Attached files
| file | filename |
|---|---|
| 8-K - 8-K - InspireMD, Inc. | v402089_8k.htm |
Exhibit 99.1

NYSE MKT: NSPR February 2015

Forward Looking Statements 2 This presentation contains “forward - looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward - looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the control of InspireMD, Inc. (the “Company”), and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward - looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with ( i ) market acceptance of the Company’s existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of the Company’s products, (iv) intense competition in the medical device industry from much larger, multi - national companies, (v) product liability claims, (vi) product malfunctions, (vii) the Company’s limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payors for the Company’s products, (ix) the Company’s efforts to successfully obtain and maintain intellectual property protection covering its products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) the Company’s reliance on single suppliers for certain product components, (xii) the fact that the Company will need to raise additional capital to meet its business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain, (xiii) the fact that the Company conducts business in multiple foreign jurisdictions, exposing it to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction and (xiv) the escalation of hostilities in Israel, which could impair the Company’s ability to manufacture its products. More detailed information about the Company and the risk factors that may affect the realization of forward - looking statements are set forth in the Company’s filings with the Securities and Exchange Commission, including the Company’s Transition Report on Form 10 - K/T and its quarterly reports on Form 10 - Q. Investors and security holders are urged to read these reports free of charge on the Securities and Exchange Commission’s web site at www.sec.gov . The Company assumes no obligation to publicly update or revise its forward - looking statements as a result of new information, future events or otherwise.

Investment Highlights 3 • 2015 return to revenue growth with improved coronary product for near term international opportunities. • Immediate product portfolio expansion: Carotid RX product with revolutionary design and strong First in Man clinical data. • Operating and financial realignment inline with development and growth initiatives. • Expanding partnerships in both coronary and carotid segments to advance adoption and accelerate revenue growth. • Advancing into Neuro and Peripheral markets to leverage technology into high growth segments. • Experienced management team and strong Chairman with track record of success in building highly valued enterprises.

Leadership 4 Solid Commercial & Clinical C apabilities Executive Team Alan Milinazzo President, CEO & Director • Medtronic • Boston Scientific Craig Shore CFO • Pfizer • General Electric Dr. James Barry COO • Boston Scientific • Howmedica Division of Pfizer Eli Bar CTO • Nicast Gwen Bame VP Corporate Development • Boston Scientific • Covidien David Blossom VP Global Marketing & Strategy • Boston Scientific • Covidien

Technology: MicroNet ™ 5 Beyond Stenting: MicroNet Mesh for Embolic Protection MGuard Embolic Protection System Combines stent and embolic protection in a single device • Stent platform provides revascularization benefit • MicroNet then acts as safety net by offering greater surface area coverage to prevent large debris flow • Mesh configuration allows perfusion to vessel wall MicroNet Platform • Proprietary circular knitted mesh wraps around stent to protect patient from plaque debris flowing downstream upon deployment • Made of a single fiber from a biocompatible polymer, widely used in medical implantations • Flexible structure • Does not promote thrombosis

Large Addressable Markets 6 Expanding the MicroNet ™ Platform MGuard ™ x $1.7B Market x CE Mark Cleared x Coronary AMI, SVG CGuard ™ x $500M Market x CE Mark Cleared x Carotid NVGuard x $125M Flow Diversion Market x $550M Aneurysm Market x Late 2015E CE Mark Planned Submission x Neurovascular PVGuard x $1.7B Market x 2016E CE Mark Planned Submission x Peripheral RGuard x $100M Market x Renal

Coronary MGuard ™ EPS 7 Improving AMI Patient Outcomes • Current stents not specifically designed for AMI • Distal embolization occurs in up to 73% of cases* • Majority of AMI market is outside of the U.S . (~ 60%) • MGuard clinical experience including two randomized trials with data showing sustained mortality rates Source: Health Research International, (June 2012 ) | *JAMA , March 2, 2005 — Vol 293, No. 9 1063 Gregg W. Stone Targeted Share Capture: Selectively scaling global reach and frequency Tier 1 • Mix of direct sales representatives, agents and distributors, with focus on KOL ’ s/high - volume AMI centers • 14 - 18 countries, primarily Europe and select Latin American and Middle East countries with favorable STEMI market factors - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Tier 2 • Country or regional partnerships with high quality local distributors or strategic partners with regional AMI focused strategies - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Tier 3 • United States - Pending successful partnership strategy • Japan - Pending successful partnership strategy 1 2 3

8

Carotid Market Opportunity 9 An Enhanced Minimally Invasive Solution • Standard of care: Open surgery: Carotid EndArterectomy (CEA) • Current stents have not improved on CEA stroke rates (CREST) • Mesh covered stent category has the potential to convert CEA to CAS • CARENET 30 - day data show CGuard device better than previous technology/therapy • Immediate commercial opportunity with revenue ramp throughout 2015 ROW $200 US $300 Global Carotid Market : $ 500M Source: JMP Securities, 2014

Carotid Solution 10 Safety Net with Greater Plaque Protection CGuard ™ Embolic Prevention System Combines stent and embolic protection in a single device • Stent platform provides revascularization benefits • MicroNet acts as safety net by offering greater plaque scaffolding to prevent prolapse related to late embolization • Allows perfusion to vessel wall, does not inhibit endothelialization • CE marked • Self - expanding nitinol stent • Global market valued at $500M* • Strong CARENET FIM data released 9/14 and 1/15 • First commercial orders (LMR) received Q4 2014 * Health Research International, 2011

Positive CGuard ™ FIM* Clinical Experience 11 * FIM , First in Man CARENET Design ( CARotid Embolic protection using microNET ) • 30 Patient Safety and efficacy clinical trial • Prospective, multi - center, multispecialty, non - randomized single arm study • Diffusion weighted MRI follow ups at 48hrs and 30 days for “ gold - standard ” neurological analysis CARENET Highlights: Results Announced at TCT 2014 • Achieved primary end point • 100% procedural success • Zero MACCE at 30 days • 50% fewer new ischemic lesions compared to historical non - mesh carotid artery stenting data • Average lesion volume per patient 10 times smaller compared to historical non - mesh carotid artery stenting data

2015 Return to Growth 12 Revenue Growth with MGuard™ and NEW CGuard ™ RX

Carotid CGuard ™ Commercialization Strategy 13 Q4 2014 – Limited Market Release • Germany, Poland, Switzerland, Belgium, Italy, and Spain • 100+ CGuard cases performed to date from 14 sites • FIM data supports full commercial release Q1/Q2 2015 – International Launch • Full launch of rapid exchange (RX) system focused on EU and LATAM • Primarily targeting high volume centers in core European markets • Revenue impact to the company in 2015 is expected to be significant and will complement coronary selling strategies CGuard Embolic Prevention System Commercial Activities to Complement MGuard Sales Activities
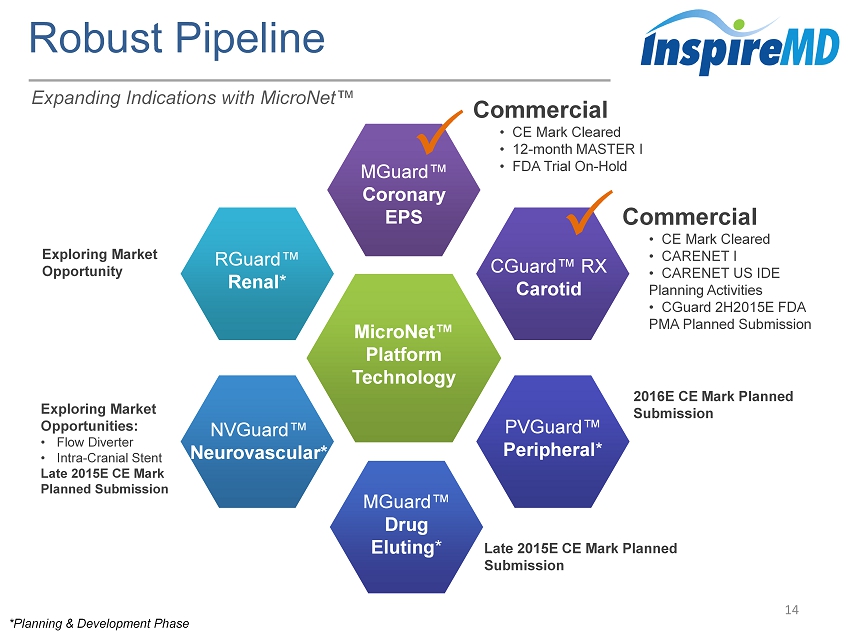
Robust Pipeline 14 Commercial • CE Mark Cleared • 12 - month MASTER I • FDA Trial On - Hold MicroNet ™ Platform Technology MGuard ™ Coronary EPS NVGuard ™ Neurovascular* RGuard™ Renal * CGuard ™ RX Carotid PVGuard™ Peripheral * MGuard ™ Drug Eluting * * Planning & Development Phase Commercial • CE Mark Cleared • CARENET I • CARENET US IDE Planning Activities • CGuard 2H2015E FDA PMA Planned Submission 2016E CE Mark Planned Submission Late 2015E CE Mark Planned Submission Exploring Market Opportunities: • Flow Diverter • Intra - Cranial Stent Late 2015E CE Mark Planned Submission Exploring Market Opportunity Expanding Indications with MicroNet ™

Neurovascular Market Opportunity 15 Innovation Leads Growth • Current designs have sub - optimal trackability and in vessel flexibility • MicroNet meets need to simultaneously manage thrombosis of the aneurysmal sac while preserving the patency of the adjacent small vessels Source: MRG Neuro Report, Ev3 Revenue Data 2014 Competitive Landscape: Relatively Fewer Players with Limited Innovation Product Company Approval Pipeline Medtronic/Covidien CE Mark / FDA 2011 Surpass Stryker CE Mark 2011 Silk Balt Extrusion CE Mark 2008 The Flow Diversion System The preferred solution for unruptured aneurysm treatment

NVGuard ™ Neurovascular 16 Differentiation Yields Increased Utility Our Significant Advantage Over Existing Flow Diverters • MicroNet aperture & size • Low metal to artery ratio • Can be placed in side branches and bifurcations, which is impossible with current technology Total Aneurysm Market Value: $946M • Aneurysm Therapy (all types): $550M • Aneurysms account for 74% of neuroendovascular disease states • Estimated that flow diverters can treat 25% of all aneurysms • Wide - neck Aneurysm Procedures: $350M • Non - coil neurovascular products: estimated 12% CAGR from 2010 - 2016 “Devices in the European neurovascular device market will face significant competition from emerging treatments, such as INR flow diversion” Source: MRG
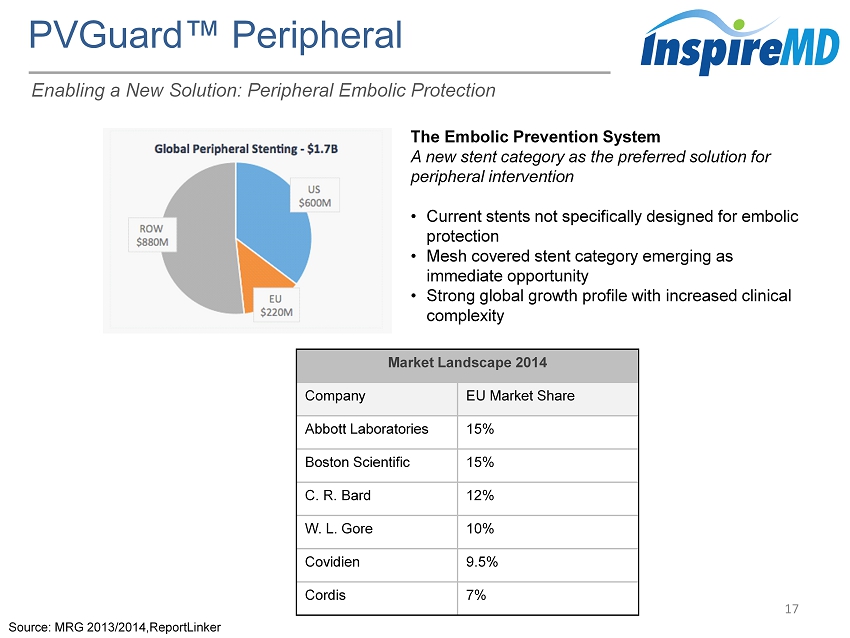
PVGuard ™ Peripheral 17 The Embolic Prevention System A new stent category as the preferred solution for peripheral intervention • Current stents not specifically designed for embolic protection • Mesh covered stent category emerging as immediate opportunity • Strong global growth profile with increased clinical complexity Source: MRG 2013/2014,ReportLinker Market Landscape 2014 Company EU Market Share Abbott Laboratories 15% Boston Scientific 15% C. R. Bard 12% W. L. Gore 10% Covidien 9.5% Cordis 7% Enabling a New Solution: Peripheral Embolic Protection

2015E 2016E 2017E R&D/ Clin / Reg Corporate Operational Commercial Target Milestones 18 Support & Execute on Growth Initiatives Strategic Partnership III Strategic Partnership V Strategic Partnership IV DES Estimated CE Mark Achieve Targeted COGS NVGuard CE Mark Submission CGuard RX Launch Neuro and Peripheral Estimated CE Mark CGuard FDA PMA Submission eMASTER Enrollment CARENET I 6M FU DES Pre Clinical Outsourced Manufacturing Facility PVGuard CE Mark Submission DES CE Mark Submission

Investment Summary 19 • 2015 return to revenue growth with improved coronary product for near term international opportunities. • Immediate product portfolio expansion: Carotid RX product with revolutionary design and strong First in Man clinical data. • Operating and financial realignment inline with development and growth initiatives. • Expanding partnerships in both coronary and carotid segments to advance adoption and accelerate revenue growth. • Advancing into Neuro and Peripheral markets to leverage technology into high growth segments. • Attractive valuation entry point with multiple near term growth drivers.

Alan Milinazzo , CEO (888) 776 - 6804 alanm@ inspiremd.com Craig Shore, CFO (888) 776 - 6804 craigs@inspiremd.com
