Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Matinas BioPharma Holdings, Inc. | v400122_8-k.htm |
Exhibit 99.1

OTCQB: MTNB BUSINESS UPDATE CONFERENCE CALL February 2, 2015 www.MatinasBioPharma.com A clinical - stage biopharmaceutical company focused on the development of lipid - based prescription therapeutics for the treatment of cardiovascular and metabolic conditions and infectious diseases 1

Forward Looking Statement This presentation contains "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including those relating to the Company’s product development, clinical and regulatory timelines, market opportunity, cash flow and other statements that are predictive in nature, that depend upon or refer to future events or conditions. All statements other than statements of historical fact are statements that could be forward - looking statements. Forward - looking statements include words such as “expects,” “anticipates,” “intends,” “plans,“ “could,” “believes,” “estimates” and similar expressions. These statements involve known and unknown risks, uncertainties and other factors which may cause actual results to be materially different from any future results expressed or implied by the forward - looking statements. Forward - looking statements are subject to a number of risks and uncertainties, including, but not limited to, our ability to obtain additional capital to meet our liquidity needs on acceptable terms, or a t all, including the additional capital which will be necessary to complete the clinical trials of our product candidates; our ability to successfully complete research and further development and commercialization of our product candidates; the uncertainties inherent in clinical testing; the timing, cost and uncertainty of obtaining regulatory approvals; our ability t o protect the Company's intellectual property; the loss of any executive officers or key personnel or consultants; competition; changes in the regulatory landscape or the imposition of regulations that affect the Company's products; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10 - K, 10 - Q and 8 - K. Investors are cautioned not to place undue reliance on such forward - looking statements, which speak only as of the date of this release. Except as may be required by law, the Company does not undertake any obligation to release publicly any revisions to such forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. Matinas BioPharma’s product candidates are all in a development stage and are not available for sale or use. 2

Agenda • MATINAS BIOPHARMA AND AQUARIUS SYNERGIES AND STRATEGY Roelof Rongen , Chief Executive Officer and Co - Founder • TECHNOLOGY, SCIENTIFIC PARTNERS, MATINAS BIOPHARMA Dr . Raphael J. Mannino , F ounder of Aquarius and an Inventor of the C ochleate B io - delivery Platform Technology • TRANSACTION SUMMARY AND FINANCIAL OUTLOOK Jerry Jabbour , Chief Business Officer and Co - Founder • Q&A • CLOSING REMARKS Roelof Rongen 3

4 Roelof Rongen Matinas BioPharma and Aquarius Biotechnologies S ynergies and Strategy

Aquarius Biotechnologies Highlights • Novel lipid - crystal nano - particle cochleate formulation delivery platform with opportunity for broad use in anti - infectives • Proprietary technology platform with broad application expected to drive a robust pipeline in high - value markets and niche, potentially orphan indications • Lead program for oral administration of Amphotericin B antifungal (MAT2203) expected to enter Phase 2a in 2015 in collaboration with NIH • Amikacin - based antibiotic potentially fulfilling significant need to treat life - threatening Gram - negative bacterial infections 5

Synergistic Lipid - Based Therapy Approach Lipid - Based Therapies Lipids as Pharmaceutically Active Compounds ▪ MAT9001 • Severe hypertriglyceridemia • Other dyslipidemia ▪ MAT8800 • Fatty Liver Disease Lipids as “ Nano - Particle” Delivery Vehicles ▪ MAT2203 – C - Amphotericin B • Broad Spectrum Fungicidal ▪ MAT2501 – C - Amikacin • Aminoglycoside Antibiotic (gram - ) Clinical Stage Program Clinical Stage Program 6

P ipeline Expansion with Additional Clinical - Stage Opportunities with Potential to Drive Sustainable Value Discovery IND Preparation Early Clinical Development Phase 3 Development MAT9001 MAT2203 MAT2501 MAT8800 Severe Hypertriglyceridemia Fungal Infections Gram - Negative Bacterial Infections Fatty Liver Disease Discovery Program 7

8 MAT9001 A Next Generation Prescription - only Omega - 3 Fatty Acid Medication

MAT9001 – Program Achievements x Promising results with DPA in pre - clinical studies x Proprietary process for high purity DPA manufacturing, at GMP 10+kg scale x Development of proprietary soft - gel formulation x Formation of prominent Scientific Advisory Board x Filed MAT9001 IND with FDA Q4 2014 x Commenced first human trial for MAT9001 in Canada Q4 2014 x Established robust MAT9001 IP estate: • Filed 22 patents across 3 families • One U.S. Patent issued Dec. 2014 9

Lead Product Candidate MAT9001 Remains a Priority Discovery IND Preparation Early Clinical Development Phase 3 Development MAT9001 Next Steps: • Comparative PK/PD crossover study ongoing in Canada – ~50 pts • Protocol responses to FDA (comparative PK and animal tox ) • Conduct comparative PK and animal tox studies • Submit results from PK and animal tox studies and Phase 3 protocol • Initiate Phase 3, pending FDA process and funding • Exploring other CV and dyslipidemia indications 10

11 MAT2203 Amphotericin B Delivered in a Lipid - Crystal Nano - Particle Cochleate Formulation -- Broad - Spectrum Fungicidal Agent --

Cochleate Technology Offers S ignificant C linical I mprovement P otential Multi - Organ Protection • Cochleates act as a shield for the body from otherwise toxic medicinal compounds Targeted Delivery • Cochleates are carried directly to infection sites Oral Administration • Efficacy demonstrated in in - vivo animal studies • Safety demonstrated in Phase 1 human study 12

Cochleate Targeted Nano - particle Delivery Mitigates the Limitations of Amphotericin B 50 - 500 nm * Phosphatidylserine PS* Bilayer Calcium Drug 1. Reduces toxicity by containing drug inside particle 2. Size and surface features facilitate targeted delivery 3. Potential for oral administration High Calcium Low Calcium 1 2 A platform drug delivery technology**… …that provides targeted delivery ** Cochleate Platform delivery technology under exclusive license from Rutgers University Nanocochleate particles open up under low calcium and deliver anti - infective intracellularly 13

Scientific Merit of Cochleate Technology and Clinical Unmet Need has Led to Several NIH Collaborations • NIH SBIR grants and research contracts towards encochleated Amphotericin B research • NIH SBIR grants and research contracts toward encochleated Aminoglycoside antibiotics research – Amikacin – Capreomycin • Discussion on Clinical Trial Agreement with NIH for Phase 2a clinical study with Amphotericin B in patients is ongoing • Other projects under discussion/review » Continuous stream of legislative initiatives to stimulate anti - infective development » Significant government funding committed towards development of new anti - infectives 14

MAT2203 – Recent Significant Advancements x Completed c ryptococcal meningitis mouse studies at NIH with C - Amphotericin B x Increasing C - Amphotericin B scale to ~800 doses/batch x Preparing for C - Amphotericin B Phase 2a efficacy trial at NIH – refractory mucocutaneous candidiasis patients 15

Invasive Fungal Infections x Cryptococcal Meningoencephalitis x Aspergillosis Significant Clinical Need for Fungicidal Agents Hematological Malignancies x Leukemias • ALL • AML Stem Cell Transplants x Autologous x Allogeneic Solid Organ Transplants x Kidney x Liver x Other Approximately 150,000 potential cases annually in the U.S. alone Potential to Address Orphan Indications Patient Populations at High Risk for Fungal Infections 16

MAT2203 – Development Overview Discovery IND Preparation Early Clinical Development Phase 3 Development MAT2203 Next Steps: x Single - Dose Phase 1 study completed ▪ Patient treatment protocols under development in collaboration with NIH/NIAID ▪ Phase 2a study expected to commence in 1H 2015 17

18 MAT2501 Amikacin Delivered in a Lipid - Crystal Nano - Particle Cochleate Formulation -- Gram - Negative Aminoglycoside Antibiotic --

MAT2501 – Development Overview MAT2501 – C - Amikacin Treating severe and hospital - acquired gram - negative bacterial infections Potential High - need Indications: • Cystic Fibrosis pulmonary infections • Ventilated patients in ICU or long - term care • Hospital acquired urinary track infections Discovery IND Preparation Early Clinical Development Phase 3 Development MAT2501 Next Steps: x Proof - of - principle testing in animal models showing in vivo efficacy of oral C - Amikacin • Formal Pre - Clinical Animal Toxicology Studies Ongoing at NIH • IND filing expected late 2015 Potential to be first oral administered Amikacin without toxicity or side effects seen with IV 19

Cochleate Nanoparticle Delivery has Broad Utility with Potential for Orphan Drug Applications Collaborations In - Vitro Animal POC IND - Prep Human Studies Amphotericin B NIH / PHRI Amikacin NIH Vaccines Ibuprofen Atovaquone NIH Capreomycin NIH Meropenem NIH Curcumin Omega - 3 FA 20
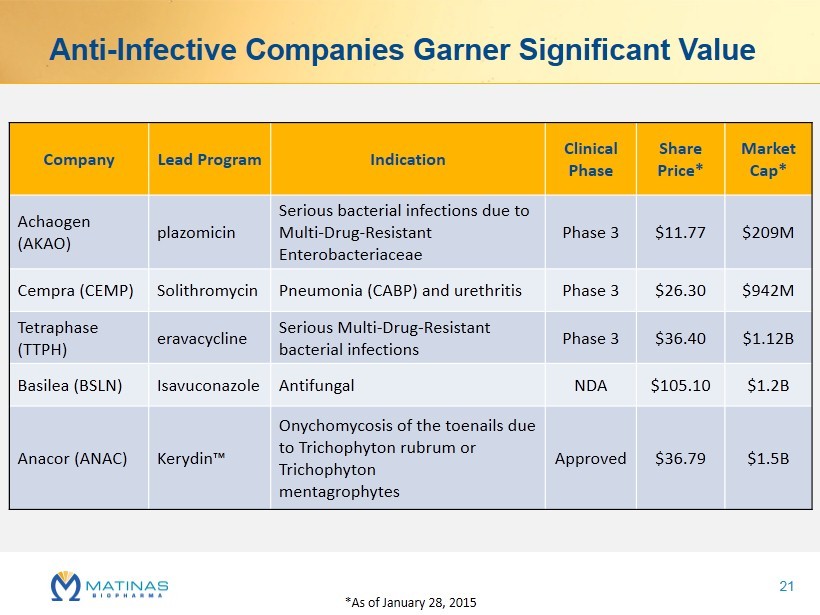
Anti - Infective Companies Garner Significant Value Company Lead Program Indication Clinical Phase Share Price* Market Cap* Achaogen (AKAO) plazomicin Serious bacterial infections due to Multi - Drug - Resistant Enterobacteriaceae Phase 3 $11.77 $209M Cempra (CEMP) Solithromycin Pneumonia (CABP) and urethritis Phase 3 $26.30 $942M Tetraphase (TTPH) eravacycline Serious Multi - Drug - Resistant bacterial infections Phase 3 $36.40 $1.12B Basilea (BSLN) Isavuconazole Antifungal NDA $105.10 $1.2B Anacor (ANAC) Kerydin ™ Onychomycosis of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes Approved $36.79 $1.5B *As of January 28, 2015 21

Recently Materialized Transactions Validate Potential in Anti - I nfectives Space Recent Acquisitions in Antibiotics Date Company Acquirer Lead Program Indication Stage at time of deal Deal Total 12/14 Cubist Merck Multiple Anti - infectives Multiple Launched/ Approved/ Phase 3 $9.5B 11/14 Durata Therapeutics Actavis Dalvance Gram - positive infection Approved in US $675M 10/14 Optimer Pharma - ceuticals Cubist fidaxomicin Clostridium difficile - associated diarrhea Launched in US $811M 8/13 Trius Therapeutics Cubist Sivextro Gram - positive infections , MRSA Phase 3 $707M 22

23 MAT8800 Fatty Liver Disease Discovery Program

MAT8800 – Development Overview MAT8800 – Proprietary Omega - 3 Discovery Program Treating Fatty Liver Disease NAFLD NASH • Common: 12% of U.S. population • A leading cause of cirrhosis • NO APPROVED TREATMENT OPTION Discovery IND Preparation Early Clinical Development Phase 3 Development MAT8800 Next Steps: • Animal studies ongoing • Composition selection or further optimization • Upon selection, pre - IND meeting with FDA and IND prep Unique Approach with omega - 3 composition; differentiated from the bile - acid approach 24

Experienced Management Team and Board Roelof Rongen – President and CEO, Director George Bobotas , PhD – Chief Scientific Officer Jerome Jabbour , JD – Chief Business Officer & General Counsel Abdel Fawzy , PhD – EVP Pharmaceutical & Supply Chain Dev. Gary Gaglione, CPA – VP Finance, Acting CFO Herbert Conrad, Chairman BOD – Roche, Reliant, Pharmasset , Celldex, Dura, Bone Care James Scibetta, Director – CFO Pacira , Bioenvision /Genzyme, Merrimack Stefano Ferrari, Director – ProSPA , Bioseutica , KD - Pharma Adam Stern, Director – CEO SternAegis Ventures Strong development and commercialization track record 25

Dyslipidemia & Cardiovascular Diseases Christie M. Ballantyne, MD, PhD, FACC, FNLA – Baylor College of Medicine , Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart and Vascular Center, Lipid Metabolism and Atherosclerosis Clinic at Houston Methodist Hospital Kevin Maki, PhD, FNLA – DePaul University, Midwest Center for Metabolic & Cardiovascular Research, Great Lakes Clinical Trials, National Lipid Association’s Expert Panel Anti - Infectives J. Carl Craft, MD; Chair – Former Chief Scientific Officer for Medicines for Malaria Venture (MMV), Former Venture Head at Abbott Laboratories Anti - Infective Development Group Raphael Mannino, PhD – Associate Professor of Pathology and Laboratory Medicine at Rutgers University, New Jersey Medical School. Founder, former President, CEO, CSO and EVP of BioDelivery Sciences, Inc . Prominent Scientific Advisory Board 26

27 Raphael Mannino , Ph.D. Technology, Scientific Partners, Matinas BioPharma

28 Jerry Jabbour Transaction Summary and Financial Update

29 Q&A

30 Roelof Rongen Closing Comments

MTNB Represents a Compelling I nvestment O pportunity x Unique and differentiated expertise in lipidomics , lipid chemistry and lipid - based delivery x Phase 2a and Phase 3 clinical development programs expected to commence in 2015 x Novel technology platform with broad application expected to drive a robust pipeline in high - value markets and niche, potentially orphan indications x Strong patent estate across platforms with decades of know - how x Multiple value - driving catalysts expected over next 12 months x Experienced management and board with strong development and commercialization track record 31

OTCQB: MTNB BUSINESS UPDATE CONFERENCE CALL February 2, 2015 www.MatinasBioPharma.com A clinical - stage biopharmaceutical company focused on the development of lipid - based prescription therapeutics for the treatment of cardiovascular and metabolic conditions and infectious diseases 32
