Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CymaBay Therapeutics, Inc. | d884055d8k.htm |
 Cowen Health Care
Conference
March 4, 2015
Boston, MA
Harold Van Wart, Ph.D.
President and CEO
Exhibit 99.1 |
 2
Safe Harbor Statement
This presentation contains "forward-looking" statements that involve risks, uncertainties
and assumptions, and actual results may differ substantially from those projected or
expected in the forward-looking statements. Forward-looking statements include, but are
not limited to: any projections of financial information; any statements about future
development, clinical or regulatory events; any statements concerning CymaBay's plans,
strategies or objectives; and any other statements of expectation or belief regarding future
events. These statements are based on estimates and information available to CymaBay at
the time of this presentation and are not guarantees of future performance. Actual results
could differ materially from CymaBay's current expectations as a result of many factors
including, but not limited to: CymaBay's ability to obtain additional financing to fund its
operations; unexpected delays or results in clinical trials; uncertainties regarding obtaining
regulatory approvals; uncertainties regarding the ability to protect CymaBay's intellectual
property; uncertainties regarding market acceptance of any products for which CymaBay is
able to obtain regulatory approval; the effects of competition; and other market and general
economic conditions. You should read CymaBay's Quarterly Report on Form 10-Q filed with
the SEC on November 14, 2014, especially under the caption “Risk Factors,” which is
available on the SEC web site at http://www.sec.gov, for a fuller discussion of these and
other risks relating to an investment in CymaBay’s common stock. CymaBay assumes no
obligation for and does not intend to update these forward-looking statements, except as
required by law.
|
 3
CymaBay Highlights
•
Arhalofenate
is
the
first
drug
in
the
Urate
Lowering
Anti-Flare
Therapy
(ULAFT)
class for the treatment of gout
Reduces gout flares and lowers serum uric acid (sUA)
Refined product profile established in recently completed Phase 2 studies
Good overall and renal safety data in over 1,000 subjects
•
MBX-8025 is a potential novel treatment for rare or serious lipid and liver
disorders, including homozygous familial hypercholesterolemia (HoFH)
Positive effects on lipids and markers of liver health demonstrated in mixed
dyslipidemia Phase 2 trial
Focusing further development in rare or orphan diseases
•
Near-term, value-driving catalysts
Arhalofenate End-of-Phase 2 meeting with FDA in 3Q 2015
Phase 2 trial initiation for MBX-8025 in HoFH expected in 1H 2015
|
 Key
Features of Gout Hyperuricemia, urate crystal deposits and flares
Hyperuricemia
Serum Uric
Acid (sUA)
Mono Sodium
Urate (MSU)
crystal deposits
Inflammatory
response
IL-1
Painful flare
Therapeutic
targets
Joint
erosion
4 |
 5
Current Treatment of Gout
Uric acid Lowering Therapies (ULTs) and anti-inflammatories
–
Treatment paradigm (ACR Guidelines)
–
Anti-inflammatory to treat the flare
–
ULT
is
initiated
to
address
the
hyperuricemia
with
a
goal
of
sUA
<
6
mg/dL
to
debulk offending MSU burden
–
Initiation
of
ULT
increases
flare
risk,
requiring
dose
titration
and
colchicine
prophylaxis
–
Anti-inflammatory drugs
–
Colchicine (Colcrys)
–
NSAIDs, steroids
–
Ilaris (anti-IL-1 biologic) approved in EU
•
ULTs
–
Xanthine oxidase (XO) inhibitors (allopurinol, febuxostat)
–
Uricosurics (probenecid, lesinurad in development)
–
Pegloticase (for severe treatment failure gout) |
 6
Gout Patients Are Poorly Served by Available Drugs
Need for more sUA lowering and better control of flares
•
More sUA lowering
–
~2
million
patients
do
not
reach
their
sUA
goal
on
their
current
ULT
•
<6 mg/dL for patients without tophi
•
<5 mg/dL for patients with tophi
–
~300 thousand patients are allopurinol intolerant
–
Inadequate responders to ULT continue to increase their MSU burden and
the disease continues to progress
•
Better flare control
–
~1 million patients experience 3 flares/year
–
Patients care about the pain, suffering and medical costs of flares
–
Colchicine,
NSAIDs
and
steroids
are
“strongly
contraindicated”
in
>40%
of
gout patients due to their comorbidities
–
Colchicine non-compliance is estimated to be ~40% |
 7
Arhalofenate
The First Urate Lowering Anti-Flare Therapy (ULAFT)
•
Lowers sUA by improving uric acid excretion in
the kidney (uricosuric effect)
–
Blocks urate reabsorption by URAT1
–
Same target as lesinurad
–
Very favorable PK for a uricosuric drug
•
50 hour half-life produces gradual
changes in serum and urinary UA with
small intraday fluctuations
•
Avoids “hyperuricosuria”
•
Reduces flares through
anti-inflammatory
properties and long plasma
half-life
–
Suppresses MSU crystal-induced
IL-1 in gouty joints
–
No systemic suppression of IL-1 and no
infection risk
Human
URAT1
Arhalofenate Acid (M)
10
-6
10
-5
10
-4
10
-3
0
20
40
60
80
100
IC
50
=
92
M |
 *
NHANES 2008 ** Source Healthcare 2012
Gout Population Segregated by sUA Status
Treating inadequate responders with a uricosuric drug
Uricosuric drug
Uricosuric drug
Uricosurics in Development:
Lesinurad (ULT)
Arhalofenate (ULAFT)
The original positioning of
arhalofenate was for the ~1M
patients flaring >3 times/year
Allopurinol
Intolerant
~300 K
Diagnosed
Gout*
8.3 M
ULT Treated**
>90% Allopurinol
~3.3 M
Inadequate
Responders
~2 M
Responders
~1.3 M
8 |
 9
Arhalofenate Phase 2 Clinical Program for Gout
•
Five Phase 2 studies completed
–
Monotherapy with and without colchicine
–
Combination with febuxostat and allopurinol
–
Arhalofenate doses of 400, 600 and 800 mg
•
Summary of results
–
Very effective sUA lowering in combination with febuxostat (40 or 80 mg)
–
Arhalofenate monotherapy is an alternative for the XOI intolerant patients
–
Arhalofenate has anti-flare activity
•
Provides the symptomatic relief that patients really want
•
Helps patients in which colchicine is contraindicated or not tolerated
–
Does not require dose titration
•
Refined product positioning
–
Recent arhalofenate Phase 2 data and lesinurad news has shifted
positioning to address the larger sUA inadequate responder segment
|
 10
Arhalofenate Febuxostat Phase 2 Study
•
Objectives
–
Assess sUA reductions of different dose combinations
–
Measure the interday and intraday fractional excretion of UA (FEUA)
–
Assess if there is a drug-drug interaction
–
Additional safety data for arhalofenate/febuxostat combination
N = 16 per cohort ; PK from cohort 2 at Weeks 2, 4 and 6
All patients received colchicine for flare prophylaxis
Cohort 1
Cohort 2
Weeks 1-2
Week 3
Week 4
Weeks 5-6
Arhalofenate 600
Febuxostat 80 +
Arhalofenate 600
Febuxostat 40 +
Arhalofenate 600
Febuxostat 40
Arhalofenate 800
Febuxostat 40 +
Arhalofenate 800
Febuxostat 80 +
Arhalofenate 800
Febuxostat 80 |
 Arhalofenate Febuxostat Phase 2 Study
Changes in sUA for treatment phases
sUA = -33%
RR = 43%
sUA = -24%
RR = 100%
Arhalofenate decrease sUA
by an additional 24% and
increases the Responder
Rate from 43 to 100%
Baseline
Fbx (40 mg)
Fbx (40 mg) +
Arhalo (800 mg)
0
2
4
6
8
10
12
11 |
 12
Arhalofenate Febuxostat Phase 2 PK/PD Study
sUA responder rate for febuxostat 40 mg treatments
< 6.0 mg/dL
< 5.0 mg/dL < 4.0 mg/dL
< 3.0 mg/dL
Febuxostat (40 mg) +
Arhalofenate (800 mg)
Febuxostat (40 mg) +
Arhalofenate (600 mg)
Febuxostat (40 mg)
N
15
14 15
p-values reflect comparisons vs. febuxostat 40 mg. McNemar’s exact
test and Fischer’s exact test were used for comparisons within and
between cohorts, respectively.
* p < .05 ** p < .01 *** p < .001
*
**
***
0
20
40
60
80
100 |
 13
< 6.0 mg/dL
< 5.0 mg/dL < 4.0 mg/dL
< 3.0 mg/dL
Febuxostat (80 mg) +
Arhalofenate (800 mg)
Febuxostat (80 mg) +
Arhalofenate (600 mg)
Febuxostat (80 mg)
N
14
16
14
Arhalofenate Febuxostat Phase 2 PK/PD Study
sUA responder rate for febuxostat 80 mg treatments
*
* p < .05 for comparison vs. febuxostat 80 mg; McNemar’s exact
test 0
20
40
60
80
100 |
 14
Time Period
*** p < 0.001
Matched pairs
t-test
Fractional Excretion of Uric Acid (FEUA)
Normal Range*
* Perez-Ruiz et al.,
Arthr. Rheum. 47, 610
(2002)
Day 0 (n = 8)
Day 14 (n = 8)
Mean ±
SE
9 am to
3 pm
3 pm to
9 pm
9 pm to
9 am
***
***
***
2
3
4
5
6
7
8
9
10
Arhalofenate Febuxostat Phase 2 PK/PD Study
Intraday and interday variation in FEUA for arhalofenate (800 mg)
14 |
 •
Design
–
Randomized double blind placebo-
and active-controlled study
–
12-week duration
–
248 gout patients who had >3 flares in prior year
•
Goal for arhalofenate
–
Establish statistically significant reduction in gout flares
–
Show anti-flare effect in the absence of colchicine
•
Primary end point
–
Mean flares/patient
•
Secondary end point
–
Reduction in sUA
Arhalofenate Phase 2b Flare Study
Arhalofenate flare study
Electronic diary
for patient flare
reporting |
 16
Arhalofenate Phase 2b Study Design
Primary Endpoint:
Reduction in flare
rate for arhalofenate
compared to allopurinol
n = 25
n = 50
n = 50
n = 50
n = 50
Allopurinol 300 mg + Colchicine
Allopurinol 300 mg
Arhalofenate 600 mg
Placebo
Flare Rescue
3 Month Treatment Phase
2 Week
Follw-up
Arhalofenate 800 mg |
 Arhalofenate Phase 2b Flare Study
Most frequent adverse events and safety summary
Placebo
Arhalofenate
600 mg
Arhalofenate
800 mg
Allopurinol
300 mg + COL
Allopurinol
300 mg
N
28
53
51
53
54
AEs
17 (60.7%)
24 (45.3%)
21 (41.2%)
24 (45.3%)
22 (40.7%)
SAEs
0
1
0
1
3
Discon due to
AE or Lab
1
1
1
5
3
CK increased
0
3 (5.7%)
2 (3.9%)
3 (5.7%)
3 (5.6%)
URT
Infection
2 (7.1%)
3 (5.7%)
2 (3.9%)
2 (3.8%)
0
Headache
1 (3.6%)
3 (5.7%)
2 (3.9%)
0
2 (3.7%)
Hypertension
2 (7.1%)
1 (1.9%)
2 (3.9%)
2 (3.8%)
1 (1.9%)
Creatinine
>1.5X and
>ULN
0
0
0
0
0
17 |
 Arhalofenate Phase 2b Flare Study
Arhaolfenate 800 mg dose met the primary flare end point
Arhalofenate (800 mg)
vs. allopurinol (300 mg) + colchicine
were not statistically different
(p = .091)
-46%
p= 0.0056
Arhalofenate
(600 mg)
Allopurinol
(300 mg)
Placebo
Allopurinol
(300 mg) +
Colchicine
Arhalofenate
(800 mg)
-68%
p < 0.0001
-41%
p = 0.049
0.00
0.25
0.50
0.75
1.00
1.25
1.50
N
53 51 53
54 28
1.13
1.04
0.66
1.24
0.40
18 |
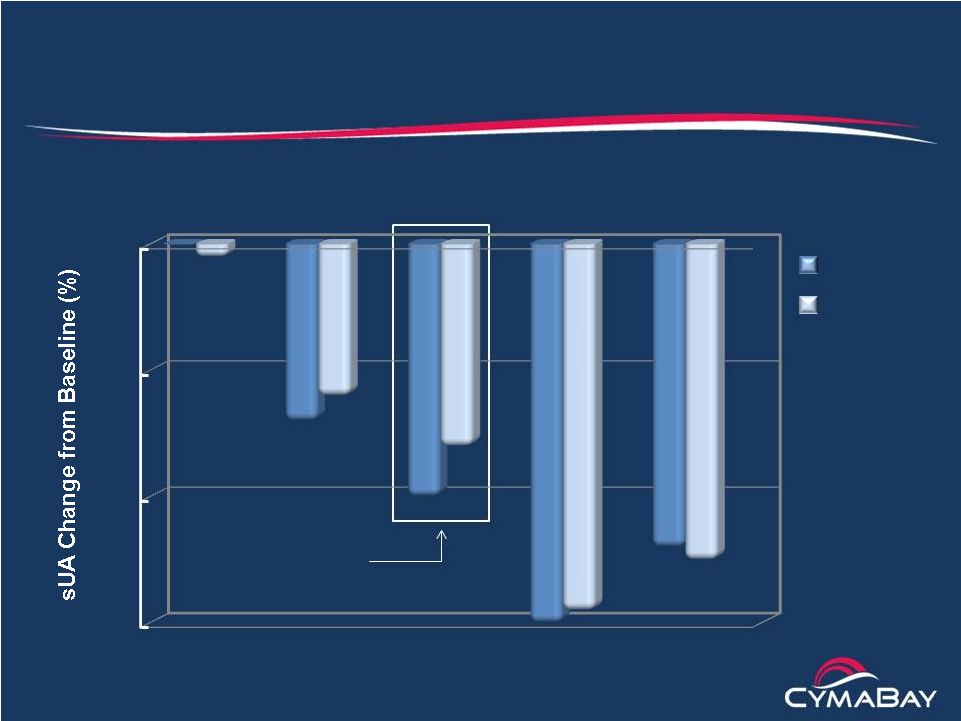 Arhalofenate Phase 2b Flare Study
Mean lowering of serum uric acid
8 weeks
p < .01 vs. Pbo
for all changes
12 weeks
Arhalofenate
(600 mg)
Allopurinol
(300 mg)
Placebo
Allopurinol
(300 mg) +
Colchicine
Arhalofenate
(800 mg)
Baseline sUA
9.1 9.0 9.1
9.2
9.1 16-20% drop in sUA
projected to give RR = 100%
in combo with Fbx
(40 mg)
-30
-20
-10
0
19
N
28 53 51
53
54 |
 20
Allopurinol
Intolerant
~300 K
Inadequate
Responders
~2 M
Arhalofenate (800 mg)
+ Febuxostat (80 mg)
Arhalofenate
(800 mg)
Arhalofenate Target Population
Major target is inadequate responders on current ULT
Patients
w/o Tophi
~1.6 M
Patients
with Tophi
~ 0.4 M
Arhalofenate (800 mg)
+ Febuxostat (40 mg)
Great majority reach their sUA goal
Experience fewer flares
Don’t need colchicine prophylaxis
No need for dose titration
A fixed dose combination
pill is in development for
patient convenience
Benefits to Patient |
 21
Comparison of Arhalofenate with Lesinurad
Arhalofenate (800 mg)
Lesinurad (200 mg)
sUA
lowering 16-24% (Ph 2)
Monotherapy
for
allopurinol intolerant
Responder Rate (< 6
mg/dL)
increase with
XOI
On top of Allo (300 mg)
On top of Fbx (40
mg)
Flare
benefit
Colchicine
Prophylaxis
needed
Renal
safety
* Ardea presentation materials **ACR presentation, 2014
No signal
No
Yes
57% (Ph 2)
Yes
No**
38% (Ph 2)*; 25% (Ph 3)**
No**
16% (Ph 2)* |
 22
Arhalofenate Clinical Studies
Safety summary
•
Completed 9 Phase 2 clinical studies (200-800 mg)
–
More than 1,000 subjects exposed to arhalofenate for up to 6 months
•
General safety
–
Adverse events similar to placebo and balanced across dose groups
–
Low incidence of asymptomatic liver transaminase elevations
–
No change in neutrophils or increase in infections
•
Renal safety
–
No kidney stones or decrease in urine pH
–
No creatinine signal
•
No dose-limiting toxicity has been identified |
 23
Arhalofenate Nonclinical Studies
Non-clinical
development
status •
Drug materials
–
Economical, proprietary synthesis
–
Tablet formulations developed
–
Fixed dose formulation with febuxostat in development
•
Completed preclinical safety package
–
Sub-chronic and chronic toxicology
–
Safety pharmacology and reproductive toxicology
–
Two-year carcinogenicity studies
–
Carcinogenicity and CV safety review by FDA completed
•
All results support further development |
 24
•
Non-tophaceous gout (n = 1,000) in allopurinol inadequate responders
–
Febuxostat (40 mg) + colchicine vs. febuxostat (40 mg) + arhalofenate (800 mg)
for 6 months + 6 month safety extension
–
Primary endpoint: sUA responder rate (< 6 mg/dL) at 6 months
–
Secondary end point: average flares/patient at 6 months
•
Tophaceous gout (n = 300) in allopurinol inadequate responders
–
Febuxostat (80 mg) + colchicine vs. febuxostat (80 mg) + arhalofenate (800 mg)
for 6 months
–
Primary endpoint: sUA responder rate (< 5 mg/dL) at 6 months
–
Secondary endpoints: flare rate and tophi resolution at 12 months
•
Patients intolerant to allopurinol (n = 200)
–
Placebo and arhalofenate (800 mg) for 6 months with 6-month safety
extension –
Primary endpoint: average flares per patient at 6 months
–
Secondary end point: sUA responder rate (< 6 mg/dL) at 6 months
Phase 3 Program for Arhalofenate
Preliminary study design |
 25
MBX-8025
•
MBX-8025
–
Potent selective PPAR-
agonist
–
Once daily orally administered
•
Status
–
Originally in development for mixed dyslipidemia
–
Phase
2
study
demonstrated
favorable
effects
on
lipids
and
liver
biomarkers
–
FDA now requiring a preapproval CV outcome study for this indication
•
Redirecting program to higher unmet need indications
–
Homozygous familial hypercholesterolemia (HoFH)
–
Primary biliary cirrhosis (PBC)
–
Severe refractory hypertriglyceridemia (SHTG)
–
Nonalcoholic steatohepatitis (NASH)
O
HO
O
S
O
O
CF
3
(R) |
 26
•
Orphan disease affecting 1 in 1 million
–
Markedly elevated LDL-C (>500 mg/dL) caused
by
loss-of-function mutations in LDL receptor (LDL-R)
–
Cardiovascular disease (MI, stroke, CAD) before the age of 20
–
Mean age of death is in the 30s
–
Current therapy is focused on lowering LDL-C
•
Therapies that raise LDL-R activity are minimally effective
–
Statins, bile acid sequestrants, cholesterol
absorption inhibitors, PCSK9
inhibitors •
Current therapeutic options
–
LDL apheresis
–
Juxtapid (lomitapide)
–
Kynamro (mipomersen)
28 year-old female with
cutaneous xanthoma
Homozygous Familial Hypercholesterolemia (HoFH)
Rare disease of impaired cholesterol metabolism |
 27
Unmet Medical Need in HoFH Remains High
Patients need more effective and better tolerated therapies
•
LDL apheresis is inconvenient and has many complications
•
Juxtapid (Microsomal Transfer Protein Inhibitor)
–
Only 28% of patients achieve LDL < 100 mg/dL
–
Dose limiting GI tolerability
–
Risk of hepatotoxicity due to increase in hepatic fat
–
Black box and REMs requiring monthly liver testing
•
Kynamro (oligonucleotide inhibitor of apo-B synthesis)
–
Risk of hepatotoxicity due to increase in hepatic fat
–
Black box and REMs requiring monthly liver testing
–
Flu-like symptoms and injection site reactions |
 28
MBX-8025 Phase 2 Mixed Dyslipidemia Study
Change in LDL-C as a function of baseline LDL-C
-60
-50
-40
-30
-20
-10
0
Placebo
MBX-8025 (50 mg)
MBX-8025 (100 mg)
LDL-C range
83-242
175
180
190
195
Mean LDL-C
159-169
186-197
189-205
195-207
197-215
N
28,27,32
8,10,11
6,9,10
3,7,9
2,5,6 |
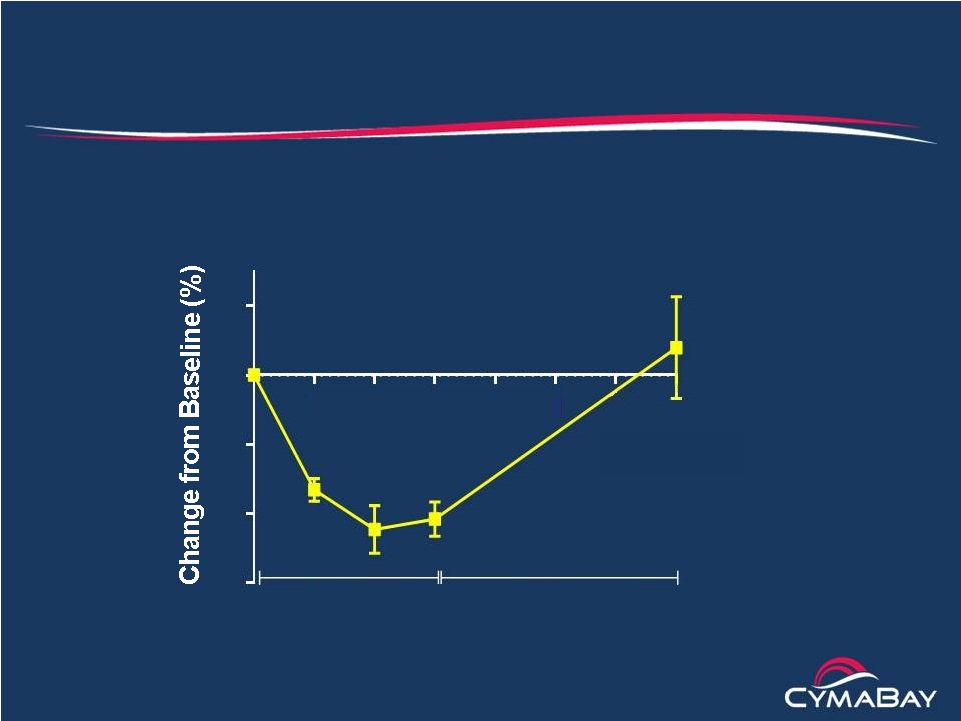 29
Effect of MBX-8025 in the WHHL Rabbit Model of HoFH
Significant and sustained reductions in LDL-C
The Watanabe
Heritable
Hyperlipidemic
(WHHL) rabbit
has low (<5%)
LDL-R activity
LDL-C
(Mean
±
SD, n = 5)
1
2
3
4
5
6
7
-60
-40
-20
0
20
MBX-8025
Time (weeks)
Washout |
 30
MBX-8025 for the Treatment of HoFH
Rationale and pilot study design
•
Rationale
–
MBX-8025 exhibited a strong anti-atherogenic profile in patients with
mixed dyslipidemia including reductions in LDL-C
–
Data from the WHHL rabbit model suggest that the decreases in
LDL-C would be retained in the setting of low LDL-R activity
characteristic of HoFH
•
Pilot study design
–
Open label dose escalation study in up to 8 patients
–
Doses are 50, 100 and 200 mg
–
Study duration of 3 months
–
Study to be conducted in 2-3 countries in Europe
–
Start of study planned for 1H 2015 |
 31
CymaBay Projected Milestones
•
Arhalofenate
–
Dose first patient in Phase 2b study
1H 2014
–
Phase 2 febuxostat combo headline data
1Q 2015
–
Phase 2b flare study headline data
2Q 2015
–
End-of-Phase 2 meeting
2H 2015
–
Start Phase 3
1H 2016
•
MBX-8025
–
Select indication for proof-of-concept
2H 2014
–
Start pilot study in HoFH
1H 2015 |
