Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Recro Pharma, Inc. | d889543d8k.htm |
| EX-99.1 - EX-99.1 - Recro Pharma, Inc. | d889543dex991.htm |
 Relieving
Pain….Improving Lives Exhibit 99.2 |
 Special Note
Regarding Forward-Looking Statements
2
This presentation contains forward-looking statements that involve risks and
uncertainties. Such forward-looking statements reflect Recro
Pharma’s expectations about its future operating results,
performance
and
opportunities
that
involve
substantial
risks
and
uncertainties.
When
used
herein,
the words "anticipate," "believe," "estimate,"
"upcoming," "plan," "target", "intend" and "expect" and
similar expressions, as they relate to Recro Pharma or its management, are intended
to identify such forward-looking statements. These forward-looking
statements are based on information available to Recro Pharma as of the date
of this presentation and are subject to a number of risks, uncertainties,
and other factors that could cause actual events to differ materially from those
expressed in the forward-looking statements set forth in this presentation
including, without limitation:
the
success
of
Recro’s
products
and
of
the
newly
acquired
products;
the
parties’
ability
to
satisfy
the
purchase
agreement
conditions
(including
required
regulatory
approvals);
Recro’s
ability to realize anticipated growth, synergies and costs savings from the
acquisition; changes in laws and regulations; Recro’s ability to
successfully integrate the acquired operations, technology and products and
to realize anticipated growth, synergies and cost savings; Recro’s ability to
successfully develop, obtain regulatory approvals or commercialize new products and
Recro’s ability to protect intellectual property rights. In
addition, the forward-looking statements in this presentation should be
considered together with the risks and uncertainties that may affect Recro
Pharma’s business and future results included in Recro Pharma’s filings
with the Securities and Exchange Commission at www.sec.gov. Recro pharma
assumes no obligation to update any such forward looking statements.
|
 Complementary Fit
3
IV / IM
Meloxicam
Manufacturing,
Royalties &
Formulation
Dex-IN / Fado |
 Transformative
Transaction •
Diversifies development risk with second, complementary hospital
acute pain product to pipeline
–
Dex-IN has upcoming interim analysis of ongoing Ph II trial
–
Meloxicam ready for Ph III –
successfully completed multiple Ph II trials
•
Manufacturing & royalty business provides manufacturing
capability and scale
–
Enhances ability for BD opportunities
–
Depending on performance, potential for cashflows to fund development
•
Depending on Dex-IN clinical success, possibility for two proprietary
compounds to enter Ph III by year end
–
Assuming sufficient capital to fund development
–
Dex-IN interim results upcoming
4 |
 Transaction
Structure •
$50 million up-front cash payment
–
non-dilutive up-front financed by senior secured loan from
OrbiMed
•
Up to $120 million in milestone payments upon
regulatory and sales milestones related to
meloxicam
•
Royalty on meloxicam net sales
•
Warrant to purchase 350,000 shares of common
stock at closing
5 |
 OrbiMed
Financing Terms Term
Summary
Amount
$50 million funded on closing date
Structure
Senior secured term loan and warrants
Security interest
First lien security on all assets
Maturity date
60 months from closing date
Interest rate
Greater of (i) LIBOR or (ii) 1.0% plus applicable margin of 14.0%
Repayment and
amortization
100% excess cash flow sweep on manufacturing & royalty business at
OrbiMed’s option
Pre-payment
penalty
Prior
to
3
rd
year:
1.5x
loan
less
all
prior
payments
of
principal
and
interest
After
3
rd
year:
10%
exit
fee
prior
to
scheduled
maturity
date
Financial covenants
Manufacturing
&
royalty
business:
Quarterly
minimum
net
sales;
Quarterly
maximum
total
leverage
ratio
&
maximum
capital
expenditures
Consolidated
business:
Minimum
liquidity
covenant
Warrant
3% of fully diluted common stock at closing, exercise price is $3.28 per share,
and 7 year term
6 |
 Acquisition Provides Scale and Diversification
•
Adds manufacturing & royalty business and Phase III ready hospital
asset –
85,000 sq. ft. facility (DEA licensed) manufactures 5 commercial
products
–
$70M+ historical revenues; manufacturing division cashflow positive
•
IV/IM meloxicam –
long acting COX-2 NSAID for moderate to severe acute
pain ready for Ph III
–
Approved, widely prescribed oral COX-2 therapeutic
–
Multiple Phase II studies successfully completed in acute pain models
–
Dosing advantages over existing acute pain therapeutics, including long
action –
Non-opioid
•
Meloxicam complementary to Dex-IN in treating moderate to severe
acute pain
–
Long
acting
formulation
has
successfully
treated
acute
pain
post
operatively
(hysterectomy)
•
Increases Company infrastructure and scale
–
Improved positioning for future BD opportunities
–
Manufacturing capacity
7 |
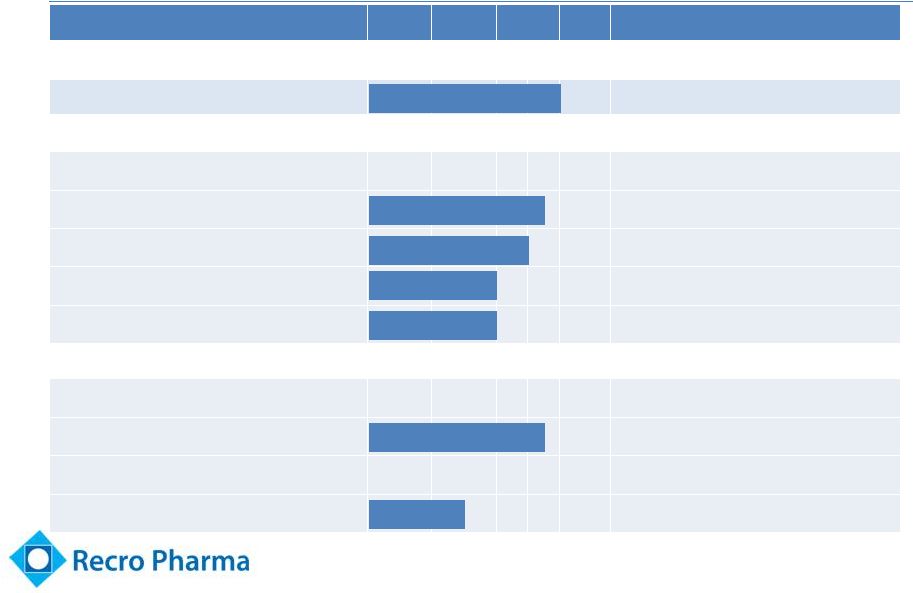 Updated
Clinical Stage Pipeline Product
PC
I
II
III
Rights
IV/IM Meloxicam
WW
Acute post-operative pain
Dexmedetomidine (“Dex”)
WW, exc. Europe, Turkey, CIS
Dex-
IN (intranasal)
Acute post-operative pain
Cancer breakthrough pain
Dex-SL (sublingual)
Transdermal
Fadolmidine (“Fado”)
WW, exc. Europe, Turkey, CIS
Intrathecal
Post-operative pain
Topical
Neuropathic pain
8 |
 IV/IM Meloxicam Overview
•
FDA approved, oral selective COX-2 inhibitor used in a
wide variety of indications
•
Proprietary long acting injectable form for moderate to
severe acute pain
–
Utilizes Alkermes’
NanoCrystal
TM
technology
•
Phase III ready –
multiple Phase II studies completed on
IV and a Phase I on IM
–
Positive Ph II hysterectomy and dental pain studies with large
effect sizes
•
IP issued through 2022 and additional IP could extend
protection through 2030
9
NanoCrystal
®
is a registered trademark of Alkermes plc. |
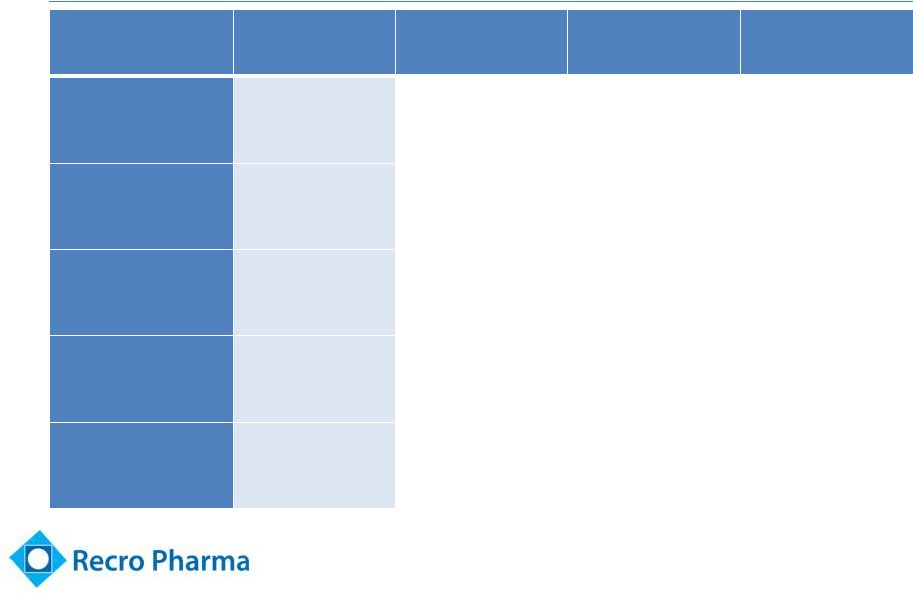 Favorable Dosing Profile
Attribute
Meloxicam
Ketorolac
Caldolor
(ibuprofen)
Ofirmev
(APAP)
Route
IV/IM
IV/IM
IV
IV
Onset of pain
relief
< 10 min
30 min
N/A
N/A
Time to peak
analgesic effect
40 min
1-2 hrs
N/A
N/A
Duration of
pain relief
18-24 hrs
4-6 hrs
4-6 hrs
4-6 hrs
Admin.
IV bolus / pre-
filled syringe
Ready to use IV
bolus (15 sec)
Dilution required,
30 min infusion
Ready to use, 15
min infusion
10 |
 Multiple
Successful IV Phase 2 Trials 11
•
Elan/ALKS have conducted 5 IV and 1 IM clinical trials
Trial
Design
Outcome
Phase II Study
N1539-02
Acute pain following dental
surgery (N = 230)
Statistically significant differences for all
doses compared to placebo were seen in
SPID24, pain relief and onset of pain relief
Phase II Study
N1539-04
Acute pain following open
abdominal hysterectomy
surgery (N = 486)
Statistically significant differences for all
doses compared to placebo were seen in
multiple efficacy analyses, including SPID24.
meloxicam 30 mg and 60 mg produced the
greatest response with no difference
between doses
Phase II Study
N1539-05
Acute pain following
laparoscopic abdominal
surgery (N =50)
Study stopped early (planned N = 250) for
business reasons. However, statistically
significant differences in SPID48 observed for
30mg QD dose despite small sample size |
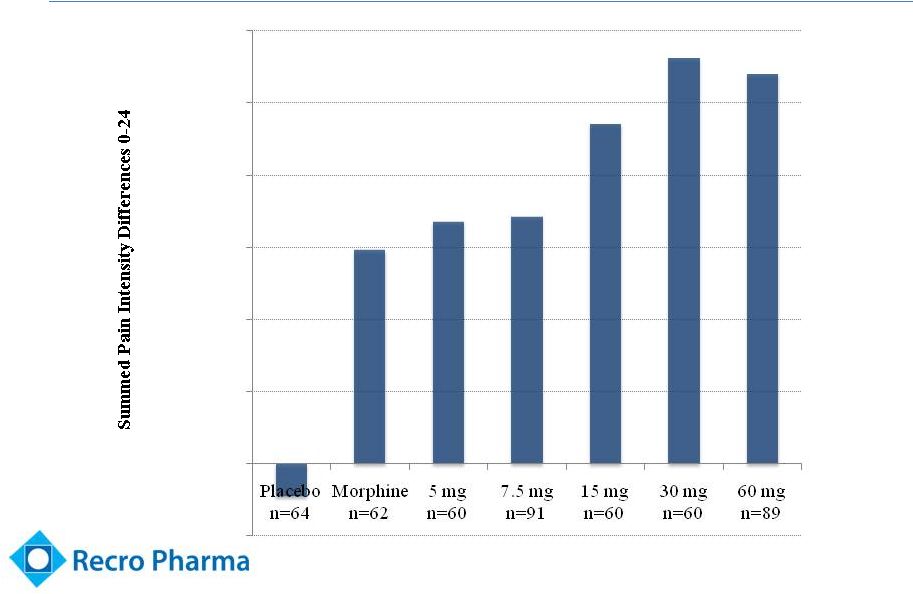 Robust
Efficacy (Abdominal Hysterectomy Trial –
IV Meloxicam)
12
*** p < 0.001 vs. Placebo
***
***
***
***
***
***
(10,000)
-
10,000
20,000
30,000
40,000
50,000
60,000 |
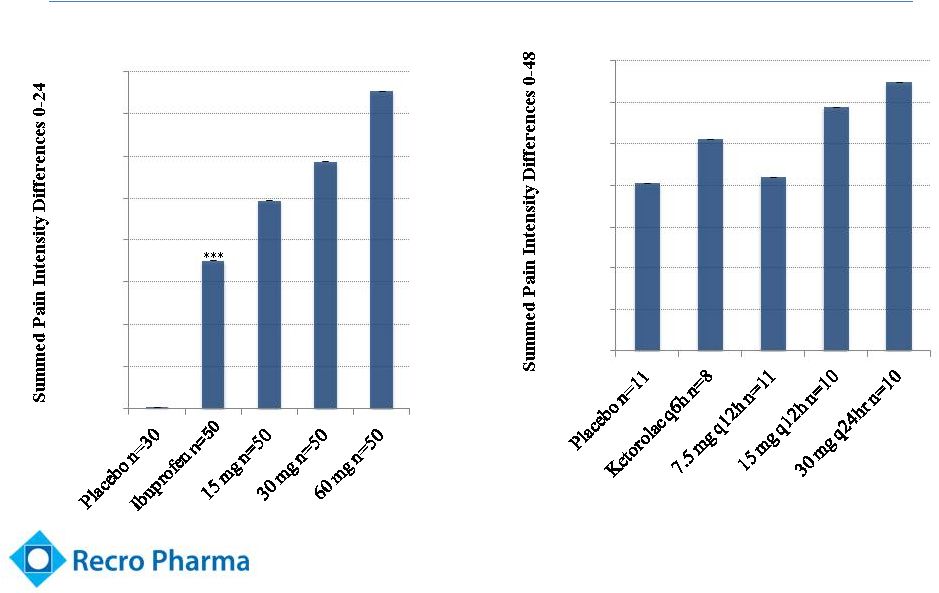 Confirmed
Efficacy in Multiple Studies Summary of Pain Intensity Differences (SPID)
13
*** p < 0.001 vs. Placebo
Dental Pain Study
Abdominal Laparoscopic Pain Study
-
10,000
20,000
30,000
40,000
50,000
60,000
70,000
80,000
***
***
***
0
200
400
600
800
1000
1200
1400
p = 0.0682
p = 0.0392 |
 Single 30 mg
Dose Maintained for 24 hrs (Abdominal Hysterectomy Trial –
IV Meloxicam)
14
Baseline
Pain
Level
60
Time (Hours) |
 Well Tolerated
(Abdominal Hysterectomy Trial –
IV Meloxicam)
15
**Reported in
3% of Subjects in any group and greater than Placebo
Meloxicam
Placebo
n=64
Morphine
n=62
5 mg
n=60
7.5 mg
n=91
15 mg
n=60
30 mg
n=60
60 mg
n=89
Anemia
3.1
4.8
3.3
13.2
3.3
1.7
10.1
Anemia Postoperative
-
1.6
-
-
-
3.3
-
Constipation
-
4.8
5.0
1.1
1.7
-
-
Flatulence
-
4.8
1.7
1.1
3.3
-
-
Hypokalaemia
-
3.2
1.7
1.1
-
1.7
-
Insomnia
4.7
8.1
10.0
4.4
5.0
5.0
4.5
Ketonuria
7.8
9.7
6.7
9.9
15
10
10.1
Leukocytosis
-
-
1.7
-
-
3.3
-
Pyrexia
1.6
3.2
3.3
2.2
-
-
-
Sinus Tachycardia
-
-
3.3
-
-
-
1.1
Percent of Subjects Reporting an Adverse Event **
|
 US Based
Manufacturing Facility 16 |
 Manufacturing
& Royalty Overview Manufacturing facility
•
87,000 sq. ft. solid oral dosage manufacturing cGMP
•
DEA licensed
•
~165 employees
Service capabilities
•
Formulation, process development and optimization
•
Process scale-up
•
Clinical supply and validation
•
Commercial supply
Ritalin LA
•
Once daily ADHD treatment marketed by Novartis
Focalin XR
•
ADHD treatment marketed by Novartis
Verelan / verapamil
•
High blood pressure treatment marketed by Actavis and
UCB
Zohydro ER
•
Extended release hydrocodone marketed by Zogenix
•
Launched in 2014
•
Abuse deterrent form expected to be launched near term
17 |
 Strong
Historical Manufacturing Performance •
Unaudited, carve-out financials –
12 mos ended 12/31/14
–
Revenues -
$73.6 million
–
EBITDA -
$26.5 million
•
Zohydro ER –
abuse deterrent form expected to be
launched in the near term
•
Additional capacity for new product opportunities
•
Positive cashflow expected to cover all debt service
obligations and excess cashflows to repay loan principal
18 |
 Illustrative
Pro Forma Capitalization Pre-transaction
Post-transaction
Common stock
7,804,063
7,804,063
Options
962,800
962,800
(1)
Existing warrants
150,000
150,000
Alkermes warrants
0
350,000
OrbiMed warrants
0
278,006
(2)
Total commons stock equiv.
8,916,863
9,544,869
19
(1) Outstanding at signing. Excludes options to be issued to acquired
employees at closing. (2) This number will be calculated based on common
stock outstanding (on a fully diluted basis) as of the Closing of the
transaction. |
 Transformative
Transaction •
Diversifies development risk with second, complementary hospital
acute pain product to pipeline
–
Dex-IN has upcoming interim analysis of ongoing Ph II trial
–
Meloxicam ready for Ph III –
successfully completed multiple Ph II trials
•
Manufacturing & royalty business provides manufacturing
capability and scale
–
Enhances ability for BD opportunities
–
Depending on performance, potential for cashflows to fund development
•
Depending on Dex-IN clinical success, possibility for two proprietary
compounds to enter Ph III by year end
–
Assuming sufficient capital to fund development
–
Dex-IN interim results upcoming
20 |
