Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Intra-Cellular Therapies, Inc. | d883062d8k.htm |
| EX-99.1 - EX-99.1 - Intra-Cellular Therapies, Inc. | d883062dex991.htm |
| EX-99.3 - EX-99.3 - Intra-Cellular Therapies, Inc. | d883062dex993.htm |
Exhibit 99.2
We are a biopharmaceutical company focused on the discovery and clinical development of innovative, small molecule drugs that address underserved medical needs in neuropsychiatric and neurological disorders by targeting intracellular signaling mechanisms within the central nervous system, or CNS. We are developing our lead drug candidate, ITI-007, for the treatment of schizophrenia, behavioral disturbances in dementia, bipolar disorder and other neuropsychiatric and neurological disorders. ITI-007 is in Phase 3 clinical development as a first-in-class treatment for schizophrenia. Current medications available for the treatment of schizophrenia do not adequately address the broad array of symptoms associated with this CNS disorder. Use of these current medications also is limited by their substantial side effects. ITI-007 is designed to be effective across a wider range of symptoms, treating both the acute and residual phases of schizophrenia, with improved safety and tolerability.
ITI-007 exhibited antipsychotic efficacy in a randomized, double-blind, placebo and active controlled Phase 2 clinical trial in patients with an acutely exacerbated episode of schizophrenia. In December 2013, we announced the clinical results from this Phase 2 trial. In this Phase 2 trial, 335 patients were randomized to receive one of four treatments: 60 mg of ITI-007, 120 mg of ITI-007, 4 mg of risperidone (active control) or placebo in a 1:1:1:1 ratio, orally once daily for 28 days. The primary endpoint for this clinical trial was change from baseline to Day 28 on the Positive and Negative Syndrome Scale, or PANSS, total score. In this study, ITI-007 met the trial’s pre-specified primary endpoint, improving symptoms associated with schizophrenia as measured by a statistically significant and clinically meaningful decrease in the PANSS total score. The trial also met key secondary outcome measures related to efficacy on PANSS subscales and safety.
We are proceeding with Phase 3 development of ITI-007 for the treatment of schizophrenia. We plan to conduct two randomized, double-blind, placebo-controlled Phase 3 clinical trials of ITI-007 in patients with acutely exacerbated schizophrenia, with over 400 patients in each trial. We initiated the first Phase 3 clinical trial in schizophrenia in the fourth quarter of 2014 and, subject to finalizing the trial protocols and arrangements with clinical trial sites, we intend to initiate a second Phase 3 clinical trial in the first half of 2015. In the first Phase 3 trial, we are randomizing patients to two doses of ITI-007 (60mg or 40mg) or placebo over a 4-week treatment duration, and the primary outcome measure is change from baseline to Day 28 on the PANSS total score. We currently expect that the second Phase 3 trial will be conducted for a 6-week treatment duration. Subject to timely enrollment, we anticipate that the results of the first Phase 3 clinical trial of ITI-007 in patients with schizophrenia could be available as early as the fourth quarter of 2015. Subject to further discussions with the U.S. Food and Drug Administration, or FDA, we also plan to initiate separate additional trials in bipolar disorder in 2015. We have not yet discussed our plans to develop ITI-007 for the treatment of bipolar disorder with the FDA. We expect that the planned trials in bipolar disorder will overlap in time with the clinical conduct of the planned trials in schizophrenia. In addition to our Phase 3 clinical trials, we will need to complete other clinical and non-clinical trials and manufacturing and pre-commercialization activities necessary to support the submission of a planned New Drug Application, or NDA, for ITI-007 in schizophrenia, which we currently expect could occur at the end of 2016 or the beginning of 2017.
In addition, in the fourth quarter of 2014, we announced the topline data from ITI-007-200, a Phase 1/2 clinical trial designed to evaluate the safety, tolerability and pharmacokinetics of low doses of ITI-007 in healthy
1
geriatric subjects and in patients with dementia, including Alzheimer’s disease. The completion of this study marks an important milestone in our strategy to develop low doses of ITI-007 for the treatment of behavioral disturbances associated with dementia and related disorders. The ITI-007-200 trial results to date indicate that ITI-007 is safe and well-tolerated across a range of low doses, has linear- and dose-related pharmacokinetics and improves cognition in the elderly. The most frequent adverse event was mild sedation at the higher doses. We believe these results further position ITI-007 as a development candidate for the treatment of behavioral disturbances in patients with dementia and other neuropsychiatric and neurological conditions. We plan to initiate additional clinical programs evaluating ITI-007 in patients with behavioral disturbances associated with dementia and related disorders, including Alzheimer’s disease, in 2015.
We are currently conducting an open-label positron emission tomography, or PET, study of ITI-007 examining brain receptor occupancy and assessing occupancy of striatal D2 receptors. In this study, patients with stable schizophrenia will be treated with ITI-007 for 14 days. We expect topline data from this study in 2015. We believe this study will further characterize ITI-007 and provide additional insight into the molecule’s unique mechanism and clinical profile.
We are also pursuing clinical development of ITI-007 for the treatment of additional CNS diseases and disorders. At the lowest doses, ITI-007 has been demonstrated to act primarily as a potent 5-HT2A serotonin receptor antagonist. As the dose is increased, additional benefits are derived from the engagement of additional drug targets, including modest dopamine receptor modulation and modest inhibition of serotonin transporters. We believe that combined interactions at these receptors may provide additional benefits above and beyond selective 5-HT2A antagonism for treating agitation, aggression and sleep disturbances in diseases that include dementia, Alzheimer’s disease, Huntington’s disease and autism spectrum disorders, while avoiding many of the side effects associated with more robust dopamine receptor antagonism. As the dose of ITI-007 is further increased, leading to moderate dopamine receptor modulation, inhibition of serotonin transporters, and indirect glutamate modulation, these actions complement the complete blockade of 5-HT2A serotonin receptors. At a dose of 60 mg, ITI-007 has been shown effective in treating the symptoms associated with schizophrenia, and we believe this higher dose range will be useful for the treatment of bipolar disorder, major depressive disorder and other neuropsychiatric diseases.
Given the potential utility for ITI-007 and follow-on compounds to treat these additional indications, we may investigate, either on our own or with a partner, agitation, aggression and sleep disturbances in additional diseases that include autism spectrum disorders; major depressive disorder; intermittent explosive disorder; non-motor symptoms and motor complications associated with Parkinson’s disease; and post-traumatic stress disorder. We hold exclusive, worldwide commercialization rights to ITI-007 and a family of compounds from Bristol-Myers Squibb Company pursuant to an exclusive license.
We have a second major program called ITI-002 that has yielded a portfolio of compounds that selectively inhibits the enzyme phosphodiesterase type 1, or PDE1. PDE1 helps regulate brain activity related to cognition, memory processes and movement/coordination. On February 25, 2011, we (through our wholly owned operating subsidiary, ITI) and Takeda Pharmaceutical Company Limited, or Takeda, entered into a license and collaboration agreement with Takeda, or the Takeda License Agreement, under which we agreed to collaborate to research, develop and commercialize our proprietary compound ITI-214 and other selected compounds that selectively inhibit PDE1 for use in the prevention and treatment of human diseases. On October 31, 2014, we entered into an agreement with Takeda terminating the Takeda License Agreement, or the Termination Agreement, pursuant to which all rights granted under the Takeda License Agreement were returned to us. Takeda will complete certain ongoing activities relating to non-clinical studies and will transfer product inventory and materials to us but will not have any other ongoing involvement or funding obligations in connection with the development program. ITI-214 is the first compound in its class to successfully advance into Phase 1 clinical trials. We intend to continue the development of ITI-214 for the treatment of CNS and other disorders. Over approximately the next 12 months, we will refine our strategy for the PDE1 inhibitor program.
2
By regaining unrestricted access to ITI-214, backups and the proprietary chemistry, we can now integrate the efforts of our internal PDE1 program to include the later stage portfolio. We do not anticipate a significant increase in our operating expenses related to our PDE development programs over the next twelve months. Other compounds in the PDE1 portfolio are also being advanced for the treatment of various indications, including non-CNS therapeutic areas.
Our pipeline also includes pre-clinical programs that are focused on advancing drugs for the treatment of cognitive dysfunction, in both schizophrenia and Alzheimer’s disease, and for disease modification and the treatment of neurodegenerative disorders, including Alzheimer’s disease.
We have assembled a management team with significant industry experience to lead the discovery and development of our product candidates. We complement our management team with a group of scientific and clinical advisors that includes recognized experts in the fields of schizophrenia and other CNS disorders, including Nobel laureate, Dr. Paul Greengard, one of our co-founders.
Our Product Candidates
Our pipeline includes two product candidates in clinical development and two product candidates in advanced pre-clinical testing. We believe that our product candidates offer innovative therapeutic approaches and may provide significant advantages relative to current therapies. The following table summarizes our product candidates and programs:
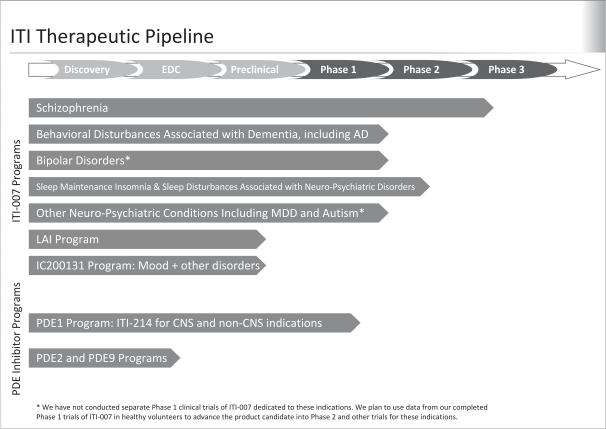
3
Our Strategy
Our goal is to discover and develop novel small molecule therapeutics for the treatment of CNS diseases in order to improve the lives of people suffering from such illnesses. Using our key understanding of intracellular signaling, we seek to accomplish our goal, using our in-house expert drug discovery and clinical development teams, in two ways:
| • | we seek to have the capability to develop first-in-class medications with novel mechanisms that have the potential to treat CNS diseases for which there are no previously marketed drugs; and |
| • | we seek to develop drugs that either can differentiate themselves in competitive markets by addressing aspects of CNS disease which are not treated by currently marketed drugs or can be effective with fewer side effects. |
The key elements of our strategy are to:
| • | complete the development of ITI-007 for its lead indication, treatment of schizophrenia, and for additional neuropsychiatric indications, such as bipolar disorder and residual symptoms in schizophrenia; |
| • | expand the commercial potential of ITI-007 by investigating its usefulness in neurological areas, such as behavioral disturbances in dementia, including Alzheimer’s disease and autism spectrum disorder, and in additional neuropsychiatric indications, such as sleep disorders associated with neuropsychiatric and neurological disorders and major depressive disorder; |
| • | continue to develop PDE inhibitor compounds, such as ITI-214, for the treatment of CNS and other disorders; and |
| • | advance earlier stage product candidates in our pipeline. |
Risks Relating to Our Business
We are a biopharmaceutical company, and our business and ability to execute our business strategy are subject to a number of significant risks of which you should be aware before you decide to buy shares of our common stock. Among these important risks are the following:
| • | We currently do not have, and may never have, any products that generate significant revenues. |
| • | There is no guarantee that our planned clinical trials for ITI-007 in schizophrenia or in other indications will be successful. |
| • | Although we have discussed our clinical development plans with the FDA, the agency may ultimately determine that our Phase 3 clinical trials and non-clinical studies, even if successfully completed, are not sufficient for regulatory approval. If we are required to conduct additional clinical trials and non-clinical studies, our development of ITI-007 for schizophrenia will be more time-consuming and costly than we presently anticipate, which would have a material adverse effect on our business, results of operations and financial condition. |
| • | If the FDA does not agree with our clinical development plans to advance ITI-007 for the treatment of schizophrenia and bipolar disorder with separate, but overlapping, well-controlled clinical trials in both indications, our development of ITI-007 for the treatment of bipolar disorder may be delayed and the costs of our development of ITI-007 would increase. |
| • | We expect our net losses to continue for at least several years and are unable to predict the extent of future losses or when we will become profitable, if ever. |
| • | We will require substantial additional funding, which may not be available to us on acceptable terms, or at all, and, if not so available, may require us to delay, limit, reduce or cease our operations. |
4
| • | Our lead product candidate, ITI-007, is only part way through the clinical trials we anticipate needing to complete before we may be able to submit an NDA to the FDA. Clinical trials are long, expensive and unpredictable, and there is a high risk of failure. |
| • | Delays, suspensions and terminations in our clinical trials could result in increased costs to us, delay our ability to generate product revenues and therefore may have a material adverse effect on our business, results of operations and future growth prospects. |
| • | Safety issues with our product candidates, or with product candidates or approved products of third parties that are similar to our product candidates, could give rise to delays in the regulatory approval process, restrictions on labeling or product withdrawal after approval. |
| • | Preliminary and interim data from our clinical studies that we may announce or publish from time to time may change as more patient data become available. |
| • | We rely on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that prevent us from successfully commercializing our product candidates. |
| • | Even if we successfully complete the clinical trials of one or more of our product candidates, the product candidates may fail for other reasons. |
| • | Following regulatory approval of any of our drug candidates, we will be subject to ongoing regulatory obligations and restrictions, which may result in significant expense and limit our ability to commercialize our potential products. |
| • | Relying on third-party manufacturers may result in delays in our clinical trials, regulatory approvals and product introductions. |
| • | We will need to continue to manage our organization and we may encounter difficulties with our staffing and any future transitions, which could adversely affect our results of operations. |
| • | Our ability to compete may be undermined if we do not adequately protect our proprietary rights. |
| • | Many of our competitors have greater resources and capital than us, putting us at a competitive disadvantage. If our competitors develop and market products that are more effective than our product candidates, they may reduce or eliminate our commercial opportunity. |
| • | Our stock price may fluctuate significantly and you may have difficulty selling your shares based on current trading volumes of our stock. In addition, numerous other factors could result in substantial volatility in the trading price of our stock. |
| • | We have broad discretion in the use of net proceeds from this offering and may not use them effectively. |
| • | Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment. |
| • | The price of our common stock could be subject to volatility related or unrelated to our operations. |
| • | Management and certain members of our board of directors beneficially own a substantial amount of our outstanding equity securities and will be able to exert substantial control over us. |
5
Recent Developments
While we have not finalized our full financial results for the fiscal year ended December 31, 2014, we expect to report that we had approximately $129.6 million of cash, cash equivalents and investment securities, available for sale, as of December 31, 2014. This amount is preliminary, has not been audited and is subject to change upon completion of the audit of our consolidated financial statements as of and for the year ended December 31, 2014. Additional information and disclosures would be required for a more complete understanding of our financial position and results of operations as of December 31, 2014.
Corporate Information
We were originally incorporated in the State of Delaware in August 2012 under the name “Oneida Resources Corp.” Oneida Resources Corp. was a “shell” company registered under the Securities Exchange Act of 1934, as amended, or the Exchange Act, with no specific business plan or purpose until it began operating the business of Intra-Cellular Therapies, Inc. (now re-named ITI, Inc., or ITI) through a reverse merger transaction on August 29, 2013 (the “Merger”). ITI was incorporated in Delaware in May 2001 to focus primarily on the development of novel drugs for the treatment of neuropsychiatric and neurologic diseases and other disorders of the central nervous system. Effective upon the Merger, a wholly-owned subsidiary of the Company merged with and into ITI, and ITI continues as the operating subsidiary of the Company.
Our corporate headquarters and laboratory are located at 430 East 29th Street, New York, New York 10016, and our telephone number is (212) 923-3344. We also have an office in Towson, Maryland. We maintain a website at www.intracellulartherapies.com, to which we regularly post copies of our press releases as well as additional information about us. The information contained on, or that can be accessed through, our website is not a part of this prospectus supplement or the accompanying prospectus. We have included our website address in this prospectus supplement solely as an inactive textual reference.
Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act are available free of charge through the investor relations page of our internet website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
All brand names or trademarks appearing in this prospectus supplement and the accompanying prospectus are the property of their respective holders. Use or display by us of other parties’ trademarks, trade dress, or products in this prospectus supplement and the accompanying prospectus is not intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners.
6
