Attached files
Exhibit 10.61
Confidential Materials Omitted and Filed Separately with the Securities and Exchange Commission Pursuant to a Request for Confidential Treatment under Rule 24b-2 under the Exchange Act of 1934, as amended. Confidential Portions are marked: [***]
SIGN AND RETURN ALL PAGES TO CIRM SP3A-07552
NOTICE OF GRANT AWARD – RFA 13-03A: CIRM Strategic Partnership III Track A Awards
California Institute for Regenerative Medicine
Issue Date: October 16, 2015
|
Grant Number:
|
SP3A-07552
|
Project Period Start:
|
10/01/2014
|
|
Grantee Name:
|
Asterias Biotherapeutics
|
Project Period End:
|
09/30/2018
|
|
Grantee ID:
|
PR-Y0035A-SF
|
||
|
Principal Investigator:
|
Jane Stephanie Lebkowski
|
Total Award Amount:
|
$14,323,318
|
|
Project Title:
|
A Phase I/IIa Dose Escalation Safety Study of AST-OPC1 in Patients with Cervical Sensorimotor Complete Spinal Cord Injury
|
||
|
Authorized Organizational Official and Address:
|
Official and Address to Receive Payments:
|
|
|
Katharine E. Spink
Chief Operating Officer
230 Constitution Drive
Menlo Park, CA 94025
|
Katharine E. Spink, Ph.D.
230 Constitution Drive
Menlo Park, CA 94025
|
The California Institute for Regenerative Medicine (CIRM) hereby awards a grant in the amount of $14,323,318 to be disbursed over a total period of 4 years to Asterias Biotherapeutics (Grantee ID: PR-Y0035A-SF) in support of the above referenced project. This award is pursuant to the California Stem Cell Research and Cures Act (Health and Safety Code section 125290.10 et. seq.) and is subject to terms and conditions referenced below. (Capitalized terms are defined in the CIRM Grants Administration Policy for For-Profit Organizations (GAP), a copy of which may be found on the CIRM website at: http://www.cirm.ca.gov/our-funding/chapter-5-grants-administration-policies
In accepting this Grant, the Grantee warrants to CIRM that any funds expended under the Award will be for the purposes set forth in the approved Application and agrees to comply with all applicable CIRM regulations and standards.
To accept this Grant, the Principal Investigator and Authorized Organizational Official must sign and return this Notice of Grant Award (NGA) to CIRM within 45 days of the issue date. Payment will be issued only after the fully signed NGA is received by CIRM. Grant funds will be sent to the organization’s address listed above under Official and Address to Receive Payments unless an updated address is provided in the box below. If the Applicant cannot accept the award, including the legal obligation to perform in accordance with the provisions of this NGA, it should notify CIRM immediately.
If you have any questions about this award, please contact the CIRM staff referenced on page 3.
|
Ellen Feigal, M.D.
Senior Vice President, Research & Development
California Institute for Regenerative Medicine
|
Updated Address to Receive Payments:
|
AWARD ACCEPTANCE: The Principal Investigator and Authorized Organizational Official must sign below and return the entire NGA to CIRM to accept the Grant award.
|
Principal Investigator
|
Authorized Organizational Official
|
|
|
Name
|
Jane Stephanie Lebkowski
|
Katharine E. Spink
|
|
Signature
|
/s/ Jane Stephanie Lebkowski
|
/s/ Katharine E. Spink
|
|
Date
|
October 16, 2014
|
October 16, 2014
|
Page 1 of 12
SIGN AND RETURN ALL PAGES TO CIRM SP3A-07552
TERMS AND CONDITIONS:
|
A.
|
This award is based on the application submitted to CIRM, and as approved by the Independent Citizens' Oversight Committee (ICOC) on the above-titled project and is subject to the terms and conditions incorporated either directly or by reference in the following:
|
| 1. | The California Stem Cell Research and Cures Act (Health and Safety Code Section 125290.10 et. seq.) and regulations adopted by the ICOC. |
| 2. | The CIRM Grants Administration Policy for Academic and Non-Profit Institutions (Title 17, California Code of Regulations, Section 100500), CIRM Intellectual Property Policy and Revenue Sharing Requirements for Non-Profit and For-Profit Grantees (Title 17, California Code of Regulations. Sections 100600-100611), the CIRM Medical and Ethical Standards Regulations (Title 17, California Code of Regulations, Sections 100010-1000120), and any subsequently adopted applicable regulations. |
| 3. | The terms and requirements detailed in RFA 13-03A: CIRM Strategic Partnership III Track A Awards. |
| 4. | The Progress Milestones and Go/No Go Milestones set out in Appendix A to this NGA. |
| 5. | Budget detail for the Principal Investigator set out below. |
| B. | CIRM-funded animal work to be carried out by Asterias Biotherapeutics, which requires IACUC protocol approval, cannot commence until such IACUC approval is provided to CIRM. |
| C. | CIRM-funded human subjects work to be carried out under this Award, which requires IRB protocol approval, cannot commence until such IRB approval is provided to CIRM. |
| D. | If CIRM determines, in its sole discretion, that Grantee has not satisfied a Progress Milestone, CIRM may suspend disbursements until such time as Grantee satisfies the Progress Milestone. Upon suspending disbursements, CIRM may convene its progress Evaluation Committee and may seek input from Grantee in order to evaluate the circumstances of the delay, including, but not limited to, its cause, impact and any mitigating factors; provided, however, that CIRM may permanently cease disbursements if Grantee does not satisfy the Progress Milestone within four (4) months of the date that the Progress Milestone was scheduled to have been satisfied, or if the delay is not addressed to CIRM's satisfaction, as determined by CIRM in its sole discretion. |
| E. | CIRM may suspend or permanently cease disbursements if CIRM determines, in its sole discretion, that the Grantee has not satisfied a Go/No Go Milestone (as defined in Appendix A to this NGA). |
| F. | Of the total award amount of $14,323,318, up to $6,737,865 is available for process development. The disbursement of these funds shall be contingent upon the negotiation of Progress and Go/No Go Milestones and a payment schedule satisfactory to CIRM. Upon CIRM’s approval of Progress and Go/No Go Milestones and a payment schedule, CIRM shall make disbursements based on the approved payment schedule. As set forth in Paragraphs C and D, CIRM reserves the right to suspend or terminate disbursements if CIRM determines, in its sole discretion, that Grantee has failed to satisfy a Progress or Go/No Go Milestone relating to process development. |
| G. | Grantee shall conduct CIRM-funded work only on the basis of the milestones and/or success criteria outlined in Appendix A that have been agreed upon by Grantee and CIRM. Throughout the progress of the grant, Grantee and CIRM may agree upon new or modified milestones, success criteria, or other funding requirements (identified as “Modified Requirements”). Such Modified Requirements shall be agreed upon in writing between Grantee and CIRM and will be incorporated by reference to the milestones document set forth in Appendix A. |
| H. | In the event that Grantee elects to terminate the award or the clinical trial or other work to be funded by the award, for reasons other than the Grantee’s failure to meet a Progress or Go/No Go Milestone and/or a decision by the Food and Drug Administration to place a clinical hold on the trial, Grantee shall reimburse CIRM for the funds received by Grantee from CIRM under the award, with interest at a rate per annum equal to the one year LIBOR rate (in effect as of the last business day of the immediately preceding calendar month) plus 1%, from the date of receipt of each disbursement to the date of repayment. |
Page 2 of 12
| I. | At least one month prior to each Q1 disbursement date (see Reporting Schedule below), Grantee will demonstrate to CIRM’s reasonable but sole satisfaction that Grantee has funds available, either in the form of cash, cash equivalents or securities listed on an automated quotation system which have no resale restrictions under federal or other applicable securities laws, sufficient to fund all of Grantee’s budgeted expenses (excluding depreciation, amortization, and other GAAP expenses which do not affect cash levels, but including overhead and administrative expenses, taxes, and the costs and expenses detailed in the Notice of Grant Award), less the costs budgeted to be covered by planned Grant disbursements, which are anticipated by Grantee to be incurred by Grantee in connection with the CIRM-Funded Project and its business operations over the next twelve (12) months, as demonstrated by Grantee’s submission of its most recent budget forecasts and other financial information reasonably requested by CIRM. CIRM reserves the right to suspend or terminate disbursements if CIRM determines, in its sole discretion, that Grantee has failed to satisfy this financial requirement. |
| J. | CIRM has the right to attend key FDA meetings regarding the funded project, including but not limited to any clinical milestone meeting, or clinical hold meeting (FDA Meetings). CIRM also has the right to review any data package(s) or other information, including confidential and/or proprietary information, provided by Grantee to the FDA in connection with such FDA Meetings, as well as any FDA Meeting minutes, and to share such information with CIRM’s confidential advisers. To facilitate CIRM’s participation in FDA Meetings, Grantee shall notify CIRM as soon as practicable after it has scheduled an FDA Meeting, and shall, upon request, provide CIRM a copy of any data package or other information it intends to provide or has provided to the FDA, as well as any FDA Meeting minutes. |
| K. | Grantee shall comply with the terms of the Communication Plan, attached hereto as Appendix B. |
| L. | If CIRM determines, in its sole discretion, that Grantee has not complied with the terms and conditions of this award, CIRM may suspend or permanently cease Disbursements, or pursue other remedies as allowed by law. |
| M. | The timing of the distribution of funds pursuant to this Grant shall be contingent upon the availability of funds in the California Stem Cell Research and Cures Fund in the State Treasury, as determined by CIRM in its sole discretion. |
Please check the following website for updated policy documents: http://www.cirm.ca.gov/cirm-operations/Regulations
AWARD DETAIL (U.S. Dollars):
|
Year 1
|
Year 2
|
Year 3
|
Year 4
|
|||||||||||||
|
Direct Project Costs
|
||||||||||||||||
|
Personnel
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
||||
|
Travel
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
||||
|
Supplies
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
||||
|
Equipment
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
||||
|
Consultants/Subcontracts
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
||||
|
Total Direct Project Costs
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
||||
|
Facilities Costs
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
||||
|
Indirect Costs
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
$
|
[***]
|
|
||||
|
TOTAL BUDGET
|
$
|
4,211,863
|
$
|
4,543,518
|
$
|
4,136,541
|
$
|
1,431,103
|
||||||||
QUARTERLY INSTALLMENTS ON GRANT PAYMENTS
Payments will be made in quarterly installments, issued at the beginning of each quarter. Quarters will be tied to the project start date. The Year 1 Budget represents a 15 month period and hence the 1st disbursement issued upon execution of the agreement represents 6/15ths of the Year 1 Budget to cover pre-award costs and the 1st 3 months of the project period.
*Any interest accrued by the Grantee from the Grant payments must be used for the CIRM Strategic Partnership III Track A Awards.
Page 3 of 12
Disbursement Schedule:
|
Payment #:
|
Type
|
Schedule Date
|
Amount
|
|||
|
1
|
Pre-Award and Year 1 Q1
|
~10/15/2014
|
$
|
916,554
|
||
|
2
|
Year 1 Q2
|
1/1/2015
|
$
|
458,277
|
||
|
3
|
Year 1 Q3
|
4/1/2015
|
$
|
458,277
|
||
|
4
|
Year 1 Q4
|
7/1/2015
|
$
|
458,277
|
||
|
5
|
Year 2 Q1
|
10/1/2015
|
$
|
541,012
|
||
|
6
|
Year 2 Q2
|
1/1/2016
|
$
|
541,012
|
||
|
7
|
Year 2 Q3
|
4/1/2016
|
$
|
541,012
|
||
|
8
|
Year 2 Q4
|
7/1/2016
|
$
|
541,012
|
||
|
9
|
Year 3 Q1
|
10/1/2016
|
$
|
489,786
|
||
|
10
|
Year 3 Q2
|
1/1/2017
|
$
|
489,786
|
||
|
11
|
Year 3 Q3
|
4/1/2017
|
$
|
489,786
|
||
|
12
|
Year 3 Q4
|
7/1/2017
|
$
|
489,786
|
||
|
13
|
Year 4 Q1
|
10/1/2017
|
$
|
292,719
|
||
|
14
|
Year 4 Q2
|
1/1/2018
|
$
|
292,719
|
||
|
15
|
Year 4 Q3
|
4/1/2018
|
$
|
292,719
|
||
|
16
|
Year 4 Q4
|
7/1/2018
|
$
|
292,719
|
||
These disbursements represent the funding for the clinical trial. Disbursements for the process development activities will be determined upon final negotiation of milestones for those activities as stated in condition F above.
PROGRESS & FINANCIAL REPORTS SCHEDULE
|
Requirement Type
|
Due Date
|
Notes
|
|
Progress Report
|
01/01/2015
|
Year 1 Q1
|
|
Financial Report
|
02/01/2015
|
Year 1 Q1
|
|
Progress Report
|
04/01/2015
|
Year 1 Q2
|
|
Financial Report
|
05/01/2015
|
Year 1 Q2
|
|
Progress Report
|
07/01/2015
|
Year 1 Q3
|
|
Financial Report
|
08/01/2015
|
Year 1 Q3
|
|
Financial Milestone Check
|
09/01/2015
|
Year 1
|
|
Annual Progress Report
|
10/01/2015
|
Year 1
|
|
Financial Report
|
01/01/2016
|
Year 1
|
|
Progress Report
|
01/01/2016
|
Year 2 Q1
|
|
Financial Report
|
02/01/2016
|
Year 2 Q1
|
|
Progress Report
|
04/01/2016
|
Year 2 Q2
|
|
Financial Report
|
05/01/2016
|
Year 2 Q2
|
|
Progress Report
|
07/01/2016
|
Year 2 Q3
|
|
Financial Report
|
08/01/2016
|
Year 2 Q3
|
|
Financial Milestone Check
|
09/01/2016
|
Year 2
|
|
Annual Progress Report
|
10/01/2016
|
Year 2
|
|
Financial Report
|
01/01/2017
|
Year 2
|
|
Progress Report
|
01/01/2017
|
Year 3 Q1
|
|
Financial Report
|
02/01/2017
|
Year 3 Q1
|
|
Progress Report
|
04/01/2017
|
Year 3 Q2
|
|
Financial Report
|
05/01/2017
|
Year 3 Q2
|
Page 4 of 12
|
Progress Report
|
07/01/2017
|
Year 3 Q3
|
|
Financial Report
|
08/01/2017
|
Year 3 Q3
|
|
Financial Milestone Check
|
09/01/2017
|
Year 3
|
|
Annual Progress Report
|
10/01/2017
|
Year 3
|
|
Financial Report
|
01/01/2018
|
Year 3
|
|
Progress Report
|
01/01/2018
|
Year 4 Q1
|
|
Financial Report
|
02/01/2018
|
Year 4 Q1
|
|
Progress Report
|
04/01/2018
|
Year 4 Q2
|
|
Financial Report
|
05/01/2018
|
Year 4 Q2
|
|
Progress Report
|
07/01/2018
|
Year 4 Q3
|
|
Financial Report
|
08/01/2018
|
Year 4 Q3
|
|
Annual Progress Report
|
10/01/2018
|
Year 4
|
|
Financial Report
|
01/01/2019
|
Year 4
|
CIRM CONTACTS:
Gabriel Thompson, Grants Management Officer
Phone: 415-396-9274 Email: gthompson@cirm.ca.gov Fax: (415) 396-9141
Kevin Whittlesey, Science Officer
Phone: 415-396-9311 Email: kwhittlesey@cirm.ca.gov Fax: (415) 396-9141
The CIRM home page is at http://www.cirm.ca.gov
CIRM Mailing Address:
California Institute for Regenerative Medicine
Attn: Gabriel Thompson, Grants Management Officer
210 King Street
San Francisco, CA 94107
Page 5 of 12
APPENDIX A - Confidential SP3A-07552
NOTICE OF GRANT AWARD – RFA 13-03A: CIRM Strategic Partnership III Track A Awards
California Institute for Regenerative Medicine
|
Grant Number:
|
SP3A-07552
|
Project Period Start:
|
10/01/2014
|
|
Grantee Name:
|
Asterias Biotherapeutics
|
Project Period End:
|
09/30/2017
|
|
Grantee ID:
|
PR-Y0035A-SF
|
||
|
Principal Investigator:
|
Jane Stephanie Lebkowski
|
Total Award Amount:
|
$14,323,318
|
|
Project Title:
|
A Phase I/IIa Dose Escalation Safety Study of AST-OPC1 in Patients with Cervical Sensorimotor Complete Spinal Cord Injury
|
||
Milestone achievement is an important indicator of progress and is a major factor in review of progress reports. Insufficient progress through Milestones may result in loss of further funding. The Milestones summarized below replace the Milestones proposed in the original Application. These Milestones will be used as a basis for review in the progress reports and progress Evaluation Meetings unless further modified with Prior Approval from CIRM.
Clinical Trial Milestones: Year 1 (Q4 2014-Q3 2015)
|
Milestone
|
Target
completion
date
|
Progress
or Go/No
Go
|
Comments
|
|
|
Clinical/
Regulatory
|
1. Execute contracts with [***] and all other contractors necessary to open all four clinical sites.
|
Q4 2014
|
Progress
|
Applies to contracts with vendors and service providers. Does not apply to contracts with all four clinical sites, which will likely extend into Q1-2 2015 for completion.
|
|
CMC
|
2. Complete final release of sufficient cGMP-grade material to supply proposed clinical plan
Success criteria: At least [***] differentiated cells meeting specified release criteria
|
Q4 2014
|
Progress milestone
|
|
|
Clinical/ Regulatory
|
3. Open first site
Success criteria: First site open for enrollment, including site initiation, site agreement signed and IRB approval and imaging CRO contract signed
|
Q1 2015
|
Progress
|
Based on previous experience, it will likely take 5-6 months to complete IRB review and site training after obtaining FDA clearance for the trial.
|
|
Clinical/ Regulatory
|
4. Execute contracts with all clinical sites
|
Q2 2015
|
Progress
|
Agreements signed with all remaining clinical sites and remaining IRB approvals obtained.
|
Page 6 of 12
|
Clinical/ Regulatory
|
5. Enroll first subject
Success criteria: First subject enrolled in first cohort
|
Q2 2015
|
Progress
|
Enrollment will likely be slow at first, but is expected to accelerate as more sites open and outreach efforts reach referral centers
|
|
Clinical/ Regulatory
|
6. Complete site initiation for remaining three clinical sites
Success criteria: Four clinical sites open for enrollment
|
Q2 2015
|
Progress
|
Currently anticipated sites are Shepherd
[***];
however, this is subject to change based on results of final site qualification visits and other considerations.
|
Clinical Trial Milestones: Year 2 (Q4 2015-Q3 2016)
|
Milestone
|
Target
completion
date
|
Progress
or Go/No
Go
|
Comments
|
|
|
Clinical/
Regulatory
|
7. Complete enrollment, DMC review of 30 day safety data from first cohort
Success criteria: Enrollment of 3 subjects within 6 months; DMC approves escalation to second dose cohort
|
Q4 2015
|
Go/no go
|
If safety concerns deemed by DMC serious enough to pose an undue risk to patients are observed in the first dose cohort, a no go decision will be made.
|
|
Clinical/ Regulatory
|
8. Enroll first subject in second dose cohort
Success criteria: Enrollment of first subject in second cohort
|
Q1 2016
|
Progress
|
Assumes acceptable safety in cohort 1. Target is to enroll first subject within two months of DMC approval of dose escalation.
|
|
Clinical/ Regulatory
|
9. Complete enrollment, DMC review of safety data from second dose cohort
Success criteria: Enrollment of 5 subjects in second cohort; DMC approves escalation to third dose cohort
|
Q3 2016
|
Go/no go
|
Enrollment should be more rapid in second dose cohort as more sites open, outreach completed. It is expected to take 4-6 months to enroll 5 patient cohort.
If safety concerns deemed by DMC serious enough to pose an undue risk to patients are observed in the second dose cohort, a no go decision will be made.
|
Page 7 of 12
Clinical Trial Milestones: Year 3 (Q4 2016-Q3 2017)
|
Milestone
|
Target
completion
date
|
Progress
or Go/No
Go
|
Comments
|
|
|
Clinical/ Regulatory
|
10. Enroll first subject in third dose cohort
Success criteria: Enrollment of first subject in the third cohort
|
Q4 2016
|
Progress
|
Assumes acceptable safety in cohort 2. Target is to enroll first subject within 1-2 months of DMC approval of dose escalation.
|
|
Clinical/ Regulatory
|
11. Initial (6 month) activity readout from second dose cohort
Success criteria: Continued demonstration of safety. If ≥2 of 5 subjects show ≥2 neurological levels improvement in motor function, this could be a possible early sign of efficacy
|
Q1 2017
|
Progress
|
Based on historical controls, anticipated spontaneous frequency of ≥2 neurological level improvement in motor function is 1 of 5 subjects (21%) at 6 months. However, given early timepoint, small number of subjects, and dose at low end of likely efficacious range, data will not be used as a go/no go decision point.
|
|
Clinical/ Regulatory
|
12. Complete enrollment of Phase 1/2a dose escalation study
Success criteria: Enrollment of 5 subjects in third dose cohort
|
Q2 2017
|
Progress
|
Target is to enroll 5 subjects in third dose cohort within 4-6 months.
|
Page 8 of 12
Clinical Trial Milestones: Year 4 (Q4 2017-Q3 2018)
|
Milestone
|
Target
completion
date
|
Progress
or Go/No
Go
|
Comments
|
|
|
Clinical/ Regulatory
|
13. Initial (6 month) activity readout from third dose cohort
Success criteria: Continued demonstration of safety. If ≥2 of 5 subjects show ≥2 neurological levels improvement in motor function, this could be a possible early sign of efficacy.
|
Q4 2017
|
Progress
|
Based on historical controls, anticipated spontaneous frequency of ≥2 neurological level improvement in motor function is 1 of 5 subjects (21%) at 6 months. If the data show a higher frequency of improvement this could be a promising early sign of efficacy. However, subjects will also be followed to 1 year as improvements may take longer than 6 months to manifest.
|
|
Clinical/ Regulatory
|
14. Complete one year follow-up of Phase 1/2a dose escalation study
|
Q2-2018
|
Progress
|
|
|
Clinical/ Regulatory
|
15. Complete study report
Success criteria: No major safety concerns identified; ≥2 of 5 subjects show two neurological levels of improvement in at least one dose cohort
|
Q3 2018
|
Go/no go
|
Go/no go decision point for subsequent clinical trials, primarily from a safety perspective. Will also be looking for evidence that are meeting TPP of >40% of patients achieving 2 neurological levels of improvement as preliminary efficacy readout for justification of investment in subsequent trials.
|
Page 9 of 12
APPENDIX B - Confidential SP3A-07552
Communication Plan for CIRM-Funded Clinical Trial
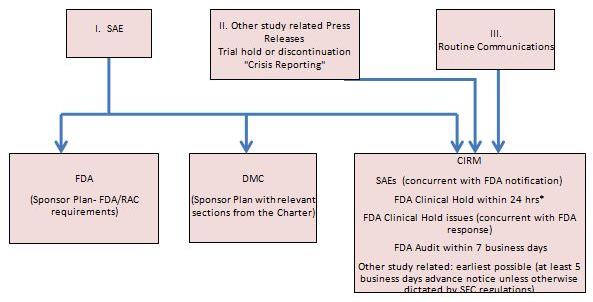
*Upon notification of an FDA Clinical Hold, Sponsor must inform CIRM within 24 hrs. of receipt of the FDA notice. In cases where the FDA communicates issues or seeks information for a potential clinical hold, Sponsor will inform CIRM concurrent with (i.e. within 24 hrs. of) their response to the FDA
| I. | SAE Reporting |
| (1) | Site Reporting to Sponsor |
The Investigator must notify the Sponsor by telephone or fax of any serious adverse event within 24 hours of the Investigator’s knowledge of the event. Telephone reports must be followed by a written report within 24 hours. Follow-up reports must be submitted in a timely fashion as additional information becomes available.
| (2) | Sponsor Reporting to the Food and Drug Administration (FDA) |
The trial Sponsor, Asterias Biotherapeutics, will report any suspected adverse reaction to study treatment that is both serious and unexpected to the FDA in an IND safety report (21 CFR 312.32(c)(1)(i)). Each report will be documented using FDA Form 3500A. Asterias will submit an IND safety report to FDA and all participating investigators no later than 15 calendar days after the Sponsor’s initial receipt of the information and determination that the suspected adverse reaction or other information qualifies for reporting. Asterias will report any unexpected fatal or life-threatening suspected adverse reaction to FDA no later than 7 calendar days after the Sponsor’s initial receipt of the information (21 CFR 312.32(c)(2)).
Reports will be completed with all available information, including a brief narrative describing the suspected adverse reaction and any other relevant information. If applicable, the Sponsor will also include identification of similar reports and an analysis of the significance of the suspected adverse reaction.
Page 10 of 12
| (3) | Review of Safety events by the Data Monitoring Committee (DMC) |
The Asterias Biotherapeutics DMC is responsible for safeguarding the interests of study participants and assessing the safety of study procedures and will manage the overall conduct of study AST-OPC1-01. This committee will provide recommendations about continuing, and stopping the study. In addition, DP Clinical will provide the DMC with summary data on adverse events, serious adverse events, and reports submitted to FDA.
Please refer to the Asterias Biotherapeutics Data Monitoring Committee Charter.
| (4) | Sponsor Reporting to CIRM |
DMC meetings will be conducted on a routine basis. During each meeting, critical issues, unexpected problems and trends will be identified and discussed. The Asterias Clinical Trial Manager (TBD reporting to Edward Wirth) will have responsibility for reporting any suspected adverse reaction to study treatment that is both serious and unexpected, and any event of impact to the clinical project to CIRM within 24 hours. In addition, Asterias will provide a summary of safety events, in coordination with the meetings of the DMC.
Asterias Safety Reporting Contacts:
| · | Edward Wirth III, MD, PhD, Chief Translational Officer |
| · | Naomi Kautz, Associate Director of Regulatory Affairs |
CIRM Safety Reporting Contacts:
| · | Kevin Whittlesey, Ph.D. |
| · | Ellen Feigal, M.D. |
| II. | Other Study Related Press Releases and Trial Hold “Crisis Reporting” |
The Asterias Project Manager,(Katharine Spink) will have primary responsibility of communication with CIRM overall project information, including study decisions and “crisis” issues or other occurrences that may impact the conduct of the trial and to do so within 24 hours of determination by the DMC. Upon notification of an FDA Clinical Hold, Asterias will inform CIRM. CIRM will also be notified of an announced FDA audit within 7 business days, and other study related issues at least 5 business days advanced notice.
Any news releases and/or public statements related to this CIRM grant will be approved by both sides to ensure consistency of message.
Asterias and CIRM agree to provide reasonable advanced notice (at least 5 business days unless otherwise dictated by SEC reporting regulations) of news releases and/or public statements related to this grant, to the other party, in order to give the other party an opportunity to review and suggest edits to the content. However, both Asterias and CIRM retain the ultimate right of authority regarding their external communications. Neither Asterias nor CIRM shall use the name of the other party in public-facing documents, statements or presentations without the prior consent of an authorized individual within the organization.
Page 11 of 12
Management and Communications Contacts:
For Asterias, the authorized individuals shall be Katharine E Spink and Jane Lebkowski.
For CIRM, the authorized individuals are the Sr. Director of Public Communications, Kevin McCormack, and Sr. Vice President of Research and Development, Dr. Ellen Feigal.
| III. | Routine Communications |
Asterias and CIRM will have a mutual commitment towards maintaining communication between quarterly reports.
Page 12 of 12
