Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ocata Therapeutics, Inc. | a15-3943_18k.htm |
Exhibit 99.1
|
|
February 9, 2015 BIO CEO Presentation |
|
|
Cautionary Statement Concerning Forward-Looking Statements Ocata Therapeutics Inc. (“Ocata” or “the Company”) has filed a registration statement (including a prospectus and a preliminary prospectus supplement) with the Securities and Exchange Commission (“SEC”) for the offering to which this presentation relates. Before you invest you should read the prospectus and the preliminary prospectus supplement in that registration statement and other documents the Company has filed with the SEC for more complete information about the Company and the offering. You may get these documents for free on the SEC’s website at http://www.sec.gov These slides and the accompanying oral presentation contain statements that are not historical facts and are considered forward-looking information. In some cases you can identify these statements by forward-looking words such as “anticipate,” “believe,” “could,” “continue,” “estimate,” “expect,” “intend,” “may,” “should,” “will”, “would,” ”plan,” ”projected,” or the negative of such words or other similar words or phrases. Investors are cautioned not to unduly rely on forward-looking statements because they involve risks and uncertainties and statements related to future events or our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. These statements are also subject to a number of material risks and uncertainties that are described more fully in the prospectus and the preliminary prospectus supplement filed with the SEC, including without limitation our most recently filed Report on Form 10-Q, as filed with the SEC. These forward-looking statements speak only as of the date on which the statements were made and are not guarantees of future performance. Except as may be required by applicable law, we do note undertake or indent to update any forward-looking statements contained herein or in our public filings wit the SEC. 2 |
|
|
Addressing Macular Degeneration with Groundbreaking RPE Therapy The Regenerative Ophthalmology™ Company 3 Safety observed, in addition to anatomical and functional evidence of repair and restoration in Phase 1 trials for dry AMD and SMD Data published in The Lancet, October 14, 2014 Initiating Phase 2 studies soon: Stargardt’s Macular Degeneration (SMD) - Q1 2015 Dry Age-related Macular Degeneration (AMD) - Q2 2015 Extensive proprietary position in major markets protecting the entire value chain of the cell therapy – from the origin of the stem cell to the delivery into patients’ eyes |
|
|
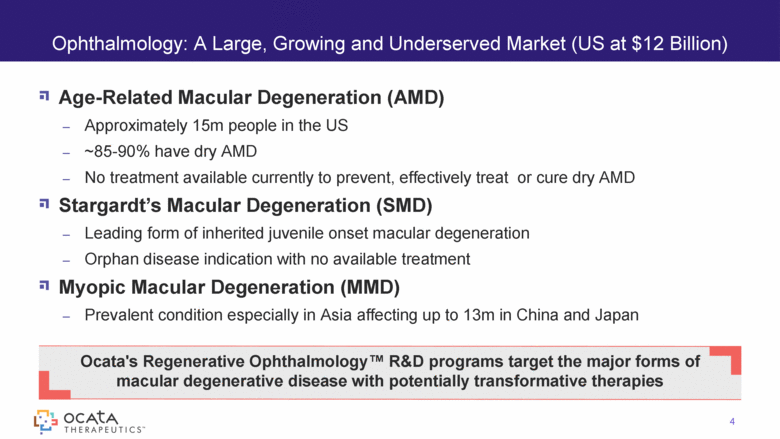
Age-Related Macular Degeneration (AMD) Approximately 15m people in the US ~85-90% have dry AMD No treatment available currently to prevent, effectively treat or cure dry AMD Stargardt’s Macular Degeneration (SMD) Leading form of inherited juvenile onset macular degeneration Orphan disease indication with no available treatment Myopic Macular Degeneration (MMD) Prevalent condition especially in Asia affecting up to 13m in China and Japan Ophthalmology: A Large, Growing and Underserved Market (US at $12 Billion) 4 Ocata's Regenerative Ophthalmology™ R&D programs target the major forms of macular degenerative disease with potentially transformative therapies |
|
|
The First Evidence of Long-term Safety and Efficacy Signal Following Transplantation of RPE Cells 5 “What we did is transplant the cells into patients who have a disease where those particular cells are dying; and we replaced those dying tissues with new tissue that's derived from these stem cells. In a way it's a retinal transplant. - Steven Schwartz, eye specialist, UCLA |
|
|
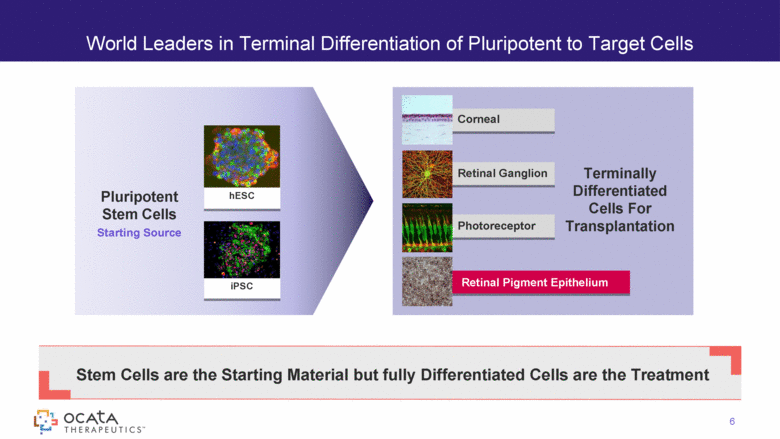
World Leaders in Terminal Differentiation of Pluripotent to Target Cells 6 Pluripotent Stem Cells Starting Source Stem Cells are the Starting Material but fully Differentiated Cells are the Treatment hESC iPSC Corneal Retinal Ganglion Photoreceptor Retinal Pigment Epithelium Terminally Differentiated Cells For Transplantation |
|
|
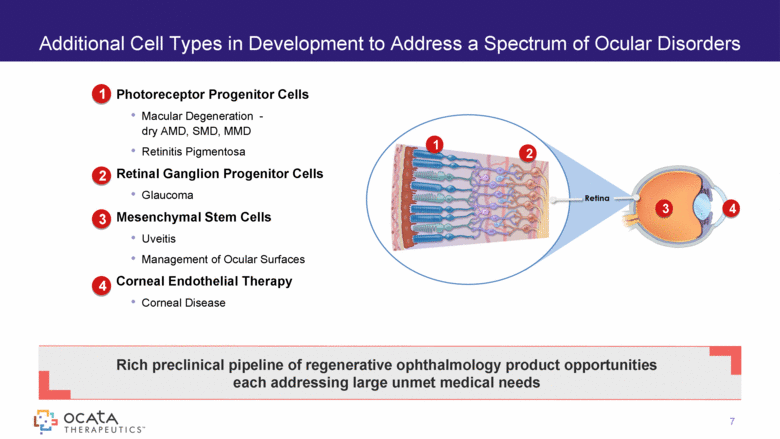
1 2 3 4 7 Additional Cell Types in Development to Address a Spectrum of Ocular Disorders Photoreceptor Progenitor Cells Macular Degeneration - dry AMD, SMD, MMD Retinitis Pigmentosa Retinal Ganglion Progenitor Cells Glaucoma Mesenchymal Stem Cells Uveitis Management of Ocular Surfaces Corneal Endothelial Therapy Corneal Disease 1 2 3 4 Rich preclinical pipeline of regenerative ophthalmology product opportunities each addressing large unmet medical needs |
|
|
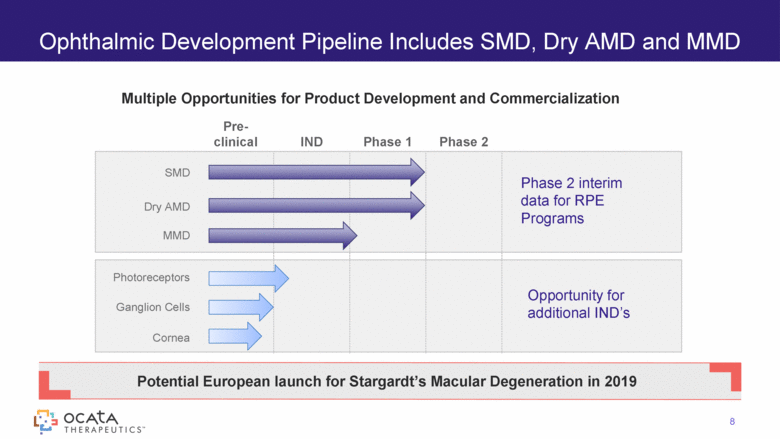
Ophthalmic Development Pipeline Includes SMD, Dry AMD and MMD Pre-clinical IND Phase 1 Phase 2 SMD Dry AMD MMD Photoreceptors Ganglion Cells Cornea Phase 2 interim data for RPE Programs Opportunity for additional IND’s Potential European launch for Stargardt’s Macular Degeneration in 2019 Multiple Opportunities for Product Development and Commercialization 8 |
|
|
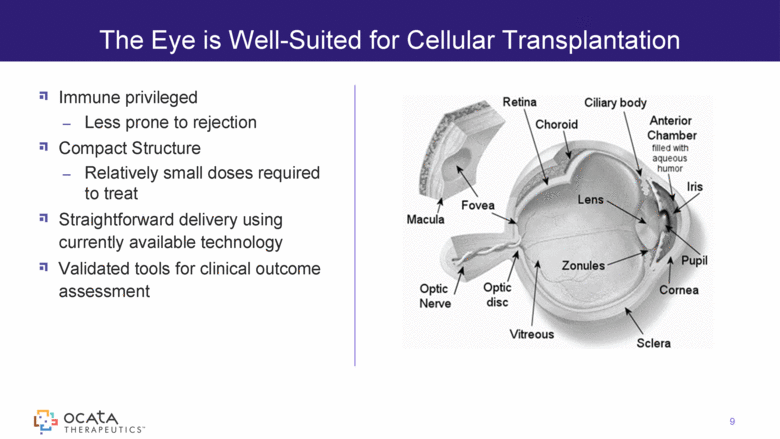
Immune privileged Less prone to rejection Compact Structure Relatively small doses required to treat Straightforward delivery using currently available technology Validated tools for clinical outcome assessment The Eye is Well-Suited for Cellular Transplantation 9 |
|
|
RPE Market Dynamics for Stargardt’s Macular Degeneration Orphan Disease with an Estimated Prevalence of 1:10,000 (~31,600 in the US) Three main subtypes of patients that physicians observe Fast deterioration Slow deterioration Rod and cone dystrophy Stargardt’s patients are primarily diagnosed and managed by retinal specialists Pipeline limited to a few product candidates in early stages of development: StarGen (Oxford Biomedica) ALK-001 (Alkeus) Emixustat (Acucela) There are no approved therapies that slow or stop the progression of Stargardt’s disease; almost all patients develop 20/200 vision or worse and are managed currently with supportive therapies 5%-10% of total Stargardt's population: could potentially benefit from aggressive treatment 60-70% of total Stargardt’s population: symptoms present early on and progress from 20/40 to 20/200 (5-10 years) 10 |
|
|
Market Dynamics Age-related Macular Degeneration ~1.8m Dry AMD Patients Diagnosed Every Year in the USA In the late form of dry AMD, patients can progress to geographic atrophy (GA) – GA progresses slowly and leads to the death of the photoreceptors. Approximately 110,000-165,000 Cases of Advanced Dry AMD are diagnosed every year with central GA – our primary target indication. Severe Dry AMD is a precursor to Wet AMD – Wet AMD is a blockbuster drug category e.g. Lucentis (Roche/Novartis) and Eylea (Regeneron) Dry AMD patients are primarily managed by general ophthalmologists who are referred to retinal specialists when there are risks for wet AMD or severe symptoms Pipeline indicative of unmet need and commercial opportunity includes: NT-501 (Neurotech), emixustat (Acucela, Lampalizumab from (Roche) and MC-1101 from MacuCLEAR There are no approved therapies that slow or stop the progression of AMD; almost all patients develop 20/100 or 20/200 vision or worse and can only be managed with supportive therapies 11 |
|
|
Well-defined prescriber base Patients are referred to retinal specialists (~2,500 in the US of which ~1,500- 2,000 are vitreoretinal [VR] surgeons) who diagnose and manage subsequent patient care Targeted marketing achievable with a small and specialized technical salesforce Ease of administration: cellular transplantation performed with current technology P1 utilizes pars plana vitrectomy and subretinal injection modalities scalable to VR surgeons Small dosage requirement Commercial scalability of manufacturing and distribution in process Significant unmet medical need – no approved treatments for dry AMD or SMD Opportunity to treat earlier stage disease SMD, Dry AMD and MMD are Specialized Opportunities and Feasible for an Emerging Biotech 12 |
|
|
Clinical Programs RPE for SMD, Dry AMD and MMD 13 |
|
|
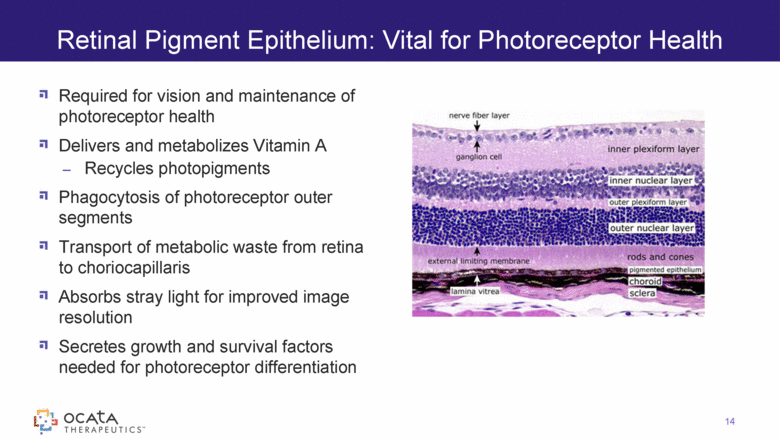
Required for vision and maintenance of photoreceptor health Delivers and metabolizes Vitamin A Recycles photopigments Phagocytosis of photoreceptor outer segments Transport of metabolic waste from retina to choriocapillaris Absorbs stray light for improved image resolution Secretes growth and survival factors needed for photoreceptor differentiation Retinal Pigment Epithelium: Vital for Photoreceptor Health 14 |
|
|
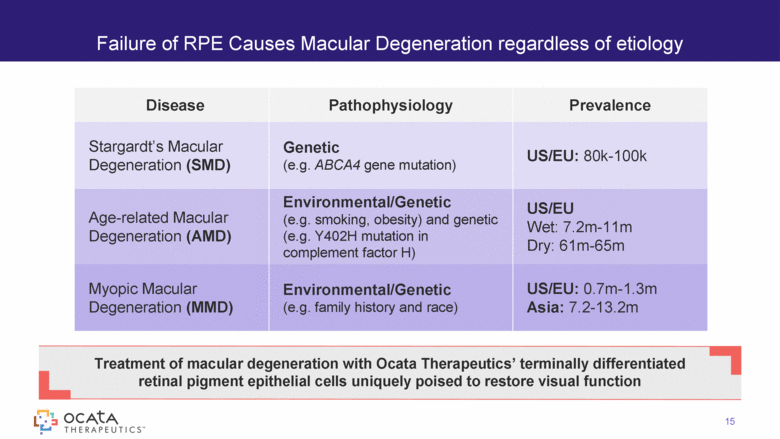
Failure of RPE Causes Macular Degeneration regardless of etiology Treatment of macular degeneration with Ocata Therapeutics’ terminally differentiated retinal pigment epithelial cells uniquely poised to restore visual function Disease Pathophysiology Prevalence Stargardt’s Macular Degeneration (SMD) Genetic (e.g. ABCA4 gene mutation) US/EU: 80k-100k Age-related Macular Degeneration (AMD) Environmental/Genetic (e.g. smoking, obesity) and genetic (e.g. Y402H mutation in complement factor H) US/EU Wet: 7.2m-11m Dry: 61m-65m Myopic Macular Degeneration (MMD) Environmental/Genetic (e.g. family history and race) US/EU: 0.7m-1.3m Asia: 7.2-13.2m 15 |
|
|
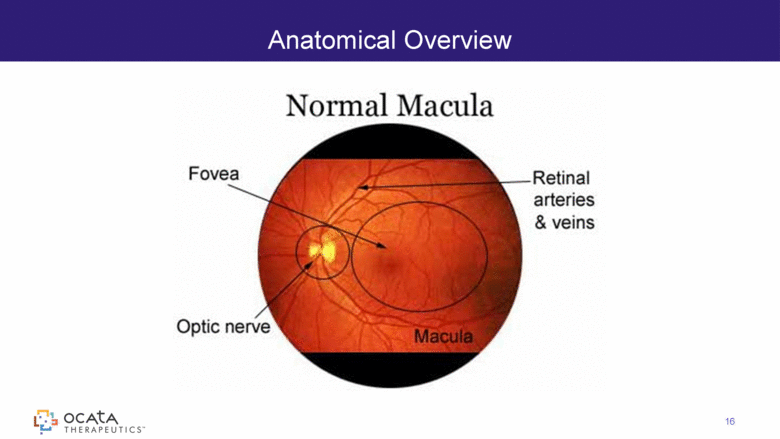
Anatomical Overview 16 |
|
|
RPE Damage and Subsequent Photoreceptor Degeneration Leads to Loss of Central Visual Acuity 17 |
|
|
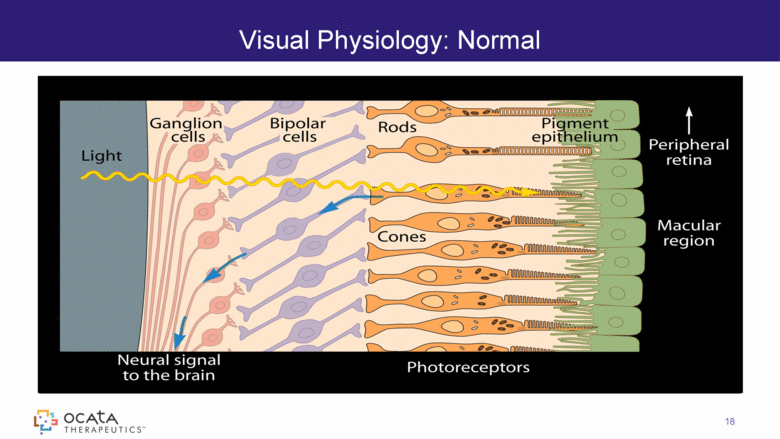
Visual Physiology: Normal 18 |
|
|
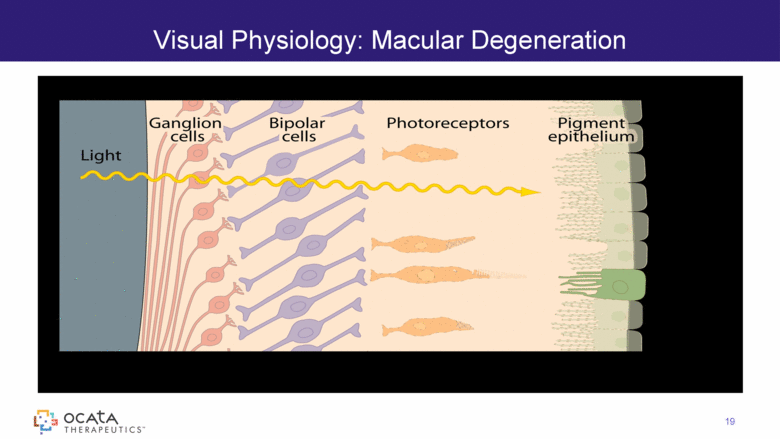
Visual Physiology: Macular Degeneration 19 |
|
|
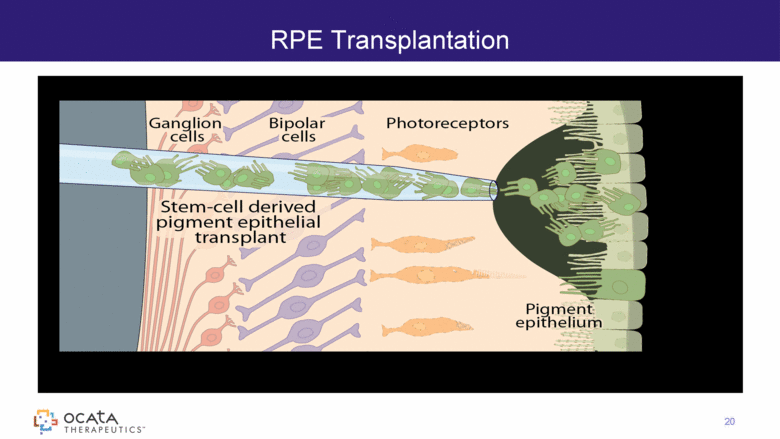
RPE Transplantation 20 |
|
|
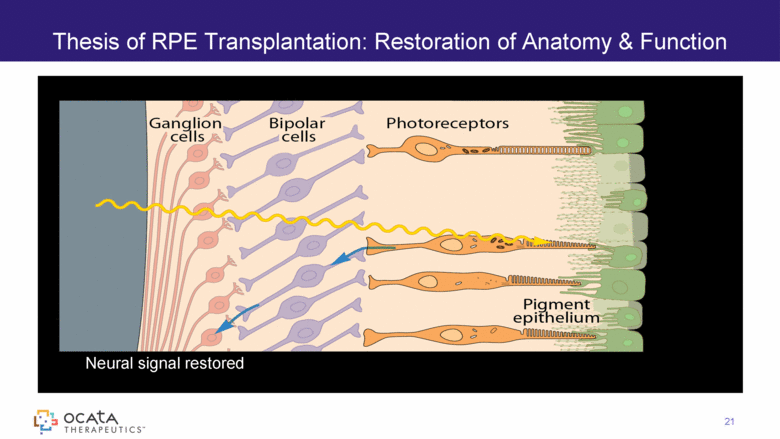
Thesis of RPE Transplantation: Restoration of Anatomy & Function 21 Neural signal restored |
|
|
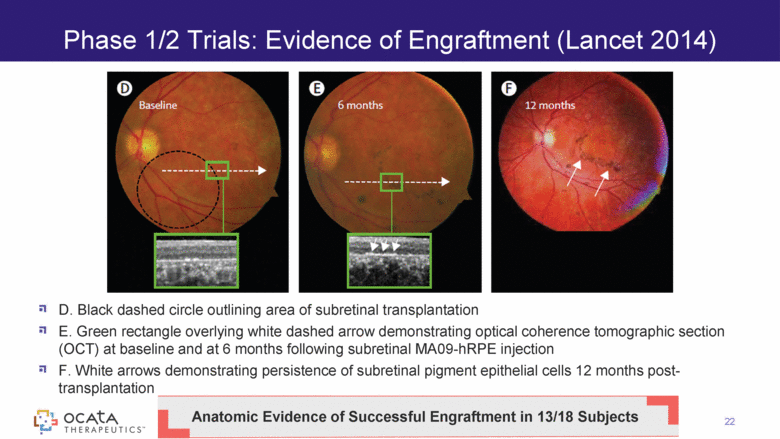
D. Black dashed circle outlining area of subretinal transplantation E. Green rectangle overlying white dashed arrow demonstrating optical coherence tomographic section (OCT) at baseline and at 6 months following subretinal MA09-hRPE injection F. White arrows demonstrating persistence of subretinal pigment epithelial cells 12 months post-transplantation Phase 1/2 Trials: Evidence of Engraftment (Lancet 2014) 22 Baseline* Month 6* Anatomic Evidence of Successful Engraftment in 13/18 Subjects |
|
|
Age-related Macular Degeneration 23 |
|
|
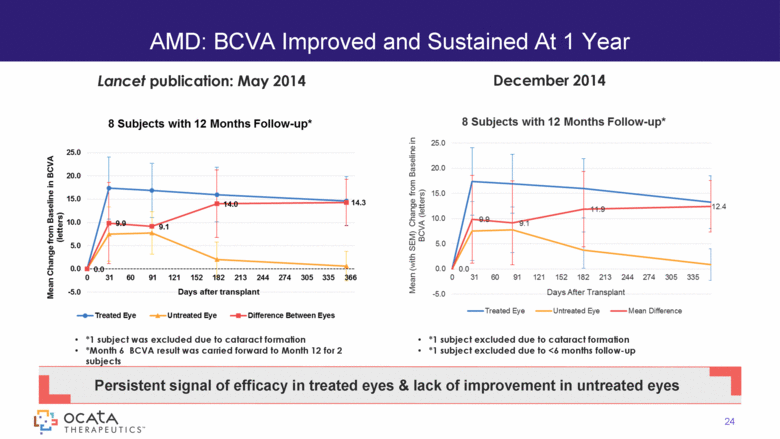
AMD: BCVA Improved and Sustained At 1 Year 24 Persistent signal of efficacy in treated eyes & lack of improvement in untreated eyes *1 subject was excluded due to cataract formation *Month 6 BCVA result was carried forward to Month 12 for 2 subjects December 2014 Lancet publication: May 2014 *1 subject excluded due to cataract formation *1 subject excluded due to <6 months follow-up 8 Subjects with 12 Months Follow-up* 8 Subjects with 12 Months Follow-up* |
|
|
Stargardt’s Macular Degeneration 25 |
|
|
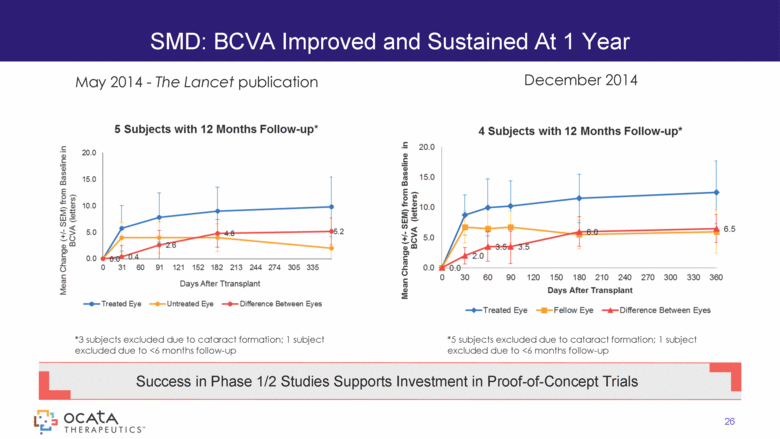
SMD: BCVA Improved and Sustained At 1 Year 26 Success in Phase 1/2 Studies Supports Investment in Proof-of-Concept Trials *3 subjects excluded due to cataract formation; 1 subject excluded due to <6 months follow-up December 2014 May 2014 - The Lancet publication *5 subjects excluded due to cataract formation; 1 subject excluded due to <6 months follow-up 5 Subjects with 12 Months Follow-up* 4 Subjects with 12 Months Follow-up* 26 |
|
|
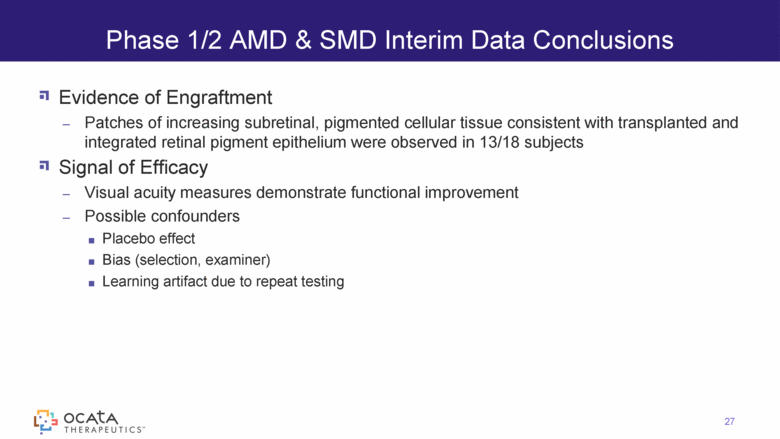
Evidence of Engraftment Patches of increasing subretinal, pigmented cellular tissue consistent with transplanted and integrated retinal pigment epithelium were observed in 13/18 subjects Signal of Efficacy Visual acuity measures demonstrate functional improvement Possible confounders Placebo effect Bias (selection, examiner) Learning artifact due to repeat testing Phase 1/2 AMD & SMD Interim Data Conclusions 27 |
|
|
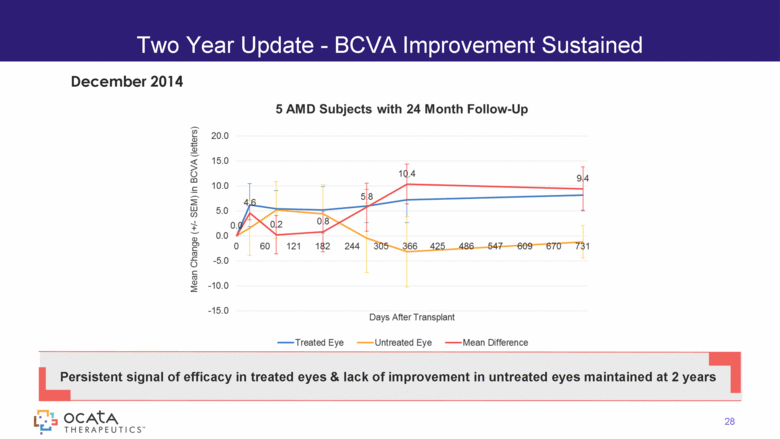
Two Year Update - BCVA Improvement Sustained 28 Persistent signal of efficacy in treated eyes & lack of improvement in untreated eyes maintained at 2 years December 2014 5 AMD Subjects with 24 Month Follow-Up |
|
|
Operations, Finance and Corporate 29 |
|
|
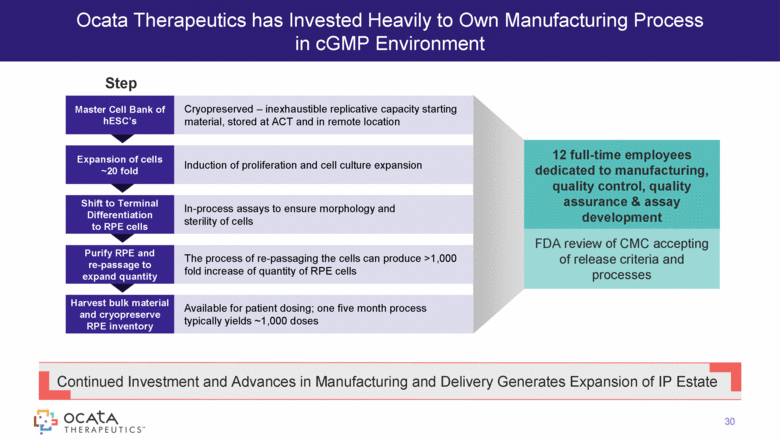
Ocata Therapeutics has Invested Heavily to Own Manufacturing Process in cGMP Environment Continued Investment and Advances in Manufacturing and Delivery Generates Expansion of IP Estate Cryopreserved – inexhaustible replicative capacity starting material, stored at ACT and in remote location Master Cell Bank of hESC’s Induction of proliferation and cell culture expansion Expansion of cells ~20 fold In-process assays to ensure morphology and sterility of cells Shift to Terminal Differentiation to RPE cells The process of re-passaging the cells can produce >1,000 fold increase of quantity of RPE cells Purify RPE and re-passage to expand quantity Available for patient dosing; one five month process typically yields ~1,000 doses Harvest bulk material and cryopreserve RPE inventory 12 full-time employees dedicated to manufacturing, quality control, quality assurance & assay development FDA review of CMC accepting of release criteria and processes Step 30 |
|
|
IP Coverage From Stem Cell Line to Patient Treatment Single Blastomere Derivation of hESCs Methods of Manufacturing hESC-derived RPE cells Product Release Assays Pharmaceutical Preparations Methods-of-Treatment 3 Patent Families 13 Issued Patents 26 Pending Applications Core Patent expiry – 2031 (with Patent Term Extension) 5 Patent Families 2 Issued Patents 34 Pending Applications Core Patents - 2031 Formulation Improvements - 2032 Shipping Medium –2035 3 Patent Families 4 Issued Patents 26 Pending Applications Core Patents - 2031 Improvements - 2032 2 Patent Family 11 Pending Applications Expiry will begin 2031 & 2032 1 Patent Family 4 Issued Patents 12 Pending Applications Expiry begins 2025 31 |
|
|
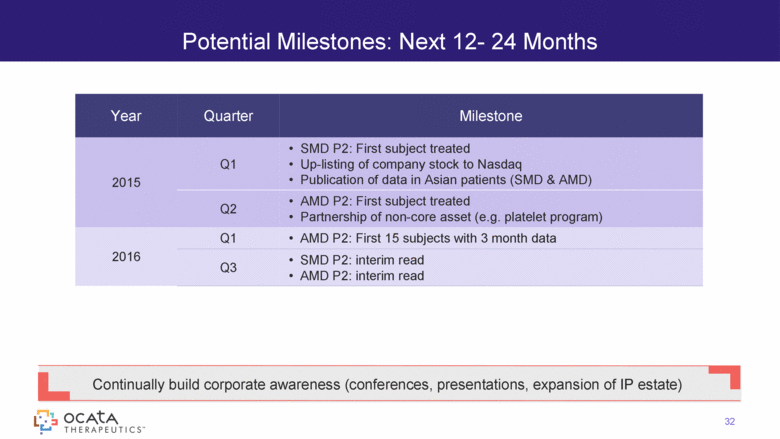
Potential Milestones: Next 12- 24 Months Year Quarter Milestone 2015 Q1 SMD P2: First subject treated Up-listing of company stock to Nasdaq Publication of data in Asian patients (SMD & AMD) Q2 AMD P2: First subject treated Partnership of non-core asset (e.g. platelet program) 2016 Q1 AMD P2: First 15 subjects with 3 month data Q3 SMD P2: interim read AMD P2: interim read 32 Continually build corporate awareness (conferences, presentations, expansion of IP estate) |
|
|
Initiating Phase 2 with novel, potentially curative therapy in areas where no approved products exist today (Dry AMD/SMD) Dry AMD is a potential blockbuster indication – a precursor to Wet AMD where Treatments include Eylea (Regeneron) and Lucentis (Novartis/Roche) Safety observed, in addition to anatomical and functional evidence of repair and restoration; data published in The Lancet, October 14, 2014 Established IP position in major markets protecting the life span of the cell therapy – from the origin of the cell to the delivery into patients’ eyes The World Leader in Regenerative Ophthalmology 33 |