Attached files
| file | filename |
|---|---|
| EX-99.1 - CORPORATE PRESENTATION - Lipocine Inc. | v403789_ex99-1.htm |
| 8-K - CURRENT REPORT - Lipocine Inc. | v403789_8k.htm |

27 th Annual Roth Conference March 11, 2015 Exhibit 99.2

2 This presentation contains and our discussions during this conference may include forward - looking statements about Lipocine Inc. (the “Company”). These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward - looking statements relate to the Company’s product candidates, clinical and regula tory processes and objectives, potential benefits of the Company’s product candidates, intellectual property and related matters, all of which involve known and unknown risks and uncertainties. Actual results may differ materially from the forward - looking statemen ts discussed in this presentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials, the potential advantages of the Company’s product candidates and the Company’s capital needs. Among other things, the projected commencement and completion of the Company’s clinical trials may be affected by difficulties or delays. In addition, the Com pan y’s results may be affected by its ability to manage its financial resources, difficulties or delays in developing manufacturing pro cesses for its product candidates, preclinical and toxicology testing and regulatory developments. Delays in clinical programs, whe the r caused by competitive developments, adverse events, patient enrollment rates, regulatory issues or other factors, could adver sel y affect the Company’s financial position and prospects. Prior clinical trial program designs and results are not necessarily pre dictive of future clinical trial designs or results. If the Company’s product candidates do not meet safety or efficacy endpoints in cl inical evaluations, they will not receive regulatory approval and the Company will not be able to market them. The Company may not b e able to enter into any strategic partnership agreements. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corporate priorities, the timing and outcomes of cli nical trials, competitive developments and the impact on expenditures and available capital from licensing and strategic collaborat ion opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to sig nif icantly delay, scale back or discontinue one or more of its drug development or discovery research programs. The Company is at an ea rly stage of development and may not ever have any products that generate significant revenue. The forward - looking statements contained in this presentation are further qualified by the detailed discussion of risks and uncertainties set forth in the d ocu ments filed by the Company with the Securities and Exchange Commission, all of which can be obtained on the Company’s website at www.lipocine.com or on the SEC website at www.sec.gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations. Forward Looking Statements
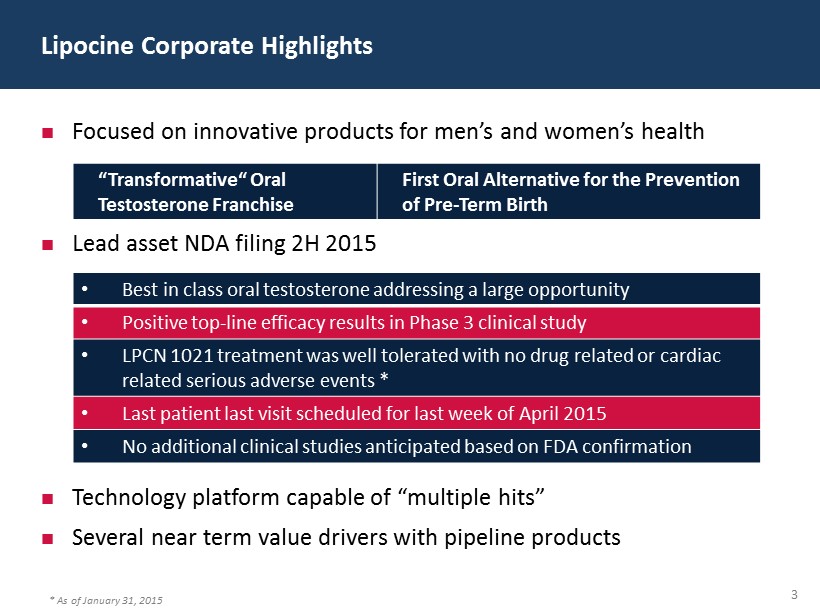
3 Lipocine Corporate Highlights Focused on innovative products for men’s and women’s health Lead asset NDA filing 2H 2015 Technology platform capable of “multiple hits” Several near term value drivers with pipeline products “Transformative“ Oral Testosterone Franchise First Oral Alternative for the Prevention of Pre - Term Birth • Best in class oral testosterone addressing a large opportunity • Positive top - line efficacy results in Phase 3 clinical study • LPCN 1021 treatment was well tolerated with no drug related or cardiac related serious adverse events * • Last patient last visit scheduled for last week of April 2015 • No additional clinical studies anticipated based on FDA confirmation * As of January 31, 2015
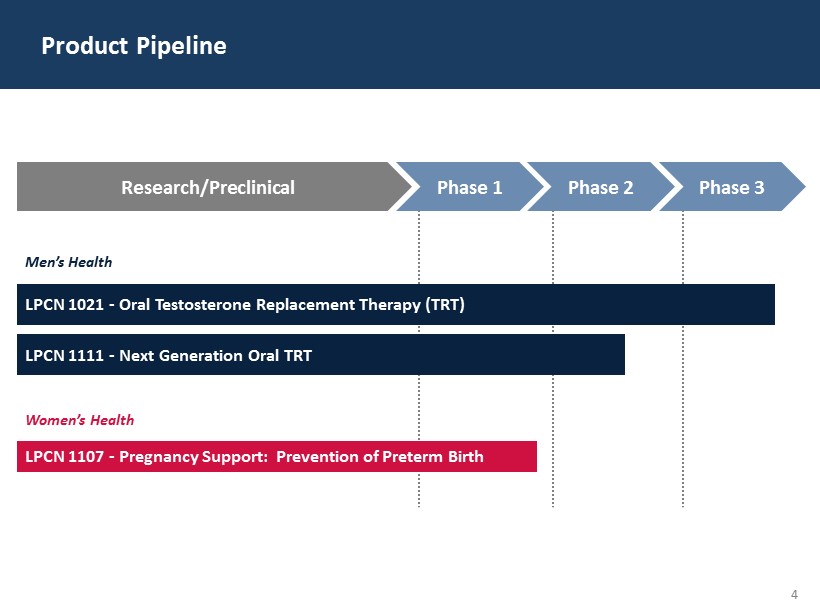
4 Product Pipeline LPCN 1021 - Oral Testosterone Replacement Therapy (TRT) Men’s Health LPCN 1111 - Next Generation Oral TRT Women’s Health LPCN 1107 - Pregnancy Support: Prevention of Preterm Birth Research/Preclinical Phase 1 Phase 2 Phase 3
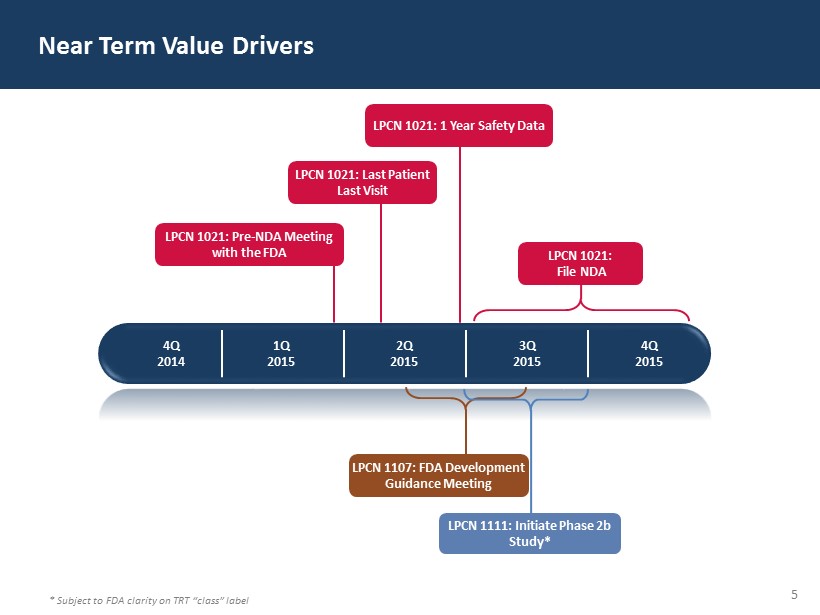
5 Near Term Value Drivers 4Q 2014 1 Q 2015 2 Q 2015 3 Q 2015 4Q 2015 LPCN 1021: 1 Year Safety Data LPCN 1021: File NDA LPCN 1107: FDA Development Guidance Meeting LPCN 1021: Pre - NDA Meeting with the FDA LPCN 1021: Last Patient Last Visit LPCN 1111: Initiate Phase 2b Study* * Subject to FDA clarity on TRT “class” label
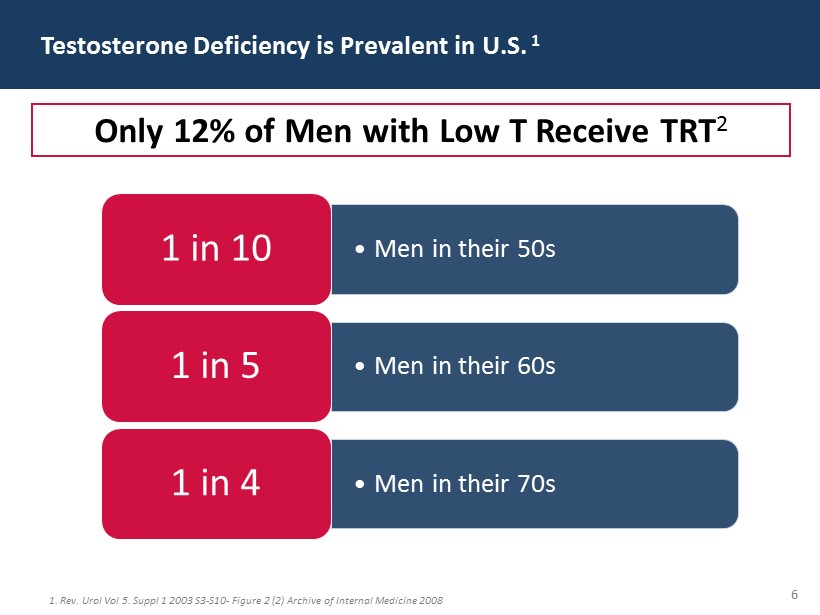
6 Testosterone Deficiency is Prevalent in U.S. 1 1. Rev. Urol Vol 5. Suppl 1 2003 S3 - S10 - Figure 2 (2) Archive of Internal Medicine 2008 • Men in their 50s 1 in 10 • Men in their 60s 1 in 5 • Men in their 70s 1 in 4 Only 12% of Men with Low T Receive TRT 2

7 US market > $2.3B in 2013 1 US market ~ 6.5M TRx in 2014 1 • 13% decrease in TRx from 2013 Current TRT “ class” label under review by FDA in US – guidance provided International markets for TRT growing Topicals dominate the market (~ 86% 1 of $ sales) • Inadvertent transference issue, black box warning • Poor compliance: 86 % discontinue at 12 months 2 Existing testosterone treatments are inadequate Testosterone Replacement Therapy Market 1. IMS data 2. Journal of Sexual Medicine 2013
8 Recent FDA TRT Communication (March 3, 2015) Changes to existing label • Narrow the intended TRT population (i.e., caused by certain medical conditions and confirmed by laboratory tests) • However, the language did not suggest contra - indicating for age related hypogonadism • Add cautionary language to label for possible cardio vascular and stroke related risk with TRT • Add cautionary language on lack of benefit and safety of TRT in age related hypogonadism Require manufacturers of approved TRT products to conduct “a” well - designed clinical study as an industry group or separately
9 Novel product primarily directed at lymphatic delivery of Testosterone Undecanoate (TU) Enhanced profile over other forms of oral testosterone (T) • Native T has short half life • Methyl T is known to have liver safety issues Maintains effective T blood levels when dosed BID Target label — consistent with TRT “class” label LPCN 1021: Oral Treatment Option for TRT
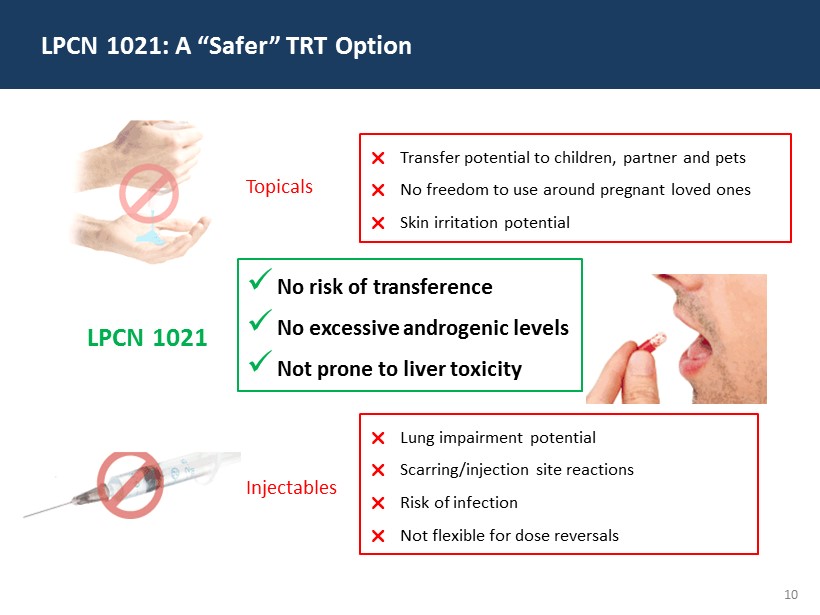
10 LPCN 1021: A “Safer” TRT Option Transfer potential to children, partner and pets No freedom to use around pregnant loved ones Skin irritation potential Lung impairment potential Scarring/injection site reactions Risk of infection Not flexible for dose reversals x No risk of transference x No excessive androgenic levels x Not prone to liver toxicity Topicals Injectables LPCN 1021
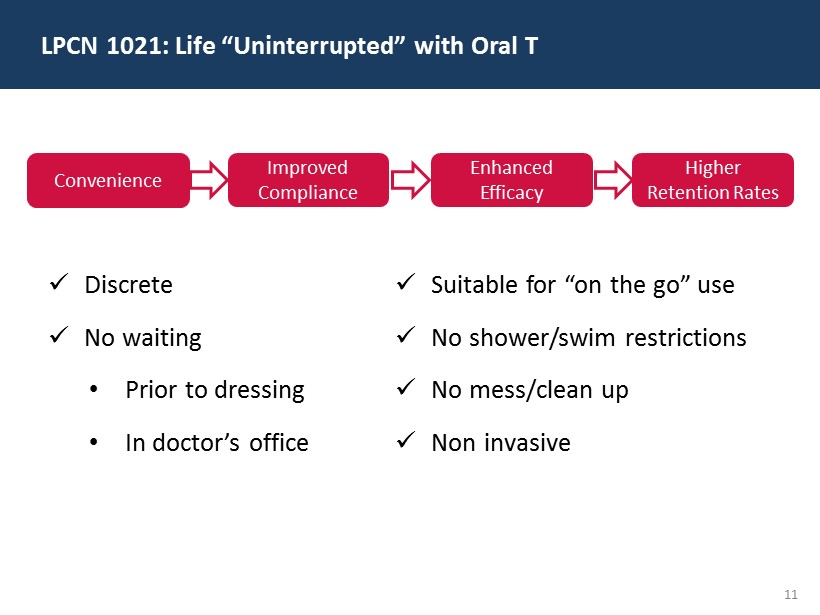
11 LPCN 1021: Life “Uninterrupted” with Oral T x Discrete x No waiting • Prior to dressing • In doctor’s office x Suitable for “on the go” use x No shower/swim restrictions x No mess/clean up x Non invasive Improved Compliance Convenience Enhanced Efficacy Higher Retention Rates
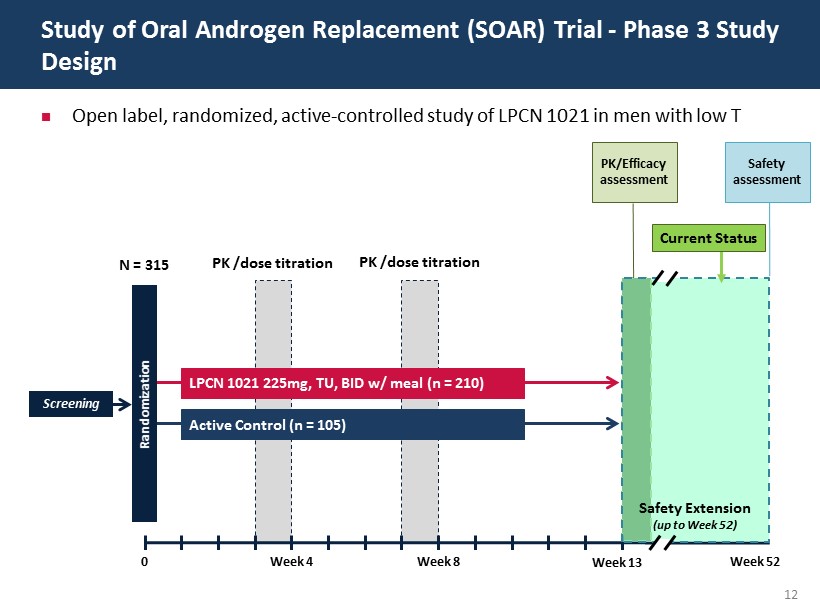
12 Open label, randomized, active - controlled study of LPCN 1021 in men with low T Study of Oral Androgen Replacement (SOAR) Trial - Phase 3 Study Design Screening N = 315 0 Week 4 Week 8 Randomization LPCN 1021 225mg, TU, BID w/ meal (n = 210) Active Control (n = 105) PK /dose titration PK /dose titration PK/Efficacy assessment Safety assessment Week 13 Week 52 Safety Extension (up to Week 52) Current Status
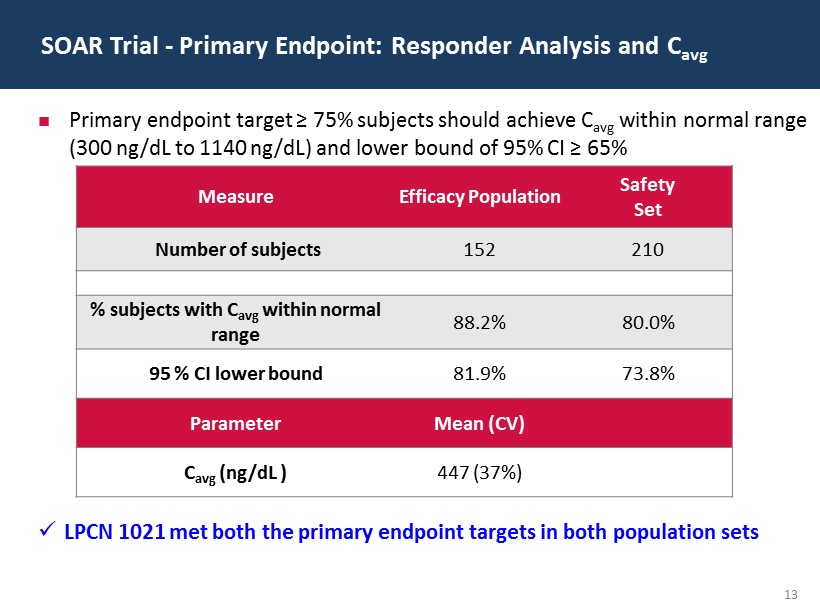
13 SOAR Trial - Primary Endpoint: Responder Analysis and C avg Measure Efficacy Population Safety Set Number of subjects 152 210 % subjects with C avg w ithin normal range 88.2% 80.0% 95 % CI lower bound 81.9% 73.8% Parameter Mean (CV) C avg ( ng / dL ) 447 (37%) Primary endpoint target ≥ 75% subjects should achieve C avg within normal range (300 ng/dL to 1140 ng/dL) and lower bound of 95% CI ≥ 65% x LPCN 1021 met both the primary endpoint targets in both population sets

14 Unequivocally met primary efficacy end point Patient friendly dosing regimen • No more than one titration required in 85% of the subjects • Starting dose was the right dose for majority of the subjects Variability consistent with or better than approved products Consistent intra/inter day performance Proportion of subjects with maximum serum concentrations generally consistent with approved products LPCN 1021 treatment was well tolerated with no drug related or cardiac related serious adverse events* Presenting results at the ENDO 2015 meeting on March 7 th SOAR Trial – LPCN 1021 Top Line Phase 3 Results Summary * as of January 31, 2015

15 Novel prodrug of testosterone for oral delivery through proprietary Lip’ral technology Maintains effective T blood levels when dosed once daily Status • Positive Phase 2a study results in hypogonadal men – Single daily oral dose provides T levels in the eugonadal range – No subject exceeded peak T levels of 1500 ng / dL • Initiate Phase 2b study - 3Q 2015 * LPCN 1111: Next Generation Oral Testosterone * Subject to FDA clarity on TRT “class” label
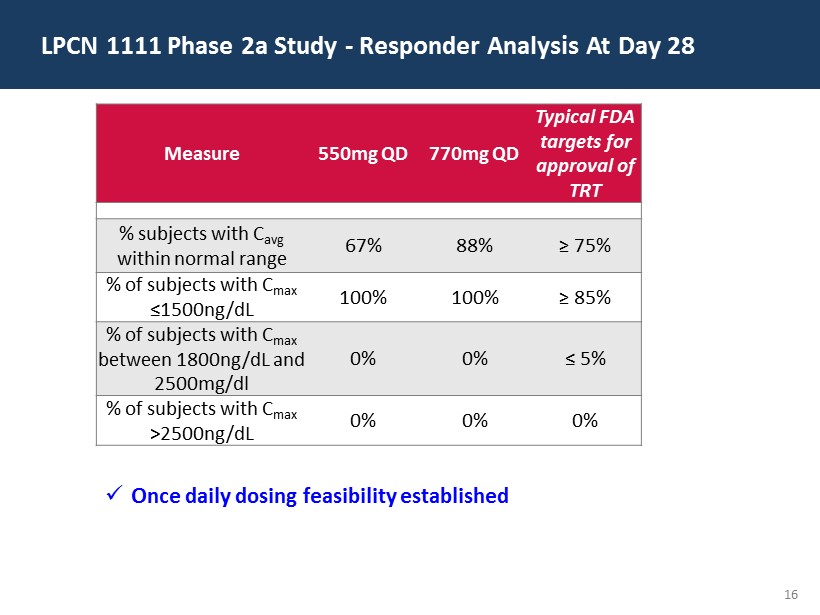
16 LPCN 1111 Phase 2a Study - Responder Analysis At Day 28 Measure 550mg QD 770mg QD Typical FDA targets for approval of TRT % subjects with C avg w ithin normal range 67% 88% ≥ 75% % of subjects with C max ≤ 1500ng/ dL 100% 100% ≥ 85% % of subjects with C max between 1800ng/ dL and 2500mg/dl 0% 0% ≤ 5% % of subjects with C max >2500ng/ dL 0% 0% 0% x Once daily dosing feasibility established

17 Preterm Birth (PTB) Represents a Significant Unmet Medical Need One Preterm Infant per Minute in the US 1 □ 11.7% of all US pregnancies 2 result in PTB - a leading cause of mortality and morbidity 3 □ ~10x more first year medical costs are for PTB infants than for full term infants 4 □ ≥ $26 billion economic impact: 4 $1 billion market opportunity 5 1. Pediatric Research (2006) 60, 775 – 776; 2. CDC (2010); 3. J. Maternal - Fetal and Neonatal Medicine, Dec. 2006, 19(12), 773 – 782; 4. Institute of Medicine of the National Academies. Jul.2006; 5. AMAG Pharmaceuticals presentation 09/29/2014
18 Potential to be the first oral standard - of care therapy • Same API as in the only approved injectable product • Elimination of 18 - 22 injections • Potential for orphan drug designation Status • Successful Phase 1a POC study in healthy non - pregnant women • Successful Phase 1b POC in pregnant women • Next step - Discuss development plan with the FDA (2Q - 3Q 2015) LPCN 1107 : Oral Candidate for Prevention of Preterm B irth
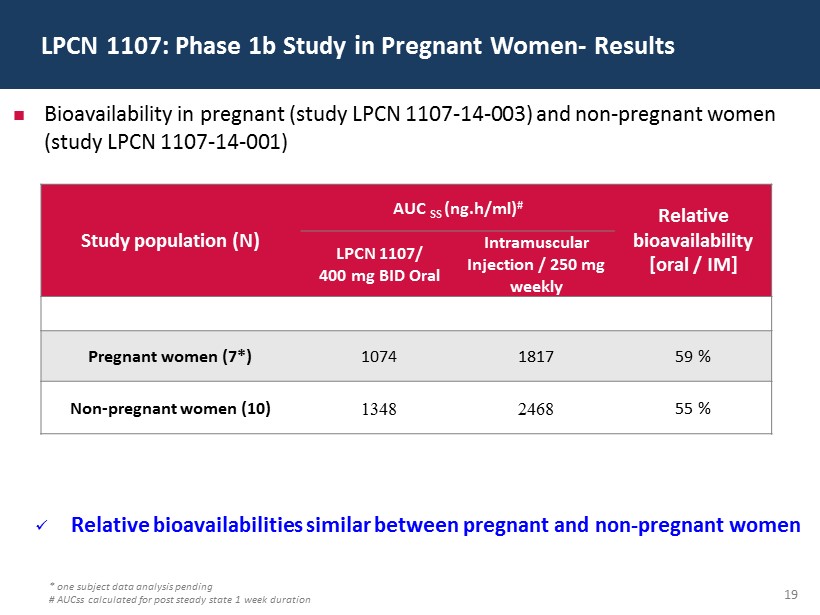
19 LPCN 1107: Phase 1b Study in Pregnant Women - Results * one subject data analysis pending # AUCss calculated for post steady state 1 week duration Bioavailability in pregnant (study LPCN 1107 - 14 - 003) and non - pregnant women (study LPCN 1107 - 14 - 001) Study population (N) AUC SS ( ng.h /ml) # Relative bioavailability [oral / IM] LPCN 1107/ 400 mg BID Oral Intramuscular Injection / 250 mg weekly Pregnant women (7*) 1074 1817 59 % Non - pregnant women (10) 1348 2468 55 % x Relative bioavailabilities similar between pregnant and non - pregnant women
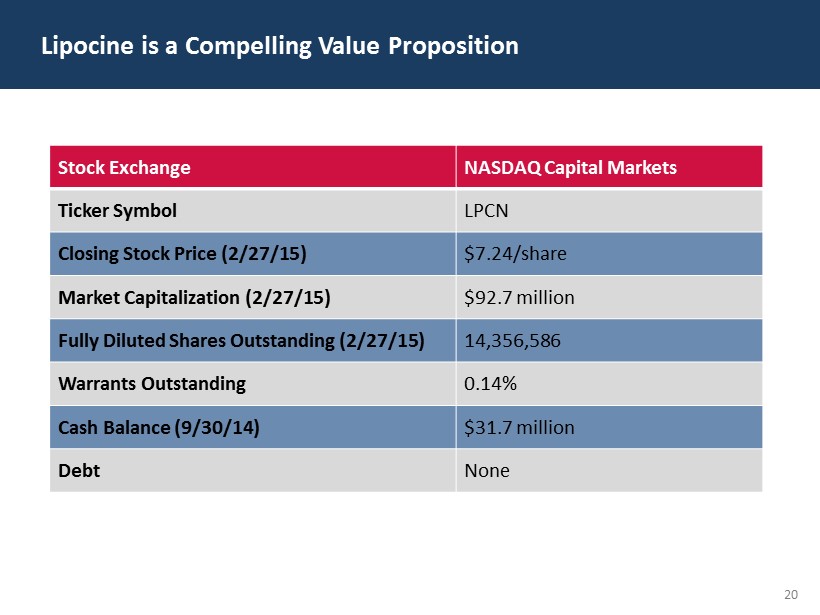
20 Lipocine is a Compelling Value Proposition Stock Exchange NASDAQ Capital Markets Ticker Symbol LPCN Closing Stock Price (2/27/15) $7.24/share Market Capitalization (2/27/15) $92.7 million Fully Diluted Shares Outstanding (2/27/15) 14,356,586 Warrants Outstanding 0.14% Cash Balance (9/30/14) $31.7 million Debt None
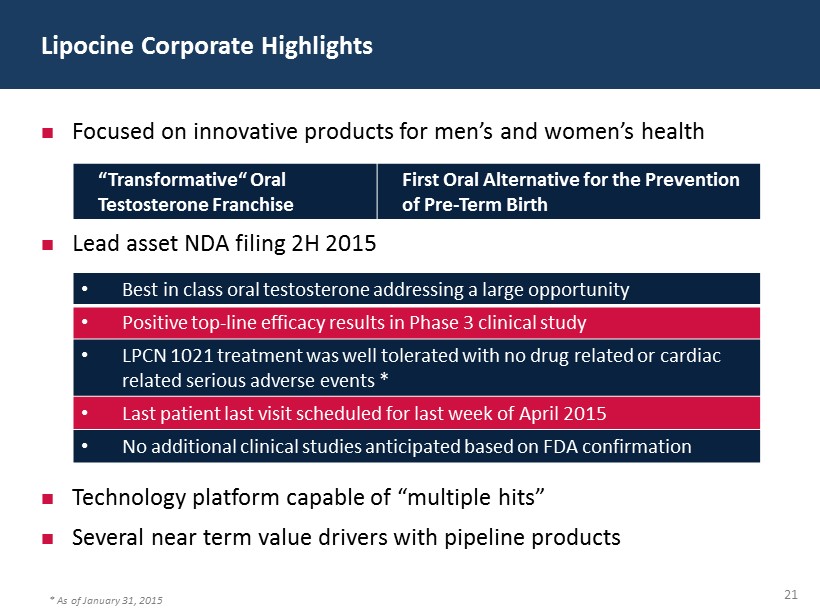
21 Lipocine Corporate Highlights Focused on innovative products for men’s and women’s health Lead asset NDA filing 2H 2015 Technology platform capable of “multiple hits” Several near term value drivers with pipeline products “Transformative“ Oral Testosterone Franchise First Oral Alternative for the Prevention of Pre - Term Birth • Best in class oral testosterone addressing a large opportunity • Positive top - line efficacy results in Phase 3 clinical study • LPCN 1021 treatment was well tolerated with no drug related or cardiac related serious adverse events * • Last patient last visit scheduled for last week of April 2015 • No additional clinical studies anticipated based on FDA confirmation * As of January 31, 2015
