Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Lipocine Inc. | v403886_8k.htm |

Efficacy and Pharmacokinetics of LPCN 1021, a Novel Oral Testosterone Replacement Therapy, in Hypogonadal Men: Study of Androgen Replacement (SOAR) Christina Wang, MD 1 ; Anthony DelConte, MD 2,5 ; Jed C. Kaminetsky, MD 3 ; Martin M. Miner, MD 4 ; Pavan Yadav, MD 1 ; Srinivasan Venkateshwaran, PhD 2 ; Nachiappan Chidambaram, PhD 2 ; Satish Nachaegari 2 ; Mahesh Patel, PhD 2 ; Adrian S. Dobs, MD, MHS 6 1 Harbor - UCLA Medical Center and Los Angeles Biomedical Research Institute, Torrance, CA; 2 Lipocine Inc., Salt Lake City, UT ; 3 University Urology Associates, New York, NY; 4 Brown University and Miriam Hospital, Providence, RI; 5 Saint Joseph’s University, Philadelphia , PA; 6 Johns Hopkins University School of Medicine, Baltimore, MD Exhibit 99.1

This study was supported by Lipocine Inc. CW – Research funding (Clinical researcher): Antares, Clarus, Lipocine, Prolor; Consulting fees: Clarus, Lipocine; Honoraria ( Advisory group ): TesoRx . AD – Consulting fees: Lipocine. JCK – Research funding (Principal investigator): AbbVie, Antares, Auxilium, Clarus, Ferring, Lipocine; Consulting fees, Antares, Auxilium; Contracted fee (Speaker): Auxilium. MMM – Research funding (Clinical r esearcher ): Forest, NERI ; Consulting fees: AbbVie, Lipocine, Repros . PY – Nothing to disclose. SV – Former employee: Lipocine . NC – Employee: Lipocine . SN – Employee: Lipocine . MP – Employee: Lipocine . ASD – Research funding (Clinical researcher ): Clarus, Endo; Honoraria (Advisory group): AbbVie, Endo, Lipocine. Disclosures 2
To understand the key efficacy outcomes from an interim analysis of the SOAR Phase 3 study of LPCN 1021, including : • Percent of subjects with an average 24 - hour serum testosterone concentration ( C avg,24h ) within the normal range after 13 weeks of treatment • Additional pharmacokinetic information • Frequency of dose adjustments Objectives 3
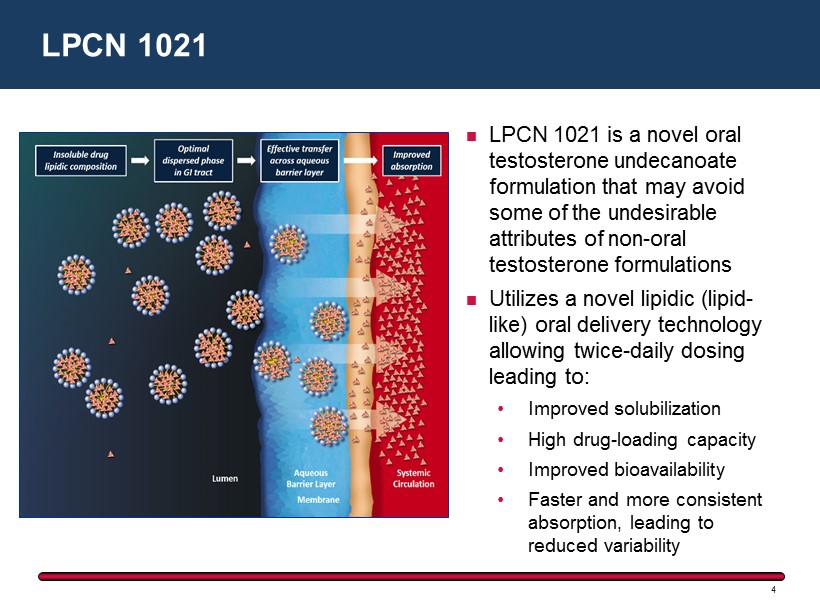
LPCN 1021 is a novel oral testosterone undecanoate formulation that may avoid some of the undesirable attributes of non - oral testosterone formulations Utilizes a novel lipidic (lipid - like ) oral delivery technology allowing twice - daily dosing leading to: • Improved solubilization • High drug - loading capacity • Improved bioavailability • Faster and more consistent absorption, leading to reduced variability LPCN 1021 4
Male 18 - 80 years of age with documented onset of hypogonadism prior to age 65 Documented diagnosis of primary hypogonadism (congenital or acquired) or hypogonadotropic hypogonadism (congenital or acquired ) Serum T <300 ng/dL based on 2 blood samples, taken approximately same time of day on 2 different days (between 6 and 10 a.m.) Naïve to androgen replacement or has discontinued current treatment and completed a washout of prior androgen therapy • Washout: 12 weeks following intramuscular androgens, 4 weeks following topical/buccal, 3 weeks following oral; or investigator discretion Key Inclusion Criteria 5
Abnormal prostate exam or I - PSS score >19 points Body mass index (BMI) ≥38 kg/m 2 Clinically significant abnormal laboratory value Concurrent medications that could affect PK measurements or patient health Partner who is concurrently pregnant or planning to become pregnant Key Exclusion Criteria 6
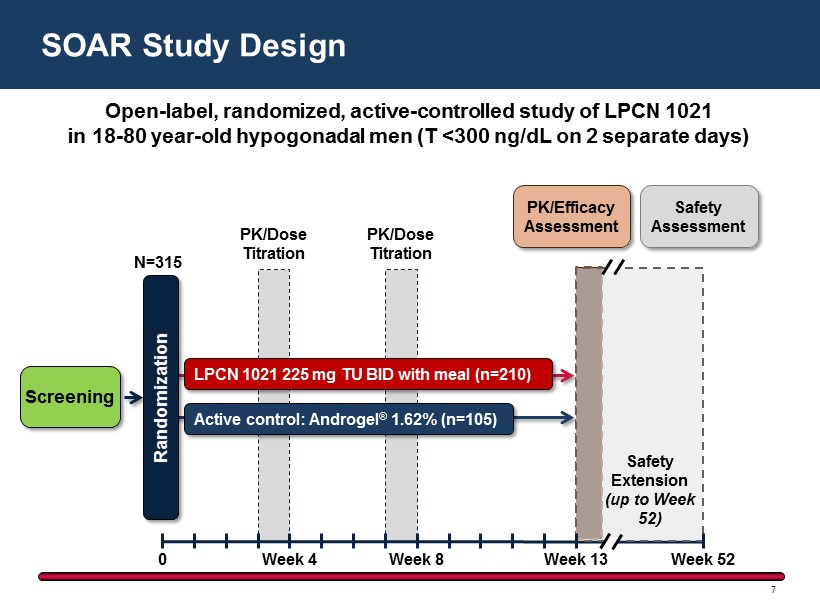
SOAR Study Design 7 Screening N=315 0 Week 4 Week 8 Randomization LPCN 1021 225 mg TU BID with meal (n=210 ) Active control: Androgel ® 1.62% (n=105 ) PK/Dose Titration PK/Dose Titration PK/Efficacy Assessment Safety Assessment Week 13 Week 52 Safety Extension (up to Week 52) Open - label, randomized, active - controlled study of LPCN 1021 in 18 - 80 year - old hypogonadal men ( T <300 ng/dL on 2 separate days )
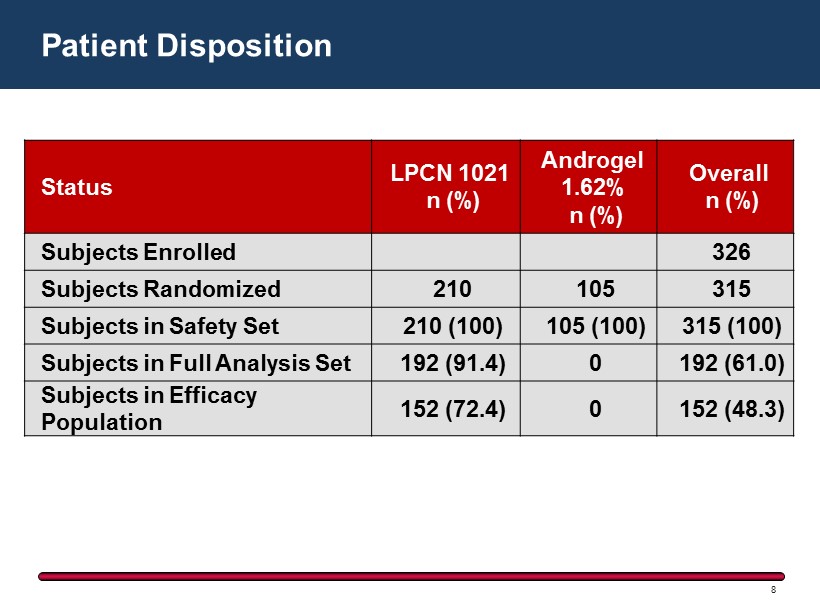
Patient Disposition 8 Status LPCN 1021 n (%) Androgel 1.62% n (%) Overall n (%) Subjects Enrolled 326 Subjects Randomized 210 105 315 Subjects in Safety Set 210 ( 100) 105 ( 100) 315 ( 100) Subjects in Full Analysis Set 192 ( 91.4) 0 192 ( 61.0) Subjects in Efficacy Population 152 ( 72.4) 0 152 ( 48.3)
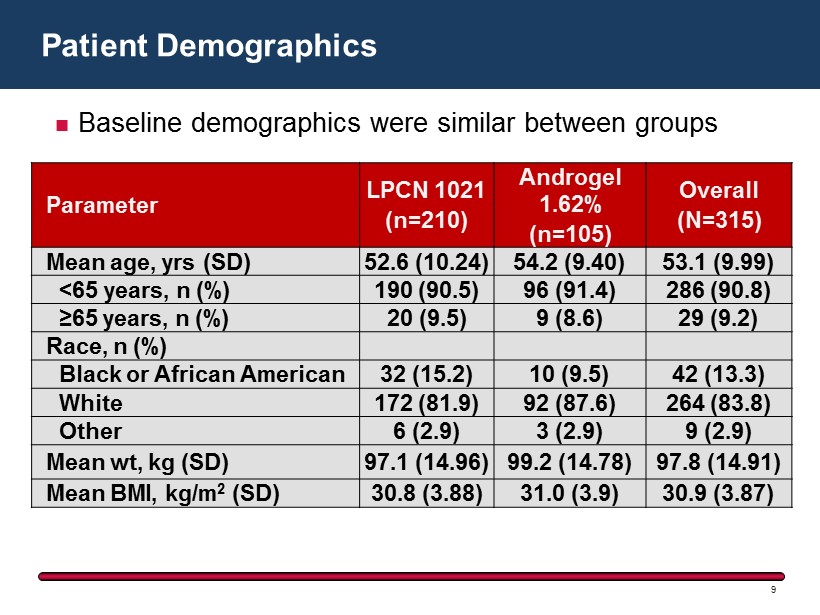
Patient Demographics 9 Parameter LPCN 1021 (n=210) Androgel 1.62 % (n=105) Overall (N=315) Mean age, yrs (SD) 52.6 (10.24) 54.2 (9.40) 53.1 (9.99) <65 years, n (%) 190 (90.5) 96 (91.4) 286 (90.8) ≥65 years, n (%) 20 (9.5) 9 (8.6) 29 (9.2) Race, n (%) Black or African American 32 (15.2) 10 (9.5) 42 (13.3) White 172 (81.9) 92 (87.6) 264 (83.8) Other 6 (2.9) 3 (2.9) 9 (2.9) Mean wt, kg (SD) 97.1 (14.96) 99.2 (14.78) 97.8 (14.91) Mean BMI, kg/m 2 (SD) 30.8 (3.88) 31.0 (3.9) 30.9 (3.87) Baseline demographics were similar between groups

The starting dose of LPCN 1021 was 225 mg TU BID taken with a standard meal The dose could be titrated up to 300 mg BID (eg , if T C avg,24h was <300 ng/dL ) or down to 150 mg BID (eg , if T C max was >1500 ng/dL and/or C avg,24h >1140 ng/dL) at weeks 4 and 8 LPCN 1021 Dosing 10
Percentage of subjects with serum total T C avg,24h within the normal range of 300 - 1140 ng/dL • The % of subjects with C avg,24h within the normal range should be ≥75% and the lower bound 95% CI ≥65% • Efficacy was assessed on week 13 based on T C avg,24h from serum samples collected over 24 hours for T assayed using LC - MS/MS • Analysis was conducted using the Efficacy Population Set (subjects with ≥1 PK profile and no major protocol deviations; n=152 LPCN 1021) Primary Outcome 11
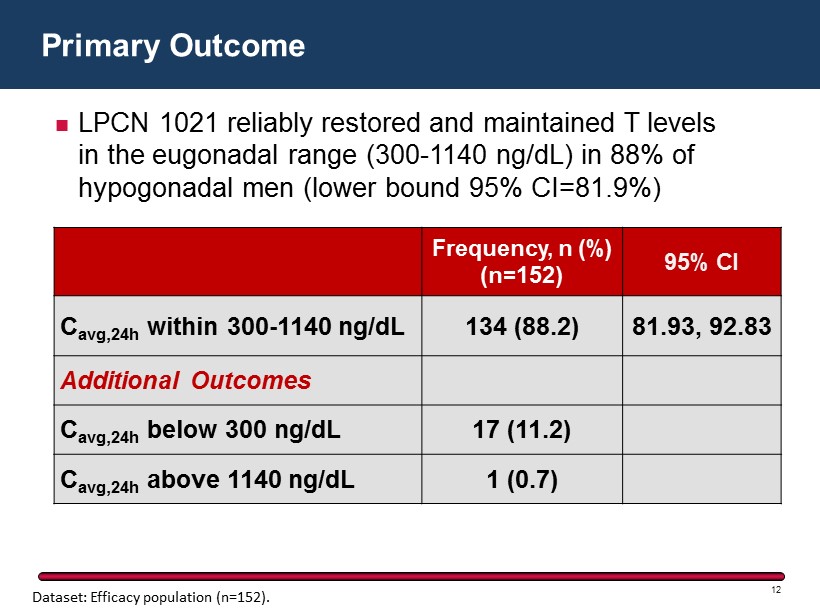
Primary Outcome 12 Frequency, n (%) (n=152) 95% CI C avg,24h within 300 - 1140 ng/dL 134 ( 88.2) 81.93, 92.83 Additional Outcomes C avg,24h below 300 ng/dL 17 ( 11.2) C avg,24h above 1140 ng/dL 1 ( 0.7) LPCN 1021 reliably restored and maintained T levels in the eugonadal range (300 - 1140 ng/dL) in 88% of hypogonadal men (lower bound 95% CI=81.9%) Dataset: Efficacy population (n=152).
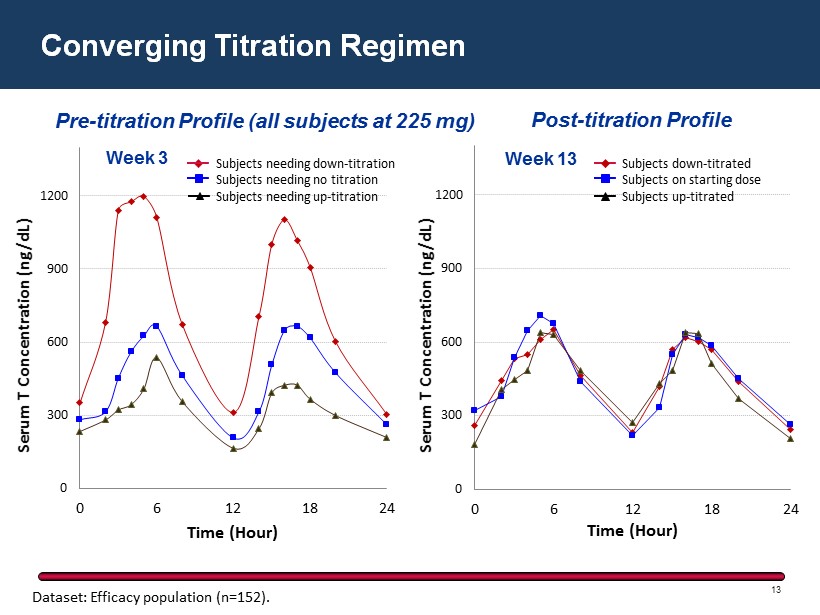
Converging Titration Regimen Dataset: Efficacy population (n=152). Pre - titration Profile (all subjects at 225 mg) Post - titration P rofile 0 300 600 900 1200 0 6 12 18 24 Serum T Concentration ( ng/dL) Time ( Hour ) Week 3 0 300 600 900 1200 0 6 12 18 24 Serum T Concentration (ng/dL) Time (Hour) Week 13 13 Subjects needing down - titration Subjects needing no titration Subjects needing up - titration Subjects down - titrated Subjects on starting dose Subjects up - titrated
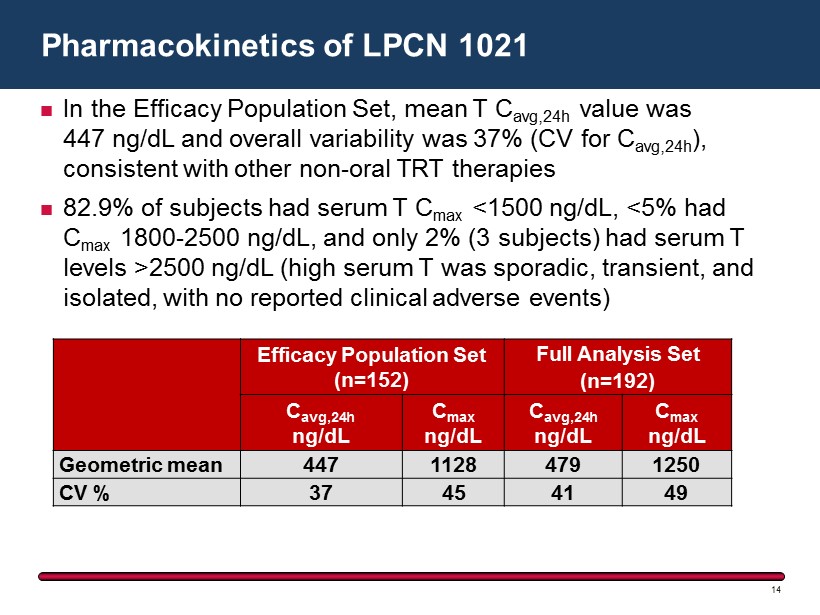
In the Efficacy Population Set, mean T C avg,24h value was 447 ng/dL and overall variability was 37 % (CV for C avg,24h ), consistent with other non - oral TRT therapies 82.9% of subjects had serum T C max <1500 ng/dL, <5% had C max 1800 - 2500 ng/dL, and only 2% (3 subjects) had serum T levels >2500 ng/ dL (high serum T was sporadic, transient, and isolated, with no reported clinical adverse events) Pharmacokinetics of LPCN 1021 14 Efficacy Population Set (n=152) Full Analysis Set (n=192) C avg,24h ng/dL C max ng/dL C avg,24h ng/dL C max ng/dL Geometric mean 447 1128 479 1250 CV % 37 45 41 49
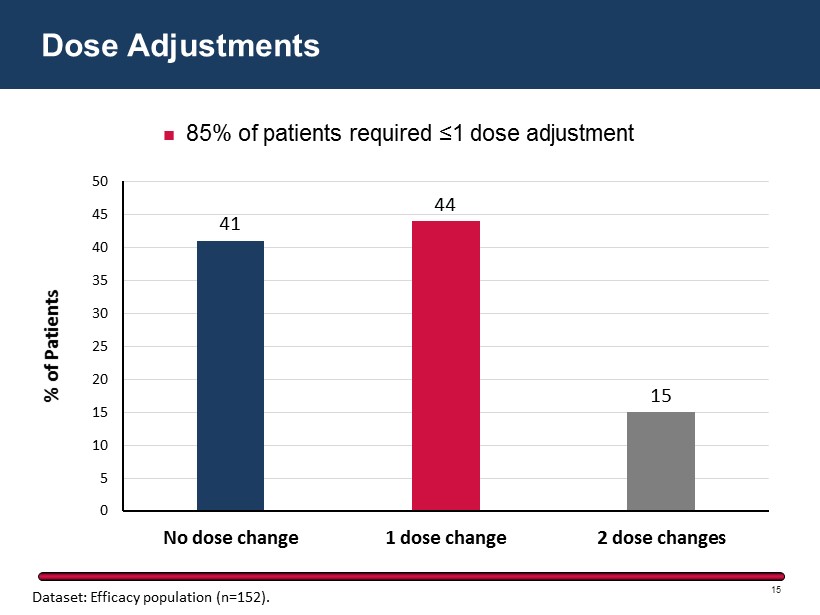
41 44 15 0 5 10 15 20 25 30 35 40 45 50 No dose change 1 dose change 2 dose changes % of Patients Dose Adjustments 15 85% of patients required ≤1 dose adjustment Dataset: Efficacy population (n=152).
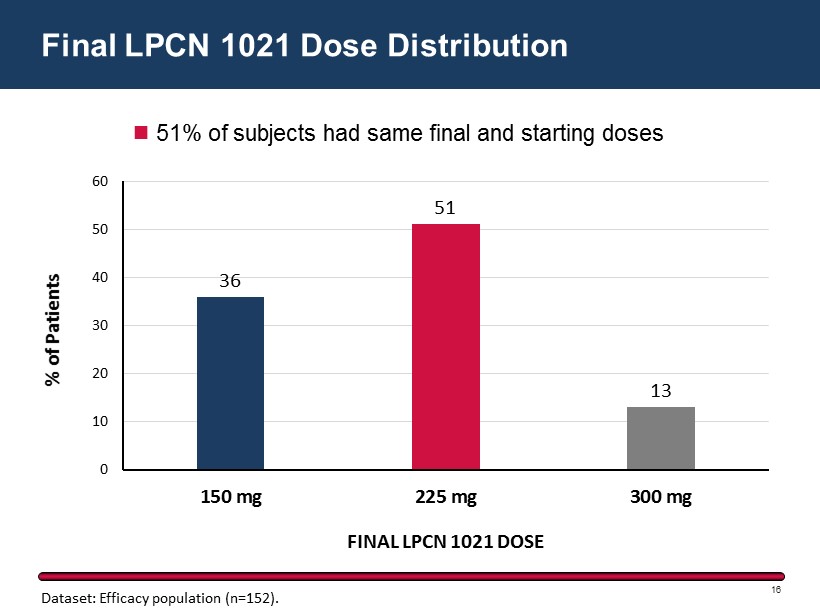
36 51 13 0 10 20 30 40 50 60 150 mg 225 mg 300 mg % of Patients FINAL LPCN 1021 DOSE Final LPCN 1021 Dose Distribution 16 51% of subjects had same final and starting doses Dataset: Efficacy population (n=152).
Serious Adverse Events (SAEs) 17 As of January 31, 2015 • No drug - related SAEs • No cardiac - related SAEs

LPCN 1021 is an orally administered product for T replacement with acceptable C avg levels, consistent with US FDA target guidelines • C max levels were generally consistent with US FDA target guidelines LPCN 1021 may improve patient compliance as a generally safe, effective, and more convenient option compared to topical, transdermal, injectable, or implanted testosterone products Conclusions 18
