Attached files
| file | filename |
|---|---|
| 8-K - 8-K - APPLIED GENETIC TECHNOLOGIES CORP | agtc-8k_20150224.htm |

Visionary Science for Life Changing Cures 2015 Exhibit 99.1

Forward Looking Statements Today’s presentation includes forward-looking statements intended to qualify for the Safe Harbor from liability established by the Private Securities Litigation Reform Act of 1995. These forward-looking statements, including statements regarding our planned pre-clinical and clinical studies, regulatory approval process and demand for our product candidates, are subject to risks, uncertainties and other factors that could cause actual results to differ materially from those suggested by our forward-looking statements. These factors include, but are not limited to, the following: we have incurred significant losses since inception and anticipate that we will continue to incur significant losses for the foreseeable future; our ability to generate revenue from product sales is highly uncertain; we may need to raise additional funding in the future, which may not be available on acceptable terms, or at all; no gene therapy products have been approved in the United States, and we may not be able to obtain regulatory approvals for our product candidates; we may encounter substantial delays in our clinical trials or fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities; we expect to rely on third parties to conduct, supervise and monitor our clinical trials and to conduct certain aspects of our product manufacturing and protocol development; the insurance coverage and reimbursement status of our product candidates is uncertain; negative public opinion and increased regulatory scrutiny of gene therapy and genetic research may adversely affect public perception of our product candidates and prospects for our business; and if we are unable to obtain and maintain adequate patent protection for our technology and products our competitors could develop and commercialize technology and products similar or identical to ours.

Company Highlights Become the world leader in ophthalmology gene therapy Clear Strategy Multiple programs providing superior long-term value to patients and providers, with key inflection points in 2015 Broad Pipeline Robust understanding of vector selection, design, manufacturing and delivery Extensive Expertise Key IP, >100 patents, protects gene, vector capsid, regulatory elements, manufacturing and/or delivery for each candidate Intellectual Property Demonstrated success in balancing resources, risks and rewards; >100 patients treated in Phase 1/2 trials Track Record of Progress Optimized AAV vectors that deliver safe and sustained expression; manufactured reproducibly at commercial scale Leading Gene Therapy Platform
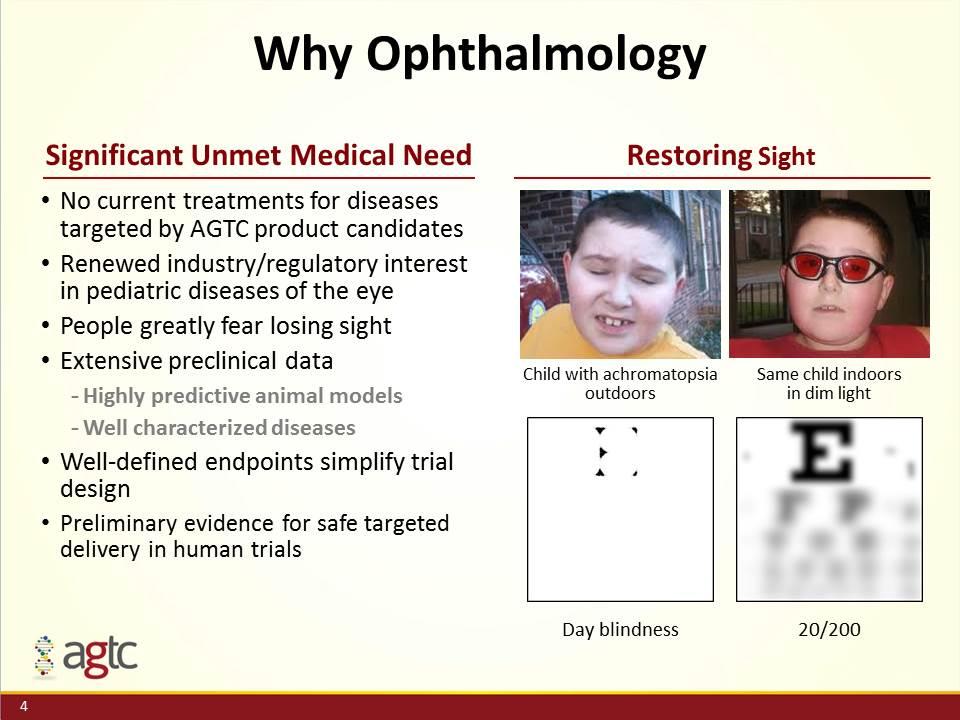
Why Ophthalmology No current treatments for diseases targeted by AGTC product candidates Renewed industry/regulatory interest in pediatric diseases of the eye People greatly fear losing sight Extensive preclinical data Highly predictive animal models Well characterized diseases Well-defined endpoints simplify trial design Preliminary evidence for safe targeted delivery in human trials Restoring Sight Significant Unmet Medical Need Child with achromatopsia outdoors Same child indoors in dim light Day blindness 20/200
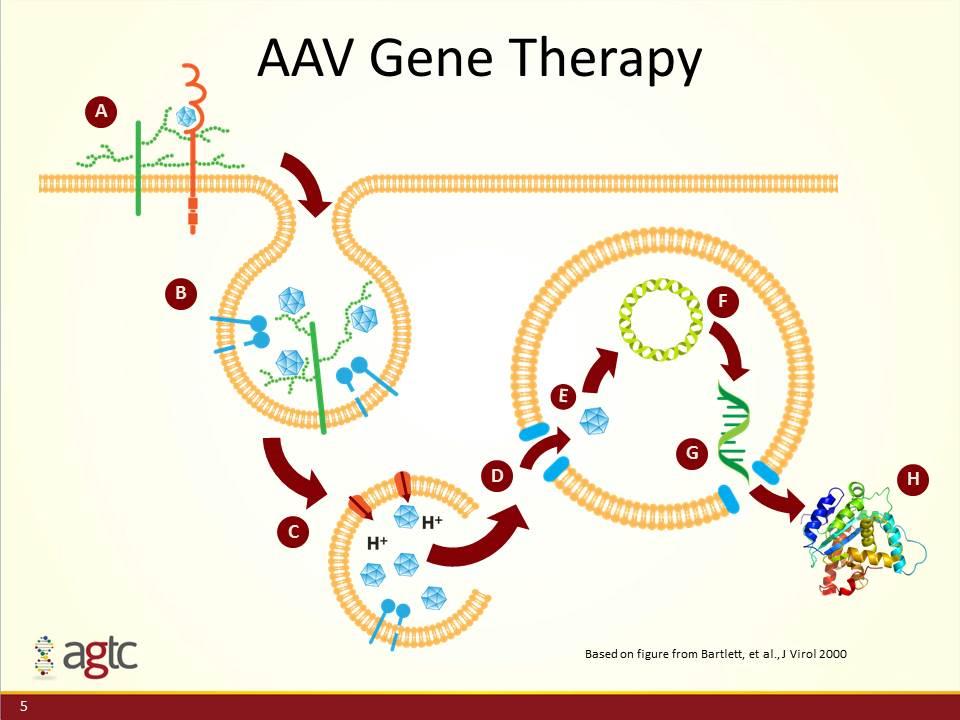
AAV Gene Therapy A B E C D F G H Based on figure from Bartlett, et al., J Virol 2000
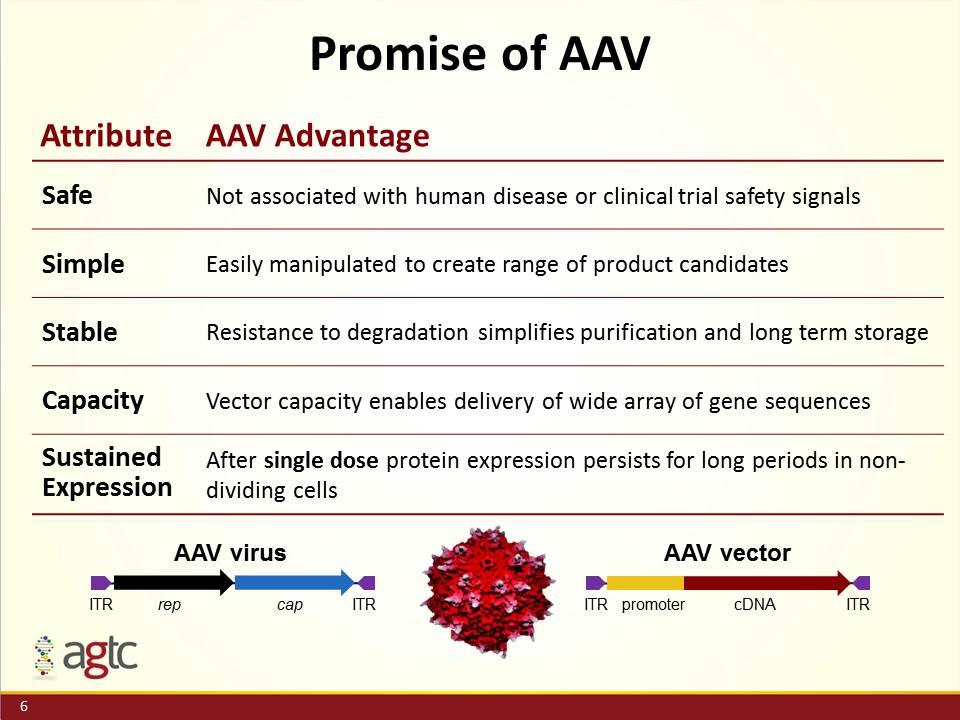
Attribute AAV Advantage Safe Not associated with human disease or clinical trial safety signals Simple Easily manipulated to create range of product candidates Stable Resistance to degradation simplifies purification and long term storage Capacity Vector capacity enables delivery of wide array of gene sequences Sustained Expression After single dose protein expression persists for long periods in non-dividing cells Promise of AAV ITR ITR rep cap AAV virus ITR ITR promoter cDNA AAV vector
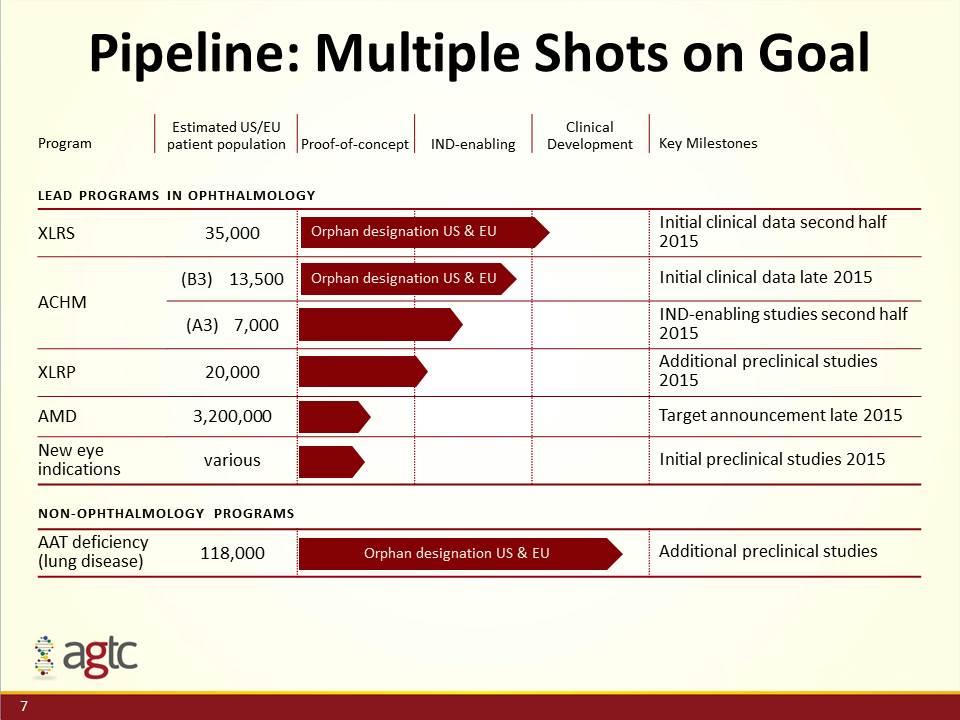
Pipeline: Multiple Shots on Goal Program Estimated US/EU patient population Proof-of-concept IND-enabling Clinical Development Key Milestones Lead programs in ophthalmology XLRS 35,000 Initial clinical data second half 2015 ACHM (B3) 13,500 Initial clinical data late 2015 (A3) 7,000 IND-enabling studies second half 2015 XLRP 20,000 Additional preclinical studies 2015 AMD 3,200,000 Target announcement late 2015 New eye indications various Initial preclinical studies 2015 NON-ophthalmology PROGRAMS AAT deficiency (lung disease) 118,000 Additional preclinical studies Orphan designation US & EU Orphan designation US & EU Orphan designation US & EU
Lead Product Candidates: Compelling business opportunities
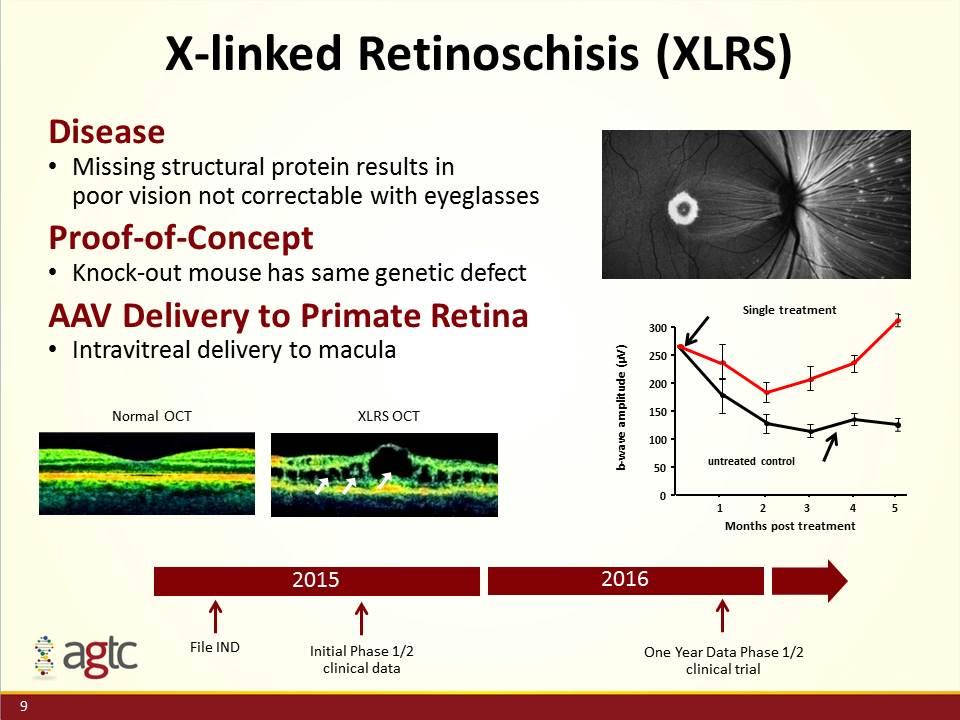
X-linked Retinoschisis (XLRS) Disease Missing structural protein results in poor vision not correctable with eyeglasses Proof-of-Concept Knock-out mouse has same genetic defect AAV Delivery to Primate Retina Intravitreal delivery to macula Months post treatment 0 50 100 150 200 250 300 b-wave amplitude (µV) 1 2 3 4 5 Single treatment untreated control Normal OCT XLRS OCT File IND Initial Phase 1/2 clinical data 2016 2015 One Year Data Phase 1/2 clinical trial
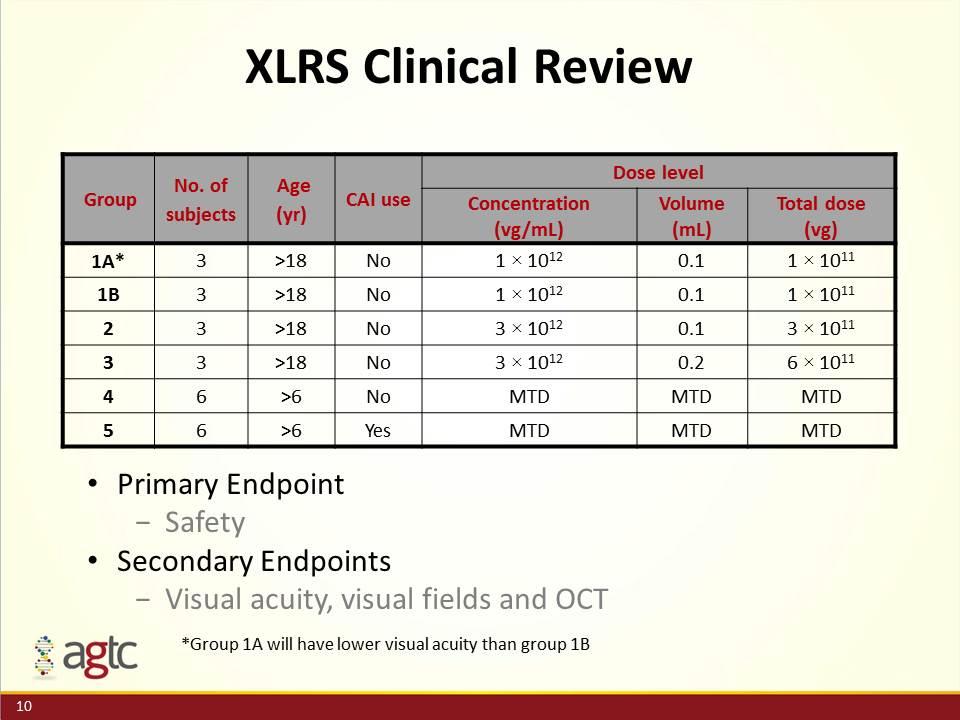
XLRS Clinical Review Group No. of subjects Age (yr) CAI use Dose level Concentration (vg/mL) Volume (mL) Total dose (vg) 1A* 3 >18 No 1 × 1012 0.1 1 × 1011 1B 3 >18 No 1 × 1012 0.1 1 × 1011 2 3 >18 No 3 × 1012 0.1 3 × 1011 3 3 >18 No 3 × 1012 0.2 6 × 1011 4 6 >6 No MTD MTD MTD 5 6 >6 Yes MTD MTD MTD Primary Endpoint Safety Secondary Endpoints Visual acuity, visual fields and OCT *Group 1A will have lower visual acuity than group 1B
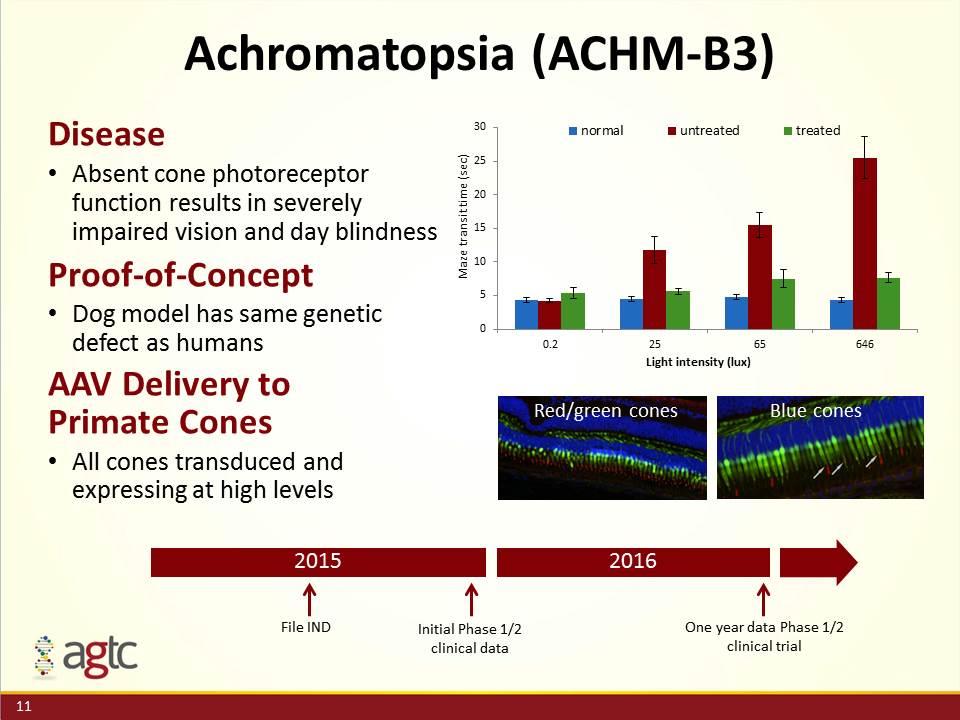
Achromatopsia (ACHM-B3) Disease Absent cone photoreceptor function results in severely impaired vision and day blindness Proof-of-Concept Dog model has same genetic defect as humans AAV Delivery to Primate Cones All cones transduced and expressing at high levels 2015 2016 Initial Phase 1/2 clinical data One year data Phase 1/2 clinical trial Red/green cones Blue cones File IND
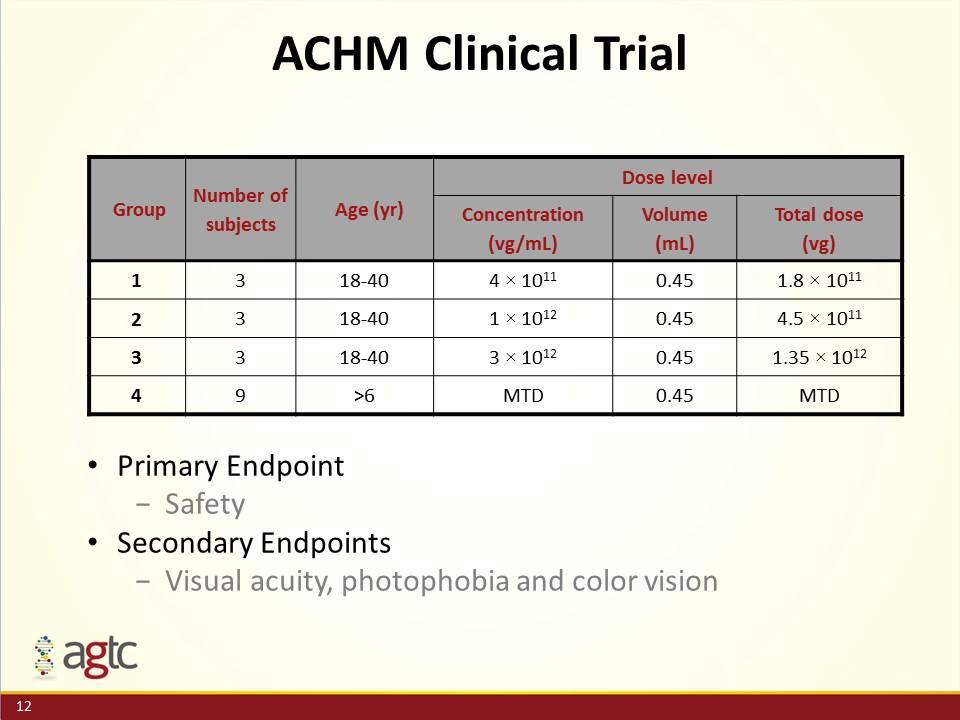
ACHM Clinical Trial Primary Endpoint Safety Secondary Endpoints Visual acuity, photophobia and color vision Group Number of subjects Age (yr) Dose level Concentration (vg/mL) Volume (mL) Total dose (vg) 1 3 18-40 4 × 1011 0.45 1.8 × 1011 2 3 18-40 1 × 1012 0.45 4.5 × 1011 3 3 18-40 3 × 1012 0.45 1.35 × 1012 4 9 >6 MTD 0.45 MTD
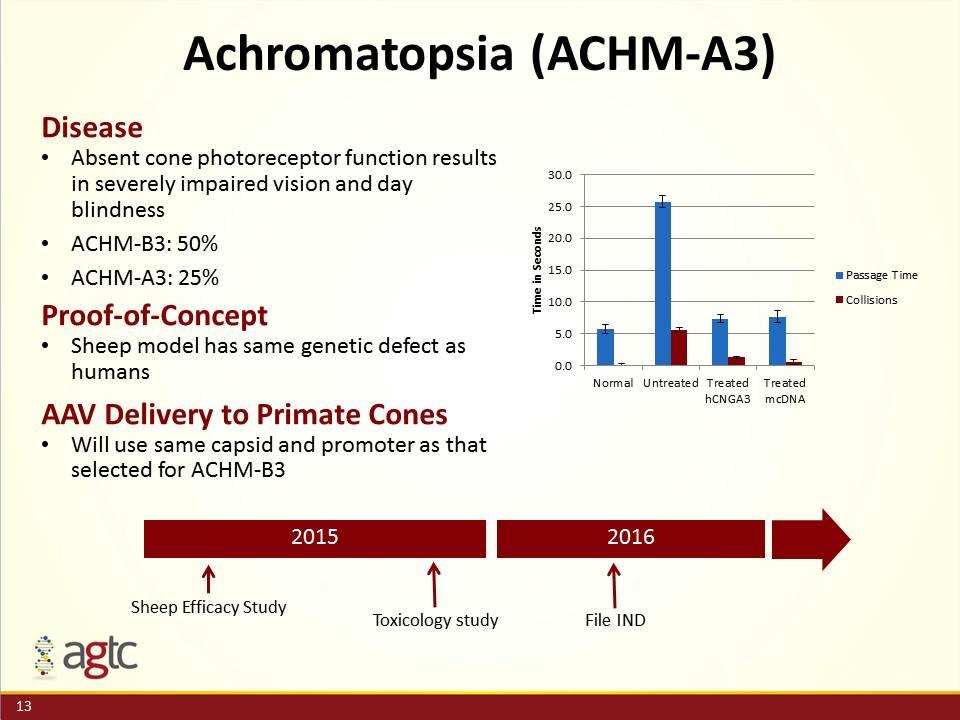
Achromatopsia (ACHM-A3) 2015 2016 Sheep Efficacy Study Toxicology study File IND Disease Absent cone photoreceptor function results in severely impaired vision and day blindness ACHM-B3: 50% ACHM-A3: 25% Proof-of-Concept Sheep model has same genetic defect as humans AAV Delivery to Primate Cones Will use same capsid and promoter as that selected for ACHM-B3
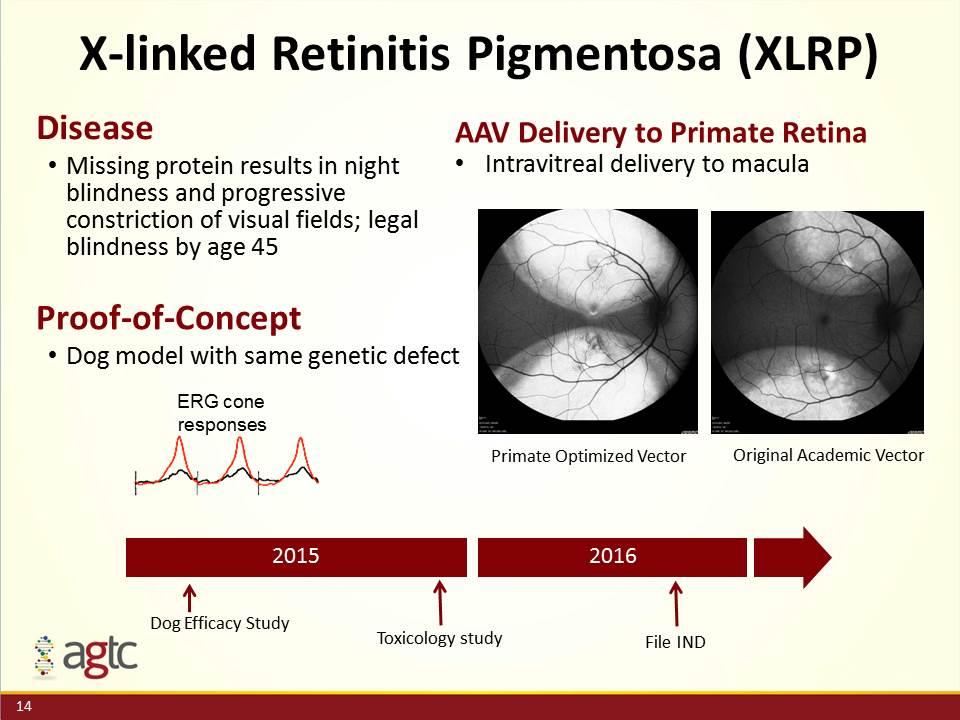
X-linked Retinitis Pigmentosa (XLRP) Disease Missing protein results in night blindness and progressive constriction of visual fields; legal blindness by age 45 2015 2016 Dog Efficacy Study Toxicology study Proof-of-Concept Dog model with same genetic defect ERG cone responses File IND AAV Delivery to Primate Retina Intravitreal delivery to macula Primate Optimized Vector Original Academic Vector
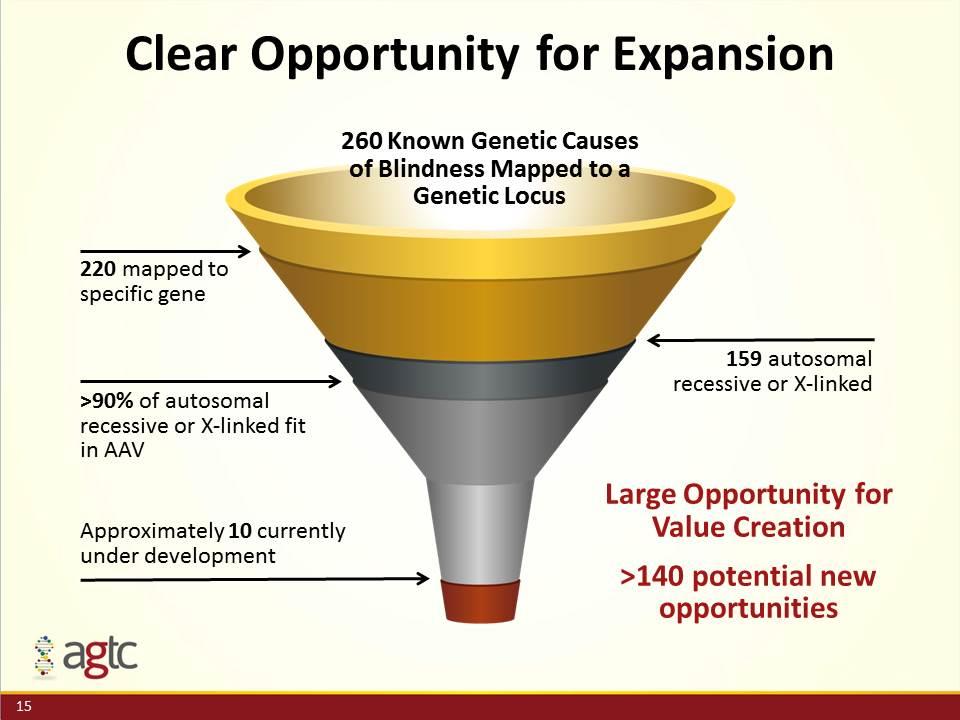
220 mapped to specific gene Clear Opportunity for Expansion 159 autosomal recessive or X-linked >90% of autosomal recessive or X-linked fit in AAV Approximately 10 currently under development Large Opportunity for Value Creation >140 potential new opportunities 260 Known Genetic Causes of Blindness Mapped to a Genetic Locus
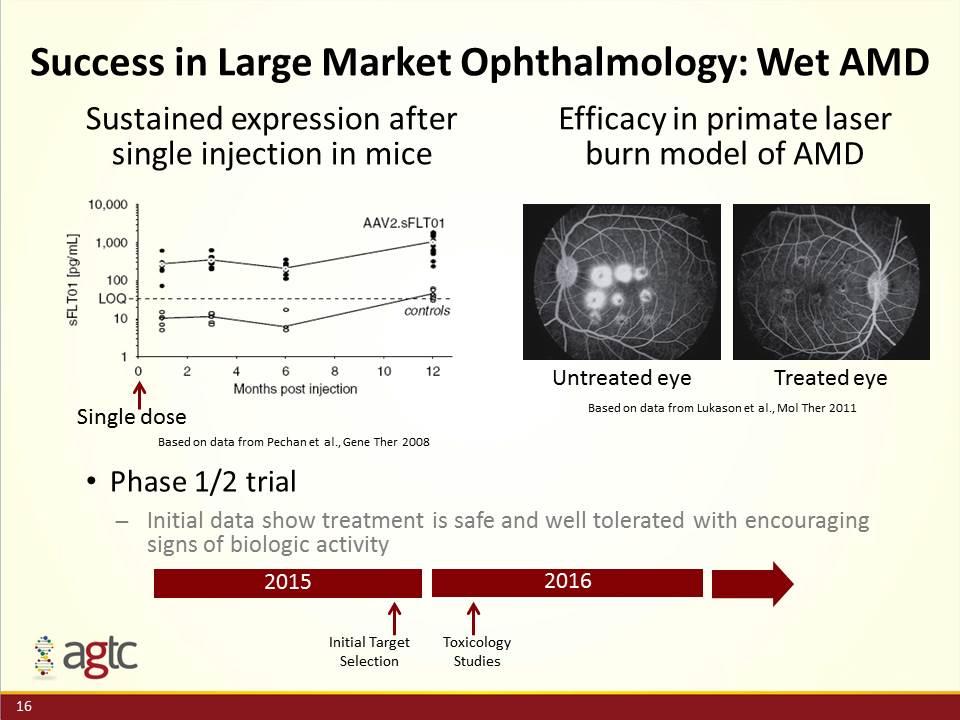
Success in Large Market Ophthalmology: Wet AMD Phase 1/2 trial Initial data show treatment is safe and well tolerated with encouraging signs of biologic activity Based on data from Lukason et al., Mol Ther 2011 Based on data from Pechan et al., Gene Ther 2008 Single dose Untreated eye Treated eye Sustained expression after single injection in mice Efficacy in primate laser burn model of AMD 2015 2016 Toxicology Studies Initial Target Selection
Realizing the Promise of Gene Therapy

Management Sue Washer Chief Executive Officer Jeff Chulay, M.D. Chief Medical Officer Stephen Potter Chief Business Officer Larry Bullock Chief Financial Officer Matt Feinsod, M.D. Product Development Officer Dave Knop, Ph.D. Sr. Director Process Development Guo-jie Ye, Ph.D. Sr. Director Research and Preclinical Development Experienced Team

KOLs / Investigators / Collaborators / Founders Expert Partners Board Members
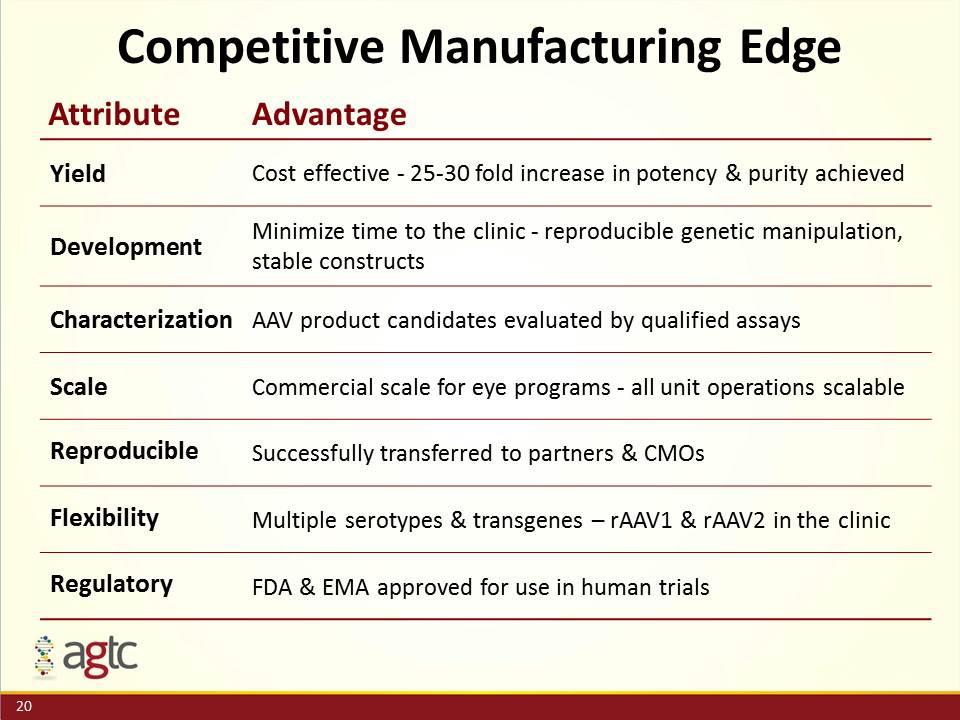
Competitive Manufacturing Edge Attribute Advantage Yield Cost effective - 25-30 fold increase in potency & purity achieved Development Minimize time to the clinic - reproducible genetic manipulation, stable constructs Characterization AAV product candidates evaluated by qualified assays Scale Commercial scale for eye programs - all unit operations scalable Reproducible Successfully transferred to partners & CMOs Flexibility Multiple serotypes & transgenes – rAAV1 & rAAV2 in the clinic Regulatory FDA & EMA approved for use in human trials
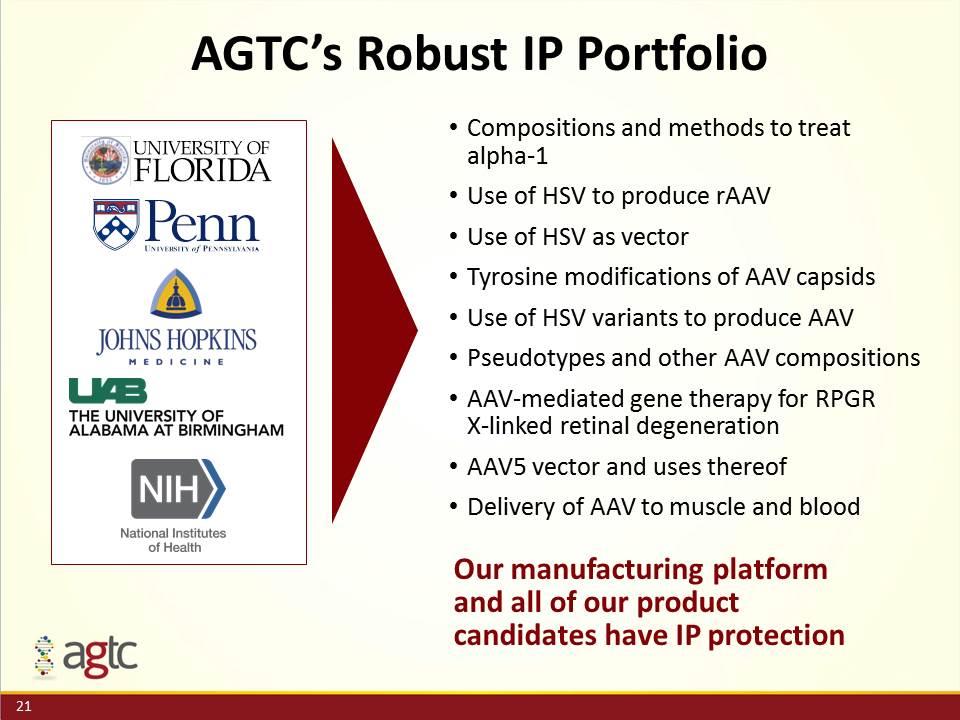
AGTC’s Robust IP Portfolio Compositions and methods to treat alpha-1 Use of HSV to produce rAAV Use of HSV as vector Tyrosine modifications of AAV capsids Use of HSV variants to produce AAV Pseudotypes and other AAV compositions AAV-mediated gene therapy for RPGR X-linked retinal degeneration AAV5 vector and uses thereof Delivery of AAV to muscle and blood Our manufacturing platform and all of our product candidates have IP protection

Financials and Key Milestones
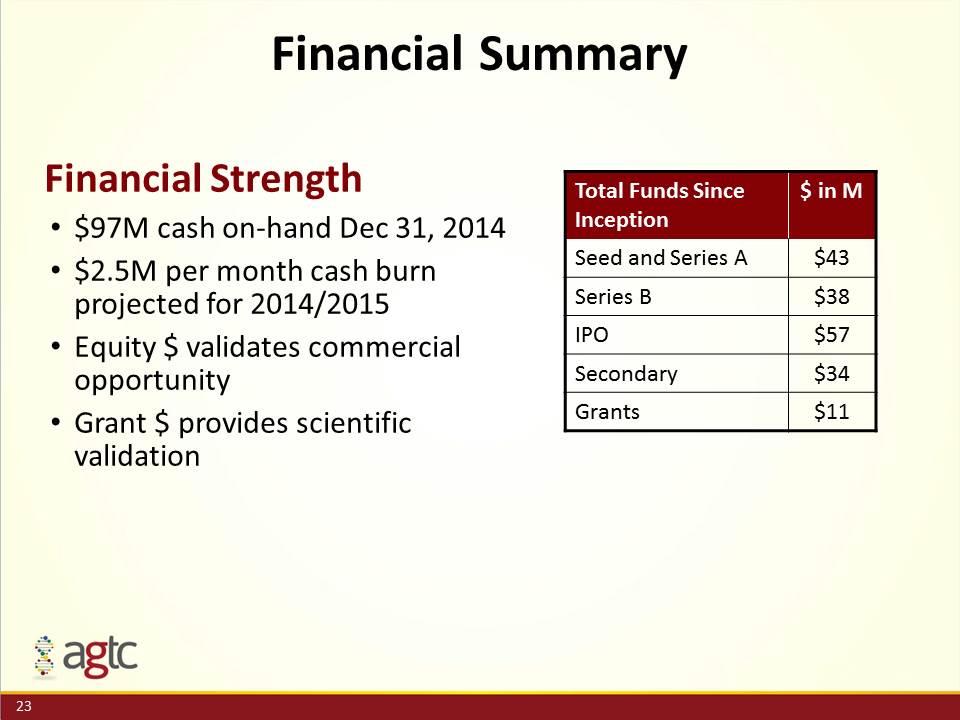
Financial Summary Financial Strength $97M cash on-hand Dec 31, 2014 $2.5M per month cash burn projected for 2014/2015 Equity $ validates commercial opportunity Grant $ provides scientific validation Total Funds Since Inception $ in M Seed and Series A $43 Series B $38 IPO $57 Secondary $34 Grants $11
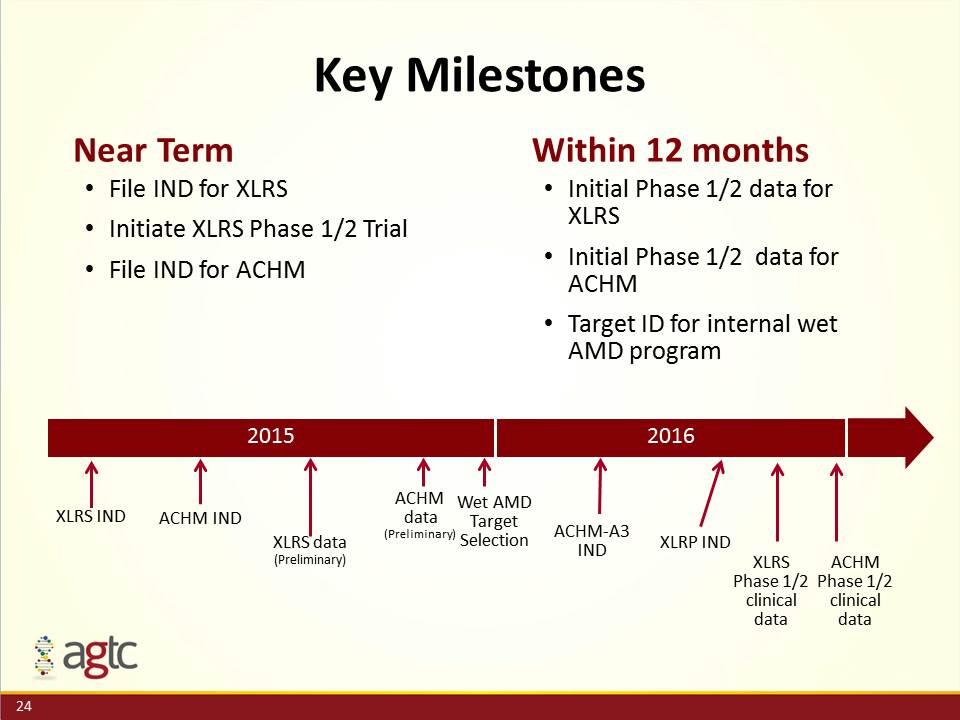
Key Milestones Near Term File IND for XLRS Initiate XLRS Phase 1/2 Trial File IND for ACHM 2015 2016 ACHM IND XLRS IND XLRS data (Preliminary) ACHM data (Preliminary) Wet AMD Target Selection Within 12 months Initial Phase 1/2 data for XLRS Initial Phase 1/2 data for ACHM Target ID for internal wet AMD program XLRP IND XLRS Phase 1/2 clinical data ACHM Phase 1/2 clinical data ACHM-A3 IND

Company Highlights Become the world leader in ophthalmology gene therapy Clear Strategy Multiple programs providing significant potential long-term value to patients & providers, key inflection points in 2015 Broad Pipeline Robust understanding of vector selection, design, manufacturing and delivery Extensive Expertise Key IP, >100 patents, protects gene, vector capsid, regulatory elements, manufacturing and/or delivery for each candidate Intellectual Property Demonstrated success in balancing resources, risks and rewards; >100 patients treated in Phase 1/2 trials Track Record of Progress Optimized AAV vectors that deliver safe and sustained expression; manufactured reproducibly at commercial scale Leading Gene Therapy Platform

Visionary Science for Life Changing Cures 2015
