Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AERIE PHARMACEUTICALS INC | d878535d8k.htm |
 Aerie Pharmaceuticals, Inc.
Company Overview
February 23, 2015
Building a Major
Ophthalmic
Pharmaceutical
Company
Exhibit 99.1 |
 2
Important Information
Any
discussion
of
the
potential
use
or
expected
success
of
our
product
candidates
is
subject
to
our
product
candidates
being
approved
by
regulatory
authorities.
In
addition,
any
discussion
of
clinical
data
results
for
our
Rhopressa™
and
Roclatan™
product
candidates
relate
to
the
results
in
our
Phase
2
clinical trials.
The information in this presentation is current only as of its date and may have
changed or may change in the future. We undertake no obligation to update
this information in light of new information, future events or otherwise. We
are not making any representation or warranty that the information in this presentation is
accurate or complete.
Certain
statements
in
this
presentation
are
“forward-looking
statements”
within
the
meaning
of
the
federal
securities laws, including beliefs, expectations, estimates, projections and
statements relating to our business plans, prospects and objectives, and the
assumptions upon which those statements are based. Words
such
as
“may,”
“will,”
“should,”
“would,”
“could,”
“believe,”
“expects,”
“anticipates,”
“plans,”
“intends,”
“estimates,”
“targets,”
“projects,”
“potential”
or similar expressions are intended to identify these forward-
looking statements. These statements are based on the Company’s current plans
and expectations. Known and unknown risks, uncertainties and other factors
could cause actual results to differ materially from those contemplated by
the statements. In evaluating these statements, you should specifically
consider various factors that may cause our actual results to differ materially
from any forward-looking statements. These risks and uncertainties are
described more fully in the quarterly and annual
reports
that
we
file
with
the
SEC,
particularly
in
the
sections
titled
“Risk
Factors”
and
“Management’s
Discussion
and
Analysis
of
Financial
Condition
and
Results
of
Operations.”
In
particular,
the preclinical research discussed in this presentation is preliminary and the
outcome of such preclinical studies may not be predictive of the outcome of
later clinical trials. Any future clinical trial results may not demonstrate
safety and efficacy sufficient to obtain regulatory approval related to the preclinical research
findings discussed in this presentation. Such forward-looking statements only
speak as of the date they are made. We undertake no obligation to publicly
update or revise any forward-looking statements, whether because of new
information, future events or otherwise, except as otherwise required by law. |
 3
Current Aerie
Products:
Once-Daily
IOP-Lowering
Eye Drops for
Glaucoma
Pre-Clinical
Research
Findings
•
Rhopressa™
shows potential to modify diseased tissue
•
May block fibrotic response in trabecular meshwork cells
•
May increase perfusion of the trabecular meshwork
•
AR-13154 shows potential for the treatment of wet AMD
•
May inhibit ROCK/JAK/PDGFR-
•
Lesion size reduction in rats exceeds market-leading product
•
Triple action Rhopressa™
•
Inhibits ROCK and NET, lowers EVP, targets diseased tissue
•
Expect P3 efficacy data mid-Q2 2015, NDA Filing mid-2016
•
Quadruple Action Roclatan™
•
Fixed combination of Rhopressa™
and latanoprost
•
P2b achieved all clinical endpoints, P3 to start mid-2015
•
Potentially most efficacious IOP-lowering therapy
Aerie
–
Building
a
Major
Ophthalmic
Pharmaceutical Company
These new preclinical discoveries represent potential breakthroughs
Full patent protection through at least 2030; Blockbuster Potential
|
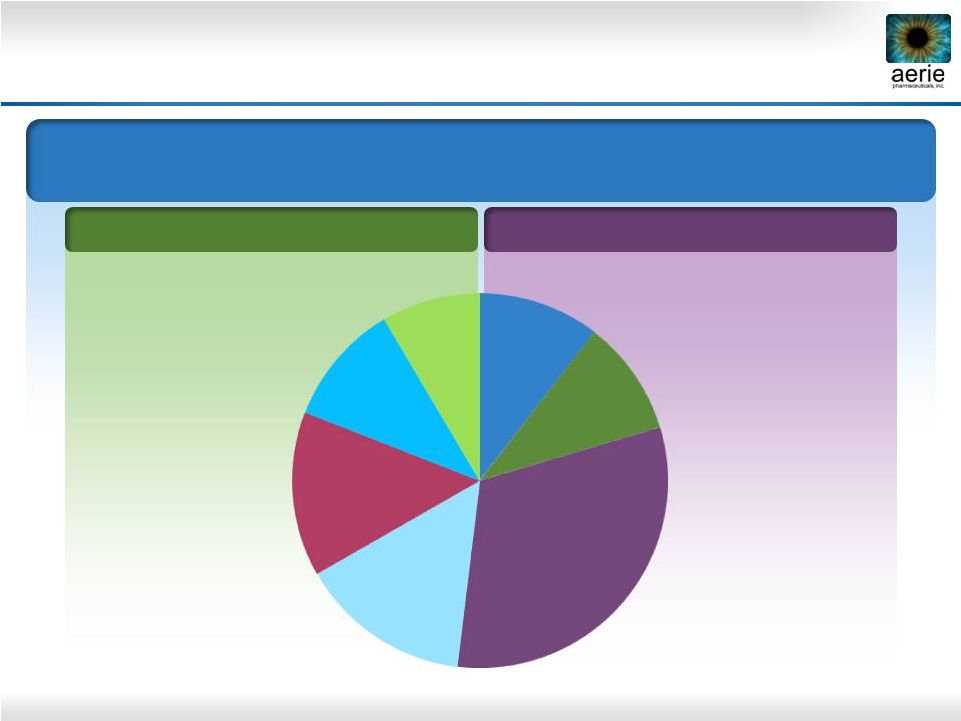 4
FY 2013 U.S. Glaucoma Market = $2.0B; 31.5M TRx
Market Share in TRx
PGA Market
Non-PGA Market
Current Product Dashboard
PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic
Anhydrase Inhibitor Once Daily
Bimatoprost
Travoprost
Latanoprost
BB
BB Fixed Combo
AA
CAI
15%
8%
10%
10%
14%
10%
31%
2-3 Times Daily |
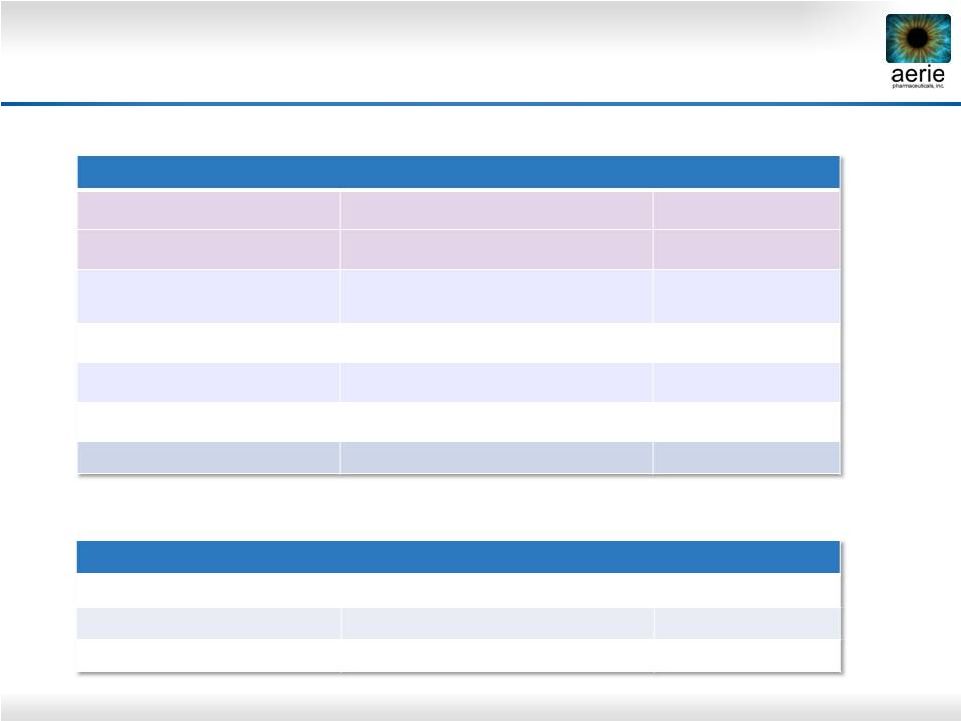 5
New MOAs
Rhopressa™
ROCK/NET inhibitor (qd)
Phase 3
Roclatan™
ROCK/NET inhibitor + PGA (qd)
Phase 3
K-115 (Kowa)
ROCK inhibitor (bid)
Approved in Japan*
Adjunctive Therapy
AMA0076 (Amakem)
ROCK inhibitor (bid)
Phase 2a
INO-8875 (Inotek)
Adenosine-A1 agonist (bid or qd)
Phase 2
OPA-6566 (Acucela)
Adenosine-A2a agonist
(bid)
Phase 1/2
SYL040012 (Sylentis)
RNAi beta blocker (qd)
Phase 2
New PGAs: not usable as add-on to current PGAs
Rhopressa™
and Roclatan™: advanced triple and quadruple MOAs
New PGAs
BOL-303259 (B+L)
NO donating latanoprost (qd)
Phase 3
DE-117 (Santen)
EP2 agonist (qd)
Phase 2
ONO-9054 (Ono)
FP/EP3 agonist (qd)
Phase 2
Glaucoma Competitors in Pipeline
* Approved in Japan 9/29/2014
(Aerie AR-13324)
(Aerie PG324) |
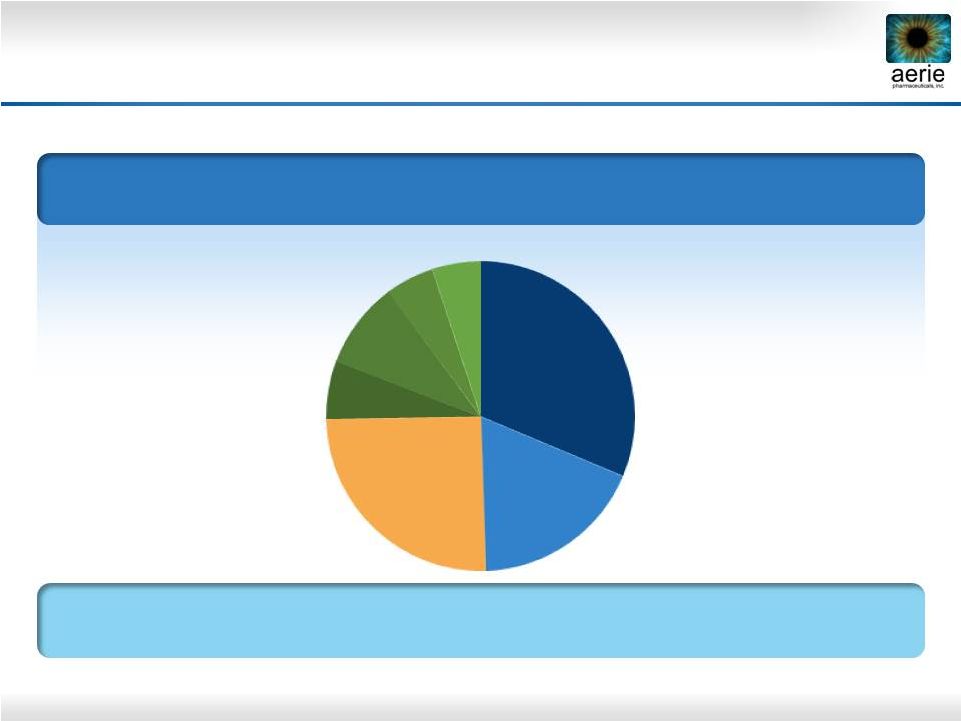 6
2023 U.S. Glaucoma Market
and Market Share Projections*
Projected Market Share in TRx
PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic
Anhydrase Inhibitor Data Source: IMS 2013 FY TRX. Internal projection
*Projected market shares compiled by an independent market research company
U.S. Glaucoma Market = $4.9B; 41.7 M TRx
TRx Growth 2013-2023 = 2.3%
Roclatan™
+
Rhopressa™
May
Capture
Over
40%
Market
Share
Based on 200 U.S. Physician Responses to Survey
PGA’s
BB
BB Fixed Combo
AA
CAI
Rhopressa™
Roclatan™
31%
18%
25%
6%
9%
5%
5% |
 7
Managed Care Market Research Summary
* Respondents ranked products on a scale of 1-7
** After pricing was disclosed and discussed
Payers’
score
on
the
overall
utility
of
Rhopressa™
was
4.7*
(Market research firm states that scores above 4 are high)
Payers’
score
on
the
overall
utility
of
Roclatan™
was
5.2*
(Market research firm states that scores above 5 are rare)
100%
of
respondents
placed
Rhopressa™
and
Roclatan™
in
Tier 2 or Tier 3 of Commercial and Medicare Part D
formularies.**
There
were
no
“Not
Covered”
recommendations.
Interviews with 12 decision makers whose companies cover over
42 million Commercial, Medicare and Medicaid lives
Payers were impressed by Roclatan™’s
unprecedented lowering of IOP Payers were receptive to
Rhopressa™’s new MOAs and efficacy at any baseline IOP |
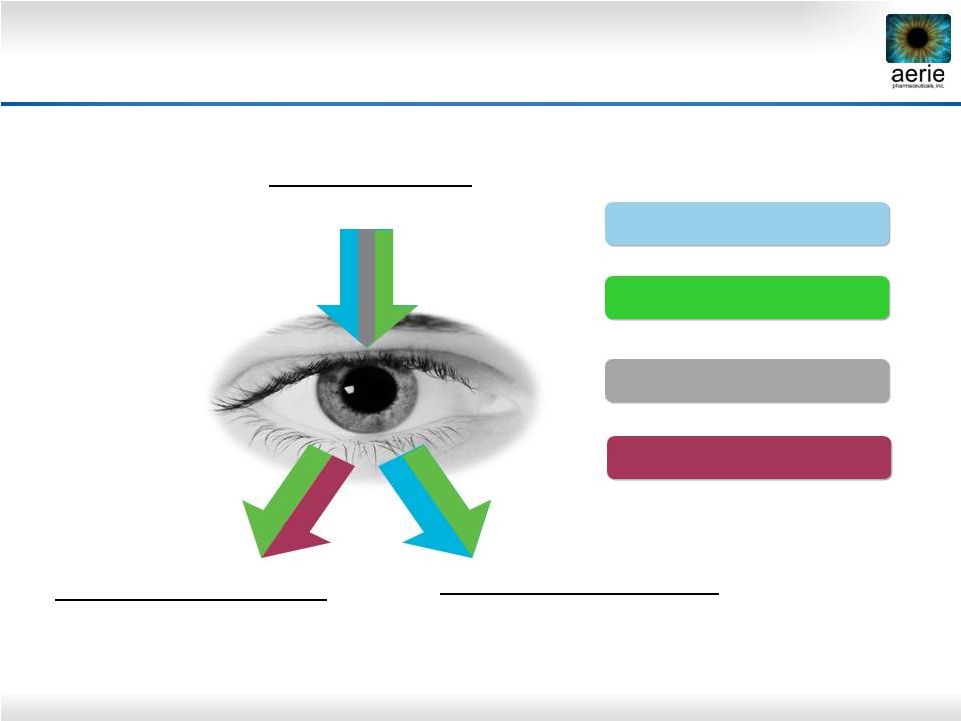 8
increase
Decreases Fluid
Inflow/Production
(Ciliary Processes)
Increases Fluid Outflow:
Secondary Drain
(Uveoscleral Pathway)
Increases Fluid Outflow:
Primary
Drain
-
Trabecular
Meshwork
(TM);
Lowers
EVP
-
(Episcleral
Venous
Pressure)
Rhopressa™
Roclatan™
AA, BB, CAI
PGAs
Aerie Products Cover the IOP-lowering Spectrum |
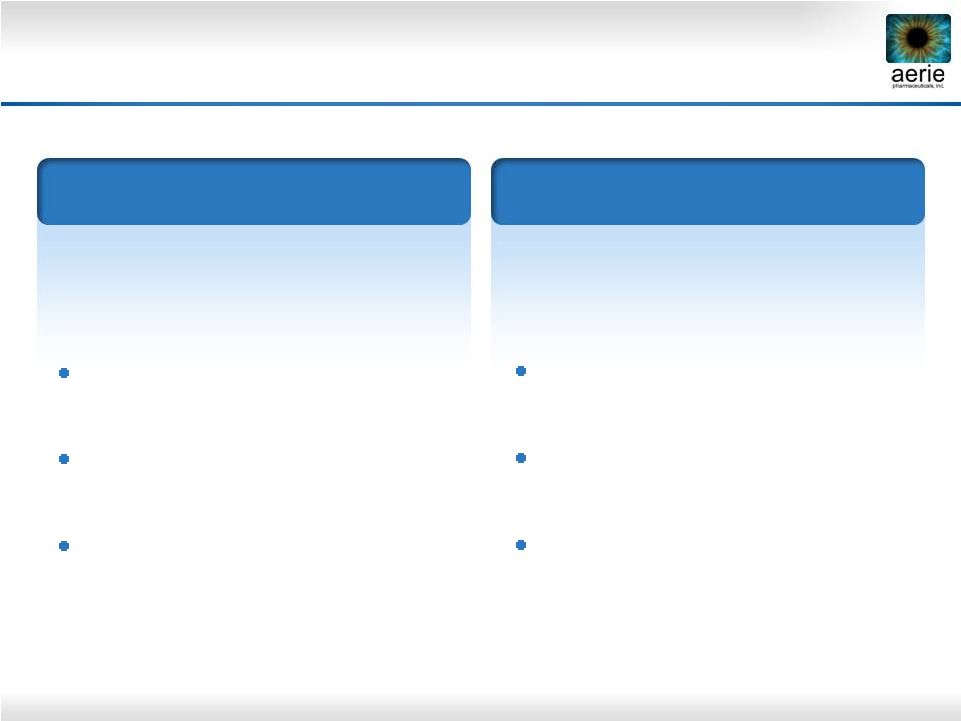 9
Aerie Product Market Positioning*
* Confirmed by Market Research
Future drug of choice for the 80%
of patients with IOP of 26 mmHg
or less
Triple-Action Rhopressa™
Future product of choice for
patients requiring maximal IOP
lowering
Quadruple-Action Roclatan™
Also for PGA users as add-on
therapy
Also for PGA non-responders and
those with tolerability concerns
Also for patients with low-tension
glaucoma
Efficacy potentially greater than
all currently marketed drugs
For patients with IOPs
above 26
mmHg Also for patients at any IOP with
significant disease progression |
 10
Rhopressa
™
NET
RKI
NET
RKI
Trabecular
Meshwork
Triple-Action Rhopressa™
Episcleral
Veins
Schlemm’s
Canal
IOP-Lowering Mechanisms
ROCK inhibition relaxes TM, increases outflow
NET inhibition reduces fluid production
ROCK inhibition lowers Episcleral Venous
Pressure (EVP)
Ciliary Processes
Uveoscleral
Outflow
IOP-Lowering Mechanisms
ROCK inhibition relaxes TM, increases outflow
NET inhibition reduces fluid production
ROCK inhibition lowers Episcleral Venous
Pressure (EVP)
Cornea |
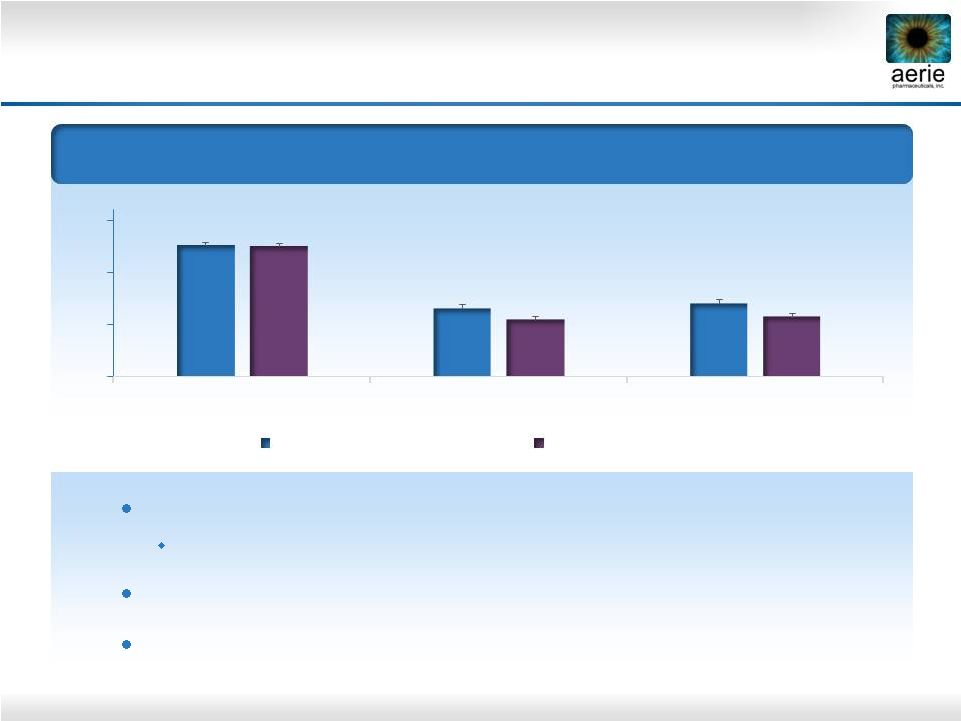 11
Rhopressa™: Powerful Compound for Physiological
Lowering of IOP –
Phase 2b Results
Mean
Diurnal
IOP
–
Entry
IOP
22-36
mmHg
(n=221)
25.5
18.4
18.7
13
18
23
28
Baseline
Day 14
Day 28
Rhopressa™
0.02% (n=71)
Latanoprost (n=76)
Once-daily
PM
dosing
of
0.02%
Rhopressa™
is
highly
effective
IOP -5.7 and -6.2 mmHg on D28 and D14
Rhopressa™
efficacy results within ~1 mmHg of latanoprost
Favorable tolerability profile with no systemic side effects
25.6
19.5
20.0 |
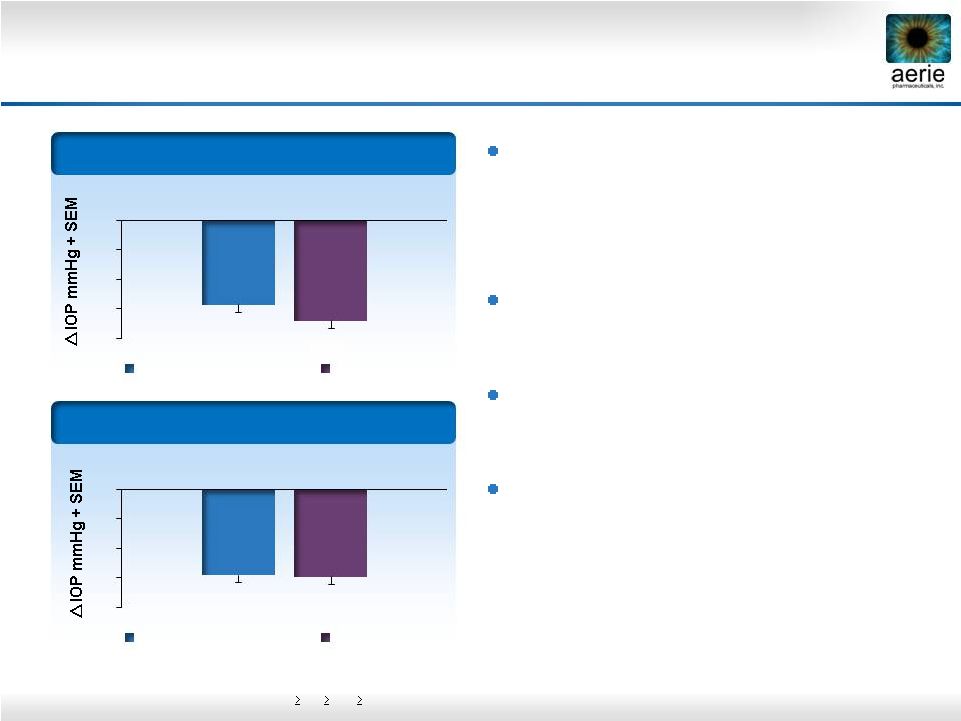 12
Rhopressa™
Differentiated Efficacy Profile
Phase 2b baseline IOP entry requirements:
24,
22,
22 mmHg (8 AM, 10 AM, 4 PM)
Rhopressa™
and latanoprost clinically
and statistically equivalent in patients
with moderately elevated IOPs of 22-26
mmHg
Latanoprost
loses
~1
mmHg
efficacy @
baselines of 22-26 mmHg
Rhopressa™
maintains consistent
efficacy
Rhopressa™; 1
st
product to treat the
diseased tissue (trabecular meshwork)
with a once-daily (QD) dose
Baseline:
22
–
36
mmHg
(n=221)
Baseline:
22
–
26
mmHg
(n=106)
-6.8
-8
-6
-4
-2
0
0.02% Rhopressa™
Latanoprost
-5.8
-5.9
-8
-6
-4
-2
0
0.02% Rhopressa™
Latanoprost
-5.7 |
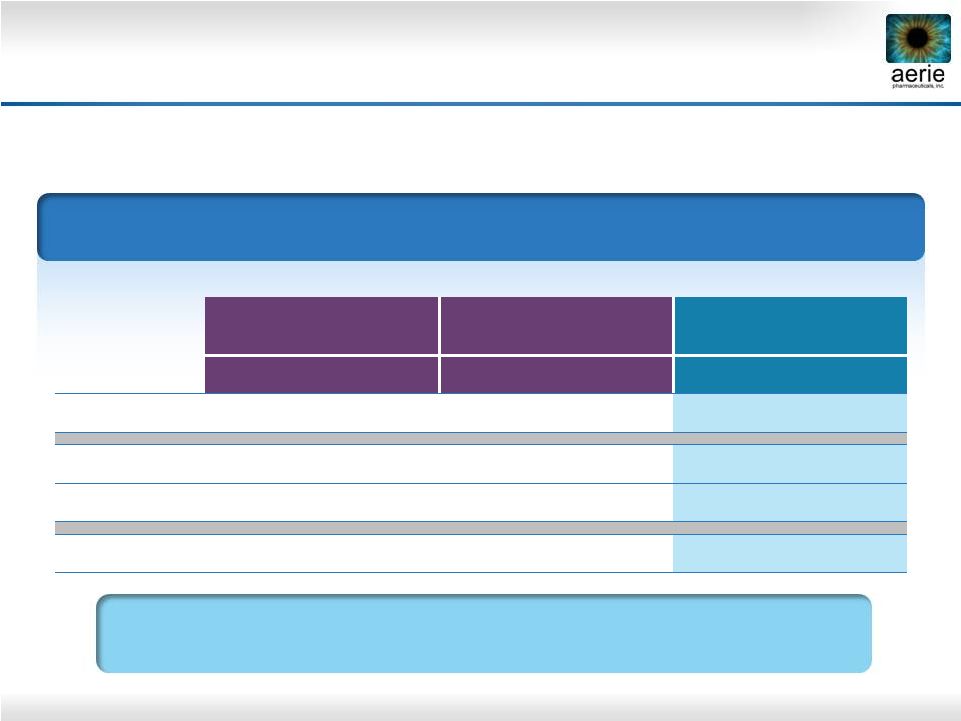 13
Sustained
Effect
of
Rhopressa™
vs.
Latanoprost
* 36 hours post dose
8 AM Mean IOP (mmHg) by Treatment Group
0.02% Rhopressa
™
Roclatan™
Phase 2b
0.02% Rhopressa
™
Rhopressa™
Phase 2b
0.005% latanoprost
Rhopressa™
Phase 2b
(n = 78)
(n = 71)
(n = 76)
Baseline
26.6
27.2
26.8
Day 8
20.0
21.1
20.0
Day 29
20.3
21.2
19.2
Day 30*
21.0
22.2
22.4
Rhopressa™
Duration
is
Superior
to
Latanoprost
36 Hours After Last Dose |
 14
Rhopressa™
EVP-Lowering
Breakthrough
Phase 2b data provided first sign of EVP-lowering:
Phase 1 study in low baseline IOP subjects:
Preclinical in vivo study:
Note: Timolol and latanoprost reported to have no effect or to increase
EVP in animal models
Consistent Efficacy Across Baseline IOPs
Lowered Average IOP by Over 30%
From 16 Down to 11 mmHg
Lowered EVP by 35% |
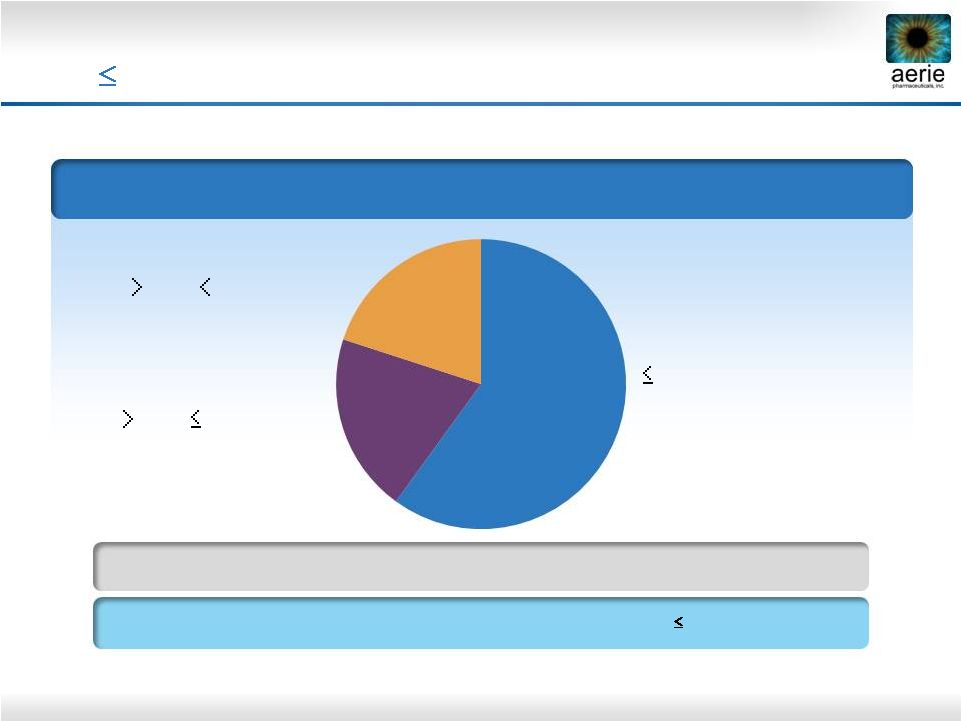 15
Baseline IOP*
~80% of U.S. Glaucoma Patients Have IOPs that
are
26 mmHg at Time of Diagnosis
The Baltimore Eye Survey
*
Sommer A, Tielsch JM, Katz J et al. Relationship between intraocular pressure and
primary open angle glaucoma among white and black Americans:
The
Baltimore
eye
survey.
Arch
Ophthalmol
1991;109:1090-1095
**
IWASE et al Tajimi study group. Japan Glaucoma Society. Ophthalmology, 2004 Sep, 111 (9): 1641-8.
21 mmHg
(Normal Tension Glaucoma)
21 -
26 mmHg
26
-
35
mmHg
10,444 Individuals Were Screened for the Prevalence of Primary Open-Angle
Glaucoma (POAG) and the IOP at Time of Diagnosis
92% of Japanese
Patients
with
POAG,
IOPs
Were
21
mmHg**
20%
20%
60% |
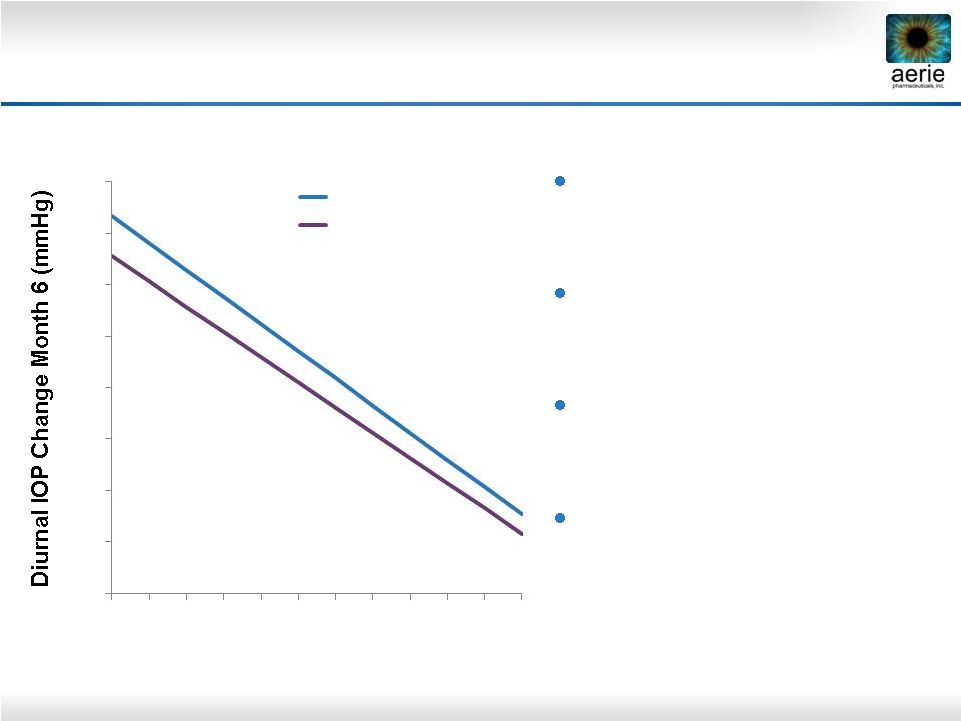 16
Latanoprost and Timolol Show Reduced Efficacy
at Lower Baseline IOPs
Latanoprost and timolol
lose efficacy as baseline
IOPs decline
Timolol at least 1 mmHg less
effective than latanoprost
across all published baselines
Rhopressa™
equivalent/
non-inferior to latanoprost
at
baselines
22–26
mmHg
Timolol is the standard
comparator for glaucoma
Phase 3 trials
-16
-14
-12
-10
-8
-6
-4
-2
0
16
18
20
22
24
26
28
30
32
34
36
38
Untreated Diurnal IOP (mmHg)
Timolol (n=369)
Latanoprost (n=460)
Pooled data from three latanoprost registration studies. Hedman and Alm; European Journal
Ophthalmology; 2000 |
 17
Rhopressa™
Registration Trial Overview
Primary efficacy endpoint: IOP at nine time points through Day 90
Phase 3 entry IOP is >20 mmHg and <27 mmHg
Non-inferiority design vs. timolol
95% CI within 1.5 mmHg at all time points, within 1.0 mmHg at a majority
of time points
Combined trials to include approximately 1,300 total patients
100 patients with 12 months of safety data needed for NDA filing
Should meet efficacy requirements for EMA filing
300 patients with 6 months safety data needed for EMA filing and
100 with
12 months |
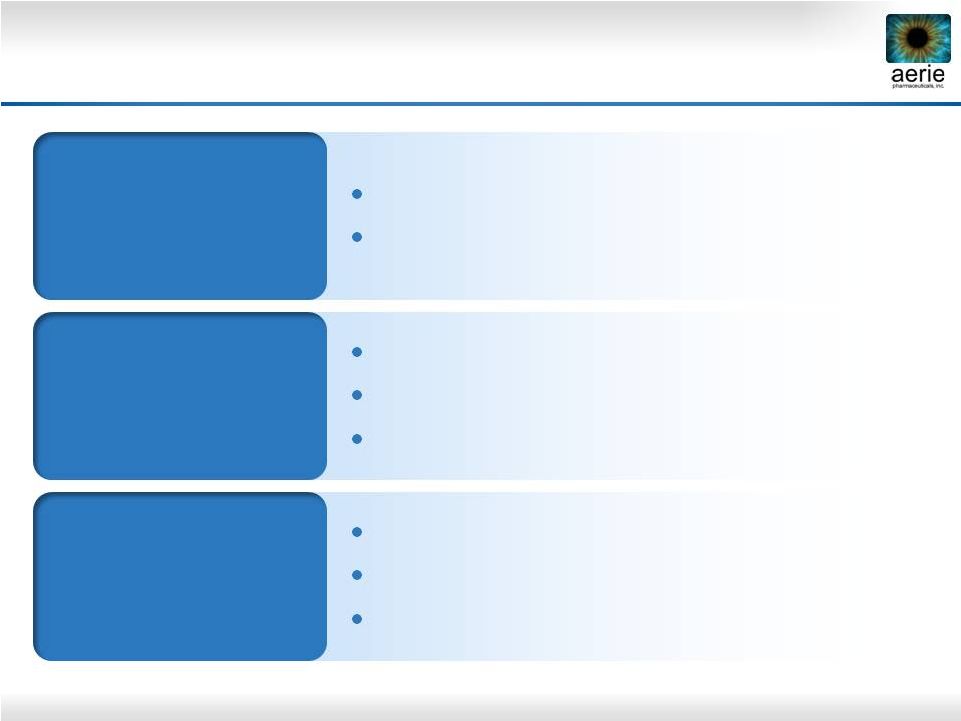 18
Rhopressa™
Registration Trial Design
*
*
PGAs have been shown to be less effective when dosed BID
PGAs have been shown to be less effective when dosed BID
“Rocket 1”
90-Day Efficacy
Registration Trial
Rhopressa™
0.02% QD
~200 patients
Timolol BID
~200 patients
“Rocket 2”
One Year Safety
(3 Mo. Interim
Efficacy)
Registration Trial
“Rocket 3”
One Year Safety
Registration Trial
Canada
Rhopressa™
0.02% QD
~230 patients
Rhopressa™
0.02% BID
*
~230 patients
Timolol BID
~230 patients
Rhopressa™
0.02% QD
~90 patients
Rhopressa™
0.02% BID
~90 patients
Timolol BID
~60 patients |
 19
Ciliary Processes
Cornea
Uveoscleral
Outflow
NET
RKI
NET
RKI
Trabecular
Meshwork
Episcleral
Veins
Schlemm’s
Canal
Latanoprost
Rhopressa
™
Quadruple-Action Roclatan™
Fixed
Combination
of
Rhopressa™
with
Latanoprost
IOP-Lowering Mechanisms
ROCK inhibition relaxes TM, increases outflow
NET inhibition reduces fluid production
ROCK inhibition lowers EVP
PGA receptor activation
increases uveoscleral outflow |
 20
Roclatan™
Phase
2b
Clinical
Trial
Design
Phase 2b Protocol
Roclatan™
0.01%
vs.
Roclatan™
0.02%
vs.
Rhopressa™
0.02%
vs.
Latanoprost
All Dosed QD PM
~300 Patients
28 Days
Primary efficacy endpoint:
Mean diurnal IOP on Day 29
Two concentrations of
Roclatan™
vs. Rhopressa™
0.02% and latanoprost
Trial design follows FDA
requirement for fixed-dose
combination
Statistically significant difference
at measured time points
Higher combo efficacy vs.
components of at least 1–3
mmHg, as previously accepted
by FDA for product approval |
 21
Roclatan™
Phase 2b Clinical Trial Performance
Achieved primary efficacy measure
Superiority over each of the components on day 29
Achieved statistical superiority over the individual components at all
time points
More efficacious than latanoprost by 1.6 –
3.2 mmHg
More efficacious than Rhopressa™
by 1.7 –
3.4 mmHg
Main adverse event was hyperemia (eye redness):
Reported in 40 percent of patients
Mild for the large majority of patients
No systemic drug-related adverse events |
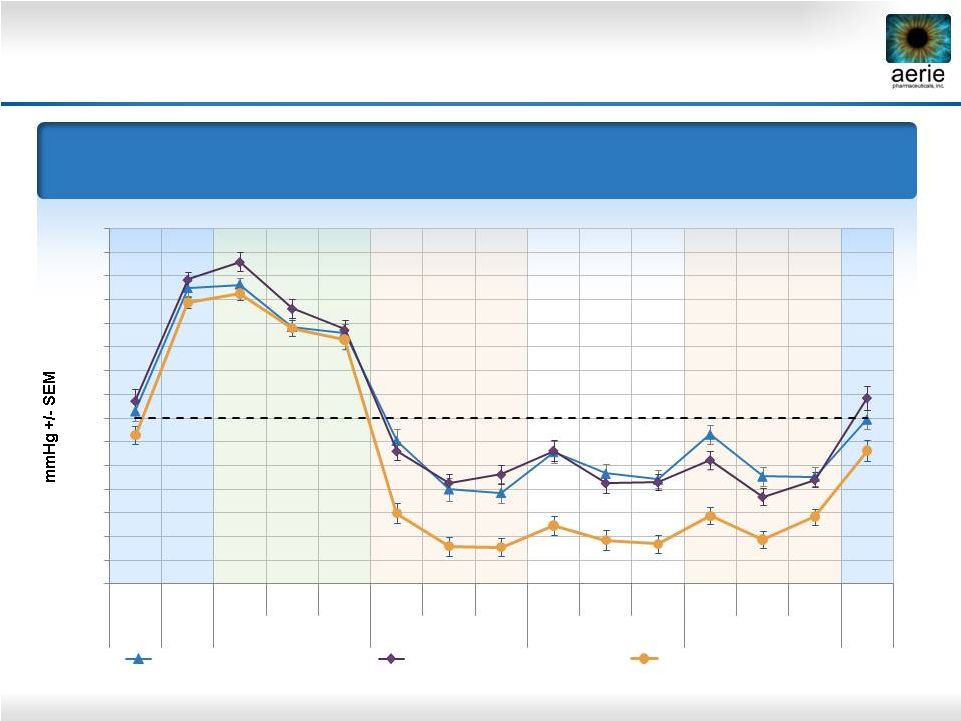 22
Mean IOP at Each Time Point
Primary Efficacy Measure
0.02% Roclatan™
Achieved Statistical Superiority Over
Individual Components at All Time Points (p<0.001)
Roclatan™
Phase 2b, Intent to Treat
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
Pre-
8AM
8AM
10AM
4PM
8AM
10AM
4PM
8AM
10AM
4PM
8AM
10AM
4PM
8AM
Study
Qual 1
Baseline
Day 8
Day 15
Day 29
Day 30
0.02% Rhopressa™
(n=78)
0.005% Latanoprost (n=73)
0.02% Roclatan™
(n=72) |
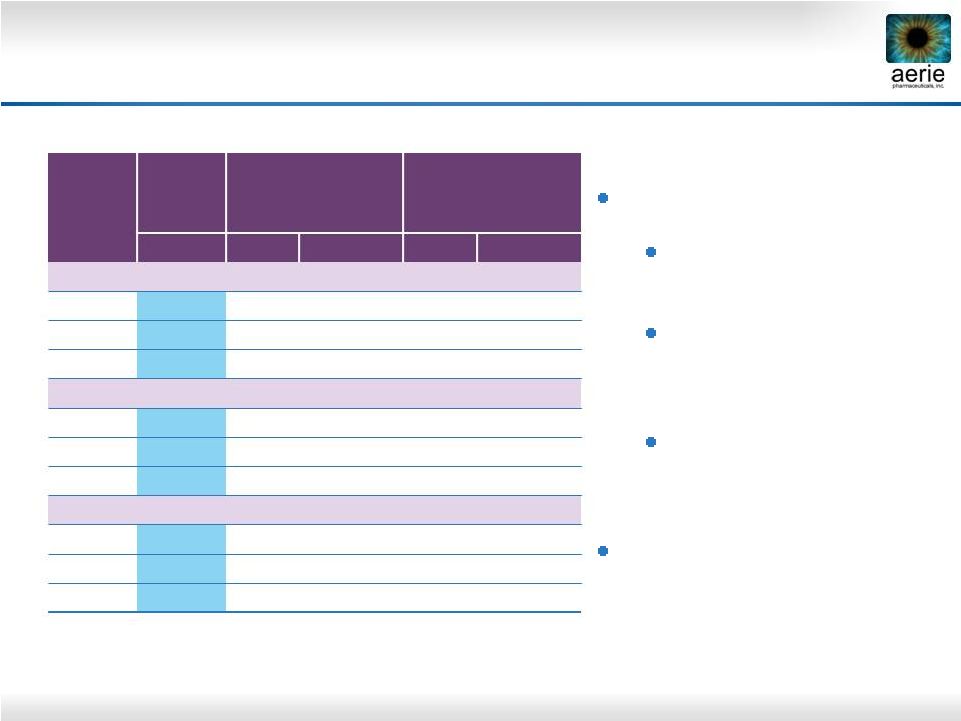 23
Roclatan™
Phase 2b, ITT
Mean IOP (mmHg)
* Difference
between
0.02%
Roclatan™
and
latanoprost
or
Rhopressa™
Roclatan™:
Produced lowest IOP
drop in any trial
Was superior to
latanoprost by 1.6–3.2
mmHg (p<0.001)
Was superior to
Rhopressa™
by 1.7–3.4
mmHg (p<0.001)
Impressive Rhopressa™
performance
0.02%
Roclatan™
(n = 72)
0.005%
latanoprost
(n = 73)
0.02%
Rhopressa™
(n = 78)
Mean
Mean
Difference*
Mean
Difference*
Day 8
8 AM
17.0
19.6
-2.6
20.0
-3.1
10 AM
15.6
18.3
-2.7
18.0
-2.4
4 PM
15.6
18.6
-3.1
17.9
-2.3
Day 15
8 AM
16.5
19.6
-3.2
19.6
-3.1
10 AM
15.8
18.3
-2.4
18.7
-2.8
4 PM
15.7
18.3
-2.6
18.4
-2.7
Day 29
8 AM
16.9
19.2
-2.4
20.3
-3.4
10 AM
15.9
17.7
-1.8
18.6
-2.7
4 PM
16.8
18.4
-1.6
18.5
-1.7 |
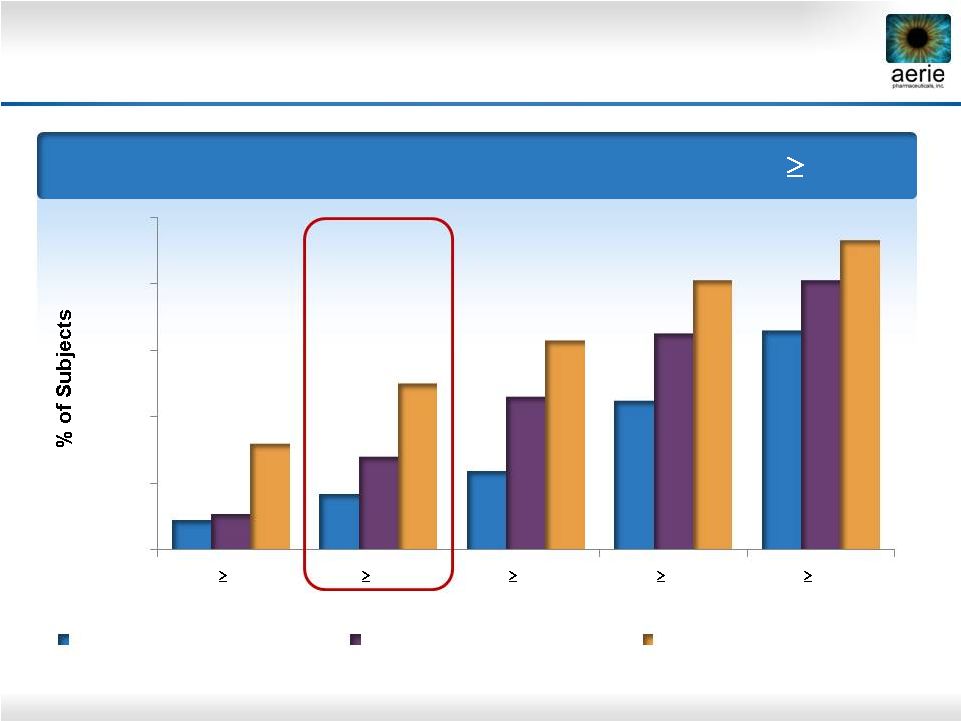 24
Day 29 –
% of Patients with IOP Reductions of
20%
Roclatan™
Phase 2b Responder Analysis:
Goal is to Achieve Lowest IOP Possible
0%
20%
40%
60%
80%
100%
40%
35%
30%
25%
20%
9%
17%
24%
45%
66%
11%
28%
46%
65%
81%
32%
50%
63%
81%
93%
Reduction
0.02% Rhopressa™
(n=78)
0.005% Latanoprost (n=73)
0.02% Roclatan™
(n=72) |
 25
Roclatan™
Phase 2b Responder Analysis:
Goal is to Achieve Lowest IOP Possible
Day 29 –
% of Subjects with IOP Reduced to <
18 mmHg
0%
20%
40%
60%
80%
100%
10%
21%
25%
40%
8%
18%
29%
47%
38%
46%
57%
69%
Reduction
0.02% Rhopressa™
(n=78)
0.005% Latanoprost (n=73)
0.02% Roclatan™
(n=72)
15 mmHg
16 mmHg
17 mmHg
8 mmHg |
 26
Diurnal
IOP in Subset Of High Responders
16 mmHg
Roclatan™
Phase 2b High Responders:
Consistent IOP Drop by Rhopressa™
and Roclatan™
-9.6
-7.2
-9.9
-9.4
-8.2
-10.6
-9.5
-9.5
-10.2
-12
-11
-10
-9
-8
-7
-6
-5
-4
-3
-2
-1
0
0.02% Rhopressa™
(n=16)
0.005% Latanoprost
(n=13)
0.02% Roclatan™
(n=31)
Day 8
Day 15
Day 29 |
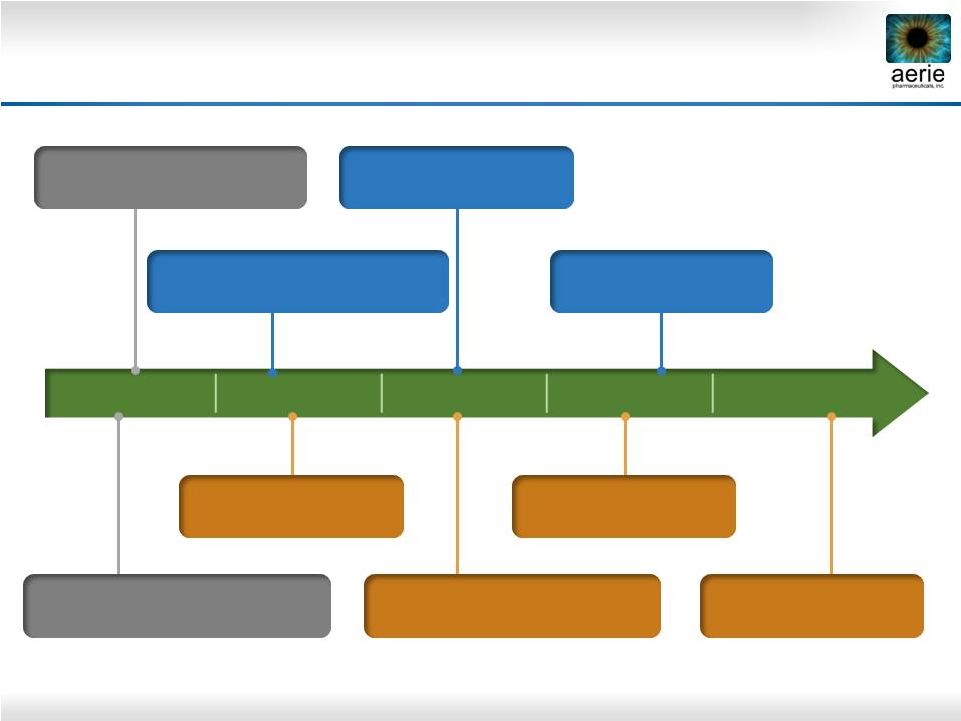 27
2014
2015
2016
2017
2018
Key Future Milestones
June 2014: Roclatan™
Phase 2b clinical trial completed
Mid-2015: Roclatan™
Phase 3 to be initiated
Mid-2016:
Roclatan™
Efficacy
results from Phase 3 expected
Mid-2017: Roclatan™
NDA filing expected
2H 2018: Roclatan™
Launch expected
3Q 2014: Rhopressa™
Phase 3 initiated
Mid-Q2 2015: Rhopressa™
Efficacy results from Phase 3
expected
Mid-2016: Rhopressa™
NDA filing expected
2H 2017: Rhopressa™
Launch expected
Nearly $200M raised -
adequate financing to fund through commercialization
|
 28
New Preclinical Research |
 29
Disease Modification: Addressing the Root
Cause of Elevated IOP and Vision Loss
Healthy TM
Less Nutrients,
Antioxidants
Cellular Stress
Fibrosis, Stiffness
Contraction
Reduced Aqueous
Perfusion Area
Elevated IOP
Cellular Stress
Progressive Degeneration of the Trabecular Meshwork Drives
Elevated IOP and Vision Loss in Glaucoma
Vision Loss
Oxidation
Aging |
 30
Rhopressa™
Has Potential to Improve Health of
TM in Patients with Glaucoma
Healthy TM
More
Nutrients,
Antioxidants
Less
Cellular Stress
Reduced
Fibrosis, Stiffness
Contraction
Increased
Aqueous
Perfusion Area
Reduced
IOP
Cellular Stress
Reducing Fibrosis, Increasing Trabecular Outflow Could Stop
Degeneration of Outflow Tissues in POAG
+ Rhopressa™
Preserve Vision
Oxidation
Aging |
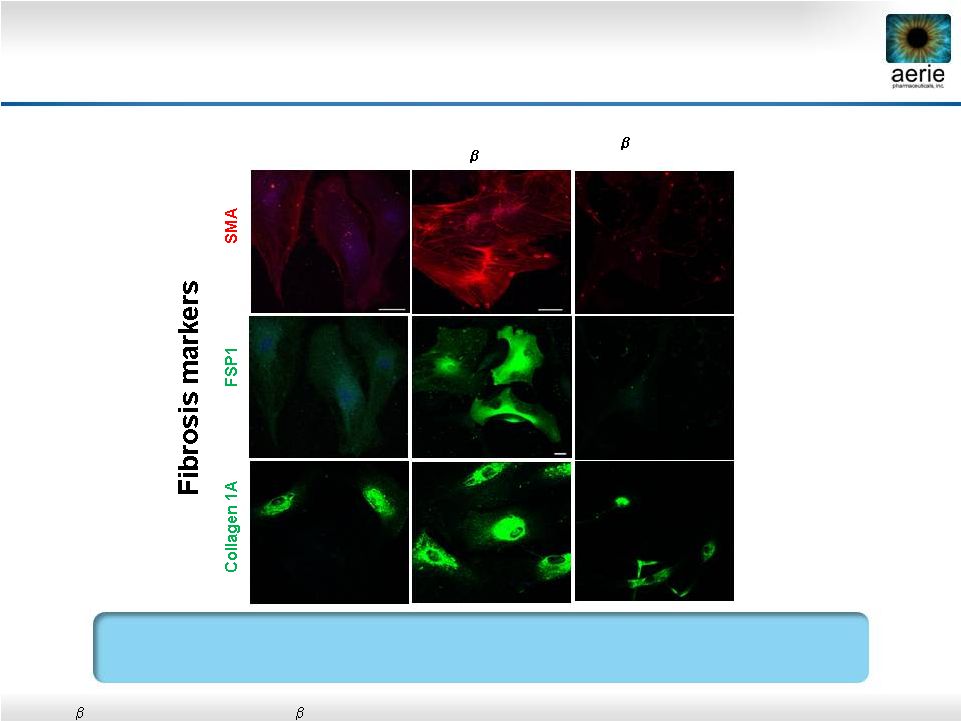 31
AR-13324* May Have Anti-Fibrotic Activity in
Human Trabecular Meshwork Cells
Control
TGF
2
(8
ng/ml) +
AR-13324 (500nM)
Vasanth Rao, Duke University
•
Active ingredient of Rhopressa™
•
TGF
2:
Transforming
growth
factor
2;
SMA:
Smooth
muscle
actin;
FSP1:
Fibroblast-specific
protein
1
AR-13324 May Block TGF-beta-Induced Expression of Fibrosis
Proteins in Human TM Cells
TGF
2
(8ng/ml) |
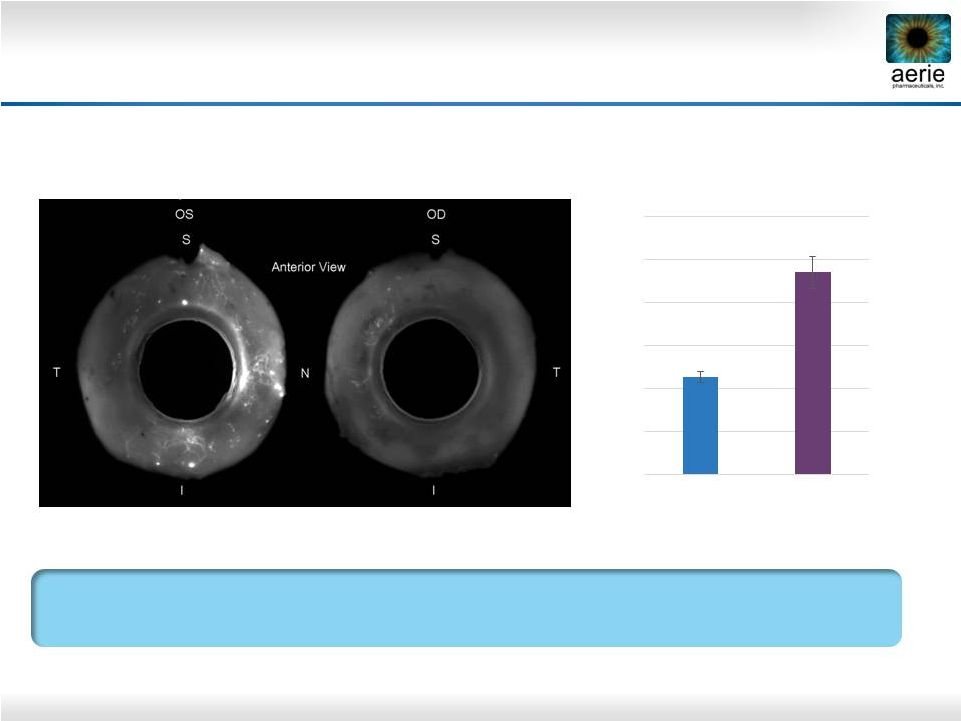 32
+ AR-13324
Control
AR-13324* May Increase Perfusion of TM Outflow
Tissues
Dan Stamer (Duke), Haiyan Gong (Boston University)
AR-13324’s Ability to Potentially Increase Perfusion of TM Should
Provide More Nutrients and Antioxidants to the TM
Left (OS) and right (OD) eyes perfused with fluorescent microbeads
Control = buffered saline solution
*Active ingredient of Rhopressa™
** Percent Effective Filtration Area
0.00
10.00
20.00
30.00
40.00
50.00
60.00
Anterior PEFA** (%)
AR-13324
Control |
 33
Selective Multi-Kinase Inhibitors for AMD/DME
•
Selective Rho kinase
inhibitors previously effective
in preclinical models of AMD
and DME
•
Compounds inhibiting
multiple disease targets
should provide best efficacy
•
Inflammation
•
Angiogenesis
•
Fibrosis
•
Screened 184 Aerie
compounds against 456
human kinases
Aerie Kinase Library Screen
Relationship tree of human kinases. TK, TKL, STE, CK1, AGC, CAMK, CMGC,
Other: Kinase superfamilies ROCK |
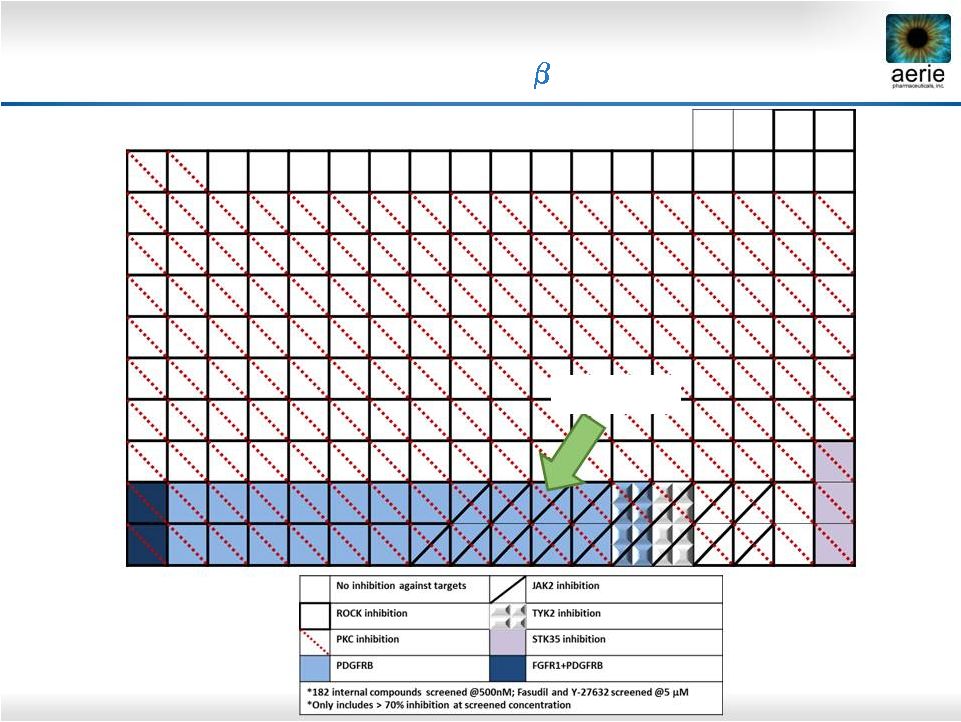 34
KINOMEscan Results: AR-13154 Inhibits AMD
Targets ROCK, JAK2,
PDGFR-
AR-13154 |
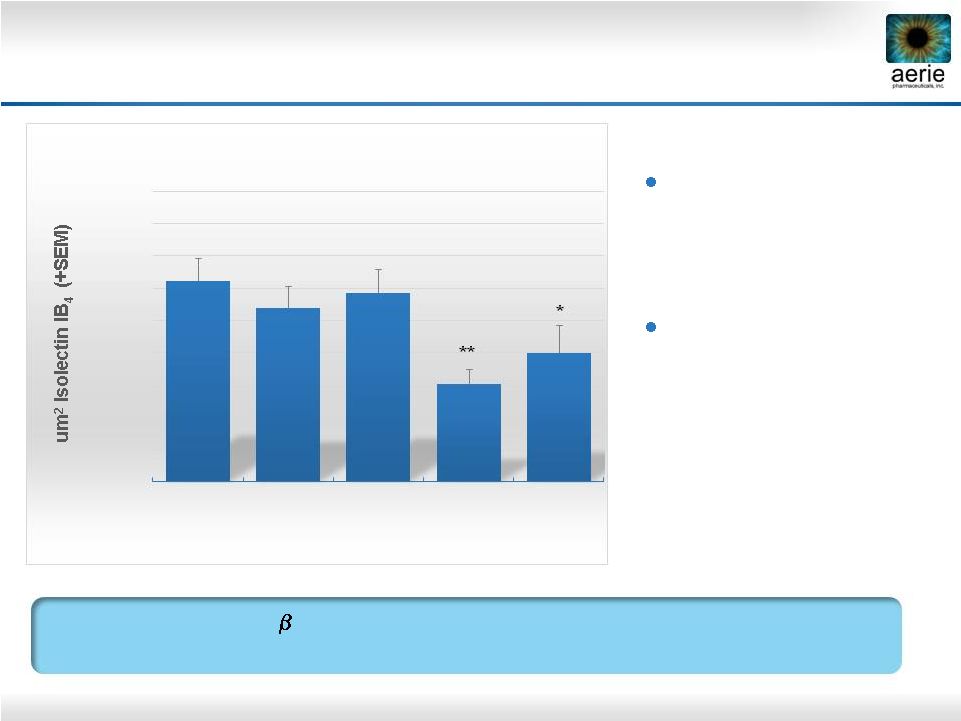 35
Laser-induced
choroidal
neovascularization
(CNV) in rats
Compounds delivered
by intravitreal injection
AR-13154 vs. Eylea in Preclinical AMD Model
ROCK/JAK2/PDGFR
Inhibitor
AR-13154
Numerically
More
Effective
than
Eylea
®
in Rat Model of AMD
20000
30000
40000
50000
60000
70000
80000
90000
100000
110000
Saline
n=49
0.06 ug/mL
AR
-13154
n=28
0.6 ug/mL
AR
-13154
n=25
6 ug/mL
AR
-13154
n=25
800 ug/mL
Eylea®
n=20
Total CNV Lesion Area (Day 21)
**
*
*
p<0.05 vs. Saline
**
p<0.001 |
 Building a Major
Ophthalmic
Pharmaceutical
Company |
