Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Sorrento Therapeutics, Inc. | d867736d8k.htm |
 Next-Generation
Cancer Therapeutics
February 2015
Exhibit 99.1 |
 2
Safe Harbor Statement NASDAQ: SRNE
This presentation contains "forward-looking statements" as that term is
defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
including statements regarding expectations, beliefs or intentions regarding our business,
technologies and products strategies or prospects. Such forward-looking
statements are characterized by future or conditional verbs such as
“may,” “will,”
“expect,”
“intend,”
“anticipate,”
“believe,”
“estimate”
and “continue”
or similar
verbs. Actual results may differ from those projected due to a number of
risks and uncertainties, including, but not limited to, the possibility that
some or all of the pending matters and transactions being considered by the Company
may not proceed as contemplated, as well as risks inherent in additional financing,
developing and obtaining regulatory approvals of new,
commercially-viable and competitive products and product candidates, including timelines, the size of
clinical trials, sufficiency of data from those trials and the requirements of the
FDA for potential approval of Cynviloq™ and
by
all
other
matters
described
in
the
Company's
filings
with
the
Securities
and
Exchange
Commission,
including
the
risk factors
set
forth
therein.
These
statements
are
made
based
upon
current
expectations that are subject to risk and
uncertainty and information available to the Company as of the date of this
presentation. The Company does not undertake to update forward-looking
statements in this presentation to reflect actual results, changes in assumptions or
changes in other factors affecting such forward-looking information.
Assumptions and other information that could cause results to differ from
those set forth in the forward-looking information can be found in the Company's filings with
the Securities and Exchange Commission, including its most recent periodic report.
We intend that all forward-looking statements be subject to the
safe-harbor provisions of the PSLRA. |
 Cytotoxics
CYNVILOQ™
Targeted Therapy
MYC inhibitor
TRAIL modulator
Immunotherapy
PD-1, PD-L1, CTLA-4
Bispecific Abs
Targeted Therapy
Anti-VEGFR2 ADC
Anti-c-MET ADC
Bispecific ADC
Adoptive Cellular
Immunotherapy
Chimeric Antigen Receptor
Tumor-attacking
Neukoplast
®
(Partnership with Conkwest)
RTX
Intractable
Cancer Pain
A Comprehensive Oncology Company
Deep and Complementary Pipeline Creates Significant Opportunities-
Novel breakthrough combination therapeutic regimens and modalities to attack
cancer Significant reduction in clinical development costs and timeline
Significant commercial edge in future drug pricing
CYNVILOQ, CAR.TNK, CAR.TNK (Chimeric Antigen Receptor Tumor-attacking
Neukoplast) are trademarks owned by Sorrento Therapeutics, Inc.
Neukoplast is a trademark owned by Conkwest, Inc.
Small
Molecules
Biologics
Cell
Therapy
Supportive
Care
3 |
 4
Corporate Events Validate and Advance Sorrento
Pipeline Unlocking Significant Value
“The
Immunotherapy
Antibody
Company”
Exclusive global partnership with Conkwest to develop next generation
anti-cancer cellular
immunotherapy
with
"Off-the-Shelf"
CAR.TNK™
(Chimeric
Antigen
Receptor
Tumor
-attacking
Neu
Koplast)
First joint venture with NantWorks and Abraxis BioScience Inc. founder, Dr.
Patrick Soon- Shiong,
to develop next generation immunotherapies for the treatment of cancer and
autoimmune disease.
G-MAB
ADC
Licensing agreement to develop and commercialize anti-PD-L1 mAb with
Lee’s Pharmaceutical for greater Chinese Market
Exclusive research and option agreement to generate and develop antibody-drug
conjugates (ADCs) with Morphotek / Eisai
CYNVILOQ
Patient enrollment in TRIBECA registration trial completed. Pilot PK suggests
bioequivalence (BE) between Cynviloq and albumin-bound paclitaxel
C
A
R
T
N
K |
 Deep
and Complementary Pipeline Creates Significant Opportunities
*
TRIBECA
505
(b)(2)
Bioequivalence
trial
versus
albumin-bound
paclitaxel
(Abraxane
®
)
(paclitaxel
albumin-bound
particles
for
injectable
suspension)
(albumin-
bound), Abraxane®
is a registered trademark of and marketed by Celgene Corp.
PDL1.TNK,
CD123.TNK,
ROR1.TNK,
PSMA.TNK
are
trademarks
owned
by
Sorrento
Therapeutics,
Inc.
CYNVILOQ™
G-MAB
Bi-Specific Ab
RTX
Immuno-oncology
> PD-L1, PD1, CD47, CD137
VEGFR2, c-MET, CXCR5
Intractable Cancer Pain
INDICATION
>
TARGET
Metastatic Breast Cancer
Non-Small Cell Lung Cancer
T R I B E C A*
Registration trial completed
PHASE 3
PHASE 2
PHASE 1
PRECLINICAL
Solid tumors and hematological malignancies
ADC
MYC Inhibitor
PD-L1.TNK, CD123.TNK, ROR1.TNK, PSMA.TNK
PD-L1/c-MET; PD-L1/CTLA-4, PD-L1/EGFR
5 |
 6
Lead Oncology Product Opportunity
Cynviloq
Registration
Trial
(Paclitaxel polymeric
micelle) |
 7
Mean size
~25 nm
Cynviloq
paclitaxel
polymeric micelle
Chemical
polymer:
Poly-lactide and
polyethylene glycol
diblock copolymer
3
>300
mg/m
2
(up
to
435
mg/m
)
Albumin-bound
paclitaxel
Mean size
130 nm
Biological
polymer:
Donor-derived human
serum albumin (HSA)
2
260
mg/m
2
Taxol
®
paclitaxel
Cremophor
EL
excipient:
Polyoxyethylated
castor oil
Formulation
Generation
1
175
mg/m
2
Maximum
Tolerated Dose
Peak
Product Sales
~ $1.6B (WW in 2000)
$ 2.2 B* (2020)
MBC, NSCLC, PC
Conversion of
paclitaxel sales +
new indications
*Celgene Presentation at JPM Healthcare Conference Jan 2015
Cynviloq: Next Generation Paclitaxel Therapy
2
st
nd
rd |
 8
Cynviloq Clinical Development Summary
Phase 1:
Trials
established
MTD
at
>300
mg/m
2
-
Dana
Farber
Cancer
Inst,
Russia,
&
S.
Korea
(total n=80)
>300
mg/m
2
(q3w)
vs.
175
mg/m
2
(Taxol;
weekly)
Phase 2:
Completed
trials
in
MBC,
NSCLC,
PC,
OC,
BC;
in
US
-
Yale
Cancer
Center,
Russia,
S.
Korea
(total
n=259)
Possible Phase 3 sNDA programs in these tumor types
Phase 2b*:
Chemo-naïve Stage IIIb/IV NSCLC vs Taxol in S. Korea (total n=276;
Cynviloq n=140) 230
mg/m²
+
cis
(q3w)
vs.
Taxol
175
mg/m
2
+
cis;
non-inferiority
established
Phase 2*:
1st line treatment of OC vs Taxol in S. Korea (total n=100; Cynviloq n=50)
260
mg/m
2
+
carbo
(q3w)
vs.
Taxol
175
mg/m
2
+
carbo;
non-inferiority
established
Phase 3:
MBC in S. Korea (total n=209; Cynviloq n=105 vs Taxol n=104)
GPMBC301.
An
Open-label,
Randomized,
Parallel,
Phase
3
Trial
to
Evaluate
the
Efficacy
and
Safety
of
Cynviloq
compared
to
Genexol®
(Paclitaxel with Cremophor EL) in Subjects with Recurrent or Metastatic Breast
Cancer) PM-Safety:
Completed for MBC and NSCLC (total n=502)
Efficacy and safety data supportive of 505(b)(2) submission
Total number of patients across all trials: 1,260
Data on file; * Investigator Initiated Study |
 9
Comparative Phase 3 MBC Clinical Results
* Trieu
et
al.
2013.
IG-001
for
Metastatic
Breast
Cancer-
Interim
Analysis
of
a
Phase
3
Trial.
4
Nanomedicine
Conference,
Sydney,
Australia.
** Gradishar et al. 2005. J Clin Oncol, 23:7794-7803.
*** Guan et al. 2007. ASCO Annual Meeting Proceedings Part I.
Jun 20;25 (Suppl 18):1038. Overall
Response
Rate (%)
th |
 10
Bioequivalence = Accelerated Pathway to Market
T R I B E C A™
Albumin-bound
paclitaxel
(n = 27)*
Cynviloq
(n = 27)*
Cynviloq
Albumin-bound
paclitaxel
Key Parameters:
Cycle 1
Cycle 2
-
Patients with MBC
Note:
Previous
trial
size
estimate
of
100
patients
was
based
on
PK
simulation
of
albumin-bound
paclitaxel
and
Cynviloq
historical
data
with
both
drugs
given
at
different
doses
and
infusion
rates.
Based
on
the
recent
positive
initial
PK
data
and
subject
to
FDA
guidance,
53
patients
may
be
sufficient
to
establish
BE.
T
R
I
B
E
C
A™
(TRIal
establishing
BioEquivalence
between
Cynviloq™
and
Albumin-bound
paclitaxel)
is
a
trademark
owned
by
Sorrento
Therapeutics,
Inc.
•
Dose:
260
mg/m
2
•
Infusion time: 30 min
•
Duration: 3 weeks +
crossover for 3 weeks
•
Endpoints:
AUC
and
C
max
(90% CI)
(TRIal establishing BioEquivalence between Cynviloq™ and Albumin-bound paclitaxel) |
 11
Pilot PK Data Analyses Suggest
BE vs.
Albumin-Bound Paclitaxel
Parameters
Ratio of Cynviloq/
Albumin-bound paclitaxel
(%)
90% CI
Ln(AUC
0 to
)
109.1
93.98 –
126.58
Ln(C
max
)
102.5
83.10 –
126.35
Point estimate
110
-
Ln(AUC
0
to
)
N = 53 with 90% power
BE Assessment and Sample Size Estimate
Log
-linear Plot (n=8) |
 12
TRIBECA Patient Enrollment Completed
54 pts
Patients from recruited from sites in East Europe, USA and Asia
Total 111 patients enrolled (expanded safety data)
Initial reported AEs consistent with historical nab-paclitaxel toxicity
profile |
 Estimated Timeline and Next Steps*
First patient dosed: March
31, 2014
Last patient in: January 2015
NDA filing:
Q3
2015
Product launch (MBC and NSCLC):
2016
BE
Study
2014
2015
NDA
Filing
2016
FDA
Approval
LAUNCH
2016
*Estimates, subject to discussions with the FDA. |
 14
Intractable
Cancer
Pain
Resiniferatoxin (RTX): A Novel, Non-opiate Analgesic
RTX |
 15
Intraganglionic:
injection
into
or
near the dorsal root ganglion
Intrathecal:
injection
into
the
cerebrospinal fluid space
Two Injection Sites =
Two Products for Human Use
Cross Sections of
spinal cord*
Absence of TRPV1-
positive cells after RTX
treatment
TRPV1-positive cells
(dark brown)
* Adapted from Karai et al., 2004
Dorsal root ganglion |
 16
Summary of Interim Data from the Phase 1/2 NIH
Sponsored Trial
No unexpected
toxicities
All 6 patients had near
complete relief post-
injection
100% of non-ambulatory
patients could walk post
injection (n=2)
MTD not reached,
additional dose
optimization being
explored
RESULTS
Clinically meaningful
improvement in QOL
Improved pain scores with
increased activity
DESIGN OVERVIEW
6 advanced cancer patients with severe refractory pain received a single injection
of RTX. Neuropathic
pain,
visceral
and
bone
pain
2
o
to
bone
metastases
(49-61 years; 4 M/ 2F, MBC, H&N, pancreatic, lymphoma, SCLC, endometrial
cancer). |
 17
Next Steps for RTX Development
~3 years for clinical development
Complete intractable cancer pain clinical Phase 1/2
trial (intrathecal injection) under Sorrento IND;
n=45-60 patients; optimization of dosing study
End of Phase 2 meeting with FDA (for intrathecal injection)
Initiate Phase 3 (intrathecal injection)
Phase 1/2 trial(s) (intraganglionic injection)
End of Phase 2 meeting with FDA (for intraganglionic injection)
OBJECTIVES
for
2015 and 2016 |
 18
Immunotherapy Programs
G-MAB
+ Neukoplast
+ Proprietary Toxins &
Conjugation Chemistries |
 19
G-MAB: Library of Therapeutic Antibodies
High Value Oncology Targets:
Immune
modulation:
PD-1,
PD-L1,
CD47
Antibody
Drug
Conjugates:
VEGFR2,
c-Met
Size of Target Antigen
Proprietary technology:
RNA amplification used for library
generation
Freedom-To-Operate
Very high library diversity:
2.1
x
10
16
distinct
antibodies
Fully human antibodies
High successful screening hit rate
(over 70 targets screened)
Ideal for CAR-Generation
Difficult Targets:
Small Peptides
Most Difficult Targets:
G Protein-Coupled Receptors (GPCRs)
No stacking royalties |
 20
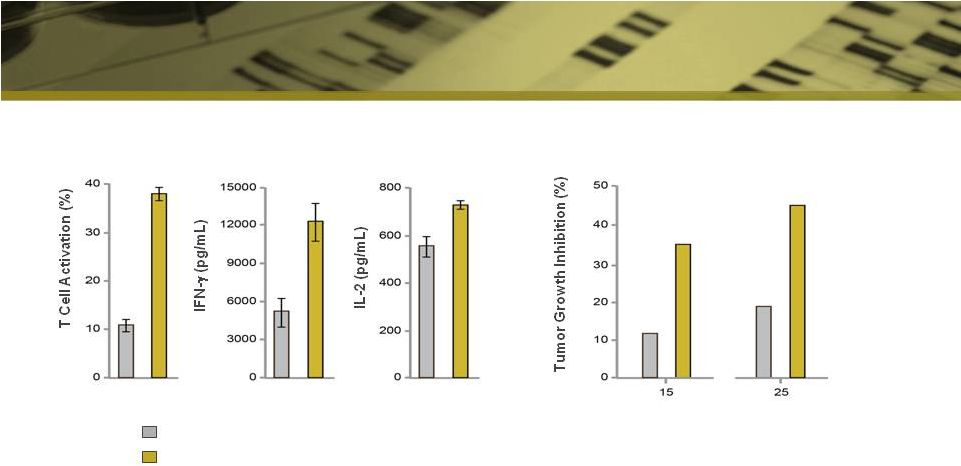
Anti-PD-L1 mAb Exhibits Potent Activity
Immune Modulation*
Tumor Mouse Model**
***
* mAbs @ 0.05 mg/mL
** xenograft model using H1975 human NSCLC cells; % inhibition relative to control
mAb treatment *** p<0.05, mean tumor volumes are significantly reduced
in STI-A1010 group versus control groups as determined by Mann-Whitney u-test
Competitor mAb
Sorrento mAb
Day |
 21
Competitor mAb
Sorrento mAb
Anti-PD1 mAb Exhibits Excellent Activity
Immune Modulation*
Target Specificity
Control
Sorrento mAb
Human
PD1
Cyno
PD1
Human
CTLA-4
Human
CD28
Human
ICOS
PBS
control |
 K-Lock
C-Lock
C-Lock conjugation
Enhances ADC stability
Prolongs PK profile
Reduces off-target effects
Maleimide
conjugation
Destabilizes antibody structure
Drug-antibody linkage not stable
Altered PK profile
Off-target drug effects
Proprietary K-Lock and C-Lock Conjugation
Chemistries Enable Homogeneous ADCs
22 |
 23
Proprietary High Potency Duostatin Toxins
EC
50
(pM)
Cancer
Her-2
DM1
MMAE
Duostatin 3
SBKR3
Breast
+++
95
72
30
HCC1954
Breast
+++
124
78
68
BT474
Breast
+++
818
126
214
MDA-MB-361
Breast
+++
218
151
35
ZR75
Breast
+++
215
298
264
HCC1419
Breast
+++
391
271
332
MDA-MB-453
Breast
++
1,877
>100,000
452
MDA-MB-175
Breast
+
>100,000
1,348
425
N87
Gastric
+++
368
139
260
OE-19
Gastric
+++
176
164
130
SKOV-3
Ovarian
+++
150
251
144
Trastuzumab was used as targeting mAb |
 24
VEGFR2-ADC STI-D0168
c-MET-ADC STI-D0602
In Vivo Proof-of-Concept of Sorrento ADCs
A431 squamous-cell carcinoma cells; ^indicates dosing
U87 xenograft; dosing twice weekly; maytansinoid drug conjugates
|
 25
“The Immunotherapy Antibody JV Company”
Independent company focused
on advancing next generation immunotherapies against cancer and auto-
immune diseases.
Both companies will contribute to its pipeline of clinical and preclinical
assets of novel and proprietary immunotherapies, ADCs, and bispecific
antibodies.
Joint venture will draw from NantWorks’
proteomic and genomic
capabilities and Sorrento’s industry-leading, highly diverse G-MAB
library. |
 AN
EXCLUSIVE JOINT PARTNERSHIP 26
CAR.TNK is a trademark owned by Sorrento Therapeutics, Inc.
Neukoplast is a trademark owned by Conkwest, Inc. |
 Advancing Cellular Immunotherapy
Beyond CAR-T Cell Therapies
Neukoplast®
NK cell line
(“off-the-shelf”)
Broad anti-cancer activity in
solid
and liquid tumors
No clinical DLTs/SAEs in
over 40 patients treated
Advanced proteomics platform
Proprietary gene insertion
(without use of lentiviruses)
‘GMP in a Box’
production
technology
G-MAB
27
High successful screening rate
(over 70 targets screened)
Proprietary technologies with
FTO
Vast diversity human antibody
library |
 28
CAR.TNK vs CAR-T: Key Differentiators
Simple:
Off-the-shelf
universal
product
CAR-modified Neukoplast cells
Cell Production
Transduction
characteristics
100%:
Master
cell
bank
with
100% of cells expressing CAR
MOA
Broad:
Multiple
MOAs,
targeting
and
killing through CAR-dependent and
innate
mechanisms
(“off-target
/
on-tumor”)
Safety
Good:
On-target
/
off-tumor
effects
limited due to short half life and lack
of IL-6 production
COGS
Low:
large
scale
bioreactor
manufacturing for many patients
Invasive:
Autologous
(patient-derived)
invasive procedure/leukapheresis
Variable
%:
Variable
CAR
transfection & expression
Limited:
Requires
co-stimulators
(CD80, CD86) not present in many
solid tumors
Poor:
Cytokine
release
syndrome,
ICU;
Prolonged bone marrow suppression;
Cardiotoxicity; Reported cases of
encephalitis; Death
High:
requires
individual
patient
processing
CAR.TNK
CAR-T |
 Unmodified Neukoplast Clinically Validated In Several
Phase 1 Studies
More than 40 patients treated
Advanced metastatic disease refractory to chemo, biologics, cytokines,
radiation, and surgery
Many patients received multiple dosing regimens (up to 6 months)
Promising activity against different cancer types, including acute myelogenous
leukemia (AML), lymphoma (NHL, HL), melanoma, renal cell cancer (RCC), and
lung cancers (SCLC, NSCLC)
No DLTs; only 1 “grade 4 SAE”
(hypoglycemia likely related to tumor lysis)
29 |
 30
-5000
0
5000
10000
15000
20000
25000
30000
35000
PHA
Allogeneic Donor
NK-92
CPM
Lymphocytes from 2 healthy donors co-cultured with each other
vigorous proliferation
Co-cultured with Neukoplast (7 days)
no proliferation
Gold: Donor 1
Aqua: Donor 2
T cell Proliferation measured using
Mixed Lymphocyte Reaction (MLR) Culture Assay
Neukoplast do not stimulate allogeneic T cells
Neukoplast
PHA
Allogeneic Donor |
 31
CAR.TNK: CAR-modified Neukoplast
Clonal cell lines expressing one or more CARs to establish a range of distinct
products Multiple
killing
mechanisms
-
CAR-targeted
as
well
as
broad
intrinsic
anti-cancer
activity of Neukoplast (“off-target / on-tumor”)
Engages the adaptive immune system through cytokine secretion and immune cell
recruitment
Titratable: repeat dosing option; controllable dose exposure to manage safety
risk |
 32
Serial Killing of Her2+ Cells by Her2.TNK Cells
Homing to Her2 expressing tumors
Inhibition of Her2+ RCC metastasis
Selective cytotoxicity (spares normal cells)
Schoenfeld et al. Mol Therapy, in press
IN VIVO PRECLINICAL MOUSE DATA
Growth inhibition and killing correlate
with Her2 expression levels
“Serial killing”
of Her2+ target cells
even after gamma radiation with 10 Gy |
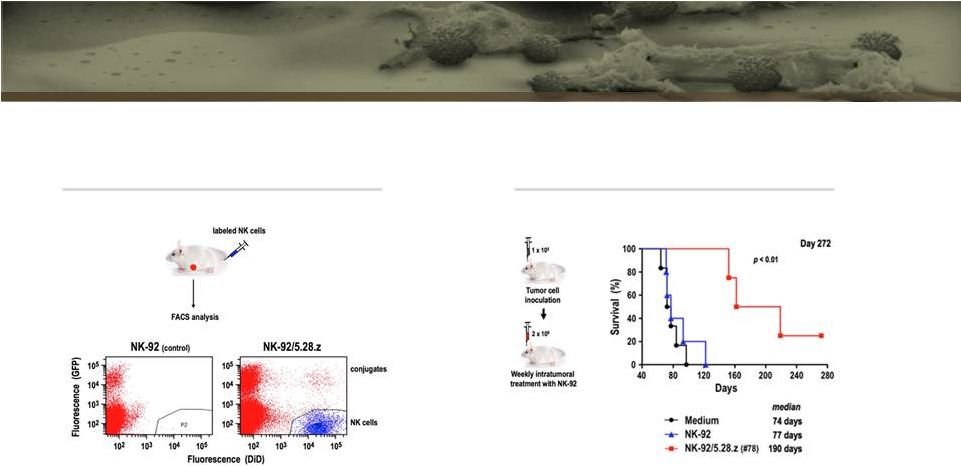 33
Her2.TNK Demonstrate Tumor Homing and Potent
Anti-Glioma Activity in Mice
Schoenfeld et al. Mol Therapy, in press
Tumor homing of CAR.TNKs
Intracranial LN-319 glioblastoma
xenografts in NSG mice |
 Prospective CAR.TNKs for Development
(Initial List)
Target
Potential Indication(s)
EGFRviii.TNK
Glioma
EphA3.TNK
Glioma, AML
L1CAM.TNK
Gastric, pancreatic, NSCLC
CSPG4.TNK
H&N, breast, mesothelioma
BCMA.TNK
Myeloma
ROR1.TNK
CLL, ALL, MCL, breast, lung, pancreas
PSMA or
PSCA.TNK
Prostate
PDL1.TNK
Myeloma, RCC, NSCLC, TNBC
CS1.TNK
Myeloma
CD123.TNK
AML
CD19.TNK
CLL, ALL
CD22.TNK
CLL, ALL
CAR targets jointly selected by the
Steering Committee
Lead company will be responsible for
all pre-clinical and clinical development,
regulatory filings, and
commercialization
Profit sharing on all CAR.TNKs
revenues proportional to contribution
EGFRviii.TNK, EphA3.TNK, L1CAM.TNK, CSPG4.TNK, BCMA.TNK, ROR1.TNK, PSMA.TNK,
PCMA.TNK, PDL1.TNK, CS1.TNK, CD123.TNK, CD19.TNK, CD22.TNK are trademarks owned by Sorrento Therapeutics, Inc.
34 |
 Next
Steps for CAR.TNK Development H1 2015
Generation of CARs
H2 2015
Generation and evaluation of stable CAR.TNK cell lines
2016
IND-enabling studies, IND submission, and initiation of Phase 1 studies
35 |
 36
Cytotoxics
CYNVILOQ™
Targeted Therapy
MYC inhibitor
TRAIL modulator
Immunotherapy
PD1, PD-L1, CTLA-4
Bispecific Abs
Targeted Therapy
Anti-VEGFR2 ADC
Anti-CMET ADC
Bispecific ADC
Adoptive Cellular
Immunotherapy
Chimeric Antigen Receptor
Tumor-attacking
Neukoplast
®
(Partnership with Conkwest)
RTX
Intractable
Cancer Pain
A Comprehensive Oncology Company
Deep and Complementary Pipeline Creates Significant Opportunities-
Novel breakthrough combination therapeutic regimens and modalities to attack
cancer Significant reduction in clinical development costs and timeline
Significant commercial edge in future drug pricing
CYNVILOQ, CAR.TNK, CAR.TNK (Chimeric Antigen Receptor Tumor-attacking
Neukoplast) are trademarks owned by Sorrento Therapeutics, Inc.
Neukoplast is a trademark owned by Conkwest, Inc.
Small
Molecules
Biologics
Cell
Therapy
Supportive
Care |
 37
Next-Generation
Cancer Therapeutics
Henry Ji, Ph.D.
President and CEO
hji@sorrentotherapeutics.com
(858) 668-6923
George Uy
Executive Vice President and CCO
guy@sorrentotherapeutics.com
(661) 607-4057
CONTACT: |
