Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REPROS THERAPEUTICS INC. | v398504_8k.htm |
Exhibit 99.1
Androxal ® January, 2015 A Mechanistically Consistent Approach to the Treatment of Secondary Hypogonadism 1

Repros Disclaimer Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to various risks, uncertainties and other factors that could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by such forward - looking statements . These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “believe,” “plan,” “seek,” “could,” “can,” “should” or similar expressions . These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current conditions, expected future developments and other factors the Company believes are appropriate in these circumstances . Forward - looking statements include, but are not limited to, those relating to anticipated milestones for Androxal® and Proellex®, the conduct of planned clinical studies and the timing and nature of the results thereof, the markets for the Company’s products and the potential success of the Company in penetrating those markets and that the Company’s need for and use of financial resources . Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factors that may cause actual events to be materially different from those expressed or implied by such forward - looking statements, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of its Androxal® and Proellex® programs, the ability to have success in the clinical development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks as are identified in the Company's most recent Annual Report on Form 10 - K and the subsequent quarterly report on Form 10 - Q and in the prospectus supplement and the accompanying prospectus included in the registration statement mentioned below . These documents are available on request from Repros or at www . sec . gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise . In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry . We obtained this information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates . Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information . While we believe that each of these studies and publications is reliable, we have not independently verified such data, and we make no any representation as to the accuracy of such information . Similarly, we believe our internal research is reliable, but it has not been verified by any independent sources . 2
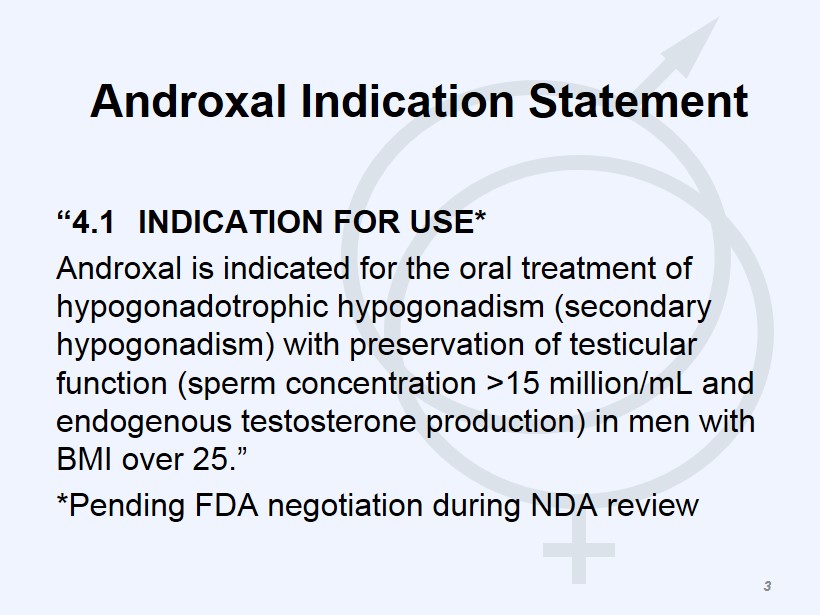
Androxal Indication Statement “4.1 INDICATION FOR USE* Androxal is indicated for the oral treatment of hypogonadotrophic hypogonadism (secondary hypogonadism) with preservation of testicular function (sperm concentration >15 million/mL and endogenous testosterone production) in men with BMI over 25.” *Pending FDA negotiation during NDA review 3
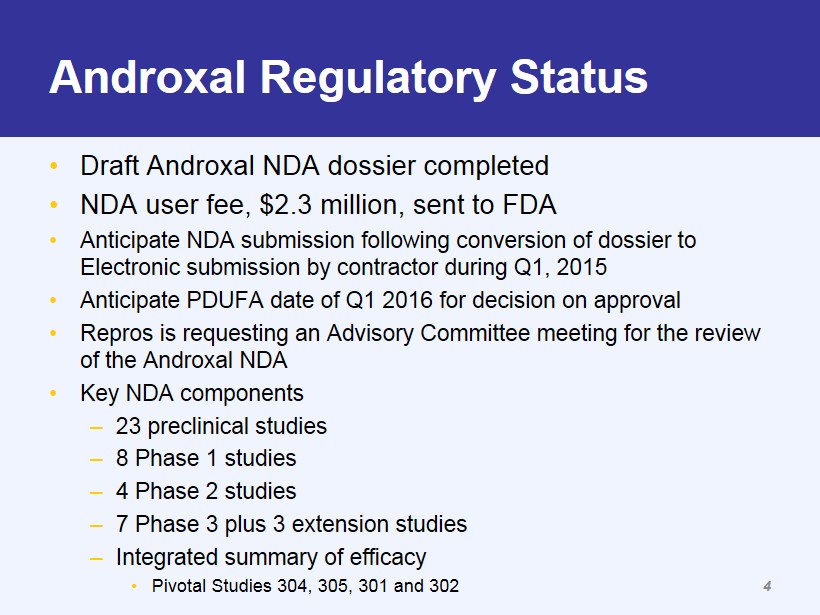
Androxal Regulatory Status • Draft Androxal NDA dossier completed • NDA user fee, $2.3 million, sent to FDA • Anticipate NDA submission following conversion of dossier to Electronic submission by contractor during Q1, 2015 • Anticipate PDUFA date of Q1 2016 for decision on approval • Repros is requesting an Advisory Committee meeting for the review of the Androxal NDA • Key NDA components – 23 preclinical studies – 8 Phase 1 studies – 4 Phase 2 studies – 7 Phase 3 plus 3 extension studies – Integrated summary of efficacy • Pivotal Studies 304, 305, 301 and 302 4
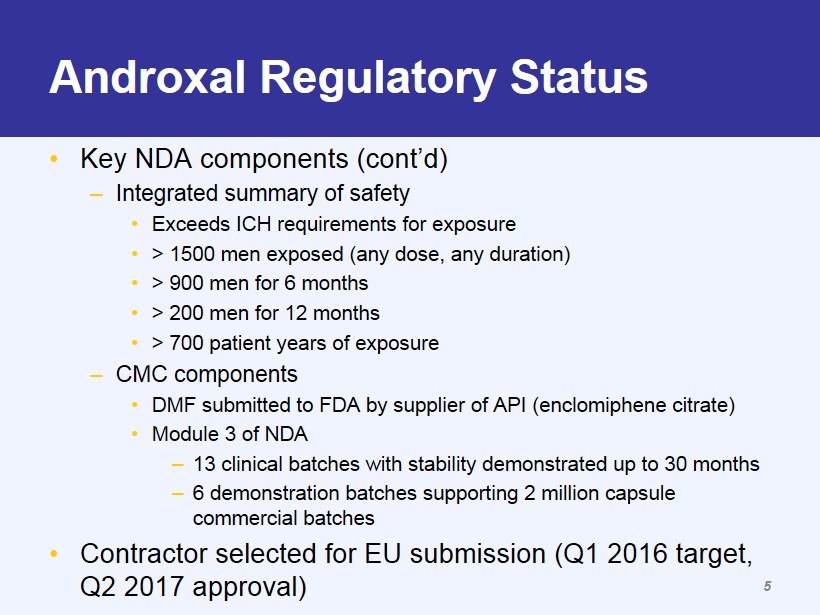
Androxal Regulatory Status • Key NDA components (cont’d) – Integrated summary of safety • Exceeds ICH requirements for exposure • > 1500 men exposed (any dose, any duration) • > 900 men for 6 months • > 200 men for 12 months • > 700 patient years of exposure – CMC components • DMF submitted to FDA by supplier of API (enclomiphene citrate) • Module 3 of NDA – 13 clinical batches with stability demonstrated up to 30 months – 6 demonstration batches supporting 2 million capsule commercial batches • Contractor selected for EU submission (Q1 2016 target, Q2 2017 approval) 5

Androxal Intellectual Property • FAMILY 1 – TRANS - CLOMIPHENE FOR TREATING TESTOSTERONE DEFICIENCY (BASE PATENT FAMILY) – US10/483,458, Granted 7,759,360, March 19, 2023 in US, July 9, 2022 elsewhere • FAMILY 2 – TRANS - CLOMIPHENE DOSING REGIMES – Priority – US 60/664,290 Filed March 22, 2005 • FAMILY 3 – TRANS - CLOMIPHENE FOR MALE INFERTILITY – Priority – US 60/650,018 Filed February 4, 2005 • FAMILY 4 – TRANS - CLOMIPHENE FOR METABOLIC SYNDROME AND TYPE 2 DIABETES – Priority – US 60/980,334 Filed October 16, 2007 • FAMILY 5 – TRANS - CLOMIPHENE METABOLITES AND PHARMACEUTICAL USES THEREOF – Priority – US 61/515,278 Filed August 4, 2011 • FAMILY 6 – SERMS WITH SHORT HALF - LIVES FOR USE IN THERAPEUTIC METHODS – Priority – US 61/598,723 Filed February 14, 2012 • FAMILY 7 – COMBINATION THERAPY FOR TREATING ANDROGEN DEFICIENCY – Priority – US 61/604,989 Filed February 29, 2012 • Recent court decision ruling core 360 patent rightfully owned by Repros 6

Key Literature Observation Regarding Secondary Hypogonadism and Repros Clinical Findings from Androxal Studies 7
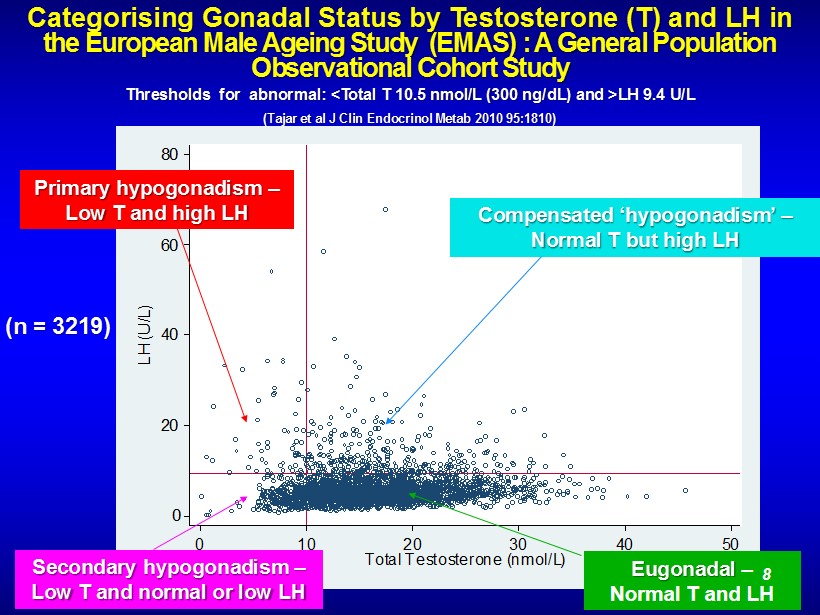
Categorising Gonadal Status by Testosterone (T) and LH in the European Male Ageing Study (EMAS) : A General Population Observational Cohort Study Thresholds for abnormal: <Total T 10.5 nmol /L (300 ng/ dL ) and >LH 9.4 U/L (Tajar et al J Clin Endocrinol Metab 2010 95:1810) 0 20 40 60 80 LH (U/L) 0 10 20 30 40 50 Total Testosterone (nmol/L) Secondary hypogonadism – Low T and normal or low LH Eugonadal – Normal T and LH Primary hypogonadism – Low T and high LH Compensated ‘ hypogonadism ’ – Normal T but high LH (n = 3219) 8
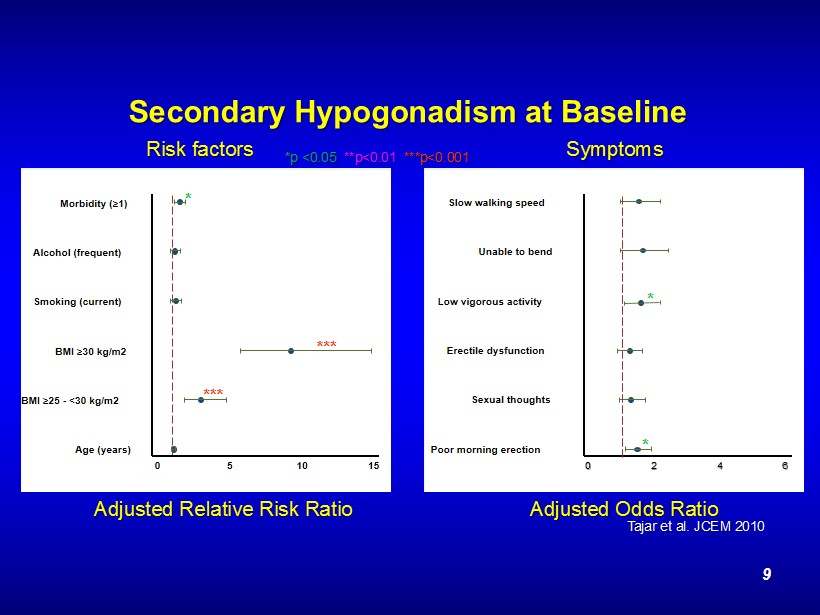
Secondary Hypogonadism at Baseline Age (years) BMI ≥ 25 - <30 kg/m2 BMI ≥ 30 kg/m2 Smoking (current) Alcohol (frequent) Morbidity ( ≥ 1) 0 5 10 15 *** *** * Poor morning erection Sexual thoughts Erectile dysfunction Low vigorous activity Unable to bend Slow walking speed 0 2 4 6 * * Adjusted Relative Risk Ratio *p <0.05 **p<0.01 ***p<0.001 Adjusted Odds Ratio Risk factors Symptoms Tajar et al. JCEM 2010 9
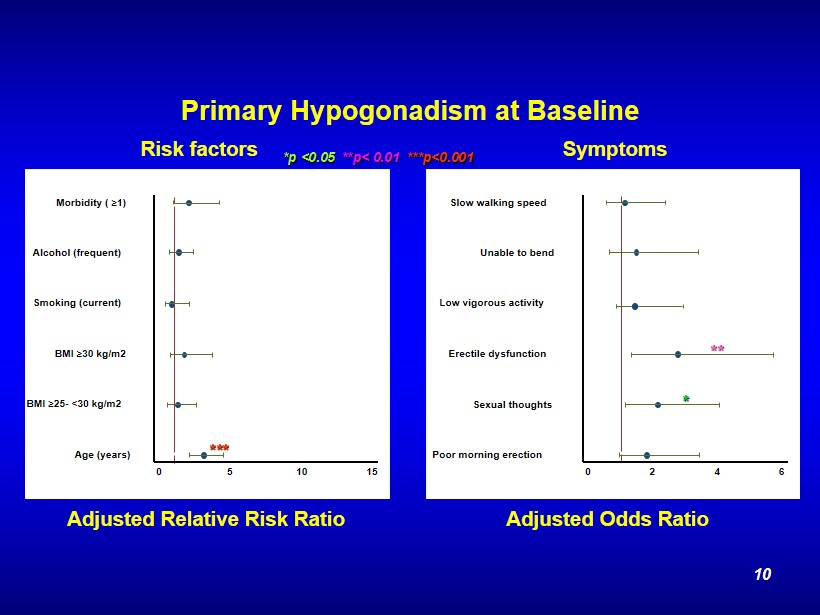
Primary Hypogonadism at Baseline Poor morning erection Sexual thoughts Erectile dysfunction Low vigorous activity Unable to bend Slow walking speed 0 2 4 6 Age (years) BMI ≥ 25 - <30 kg/m2 BMI ≥ 30 kg/m2 Smoking (current) Alcohol (frequent) Morbidity ( ≥ 1) 0 5 10 15 *** * ** *p <0.05 **p< 0.01 ***p<0.001 Adjusted Relative Risk Ratio Adjusted Odds Ratio Risk factors Symptoms 10
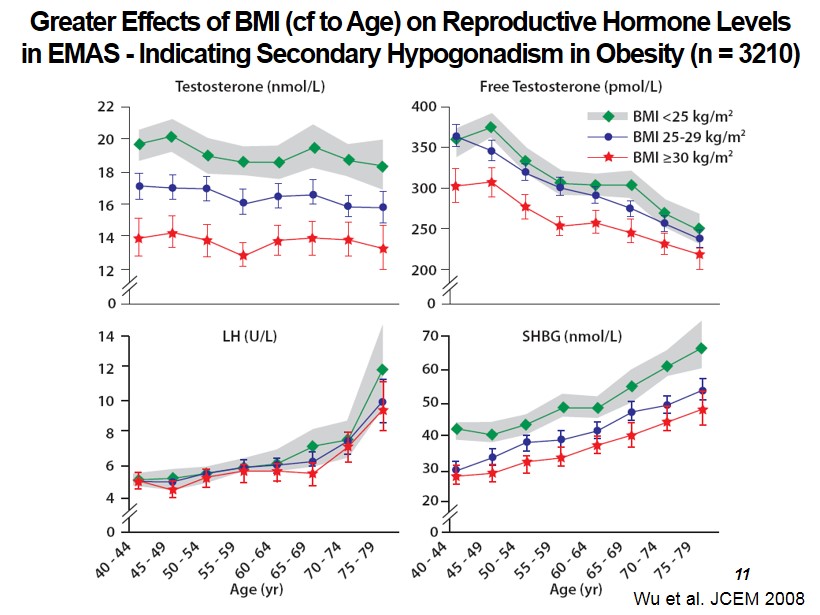
Wu et al. JCEM 2008 Greater Effects of BMI ( cf to Age) on Reproductive Hormone Levels in EMAS - Indicating Secondary Hypogonadism in Obesity (n = 3210) 11
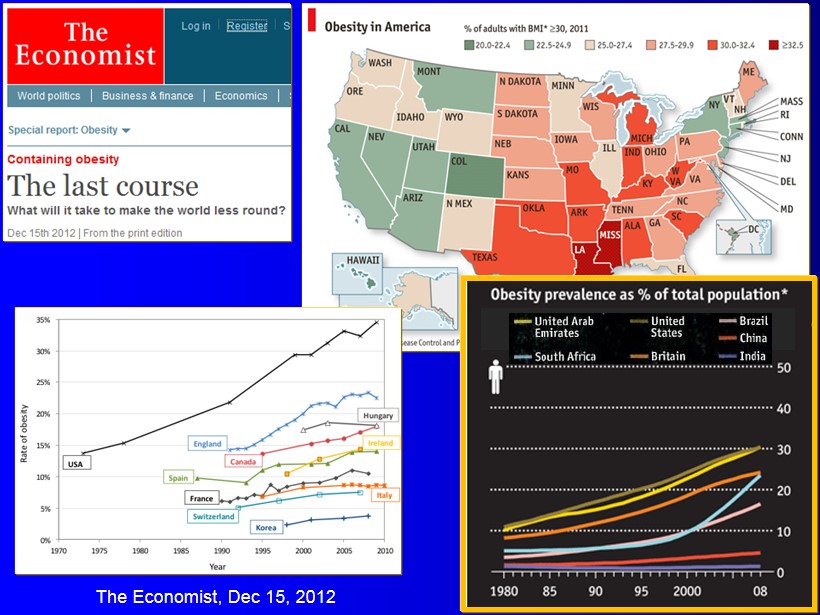
The Economist, Dec 15, 2012
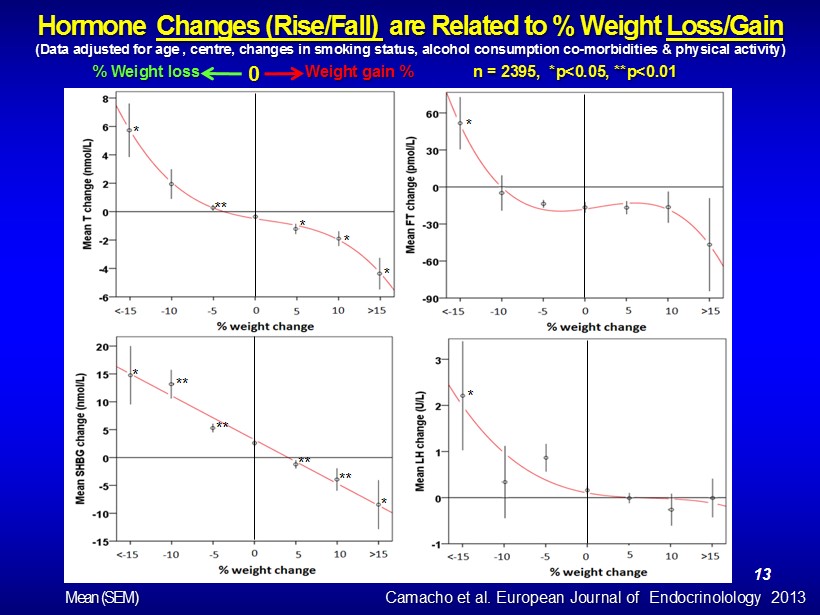
Hormone Changes (Rise/Fall) are Related to % Weight Loss/Gain (Data adjusted for age , centre, changes in smoking status, alcohol consumption co - morbidities & physical activity) n = 2395, *p<0.05, **p<0.01 % Weight loss Weight gain % 0 * ** * * * * * * ** ** ** ** * Camacho et al. European Journal of Endocrinolology 2013 Mean (SEM) 13
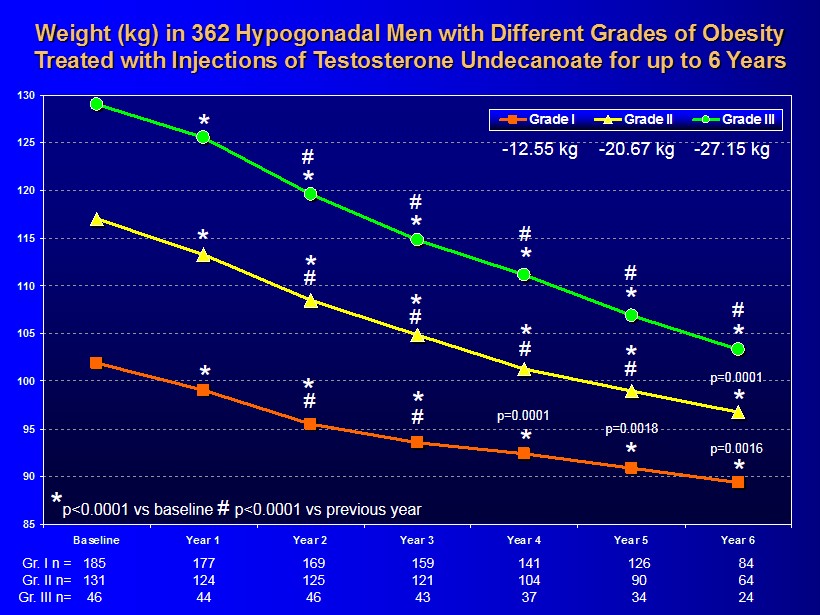
85 90 95 100 105 110 115 120 125 130 Baseline Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Grade I Grade II Grade III Weight (kg) in 362 Hypogonadal Men with Different Grades of Obesity Treated with Injections of Testosterone Undecanoate for up to 6 Years * * * * 185 159 141 126 84 177 169 Gr. I n = * 131 121 104 90 64 124 125 Gr. II n= 46 43 37 34 24 44 46 Gr. III n= * * * * * * * * * * * * # # # # # # # # # # p=0.0001 p=0.0018 * # p=0.0001 p=0.0016 - 12.55 kg - 20.67 kg - 27.15 kg * p<0.0001 vs baseline # p<0.0001 vs previous year
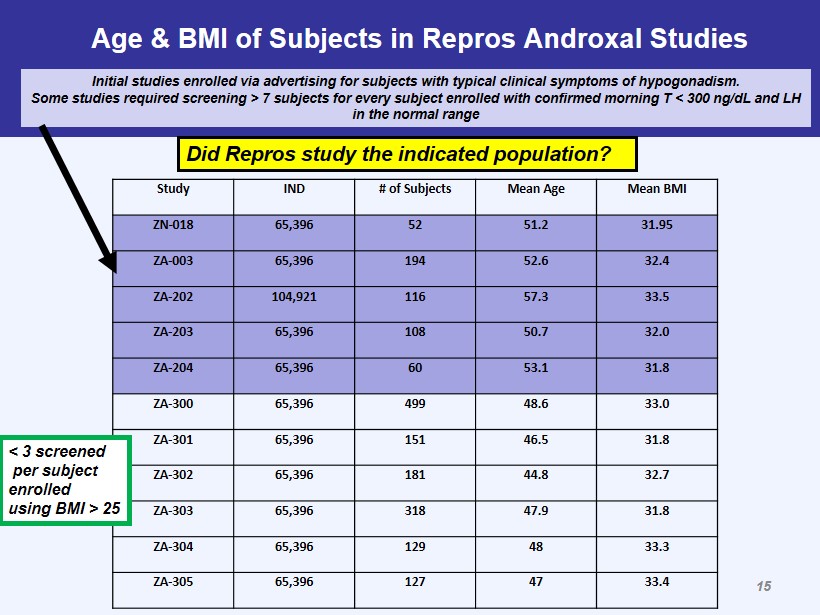
Age & BMI of Subjects in Repros Androxal Studies Study IND # of Subjects Mean Age Mean BMI ZN - 018 65,396 52 51.2 31.95 ZA - 003 65,396 194 52.6 32.4 ZA - 202 104,921 116 57.3 33.5 ZA - 203 65,396 108 50.7 32.0 ZA - 204 65,396 60 53.1 31.8 ZA - 300 65,396 499 48.6 33.0 ZA - 301 65,396 151 46.5 31.8 ZA - 302 65,396 181 44.8 32.7 ZA - 303 65,396 318 47.9 31.8 ZA - 304 65,396 129 48 33.3 ZA - 305 65,396 127 47 33.4 Initial studies enrolled via advertising for subjects with typical clinical symptoms of hypogonadism. Some studies required screening > 7 subjects for every subject enrolled with confirmed morning T < 300 ng/ dL and LH in the normal range < 3 screened per subject enrolled using BMI > 25 Did Repros study the indicated population? 15

Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Two Separate Tracks of Hypogonadism 1. Secondary hypogonadism associated with obesity , independent of age and reversible 2. Primary hypogonadism - associated with ageing , independent of obesity, largely irreversible • Distinct clinical features and outcomes between tracks/categories • Require different management strategies
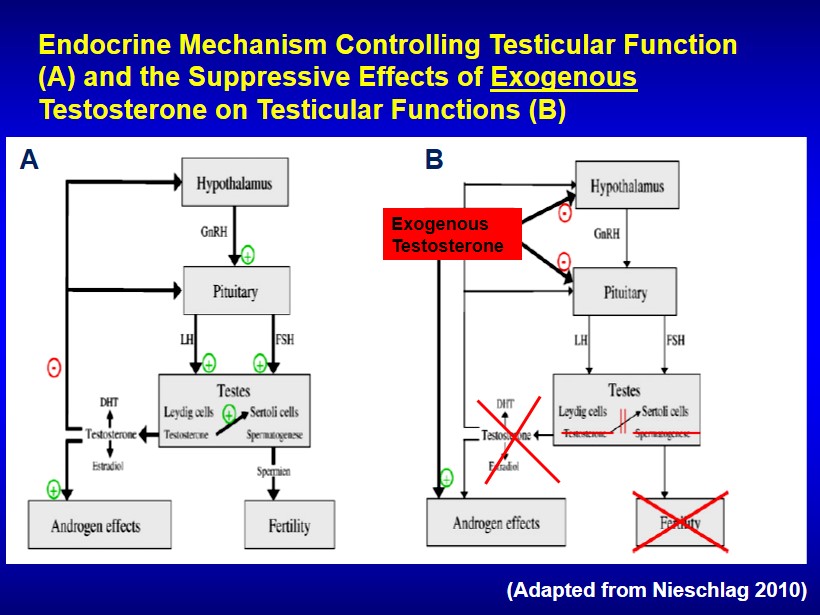
A B Endocrine Mechanism Controlling Testicular Function (A) and the Suppressive Effects of Exogenous Testosterone on Testicular Functions (B) (Adapted from Nieschlag 2010) Exogenous Testosterone

Androxal ® Efficacy A Mechanistically Consistent Approach to the Treatment of Secondary Hypogonadism 18
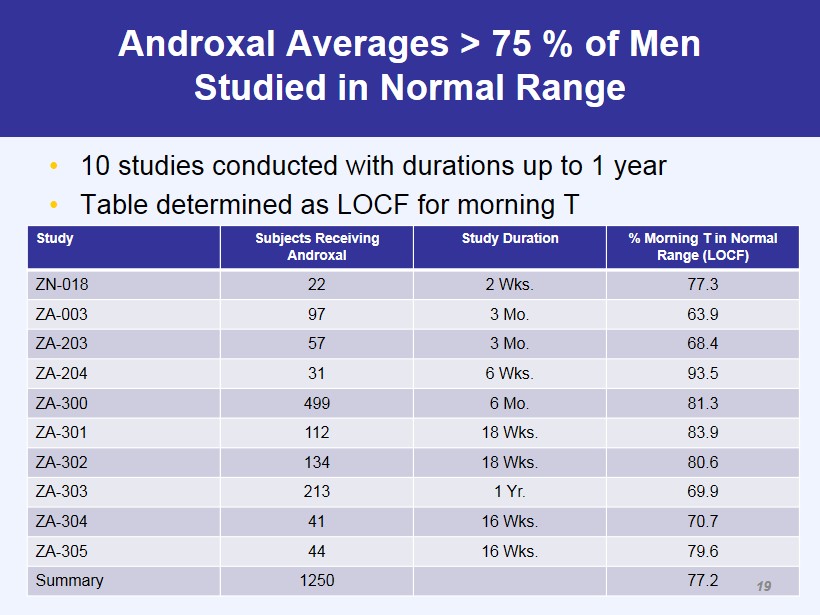
Androxal Averages > 75 % of Men Studied in Normal Range • 10 studies conducted with durations up to 1 year • Table determined as LOCF for morning T Study Subjects Receiving Androxal Study Duration % Morning T in Normal Range (LOCF) ZN - 018 22 2 Wks. 77.3 ZA - 003 97 3 Mo. 63.9 ZA - 203 57 3 Mo. 68.4 ZA - 204 31 6 Wks. 93.5 ZA - 300 499 6 Mo. 81.3 ZA - 301 112 18 Wks. 83.9 ZA - 302 134 18 Wks. 80.6 ZA - 303 213 1 Yr. 69.9 ZA - 304 41 16 Wks. 70.7 ZA - 305 44 16 Wks. 79.6 Summary 1250 77.2 19
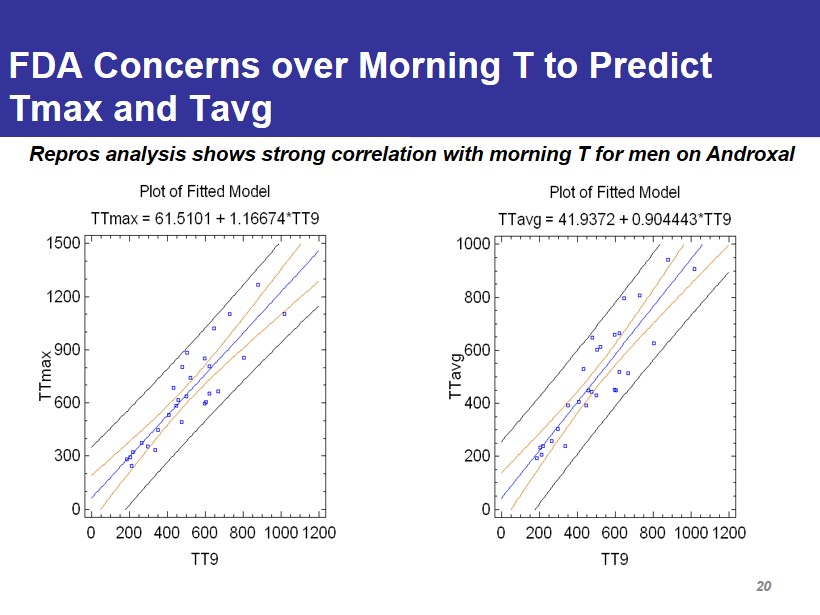
FDA Concerns over Morning T to Predict Tmax and Tavg Repros analysis shows strong correlation with morning T for men on Androxal 20
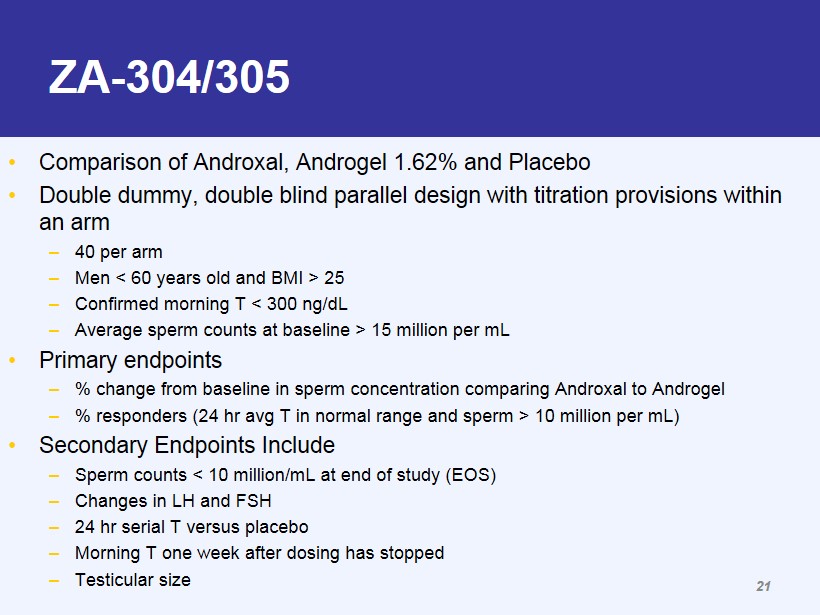
ZA - 304/305 • Comparison of Androxal, Androgel 1.62% and Placebo • Double dummy, double blind parallel design with titration provisions within an arm – 40 per arm – Men < 60 years old and BMI > 25 – Confirmed morning T < 300 ng/dL – Average sperm counts at baseline > 15 million per mL • Primary endpoints – % change from baseline in sperm concentration comparing Androxal to Androgel – % responders (24 hr avg T in normal range and sperm > 10 million per mL) • Secondary Endpoints Include – Sperm counts < 10 million/mL at end of study (EOS) – Changes in LH and FSH – 24 hr serial T versus placebo – Morning T one week after dosing has stopped – Testicular size 21
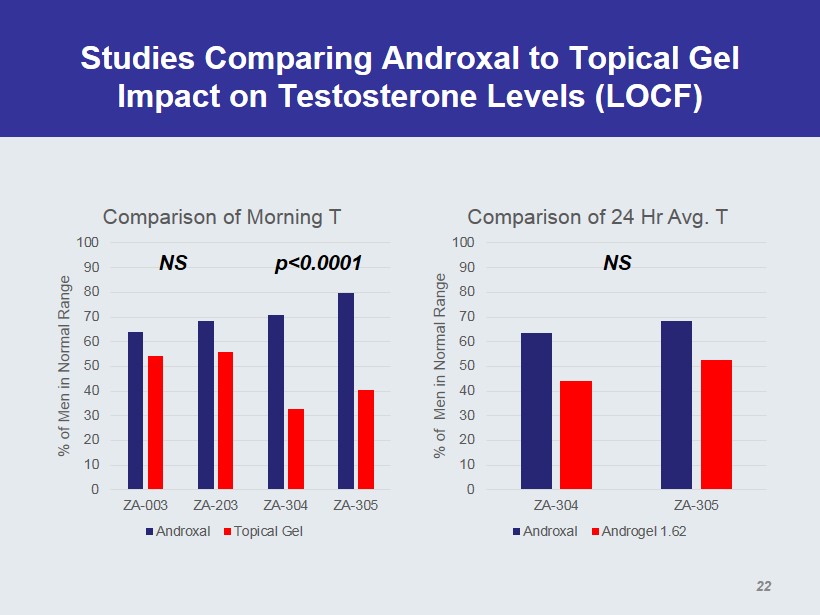
Studies Comparing Androxal to Topical Gel Impact on Testosterone Levels (LOCF) 0 10 20 30 40 50 60 70 80 90 100 ZA-003 ZA-203 ZA-304 ZA-305 % of Men in Normal Range Comparison of Morning T Androxal Topical Gel 0 10 20 30 40 50 60 70 80 90 100 ZA-304 ZA-305 % of Men in Normal Range Comparison of 24 Hr Avg. T Androxal Androgel 1.62 p<0.0001 NS NS 22
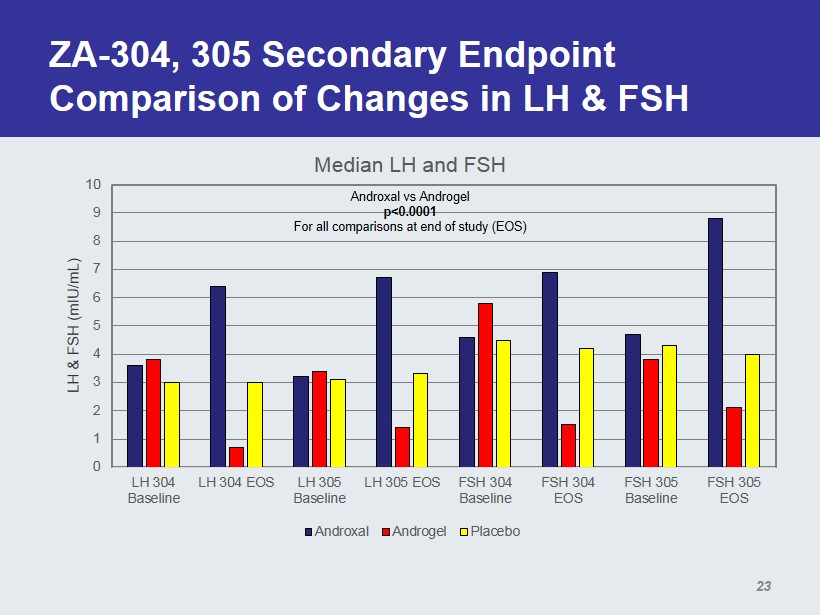
ZA - 304, 305 Secondary Endpoint Comparison of Changes in LH & FSH 0 1 2 3 4 5 6 7 8 9 10 LH 304 Baseline LH 304 EOS LH 305 Baseline LH 305 EOS FSH 304 Baseline FSH 304 EOS FSH 305 Baseline FSH 305 EOS LH & FSH (mIU/mL) Median LH and FSH Androxal Androgel Placebo Androxal vs Androgel p<0.0001 For all comparisons at end of study (EOS) 23
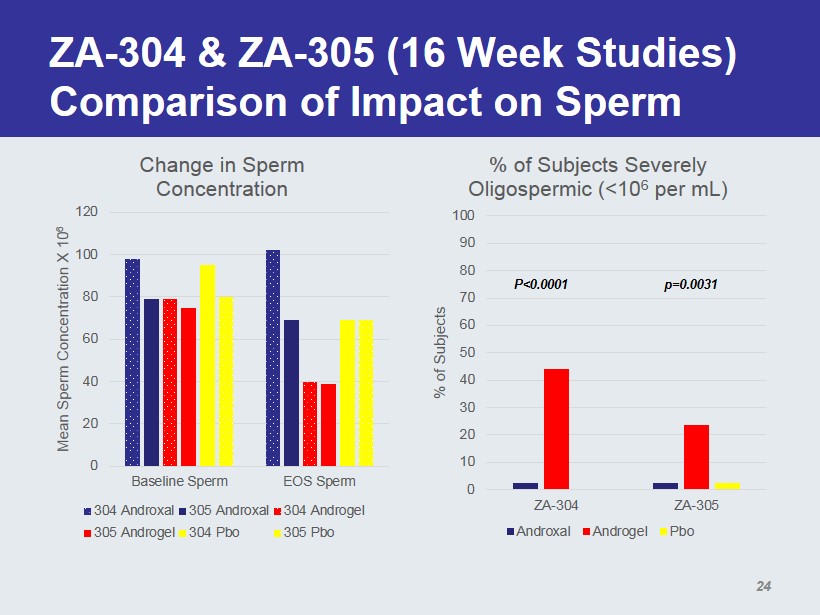
ZA - 304 & ZA - 305 (16 Week Studies) Comparison of Impact on Sperm 0 20 40 60 80 100 120 Baseline Sperm EOS Sperm Mean Sperm Concentration X 10 6 Change in Sperm Concentration 304 Androxal 305 Androxal 304 Androgel 305 Androgel 304 Pbo 305 Pbo 0 10 20 30 40 50 60 70 80 90 100 ZA-304 ZA-305 % of Subjects % of Subjects Severely Oligospermic (<10 6 per mL) Androxal Androgel Pbo p=0.0031 P<0.0001 24
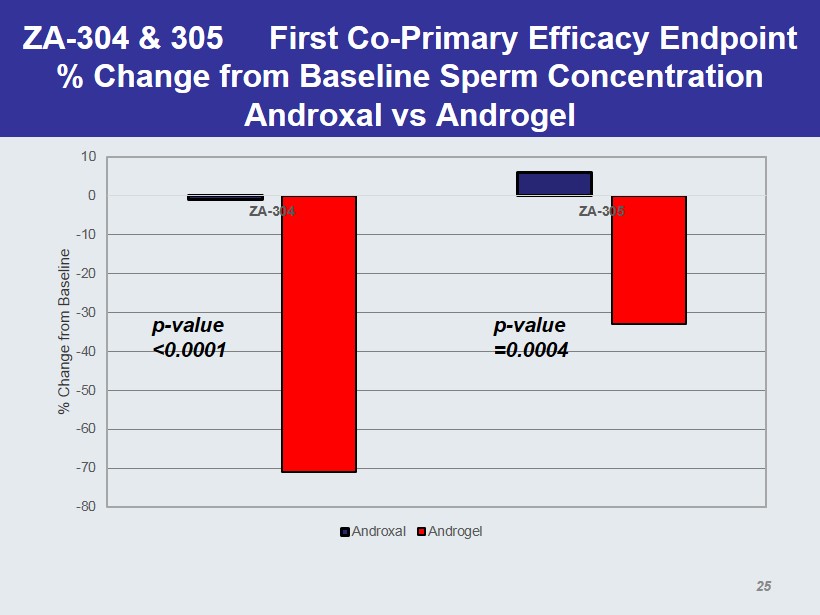
ZA - 304 & 305 First Co - Primary Efficacy Endpoint % Change from Baseline Sperm Concentration Androxal vs Androgel -80 -70 -60 -50 -40 -30 -20 -10 0 10 ZA-304 ZA-305 % Change from Baseline Androxal Androgel p - value <0.0001 p - value =0.0004 25
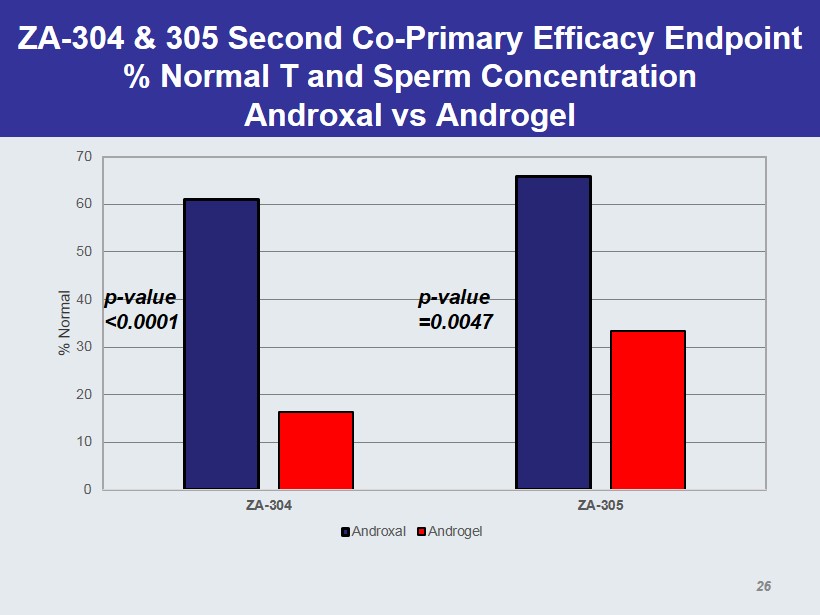
ZA - 304 & 305 Second Co - Primary Efficacy Endpoint % Normal T and Sperm Concentration Androxal vs Androgel 0 10 20 30 40 50 60 70 ZA-304 ZA-305 % Normal Androxal Androgel p - value <0.0001 p - value =0.0047 26
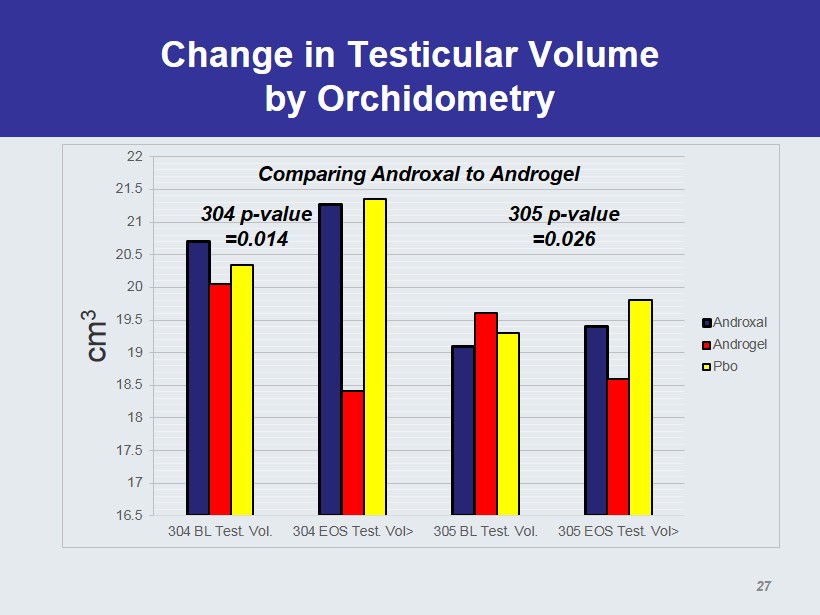
Change in Testicular Volume by Orchidometry 16.5 17 17.5 18 18.5 19 19.5 20 20.5 21 21.5 22 304 BL Test. Vol. 304 EOS Test. Vol> 305 BL Test. Vol. 305 EOS Test. Vol> cm 3 Androxal Androgel Pbo 304 p - value =0.014 305 p - value =0.026 Comparing Androxal to Androgel 27
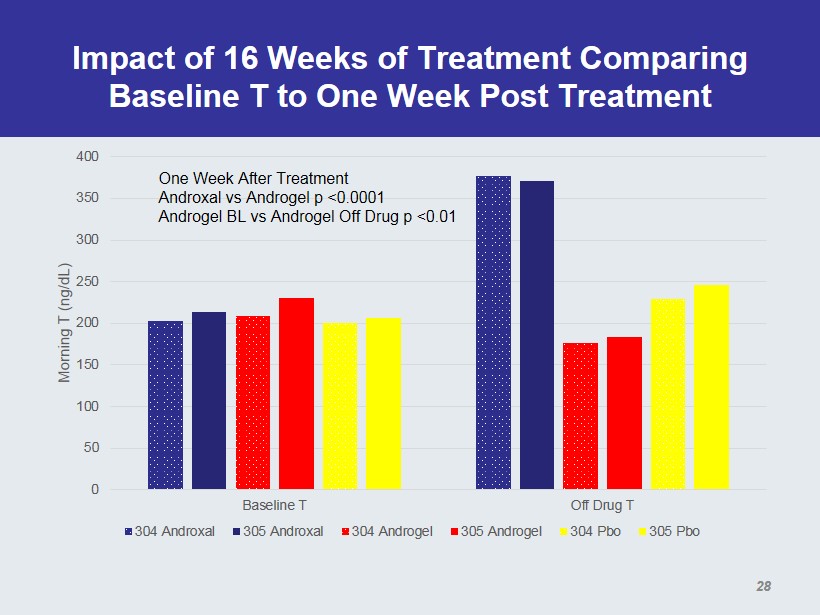
Impact of 16 Weeks of Treatment Comparing Baseline T to One Week Post Treatment 0 50 100 150 200 250 300 350 400 Baseline T Off Drug T Morning T (ng/ dL ) 304 Androxal 305 Androxal 304 Androgel 305 Androgel 304 Pbo 305 Pbo One Week After Treatment Androxal vs Androgel p <0.0001 Androgel BL vs Androgel Off Drug p <0.01 28
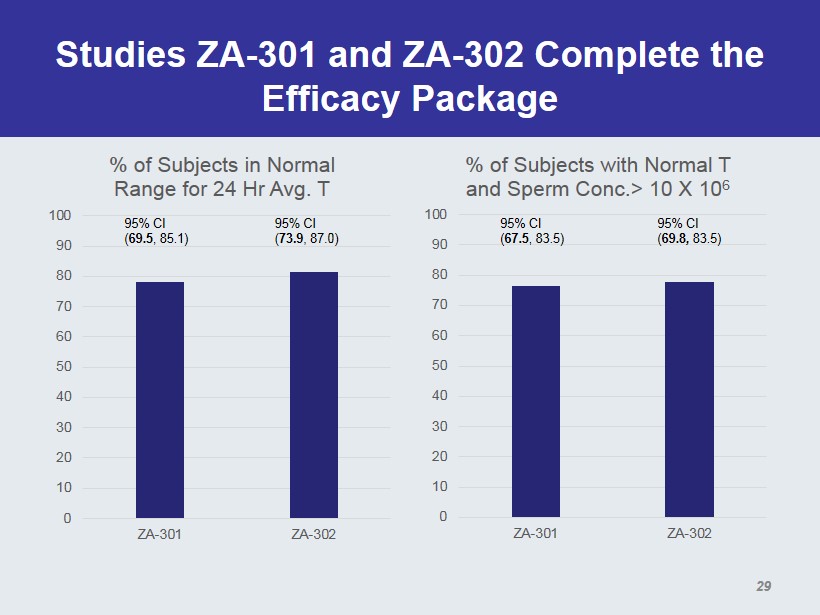
Studies ZA - 301 and ZA - 302 Complete the Efficacy Package 0 10 20 30 40 50 60 70 80 90 100 ZA-301 ZA-302 % of Subjects in Normal Range for 24 Hr Avg. T 95% CI ( 69.5 , 85.1) 95% CI ( 73.9 , 87.0) 0 10 20 30 40 50 60 70 80 90 100 ZA-301 ZA-302 % of Subjects with Normal T and Sperm Conc.> 10 X 10 6 95% CI ( 67.5 , 83.5) 95% CI ( 69.8 , 83.5) 29

Androxal ® Safety A Generally Well Tolerated Approach to the Treatment of Secondary Hypogonadism 30
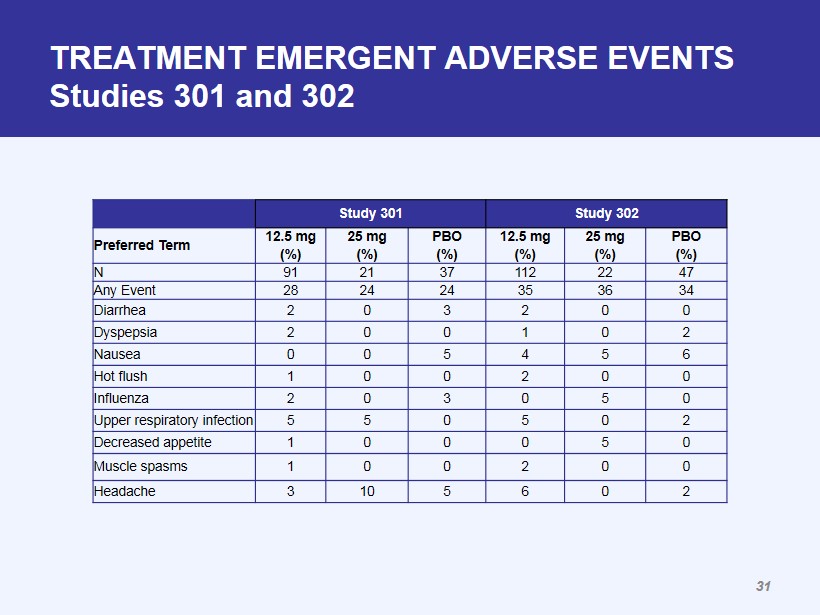
TREATMENT EMERGENT ADVERSE EVENTS Studies 301 and 302 Study 301 Study 302 Preferred Term 12.5 mg (%) 25 mg (%) PBO (%) 12.5 mg (%) 25 mg (%) PBO (%) N 91 21 37 112 22 47 Any Event 28 24 24 35 36 34 Diarrhea 2 0 3 2 0 0 Dyspepsia 2 0 0 1 0 2 Nausea 0 0 5 4 5 6 Hot flush 1 0 0 2 0 0 Influenza 2 0 3 0 5 0 Upper respiratory infection 5 5 0 5 0 2 Decreased appetite 1 0 0 0 5 0 Muscle spasms 1 0 0 2 0 0 Headache 3 10 5 6 0 2 31
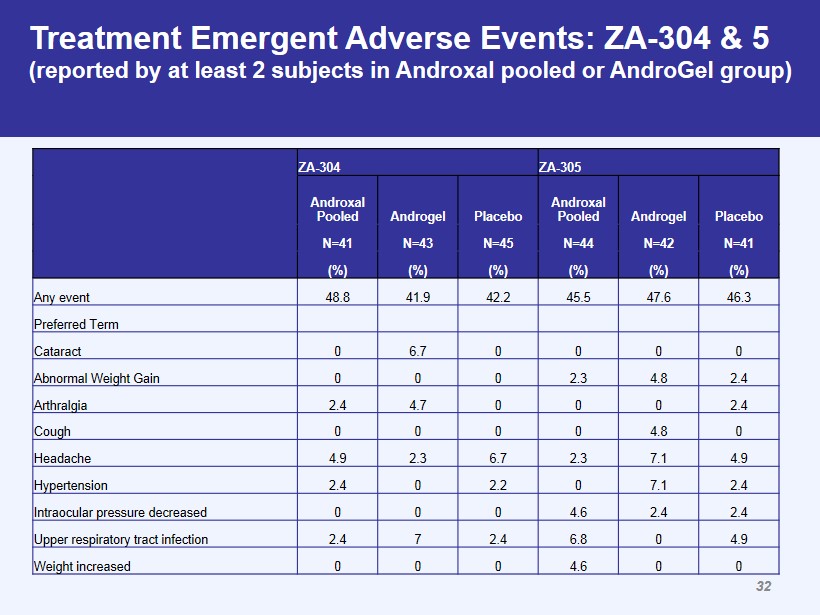
ZA - 304 ZA - 305 Androxal Pooled Androgel Placebo Androxal Pooled Androgel Placebo N=41 N=43 N=45 N=44 N=42 N=41 (%) (%) (%) (%) (%) (%) Any event 48.8 41.9 42.2 45.5 47.6 46.3 Preferred Term Cataract 0 6.7 0 0 0 0 Abnormal Weight Gain 0 0 0 2.3 4.8 2.4 Arthralgia 2.4 4.7 0 0 0 2.4 Cough 0 0 0 0 4.8 0 Headache 4.9 2.3 6.7 2.3 7.1 4.9 Hypertension 2.4 0 2.2 0 7.1 2.4 Intraocular pressure decreased 0 0 0 4.6 2.4 2.4 Upper respiratory tract infection 2.4 7 2.4 6.8 0 4.9 Weight increased 0 0 0 4.6 0 0 Treatment Emergent Adverse Events: ZA - 304 & 5 (reported by at least 2 subjects in Androxal pooled or AndroGel group) 32
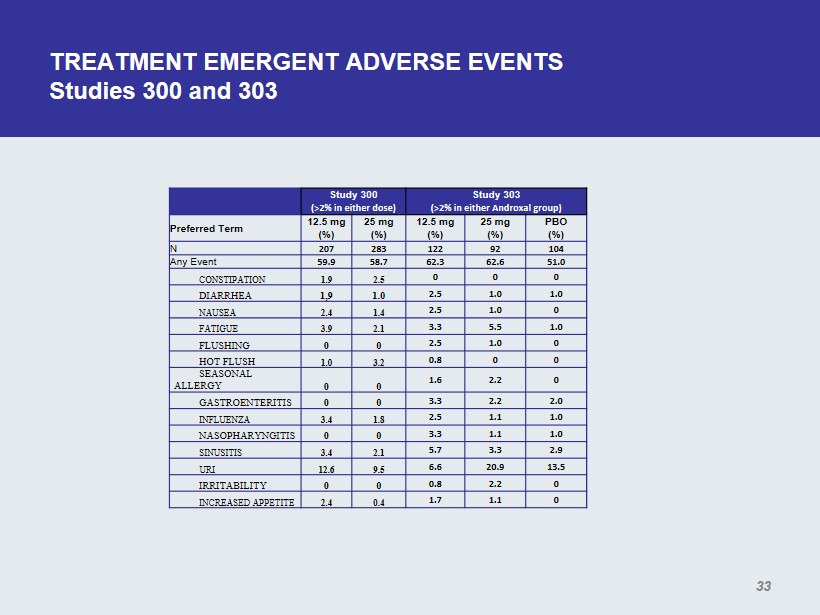
TREATMENT EMERGENT ADVERSE EVENTS Studies 300 and 303 Study 300 (>2% in either dose) Study 303 (>2% in either Androxal group ) Preferred Term 12.5 mg (%) 25 mg (%) 12.5 mg (%) 25 mg (%) PBO (%) N 207 283 122 92 104 Any Event 59.9 58.7 62.3 62.6 51.0 CONSTIPATION 1.9 2.5 0 0 0 DIARRHEA 1,9 1.0 2.5 1.0 1.0 NAUSEA 2.4 1.4 2.5 1.0 0 FATIGUE 3.9 2.1 3.3 5.5 1.0 FLUSHING 0 0 2.5 1.0 0 HOT FLUSH 1.0 3.2 0.8 0 0 SEASONAL ALLERGY 0 0 1.6 2.2 0 GASTROENTERITIS 0 0 3.3 2.2 2.0 INFLUENZA 3.4 1.8 2.5 1.1 1.0 NASOPHARYNGITIS 0 0 3.3 1.1 1.0 SINUSITIS 3.4 2.1 5.7 3.3 2.9 URI 12.6 9.5 6.6 20.9 13.5 IRRITABILITY 0 0 0.8 2.2 0 INCREASED APPETITE 2.4 0.4 1.7 1.1 0 33
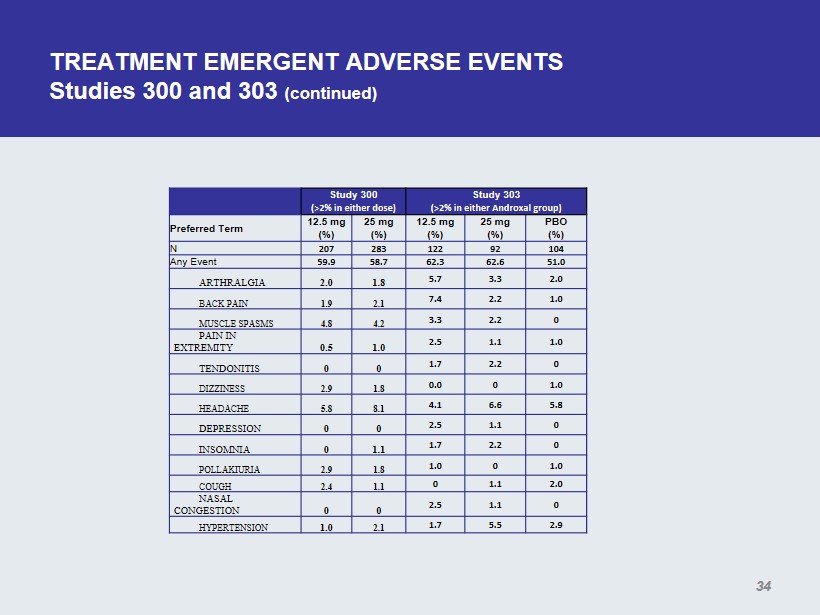
TREATMENT EMERGENT ADVERSE EVENTS Studies 300 and 303 (continued) Study 300 (>2% in either dose) Study 303 (>2% in either Androxal group ) Preferred Term 12.5 mg (%) 25 mg (%) 12.5 mg (%) 25 mg (%) PBO (%) N 207 283 122 92 104 Any Event 59.9 58.7 62.3 62.6 51.0 ARTHRALGIA 2.0 1.8 5.7 3.3 2.0 BACK PAIN 1.9 2.1 7.4 2.2 1.0 MUSCLE SPASMS 4.8 4.2 3.3 2.2 0 PAIN IN EXTREMITY 0.5 1.0 2.5 1.1 1.0 TENDONITIS 0 0 1.7 2.2 0 DIZZINESS 2.9 1.8 0.0 0 1.0 HEADACHE 5.8 8.1 4.1 6.6 5.8 DEPRESSION 0 0 2.5 1.1 0 INSOMNIA 0 1.1 1.7 2.2 0 POLLAKIURIA 2.9 1.8 1.0 0 1.0 COUGH 2.4 1.1 0 1.1 2.0 NASAL CONGESTION 0 0 2.5 1.1 0 HYPERTENSION 1.0 2.1 1.7 5.5 2.9 34

Financial Summary • Cash and equivalents (unaudited year end 2014) $46.6 M • Estimated Burn in 2015 : $22.0 M • Cash runway: Through 2016 – Anticipate Androxal approved • Current shares outstanding: 24.3 M shares – Warrants Outstanding – Series A – 39,595 (purchased in unit deal @ $2.45); Series B – 429,704 @ $2.49 exercise price.
CONCLUSIONS • Androxal is well tolerated • Findings are consistent with known pharmacology • Thrombotic events (with or without Hct increase) are an established risk (similar to topical testosterone) • No unexpected safety issues 36

Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Clinical Implications • Secondary hypogonadism is closely associated with obesity and reversible suppression of hypothalamic/pituitary function • Diagnostic differentiation of secondary from primary hypogonadism is essential – measure T and LH • First line standard of care for obese men with low T should be weight loss • Strategies to mitigate gonadotrophin suppression thereby raising endogenous T and preserving fertility are preferable to exogenous T treatment in men with secondary hypogonadism

Summary of Provider and Payer Perspectives Regarding Androxal October 31, 2014
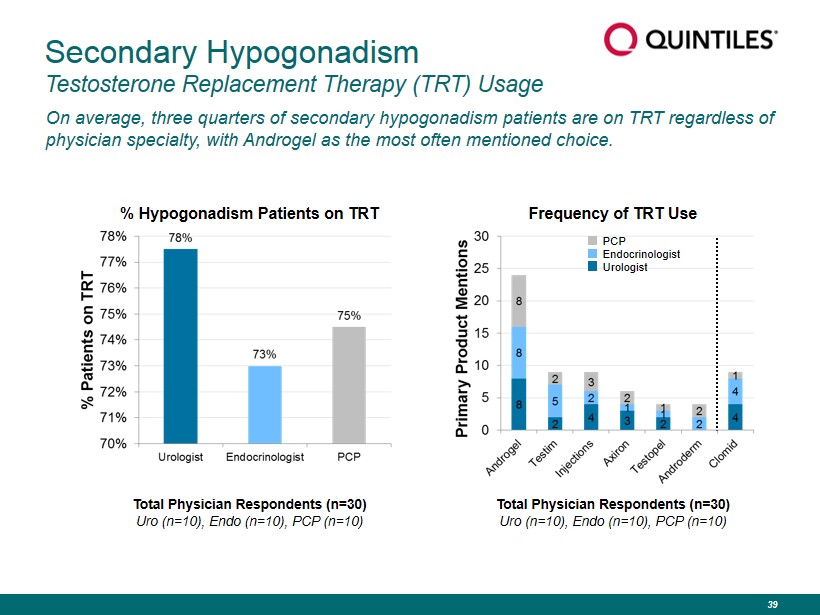
Secondary Hypogonadism Testosterone Replacement Therapy (TRT) Usage On average, three quarters of secondary hypogonadism patients are on TRT regardless of physician specialty, with Androgel as the most often mentioned choice. Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) Frequency of TRT Use % Hypogonadism Patients on TRT PCP Endocrinologist Urologist Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) 39
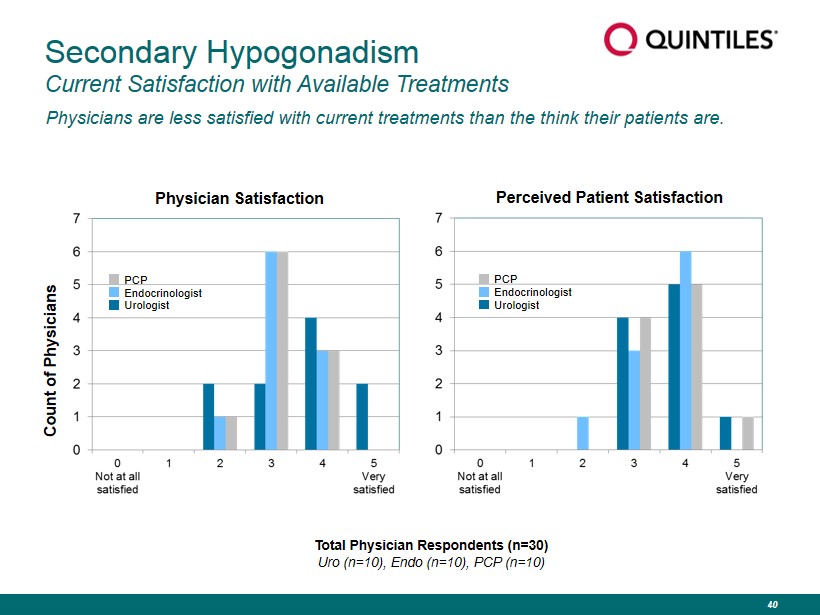
Secondary Hypogonadism Current Satisfaction with Available Treatments Physicians are less satisfied with current treatments than the think their patients are. Physician Satisfaction Perceived Patient Satisfaction Count of Physicians Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) PCP Endocrinologist Urologist PCP Endocrinologist Urologist 40
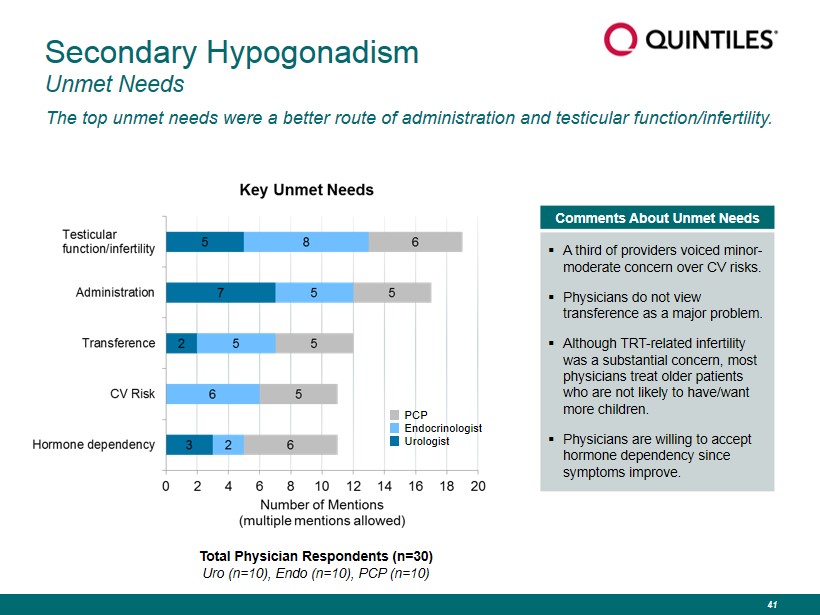
Secondary Hypogonadism Unmet Needs The top unmet needs were a better route of administration and testicular function/infertility. Comments About Unmet Needs ▪ A third of providers voiced minor - moderate concern over CV risks. ▪ Physicians do not view transference as a major problem. ▪ Although TRT - related infertility was a substantial concern, most physicians treat older patients who are not likely to have/want more children. ▪ Physicians are willing to accept hormone dependency since symptoms improve. PCP Endocrinologist Urologist Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) 41
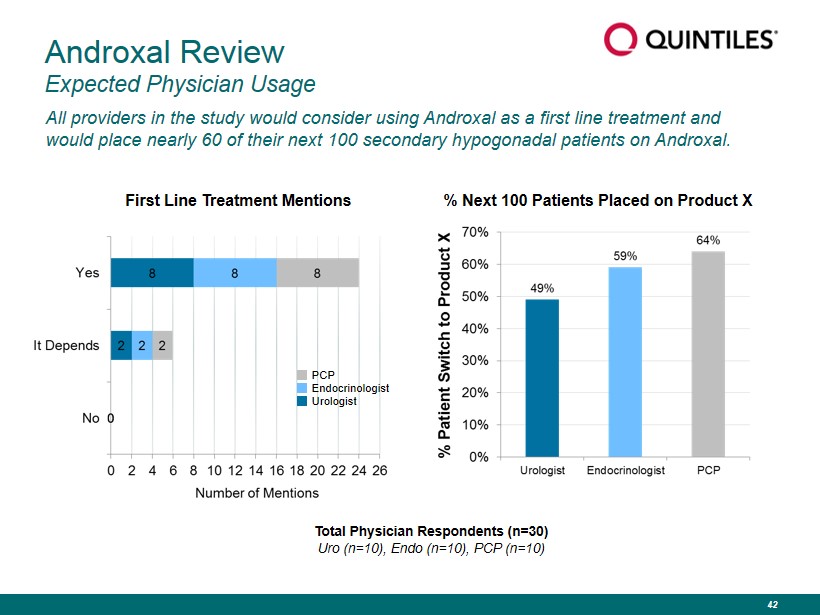
Androxal Review Expected Physician Usage All providers in the study would consider using Androxal as a first line treatment and would place nearly 60 of their next 100 secondary hypogonadal patients on Androxal. First Line Treatment Mentions Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) PCP Endocrinologist Urologist % Next 100 Patients Placed on Product X 42
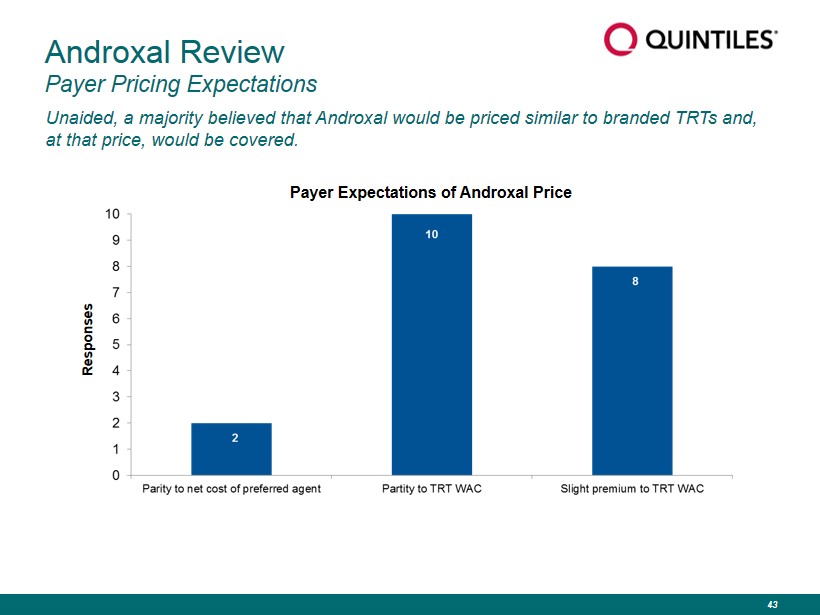
Unaided, a majority believed that Androxal would be priced similar to branded TRTs and, at that price, would be covered. Androxal Review Payer Pricing Expectations Responses Payer Expectations of Androxal Price 43
Conclusions Key Commercial Conclusions ▪ Payers and providers recognize room for improvement with current treatment options and are becoming more aware of the downsides of TRTs. ▪ Additional education will be important to inform physicians and payers as to the issues associated with testosterone replacement therapy. ▪ Both payers and providers reacted very positively to the Androxal profile and believe it would make a nice addition to the treatment of secondary hypogonadism and replace TRT use in many cases. ▪ Providers would expect to use it as a first line agent and would anticipate writing it for a majority of their new secondary hypogonadal patients. ▪ Payers would expect Androxal to be priced in line with other TRTs and would cover it at that price, with preferred positioning possible depending on contracting. 44

Proellex ® 45
Proellex Overview • Selective progesterone receptor modulator (SPRM) • Potential indications: uterine fibroids, endometriosis, breast cancer • Potential significant advantages over existing GnRHa therapies – No loss of bone mineral density in clinical trials – No chronic drug treatments available • Composition of matter patent issued in the US – Patent life to 2017 • Potential for Hatch - Waxman extension – Potential use applications with life to 2027 • Licensed from the NIH – 44 compounds included in license – Worldwide exclusive rights • Program stalled in 2009 due to liver toxicity at high doses • All pre - clinical studies including long term carcinogenicity completed • Uterine fibroid and endometriosis programs now at Phase 2b using low doses and alternative delivery (vaginal) • Move to Phase 3 post completion of Phase 2b studies 46
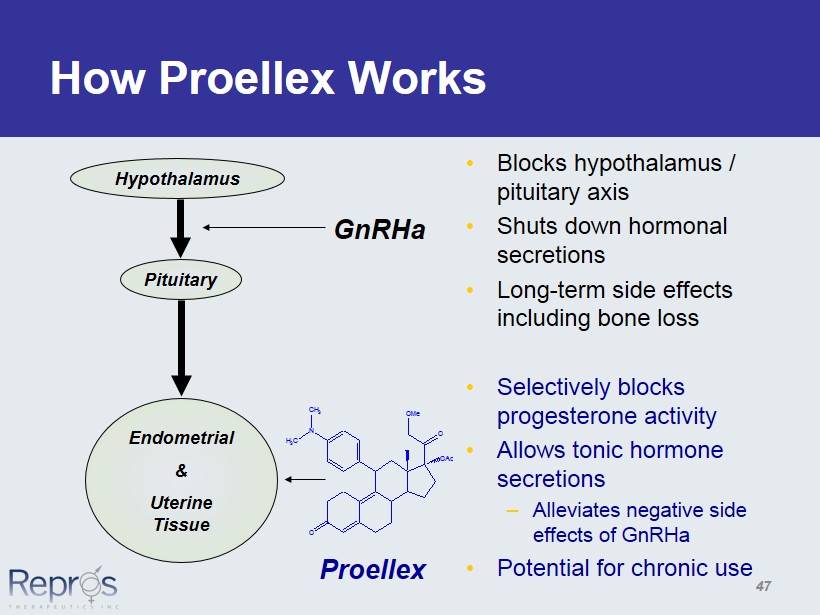
O O OMe N OAc CH 3 H 3 C How Proellex Works • Blocks hypothalamus / pituitary axis • Shuts down hormonal secretions • Long - term side effects including bone loss • Selectively blocks progesterone activity • Allows tonic hormone secretions – Alleviates negative side effects of GnRHa • Potential for chronic use GnRHa Proellex Hypothalamus Pituitary Endometrial & Uterine Tissue 47
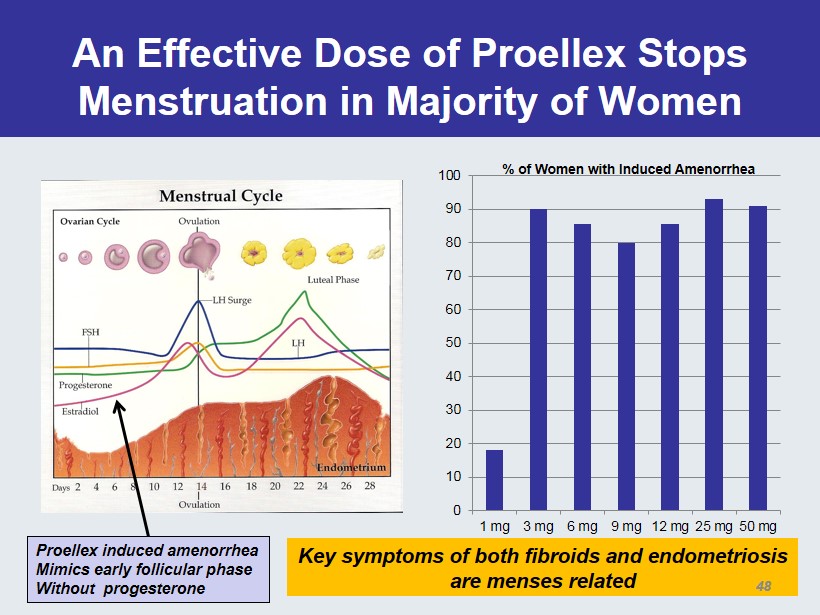
An Effective Dose of Proellex Stops Menstruation in Majority of Women 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg % of Women with Induced Amenorrhea Proellex induced amenorrhea Mimics early follicular phase Without progesterone Key symptoms of both fibroids and endometriosis are menses related 48
Uterine Fibroids • Benign, monoclonal, hormone sensitive, smooth muscle tumors of the uterus • Most common tumor of the female reproductive tract – Heavy bleeding / anemia – Abdominal pressure / pain / urinary frequency • Affect 20 - 77% of women age 35 – 55 • 600,000 hysterectomies conducted annually in the US Courtesy of Jay Goldberg, MD, MSCP Director, Jefferson Fibroid Center Director, Division of General OB/GYN Jefferson Medical College, Philadelphia, PA 49
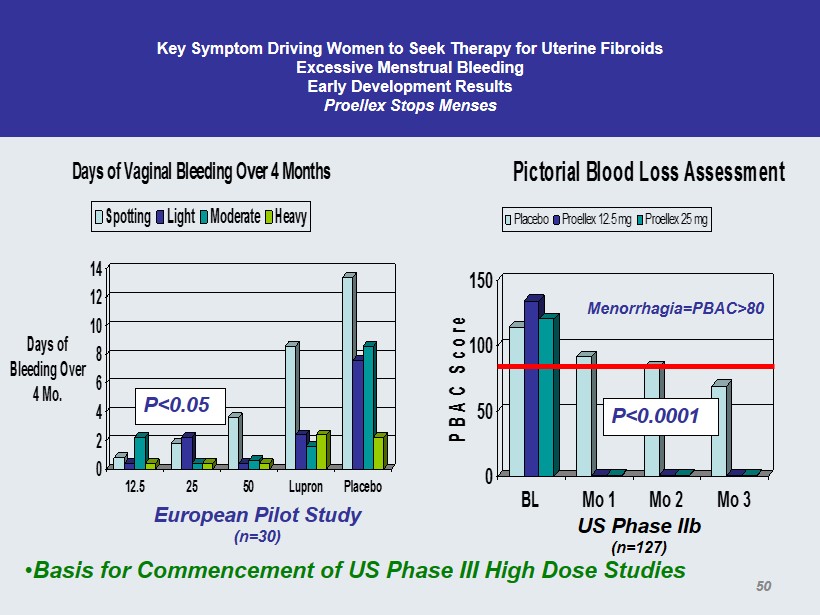
Key Symptom Driving Women to Seek Therapy for Uterine Fibroids Excessive Menstrual Bleeding Early Development Results Proellex Stops Menses 0 2 4 6 8 10 12 14 Days of Bleeding Over 4 Mo. 12.5 25 50 Lupron Placebo Days of Vaginal Bleeding Over 4 Months Spotting Light Moderate Heavy European Pilot Study (n=30) 0 50 100 150 PBAC Score BL Mo 1 Mo 2 Mo 3 Pictorial Blood Loss Assessment Placebo Proellex 12.5 mg Proellex 25 mg P<0.05 US Phase IIb (n=127) P<0.0001 Menorrhagia=PBAC>80 • Basis for Commencement of US Phase III High Dose Studies 50
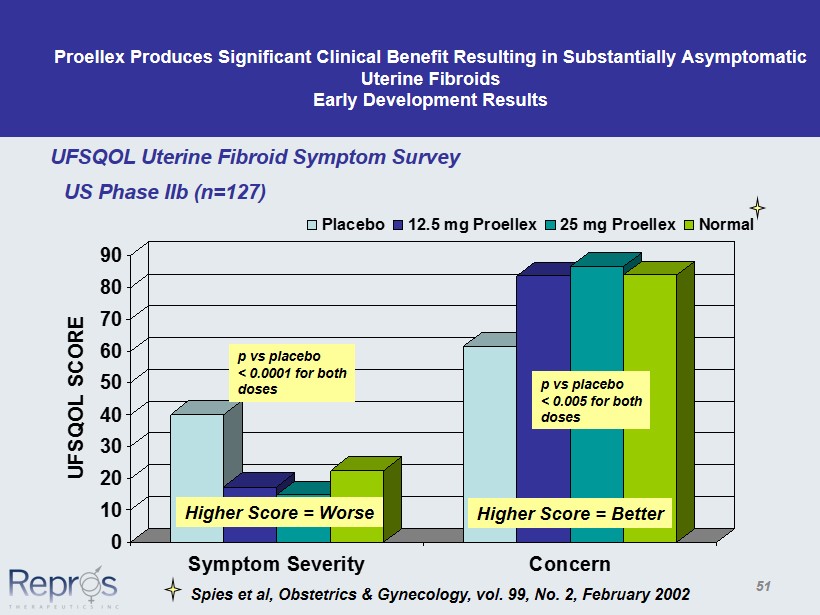
Proellex Produces Significant Clinical Benefit Resulting in Substantially Asymptomatic Uterine Fibroids Early Development Results 0 10 20 30 40 50 60 70 80 90 UFSQOL SCORE Symptom Severity Concern Placebo 12.5 mg Proellex 25 mg Proellex Normal UFSQOL Uterine Fibroid Symptom Survey p vs placebo < 0.0001 for both doses p vs placebo < 0.005 for both doses Spies et al, Obstetrics & Gynecology, vol. 99, No. 2, February 2002 Higher Score = Worse Higher Score = Better US Phase IIb (n=127) 51
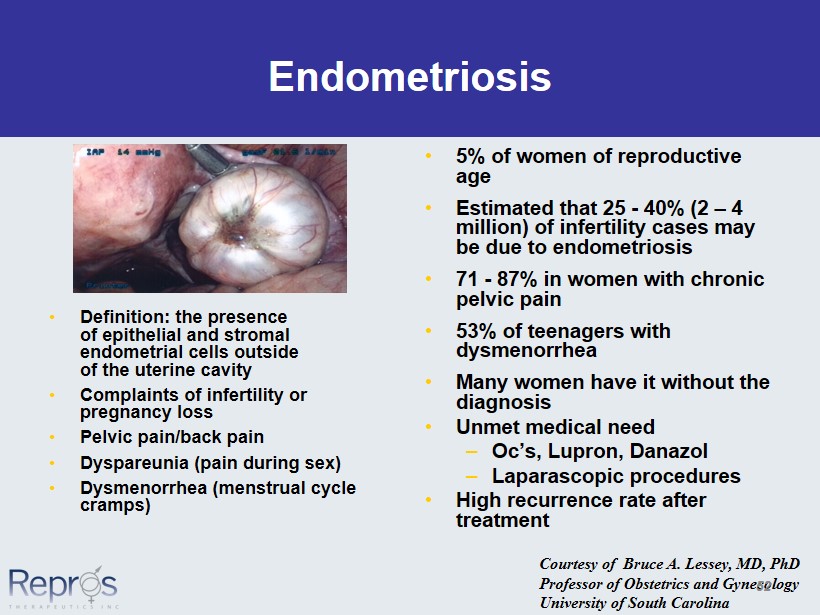
• Definition: the presence of epithelial and stromal endometrial cells outside of the uterine cavity • Complaints of infertility or pregnancy loss • Pelvic pain/back pain • Dyspareunia (pain during sex) • Dysmenorrhea (menstrual cycle cramps) Endometriosis • 5% of women of reproductive age • Estimated that 25 - 40% (2 – 4 million) of infertility cases may be due to endometriosis • 71 - 87% in women with chronic pelvic pain • 53% of teenagers with dysmenorrhea • Many women have it without the diagnosis • Unmet medical need – Oc’s, Lupron, Danazol – Laparascopic procedures • High recurrence rate after treatment Courtesy of Bruce A. Lessey, MD, PhD Professor of Obstetrics and Gynecology University of South Carolina 52
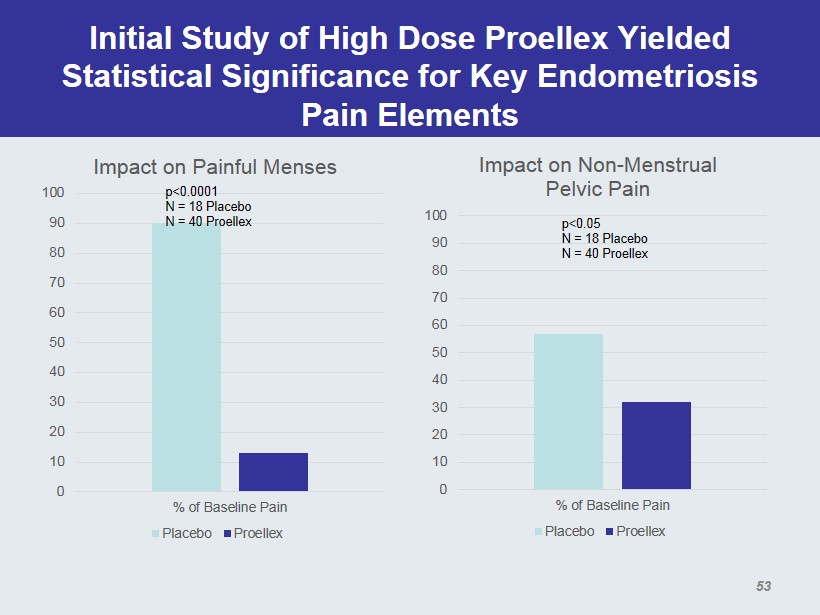
Initial Study of High Dose Proellex Yielded Statistical Significance for Key Endometriosis Pain Elements 0 10 20 30 40 50 60 70 80 90 100 % of Baseline Pain Impact on Painful Menses Placebo Proellex p<0.0001 N = 18 Placebo N = 40 Placebo 0 10 20 30 40 50 60 70 80 90 100 % of Baseline Pain Impact on Non - Menstrual Pelvic Pain Placebo Proellex p<0.05 N = 18 Placebo N = 40 Placebo 53
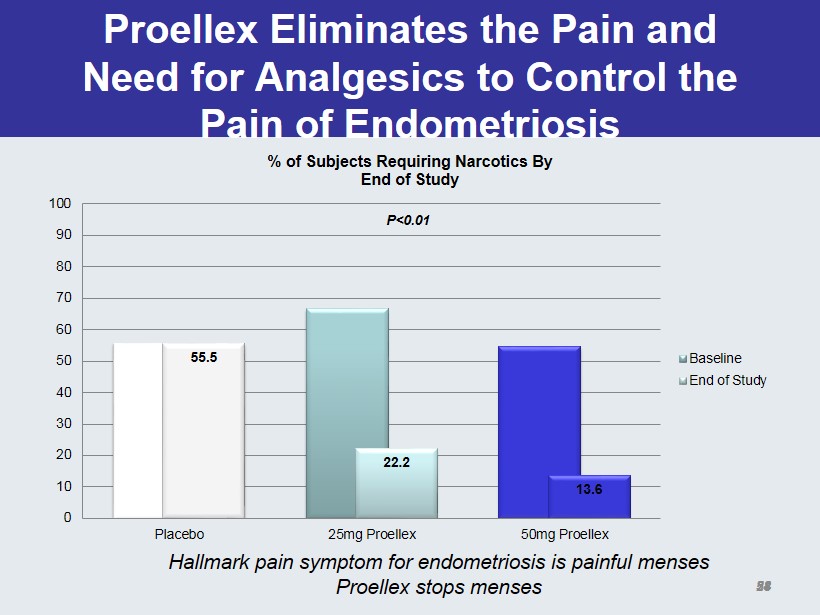
Proellex Eliminates the Pain and Need for Analgesics to Control the Pain of Endometriosis 55.5 22.2 13.6 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotics By End of Study Baseline End of Study P<0.01 28 Hallmark pain symptom for endometriosis is painful menses Proellex stops menses 54
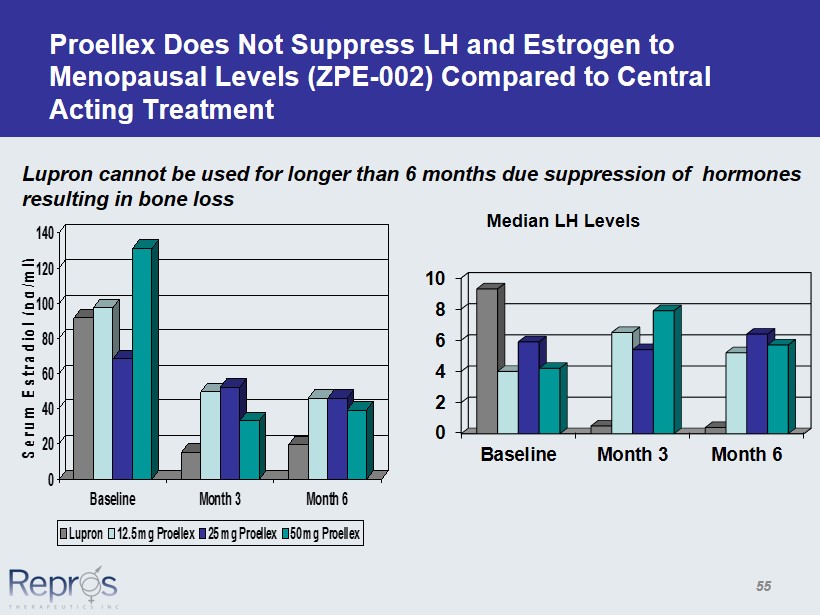
Proellex Does Not Suppress LH and Estrogen to Menopausal Levels (ZPE - 002) Compared to Central Acting Treatment 0 20 40 60 80 100 120 140 Serum Estradiol (pg/ml) Baseline Month 3 Month 6 Lupron 12.5 mg Proellex 25 mg Proellex 50 mg Proellex 0 2 4 6 8 10 Baseline Month 3 Month 6 Median LH Levels Lupron cannot be used for longer than 6 months due suppression of hormones resulting in bone loss 55
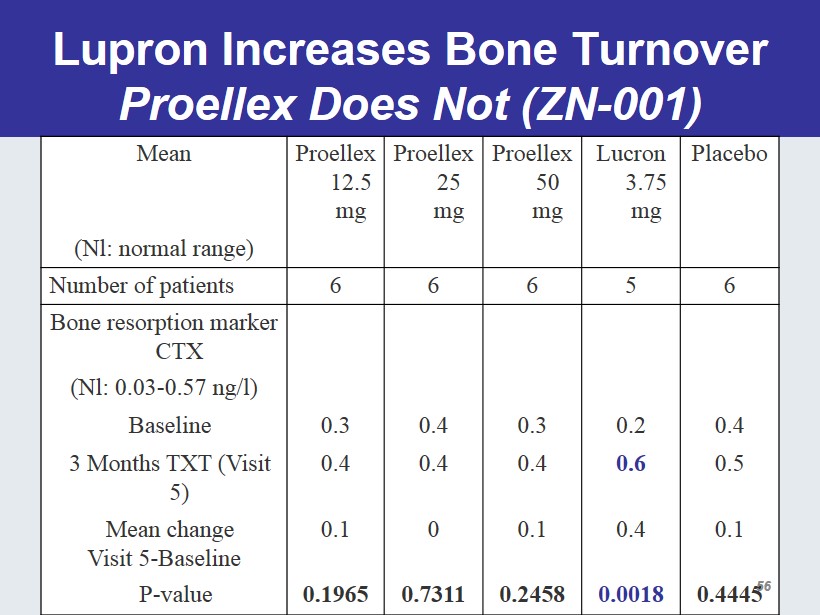
Lupron Increases Bone Turnover Proellex Does Not (ZN - 001) Mean Proellex 12.5 mg Proellex 25 mg Proellex 50 mg Lucron 3.75 mg Placebo (Nl: normal range) Number of patients 6 6 6 5 6 Bone resorption marker CTX (Nl: 0.03 - 0.57 ng/l) Baseline 0.3 0.4 0.3 0.2 0.4 3 Months TXT (Visit 5) 0.4 0.4 0.4 0.6 0.5 Mean change Visit 5 - Baseline 0.1 0 0.1 0.4 0.1 P - value 0.1965 0.7311 0.2458 0.0018 0.4445 56

Vaginal Delivery of Proellex • Avoid 1 st - pass liver effects • Achieve high local concentrations • Formulation Developed (using standard excipients) Completed Phase 2 study in uterine fibroids • FDA recommends Phase 2b before proceeding to Phase 3 • Phase 2b study initiated 57
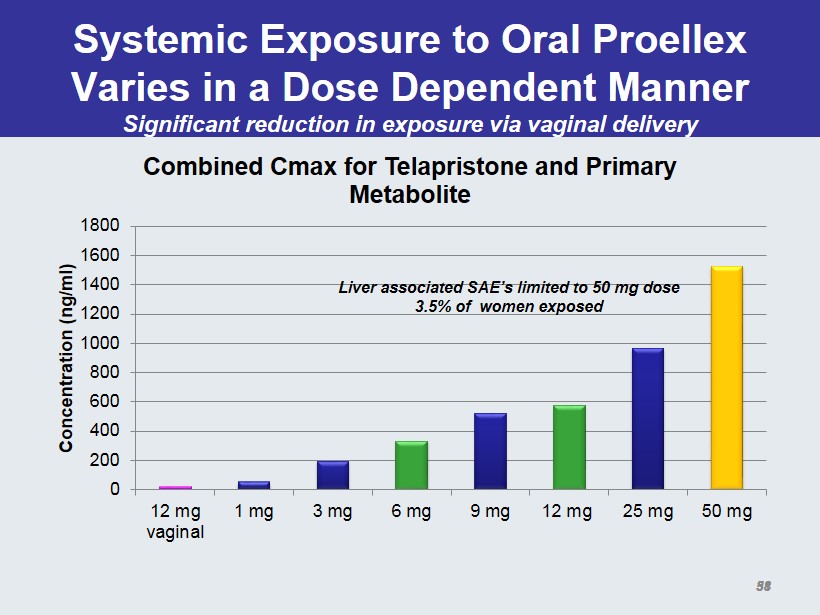
Systemic Exposure to Oral Proellex Varies in a Dose Dependent Manner Significant reduction in exposure via vaginal delivery 0 200 400 600 800 1000 1200 1400 1600 1800 12 mg vaginal 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg Concentration ( ng /ml) Combined Cmax for Telapristone and Primary Metabolite Liver associated SAE’s limited to 50 mg dose 3.5% of women exposed 31 58
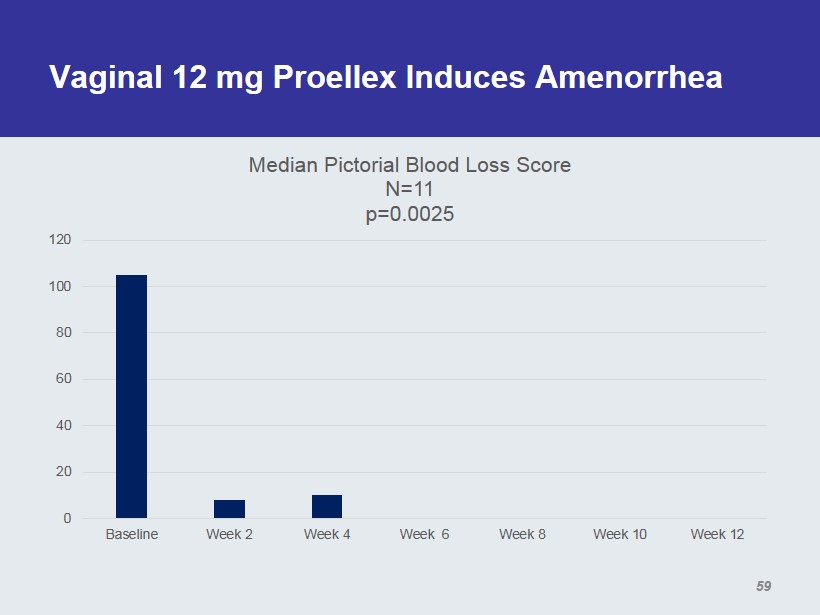
Vaginal 12 mg Proellex Induces Amenorrhea 0 20 40 60 80 100 120 Baseline Week 2 Week 4 Week 6 Week 8 Week 10 Week 12 Median Pictorial Blood Loss Score N=11 p=0.0025 59
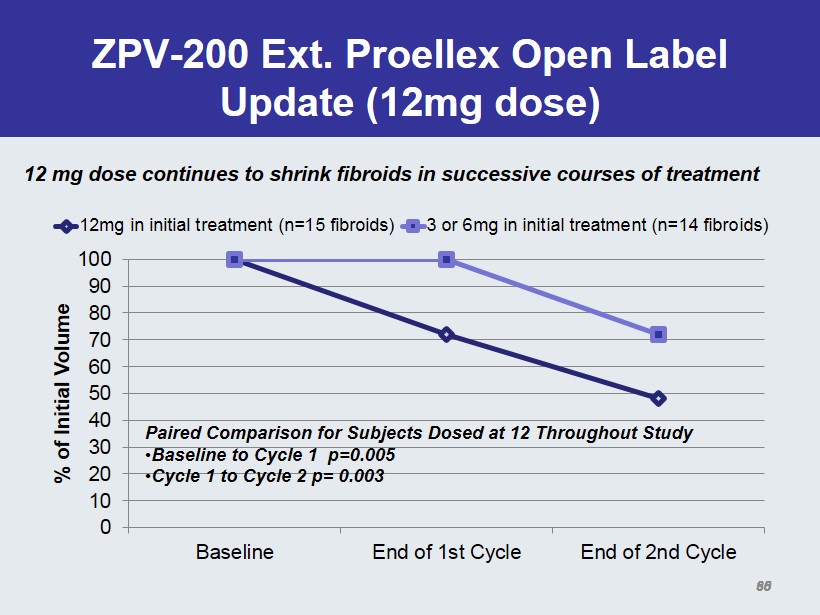
ZPV - 200 Ext. Proellex Open Label Update (12mg dose) 0 10 20 30 40 50 60 70 80 90 100 Baseline End of 1st Cycle End of 2nd Cycle % of Initial Volume 12mg in initial treatment (n=15 fibroids) 3 or 6mg in initial treatment (n=14 fibroids) Paired Comparison for Subjects Dosed at 12 Throughout Study • Baseline to Cycle 1 p=0.005 • Cycle 1 to Cycle 2 p= 0.003 35 12 mg dose continues to shrink fibroids in successive courses of treatment 60
Current Clinical Activity Uterine Fibroids • Phase 2b Programs – Initiated Low Dose Oral Phase 2b study – Initiated Vaginal Administration Phase 2b study • Identical designs – Placebo, 12 mg and 6 mg doses – 3 arm parallel design (15 subjects per arm) • All subjects with confirmed fibroids and menstrual blood loss > 80 ml of blood – 2 x 4 month courses of drug treatment – Primary endpoint requested by FDA reduction of menstrual bleeding compared to placebo 61
Current Clinical Activity Endometriosis • Low Dose Oral Program – Ongoing 60 subjects Phase 2 study – 20 each @ placebo, 6mg and 12 mg – 4 month dosing – Primary endpoint: change in BBSS score – Secondary endpoint: reduction in analgesic use • Stalled enrollment – Anticipate enrollment completed during 2015 62
Overall Conclusions and Plans Going Forward • Proellex is an effective medical treatment for – Uterine fibroids • Rapid and maintained reduction in bleeding • Significant restoration of QOL • Rapid recovery of anemia • Reduction of fibroid bulk • Reduction of bulk associated symptoms – Endometriosis • Rapid reduction in menstrual and non - menstrual pain • Significant reduction in narcotic and non - narcotic analgesic use • Proellex safety profile very encouraging at lower doses – General adverse events are mild to moderate and transient • Request Type C meeting with FDA to discuss overall exposure for safety late 2015 pending enrollment and completion of first course of treatment 63
