Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a15-2163_18k.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a15-2163_1ex99d1.htm |
Exhibit 99.2
|
|
AMAG Pharmaceuticals JP Morgan Healthcare January 2015 Conference AMAG Pharmaceuticals, Inc. 1100 Winter Street Waltham, MA 02451 617.498.3300 A SPECIALTY PHARMACEUTICAL COMPANY DEDICATED TO BRINGING TO MARKET THERAPIES THAT IMPROVE PATIENTS’ LIVES www.amagpharma.com Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including among others, statements regarding beliefs about AMAG’s cash flow, earnings, market segments, position for portfolio expansion and future effective tax rate; the potential timing of regulatory approval for the Makena 1mL preservative-free vial; the Makena market opportunity; beliefs about market dynamics and reimbursement trends for Makena; Makena’s patient-centric approach; the competitive landscape of Feraheme; Feraheme growth opportunities, including for the current indication and if a broader indication is pursued and obtained, as well as potential future call points; expectations for MuGard; AMAG’s future expansion opportunities, including business development, product growth and Makena line extension/lifecycle management; AMAG’s business development targeting strategy, including its criteria and considerations; plans to further diversify AMAG’s product portfolio, opportunities with maturing late-stage development pipeline and expected cash generation; expected 2014 preliminary results, including revenues and operating expenses; 2015 financial guidance, including expected Makena sales, Feraheme sales (which projected range takes into account expected label changes), adjusted EBITDA and cash earnings; and AMAG’s being well-positioned for success in 2015 and beyond are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others: (1) demand for Feraheme and AMAG’s ability to successfully compete in the intravenous iron replacement market as a result of the FDA’s recommended label changes, including a boxed warning which would provide, among other things, (i) that Feraheme be administered only when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions, (ii) observation for signs or symptoms of hypersensitivity reactions during and for at least 30 minutes following infusion and (iii) that hypersensitivity reactions have occurred in patients in whom a previous Feraheme dose was tolerated; (2) the outcome and timing of the process in accordance with Section 505(o) of the Federal Food, Drug and Cosmetic Act whereby the FDA is authorized to require AMAG to make safety-related label changes, including prescribed periods for submitting proposed changes to the label recommended by the FDA; (3) the impact on sales if AMAG disseminates future Dear Healthcare Provider letters; (4) the ability of AMAG to invest in the development and commercialization of Feraheme/Rienso outside the U.S., and the level of commercial success of any of such efforts, given the December 2014 arrangement to terminate AMAG’s and Takeda’s license arrangement; (5) uncertainties regarding the likelihood and timing of potential approval of Feraheme/Rienso in the U.S., the EU and Canada in the broader IDA indication; (6) the possibility that following review of new safety information, the FDA or regulators in Europe and Canada will request additional technical or scientific information, new studies or reanalysis of existing data, on-label warnings, post-marketing requirements/commitments or risk evaluation and mitigation strategies (REMS) in the current CKD indication for Feraheme/Rienso, or cause Feraheme/Rienso to be withdrawn from the market, and the additional costs and expenses that will or may be incurred in connection with such activities; (7) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso or Makena and in turn affect sales or AMAG’s ability to market such product; (8) AMAG’s patents and proprietary rights; (9) maintaining the benefits associated with Makena’s orphan drug exclusivity status; (10) the risk of an Abbreviated New Drug Application (ANDA) filing, especially (i) as to Feraheme following the FDA’s draft bioequivalence recommendation for ferumoxytol published in December 2012 and (ii) as to Makena given the history of the formerly FDA-approved drug Delalutin (the original version of 17-alpha-hydroxyprogesterone caproate) for conditions other than reducing the risk of preterm birth; (11) AMAG’s ability to execute on, or to realize the expected results from, its long-term strategic plan; (12) the possibility that AMAG will not realize expected synergies and other benefits from its acquisition of Lumara Health, as well as AMAG’s ability to pursue additional business development opportunities, especially in light of AMAG’s being highly leveraged; (13) the impact on sales of Makena from competitive, commercial payor, government (including federal and state Medicaid reimbursement policies), physician, patient or public responses with respect to product pricing, product access and sales and marketing initiatives, as well as patient compliance and the number of preterm birth risk pregnancies for which Makena may be prescribed; (14) the likelihood that labeling changes may be used to support product liability claims that the prior product labeling did not adequately disclose the risk of adverse events; (15) compliance with restrictive and affirmative covenants with respect to substantial indebtedness incurred to finance the acquisition of Lumara Health, including a requirement that AMAG reduce its leverage over time; (16) the possibility that AMAG will need to raise additional capital from the sale of its common stock, which will cause significant dilution to its stockholders, in order to satisfy its contractual obligations, including its debt service, milestone payments that may become payable to Lumara Health’s stockholders, or in order to pursue business development activities; (17) the availability and timing of tax net operating loss carryforwards; (18) the manufacture of AMAG’s products, including any significant interruption in the supply of raw materials or finished product and (19) other risks identified in AMAG’s filings with the U.S. Securities and Exchange Commission (SEC), including its Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and subsequent filings with the SEC. Any of the above risks and uncertainties could materially and adversely affect AMAG’s results of operations, its profitability and its cash flows, which would, in turn, have a significant and adverse impact on AMAG’s stock price. Use of the term “including” in the two paragraphs above shall mean in each case “including, but not limited to.” AMAG cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals® and Feraheme® are registered trademarks of AMAG Pharmaceuticals, Inc. MuGard® is a registered trademark of PlasmaTech Biopharmaceuticals, Inc. (formerly known as Access Pharmaceuticals, Inc.). Rienso™ is a trademark of Takeda Pharmaceutical Company Limited. Lumara Health™ is a trademark of Lumara Health Inc. Makena® is a registered trademark of Lumara Health Inc. Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
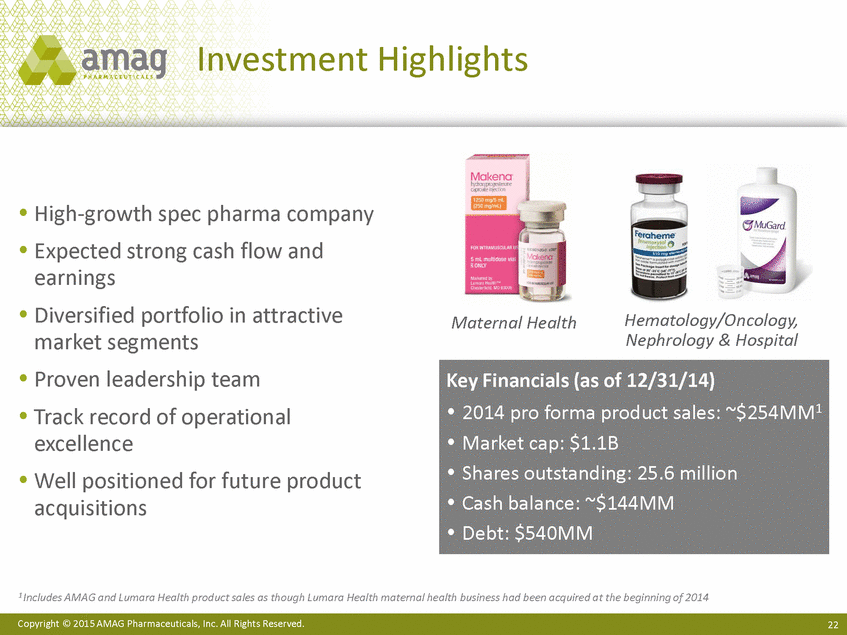
Investment Highlights High-growth spec pharma company Expected strong cash flow and earnings Diversified portfolio in attractive market segments Proven leadership team Track record of operational excellence Well positioned for future product acquisitions Hematology/Oncology, Nephrology & Hospital Maternal Health 1 Includes AMAG and Lumara Health product sales as though Lumara Health maternal health business had been acquired at the beginning of 2014 Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 2 Key Financials (as of 12/31/14) 2014 pro forma product sales: ~$254MM1 Market cap: $1.1B Shares outstanding: 25.6 million Cash balance: ~$144MM Debt: $540MM |
|
|
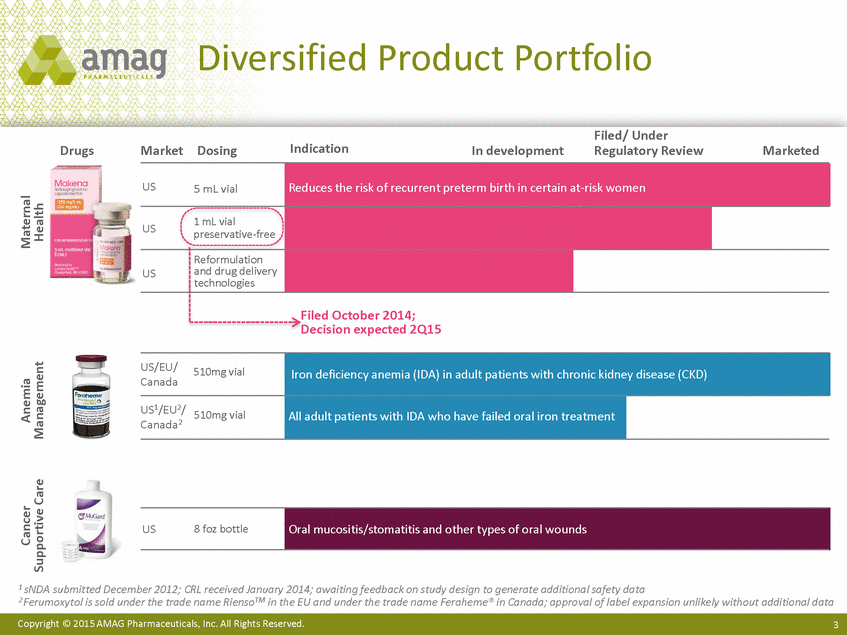
Diversified Product Portfolio Filed/ Under Regulatory Review Indication Drugs Market Dosing In development Marketed US preservative-free and drug delivery US Filed October 2014; Decision expected 2Q15 Canada Canada2 1 sNDA submitted December 2012; CRL received January 2014; awaiting feedback on study design to generate additional safety data 2 Ferumoxytol is sold under the trade name RiensoTM in the EU and under the trade name Feraheme® in Canada; approval of label expansion unlikely without additional data Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 3 Cancer Supportive Care Anemia Management Maternal Health US8 foz bottle Oral mucositis/stomatitis and other types of oral wounds US/EU/510mg vial Iron deficiency anemia (IDA) in adult patients with chronic kidney disease (CKD) US1/EU2/ 510mg vial All adult patients with IDA who have failed oral iron treatment US5 mL vial Reduces the risk of recurrent preterm birth in certain at-risk women 1 mL vial Reformulation technologies |
|
|
2014 – Transformational Year Solid execution of plan and strong financial performance vs. 2013 Feraheme revenue +19% In-market physician demand +9.2% Evolution index1 of 102, gaining share of a growing market (+7%) Acquisition of Lumara Health financially transforms company Added high-growth Makena and high-performing commercial team Rapid integration in progress; $20MM expected annual synergy cost savings Lower potential near-term effective tax rate due to availability of NOLs (AMAG + Lumara) Enterprise value +350% and market cap +100% since announcement on Sept. 29, 2014 Expanded and strengthened executive team Hired new heads of business development, sales & marketing and human resources Key Lumara Health executives joined AMAG 1 Evolution Index = Product growth divided by market growth; E.I. of 102 means Feraheme is growing 2% faster than the market in 2014 vs. 2013 Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 4 |
|
|
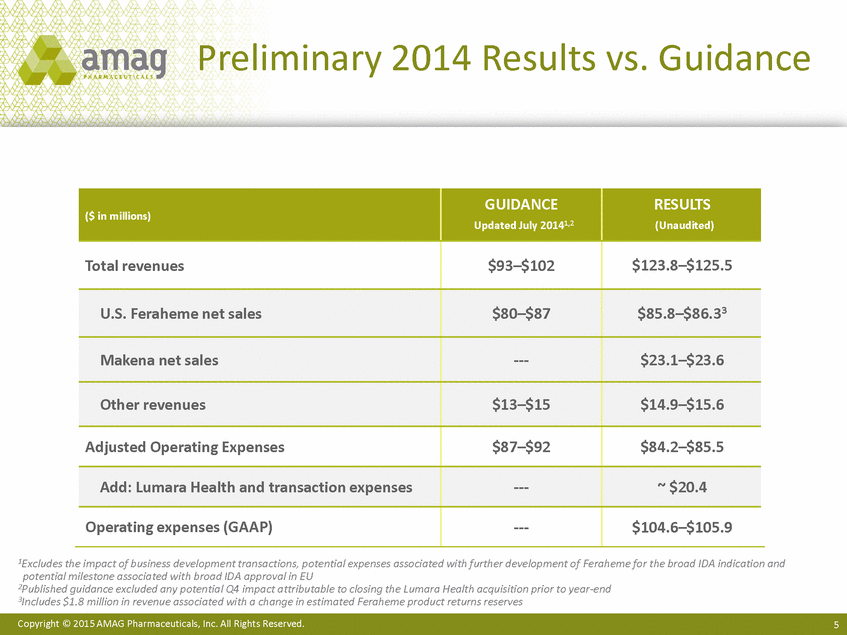
Preliminary 2014 Results vs. Guidance 1Excludes the impact of business development transactions, potential expenses associated with further development of Feraheme for the broad IDA indication and potential milestone associated with broad IDA approval in EU 2Published guidance excluded any potential Q4 impact attributable to closing the Lumara Health acquisition prior to year-end 3Includes $1.8 million in revenue associated with a change in estimated Feraheme product returns reserves Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 5 ($ in millions) GUIDANCE Updated July 20141,2 RESULTS (Unaudited) Total revenues $93–$102 $123.8–$125.5 U.S. Feraheme net sales $80–$87 $85.8–$86.33 Makena net sales ---$23.1–$23.6 Other revenues $13–$15 $14.9–$15.6 Adjusted Operating Expenses $87–$92 $84.2–$85.5 Add: Lumara Health and transaction expenses ---~ $20.4 Operating expenses (GAAP) ---$104.6–$105.9 |
|
|
Makena Copyright © 2015 AMAG Pharmaceuticals,Inc. All Rights Reserved. |
|
|
Overview of Recently acquired Lumara Health, a privately held specialty pharmaceutical company focused on maternal health Makena commercial team dedicated exclusively to OB/GYN subspecialty o 75-person sales force targets more than 9,000 OB/GYNs Copyright © 2015 AMAG Pharmaceuticals., Inc. All Rights Reserved. 7 Flagship product, Makena Only FDA-approved drug to lower the risk of preterm birth in women who are pregnant with one baby and who have spontaneously delivered one preterm baby in the past Weekly intramuscular injections from 16 until 37 weeks of pregnancy, 21 maximum possible injections |
|
|
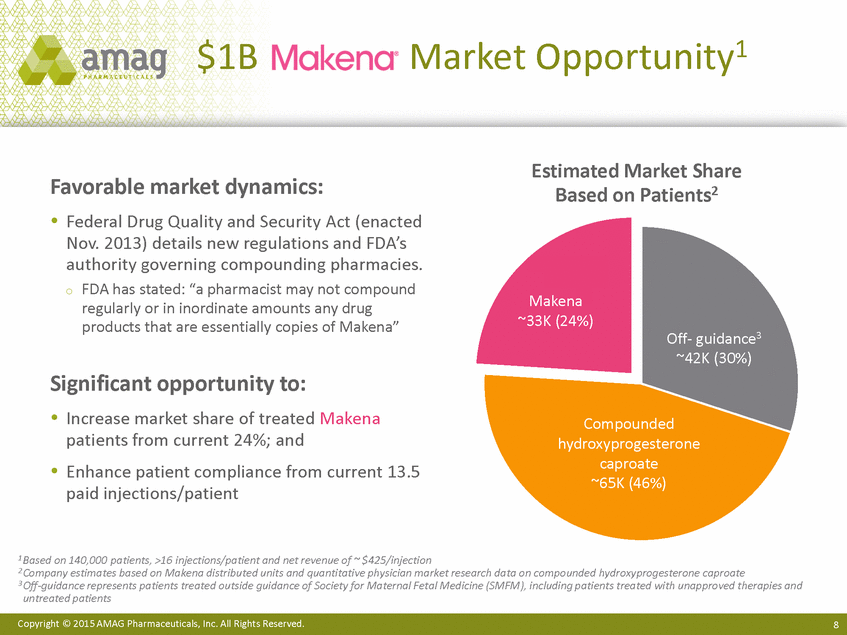
Market Opportunity1 $1B Estimated Market Share Based on Patients2 Favorable market dynamics: Federal Drug Quality and Security Act (enacted Nov. 2013) details new regulations and FDA’s authority governing compounding pharmacies. o FDA has stated: “a pharmacist may not compound regularly or in inordinate amounts any drug products that are essentially copies of Makena” Makena ~33K (24%) Off-guidance3 ~42K (30%) Significant opportunity to: Increase market share of treated Makena patients from current 24%; and Enhance patient compliance from current 13.5 paid injections/patient Compounded hydroxyprogesterone caproate ~65K (46%) 1 Based on 140,000 patients, >16 injections/patient and net revenue of ~ $425/injection 2 Company estimates based on Makena distributed units and quantitative physician market research data on compounded hydroxyprogesterone caproate 3 Off-guidance represents patients treated outside guidance of Society for Maternal Fetal Medicine (SMFM), including patients treated with unapproved therapies and untreated patients Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 8 |
|
|
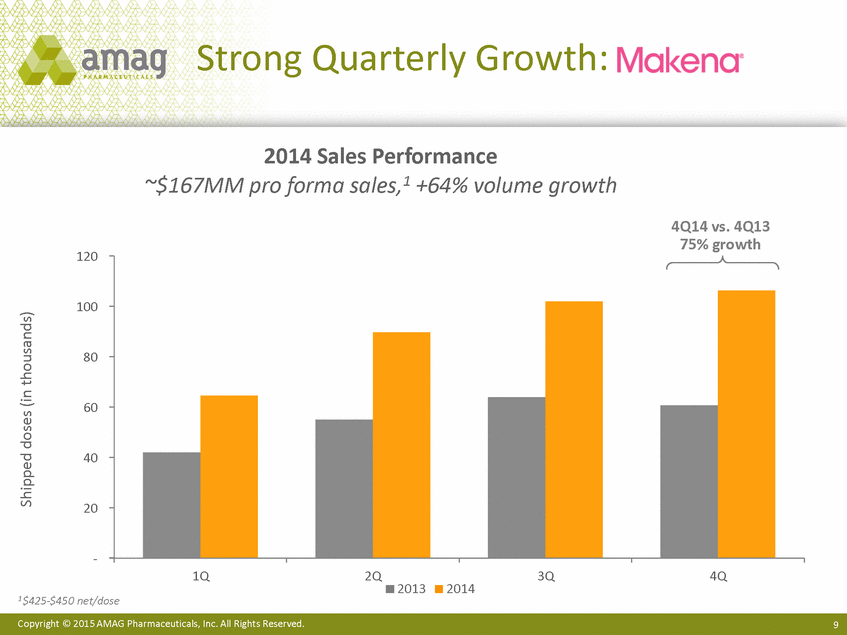
Strong Quarterly Growth: 2014 Sales Performance ~$167MM pro forma sales,1 +64% volume growth 4Q14 vs. 4Q13 75% growth 120 100 80 60 40 20 - 1Q 2Q 3Q 4Q 2013 2014 1 $425-$450 net/dose Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 9 Shipped doses (in thousands) |
|
|
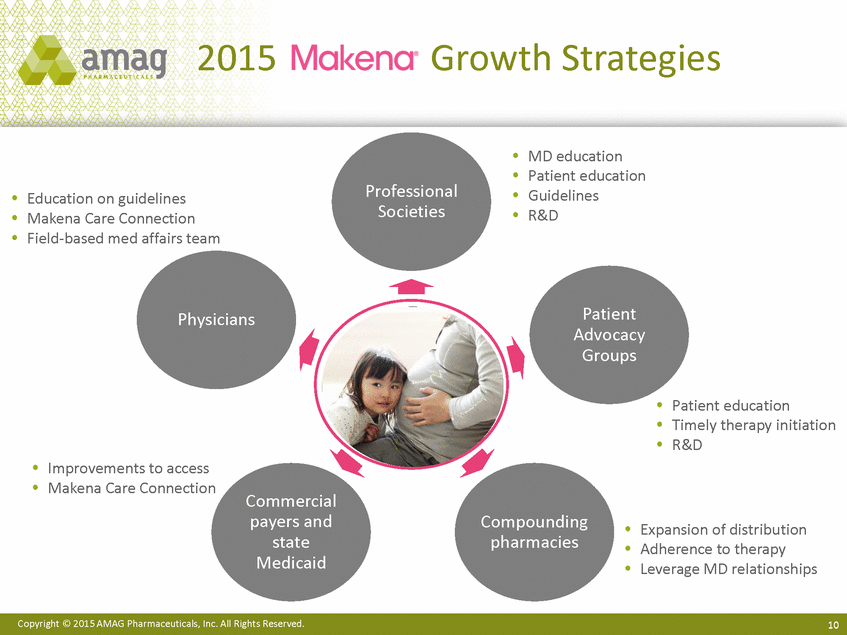
2015 Growth Strategies MD education Patient education Guidelines R&D Professional Societies Education on guidelines Makena Care Connection Field-based med affairs team Patient Advocacy Groups Physicians Patient education Timely therapy initiation R&D Improvements to access Makena Care Connection Commercial payers and state Medicaid Compounding pharmacies Expansion of distribution Adherence to therapy Leverage MD relationships Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 10 |
|
|
Feraheme and MuGard 11 Copyright© 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
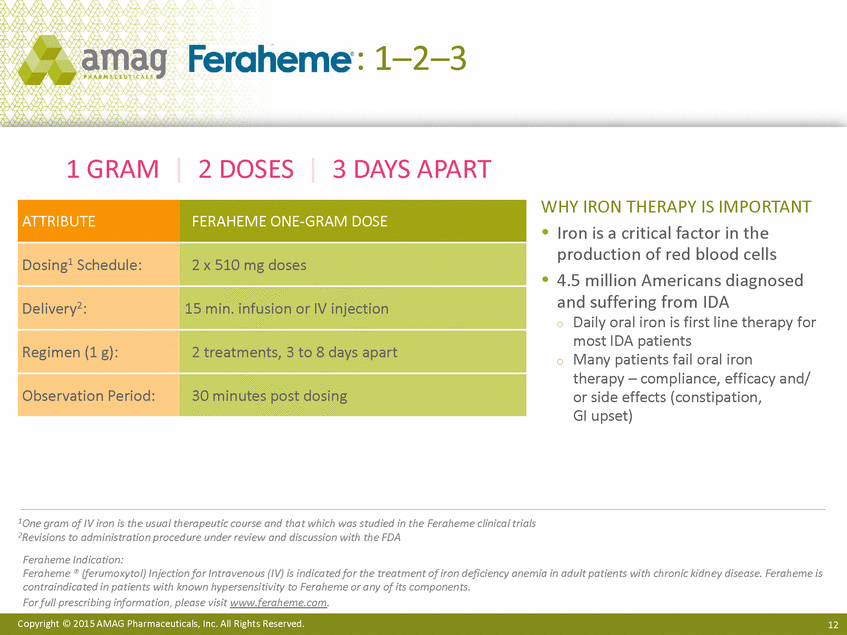
Feraheme: 1–2–3 1 GRAM | 2 DOSES | 3 DAYS APART WHY IRON THERAPY IS IMPORTANT Iron is a critical factor in the production of red blood cells 4.5 million Americans diagnosed and suffering from IDA Daily oral iron is first line therapy for most IDA patients Many patients fail oral iron therapy – compliance, efficacy and/ or side effects (constipation, GI upset) o o 1One gram of IV iron is the usual therapeutic course and that which was studied in the Feraheme clinical trials 2Revisions to administration procedure under review and discussion with the FDA Feraheme Indication: Feraheme ® (ferumoxytol) Injection for Intravenous (IV) is indicated for the treatment of iron deficiency anemia in adult patients with chronic kidn ey disease. Feraheme is contraindicated in patients with known hypersensitivity to Feraheme or any of its components. For full prescribing information, please visit www.feraheme.com. Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 12 ATTRIBUTE FERAHEME ONE-GRAM DOSE Dosing1 Schedule: 2 x 510 mg doses Delivery2: 15 min. infusion or IV injection Regimen (1 g): 2 treatments, 3 to 8 days apart Observation Period: 30 minutes post dosing |
|
|
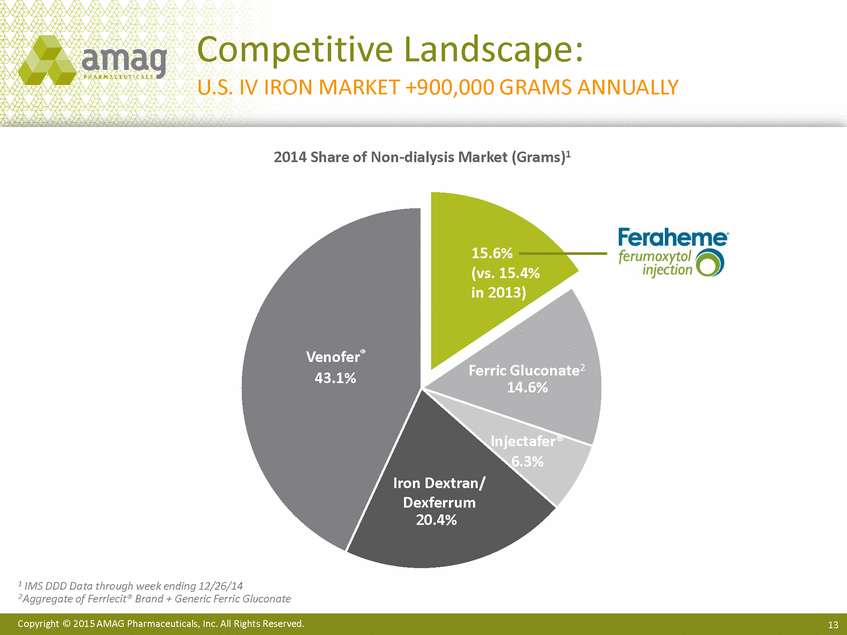
Competitive Landscape: U.S. IV IRON MARKET +900,000 GRAMS ANNUALLY 2014 Share of Non-dialysis Market (Grams)1 15.6% (vs. 15.4% in 2013) Venofer® 43.1% Ferric Gluconate2 Injectafer® 6.3% Iron Dextran/ Dexferrum 20.4% 1 IMS DDD Data through week ending 12/26/14 2 Aggregate of Ferrlecit® Brand + Generic Ferric Gluconate Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 13 |
|
|
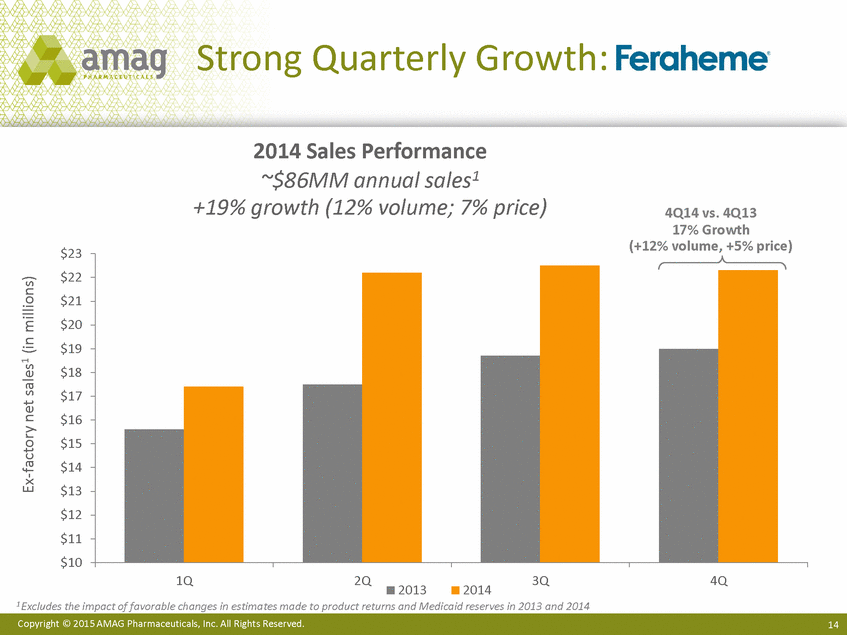
Strong Quarterly Growth: 2014 Sales Performance ~$86MM annual sales1 +19% growth (12% volume; 7% price) 4Q14 vs. 4Q13 17% Growth (+12% volume, +5% price) $23 $22 $21 $20 $19 $18 $17 $16 $15 $14 $13 $12 $11 $10 1Q 2Q 3Q 4Q 2013 2014 1 Excludes the impact of favorable changes in estimates made to product returns and Medicaid reserves in 2013 and 2014 Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 14 Ex-factory net sales1 (in millions) |
|
|
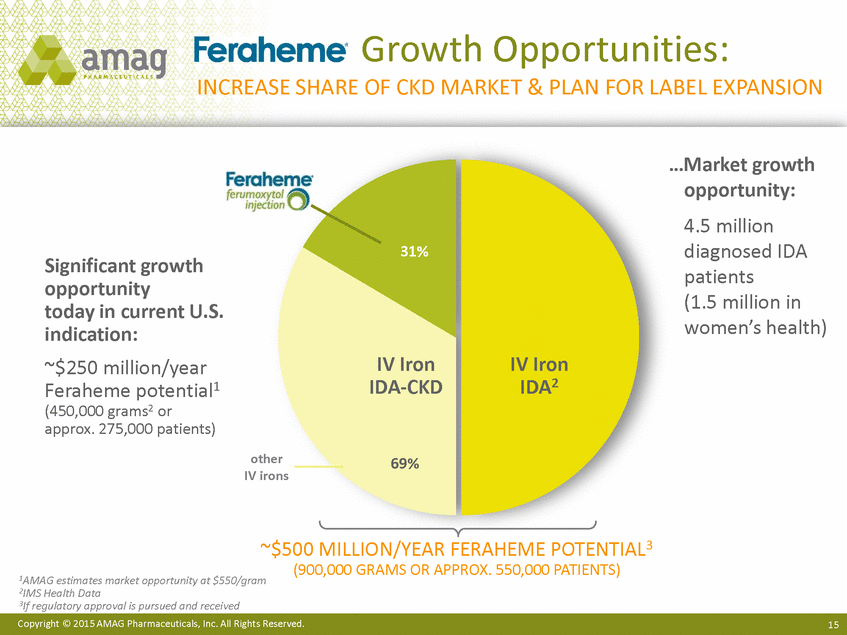
Growth Opportunities: INCREASE SHARE OF CKD MARKET & PLAN FOR LABEL EXPANSION …Market growth opportunity: 4.5 million diagnosed IDA patients (1.5 million in women’s health) 31% Significant growth opportunity today in current U.S. indication: ~$250 million/year Feraheme potential1 (450,000 grams2 or approx. 275,000 patients) IV Iron IDA-CKD IV Iron IDA2 other 69% IV irons ~$500 MILLION/YEAR FERAHEME POTENTIAL3 (900,000 GRAMS OR APPROX. 550,000 PATIENTS) 1AMAG estimates market opportunity at $550/gram 2IMS Health Data 3If regulatory approval is pursued and received Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 15 |
|
|
MuGard Overview Overview 2014 Highlights Study demonstrating strong product differentiation featured on cover of journal Cancer in May Expanding patient access to MuGard Prescription mucoadhesive for the management of oral mucositis (OM) OM is a common, painful, and debilitating side effect of multiple types of cancer treatments. o 400,000 cancer patients with oral mucositis in U.S. annually1 o Incremental medical care cost of severe OM in head and neck cancer patients is ~$18,0002 MuGard forms a protective coating over the oral mucosa to shield the membranes of the mouth and tongue Increased payer coverage through additional managed care formulary acceptances Established new GPO contracting strategy o o Strong market and market share growth3 o Total MuGard prescriptions +9.2% vs. 2013 1Sonis ST. Oral Oncol. 2009 Dec;45(12):1015-20 2Treister N, Sonis S. Mucositis: biology and management. Curr Opin Otolaryngol Head Neck Surg. 2007 Apr;15(2):123-9 3IMS NPA Data through November 2014 Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 16 |
|
|
Future Expansion Opportunities Copyright © 2015 AMAG Pharmaceuticals,Inc. All Rights Reserved. 17 |
|
|
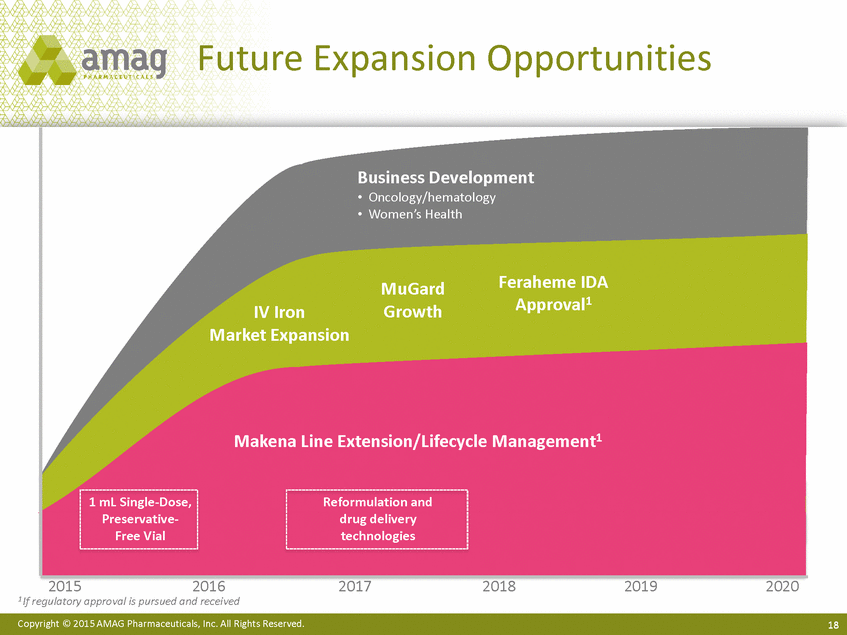
Future Expansion Opportunities Approval1 Growth IV Iron 2015 2016 2017 2018 2019 2020 1 If regulatory approval is pursued and received Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 18 Business Development • Oncology/hematology • Women’s Health MuGardFeraheme IDA Market Expansion Makena Line Extension/Lifecycle Management1 1 mL Single-Dose,Reformulation and Preservative-drug delivery Free Vialtechnologies |
|
|
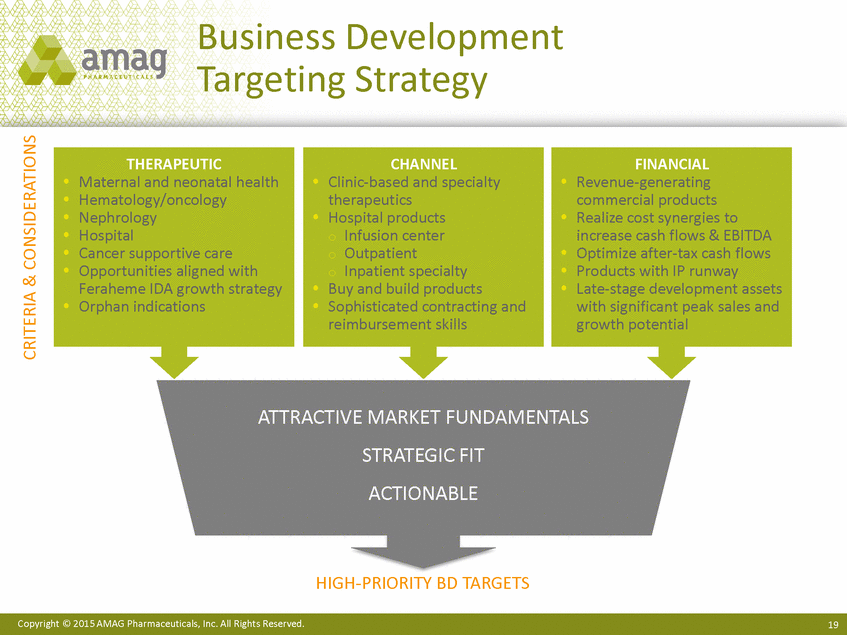
Business Development Targeting Strategy THERAPEU Maternal and neonatal health Hematology/oncology Nephrology Hospital Cancer supportive care Opportunities aligned with Feraheme IDA growth strategy Orphan indications CHA Clinic-based and specialty therapeutics Hospital products o Infusion center o Outpatient o Inpatient specialty Buy and build products Sophisticated contracting and reimbursement skills FINANCIAL Revenue-generating commercial products Realize cost synergies to increase cash flows & EBITDA Optimize after-tax cash flows Products with IP runway Late-stage development assets with significant peak sales and growth potential ATTRACTIVE MARKET FUNDAMENTALS STRATEGIC FIT ACTIONABLE HIGH-PRIORITY BD TARGETS Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 19 CRITERIA & CONSIDERATIONS |
|
|
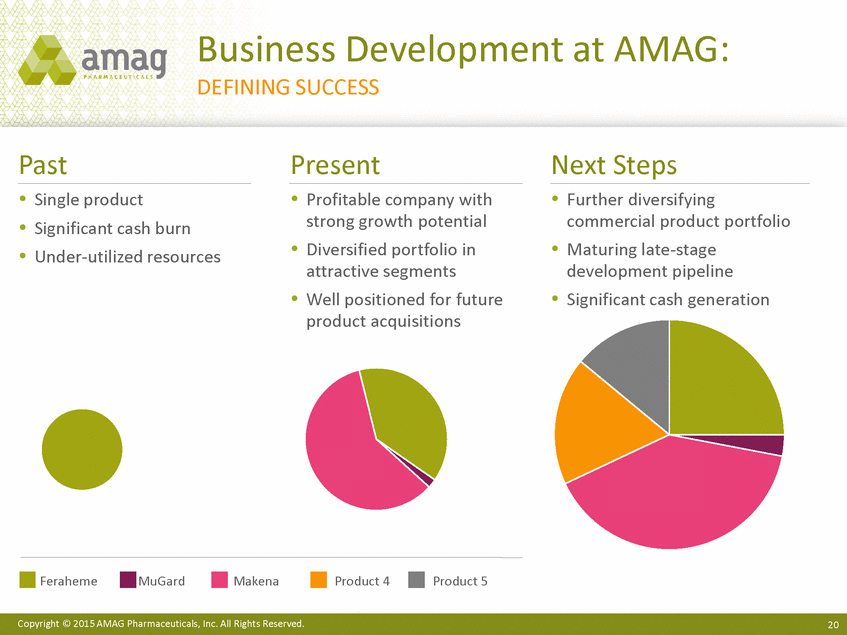
Business Development at AMAG: DEFINING SUCCESS Past Present Next Steps Single product Significant cash burn Under-utilized resources Profitable company with strong growth potential Diversified portfolio in attractive segments Well positioned for future product acquisitions Further diversifying commercial product portfolio Maturing late-stage development pipeline Significant cash generation Feraheme MuGard Makena Product 4 Product 5 Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 20 |
|
|
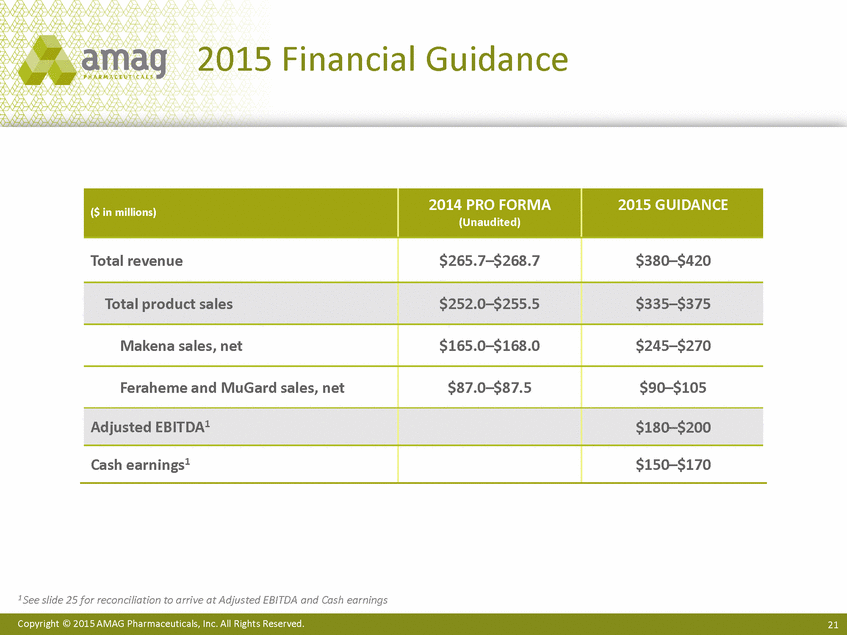
2015 Financial Guidance 1 See slide 25 for reconciliation to arrive at Adjusted EBITDA and Cash earnings Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 21 ($ in millions) 2014 PRO FORMA (Unaudited) 2015 GUIDANCE Total revenue $265.7–$268.7 $380–$420 Total product sales $252.0–$255.5 $335–$375 Makena sales, net $165.0–$168.0 $245–$270 Feraheme and MuGard sales, net $87.0–$87.5 $90–$105 Adjusted EBITDA1 $180–$200 Cash earnings1 $150–$170 |
|
|
Investment Highlights High-growth spec pharma company Expected strong cash flow and earnings Diversified portfolio in attractive market segments Proven leadership team Track record of operational excellence Well positioned for future product acquisitions Hematology/Oncology, Nephrology & Hospital Maternal Health 1 Includes AMAG and Lumara Health product sales as though Lumara Health maternal health business had been acquired at the beginning of 2014 Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 22 Key Financials (as of 12/31/14) 2014 pro forma product sales: ~$254MM1 Market cap: $1.1B Shares outstanding: 25.6 million Cash balance: ~$144MM Debt: $540MM |
|
|
Well positioned for success in 2015 … and beyond AMAG Pharmaceuticals, Inc. 1100 Winter Street Waltham, MA 02451 617.498.3300 A SPECIALTY PHARMACEUTICAL COMPANY DEDICATED TO BRINGING TO MARKET THERAPIES THAT IMPROVE PATIENTS’ LIVES www.amagpharma.com Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
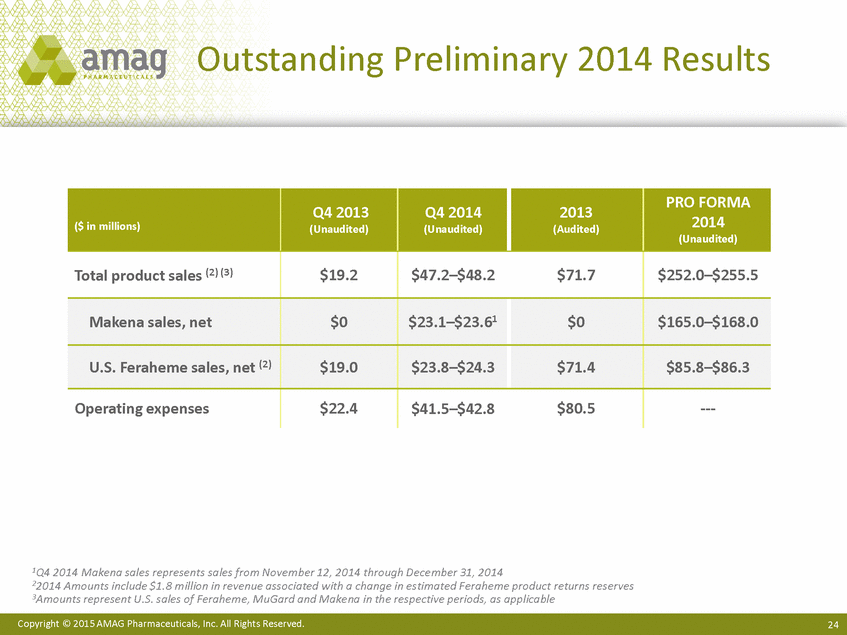
Outstanding Preliminary 2014 Results 2014 1Q4 2014 Makena sales represents sales from November 12, 2014 through December 31, 2014 22014 Amounts include $1.8 million in revenue associated with a change in estimated Feraheme product returns reserves 3Amounts represent U.S. sales of Feraheme, MuGard and Makena in the respective periods, as applicable Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 24 ($ in millions) Q4 2013 (Unaudited) Q4 2014 (Unaudited) 2013 (Audited) PRO FORMA (Unaudited) Total product sales (2) (3) $19.2 $47.2–$48.2 $71.7 $252.0–$255.5 Makena sales, net $0 $23.1–$23.61 $0 $165.0–$168.0 U.S. Feraheme sales, net (2) $19.0 $23.8–$24.3 $71.4 $85.8–$86.3 Operating expenses $22.4 $41.5–$42.8 $80.5 --- |
|
|
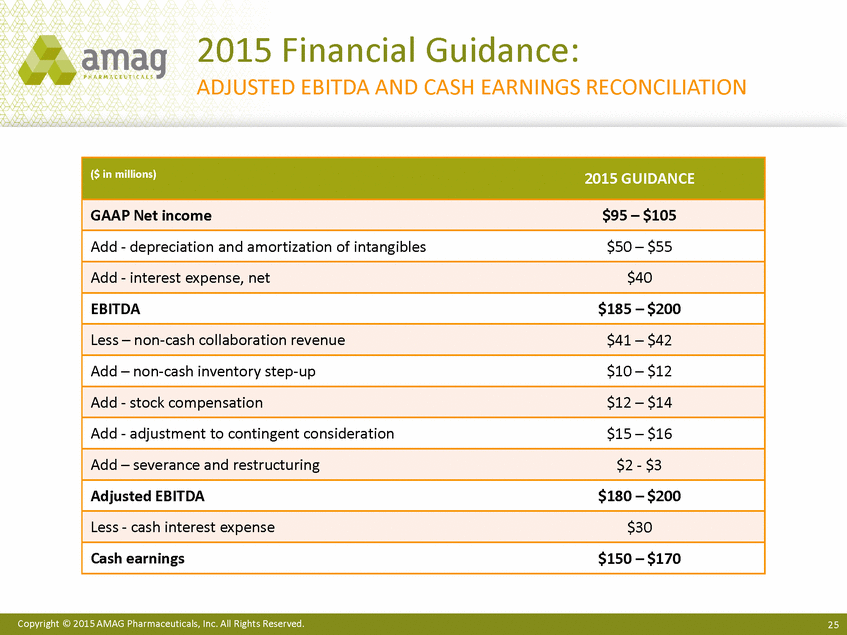
2015 Financial Guidance: ADJUSTED EBITDA AND CASH EARNINGS RECONCILIATION Copyright © 2015 AMAG Pharmaceuticals, Inc. All Rights Reserved. 25 ($ in millions)2015 GUIDANCE GAAP Net income$95 – $105 Add - depreciation and amortization of intangibles$50 – $55 Add - interest expense, net$40 EBITDA$185 – $200 Less – non-cash collaboration revenue$41 – $42 Add – non-cash inventory step-up$10 – $12 Add - stock compensation$12 – $14 Add - adjustment to contingent consideration$15 – $16 Add – severance and restructuring$2 - $3 Adjusted EBITDA$180 – $200 Less - cash interest expense$30 Cash earnings$150 – $170 |