Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REVA Medical, Inc. | a15-2434_18k.htm |
Exhibit 99.1
|
|
January 15, 2015 J.P. Morgan 2015 Healthcare Conference |
|
|
Important Notice Information is a Synopsis Only This presentation only contains a synopsis of information on the Company and, accordingly, no reliance may be placed for any purpose whatsoever on the sufficiency or completeness of such information. Information presented in this presentation is subject to change without notice and REVA does not have any responsibility or obligation to inform you of any matter arising or coming to their notice after the date of this presentation, which may affect any matter in the presentation. Currency References Financial amounts in this presentation are expressed in US Dollars, except where specifically noted. Forward-Looking Statements This presentation contains or may contain forward-looking statements that are based on management’s beliefs, assumptions, and expectations and on information currently available to management. All statements that are not statements of historical fact, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements, such as those statements regarding our ability to obtain regulatory approvals, timely and successfully complete our clinical trials, protect our intellectual property position, commercialize our products if and when approved, develop and commercialize new products, and estimates regarding our capital requirements and financial performance, including profitability. You should not place undue reliance on these forward-looking statements. Although management believes these forward-looking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause actual results to vary materially from those expressed in the forward-looking statements, which risks and uncertainties are described in the “Risk Factors” section of our Annual Report on Form 10-K filed with the US Securities and Exchange Commission (the “SEC”) on March 17, 2014, and as updated in our periodic reports since then.. Any forward-looking statements in this presentation speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. Disclaimer This presentation and any supplemental materials have been prepared by the Company based on available information. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness, or correctness of such information and opinions and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of the Company, or any of its members, directors, officers, employees, or agents or advisers, nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of the Company or any of its directors, officers, employees, or agents. |
|
|
Corporate Summary Pre-revenue, clinical-stage company Focused on development of bioresorbable scaffolds for the treatment of coronary artery disease Single facility located in San Diego, CA Development labs Manufacturing facilities Corporate offices ASX listed (RVA.AX); SEC registered ISO 13485:2012 certified 47 employees Experienced and invested executive management and board of directors Strong patent portfolio |
|
|
Key 2014 Accomplishments Developed Fantom™, a novel bioresorbable scaffold to offer the desired advancements for a second generation product Completed Q4’14 $25 million financing to support REVA’s timeframe to CE Mark approval of Fantom Goldman Sachs and Senrigan Other existing key investors include Domain Partners, Elliott Associates, Saints Capital, Brookside Capital, Cerberus and Medtronic Initiated first human implants of Fantom Q4’14 Data to be presented at industry conferences beginning May 2015 (EuroPCR) |
|
|
CORE PRODUCT VALUES REVA Core Product Values REstore VAsomotion Innovation Patient Safety Procedural Ease |
|
|
Product Development Approach Minimize risk through the use of proven technologies Known device: standard deformable design Proven drug: Sirolimus Standard rapid exchange delivery system Create valuable product advancements through innovative technology Well-studied REVA polycarbonate polymer Enables the only fully visible bioresorbable scaffold Allows for ease-of-use features including single-step inflation and scaffold expansion Polymer family has 10-year proven safety history |
|
|
Bioresorbable Scaffold Market |
|
|
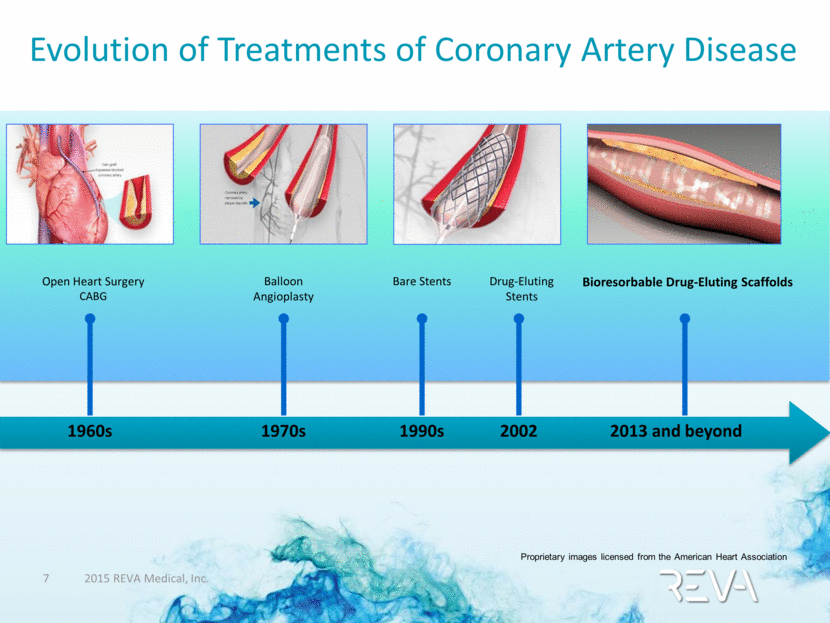
Evolution of Treatments of Coronary Artery Disease Proprietary images licensed from the American Heart Association Open Heart Surgery CABG 1960s Bioresorbable Drug-Eluting Scaffolds 2013 and beyond Balloon Angioplasty 1970s Bare Stents 1990s 2002 Drug-Eluting Stents |
|
|
The Appeal of a Bioresorbable Scaffold (“BRS”) Clinically preferable Preserves maximum flexibility for future treatment options (bypass grafting, distal stenting) May delay disease progression and reduce the rate of future clinical events May enable future preventative therapy Intuitively preferred by patients Allows artery to return to its natural state to restore freedom of movement (vasomotion) Suited for patients needing multiple treatments, especially young patients Internet-enabled patients may help drive market |
|
|
Bioresorbable Scaffolds Today’s Snapshot Clinical data continues to demonstrate safety and additional potential BRS benefits Comparable MACE (7.3% vs. 9.1%)* and low stent thrombosis (0.6%)* Restoration of natural vasomotion Signal for reduced angina (recurrent chest pain) in BRS patients BRS being used in complex cases (CTOs, bifurcations, left main) Physicians seeking improvements in 2nd generation devices Thinner, more deliverable, easier to use; no compromise on strength * Absorb II Study of 500+ patients, Absorb vs. Xience DES, one-year data published by Abbott Vascular September 14, 2014 |
|
|
“Bioresorbable scaffolds have the potential to transform the way coronary artery disease is treated. With further advancements bioresorbable scaffold usage may be as high as 80% within the next ten years.“ Dean Kereiakes, M.D., FACC, FSCAI Principal Investigator, Absorb III and IV US Clinical Trials Medical Director, The Christ Hospital Heart & Vascular Center and the Lindner Research Center in Cincinnati, OH Professor of clinical medicine at Ohio State University |
|
|
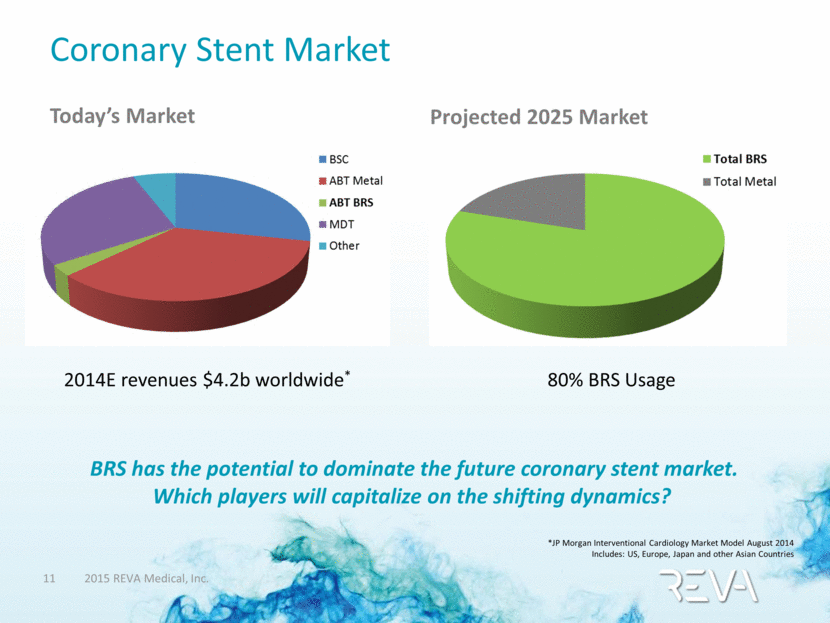
Coronary Stent Market Today’s Market Projected 2025 Market *JP Morgan Interventional Cardiology Market Model August 2014 Includes: US, Europe, Japan and other Asian Countries 2014E revenues $4.2b worldwide* 80% BRS Usage BRS has the potential to dominate the future coronary stent market. Which players will capitalize on the shifting dynamics? |
|
|
Fantom™ Sirolimus-Eluting Bioresorbable Scaffold |
|
|
Disappears to restore natural vasomotion Fantom Delivering the Next Advancement in Bioresorbable Scaffolds FANTOM Visible Deliverable Thin Struts Drug Eluting Easy to Use Strong |
|
|
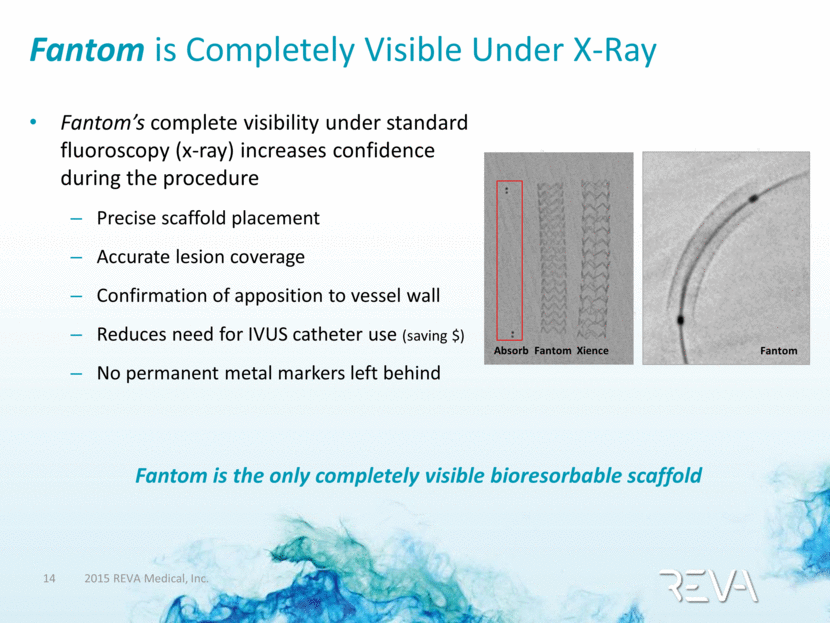
Fantom is Completely Visible Under X-Ray Fantom’s complete visibility under standard fluoroscopy (x-ray) increases confidence during the procedure Precise scaffold placement Accurate lesion coverage Confirmation of apposition to vessel wall Reduces need for IVUS catheter use (saving $) No permanent metal markers left behind Fantom is the only completely visible bioresorbable scaffold Absorb Fantom Xience Fantom |
|
|
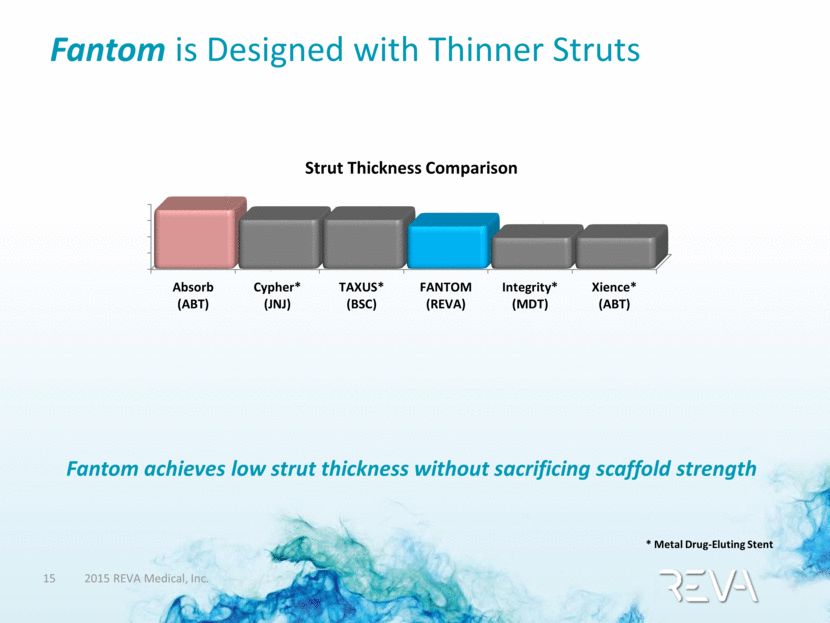
Fantom is Designed with Thinner Struts Fantom achieves low strut thickness without sacrificing scaffold strength Strut Thickness Comparison * Metal Drug-Eluting Stent Absorb (ABT) Cypher* (JNJ) TAXUS* (BSC) FANTOM (REVA) Integrity* (MDT) Xience* (ABT) |
|
|
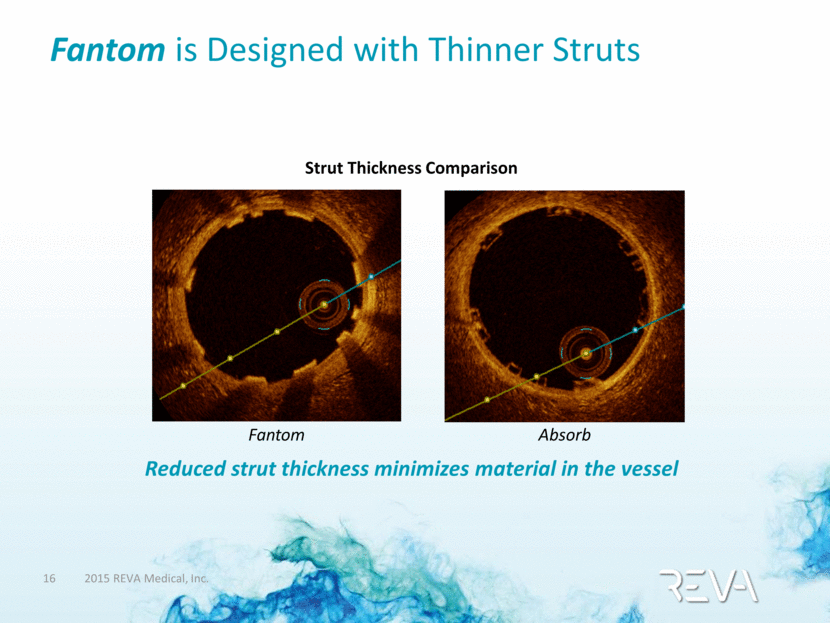
Fantom is Designed with Thinner Struts Reduced strut thickness minimizes material in the vessel Strut Thickness Comparison Fantom Absorb |
|
|
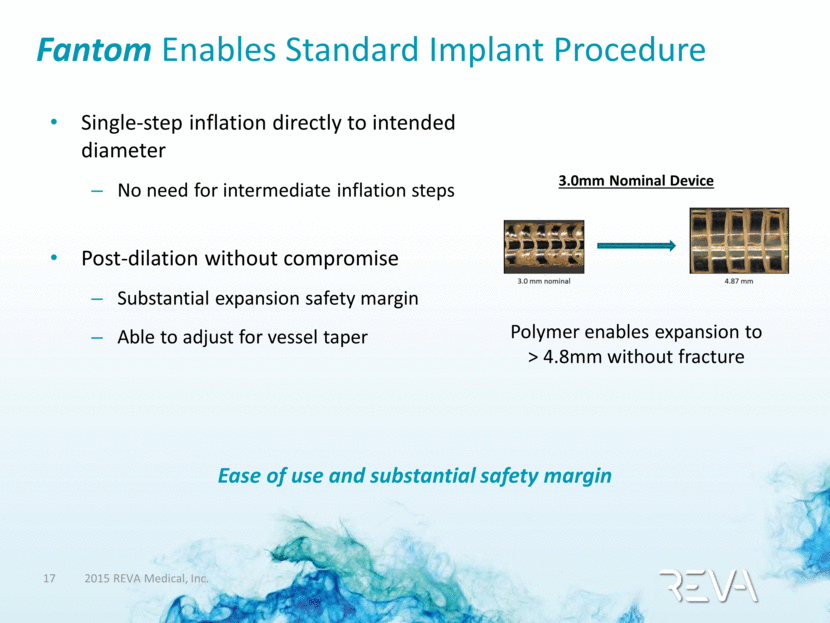
Fantom Enables Standard Implant Procedure Single-step inflation directly to intended diameter No need for intermediate inflation steps Post-dilation without compromise Substantial expansion safety margin Able to adjust for vessel taper 3.0mm Nominal Device Polymer enables expansion to > 4.8mm without fracture Ease of use and substantial safety margin |
|
|
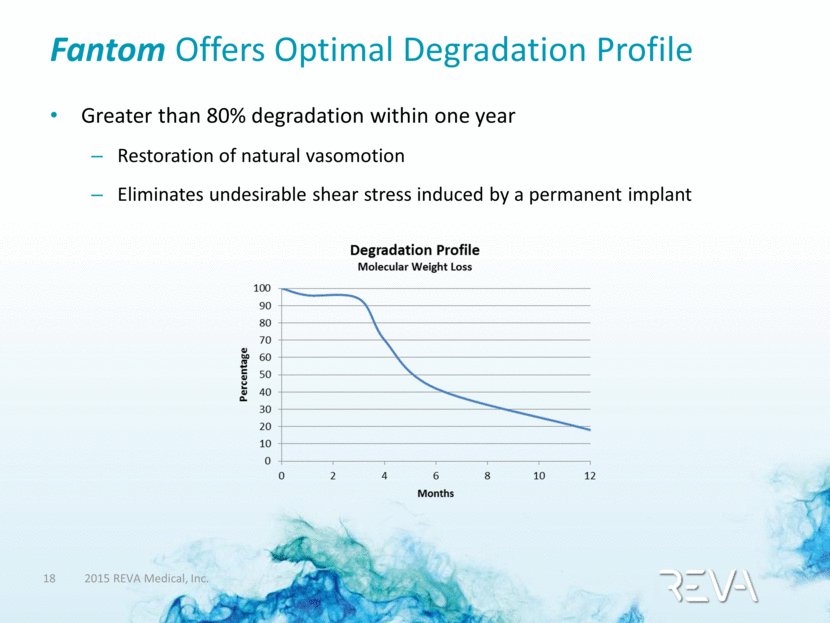
Fantom Offers Optimal Degradation Profile Greater than 80% degradation within one year Restoration of natural vasomotion Eliminates undesirable shear stress induced by a permanent implant |
|
|
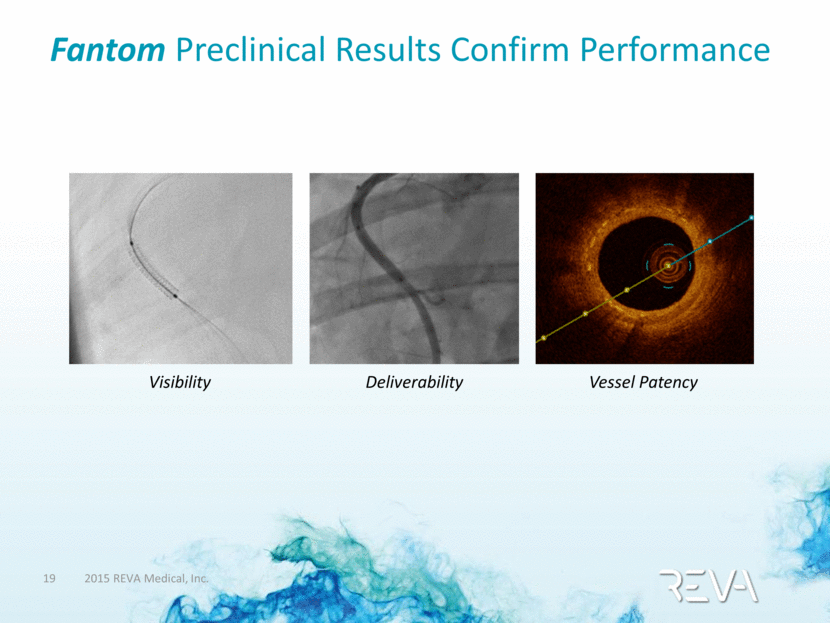
Fantom Preclinical Results Confirm Performance Deliverability Vessel Patency Visibility |
|
|
Commercializing Fantom |
|
|
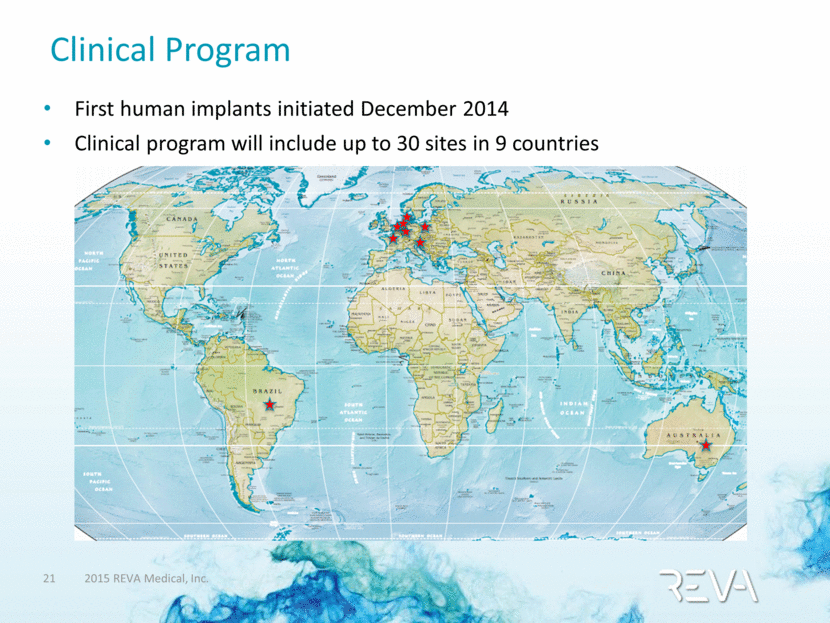
Clinical Program First human implants initiated December 2014 Clinical program will include up to 30 sites in 9 countries |
|
|
Commercialization CE Mark approval anticipated mid-2016 Actively planning early commercialization efforts First launch to target EU Follow-on launch planning underway Direct sales force or distribution partnership with Boston Scientific |
|
|
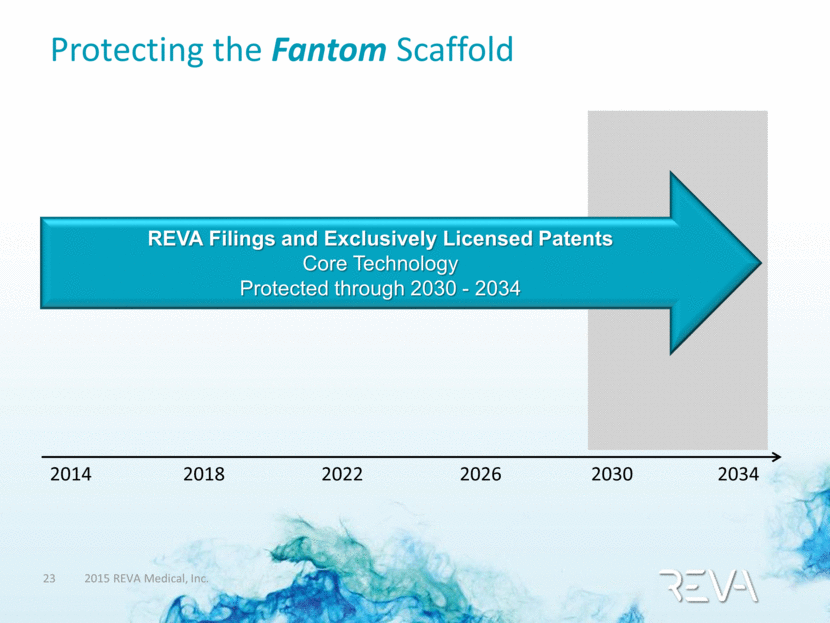
Protecting the Fantom Scaffold REVA Filings and Exclusively Licensed Patents Core Technology Protected through 2030 - 2034 2014 2018 2022 2026 2030 2034 |
|
|
REVA Summary Financial resources secured to meet clinical and commercialization objectives In clinical trials with competitive and differentiated bioresorbable scaffold product Planning of early commercialization efforts underway Market potential for bioresorbable scaffolds is substantial and will be fueled by advancements in technology Stay tuned for Fantom clinical data results at EuroPCR 2015 |