Attached files
| file | filename |
|---|---|
| EX-99.5 - EX-99.5 - Acer Therapeutics Inc. | d848715dex995.htm |
| EX-4.13 - EX-4.13 - Acer Therapeutics Inc. | d848715dex413.htm |
| EX-23.1 - EX-23.1 - Acer Therapeutics Inc. | d848715dex231.htm |
| EX-4.10 - EX-4.10 - Acer Therapeutics Inc. | d848715dex410.htm |
| EX-99.6 - EX-99.6 - Acer Therapeutics Inc. | d848715dex996.htm |
Table of Contents
As filed with the Securities and Exchange Commission on February 24, 2015
Registration No. 333-201731
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2
to
FORM S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
OPEXA THERAPEUTICS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Texas | 2834 | 76-0333165 | ||
| (State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
2635 Technology Forest Blvd.
The Woodlands, Texas 77381
(281) 272-9331
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Neil K. Warma
President and Chief Executive Officer
OPEXA THERAPEUTICS, INC.
2635 Technology Forest Blvd.
The Woodlands, Texas 77381
(281) 272-9331
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent for Service)
Copies to:
| Mike Hird, Esq. Gabriella A. Lombardi, Esq. Patty M. DeGaetano, Esq. Pillsbury Winthrop Shaw Pittman LLP 12255 El Camino Real, Suite 300 San Diego, CA 92130 (619) 234-5000 |
Mitchell S. Nussbaum, Esq. Norwood P. Beveridge, Jr., Esq. Lili Taheri, Esq. Loeb & Loeb LLP 345 Park Avenue New York, NY 10154 (212) 407-4000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ |
Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company x | |||
| (Do not check if a smaller reporting company) | ||||||
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title of each class of securities to be registered |
Amount to be Registered |
Proposed Maximum Per Unit |
Proposed Maximum Aggregate Offering Price(1) |
Amount of Registration Fee(5) | ||||
| Units, each consisting of one share of common stock, par value $0.01 per share (“Common Stock”) and one warrant to purchase one share of common stock (“Units”) |
28,776,419 | $0.70 | $20,143,493 | $2,340.67 | ||||
| Non-transferable Rights to purchase Units(2) |
– | – | – | – | ||||
| Common Stock included as part of the Units |
28,776,419 | Included with Units above |
Included with Units above |
– | ||||
| Warrants included as part of the Units(3) |
28,776,419 | Included with Units above |
Included with Units above |
– | ||||
| Common Stock issuable upon exercise of the Warrants included in the Units(1)(4) |
28,776,419 | $1.50 | $43,164,629 | $5,015.73 | ||||
| Total |
$63,308,122 | $7,356.40 | ||||||
|
| ||||||||
|
| ||||||||
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) of the Securities Act of 1933 (the “Act”). |
| (2) | Non-transferable Rights to subscribe for Units are being issued without consideration. Pursuant to Rule 457(g) under the Act, no separate registration fee is required for the Rights because the Rights are being registered in the same registration statement as the common stock of the Registrant underlying the Rights. |
| (3) | Pursuant to Rule 457(g) of the Act, no separate registration fee is required for the Warrants because the Warrants are being registered in the same registration statement as the common stock of the Registrant issuable upon exercise of the Warrants. |
| (4) | In addition to the shares of Common Stock set forth in this table, pursuant to Rule 416 under the Act, this registration statement also registers such indeterminate number of shares of Common Stock as may become issuable upon exercise of the Warrants as the same may be adjusted as a result of their anti-dilution provisions. |
| (5) | Previously paid. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED FEBRUARY 24, 2015 |

We are distributing to holders of our common stock and to holders of certain of our outstanding warrants who are entitled to participate in this offering pursuant to the terms of such warrants (Series L), at no charge, non-transferable subscription rights to purchase units. Each unit, which we refer to as a Unit, consists of one share of common stock and one tradable warrant representing the right to purchase one share of common stock, which we refer to as the Warrants. We refer to the offering that is the subject of this prospectus as the Rights Offering. In the Rights Offering, you will receive one subscription right for each share of common stock or each share of common stock underlying our Series L warrants owned at 5:00 p.m., Eastern Time, on March 13, 2015, the record date of the Rights Offering, or the Record Date. The common stock and the Warrants comprising the Units will separate upon the exercise of the rights and the Units will not trade as a separate security. The subscription rights will not be tradable.
Each subscription right will entitle you to purchase one Unit, which we refer to as the Basic Subscription Right, at a subscription price per Unit of $0.70, which we refer to as the Subscription Price. The Warrants entitle the holder to purchase one share of common stock at an exercise price of (i) $0.50 per share from the date of issuance through June 30, 2016 and (ii) $1.50 per share from July 1, 2016 through their expiration three years from the date of issuance. If you exercise your Basic Subscription Rights in full, and other shareholders or warrant holders do not fully exercise their Basic Subscription Rights, you will be entitled to an over-subscription privilege to purchase a portion of the unsubscribed Units at the Subscription Price, subject to proration and ownership limitations, which we refer to as the Over-Subscription Privilege. Each subscription right consists of a Basic Subscription Right and an Over-Subscription Privilege, which we refer to as the Subscription Right.
The Subscription Rights will expire if they are not exercised by 5:00 p.m., Eastern Time, on April 8, 2015. We may extend the Rights Offering for additional periods in our sole discretion. Once made, all exercises of Subscription Rights are irrevocable.
We have not entered into any standby purchase agreement or other similar arrangement in connection with the Rights Offering. The Rights Offering is being conducted on a best-efforts basis and there is no minimum amount of proceeds necessary to be received in order for us to close the Rights Offering.
We have engaged Maxim Group LLC and National Securities Corporation to act as dealer-managers in the Rights Offering.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 21 of this prospectus. You should carefully consider these risk factors, as well as the information contained in this prospectus, before you invest.
Continental Stock Transfer & Trust Company will serve as the Subscription Agent for the Rights Offering. The Subscription Agent will hold the funds we receive from subscribers until we complete, abandon or terminate the Rights Offering. Advantage Proxy, Inc. will serve as Information Agent for the Rights Offering. If you want to participate in this Rights Offering and you are the record holder of your shares or Series L warrants, we recommend that you submit your subscription documents to the Subscription Agent well before the deadline. If you want to participate in this Rights Offering and you hold shares through your broker, dealer, bank, or other nominee, you should promptly contact your broker, dealer, bank, or other nominee and submit your subscription documents in accordance with the instructions and within the time period provided by your broker, dealer, bank, or other nominee. For a detailed discussion, see “The Rights Offering – The Subscription Rights.”
Our board of directors reserves the right to terminate the Rights Offering for any reason any time before the closing of the Rights Offering. If we terminate the Rights Offering, all subscription payments received will be returned within 10 business days, without interest or penalty. We expect the Rights Offering to expire on or about April 8, 2015, subject to our right to extend the Rights Offering as described above.
Our common stock is listed on The NASDAQ Capital Market, or NASDAQ, under the symbol “OPXA.” On February 20, 2015, the last reported sale price of our common stock was $0.73 per share. We have applied to list the Warrants on NASDAQ following their issuance under the symbol “OPXAW.” The Subscription Rights are non-transferrable and will not be listed for trading on NASDAQ or any other stock exchange or market. You are urged to obtain a current price quote for our common stock before exercising your Subscription Rights.
| Per Unit | Total(2) | |||||||
| Subscription price |
$ | 0.70 | $ | 20,143,493 | ||||
| Dealer-Manager fees and expenses (1) |
$ | 0.05 | $ | 1,560,403 | ||||
| Proceeds to us, before expenses |
$ | 0.65 | $ | 18,583,090 | ||||
| (1) | In connection with this Rights Offering, we have agreed to pay fees to the dealer-managers as follows: (i) to Maxim Group LLC, a cash fee equal to (a) 1% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is less than $6 million; (b) 4% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million but less than $10 million; and (c) 4.5% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $10 million; (ii) to National Securities Corporation, a cash fee equal to 2.75% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million. We have also agreed to reimburse the dealer-managers for their expenses as follows: (i) up to $50,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is less than $6 million; and (ii) up to $100,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is at least $6 million. See “Plan of Distribution.” |
| (2) | Assumes the Rights Offering is fully subscribed, but excludes proceeds from the exercise of Warrants included within the Units. |
Our board of directors is making no recommendation regarding your exercise of the Subscription Rights. You should carefully consider whether to exercise your Subscription Rights before the expiration date. You may not revoke or revise any exercises of Subscription Rights once made unless we terminate the Rights Offering.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Dealer-Managers
| Maxim Group LLC | National Securities Corp. |
The date of this Prospectus is , 2015
Table of Contents
The Root Cause of Multiple Sclerosis: Activated T-cells
Degrade Myelin and Damage Myelin Producing Cells
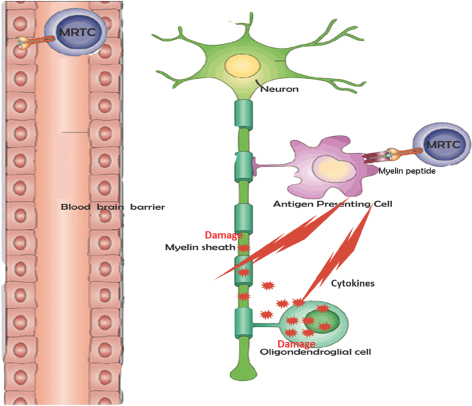
Adapted by permission from Macmillan Publishers Ltd: NATURE REVIEWS
IMMUNOLOGY 3, 483-492 (June 2003), copyright (2003)
| • | In MS patients, the faulty immune system is not able to prevent the attack of a small sub-population of myelin reactive T-cells (MRTCs) leading to: |
| ¡ | Destruction of myelin sheath, the protective coating of nerve fibers |
| ¡ | Destruction of oligodendroglial cells, which are responsible for producing myelin |
| • | Therapeutic dose of Tcelna (attenuated T-cell clones) is injected subcutaneously |
| • | This triggers an immune response specifically targeting circulating MRTCs |
| • | Immune cells, including regulatory T cells (Tregs), have been primed, or sensitized to specifically target the pathogenic MRTCs for elimination or regulation |
|
Opexa’s Strategy Tcelna programs the immune system to specifically recognize MRTCs as pathogenic thereby inhibiting further destruction of the myelin sheath and potentially enabling remyelination
|
Table of Contents
| Page |
| 1 |
| 8 |
| 17 | ||||
| 21 | ||||
| 49 | ||||
| 51 | ||||
| 52 | ||||
| 53 | ||||
| Market Price of our Common Stock and Related Stockholder Matters |
54 | |||
| 54 | ||||
| 55 | ||||
| 63 | ||||
| 70 | ||||
| 73 | ||||
| 75 | ||||
| 75 | ||||
| 75 | ||||
| 75 | ||||
| 76 |
ABOUT THIS PROSPECTUS
The registration statement we filed with the Securities and Exchange Commission, or SEC, includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC, together with the additional information described under the headings “Where You Can Find More Information” and “Incorporation by Reference” before making your investment decision.
You should rely only on the information provided in this prospectus or in a prospectus supplement or amendment thereto. We have not authorized anyone else to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any state where the offer or sale is not permitted. You should assume that the information in this prospectus is accurate only as of the date hereof. Our business, financial condition, results of operations and prospects may have changed since that date.
Unless the context otherwise requires, references in this prospectus to “Opexa,” “the Company,” “we,” “us” and “our” refer to Opexa Therapeutics, Inc. Tcelna® is a registered trademark of Opexa and ImmPath® and Precision Immunotherapy® are service marks of Opexa. All other product and company names are trademarks of their respective owners.
i
Table of Contents
QUESTIONS AND ANSWERS RELATING TO THE RIGHTS OFFERING
The following are examples of what we anticipate will be common questions about the Rights Offering. The answers are based on selected information included elsewhere in this prospectus. The following questions and answers do not contain all of the information that may be important to you and may not address all of the questions that you may have about the Rights Offering. This prospectus and the documents incorporated by reference into this prospectus contain more detailed descriptions of the terms and conditions of the Rights Offering and provides additional information about us and our business, including potential risks related to the Rights Offering, the Units offered hereby, and our business. We urge you to read this entire prospectus and the documents incorporated by reference into this prospectus.
Why are we conducting the Rights Offering?
We are conducting the Rights Offering to raise additional capital:
| • | to continue funding the ongoing Phase IIb “Abili-T” clinical study of Tcelna in patients with secondary progressive multiple sclerosis (SPMS); |
| • | to continue preclinical and manufacturing activities for OPX-212 in patients with neuromyelitis optica (NMO), and if such activities are successful, to file an investigational new drug application with the U.S. Food and Drug Administration to initiate a Phase 1/2 proof-of-concept study; and |
| • | for general corporate purposes, including working capital, research and development, business development and operational purposes. |
What is the Rights Offering?
We are distributing, at no charge, to record holders of our common stock and to holders of certain of our outstanding warrants (Series L) who are entitled to participate in this Rights Offering pursuant to the terms of such warrants, non-transferable Subscription Rights to purchase Units at a price of $0.70 per whole Unit. The Subscription Rights will not be tradable. Each Unit consists of one share of common stock and one Warrant representing the right to purchase one share of common stock. Upon closing of the Rights Offering, the common stock and Warrants will immediately separate. We have applied to list the Warrants on NASDAQ under the symbol “OPXAW.” You will receive one Subscription Right for each share of common stock or each share of common stock underlying our Series L warrants that you owned as of 5:00 p.m., Eastern Time, on the Record Date. Each Subscription Right entitles the record holder or holder of a Series L warrant to a Basic Subscription Right and an Over-Subscription Privilege.
What are the Basic Subscription Rights?
For each whole share you owned or whole share underlying our Series L warrants you owned as of the Record Date, you will receive one Basic Subscription Right, which gives you the opportunity to purchase one share of our common stock and to receive one Warrant to purchase one additional share of our common stock for a price of $0.70 per Unit. For example, if you owned 100 shares of common stock as of the Record Date, you will receive 100 Subscription Rights and will have the right to purchase 100 shares of our common stock and Warrants to purchase 100 shares of our common stock for $0.70 per whole Unit (or a total payment of $70.00). You may exercise all or a portion of your Basic Subscription Rights or you may choose not to exercise any Basic Subscription Rights at all.
If you are a record holder or a holder of Series L warrants, the number of shares you may purchase pursuant to your Basic Subscription Rights is indicated on the enclosed Rights Certificate. If you hold your
1
Table of Contents
shares in the name of a broker, dealer, bank, or other nominee who uses the services of the Depository Trust Company, or DTC, you will not receive a Rights Certificate. Instead, DTC will issue one Subscription Right to your nominee record holder for each share of our common stock that you own as of the Record Date. If you are not contacted by your nominee, you should contact your nominee as soon as possible.
What is the Over-Subscription Privilege?
If you exercise your Basic Subscription Rights in full, you may also choose to exercise your Over-Subscription Privilege to purchase a portion of any Units that the other record holders and applicable warrant holders do not purchase through the exercise of their Basic Subscription Rights. You should indicate on your Rights Certificate, or the form provided by your nominee if your shares are held in the name of a nominee, how many additional Units you would like to purchase pursuant to your Over-Subscription Privilege.
Subject to stock ownership limitations, if sufficient Units are available, we will seek to honor your Over-Subscription request in full. If Over-Subscription requests exceed the number of Units available, however, we will allocate the available Units pro-rata among the record holders and applicable warrant holders exercising the Over-Subscription Privilege in proportion to the number of shares of our common stock each of those record holders owned or the number of shares of our common stock underlying our Series L warrants held by each of those warrant holders on the Record Date, relative to the number of shares owned or shares underlying Series L warrants on the Record Date by all record holders and Series L warrant holders exercising the Over-Subscription Privilege. If this pro-rata allocation results in any record holders or Series L warrant holders receiving a greater number of Units than the record holder or Series L warrant holder subscribed for pursuant to the exercise of the Over-Subscription Privilege, then such record holder or Series L warrant holder will be allocated only that number of Units for which the record holder or Series L warrant holder oversubscribed, and the remaining Units will be allocated among all other record holders and Series L warrant holders exercising the Over-Subscription Privilege on the same pro rata basis described above. The proration process will be repeated until all Units have been allocated. See “The Rights Offering—Limitation on the Purchase of Units” for a description of certain stock ownership limitations.
To properly exercise your Over-Subscription Privilege, you must deliver to the Subscription Agent the subscription payment related to your Over-Subscription Privilege before the Rights Offering expires. See “The Rights Offering—The Subscription Rights—Over-Subscription Privilege.” To the extent you properly exercise your Over-Subscription Privilege for an amount of Units that exceeds the number of unsubscribed Units available to you, any excess subscription payments will be returned to you within 10 business days after the expiration of the Rights Offering, without interest or penalty.
Continental Stock Transfer & Trust Company, our Subscription Agent for the Rights Offering, will determine the Over-Subscription allocation based on the formula described above.
What are the terms of the Warrants?
Each Warrant entitles the holder to purchase one share of common stock at an exercise price of (i) $0.50 per share from the date of issuance through June 30, 2016 and (ii) $1.50 per share from July 1, 2016 through their expiration three years from the date of issuance. The Warrants will be exercisable for cash, or, solely during any period when a registration statement for the exercise of the Warrants is not in effect, on a cashless basis. We may redeem the Warrants for $0.01 per Warrant if our common stock closes above $2.50 per share for 10 consecutive trading days.
Will fractional shares be issued upon exercise of Subscription Rights or upon the exercise of Warrants?
No. We will not issue fractional shares of common stock in the Rights Offering. Rights holders will only be entitled to purchase a number of Units representing a whole number of shares of common stock, rounded down to the nearest whole number of Units a holder would otherwise be entitled to purchase. Any excess
2
Table of Contents
subscription payments received by the Subscription Agent will be returned within 10 business days after expiration of the Rights Offering, without interest or penalty. Similarly, no fractional shares of common stock will be issued in connection with the exercise of a Warrant. Instead, for any such fractional share that would otherwise have been issuable upon exercise of the Warrant, the holder will be entitled to a cash payment equal to the pro-rated per share market price of the common stock on the last trading day preceding the exercise.
What effect will the Rights Offering have on our outstanding common stock?
Based on 28,234,751 shares of common stock outstanding as of February 20, 2015, as well as Series L warrants to purchase 541,668 shares of common stock outstanding on that date, assuming no other transactions by us involving our common stock prior to the expiration of the Rights Offering, if the Rights Offering is fully subscribed, 57,011,170 shares of our common stock will be issued and outstanding and Warrants to purchase an additional 28,776,419 shares of our common stock will be outstanding stock (excluding the 3,046,801 currently outstanding warrants). The exact number of shares and Warrants that we will issue in this Rights Offering will depend on the number of Units that are subscribed for in the Rights Offering.
How was the Subscription Price determined?
In determining the Subscription Price, the directors considered, among other things, the following factors:
| • | the current and historical trading prices of our common stock; |
| • | the price at which shareholders might be willing to participate in the Rights Offering; |
| • | the value of the Warrant being issued as a component of the Unit; |
| • | our need for additional capital and liquidity; |
| • | the cost of capital from other sources; and |
| • | comparable precedent transactions, including the percentage of shares offered, the terms of the subscription rights being offered, the subscription price and the discount that the subscription price represented to the immediately prevailing closing prices for those offerings. |
In conjunction with the review of these factors, the board of directors also reviewed our history and prospects, including our past and present earnings and cash requirements, our prospects for the future, the outlook for our industry and our current financial condition. The board of directors also believed that the Subscription Price should be designed to provide an incentive to our current shareholders and holders of our Series L warrants to participate in the Rights Offering and exercise their Basic Subscription Right and their Over-Subscription Privilege.
The Subscription Price does not necessarily bear any relationship to any established criteria for value. You should not consider the Subscription Price as an indication of actual value of our company or our common stock. We cannot assure you that the market price of our common stock will not decline during or after the Rights Offering. You should obtain a current price quote for our common stock before exercising your Subscription Rights and make your own assessment of our business and financial condition, our prospects for the future, and the terms of this Rights Offering. Once made, all exercises of Subscription Rights are irrevocable.
Am I required to exercise all of the Basic Subscription Rights I receive in the Rights Offering?
No. You may exercise any number of your Basic Subscription Rights, or you may choose not to exercise any Basic Subscription Rights. If you do not exercise any Basic Subscription Rights, the number of shares of our common stock or number of shares underlying our Series L warrants you own will not change. However, if you
3
Table of Contents
choose to not exercise your Basic Subscription Rights in full, your proportionate ownership interest in our company will decrease. If you do not exercise your Basic Subscription Rights in full, you will not be entitled to exercise your Over-Subscription Privilege.
How soon must I act to exercise my Subscription Rights?
If you received a Rights Certificate and elect to exercise any or all of your Subscription Rights, the Subscription Agent must receive your completed and signed Rights Certificate and payment for both your Basic Subscription Rights and any Over-Subscription Privilege you elect to exercise, including final clearance of any uncertified check, before the Rights Offering expires on April 8, 2015, at 5:00 p.m., Eastern Time. If you hold your shares in the name of a broker, dealer, custodian bank, or other nominee, your nominee may establish a deadline before the expiration of the Rights Offering by which you must provide it with your instructions to exercise your Subscription Rights, along with the required subscription payment.
May I transfer my Subscription Rights?
No. The Subscription Rights may be exercised only by the shareholders to whom they are distributed, and they may not be sold, transferred, assigned or given away to anyone else, other than by operation of law. As a result, Rights Certificates may be completed only by the shareholder who receives the certificate. The Subscription Rights will not be listed for trading on any stock exchange or market.
Will our directors and executive officers participate in the Rights Offering?
To the extent they hold common stock as of the Record Date, our directors and executive officers will be entitled to participate in the Rights Offering on the same terms and conditions applicable to other Rights holders. While none of our directors or executive officers has entered into any binding commitment or agreement to exercise Subscription Rights received in the Rights Offering, certain directors and executive officers have indicated an interest in participating (although the number of Units represented by such indications of interest would not be material to the overall Rights Offering).
Has the board of directors made a recommendation to shareholders and warrant holders regarding the Rights Offering?
No. Our board of directors is not making a recommendation regarding your exercise of the Subscription Rights. Stockholders and holders of our Series L warrants who exercise Subscription Rights will incur investment risk on new money invested. We cannot predict the price at which our shares of common stock will trade after the Rights Offering. On February 20, 2015, the closing price of our common stock was $0.73 per share. The market price for our common stock may be above the Subscription Price or may be below the Subscription Price. If you exercise your Subscription Rights, you may not be able to sell the underlying shares of our common stock or Warrants in the future at the same price or a higher price. You should make your decision based on your assessment of our business and financial condition, our prospects for the future, the terms of the Rights Offering and the information contained in this prospectus. See “Risk Factors” for discussion of some of the risks involved in investing in our securities.
How do I exercise my Subscription Rights?
If you are a shareholder of record (meaning you hold your shares of our common stock in your name and not through a broker, dealer, bank, or other nominee) or a holder of our Series L warrants and you wish to participate in the Rights Offering, you must deliver a properly completed and signed Rights Certificate, together with payment of the Subscription Price for both your Basic Subscription Rights and any Over-Subscription Privilege you elect to exercise, to the Subscription Agent before 5:00 p.m., Eastern Time, on April 8, 2015. If you are exercising your Subscription Rights through your broker, dealer, bank, or other nominee, you should promptly contact your broker, dealer, bank, or other nominee and submit your subscription documents and
4
Table of Contents
payment for the Units subscribed for in accordance with the instructions and within the time period provided by your broker, dealer, bank or other nominee.
What if my shares are held in “street name”?
If you hold your shares of our common stock in the name of a broker, dealer, bank, or other nominee, then your broker, dealer, bank, or other nominee is the record holder of the shares you own. The record holder must exercise the Subscription Rights on your behalf. Therefore, you will need to have your record holder act for you.
If you wish to participate in this Rights Offering and purchase Units, please promptly contact the record holder of your shares. We will ask the record holder of your shares, who may be your broker, dealer, bank, or other nominee, to notify you of this Rights Offering.
What form of payment is required?
You must timely pay the full Subscription Price for the full number of Units you wish to acquired pursuant to the exercise of Subscription Rights by delivering to the Subscription Agent a:
| • | personal check drawn on a U.S. bank; |
| • | cashier’s or certified check drawn on a U.S. bank; |
| • | U.S. Postal money order; or |
| • | wire transfer. |
If you send payment by personal uncertified check, payment will not be deemed to have been delivered to the Subscription Agent until the check has cleared.
If you send a payment that is insufficient to purchase the number of Units you requested, or if the number of Units you requested is not specified in the forms, the payment received will be applied to exercise your Subscription Rights to the fullest extent possible based on the amount of the payment received.
When will I receive my new shares of common stock and Warrants?
The Subscription Agent will arrange for the issuance of the common stock and Warrants as soon as practicable after the expiration of the Rights Offering, payment for the Units subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected. All shares and Warrants that you purchase in the Rights Offering will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of these securities if you are a holder of record of shares or Series L warrants. If you hold your shares in the name of a broker, dealer, bank, or other nominee, DTC will credit your account with your nominee with the securities you purchase in the Rights Offering.
After I send in my payment and Rights Certificate to the Subscription Agent, may I cancel my exercise of Subscription Rights?
No. Exercises of Subscription Rights are irrevocable unless the Rights Offering is terminated, even if you later learn information that you consider to be unfavorable to the exercise of your Subscription Rights. You should not exercise your Subscription Rights unless you are certain that you wish to purchase Units at the Subscription Price.
5
Table of Contents
How much will our company receive from the Rights Offering?
Assuming that all 28,776,419 Units are sold in the Rights Offering, we estimate that the proceeds from the Rights Offering will be approximately $17.8 million, based on the Subscription Price of $0.70 per Unit, after deducting fees and expenses payable to the dealer-managers, and after deducting other expenses payable by us and excluding any proceeds received upon exercise of any Warrants.
Are there risks in exercising my Subscription Rights?
Yes. The exercise of your Subscription Rights involves risks. Exercising your Subscription Rights involves the purchase of additional shares of our common stock and Warrants to purchase common stock and you should consider this investment as carefully as you would consider any other investment. We cannot assure you that the market price of our common stock will exceed the Subscription Price, nor can we assure you that the market price of our common stock will not further decline during or after the Rights Offering. We also cannot assure you that you will be able to sell shares of our common stock or Warrants purchased in the Rights Offering at a price equal to or greater than the Subscription Price. In addition, you should carefully consider the risks described under the heading “Risk Factors” for discussion of some of the risks involved in investing in our securities.
Can the board of directors terminate or extend the Rights Offering?
Yes. Our board of directors may decide to terminate the Rights Offering at any time and for any reason before the expiration of the Rights Offering. We also have the right to extend the Rights Offering for additional periods in our sole discretion. We do not presently intend to extend the Rights Offering. We will notify shareholders and warrant holders if the Rights Offering is terminated or extended by issuing a press release.
If the Rights Offering is not completed or is terminated, will my subscription payment be refunded to me?
Yes. The Subscription Agent will hold all funds it receives in a segregated bank account until completion of the Rights Offering. If we do not complete the Rights Offering, all subscription payments received by the Subscription Agent will be returned within 10 business days after the termination or expiration of the Rights Offering, without interest or penalty. If you own shares in “street name,” it may take longer for you to receive your subscription payment because the Subscription Agent will return payments through the record holder of your shares.
How do I exercise my Rights if I live outside the United States?
The Subscription Agent will hold Rights Certificates for shareholders having addresses outside the United States. To exercise Subscription Rights, foreign shareholders must notify the Subscription Agent and timely follow other procedures described in the section entitled “The Rights Offering – Foreign Shareholders.”
What fees or charges apply if I purchase shares in the Rights Offering?
We are not charging any fee or sales commission to issue Subscription Rights to you or to issue shares or Warrants to you if you exercise your Subscription Rights. If you exercise your Subscription Rights through a broker, dealer, custodian bank, or other nominee, you are responsible for paying any fees your broker, dealer, bank, or other nominee may charge you.
What are the U.S. federal income tax consequences of exercising my Subscription Rights?
For U.S. federal income tax purposes, we do not believe you should recognize income or loss in connection with the receipt or exercise of Subscription Rights in the Rights Offering. You should consult your tax advisor as to the tax consequences of the Rights Offering in light of your particular circumstances. For a detailed discussion, see “Material U.S. Federal Income Tax Consequences.”
6
Table of Contents
To whom should I send my forms and payment?
If your shares are held in the name of a broker, dealer, bank, or other nominee, then you should send your subscription documents and subscription payment to that broker, dealer, bank, or other nominee. If you are the record holder or a holder of our Series L warrants, then you should send your subscription documents, Rights Certificate, and subscription payment to the Subscription Agent hand delivery, first class mail or courier service to:
Continental Stock Transfer & Trust Company
17 Battery Place—8th Floor
New York, NY 10004
Attn: Corporate Actions Department
Telephone: (917) 262-2378
You or, if applicable, your nominee are solely responsible for completing delivery to the Subscription Agent of your subscription documents, Rights Certificate and payment. You should allow sufficient time for delivery of your subscription materials to the Subscription Agent and clearance of payment before the expiration of the Rights Offering at 5:00 p.m. Eastern Time on April 8, 2015.
Whom should I contact if I have other questions?
If you have other questions or need assistance, please contact the Information Agent:
Advantage Proxy, Inc.
(877) 870-8565 (toll free)
(206) 870-8565 (collect)
Who are the dealer-managers?
Maxim Group LLC and National Securities Corporation will act as dealer-managers for the Rights Offering. Under the terms and subject to the conditions contained in the dealer-manager agreement, the dealer-managers will use their best efforts to solicit the exercise of Subscription Rights. We have agreed to pay the dealer-managers for certain fees for acting as dealer-managers and to reimburse the dealer-managers for certain out-of-pocket expenses incurred in connection with this offering. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants being issued in this offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares of common stock or Warrants.
7
Table of Contents
This summary contains basic information about us and this offering. Because it is a summary, it does not contain all of the information that you should consider before investing. Before you decide to invest in our Units, you should read this entire prospectus carefully, including the section entitled “Risk Factors” and any information incorporated by reference herein.
OUR COMPANY
Our Business
Opexa is a biopharmaceutical company developing a personalized immunotherapy with the potential to treat major illnesses, including multiple sclerosis (MS) as well as other autoimmune diseases such as neuromyelitis optica (NMO). These therapies are based on our proprietary T-cell technology. Our mission is to lead the field of Precision Immunotherapy® by aligning the interests of patients, employees and shareholders. Information related to our product candidates, Tcelna® and OPX-212, is preliminary and investigative. Tcelna and OPX-212 have not been approved by the U.S. Food and Drug Administration (FDA) or other global regulatory agencies for marketing.
MS is an inflammatory autoimmune disease of the central nervous system (CNS), which is made up of the brain, spinal cord and optic nerves, with a clinically heterogeneous and unpredictable course that persists for decades. MS attacks the covering surrounding nerve cells, or myelin sheaths, leading to loss of myelin (demyelination) and nerve damage. In addition to demyelination, the neuropathology of MS is characterized by variable loss of oligodendroglial cells and axonal degeneration and manifests in neurological deficits. Symptoms may be mild, such as numbness in the limbs, or severe, such as paralysis or loss of vision. This inflammatory, demyelinating, autoimmune disease has varied clinical presentations, ranging from relapses and remissions (relapsing remitting MS, or RRMS) to slow accumulation of disability with or without relapses (secondary progressive MS, or SPMS). There are approximately 450,000 MS patients in North America and over 2,000,000 patients worldwide according to estimates from The National MS Society. The portion of the MS patient population that can be classified as SPMS is estimated by various industry sources to be between 30-45% of the total MS patient population.
We believe that our lead product candidate, Tcelna, has the potential to fundamentally address the root cause of MS by stopping the demyelination process and supporting the generation of new myelin sheaths where demyelination has occurred (remyelination). Tcelna is an autologous T-cell immunotherapy that is currently being developed for the treatment of SPMS and is specifically tailored to each patient’s immune response profile to myelin. Tcelna is designed to reduce the number and/or functional activity of specific subsets of myelin-reactive T-cells (MRTCs) known to attack myelin. This technology was originally licensed from Baylor College of Medicine in 2001.
Tcelna is manufactured using our proprietary method for the production of an autologous T-cell product, which comprises the collection of blood from the MS patient and the expansion of MRTCs from the blood. Upon completion of the manufacturing process, an annual course of therapy consisting of five doses is cryopreserved. At each dosing time point, a single dose of Tcelna is formulated and attenuated by irradiation before returning the final product to the clinical site for subcutaneous administration to the patient.
Tcelna has received Fast Track designation from the FDA in SPMS, and we believe it is positioned as a potential first-to-market personalized T-cell therapy for MS patients. The FDA’s Fast Track program is designed to facilitate the development and expedite the review of drug candidates intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
8
Table of Contents
In addition to our ongoing clinical development of Tcelna, we announced on September 8, 2014, that we are also developing OPX-212 as an autologous T-cell immunotherapy for the treatment of NMO. NMO is an autoimmune disorder in which immune system cells and antibodies attack and destroy astrocytic/myelin cells in the optic nerves and the spinal cord leading to demyelination and loss of axons. There are currently no FDA-approved therapies for NMO, other than to treat an attack while it is happening, to reduce symptoms and to prevent relapses. OPX-212 is specifically tailored to each patient’s immune response to a protein, aquaporin-4, which is the targeted antigen in NMO. In NMO, the immune system recognizes aquaporin-4 as foreign, thus triggering the attack. We believe a mechanism of action of OPX-212 may be to reduce the number and/or regulate aquaporin-4 reactive T-cells (ARTCs), thereby reducing the frequency of clinical relapses and subsequent progression in disability. See “—NMO – OPX-212” below for more information on our development plans for OPX-212 in NMO.
Multiple Sclerosis—Background
MS is a disease that is more common in females than males (2:1) between the ages of 20 and 40, with a peak onset of approximately 25 years of age. MS frequently causes impairment of motor, sensory, coordination and balance, visual, and/or cognitive functions, as well as urinary (bladder) or bowel dysfunction and symptoms of fatigue. The identified autoimmune mechanisms directed at myelin tissue of the CNS may play an important role in the pathogenesis of MS. Epidemiologic studies suggest that a variety of genetic, immunologic, and environmental factors including viral infections may play a role in defining the etiology and in triggering the onset and progression of MS.
At the onset of MS, approximately 85% of MS patients have RRMS. Without disease-modifying medication, one-half of these RRMS patients will develop steadily progressive disease, SPMS, within 10 years, increasing to 90% within 25 years of MS diagnosis. The MS drug market was approximately $13 billion in 2012 and is forecasted to reach as much as $16 billion by 2015.
MS remains a challenging autoimmune disease to treat because the pathophysiologic mechanisms are diverse, and the chronic, unpredictable course of the disease makes it difficult to determine whether the favorable effects of short-term treatment will be sustained. Therapies that are easy to use and can safely prevent or stop the progression of disease represent the greatest unmet need in MS.
In recent years, the understanding of MS pathogenesis has evolved to comprise an initial, T-cell-mediated inflammatory activity followed by selective demyelination (erosion of the myelin coating of the nerve fibers) and then neurodegeneration. The discovery of disease-relevant immune responses has accelerated the development of targeted therapeutic products for the treatment of the early stages of MS. Some subjects, who have the appropriate genetic background, have increased susceptibility for the in vivo activation and expansion of MRTCs. These MRTCs may remain dormant, but at some point they are activated in the periphery, thus enabling them to cross the blood-brain barrier and infiltrate the healthy tissue of the brain and spinal cord. The cascade of pathogenic events leads to demyelination of protrusions from nerve cells called axons, which causes nerve impulse transmissions to diffuse into the tissue resulting in disability to the individual.
Tcelna for MS
We believe that Tcelna works selectively on the MRTCs by harnessing the body’s natural immune defense system and feedback mechanisms to deplete these T-cells and induce favorable immune regulatory responses by rebalancing the immune system. Tcelna is a personalized immunotherapy that is specifically tailored to each patient’s disease profile. Tcelna is manufactured by using ImmPath®, our proprietary method for the production of a patient-specific T-cell immunotherapy which encompasses the collection of blood from the MS patient, isolation of peripheral blood mononuclear cells, generation of an autologous pool of MRTCs raised against selected peptides from myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and
9
Table of Contents
proteolipid protein (PLP), expanding these MRTCs to a therapeutic dose ex-vivo, and attenuating them with gamma irradiation to prevent DNA replication and thereby cellular proliferation. These attenuated MRTCs are then injected subcutaneously into the body in therapeutic dosages. The body recognizes specific T-cell receptor molecules of these MRTCs as immunogenic and initiates an immune response reaction against them, resulting in the depletion and/or immunosuppression of circulating MRTCs carrying the peptide-specific T-cell receptor molecules. In addition, we believe that T-cell activation molecules on the surface of the activated MRTCs promote anti-inflammatory responses. We believe that because the therapy uses an individual’s own cells, the only direct identifiable side effect observed thus far is injection site reactions which typically are minor and generally clear within 24 hours.
Tcelna Clinical Development Program
Tcelna is a novel T-cell immunotherapy in Phase IIb clinical development for the treatment of patients with SPMS. It is also positioned to enter Phase III clinical development for the treatment of patients with RRMS, subject to the availability of sufficient resources or a strategic partnering commitment.
The Tcelna clinical development program spans studies conducted by Baylor College of Medicine and by Opexa.
Summary of Phase I Dose Escalation Study in MS
A Phase 1 dose escalation study completed in 2006 was conducted in patients with both RRMS and SPMS who were intolerant or unresponsive to current approved therapies for MS. The open-label, dose escalation study evaluated safety and clinical benefit by administering a primary series of four treatments at one of three dose levels administered at baseline and weeks 4, 8 and 12. Results of the efficacy analyses provide some evidence of the effectiveness of Tcelna in the treatment of MS. Data from the Phase I study evaluating the Expanded Disability Status Scale (EDSS) showed improvements in some subjects in comparison to baseline for weeks 20 and 28.
Subjects showed statistically significant improvement in overall reduction of MRTC counts over baseline at all visits through week 52 for subjects receiving 30-45 million cells per dose, as assessed by total MRTC count percentage changes. These data indicate that Tcelna treatment causes a depletion or immunomodulation of these cells, most obvious at time points closer to the injections. These findings were published in Clinical Immunology (2009) 131, 202-215.
Overall, results of the safety analyses indicate that treatment with Tcelna is well-tolerated. Reported adverse events were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. In conclusion, data from this study suggest that Tcelna is safe for the treatment of MS.
Summary of Phase I/IIA Clinical Trial Data in MS
The second clinical study performed by Opexa was an open-label extension study completed in 2007 to treat patients who were previously treated with T-cell immunotherapy but who saw a rebound in MRTC activity. The purpose of this extension study was to continue evaluating the efficacy, safety and tolerability of Tcelna in patients with RRMS and SPMS with repeated administration of Tcelna. Results of the study provide evidence of the effectiveness of Tcelna in the treatment of MS with repeated dosing. Improvements from baseline at both week 28 and week 52 of the extension study were observed for the frequency of MS exacerbations, or annualized relapse rate (ARR). Evaluation of the Multiple Sclerosis Impact Scale (MSIS-29) component scores suggests a trend for Tcelna therapy in the improvement of physical and psychological parameters assessed by the MSIS-29. The EDSS score analysis revealed an upward trend for the percentage of subjects that reported improvement and sustained improvement over baseline as a result of Tcelna treatment.
10
Table of Contents
Subjects showed statistically significant reduction over baseline in the MRTC counts for each time point through month nine of the extension study. Overall, results of the safety analyses indicate that repeated treatment with Tcelna is well-tolerated. Reported adverse events (AEs) were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. Furthermore, results from this study suggest that repeated dosing of Tcelna has a substantive effect in reduction of ARR in subjects with MS and was well-tolerated.
Summary of TERMS Phase IIb Clinical Trial Data in RRMS
Tovaxin for Early Relapsing Multiple Sclerosis (TERMS) was a Phase IIb clinical study of Tcelna in RRMS patients completed in 2008. Although the study did not show statistical significance in its primary endpoint (the cumulative number of gadolinium-enhanced brain lesions using magnetic resonance imaging (MRI) scans summed at various points in the study), the study showed compelling evidence of efficacy in various clinical and other MRI endpoints.
The TERMS study was a multi-center, randomized, double blind, placebo-controlled trial in 150 patients with RRMS or high risk Clinically Isolated Syndrome. The inclusion criteria for TERMS was an EDSS score of 0 to 5.5. Patients received a total of five subcutaneous injections at weeks 0, 4, 8, 12 and 24. Key results from the TERMS trial included:
| • | In the modified intent to treat patient population consisting of all patients who received at least one dose of study product and had at least one MRI scan at week 28 or later (n=142), the ARR for Tcelna-treated patients was 0.214 as compared to 0.339 for placebo-treated patients, which represented a 37% decrease in ARR for Tcelna as compared to placebo in the general population; |
| • | In a prospective group of patients with more active disease (ARR>1, n=50), Tcelna demonstrated a 55% reduction in ARR as compared to placebo, an 88% reduction in whole brain atrophy and a statistically significant improvement in disability (EDSS) compared to placebo (p<0.045) at week 52 during the 24-week period following the administration of the full course of treatment; and |
| • | In a retrospective analysis in patients naïve to previous disease modifying treatment, the results showed that patients, when treated with Tcelna, had a 56% to 73% reduction in ARR versus placebo for the various subsets and p values ranged from 0.009 to 0.06. |
We remain committed to further advancing Tcelna in RRMS at a later date assuming the availability of sufficient resources or a strategic partnering commitment. For Opexa, however, SPMS is an area which we believe represents a higher unmet medical need.
SPMS Overview and Tcelna Mechanism of Action
SPMS is characterized by a steady accrual of irreversible disability, despite, in some cases, relapses followed by remissions or clinical plateaus. Older age at onset of MS diagnosis is the strongest predictor of conversion to SPMS. Males have a shorter time to conversion to SPMS compared with females. Available immunomodulating and immunosuppressive therapies used for RRMS have not been effective in SPMS. In clinical trials, these therapies have demonstrated anti-inflammatory properties as measured by the reduction in number and volume of contrast-enhancing or acutely inflammatory CNS lesions most commonly seen in patients with RRMS. The typical SPMS patient, however, has little or no radiographic evidence of acute inflammation. It is commonly observed that contrast-enhancing CNS lesions are uncommon among these patients, despite a clearly deteriorating neurologic course.
11
Table of Contents
The lack of effect of conventional MS therapeutics in SPMS suggests that the cerebral deterioration characterizing progressive disease may be driven by factors other than acute inflammation. For instance, the immunopathology of SPMS is more consistent with a transition to a chronic T-cell dependent inflammatory type, which may encompass the innate immune response and persistent activation of microglia cells. Meningeal follicles close to cortical gray matter lesions suggests that adaptive immune responses involving antibody and complement contribute to progression in SPMS. Furthermore, chronic MRTCs may be contributing to the development of both innate and adaptive immune responses persisting in the CNS.
Radiographic features that stand out among patients with SPMS include significantly more atrophy of gray matter compared with RRMS patients. Of note, long-term disability in MS in general appears more closely correlated to gray matter atrophy than to white matter inflammation. Such atrophy may be suggestive of progressive clinical disability. Both clinically and radiographically, SPMS represents a disease process with certain features distinct from those of RRMS, and one with extremely limited treatment options.
Tcelna immunotherapy in SPMS may reduce the drivers of this chronic disease by down-regulating anti-myelin immunity through priming regulatory responses that may act in the periphery as well as within the CNS. We believe that our clinical results show therapeutic subcutaneous dosing of 30-45 million cells of Tcelna stimulates host reactivity to the over-represented MRTCs and, as a consequence, a dominant negative regulatory T-cell response is induced leading to down-regulation of similar endogenous disease-causing MRTCs.
We believe that Tcelna has the potential to induce an up-regulation of regulatory cells, such as Foxp3+ Treg cells and IL-10 secreting Tr1 cells, which may effect a reduction in inflammation and provide neuroprotection should they gain entry to the CNS. Data from our TERMS study showed statistically significant changes from baseline (p=0.02) in Foxp3+ Treg cells for the subset of Tcelna patients who had ARR >1. The placebo arm for this subset was not statistically different from its baseline levels. Three SPMS patients from prior clinical studies, whose blood samples were analyzed to measure Tr1 cells prior to treatment and post treatment, showed an increase in the levels of Tr1 cells from non-detectable levels to the range of healthy donor samples. These three patients who had relapses in the preceding 12-24 month period remained relapse free during the 52-week assessment period and also showed a 57% to 67% reduction in MRTCs.
Current Treatment Options for SPMS
Only one product, mitoxantrone, is currently approved for the indication of SPMS in the U.S. However, since 2005, this drug carries a black box warning, due to significant risks of decreased systolic function, heart failure, and leukemia. The American Academy of Neurology has issued a report indicating that these risks are even higher than suggested in the original report leading to the black box warning. Hence, a safe and effective treatment for SPMS remains a significant unmet medical need.
Tcelna Clinical Overview in SPMS
In multiple previously conducted clinical trials for the treatment of patients with MS (which have been weighted significantly toward patients with RRMS), Tcelna has demonstrated one of the safest side effect profiles for any marketed or development-stage MS therapy, as well as encouraging efficacy signals. A total of 144 MS patients have received Tcelna in previously conducted Opexa trials for RRMS and SPMS. The therapy has been well-tolerated in all subjects and has demonstrated an excellent overall safety profile. The most common side effect is mild to moderate irritation at the site of injection, which is typically resolved in 24 hours. Tcelna has been administered to a total of 36 subjects with SPMS across three previous clinical studies.
In a pooled assessment of data from 36 SPMS patients treated in Phase I open label studies at the Baylor College of Medicine completed in 1998 and in Opexa-sponsored studies completed in 2006 and 2007, approximately 80% of the 35 SPMS patients who completed two years of treatment showed disease stabilization
12
Table of Contents
as measured by EDSS following two years of treatment with Tcelna, with the other 20% showing signs of progression. This compares to historical control data which showed a progression rate of 40% in SPMS patients (as reported in ESIMS Study published in Hommes Lancet 2004). The 10 SPMS patients in Opexa sponsored studies showed a substantial reduction in ARR at two years from 0.5 to an ARR less than 0.1. Only 1 out of the 10 patients experienced one episode of relapse during the two years of assessment. This same cohort showed no worsening of physical or psychological condition (key quality of life indicators as measured by the MS Impact Scale) after two years of treatment with Tcelna. Additionally, there were no reported serious adverse events (SAEs) in any of the patients. Based on preliminary data suggesting stabilized or improved disability among SPMS subjects receiving Tcelna, we believe that further development of this product candidate in SPMS is warranted.
Abili-T Trial: Phase IIb Clinical Study in Patients with SPMS
In September 2012, we announced the initiation of a Phase IIb clinical trial of Tcelna in patients with SPMS. The trial is entitled: A Phase II Double-Blind, Placebo Controlled Multi-Center Study to Evaluate the Efficacy and Safety of Tcelna in Subjects with Secondary Progressive Multiple Sclerosis and has been named the “Abili-T” trial. The Abili-T trial is a double-blind, 1:1 randomized, placebo-controlled study in SPMS patients who demonstrate evidence of disease progression with or without associated relapses. The trial is being conducted at approximately 35 leading clinical sites in the U.S. and Canada and has enrolled patients who have Expanded Disability Status Scale (EDSS) scores between 3.0 and 6.0. According to the study protocol, patients are receiving two annual courses of Tcelna treatment consisting of five subcutaneous injections per year at weeks 0, 4, 8, 12 and 24.
The primary efficacy endpoint of the trial is the percentage of brain volume change (whole brain atrophy) at 24 months. Study investigators will also measure several important secondary outcomes commonly associated with MS including sustained disease progression as measured by EDSS, changes in EDSS, time to sustained progression, ARR, change in Multiple Sclerosis Functional Composite (MSFC) assessment of disability and change in Symbol Digit Modality Test. Data on certain exploratory endpoints such as quality of life metrics as measured by the Multiple Sclerosis Quality of Life Inventory (MSQLI), MRI measures and immune monitoring metrics are also being collected.
As part of the Abili-T trial, we are undertaking a comprehensive immune monitoring program for all patients enrolled in the study. The goals of this program are to further understand the biology behind the mechanism of action for Tcelna and to possibly identify novel biomarkers that are dominant in the pathophysiology of SPMS patients. The program encompasses an analysis of various pro-inflammatory and anti-inflammatory biomarkers and biomarker data is being gathered during the course of the trial on a blinded basis. We believe that directional movement of certain biomarkers, when corroborated with final clinical trial data, may be indicative of responders and disease stabilization or progression.
A scheduled Data Safety Monitoring Board meeting took place during the week of October 6, 2014, and a recommendation was made to continue the study. We reached our enrollment target for the Abili-T trial in June 2014, and a total of 190 patients have been enrolled in this two-year study. We expect top-line data for Tcelna to be available in the second half of 2016.
Option and License Agreement with Merck Serono
On February 4, 2013, we entered into an Option and License Agreement with Ares Trading SA (“Merck Serono”), a wholly owned subsidiary of Merck Serono S.A. Pursuant to the agreement, Merck Serono has an option (the “Option”) to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck Serono prior to or upon completion of our ongoing Abili-T trial of Tcelna in patients with SPMS. Under the terms of the agreement, we received an upfront payment
13
Table of Contents
of $5 million for granting the Option. If the Option is exercised, Merck Serono would pay us an upfront license fee of $25 million unless Merck Serono is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck Serono), in which event the upfront license fee would be $15 million. After exercising the Option, Merck Serono would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights to use for other indications outside of MS.
Based upon the achievement of development milestones by Merck Serono for Tcelna in SPMS, we would be eligible to receive one-time milestone payments totaling up to $70 million as follows: (i) milestone payments aggregating $35 million if Tcelna is submitted for regulatory approval and commercialized in the United States; (ii) milestone payments aggregating $30 million if Tcelna is submitted for regulatory approval in Europe and commercialized in at least three major countries in Europe; and (iii) a milestone payment of $5 million if Tcelna is commercialized in certain markets outside of the United States and Europe. If Merck Serono elects to develop and commercialize Tcelna in RRMS, we would be eligible to receive milestone payments aggregating up to $40 million based upon the achievement by Merck Serono of various development, regulatory and first commercial sale milestones.
If Tcelna receives regulatory approval and is commercialized by Merck Serono, we would be eligible to receive royalties pursuant to a tiered structure at rates ranging from 8% to 15% of annual net sales, with step-ups over such range occurring when annual net sales exceed $500 million, $1 billion and $2 billion. Any royalties would be subject to offset or reduction in various situations, including if third party rights are required or if patent protection is not available in an applicable jurisdiction. We would also be responsible for royalty obligations to certain third parties, such as Baylor College of Medicine from which we originally licensed related technology. If we were to exercise an option to co-fund certain of Merck Serono’s development, the royalty rates payable by Merck Serono would be increased to rates ranging from 10% to 18%. In addition to royalty payments, we would be eligible to receive one-time commercial milestones totaling up to $85 million, with $55 million of such milestones achievable at annual net sales targets in excess of $1 billion.
NMO – OPX-212
In addition to our ongoing clinical development of Tcelna, we announced on September 8, 2014 that we are also developing OPX-212 as an autologous T-cell immunotherapy for the treatment of NMO. NMO is an autoimmune disorder in which immune system cells and antibodies attack astrocytes leading to the secondary destruction of nerve cells (axons) in the optic nerves and the spinal cord. OPX-212 is specifically tailored to each patient’s immune response to a protein, aquaporin-4 expressed by astrocytes, which is the targeted antigen in NMO. In NMO, the immune system recognizes aquaporin-4 as foreign, thus triggering the attack. We believe a mechanism of action of OPX-212 may be to reduce the number and/or regulate aquaporin-4 reactive T-cells (ARTCs), thereby reducing the frequency of clinical relapses and subsequent progression in disability.
Patients with NMO present with acute, often severe, attacks of blindness in one or both eyes followed within days or weeks by varying degrees of paralysis in the arms and legs. Most patients have relapsing attacks (separated by months or years with partial recovery), with usually sequential index episodes of optic neuritis (ON) and myelitis. A relapsing course is more frequent in women, and nearly 90% of patients are female (typically late middle-aged). It is estimated that there are approximately 4,800 cases of NMO in the U.S. NMO has a worldwide estimated prevalence of 1-2 people per 100,000 population.
There are currently no FDA-approved therapies for NMO. An initial attack is usually treated with a combination of corticosteroids and/or by plasma exchange to limit the severity of the attack. Although not approved for NMO, some physicians may utilize an immunosuppressant such as Rituximab as long-term therapy to provide protection from increasing neurological impairments through relapse.
14
Table of Contents
We expect to manufacture OPX-212 using ImmPath, our proprietary method for the production of an autologous T-cell product, which comprises the collection of a blood product from the NMO patient and the expansion of ARTCs from the blood product. Upon completion of the manufacturing process, ARTCs are cryopreserved in dose-equivalents until required for use. On demand, a dose-equivalent is thawed, formulated and attenuated by irradiation before being returned to the patient for subcutaneous injection, with the express purpose of inducing a regulatory immune response to reduce the frequency and/or function of pathogenic ARTCs.
We initiated development activities for OPX-212, our drug development candidate for NMO, earlier this year and have achieved a number of regulatory and early development milestones to date. These include conducting a pre-Investigational New Drug application (pre-IND) meeting with the U.S. FDA and performing in-house manufacturing runs with NMO patient samples. We are continuing with preclinical development activities and expect to file an IND for OPX-212 with the FDA by mid-2015. We believe OPX-212 for NMO will qualify for Orphan drug designation, and we also expect to apply for Fast Track designation.
We believe part of the value of our T-cell platform comes from the ability to move relatively quickly and cost effectively into new autoimmune diseases. We do not expect our ongoing preclinical development activities related to the NMO program to materially affect the Company’s cash burn through IND submission. Assuming successful completion of the preclinical development activities and submission and acceptance of the IND by the FDA and/or CTA by Health Canada, we may thereafter advance into clinical development with a Phase 1/2 proof-of-concept study. We intend to evaluate various options to fund such clinical study, including public or private capital raises as well as potential partnership and out-licensing activities.
Other Opportunities
Our proprietary T-cell technology has enabled us to develop intellectual property and a comprehensive sample database that may enable discovery of novel biomarkers associated with MS. Depending upon the outcome of further feasibility analysis, the T-cell platform may have applications in developing treatments for other autoimmune disorders. While the primary focus of Opexa remains the development of Tcelna in SPMS, as well as our development plans for OPX-212 in NMO, we continue to investigate the expansion of the T-cell platform into other autoimmune diseases as well as potential in-licensing of other novel technologies.
Corporate Information
We were incorporated in Texas in March 1991. Our principal executive offices are located at 2653 Technology Forest Blvd., The Woodlands, Texas 77381, and our telephone number is (281) 775-0600. Our website address is www.opexatherapeutics.com. The information contained on, or that can be accessed through, our website is not part of this prospectus.
15
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL DATA
The following summary consolidated statements of operations data for the years ended December 31, 2014 and 2013 have been derived from our audited consolidated financial statements that are included in the documents incorporated by reference into this prospectus. The historical financial data presented below is not necessarily indicative of our financial results in future periods. You should read the summary consolidated financial data in conjunction with those financial statements and the accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the documents incorporated by reference into this prospectus. Our consolidated financial statements are prepared and presented in accordance with United States generally accepted accounting principles, or U.S. GAAP.
| For the year ended December 31, |
||||||||
| 2014 | 2013 | |||||||
| Consolidated Statements of Operations Data: |
||||||||
| Revenue: |
||||||||
| Option revenue |
$ | 1,271,895 | $ | 1,266,611 | ||||
| Research and development |
12,118,629 | 9,181,090 | ||||||
| General and administrative |
3,833,370 | 3,670,769 | ||||||
| Depreciation and amortization |
387,779 | 335,597 | ||||||
| Loss on disposal of fixed assets |
— | 2,161 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(15,067,883) | (11,923,006) | ||||||
| Interest income |
15,456 | 14,985 | ||||||
| Other income and expense, net |
2,147 | 37,910 | ||||||
| Loss on extinguishment of debt |
— | (2,518,912) | ||||||
|
|
|
|
|
|||||
| Interest expense |
(1,983) | (2,267,302) | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (15,052,263) | $ | (16,656,325) | ||||
|
|
|
|
|
|||||
| Basic and diluted loss per share |
$ | (0.54) | $ | (1.25) | ||||
| Weighted average shares outstanding |
27,821,056 | 13,332,350 | ||||||
| December 31, 2014 | ||||
| Consolidated Balance Sheet Data: |
||||
| Cash and cash equivalents |
$ | 9,906,373 | ||
| Other current assets |
758,943 | |||
| Property & equipment, net |
1,098,104 | |||
| Other long-term assets |
38,939 | |||
| Total assets |
11,802,359 | |||
| Total current liabilities |
3,132,424 | |||
| Deferred revenue |
1,230,748 | |||
| Total stockholders’ equity |
7,439,187 | |||
16
Table of Contents
SUMMARY OF THE RIGHTS OFFERING
| Securities to be offered |
We are distributing to you, at no charge, one non-transferable Subscription Right to purchase one Unit for every share of our common stock that you owned on the Record Date, either as a holder of record or, in the case of shares held of record by brokers, banks, or other nominees, on your behalf, as a beneficial owner of such shares. Each Unit consists of one share of common stock and one warrant representing the right to purchase one share of common stock. |
| Holders of our currently outstanding Series L warrants (which expire February 11, 2017) will also be entitled, pursuant to the terms of such Series L warrants, to receive one Subscription Right to purchase one Unit for every share of common stock with respect to which such Series L warrants are exercisable as of the Record Date. |
| Size of offering |
28,776,419 Units, each consisting of one share of common stock and one Warrant representing the right to purchase one share of common stock. |
| Subscription Price |
$0.70 per Unit. |
| Warrants |
Each Warrant entitles the holder to purchase one share of common stock at an exercise price of (i) $0.50 per share from the date of issuance through June 30, 2016 and (ii) $1.50 per share from July 1, 2016 through their expiration three years from the date of issuance. The Warrants will be exercisable for cash, or, solely during any period when a registration statement for the exercise of the Warrants is not in effect, on a cashless basis. We have applied to list, the Warrants on NASDAQ under the symbol “OPXAW,” although there is no assurance that a sufficient number of Subscription Rights will be exercised so that the Warrants will meet the minimum listing criteria to be accepted for listing on NASDAQ. We may redeem the Warrants for $0.01 per Warrant if our common stock closes above $2.50 per share for 10 consecutive trading days. |
| Record Date |
5:00 p.m., Eastern Time, March 13, 2015. |
| Subscription Right |
Each Subscription Right consists of a Basic Subscription Right and an Over-Subscription Privilege. |
17
Table of Contents
| Basic Subscription Rights |
Basic Subscription Right will entitle you to purchase one Unit at the Subscription Price. |
| Over-Subscription Privilege |
If you exercise your Basic Subscription Rights in full, you may also choose to purchase a portion of any Units that are not purchased by our other shareholders or holders of Series L warrants through the exercise of their Basic Subscription Rights. You may subscribe for additional Units pursuant to this Over-Subscription Privilege, subject to proration and stock ownership limitations described elsewhere. |
| Expiration date |
The Subscription Rights will expire at 5:00 p.m., Eastern Time, on April 8, 2015. We reserve the right to extend the expiration date in our sole discretion. |
| Procedure for exercising Subscription Rights |
To exercise your Subscription Rights, you must take the following steps: |
| If you are a record holder of our common stock or a holder of Series L warrants, you must deliver payment and a properly completed Rights Certificate to the Subscription Agent to be received before 5:00 p.m., Eastern Time, on April 8, 2015. You may deliver the documents and payments by first class mail or courier service. If you use first class mail for this purpose, we recommend using registered mail, properly insured, with return receipt requested. |
| If you are a beneficial owner of shares that are registered in the name of a broker, dealer, custodian bank, or other nominee, you should instruct your broker, dealer, custodian bank, or other nominee to exercise your Subscription Rights on your behalf. Please follow the instructions of your nominee, who may require that you meet a deadline earlier than 5:00 p.m., Eastern Time, on April 8, 2015. |
| Delivery of shares and Warrants |
As soon as practicable after the expiration of the Rights Offering, the Subscription Agent will arrange for the issuance of the shares of common stock and Warrants purchased pursuant to the Rights Offering. All shares and Warrants that are purchased in the Rights Offering will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of these securities if you are a holder of record of shares or Series L warrants. If you hold your shares in the name of a custodian bank, broker, dealer, or other nominee, |
18
Table of Contents
| DTC will credit your account with your nominee with the securities you purchased in the Rights Offering. |
| Non-transferability of Subscription Rights |
The Subscription Rights may not be sold, transferred, assigned or given away to anyone. The Subscription Rights will not be listed for trading on any stock exchange or market. |
| Transferability of Warrants |
The Warrants will be separately transferable following their issuance and through their expiration three years from the issuance date. |
| No board recommendation |
Our board of directors is not making a recommendation regarding your exercise of the Subscription Rights. You are urged to make your decision to invest based on your own assessment of our business and the Rights Offering. Please see “Risk Factors” for a discussion of some of the risks involved in investing in our securities. |
| No revocation |
All exercises of Subscription Rights are irrevocable, even if you later learn of information that you consider to be unfavorable to the exercise of your Subscription Rights. |
| Use of proceeds |
Assuming the exercise of Subscription Rights to purchase all 28,776,419 Units of the Rights Offering, after deducting fees and expenses and excluding any proceeds received upon exercise of any Warrants, we estimate the net proceeds of the Rights Offering will be approximately $17.8 million, based on the Subscription Price of $0.70 per Unit. We intend to use the net proceeds we receive from the offering (i) to continue funding the ongoing Phase IIb “Abili-T” clinical study of Tcelna in patients with secondary progressive multiple sclerosis (SPMS), (ii) to continue preclinical and manufacturing activities for OPX-212 in patients with neuromyelitis optica (NMO), and if such activities are successful, to file an investigational new drug application with the U.S. Food and Drug Administration to initiate a Phase 1/2 proof-of-concept study, and (iii) for other general corporate purposes. See “Use of Proceeds.” |
Material U.S. federal income tax consequences
For U.S. federal income tax purposes, we do not believe you should recognize income or loss upon receipt or exercise of a Subscription Right. You should consult your own tax advisor as to the tax
19
Table of Contents
| consequences of the Rights Offering in light of your particular circumstances. See “Material U.S. Federal Income Tax Consequences.” |
| Extension and termination |
Although we do not presently intend to do so, we may extend the Rights Offering for additional time in our sole discretion. Our board of directors may for any reason terminate the Rights Offering at any time before the completion of the Rights Offering. |
| Subscription Agent |
Continental Stock Transfer & Trust Company. |
| Information Agent |
Advantage Proxy, Inc. |
| Questions |
If you have any questions about the Rights Offering, please contact the Subscription Agent, Continental Stock Transfer & Trust Company, at (917) 262-2378, or the Information Agent, Advantage Proxy, Inc. at (877) 870-8565 (toll free) or (206) 870-8565 (collect). |
| Market for common stock |
Our common stock is listed on NASDAQ under the symbol “OPXA.” See “Market Price of our Common Stock and Related Stockholder Matters.” |
| Risk factors |
Before you exercise your Subscription Rights to purchase Units, you should be aware that there are risks associated with your investment, and you should carefully read and consider risks described in the section captioned “Risk Factors” together with all of the other information included in this prospectus. |
| Dealer-Managers |
Maxim Group LLC and National Securities Corporation. |
| Distribution arrangements |
Under the terms and subject to the conditions contained in the dealer-manager agreement, the dealer-managers will use their best efforts to solicit the exercise of Subscription Rights. We have agreed to pay the dealer-managers certain fees for acting as dealer-managers and to reimburse the dealer-managers for certain out-of-pocket expenses incurred in connection with this offering. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants being issued in this offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares of common stock or Warrants. |
20
Table of Contents
Investing in our securities involves a high degree of risk. You should consider the following risk factors, as well as other information contained in this prospectus, before deciding to invest in our securities. The following factors affect our business, our intellectual property, the industry in which we operate and our securities. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known or which we consider immaterial as of the date hereof may also have an adverse effect on our business. If any of the matters discussed in the following risk factors were to occur, our business, financial condition, results of operations, cash flows or prospects could be materially adversely affected, the market price of our securities could decline and you could lose all or part of your investment in our securities.
Risks Related to Our Business
We will be required to raise significant additional capital, and our ability to obtain funding is uncertain. If sufficient capital is not available, we may not be able to continue our operations as proposed (including the Phase IIb clinical trial ongoing for Tcelna, or any study planned for OPX-212 in NMO), which may require us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws.
As of December 31, 2014, we had cash and cash equivalents of approximately $9.9 million. During 2012, we closed a private offering in July 2012 consisting of convertible secured notes and warrants to purchase common stock which generated approximately $4.1 million in gross proceeds. These convertible secured notes were converted into equity during 2013 and an aggregate of 2,002,926 shares of common stock were issued. From November 2012 through January 2013, we sold an aggregate of 390,000 shares of our common stock to Lincoln Park for gross proceeds of $523,709 pursuant to our $1.5 million purchase agreement with Lincoln Park. We closed a private offering of unsecured convertible promissory notes and warrants to purchase common stock in January 2013 which generated $650,000 in gross proceeds. Upon receipt of the upfront payment from Merck Serono in February 2013, we repaid $550,000 principal amount plus accrued interest of the January 2013 notes and converted the remaining $100,000 principal amount into shares of common stock pursuant to the investor’s election to convert into equity. In February 2013, we sold an aggregate of 167,618 shares of our common stock pursuant to a sales agreement executed on September 6, 2012 with Brinson Patrick Securities Corporation acting as sales agent under an “at-the-market” (ATM) program, for gross proceeds of $536,417. On February 4, 2013, we entered into an Option and License Agreement with Merck Serono pursuant to which we granted the Option to Merck Serono to acquire an exclusive, worldwide (excluding Japan) license to our Tcelna program for the treatment of MS in consideration for an upfront payment of $5 million. On February 11, 2013, we closed an offering of 1,083,334 shares of common stock and warrants to purchase 541,668 shares of common stock for gross proceeds of $3.25 million, or net proceeds of approximately $3.0 million after deducting commissions and offering expenses. On August 13, 2013, we closed an offering of 12 million shares of common stock for gross proceeds of $18 million, or net proceeds of approximately $16.2 million after deducting underwriting discounts and commissions and offering expenses, and we granted the underwriters a 30-day option to purchase up to an additional 1.8 million shares of common stock to cover over-allotments. During September 2013, the underwriters of the August 2013 underwritten public offering exercised the over-allotment option granted to them which resulted in the issuance of an additional 900,000 shares of common stock for gross proceeds of $1.35 million, or net proceeds of approximately $1.2 million after deducting underwriting discounts and commissions and offering expenses. On December 23, 2013, we closed an offering of 4,738,000 shares of common stock including the full exercise of the over-allotment option granted to the underwriters, raising gross proceeds of approximately $8.1 million or net proceeds of approximately $7.3 million after deducting the underwriting discounts and commission and offering expenses. Between March 5, 2014 and December 31, 2014, we generated gross and net proceeds including amortization of deferred financing costs of $674,126 and $648,175, respectively, on sales of an aggregate of 518,412 shares of our common stock under an amendment to our ATM sales agreement pursuant to which Meyers Associates, L.P. (doing business as Brinson Patrick, a division of Meyers Associates, L.P.) became the new sales agent.
21
Table of Contents
Our operating cash burn rate during the 12 months ended December 31, 2014 was approximately $1.2 million per month. We reached our enrollment target for the Abili-T trial in June 2014, and a total of 190 patients have been enrolled in this two-year study. Costs associated with the ongoing Abili-T trial and the potential commencement of a Phase 1/2 proof-of-concept study of OPX-212 in NMO may result in an increase in our monthly operating cash burn in 2015. However, costs associated with our ongoing preclinical and manufacturing activities for OPX-212 should not materially affect our cash burn through IND submission, which is expected by mid-2015. We will need to secure significant additional resources to complete the Abili-T trial of Tcelna in SPMS and, if initiated, to conduct a Phase 1/2 proof-of-concept study of OPX-212 in NMO, as well as to support our operations during the course of the trials. We believe that we have sufficient liquidity to support our current clinical activities for the Abili-T trial of Tcelna in SPMS, to continue planned preclinical development activities for OPX-212 in NMO with a possible IND submission by mid-2015, and for general operations to sustain the Company and support such activities into the fourth quarter of 2015. Any other costs, however, such as costs associated with the initiation and completion of a Phase 1/2 proof-of-concept study of OPX-212 in NMO or with pursuing additional disease indications for our T-cell technology or other research or development programs, would of course shorten this period.
Given our need for substantial amounts of capital to complete the Abili-T clinical study of Tcelna in SPMS and to initiate and complete a Phase 1/2 proof-of-concept study of OPX-212 in NMO, we intend to continue to explore potential opportunities and alternatives to obtain the significant additional resources, including one or more additional financing transactions, that will be necessary to complete the studies and to support our operations during the pendency of such studies. There can be no assurance that any such financings or potential opportunities and alternatives can be consummated on acceptable terms, if at all. If we are unable to obtain additional funding for operations beyond the projected runway, we will be forced to suspend or terminate any ongoing clinical trials, which may require us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws.
Other than the $1.5 million purchase agreement and the $15.0 million purchase agreement we entered into with Lincoln Park on November 5, 2012 and November 2, 2012, respectively, each of which is subject to certain limitations and conditions, we have no sources of debt or equity capital committed for funding and we must rely upon best efforts third-party debt or equity funding. We can provide no assurance that we will be successful in any funding effort. The timing and degree of any future capital requirements will depend on many factors, including:
| • | our ability to establish, enforce and maintain strategic arrangements for research, development, clinical testing, manufacturing and marketing; |
| • | the accuracy of the assumptions underlying our estimates for capital needs in 2014 and beyond as well as for the clinical study of Tcelna; |
| • | scientific progress in our research and development programs; |
| • | the magnitude and scope of our research and development programs; |
| • | our progress with preclinical development and clinical trials; |
| • | the time and costs involved in obtaining regulatory approvals; |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; and |
| • | the number and type of product candidates that we pursue. |
If we raise additional funds by issuing equity securities, shareholders may experience substantial dilution. Debt financing, if available, may involve restrictive covenants that may impede our ability to operate our business. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our shareholders. There is no assurance that our capital raising efforts will be able to attract the capital needed to execute on our business plan and sustain our operations.
If we are unable to obtain additional funding to support our current clinical trial activities beyond the projected runway, we may not be able to continue or complete the Phase IIb clinical study of Tcelna in SPMS
22
Table of Contents
and, if initiated, to continue or complete a Phase 1/2 proof-of-concept study of OPX-212 in NMO, or otherwise continue our operations as proposed, which may require us to modify our business plan or curtail various aspects of our operations. If we are unable to maintain an adequate level of capital, it may be necessary to cease operations or seek relief under applicable bankruptcy laws. In such event, our shareholders may lose a portion or even all of their investment.
We may make changes to discretionary R&D investments that may have an impact on costs.
We are presently complementing the Abili-T clinical trial with an immune monitoring program. Expenses associated with the immune monitoring program are incurred at our discretion and are not required to satisfy any FDA-mandated criteria. Consequently, we may make changes to the parameters that are being analyzed, and these changes may result in either increased or decreased expenses for the study.
We may also incur discretionary expenses related to Phase I, Phase II and/or Phase III development programs, manufacturing scale-up/automation and technology transfer, research on additional indications and business development activities. There is no assurance that any such future expenses would be recovered by us.
Funding from our purchase agreements with Lincoln Park and our ATM facility may be limited or be insufficient to fund our operations or to implement our strategy.
Under our $1.5 million purchase agreement and our $15.0 million purchase agreement with Lincoln Park, we may direct Lincoln Park to purchase up to $16.5 million of shares of common stock, subject to certain limitations and conditions, over a 30-month period. From November 2012 through January 2013, we sold an aggregate of 390,000 shares to Lincoln Park pursuant to the $1.5 million purchase agreement, and we issued an aggregate of 56,507 initial commitment shares and 3,585 additional commitment shares in connection therewith. There can be no assurance that we will be able to receive any or all of the additional funds from Lincoln Park because the $1.5 million purchase agreement and the $15.0 million purchase agreement contain limitations, restrictions, requirements, events of default and other provisions that could limit our ability to cause Lincoln Park to buy common stock from us, including that the closing price of our stock is at least $1.00 and that Lincoln Park own no more than 4.99% of our common stock under the $1.5 million purchase agreement or no more than 9.99% of our common stock under the $15.0 million purchase agreement, and the requirement to keep current the prospectus included as part of the Form S-1 registration statement relating to the $15.0 million purchase agreement (which is not current as of this date). In addition, under the applicable rules of the NASDAQ Capital Market, if we seek to issue shares which may be aggregated with shares sold to Lincoln Park under the $1.5 million purchase agreement and the $15.0 million purchase agreement in excess of 1,151,829 shares or 19.99% of the total common stock outstanding as of the date of the $15.0 million purchase agreement, we may be required to seek shareholder approval in order to be in compliance with the NASDAQ Capital Market rules.
The extent to which we rely on Lincoln Park as a source of funding will depend on a number of factors, including the amount of working capital needed, the prevailing market price of our common stock and the extent to which we are able to secure working capital from other sources. If obtaining sufficient funding from Lincoln Park were to prove unavailable or prohibitively dilutive, we would need to secure another source of funding.
We will need to keep current the offering prospectus relating to the new ATM Agreement with Brinson Patrick, a division of Meyers Associates, L.P., in order to use the program to sell shares of our common stock. The number of shares and price at which we may be able to sell shares under the new ATM Agreement may be limited due to market conditions and other factors beyond our control.
We have a history of operating losses and do not expect to be profitable in the foreseeable future.
We have not generated any profits since our entry into the biotechnology business and we have incurred significant operating losses. We expect to incur additional operating losses for the foreseeable future. We have
23
Table of Contents
not received, and we do not expect to receive for at least the next several years, any revenues from the commercialization of any potential products. We do not currently have any sources of revenues and may not have any in the foreseeable future.
Our business is at an early stage of development. We are largely dependent on the success of our lead product candidate, Tcelna, and we cannot be certain that Tcelna will receive regulatory approval or be successfully commercialized.
Our business is at an early stage of development. We do not have any product candidates that have completed late-stage clinical trials nor do we have any products on the market. We have only one product candidate, Tcelna, which has progressed to the stage of being studied in human clinical trials in the United States. We announced the initiation of early development activities with our second pipeline candidate, OPX-212 in NMO. Tcelna, and any other potential products, including OPX-212, will require regulatory approval prior to marketing in the United States and other countries. Obtaining such approval requires significant research and development and preclinical and clinical testing. We may not be able to develop any products, to obtain regulatory approvals, to continue clinical development of Tcelna, to enter clinical trials (or any development activities) for any other product candidates (such as OPX-212) or to commercialize any products. Tcelna, and any other potential products (such as OPX-212), may prove to have undesirable or unintended side effects or other characteristics adversely affecting their safety, efficacy or cost-effectiveness that could prevent or limit their use. Any product using any of our technology may fail to provide the intended therapeutic benefits or to achieve therapeutic benefits equal to or better than the standard of treatment at the time of testing or production.
We have provided Merck Serono with the Option, which provides Merck Serono with the opportunity, if exercised, to control the development and commercialization of Tcelna in MS.
In February 2013, we granted the Option to Merck Serono. The Option permits Merck Serono to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck Serono prior to or upon completion of our ongoing Phase IIb trial of Tcelna in patients with SPMS. If Merck Serono exercises the Option, Merck Serono would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS. In consideration for the Option, we received an upfront payment of $5 million and may be eligible to receive an option exercise fee as well as milestone and royalty payments based on achievement of development and commercialization milestones. The rights we have relinquished to our product candidate Tcelna, including development and commercialization rights, may harm our ability to generate revenues and achieve or sustain profitability.
If Merck Serono exercises the Option, we would become reliant on Merck Serono’s resources and efforts with respect to Tcelna in MS. In such an event, Merck Serono may fail to develop or effectively commercialize Tcelna for a variety of reasons, including that Merck Serono:
| • | does not have sufficient resources or decides not to devote the necessary resources due to internal constraints such as limited cash or human resources; |
| • | decides to pursue a competitive potential product; |
| • | cannot obtain the necessary regulatory approvals; |
| • | determines that the market opportunity is not attractive; or |
| • | cannot manufacture or obtain the necessary materials in sufficient quantities from multiple sources or at a reasonable cost. |
If Merck Serono does not exercise the Option, we may be unable to enter into a collaboration with any other potential partner on acceptable terms, if at all. We face competition in our search for partners from other
24
Table of Contents
organizations worldwide, many of whom are larger and are able to offer more attractive deals in terms of financial commitments, contribution of human resources, or development, manufacturing, regulatory or commercial expertise and support.
If Merck Serono does not exercise the Option, and we are not successful in attracting another partner and entering into collaboration on acceptable terms, we may not be able to complete development of or commercialize any product candidate, including Tcelna. In such event, our ability to generate revenues and achieve or sustain profitability would be significantly hindered and we may not be able to continue operations as proposed, requiring us to modify our business plan, curtail various aspects of our operations or cease operations.
We will need regulatory approvals for any product candidate, including Tcelna, prior to introduction to the market, which will require successful testing in clinical trials. Clinical trials are subject to extensive regulatory requirements, and are very expensive, time-consuming and difficult to design and implement. Any product candidate, such as Tcelna, may fail to achieve necessary safety and efficacy endpoints during clinical trials in which case we will be unable to generate revenue from the commercialization and sale of our products.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous FDA requirements, and must otherwise comply with federal, state and local requirements and policies of the medical institutions where they are conducted. The clinical trial process is also time-consuming. We reached our enrollment target for the Abili-T trial in June 2014, and a total of 190 patients have been enrolled in this two-year study. We expect top-line data for Tcelna to be available in the second half of 2016. In addition, we anticipate that at least a pivotal Phase III clinical trial would be necessary before an application could be submitted for approval of Tcelna for SPMS. Failure can occur at any stage of the trials, and problems could be encountered that would cause us or Merck Serono (in the event the Option is exercised) to be unable to initiate a trial, or to abandon or repeat a clinical trial.
The commencement and completion of clinical trials, including the continuation and completion of the Phase IIb clinical trial of Tcelna in SPMS, may be delayed or prevented by several factors, including:
| • | FDA or IRB objection to proposed protocols; |
| • | discussions or disagreement with the FDA over the adequacy of trial design to potentially demonstrate effectiveness, and subsequent design modifications; |
| • | unforeseen safety issues; |
| • | determination of dosing issues, epitope profiles, and related adjustments; |
| • | lack of effectiveness during clinical trials; |
| • | slower than expected rates of patient recruitment; |
| • | product quality problems (e.g., sterility or purity); |
| • | challenges to patient monitoring, retention and data collection during or after treatment (e.g., patients’ failure to return for follow-up visits or to complete the trial, detection of epitope profiles in subsequent visits, etc.); and |
| • | failure of medical investigators to follow our clinical protocols. |
In addition, we, Merck Serono with respect to Tcelna (if the Option is exercised) or the FDA (based on its authority over clinical studies) may delay a proposed investigation or suspend clinical trials in progress at any time if it appears that the study may pose significant risks to the study participants or other serious deficiencies are identified. Prior to approval of any product candidate, the FDA must determine that the data demonstrate safety and effectiveness. The large majority of drug candidates that begin human clinical trials fail to demonstrate the desired safety and efficacy characteristics.
Furthermore, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols, or otherwise modify our intended course of clinical development, to reflect these changes. This, too, may impact the costs, timing or successful completion of a clinical trial. In light of widely publicized
25
Table of Contents
events concerning the safety risk of certain drug products, regulatory authorities, members of Congress, the U.S. Government Accountability Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products, and establishment of risk management programs that may, for instance, restrict distribution of drug products. The increased attention to drug safety issues may result in a more cautious approach by the FDA to clinical trials. Data from clinical trials may receive greater scrutiny with respect to safety, which may make the FDA or other regulatory authorities more likely to terminate clinical trials before completion or require longer or additional clinical trials that may result in substantial additional expense and a delay or failure in obtaining approval or approval for a more limited indication than originally sought.
Even if regulatory approval is obtained for any product candidate, such as Tcelna, any such approval may be subject to limitations on the indicated uses for which it may be marketed. Our ability to generate revenues from the commercialization and sale of any potential products, whether directly or through any development arrangement (such as where Merck Serono exercises the Option) will be limited by any failure to obtain or limitation on necessary regulatory approvals.
If Merck Serono exercises the Option, Merck Serono would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates.
We will rely on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that may hamper our ability to successfully develop and commercialize any product candidate, including Tcelna.
Although we have participated in the design and management of our past clinical trials, we do not have the ability to conduct clinical trials directly for any product candidate, including Tcelna. We will need to rely on contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct our clinical trials and to perform data collection and analysis, including the Phase IIb trial of Tcelna in patients with SPMS.
Our clinical trials may be delayed, suspended or terminated if:
| • | any third party upon whom we rely does not successfully carry out its contractual duties or regulatory obligations or meet expected deadlines; |
| • | licenses needed from third parties for manufacturing in order to conduct Phase III trials or to conduct commercial manufacturing, if applicable, are not obtained; |
| • | any such third party needs to be replaced; or |
| • | the quality or accuracy of the data obtained by the third party is compromised due to its failure to adhere to clinical protocols or regulatory requirements or for other reasons. |
Failure to perform by any third party upon whom we rely may increase our development costs, delay our ability to obtain regulatory approval and prevent the commercialization of any product candidate, including Tcelna. While we believe that there are numerous alternative sources to provide these services, we might not be able to enter into replacement arrangements without delays or additional expenditures if we were to seek such alternative sources.
If we fail to identify and license or acquire other product candidates, we will not be able to expand our business over the long term.
We have focused on MS as the first disease to be pursued off our T-cell platform technology, and we announced the initiation of NMO as the second disease we are pursuing. As a platform technology, there exists the potential to address other autoimmune diseases with the technology. Early development activities including
26
Table of Contents
preclinical and manufacturing activities have been initiated in OPX-212. The work in NMO is modest compared to the effort that has been committed to the lead MS indication. Our business over the long term is substantially dependent on our ability to develop, license or acquire product candidates and further develop them for commercialization. The success of this strategy depends upon our ability to expand our existing platform or identify, select and acquire the right product candidates. We have limited experience identifying, negotiating and implementing economically viable product candidate acquisitions or licenses, which is a lengthy and complex process. Also, the market for licensing and acquiring product candidates is intensely competitive, and many of our competitors have greater resources than we do. We may not have the requisite capital resources to consummate product candidate acquisitions or licenses that we identify to fulfill our strategy.
Moreover, any product candidate acquisition that we do complete will involve numerous risks, including:
| • | difficulties in integrating the development program for the acquired product candidate into our existing operations; |
| • | diversion of financial and management resources from existing operations; |
| • | risks of entering new potential markets or technologies; |
| • | inability to generate sufficient funding to offset acquisition costs; and |
| • | delays that may result from our having to perform unanticipated preclinical trials or other tests on the product candidate. |
We are dependent upon our management team and a small number of employees.
Our business strategy is dependent upon the skills and knowledge of our management team. If any critical employee leaves, we may be unable on a timely basis to hire suitable replacements to operate our business effectively. We also operate with a very small number of employees and thus have little or no backup capability for their activities. The loss of the services of any member of our management team or the loss of just a few other employees could have a material adverse effect on our business and results of operations.
If we fail to meet our obligations under our license agreements, we may lose our rights to key technologies on which our business depends.
Our business depends on licenses from third parties. These third party license agreements impose obligations on us, such as payment obligations and obligations diligently to pursue development of commercial products under the licensed patents. We may also need to seek additional licenses as we move into Phase III trials and, if applicable, the commercial stage of operations. These licenses may require increased payments to the licensors. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation, our ability to carry out the development and commercialization of potential products could be significantly and negatively affected. If our license rights were restricted or ultimately lost, our ability to continue our business based on the affected technology platform could be adversely affected.
Our research and manufacturing facility is not large enough to manufacture product candidates, such as Tcelna, for certain clinical trials or, if such clinical trials are successful, commercial applications.
We conduct our research and development in a 10,200 square foot facility in The Woodlands, Texas, which includes an approximately 1,200 square foot suite of three rooms for the manufacture of T-cell therapies. We believe our facility should have the capacity to support full clinical development of Tcelna in North American trials for SPMS and, if applicable, a Phase 1/2 proof-of-concept study of OPX-212 in NMO. It is not sufficient, however, to support clinical trials outside North America including Europe and Asia, if required, or the commercial launch of Tcelna or any other product candidate. In this case, we would need to expand our
27
Table of Contents
manufacturing staff and facility, obtain a new facility, contract with corporate collaborators or other third parties to assist with future drug production and commercialization, or defer to Merck Serono (in the event the Option is exercised) to address manufacturing requirements.
In the event that we decide to establish a commercial-scale manufacturing facility, we will require substantial additional funds and will be required to hire and train significant numbers of employees and comply with applicable regulations, which are extensive. We do not have funds available for building a manufacturing facility, and we may not be able to build a manufacturing facility that both meets regulatory requirements and is sufficient for our commercial-scale manufacturing.
We may arrange with third parties for the manufacture of our future products, if any. However, our third-party sourcing strategy may not result in a cost-effective means for manufacturing our future products. If we employ third-party manufacturers, we will not control many aspects of the manufacturing process, including compliance by these third parties with cGMP and other regulatory requirements. We further may not be able to obtain adequate supplies from third-party manufacturers in a timely fashion for development or commercialization purposes, and commercial quantities of products may not be available from contract manufacturers at acceptable costs.
Problems with our manufacturing process or with a manufacturing facility (whether ours or a third party’s) could result in the failure to produce, or a delay in producing, adequate supplies of a product candidate such as Tcelna. A number of factors could cause interruptions or delays, including equipment malfunctions or failures, destruction or damage to a manufacturing facility due to natural disasters or otherwise, contamination of materials, changes in regulatory requirements or standards that require modifications to our manufacturing process, action by a regulatory agency or by a manufacturer (whether us or a third party) that results in the halting or slowdown of production due to regulatory issues, any third-party manufacturer going out of business or failing to produce as contractually required, or other similar factors.
Difficulties, delays or interruptions in the manufacture and supply of a product candidate such as Tcelna could require us to stop treating patients in our clinical development of such product candidate and/or require a halt to or suspension of, or otherwise adversely affect, a clinical trial, thus increasing our costs and damaging our reputation. If a product candidate such as Tcelna is approved, difficulties, delays or interruptions in the manufacture and supply of such product candidate could cause a delay in or even halt or suspend the commercialization of such product candidate, potentially causing a partial or complete loss of revenue or market share.
Tcelna is manufactured using our proprietary ImmPath® technology for the production of an autologous T-cell immunotherapy utilizing a patient’s own blood. Our manufacturing process may raise development issues that may not be resolvable, regulatory issues that could delay or prevent approval, or personnel issues that may prevent the further development or commercialization, if approved, of any product candidate such as Tcelna.
Tcelna is based on our novel T-cell immunotherapy platform, ImmPath, which produces an autologous T-cell immunotherapy utilizing a patient’s own blood. OPX-212 is expected to be similarly produced. The manufacture of living T-cell products requires specialized facilities, equipment and personnel which are different than the resources required for manufacturing chemical or biologic compounds. Scaling-out the manufacture of living cell products to meet demands for commercialization will require substantial amounts of such specialized facilities, equipment and personnel, especially where, as is the case for Tcelna and expected to be the case for OPX-212, the products are personalized and must be made for each patient individually. Because our manufacturing processes are complex, require facilities and personnel that are not widely available in the industry, involve equipment and training with long lead times, and the establishment of new manufacturing facilities is subject to a potentially lengthy regulatory approval process, alternative qualified production capacity may not be available on a timely basis or on reasonably terms, if at all. In addition, not many consultants or
28
Table of Contents
advisors in the industry have relevant experience and can provide guidance or assistance because active immune therapies such as Tcelna are fundamentally a new category of product in two major ways: (i) the product consists of living T-cells, not chemical or biologic compounds; and (ii) the product is personalized. There can be no assurance that manufacturing problems will not arise in the future which we may not be able to resolve or which may cause significant delays in development or, if any product candidate such as Tcelna is approved, commercialization.
Regulatory approval of product candidates such as Tcelna that are manufactured using novel manufacturing processes such as ours can be more expensive and take longer than other, more well-known or extensively studied pharmaceutical or biopharmaceutical products, due to a lack of experience with them. FDA approval of personalized immunotherapy products has been limited to date. This lack of experience and precedent may lengthen the regulatory review process, require that additional studies or clinical trials be conducted, increase development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization, or lead to significant post-approval limitations or restrictions.
In addition, the novel nature of product candidates such as Tcelna also means that fewer people are trained in or experienced with product candidates of this type, which may make it difficult to find, hire and retain capable personnel for research, development and manufacturing positions.
If any product we may eventually have is not accepted by the market or if users of any such product are unable to obtain adequate coverage of and reimbursement for such product from government and other third-party payors, our revenues and profitability will suffer.
In the instance of Tcelna, if Merck Serono exercises the Option then our ability to achieve revenue will be dependent upon the efforts and success of Merck Serono in developing and commercializing Tcelna. Our ability to successfully commercialize any product we may eventually have, to the extent applicable, and/or our ability to receive any revenue associated with Tcelna in the event Merck Serono exercises the Option, will depend in significant part on the extent to which appropriate coverage of and reimbursement for such product and any related treatments are obtained from governmental authorities, private health insurers and other organizations, such as health maintenance organizations, or HMOs. Third-party payors are increasingly challenging the prices charged for medical products and services. We cannot provide any assurances that third-party payors will consider any product cost-effective or provide coverage of and reimbursement for such product, in whole or in part.
Uncertainty exists as to the coverage and reimbursement status of newly approved medical products and services and newly approved indications for existing products. Third-party payors may conclude that any product is less safe, less clinically effective, or less cost-effective than existing products, and third-party payors may not approve such product for coverage and reimbursement. If adequate coverage of and reimbursement for any product from third-party payors cannot be obtained, physicians may limit how much or under what circumstances they will prescribe or administer them. Such reduction or limitation in the use of any such product would cause sales to suffer. Even if third-party payors make reimbursement available, payment levels may not be sufficient to make the sale of any such product profitable.
In addition, the trend towards managed health care in the United States and the concurrent growth of organizations such as HMOs, which could control or significantly influence the purchase of medical services and products, may result in inadequate coverage of and reimbursement for any product we may eventually have. Many third-party payors, including in particular HMOs, are pursuing various ways to reduce pharmaceutical costs, including, for instance, the use of formularies. The market for any product depends on access to such formularies, which are lists of medications for which third-party payors provide reimbursement. These formularies are increasingly restricted, and pharmaceutical companies face significant competition in their efforts to place their products on formularies of HMOs and other third-party payors. This increased competition has led to a downward pricing pressure in the industry. The cost containment measures that third-party payors are instituting could have a material adverse effect on our ability to operate profitably.
29
Table of Contents
Any product candidate, such as Tcelna, if approved for sale, may not gain acceptance among physicians, patients and the medical community, thereby limiting our potential to generate revenues.
Even if a product candidate, such as Tcelna, is approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product candidate by physicians, healthcare professionals and third-party payors, and our profitability and growth, will depend on a number of factors, including:
| • | demonstration of efficacy; |
| • | relative convenience and ease of administration; |
| • | the prevalence and severity of any adverse side effects; |
| • | availability and cost of alternative treatments, including cheaper generic drugs; |
| • | pricing and cost effectiveness, which may be subject to regulatory control; |
| • | effectiveness of sales and marketing strategies for the product and competition for such product; |
| • | the product labeling or product insert required by the FDA or regulatory authority in other countries; and |
| • | the availability of adequate third-party insurance coverage or reimbursement. |
If any product candidate does not provide a treatment regimen that is as beneficial as the current standard of care or otherwise does not provide patient benefit, that product candidate, if approved for commercial sale by the FDA or other regulatory authorities, likely will not achieve market acceptance and our ability to generate revenues from that product candidate would be substantially reduced.
We have incurred, and expect to continue to incur, increased costs and risks as a result of being a public company.
As a public company, we are required to comply with the Sarbanes-Oxley Act of 2002, or SOX, as well as rules and regulations implemented by the SEC and The NASDAQ Stock Market (NASDAQ). Changes in the laws and regulations affecting public companies, including the provisions of SOX and rules adopted by the SEC and by NASDAQ, have resulted in, and will continue to result in, increased costs to us as we respond to their requirements. Given the risks inherent in the design and operation of internal controls over financial reporting, the effectiveness of our internal controls over financial reporting is uncertain. If our internal controls are not designed or operating effectively, we may not be able to conclude an evaluation of our internal control over financial reporting as required or we or our independent registered public accounting firm may determine that our internal control over financial reporting was not effective. In addition, our registered public accounting firm may either disclaim an opinion as it relates to management’s assessment of the effectiveness of our internal controls or may issue an adverse opinion on the effectiveness of our internal controls over financial reporting. Investors may lose confidence in the reliability of our financial statements, which could cause the market price of our common stock to decline and which could affect our ability to run our business as we otherwise would like to. New rules could also make it more difficult or more costly for us to obtain certain types of insurance, including directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the coverage that is the same or similar to our current coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our Board committees and as executive officers. We cannot predict or estimate the total amount of the costs we may incur or the timing of such costs to comply with these rules and regulations.
Under the corporate governance standards of NASDAQ, a majority of our Board of Directors and each member of our Audit and Compensation Committees must be an independent director. If any vacancies on our Board or our Audit or Compensation Committees occur that need to be filled by independent directors, we may encounter difficulty in attracting qualified persons to serve on our Board and, in particular, our Audit Committee. If we fail to attract and retain the required number of independent directors, we may be subject to SEC enforcement proceedings and delisting of our common stock from the NASDAQ Capital Market.
30
Table of Contents
Any acquisitions that we make could disrupt our business and harm our financial condition.
We expect to evaluate potential strategic acquisitions of complementary businesses, products or technologies on a global geographic footprint. We may also consider joint ventures, licensing and other collaborative projects. We may not be able to identify appropriate acquisition candidates or strategic partners, or successfully negotiate, finance or integrate acquisitions of any businesses, products or technologies. Furthermore, the integration of any acquisition and management of any collaborative project may divert our management’s time and resources from our core business and disrupt our operations. We do not have any experience with acquiring companies, or with acquiring products outside of the United States. Any cash acquisition we pursue would potentially divert the cash we have on our balance sheet from our present clinical development programs. Any stock acquisitions would dilute our shareholders’ ownership. While we from time to time evaluate potential collaborative projects and acquisitions of businesses, products and technologies, and anticipate continuing to make these evaluations, we have no present commitments or agreements with respect to any acquisitions or collaborative projects.
Risks Related to Doing Business Internationally
We plan to do business internationally, which may prove to be difficult and fraught with economic, regulatory and political issues. We may acquire or in-license foreign companies or technologies or commercialize our T-cell or stem cell platform in countries where the business, economic and political climates are very different from those of the United States. We may not be aware of some of these issues and it may be difficult for a U.S. company to overcome these issues and ultimately become profitable. Certain foreign countries may favor businesses that are owned by nationals of those countries as opposed to foreign-owned business operating locally. As a small company, we may not have the resources to engage in the negotiation and time-consuming work needed to overcome some of these potential issues.
Risks Related to Our Intellectual Property
Patents obtained by other persons may result in infringement claims against us that are costly to defend and which may limit our ability to use the disputed technologies and prevent us from pursuing research and development or commercialization of potential products, such as Tcelna.
If third party patents or patent applications contain claims infringed by either our licensed technology or other technology required to make or use our potential products, such as Tcelna, and such claims are ultimately determined to be valid, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost, if at all, or be able to develop or obtain alternative technology. If we are unable to obtain such licenses at a reasonable cost, we (or, in the event the Option is exercised, Merck Serono with respect to Tcelna) may not be able to develop any affected product candidate commercially. There can be no assurance that we will not be obliged to defend ourselves (or, in the event the Option is exercised, Merck Serono with respect to Tcelna) in court against allegations of infringement of third party patents. Patent litigation is very expensive and could consume substantial resources and create significant uncertainties. An adverse outcome in such a suit could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease using such technology.
If we are unable to obtain patent protection and other proprietary rights, our operations will be significantly harmed.
Our ability to compete effectively is dependent upon obtaining patent protection relating to our technologies. The patent positions of pharmaceutical and biotechnology companies, including ours, are uncertain and involve complex and evolving legal and factual questions. The coverage sought in a patent application can be denied or significantly reduced before or after the patent is issued. Consequently, we do not know whether pending patent applications for our technology will result in the issuance of patents, or if any issued patents will
31
Table of Contents
provide significant protection or commercial advantage or will be circumvented by others. Since patent applications are secret until the applications are published (usually 18 months after the earliest effective filing date), and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that the inventors of our owned or licensed intellectual property rights were the first to make the inventions at issue or that any patent applications at issue were the first to be filed for such inventions. There can be no assurance that patents will issue from pending patent applications or, if issued, that such patents will be of commercial benefit to us, afford us adequate protection from competing products, or not be challenged or declared invalid.
Issued U.S. patents require the payment of maintenance fees to continue to be in force. We rely on a third party payor to do this and their failure to do so could result in the forfeiture of patents not timely maintained. Many foreign patent offices also require the payment of periodic annuities to keep patents and patent applications in good standing. As we may not maintain direct control over the payment of all such annuities, we cannot assure you that our third party payor will timely pay such annuities and that the granted patents and pending patent applications will not become abandoned. In addition, we or our licensors may have selected a limited amount of foreign patent protection, and therefore applications have not been filed in, and foreign patents may not have been perfected in, all commercially significant countries.
The patent protection of product candidates, such as Tcelna, involves complex legal and factual questions. To the extent that it would be necessary or advantageous for any of our licensors to cooperate or lead in the enforcement of our licensed intellectual property rights, we cannot control the amount or timing of resources such licensors devote on our behalf or the priority they place on enforcing such rights. We may not be able to protect our intellectual property rights against third party infringement, which may be difficult to detect. Additionally, challenges may be made to the ownership of our intellectual property rights, our ability to enforce them, or our underlying licenses.
We cannot be certain that any of the patents issued to us or to our licensors will provide adequate protection from competing products. Our success will depend, in part, on whether we or our licensors can:
| • | obtain and maintain patents to protect our product candidates such as Tcelna; |
| • | obtain and maintain any required or desirable licenses to use certain technologies of third parties, which may be protected by patents; |
| • | protect our trade secrets and know-how; |
| • | operate without infringing the intellectual property and proprietary rights of others; |
| • | enforce the issued patents under which we hold rights; and |
| • | develop additional proprietary technologies that are patentable. |
The degree of future protection for our proprietary rights (owned or licensed) is uncertain. For example:
| • | we or our licensor might not have been the first to make the inventions covered by pending patent applications or issued patents owned by, or licensed to, us; |
| • | we or our licensor might not have been the first to file patent applications for these inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of the technologies owned by, or licensed to, us; |
| • | it is possible that none of the pending patent applications owned by, or licensed to, us will result in issued patents; |
| • | any patents under which we hold rights may not provide us with a basis for commercially viable products, may not provide us with any competitive advantages or may be challenged by third parties as invalid, or unenforceable under U.S. or foreign laws; or |
| • | any of the issued patents under which we hold rights may not be valid or enforceable or may be circumvented successfully in light of the continuing evolution of domestic and foreign patent laws. |
32
Table of Contents
Confidentiality agreements with employees and others may not adequately prevent disclosure of our trade secrets and other proprietary information and may not adequately protect our intellectual property, which could limit our ability to compete.
We rely in part on trade secret protection in order to protect our proprietary trade secrets and unpatented know-how. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with our employees, consultants, outside scientific collaborators and other advisors, to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. We also typically obtain agreements from these parties which provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Further, we have limited control, if any, over the protection of trade secrets developed by our licensors. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
A dispute concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and costly, and an unfavorable outcome could harm our business.
A number of pharmaceutical, biotechnology and other companies, universities and research institutions have filed patent applications or have been issued patents relating to cell therapy, T-cells, and other technologies potentially relevant to or required by our product candidates such as Tcelna. We cannot predict which, if any, of such applications will issue as patents or the claims that might be allowed. We are aware of a number of patent applications and patents claiming use of modified cells to treat disease, disorder or injury.
There is significant litigation in our industry regarding patent and other intellectual property rights. While we are not currently subject to any pending intellectual property litigation, and are not aware of any such threatened litigation, we may be exposed to future litigation by third parties based on claims that our product candidates, such as Tcelna, or their methods of use, manufacturing or other technologies or activities infringe the intellectual property rights of such third parties. If our product candidates, such as Tcelna, or their methods of manufacture are found to infringe any such patents, we may have to pay significant damages or seek licenses under such patents. We have not conducted comprehensive searches of patents issued to third parties relating to Tcelna or OPX-212. Consequently, no assurance can be given that third-party patents containing claims covering Tcelna or OPX-212, their methods of use or manufacture do not exist or have not been filed and will not be issued in the future. Because some patent applications in the United States may be maintained in secrecy until the patents are issued, and because patent applications in the United States and many foreign jurisdictions are typically not published until 18 months after filing, we cannot be certain that others have not filed patent applications that will mature into issued patents that relate to our current or future product candidates that could have a material effect in developing and commercializing one or more of our product candidates. A patent holder could prevent us from importing, making, using or selling the patented compounds. We may need to resort to litigation to enforce our intellectual property rights or to determine the scope and validity of third-party proprietary rights. Similarly, we may be subject to claims that we have inappropriately used or disclosed trade secrets or other proprietary information of third parties. If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources, regardless of whether we win or lose. Some of our competitors may be able to sustain the costs of complex intellectual property litigation more effectively than we can because they have substantially greater resources. We may not be able to afford the costs of litigation. Any legal action against us or our collaborators could lead to:
| • | payment of actual damages, royalties, lost profits, potentially treble damages and attorneys’ fees, if we are found to have willfully infringed a third party’s patent rights; |
33
Table of Contents
| • | injunctive or other equitable relief that may effectively block our ability to further develop, commercialize and sell our products; |
| • | we or our collaborators having to enter into license arrangements that may not be available on commercially acceptable terms if at all; or |
| • | significant cost and expense, as well as distraction of our management from our business. |
As a result, we could be prevented from commercializing current or future product candidates.
Risks Related to Our Industry
We are subject to stringent regulation of our product candidates, such as Tcelna, which could delay development and commercialization.
We, our third-party contractors, suppliers and partners (such as Merck Serono, in the event the Option is exercised, with respect to Tcelna), and our product candidates, such as Tcelna, are subject to stringent regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. None of our product candidates can be marketed in the United States until it has been approved by the FDA. No product candidate of ours has been approved, and we may never receive FDA approval for any product candidate. Obtaining FDA approval typically takes many years and requires substantial resources. Even if regulatory approval is obtained, the FDA may impose significant restrictions on the indicated uses, conditions for use and labeling of such products. Additionally, the FDA may require post-approval studies, including additional research and development and clinical trials. These regulatory requirements may limit the size of the market for the product or result in the incurrence of additional costs. Any delay or failure in obtaining required approvals could substantially reduce our ability to generate revenues.
In addition, both before and after regulatory approval, we, our partners and our product candidates, such as Tcelna, are subject to numerous FDA requirements covering, among other things, testing, manufacturing, quality control, labeling, advertising, promotion, distribution and export. The FDA’s requirements may change and additional government regulations may be promulgated that could affect us, our partners and our product candidates, such as Tcelna. Given the number of recent high profile adverse safety events with certain drug products, the FDA may require, as a condition of approval, costly risk management programs, which may include safety surveillance, restricted distribution and use, patient education, enhanced labeling, special packaging or labeling, expedited reporting of certain adverse events, preapproval of promotional materials and restrictions on direct-to-consumer advertising. Furthermore, heightened Congressional scrutiny on the adequacy of the FDA’s drug approval process and the agency’s efforts to assure the safety of marketed drugs resulted in the enactment of legislation addressing drug safety issues, the FDA Amendments Act of 2007. This legislation provides the FDA with expanded authority over drug products after approval and the FDA’s exercise of this authority could result in delays or increased costs during the period of product development, clinical trials and regulatory review and approval, and increased costs to assure compliance with new post-approval regulatory requirements. We cannot predict the likelihood, nature or extent of government regulation that may arise from this or future legislation or administrative action, either in the United States or abroad.
In order to market any of our products outside of the United States, we and our strategic partners and licensees must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods and the time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States. Approval by the FDA does not automatically lead to the approval of authorities outside of the United States and, similarly, approval by other regulatory authorities outside the United States will not automatically lead to FDA approval. In addition, regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. Our product
34
Table of Contents
candidates, such as Tcelna, may not be approved for all indications that we request, which would limit uses and adversely impact our potential royalties and product sales. Such approval may be subject to limitations on the indicated uses for which any potential product may be marketed or require costly, post-marketing follow-up studies.
If we fail to comply with applicable regulatory requirements in the United States and other countries, among other things, we may be subject to fines and other civil penalties, delays in approving or failure to approve a product, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, interruption of manufacturing or clinical trials, injunctions and criminal prosecution, any of which would harm our business.
We may need to change our business practices to comply with health care fraud and abuse regulations, and our failure to comply with such laws could adversely affect our business, financial condition and results of operations.
If Merck Serono exercises the Option, Merck Serono would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. Otherwise, if we are successful in achieving approval to market one or more of our product candidates, our operations will be directly, or indirectly through our customers, subject to various state and federal fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and False Claims Act. These laws may impact, among other things, our proposed sales, marketing, and education programs.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Department of Health and Human Services, Office of Inspector General, or OIG, to issue a series of regulations, known as the “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The federal False Claims Act prohibits persons from knowingly filing or causing to be filed a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, sometimes known as “relators” or, more commonly, as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The frequency of filing of qui tam actions has increased significantly in recent years, causing greater numbers of healthcare companies to have to defend a False Claims Act action. When an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties. Various states have also enacted laws modeled after the federal False Claims Act.
35
Table of Contents
In addition to the laws described above, the Health Insurance Portability and Accountability Act of 1996 created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment.
Beginning August 1, 2013, the Physician Payments Sunshine Act (the “Sunshine Act”), which is part of the Patient Protection and Affordable Care Act, requires manufacturers of drugs, medical devices, biologicals or medical supplies that participate in U.S. federal health care programs to track and then report certain payments and items of value given to U.S. physicians and U.S. teaching hospitals (defined as “Covered Recipients”). The Sunshine Act requires that manufacturers collect this information on a yearly basis and then report it to Centers for Medicare & Medicaid Services by the 90th day of each subsequent year.
If our operations are found to be in violation of any of the laws described above and other applicable state and federal fraud and abuse laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from government healthcare programs, and the curtailment or restructuring of our operations.
If our competitors develop and market products that are more effective than our product candidates, they may reduce or eliminate our commercial opportunities.
Competition in the pharmaceutical industry, particularly the market for MS products, is intense, and we expect such competition to continue to increase. We face competition from pharmaceutical and biotechnology companies, as well as numerous academic and research institutions and governmental agencies, in the United States and abroad. Our competitors have products that have been approved or are in advanced development and may succeed in developing drugs that are more effective, safer and more affordable or more easily administered than ours, or that achieve patent protection or commercialization sooner than our products. Our most significant competitors are fully integrated pharmaceutical companies and more established biotechnology companies. These companies have significantly greater capital resources and expertise in research and development, manufacturing, testing, obtaining regulatory approvals, and marketing than we currently do. However, smaller companies also may prove to be significant competitors, particularly through proprietary research discoveries and collaboration arrangements with large pharmaceutical and established biotechnology companies. In addition to the competitors with existing products that have been approved, many of our competitors are further along in the process of product development and also operate large, company-funded research and development programs. As a result, our competitors may develop more competitive or affordable products, or achieve earlier patent protection or further product commercialization than we are able to achieve. Competitive products may render any products or product candidates that we develop obsolete.
Our competitors may also develop alternative therapies that could further limit the market for any products that we may develop.
Rapid technological change could make our products obsolete.
Biopharmaceutical technologies have undergone rapid and significant change, and we expect that they will continue to do so. As a result, there is significant risk that our product candidates, such as Tcelna, may be rendered obsolete or uneconomical by new discoveries before we recover any expenses incurred in connection with their development. If our product candidates, such as Tcelna, are rendered obsolete by advancements in biopharmaceutical technologies, our future prospects will suffer.
36
Table of Contents
Consumers may sue us for product liability, which could result in substantial liabilities that exceed our available resources and damage our reputation.
Developing and commercializing drug products entails significant product liability risks. Liability claims may arise from our and our collaborators’ use of products in clinical trials and the commercial sale of those products.
In the event that any of our product candidates becomes an approved product and is commercialized, consumers may make product liability claims directly against us and/or our partners (such as Merck Serono, in the event the Option is exercised, with respect to Tcelna), and our partners or others selling these products may seek contribution from us if they incur any loss or expenses related to such claims. We have insurance that covers clinical trial activities. We believe our insurance coverage as of the date hereof is reasonably adequate at this time. However, we will need to increase and expand this coverage as we commence additional clinical trials, as well as larger scale trials, and if any product candidate is approved for commercial sale. This insurance may be prohibitively expensive or may not fully cover our potential liabilities. Our inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the regulatory approval or commercialization of products that we or one of our collaborators develop. Product liability claims could have a material adverse effect on our business and results of operations. Liability from such claims could exceed our total assets if we do not prevail in any lawsuit brought by a third party alleging that an injury was caused by one or more of our products.
Government controls and health care reform measures could adversely affect our business.
The business and financial condition of pharmaceutical and biotechnology companies are affected by the efforts of governmental and third-party payors to contain or reduce the costs of health care. In the United States and in foreign jurisdictions, there have been, and we expect that there will continue to be, a number of legislative and regulatory proposals aimed at changing the health care system. For example, in some foreign countries, particularly in Europe, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct additional clinical trials that compare the cost-effectiveness of any product candidate, such as Tcelna, to other available therapies. If reimbursement of any product candidate such as Tcelna, if approved, is unavailable or limited in scope or amount in a particular country, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability in such country. In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) changed the way Medicare covers and pays for pharmaceutical products. The legislation established Medicare Part D, which expanded Medicare coverage for outpatient prescription drug purchases by the elderly but provided authority for limiting the number of drugs that will be covered in any therapeutic class. The MMA also introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. Any negotiated prices for any product candidate such as Tcelna, if approved, covered by a Part D prescription drug plan will likely be lower than the prices that might otherwise be obtained outside of the Medicare Part D prescription drug plan. Moreover, while Medicare Part D applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment under Medicare Part D may result in a similar reduction in payments from non-governmental payors.
The United States and several other jurisdictions are considering, or have already enacted, a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell any product candidate such as Tcelna, if approved. Among policy-makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access to healthcare. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. There have been, and likely will continue to be, legislative and regulatory proposals at the
37
Table of Contents
federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare may adversely affect: the demand for any product candidate such as Tcelna, if approved; the ability to set a price that we believe is fair for any product candidate such as Tcelna, if approved; our ability to generate revenues and achieve or maintain profitability; the level of taxes that we are required to pay; and the availability of capital.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the ACA), became law in the United States. The goal of the ACA is to reduce the cost of healthcare and substantially change the way healthcare is financed by both governmental and private insurers. The ACA may result in downward pressure on pharmaceutical reimbursement, which could negatively affect market acceptance of any product candidate such as Tcelna, if approved. Provisions of the ACA relevant to the pharmaceutical industry include the following: an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively; a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts on negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level beginning in 2014, thereby potentially increasing manufacturers’ Medicaid rebate liability; expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; new requirements under the federal Open Payments program and its implementing regulations; and expansion of healthcare fraud and abuse laws, including the federal False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance. In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several types of providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding.
Another example of reform that could affect our business is drug reimportation into the United States (i.e., the reimportation of approved drugs originally manufactured in the United States back into the United States from other countries where the drugs were sold at lower prices). Initiatives in this regard could decrease the price we or any potential collaborators receive for our product candidates if they are ever approved for sale, adversely affecting our future revenue growth and potential profitability. Moreover, the pendency or approval of such proposals could result in a decrease in our stock price or adversely affect our ability to raise capital or to obtain strategic partnerships or licenses.
Risks Related to Our Securities
There is currently a limited market for our securities, and any trading market that exists in our securities may be highly illiquid and may not reflect the underlying value of our net assets or business prospects.
Although our common stock is traded on the NASDAQ Capital Market, there is currently a limited market for our securities and there can be no assurance that an active market will ever develop. Investors are cautioned not to rely on the possibility that an active trading market may develop.
38
Table of Contents
Our stock may be delisted from NASDAQ, which could affect its market price and liquidity.
We are required to meet certain qualitative and financial tests (including a minimum bid price for our common stock of $1.00 per share and a minimum stockholders’ equity of $2.5 million), as well as certain corporate governance standards, to maintain the listing of our common stock on the NASDAQ Capital Market. It is possible that we could fail to satisfy one or more of these requirements. Since November 2014, our stock has continued to trade below the minimum bid price continued listing requirement, and our common stock is in jeopardy of being delisted. On December 5, 2014, we received a staff deficiency letter from NASDAQ indicating that our common stock failed to comply with the minimum bid price requirement because it closed below the $1.00 minimum closing bid price for 30 consecutive trading days. The notice further stated that we will be provided a period of 180 calendar days to regain compliance. If our common stock maintains a closing bid price of $1.00 per share or more for a minimum of 10 consecutive business days (or such longer period of time as the NASDAQ staff may require in some circumstances, but generally not more than 20 consecutive business days) before June 3, 2015, we will achieve compliance with the listing standards. If our common stock does not achieve compliance with the minimum bid price by June 3, 2015, we may be eligible for an additional 180 day grace period so long as we continue to meet the other listing standards and provide timely notice of our intention to cure the deficiency. However, if it appears to the NASDAQ staff that we will not be able to cure the deficiency, or if we do not meet the other listing standards, NASDAQ could provide notice that our stock will become subject to delisting.
We previously received a similar staff deficiency letter in February 2012 indicating that our common stock failed to comply with the minimum bid price requirement because it traded below the $1.00 minimum closing bid price for 30 consecutive trading days, and after an initial and an extended grace period, and implementation of a one-for-four reverse stock split of our common stock on December 14, 2012, we regained compliance with the $1.00 minimum closing bid price listing standard and NASDAQ notified us that the matter was closed in January 2013. We also received a staff deficiency letter in November 2012 notifying us that the stockholders’ equity of $2,339,285 as reported in our Quarterly Report on Form 10-Q for the period ended September 30, 2012 was below the minimum stockholders’ equity of $2.5 million required for continued listing on NASDAQ. We were provided 45 calendar days, or until January 10, 2013, to submit a plan to regain compliance with the minimum stockholders’ equity standard. We submitted such a plan and it was accepted, with NASDAQ thus granting us an extension until May 15, 2013 to evidence compliance with the minimum stockholders’ equity standard. Upon executing the plan, we attained the necessary stockholders’ equity level and subsequently received notice from NASDAQ that we had regained compliance with the listing standard and the matter was closed in May 2013.
While we are exercising diligent efforts to maintain the listing of our common stock on NASDAQ, there can be no assurance that we will be able to maintain compliance with the minimum bid price, stockholder’s equity or other listing standards in the future. We may receive additional future notices from NASDAQ that we have failed to meet its requirements, and proceedings to delist our stock could be commenced. In such event, NASDAQ rules permit us to appeal any delisting determination to a NASDAQ Hearings Panel. If we are unable to maintain or regain compliance in a timely manner and our common stock is delisted, it could be more difficult to buy or sell our common stock and obtain accurate quotations, and the price of our stock could suffer a material decline. Delisting may also impair our ability to raise capital.
Our share price is volatile, and you may not be able to resell our shares at a profit or at all.
The market prices for securities of biopharmaceutical and biotechnology companies, and early-stage drug discovery and development companies like us in particular, have historically been highly volatile and may continue to be highly volatile in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
| • | the development status of any drug candidates, such as Tcelna, including clinical study results and determinations by regulatory authorities with respect thereto; |
39
Table of Contents
| • | the initiation, termination, or reduction in the scope of any collaboration arrangements (such as developments involving Merck Serono and the Option, including a decision by Merck Serono to exercise or not exercise the Option) or any disputes or developments regarding such collaborations; |
| • | announcements of technological innovations, new commercial products or other material events by our competitors or us; |
| • | disputes or other developments concerning our proprietary rights; |
| • | changes in, or failure to meet, securities analysts’ or investors’ expectations of our financial performance; |
| • | additions or departures of key personnel; |
| • | discussions of our business, products, financial performance, prospects or stock price by the financial and scientific press and online investor communities; |
| • | public concern as to, and legislative action with respect to, the pricing and availability of prescription drugs or the safety of drugs and drug delivery techniques; |
| • | regulatory developments in the United States and in foreign countries; or |
| • | dilutive effects of sales of shares of common stock by us or our shareholders, and sales of common stock acquired upon exercise or conversion by the holders of warrants, options or convertible notes. |
Broad market and industry factors, as well as economic and political factors, also may materially adversely affect the market price of our common stock.
We may be or become the target of securities litigation, which is costly and time-consuming to defend.
In the past, following periods of market volatility in the price of a company’s securities or the reporting of unfavorable news, security holders have often instituted class action litigation. If the market value of our securities experience adverse fluctuations and we become involved in this type of litigation, regardless of the outcome, we could incur substantial legal costs and our management’s attention could be diverted from the operation of our business, causing our business to suffer.
Our “blank check” preferred stock could be issued to prevent a business combination not desired by management or our majority shareholders.
Our charter authorizes the issuance of “blank check” preferred stock with such designations, rights and preferences as may be determined by our Board of Directors without shareholder approval. Our preferred stock could be utilized as a method of discouraging, delaying, or preventing a change in our control and as a method of preventing shareholders from receiving a premium for their shares in connection with a change of control.
Future sales of our securities could cause dilution, and the sale of such securities, or the perception that such sales may occur, could cause the price of our stock to fall.
In July 2012, we closed a private offering consisting of convertible secured notes and warrants to purchase common stock which generated approximately $4.1 million in gross proceeds, of which notes in the aggregate principal amount of $900,000 were converted into shares of Series A convertible preferred stock which, in turn, were converted into an aggregate of 288,229 shares of common stock during February 2013. The remaining notes were converted into an aggregate of 1,714,697 shares of common stock at $1.91 per share on September 24, 2013. From November 2012 through January 2013, we sold an aggregate of 390,000 shares to Lincoln Park pursuant to the $1.5 million purchase agreement and issued an additional 56,507 shares as initial commitment shares and 3,585 shares as additional commitment shares. In January 2013, we issued $650,000 principal amount of unsecured convertible promissory notes of which $100,000 was converted into 77,034 shares of common stock at $1.298125 per share during February 2013 and the remaining $550,000 of principal amount plus accrued interest was repaid during February 2013. Purchasers of such notes also received five-year warrants
40
Table of Contents
to acquire an aggregate of 243,750 shares of our common stock at an exercise price of $1.24 per share. In February 2013, we sold an aggregate of 167,618 shares of our common stock pursuant to the ATM Agreement for gross proceeds of $536,417. On February 11, 2013, we closed on an offering of 1,083,334 shares of common stock and warrants to purchase 541,668 shares of common stock for gross proceeds of $3.25 million, or net proceeds of approximately $3.0 million after deducting commissions and offering expenses. On August 13, 2013, we closed an offering of 12 million shares of common stock for gross proceeds of $18 million, or net proceeds of approximately $16.2 million after deducting underwriting discounts and commissions and offering expenses, and we granted the underwriters a 30-day option to purchase up to an additional 1.8 million shares of common stock to cover over-allotments. During September 2013, the underwriters of the August 2013 underwritten public offering exercised the over-allotment option granted to them which resulted in the issuance of an additional 900,000 shares of common stock for gross proceeds of $1.35 million, or net proceeds of approximately $1.2 million after deducting underwriting discounts and commissions and offering expenses. On December 23, 2013, we closed an offering of 4,738,000 shares of common stock, including the full exercise of the over-allotment option granted to the underwriters, raising gross proceeds of $8.1 million or net proceeds of approximately $7.3 million after deducting the underwriting discounts and commission and offering expenses. Between March 5, 2014 and December 31, 2014, we generated gross and net proceeds including amortization of deferred financing costs of $674,126 and $648,175, respectively, on sales of an aggregate of 518,412 shares of our common stock under the new ATM Agreement.
Sales of additional shares of our common stock, as well as securities convertible into or exercisable for common stock, could result in substantial dilution to our shareholders and cause the market price of our common stock to decline. An aggregate of 28,234,751 shares of common stock were outstanding as of December 31, 2014. As of such date, another (i) 2,423,253 shares of common stock were issuable upon exercise of outstanding options and (ii) 3,046,801 shares of common stock were issuable upon the exercise of outstanding warrants. A substantial majority of the outstanding shares of our common stock are freely tradable without restriction or further registration under the Securities Act of 1933.
We may sell additional shares of common stock, as well as securities convertible into or exercisable for common stock, in subsequent public or private offerings. We may also issue additional shares of common stock, as well as securities convertible into or exercisable for common stock, to finance future acquisitions. Among other requirements, we will need to raise significant additional capital in order to complete the Phase IIb clinical study of Tcelna in SPMS, and, if initiated, to complete a Phase 1/2 proof-of-concept study of OPX-212 in NMO, and this may require us to issue a substantial amount of securities (including common stock as well as securities convertible into or exercisable for common stock). There can be no assurance that our capital raising efforts will be able to attract the capital needed to execute on our business plan and sustain our operations. Moreover, we cannot predict the size of future issuances of our common stock, as well as securities convertible into or exercisable for common stock, or the effect, if any, that future issuances and sales of our securities will have on the market price of our common stock. Sales of substantial amounts of our common stock, as well as securities convertible into or exercisable for common stock, including shares issued in connection with an acquisition or securing funds to complete our clinical trial plans, or the perception that such sales could occur, may result in substantial dilution and may adversely affect prevailing market prices for our common stock.
Under the $1.5 million purchase agreement and $15.0 million purchase agreement with Lincoln Park, we may direct Lincoln Park to purchase up to $16.5 million of shares of common stock, subject to certain limitations and conditions, over a 30-month period. We have sold an aggregate of 390,000 shares to date under the $1.5 million purchase agreement. Additionally, we issued Lincoln Park 56,507 shares of common stock as initial commitment shares and have issued an aggregate of 3,585 additional commitment shares, and may in the future issue up to an additional 109,428 shares of common stock as additional commitment shares, as a fee for its commitment to purchase the shares under the $1.5 million purchase agreement and the $15.0 million purchase agreement. The purchase price for the shares that we may sell to Lincoln Park will fluctuate based on the price of our common stock and other factors determined by us. As such, Lincoln Park may ultimately purchase all, some or none of the shares of our common stock issuable pursuant to the purchase agreements after the date hereof
41
Table of Contents
and, after it has acquired shares, Lincoln Park may sell all, some or none of those shares. Therefore, sales to Lincoln Park by us pursuant to either or both of the purchase agreements could result in substantial dilution to the interests of other holders of our common stock. Additionally, the sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could cause the trading price of our common stock to decline and could make it more difficult for us to sell equity or equity-related securities in the future.
We presently do not intend to pay cash dividends on our common stock.
We currently anticipate that no cash dividends will be paid on the common stock in the foreseeable future. While our dividend policy will be based on the operating results and capital needs of the business, it is anticipated that all earnings, if any, will be retained to finance the future expansion of our business.
Our shareholders may experience substantial dilution in the value of their investment if we issue additional shares of our capital stock.
Our charter allows us to issue up to 100,000,000 shares of our common stock and to issue and designate the rights of, without shareholder approval, up to 10,000,000 shares of preferred stock. In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share paid by other investors, and dilution to our shareholders could result. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors, and investors purchasing shares or other securities in the future could have rights superior to existing shareholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by other investors.
We may issue debt and equity securities or securities convertible into equity securities, any of which may be senior to our common stock as to distributions and in liquidation, which could negatively affect the value of our common stock.
In the future, we may attempt to increase our capital resources by entering into debt or debt-like financing that is unsecured or secured by up to all of our assets, or by issuing additional debt or equity securities, which could include issuances of secured or unsecured commercial paper, medium-term notes, senior notes, subordinated notes, guarantees, preferred stock, hybrid securities, or securities convertible into or exchangeable for equity securities. In the event of our liquidation, our lenders and holders of our debt and preferred securities would receive distributions of our available assets before distributions to the holders of our common stock. Because our decision to incur debt and issue securities in future offerings may be influenced by market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future offerings or debt financings. Further, market conditions could require us to accept less favorable terms for the issuance of our securities in the future.
Our management has significant flexibility in using our current available cash and the net proceeds from this offering
In addition to general corporate purposes (including working capital, research and development, business development and operational purposes), we currently intend to use our available cash and the net proceeds from this offering (i) to continue funding the ongoing Abili-T clinical study of Tcelna in patients with SPMS, and (ii) to continue preclinical and manufacturing activities for OPX-212 in patients with NMO, and if such activities are successful, to file an IND application with the FDA to initiate a Phase 1/2 proof-of-concept study. We reached our enrollment target for the Abili-T trial in June 2014, and a total of 190 patients have been enrolled in this two-year study. We expect top-line data for Tcelna to be available in the second half of 2016. Our existing resources are not adequate to permit us to complete the ongoing Abili-T clinical study of Tcelna in patients with SPMS or, if initiated, to conduct a Phase 1/2 proof-of-concept study of OPX-212 in NMO. We will
42
Table of Contents
need to secure significant additional resources to complete the ongoing Abili-T clinical study of Tcelna in patients with SPMS and, if initiated, to conduct a Phase 1/2 proof-of-concept study of OPX-212 in NMO and to support our operations during the course of the trials.
Depending on future developments and circumstances, we may use some of our available cash or the net proceeds from this offering for other purposes which may have the potential to decrease the forecasted cash runway. Notwithstanding our current intentions regarding use of our available cash and the net proceeds from this offering, our management will have significant flexibility with respect to such use. The actual amounts and timing of expenditures will vary significantly depending on a number of factors, including the amount and timing of cash used in our operations and our research and development efforts. Management’s failure to use these funds effectively would have an adverse effect on the value of our common stock and could make it more difficult and costly to raise funds in the future.
Risks Related to the Rights Offering
We will incur substantial expenses in connection with the Rights Offering, which may not return adequate value if the Rights Offering is ultimately not consummated or successful.
The estimated expenses for the Rights Offering are approximately $750,000, excluding fees and expenses of the dealer-managers that we have engaged to assist us with the Rights Offering. If the registration statement of which this prospectus is a part is not declared effective, the Rights Offering is not commenced or the Rights Offering is not ultimately consummated or successful, we will incur these expenses nonetheless. In addition, we have agreed to pay fees to the dealer-managers as follows: (i) to Maxim Group LLC, a cash fee equal to (a) 1% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is less than $6 million; (b) 4% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million but less than $10 million; and (c) 4.5% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $10 million; and (ii) to National Securities Corporation, a cash fee equal to 2.75% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million. We have also agreed to reimburse the dealer-managers for their expenses as follows: (i) up to $50,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is less than $6 million; and (ii) up to $100,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is at least $6 million.
Your interest in our company may be diluted as a result of this Rights Offering.
Shareholders who do not fully exercise their Subscription Rights should expect that they will, at the completion of this offering, own a smaller proportional interest in our company than would otherwise be the case had they fully exercised their Basic Subscription Right and Over-Subscription Privilege. Further, the shares issuable upon the exercise of the Warrants to be issued pursuant to the Rights Offering will dilute the ownership interest of shareholders not participating in this offering or holders of Warrants issued pursuant to this offering who have not exercised them.
Further, because the price per Unit being offered may be substantially higher than the net tangible book value per share of our common stock, you may suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering. If you purchase Units in this offering at the Subscription Price, you may suffer immediate and substantial dilution in the net tangible book value of the common stock. See “Dilution” in this prospectus for a more detailed discussion of the dilution which may incur in connection with this offering.
Completion of the Rights Offering is not subject to us raising a minimum offering amount.
Completion of the Rights Offering is not subject to us raising a minimum offering amount and therefore proceeds may be insufficient to meet our objectives, thereby increasing the risk to investors in this offering, including investing in a company that continues to require capital.
43
Table of Contents
This Rights Offering may cause the trading price of our common stock to decrease.
The Subscription Price, together with the number of shares of common stock we propose to issue and ultimately will issue if this Rights Offering is completed, may result in an immediate decrease in the market price of our common stock. This decrease may continue after the completion of this Rights Offering. If that occurs, you may have committed to buy shares of common stock in the Rights Offering at a price greater than the prevailing market price. We cannot predict the effect, if any, that the availability of shares for future sale represented by the Warrants issued in connection with the Rights Offering will have on the market price of our common stock from time to time. Further, if a substantial number of Subscription Rights are exercised and the holders of the shares received upon exercise of those Subscription Rights or the related Warrants choose to sell some or all of the shares underlying the Subscription Rights or the related Warrants, the resulting sales could depress the market price of our common stock. Following the exercise of your Subscription Rights you may not be able to sell your common stock at a price equal to or greater than the Subscription Price.
You could be committed to buying shares of common stock above the prevailing market price.
Once you exercise your Subscription Rights, you may not revoke such exercise even if you later learn information that you consider to be unfavorable to the exercise of your Subscription Rights. The market price of our shares of common stock may decline prior to the expiration of this offering or a Subscribing Rights holder may not be able to sell shares of common stock purchased in this offering at a price equal to or greater than the Subscription Price. Until shares of our common stock are delivered upon expiration of the Rights Offering, you will not be able to sell or transfer the shares of our common stock that you purchase in the Rights Offering. Any such delivery will occur as soon as practicable after the Rights Offering has expired, payment for the shares of common stock and attached Warrants subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected.
If we terminate this offering for any reason, we will have no obligation other than to return subscription monies as soon as practicable.
We may decide, in our sole discretion and for any reason, to cancel or terminate the Rights Offering at any time prior to the expiration date. If this offering is cancelled or terminated, we will have no obligation with respect to Subscription Rights that have been exercised except to return as soon as practicable, without interest, the subscription payments deposited with the Subscription Agent. If we terminate this offering and you have not exercised any Subscription Rights, such Subscription Rights will expire worthless.
Our common stock price may be volatile as a result of this Rights Offering.
The trading price of our common stock may fluctuate substantially. The price of the common stock that will prevail in the market after this offering may be higher or lower than the Subscription Price depending on many factors, some of which are beyond our control and may not be directly related to our operating performance. These factors include, but are not limited to, the following:
| • | the development status of any drug candidates, such as Tcelna, including clinical study results and determinations by regulatory authorities with respect thereto; |
| • | the initiation, termination, or reduction in the scope of any collaboration arrangements (such as developments involving Merck Serono and the Option, including a decision by Merck Serono to exercise or not exercise the Option) or any disputes or developments regarding such collaborations; |
| • | announcements of technological innovations, new commercial products or other material events by our competitors or us; |
| • | disputes or other developments concerning our proprietary rights; |
| • | changes in, or failure to meet, securities analysts’ or investors’ expectations of our financial performance; |
44
Table of Contents
| • | additions or departures of key personnel; |
| • | discussions of our business, products, financial performance, prospects or stock price by the financial and scientific press and online investor communities; |
| • | public concern as to, and legislative action with respect to, the pricing and availability of prescription drugs or the safety of drugs and drug delivery techniques; |
| • | regulatory developments in the United States and in foreign countries; or |
| • | dilutive effects of sales of shares of common stock by us or our shareholders, and sales of common stock acquired upon exercise or conversion by the holders of warrants, options or convertible notes. |
Additionally, the stock market historically has experienced significant price and volume fluctuations. These fluctuations are often unrelated to the operating performance of particular companies. These broad market fluctuations may cause declines in the trading price and market value of our common stock.
We cannot assure you that the trading price of our common stock will not decline after you elect to exercise your Subscription Rights. If that occurs, you may have committed to buy shares of common stock in the Rights Offering at a price greater than the prevailing market price and could have an immediate unrealized loss. Moreover, we cannot assure you that, following the exercise of your Subscription Rights, you will be able to sell your common stock at a price equal to or greater than the Subscription Price, and you may lose all or part of your investment in our common stock. Until shares are delivered upon expiration of the Rights Offering, you will not be able to sell the shares of our common stock that you purchase in the Rights Offering. Shares of our common stock purchased will be issued as soon as practicable after the Rights Offering has expired, payment for the shares of common stock and attached Warrants subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected. We will not pay you interest on funds delivered to the Subscription Agent pursuant to your exercise of Subscription Rights.
Because we do not have any formal commitments from any of our shareholders to participate in the Rights Offering, the net proceeds we receive from the Rights Offering may be lower than we currently anticipate.
We do not have any formal commitments from any of our shareholders to participate in the Rights Offering, and we cannot assure you that any of our shareholders or warrant holders will exercise all or any part of their Basic Subscription Rights or their Over-Subscription Privilege. If our shareholders or warrantholders that may acquire Subscription Rights subscribe for fewer shares of our common stock than we currently anticipate, the net proceeds we receive from the Rights Offering could be significantly lower than we currently expect.
The Subscription Price determined for this offering is not an indication of the fair value of our common stock.
In determining the Subscription Price, our board of directors considered a number of factors, including, but not limited to, the price at which our stockholders might be willing to participate in the Rights Offering, the value of the Warrant being issued as a component of the Unit, historical and current trading prices for our common stock, the amount of proceeds desired, the potential need for liquidity and capital, potential market conditions, and the desire to provide an opportunity to our shareholders to participate in the Rights Offering. In conjunction with its review of these factors, our board of directors also reviewed a range of discounts to market value represented by the subscription prices in various prior Rights Offerings by other public companies. The Subscription Price does not necessarily bear any relationship to the book value of our assets, results of operations, cash flows, losses, financial condition or any other established criteria for value. You should not consider the Subscription Price as an indication of the fair value of our common stock. After the date of this prospectus, our common stock may trade at prices above or below the Subscription Price.
45
Table of Contents
If you do not act on a timely basis and follow subscription instructions, your exercise of Subscription Rights may be rejected.
Holders of Subscription Rights who desire to purchase shares of our common stock and attached Warrants in this offering must act on a timely basis to ensure that all required forms and payments are actually received by the Subscription Agent prior to 5:00 p.m., New York City time, on the expiration date, unless extended. If you are a beneficial owner of shares of common stock and you wish to exercise your Subscription Rights, you must act promptly to ensure that your broker, dealer, custodian bank, trustee or other nominee acts for you and that all required forms and payments are actually received by your broker, dealer, custodian bank, trustee or other nominee in sufficient time to deliver such forms and payments to the Subscription Agent to exercise the Subscription Rights granted in this offering that you beneficially own prior to 5:00 p.m., New York City time on the expiration date, as may be extended. We will not be responsible if your broker, dealer, custodian bank, trustee or other nominee fails to ensure that all required forms and payments are actually received by the Subscription Agent prior to 5:00 p.m., New York City time, on the expiration date.
If you fail to complete and sign the required subscription forms, send an incorrect payment amount, or otherwise fail to follow the subscription procedures that apply to your exercise in this Rights Offering, the Subscription Agent may, depending on the circumstances, reject your subscription or accept it only to the extent of the payment received. Neither we nor the Subscription Agent undertakes to contact you concerning an incomplete or incorrect subscription form or payment, nor are we under any obligation to correct such forms or payment. We have the sole discretion to determine whether a subscription exercise properly follows the subscription procedures.
You may not receive all of the Units for which you subscribe.
Holders who fully exercise their Basic Subscription Rights will be entitled to subscribe for an additional number of Units. Over-Subscription Privileges will be allocated pro rata among Rights holders who over-subscribed, based on the number of over-subscription Units to which they have subscribed. We cannot guarantee that you will receive any or the entire amount of Units for which you over-subscribed. If the prorated amount of Units allocated to you in connection with your Over-Subscription Privilege is less than your Over-Subscription Request, then the excess funds held by the Subscription Agent on your behalf will be returned to you, without interest, as soon as practicable after the Rights Offering has expired and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected, and we will have no further obligations to you.
Unless we otherwise agree in writing, a person or entity, together with related persons or entities, may not exercise Subscription Rights (including Over-Subscription Privileges) to purchase Units that, when aggregated with their existing ownership, would result in such person or entity, together with any related persons or entities, owning in excess of 19.9% of our issued and outstanding shares of common stock following the closing of the transactions contemplated by this Rights Offering. If the amount of shares allocated to you is less than your subscription request, then the excess funds held by the Subscription Agent on your behalf will be returned to you, without interest, as soon as practicable after the Rights Offering has expired and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected, and we will have no further obligations to you.
If you make payment of the Subscription Price by personal check, your check may not clear in sufficient time to enable you to purchase shares in this Rights Offering.
Any personal check used to pay for shares and Warrants to be issued in this Rights Offering must clear prior to the expiration date of this Rights Offering, and the clearing process may require five or more business days. If you choose to exercise your Subscription Rights, in whole or in part, and to pay for shares and Warrants by personal check and your check has not cleared prior to the expiration date of this Rights Offering, you will not have satisfied the conditions to exercise your Subscription Rights and will not receive the shares and Warrants you wish to purchase.
46
Table of Contents
The receipt of Subscription Rights may be treated as a taxable distribution to you.
We believe the distribution of the Subscription Rights in this Rights Offering should be a non-taxable distribution to holders of shares of common stock and our Series L warrants under Section 305(a) of the Internal Revenue Code of 1986, as amended (the “Code”). Please see the discussion on the “Material U.S. Federal Income Tax Consequences” below. This position is not binding on the IRS, or the courts, however. If this Rights Offering is deemed to be part of a “disproportionate distribution” under Section 305 of the Code, your receipt of Subscription Rights in this offering may be treated as the receipt of a taxable distribution to you equal to the fair market value of the Subscription Rights. Any such distribution would be treated as dividend income to the extent of our current and accumulated earnings and profits, if any, with any excess being treated as a return of capital to the extent thereof and then as capital gain. Each holder of shares of common stock and each holder of a Series L warrant is urged to consult his, her or its own tax advisor with respect to the particular tax consequences of this Rights Offering.
The Subscription Rights are not transferable, and there is no market for the Subscription Rights.
You may not sell, transfer, assign or give away your Subscription Rights. Because the Subscription Rights are non-transferable, there is no market or other means for you to directly realize any value associated with the Subscription Rights. You must exercise the Subscription Rights to realize any potential value from your Subscription Rights.
Absence of a public trading market for the Warrants may limit your ability to resell the Warrants.
There is no established trading market for the Warrants to be issued pursuant to this offering, and the Warrants may not be widely distributed. We have applied to list the Warrants for trading on NASDAQ under the symbol “OPXAW,” but there can be no assurance that a sufficient number of Subscription Rights will be exercised so that the Warrants will meet minimum listing criteria to be accepted for listing on NASDAQ or that a market will develop for the Warrants. Even if a market for the Warrants does develop, the price of the Warrants may fluctuate and liquidity may be limited. If the Warrants are not accepted for listing on NASDAQ or if a market for the Warrants does not develop, then purchasers of the Warrants may be unable to resell the Warrants or sell them only at an unfavorable price for an extended period of time, if at all. Future trading prices of the Warrants will depend on many factors, including:
| • | our operating performance and financial condition; |
| • | our ability to continue the effectiveness of the registration statement, of which this prospectus is a part, covering the Warrants and the common stock issuable upon exercise of the Warrants; |
| • | the interest of securities dealers in making a market; and |
| • | the market for similar securities. |
The market price of our common stock may never exceed the exercise price of the Warrants issued in connection with this offering.
The Warrants being issued in connection with this offering become exercisable upon issuance and will expire three years after issuance. We cannot provide you any assurance that the market price of our common stock will ever exceed the exercise price of the Warrants prior to their date of expiration. Any Warrants not exercised by their date of expiration will expire worthless and we will be under no further obligation to the Warrant holder.
The Warrants may be redeemed on short notice. This may have an adverse impact on their price.
We may redeem the Warrants for $0.01 per Warrant once the closing price of our common stock has equaled or exceeded $2.50 per share, subject to adjustment, for 10 consecutive trading days. If we give notice of redemption, you will be forced to sell or exercise your Warrants or accept the redemption price. The notice of redemption could come at a time when it is not advisable or possible for you to exercise the Warrants. As a result, you would be unable to benefit from owning the Warrants being redeemed.
47
Table of Contents
The dealer-managers are not underwriting, nor acting as placement agents of, the Subscription Rights or the securities underlying the Subscription Rights.
Maxim Group LLC and National Securities Corporation will act as dealer-managers for this Rights Offering. Under the terms and subject to the conditions contained in the dealer-manager agreement, the dealer-managers will provide marketing assistance in connection with this offering. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants being issued in this offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares or Warrants. The dealer-managers will not be subject to any liability to us in rendering the services contemplated by the dealer-manager agreement except for any act of bad faith or gross negligence by the dealer-managers. The services of the dealer-managers to us in this connection cannot be construed as any assurance that this offering will be successful.
Our ability to use net operating loss carryovers to reduce future tax payments may be limited.
As of December 31, 2014, we had net operating loss carryforwards (NOLs) for federal income tax purposes of approximately $70 million. We generally are able to carry NOLs forward to reduce taxable income in future years. These NOLs begin to expire at December 31, 2025. However, our ability to utilize the NOLs is subject to the rules of Section 382 of the Internal Revenue Code. Section 382 generally restricts the use of NOLs after an “ownership change.” An ownership change occurs if, among other things, the stockholders (or specified groups of stockholders) who own or have owned, directly or indirectly, five (5%) percent or more of our common stock or are otherwise treated as five (5%) percent stockholders under Section 382 and the regulations promulgated thereunder increase their aggregate percentage ownership of our stock by more than 50 percentage points over the lowest percentage of the stock owned by these stockholders over a three-year rolling period. In the event of an ownership change, Section 382 imposes an annual limitation on the amount of taxable income a corporation may offset with NOL carryforwards. This annual limitation is generally equal to the product of the value of our stock on the date of the ownership change, multiplied by the long-term tax-exempt rate published monthly by the Internal Revenue Service. Any unused annual limitation may be carried over to later years until the applicable expiration date for the respective NOL carryforwards.
The rules of Section 382 are complex and subject to varying interpretations. Because of our numerous capital raises, which have included the issuance of various classes of convertible securities and warrants, uncertainty exists as to whether we may have undergone an ownership change in the past or will undergo one as a result of the Rights Offering. Even if the Rights Offering does not cause an ownership change, it may increase the likelihood that we may undergo an ownership change in the future. Based on our recent stock prices, we believe any ownership change would severely limit our ability to utilize the NOLs. Accordingly, no assurance can be given that our NOLs will be fully available. As a result, we could pay taxes earlier and in larger amounts than would be the case if the NOLs were available to reduce the federal income taxes without restriction.
Since the Warrants are executory contracts, they may have no value in a bankruptcy or reorganization proceeding.
In the event a bankruptcy or reorganization proceeding is commenced by or against us, a bankruptcy court may hold that any unexercised Warrants are executory contracts that are subject to rejection by us with the approval of the bankruptcy court. As a result, holders of the Warrants may, even if we have sufficient funds, not be entitled to receive any consideration for their Warrants or may receive an amount less than they would be entitled to if they had exercised their Warrants prior to the commencement of any such bankruptcy or reorganization proceeding.
48
Table of Contents
This prospectus and the documents incorporated by reference herein contain “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. When used in this prospectus, the words “expects,” “believes,” “hopes,” “anticipates,” “estimates,” “may,” “could,” “intends,” “exploring,” “evaluating,” “progressing,” “proceeding” and similar expressions are intended to identify forward-looking statements. These forward-looking statements do not constitute guarantees of future performance. Investors are cautioned that statements which are not strictly historical statements, including, without limitation, statements regarding current or future financial payments, costs, returns, royalties, performance and position, plans and objectives for future operations, plans and objectives for product development, plans and objectives for present and future clinical trials and results of such trials, plans and objectives for regulatory approval, litigation, intellectual property, product development, manufacturing plans and performance, management’s initiatives and strategies, and the development of the Company’s product candidates, Tcelna (imilecleucel-T) and OPX-212, constitute forward-looking statements. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated. These risks and uncertainties include, but are not limited to, those risks discussed in “Risk Factors,” as well as, without limitation, risks associated with:
| • | market conditions; |
| • | our capital position; |
| • | our ability to compete with larger, better financed pharmaceutical and biotechnology companies; |
| • | new approaches to the treatment of our targeted diseases; |
| • | our expectation of incurring continued losses; |
| • | our uncertainty of developing a marketable product; |
| • | our ability to raise additional capital to continue our development programs (including to undertake and complete any ongoing or further clinical studies for Tcelna or OPX-212); |
| • | our ability to maintain compliance with NASDAQ listing standards; |
| • | the success of our clinical trials (including the Phase IIb trial for Tcelna in SPMS which, depending upon results, may determine whether Merck Serono elects to exercise its Option to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS; |
| • | whether Merck Serono exercises its Option and, if so, whether we receive any development or commercialization milestone payments or royalties from Merck Serono pursuant to the Option; |
| • | our dependence (if Merck Serono exercises its Option) on the resources and abilities of Merck Serono for the further development of Tcelna; |
| • | the efficacy of Tcelna for any particular indication, such as for relapsing remitting MS or secondary progressive MS, and the efficacy of OPX-212 for neuromyelitis optica (NMO); |
| • | our ability to develop and commercialize products; |
| • | our ability to obtain required regulatory approvals; |
| • | our compliance with all Food and Drug Administration regulations; |
| • | our ability to obtain, maintain and protect intellectual property rights (including for Tcelna and OPX-212); |
| • | the risk of litigation regarding our intellectual property rights or the rights of third parties; |
| • | the success of third party development and commercialization efforts with respect to products covered by intellectual property rights that we may license or transfer; |
| • | our limited manufacturing capabilities; |
| • | our dependence on third-party manufacturers; |
| • | our ability to hire and retain skilled personnel; |
| • | our volatile stock price; and |
| • | other risks detailed in our filings with the SEC. |
49
Table of Contents
These forward-looking statements speak only as of the date made. We assume no obligation or undertaking to update any forward-looking statements to reflect any changes in expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. You should, however, review additional disclosures we make in our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the SEC.
50
Table of Contents
Assuming that all 28,776,419 Units are subscribed for in the Rights Offering, we estimate that the net proceeds from the Rights Offering will be approximately $17.8 million, based on the Subscription Price of $0.70 per Unit, after deducting expenses relating to this offering payable by us estimated at approximately $2.3 million, including dealer-manager fees and expenses and excluding any proceeds received upon exercise of any Warrants.
We intend to use the net proceeds from the exercise of Subscription Rights:
| • | to continue funding the ongoing Phase IIb “Abili-T” clinical study of Tcelna in patients with secondary progressive multiple sclerosis (SPMS); |
| • | to continue preclinical and manufacturing activities for OPX-212 in patients with neuromyelitis optica (NMO), and if such activities are successful, to file an investigational new drug application with the U.S. Food and Drug Administration to initiate a Phase 1/2 proof-of-concept study; and |
| • | for other general corporate purposes, including working capital, research and development, business development and operational purposes. |
Our management will retain broad discretion as to the allocation of the net proceeds from this offering. Until we use the net proceeds of this offering, we intend to invest the funds in short-term, interest bearing investments. Any proceeds received by us from the exercise of Warrants will be used for the same purposes and in the same manner.
51
Table of Contents
The following table presents our cash, cash equivalents and capitalization, as of December 31, 2014:
| • | on an actual basis; and |
| • | on a pro forma as adjusted basis to give effect to the sale by us in this Rights Offering of maximum of 28,776,419 Units (consisting of 28,776,419 shares of our common stock and Warrants to purchase an aggregate of 28,776,419 shares of common stock upon exercise), at the Subscription Price of $0.70, and our receipt of the net proceeds from that sale after deducting estimated offering expenses. |
The table below does not reflect the exercise of Warrants issued in connection with the Rights Offering. The pro forma as adjusted information set forth below is illustrative only and will be adjusted based on the number of Units sold. You should read this information in conjunction with our consolidated financial statements and notes thereto incorporated by reference into this prospectus.
| As of December 31, 2014 | ||||||||
| Actual | Pro Forma As Adjusted |
|||||||
| (Unaudited) | ||||||||
| Cash and cash equivalents |
$ | 9,906,373 | $ | 27,741,727 | ||||
|
|
|
|
|
|||||
| Long-term liabilities |
$ | 1,230,748 | $ | 1,230,748 | ||||
| Stockholders’ equity: |
||||||||
| Preferred stock, no par value, 10,000,000 shares authorized; none issued and outstanding, actual or pro forma as adjusted |
— | — | ||||||
| Common stock, $0.01 par value, 100,000,000 shares authorized; |
282,348 | 570,112 | ||||||
| Actual – 28,234,751 shares issued and outstanding; |
||||||||
| Pro forma as adjusted – 57,011,170 shares issued and outstanding |
||||||||
| Additional paid-in capital |
148,477,047 | 166,024,637 | ||||||
| Accumulated deficit |
(141,320,208) | (141,320,208) | ||||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
$ | 7,439,187 | $ | 25,274,541 | ||||
|
|
|
|
|
|||||
The information above is as of December 31, 2014 and excludes:
| • | 2,423,253 shares of common stock issuable upon the exercise of stock options outstanding at December 31, 2014 with a weighted average exercise price of $2.92 per share; |
| • | 3,046,801 shares of common stock issuable upon the exercise of outstanding warrants at December 31, 2014 with a weighted average exercise price of $4.08 per share; |
| • | 1,509,479 shares of common stock available for future grants under our 2010 Stock Incentive Plan at December 31, 2014; and |
| • | any additional shares of common stock that we may issue to Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to a $1,500,000 purchase agreement and a $15,000,000 purchase agreement we entered into on November 5, 2012 and November 2, 2012, respectively, which provides that, upon the terms and subject to the conditions and limitation set forth therein, Lincoln Park is committed to purchase up to an aggregate of an additional $15.9 million of shares of our common stock over the term of the purchase agreements, should we elect to sell shares to Lincoln Park. |
52
Table of Contents
Purchasers of our common stock in the Rights Offering (and upon exercise of the Warrants issued pursuant to this Rights Offering) will experience an immediate dilution of the net tangible book value per share of our common stock. Our net tangible book value as of December 31, 2014 was approximately $5,543,201, or $0.20 per share of our common stock (based upon 28,234,751 shares of our common stock outstanding). Net tangible book value per share is equal to our total net tangible book value, which is our total tangible assets less our total liabilities, divided by the number of shares of our outstanding common stock. Dilution per share equals the difference between the amount per share paid by purchasers of shares of common stock in the Rights Offering and the net tangible book value per share of our common stock immediately after the Rights Offering.
Based on the sale by us in this Rights Offering of a maximum of 28,776,419 Units (consisting of 28,776,419 shares of our common stock and Warrants to purchase an aggregate of 28,776,419 shares of common stock upon exercise), at the Subscription Price of $0.70 per Unit, and after deducting estimated offering expenses and dealer-manager fees and expenses payable by us of $2,308,139, and the application of the estimated $17,835,354 of net proceeds from the Rights Offering, our pro forma net tangible book value as of December 31, 2014 would have been approximately $23,378,555, or $0.41 per share. This represents an immediate increase in pro forma net tangible book value to existing shareholders of $0.21 per share and an immediate dilution to purchasers in the Rights Offering of $0.29 per share.
The following table illustrates this per-share dilution (assuming a fully subscribed for Rights Offering of 28,776,419 Units at the Subscription Price of $0.70 per Unit, but excluding any issuance of shares of common stock upon exercise of Warrants):
| Subscription Price |
$ | 0.70 | ||||||
| Net tangible book value per share as of December 31, 2014, before Rights Offering |
$ | 0.20 | ||||||
| Increase in net tangible book value per share attributable to Rights Offering |
0.21 | |||||||
| Pro forma net tangible book value per share as of December 31, 2014, after giving effect to Rights Offering |
0.41 | |||||||
| Dilution in net tangible book value per share to purchasers |
$ | 0.29 | ||||||
The information above is as of December 31, 2014 and excludes:
| • | 2,423,253 shares of common stock issuable upon the exercise of stock options outstanding at December 31, 2014 with a weighted average exercise price of $2.92 per share; |
| • | 3,046,801 shares of common stock issuable upon the exercise of outstanding warrants at December 31, 2014 with a weighted average exercise price of $4.08 per share; |
| • | 1,509,479 shares of common stock available for future grants under our 2010 Stock Incentive Plan at December 31, 2014; and |
| • | any additional shares of common stock that we may issue to Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to a $1,500,000 purchase agreement and a $15,000,000 purchase agreement we entered into on November 5, 2012 and November 2, 2012, respectively, which provides that, upon the terms and subject to the conditions and limitation set forth therein, Lincoln Park is committed to purchase up to an aggregate of an additional $15.9 million of shares of our common stock over the term of the purchase agreements, should we elect to sell shares to Lincoln Park. |
53
Table of Contents
MARKET PRICE OF OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Our common stock is traded on The NASDAQ Capital Market under the symbol “OPXA.” Our common stock has, from time to time, traded on a limited, sporadic and volatile basis. The table below shows the high and low sales prices for our common stock for the periods indicated, as reported by Bloomberg.
| Price Ranges | ||||
| High | Low | |||
| Fiscal Quarter Ended March 31, 2015 |
||||
| First Quarter (through February 20, 2015) |
$0.90 | $0.68 | ||
| Fiscal Year Ended December 31, 2014 |
||||
| First Quarter |
$2.20 | $1.64 | ||
| Second Quarter |
1.93 | 1.26 | ||
| Third Quarter |
1.71 | 0.85 | ||
| Fourth Quarter |
1.07 | 0.70 | ||
| Fiscal Year Ended December 31, 2013 |
||||
| First Quarter |
$5.19 | $1.09 | ||
| Second Quarter |
2.44 | 1.44 | ||
| Third Quarter |
3.70 | 1.25 | ||
| Fourth Quarter |
2.56 | 1.65 | ||
The closing price of our common stock on February 20, 2015 was $0.73 per share. As of February 20, 2015, there were approximately 175 holders of record of our common stock, excluding shareholders for whom shares are held in “nominee” or “street name.”
We have never declared or paid any cash dividends on our common stock and we do not intend to pay cash dividends in the foreseeable future. We expect as of the date hereof to retain any future earnings to fund the operation and expansion of our business.
54
Table of Contents
The Subscription Rights
We are distributing to the record holders and to holders of our outstanding Series L warrants (who are entitled to participate in this offering pursuant to the terms of such warrants), at no charge, non-transferable Subscription Rights to purchase one Unit at a subscription price of $0.70 per Unit. Each Basic Subscription Right will entitle you to purchase one share of our common stock and a Warrant for the purchase of one additional share of our common stock at an exercise price of (i) $0.50 per share from the date of issuance through June 30, 2016 and (ii) $1.50 per share from July 1, 2016 through the expiration three years from the date of issuance. Each record holder will receive one Subscription Right for each whole share of our common stock owned by such record holder as of the Record Date. Each holder of an outstanding Series L warrant will receive one Subscription Right for each whole share of our common stock into which such Series L warrant is exercisable on the Record Date. Each Subscription Right entitles the record holder or holder of a Series L warrant to a Basic Subscription Right and an Over-Subscription Privilege.
Basic Subscription Rights
Your Basic Subscription Rights will entitle you to purchase one share of our common stock and a Warrant to purchase one share of our common stock. For example, if you owned 100 shares of common stock as of the Record Date, you will receive 100 Subscription Rights and will have the right to purchase 100 shares of our common stock and Warrants to purchase 100 shares of our common stock for $0.70 per whole Unit, or a total payment of $70.00. You may exercise all or a portion of your Basic Subscription Rights, or you may choose not to exercise any of your Basic Subscription Rights. If you do not exercise your Basic Subscription Rights in full, you will not be entitled to exercise your Over-Subscription Privilege.
Over-Subscription Privilege
If you exercise your Basic Subscription Rights in full, you may also choose to exercise your Over-Subscription Privilege. Subject to proration and stock ownership limitations, if applicable, we will seek to honor the Over-Subscription Privilege requests in full. If Over-Subscription Privilege requests exceed the number of Units available, however, we will allocate the available Units pro rata among the record holders and Series L warrant holders exercising the Over-Subscription Privilege in proportion to the number of shares of our common stock each of those record holders owned or the number of shares underlying Series L warrants held by each of those warrant holders on the Record Date, relative to the number of shares owned or underlying Series L warrants on the Record Date by all record holders and Series L warrant holders exercising the Over-Subscription Privilege. If this pro rata allocation results in any record holder or Series L warrant holder receiving a greater number of Units than the record holder or Series L warrant holder subscribed for pursuant to the exercise of the Over-Subscription Privilege, then such record holder or Series L warrant holder will be allocated only that number of Units for which the record holder or Series L warrant holder oversubscribed, and the remaining Units will be allocated among all other record holders and Series L warrant holders exercising the Over-Subscription Privilege on the same pro rata basis described above. The proration process will be repeated until all Units have been allocated.
Continental Stock Transfer & Trust Company, the Subscription Agent for the Rights Offering, will determine the over-subscription allocation based on the formula described above.
To the extent the aggregate subscription payment of the actual number of unsubscribed Units available to you pursuant to the Over-Subscription Privilege is less than the amount you actually paid in connection with the exercise of the Over-Subscription Privilege, you will be allocated only the number of unsubscribed Units available to you, and any excess subscription payments will be returned to you, without interest or penalty, with 10 business days after expiration of the Rights Offering.
55
Table of Contents
We can provide no assurances that you will actually be entitled to purchase the number of Units issuable upon the exercise of your Over-Subscription Privilege in full at the expiration of the Rights Offering. We will not be able to satisfy any requests for Units pursuant to the Over-Subscription Privilege if all of our shareholders and Series L warrant holders exercise their Basic Subscription Rights in full, and we will only honor an Over-Subscription Privilege to the extent sufficient Units are available following the exercise of Basic Subscription Rights.
Our Participating Warrant Holders
The terms and conditions of our currently outstanding Series L warrants to purchase shares of our common stock entitle the holders of these warrants to participate in this Rights Offering. None of our other currently outstanding warrants is entitled to receive Subscription Rights in this offering.
The Series L warrants were originally issued in a private placement of shares of our common stock and warrants on February 11, 2013. Series L warrants to purchase an aggregate of 541,668 shares of common stock at an exercise price of $3.00 per share are currently outstanding. These warrants expire on February 11, 2017. The Series L warrants provide that the holders are entitled to participate in a rights offering as if each of such warrants had been exercised immediately prior to the record date for a rights offering. As a result, holders of our Series L warrants are receiving Subscription Rights for an aggregate of 541,668 Units in connection with this Rights Offering.
Limitation on the Purchase of Units
You may only purchase the number of whole Units purchasable upon exercise of the number of Basic Subscription Rights distributed to you in the Rights Offering, plus the Over-Subscription Privilege, if any. Accordingly, the number of Units that you may purchase in the Rights Offering is limited by the number of shares of our common stock you held on the Record Date or the number of shares underlying your outstanding warrants and by the extent to which other shareholders and warrant holders exercise their Basic Subscription Rights and Over-Subscription Privileges, which we cannot determine prior to completion of the Rights Offering. However, due to stock exchange restrictions, we will not issue Units in the Rights Offering to the extent that a holder would beneficially own, together with any other person with whom such holder’s securities may be aggregated under applicable law, more than 19.9% of our outstanding shares of common stock.
Subscription Price
The Subscription Price is $0.70 per Unit. The Subscription Price does not necessarily bear any relationship to our past or expected future results of operations, cash flows, current financial condition, or any other established criteria for value. No change will be made to the Subscription Price by reason of changes in the trading price of our common stock or other factor prior to the expiration of this Rights Offering.
Determination of Subscription Price
In the determining the Subscription Price, the board of directors considered a variety of factors including those listed below:
| • | our need to raise capital in the near term to continue our operations; |
| • | the current and historical trading prices of our common stock; |
| • | a price that would increase the likelihood of participation in the Rights Offering; |
| • | the cost of capital from other sources; |
| • | the value of the Warrant being issued as a component of the Unit; |
56
Table of Contents
| • | comparable precedent transactions, including the percentage of shares offered, the terms of the subscription rights being offered, the subscription price and the discount that the subscription price represents to the immediately prevailing closing prices for these offerings; |
| • | an analysis of stock price trading multiples for companies similar to us that, among other things, did not need to raise capital in the near-term; and |
| • | our most recently forecasted revenue relative to our peer group. |
The Subscription Price does not necessarily bear any relationship to any established criteria for value. No valuation consultant or investment banker has opined upon the fairness or adequacy of the Subscription Price. You should not consider the Subscription Price as an indication of actual value of our company or our common stock. You should not assume or expect that, after the Rights Offering, our shares of common stock will trade at or above the Subscription Price in any given time period. The market price of our common stock may decline during or after the Rights Offering. We cannot assure you that you will be able to sell the shares of our common stock purchased during the Rights Offering at a price equal to or greater than the Subscription Price. You should obtain a current price quote for our common stock before exercising your Subscription Rights and make your own assessment of our business and financial condition, our prospects for the future, and the terms of this Rights Offering. Once made, all exercises of Subscription Rights are irrevocable.
No Recombination
The common stock and Warrants comprising the Units will separate upon the exercise of the Subscription Rights, and the Units will not trade as a separate security. Holders may not recombine shares of common stock and Warrants to receive a Unit.
Non-Transferability of Subscription Rights
The Subscription Rights are non-transferable (other than by operation of law) and, therefore, you may not sell, transfer, assign or give away your Subscription Rights to anyone. The Subscription Rights will not be listed for trading on any stock exchange or market.
Expiration Date; Extension
The subscription period, during which you may exercise your Subscription Rights, expires at 5:00 p.m., Eastern Time, on April 8, 2015, which is the expiration of the Rights Offering. If you do not exercise your Subscription Rights before that time, your Subscription Rights will expire and will no longer be exercisable. We will not be required to issue shares to you if the Subscription Agent receives your Rights Certificate or your subscription payment after that time. We have the option to extend the Rights Offering in our sole discretion, although we do not presently intend to do so. We may extend the Rights Offering by giving oral or written notice to the Subscription Agent before the Rights Offering expires. If we elect to extend the Rights Offering, we will issue a press release announcing the extension no later than 9:00 a.m., Eastern Time, on the next business day after the most recently announced expiration date of the Rights Offering.
If you hold your shares of common stock in the name of a broker, dealer, custodian bank or other nominee, the nominee will exercise the Subscription Rights on your behalf in accordance with your instructions. Please note that the nominee may establish a deadline that may be before 5:00 p.m., Eastern Time, on April 8, 2015, which is the expiration date that we have established for the Rights Offering.
Termination
We may terminate the Rights Offering at any time and for any reason prior to the completion of the Rights Offering. If we terminate the Rights Offering, we will issue a press release notifying shareholders, warrant holders and the public of the termination.
57
Table of Contents
Return of Funds upon Completion or Termination
The Subscription Agent will hold funds received in payment for shares in a segregated account pending completion of the Rights Offering. The Subscription Agent will hold this money until the Rights Offering is completed or is terminated. To the extent you properly exercise your Over-Subscription Privilege for an amount of Units that exceeds the number of unsubscribed Units available to you, any excess subscription payments will be returned to you within 10 business days after the expiration of the Rights Offering, without interest or penalty. If the Rights Offering is terminated for any reason, all subscription payments received by the Subscription Agent will be returned within 10 business days, without interest or penalty.
Shares of Our Common Stock Outstanding After the Rights Offering
Based on 28,234,751 shares of common stock outstanding as of December 31, 2014, as well as Series L warrants to purchase 541,668 shares of common stock outstanding on that date, assuming no other transactions by us involving our common stock prior to the expiration of the Rights Offering, and if the Rights Offering is fully subscribed, we will have 57,011,170 shares of common stock issued and outstanding, and Warrants to purchase an additional 28,776,419 shares of our common stock (excluding the 3,046,801 currently outstanding warrants). The exact number of shares and Warrants that we will issue in this offering will depend on the number of Units that are subscribed for in the Rights Offering.
Methods for Exercising Subscription Rights
The exercise of Subscription Rights is irrevocable and may not be cancelled or modified. You may exercise your Subscription Rights as follows:
Subscription by Record Holders
If you are a shareholder of record or a holder of Series L warrants, the number of Units you may purchase pursuant to your Subscription Rights in indicated on the enclosed Rights Certificate. You may exercise your Subscription Rights by properly completing and executing the Rights Certificate and forwarding it, together with your full payment, to the Subscription Agent at the address given below under “Subscription Agent,” to be received before 5:00 p.m., Eastern Time, on April 8, 2015.
Subscription by Beneficial Owners
If you are a beneficial owner of shares of our common stock that are registered in the name of a broker, dealer, custodian bank, or other nominee, you will not receive a Rights Certificate. Instead, we will issue one Subscription Right to such nominee record holder for all shares of our common stock held by such nominee at the Record Date. If you are not contacted by your nominee, you should promptly contact your nominee in order to subscribe for shares in the Rights Offering and follow the instructions provided by your nominee.
To properly exercise your Over-Subscription Privilege, you must deliver the subscription payment related to your Over-Subscription Privilege before the Rights Offering expires. Because we will not know the total number of unsubscribed Units before the Rights Offering expires, if you wish to maximize the number of shares you purchase pursuant to your Over-Subscription Privilege, you will need to deliver payment in an amount equal to the aggregate subscription payment for the maximum number of Units that you wish to purchase.
Payment Method
Payments must be made in full in U.S. currency by personal check, certified check or bank draft, or by wire transfer, and payable to “Continental Stock Transfer & Trust Company, as Subscription Agent for Opexa Therapeutics, Inc.” You must timely pay the full subscription payment, including payment for the Over-
58
Table of Contents
Subscription Privilege, for the full number of shares of our common stock you wish to acquired pursuant to the exercise of Subscription Rights by delivering a:
| • | cashier’s, certified or personal check drawn against a U.S. bank payable to “Continental Stock Transfer & Trust Company, as Subscription Agent for Opexa Therapeutics, Inc.”; |
| • | U.S. Postal money order payable to “Continental Stock Transfer & Trust Company, as Subscription Agent for Opexa Therapeutics, Inc.”; or |
| • | wire transfer of immediately available funds directly to the account maintained by Continental Stock Transfer & Trust Company, as Subscription Agent, for purposes of accepting subscriptions in this Rights Offering at JP Morgan Chase, ABA, #021000021, Account # 475-583698 FBO Opexa Therapeutics, Inc., with reference to the name of the Rights holder. |
If you elect to exercise your Subscription Rights, you should consider using a wire transfer or certified check drawn on a U.S. bank to ensure that the Subscription Agent receives your funds before the Rights Offering expires. If you send a personal check, payment will not be deemed to have been received by the Subscription Agent until the check has cleared. The clearinghouse may require five or more business days to clear a personal check. Accordingly, holders who wish to pay the Subscription Price by means of a personal check should make payment sufficiently in advance of the expiration of the Rights Offering to ensure that the payment is received and clears by that date. If you send a certified check, payment will be deemed to have been received by the Subscription Agent immediately upon receipt of such instrument.
You should read the instruction letter accompanying the Rights Certificate carefully and strictly follow it. DO NOT SEND RIGHTS CERTIFICATES OR PAYMENTS DIRECTLY TO US. We will not consider your subscription received until the Subscription Agent has received delivery of a properly completed and duly executed Rights Certificate and payment of the full subscription payment.
The method of delivery of Rights Certificates and payment of the subscription payment to the Subscription Agent will be at the risk of the holders of Subscription Rights. If sent by mail, we recommend that you send those certificates and payments by registered mail, properly insured, with return receipt requested, or by overnight courier, and that you allow a sufficient number of days to ensure delivery to the Subscription Agent and clearance of payment before the Rights Offering expires.
Missing or Incomplete Subscription Forms or Payment
If you fail to complete and sign the Rights Certificate or otherwise fail to follow the subscription procedures that apply to the exercise of your Subscription Rights before the Rights Offering expires, the Subscription Agent will reject your subscription or accept it to the extent of the payment received. Neither we nor our Subscription Agent undertakes any responsibility or action to contact you concerning an incomplete or incorrect subscription form, nor are we under any obligation to correct such forms. We have the sole discretion to determine whether a subscription exercise properly complies with the subscription procedures.
If you send a payment that is insufficient to purchase the number of shares you requested, or if the number of shares you requested is not specified in the forms, the payment received will be applied to exercise your Subscription Rights to the fullest extent possible based on the amount of the payment received. Any excess subscription payments received by the Subscription Agent will be returned, without interest or penalty, within 10 business days following the expiration of the Rights Offering.
Issuance of common stock and Warrants
The shares of common stock and Warrants that are purchased in the Rights Offering as part of the Units will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of these securities if you are a holder of record of
59
Table of Contents
shares or Series L warrants. If you hold your shares of common stock in the name of a custodian bank, broker, dealer, or other nominee, DTC will credit your account with your nominee with the securities you purchased in the Rights Offering.
Subscription Agent
The Subscription Agent for the Rights Offering is Continental Stock Transfer & Trust Company. The address to which Rights Certificates and payments should be mailed or delivered by overnight courier is provided below. If sent by mail, we recommend that you send documents and payments by registered mail, properly insured, with return receipt requested, and that you allow a sufficient number of days to ensure delivery to the Subscription Agent and clearance or payment before the Rights Offering expires. Do not send or deliver these materials to us.
Continental Stock Transfer & Trust Company
17 Battery Place—8th Floor
New York, NY 10004
Attn: Corporate Actions Department
Telephone: (917) 262-2378
If you deliver the Rights Certificates in a manner different than that described in this prospectus, we may not honor the exercise of your Subscription Rights.
Information Agent
You should direct any questions or requests for assistance concerning the method of subscribing for the shares of our common stock or for additional copies of this prospectus to the Information Agent as follows:
Advantage Proxy, Inc.
(877) 870-8565 (toll free)
(206) 870-8565 (collect)
No Fractional Shares
We will not issue fractional shares of common stock in the Rights Offering. Rights holders will only be entitled to purchase a number of Units representing a whole number of shares of common stock, rounded down to the nearest whole number of Units a holder would otherwise be entitled to purchase. Any excess subscription payments received by the Subscription Agent will be returned within 10 business days after expiration of the Rights Offering, without interest or penalty. Similarly, no fractional shares of common stock will be issued in connection with the exercise of a Warrant. Instead, for any such fractional share that would otherwise have been issuable upon exercise of the Warrant, the holder will be entitled to a cash payment equal to the pro-rated per share market price of the common stock on the last trading day preceding the exercise.
Notice to Brokers and Nominees
If you are a broker, dealer, bank, or other nominee holder that holds shares of our common stock for the account of others on the Record Date, you should notify the beneficial owners of the shares for whom you are the nominee of the Rights Offering as soon as possible to learn their intentions with respect to exercising their Subscription Rights. If a beneficial owner of our common stock so instructs, you should complete the Rights Certificate and submit it to the Subscription Agent with the proper subscription payment by the expiration date. You may exercise the number of Subscription Rights to which all beneficial owners in the aggregate otherwise would have been entitled had they been direct holders of our common stock on the Record Date, provided that you, as a nominee record holder, make a proper showing to the Subscription Agent by submitting the form entitled “Nominee Holder Certification,” which is provided with your Rights Offering materials. If you did not receive this form, you should contact our Subscription Agent to request a copy.
60
Table of Contents
Validity of Subscriptions
We will resolve all questions regarding the validity and form of the exercise of your Subscription Rights, including time of receipt and eligibility to participate in the Rights Offering. Our determination will be final and binding. Once made, subscriptions are irrevocable; we will not accept any alternative, conditional, or contingent subscriptions. We reserve the absolute right to reject any subscriptions not properly submitted or the acceptance of which would be unlawful. You must resolve any irregularities in connection with your subscriptions before the expiration date of the Rights Offering, unless we waive them in our sole discretion. Neither we nor the Subscription Agent is under any duty to notify you or your representative of defects in your subscriptions. A subscription will be considered accepted, subject to our right to withdraw or terminate the Rights Offering, only when the Subscription Agent receives a properly completed and duly executed Rights Certificate and any other required documents and the full subscription payment including final clearance of any personal check. Our interpretations of the terms and conditions of the Rights Offering will be final and binding.
Stockholder Rights
You will have no rights as a holder of the shares of our common stock you purchase in the Rights Offering until shares are issued in book-entry form or your account at your broker, dealer, bank, or other nominee is credited with the shares of our common stock purchased in the Rights Offering. Holders of Warrants issued in connection with the Rights Offering will not have rights as holders of our common stock until such Warrants are exercised and the shares of common stock underlying the Warrants are issued to the holder.
Foreign Shareholders
We will not mail this prospectus or Rights Certificates to shareholders with addresses that are outside the United States or that have an army post office or foreign post office address. The Subscription Agent will hold these Rights Certificates for their account. To exercise Subscription Rights, our foreign shareholders must notify the Subscription Agent prior to 5:00 p.m., Eastern Time, on April 3, 2015, the third business day prior to the expiration date, of your exercise of Subscription Rights and provide evidence satisfactory to us, such as a legal opinion from local counsel, that the exercise of such Subscription Rights does not violate the laws of the jurisdiction in which such shareholder resides and payment by a U.S. bank in U.S. dollars before the expiration of the offer. If no notice is received by such time or the evidence presented is not satisfactory to us, the Subscription Rights represented thereby will expire.
No Revocation or Change
Once you submit the Rights Certificate or have instructed your nominee of your subscription request, you are not allowed to revoke or change the exercise or request a refund of monies paid. All exercises of Subscription Rights are irrevocable, even if you learn information about us that you consider to be unfavorable. You should not exercise your Subscription Rights unless you are certain that you wish to purchase shares at the Subscription Price.
U.S. Federal Income Tax Treatment of Rights Distribution
For U.S. federal income tax purposes, we do not believe holders of shares of our common stock or Series L warrants should recognize income or loss upon receipt or exercise of a Subscription Right. See “Material U.S. Federal Income Tax Consequences.”
No Recommendation to Rights Holders
Our board of directors is not making a recommendation regarding your exercise of the Subscription Rights. Stockholders who exercise Subscription Rights risk investment loss on money invested. We cannot assure you that the market price of our common stock will reach or exceed the Subscription Price, and even if it does so, that it will not decline during or after the Rights Offering. We also cannot assure you that you will be
61
Table of Contents
able to sell shares of our common stock or Warrants purchased in the Rights Offering at a price equal to or greater than the Subscription Price. You should make your investment decision based on your assessment of our business and financial condition, our prospects for the future and the terms of this Rights Offering. Please see “Risk Factors” for a discussion of some of the risks involved in investing in our common stock.
Fees and Expenses
We will pay all fees charged by the Subscription Agent and the Information Agent, and by the dealer-managers. You are responsible for paying any other commissions, fees, taxes or other expenses incurred in connection with the exercise of your Subscription Rights.
Listing
The Subscription Rights may not be sold, transferred, assigned or given away to anyone, and will not be listed for trading on any stock exchange or market. We have applied to have the Warrants listed for trading on NASDAQ under the symbol “OPXAW,” however, there is no assurance that a sufficient number of Subscription Rights will be exercised so that the Warrants will meet the minimum listing criteria to be accepted for listing on NASDAQ. The shares of our common stock, including the shares to be issued in the Rights Offering and the shares underlying the Warrants to be issued in the Rights Offering, are traded on NASDAQ under the symbol “OPXA.”
Important
Do not send Rights Certificates directly to us. You are responsible for choosing the payment and delivery method for your Rights Certificate and you bear the risks associated with such delivery. If you choose to deliver your Rights Certificate and payment by mail, we recommend that you use registered mail, properly insured, with return receipt requested. We also recommend that you allow a sufficient number of days to ensure delivery to the Subscription Agent and clearance of payment prior to the expiration time.
Distribution Arrangements
Maxim Group LLC and National Securities Corp. are the dealer-managers for the Rights Offering. The dealer-managers will provide marketing assistance and advice to us in connection with the Rights Offering and will use their best efforts to solicit the exercise of Subscription Rights and participation in the Over-Subscription Privilege. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants to be issued in the Rights Offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares or Warrants. We have agreed to pay the dealer-managers certain fees and to reimburse the dealer-managers for certain out-of-pocket expenses incurred in connection with this offering. See “Plan of Distribution.”
62
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following discussion is a summary of material U.S. federal income tax consequences relating to the receipt and exercise (or expiration) of the Subscription Rights acquired through the Rights Offering and the ownership and disposition of shares of our common stock and Warrants received upon exercise of the Subscription Rights or Warrants.
This summary deals only with Subscription Rights acquired through the Rights Offering, shares of our common stock and Warrants acquired upon exercise of Subscription Rights and shares of our common stock acquired upon exercise of the Warrants, in each case, that are held as capital assets by a beneficial owner. This discussion does not address all aspects of U.S. federal income taxation that may be relevant to such beneficial owners in light of their personal circumstances. This discussion also does not address tax consequences to holders that may be subject to special tax rules, including, without limitation, insurance companies, real estate investment trusts, regulated investment companies, grantor trusts, tax-exempt organizations, employee stock purchase plans, partnerships and other pass-through entities, persons holding Subscription Rights, shares of our common stock, Series L warrants or Warrants as part of a hedging, integrated, conversion or constructive sale transaction or a straddle, financial institutions, brokers, dealers in securities or currencies, traders that elect to mark-to-market their securities, persons that acquired Subscription Rights, shares of our common stock, Series L warrants or Warrants in connection with employment or other performance of services, U.S. Holders (as defined below) that have a functional currency other than the U.S. dollar, U.S. expatriates, and certain former citizens or residents of the United States. In addition, the discussion does not describe any tax consequences arising out of the tax laws of any state, local or foreign jurisdiction, or any U.S. federal tax considerations other than income taxation (such as Medicare contribution taxation or estate, generation skipping or gift taxation).
The discussion below is based upon the provisions of the Internal Revenue Code of 1986, as amended, or the Code, and regulations, rulings and judicial decisions thereunder, as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively. We have not sought, and will not seek, any rulings from the Internal Revenue Service, or the IRS, regarding the matters discussed below. There can be no assurance that the IRS or a court will not take positions concerning the tax consequences of the receipt of Subscription Rights acquired through the Rights Offering by persons holding shares of our common stock or Series L warrants, the exercise (or expiration) of the Subscription Rights, the acquisition, ownership and disposition of shares of our common stock and the acquisition, ownership and disposition (or expiration) of Warrants acquired upon exercise of the Subscription Rights that are different from those discussed below.
As used herein, a “U.S. Holder” means a beneficial owner of shares of our common stock, Series L warrants, Subscription Rights, shares of our common stock and Warrants acquired upon exercise of Subscription Rights or shares of our common stock acquired upon exercise of Warrants, as the case may be, that is for U.S. federal income tax purposes: (1) an individual who is a citizen or resident of the United States; (2) a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States or any state thereof or the District of Columbia; (3) an estate the income of which is subject to U.S. federal income taxation regardless of its source; or (4) a trust (a) the administration of which is subject to the primary supervision of a court within the United States and one or more United States persons as described in Section 7701(a)(30) of the Code have authority to control all substantial decisions of the trust, or (b) that has a valid election in effect to be treated as a United States person. A “Non-U.S. Holder” is such a beneficial owner (other than an entity or arrangement that is treated as a partnership for U.S. federal income tax purposes) that is not a U.S. Holder.
If any entity or arrangement that is treated as a partnership for U.S. federal income tax purposes is such a beneficial owner, the U.S. federal income tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. Holders that are partnerships (and partners in such partnerships) are urged to consult their own tax advisors.
63
Table of Contents
HOLDERS OF SHARES OF OUR COMMON STOCK AND SERIES L WARRANTS SHOULD CONSULT THEIR OWN TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AND THE CONSEQUENCES UNDER FEDERAL ESTATE AND GIFT TAX LAWS, FOREIGN, STATE, AND LOCAL LAWS AND TAX TREATIES OF THE RECEIPT, OWNERSHIP AND EXERCISE OF SUBSCRIPTION RIGHTS AND THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF SHARES OF OUR COMMON STOCK AND WARRANTS ACQUIRED UPON EXERCISE OF SUBSCRIPTION RIGHTS AND SHARES OF OUR COMMON STOCK ACQUIRED UPON EXERCISE OF WARRANTS.
Tax Consequences to U.S. Holders
Taxation of Subscription Rights
Receipt of Subscription Rights
Although the authorities governing transactions such as this Rights Offering are complex and do not speak directly to the consequences of certain aspects of this Rights Offering, including the inclusion of the right to purchase Warrants in the Subscription Rights (rather than the right to purchase only shares of our common stock), the distribution of Subscription Rights to Series L warrant holders and the effects of the Over-Subscription Privilege, we do not believe your receipt of Subscription Rights pursuant to the Rights Offering should be treated as a taxable distribution with respect to your existing shares of common stock or Series L warrants for U.S. federal income tax purposes. Section 305(a) of the Code states that a shareholder’s taxable income does not include in-kind stock dividends; however, the general non-recognition rule in Section 305(a) is subject to exceptions in Section 305(b), which include “disproportionate distributions.” A disproportionate distribution is a distribution or a series of distributions, including deemed distributions, that has the effect of the receipt of cash or other property by some shareholders or holders of debt instruments convertible into stock and an increase in the proportionate interest of other shareholders in a corporation’s assets or earnings and profits. During the last 36 months, we have not made any distributions of cash or non-stock property with respect to: (i) our common stock or (ii) our options or warrants to acquire common stock. Within the past 36 months, we did, however, make a payment in cash of the accrued interest on certain previously outstanding convertible notes upon their retirement. Currently we do not have any convertible debt outstanding nor do we currently intend to issue any convertible debt or pay any dividends on our common stock (other than the issuance of the Subscription Rights in connection with this offering). Further, the payment of cash interest upon retirement of the previously outstanding convertible notes and the distribution of the Subscription Rights hereunder are unrelated isolated transactions which are not part of a plan to increase any shareholder’s proportionate interest in our earnings and profits or assets.
Our position regarding the tax-free treatment of the Subscription Right distribution is not binding on the IRS, or the courts. If this position is finally determined by the IRS or a court to be incorrect, whether on the basis that the issuance of the Subscription Rights is a “disproportionate distribution” or otherwise, the fair market value of the Subscription Rights would be taxable to holders of our common stock as a dividend to the extent of the holder’s pro rata share of our current and accumulated earnings and profits, if any, with any excess being treated as a return of capital to the extent thereof and then as capital gain. Although no assurance can be given, it is anticipated that we will not have current and accumulated earnings and profits through the end of 2015. Further, if our position is incorrect, the treatment of holders of Series L warrants in that case is not clear, and it may differ from the treatment of the Subscription Right distribution to the holders of our common stock. The Series L warrant holders may be treated in a manner similar to holders of our common stock but it is possible that they may be subject to different and adverse U.S. federal income tax consequences.
The following discussion is based upon the treatment of the Subscription Right issuance as a non-taxable distribution with respect to your existing shares of common stock or Series L warrants for U.S. federal income tax purposes.
64
Table of Contents
Tax Basis in the Subscription Rights
If the fair market value of the Subscription Rights you receive is less than 15% of the fair market value of your existing shares of common stock or Series L warrants (with respect to which the Subscription Rights are distributed) on the date you receive the Subscription Rights, the Subscription Rights will be allocated a zero basis for U.S. federal income tax purposes, unless you elect to allocate your basis in your existing shares of common stock or Series L warrants between your existing shares of common stock or Series L warrants and the Subscription Rights in proportion to the relative fair market values of the existing shares of common stock or Series L warrants and the Subscription Rights determined on the date of receipt of the Subscription Rights. If you choose to allocate basis between your existing common shares or Series L warrants and the Subscription Rights, you must make this election on a statement included with your timely filed tax return (including extensions) for the taxable year in which you receive the Subscription Rights. Such an election is irrevocable.
However, if the fair market value of the Subscription Rights you receive is 15% or more of the fair market value of your existing shares of common stock or Series L warrants on the date you receive the Subscription Rights, then you must allocate your basis in your existing shares of common stock or Series L warrants between those shares or Series L warrants and the Subscription Rights you receive in proportion to their fair market values determined on the date you receive the Subscription Rights.
The fair market value of the Subscription Rights on the date that the Subscription Rights are distributed is uncertain, and we have not obtained, and do not intend to obtain, an appraisal of the fair market value of the Subscription Rights on that date. In determining the fair market value of the Subscription Rights, you should consider all relevant facts and circumstances, including any difference between the Subscription Price of the Subscription Rights and the trading price of our shares of common stock on the date that the Subscription Rights are distributed, the exercise price of the Series L warrants, the length of the period during which the Subscription Rights may be exercised and the fact that the Subscription Rights are non-transferable.
Exercise of Subscription Rights
Generally, you will not recognize gain or loss upon the exercise of a Subscription Right in the Rights Offering. Your adjusted tax basis, if any, in the Subscription Right plus the Subscription Price should be allocated between the new common share and Warrant acquired upon exercise of the Subscription Right in proportion to their relative fair market values on the exercise date. This allocation will establish your initial tax basis for U.S. federal income tax purposes in your new common shares and Warrants. The holding period of a share of common stock or Warrant acquired upon exercise of a Subscription Right in the Rights Offering will begin on the date of exercise.
If you exercise a Subscription Right received in the Rights Offering after disposing of the shares of our common stock or Series L warrants with respect to which such Subscription Right is received, then certain aspects of the tax treatment of the exercise of the Subscription Right are unclear, including (1) the allocation of the tax basis between the shares of common stock or Series L warrants previously sold and the Subscription Right, (2) the impact of such allocation on the amount and timing of gain or loss recognized with respect to the shares of our common stock or Series L warrants previously sold, and (3) the impact of such allocation on the tax basis of the shares of our common stock and Series L warrants acquired upon exercise of the Subscription Right. If you exercise a Subscription Right received in the Rights Offering after disposing of shares of our common stock or Series L warrants with respect to which the Subscription Right is received, you should consult with your tax advisor.
Expiration of Subscription Rights
If you allow Subscription Rights received in the Rights Offering to expire, you should not recognize any gain or loss for U.S. federal income tax purposes, and you should re-allocate any portion of the tax basis in your existing common shares or Series L warrants previously allocated to the Subscription Rights that have expired to the existing common shares or Series L warrants.
65
Table of Contents
Taxation of Warrants
Sale or other Taxable Disposition of Warrants
Upon the sale, exchange or other taxable disposition of a Warrant, in general, you will recognize taxable gain or loss measured by the difference, if any, between (i) the amount of cash and the fair market value of any property received upon such taxable disposition, and (ii) your adjusted tax basis in the Warrant as allocated pursuant to the rules discussed above. Your gain or loss generally will be capital gain or loss and generally will be long-term capital gain or loss if, at the time of the sale or other disposition, your holding period for the Warrant is more than one year. The deductibility of capital losses is subject to limitations.
Exercise of Warrants
Upon the exercise of a Warrant for cash, in general, you will not recognize gain or loss for U.S. federal income tax purposes, except to the extent you receive a cash payment for any such fractional share that would otherwise have been issuable upon exercise of the Warrant. Your initial tax basis in common stock received will equal your adjusted tax basis in the Warrant exercised, increased by the amount of cash paid to exercise the Warrant and decreased by the adjusted tax basis allocable to any fractional share that would otherwise have been issuable upon exercise of the Warrant. Your holding period for the shares of our common stock received on exercise generally will commence on the day of exercise.
In certain circumstances, the Warrants will be exercisable on a cashless basis. The tax consequences of a cashless exercise are not clear and could differ from the consequences described above, including the possibility that a cashless exercise could be a taxable event. You should consult your tax advisor regarding the tax consequences of a cashless exercise of a Warrant.
Expiration of Warrants
If you allow a Warrant to expire, you will generally recognize a loss for U.S. federal income tax purposes equal to the adjusted tax basis of the Warrant. In general, such a loss will be a capital loss, and will be a short-term or long-term capital loss depending on your holding period for the Warrant.
Certain Adjustments to the Warrants
Under Section 305 of the Code, an adjustment to the number of Warrant shares that will be issued on the exercise of the Warrants, or an adjustment to the exercise price of the Warrants, may be treated as a constructive distribution to you if, and to the extent that, such adjustment has the effect of increasing your proportionate interest in our earnings and profits or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of Warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the Warrants should generally not be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property. See the more detailed discussion of the rules applicable to distributions made by us under the heading “Taxation of Common Shares – Distributions” below.
Taxation of Common Shares
Distributions
Distributions with respect to shares of our common stock acquired upon exercise of Subscription Rights or upon exercise of Warrants will be taxable as dividend income when actually or constructively received to the extent of our current or accumulated earnings and profits as determined for U.S. federal income tax purposes.
66
Table of Contents
Dividend income received by certain non-corporate U.S. Holders with respect to shares of our common stock generally will be “qualified dividends” subject to preferential rates of U.S. federal income tax, provided that the U.S. Holder meets applicable holding period and other requirements. Subject to similar exceptions for short-term and hedged positions, dividend income on our shares of common stock paid to U.S. Holders that are domestic corporations generally will qualify for the dividends-received deduction. To the extent that the amount of a distribution exceeds our current and accumulated earnings and profits, such distribution will be treated first as a tax-free return of capital to the extent of your adjusted tax basis in such shares of our common stock and thereafter as capital gain.
Dispositions
If you sell or otherwise dispose of shares of common stock acquired upon exercise of Subscription Rights or upon exercise of Warrants in a taxable transaction, you will generally recognize capital gain or loss equal to the difference between the amount realized and your adjusted tax basis in the shares. Such capital gain or loss will be long-term capital gain or loss if your holding period for such shares is more than one year at the time of disposition. Long-term capital gain of a non-corporate U.S. Holder is generally taxed at preferential rates of U.S. federal income tax. The deductibility of capital losses is subject to limitations.
Information Reporting and Backup Withholding
You may be subject to information reporting and/or backup withholding with respect to the gross proceeds from the disposition of Warrants, shares of our common stock acquired through the exercise of Subscription Rights or through the exercise of Warrants, or dividend payments. Backup withholding (currently at the rate of 28%) may apply under certain circumstances if you (1) fail to furnish your social security or other taxpayer identification number, or TIN, (2) furnish an incorrect TIN, (3) fail to report interest or dividends properly, or (4) fail to provide a certified statement, signed under penalty of perjury, that the TIN provided is correct, that you are not subject to backup withholding and that you are a U.S. person on IRS Form W-9 or Substitute Form W-9. Any amount withheld from a payment under the backup withholding rules is allowable as a credit against (and may entitle you to a refund with respect to) your U.S. federal income tax liability, provided that the required information is timely furnished to the IRS. Certain persons are exempt from information reporting and backup withholding, including corporations and financial institutions, provided that they demonstrate this fact, if requested. You are urged to consult your own tax advisor as to your qualification for exemption from backup withholding and the procedure for obtaining such exemption.
Tax Consequences to Non-U.S. Holders
Taxation of the Subscription Rights
Receipt, Exercise and Expiration of the Subscription Rights
The discussion assumes that the receipt of Subscription Rights will be treated as a nontaxable distribution. See “Tax Consequences to U.S. Holders – Taxation of Subscription Rights – Receipts of Subscription Rights” above. You will not be subject to U.S. federal income tax (or any withholding thereof) on the receipt, exercise or expiration of the Subscription Rights.
Exercise and Expiration of Warrants and Certain Adjustments to Warrants
Exercise of Warrants
In general, a Non-U.S. Holder will not recognize gain or loss for U.S. federal income tax purposes upon exercise of a Warrant, except to the extent the Non-U.S. Holder receives a cash payment for any such fractional share that would otherwise have been issuable upon exercise of the Warrant, which will be treated as a sale subject to the rules described under “Sale or Other Disposition of Common Stock or Warrants” below.
67
Table of Contents
Expiration of Warrants
In general, a Non-U.S. Holder will not be able to utilize a loss recognized upon expiration of a Warrant against the Non-U.S. Holder’s U.S. federal income tax liability unless the loss is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment in the United States) or is treated as a U.S.-source loss and the Non-U.S. Holder is present 183 days or more in the taxable year of disposition and certain other conditions are met.
Certain Adjustments to the Warrants
Under Section 305 of the Code, an adjustment to the number of Warrant shares that will be issued on the exercise of the Warrants, or an adjustment to the exercise price of the Warrants, may be treated as a constructive distribution to a Non-U.S. Holder of the Warrants if, and to the extent that, such adjustment has the effect of increasing such Non-U.S. Holder’s proportionate interest in our “earnings and profits” or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of Warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the Warrants should generally not be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property. See the more detailed discussion of the rules applicable to distributions made by us under the heading “– Taxation of Distributions on Common Shares” below.
Taxation of Distributions on Common Shares
Any distributions of cash or property made with respect to our common stock generally will be subject to withholding tax to the extent paid out of our current or accumulated earnings and profits as determined for U.S. federal income tax purposes, if any, at a rate of 30% (or a lower rate prescribed in an applicable income tax treaty). In order to obtain a reduced withholding tax rate, if applicable, you will be required to provide an IRS Form W-8BEN or IRS Form W-8BEN-E, as applicable, certifying your entitlement to benefits under a treaty. In addition, you will not be subject to withholding tax if you provide an IRS Form W-8ECI certifying that the distributions are effectively connected with your conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment within the United States); instead, you generally will be subject to U.S. federal income tax, net of certain deductions, with respect to such income at the same rates applicable to U.S. persons, and if you are a corporation, a “branch profits tax” of 30% (or a lower rate prescribed in an applicable income tax treaty) also may apply to such effectively connected income.
Non-U.S. Holders may be required to periodically update their IRS Forms W-8.
Sale or Other Disposition of Our Common Stock or Warrants
In general, you will not be subject to U.S. federal income tax on any gain realized on a sale of shares of our common stock, or Warrants unless:
| • | the gain is effectively connected with your conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment in the United States); |
| • | you are an individual, you hold your Subscription Rights, shares of common stock or Warrants as capital assets, you are present in the United States for 183 days or more in the taxable year of disposition and certain other conditions are met; or |
| • | we are or have been a “United States real property holding corporation,” or USRPHC, for U.S. federal income tax purposes unless an exception for 5% or less shareholders applies. |
Gain that is effectively connected with your conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment within the United States) generally
68
Table of Contents
will be subject to U.S. federal income tax, net of certain deductions, at the same rates applicable to U.S. persons. If you are a corporation, a “branch profits tax” of 30% (or a lower rate prescribed in an applicable income tax treaty) also may apply to such effectively connected gain.
A domestic corporation is treated as a USRPHC if the fair market value of its United States real property interests equals or exceeds 50% of the sum of (1) the fair market value of its United States real property interests, (2) the fair market value of its non-United States real property interests and (3) the fair market value of any other of its assets which are used or held for use in a trade or business. We believe that we are not currently, and have not been within the relevant testing period, a USRPHC. However, no assurance can be given that we will not become a USRPHC in the future. If we are a USRPHC or become a USRPHC in the future, a Non-U.S. Holder may still not be subject to U.S. federal income tax on a sale or other disposition if an exception for 5% or less shareholders applies. You are urged to consult your own tax advisor regarding the U.S. federal income tax considerations that could result if we are, or become, a USRPHC and with respect to the exception for 5% or less shareholders.
Information Reporting and Backup Withholding
Distributions on our common stock and the amount of tax withheld, if any, with respect to such distributions will generally be subject to information reporting. If you comply with certification procedures to establish that you are not a United States person, additional information reporting and backup withholding should not apply to distributions on our common stock and information reporting and backup withholding should not apply to the proceeds from a sale or other disposition of Warrants or shares of our common stock. The amount of any backup withholding will generally be allowed as a refund or credit against your U.S. federal income tax liability, provided that the required information is timely furnished to the IRS.
FATCA
Legislation enacted in 2010 and commonly referred to as FATCA may impose withholding taxes on certain types of payments made to “foreign financial institutions” and certain other non-U.S. entities. The legislation imposes a 30% withholding tax on dividends on shares of our common stock and on or after January 1, 2017, the gross proceeds from the sale or other disposition of our shares of common stock or Warrants received by a foreign financial institution unless the foreign financial institution enters into an agreement with the U.S. Treasury to among other things, undertake to identify accounts held by certain U.S. persons or U.S.-owned foreign entities, annually report certain information about such accounts and withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements. In addition, the legislation imposes a 30% withholding tax on the same types of payments to a non-financial foreign entity unless the entity certifies that it does not have any substantial U.S. owners or furnishes identifying information regarding each substantial U.S. owner. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules. Depending on your circumstances, you may be entitled to a refund or credit in respect of some or all of this withholding. However, even if you are entitled to have any such withholding refunded, the required procedures could be cumbersome and significantly delay your receipt of any withheld amounts. Prospective investors should consult their tax advisors regarding this legislation.
THE PRECEDING DISCUSSION OF MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES IS NOT TAX ADVICE. HOLDERS OF SHARES OF OUR COMMON STOCK AND SERIES L WARRANTS SHOULD CONSULT THEIR OWN TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AND THE CONSEQUENCES UNDER FEDERAL ESTATE AND GIFT TAX LAWS, FOREIGN, STATE, AND LOCAL LAWS AND TAX TREATIES OF THE RECEIPT, OWNERSHIP AND EXERCISE OF SUBSCRIPTION RIGHTS AND THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF SHARES OF OUR COMMON STOCK AND WARRANTS ACQUIRED UPON EXERCISE OF SUBSCRIPTION RIGHTS AND SHARES OF OUR COMMON STOCK ACQUIRED UPON EXERCISE OF WARRANTS.
69
Table of Contents
Common Stock
This section describes the general terms and provisions of the shares of our common stock, $0.01 par value. This description is only a summary and is qualified in its entirety by reference to the description of our common stock included in our restated certificate of formation and our amended and restated bylaws which have been filed as exhibits to the registration statement of which this prospectus is a part. You should read our restated certificate of formation and our amended and restated bylaws for additional information before you buy any of our common stock or other securities. See “Where You Can Find More Information” and “Incorporation by Reference.”
We have 100,000,000 shares of authorized common stock. As of December 31, 2014, there were 28,234,751 shares of common stock issued and outstanding, as well as Series L warrants to purchase 541,668 shares of common stock outstanding on that date. Each holder of common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of shareholders. We have not provided for cumulative voting for the election of directors in our restated certificate of formation. This means that the holders of a majority of the shares voted can elect all of the directors then standing for election. Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of assets legally available at the times and in the amounts that our board of directors may determine from time to time. Upon our liquidation, dissolution or winding-up, the holders of common stock are entitled to share ratably in all assets remaining after payment of all liabilities and the liquidation preferences of any outstanding preferred stock. Holders of common stock have no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and nonassessable, and the shares of common stock offered, when issued, will be fully paid and nonassessable.
The shares of common stock that are purchased in the Rights Offering as part of the Units will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of shares if you are a holder of record of shares or Series L warrants. The Subscription Agent will arrange for the issuance of the common stock as soon as practicable after the expiration of the Rights Offering, payment for the Units subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected. If you hold your shares of common stock in the name of a custodian bank, broker, dealer, or other nominee, DTC will credit your account with your nominee with the common stock you purchased in the Rights.
Preferred Stock
We have 10,000,000 shares of authorized preferred stock, no par value, none of which were issued or outstanding as of December 31, 2014. Of this amount, 80,000 shares have been designated Series A convertible preferred stock. We may issue preferred stock, in series, with such designations, powers, preferences and other rights and qualifications, limitations or restrictions as our board of directors may authorize, without further action by our stockholders.
Warrants
Warrants Included in Units Issuable in the Rights Offering
The Warrants to be issued as a part of this Rights Offering will be designated as our “Series M” warrants. These Warrants will be separately transferable following their issuance and through their expiration three years from the date of issuance. The Warrants entitle the holder to purchase one share of common stock at an exercise price of (i) $0.50 per share from the date of issuance through June 30, 2016 and (ii) $1.50 per share
70
Table of Contents
from July 1, 2016 through their expiration. We have applied to list the Warrants for trading on NASDAQ under the symbol “OPXAW,” however, there is no assurance that a sufficient number of Subscription Rights will be exercised so that the Warrants will meet the minimum listing criteria to be accepted for listing on NASDAQ. The common stock underlying the Warrants, upon issuance, will also be traded on NASDAQ under the symbol “OPXA.”
All Warrants that are purchased in the Rights Offering as part of the Units will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of Warrants if you are a holder of record of shares or Series L warrants. The Subscription Agent will arrange for the issuance of the Warrants as soon as practicable after the expiration of the Rights Offering, payment for the Units subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected. If you hold your shares of common stock in the name of a custodian bank, broker, dealer, or other nominee, DTC will credit your account with your nominee with the Warrants you purchased in the Rights Offering.
The Warrants will be exercisable for cash, or solely during any period when a registration statement for the exercise of the Warrants is not in effect, on a cashless basis.
We may call the Warrants for redemption, in whole and not in part, at a price of $.01 per Warrant at any time while the Warrants are exercisable, upon not less than 30 days’ prior written notice of redemption to each Warrant holder, and if, and only if, the closing price of the common stock equals or exceeds $2.50 per share, subject to adjustment, for 10 consecutive trading days.
The exercise price of the Warrants and the number of shares of common stock issuable upon exercise of the Warrants are subject to adjustment in certain circumstances, including a stock split of, stock dividend on, or a subdivision, combination or recapitalization of the common stock. Upon the merger, consolidation, sale of substantially all of our assets, or other similar transaction, the holders of Warrants shall, at the option of the company, be required to exercise the Warrants immediately prior to the closing of the transaction, or such Warrants shall automatically expire. Upon such exercise, the holders of Warrants shall participate on the same basis as the holders of common stock in connection with the transaction.
The Warrants do not confer upon the holder any voting or any other rights of a shareholder of the Company. Upon notice to the Warrants holders, we have the right at any time and from time to time, to reduce the exercise price or to extend the Warrants termination date.
The Warrants will be issued pursuant to a warrant agreement by and between us and Continental Stock Transfer & Trust Company, the warrant agent.
71
Table of Contents
Other Currently Outstanding Warrants
We have the following series of warrants currently outstanding, some of which contain a provision for adjustment of the warrants upon completion of this Rights Offering:
| Series |
Expiration Date |
Shares of Common Stock Underlying Warrants |
Exercise Price |
Potential Warrant
Adjustment | ||||||
| Series A | 6-15-15 | 223,119 | $10.20 | Warrant exercise price is subject to adjustment if the Subscription Price is less than the daily volume weighted average price (VWAP) of the common stock on the Record Date, based on the number of shares outstanding, the number of additional shares offered and the VWAP. | ||||||
| Series H | 2-11-16 | 414,649 | $10.44 | None | ||||||
| Series I | 7-25-17 | 1,436,115 | $2.56 | None | ||||||
| Series J | 1-23-18 | 243,750 | $1.24 | Warrant exercise price is subject to adjustment, based on fair market value of the Subscription Rights, market price of the common stock prior to the Record Date, and number of shares outstanding. | ||||||
| Series K | 1-29-18 | 187,500 | $1.21 | Warrant exercise price is subject to adjustment, based on fair market value of the Subscription Rights, market price of the common stock prior to the Record Date, and number of shares outstanding. | ||||||
| Series L | 2-11-17 | 541,668 | $3.00 | Warrant exercise price and number of shares issuable upon exercise of the warrant are subject to adjustment, based on the number of shares outstanding before and after the offering. | ||||||
|
|
|
|||||||||
| TOTAL |
3,046,801 | |||||||||
|
|
|
|||||||||
Certain Provisions of our Charter and Bylaws
Certain provisions of our restated certificate of formation and our amended and restated bylaws described below may have the effect of delaying, deferring or discouraging another party from acquiring control of us. Our restated certificate of formation and amended and restated bylaws provided that:
| • | Our board of directors is authorized to issue preferred stock without shareholder approval; and |
| • | We will indemnify officers and directors against losses that they may incur in investigations and legal proceedings resulting from their services to us, which may include services in connection with takeover defense measures. |
Transfer Agent
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company, 17 Battery Place, New York, New York 10004.
72
Table of Contents
On or about March 16, 2015, we will distribute the Subscription Rights, Rights Certificates and copies of this prospectus to the holders of our common stock and Series L warrants on the Record Date. Subscription Rights holders who wish to exercise their Subscription Rights and purchase Units must complete the Subscription Rights Certificate and return it with payment for the shares to the Subscription Agent at the following address:
Continental Stock Transfer & Trust Company
17 Battery Place—8th Floor
New York, NY 10004
Attn: Corporate Actions Department
See “The Rights Offering—Methods for Exercising Subscription Rights.”
If you have any questions, you should contact our Information Agent for the Rights Offering:
Advantage Proxy, Inc.
(877) 870-8565 (toll free)
(206) 870-8565 (collect)
Other than as described in this prospectus, we do not know of any existing agreements between any shareholder, broker, dealer, underwriter or agent relating to the sale or distribution of the underlying common stock.
Maxim Group LLC and National Securities Corp. are the dealer-managers of this Rights Offering. In such capacity, such dealer-managers will provide marketing assistance and advice to us in connection with this offering and will solicit the exercise of Subscription Rights and participation in the Over-Subscription Privilege. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants being issued in this offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares or Warrants.
In connection with this Rights Offering, we have agreed to pay fees to the dealer-managers as follows: (i) to Maxim Group LLC, a cash fee equal to (a) 1% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is less than $6 million; (b) 4% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million but less than $10 million; and (c) 4.5% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $10 million; and (ii) to National Securities Corporation, a cash fee equal to 2.75% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million. We have also agreed to reimburse the dealer-managers for their expenses (including, without limitation, legal fees) as follows: (i) up to $50,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is less than $6 million; and (ii) up to $100,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is at least $6 million. We advanced $20,000 to Maxim Group LLC against reimbursement of accountable expenses upon their engagement as a dealer-manager (the “Advance”). Any portion of the Advance will be returned to us to the extent it is not actually incurred.
We have also agreed to indemnify the dealer-managers and their respective affiliates against certain liabilities arising under the Securities Act of 1933. The dealer-managers’ participation in this offering is subject to customary conditions contained in the dealer-manager agreement, including the receipt by the dealer-managers of an opinion of our counsel. The dealer-managers and their affiliates may provide to us from time to time in the future in the ordinary course of their business certain financial advisory, investment banking and other services for which they will be entitled to receive fees.
73
Table of Contents
Each of Maxim Group LLC and National Securities Corporation is a broker-dealer and member of the Financial Industry Regulatory Authority, Inc. The principal business address of Maxim Group LLC is 405 Lexington Avenue, New York, New York 10174, and the principal business address of National Securities Corporation is 410 Park Avenue, 14th Floor, New York, New York 10022.
74
Table of Contents
The financial statements of Opexa as of December 31, 2014, and for the years ended December 31, 2014 and 2013, incorporated in this prospectus by reference to our Annual Report on Form 10-K for the year ended December 31, 2014 have been audited by MaloneBailey, LLP, an independent registered public accounting firm, and are incorporated by reference in reliance upon their report dated February 20, 2015, given upon such firm’s authority as experts in auditing and accounting.
The validity of any securities offered by this prospectus will be passed upon for us by Pillsbury Winthrop Shaw Pittman LLP, San Diego, California. The dealer-managers are being represented by Loeb & Loeb LLP, New York, New York.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 with the SEC under the Securities Act of 1933, as amended. This prospectus is part of the registration statement but the registration statement includes additional information and exhibits. We file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy the registration statement and any document we file with the SEC at the public reference room maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a web site that contains reports, proxy and information statements and other information regarding companies, such as ours, that file documents electronically with the SEC. The website address is www.sec.gov. The information on the SEC’s website is not part of this prospectus, and any references to this website or any other website are inactive textual references only.
The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC, which means that we can disclose important information to you by referring you to those documents rather than by including them in this prospectus. Information that is incorporated by reference is considered to be part of this prospectus and you should read it with the same care that you read this prospectus. We have filed with the SEC, and incorporate by reference in this prospectus:
| • | our Annual Report on Form 10-K for the year ended December 31, 2014; |
| • | the section entitled “Transactions with Related Persons” of “Item 13. Certain Relationships and Related Transactions, and Director Independence” and the disclosure included in footnote 3 to the “Beneficial Ownership Table” appearing in “Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” in our Annual Report on Form 10-K for the year ended December 31, 2013; and |
| • | our Current Report on Form 8-K filed January 28, 2015. |
We are not, however, incorporating, in each case, any documents or information that we are deemed to furnish and not file in accordance with SEC rules.
You may request a copy of any or all of the documents incorporated by reference but not delivered with this prospectus, at no cost, by writing or telephoning us at the following address and number: Investor Relations, Opexa Therapeutics, Inc., 2635 Technology Forest Blvd., The Woodlands, Texas 77381, telephone (281) 775-0600. We will not, however, send exhibits to those documents, unless the exhibits are specifically incorporated by reference in those documents. We also maintain a website at www.opexatherapeutics.com. However, the information on our website is not part of this prospectus and should not be relied upon with respect to this offering.
75
Table of Contents
| AEs | Adverse events. | |
| ARR | Annualized relapse rate. | |
| Attenuation | Process of irradiation that renders cell replication incompetent but viable for a short period of time. | |
| Axon | The long projection of nerve cells. | |
| Biomarker | Is a molecule that is present (or absent) from a particular cellular type. This facilitates the characterization of a cell type and their identification. A biomarker can be used to identify a cell population, make a diagnostic, measure the progress of disease or the effects of treatment. | |
| BLA | Biologics license application. | |
| Blood-brain barrier | Is a separation of circulating blood from the brain extracellular fluid in the central nervous system. | |
| cGMP | Current good manufacturing practice. | |
| CNS | Central nervous system. | |
| Cryopreserved | Storage of cells in a frozen state in liquid nitrogen. | |
| Demyelination | Destruction of myelin sheath. | |
| Dose | Number of attenuated MRTCs per vial. | |
| EDSS | Expanded disability status scale. | |
| EPA | An Epitope Profiling Essay is a proprietary assay developed by Opexa to identify the patient specific antigens that might be responsible for triggering the myelin sheath destruction pathway. | |
| Epitope | Also known as antigenic determinant is the part of an antigen that is recognized by the immune system, specifically by antibodies, B-cells, or T-cells. | |
| FDA | U.S. Food and Drug Administration. | |
| Immunotherapy | Is a medical term defined as the “treatment of disease by inducing, enhancing, or suppressing an immune response.” | |
| ImmPath® | Our proprietary method for the production of a patient-specific T-cell immunotherapy which encompasses the collection of blood from the MS patient, isolation of peripheral blood mononuclear cells, generation of an autologous pool of MRTCs raised against selected peptides from MBP, MOG and PLP, expanding these MRTCs to a therapeutic dose ex-vivo, and attenuating them with gamma irradiation to prevent DNA replication and thereby cellular proliferation. | |
| Lymphocytes | A kind of white blood cell in the vertebrate immune system, specifically, landmark of the adaptive immune system. Lymphocytes can be divided into large lymphocytes and small lymphocytes. Large granular lymphocytes include natural killer cells (NK cells). Small lymphocytes consist of T-cells and B-cells. | |
| MBP | Myelin basic protein is a protein believed to be important in the process of myelination of nerves in the nervous system. MBP maintain the correct structure of myelin, interacting with the lipids in the myelin membrane. | |
76
Table of Contents
| MS | Multiple sclerosis is a disease that involves an immune system attack against the central nervous system (brain, spinal cord, and optic nerves). The disease is thought to be triggered in a genetically susceptible individual by a combination of one or more environmental factors. The day-to-day actions of humans are facilitated by neurons as they send electrical impulses at very high speeds to different parts of the body. The ability of the neurons to function properly is determined by the fatty tissue insulation surrounding the axons known as myelin or myelin sheath. In the case of multiple sclerosis, the body’s own immune system attacks the myelin sheath. When this happens the electrical impulses from the brain could be slowed down, distorted or stopped resulting in weakness, lack of coordination, fatigue, vision problems, numbness or paralysis. The diagnosis of multiple sclerosis is usually through an MRI scan which helps identify any scar tissue in the brain or the de-myelinated neurons. | |
| MOG | Myelin oligodendrocyte glycoprotein is a glycoprotein believed to be important in the process of myelinization of nerves in the central nervous system. In humans this protein is encoded by the MOG gene. It is speculated to serve as a necessary “adhesion molecule” to provide structural integrity to the myelin sheath and is known to develop late on the oligodendrocyte. | |
| MRTCs | Myelin-reactive T-cells are a subset of T-cells that are associated with the destruction of the myelin sheath. | |
| MSFC | Multiple sclerosis functional composite. | |
| MSIS | Multiple sclerosis impact scale. | |
| MSQLI | Multiple sclerosis quality of life inventory. | |
| Myelin or myelin sheath | The ability of the neurons to function properly is determined by the fatty tissue insulation surrounding the axons known as myelin or myelin sheath. | |
| NDA | New drug application. | |
| Oligodendroglial cells | Are a type of brain cell whose main functions are to provide support and to insulate the axons in the central nervous system. | |
| PBMC | Peripheral blood mononuclear cell. | |
| Peptide | Short chains of amino acids linked by amide bonds. | |
| PLP | In a myelin sheath, as the layers of myelin wraps come together, proteolipid protein will bind itself and tightly hold the cellular membranes together. | |
| Precision Immunotherapy® | A service mark owned by Opexa. | |
| Regulatory T-Cells | Treg cells are a sub population of T cells which modulate the immune system. Animal models have suggested that modulation of Tregs can treat autoimmne diseases. | |
| Remyelination | Remyelination is the process propagating oligodendrocyte precursor cells to form oligodendrocytes to create new myelin sheaths on demyelinated axons in the CNS. This is a process naturally regulated in the body and tends to be very efficient in a healthy CNS. | |
| RRMS | Relapsing remitting multiple sclerosis. People with this type of MS experience clearly defined attacks of worsening neurologic function. These attacks—which are called relapses, flare-ups, or exacerbations—are followed by partial or complete recovery periods (remissions), during which no disease progression occurs. Approximately 85% of people are initially diagnosed with relapsing-remitting MS. | |
77
Table of Contents
| SAEs | Serious adverse events. | |
| SPMS | Secondary progressive multiple sclerosis. Following an initial period of relapsing-remitting MS, many people develop a secondary-progressive disease course in which the disease worsens more steadily, with or without occasional flare-ups, minor recoveries (remissions), or plateaus. Before the disease-modifying medications became available, approximately 50% of people with relapsing-remitting MS developed this form of the disease within 10 years. | |
| T-cells or T-lymphocytes | Are a type of lymphocytes (itself a type of white blood cells) that play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B-cells and natural killer cells (NK-cells), by the presence of a T-cell receptor on the cell surface. They are called T-cells because they mature in the thymus. There are several subsets of T-cells, each with a distinct function. | |
| Tcelna® | Imilecleucel-T, formerly known as Tovaxin®. | |
| TERMS | Tovaxin for Early Relapsing Multiple Sclerosis was a Phase IIb clinical study of Tcelna in relapsing remitting multiple sclerosis patients completed in 2008. | |
| Whole brain atrophy | Brain atrophy is a condition in which cells in the brain are lost, or the connections between them are damaged. In the brain, losing neurons is highly undesirable, as loss of brain tissue can cause a variety of neurological and cognitive problems. Patients with brain atrophy may develop seizures, dementia, and aphasias. In focal cerebral atrophy, the damage is concentrated on a particular area of the brain, which means that the functions of that area of the brain can become impaired. Generalized brain atrophy involves the whole brain, and may be associated with a range of problems. | |
Sources: National MS Society, Wikipedia, Opexa documents
78
Table of Contents

PROSPECTUS
Subscription Rights to Purchase Up to 28,776,419 Units
Consisting of an Aggregate of Up to 28,776,419 Shares of Common Stock
and Warrants to Purchase Up to 28,776,419 Shares of Common Stock
at a Subscription Price of $0.70 Per Unit
Dealer-Managers
Maxim Group LLC
National Securities Corp.
, 2015
Table of Contents
PART II
Information Not Required In Prospectus
Item 13. Other Expenses of Issuance and Distribution.
The following is a statement of estimated expenses in connection with the issuance and distribution of the securities being registered, excluding dealer-manager fees. All expenses incurred with respect to the registration of the common stock will be borne by us. All amounts are estimates except the SEC registration fee and the FINRA filing fee.
|
Amount to be Paid | ||
| SEC Registration Fee |
$ 7,524 | |
| FINRA Filing Fee |
10,212 | |
| NASDAQ Fee |
76,000 | |
| Printing Expenses |
125,000 | |
| Legal Fees and Expenses |
285,000 | |
| Accounting Fees and Expenses |
16,500 | |
| Subscription Agent, Information Agent and Warrant Agent Fees and Expenses |
27,500 | |
| Miscellaneous Expenses |
175,000 | |
|
| ||
| $747,736 | ||
|
|
Item 14. Indemnification of Directors and Officers.
Section 8.101 of the Texas Corporation Law (or the TCA) authorizes the Registrant to indemnify certain persons, including any person who was, is or is threatened to be made a named defendant or respondent in a threatened, pending or completed action or other proceeding, because the person is or was a director or officer, against judgments and reasonable expenses actually incurred by the person in connection with the threatened, pending or completed action or other proceeding. The Registrant is required by Section 8.051 of the TCA to indemnify a director or officer against reasonable expenses actually incurred by him or her in connection with a threatened, pending, or completed action or other proceeding in which he or she is a named defendant or respondent because he or she is or was a director or officer if he or she has been wholly successful, on the merits or otherwise, in the defense of the action or proceeding.
The Registrant’s restated certificate of formation provides that none of its directors shall be personally liable to the Registrant or its shareholders for monetary damages for an act or omission in such director’s capacity as a director; provided, however, that the liability of such director is not limited to the extent that such director is found liable for (i) a breach of the director’s duty of loyalty to the Registrant or its shareholders, (ii) an act or omission not in good faith that constitutes a breach of duty of the director to the Registrant or an act or omission that involves intentional misconduct or a knowing violation of the law, (iii) a transaction from which the director received an improper benefit, whether or not the benefit resulted from an action taken within the scope of the director’s office, or (iv) an act or omission for which the liability of the director is expressly provided by an applicable statute.
The Registrant’s restated certificate of formation and amended and restated bylaws provide that the Registrant shall indemnify its officers, directors, agents and any other persons to the fullest extent permitted by applicable law. The Registrant’s directors and officers are covered by insurance indemnifying them against certain liabilities which might be incurred by them in their capacities as such. Pursuant to terms of their employment contracts, certain of the Registrant’s officers are entitled to indemnification in their capacity as such and to the fullest extent permitted by applicable law.
At the present time, there is no pending litigation or proceeding involving a director, officer, employee or other agent of the Registrant in which indemnification would be required or permitted. The Registrant is not aware of any threatened litigation or proceeding which may result in a claim for such indemnification.
II-1
Table of Contents
Item 15. Recent Sales of Unregistered Securities.
The following is a summary of all securities that we have sold within the past three years without registration under the Securities Act of 1933, as amended.
| • | On July 25, 2012, we completed a private offering of $4,085,000 in principal amount of convertible secured promissory notes and Series I warrants to purchase an aggregate of 1,436,121 shares of common stock, subject to certain adjustments, with an exercise price of $2.56 per share. The offers and sales were made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor. In February 2013, we issued an aggregate of 288,229 shares of common stock to three noteholders who elected to convert such notes into shares of Series A convertible preferred stock with further immediate conversion into common stock. |
| • | On November 2, 2012, we completed a private placement to Lincoln Park Capital Fund, LLC pursuant to which we have the right to sell to Lincoln Park up to $15,000,000 in shares of common stock, subject to certain limitations, from time to time over the 30-month period commencing on the date that a registration statement covering the resale of the shares is declared effective by the SEC. The issuance and sale was made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based on the offering of such securities to one investor, the lack of any general solicitation or advertising in connection with such issuance, and the representations of such investor that it was an accredited investor and that it was purchasing the shares for its own account and without a view to distribute them. |
| • | On January 23, 2013, we completed a private offering of $650,000 in principal amount of unsecured convertible promissory notes and Series J warrants to purchase an aggregate of 243,750 shares of common stock, subject to certain adjustments, with an exercise price of $1.24 per share. The offers and sales were made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor. On February 26, 2013, we issued 77,034 shares of common stock to a noteholder who elected to convert such note into common stock. |
| • | On January 30, 2013, we issued Series K warrants to purchase an aggregate of 187,500 shares of common stock, at an exercise price of $1.21 per share, to the holders of our July 2012 convertible secured promissory notes in exchange for a waiver and reduction in the cash held in a controlled account as security for repayment of the notes. The issuance was made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor. |
| • | On October 21, 2014, we issued 54,664 shares of common stock to a consultant as compensation for services rendered in lieu of a cash payment of $50,000. The issuance and sale was made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based on the offering of such securities to one investor, the lack of any general solicitation or advertising in connection with such issuance, and the representations of such investor that it was an accredited investor and that it was purchasing the shares for its own account and without a view to distribute them. |
II-2
Table of Contents
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
The exhibits to the registration statement are listed in the Exhibit Index to this registration statement and are incorporated herein by reference.
(b) Financial statement schedules
All schedules have been omitted because either they are not required, are not applicable or the information is otherwise set forth in the financial statements and related notes thereto.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the
II-3
Table of Contents
registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to any charter provision, by law or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(7) The undersigned registrant hereby undertakes that:
(i) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(I) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and
(ii) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of The Woodlands, State of Texas, on February 24, 2015.
| OPEXA THERAPEUTICS, INC. | ||
| By: | /s/ Neil K. Warma | |
| Neil K. Warma President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Name |
Title |
Date | ||
| /s/ Neil K. Warma Neil K. Warma |
President, Chief Executive Officer and Director (Principal Executive Officer) |
February 24, 2015 | ||
| /s/ Karthik Radhakrishnan Karthik Radhakrishnan |
Chief Financial Officer (Principal Financial and Accounting Officer) |
February 24, 2015 | ||
| * Timothy C. Barabe |
Director | February 24, 2015 | ||
| * Hans-Peter Hartung, M.D. |
Director | February 24, 2015 | ||
| * Gail J. Maderis |
Director | February 24, 2015 | ||
| * Michael S. Richman |
Director | February 24, 2015 | ||
| * Scott B. Seaman |
Director | February 24, 2015 | ||
| * By: | /s/ Neil K. Warma | |
| Neil K. Warma Attorney-in-Fact |
II-5
Table of Contents
EXHIBIT INDEX
| Exhibit No. |
Description | |
| 1.1** | Form of Dealer-Manager Agreement by and between Opexa Therapeutics, Inc., Maxim Group LLC and National Securities Corporation. | |
| 3.1 | Restated Certificate of Formation of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 3.2 | Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 3.3 | Certificate of Amendment of the Restated Certificate of Formation of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on December 14, 2012). | |
| 3.4 | Amended and Restated By-laws, as amended (incorporated by reference to Exhibit 3.3 to the Company’s Annual Report on form 10-K filed on March 8, 2011). | |
| 4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 4.7 to the Company’s Registration Statement on Form S-3 filed on November 13, 2009, File No. 333-163108). | |
| 4.2 | Form of Securities Purchase Agreement dated as of December 9, 2009 by and between Opexa Therapeutics, Inc. and each investor signatory thereto for Unit offering of Common Stock and Series A and Series B Warrants (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed December 10, 2009). | |
| 4.3 | Form of Common Stock Purchase Warrant for Series A and Series B Warrants (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed December 10, 2009). | |
| 4.4 | Form of Series H Warrant issued on February 11, 2011 (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed February 8, 2011). | |
| 4.5 | Form of Series I Warrant issued on July 25, 2012 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 4.6 | Form of Series J Warrant issued on January 23, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on January 23, 2013). | |
| 4.7 | Form of Series K Warrant issued on January 30, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on January 30, 2013). | |
| 4.8 | Form of Series L Warrant issued on February 11, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on February 7, 2013). | |
| 4.9 | Form of Securities Purchase Agreement, dated as of February 7, 2013, by and between Opexa Therapeutics, Inc. and each investor signatory thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 7, 2013). | |
| 4.10* | Form of Non-Transferable Subscription Rights Certificate. | |
| 4.11** | Form of Series M Warrant underlying the Units. | |
| 4.12** | Form of Warrant Agreement between Opexa Therapeutics, Inc. and Continental Stock Transfer & Trust Company. | |
| 4.13* | Form of Subscription Agent Agreement between Opexa Therapeutics, Inc. and Continental Stock Transfer & Trust Company. | |
| 5.1** | Opinion of Pillsbury Winthrop Shaw Pittman LLP. | |
Table of Contents
| Exhibit No. |
Description | |
| 10.1+ | Opexa Therapeutics, Inc. June 2004 Compensatory Stock Option Plan (incorporated by reference to Exhibit B to the Company’s Definitive Information Statement on Schedule 14C filed on June 29, 2004, File No. 000-25513). | |
| 10.2+ | Certificate of Amendments to the Opexa Therapeutics, Inc. June 2004 Compensatory Stock Option Plan (incorporated by reference to Exhibit 10.15 of the Company’s Annual Report on Form 10-K filed March 5, 2010). | |
| 10.3+ | Opexa Therapeutics, Inc. 2010 Stock Incentive Plan, as amended and restated (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 12, 2013). | |
| 10.4+ | Form of Notice of Stock Option Grant and Stock Option Agreement for awards granted under the 2010 Stock Incentive Plan, as amended and restated (incorporated by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q filed August 14, 2014). | |
| 10.5+ | Employment Agreement dated June 16, 2008 by and between Opexa Therapeutics, Inc. and Neil K. Warma (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 18, 2008). | |
| 10.6+ | Amended and Restated Employment Agreement entered into on April 21, 2010 by and between Opexa Therapeutics, Inc. and Donna R. Rill (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed April 27, 2010). | |
| 10.7+ | Offer Letter, effective March 29, 2013, by and between Opexa Therapeutics, Inc. and Karthik Radhakrishnan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 1, 2013). | |
| 10.8 | License Agreement dated September 5, 2001 by and between Opexa Therapeutics, Inc. and Baylor College of Medicine (incorporated by reference to Exhibit 10.14 to the Company’s Annual Report on Form 10-KSB filed April 15, 2005, File No. 000-25513). | |
| 10.9 | Lease dated August 19, 2005 by and between Opexa Therapeutics, Inc. and Dirk D. Laukien (incorporated by reference to Exhibit 10.13 to the Company’s Annual Report on Form 10-KSB filed March 31, 2006, File No. 000-25513). | |
| 10.10 | License Agreement dated January 13, 2006 by and between Opexa Therapeutics, Inc. and Shanghai Institute for Biological Services (incorporated by reference to Exhibit 10.23 to the Company’s Registration Statement on Form SB-2 (Amendment No. 1) filed February 9, 2006, File No. 333-126687). | |
| 10.11 | Sales Agreement, dated September 6, 2012, by and between Opexa Therapeutics, Inc. and Brinson Patrick Securities Corporation (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 7, 2012). | |
| 10.12 | First Amendment to Sales Agreement, dated March 5, 2014, by and among Opexa Therapeutics, Inc., Meyers Associates, L.P. (doing business as Brinson Patrick, a division of Meyers Associates, L.P.) and Brinson Patrick Securities Corporation (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K files on March 5, 2014). | |
| 10.13 | $15.0 million Purchase Agreement, dated as of November 2, 2012, by and between Opexa Therapeutics, Inc. and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 5, 2012). | |
| 10.14 | $1.5 million Purchase Agreement, dated as of November 5, 2012, by and between Opexa Therapeutics, Inc. and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on November 5, 2012). | |
| 10.15 | Registration Rights Agreement, dated as of November 2, 2012, by and between Opexa Therapeutics, Inc. and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed November 5, 2012). | |
Table of Contents
| Exhibit No. |
Description | |
| 10.16# | Option and License Agreement, dated February 4, 2013, by and between Ares Trading SA, a wholly owned subsidiary of Merck Serono S.A., and Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 5, 2013). | |
| 21.1 | List of Subsidiaries (incorporated by reference to Exhibit 21.1 to the Company’s Annual Report on Form 10-K filed on March 29, 2013). | |
| 23.1* | Consent of Independent Registered Public Accounting Firm MaloneBailey, LLP. | |
| 23.2** | Consent of Pillsbury Winthrop Shaw Pittman LLP, included in Exhibit 5.1 hereto. | |
| 24.1** | Power of attorney. | |
| 99.1** | Form of Instructions as to Use of Subscription Rights Certificates. | |
| 99.2** | Form of Letter to Shareholders who are Record Holders. | |
| 99.3** | Form of Letter to Brokers, Dealers, Banks and Other Nominees. | |
| 99.4** | Form of Broker Letter to Clients Who are Beneficial Holders. | |
| 99.5* | Form of Beneficial Owner Election Form. | |
| 99.6* | Form of Nominee Holder Certification. | |
| 99.7** | Form of Notice of Important Tax Information. | |
| * | Filed herewith |
| ** | Previously filed |
| + | Management contract or compensatory plan or arrangement |
| # | Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
