Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TESARO, Inc. | a15-4883_18k.htm |
| EX-99.1 - EX-99.1 - TESARO, Inc. | a15-4883_1ex99d1.htm |
Exhibit 99.2
|
|
Fourth-Quarter and Full-Year 2014 Results February 19, 2015 |
|
|
Safe Harbor Statement Statements made in this presentation about TESARO, Inc. that are not descriptions of historical facts are forward-looking statements reflecting the current beliefs and expectations of management. Forward-looking statements are sometimes identified by words such as “plan,” “may,” “will,” “expect,” and similar expressions referencing future events, conditions or circumstances. These forward-looking statements involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements, as a result of various factors, including, without limitation, the risks described in our Annual Report on Form 10-K for the year ended December 31, 2013 and our subsequent filings with the SEC. TESARO, Inc. undertakes no obligation to update or revise any forward-looking statement for any reason. |
|
|
Lonnie Moulder Chief Executive Officer |
|
|
CINV: chemotherapy-induced nausea & vomiting Market opportunity projections are based upon Company estimates IND: Investigational new drug application KOL: Key opinion leader TESARO Is Positioned for Success Cash and equivalents of ~$257M as of 12/31/2014 Approximately 36.1 million shares outstanding as of 12/31/2014 Well Capitalized Commercial, development and regulatory experience in oncology Commercial leadership team now in place Relationships with KOLs and national oncology networks Experienced Team Differentiated Pipeline Rolapitant: Unmet market need that can be addressed via education and adherence to existing guidelines Niraparib: Broad development program underway with initial Phase 3 data expected in 2015 TSR-011: Phase 1 activity and favorable tolerability in ALK-positive NSCLC patients TSR-042: IND filing planned for late 2015 Anti-TIM-3, anti-LAG-3 and other mAb programs advancing Large Peak Target Market Opportunities Rolapitant: $1.5B U.S. CINV market Niraparib: $6B WW initial indications |
|
|
Tim Pearson Chief Financial Officer |
|
|
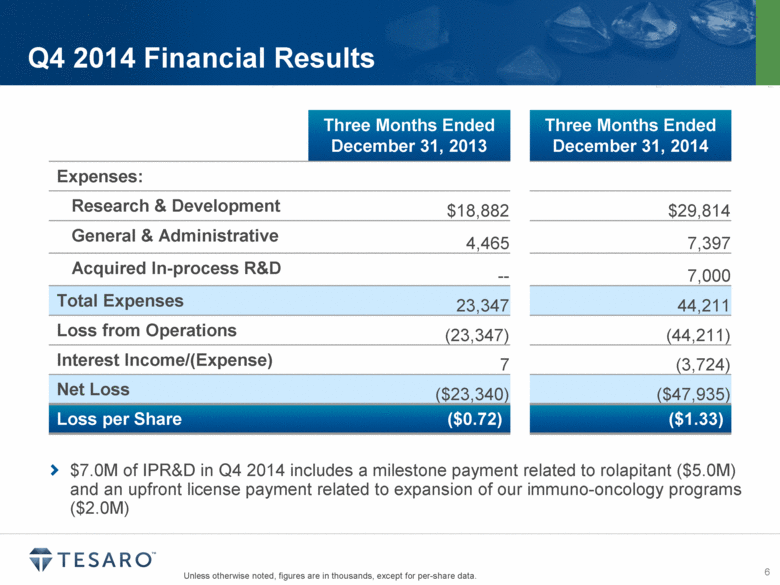
Q4 2014 Financial Results Three Months Ended December 31, 2013 Three Months Ended December 31, 2014 Expenses: Research & Development $18,882 $29,814 General & Administrative 4,465 7,397 Acquired In-process R&D -- 7,000 Total Expenses 23,347 44,211 Loss from Operations (23,347) (44,211) Interest Income/(Expense) 7 (3,724) Net Loss ($23,340) ($47,935) Loss per Share ($0.72) ($1.33) $7.0M of IPR&D in Q4 2014 includes a milestone payment related to rolapitant ($5.0M) and an upfront license payment related to expansion of our immuno-oncology programs ($2.0M) Unless otherwise noted, figures are in thousands, except for per-share data. |
|
|
FY 2014 Financial Results Year Ended December 31, 2013 Year Ended December 31, 2014 Expenses: Research & Development $75,725 $118,425 General & Administrative 14,780 23,935 Acquired In-process R&D 1,940 24,900 Total Expenses 92,445 167,260 Loss from Operations (92,445) (167,260) Interest Income/(Expense) 83 (3,752) Net Loss ($92,362) ($171,012) Loss per Share ($2.93) ($4.79) As of December 31, 2014: Cash & equivalents balance totaled approximately $257 million Approximately 36.1 million shares outstanding of common stock Unless otherwise noted, figures are in thousands, except for per-share data. |
|
|
Mary Lynne Hedley, Ph.D. President and COO |
|
|
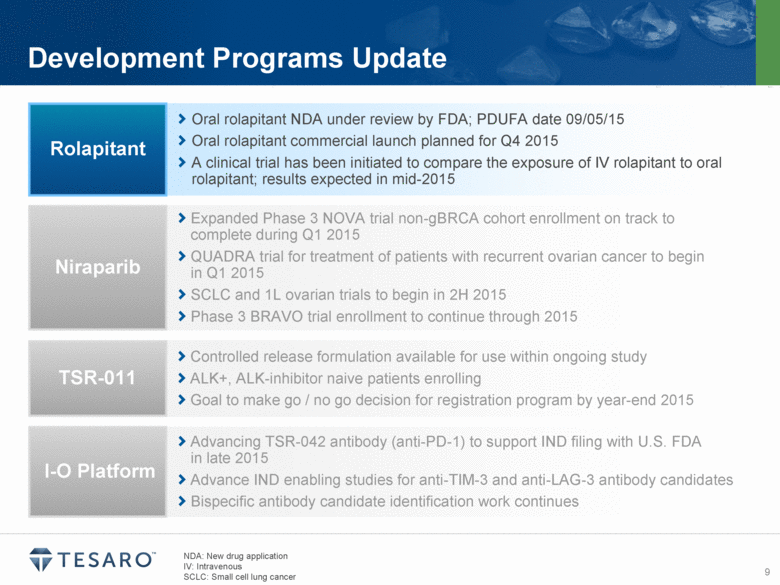
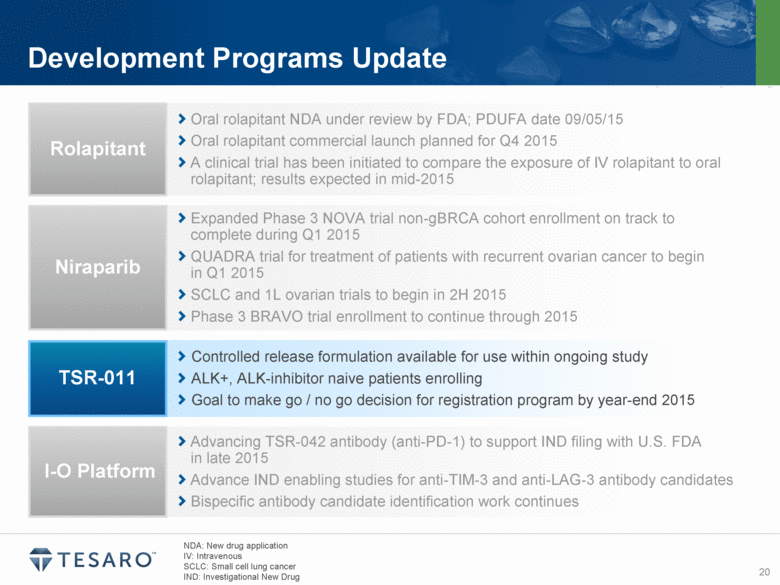
Development Programs Update Oral rolapitant NDA under review by FDA; PDUFA date 09/05/15 Oral rolapitant commercial launch planned for Q4 2015 A clinical trial has been initiated to compare the exposure of IV rolapitant to oral rolapitant; results expected in mid-2015 Rolapitant Expanded Phase 3 NOVA trial non-gBRCA cohort enrollment on track to complete during Q1 2015 QUADRA trial for treatment of patients with recurrent ovarian cancer to begin in Q1 2015 SCLC and 1L ovarian trials to begin in 2H 2015 Phase 3 BRAVO trial enrollment to continue through 2015 Niraparib Controlled release formulation available for use within ongoing study ALK+, ALK-inhibitor naive patients enrolling Goal to make go / no go decision for registration program by year-end 2015 TSR-011 Advancing TSR-042 antibody (anti-PD-1) to support IND filing with U.S. FDA in late 2015 Advance IND enabling studies for anti-TIM-3 and anti-LAG-3 antibody candidates Bispecific antibody candidate identification work continues I-O Platform NDA: New drug application IV: Intravenous SCLC: Small cell lung cancer |
|
|
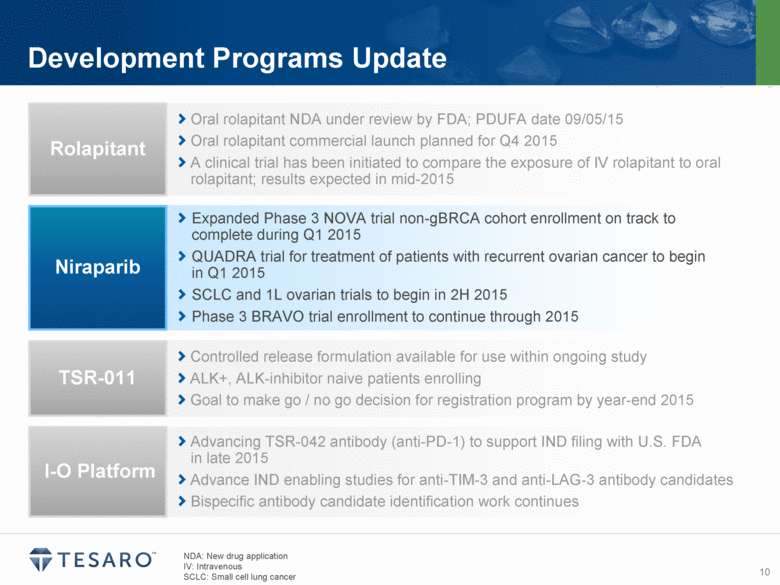
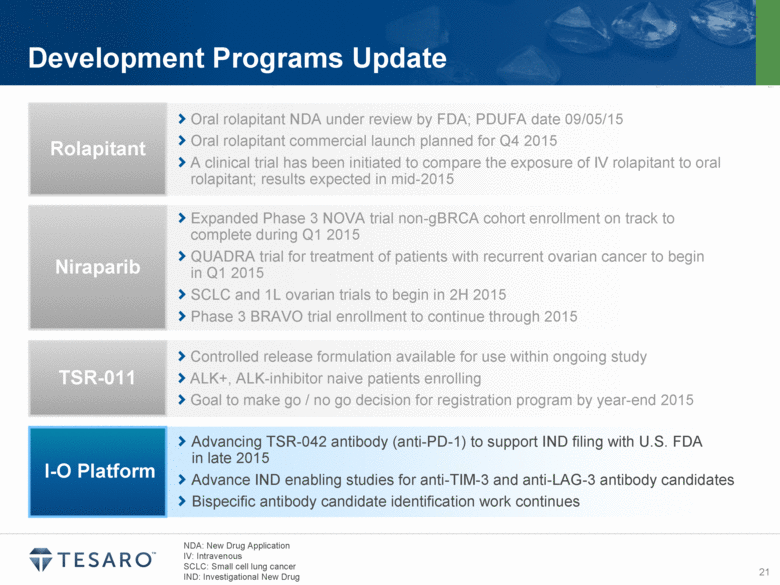
Development Programs Update NDA: New drug application IV: Intravenous SCLC: Small cell lung cancer Oral rolapitant NDA under review by FDA; PDUFA date 09/05/15 Oral rolapitant commercial launch planned for Q4 2015 A clinical trial has been initiated to compare the exposure of IV rolapitant to oral rolapitant; results expected in mid-2015 Rolapitant Expanded Phase 3 NOVA trial non-gBRCA cohort enrollment on track to complete during Q1 2015 QUADRA trial for treatment of patients with recurrent ovarian cancer to begin in Q1 2015 SCLC and 1L ovarian trials to begin in 2H 2015 Phase 3 BRAVO trial enrollment to continue through 2015 Niraparib Controlled release formulation available for use within ongoing study ALK+, ALK-inhibitor naive patients enrolling Goal to make go / no go decision for registration program by year-end 2015 TSR-011 Advancing TSR-042 antibody (anti-PD-1) to support IND filing with U.S. FDA in late 2015 Advance IND enabling studies for anti-TIM-3 and anti-LAG-3 antibody candidates Bispecific antibody candidate identification work continues I-O Platform |
|
|
Niraparib Value Proposition Compelling RECIST response rate and durability in heavily pre-treated patients 1 Potentially differentiated tolerability profile 2 Convenient, once per day dosing (3 capsules) 3 Broadest PARPi program in ovarian cancer 4 Biomarker incorporation potentially allows for patient selection 5 Potential for combinations with immuno-oncology and other targeted agents 6 |
|
|
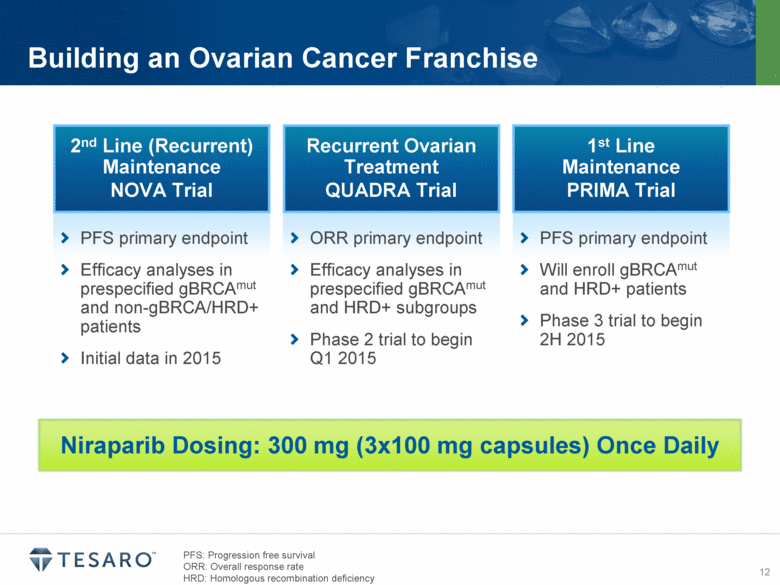
Niraparib Dosing: 300 mg (3x100 mg capsules) Once Daily Building an Ovarian Cancer Franchise Recurrent Ovarian Treatment QUADRA Trial ORR primary endpoint Efficacy analyses in prespecified gBRCAmut and HRD+ subgroups Phase 2 trial to begin Q1 2015 1st Line Maintenance PRIMA Trial PFS primary endpoint Will enroll gBRCAmut and HRD+ patients Phase 3 trial to begin 2H 2015 PFS: Progression free survival ORR: Overall response rate HRD: Homologous recombination deficiency |
|
|
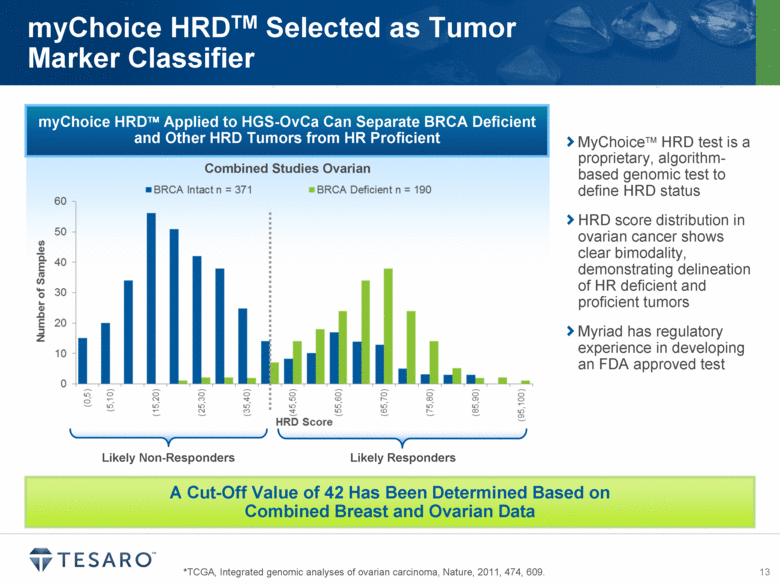
*TCGA, Integrated genomic analyses of ovarian carcinoma, Nature, 2011, 474, 609. myChoice HRDTM Selected as Tumor Marker Classifier myChoice HRD Applied to HGS-OvCa Can Separate BRCA Deficient and Other HRD Tumors from HR Proficient A Cut-Off Value of 42 Has Been Determined Based on Combined Breast and Ovarian Data Combined Studies Ovarian Likely Non-Responders Likely Responders MyChoice HRD test is a proprietary, algorithm-based genomic test to define HRD status HRD score distribution in ovarian cancer shows clear bimodality, demonstrating delineation of HR deficient and proficient tumors Myriad has regulatory experience in developing an FDA approved test Number of Samples Likely Non-Responders Likely Responders |
|
|
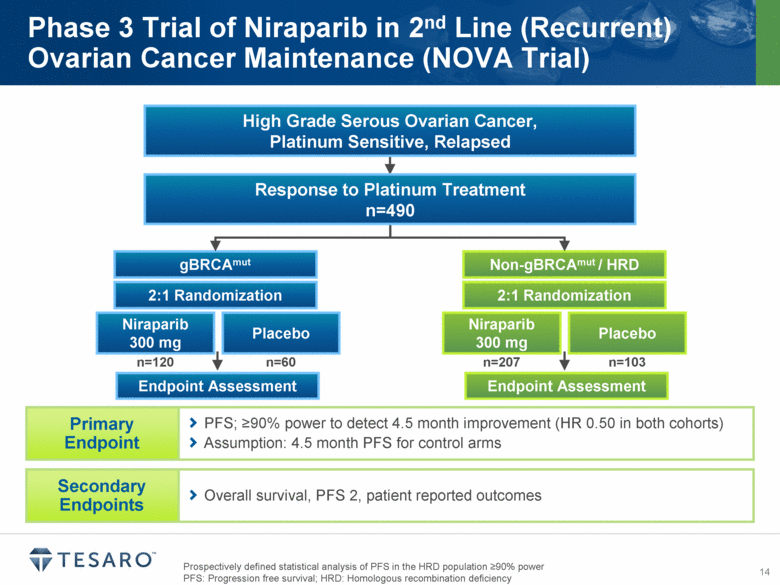
Prospectively defined statistical analysis of PFS in the HRD population >90% power PFS: Progression free survival; HRD: Homologous recombination deficiency Phase 3 Trial of Niraparib in 2nd Line (Recurrent) Ovarian Cancer Maintenance (NOVA Trial) gBRCAmut Endpoint Assessment Niraparib 300 mg Placebo Non-gBRCAmut / HRD Endpoint Assessment Niraparib 300 mg Placebo 2:1 Randomization 2:1 Randomization n=120 n=60 n=207 n=103 Overall survival, PFS 2, patient reported outcomes PFS; >90% power to detect 4.5 month improvement (HR 0.50 in both cohorts) Assumption: 4.5 month PFS for control arms High Grade Serous Ovarian Cancer, Platinum Sensitive, Relapsed Response to Platinum Treatment n=490 |
|
|
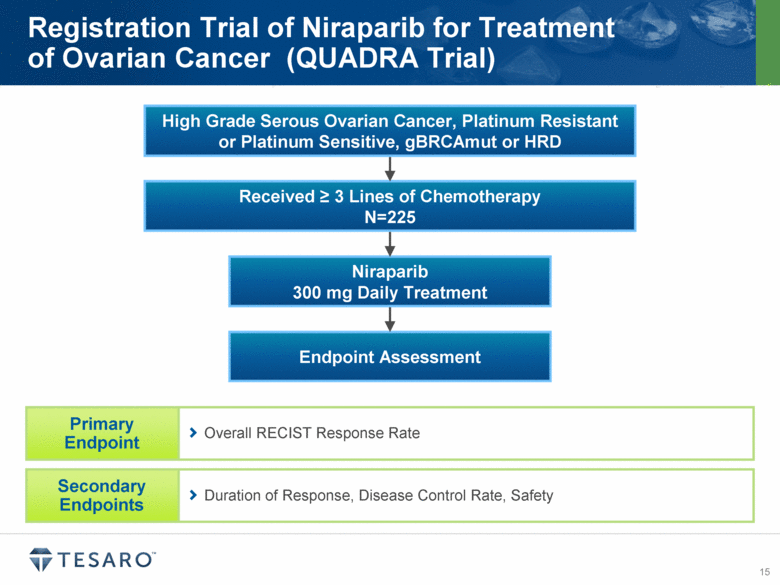
Endpoint Assessment Niraparib 300 mg Daily Treatment Received > 3 Lines of Chemotherapy N=225 Registration Trial of Niraparib for Treatment of Ovarian Cancer (QUADRA Trial) Duration of Response, Disease Control Rate, Safety Overall RECIST Response Rate High Grade Serous Ovarian Cancer, Platinum Resistant or Platinum Sensitive, gBRCAmut or HRD |
|
|
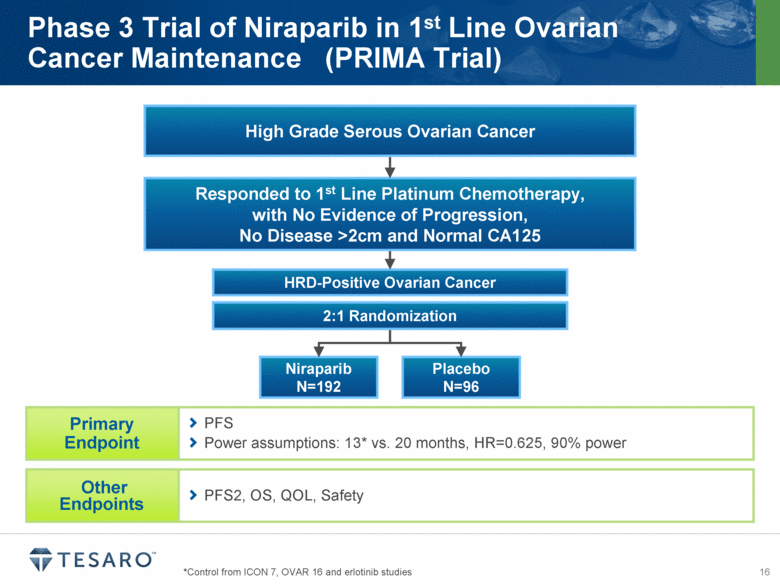
Placebo N=96 2:1 Randomization *Control from ICON 7, OVAR 16 and erlotinib studies Phase 3 Trial of Niraparib in 1st Line Ovarian Cancer Maintenance (PRIMA Trial) PFS Power assumptions: 13* vs. 20 months, HR=0.625, 90% power PFS2, OS, QOL, Safety Responded to 1st Line Platinum Chemotherapy, with No Evidence of Progression, No Disease >2cm and Normal CA125 High Grade Serous Ovarian Cancer |
|
|
1L: 1st Line; 2L: 2nd Line; Treatment: 3 or more lines of previous therapy Dollar figures reflect current PARP inhibitor pricing. Source: Company estimates. PARP Inhibitor Peak Market Opportunity in Ovarian Cancer Approximates $4 Billion gBRCAmut non-gBRCAmut/HRD+ Non-gBRCAmut / HRD- United States Europe Approximately 40,000 Eligible Patients in the U.S. and Europe Other/Ineligible |
|
|
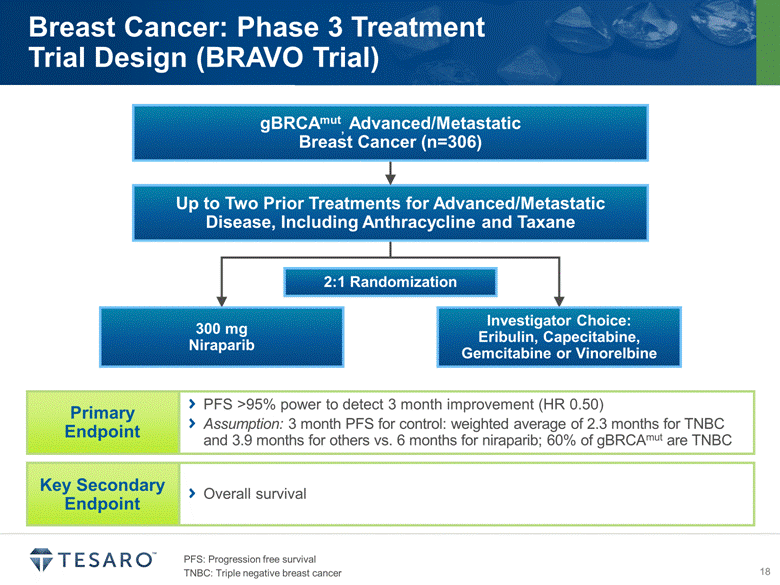
Breast Cancer: Phase 3 Treatment Trial Design (BRAVO Trial) 2:1 Randomization 300 mg Niraparib Investigator Choice: Eribulin, Capecitabine, Gemcitabine or Vinorelbine PFS: Progression free survival TNBC: Triple negative breast cancer PFS >95% power to detect 3 month improvement (HR 0.50) Assumption: 3 month PFS for control: weighted average of 2.3 months for TNBC and 3.9 months for others vs. 6 months for niraparib; 60% of gBRCAmut are TNBC Overall survival Up to Two Prior Treatments for Advanced/Metastatic Disease, Including Anthracycline and Taxane gBRCAmut, Advanced/Metastatic Breast Cancer (n=306) |
|
|
Dollar figures reflect current PARP inhibitor pricing. Source: Company estimates PARP Inhibitor Peak Market Opportunity in gBRCAmut Breast Cancer Exceeds $1 Billion Approximately 20,000 Eligible Patients in the U.S. and Europe 1L Patients 2L Patients 3L Patients |
|
|
Development Programs Update NDA: New drug application IV: Intravenous SCLC: Small cell lung cancer IND: Investigational New Drug Oral rolapitant NDA under review by FDA; PDUFA date 09/05/15 Oral rolapitant commercial launch planned for Q4 2015 A clinical trial has been initiated to compare the exposure of IV rolapitant to oral rolapitant; results expected in mid-2015 Rolapitant Expanded Phase 3 NOVA trial non-gBRCA cohort enrollment on track to complete during Q1 2015 QUADRA trial for treatment of patients with recurrent ovarian cancer to begin in Q1 2015 SCLC and 1L ovarian trials to begin in 2H 2015 Phase 3 BRAVO trial enrollment to continue through 2015 Niraparib Controlled release formulation available for use within ongoing study ALK+, ALK-inhibitor naive patients enrolling Goal to make go / no go decision for registration program by year-end 2015 TSR-011 Advancing TSR-042 antibody (anti-PD-1) to support IND filing with U.S. FDA in late 2015 Advance IND enabling studies for anti-TIM-3 and anti-LAG-3 antibody candidates Bispecific antibody candidate identification work continues I-O Platform |
|
|
Development Programs Update NDA: New Drug Application IV: Intravenous SCLC: Small cell lung cancer IND: Investigational New Drug Oral rolapitant NDA under review by FDA; PDUFA date 09/05/15 Oral rolapitant commercial launch planned for Q4 2015 A clinical trial has been initiated to compare the exposure of IV rolapitant to oral rolapitant; results expected in mid-2015 Rolapitant Expanded Phase 3 NOVA trial non-gBRCA cohort enrollment on track to complete during Q1 2015 QUADRA trial for treatment of patients with recurrent ovarian cancer to begin in Q1 2015 SCLC and 1L ovarian trials to begin in 2H 2015 Phase 3 BRAVO trial enrollment to continue through 2015 Niraparib Controlled release formulation available for use within ongoing study ALK+, ALK-inhibitor naive patients enrolling Goal to make go / no go decision for registration program by year-end 2015 TSR-011 Advancing TSR-042 antibody (anti-PD-1) to support IND filing with U.S. FDA in late 2015 Advance IND enabling studies for anti-TIM-3 and anti-LAG-3 antibody candidates Bispecific antibody candidate identification work continues I-O Platform |
|
|
Lonnie Moulder Chief Executive Officer |
|
|
Rolapitant Value Proposition 1 Provides 5 continuous days of protection from CINV 2 Convenient single dose administered just prior to chemotherapy 3 4 5 6 Customer partnerships enable support of CINV educational initiatives Reduced risk of CYP3A4-mediated drug interactions Oral and IV formulations in development to address entire market May be combined with any approved 5-HT3 RA |
|
|
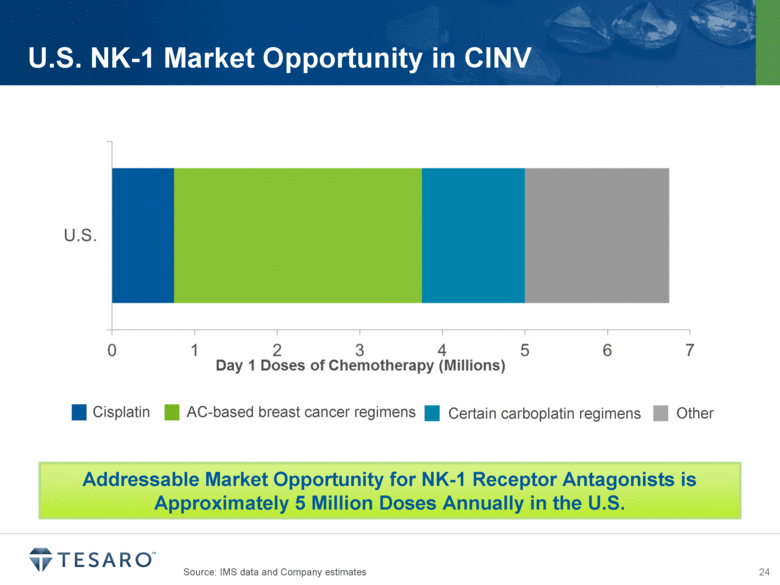
Source: IMS data and Company estimates U.S. NK-1 Market Opportunity in CINV Cisplatin AC-based breast cancer regimens Certain carboplatin regimens Other Addressable Market Opportunity for NK-1 Receptor Antagonists is Approximately 5 Million Doses Annually in the U.S. |
|
|
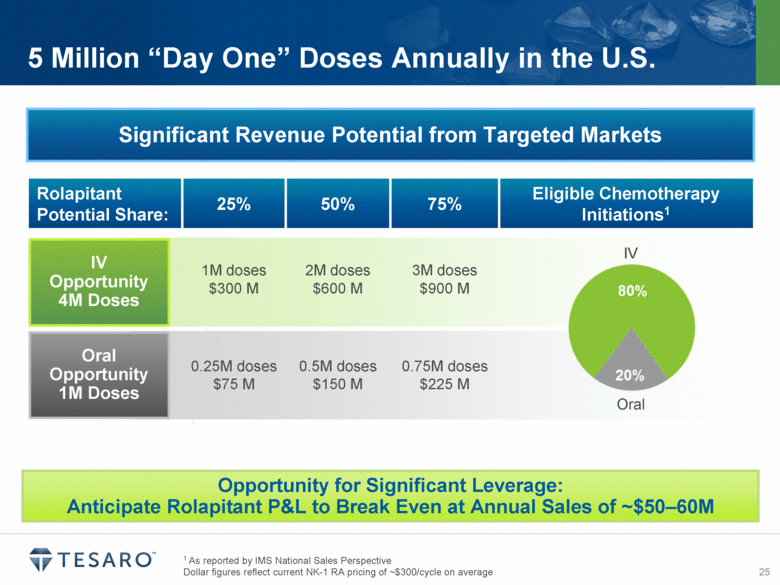
1 As reported by IMS National Sales Perspective Dollar figures reflect current NK-1 RA pricing of ~$300/cycle on average 5 Million “Day One” Doses Annually in the U.S. Significant Revenue Potential from Targeted Markets Rolapitant Potential Share: 25% 50% 75% Eligible Chemotherapy Initiations1 1M doses $300 M 2M doses $600 M 3M doses $900 M 0.25M doses $75 M 0.5M doses $150 M 0.75M doses $225 M IV Opportunity 4M Doses Oral Opportunity 1M Doses IV Oral Opportunity for Significant Leverage: Anticipate Rolapitant P&L to Break Even at Annual Sales of ~$50–60M |
|
|
[LOGO] |
|
|
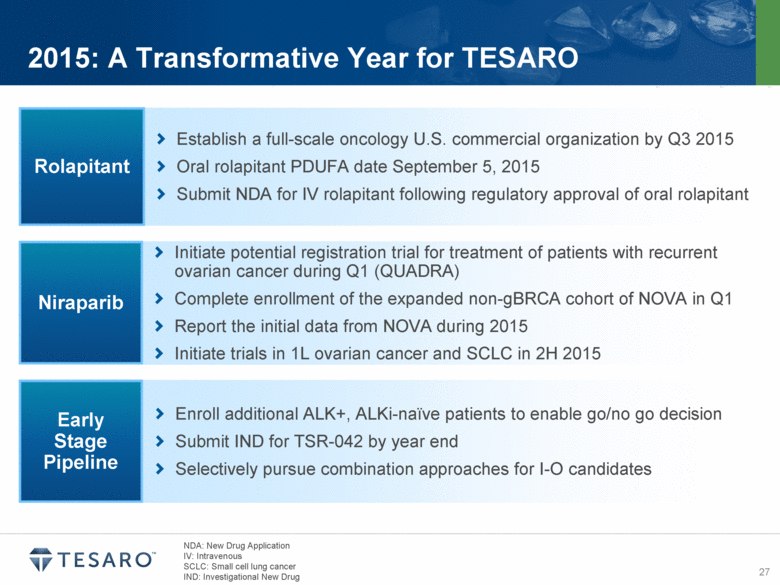
2015: A Transformative Year for TESARO Initiate potential registration trial for treatment of patients with recurrent ovarian cancer during Q1 (QUADRA) Complete enrollment of the expanded non-gBRCA cohort of NOVA in Q1 Report the initial data from NOVA during 2015 Initiate trials in 1L ovarian cancer and SCLC in 2H 2015 Niraparib Establish a full-scale oncology U.S. commercial organization by Q3 2015 Oral rolapitant PDUFA date September 5, 2015 Submit NDA for IV rolapitant following regulatory approval of oral rolapitant Rolapitant Enroll additional ALK+, ALKi-naïve patients to enable go/no go decision Submit IND for TSR-042 by year end Selectively pursue combination approaches for I-O candidates Early Stage Pipeline NDA: New Drug Application IV: Intravenous SCLC: Small cell lung cancer IND: Investigational New Drug |
|
|
Fourth-Quarter and Full-Year 2014 Results February 19, 2015 |