Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AVEO PHARMACEUTICALS, INC. | d850777d8k.htm |
 JP
Morgan 2015 Global Healthcare Conference
JANUARY 15, 2015
Exhibit 99.1 |
 Forward-Looking Statements
This presentation contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. All statements, other than
statements of historical facts, contained in this presentation
are
forward-looking
statements.
The
words
“anticipate,”
“believe,”
“estimate,”
“expect,”
“intend,”
“may,”
“plan,”
“predict,”
“project,”
“target,”
“potential,”
“will,”
“would,”
“could,”
“should,”
“continue,”
“contemplate,”
or
the
negative
of
these
terms
or
other
similar
expressions
are
intended
to
identify
forward-looking statements, although not all forward-looking statements
contain these identifying words. These forward-looking statements
include, among others, statements about: AVEO’s estimates for its 2014
year-end cash balance; AVEO’s goals and business strategy and its ability to optimize its
resources; the timing and results of preclinical and clinical trials; AVEO’s
approach to treat cachexia; and AVEO’s
plans
to
leverage
biomarkers
and
pursue
strategic
partnerships
for
certain
of
its
assets.
Actual
results
or
events
could
differ
materially
from
the
plans,
intentions
and
expectations
disclosed
in
the
forward-looking statements AVEO makes due to a number of important factors,
including substantial risks and uncertainties relating to: AVEO’s
ability to successfully implement its restructuring and strategic
plans;
AVEO’s
ability
to
successfully
develop,
test
and
gain
regulatory
approval
of
its
product
candidates;
AVEO’s ability to obtain necessary financing; AVEO’s ability to establish
and maintain new strategic partnerships; AVEO’s ability to obtain,
maintain and enforce intellectual property rights; competition; AVEO’s
dependence on its strategic partners and other third parties; adverse economic conditions; and
those
risk
factors
discussed
in
the
“Risk
Factors”
and
elsewhere
in
AVEO’s
Quarterly
Report
on
Form
10-
Q
for
the
quarter
ended
September
30,
2014,
and
other
periodic
filings
AVEO
makes
with
the
SEC.
All
forward-looking statements contained in this presentation speak only as of the
date of this presentation, and AVEO undertakes no obligation to update any
of these statements, except as required by law. 2
|
 AVEO
Path Forward: Goals and Milestones January 12, 2015
3
AVEO Has a Robust Pipeline and Streamlined
Organization
Tivozanib
VEGFR 1,2,3 TKI
Evaluate potential development strategies for registration
-
EU MAA (RCC)
-
CRC biomarker-driven studies
-
RCC studies
Present biomarker data
Ficlatuzumab
HGF MAb
Enroll Biodesix-funded Phase 2 FOCAL study in NSCLC
Target Phase 3 readiness by 2017
AV-203
ERBB3 MAb
Leverage strong biomarker data to secure a partnership
AV-380
GDF-15 MAb
Leverage preclinical proof of concept data to determine optimal go-
forward strategy, including partnership or internal development
|
 AVEO
Go Forward Strategy Optimally Leveraging Biomarker
Insights and Partnership Resources
to Advance Our Portfolio of
Development-Stage Assets
January 12, 2015
4 |
 AVEO
is Leveraging Biomarkers and Partnerships to Advance its Robust Pipeline of
Assets 5
Substantial Ownership of all Programs
Program
Pathway
Development
Candidate
Preclinical
Phase 1
Phase 2
Phase 3
Rights
Tivozanib
VEGFR 123 TKI
Own ex-Asian
rights
Ficlatuzumab
HGF MAb
AV-203
ERBB3 MAb
Wholly Owned
AV-380
GDF15 MAb
Wholly Owned
Most advanced stage of development
Streamlined Organization to Focus on
Clinical and Business Development Operations
GDF-15 Biomarker Cachexia
Potential EU File RCC
NRG-1 Biomarker Solid Tumors
Serum Proteomic Biomarker NSCLC
Angiogenesis Biomarkers CRC
Ocular Diseases |
 Tivozanib
A
POTENT
,
SELECTIVE
,
LONG HALF
-LIFE INHIBITOR OF
VASCULAR ENDOTHELIAL
GROWTH FACTOR
(VEGF) 1, 2, AND 3 RECEPTORS 6 |
 Tivozanib –
VEGFR 1, 2 & 3 Tyrosine Kinase Inhibitor
•
Potent, selective inhibitor of VEGFRs 1, 2, and 3
with a long half-life that is designed to optimize
blockade while minimizing off-target toxicities
1,2
•
Patent term: 2022 (composition of matter) with
potential extension to 2027
•
Orphan drug designation in Europe for RCC
1. Nakamura K et al. Cancer Res 2006;66:9134–9142.
2. Eskens FA et al. Clin Cancer Res 2011;17:7156–7163.
7
1.
Reacquired worldwide rights (Aug 2014)
2.
Ophthotech option agreement to evaluate in ocular indications (Nov 2014)
3.
Received confirmation of eligibility for MAA from the EMA for RCC (Jan 2015)
4.
Ongoing evaluation of novel angiogenesis biomarkers from BATON studies
to explore advancing tivozanib clinical development |
 Ophthotech Research and Option Agreement: Nov. 2014
January 12, 2015
8
8
Agreement
Research and Exclusive Option Agreement for
Non-Oncology Disease of the Eye
Territory
Worldwide ex-Asia
Upfront
$500,000
Option Term Milestones
$2M at IND filing and $6M based upon the
achievement of specified R&D, business goals
Option Payment*
$2M
Clinical and Regulatory Milestones
$50M
Sales-based Milestones
$45M
Royalties
Tiered, double digit royalties, up to the mid-
teens, on net sales
Agreement monetizes tivozanib while retaining oncology indications and
providing significant potential downstream value for AVEO
All payments received by AVEO subject to sublicense revenue obligation to Kyowa
Hakko Kirin * Assumes execution of option by Ophthotech
|
 Tivozanib Preclinical Activity in Ocular Models
January 12, 2015
9
Compared to the vehicle-treatment, tivozanib
suppressed
the
development
of
CNV
lesions
and
led
to
a
significant
regression
of
established
CNV
reducing the affected areas by 80.7% and 67.7% respectively |
 |
 Evaluating Potential for MAA Filing for RCC
January 12, 2015
•
Met primary PFS endpoint
of superiority vs sorafenib
•
First H2H RCC pivotal trial
to show superiority over
another VEGF TKI
•
Demonstrated
differentiated safety profile
•
OS confounded by
crossover
•
Median OS similar to
historical TKI agents,
despite lack of subsequent
therapy |
 January 12, 2015
•
Interim results (ESMO 2014) suggest that tivozanib+mFOLFOX6 is comparable to
bevacizumab+mFOLFOX6 in the ITT population, with an acceptable safety profile.
•
PFS: 9.4 (Tivo) vs 10.7 (Bev) months; HR=1.091, p=0.706
•
ORR: 45% (Tivo) vs. 43% (Bev), p=0.718
•
Final pre-specified biomarker analysis to be presented at upcoming scientific
meeting •
Biomarker IP to be filed in January
* Interim ITT PFS analysis (after full enrollment) indicated futility for
superiority of Tivo vs. Bev Tivozanib Biomarker Study
tivozanib
+
mFOLFOX6
bevacizumab
+
mFOLFOX6
N = 265
•
1
st
line Stage IV mCRC
•
No fluorouracil adj tx
<6 months
•
ECOG PS 0 or 1
•
1
O
: PFS*
•
2
O
: OS, ORR, DoR,
TTF, HRQol, Safety
and tolerability for
ITT & Pre-Specified
Biomarkers
|
 Ficlatuzumab
13 |
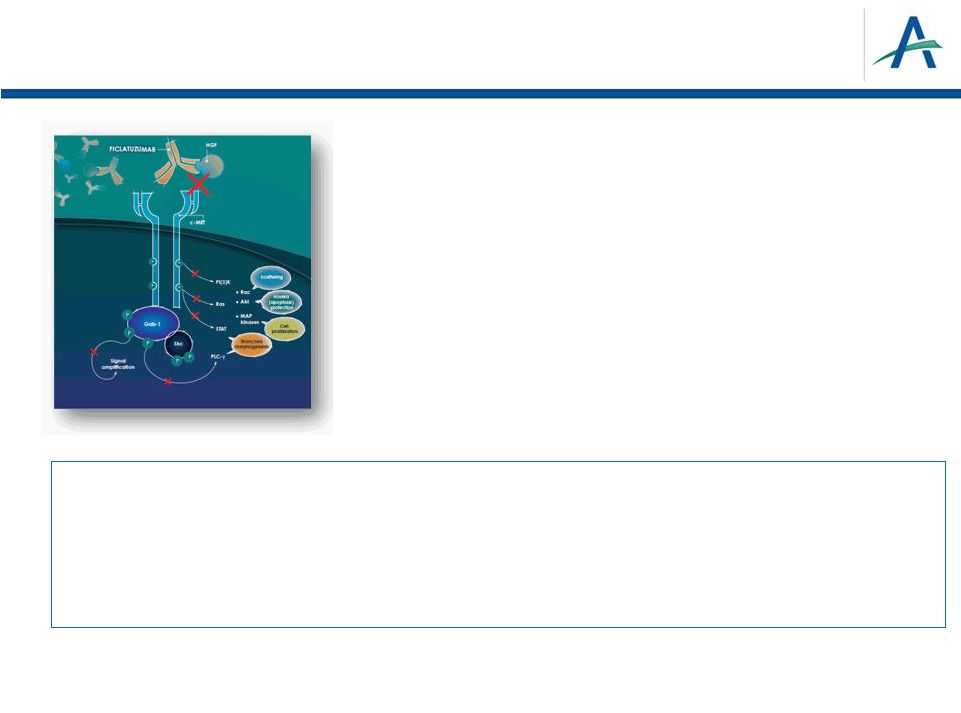 •
A Favorable Profile to In-Class Antibodies
–
High affinity (pM) and slow off-rate for HGF
–
High potency (nM) inhibiting all biological activities of
HGF, including autocrine/paracrine activation loops
–
In vivo preclinical efficacy superior to in-class
antibodies
Ficlatuzumab –
Humanized IgG1k MAb Inhibitor of HGF
14
Data on file
* January 2028 expiry in US and June 2027 expiry in Europe and Japan
1.
Identified potential serum proteomic biomarker: Biodesix VeriStrat
®
2.
Finalized Biodesix partnership to fund confirmatory phase 2 study (April
2014) 3.
Presented Phase 2 Biodesix VeriStrat
®
analysis (ESMO 2014)
4.
Confirmatory phase 2 NSCLC study open
•
Patent Term: 2027-28 (composition of matter) with
potential extension to 2032-2033* |
 Biodesix Partnership: April 2014
AVEO and Biodesix Partner to Co-Develop and Commercialize
Ficlatuzumab with a Companion Diagnostic
–
Unique collaboration where the diagnostic company provides funding for
POC clinical development in NSCLC
•
Biodesix will fund up to $15 million of the cost of the confirmatory phase 2
study in NSCLC and all companion diagnostic development costs
•
50/50 sharing of ficlatuzumab development and commercialization
expenses beyond confirmatory phase 2
•
50/50 sharing of potential profits from ficlatuzumab
•
Biodesix retains revenue from companion diagnostic
•
AVEO to lead worldwide commercialization of ficlatuzumab
–
Agreement advances ficlatuzumab with external funding while retaining
significant downstream value for AVEO
January 12, 2015
15 |
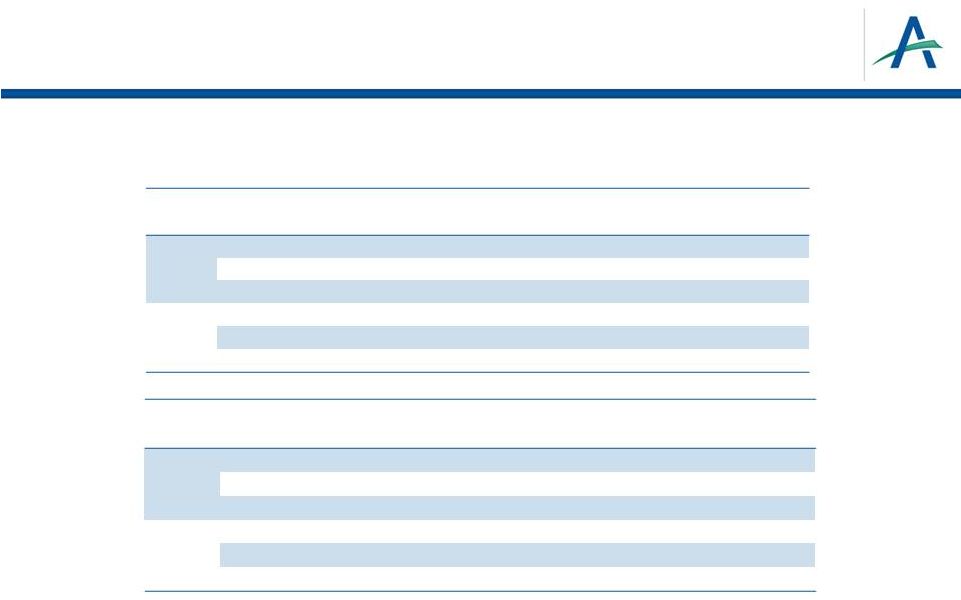 Ficlatuzumab Randomized Phase 2 Study (P06162):
Exploratory Analysis Results*
VeriStrat
may
be
a
predictive
biomarker
for
the
combination
of
ficlatuzumab
EGFR
TKI
over
EGFR
TKI
alone
Observation to be confirmed in the Phase 2 FOCAL study, initiated in 2014
16
* Mok et al ESMO 2014
VeriStrat Poor (VSP)
ficlatuzumab + gefitinib
n=18
gefitinib alone
n=17
OS
Median
23.9 months
5.8 months
Hazard Ratio
0.41
p-value
0.032
PFS
Median
7.4 months
2.3 months
Hazard Ratio
0.41
p-value
0.014
VSP & EGFRm
ficlatuzumab + gefitinib
n=5
gefitinib alone
n=6
OS
Median
17.8 months
10.4 months
Hazard Ratio
0.3
p-value
0.09
PFS
Median
11.1 months
2.3 months
Hazard Ratio
<0.01
p-value
<0.01
+
® |
 FOCAL
Study – Confirmatory Phase 2
January 12, 2015
17
A Phase 2, Multicenter, Randomized, Double-blind Study of
Ficlatuzumab Plus Erlotinib Versus Placebo Plus Erlotinib in Subjects Who
Have Previously Untreated Metastatic, EGFR-mutated Non-small Cell Lung
Cancer (NSCLC) and BDX004 Positive Label
1
st
Line
Stage
IV
NSCLC
EGFR Mutation+
BX004 Positive
ECOG PS 0 or 1
N
= 86
ficlatuzumab
+
erlotinib
placebo
+
erlotinib
1
O
: PFS
2
O
: OS, ORR, Disease
Control, PK, Safety
and tolerability |
 AV-203
18 |
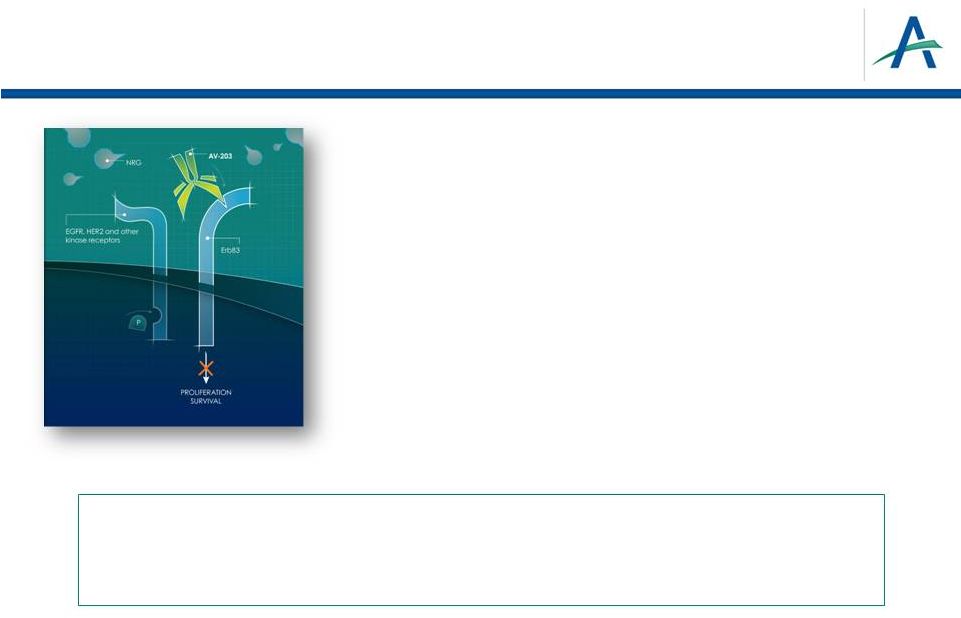 AV-203:
Superhumanized
IgG1k
MAb
Inhibitor
of
ERBB3
•
Potent high affinity NRG-1/HRG binding
inhibition
–
Exclusively blocks ERBB3 signaling
–
Potential for inducing ADCC
–
Prevents NRG-1/HRG induced proliferation
–
Opportunity to explore combinations with HER2
and EGFR Targeted Therapies
•
Patent Term: 2031 (composition of matter) and
2032 (NRG-1 biomarker) with potential for
extensions
January 12, 2015
19
1.
Reacquired worldwide rights from Biogen Idec (March 2014)
2.
Presented Phase 1 monotherapy data showing encouraging early
support for NRG-1/HRG biomarker hypothesis (ASCO 2014)
TM |
 Biogen Idec Agreement: March 2014
January 12, 2015
20
•
Actively pursuing partnership to advance development
•
AVEO reacquired AV-203 worldwide rights from Biogen Idec
–
NRG-1/HRG positive tumors
–
Combinations with EGFR and HER2 targeted inhibitors
–
Combination with chemotherapy in chemotherapy resistant/refractory tumors
•
Multiple, attractive opportunities for clinical development leveraging
NRG- 1/HRG Biomarker
–
AVEO owes a percentage of milestones from a future partnership and low single
digit royalties on net sales up to a fixed cap of $50M
Biogen Idec agreement allows for AVEO to find a worldwide partner to
fund further development for AV-203 |
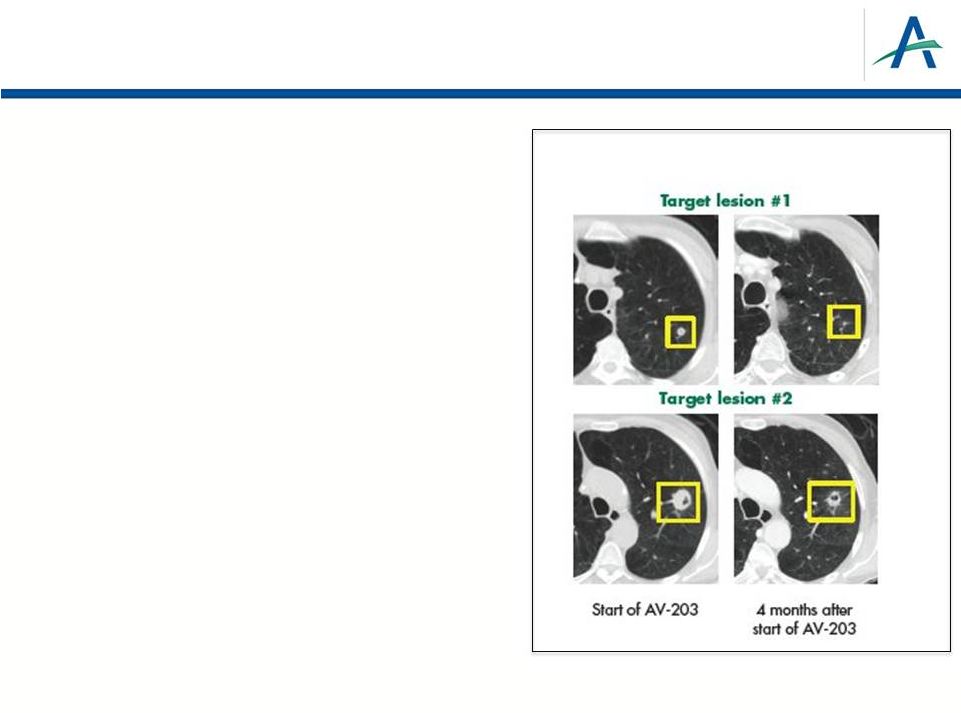 Phase
1 AV-203 Dose-Escalation, Monotherapy Study Completed*
•
22 subjects enrolled
–
Tumor types: 4 NSCLC (3 sq, 1 adeno); 4 CRC;
2 sq cell skin; one each ovarian, peritoneal,
SCLC, endometrioid, osteosarcoma, HCC,
pancreatic, neuroendocrine, bladder,
mesothelioma, SCCHN, cervical
•
Recommended P2 dose is 20mg/kg every
2 weeks (maximum dose tested)
–
Most common treatment-related AEs were
diarrhea (59%), dry skin and decreased appetite
(32% each); 1 DLT observed
•
Encouraging early efficacy and
support for NRG-1/HRG biomarker
hypothesis
–
Of 22 patients, 8 SD and 1 PR
–
1 of 2 NRG-1/HRG+ patients experienced a PR
January 12, 2015
* Sarantopoulos, ASCO 2014. Abs#11113.
NSCLC with partial response (9 mg/kg)
(NRG-1/HRG Positive)
21 |
 22
AV-380 |
 23
associated with several underlying chronic
illnesses and characterized by loss of
muscle mass and strength, loss of whole
body fat, inflammation, anemia, etc.
~400,000 pts
In US
~960,000 pts
In US
~150,000 pts
In US
~3,200,000 pts
In US
Morley
et
al;
Am
J
Clin
Nutr
2006;83:735–
43
Cachexia Represents a Significant Unmet Medical Need
Cancer
Congestive
Heart
Failure
Chronic
Kidney
Disease
Chronic
Obstructive
Pulmonary Disease
Cachexia
(Wasting Syndrome)
Cachexia
Complex
metabolic
syndrome
: |
 GDF15
– Driver of Cachexia (Wasting Syndrome)
24
Proof-of-Concept:
•
Elevated in cachectic
models and patients
(vs. non-cachectic)
•
Administration of
GDF15 induces
cachexia
•
Inhibition of GDF15
reverses cachexia
24 |
 GDF15
– Driver of Cachexia (Wasting Syndrome)
25
Proof-of-Concept:
25
GDF15 Levels –
Patient Samples
GDF15 Levels –
Murine Models
Inhibition of GDF15
reverses cachexia
Administration of
GDF15 induces
cachexia
Elevated in cachectic
models and patients
(vs. non-cachectic) |
 GDF15
– Driver of Cachexia (Wasting Syndrome)
26
Proof-of-Concept:
Elevated in cachectic
models and patients
(vs. non-cachectic)
Administration of
GDF15 induces
cachexia
Inhibition of GDF15
reverses cachexia
26
*** |
 GDF15
– Driver of Cachexia (Wasting Syndrome)
27
Proof-of-Concept:
Elevated in cachectic
models and patients
(vs. non-cachectic)
Administration of
GDF15 induces
cachexia
Inhibition of GDF15
reverses cachexia
27 |
 GDF15
– Driver of Cachexia (Wasting Syndrome)
28
Proof-of-Concept:
28
Inhibition of GDF15
reverses cachexia
Administration of
GDF15 induces
cachexia
Elevated in cachectic
models and patients
(vs. non-cachectic) |
 First-in-Class, Differentiated Approach to Cachexia
•
AV-380: Potent humanized GDF15 inhibitory MAb
–
Unique mechanism of action
•
Increase
calorie/food intake
•
Reverse
body weight loss
•
Restore
normal body composition
•
Mechanism of action different from:
–
Hormonal/Metabolic agents (i.e. ghrelin, SARMs)
•
Focus on stimulation of appetite or muscle protein synthesis
–
Muscle regulation-directed agents (i.e. myostatin, activin)
•
Address the muscle wasting aspect of the diseases
–
Early
cytokine
inhibitors
(i.e.TNF
,IL-6,
IL-8)
•
Mechanistic link to the disease not well established
•
Strong Intellectual Property Position
–
Composition of matter patent (filed)
–
Method of use patent (granted in US and EU)
29 |
 Financials and Summary
January 12, 2015
30 |
 Financials Highlights
•
Streamlined operations
–
Exited long term facilities lease
–
Eliminated research function to focus on clinical and BD functions
•
Headcount reduction from 60 to 20
•
Expected to reduce annual compensation expenses by ~$6 million
•
Will reduce AVEO’s facilities requirements by up to 80% of its current
space, including the elimination of lab and vivarium needs
•
Estimated 4Q14 cash and securities of ~$52M*
•
Shares outstanding: ~52M
31
* Unaudited 4Q financial results as of 12/31/14 |
 AVEO
is Poised to Unlock the Potential of its Robust Pipeline of Assets by Leveraging
Biomarkers and Partnerships 32
Substantial Ownership of all Programs
Program
Pathway
Development
Candidate
Preclinical
Phase 1
Phase 2
Phase 3
Rights
Tivozanib
VEGFR 123 TKI
Own ex-Asian
rights
Ficlatuzumab
HGF MAb
AV-203
ERBB3 MAb
Wholly Owned
AV-380
GDF15 MAb
Wholly Owned
Most advanced stage of development
Streamlined Organization to Focus on
Clinical and Business Development Operations
GDF-15 Biomarker Cachexia
Potential EU File RCC
NRG-1 Biomarker Solid Tumors
Serum Proteomic Biomarker NSCLC
Angiogenesis Biomarkers CRC
Ocular Diseases |
 33
JP Morgan 2015 Global
Healthcare Conference
JANUARY 15, 2014 |
