Attached files
| file | filename |
|---|---|
| 8-K - ARIAD PHARMACEUTICALS, INC. 8-K - ARIAD PHARMACEUTICALS INC | a51018777.htm |
| EX-99.1 - EXHIBIT 99.1 - ARIAD PHARMACEUTICALS INC | a51018777ex99_1.htm |
Exhibit 99.2
 Path to Value and
Profitability Elsa So Non-small cell lung cancer ARIAD clinical trial
patient Harvey J. Berger M.D. Chairman and Chief Executive Officer ARIAD
Pharmaceuticals, Inc.
Path to Value and
Profitability Elsa So Non-small cell lung cancer ARIAD clinical trial
patient Harvey J. Berger M.D. Chairman and Chief Executive Officer ARIAD
Pharmaceuticals, Inc.
 01.14.2015 J.P.
Morgan Healthcare Conference Some of the statements in this presentation
constitute “forward looking statements” under the Private Securities
Litigation Reform Act of 1995. Such statements are subject to factors,
risks and uncertainties (such as those detailed in the Company’s
periodic filings with the SEC) that may cause actual results to differ
materially from those expressed or implied by such forward looking
statements.
01.14.2015 J.P.
Morgan Healthcare Conference Some of the statements in this presentation
constitute “forward looking statements” under the Private Securities
Litigation Reform Act of 1995. Such statements are subject to factors,
risks and uncertainties (such as those detailed in the Company’s
periodic filings with the SEC) that may cause actual results to differ
materially from those expressed or implied by such forward looking
statements.
 01.14.2015 J.P.
Morgan Healthcare Conference ARIAD 2014: key areas of progress
Re-launched Iclusig in the U.S. with ~800 patients treated Successfully
completed EMA review - Iclusig indication statement unchanged Improved
understanding of benefit/risk profile of ponatinib Achieved positive P&R
outcomes in key European countries Concluded consultations with FDA and
EMA on Iclusig dose-ranging trial Secured experienced Japanese partner
for Iclusig Advanced brigatinib (AP26113) into pivotal trial and
designated as Breakthrough Therapy Nominated new development candidate
(AP32788)
01.14.2015 J.P.
Morgan Healthcare Conference ARIAD 2014: key areas of progress
Re-launched Iclusig in the U.S. with ~800 patients treated Successfully
completed EMA review - Iclusig indication statement unchanged Improved
understanding of benefit/risk profile of ponatinib Achieved positive P&R
outcomes in key European countries Concluded consultations with FDA and
EMA on Iclusig dose-ranging trial Secured experienced Japanese partner
for Iclusig Advanced brigatinib (AP26113) into pivotal trial and
designated as Breakthrough Therapy Nominated new development candidate
(AP32788)
 01.14.2015 J.P.
Morgan Healthcare Conference 4 ARIAD 2015: our strategic focus Expand
the Iclusig global commercial opportunity Leverage existing commercial
infrastructure and investment Secure a broad partnership for brigatinib
Invest in randomized trials to advance Iclusig and brigatinib into
earlier lines of treatment, expand addressable market Achieve sustained
profitability in 2018 by reaching global product revenue of >$400M
01.14.2015 J.P.
Morgan Healthcare Conference 4 ARIAD 2015: our strategic focus Expand
the Iclusig global commercial opportunity Leverage existing commercial
infrastructure and investment Secure a broad partnership for brigatinib
Invest in randomized trials to advance Iclusig and brigatinib into
earlier lines of treatment, expand addressable market Achieve sustained
profitability in 2018 by reaching global product revenue of >$400M
 01.14.2015 J.P.
Morgan Healthcare Conference 5 Iclusig: seeking to expand the global
opportunity in resistant Ph+ leukemias Early-switch 2nd line 2nd line
3rd line No other options T315I
01.14.2015 J.P.
Morgan Healthcare Conference 5 Iclusig: seeking to expand the global
opportunity in resistant Ph+ leukemias Early-switch 2nd line 2nd line
3rd line No other options T315I
 01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: seeking to expand the global
opportunity in resistant Ph+ leukemias Chronic Phase Accelerated Phase
Blast Phase Ph+ ALL
01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: seeking to expand the global
opportunity in resistant Ph+ leukemias Chronic Phase Accelerated Phase
Blast Phase Ph+ ALL
 01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: leverage existing infrastructure
and investment U.S. and Europe Further expand into chronic-phase disease
and earlier lines of therapy Advance to market leader in 3rd line
chronic-phase CML Continue launching throughout EU28 countries* Japan
Otsuka partnership in Japan and select Asian countries Commercial launch
expected in 2016 Rest of World Additional regional distribution
agreements in 2015 *Includes Central and Eastern Europe distributorship
with CSC Pharmaceuticals, a subsidiary of Angelini Pharma.
01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: leverage existing infrastructure
and investment U.S. and Europe Further expand into chronic-phase disease
and earlier lines of therapy Advance to market leader in 3rd line
chronic-phase CML Continue launching throughout EU28 countries* Japan
Otsuka partnership in Japan and select Asian countries Commercial launch
expected in 2016 Rest of World Additional regional distribution
agreements in 2015 *Includes Central and Eastern Europe distributorship
with CSC Pharmaceuticals, a subsidiary of Angelini Pharma.
 01.14.2015 J.P.
Morgan Healthcare Conference ARIAD and Otsuka: a strategic Iclusig
partnership in Japan and Asia Existing hematology sales force with CML
TKI experience Exclusive commercialization by Otsuka in ten Asian
countries Building major hematology/oncology business in Territory $77.5
million upfront, future milestones and substantial portion of net
product sales to ARIAD Joint development in Territory with Otsuka
funding additional agreed-upon clinical studies ARIAD rights to
co-promotion in Japan and China beginning in third year of
commercialization* Approval and launch of Iclusig in Japan expected in
2016 *ARIAD to bear its own cost for the co-promotion and receive a
higher share of incremental sales above a baseline forecast
01.14.2015 J.P.
Morgan Healthcare Conference ARIAD and Otsuka: a strategic Iclusig
partnership in Japan and Asia Existing hematology sales force with CML
TKI experience Exclusive commercialization by Otsuka in ten Asian
countries Building major hematology/oncology business in Territory $77.5
million upfront, future milestones and substantial portion of net
product sales to ARIAD Joint development in Territory with Otsuka
funding additional agreed-upon clinical studies ARIAD rights to
co-promotion in Japan and China beginning in third year of
commercialization* Approval and launch of Iclusig in Japan expected in
2016 *ARIAD to bear its own cost for the co-promotion and receive a
higher share of incremental sales above a baseline forecast
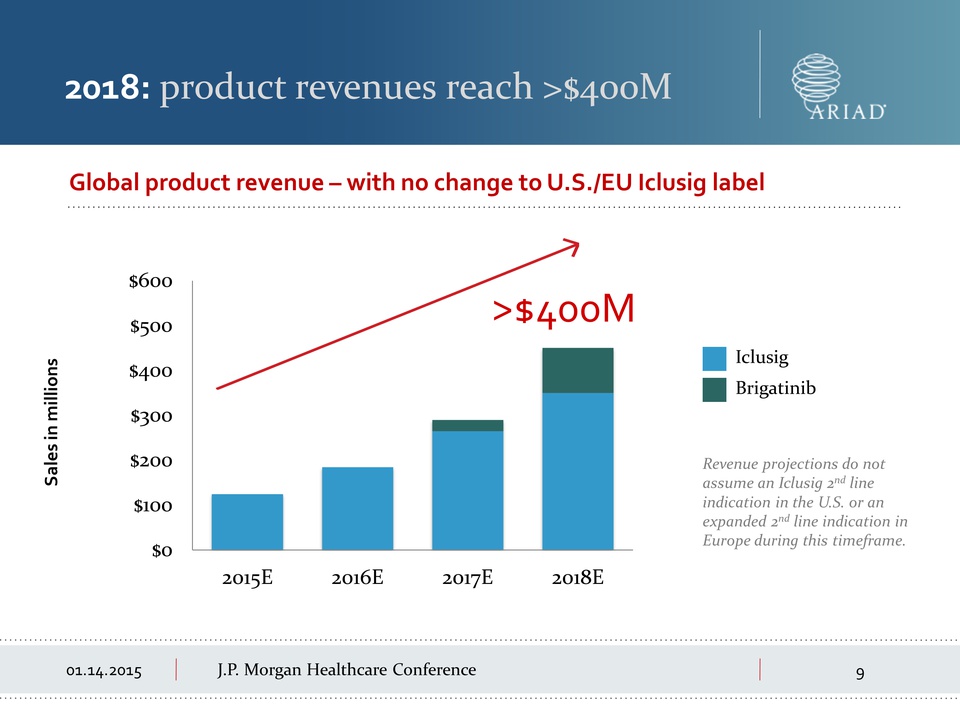 01.14.2015 J.P.
Morgan Healthcare Conference 2018: product revenues reach >$400M
Revenue projections do not assume an Iclusig 2nd line indication in the
U.S. or an expanded 2nd line indication in Europe during this timeframe.
Global product revenue – with no change to U.S./EU Iclusig label $0 $100
$200 $300 $400 $500 $600 2015E 2016E 2017E 2018E >$400M Sales in
millions Brigatinib Iclusig
01.14.2015 J.P.
Morgan Healthcare Conference 2018: product revenues reach >$400M
Revenue projections do not assume an Iclusig 2nd line indication in the
U.S. or an expanded 2nd line indication in Europe during this timeframe.
Global product revenue – with no change to U.S./EU Iclusig label $0 $100
$200 $300 $400 $500 $600 2015E 2016E 2017E 2018E >$400M Sales in
millions Brigatinib Iclusig
 01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: potential 2nd line indication more
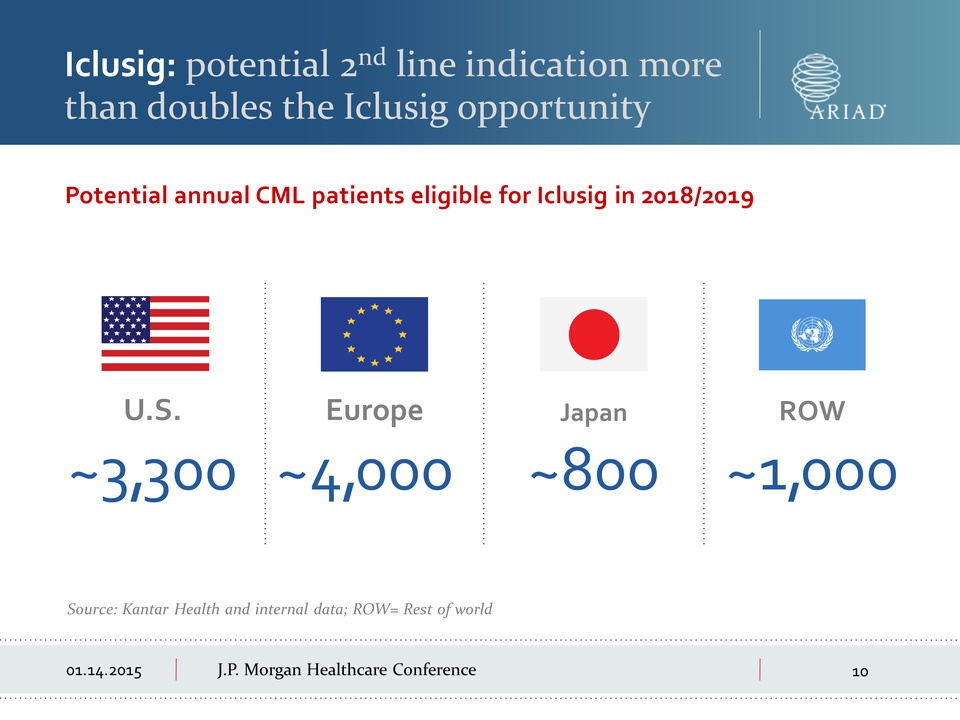
than doubles the Iclusig opportunity Potential annual CML patients
eligible for Iclusig in 2018/2019 ~3,300 U.S. Source: Kantar Health and
internal data; ROW= Rest of world Europe ~4,000 Japan ~800 ROW ~1,000
01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: potential 2nd line indication more
than doubles the Iclusig opportunity Potential annual CML patients
eligible for Iclusig in 2018/2019 ~3,300 U.S. Source: Kantar Health and
internal data; ROW= Rest of world Europe ~4,000 Japan ~800 ROW ~1,000
 01.14.2015 J.P.
Morgan Healthcare Conference Brigatinib – ALK+ NSCLC AP32788 – NSCLC
Lung Cancer: two distinct opportunities - Potential best-in-class orally
active TKI - Highly competitive profile expected to garner significant
market share - Global market approaches $2B by 2020 - Orally active TKI
- Unique profile against a validated class of mutated targets -
Addresses unmet medical need
01.14.2015 J.P.
Morgan Healthcare Conference Brigatinib – ALK+ NSCLC AP32788 – NSCLC
Lung Cancer: two distinct opportunities - Potential best-in-class orally
active TKI - Highly competitive profile expected to garner significant
market share - Global market approaches $2B by 2020 - Orally active TKI
- Unique profile against a validated class of mutated targets -
Addresses unmet medical need
 01.14.2015 J.P.
Morgan Healthcare Conference Brigatinib: maximizing the full value
Secure a broad partnership to co-develop and co-commercialize brigatinib
in 2015 - Leverage existing infrastructure and capabilities - Accelerate
randomized, 1st line trial against crizotinib - Explore new combination
therapies - Mitigate risk ALTA trial forms basis for initial approval;
filing expected mid-2016 Breakthrough therapy designation may accelerate
approval timeline
01.14.2015 J.P.
Morgan Healthcare Conference Brigatinib: maximizing the full value
Secure a broad partnership to co-develop and co-commercialize brigatinib
in 2015 - Leverage existing infrastructure and capabilities - Accelerate
randomized, 1st line trial against crizotinib - Explore new combination
therapies - Mitigate risk ALTA trial forms basis for initial approval;
filing expected mid-2016 Breakthrough therapy designation may accelerate
approval timeline
 01.14.2015 J.P.
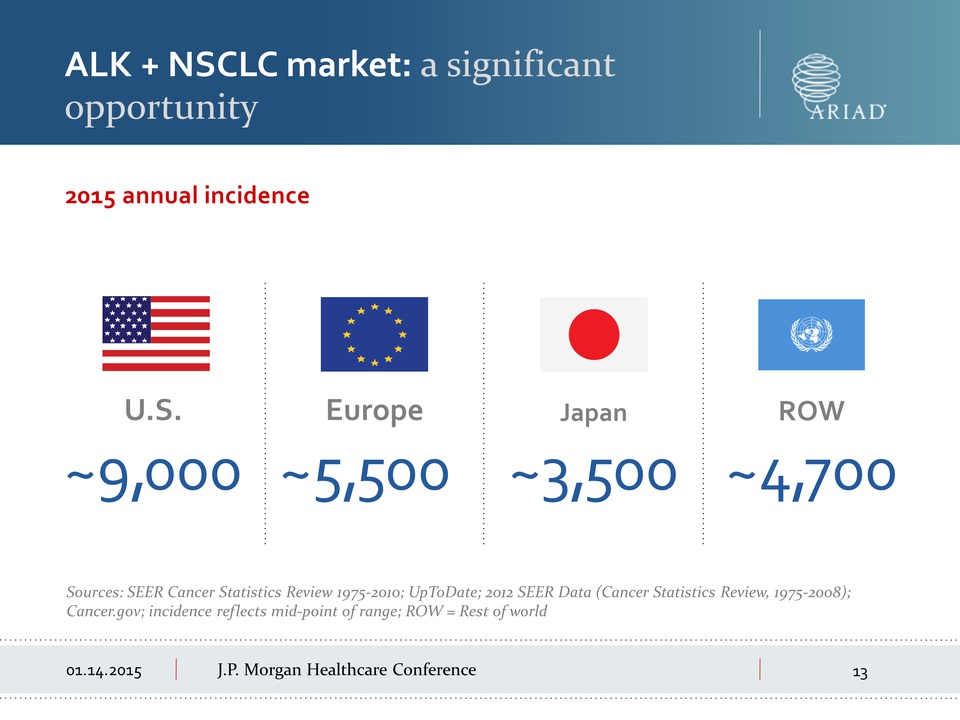
Morgan Healthcare Conference ALK + NSCLC market: a significant
opportunity 2015 annual incidence ~9,000 U.S. Europe ~5,500
Japan ~3,500 ROW ~4,700 Sources: SEER Cancer Statistics Review
1975-2010; UpToDate; 2012 SEER Data (Cancer Statistics Review,
1975-2008); Cancer.gov; incidence reflects mid-point of range; ROW =
Rest of world
01.14.2015 J.P.
Morgan Healthcare Conference ALK + NSCLC market: a significant
opportunity 2015 annual incidence ~9,000 U.S. Europe ~5,500
Japan ~3,500 ROW ~4,700 Sources: SEER Cancer Statistics Review
1975-2010; UpToDate; 2012 SEER Data (Cancer Statistics Review,
1975-2008); Cancer.gov; incidence reflects mid-point of range; ROW =
Rest of world
 01.14.2015 J.P.
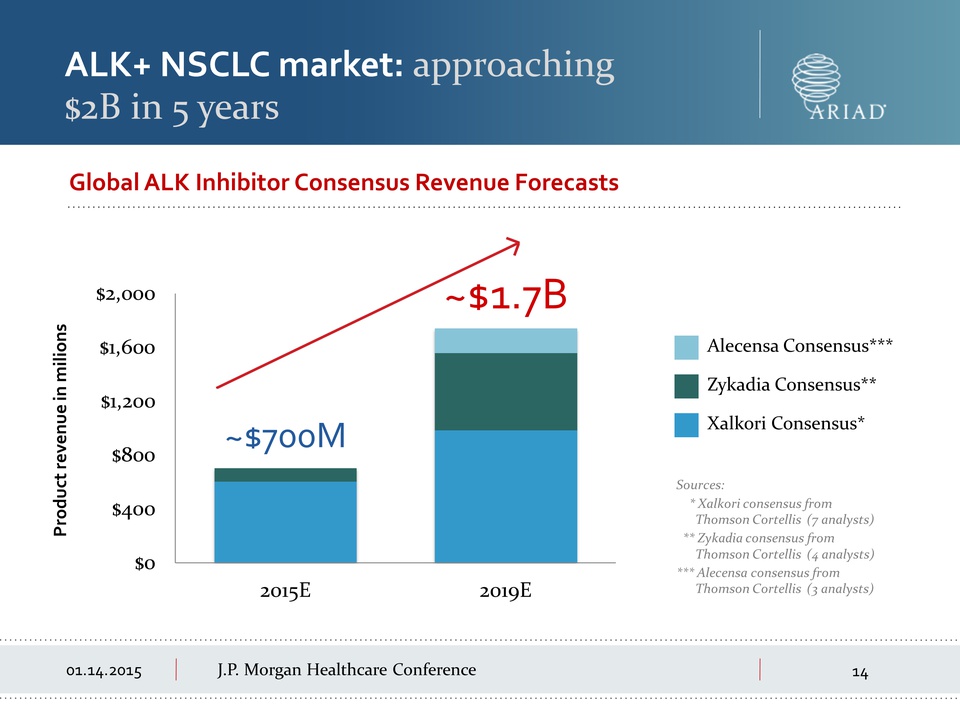
Morgan Healthcare Conference ALK+ NSCLC market: approaching $2B in 5
years Sources: * Xalkori consensus from Thomson Cortellis (7 analysts)
** Zykadia consensus from Thomson Cortellis (4 analysts) *** Alecensa
consensus from Thomson Cortellis (3 analysts) Global ALK Inhibitor
Consensus Revenue Forecasts $0 $400 $800 $1,200 $1,600 $2,000 2015E
2019E ~$700M ~$1.7B Product revenue in millions Alecensa Consensus***
Zykadia Consensus** Xalkori Consensus*
01.14.2015 J.P.
Morgan Healthcare Conference ALK+ NSCLC market: approaching $2B in 5
years Sources: * Xalkori consensus from Thomson Cortellis (7 analysts)
** Zykadia consensus from Thomson Cortellis (4 analysts) *** Alecensa
consensus from Thomson Cortellis (3 analysts) Global ALK Inhibitor
Consensus Revenue Forecasts $0 $400 $800 $1,200 $1,600 $2,000 2015E
2019E ~$700M ~$1.7B Product revenue in millions Alecensa Consensus***
Zykadia Consensus** Xalkori Consensus*
 01.14.2015 J.P.
Morgan Healthcare Conference Research & Development Key Investments J.P.
01.14.2015 J.P.
Morgan Healthcare Conference Research & Development Key Investments J.P.
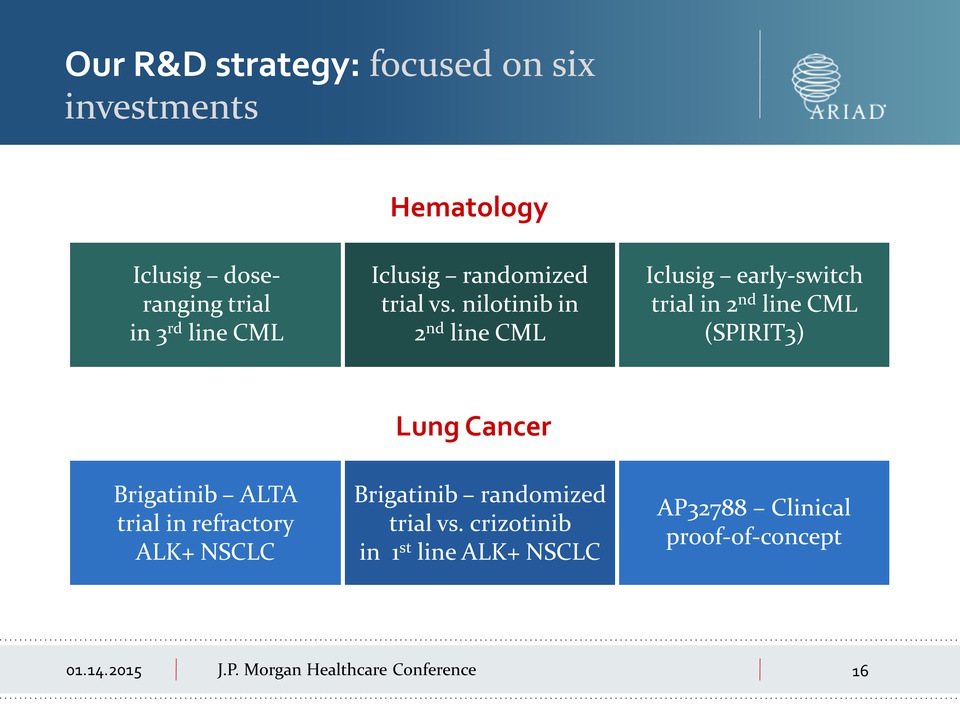 01.14.2015 Morgan
Healthcare Conference Iclusig – doseranging trial in 3rd line CML
Iclusig – randomized trial vs. nilotinib in 2nd line CML Iclusig –
early-switch trial in 2nd line CML (SPIRIT3) Brigatinib – ALTA trial in
refractory ALK+ NSCLC Brigatinib – randomized trial vs. crizotinib in
1st line ALK+ NSCLC AP32788 – Clinical proof-of-concept Hematology Lung
Cancer Our R&D strategy: focused on six investments
01.14.2015 Morgan
Healthcare Conference Iclusig – doseranging trial in 3rd line CML
Iclusig – randomized trial vs. nilotinib in 2nd line CML Iclusig –
early-switch trial in 2nd line CML (SPIRIT3) Brigatinib – ALTA trial in
refractory ALK+ NSCLC Brigatinib – randomized trial vs. crizotinib in
1st line ALK+ NSCLC AP32788 – Clinical proof-of-concept Hematology Lung
Cancer Our R&D strategy: focused on six investments
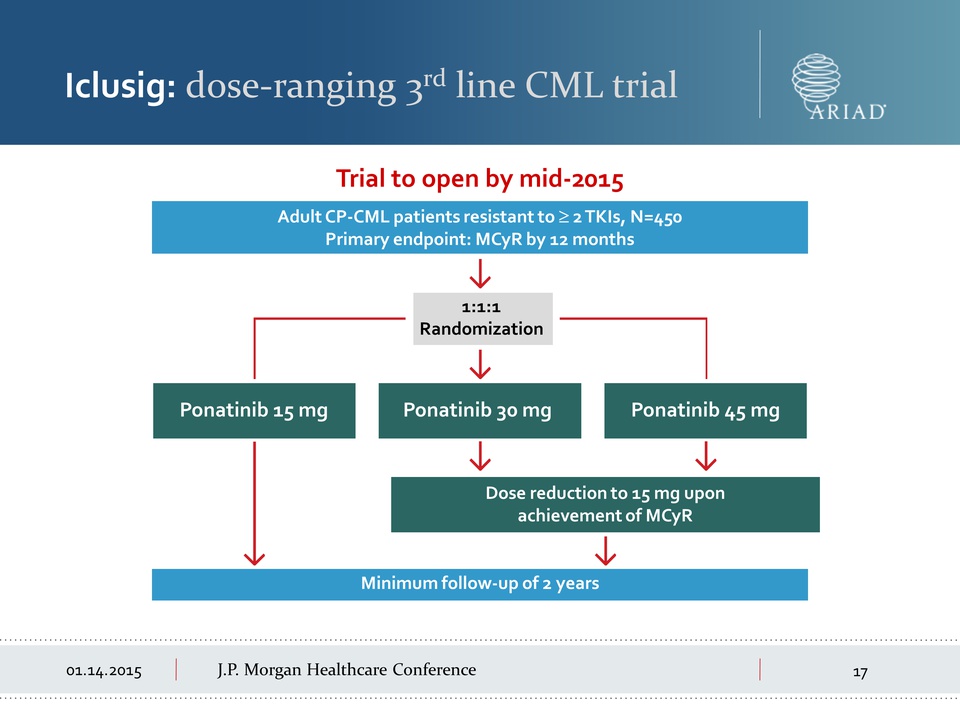 01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: dose-ranging 3rd line CML trial
Trial to open by mid-2015 Adult CP-CML patients resistant to ≥ 2 TKIs,
N=450 Primary endpoint: MCyR by 12 months Minimum follow-up of 2 years
1:1:1 Randomization Dose reduction to 15 mg upon achievement of MCyR
Ponatinib 15 mg Ponatinib 30 mg Ponatinib 45 mg
01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: dose-ranging 3rd line CML trial
Trial to open by mid-2015 Adult CP-CML patients resistant to ≥ 2 TKIs,
N=450 Primary endpoint: MCyR by 12 months Minimum follow-up of 2 years
1:1:1 Randomization Dose reduction to 15 mg upon achievement of MCyR
Ponatinib 15 mg Ponatinib 30 mg Ponatinib 45 mg
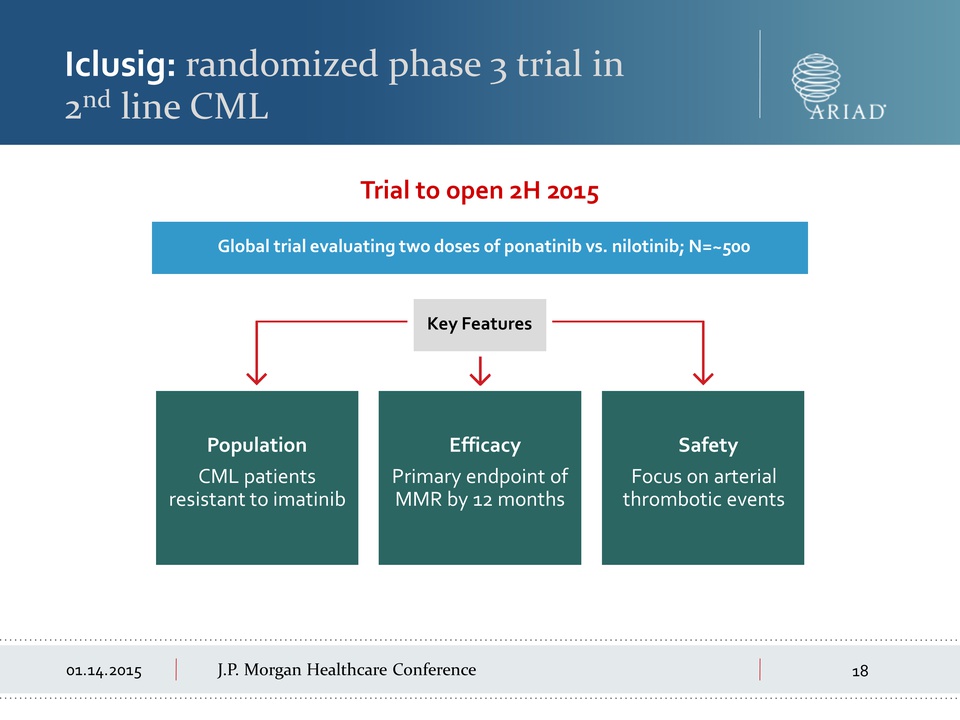 01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: randomized phase 3 trial in 2nd
line CML Global trial evaluating two doses of ponatinib vs. nilotinib;
N=~500 Key Features CML patients resistant to imatinib Population
Primary endpoint of MMR by 12 months Efficacy Focus on arterial
thrombotic events Safety Trial to open 2H 2015
01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: randomized phase 3 trial in 2nd
line CML Global trial evaluating two doses of ponatinib vs. nilotinib;
N=~500 Key Features CML patients resistant to imatinib Population
Primary endpoint of MMR by 12 months Efficacy Focus on arterial
thrombotic events Safety Trial to open 2H 2015
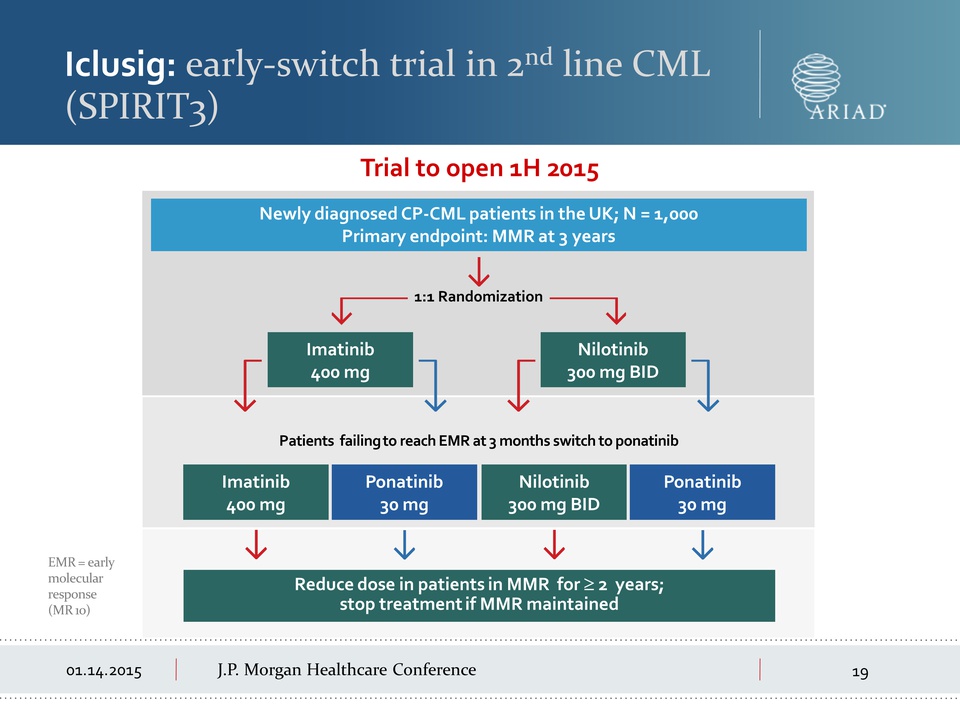 01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: early-switch trial in 2nd line
CML (SPIRIT3) EMR = early molecular response (MR 10) Trial to open 1H
2015 Patients failing to reach EMR at 3 months switch to ponatinib
Reduce dose in patients in MMR for ≥ 2 years; stop treatment if MMR
maintained 1:1 Randomization Newly diagnosed CP-CML patients in the UK;
N = 1,000 Primary endpoint: MMR at 3 years Imatinib 400 mg Nilotinib 300
mg BID Imatinib 400 mg Ponatinib 30 mg Ponatinib 30 mg Nilotinib 300 mg
BID
01.14.2015 J.P.
Morgan Healthcare Conference Iclusig: early-switch trial in 2nd line
CML (SPIRIT3) EMR = early molecular response (MR 10) Trial to open 1H
2015 Patients failing to reach EMR at 3 months switch to ponatinib
Reduce dose in patients in MMR for ≥ 2 years; stop treatment if MMR
maintained 1:1 Randomization Newly diagnosed CP-CML patients in the UK;
N = 1,000 Primary endpoint: MMR at 3 years Imatinib 400 mg Nilotinib 300
mg BID Imatinib 400 mg Ponatinib 30 mg Ponatinib 30 mg Nilotinib 300 mg
BID
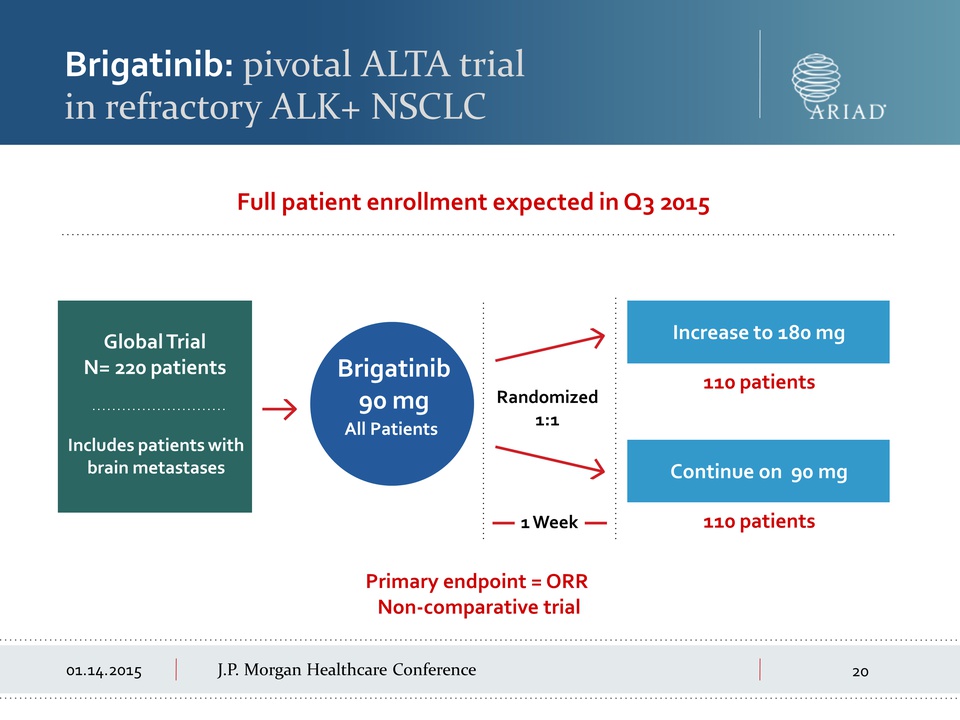 01.14.2015 J.P.
Morgan Healthcare Conference Brigatinib: pivotal ALTA trial in
refractory LK+ NSCLC Primary endpoint = ORR Non-comparative trial Full
patient enrollment expected in Q3 2015 Brigatinib 90 mg All Patients
Global Trial N= 220 patients Includes patients with brain metastases
Randomized 1:1 1 Week Continue on 90 mg 110 patients Increase to 180 mg
110 patients
01.14.2015 J.P.
Morgan Healthcare Conference Brigatinib: pivotal ALTA trial in
refractory LK+ NSCLC Primary endpoint = ORR Non-comparative trial Full
patient enrollment expected in Q3 2015 Brigatinib 90 mg All Patients
Global Trial N= 220 patients Includes patients with brain metastases
Randomized 1:1 1 Week Continue on 90 mg 110 patients Increase to 180 mg
110 patients
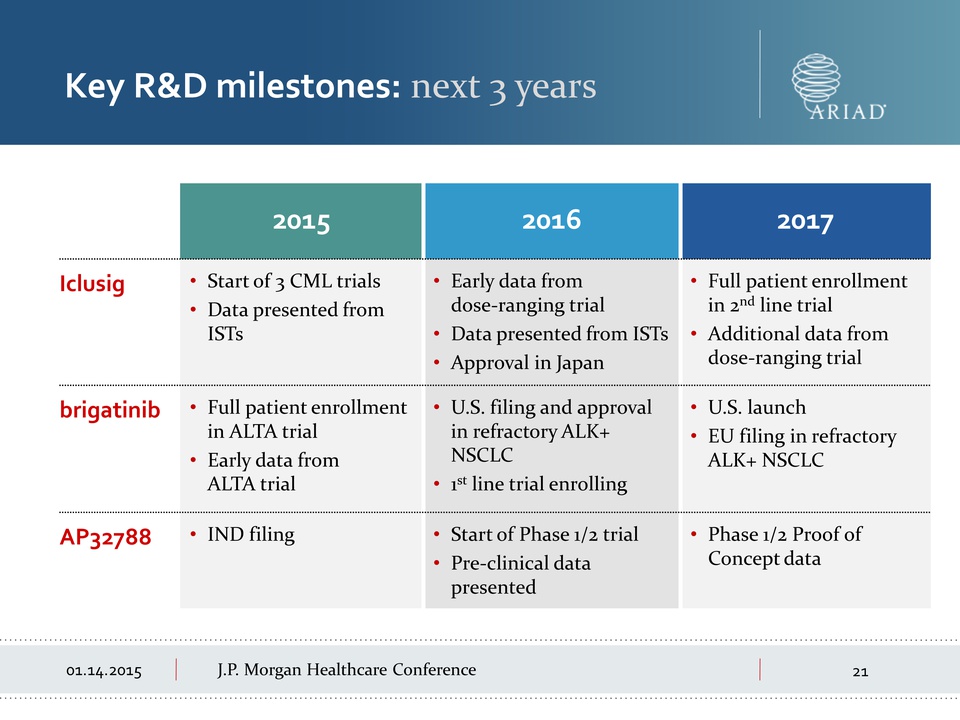 01.14.2015 J.P.
Morgan Healthcare Conference 2015 2016 2017 Iclusig • Start of 3 CML
trials • Data presented from ISTs • Early data from dose-ranging trial
Data presented from ISTs Approval in Japan Full patient enrollment in
2nd line trial Additional data from dose-ranging trial brigatinib Full
patient enrollment in ALTA trial Early data from ALTA trial U.S. filing
and approval in refractory ALK+ NSCLC 1st line trial enrolling U.S.
launch EU filing in refractory ALK+ NSCLC AP32788 IND filing Start of
Phase 1/2 trial Pre-clinical data presented Phase 1/2 Proof of Concept
data Key R&D milestones: next 3 years
01.14.2015 J.P.
Morgan Healthcare Conference 2015 2016 2017 Iclusig • Start of 3 CML
trials • Data presented from ISTs • Early data from dose-ranging trial
Data presented from ISTs Approval in Japan Full patient enrollment in
2nd line trial Additional data from dose-ranging trial brigatinib Full
patient enrollment in ALTA trial Early data from ALTA trial U.S. filing
and approval in refractory ALK+ NSCLC 1st line trial enrolling U.S.
launch EU filing in refractory ALK+ NSCLC AP32788 IND filing Start of
Phase 1/2 trial Pre-clinical data presented Phase 1/2 Proof of Concept
data Key R&D milestones: next 3 years
 01.14.2015 J.P.
Morgan Healthcare Conference Marcele Wilson Chronic myeloid leukemia
ARIAD clinical trial patient Achieving Profitability in 2018
01.14.2015 J.P.
Morgan Healthcare Conference Marcele Wilson Chronic myeloid leukemia
ARIAD clinical trial patient Achieving Profitability in 2018
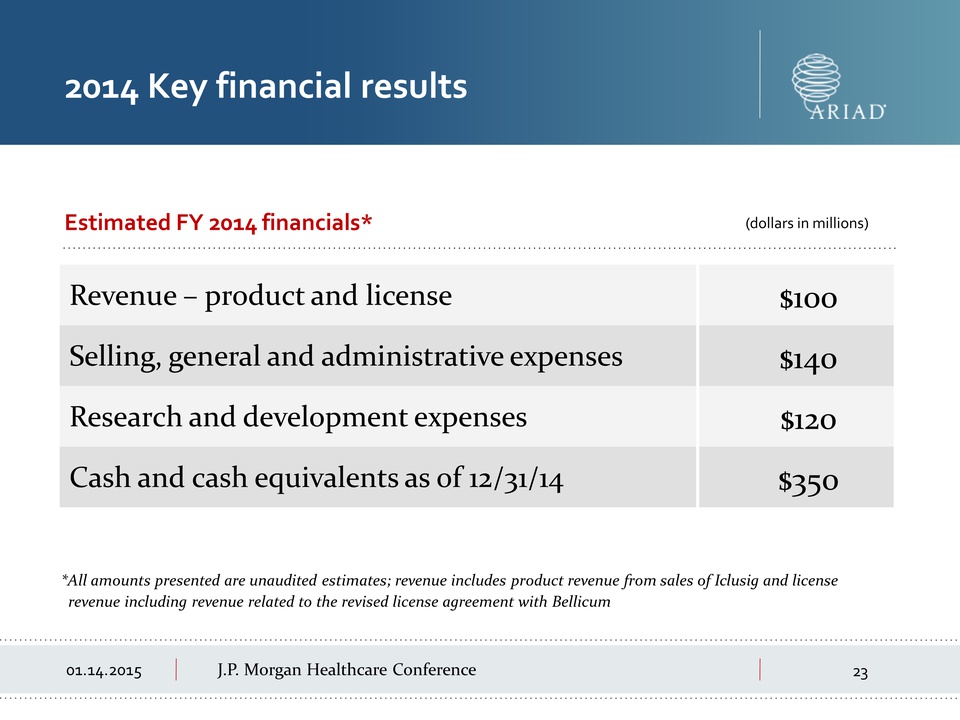 01.14.2015 J.P.
Morgan 01.14.2015 Healthcare Conference Revenue – product and license
$100 Selling, general and administrative expenses $140 Research and
development expenses $120 Cash and cash equivalents as of 12/31/14 $350
2014 Key financial results *All amounts presented are unaudited
estimates; revenue includes product revenue from sales of Iclusig and
license revenue including revenue related to the revised license
agreement with Bellicum Estimated FY 2014 financials* (dollars in
millions)
01.14.2015 J.P.
Morgan 01.14.2015 Healthcare Conference Revenue – product and license
$100 Selling, general and administrative expenses $140 Research and
development expenses $120 Cash and cash equivalents as of 12/31/14 $350
2014 Key financial results *All amounts presented are unaudited
estimates; revenue includes product revenue from sales of Iclusig and
license revenue including revenue related to the revised license
agreement with Bellicum Estimated FY 2014 financials* (dollars in
millions)
 01.14.2015 J.P.
Morgan Healthcare Conference Path to sustained profitability 2015 2016
2017 2018 Revenue growth and partnerships drive increased cash flow
Iclusig revenue growth in U.S. and Europe from current label Iclusig
revenue from Japan and new geographies Brigatinib revenue and
partnership payments Sustained profitability achieved on >$400M revenues
in 2018 Strategic investment to support long-term growth Commercial
presence in U.S. and Europe Iclusig development in earlier lines of
therapy Brigatinib and AP32788 development No requirement for additional
equity capital Broad co-development and co-commercialization partnership
for brigatinib
01.14.2015 J.P.
Morgan Healthcare Conference Path to sustained profitability 2015 2016
2017 2018 Revenue growth and partnerships drive increased cash flow
Iclusig revenue growth in U.S. and Europe from current label Iclusig
revenue from Japan and new geographies Brigatinib revenue and
partnership payments Sustained profitability achieved on >$400M revenues
in 2018 Strategic investment to support long-term growth Commercial
presence in U.S. and Europe Iclusig development in earlier lines of
therapy Brigatinib and AP32788 development No requirement for additional
equity capital Broad co-development and co-commercialization partnership
for brigatinib
 01.14.2015 J.P.
Morgan Healthcare Conference ARIAD 2015: key catalysts Accelerate
Iclusig sales in U.S. and Europe Initiate key Iclusig trials
Dose-ranging trial Randomized 2nd line trial vs. nilotinib Early- switch
2nd line trial (SPIRIT3) Secure broad partnership for brigatinib Present
early data from brigatinib pivotal ALTA trial File for approval of
Iclusig in Japan with Otsuka File IND for new development candidate
AP32788
01.14.2015 J.P.
Morgan Healthcare Conference ARIAD 2015: key catalysts Accelerate
Iclusig sales in U.S. and Europe Initiate key Iclusig trials
Dose-ranging trial Randomized 2nd line trial vs. nilotinib Early- switch
2nd line trial (SPIRIT3) Secure broad partnership for brigatinib Present
early data from brigatinib pivotal ALTA trial File for approval of
Iclusig in Japan with Otsuka File IND for new development candidate
AP32788
 ARIAD®
ARIAD®
