Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - SALIX PHARMACEUTICALS LTD | d867379dex321.htm |
| EX-31.2 - EX-31.2 - SALIX PHARMACEUTICALS LTD | d867379dex312.htm |
| EX-32.2 - EX-32.2 - SALIX PHARMACEUTICALS LTD | d867379dex322.htm |
| EX-31.1 - EX-31.1 - SALIX PHARMACEUTICALS LTD | d867379dex311.htm |
| EX-23.1 - EX-23.1 - SALIX PHARMACEUTICALS LTD | d867379dex231.htm |
| EX-21.1 - EX-21.1 - SALIX PHARMACEUTICALS LTD | d867379dex211.htm |
| EXCEL - IDEA: XBRL DOCUMENT - SALIX PHARMACEUTICALS LTD | Financial_Report.xls |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K/A
(Amendment No. 1)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year ended December 31, 2013
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period from to
Commission File Number: 000-23265
Salix Pharmaceuticals, Ltd.
(Exact name of Registrant as specified in its charter)
| Delaware | 94-3267443 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
8510 Colonnade Center Drive
Raleigh, North Carolina 27615
(Address of principal executive offices, including zip code)
(919) 862-1000
(Registrant’s telephone number, including area code)
Securities Registered Pursuant to Section 12(b) of the Act:
| Title of Each Class |
Name of Exchange on which Registered | |
| Common Stock, $0.001 Par Value | Nasdaq Global Market |
Securities Registered Pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES x NO ¨
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ¨ NO x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES x NO ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES x NO ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Act (Check one):
| Large accelerated filer | x | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ | Smaller reporting company | ¨ | |||
Indicate by check mark whether registrant is a shell company (as defined) in Rule 12b-2 of the Exchange Act. YES ¨ NO x
The aggregate market value of the Registrant’s common stock held by non-affiliates of the Registrant on June 28, 2013 (based on the closing sale price of U.S. $66.15 of the Registrant’s common stock, as reported on The Nasdaq Global Market on such date) was approximately U.S. $3,367,145,603. Common stock held by each officer and director and by each person known to the Company who owned 10% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The number of shares of the Registrant’s common stock outstanding at February 24, 2014 was 63,334,392.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement to be filed for its 2014 Annual Meeting of Stockholders currently scheduled to be held June 12, 2014 are incorporated by reference into Part III of this report.
Table of Contents
Explanatory Note
We are filing this amended Form 10-K/A (“Form 10-K/A”) to amend our Annual Report on Form 10-K for the year ended December 31, 2013, originally filed with the Securities and Exchange Commission (the “SEC”) on February 28, 2014 (“Original Filing”), to restate our consolidated financial statements and related footnote disclosures for the year ended December 31, 2013. This Form 10-K/A also includes certain restated quarterly information under the Supplementary Information heading in Item 8 of this Form 10-K/A. This Form 10-K/A also amends certain other Items in the Original Filing, as listed in “Items Amended in this Form 10-K/A” below.
Restatement Background
On January 22, 2015, the Audit Committee of the Board of Directors (the “Audit Committee”) of Salix Pharmaceuticals, Ltd. (the “Company”), after discussion with management and the Company’s independent registered public accounting firm, Ernst & Young LLP (“EY”), concluded that the Company’s previously issued audited consolidated financial statements for the year ended December 31, 2013 and the previously issued unaudited consolidated financial statements for the fiscal quarters ended March 31, June 30, and September 30, 2014, and the disclosures and related communications for each of those periods, required correction of certain errors and should no longer be relied upon. The Audit Committee also concluded that management’s report on internal controls over financial reporting for the year ended December 31, 2013 should no longer be relied upon. Additionally, EY concluded that EY’s opinion on the consolidated financial statements for the year ended December 31, 2013, as well as EY’s opinion on the effectiveness of the Company’s internal controls over financial reporting as of December 31, 2013 should no longer be relied upon.
In connection with the Audit Committee’s previously announced review of prior public disclosures of inventory amounts in the distribution channel and related issues in press releases, on analyst calls, and in the Company’s various SEC filings, outside counsel, reporting to the Audit Committee, engaged BDO Consulting, a division of BDO USA, LLP (“BDO”), to assist in the review of accounting and financial reporting issues associated with sales to wholesalers and other wholesaler-related transactions. The Audit Committee’s review also prompted the Company to reevaluate its classification of certain expenses. Based on this review, the Audit Committee determined that the Company had made certain errors in its accounting, which are primarily associated with the timing of recognition of certain revenue, revenue-reducing returns and discounts, and expenses.
As a result of these errors, we have restated our audited consolidated financial statements for the year ended December 31, 2013 and our unaudited consolidated financial statements for the fiscal quarters ended March 31, June 30, and September 30, 2014. The correction of these errors resulted in a reduction of net revenue of approximately $20.1 million and a reduction of net income and earnings per share of approximately $11.8 million and $0.18 per share, respectively, for the year ended December 31, 2013. Reference is made to Note 1A of the consolidated financial statements for a schedule detailing each change by category.
The Company recognized revenue for certain shipments to a wholesale customer with FOB Destination shipping terms, as set forth in a contract entered between the Company and the wholesale customer during the quarter ended December 31, 2013, that were not received by the customer by the close of the fiscal period. Of those shipments that were delivered to the wholesaler after the end of the fiscal period, nearly all were shipped four or more days prior to the end of the fiscal period but arrived at the wholesaler a few days into the next fiscal period. As a result, the Company incorrectly recognized gross revenue of approximately $14.4 million in the fiscal quarter ended December 31, 2013 that should have been recognized in the fiscal quarter ended March 31, 2014, based upon delivery in early January 2014. This $14.4 million of gross revenue was adjusted by reserves for returns, discounts and other allowances of approximately $2.5 million. There were also minor net adjustments required to cost of sales and royalties that decreased cost of sales by $1.6 million. The Company also recorded an increase of approximately $1.0 million in Research and Development (“R&D”) expenses in the fiscal quarter ended December 31, 2013 related to holding fees that were erroneously capitalized as inventory for a new product. This error relates to raw material inventory that should not have been capitalized based on a change in the stage of development of the product.
The following is a summary description of the errors that resulted in the $11.8 million decrease in net income. The Company incorrectly recognized a reserve for product returns of only $8.7 million at December 31, 2013 for certain out-of-policy returns of GIAZO® when it should have recognized a reserve of approximately $16.9 million, resulting in an understatement of the reserve balance of approximately $8.2 million in the fourth quarter of 2013. The Company had agreed to accept such returns in the fourth quarter of 2013. This correction resulted in an approximately $8.2 million reduction of net revenue in the fourth quarter of 2013 and a corresponding increase of approximately $8.2 million in net revenue for the first quarter of 2014, as the returns were originally recorded in the first quarter of 2014.
The above adjustments resulted in a reduction of income tax expense of approximately $7.6 million.
The Company incorrectly classified certain expenses as related to R&D instead of Selling, General, and Administrative (“SG&A”) expenses. These errors largely were a result of the Company’s historical practice of aligning certain expenses along organizational hierarchy, rather than the business activity. As a result, the Company incorrectly recorded expenses of approximately $38.2 million, $38.3 million and $32.5 million as R&D that should have been recognized as SG&A for the fiscal years ended
i
Table of Contents
December 31, 2013, 2012 and 2011, respectively. The corresponding corrections for the fiscal quarters in 2013 would be a reduction in R&D and an increase of SG&A of $8.7 million for the quarter ended March 31, 2013, $10.1 million for the quarter ended June 30, 2013, $9.0 million for the quarter ended September 30, 2013 and $10.4 million for the quarter ended December 31, 2013. The foregoing reclassifications do not impact previously reported revenue or net income (loss).
The revisions to the Company’s audited consolidated financial statements for the year ended December 31, 2013 are considered to be a “restatement” under U.S. generally accepted accounting principles. Accordingly, this revised financial information included in this Annual Report on Form 10-K/A has been identified as “restated”.
Restatement of Other Financial Statements
In addition to this Form 10-K/A, we are concurrently filing an amendment to our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014 (the “Form 10-Q/As”). We are filing the Form 10-Q/As to restate our unaudited condensed consolidated financial statements and related financial information for the periods contained in those reports and to amend certain other items within those reports.
Internal Control Consideration
Our management has determined that there were deficiencies in our internal control over financial reporting that constitute material weaknesses, as defined by SEC regulations, at December 31, 2013, as discussed in Part II, Item 9A of this Form 10-K/A.
Items Amended in this Form 10-K/A
For the convenience of the reader, this Form 10-K/A sets forth the Original Filing, in its entirety, as modified and superseded as necessary to reflect the restatement. The following items in the Original Filing have been amended as a result of, and to reflect, the restatement:
| A. | Part I, Forward-Looking Statements |
| B. | Part I, Item 1. Business. |
| C. | Part I, Item 1A. Risk Factors. |
| D. | Part II, Item 6. Selected Financial Data |
| E. | Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations |
| F. | Part II, Item 8. Financial Statements and Supplementary Data |
| G. | Part II, Item 9A. Controls and Procedures |
| H. | Part IV, Item 15. Exhibits, Financial Statement Schedules |
This report on Form 10-K/A is presented as of the filing date of the Original Form 10-K and does not reflect events occurring after that date, or modify or update the information contained therein other than as required to correct the error and record the adjustments described above.
In particular, the information in Part I, Executive Officers of the Registrant, has not been updated to reflect events occurring after the original filing date of the Form 10-K and investors should refer to the Company’s most recent filings with the SEC for current information regarding the Company’s executive officers, including the Company’s Current Reports on Form 8-K filed on November 7, 2014, January 5, 2015 and February 5, 2015.
In accordance with applicable SEC rules, this Form 10-K/A includes new certifications required by Sections 302 and 906 of the Sarbanes-Oxley Act of 2002, as amended, from our Acting Chief Executive Officer and Acting Chief Financial Officer dated as of the filing date of this Form 10-K/A.
ii
Table of Contents
ANNUAL REPORT ON FORM 10-K/A
TABLE OF CONTENTS
| Page | ||||||
| PART I | ||||||
| Item 1. | 2 | |||||
| Item 1A. | 36 | |||||
| Item 1B. | 47 | |||||
| Item 2. | 48 | |||||
| Item 3. | 48 | |||||
| Item 4. | 52 | |||||
| 52 | ||||||
| PART II | ||||||
| Item 5. | 54 | |||||
| Item 6. | 56 | |||||
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
57 | ||||
| Item 7A. | 79 | |||||
| Item 8. | 80 | |||||
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
81 | ||||
| Item 9A. | 81 | |||||
| Item 9B. | 82 | |||||
| PART III | ||||||
| Item 10. | 83 | |||||
| Item 11. | 83 | |||||
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
83 | ||||
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
84 | ||||
| Item 14. | 84 | |||||
| PART IV | ||||||
| Item 15. | 85 | |||||
| SIGNATURES | 94 | |||||
i
Table of Contents
PART I
Forward-Looking Statements
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Such statements are generally accompanied by words such as “anticipate,” “assume,” “believe,” “could,” “estimate,” “expect,” “foresee,” “goal,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “seek,” “target,” “will” or similar expressions that convey uncertainty as to future events or outcomes. Forward-looking statements are based on assumptions and beliefs that we believe to be reasonable; however, assumed facts almost always vary from actual results, and the differences between assumed facts and actual results can be material depending upon the circumstances. Where we or our management express an expectation or belief as to future results, that expectation or belief is expressed in good faith and based on assumptions believed to have a reasonable basis. We cannot assure you, however, that the stated expectation or belief will occur or be achieved or accomplished. Among the factors that could cause our results to differ materially from those indicated by forward-looking statements are risks and uncertainties inherent in our business including, without limitation:
| • | our dependence on our pharmaceutical products, particularly Xifaxan® (rifaximin) tablets 550 mg, and the uncertainty of market acceptance of our products; |
| • | our level of indebtedness could require us to devote a substantial portion of our cash flow to debt service, reducing funds available for other purposes or otherwise constraining our financial flexibility; |
| • | our status as a holding company, which makes us reliant on our subsidiaries to meet our obligations; |
| • | restrictive covenants in our debt agreements could limit our operational and financial flexibility; |
| • | our ability to realize the full extent of the anticipated benefits of our acquisition of Santarus, including achieving operational cost savings and synergies, in light of potential delays we may encounter in the integration process and additional unforeseen expenses; |
| • | the elevated levels of inventory of our key products held by our wholesale customers, which may result in decreased future sales and/or increased pressure by wholesalers for us to extend greater pricing discounts in order to generate sales; |
| • | intense competition, including from generics, in an increasingly global market; |
| • | the possible impairment of, or inability to obtain intellectual property rights and the costs of obtaining such rights from third parties in an increasingly global market; |
| • | the high cost and uncertainty of the research, clinical trials and other development activities involving pharmaceutical products; |
| • | the unpredictability of the duration and results of regulatory review of New Drug Applications, or NDAs, and Investigational New Drug Applications, or INDs; |
| • | general economic and business conditions; |
| • | our need to maintain profitability and acquire new products; |
| • | the uncertainty of obtaining, and our dependence on, third parties to supply and manufacture our products; |
| • | post-marketing approval regulation, including the ongoing Department of Justice investigation of our marketing practices; |
| • | revenue recognition and other critical accounting policies; and |
| • | results of ongoing and any future litigation. |
Our forward-looking statements are expressly qualified by these cautionary statements, which you should consider carefully, along with the risks discussed under the heading “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this report, that could cause actual results to differ materially from historical results or anticipated results. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law.
1
Table of Contents
Corporate Information
Salix Pharmaceuticals, Ltd., or Salix, is a Delaware corporation organized in 2001. Our website address is www.salix.com. Information on our website, Twitter feed and Facebook page are not incorporated herein by reference. We make available free of charge through our website our press releases, Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, proxy statements and all amendments to those documents as soon as reasonably practicable after they are electronically filed with or furnished to the Securities and Exchange Commission.
Unless otherwise indicated or required by the context, the terms “we,” “our,” “us” and the “Company” refer to Salix Pharmaceuticals, Ltd. and all of its subsidiaries.
OVERVIEW
We are a specialty pharmaceutical company dedicated to acquiring, developing and commercializing prescription drugs and medical devices used in the treatment of a variety of gastrointestinal disorders, which are those affecting the digestive tract. Our strategy is to:
| • | identify and acquire rights to products that we believe have potential for near-term regulatory approval or are already approved; |
| • | apply our regulatory, product development, and sales and marketing expertise to commercialize these products; and |
| • | market our products through our specialty sales and marketing team by primarily focusing on high-prescribing U.S. physicians in the following specialties: gastroenterologists, who are doctors who specialize in gastrointestinal disorders; hepatologists, who are doctors who specialize in liver disease; colorectal surgeons, who are doctors who specialize in disorders of the colon and rectum and primary care doctors. |
Significant Developments in 2014
| • | On January 2, 2014, we completed our acquisition of Santarus, a specialty biopharmaceutical company focused on acquiring, developing and commercializing proprietary products that address the needs of patients treated by physician specialists in gastroenterology and endocrinology, for aggregate consideration of approximately $2.6 billion. As a result of the acquisition, Santarus became one of our indirect wholly owned subsidiaries. We financed the acquisition through a combination of (i) a term loan facility in the principal amount of $1.2 billion, (ii) The net proceeds from our issuance of $750 million of 6.00% senior notes due 2021, or the 2021 Notes, and (iii) cash on hand of approximately $800.4 million. |
| • | On January 30, 2014, the Food and Drug Administration, or FDA, accepted for filing an NDA for Budesonide 2 mg rectal foam for the induction of remission in patients with active mild to moderate ulcerative proctitis, extending up to 40 cm from the anal verge. The FDA has issued an action date of September 15, 2014 under the Prescription Drug User Fee Act, or PDUFA. |
| • | On February 25, 2014 a New York Supreme Court entered a verdict in favor of Salix in a lawsuit filed by Napo Pharmaceuticals related to the FULYZAQ® (crofelemer) collaboration agreement. See Part I. Item 3. Legal Proceedings. |
Introduction to Products
After the integration of Santarus, we will execute our strategy by:
| • | marketing our currently marketed products (including Santarus’s product portfolio) through our established channels and Santarus’s network of high volume specialty and primary care physician-focused sales representatives; |
| • | utilizing our expertise to progress pipeline products and indications through late-stage development and approval; and |
| • | making select acquisitions to further diversify our product portfolio and improve profitability. |
2
Table of Contents
In connection with the execution of this strategy, we now offer over 20 marketed products. We believe that these product offerings place us in a premier position in gastroenterology and create the opportunity for us to expand our digestive disease expertise into other key specialties.
As of December 31, 2013, our products were:
| • | XIFAXAN550® (rifaximin) tablets 550 mg, indicated for the reduction in risk of overt hepatic encephalopathy, or HE, recurrence in patients 18 years of age and older; |
| • | XIFAXAN (rifaximin) tablets 200 mg, indicated for the treatment of patients 12 years of age and older with travelers’ diarrhea, or TD, caused by noninvasive strains of Escherichia coli, or E. coli; |
| • | APRISO® (mesalamine) extended-release capsules, indicated for the maintenance of remission of ulcerative colitis, or UC; |
| • | MOVIPREP® (polyethylene glycol-salt, or PEG, 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate and ascorbic acid for oral solution), indicated for cleansing of the colon as a preparation for colonoscopy in adults 18 years of age or older; |
| • | RELISTOR® (methylnaltrexone bromide) subcutaneous injection, or SI, indicated for the treatment of opioid-induced constipation, or OIC, in patients with advanced illness who are receiving palliative care, when the response to laxative therapy has not been sufficient; |
| • | OSMOPREP® (sodium phosphate monobasic monohydrate, USP and sodium phosphate dibasic anhydrous, USP) tablets, indicated for cleansing of the colon as a preparation for colonoscopy in adults 18 years of age or older; |
| • | SOLESTA®, a biocompatible tissue-bulking agent indicated for the treatment of fecal incontinence in patients 18 years or older who have failed conservative therapy; |
| • | DEFLUX®, a biocompatible tissue-bulking agent indicated for the treatment of vesicoureteral reflux, or VUR, grades II-IV; |
| • | FULYZAQ® (crofelemer) delayed-release tablets, indicated for the symptomatic relief of non-infectious diarrhea in adult patients with HIV/AIDS on anti-retroviral therapy; |
| • | GIAZO® (balsalazide disodium) tablets, indicated for the treatment of mildly to moderately active UC in male patients 18 years of age and older; |
| • | METOZOLV® ODT (metoclopramide hydrochloride) 5 mg and 10 mg orally disintegrating tablets, indicated for short-term (4 to 12 weeks) use in adults for treatment of refractory gastroesophageal reflux disease, or GERD, that fails to respond to conventional therapy, and for relief of symptoms of acute and recurrent diabetic gastroparesis; |
| • | AZASAN® (azathioprine, USP), 75 mg and 100 mg tablets, indicated as an adjunct for the prevention of rejection in renal homotransplantations and to reduce signs and symptoms of severe active rheumatoid arthritis; |
| • | ANUSOL-HC® (hydrocortisone, USP) 2.5% cream and ANUSOL-HC® (hydrocortisone acetate) 25 mg suppositories, indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses; |
| • | PROCTOCORT® (hydrocortisone, USP) 1% cream and PROCTOCORT® (hydrocortisone acetate) 30 mg suppositories, indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses; |
| • | PEPCID® (famotidine) for oral suspension, indicated for the short-term treatment of GERD, active duodenal ulcer, active benign gastric ulcer, erosive esophagitis due to GERD, and peptic ulcer disease; |
| • | DIURIL® (chlorothiazide) oral suspension, indicated for the treatment of hypertension and also as adjunctive therapy in edema associated with congestive heart failure, cirrhosis of the liver, corticosteroid and estrogen therapy, and kidney disease; and |
| • | COLAZAL® (balsalazide disodium) capsules, indicated for the treatment of mildly to moderately active UC in patients 5 years of age and older. |
3
Table of Contents
As of December 31, 2013, Santarus’s products were:
| • | UCERIS® (budesonide MMX) extended release tablets, indicated for the induction of remission in patients with active, mild to moderate UC; |
| • | ZEGERID® (omeprazole/sodium bicarbonate) capsules and powder for oral suspension, indicated for the treatment of certain upper gastrointestinal conditions, including active duodenal ulcer, active benign gastric ulcer and GERD; |
| • | GLUMETZA® (metformin hydrochloride) extended release tablets, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus; |
| • | CYCLOSET® (bromocriptine mesylate) tablets, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus; and |
| • | FENOGLIDE® (fenofibrate) tablets, indicated as an adjunct to diet to reduce elevated low-density lipoprotein-cholesterol, or LDL-C, total cholesterol, triglycerides and apolipoprotein B, or Apo B, and to increase high-density lipoprotein-cholesterol, or HDL-C, in adult patients with primary hyperlipidemia or mixed dyslipidemia. Fenoglide also is indicated as an adjunct to diet for treatment of adult patients with hypertriglyceridemia. |
Our and Santarus’s primary product candidates currently under development and their status are as follows:
| Compound |
Condition |
Status | ||
| Rifaximin |
Irritable bowel syndrome, or IBS | Supplemental New Drug Application, or sNDA, submitted June 7, 2010; Complete Response Letter, or CRL, received on March 7, 2011; FDA meeting held on June 20, 2011; Advisory Committee meeting held on November 16, 2011; currently in Phase 3 retreatment study, with top-line data expected in July 2014 | ||
| Methylnaltrexone bromide SI |
OIC, in patients with chronic non-malignant pain; subcutaneous injection | sNDA accepted for filing; CRL received July 27, 2012; FDA Advisory Committee proposed to occur March 10-11, 2014 postponed | ||
| Budesonide foam |
Ulcerative proctitis | NDA accepted for filing January 2014; PDUFA action date September 15, 2014 | ||
| Methylnaltrexone bromide oral |
OIC in patients with chronic non-malignant pain; oral | Phase 3 | ||
| Rifaximin extended intestinal release (EIR) |
Crohn’s disease | Phase 3 | ||
| Rifaximin soluble solid dispersion (SSD)/next generation |
Prevention of complications in compensated liver cirrhosis | Phase 2 | ||
| Ruconest |
Hereditary angioedema (HAE) | Biologics License Application, or BLA, submitted April 2013; PDUFA action date extended to July 16, 2014 | ||
4
Table of Contents
Introduction to Sales Force
We have historically generated revenue primarily by selling our products to pharmaceutical wholesalers. These direct customers resell and distribute our products to and through pharmacies to patients who have had our products prescribed by doctors. Because demand for our products originates with doctors, we currently market our products, and intend to market future products, if approved by the FDA, to U.S. gastroenterologists, hepatologists, endocrinologists, colorectal surgeons, primary care and other physicians through our own direct sales force. We monitor new and total prescriptions for our products as key performance indicators for our business. Prescriptions result in our products being used by patients, requiring our direct pharmaceutical wholesaler customers to purchase more products to replenish their inventory. However, our revenue might fluctuate from quarter to quarter due to factors other than changes in prescriptions written, such as increased buying by wholesalers in anticipation of a price increase or because of the introduction of new products, or decreased buying by wholesalers (or wholesaler demands for increased product discounts) as a result of wholesalers holding a significant number of months of inventory of our key products. In such circumstances, revenue could be less than anticipated in subsequent quarters as wholesalers’ increased inventory is consumed.
For additional information regarding our sales force, see Part I. Item 1. “Business—Sales and Marketing.”
PRODUCTS
Salix products
Xifaxan (rifaximin) tablets
Xifaxan, our largest product, is a gastrointestinal-specific oral antibiotic. Xifaxan 550 mg was approved by the FDA in March 2010 for reduction in risk of overt HE recurrence in patients 18 years of age or older. HE is a chronic worsening of brain function that occurs when the liver is not able to remove toxic substances from the blood. We believe that there are approximately 400,000 patients in the United States who currently suffer from episodic overt HE. Additionally, the FDA approved Xifaxan 200 mg in May 2004 for the treatment of patients 12 years of age and older with TD caused by noninvasive strains of E coli. Xifaxan sales accounted for approximately 70%, 70%, and 69% of our consolidated revenue in 2013, 2012, 2011, respectively.
We are exploring potential additional indications, formulations, and clinical trials and co-promotion arrangements to capitalize on the potential for Xifaxan, including our development programs in IBS, Crohn’s disease and liver disease.
Xifaxan 550 mg has orphan drug exclusivity, granted by the FDA, for HE through March 24, 2017. We have 15 patents potentially providing combined protection on the Xifaxan molecule until 2027 and method of use patent protection for the TD, HE and IBS indications until 2029.
We filed in Canada in January 2013 to market Xifxan550 to treat hepatic encephalopathy and received marketing approval in August 2013.
Apriso (mesalamine) extended-release capsules
Apriso is a locally-acting aminosalicylate that was approved by the FDA in October 2008. It is the only delayed and extended release mesalamine product that is FDA-approved for the maintenance of remission of UC in adults. Apriso is designed to provide for the distribution of the active ingredient beginning in the small bowel and continuing throughout the colon. The product’s unique prolonged release mechanism might allow us to expand the range of treatment options for UC.
We expect that both the Apriso formulation and methods of use will be patent-protected until 2018. See Part I. Item 3. Legal Proceedings and Note 13 to Consolidated Financial Statements for information about our ongoing litigation against Lupin Ltd. and Lupin Pharmaceuticals, Inc., collectively, Lupin, and against Novel Laboratories, Inc., or Novel, regarding infringement of the Apriso patents.
Apriso sales, together with sales from Giazo and Colazal, two of our other products used to treat UC, accounted for approximately 13%, 12%, and 9% of our consolidated revenue in 2013, 2012, and 2011, respectively.
5
Table of Contents
MoviPrep (PEG 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate and ascorbic acid) oral solution
MoviPrep is a patent-protected, liquid PEG bowel cleansing product that the FDA approved in August 2006. MoviPrep competes with a number of liquid PEG bowel cleansing products, but it is differentiated from these other products by the inclusion of ascorbic acid in its formulation. MoviPrep is indicated for bowel cleansing prior to colonoscopy, intestinal surgery and barium enema X-ray examinations.
The patents protecting our MoviPrep product do not expire until September 2024. In August 2010, however, we entered into a sublicense agreement that granted Novel, a license under the patents covering MoviPrep permitting Novel to launch a generic version of MoviPrep no later than September 24, 2018.
MoviPrep and OsmoPrep, our other bowel-cleaning product, together accounted for approximately 8%, 9%, and 16% of our consolidated revenue in 2013, 2012, and 2011, respectively.
Relistor (methylnaltrexone bromide)
Relistor SI is currently indicated for the treatment of OIC in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient. Relistor was approved by the FDA in 2008, and currently it is approved for use in over 55 countries worldwide. In 2010, Relistor single–use, pre–filled syringes were approved for use in the United States, Canada and the European Union.
There are multiple patents protecting our Relistor products that give our Relistor products patent protection for the period ranging from 2017 through 2031. Our pre-filled syringe Relistor product has patent protection until December 2030. There are also pending applications on our Relistor products that if issued will provide further protection until 2024 and 2031.
OsmoPrep (sodium phosphate monobasic monohydrate, USP, sodium phosphate dibasic anhydrous, USP) tablets
OsmoPrep tablets are indicated for cleansing of the colon as a preparation for colonoscopy in adults 18 years of age or older. OsmoPrep, approved by the FDA in 2006, is the only tablet bowel cleansing product currently marketed in the United States.
The patents protecting our OsmoPrep product do not expire until June 2028. In September 2010, however, we entered into a sublicense agreement that granted Novel a license under the patents covering OsmoPrep permitting Novel to launch a generic version of OsmoPrep no later than November 16, 2019.
Solesta
Solesta is a biocompatible tissue bulking agent, consisting of dextranomer microspheres and stabilized sodium hyaluronate. Solesta is indicated for the treatment of fecal incontinence in patients 18 years and older who have failed conservative therapy, such as diet, fiber therapy and/or anti-motility medications. It is the only injectable gel for this indication to be administered in an outpatient setting without the need for surgery or anesthesia. The FDA approved Solesta through the premarket approval, or PMA, process as a Class III Medical Device in May 2011. In December 2011, we acquired an exclusive worldwide license to Solesta with the completion of our acquisition of Oceana Therapeutics, Inc., or Oceana. Solesta also is CE Mark-approved for distribution in Europe and is marketed throughout Europe. Solesta should be protected until May 2014 by a composition and method claims patent, and until July 2015 by a methods of manufacturing patent.
Deflux
Deflux is a biocompatible tissue bulking agent, consisting of dextranomer microspheres and stabilized sodium hyaluronate. It is indicated for children affected by VUR grades II-IV, a malformation of the urinary bladder that can result in severe infections of the kidneys and irreversible kidney damage. The Deflux PMA application was granted by the FDA in September 2001. In December 2011, we acquired an exclusive worldwide license to Deflux with the completion of our acquisition of Oceana. Deflux should be protected until May 2014 by a composition and method claims patent, and until July 2015 by a methods of manufacturing patent.
6
Table of Contents
Fulyzaq (crofelemer) delayed-release tablets
Fulyzaq is indicated for the symptomatic relief of non-infectious diarrhea in adult patients with HIV/AIDS on anti-retroviral therapy. In December 2008, we acquired rights to crofelemer, the Fulyzaq compound, from Napo Pharmaceuticals, Inc., or Napo. On December 31, 2012, the FDA granted marketing approval for Fulyzaq. See Part I. Item 3. Legal Proceedings and Note 13 to Consolidated Financial Statements for information about the ongoing litigation with Napo related to this product.
Patents for Fulyzaq should provide intellectual property protection to 2018. Fulyzaq should also be eligible for five years of marketing exclusivity from the date of FDA approval, and the product might be entitled to patent term restoration.
Giazo (balsalazide disodium) tablets
Giazo is indicated for the treatment of mildly to moderately active UC in male patients 18 years of age and older. On February 3, 2012, the FDA granted marketing approval for this product under the trade name Giazo. We shipped Giazo to wholesalers in the fourth quarter of 2012 and launched Giazo to physicians in the first quarter of 2013.
The patent for balsalazide disodium tablets will expire in 2018.
Metozolv ODT (metoclopramide hydrochloride) 5 mg and 10 mg orally disintegrating tablets
Metozolv ODT is indicated for short-term (4 to 12 weeks) use in adults for treatment of GERD that fails to respond to conventional therapy and for the relief of symptoms in adults associated with acute and recurrent diabetic gastroparesis. The patent for Metozolv expires in 2023.
On November 3, 2010, we received a paragraph IV notification from Novel stating that Novel had filed an ANDA to seek approval to market a generic version of Metozolv ODT, 5 mg and 10 mg. The notification letter asserted non-infringement of U.S. Patent No. 6,413,549, or the ‘549 patent. Upon examination of the relevant sections of the ANDA, we concluded that the ‘549 patent would not be enforced against Novel. On March 15, 2010 we received a paragraph IV notification from Zydus Pharmaceuticals, or Zydus, stating that Zydus had filed an ANDA to seek approval to market a generic version of Metozolv ODT, 5 mg and 10 mg. The notification letter asserted non-infringement of the ‘549 patent. Upon examination of the relevant sections of the ANDA, we concluded that the ‘549 patent would not be enforced against Zydus.
Azasan (azathioprine) tablets
Azasan is an FDA-approved drug that suppresses immune system responses and is indicated for preventing rejection of kidney transplants and treatment of severe arthritis. In November 2003, we acquired from aaiPharma LLC, or aaiPharma, the exclusive right to sell 25, 75 and 100 mg dosage strengths of Azasan in North America, and in February 2004, we launched the 75 and 100 mg dosage strengths of Azasan in the United States. The patents and data exclusivity for Azasan have expired.
Anusol-HC and Proctocort (hydrocortisone) creams and suppositories
Anusol-HC (hydrocortisone) 2.5% cream and Proctocort (hydrocortisone) 1% cream are topical corticosteroids indicated for relief of the inflammatory and pruritic, or itching, manifestations of corticosteroid-responsive dermatoses.
Anusol-HC (hydrocortisone acetate) 25 mg rectal suppositories and Proctocort (hydrocortisone acetate) 30 mg rectal suppositories are indicated for use in inflamed hemorrhoids and postirradiation proctitis, as well as an adjunct in the treatment of chronic UC and other inflammatory conditions.
The patents and data exclusivity for Anusol-HC and Proctocort have expired.
7
Table of Contents
Pepcid (famotidine) for oral suspension
Pepcid Oral Suspension is a widely known prescription pharmaceutical product indicated for several gastrointestinal indications, including the treatment of duodenal ulcer, benign gastric ulcer and GERD. In February 2007, we purchased the U.S. prescription pharmaceutical product rights to Pepcid Oral Suspension from Merck & Co., Inc., or Merck. The patents and data exclusivity for Pepcid Oral Suspension have expired. The FDA approved two generic famotidine oral suspension products in May and June 2010, respectively, and we launched an authorized generic famotidine product in May 2010 to compete with these generic products.
Diuril (Chlorothiazide) for oral suspension
Diuril Oral Suspension is indicated for the treatment of high blood pressure and also as adjunctive therapy in edema associated with congestive heart failure, cirrhosis of the liver, corticosteroid and estrogen therapy, and kidney disease. In connection with our purchase from Merck of the U.S. prescription pharmaceutical product rights to Pepcid Oral Suspension in February 2007, we also purchased the U.S. prescription pharmaceutical product rights to Diuril Oral Suspension. The patents and data exclusivity for Diuril Oral Suspension have expired.
Colazal (balsalazide disodium) capsules
Colazal, was approved by the FDA in July 2000 for the treatment of mildly to moderately active UC. In December 2006, the FDA approved Colazal for use in pediatric patients between 5 to 17 years of age with UC. The pediatric use of Colazal was granted orphan drug designation at the time of its approval, but it has now expired. On December 28, 2007, the Office of Generic Drugs of the FDA, or OGD, approved three generic balsalazide disodium capsule products. We do not anticipate significant Colazal sales in future periods.
Santarus Products
Uceris (budesonide) extended release tablets
Uceris is a locally acting corticosteroid in a once-daily oral tablet formulation that utilizes proprietary multimatrix system (MMX®) colonic delivery technology. Uceris is indicated for the induction of remission in patients with active, mild to moderate UC. Santarus received FDA approval of Uceris in January 2013 and launched this product commercially in February 2013.
Currently, there are four patents that we believe provide coverage for Uceris, with expiration dates in 2020. There are also pending applications that if issued will provide further protection until 2031 and 2033.
Zegerid (omeprazole/sodium bicarbonate) capsules and powder for oral suspension
Zegerid is an immediate-release formulation of the proton pump inhibitor omeprazole and is indicated for short-term treatment of active duodenal ulcer, short-term treatment of active benign gastric ulcer, treatment of GERD, maintenance of healing of erosive esophagitis and reduction of risk of upper gastrointestinal bleeding in critically ill patients.
Santarus determined in late June 2010 to cease promotion of the Zegerid products in connection with the launch of a generic version of Zegerid capsules that occurred after an April 2010 lower court decision that the patents covering the Zegerid products were invalid due to obviousness. At this same time, Santarus also launched an authorized generic version of its Zegerid capsules product under a distribution and supply agreement with Prasco LLC, or Prasco, as further described below. In September 2012, the U.S. Court of Appeals for the Federal Circuit reversed in part the April 2010 decision of the lower court and found that certain of the asserted patent claims were valid. In December 2012, the Federal Circuit issued an order denying a combined petition for panel and en banc rehearing filed by Par Pharmaceutical, Inc., or Par, and issued its mandate, remanding the case to the lower court for further proceedings pertaining to damages. A jury trial on damages has been scheduled for November 2014. Following the Federal Circuit’s decision and subsequent denials of a petition for rehearing, Santarus resumed promotion of Zegerid in February 2013.
8
Table of Contents
Currently, there are three patents that we believe provide coverage for our Zegerid products, with expiration dates in mid-2016. There are also pending applications that if issued will provide further protection until 2016. Under certain circumstances, however, Zegerid could lose patent protection at an earlier date. See Part I. Item 1. Legal Proceedings for information about patent litigation relating to Zegerid.
To leverage Santarus’s proton pump inhibitor, or PPI, technology and diversify its sources of revenue, Santarus licensed certain exclusive rights to MSD Consumer Products, Inc., which we will refer to as MSD, a subsidiary of Merck, to develop, manufacture and sell over-the-counter, or OTC, Zegerid products in the U.S. and Canada. Santarus has also licensed certain exclusive rights to its PPI technology to Glaxo Group Limited, an affiliate of GlaxoSmithKline, plc, or GSK, to develop, manufacture and commercialize prescription and OTC immediate-release omeprazole products in more than 100 specified countries (including markets within Africa, Asia, the Middle-East, and Latin America).
Glumetza (metformin hydrochloride) extended release tablets
Glumetza is a once-daily, extended-release formulation of metformin in 500 mg and 1000 mg dosage strengths that incorporates patented drug delivery technology and is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. Metformin is one of the most commonly prescribed oral medications for the treatment of type 2 diabetes.
Currently, there are four patents for Glumetza 500 mg dose product, with expiration dates in 2016, 2020 and 2021. There are three patents for Glumetza 1000 mg dose product, with expiration dates in 2020 and 2025. There are also pending applications that if issued will provide further protection until 2025.
In connection with previously entered settlement agreements, a first generic filer was granted the right to sell a generic version of Glumetza in February 2016 and two subsequent generic filers were granted the right to sell a generic version of Glumetza in August 2016. Under certain circumstances, however, Glumetza could face generic competition at an earlier date. See Part I. Item 3. Legal Proceedings for information about the patent litigation relating to Glumetza.
Cycloset (bromocriptine mesylate) tablets
Cycloset (bromocriptine mesylate) 0.8 mg tablets is a novel formulation of bromocriptine, and is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes both as mono-therapy and in combination with other oral antidiabetic agents.
Santarus began promoting Cycloset in November 2010 under a distribution and license agreement with S2 Therapeutics, Inc., or S2, and VeroScience, LLC, or VeroScience.
Currently, there are eight patents that Santarus licensed from S2 and VeroScience that we believe provide coverage for Cycloset, with expiration dates in 2014, 2015, 2023 and 2032.
Fenoglide (fenofibrate) tablets
Fenoglide (fenofibrate) 40 mg and 120 mg tablets is a proprietary formulation of fenofibrate and is indicated as an adjunct to diet to reduce elevated LDL-C, total cholesterol, triglycerides and Apo B, and to increase HDL-C, in adult patients with primary hyperlipidemia or mixed dyslipidemia. Fenoglide also is indicated as an adjunct to diet for treatment of adult patients with hypertriglyceridemia.
Santarus began promoting Fenoglide in February 2012, under the terms of a license agreement with Cowen Healthcare Royalty Partners, L.P. and Shore Therapeutics, Inc., or Shore, as further described below.
Currently, there are three patents that we believe provide coverage for the Fenoglide products, with expiration dates in 2024. There are also pending applications that if issued will provide further protection until 2024.
9
Table of Contents
In connection with a settlement agreement entered into in December 2011, Shore granted a generic filer the right to begin selling a generic version of Fenoglide in October 2015, or earlier under certain circumstances. See Part I. Item 3. Legal Proceedings for information about patent litigation relating to Fenoglide.
DEVELOPMENT PROGRAMS
Xifaxan (rifaximin) tablets
IBS, characterized by abdominal pain, bloating and altered bowel habits, is a common chronic medical condition and is associated with substantial medical costs. In September 2009, we announced the successful completion and outcome of two identical pivotal Phase 3 trials to evaluate the efficacy and safety of rifaximin 550 mg dosed three times a day in the treatment of non-constipation IBS, or non-C IBS. In each trial, rifaximin versus placebo treated patients demonstrated a statistically significant improvement for the primary endpoint of the adequate relief of IBS symptoms as assessed over a one-month period (weeks 3, 4, 5 and 6) following completion of a 14-day course of therapy (weeks 1 and 2). Consistent with the primary endpoint in each trial, the key secondary endpoint of relief of IBS-related bloating also demonstrated statistical significance of rifaximin versus placebo in each trial. We received a CRL to our NDA on March 7, 2011. The FDA issues a CRL to indicate that the review cycle for an application is complete and that the application is not ready for approval. In the CRL, the FDA deemed that the Xifaxan 550 mg sNDA was not ready for approval primarily due to a newly expressed need for retreatment information. On November 16, 2011, the Gastrointestinal Drugs Advisory Committee of the FDA held a meeting where it supported the Salix/FDA-developed proposed design of a clinical trial to evaluate the safety, efficacy and durability of response with repeat treatment cycles of Xifaxan 550 mg for IBS with diarrhea. We initiated enrollment in this trial in the first quarter of 2012 and completed enrollment in the double-blind phase of the trial in January 2014. We anticipate top-line data for this trial will be available during July 2014.
Relistor (methylnaltrexone bromide)
We currently have two development programs for methylnaltrexone bromide: (1) for OIC in patients with chronic non-malignant pain administered via SI, or OIC Chronic Pain; and (2) for OIC in patients with chronic non-malignant pain administered orally, or OIC Oral.
OIC Chronic Pain
On August 30, 2011, we announced that the FDA accepted for filing our sNDA for Relistor (methylnaltrexone bromide) SI to treat OIC in patients with chronic, non–cancer pain. The FDA issued an action date of April 27, 2012, and subsequently extended the date to July 27, 2012. On July 27, 2012, the FDA issued a CRL following its review of the sNDA. The CRL requested additional clinical data. In October 2012, Salix and Progenics Pharmaceuticals, Inc., or Progenics, the entity from whom Salix acquired the license to develop Relistor products, held an End-of-Review meeting with the FDA’s Division of Gastroenterology and Inborn Errors Products, or the Division, to better understand the contents of the CRL. The Division expressed a concern that there might be a risk associated with the chronic use of mu-opioid receptor antagonists in patients that are taking opioids for chronic pain. In order to understand this potential risk, the Division has communicated that a very large, well-controlled, chronic administration trial will have to be conducted to assess the safety of any mu-opioid receptor antagonist prior to market approval for the treatment of patients with OIC who are taking opioids for chronic, non-cancer pain. We have since had additional discussions with the Division and expressed the view that the post-marketing, clinical and preclinical data currently available adequately demonstrate an appropriate and expected safety profile sufficient to permit the approval of the sNDA. The FDA initially planned to convene an Advisory Committee on March 10-11, 2014 regarding this sNDA, but postponed the date of the meeting due to scheduling conflicts. The FDA intends to seek input from this Advisory Committee prior to answering our formal appeal to the CRL and has stated that it will take action under the appeal within 30 days after receiving input from the Advisory Committee. We remain hopeful that a path forward can lead to the expansion of the use of this new and alternative therapy to treat OIC in patients suffering from chronic pain; however, depending on the results of our discussions with the Division, we believe we might terminate our development program for Relistor SI for the treatment of OIC in chronic non-cancer pain.
10
Table of Contents
OIC Oral
On December 20, 2011, we announced the successful outcome of the Phase 3 trial to evaluate the efficacy and safety of oral methylnaltrexone for the treatment of OIC in subjects with chronic, non–cancer pain. This trial, evaluating three once-daily oral methylnaltrexone dosing regimens (150, 300 and 450 mg), demonstrated highly statistically significant results for the primary endpoint in two of the three treatment arms when compared to the placebo treatment arm. Overall, efficacy of oral methylnaltrexone in this study was comparable to that reported in clinical studies of subcutaneous methylnaltrexone in subjects with chronic, non-cancer pain. The overall observed safety profile seen in patients treated with oral methylnaltrexone was comparable to placebo in this study. Based on the CRL discussed above for OIC Chronic Pain, we are currently evaluating the oral OIC development program and believe we will continue this program. However, additional information and additional guidance from the FDA could result in the termination of the oral OIC development program.
Budesonide Foam
In March 2008, we acquired a license from Dr. Falk Pharma GmbH, or Dr. Falk Pharma, to a family of budesonide products, including a budesonide rectal foam, in the United States. In November 2009, we initiated two Phase 3 trials to evaluate the effectiveness and safety of budesonide rectal foam for the treatment of mild to moderate ulcerative proctitis or proctosigmoiditis. The rectal foam product should have patent coverage in the United States until 2015. In May 2013, we announced positive Phase 3 data from our Phase 3 trials. The FDA accepted for filing an NDA for budesonide foam in January 2014 and has issued a PDUFA action date of September 15, 2014.
Rifaximin EIR
In August 2012, we acquired from Alfa Wassermann S.p.A., or Alfa Wassermann, an exclusive license in the United States and Canada to develop an EIR formulation of rifaximin for gastrointestinal and respiratory indications, including Crohn’s disease. The EIR formulation of rifaximin has been designed to release the active drug following passage through the stomach and provide a homogeneous distribution of rifaximin in the intestinal tract. The EIR formulation of rifaximin was designed to provide an efficient delivery of rifaximin and will be studied for its potential to target difficult-to-treat diseases of the intestinal tract such as Crohn’s disease. The FDA has approved our trial protocol, and we expect to initiate Phase 3 trials in Crohn’s disease in early 2014. The rifaximin EIR product should have patent coverage in the United States until 2027.
Rifaximin SSD/Next Generation
Rifaximin SSD was designed to prevent complications of early decompensated liver cirrhosis. We initiated a Phase 2 trial in June 2013.
Ruconest
Ruconest is a recombinant version of the human protein C1 esterase inhibitor, which is produced using proprietary transgenic technology. Ruconest was granted orphan drug designation by the FDA for the treatment of acute attacks of angioedema in patients with hereditary angioedema. Santarus has the rights to commercialize Ruconest in North America under a license agreement with Pharming Group NV, or Pharming. Santarus filed a BLA with the FDA for Ruconest and was originally granted a PDUFA action date of April 16, 2014 which has been extended to July 16, 2014.
Currently, there are two issued U.S. patents that should provide coverage for the Ruconest investigational drug through 2022 and 2024, respectively.
11
Table of Contents
COLLABORATIVE AND PRODUCT ACQUISITION AGREEMENTS
We have and plan to continue to enter into various collaborations and product acquisition agreements with licensors, licensees and others. To date, we and Santarus have entered into the following material agreements:
Salix Product Acquisitions and In-License Agreements
Rifaximin (Xifaxan)
Alfa Wassermann
In June 1996, we entered into a license agreement, or the 1996 Agreement, with Alfa Wassermann, a privately held pharmaceutical company headquartered in Italy, pursuant to which Alfa Wassermann licensed to us the exclusive rights to make, use and sell rifaximin (Xifaxan) in the United States and Canada for the treatment of gastrointestinal and respiratory tract diseases. Pursuant to the 1996 Agreement, we agreed to pay Alfa Wassermann a single-digit percentage royalty based on annual rifaximin usage, as well as milestone payments. We made annual milestone payments in varying amounts to Alfa Wassermann until the commercial launch of Xifaxan in July 2004. No more milestone payments remain under this 1996 Agreement. Our obligation to pay royalties commenced upon the commercial launch of the product and continues until the later of (1) the expiration of the period in which the manufacture, use or sale of the products by an unlicensed third party would constitute an infringement on the patent covering the product and there is no regulatory exclusivity or (2) 10 years from commercial launch. The last patent is currently scheduled to expire in 2027. Thereafter, the licenses granted to us continue as irrevocable royalty-free paid-up licenses. However, we would remain obligated to pay a net sales based royalty for use of the product trademark if we choose to continue using it after the other licenses expired. Because the amount of the royalty payment is based on sales during a quarter, with no minimum royalty amount, we are unable to prospectively disclose the absolute amount of such royalty payments. Royalties are only incurred if there is associated revenue, and then are included in “Cost of products sold” in the Consolidated Statements of Comprehensive Income.
Alfa Wassermann has agreed separately to supply us with bulk active ingredient rifaximin at a fixed price. Salix is committed to purchase a percentage of its rolling 12-month forecast that is updated monthly, until July 2014 or introduction of a generic product, whichever occurs first, and these amounts are included in the “Purchase Commitments” line of our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations.
In August 2012, we entered into an agreement, which we refer to as the Amended Agreement, that amended and replaced our 1996 Agreement with Alfa Wassermann to develop and commercialize rifaximin. The Amended Agreement does not alter any of the terms for the TD and HE indications developed under the 1996 Agreement and IBS. We are obligated to pay Alfa Wassermann royalties, at the same range of rates as under the 1996 Agreement, on net sales of such products. In addition, the Amended Agreement provides us with an exclusive license to develop and commercialize rifaximin products for Crohn’s disease in the United States and Canada and a non-exclusive license to develop such products worldwide. We paid Alfa Wassermann a non-refundable upfront fee of $10.0 million in August 2012, and are obligated to make a $25.0 million milestone payment upon receipt of marketing authorization in the United States for an EIR formulation product for Crohn’s disease, and additional milestones based on net sales of EIR formulation products for Crohn’s disease of up to $200.0 million. In addition, we are required to pay Alfa Wassermann royalties on sales of EIR formulation rifaximin products for Crohn’s disease at percentage rates ranging from the low teens to low double digits. Our obligation to pay royalties would commence upon the commercial launch of the product and continue until the later of (1) the expiration of the period in which the manufacture, use or sale of the products by an unlicensed third party would constitute an infringement on the patent covering the product and there is no regulatory exclusivity or (2) 10 years from commercial launch.
The Amended Agreement does not have a fixed expiration date, but continues until neither party remains obligated to pay royalties. Either party may terminate following a material breach by the other party that is not cured within 60 days, a challenge by the other party of the first party’s relevant patents, or if the other party becomes insolvent or the subject of bankruptcy proceedings. Alfa has the right to terminate in respect of EIR formulation rifaximin products if Salix abandons development of EIR formulation of rifaximin products for Crohn’s disease and does not recommence development within a specified period after notice from Alfa. We may terminate the Amended Agreement if the FDA requires or causes the withdrawal of a rifaximin product marketed by us or our sublicensees or in respect of the EIR formulation rifaximin Crohn’s disease product upon 60 days’ notice. In addition, Alfa Wassermann can also terminate if we become involved in bankruptcy, liquidation or similar proceedings.
12
Table of Contents
Cedars-Sinai Medical Center
On June 28, 2006, we entered into a license agreement with Cedars-Sinai Medical Center, or CSMC, for the right to use a patent and a patent application relating to methods of diagnosing and treating IBS and other disorders caused by small intestinal bacterial overgrowth. Under the agreement, CSMC grants us the right to use its patent and patent application relating to methods of treating IBS and other disorders caused by small intestinal bacterial overgrowth. CSMC also grants us a nonexclusive license to use any unpublished research and development information, know-how and technical data of CSMC as necessary to exploit all rights granted to us with respect to rifaximin, with a right to sublicense. CSMC holds three patents that will provide protection for rifaximin for treating IBS through August 2019. We have an exclusive license to these patents from CSMC to make, have made, use, sell and have sold and import licensed products related to the use of rifaximin. As of December 31, 2013, we had paid the aggregate $1.2 million license fee. A portion of the $1.2 million was considered an up-front, non-refundable and irrevocable licensing fee. The balance was considered a prepaid, non-refundable and irrevocable royalty applicable as credit towards royalty amounts due and payable to CSMC, if any, under the agreement. At such time as the use of rifaximin is approved by the FDA as a treatment for IBS, we will be required to pay CSMC low single digit percentage royalties on net sales of licensed products. An additional term of the license agreement provides that we will expend a minimum amount per calendar year to seek and obtain regulatory approval and develop and commercialize licensed products. Because the license agreement provides the ability for us to terminate the agreement upon giving written notice of not less than 90 days, we do not include amounts payable under the license agreement as a purchase obligation in our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations. The license agreement does not have a fixed expiration date, but continues until terminated in accordance with its terms or until the last patent expires, which is currently in 2019. Royalty obligations terminate with the related patents on a country-by-country basis and when the license agreement terminates. The agreement will terminate automatically if we are declared insolvent. The agreement and royalty obligations may be terminated by CSMC if we materially breach the agreement and fail to cure the breach after notice.
Lupin, Ltd.
In September 2009, we entered into a Development, Commercialization and License Agreement with Lupin Ltd. for its proprietary drug delivery technology for rifaximin. We made an up-front payment of $5.0 million to Lupin upon execution of this agreement. In March 2011, we entered into an Amended and Restated Development, Commercialization and License Agreement with Lupin Ltd., and further amended it in February 2013, as so amended, the Amended License Agreement. The Amended License Agreement replaces in its entirety the September 2009 agreement. The Amended License Agreement provides that we are obligated to make additional upfront payments of $10.0 million, milestone payments to Lupin Ltd. that could total up to $53.0 million over the term of the agreement, and low double digit royalties in connection with commercialization of relevant products. As of December 31, 2013, we had paid $15.0 million of upfront payments related to the Amended License Agreement. In addition, during the portion of the term of the Amended License Agreement ending on September 30, 2019, we must pay Lupin Ltd. a minimum quarterly payment unless specified payments by us to Lupin Ltd. during that quarter exceed that amount. Beginning January 1, 2012, we are permitted to charge against such minimum quarterly payments the purchase price for certain rifaximin to be supplied to us by Lupin Ltd. pursuant to a Rifaximin Manufacturing and Supply Agreement that we and Lupin Ltd. entered into in September 2009 and amended in November 2009 and February 2013, as so amended, the Amended Supply Agreement. In the event we exercise our right to terminate the Amended License Agreement for convenience, we must pay Lupin Ltd. an early termination fee equal to a specified portion of the minimum quarterly payments payable by us to Lupin Ltd. through September 30, 2019, that we have not paid or otherwise satisfied as of the date of termination. The remaining milestone payments are contingent upon achievement of certain clinical and regulatory milestones. Because these milestone payments are conditioned upon events that might never occur, we do not consider the potential milestone payments as purchase obligations nor as a commitment to be reported in our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations. The term of the Amended License Agreement continues until the later of the expiration of our obligations to pay royalties in respect of specified products (which is triggered by certain events relating to the introduction by third parties of competitive products, the loss of patent or regulatory exclusivity, and the lapse of time since commercial launch, depending the specified product) and March 31, 2021. Currently, if issued, the last patent would be scheduled to expire in June 2028, or later if patent term adjustments are granted.
13
Table of Contents
The agreement may be terminated by either party if the other materially breaches the agreement and fails to cure the breach after notice or if the other party is declared insolvent. We may terminate the agreement if any regulatory authority in the United States requires or causes the withdrawal of one of our prescription rifaximin products, in the event of a generic product entry, for convenience (subject to payment of the early termination fee described above in relevant situations), or if we determine that it is not feasible or desirable to pursue development or commercialization of products licensed under the agreement for reasons relating to the identity of any successor to Lupin Ltd. or our relations with any such successor. Lupin Ltd. may terminate the agreement if the Supply Agreement described below is terminated for any reason other than its breach.
Under the Amended Supply Agreement, Lupin agrees to manufacture and supply us with rifaximin at a set price pursuant to rolling monthly forecasts and quarterly firm forecasts. We must take or pay for rifaximin in an amount equal to not less than 50% of our requirements of rifaximin for the manufacture of our current Xifaxan products or new immediate release forms thereof or generic forms of the foregoing for sale in the United States, subject to certain minimum purchase requirements, and these amounts are included in the “Purchase Commitments” line of our contractual commitments table in our Management’s Discussion and Analysis of Financial Condition and Results of Operations. The agreement terminates 10 years from the date we first required supply from Lupin thereunder, unless we extend it for additional periods. We may terminate the agreement if any regulatory authority in the United States requires or causes the withdrawal of one of our rifaximin products covered by the agreement. We may also terminate the agreement after the commercial sale or distribution in the United States of a generic version of a product covered by the agreement by a third party, or at our discretion after September 30, 2019. The agreement may be terminated by either party if the other materially breaches the agreement and fails to cure the breach after notice or if the other party is declared insolvent.
Under the Amended License Agreement and the Amended Supply Agreement, we agreed that if, after good faith negotiation by Lupin Ltd., we and Lupin Ltd. failed by April 12, 2013, to enter into an agreement pursuant to which Lupin Ltd. would serve as a distributor of authorized generic versions of certain of our products we would relinquish the right to terminate the agreement as a result of the sale or distribution of a generic version of a product covered by the agreement by a third party. The parties did not enter into such an agreement by April 12, 2013, but they have agreed that Salix may terminate the existing supply agreement upon 30 days’ notice.
Apriso
Pursuant to our license agreement, as amended, with Dr. Falk Pharma, we acquired the rights to develop and market a granulated formulation of mesalamine. The agreement obligates us to make milestone payments in an aggregate amount of up to $11.0 million to Dr. Falk Pharma upon certain events prior to the commercial launch of the product, and quarterly low double-digit percentage royalty payments thereafter. As of December 31, 2013, we had made all of these milestone payments. Royalties are only incurred if there is associated revenue, and then are included in “Cost of products sold” in the Consolidated Statements of Comprehensive Income. The agreement and our obligation to pay royalties continue until the later of expiration of the last patent, which is currently scheduled in 2018, or 15 years from commercial launch, which would be December 2023 because we launched the product in December 2008. The agreement and royalty obligations may be terminated by either party if the other materially breaches the agreement and fails to cure the breach after notice. Dr. Falk Pharma may terminate the agreement if we sell all or substantially all of its assets or stock without notifying Dr. Falk Pharma and making a non-termination payment to Dr. Falk Pharma.
MoviPrep
In December 2005, we acquired from Norgine B.V., or Norgine, the exclusive rights to sell NRL944 (now marketed by us under the trade name MoviPrep), a proprietary, liquid PEG bowel cleansing product, in the United States. Our agreement with Norgine was amended in August 2010. The agreement obligates us to make an upfront payment and milestone payments to Norgine that could total up to $37.0 million. As of December 31, 2013, we had made $27.0 million of upfront and milestone payments. The remaining milestone payment is contingent upon reaching a sales threshold. Because this milestone payment is conditioned upon an event that might never occur, we do not consider the potential milestone payment as a purchase obligation or a commitment to be reported in our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations. We
14
Table of Contents
pay Norgine a royalty in the low teens as a percentage of net sales, and we purchase finished product from Norgine. The royalty obligations terminate, subject to a continuing trademark royalty in the single digits, upon the expiration of the last patent and are reduced to a single-digit rate upon the first commercial sale by an unauthorized third party of a generic version of the product. The last patent is currently scheduled to expire in 2024. The license agreement does not have a fixed expiration date, but continues until terminated in accordance with its terms. The agreement may be terminated by either party if the other materially breaches the agreement and fails to cure the breach after notice or becomes insolvent. We may terminate the agreement with 12 months’ written notice. The August 2010 amendment modified our obligation to source from Norgine, and Norgine’s obligation to supply, our requirements for MoviPrep. The August 2010 amendment also provides for Norgine to reimburse us for one-half of the facilities improvement and expansion payments that we are to make to Novel, up to a specified amount.
Relistor
In February 2011, we acquired an exclusive worldwide license to develop and commercialize the products containing methylnaltrexone bromide, or the MNTX Compound, marketed under the name Relistor, from Progenics and a non-exclusive license to manufacture the MNTX Compound and products containing that compound. These licenses are worldwide, except in Japan, where Ono Pharmaceutical Co. Ltd., or Ono Pharmaceutical, has licensed the subcutaneous formulation of the drug from Progenics. We paid Progenics an up-front license fee payment of $60.0 million. In addition, we are obligated to pay Progenics up to $90.0 million contingent upon achieving specified regulatory approvals and up to $200.0 million contingent upon achieving specified targets for net sales over the term of the agreement. None of the milestones had been achieved, and therefore none of these payments had been made, as of December 31, 2013. Because these milestone payments are conditioned upon events that might never occur, we do not consider the potential milestone payments as purchase obligations or a commitment to be reported in our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations. We must pay Progenics 60% of any revenue received from sublicensees in respect of any country outside the United States. We must pay Progenics royalties based on a percentage ranging from the mid- to high-teens of net sales of any product containing the MNTX Compound. We are responsible for the future costs of the development programs for MNTX Compounds (excluding sales by ex-U.S. sublicensees). The royalty period generally runs until the later of (i) the expiration of the last valid relevant patent claim, (ii) the date on which there is no marketing exclusivity right with respect to the product, and (iii) the 15th anniversary of the first commercial sale subject, in the case of clause (iii), to earlier termination if unauthorized generic competition exceeds specified thresholds. Either party may terminate the license agreement upon an uncured material branch or specified bankruptcy events. In addition, we may terminate the agreement for safety or efficiency issues, or upon specified prior notice at any time on or after the first anniversary of the agreement, subject to Progenics’ right to postpone such latter termination in certain circumstances. Upon the termination of the agreement, all licenses granted to Salix by Progenics will terminate other than respecting any product the royalty period for which has expired in a particular country.
OsmoPrep
In connection with our acquisition of InKine Pharmaceutical Company, Inc., or InKine, in September 2005, we assumed a license agreement with ALW Partnership for the worldwide rights, in perpetuity, to develop, use, market, sell, manufacture, have manufactured and sub-license Visicol (our first-generation tablet bowel-cleansing product) and improvements, including OsmoPrep, in the field of colonic purgatives, along with ALW Partnership’s body of proprietary technical information, trade secrets and related know-how. Pursuant to this license agreement, we pay to Clinical Development Capital Partnership, or CDC, ALW’s successor, on a quarterly basis, a single-digit percentage royalty payment based on our net sales of these products. Because the amounts of the royalty payments are based on net sales during a quarter, we are unable to prospectively disclose the amount of such royalty payments. The agreement requires a minimum annual royalty payment of $0.1 million. Additional royalties are only incurred if there is associated revenue, and then are included in “Cost of products sold” in the Consolidated Statements of Comprehensive Income. The license agreement does not have a fixed expiration date, but continues until terminated in accordance with its terms or until the last patent expires, which is currently in 2028. The agreement and royalty obligations may be terminated by either party if the other materially breaches the agreement and fails to cure the breach after notice. We may terminate the agreement with 60 days’ written notice to CDC. CDC has the right to terminate the agreement in the event we are declared insolvent.
15
Table of Contents
Solesta and Deflux
In connection with our acquisition of Oceana in December 2011, we acquired two license agreements with Q-Med AB, or Q-Med, which provide us the worldwide right to commercialize Solesta and Deflux. Under a stock purchase agreement with Q-Med that we assumed in connection with the Oceana acquisition, we are obligated to pay up to $45.0 million contingent upon achieving specified targets for net sales of Solesta over the term of the agreement. No milestone payments had been made as of December 31, 2013. Because these milestone payments are conditioned upon events that might never occur, we do not consider the potential milestone payments as purchase obligations or a commitment to be reported in our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations. Additionally, we must pay Q-Med royalties based on a percentage in the low double-digits of net sales of Solesta and Deflux by Salix and its affiliates in the U.S. and a fixed per-unit royalty on sales of these products outside the U.S. The royalty obligations continue until the agreements are terminated, but may be reduced if certain conditions are met. Unless previously terminated, the license agreements will expire June 30, 2030, although they may be extended if certain conditions are met. Q-Med may terminate the agreements for our material breach if not cured within 90 days of notice. In addition, we have supply agreements under which we purchase our entire requirements of Deflux and Solesta from Q-Med.
Fulyzaq
In December 2008 we acquired certain rights to crofelemer from Napo for the United States and certain other countries. On December 31, 2012, the FDA granted marketing approval for a crofelemer product, under the trade name Fulyzaq.
During our December 2008 collaboration agreement with Napo, we made an initial payment of $5.0 million, consisting of $4.5 million in an upfront license fee, and a $0.5 million equity investment in Napo. In addition, we will make up to $50.0 million in milestone payments to Napo contingent on regulatory approvals and up to $250.0 million in milestone payments contingent on reaching certain sales thresholds. We will be responsible for development costs of crofelemer, but costs exceeding $12.0 million for development of crofelemer for the HIV-associated diarrhea indication will be credited towards regulatory milestones and thereafter against sales milestones. Through December 31, 2012, development costs exceeded $12.0 million by more than the amount of the milestone payment due upon the FDA approval received on December 31, 2012. Therefore, no payment was due for achievement of that milestone. Additionally, we will pay tiered royalties, ranging from the low teens to 20 percent, depending on annual sales levels, on net sales of crofelemer. Because the milestone payments are conditioned upon events that might never occur, we do not consider the potential milestone payments as purchase obligations or a commitment to be reported in our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations. The license and royalty obligations do not have a fixed expiration date, but continue until terminated in accordance with the terms of the collaboration agreement or until the last patent expires. The last patent is currently scheduled to expire in 2018. The agreement and royalty obligations may be terminated by either party if the other materially breaches the agreement and fails to cure the breach after notice or is declared insolvent. We may terminate the agreement if we determine development of the product is not commercially feasible.
On May 5, 2011, Napo filed a lawsuit against us in the Supreme Court of the State of New York, County of New York, alleging that we breached our collaboration agreement with Napo and breached our duty of good faith and fair dealing. For more details, see Part I. Item 3. Legal Proceedings and Note 13 to Consolidated Financial Statements. We continue to advance our development and commercialization plans for crofelemer in accordance with the existing collaboration agreement.
Budesonide
In March 2008, we acquired a license from Dr. Falk Pharma to a family of budesonide products, including a budesonide rectal foam and budesonide gastro-resistant capsules, in the United States. The rectal foam product has patent coverage in the U.S. until 2015. The agreement requires us to make an upfront payment and regulatory milestone payments that could total up to $23.0 million to Dr. Falk Pharma, with the majority contingent upon achievement of U.S. regulatory approval. At such time as the use of the rectal foam product is approved by the FDA,
16
Table of Contents
Salix will be required to pay Dr. Falk Pharma low double-digit percentage royalties on net sales of licensed products. As of December 31, 2013, $1.5 million of upfront and milestone payments had been made. The remaining milestone payments are contingent upon the filing of NDAs and achievement of regulatory approvals. We have included the milestone payment to be paid upon filing an NDA for our rectal foam product in our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations. Because the milestone payments relating to achievement of regulatory approvals are conditioned upon events that might never occur, we do not consider these potential milestone payments as purchase obligations or a commitment to be reported in our contractual commitments table in Management’s Discussion and Analysis of Financial Condition and Results of Operations. The agreement term continues until the later of expiration of the last patent or 17 years from commercial launch. The last patent is currently scheduled to expire in 2015, and we have yet to launch this product. The agreement may be terminated if either party materially breaches the agreement and fails to cure the breach after notice. We may terminate the agreement if development milestones are not achieved. Dr. Falk Pharma may terminate the agreement if development milestones are not achieved or if we sell all or substantially all of its assets or stock without notifying Dr. Falk Pharma and making a non-termination payment to Dr. Falk Pharma.
Santarus Product Acquisitions and In-License Agreements
Uceris
In December 2008, Santarus entered into a strategic collaboration with Cosmo Technologies Limited, or Cosmo, including a license agreement, which we will refer to as the 2008 License Agreement, stock issuance agreement and registration rights agreement, under which Santarus was granted exclusive rights in the U.S. to develop and commercialize Uceris and rifamycin SV MMX. In November 2013, Santarus, Cosmo and Salix entered into an amendment to the 2008 License Agreement pursuant to which, among other things, (1) Santarus agreed to return to Cosmo all rights to rifamycin SV MMX acquired by Santarus under the 2008 License Agreement and all regulatory approvals, filings and study data relating to rifamycin SV MMX, (2) Cosmo consented to the development, promotion and marketing in the United States by Salix, Santarus and any of their subsidiaries of budesonide products; provided that Salix, Santarus and their subsidiaries are prohibited from developing, promoting or marketing an oral formulation budesonide product other than Uceris, and (3) the stock issuance agreement and the registration rights agreement were terminated. We will refer to the 2008 License Agreement, as amended, as the Cosmo License Agreement.
Under the Cosmo License Agreement, Cosmo has granted Santarus the exclusive right to develop, market and commercialize Uceris in the U.S. As upfront consideration for the rights granted under the 2008 License Agreement, Santarus issued 6,000,000 shares of its common stock and made a cash payment of $2.5 million to Cosmo. In addition, following the completion of the phase III studies for Uceris, Cosmo elected to receive payment of a $3.0 million clinical milestone through the issuance of 972,132 shares of Santarus’s common stock. Following FDA acceptance for filing of the NDA for Uceris, Cosmo elected to receive payment of a $4.0 million regulatory milestone through the issuance of 906,412 shares of Santarus’s common stock. Following the first commercial sale of Uceris which occurred in February 2013, Cosmo had the option to elect, on or before April 15, 2013, whether to receive payment of a $7.0 million commercial milestone in cash or through the issuance of 565,793 shares of Santarus’s common stock. It elected to receive the milestone in cash. In the future, Santarus may be required to pay Cosmo commercial milestones of up to $22.5 million for Uceris. These milestones are payable only in cash.
Santarus is required to pay tiered royalties to Cosmo equal to 12% on annual net sales of Uceris up to $120 million and 14% on annual net sales of Uceris in excess of $120 million. Such royalties are subject to reduction in certain circumstances, including in the event of market launch in the U.S. of a generic version of Uceris. Santarus’s obligation to pay the specified royalties under the Cosmo License Agreement will continue for the life of the patents (including certain patent applications) covering Uceris. Following that period, the parties have agreed to negotiate in good faith a reduced royalty arrangement for the continued use of Cosmo’s know-how and trademarks related to Uceris.
The term of the Cosmo License Agreement will continue until 50 years following the expiration of the licensed patent rights, but either party may terminate the Cosmo License Agreement in the event of the other party’s uncured material breach.
17
Table of Contents
Zegerid
In January 2001, Santarus entered into an exclusive, worldwide license agreement with the University of Missouri for patents and pending patent applications relating to specific formulations of PPIs with antacids and other buffering agents and methods of using these formulations. Pursuant to the terms of the license agreement, Santarus paid the University of Missouri an upfront licensing fee of $1.0 million in 2001, a one-time $1.0 million milestone fee in 2003 following the filing of its first NDA and a one-time $5.0 million milestone fee in July 2004 following the FDA’s approval of Zegerid powder for oral suspension 20 mg. Santarus is required to make additional milestone payments to the University of Missouri upon initial commercial sale in specified territories outside the U.S., which may total up to $3.5 million in the aggregate. Santarus is also required to make milestone payments, up to a maximum of $86.3 million, based on first-time achievement of significant sales thresholds, the first of which was a one-time $2.5 million milestone payment upon initial achievement of $100.0 million in annual calendar year net product sales, which was paid to the University of Missouri in the first quarter of 2009, and the next of which is a one-time $7.5 million milestone payment upon initial achievement of $250.0 million in annual calendar year net product sales. Santarus must pay mid-single digit royalties to the University of Missouri on net sales of its products and any products sold by Prasco, Merck and GSK under its existing license and distribution agreements.
The license from the University of Missouri expires in each country when the last patent for licensed technology expires in that country and the last patent application for licensed technology in that country is abandoned, provided that Santarus’s obligation to pay certain minimum royalties in countries in which there are no pending patent applications or existing patents terminates on a country-by-country basis on the 15th anniversary of its first commercial sale in such country. If Santarus fails to meet certain diligence obligations following commercialization in specified countries, the University of Missouri can terminate the license or render it non-exclusive with respect to those countries. Santarus’s rights under this license are also generally subject to early termination under specified circumstances, including our material and uncured breach or our bankruptcy or insolvency. Santarus can terminate the license at any time, in whole or in part, with 60 days’ prior written notice.
OTC License Agreement with Merck
In October 2006, Santarus licensed exclusive rights to Merck under its PPI technology to develop, manufacture, market and sell Zegerid brand OTC products in the lower dosage strength of 20 mg of omeprazole in the U.S. and Canada. Under the license agreement, Merck is required to use active, sustained and diligent efforts to conduct and complete in a timely manner all activities required to develop licensed products, receive marketing approval for licensed products and market, sell and generate and meet market demand for licensed products in the licensed territories. Merck commenced commercial sales of Zegerid OTC (omeprazole 20 mg/sodium bicarbonate 1100 mg capsules) in March 2010.
Under the license agreement, Santarus received a $15.0 million upfront license fee in November 2006 and have received $27.5 million in milestone payments to date. Santarus may receive up to an additional $37.5 million in aggregate milestone payments upon the achievement of specified sales milestones. It is also entitled to receive low double-digit royalties, subject to adjustment in certain circumstances, on net sales of any OTC products sold by Merck under the license agreement. In turn, Santarus is obligated under its license agreement with the University of Missouri to pay royalties to the University of Missouri based on net sales of any OTC products sold by Merck.
The license agreement remains in effect as long as Merck is marketing products under the license agreement. Merck may terminate the agreement at any time on 180 days’ prior written notice to us. In addition, either party may terminate the license agreement in the event of uncured material breach of a material obligation, subject to certain limitations, or in the event of bankruptcy or insolvency.
License Agreement with GSK
In November 2007, Santarus entered into a license agreement granting exclusive rights to GSK under its PPI technology to commercialize prescription and OTC immediate-release omeprazole products in a number of international markets. Under the license agreement, Santarus granted GSK the exclusive right to develop, manufacture and commercialize prescription and OTC immediate-release omeprazole products for sale in more than 100 countries within Africa, Asia, the Middle-East and Latin America. GSK is required to use commercially reasonable efforts to
18
Table of Contents
seek regulatory approval for, and to launch, market and sell licensed products in the licensed territories and is required to do so within specified time frames in certain “major countries,” defined in the license agreement as Brazil, China, Mexico, South Africa, South Korea, Taiwan and Turkey. To date, GSK has elected not to pursue development in China and Taiwan and has returned the rights to those territories to Santarus. Currently, GSK has launched licensed products in Mexico, Ecuador, Kenya, Nigeria, French W. Africa and Tanzania and has made regulatory filings in other selected countries in Africa, Asia and Latin America. GSK is continuing work to prepare the regulatory filings necessary to obtain marketing approval authorization in additional countries covered by the license agreement.
Pursuant to the license agreement, Santarus received an $11.5 million upfront fee from GSK. Santarus will also receive tiered royalties equal to a percentage of net sales, ranging from the mid-teens to mid-twenties, of any licensed products sold by GSK under the license agreement. The royalties are subject to reduction on a country-by-country basis in the event that sales of any generic products achieve a specific level of market share. In turn, Santarus will be obligated under its license agreement with the University of Missouri to pay royalties to the University of Missouri based on net sales of any licensed products sold by GSK. GSK’s obligation to pay royalties under the license agreement will continue as long as GSK is selling licensed products, unless the license agreement is terminated earlier or in the event GSK exercises its option to make a buy-out payment in 2027, the 20th anniversary of the license agreement. To support GSK’s initial launch costs, Santarus agreed to waive the initial $2.5 million of aggregate royalties payable by GSK.
The license agreement will remain in effect as long as GSK is obligated to pay royalties under the license agreement for one or more licensed territories. GSK may terminate the license agreement on six months’ prior written notice to Santarus at any time. Santarus may terminate the license agreement on a country-by-country basis in the event that GSK fails to satisfy its diligence obligations applicable to such country. In addition, either party may terminate the license agreement in the event of the other party’s uncured material breach or bankruptcy or insolvency. Following termination, the rights associated with licensed products revert to Santarus.
Glumetza
In August 2011, Santarus entered into a commercialization agreement with Depomed granting Santarus exclusive rights to manufacture and commercialize Glumetza prescription products in the U.S., including its territories and possessions and Puerto Rico. The commercialization agreement replaced an existing promotion agreement between the parties entered into in July 2008, pursuant to which Santarus has promoted Glumetza in the U.S.
Santarus was required to pay to Depomed royalties on net product sales in the territory of 26.5% in 2011, 29.5% in 2012 and 32.0% in 2013, and it is required to pay to Depomed royalties on net product sales in the territory of 32.0% in 2014 and 34.5% in 2015 and beyond prior to generic entry of a Glumetza product. Santarus has the exclusive right to commercialize authorized generic versions of the Glumetza products. In the event of generic entry of a Glumetza product in the territory, the parties will equally share proceeds based on a gross margin split. Santarus will pay no additional sales milestones to Depomed, as was required under the prior promotion agreement.
The commercialization agreement will continue in effect for so long as Santarus commercializes branded Glumetza or authorized generic products, unless terminated sooner. Subject to 120 days’ prior written notice to Depomed, Santarus has the right to terminate the agreement at any time. Subject to 60 days’ prior written notice to Santarus, Depomed may terminate the agreement if Santarus fails to meet its obligations with respect to minimum promotion and expenditure obligations and fail to cure such breach within a specified time period. Either party may terminate the agreement if the other party fails to perform any material term of the agreement and fails to cure such breach, subject to prior written notice within a specified time period. In addition, either party may terminate the agreement if a force majeure event prevents the other party from carrying out its material obligations under the agreement for a period of at least six months. Finally, either party may terminate the agreement if the other party becomes insolvent, files or consents to the filing of a petition under any bankruptcy or insolvency law or has any such petition filed against it, and within a specified time period, such filing has not been dismissed.
Cycloset
In September 2010, Santarus entered into a distribution and license agreement with S2 and VeroScience, granting it exclusive rights to manufacture and commercialize the Cycloset prescription product in the U.S., subject to the right
19
Table of Contents
of S2 to co-promote Cycloset as described below. Under the terms of the distribution and license agreement, Santarus paid S2 and VeroScience an upfront fee totaling $5 million. Santarus records sales of Cycloset and pays a product royalty to S2 and VeroScience of 35% of the gross margin associated with net sales of Cycloset up to $100 million of cumulative total gross margin, increasing to 40% thereafter. Gross margin is defined as net sales less cost of goods sold. In the event net sales of Cycloset exceed $100 million in a calendar year, Santarus will pay an additional 3% of the gross margin to S2 and VeroScience on incremental net sales over $100 million.
The distribution and license agreement will continue in effect until Santarus ceases to market or sell Cycloset in the U.S., unless terminated sooner. Santarus may terminate the agreement at any time subject to 120 days’ prior written notice. Santarus may also terminate the agreement immediately in specified circumstances relating to a significant recall or market withdrawal of Cycloset, in the event of certain regulatory or governmental actions that would prevent it from performing our obligations under the agreement or in the event of FDA approval of a third party ANDA for an “AB” rated equivalent of Cycloset. Either Santarus on the one hand or S2 and VeroScience on the other hand may terminate the agreement in the following circumstances: (a) if the other party fails to perform any material term of the agreement and fails to cure such breach, subject to prior written notice within a specified time period; (b) if a force majeure event prevents the carrying out of material obligations of the other party under the agreement for a period of at least six months; or (c) upon the insolvency or occurrence of other specified bankruptcy events.
Ruconest
In September 2010, Santarus entered into a license agreement and a supply agreement with Pharming, under which it was granted certain non-exclusive rights to develop and manufacture, and certain exclusive rights to commercialize Ruconest in the U.S., Canada and Mexico for the treatment of HAE and other future indications.
In partial consideration of the licenses granted under the license agreement, Santarus paid Pharming a $15 million upfront fee. In addition, in November 2012, Santarus paid Pharming a $10 million milestone following successful achievement of the primary endpoint of the phase 3 clinical study. Santarus paid a $5 million milestone to Pharming in July 2013 upon FDA acceptance for review of a BLA for Ruconest. Santarus may also be required to pay Pharming additional success-based regulatory and commercial milestones totaling up to an aggregate of $25 million, including a $20 million milestone upon the earlier of first commercial sale of Ruconest in the U.S. or 90 days following receipt of FDA approval. In addition, Santarus will be required to pay the following one-time performance milestones if it achieves certain aggregate net sales levels of Ruconest: a $20 million milestone if calendar year net sales exceed $300 million and a $25 million milestone if calendar year net sales exceed $500 million.
The license agreement will continue in effect until Santarus ceases to sell Ruconest in the U.S., Canada and Mexico, unless terminated sooner. Either party may terminate the agreement in the following circumstances: (a) if the other party breaches any material term of the agreement and fails to cure such breach within a specified time period following written notice; or (b) upon the insolvency or occurrence of other specified bankruptcy events. Santarus may also terminate the license agreement at any time subject to 12 months’ prior written notice.
Following termination by Pharming or by Santarus at will, the rights associated with Ruconest revert to Pharming. Following termination by Santarus for uncured material breach, bankruptcy or insolvency, the licenses granted to Santarus will survive.
MANUFACTURING AND DISTRIBUTION
We own no manufacturing facilities. We have in the past used and plan to continue to use third-party vendors to produce material for use in clinical trials and for commercial product. This manufacturing strategy enables us to direct our financial resources to product in-licensing and acquisition, product development, and sales and marketing efforts, without devoting resources to the time and cost associated with building and maintaining manufacturing or packaging facilities. Santarus similarly relies on third parties for the manufacture of both clinical and commercial quantities of its products and for product distribution, and it does not currently have any manufacturing or distribution facilities. Descriptions of our and Santarus’s principal manufacturing and distribution arrangements are set forth below.
20
Table of Contents
Salix Manufacturing and Distribution
Rifaximin
Under our supply agreement with Alfa Wassermann, Alfa Wassermann is obligated to supply us with bulk rifaximin drug substance, the active pharmaceutical ingredient in Xifaxan 200 mg rifaximin tablets and Xifaxan 550 mg rifaximin tablets, until July 2014 or introduction of a generic product, whichever occurs first. Our supply of rifaximin drug substance supplied by Alfa Wassermann is manufactured by ZaCh Systems in Lonigo, Italy, and Sanofi-Aventis in Brindisi, Italy.
Under our supply agreement with Lupin, we are obligated to purchase 50% of our annual requirements of bulk rifaximin drug substance from Lupin Ltd., subject to certain minimum purchase requirements.
Under a long-term supply agreement, rifaximin is converted into Xifaxan drug product for us by Patheon, Inc. in Whitby, Ontario. Bulk Xifaxan tablets are packaged into finished Xifaxan commercial bottles by Patheon and packaged into Xifaxan commercial blister packs by Pharma Packaging Solutions in Norris, Tennessee.
Apriso
Bayer AG, or Bayer, in Wuppertal, Germany and PharmaZell GmbH, or Pharmazell, in Raubling, Germany supply us with bulk mesalamine active ingredient. Under a long-term supply agreement with Catalent Pharma Solutions, or Catalent, in Winchester, Kentucky, Catalent converts this mesalamine into our commercial supply of bulk Apriso, 375 mg mesalamine capsules. Bulk Apriso capsules are then packaged into finished Apriso commercial bottles by Pharma Packaging Solutions in Norris, Tennessee.
Purgatives
Under our supply agreement with Actavis, Inc. and Novel, Novel produces our commercial supply of finished MoviPrep kits. Under our supply agreement with Novel in Somerset, New Jersey, Novel produces our commercial supply of bulk OsmoPrep tablets, which are then packaged into finished OsmoPrep commercial bottles by Pharma Packaging Solutions in Norris, Tennessee.
Relistor
Relistor SI in a vial presentation is produced in bulk by DSM Pharmaceutical Products in Greenville, North Carolina and then packaged into finished Relistor single vials or vial kits by Packaging Coordinators, Inc. in Philadelphia, Pennsylvania.
Relistor SI in a pre-filled syringe presentation is produced and packaged into finished Relistor kits by Vetter Pharma International GmbH in Ravensburg, Germany.
The drug substance for these Relistor SI presentations is supplied by Mallinckrodt Pharmaceuticals in St. Louis, Missouri.
Solesta and Deflux
Both Solesta and Deflux are produced and packaged into finished Solesta and Deflux kits, respectively, by Q-Med, a division of Galderma, in Uppsala, Sweden.
Fulyzaq
We obtain a raw material from several suppliers in South America that is then provided to Glenmark. Under our supply agreement with Glenmark, in Mumbai, India, Glenmark supplies us with Fulyzaq drug substance. Under a long term supply agreement with Patheon in Cincinnati, Ohio, crofelemer drug substance is converted into Fulyzaq tablets and then packaged into commercial bottles.
21
Table of Contents
Products in Development
With respect to our budesonide foam formulation, our methylnaltrexone bromide tablet formulation, and our methylnaltrexone bromide multi-dose pen SI formulation, all of which are currently under development, we plan to negotiate commercial supply agreements with the manufacturers who produced the drug substance and drug product for the Phase 3 clinical trial material, or the manufacturers who produced the pivotal registration batches, if these products receive FDA approval.
Santarus Manufacturing and Distribution
Uceris
Santarus relies on Cosmo, located in Italy, to manufacture and supply all of its bulk drug product requirements for Uceris. Santarus also relies on Packaging Coordinators, Inc. for the commercial bottling and blister packing for Uceris. Santarus has agreed to purchase such requirements exclusively from Cosmo during the term of its license agreement with Cosmo. Under the terms of its supply agreement with Cosmo relating to the commercial supply of Uceris, Santarus has agreed to pay Cosmo a supply price equal to 10% of net sales. Cosmo is required to reimburse Santarus for costs associated with packaging, which is handled by a third party. The term of the supply agreement continues for so long as the license agreement with Cosmo remains in effect. The agreement may also be terminated under certain other specified circumstances. Santarus also relies on Packaging Coordinators, Inc. for the commercial bottling and blister packing for Uceris.
Zegerid
Santarus currently relies on Norwich Pharmaceuticals, Inc., or Norwich, located in New York, as the sole third-party manufacturer of the brand and related authorized generic Zegerid capsules product. Santarus has entered into a supply agreement with Norwich that continues in force indefinitely unless terminated by either party with 18 months’ prior written notice. The agreement may also be terminated under certain other specified circumstances.
Santarus currently relies on Patheon in Whitby, Ontario for the manufacture of Zegerid powder for oral suspension. The agreement, as amended, has an initial five-year term, which expires in October 2014. Thereafter, the term of the agreement continues in force indefinitely, unless terminated by either party with 18 months’ prior written notice. The agreement may also be terminated under certain other specified circumstances.
Under Santarus’s authorized generic agreement with Prasco, Santarus supplies authorized generic of prescription Zegerid capsules to Prasco, and Prasco is responsible for invoicing and distribution to pharmaceutical wholesalers and other customers. In April 2010, as part of its contingency plan to prepare for a possible launch of a generic version of our Zegerid prescription products, Santarus entered into a distribution and supply agreement with Prasco that granted Prasco the right to distribute and sell an authorized generic version of Santarus’s Zegerid prescription products in the U.S. Under the terms of the agreement, which was amended in November 2012, Prasco is obligated to use commercially reasonable efforts to distribute and sell such products in the U.S. Prasco agreed to purchase all of its authorized generic product requirements from Santarus and pays Santarus a specified invoice supply price for such products. Prasco is also obligated to pay Santarus a significant percentage of the gross margin on sales of the authorized generic products. The term of the distribution and supply agreement will continue until June 2015, five years after the date of launch of the first authorized generic product, with automatic one year renewals thereafter unless either party elects not to renew by giving notice at least six months prior to the expiration of the applicable renewal period. The distribution and supply agreement may also be terminated under certain other specified circumstances. In the event of termination, the rights granted by Santarus to Prasco associated with the authorized generic products will cease.
Glumetza
For Glumetza 500 mg, Santarus assumed from Depomed a commercial manufacturing agreement with Patheon, and, accordingly, it relies on a Patheon facility located in Puerto Rico as the sole third party manufacturer of Glumetza 500 mg. The current term of the manufacturing agreement with Patheon expires in June 2014. Thereafter, the manufacturing agreement automatically renews for additional terms of two years each, unless one party gives notice to terminate 12 months prior to the expiration of the current term. Neither party to the manufacturing agreement has given notice to terminate. The agreement may also be terminated under certain other specified circumstances.
22
Table of Contents
For Glumetza 1000 mg, Santarus currently relies on Depomed to oversee product manufacturing and supply. Depomed, in turn, relies on a Valeant Pharmaceuticals International, Inc. facility located in Canada as the sole third party manufacturer of Glumetza 1000 mg.
Cycloset
In connection with the license of rights to Cycloset, Santarus assumed a manufacturing services agreement with Patheon, and, accordingly, it relies on a Patheon in Cincinnati, Ohio as the sole third-party commercial manufacturer and packager for Cycloset. The manufacturing services agreement with Patheon is non-exclusive and although Santarus is not required to purchase any minimum quantity of the product under the agreement, Santarus has agreed to purchase not less than a specified percentage of the total amount of Cycloset offered for sale by us in the U.S. For so long as Patheon manufactures the required percentage for Santarus, Patheon has agreed not to manufacture bromocriptine mesylate products, regardless of dosage form, for any other third party without Santarus’s express written consent. The agreement expires in December 2016, and thereafter automatically continues for two-year renewal terms, unless 18 months’ prior written notice is provided by either party. The agreement may also be terminated under certain other specified circumstances.
Ruconest
Santarus relies on Pharming to oversee product manufacturing and supply. In turn, Pharming utilizes certain of its own facilities as well as third-party manufacturing facilities for supply, all of which are located in Europe. Following at-will termination by Pharming or by Santarus of the license agreement between the parties, the supply agreement will terminate. Following termination by Santarus for uncured material breach, bankruptcy or insolvency, Santarus will have a right to reduce the supply price, and the supply agreement will remain in effect.
SALES AND MARKETING
Our sales force cultivates relationships of trust and confidence with the high prescribing physicians whom it contacts in the United States. We use a variety of marketing techniques to promote our products including sampling, journal advertising, promotional materials, specialty publications, coupons, money-back or product replacement guarantees, educational conferences and informational websites.
As of December 31, 2013 (before our acquisition of Santarus), our sales and marketing staff, including our sales representatives, consisted of approximately 335 people.
As of December 31, 2013 (before our acquisition of Santarus), our sales force had approximately 235 sales representatives in the field marketing our approved products. Although the creation of an independent sales organization involved substantial costs, we believe that the financial returns from our direct product sales have been and will continue to be more favorable to us than those from the indirect sale of products through marketing partners. We generally enter into distribution or licensing relationships outside the United States. As a result of our acquisition of Oceana in December 2011, we have approximately ten sales representatives based in Europe who sell Solesta and Deflux there. We also sell Deflux through distributors in approximately 20 countries outside the United States and Europe.
Going forward after the Santarus acquisition, we believe our sales effort will benefit from the addition of Santarus sales representatives. As a result our sales force structure will consist of three 97-member gastrointestinal-focused sales forces, a 160-member digestive disease specialty sales force, and a 48-member government and institutional sales force. We expect this sales force structure will provide greater reach and frequency for our promoted products with gastroenterologists, hepatologists and colorectal surgeons. We believe the digestive disease specialty sales force may enable us to expand our specialty sales effort to target endocrinologists, infectious disease and pain specialists and high-volume primary care physicians – prescribers formerly not generally accessed by our gastrointestinal-focused sales effort. We believe these strategic additions to our sales effort should increase sales of our current products in the short term and begin to position us to maximize the sales of potential products over the longer term.
23
Table of Contents
PATENTS AND PROPRIETARY RIGHTS
Our goal is to obtain, maintain and enforce patent protection for our products, compounds, formulations, processes, methods and other proprietary technologies invented, developed, licensed or acquired by us, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the U.S. and in other countries. Our policy is to actively seek to obtain, where appropriate, intellectual property protection for our products, proprietary information and proprietary technology through a combination of contractual arrangements and laws, including patents, both in the U.S. and elsewhere in the world.
Due to the length of time and expense associated with bringing new pharmaceutical products to market, we recognize that there are considerable benefits associated with developing, licensing or acquiring products that are protected by existing patents or for which patent protection can be obtained. In addition, we have applied and intend to continue to apply for patent protection for new technology we develop whenever we determine that the benefit of patent protection outweighs the cost of obtaining patent protection.
We also depend upon the skills, knowledge and experience of our scientific and technical personnel, as well as that of our advisors, consultants and other contractors. To help protect our proprietary know-how that is not patentable, and for inventions for which patents may be difficult to enforce, we rely on trade secret protection and confidentiality agreements to protect our interests. To this end, we require our employees, consultants, advisors and certain other contractors to enter into confidentiality agreements which prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business. Additionally, these confidentiality agreements require that our employees, consultants and advisors do not bring to us, or use without proper authorization, any third party’s proprietary technology or information.
Our and Santarus’s principal intellectual property is discussed below.
Salix Intellectual Property
Rifaximin
General
The patents for the rifaximin composition of matter (also covering a process of making rifaximin and using rifaximin to treat gastrointestinal infectious diseases) expired in May 2001 in the United States and Canada. Rifaximin 550 mg has orphan exclusivity through March 2017. Patents covering several physical states, or polymorphic forms, of rifaximin that provide protection for all indications currently marketed and being assessed are listed below in the table. Alfa Wasserman, the owner of these patents, has licensed the rights to Salix in the United States. In July 2006, Salix entered into an agreement with CSMC for the right to use its patent and patent applications relating to methods of diagnosis and treating IBS and other disorders caused by small intestinal bacterial overgrowth. The CSMC agreement provides Salix the right to use the patents listed below as well as other members of the patent family indicated as licensed from CSMC.
| U.S. Patent No. | Issue Date | Expiration | Subject | |||||||||
| 7,045,620 | * | May-06 | Jun-24 | Composition of matter and process patent covering several physical states of rifaximin*** | ||||||||
| 7,612,199 | * | Nov-09 | Jun-24 | Covers several physical states, or polymorphous forms of rifaximin*** | ||||||||
| 7,906,542 | * | May-11 | Jun-25 | Covers several physical states, or polymorphous forms of rifaximin in pharmaceutical formulations*** | ||||||||
| 7,902,206 | * | Mar-11 | Jun-24 | Covers several physical states, or polymorphous forms of rifaximin*** | ||||||||
| 7,452,857 | ** | Nov-08 | Aug-19 | Use of rifaximin for treating IBS | ||||||||
| 7,605,240 | ** | Oct-09 | Aug-19 | Treatment of bloating caused by small intestinal bacterial overgrowth associated with IBS | ||||||||
| 7,718,608 | ** | May-10 | Aug-19 | Use of rifaximin for treating IBS | ||||||||
| U.S. Patent No. | Issue Date | Expiration | Subject | |||||||||
| 7,935,799 | ** | May-11 | Aug-19 | Use of rifaximin for treating diarrhea | ||||||||
| 7,928,115 | Apr-11 | Jul-29 | Use of rifaximin for treating TD*** | |||||||||
| 8,158,781 | * | Apr-12 | Jun-24 | Covers physical states, or polymorphous forms of rifaximin*** | ||||||||
| 8,158,644 | * | Apr-12 | Jun-24 | Covers physical states, or polymorphous forms of rifaximin*** | ||||||||
| 8,193,196 | * | Jun-12 | Sept-27 | Covers physical states, or polymorphous forms of rifaximin*** | ||||||||
| 8,309,569 | Nov-12 | Jul-29 | Use of rifaximin for treating IBS | |||||||||
| 8,518,949 | * | Aug-13 | Feb-26 | Pharmaceutical composition comprising rifaximin*** | ||||||||
24
Table of Contents
| U.S. Patent No. | Issue Date | Expiration | Subject | |||||||||
| 8,217,054 | * | Jul-12 | Dec-27 | Pharmaceutical composition and formulation related to the EIR formulation | ||||||||
| 8,568,782 | * | Oct-13 | Feb-27 | Pharmaceutical composition and formulation related to the EIR formulation | ||||||||
| 8,642,573 | Feb-14 | Oct-29 | Use of rifaximin for treating HE*** | |||||||||
| * | Licensed from Alfa Wasserman |
| ** | Licensed from CSMC |
| *** | Listed in the Orange Book |
In addition, we have filed applications for patents relating to additional indications using rifaximin and related chemical substances. In September 2009, Lupin Ltd. granted Salix the exclusive right in the United States to its bioadhesive drug delivery technology for use with rifaximin. In March 2011, Lupin granted Salix an exclusive license to exploit any product containing rifaximin and covered by the Lupin technology or joint technology for all uses in humans worldwide except India. In October 2009, Cipla, Limited, or Cipla, granted Salix the exclusive rights in the United States to its amorphous rifaximin application PCT Patent Application No. PCT/GB2007/003629; WO 2008/035109. In October 2012, the United States Patent and Trademark Office, or USPTO, declared in interference to determine the priority of invention between Cipla’s application related to amorphous rifaximin and the application from Olon S.p.A. (formerly known as Solmag S.p.A.), or Olon, directed to amorphous rifaximin. The USPTO had also declared an interference between the same Olon application and an Apotex-issued patent related to the same technology. Olon subsequently acquired the Apotex patent and in 2013, Salix acquired the rights to Olon’s amorphous rifaximin intellectual property, which included the rights to the Apotex patent. The USPTO resolved the two interferences, wherein Cipla was granted amorphous rifaximin composition of matter claims, the Apotex and Olon claims to the amorphous rifaximin composition of matter were canceled, and the Apotex patent claims directed to methods of making amorphous rifaximin remained valid. We continue to seek novel method claims for amorphous rifaximin from both the Olon and Cipla applications.
Data Exclusivity
Rifaximin had data exclusivity for the TD indication through May 2009. Upon expiration of this data exclusivity period, the OGD could approve an ANDA for Xifaxan 200 mg. We submitted a Citizen’s Petition in May 2008 requesting that the director of OGD impose scientifically appropriate standards for the demonstration of bioequivalence for ANDAs citing Xifaxan as the reference listed drug. In November 2011, the FDA posted draft bioequivalence guidance for rifaximin 200 mg tablets for the treatment of TD. This guidance recommends successful completion of a randomized, double blind, parallel placebo-controlled clinical trial in humans with clinical endpoints in order to file an ANDA for approval of a generic rifaximin 200 mg tablet for the treatment of TD.
Xifaxan 550 mg has orphan exclusivity through March 2017. Accordingly, although a competitor could file an ANDA for rifaximin 550 mg at any time, the OGD would be unable to approve the ANDA until March 2017. We are not aware of any ANDA that has been filed for rifaximin 550 mg. In February 2012, the FDA posted draft bioequivalence guidance for Xifaxan 550 mg tablets. The draft guidance recommends that, in addition to conducting the program outlined by the FDA for rifaximin 200 mg, the performance of a single-dose, three-way crossover in-vivo study of fasting bioequivalence with pharmacokinetic endpoints in both fasting and fed states. Additionally, with regard to the filing of an ANDA approval of a generic rifaximin 550 mg tablet for the treatment of HE, the guidance stipulated that the formulation of the 550 mg strength should be proportionally similar to that of the 200 mg strength.
25
Table of Contents
Apriso
Currently, there are four patents that we believe provide coverage for Apriso, including for methods of production and use, until 2018 (U.S. Patent Nos. 6,551,620, or the ‘620 patent, 7,547,451, or the ‘451 patent, 8,337,886, or the ‘886 patent, and 8,496,965, or the ’965 patent). See Part I. Item 3. Legal Proceedings and Note 13 to Consolidated Financial Statements for information about our ongoing litigation against Lupin and Novel for infringement of these patents.
Apriso is not a new chemical entity, but it was entitled to three years of exclusivity from the time of its approval based on the new clinical investigations required during the approval process. This exclusivity period has now expired.
MoviPrep
There are two patents for MoviPrep that we expect to provide patent protection until 2024 (U.S. Patent Nos. 7,169,381, or the ‘381 patent, and 7,658,914, or the ‘914 patent). The patents were issued to Norgine (including Norgine Europe, B.V.) by the USPTO in January 2007 and February 2010, respectively, and contain composition of matter and kit claims, and Norgine has licensed these patents to us.
Novel filed an ANDA with the FDA, which was accepted for filing in April 2008, seeking approval to market a generic version of MoviPrep in the United States prior to the September 2024 expiration of the ‘381 patent. On May 14, 2008, we and Norgine filed a lawsuit in the U.S. District Court for the District of New Jersey against Novel for infringement of this ‘381 patent. In August 2010, we entered into a settlement with the parties to the litigation and Actavis, Inc., resolving all of the parties’ outstanding claims and defenses in the lawsuit. The Consent Judgment entered by the court in connection with the settlement provides that Novel’s proposed generic product, absent a license as granted to Novel under the settlement, would infringe the ‘381 patent and further provides that Novel acknowledges and agrees not to contest the validity and enforceability of the ‘381 patent. In connection with the settlement, we entered into a Sublicense Agreement with Norgine and Novel, as well as a Supply Agreement with Novel and Actavis and a First Amendment to License and Supply Agreement with Norgine. Under the terms of the Sublicense Agreement, we and Norgine have granted Novel a fully paid-up license under the MoviPrep patents such that Novel is permitted to launch a generic MoviPrep product no later than September 24, 2018.
Relistor
Patents covering Relistor that provide protection for all indications currently marketed and being assessed are listed below in the table. Progenics, the owner of these patents, has licensed the worldwide rights to these patents to Salix.
| U.S. Patent No. | Issue Date | Expiration | Subject | |||||||||
| 6,559,158 | May-03 | Nov-17 | Covers Relistor SI | |||||||||
| 8,247,425 | Aug-12 | Dec-30 | Covers Relistor pre-filled syringe | |||||||||
| 8,420,663 | Apr-13 | Sept-29 | Covers all Relistor subcutaneous products | |||||||||
| 8,524,276 | Sept-13 | Mar-31 | Covers oral Relistor | |||||||||
| 8,552,025 | Oct-13 | Apr-24 | Covers Relistor syringe, vial, prefilled syringe and multi-dose pen products | |||||||||
There are pending applications on both the subcutaneous and oral formulations that, if issued, will provide further protection until 2024 and 2031, respectively.
In addition to the patent protection noted above, our Relistor SI product had five-year new chemical entity exclusivity from the date it received FDA approval. This exclusivity period expired on April 24, 2013. With the expiration of this data exclusivity period, the OGD may now approve an ANDA for Relistor, should one be filed. We are not aware of any ANDAs that have been filed with regard to our Relistor products.
OsmoPrep
The patent for OsmoPrep provides patent coverage until June 2028 (U.S. Patent No. 7,687,075, or the ‘075 patent). We own the ‘075 patent, and had licensed U.S. Patent No. 5,616,346, or the ‘346 patent, from CDC for commercialization of OsmoPrep in the United States. The ‘346 patent expired in May 2013.
26
Table of Contents
Novel filed an ANDA, which was accepted for filing in 2008, with the FDA seeking approval to market a generic version of OsmoPrep in the United States prior to the May 2013 expiration of the ‘346 patent and the 2028 expiration of the ‘075 patent. On September 8, 2008, we filed a lawsuit in the U.S. District Court for the District of New Jersey against Novel for the infringement of the ‘346 patent and seeking a declaratory judgment confirming the validity of the patent. The lawsuit also joined CDC as a party. In September 2010, we entered into a settlement with the parties to the litigation and Actavis, Inc., resolving all of the parties’ outstanding claims and defenses in the lawsuit. The Consent Judgment entered by the court in connection with the settlement provides that Novel’s proposed generic product, absent a license as granted to Novel under the Sublicense Agreement described below, would infringe the ‘346 patent and further provides that Novel acknowledges and agrees not to contest the validity and enforceability of the ‘346 patent. In connection with the settlement, we entered into: a Settlement Agreement with CDC, Novel, Actavis Inc. and the general partnership of Craig Aronchick, William H. Lipshutz and Scott H. Wright, or the General Partnership, that was the initial licensor of the ‘346 patent to Salix; a Sublicense Agreement with CDC, the General Partnership and Novel; a Supply Agreement with Novel; and, a Second Amendment to Supply Agreement with CDC and the General Partnership. Under the terms of the Sublicense Agreement, we, CDC and the General Partnership have granted Novel a fully paid-up, non-exclusive license under the OsmoPrep patents such that it is permitted to launch a generic OsmoPrep product no later than November 16, 2019.
Solesta and Deflux
In December 2011, Salix acquired licensed rights to Solesta and Deflux through the acquisition of Oceana. Solesta and Deflux are protected by U.S. Patent No. 5,633,001, which is directed to composition and method claims and provides protection until May 2014, and U.S. Patent No. 5,827,937, which is directed to methods of manufacturing and provides protection until July 2015.
Deflux was granted PMA approval on September 24, 2001. Solesta was granted PMA approval on May 27, 2011, and it should receive six years of data exclusivity from that date.
Fulyzaq
The patents related to the formulation of Fulyzaq provide protection until 2018 (U.S. Patent Nos. 7,323,195 and 7,341,744), and the patent related to methods of treating diarrhea with Fulyzaq, which was issued in November 2013 and subsequently listed in the Orange Book, provides protection until 2018 (U.S. Patent No. 8,573,634). In addition, we are seeking applications for patents relating to additional indications using Fulyzaq and related chemical substances. As a new molecular entity, we believe Fulyzaq may be entitled to patent term restoration, applications for which are pending at the USPTO.
Fulyzaq is also eligible for market exclusivity for five years from the date of FDA approval, or until December 31, 2017.
Giazo
The patent related to the formulation of Giazo provides protection until 2018 (U.S. Patent No. 6,197,341). Two method of use patents related to increasing the bioavailability of the drug provide protection until August 2026 (U.S. Patent Nos. 7,452,872 and 7,625,884). A third method of use patent for Giazo that was issued in July 2013 provides coverage until June 2031 (U.S. Patent No. 8,497,256).
Budesonide foam
The budesonide rectal foam product has patent coverage in the U.S. until 2015 (U.S. Patent Nos. 5,914,122).
Santarus Intellectual Property
Uceris
Santarus has exclusive rights to develop and commercialize Uceris in the U.S. under its strategic collaboration with Cosmo. Currently, there are four patents that are owned by Cosmo and licensed to Santarus that should provide coverage for Uceris (U.S. Patent Nos. 7,431,943, 7,410,651, RE43,799 and 8,293,273), each of which expires in 2020. There are pending patent applications that could potentially provide protection, if issued, until 2031 and 2033.
27
Table of Contents
Zegerid
Santarus has entered into an exclusive, worldwide license agreement with the University of Missouri for patents and pending patent applications relating to specific formulations of PPIs with antacids and other buffering agents and methods of using these formulations. Currently, there are three patents that we believe provide coverage for Zegerid (U.S. Patent Nos. 5,840,73, 6,780,882, and 7,399,772), with expiration dates in mid-2016. In addition to the issued U.S. patent coverage described above, several international patents have been issued for Zegerid. There are pending patent applications that could potentially provide protection, if issued, until 2016. Under certain circumstances, however, Zegerid could lose patent protection at an earlier date.
See Part I. Item 3. Legal Proceedings for information about patent litigation relating to Zegerid.
Glumetza
Santarus has exclusive rights to manufacture and commercialize the Glumetza products in the U.S., including its territories and possessions and Puerto Rico, under its commercialization agreement with Depomed. Currently, there are four patents that are owned or licensed by Depomed that should provide coverage for the Glumetza 500 mg dose product (U.S. Patent Nos. 6,340,475, 6,635,280, 6,488,962, and 6,723,340), with expiration dates in 2016, 2020 and 2021. There are three patents that are owned or licensed by Depomed that we believe provide coverage for the Glumetza 1000 mg dose product (U.S. Patent Nos. 6,488,962, 7,780,987, and 8,323,692), with expiration dates in 2020 and 2025. There are pending patent applications that could potentially provide protection, if issued, until 2025.
In connection with previously entered settlement agreements, a first generic filer was granted the right to sell a generic version of Glumetza in February 2016 and two subsequent generic filers were granted the right to sell a generic version of Glumetza in August 2016. Under certain circumstances, however, Glumetza could face generic competition at an earlier date.
See Part I. Item 3. Legal Proceedings for information about patent litigation relating to Glumetza.
Cycloset
Santarus has exclusive rights to manufacture and commercialize Cycloset in the U.S. under its distribution and license agreement with S2 and VeroScience. Currently, there are eight patents that Santarus has licensed from S2 and VeroScience that should provide coverage for Cycloset (U.S. Patent Nos. 5,679,685, 5,716,957, 7,888,310, 8,137,992, 8,137,993, 8,137,994, and 8,431,155), with expiration dates in 2014, 2015, 2023 and 2032.
Ruconest
Santarus has exclusive rights to develop and commercialize the Ruconest investigational drug in the U.S., Canada and Mexico under license and supply agreements with Pharming. Currently, there are patents owned by Pharming and licensed to Santarus that should provide coverage for Ruconest (U.S. Patent Nos. 7,067,713 and RE43,691), which expire in 2022 and 2024. In addition, as a biological product, Ruconest should be entitled under the Patient Protection and Affordable Care Act of 2010, or the Affordable Care Act, to a period of 12 years of regulatory exclusivity in the U.S.
GOVERNMENT REGULATION
Government authorities at the federal, state, and local level, and in countries outside of the United States, extensively regulate, among other things, the development, testing, manufacture, quality, approval, distribution, labeling, packaging, storage, record keeping, marketing, import/export, and promotion of drugs, biologics, and medical devices, as well as other types of medical products.
Requirements Applicable to Drugs and Biologics in the United States
In the United States, the FDA regulates drugs and biologics under the Federal Food, Drug and Cosmetic Act, or FDCA, the Public Health Service Act, or PHSA, and implementing regulations. Section 505 of the FDCA prohibits the introduction of a new drug into interstate commerce without an FDA approved application for marketing authorization under either section 505(b) or section 505(j). Applications under FDCA section 505 include the NDA, the ANDA and the “505(b)(2)” application. Section 351 of the PHSA imposes a similar requirement for biological products. Applications under PHSA section 351 include the BLA, and the biosimilar application.
28
Table of Contents
The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the development process, approval process, or after approval may subject a company to significant sanctions, including refusal to approve pending applications, withdrawal of an approval, clinical holds, warning letters, product recalls, product seizures, injunctions, fines, refusals of government contracts, restitution, disgorgement, or other civil or criminal penalties.
Preapproval Regulation
The process required by FDA before a drug or biologic may be marketed in the United States requires numerous steps, including the following:
| • | Completion of pre-clinical laboratory tests, animal studies, and other studies in accordance with current good laboratory practices, or cGLPs, or other applicable regulations; |
| • | Submission to FDA of an IND, which must become effective before the product can be tested in humans, and which must contain pre-clinical data, together with manufacturing information, analytical data, and any available clinical data or literature; |
| • | Performance of adequate and well-controlled clinical trials in humans according to FDA’s current good clinical practices, or cGCPs, to establish the safety and efficacy (or safety, purity, and potency in the case of a biologic) of the product for its intended use(s); |
| • | Submission to FDA of an NDA for a new drug, or a BLA for a biological product; |
| • | Satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the product is produced to assess compliance with FDA’s current good manufacturing practices, or cGMPs, and to assure that the facilities, methods, and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| • | FDA review and approval of the NDA or BLA. |
Before testing new drugs or biologics in humans, the product enters the pre-clinical testing phase. Pre-clinical tests include laboratory evaluations of the product’s chemical and/or biological characteristics, as well as animal studies that assess various aspects of the product candidate’s safety and efficacy. Before commencing clinical trials, the sponsor must submit the results of the pre-clinical tests, together with manufacturing information, analytical data, and any available clinical data or literature, to FDA as part of the IND. The sponsor must also include an initial protocol detailing the first phase of the proposed clinical investigation and must update the IND with subsequent protocols if the clinical program advances beyond early testing. The IND automatically becomes effective 30 days after receipt by FDA unless the FDA imposes a clinical hold within that 30-day time period. If FDA institutes a clinical hold, the sponsor must resolve any and all concerns to FDA’s satisfaction before clinical trials under the IND can begin. The FDA may also impose clinical holds on a product candidate at any time before or during clinical trials due to safety concerns or non-compliance with GCPs or other regulations.
Each clinical protocol must be submitted to the IND for FDA review, and to an Institutional Review Board (IRB) for approval. Protocols detail, among other things, the objectives of the clinical trial, dosing procedures, subject inclusion and exclusion criteria, and the parameters to be used to monitor subject safety. An IRB is charged with protecting the welfare and rights of study participants, giving consideration to whether the risks to individual study participants are minimized and reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative, and must monitor the clinical trial until completed.
29
Table of Contents
Clinical testing of a new drug or biologic generally proceeds in three phases, though in some cases they may overlap and/or be combined:
| • | Phase 1. Phase 1 includes the initial introduction of an investigational new drug or biologic in humans with the targeted disease or healthy volunteers. These studies are designed to evaluate the safety, dosage tolerance, and other characteristics of the investigational product, and if possible, to gain initial evidence of efficacy. Phase 1 trials typically include 20 to 80 participants. |
| • | Phase 2. Phase 2 testing includes clinical trials designed to evaluate the effectiveness of the investigational product for a particular indication(s) in patients with the targeted disease, to determine optimal dosage, and to identify possible adverse side effects. Phase 2 clinical trials are typically conducted in a small patient population, usually involving no more than several hundred participants. |
| • | Phase 3. Phase 3 clinical trials are conducted in an expanded patient population and are typically conducted at geographically dispersed sites. They are intended to further evaluate dosage, clinical effectiveness, and safety, as well as to establish the overall benefit-risk ratio of the product, and to provide the primary basis for approval. Phase 3 clinical trials usually involve several hundred to several thousand participants. |
Sponsors must submit progress reports at least annually to FDA, detailing the results of the clinical trials. The sponsor must also submit written IND safety reports to FDA and to the investigators describing serious and unexpected adverse events or any finding from tests in laboratory animals that suggests a significant risk for human subjects. FDA, the sponsor, or the sponsor’s data safety monitoring board may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to unacceptable health risks. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution for a variety of reasons, including that if the clinical trial is not being conducted in accordance with the IRB’s requirements, that patient rights are not being adequately protected, or if the drug or biologic has been associated with unexpected serious harm to patients. Additionally, sponsors of clinical trials, except Phase 1 trials, are required to submit certain registry and results information for inclusion in a publicly available registry data bank.
U.S. Review and Approval Process
The results of pre-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the product, proposed labeling, and other relevant information are submitted to FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is typically subject to the payment of substantial user fees. In addition, under the Pediatric Research Equity Act of 2003, or PREA, an NDA or BLA must contain data to assess the safety and effectiveness of the drug or biologic for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. FDA may grant deferrals for submission of data or full or partial waivers.
FDA reviews all NDAs and BLAs submitted to ensure that they are sufficiently complete for substantive review before accepting them for filing. FDA may request additional information rather than accept an application for filing. Once an application is accepted for filing, FDA begins an in-depth substantive review to determine, among other things, whether a drug is safe and effective (or, for biologics, whether the product is safe, pure, and potent) for its intended use(s) and whether the manufacturing controls are adequate to assure and preserve the product’s identity, strength, quality, and purity. Before approving an application, FDA typically inspects the facility where the product is manufactured in order to ensure compliance with cGMPs. FDA will also determine whether a REMS is necessary to assure the safe use of the product. If the FDA concludes that a REMS is necessary, the applicant must submit a proposed REMS. The FDA will not approve a marketing application without a REMS, if required.
FDA may refer an application to an Advisory Committee for a recommendation as to whether the application should be approved. An Advisory Committee is a panel of experts who provide advice and recommendations to the agency. FDA is not required to follow an Advisory Committee’s recommendations.
The development and approval process is lengthy and resource-intensive. For additional information regarding our research and development costs, see “Critical Accounting Policies—Research and Development” in “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Ultimately, FDA may refuse to allow a
30
Table of Contents
clinical program to begin, terminate a clinical development program, require submission of additional pre-clinical or clinical data, and/or refuse to approve an application for numerous reasons. Even if an applicant submits all of the data requested by FDA, the agency may ultimately decide that the application does not satisfy the criteria for approval. In addition, even if a product is approved, the scope of the approval may be significantly limited in terms of patient populations, indications, other conditions of use, and/or restrictions on distribution and use (e.g., through a REMS). FDA could also require significant contraindications, warnings, or precautions be included in the product labeling.
Patent Term Restoration and Exclusivity
Under the Hatch-Waxman Act, certain U.S. patents are eligible for a limited patent term extension of up to five years in order to compensate the sponsor of a new drug or biologic for patent term lost during product testing and FDA review. Only one patent per drug or biologic is eligible for the extension.
Also under Hatch-Waxman, drugs that are new chemicals entities, or NCEs, are eligible for a five-year data exclusivity period. During this period, FDA may not accept for review an ANDA or a 505(b)(2) application submitted by another company that relies on any of the data submitted by the innovator company. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator NDA holder. Hatch-Waxman also provides three years of data exclusivity for an NDA, 505(b)(2) application, or NDA supplement that is not an NCE if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed essential to approval. During this period, FDA will not approve an application filed by a third party for the protected conditions of use that relies on any of the data that was submitted by the innovator company. Neither exclusivity period blocks the approval of full applications submitted to FDA that do not rely on the pioneer’s data.
The Biologics Price Competition and Innovation Act of 2009, or BPCIA, created a 12-year exclusivity period for innovator biologics. FDA therefore cannot approve a biosimilar application relying on a specific reference product until 12 years after the reference product is first licensed. BLA supplements are not eligible for any additional exclusivity. In addition, a BLA is not entitled to the 12-year exclusivity period if it is a subsequent application filed by the same manufacturer or sponsor as an earlier application (or a licensor, predecessor in interest, or other related entity), if the subsequent application relates to: (1) a change (not including a modification to the structure of the biological product) that results in a new indication, route of administration, dosing schedule, dosage form, delivery system, delivery device, or strength, or (2) a modification to the structure of the biological product that does not result in a change in safety, purity, or potency. FDA has yet to define the key terms in this exclusivity provision, so the robustness of exclusivity for biologics is somewhat uncertain.
Under the Orphan Drug Act, special incentives exist for companies to develop products for diseases or conditions that affect fewer than 200,000 people in the United States. Products designated as orphan drugs are eligible for special incentives. Companies must submit their request that the FDA grant a drug orphan designation prior to submission of an NDA or BLA for that product. If a product that has orphan drug designation is the first to subsequently receive FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusivity. Orphan drug exclusivity prevents FDA approval of applications by others for the same drug for the designated orphan disease or condition for a period of seven years. Orphan drug exclusivity does not prevent FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. In addition, FDA may approve a subsequent application from another entity for the same drug for the same orphan disease or condition if the FDA determines that the subsequent product is clinically superior, or that the holder of the orphan drug exclusivity cannot assure the availability of sufficient quantities of the drug to meet the public’s need.
Post-Approval Requirements
Any FDA-approved drug or biologic is subject to continuing regulation by FDA. Post-approval requirements include, among other things, record keeping and reporting requirements, packaging requirements, requirements for reporting of adverse drug experiences, import/export controls, restrictions on advertising and promotion, and cGMP requirements. FDA strictly regulates labeling, advertising, and promotion of drugs and biologics. Such products may be promoted only for the FDA-approved indications and in accordance with the provisions of the FDA-approved label. FDA periodically inspects manufacturing facilities to ensure that the product is being manufactured in accordance with cGMPs and the specifications outlined in the NDA or BLA. Manufacturing facilities must be registered with FDA, and
31
Table of Contents
companies must list all of the drugs and biologics they manufacture with FDA. As a condition of approval, FDA could also impose a Phase 4 study or trial to further assess the benefit/risk profile of the product, which could require the expenditure of significant time and resources. Postmarket data may cause the agency to seek significant changes in the labeling for the product including new warnings or a REMS. Even if it does not impose a Phase 4 study or trial on a sponsor, FDA may withdraw approval for the product if it determines that the benefits of the product no longer outweigh the risks.
Many states also regulate the manufacture and distribution of drugs and biologics and require companies to register in order to manufacture or distribute products in the state. Failure to comply with these federal and state requirements could subject a company to significant sanctions, including withdrawal of an approval, warning letters, product recalls, product seizures, injunctions, fines, refusals of government contracts, or civil or criminal penalties.
Requirements Applicable to Medical Devices in the United States
FDA regulates, among other things, the development, testing, manufacturing, labeling, marketing, and distribution of medical devices. The level of regulation applied by FDA generally depends on the class into which the medical device falls: Class I, II, or III. Class I medical devices present the lowest risk, and Class III medical devices present the highest risk. In general, the higher class of device, the greater the degree of regulatory control. All devices, for example, are subject to “General Controls,” which include:
| • | Establishment registration by manufacturers, distributors, re-packagers, and re-labelers; |
| • | Device listing with FDA; |
| • | Good manufacturing practices; |
| • | Labeling regulations; and |
| • | Reporting of adverse events. |
Class II medical devices are subject to General Controls, but also Special Controls, including special labeling requirements, mandatory performance standards, additional postmarket surveillance, and specific FDA guidance.
Most Class I devices are exempt from FDA premarket review or approval. With some exceptions, Class II devices may be marketed only if FDA “clears” the medical device through the 510(k) process, which requires a company to show that the device is “substantially equivalent” to certain devices already on the market. Again with some exceptions, Class III devices are approved through a PMA which generally requires an applicant to submit data from clinical trials that establish the safety and effectiveness of the device. Clinical data is sometimes required for a 510(k) application as well. Manufacturers conducting clinical trials with medical devices are subject to similar requirements as those conducting clinical trials with drugs or biologics. For example, a manufacturer must obtain an investigational device exemption, or IDE to test a device in humans, must comply with GCPs (which for devices are called quality system regulations, or QSRs), and must obtain IRB approval.
FDA has broad post-market regulatory and enforcement powers with respect to medical devices, similar to those for drugs and biologics. For example, medical devices are subject to detailed manufacturing standards under FDA’s QSRs and specific rules regarding labeling and promotion. Medical device manufacturers must also register their establishments and list their products with FDA.
States also impose regulatory requirements on medical device manufacturers and distributors, including registration and record-keeping requirements. Failure to comply with the applicable federal and state medical device requirements could result in, among other things, refusal to approve or clear pending applications, withdrawal of an approval or clearance, warning letters, product recalls, product seizures, total or partial suspension of production, fines, refusals of government contracts, restitution, disgorgement, or other civil or criminal penalties.
Other U.S. Healthcare Laws and Compliance Regulations
In the U.S., our activities are subject to regulation by various federal, state, and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, or CMS, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), and the U.S. Department of Justice and individual
32
Table of Contents
U.S. Attorney offices within the Department of Justice, as well as state and local governments. For example, sales and marketing activities as well as scientific/educational grant programs and other interactions with health care providers must comply with the anti-fraud and abuse provisions of the Social Security Act, the False Claims Act, the privacy provisions of the Health Insurance Portability and Accountability Act, or HIPAA, and similar state laws, each as amended. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veterans Health Care Act of 1992, each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements may apply.
Under the Veterans Health Care Act, or VHCA, companies are required to offer innovator drugs and biological products at reduced price to a number of federal agencies, including the U.S. Department of Veterans Affairs and the U.S. Department of Defense, the Public Health Service, and certain private Public Health Service-designated entities in order to participate in other federal funding programs including Medicare and Medicaid. Participation under the VHCA requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations. In addition, legislative changes require that VHCA discounted prices be offered via a rebate system for products distributed to retail pharmacies dispensed to TRICARE beneficiaries. Participation under the VHCA requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations. In order for a manufacturer’s outpatient drug and biological products to be covered under Medicaid, the manufacturer must enter into a rebate agreement with CMS, which requires the payment of substantial rebates to state Medicaid programs and the reporting of certain pricing information to CMS on a monthly and quarterly basis. For drugs that are covered under Medicare Part B, the manufacturer must report average sales price, or ASP, to CMS on a quarterly basis. Failure to report this information in a timely and accurate manner can lead to substantial civil and criminal penalties and to liability under the False Claims Act. In order for a manufacturer’s products to be covered under Medicare Part D, the manufacturer must enter into a Medicare Coverage Gap Discount Agreement, which requires the manufacturer to reimburse a Part D plan for 50% of the negotiated price of any prescription for one of its products dispensed to a Medicare beneficiary in the Part D coverage gap or “donut hole.”
The Affordable Care Act includes new reporting and disclosure requirements for pharmaceutical and device manufacturers with regard to payments or other transfers of value made to health care providers. The first report, covering August to December 2013, is due in March 2014. Reports submitted under these new requirements will be placed on a public database. If we fail to provide these reports, or if the reports we provide are not accurate, we could be subject to significant penalties.
We are subject to strict regulation by federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback laws, physician self-referral laws, false claims laws, and others. The federal Anti-Kickback Statute, for example, prohibits persons from soliciting, offering, receiving, or providing remuneration to induce the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as Medicare or Medicaid. The federal False Claims Act prohibits presenting claims that seek payment from Medicare, Medicaid, or other federal government payors that are false or fraudulent.
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical and biological products in a state. In certain states, this includes manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Several states have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials, and other activities, or register their sales representatives. Many of these laws also prohibit certain sales and marketing practices. In addition, all of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
In addition to those laws and regulations described above, other federal and state laws that could affect our operations include:
| • | The U.S. Foreign Corrupt Practices Act, or FCPA, which prohibits companies from making certain improper payments to foreign officials, and which requires companies to maintain certain record keeping procedures; |
33
Table of Contents
| • | State and federal laws governing human subject research and animal testing; |
| • | Occupational Safety and Health, or OSHA, requirements; and |
| • | State and local laws and regulations on the handling and disposal of medical waste. |
Foreign Regulatory Requirements
In addition to laws and regulations in the U.S., we will be subject to a variety of foreign laws and regulations governing clinical trials and commercial sales and distribution of our products to the extent we choose to sell those products outside of the U.S. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required to obtain FDA approval. The requirements governing other aspects of drug, biologic, and medical device development, testing, licensing, manufacturing, sales and marketing, distribution, pricing and reimbursement vary greatly from country to country.
COMPETITION
Competition in our business is intense and characterized by extensive research efforts, rapid technological progress and an increasing rate of generic product approvals. Technological developments by competitors, earlier regulatory approval for marketing competitive products, including generic versions of our products, such as those launched against Colazal in December 2007 and Pepcid in 2010, or superior marketing capabilities possessed by competitors could adversely affect the commercial potential of our products and could have a material adverse effect on our revenue and results of operations. We believe that there are numerous pharmaceutical and biotechnology companies, including large well-known pharmaceutical companies and generic manufacturers, as well as academic research groups throughout the world, engaged in research and development efforts with respect to pharmaceutical products targeted at gastrointestinal diseases and conditions addressed by our current and potential products. In particular, we are aware of products in research or development by competitors that address the conditions being targeted by our products. Developments by others might render our current and potential products obsolete or non-competitive. Competitors might be able to complete the development and regulatory approval process sooner and, therefore, market their products earlier than us. Many of our competitors have greater financial, marketing and personnel resources and development capabilities than we do.
In addition, certain of our competitors may market lower-priced products from foreign countries that have placed price controls on pharmaceutical products. Proposed federal legislative changes may expand consumers’ ability to import lower-priced versions of its products and competing products from Canada and other developed countries. Further, several states and local governments have implemented importation schemes for their citizens and, in the absence of federal action to curtail such activities, other states and local governments may launch importation efforts. The importation of foreign products that compete with our own products could negatively impact our business and prospects.
The existence of numerous competitive products may put downward pressure on pricing and market share, which in turn may adversely affect our business, financial condition and results of operations.
In addition, if approved, our investigational drugs will compete with many other drug and biologic products that are already entrenched in the marketplace, and face competition from other product candidates currently under development by our competitors.
Competitors for Principal Salix Products
Xifaxan 550 mg competes with lactulose, which is offered by Cumberland Pharmaceuticals, Inc., or Cumberland, and various generic manufacturers.
Xifaxan 200 mg competes with many products, including ciprofloxacin, commonly known as “Cipro®” and marketed by Bayer.
34
Table of Contents
Apriso competes with many products, including:
| • | branded 5-aminosalicylate, or 5-ASA, prescription products (such as Asacol®, Delzicol®, Lialda®, Pentasa®, and Dipentum®); and |
| • | generic 5-ASA prescription products (such as sulfasalzine, mesalamine, balsalazide disodium and olsalazine). |
MoviPrep competes with many products, including:
| • | branded liquid PEG prescription products (such as Colyte, Golytely, Halflytely, SuPrep and Nulytely, Trilyte and Prepopik); |
| • | generic liquid PEG prescription products (such as PEG 3350 and Electrolytes marketed by Novel and Mylan); and |
| • | Various over-the-counter, or OTC, products. |
Our OsmoPrep is currently the only FDA-approved tablet bowel cleansing product being marketed in the U.S.
Relistor competes with many products in the OIC market for advanced illness/palliative care, although these products are not indicated for this condition:
| • | branded prescription products (such as Amitiza, Kristalose, Linzess and Entereg); |
| • | OTC products (such as Kondremul, Miralax, Fleets Phosphosoda and Milk of Magnesia saline laxatives, Dulcolax, Senokot and Colace). |
Competitors for Principal Santarus Products
Uceris competes with many other products, including:
| • | branded 5-ASA prescription products (such as Asacol, Delzicol, Lialda and Pentasa); |
| • | generic 5-ASA prescription products (such as sulfasalzine, mesalamine and balsalazide disodium); |
| • | generic prescription corticosteroids (such as prednisone and hydrocortisone); |
| • | branded and generic prescription immunosuppressive products (such as aziothioprine and 6-mercaptopurine); and |
| • | branded anti-TNF-a prescription products (such as Remicade® and Humira®). |
Zegerid (branded and authorized generic) competes with many other products, including:
| • | branded PPI prescription products (such as Nexium®, Aciphex® and Dexilant®); |
| • | generic PPI prescription products (such as delayed-release omeprazole, delayed-release lansoprazole and delayed-release pantoprazole); |
| • | OTC PPI products (such as Prilosec OTC® , Prevacid® 24HR and store-brand versions); and |
| • | other prescription and/or OTC acid-reducing agents (such as histamine-2 receptor antagonists and antacids). |
Glumetza and Cycloset compete with many products, including:
| • | other branded immediate-release and extended-release metformin products (such as Fortamet®, Glucophage® and Glucophage XR®); |
| • | branded extended-release metformin combination products (such as Janumet®XR and Kombiglyze®XR); |
| • | generic immediate-release and extended-release metformin products; and |
| • | other prescription diabetes treatments. |
35
Table of Contents
In addition, various companies are developing new products that may compete with Glumetza and Cycloset in the future. For example, Depomed has licensed rights to use its extended-release patents in combination with canagliflozin, a sodium-glucose transporter-2, or SGLT2, compound being developed by Janssen Biotech, Inc., or Janseen. Depomed has also licensed rights to use its extended-release metformin patents to Boehringer Ingelheim for use with certain fixed dose combination products that include proprietary Boehringer Ingelheim compounds. Like Glumetza, Cycloset competes with many other products, including:
| • | dipeptidyl peptidase IV inhibitors, or DPP-4, products (such as Januvia® and Onglyza®); |
| • | glucagon-like peptide 1, or GLP-1, receptor agonist products (such as Byetta®, Victoza® and Bydureon®); |
| • | thiazolidinedione, or TZD, products (such as Avandia® and Actos®); |
| • | sulfonylureas products (such as Amaryl® and Glynase®); and |
| • | branded and generic metformin products. |
EMPLOYEES
As of December 31, 2013, we had approximately 555 full-time employees, and Santarus had 332 full-time employees. We believe that our future success will depend in part on our continued ability to attract, hire, and retain qualified personnel, including sales and marketing personnel in particular. Competition for such personnel is intense, and there can be no assurance that we will be able to identify, attract, and retain such personnel in the future. None of our or Santarus’s employees is represented by a labor union. We have not experienced any work stoppages and consider our relations with our and Santarus’s employees to be good.
This report contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed in this report. Factors that could cause or contribute to these differences include, but are not limited to, those discussed below and elsewhere in this report and in any documents incorporated in this report by reference.
If any of the following risks, or other risks not presently known to us or that we currently believe to not be significant, develop into actual events, then our business, financial condition, results of operations or prospects could be materially adversely affected. If that happens, the market price of our common stock could decline, and stockholders might lose all or part of their investment.
General Risks
Future sales of Xifaxan and our other marketed products might be less than expected.
Following completion of our acquisition of Santarus, we currently actively market and sell more than 20 primary products. We expect Xifaxan, which was launched in mid-2004 for the treatment of TD, and approved and launched in March 2010 for the treatment of HE, to continue to be our most significant source of revenue in the future. If sales of our marketed products decline or if we experience product returns significantly in excess of estimated amounts recorded, particularly with respect to Xifaxan, it would have a material adverse effect on our business, financial condition and results of operations.
The degree of market acceptance of our products among physicians, patients, healthcare payors and the medical community will depend upon a number of factors including:
| • | the timing of regulatory approvals and product launches by us or competitors, and including any generic or OTC competitors; |
| • | perceptions by physicians and other members of the healthcare community regarding the safety and efficacy of our products; |
| • | price increases, and the price of our products relative to other drugs or competing treatments; |
| • | patient and physician demand; |
36
Table of Contents
| • | adverse side effects or unfavorable publicity concerning our products or other drugs in our class; |
| • | the results of product development efforts for new indications; |
| • | the scope and timing of additional marketing approvals and favorable reimbursement programs for expanded uses; |
| • | the availability of sufficient commercial quantities of the products; and |
| • | our success in getting other companies to distribute our products outside of the U.S. gastroenterology, hepatology and colorectal surgery markets. |
Regulatory approval of our product candidates is time-consuming, expensive and uncertain, and could result in unexpectedly high expenses.
Development of our products is subject to extensive regulation by governmental authorities in the United States and other countries. To market a new drug in the U.S., we must submit to the FDA and obtain FDA approval of an NDA or a BLA. An NDA or BLA must be supported by extensive clinical and preclinical data, as well as extensive information regarding chemistry, manufacturing and controls, or CMC, to demonstrate the safety and effectiveness of the applicable product candidate. The FDA’s regulatory review of NDAs and BLAs is becoming increasingly focused on product safety attributes, and even if approved, our product candidates may not be approved for all indications requested and such approval may be subject to limitations on the indicated uses for which the product candidate may be marketed, restricted distribution methods or other limitations. In addition, the FDA’s large workload has led to delays in its review of NDA submissions, which could require us to incur significant unexpected expenses or delay or limit our ability to sell product candidates for which we have not yet received regulatory approval.
Failure can occur at any stage of clinical testing. The clinical study process may fail to demonstrate that our product candidates are safe for humans or effective for their intended uses. Our clinical tests must comply with FDA and other applicable U.S. and foreign regulations, including a requirement that they be conducted in accordance with good clinical practices. We may encounter delays based on our inability to timely enroll enough patients to complete our clinical studies. We may suffer significant setbacks in advanced clinical studies, even after showing promising results in earlier studies. Based on results at any stage of clinical studies, we may decide to discontinue development of a product candidate. In addition, we or the FDA may suspend clinical studies at any time if the patients participating in the studies are exposed to unacceptable health risks or if the FDA finds deficiencies in our applications to conduct the clinical studies or in the conduct of our studies.
Regulatory approval of an NDA or a BLA is difficult, time-consuming and expensive to obtain. The number and types of preclinical studies and clinical trials that will be required for NDA or BLA approval varies depending on the drug, the disease or the condition that the drug is designed to target and the regulations applicable to any particular drug. We could encounter problems that cause us to repeat or perform additional preclinical studies, CMC studies or clinical studies. Our clinical studies might be delayed or halted, or additional studies might be required, for various reasons, including:
| • | the drug is not effective; |
| • | patients experience severe side effects during treatment; |
| • | patients do not enroll in the studies at the rate expected; |
| • | drug supplies are not sufficient to treat the patients in the studies; or |
| • | we decide to modify the drug during testing. |
If regulatory approval of any product is granted, it will be limited to those indications for which the product has been shown to be safe and effective, as demonstrated to the FDA’s satisfaction through clinical studies. In addition, before the FDA approves one of our investigational drugs, the FDA may choose to conduct an inspection of one or more clinical or manufacturing sites. These inspections may be conducted by the FDA both at U.S. sites as well as overseas. Any restrictions on the ability of FDA investigators to travel overseas to conduct such inspections, either because of financial or other reasons including political unrest, disease outbreaks or terrorism, could delay the inspection of overseas sites and consequently delay FDA approval of our investigational drugs.
37
Table of Contents
To market drugs outside the U.S., we and current or future collaborators must comply with numerous and varying regulatory requirements of other countries. Regulatory approval procedures vary among countries and can involve additional product testing and additional administrative review periods, including obtaining reimbursement approval in select markets. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others.
The FDA may require significant additional clinical testing for our product candidates, and we may not receive regulatory approval for some or all of these product candidates.
In addition to the general development and regulatory risks described above, each of our investigational drugs is subject to additional risks that may cause us to incur significant additional costs and the FDA, or applicable foreign regulator, may ultimately refuse to approve of one or more of our product candidates. If we experience delays or setbacks for any reason, our product development costs will increase and we may decide to abandon a product candidate entirely. If any of our product candidates fail to receive regulatory approval, we will have incurred significant expenses without the possibility of generating revenues, which could have a material adverse effect on our business.
In August 2010, the FDA accepted our NDA for rifaximin for IBS, and gave us an action date of December 7, 2010. In October 2010, the FDA informed us it was extending the action date by three months to provide for a full review and extended our action date to March 7, 2011. We received a CRL on March 7, 2011. The FDA deemed that the Xifaxan 550 mg sNDA was not ready for approval, primarily due to a newly expressed need for retreatment information. We initiated enrollment in a retreatment trial in the first quarter of 2012, but there is no assurance that the FDA will approve rifaximin for IBS in a timely manner, or at all.
On July 27, 2012, the FDA issued a CRL following the FDA’s review of an sNDA for Relistor SI for the treatment of OIC in adult patients with chronic, non-cancer pain. The CRL requested additional clinical data. In October 2012, Salix and Progenics held an End-of-Review meeting with the Division of Gastroenterology and Inborn Errors Products to better understand the contents of the CRL. The Division expressed a concern that there might be a risk associated with the chronic use of mu-opioid antagonists in patients that are taking opioids for chronic pain. In order to understand this potential risk, the Division has communicated that a very large, well-controlled, chronic administration trial will have to be conducted to assess the safety of any mu-opioid antagonist prior to market approval for the treatment of patients with OIC who are taking opioids for chronic, non-cancer pain. We have since had additional discussions with the Division and expressed the view that the post-marketing, clinical and preclinical data currently available adequately demonstrate an appropriate and expected safety profile sufficient to permit the approval of the sNDA and filed a formal appeal to that effect. The FDA initially planned to convene an Advisory Committee on March 10-11, 2014 regarding this sNDA, but postponed the date of the meeting due to scheduling conflicts. The FDA intends to seek input from this Advisory Committee prior to answering our formal appeal to the CRL and has stated that it will take action under the appeal within 30 days after receiving input from the Advisory Committee. Based on the results of this meeting, we believe we might terminate our development program for Relistor SI for the treatment of OIC in chronic non-cancer pain. We remain hopeful that a path forward can lead to the expansion of the use of this new and alternative therapy to treat OIC in patients suffering from chronic pain; however, depending on the results of our discussions with the Division, we believe we might terminate our development program for Relistor SI for the treatment of OIC in chronic non-cancer pain. We are currently evaluating the Relistor Oral program and currently believe we will continue this program. However, additional information and additional guidance from the FDA could result in the termination of the Relistor Oral program. There is no assurance the FDA will approve Relistor SI or Relistor Oral for the treatment of OIC in adult patients with chronic, non-cancer pain in a timely manner, or at all.
Regulatory approvals, even if granted, might entail ongoing requirements or restrictions on marketing. These requirements or restrictions, or inquiries into our marketing practices, could increase our expenses and limit revenue.
Regulatory approvals might entail ongoing requirements for post-marketing studies or limit how or to whom we can sell our products. Even if we obtain regulatory approvals, labeling and promotional activities are subject to continual scrutiny by the FDA and other federal and state authorities. For example, in 2008, the FDA required us to put
38
Table of Contents
a “black box” warning on the OsmoPrep and Visicol labels regarding potential kidney damage that could result from their use, and a “black box” warning for Metozolv regarding tardive dyskensia which could result from its use. We believe these warnings contributed to reduced sales of those products, and they could limit future sales of those products. With regard to OsmoPrep and Visicol, following consultation with the FDA, we also developed a risk evaluation and mitigation strategy, or REMS, including a medication guide. We have conducted post-marketing clinical trials as part of this strategy. In December 3011, the FDA agreed that a REMS was no longer required for OsmoPrep and Visicol.
In addition, we periodically receive inquiries from authorities, including specifically the Office of Prescription Drug Promotion of the FDA, or OPDP, formerly known as the Division of Drug Marketing, Advertising, and Communications, regarding compliance with marketing and other regulations. Responding to inquiries from authorities can be costly and divert the time and attention of our senior management from our business operations and result in increased legal expenses. The laws and regulations regarding off-label promotion and the authorities’ interpretation of them might increase our expenses, impair our ability to effectively market our products, and limit our revenue.
On February 1, 2013, our wholly owned subsidiary Salix Pharmaceuticals, Inc. received a subpoena from the U.S. Attorney’s Office for the Southern District of New York requesting documents regarding our sales and promotional practices for Xifaxan, Relistor and Apriso. The Company is in the process of responding to the subpoena and intends to cooperate fully with the subpoena and related government investigation, which has and will continue to increase our legal expenses, and might require management time and attention. Currently, we cannot predict or determine the timing or outcome of this inquiry or its impact on financial condition or results of operations.
Our intellectual property rights might not afford us with meaningful protection, which could result in substantial costs to us and negatively affect our revenues by impacting our pricing and sales volume as well as royalties and other payments owed to us by third parties.
The intellectual property rights protecting our products might not afford us with meaningful protection from generic and other competition. In addition, because our strategy is to in-license or acquire pharmaceutical products which typically have been discovered and initially researched by others, future products might have limited or no remaining patent protection due to the time elapsed since their discovery. Competitors could also design around any of our intellectual property or otherwise design competitive products that do not infringe our intellectual property.
Any litigation in which we become involved to enforce intellectual property rights could result in substantial cost to us. In addition, claims by others that we infringe their intellectual property could be costly. Our patent or other proprietary rights related to our products might conflict with the current or future intellectual property rights of others. Litigation or patent interference proceedings, either of which could result in substantial cost to us, might be necessary to defend any patents to which we have rights and our other proprietary rights or to determine the scope and validity of other parties’ proprietary rights. The defense of patent and intellectual property claims is both costly and time-consuming, even if the outcome is favorable. Any adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease selling one or more of our products. We might not be able to obtain a license to any third-party technology that we require to conduct our business, or, if obtainable, that technology might not be available at a reasonable cost.
Upon patent expiration, our drugs could be subject to generic competition, which could negatively affect our pricing and sales volume. As previously disclosed, this has already happened to Colazal, which had been our largest selling drug prior to 2008. See Part I. Item 1. “Business—Patents and Proprietary Rights” for additional information about the existing patent protection for our and Santarus’s products.
We also rely on trade secrets, proprietary know-how and technological advances, which we seek to protect, in part, through confidentiality agreements with collaborative partners, employees and consultants. These agreements might be breached and we might not have adequate remedies for any such breach. In addition, our trade secrets and proprietary know-how might otherwise become known or be independently developed by others.
39
Table of Contents
Intense competition might render our products noncompetitive or obsolete.
Competition in our business is intense and characterized by extensive research efforts and rapid technological progress. Technological developments by competitors, regulatory approval for marketing competitive products, including potential generic or over-the-counter products, or superior marketing resources possessed by competitors could adversely affect the commercial potential of our products and could have a material adverse effect on our revenue and results of operations. Generic competition is an increasing risk, as we have experienced with Colazal and Pepcid, and with challenges to our bowel-cleansing products’ intellectual property noted above. We believe that there are numerous pharmaceutical and biotechnology companies, including large well-known pharmaceutical companies, as well as academic research groups throughout the world, engaged in research and development efforts with respect to pharmaceutical products targeted at gastrointestinal diseases and conditions addressed by our current and potential products. In particular, we are aware of products in research or development by competitors that address the diseases being targeted by our products. Developments by others might render our current and potential products obsolete or noncompetitive. Competitors might be able to complete the development and regulatory approval process sooner and, therefore, market their products earlier than we can.
Many of our competitors have greater financial, marketing and personnel resources and development capabilities than we do. For example, many large, well-capitalized companies already offer products in the United States and Europe that target the indications for:
| • | Xifaxan for HE, including lactulose (various manufacturers); |
| • | Xifaxan for TD, including ciprofloxacin, commonly known as Cipro (Bayer); |
| • | Apriso, including Asacol and Delzicol (Warner Chilcott plc, or Warner Chilcott), sulfasalazine (Pfizer Pharmaceuticals, or Pfizer), Dipentum (Alaven), Pentasa and once-a-day Lialda (Shire Pharmaceuticals Group, or Shire) and three generic balsalazide disodium capsule products; |
| • | OsmoPrep and MoviPrep, including Colyte (Meda Pharmaceuticals), Golytely (Braintree Laboratories, Inc., or Braintree), Halflytely (Braintree), SuPrep (Braintree), and Nulytely (Braintree), Trilyte (Alaven Pharmaceutical LLC, or Alaven) and Prepopik (Ferring Pharmaceuticals, Inc.), as well as potential generics from Novel or others; |
| • | Relistor for OIC, including OTC laxatives (various manufacturers), Amitiza (Sucampo), Kristalose (Cumberland), and Entereg (Cubist Pharmaceuticals, Inc.); |
| • | Solesta, including various OTC antidiarrheals, fiber, stool softeners and laxatives (various manufacturers), biofeedback, the medical device Inter Stim (Medtronic) and sphincteroplasty surgery; |
| • | Metozolv ODT, including Reglan (Ani Pharmaceuticals, Inc.), and various generics; |
| • | Uceris, including Asacol and Delzicol (Warner Chilcott), Lialda and Pentasa (Shire), Remicade (Janssen) and Humira (AbbVie Inc., or AbbVie); |
| • | Zegerid, including Nexium (AstraZeneca plc), Aciphex (Eisai Inc.) and Dexilant (Takeda Pharmaceuticals, Inc., or Takeda) and various generics and OTI PPI products; |
| • | Glumetza, including Fortamet (Andrx Laboratories LLC), Glucophage and Glucophage XR (Bristol Myers Squibb, or BMS), various generics and other prescription diabetes treatments; |
| • | Cycloset, including Januvia (Merck), Onglyza (BMS), Byetta (Amylin Pharmaceuticals, Inc., or Amylin), Victoza (Novo Nordisk Inc.), Bydureon (Amylin), Avandia (SB PharmCo), Actos (Takeda), Amaryl (Sanofi Aventis), Glynase (Pharmacia & Upjohn, Inc.) and various branded and generic metformin products; and |
| • | Fenoglide, including Trico (AbbVie), Antara (Lupin Atlantis), Lipofen (Cipher Pharmaceuticals, Inc.), Lopid (Pfizer), Trilipix (AbbVie) and other prescription treatments for primary hyperlipidemia, mixed dyslipidemia and hypertriglyceridemia (such as statins and niacin). |
In addition, other products are in research or development by competitors that address the diseases and diagnostic procedures being targeted by these and our other products.
40
Table of Contents
Failure to integrate Santarus or other acquired businesses into our operations successfully could adversely affect our business.
Our strategy is to identify and acquire rights to products that we believe have potential for near-term regulatory approval or are already approved, through the purchase or license of products and purchase of companies. Our integration of the operations of acquired products and businesses, including Santarus, which we acquired on January 2, 2014, and Oceana, which we acquired in December 2011 and which includes foreign employees and operations, requires significant efforts, including the coordination of information technologies, research and development, sales and marketing, operations, manufacturing and finance. These efforts result in additional expenses and involve significant amounts of management’s time. In addition, acquisitions may result in our assumption of unknown and/or unexpected, and perhaps material, liabilities. Factors that will affect the success of our acquisitions, including the acquisition of Santarus, include the strength of the acquired companies’ or products’ underlying technology, our ability to execute our business strategy, results of clinical trials, regulatory approvals and reimbursement levels of the acquired products and related procedures, our ability to adequately fund acquired in-process research and development projects and retain key employees, and our ability to achieve financial and operational synergies with our acquired companies and products, such as by increasing sales of our products, achieving cost savings and effectively combining technologies to develop new products. Our failure to manage successfully and coordinate the growth of these acquisitions could have a material adverse impact on our business. In addition, we cannot be certain that the businesses or products we acquire will become profitable or remain so or that we will realize that operational cost savings or other expected synergies of an acquisition. If an acquisition is not successful, we may record related asset impairment charges in the future.
We have incurred, and will continue to incur, significant costs in connection with our acquisition of Santarus.
We have incurred and expect to continue to incur a number of non-recurring costs associated with combining our operations with Santarus’s operations. These costs and expenses include the incurrence of $1.95 billion of new indebtedness, financial advisory, legal, accounting, consulting and other advisory fees and expenses, reorganization and restructuring costs, severance/employee benefit-related expenses, filing fees, printing expenses and other related charges. There are also a large number of processes, policies, procedures, operations, technologies and systems that must be integrated in connection with the acquisition. While both we and Santarus have assumed that a certain level of expenses would be incurred in connection with our acquisition of Santarus, there are many factors beyond our control that could affect the total amount or the timing of the integration and implementation expenses.
We also may incur additional unanticipated costs in connection with the acquisition of Santarus that we may not recoup. These costs and expenses could, particularly in the near term, exceed the cost savings that we expect to achieve from the elimination of duplicative expenses and the realization of economies of scale, other efficiencies and cost savings. Although we expect that these savings will offset these integration and implementation costs over time, this net benefit may not be achieved in the near term or at all.
Uncertainties associated with our acquisition of Santarus may cause a loss of employees and may otherwise affect our future operations.
Our success will depend in part upon our ability to retain our key employees. Key employees may depart because of issues relating to the uncertainty and difficulty of integration or a desire not to remain us. As a result, we may not retain key employees to the same extent that we and Santarus have been able to retain their own employees in the past, which could have a negative impact on the our business. If key employees depart, the integration of Santarus may be more difficult and our business and results of operations could be harmed.
We could be exposed to significant product liability claims that could prevent or interfere with our product commercialization efforts.
We have been in the past and might continue to be subjected to product liability claims that arise through the testing, manufacturing, marketing and sale of our products. For example, we are currently and might continue to be subject to a number of product liability claims relating to OsmoPrep and Visicol in connection with their “black box”
41
Table of Contents
label warnings. We intend to defend these claims vigorously. During the fourth quarter of 2011, we settled a number of outstanding OsmoPrep and Visicol lawsuits and were notified by our insurer that settlement of these claims exceeded the limits of the policies related to these claims, making us responsible for additional damages, fees and expenses, if any. As a result, we recorded a $3.5 million reserve, which is our estimate of the costs of the remaining claims of which we are aware. However, the eventual settlement of these claims could exceed this estimate, and additional claims of which we are not currently aware could be filed.
We currently maintain liability coverage for both clinical trials and the commercialization of our products other than the claims discussed above, but it is possible that this coverage and any future coverage will be insufficient to satisfy any liabilities that arise. We would have to assume defense of the lawsuits and be responsible for damages, fees and expenses, if any, that are awarded against us or for amounts in excess of our product liability coverage. These claims could expose us to significant liabilities that could prevent or interfere with our product commercialization efforts. Product liability claims could require us to spend significant time and money in litigation or to pay significant damages. In the future, we might not be able to obtain adequate coverage at an acceptable cost or might be unable to obtain adequate coverage at all.
If government and other third-party payors do not provide coverage or reimburse patients for our products, our ability to derive revenues might suffer.
Our success will depend in part on the extent to which government and health administration authorities, private health insurers and other third-party payors will pay for our products. Reimbursement for newly approved healthcare products is uncertain. We acquired our first medical devices in December 2011, one of which was launched in 2011, and we are navigating the complex medical device reimbursement system.
In the United States and elsewhere, third-party payors, such as Medicaid, are increasingly challenging the prices charged for medical products and services. Government and other third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new therapeutic products. In the United States, a number of legislative and regulatory proposals aimed at changing the healthcare system have been passed in recent years, including the Affordable Care Act. Many significant changes in this legislation do not take effect until 2014. These changes to the healthcare system could increase our costs and reduce the amount we can charge for our products. In addition, an increasing emphasis on managed care in the United States has and will continue to increase pressure on pharmaceutical and medical device pricing. While we cannot predict whether legislative or regulatory proposals will be adopted or what effect those proposals or managed care efforts, including those relating to Medicaid payments, might have on our business, the announcement and/or adoption of such proposals or efforts could increase costs and reduce or eliminate profit margins, which could have a material adverse effect on our business, financial condition and results of operations. Third-party insurance coverage might not be available to patients for our products. If government and other third-party payors do not provide adequate coverage and reimbursement levels for our products, the market acceptance of these products might be reduced.
Our ability to increase revenue in the future will depend in part on our success in in-licensing or acquiring additional pharmaceutical products or medical devices.
We currently intend to in-license or acquire additional pharmaceutical products or medical devices, as we did with crofelemer and budesonide, that have been developed beyond the initial discovery phase and for which late-stage human clinical data is already available, or as we did with Relistor, Deflux and Solesta, that have already received regulatory approval. As we have grown, there are fewer of these opportunities that are large enough to have a material impact on our revenues, and we might encounter more competition from larger companies for these opportunities. These kinds of pharmaceutical products and medical devices might not be available to us on attractive terms or at all. To the extent we acquire rights to additional products, we might incur significant additional expense in connection with the development and, if approved by the FDA, marketing of these products.
We are dependent on third parties to supply us with products.
We rely entirely on third parties to supply us with our commercially marketed products and our products under development, and it may be difficult or impossible to obtain these products or the raw materials used to produce them.
42
Table of Contents
The raw material used in production of the crofelemer drug substance, our anti-secretory agent that is approved for marketing in the United States under the trade name Fulyzaq for the treatment of HIV-associated diarrhea, grows in select countries in South America. In addition, a key raw material for Relistor grows in Tasmania. Our ability to successfully obtain raw materials is not within our control. Failure to obtain these raw materials, whether due to international, political or economic conditions or otherwise, could delay development, increase expenses, delay regulatory approval, or eventually prevent us from generating revenue from additional indications for crofelemer or Relistor, if approved, which could have a material adverse effect on our business. Likewise, interruption of supply of any of our other products, whether for clinical use or commercial use, could have a material adverse effect on our business.
We do not have any manufacturing facilities and are dependent on third parties to manufacture our products.
We own no manufacturing facilities, and we have limited capabilities in manufacturing pharmaceutical products. We do not generally expect to engage directly in the manufacturing of products, but instead contract with and rely on third-party vendors for these services. A limited number of contract manufacturers exist which are capable of manufacturing our marketed products and our product candidates. Our manufacturers must comply with U.S. regulations, including cGMP regulations relating to manufacturing, packaging, documentation, quality control and quality assurance, and their facilities must be inspected and approved by the FDA and other regulatory agencies on an ongoing basis. We may be subject to serious consequences if our manufacturers are found to have deficiencies in their manufacturing processes, including potential delays in the regulatory approval process for our drug candidates and recalls of our commercialized products. For example, in April 2010 we received a CRL from the FDA related to our NDA for balsalazide disodium tablets. The sole issue raised in this letter concerned a deficiency of the manufacturing facility for this application, which delayed FDA approval almost two years. Given our ongoing dependence on third-party vendors for supply of material for use in clinical trials and for commercial product, our manufacturing strategy presents the following risks:
| • | the manufacture of products might be difficult to scale up when required and result in delays, inefficiencies and poor or low yields of quality products; |
| • | some of our contracts contain purchase commitments that require us to make minimum purchases that might exceed our needs or limit our ability to negotiate with other manufacturers, which might increase costs; |
| • | the cost of manufacturing certain products might make them prohibitively expensive; |
| • | delays in scale-up to commercial quantities and any change in manufacturers could delay clinical studies, regulatory submissions and commercialization of our products; |
| • | manufacturers are subject to the FDA’s cGMP regulations and similar foreign standards, and we do not have control over compliance with these regulations by the third-party manufacturers; |
| • | if we need to change manufacturers, transfers of technical expertise would be required which would include educating the new manufacturer in the processes necessary for the production of our products, which might not be successful; and |
| • | if we need to change manufacturers, FDA and comparable foreign regulators might require additional testing and compliance inspections prior to the new manufacturer being qualified for the production of our products. |
Any manufacturing defect or error discovered after products have been produced and distributed could result in even more significant consequences, including:
| • | delays, warning letters and fines; |
| • | product recalls or seizures and injunctions on sales; |
| • | refusal of the FDA to review pending applications; |
| • | total or partial suspension of production; |
| • | withdrawals of previously approved marketing applications; |
| • | damage to our reputation; and |
| • | product liability claims, civil penalties and criminal prosecutions. |
43
Table of Contents
In addition, the occurrence of manufacturing-related compliance issues could require subsequent withdrawal of the drug approval, reformulation of the drug product, additional testing or changes in labeling of the finished product. Any delay, interruption or cessation of production by our third-party manufacturers or strategic partners of our commercial products or product candidates, or their respective materials and components, as a result of any of the above factors or otherwise, may limit our ability to meet demand for commercial products and/or delay ongoing clinical trials, either of which could have a material adverse effect on our business, results of operations and financial condition.
Because our business and industry are highly regulated and scrutinized, any failure to follow such regulations could result in litigation or government enforcement actions that could have a material adverse effect on our business and results of operations.
Our business and industry are highly regulated and scrutinized, and subject to litigation risks, including product liability risks described above and the risk of government enforcement actions. We are subject to extensive and complex laws and regulations, including but not limited to, health care “fraud and abuse” laws, such as the federal False Claims Act, the federal Anti-Kickback Statute, other state and federal laws and regulations, and, with respect to our international operations, U.S. laws such as the FCPA, local laws such as the UK Bribery Act 2010 and various foreign laws and regulations. While we have developed and implemented a corporate compliance program designed to promote compliance with applicable laws and regulations, we cannot guarantee that this program will protect us from governmental investigations or other actions or lawsuits stemming from a failure or alleged failure to be in compliance with such laws or regulations. In recent years, there has been a heightened risk of governmental investigations into pharmaceutical companies’ sales and promotional practices for their products, including off-label uses, as evidenced by recent enforcement activity and/or pronouncements by the Office of Inspector General of the Department of Health and Human Services, the Department of Justice and state attorneys general. Matters underlying governmental investigations may also be the subject of private litigation. See the risk factor entitled “Regulatory approvals, even if granted, might entail ongoing requirements or restrictions on marketing. These requirements or restrictions, or inquiries into our marketing practices, could increase our expenses and limit revenue” above, and Part I. Item 3. Legal Proceedings for information about a pending federal government investigation concerning our sales and promotional practices for Xifaxan, Relistor and Apriso. If we are not successful in defending ourselves or asserting our rights in this investigation, or any other investigation or litigation, we could incur significant damages, fines or other penalties, which could have a material adverse effect on our business and results of operations.
We are subject to numerous environmental laws and regulations and any failure to comply with such laws and regulations could have a material adverse effect on our business and results of operations.
Our research, development and manufacturing efforts, and those of third parties that research, develop and manufacture our products and product candidates on our behalf or in collaboration with us, involve the controlled use of hazardous materials, including chemicals, viruses, bacteria and various radioactive compounds, and are therefore subject to numerous U.S. and international environmental and safety laws and regulations and to periodic inspections for possible violations of these laws and regulations. In addition, we, and our collaborators and third-party manufacturers may also become subject to laws and regulations related to climate change, including the impact of global warming. The costs of compliance with environmental and safety laws and regulations are significant, and the costs of complying with climate change laws could also be significant. Any violations, even if inadvertent or accidental, of current or future environmental, safety or climate change laws or regulations could subject us to substantial fines, penalties or environmental remediation costs, or cause us to lose permits or other authorizations to operate affected facilities, any of which could adversely affect our operations.
We are subject to complex laws and regulations governing our employees and contractors and any failure to comply with such laws and regulations could have a material adverse effect on our business and results of operations.
The laws and regulations applicable to our relationships with our employees and contractors are complex, extensive and fluid, and are subject to evolving interpretations by regulatory and judicial authorities. Failure to comply with these laws and regulations could result in significant damages, orders and/or fines and therefore could adversely affect our operations.
44
Table of Contents
Our results of operations might fluctuate from period to period, and a failure to meet the expectations of investors or the financial community at large could result in a decline in our stock price.
As they have in the past, our results of operations might fluctuate significantly on a quarterly and annual basis due to, among other factors:
| • | the timing of regulatory approvals and product launches by us or competitors, including potential generic or over-the-counter competitors; |
| • | the level of revenue generated by commercialized products, including potential (1) increased purchases of inventory by wholesalers in anticipation of potential price increases or introductions of new dosages or bottle sizes, and subsequent lower than expected revenue as the inventory is used, or (2) decreased purchases of inventory by wholesalers (or wholesaler demands for increased product discounts) as a result of wholesalers holding a significant number of months of inventory of our key products; |
| • | the timing of any up-front payments that might be required in connection with any future acquisition of product rights; |
| • | the timing of milestone payments that might be required to our current or future licensors; |
| • | fluctuations in our development and other costs in connection with ongoing product development programs; |
| • | the level of marketing and other expenses required in connection with product launches and ongoing product growth; |
| • | the timing of the acquisition and integration of businesses, assets, products and technologies; and |
| • | general and industry-specific business and economic conditions. |
We may not be able to generate sufficient cash to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or to refinance our debt obligations depends on our financial condition and operating performance, which is subject to prevailing economic and competitive conditions and to certain financial, business and other factors beyond our control. We may be unable able to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our indebtedness, including the notes.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay investments and capital expenditures, or to sell assets, seek additional capital or restructure or refinance our indebtedness. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. The terms of our credit agreement, other existing or future debt instruments and the indenture governing the 2021 Notes may restrict us from adopting some of these alternatives. In the absence of such operating results and resources, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. The credit agreement and the indenture governing the 2021 Notes restrict our ability to dispose of assets and use the proceeds from the disposition. We may not be able to consummate those dispositions or to obtain the proceeds that we could realize from them, and these proceeds may not be adequate to meet any debt service obligations then due. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations.
We are a holding company that depends on cash flows from our wholly owned subsidiaries to meet our obligations.
We are a holding company conducting substantially all of our operations through our subsidiaries, and all of our consolidated operating assets are held by our subsidiaries. Accordingly, we rely on the operations of our subsidiaries to fund payments on our indebtedness. Our subsidiaries are legally distinct from us and, under certain circumstances, legal and contractual restrictions may limit our ability to obtain cash from them. We and certain of our subsidiaries have entered into agreements limiting the ability of these subsidiaries to incur consensual encumbrances or restrictions on their ability to pay dividends or make other intercompany payments to us, but these limitations are subject to certain qualifications and exceptions. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness.
45
Table of Contents
Our stock price is volatile.
Our stock price has been extremely volatile and might continue to be, making owning our stock risky. Between January 1, 2011 and February 24, 2014, the price of a share of our common stock varied from a low of $25.64 to a high of $108.87. Our stock price increased or decreased by 5% or more on 15 days in 2011, 4 days in 2012 and 5 days in 2013.
The securities markets have experienced significant price and volume fluctuations unrelated to the performance of particular companies, including as a result of the current credit and economic crisis. In addition, the market prices of the common stock of many publicly traded pharmaceutical and biotechnology companies have in the past been and can in the future be expected to be especially volatile. Announcements of strategic transactions, prescription trends, technological innovations or new products by us or our competitors, generic approvals, developments or disputes concerning proprietary rights, publicity regarding actual or potential medical results relating to products under development by us or our competitors, regulatory developments in both the United States and other countries, public concern as to the safety of pharmaceutical products, economic and other external factors, period-to-period fluctuations in financial results, and stock market speculation regarding any of these factors, might have a significant impact on the market price of our common stock.
Provisions in our charter documents and under Delaware law could discourage a takeover or changes in our current directors or management that stockholders consider favorable.
Provisions in our certificate of incorporation and amended and restated bylaws could have the effect of discouraging, delaying or preventing a takeover or other change of control of us or the removal of our current directors and management, even if these events could be beneficial to stockholders. These provisions, which could also limit the price that investors might be willing to pay for our common stock, include the following:
| • | Our stockholders may not act by written consent. As a result, a stockholder, or stockholders, controlling a majority of our common stock would not be able to take certain actions without holding a stockholders’ meeting. |
| • | Our board of directors may issue, without stockholder approval, up to 5,000,000 shares of undesignated preferred stock. The ability to issue undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to acquire us. |
| • | Only our board of directors has the right to elect directors to fill vacancies created by the expansion of the board of directors or the resignation, death, or removal of directors, which prevents stockholders from being able to fill vacancies on our board of directors. |
| • | Stockholders must provide advance notice to nominate individuals for election to our board of directors or to propose matters that can be acted upon at a stockholders’ meeting. These provisions might discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company. |
As a Delaware corporation, we are also subject to certain Delaware anti-takeover provisions. Under Delaware law, a corporation may not engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Our board of directors could rely on Delaware law to prevent or delay an acquisition of us.
46
Table of Contents
Risks Relating to the Restatement
Implementing our plan to decrease wholesaler inventory levels will adversely affect our revenues.
In connection with the Audit Committee’s review, which began in the Fall 2014, of Salix’s financial statements and related disclosures, our Audit Committee and management determined that wholesaler inventory levels of XIFAXAN® 550, APRISO® and UCERIS® were greater, at approximately 9 months, 9 months and 5 months, respectively, as of September 30, 2014, than our target (established in Fall 2014) of approximately 3 months. In order to reduce wholesaler inventory levels to meet this target by the end of 2015, we intend to sell to our wholesalers amounts of XIFAXAN® 550, APRISO® and UCERIS® that are less than end-user demand until the target levels are reached. As a result, our revenue and cash flows may be decreased in the fourth quarter of 2014 and the full year 2015, compared to prior periods. In addition, wholesalers may demand increased discounts on our products, which could further decrease revenue and cash flows, and it may take longer than anticipated to reach our target wholesaler inventory levels, which could result in decreased revenues and cash flows for a longer period than anticipated.
We are restating certain prior consolidated financial statements, which may lead to additional risks and uncertainties, including shareholder litigation and governmental investigations, loss of investor confidence, and negative impacts on our stock price.
As discussed in the Explanatory Note to this Form 10-K/A and Note 1A to the Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K/A, we are restating our audited consolidated financial statements for the year ended December 31, 2013, and our unaudited consolidated financial statements for the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014. The determination to restate these financial statements was made by our Audit Committee, after discussion with management and EY, following the identification of certain errors in its accounting, which are primarily associated with the timing of recognition of certain revenue, revenue-reducing returns and discounts, and expenses. As a result of these events, we have become subject to a number of additional risks and uncertainties, including substantial unanticipated costs for accounting and legal fees in connection with or related to the restatement and potential shareholder litigation and governmental investigations. We will incur substantial defense and investigation costs regardless of the outcome of any such litigation or governmental investigation. Likewise, such events may cause a diversion of our management’s time and attention. If we do not prevail in any such litigation or governmental investigation, we could be required to pay substantial damages or settlement costs. In addition, the fact that we have completed a restatement may lead to a loss of investor confidence and have negative impacts on the trading price of our common stock.
If we are unable to maintain effective internal control over financial reporting in the future, the accuracy and timeliness of our financial reporting may be adversely affected.
Maintaining effective internal control over financial reporting is necessary for us to produce reliable financial statements. In connection with the restatement of our consolidated financial statements in this Form 10-K/A, management, including our Acting Chief Executive Officer and Acting Chief Financial Officer, reassessed the effectiveness of our internal control over financial reporting as of December 31, 2013. Based on this reassessment using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control – Integrated Framework (1992 framework), management has concluded that we did not maintain effective internal control over financial reporting as of December 31, 2013 because we did not (1) establish and maintain adequate procedures and controls for (a) product returns and for the communications between our sales and accounting/finance functions to record agreed upon returns and (b) the recognition of revenue for sales to customers with FOB Destination shipping terms, (2) comply with established policies to properly obtain, evaluate, review and approve agreements with customers and (3) periodically review and assess our account classification policies in light of changes in our organization, management and personnel over time, and the effect of non-routine transactions. These control deficiencies resulted in the restatement of our audited consolidated financial statements as of December 31, 2013 and for the year then ended (and the restatement of the unaudited quarterly consolidated financial information for the quarters ended March 31, 2014, June 30, 2014, and September 30, 2014), as discussed in further detail in the Explanatory Note to this Form 10-K/A and Note 1A to the Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K/A. We are actively engaged in developing and implementing remediation plans designed to address these material weaknesses. If we are unable to effectively remediate these material weaknesses or we are otherwise unable to maintain effective internal control over financial reporting, it could result in another material misstatement of our financial statements that would require a restatement, investor confidence in the accuracy and timeliness of our financial reports may be impacted, and the market price of our common stock could be negatively impacted.
Item 1B. Unresolved Staff Comments
None.
47
Table of Contents
We currently lease approximately 215,000 square feet of office space in Raleigh, North Carolina. We also lease a small amount of additional space in Palo Alto, California and Edison, New Jersey. Santarus’s primary office facility consists of approximately 40,000 square feet of leased office space in San Diego, California. We believe these facilities are adequate for our current needs and that suitable additional or alternative space will be available in the future on commercially reasonable terms as needed.
From time to time, we are party to various legal proceedings or claims, either asserted or unasserted, which arise in the ordinary course of business. Management has reviewed pending legal matters and believes that the resolution of such matters will not have a significant adverse effect on our financial condition or results of operations, except as otherwise discussed below.
Claims related to OsmoPrep, Visicol and Metozolv ODT
We are currently and might continue to be subject to product liability claims that arise through the testing, manufacturing, marketing and sale of our products, including a number of claims relating to OsmoPrep, Visicol and Metozolv ODT in connection with their respective “box” label warnings. Following consultation with the FDA, we revised the labels for these products to provide information about the risk of potential side effects that could result from their use. We are vigorously defending claims relating to these warnings and intend to continue to do so. During the fourth quarter of 2011, we recorded a $3.5 million reserve, which is our estimate of the settlements of the remaining claims of which we are aware, after being notified by our insurer that settlement of certain OsmoPrep and Visicol lawsuits exceeded the limits of the policies related to these claims. However, the eventual settlement of these claims could exceed this estimate, and we might receive additional claims as to which we are not currently aware.
Napo Litigation
On May 5, 2011, Napo Pharmaceuticals, Inc. filed a lawsuit against us in the Supreme Court of the State of New York, County of New York, alleging that we had engaged in fraudulent conduct, breached our collaboration agreement with Napo dated December 9, 2008, and breached our duty of good faith and fair dealing. Napo also sought a declaratory judgment that Napo had the right to terminate the collaboration agreement and sought unspecified damages in excess of $150 million. On or about December 28, 2011, Napo filed an amended complaint seeking an unspecified amount of damages for alleged breaches of the collaboration agreement by the Company and replacing Napo’s original complaint. Napo’s amended complaint no longer seeks a declaratory judgment that Napo has the right to terminate the collaboration agreement and removed the need for the court to rule on the Company’s motion to dismiss the original complaint. We believe that Napo’s allegations continue to be without merit and its lawsuit baseless. We filed an answer to the amended complaint and counterclaims on or about January 17, 2012, and are vigorously defending against the lawsuit. Discovery concluded last year, and, on May 31, 2013 we filed a motion for partial summary judgment. The court heard oral arguments on the motion in August 2013. On December 24, 2013, the court entered a short-form order granting our motion for partial summary judgment, narrowing the issues in the case. Napo filed an appeal of that decision on January 27, 2014 to the Appellate Division of the Supreme Court of the State of New York. On January 29, 2014 the Court vacated and replaced portions of the short-form order with an order continuing to grant our motion for partial summary judgment, narrowing the issues in the case. Trial on the claims remaining in the case commenced on February 10, 2014 and the jury decided on February 25, 2014 that Salix complied with its contractual obligations in commercializing Fulyzaq in the United States, and thus did not breach the collaboration agreement between the parties. We continue to advance our development and commercialization plans for crofelemer in accordance with the existing collaboration agreement with Napo.
Lupin Litigation
On September 7, 2012, we and Dr. Falk Pharma filed a patent infringement complaint against Lupin in the U.S. District Court for the District of Delaware. The complaint alleges infringement of the ‘620 patent, based on Lupin’s filing of an ANDA seeking approval to market and sell a generic version of Apriso before the expiration of the
48
Table of Contents
‘620 patent. The filing of this suit within the 45 day response period provided by the Hatch Waxman Act imposes an automatic 30 month stay of approval of Lupin’s ANDA. On March 4, 2013, we and Dr. Falk Pharma filed an amended complaint to also enforce the issued ‘886 patent in the pending suit. On July 30, 2013 the USPTO issued the ‘965 patent, which further protects the Apriso product. On August 19, 2013 we and Dr. Falk Pharma filed a second amended complaint to enforce the ‘965 patent and the ‘451 patent against Lupin. The court conducted a pretrial evidentiary hearing, known as a Markman hearing, on November, 21, 2013. The judge adopted each of Salix’s claim construction positions in his order issued December 17, 2013. The trial is scheduled for September, 2014. We continue to evaluate our intellectual property protecting Apriso, in which we have full confidence. We intend to vigorously enforce our intellectual property rights.
Novel Litigation
On February 18, 2014, we and Dr. Falk Pharma filed a patent infringement complaint against Novel in the U.S. District Court for the District of Delaware. The complaint alleges infringement of the ‘620 patent, the ‘886 patent, and the ‘965 patent based on Novel’s filing of an ANDA seeking approval to market and sell a generic version of Apriso before the expiration of these patents. The filing of this suit within the 45-day response period provided by the Hatch-Waxman Act imposes an automatic 30-month stay of approval of Novel’s ANDA. We continue to evaluate our intellectual property protecting Apriso, in which we have full confidence. We intend to vigorously enforce our intellectual property rights.
DOJ Subpoena
On February 1, 2013, our wholly owned subsidiary Salix Pharmaceuticals, Inc. received a subpoena from the U.S. Attorney’s Office for the Southern District of New York requesting documents regarding our sales and promotional practices for Xifaxan, Relistor and Apriso. The Company is continuing to respond to the subpoena and intends to cooperate fully with the subpoena and related government investigation. Currently, we cannot predict or determine the timing or outcome of this inquiry or its impact on financial condition or results of operations.
Santarus Shareholder Litigation
Beginning on November 12, 2013, eleven putative class action lawsuits were filed by shareholders of Santarus seeking to challenge our proposed acquisition of Santarus, which was announced on November 7, 2013. Nine of these actions were filed in the Delaware Court of Chancery, one was filed in California Superior Court (San Diego County) and one was filed in the U.S. District Court for the Southern District of California. These actions generally allege that the members of the Santarus board of directors breached their fiduciary duties to Santarus’s shareholders by failing to maximize the value of Santarus and by making inadequate or misleading disclosures regarding the proposed merger, and that Santarus, we and certain of our subsidiaries aided and abetted those breaches of fiduciary duty. The complaint in the action pending in California federal court also asserts causes of action on behalf of the individual plaintiff for alleged violations of certain sections of the Exchange Act. These actions generally sought, among other things, to enjoin the merger, unspecified damages and fees. On December 9, 2013, Santarus and its directors filed a motion to stay the action pending in California Superior Court. On December 11, 2013, the Delaware Court of Chancery consolidated the nine actions pending in that court, appointed lead counsel for the plaintiffs, and designated the amended complaint filed by plaintiff Imad Ahmad Khalil on December 9, 2013 as the operative complaint in the consolidated Delaware litigation. On December 20, 2013, the parties in the Delaware litigation reached an agreement in principle, subject to full documentation, to resolve the plaintiffs’ claims in that action in exchange for certain supplemental disclosures that Santarus included in an amended Schedule 14D-9 it filed on that date. We completed our merger with Santarus on January 2, 2014. The parties in the Delaware litigation executed a Memorandum of Understanding reflecting the terms of their agreement in principle on January 17, 2014 and are currently drafting full settlement documentation and engaging in confirmatory discovery. The settlement of the Delaware litigation will be subject to approval by the Delaware Court of Chancery. The plaintiffs’ counsel in the Delaware litigation has also indicated that the plaintiffs intend to request an award of an unspecified amount attorneys’ fees from the Delaware Court of Chancery. On January 23, 2014, Santarus and its directors filed a renewed motion to stay the action pending in California Superior Court, and we filed a separate motion to stay that action in favor of the Delaware litigation. On February 12, 2014, the parties in the action pending in California federal court filed a joint motion to stay that action
49
Table of Contents
pending a decision by the Delaware Court of Chancery regarding final approval of the proposed settlement of the Delaware litigation, and the California federal court granted that motion on February 13, 2014. We are vigorously defending the action pending in California Superior Court and attempting to finalize the settlement of the consolidated Delaware litigation as described above. We believe that all of the claims asserted against us by Santarus shareholders lack merit.
Zegerid Rx and Zegerid OTC Patent Litigation
Zegerid Rx Litigation
In April 2010, the U.S. District Court for the District of Delaware ruled that five patents covering Zegerid capsules and Zegerid powder for oral suspension (U.S. Patent Nos. 6,489,346; 6,645,988; 6,699,885; 6,780,882; and 7,399,772) were invalid due to obviousness. These patents were the subject of lawsuits we filed in 2007 against Par, in response to ANDAs filed by Par with the FDA. The University of Missouri, licensor of the patents, is joined in the litigation as a co-plaintiff. In May 2010, we filed an appeal of the District Court’s ruling to the U.S. Court of Appeals for the Federal Circuit. Following the District Court’s decision, Par launched its generic version of Zegerid capsules in late June 2010.
In September 2012, the U.S. Court of Appeals for the Federal Circuit reversed in part the April 2010 decision of the District Court. The Federal Circuit found that certain claims of asserted U.S. Patent Nos. 6,780,882 and 7,399,772, which Par had been found to infringe, were not invalid due to obviousness. The Federal Circuit affirmed the District Court’s finding of invalidity for the asserted claims from the remaining three patents. Following the Federal Circuit’s decision, Par announced that it had ceased distribution of its generic Zegerid capsules product in September 2012. In December 2012, the Federal Circuit remanded the case to the District Court for further proceedings pertaining to damages. In February 2013, we filed an amended complaint with the District Court for infringement of U.S. Patent Nos. 6,780,882 and 7,399,772 and requested a jury trial with respect to the issue of damages in connection with Par’s launch of its generic version of Zegerid capsules in June 2010. In March 2013, Par filed its amended answer, which alleges, among other things, failure to state a claim upon which relief can be granted and non-infringement based on purported invalidity of the two asserted patents. In addition, Par filed a motion for a judgment on the pleadings, alleging, among other things, that the two asserted patents are invalid because the Federal Circuit purportedly did not expressly address certain prior art references considered by the District Court. The trial on Santarus’s damages claim against Par is currently scheduled for November 2014. Although we do not believe that Par has a meritorious basis upon which to further challenge validity of the asserted patents in this proceeding, we cannot be certain of the outcome of this or any other proceedings.
In December 2011, Santarus filed a lawsuit in the U.S. District Court for the District of New Jersey against Zydus Pharmaceuticals USA, Inc., or Zydus, for infringement of the patents listed in the Orange Book for Zegerid capsules. The University of Missouri, licensor of the patents, is joined in the litigation as a co-plaintiff. Zydus had filed an ANDA with the FDA regarding its intent to market a generic version of Zegerid capsules prior to the expiration of the listed patents. In September 2012, Santarus amended its complaint to be limited to U.S. Patent No. 7,399,772, which patent was found not to be invalid in the September 2012 Federal Circuit decision. In October 2012, Zydus filed its answer, which alleges, among other things, failure to state a claim upon which relief can be granted. The lawsuit was commenced within the requisite 45-day time period, resulting in an FDA stay on the approval of Zydus’ proposed product for 30 months or until a decision is rendered by the District Court, which is adverse to the asserted patent. In December 2013, the District Court entered an order staying the case for 60 days, rescheduling the trial for April 2014, and extending the 30-month stay from May 2014 to July 2014. In February 2014, Santarus and MSD agreed to settle this case and the related Zydus OTC litigation, discussed below. Zydus may begin selling a generic version of prescription Zegerid capsules upon expiration of the applicable patent (or earlier under certain circumstances). We expect the District Court to enter an order dismissing this case soon.
In August 2012, Santarus filed a lawsuit in the U.S. District Court for the District of New Jersey against Dr. Reddy’s Laboratories, Ltd. and Dr. Reddy’s Laboratories, Inc., collectively referred to herein as Dr. Reddy’s, for infringement of the patents listed in the Orange Book for Zegerid capsules. The University of Missouri, licensor of the patents, was joined in the litigation as a co-plaintiff. Dr. Reddy’s had filed an ANDA with the FDA regarding its intent
50
Table of Contents
to market a generic version of Zegerid capsules prior to the expiration of the listed patents. In October 2012, Santarus amended its complaint to be limited to U.S. Patent No. 7,399,772, which patent was found not to be invalid in the September 2012 Federal Circuit decision. Also in October 2012, Dr. Reddy’s filed its answer, which alleges, among other things, non-infringement, invalidity, failure to state a claim upon which relief can be granted and estoppel. The lawsuit was commenced within the requisite 45-day time period, resulting in an FDA stay on the approval of Dr. Reddy’s proposed product for 30 months or until a decision is rendered by the District Court, which is adverse to the asserted patent. In June 2013, this case was settled allowing Dr. Reddy’s to begin selling a generic version of prescription Zegerid capsules upon expiration of the applicable patent (or earlier under certain circumstances), and the District Court entered an order dismissing the case with prejudice.
Zegerid OTC Litigation
In September 2010, MSD filed a lawsuit in the U.S. District Court for the District of New Jersey against Par for infringement of the patents listed in the Orange Book for Zegerid OTC. Santarus and the University of Missouri, licensors of the listed patents, are joined in the lawsuit as co-plaintiffs. Par had filed an ANDA with the FDA regarding its intent to market a generic version of Zegerid OTC prior to the expiration of the listed patents. In October 2012, MSD amended its complaint to be limited to U.S. Patent No. 7,399,772, which patent was found not to be invalid in the September 2012 Federal Circuit decision disclosed above. Also in October 2012, Par filed its answer, which alleges, among other things, failure to state a claim upon which relief can be granted, non-infringement and invalidity. Par has received tentative approval of its proposed generic Zegerid OTC product. Although the 30-month stay expired in February 2013, the parties have agreed that Par will not launch its generic Zegerid OTC product unless there is a District Court judgment favorable to Par or in certain other specified circumstances. The Markman hearing for this matter took place in July 2013, and the District Court issued a Markman order in October 2013 in which the District Court adopted MSD’s proposed construction of several claim terms that were consistent with those adopted by the U.S. District Court for the District of Delaware. In February 2014, the case was settled, allowing Par to begin selling a generic version of Zegerid OTC capsules upon expiration of the applicable patent (or earlier under certain circumstances). We expect the District Court to enter an order dismissing this case soon.
In September 2010, MSD filed a lawsuit in the U.S. District Court for the District of New Jersey against Perrigo Company and Perrigo Research and Development Company, collectively referred to herein as Perrigo, for infringement of the patents listed in the Orange Book for Zegerid OTC. Santarus and the University of Missouri, licensors of the listed patents, were joined in the lawsuit as co-plaintiffs. Perrigo had filed an ANDA with the FDA regarding its intent to market a generic version of Zegerid OTC prior to the expiration of the listed patents. Shortly after the stay was lifted in October 2012, the case was settled, allowing Perrigo to begin selling a generic version of Zegerid OTC capsules upon expiration of the applicable patent (or earlier under certain circumstances). In January 2013, the case was voluntarily dismissed with prejudice.
In December 2011, MSD filed a lawsuit in the U.S. District Court for the District of New Jersey against Zydus for infringement of the patents listed in the Orange Book for Zegerid OTC. Santarus and the University of Missouri, licensors of the listed patents, are joined in the litigation as co-plaintiffs. Zydus had filed an ANDA with the FDA regarding its intent to market a generic version of Zegerid OTC prior to the expiration of the listed patents. In September 2012, MSD amended its complaint to be limited to U.S. Patent No. 7,399,772, which patent was found not to be invalid in the September 2012 Federal Circuit decision. In October 2012, Zydus filed its answer, which alleges, among other things, failure to state a claim upon which relief can be granted. The lawsuit was commenced within the requisite 45-day time period, resulting in an FDA stay on the approval of Zydus’ proposed product for 30 months or until a decision is rendered by the District Court, which is adverse to the asserted patent. In December 2013, the District Court entered an order staying the case for 60 days, rescheduling the trial for April 2014, and extending the 30-month stay from May 2014 to July 2014. As discussed in connection with the lawsuit against Zydus for infringement of the patents covering prescription Zegerid, in February 2014, Santarus and MSD agreed to settle this case and the related Zydus litigation. Zydus may begin selling a generic version of Zegerid OTC capsules upon expiration of the applicable patent (or earlier under certain circumstances). We expect the District Court to enter an order dismissing this case soon.
51
Table of Contents
Glumetza Patent Litigation
In June 2011, Depomed filed a lawsuit in the U.S. District Court for the District of New Jersey against Sun Pharma Global FZE, Sun Pharmaceutical Industries Ltd. and Sun Pharmaceutical Industries Inc., collectively referred to herein as Sun, for infringement of the patents listed in the Orange Book for Glumetza. Valeant International Bermuda, or Valeant, was joined in the lawsuit as a co-plaintiff. The lawsuit was filed in response to an ANDA filed with the FDA by Sun regarding Sun’s intent to market generic versions of Glumetza 500 mg and 1000 mg tablets prior to the expiration of the listed patents. In January 2013, Santarus, Depomed and Valeant entered into a settlement agreement with Sun that grants Sun the right to begin selling a generic version of Glumetza in August 2016, or earlier under certain circumstances. In January 2013, the District Court dismissed the lawsuit without prejudice in view of the settlement agreement. The settlement agreement is subject to review by the U.S. Department of Justice and the Federal Trade Commission.
In April 2012 and February 2013, Depomed filed lawsuits in the U.S. District Court for the District of Delaware against Watson Laboratories, Inc.—Florida, Actavis, Inc. and Watson Pharma, Inc., collectively referred to herein as Watson, for infringement of the patents listed in the Orange Book for Glumetza. Valeant was joined in one of the lawsuits as a co-plaintiff. The lawsuits were filed in response to an ANDA filed with the FDA by Watson regarding Watson’s intent to market generic versions of Glumetza 500 mg and 1000 mg tablets prior to the expiration of the listed patents. In November 2013, the case was settled allowing Watson to begin selling a generic version of Glumetza in August 2016, or earlier under certain circumstances, and the District Court entered an order dismissing the case without prejudice.
In addition, certain of the patents that provide coverage for Glumetza are utilized in other products developed by Depomed or Depomed licensees and are subject to ongoing patent infringement litigation and may be subject to patent infringement litigation in the future. Any adverse outcome in such patent infringement litigation could negatively impact the value of the patent coverage for Glumetza.
Fenoglide Patent Litigation
In January 2013, Santarus filed a lawsuit in the U.S. District Court for the District of Delaware against Mylan Inc. and Mylan Pharmaceuticals Inc., collectively referred to herein as Mylan, for infringement of the patents listed in the Orange Book for Fenoglide 120 mg and 40 mg (U.S. Patent Nos. 7,658,944, and 8,124,125). Veloxis Pharmaceuticals A/S, or Veloxis, is joined in the lawsuit as a co-plaintiff. The lawsuit was filed in response to an ANDA filed with the FDA by Mylan regarding Mylan’s intent to market a generic version of Fenoglide 120 mg and 40 mg tablets prior to the expiration of the listed patents. Santarus commenced the lawsuit within the requisite 45-day time period, resulting in an FDA stay on the approval of Mylan’s proposed product for 30 months or until a decision is rendered by the District Court, which is adverse to the asserted patents, whichever may occur earlier. Absent a court decision, the 30-month stay is expected to expire in June 2015. Mylan has filed an answer in the case that asserts, among other things, non-infringement, invalidity, and failure to state a claim, and has also filed counterclaims. We are not able to predict the timing or outcome of this lawsuit.
Item 4. Mine Safety Disclosures
Not applicable.
Executive Officers of the Registrant
The following table sets forth information concerning our executive officers as of February 28, 2014:
| Name |
Age | Position | ||||
| Carolyn J. Logan | 65 | President, Chief Executive Officer, and Director | ||||
| Adam C. Derbyshire | 48 | Executive Vice President, Finance and Administration, and Chief Financial Officer | ||||
| William P. Forbes | 52 | Executive Vice President, Research and Development and Chief Development Officer | ||||
| Rick D. Scruggs | 54 | Executive Vice President, Business Development | ||||
52
Table of Contents
Carolyn J. Logan has served as President and Chief Executive Officer and as a member of the Board of Directors since July 2002. She previously served as Senior Vice President, Sales and Marketing from June 2000 to July 2002. Prior to joining us, Ms. Logan served as Vice President, Sales and Marketing of the Oclassen Dermatologics division of Watson Pharmaceuticals, Inc. from May 1997 to June 2000, and as Vice President, Sales from February 1997 to May 1997. Prior to that date, she served as Director, Sales of Oclassen Pharmaceuticals, Inc., or Oclassen, from January 1993 to February 1997. Prior to joining Oclassen, Ms. Logan held various sales and marketing positions with Galderma Laboratories, Ulmer Pharmacal and Westwood Pharmaceuticals. Ms. Logan holds a B.S. degree in Biology and Dental Hygiene from the University of North Carolina at Chapel Hill.
Adam C. Derbyshire has served as Executive Vice President, Finance and Administration and Chief Financial Officer since January 2009. Mr. Derbyshire previously served as Senior Vice President, Finance and Administration and Chief Financial Officer from June 2003 to January 2009 and as Vice President, Finance and Administration and Chief Financial Officer from June 2000 to June 2003. From June 1999 to June 2000, Mr. Derbyshire was Vice President, Corporate Controller and Secretary of Medco Research, Inc., or Medco, acquired by King Pharmaceuticals, Inc. or King Pharmaceuticals, in February 2000, Corporate Controller and Secretary of Medco from September 1995 to June 1999 and Assistant Controller of Medco from October 1993 to September 1995. Mr. Derbyshire holds a B.S. degree from the University of North Carolina at Wilmington and an MBA from the University of North Carolina at Charlotte.
William P. Forbes has served as Executive Vice President, Research and Development and Chief Medical Officer since January 2010. Dr. Forbes previously served as Senior Vice President, Research and Development and Chief Medical Officer from January 2009 to January 2010. Dr. Forbes previously served as Vice President, Research and Development, and Chief Medical Officer from January 2005 to January 2009. From 2002 through 2004, Dr. Forbes was Vice President, Clinical Development and Regulatory Affairs of Metabasis Therapeutics, Inc. He has also worked for Otsuka America Pharmaceutical, Inc. in a variety of roles of increasing responsibility from 1991 to 2002 and Glaxo, Inc. from 1989 through 1991. Dr. Forbes holds a Doctor of Pharmacy degree from Creighton University.
Rick D. Scruggs has served as Executive Vice President, Business Development since January 2011. Mr. Scruggs previously served as Senior Vice President, Business Development from January 2009 to January 2011. Mr. Scruggs previously served as Vice President, Business Development from January 2006 to January 2009. From January 2004 to January 2006, Mr. Scruggs served as Vice President, Commercial Development. From 2000 to 2006, Mr. Scruggs was in various sales and commercial trade related positions at Salix. Before joining Salix, he served as Director of Managed Markets at Watson from May 1997 to July 2000. Prior to that, Mr. Scruggs served as Director, Managed Markets and Trade at Oclassen from January 1995 to February 1997. Before joining Oclassen, Mr. Scruggs held various sales and marketing positions of increasing responsibility with Johnson & Johnson and Ciba-Geigy. Mr. Scruggs holds a B.S. degree in Criminal Justice from Appalachian State University.
53
Table of Contents
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is traded on the Nasdaq Global Market under the symbol “SLXP”. The following table sets forth the high and low sales prices of our common stock, as reported on the Nasdaq Global Market for the eight quarters ended December 31, 2013.
| High | Low | |||||||
| Fiscal year ended December 31, 2013 |
||||||||
| Fourth quarter |
$ | 90.01 | $ | 68.30 | ||||
| Third quarter |
75.54 | 65.73 | ||||||
| Second quarter |
66.83 | 47.72 | ||||||
| First quarter |
51.37 | 42.48 | ||||||
| Fiscal year ended December 31, 2012 |
||||||||
| Fourth quarter |
$ | 43.24 | $ | 37.52 | ||||
| Third quarter |
55.99 | 41.51 | ||||||
| Second quarter |
54.57 | 47.45 | ||||||
| First quarter |
53.99 | 44.52 | ||||||
On February 24, 2013 the closing price for the common stock as reported on the Nasdaq Global Market was $108.26. As of February 24, 2013 there were 220 stockholders of record, which excludes stockholders whose shares were held in nominee or street name by brokers.
Performance Graph
The following graph compares our cumulative total stockholder return from December 31, 2008 with those of the Nasdaq Composite Index and the Nasdaq Biotech Index and assumes that all dividends were reinvested. The graph assumes that U.S. $100 was invested on December 31, 2008 in (1) our common stock, (2) the Nasdaq Composite Index and (3) the Nasdaq Biotech Index. The measurement points utilized in the graph consist of the last trading day in each calendar year, which closely approximates the last day of the respective fiscal year of the Company. The historical stock performance presented below is not intended to and may not be indicative of future stock performance.
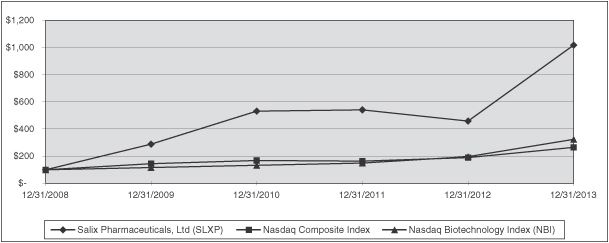
| 12/31/08 | 12/31/09 | 12/31/10 | 12/31/11 | 12/31/12 | 12/31/13 | |||||||||||||||||||
| SLXP |
$ | 100 | $ | 288 | $ | 532 | $ | 542 | $ | 458 | $ | 1,019 | ||||||||||||
| Nasdaq Composite Index |
$ | 100 | $ | 144 | $ | 168 | $ | 165 | $ | 191 | $ | 265 | ||||||||||||
| Nasdaq Biotech Index |
$ | 100 | $ | 116 | $ | 133 | $ | 149 | $ | 196 | $ | 325 | ||||||||||||
54
Table of Contents
Dividend Policy
We have never declared or paid cash dividends on our common stock. We currently expect to retain future earnings, if any, for use in the operation and expansion of business and do not anticipate paying any cash dividends in the foreseeable future.
Equity Compensation Plans
The information required by Item 5 of Form 10-K regarding securities authorized for issuance under our equity compensation plans is incorporated herein by reference to “Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Recent Sales of Unregistered Securities
On December 27, 2013, we closed an offering of $750 million in 2021 Notes. The Notes were issued at 100% of face value in accordance with the terms of a purchase agreement, dated December 12, 2013, by and among us, Oceana and Salix Pharmaceuticals, Inc., and Jefferies LLC, as the representative of the several initial purchasers named therein. The 2021 Notes were issued in a transaction exempt from the registration requirements under the Securities Act, in reliance on Rule 144A and Regulation S thereunder. We used the net proceeds from the sale of the 2021 Notes to finance a portion of the approximately $2.6 billion that was payable as consideration for our acquisition of Santarus, which closed on January 2, 2014, and to pay the related fees and expenses in connection therewith. For additional information, see “Liquidity and Capital Resources—2021 Notes” in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Note 6 of Notes to Consolidated Financial Statements.
55
Table of Contents
Item 6. Selected Financial Data (Restated)
As discussed in the Explanatory Note to this Form 10-K/A and in Note 1A of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K/A, we are restating our audited consolidated financial statements and related disclosures for the year ended December 31, 2013. The following selected financial data should be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this Form 10-K/A. Our historical results are not necessarily indicative of the results that should be expected in the future and the selected financial data is not intended to replace the consolidated financial statements.
Consolidated Statements of Operations Data:
| Year Ended December 31, | ||||||||||||||||||||
| 2013 (Restated) |
2012 | 2011 | 2010 | 2009 | ||||||||||||||||
| (U.S. dollars, in thousands, except per share data) | ||||||||||||||||||||
| Revenues: |
||||||||||||||||||||
| Net product revenues |
$ | 913,781 | $ | 735,444 | $ | 540,488 | $ | 336,973 | $ | 232,890 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Costs and expenses: |
||||||||||||||||||||
| Cost of products sold |
177,776 | 124,597 | 95,369 | 68,677 | 52,025 | |||||||||||||||
| Amortization of product rights and intangible assets |
44,744 | 45,351 | 10,908 | 10,370 | 11,485 | |||||||||||||||
| Intangible impairment charge |
— | 41,600 | — | 34,656 | — | |||||||||||||||
| Research and development (as revised) |
112,791 | 84,963 | 71,861 | 40,969 | 64,769 | |||||||||||||||
| Selling, general and administrative (as revised) |
342,359 | 296,458 | 219,477 | 188,478 | 144,717 | |||||||||||||||
| Change in acquisition-related contingent consideration |
(16,200 | ) | (29,598 | ) | 27,000 | — | — | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
661,470 | 563,371 | 424,615 | 343,150 | 272,996 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
252,311 | 172,073 | 115,873 | (6,177 | ) | (40,106 | ) | |||||||||||||
| Loss on extinguishment of debt |
— | (15,580 | ) | — | — | — | ||||||||||||||
| Interest expense |
(62,026 | ) | (55,518 | ) | (32,121 | ) | (20,652 | ) | (6,746 | ) | ||||||||||
| Interest and other income |
2,003 | 10,853 | 2,349 | 2,626 | 1,221 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before income tax expense |
192,288 | 111,828 | 86,101 | (24,203 | ) | (45,631 | ) | |||||||||||||
| Income tax (expense) benefit |
(61,477 | ) | (47,582 | ) | 1,298 | (2,858 | ) | 2,012 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
$ | 130,811 | $ | 64,246 | $ | 87,399 | $ | (27,061 | ) | $ | (43,619 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) per share, basic(1) |
$ | 2.12 | $ | 1.09 | $ | 1.49 | $ | (0.47 | ) | $ | (0.88 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) per share, diluted(1) |
$ | 1.99 | $ | 1.01 | $ | 1.44 | $ | (0.47 | ) | $ | (0.88 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Shares used in computing net income (loss) per share, basic(1) |
61,792 | 58,747 | 58,718 | 57,300 | 49,350 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Shares used in computing net income (loss) per share, diluted(1) |
65,692 | 63,699 | 65,483 | 57,300 | 49,350 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Consolidated Balance Sheet Data:
| As of December 31, | ||||||||||||||||||||
| 2013 (Restated) |
2012 | 2011 | 2010 | 2009 | ||||||||||||||||
| Cash and cash equivalents |
$ | 1,157,850 | $ | 751,006 | $ | 292,814 | $ | 518,030 | $ | 192,512 | ||||||||||
| Working capital |
1,865,432 | 927,661 | 356,449 | 560,110 | 217,537 | |||||||||||||||
| Total assets |
2,925,951 | 1,874,784 | 1,304,255 | 851,543 | 543,040 | |||||||||||||||
| Borrowings under credit facility |
— | — | — | — | 15,000 | |||||||||||||||
| Convertible senior notes |
882,050 | 857,209 | 340,283 | 323,005 | 47,299 | |||||||||||||||
| Long term portion of capital lease obligations |
— | — | — | 90 | 499 | |||||||||||||||
| Senior notes |
750,000 | — | — | — | — | |||||||||||||||
| Retained earnings (deficit) |
59,431 | (71,380 | ) | (135,626 | ) | (223,025 | ) | (195,964 | ) | |||||||||||
| Stockholders’ equity |
728,643 | 560,501 | 549,637 | 401,919 | 370,024 | |||||||||||||||
| (1) | See Note 2 of Notes to Consolidated Financial Statements for an explanation of shares used in computing net income (loss) per share. |
56
Table of Contents
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
RESTATEMENT
As discussed in the Explanatory Note to this Form 10-K/A and in Note 1A of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K/A, we are restating our audited consolidated financial statements and related disclosures for the year ended December 31, 2013. The following discussion and analysis of our financial condition and results of operations incorporates the restated amounts. For this reason, the data set forth in this Item 7 may not be comparable to the discussion and data in our previously filed Annual Report on Form 10-K for the year ended December 31, 2013.
OVERVIEW
We are a specialty pharmaceutical company dedicated to acquiring, developing and commercializing prescription drugs and medical devices used in the treatment of a variety of gastrointestinal disorders, which are those affecting the digestive tract. Our strategy is to:
| • | identify and acquire rights to products that we believe have potential for near-term regulatory approval or are already approved; |
| • | apply our regulatory, product development, and sales and marketing expertise to commercialize these products; and |
| • | market our products through our specialty sales and marketing team by primarily focusing on high-prescribing U.S. physicians in the following specialties: gastroenterologists, who are doctors who specialize in gastrointestinal disorders; hepatologists, who are doctors who specialize in liver disease; and colorectal surgeons, who are doctors who specialize in disorders of the colon and rectum, and primary care doctors. |
Significant Developments in 2014
| • | On January 2, 2014, we completed our acquisition of Santarus, a specialty biopharmaceutical company focused on acquiring, developing and commercializing proprietary products that address the needs of patients treated by physician specialists in gastroenterology and endocrinology, for aggregate consideration of approximately $2.6 billion. As a result of the acquisition, Santarus became one of our indirect wholly owned subsidiaries. We financed the acquisition through a combination of (i) a term loan facility in the principal amount of $1.2 billion, (ii) the net proceeds from our issuance of $750 million of 2021 Notes, and (iii) cash on hand of approximately $800.4 million. |
| • | On January 30, 2014, the FDA accepted for filing an NDA for our Budesonide 2 mg rectal form product. The FDA has issued a PDUFA action date of September 15, 2014. |
| • | On February 25, 2014 a New York Supreme Court entered a verdict in favor of Salix in a lawsuit filed by Napo Pharmaceuticals related to the FULYZAQ® (crofelemer) collaboration agreement. See Part I. Item 3. Legal Proceedings. |
Introduction to Products
After the integration of Santarus, we will execute our strategy by:
| • | marketing our currently marketed products (including Santarus’s product portfolio) through our established channels and Santarus’s network of high-volume specialty and primary care physician-focused sales representatives; |
| • | utilizing our expertise to progress pipeline products and indications through late-stage development and approval; and |
| • | making select acquisitions to further diversify our product portfolio and improve profitability. |
In connection with the execution of this strategy, we now offer over 20 marketed products. We believe that these product offerings place us in a premier position in gastroenterology and create the opportunity for us to expand our digestive disease expertise into other key specialties.
As of December 31, 2013, our products were:
| • | XIFAXAN550 (rifaximin) tablets 550 mg, indicated for the reduction in risk of overt HE recurrence in patients 18 years of age and older; |
| • | XIFAXAN (rifaximin) tablets 200 mg, indicated for the treatment of patients 12 years of age and older with TD caused by noninvasive strains of E. coli; |
| • | APRISO (mesalamine) extended-release capsules, indicated for the maintenance of remission of UC; |
| • | MOVIPREP (PEG 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate and ascorbic acid for oral solution), indicated for cleansing of the colon as a preparation for colonoscopy in adults 18 years of age or older; |
57
Table of Contents
| • | RELISTOR (methylnaltrexone bromide) SI indicated for the treatment of OIC in patients with advanced illness who are receiving palliative care, when the response to laxative therapy has not been sufficient; |
| • | OSMOPREP (sodium phosphate monobasic monohydrate, USP and sodium phosphate dibasic anhydrous, USP) tablets, indicated for cleansing of the colon as a preparation for colonoscopy in adults 18 years of age or older; |
| • | SOLESTA, a biocompatible tissue-bulking agent indicated for the treatment of fecal incontinence in patients 18 years or older who have failed conservative therapy; |
| • | DEFLUX, a biocompatible tissue-bulking agent indicated for the treatment of VUR, grades II-IV; |
| • | FULYZAQ (crofelemer) delayed-release tablets, indicated for the symptomatic relief of non-infectious diarrhea in adult patients with HIV/AIDS on anti-retroviral therapy; |
| • | GIAZO (balsalazide disodium) tablets, indicated for the treatment of mildly to moderately active UC in male patients 18 years of age and older; |
| • | METOZOLV ODT (metoclopramide hydrochloride) 5 mg and 10 mg orally disintegrating tablets, indicated for short-term (4 to 12 weeks) use in adults for treatment of GERD that fails to respond to conventional therapy, and for relief of symptoms of acute and recurrent diabetic gastroparesis; |
| • | AZASAN (azathioprine, USP), 75 mg and 100 mg tablets, indicated as an adjunct for the prevention of rejection in renal homotransplantations and to reduce signs and symptoms of severe active rheumatoid arthritis; |
| • | ANUSOL-HC (hydrocortisone, USP) 2.5% cream and ANUSOL-HC (hydrocortisone acetate) 25 mg suppositories, indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses; |
| • | PROCTOCORT (hydrocortisone, USP) 1% cream and PROCTOCORT (hydrocortisone acetate) 30 mg suppositories, indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses; |
| • | PEPCID (famotidine) for oral suspension, indicated for the short-term treatment of GERD, active duodenal ulcer, active benign gastric ulcer, erosive esophagitis due to GERD, and peptic ulcer disease; |
| • | DIURIL (chlorothiazide) oral suspension, indicated for the treatment of hypertension and also as adjunctive therapy in edema associated with congestive heart failure, cirrhosis of the liver, corticosteroid and estrogen therapy, and kidney disease; and |
| • | COLAZAL (balsalazide disodium) capsules, indicated for the treatment of mildly to moderately active UC in patients 5 years of age and older. |
As of December 31, 2013, Santarus’s products were:
| • | UCERIS (budesonide MMX) extended release tablets, indicated for the induction of remission in patients with active, mild to moderate UC; |
| • | ZEGERID (omeprazole/sodium bicarbonate) capsules and powder for oral suspension, indicated for the treatment of certain upper gastrointestinal conditions, including active duodenal ulcer, active benign gastric ulcer and GERD; |
| • | GLUMETZA (metformin hydrochloride) extended release tablets, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus; |
| • | CYCLOSET (bromocriptine mesylate) tablets, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus; and |
| • | FENOGLIDE (fenofibrate) tablets, indicated as an adjunct to diet to reduce elevated LDL-C. total cholesterol, triglycerides and Apo B, and to increase HDL-C in adult patients with primary hyperlipidemia or mixed dyslipidemia. Fenoglide also is indicated as an adjunct to diet for treatment of adult patients with hypertriglyceridemia. |
58
Table of Contents
Our and Santarus’s primary product candidates currently under development and their status are as follows:
| Compound |
Condition |
Status | ||
| Rifaximin |
Irritable bowel syndrome, or IBS | sNDA submitted June 7, 2010; CRL received on March 7, 2011; FDA meeting held on June 20, 2011; Advisory Committee meeting held on November 16, 2011; currently in Phase 3 retreatment study, with top-line data expected in July 2014 | ||
| Methylnaltrexone bromide SI |
OIC, in patients with chronic non-malignant pain; subcutaneous injection | sNDA accepted for filing; CRL received July 27, 2012; FDA Advisory Committee proposed to occur March 10-11, 2014 postponed | ||
| Budesonide foam |
Ulcerative proctitis | NDA accepted for filing January 2014; PDUFA action date September 15, 2014 | ||
| Methylnaltrexone bromide oral |
OIC in patients with chronic non-malignant pain; oral | Phase 3 | ||
| Rifaximin EIR |
Crohn’s disease | Phase 3 | ||
| Rifaximin SSD/next generation |
Prevention of complications in compensated liver cirrhosis | Phase 2 | ||
| Ruconest |
HAE | BLA submitted April 2013; PDUFA action date extended to July 16, 2014 | ||
APPLICATION OF CRITICAL ACCOUNTING ESTIMATES
General
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to sales of our products, bad debts, inventories, investments, intangible assets and legal issues. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results might differ materially from these estimates under different assumptions or conditions.
Methodologies used and assumptions selected by management in making these estimates, as well as the related disclosures, have been reviewed by and discussed with the Audit Committee of our Board of Directors.
We believe the following critical accounting estimates affect how we apply our more significant policies used in the preparation of our consolidated financial statements.
Revenue Recognition
We recognize revenue when it is realized or realizable and earned. Revenue is realized or realizable and earned when all of the following criteria are met: (a) persuasive evidence of an arrangement exists; (b) delivery has occurred or services have been rendered; (c) the Company’s price to the buyer is fixed or determinable; and (d) collectability is reasonably assured.
We recognize revenues for product sales at the time title and risk of loss are transferred to the customer, which is generally at the time products are shipped (unless products are shipped under FOB Destination shipping terms, in which case risk of loss is transferred to the customer upon delivery). We recognize revenue from sales transactions where the buyer has the right to return the product at the time of sale only if (1) our price to the buyer is substantially fixed or determinable at the date of sale, (2) the buyer has paid us, or the buyer is obligated to pay us and the obligation is not contingent on resale of the product, (3) the buyer’s obligation to us would not be changed in the event of theft or physical destruction or damage of the product, (4) the buyer acquiring the product for resale has economic substance apart from any provided by us, (5) we do not have significant obligations for future performance to directly bring about resale of the product by the buyer, and (6) the amount of future returns can be reasonably estimated. The Company’s net product revenue represents the Company’s total revenues less allowances for customer credits, including wholesaler discounts, estimated rebates, chargebacks, patient-focused promotional programs and product returns.
59
Table of Contents
We establish allowances for estimated rebates, chargebacks and product returns based on numerous quantitative and qualitative factors, including:
| • | the number of and specific contractual terms of agreements with customers; |
| • | estimated levels of inventory in the distribution channel; |
| • | historical rebates, chargebacks and returns of products; |
| • | direct communication with customers; |
| • | anticipated introduction of competitive products or generics; |
| • | anticipated pricing strategy changes by us and/or our competitors; |
| • | analysis of prescription data gathered by a third-party prescription data provider; |
| • | the impact of changes in state and federal regulations; and |
| • | estimated remaining shelf life of products. |
In our analyses, we use prescription data purchased from a third-party data provider to develop estimates of historical inventory channel pull-through. We utilize an internal analysis to compare historical net product shipments to estimated historical prescriptions written. Based on that analysis, we develop an estimate of the quantity of product in the channel that might be subject to various rebate, chargeback and product return exposures. At least quarterly for each product line, we prepare an internal estimate of ending inventory units in the distribution channel by adding estimated inventory in the channel at the beginning of the period, plus net product shipments for the period, less estimated prescriptions written for the period. To estimate months of ending inventory in the distribution channel the Company divides estimated ending inventory in the distribution channel by the Company’s estimate of the succeeding quarter’s demand, not taking into account any future anticipated demand growth beyond the succeeding quarter. Based on that analysis, we develop an estimate of the quantity of product in the channel that might be subject to various rebate, chargeback and product return exposures. This is done for each product line by applying a rate of historical activity for rebates, chargebacks and product returns, adjusted for relevant quantitative and qualitative factors discussed above, to the potential exposed product estimated to be in the distribution channel. Internal forecasts that are utilized to calculate the estimated number of months in the channel are regularly adjusted based on input from members of our sales, marketing and operations groups. The adjusted forecasts take into account numerous factors including, but not limited to, new product introductions, direct communication with customers and potential product expiry issues. Adjustments to estimates are recorded in the period when significant events or changes in trends are identified.
We offer discounts to our wholesalers and other customers. These discounts are calculated as a percentage of the current published list price and are treated as off-invoice allowances. Accordingly, we record the discounts as a reduction of revenue in the period that we offer the discounts. In addition to these discounts, at the time that we implement a price increase, we generally offer our existing customers an opportunity to purchase a limited quantity of product at the previous list price. Shipments resulting from these offers generally are not in excess of ordinary levels, therefore, we recognize the related revenue upon shipment and include the shipments in estimating our various product related allowances. In the event we determine that these shipments represent purchases of inventory in excess of ordinary levels for a given wholesaler, the potential impact on product returns exposure would be specifically evaluated and reflected as a reduction in revenue at the time of such shipments.
Allowances for estimated rebates, chargebacks and patient-focused promotional programs were $184.6 million and $103.8 million as of December 31, 2013 and 2012, respectively. These allowances reflect an estimate of our liability for items such as rebates due to various governmental organizations under the Medicare/Medicaid regulations, rebates due to managed care organizations under specific contracts and chargebacks due to various organizations purchasing certain of our products through federal contracts and/or group purchasing agreements. We estimate our liability for rebates, chargebacks and patient-focused promotional programs at each reporting period based on a methodology of applying relevant quantitative and qualitative assumptions. Due to the subjectivity of our accrual estimates for rebates and chargebacks, we prepare various sensitivity analyses to ensure our final estimate is within a reasonable range as well as review prior period activity to ensure that our methodology is still reasonable. Had a change in one or more variables in the analyses (utilization rates, contract modifications, etc.) resulted in an additional percentage point change in the trailing average of estimated chargeback and rebate activity for the year ended December 31, 2013, we would have recorded an adjustment to revenues of approximately $12.6 million, or 1.4%, for the year.
Allowances for product returns were $68.2 million and $36.4 million as of December 31, 2013 and 2012, respectively. These allowances reflect an estimate of our liability for product that may be returned by the original purchaser in accordance with our stated return policy. We estimate our liability for product returns at each reporting period based on historical return rates, the estimated inventory in the channel, and the other factors discussed above. Due to the subjectivity of our accrual estimates for product returns, we prepare various sensitivity analyses as well as review prior period activity to ensure that our methodology is still reasonable.
60
Table of Contents
For the years ended December 31, 2013, 2012 and 2011, our provision for rebates, chargebacks, patient-focused promotional programs and product returns (which items do not include wholesaler discounts) grew primarily as a result of increased sales of our existing products, the approval of new products and the acquisition of products. Accordingly, reductions to revenue and corresponding increases to allowance accounts have likewise increased. The provision for rebates, chargebacks, patient-focused promotional programs and product returns as a percentage of gross product revenue in the years ended December 31, 2013, 2012 and 2011 was 20.3%, 15.7% and 14.6% for rebates, chargebacks and patient-focused promotional programs and was 3.9%, 2.3% and 3.9% for product returns, respectively.
During the second quarter of 2010 we recognized product revenue related to initial shipments to wholesalers of Xifaxan 550mg, which the FDA approved on March 24, 2010 for reduction in risk of overt hepatic encephalopathy, or HE, recurrence in patients 18 years of age or older, and we launched to physicians in May 2010. Based on our historical experience with Xifaxan 200mg, which we distribute through the same distribution channels and is prescribed by the same physicians as Xifaxan 550mg, we have the ability to estimate returns for Xifaxan 550mg, and therefore we recognize revenue upon meeting the shipping or delivery criteria set forth in the underlying purchase agreement.
During the second quarter of 2011, we recognized product revenue related to shipments to wholesalers of Relistor, which we acquired from Progenics in February 2011. Based on historical experience with Relistor obtained from Progenics, and historical experience with our products, specifically Xifaxan 200mg, Xifaxan 550mg and Apriso, which we distribute through the same distribution channels and are prescribed by the same physicians as Relistor, management has the ability to estimate returns for Relistor, and therefore we recognize revenue upon meeting the shipping or delivery criteria set forth in the underlying purchase agreement.
In December 2011 we acquired an exclusive worldwide license to Solesta and Deflux with the completion of our acquisition of Oceana. Solesta and Deflux are medical devices that we sell to specialty distributors who then sell the products to end users, primarily hospitals, surgical centers and physicians. The specialty distributors generally do not purchase these products until an end user is identified. Based on historical experience with these products obtained from Oceana, and historical experience with our products, specifically Xifaxan 200mg, Xifaxan 550mg and Apriso, which are prescribed by the same physicians as Solesta, management has the ability to estimate returns for Solesta and Deflux, and therefore we recognize revenue upon meeting the shipping or delivery criteria set forth in the underlying purchase agreement.
The enactment of the “Patient Protection and Affordable Care Act” and “The Health Care and Education Reconciliation Act of 2010” in March 2010 brings significant changes to U.S. health care. These changes began to take effect in the first quarter of 2010. Changes to the rebates for prescription drugs sold to Medicaid beneficiaries, which increase the minimum statutory rebate for branded drugs from 15.1 percent to 23.1 percent, were generally effective in the first quarter of 2010. This rebate has been expanded to managed Medicaid, a program that provides for the delivery of Medicaid benefits via managed care organizations, under arrangements between those organizations and state Medicaid agencies. Additionally, a prescription drug discount program for outpatient drugs in health care facilities that serve low-income and uninsured patients, known as 340B facilities, has been expanded. The effect of these changes was not material to our 2013, 2012 or 2011 financial results. Based on our current product and payor mix, we believe the effect of these changes should not be material to our future financial results.
Also, there are changes to the tax treatment of subsidies paid by the government to employers who provide their retirees with a drug benefit at least equivalent to the Medicare Part D drug benefit. Beginning in 2013, the federal government will tax the subsidy it provides to such employers. We do not provide retirees with any drug benefits, therefore this change should not affect our financial results.
61
Table of Contents
Beginning in 2011, drug manufacturers will provide a discount of 50 percent of the cost of branded prescription drugs for Medicare Part D participants who are in the “doughnut hole” coverage gap in Medicare prescription drug coverage. The doughnut hole will be phased out by the federal government between 2011 and 2020. Based on our current product and payor mix, the cost of this discount was approximately 1% of our gross revenue for the year ended December 31, 2013, however, the cost of this discount might have a material effect on our results of operations in future periods.
Beginning in 2011, pharmaceutical manufacturers and importers that sell branded prescription drugs to specified government programs had to pay a non-tax deductible annual fee to the federal government. Companies have to pay an amount based on their prior calendar year market share for branded prescription drug sales into these government programs. Based on our current product and payor mix, the effect of this tax was not material to our financial results in 2013, 2012 or 2011 and we do not believe the effect of this tax will be material to future periods.
Additionally, the 2010 healthcare reform legislation imposes a 2.3 percent excise tax on U.S. sales of Class I, II and III medical devices beginning in 2013. This tax had no effect on our financial statements for 2012, and did not have a material effect on our financial results for 2013. We do not believe the effect of this tax will be material to future periods.
Inventories
Inventory at December 31, 2013 consisted of $61.0 million of raw materials, $11.7 million of work-in-process and $31.5 million of finished goods. Inventory at December 31, 2012 consisted of $40.1 million of raw materials, $17.6 million of work-in-process and $32.8 million of finished goods.
We state raw materials, work-in-process and finished goods inventories at the lower of cost (which approximates actual cost on a first-in, first-out cost method) or market value. In evaluating whether inventory is stated at the lower of cost or market, management considers such factors as the amount of inventory on hand and in the distribution channel, estimated time required to sell such inventory, remaining shelf life, and current and expected market conditions, including levels of competition, including generic competition. Inventory adjustments are measured as the difference between the cost of the inventory and estimated market value based upon assumptions about future demand and charged to the provision for inventory, which is a component of cost of sales. At the point of the loss recognition, a new, lower-cost basis for that inventory is established, and any subsequent improvements in facts and circumstances do not result in the restoration or increase in that newly established cost basis.
We expense pre-approval inventory unless we believe it is probable that the inventory will be saleable. We capitalize inventory costs associated with marketed products and certain products prior to regulatory approval and product launch, based on management’s judgment of probable future commercial use and net realizable value. Capitalization of this inventory does not begin until the product candidate is considered to have a high probability of regulatory approval, which is generally after we have analyzed Phase 3 data or filed a New Drug Application, or NDA. If we are aware of any specific risks or contingencies that are likely to impact the expected regulatory approval process or if there are any specific issues identified during the research process relating to safety, efficacy, manufacturing, marketing or labeling of the product candidate, we do not capitalize the related inventory. Once we capitalize inventory for a product candidate that is not yet approved, we monitor, on a quarterly basis, the status of this candidate within the regulatory approval process. We could be required to expense previously capitalized costs related to pre-approval inventory upon a change in our judgment of future commercial use and net realizable value, due to a denial or delay of approval by regulatory bodies, a delay in the timeline for commercialization or other potential factors. On a quarterly basis, we evaluate all inventory, including inventory capitalized for which regulatory approval has not yet been obtained, to determine if any lower of cost or market adjustment is required. As it relates to pre-approval inventory, we consider several factors including expected timing of FDA approval, projected sales volume and estimated selling price. At December 31, 2013 and 2012, there were no amounts included in inventory related to pre-approval inventory.
Valuation of Intangible Assets and Contingent Consideration Liabilities Acquired in Business Combinations
We have acquired and expect to continue to acquire intangible assets in connection with business combinations. These intangible assets consist primarily of technology associated with currently marketed prescription drugs and
62
Table of Contents
medical devices, as well as product candidates. We typically use discounted cash flow models to determine the fair values of these intangible assets for purposes of allocating consideration paid to the net assets acquired in a business combination. These models require the use of significant estimates and assumptions, including, but not limited to:
| • | determining the timing and expected costs to complete in-process projects taking into account the stage of completion at the acquisition date; |
| • | projecting the probability and timing of obtaining marketing approval from the FDA and other regulatory agencies for product candidates; |
| • | estimating the timing of and future net cash flows from product sales resulting from completed products and in-process projects; and |
| • | developing appropriate discount rates to calculate the present values of those cash flows. |
Significant estimates and assumptions are also required to determine the acquisition date fair values of any contingent consideration liabilities incurred in connection with business combinations. In addition, we must revalue these liabilities each subsequent reporting period until the related contingencies are resolved and record changes in their fair values in earnings. We determined the acquisition date fair values of the various contingent consideration liabilities incurred in our business acquisitions using a combination of valuation techniques. Significant estimates and assumptions required for these valuations included, but were not limited to, the probability of achieving regulatory milestones, product sales projections under various scenarios and discount rates used to calculate the present value of the required payments. We update these estimates and assumptions each reporting period in order to revalue these contingent consideration liabilities. Accordingly, subsequent changes in underlying facts and circumstances could result in changes in these estimates and assumptions, which could have a material impact on the estimated future fair values of these liabilities.
We believe the fair values used to record intangible assets acquired and contingent consideration liabilities incurred in connection with business combinations are based upon reasonable estimates and assumptions given the facts and circumstances as of the related valuation dates.
Intangible Assets and Goodwill
Our intangible assets consist of license agreements, product rights and other identifiable intangible assets, which result from product and business acquisitions. Goodwill represents the excess purchase price over the fair value of assets acquired and liabilities assumed in a business combination.
When the Company makes product acquisitions that include license agreements, product rights and other identifiable intangible assets, it records the purchase price of such intangibles as intangible assets, in addition to recording the value of the product related liabilities that it assumes in connection with the product acquisition. We allocate the aggregate purchase price to the fair value of the various tangible and intangible assets in order to determine the appropriate carrying value of the acquired assets and then amortize the cost of the intangible assets as an expense in the consolidated statements of comprehensive income over the estimated economic useful life of the related assets. We assess the impairment of identifiable intangible assets whenever events or changes in circumstances indicate that the carrying value might not be recoverable. We believe the following factors could trigger an impairment review: significant underperformance relative to expected historical or projected future operating results; significant changes in the manner of use of the acquired assets or the strategy for our overall business; approval of generic products; and significant negative industry or economic trends.
In assessing the recoverability of our intangible assets, we must make assumptions regarding estimated future cash flows and other factors. If the estimated undiscounted future cash flows do not exceed the carrying value of the intangible assets, we must determine the fair value of the intangible assets. If the fair value of the intangible assets is less than the carrying value, we will recognize an impairment loss equal to the difference. We review goodwill for impairment on an annual basis, and goodwill and other intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
63
Table of Contents
In September 2005, we acquired InKine for $210.0 million. We allocated $74.0 million of the purchase price to in-process research and development, $9.3 million to net assets acquired and $37.0 million to specifically identifiable product rights and related intangibles with an ongoing economic benefit to us. We allocated the remaining $89.7 million to goodwill, which is not being amortized. The InKine product rights and related intangibles are being amortized over an average period of 14 years, which we believed was an appropriate amortization period due to the product’s patent protection and the estimated economic lives of the product rights and related intangibles. In September 2010 we entered into a sublicense agreement which granted Novel Laboratories, Inc. a license under the patents covering OsmoPrep permitting Novel to launch a generic version of OsmoPrep no later than November 16, 2019. As a result of this agreement, we adjusted the amortization period prospectively, and we are amortizing the remaining net book value of the intangible asset through November 16, 2019, which is our revised estimate of its remaining economic life. As a result of this agreement, we assessed whether there was an impairment to the carrying value of the related intangible asset due to its reduced economic life and determined that there was no impairment. At December 31, 2013 and 2012, accumulated amortization for the InKine intangibles was $22.9 million and $20.4 million, respectively. There is no future research or development planned for the products purchased from InKine.
In December 2005, we entered into a License and Supply Agreement with Norgine B.V., granting Salix the exclusive right to sell a patented-protected, liquid PEG bowel cleansing product, NRL 944, in the United States. In August 2006, we received FDA marketing approval for NRL 944 under the branded name of MoviPrep. In January 2007 the USPTO issued a patent providing coverage to September 1, 2024. Pursuant to the terms of the Agreement, Salix paid Norgine milestone payments of $15.0 million in August 2006, $5.0 million in December 2008 and $5.0 million in December 2009. We were amortizing these milestone payments over a period of 17.3 years through 2022, which we believed was an appropriate amortization period due to the product’s patent protection and the estimated economic life of the related intangible. In August 2010 we entered into a sublicense agreement that granted Novel Laboratories, Inc. a license to the patents covering MoviPrep permitting Novel to launch a generic version of MoviPrep no later than September 24, 2018. As a result of this agreement, we adjusted the amortization period prospectively, and we are now amortizing the remaining net book value of the intangible asset through September 24, 2018, which is our revised estimate of its remaining economic life. As a result of this agreement, we assessed whether there was an impairment to the carrying value of the related intangible asset due to its reduced economic life and determined that there was no impairment. At December 31, 2013 and 2012, accumulated amortization for the MoviPrep intangible was $13.9 million and $11.5 million, respectively.
In February 2007, we entered into a Master Purchase and Sale and License Agreement with Merck & Co. Inc., to purchase the U.S prescription pharmaceutical product rights to Pepcid Oral Suspension and Diuril Oral Suspension from Merck. We paid Merck $55.0 million at the closing of this transaction. The purchase price was fully allocated to product rights and related intangibles, and is being amortized over a period of 15 years. Although Pepcid and Diuril do not have patent protection, we believe 15 years was an appropriate amortization period based on established product history and management experience. The FDA approved two generic famotidine oral suspension products in May and June 2010, and we launched an authorized generic version of our Pepcid product in May 2010 to compete with these generic products. As a result of these events, we assessed whether there was an impairment to the carrying value of the related intangible asset. Based on this analysis, we recorded a $30 million impairment charge to reduce the carrying value of the intangible asset to its estimated fair value during the three-month period ended June 30, 2010. At December 31, 2013 and 2012, accumulated amortization for the Merck products was $16.2 million and $15.1 million, respectively.
In July 2002, we acquired the rights to develop and market a granulated formulation of mesalamine from Dr. Falk Pharma GmbH. On October 31, 2008, the FDA granted marketing approval for Apriso for the maintenance of remission of ulcerative colitis in adults. In November 2008, we made a $8.0 million milestone payment to Dr. Falk. In December 2009, we made a $2.0 million milestone payment to Dr. Falk. We are amortizing these milestone payments over a period of 9.5 years, which we believe is an appropriate amortization period due to the product’s patent protection and the estimated economic life of the related intangible. At December 31, 2013 and 2012, accumulated amortization for the Apriso intangible was $4.4 million and $3.5 million, respectively.
In September 2007, we acquired the exclusive, worldwide right to sell metoclopramide–Zydis® (trade name Metozolv) from Wilmington Pharmaceuticals, LLC, or Wilmington. On September 8, 2009 the FDA granted marketing
64
Table of Contents
approval for Metozolv™ ODT (metoclopramide HCl) 5 mg and 10 mg orally disintegrating tablets. In October 2009, we made a $7.3 million milestone payment to Wilmington. We are amortizing this milestone payment over a period of eight years, which we believe is an appropriate amortization period due to the product’s patent protection and the estimated economic life of the related intangible. On November 3, 2010, we received a paragraph IV notification from Novel stating that Novel had filed an ANDA to seek approval to market a generic version of Metozolv ODT, 5 mg and 10 mg. The notification letter asserted non-infringement of U.S. Patent No. 6,413,549 (“the ‘549 patent”). Upon examination of the relevant sections of the ANDA, we concluded that the ‘549 patent would not be enforced against Novel Laboratories. As a result of this event, we assessed whether there was an impairment to the carrying value of the related intangible asset. Based on this analysis, we recorded a $4.6 million impairment charge to reduce the carrying value of the intangible asset to its estimated fair value during the three-month period ended December 31, 2010. At December 31, 2013 and 2012 accumulated amortization for the Metozolv intangible was $2.6 million and $2.6 million, respectively.
In February 2011, we acquired an exclusive worldwide license to develop and commercialize the products containing the MNTX Compound, marketed under the name Relistor®, from Progenics (except in Japan, where Ono Pharmaceutical has previously licensed the subcutaneous formulation of the drug from Progenics) and a non-exclusive license to manufacture the MNTX Compound and products containing that compound in the same territory. We paid Progenics an up-front license fee payment of $60.0 million. We also agreed to pay development milestone payments of up to $90.0 million contingent upon achieving specified regulatory approvals and commercialization milestone payments of up to $200.0 million contingent upon achieving specified targets for net sales. We must pay Progenics 60% of any revenue received from sublicensees in respect of any country outside the United States. Additionally, we must pay Progenics royalties based on a percentage ranging from the mid- to high-teens of net sales by the Company and its affiliates of any product containing the MNTX Compound.
We accounted for the Progenics transaction as a business combination under the acquisition method of accounting. Under the acquisition method of accounting, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date in our consolidated financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. As of the acquisition date, the estimated fair value of the assets acquired was $113.0 million. We estimated the fair value of the contingent consideration related to this transaction at $53.0 million, which was booked as a long-term liability on the consolidated balance sheet. We determined this liability amount using a probability-weighted discounted cash flow model based on the current regulatory status of the methylnaltrexone bromide development programs. We assess the fair value of the contingent consideration quarterly, or whenever events or changes in circumstances indicate that the fair value may have changed, primarily as a result of clinical or regulatory results in the related in-process development programs. In December 2011, we announced positive Phase 3 data from the OIC Oral development program. Based on this information, we reassessed the fair value of the contingent consideration and recorded a $27.0 million increase in the contingent consideration and a corresponding charge to earnings in the fourth quarter of 2011. At December 31, 2013 and 2012, accumulated amortization for the intangible related to the currently approved indication for Relistor was $7.2 million and $4.6 million, respectively.
On July 27, 2012 we received a CRL from the FDA following its review of a sNDA for methylnaltrexone bromide injection for subcutaneous use for the treatment of OIC in adult patients with chronic, non-cancer pain. The CRL requested additional clinical data. In October 2012 Salix and Progenics held an End-of-Review meeting with the Division of Gastroenterology and Inborn Errors Products to better understand the contents of the CRL. Based on the results of this meeting, we reassessed the value of the indefinite lived intangible asset related to methylnaltrexone bromide injection for subcutaneous use for the treatment of OIC in chronic non-cancer pain and recorded a non-cash benefit to earnings of $41.6 million in the three-month period ended September 30, 2012. Based on these events, we reassessed the fair value of the contingent consideration related to the Progenics transaction and recorded a $33.0 million decrease in the contingent consideration and a corresponding non-cash charge to earnings in the three-month period ended September 30, 2012. We are currently evaluating the oral OIC development program and currently believe we will continue this program. However, additional information and additional guidance from the FDA could result in the termination of the oral OIC development program, which would result in impairment of the related intangible asset and a decrease in the related contingent consideration.
65
Table of Contents
In December 2011 we acquired Oceana Therapeutics, Inc. Oceana has two products, Deflux and Solesta. Oceana has license agreements with Q-Med, which provide us the worldwide right to commercialize Deflux and Solesta. Under a stock purchase agreement with Q-Med that we have assumed, we are obligated to pay up to $45.0 million contingent upon achieving specified targets for net sales of Solesta over the term of the agreement.
We accounted for the Oceana acquisition as a business combination under the acquisition method of accounting. Under the acquisition method of accounting, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date in its consolidated financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. As of the acquisition date, the estimated fair value of the assets acquired was $342.8 million. We estimated the fair value of the contingent consideration related to this transaction at $39.7 million, which was booked as a long-term liability on the consolidated balance sheet. We determined this liability amount using a probability-weighted discounted cash flow model. We assess the fair value of the contingent consideration whenever events or changes in circumstances indicate that the fair value may have changed, primarily as a result of significant changes in our forecast of net sales for Solesta. At December 31, 2013, accumulated amortization for the intangible related to Deflux was $9.3 million and $58.1 million for the intangible related to Solesta. At December 31, 2012, accumulated amortization for the intangible related to Deflux was $4.7 million and $29.0 million for the intangible related to Solesta.
In August 2012 we amended our 1996 Agreement with Alfa Wassermann to develop rifaximin. The Amended Agreement does not alter any of the terms of the TD and HE indications developed under the 1996 Agreement or IBS. We are obligated to pay Alfa royalties, at the same range of rates as under the 1996 Agreement, on net sales of such products. In addition, the Amended Agreement provides us with an exclusive license to develop and commercialize rifaximin products for Crohn’s disease (CD) in the United States and Canada and a non-exclusive license to develop such products worldwide. We paid Alfa a non-refundable upfront fee of $10.0 million in August 2012, and are obligated to make a $25.0 million milestone payment upon receipt of marketing authorization in the United States for an extended intestinal release, or EIR, formulation product for CD, and additional milestones based on net sales of EIR formulation products for CD of up to $200.0 million. In addition, we are required to pay Alfa royalties on sales of rifaximin products for Crohn’s at percentage rates ranging from the low teens to low double digits.
We accounted for the Alfa Wassermann transaction as a business combination under the acquisition method of accounting. Under the acquisition method of accounting, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date in its consolidated financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. As of the acquisition date, the estimated fair value of the assets acquired was $23.4 million which is included as an indefinite lived intangible asset on the consolidated balance sheet. We estimated the fair value of the contingent consideration related to this transaction at $13.4 million, which was booked as a long-term liability on the consolidated balance sheet. We determined this liability amount using a probability-weighted discounted cash flow model based on the current regulatory status of the EIR development program. We assess the fair value of the contingent consideration quarterly, or whenever events or changes in circumstances indicate that the fair value may have changed, primarily as a result of clinical or regulatory results in the related in-process development programs.
Research and Development
We expense research and development costs, both internal and externally contracted, as incurred. For nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities, we initially capitalize the advance payment. We then recognize such amounts as an expense as the related goods are delivered or the related services are performed. At December 31, 2013 and 2012, the net liability related to on-going research and development activities was $11.8 million and $11.8 million, respectively.
Due to the significant risks and uncertainties inherent in the clinical development and regulatory approval processes, the cost to complete projects and development timelines for their completion cannot be reasonably estimated. Enrollment in clinical trials might be delayed for reasons beyond our control, requiring additional cost and time. Results from clinical trials might not be favorable, or might require us to perform additional unplanned clinical
66
Table of Contents
trials, requiring additional cost and time, or resulting in termination of the project. Further, data from clinical trials is subject to varying interpretation, and might be deemed insufficient by the regulatory bodies reviewing applications for marketing approvals, requiring additional cost and time, or resulting in termination of the project. Process development and manufacturing scale-up for production of clinical and commercial product supplies might take longer and cost more than our forecasts. As such, clinical development and regulatory programs are subject to risks and changes that might significantly impact cost projections and timelines.
The following table summarizes costs incurred for our significant projects, in thousands. We consider a project significant if expected spend for any year exceeds 10% of our development project budget for that year.
| Project |
Year ended December 31, | Cumulative through December 31, 2013 (as revised) |
||||||||||||||
| 2013 (as revised) |
2012 | 2011 | ||||||||||||||
| Rifaximin for HE |
$ | 5,896 | $ | 3,700 | $ | 5,112 | $ | 50,206 | ||||||||
| Rifaximin for IBS |
42,844 | 21,209 | 3,415 | 113,873 | ||||||||||||
| Crofelemer for HIV-associated diarrhea |
1,163 | 8,052 | 4,206 | 36,005 | ||||||||||||
| Budesonide foam for ulcerative proctitis |
5,970 | 7,560 | 9,407 | 38,740 | ||||||||||||
| Methylnaltrexone bromide for OIC in patients with chronic pain |
6,936 | 14,405 | 19,054 | 40,395 | ||||||||||||
| Rifaximin SSD / next generation for chronic liver disease |
7,991 | — | — | 7,991 | ||||||||||||
| Other non-significant rifaximin clinical projects (2 in 2013, 2 in 2012 and 4 in 2011) |
1,172 | 5,431 | 5,985 | N/A | ||||||||||||
| All other clinical programs (6 in 2013, 4 in 2012 and 6 in 2011) |
6,815 | 9,312 | 4,301 | N/A | ||||||||||||
Convertible Debt Transactions
We separately account for the liability and equity components of convertible debt instruments by allocating the proceeds from issuance between the liability component and the embedded conversion option, or equity component, in accordance with accounting for convertible debt instruments that may be settled in cash (including partial cash settlement) upon conversion. The value of the equity component is calculated by first measuring the fair value of the liability component, using the interest rate of a similar liability that does not have a conversion feature, as of the issuance date. The difference between the proceeds from the convertible debt issuance and the amount measured as the liability component is recorded as the equity component. We recognize the accretion of the resulting discount as part of interest expense in our consolidated statements of income.
Upon settlement of our convertible senior notes, we revalue the liability component, utilizing an interest rate of comparable nonconvertible debt. We allocate a portion of the consideration transferred to the liability component equal to the fair value of that component immediately prior to repurchase. Any difference between the consideration attributed to the liability component and the net carrying amount of the liability component is recognized as a gain or loss in the statement of income. Any remaining consideration is allocated to the reacquisition of the equity component and is recognized as a reduction of stockholders’ equity.
Deferred Tax Asset Valuation Allowance
We calculate the valuation allowance in accordance with the provisions of ASC 740, “Income Taxes,” which requires an assessment of both positive and negative evidence regarding the realizability of these deferred tax assets, when measuring the need for a valuation allowance. Significant management judgment is required in determining any valuation allowance recorded against deferred tax assets. Due to a history of operating losses, resulting in a cumulative loss position, management concluded that a full valuation allowance was needed to offset all of the deferred tax assets, net of deferred tax liabilities, as of December 31, 2010. At December 31, 2011, management concluded that it was more likely than not that a majority of our deferred tax assets would be realized through future taxable income. This conclusion was based, in part, on our achieving sustained profitability in 2011 and projections of positive future earnings. Therefore, we released a significant portion of the valuation allowances related to these deferred tax assets in the fourth quarter of 2011. The release of these valuation allowances resulted in an income tax benefit of $41.4 million, which was recorded as a discrete item during the year ending December 31, 2011. The release of the valuation
67
Table of Contents
allowance did not affect the amount of cash paid for income taxes. We continue to assess our the ability to realize our deferred tax benefits on a quarterly basis. If it is more likely than not that we will not realize the deferred tax benefits, then all or a portion of the valuation allowance may need to be re-established, which would result in a charge to tax expense. Management did not release any portion of the valuation allowance in 2013. We continue to provide a valuation allowance for net deferred tax assets related to several state net operating loss carryforwards.
RESULTS OF OPERATIONS
Years Ended December 31, 2013, 2012 and 2011
Revenues
The following table summarizes net product revenues, in thousands, for the years ended December 31, 2013, 2012 and 2011:
| Year ended December 31, | ||||||||||||
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Xifaxan |
$ | 635,941 | $ | 514,480 | $ | 371,653 | ||||||
| % of net product revenues |
70 | % | 70 | % | 69 | % | ||||||
| Purgatives—MoviPrep/OsmoPrep |
76,390 | 64,932 | 88,746 | |||||||||
| % of net product revenues |
8 | % | 9 | % | 16 | % | ||||||
| Inflammatory Bowel Disease—Colazal/Apriso/Giazo |
117,914 | 85,504 | 49,736 | |||||||||
| % of net product revenues |
13 | % | 12 | % | 9 | % | ||||||
| Other—Anusol/Azasan/Diuril/Pepcid/Proctocort/Relistor/ |
83,536 | 70,528 | 30,353 | |||||||||
| % of net product revenues |
9 | % | 9 | % | 6 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Net product revenues |
$ | 913,781 | $ | 735,444 | $ | 540,488 | ||||||
|
|
|
|
|
|
|
|||||||
Net product revenues for 2013 were $913.8 million, compared to $735.4 million for 2012. The net product revenue increase from 2012 to 2013 was primarily due to:
| • | increased unit sales of Xifaxan, MoviPrep and Apriso; |
| • | increased unit sales of Relistor; and |
| • | price increases on our products. |
These increases were partially offset by reduced unit sales of OsmoPrep and Giazo returns.
Total milligrams of Xifaxan 550mg prescribed during 2013 increased 22% compared to 2012. Prescriptions for our purgatives as a group decreased 2% for 2013 when compared to 2012. Prescriptions for MoviPrep increased 1% for 2013 compared to prescriptions for 2012. Prescriptions for OsmoPrep for 2013 declined 25% compared to prescriptions for 2012. Prescriptions for Apriso increased 36% for 2013 compared to prescriptions for 2012.
Net product revenues for 2012 were $735.4 million, compared to $540.5 million for 2011. The net product revenue increase from 2011 to 2012 was primarily due to:
| • | increased unit sales of Xifaxan and Apriso; |
| • | increased unit sales of Relistor, which we began promoting in the second quarter of 2011; |
| • | sales of Deflux, which we acquired in connection with our acquisition of Oceana in December 2011; and |
| • | price increases on our products. |
These increases were partially offset by reduced unit sales of OsmoPrep and MoviPrep.
68
Table of Contents
Total milligrams of Xifaxan prescribed during 2012 increased 24% compared to 2011. Prescriptions for our purgatives as a group decreased 5% for 2012 when compared to 2011. Prescriptions for MoviPrep decreased 3% for 2012 compared to prescriptions for 2011. Prescriptions for OsmoPrep for 2012 declined 21% compared to prescriptions for 2011. Prescriptions for Apriso increased 29% for 2012 compared to prescriptions for 2011.
Costs and Expenses
Total costs and expenses were $661.5 million, $563.4 million and $424.6 million for 2013, 2012 and 2011, respectively. Higher operating expenses in absolute terms for 2013 compared to 2012 were due primarily to increased research and development costs due primarily to higher expenses for certain development programs, increased selling, general and administrative costs due to a full year of personnel and operating costs in 2013 due to the acquisition of Oceana in December 2011 and the expansion of our sales force in 2012, and increased cost of products sold related to the corresponding increase in product revenue, offset by the intangible impairment charge recorded in the three-month period ended September 30, 2012, net of the change in acquisition-related contingent consideration recorded in the three-month period ended September 30, 2012. Higher operating expenses in absolute terms for 2012 compared to 2011 were due primarily to increased selling, general and administrative costs due to the acquisition of Oceana in December 2011 and an expansion of our sales force in 2012, increased cost of products sold related to the corresponding increase in product revenue and the intangible impairment charge recorded in the three-month period ended September 30, 2012, partially offset by the change in acquisition-related contingent consideration recorded in the three-month period ended September 30, 2012.
Cost of Products Sold
Cost of products sold were $177.8 million, $124.6 million and $95.4 million for 2013, 2012 and 2011, respectively. Gross margin on total product revenue, excluding $44.7 million, $45.4 million and $10.9 million in amortization of product rights and intangible assets for 2013, 2012 and 2011, respectively, was 81%, 83% and 82% in 2013, 2012 and 2011, respectively. The increase in cost of products sold in absolute terms was due to the increase in net product revenues discussed above. The period-to-period changes in gross margin are due to the product revenue mix in the respective periods, primarily due to increasing sales of Xifaxan and Apriso. Cost of products sold does not include amortization of product rights and intangibles. Refer to “Critical Accounting Policies—Intangible Assets and Goodwill” above.
Amortization of Product Rights and Intangible Assets
Amortization of product rights and intangible assets consists of amortization of the costs of license agreements, product rights and other identifiable intangible assets, which result from product and business acquisitions. The increase for 2012 compared to 2011 was primarily due to amortization of intangibles related to Deflux and Solesta, which we acquired in connection with our acquisition of Oceana in December 2011.
Intangible Impairment Charges
On July 27, 2012 we received a CRL from the FDA following its review of an sNDA for methylnaltrexone bromide injection for subcutaneous use for the treatment of OIC in adult patients with chronic, non-cancer pain. The CRL requested additional clinical data. Salix and Progenics held an End-of-Review meeting with the Division of Gastroenterology and Inborn Errors Products to better understand the contents of the CRL in October 2012. Based on the results of this meeting, we reassessed the value of the indefinite lived intangible asset related to methylnaltrexone bromide injection for subcutaneous use for the treatment of OIC in chronic non-cancer pain and recorded a non-cash charge to earnings of $41.6 million in the three-month period ended September 30, 2012.
We did not incur any intangible impairment charges in 2011 or 2013.
Research and Development
Research and development expense was $112.8 million, $85.0 million and $71.9 million for 2013, 2012 and 2011, respectively. This represents an increase in research and development expenses as a percentage of net product revenues to 12.3% for 2013 from 11.6% for 2012, and a reduction in research and development expenses as a percentage of net product revenues to 11.5% for 2012 from 13.3% for 2011.
69
Table of Contents
The increase in research and development expenses in absolute terms from 2012 to 2013 was due primarily to:
| • | increased expenses of approximately $31.8 million related to certain development programs, including rifaximin for irritable bowel syndrome, rifaximin SSD, and rifaximin for hepatic encephalopathy; |
| • | the $10.0 million payment to Olon S.p.A. for the purchase of intellectual property; and |
| • | increased personnel costs of approximately $3.4 million. |
These increases were partially offset by:
| • | a $4.5 million development milestone payment for LumacanTM, a Phase 1 product candidate for diagnosing, staging, or monitoring gastrointestinal dysplasia or cancer, to Photocure ASA, the entity from which we licensed the worldwide exclusive rights to Lumacan, that occurred during the third quarter of 2012; and |
| • | decreased expenses of approximately $12.8 million related to certain development programs, including crofelemer for HIV-associated diarrhea, which the FDA approved in December 2012, and methylnaltrexone bromide for opioid induced constipation, and other items. |
The increase in research and development expenses in absolute terms from 2011 to 2012 was due primarily to:
| • | increased expenses related to our development programs for crofelemer and rifaximin for irritable bowel syndrome; and |
| • | increased personnel costs. |
These increases were partially offset by:
| • | the $10.0 million upfront payment for our amended license agreement with Lupin in March 2011, which did not recur in 2012; |
| • | decreased expenses related to investigator-initiated studies; and |
| • | decreased expenses related to our development programs for methylnaltrexone bromide, rifaximin for HE and budesonide foam. |
Since inception through December 31, 2013, we have incurred research and development expenditures of approximately $41.7 million for balsalazide, $223.9 million for rifaximin, $32.1 million for granulated mesalamine, $36.1 million for crofelemer, $40.4 million for methylnaltrexone bromide and $38.8 million for budesonide foam.
Due to the significant risks and uncertainties inherent in the clinical development and regulatory approval processes, we cannot reasonably estimate the cost to complete projects and development timelines for their completion. Enrollment in clinical trials might be delayed or occur faster than anticipated for reasons beyond our control, requiring additional cost and time or accelerating spending. Results from clinical trials might not be favorable, or might require us to perform additional unplanned clinical trials, accelerating spending, requiring additional cost and time, or resulting in termination of the project. Further, as evidenced by the Complete Response Letter for rifaximin as a treatment for IBS, data from clinical trials is subject to varying interpretation, and might be deemed insufficient by the regulatory bodies reviewing applications for marketing approvals, requiring additional cost and time, or resulting in termination of the project. Regulatory reviews can also be delayed. For example the PDUFA action dates for Relistor and crofelemer were each extended by three months. Process development and manufacturing scale-up for production of clinical and commercial product supplies might take longer and cost more than our forecasts. As a result, clinical development and regulatory programs are subject to risks and changes that might significantly impact cost projections and timelines.
Selling, General and Administrative
Selling, general and administrative expenses were $342.4 million, $296.5 million and $219.5 million for 2013, 2012 and 2011, respectively. Selling, general and administrative expenses as a percentage of net product revenues was 37.5% for 2013 compared to 40.3% for 2012 and 40.6% for 2011.
70
Table of Contents
The increase from 2012 to 2013 in absolute terms was primarily due to:
| • | increased personnel costs of approximately $13.0 million, including a full year of expenses in 2013 due to our acquisition of Oceana in December 2011 and the expansion of our sales force in 2012; |
| • | increased marketing costs of approximately $2.8 million, due to costs associated with Fulyzaq, which received FDA approval in December 2012; |
| • | transaction costs of approximately $8.7 million related to our acquisition of Santarus, which closed in January 2014; |
| • | increased legal expenses of approximately $15.2 million primarily related to the Department of Justice subpoena and other general corporate legal matters including our litigation with Napo Pharmaceuticals; and |
| • | increased operating costs of approximately $6.2 million, including a full year of expenses in 2013 due to the acquisition of Oceana in December 2011 and the expansion of our sales force in 2012; |
The increase from 2011 to 2012 was primarily due to:
| • | increased personnel costs, including costs related to the addition of 25 key account managers in April 2011, our acquisition of Oceana in December 2011 and the addition of 10 sales representatives in the second quarter of 2012 and 22 sales representatives in the third quarter of 2012; |
| • | increased marketing expenses related to Relistor, which we in-licensed in February 2011 and launched in April 2011, Solesta, which we acquired in December 2011, and Xifaxan; |
| • | pre-marketing expenses related to Giazo and Fulyzaq, which we began promoting in 2013; and |
| • | increased legal expenses related to our product liability litigation, which was previously covered by our products liability insurance, the limits of which we exceeded in the fourth quarter of 2011. |
These increases were partially offset by reduced pre-marketing expenses related to Xifaxan 550mg for irritable bowel syndrome incurred in 2011 prior to our receipt of the CRL from the FDA.
We expect selling, general and administrative expenses to continue to increase in absolute terms as we expand our sales and marketing efforts for our current products, including Relistor, Solesta and Deflux, and other indications for rifaximin and methylnaltrexone bromide, if approved and the products we acquired from Santarus in January 2014.
Change in Acquisition-Related Contingent Consideration
We accounted for the Progenics transaction as a business combination under the acquisition method of accounting. Under the acquisition method of accounting, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date in our consolidated financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. As of the acquisition date, the estimated fair value of the contingent consideration related to the transaction was $53.0 million, which we included as a long-term liability on the consolidated balance sheet. We determined this liability amount using a probability-weighted discounted cash flow model based on the current regulatory status of the methylnaltrexone bromide development programs. We assess the fair value of the contingent consideration quarterly, or whenever events or changes in circumstances indicate that the fair value might have changed, primarily as a result of clinical or regulatory results in the related in-process development programs. In December 2011 we announced the successful outcome of the Phase 3 trial to evaluate the efficacy and safety of oral methylnaltrexone for the treatment of opioid–induced constipation in subjects with chronic, non–cancer pain. As a result of this event, we reassessed the fair value of the contingent consideration and recorded an increase of $27.0 million and a corresponding charge in the fourth quarter of 2011.
On July 27, 2012 we received a CRL from the FDA following its review of an sNDA for methylnaltrexone bromide SI for the treatment of OIC in adult patients with chronic, non-cancer pain. The CRL requested additional clinical data. Salix and Progenics held an End-of-Review meeting with the Division of Gastroenterology and Inborn Errors Products to better understand the contents of the CRL in October 2012. Based on the results of this meeting, we reassessed the value of the indefinite lived intangible asset related to methylnaltrexone bromide SI for the treatment of
71
Table of Contents
OIC in chronic non-cancer pain and recorded a non-cash charge to earnings of $41.6 million in the three-month period ended September 30, 2012. Based on these events, we reassessed the fair value of the contingent consideration related to the Progenics transaction and recorded a $33.0 million decrease in the contingent consideration and a corresponding non-cash benefit to earnings in the three-month period ended September 30, 2012.
For the year ended December 31, 2013, we reassessed the fair value of the Progenics, Oceana and EIR contingent considerations and recorded a $16.2 million net decrease in the fair values primarily driven by a decrease in the fair value of the Oceana contingent consideration of $24.6 million due to a change in the sales forecast, offset by an increase in the fair value of the Progenics and EIR contingent considerations of $8.4 million due to the passage of time.
For the year ended December 31, 2012, we reassessed the fair values of the Progenics, Oceana and EIR contingent considerations and recorded a $3.4 million increase in the fair values due to the passage of time.
Loss on Extinguishment of Debt
In March 2012, we entered into a note repurchase agreement with the holder of a majority in principal amount of the 2028 Notes. We used a portion of the proceeds from the offering of the 2019 Notes to purchase from this holder and another holder approximately 42.1% of the 2028 Notes for an aggregate purchase price of approximately $137.2 million. In addition, for a period of 90 days after March 12, 2012, the majority holder had the option to require us to purchase its remaining 2028 Notes at the same price, which represents approximately 37.1% of the 2028 Notes. This option expired unexercised in June 2012.
In December 2012 one of the holders of the 2028 Notes converted notes with a par value of $22.3 million under the terms of the note indenture, and received cash equal to the par value of the notes and interest on these notes through February 15, 2013, and 1.9 million shares of common stock.
Loss on extinguishment of debt primarily consists of $9.3 million in estimated fair market value of the put option granted to the majority holder, $3.6 million in estimated fair market value of the notes extinguished over their book value at the extinguishment date, and $2.2 million paid to the note holders for interest that the note holders would have been due subsequent to the extinguishment date.
We did not incur any loss on extinguishment of debt in 2011 or 2013.
Interest Expense
Interest expense was $62.0 million, $55.5 million and $32.1 million in 2013, 2012 and 2011, respectively.
Interest expense for 2013 consisted of:
| • | $27.8 million of interest expense on our 2015 convertible notes issued in June 2010, including $16.6 million of amortization of debt discount; |
| • | $1.1 million of interest expense on our 2028 convertible notes issued in August 2008, including $0.6 million of amortization of debt discount; and |
| • | $32.8 million of interest expense on our 2019 convertible notes issued in March 2012, including $20.1 million of amortization of debt discount. |
Interest expense for 2012 consisted of:
| • | $26.4 million of interest expense on our 2015 convertible notes issued in June 2010, including $15.2 million of amortization of debt discount; |
| • | $5.1 million of interest expense on our 2028 convertible notes issued in August 2008, including $2.4 million of amortization of debt discount; and |
| • | $24.0 million of interest expense on our 2019 convertible notes issued in March 2012, including $14.0 million of amortization of debt discount. |
72
Table of Contents
Interest expense for 2011 consisted of:
| • | $25.2 million of interest expense on our 2015 convertible notes issued in June 2010, including $14.0 million of amortization of debt discount; and |
| • | $6.9 million of interest expense on our 2028 convertible notes issued in August 2008, including $3.3 million of amortization of debt discount. |
Interest and Other Income
Interest and other income was $2.0 million, $10.9 million and $2.3 million for 2013, 2012 and 2011, respectively. Other income for 2012 includes $9.3 million related to the put option granted to the majority holder of the 2028 notes discussed above, which expired unexercised in June 2012.
Due to the current economic climate, we expect 2014 interest rates paid to us on our cash and cash equivalents will be equal to or lower than we experienced during 2013. We expect cash balances in 2014 to be lower as a result of our acquisition of Santarus.
Provision for Income Tax
Income tax (benefit) expense was $61.5 million, $47.6 million and ($1.3) million in 2013, 2012 and 2011, respectively. Our effective tax rate was 31.9%%, 42.6% and (1.5)% in 2013, 2012 and 2011, respectively.
The significant change in the effective tax rate between 2013 and 2012 was primarily related to the reinstatement of the federal research and development tax credit in January 2013 and decreases in reserves for uncertain tax positions related to debt financing costs and state tax issues.
Our 2013 effective tax rate of 31.9% varies from the federal statutory rate of 35% due primarily to: state income taxes, net of federal income tax benefit, of 4.0%; reserve for uncertain tax positions of (4.9)%; and federal research and development tax credits for tax years 2012 and 2013 of (3.1%).
Our 2012 effective tax rate of 42.6% varies from the federal statutory rate of 35% due primarily to: state income taxes, net of federal income tax benefit, of 3.5%; reserve for uncertain tax positions of 11.4%; debt costs of (8.5%); various non-deductible expenses 2.5%; and the change in valuation allowance related to certain state deferred tax assets (2.0%).
At December 31, 2012 and 2011, we had U.S. federal net operating loss carryforwards of approximately $3.6 million and $12.8 million. At December 31, 2013, we did not have any remaining federal net operating loss carryforwards available to offset future taxable income. At December 31, 2013, and 2012 our non-U.S. tax losses totaled approximately $11.8 million and $11.8 million, respectively. The Company provides a full valuation allowance against these non-U.S. tax losses. At December 31, 2013, 2012 and 2011, we also had state net operating loss carryforwards available to offset future taxable income of approximately $151.5 million, $162.2 million and $149.9 million, respectively. Certain of these state net operating loss carryforwards have full valuation allowances set up against them.
Quarterly Results of Operations
See Note 12 of Notes to Consolidated Financial Statements for a presentation of our unaudited quarterly results of operations for the years ended December 31, 2013 and 2012.
73
Table of Contents
LIQUIDITY AND CAPITAL RESOURCES
From inception until first achieving profitability in the third quarter of 2004, we financed product development, operations and capital expenditures primarily from public and private sales of equity securities and from funding arrangements with collaborators. Since launching Colazal in January 2001, net product revenue has been a growing source of cash. In August 2008, we closed an offering of $60.0 million in convertible senior notes due 2028 (“2028 Notes”), with net proceeds of $57.3 million. None of the 2028 Notes currently are outstanding. On June 3, 2010, we closed an offering of $345.0 million in convertible senior notes due May 15, 2015 (“2015 Notes”), with net proceeds of approximately $334.2 million. On March 16, 2012, we closed an offering of $690.0 million in convertible senior notes due March 15, 2019 (“2019 Notes”), with net proceeds of approximately $668.3 million. On December 27, 2013, we closed an offering of the 2021 Notes.
As of December 31, 2013 we had $1.2 billion in cash and cash equivalents, compared to $751.0 million as of December 31, 2012. In addition, on December 31, 2013, $750 million in restricted cash represented the gross proceeds from the sale of our 2021 Notes on December 27, 2013, which was held in escrow to finance a portion of the consideration for our acquisition of Santarus. We expect our future cash balances to be lower due to our acquisition of Santarus.
On January 2, 2014, we completed our acquisition of Santarus. We financed the acquisition transaction through a combination of (i) a term loan facility in the principal amount of $1.2 billion, (ii) the net proceeds from our issuance of the 2021 Notes and (iii) $800.4 million of cash on hand.
At December 31, 2013, our cash and cash equivalents consisted primarily of demand deposits, certificates of deposit, overnight investments in Eurodollars and money market funds at reputable financial institutions, and did not include any auction rate securities. We have not realized any material loss in principal or liquidity in any of our investments to date. However, declines in the stock market and deterioration in the overall economy could lead to a decrease in demand for our marketed products, which could have an adverse effect on our business, financial condition and results of operations. Based on our current projections, we believe that our cash flow from operations should be sufficient to satisfy our expected cash requirements, including debt service for our current debt and debt issued in connection with our acquisition of Santarus on January 2, 2014, without requiring additional capital. However, we might seek additional debt or equity financing or both to fund our operations or acquisitions, and our actual cash needs might vary materially from those now planned because of a number of factors including: whether we acquire additional products or companies; risk associated with acquisitions; FDA and foreign regulation and regulatory processes; the status of competitive products, including potential generics in an increasingly global industry; intellectual property and related litigation risks in an increasingly global industry; product liability litigation; our success selling products; the results of research and development activities; establishment of and change in collaborative relationships; general economic conditions; and technological advances by us and other pharmaceutical companies. The Credit Agreement and the 2021 Notes discussed below contain covenants that restrict our ability to issue additional debt or additional capital, and any additional debt we issue might be subject to financial and restrictive covenants. If we issue additional equity, our stockholders could suffer dilution. We might also enter into additional collaborative arrangements that could provide us with additional funding in the form of equity, debt, licensing, milestone and/or royalty payments. We might not be able to enter into such arrangements or raise any additional funds on terms favorable to us or at all.
In connection with the Audit Committee’s review, which began in Fall 2014, of Salix’s financial statements and related disclosures, our Audit Committee and management determined that wholesaler inventory levels of XIFAXAN® 550, APRISO® and UCERIS® were greater, at approximately 9 months, 9 months and 5 months, respectively, as of September 30, 2014, than our target (established in Fall 2014) of approximately 3 months. In order to reduce wholesaler inventory levels to meet this target by the end of 2015, we intend to sell to our wholesalers amounts of XIFAXAN® 550, APRISO® and UCERIS® that are less than end-user demand until the target levels are reached. As a result, our revenue and cash flow may be decreased in the fourth quarter of 2014 and the full year 2015, compared to prior periods. In addition, wholesalers may demand increased discounts on our products, which could further decrease revenue and cash flow, and it may take longer than planned to reach our target wholesaler inventory levels, which could result in decreased revenue and cash flow for a longer period than anticipated. If our revenue and cash flow from operations are less than expected, we may need to seek additional debt or equity financing to satisfy our expected cash requirements, and such financing may not be available on satisfactory terms or at all.
Cash Flows
Net cash provided by operating activities was $415.9 million in 2013 and was primarily attributable to our net income for the period, net of non-cash charges, decreased accounts receivable due to increased cash collections, increased reserves for product returns, rebates and chargebacks due to the timing of quarterly payments, and non-cash depreciation and amortization expense. Net cash provided by operating activities was $87.6 million in 2012 and was primarily attributable to our net income for the period, net of non-cash charges, offset by an increase in accounts receivable and inventory. Net cash provided by operating activities was $119.2 million in 2011 and was primarily attributable to our net income for the period, net of non-cash charges, offset by an increase in accounts receivable and inventory.
74
Table of Contents
Net cash used in investing activities was $6.9 million in 2013 and was primarily due to the purchase of property and equipment. Net cash used in investing activities was $15.4 million in 2012 and was primarily due to the purchase of Rifaximin EIR in August 2012 and purchases of property and equipment. Net cash used in investing activities was $388.6 million in 2011 and was primarily due to the purchase of Relistor in February 2011, the purchase of Oceana in December 2011 and purchases of property and equipment.
Net cash used by financing activities of $2.6 million in 2013 and was primarily due to the repurchase of the remaining portion of our 2028 convertible senior notes and payments related to net settlement of stock-based compensation, offset by excess tax benefits from stock-based compensation and proceeds from the issuance of common stock upon the exercise of stock options. Net cash provided by financing activities of $385.6 million in 2012 related primarily to our offering of $690.0 million in convertible senior notes, including net proceeds of $669.0 million and the receipt of $99.0 million from the sale of warrants, offset by $167.0 million for the purchase of call options, $159.6 million for the repurchase of a portion of our 2028 convertible senior notes, and $74.8 million for the repurchase of common stock. Net cash provided by financing activities of $44.4 million in 2011 consisted primarily of proceeds from the exercise of stock options and tax benefits from stock-based compensation.
Commitments
As of December 31, 2013, we had non-cancelable purchase order commitments for inventory purchases of approximately $217.4 million, which included any minimum purchase commitments under our manufacturing agreements. We anticipate significant expenditures related to our on-going sales, marketing, product launch efforts and our on-going development efforts for rifaximin, our budesonide product candidates, methylnaltrexone bromide and crofelemer. To the extent we acquire rights to additional products, we will incur additional expenditures.
Our contractual commitments for non-cancelable purchase commitments of inventory, minimum lease obligations for all non-cancelable operating leases, debt and minimum capital lease obligations (including interest) as of December 31, 2013, which do not include any contractual commitments we assumed as part of the acquisition of Santarus or borrowings under the Credit Agreement, were as follows (in thousands):
| Total | < 1 year | 1-3 years | 3-5 years | > 5 years | ||||||||||||||||
| Operating leases |
$ | 48,911 | $ | 4,506 | $ | 9,947 | $ | 9,657 | $ | 24,801 | ||||||||||
| Purchase commitments |
217,394 | 107,394 | 44,000 | 44,000 | 22,000 | |||||||||||||||
| 2015 Notes(1) |
358,046 | 9,488 | 348,558 | — | — | |||||||||||||||
| 2019 Notes(2) |
743,906 | 10,350 | 20,700 | 20,700 | 692,156 | |||||||||||||||
| 2021 Notes(3) |
1,065,000 | 45,000 | 90,000 | 90,000 | 840,000 | |||||||||||||||
| Capital lease obligations |
47 | 47 | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 2,433,304 | $ | 176,785 | $ | 513,205 | $ | 164,357 | $ | 1,578,957 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Contractual interest and principal obligations related to our 2015 Notes total $358.0 million at December 31, 2013, including $9.5 million and $348.6 million due in one year or less and one to three years, respectively. If these notes had been converted at December 31, 2013 based on the closing price of our stock of $89.94 per share on that date and we chose to settle them in cash, the settlement amount would have been approximately $669.0 million. |
| (2) | Contractual interest and principal obligations related to our 2019 Notes total $743.9 million at December 31, 2013, including $10.3 million, $20.7 million, $20.7 million and $692.2 million due in one year or less, one to three years, and three to five years, and greater than five years, respectively. If these notes had been converted at December 31, 2013 based on the closing price of our stock of $89.94 per share on that date and we chose to settle them in cash, the settlement amount would have been approximately $943.0 million. |
| (3) | Contractual interest and principal obligations related to our 2021 senior notes total $1,065 million at December 31, 2013, including $45.0 million, $90.0 million, $90.0 million and $840.0 million due in one year or less, one to three years, and three to five years, and greater than five years, respectively. |
We enter into license agreements with third parties that sometimes require us to make royalty, milestone or other payments contingent upon the occurrence of certain future events linked to the successful development and
75
Table of Contents
commercialization of pharmaceutical products. Some of the payments are contingent upon the successful achievement of an important event in the development life cycle of these pharmaceutical products, which might or might not occur. If required by the agreements, we will make royalty payments based upon a percentage of the sales of a pharmaceutical product if regulatory approval to market this product is obtained and the product is commercialized. Because of the contingent nature of these payments, we have not attempted to predict the amount or period in which such payments would possibly be made and thus they are not included in the table of contractual obligations.
2028 Notes
On August 22, 2008 we closed an offering of $60.0 million in 2028 Notes. Net proceeds from the offering were $57.3 million. The 2028 Notes were governed by an indenture, dated as of August 22, 2008, between us and U.S. Bank National Association, as trustee.
In March 2012, we entered into a note repurchase agreement with the holder of a majority in principal amount of the 2028 Notes. We used a portion of the proceeds from our offering of the 2019 Notes discussed below to purchase from this holder and another holder approximately 42.1% of the 2028 Notes for an aggregate purchase price of approximately $137.2 million. In addition, for a period of 90 days after March 12, 2012, the majority holder had the option to require us to purchase at the same price its remaining 2028 Notes, which represented approximately 37.1% of the 2028 Notes. This put option expired unexercised in June 2012. In December 2012 one of the holders of the 2028 Notes converted notes with a par value of $22.3 million under the terms of the note indenture, and received cash equal to the par value of the notes and interest on these notes through February 15, 2013, and 1.9 million shares of common stock.
We had the right to redeem the 2028 Notes, in whole or in part, at any time after August 15, 2013 for cash equal to the principal amount of the 2028 Notes to be redeemed, plus any accrued and unpaid interest. We called the 2028 Notes for redemption in September 2013 but before the redemption date, the holders elected to convert the remaining 2028 Notes with a par value of $12.5 million under the terms of the note indenture, and the holders received cash equal to the par value of the notes and interest on these notes through August 15, 2013, and 1.2 million shares of common stock.
2015 Notes
On June 3, 2010 we closed an offering of $345.0 million in 2015 Notes. Net proceeds from the offering were $334.2 million. The 2015 Notes are governed by an indenture, dated as of June 3, 2010, between us and U.S. Bank National Association, as trustee. The 2015 Notes bear interest at a rate of 2.75% per year, payable semiannually in arrears on May 15 and November 15 of each year, beginning on November 15, 2010. The 2015 Notes will mature on May 15, 2015, unless previously converted or repurchased in accordance with their terms prior to such date. The 2015 Notes are senior unsecured obligations, and rank (i) equally to any of our existing and future unsecured senior debt, (ii) senior to any of our future indebtedness that is expressly subordinated to these 2015 Notes, and (iii) effectively junior to any secured indebtedness to the extent of the value of the assets securing such indebtedness.
The 2015 Notes are convertible into approximately 7,439,000 shares of our common stock under certain circumstances prior to maturity at a conversion rate of 21.5592 shares per $1,000 principal amount of 2015 Notes, which represents a conversion price of approximately $46.38 per share, subject to adjustment under certain conditions. Holders may convert their 2015 Notes at their option at specified times prior to the maturity date of May 15, 2015 only if: (1) during any fiscal quarter commencing after June 30, 2010, if the last reported sale price of our common stock for at least 20 trading days in the period of 30 consecutive trading days ending on the last trading day of the immediately preceding fiscal quarter is equal to or more than 130% of the conversion price of the 2015 Notes on the last day of such preceding fiscal quarter; (2) a Holder submits its 2015 Notes for conversion during the five business day period following any five consecutive trading day period in which the trading price for the 2015 Notes, per $1,000 principal amount of the 2015 Notes, for each such trading day was less than 98% of the product of the last reported sale price of our common stock and the conversion rate of the 2015 Notes on such date; or (3) we enter into specified corporate transactions. The first of these conditions was met as of December 31, 2013. The 2015 Notes will be convertible, at the option of the noteholders, regardless of whether any of the foregoing conditions have been satisfied, on or after January 13, 2015 at any time prior to the close of business on the second scheduled trading day immediately preceding the stated maturity date of May 15, 2015. Upon conversion, we may pay cash, shares of our common stock or a combination of cash and stock, as determined by us at our discretion.
76
Table of Contents
In connection with the issuance of the 2015 Notes, we entered into capped call transactions covering approximately 7,439,000 shares of our common stock. The capped call transactions have a strike price of $46.38 and a cap price of $62.44, and are exercisable when and if the 2015 Notes are converted. If upon conversion of the 2015 Notes, the price of our common stock is above the strike price of the capped calls, the counterparties will deliver shares of our common stock and/or cash with an aggregate value approximately equal to the difference between the price of our common stock at the conversion date (as defined, with a maximum price for purposes of this calculation equal to the cap price) and the strike price, multiplied by the number of shares of our common stock related to the capped call transactions being exercised. We paid $44.3 million for these capped calls, and charged that amount to additional paid-in capital.
2019 Notes
On March 16, 2012 we closed an offering of $690.0 million in 2019 Notes. Net proceeds from the offering were approximately $668.3 million. The 2015 Notes are governed by an indenture, dated as of March 16, 2012 between the Company and U.S. Bank National Association, as trustee. The 2019 Notes bear interest at a rate of 1.50% per year, payable semiannually in arrears on March 15 and September 15 of each year, beginning on September 15, 2012. The 2019 Notes will mature on March 15, 2019, unless earlier converted or repurchased in accordance with their terms prior to such date. The 2019 Notes are senior unsecured obligations, and rank (i) equally to any of our existing and future unsecured senior debt, (ii) senior to any of our future indebtedness that is expressly subordinated to them, and (iii) effectively junior to any secured indebtedness to the extent of the value of the assets securing such indebtedness.
The 2019 Notes are convertible into approximately 10,484,000 shares of our common stock under certain circumstances prior to maturity at a conversion rate of 15.1947 shares per $1,000 principal amount of 2019 Notes, which represents a conversion price of approximately $65.81 per share, subject to adjustment under certain conditions. Holders may convert their 2019 Notes at their option at specified times prior to the maturity date of March 15, 2019 only if: (1) during any fiscal quarter commencing after June 30, 2012, if the last reported sale price of our common stock for at least 20 trading days in the period of 30 consecutive trading days ending on the last trading day of the immediately preceding fiscal quarter is equal to or more than 130% of the conversion price of the 2019 Notes on the last day of such preceding fiscal quarter; (2) a Holder submits its 2019 Notes for conversion during the five business day period following any five consecutive trading day period in which the trading price for the 2019 Notes, per $1,000 principal amount of the 2019 Notes, for each such trading day was less than 98% of the product of the last reported sale price of our common stock and the conversion rate of the 2019 Notes on such date; or (3) we enter into specified corporate transactions. None of these conditions had been met as of December 31, 2013. The 2019 Notes will be convertible, at the option of the noteholders, regardless of whether any of the foregoing conditions have been satisfied, on or after November 9, 2018 at any time prior to the close of business on the second scheduled trading day immediately preceding the stated maturity date of March 15, 2019. Upon conversion, we may pay cash, shares of our common stock or a combination of cash and stock, as determined by us at our discretion.
In connection with the issuance of the 2019 Notes, we entered into convertible bond hedge transactions with certain counterparties covering approximately 10,484,000 shares of our common stock. The convertible bond hedge transactions have a strike price of $65.81 and are exercisable when and if the 2019 Notes are converted. If upon conversion of the 2019 Notes, the price of our common stock is above the strike price of the convertible bond hedge transactions, the counterparties will deliver shares of our common stock and/or cash with an aggregate value approximately equal to the difference between the price of our common stock at the conversion date and the strike price, multiplied by the number of shares of our common stock related to the convertible bond hedge transaction being exercised. We paid $167.0 million for these convertible bond hedge transactions and charged this to additional paid-in capital.
Simultaneously with entering into the convertible bond hedge transactions, we entered into privately negotiated warrant transactions whereby we sold the counterparties to these transactions warrants to acquire, subject to customary adjustments, approximately 10,484,000 shares of our common stock at a strike price of $85.31 per share, also subject to adjustment. We received $99.0 million for these warrants and credited this amount to additional paid-in capital.
77
Table of Contents
2021 Notes
On December 27, 2013, we closed an offering of $750.0 million in 2021 Notes. The Notes were issued pursuant to an indenture, dated as of December 27, 2013, between us and U.S. Bank National Association, as trustee. We used the net proceeds from the sale of the 2021 Notes to finance a portion of the approximately $2.6 billion that was payable as consideration for our acquisition of Santarus, which closed on January 2, 2014, and to pay the related fees and expenses in connection therewith. The 2021 Notes will mature on January 15, 2021 and bear interest at a rate of 6.00% per annum, accruing from December 27, 2013. The 2021 Notes are senior unsecured obligations, and rank (i) equally to any of our existing and future unsecured senior debt, (ii) senior to any of our future indebtedness that is expressly subordinated to them, and (iii) effectively junior to any secured indebtedness to the extent of the value of the assets securing such indebtedness. Following the acquisition of Santarus, Santarus, Salix Pharmaceuticals, Inc. and Oceana became guarantors of the 2021 Notes. The 2021 Notes are governed by terms contained in an Indenture.
At any time prior to January 15, 2017, we are entitled, at our option, to redeem some or all of the 2021 Notes at a redemption price of 100% of the principal amount thereof, plus a make-whole premium set forth in the indenture and accrued and unpaid interest, if any, to the redemption date. On and after January 15, 2017, we may redeem the 2021 Notes, in whole or in part, at redemption prices (expressed as percentages of principal amount thereof) equal to 104.50% for the 12-month period beginning on January 15, 2017, 103.00% for the 12-month period beginning January 15, 2018, 101.50% for the 12-month period beginning on January 15, 2019 and 100.00% for the period beginning on January 15, 2020 and thereafter, plus accrued and unpaid interest, if any. At any time prior to January 15, 2017, we also may redeem up to 35% of the principal amount of the 2021 Notes at a redemption price equal to 106.00% of the principal amount thereof plus accrued and unpaid interest, if any, with the net cash proceeds of certain equity offerings.
The Indenture contains covenants that restrict the ability of us and certain of our subsidiaries to, among other things: (i) borrow money or issue preferred stock; (ii) pay dividends or make other payments or distributions on equity or purchase, redeem or otherwise acquire equity; (iii) make principal payments on, or purchase or redeem subordinated indebtedness prior to any scheduled principal payment or maturity; (iv) make certain investments; (v) create liens on their assets; (vi) sell their assets; (vii) enter into certain transactions with affiliates; (viii) engage in unrelated businesses and (ix) consolidate, merge or sell substantially all of the Company’s assets. These covenants are subject to a number of exceptions and qualifications, including the fall away of certain of these covenants if the 2021 Notes receive an investment grade credit rating in the future. The Indenture also requires us to make an offer to repurchase the 2021 Notes upon the occurrence of certain events constituting either a change of control that reduces our credit rating or an asset sale.
Credit Agreement
On January 2, 2014, we entered into a credit agreement, or the Credit Agreement, with Jefferies Finance LLC, as collateral agent and administrative agent, and the lenders party thereto, providing for (i) a $1.2 billion six year senior secured term loan facility, or the Senior Term Loan Facility, and (ii) a $150 million five year senior secured revolving credit facility, or the Revolving Credit Facility. We refer to the Senior Term Loan Facility and the Revolving Credit Facility, collectively, as the Senior Secured Facilities. The proceeds of the Senior Term Loan Facility were used to fund a portion of the purchase price of the acquisition of Santarus. The proceeds of the Revolving Credit Facility can be used in the future for working capital and general corporate purposes, including permitted investments and acquisitions. Santarus, Salix Pharmaceuticals, Inc. and Oceana, each a guarantor, have guaranteed our obligations under the Credit Agreement and the obligations of each of the other guarantors under the loan documents. Additionally, we and the guarantors have granted to the collateral agent, for the benefit of the lenders under the Credit Agreement, a first priority security interest in substantially all of our and their respective assets.
The term loans under the Senior Term Loan Facility are subject to quarterly amortization equal to 1.25% of the original aggregate principal amount thereof and the remaining principal balance is due and payable on January 2, 2020, unless earlier prepaid. The Senior Secured Facilities bear interest at an annual rate of, at our option, either (i) Adjusted LIBOR (as defined by the Credit Agreement), with a floor of 1.00%, plus a margin of 3.25% or (ii) the highest of (A) the Wall Street Journal’s published “U.S. Prime Lending Rate,” (B) the Federal Funds Effective Rate (as defined by the credit agreement) in effect on such day plus 1/2 of 1%, (C) one-month Adjusted LIBOR plus 1.00% per annum and (D) 2.00%, in each case plus a margin of 2.25%. If the ratio of the our consolidated total debt to consolidated EBITDA is less than 3.75 to 1.00, the margins will be reduced by 25 basis points.
78
Table of Contents
We are required to prepay term loans under the Senior Term Loan Facility with (i) 100% of the proceeds of asset sales not reinvested within generally one year, (ii) 100% of the proceeds from certain debt financings and (iii) 50% of Excess Cash Flow (as defined in the Credit Agreement). The percentage of Excess Cash Flow that must be used to prepay the Senior Term Loan Facility decreases to 25% if the Total Leverage Ratio is less than 3:50 to 1:00 and to zero if the Total Leverage Ratio is less than 2:50 to 1:00.
The Credit Agreement includes customary affirmative and negative covenants, including restrictions on additional indebtedness, liens, investments, asset sales, stock buybacks and dividends, mergers, consolidations, and transactions with affiliates and capital expenditures. The negative covenants are generally subject to various exceptions. The Credit Agreement does not include any financial maintenance covenants, with the exception that if 25% or more of the Revolving Credit Facility is being utilized, a Total Leverage Ratio requirement (measured as of the last day of each quarter), which decreases over time, must be satisfied.
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS
In February 2013, the Financial Accounting Standards Board (“FASB”) issued an Accounting Standards Update to the Comprehensive Income Topic in the Accounting Standards Codifications (“ASC”). This update requires separate presentation of the components that are reclassified out of accumulated other comprehensive income either on the face of the financial statements or in the notes to the financial statements. This update also requires companies to disclose the income statement line items impacted by any significant reclassifications, such as the realization of foreign currency translation gains and losses. These items are required for both interim and annual reporting for public companies and have an immaterial effect on the Company’s financial statements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Our purchases of raw materials and finished goods are denominated primarily in U.S. dollars and purchases denominated in currencies other than the U.S. dollar are insignificant. Additionally, our net assets denominated in currencies other than the U.S. dollar are insignificant and have not historically exposed us to material risk associated with fluctuations in currency rates. Given these facts, we have not considered it necessary to use foreign currency contracts or other derivative instruments to manage changes in currency rates. However, these circumstances might change.
We have outstanding $345.0 million of 2.75% convertible senior notes due 2015, $690.0 million of 1.5% convertible senior notes due 2019 and $750 million of 6.00% senior notes due 2021. The interest rates on these notes are fixed and therefore they do not expose us to risk related to rising interest rates.
In connection with the June 2010 offering of the 2015 Notes, we paid $44.3 million to purchase capped call options covering approximately 7,439,000 shares of our common stock. If the per share price of our common stock remains below $46.38, these call options will be worthless. If the per share price of our common stock exceeds $62.44, then to the extent of the excess, these call options will not provide us protection against dilution from conversion of the 2015 Notes.
In connection with the March 2012 offering of the 2019 Notes, we paid $167.0 million to purchase convertible bond hedge transactions covering approximately 10,484,000 shares of our common stock. If the per share price of our common stock remains below $65.81, these call options will be worthless. Simultaneously with entering into the convertible bond hedge transactions, we sold warrants to acquire, subject to customary adjustments, approximately 10,484,000 shares of our common stock at a strike price of $85.31 per share, also subject to adjustment. If the per share price of our common stock exceeds $85.31, then to the extent of the excess, these warrants will counter any benefit of the convertible bond hedges we purchased.
79
Table of Contents
The Credit Agreement we entered into in January 2014 in connection with our acquisition of Santarus consists of a $1.2 billion six year senior Term Loan Facility, and a $150 million five year senior secured Revolving Credit Facility. Both of these facilities bear interest at variable rates based on either LIBOR or the Federal Funds Effective Rate plus a margin, with a minimum rate for each of the indexes.
Item 8. Financial Statements and Supplementary Data (Restated)
The information required by this Item is set forth in the Consolidated Financial Statements and Notes thereto beginning at page F-1 of this Report.
80
Table of Contents
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Control and Procedures
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Disclosure controls and procedures (as defined in Exchange Act Rule 13a-15(e)) are designed only to provide reasonable assurance that information to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and accumulated and communicated to the issuer’s management, including its principal financial officer, or persons performing similar functions, as appropriate to allow timely decision regarding required disclosure. As of the end of the period covered by this Form 10-K/A, the Company carried out an evaluation, under the supervision and with the participation of the Company’s management, including the Company’s Acting Chief Executive Officer and Senior Vice President, Finance and Administration and Acting Chief Financial Officer, of the effectiveness of the Company’s disclosure controls and procedures pursuant to Exchange Act Rule 13a-15(e). Based upon this evaluation, and as further discussed below, the Company’s Acting Chief Executive Officer and Senior Vice President, Finance and Administration and Acting Chief Financial Officer have concluded that our disclosure controls and procedures are not effective to provide the reasonable assurance discussed above.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Our internal control over financial reporting is designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. A control system, no matter how well designed and operated, can only provide reasonable, not absolute, assurance that the objectives of the control system are met. Because of these inherent limitations, management does not expect that our internal controls over financial reporting will prevent all error and all fraud. Under the supervision and with the participation of our management, including our former President and Chief Executive Officer and our former Executive Vice President, Finance and Administration and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework (the 1992 framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control—Integrated Framework (the 1992 framework), our management concluded that our internal control over financial reporting was effective as of December 31, 2013.
In connection with the restatement discussed in the Explanatory Note to this Form 10-K/A and Note 1A of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K/A, management, including our Acting Chief Executive Officer, and Senior Vice President, Finance and Administrative and Acting Chief Financial Officer, reassessed the effectiveness of our internal control over financial reporting as of December 31, 2013. Based on this reassessment using the COSO criteria, management has concluded that we did not maintain effective internal control over financial reporting as of December 31, 2013 because we did not (1) establish and maintain adequate procedures and controls for (a) product returns and for communications between our sales and accounting/finance functions to record agreed upon returns and (b) the recognition of revenue for sales to customers with FOB Destination shipping terms, (2) comply with established policies to properly obtain, evaluate, review and approve agreements with customers and (3) periodically review and assess our account classification policies in light of changes in our organization, management and personnel over time, and the effect of non-routine transactions. Management concluded that these deficiencies were material weaknesses as defined in the Securities and Exchange Commission regulations. These material weaknesses resulted in the misstatement of our financial statements and disclosures for the year ended December 31, 2013 (and the restatement of the unaudited consolidated financial information for the quarters ended March 31, 2014, June 30, 2014, and September 30, 2014) as more fully discussed in Note 1A to the Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K/A.
The effectiveness of our internal control over financial reporting as of December 31, 2013 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in its report which is included in this Form 10-K/A.
Remediation Plan
We are actively engaged in developing and implementing remediation plans designed to address these material weaknesses. The actions that we are taking are subject to ongoing senior management review and Audit Committee oversight. Management believes the foregoing efforts will effectively remediate the material weaknesses in the first quarter of 2015. As we continue to evaluate and work to improve our internal control over financial reporting, management may execute additional measures to address potential control deficiencies or modify its remediation plans and will continue to review and make necessary changes to the overall design of our internal controls.
81
Table of Contents
Changes in Internal Control over Financial Reporting
There was no change in our internal controls over financial reporting during the fourth quarter of the period covered by this Annual Report that has materially affected, or is reasonably likely to materially affect, our internal controls over financial reporting.
On February 26, 2014, upon the recommendation of the Compensation Committee, the Board of Directors approved revisions to change in control payments payable under the Employment Agreements with each of its named executive officers in the event of a qualifying termination of employment that occurs within 12 months after a change in control of Salix. A qualifying termination includes a termination by Salix without reasonable cause or by the officer with good reason, in each case, within 12 months after a change in control of Salix (defined as any sale, transfer, merger, and/or similar disposition of Salix’s business, whether direct or indirect and whether by purchase, lease, merger, consolidation, liquidation or otherwise). As amended, in the event of a qualifying termination, each named executive officer of Salix will be paid his or her base salary as of the date of termination for a period of 36 months and will be paid a bonus equal to a multiple of 3 times his or her maximum annual target bonus. In addition, each named executive officer will be paid benefits for 36 months, will be provided outplacement benefits for 6 months following such termination and will receive assistance moving back to his or her original place of residence if such executive had relocated at the request of Salix within 18 months prior to the change in control.
82
Table of Contents
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information required by this Item concerning our directors is incorporated by reference from the section captioned “Proposal One—Election of Directors” contained in our proxy statement related to the 2014 Annual Meeting of Stockholders scheduled to be held on June 12, 2014, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
The Board of Directors has determined that the members of the Audit Committee are independent as defined in Rule 5605(c)(2)(A) of the Nasdaq listing standards, and the members of the Compensation Committee meet the standards set forth in Nasdaq Rule 5605 (d)(2)(A). The Board of Directors has also determined that John F. Chappell, Thomas W. D’Alonzo, Mark A. Sirgo and William P. Keane are “audit committee financial experts” as defined in Item 407 (d)(5) of Regulation S-K.
Our Board of Directors adopted a code of conduct that applies to all of our directors and employees. Our Board also adopted a separate code of ethics for our Chief Executive Officer, Chief Financial Officer, Chief Accounting Officer and Controller, or persons performing similar functions. We will provide copies of our code of conduct and code of ethics without charge upon request. To obtain a copy of our code of conduct and code of ethics, please send your written request to Salix Pharmaceuticals, Ltd., 8510 Colonnade Center Drive, Raleigh, NC 27615, Attn: General Counsel. In addition, you can find those codes on our website at www.salix.com/assets/pdf/about/Salix_Code_Bus_Cond.pdf.
The information required by this Item concerning our executive officers is set forth at the end of Part I of this report.
The information required by this Item concerning compliance with Section 16(a) of the Exchange Act is incorporated by reference from the section of the proxy statement captioned “—Section 16(a) Beneficial Ownership Reporting Compliance.”
Item 11. Executive Compensation
The information required by this Item is incorporated by reference to the information under the sections captioned “—Grant of Plan Based Awards for 2013,” “—Outstanding Equity Awards at 2013 Fiscal Year End,” “—Option Exercises and Stock Vested in 2013,” “—Director Compensation For 2013,” “—Compensation Discussion and Analysis,” “—Summary Compensation Table,” “—Compensation Committee Report,” and “—Compensation Committee Interlocks and Insider Participation” contained in the proxy statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth the indicated information as of December 31, 2013 with respect to our equity compensation plans:
| Plan Category |
(a) Number of Securities to be issued Upon Exercise of Outstanding Options, Warrants and Rights, or Vesting of Restricted Shares |
(b) Weighted Average Exercise Price of Outstanding Options, Warrants, Rights and Restricted Shares |
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Related in Column (a)) |
|||||||||
| Equity Compensation Plans Approved by Security Holders |
1,970,748 | $ | 39.66 | 3,031,539 | ||||||||
| Equity Compensation Plans Not Approved by Security Holders |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
1,970,748 | $ | 39.66 | 3,031,539 | ||||||||
|
|
|
|
|
|
|
|||||||
83
Table of Contents
The other information required by this Item is incorporated by reference to the information under the section captioned “—Security Ownership of Management and Certain Beneficial Owners” contained in the proxy statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated by reference to the information under the section captioned “—Transactions with Related Persons,” “Proposal One—Election of Directors” and “—Corporate Governance Matters” contained in the proxy statement.
Item 14. Principal Accounting Fees and Services
The information required by this Item is incorporated by reference to the information under the section captioned “—Audit Committee Report” contained in the proxy statement.
84
Table of Contents
PART IV
Item 15. Exhibits and Financial Statement Schedules (Restated)
(a) 1. Financial Statements (as restated)
The following statements are filed as part of this report:
| Page | ||||
| Report of Independent Registered Public Accounting Firm |
F-2 | |||
| Consolidated Balance Sheets (as restated) |
F-4 | |||
| Consolidated Statements of Comprehensive Income (as restated) |
F-5 | |||
| Consolidated Statements of Stockholders’ Equity (as restated) |
F-6 | |||
| Consolidated Statements of Cash Flows (as restated) |
F-7 | |||
| Notes to Consolidated Financial Statements (as restated) |
F-8 | |||
2. Financial Statement Schedules (as restated)
| Schedule II—Valuation and Qualifying Accounts (as restated) |
F-41 |
Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
3. Exhibits
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed | |||||||||||
| 2.1* | Stock Purchase Agreement, dated as of April 22, 2009, by and between Oceana Therapeutics LLC and Q-Med AB | 10-K | 02/28/12 | 2.4 | ||||||||||||
| 2.2* | Stock Purchase Agreement, dated as of November 8, 2011, by and between Salix Pharmaceuticals, Inc. and Oceana Therapeutics, LLC | 10-K | 02/28/12 | 2.5 | ||||||||||||
| 2.3* | Agreement and Plan of Merger, dated September 10, 2010, among Santarus, Inc., SAN Acquisition Corp., Covella Pharmaceuticals, Inc. and Lawrence C. Fritz, as the Stockholder Representative | 10-Q/A+ | 03/08/11 | 2.1 | ||||||||||||
| 3.1 | Certificate of Incorporation, as amended. | 10-Q | 05/10/11 | 3.1 | ||||||||||||
| 3.2 | Amended and Restated Bylaws. | 8-K | 12/23/13 | 3.1 | ||||||||||||
| 4.1 | Indenture dated August 22, 2008 by and between Salix Pharmaceuticals, Ltd. and U.S. Bank National Association. | 8-K | 08/22/08 | 4.1 | ||||||||||||
| 4.2 | Form of 5.5% Convertible Senior Note due 2028 (included in Exhibit 4.1). | 8-K | 08/22/08 | 4.1 | ||||||||||||
| 4.3 | Form of Convertible Senior Note due 2015. | S-3 | 05/27/10 | 4.1 | ||||||||||||
| 4.4 | Form of Indenture for Convertible Senior Notes due 2015 by and between Salix Pharmaceuticals, Ltd. and U.S. Bank National Association | S-3 | 05/27/10 | 4.2 | ||||||||||||
| 4.5 | First Supplemental Indenture dated March 14, 2012 by and between Salix Pharmaceuticals, Ltd. and U.S. Bank National Association for 5.5% Convertible Senior Notes due 2028. | 8-K | 03/16/12 | 4.3 | ||||||||||||
| 4.6 | Indenture dated March 16, 2012 by and between Salix Pharmaceuticals, Ltd. and U.S. Bank National Association for 1.5% Convertible Senior Notes due 2019. | 8-K | 03/16/12 | 4.4 | ||||||||||||
85
Table of Contents
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed | |||||||||||
| 4.7 | Form of 1.5% Convertible Senior Note due 2019 (included in Exhibit 4.4). | 8-K | 03/16/12 | 4.5 | ||||||||||||
| 4.8 | Indenture, dated as of December 27, 2013 between Salix Pharmaceuticals Ltd. and U.S. Bank National Association for 6.00% Senior Notes due 2021 | 8-K | 12/27/13 | 4.1 | ||||||||||||
| 4.9 | Form of 6.00% Senior Note due 2021 (included in Exhibit 4.1) | 8-K | 12/27/13 | 4.1 | ||||||||||||
| 10.1 | Form of 1996 Stock Plan for Salix Holdings, Ltd., as amended through July 1, 2004. | 10-Q | 08/09/04 | 10.3 | ||||||||||||
| 10.2* | Supply Agreement, dated June 24, 1996, between Alfa Wassermann S.p.A. and Salix Pharmaceuticals, Ltd. | S-1 | 08/15/97 | 10.13 | ||||||||||||
| 10.3 | Lease Agreement dated June 30, 2000 by and between Colonnade Development, LLC and Salix Pharmaceuticals, Inc. | 10-Q | 08/14/01 | 10.29 | ||||||||||||
| 10.3.1* | Second Amendment to Lease dated February 11, 2011 between EOS Acquisition I LLC and Salix Pharmaceuticals, Inc. | 10-Q | 05/10/11 | 10.29.1 | ||||||||||||
| 10.3.2 | Third Amendment to Lease dated October 18, 2011 between EOS Acquisition I LLC and Salix Pharmaceuticals, Inc. | 10-Q | 11/09/11 | 10.29.2 | ||||||||||||
| 10.4 | Form of Employment Agreement for executive officers. | 10-Q | 11/14/01 | 10.31 | ||||||||||||
| 10.5* | License Agreement by and between Salix Pharmaceuticals, Inc. and Dr. Falk Pharma GmbH dated July 15, 2002. | 10-Q | 11/14/02 | 10.32 | ||||||||||||
| 10.6* | Amendment Agreement dated November 24, 2003 between Salix Pharmaceuticals, Inc. and Dr. Falk Pharma GmbH. | 10-K | 03/12/04 | 10.40 | ||||||||||||
| 10.7* | Supply Agreement dated June 30, 2004 between King Pharmaceuticals, Inc., Parkedale Pharmaceuticals, Inc., Salix Pharmaceuticals, Inc. and Salix Pharmaceuticals, Ltd. | 10-Q | 08/09/04 | 10.44 | ||||||||||||
| 10.8 | License Assignment and Consent Agreement dated June 30, 2004 between Parkedale Pharmaceuticals, Inc., King Pharmaceuticals, Inc., Salix Pharmaceuticals, Inc., Salix Pharmaceuticals, Ltd., Warner-Lambert Company LLC and Parke, Davis & Company LLC. | 10-Q | 08/09/04 | 10.45 | ||||||||||||
| 10.9 | Assignment of Trademarks Agreement dated June 30, 2004 between Monarch Pharmaceuticals, Inc., Parkedale Pharmaceuticals, Inc. and Salix Pharmaceuticals, Inc. | 10-Q | 08/09/04 | 10.46 | ||||||||||||
| 10.10 | License Agreement dated June 30, 2004 between Monarch Pharmaceuticals, Inc., Parkedale Pharmaceuticals, Inc., King Pharmaceuticals, Inc., Salix Pharmaceuticals, Inc. and Salix Pharmaceuticals, Ltd. | 10-Q | 08/09/04 | 10.47 | ||||||||||||
| 10.11 | 2005 Stock Plan, as amended. | 8-K | 06/11/09 | 10.50 | ||||||||||||
| 10.12* | License and Supply Agreement dated as of December 7, 2005 between Salix Pharmaceuticals, Inc. and Norgine B.V. | 8-K | 12/13/05 | 10.53 | ||||||||||||
| 10.13 | Form of Restricted Stock Grant to be granted pursuant to the 2005 Stock Plan. | 10-K | 03/16/06 | 10.54 | ||||||||||||
86
Table of Contents
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed | |||||||||||
| 10.14* | License Agreement entered into on June 22, 2006 between Cedars-Sinai Medical Center and Salix Pharmaceuticals, Inc. | 8-K | 07/05/06 | 10.55 | ||||||||||||
| 10.15* | Master Purchase and Sale and License Agreement dated February 22, 2007 between Merck & Co., Inc. and Salix Pharmaceuticals, Ltd. | 10-Q | 5/10/07 | 10.58 | ||||||||||||
| 10.16* | License Agreement dated April 16, 2007 between Salix Pharmaceuticals, Inc. and Dr. Falk Pharma GmbH. | 10-Q | 5/10/07 | 10.59 | ||||||||||||
| 10.17* | License Agreement dated March 13, 2008 between Dr. Falk Pharma GmbH and Salix Pharmaceuticals, Inc. | 10-Q | 5/07/08 | 10.62 | ||||||||||||
| 10.18* | Collaboration Agreement, dated December 9, 2008, between Napo Pharmaceuticals, Inc. and Salix Pharmaceuticals, Inc. | 10-K | 3/11/09 | 10.64 | ||||||||||||
| 10.19* | Commercial Manufacturing Agreement, dated September 9, 2008, between Salix Pharmaceuticals, Inc. and Catalent Pharma Solutions, LLC | 10-Q | 8/17/09 | 10.66 | ||||||||||||
| 10.20* | Rifaximin Manufacturing and Supply Agreement, dated September 30, 2009, between Salix Pharmaceuticals, Inc. and Lupin Ltd. | 10-Q | 11/09/09 | 10.68 | ||||||||||||
| 10.21.1* | First Amendment to Rifaximin Manufacturing and Supply Agreement dated March 31, 2011 between Salix Pharmaceuticals, Inc. and Lupin Ltd. | 10-Q | 05/10/11 | 10.68.1 | ||||||||||||
| 10.21.2* | Second Amendment to Rifaximin Manufacturing and Supply Agreement dated February 22, 2013 between Salix Pharmaceuticals, Inc. and Lupin Ltd. | 10-Q | 05/10/13 | 10.68.2 | ||||||||||||
| 10.21.3* | Third Amendment to Rifaximin Manufacturing and Supply Agreement dated May 15, 2013 between Salix Pharmaceuticals, Inc. and Lupin Ltd. | 10-Q | 08/09/13 | 10.68.3 | ||||||||||||
| 10.22 | Confirmation for base capped call transaction dated as of May 27, 2010, between Bank of America, N.A. and Salix Pharmaceuticals, Ltd. | 8-K | 6/03/10 | 10.69 | ||||||||||||
| 10.23 | Confirmation for additional capped call transaction dated as of June 2, 2010, between Bank of America N.A. and Salix Pharmaceuticals, Ltd. | 8-K | 6/03/10 | 10.70 | ||||||||||||
| 10.24* | Settlement Agreement (MoviPrep®) dated August 27, 2010, by and among Salix Pharmaceuticals, Inc., Norgine B.V., Norgine Europe B.V., Novel Laboratories, Inc., and Actavis Inc. | 10-Q | 11/09/10 | 10.71 | ||||||||||||
| 10.25* | Sublicense Agreement (MoviPrep®) dated August 27, 2010, by and among Salix Pharmaceuticals, Inc., Norgine B.V., Norgine Europe B.V., and Novel Laboratories, Inc. | 10-Q | 11/09/10 | 10.72 | ||||||||||||
| 10.26* | Supply Agreement dated August 27, 2010, by and among Salix Pharmaceuticals, Inc, Actavis Inc., and Novel Laboratories, Inc. | 10-Q | 11/09/10 | 10.73 | ||||||||||||
| 10.27* | First Amendment to License and Supply Agreement dated August 27, 2010, by and between Norgine B.V. and Salix Pharmaceuticals, Inc. | 10-Q | 11/09/10 | 10.74 | ||||||||||||
87
Table of Contents
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed | |||||||||||
| 10.28* | Settlement Agreement (OsmoPrep®) dated September 29, 2010, by and among Salix Pharmaceuticals, Inc., CDC III, LLC, a general partnership of Craig Aronchick, William H. Lipshutz and Scott H. Wright, Novel Laboratories, Inc., and Actavis Inc. | 10-Q | 11/09/10 | 10.75 | ||||||||||||
| 10.29* | Sublicense Agreement (OsmoPrep®) dated September 29, 2010, by and among Salix Pharmaceuticals, Inc., CDC III, LLC, a general partnership of Craig Aronchick, William H. Lipshutz and Scott H. Wright, and Novel Laboratories, Inc. | 10-Q | 11/09/10 | 10.76 | ||||||||||||
| 10.30* | Supply Agreement dated September 29, 2010, by and between Salix Pharmaceuticals, Inc. and Novel Laboratories, Inc. | 10-Q | 11/09/10 | 10.77 | ||||||||||||
| 10.31* | Second Amendment to License Agreement dated September 29, 2010, by and among CDC III, LLC, a general partnership of Craig Aronchick, William H. Lipshutz and Scott H. Wright, and Salix Pharmaceuticals, Inc. | 10-Q | 11/09/10 | 10.78 | ||||||||||||
| 10.32* | License Agreement dated October 19, 2010 by and between Photocure ASA and Salix Pharmaceuticals, Inc. | 10-K | 03/01/11 | 10.79 | ||||||||||||
| 10.33* | License Agreement dated February 3, 2011, between Salix Pharmaceuticals, Inc. and Progenics Pharmaceuticals, Inc., Progenics Pharmaceuticals Nevada, Inc. and Excelsior Life Sciences Ireland Limited | 10-Q | 05/10/11 | 10.80 | ||||||||||||
| 10.34* | 2010 Agreement Related to Progenics’s MNTX In-License dated February 3, 2011, by and among Salix Pharmaceuticals, Inc., the University of Chicago, Progenics Pharmaceuticals, Inc. and Progenics Pharmaceuticals Nevada, Inc. | 10-Q | 05/10/11 | 10.81 | ||||||||||||
| 10.35* | Office Lease dated February 14, 2011 between Cornerstone Colonnade LLC and Salix Pharmaceuticals, Inc. | 10-Q | 05/10/11 | 10.82 | ||||||||||||
| 10.36* | Amended and Restated Development, Commercialization and License Agreement dated March 31, 2011 between Lupin Ltd. and Salix Pharmaceuticals, Inc. | 10-Q | 05/10/11 | 10.83 | ||||||||||||
| 10.36.1* | First Amendment to Amended and Restated Development, Commercialization, and License Agreement dated February 22, 2013 between Lupin Ltd. and Salix Pharmaceuticals, Inc. | 10-Q | 05/10/13 | 10.83.1 | ||||||||||||
| 10.37* | Finished Product Manufacturing and Supply Agreement dated March 31, 2011 between Salix Pharmaceuticals, Inc. and Lupin Ltd. | 10-Q | 05/10/11 | 10.84 | ||||||||||||
| 10.38* | Amended and Restated Manufacturing and Supply Agreement dated July 18, 2011, between Salix Pharmaceuticals, Inc. and Glenmark Pharmaceuticals Ltd. | 10-Q | 11/09/11 | 10.85 | ||||||||||||
| 10.39* | Agreement for Advance Against Commitment Fee dated July 18, 2011 between Salix Pharmaceuticals, Inc. and Glenmark Pharmaceuticals Ltd. | 10-Q | 11/09/11 | 10.86 | ||||||||||||
| 10.40* | License Agreement, dated as of June 2, 2009, by and between Q-Med AB and Q-Med Scandinavia Inc. (n/k/a Oceana Therapeutics, Inc.) | 10-K | 02/28/12 | 10.87 | ||||||||||||
88
Table of Contents
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed | |||||||||||
| 10.41* | License Agreement, dated as of June 2, 2009, by and between Q-Med AB and Cetacea Limited (n/k/a Oceana Therapeutics Limited) | 10-K | 02/28/12 | 10.88 | ||||||||||||
| 10.42* | Supply Agreement, dated as of June 2, 2009, by and between Q-Med AB and Q-Med Scandinavia Inc. (n/k/a Oceana Therapeutics, Inc.) | 10-K | 02/28/12 | 10.89 | ||||||||||||
| 10.43* | Supply Agreement, dated as of June 2, 2009, by and between Q-Med AB and Cetacea Limited (n/k/a Oceana Therapeutics Limited) | 10-K | 02/28/12 | 10.90 | ||||||||||||
| 10.44* | Manufacturing and Supply Agreement, dated as of October 20, 2010, by and between Oceana Therapeutics Limited and Bio Hospital AB | 10-K | 02/28/12 | 10.91 | ||||||||||||
| 10.45 | Form of Letter Agreements relating to the Base Convertible Bond Hedge Transactions dated March 13, 2012 | 8-K | 03/16/12 | 10.92 | ||||||||||||
| 10.46 | Form of Letter Agreements relating to the Base Issuer Warrant Transactions dated March 13, 2012 | 8-K | 03/16/12 | 10.93 | ||||||||||||
| 10.47 | Note Repurchase Agreement dated March 12, 2012 by and between Salix Pharmaceuticals, Ltd. and 14159, L.P., 667, L.P. and Baker Brothers Life Sciences, L.P. | 8-K | 03/16/12 | 10.94 | ||||||||||||
| 10.48* | Amended and Restated License Agreement, dated August 6, 2012, by and between Alfa Wassermann S.p.A. and Salix Pharmaceuticals, Inc. | 10-Q | 11/08/12 | 10.95 | ||||||||||||
| 10.49* | EIR Supply Agreement, dated August 6, 2012, by and between Alfa Wassermann S.p.A. and Salix Pharmaceuticals, Inc. | 10-Q | 11/08/12 | 10.96 | ||||||||||||
| 10.50.1* | Amendment Number Two to Supply Agreement, dated August 6, 2012, by and between Alfa Wassermann S.p.A. and Salix Pharmaceuticals, Inc. | 10-Q | 11/08/12 | 10.97 | ||||||||||||
| 10.51* | Trademark License Agreement (Alfa to Salix), dated August 6, 2012, by and between Alfa Wassermann Hungary Kft. and Salix Pharmaceuticals, Inc. | 10-Q | 11/08/12 | 10.98 | ||||||||||||
| 10.52* | Trademark License Agreement (Salix to Alfa), dated August 6, 2012, by and between Alfa Wassermann S.p.A. and Salix Pharmaceuticals, Inc. | 10-Q | 11/08/12 | 10.99 | ||||||||||||
| 10.53* | Letter Amendment, dated September 5, 2012, by and between Alfa Wassermann S.p.A. and Salix Pharmaceuticals, Inc. | 10-Q | 11/08/12 | 10.100 | ||||||||||||
| 10.54** | Exclusive License Agreement, dated January 26, 2001, between Santarus, Inc. and The Curators of the University of Missouri | 10-K | 02/28/14 | 10.54 | ||||||||||||
| 10.54.1** | Amendment No. 1 to Exclusive License Agreement, dated February 21, 2003, between Santarus, Inc. and The Curators of the University of Missouri | S-1 + | 12/23/03 | 10.3 | ||||||||||||
| 10.54.2** | Amendment No. 2 to Exclusive License Agreement, dated August 20, 2007, between Santarus, Inc. and The Curators of the University of Missouri | 10-Q + | 11/02/07 | 10.1 | ||||||||||||
89
Table of Contents
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed | |||||||||||
| 10.55* | Omeprazole Supply Agreement, dated September 25, 2003, among Santarus, Inc., InterChem Trading Corporation and Union Quimico Farmaceutica, S.A. | S-1 + | 12/23/03 | 10.5 | ||||||||||||
| 10.55.1* | Amendment No. 1 to Omeprazole Supply Agreement, dated November 1, 2004, among Santarus, Inc., InterChem Trading Corporation and Union Quimico Farmaceutica, S.A. | 10-Q + | 08/6/07 | 10.4 | ||||||||||||
| 10.55.2* | Amendment No. 2 to Omeprazole Supply Agreement, dated July 11, 2007, among Santarus, Inc., InterChem Trading Corporation and Union Quimico Farmaceutica, S.A. | 10-Q + | 08/6/07 | 10.5 | ||||||||||||
| 10.55.3* | Amendment No. 3 to Omeprazole Supply Agreement, dated December 17, 2008, among Santarus, Inc., InterChem Trading Corporation and Union Quimico Farmaceutica, S.A. | 10-K + | 03/06/09 | 10.8 | ||||||||||||
| 10.55.4* | Amendment No. 4 to Omeprazole Supply Agreement, dated October 30, 2009, among Santarus, Inc., InterChem Trading Corporation and Union Quimico Farmaceutica, S.A. | 10-K + | 03/04/10 | 10.9 | ||||||||||||
| 10.56* | Second Amended and Restated Manufacturing and Supply Agreement, dated October 20, 2009, between Santarus, Inc. and Patheon Inc. | 10-K + | 03/04/10 | 10.10 | ||||||||||||
| 10.57* | Manufacturing and Supply Agreement, dated September 27, 2004, between Santarus, Inc. and OSG Norwich Pharmaceuticals Inc. | 10-Q + | 11/12/04 | 10.4 | ||||||||||||
| 10.58* | OTC License Agreement, dated October 17, 2006, between Santarus, Inc. and Schering-Plough Healthcare Products, Inc. | 8-K+ | 10/18/06 | 10.1 | ||||||||||||
| 10.58.1* | Amendment No. 1 to OTC License Agreement, dated July 24, 2009, between Santarus, Inc. and Schering-Plough Healthcare Products, Inc. | 10-Q+ | 11/05/09 | 10.1 | ||||||||||||
| 10.58.2 | Amendment No. 2 to OTC License Agreement, dated August 6, 2010, between Santarus, Inc. and Schering-Plough Healthcare Products, Inc. | 10-Q+ | 11/09/10 | 10.2 | ||||||||||||
| 10.58.3* | Amendment No. 3 to OTC License Agreement, dated April 1, 2011, between Santarus, Inc. and Schering-Plough Healthcare Products, Inc. | 10-Q+ | 05/05/11 | 10.3 | ||||||||||||
| 10.58.4 | Amendment No. 4 to OTC License Agreement, dated September 23, 2011, between Santarus, Inc. and MSD Consumer Care, Inc. (formerly known as Schering-Plough Healthcare Products, Inc.) | 10-Q+ | 11/07/11 | 10.3 | ||||||||||||
| 10.59 | License Agreement, dated November 30, 2007, between Santarus, Inc. and Glaxo Group Limited | 10-K | 02/28/14 | 10.59 | ||||||||||||
| 10.60* | License Agreement, dated December 10, 2008, between Santarus, Inc. and Cosmo Technologies Limited | 10-K+ | 03/06/09 | 10.25 | ||||||||||||
| 10.60.1 | Agreement, dated November 7, 2013, between Santarus, Inc., Salix Pharmaceuticals, Ltd., and Cosmo Technologies Limited | 14D-9+ | 12/03/13 | 99.E.26 | ||||||||||||
90
Table of Contents
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed | |||||||||||
| 10.61* | Promotion Agreement, dated July 21, 2008, between Santarus, Inc. and Depomed, Inc. | 10-Q+ | 08/05/08 | 10.2 | ||||||||||||
| 10.62* | Amended and Restated Distribution and Supply Agreement, dated November 30, 2012, between Santarus, Inc. and Prasco, LLC | 10-K+ | 03/04/13 | 10.35 | ||||||||||||
| 10.63* | Distribution and License Agreement, dated September 3, 2010, among Santarus, Inc., VeroScience, LLC and S2 Therapeutics, Inc. | 10-Q/A+ | 03/08/11 | 10.3 | ||||||||||||
| 10.63.1* | First Amendment to Distribution and License Agreement, dated March 10, 2011, among Santarus, Inc., VeroScience, LLC and S2 Therapeutics, Inc. | 10-Q+ | 05/05/11 | 10.2 | ||||||||||||
| 10.64* | Manufacturing Services Agreement, dated May 26, 2010, between Patheon Pharmaceuticals Inc. and S2 Therapeutics, Inc. | 10-Q/A+ | 03/08/11 | 10.4 | ||||||||||||
| 10.65 | Assignment and Assumption Agreement, dated September 3, 2010, between Santarus, Inc. and S2 Therapeutics, Inc. | 10-Q+ | 11/09/10 | 10.5 | ||||||||||||
| 10.66* | License Agreement, dated September 10, 2010, among Santarus, Inc., Pharming Group N.V., on behalf of itself and each of its affiliates, including Pharming Intellectual Property B.V. and Pharming Technologies B.V. | 10-Q/A+ | 03/08/11 | 10.6 | ||||||||||||
| 10.66.1 | Amendment to License Agreement, dated June 18, 2012, among Santarus, Inc., Pharming Group N.V., on behalf of itself and each of its affiliates, including Pharming Intellectual Property B.V. and Pharming Technologies B.V. | 10-Q+ | 08/07/12 | 10.2 | ||||||||||||
| 10.66.2 | Second Amendment to License Agreement, dated May 1, 2013, among Santarus, Inc., Pharming Group N.V., on behalf of itself and each of its affiliates, including Pharming Intellectual Property B.V. and Pharming Technologies B.V. | 10-Q+ | 08/06/13 | 10.1 | ||||||||||||
| 10.67* | Supply Agreement, dated September 10, 2010, among Santarus, Inc., Pharming Group N.V., on behalf of itself and each of its affiliates, including Pharming Intellectual Property B.V. and Pharming Technologies B.V. | 10-Q+ | 11/09/10 | 10.7 | ||||||||||||
| 10.68* | License Agreement, dated January 22, 2009, between Covella Pharmaceuticals, Inc. and Biogen Idec MA Inc. | 10-Q/A+ | 03/08/11 | 10.8 | ||||||||||||
| 10.69* | Amendment to License Agreement, dated September 10, 2010, among Santarus, Inc., Covella Pharmaceuticals, Inc. and Biogen Idec MA Inc. | 10-Q/A+ | 03/08/11 | 10.9 | ||||||||||||
| 10.70* | Amended and Restated Services and Supply Agreement, dated September 10, 2010, among Santarus, Inc., Covella Pharmaceuticals, Inc. and Biogen Idec MA Inc. | 10-K+ | 03/05/12 | 10.44 | ||||||||||||
| 10.70.1 | First Amendment to Amended and Restated Services and Supply Agreement, dated November 4, 2011, among Santarus, Inc., Covella Pharmaceuticals, Inc. and Biogen Idec MA Inc. | 10-K+ | 03/05/12 | 10.45 | ||||||||||||
| 10.70.2* | Second Amendment to Amended and Restated Services and Supply Agreement, dated December 17, 2012, among Santarus, Inc., Covella Pharmaceuticals, Inc. and Biogen Idec MA Inc. | 10-K+ | 03/04/13 | 10.47 | ||||||||||||
91
Table of Contents
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed | |||||||||||
| 10.71* | Third Amendment to Amended and Restated Services and Supply Agreement, dated June 4, 2013, among Santarus, Inc., Covella Pharmaceuticals, Inc. and Biogen Idec MA Inc. | 10-Q+ | 08/06/13 | 10.2 | ||||||||||||
| 10.72* | Commercialization Agreement, dated August 22, 2011, between Santarus, Inc. and Depomed, Inc. | 10-Q+ | 11/07/11 | 10.2 | ||||||||||||
| 10.73* | Commercial Manufacturing Agreement, dated December 19, 2006, between Patheon Puerto Rico, Inc. (f/k/a MOVA Pharmaceutical Corporation) and Depomed, Inc. | 10-K+ | 03/05/12 | 10.47 | ||||||||||||
| 10.74 | Assignment and Assumption Agreement, dated November 3, 2011, between Santarus, Inc. and Depomed, Inc. | 10-K+ | 03/05/12 | 10.48 | ||||||||||||
| 10.75* | License Agreement, dated December 21, 2011, among Santarus, Inc., Cowen Healthcare Royalty Partners, L.P. and Shore Therapeutics, Inc. | 10-K+ | 03/05/12 | 10.49 | ||||||||||||
| 10.76* | Manufacturing and Supply Agreement, dated May 18, 2012, between Santarus, Inc. and Cosmo Technologies Limited | 10-Q+ | 08/07/12 | 10.1 | ||||||||||||
| 10.77 | Sublease, dated December 11, 2007, between Santarus, Inc. and Avnet, Inc. | 8-K+ | 12/13/07 | 10.1 | ||||||||||||
| 10.78 | First Amendment to Sublease, dated August 5, 2011, between Santarus, Inc. and Avnet, Inc. | 10-Q+ | 11/07/11 | 10.1 | ||||||||||||
| 10.79 | Kilroy Center Del Mar Office Lease, dated October 5, 2012, between Santarus, Inc. and Kilroy Realty, L.P. | 8-K+ | 10/10/12 | 10.1 | ||||||||||||
| 10.80 | Form of Indemnification Agreement between Santarus, Inc. and each of its directors and officers | S-1/A+ | 02/04/04 | 10.10 | ||||||||||||
| 10.81 | Executive Non-Qualified “Excess” Plan Adoption Agreement | 10-Q+ | 08/07/12 | 10.3 | ||||||||||||
| 10.82 | 2013 Bonus Plan | 8-K+ | 02/22/13 | 10.1 | ||||||||||||
| 10.83 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Gerald T. Proehl | 10-Q+ | 05/06/13 | 10.1 | ||||||||||||
| 10.84 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Debra P. Crawford | 10-Q+ | 05/06/13 | 10.2 | ||||||||||||
| 10.85 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and E. David Ballard, II, M.D. | 10-Q+ | 05/06/13 | 10.3 | ||||||||||||
| 10.86 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Maria Bedoya-Toro | 10-Q+ | 05/06/13 | 10.4 | ||||||||||||
| 10.87 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Julie A. DeMeules | 10-Q+ | 05/06/13 | 10.5 | ||||||||||||
92
Table of Contents
| Exhibit |
Description of Document |
Form | Dated | Exhibit Number |
Filed |
|||||||||||||
| 10.88 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and William C. Denby, III | 10-Q+ | 05/06/13 | 10.6 | ||||||||||||||
| 10.89 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Carey J. Fox | 10-Q+ | 05/06/13 | 10.7 | ||||||||||||||
| 10.90 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Warren E. Hall | 10-Q+ | 05/06/13 | 10.8 | ||||||||||||||
| 10.91 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Michael D. Step | 10-Q+ | 05/06/13 | 10.9 | ||||||||||||||
| 10.92 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Mark Totoritis | 10-Q+ | 05/06/13 | 10.10 | ||||||||||||||
| 10.93 | Amended and Restated Employment Agreement, dated March 22, 2013, between Santarus, Inc. and Wendell Wierenga, Ph.D. | 10-Q+ | 05/06/13 | 10.11 | ||||||||||||||
| 10.94 | Memorandum Regarding Amendment to Amended and Restated Employment Agreement, dated November 4, 2013, from Gerald T. Proehl to Executive Officers of Santarus, Inc. | 14D-9+ | 12/03/13 | 99.E.25 | ||||||||||||||
| 21.1 | Subsidiaries of the Registrant. | X | ||||||||||||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm. | X | ||||||||||||||||
| 31.1 | Certification by the Acting Chief Executive Officer pursuant to Section 240.13a-14 or Section 240.15d-14 of the Securities Exchange Act of 1934, as amended. | X | ||||||||||||||||
| 31.2 | Certification by the Acting Chief Financial Officer pursuant to Section 240.13a-14 or Section 240.15d-14 of the Securities Exchange Act of 1934, as amended. | X | ||||||||||||||||
| 32.1 | Certification by the Acting Chief Executive Officer pursuant to 18 U.S.C. 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X | ||||||||||||||||
| 32.2 | Certification by the Acting Chief Financial Officer pursuant to 18 U.S.C. 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X | ||||||||||||||||
| 101 | Financials in XBRL format | X | ||||||||||||||||
| * | Salix Pharmaceuticals, Ltd. or Santarus, Inc., as applicable, has received confidential treatment with respect to portions of this exhibit. Those portions have been omitted from this exhibit and filed separately with the U.S. Securities and Exchange Commission. |
| ** | Salix Pharmaceuticals, Ltd. or Santarus, Inc., as applicable, has requested confidential treatment with respect to portions of this exhibit. Those portions have been omitted from the exhibit and filed separately with the U.S. Securities and Exchange Commission. |
| + | This filing was made by Santarus, prior to the acquisition. |
(b) Exhibits
See Item 15(a)(3) above.
(c) Financial Statement Schedules
See Item 15(a)(1) above.
93
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Form 10-K/A to be signed on its behalf by the undersigned, thereunto duly authorized.
| SALIX PHARMACEUTICALS, LTD. | ||
| /s/ THOMAS W. D’ALONZO | ||
| Thomas W. D’Alonzo | ||
| Acting Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Form 10-K/A has been signed below by the following persons on behalf of the Registrant and on the dates indicated.
| Date: March 2, 2015 |
/s/ THOMAS W. D’ALONZO | |||
| Thomas W. D’Alonzo, Acting Chief Executive Officer | ||||
| and Chairman of the Board (Principal Executive Officer) | ||||
| Date: March 2, 2015 |
/s/ TIMOTHY J. CREECH | |||
| Timothy J. Creech, | ||||
| Senior Vice President, Finance & Administration and Acting Chief Financial Officer | ||||
| (Principal Financial and Accounting Officer) | ||||
| Date: March 2, 2015 |
/s/ JOHN F. CHAPPELL | |||
| John F. Chappell | ||||
| Director | ||||
| Date: March 2, 2015 |
/s/ WILLIAM P. KEANE | |||
| William P. Keane | ||||
| Director | ||||
| Date: March 2, 2015 |
/s/ MARK A. SIRGO | |||
| Mark A. Sirgo | ||||
| Director | ||||
94
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Index to Consolidated Financial Statements
| PAGE | ||||
| AUDITED CONSOLIDATED FINANCIAL STATEMENTS |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| Consolidated Statements of Comprehensive Income (as restated) |
F-5 | |||
| Consolidated Statements of Stockholders’ Equity (as restated) |
F-6 | |||
| F-7 | ||||
| F-8 | ||||
| CONSOLIDATED FINANCIAL STATEMENT SCHEDULE |
||||
| F-44 | ||||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Salix Pharmaceuticals, Ltd.
We have audited Salix Pharmaceuticals, Ltd.’s internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework) (the COSO criteria). Salix Pharmaceuticals, Ltd.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting appearing in Item 9A. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in management’s assessment. Management has identified material weaknesses in controls related to product returns and communications between trade relations and accounting/finance to record agreed-upon returns by trade personnel; controls for the recognition of revenue for sales to customers with FOB Destination shipping terms; controls to comply with established policies and procedures to obtain, evaluate, review, and approve agreements with customers; and controls around the classification of balances within the consolidated financial statements. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Salix Pharmaceuticals, Ltd. as of December 31, 2013 and 2012, and the related consolidated statements of comprehensive income, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2013 of Salix Pharmaceuticals, Ltd. and our report dated March 2, 2015 expressed an unqualified opinion thereon. These material weaknesses were considered in determining the nature, timing and extent of audit tests applied in our audit of the 2013 financial statements, and this report does not affect our report dated March 2, 2015, which expressed an unqualified opinion on those financial statements.
In our opinion, because of the effect of the material weaknesses described above on the achievement of the objectives of the control criteria, Salix Pharmaceuticals, Ltd. has not maintained effective internal control over financial reporting as of December 31, 2013, based on the COSO criteria.
/s/ Ernst & Young LLP
Raleigh, North Carolina
March 2, 2015
F-2
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Salix Pharmaceuticals, Ltd.
We have audited the accompanying consolidated balance sheets of Salix Pharmaceuticals, Ltd. as of December 31, 2013 and 2012, and the related consolidated statements of comprehensive income, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2013. Our audits also included the financial statement schedule listed in the Index at Item 15(a)(2). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Salix Pharmaceuticals, Ltd. at December 31, 2013 and 2012, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2013, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Salix Pharmaceuticals, Ltd.’s internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework) and our report dated March 2, 2015 expressed an adverse opinion thereon.
As discussed in Note 1A to the consolidated financial statements, the 2013 financial statements and schedule have been restated to correct previously reported amounts.
/s/ Ernst & Young LLP
Raleigh, North Carolina
March 2, 2015
F-3
Table of Contents
SALIX PHARMACEUTICALS, LTD.
(U.S. dollars, in thousands, except share and per share amounts)
| December 31, | ||||||||
| 2013 (Restated) |
2012 | |||||||
| A S S E T S |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 1,157,850 | $ | 751,006 | ||||
| Restricted cash |
750,000 | — | ||||||
| Accounts receivable, net |
133,581 | 268,239 | ||||||
| Inventory |
104,209 | 90,533 | ||||||
| Deferred tax assets |
85,788 | 57,050 | ||||||
| Prepaid expenses and other current assets |
51,241 | 21,753 | ||||||
|
|
|
|
|
|||||
| Total current assets |
2,282,669 | 1,188,581 | ||||||
| Property and equipment, net |
27,312 | 27,878 | ||||||
| Goodwill |
180,909 | 180,905 | ||||||
| Product rights and intangibles, net |
397,510 | 441,506 | ||||||
| Other assets |
37,551 | 35,914 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 2,925,951 | $ | 1,874,784 | ||||
|
|
|
|
|
|||||
|
L I A B I L I T I E S A N D S T O C K H O L D E R S’ E Q U I T Y |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 32,632 | $ | 31,110 | ||||
| Accrued liabilities |
96,909 | 81,062 | ||||||
| Income taxes payable |
34,820 | 8,507 | ||||||
| Reserve for product returns, rebates, chargebacks and patient-focused promotional programs |
252,829 | 140,191 | ||||||
| Current portion of capital lease obligations |
47 | 50 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
417,237 | 260,920 | ||||||
| Long-term liabilities: |
||||||||
| Convertible senior notes |
882,050 | 857,209 | ||||||
| Lease incentive obligation |
8,610 | 7,554 | ||||||
| Senior notes |
750,000 | — | ||||||
| Acquisition-related contingent consideration |
87,300 | 103,500 | ||||||
| Deferred tax liabilities |
42,446 | 64,255 | ||||||
| Other long-term liabilities |
9,665 | 20,845 | ||||||
|
|
|
|
|
|||||
| Total long-term liabilities |
1,780,071 | 1,053,363 | ||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.001 par value; 5,000,000 shares authorized, issuable in series, none outstanding |
— | — | ||||||
| Common stock, $0.001 par value; 150,000,000 shares authorized, 62,937,966 and 60,918,391 shares issued and outstanding at December 31, 2013 and 2012, respectively |
63 | 61 | ||||||
| Additional paid-in-capital |
667,428 | 631,364 | ||||||
| Accumulated other comprehensive income |
1,721 | 456 | ||||||
| Retained earnings (deficit) |
59,431 | (71,380 | ) | |||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
728,643 | 560,501 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 2,925,951 | $ | 1,874,784 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Consolidated Statements of Comprehensive Income
(U.S. dollars, in thousands, except per share data)
| Year Ended December 31, | ||||||||||||
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Revenues: |
||||||||||||
| Net product revenues |
$ | 913,781 | $ | 735,444 | $ | 540,488 | ||||||
|
|
|
|
|
|
|
|||||||
| Costs and expenses: |
||||||||||||
| Cost of products sold (excluding $44,744, $45,351 and $10,908 in amortization of product rights and intangible assets for the years ended December 31, 2013, 2012 and 2011, respectively) |
177,776 | 124,597 | 95,369 | |||||||||
| Amortization of product rights and intangible assets |
44,744 | 45,351 | 10,908 | |||||||||
| Intangible impairment charges |
— | 41,600 | — | |||||||||
| Research and development (as revised) |
112,791 | 84,963 | 71,861 | |||||||||
| Selling, general and administrative (as revised) |
342,359 | 296,458 | 219,477 | |||||||||
| Change in acquisition-related contingent consideration |
(16,200 | ) | (29,598 | ) | 27,000 | |||||||
|
|
|
|
|
|
|
|||||||
| Total costs and expenses |
661,470 | 563,371 | 424,615 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income from operations |
252,311 | 172,073 | 115,873 | |||||||||
| Loss on extinguishment of debt |
— | (15,580 | ) | — | ||||||||
| Interest expense |
(62,026 | ) | (55,518 | ) | (32,121 | ) | ||||||
| Interest and other income |
2,003 | 10,853 | 2,349 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income before provision for income tax |
192,288 | 111,828 | 86,101 | |||||||||
| Income tax (expense) benefit |
(61,477 | ) | (47,582 | ) | 1,298 | |||||||
|
|
|
|
|
|
|
|||||||
| Net income |
$ | 130,811 | $ | 64,246 | $ | 87,399 | ||||||
|
|
|
|
|
|
|
|||||||
| Net income per share, basic |
$ | 2.12 | $ | 1.09 | $ | 1.49 | ||||||
|
|
|
|
|
|
|
|||||||
| Net income per share, diluted |
$ | 1.99 | $ | 1.01 | $ | 1.44 | ||||||
|
|
|
|
|
|
|
|||||||
| Shares used in computing net income per share, basic |
61,792 | 58,747 | 58,718 | |||||||||
|
|
|
|
|
|
|
|||||||
| Shares used in computing net income per share, diluted |
65,692 | 63,699 | 65,483 | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive income |
$ | 132,076 | $ | 64,813 | $ | 87,288 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Consolidated Statements of Stockholders’ Equity
(U.S. dollars, in thousands, except share amounts)
| Common Stock | Additional Paid-in- capital |
Accumulated Other Comprehensive Income (Loss) |
Retained Earnings (Deficit) |
Total Stockholders’ Equity |
||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Balance at December 31, 2010 |
58,139,941 | $ | 58 | $ | 624,886 | $ | — | $ | (223,025 | ) | $ | 401,919 | ||||||||||||
| Issuance of common stock upon exercise of stock options |
370,874 | — | 5,396 | — | — | 5,396 | ||||||||||||||||||
| Payments related to net settlement of stock-based awards |
— | — | (3,030 | ) | — | — | (3,030 | ) | ||||||||||||||||
| Issuance of common stock upon vesting of restricted stock |
694,444 | 1 | (1 | ) | — | — | — | |||||||||||||||||
| Income tax benefit from non-qualified stock option exercises |
— | — | 42,272 | — | — | 42,272 | ||||||||||||||||||
| Foreign translation adjustments |
— | — | — | (111 | ) | — | (111 | ) | ||||||||||||||||
| Compensation expense related to restricted stock awards |
— | — | 15,792 | — | — | 15,792 | ||||||||||||||||||
| Net income |
— | — | — | 87,399 | 87,399 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2011 |
59,205,259 | 59 | 685,315 | (111 | ) | (135,626 | ) | 549,637 | ||||||||||||||||
| Issuance of common stock upon exercise of stock options |
850,204 | 1 | 8,531 | — | — | 8,532 | ||||||||||||||||||
| Payments related to net settlement of stock-based awards |
— | — | (2,926 | ) | — | — | (2,926 | ) | ||||||||||||||||
| Issuance of common stock upon vesting of restricted stock |
536,981 | — | — | — | — | — | ||||||||||||||||||
| Income tax benefit from non-qualified stock option exercises |
— | — | 10,971 | — | — | 10,971 | ||||||||||||||||||
| Foreign translation adjustments |
— | — | — | 567 | — | 567 | ||||||||||||||||||
| Issuance of convertible debt, net of tax |
— | — | 92,153 | — | — | 92,153 | ||||||||||||||||||
| Sale of warrants |
— | — | 98,994 | — | — | 98,994 | ||||||||||||||||||
| Purchase of convertible note hedge, net of tax |
(98,702 | ) | (98,702 | ) | ||||||||||||||||||||
| Repurchase of common stock |
(1,534,800 | ) | (1 | ) | (74,821 | ) | — | — | (74,822 | ) | ||||||||||||||
| Compensation expense related to restricted stock awards |
— | — | 21,202 | — | — | 21,202 | ||||||||||||||||||
| Extinguishment of 2028 Notes |
— | — | (184,507 | ) | — | — | (184,507 | ) | ||||||||||||||||
| Issuance of common stock upon conversion of 2028 Notes |
1,860,747 | 2 | 75,154 | — | — | 75,156 | ||||||||||||||||||
| Net income |
— | — | — | — | 64,246 | 64,246 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2012 |
60,918,391 | 61 | 631,364 | 456 | (71,380 | ) | 560,501 | |||||||||||||||||
| Issuance of common stock upon exercise of stock options |
263,754 | — | 4,218 | — | — | 4,218 | ||||||||||||||||||
| Payments related to net settlement of stock-based awards |
— | — | (4,037 | ) | — | — | (4,037 | ) | ||||||||||||||||
| Issuance of common stock upon vesting of restricted stock |
585,238 | 1 | (1 | ) | — | — | — | |||||||||||||||||
| Income tax benefit from non-qualified stock option exercises |
— | — | 9,731 | — | — | 9,731 | ||||||||||||||||||
| Foreign translation adjustments |
— | — | — | 1,265 | — | 1,265 | ||||||||||||||||||
| Compensation expense related to restricted stock awards |
— | — | 26,154 | — | — | 26,154 | ||||||||||||||||||
| Issuance of common stock upon conversion of 2028 Notes |
1,170,583 | 1 | (1 | ) | — | — | — | |||||||||||||||||
| Net income (restated) |
— | — | — | — | 130,811 | 130,811 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2013(restated) |
62,937,966 | $ | 63 | $ | 667,428 | $ | 1,721 | $ | 59,431 | $ | 728,643 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Other comprehensive income is composed entirely of adjustments resulting from the translation of the financial statements of the Company’s foreign subsidiary into U.S. dollars.
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Consolidated Statements of Cash Flows
(U.S. dollars, in thousands)
| Year Ended December 31, | ||||||||||||
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Cash Flows from Operating Activities |
||||||||||||
| Net income |
$ | 130,811 | $ | 64,246 | $ | 87,399 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||||||
| Loss on disposal of property and equipment |
113 | 233 | 534 | |||||||||
| Depreciation and amortization |
52,133 | 52,150 | 15,247 | |||||||||
| Intangible impairment charge |
— | 41,600 | — | |||||||||
| Amortization of debt discount |
37,341 | 31,655 | 17,278 | |||||||||
| Loss on extinguishment of debt |
— | 12,842 | — | |||||||||
| Gain on adjustment of put option to fair market value |
— | (9,325 | ) | — | ||||||||
| Stock-based compensation expense |
26,154 | 21,202 | 15,792 | |||||||||
| Deferred income taxes |
(50,547 | ) | (7,460 | ) | (76,239 | ) | ||||||
| Change in acquisition-related contingent consideration |
(16,200 | ) | (29,598 | ) | 27,000 | |||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable, inventory, prepaid expenses and other assets |
89,857 | (154,696 | ) | (60,427 | ) | |||||||
| Accounts payable, accrued and other liabilities |
33,555 | 22,431 | 55,327 | |||||||||
| Reserve for product returns, rebates, chargebacks and patient-focused promotional programs |
112,638 | 42,312 | 37,239 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by operating activities |
415,855 | 87,592 | 119,150 | |||||||||
| Cash Flows from Investing Activities |
||||||||||||
| Purchases of property and equipment |
(6,936 | ) | (5,370 | ) | (26,139 | ) | ||||||
| Business acquisitions, net of cash acquired |
— | (10,000 | ) | (362,482 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used by investing activities |
(6,936 | ) | (15,370 | ) | (388,621 | ) | ||||||
| Cash Flows from Financing Activities |
||||||||||||
| Proceeds from convertible senior note offering |
— | 690,000 | — | |||||||||
| Debt issuance costs |
— | (21,159 | ) | — | ||||||||
| Purchase of call options |
— | (166,980 | ) | — | ||||||||
| Proceeds from sale of warrants |
— | 98,994 | — | |||||||||
| Repurchase of common stock |
— | (74,822 | ) | — | ||||||||
| Extinguishment of 2028 convertible senior notes |
(12,500 | ) | (156,851 | ) | — | |||||||
| Principal payments on capital lease obligations |
— | (138 | ) | (272 | ) | |||||||
| Excess tax benefit from stock-based compensation |
9,731 | 10,971 | 42,272 | |||||||||
| Payments related to net settlement of stock-based awards |
(4,037 | ) | (2,926 | ) | (3,030 | ) | ||||||
| Proceeds from issuance of common stock upon exercise of stock awards |
4,218 | 8,532 | 5,396 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash (used) provided by financing activities |
(2,588 | ) | 385,621 | 44,366 | ||||||||
| Effect of exchange rate changes on cash |
513 | 349 | (111 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
406,844 | 458,192 | (225,216 | ) | ||||||||
| Cash and cash equivalents at beginning of year |
751,006 | 292,814 | 518,030 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of year |
$ | 1,157,850 | $ | 751,006 | $ | 292,814 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental Disclosure of Cash Flow Information |
||||||||||||
| Cash paid for income taxes |
$ | 87,311 | $ | 33,811 | $ | 14,855 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash paid for interest |
$ | 20,525 | $ | 17,239 | $ | 12,788 | ||||||
|
|
|
|
|
|
|
|||||||
| Acquisition-related contingent consideration |
$ | 87,300 | $ | 103,500 | $ | 119,698 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements
December 31, 2013
(1A) RESTATEMENT OF PRIOR PERIOD FINANCIAL STATEMENTS
The Company has restated its previously reported consolidated financial statements for the year ended December 31, 2013 in order to correct certain previously reported amounts.
During the first quarter of 2015, the Company determined that it had incorrectly recognized gross revenue related to sales with FOB Destination shipping terms of approximately $14.4 million in the fiscal quarter ended December 31, 2013 that should have been recognized in the fiscal quarter ended March 31, 2014 based upon a delivery in early January 2014. This cutoff error impacted the fourth quarter and year ended December 31, 2013 consolidated financial statements. This gross revenue was offset by related adjustments to reserves for returns, discounts and allowances of approximately $2.5 million. There were also net adjustments required to cost of sales and royalties that decreased cost of sales by $1.6 million. In addition, the Company incorrectly recognized a reserve of only $8.7 million for product returns at December 31, 2013 for certain out-of-policy returns of GIAZO® when it should have recognized a reserve of approximately $16.9 million, resulting in an understatement of the reserve balance of approximately $8.2 million in the fourth quarter 2013. The Company had agreed to accept such returns in the fourth quarter of 2013. This correction resulted in an approximately $8.2 million reduction of net revenue in the fourth quarter of 2013 and a corresponding increase of approximately $8.2 million in net revenue for the first quarter of 2014, as the returns were originally recorded in the first quarter of 2014. The Company also recorded an increase of $1.1 million for Research and Development (“R&D”) expenses in the fiscal quarter ended December 31, 2013 related to holding fees that were erroneously capitalized as inventory for a new product. On a combined basis these adjustments resulted in a reduction of income tax expense of approximately $7.6 million for the year ended December 31, 2013.
In addition, the Company incorrectly classified certain expenses as related to R&D instead of Selling, General & Administrative (SG&A) expenses. These errors were largely a result of the Company’s historical practice of aligning certain expenses along organizational lines rather than business activity. As a result, the Company incorrectly recorded expenses of approximately $38.2 million, $38.3 million and $32.5 million as R&D that should have been recognized as SG&A for the fiscal years ended December 31, 2013, 2012 and 2011, respectively. The foregoing reclassifications do not impact previously reported revenue or net income (loss).
The following table sets forth the corrections to each of the individual line items affected in the consolidated financial statements for the year ended December 31, 2013.
F-8
Table of Contents
Consolidated Balance Sheet
(U.S. dollars, in thousands, except share amounts)
| At December 31, 2013 | ||||||||||||
| As previously reported |
Error correction |
Restated | ||||||||||
| ASSETS |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 1,157,850 | $ | 1,157,850 | ||||||||
| Restricted cash |
750,000 | 750,000 | ||||||||||
| Accounts receivable, net |
147,933 | (14,352 | )(a) | 133,581 | ||||||||
| Inventory |
104,395 | (186 | )(b) | 104,209 | ||||||||
| Deferred tax assets |
86,693 | (905 | )(c) | 85,788 | ||||||||
| Prepaid expenses and other current assets |
51,241 | 51,241 | ||||||||||
|
|
|
|
|
|||||||||
| Total current assets |
2,298,112 | 2,282,669 | ||||||||||
| Property and equipment, net |
27,312 | 27,312 | ||||||||||
| Goodwill |
180,909 | 180,909 | ||||||||||
| Product rights and intangibles, net |
397,510 | 397,510 | ||||||||||
| Other assets |
37,551 | 37,551 | ||||||||||
|
|
|
|
|
|||||||||
| Total assets |
$ | 2,941,394 | $ | 2,925,951 | ||||||||
|
|
|
|
|
|||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 32,632 | $ | 32,632 | ||||||||
| Accrued liabilities |
97,661 | (752 | )(d) | 96,909 | ||||||||
| Income taxes payable |
43,354 | (8,534 | )(e) | 34,820 | ||||||||
| Reserve for product returns, rebates, chargebacks and patient-focused promotional programs |
246,838 | 5,991 | (f) | 252,829 | ||||||||
| Current portion of capital lease obligations |
47 | 47 | ||||||||||
|
|
|
|
|
|||||||||
| Total current liabilities |
420,532 | 417,237 | ||||||||||
| Long-term liabilities: |
||||||||||||
| Convertible senior notes |
882,050 | 882,050 | ||||||||||
| Lease incentive obligation |
8,610 | 8,610 | ||||||||||
| Senior notes |
750,000 | 750,000 | ||||||||||
| Acquisition-related contingent consideration |
87,300 | 87,300 | ||||||||||
| Deferred tax liabilities |
42,371 | 75 | (g) | 42,446 | ||||||||
| Other long-term liabilities |
9,665 | 9,665 | ||||||||||
|
|
|
|
|
|||||||||
| Total long-term liabilities |
1,779,996 | 1,780,071 | ||||||||||
| Stockholders’ equity: |
||||||||||||
| Preferred stock, $0.001 par value; 5,000,000 shares authorized, issuable in series, none outstanding |
— | — | ||||||||||
| Common stock, $0.001 par value; 150,000,000 shares authorized, 62,937,966 and 60,918,391 shares issued and outstanding at December 31, 2013 and 2012, respectively |
63 | 63 | ||||||||||
| Additional paid-in-capital |
667,428 | 667,428 | ||||||||||
| Accumulated other comprehensive income |
1,721 | 1,721 | ||||||||||
| Retained earnings (deficit) |
71,654 | (12,223 | )(h) | 59,431 | ||||||||
|
|
|
|
|
|||||||||
| Total stockholders’ equity |
740,866 | 728,643 | ||||||||||
|
|
|
|
|
|||||||||
| Total liabilities and stockholders’ equity |
$ | 2,941,394 | $ | 2,925,951 | ||||||||
|
|
|
|
|
|||||||||
| (a) | Adjustment to accounts receivable for incorrectly recognized revenue in the fiscal quarter ended December 31, 2013. |
| (b) | Net effect of increase in inventory for incorrectly recognized revenue in the fiscal quarter ended December 31, 2013 offset by erroneously capitalized holding fees as inventory for a new product. |
| (c) | Net impact on deferred tax assets related to correction of errors. |
| (d) | Decrease in accrued liabilities (discounts and royalties) due to the reversal of incorrectly recognized revenue in the fiscal quarter ended December 31, 2013, impact of interest accrual, and a minor adjustment to selling, general, and administrative expenses. |
| (e) | Net impact on income taxes payable related to correction of errors. |
| (f) | Increase in reserve for product returns, rebates and chargebacks related to accrual for returns of GIAZO offset by a decrease resulting from the reversal of incorrectly recognized revenue in the fiscal quarter ended December 31, 2013. |
| (g) | Net impact on deferred tax liabilities related to correction of errors. |
| (h) | Net impact of correction of errors on retained earnings. |
F-9
Table of Contents
Consolidated Statement of Comprehensive Income
(U.S. dollars, in thousands, except per share data)
| Year ended December 31, 2013 | ||||||||||||
| As previously reported |
Error correction |
Restated | ||||||||||
| Revenues: |
||||||||||||
| Net product revenues |
$ | 933,838 | (20,057 | )(a) | $ | 913,781 | ||||||
|
|
|
|
|
|||||||||
| Costs and expenses: |
||||||||||||
| Cost of products sold (excluding $44,744, $45,351 and $10,908 in amortization of product rights and intangible assets for the years ended December 31, 2013, 2012 and 2011, respectively) |
179,392 | (1,616 | )(b) | 177,776 | ||||||||
| Amortization of product rights and intangible assets |
44,744 | 44,744 | ||||||||||
| Intangible impairment charges |
— | — | ||||||||||
| Research and development (as revised) |
149,974 | (37,183 | )(c) | 112,791 | ||||||||
| Selling, general and administrative (as revised) |
304,215 | 38,144 | (d) | 342,359 | ||||||||
| Change in acquisition-related contingent consideration |
(16,200 | ) | (16,200 | ) | ||||||||
|
|
|
|
|
|||||||||
| Total cost and expenses |
662,125 | (655 | )(e) | 661,470 | ||||||||
|
|
|
|
|
|||||||||
| Income from operations |
271,713 | (19,402 | )(f) | 252,311 | ||||||||
| Loss on extinguishment of debt |
— | — | ||||||||||
| Interest expense |
(61,651 | ) | (375 | )(g) | (62,026 | ) | ||||||
| Interest and other income |
2,003 | 2,003 | ||||||||||
|
|
|
|
|
|||||||||
| Income before provision for income tax |
212,065 | (19,777 | )(h) | 192,288 | ||||||||
| Income tax (expense) benefit |
(69,031 | ) | 7,554 | (i) | (61,477 | ) | ||||||
|
|
|
|
|
|||||||||
| Net income |
$ | 143,034 | (12,223 | )(j) | $ | 130,811 | ||||||
|
|
|
|
|
|||||||||
| Net income (loss) per share, basic |
$ | 2.31 | (0.19 | )(k) | $ | 2.12 | ||||||
|
|
|
|
|
|||||||||
| Net income (loss) per share, diluted |
$ | 2.18 | (0.19 | )(l) | $ | 1.99 | ||||||
|
|
|
|
|
|||||||||
| Shares used in computing net income (loss) per share, basic |
61,792 | 61,792 | ||||||||||
|
|
|
|
|
|||||||||
| Shares used in computing net income (loss) per share, diluted |
65,692 | 65,692 | ||||||||||
|
|
|
|
|
|||||||||
| Comprehensive income (loss) |
$ | 144,299 | (12,223 | ) | $ | 132,076 | ||||||
|
|
|
|
|
|||||||||
| (a) | Reduction in net product revenues for incorrectly recognized revenue of approximately $14,353 offset by a related reduction in reserves for returns, discounts and other allowances of approximately $2,474 and decrease in net product revenues for additional reserve required for Giazo product returns of approximately $8,178. |
| (b) | Reduction in cost of products sold related to cost of sales ($872) and royalties ($744) for reduction in net product revenues for incorrectly recognized revenue. |
| (c) | Reclassification of Research and Development (R&D) expenses of approximately $38,241 to Selling, General & Administrative (SG&A) expenses offset by an increase of approximately $1,058 in R&D related to holding fees erroneously capitalized as inventory for a new product. |
| (d) | Reclassification of R&D expenses of approximately $38,241 to SG&A expenses offset by a minor adjustment of approximately $97 related to incorrectly recognized revenue. |
| (e) | Net impact of adjustments to total costs and expenses. |
| (f) | Net impact of adjustments to income from operations. |
| (g) | Increase in interest expense due to failure to accrue interest on net proceeds from the Company’s issuance of $750.0 million of 6.00% senior notes due 2021 |
| (h) | Net impact of adjustments to income before provision for income tax. |
| (i) | Net impact of adjustments to income tax expense. |
| (j) | Net impact of adjustments to net income. |
| (k) | Net impact of adjustments to net income per share, basic. |
| (l) | Net impact of adjustments to net income per share, diluted. |
The following table presents the effects of the reclassifications made to the Company’s previously reported research and development (“R&D”) expenses and selling, general, and administrative (“SG&A”) expenses for the R&D expenses that should have been classified as SG&A expenses for the fiscal years ended December 31, 2012 and 2011:
| Year ended December 31 | ||||||||||||
| As previously reported |
Reclassifications | Revised | ||||||||||
| 2012 |
||||||||||||
| Research and development |
$ | 123,234 | $ | (38,271 | ) | $ | 84,963 | |||||
| Selling, general and administrative |
$ | 258,187 | $ | 38,271 | $ | 296,458 | ||||||
| 2011 |
||||||||||||
| Research and development |
$ | 104,350 | $ | (32,489 | ) | $ | 71,861 | |||||
| Selling, general and administrative |
$ | 186,988 | $ | 32,489 | $ | 219,477 | ||||||
The Company did not present tables for the adjustments within the consolidated statement of cash flows since all of the foregoing adjustments were within the operating activities section of the consolidated statement of cash flows. These adjustments did not affect total cash flows from operating activities, financing activities or investing activities for any period presented.
(1) ORGANIZATION AND BASIS OF PRESENTATION
Salix Pharmaceuticals, Ltd., a Delaware corporation (“Salix” or the “Company”), is a specialty pharmaceutical company dedicated to acquiring, developing and commercializing prescription drugs and medical devices used in the treatment of a variety of gastrointestinal diseases, which are those affecting the digestive tract.
F-10
Table of Contents
These consolidated financial statements are stated in U.S. dollars and are prepared under accounting principles generally accepted in the United States. The accompanying consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant inter-company balances and transactions have been eliminated in consolidation.
The accompanying consolidated financial statements include all adjustments that, in the opinion of management, are necessary for a fair presentation of financial position, results of operations, and cash flows.
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts of assets and liabilities reported at the date of the financial statements, the disclosure of contingent assets and liabilities, and the reported amounts of revenues and expenses recognized during the reporting periods. On an ongoing basis, the Company evaluates its estimates, including but not limited to those related to product returns, rebates, chargebacks, collectability of receivables, inventory, intangible assets, income taxes and contingencies and litigation. Actual results could differ materially from those estimates.
Revenue Recognition
The Company recognizes revenue when it is realized or realizable and earned. Revenue is realized or realizable and earned when all of the following criteria are met: (a) persuasive evidence of an arrangement exists; (b) delivery has occurred or services have been rendered; (c) the Company’s price to the buyer is fixed or determinable; and (d) collectability is reasonably assured.
The Company recognizes revenues for product sales at the time title and risk of loss are transferred to the customer, which is generally at the time products are shipped (unless products are shipped under FOB Destination shipping terms, in which case risk of loss is transferred to the customer upon delivery). The Company recognizes revenue from sales transactions where the buyer has the right to return the product at the time of sale only if (1) the Company’s price to the buyer is substantially fixed or determinable at the date of sale, (2) the buyer has paid the Company, or the buyer is obligated to pay the Company and the obligation is not contingent on resale of the product, (3) the buyer’s obligation to the Company would not be changed in the event of theft or physical destruction or damage of the product, (4) the buyer acquiring the product for resale has economic substance apart from any provided by the Company, (5) the Company does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and (6) the amount of future returns can be reasonably estimated. The Company’s net product revenue represents the Company’s total revenues less allowances for customer credits, including wholesaler discounts, estimated rebates, chargebacks, patient-focused promotional programs and product returns.
The Company establishes allowances for estimated rebates, chargebacks and product returns based on numerous qualitative and quantitative factors, including:
| • | the number of and specific contractual terms of agreements with customers; |
| • | estimated levels of inventory in the distribution channel; |
F-11
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
| • | historical rebates, chargebacks and returns of products; |
| • | direct communication with customers; |
| • | anticipated introduction of competitive products or generics; |
| • | anticipated pricing strategy changes by the Company and/or its competitors; |
| • | analysis of prescription data gathered by a third-party prescription data provider; |
| • | the impact of changes in state and federal regulations; and |
| • | estimated remaining shelf life of products. |
In its analyses, the Company uses prescription data purchased from a third-party data provider to develop estimates of historical inventory channel pull-through. The Company utilizes an internal analysis to compare historical net product shipments to estimated historical prescriptions written. Based on that analysis, management develops an estimate of the quantity of product in the channel which may be subject to various rebate, chargeback and product return exposures. At least quarterly for each product line, the Company prepares an internal estimate of ending inventory units in the distribution channel by adding estimated inventory in the channel at the beginning of the period, plus net product shipments for the period, less estimated prescriptions written for the period. To estimate months of ending inventory in the distribution channel the Company divides estimated ending inventory in the distribution channel by the Company’s estimate of the succeeding quarter’s demand, not taking into account any future anticipated demand growth beyond the succeeding quarter. Based on that analysis, the Company develops an estimate of the quantity of product in the channel that might be subject to various rebate, chargeback and product return exposures. This is done for each product line by applying a rate of historical activity for rebates, chargebacks and product returns, adjusted for relevant quantitative and qualitative factors discussed above, to the potential exposed product estimated to be in the distribution channel. The Company regularly adjusts internal forecasts that are utilized to calculate the estimated number of months in the channel based on input from members of the Company’s sales, marketing and operations groups. The adjusted forecasts take into account numerous factors including, but not limited to, new product introductions, direct communication with customers and potential product expiry issues. Adjustments to estimates are recorded in the period when significant events or changes in trends are identified.
The Company offers discounts to the Company’s wholesalers and other customers. These discounts are calculated as a percentage of the current published list price and are treated as off-invoice allowances. Accordingly, the discounts are recorded as a reduction of revenue in the period that the discounts are offered. In addition to these discounts, at the time that the Company implements a price increase, it generally offers its existing customer base an opportunity to purchase a limited quantity of product at the previous list price. Shipments resulting from these offers generally are not in excess of ordinary levels, therefore, the Company recognizes the related revenue upon shipment and includes the shipments in estimating various product related allowances. In the event the Company determines that these shipments represent purchases of inventory in excess of ordinary levels for a given wholesaler, the potential impact on product returns exposure would be specifically evaluated and reflected as a reduction in revenue at the time of such shipments.
Allowances for estimated rebates, chargebacks and patient-focused promotional programs were $184.6 million and $103.8 million as of December 31, 2013 and 2012, respectively. These allowances reflect an estimate of the Company’s liability for items such as rebates due to various governmental organizations under the Medicare/Medicaid regulations, rebates due to managed care organizations under specific contracts and chargebacks due to various organizations purchasing products through federal contracts and/or group purchasing agreements. The Company estimates its liability for rebates, chargebacks and patient-focused promotional programs at each reporting period based on a methodology of applying quantitative and qualitative assumptions. Due to the subjectivity of the Company’s accrual estimates for rebates and chargebacks, the Company prepares various sensitivity analyses to ensure the Company’s final estimate is within a reasonable range as well as review prior period activity to ensure that the Company’s methodology continues to be appropriate.
Allowances for product returns were $68.2 million and $36.4 million as of December 31, 2013 and 2012, respectively. These allowances reflect an estimate of the Company’s liability for products that may be returned by the original purchaser in accordance with the Company’s stated return policy. The Company estimates its liability for
F-12
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
product returns at each reporting period based on historical return rates, estimated inventory in the channel and the other factors discussed above. Due to the subjectivity of the Company’s accrual estimates for product returns, the Company prepares various sensitivity analyses and also reviews prior period activity to ensure that the Company’s methodology is still reasonable.
The Company’s provision for rebates, chargebacks, patient-focused promotional programs and product returns (which items do not include wholesaler discounts) as a percentage of gross product revenue in the years ended December 31, 2013, 2012 and 2011 was 20.3%, 15.7% and 14.6% for rebates, chargebacks and patient-focused promotional programs and was 3.9%, 2.3% and 3.9% for product returns, respectively.
During the second quarter of 2010 the Company recognized product revenue related to initial shipments to wholesalers of Xifaxan 550mg, which the FDA approved on March 24, 2010 for reduction in risk of overt hepatic encephalopathy, or HE, recurrence in patients 18 years of age or older, and launched to physicians in May 2010. Based on our historical experience with Xifaxan 200mg, which the Company distributes through the same distribution channels and is prescribed by the same physicians as Xifaxan 550mg, we have the ability to estimate returns for Xifaxan 550mg and therefore recognized revenue upon meeting the shipping or delivery criteria set forth in the underlying purchase agreement.
During the second quarter of 2011, the Company began recognizing product revenue related to shipments to wholesalers of Relistor, which the Company acquired from Progenics Pharmaceuticals, Inc. in February 2011. Based on historical experience with Relistor obtained from Progenics, and historical experience with the Company’s products, specifically Xifaxan 200mg, Xifaxan 500mg and Apriso, which the Company distributes through the same distribution channels and are prescribed by the same physicians as Relistor, management has the ability to estimate returns for Relistor and therefore recognized revenue upon meeting the shipping or delivery criteria set forth in the underlying purchase agreement.
In December 2011, the Company acquired an exclusive worldwide license to Solesta and Deflux with the completion of its acquisition of Oceana Therapeutics, Inc. Solesta and Deflux are medical devices that the Company sells to specialty distributors who then sell the products to end users, primarily hospitals, surgical centers and physicians. The specialty distributors generally do not purchase these products until an end user is identified. Based on historical experience with these products obtained from Oceana, and historical experience with the Company’s products, specifically Xifaxan 200mg, Xifaxan 550mg and Apriso, which are prescribed by the same physicians as Solesta, management has the ability to estimate returns for Solesta and Deflux and therefore recognized revenue upon meeting the shipping or delivery criteria set forth in the underlying purchase agreement.
Research and Development
The Company expenses research and development costs, both internal and externally contracted, as incurred. For nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities, the Company initially capitalizes the advance payment. The Company then recognizes such amounts as an expense as the related goods are delivered or the related services are performed. At December 31, 2013 and 2012, the net liability related to on-going research and development activities was $11.8 million and $11.8 million, respectively.
Reclassifications
Certain prior year amounts have been reclassified to conform to current period presentation.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities from date of purchase of three months or less to be cash equivalents. The Company maintains its cash and cash equivalents in several different financial instruments with various banks and brokerage houses. This diversification of risk is consistent with Company policy to maintain liquidity and ensure the safety of principal. At December 31, 2013, cash and cash equivalents consisted primarily of demand deposits, overnight investments in Eurodollars, certificates of deposit and money market funds at reputable financial institutions and did not include any auction rate securities.
F-13
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Accounts Receivable
The Company extends credit on an uncollateralized basis primarily to wholesale drug distributors and retail pharmacy chains throughout the United States. The Company is required to estimate the level of accounts receivable which ultimately will be uncollectible. The Company calculates this estimate based on a review of specific customer balances, industry experience and the current economic environment. Currently, the Company reserves for specific accounts plus a percentage of the Company’s outstanding trade accounts receivable balance as an allowance for uncollectible accounts. The allowance for uncollectible accounts at December 31, 2013 and 2012 was $2.8 million and $2.5 million, respectively, which includes $1.0 million of royalties due.
Financial Instruments, Recurring and Nonrecurring Fair Value Measurements
Recurring Fair Value Measurements
The carrying amounts of the Company’s financial instruments, which include cash and cash equivalents, approximated their fair values as of December 31, 2013 and 2012 due to the short-term nature of these financial instruments and they are considered Level 1 investments. Level 1 investments are investments where there are quoted prices in active markets available for identical assets or liabilities. Accounts receivable, accounts payable, accrued liabilities and capital lease obligations approximated their fair values at December 31, 2013 and 2012 due to the short-term nature of these financial instruments.
The Company’s convertible senior notes are considered Level 2 instruments, which are defined as those with significant other observable inputs. The fair value of the convertible senior notes was estimated using a Black-Scholes model incorporating the period-ending price of the Company’s common stock and other inputs.
The fair value of the contingent consideration liability, consisting of future potential milestone payments related to the Oceana, Progenics and Alfa Wassermann EIR acquisitions was $87.3 million and $103.5 million at December 31, 2013 and 2012, respectively. The Company considers this liability a Level 3 instrument in the fair value hierarchy, which is defined as one with significant unobservable inputs. The Company determined fair values based on the income approach using probability-weighted discounted cash flows that included probability assessments of occurrence of triggering events appropriately discounted considering the uncertainties associated with the obligation, calculated in accordance with the terms of the acquisition agreement based on management’s forecasts, and Monte-Carlo simulation models. The most significant unobservable inputs are the probability of receiving FDA approval for the relevant compounds and the subsequent commercial success of these compounds, if approved. The fair value of the related contingent consideration would be minimal if a compound does not receive FDA approval. The Company reviews the fair value of contingent consideration quarterly or whenever events or changes in circumstances occur that indicate there has been a change in the fair value.
The following table summarizes the activity related to the Company’s contingent consideration liability for the years ended December 31:
| 2013 | 2012 | |||||||
| Balance at January 1 |
$ | 103,500 | $ | 119,698 | ||||
| Increase related to Alfa Wassermann EIR acquisition |
— | 13,400 | ||||||
| Other changes in contingent consideration value |
(16,200 | ) | (29,598 | ) | ||||
|
|
|
|
|
|||||
| Balance at December 31 |
$ | 87,300 | $ | 103,500 | ||||
|
|
|
|
|
|||||
Nonrecurring Fair Value Measurements
The fair value of the put option granted to the majority holder of the Company’s 2028 Notes, a Level 3 instrument in the fair value hierarchy, which is defined as one with significant unobservable inputs, was $5.6 million at March 31, 2012. The Company determined the fair value based on a Black-Scholes model incorporating the period-ending price of the Company’s common stock and other inputs. The put option expired unexercised in June 2012.
F-14
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
The Company’s non-financial assets, such as intangible assets and property and equipment, are measured at fair value when there is an indicator of impairment and recorded at fair value only when an impairment charge is recognized. The Company reassessed the value of the indefinite lived intangible asset related to methylnaltrexone bromide injection for subcutaneous use for the treatment of opioid-induced constipation, or OIC, in adult patients with chronic, non-cancer pain and recorded a non-cash charge to earnings of $41.6 million in the three-month period ended September 30, 2012. The Company determined the fair value of the indefinite lived intangible asset using a discounted cash flow approach, which contains significant unobservable inputs and therefore is considered a Level 3 fair value measurement. The unobservable inputs in the analysis included future cash flow projections and a discount rate.
Inventories
The Company states raw materials, work-in-process and finished goods inventories at the lower of cost (which approximates actual cost on a first-in, first-out cost method) or market value. In evaluating whether inventory is stated at the lower of cost or market, management considers such factors as the amount of inventory on hand and in the distribution channel, estimated time required to sell such inventory, remaining shelf life, and current and expected market conditions, including levels of competition, including generic competition. The Company measures inventory adjustments as the difference between the cost of the inventory and estimated market value based upon assumptions about future demand and charged to the provision for inventory, which is a component of cost of sales. At the point of the loss recognition, the Company establishes a new, lower-cost basis for that inventory, and any subsequent improvements in facts and circumstances do not result in the restoration or increase in that newly established cost basis.
The Company expenses pre-approval inventory unless the Company believes it is probable that the inventory will be saleable. The Company capitalizes inventory costs associated with marketed products and certain products prior to regulatory approval and product launch, based on management’s judgment of probable future commercial use and net realizable value. Capitalization of this inventory does not begin until the product candidate is considered to have a high probability of regulatory approval, which is generally after the Company has analyzed Phase 3 data or filed an NDA. If the Company is aware of any specific risks or contingencies that are likely to impact the expected regulatory approval process or if there are any specific issues identified during the research process relating to safety, efficacy, manufacturing, marketing or labeling of the product candidate, the Company does not capitalize the related inventory. Once the Company capitalizes inventory for a product candidate that is not yet approved, the Company monitors, on a quarterly basis, the status of this candidate within the regulatory approval process. The Company could be required to expense previously capitalized costs related to pre-approval inventory upon a change in its judgment of future commercial use and net realizable value, due to a denial or delay of approval by regulatory bodies, a delay in the timeline for commercialization or other potential factors. On a quarterly basis, the Company evaluates all inventory, including inventory capitalized for which regulatory approval has not yet been obtained, to determine if any lower of cost or market adjustment is required. As it relates to pre-approval inventory, the Company considers several factors including expected timing of FDA approval, projected sales volume and estimated selling price. At December 31, 2013 and 2012, there were no amounts included in inventory related to pre-approval inventory.
Inventory at December 31, 2013 consisted of $61.0 million of raw materials, $11.7 million of work-in-process, and $31.5 million of finished goods. Inventory at December 31, 2012 consisted of $40.1 million of raw materials, $17.6 million of work-in-process, and $32.8 million of finished goods.
Property and Equipment
Property and equipment are stated at cost and depreciated over the estimated useful lives of the assets, generally three to five years, using the straight-line method.
F-15
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Intangible Assets and Goodwill
The Company’s intangible assets consist of license agreements, product rights and other identifiable intangible assets, which result from product and business acquisitions. Goodwill represents the excess purchase price over the fair value of assets acquired and liabilities assumed in a business combination.
When the Company makes product acquisitions that include license agreements, product rights and other identifiable intangible assets, it records the purchase price of such intangibles as intangible assets, in addition to recording the value of the product related liabilities that it assumes in connection with the product acquisition. The Company allocates the aggregate purchase price to the fair value of the various tangible and intangible assets in order to determine the appropriate carrying value of the acquired assets and then amortizes the cost of finite lived intangible assets as an expense in its consolidated statements of comprehensive income over the estimated economic useful life of the related assets. Finite lived intangible assets consist primarily of product rights for currently marketed products and are amortized over their expected economic life. The Company accounts for acquired in-process research and development as indefinite lived intangible assets until regulatory approval or discontinuation. The Company assesses the impairment of identifiable intangible assets whenever events or changes in circumstances indicate that the carrying value might not be recoverable. The Company believes that the following factors could trigger an impairment review: significant underperformance relative to expected historical or projected future operating results; significant changes in the manner of the Company’s use of the acquired assets or the strategy for the Company’s overall business; approval of generic products; and significant negative industry or economic trends.
In assessing the recoverability of its intangible assets, the Company must make assumptions regarding estimated future cash flows and other factors. If the estimated undiscounted future cash flows do not exceed the carrying value of the intangible assets, the Company must determine the fair value of the intangible assets. If the fair value of the intangible assets is less than the carrying value, the Company will recognize an impairment loss in an amount equal to the difference. The Company reviews goodwill and indefinite lived intangibles for impairment on an annual basis in the fourth quarter, and goodwill and other intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. As discussed below, the Company reassessed the value of the indefinite lived intangible asset related to methylnaltrexone bromide injection for subcutaneous use for the treatment of opioid-induced constipation, or OIC, in adult patients with chronic, non-cancer pain and recorded a non-cash charge to earnings of $41.6 million in the three-month period ended September 30, 2012. At December 31, 2013 there was no impairment to goodwill.
The following table reflects the components of all specifically identifiable intangible assets as of December 31 (in thousands):
| 2013 | 2012 | |||||||||||||||||||||||||||||||
| Gross Amount |
Accumulated Amortization |
Foreign Exchange Translation |
Net Carrying Value |
Gross Amount |
Accumulated Amortization |
Foreign Exchange Translation |
Net Carrying Value |
|||||||||||||||||||||||||
| Goodwill |
$ | 180,905 | $ | — | $ | 4 | $ | 180,909 | $ | 180,905 | $ | — | $ | — | $ | 180,905 | ||||||||||||||||
| Finite lived intangible assets |
490,367 | (149,322 | ) | 865 | 341,910 | 490,367 | (104,679 | ) | 218 | 385,906 | ||||||||||||||||||||||
| Indefinite lived intangible assets |
55,600 | — | — | 55,600 | 55,600 | — | — | 55,600 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total |
$ | 726,872 | $ | (149,322 | ) | $ | 869 | $ | 578,419 | $ | 726,872 | $ | (104,679 | ) | $ | 218 | $ | 622,411 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
The weighted-average remaining life of our finite lived intangible assets was eight years and nine years at December 31, 2013 and 2012, respectively.
F-16
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
The following table summarizes the activity related to the Company’s goodwill:
| Balance at January 1, 2011 |
$ | 85,257 | ||
| Increase related to Oceana acquisition |
101,775 | |||
|
|
|
|||
| Balance at December 31, 2011, as initially reported |
$ | 187,032 | ||
| Measurement period adjustment for Oceana acquisition |
(6,127 | ) | ||
|
|
|
|||
| Balance at December 31, 2011, revised and December 31, 2012 |
$ | 180,905 | ||
| Foreign exchange translation |
4 | |||
|
|
|
|||
| Balance at December 31, 2013 |
$ | 180,909 | ||
|
|
|
The Company recorded goodwill of $101.8 million in connection with the Oceana acquisition in December 2011, which was decreased by $6.1 million in 2012, upon completion of purchase accounting.
Amortization expense is calculated on a straight-line basis over the estimated useful life of the asset. Amortization expense for the years ended December 31, 2013, 2012 and 2011was $44.7 million, $45.4 million and $10.9 million, respectively. Estimated amortization expense related to intangible assets existing as of December 31, 2013 is between approximately $42 million and $44 million annually for each of the succeeding five years.
In November 2003, the Company acquired from aaiPharma LLC for $2.0 million the exclusive right to sell 25, 75 and 100 milligram dosage strengths of azathioprine tablets in North America under the name Azasan. The purchase price was fully allocated to product rights and related intangibles and is being amortized over a period of ten years. Although Azasan does not have any patent protection, the Company believes ten years is an appropriate amortization period based on established product sales history and management’s experience. At December 31, 2013 and 2012, accumulated amortization for the Azasan intangible was $2.0 million and $1.8 million, respectively.
In June 2004, the Company acquired the exclusive U.S. rights to Anusol-HC (hydrocortisone USP) 2.5% cream, Anusol-HC (hydrocortisone acetate) 25 mg suppository, Proctocort (hydrocortisone USP) 1% cream and Proctocort (hydrocortisone acetate) 30 mg suppositories from King Pharmaceuticals, Inc. for $13.0 million. The purchase price was fully allocated to product rights and related intangibles and is being amortized over a period of ten years. Although Anusol-HC and Proctocort do not have any patent protection, the Company believes ten years is an appropriate amortization period based on established product sales history and management’s experience. At December 31, 2013 and 2012, accumulated amortization for the King product intangibles was $12.4 million and $11.0 million, respectively.
In September 2005, the Company acquired InKine Pharmaceutical Company, Inc. for $210.0 million. The Company allocated $74.0 million of the purchase price to in-process research and development, $9.3 million to net assets acquired and $37.0 million to specifically identifiable product rights and related intangibles with an ongoing economic benefit to the Company. The Company allocated the remaining $89.7 million to goodwill, which is not being amortized. The InKine product rights and related intangibles were being amortized over an average period of 14 years, which the Company believed was an appropriate amortization period due to the product’s patent protection and the estimated economic lives of the product rights and related intangibles. In September 2010, the Company entered into a Sublicense Agreement which granted Novel Laboratories, Inc. a license under the patents covering OsmoPrep such permitting Novel to launch a generic version of OsmoPrep no later than November 16, 2019. As a result of this agreement the amortization period was adjusted prospectively, and the remaining net book value of the intangible asset will be amortized through November 16, 2019, which is the Company’s revised estimate of its remaining economic life. The Company assessed whether there was an impairment to the carrying value of the related intangible asset due to its reduced economic life and determined that there was no impairment. At December 31, 2013 and 2012, accumulated amortization for the InKine intangibles was $22.9 million and $20.4 million, respectively.
F-17
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
In December 2005, the Company entered into a License and Supply Agreement with Norgine B.V., granting Salix the exclusive right to sell a patented-protected, liquid PEG bowel cleansing product, NRL 944, in the United States. In August 2006, the Company received Food and Drug Administration marketing approval for NRL 944 under the branded name of MoviPrep. In January 2007 the United States Patent Office issued a patent providing coverage to September 1, 2024. Pursuant to the terms of the Agreement, Salix paid Norgine milestone payments of $15.0 million in August 2006, $5.0 million in December 2008 and $5.0 million in December 2009. The Company was amortizing these milestone payments over a period of 17.3 years through 2022, which the Company believed was an appropriate amortization period due to the product’s patent protection and the estimated economic life of the related intangible. In August 2010 the Company entered into a Sublicense Agreement that granted Novel Laboratories, Inc. a license to the patents covering MoviPrep permitting Novel to launch a generic version of MoviPrep no later than September 24, 2018. As a result of this agreement the amortization period was adjusted prospectively, and the remaining net book value of the intangible asset will be amortized through September 24, 2018, which is the Company’s revised estimate of its remaining economic life. The Company assessed whether there was an impairment to the carrying value of the related intangible asset due to its reduced economic life and determined that there was no impairment. At December 31, 2013 and 2012, accumulated amortization for the MoviPrep intangible was $13.9 million and $11.5 million, respectively.
In February 2007, the Company entered into a Master Purchase and Sale and License Agreement with Merck & Co. Inc., to purchase the U.S prescription pharmaceutical product rights to Pepcid Oral Suspension and Diuril Oral Suspension from Merck. The Company paid Merck $55.0 million at the closing of this transaction. The Company fully allocated the purchase price to product rights and related intangibles, and it is being amortized over a period of 15 years. Although Pepcid and Diuril do not have patent protection, the Company believes 15 years was an appropriate amortization period based on established product history and management experience. The FDA approved two generic famotidine oral suspension products in May and June 2010, respectively, and we launched an authorized generic version of our Pepcid product in May 2010 to compete with these generic products. As a result of these events, the Company assessed whether there was an impairment to the carrying value of the related intangible asset. Based on this analysis, the Company recorded a $30 million impairment charge to reduce the carrying value of the intangible asset to its estimated fair value during the three-month period ended June 30, 2010. At December 31, 2013 and 2012, accumulated amortization for the Merck products was $16.2 million and $15.1 million, respectively and the carrying value was $8.8 million and $9.9 million at December 31, 2013 and 2012, respectively.
In July 2002, the Company acquired the rights to develop and market a granulated formulation of mesalamine from Dr. Falk Pharma GmbH. On October 31, 2008, the FDA granted marketing approval for Apriso for the maintenance of remission of ulcerative colitis in adults. In November 2008, the Company made a $8.0 million milestone payment to Dr. Falk. The Company is amortizing this milestone payment over a period of 9.5 years, which the Company believes is an appropriate amortization period due to the product’s patent protection and the estimated economic life of the related intangible. At December 31, 2013 and 2012, accumulated amortization for the Apriso intangible was $4.4 million and $3.5 million, respectively.
In September 2007, the Company acquired the exclusive, worldwide right to sell metoclopramide-Zydis® (trade name Metozolv) from Wilmington Pharmaceuticals, LLC. On September 8, 2009 the FDA granted marketing approval for Metozolv™ ODT (metoclopramide HCl) 5 mg and 10 mg orally disintegrating tablets. In October 2009, the Company made a $7.3 million milestone payment to Wilmington. The Company was amortizing this milestone payment over a period of eight years, which the Company believed was an appropriate amortization period due to the product’s patent protection and the estimated economic life of the related intangible. On November 3, 2010, the Company received a paragraph IV notification from Novel stating that Novel had filed an ANDA to seek approval to market a generic version of Metozolv ODT, 5 mg and 10 mg. The notification letter asserted non-infringement of U.S. Patent No. 6,413,549 (the ‘549 patent). Upon examination of the relevant sections of the ANDA, the Company concluded that the ‘549 patent would not be enforced against Novel Laboratories. As a result of this event, the Company assessed whether there was an impairment to the carrying value of the related intangible asset. Based on this analysis, the Company recorded a $4.6 million impairment charge to reduce the carrying value of the intangible asset to its estimated fair value during the three-month period ended December 31, 2010. At December 31, 2013 and 2012 accumulated amortization for the Metozolv intangible was $2.6 million and $2.6 million, respectively and the carrying value was $0.0 million.
F-18
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
In February 2011, the Company acquired an exclusive worldwide license to develop and commercialize the products containing methylnaltrexone bromide, or the MNTX Compound, marketed under the name Relistor, from Progenics Pharmaceuticals, Inc. (except in Japan, where Ono Pharmaceutical Co. Ltd. has previously licensed the subcutaneous formulation of the drug from Progenics) and a non-exclusive license to manufacture the MNTX Compound and products containing that compound in the same territory. The Company paid Progenics an up-front license fee payment of $60.0 million. The Company also agreed to pay development milestone payments of up to $90.0 million contingent upon achieving specified regulatory approvals and commercialization milestone payments of up to $200.0 million contingent upon achieving specified targets for net sales. The Company must pay Progenics 60% of any revenue received from sublicensees in respect of any country outside the United States. The Company must pay Progenics royalties based on a percentage ranging from the mid- to high-teens of net sales by the Company and its affiliates of any product containing the MNTX Compound (excluding sales by ex-U.S. sublicensees).
The Company accounted for the Progenics transaction as a business combination under the acquisition method of accounting. Under the acquisition method of accounting, the Company recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date in its consolidated financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. As of the acquisition date, the estimated fair value of the assets acquired was $113.0 million. The Company estimated the fair value of the contingent consideration related to this transaction at $53.0 million, which was booked as a long-term liability on the consolidated balance sheet. The Company determined this liability amount using a probability-weighted discounted cash flow model based on the current regulatory status of the methylnaltrexone bromide development programs. The Company assesses the fair value of the contingent consideration quarterly, or whenever events or changes in circumstances indicate that the fair value may have changed, primarily as a result of clinical or regulatory results in the related in-process development programs. In December 2011, the Company announced positive Phase 3 data from the OIC Oral development program. Based on this information, the Company reassessed the fair value of the contingent consideration and recorded a $27.0 million increase in the contingent consideration and a corresponding charge to earnings in the fourth quarter of 2011. At December 31, 2013 and 2012, accumulated amortization for the intangible related to the currently approved indication for Relistor was $7.2 million and $4.6 million, respectively.
On July 27, 2012 the Company received a Complete Response Letter, or CRL, from the FDA following its review of a Supplemental New Drug Application (sNDA) for methylnaltrexone bromide injection for subcutaneous use for the treatment of OIC in adult patients with chronic, non-cancer pain. The CRL requested additional clinical data. In October 2012 the Company and Progenics held an End-of-Review meeting with the Division of Gastroenterology and Inborn Errors Products to better understand the contents of the CRL. Based on the results of this meeting, the Company reassessed the value of the indefinite lived intangible asset related to methylnaltrexone bromide injection for subcutaneous use for the treatment of OIC in chronic non-cancer pain and recorded a non-cash charge to earnings of $41.6 million in the three-month period ended September 30, 2012. Based on these events, the Company reassessed the fair value of the contingent consideration related to the Progenics transaction and recorded a $33.0 million decrease in the contingent consideration and a corresponding non-cash benefit to earnings in the three-month period ended September 30, 2012. The Company is currently evaluating the oral OIC development program and currently believes it will continue this program. However, additional information and additional guidance from the FDA could result in the termination of the oral OIC development program, which would result in impairment of the related intangible asset and a decrease in the related contingent consideration.
In December 2011, the Company completed its acquisition of Oceana Therapeutics, Inc. for a purchase price of approximately $303 million. Oceana has license agreements with Q-Med that provide us the worldwide right to commercialize Deflux and Solesta. Under a stock purchase agreement with Q-Med that was assumed in connection with this transaction, the Company is obligated to pay commercialization milestone payments of up to $45.0 million contingent upon achieving specified targets for net sales of Solesta. Additionally, the Company must pay low double-digit royalties under these license agreements based on a percentage of net sales of both Deflux and Solesta by the Company and its affiliates in the U.S. and a fixed per-unit royalty of the products outside the U.S.
F-19
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
The Company accounted for the Oceana transaction as a business combination under the acquisition method of accounting. Under the acquisition method of accounting, the Company recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date in its consolidated financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. As of the acquisition date, the estimated fair value of the assets acquired was approximately $342.8 million. The Company estimated the fair value of the contingent consideration related to this transaction at $39.7 million, which was booked as a long-term liability on the consolidated balance sheet. The Company determined this liability amount using a probability-weighted discounted cash flow model. The Company assesses the fair value of the contingent consideration quarterly, or whenever events or changes in circumstances indicate that the fair value may have changed, primarily as a result of significant changes in our forecast of net sales for Solesta. At December 31, 2013 accumulated amortization for the Deflux intangible was $9.3 million and $58.1 million for the Solesta intangible. At December 31, 2012 accumulated amortization for the Deflux intangible was $4.7 million and $29.0 million for the Solesta intangible.
In August 2012 the Company amended its 1996 Agreement with Alfa Wassermann. The Amended Agreement does not alter any of the terms for the TD or HE indications developed under the 1996 Agreement or IBS. The Company remains obligated to pay Alfa royalties, at the same range of rates as under the 1996 Agreement, on net sales of such products. In addition, the Amended Agreement provides the Company with an exclusive license to develop and commercialize rifaximin products for Crohn’s disease in the United States and Canada and a non-exclusive license to develop such products worldwide. The Company paid Alfa a non-refundable upfront fee of $10.0 million in August 2012, and is obligated to make a $25.0 million milestone payment upon receipt of marketing authorization in the United States for an extended intestinal release, or EIR, formulation product for CD, and additional milestones based on net sales of EIR formulation products for CD of up to $200.0 million. In addition, the Company is required to pay Alfa royalties on sales of rifaximin products for Crohn’s at percentage rates ranging from the low to mid-double digits.
The Company accounted for the Alfa Wassermann transaction as a business combination under the acquisition method of accounting. Under the acquisition method of accounting, the Company recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date in its consolidated financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. As of the acquisition date, the estimated fair value of the assets acquired was $23.4 million which is included as an indefinite lived intangible asset on the consolidated balance sheet. The Company estimated the fair value of the contingent consideration related to this transaction at $13.4 million, which was booked as a long-term liability on the consolidated balance sheet. The Company determined this liability amount using a probability-weighted discounted cash flow model based on the current regulatory status of the EIR development program. The Company assesses the fair value of the contingent consideration quarterly, or whenever events or changes in circumstances indicate that the fair value may have changed, primarily as a result of clinical or regulatory results in the related in-process development programs.
Shipping and Handling Costs
The Company does not charge its customers for freight costs. The amounts of such costs are included in selling, general and administrative expenses and are not material.
Advertising Costs
The Company charges advertising costs to expense as incurred. Advertising expenses were approximately $19.1 million, $21.4 million and $11.3 million for the years ended December 31, 2013, 2012 and 2011, respectively.
F-20
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Segment Reporting
The Company operates in a single industry and segment acquiring, developing and commercializing prescription drugs used in the treatment of a variety of gastrointestinal diseases, which are those affecting the digestive tract. Accordingly, the Company’s business is classified as a single reportable segment.
The following table presents net product revenues by product (in thousands):
| Year ended December 31, | ||||||||||||
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Xifaxan |
$ | 635,941 | $ | 514,480 | $ | 371,653 | ||||||
| Purgatives—OsmoPrep/MoviPrep |
76,390 | 64,932 | 88,746 | |||||||||
| Inflammatory Bowel Disease—Colazal/Apriso/Giazo |
117,914 | 85,504 | 49,736 | |||||||||
| Other—Anusol/Azasan/Diuril/Pepcid/Proctocort/Relistor/Deflux/Solesta/Metozolv/ Fulyzaq |
83,536 | 70,528 | 30,353 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net product revenues |
$ | 913,781 | $ | 735,444 | $ | 540,488 | ||||||
|
|
|
|
|
|
|
|||||||
Other Comprehensive Income
Other comprehensive income is composed entirely of adjustments resulting from the translation of the financial statements of the Company’s foreign subsidiary, Ocean Therapeutics, Limited, which the Company acquired in December 2011, into U.S. dollars.
Stock-Based Compensation
At December 31, 2013, the Company had one active share-based compensation plan, the 2005 Stock Plan, allowing for the issuance of stock options and restricted stock. The Company estimates the fair value of share-based payment awards on the date of the grant. The cost is to be recognized over the period during which an employee is required to provide service in exchange for the award.
Translation of Foreign Currencies
The functional currency of the Company’s foreign subsidiary, Oceana Therapeutics Limited, is the Euro. The Company translates its assets and liabilities using the current exchange rate as of the consolidated balance sheet date. The Company translates its stockholders’ equity using historical rates at the consolidated balance sheet date. The Company translates its expenses and items of income using a weighted-average exchange rate over the period ended on the consolidated balance sheet date. Adjustments resulting from the translation of the financial statements of the Company’s foreign subsidiary into U.S. dollars are excluded from the determination of net income and are accumulated in a separate component of stockholders’ equity. The Company includes foreign exchange transaction gains and losses in its consolidated results of operations.
Pharmaceutical Manufacturers Fee
Effective January 1, 2011 the Company adopted Accounting Standard Update (“ASU”) No. 2010-27, Other Expenses (Topic 720): Fees Paid to the Federal Government by Pharmaceutical Manufacturers. This ASU provides guidance on how pharmaceutical manufacturers should recognize and classify in their income statements fees mandated by the Patient Protection and Affordable Care Act (PPACA) and the Health Care and Education Reconciliation Act, both enacted in March 2010, referred to in this Note as the “Acts”. The Acts impose an annual fee on the pharmaceutical manufacturing industry for each calendar year beginning on or after January 1, 2011, payable no later than September 30 of the applicable calendar year and not tax deductible. The amount payable by a company is based on its brand prescription drug sales (including authorized generic product sales) for the preceding year as a
F-21
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
percentage of the industry’s brand prescription drug sales (including authorized generic product sales) for the same period. The ASU specifies that the liability for the fee should be estimated and recorded in full upon the first qualifying sale with a corresponding deferred cost that is amortized to expense using a straight-line method of allocation unless another method better allocates the fee over the calendar year that it is payable. The annual fee is an operating expense in the consolidated statement of comprehensive income. The annual impact of this fee on the Company will be highly variable depending on the volume of product sales. There was no material impact of the adoption of this guidance on the consolidated financial statements of the Company.
Income Taxes
The Company provides for income taxes under the liability method. This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of differences between the tax basis of assets or liabilities and their carrying amounts in the consolidated financial statements. The Company provides a valuation allowance for deferred tax assets if it is more likely than not that these items will either expire before the Company is able to realize their benefit or if future deductibility is uncertain. During the year ended December 31, 2011, management concluded that it was more likely than not that a majority of our deferred tax assets will be realized through future taxable income and released a significant portion of the valuation allowances related to these deferred tax assets during 2011.
The Company applies the provisions of ASC 740-10, “Income Taxes” with respect to accounting for uncertainty in income taxes. The Company’s net unrecognized tax benefits could change significantly due to tax benefits and liabilities that may be effectively settled within the next twelve months. The Company recognizes any interest accrued related to unrecognized tax benefits and penalties in income tax expense. During the twelve-month periods ended December 31, 2013, 2012 and 2011 there was no such interest or penalties.
The Company files a consolidated U.S. federal income tax return and consolidated and separate company income tax returns in many U.S. state jurisdictions. Generally, the Company is no longer subject to federal and state income tax examinations by U.S. tax authorities for years prior to 1993.
Net Income (Loss) Per Share
The Company computes basic net income (loss) per share by dividing net income (loss) by the weighted average number of common shares outstanding. The Company computes diluted net income (loss) per share by dividing net income (loss) by the weighted average number of common shares and dilutive common share equivalents then outstanding. Common share equivalents consist of the incremental common shares issuable upon the exercise of stock options and the impact of unvested restricted stock grants. The Company accounts for the effect of the convertible notes on diluted net income (loss) per share using the treasury stock method. As a result, the convertible notes have no effect on diluted net income (loss) per share until the Company’s stock price exceeds the conversion price of $9.25 per share for the 2028 Notes, $46.38 for the 2015 Notes, and $65.81 for the 2019 Notes. For the year ended December 31, 2011, net income used to calculate diluted earnings per share includes in weighted average common shares, diluted, the effect of approximately 6,486,000 share issuable upon conversion of the 2028 Notes calculated using the treasury stock method, since the Company’s average stock price exceeded $9.25 during that period. For the year ended December 31, 2011, the effect of the approximately 7,439,000 shares issuable upon conversion of the 2015 Notes were excluded from the diluted net income per share calculation, because the Company’s average stock price did not exceed $46.38 during those periods. For the year ended December 31, 2012, weighted average common shares, diluted, includes the effect of approximately 6,486,000 shares issuable upon conversion of the 2028 Notes calculated using the treasury stock method, taking into effect the repurchase in March and December 2012 of 2028 Notes convertible into approximately 2,730,000 and 2,405,000 shares, respectively, since the Company’s average stock price exceeded $9.25 during the period. For the year ended December 31, 2012, weighted average common shares, diluted, includes the effect of the approximately 7,439,000 shares issuable upon conversion of the 2015 Notes, since the Company’s average stock price exceeded $46.38 during the period. For the year ended December 31, 2012, the effect of the approximately 10,484,000
F-22
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
shares issuable upon conversion of the 2019 Notes issued in March 2012, were excluded from the diluted net income per share calculation, because the Company’s average stock price did not exceed $65.81 during that period. For the year ended December 31, 2013, the effect of the approximately 10,484,000 shares issuable upon conversion of the 2019 Notes issued in March 2012, were excluded from the diluted net income per share calculation, because the Company’s average stock price did not exceed $65.81 during that period.
For the years ended 2013, 2012 and 2011, there were 43,811, 33,771, and 129,671, respectively, potential common shares outstanding that were excluded from the diluted net income (loss) per share calculation because their effect would have been anti-dilutive. For the years ended 2013, 2012 and 2011 there were 342,197, 4,214,888 and 4,850,459 potential common shares outstanding, respectively, as a result of our convertible debt that were excluded from the diluted net income (loss) per share calculation because their effect would have been anti-dilutive.
The following table reconciles the numerator and denominator used to calculate diluted net income per share (in thousands):
| Year ended December 31, | ||||||||||||
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Numerator: |
||||||||||||
| Net income |
$ | 130,811 | $ | 64,246 | $ | 87,399 | ||||||
|
|
|
|
|
|
|
|||||||
| Denominator: |
||||||||||||
| Weighted average common shares, basic |
61,792 | 58,747 | 58,718 | |||||||||
| Dilutive effect of restricted stock |
600 | 606 | 782 | |||||||||
| Dilutive effect of convertible debt |
2,866 | 3,521 | 4,856 | |||||||||
| Dilutive effect of stock options |
434 | 825 | 1,127 | |||||||||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares, diluted |
65,692 | 63,699 | 65,483 | |||||||||
|
|
|
|
|
|
|
|||||||
Recently Issued Accounting Pronouncements
In February 2013, the Financial Accounting Standards Board (“FASB”) issued an Accounting Standards Update to the Comprehensive Income Topic in the Accounting Standards Codifications (“ASC”). This update requires separate presentation of the components that are reclassified out of accumulated other comprehensive income either on the face of the financial statements or in the notes to the financial statements. This update also requires companies to disclose the income statement line items impacted by any significant reclassifications, such as the realization of foreign currency translation gains and losses. These items are required for both interim and annual reporting for public companies and have an immaterial effect on the Company’s financial statements.
F-23
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
(3) PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at December 31 (in thousands):
| 2013 | 2012 | |||||||
| Cost: |
||||||||
| Furniture and equipment |
$ | 24,088 | $ | 24,430 | ||||
| Computer equipment |
24,186 | 17,542 | ||||||
| Assets under capital lease |
382 | 951 | ||||||
|
|
|
|
|
|||||
| 48,656 | 42,923 | |||||||
|
|
|
|
|
|||||
| Accumulated depreciation: |
||||||||
| Furniture and equipment |
(7,981 | ) | (5,363 | ) | ||||
| Computer equipment |
(12,981 | ) | (8,731 | ) | ||||
| Assets under capital lease |
(382 | ) | (951 | ) | ||||
|
|
|
|
|
|||||
| (21,344 | ) | (15,045 | ) | |||||
|
|
|
|
|
|||||
| Net property and equipment |
$ | 27,312 | $ | 27,878 | ||||
|
|
|
|
|
|||||
Depreciation expense was approximately $7.4 million, $6.8 million and $4.3 million in 2013, 2012 and 2011, respectively.
(4) OTHER ASSETS
In July 2011 the Company entered into an Amended and Restated Manufacturing and Supply Agreement with Glenmark Pharmaceuticals for the manufacture of crofelemer. The Amended Agreement replaced the agreement entered into in December 2008. Simultaneously upon entering the Amended Agreement, the Company entered into an Agreement for Advance Against Commitment Fee and provided a $15.0 million advance to Glenmark in order for them to meet the potential commercial demands for Crofelemer. This advance is included in other assets. The Company also agreed to fund an additional $1.3 million advance annually beginning with the first anniversary of the Amended Agreement, as long as the Amended Amendment has not been terminated.
On December 31, 2012, the FDA granted marketing approval for this product, under the trade name Fulyzaq. Therefore the Company believes this advance will have future commercial use. The Company is amortizing the advance over its estimated economic life as a component of the cost of commercial supply of crofelemer upon approval. Consistent with the Company’s policy for capitalizing pre-approval inventory, the Company will monitor, on a quarterly basis, the status of crofelemer within the regulatory approval process. The Company could be required to expense this advance upon a change in its judgment of future commercial use and net realizable value, due to a denial or delay of approval by regulatory bodies, a delay in the timeline for commercialization or other potential factors. On a quarterly basis, the Company will evaluate this advance to determine if any lower of cost or market adjustment is required. The Company considers several factors in this evaluation, including expected timing of FDA approval, projected sales volume, estimated cost of goods and estimated selling price.
F-24
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
(5) ACCRUED LIABILITIES
Accrued liabilities consisted of the following at December 31 (in thousands):
| 2013 (Restated) |
2012 | |||||||
| Accrued expenses |
$ | 56,304 | $ | 48,601 | ||||
| Accrued clinical expenses |
13,520 | 14,206 | ||||||
| Accrued terms discounts |
2,623 | 5,587 | ||||||
| Accrued royalties |
24,462 | 12,668 | ||||||
|
|
|
|
|
|||||
| Total accrued liabilities |
$ | 96,909 | $ | 81,062 | ||||
|
|
|
|
|
|||||
(6) NOTES
Convertible Senior Notes Due 2028
On August 22, 2008 the Company closed an offering of $60.0 million in Convertible Senior Notes due 2028 (“2028 Notes”). Net proceeds from the offering were $57.3 million. The 2028 Notes were governed by an indenture, dated as of August 22, 2008, between the Company and U.S. Bank National Association, as trustee.
The 2028 Notes bore interest at a rate of 5.5% per year, payable semiannually in arrears on February 15 and August 15 of each year, beginning on February 15, 2009. The 2028 Notes were to mature on August 15, 2028, unless previously converted or repurchased in accordance with their terms prior to such date.
The 2028 Notes were senior unsecured obligations, and ranked (i) equally to any of the Company’s existing and future unsecured senior debt, (ii) senior to any of the Company’s future indebtedness that is expressly subordinated to these 2028 Notes, and (iii) effectively junior to any secured indebtedness to the extent of the value of the assets securing such indebtedness.
On August 15, 2013, August 15, 2018 and August 15, 2023 or upon the occurrence of a “fundamental change”, as defined in the indenture, the holders may require the Company to repurchase all or a portion of the 2028 Notes for cash at 100% of the principal amount of the Notes being purchased, plus any accrued and unpaid interest.
In March 2012, the Company entered into a note repurchase agreement with the holder of a majority in principal amount of the 2028 Notes. The Company used a portion of the proceeds from its offering of the 2019 Notes discussed below to purchase from this holder and another holder approximately 42.1% of the 2028 Notes for an aggregate purchase price of approximately $137.2 million. In addition, for a period of 90 days after March 12, 2012, the majority holder had the option to require the Company to purchase its remaining 2028 Notes at the same price, which represented approximately 37.1% of the 2028 Notes. This option expired unexercised in June 2012. The Company incurred a loss on extinguishment of debt during the three-month period ended March 31, 2012 of $14.4 million, which primarily consists of $9.3 million in estimated fair market value of the put option granted to the majority holder, $2.5 million in estimated fair market value of the notes extinguished over their book value at the extinguishment date, and $2.0 million paid to the note holder for interest that the note holders would have received through August 2013, the first date the Company could call the debt under the original debt indenture. In December 2012 one of the holders of the 2028 Notes converted notes with a par value of $22.3 million under the terms of the note indenture, and received cash equal to the par value of the notes and interest on these notes through February 15, 2013, and 1.9 million shares of common stock. The Company incurred a loss on extinguishment of debt during the three-month period ended December 31, 2012 of $1.2 million, which primarily consists of $1.1 million in estimated fair market value of the notes extinguished over their book value at the extinguishment date, and $0.1 million paid to the note holder for interest that the note holders would have received through February 2013.
F-25
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
In connection with the issuance of the 2028 Notes, the Company incurred $2.7 million of issuance costs, which primarily consisted of investment banker, legal and other professional fees. These costs are being amortized and were recorded as additional interest expense through August 2013, the first scheduled date on which holders have the option to require the Company to repurchase the 2028 Notes.
The Company has separately accounted for the liability and equity components of the convertible debt instrument by allocating the proceeds from issuance of the 2028 Notes between the liability component and the embedded conversion option, or equity component. This allocation was done by first estimating an interest rate at the time of issuance for similar notes that do not include the embedded conversion option. This interest rate of 12.5% was used to compute the initial fair value of the liability component of $44.1 million.
The excess of the initial proceeds received from the convertible 2028 Notes over the initial amount allocated to the liability component, of $15.9 million, is allocated to the embedded conversion option, or equity component. This excess is reported as a debt discount and subsequently amortized as interest cost, using the interest method, through August 2013, the first scheduled date on which the holders have the option to require the Company to repurchase the 2028 Notes.
The Company had the right to redeem the 2028 Notes, in whole or in part, at any time after August 15, 2013 for cash equal to the principal amount of the Notes to be redeemed, plus any accrued and unpaid interest. The Company called the 2028 Notes for redemption in September 2013, but before the redemption date, the holders elected to convert the remaining 2028 Notes with a par value of $12.5 million under the terms of the note indenture, and the holders received cash equal to the par value of the notes and interest on these notes through August 15, 2013, and 1.2 million shares of common stock.
The carrying value of the equity component at December 31, 2013 and 2012 was $6.6 million. The effective interest rate on the liability component for the years ended December 31, 2013, 2012 and 2011 was 12.6%. Total interest cost of $1.1 million, $5.2 million and $7.0million was recognized during the years ended December 31, 2013, 2012 and 2011, respectively, including $0.6 million, $2.5 million, and $3.3 million of amortization of debt discount, respectively.
Convertible Senior Notes Due 2015
On June 3, 2010 the Company closed an offering of $345.0 million in Convertible Senior Notes due May 15, 2015 (“2015 Notes”). Net proceeds from the offering were approximately $334.2 million. The 2015 Notes are governed by an indenture, dated as of June 3, 2010 between the Company and U.S. Bank National Association, as trustee.
The 2015 Notes bear interest at a rate of 2.75% per year, payable semiannually in arrears on May 15 and November 15 of each year, beginning on November 15, 2010. The 2015 Notes will mature on May 15, 2015, unless earlier converted or repurchased in accordance with their terms prior to such date.
The 2015 Notes are senior unsecured obligations, and rank (i) equally to any of the Company’s existing and future unsecured senior debt, (ii) senior to any of the Company’s future indebtedness that is expressly subordinated to these 2015 Notes, and (iii) effectively junior to any secured indebtedness to the extent of the value of the assets securing such indebtedness.
The 2015 Notes are convertible into approximately 7,439,000 shares of the Company’s common stock under certain circumstances prior to maturity at a conversion rate of 21.5592 shares per $1,000 principal amount of 2015 Notes, which represents a conversion price of approximately $46.38 per share, subject to adjustment under certain conditions. Holders may convert their 2015 Notes at their option at specified times prior to the maturity date of May 15, 2015 only if: (1) during any fiscal quarter commencing after June 30, 2010, if the last reported sale price of the Company’s common stock for at least 20 trading days in the period of 30 consecutive trading days ending on the
F-26
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
last trading day of the immediately preceding fiscal quarter is equal to or more than 130% of the conversion price of the 2015 Notes on the last day of such preceding fiscal quarter; (2) a Holder submits its 2015 Notes for conversion during the five business day period following any five consecutive trading day period in which the trading price for the 2015 Notes, per $1,000 principal amount of the 2015 Notes, for each such trading day was less than 98% of the product of the last reported sale price of the Company’s common stock and the conversion rate of the 2015 Notes on such date; or (3) the Company enters into specified corporate transactions. The first of these conditions was met as of December 31, 2012. The 2015 Notes will be convertible, at the option of the noteholders, regardless of whether any of the foregoing conditions have been satisfied, on or after January 13, 2015 at any time prior to the close of business on the second scheduled trading day immediately preceding the stated maturity date of May 15, 2015. Upon conversion, the Company may pay cash, shares of the Company’s common stock or a combination of cash and stock, as determined by the Company in its discretion.
The Company is required to separately account for the liability and equity components of the convertible debt instrument by allocating the proceeds from issuance of the 2015 Notes between the liability component and the embedded conversion option, or equity component. This allocation was done by first estimating an interest rate at the time of issuance for similar notes that do not include the embedded conversion option. This interest rate of 8.35% was used to compute the initial fair value of the liability component of $265.6 million. The excess of the initial proceeds received from the convertible 2015 Notes over the initial amount allocated to the liability component, of $79.4 million, is allocated to the embedded conversion option, or equity component. This excess is reported as a debt discount and subsequently amortized as interest cost, using the interest method, through May 2015, the maturity date of the 2015 Notes.
In connection with the issuance of the 2015 Notes, the Company incurred $10.8 million of issuance costs, which primarily consisted of investment banker, legal and other professional fees. The portion of these costs related to the equity component of $2.5 million was charged to additional paid-in capital. The portion of these costs related to the debt component of $8.3 million is being amortized and are recorded as additional interest expense through May 2015, the maturity date of the 2015 Notes.
In connection with the issuance of the 2015 Notes, the Company entered into capped call transactions with certain counterparties covering approximately 7,439,000 shares of the Company’s common stock. The capped call transactions have a strike price of $46.38 and a cap price of $62.44, and are exercisable when and if the 2015 Notes are converted. If upon conversion of the 2015 Notes, the price of the Company’s common stock is above the strike price of the capped calls, the counterparties will deliver shares of the Company’s common stock and/or cash with an aggregate value approximately equal to the difference between the price of the Company’s common stock at the conversion date (as defined, with a maximum price for purposes of this calculation equal to the cap price) and the strike price, multiplied by the number of shares of the Company’s common stock related to the capped call transactions being exercised. The Company paid $44.3 million for these capped calls and charged this to additional paid-in capital.
The carrying value of the equity component related to the 2015 Notes at December 31, 2013 and 2012 was $79.4 million. The effective interest rate on the liability component for the years ended December 31, 2013, 2012 and 2011 was 8.35%. Total interest cost of $27.7 million, $26.4 million and $25.2 million was recognized during the years ended December 31, 2013, 2012 and 2011, respectively, including $16.6 million, $15.2 million and $14.0 million of amortization of debt discount, respectively. The fair value of the 2015 Notes was approximately $668.4 million at December 31, 2013.
Convertible Senior Notes Due 2019
On March 16, 2012 the Company closed an offering of $690.0 million in Convertible Senior Notes due March 15, 2019 (“2019 Notes”). Net proceeds from the offering were approximately $668.3 million. The 2019 Notes are governed by an indenture, dated as of March 16, 2012 between the Company and U.S. Bank National Association, as trustee.
F-27
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
The 2019 Notes bear interest at a rate of 1.50% per year, payable semiannually in arrears on March 15 and September 15 of each year, beginning on September 15, 2012. The 2019 Notes will mature on March 15, 2019, unless earlier converted or repurchased in accordance with their terms prior to such date.
The 2019 Notes are senior unsecured obligations, and rank (i) equally to any of the Company’s existing and future unsecured senior debt, (ii) senior to any of the Company’s future indebtedness that is expressly subordinated to these 2019 Notes, and (iii) effectively junior to any secured indebtedness to the extent of the value of the assets securing such indebtedness.
The 2019 Notes are convertible into approximately 10,484,000 shares of the Company’s common stock under certain circumstances prior to maturity at a conversion rate of 15.1947 shares per $1,000 principal amount of 2019 Notes, which represents a conversion price of approximately $65.81 per share, subject to adjustment under certain conditions. Holders may convert their 2019 Notes at their option at specified times prior to the maturity date of March 15, 2019 only if: (1) during any fiscal quarter commencing after June 30, 2012, if the last reported sale price of the Company’s common stock for at least 20 trading days in the period of 30 consecutive trading days ending on the last trading day of the immediately preceding fiscal quarter is equal to or more than 130% of the conversion price of the 2019 Notes on the last day of such preceding fiscal quarter; (2) a Holder submits its 2019 Notes for conversion during the five business day period following any five consecutive trading day period in which the trading price for the 2019 Notes, per $1,000 principal amount of the 2019 Notes, for each such trading day was less than 98% of the product of the last reported sale price of the Company’s common stock and the conversion rate of the 2019 Notes on such date; or (3) the Company enters into specified corporate transactions. None of these conditions had been met as of December 31, 2012. The 2019 Notes will be convertible, at the option of the noteholders, regardless of whether any of the foregoing conditions have been satisfied, on or after November 9, 2018 at any time prior to the close of business on the second scheduled trading day immediately preceding the stated maturity date of March 15, 2019. Upon conversion, the Company may pay cash, shares of the Company’s common stock or a combination of cash and stock, as determined by the Company in its discretion.
The Company is required to separately account for the liability and equity components of the convertible debt instrument by allocating the proceeds from issuance of the 2019 Notes between the liability component and the embedded conversion option, or equity component. This allocation was done by first estimating an interest rate at the time of issuance for similar notes that do not include the embedded conversion option. This interest rate of 5.50% was used to compute the initial fair value of the liability component of $529.3 million. The excess of the initial proceeds received from the convertible 2019 Notes over the initial amount allocated to the liability component, of $160.7 million, is allocated to the embedded conversion option, or equity component. This excess is reported as a debt discount and subsequently amortized as interest cost, using the interest method, through March 2019, the maturity date of the 2019 Notes.
In connection with the issuance of the 2019 Notes, the Company incurred $21.7 million of issuance costs, which primarily consisted of investment banker, legal and other professional fees. The portion of these costs related to the equity component of $5.1 million was charged to additional paid-in capital. The portion of these costs related to the debt component of $16.6 million is being amortized and is recorded as additional interest expense through March 2019, the maturity date of the 2019 Notes.
In connection with the issuance of the 2019 Notes, the Company entered into convertible bond hedge transactions with certain counterparties covering approximately 10,484,000 shares of the Company’s common stock. The convertible bond hedge transactions have a strike price of $65.81 and are exercisable when and if the 2019 Notes are converted. If upon conversion of the 2019 Notes, the price of the Company’s common stock is above the strike price of the convertible bond hedge transactions, the counterparties will deliver shares of the Company’s common stock and/or cash with an aggregate value approximately equal to the difference between the price of the Company’s common stock at the conversion date and the strike price, multiplied by the number of shares of the Company’s common stock related to the convertible bond hedge transaction being exercised. The Company paid $167.0 million for these convertible bond hedge transactions and charged this to additional paid-in capital.
F-28
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Simultaneously with entering into the convertible bond hedge transactions, the Company entered into privately negotiated warrant transactions whereby the Company sold the counterparties to these transactions warrants to acquire, subject to customary adjustments, approximately 10,484,000 shares of the Company’s common stock at a strike price of $85.31 per share, also subject to adjustment. The Company received $99.0 million for these warrants and credited this amount to additional paid-in capital.
The carrying value of the equity component related to the 2019 Notes at December 31, 2013 and 2012 was $160.7 million. The effective interest rate on the liability component for the years ended December 31, 2013 and 2012 was 5.50%. Total interest cost of $32.8 million and $24.0 million was recognized during the years ended December 31, 2013 and 2012, respectively, including $20.2 million and $14.0 million of amortization of debt discount, respectively. The fair value of the 2019 Notes was approximately $1,024.6 million at December 31, 2013.
The following table summarizes information on the convertible debt at December 31 (in thousands):
| 2013 | 2012 | |||||||
| Convertible Notes due 2028: |
||||||||
| Principal amount of the liability component |
$ | — | $ | 12,500 | ||||
| Unamortized discount |
— | (609 | ) | |||||
|
|
|
|
|
|||||
| Net carrying amount |
$ | — | $ | 11,891 | ||||
|
|
|
|
|
|||||
| Convertible Notes due 2015: |
||||||||
| Principal amount of the liability component |
$ | 345,000 | $ | 345,000 | ||||
| Unamortized discount |
(26,356 | ) | (42,914 | ) | ||||
|
|
|
|
|
|||||
| Net carrying amount |
$ | 318,644 | $ | 302,086 | ||||
|
|
|
|
|
|||||
| Convertible Notes due 2019: |
||||||||
| Principal amount of the liability component |
$ | 690,000 | $ | 690,000 | ||||
| Unamortized discount |
(126,594 | ) | (146,768 | ) | ||||
|
|
|
|
|
|||||
| Net carrying amount |
$ | 563,406 | $ | 543,232 | ||||
|
|
|
|
|
|||||
| Total Convertible Senior Notes |
||||||||
| Principal amount of the liability component |
$ | 1,035,000 | $ | 1,047,500 | ||||
| Unamortized discount |
(152,950 | ) | (190,291 | ) | ||||
|
|
|
|
|
|||||
| Net carrying amount |
$ | 882,050 | $ | 857,209 | ||||
|
|
|
|
|
|||||
Notes Due 2021
On December 27, 2013, the Company, completed the issuance and sale of $750,000,000 in aggregate principal amount of 6.00% senior notes due 2021 (the “2021 Notes”) in a private placement. The 2021 Notes will mature on January 15, 2021 and bear interest at a rate of 6.00% per annum, accruing from December 27, 2013. Interest is payable on the 2021 Notes on each January 15 and July 15, commencing July 15, 2014. The 2021 Notes were issued at 100% of face value and the net proceeds to the Company from the sale of the 2021 Notes were $723.0 million after deducting the initial purchasers’ discounts and offering expenses, which were recorded in 2014 upon the completion of our acquisition of Santarus. The 2021 Notes are governed by terms contained in an Indenture.
Upon closing, the Company placed the gross proceeds from the sale of the 2021 Notes into a secured escrow account, and they are recorded as restricted cash on the consolidated balance sheet. As discussed in Note 14, the Company completed its previously announced tender offer for all outstanding shares of common stock of Santarus, Inc., at a purchase price of $32.00 per share on January 2, 2014. Concurrently with completion of the tender offer, the proceeds in the escrow account were released to fund the acquisition. The 2021 Notes are unsecured obligations of the Company. Promptly following the acquisition of Santarus, Santarus and certain current subsidiaries of the Company became guarantors of the 2021 Notes on a senior unsecured basis.
F-29
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
At any time prior to January 15, 2017, the Company may, at its option, redeem some or all of the 2021 Notes at a redemption price of 100% of the principal amount thereof, plus a make-whole premium set forth in the Indenture and accrued and unpaid interest, if any, to the redemption date. Beginning January 15, 2017 the Company may redeem the 2021 Notes, in whole or in part, at redemption prices (expressed as percentages of principal amount) equal to 104.5%, 103.0%, 101.5% and 100.0% for the 12-month periods beginning on January 15, 2017, January 15, 2018, January 15, 2019 and January 15, 2020, respectively, plus accrued and unpaid interest, if any. At any time prior to January 15, 2017, the Company also may redeem up to 35% of the principal amount of the 2021 Notes at a redemption price equal to 106.00% of the principal amount thereof plus accrued and unpaid interest, if any, with the net cash proceeds of certain equity offerings.
The Indenture contains covenants that restrict the ability of the Company and certain of its subsidiaries to, among other things: (i) borrow money or issue preferred stock; (ii) pay dividends or make other payments or distributions on equity or purchase, redeem or otherwise acquire equity; (iii) make principal payments on, or purchase or redeem subordinated indebtedness prior to any scheduled principal payment or maturity; (iv) make certain investments; (v) create liens on their assets; (vi) sell their assets; (vii) enter into certain transactions with affiliates; (viii) engage in unrelated businesses and (ix) consolidate, merge or sell substantially all of the Company’s assets. These covenants are subject to a number of exceptions and qualifications, including the fall away of certain of these covenants if the 2021 Notes receive an investment grade credit rating in the future. The Indenture also requires the Company to make an offer to repurchase the 2021 Notes upon the occurrence of certain events constituting either a change of control that reduces the Company’s credit rating or an asset sale.
(7) STOCKHOLDERS’ EQUITY
Preferred Stock
A total of 5,000,000 shares of preferred stock are authorized and issuable. No shares of preferred stock were issued or outstanding as of December 31, 2013 or 2012.
Common Stock
As of December 31, 2013 the Company was authorized to issue up to 150,000,000 shares of $0.001 par value common stock. As of December 31, 2013 and 2012, there were 62,937,966 and 60,918,391 shares of common stock issued and outstanding, respectively.
Stockholder Rights Plan
On January 9, 2003, the Company’s board of directors adopted an updated stockholder rights plan. Consequently, the Board authorized the redemption, effective on January 20, 2003, of rights under its existing stockholder rights plan for $0.0001 per right. Pursuant to the updated plan, stock purchase rights were distributed to stockholders at the rate of one right with respect to each share of common stock held of record as of January 20, 2003. The rights plan was designed to enhance the Board’s ability to prevent an acquirer from depriving stockholders of the long-term value of their investment and to protect stockholders against attempts to acquire the Company by means of unfair or abusive takeover tactics. The rights would only have become exercisable based upon certain limited conditions related to acquisitions of stock, tender offers and business combinations involving the Company. This stockholder rights plan expired, in accordance with its terms, on January 9, 2013.
Stock Plans
The Company’s 1994 Stock Plan, or the 1994 Plan, was adopted by the Board of Directors in March 1994 and approved by the stockholders in March 1995. The Company’s 1996 Stock Plan, or the 1996 Plan, was adopted by the Board of Directors and approved by the Company’s stockholders in February 1996 and amended, with stockholder approval, in July 2004. The Company’s 2005 Stock Plan (the “2005 Plan”) was adopted by the Board of Directors in
F-30
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
April 2005, approved by the stockholders in June 2005 and amended, with stockholder approval, in June 2009. Under both the 1994 Plan and the 2005 Plan, the Company is authorized to grant either stock options or restricted shares, while the 1996 Plan authorizes only the grant of stock options. Stock options expire no later than ten years from the date of grant.
Option exercise prices must be at least 100% of the fair market value on the date of grant. If, at the time the Company grants an option, the optionee directly or by attribution owns stock possessing more than 10% of the total combined voting power of all classes of stock of the Company, the exercise price for an incentive stock option must be at least 110% of the fair market value and the option may not be exercisable more than five years after the date of grant. The options generally become exercisable over a four year period, with 1/4 of the shares vesting on each grant date anniversary during this period. Options may be granted with different vesting terms as determined by the board of directors. Since inception of the Company’s 1994 Plan, 1996 Plan and 2005 Plan, the Company’s stock option grants were all at fair market value. See “Stock Based Compensation” below for information about the terms of our restricted stock grants.
Stock-Based Compensation
At December 31, 2013, the Company had one active share-based compensation plan, the 2005 Stock Plan, allowing for the issuance of stock options and restricted stock. The Company estimates the fair value of share-based payment awards on the date of the grant. The Company recognizes cost over the period during which an employee is required to provide service in exchange for the award.
Starting in 2006, the Company began issuing restricted shares to employees, executives and directors of the Company. The restrictions on the restricted stock lapse according to one of two schedules. For employees and executives of the Company, restrictions lapse 25% annually over four years or 33% over 3 years. For Board members of the Company, restrictions lapse 100% after one year. The fair value of the restricted stock was estimated using an assumed forfeiture rate of 9.5% and is being expensed on a straight-line basis over the period during which the restrictions lapse. For the years ended December 31, 2013, 2012 and 2011, the Company recognized $26.2 million, $21.2 million and $15.8 million in share based compensation expense related to the restricted shares, respectively. As of December 31, 2013, the total amount of unrecognized compensation cost related to nonvested restricted stock awards, to be recognized as expense subsequent to December 31, 2013, was approximately $40.5 million, and the related weighted-average period over which it is expected to be recognized is approximately 2.5 years.
F-31
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Aggregate stock plan activity is as follows:
| Total Shares Available For Grant |
Stock Options | Restricted Shares | Stock Options and Restricted Shares |
|||||||||||||||||||||||||
| Number | Weighted Average Price |
Number Subject to Issuance |
Weighted Average Price |
Number | Weighted Average Price |
|||||||||||||||||||||||
| Balance at December 31, 2010 |
1,551,571 | 2,008,512 | $ | 14.11 | 1,743,699 | $ | 18.81 | 3,752,211 | $ | 16.30 | ||||||||||||||||||
| Granted |
(774,409 | ) | — | — | 774,409 | $ | 37.38 | 774,409 | $ | 37.38 | ||||||||||||||||||
| Exercised |
— | (370,874 | ) | $ | 14.55 | — | — | (370,874 | ) | $ | 14.55 | |||||||||||||||||
| Vested |
— | — | — | (694,444 | ) | $ | 12.36 | (694,444 | ) | $ | 12.36 | |||||||||||||||||
| Canceled/forfeited |
228,691 | (25,187 | ) | $ | 44.08 | (203,504 | ) | $ | 29.68 | (228,691 | ) | $ | 31.27 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2011 |
1,005,853 | 1,612,451 | $ | 13.54 | 1,620,160 | $ | 29.08 | 3,232,611 | $ | 21.33 | ||||||||||||||||||
| Granted |
(699,204 | ) | — | — | 699,204 | $ | 50.18 | 699,204 | $ | 50.18 | ||||||||||||||||||
| Additional shares authorized |
3,000,000 | — | — | — | — | — | — | |||||||||||||||||||||
| Exercised |
— | (850,204 | ) | $ | 10.03 | — | — | (850,204 | ) | $ | 10.03 | |||||||||||||||||
| Vested |
— | — | — | (536,981 | ) | $ | 22.75 | (536,981 | ) | $ | 22.75 | |||||||||||||||||
| Canceled/forfeited |
192,809 | — | — | (192,809 | ) | $ | 41.91 | (192,809 | ) | $ | 41.91 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2012 |
3,499,458 | 762,247 | $ | 17.46 | 1,589,574 | $ | 38.94 | 2,351,821 | $ | 31.98 | ||||||||||||||||||
| Granted |
(657,959 | ) | — | — | 657,959 | $ | 50.87 | 657,959 | $ | 50.87 | ||||||||||||||||||
| Exercised |
— | (263,754 | ) | $ | 16.00 | — | — | (263,754 | ) | $ | 16.00 | |||||||||||||||||
| Vested |
— | — | — | (585,238 | ) | $ | 29.42 | (585,238 | ) | $ | 29.42 | |||||||||||||||||
| Canceled/forfeited |
190,040 | — | — | (190,040 | ) | $ | 47.81 | (190,040 | ) | $ | 47.81 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2013 |
3,031,539 | 498,493 | $ | 18.23 | 1,472,255 | $ | 46.91 | 1,970,748 | $ | 39.66 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Exercise prices for options outstanding as of December 31, 2013 ranged from $14.21 to $24.18 per share.
| Options Outstanding and Exercisable | ||||||||||||
| Exercise Price |
Number Outstanding | Weighted Average Remaining Contractual Life (Yrs) |
Weighted Average Exercise Price |
|||||||||
| $11.94 – 14.46 |
22,813 | 0.01 | 0.07 | |||||||||
| $15.02 – 17.77 |
204,381 | 0.59 | 7.22 | |||||||||
| $18.46 – 19.68 |
258,246 | 0.31 | 9.78 | |||||||||
| $20.55 – 24.18 |
13,053 | 0.00 | 0.59 | |||||||||
|
|
|
|||||||||||
| 498,493 | 0.91 | $ | 17.66 | |||||||||
|
|
|
|||||||||||
At December 31, 2013, there were 498,493 exercisable options with a weighted average exercise price of $17.66. At December 31, 2012, there were 762,247 exercisable options with a weighted average exercise price of $17.47. At December 31, 2011, there were 1,612,451 exercisable options with a weighted average exercise price of $13.55.
For the year ended December 31, 2013, 0.3 million shares of the Company’s outstanding stock with a market value of $15.6 million were issued upon the exercise of stock options. For the year ended December 31, 2012, 0.9 million shares of the Company’s outstanding stock with a market value of $41.3 million were issued upon the exercise of stock options. For the year ended December 31, 2011, 0.4 million shares of the Company’s outstanding stock with a market value of $13.5 million were issued upon the exercise of stock options. The Company recognized no share-based compensation expense related to stock options for the years ended December 31, 2013, 2012 or 2011, nor any income tax benefit. The total intrinsic value of options exercised for the years ended December 31, 2013, 2012 and 2011 was $11.4 million, $32.8 million, and $8.1 million, respectively. As of December 31, 2013, there was no unrecognized compensation cost for stock options due to the fact that all stock options were fully vested as noted above. For the years ended December 31, 2013, 2012 and 2011, the Company received $4.2 million, $8.5 million and $5.4million in cash from stock option exercises, respectively.
F-32
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
The following table summarizes stock-based compensation expense incurred for the years ended December 31, in thousands:
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Research and development (as revised) |
$ | 3,480 | $ | 2,614 | $ | 1,876 | ||||||
| Selling, general and administrative (as revised) |
22,674 | 18,588 | 13,916 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 26,154 | $ | 21,202 | $ | 15,792 | ||||||
|
|
|
|
|
|
|
|||||||
(8) INCOME TAXES
The provision for (benefit from) income taxes in the accompanying consolidated statements of comprehensive income for the years ended December 31, 2013, 2012 and 2011 consisted of the following (in thousands):
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Current: |
||||||||||||
| Federal |
$ | 93,403 | $ | 38,414 | $ | 37,539 | ||||||
| State |
19,413 | 13,751 | 13,423 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current taxes |
112,816 | 52,165 | 50,962 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred: |
||||||||||||
| Federal |
(42,825 | ) | 4,237 | (47,843 | ) | |||||||
| State |
(8,514 | ) | (8,820 | ) | (4,417 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total deferred taxes |
(51,339 | ) | (4,583 | ) | (52,260 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total tax expense (benefit) |
$ | 61,477 | $ | 47,582 | $ | (1,298 | ) | |||||
|
|
|
|
|
|
|
|||||||
A reconciliation of the statutory income tax rate to the effective income tax rate is as follows:
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Federal statutory income tax rate |
35.0 | % | 35.0 | % | 35.0 | % | ||||||
| State income taxes, net of federal income tax benefit |
4.0 | % | 3.5 | % | 3.8 | % | ||||||
| Federal research and development credit |
(3.1 | ) % | — | (2.0 | )% | |||||||
| Change in deferred tax estimate |
(0.6 | )% | (1.1 | )% | (0.6 | )% | ||||||
| Debt costs |
(0.7 | ) % | (8.5 | )% | — | |||||||
| Change in tax status |
— | 0.8 | % | — | ||||||||
| Increase/(Decrease) in reserve for uncertain tax positions |
(4.9 | ) % | 11.4 | % | 4.5 | % | ||||||
| Non-deductible section 162(m) limitation |
0.8 | % | 1.2 | % | 2.6 | % | ||||||
| Non-deductible meals and entertainment |
0.8 | % | 1.3 | % | 2.2 | % | ||||||
| Non-deductible transaction costs |
— | — | 0.9 | % | ||||||||
| Provision to return adjustments |
0.5 | % | 0.9 | % | — | |||||||
| Change in valuation allowance |
0.1 | % | (2.0 | )% | (48.1 | )% | ||||||
| Other non-deductible expenses |
— | 0.1 | % | 0.2 | % | |||||||
|
|
|
|
|
|
|
|||||||
| 31.9 | % | 42.6 | % | (1.5 | )% | |||||||
|
|
|
|
|
|
|
|||||||
F-33
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
The tax effects of temporary differences that give rise to significant portions of the net deferred tax asset at December 31, 2013 and 2012 are as follows (in thousands):
| 2013 (Restated) |
2012 | |||||||
| Deferred Tax Assets: |
||||||||
| Research and development credits |
$ | — | $ | 2,841 | ||||
| Net operating loss carryforwards |
9,148 | 11,754 | ||||||
| Capitalized research and development expenses |
81 | 140 | ||||||
| Other credits |
102 | 31 | ||||||
| Intangible assets |
28,846 | 26,112 | ||||||
| Timing differences, including reserves, accruals, and writeoffs |
116,242 | 85,719 | ||||||
|
|
|
|
|
|||||
| Total gross deferred tax assets |
154,419 | 126,597 | ||||||
| Less: Valuation allowance |
(6,005 | ) | (6,913 | ) | ||||
|
|
|
|
|
|||||
| Deferred tax assets |
148,414 | 119,684 | ||||||
|
|
|
|
|
|||||
| Deferred Tax Liabilities: |
||||||||
| Property, plant & equipment |
(8,362 | ) | (10,156 | ) | ||||
| Debt discount and issuance costs |
(7,565 | ) | (13,919 | ) | ||||
| 481 (a) adjustments |
(971 | ) | (2,033 | ) | ||||
| Intangible assets acquired in Oceana transaction |
(85,692 | ) | (98,386 | ) | ||||
| Timing differences, including reserves, accruals, and writeoffs |
(2,482 | ) | (2,395 | ) | ||||
|
|
|
|
|
|||||
| Deferred tax liabilities |
(105,072 | ) | (126,889 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax asset (liability) |
$ | 43,342 | $ | (7,205 | ) | |||
|
|
|
|
|
|||||
The following table presents the breakdown between current and non-current deferred tax assets (liabilities) (in thousands):
| 2013 (Restated) |
2012 | |||||||
| Current deferred tax asset |
$ | 85,788 | $ | 57,050 | ||||
| Non current deferred tax liability |
(42,446 | ) | (64,255 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax asset (liability) |
$ | 43,342 | $ | (7,205 | ) | |||
|
|
|
|
|
|||||
The amounts recorded as gross deferred tax assets as of December 31, 2013 and 2012 represent the amount of tax benefits of existing deductible temporary differences and carryforwards that are more likely than not to be realized through the generation of sufficient future taxable income within the carryforward period. Significant management judgment is required in determining any valuation allowance recorded against deferred tax assets. The Company reassesses the ability to realize the deferred tax benefits on a quarterly basis. If it is more likely than not that the Company will not realize the deferred tax benefits, then all or a portion of the valuation allowance might need to be re-established, which would result in a charge to tax expense. The Company will continue to provide a valuation allowance for a portion of the net deferred tax assets related to certain state net operating loss carryforwards.
At December 31, 2013, the Company had no remaining federal net operating loss carryforwards available to offset future taxable income. The Company has non-U.S. tax losses, which at December 31, 2013 totaled approximately $11.8 million. The Company provides a full valuation allowance against the non-U.S. tax losses. At December 31, 2013, the Company also had state net operating loss carryforwards available to offset future taxable income of approximately $151.5 million. The operating loss carryforwards will begin to expire in 2017. As mentioned above, certain of these state net operating loss carryforwards have full valuation allowances set up against them. The Company also has minimum tax credit carryforwards of approximately $0.1 million.
F-34
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
At December 31, 2013, the Company realized excess tax benefits related to stock based compensation totaling $9.8 million. In accordance with ASC 718, the amount realized as excess stock based compensation was credited against additional paid in capital.
The following table summarizes the activity related to the Company’s unrecognized tax benefits for the years ended December 31, 2013, 2012 and 2011 (in thousands):
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Balance at January 1 |
$ | 20,620 | $ | 7,833 | $ | 3,641 | ||||||
| Increases related to prior year tax positions |
857 | 527 | 3,516 | |||||||||
| Increases related to current year tax positions |
852 | 12,440 | 676 | |||||||||
| Decreases related to prior year tax positions |
(12,666 | ) | (180 | ) | — | |||||||
| Decreases related to tax settlements with taxing authorities |
0 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31 |
$ | 9,663 | $ | 20,620 | $ | 7,833 | ||||||
|
|
|
|
|
|
|
|||||||
The Company’s unrecognized tax benefits as of December 31, 2013, which, if recognized, would affect the Company’s effective tax rate are approximately $9.4 million. The Company recognizes interest and penalties related to unrecognized tax benefits in income tax expense. The Company has recorded $0.4 million of interest expense and no penalties have been recorded by the Company through December 31, 2013. During 2013, the Company reduced its unrecognized tax benefit by $12.7 million. The reduction was primarily related to certain federal tax deductions from the repurchase of outstanding 2008 convertible notes in 2012. During September 2013, the Company entered into the IRS’ pre-filing agreement (“PFA”) program. As a result of this program, the Company received the final executed IRS pre-filing closing agreement, which allowed the Company to release $10.8 million of unrecognized tax benefit due to the fact that the position is effectively settled. Of the $10.8 million, approximately $9.6 million was recognized and affected the Company’s effective tax rate. The remaining $1.9 million of reversals was related to state tax issues which were effectively settled during 2013. Of the $1.9 million, approximately $0.4 million was recognized and affected the Company’s effective tax rate. The Company’s net unrecognized tax benefits could change significantly due to tax benefits and liabilities that may be effectively settled within the next 12 months. The results and timing of the settlements is highly uncertain and the Company is unable to estimate the range of the possible changes to the balance of unrecognized tax benefits.
The Company files a consolidated U.S. federal income tax return, Irish Corporate Tax return for the Company’s Irish subsidiary, and consolidated and separate company income tax returns in many U.S. state jurisdictions. Generally, the Company is no longer subject to federal and state income tax examinations by U.S. tax authorities for years prior to 1994. The Company received a notice in January 2014 that the Internal Revenue Service will be auditing the 2011 tax year.
(9) SIGNIFICANT CONCENTRATIONS
The Company operates in a single industry acquiring, developing and commercializing prescription drugs and medical devices used in the treatment of a variety of gastrointestinal diseases, which are those affecting the digestive tract. The Company’s principal financial instruments subject to potential concentration of credit risk are accounts receivable, which are unsecured, and cash equivalents. The Company’s cash equivalents consist primarily of money market funds. The amount of bank deposits might at times exceed the FDIC insurance limits.
The Company’s primary customers are wholesale pharmaceutical distributors and retail pharmacy chains in the United States.
F-35
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Total revenues from customers representing 10% or more of total revenues for the respective years, are summarized as follows:3
| Year Ended December 31, | ||||||||||||
| 2013 (Restated) |
2012 | 2011 | ||||||||||
| Customer 1 |
33 | % | 37 | % | 47 | % | ||||||
| Customer 2 |
28 | % | 36 | % | 20 | % | ||||||
| Customer 3 |
16 | % | 16 | % | 13 | % | ||||||
| Customer 4 |
14 | % | — | 12 | % | |||||||
Additionally, 95% (as restated) and 94% of the Company’s accounts receivable balances were due from these four customers at December 31, 2013 and 2012, respectively.
Under the Company’s supply agreement with Alfa Wassermann, the Company is obligated to purchase from Alfa Wassermann bulk rifaximin drug substance, the active pharmaceutical ingredient in Xifaxan 200mg rifaximin tablets and Xifaxan 550mg rifaximin tablets, until July 2014 or introduction of a generic product, whichever occurs first. The Company’s supply of rifaximin drug substance supplied by Alfa Wassermann is manufactured by ZaCh Systems in Lonigo, Italy, and Sanofi-Aventis in Brindisi, Italy. Under a supply agreement with Lupin, the Company is obligated to purchase 50% of its annual requirements of bulk rifaximin drug substance from Lupin, subject to certain minimum purchase requirements. Under a long-term supply agreement, rifaximin is converted into Xifaxan drug product by Patheon, Inc. in Whitby, Ontario. Bulk Xifaxan tablets are packaged into finished Xifaxan commercial bottles by Patheon and packaged into Xifaxan commercial blister packs by Pharma Packaging Solutions in Norris, Tennessee.
In September 2010 the Company entered into a supply agreement with Novel Laboratories, Inc. in Somerset, New Jersey. In October 2011 Novel began producing commercial supply of bulk OsmoPrep tablets which are then packaged into finished OsmoPrep commercial bottles by Pharma Packaging Solutions in Norris, Tennessee.
In August 2010 the Company entered into a supply agreement with Novel Laboratories, Inc. and Actavis Inc. Under this supply agreement the Company agreed to purchase from Novel all of its requirements in excess of a certain amount of MoviPrep in 2011 and all of its requirements of MoviPrep beginning in 2012.
Bayer AG in Wuppertal, Germany and Pharmazell in Raubling, Germany supply the Company with bulk mesalamine active ingredient. Under a long-term supply agreement with Catalent Pharma Solutions in Winchester, Kentucky, Catalent converts this mesalamine into the Company’s commercial supply of bulk Apriso, 375mg mesalamine capsules. The bulk Apriso capsules are then packaged into finished Apriso commercial bottles by Pharma Packaging Solutions in Norris, Tennessee.
Relistor subcutaneous injection in a vial presentation is produced in bulk by DSM Pharmaceutical Products in Greenville, North Carolina and then packaged into finished Relistor single vials or vial kits by Packaging Coordinators, Inc. in Philadelphia, Pennsylvania. Relistor subcutaneous injection in a pre-filled syringe presentation is produced and packaged into finished Relistor kits by Vetter Pharma International GmbH in Ravensburg, Germany. The drug substance for these Relistor subcutaneous injection presentations is supplied by Mallinckrodt, a subsidiary of Covidien, in St. Louis, Missouri.
Both Deflux and Solesta are produced and packaged into finished Deflux and Solesta kits, respectively, by Q-Med in Uppsala, Sweden.
Under the Company’s supply agreement with Glenmark Pharmaceuticals, Ltd. in Mumbai, India, Glenmark supplies the Company with crofelemer drug substance. With respect to the Company’s budesonide foam formulation, the Company’s methylnaltrexone bromide tablet formulation, and the Company’s methylnaltrexone bromide multi-dose pen subcutaneous injection formulation, all of which are currently under development, the Company plans to negotiate commercial supply agreements with the manufacturers who produced the drug substance and drug product for the Phase 3 clinical trial material, or the manufacturers who produced the pivotal registration batches, if these products receive FDA approval.
| 3 | Company to confirm no impact from FOB Destination issue. |
F-36
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
(10) 401(k) PLAN
In 1996, the Company adopted the Salix Pharmaceuticals, Inc. 401(k) Retirement Plan. Eligible participants may elect to defer a percentage of their compensation. From inception through June 2006, the Company matched up to 50% of participant deferrals up to 6% of the participant’s compensation. Effective July 2006, the Company matches up to 75% of participant deferrals up to 6% of the participant’s compensation. The Company’s total matching contributions for all participants were approximately $2.4 million, $2.1 million and $1.7 million in 2013, 2012 and 2011, respectively. Additional discretionary employer contributions may be made on an annual basis.
(11) COMMITMENTS
At December 31, 2013, the Company had binding purchase order commitments for inventory purchases aggregating approximately $217.4 million over the next five years, which includes any minimum purchase commitments under our manufacturing agreements. Actual payments for the purchases related to these manufacturing agreements were $181.9 million, $145.6 million and $84.1 million for the years ended December 31, 2013, 2012 and 2011, respectively.
Lease Agreements
In February 2011 the Company notified the landlord for its corporate headquarters in Morrisville, NC that it was terminating this lease as of October 2011. In February 2011 the Company entered into a lease for approximately 127,000 square feet for a corporate headquarters in Raleigh, North Carolina, which began in September 2011 and expires in 2023. In February 2011 the Company extended the lease on its former corporate headquarters located in Raleigh, North Carolina, of approximately 26,000 square feet of office space to 2018, and in October 2011 the Company leased an additional 12,000 square feet in this building. In 2012 the Company leased an additional 5,000 square feet in this building, and an additional 45,000 square feet in 2013. The Company leases office facilities, primarily its headquarters discussed above, under various non-cancelable operating leases, the last of which expires in 2023. Certain of these leases contain future payment obligations that escalate over time. Rent expense was approximately $3.7 million, $3.1 million and $2.3 million for the years ended December 31, 2013, 2012 and 2011, respectively.
As of December 31, 2013, future minimum payments for leases were as follows (in thousands):
| Years ending December 31, |
Operating Leases |
Capital Leases |
||||||
| 2014 |
$ | 4,506 | $ | 47 | ||||
| 2015 |
4,862 | — | ||||||
| 2016 |
5,085 | — | ||||||
| 2017 |
5,049 | — | ||||||
| 2018 |
4,608 | — | ||||||
| Thereafter |
24,801 | — | ||||||
|
|
|
|
|
|||||
| Total future minimum payments required |
$ | 48,911 | 47 | |||||
|
|
|
|||||||
| Less: Amount representing interest |
— | |||||||
|
|
|
|||||||
| Present value of net minimum lease payments |
47 | |||||||
| Less: Current portion of capital lease obligations |
47 | |||||||
|
|
|
|||||||
| Long term portion of capital lease obligations |
$ | — | ||||||
|
|
|
|||||||
F-37
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Potential Milestone Payments
The Company has entered into collaborative agreements with licensors, licensees and others. Pursuant to the terms of these collaborative agreements, the Company is obligated to make one or more payments upon the occurrence of certain milestones. The following is a summary of the material payments that the Company might be required to make under its collaborative agreements if certain milestones are satisfied.
Amended and Restated License Agreement with Alfa Wassermann S.p.A—In August 2012 the Company amended its 1996 License Agreement with Alfa Wassermann to develop rifaximin. The Restated Agreement provides the Company with an exclusive license to develop and commercialize rifaximin products for Crohn’s disease in the United States and Canada and a non-exclusive license to develop such products worldwide. The Company paid Alfa a non-refundable upfront fee of $10.0 million in August 2012, and is obligated to make a $25.0 million milestone payment upon receipt of marketing authorization in the United States for a rifaximin product for Crohn’s, and additional milestone payments of up to $200.0 million based on net sales of rifaximin products for Crohn’s. No milestone payments had been earned or made as of December 31, 2013.
License Agreement with Dr. Falk Pharma GmbH for budesonide—In March 2008, the Company entered into a license agreement with Dr. Falk Pharma. The agreement provides the Company with an exclusive license to develop and commercialize in the United States Dr. Falk Pharma’s budesonide products. The products covered in the license agreement include U.S. patent-protected budesonide rectal foam and budesonide gastro-resistant capsule, patents for which expire in 2015 and 2016, respectively. Pursuant to the license agreement the Company is obligated to make an upfront payment and regulatory milestone payments that could total up to $23.0 million to Dr. Falk Pharma, with the majority contingent upon achievement of U.S. regulatory approval. As of December 31, 2013, the Company had paid $1.0 million of these milestone payments.
Development, Commercialization and License Agreement with Lupin Ltd—In September 2009, the Company entered into a Development, Commercialization and License Agreement with Lupin for Lupin’s proprietary drug delivery technology for rifaximin. The Company made an upfront payment of $5.0 million to Lupin upon execution of this agreement.
In March 2011, the Company entered into an amendment and restatement of its Development, Commercialization and License Agreement with Lupin, and further amended it in February 2013, as so amended, the Amended License Agreement. The Amended License Agreement replaces in its entirety the September 2009 agreement. This agreement provides that the Company is obligated to pay Lupin an additional upfront payment of $10.0 million, milestone payments that could total up to $53.0 million over the term of the agreement and royalties in connection with the commercialization of relevant products. During the portion of the term of the Amended License Agreement ending September 30, 2019, the Company must pay Lupin a minimum quarterly payment unless specified payments by the Company to Lupin during that quarter exceed that amount. The Company is permitted to charge against such minimum quarterly payments as it makes in respect of quarters beginning on or after January 1, 2012, the purchase price for certain rifaximin to be supplied to it by Lupin pursuant to a Rifaximin Manufacturing and Supply Agreement into which the Company and Lupin entered in September 2009 and subsequently amended. In the event the Company should exercise its right to terminate the Amended License Agreement for convenience, it must pay Lupin as an early termination fee a specified portion of the minimum quarterly payments payable by it to Lupin through September 30, 2019, that have not been paid or otherwise satisfied as of the date of termination. As of December 31, 2013, the Company had paid the additional $10.0 million upfront payment. The milestone payments are contingent upon achievement of certain clinical and regulatory milestones.
License Agreement with Napo Pharmaceuticals, Inc.—In December 2008 the Company entered into a collaboration agreement with Napo. Pursuant to the agreement, the Company has an exclusive, royalty-bearing license to crofelemer for the treatment of HIV-associated diarrhea and additional indications of pediatric diarrhea and acute infectious diarrhea in a specified territory. The Company also has a non-exclusive, worldwide, royalty-bearing license
F-38
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
to use Napo-controlled trademarks associated with crofelemer. The Company has made an initial payment of $5.0 million to Napo and will make up to $50.0 million in milestone payments to Napo contingent on regulatory approvals and up to $250.0 million in milestone payments contingent on reaching certain sales thresholds. The Company is responsible for the development costs of crofelemer, but costs exceeding $12.0 million for development of crofelemer used for the HIV-associated diarrhea indication will be credited towards regulatory milestones and thereafter against sales milestones. On December 31, 2012, the FDA granted marketing approval for this product, under the trade name Fulyzaq. At December 31, 2013 development costs exceeded $12.0 million by more than the amount of the milestone due upon FDA marketing approval, therefore there was no payment due to Napo.
License and Supply Agreement with Norgine B.V.—In December 2005, the Company entered into a license and supply agreement with Norgine for the rights to sell NRL944, a bowel cleansing product the Company now markets in the United States under the trade name MoviPrep. Pursuant to the terms of this agreement, the Company is obligated to make upfront and milestone payments to Norgine that could total up to $37.0 million over the term of the agreement. As of December 31, 2013, the Company had paid $27.0 million of milestone payments. The remaining milestone payments are contingent upon reaching sales thresholds.
License Agreement with Photocure ASA—In October 2010, the Company entered into a license agreement with Photocure for the worldwide exclusive rights, excluding the Nordic region, to develop and commercialize LumacanTM for diagnosing, staging or monitoring gastrointestinal dysplasia or cancer. The Company made an initial payment of $4.0 million to Photocure, and will be responsible for development costs of Lumacan, but Photocure will reimburse the Company up to $3.0 million for certain out-of-pocket costs. In December 2012 the Company made a $4.5 million milestone payment. In addition, the Company is obligated to make up to $72.0 million in milestone payments to Photocure contingent on development and regulatory milestones, and up to $50.0 million in milestone payments contingent on reaching certain sales thresholds over the term of the agreement. No milestone payments had been earned or made as of December 31, 2013.
License Agreement with Progenics Pharmaceuticals, Inc.—In February 2011, the Company acquired an exclusive worldwide license to develop and commercialize the products containing methylnaltrexone bromide, or the MNTX Compound, marketed under the name Relistor®, from Progenics. The Company paid Progenics an up-front license fee payment of $60.0 million. In addition, the Company is obligated to pay development milestone payments of up to $90.0 million contingent upon achieving specified regulatory approvals and commercialization milestone payments of up to $200.0 million contingent upon achieving specified targets for net sales over the term of the agreement. No milestone payments had been earned or made as of December 31, 2013.
License Agreements and Stock Purchase Agreement with Q-Med—In connection with the Company’s acquisition of Oceana Therapeutics, Inc. in December 2011, the Company acquired two license agreements with Q-Med, which provide it the worldwide right to commercialize Deflux and Solesta. Under a stock purchase agreement with Q-Med that was assumed in connection with the Oceana transaction, the Company is obligated to pay commercialization milestone payments of up to $45.0 million contingent upon achieving specified targets for net sales of Solesta over the term of the agreement. No milestone payments had been earned or made as of December 31, 2013.
F-39
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
(12) QUARTERLY RESULTS OF OPERATIONS (UNAUDITED)
The following is a summary of the unaudited quarterly results of operations for the years ended December 31, 2013 and 2012:
| Mar 31 | June 30 | Sept 30 | Dec 31 (Restated) |
|||||||||||||
| (in thousands, except per share amounts) | (unaudited) | |||||||||||||||
| 2013 |
||||||||||||||||
| Net product revenue |
$ | 202,601 | $ | 235,441 | $ | 238,184 | $ | 237,555 | ||||||||
| Cost of products sold, excluding amortization of product rights and intangible assets |
33,072 | 46,489 | 42,899 | 55,316 | ||||||||||||
| Net income |
22,441 | 21,000 | 47,331 | 40,039 | ||||||||||||
| Net income per share, basic(1) |
0.37 | 0.34 | 0.77 | 0.64 | ||||||||||||
| Net income per share, diluted(1) |
0.35 | 0.32 | 0.71 | 0.58 | ||||||||||||
| Mar 31 | June 30 | Sept 30 | Dec 31 | |||||||||||||
| (in thousands, except per share amounts) | (unaudited) | |||||||||||||||
| 2012 |
||||||||||||||||
| Net product revenue |
$ | 181,006 | $ | 171,133 | $ | 185,132 | $ | 198,173 | ||||||||
| Cost of products sold, excluding amortization of product rights and intangible assets |
33,257 | 34,190 | 26,471 | 30,679 | ||||||||||||
| Net income |
20,134 | 9,953 | 16,536 | 17,623 | ||||||||||||
| Net income per share, basic(1) |
0.35 | 0.17 | 0.28 | 0.30 | ||||||||||||
| Net income per share, diluted(1) |
0.32 | 0.15 | 0.26 | 0.28 | ||||||||||||
| (1) | The sum of per share earnings by quarter may not equal earnings per share for the year due to the changes in average share calculations. This is in accordance with prescribed reporting requirements. |
The following table presents the effects of the correction and other adjustments made to the Company’s previously unaudited quarterly financial information for the quarter ended December 31, 2013:
| Quarter Ended December 31, 2013 | ||||||||||||
| As previously reported |
Adjustments (see Note 1A) |
As restated |
||||||||||
| (in thousands, except per share amounts) | ||||||||||||
| Net product revenue |
$ | 257,612 | ($ | 20,057 | ) | $ | 237,555 | |||||
| Cost of products sold, excluding amortization of product rights and intangible assets |
56,932 | (1,616 | ) | $ | 55,316 | |||||||
| Net income |
52,262 | (12,223 | ) | $ | 40,039 | |||||||
| Net income per share, basic(1) |
0.83 | (0.19 | ) | 0.64 | ||||||||
| Net income per share, diluted(1) |
0.76 | (0.18 | ) | 0.58 | ||||||||
(13) LEGAL PROCEEDINGS
From time to time, the Company is involved in various litigation matters that are being defended and handled in the ordinary course of business. The assessment of whether a loss is probable or reasonably possible, and whether the loss or a range of loss is estimable, often involves a series of complex judgments about future events. Management maintains accruals for such losses that are probable of being incurred and subject to reasonable estimation. For current matters not specifically reported herein, management does not anticipate that the ultimate liabilities, if any, arising from the resolution of such current matters would have a material effect on the Company’s financial condition or results of operations. It is possible, however, that future results of operations for any particular period could be materially affected by changes in the Company’s assessment related to any of these matters.
The Company is currently and might continue to be subject to product liability claims that arise through the testing, manufacturing, marketing and sale of its products. The Company is vigorously defending these claims and intends to continue to do so. The Company currently maintains liability coverage for its products but it is possible that this coverage, and any future coverage, will be insufficient to satisfy any liabilities that arise. The Company would have to assume defense of the lawsuits and be responsible for damages, fees and expenses, if any, that are awarded against it or for amounts in excess of the Company’s product liability coverage.
On May 5, 2011, Napo filed a lawsuit against the Company in the Supreme Court of the State of New York, County of New York, alleging that the Company had engaged in fraudulent conduct, breached its collaboration agreement with Napo dated December 9, 2008, and breached its duty of good faith and fair dealing. Napo also sought a declaratory judgment that Napo had the right to terminate the collaboration agreement and sought unspecified damages in excess of $150 million. On or about December 28, 2011, Napo filed an amended complaint seeking an unspecified amount of damages for alleged breaches of the collaboration agreement by the Company and replacing Napo’s original complaint. Napo’s amended complaint no longer seeks a declaratory judgment that Napo has the right to terminate the collaboration agreement and removed the need for the court to rule on the Company’s motion to dismiss the original complaint. The Company believes that Napo’s allegations continue to be without merit and its lawsuit baseless. The
F-40
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Company filed an answer to the amended complaint and counterclaims on or about January 17, 2012, and is vigorously defending against the lawsuit. Discovery concluded last year, and, on May 31, 2013, the Company filed a motion for partial summary judgment. The court heard oral arguments on the motion in August 2013. On December 24, 2013, the court entered a short-form order granting the Company’s motion for partial summary judgment, narrowing the issues in the case. Napo filed an appeal of that decision on January 27, 2014 to the Appellate Division of the Supreme Court of the State of New York. On January 29, 2014 the Court vacated and replaced portions of the short-form order with an order continuing to grant the Company’s motion for partial summary judgment, narrowing the issues in the case. Trial on the claims remaining in the case commenced on February 10, 2014 and the jury decided on February 25, 2014 that Salix complied with its contractual obligations in commercializing Fulyzaq in the United States, and thus did not breach the collaboration agreement between the parties. The Company continues to advance its development and commercialization plans for crofelemer in accordance with the existing collaboration agreement with Napo.
On September 7, 2012, the Company and Dr. Falk Pharma filed a patent infringement complaint against Lupin in the U.S. District Court for the District of Delaware. The complaint alleges infringement of the ‘620 patent, based on Lupin’s filing of an ANDA seeking approval to market and sell a generic version of Apriso before the expiration of the ‘620 patent. The filing of this suit within the 45 day response period provided by the Hatch Waxman Act imposes an automatic 30 month stay of approval of Lupin’s ANDA. On March 4, 2013, the Company and Dr. Falk Pharma filed an amended complaint to also enforce the issued ‘886 patent in the pending suit. On July 30, 2013 the USPTO issued the ‘965 patent, which further protects the Apriso product. On August 19, 2013, the Company and Dr. Falk Pharma filed a second amended complaint to enforce the ‘965 patent and the ‘451 patent against Lupin. The court conducted a pretrial evidentiary hearing, known as a Markman hearing, on November, 21, 2013. The judge adopted each of Salix’s claim construction positions in his order issued December 17, 2013. The trial is scheduled for September, 2014. The Company continues to evaluate its intellectual property protecting Apriso, in which it has full confidence. The Company intends to vigorously enforce its intellectual property rights. Currently the Company cannot predict or determine the timing or outcome of this matter or its impact on financial condition or results of operations.
On February 18, 2014, the Company and Dr. Falk Pharma filed a patent infringement complaint against Novel in the U.S. District Court for the District of Delaware. The complaint alleges infringement of the ‘620 patent, the ‘886 patent, and the ‘965 patent based on Novel’s filing of an ANDA seeking approval to market and sell a generic version of Apriso before the expiration of these patents. The filing of this suit within the 45-day response period provided by the Hatch-Waxman Act imposes an automatic 30-month stay of approval of Novel’s ANDA. The Company continues to evaluate its intellectual property protecting Apriso, in which it has full confidence. The Company intends to vigorously enforce its intellectual property rights. Currently the Company cannot predict or determine the timing or outcome of this matter or its impact on financial condition or results of operations.
On February 1, 2013, the Company’s wholly owned subsidiary Salix Pharmaceuticals, Inc. received a subpoena from the U.S. Attorney’s Office for the Southern District of New York requesting documents regarding the Company’s sales and promotional practices for Xifaxan, Relistor and Apriso. The Company is continuing to respond to the subpoena and intends to cooperate fully with the subpoena and related government investigation. Currently, the Company cannot predict or determine the timing or outcome of this inquiry or its impact on financial condition or results of operations.
Beginning on November 12, 2013, eleven putative class action lawsuits were filed by shareholders of Santarus seeking to challenge the Company’s proposed acquisition of Santarus, which was announced on November 7, 2013. Nine of these actions were filed in the Delaware Court of Chancery, one was filed in California Superior Court (San Diego County) and one was filed in the U.S. District Court for the Southern District of California. These actions generally allege that the members of the Santarus board of directors breached their fiduciary duties to Santarus’s shareholders by failing to maximize the value of Santarus and by making inadequate or misleading disclosures regarding the proposed merger, and that Santarus, Salix and certain of the Company’s subsidiaries aided and abetted those breaches of fiduciary duty. The complaint in the action pending in California federal court also asserts causes of action on behalf of the individual plaintiff for alleged violations of certain sections of the Exchange Act. These actions generally sought, among other things, to enjoin the merger, unspecified damages and fees. On December 9, 2013,
F-41
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
Santarus and its directors filed a motion to stay the action pending in California Superior Court. On December 11, 2013, the Delaware Court of Chancery consolidated the nine actions pending in that court, appointed lead counsel for the plaintiffs, and designated the amended complaint filed by plaintiff Imad Ahmad Khalil on December 9, 2013 as the operative complaint in the consolidated Delaware litigation. On December 20, 2013, the parties in the Delaware litigation reached an agreement in principle, subject to full documentation, to resolve the plaintiffs’ claims in that action in exchange for certain supplemental disclosures that Santarus included in an amended Schedule 14D-9 it filed on that date. The Company completed its merger with Santarus on January 2, 2014. The parties in the Delaware litigation executed a Memorandum of Understanding reflecting the terms of their agreement in principle on January 17, 2014 and are currently drafting full settlement documentation and engaging in confirmatory discovery. The settlement of the Delaware litigation will be subject to approval by the Delaware Court of Chancery. The plaintiffs’ counsel in the Delaware litigation have also indicated that they intend to request an award of an unspecified amount attorneys’ fees from the Delaware Court of Chancery. On January 23, 2014, Santarus and its directors filed a renewed motion to stay the action pending in California Superior Court, and Salix filed a separate motion to stay that action in favor of the Delaware litigation. On February 12, 2014, the parties in the action pending in California federal court filed a joint motion to stay that action pending a decision by the Delaware Court of Chancery regarding final approval of the proposed settlement of the Delaware litigation, and the California federal court granted that motion on February 13, 2014. Salix is vigorously defending the action pending in California Superior Court and attempting to finalize the settlement of the consolidated Delaware litigation as described above. The Company believes that all of the claims asserted against it by Santarus shareholders lack merit. Currently the Company cannot predict the timing or outcome of this matter or its impact on financial condition or results of operations.
(14) SUBSEQUENT EVENTS
On January 2, 2014, the Company completed a tender offer for all outstanding shares of common stock par value $0.0001 per share, including the associated rights to purchase shares of Series A Junior Participating Preferred Stock, par value $0.0001 per share, of Santarus, Inc., at a purchase price of $32.00 per share. Following the tender offer, Salix completed the acquisition of Santarus through a merger under Section 251(h) of the General Corporation Law of the State of Delaware.
The Company believes the transaction will create a leader in the gastroenterology pharmaceuticals space in the United States, offering a highly complementary portfolio of well-known and profitable drugs, and allow the Company to expand the size and reach of its sales force. The Company expects the Santarus acquisition to be accounted for as a business combination and the Company will record the assets acquired and the estimated future consideration obligations at their respective fair values as of the acquisition date during the first quarter of 2013. A determination of the acquisition-date fair values of the assets acquired and the liabilities assumed is pending the completion of an independent appraisal and other evaluations.
On January 2, 2014, the Company entered into a Credit Agreement (the “Credit Agreement”) with Jefferies Finance LLC, as collateral agent (the “Collateral Agent”) and administrative agent, and the lenders party thereto, providing for (i) a $1,200 million six year senior secured term loan facility (the “Senior Term Loan Facility”) and (ii) a $150 million five year senior secured revolving credit facility (the “Revolving Credit Facility” and together with the Senior Term Loan Facility, the “Senior Secured Facilities”). The proceeds of the Senior Term Loan Facility were used to fund a portion of the purchase price of the tender offer for Santarus. The proceeds of the Revolving Credit Facility can be used in the future for working capital and general corporate purposes, including permitted investments and acquisitions.
In connection with the entry by the Company into the Credit Agreement, the Company and the Guarantors have entered into a Guarantee and Collateral Agreement, dated January 2, 2014 (the “Guarantee and Collateral Agreement”), with the Collateral Agent, pursuant to which (i) each of the Guarantors has guaranteed the obligations of the Company under the Credit Agreement and the obligations of each of the other Guarantors under the Guarantee and Collateral Agreement and (ii) the Company and each of the Guarantors has granted to the Collateral Agent, for the benefit of the lenders under the Credit Agreement, a first priority security interest in substantially all of its assets.
F-42
Table of Contents
SALIX PHARMACEUTICALS, LTD.
Notes to Consolidated Financial Statements — Continued
The term loans under the Senior Term Loan Facility are subject to quarterly amortization equal to 1.25% of the original aggregate principal amount thereof and the remaining principal balance is due and payable on January 2, 2020 unless earlier prepaid. The Senior Secured Facilities bear interest at an annual rate of, at the Company’s option, either (i) Adjusted LIBOR (as defined by the Credit Agreement), with a floor of 1.00%, plus a margin of 3.25% or (ii) the highest of (A) the Wall Street Journal’s published “U.S. Prime Lending Rate,” (B) the Federal Funds Effective Rate (as defined by the Credit Agreement) in effect on such day plus 1/2 of 1%, (C) one-month Adjusted LIBOR plus 1.00% per annum and (D) 2.00%, in each case plus a margin of 2.25%. If the ratio of the Company’s consolidated total debt to consolidated EBITDA (the “Total Leverage Ratio”) is less than 3.75 to 1.00, the margins will be reduced by 25 basis points.
The Company is required to prepay term loans under the Senior Term Loan Facility with (i) 100% of the proceeds of asset sales not reinvested within generally one year, (ii) 100% of the proceeds from certain debt financings and (iii) 50% of Excess Cash Flow (as defined in the Credit Agreement). The percentage of Excess Cash Flow that must be used to prepay the Senior Term Loan Facility decreases to 25% if the Total Leverage Ratio is less than 3:50 to 1:00 and to zero if the Total Leverage Ratio is less than 2:50 to 1:00.
The Credit Agreement includes customary affirmative and negative covenants, including restrictions on additional indebtedness, liens, investments, asset sales, stock buybacks and dividends, mergers, consolidations, and transactions with affiliates and capital expenditures. The negative covenants are generally subject to various exceptions. The Credit Agreement does not include any financial maintenance covenants, with the exception that if 25% or more of the Revolving Credit Facility is being utilized, a Total Leverage Ratio requirement (measured as of the last day of each quarter), which decreases over time, must be satisfied.
On February 22, 2015 the Company announced that it had entered into a definitive agreement with Valeant Pharmaceuticals International, Inc., under which Valeant will acquire all of the outstanding common stock of the Company. The acquisition is structured as an all-cash tender offer at a price of $158.00 per share followed by a merger in which each remaining untendered share of Salix common stock would be converted into the right to receive the same $158.00 cash per share consideration as in the tender offer. The transaction, which is expected to close in the second quarter of 2015, is subject to customary closing conditions and regulatory approval.
F-43
Table of Contents
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
Allowance for Rebates, and Chargebacks
| Beginning Balance |
Additions | Deductions | Ending Balance |
|||||||||||||||||
| Year ended December 31, |
Provision Related to Current Period Sales |
Provision Related to Period Prior Sales |
Rebates, Chargebacks and Discounts or Credits |
|||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| 2013(Restated) |
$ | 103,819 | $ | 249,885 | $ | 5,252 | $ | 174,372 | $ | 184,584 | ||||||||||
| 2012 |
$ | 69,167 | $ | 155,219 | $ | 5,104 | $ | 125,671 | $ | 103,819 | ||||||||||
| 2011 |
$ | 40,833 | $ | 100,433 | $ | 5,481 | $ | 77,580 | $ | 69,167 | ||||||||||
Allowance for Returns
| Beginning Balance |
Additions | Deductions | Ending Balance |
|||||||||||||||||
| Year ended December 31, |
Provision Related to Current Period Sales |
Provision Related to Prior Period Sales |
Returns or Credits Related to Prior Period Sales |
|||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| 2013(Restated) |
$ | 36,372 | $ | 34,513 | $ | 15,630 | $ | 18,270 | $ | 68,245 | ||||||||||
| 2012 |
$ | 28,712 | $ | 22,192 | $ | 296 | $ | 14,828 | $ | 36,372 | ||||||||||
| 2011 |
$ | 19,705 | $ | 17,933 | $ | 10,638 | $ | 19,564 | $ | 28,712 | ||||||||||
Allowance for Uncollectible Accounts
| Beginning Balance |
Additions | Deductions | Ending Balance |
|||||||||||
| Year ended December 31, |
Charged to Costs and Expenses |
Accounts Written Off During Period |
||||||||||||
| (in thousands) | ||||||||||||||
| 2013(Restated) |
$ | 1,495 | $ | 317 | $ | 1,812 | ||||||||
| 2012 |
$ | 831 | $ | 664 | $ | 1,495 | ||||||||
| 2011 |
$ | 508 | $ | 323 | $ | 831 | ||||||||
Valuation Allowance on Deferred Tax Assets
| Beginning Balance |
Additions | Deductions | Ending Balance |
|||||||||||||
| Year ended December 31, |
Provisions for Valuation Allowance |
Release of Valuation Allowance /Other |
||||||||||||||
| (in thousands) | ||||||||||||||||
| 2013 (Restated) |
$ | 6,913 | $ | 293 | $ | 1,201 | $ | 6,005 | ||||||||
| 2012 |
$ | 7,711 | $ | 1,694 | $ | 2,492 | $ | 6,913 | ||||||||
| 2011 |
$ | 48,727 | — | $ | 41,016 | $ | 7,711 | |||||||||
F-44
