7.50, for an additional subscription amount of up to $17.05 million. The Over-allotment Right was superseded by a warrant issued in connection with a subsequent financing transaction involving the same investor that was completed in October 2014 as more fully described below.
| F-8 |
On August 19, 2014, the Company completed a private offering of $17.5 million aggregate principal amount of 5% Senior Secured Convertible Promissory Notes (the “Senior Notes”) with an initial conversion price of $7.30 per share, for total net proceeds to the Company of approximately $16.2 million after deducting placement agent fees and other expenses. The Company capitalized these placement agent fees as deferred financing costs. The primary purpose of the financing was for expansion of manufacturing capacity for the Company’s products in Europe. As part of this transaction the Company transferred $2.6 million to interest payments held in escrow.
On August 19, 2014, the Company completed the purchase of a facility and property in the U.K (“UK Facility”). For several years, Cognate had undertaken to identify, inspect and evaluate potential manufacturing facilities and sites in Europe for the Company, with such services to be compensated following successful completion of an acquisition and facility plans. A large number of diverse sites and facilities, in diverse locations, were identified, inspected and evaluated. Due diligence work was undertaken by Cognate throughout 2013 on one of the candidate sites, which the Company ultimately decided not to acquire. Such work was again undertaken throughout 2014, culminating in the Company’s purchase of the UK facility on August 19, and purchase of an additional portion of that site on December 9 as described below. The purchase price of the UK facility was £13 million (approximately $20.8 million, excluding professional fees and other capitalized items of $2.6 million associated with the purchase of the U.K Facility). The facility is an existing building of approximately 65,000 square feet. The Company plans to re-purpose the facility and have it built out as part of the expansion of manufacturing capacity for its products in Europe, potentially doubling the building’s square footage. Such re-purposing requires approval of the applicable Planning Commission. If re-purposing is approved, then the specific design and engineering of the proposed build out will also have to be approved. In addition to the facility, the acquisition included about 25 acres of potentially developable land (as well as non-developable land). Any future development for business use will require removal of certain existing structures, permission from the Planning Commission for the intended purpose, and then permission from the Planning Commission for the specific designs and engineering.
On October 6, 2014, the Company entered into a Stock Purchase, Amendment and Issuance Agreement (the “October Offering”) with an existing single institutional investor for the sale of 2,272,727 shares of common stock at a purchase price of $5.05 per share, for a total purchase price of about $11.5 million. In the Agreement, the Company terminated the investor’s existing contractual over-allotment purchase right issued in the April Offering, which had provided for the purchase of up to $17,045,452.50 worth of shares of common stock for $7.50 per share at any time prior to April 14, 2015, and agreed to issue the purchaser a warrant to purchase up to 14,085,250 worth of shares at an exercise price of $5.15 per share (2,735,000 warrants) exercisable commencing six months after issuance and with an exercise period of 30 months. The offering closed on October 9, 2014.
On November 17, 2014, the Company entered into a private offering of $25 million of unregistered shares of common stock (the “November Offering”) of the Company, at a price of $5.79 per share (the closing price of the stock on the trading day prior to the sale of shares). The shares were purchased by C.F. Woodford Equity Income Fund of the U.K. The Company agreed to use best efforts to file a registration statement within two weeks after the closing, and to use best efforts to complete the registration within sixty days thereafter. No registration has been filed as of March 15, 2015. There are no warrants, overallotment rights, pre-emptive rights or other securities or rights entitling the investor to purchase or obtain additional shares.
Also on November 17, 2014, the Company completed a $10 million mortgage secured solely by the UK Property. The Company plans to use the proceeds in connection with its expansion of manufacturing capacity in Europe. The mortgage has a term of 2 years, interest only payments until maturity, and an interest rate of 12% annually.
On December 9, 2014, the Company completed the purchase of the second portion of the UK Property intended for the manufacturing expansion for DCVax products in the UK. This property is located within and surrounded by the first portion of the property already acquired by the Company, as previously reported. The purchase price for the additional property was £5 million (approximately $7.9 million and other capitalized costs of $2.4 million). The additional property includes approximately 12 acres of potentially developable land (as well as non-developable land), and certain existing buildings. Development of the property for DCVax manufacturing will require approval by the Planning Commission to re-classify the property, removal of certain existing structures, making certain site improvements, Planning Commission approval of the intended use after re-classification, and Planning Commission approval of the specific designs and engineering. The Company plans to explore various structures and approaches for financing and/or development of the property that may enable the Company to withdraw its capital from the property.
The Company had cash and cash equivalents of $13.4 million as of December 31, 2014, and a deficit in current assets less accounts payable and accrued expenses, derivative and environmental liabilities and notes payable of approximately $54.2 million at December 31, 2014. The non-cash derivative liabilities comprised $44.7 of the $54.2 million total. The Company owes an aggregate of $5.7 million of trade liabilities and convertible notes to related parties. The Company has not yet generated any material revenue from the sale of its products and is subject to all of the risks and uncertainties that are typically faced by biotechnology companies that devote substantially all of their efforts to developing products that have not yet been commercialized. The Company expects to continue incurring losses until such time. The Company’s existing liquidity is not sufficient to fund its operations, anticipated capital expenditures, working capital and other financing requirements until the Company reaches significant revenues. Until that time. the Company will need to obtain additional equity and/or debt financing, especially if the Company experiences downturns in its business that are more severe or longer than anticipated, or if the Company experiences significant increases in expense levels resulting from being a publicly-traded company or from expansion of operations. If the Company attempts to obtain additional equity or debt financing, the Company cannot assume that such financing will be available to the Company on favorable terms, or at all.
Because of recurring operating losses, net operating cash flow deficits, and an accumulated deficit, there is substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements have been prepared assuming that the Company will continue as a going concern, and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
| F-9 |
| 3. | Summary of Significant Accounting Policies |
Basis of Presentation
The accompanying consolidated financial statements of the Company were prepared in accordance with generally accepted accounting principles in the U.S. (“U.S. GAAP”) and include the assets, liabilities, revenues and expenses of the wholly owned subsidiaries in Germany, Switzerland and the United Kingdom. All intercompany transactions and accounts have been eliminated in consolidation.
Cash and Cash Equivalents
Cash consists of funds deposited in checking accounts. While cash held by financial institutions may at times exceed federally insured limits, management believes that no material credit or market risk exposure exists due to the high credit quality of the institutions that have custody of the Company’s funds. The Company has not incurred any losses on such accounts.
Property, Plant and Equipment
Property and equipment are stated at cost. Depreciation and amortization are provided for using straight-line methods, in amounts sufficient to charge the cost of depreciable assets to operations over their estimated service lives. The UK Property’s building will be depreciated over 15 years once the UK property is fully functional. Repairs and maintenance costs are charged to operations as incurred. For more details see Note 6.
Fair Value of Financial Instruments
The fair value of financial instruments other than liabilities payable to related parties approximate the recorded value based on the short term nature of these financial instruments. The fair value of derivative liabilities is measured using a binomial model or Monte Carlo simulation depending on the complexity of the derivative (Note 4).
Deferred Financing Costs
The Company capitalizes costs related to the issuance of debt which are included on the accompanying consolidated balance sheets. Deferred financing costs are amortized using a method that approximates the interest method over the life of the related loan and are included as a component of interest expense on the accompanying consolidated statements of operations. The Company recorded $1.3 million of deferred financing costs related to convertible notes issued in August 2014 and $1.1 million related to the UK mortgage loan issued in November 2014. As of December 31, 2014, deferred financing costs were $2.0 million, net of accumulated amortization expense of $0.2 million.
Warrant Liability
The Company accounts for the 12,555,198 common stock warrants outstanding in accordance with the guidance contained in ASC 815-40-15-7D, "Contracts in Entity's Own Equity" whereby under that provision they do not meet the criteria for equity treatment and must be recorded as a liability. Accordingly, the Company classifies the warrant instrument as a liability at its fair value and adjusts the instrument to fair value at each reporting period. This liability is subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in the Company's statements of operations. The fair value of the warrants issued by the Company in connection the Conversion Transaction has been estimated using a Monte Carlo simulation.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. In performing a review for impairment, the Company compares the carrying value of the assets with its estimated future undiscounted cash flows from the use of the asset and eventual disposition. If the estimated undiscounted future cash flows are less than carrying value, an impairment loss is charged to operations based on the difference between the carrying amount and the fair value of the asset.
Redeemable Securities
We account for redeemable common stock in accordance with ASC 480-10-S99-3A, “Classification and Measurement of Redeemable Securities”, which provides that securities that are optionally redeemable by the holder for cash or other assets are classified outside of permanent equity in temporary equity.
Environmental Remediation Liabilities
The Company records environmental remediation liabilities for properties acquired. The environmental remediation liabilities are initially recorded at fair value. The liability is reduced for actual costs incurred in connection with the clean-up activities for each property. Upon completion of the clean-up, the environmental remediation liability is adjusted to equal the fair value of the remaining operation, maintenance and monitoring activities to be performed for the property. The reduction in the liability resulting from the completion of the clean-up is included in other income. As of December 31, 2014, we estimate that the total environmental remediation costs associated with the purchase of the UK Facility will amount to approximately $6.2 million. Contamination clean-up costs that improve the property from its original acquisition state are capitalized as part of the property’s overall development costs. The Company engaged a third party specialist to conduct certain surveys of the condition of the property which included, among other things, a preliminary analysis of potential environmental remediation exposures. The Company determined, based on information contained in the specialist’s report, that it would be required to estimate the fair value of an unconditional obligation to remediate specific ground contamination at an estimated fair value of approximately $6.2 million. The Company computed its preliminary estimate of the fair value of this obligation using a probability weighted approach that measures the likelihood of the following two potential outcomes: (i) a higher probability requirement of erecting a protective barrier around the affected area at an estimated cost of approximately $4.5 million, and (ii) a lower probability requirement of having to excavate the affected area at an estimated cost of approximately $32.0 million. The Company’s estimate is preliminary and therefore subject to change as further studies are conducted, and as additional facts come to the Company’s attention. Environmental remediation efforts are complex, technical and subject to various uncertainties. Accordingly, it is at least reasonably possible that any changes in the Company’s estimate could materially differ from the management’s preliminary assessment discussed herein.
| F-10 |
Foreign Currency Translation
For certain operations outside the U.S. that prepare financial statements in currencies other than U.S. dollars, we translate the financial statements into U.S. dollars. Results of operations and cash flows are translated at average exchange rates during the period, and assets and liabilities are translated at end of period exchange rates, except for equity transactions and advances not expected to be repaid in the foreseeable future, which are translated at historical cost. During 2013 and 2014 foreign currency translation gains and losses were insignificant.
Foreign Currency Transactions
The mortgage loan, which is denominated in a foreign currency (British Pounds), is converted into the Company’s functional currency (the United States dollar) at the exchange rate on the balance sheet date. The foreign currency exchange gain or loss was insignificant.
Comprehensive loss
Comprehensive loss equals net loss for all periods presented.
Revenue Recognition
The Company recognizes revenue in accordance with the terms stipulated under the patient service contract. In various situations, the Company receives certain payments for DCVax®-L for patient treatment. These payments are non-refundable, and are not dependent on the Company’s ongoing future performance. The Company has adopted a policy of recognizing these payments as revenue when received.
Accrued Outsourcing Costs
Substantial portions of our preclinical studies and clinical trials are performed by third-party laboratories, medical centers, contract research organizations and other vendors (collectively "CROs"). These CROs generally bill monthly or quarterly for services performed, or bill based upon milestones achieved. For clinical studies, expenses are accrued based upon the number of patients enrolled and the duration of the study. The Company monitors patient enrollment, the progress of clinical studies and related activities through internal reviews of data that is tracked by the CROs under contractual arrangements, correspondence with the CROs and visits to clinical sites.
Research and Development Costs
Research and development costs are charged to operations as incurred and consist primarily of clinical trial costs, related party manufacturing costs, consulting costs, contract research and development costs, and compensation costs.
For the years ended December 31, 2014 and 2013, the Company recognized research and development costs (cash and non-cash combined) of $85.6 million and $43.9 million, respectively. Included in research and development expense was $10.0 million and $3.8 million, respectively, related to clinical site expenses such as CRO fees and site fees.
| F-11 |
For the years ended December 31, 2014 and 2013, the Company made cash payments of approximately $18.7 million, and $12.4 million, respectively, to Cognate. A portion of the $18.7 million paid in 2014 was payment for unpaid invoices from 2013. At December 31, 2014 and 2013, the Company owed Cognate $5.7 million and $3.6 million, respectively, for unpaid invoices for services performed by Cognate (including manufacturing for both the Phase III and Phase I/II clinical trials, ongoing product and process development, expansion of several company programs and services related to expansion of manufacturing capacity).
During the years ended December 31, 2014 and 2013, the Company incurred non-cash equity based compensation (restricted common stock and warrants) related to Cognate BioServices of $21.3 million and $0, respectively. This equity compensation primarily involved one-time initiation payments of shares and warrants relating to the four new agreements the Company entered into with Cognate in January, 2014. The shares are vesting over a period of three years from the date of the agreements. The fair value calculation of these shares was determined using the market price for tradable shares; however the shares issued to Cognate were unregistered restricted shares. The equity compensation also included lock-up warrants (for the lock-up of Cognate shares) and most favored nation shares and warrants.
For the years ended December 31, 2014 and 2013, Cognate’s unpaid payables and accrued expenses increased by $19.3 million (increase from 2013 to 2014) and $13.8 million (increase from 2012 to 2013), respectively, as the Company launched and carried out its DCVax-Direct program, and expanded its DCVax-L program.
Income Taxes
The Company recognizes income taxes on an accrual basis based on tax positions taken or expected to be taken in its tax returns. A tax position is defined as a position in a previously filed tax return or a position expected to be taken in a future tax filing that is reflected in measuring current or deferred income tax assets and liabilities. Tax positions are recognized only when it is more likely than not (i.e., likelihood of greater than 50%), based on technical merits, that the position would be sustained upon examination by taxing authorities. Tax positions that meet the more likely than not threshold are measured using a probability-weighted approach as the largest amount of tax benefit that is greater than 50% likely of being realized upon settlement. Income taxes are accounted for using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. A valuation allowance is established to reduce deferred tax assets if all, or some portion, of such assets will more than likely not be realized. Should they occur, our policy is to classify interest and penalties related to tax positions as income tax expense. Since our inception, no such interest or penalties have been incurred, however prior to 1998 the Company was a limited liability company and the Company’s tax losses and credits generally flowed directly to the members.
Non-Employee Stock Based Compensation
The Company accounts for stock based compensation awards issued to non-employees for services, as prescribed by ASC 505, at either the fair value of the services or the instruments issued in exchange for such services (based on the same methodology described for employee stock based compensation), whichever is more readily determinable. Subsequent to the measurement date, the Company recognizes and classifies any future changes in the fair value in accordance with the relevant accounting literature on financial instruments ASC 815-40.
Use of Estimates
In preparing financial statements in conformity with U.S. GAAP, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of expenses during the reporting period. Due to inherent uncertainty involved in making estimates, actual results reported in future periods may be affected by changes in these estimates. On an ongoing basis, the Company evaluates its estimates and assumptions. These estimates and assumptions include valuing equity securities in share-based payment arrangements, estimating the fair value of equity instruments recorded as derivative liabilities, useful lives of depreciable assets and whether impairment charges may apply, and the fair value of environmental remediation liabilities.
Loss per Share
Basic loss per share is computed on the basis of the weighted average number of shares outstanding for the reporting period. Diluted loss per share is computed on the basis of the weighted average number of common shares (including redeemable shares) plus dilutive potential common shares outstanding using the treasury stock method. Any potentially dilutive securities are anti-dilutive due to the Company’s net losses. For the years presented, there is no difference between the basic and diluted net loss per share.
Segments
The Company operates in one reportable segment and, accordingly, no segment disclosures have been presented herein.
| F-12 |
Subsequent Events
The Company follows the provisions of ASC Topic 855-10, “Subsequent Events,” relating to subsequent events. This guidance establishes principles and requirements for subsequent events. This guidance defines the period after the balance sheet date during which events or transactions that may occur would be required to be disclosed in a company’s financial statements. The Company has evaluated subsequent events up to the date of filing of its Annual Report on Form 10-K for the year ended December 31, 2014 (see Note 14).
Recent and Adopted Accounting Pronouncements
In June 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No. 2014-10, Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation. The amendments in this update remove the definition of a development stage entity from the Master Glossary of the Accounting Standards Codification, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, the amendments eliminate the requirements for development stage entities to (1) present inception-to-date information in the statements of income, cash flows and shareholder equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. A public entity is required to apply the amendments for annual reporting periods beginning after December 15, 2014, and interim periods therein. An entity should apply the amendments retrospectively for all comparative periods presented. Early adoption is permitted. The Company adopted the guidance during the second quarter of 2014. Adoption of this standard did not have a material impact on the Company’s financial position, results of operations, or cash flows.
In August 2014, the FASB issued ASU 2014-15, “Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” (“ASU 2014-15”). ASU 2014-15 provides guidance on management’s responsibility in evaluating whether there is substantial doubt about a company’s ability to continue as a going concern and about related footnote disclosures. For each reporting period, management will be required to evaluate whether there are conditions or events that raise substantial doubt about a company’s ability to continue as a going concern within one year from the date the financial statements are issued. The amendments in ASU 2014-15 are effective for annual reporting periods ending after December 15, 2016, and for annual and interim periods thereafter. Early adoption is permitted. The Company will adopt the methodologies prescribed by ASU 2014-15 by the date required, and does not anticipate that the adoption of ASU 2014-15 will have a material effect on its financial position or results of operations.
In June 2014, the FASB issued ASU 2014-12, Compensation-Stock Compensation (Topic 718). The ASU clarifies how entities should treat performance targets that can be achieved after the requisite service period of a share-based payment award. The accounting standard is effective for interim and annual periods beginning after December 15, 2015. The Company is currently in the process of evaluating the impact of the guidance on its financial position, results of operation, and cash flows.
| 4. | Fair Value Measurements |
The Company’s assets and liabilities recorded at fair value have been categorized based upon a fair value hierarchy.
The following table presents information about the Company’s liabilities measured at fair value on a recurring basis and the Company’s estimated level within the fair value hierarchy of those assets and liabilities as of December 31, 2014 and December 31, 2013 (in thousands):
| Fair value measured at December 31, 2014 | ||||||||||||||||
| Quoted prices in | Significant other | Significant | ||||||||||||||
| Fair value at | active markets | observable inputs | unobservable inputs | |||||||||||||
| December 31, 2014 | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| Warrant liability | $ | 44,742 | $ | - | $ | - | $ | 44,742 | ||||||||
| Fair value measured at December 31, 2013 | ||||||||||||||||
| Quoted prices in | Significant other | Significant | ||||||||||||||
| Fair value at | active markets | observable inputs | unobservable inputs | |||||||||||||
| December 31, 2013 | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| Warrant liability | $ | 8,688 | $ | - | $ | - | $ | 8,688 | ||||||||
There were no transfers between Level 1, 2 or 3 during the year ended December 31, 2014.
The following table presents additional information about Level 3 assets and liabilities measured at fair value. Both observable and unobservable inputs may be used to determine the fair value of positions that the Company has classified within the Level 3 category. As a result, the unrealized gains and losses for assets and liabilities within the Level 3 category may include changes in fair value that were attributable to both observable (e.g., changes in market interest rates) and unobservable (e.g., changes in unobservable long-dated volatilities) inputs.
| F-13 |
Changes in Level 3 liabilities measured at fair value for the period ended December 31, 2014 and at December 31, 2013 were as follows (dollars in thousands):
| Warrant | ||||
| Liability | ||||
| Balance – December 31, 2012 | $ | - | ||
| Additions during the period | 9,845 | |||
| Change in fair value | (1,157 | ) | ||
| Balance – December 31, 2013 | 8,688 | |||
| Additions during the period | 20,992 | |||
| Change in fair value | 15,062 | |||
| Balance – December 31, 2014 | $ | 44,742 | ||
A summary of quantitative information with respect to valuation methodology, estimated using a Monte Carlo simulation, and significant unobservable inputs used for the Company’s warrant liabilities that are categorized within Level 3 of the fair value hierarchy for the years ended 2013 (including the inputs from the revaluation of warrants as of December 31, 2013) is as follows:
| Date of valuation | July 31, 2013 | November 25, 2013 | December 2, 2013 | December 19, 2013 | December 31, 2013 | |||||||||||||||||||
| Dividend yield (per share) | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||||||||
| Strike price | $ | 4.00 | $ | 6.00 | $ | 4.00 | $ | 4.00 | $2.40 - $6.00 | |||||||||||||||
| Volatility (annual) | 80.26 | % | 77.18 | % | 77.18 | % | 77.66 | % | 77.66 | % | ||||||||||||||
| Risk-free rate | 1.39 | % | 1.37 | % | 1.37 | % | 1.55 | % | 0.75% to 1.71% | |||||||||||||||
| Contractual term (years) | 5.0 | 5.0 | 5.00 | 5.00 | 2.66 - 4.97 | |||||||||||||||||||
| Total | ||||||||||||||||||||||||
| Number of warrants issued | 2,116 | 2,815 | 112 | 129 | 117 | 5,289 | ||||||||||||||||||
| Fair value of warrants at issuance date | $ | 3,576 | $ | 5,602 | $ | 254 | $ | 196 | $ | 217 | $ | 9,845 | ||||||||||||
The Warrants issued on July 31, 2013, December 2, 2013, December 19, 2013, and December 31, 2013, originated in connection with settlement of trade liabilities due to Cognate. The warrants issued on November 25, 2013 originated in connection with in the Company’s underwritten public offering.
The following warrant tables for the year ended December 31, 2014, relate to warrants issued in connection with settlement of trade liabilities due to Cognate (including the inputs from the revaluation of warrants as of December 31, 2013):
| Date of valuation | January 6, 2014 | January 17, 2014 | January 31, 2014 | February 3, 2014 | February 28, 2014 | March 31, 2014 | ||||||||||||||||||||||
| Dividend yield (per share) | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||||||||||
| Strike price | $ | 4.00 | $ | 4.00 | $ | 4.00 | $ | 4.00 | $ | 4.00 | $2.40 - $6.00 | |||||||||||||||||
| Volatility (annual) | 92.13 | % | 93.12 | % | 96.72 | % | 91.79 | % | 105.03 | % | 74.43%-88.03% | |||||||||||||||||
| Risk-free rate | 1.73 | % | 1.66 | % | 1.55 | % | 1.49 | % | 1.49 | % | 1.73 | % | ||||||||||||||||
| Contractual term (years) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 4.33-4.85 | ||||||||||||||||||||||
| Total | ||||||||||||||||||||||||||||
| Number of warrants issued | 139 | 2,434 | 143 | 119 | 195 | 145 | 3,175 | |||||||||||||||||||||
| Fair value of warrants at issuance date | $ | 308 | $ | 5,501 | $ | 383 | $ | 327 | $ | 844 | $ | 589 | $ | 7,952 | ||||||||||||||
| Date of valuation | April 30, 2014 | May 30, 2014 | June 30, 2014 | |||||||||||||
| Dividend yield (per share) | 0 | % | 0 | % | 0 | % | ||||||||||
| Strike price | $ | 4.00 | $ | 4.00 | $2.40 - $6.00 | |||||||||||
| Volatility (annual) | 84.18 | % | 83.48 | % | 79.79%-82.90% | |||||||||||
| Risk-free rate | 1.74 | % | 1.52 | % | 1.62%-1.64% | |||||||||||
| Contractual term (years) | 5.0 | 5.0 | 5.00 | |||||||||||||
| Total | ||||||||||||||||
| Number of warrants issued | 171 | 193 | 281 | 645 | ||||||||||||
| Fair value of warrants at issuance date | $ | 527 | $ | 621 | $ | 990 | $ | 2,138 | ||||||||
| F-14 |
| Date of valuation | July 1, 2014 | July 17, 2014 | August 1, 2014 | September 1, 2014 | September 30, 2014 | |||||||||||||||
| Dividend yield (per share) | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||||
| Strike price | $ | 4.00 | $ | 4.00 | $ | 4.00 | $ | 4.00 | $2.40-$6.00 | |||||||||||
| Volatility (annual) | 79.97 | % | 78.25 | % | 74.45 | % | 85.73 | % | 70.89 | % | ||||||||||
| Risk-free rate | 1.62 | % | 1.71 | % | 1.67 | % | 1.63 | % | 1.78 | % | ||||||||||
| Contractual term (years) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | |||||||||||||||
| Total | ||||||||||||||||||||
| Number of warrants issued | 473 | 2,325 | 329 | 320 | 3,447 | |||||||||||||||
| Fair value of warrants at issuance date | $ | 1,671 | $ | 7,162 | $ | 1,071 | $ | 997 | $ | 10,901 | ||||||||||
| Date of valuation | December 31, 2014 | ||
| Dividend yield (per share) | 0 | % | |
| Strike price | $ | 2.40-$6.00 | |
| Volatility (annual) | 64.09%-67.72 | % | |
| Risk-free rate | 0.67%-1.65 | % | |
| Contractual term (years) | 5.0 |
The development and determination of the unobservable inputs for Level 3 fair value measurements and fair value calculations are the responsibility of the Company’s Management. The Company values such warrants using the Monte Carlo simulation. The model requires management to use five inputs: price, risk-free interest rate, and exercise price, time remaining on the warrant and expected price volatility. The volatility factor is derived primarily from the implied volatility of traded warrants, when available, or the historical prices of its underlying common stock and a comparison of average volatility rates of similar companies. The expected term for the warrants equals the contractual term.
| 5. | Stock-based Compensation |
Compensation expense for all stock-based awards is measured on the grant date based on the fair value of the award and is recognized as an expense, on a straight-line basis, over the employee's requisite service period (generally the vesting period of the equity award). The fair value of each option award is estimated on the grant date using a Black-Scholes option valuation model. Stock-based compensation expense is recognized only for those awards that are expected to vest using an estimated forfeiture rate. The Company estimates pre-vesting option forfeitures at the time of grant and reflects the impact of estimated pre-vesting option forfeitures in compensation expense recognized. For options and warrants issued to non-employees, the Company recognizes stock compensation costs utilizing the fair value methodology over the related period of benefit.
On January 17, 2014, in connection with the four Cognate BioServices Agreements, the Company issued one-time aggregate initiation payments of 5,101,366 shares of common stock. The common stock is vesting over thirty-six months from the closing date. Stock-based compensation expense related to Cognate BioServices, with respect to these shares, was $8.7 million for the year ended December 31, 2014.
The Company also entered into a Lock-Up Agreement with Cognate BioServices on January 17, 2014, under which Cognate BioServices agreed to have all of the shares that are issued as part of the Cognate BioServices Agreements (“Lock-Up Shares”) locked up for up to 36 months, in return for 15% warrant coverage for each 6-month period of lock-up, on the same terms as the warrants in the Cognate BioServices Agreements. During the lock-ups, the Lock-Up Shares may not be sold or traded on the market. These lock-up terms are subject to the same most favored nation treatment as provided in the Cognate BioServices Agreements, as described in Note 1. On July 17, 2014, the Company issued 2,325,467 warrants in connection with the lock-up. The warrants had a term of 5.0 years and an initial exercise price of $4.00. Using a Monte Carlo simulation model, the Company valued the warrants at $3.08 and $3.80 per share on July 17, 2014 and December 31, 2014, respectively. The fair value of the warrants granted is based on the Monte Carlo simulation model using the following assumptions:
| Date of valuation | July 17, 2014 | December 31, 2014 | ||||||
| Dividend yield (per share) | 0 | % | 0 | % | ||||
| Strike price | $ | 4.00 | $ | 3.35 | ||||
| Volatility (annual) | 78.25 | % | 67.72 | % | ||||
| Risk-free rate | 1.71 | % | 1.65 | % | ||||
| Contractual term (years) | 5.0 | 4.5 | ||||||
The Company has evaluated the terms of the warrants with reference to the guidance provided in ASC 815-40-15. The Company has concluded that these warrants are not indexed to the Company’s own stock due to the most favored nation provision. Therefore, these warrants have been classified as a derivative liability as of July 17, 2014 and December 31, 2014.
| F-15 |
| 6. | Property and Equipment |
Property and equipment consist of the following at December 31, 2014 and December 31, 2013 (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Leasehold improvements | $ | 69 | $ | 69 | ||||
| Office furniture and equipment | 25 | 25 | ||||||
| Computer equipment and software | 137 | 137 | ||||||
| Property | 39,928 | - | ||||||
| 40,159 | 231 | |||||||
| Less: accumulated depreciation | (160 | ) | (148 | ) | ||||
| $ | 39,999 | $ | 83 | |||||
On August 19, 2014, the Company completed the first acquisition of a facility and property in the UK (“UK Facility”). The purchase price of the property was £13 million (approximately $20.8 million, excluding acquisition related and other capitalized costs of $2.6 million). The Company plans to re-purpose the facility and have it built out as part of the expansion of manufacturing capacity for its products in Europe. Such re-purposing requires approval of the applicable Planning Commission. If re-purposing is approved, then the specific design and engineering of the proposed build-out will also have to be approved. In addition to the facility, the acquisition included about 25 acres of potentially developable land (as well as non-developable land). Any future development for business use will require removal of certain existing structures, permission from the Planning Commission for the intended purpose, and then permission from the Planning Commission for the specific designs and engineering. The amount of development costs that the Company expects to incur in order to place this asset in service has not yet been quantified.
Depreciation expense amounted to approximately $0.01 million for the year ended December 31, 2014. The Company also capitalized a $6.2 million environmental liability, which represents the Company’s estimated costs of remediating certain ground contamination that was known to exist on the property at the time of the purchase. The building is not yet under construction and the Company will incur various development costs in the future. For further information on the environmental liability please see Note 3.
On December 9, 2014, the Company completed the purchase of the second portion of the property intended for the manufacturing expansion for DCVax products in the UK. This property is located within and surrounded by the first portion of the property already acquired by the Company as previously reported. The purchase price for the additional property was £5 million (approximately $7.9 million and other capitalized costs of $2.4 million). The additional property includes approximately 12 acres of potentially developable land (as well as non-developable land), and certain existing buildings. Development of the property for DCVax manufacturing will require approval by the Planning Commission to re-classify the property, remove certain existing structures, and make certain site improvements, as well as Planning Commission approval of the intended use after re-classification, and Planning Commission approval of the specific designs and engineering. The Company plans to explore various structures and approaches for financing and/or development of the property that may enable the Company to withdraw its capital from the property. The amount of development costs that the Company expects to incur in order to place this asset in service has not yet been quantified.
| 7. | Notes Payable |
2014 Convertible Senior Notes
Overview
On August 19, 2014, the Company completed a private offering of $17.5 million aggregate principal amount of Senior Notes with an initial conversion price of $7.30 per share, for total net proceeds to the Company of approximately $16.2 million after deducting placement agent fees and other offering costs. The Company capitalized these placement agent fees and other offering costs as deferred financing cost. Interest expense amounted to $0.5 million which included $0.3 million related to the 5% coupon and $0.2 million related to the deferred offering costs for the year ended December 31, 2014.
The Senior Notes are due on August 15, 2017, and are not convertible during the first three months, unless the current stock price is greater than 150% of the conversion price. Thereafter, the Senior Notes are convertible at any time. Pursuant to a one-time potential price reset provision, the conversion price was reset from $7.30 per share to $6.60 per share, as described below. The initial investors had a 3-month right to purchase an additional 30% on the same terms and conditions as the initial purchase, but did not exercise it. The Company deposited approximately $2.6 million from the total proceeds in an escrow account. This funding is sufficient to fund, when due, the total aggregate amount of the six scheduled semi-annual interest payments during the term of the notes, excluding additional interest, if any.
| F-16 |
Conversion Price
Pursuant to a one-time potential price reset provision, on February 15, 2015, the initial conversion price of $7.30 was reset (“Reset”) to the lower of (a) the initial conversion price or (b) 110% of the common stock price on the 10 trading days ending on February 15, 2015. The adjustment resulted in reduction of the conversion price from $7.30 to $6.60 per share (151.4142 shares per $1,000). In the future, the conversion price will be adjusted if the Company issues to all or substantially all of its stockholders any equity or convertible debt at a purchase price that is less than the current exercise price or in certain fundamental transactions, with certain exceptions. The conversion price will also adjust on any subsequent issuance of shares of common stock or common stock equivalents based on a formula intended to maintain the fully diluted percentage ownership on an “if converted” basis. The Company determined that the Reset is an input to the fair value of a “fixed-for-fixed” conversion option. As a result, this conversion option is indexed to the Company’s own stock.
The conversion price is subject to standard adjustment for stock splits, subdivisions, reclassifications or combinations, etc. If the holders elect to convert the Notes upon a change of control, the conversion rate will increase by a number of additional shares as determined by reference to an adjustment schedule based on the date on which the change in control becomes effective and the price paid per common share in the transaction (referred to as the "Fundamental Change Make-Whole Premium"). The Fundamental Make-Whole Premium is intended to compensate holders for the loss of time value upon early exercise. The standard anti-dilution provisions and Fundamental Make-Whole Premiums are inputs to the fair value of “fixed for fixed” conversion option.
The convertible notes contain an interest make-whole payment provision pursuant to which holders who convert their notes prior to maturity will receive all interest due through the term of the note. The interest make-whole payment is considered an embedded derivative and is separated from the host contract; the convertible notes are carried at fair value. As of December 31, 2014, the carrying amount of the interest make-whole payment provision was insignificant.
Mortgage Loan
On November 17, 2014, the Company entered into a bridge loan agreement (“the Mortgage”) with Lancashire Mortgage Corporation Limited in UK for approximately $10 million (£6.25 million). The Mortgage has a 2 year term with a 12% annual interest rate. The Company initially received the first tranche of approximately $7 million (£4.5 million), and this amount was netted by approximately $0.3 million of a related financing charge, which was capitalized as deferred financing cost that is being amortized over the term of the Mortgage. Interest expense amounted to $0.2 million which included $0.1 million related to the 12% coupon and $0.1 million related to the amortization of deferred offering financing costs on the mortgage loan.
Other Notes Payable
Notes payable consist of the following at December 31, 2014 and December 31, 2013:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Notes payable - current | ||||||||
| 12% unsecured originally due July 2011 - in dispute (1) | 934 | 934 | ||||||
| 934 | 934 | |||||||
| Convertible notes payable, net - current | ||||||||
| 6% unsecured (2) | 135 | 160 | ||||||
| 8% unsecured note due 2014 (3) | 53 | 53 | ||||||
| 188 | 213 | |||||||
| Convertible Notes payable related party, net - current | ||||||||
| 6% due on demand (4) | 50 | 75 | ||||||
| 50 | 75 | |||||||
| Total notes payable, net | $ | 1,172 | $ | 1,222 | ||||
(1) This $0.934 million note, which was originally due in July 2011 is currently under dispute with the creditor as to the validity of the note payable balance, which the Company believes has already been paid in full and is not outstanding.
(2) The balance of $0.160 million as of December 31, 2013 consists of three separate 6% notes in the amounts of $0.110 million, $0.025 million and $0.025 million. In regard to the $0.110 million note, the Company has made ongoing attempts to locate the creditor to repay or convert this note, but has been unable to locate the creditor to date. In regard to the $0.025 million note, the holder has elected to convert these notes into equity. The Company has delivered the applicable conversion documents to the holder, and the Company is waiting for the holder to execute and return the documents. Until such documents are executed the Company will continue to include this convertible note as outstanding. In regard to the other $0.025 million note, the holder converted this note and related accrued interest into equity in March 2014.
(3) This $0.530 million note was due May 25, 2014, and is currently past due.
(4) This $0.050 million demand note as of December 31, 2014 is held by an officer of the Company. The holder has made no demand for payment, but reserves the right to make a demand at any time.
For the year ended December 31, 2014, the Company converted notes and relevant accrued interest of $0.2 million into approximately 0.07 million shares of common stock.
For the year ended December 31, 2014, the Company made a repayment of $0.02 million to one of the debt holders.
| F-17 |
| 8. | Net Loss per Share Applicable to Common Stockholders |
Options, warrants, and convertible debt outstanding were all considered anti-dilutive for the years ended December 31, 2014 and 2013, due to net losses.
The following securities were not included in the diluted net loss per share calculation because their effect was anti-dilutive as of the periods presented (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Common stock options | 1,551 | 1,551 | ||||||
| Common stock warrants - equity treatment | 16,830 | 15,321 | ||||||
| Common stock warrants - liability treatment | 12,555 | 2,233 | ||||||
| Convertible notes | 2,479 | 127 | ||||||
| Excluded potentially dilutive securities | 33,415 | 19,232 | ||||||
| 9. | Related Party Transactions |
a. Cognate BioServices
For the years ended December 31, 2014 and 2013, the Company made cash payments of approximately $18.7 million, and $12.4 million, respectively, to Cognate BioServices. A portion of the $18.7 million paid in 2014 was payment for unpaid invoices from 2013. At December 31, 2014 and 2013, the Company owed Cognate BioServices $5.7 million and $3.6 million, respectively, for unpaid invoices for services performed by Cognate BioServices (including manufacturing for both the Phase III and Phase I/II clinical trials, ongoing product and process development, expansion of several company programs and services related to expansion of manufacturing capacity).
During the years ended December 31, 2014 and 2013, the Company incurred non-cash equity based compensation (restricted common stock and warrants) related to Cognate BioServices of $21.3 million and $0, respectively. This equity compensation primarily involved one-time initiation payments of shares and warrants relating to the four new agreements the Company entered into with Cognate in January, 2014. The shares are vesting over a period of three years from the date of the agreements. The fair value calculation of these shares was determined using the market price for tradable shares; however the shares issued to Cognate BioServices were unregistered restricted shares. The equity compensation also included lock-up warrants (for the lock-up of Cognate shares) and most favored nation shares and warrants.
For the years ended December 31, 2014 and 2013, Cognate BioServices unpaid payables and accrued expenses increased by $19.3 million (increase from 2013 to 2014) and $13.1 million (increase from 2012 to 2013), respectively, as the Company launched and carried out its DCVax-Direct program, and expanded its DCVax-L program.
The Company and Cognate BioServices entered into a DCVax-L Manufacturing Services Agreement effective January 17, 2014, and that Agreement followed and superseded Manufacturing Services Agreements in 2011 and 2007. The 2007 and 2011 had provided for baseline charges to the Company per month for dedicated manufacturing capacity, and the 2014 DCVax-L and DCVax-Direct Manufacturing Services Agreements also provide for such baseline charges. These minimum charges reflect the fact that the manufacturing suites and capacity that are going to be used for production of the Company’s DCVax products must be dedicated exclusively to the DCVax products and cannot be used to produce numerous different clients’ products in batches on a campaign basis, as is the usual case in contract manufacturing facilities. See description in Note 1 above. The capacity charges in the DCVax-L and DCVax-Direct Agreements entered into in January, 2014, were increased in accordance with the expansion of DCVax-L and DCVax-Direct production needed for the Company’s growing programs and requested by the Company.
The 2007 and 2011 Agreements also contained certain provisions for fees in the event that the Company shuts down or suspends its DCVax-L clinical trial program during the Term of the Agreement.
Under the April 2011 Agreement, the Company was contingently obligated to pay a $2 million fee if the Company did stop or suspend its DCVax-L program. This provision terminated with the January 17, 2014 DCVax®-L Manufacturing Services Agreement.
Under the January 17, 2014 DCVax®-L Manufacturing Services Agreement and the DCVax-Direct Agreement, a new set of provisions apply going forward to any shut down or suspension. Under these provisions, the Company will be contingently obligated to pay certain fees to Cognate BioServices (in addition to any other remedies) if the Company shuts down or suspends its DCVax-L program or DCVax-Direct program. For a shut down or suspension of the DCVax-L program, the fees will be as follows:
| • | Prior to the last dose of the last patient enrolled in the Phase III trial for DCVax®-L or After the last dose of the last patient enrolled in the Phase III clinical trial for DCVax®-L but before any submission for product approval in any jurisdiction or After the submission of any application for market authorization but prior to receiving a marketing authorization approval: in any of these cases, the fee shall be $3 million. |
| • | At any time after receiving the equivalent of a marketing authorization for DCVax®-L in any jurisdiction, the fee shall be $5 million. |
| F-18 |
In July 2013, the Company received a short-term loan of $0.6 million from Cognate BioServices. The short-term loan was repaid during the third quarter of 2013.
In October and November 2013, the Company received short-term loans of $0.6 million from Cognate BioServices. The short-term loans were repaid during December of 2013.
Cognate BioServices Accounts Payable Conversions and Inducement Charge
On July 31, 2013, Cognate BioServices, a related party supplier, agreed to convert an aggregate of $11.6 million of accounts payable into shares of common stock (“Conversion Transaction”) at an initial conversion price of $4.00 per share, which resulted in the issuance of 2.9 million shares of common stock, subject to most favored nation treatment with respect to the terms provided to any other investors or creditors (including with respect to any warrants). The conversion shares are subject to a lock-up period of at least 18 months from the date of their issuance, on market based terms. Under the lock-up, the shares cannot be sold or traded on the market. The conversions and the lock-up terms are subject to most favored nation treatment with respect to the terms provided to any other investors or creditors (including with respect to any warrants).
During the year ended December 31, 2014, $16.8 million of accounts payable owed to Cognate BioServices was settled for 4.2 million shares and 2.2 million warrants. The non-cash inducement charge was $16.0 million related to these transactions. The inducement charge was based upon the market price for tradable shares; however, the shares issued to Cognate were unregistered, restricted shares. In addition, upon issuance the shares were subject to a lock-up for 3 years as described below. The fair value amounts used to determine the inducement charge did not take account of any liquidity discounts relating to the restricted nature of the stock or the 3-year lock-up.
During the year ended December 31, 2013, $13.5 million of accounts payable owed to Cognate BioServices (including the July 31, 2013 transaction discussed above) was settled for 4.7 million shares and 2.4 million warrants. The inducement charge was $7.5 million related to these transactions. The inducement charge was based upon the market price for tradable shares; however, the shares issued to Cognate were unregistered, restricted shares. In addition, upon issuance the shares were subject to a lock-up for 3 years as described below. The fair value amounts used to determine the inducement charge did not take account of any liquidity discounts relating to the restricted nature of the stock or the 3-year lock-up.
The conversion shares are subject to a lock-up period of 36 months from the date of their issuance. Under the lock-up, the shares cannot be sold or traded. The fair value of the shares does not take account of any liquidity discounts related to the 36-month lock-up period as such liquidity discount.
Inducement expense is calculated based upon the difference between the carrying value of the accounts payable and the fair value of the restricted stock and warrants on the date such shares and warrants are issued (using the market price of unrestricted, tradable shares).
The Company classified the warrants as liabilities measured at fair value, and re-measured the instruments at fair value each reporting period.
Cognate BioServices’ cash purchase of common stock
On June 30, 2014, Cognate BioServices purchased 562,500 shares of common stock and 281,250 warrants from the Company for a cash purchase price of $2.3 million. The shares and warrants are subject to most favored nation treatment with respect to the terms (including in regards to warrants) provided to any other investors or creditors, including share issuances upon the exercise of previously issued derivative securities. The inducement charge was $2.4 million related to this transaction.
"Short swing" profit rules under Section 16(b)
The Company and Cognate BioServices, an affiliate of the Company, recently conducted a review of the Company securities held by Cognate BioServices, the issuances to date, and any actions or transactions involving Company securities held by Cognate BioServices. In the course of the review, the parties noted that Cognate BioServices awarded some shares of Company stock that were owned by Cognate BioServices to two of Cognate BioServices’ managers, as part of the two managers’ compensation (the “Equity Awards”). The parties further noted that these Equity Awards may be deemed to be “dispositions” of those Company shares for purposes of Section 16(b) of the Securities Exchange Act of 1934 (“Section 16(b)”). Since the Equity Awards were made within six months of the time that various issuances of Company stock were made by the Company to Cognate BioServices in payment of accounts payable, as previously disclosed, the Equity Awards may be deemed to have resulted in “short swing” profits under Section 16(b).
| F-19 |
Also in the course of the review, the parties noted that Cognate BioServices had entered into two convertible debt financings with unrelated third party investors, secured by Cognate BioServices assets, and provided the proceeds of the financings for NWBO’s programs. The debt was convertible, at the investors’ election, into shares of Company stock owned by Cognate BioServices. The third party investors elected to convert the debt and receive repayment in shares of Company stock rather than in cash. In the review, the Company and Cognate BioServices noted that the debt conversions related to these two financings may be deemed to be “dispositions” of those Company shares for purposes of Section 16(b). Since the debt conversions by the third party investors were made within six months of the time that various issuances of Company stock were made by the Company to Cognate BioServices in payment of accounts payable, as previously disclosed, the debt conversions may be deemed to have resulted in “short swing” profits under Section 16(b).
To resolve any uncertainty regarding whether these transactions constituted dispositions that were subject to Section 16(b), during the fourth quarter Cognate BioServices agreed to pay the Company $0.448 million, and that payment was effectuated.
Short term loans
During July, 2013, Cognate provided $1.2 million to the Company from short term loan proceeds. This short term loan was repaid during the third quarter of 2013.
b. Toucan Capital and Toucan Partners
In March 2013, the Company received a short-term loan of $0.2 million from Toucan. The loan was repaid during the second quarter of 2013.
c. Other Related Parties
In March 2013, the Company received a short-term loan of $0.2 million from an executive officer. The loan was repaid during the second quarter of 2013.
| 10. | Redeemable Common Stock |
In October and November 2012, the Company issued 1.9 million shares of redeemable common stock, at a purchase price of $4.80 per share to accredited investors (collectively, the “Investors”) in separate private placement transactions and as part of conversions of most of the Company’s debt into equity. Total cash received amounted to $5.3 million and total conversions of debt into redeemable common stock amounted to $4.6 million (out of more than $36 million of debt converted at that time). These transactions were completed pursuant to a Securities Purchase Agreement (the “Agreement”) which the Company entered into with each respective investor. The Agreements for the redeemable common stock provide that such Investors can redeem the securities for cash at a premium to the original issuance ranging from 15% to 25%. The redemption provisions occurred within 9 months from the date of issuance.
The Company first assessed the redeemable common stock to determine if the instrument should be accounted for as a liability in accordance with ASC 480. As the put option is optionally redeemable by the holder, the common stock was not required to be accounted for as a liability. Next, the Company assessed the put option within the redeemable common stock as a potential embedded derivative pursuant to the provisions of ASC 815, “Derivatives and Hedging” , and concluded that the put option did not meet the net settlement criteria within the definition of a derivative. Therefore, the Company has accounted for the common stock issued pursuant to the Agreement in accordance with ASC 480-10-S99-3A, “Classification and Measurement of Redeemable Securities” , which provides that securities that are optionally redeemable by the holder for cash or other assets are classified outside of permanent equity in temporary equity. The 1.9 million shares of common stock issued pursuant to the Agreement were recorded as redeemable common stock at an initial carrying value of $8.9 million. The Company elected to record the common stock at its redemption value of $11.0 million, as if it were redeemable immediately and accordingly recorded accretion of $2.0 million to redeemable securities expense during the year ended December 31, 2012.
During the third quarter of 2013, the Company entered into a series of extension agreements, with a counterparty owning 520,833 redeemable shares, to extend the redemption date to October 4, 2013. In connection with these extensions, the Company issued 72,825 warrants with an exercise price of $4.00 and a 5 year term. The fair value of the warrants was determined using the Black-Scholes model with the following assumptions: risk free interest rate - 1.72%, volatility - 98.04%, expected term - 5 years, expected dividends- N/A. The fair value of the warrants amounted to $0.2 million and was charged to inducement expense. The warrants issued resulted in stockholders’ equity classification in accordance with the guidance contained in ASC 815-40-15-7D, "Contracts in Entity's Own Equity".
During the third quarter of 2013, the Company entered into a series of extension agreements, with a counterparty owning 366,667 redeemable shares, to extend the redemption date to November 20, 2013. In connection with this extension, the Company issued 30,000 restricted shares at a fair value of $3.46 per share based on the publicly quoted price of the Company’s common stock and recorded a conversion inducement expense of approximately $0.1 million.
During the third quarter of 2013, the Company entered into a series of extension agreements, with a counterparty owning 398,920 redeemable shares, to extend the redemption date to January 31, 2014.
| F-20 |
During the quarter ended December 31, 2013, the Company issued an aggregate of 733,104 restricted shares of common stock in exchange for the extension of the redemption period. The fair value of the restricted shares was $3.68 per share based on the publicly quoted price of the Company’s common stock and the Company recorded a conversion inducement expense of approximately $2.7 million.
During the quarter ended December 31, 2013, the redemption provision on 20,850 redeemable shares with a carrying value of $0.1 million lapsed. Accordingly, the shares were reclassified to stockholders’ equity.
During the fourth quarter of 2013, the Company entered into a series of extension agreements, with a counterparty owning 520,833 redeemable shares, to extend the redemption dates to February 25, 2014. In connection with these extensions, the Company issued 68,750 restricted shares. If the Company’s stock price is above the redemption price, then the redemption provision no longer applies. The fair value of the restricted shares which amounted to $0.2 million and was charged to inducement expense.
During the fourth quarter of 2013, the Company redeemed 41,667 redeemable shares for $0.2 million. The common shares have not been cancelled as of December 31, 2014.
During the fourth quarter of 2013, the redemption provision on 383,315 redeemable shares with a carrying value of $1.9 million lapsed. Accordingly, the shares were reclassified to stockholders’ equity.
During the first quarter of 2014, the redemption provision on all 1.4 million redeemable shares outstanding as of December 31, 2013 lapsed, and $8.9 million was transferred from redeemable common stock to stockholders’ deficit.
| 11. | Stockholders’ Deficit |
Common Stock Issuances
First Quarter 2014
During the quarter ended March 31, 2014, the Company issued in aggregate 238,496 shares of common stock in exchange for consulting services for which performance was complete. The fair value of the common stock recognized was $1.6 million.
On January 17, 2014, in connection with the four Cognate BioServices Agreements, the Company issued one-time initiation payments of 5,101,366 shares of common stock, subject to most favored nation treatment. The common stock is vesting over thirty six months from the closing date. Stock-based compensation expense related to Cognate BioServices with respect to these shares was $8.7 million for the year ended December 31, 2014. See Note 5 for more information.
During the quarter ended March 31, 2014, the Company issued in aggregate 32,000 shares of common stock for cash. The fair value of the common stock recognized was $0.2 million.
During the quarter ended March 31, 2014, the Company converted accounts payable due to Cognate BioServices of approximately $5.9 million into 1,481,644 shares, subject to most favored nation treatment. The Company recorded $2.8 million of inducement expense associated with the issuance of the common shares. In addition, the Company issued warrants that were valued at $2.5 million at the date of issuance related to the conversion of accounts payable. Total inducement charge was $5.3 million.
During the quarter ended March 31, 2014, the Company converted notes and relevant accrued interest of $0.2 million into approximately 0.07 million shares of common stock.
During the quarter ended March 31, 2014, the Company issued an aggregate of 721,827 shares of common stock from the exercise of warrants previously issued. The Company received proceeds of approximately $2.7 million from the exercise of these warrants.
During the quarter ended March 31, 2014, 1,444,788 redeemable shares with a carrying value of $8.9 million were no longer redeemable and were reclassified to stockholders’ equity.
During the quarter ended March 31, 2014, the Company issued an aggregate of 41,310 shares of common stock from the cashless exercise of warrants previously issued.
During the quarter ended March 31, 2014, the Company issued 20,833 shares of common stock to an individual investor. However the cash proceeds of $0.1 million was received in 2012.
| F-21 |
Second Quarter 2014
On April 9, 2014, the Company entered into a Securities Purchase Agreement with a single institutional investor for the sale of 2,272,727 shares of common stock at a purchase price of $6.60 per share, for a total purchase price of $15.0 million. Additionally, for one year after the closing date, the investor had a non-transferable contractual over-allotment right to purchase up to 2,272,727 additional shares of common stock at a price per share of $7.50, for an additional subscription amount of up to $17.05 million. This over-allotment right was cancelled on October 9, 2014. See Note 12 for further explanation.
On June 30, 2014, the Company issued Cognate BioServices 562,500 shares of common stock and 281,250 warrants for proceeds of $2.3 million. The shares and warrants are subject to most favored nation treatment with respect to the terms (including in regards to warrants) provided to any other investors or creditors, including share issuances upon the exercise of previously issued derivative securities.
During the quarter ended June 30, 2014, the Company issued 200,000 shares of common stock to an individual investor at $7.00 per share. The total proceeds of $1.4 million were received by the Company during the first quarter in 2014, and were recorded as shares payable on the balance sheet as of March 31, 2014. The $1.4 million shares payable were re-classed to stockholders’ deficit during the second quarter in 2014.
During the quarter ended June 30, 2014, the Company issued 58,614 shares of common stock for cash to an individual investor for proceeds of $435,540.
During the quarter ended June 30, 2014, the Company issued 16,200 shares of common stock for $125,550 of cash.
During the quarter ended June 30, 2014, the Company issued an aggregate of 92,100 shares of common stock from the exercise of warrants previously issued. The Company received proceeds of $394,925 from the exercise of these warrants.
During the quarter ended June 30, 2014, the Company issued an aggregate of 12,533 shares of common stock from the cashless exercise of warrants previously issued.
During the quarter ended June 30, 2014, the Company issued an aggregate of 24,924 shares of common stock in exchange for consulting services. The fair value of the common stock recognized was $155,607.
During the quarter ended June 30, 2014, the Company converted accounts payable due to Cognate BioServices of approximately $2.9 million into 727,291 shares of common stock and 363,646 warrants, subject to most favored nation treatment. The Company recorded $1.4 million of inducement expense associated with the issuance of the common shares. In addition, as noted in Note 4 the Company issued warrants that were valued at $1.1 million at the date of issuance related to the conversion of accounts payable. Total inducement charge was $2.5 million.
Third Quarter 2014
During the quarter ended September 30, 2014, the Company entered into a Securities Purchase Agreement with an individual investor for the sale of 435,202 shares of restricted common stock at purchase price of $5.17 per share and 448,207 shares of restricted common stock at a purchase price of $5.02 per share, for aggregate proceeds of $4.5 million. Additionally, the Company also agreed to reset the expiration date of the investor’s 1,398,625 warrants to September 15, 2018. The modification of the warrants in connection with this transaction was accounted for as a component of equity.
During the quarter ended September 30, 2014, the Company issued an aggregate of 497,133 shares of common stock from the exercise of warrants previously issued. The Company received proceeds of $1.7 million from the exercise of these warrants.
During the quarter ended September 30, 2014, the Company converted accounts payable due to Cognate BioServices of approximately $7.9 million into 1,986,205 shares of common stock and 1.1 million warrants, subject to most favored nation treatment. The Company recorded $4.5 million of inducement expense associated with the issuance of the common shares. In addition, as noted in Note 4, the warrants were valued at $3.8 million at the date of issuance, resulting in a total inducement charge for the quarter of $8.3 million.
During the quarter ended September 30, 2014, the Company issued an aggregate of 14,519 shares of common stock in exchange for consulting services. The fair value of the common stock recognized was $94,999.
On August 12, 2014, the Company issued 3,013 common shares at a purchase price of $5.60 per share and 1,507 warrants to an individual investor. The proceeds of $15,000 were received by the Company in August 2012. Therefore the Company made a reclassification between additional paid-in-capital and common stock par value during the quarter ended September 30, 2014.
On September 19, 2014, the Company issued 49,107 shares of common stock at purchase price of $5.60 per share and 14,732 warrants to an individual investor. The proceeds of $275,000 were received by the Company in August 2012. Therefore the Company made a reclassification between additional paid-in-capital and common stock par value during the quarter ended September 30, 2014.
During the quarter ended September 30, 2014, the Company issued 52,120 shares of common stock to multiple investors. However the cash proceeds of $0.3 million was received in 2012.
| F-22 |
Fourth Quarter 2014
On October 6, 2014, the Company entered into a Stock Purchase, Amendment and Issuance Agreement (the “Agreement”) with an existing single institutional investor for the sale of 2,272,727 shares of common stock at a purchase price of $5.05 per share, for a total purchase price of about $11.5 million. In the Agreement, the Company terminated the investor’s existing contractual over-allotment purchase right to purchase 2,272,727 shares of our common stock for $7.50 per share at any time prior to April 14, 2015, and agreed to issue the purchaser a warrant to purchase up 2,735,000 shares at an exercise price of $5.15 per share, exercisable commencing six months after issuance and with an exercise period of 30 months.
During the quarter ended December 31, 2014, the Company issued 109,868 shares of common stock to an individual investor as settlement of redemption of redeemable securities. The total settlement expense was $0.4 million and was recorded as inducement expense.
On November 17, 2014, the Company entered into a private offering of $25 million to purchase 4,317,789 shares of common stock of the Company, at a price of $5.79 per share (the closing price of the stock on November 14, 2014, the trading day prior to the sale of shares). The shares were purchased by C.F. Woodford Equity Income Fund of the UK. The Company agreed to file a registration statement within two weeks after the closing, and to use best efforts to complete the registration within sixty days thereafter. There were no warrants, overallotment rights, pre-emptive rights or other securities or rights entitling the investor to purchase or obtain additional shares.
During the quarter ended December 31, 2014, the Company entered into a service agreement with an agent for services provided on the UK mortgages. On November 19, 2014, the Company issued total 38,400 shares of common stock at fair value of $5.15 per share to the agent and related finders. Total amount of $197,376 was recorded as deferred financing cost and amortized though the Mortgage’s life.
First Quarter 2013
During the quarter ended March 31, 2013, the Company issued 235,593 shares of common stock to existing stockholders in connection with an agreement with the stockholders and a consultant providing for advisory services. The shares of common stock were valued at the closing price of the Company’s common stock on the date issued and amounted to approximately $0.9 million.
For the quarter ended March 31, 2013, the Company converted $0.9 million of notes payable into approximately 359,000 shares. The fair value of the common stock on the date of these transactions was approximately $2.60 per share.
Second Quarter 2013
During the quarter ended June 30, 2013, the Company issued a total of 178,504 shares of common stock to non-employees in exchange for services. The shares were fully vested and non-forfeitable on the date of issue and were therefore recorded as a charge to operations at their grant date fair value of $0.6 million.
In April 2013, the Company entered into an agreement with an institutional investor for $1.0 million in exchange for 281,690 shares of common stock at the closing market price of $3.55 per share.
In April 2013, the Company entered into an agreement with one healthcare-dedicated institutional investor for a registered direct placement of $10.0 million of common stock at $3.90 per share. The Company issued to the investor 2,564,103 shares of common stock and 1,025,641 common stock purchase warrants. The warrants have an exercise price of $4.29 per share and are exercisable beginning six months after closing, with a term of five years. The Company incurred offering costs amounting to $0.8 million in connection with this financing transaction. The warrants issued resulted in stockholders’ equity classification in accordance with the guidance contained in ASC 815-40-15-7D, "Contracts in Entity's Own Equity".
During the quarter ended June 30, 2013, the Company converted $0.9 million of notes payable into approximately 316,000 shares. The fair value of the common stock on the date of these transactions was approximately $2.90 per share.
During the quarter ended June 30, 2013, the Company issued an aggregate of 168,354 shares of common stock from the cashless exercise of warrants. The Company did not receive any proceeds from this cashless exercise.
Third Quarter 2013
During the quarter ended September 30, 2013, the Company issued an aggregate of 14,326 shares of common stock to non-employees in exchange for services. The shares were fully vested and non-forfeitable on their dates of issue and were therefore recorded as a charge to operations at their grant date fair values. The aggregate grant date fair value of these shares amounted to approximately $0.1 million.
During the third quarter of 2013, the Company entered into a series of extension agreements, with a counterparty owning 366,667 redeemable shares, to extend the redemption date to November 20, 2013. In connection with this extension, the Company issued 30,000 restricted shares at a fair value of $3.46 per share based on the publicly quoted price of the Company’s common stock and recorded a conversion inducement expense of approximately $0.1 million.
| F-23 |
On August 8, 2013, the Company entered into a securities purchase agreement with institutional investors for the sale of an aggregate of $15.0 million of units in a registered direct offering. Each unit consists of one share of common stock, warrant to purchase 0.25 of a share of common stock and one over-allotment warrant to purchase 0.25 of a share of common stock. The Company issued to the investors 4,477,612 shares of common stock and long-term warrants exercisable for 1,119,403 shares of common stock, with an exercise price of $4.00 per share, exercisable six months after closing with a term of five years after they are first exercisable. The Company also issued to the investor’s over-allotment warrants exercisable for 1,119,403 shares of common stock, with an exercise price of $3.35 per share and exercisable immediately with a term of one year. The Company incurred offering costs amounting to $1.2 million in connection with this financing transaction. The warrants issued resulted in stockholders’ equity classification in accordance with the guidance contained in ASC 815-40-15-7D, "Contracts in Entity's Own Equity".
During the third quarter ended September 30, 2013, the Company converted $10.3 million of accounts payable to Cognate BioServices into approximately 2,902,072 shares and fifty percent warrant coverage, subject to most favored nation treatment. The fair value of the common stock on the date of these transactions was approximately $3.55 per share.
During the third quarter ended September 30, 2013, the Company converted $0.5 million of notes payable into approximately 184,600 shares. The fair value of the common stock on the date of these transactions was approximately $2.60 per share. The Company also issued 164,155 shares for notes payable that were converted in 2012.
Fourth Quarter 2013
During the quarter ended December 31, 2013 the Company issued in aggregate 141,330 shares of common stock in exchange for consulting services. The fair value of the common stock on the date of this transaction was approximately $0.7 million.
During the quarter ended December 31, 2013, the Company issued an aggregate of 733,104 restricted shares of common stock in exchange for the extension of the redemption period. The fair value of the restricted shares was $3.68 per share based on the publicly quoted price of the Company’s common stock and the Company recorded a conversion inducement expense of approximately $2.7 million.
During the fourth quarter of 2013, the Company redeemed 41,667 redeemable shares for $0.2 million. The common shares have not been cancelled as of December 31, 2014.
During the fourth quarter of 2013, 383,315 redeemable shares with a carrying value of $1.9 million were no longer redeemable and were reclassed to stockholders’ deficit.
During the quarter ended December 31, 2013, the Company issued an aggregate of 179,094 shares of common stock from the exercise of warrants previously issued. The Company received proceeds of approximately $0.6 million from the exercise of these warrants.
In November 2013, the Company entered into an underwriting agreement for a registered direct placement of $27.0 million of common stock at the closing market price of $4.80 per share. The Company issued 5,630,208 shares of common stock at $4.80 per share for gross proceeds of approximately $27.0 million. In connection with the offering, the Company issued warrants exercisable for 2,815,104 shares of common stock. The warrants have an exercise price of $6.00 per share and are exercisable beginning six months after closing, with a term of five years. The Company incurred offering costs amounting to $2.0 million in connection with this financing transaction. Net proceeds to the Company amounted to $25.0 million. The warrants have a five year term and the original exercise price was $6.00. The warrants contain “down round protection.” Accordingly, the Company allocated approximately $5.6 million of the proceeds received in this transaction to the warrant instruments, which have been recorded as a liability stated at fair value and. This liability is subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in the Company's statements of operations. The fair value of the warrants issued by the Company in connection with this transaction have been estimated using a Monte Carlo simulation (Note 4).
During the quarter ended December 31, 2013, the Company converted accounts payable to Cognate BioServices into approximately 1,818,000 shares, with fifty percent warrant coverage, subject to most favored nation treatment (including with respect to warrants). The Company also converted notes payable to Cognate into 150,000 shares. The fair value of the common stock on the date of these transactions was approximately $6.7 million and $0.5 million, respectively. The Company recorded $1.5 million of inducement expense related to the conversion of accounts payable.
During the quarter ended December 31, 2013, the Company converted $0.5 million of notes payable into 149,732 shares of common stock.
| F-24 |
c. Stock Purchase Warrants
Stock Purchase Warrants
The following is a summary of warrant activity for the year ended December 31, 2013 and December 31, 2014:
| Number of | Weighted Average | |||||||
| Warrants | Exercise Price | |||||||
| Outstanding as of December 31, 2012 | 12,086 | $ | 6.18 | |||||
| January 2013, warrants issued in exchange for services | 109 | 6.40 | ||||||
| Expired in first quarter of 2013 | (18 | ) | 15.45 | |||||
| Outstanding as of March 31, 2013 | 12,177 | 6.82 | ||||||
| April 2013, warrants issued in connection with registered direct offering | 1,026 | 4.29 | ||||||
| April 2013, warrants issued to placement agent in connection with registered direct offering | 128 | 4.29 | ||||||
| Exercised on a cashless basis in second quarter of 2013 | (168 | ) | 5.60 | |||||
| Expired in second quarter of 2013 | (18 | ) | 12.00 | |||||
| Outstanding as of June 30, 2013 | 13,145 | 6.78 | ||||||
| July 2013, warrants issued in connection with conversion of Cognate accounts payable | 2,116 | 4.00 | ||||||
| August 2013, warrants issued in connection with registered direct offering | 2,239 | 4.00 | ||||||
| September 2013, warrants issued for extension of redeemable securities | 73 | 4.00 | ||||||
| Expired in third quarter of 2013 | (19 | ) | 12.00 | |||||
| Outstanding as of September 30, 2013 | 17,554 | 6.07 | ||||||
| Warrants issued for service | 74 | 5.00 | ||||||
| October 2013, warrants issued in connection with registered direct offering* | 2,815 | 6.00 | ||||||
| September 2013, warrants issued for extension of redeemable securities | 53 | 6.40 | ||||||
| December 2013, warrants issued in connection with conversion of Cognate accounts payable* | 240 | 4.00 | ||||||
| Expired in fourth quarter of 2013 | (441 | ) | 11.94 | |||||
| Exercised in fourth quarter of 2013 | (179 | ) | 3.35 | |||||
| Outstanding as of December 31, 2013 | 20,116 | 5.23 | ||||||
| Warrants issued in connection with conversion of Cognate accounts payable* | 741 | 4.00 | ||||||
| Warrants issued in exchange for services* | 2,434 | 4.00 | ||||||
| Warrants issued in connection with common stock issued | 150 | 5.00 | ||||||
| Warrants exercised on a cashless basis | (73 | ) | - | |||||
| Warrants exercised for cash | (722 | ) | 3.66 | |||||
| Expired in first quarter of 2014 | (6 | ) | 9.54 | |||||
| Outstanding as of March 31, 2014 | 22,640 | 5.12 | ||||||
| Warrants issued in connection with conversion of Cognate accounts payable* | 364 | 4.00 | ||||||
| Warrants issued to Cognate in connection with common stock issued for cash* | 281 | 4.00 | ||||||
| Warrants exercised for cash | (90 | ) | 4.25 | |||||
| Over-allotment rights issued in connection with registered direct offering | 2,273 | 7.50 | ||||||
| Warrants issued to placement agent in connection with registered direct offering | 113 | 8.25 | ||||||
| Outstanding as of June 30, 2014 | 25,581 | 5.32 | ||||||
| Warrants issued in connection with conversion of Cognate accounts payable* | 1,121 | 4.00 | ||||||
| Warrants issued to Cognate for services* | 2,326 | 4.00 | ||||||
| Warrants issued for services | 17 | 6.33 | ||||||
| Warrants exercised for cash | (497 | ) | 3.48 | |||||
| Expired in third quarter of 2014 | (1 | ) | 7.63 | |||||
| Adjustment related to prior issued warrants | 415 | 6.44 | ||||||
| Outstanding as of September 30, 2014 ** | 28,962 | 4.90 | ||||||
| Warrants issued in connection with registered direct offering | 2,735 | 5.15 | ||||||
| Warrants canceled | (2,273 | ) | 7.50 | |||||
| Warrants adjustment for prior period | 4 | 5.60 | ||||||
| Expired and canceled in fourth quarter 2014 | (43 | ) | 8.14 | |||||
| Outstanding as of December 31, 2014 ** | 29,385 | $ | 4.72 | |||||
*The warrants contain “down round protection” and the Company classifies these warrant instruments as liabilities at their fair value and adjusts the instruments to fair value at each reporting period.
** Approximately 14,261,000 warrants issued to Cognate BioServices, during the 7 years 2008 through 2014, with a weighted average exercise price and remaining contractual term of $4.00 and 3.8 years, respectively. The weighted average exercise price in part gives effect to adjustments related to the most favored nation clause that occurred during the period, based upon terms provided by the Company to other unrelated investors and creditors.
| F-25 |
| 12. | Commitments and Contingencies |
On July 31, 2012, the Company entered into a non-cancelable operating lease for 7,097 square feet of office space in Bethesda, Maryland, which expires in March 2018. Rent expense for 2014 and 2013 amounted to $0.3 million and $0.2 million, respectively.
On October 28, 2013, the Company entered into a non-cancelable operating lease for 4,251 square feet of office space in Germany, which expires in December 2017.
The Company’s future minimum lease payments are as follows as of December 31, 2014 (in thousands):
| Office Leases | |||||||||
| Germany | U.S. | ||||||||
| 2015 | $ | 74 | $ | 300 | |||||
| 2016 | 74 | 309 | |||||||
| 2017 | 74 | 318 | |||||||
| Thereafter | 74 | 81 | |||||||
| Total | $ | 296 | $ | 1,008 | |||||
Shareholder Books and Records Demands
The Company has received demand letters from three purported individual shareholders seeking to inspect our corporate books and records pursuant to Section 220 of the Delaware General Corporation Law. The demand letters are all substantially the same, and claim that their purpose is to investigate possible mismanagement and breaches of fiduciary duty by our directors and officers. They request a range of documents, including books and records relating to agreements and interactions with related parties, stock ownership, and the independence of our Board members. One of the individual shareholders filed a complaint in the Delaware Court of Chancery seeking to enforce her books and records demand. The parties reached a negotiated settlement about limited records to be provided, under a confidentiality agreement. Discussions with counsel for the other two individual demanding shareholders are ongoing.
| 13. | Income Taxes |
There was no income tax benefit attributable to net losses for 2014 and 2013. The difference between actual tax provisions and taxes computed by applying the corporate rate of 40% in 2014 and 2013, respectively, is primarily the result of establishing a valuation allowance on the Company’s deferred tax assets arising primarily from tax loss carry forwards.
The tax effects of temporary differences and tax loss and credit carry forwards that give rise to significant portions of deferred tax assets and liabilities at December 31, 2014 and 2013 are comprised of the following (in thousands):
| As of December 31, 2014 | As of December 31, 2013 | |||||||
| Deferred tax asset | ||||||||
| Net operating loss carryforward | $ | 133,684 | $ | 99,058 | ||||
| Research and development credit carry forwards | 9,911 | $ | 4,048 | |||||
| Stock based compensation and other | 26,510 | 9,094 | ||||||
| Total deferred tax assets | 170,105 | 112,200 | ||||||
| Valuation Allowance | (170,105 | ) | (112,200 | ) | ||||
| Deferred tax asset, net of allowance | $ | - | $ | - | ||||
At December 31, 2014, the Company had net operating loss carry forwards for income tax purposes of approximately $339 million and unused research and development tax credits of approximately $9.9 million available to offset future taxable income and income taxes, respectively, expiring in 2018 through 2034. The Company has not performed a detailed analysis to determine whether an ownership change under Section 382 of the IRC has occurred. The effect of an ownership change would be the imposition of an annual limitation on the use of net operating loss carryforwards attributable to periods before the change. Any limitation may result in expiration of a portion of the NOL or research and development credit carryforwards before utilization. The tax years 2010 through 2014 remain open to examination by federal agencies and other jurisdictions in which the Company operates.
During 2014 the company reevaluated the pricing/deductibility of stock options granted and the value of warrants issued, resulting in the increase in the potential future tax deduction from those instruments.
During 2014 and 2013, the company has adjusted/reevaluated its R&D credit calculations, resulting in the increase in the possible future credit against tax.
| F-26 |
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the period in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and taxing strategies in making this assessment. In case the deferred tax assets will not be realized in future periods, the Company has provided a valuation allowance for the full amount of the deferred tax assets at December 31, 2014 and 2013.
The expected tax expense (benefit) based on the U.S. federal statutory rate is reconciled with actual tax expense (benefit) as follows:
(dollars in thousands)
| For year ended December 31, 2014 | For year ended December 31, 2013 | |||||||
| Statutory federal income tax rate | 34.0 | % | 34.0 | % | ||||
| State taxes, net of federal tax benefit | 5.4 | % | 5.4 | % | ||||
| Other, net | -0.4 | % | -0.7 | % | ||||
| Research and development credits | 4.4 | % | 2.6 | % | ||||
| Change in valuation allowance | -43.4 | % | -41.3 | % | ||||
| Income tax provision (benefit) | 0.0 | % | 0.0 | % | ||||
| For year ended December 31, 2014 | For year ended December 31, 2013 | |||||||
| Federal | ||||||||
| Current | $ | - | $ | - | ||||
| Deferred | 49,912 | 21,189 | ||||||
| State | ||||||||
| Current | - | - | ||||||
| Deferred | 7,993 | 3,393 | ||||||
| Change in valuation allowance | (57,905 | ) | (24,582 | ) | ||||
| Income tax provision (benefit) | $ | - | $ | - | ||||
| 14. | Subsequent Events |
On February 3, 2015, the Company entered into a bridge loan agreement (“the Mortgage”) with Lancashire Mortgage Corporation Limited in UK for approximately $4.9 million. The Mortgage has a 18 month term with a 12% annual interest rate.
| F-27 |
