Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Aevi Genomic Medicine, Inc. | v401436_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Aevi Genomic Medicine, Inc. | v401436_ex99-1.htm |
Exhibit 99.2

Q4 2014 Results February 13, 2015

Forward Looking Statement This presentation includes certain estimates and other forward - looking statements within the meaning of Section 21 E of the Securities Exchange Act of 1934 , as amended, including statements with respect to anticipated operating and financial performance, clinical results, potential partnerships, licensing opportunities and other statements of expectation . Words such as “ expects, ” “ anticipates, ” “ intends, ” “ plans, ” “ believes, ” “ assumes, ” “ seeks, ” “ estimates, ” “ should ” and variations of these words and similar expressions, are intended to identify these forward - looking statements . While we believe these statements are accurate, forward - looking statements are inherently uncertain and we cannot assure you that these expectations will occur and our actual results may be significantly different . These statements by the Company and its management are based on estimates, projections, beliefs and assumptions of management and are not guarantees of future performance . Important factors that could cause actual results to differ from those in the forward - looking statements include the factors described in the Company ’ s filings with the U . S . Securities and Exchange Commission . The Company disclaims any obligation to update or revise any forward - looking statement based on the occurrence of future events, the receipt of new information, or otherwise . 2
Agenda 3 • 2014 Recap • MDGN - 201 Clinical Update • Early Stage Pipeline • Collaborations • Q4 Financial Update • 2015 Milestones
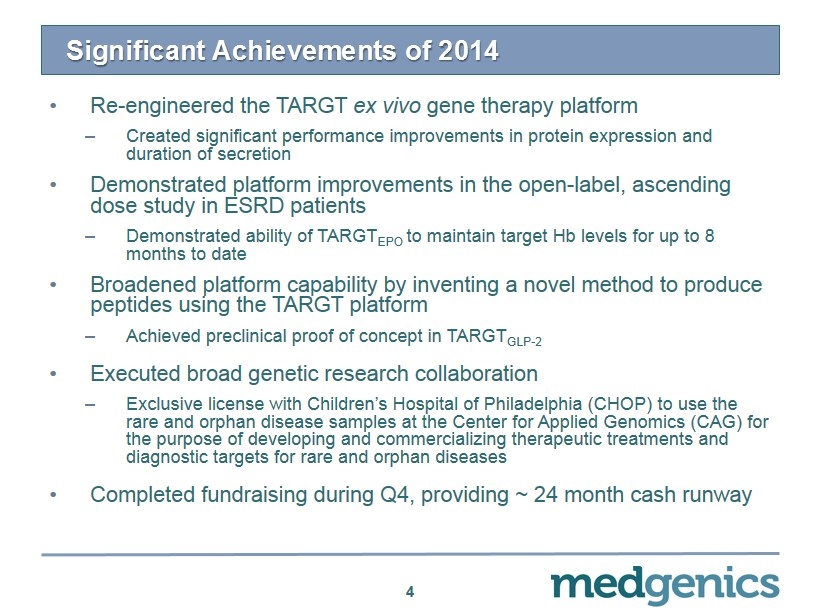
Significant Achievements of 2014 4 • Re - engineered the TARGT ex vivo gene therapy platform – Created significant performance improvements in protein expression and duration of secretion • Demonstrated platform improvements in the open - label, ascending d ose study in ESRD patients – Demonstrated ability of TARGT EPO to maintain target Hb levels for up to 8 months to date • Broadened platform capability by inventing a novel method to produce peptides using the TARGT platform – Achieved preclinical proof of concept in TARGT GLP - 2 • Executed broad genetic research collaboration – Exclusive license with Children’s Hospital of Philadelphia (CHOP) to use the rare and orphan disease samples at the Center for Applied Genomics (CAG) for the purpose of developing and commercializing therapeutic treatments and diagnostic targets for rare and orphan diseases • Completed fundraising during Q4, providing ~ 24 month cash runway

MDGN - 201 Study Update 5
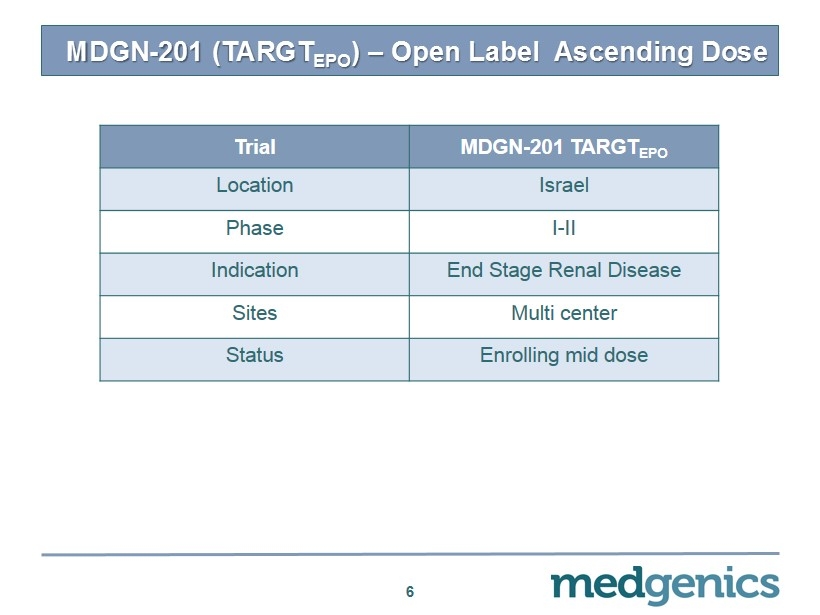
6 Trial MDGN - 201 TARGT EPO Location Israel Phase I - II Indication End Stage Renal Disease Sites Multi center Status Enrolling mid dose MDGN - 201 (TARGT EPO ) – Open Label Ascending Dose
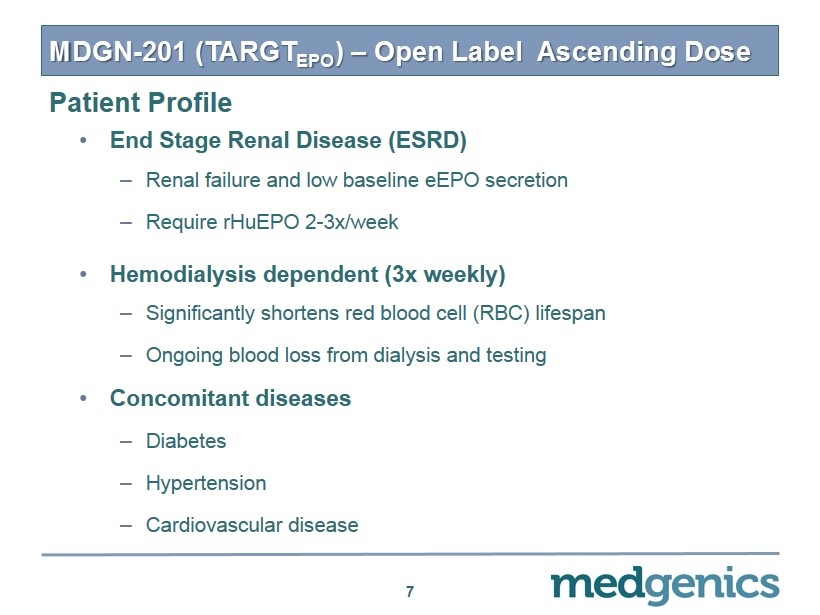
MDGN - 201 (TARGT EPO ) – Open Label Ascending Dose • End Stage Renal Disease (ESRD) – Renal failure and low baseline eEPO secretion – Require rHuEPO 2 - 3x/week • Hemodialysis dependent (3x weekly) – Significantly shortens red blood cell (RBC) lifespan – Ongoing blood loss from dialysis and testing • Concomitant diseases – Diabetes – Hypertension – Cardiovascular disease 7 Patient Profile
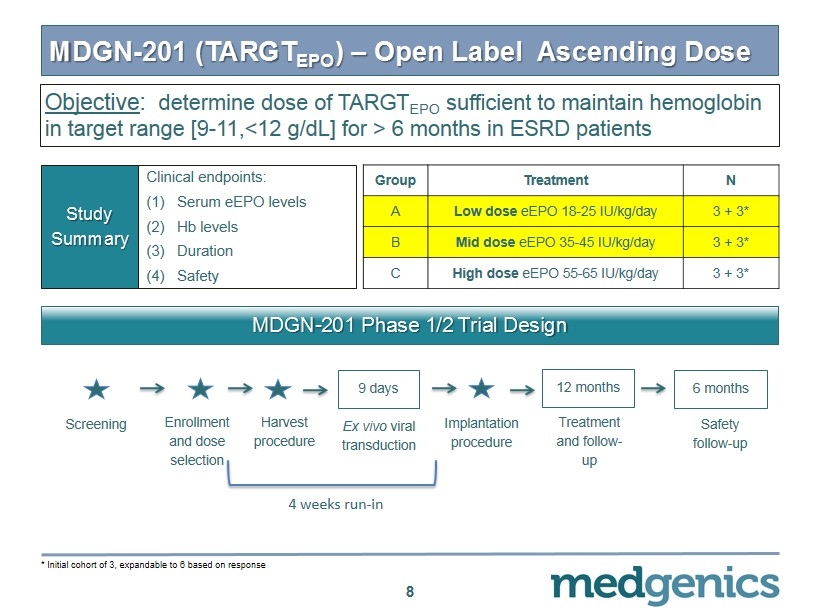
MDGN - 201 (TARGT EPO ) – Open Label Ascending Dose 8 Clinical endpoints: (1) Serum eEPO levels (2) Hb levels (3) Duration (4) Safety Study Summary Group Treatment N A Low dose eEPO 18 - 25 IU/kg/day 3 + 3 * B Mid dose eEPO 35 - 45 IU/kg/day 3 + 3 * C High dose eEPO 55 - 65 IU/kg/day 3 + 3 * MDGN - 201 Phase 1/2 Trial Design Enrollment and dose selection Screening Harvest procedure Ex vivo viral transduction 9 days Treatment and follow - up 12 months Safety follow - up 6 months Implantation procedure 4 weeks run - in * Initial cohort of 3 , expandable to 6 based on response Objective : determine dose of TARGT EPO sufficient to maintain hemoglobin in target range [9 - 11,<12 g/ dL ] for > 6 months in ESRD patients
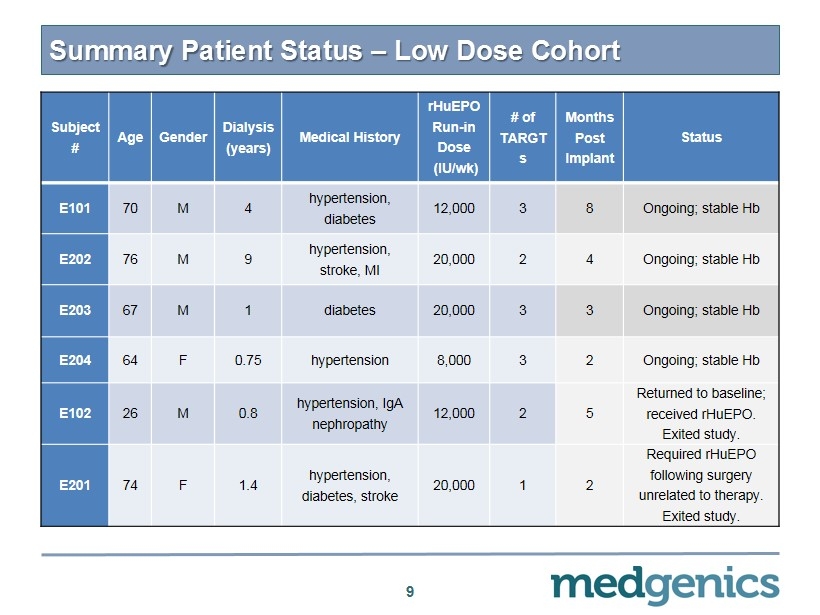
9 Subject # Age Gender Dialysis (years) Medical History rHuEPO Run - in Dose (IU/ wk ) # of TARGT s Months Post Implant Status E101 70 M 4 hypertension, diabetes 12,000 3 8 Ongoing ; s table Hb E202 76 M 9 hypertension, stroke, MI 20,000 2 4 Ongoing; stable Hb E203 67 M 1 diabetes 20,000 3 3 Ongoing; stable Hb E204 64 F 0.75 hypertension 8,000 3 2 Ongoing; stable Hb E102 26 M 0.8 hypertension, IgA nephropathy 12,000 2 5 Returned to baseline; received rHuEPO . Exited study. E201 74 F 1.4 hypertension, diabetes, stroke 20,000 1 2 Required rHuEPO following surgery unrelated to therapy. Exited study. Summary Patient Status – Low Dose Cohort
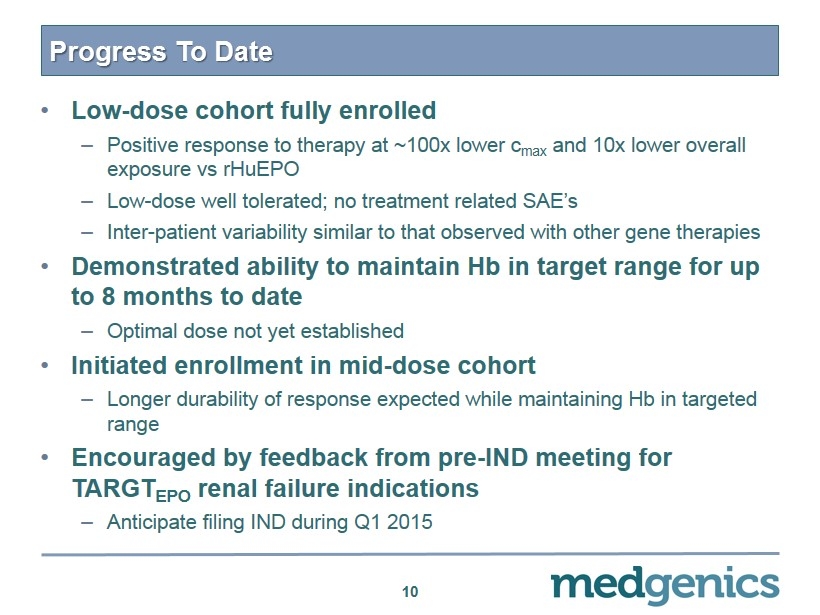
Progress To Date • Low - dose cohort fully enrolled – P ositive response to therapy at ~ 100x lower c max and 10x lower overall exposure vs rHuEPO – Low - dose well tolerated; no treatment related SAE’s – Inter - patient variability similar to that observed with other gene therapies • Demonstrated ability to maintain Hb in target range for up to 8 months to date – Optimal dose not yet established • Initiated enrollment in mid - dose cohort – L onger durability of response expected while maintaining Hb in targeted range • Encouraged by feedback from pre - IND meeting for TARGT EPO renal failure indications – Anticipate filing IND during Q1 2015 10
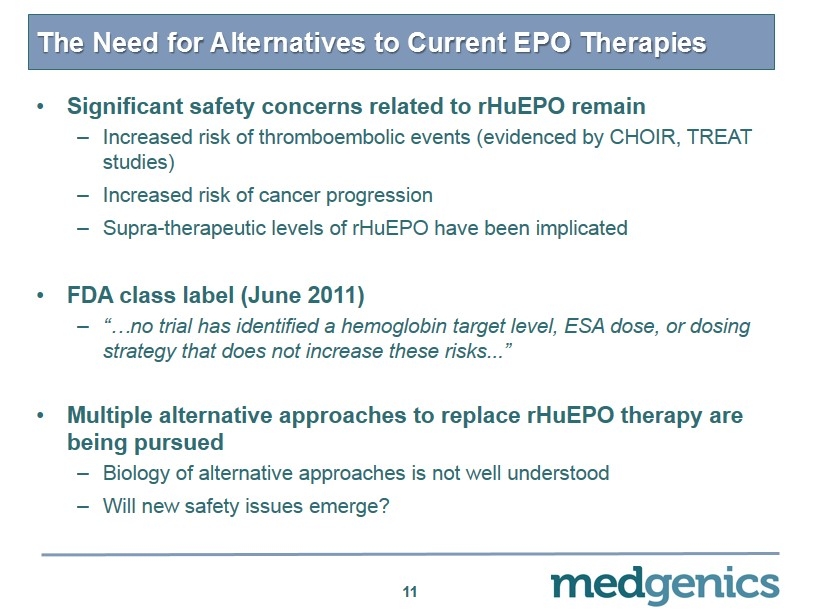
The Need for Alternatives to Current EPO Therapies 11 • Significant safety concerns related to rHuEPO remain – Increased risk of thromboembolic events (evidenced by CHOIR, TREAT studies) – Increased risk of cancer progression – Supra - therapeutic levels of rHuEPO have been implicated • FDA class label (June 2011) – “…no trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks... ” • Multiple alternative approaches to replace rHuEPO therapy are being pursued – Biology of alternative approaches is not well understood – Will new safety issues emerge?
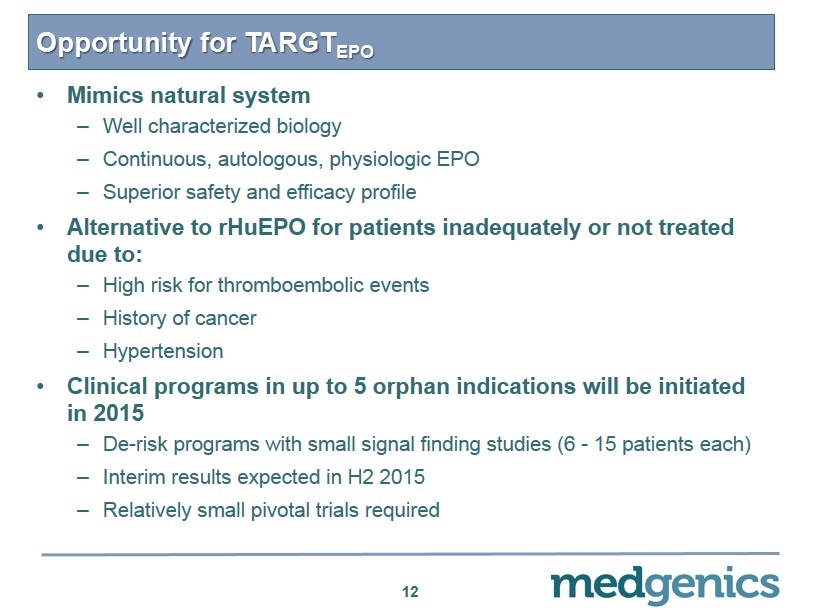
Opportunity for TARGT EPO 12 • M imics natural system – Well characterized biology – Continuous, autologous, physiologic EPO – Superior safety and efficacy profile • Alternative to rHuEPO for patients inadequately or not treated due to: – H igh risk for thromboembolic events – History of cancer – Hypertension • Clinical programs in up to 5 orphan indications will be initiated in 2015 – De - risk programs with small signal finding studies (6 - 15 patients each) – Interim results expected in H2 2015 – R elatively small pivotal trials required
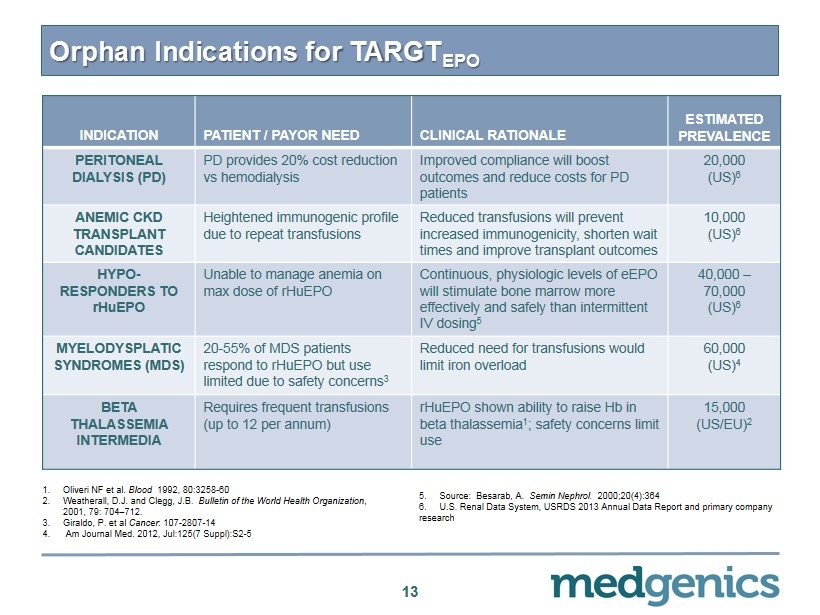
Orphan Indications for TARGT EPO 13 INDICATION PATIENT / PAYOR NEED CLINICAL RATIONALE ESTIMATED PREVALENCE PERITONEAL DIALYSIS (PD) PD provides 20% cost reduction vs hemodialysis Improved compliance will boost outcomes and reduce costs for PD patients 20,000 (US) 6 ANEMIC CKD TRANSPLANT CANDIDATES Heightened immunogenic profile due to repeat transfusions Reduced transfusions will prevent increased immunogenicity, shorten wait times and improve transplant outcomes 10,000 (US) 6 HYPO - RESPONDERS TO rHuEPO Unable to manage anemia on max dose of rHuEPO Continuous, physiologic levels of eEPO will stimulate bone marrow more effectively and safely than intermittent IV dosing 5 40,000 – 70,000 (US) 6 MYELODYSPLATIC SYNDROMES (MDS) 20 - 55% of MDS patients respond to rHuEPO but use limited due to safety concerns 3 Reduced need for transfusions would limit iron overload 60,000 (US) 4 BETA THALASSEMIA INTERMEDIA Requires frequent transfusions (up to 12 per annum) rHuEPO shown ability to raise Hb in beta thalassemia 1 ; safety concerns limit use 15,000 (US/EU) 2 1. Oliveri NF et al. Blood 1992 , 80:3258 - 60 2. Weatherall , D.J. and Clegg, J.B. Bulletin of the World Health Organization , 2001 , 79 : 704 – 712 . 3. Giraldo , P. et al Cancer : 107 - 2807 - 14 4. Am Journal Med. 2012 , Jul: 125 ( 7 Suppl ):S 2 - 5 5 . Source : Besarab , A. Semin Nephrol . 2000 ; 20 ( 4 ): 364 6 . U.S . Renal Data System, USRDS 2013 Annual Data Report and primary company research
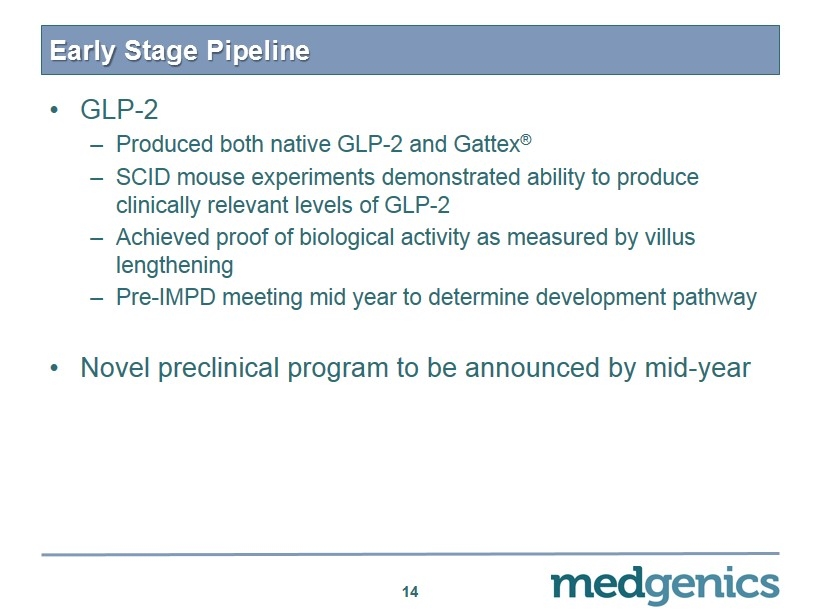
Early S tage P ipeline • GLP - 2 – Produced both native GLP - 2 and Gattex ® – SCID mouse experiments demonstrated ability to produce clinically relevant levels of GLP - 2 – Achieved proof of biological activity as measured by villus lengthening – Pre - IMPD meeting mid year to determine development pathway • Novel preclinical program to be announced by mid - year 14
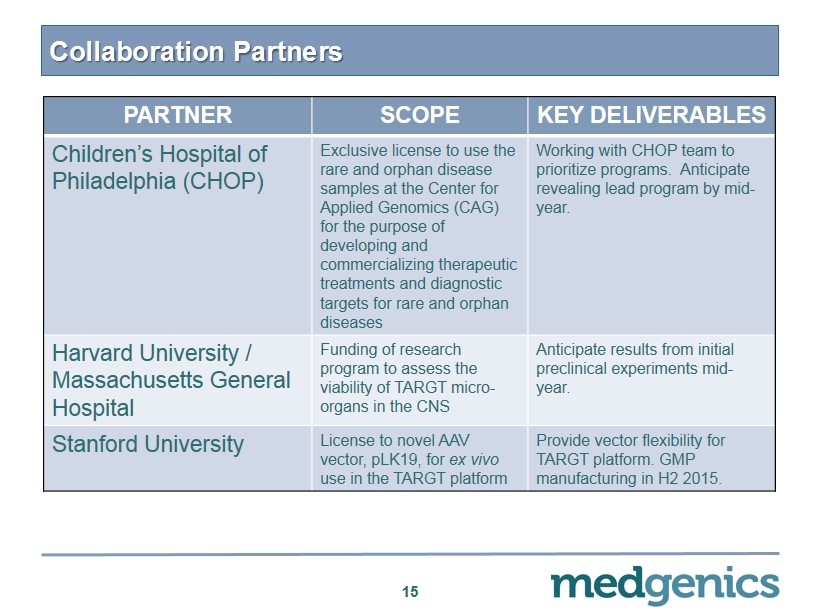
Collaboration Partners 15 PARTNER SCOPE KEY DELIVERABLES Children’s Hospital of Philadelphia (CHOP) Exclusive license to use the rare and orphan disease samples at the Center for Applied Genomics (CAG) for the purpose of developing and commercializing therapeutic treatments and diagnostic targets for rare and orphan diseases Working with CHOP team to prioritize programs . Anticipate revealing lead program by mid - year. Harvard University / Massachusetts General Hospital Funding of research program to a ssess the viability of TARGT micro - organs in the CNS Anticipate results from initial preclinical experiments mid - year. Stanford University License to novel AAV vector, pLK19, for ex vivo use in the TARGT platform Provide vector flexibility for TARGT platform . GMP manufacturing in H2 2015.

Financial Update 16
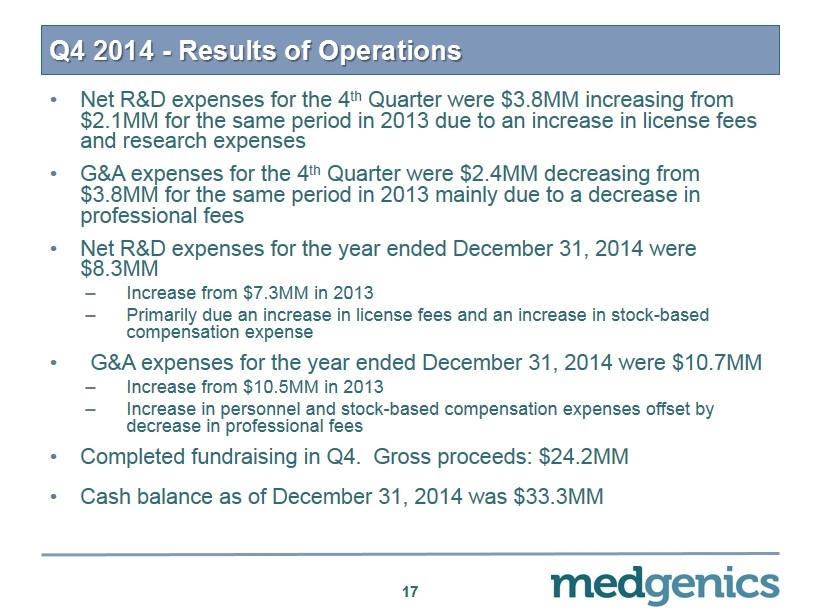
Q4 2014 - Results of Operations • Net R&D expenses for the 4 th Quarter were $3.8MM increasing from $2.1MM for the same period in 2013 due to an increase in license fees and research expenses • G&A expenses for the 4 th Quarter were $2.4MM decreasing from $3.8MM for the same period in 2013 mainly due to a decrease in professional fees • Net R&D expenses for the year ended December 31, 2014 were $8.3MM – Increase from $7.3MM in 2013 – Primarily due an increase in license fees and an increase in stock - based compensation expense • G&A expenses for the year ended December 31, 2014 were $ 10.7MM – Increase from $10.5MM in 2013 – Increase in personnel and stock - based compensation expenses offset by decrease in professional fees • Completed fundraising in Q4. Gross proceeds: $ 24.2MM • Cash balance as of December 31, 2014 was $ 33.3MM 17
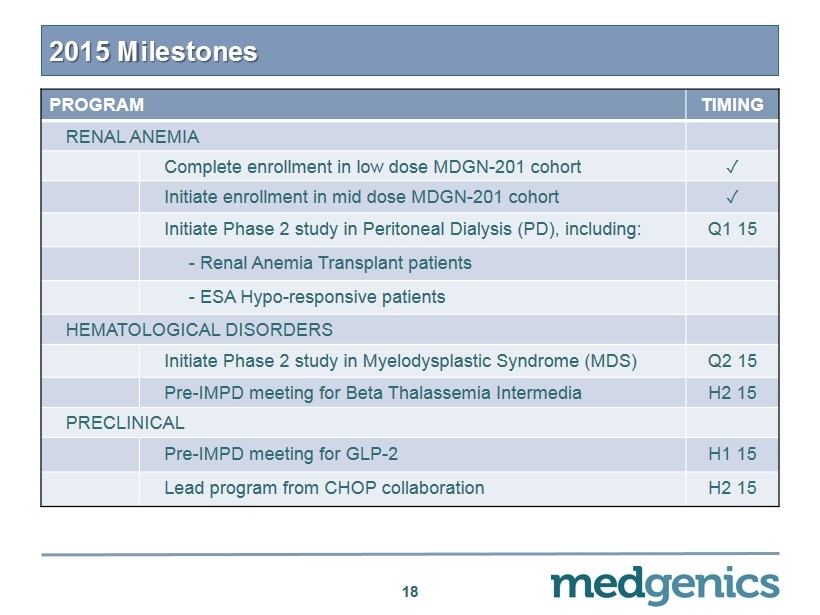
2015 Milestones 18 PROGRAM TIMING RENAL ANEMIA Complete enrollment in low dose MDGN - 201 cohort ✓ Initiate enrollment in mid dose MDGN - 201 cohort ✓ Initiate Phase 2 study in Peritoneal Dialysis (PD), including: Q1 15 - Renal Anemia Transplant patients - ESA Hypo - responsive patients HEMATOLOGICAL DISORDERS Initiate Phase 2 study in Myelodysplastic Syndrome (MDS) Q2 15 Pre - IMPD meeting for Beta Thalassemia Intermedia H2 15 PRECLINICAL Pre - IMPD meeting for GLP - 2 H1 15 Lead program from CHOP collaboration H2 15
