Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CymaBay Therapeutics, Inc. | d850210d8k.htm |
| EX-99.2 - EX-99.2 - CymaBay Therapeutics, Inc. | d850210dex992.htm |
 CymaBay Therapeutics
Arhalofenate Febuxostat
Phase 2 PK/PD Study
Preliminary Top Line Data
1
Exhibit 99.1 |
 2
Safe Harbor Statement
This presentation contains "forward-looking" statements that involve risks, uncertainties
and assumptions, and actual results may differ substantially from those projected or expected in
the forward-looking statements. Forward-looking statements include, but are not
limited to: any projections of financial information; any statements about future development,
clinical or regulatory events; any statements concerning CymaBay's plans, strategies or
objectives; and any other statements of expectation or belief regarding future events. These
statements are based on estimates and information available to CymaBay at the time of this
presentation and are not guarantees of future performance. Actual results could differ materially
from CymaBay's current expectations as a result of many factors including, but not limited to:
CymaBay's ability to obtain additional financing to fund its operations; unexpected delays or
results in clinical trials; uncertainties regarding obtaining regulatory approvals; uncertainties
regarding the ability to protect CymaBay's intellectual property; uncertainties regarding market
acceptance of any products for which CymaBay is able to obtain regulatory approval; the effects
of competition; and other market and general economic conditions. You should read CymaBay's
Quarterly Report on Form 10-Q filed with the SEC on November 14, 2014, especially under the
caption “Risk Factors,” which is available on the SEC web site at http://www.sec.gov,
for a fuller discussion of these and other risks relating to an investment in CymaBay’s
common stock. CymaBay assumes no obligation for and does not intend to update these
forward-looking statements, except as required by law. |
 3
Arhalofenate Febuxostat Phase 2 PK/PD Study
•
Objectives
–
Assess sUA reductions of different dose combinations
–
Measure the inter-
and intraday fractional excretion of UA (FEUA)
–
Assess if there is a drug-drug interaction
–
Additional safety data for arhalofenate/febuxostat combination
Weeks 1-2
Arhalofenate 600
Week 3
Febuxostat 80 +
Arhalofenate 600
Week 4
Febuxostat 40 +
Arhalofenate 600
N = 16 per cohort ; PK from cohort 2 at Weeks 2, 4 and 6
All patients received colchicine for flare prophylaxis
Weeks 5-6
Febuxostat 80
Arhalofenate 800
Febuxostat 40 +
Arhalofenate 800
Febuxostat 80 +
Arhalofenate 800
Febuxostat 40
Cohort 1
Cohort 2 |
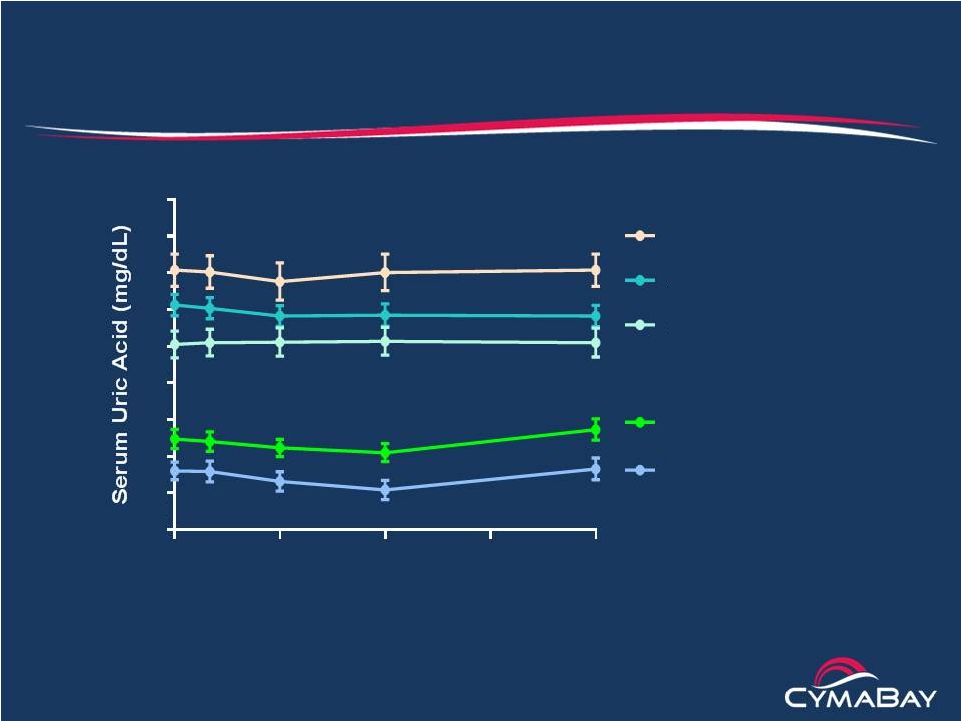 4
Arhalofenate Febuxostat Phase 2 PK/PD Study
Intraday variation of sUA for selected dose combinations
Mean ±
SE
Arh 800 mg (Day 14, n = 8)
Arh 800 mg + Fbx 80 mg
(Day 28, n = 7)
Fbx 80 mg (Day 42, n = 7)
Arh 800 mg (Day 7, n = 8)
Baseline (Day 0, n = 8)
Time (hours)
0
6
12
18
24
2
3
4
5
6
7
8
9
10
11 |
 5
Arhalofenate Febuxostat Phase 2 PK/PD Study
Intra-
and interday variation in FEUA for arhalofenate (800 mg)
Mean ±
SE
Time Period
***p <0.001
Matched pairs t-test
***
***
***
0
1
2
3
4
5
6
7
8
Day 0 (n = 8)
Day 14 (n = 8)
9 am to
3 pm
3 pm to
9 pm
9 pm to
9 am |
 6
Arhalofenate Febuxostat Phase 2 PK/PD Study
Mean lowering of serum uric acid
Fbx alone Fbx + Arhalofenate 600
mg Fbx + Arhalofenate 800 mg
Febuxostat 40
mg Febuxostat 80 mg
-33%
-45%
-54%
-51%
-55%
-63%
****
****
****
**** p < .0001 vs. febuxostat alone groups
-70
-60
-50
-40
-30
-20
-10
0 |
 7
Arhalofenate Febuxostat Phase 2 PK/PD Study
sUA responder rate for febuxostat 40 mg treatments
< 6.0 mg/dL
< 5.0 mg/dL < 4.0 mg/dL
< 3.0 mg/dL
Febuxostat (40 mg) +
Arhalofenate (800 mg)
Febuxostat (40 mg) +
Arhalofenate (600 mg)
Febuxostat (40 mg)
N
15
14 15
p-values reflect comparisons vs. febuxostat 40 mg. McNemar’s exact
test and Fischer’s exact test were used for comparisons within and
between cohorts, respectively.
* p < .05 ** p < .01 *** p < .001
*
**
***
0
20
40
60
80
100 |
 8
< 6.0 mg/dL
< 5.0 mg/dL < 4.0 mg/dL
< 3.0 mg/dL
Arhalofenate Febuxostat Phase 2 PK/PD Study
sUA responder rate for febuxostat 80 mg treatments
Febuxostat (80 mg) +
Arhalofenate (800 mg)
Febuxostat (80 mg) +
Arhalofenate (600 mg)
Febuxostat (80 mg)
N
14
16
14
p-values reflect comparisons vs. febuxostat 80 mg. McNemar’s exact
test and Fischer’s exact test were used for comparisons within and
between cohorts, respectively.
* p < .05
*
0
20
40
60
80
100 |
 9
Arhalofenate Febuxostat Phase 2 PK/PD Study
Safety overview
•
Noncompleters
–
Uncontrolled hypertension deemed unrelated to study drugs by PI
–
GI symptoms, myalgia, headache attributed to colchicine by PI
–
One patient terminated due to protocol non-compliance
•
Adverse events
–
No serious adverse events
–
1 Severe AE (uncontrolled hypertension)
–
1 flare during the run-in and 3 during the treatment phase
•
Laboratory findings
–
One case of transaminase elevation that emerged after initiation
of
febuxostat
–
No patient had a creatinine increase of >1.5X or a creatinine value
greater than the upper limit of normal |
