Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Wright Medical Group N.V. | d850054dex991.htm |
| 8-K - 8-K - Wright Medical Group N.V. | d850054d8k.htm |
 Investor Presentation
4Q 2014
Exhibit 99.2 |
 Forward Looking Statements
Forward-Looking Statements
Statements
contained
in
this
presentation
that
relate
to
future,
not
past,
events
are
forward-looking
statements
under
the
Private
Securities
Litigation
Reform
Act
of
1995.
Forward-looking
statements
are
based
on
current
expectations
of
future
events
and
often
can
be
identified
by
words
such
as
“expect,”
“anticipate,”
“project,”
“intend,”
“will,”
“may,”
“believe,”
“could,”
“continue,”
“estimate,”
“outlook,”
“plan,”
“guidance,”
“tomorrow”,
“desired
state,”
other
words
of
similar
meaning
or
the
use
of
future
dates.
Forward-looking
statements
by
their
nature
address
matters
that
are,
to
different
degrees,
uncertain.
Uncertainties
and
risks
may
cause
Tornier’s
actual
results
to
be
materially
different
than
those
expressed
in
or
implied
by
Tornier’s forward-
looking
statements.
For
Tornier,
such
uncertainties
and
risks
include,
among
others,
risks
relating
to
Tornier’s
proposed
merger
with
Wright
Medical
Group,
Inc.,
including
the
timing
of
the
transaction;
uncertainties
as
to
whether
Tornier
shareholders
and
Wright
shareholders
will
approve
the
transaction;
the
risk
that
competing
offers
will
be
made;
the
possibility
that
various
closing
conditions
for
the
transaction
may
not
be
satisfied
or
waived,
including
that
a
governmental
entity
may
prohibit,
delay
or
refuse
to
grant
approval
for
the
consummation
of
the
transaction,
or
the
terms
of
such
approval;
the
effects
of
disruption
from
the
transaction
making
it
more
difficult
to
maintain
relationships
with
employees,
customers,
vendors
and
other
business
partners;
the
risk
that
shareholder
litigation
in
connection
with
the
transaction
may
result
in
significant
costs
of
defense,
indemnification
and
liability;
other
business
effects,
including
the
effects
of
industry,
economic
or
political
conditions
outside
of
Wright’s
or
Tornier’s
control;
the
failure
to
realize
synergies
and
cost-savings
from
the
transaction
or
delay
in
realization
thereof;
the
businesses
of
Wright
and
Tornier
may
not
be
combined
successfully,
or
such
combination
may
take
longer,
be
more
difficult,
time-consuming
or
costly
to
accomplish
than
expected;
operating
costs
and
business
disruption
following
completion
of
the
transaction,
including
adverse
effects
on
employee
retention
and
on
Wright’s
and
Tornier’s
respective
business
relationships
with
third
parties;
transaction
costs;
actual
or
contingent
liabilities;
the
adequacy
of
the
combined
company’s
capital
resource;
and
other
risks
and
uncertainties,
including
Tornier’s
future
operating
results
and
financial
performance;
the
success
of
and
possible
disruption
from
Tornier’s
recently
completed
transition
to
dedicated
upper
and
lower
extremities
sales
forces;
fluctuations
in
foreign
currency
exchange
rates;
the
effect
of
global
economic
conditions;
the
timing
of
regulatory
approvals
and
introduction
of
new
products;
physician
acceptance,
endorsement,
and
use
of
new
products;
and
the
effect
of
regulatory
actions,
changes
in
and
adoption
of
reimbursement
rates,
product
recalls
and
competitor
activities.
More
detailed
information
on
these
and
other
factors
that
could
affect
Tornier’s
actual
results
are
described
in
Tornier’s
filings
with
the
U.S.
Securities
and
Exchange
Commission,
including
its
most
recent
annual
report
on
Form
10-K,
subsequent
quarterly
reports
on
Form
10-Q
and
registration
statement
on
Form
S-4
filed
in
connection
with
its
proposed
merger
with
Wright.
Tornier
undertakes
no
obligation
to
update
its
forward-looking
statements. |
 Non-GAAP Financial Measures
Non-GAAP Financial Measures
Tornier
uses
certain
non-GAAP
financial
measures
in
this
presentation,
such
as
adjusted
EBITDA,
adjusted
gross
margin
and
constant
currency.
Tornier
uses
non-GAAP
financial
measures
as
supplemental
measures
of
performance
and
believes
these
measures
provide
useful
information
to
investors
in
evaluating
Tornier’s
operations,
period
over
period.
However,
non-GAAP
financial
measures
have
limitations
as
analytical
tools,
and
should
not
be
considered
in
isolation
or
as
a
substitute
for
Tornier’s
financial
results
prepared
in
accordance
with
GAAP.
In
addition,
investors
should
note
that
any non-
GAAP
financial
measure
Tornier
uses
may
not
be
the
same
non-GAAP
financial
measure,
and
may
not
be
calculated
in
the
same
manner,
as
that
of
other
companies.
A
reconciliation
of
the
non-GAAP
financial
measures
used
in
the
presentation
to
the
most
directly
comparable
GAAP
financial
measures
can
be
found
on
Tornier’s
website
www.tornier.com
under
the
“Non-GAAP
Measure
Reconciliation
Tables”
section
of
the
“Investor
Relations”
page.
Important
Additional
Information
and
Where
to
Find
It
In
connection
with
the
proposed
merger,
Tornier
has
filed
with
the
U.S.
Securities
and
Exchange
Commission
(SEC)
a
registration
statement
on
Form
S-4
that
includes
a
preliminary
joint
proxy
statement
of
Wright
and
Tornier
that
also
constitutes
a
preliminary
prospectus
of
Tornier.
The
registration
statement
is
not
complete
and
will
be
amended.
Once
finalized,
Wright
and
Tornier
will
make
the
final
joint
proxy
statement/prospectus
available
to
their
respective
shareholders.
Investors
are
urged
to
read
the
final
joint
proxy
statement/prospectus
when
it
becomes
available,
because
it
will
contain
important
information.
The
registration
statement,
definitive
joint
proxy
statement/prospectus
and
other
documents
filed
by
Tornier
and
Wright
with
the
SEC
will
be
available
free
of
charge
at
the
SEC’s
website
(www.sec.gov)
and
from
Tornier
and
Wright.
Requests
for
copies
of
the
joint
proxy
statement/prospectus
and
other
documents
filed
by
Wright
with
the
SEC
may
be
made
by
contacting
Julie
D.
Tracy,
Senior
Vice
President
and
Chief
Communications
Officer
by
phone
at
(901)
290-5817
or
by
email
at
julie.tracy@wmt.com,
and
request
for
copies
of
the
joint
proxy
statement/prospectus
and
other
documents
filed
by
Tornier
may
be
made
by
contacting
Shawn
McCormick,
Chief
Financial
Officer
by
phone
at
(952)
426-7646
or
by
email
at
shawn.mccormick@tornier.com. Wright,
Tornier,
their
respective
directors,
executive
officers
and
employees
may
be
deemed
to
be
participants
in
the
solicitation
of
proxies
from
Wright’s
and
Tornier’s
shareholders
in
connection
with
the
proposed
transaction.
Information
about
the
directors
and
executive
officers
of
Wright
and
their
ownership
of
Wright
stock
is
set
forth
in
Wright’s
annual
report
on
Form
10-K
for
the
fiscal
year
ended
December
31,
2013,
which
was
filed
with
the
SEC
on
February
27,
2014
and
its
proxy
statement
for
its
2014
annual
meeting
of
stockholders,
which
was
filed
with
the
SEC
on
March
31,
2014.
Information
regarding
Tornier’s
directors
and
executive
officers
is
contained
in
Tornier’s
annual
report
on
Form
10-K
for
the
fiscal
year
ended
December
29,
2013,
which
was
filed
with
the
SEC
on
February
21,
2014,
and
its
proxy
statement
for
its
2014
annual
general
meeting
of
shareholders,
which
was
filed
with
the
SEC
on
May
16,
2014.
These
documents
can
be
obtained
free
of
charge
from
the
sources
indicated
above.
Certain
directors,
executive
officers
and
employees
of
Wright
and
Tornier
may
have
direct
or
indirect
interest
in
the
transaction
due
to
securities
holdings,
vesting
of
equity
awards
and
rights
to
severance
payments.
Additional
information
regarding
the
participants
in
the
solicitation
of
Wright
and
Tornier
shareholders
will
be
included
in
the
joint
proxy
statement/prospectus. |
 Tornier is…
A global medical device company,
focused on providing superior surgical solutions
treating orthopaedic extremities injuries & disorders.
|
 Tornier is Well-Positioned for Long-Term Growth & Margin
Expansion Based upon management estimates
RESULTS DRIVEN TEAM |
 Tornier is Well-Positioned for Long-Term Growth & Margin
Expansion Based upon management estimates
RESULTS DRIVEN TEAM |
 LOWER
EXTREMITIES
Tornier is Well-balanced across Upper and Lower Extremities
UPPER
EXTREMITIES
Leveraging dedicated sales reps and a broad & innovative portfolio,
Tornier plans to drive above market growth in both upper and lower
extremities |
 Extremities Market Growth driven by Demographics,
Clinical Practice Patterns, and Design Enhancement
•
Unmet need for Early-intervention
•
Revision of existing Shoulders & Ankles
•
Less-invasive and Revise-able Devices
•
Aging population –
Aging Workforce
•
Awareness to treatment options
•
“Quality of life”
expectations
Demographics
Volume Expansion
Practice Patterns
Mix Shift
•
Shift from Anatomic to Reversed Shoulder
•
Shift from Ankle Fusion to Arthroplasty
•
Increased Implant use in F&A Surgeries
Designs
Market Expansion
Simpliciti Ultra Short Stem –
Total Shoulder Platform |
 Global Upper Extremity Market driven by US Shoulder Volumes and
Surgeon Practice Patterns
US will remain > 70% of Global Market Size through ’19
•
Additional Reversed Shoulder Arthroplasty adoption
•
Increased volumes through General Orthopaedic surgeons
•
Market expansion to early intervention and later-stage revision
INT’L Markets growing at 7 –
8% CAGR through ‘19
•
Geographic expansion into emerging markets
•
New technology introduction into more mature markets
Global Shoulder Arthroplasty Market
Geographic Split
Global Shoulder Arthroplasty Market
By Segment
Ultra Short Stem Segment expected to grow > 20% CAGR
•
More reproducible / revision-friendly solution for younger pts.
•
Increased glenoid access to perform a total shoulder
•
Next generations focused on avoiding glenoid replacement
Continued uptake of reversed shoulder with 12 –
14% CAGR
•
Proven treatment options for Torn Rotator Cuffs and severe OA
•
Increased
surgeon
users
-
US
Orthopaedic
generalist
training
•
Increased access (regulatory) to product in key OUS geographies
|
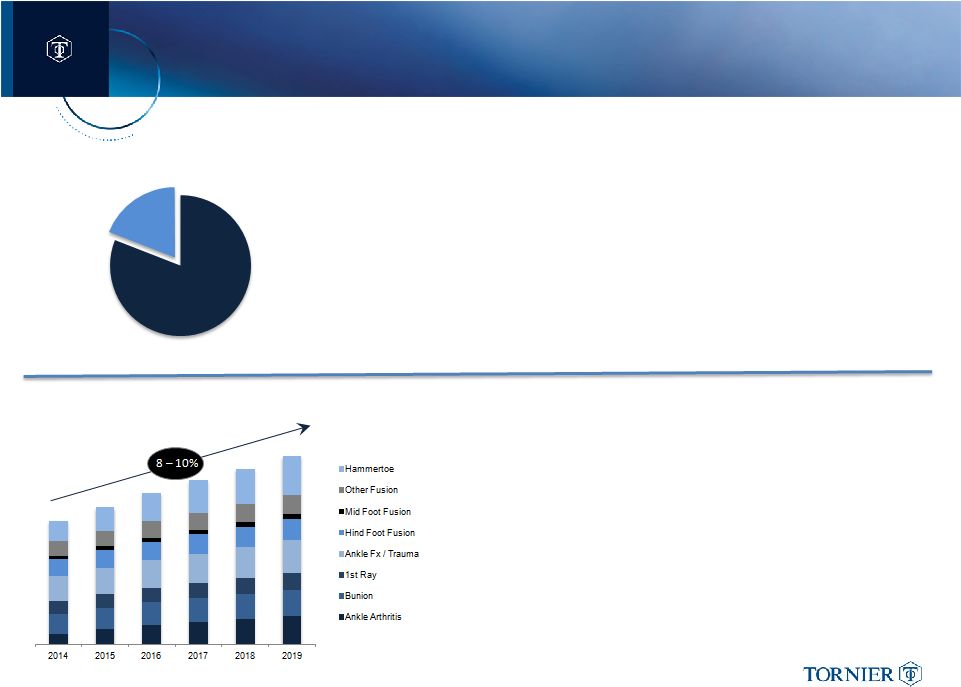 Global Lower Extremity Market Driven by US Foot & Ankle Volumes,
Indication Specific Designs and Shifts in Practice
Global Foot & Ankle Market
By Segment
Ankle Arthritis market expected to double within 4 –
5 yrs.
•
Shift in practice to TAA with ASP’s at 4x Fusion
•
Clinical data and Ease of Use driving increase adoption
Core Foot & Ankle Fusion Markets remain healthy
•
Steady volume increase expected in Hind/Mid-foot fusions
•
Increased indication specific solutions drive increased ASPs
•
“Add-on”
Biologics for fusions cases will increase market size
Hammertoe Correction Markets growing 12 –
14% CAGR
•
Steady volume increase expected
•
New Materials and designs driving higher ASPs
US represents ~80% of Global Foot and Ankle Market
•
Increasing case volumes for Orthopaedic Surgeon & Surgical
Podiatrist
•
Mix shift benefit from Ankle Fusion to Ankle Arthroplasty
•
Availability
of
“on-indication”
biologics
for
better
fusion
outcomes
INT’L Foot & Ankle Markets still early in growth curve
•
Geographic expansion into emerging markets
•
Mix shift from Ankle Fusion to Arthroplasty in more mature markets
Global Foot & Ankle Market
Geographic Split
US
INTL |
 Tornier is Well-Positioned for Long-Term Growth & Margin
Expansion Based upon management estimates
RESULTS DRIVEN TEAM |
 Tornier’s Comprehensive Portfolio Provides
Full-line Support to the Extremity Surgeon
PROCEDURES
Number of
Extremities
Products:
JOINT
REPLACEMENTS
BONE
REPAIR
BIOLOGICS
SOFT TISSUE
REPAIR
UPPER
Shoulder,
Elbow, Hand &
Wrist
LOWER
Foot & Ankle |
  Ascend
Flex
Platform
–
TSA
/
RSA
-
Flex Convertible Stem
-
PerFORM
Glenoid
-
Reversed Threaded Post Baseplate
Phantom
Fiber
–
Soft
Tissue
Fixation
Salto
Talaris
v2.1
–
Ankle
Arthroplasty
Tornier’s Current Portfolio has Products to Drive Growth and
Innovative Designs to Fuel Market Expansion
Best-in-Class Products
Current Examples in Portfolio
Addressing Unmet Needs
Products in Development Pipeline
Simpliciti
Shoulder
Ultra Short Stem Total Shoulder Arthroplasty
Pyrolytic
Carbon
Aequalis
Humeral
Head
Improved
Articulating
Surface
Salto
Talaris
XT
Total
Ankle
Arthroplasty
Revision
System
•
Improve Clinical Outcomes
•
Improve Ease-of-use / Repeatability for
Extremities Procedures
•
Offer Value-differentiated Products
Portfolio Development
Strategic Levers
I. Drive Growth:
II. Expand Extremities Markets:
•
Provide Early Intervention Solutions
•
Create Improved Revision Solutions
•
Fill Product Gaps associated with geographic
specific requirements |
 Tornier anticipates a Mid-2015 US Launch of Simpliciti,
Ultra Short Stem Total Shoulder
Ultra
Short
Stem
Estimated
Market
Opportunity:
$200m
-
$250m
•
TSA penetration of Ultra Short Stem expected to reach 25% by 2019
•
Ultra Short Stem will enable shift from Hemi-Shoulder to TSA
•
Ultra Short Stem offers TSA outcomes with less-invasive procedures
Tornier is 18 mos. ahead of Competitive US Market Entries
•
510k submission with IDE study data early 1Q 2015
•
Expected US Launch 2H15
•
2 Competitive IDEs in-progress, tracking toward 2017 FDA approvals
US Total Shoulder Arthroplasty Market
Conversion Candidates for Simpliciti
Total Shoulder Arthroplasty (TSA)
Hemi-Shoulder Arthroplasty
Resurfacing
More than 2700 cases performed OUS with excellent clinical feedback
Simpliciti Market Position
•
1
st
truly
bone
sparing
system
in
US
–
opens
new
market
category
•
Provides
Clinical
Benefits
–
simplifying
surgery
&
reducing
variables
•
Designed
for
simple
revision
/
removal
•
Expands
patient
pool
surgeons
willing
to
treat
Simpliciti Device provides economic benefits
•
Reduced
inventory
required
for
case
(40%
less
than
standard
TSA)
•
Hospital
enjoys
cost
benefits
of
less
blood
loss
and
shorter
OR
time
•
Capital
reduction
–
Instruments
approx.
50%
of
standard
TSA
sets
Simpliciti Ultra Short Stem TSA
Platform |
 Tornier is Well-Positioned for Long-Term Growth & Margin
Expansion Based upon management estimates
RESULTS DRIVEN TEAM |
 TODAY EXITING 2014
The US Sales Channel has been Restructured with
a Significant Portion now Direct Sales Territories
EXITING 2012
2014 US Revenue: ~ $200 Million
2014 US Growth Rate: 10%
US Revenues represent ~ 58% of Total Revenue |
 While Work Remains, Tornier is Focused on
Transforming to a Competitively Superior Sales Force
Timing
2012
Desired State
Agreements
& Alignment
Rep Training
& Education
Performance &
Productivity
Distributor
Negotiation
US Sales Channel Transformation
Territory
Staffing
Sales Mgmt. &
Training Org.
Uncertainty, Limited
Accountability
Uncertainty, Limited
Accountability
Strong, Reliable & Above
Market Performance |
 Tornier has a Strong International Footprint with
Direct Sales in most of the Major Geographies
Primarily direct sales
Primarily distributor
Mixed Model
Country Office
2014 OUS Revenue: ~145 Million
2014 OUS Growth Rate: ~13%
OUS Revenue represents ~ 42% of Total Revenue |
 Our International Commercial Team plans to Leverage
Three Drivers to Sustain Above-market Extremities Growth
Selectively Invest in Emerging Markets
•
Establish
Emerging
Market
Presence
with
Select
Investments
–
Set-up
a
Brazil
Office
•
Evaluate
Expansion
Plans
in
other
Emerging
Geographies
–
Evaluating
India
Options
3
Introduce New Technologies in key geographies
2
•
Launch
New
Platform
Products
Globally
–
Deploy
additional
Ascend
Flex
&
Salto-Talaris
sets
•
Bring
Technologies
to
Underpenetrated
Markets
–
Continue
Japanese
Rev.
Shoulder
Introduction
•
Broaden
Product
Offering
in
Existing
Markets
–
Expand
PyC
Humeral
Head
in
EU
&
Australia
Increased Penetration in existing geographies
•
Strengthen
and
further
focus
our
OUS
Sales
Force
-
Adding
Direct
Reps
in
Key
Markets
•
Invest
in
Strategic
Development
Activities
–
European
Foot
&
Ankle
Markets
1 |
 Tornier is Well-Positioned for Long-Term Growth & Margin
Expansion Based upon management estimates
RESULTS DRIVEN TEAM |
 Tornier plans to Expand Gross Margin and
Leverage Expenses to Deliver >20% EBITDA by FY19
Gross Margin History & Expansion Plans
Improvement Levers
EBITDA History & Improvement Plans
Improvement Drivers (in addition to GM expansion)
•
Manufacturing Efficiencies
•
Product Process Improvements
•
Insourcing / Vendor Cost Reductions
-
Overhead reductions
-
Leverage higher production volumes
-
Innovative manufacturing processes
-
Scrap reductions and lower cost materials
-
Equipment / Inspection Automation
-
Insourcing F&A Plates & Screws
-
Increased
vendor
leverage
Higher
Volumes
•
Leverage G&A Expenses
•
Sales Force Productivity Improvements
•
Leverage Commercial Infrastructure Investments
-
Training Effectiveness and Sales Channel Maturity
-
Increased revenue per procedure on covered cases
-
Fixed Expenses for Sales Training & Education
-
Fixed Expenses for National Accounts and Pricing
-
Fixed Expenses for Sales Management
-
Fixed Expenses –
Public Company costs & Sr. Management
-
ERP System enables greater process efficiency/improvement
|
 FY14
Financial Update (1)
Revenue
(2)
:
4Q:
~$96 million,
14.6% growth
FY:
~$345 million,
11.3% growth
Extremities revenue
(2)
:
4Q: ~$79 million,
16.0% growth
FY:
~$288 million,
11.7% growth
Full Year 2014 Financial and Key Metrics Update
(1) Preliminary and unaudited
(2) Constant currency, which is a non-GAAP financial measure
Key Metrics
(1) |
 Wright and Tornier Agree to Merge Creating Premier
High-Growth Extremities-Biologics Company
Combination
Offers
Comprehensive
Upper
&
Lower
Extremity
Product
Portfolio
with
Broad
Global
Reach
Further
Accelerates
Growth
Opportunities
in
Three
of
the
Fastest
Growing
Areas
in
Orthopaedics
Adds
Significant
Scale
and
Scope
to
Provide
Accelerated
Path
to
Profitability
and
Stronger
Financial
Profile
Wright
Receives
Approvable
Letter
from
FDA
for
Augment®
Bone
Graft
+
Wright Medical and Tornier agree to merge…
Announced October 27, 2014 |
 Tornier is Well-Positioned for Long-Term Growth & Margin
Expansion Based upon management estimates
RESULTS DRIVEN TEAM |
 Thank You |
