Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - SENOMYX INC | Financial_Report.xls |
| EX-31.2 - EXHIBIT 31.2 - SENOMYX INC | ex31-2.htm |
| EX-32.1 - EXHIBIT 32.1 - SENOMYX INC | ex32-1.htm |
| EX-23.1 - EXHIBIT 23.1 - SENOMYX INC | ex23-1.htm |
| EX-31.1 - EXHIBIT 31.1 - SENOMYX INC | ex31-1.htm |
| EX-10.22 - EXHIBIT 10.22 - SENOMYX INC | ex10-22.htm |
| EX-10.11 - EXHIBIT 10.11 - SENOMYX INC | ex10-11.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2014
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number 000-50791
SENOMYX, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
33-0843840 |
|
(State or other jurisdiction of incorporation |
(I.R.S. Employer Identification No.) |
|
4767 Nexus Centre Drive |
92121 |
|
(Address of principal executive offices) |
(Zip Code) |
(858) 646-8300
Registrant’s telephone number, including area code
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Name of Exchange on Which Registered |
|
Common Stock, par value $0.001 per share |
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer ☐ |
Accelerated filer ☒ |
| |
|
Non-accelerated filer ☐ |
Smaller reporting company ☐ |
| |
|
(Do not check if a smaller reporting company) |
| ||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
As of June 30, 2014, the aggregate market value of the voting stock held by non–affiliates of the registrant, computed by reference to the last sale price of such stock as of such date on the NASDAQ Stock Market LLC, was approximately $288,612,000. Excludes an aggregate of 9,506,168 shares of common stock held by officers and directors and by each person known by the registrant to own 5% or more of the outstanding common stock as of June 30, 2014. Exclusion of shares held by any of these persons should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant, or that such person is controlled by or under common control with the registrant.
As of February 19, 2015, there were 43,411,192 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement for the 2015 Annual Meeting of Stockholders, to be filed with the Securities and Exchange Commission pursuant to Regulation 14A not later than 120 days after the end of the fiscal year covered by this Form 10-K, are incorporated by reference in Part III, Items 10-14 of this Form 10-K.
SENOMYX, INC.
Annual Report on Form 10-K
For the Fiscal Year Ended December 31, 2014
TABLE OF CONTENTS
|
Page | ||
|
PART I |
3 | |
|
Item 1. |
Business |
3 |
|
Item 1A. |
Risk Factors |
22 |
|
Item 1B. |
Unresolved Staff Comments |
36 |
|
Item 2. |
Properties |
36 |
|
Item 3. |
Legal Proceedings |
36 |
|
Item 4. |
Mine Safety Disclosures |
36 |
|
PART II |
37 | |
|
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
37 |
|
Item 6. |
Selected Financial Data |
39 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
40 |
|
Item 7A. |
Quantitative and Qualitative Disclosures About Market Risk |
49 |
|
Item 8. |
Financial Statements and Supplementary Data |
50 |
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
72 |
|
Item 9A. |
Controls and Procedures |
72 |
|
Item 9B. |
Other Information |
74 |
|
PART III |
74 | |
|
Item 10. |
Directors, Executive Officers and Corporate Governance |
74 |
|
Item 11. |
Executive Compensation |
74 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
74 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
74 |
|
Item 14. |
Principal Accountant Fees and Services |
74 |
|
PART IV |
75 | |
|
Item 15. |
Exhibits and Financial Statement Schedules |
75 |
|
Signatures |
78 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements included or incorporated by reference in this annual report on Form 10-K other than statements of historical fact, are forward-looking statements. You can identify these and other forward-looking statements by the use of words such as “may,” “will,” “could,” “anticipate,” “expect,” “intend,” “believe,” “continue” or the negative of such terms, or other comparable terminology. Forward-looking statements also include the assumptions underlying or relating to such statements.
Our actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth below under the caption “Risk Factors” in Part I Item 1A and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part II Item 7 of this annual report on Form 10-K and elsewhere in this annual report on Form 10-K. Readers are cautioned not to place undue reliance on forward-looking statements. The forward-looking statements speak only as of the date on which they are made and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they are made except as required by law.
PART I
Item 1. Business
Overview
We are a leading company using proprietary taste receptor technologies to discover, develop and commercialize innovative flavor ingredients for the packaged food, beverage and ingredient supply industries. We consider flavor ingredients to include flavors, such as savory and cooling, and flavors with modifying properties, such as sweet and salt modifiers and bitter blockers. Flavors with modifying properties is a term used by the flavor industry to describe ingredients that function as part of a flavor system to modify or enhance the flavor profile of a variety of food and beverages. We also have an ongoing effort to discover and develop natural high intensity sweeteners. We believe our flavor ingredients, when added as part of a flavor system, will enable packaged food, beverage and ingredient supply companies to improve the nutritional profile of their products while maintaining or improving taste and, in certain cases, generating cost of goods savings.
We have historically derived our revenues from collaborative agreements. We license our flavor ingredients to our collaborators on an exclusive or co-exclusive basis, which we believe will provide these companies with a competitive advantage. We currently have collaborative agreements with several of the world’s leading packaged food, beverage and ingredient companies, including Ajinomoto Co., Inc., or Ajinomoto, Firmenich SA, or Firmenich, Nestlé SA, or Nestlé, and PepsiCo, Inc., or PepsiCo. Depending upon the collaboration, our collaboration agreements generally provide for license fees, research and development funding, reimbursement of certain costs, milestone payments based upon our achievement of research or development goals and, in the event of commercialization, commercial milestones, minimum periodic royalties and royalties on sales of products incorporating our flavor ingredients.
In addition to revenues from collaborative agreements, we have a complementary commercialization strategy whereby we sell certain of our flavor ingredients directly to flavor companies for re-sale to their food and beverage company customers. The flavor companies add value by incorporating our ingredients into proprietary flavor systems, which include a combination or variety of flavor ingredients, for their customers. To support this direct sales program, we have established relationships with third party manufacturers. Our commercial revenues under the direct sales program are generated from actual sales of our flavor ingredients to flavor companies and other customers.
Individuals experience the sensation of taste when flavor ingredients in food and beverage products interact with taste receptors in the mouth. A taste receptor functions either by physically binding to a flavor ingredient in a process analogous to the way a key fits into a lock or by acting as a channel to allow ions to flow directly into a taste cell. As a result of these interactions, signals are sent to the brain where a specific taste sensation is registered. There are currently five recognized primary tastes: sweet, umami (which is the savory taste of glutamate), bitter, salt and sour. In addition, there are secondary taste sensations, such as cool, hot and fat.
We are currently pursuing the discovery, development and/or commercialization of flavor ingredients through five programs focused on sweet, savory, bitter, cooling and salt taste areas. The goal of our Sweet Taste Program is to grow our portfolio of new flavors with modifying properties that, when added to a flavor system, allow our customers to maintain the desired sweet taste to food and beverage products that have a significant reduction in sweeteners. The goals of our Savory Flavor Program are to utilize our portfolio of new flavor ingredients that mimic the taste of naturally occurring glutamate to enable the reduction or replacement of added monosodium glutamate, or MSG, and to provide new savory tastes to foods by combining our savory flavor ingredients with other ingredients to create unique new flavors. The goal of our Bitter Blocker Program is to reduce or block bitter taste to improve the overall taste characteristics of packaged foods, beverages, over the counter, or OTC, health care products and pharmaceutical products. The goal of our Cooling Taste Program is to support the commercialization of novel cooling flavor ingredients that have advantages over currently available flavor ingredients for a variety of applications. The goal of our Salt Taste Program is to validate a taste-related protein as a screening target to identify flavor ingredients that allow a significant reduction of sodium in foods and beverages yet maintain the salty taste desirable to consumers.
We were incorporated in September 1998 in Delaware. Our internet address is www.senomyx.com. We post links on our website to the following filings as soon as reasonably practicable after they are electronically filed with or furnished to the Securities and Exchange Commission, or SEC: annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendment to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended. All such filings are available through our website free of charge. Our filings may also be read and copied at the SEC’s Public Reference Room at 100 F Street, NE Washington, DC 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that website is www.sec.gov.
Industry Background
Flavor Industry Overview
Flavor ingredients are used in a variety of packaged food, beverage and ingredient products throughout the world. Flavor ingredients can be either natural or artificial compounds. Flavor ingredients are generally sold as part of a flavor system developed by a flavor company. Flavor systems are then sold for use by packaged food and beverage companies or companies that make pharmaceutical or healthcare products.
While a few packaged food, beverage and ingredient companies have their own internal flavor research and development programs, most have traditionally relied on purchases of flavor systems from third parties. Historically, most third party flavor companies have purchased or manufactured flavor ingredients on a commodity basis and then used these to create flavor systems for their packaged food and beverage customers. The ability to incorporate unique flavors that deliver a great taste profile in end products is a key consideration of packaged food and beverage companies as they strive to differentiate their brands in the marketplace. As such, flavor companies are constantly looking to identify unique, value-added ingredients that will help to meet their customers’ needs for innovative flavor solutions.
Traditionally, companies have discovered new flavor ingredients primarily by using inefficient, non-automated and labor-intensive trial and error processes involving a limited number of trained taste testers. Using this approach, taste testers must physically taste each potential flavor ingredient to assess the taste characteristics of the compound. Taste testers can assess only a limited number of potential flavor ingredients at one time due to the sensory fatigue that results from repeated tasting. In addition, the human tongue has a limited ability to identify distinct flavors. As a result, only a small fraction of the available universe of ingredients can be tested economically.
Flavor ingredients are regulated in the United States under provisions of the Food, Drug and Cosmetic Act, or FD&C Act. Flavor ingredients sold in countries and regions outside the United States are also subject to regulations imposed by national governments or regional regulatory authorities, as is the case in the European Union. For further discussion of the regulatory regime for flavor ingredients, please see the “Regulatory” section below.
Packaged Food and Beverage Industry
Packaged food and beverage products include carbonated and non-carbonated beverages, baked goods, dairy products, frozen foods, snack foods, main meal and side dish mixes, canned soup and numerous other products. According to recent data from Euromonitor International, an independent research organization, approximately 744 million metric tons of packaged food was sold worldwide in 2013. This figure is comprised of the weight of ready-to-eat finished food products (e.g., yogurt) and the weight of the concentrated forms of products before adding water or other components required for reconstitution (e.g., condensed soup). The worldwide sales of packaged food and beverage products in 2013 were approximately $3.2 trillion, of which approximately $547 billion was generated in the United States. These figures represent annual growth rates of approximately 5% and 2%, respectively, over 2008 amounts. Based on these estimates, of the worldwide total, sales of packaged foods were approximately $2.2 trillion and sales of non-alcoholic beverages were approximately $955 billion.1
Flavor Ingredients as a Source of Competitive Advantage
The packaged food, beverage and ingredient industries are comprised of a number of large and highly competitive market segments. Small market share gains in specific large market segments can translate into significant additional revenue for packaged food, beverage and ingredient companies. For example, according to recent Euromonitor data, estimated 2013 worldwide sales of non-alcoholic beverages were approximately $955 billion. Thus, an increase of a tenth of a percentage point in overall worldwide market share would result in additional revenues of approximately $955 million.
As a result of these market opportunities, packaged food, beverage and ingredient companies are constantly seeking ways to differentiate their products, demand for which can be greatly affected by very small actual or perceived improvements in flavor or health profiles. Flavor ingredients can potentially provide an important way to differentiate a particular product through improvements in nutritional profile, flavor ingredient innovation and cost of goods savings while maintaining desired taste.
Our Solution
We use our proprietary taste receptor-based assays and screening technologies to discover and develop novel flavor ingredients. We have developed proprietary taste receptor-based assays for use in our high-throughput screening systems to rapidly and efficiently screen our compound libraries and identify large numbers of novel potential flavor ingredients. We believe our approach improves the likelihood that ingredients with the desired characteristics can be discovered and then optimized into novel flavor ingredients.
We believe our approach will result in the discovery and development of flavor ingredients that will address the following key challenges faced by the packaged food, beverage and ingredient industries:
|
• |
Maintaining and Improving Taste. Our goal is to discover, develop and commercialize flavor ingredients that will enable food and beverage manufacturers to improve or maintain the taste of their offerings while improving the nutritional profile of packaged food, beverage and ingredient products. |
|
• |
Reducing Sweeteners, MSG and Salt in Packaged Food and Beverage Products. Our resources are focused on discovering, developing and commercializing flavor ingredients that, when added as part of a flavor system, will enable food and beverage manufacturers to significantly reduce the levels of sugar, fructose, artificial sweeteners, MSG and salt in packaged food and beverage products while maintaining or improving taste. We believe reducing the levels of such ingredients will improve the nutritional profile and/or taste of packaged food and beverage products. |
|
• |
Blocking Undesirable Tastes. We have developed flavor ingredients that we believe will be useful in blocking bitter and other unwanted tastes associated with certain packaged food and beverage products. Our technology may also be useful to improve the taste of products outside of food and beverages, such as OTC health care products and pharmaceutical products. |
1 Amount does not include fountain or foodservice sales in certain beverage categories
|
• |
Identifying Natural Ingredients. We are using our technologies to screen libraries of natural samples isolated from plants and other natural sources to discover new natural ingredients. |
|
• |
Obtaining Exclusive or Co-Exclusive Use of Proprietary Flavor Ingredients. We are able to offer our current and future collaborators exclusive or co-exclusive use of certain proprietary flavor ingredients in defined packaged food, beverage and ingredient product categories and geographies. We believe this approach will assist our collaborators in differentiating their products from those of their competitors. This strategy has been executed with our existing collaborative agreements. |
|
• |
Accessing Proprietary Flavor Ingredients. Through our direct sales program, we are able to offer flavor companies our proprietary flavor ingredients for incorporation into their flavor systems for re-sale to food and beverage manufacturers. We believe this approach will allow numerous food and beverage manufacturers to improve or maintain the taste of their offerings while improving the nutritional profile of packaged food, beverage and ingredient products. |
|
• |
Reducing Cost of Goods. We believe our proprietary flavor ingredients will enable food and beverage manufacturers to reduce overall raw material ingredient costs in certain cases, particularly for those products containing high levels of carbohydrate sweeteners and MSG. |
|
• |
Reducing Environmental Impact. Flavor ingredients such as our sweet taste modifiers can be used as part of a flavor system to reduce the use of sweeteners such as sucrose, or common table sugar, and high fructose corn syrup. This can reduce the cost and environmental impact of transporting the goods, as well as the use of water and fertilizers to grow the associated crops. In addition, in the case of powdered or concentrated products, a reduction in the use of added sweeteners may allow manufacturers to reduce package sizes and therefore use less packaging materials without impacting serving sizes or the number of servings in a single package. |
Our Strategy
Our goal is to become the leader in the discovery, development and commercialization of new and improved proprietary flavor ingredients that are commercialized either through collaborations with leading companies or through direct sales by us. Key elements of our strategy include:
|
• |
Maintaining and Expanding Our Technology Position. We believe our proprietary taste receptor-based technologies, including our receptor discovery, assay development and high-throughput screening technologies, and our natural and artificial compound libraries provide us and our collaborators with significant competitive advantages. We intend to continue to add to our compound libraries and develop and acquire proprietary technologies and related intellectual property rights to expand and enhance our ability to discover, develop and commercialize new proprietary flavor ingredients. |
|
• |
Developing Flavor Ingredients that are Eligible for Flavor and Extract Manufacturers Association, or FEMA, Generally Recognized as Safe, or GRAS, Determination. Our primary focus is on the development of flavors and flavors with modifying properties that will qualify for a FEMA GRAS determination. Fourteen flavor ingredients developed as part of our savory, sweet, bitter and cool programs have received FEMA GRAS determination. Upon the GRAS determination, our collaborators and customers can begin to test market and commercialize retail products or flavor systems incorporating our flavor ingredients in several key markets. |
|
• |
Collaborating With Leading Packaged Food, Beverage and Ingredient Companies. We are collaborating with leading packaged food, beverage, flavor and ingredient companies to develop and commercialize certain of our product candidates. Under our license agreements, our collaborators are allowed exclusive use, co-exclusive use or a limited period of exclusive use of certain of our proprietary flavor ingredients in defined packaged food, beverage and ingredient product categories and geographies. In general, our collaborators are responsible for manufacturing, marketing, selling and distributing their products incorporating flavor ingredients discovered and developed by us. In addition, our collaborators are responsible for all costs of manufacturing our flavor ingredients for their own use. As a result, we expect that certain of our flavor ingredients will be commercialized without our incurring significant sales, marketing, manufacturing and distribution costs. As appropriate, we seek to establish additional collaborations with leading packaged food, beverage and ingredient companies to use flavor ingredients developed through our existing programs for use within new packaged food, beverage and ingredient product fields. |
|
• |
Pursuing Market Opportunities through Direct Sales. We seek to optimize the value of our flavor ingredients by creating new opportunities to increase their usage by food and beverage companies. In some cases, we may be able to commercialize our flavor ingredients in select product categories and geographies that have been licensed to collaborators. In these instances, we may elect to commercialize those flavor ingredients ourselves through our direct sales program in available product categories or geographies. We also have discovered and developed flavor ingredients which have not been licensed under collaborative agreements. Our direct sales efforts are focused almost exclusively on selling certain of our flavor ingredients directly to flavor companies. We expect the flavor companies will add value by incorporating our ingredients into proprietary flavor systems for re-sale to their food and beverage company customers. We believe the direct sales program enables a deeper and broader penetration of the food and beverage industry and accelerates commercialization by expanding the market for our products. |
Our Discovery and Development Process
The following diagram summarizes our discovery and development process.
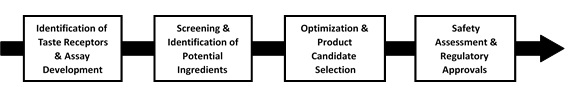 |
The key elements of our Discovery and Development process are:
|
• |
Identification of Relevant Taste Receptors and Proprietary Taste Receptor-Based Assay Development. The first steps in our discovery and development process are to identify the relevant taste receptors and to develop proprietary assays based on the identified receptors. Our assays are tests that measure interactions between the taste receptors and potential flavor ingredients. To date, we have developed assays to test for compounds that interact with receptors associated with savory, sweet, bitter and cooling tastes. |
|
• |
High-Throughput Screening and Identification of Potential Ingredients. The next step in our discovery and development process is to use our proprietary taste receptor-based assays to identify compounds that bind to taste receptors, known as hits. We use automated high-throughput screening to rapidly evaluate our libraries of diverse artificial and natural compounds. A panel of taste testers then evaluates the taste effect of the most potent hits. Based on this evaluation, we designate hits that exhibit a positive taste effect as proof-of-concept compounds. We then select the most promising of those proof-of-concept compounds, which we call lead compounds, for optimization. |
|
• |
Optimization of Lead Compounds and Selection of Product Candidates. The next step in our discovery and development process is to chemically modify our lead compounds to allow lower amounts of the compound to be used in the finished product or improve the activity to meet the taste attribute goals of our collaborators. Optimization may also be required to improve the safety profile and physical properties of a compound so that it is stable under manufacturing, storage and food preparation conditions. We refer to optimized compounds that provide desirable taste attributes in packaged food, beverage and ingredient product prototypes as product candidates. When screening natural libraries, optimization involves selecting the appropriate source and developing the most efficient process, such as fermentation, to obtain the active compound. |
|
• |
Safety Studies and Regulatory Approval of Product Candidates. The next step in our discovery and development process is to select one or more product candidates for development and potential commercialization. We then evaluate the selected product candidate for safety in accordance with flavor industry standards, including preliminary in-vitro evaluation and additional in-vivo studies to confirm an acceptable safety profile. Following this evaluation, we submit the safety data along with the physical and chemical properties of the product candidate and a description of manufacturing and conditions of intended use to FEMA for review. The FEMA review is conducted by an independent Expert Panel identified and convened by FEMA. If the Expert Panel determines the product candidate to be GRAS, data from the safety studies supporting the GRAS determination are provided to the U.S. Food and Drug Administration, or FDA, and published in the journal Food Technology. The FEMA GRAS determination allows the flavor ingredients to be commercialized in the United States and several other countries and regions. The FEMA GRAS determination also facilitates regulatory determinations in a number of additional countries. |
Fourteen flavor ingredients developed as part of our savory, sweet, bitter and cool programs have received FEMA GRAS determinations. The process from selection for development until receipt of that determination has historically ranged from 12 to 18 months. Costs associated with the FEMA GRAS process, including external third-party safety studies and preparation of the application, ranged up to approximately $1.5 million per flavor ingredient. We expect that most of the flavor ingredients we develop in the future will require a similar amount of time and cost. However, the length of time and cost may vary depending on the properties of the flavor ingredient. In addition, regulatory approval of the flavor ingredients in other jurisdictions may require that we conduct additional studies or incur additional expenses. Furthermore, the regulatory approval of natural high intensity sweeteners will likely require submission through the Food Additive Petition process.
We are currently pursuing the discovery and development of flavor ingredients through three programs focused on sweet and salt taste. We may also investigate other areas of taste as part of our broader research related to human taste biology.
The goal of the sweet modifier component of our Sweet Taste Program is to restore the desired taste profile of products in which natural and artificial sweeteners have been reduced. We continue to optimize and evaluate promising artificial sweet taste modifiers of sucrose and/or high fructose corn syrup, which are widely used as sweeteners for beverages and other products. In addition, we continue to make progress in our efforts to discover and develop natural sweet taste modifiers, including the achievement of a taste proof-of-concept for a natural sucrose modifier.
The goal of the natural high intensity sweetener component of our Sweet Taste Program is to discover and develop novel no- or low-calorie natural high intensity sweeteners. We continue to make progress in our efforts to discover and develop natural high-potency sweeteners, including the achievement of a taste proof-of-concept for a natural high-potency sweetener. We have also discovered a novel plant-derived sweetener that is more potent than rebaudioside-A and other stevia derivatives in blinded taste tests. Further evaluation of the taste properties, physical attributes and potential manufacturing scale-up paths are being investigated before we pursue full development of this sweetener. Other ongoing activities include further expansion of our natural products library, high-throughput screening of these plant-derived samples, and taste tests of samples of interest.
The goal of the Salt Taste Program is to identify flavor ingredients that allow a significant reduction of sodium in foods and beverages yet maintain the salty taste desirable to consumers. This program is an important research focus for our longer-term pipeline. We are using analytical approaches to evaluate a large proprietary database of proteins found in taste buds in order to prioritize and find the specific protein or group of proteins that function to detect salt in foods. We are currently screening target proteins with a goal of validating the protein(s) that function in human salty taste perception, supporting our use of the protein(s) as research targets to discover new salt flavor modifiers using our novel flavor technology.
Regulatory Process
Flavoring substances, including flavor ingredients intended for use in foods and beverages in the United States, are regulated under provisions of the FD&C Act. Flavor ingredients sold in countries and regions outside of the United States are also subject to regulations imposed by national governments or regional regulatory authorities, as is the case in the European Union. These regulations are subject to frequent revisions and interpretation.
Regulation of Flavor Ingredients in the United States. In the United States, flavor ingredients are regulated by the FDA as approved food additives, or as GRAS ingredients under the FD&C Act. The Food Additive Amendments of 1958 prompted the flavor industry to establish in 1960 the FEMA Expert Panel. FEMA has administered the GRAS program for flavor ingredients on behalf of the industry for over 50 years. Several countries outside of the United States, including Canada, Brazil, Argentina and the Philippines, allow flavor use in food based on GRAS determination by the FEMA Expert Panel. Several other countries either add new FEMA GRAS ingredients to their positive lists of approved flavoring agents or add the new FEMA GRAS lists to their flavor regulations by specific reference. The other possible route for approval of a flavor-modifying ingredient is a GRAS self-determination (independent of FEMA) with or without FDA notification. Our goal is that the flavor ingredients we discover will achieve GRAS determination by the FEMA Expert Panel.
GRAS Review Process. Flavor ingredients that qualify for the GRAS review process are generally intended to be consumed in small quantities and have data supporting their safety under conditions of intended use. An independent Expert Panel, convened to undertake a GRAS review, determines whether an ingredient is generally recognized as safe under the conditions of its intended use. These experts are qualified by scientific training and experience to evaluate the safety of certain new ingredients used in food and may declare them as having been adequately shown through scientific procedures to be generally recognized as safe under the conditions of their intended use. Under the GRAS process, manufacturers are required to obtain safety data from the scientific literature or through the conduct of safety studies, determine the estimated daily intake of the flavor ingredient per person and submit a report to the GRAS review panel describing the physical, structural, safety, and metabolic properties of the flavor ingredient. The entire GRAS determination process, including the safety and metabolic studies, application preparation and GRAS panel review, can take up to two years or longer. However, if there are prior safety data on the ingredient or an ingredient with a related structure, then fewer safety studies may be required for the GRAS review and the GRAS review process can be shorter than two years.
The most common types of GRAS reviews are:
|
• |
FEMA Expert Panel. The FEMA Expert Panel is an independent panel of experts for which FEMA provides administrative assistance. The FEMA Expert Panel, which may be used by FEMA members and certain other parties, meets up to three times per year. If the FEMA Expert Panel determines a flavor ingredient to be GRAS, data from the safety studies supporting the GRAS determination are provided to the FDA and published in the journal Food Technology. To our knowledge, the FDA has not challenged the FEMA Expert Panel’s conclusion that the use of a flavoring substance is GRAS. Fourteen of our flavor ingredients have been determined to be GRAS by the FEMA Expert Panel. |
|
• |
Specifically Convened Independent Panel. An independent, qualified panel of experts in pertinent scientific disciplines may be formed by the manufacturer to evaluate the safety of a specific ingredient for GRAS status. This process is known as “self-determination of GRAS status.” The basis for the GRAS self-determination is not required to be submitted to the FDA. However, the FDA may request information on ingredients that have been self-determined to be GRAS, or the information may be provided voluntarily. |
Regulation of Flavor Ingredients outside of the United States. Outside of the United States, flavor approvals vary by country. There is, however, some commonality in approach in many countries. As explained above, several countries either accept GRAS determination by the FEMA Expert Panel as the basis for approval or GRAS determination facilitates country approval. Many countries, particularly developing countries in Latin America and Africa, accept favorable review by the WHO/FAO Joint Expert Committee on Food Additives, or JECFA, as the basis for approval or to facilitate approval. FEMA automatically submits new GRAS ingredients to the Codex Alimentarius through the FDA for JECFA review. Some other countries have their own unique approval processes. The European Union, or EU, has established EU-wide regulations for flavor ingredient use based on safety evaluations by the European Food Safety Authority, or EFSA, and risk management (i.e. regulation development) by the Directorate General for Health. A number of countries in eastern Europe or in Africa accept EU approval as the basis for approved use. China, Japan, Indonesia and Russia also have independent regulatory review processes for flavors. We have received regulatory approvals for several of our flavor ingredients from JECFA, EFSA, the Chinese Ministry of Health and other authorities outside of the United States.
Food Additive Petition Process. Food ingredients may be evaluated through a food additive petition. If there is insufficient general knowledge or for a variety of other reasons, including unusual conditions of intended use, a food additive petition may be filed with the FDA. Food additive petitions contain information on the structural nature of the ingredient, the manufacturing process, information on use in food, estimates of human exposure from use of the ingredient in food and all known information related to the safety of the ingredient. The FDA reviews the petition content, requests additional information if necessary, publishes a proposed rule for use in food, reviews comments on the proposed rule and publishes a final rule, if the use is determined to be acceptable. The safety data requirements for food additives are the same as for GRAS substances, although high use substances will require additional safety data. We estimate that a food additive petition could cost up to $10 million and may take up to four years to complete for submission. Furthermore, additional studies adding cost and time to approval may be required depending on the results of the initial safety studies. Examples of ingredients that have gone through a food additive petition process include the artificial sweeteners aspartame, acesulfame K and sucralose. It may be necessary for any high intensity sweeteners that we discover or develop to follow this regulatory route.
Patents and Proprietary Rights
Our policy is to file patent applications and to protect technologies, inventions and improvements to inventions that are commercially important to the development of our business. For example, we may seek patent protection for receptors and nucleic acid sequences encoding receptors that are involved in taste and the use of such receptors to identify ingredients that modulate taste. As of December 31, 2014 we are the owner or the exclusive licensee of 221 issued United States patents, 39 pending United States patent applications, 257 issued foreign patents and 232 pending foreign applications covering various aspects of our proprietary technology. Our issued patents have terms that expire between 2015 and 2033. The earliest patent expiration for our approved flavor ingredients is in 2024 for certain savory flavor ingredients. We also rely on trademarks to protect our proprietary technology. Generally, United States patents have a term of 17 years from the date of issue or 20 years from the earliest claimed priority date, whichever is later, for patents issued from applications filed with the United States Patent and Trademark Office prior to June 8, 1995 or 20 years from the application filing date or earlier claimed priority date in the case of patents issued from applications filed on or after June 8, 1995. Patents in most other countries have a term of 20 years from the date of filing the patent application. We intend to continue to file patent applications as we discover and develop new flavor ingredients and technologies.
Commercialization
Following regulatory approval in a given country, foods and beverages containing our proprietary flavor ingredients can be commercialized immediately. Our collaborators and customers have ultimate responsibility for the commercialization of our flavor ingredients in their end product and flavor system offerings, respectively. Prior to commercialization, collaborators and customers complete extensive product formulation work on their targeted product and flavor system offerings. Food and beverage manufacturers may validate final formulations for products through in-house sensory evaluation as well as external in-market taste tests by consumers. Upon confirming consumer acceptance of target products, the companies complete activities such as the development of packaging and sales materials, and initial manufacturing scale-up of the products, enabling their market launch.
Market Overview
Each of our programs focuses on flavor ingredients that address large, potentially overlapping markets. Our Sweet Taste Program is aimed at developing flavor ingredients or discovering natural high intensity sweeteners that could be used in product categories such as beverages, dairy products and baked goods. Our Savory Flavor Program is aimed at flavor ingredients that could be used in product categories such as ready meals, sauces, soups, pastas, dried foods and snack foods. Our Bitter Blocker Program is focused on flavor ingredients that could be used in products that contain bitter tastants, including beverages, ready meals, canned foods and soups, and products which utilize certain artificial sweeteners. Our Cooling Taste Program is aimed at flavor ingredients that could be used in products that incorporate cooling agents, such as cough medicines, confectionaries and OTC oral hygiene products. Our Salt Taste Program is aimed at developing flavor ingredients that could be used in virtually all food product categories.
Our commercial revenues to date are primarily based on predefined terms within our collaborative agreements, generally royalties under a retail or ingredient supply model. In our retail-based agreements, royalties are calculated as a percentage of the net sales price of a manufacturer’s finished products or are based on the volume of a manufacturer’s finished product that it sells. In our ingredient supply-based agreements, royalties are calculated as a percentage of the sales price of either our flavor ingredient itself or the flavor system in which our flavor ingredient is contained or are based on the volume of the flavor ingredient itself used by a manufacturer in a finished product.
We sell certain novel flavor ingredients to targeted flavor companies for their use in proprietary flavor systems. Some market opportunities are represented in the tables below as direct sales; however, we may still establish collaborative agreements for select flavor ingredients to optimize their commercial value.
The market potential, partners and licenses for our key programs are described below.
Sweet Taste Program
Our Sweet Taste Program is focused on developing flavor ingredients or discovering natural high intensity sweeteners that address large global markets. We have identified S2383, a novel modifier of the high-intensity sweetener sucralose; S6973 and S9632, two modifiers of sucrose, or common table sugar; and S617, a modifier of sucrose and high fructose corn syrup.
The worldwide market for sucrose and fructose is estimated to be $82.4 billion at a volume of 171.8 million metric tons. The sucralose market is approximately $470 million at a volume of 4,756 metric tons. These estimates are based on 2013 data from Euromonitor, Leatherhead Food Research, LMC International, Milling & Baking News and the USDA Economic Research Service.
The most important product category for our Sweet Taste Program is the non-alcoholic beverage market. According to Beverage Digest, 2013 global sales at the retail consumer level exceeded $1 trillion with global volume of almost 136 billion unit cases (each unit case is 192 ounces). The largest segment of this market is carbonated soft drinks with global sales of nearly $374 billion and volume of almost 40 billion unit cases. The following table provides additional detail for 2013 on the key segments of the global non-alcoholic beverage market.
|
Beverage Type |
US Dollars (billions) |
Unit Cases (billions) |
||||||||||||||
|
Carbonated Soft Drinks |
$ | 373.9 | 36 | % | 40.0 | 29 | % | |||||||||
|
Bottle water |
207.4 | 20 | % | 36.5 | 27 | % | ||||||||||
|
Juice drinks |
213.3 | 20 | % | 17.1 | 13 | % | ||||||||||
|
Sports / Energy Drinks |
87.2 | 8 | % | 3.8 | 3 | % | ||||||||||
|
Other1 |
171.8 | 16 | % | 38.6 | 28 | % | ||||||||||
|
Totals |
$ | 1,053.6 | 100 | % | 136.0 | 100 | % | |||||||||
Based on our review of the available third party data, market intelligence and our internal assumptions, we estimate that approximately 10% of the unit cases in the non-alcoholic beverage market are represented in our PepsiCo collaborative agreement. Approximately 65% of this total incorporate a carbohydrate sweetener (e.g. sucrose) and are eligible for the use of our sweet flavor modifier(s).
1 “Other” includes bulk water, squash/syrups, fruit powders, ready-to-drink tea and ready-to-drink coffee.
The commercialization strategy and licensed rights by company for flavor ingredients from our Sweet Taste Program are summarized in the following table.
|
Commercialization Strategy and Licensed Rights by Company | ||||||
|
Sweet Flavor Ingredient / Regulatory Approvals |
Firmenich (Ingredient Supply) |
PepsiCo |
Senomyx | |||
|
Fructose/sucrose modifier S617
Regulatory approval: FEMA GRAS |
Period of exclusivity for all foods and alcoholic beverages; co-exclusive with PepsiCo for powdered beverages |
Exclusive for all non-alcoholic beverages, except co-exclusive with Firmenich for powdered beverages |
Effective March 2018, available for direct sales to flavor companies for use in all foods and alcoholic beverages | |||
|
Sucrose modifier S6973 (Sweetmyx® SR69)
Regulatory approvals: FEMA GRAS, JECFA and EU |
Non-exclusive for all foods and certain beverages |
None |
Currently available for direct sales to flavor companies for use in all foods and certain beverages | |||
|
Sucrose modifier S9632 (Sweetmyx SR96)
Regulatory approvals: FEMA GRAS and JECFA |
Period of exclusivity for all foods and alcoholic beverages; non-exclusive for powdered beverages |
None |
Currently available for direct sales for use in all non-alcoholic beverages; effective September 2015, available for direct sales to flavor companies for use in all foods and alcoholic beverages | |||
|
Sucralose modifier S2383
Regulatory approvals: FEMA GRAS, JECFA and EU |
Exclusive for all foods and beverages |
None |
None | |||
|
New artificial sweet modifiers |
Period of exclusivity for all foods and alcoholic beverages; co-exclusive with PepsiCo for powdered beverages |
Exclusive for all non-alcoholic beverages, except co-exclusive with Firmenich for powdered beverages |
Following Firmenich period of exclusivity, available for direct sales to flavor companies for use in all foods and alcoholic beverages | |||
|
New natural sweet modifiers |
Exclusive for all food and alcoholic beverages; co-exclusive with PepsiCo for powdered beverages |
Exclusive for all non-alcoholic beverages, except co-exclusive with Firmenich for powdered beverages |
None | |||
|
New natural sweeteners |
None |
Exclusive for all non-alcoholic beverages, except co-exclusive with Senomyx for powdered beverages; royalties based on ingredient supply model |
Potential direct sales for use in all foods, alcoholic beverages and powdered beverages | |||
Savory Flavor Program
The goals of our Savory Flavor Program are to mimic the taste of naturally occurring glutamate to enable the reduction or removal of added MSG and to provide new savory tastes to foods by combining our savory flavor ingredients with other ingredients to create unique new flavors. We have received FEMA GRAS determinations for several of our savory flavor ingredients, including S336 and S807. The Chinese Ministry of Health has granted official regulatory approval in China for S336 and S807, and these flavor ingredients have also received positive reviews by JECFA and regulatory approval in the EU.
Our Savory Flavor Program is aimed at flavor ingredients that address a worldwide market for monosodium glutamate (MSG) with a value of $3.6 billion and consumption of 2.6 million metric tons, based on 2013 data from Leatherhead Food Research. Additionally, these flavor ingredients may be used to create unique savory flavor systems. The incremental opportunity for the entire savory flavors market segment is approximately $1.9 billion in global sales based on 2012 data from RTS Resource, Ltd.
We currently have one partner for this program with exclusive rights to our S336 flavor ingredient for virtually all product categories in Asia (except Japan), operating under a retail and ingredient supply-based agreement. This partner also has non-exclusive rights to our S807 flavor ingredient for all food and beverage categories in Asia (except Japan). Outside of the rights exclusively licensed to this partner, we retain the rights for direct sales of all savory flavor ingredients for use in all product categories worldwide.
Bitter Blocker Program
Our bitter taste modifiers are intended to reduce the bitterness associated with certain bitter tastants such as certain high intensity sweeteners, whey and other dairy proteins, cocoa, caffeine and menthol. These bitter tastants may be used in a wide variety of products including tea, nutritional beverages, protein bars, confectionary, ice cream, yogurt, desserts and baked goods. Two of our bitter blockers, S6821 and S7958, have received FEMA GRAS determinations. S6821 has demonstrated activity against bitter tasting foods and beverages that include soy and whey proteins, menthol, caffeine, cocoa, and Rebaudioside A (stevia). S7958, a related bitter blocker with similar functionality, has alternative desirable physical properties that may be useful for these or other product applications. We currently have one partner on this program with exclusive rights in coffee and coffee whitener products, in all geographies except North and South America, operating under an ingredient supply-based agreement with royalty payments based on the quantity of our flavor ingredient manufactured during the royalty period. For the open product categories, we have a direct sales program to sell our novel flavor ingredients to targeted flavor companies for their use in proprietary flavor systems.
Cooling Taste Program
Our cooling taste agents are intended to improve upon the properties of existing cooling agents with benefits which may include odorless compared to menthol, greater potency or improved temporal sensory profiles. Existing cooling agents include menthol, WS-3, Frescolat ML & MGA, Cooler 1 & 2, and Evercool. These cooling agents may be used in a wide variety of products including tea, flavored waters, confectionary products such as chewing gum and mints, toothpaste and mouthwash. We have identified several sample classes of new cooling agents that demonstrate a taste proof-of-concept and display preferred cooling properties. In July 2014, we announced receipt of GRAS determination by the FEMA Expert Panel for S2227, a new cooling ingredient. We currently have one exclusive partner, Firmenich, for S2227 operating under an ingredient supply-based agreement with royalty payments based on their flavor system sales incorporating this cool flavor ingredient.
Salt Taste Program
Our salt taste modifier program is intended to reduce the level of salt contained in packaged food and beverage products. High salt levels are used in a wide variety of products including ready meals, sauces, soups, snack foods, frozen foods, canned foods, dried foods, processed meats and certain baked goods. The actual cost of salt (sodium chloride) is extremely low but the annual usage of food grade salt is estimated at over 1.4 million metric tons (per K+S Group Financial Report 2013).
We have identified a small group of proteins that meet certain criteria for potentially being involved in salt taste, and we have begun more advanced tests to determine if they function to detect salt. Each of these candidate targets is being further evaluated to see if it modulates salty taste, which would provide a taste proof-of-concept. Currently, we have one partner, PepsiCo, for this program.
Commercialization Status
Through December 31, 2014, six of our flavor ingredients have been commercialized through one of our collaborators or direct sales customers. This total includes two of our savory flavors, three products from our sweet taste program, and a bitter blocker that have been incorporated into packaged food or beverage products. Precise information is limited, primarily due to confidentiality agreements. Based on market intelligence, including the royalty reports we receive from our collaborators and the estimated use level of our flavor ingredients, we estimate that finished products of almost 10.4 billion 8 fluid ounce servings or 433 million unit cases of beverages, and over 8.3 million metric tons of food products, have been manufactured or prepared using Senomyx’s novel flavor ingredients. Our estimates are based on the volume or weight of finished products, which include ready-to-drink beverages and ready-to-eat food products along with the volume of finished beverages and weight of food products which are sold as concentrates and require reconstitution prior to consumption. Historically, these products have been marketed in North America, Latin America, Asia, Europe, Africa, Australia and the Middle East.
Royalty-based Collaborations
Our collaborators have commercialized products containing flavor ingredients from three of our programs: Sweet Taste Program, Savory Taste Program and Bitter Blockers Program.
Firmenich has exclusive worldwide rights to market S2383, our modifier of sucralose, in all food and beverage product categories. Retail products incorporating S2383 are currently being marketed in North America, Latin America, Asia and Europe. Firmenich continues to work with other clients evaluating S2383 in a variety of products.
Firmenich has non-exclusive worldwide rights to commercialize our S6973 sucrose modifier for virtually all food and specified beverage categories. Retail products incorporating S6973 are being marketed in the United States, Latin America, Asia, Australia and Africa. These products span a variety of categories including ready-to-drink and powdered beverages, dairy products and baked goods. Products launched with S6973 are showing promising performance based on re-order patterns and additional launches of products containing S6973 are expected during 2015.
Firmenich also has exclusive worldwide rights for a limited time to commercialize our S9632 sucrose modifier in virtually all food categories and alcoholic beverages.
Nestlé’s exclusive rights to our S336 savory flavor ingredient were returned to us effective at the end of 2014. Nestlé’s commercialization of their products incorporating S336 into established products that contain MSG in the Asia, Latin America, Africa, and Middle East regions also ended at the end of 2014. In the future, Nestlé can gain access to S336 through purchases from flavor houses which may purchase S336 from us through our direct sales program.
Ajinomoto has been introducing products that contain one of our flavor ingredients in several Asian countries including China. Ajinomoto has continued to explore additional opportunities to expand their customer base and the number of product offerings within Asia.
A collaborator initiated its first market launch of a retail product containing our S6821 bitter blocker in a Southeast Asian country in 2012 and continues to evaluate the use of S6821 in additional products and geographies.
Sales and Marketing under Royalty-based Collaborations
Under our current collaboration agreements, our collaborators are responsible for sales, marketing, and distribution of any packaged food or beverage product incorporating our flavor ingredients for their own use. As a result, in the past our flavor ingredients were commercialized without our incurring significant sales, marketing and distribution costs and we expect our collaborators to continue to assume those same responsibilities for the use of any flavor ingredients in fields of use that they have licensed from us. Our current collaborators, Ajinomoto, Firmenich, Nestlé and PepsiCo, are recognized leaders in the sales, marketing and distribution of packaged food, beverage and ingredient products.
Manufacturing under Royalty-based Collaborations
Under the majority of our existing collaborative agreements, our collaborator may, in its sole discretion, manufacture directly or through a third party manufacturer the flavor ingredients it licenses from us.
Direct Sales to Flavor Companies
Rather than relying solely on royalty-based collaborations for commercialization, we have a direct sales program to sell certain of our pure flavor ingredients to flavor companies for their use in the development of proprietary flavor systems for their customers. We are marketing these flavor ingredients under the Complimyx® umbrella brand, which we selected to connote the benefits of using these offerings to add value to flavor systems. Additionally, we have developed sub-brands to identify each taste area separately, such as Sweetmyx for our sweet taste modifiers and Savorymyx® for our savory flavors. Specific ingredients within a taste category are referenced using a category code, such as Sweetmyx SR96 which is also known as the sucrose modifier S9632.
Sweetmyx SR96, a sucrose modifier, is currently being evaluated by flavor companies for use in flavor systems that may be incorporated into their customers’ non-alcoholic beverage product offerings. In addition, certain flavor companies have used Sweetmyx SR96 in product demonstrations and/or responses to client briefs, which outline objectives, criteria and deliverables for a new or reformulated flavor system development project. Under terms of the agreement with Firmenich that was amended in April 2013, after a period of exclusivity for Firmenich that ends in September 2015, we can then also begin selling Sweetmyx SR96 for use in food and alcoholic beverage product categories.
We believe our current market potential for Sweetmyx SR96 is based on a number of targeted non-alcoholic beverage product categories, including ready-to-drink and powdered forms of coffee and tea, powdered soft drinks, flavored milk and dairy beverages. According to Euromonitor, these target product categories utilize 5.4 million metric tons of sucrose on an annual basis. Based on our assumptions for average sucrose content of the targeted beverages, the anticipated use level of Sweetmyx SR96 and our anticipated selling price to flavor companies, we estimate that each global market share point is worth $8.3 million to us in direct sales revenues. Furthermore, based on Euromonitor data, we estimate that our current regulatory approvals allow for the commercialization of Sweetmyx SR96 in approximately 50% of the target market opportunity at this time.
Under terms of the agreement with Firmenich as amended in April 2013, their period of exclusivity for Sweetmyx SR69 (S6973), a sucrose modifier, expired on March 31, 2014. Flavor companies are conducting evaluations of Sweetmyx SR69 in their flavor systems for end-use in food and certain beverage product categories. In addition, certain flavor companies have demonstrated and/or responded to client briefs by using Sweetmyx SR69 in some of their flavor solutions for consumer product customers. We received initial commercial quantities of Sweetmyx SR69 from our contract manufacturer, Firmenich, in mid-2014.
The addition of Sweetmyx SR69 to our direct sales program portfolio provides a flavor ingredient offering in food product categories. According to Euromonitor, global use of sucrose across all food categories is 26.4 million metric tons on an annual basis. Key targeted food categories for using Sweetmyx SR69, including baked goods, confectionary, dairy products and sweet sauces and condiments, represent around 65% of this volume. The average sucrose content of targeted food products, anticipated use level of Sweetmyx SR69 and the potential sucrose reduction level vary considerably by product category, making it difficult to calculate a generalized dollar value of direct sales revenue for each global market share point achieved. We estimate that our current regulatory approvals allow for the commercialization of Sweetmyx SR69 in over 90% of the target market opportunity.
Savorymyx UM80 (S807) is also being evaluated by flavor companies in their flavor systems for use in numerous food and beverage categories. In addition, certain flavor companies have demonstrated and/or responded to client briefs by using Savorymyx UM80 in some of their flavor solutions for consumer product customers. We received commercial-scale quantities of Savorymyx UM80 from our contract manufacturer in the first quarter of 2014. We also added Savorymyx UM33 (S336) to our direct sales portfolio in the first quarter of 2015. We currently expect commercial-scale quantities of Savorymyx UM33 to be available for potential sales outside of Asia (except Japan) by the end of the first quarter of 2015.
We estimate the direct sales potential of Savorymyx UM80 based on the global MSG market. According to Leatherhead, 2013 MSG consumption was 2.6 million metric tons. Based on our assumptions for average MSG content, the anticipated use level of Savorymyx UM80 and our anticipated selling price to flavor companies, we estimate that each global market share point is worth $2.0 million to us in direct sales revenues. Our estimate does not include direct sales revenues from the $1.9 billion global savory flavors market. We estimate that our current regulatory approvals allow for the commercialization of Savorymyx UM80 in over 90% of the target market opportunity.
Bittermyx® BB68 (S6821), a bitter blocker, is currently being evaluated by flavor companies for multiple potential usages. In addition, certain flavor companies have demonstrated and/or responded to client briefs by using Bittermyx BB68 in some of their flavor solutions for consumer product customers. We received commercial quantities of Bittermyx BB68 from our contract manufacturer in the third quarter of 2014.
Sales and Marketing under Direct Sales Program
We have developed sales and marketing capabilities within our internal organization to support our direct sales program. Our initial focus is directed toward global flavor companies with operations in the United States and other U.S. based flavor companies. We anticipate that global flavor companies will commercialize our flavor ingredients outside the United States in geographies where we have appropriate regulatory authorization, and in the future we may also market our flavor ingredients directly to more region specific flavor companies outside the United States.
Manufacturing under Direct Sales Program
Our internal commercial organization is also overseeing supply chain requirements, including management of third party manufacturers of our flavor ingredients. These third parties are responsible for the purchase of raw materials and manufacture of finished products. We may also utilize third parties to provide inventory warehousing, distribution, importation, and other supply chain support.
Our Collaborative Agreements
We pursue collaborations with leaders in the packaged food, beverage and ingredient supply markets. Under each of our current collaborative agreements, we have agreed to conduct research and develop flavor ingredients in one or more specified taste areas, such as sweet, savory, bitter, cool or salt. These collaborations are generally focused on one or more specific product categories, such as non-alcoholic beverages, confectionary products or frozen foods. We currently have collaborative agreements with Ajinomoto, Firmenich, Nestlé and PepsiCo.
Our current collaboration agreements generally provide for royalty payments in the event the collaborator commercializes a product incorporating our flavor ingredients. The specific type of royalty and method for calculating royalty payments varies by agreement and many factors may affect the potential royalty payments under our agreements. In addition, royalty rates under some of our agreements may vary from period to period. Accordingly, any estimates of future royalties are uncertain and difficult to predict. We generally describe our collaborative agreements as retail-based agreements or ingredient supply-based agreements. Under our retail-based royalty agreements, any potential royalty payable to us is calculated as a percentage of the net sales price of a manufacturer’s finished products at the distribution level or is based on the volume of a manufacturer’s finished product that it sells. Our retail-based royalty agreements provide for an effective royalty of up to 4%. Our agreements with PepsiCo (Sweet) and Ajinomoto are partially retail-based royalty agreements. Under our ingredient supply-based agreements, any potential royalty payable to us is calculated as a percentage of the sales price of either our flavor ingredient itself or the flavor system in which our flavor ingredient is contained or is based on the volume of the flavor ingredient itself used by a manufacturer in a finished product. Our ingredient supply royalty agreements specify royalty rates that are typically greater than the rates specified by our retail-based agreements. Our agreements with Ajinomoto, Firmenich, Nestlé and PepsiCo (Sweet) are either exclusively or partially ingredient supply-based royalty agreements.
Most of our current collaboration agreements provide for research and development funding, milestone payments upon achievement of pre-defined research or development targets and cost reimbursement. Certain of our current collaboration agreements also provide for license fees and minimum periodic royalties. The research and development funding under each of these agreements is typically paid according to a fixed payment schedule, but may vary from period to period upon mutual agreement of the parties. Each of these collaborations provides us with a portion of the funding we require to pursue the discovery and development of flavor ingredients for the applicable program. Under each of these agreements, we are primarily responsible for the discovery and development phases and any associated expenses, while our collaborator is primarily responsible for selecting the products that may incorporate our flavor ingredients. Under most of our agreements, our collaborator is also responsible for manufacturing, marketing, selling and distributing any of these products for their own use, and any associated expenses. Under our agreements, we are primarily responsible for the development and regulatory approval phase for flavor ingredients, as well as the prosecution and maintenance of the underlying intellectual property for our flavor ingredients, and a portion of the associated expenses for all of those activities.
We believe our collaborations will allow us to benefit from our collaborators’ well-established brand recognition, global market presence, established sales and distribution channels and other industry-specific expertise. Each of our collaborations is governed by a joint steering committee, consisting of an equal number of representatives of the collaborator and us. The steering committees provide strategic direction and establish performance criteria for the research, development and commercialization of our flavor ingredients. In most instances, decisions of the steering committees must be unanimous.
Our collaborative agreements provide that we will conduct research and development on flavor ingredients for use within clearly defined packaged food, beverage and ingredient product fields, typically on an exclusive or co-exclusive basis for the collaborator during the collaborative period specified in each of the agreements. Our current product discovery and development collaborators are not prohibited from entering into research and development collaboration agreements with third parties in any product field. Under the terms of each agreement, we will retain rights to flavor ingredients that we discover during the collaboration for use with the collaborator, or for our use with other collaborators outside of the defined product fields of that agreement. We will also generally retain rights to any flavor ingredients that we discover after the respective collaborative period. However, in some instances we have agreed to arrangements where we would not launch competing products that one of our collaborators has selected for development and commercialization. We have also agreed under the terms of one of our collaboration agreements that we would not license similar flavor ingredients to the collaborator’s competitor for the same intended uses and product categories that we have licensed to our collaborator. In addition to the collaborative agreements, we have a commercialization and license agreement with Firmenich regarding our S2383 sucralose modifier. In the case of certain of our agreements, if the collaborator terminates the agreement or fails after a reasonable time following regulatory approval or FEMA GRAS determination to incorporate one or more of our flavor ingredients into a product, it will no longer be entitled to use, and we will have the right to sell or license the flavor ingredients to other packaged food, beverage and ingredient companies for use in any product field covered in the agreement.
Most of our agreements expire when we are no longer entitled to royalty payments under the agreement. In addition, each agreement may be terminated earlier by mutual agreement or by either party in the event of a breach by the other party of its obligations under the agreement. Furthermore, following the collaborative research period under a given agreement, our collaborators may elect to discontinue the commercialization of one of our flavor ingredients and unilaterally terminate the agreement. In the event of termination of an agreement prior to expiration, rights to sell or license flavor ingredients generally revert to us.
Key Collaborative Agreements
Firmenich (Sweet)
In July 2009, we entered into a collaboration agreement with Firmenich to work for a minimum two-year collaborative period to discover novel flavor ingredients intended to modify the sweet taste of sucrose, fructose, or various forms of rebaudioside. The agreement included three consecutive options to extend the collaborative research funding period by one year each. Under the agreement, Firmenich agreed to pay a license fee in three installments, research and development fees, cost reimbursements and specified payments upon the achievement of milestones. In January 2010, Firmenich elected to proceed with commercial development of S6973. In October 2010, we amended the agreement with Firmenich to include commercial development of S6973 for specific beverage applications in exchange for an incremental license fee, additional milestones and minimum annual royalties, in addition to royalties on sales of products containing S6973. In February 2011, Firmenich elected to exercise their first one-year option to extend the collaborative research funding period through July 2012. In April 2012, Firmenich elected to exercise their second one-year option to extend the collaborative research funding period through July 2013. In April 2013, we amended and restated the collaboration agreement with Firmenich to extend the collaborative research period through July 2016, subject to limited termination rights. Firmenich agreed to pay an additional non-refundable license fee of $5 million, of which $4 million was paid in 2013. The remaining $1 million will be paid at the earlier of the achievement of a certain milestone or the end of the research period.
Under this agreement, through December 31, 2014, we have received $46.0 million in license fees, research and development funding and cost reimbursements and six milestones totaling $3.4 million. If all milestones are achieved, and including the remaining license fees and research and development funding, we may be entitled to an additional $21.3 million for a total of $70.7 million. There is no guarantee that we will receive any further milestone payments under this collaboration. We are entitled to receive royalties on future net sales of products containing a discovered flavor ingredient from the date of introduction of each product in each country until the expiration of relevant patents. Any future royalties under this collaboration are uncertain and difficult to predict. If our collaborative agreement with Firmenich were to terminate earlier than currently anticipated, or if Firmenich were to discontinue or reduce its actual commercialization efforts related to currently marketed Senomyx flavor ingredients, we may experience a material decline in our revenues.
PepsiCo (Sweet)
In August 2010, we entered into a collaboration agreement with PepsiCo. The agreement relates to a four-year research program to discover and develop (i) novel natural and artificial flavor ingredients intended to modify the sweet taste of sucrose and fructose, including high fructose corn syrup, and (ii) natural high intensity sweeteners, in each case for use in non-alcoholic beverage product categories on a worldwide basis. Under the agreement, we received an upfront payment of $30.0 million from PepsiCo, $7.5 million of which was paid in the second quarter of 2010 in connection with the signing of a letter agreement between the parties and $22.5 million of which was paid in the third quarter of 2010. We received $32.0 million in research and development payments over the initial four-year research period, which ended in August 2014. In May 2014, PepsiCo exercised its option to extend the research period for an additional two years through August 2016. We are entitled to $17.6 million in committed research funding to be paid in quarterly installments over the additional two-year period. We are also entitled to milestone payments and reimbursement of certain out-of-pocket expenses. Upon commercialization, we are entitled to minimum annual royalties and royalty payments on products that incorporate selected flavor ingredients and/or natural high intensity sweeteners. Royalties on products sold by PepsiCo or its affiliates that incorporate a selected flavor ingredient will be equal to a base amount plus a portion of the cost savings, if any, derived from the use of the flavor ingredient in the applicable product. Royalties on products sold by PepsiCo or its affiliates that incorporate a selected natural high intensity sweetener will be equal to a portion of the cost of the sweetener. PepsiCo has the unilateral right to terminate the agreement in the event that a direct competitor of PepsiCo acquires more than 30% of our outstanding voting securities.
With respect to the sweet taste modifiers and natural high intensity sweeteners that PepsiCo selects for commercial development, generally PepsiCo will have (i) exclusive rights to use the flavor ingredient or natural high intensity sweetener, as the case may be, for all forms of non-alcoholic beverages other than powdered beverages for so long as PepsiCo continues to pay the applicable minimum annual royalty, and (ii) co-exclusive rights to use the flavor ingredient or natural high intensity sweetener, as the case may be, for all powdered non-alcoholic beverages. However, PepsiCo has agreed to sublicense its rights to any flavor ingredients that they select for development to one or more third party ingredient suppliers that will be authorized to supply the flavor ingredient, either alone or in combination with other flavors, to any third party manufacturer of non-alcoholic beverages for use in the categories of: functional beverages, including meal replacement drinks and energy drinks, coffee based drinks, milk and soy based beverages and sour-milk beverages. We will receive royalties based on a percentage of the net sales price of products that are sold by a sublicensee ingredient supplier and that incorporate a selected flavor ingredient. PepsiCo was not granted a right to sublicense its rights to selected flavor ingredients for use in the categories of non-alcoholic carbonated beverages, fruit drinks, teas, waters and sports drinks. Also, PepsiCo was not granted a right to sublicense its rights to natural high intensity sweeteners for use in any non-alcoholic beverages. Accordingly, we anticipate that PepsiCo will use flavor ingredients and natural high intensity sweeteners in those categories on an exclusive basis unless PepsiCo and we mutually agree to other arrangements at some time in the future.
Under this agreement, through December 31, 2014, we have received $69.5 million in upfront fees, research and development funding and cost reimbursements and four milestones totaling $3.0 million. If all milestones are achieved and including the remaining research and development funding, we may be entitled to an additional $25.6 million for a total of $98.1 million. There is no guarantee that we will receive any additional milestone payments under this collaboration. In the event of commercialization, we are entitled to receive royalties on future net sales of products containing a discovered flavor ingredient from the date of introduction of each product in each country until the expiration of relevant patents. We cannot assure you that we will receive any royalties under this collaboration. If our collaborative agreement with PepsiCo were to terminate earlier than currently anticipated, we may experience a material decline in our revenues.
Other Collaborative Agreements
Ajinomoto
In March 2006, we entered into a collaborative research, development, commercialization and license agreement with Ajinomoto for the discovery and commercialization of novel flavor ingredients on an exclusive basis in the soup, sauce and culinary aids, and noodle product categories, and on a co-exclusive basis in the bouillon product category within certain Asian markets. Under the terms of the initial collaboration, Ajinomoto agreed to pay us an upfront license fee and research and development funding for up to three years. In addition, we are eligible to receive milestone payments upon achievement of specific product discovery and development goals and reimbursement for certain expenses that we incur. In April 2007, we amended the agreement to expand Ajinomoto’s rights into North America. In August 2007, we further amended the agreement to expand Ajinomoto’s rights into additional product categories and geographies that were not previously licensed by us. In January 2013, Ajinomoto relinquished and returned to us the rights to the previously licensed flavor ingredients in North America and in the other geographies granted in the August 2007 amendment. Ajinomoto is continuing to commercialize products that include one of our flavor ingredients in Asia. We received research and development funding through March 2010. In addition, we have received milestone payments, cost reimbursements and minimum periodic royalty payments from Ajinomoto.
Firmenich (Cool)
In December 2007, we entered into a collaboration agreement with Firmenich to work for a three-year collaborative period to discover and develop novel flavor ingredients that may be used by Firmenich on an exclusive basis worldwide to impart a cool taste in flavor systems. In November 2010, we amended the agreement to extend the collaborative period until December 2012. In December 2012, we amended the agreement to extend the collaborative period until June 2013, which marked the end of the collaborative period. Under the agreement, Firmenich agreed to pay research fees and specified payments upon the achievement of milestones. Firmenich also agreed to reimburse us for a portion of the costs associated with the development and regulatory approval process of flavor ingredients that it selects for development, as well as a portion of certain expenses that we incur related to the research program. In addition, based on the regulatory approval in 2014 of a discovered flavor ingredient, we are entitled to minimum periodic royalties; in the event of commercialization, we are entitled to royalties on future sales of products containing the discovered flavor ingredient until the expiration of relevant patents. We cannot assure you that we will receive any future milestone payments or royalties under this collaboration.
Firmenich (Sucralose)
In November 2008, we entered into a second collaboration agreement with Firmenich for S2383, our novel modifier of the high-intensity sweetener sucralose. Under the agreement, Firmenich has agreed to pay to us royalty payments based on sales of S2383 when it is sold on either a stand-alone basis or within a flavor system. We have received royalty payments under this collaboration.
Nestlé SA (2002 Collaboration)
In April 2002, we entered into an initial collaboration agreement with Nestlé to discover specified flavor ingredients in the food and beverage product fields of dehydrated and culinary food, frozen food and wet soup. In April 2005, we amended the agreement to provide for an extension of the collaborative research phase. In March 2006, we further amended the agreement to include commercialization of novel flavor ingredients in the pet food category on a worldwide, co-exclusive basis. In addition to the expansion, under the amendment we reacquired rights to certain of our flavor ingredients in certain geographic regions. As a result of this amendment, Nestlé had rights to flavor ingredients in Europe, Asia, Israel, Oceania, Africa, the Middle East and Latin America in specified product categories within the dehydrated and culinary food, frozen food, and/or wet soup product categories, as well as worldwide rights for the pet food category. In April 2008, we further amended the agreement to extend the collaborative period through April 2010. In September 2008, we further amended the agreement to provide for a specified escalating payment arrangement in lieu of a sales-based royalty for a limited period of time. In July 2010, we again amended the agreement to provide for an alternative methodology for calculating payments to us in lieu of a sales-based royalty. This amendment, among other things, also granted Nestlé additional non-exclusive rights to commercialize certain retail products containing S336, our savory flavor ingredient, in additional countries in Asia. Effective December 31, 2014, the agreement concluded and consequently we regained rights previously licensed to Nestlé for all flavor ingredients in all product categories worldwide. Under the agreement, we received research and development funding through April 2010 and we received milestone payments, cost reimbursements, royalty payments and minimum periodic royalty payments. We received cumulative payments from Nestlé under this agreement totaling $26.2 million, including $2.6 million of minimum annual royalties and minimum volume requirement payments reported as commercial revenues in 2014.
Nestlé SA (2004 Collaboration)
In October 2004, we entered into a collaborative agreement with Nestlé focused on the discovery and commercialization of specified novel flavor ingredients in the coffee and coffee whiteners field. Under the terms of the agreement, Nestlé paid certain research and development funding through July 2010 and specified payments upon the achievement of milestones. In May 2012, we amended the agreement such that, in lieu of a royalty based on sales of Nestlé products, royalty payments would be based on the quantity of our flavor ingredient manufactured by or on behalf of Nestlé during the applicable royalty period.
PepsiCo (Salt)
In April 2014, we entered into a collaborative research agreement with PepsiCo focused on the identification of flavors with modifying properties intended to restore the desired salty taste in products with reduced salt. Under the terms of the agreement, PepsiCo provided certain research funding for our Salt Taste Program through December 2014, with options to continue the research funding period for up to three years. In January 2015, we entered into an amendment of the agreement whereby PepsiCo will provide certain additional research funding through December 2015. Under the agreement, we will determine whether to advance into commercial development any salt flavor modifiers discovered during the research funding period. PepsiCo will have non-exclusive rights to selected salt flavor modifiers discovered during the research funding period for use in all PepsiCo products worldwide. In addition, PepsiCo will receive a two-year period of exclusivity for the use of each of these flavor modifiers in snack foods and snack dips. In the event that PepsiCo pursues commercialization of any salt flavor modifiers discovered during the research funding period, PepsiCo will purchase the salt flavor modifiers directly from us. PepsiCo has received customary protection provisions to ensure supply if we do not meet PepsiCo’s supply requirements; in such case, PepsiCo will have the right to contract with third party manufacturers and we will receive a royalty based on PepsiCo’s purchases from these third party manufacturers. We cannot assure you that we will receive any future commercial payments under this collaboration.
Competition
Our goal is to be the leader in discovering novel flavor ingredients for use in a wide range of packaged food, beverage and ingredient products. Other companies are possibly pursuing similar technologies and the commercialization of products and services relevant to flavor ingredients. Although we are not aware of any other companies that have the scope of proprietary technologies and processes that we have developed in our field, there are a number of competitors who possess capabilities relevant to the flavor ingredient field.
In particular, we face substantial competition from companies pursuing the commercialization of products and services relevant to taste using more traditional methods for the discovery of flavor ingredients, or for the reduction of salt, sugar, monosodium glutamate, or MSG, or bitter taste. These competitors include leading flavor companies, such as Firmenich, Givaudan SA, International Flavors & Fragrances Inc., Symrise and Takasago. These companies provide flavors and other products, such as oils, extracts and distillates, to consumer products companies for use in a wide variety of products including foods, beverages, confectionaries, dairy products and pharmaceuticals. Competitors currently developing or marketing high intensity sweeteners include Ajinomoto, BRAIN AG or Biotechnology Research and Information Network AG, Cargill, GLG Life Tech, Natur Research Ingredients, Nutrasweet, Nutrinova GMBH, PureCircle Limited, Symrise and Tate & Lyle. Competitors currently developing or marketing menthol or cooling agents include International Flavors and Fragrances, Givaudan, Jindal Drugs, Mentha & Allied, Sharp Menthol, Symrise, Takasago and Renessenz LLC. We will continue to compete in the future with these companies in collaborating with and selling flavor ingredients and technologies to manufacturers of food, beverage and ingredient products. Many of these companies have substantially greater capital resources, research and development resources and experience, manufacturing capabilities, regulatory expertise, sales and marketing resources, and more established relationships with consumer products companies than we do.
We may in the future face competition from life sciences and other technology companies and other commercial enterprises. These entities engage as we do in biotechnology, biology or chemistry and could apply this technology to the discovery and development of flavor ingredients. We are aware of another company, Chromocell, Inc., that is involved in research for the discovery and development of sweet enhancers and salt substitutes. Products developed as a result of our competitors’ existing or future collaborations may compete with our flavor ingredients.
Universities and public and private research institutions are also potential competitors. While these organizations primarily have educational objectives, they may develop proprietary technologies related to the sense of taste or secure patent protection that we may need for the development of our technologies and products. We may attempt to license these proprietary technologies, but these licenses may not be available to us on acceptable terms, if at all.
Methods for reducing sodium include the use of potassium chloride in combination with flavors and masking agents. Although savory flavor enhancers are commercially available, they are not very potent, are not patent protected and are sold as a commodity. The blocking of bitter taste is typically accomplished by attempting to mask the bitter taste with a sweetener or another flavor ingredient. Existing cooling agents, such as menthol and WS-3, are currently in use. However, our competitors, either alone or with their collaborative partners, may succeed in developing technologies or discovering flavor ingredients that are similar or preferable in the areas of, among others, effectiveness, safety, cost and ease of commercialization, and our competitors may obtain intellectual property protection or commercialize such products sooner than we do. Developments by others may render our product candidates or our technologies obsolete. In addition, our current product discovery and development collaborators are not prohibited from entering into research and development agreements with third parties in any particular field.
Employees
As of December 31, 2014, we had 110 full-time employees, including 27 with Ph.D. degrees. Of our full-time workforce, 83 employees are engaged in research and development and 27 are engaged in sales, marketing, intellectual property management, finance and administration. We also retain outside consultants. None of our employees are covered by collective bargaining arrangements, and our management considers its relationships with our employees to be good.
In January 2015, we announced a restructuring plan designed to improve operating efficiencies whereby we reduced our work force by 16 full-time equivalent employees, comprised of 15 full-time employees and two part-time employees.
ITEM 1A. RISK FACTORS
You should consider carefully the following information about the risks described below, together with the other information contained in this annual report on Form 10-K and in our other public filings, in evaluating our business. If any of the following risks actually occurs, our business, financial condition, results of operations and future growth prospects would likely be materially and adversely affected. In these circumstances, the market price of our common stock would likely decline.
Risks Related To Our Business
We are substantially dependent on our current and any future product discovery and development collaborators to develop and commercialize any flavor ingredients we may discover.
Under our current royalty-based business model, we are substantially dependent on our current and any other possible future collaborators to commercialize any flavor ingredients that we successfully develop and to provide the sales, marketing and distribution capabilities required for the success of our business. We have limited or no control over the amount and timing of resources that our current or any future collaborators may devote to our programs or potential products. Our collaborators may decide not to devote the necessary resources to the commercialization of our flavor ingredients and may choose not to incorporate our flavor ingredients into any or all of their products within their exclusive or co-exclusive product fields on a timely basis or at all. Although our collaboration agreements vary, in some situations a collaborator may have the ability to return rights to one or more of our licensed flavor ingredients in some or all product categories or licensed territories and discontinue any associated minimum annual royalty obligations for those flavor ingredients, product categories or territories, as the case may be. A collaborator may elect to take any of these actions for any number of reasons, including as a result of unfavorable publicity regarding our flavor ingredients or our research methods, or if our flavor ingredients do not have the characteristics desired by the collaborator. These characteristics include, among other things, modifying properties, stability under various manufacturing and use conditions, solubility, taste, cost and an adequate safety profile. If these collaborators fail to conduct their commercialization, sales and marketing or distribution activities successfully and in a timely manner, or if our existing collaborators terminate their collaboration agreements with us prior to the expiration of the agreements, it will delay our ability to commercialize our flavor ingredients, we will earn little or no royalty revenues from our flavor ingredients and we will not be able to achieve our objectives or build a sustainable or profitable business.
A substantial portion of our revenues is derived from only two collaborators. If our agreements with these two collaborators terminate earlier than anticipated for any reason, our revenues may materially decline.
During fiscal year 2014, 87% of our revenues were derived from two collaborators, with PepsiCo comprising $14.5 million, or 53%, of revenues and Firmenich comprising $9.4 million, or 34%, of revenues.
Our current collaborative agreements with PepsiCo and Firmenich related to our Sweet Taste Program are scheduled to expire in August 2016 and July 2016, respectively. If either collaborative agreement were to terminate earlier than currently anticipated, or if Firmenich were to discontinue or reduce its commercialization efforts related to our currently marketed flavor ingredients, we may experience a decline in our revenues and we may not be able to achieve our financial projections.
Even if we or our collaborators receive regulatory approval and incorporate our flavor ingredients into products, those products may never be commercially successful.
Even if we discover and develop flavor ingredients with appropriate attributes required for use in commercial products and we obtain the necessary GRAS determinations or other regulatory approvals, the commercial utility for a novel flavor ingredient that we develop may ultimately be more limited than we expect. Our success depends to a significant degree upon successful commercial launches of food, beverage and ingredient products incorporating our flavor ingredients. If these products fail to achieve or subsequently maintain market acceptance or commercial viability, our business would be significantly harmed because our commercial revenues are dependent upon consumer sales of these products. In addition, we may be unable to maintain our existing collaborations or attract new product discovery and development collaborators or new customers for our direct sales program. Many factors may affect the willingness of food and beverage companies to launch new or reformulated products incorporating our flavor ingredients and the market acceptance and commercial success of any potential products incorporating flavor ingredients, including:
|
● |
health concerns, whether actual or perceived, regarding our flavor ingredients or those of our competitors; |
|
● |
unfavorable publicity regarding our flavor ingredients or our research methods; |
|
● |
the timing of market entry as compared to competitive products; |
|
● |
whether our collaborators devote sufficient financial and other resources to promote our flavor ingredients; |
|
● |
the pricing of products that contain our flavor ingredients relative to other competing products; |
|
● |
the costs and market risks of reformulating existing products; |
|
● |
the rate of adoption of products by our collaborators and other companies in the flavor industry; and |
|
● |
any product labeling that may be required by United States or foreign regulatory agencies for products incorporating our flavor ingredients. |
We may not be able to commercialize the flavor ingredients in our portfolio that we currently control, which could negatively impact our results of operations and market share.
We have several flavor ingredients in our portfolio that we have discovered and developed but that are not currently exclusively licensed to a third party collaborator for one or more product categories and/or geographies, including, but not limited to, our Sweetmyx® SR96 (S9632) flavor ingredient for which we have rights for use in non-alcoholic beverages, Savorymyx® UM80 (S807) for which we have worldwide rights in all products, Sweetmyx SR69 (S6973) for which we have worldwide rights in all products, Bittermyx® BB68 (S6821) for which we have worldwide rights in certain products, and Savorymyx UM33 (S336), for which we have certain rights in Japan and worldwide rights, outside of Asia, in all products. We currently intend to commercialize these and potentially other flavor ingredients under our direct sales program; however, we also retain the flexibility to consider licensing the rights to any flavor ingredients that we control to a third party collaborator.
There can be no assurance that our direct sales program will be successful or that we will enter into any new business arrangements for any of our flavor ingredients that are not currently exclusively licensed to a third party collaborator. We may encounter difficulties or delays in implementing our direct sales program or entering into any new business arrangements that we elect to pursue. Any of these events could also delay our anticipated timelines, prevent the successful commercialization of our flavor ingredients, negatively impact our financial results, and delay or prevent us from ever achieving or sustaining profitability.
We have a history of operating losses and we may not achieve or maintain profitability.
We have not been profitable and have generated substantial operating losses since we were incorporated in September 1998. We expect to incur additional losses in the future. The extent and duration of our future losses will depend, in part, on the rate of increase in our operating expenses and the rate of growth, if any, in our revenues from our existing and any future product discovery and development collaborations as well as from our direct sales program and other sources that may become available to us in the future. To date, substantially all of our revenues have come from research and development funding, license fees, cost reimbursement and milestone payments under our product discovery and development collaborations. In order for us to generate further royalty revenues and become profitable, we must successfully retain our existing product discovery and development collaborations and our collaborators must further commercialize products incorporating one or more of our flavor ingredients, from which we can derive additional royalty revenues, or we must successfully implement our direct sales program or alternative strategies where we receive revenues from other sources. Our ability to generate commercial revenue is uncertain and will depend upon, among other things, our ability to meet particular commercialization, research and development objectives.
We may seek additional capital to fund our operations.
If we are unable to successfully commercialize our flavor ingredients, maintain our existing product discovery and development collaborations or enter into new collaborations, we will likely need to obtain additional capital, reduce our ongoing expenses and/or modify our strategy to continue our operations. In addition, our business and operations may change in a manner that would consume available resources at a greater rate than anticipated, or we may decide that for other reasons it is in our best interests to seek additional capital. In such an event, we may need to raise substantial additional capital to, among other things:
|
● |
fund research, discovery or development programs; |
|
● |
advance product candidates into and through the safety evaluation and regulatory approval process; |
|
● |
acquire rights to products or product candidates, technologies or businesses; |
|
● |
support the commercialization of our flavor ingredients; and |
|
● |
prosecute, maintain and enforce our intellectual property rights. |
If we pursue additional capital to continue our operations, we cannot assure you that additional financing will be available on terms acceptable to us, or at all. If adequate funds are not available or are not available on acceptable terms, our ability to fund our operations, take advantage of opportunities, identify and develop flavor ingredients, develop technologies or otherwise respond to competitive pressures could be significantly limited. In addition, if financing is not available, we may need to alter our strategies, reduce our ongoing expenses or cease operations. In addition, issuances of debt or additional equity could impact the rights of the holders of our common stock, may dilute our stockholders’ ownership and may impose restrictions on our operations. These restrictions could include limitations on additional borrowing, specific restrictions on the use of our assets as well as prohibitions on our ability to create liens, pay dividends, redeem our stock or make investments.
Disagreements or disputes with a collaborator or customer of our direct sales program could adversely impact our business operations and prospects.
From time to time we have disagreements or disputes with our collaborators regarding various subject matters, such as the interpretation of contractual rights and obligations under our agreements, the design of development studies for our flavor ingredients and intellectual property matters. Because we depend on our collaborators to fund our research and development programs and commercialize our flavor ingredients, any disputes or disagreements with our collaborators could disrupt our business operations and adversely impact our ability to maintain existing collaborations or secure new collaborations. We may also have disagreements or disputes with customers of our direct sales program regarding various subject matters such as the interpretation of contractual rights and obligations under our terms and conditions of sale. Whenever we become involved in a dispute or litigation with any collaborator or customer, we might have to spend significant amounts of money, time and effort to defend our position and we may not be successful. Even if we are successful, any dispute could divert management attention and resources from other strategic, commercial and research priorities.
We must secure and maintain regulatory approvals of our flavor ingredients through various governmental bodies outside the United States. The applicable regulations are complex and subject to change, which may adversely impact our ability to commercialize our flavor ingredients internationally.
Sales of our flavor ingredients outside of the United States will be subject to foreign regulatory requirements, which are determined by multiple governing bodies, such as the Joint FAO/WHO Expert Committee on Food Additives, or JECFA, and the European Food Safety Authority, or EFSA, and in some instances individual countries, such as China, Indonesia and Japan. These foreign regulatory requirements are complex and constantly changing, sometimes quite unpredictably, due, in part, to changes in agendas of political, business and social activist groups as well as government priorities. We may be required to incur substantial costs to comply with current or future laws and regulations, or new interpretations of existing laws and regulations, and our operations, business or financial condition could be adversely affected by such future requirements or interpretations of existing requirements.
A Generally Recognized as Safe, or GRAS, determination in the United States or in any other jurisdiction does not ensure approval in other jurisdictions because the requirements from jurisdiction to jurisdiction may vary widely and may change over time. In most cases, whether or not a GRAS determination has been obtained in the United States, approval of a product by the applicable regulatory authorities for a foreign country must still be obtained prior to manufacturing or marketing the product in that country. For example, we are aware of ongoing activities that are intended to clarify the regulatory approval process for flavor ingredients within the EU. Because of the inherent uncertainty associated with the regulatory approval process outside the United States, predicting the outcome or timing of review of any of our submissions to foreign regulatory authorities, present or in the future, is difficult. Accordingly, our estimates and forecasts for those submissions and potential approvals may not be accurate. The process of obtaining foreign approvals could result in significant delays, difficulties and costs for us and require additional safety studies and additional expenses. If we experience delays or if we fail to comply with these regulatory requirements or to obtain and maintain required approvals, our ability to generate revenue will be diminished.
We and our collaborators may not be successful in overcoming these regulatory hurdles, which could result in product launch delays, unanticipated expenses, termination of collaborations and flavor ingredients not being approved for incorporation into consumer products in one or more geographies. In addition, even after regulatory approval of our flavor ingredients, we may become aware of new information that suggests our flavor ingredients are unsuitable for consumer use, in which case our regulatory approvals may be revoked or we may elect to voluntarily cease the commercialization of those ingredients. These consequences would have a material adverse effect on our business financial condition and results of operations.
If we or our collaborators are unable to obtain and maintain the GRAS determination by FEMA or other regulatory approval required before certain of our flavor ingredients can be incorporated into products that are sold, we would be unable to commercialize our flavor ingredients and our business would be adversely affected.
In the United States, flavor ingredients are regulated by the Food and Drug Administration, or FDA, as approved food additives or GRAS ingredients under the Food, Drug and Cosmetic Act, or FD&C Act. Flavor ingredients that qualify for the GRAS review process are generally intended to be consumed in small quantities and have data supporting their safety under conditions of intended use. The Food Additive Amendments of 1958 prompted the flavor industry to establish in 1960 the FEMA Expert Panel whose purpose is to administer the FEMA GRAS review program for flavor ingredients.
Depending on the amount or intended use of a particular flavor ingredient added to a product and the number of product categories in which the flavor ingredient will be incorporated, specific safety assessment protocols and regulatory processes must be satisfied before we or our collaborators can commercially market and sell products containing any flavor ingredients that we may discover. A key element of our strategy is to develop flavor ingredients that may be subject to review under the FEMA GRAS process. Obtaining and maintaining a GRAS determination or other regulatory approval can be costly and take many years.
In our experiences with the savory, sweet, bitter and cool programs, safety studies, preparation and FEMA GRAS review has historically ranged from 12 to 18 months and cost up to approximately $1.5 million per flavor ingredient. This experience may not be representative of the timing and cost for current and future programs. This approach is less expensive than the alternative of filing a food additive petition with the FDA, approval of which can take up to four years. The FEMA GRAS process may take longer than 12 to 18 months and cost more than $1.5 million depending on the properties of the flavor ingredient, and if we elect to perform additional safety studies or if additional safety studies are requested by the FEMA Expert Panel or one of our collaborators or are necessary to explain unexpected safety study findings. There is a risk that one or more of our product candidates for which we seek FEMA GRAS determination may not qualify for a FEMA GRAS determination for specific categories or at all. This may occur for a variety of reasons, including the flavor ingredient’s intended use, the amount of the flavor ingredient intended to be added to foods and beverages, the number of product categories in which the flavor ingredient will be incorporated, whether the flavor ingredient imparts sweetness, the safety profile of the flavor ingredient and the FEMA Expert Panel’s interpretation of the safety data. For example, we do not believe that any natural high intensity sweetener that we discover would qualify for a FEMA GRAS determination for its use as a sweetener. Even if we obtain a GRAS determination with respect to a flavor ingredient, the FDA has the ability to challenge such determination or one or more of our collaborators may insist on additional studies, which could materially adversely affect our ability to market products on schedule or at all. In the event that a particular flavor ingredient does not qualify for FEMA GRAS determination or if one or more of our collaborators requires additional studies, we could be required to pursue a lengthy FDA approval process or dedicate our development efforts to alternative ingredients, which would further delay commercialization. In addition, laws, regulations or FDA practice governing the regulatory approval process, the availability of the GRAS determination process or the manufacture or labeling of such products, may change in a manner that could adversely affect our ability to commercialize products on schedule or at all and may also harm our ability to maintain our existing collaboration agreements or enter into new collaborations.
We expect that our results of operations will fluctuate from period to period, and this fluctuation could cause our stock price to decline.
Our operating results have fluctuated in the past and are likely to vary significantly in the future based upon a number of factors, many of which we have little or no control over. We operate in a highly dynamic industry and future results could be subject to significant fluctuations. These fluctuations could cause us to fail to meet or exceed our published guidance or financial expectations of securities analysts or investors, which could cause our stock price to decline rapidly and significantly. Revenue and expenses in future periods may be greater or less than revenue and expenses in the immediately preceding period or in the comparable period of the prior year. Therefore, period-to-period comparisons of our operating results are not necessarily a good indication of our future performance. Some of the factors that could cause our operating results to fluctuate include:
|
● |
the ability of our product discovery and development collaborators to incorporate our flavor ingredients into food, beverage and ingredient products, on expected timelines, if at all; |
|
● |
our ability to implement our direct sales program; |
|
● |
the ability and willingness of food and beverage companies to commercialize products incorporating our flavor ingredients in a timely manner, if at all; |
|
● |
the demand for our collaborators’ and other customers’ products containing our flavor ingredients; |
|
● |
the termination, expiration or amendment of any of our product discovery and development collaboration agreements; |
|
● |
our receipt of milestone payments in any particular period; |
|
● |
our ability to enter into new product discovery and development collaborations and technology collaborations or to extend the terms of our existing collaboration agreements and our payment obligations, expected revenue and other terms of any of our agreements; |
|
● |
our ability, or our collaborators’ ability, to successfully satisfy all pertinent regulatory requirements; and |
|
● |
general and industry specific economic conditions which may affect our collaborators’ research and development expenditures and commercialization efforts. |
We are dependent on our current and any future product discovery and development collaborators for our research and development funding.
A key element of our current strategy is to commercialize our flavor ingredients through collaborative agreements. To date, substantially all of our research and development funding has been derived solely from research and development payments, license fees, milestone payments and cost reimbursement payments received under our collaborations. Substantially all of our research and development funding in the foreseeable future will result from these types of payments from these collaborations until such time, if ever, that we earn more significant royalties on future sales of consumer products incorporating our flavor ingredients or begin to generate meaningful revenues from our direct sales program.
Our current collaborators may amend or not renew their agreements with us or, if they do, they may not be on terms that are as favorable to us as our current agreements. If any or all of our current material agreements with our collaborators are amended, expire or are terminated, or if we are unable to, or elect not to, renew or enter into new collaborative agreements, our research and development funding could significantly decline or be substantially eliminated, which would have a material adverse effect on our business, financial condition and results of operations.
We may not be able to negotiate additional collaboration agreements having terms satisfactory to us or at all.
We may not be able to enter into additional collaborative agreements due to the exclusive nature of our current product discovery and development collaborations. Each of our current collaboration agreements provides for the use of flavor ingredients within one or more defined food, beverage and ingredient product fields on an exclusive, co-exclusive or non-exclusive basis for the respective collaborator during the collaborative period specified in the agreement. In the case of exclusive agreements, or co-exclusive agreements where all fields and geographies are granted, we will not be able to enter into additional collaborations with any other food, beverage and ingredient company covering the same product field during the applicable collaborative period. In addition, our collaborators’ competitors may not wish to do business with us at all due to our relationship with our collaborators and under some agreements we have agreed to arrangements where we would not launch competing products or collaborate with a collaborator’s competitor for a limited period of time even after the conclusion of the applicable collaborative period. Consolidation in our target markets may also limit the number of potential collaborators. Further, if we do not achieve our research and development objectives under our existing collaboration agreements prior to the expiration of the collaborative period, our collaborators may elect not to renew these agreements on terms that are acceptable to us. If we are unable to enter into additional product discovery and development collaborations on satisfactory terms, our ability to sustain or expand our business may be significantly diminished.
Our business and operating results may be adversely affected by unfavorable economic and market conditions.
A significant portion of our current business model depends on our ability to maintain and enter into new collaborative research, development and commercialization agreements with leading food, beverage and ingredient companies. Our collaborative agreements typically require our collaborators to make a significant commitment of capital and other resources. In most instances these investments are discretionary on the part of our collaborators. The current weak global economic conditions may reduce the amount of discretionary investment that our current and prospective collaborators may be willing to make in our programs as well as the demand for our flavor ingredients in general. In some instances the result may be that companies elect to defer or delay entering into a collaborative agreement with us, or existing collaborators may amend, terminate or not renew an existing program when it expires. Therefore, weak economic conditions, or a reduction in research and development funding, even if economic conditions improve, would likely adversely impact our business, operating results and financial condition in a number of ways, including longer business development cycles, unfavorable financial or other commercial terms, and longer development timelines.
If we lose our key personnel or are unable to attract and retain qualified personnel, it could adversely affect our business.
Our success depends to a significant degree upon the continued contributions of our executive officers, management and scientific staff. We have entered into employment letter agreements with each of our executive officers; however, all of our employees are at-will employees, which means that either we or the employee may terminate their employment at any time. In addition, we currently have no key person insurance. If we are not able to attract and retain the necessary personnel to accomplish all of our business objectives, we may experience constraints that will adversely affect our ability to meet the demands of our current or any future product discovery and development collaborators in a timely fashion, to support our independent discovery and development programs or to pursue our direct sales program. In addition, we may be delayed or unable to develop new product candidates, commercialize our existing product candidates or achieve our other business objectives as a result of any future loss of our other executive officers or key members of our management or scientific staff, which could cause our stock price to decline. Moreover, the loss of the services of one or more of our executive officers or key members of our management or scientific staff could negatively impact the relationships we have with our collaborators.
We may encounter difficulties managing our growth, which could adversely affect our business.
Our strategy includes entering into and working on simultaneous flavor ingredient discovery and development programs across multiple markets. We may choose to increase headcount in the future in order to meet our strategic objectives. If our growth continues, it will continue to place a strain on us, our management and our resources. Our ability to effectively manage our operations, growth and various projects requires us to continue to improve our operational, financial and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented employees. We may not be able to successfully implement these tasks on a larger scale and, accordingly, we may not achieve our research, development and commercialization goals. If we fail to improve our operational, financial and management information systems, or fail to effectively monitor or manage our new and future employees or our growth, our business would suffer significantly. In addition, no assurance can be made that we will be able to maintain adequate facilities to house our staff, conduct our research or achieve our business objectives.
If we acquire products, technologies or other businesses, we will incur a variety of costs, may have integration difficulties and may experience numerous other risks that could adversely affect our business.
If appropriate opportunities become available, we may consider acquiring businesses, technologies or products that we believe are a strategic fit with our business. We may also consider reacquiring rights to flavor ingredients that are currently licensed to one or more of our collaborators. We have limited, if any, experience in identifying acquisition targets, successfully acquiring them and integrating them into our current infrastructure. We may not be able to successfully integrate any businesses, products, technologies or personnel that we might acquire in the future without a significant expenditure of operating, financial and management resources, if at all. In addition, future acquisitions might be funded by issuances of debt or additional equity, which could impact your rights as a holder of our common stock and may dilute your ownership percentage. Any of the foregoing could have a significant adverse effect on our business, financial condition and results of operations.
Risks Related To Production and Supply
We rely on third parties to manufacture our flavor ingredients on a commercial scale.
We do not have experience in manufacturing nor do we have the resources or facilities to manufacture flavor ingredients on a commercial scale. Therefore, the commercialization of our flavor ingredients depends in part on our or our collaborators’ ability to manufacture, or to contract with third-party manufacturers of our flavor ingredients, on a large scale, at a competitive cost, with the specified quality and in accordance with relevant food, beverage and ingredient regulatory requirements. Any such collaborators or third-party manufacturers may encounter manufacturing difficulties at any time that could result in delays in the commercialization of potential flavor ingredients.
Our inability to find capable manufacturing capacity or to enter into agreements on acceptable terms with third party manufacturers could delay commercialization of any products we may develop and may harm our relationships with our existing and any future product discovery and development collaborators and our customers. Moreover, if we or our collaborators are required to change from one third-party manufacturer to another for any reason, the commercialization of our products may be delayed further. In addition, if any manufacturer of our flavor ingredients fail to comply with the FDA’s good manufacturing practice regulations or similar regulations in other countries, then we or our collaborators may be subject to adverse regulatory action including product recalls, warning letters and withdrawal of our products, or our collaborators’ or customers’ products, from the market, any of which may harm our reputation and our business.
Further, because our flavor ingredients are regulated as food products under the FD&C Act, we and the third parties with which we collaborate or contract to manufacture, process, pack, import or otherwise handle our products or our product ingredients, may be required to comply with certain registration, prior notice submission, recordkeeping and other regulatory requirements. Failure of any party in the chain of distribution to comply with any applicable requirements under the FD&C Act or any new regulations implemented by the FDA, or similar regulations in other countries, may adversely affect the manufacture and/or distribution of our products in commerce.
We currently expect to rely on outside suppliers for our flavor ingredients to support our direct sales program, including Firmenich as sole supplier of our sweet flavor ingredients. If Firmenich or other suppliers are unable to supply us with our required amounts of our flavor ingredients on a timely basis, our results of operations may be adversely affected.
We have agreed to utilize Firmenich as our exclusive manufacturer of any sweet flavor ingredient for our direct sales program that Firmenich has selected to develop under the terms of our collaboration agreement. We have also entered into supply agreements with manufacturers for our savory flavor ingredients and bitter blockers and may enter into additional manufacturing arrangements in the future. Because Firmenich and our other suppliers are third party manufacturers, we have only limited control over the timing and level of their production volumes. If Firmenich or our other suppliers fail to supply us with required amounts of our flavor ingredients under our agreements, we would not be able to meet our customers’ demands unless we were able to utilize alternative sources of supply, which may be more costly and may not even be available on acceptable terms or within an acceptable timeframe. Accordingly, if Firmenich or our other suppliers are unable to supply us with our required amounts of flavor ingredients on a timely basis and with acceptable quality, it may have a material adverse effect on our results of operations.
We hold inventory and have committed to future inventory purchases to support our direct sales program. If our inventory on-hand or committed amounts cannot be sold, our results of operations and/or financial position may be adversely affected.
We hold inventory of commercial quantities of certain of our flavor ingredients for use in our direct sales program. We have committed and will continue to commit to future purchases of our flavor ingredients in advance of customer orders. We have no prior experience in managing inventory for the commercialization of our flavor ingredients. In particular, we may have excess inventory on-hand, we may be forced to purchase excessive amounts of flavor ingredients in order to establish manufacturing relationships with third parties or to give potential customers greater confidence in the reliability of our supply, which may have a material adverse effect on our results of operations and/or financial position.
Risks Related To Our Industry
Many potential competitors, including those who have greater resources and experience than we do, may develop products or technologies that make ours obsolete or noncompetitive.
The life sciences and other technology industries are characterized by rapid technological change, and the area of sensory or taste receptor research is a rapidly evolving field. Our future success will depend on our ability to maintain a competitive position with respect to technological advances. Technological developments by others may result in our flavor ingredients and technologies becoming obsolete.
In particular, we face substantial competition from companies pursuing the commercialization of products and services relevant to taste using more traditional methods for the discovery of flavor ingredients, or for the reduction of salt, sugar, monosodium glutamate, or MSG, or bitter taste. These competitors include leading flavor companies, such as Firmenich, Givaudan SA, International Flavors & Fragrances Inc., Symrise and Takasago. These companies provide flavors and other ingredients, such as essential oils, extracts and distillates, to consumer products companies for use in a wide variety of products including foods, beverages, confectionaries, dairy products and pharmaceuticals. Competitors currently developing or marketing high intensity sweeteners include Ajinomoto, BRAIN AG, or Biotechnology Research and Information Network AG, Cargill, GLG Life Tech, Natur Research Ingredients, Nutrasweet, Nutrinova GMBH, PureCircle Limited and Tate & Lyle. Competitors currently developing or marketing menthol or cooling agents include International Flavors and Fragrances, Givaudan, Jindal Drugs, Mentha & Allied, Sharp Menthol, Symrise, Takasago and Renessenz LLC. We currently compete and will continue to compete in the future with these companies in collaborating with and selling flavor ingredients for incorporation in food and beverage products. Many of these companies have substantially greater capital resources, research and development resources and experience, manufacturing capabilities, regulatory expertise, sales and marketing resources, established relationships with consumer products companies and production facilities.
Savory flavor ingredients, particularly inosine monophosphate, or IMP, are commercially available, and we will compete with the companies that produce these flavor ingredients. IMP is widely available and is a generally accepted food additive by the food, beverage and ingredient industries. As a result, our existing and future collaborators may choose to incorporate IMP or similar savory flavor ingredients into their food, beverage and ingredient products instead of our savory flavor ingredients. We may compete with bitter masking or bitter blocking compounds, such as adenosine 5’ monophosphate, or AMP. We may also compete with known methods for reducing sodium, such as the use of potassium chloride in combination with flavors and masking agents. In addition, we may compete with existing cooling agents, such as menthol and WS-3, which are currently in use.
We may in the future face competition from life sciences and other technology companies and other commercial enterprises. These entities engage as we do in biotechnology, biology or chemistry research and could apply this technology to the discovery and development of flavor ingredients. We are aware of one other company, Chromocell Corp., which is involved in research for the discovery and development of sweet flavor modifiers, bitter blockers and salt substitutes. We cannot guarantee that products developed as a result of our competitors’ existing or future collaborations will not compete with our flavor ingredients.
Universities and public and private research institutions are also potential competitors. While these organizations primarily have educational objectives, they may develop proprietary technologies related to the sense of taste or secure patent protection that we may need for the development of our technologies and products. We may attempt to license these proprietary technologies, but these licenses may not be available to us on acceptable terms, if at all.
Our competitors, either alone or with their collaborative partners, may succeed in developing technologies or discovering flavor ingredients that are more effective, more affordable or more easily commercialized than ours, and our competitors may obtain intellectual property protection or commercialize products sooner than we do. Developments by others may render our product candidates or our technologies obsolete. In addition, our current product discovery and development collaborators are not prohibited from entering into research and development collaboration agreements with third parties in any product field. Our failure to compete effectively would have a significant adverse effect on our business, financial condition and results of operations
Our ability to compete in the flavor ingredient market may decline if we do not adequately protect our proprietary technologies.
Our success depends in part on our ability to obtain and maintain intellectual property that protects our technologies and flavor ingredients. Patent positions may be highly uncertain and may involve complex legal and factual questions, including the ability to establish patentability of sequences relating to taste receptors, proteins, chemical synthesis techniques, compounds and methods for using them to modulate taste for which we seek patent protection. No consistent standard regarding the allowability or enforceability of claims in many of our pending patent applications has emerged to date. As a result, we cannot predict the breadth of claims that will ultimately be allowed in our patent applications, if any, including those we have in-licensed or the extent to which we may enforce these claims against our competitors. The degree of future protection for our proprietary rights is therefore highly uncertain and we cannot assure you that:
|
● |
we were or will be the first to file patent applications or to invent the subject matter claimed in patent applications relating to the technologies upon which we rely; |
|
● |
others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
|
● |
others did not publicly disclose our claimed technology before we conceived the subject matter included in any of our patent applications; |
|
● |
any of our patent applications will result in issued patents; |
|
● |
any of our patent applications will not result in interferences or disputes with third parties regarding priority of invention or the validity of any issued patent; |
|
● |
any patents that have issued or may be issued to us, our collaborators or our licensors will provide a basis for commercially viable products or will provide us with any competitive advantages or will not be challenged by third parties; |
|
● |
we will develop additional proprietary technologies that are patentable; |
|
● |
the patents of others will not have an adverse effect on our ability to do business; or |
|
● |
new proprietary technologies from third parties, including existing licensors, will be available for licensing to us on reasonable commercial terms, if at all. |
In addition, patent law outside the United States is uncertain and in many countries intellectual property laws are undergoing review and revision. The laws of some countries do not protect intellectual property rights to the same extent as domestic laws. It may be necessary or useful for us to participate in opposition proceedings to determine the validity of our competitors’ patents, litigation to enforce our or our licensed intellectual property against others or to defend the validity of any of our or our licensors’ future patents, which could result in substantial costs and would divert our efforts and attention from other aspects of our business. We cannot be certain of the outcome of any such proceedings or litigation.
Technologies licensed to us by others, or in-licensed technologies, are important to our business. In particular, we depend on technology related to certain taste receptor sequences that we license from the University of California and others and technology related to compound libraries that we license from third parties. In addition, we may in the future acquire rights to additional technologies by licensing such rights from existing licensors or from third parties. Such in-licenses may be costly. Also, we generally do not control the patent prosecution, maintenance or enforcement of in-licensed technologies. Accordingly, we are unable to exercise the same degree of control over this intellectual property as we do over our internally developed technologies. Moreover, some of our academic institution licensors, collaborators and scientific advisors have rights to publish data and information to which we have rights. If we cannot maintain the confidentiality of our technologies and other confidential information in connection with our collaborations, our ability to protect our proprietary information or obtain patent protection in the future may be impaired, which could have a significant adverse effect on our business, financial condition and results of operations.
Many of the patent applications we and our licensors have filed have not yet been substantively examined and may not result in patents being issued.
Many of the patent applications filed by us and our licensors were filed recently with the United States Patent and Trademark Office and in foreign patent offices and some have not been substantively examined and may result in granted patents with claims of narrow scope that may not sufficiently deter competitors or may not result in patents being issued. Some of these patent applications claim sequences that were identified from different publicly available sequence information sources such as the High-Throughput Genomic Sequences division of GenBank. It is difficult to predict whether all of our or our licensors’ applications will ultimately be found to be patentable or, if so, to predict the scope of any allowed claims. In addition, the disclosure in our or our licensors’ patent applications, particularly in respect of the utility of our claimed inventions, may not be sufficient to meet the statutory requirements for patentability in all cases. As a result, it is difficult to predict whether all of our or our licensors’ applications will be allowed, or, if so, to predict the scope of any allowed claims or the enforceability of the patents. Even if enforceable, others may be able to design around any patents or develop similar technologies that are not within the scope of such patents. Our and our licensors’ patent applications may not issue as patents that will provide us with any protection or competitive advantage, which may have a material adverse effect on our business.
The Supreme Court recently in the Myriad decision determined that some isolated naturally occurring nucleic acids are ineligible for United States patent protection. This decision may raise concerns as to the validity of some or all of our granted claims, and those in our licensors’ patents, and the patentability of our or our licensors’ pending claims, which are directed to isolated, naturally occurring nucleic acids. Also, while the Supreme Court in the Myriad decision did not address the patentability of other isolated, naturally occurring materials (i.e., non-nucleic acids), it is possible that later courts may determine that other isolated naturally occurring materials are similarly ineligible for United States patent protection. This may raise concerns as to the validity of some or all of our granted claims, and those in our licensors’ patents, which are directed to isolated naturally occurring materials such as isolated, naturally occurring taste receptor polypeptides or isolated, naturally occurring flavor ingredients. In recently issued interim guidance, the United States Patent and Trademark Office has interpreted the Myriad decision as applying to any isolated naturally occurring material including non-nucleic acids. Thus, pending patent applications filed by us or our licensors that are directed to isolated naturally occurring materials may not issue in the United States, which could have a significant adverse effect on our business, financial condition and results of operations.
Disputes concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and extremely costly and could delay our research and development efforts.
Our commercial success, if any, will be significantly harmed if we infringe the patent rights of third parties or if we breach any license or other agreements that we have entered into with regard to our technology or business. Our success will also depend, in part, on our ability to prevent others from infringing our patent rights.
We are aware of other companies and academic institutions that have been performing research in the areas of taste modulation and flavor ingredients. In particular, other companies, academic institutions and inventor applicants have announced that they have conducted taste-receptor or ion channel research and have published data on taste receptor sequence information and taste receptors or filed patent applications or obtained patent protection on taste modulation or taste receptors and their uses, including Ajinomoto, California Institute of Technology, Cargill, Chromocell Corp., Colorado State University, Columbia University, Dendreon, Duke University, the German Institute of Human Nutrition, Givaudan SA, International Flavors & Fragrances Inc., Johannes Gutenberg University, Kyushu University, Monell Chemical Senses Corp., Mount Sinai School of Medicine, the National Institutes of Health, Nestlé, Novartis, NutraSweet, Nutrinova GMBH, Pfizer, Inc., Sloan Kettering, Symrise, Tate & Lyle, The Scripps Research Institute, Unilever, the University of California, the University of Miami, the University of Tokyo, the University of Wisconsin, Virginia Commonwealth University and Wiessenbach. To the extent any of these companies, academic institutions or inventor applicants currently have, or obtain in the future, broad patent claims, such patents could block our ability to use various aspects of our discovery and development process and might prevent us from developing or commercializing newly discovered flavor ingredients or otherwise conducting our business. In addition, it is possible that some of the flavor ingredients that are discovered using our technology may not be patentable or may be covered by intellectual property of third parties.
The life sciences and other technology industries are characterized by extensive litigation regarding patents and other intellectual property rights. Many life sciences and other technology companies have employed intellectual property litigation as a way to gain a competitive advantage. We may become involved in litigation, interference proceedings, derivative proceedings, oppositions, reexamination, protest or other potentially adverse intellectual property proceedings as a result of alleged infringement by us of the rights of others or as a result of priority of invention disputes with third parties. Third parties may also challenge the validity of any of our issued patents in litigation or in opposition, reexamination, or inter partes review proceedings. Similarly, we may initiate proceedings to enforce our patent rights and prevent others from infringing our or our licensed intellectual property rights. In any of these circumstances, we might have to spend significant amounts of money, time and effort defending our position and we may not be successful. In addition, any claims relating to the infringement of third-party proprietary rights or proprietary determinations, or validity determinations, even if not meritorious, could result in costly litigation, lengthy governmental proceedings, divert management’s attention and resources, or require us to enter into royalty or license agreements that are not advantageous to us.
Should any person have filed patent applications or obtained patents that claim inventions also claimed by us, we may have to participate in an interference proceeding or derivative proceeding declared by the relevant patent regulatory agency to determine priority of invention or derivation and, thus, the right to a patent for these inventions in the United States. Such a proceeding could result in substantial cost to us even if the outcome is favorable. Even if successful on priority grounds, an interference action may result in loss of claims based on patentability grounds raised in the interference action. Litigation, interference proceedings, derivative proceedings, or other proceedings could divert management’s time and efforts. Even unsuccessful claims could result in significant legal fees and other expenses, diversion of management’s time and disruption in our business. Uncertainties resulting from initiation and continuation of any patent proceeding or related litigation could harm our ability to compete and could have a significant adverse effect on our business, financial condition and results of operations.
An adverse ruling arising out of any intellectual property dispute, including an adverse decision as to the priority of our inventions or invalidity of our patents, could undercut or invalidate our intellectual property position. An adverse ruling could also subject us to significant liability for damages, including possible treble damages, prevent us from using technologies or developing products, or require us to negotiate licenses to disputed rights from third parties. Although patent and intellectual property disputes in the technology area are often settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include license fees and ongoing royalties. Furthermore, necessary licenses may not be available to us on satisfactory terms, if at all. Failure to obtain a license in such a case could have a significant adverse effect on our business, financial condition and results of operations.
If we are unable to protect our trade secrets and other proprietary information, we could lose any competitive advantage we may have, which could adversely affect our business.
We rely in part on trade secret protection for our confidential and proprietary information, knowhow and processes. Our policy is to execute proprietary information and invention agreements with our employees and consultants upon the commencement of an employment or consulting relationship. These agreements generally require that all confidential information developed by the individual or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not be disclosed to third parties. These agreements also generally provide that inventions conceived by the individual in the course of their employment shall be our exclusive property. Similarly, in the course of our collaborations or in the negotiation of potential collaborations we often disclose confidential and proprietary information under written agreements that obligate those third parties to keep our information confidential and to use our confidential information only for the purposes that we specify. There can be no assurance that we will be able to effectively enforce these agreements or that proprietary information is our exclusive property. There can be no assurance that the subject proprietary information will not be disclosed, that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or that we can meaningfully protect our trade secrets. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Risks Related to Quality and Safety
Concerns with safety and quality could cause customers to avoid products that contain our flavor ingredients.
Adverse publicity about the safety of certain foods due to the actual or potential existence of certain artificial flavors or other ingredients has heightened the sensitivities of many consumers. These safety and quality issues, whether real or perceived, may discourage customers from buying products containing or perceived to contain the ingredients which give rise to such concerns. We could be adversely affected if our customers or the ultimate consumers of our products lose confidence in the safety and quality of our flavor ingredients. Any negative perceptions about the safety and quality of our flavor ingredients could adversely affect our business and financial condition.
We may be sued for product liability and exposed to other product safety-related risks, which could adversely affect our business and harm our reputation.
Because our business strategy involves the development and sale of commercial products incorporating our flavor ingredients, we may be sued for product liability and we may also be the subject of product recalls, product seizures and related adverse publicity. Product liability claims and recalls of products that contain any of our flavor ingredients could result from such things as contamination, spoilage, product misbranding or product tampering, whether real or perceived.
From time to time we receive reports of observed effects after individuals taste solutions or products that include novel flavor ingredients that we are testing or developing, including reports such as irritation of the mouth, tingling of the tongue, lips or gums, and modulation or loss of taste sensation. Our practice is to track reports of any observed effects and, in particular, to evaluate whether any adverse effect may be related to our novel flavor ingredient or whether another cause is determinable. In some instances, these effects may be observed only at higher levels of use or exposure, in which case we may elect to proceed with development, and subsequent commercialization, of a novel flavor ingredient at use levels that we believe are appropriate for only a subset of all potential applications. Nevertheless, we may be held liable if any flavor ingredient we test, develop or commercialize, or any product our collaborators test, develop or commercialize that incorporates any of our flavor ingredients, causes injury or illness or is found otherwise unsuitable during product testing, manufacturing, marketing, sale or consumer use. In addition, the safety studies we must perform and the regulatory approvals we must obtain prior to incorporating our flavor ingredients into a commercial product will not protect us from any such liability.
Any alleged illness or injury associated with any of our flavor ingredients, product defect, product liability judgment or product recall may negatively impact our financial results depending on the reaction of our collaborators, scope, competitive reaction, and consumer attitudes. Even if such an allegation or product liability claim lacks merit, cannot be substantiated, is unsuccessful or is not fully pursued, the negative publicity surrounding any assertion that our flavor ingredients or products that incorporate our flavor ingredients caused illness, injury or death could adversely affect our reputation with existing and potential collaborators and licensees and our corporate image and could cause a decline in our stock price.
Our product liability insurance may not be sufficient to cover our potential liabilities in the case of a product recall or other safety-related claims.
Our product liability insurance may not fully cover our potential liabilities associated with the sale of commercial products incorporating any of our flavor ingredients. Insurance coverage for such risks may be expensive and difficult to obtain, and we may be unable to obtain coverage in the future on acceptable terms, if at all. Our inability to obtain sufficient insurance coverage to protect against potential product liability claims could prevent or inhibit the commercialization of products developed by us or our product discovery and development collaborators. We may be obligated to indemnify our product discovery and development collaborators or customers of our direct sales program for product liability or other losses they incur as a result of our flavor ingredients. Any indemnification we receive from such collaborators or customers for product liability that does not arise from our flavor ingredients may not be sufficient to satisfy our liability to injured parties. If we are sued for any injury caused by our flavor ingredients or products incorporating our flavor ingredients, our liability could exceed our total assets.
We use hazardous materials. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our discovery and development process requires our employees to routinely handle hazardous chemical, radioactive and biological materials. Our operations also produce hazardous waste products. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these materials. As a result of the increase in size of our operations, we are now classified as a large quantity generator of hazardous waste. This classification may result in increased scrutiny of our operations by the Environmental Protection Agency. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental regulations may impair our discovery and development efforts.
In addition, we cannot entirely eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. Our insurance policies have limited coverage for damages or cleanup costs related to hazardous waste disposal or contamination. We may be forced to curtail operations or be sued for any injury or contamination that results from our use or the use by others of these materials, and our liability may exceed our total assets.
Risks Related To Our Common Stock
The price of our common stock is volatile.
The market prices for securities of biotechnology companies historically have been highly volatile, and the market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. Since our initial public offering in June 2004, the price of our common stock has ranged from approximately $1 per share to approximately $23 per share. The market price of our common stock may fluctuate in response to many factors, including:
|
● |
delays in commercialization of our flavor ingredients; |
|
● |
our ability to generate significant revenues from our direct sales program; |
|
● |
developments concerning our collaborative agreements; |
|
● |
failure of any of our flavor ingredients, if approved, to achieve commercial success; |
|
● |
fluctuations in our operating results; |
|
● |
public concern as to the safety of our flavor ingredients or other unfavorable publicity regarding our flavor ingredients or our research methods; |
|
● |
developments related to the United States and international regulatory approval of our products; |
|
● |
results of safety evaluation of our flavor ingredients; |
|
● |
government regulation; |
|
● |
the discovery of a product defect or the commencement of a product recall; |
|
● |
an allegation of illness or injury relating to our flavor ingredients, whether meritorious or not, or any product liability judgment; |
|
● |
developments in patent or other proprietary rights; |
|
● |
announcements of technological innovations by us or others; |
|
● |
changes in our management, key personnel or members of our Board of Directors; |
|
● |
future sales of our common stock by existing stockholders; |
|
● |
comments by securities analysts; and |
|
● |
general market conditions. |
Anti-takeover provisions in our charter documents and under Delaware law may make an acquisition of us more complicated and the removal and replacement of our directors and management more difficult.
Provisions of our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions may also make it difficult for stockholders to remove and replace our board of directors and management. These provisions:
|
● |
authorize the issuance of “blank check” preferred stock by our board of directors, without stockholder approval, which could increase the number of outstanding shares and prevent or delay a takeover attempt; |
|
● |
limit who may call a special meeting of stockholders; |
|
● |
prohibit stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; and |
|
● |
establish advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
In addition, the requirements of Section 203 of the Delaware General Corporation Law may discourage, delay or prevent a third party from acquiring us.
We have never paid cash dividends on our capital stock and we do not anticipate paying dividends in the foreseeable future.
We have paid no cash dividends on any of our classes of capital stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. In addition, the terms of any future debt or credit facility may preclude us from paying any dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of potential gain for the foreseeable future.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We currently lease approximately 65,000 square feet of laboratory and office space at 4767 Nexus Centre Drive, San Diego, California, 92121. In February 2015, we entered into an amendment to the lease agreement to extend the lease period by seven years from February 2017 to February 2024. We believe that our facilities are adequate to meet our business requirements for the near-term and that additional space will be available on commercially reasonable terms, if required.
Item 3. Legal Proceedings
We are not a party to any material legal proceedings at this time.
Item 4. Mine Safety Disclosures
None.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Common Stock Market Price
Our common stock commenced trading on the NASDAQ Stock Market on June 22, 2004 under the symbol “SNMX.” The following table sets forth the high and low sales prices per share of our common stock as traded on the NASDAQ Stock Market for the periods indicated.
|
Fiscal 2014 Quarter ended |
March 31, |
June 30, |
September 30, 2014 |
December 31, |
||||||||||||
|
High |
$ | 12.74 | $ | 12.44 | $ | 10.11 | $ | 8.62 | ||||||||
|
Low |
$ | 5.10 | $ | 6.25 | $ | 6.42 | $ | 4.77 | ||||||||
|
Fiscal 2013 Quarter ended |
March 31, |
June 30, |
September 30, 2013 |
December 31, |
||||||||||||
|
High |
$ | 2.94 | $ | 2.26 | $ | 3.84 | $ | 5.38 | ||||||||
|
Low |
$ | 1.53 | $ | 1.81 | $ | 2.04 | $ | 3.27 | ||||||||
The last sale price for our common stock as reported by the NASDAQ Stock Market on February 19, 2015 was $6.32 per share. As of February 19, 2015, there were approximately 50 stockholders of record of our common stock.
We have never declared or paid any cash dividends to our stockholders. We do not presently plan to pay cash dividends in the foreseeable future and intend to retain any future earnings for reinvestment in our business.
Information about our equity compensation plans is included in Item 12 of Part III of this annual report on Form 10-K.
Repurchases of Equity Securities
There were no repurchases of equity securities in 2014.
Performance Measurement Comparison (1)
The following graph compares the cumulative five-year total return to stockholders on Senomyx, Inc.'s common stock relative to the cumulative total returns of the NASDAQ Composite index and the NASDAQ Biotechnology index. The graph assumes that the value of the investment in our common stock and in each of the indexes (including reinvestment of dividends) was $100 on December 31, 2009 and tracks it through December 31, 2014. To date, we have not declared any dividends. The comparisons in the graph are required by the SEC and are not intended to forecast or be indicative of the possible future performance of our common stock.
 |
|
12/2009 |
12/2010 |
12/2011 |
12/2012 |
12/2013 |
12/2014 |
|||||||||||||||||||
|
Senomyx, Inc. |
100.00 | 189.12 | 92.31 | 44.56 | 134.22 | 159.42 | ||||||||||||||||||
|
NASDAQ Composite |
100.00 | 117.61 | 118.70 | 139.00 | 196.83 | 223.74 | ||||||||||||||||||
|
NASDAQ Biotechnology |
100.00 | 106.73 | 122.40 | 166.72 | 286.55 | 379.71 | ||||||||||||||||||
(1) This section is not “soliciting material,” is not deemed “filed” with the SEC, is not subject to the liabilities of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference in any of our filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
Item 6. Selected Financial Data
The Statement of Operations Data and Balance Sheet Data presented below should be read in conjunction with Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, and Item 8, Financial Statements and Supplementary Data included in this annual report on Form 10-K. Amounts are in thousands, except share and per share amounts.
|
Years ended December 31, |
||||||||||||||||||||
|
2014 |
2013 |
2012 |
2011 |
2010 |
||||||||||||||||
|
Statements of Operations Data: |
||||||||||||||||||||
|
Revenues: |
||||||||||||||||||||
|
Development revenues |
$ | 21,827 | $ | 24,657 | $ | 26,981 | $ | 28,182 | $ | 26,153 | ||||||||||
|
Commercial revenues |
5,835 | 4,630 | 4,332 | 3,372 | 2,709 | |||||||||||||||
|
Total revenues |
27,662 | 29,287 | 31,313 | 31,554 | 28,862 | |||||||||||||||
|
Operating expenses: |
||||||||||||||||||||
|
Cost of commercial revenues |
644 | 325 | 303 | 219 | 154 | |||||||||||||||
|
Research, development and patents |
26,627 | 28,612 | 28,644 | 28,687 | 26,636 | |||||||||||||||
|
Selling, general and administrative |
12,578 | 12,283 | 11,619 | 11,500 | 13,077 | |||||||||||||||
|
Total operating expenses |
39,849 | 41,220 | 40,566 | 40,406 | 39,867 | |||||||||||||||
|
Loss from operations |
(12,187 |
) |
(11,933 |
) |
(9,253 |
) |
(8,852 |
) |
(11,005 |
) | ||||||||||
|
Other income |
37 | 35 | 67 | 128 | 339 | |||||||||||||||
|
Net loss |
$ | (12,150 |
) |
$ | (11,898 |
) |
$ | (9,186 |
) |
$ | (8,724 |
) |
$ | (10,666 |
) | |||||
|
Basic and diluted net loss per share |
$ | (0.29 |
) |
$ | (0.29 |
) |
$ | (0.23 |
) |
$ | (0.22 |
) |
$ | (0.28 |
) | |||||
|
Shares used to compute basic and diluted net loss per share |
42,611,904 | 40,662,640 | 39,956,283 | 39,571,094 | 37,726,285 | |||||||||||||||
|
As of December 31, |
||||||||||||||||||||
|
2014 |
2013 |
2012 |
2011 |
2010 |
||||||||||||||||
|
Balance Sheet Data: |
||||||||||||||||||||
|
Cash, cash equivalents and investments available-for-sale |
$ | 28,738 | $ | 32,962 | $ | 41,823 | $ | 55,106 | $ | 71,612 | ||||||||||
|
Working capital |
19,800 | 15,611 | 22,051 | 36,255 | 33,323 | |||||||||||||||
|
Total assets |
38,161 | 41,614 | 53,280 | 67,646 | 84,672 | |||||||||||||||
|
Accumulated deficit |
(254,837 |
) |
(242,687 |
) |
(230,789 |
) |
(221,603 |
) |
(212,879 |
) | ||||||||||
|
Total stockholders’ equity |
23,558 | 19,678 | 25,728 | 29,721 | 32,182 | |||||||||||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion of our financial condition and results of operations should be read in conjunction with our Financial Statements and the related Notes to Financial Statements in Item 8, “Financial Statements and Supplementary Data” in this annual report on Form 10-K.
Certain statements contained in this annual report on Form 10-K, including statements regarding the development, growth and expansion of our business, our intent, belief or current expectations, primarily with respect to our future operating performance, and the products we expect to offer and other statements regarding matters that are not historical facts, are “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended, and are subject to the “safe harbor” created by these sections. Future filings with the SEC, future press releases and future oral or written statements made by us or with our approval, which are not statements of historical fact, may also contain forward-looking statements. Because such statements include risks and uncertainties, many of which are beyond our control, actual results may differ materially from those expressed or implied by such forward-looking statements. Some of the factors that could cause actual results to differ materially from those expressed or implied by such forward-looking statements can be found under the caption “Risk Factors” and elsewhere in this annual report on Form 10-K. Readers are cautioned not to place undue reliance on forward-looking statements. The forward-looking statements speak only as of the date on which they are made, and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they are made.
Overview and Recent Developments
We are a leading company using proprietary taste receptor technologies to discover, develop and commercialize innovative flavor ingredients for the packaged food, beverage and ingredient supply industries. We consider flavor ingredients to include flavors, such as savory and cooling, and flavors with modifying properties, such as sweet and salt modifiers and bitter blockers. We also have an ongoing effort to discover and develop natural high intensity sweeteners. We believe our flavor ingredients when added as part of a flavor system will enable packaged food, beverage and ingredient companies to improve the nutritional profile of their products and generate cost of goods savings while maintaining or improving taste.
We have historically licensed our flavor ingredients to our collaborators on an exclusive or co-exclusive basis. We currently have product discovery, development and commercialization collaborations with several of the world’s leading packaged food, beverage and ingredient companies: Ajinomoto, Firmenich, Nestlé and PepsiCo. Depending upon the collaboration, our collaboration agreements provide for license fees, research and development funding, reimbursement of certain costs, development milestones based upon our achievement of research or development goals and, in the event of commercialization, commercial milestones, minimum periodic royalties and royalties on sales of products incorporating our flavor ingredients. We anticipate that we will derive all of our revenues from existing and future collaborations and from a direct sales program whereby we sell certain of our flavor ingredients directly to flavor companies for re-sale to food and beverage companies.
In March 2014, we announced that a flavor ingredient from our Sweet Taste Program was determined Generally Recognized As Safe, or GRAS, by the Expert Panel of the Flavor and Extract Manufacturers Association of the United States. The new Sweetmyx® S617 ingredient is a flavor with modifying properties that is used as part of a flavor system to maintain the taste in a wide variety of foods and beverages in which a sweetener such as sugar has been reduced. The GRAS determination allows our partners, PepsiCo and Firmenich, to pursue commercialization of the Sweetmyx S617 flavor ingredient in the United States and a number of other countries. The GRAS determination also assists with regulatory approvals in other regions of the world. PepsiCo has exclusive rights to use the new Sweetmyx S617 flavor ingredient worldwide in non-alcoholic beverages. Firmenich has rights to commercialize this Sweetmyx S617 flavor ingredient for food product categories and alcoholic beverages, with exclusive rights until March 2018. We will receive royalties from each of our partners based on sales of products that incorporate the new flavor ingredient.
In April 2014, we announced that we entered into a collaborative research agreement with PepsiCo focused on the identification of flavors with modifying properties intended to restore the desired salty taste in products with reduced salt. Under the terms of the agreement, PepsiCo provided certain research funding for our Salt Taste Program through December 2014, with options to continue the research funding period for up to three years. In January 2015, we entered into an amendment of the agreement whereby PepsiCo will provide certain additional research funding through December 2015.
In May 2014, PepsiCo, Inc. exercised its option to extend the collaboration agreement dated August 16, 2010 related to our Sweet Taste Program for an additional two years, through August 2016. The collaboration will continue to be focused on the discovery and development of new flavor ingredients and new natural high-potency sweeteners. We are entitled to $17.6 million in committed research funding to be paid in quarterly installments over the additional two-year period.
We have incurred significant losses since our inception in 1998 and, as of December 31, 2014 our accumulated deficit was $255 million. Our results of operations have fluctuated from period to period and likely will continue to fluctuate substantially in the future based upon:
|
● |
the ability of our product discovery and development collaborators to incorporate our flavor ingredients into food, beverage and ingredient products, on expected timelines, if at all; |
|
● |
our ability to implement our direct sales program; |
|
● |
the ability and willingness of food and beverage companies to commercialize products incorporating our flavor ingredients in a timely manner, if at all; |
|
● |
the demand for our collaborators’ and other customers’ products containing our flavor ingredients |
|
● |
the termination, expiration or amendment of any of our product discovery and development collaboration agreements; |
|
● |
our receipt of milestone payments in any particular period; |
|
● |
our ability to enter into new product discovery and development collaborations and technology collaborations or to extend the terms of our existing collaboration agreements and our payment obligations, expected revenue and other terms of any of our agreements; |
|
● |
our ability, or our collaborators’ ability, to successfully satisfy all pertinent regulatory requirements; and |
|
● |
general and industry specific economic conditions which may affect our collaborators’ research and development expenditures and commercialization efforts. |
Revenues
Our revenues to date have come from license fees, research and development funding, reimbursement of certain patent and regulatory costs, milestone payments and royalty payments under our collaborative agreements and from sales of our flavor ingredients directly to flavor companies for re-sale to food and beverage companies.
As of December 31, 2014, we have recognized cumulative revenues under our collaborations of $248 million. If any of our collaborative agreements were to terminate earlier than currently anticipated, we may experience a significant decline in our revenues. Based on current collaborations, we anticipate that a significant portion of our revenues in the near future will be derived from research and development payments and license fees. We may receive additional milestone payments in the future upon the achievement of certain goals set forth in our collaboration agreements.
As our collaborators launch products incorporating our flavor ingredients, we receive minimum periodic royalties and royalty payments based upon the sales of those products. In addition, we generate revenues from flavor ingredient sales directly to flavor companies for re-sale to food and beverage companies. Such royalty payments and flavor ingredient sales in the future could be significantly larger than research and development funding, license fees or milestone payments. In order for us to generate significant royalty revenues and flavor ingredient sales and to become profitable, we must retain our existing or establish new product discovery, development and commercialization collaborations and our collaborators and other customers must successfully commercialize products incorporating one or more of our flavor ingredients. Our ability to generate significant royalty revenues or flavor ingredient sales is inherently uncertain and will depend upon our ability to meet particular research, development and commercialization objectives and the ability and willingness of food and beverage companies to commercialize products incorporating our flavor ingredients on expected timelines.
Cost of Commercial Revenues
Cost of commercial revenues consists of royalties payable under our third-party licensing agreements and cost of goods sold for sales of our flavor ingredients under our direct sales program.
Research, Development and Patents
Our research, development and patent expenses consist primarily of costs associated with our discovery and development efforts in connection with the development of savory flavors, sweet and salt modifiers, bitter blockers and cooling agents. We track research, development and patent costs by the type of cost incurred rather than by project. Research, development and patent costs are comprised of salaries and other personnel-related expenses, facilities and depreciation, research and development supplies, patents and licensing, outside services and non-cash stock-based compensation expenses. We charge research, development and patent expenses to operations as incurred.
The research, development and patents payments we have received from our collaboration agreements historically have not covered all of our research, development and patents expenses.
At this time, due to the risks inherent in the discovery of flavor ingredients, we are unable to estimate with any certainty the costs we will incur in the continued development of our flavor ingredients for commercialization. We anticipate that we will make determinations regarding the research and development projects to pursue and the funding of each project on an ongoing basis in response to the progress of each discovery and development program, as well as an ongoing assessment of its market potential. We cannot be certain when any material net cash inflow from the commercialization of our flavor ingredients will commence.
Our ability to complete the development of our current product candidates is subject to many risks and uncertainties. These risks include the risks, among others, that:
|
• |
we are substantially dependent upon our collaborators for research and development funding; |
|
• |
our collaborators may terminate their respective collaboration programs early; |
|
• |
we may not be able to discover flavor ingredients with the desired taste attributes; |
|
• |
we may not be successful in developing flavor ingredients with other attributes required for use in commercial products; and |
|
• |
we may be unable to obtain and maintain FEMA GRAS determination for our flavor ingredients, or equivalent regulatory approvals in other geographies. |
If we do not complete the development of our flavor ingredients on a timely basis, our collaborators may terminate or not renew our collaboration agreements, we may begin receiving revenue from the commercialization of products incorporating our flavor ingredients later than anticipated, or not at all, and it may be more difficult to enter into new collaboration agreements. In any of these cases, we may require substantial additional funding in order to continue development of our flavor ingredients.
Selling, General and Administrative
Selling, general and administrative expenses consist primarily of salaries and other personnel-related expenses related to business development, legal, financial, sales and commercial development, supply chain management and other administrative functions. Selling, general and administrative expenses also include non-cash stock-based compensation expenses.
Results of Operations
Years Ended December 31, 2014, 2013 and 2012
Revenues
We recorded revenues of $27.7 million, $29.3 million and $31.3 million during the years ended December 31, 2014, 2013 and 2012, respectively. Research and development payments, license fees, milestone payments and royalty revenues under our material collaborations with Firmenich and PepsiCo accounted for 77% of the total revenues for the year ended December 31, 2014. The decrease of $1.6 million from 2013 to 2014 resulted from a $2.8 million decrease in development revenues and a $1.2 million increase in commercial revenues in 2014.
The $2.8 million decrease in development revenues from 2013 to 2014 primarily reflects a $3.9 million decrease in license fee revenues from our Sweet Taste Program collaboration with PepsiCo due to a change in the service period over which the upfront license fee related to this collaboration is being recognized. In the second quarter of 2014, PepsiCo elected, in accordance with our agreement, to extend the service period of the collaboration an additional two years through August 2016. Partially offsetting the decrease in license fee revenues was an increase in research funding revenues related to our Salt Taste Program collaboration with PepsiCo that began in 2014 and resulted in $1.5 million of development revenues for the year.
Commercial revenues increased $1.2 million as a result of increases in royalties and commercial milestone payments related to our Sweet Taste Program and increases in direct sales during 2014.
For 2013, research and development payments, license fees, milestone payments and royalty revenues under our material collaborations with Firmenich and PepsiCo accounted for 82% of total revenues. The decrease of $2.0 million from 2012 to 2013 was primarily due to a $2.1 million decrease in revenues from development milestones.
Development revenues from our Sweet Taste Program collaboration with PepsiCo decreased $1.5 million to $16.2 million in 2013 from $17.7 million in 2012 primarily due to $1.5 million in development milestones revenues earned in 2012 compared to none in 2013. Revenues from development cost reimbursements across all programs increased by $1.0 million from 2012 to 2013. Our Cooling Taste Program revenues decreased $875,000 as a result of the June 2013 expiration of the research funding period under our collaboration with Firmenich and decreased $500,000 due to a development milestone earned in 2012 compared to none in 2013.
Commercial revenues increased $298,000 from 2012 to 2013, reflecting increased revenues from our Sweet Taste Program, partially offset by decreased revenues from our Savory Taste Program.
For 2015, we expect total revenues to remain relatively constant compared to 2014, with increases in commercial revenues and decreases in development revenues.
Cost of Commercial Revenues
Our costs of commercial revenues were $644,000, $325,000 and $303,000 for the years ended December 31, 2014, 2013 and 2012, respectively. The increases from 2012 to 2014 are consistent with corresponding increases in commercial revenues.
Research, Development and Patents Expenses
Our research, development and patents expenses, which include stock-based compensation expenses associated with research and development personnel, were $26.6 million, $28.6 million and $28.6 million for the years ended December 31, 2014, 2013 and 2012, respectively. A comparison of research, development and patents expenses by category is as follows (in thousands):
|
Years Ended |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Salaries and personnel |
$ | 12,670 | $ | 13,237 | $ | 13,131 | ||||||
|
Facilities and depreciation |
4,988 | 5,543 | 5,510 | |||||||||
|
Non-cash stock-based compensation |
2,575 | 1,688 | 1,876 | |||||||||
|
Research and development supplies |
2,131 | 2,123 | 2,758 | |||||||||
|
Patents and licensing |
2,020 | 1,943 | 2,144 | |||||||||
|
Outside services |
1,364 | 3,265 | 2,391 | |||||||||
|
Miscellaneous |
879 | 813 | 834 | |||||||||
|
Total research, development and patents expenses |
$ | 26,627 | $ | 28,612 | $ | 28,644 | ||||||
Salaries and Personnel. Our expenses for research and development personnel, including consultants, were $12.7 million, $13.2 million and $13.1 million for the years ended December 31, 2014, 2013 and 2012, respectively. The $567,000 decrease in salaries and personnel expenses from 2013 to 2014 resulted primarily from a decrease in employee bonuses. The $106,000 increase from 2012 to 2013 was primarily due to annual salary increases. For 2015, we expect salaries and personnel expenses to decrease slightly from 2014 levels.
Facilities and Depreciation. Our facilities and depreciation expenses were $5.0 million, $5.5 million and $5.5 million for the years ended December 31, 2014, 2013 and 2012, respectively. The $555,000 decrease from 2013 to 2014 reflects lower depreciation expenses as certain scientific equipment became fully depreciated during 2014. For 2015, we expect a slight increase in facilities and depreciation expenses as compared to 2014.
Non-cash Stock-based Compensation. Our non-cash stock-based compensation expenses were $2.6 million, $1.7 million and $1.9 million for the years ended December 31, 2014, 2013 and 2012, respectively. The $887,000 increase in stock-based compensation expenses was due to higher valuations of stock options granted to employees in 2014 based on the higher price of our common stock during the year. The decrease of $188,000 from 2012 to 2013 was primarily due to decreased valuations of stock options granted to employees. For 2015, we expect a decrease in non-cash stock-based compensation expenses in comparison to 2014.
Research and Development Supplies. Our expenses for supplies used in research and development were $2.1 million, $2.1 million and $2.8 million for the years ended December 31, 2014, 2013 and 2012, respectively. The decrease of $635,000 from 2012 to 2013 was primarily due to decreased purchases of compounds and decreases in expensed supplies used in high-throughput screening. For 2015, we expect a decrease in research and development supplies expenses compared to 2014 levels.
Patents and Licensing. Our patents and licensing expenses were $2.0 million, $1.9 million and $2.1 million for the years ended December 31, 2014, 2013 and 2012, respectively. The increase of $77,000 from 2013 to 2014 was primarily due to varying patent legal expenses due to the timing of filings and other activities. The decrease of $201,000 from 2012 to 2013 was due to decreased licensing costs related to software used in our research programs and varying patent legal expenses due to the timing of filings and other activities. For 2015, we expect our patents and licensing expenses to increase compared to 2014.
Outside Services. Our outside services expenses were $1.4 million, $3.3 million and $2.4 million for the years ended December 31, 2014, 2013 and 2012, respectively. The $874,000 increase from 2012 to 2013 and the $1.9 million decrease from 2013 to 2014 in outside services expense reflects increased costs for safety studies during 2013 for product candidates advancing in development such as S617, for which we announced receipt of FEMA GRAS determination in March 2014. For 2015, we expect a slight decrease in outside services expenses compared to 2014 due to reduced costs for safety studies associated with product candidates under development.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses, which include non-cash stock-based compensation expenses associated with selling, general and administrative personnel, were $12.6 million, $12.3 million and $11.6 million for the years ended December 31, 2014, 2013 and 2012, respectively.
The $295,000 increase in expenses from 2013 to 2014 was attributable to a $1.3 million increase in non-cash stock-based compensation expenses and a $1.1 million decrease in personnel-related expenses, partly due to reduced bonuses in 2014. The $664,000 increase in expenses from 2012 to 2013 primarily resulted from personnel-related expenses, including recruiting and relocation expenses related to hiring of employees dedicated to our direct sales program. Also contributing to the increase in 2013 was higher legal expenses related to collaboration agreements and other matters.
For 2015, we expect an increase in our selling, general and administrative expenses in comparison to 2014, driven partly by bonuses returning to levels consistent with the years prior to 2014.
Other Income
Other income was $37,000, $35,000 and $67,000 for the years ended December 31, 2014, 2013 and 2012, respectively. The $32,000 decrease from 2012 to 2013 was primarily due to decreased interest income from lower average investable balances.
Liquidity and Capital Resources
Since our inception, we have financed our business primarily through our product discovery and development collaborations, private and public placements of stock, royalties and interest income. As of December 31, 2014, we had received $232 million in non-refundable license fees, research and development payments, cost reimbursements and milestone payments from our collaboration agreements. In addition, we had received $205 million in proceeds from the sales of common and preferred stock, $20 million in royalties and commercial milestone payments and $13 million in interest income.
At December 31, 2014, we had $28.7 million in cash, cash equivalents and investments available-for-sale as compared to $33.0 million at December 31, 2013, a decrease of $4.3 million. This decrease resulted primarily from the use of cash to fund our operations, offset by proceeds from the issuance of common stock upon the exercise of options.
Operating Activities
Operating activities used cash of $13.6 million, $9.7 million and $12.4 million in 2014, 2013 and 2012, respectively. The use of cash was driven by net losses and changes in operating assets and liabilities.
The $3.9 million increase in cash used in operating activities from 2013 to 2014 resulted primarily from a $5.2 million increase in cash used for net changes in operating assets and liabilities, including a $1.2 million increase related to inventories and a $2.7 million increase related to accounts payable, accrued expenses and other current liabilities. Partially offsetting the impact of operating assets and liabilities was a $1.5 million net increase in non-cash expenses, including a $2.1 million increase in stock-based compensation expenses and a $608,000 decrease in depreciation expenses.
The $2.7 million decrease in cash used in operating activities from 2012 to 2013 resulted primarily from a $5.7 million decrease in net changes in operating assets and liabilities, partially offset by a $2.7 million increase in net loss. The decreased net use of cash from changes in operating assets and liabilities reflects deferred revenues for $4 million of license fee payments received in 2013 under the Firmenich Sweet Taste Program collaboration.
Investing Activities
Investing activities used cash of $3.6 million in 2014 and provided cash of $3.8 million and $11.1 million in 2013 and 2012, respectively. The sources of cash flow reflect changes in the timing of maturities and purchases of available-for-sale securities and purchases of property and equipment. The net use of cash in 2014 largely results from investment portfolio changes to hold more short-term investments and less cash equivalents. Purchases of property and equipment decreased from $1.5 million in 2012 to $623,000 in 2013 and $328,000 in 2014, reflecting certain significant purchases of scientific equipment in 2012.
Financing Activities
Financing activities provided cash of $10.0 million, $1.7 million and $746,000 in 2014, 2013 and 2012, respectively. Cash provided by financing activities reflects the net proceeds from the issuance or sale of common stock from our employee stock purchase program and the exercise of employee stock options. Such totals generally fluctuate based on changes in the price of our common stock and the higher price of our common stock through most of 2014 resulted in increased exercises of employee stock options and greater participation in the employee stock purchase program.
In February 2015, we amended the lease agreement for our laboratory and office facility in San Diego, California. The amended lease expires on February 28, 2024, a seven-year extension of the previous lease period. Including the amended lease, as of December 31, 2014, the future minimum payments due under our contractual obligations are as follows (in thousands):
|
Payments Due by Period |
||||||||||||||||||||
|
Total |
Less than 1 |
1-3 years |
4-5 years |
After 5 years |
||||||||||||||||
|
Operating leases |
$ | 24,171 | $ | 1,075 | $ | 4,958 | $ | 5,749 | $ | 12,389 | ||||||||||
|
License payments |
249 | 225 | 24 | — | — | |||||||||||||||
|
Total |
$ | 24,420 | $ | 1,300 | $ | 4,982 | $ | 5,749 | $ | 12,389 | ||||||||||
As of December 31, 2014, we had no long-term debt obligations.
As of December 31, 2014, we have net open purchase orders (defined as total open purchase orders at year end less any accruals or invoices charged to or amounts paid against such purchase orders) totaling approximately $700,000. In the next 12 months, we also plan to spend approximately $1.4 million on capital expenditures.
Our license agreement with the University of California calls for annual maintenance fees, which commenced in 2006, or royalties or service revenues on sales of any products developed using technologies licensed under the agreement. Royalties are calculated as a percentage of covered sales and are included in cost of commercial revenues. The agreement specifies minimum annual royalty payments which commenced in 2014 and continue through the expiration of the last to expire patent licensed under the agreement. Royalties paid under the agreement in 2014 exceeded the minimum annual royalty.
Our future capital uses and requirements depend on numerous forward-looking factors. These factors may include, but are not limited to, the following:
|
● |
our ability to generate flavor ingredient sales under our direct sales program; |
|
● |
our ability to maintain product discovery, development and commercialization collaborations; |
|
● |
the rate of progress and cost of research and development activities; |
|
● |
the terms and timing of any collaborative, licensing and other arrangements that we may establish; |
|
● |
the cost of implementation of our direct sales program, including purchases of inventory; |
|
● |
the number and scope of our research activities; |
|
● |
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
|
● |
the proceeds from the issuance of common stock upon the exercise of stock options; |
|
● |
the effect of competing technological and market developments; and |
|
● |
the extent to which we acquire or in-license new products, technologies or businesses. |
We believe our available cash, cash equivalents, investments and existing sources of funding will be sufficient to satisfy our anticipated operating and capital requirements through at least the next 12 months.
Until we can generate significant cash from our operations, we expect to continue to fund our operations with existing cash resources that were primarily generated from the proceeds of offerings of our equity securities and license payments, research and development payments and milestone payments under our product discovery and development collaborations. From time to time we may also consider raising additional cash from the sale of equity or other securities.
As of January 1, 2015, we are entitled to receive $19.8 million in non-refundable license fees and research and development payments from our collaborators over the remaining life of our current collaboration agreements. Assuming all milestones are achieved for all program goals for all collaborations and we receive all license fees and research and development funding, we may be entitled to receive up to $47.4 million.
In the next twelve months (through December 31, 2015), we anticipate receiving $13.2 million in research and development funding. This does not include any additional payments we may receive related to the following events:
|
• |
the achievement of additional milestones; |
|
• |
the earning of royalties from the sale of products containing our flavor ingredients; |
|
• |
the earning of any minimum periodic royalty payments; |
|
• |
direct sales of flavor ingredients; |
|
• |
the earning of any cost reimbursements; and |
|
• |
the signing of new collaborations or extensions of existing collaborations. |
We may not receive the payments if the collaborations are terminated, amended or not renewed, or if we do not achieve the milestones set forth in the collaboration agreements. In addition, the timing of the receipt of milestone payments in particular is uncertain, as we may achieve milestones significantly earlier or later than we currently expect. We cannot predict at this time the level of our collaborators’ royalty-generating sales, as these sales to date have been based on launches of new products without established sales histories, or the level of flavor ingredient sales under our direct sales program.
We continue to pursue additional collaborations which could result in additional revenue. We may not recognize revenues for license fees, research and development funding, milestones, minimum periodic royalties or royalties if the collaborations are terminated or amended, or if we do not achieve the milestones set forth in the collaboration agreements. Our expenses will vary based upon the forward-looking factors listed above.
Off-Balance Sheet Arrangements
As of December 31, 2014 and 2013, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as special purpose or structured finance entities, which would have been established for the purposes of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As such, we are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in these relationships.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with United States generally accepted accounting principles, or GAAP. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate these estimates, including those related to revenue recognition, stock-based compensation, uncollectible receivables, excess and obsolete inventories, long-lived assets, accrued liabilities and income taxes. These estimates are based on historical experience and various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. While our significant accounting policies are described in more detail in Note 1 to our financial statements, we believe the following accounting policy to be critical to the judgments and estimates used in the preparation of our financial statements:
Revenue Recognition
Development Revenues
Development revenues are based on our product discovery, development and commercialization collaboration agreements. Some of our collaboration agreements contain multiple elements, including technological and territorial licenses and research and development services. In accordance with these agreements, we may be eligible for license fees, research and development funding, development milestone payments, cost reimbursements, royalty payments, minimum periodic royalty payments and commercial milestone payments. Development revenues include revenues from license fees, research and development funding, the achievement of development milestones and cost reimbursements.
Pursuant to the Revenue Recognition – Multiple-Element Arrangements Topic of the Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, each required deliverable is evaluated to determine if it qualifies as a separate unit of accounting. For us, this determination is generally based on whether the deliverable has “stand-alone value” to the customer. The arrangement’s consideration is then allocated to each separate unit of accounting based on the relative selling price of each deliverable. The estimated selling price of each deliverable is determined using the following hierarchy of values: (i) vendor-specific objective evidence of fair value; (ii) third-party evidence of selling price; and (iii) best estimate of selling price, or BESP. The BESP reflects our best estimate of what the selling price would be if the deliverable was regularly sold by us on a stand-alone basis. We expect, in general, to use the BESP for allocating consideration to each deliverable. In general, the consideration allocated to each unit of accounting is then recognized as the related goods or services are delivered, limited to the consideration that is not contingent upon future deliverables. For multiple-element arrangements entered into prior to January 1, 2011 and not materially modified thereafter, we continue to apply our prior accounting policy with respect to such arrangements.
Non-refundable license fees, if not associated with our future performance, are recognized when received. Non-refundable license fees, if associated with our future performance obligations, are attributed to a specific program or collaboration and recognized over the period of service for that specific program or collaboration. Amounts received for research funding are recognized as revenues as the services are performed. Revenue is deferred for fees received before earned. Revenues from development milestones are accounted for in accordance with the Revenue Recognition – Milestone Method Topic of the FASB ASC. Milestones are recognized when earned, as evidenced by written acknowledgment from the collaborator or other persuasive evidence that the milestone has been achieved, provided that the milestone event is substantive. A milestone event is considered to be substantive if its achievability was not reasonably assured at the inception of the agreement and our efforts led to the achievement of the milestone or the milestone was due upon the occurrence of a specific outcome resulting from our performance. If both of these criteria are not met, the milestone payment is recognized over the remaining minimum period of our performance obligations under the agreement. We assess whether a milestone is substantive at the inception of each agreement. Revenues from cost reimbursement are recognized when earned, as evidenced by written acknowledgment from the collaborator or other persuasive evidence.
Commercial Revenues
Commercial revenues from collaboration agreements include royalties on sales made by our collaborators of products incorporating our flavor ingredients, minimum periodic royalty payments and revenues from the achievement of commercial milestones. Commercial revenues also include direct sales of our flavor ingredients to flavor companies.
Royalties on sales made by our collaborators of products incorporating our flavor ingredients are recognized when a royalty report or other persuasive evidence is received, which is generally one quarter in arrears. Non-refundable minimum periodic royalty payments are recognized as revenues over the related royalty periods. Royalty terms are specific to each collaboration and collaborator and can vary from year to year. These terms vary based on factors such as the characteristics of the flavor ingredient and the product categories and geographies licensed by the collaborator. Periodically, as contractually specified, our collaborators are required to provide a report detailing all sales of products containing our flavor ingredients. To the extent that calculated royalties on sales of such products exceed the minimum periodic royalty payments made to date, the collaborators are required to remit to us the difference between royalties calculated and minimum periodic royalty payments made to date. We recognize this difference as royalties on product sales at the time the report is received. To the extent that minimum periodic royalty payments through the end of any applicable period exceed calculated royalties, we are not required to refund the difference. Although we currently do not have any collaborations that include refundable minimum periodic royalty payments, in such a case, revenues would be deferred for refundable minimum periodic royalty payments received before earned. Revenues from commercial milestones are recognized when earned, as evidenced by written acknowledgment from the collaborator or other persuasive evidence that the milestone has been achieved, because we have no further performance obligations related to the commercial milestones.
Revenues from direct sales of our flavor ingredients are recorded when there is persuasive evidence that an arrangement exists, title has passed, collection is reasonably assured and the price is fixed or determinable. Revenues are recorded following any general right of return period, which typically is no longer than five days. We generally do not offer discounts or rebates on sales of flavor ingredients.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of United States interest rates. Due to the nature of our investments, we believe that we are not subject to any material market risk exposure. We do not have any foreign currency or other derivative financial instruments.
Item 8. Financial Statements and Supplementary Data
Index to Financial Statements
|
Description |
Page | |
|
Report of Independent Registered Public Accounting Firm |
51 | |
|
Balance Sheets |
52 | |
|
Statements of Operations and Comprehensive Loss |
53 | |
|
Statements of Stockholders’ Equity |
54 | |
|
Statements of Cash Flows |
55 | |
|
Notes to Financial Statements |
56 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Senomyx, Inc.
We have audited the accompanying balance sheets of Senomyx, Inc. as of December 31, 2014 and 2013, and the related statements of operations and comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Senomyx, Inc. at December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Senomyx Inc.’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 26, 2015 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
San Diego, California
February 26, 2015
Senomyx, Inc.
Balance Sheets
(In thousands, except share and per share data)
|
December 31, |
||||||||
|
2014 |
2013 |
|||||||
|
Assets: |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 3,961 | $ | 11,201 | ||||
|
Short-term investments available-for-sale |
22,630 | 18,962 | ||||||
|
Accounts receivable |
2,276 | 2,126 | ||||||
|
Inventories |
2,319 | 196 | ||||||
|
Other current assets |
993 | 677 | ||||||
|
Total current assets |
32,179 | 33,162 | ||||||
|
Long-term investments available-for-sale |
2,147 | 2,799 | ||||||
|
Property and equipment, net |
3,835 | 5,653 | ||||||
|
Total assets |
$ | 38,161 | $ | 41,614 | ||||
|
Liabilities and stockholders’ equity |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable, accrued expenses and other current liabilities |
$ | 5,903 | $ | 7,119 | ||||
|
Deferred rent |
354 | 267 | ||||||
|
Leasehold incentive obligation |
987 | 987 | ||||||
|
Deferred revenues |
5,135 | 9,178 | ||||||
|
Total current liabilities |
12,379 | 17,551 | ||||||
|
Deferred rent |
516 | 871 | ||||||
|
Leasehold incentive obligation |
1,152 | 2,139 | ||||||
|
Deferred revenues |
556 | 1,375 | ||||||
|
Commitments |
||||||||
|
Stockholders’ equity: |
||||||||
|
Preferred stock, $0.001 par value, 7,500,000 shares authorized; no shares issued or outstanding at December 31, 2014 and 2013 |
— | — | ||||||
|
Common stock, $0.001 par value, 120,000,000 shares authorized; 43,370,309 and 41,095,917 shares issued and outstanding at December 31, 2014 and 2013, respectively |
43 | 41 | ||||||
|
Additional paid-in-capital |
278,362 | 262,317 | ||||||
|
Accumulated other comprehensive income |
(10 |
) |
7 | |||||
|
Accumulated deficit |
(254,837 |
) |
(242,687 |
) | ||||
|
Total stockholders’ equity |
23,558 | 19,678 | ||||||
|
Total liabilities and stockholders’ equity |
$ | 38,161 | $ | 41,614 | ||||
See accompanying notes to financial statements.
Senomyx, Inc.
Statements of Operations and Comprehensive Loss
(In thousands, except share and per share data)
|
Years Ended December 31, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Revenues: |
||||||||||||
|
Development revenues |
$ | 21,827 | $ | 24,657 | $ | 26,981 | ||||||
|
Commercial revenues |
5,835 | 4,630 | 4,332 | |||||||||
|
Total revenues |
27,662 | 29,287 | 31,313 | |||||||||
|
Operating expenses: |
||||||||||||
|
Cost of commercial revenues |
644 | 325 | 303 | |||||||||
|
Research, development and patents |
26,627 | 28,612 | 28,644 | |||||||||
|
Selling, general and administrative |
12,578 | 12,283 | 11,619 | |||||||||
|
Total operating expenses |
39,849 | 41,220 | 40,566 | |||||||||
|
Loss from operations |
(12,187 |
) |
(11,933 |
) |
(9,253 |
) | ||||||
|
Other income |
37 | 35 | 67 | |||||||||
|
Net loss |
(12,150 |
) |
(11,898 |
) |
(9,186 |
) | ||||||
|
Other comprehensive income (loss): |
||||||||||||
|
Unrealized loss on investments |
(17 |
) |
— | (23 |
) | |||||||
|
Comprehensive loss |
$ | (12,167 |
) |
$ | (11,898 |
) |
$ | (9,209 |
) | |||
|
Net loss per share, basic and diluted |
$ | (0.29 |
) |
$ | (0.29 |
) |
$ | (0.23 |
) | |||
|
Shares used in calculating net loss per share, basic and diluted |
42,611,904 | 40,662,640 | 39,956,283 | |||||||||
See accompanying notes to financial statements.
Senomyx, Inc.
Statements of Stockholders’ Equity
(In thousands, except for share data)
|
Common Stock |
Additional paid-in |
Accumulated other comprehensive |
Accumulated |
Total stockholders’ |
||||||||||||||||||||
|
Shares |
Amount |
capital |
income/(loss) |
deficit |
equity |
|||||||||||||||||||
|
Balance at December 31, 2011 |
39,759,137 | 40 | 251,254 | 30 | (221,603 |
) |
29,721 | |||||||||||||||||
|
Issuance of common stock related to the exercise of options |
17,068 | — | 26 | — | — | 26 | ||||||||||||||||||
|
Issuance of common stock related to employee stock plan purchases |
324,278 | — | 720 | — | — | 720 | ||||||||||||||||||
|
Compensation related to stock options granted to employees and non-employee directors |
— | — | 4,464 | — | — | 4,464 | ||||||||||||||||||
|
Compensation related to stock options granted to consultants |
— | — | 6 | — | — | 6 | ||||||||||||||||||
|
Unrealized loss on investments |
— | — | — | (23 |
) |
— | (23 |
) | ||||||||||||||||
|
Net loss |
— | — | — | — | (9,186 |
) |
(9,186 |
) | ||||||||||||||||
|
Balance at December 31, 2012 |
40,100,483 | $ | 40 | $ | 256,470 | $ | 7 | $ | (230,789 |
) |
$ | 25,728 | ||||||||||||
|
Issuance of common stock related to the exercise of options |
715,440 | 1 | 1,366 | — | — | 1,367 | ||||||||||||||||||
|
Issuance of common stock related to employee stock plan purchases |
279,994 | — | 452 | — | — | 452 | ||||||||||||||||||
|
Compensation related to stock options granted to employees and non-employee directors |
— | — | 4,005 | — | — | 4,005 | ||||||||||||||||||
|
Compensation related to stock options granted to consultants |
— | — | 24 | — | — | 24 | ||||||||||||||||||
|
Unrealized gain on investments |
— | — | — | — | — | — | ||||||||||||||||||
|
Net loss |
— | — | — | — | (11,898 |
) |
(11,898 |
) | ||||||||||||||||
|
Balance at December 31, 2013 |
41,095,917 | $ | 41 | $ | 262,317 | $ | 7 | $ | (242,687 |
) |
$ | 19,678 | ||||||||||||
|
Issuance of common stock related to the exercise of options |
1,802,295 | 2 | 8,564 | — | — | 8,566 | ||||||||||||||||||
|
Issuance of common stock related to employee stock plan purchases |
472,097 | — | 1,300 | — | — | 1,300 | ||||||||||||||||||
|
Compensation related to stock options granted to employees and non-employee directors |
— | — | 6,142 | — | — | 6,142 | ||||||||||||||||||
|
Compensation related to stock options granted to consultants |
— | — | 39 | — | — | 39 | ||||||||||||||||||
|
Unrealized loss on investments |
— | — | — | (17 |
) |
— | (17 |
) | ||||||||||||||||
|
Net loss |
— | — | — | — | (12,150 |
) |
(12,150 |
) | ||||||||||||||||
|
Balance at December 31, 2014 |
43,370,309 | $ | 43 | $ | 278,362 | $ | (10 |
) |
$ | (254,837 |
) |
$ | 23,558 | |||||||||||
See accompanying notes to financial statements.
Senomyx, Inc.
Statements of Cash Flows
(In thousands)
|
Years Ended December 31. |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Operating Activities |
||||||||||||
|
Net loss |
$ | (12,150 |
) |
$ | (11,898 |
) |
$ | (9,186 |
) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
|
Depreciation |
2,239 | 2,847 | 2,789 | |||||||||
|
Accretion of premium on available-for-sale securities |
220 | 258 | 181 | |||||||||
|
Amortization of leasehold incentive obligation |
(987 |
) |
(987 |
) |
(987 |
) | ||||||
|
Stock-based compensation for employees and non-employee directors |
6,142 | 4,005 | 4,464 | |||||||||
|
Stock-based compensation for non-employees |
39 | 24 | 6 | |||||||||
|
Change in operating assets and liabilities: |
||||||||||||
|
Accounts receivable |
(150 |
) |
576 | (268 |
) | |||||||
|
Inventories |
(1,361 |
) |
(196 |
) |
— | |||||||
|
Other current assets |
(438 |
) |
235 | (199 |
) | |||||||
|
Accounts payable, accrued expenses and other current liabilities |
(2,071 |
) |
614 | 592 | ||||||||
|
Deferred revenues |
(4,862 |
) |
(5,027 |
) |
(9,702 |
) | ||||||
|
Deferred rent |
(268 |
) |
(183 |
) |
(105 |
) | ||||||
|
Net cash used in operating activities |
(13,647 |
) |
(9,732 |
) |
(12,415 |
) | ||||||
|
Investing activities |
||||||||||||
|
Purchases of property and equipment |
(328 |
) |
(623 |
) |
(1,470 |
) | ||||||
|
Purchases of available-for-sale securities |
(22,039 |
) |
(20,885 |
) |
(30,408 |
) | ||||||
|
Maturities of available-for-sale securities |
18,815 | 25,295 | 43,010 | |||||||||
|
Net cash provided by (used in) investing activities |
(3,552 |
) |
3,787 | 11,132 | ||||||||
|
Financing activities |
||||||||||||
|
Proceeds from issuance of common stock |
9,959 | 1,719 | 746 | |||||||||
|
Net cash provided by financing activities |
9,959 | 1,719 | 746 | |||||||||
|
Net change in cash and cash equivalents |
(7,240 |
) |
(4,226 |
) |
(537 |
) | ||||||
|
Cash and cash equivalents at beginning of year |
11,201 | 15,427 | 15,964 | |||||||||
|
Cash and cash equivalents at end of year |
$ | 3,961 | $ | 11,201 | $ | 15,427 | ||||||
|
Supplemental disclosure of cash flow information: |
||||||||||||
|
Purchases of inventories included in accounts payable, accrued expenses and other current liabilities at year-end |
$ | 762 | $ | — | $ | — | ||||||
|
Purchases of property and equipment included in accounts payable, accrued expenses and other current liabilities at year-end |
$ | 93 | $ | — | $ | 33 | ||||||
See accompanying notes to financial statements.
Senomyx, Inc.
Notes to Financial Statements
1. Organization and Summary of Significant Accounting Policies
Organization and Business
Senomyx, Inc. (“we”, “us” or “our”) was incorporated on September 16, 1998 in Delaware and commenced operations in January 1999. We use proprietary taste receptor technologies to discover, develop and commercialize innovative flavor ingredients for the packaged food, beverage and ingredient supply industries to improve the nutritional profile of their products and generate cost of goods savings while maintaining or improving taste. Our current programs focus on the development and commercialization of sweet, savory and salt flavor ingredients, bitter blockers and cooling agents.
We currently have product discovery, development and commercialization collaborations with several of the world’s leading packaged food, beverage and ingredient companies: Ajinomoto Co., Inc. (“Ajinomoto”), Firmenich SA (“Firmenich”), Nestlé SA (“Nestlé”) and PepsiCo, Inc. (“PepsiCo”). Our collaboration agreements generally provide for license fees, research and development funding, reimbursement of certain costs, development milestones based upon our achievement of research or development goals and, in the event of commercialization, commercial milestones, minimum periodic royalties and royalties on sales of products incorporating our flavor ingredients. We also sell certain flavor ingredients directly to flavor companies for inclusion in a flavor system for re-sale to food and beverage companies.
Use of Estimates
The preparation of financial statements in conformity with United States generally accepted accounting principles (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
We consider all highly liquid investments with a remaining maturity of three months or less when purchased to be cash equivalents. Cash equivalents are recorded at cost, which approximates market value.
Investments Available-for-Sale
Our surplus cash is invested in United States Treasuries, United States government agency bonds and corporate bonds with maturity dates of two years or less from the settlement date. The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity with all amortization and accretion included in interest income. Our investments are classified as available-for-sale and carried at estimated fair value, with unrealized gains and losses reported in a separate component of accumulated other comprehensive income (loss). Realized gains and losses and declines in value judged to be other-than-temporary, if any, on available-for-sale securities are included in interest income. The cost of securities sold is based on the specific identification method. Interest on securities classified as available-for-sale is included in interest income.
Fair Value of Financial Instruments other than Investments Available-for-Sale
The carrying amount of cash and cash equivalents, accounts receivables, accounts payable and accrued expenses are considered to be representative of their respective fair value because of the short-term nature of those items.
Concentration of Credit Risk and Major Collaborations
Financial instruments, which potentially subject us to concentrations of credit risk, consist of cash, cash equivalents and investments available-for-sale. We limit our exposure to credit loss by placing our cash, cash equivalents, and investments with high credit quality financial institutions in instruments with short maturities.
We derive significant portions of our revenues from a relatively small number of collaborators. For the years ended December 31, 2014, 2013 and 2012, revenues from any single collaborator that contributed 10% or more of revenues for the period were as follows:
|
Years Ended December 31, | |||||
|
2014 |
2013 |
2012 | |||
|
PepsiCo |
$14.5 million (53%) |
$16.2 million (55%) |
$17.7 million (56%) | ||
|
Firmenich |
$9.4 million (34%) |
$9.4 million (32%) |
$9.6 million (31%) | ||
Accounts Receivable
We extend credit to our customers in the normal course of business based upon an evaluation of the customer’s credit history, financial condition and other factors. Estimates of allowances for uncollectible receivables are determined by evaluating individual customer circumstances, historical payment patterns, length of time past due and other factors. At December 31, 2014 and 2013, we did not have any allowances for uncollectible receivables.
Accounts receivable from collaborators which contributed 10% or more of accounts receivable at December 31, 2014 and 2013 were as follows:
|
December 31, | |||
|
2014 |
2013 | ||
|
Firmenich |
$1.2 million (55%) |
$1.7 million (78%) | |
|
Nestlé |
$0.6 million (26%) |
— | |
|
PepsiCo |
— |
$0.3 million (14%) | |
Inventories
Inventories consist entirely of purchased finished goods. Inventories are valued at lower of cost (on a moving average basis) or market value. We are required to make assumptions regarding the level of reserves required to value items at the lower of cost or market. These assumptions require us to analyze forecasted demand, pricing trends and margins, and to make judgments and estimates regarding excess or obsolete inventory. At December 31, 2014 and 2013, we did not have any reserves for lower of cost or market, excess or obsolete inventory.
Inventories amounts from prior years have been reclassified from other current assets to conform to the current year presentation.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and are depreciated over the estimated useful lives of the assets (ranging from three to five years) using the straight-line method. Leasehold improvements are amortized over the estimated useful life of the asset or the lease term, whichever is shorter.
Impairment of Long-Lived Assets
In accordance with the Property, Plant and Equipment Topic of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”), if indicators of impairment exist, we assess the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If impairment is indicated, we measure the amount of such impairment by comparing the fair value to the carrying value. There have been no indicators of impairment through December 31, 2014.
Deferred Rent
Rent expense is recorded on a straight-line basis over the initial term of any lease. The difference between rent expense accrued and amounts paid under any lease agreement is recorded as deferred rent in the accompanying balance sheets.
Leasehold Incentive Obligation
In conjunction with the lease agreement covering the facility occupied by us, we received a tenant improvement allowance of $155 per square foot leased, or $10.1 million. As the tenant improvements were constructed, we recorded both the covered tenant improvements (as property and equipment) and an offsetting leasehold incentive obligation on our balance sheet. Through the term of the lease, we record depreciation expense to depreciate the tenant improvements and record an offsetting reduction to rent expense to amortize the leasehold incentive obligation.
Revenue Recognition
Development Revenues
Development revenues are based on our product discovery, development and commercialization collaboration agreements. Some of our collaboration agreements contain multiple elements, including technological and territorial licenses and research and development services. In accordance with these agreements, we may be eligible for license fees, research and development funding, development milestone payments, cost reimbursements, royalty payments, minimum periodic royalty payments and commercial milestone payments. Development revenues include revenues from license fees, research and development funding, the achievement of development milestones and cost reimbursements.
Pursuant to the Revenue Recognition – Multiple-Element Arrangements Topic of the FASB ASC, each required deliverable is evaluated to determine if it qualifies as a separate unit of accounting. For us, this determination is generally based on whether the deliverable has “stand-alone value” to the customer. The arrangement’s consideration is then allocated to each separate unit of accounting based on the relative selling price of each deliverable. The estimated selling price of each deliverable is determined using the following hierarchy of values: (i) vendor-specific objective evidence of fair value; (ii) third-party evidence of selling price; and (iii) best estimate of selling price, or BESP. The BESP reflects our best estimate of what the selling price would be if the deliverable was regularly sold by us on a stand-alone basis. We expect, in general, to use the BESP for allocating consideration to each deliverable. In general, the consideration allocated to each unit of accounting is then recognized as the related goods or services are delivered, limited to the consideration that is not contingent upon future deliverables. For multiple-element arrangements entered into prior to January 1, 2011 and not materially modified thereafter, we continue to apply our prior accounting policy with respect to such arrangements.
Non-refundable license fees, if not associated with our future performance, are recognized when received. Non-refundable license fees, if associated with our future performance obligations, are attributed to a specific program or collaboration and recognized over the period of service for that specific program or collaboration. Amounts received for research funding are recognized as revenues as the services are performed. Revenue is deferred for fees received before earned. Revenues from development milestones are accounted for in accordance with the Revenue Recognition – Milestone Method Topic of the FASB ASC. Milestones are recognized when earned, as evidenced by written acknowledgment from the collaborator or other persuasive evidence that the milestone has been achieved, provided that the milestone event is substantive. A milestone event is considered to be substantive if its achievability was not reasonably assured at the inception of the agreement and our efforts led to the achievement of the milestone or the milestone was due upon the occurrence of a specific outcome resulting from our performance. If both of these criteria are not met, the milestone payment is recognized over the remaining minimum period of our performance obligations under the agreement. We assess whether a milestone is substantive at the inception of each agreement. Revenues from cost reimbursement are recognized when earned, as evidenced by written acknowledgment from the collaborator or other persuasive evidence.
Commercial Revenues
Commercial revenues from collaboration agreements include royalties on sales made by our collaborators of products incorporating our flavor ingredients, minimum periodic royalty payments and revenues from the achievement of commercial milestones. Commercial revenues also include direct sales of our flavor ingredients to flavor companies.
Royalties on sales made by our collaborators of products incorporating our flavor ingredients are recognized when a royalty report or other persuasive evidence is received, which is generally one quarter in arrears. Non-refundable minimum periodic royalty payments are recognized as revenues over the related royalty periods. Royalty terms are specific to each collaboration and collaborator and can vary from year to year. These terms vary based on factors such as the characteristics of the flavor ingredient and the product categories and geographies licensed by the collaborator. Periodically, as contractually specified, our collaborators are required to provide a report detailing all sales of products containing our flavor ingredients. To the extent that calculated royalties on sales of such products exceed the minimum periodic royalty payments made to date, the collaborators are required to remit to us the difference between royalties calculated and minimum periodic royalty payments made to date. We recognize this difference as royalties on product sales at the time the report is received. To the extent that minimum periodic royalty payments through the end of any applicable period exceed calculated royalties, we are not required to refund the difference. Although we currently do not have any collaborations that include refundable minimum periodic royalty payments, in such a case, revenues would be deferred for refundable minimum periodic royalty payments received before earned. Revenues from commercial milestones are recognized when earned, as evidenced by written acknowledgment from the collaborator or other persuasive evidence that the milestone has been achieved, because we have no further performance obligations related to the commercial milestones.
Revenues from direct sales of our flavor ingredients are recorded when there is persuasive evidence that an arrangement exists, title has passed, collection is reasonably assured and the price is fixed or determinable. Revenues are recorded following any general right of return period, which typically is no longer than five days. We generally do not offer discounts or rebates on sales of flavor ingredients.
New Accounting Guidance
In May 2014, the FASB issued accounting guidance on the recognition of revenue from customers. The guidance will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. Under this guidance, an entity will recognize revenue when it transfers promised goods or services to customers in an amount that reflects that the entity expects in exchange for the goods or services. This guidance also requires more detailed disclosures to enable users of the financial statements to understand the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The guidance is effective for fiscal years, and interim periods within those years, beginning after December 15, 2016, and early application is not permitted. The guidance allows entities to select one of two methods of adoption, either the full retrospective approach, meaning the guidance would be applied to all periods presented, or modified retrospective, meaning the cumulative effect of applying the guidance would be recognized as an adjustment to our opening retained earnings new balance at January 1, 2017, along with providing certain additional disclosures. We will adopt this guidance in our fiscal year beginning January 1, 2017. We have not yet selected a transition method nor have we determined the effect of the standard on our ongoing financial reporting.
Cost of Commercial Revenues
Cost of commercial revenues represents royalties payable under our third-party licensing agreements and the cost of goods sold related to direct sales, including related shipping and handling costs.
Research, Development and Patents
Research and development costs, including those incurred in relation to our collaborative agreements, are expensed in the period incurred. Research and development costs primarily consist of salaries and related expenses for personnel, facilities and depreciation, research and development supplies, licenses and outside services. Such research and development costs totaled $24.9 million, $27.1 million and $27.0 million for the years ended December 31, 2014, 2013 and 2012, respectively.
Costs related to filing and pursuing patent applications are expensed as incurred, as recoverability of such expenditures is uncertain. We include all external costs related to the filing of patents on developments in Research, Development and Patents expenses. Such patent-related expenses totaled $1.7 million, $1.5 million and $1.6 million for the years ended December 31, 2014, 2013 and 2012, respectively.
Employee Benefit Plans
We have a defined contribution plan under Section 401(k) of the Internal Revenue Code covering employees who meet certain eligibility requirements. Eligible employees may defer their pre-tax compensation up to the maximum allowed by the Internal Revenue Service, or IRS. Under the plan, we may match a portion of employee contributions up to a defined maximum. Such matching contributions become vested and non-forfeitable according to a defined vesting schedule. We made matching contributions of $199,000, $187,000 and $165,000 for the years ended December 31, 2014, 2013 and 2012, respectively.
Stock-Based Compensation
Compensation cost for stock-based awards is based on the estimated grant-date fair value. We use the Black-Scholes option-pricing model for determining the estimated fair value for stock-based awards with the following weighted average assumptions (annualized percentages):
|
Years Ended December 31, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Stock Options |
||||||||||||
|
Expected volatility |
71.8 | % | 70.2 | % | 69.6 | % | ||||||
|
Risk-free interest rate |
1.82 | % | 1.54 | % | 1.22 | % | ||||||
|
Dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
|
Expected term (years) |
6.2 | 6.1 | 6.0 | |||||||||
|
Employee Stock Purchase Plan |
||||||||||||
|
Expected volatility |
68.9 | % | 62.8 | % | 69.8 | % | ||||||
|
Risk-free interest rate |
0.23 | % | 0.10 | % | 0.16 | % | ||||||
|
Dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
|
Expected term (years) |
1.3 | 1.2 | 1.3 | |||||||||
Expected volatility is based on our historical volatility. The risk-free interest rate for the expected term of the option is based on the average United States Treasury yield curve at the balance sheet date. The assumed dividend yield is based on our expectation of not paying dividends in the foreseeable future. The expected term of options granted is based on historical exercise data. The assumptions related to expected volatility and risk-free interest rate used for the valuation of stock options differ from those used for the valuation of employee stock purchase plan rights primarily due to the difference in their respective expected terms.
The weighted-average estimated fair value of stock options granted during the years ended December 31, 2014, 2013 and 2012 were $5.53, $1.36 and $2.01 per share, respectively. The weighted-average estimated fair value of employee stock purchase plan rights granted during the years ended December 31, 2014, 2013 and 2012 were $4.21, $1.32 and $0.96 per share, respectively.
Stock-based compensation expenses recognized in the Statements of Operations are based on awards ultimately expected to vest; accordingly, these expenses are reduced for estimated forfeitures. The Compensation-Stock Compensation Topic of the FASB ASC requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Pre-vesting forfeitures were estimated to be approximately 4.0%, 6.1% and 6.3% for the years ended December 31, 2014, 2013 and 2012, respectively, based on historical experience.
Stock-based compensation expenses for options and awards are recognized on a straight-line basis. Stock-based compensation expenses are allocated to research, development and patents or selling, general and administrative based upon the department to which the associated employee or non-employee reports.
Total stock-based compensation expenses recognized for the years ended December 31, 2014, 2013 and 2012 was comprised as follows (in thousands):
|
Years Ended December 31, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Research, development and patents |
$ | 2,575 | $ | 1,688 | $ | 1,876 | ||||||
|
Selling, general and administrative |
3,606 | 2,341 | 2,594 | |||||||||
|
Total stock-based compensation expenses |
$ | 6,181 | $ | 4,029 | $ | 4,470 | ||||||
The total fair value of options that vested during the years ended December 31, 2014, 2013 and 2012 was $3.7 million, $3.9 million and $4.7 million, respectively. At December 31, 2014, total unrecognized estimated compensation expenses related to non-vested stock options granted prior to that date were $8.9 million, expected to be recognized over a weighted average period of 1.7 years.
Income Taxes
We account for income taxes in accordance with provisions which set forth an asset and liability approach that requires the recognition of deferred tax assets and deferred tax liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities. Valuation allowances are established when necessary to reduce deferred tax assets to the amount that is more likely than not expected to be realized. In making such a determination, a review of all available positive and negative evidence is considered, including scheduled reversal of deferred tax liabilities, projected future taxable income, tax planning strategies and recent financial performance.
The provisions of the Income Taxes Topic of the FASB ASC defines a recognition threshold and measurement attributes for financial statement recognition and measurement of a tax provision taken or expected to be taken in a tax return. The Topic also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. Under the Topic, the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. We recognize interest and penalties related to uncertain tax positions as a component of the income tax provision.
Net Loss Per Share
Basic earnings per share (“EPS”) is calculated by dividing the net loss by the weighted average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted EPS is computed by dividing the net income or loss by the weighted average number of common share equivalents outstanding for the period determined using the treasury-stock method. Dilutive common share equivalents include the dilutive effect of in-the-money shares, which is calculated based on the average share price for each period using the treasury stock method. Under the treasury stock method, the exercise price of a share, the amount of compensation cost, if any, for future service that we have not yet recognized, and the amount of estimated tax benefits that would be recorded in paid-in capital, if any, when the share is exercised are assumed to be used to repurchase shares in the current period. For purposes of this calculation, options to purchase common stock are considered to be common stock equivalents and are only included in the calculation of diluted earnings per share when their effect is dilutive.
The following table sets forth the computation of basic and diluted net loss per share for the respective periods.
|
Years Ended December 31, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Numerator: |
||||||||||||
|
Net loss (in thousands) |
$ | (12,150 |
) |
$ | (11,898 |
) |
$ | (9,186 |
) | |||
|
Denominator: |
||||||||||||
|
Weighted average common shares |
42,611,904 | 40,662,640 | 39,956,283 | |||||||||
|
Basic and diluted net loss per share |
$ | (0.29 |
) |
$ | (0.29 |
) |
$ | (0.23 |
) | |||
|
Years Ended December 31, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Outstanding antidilutive securities not included in diluted net loss per share calculation: |
||||||||||||
|
Options to purchase common stock |
11,256,053 | 11,578,438 | 10,574,368 | |||||||||
Comprehensive Income (Loss)
The Comprehensive Income Topic of the FASB ASC requires that all components of comprehensive income (loss), including net income (loss), be reported in the financial statements in the period in which they are recognized. Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Our accumulated other comprehensive income as of December 31, 2014, 2013 and 2012 consisted of unrealized gains or losses on investments available-for-sale and is reported in stockholders’ equity.
Segment Reporting
We operate in one business segment, which is the development and commercialization of flavor ingredients.
2. Balance Sheet Details
Investments Available-for-Sale
The following is a summary of investments available-for-sale securities at December 31, 2014 (in thousands):
|
Amortized Cost |
Unrealized Gain |
Unrealized Loss |
Estimated |
|||||||||||||
|
United States Treasuries |
$ | 1,500 | $ | — | $ | — | $ | 1,500 | ||||||||
|
United States government agency bonds |
16,341 | — | (6 |
) |
16,335 | |||||||||||
|
Corporate bonds |
6,946 | — | (4 |
) |
6,942 | |||||||||||
| $ | 24,787 | $ | — | $ | (10 |
) |
$ | 24,777 | ||||||||
The following is a summary of investments available-for-sale securities at December 31, 2013 (in thousands):
|
Amortized Cost |
Unrealized Gain |
Unrealized Loss |
Estimated |
|||||||||||||
|
United States Treasuries |
$ | 7,505 | $ | 3 | $ | — | $ | 7,508 | ||||||||
|
United States government agency bonds |
14,249 | 4 | — | 14,253 | ||||||||||||
| $ | 21,754 | $ | 7 | $ | — | $ | 21,761 | |||||||||
Investments we consider to be temporarily impaired at December 31, 2014 were as follows (in thousands, except for number of investments):
|
Less than 12 Months |
||||||||||||
|
Description |
Number of investments |
Estimated Fair Value |
Unrealized Losses |
|||||||||
|
United States government agency bonds |
9 | $ | 10,632 | $ | (6 |
) | ||||||
|
Corporate bonds |
6 | 6,942 | (4 |
) | ||||||||
|
Total temporarily impaired securities |
15 | $ | 17,574 | $ | (10 |
) | ||||||
We believe that the decline in value of these securities is temporary and primarily related to the change in market interest rates since purchase and that it is more likely than not that we will be able to hold these securities to maturity. Therefore we anticipate full recovery of their amortized cost basis at maturity.
Gross realized gains and losses on available-for-sale securities were immaterial during the years ended December 30, 2014 and 2013. As of December 31, 2014, we held $22.6 million of available-for-sale securities with maturity dates within one year and $2.1 million with maturity dates over one year and less than two years.
Property and Equipment
Property and equipment consists of the following (in thousands):
|
As of December 31, |
||||||||
|
2014 |
2013 |
|||||||
|
Scientific equipment |
$ | 12,546 | $ | 12,645 | ||||
|
Computer equipment |
3,423 | 3,463 | ||||||
|
Furniture and fixtures |
1,099 | 1,099 | ||||||
|
Leasehold improvements |
13,211 | 13,204 | ||||||
| 30,279 | 30,411 | |||||||
|
Less accumulated depreciation and amortization |
(26,444 |
) |
(24,758 |
) | ||||
| $ | 3,835 | $ | 5,653 | |||||
Depreciation expense was $2.2 million, $2.8 million and $2.8 million for the years ended December 31, 2014, 2013 and 2012, respectively.
Accounts Payable, Accrued Expenses and Other Current Liabilities
Accounts payable and accrued expenses consist of the following (in thousands):
|
As of December 31, |
||||||||
|
2014 |
2013 |
|||||||
|
Accounts payable |
$ | 1,064 | $ | 663 | ||||
|
Accrued employee benefits |
3,192 | 5,320 | ||||||
|
Other accrued expenses |
1,647 | 1,136 | ||||||
| $ | 5,903 | $ | 7,119 | |||||
3. Fair Value Disclosures
The following table presents information about our financial assets and financial liabilities measured at fair value on a recurring basis as of December 31, 2014 and indicates the fair value hierarchy of the valuation techniques we use to determine such fair value. In general, fair values determined by Level 1 inputs use quoted prices (unadjusted) in active markets for identical assets or liabilities that we have the ability to access. We classify money market funds and United States Treasuries as Level 1 assets.
Fair values determined by Level 2 inputs utilize inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly. Level 2 inputs include quoted prices for similar assets and liabilities in active markets, and inputs other than quoted prices that are observable for the asset or liability, such as interest rates and yield curves that are observable at commonly quoted intervals. We obtain the fair value of Level 2 financial instruments from a third-party professional pricing service using quoted market prices for identical or comparable instruments. The professional pricing service gathers market prices from a variety of industry standard data providers, security master files from large financial institutions and other third-party sources. The service uses these multiple prices as inputs into a distribution-curve based algorithm to determine a fair value. We then validate the quoted fair values provided by the professional pricing service by comparing the service’s assessment of the fair values of our Level 2 investment portfolio balance against the fair values of our Level 2 investment portfolio balance provided by our investment managers. We classify United States government agency securities and corporate bonds as Level 2 assets. There were no transfers between Level 1 and Level 2 during 2014 or 2013.
Level 3 inputs are unobservable inputs for the asset or liability, and include situations where there is little, if any, market activity for the asset or liability. We do not hold any Level 3 assets. In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, the level in the fair value hierarchy within which the fair value measurement in its entirety falls has been determined based on the lowest level input that is significant to the fair value measurement in its entirety. Our assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment, and we consider factors specific to the asset or liability.
The fair value as of December 31, 2014 and 2013 for assets that have recurring measurements are shown below (in thousands):
|
Fair Value Measurement at Reporting Date Using |
||||||||||||||||
|
Description |
Balance as of December 31, 2014 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
|
Financial instruments owned: |
||||||||||||||||
|
Money market funds |
$ | 3,088 | $ | 3,088 | $ | — | $ | — | ||||||||
|
U.S. Treasuries |
1,500 | 1,500 | — | — | ||||||||||||
|
U.S. government agency bonds |
16,335 | — | 16,335 | — | ||||||||||||
|
Corporate bonds |
6,942 | — | 6,942 | — | ||||||||||||
|
Total financial instruments owned |
$ | 27,865 | $ | 4,588 | $ | 23,277 | $ | — | ||||||||
|
Fair Value Measurement at Reporting Date Using |
||||||||||||||||
|
Description |
Balance as of December 31, 2013 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
|
Financial instruments owned: |
||||||||||||||||
|
Money market funds |
$ | 10,545 | $ | 10,545 | $ | — | $ | — | ||||||||
|
U.S. Treasuries |
7,508 | 7,508 | — | — | ||||||||||||
|
U.S. government agency bonds |
14,253 | — | 14,253 | — | ||||||||||||
|
Total financial instruments owned |
$ | 32,306 | $ | 18,053 | $ | 14,253 | $ | — | ||||||||
4. Product Discovery, Development and Commercialization Collaborations
Our current collaboration agreements generally provide for research and development funding, milestone payments, cost reimbursement and royalty payments in the event the collaborator commercializes a product incorporating our flavor ingredients.
For our agreements that include development milestones and commercial milestones, development milestones generally range from $500,000 to $1.0 million each. Development milestones are generally due upon selection and regulatory events. Development milestones are considered to be due to our performance and are accounted for as substantive milestones in accordance with the Revenue Recognition – Milestone Method Topic of the FASB ASC. Development milestones are recorded as development revenues. An example of a selection event would be a collaborator selecting a compound for development. Our efforts that support the compound selection process include the identification of relevant taste receptors and the development of proprietary taste receptor-based assays based in the identified receptor, the screening and identification of compounds that bind to the identified receptor and optimization of compounds for selection. An example of a regulatory event would be a selected compound obtaining either U.S. or foreign regulatory approval. As of December 31, 2014, our agreements contain 21 unearned potential development milestones totaling $18.9 million. There are circumstances under which we and our collaborators in the future may mutually agree to pursue additional program goals, in which case we could then be eligible to earn additional milestones. Any such additional milestones remain uncertain at this time. We do not consider any individual development milestone to be material due to the relatively small size of any individual development milestone payment in relation to our annual revenues, and due to the uncertainty associated with the scientific progress required for us to earn such milestones.
Commercial milestones generally range from $250,000 to $1.5 million each and are due upon commercial events. We do not consider commercial events to be due to our performance, and as such, are not accounted for as substantive milestones in accordance with the Revenue Recognition – Milestone Method Topic of the FASB ASC. Examples of commercial events would be the first commercial sale of a product containing a developed compound or upon sales of a product containing a developed compound reaching a certain level. As of December 31, 2014, our agreements contain eight unearned potential commercial milestones totaling $8.5 million. There are circumstances under which we and our collaborators in the future may mutually agree to pursue additional program goals, in which case we could then be eligible to earn additional milestones. Any such additional milestones remain uncertain at this time. We consider milestones for commercial events to be commercial revenues.
The specific type of royalty and method for calculating royalty payments varies by agreement. We have retail-based royalty agreements, where any potential royalty payable to us is calculated as a percentage of the net sales price of a manufacturer’s finished products or is based on the volume of a manufacturer’s finished product that it sells. Our retail-based royalty agreements provide for an effective royalty rate of up to 4%. Our agreements with Ajinomoto and PepsiCo are either exclusively or partially retail-based royalty agreements. We have ingredient supply agreements, where any potential royalty payable to us is calculated as a percentage of the sales price of either our ingredient itself or the flavor system in which our ingredient is contained or is based on the volume of the ingredient itself used by a manufacturer in a finished product. Our ingredient supply royalty agreements specify royalty rates that are typically greater than the rates specified by our retail-based agreements. Our agreements with Firmenich, Nestlé and PepsiCo are either exclusively or partially ingredient supply-based royalty agreements. Certain of our current collaboration agreements also provide for upfront license fees and minimum periodic royalties. Below is a discussion of our material agreements.
Material Agreements
Firmenich. In July 2009, we entered into a collaboration agreement with Firmenich to work for a minimum two-year collaborative period to discover novel flavor ingredients intended to modify the sweet taste of sucrose, fructose or various forms of rebaudioside. The agreement includes three consecutive options of one year each that could further extend the collaborative research funding period. The agreement was subsequently amended in October 2009. Under the agreement as amended, Firmenich agreed to pay a license fee, payable in three installments, research and development fees and specified payments upon the achievement of milestones. In the event of commercialization, we are entitled to receive royalties on future sales of products containing a discovered flavor ingredient.
In October 2010 we and Firmenich further amended the agreement to include, among other things, commercial development of S6973, our novel sucrose modifier, for specific beverage applications. The amendment also converts Firmenich’s license for use of S6973 in powdered beverages from co-exclusive to exclusive and grants Firmenich an exclusive right to commercialize any compound that they select for development for use in confectionary food products. In return, under the terms of the amendment we received an additional license fee, and incremental milestone payments and minimum annual royalties.
In November 2010, Firmenich exercised its option to expand the companies’ agreement to include the discovery, development, and worldwide commercialization of natural flavor ingredients that modify the taste of sucrose, fructose, and various forms of rebaudioside. In consideration of the expansion of the agreement, Firmenich paid us additional research funding as well as milestones, minimum annual royalties, and royalties on sales of natural sweet enhancers developed under the collaboration.
In April 2013, we amended and restated the collaboration agreement with Firmenich to extend the collaborative research period through July 2016, subject to limited termination rights. Firmenich will pay an additional non-refundable license fee of $5 million, of which $4 million was paid in 2013. The remaining $1 million will be paid at the earlier of the achievement of a certain milestone or the end of the research period. Firmenich will also pay approximately $13 million in research funding over the three-year extension period from July 2013 through July 2016, payable on a quarterly basis. Firmenich continues to have obligations for other milestone payments, cost reimbursements and royalty payments under an increased royalty rate structure based upon Firmenich sales of flavor ingredients developed under the collaboration.
In connection with this collaborative agreement, we recognized development revenues of $6.5 million, $6.9 million and $6.8 million for the years ended December 31, 2014, 2013 and 2012, respectively. Development revenues for the years ended 2014, 2013 and 2012 included $500,000, $667,000 and $750,000 related to the earning of one, one and two development milestones, respectively. We also recognized commercial revenues of $1.7 million, $1.1 million and $553,000 for the years ended December 31, 2014, 2013 and 2012, respectively. As of December 31, 2014 and 2013, we had deferred revenues of $1.7 million and $3.2 million, respectively.
Under this agreement, through December 31, 2014, we have received $46.0 million in license fees, research and development funding and cost reimbursements and $3.4 million in milestone payments. If all milestones are achieved, and including the remaining license fees and research and development funding, we may be entitled to an additional $21.3 million for a total of $70.7 million. There is no guarantee that we will receive any further milestone payments under this collaboration.
PepsiCo. In August 2010 we entered into a collaboration agreement with PepsiCo. The agreement relates to a research program to discover and develop (1) novel natural and artificial flavor ingredients intended to modify the sweet taste of sucrose and fructose, including high fructose corn syrup, and (2) natural high intensity sweeteners, in each case for use in non-alcoholic beverage product categories on a worldwide basis. Under the agreement, we received an upfront payment of $30.0 million from PepsiCo, $7.5 million of which was paid in the second quarter of 2010 in connection with the signing of a letter agreement between the parties and $22.5 million of which was paid in the third quarter of 2010. The upfront payment is being recognized over the entire research period of the agreement. We received $32.0 million in research and development payments over the initial four-year research period. We are also entitled to milestone payments and reimbursement of certain out-of-pocket expenses. Upon commercialization, we are entitled to minimum annual royalties and royalty payments on products that incorporate selected flavor ingredients and/or natural high intensity sweeteners.
In May 2014, PepsiCo exercised its option to extend the research period for an additional two years through August 2016. We are entitled to $17.6 million in committed research funding to be paid in quarterly installments over the additional two-year period.
In connection with this agreement, we recognized development revenues of $13.0 million, $16.2 million and $17.7 million for the years ended December 31, 2014, 2013 and 2012, respectively. Included in development revenues was $750,000, zero, and $1.5 million related to the earnings of one, zero and two development milestones for the years ended December 31, 2014, 2013 and 2012, respectively. As of December 31, 2014 and 2013, we had deferred revenues of $4.0 million and $7.3 million, respectively.
Under this agreement, through December 31, 2014, we have received $69.5 million in upfront fees, research and development funding and cost reimbursements and $3.0 million in milestones. If all milestones are achieved, and including the remaining research and development funding, we may be entitled to an additional $25.6 million for a total of $98.1 million. There is no guarantee that we will receive any further milestone payments under this collaboration.
This footnote only discloses amounts recognized for individually material collaboration agreements during the years ended December 31, 2014, 2013 and 2012, and does not represent the entire amount of development and commercial revenues recognized during those periods.
5. Commitments
Leases and Loans
We lease our primary office facility under a lease that expires on February 28, 2017. The lease provides for an annual 3% rent increase. Rent expense for the years ended December 31, 2014, 2013 and 2012 was $1.7 million for all years. We have also entered into various operating lease agreements for office equipment.
The estimated annual future minimum rental payments under our operating leases in effect at December 31, 2014, which expire through 2017, for the years ending December 31 are as follows (in thousands):
|
Operating |
||||
|
2015 |
$ | 3,052 | ||
|
2016 |
3,131 | |||
|
2017 |
538 | |||
|
Total minimum lease payments |
$ | 6,721 | ||
In connection with certain license agreements, our annual future minimum obligation payments are $225,000 and $24,000 for the years ending December 31, 2015 and 2016, respectively.
6. Stockholders’ Equity
Convertible Preferred Stock
Our certificate of incorporation, as amended and restated, authorizes us to issue up to 7,500,000 shares of preferred stock, with a par value of $0.001, in one or more series. Our Board of Directors may authorize the issuance of convertible preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of convertible preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of the company and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock. No shares of convertible preferred stock were outstanding as of December 31, 2014 or 2013.
Equity Incentive Plan
We adopted the 2004 Equity Incentive Plan in connection with our initial public offering and adopted the 2013 Equity Incentive Plan effective for grants beginning January 1, 2014 (collectively, the “Plan”). The Plan provides for the grant of incentive and non-statutory stock options and restricted stock purchase rights to our employees, directors and consultants. The Plan, as amended, authorizes us to issue up to 18,935,450 shares of common stock. At December 31, 2014, 2,624,114 shares remain available for grant under the Plan. We issue new shares of common stock upon the exercise of stock options.
The Plan allows us to grant restricted stock purchase rights at no less than 85% of the fair value of our common stock as determined by our Board of Directors at the date of the grant. All restricted stock purchase rights vest in accordance with a vesting schedule determined by our Board of Directors, typically over a four-year period. Since the Plan’s inception, 457,069 restricted stock purchase rights have been granted at exercise prices ranging from $0.35 to $0.94 per share, all of which have been exercised as of December 31, 2014. We have repurchased a total of 131,152 shares since the Plan’s inception and no shares are subject to repurchase at December 31, 2014. No restricted stock purchase rights were granted during the three years ended December 31, 2014.
Options granted under the Plan generally expire no later than 10 years from the date of grant (five years for a 10% stockholder). Options generally vest and become fully exercisable over a period of four years. In certain cases, grants to officers, directors and consultants can be made fully exercisable at the date of grant. The exercise price of incentive stock options must be equal to at least the fair value of our common stock on the date of grant, and the exercise price of non-statutory stock options may be no less than 85% of the fair value of our common stock on the date of grant. The exercise price of any option granted to a 10% stockholder may be no less than 110% of the fair value of our common stock on the date of grant. We have an option to repurchase all unvested shares at the original purchase price for any reason upon the voluntary or involuntary termination of employment with us or consulting services provided to us. At December 31, 2014, no shares of common stock were unvested and subject to repurchase.
The following is a summary of stock option activity under the Plan through December 31, 2014:
|
Shares |
Weighted Average |
Weighted Average Remaining Contractual Term (years) |
Aggregate Intrinsic Value (in thousands) |
|||||||||||||
|
Outstanding at December 31, 2013 |
11,578,438 | $ | 5.83 | |||||||||||||
|
Granted |
1,704,860 | $ | 8.53 | |||||||||||||
|
Exercised |
(1,802,295 |
) |
$ | 4.78 | ||||||||||||
|
Cancelled |
(224,950 |
) |
$ | 6.28 | ||||||||||||
|
Outstanding at December 31, 2014 |
11,256,053 | $ | 6.40 | 5.5 | $ | 16,938 | ||||||||||
|
Vested or expected to vest at December 31, 2014 |
11,127,806 | $ | 6.40 | 5.5 | $ | 16,753 | ||||||||||
|
Exercisable at December 31, 2014 |
8,243,626 | $ | 6.60 | 4.4 | $ | 12,253 | ||||||||||
The aggregate intrinsic value represents the difference between the closing market price of our common stock at December 31, 2014 of $6.01 and the exercise price of in-the-money options. The total intrinsic value of options exercised was $8.1 million, $844,000, and $29,000 during the years ended December 31, 2014, 2013 and 2012, respectively. We received $8.6 million, $1.4 million and $26,000 in proceeds from the exercise of stock options during the years ended December 31, 2014, 2013 and 2012, respectively.
Employee Stock Purchase Plan
During 2004, we adopted the 2004 Employee Stock Purchase Plan (the “Purchase Plan”), which allows all eligible employees to purchase shares of our common stock at the lower of: (i) 85% of the fair market value on the first day of a two-year offering period; or (ii) 85% of the fair market value on the last date of each six-month purchase period within the two-year offering period. Employees may authorize us to withhold up to 15% of their compensation during any purchase period, subject to certain limitations. The Purchase Plan authorizes up to 5,633,096 shares to be granted. At December 31, 2014, 3,105,154 shares of common stock have been issued under the Purchase Plan at an average price of $2.63 per share.
Shares Reserved for Future Issuance
The following shares of common stock are reserved for future issuance:
|
December 31, 2014 |
||||
|
Common stock options granted and outstanding |
11,256,053 | |||
|
Common stock options reserved for future grant |
2,624,114 | |||
|
Common stock reserved under Purchase Plan |
2,527,942 | |||
|
Total common stock shares reserved for future issuance |
16,408,109 | |||
7. Income Taxes
Significant components of our net deferred tax assets at December 31, 2014 and 2013 are shown below (in thousands). A valuation allowance of $92.0 million and $87.2 million has been established to offset the net deferred tax assets as of December 31, 2014 and 2013, respectively, due to uncertainties surrounding our ability to generate future taxable income to realize these assets.
|
Years ended |
||||||||
|
2014 |
2013 |
|||||||
|
Deferred tax assets: |
||||||||
|
Net operating loss carryforwards |
$ | 39,900 | $ | 37,500 | ||||
|
Capitalized research and development |
27,200 | 23,600 | ||||||
|
Research and development credits |
10,700 | 9,800 | ||||||
|
Stock compensation |
9,600 | 8,900 | ||||||
|
Deferred revenues |
2,300 | 4,300 | ||||||
|
Other, net |
2,300 | 3,100 | ||||||
|
Total deferred tax assets |
92,000 | 87,200 | ||||||
|
Total deferred tax liabilities |
— | — | ||||||
|
Net deferred tax assets |
92,000 | 87,200 | ||||||
|
Valuation allowance for deferred tax assets |
(92,000 |
) |
(87,200 |
) | ||||
|
Net deferred tax assets |
$ | — | $ | — | ||||
The provision for income taxes on earnings subject to income taxes differs from the statutory federal rate at December 31, 2014, 2013 and 2012, due to the following (in thousands):
|
2014 |
2013 |
2012 |
||||||||||
|
Federal income taxes at 35% |
$ | (4,252 |
) |
$ | (4,164 |
) |
$ | (3,215 |
) | |||
|
State income tax, net of Federal benefit |
(622 |
) |
(581 |
) |
(409 |
) | ||||||
|
Tax effect on non-deductible expenses and credits |
(779 |
) |
(5 |
) |
542 | |||||||
|
Expiring state net operating losses |
218 | |||||||||||
|
Other |
566 | (350 |
) |
(28 |
) | |||||||
|
Increase in valuation allowance |
4,869 | 5,100 | 3,110 | |||||||||
| $ | — | $ | — | $ | — | |||||||
We recognize windfall tax benefits associated with the exercise of stock options directly to stockholders’ equity only when realized. Accordingly, deferred tax assets are not recognized for net operating loss carryforwards resulting from windfall tax benefits occurring from January 1, 2006 onward. At December 31, 2014 deferred tax assets do not include excess tax benefits from stock-based compensation of approximately $3.7 million.
At December 31, 2014, we had Federal and California tax net operating loss carryforwards of approximately $109.1 million and $94.1 million, respectively. The Federal and California tax loss carryforwards will begin to expire in 2019 and 2015, respectively, unless previously utilized.
At December 31, 2014, we also had Federal and California research and development tax credit carryforwards of approximately $9.4 million and $7.7 million, respectively. The Federal credit carryforward will begin to expire in 2019 unless previously utilized and the California credit will carry forward indefinitely until utilized.
We have analyzed filing positions in all of the Federal and state jurisdictions where we are required to file income tax returns for all open tax years in these jurisdictions. Our analysis concluded that, due to past ownership changes, our deferred tax assets for net operating losses and research and development credits will be subject to an annual limitation. As such, $3.1 million of net operating losses will expire and $944,000 of research and development credits will expire for Federal purposes as a result of the multiple ownership changes which occurred in prior years. As a result of the completion of the analysis, we have included the net operating loss and research and development credit carryforward net of the amount to be expired as a deferred tax asset. However, we have determined that sufficient future taxable income may not be available to realize the deferred tax assets for net operating loss and research and development credit carryforwards. Therefore, we have recognized a full valuation allowance for these deferred tax assets.
We updated our Section 382 and 383 analyses through December 31, 2014 and determined that there has not been a subsequent ownership change since February 2007. The completion of our Section 382 and 383 analyses through December 31, 2014 does not prevent further limitations to our net operating loss and research and development credit carryforwards. Additional limitations may arise if we experience an ownership change in subsequent periods.
A rollforward of changes in our unrecognized tax benefits is shown below (in thousands).
|
2014 |
2013 |
2012 |
||||||||||
|
Balance at beginning of year |
$ | 3,989 | $ | 3,629 | $ | 3,521 | ||||||
|
Additions based on tax positions related to the current year |
371 | 242 | 94 | |||||||||
|
Additions based on tax positions of prior years |
— | 118 | 14 | |||||||||
|
Balance at end of year |
$ | 4,360 | $ | 3,989 | $ | 3,629 | ||||||
Due to the existence of the valuation allowance, future changes in unrecognized tax benefits will not impact our effective tax rate.
We are subject to taxation in the United States and state jurisdictions. Our tax years for 2011 and forward are subject to examination by the IRS and tax years 2010 and forward are subject to examination by California tax authorities. Due to the carryforward of unutilized net operating losses and research and development credits, the IRS and the California tax authorities may go back to the taxable years in which the net operating losses and research and development credits became available to recompute such amounts, but not redetermine the tax liability for such years. We are currently not under examination by any taxing authorities.
We recognize interest and penalties related to income tax matters in income tax expense. For the three years ended December 31, 2014, we did not recognize any interest or penalties.
8. Summary of Quarterly Financial Data (unaudited)
The following is a summary of the unaudited quarterly results of operations for the years ended December 31, 2014 and 2013 (in thousands, except per share amounts):
|
Year Ended December 31, 2014 |
||||||||||||||||
|
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
|||||||||||||
|
Selected Quarterly Financial Data: |
||||||||||||||||
|
Revenues |
$ | 8,204 | $ | 7,343 | $ | 5,858 | $ | 6,257 | ||||||||
|
Total operating expenses |
10,220 | 9,992 | 10,275 | 9,362 | ||||||||||||
|
Net loss |
(2,008 |
) |
(2,641 |
) |
(4,406 |
) |
(3,095 |
) | ||||||||
|
Basic and diluted net loss per common share |
$ | (0.05 |
) |
$ | (0.06 |
) |
$ | (0.10 |
) |
$ | (0.07 |
) | ||||
|
Year Ended December 31, 2013 |
||||||||||||||||
|
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
|||||||||||||
|
Selected Quarterly Financial Data: |
||||||||||||||||
|
Revenues |
$ | 7,482 | $ | 7,667 | $ | 6,729 | $ | 7,409 | ||||||||
|
Total operating expenses |
10,501 | 10,005 | 10,212 | 10,502 | ||||||||||||
|
Net loss |
(3,008 |
) |
(2,329 |
) |
(3,475 |
) |
(3,086 |
) | ||||||||
|
Basic and diluted net loss per common share |
$ | (0.07 |
) |
$ | (0.06 |
) |
$ | (0.09 |
) |
$ | (0.08 |
) | ||||
9. Subsequent Events
In February 2015, we amended the lease agreement for our laboratory and office facility in San Diego, California. The amended lease expires on February 28, 2024, a seven-year extension of the previous lease period. The amended lease provides for certain rent-free periods beginning in 2015 and an annual 1% rent increase during the extended lease period. The estimated annual future minimum rental payments under all of our operating leases, including the amended property lease, for the years ending December 31 are as follows (in thousands):
|
Operating |
||||
|
2015 |
$ | 1,075 | ||
|
2016 |
3,131 | |||
|
2017 |
1,827 | |||
|
2018 |
2,860 | |||
|
2019 |
2,889 | |||
|
Thereafter |
12,389 | |||
|
Total minimum lease payments |
$ | 24,171 | ||
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our Chief Executive Officer and our Senior Vice President and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on our evaluation under the framework in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2014.
The effectiveness of our internal control over financial reporting as of December 31, 2014 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which is included herein.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal controls over financial reporting.
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Senomyx, Inc.
We have audited Senomyx, Inc.’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Senomyx, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Senomyx, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Senomyx, Inc. as of December 31, 2014 and 2013, and the related statements of operations and comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2014 of Senomyx, Inc. and our report dated February 26, 2015 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
San Diego, California
February 26, 2015
Item 9B. Other Information
None.
PART III
Certain information required by Part III of this Form 10-K is omitted from this report because registrant will file a definitive Proxy Statement within 120 days after the end of its fiscal year pursuant to Regulation 14A for its 2015 Annual Meeting of Stockholders to be held on May 20, 2015, referred to as the Proxy Statement, and the information included therein is incorporated herein by reference.
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item is incorporated by reference to the Proxy Statement under the sections entitled “Election of Directors,” “Section 16(a) Beneficial Ownership Reporting Compliance,” “Code of Business Conduct and Ethics,” and “Information Regarding the Board of Directors and its Committees.”
Item 11. Executive Compensation
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Executive Compensation,” “Compensation Committee Report” and “Compensation Committee Interlocks and Insider Participation.”
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information.”
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Election of Directors” and “Certain Relationships and Related Transactions.”
Item 14. Principal Accountant Fees and Services
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the section entitled “Principal Accountant Fees and Services.”
PART IV
Item 15. Exhibits and Financial Statement Schedules
|
(a) |
1. Financial Statements |
See Index to Financial Statements in Item 8 of this annual report on Form 10-K, which is incorporated herein by reference.
2. Financial Statement Schedules
All schedules have been omitted because they are not applicable or required, or the information required to be set forth therein is included in the Financial Statements or notes thereto included in Item 8 of this annual report on Form 10-K.
3. Exhibits
|
Exhibit |
Exhibit |
Description of Document | ||
|
(1) |
3.1 |
Amended and Restated Certificate of Incorporation as currently in effect. | ||
|
(2) |
3.2 |
Amended and Restated Bylaws as currently in effect. | ||
|
(1) |
4.1 |
Form of Common Stock Certificate. | ||
|
(1) |
10.1+ |
Form of Indemnity Agreement. | ||
|
(1) |
10.2+ |
1999 Equity Incentive Plan and Form of Stock Option Agreement thereunder. | ||
|
(1) |
10.3+ |
Amended and Restated 2004 Equity Incentive Plan and Form of Employee and Consultant Stock Option Agreement thereunder. | ||
|
(3) |
10.4+ |
Form of Non-Employee Director Stock Option Agreement under the 2004 Equity Incentive Plan. | ||
|
(4) |
10.5+ |
2013 Equity Incentive Plan. | ||
|
(5) |
10.6+ |
Form of Employee Stock Option Agreement under the 2013 Equity Incentive Plan. | ||
|
(5) |
10.7+ |
Form of Non-Employee Director Stock Option Agreement under the 2013 Equity Incentive Plan. | ||
|
(1) |
10.8+ |
2004 Employee Stock Purchase Plan and Form of Offering Document thereunder. | ||
|
(6) |
10.9+ |
Amendment No. 1 to Senomyx, Inc. 2004 Employee Stock Purchase Plan. | ||
|
(7) |
10.10+ |
Senomyx, Inc. 2015 Executive Bonus Plan. | ||
|
10.11+ |
Non-Employee Director Compensation Policy effective January 1, 2015. | |||
|
(8) |
10.12+ |
Description of director compensation provided to Jay Short. | ||
|
(9) |
10.13+ |
Letter agreement dated September 20, 2013 between Senomyx, Inc. and Kent Snyder. | ||
|
(9) |
10.14+ |
Letter agreement dated September 20, 2013 between Senomyx, Inc. and John Poyhonen. | ||
|
(9) |
10.15+ |
Second Amendment to Change in Control Agreement dated September 20, 2013 between Senomyx, Inc. and John Poyhonen. | ||
|
(10) |
10.16+ |
Employment letter agreement dated March 14, 2006 between Senomyx, Inc. and Sharon Wicker. | ||
|
(11) |
10.17+ |
First Amendment to Employment Agreement dated March 14, 2006, as amended effective December 31, 2008, between Senomyx, Inc. and Sharon Wicker. | ||
|
(12) |
10.18+ |
Employment letter agreement dated April 13, 2007 between Senomyx, Inc. and Donald S. Karanewsky, Ph.D. | ||
|
(13) |
10.19+ |
Employment letter agreement dated February 20, 2002 between Senomyx, Inc. and Antony E. Rogers. | ||
|
(11) |
10.20+ |
Form of Amended and Restated Change in Control Agreement. | ||
|
(14) |
10.21* |
Lease Agreement dated January 12, 2006 between ARE-NEXUS CENTRE II, LLC and Senomyx, Inc. | ||
|
10.22 |
First Amendment dated February 19, 2015 to Lease Agreement dated January 12, 2006 between ARE-NEXUS CENTRE II, LLC and Senomyx, Inc. | |||
|
(15) |
10.23 |
Subordination, Non-Disturbance and Attornment Agreement dated October 23, 2009 by and between Pacific Life Insurance Co., Senomyx, Inc. and ARE-Nexus Centre II, LLC. |
|
(10) |
10.24* |
License Agreement dated October 11, 2006 between Senomyx, Inc. and The Regents of the University of California. | ||
|
(16) |
10.25* |
First Amendment dated February 7, 2007 to the License Agreement dated October 11, 2006 between Senomyx, Inc. and The Regents of the University of California. | ||
|
(15) |
10.26* |
Second Amendment dated November 20, 2009 to the License Agreement between Senomyx, Inc. and The Regents of the University of California dated October 11, 2006. | ||
|
(17) |
10.27* |
Collaborative Research, Development, Commercialization and License Agreement dated August 16, 2010 between Senomyx, Inc. and PepsiCo, Inc. | ||
|
(18) |
10.28* |
First Amendment dated August 2, 2012 to the Collaborative Research, Development, Commercialization and License Agreement dated August 16, 2010, by and between Senomyx, Inc. and PepsiCo, Inc. | ||
|
(6) |
10.29* |
Amended and Restated Collaborative Research, Development, Commercialization and License Agreement dated April 9, 2013 between Senomyx, Inc. and Firmenich SA. | ||
|
23.1 |
Consent of Independent Registered Public Accounting Firm. | |||
|
24.1 |
Power of Attorney. Reference is made to the signature page. | |||
|
31.1 |
Certification of John Poyhonen, Chief Executive Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |||
|
31.2 |
Certification of Antony Rogers, Chief Financial Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |||
|
32.1 |
Certification of John Poyhonen, Chief Executive Officer, and Antony Rogers, Chief Financial Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |||
|
101 |
The following financial statements from the Senomyx, Inc. Annual Report on Form 10-K for the year ended December 31, 2014, formatted in Extensive Business Reporting Language (XBRL): (i) balance sheets, (ii) statements of operations, (iii) statements of stockholders’ equity, (iv) statements of cash flows, and (v) notes to financial statements (tagged as blocks of text). |
|
+ |
Indicates management contract or compensatory plan. |
|
* |
Confidential treatment has been granted or requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
|
(1) |
Filed as an exhibit to Registration Statement File No. 333-113998 and incorporated herein by reference. |
|
(2) |
Filed as an exhibit to our Current Report on Form 8-K filed with the Securities and Exchange Commission on December 20, 2007 and incorporated herein by reference. |
|
(3) |
Filed as an exhibit to our Current Report on Form 8-K filed with the Securities and Exchange Commission on September 25, 2009 and incorporated herein by reference. |
|
(4) |
Filed as an exhibit to Registration Statement File No. 333-193306 and incorporated herein by reference. |
|
(5) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2013 and incorporated herein by reference. |
|
(6) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by reference. |
|
(7) |
Filed as an exhibit to our Current Report on Form 8-K filed with the Securities and Exchange Commission on February 26, 2015 and incorporated herein by reference. |
|
(8) |
Filed as our Current Report on Form 8-K filed with the Securities and Exchange Commission on July 13, 2006 and incorporated herein by reference. |
|
(9) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference. |
|
(10) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2006 and incorporated herein by reference. |
|
(11) |
Filed as an exhibit to our Current Report on Form 8-K filed with the Securities and Exchange Commission on December 23, 2008 and incorporated herein by reference. |
|
(12) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2010 and incorporated herein by reference. |
|
(13) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2009 and incorporated herein by reference. |
|
(14) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2005 and incorporated herein by reference. |
|
(15) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2009 and incorporated herein by reference. |
|
(16) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2007 and incorporated herein by reference. |
|
(17) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2010 and incorporated herein by reference. |
|
(18) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2012 and incorporated herein by reference. |
(b) Exhibits
See Item 15(a) above.
(c) Financial Statement Schedules
See Item 15(a) above.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Senomyx, Inc. | |||
|
By: |
/S/ JOHN POYHONEN |
||
|
John Poyhonen |
|||
|
President, Chief Executive Officer and Director |
|||
|
Dated: February 26, 2015 |
|||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints John Poyhonen and Antony Rogers, and each of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their or his substitute or substituted, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Signature |
Title |
Date | ||
|
/S/ JOHN POYHONEN |
President, Chief Executive Officer and Director |
|
February 26, 2015 | |
|
John Poyhonen |
(Principal Executive Officer) |
|||
|
/S/ ANTONY ROGERS |
Senior Vice President and Chief Financial Officer |
|
February 26, 2015 | |
|
Antony Rogers |
(Principal Financial and Accounting Officer) |
|||
|
/S/ ROGER D. BILLINGSLEY |
Director |
|
February 26, 2015 | |
|
Roger D. Billingsley, Ph.D. |
||||
|
/S/ STEPHEN A. BLOCK |
Director |
February 26, 2015 | ||
|
Stephen A. Block, Esq. |
||||
|
/S/ MARY ANN GRAY |
Director |
February 26, 2015 | ||
|
Mary Ann Gray, Ph.D. |
||||
|
/S/ MICHAEL E. HERMAN |
Director |
February 26, 2015 | ||
|
Michael E. Herman |
||||
|
/S/ JAY M. SHORT |
Director |
February 26, 2015 | ||
|
Jay M. Short, Ph.D. |
|
|||
|
/S/ KENT SNYDER |
Chairman of the Board of Directors |
February 26, 2015 | ||
|
Kent Snyder |
|
|||
|
/S/ CHRISTOPHER TWOMEY |
Director |
February 26, 2015 | ||
|
Christopher Twomey |
EXHIBIT INDEX
|
Exhibit |
Exhibit |
Description of Document | ||
|
(1) |
3.1 |
Amended and Restated Certificate of Incorporation as currently in effect. | ||
|
(2) |
3.2 |
Amended and Restated Bylaws as currently in effect. | ||
|
(1) |
4.1 |
Form of Common Stock Certificate. | ||
|
(1) |
10.1+ |
Form of Indemnity Agreement. | ||
|
(1) |
10.2+ |
1999 Equity Incentive Plan and Form of Stock Option Agreement thereunder. | ||
|
(1) |
10.3+ |
Amended and Restated 2004 Equity Incentive Plan and Form of Employee and Consultant Stock Option Agreement thereunder. | ||
|
(3) |
10.4+ |
Form of Non-Employee Director Stock Option Agreement under the 2004 Equity Incentive Plan. | ||
|
(4) |
10.5+ |
2013 Equity Incentive Plan. | ||
|
(5) |
10.6+ |
Form of Employee Stock Option Agreement under the 2013 Equity Incentive Plan. | ||
|
(5) |
10.7+ |
Form of Non-Employee Director Stock Option Agreement under the 2013 Equity Incentive Plan. | ||
|
(1) |
10.8+ |
2004 Employee Stock Purchase Plan and Form of Offering Document thereunder. | ||
|
(6) |
10.9+ |
Amendment No. 1 to Senomyx, Inc. 2004 Employee Stock Purchase Plan. | ||
|
(7) |
10.10+ |
Senomyx, Inc. 2015 Executive Bonus Plan. | ||
|
10.11+ |
Non-Employee Director Compensation Policy effective January 1, 2015. | |||
|
(8) |
10.12+ |
Description of director compensation provided to Jay Short. | ||
|
(9) |
10.13+ |
Letter agreement dated September 20, 2013 between Senomyx, Inc. and Kent Snyder. | ||
|
(9) |
10.14+ |
Letter agreement dated September 20, 2013 between Senomyx, Inc. and John Poyhonen. | ||
|
(9) |
10.15+ |
Second Amendment to Change in Control Agreement dated September 20, 2013 between Senomyx, Inc. and John Poyhonen. | ||
|
(10) |
10.16+ |
Employment letter agreement dated March 14, 2006 between Senomyx, Inc. and Sharon Wicker. | ||
|
(11) |
10.17+ |
First Amendment to Employment Agreement dated March 14, 2006, as amended effective December 31, 2008, between Senomyx, Inc. and Sharon Wicker. | ||
|
(12) |
10.18+ |
Employment letter agreement dated April 13, 2007 between Senomyx, Inc. and Donald S. Karanewsky, Ph.D. | ||
|
(13) |
10.19+ |
Employment letter agreement dated February 20, 2002 between Senomyx, Inc. and Antony E. Rogers. | ||
|
(11) |
10.20+ |
Form of Amended and Restated Change in Control Agreement. | ||
|
(14) |
10.21* |
Lease Agreement dated January 12, 2006 between ARE-NEXUS CENTRE II, LLC and Senomyx, Inc. | ||
|
10.22 |
First Amendment dated February 19, 2015 to Lease Agreement dated January 12, 2006 between ARE-NEXUS CENTRE II, LLC and Senomyx, Inc. | |||
|
(15) |
10.23 |
Subordination, Non-Disturbance and Attornment Agreement dated October 23, 2009 by and between Pacific Life Insurance Co., Senomyx, Inc. and ARE-Nexus Centre II, LLC. | ||
|
(10) |
10.24* |
License Agreement dated October 11, 2006 between Senomyx, Inc. and The Regents of the University of California. | ||
|
(16) |
10.25* |
First Amendment dated February 7, 2007 to the License Agreement dated October 11, 2006 between Senomyx, Inc. and The Regents of the University of California. | ||
|
(15) |
10.26* |
Second Amendment dated November 20, 2009 to the License Agreement between Senomyx, Inc. and The Regents of the University of California dated October 11, 2006. | ||
|
(17) |
10.27* |
Collaborative Research, Development, Commercialization and License Agreement dated August 16, 2010 between Senomyx, Inc. and PepsiCo, Inc. | ||
|
(18) |
10.28* |
First Amendment dated August 2, 2012 to the Collaborative Research, Development, Commercialization and License Agreement dated August 16, 2010, by and between Senomyx, Inc. and PepsiCo, Inc. | ||
|
(6) |
10.29* |
Amended and Restated Collaborative Research, Development, Commercialization and License Agreement dated April 9, 2013 between Senomyx, Inc. and Firmenich SA. |
|
23.1 |
Consent of Independent Registered Public Accounting Firm. | |||
|
24.1 |
Power of Attorney. Reference is made to the signature page. | |||
|
31.1 |
Certification of John Poyhonen, Chief Executive Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |||
|
31.2 |
Certification of Antony Rogers, Chief Financial Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |||
|
32.1 |
Certification of John Poyhonen, Chief Executive Officer, and Antony Rogers, Chief Financial Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |||
|
101 |
The following financial statements from the Senomyx, Inc. Annual Report on Form 10-K for the year ended December 31, 2014, formatted in Extensive Business Reporting Language (XBRL): (i) balance sheets, (ii) statements of operations, (iii) statements of stockholders’ equity, (iv) statements of cash flows, and (v) notes to financial statements (tagged as blocks of text). |
|
+ |
Indicates management contract or compensatory plan. |
|
* |
Confidential treatment has been granted or requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
|
(1) |
Filed as an exhibit to Registration Statement File No. 333-113998 and incorporated herein by reference. |
|
(2) |
Filed as an exhibit to our Current Report on Form 8-K filed with the Securities and Exchange Commission on December 20, 2007 and incorporated herein by reference. |
|
(3) |
Filed as an exhibit to our Current Report on Form 8-K filed with the Securities and Exchange Commission on September 25, 2009 and incorporated herein by reference. |
|
(4) |
Filed as an exhibit to Registration Statement File No. 333-193306 and incorporated herein by reference. |
|
(5) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2013 and incorporated herein by reference. |
|
(6) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by reference. |
|
(7) |
Filed as an exhibit to our Current Report on Form 8-K filed with the Securities and Exchange Commission on February 26, 2015 and incorporated herein by reference. |
|
(8) |
Filed as our Current Report on Form 8-K filed with the Securities and Exchange Commission on July 13, 2006 and incorporated herein by reference. |
|
(9) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference. |
|
(10) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2006 and incorporated herein by reference. |
|
(11) |
Filed as an exhibit to our Current Report on Form 8-K filed with the Securities and Exchange Commission on December 23, 2008 and incorporated herein by reference. |
|
(12) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2010 and incorporated herein by reference. |
|
(13) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2009 and incorporated herein by reference. |
|
(14) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2005 and incorporated herein by reference. |
|
(15) |
Filed as an exhibit to our Annual Report on Form 10-K for the year ended December 31, 2009 and incorporated herein by reference. |
|
(16) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2007 and incorporated herein by reference. |
|
(17) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2010 and incorporated herein by reference. |
|
(18) |
Filed as an exhibit to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2012 and incorporated herein by reference. |
81
