Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AERIE PHARMACEUTICALS INC | d850110d8k.htm |
| EX-99.1 - EX-99.1 - AERIE PHARMACEUTICALS INC | d850110dex991.htm |
 Aerie Pharmaceuticals, Inc.
Company Overview
January 12 -
15, 2015
Building a Major
Ophthalmic
Pharmaceutical
Company
Exhibit 99.2 |
 2
Important Information
Any discussion of the potential use or expected success of our product candidates
is subject to our product candidates being approved by regulatory
authorities. In addition, any discussion of clinical data results for our
Rhopressa ™
and Roclatan
™
product candidates relate to the results in our Phase 2 clinical
trials.
The information in this presentation is current only as of its date and may have
changed or may change in the future. We undertake no obligation to update
this information in light of new information, future events or otherwise. We
are not making any representation or warranty that the information in this
presentation is accurate or complete.
Certain
statements
in
this
presentation
are
“forward-looking
statements”
within
the
meaning
of
the
federal
securities laws, including beliefs, expectations, estimates, projections and
statements relating to our business plans, prospects and objectives, and the
assumptions upon which those statements are based. Words such as
“may,” “will,”
“should,”
“would,”
“could,”
“believe,”
“expects,”
“anticipates,”
“plans,”
“intends,”
“estimates,”
“targets,”
“projects”
or similar expressions are intended to identify these forward-
looking statements. These statements are based on the Company’s current plans
and expectations. Known and unknown risks, uncertainties and other factors
could cause actual results to differ materially from those contemplated by
the statements. In evaluating these statements, you should specifically
consider various factors that may cause our actual results to differ materially
from any forward-looking statements. These risks and uncertainties are
described more fully in the quarterly and annual reports that
we
file
with
the
SEC,
particularly
in
the
sections
titled
“Risk
Factors”
and
“Management’s
Discussion
and
Analysis
of
Financial
Condition
and
Results
of
Operation.”
Such
forward-looking
statements
only
speak as of the date they are made. We undertake no obligation to publicly update
or revise any forward- looking statements, whether because of new
information, future events or otherwise, except as otherwise required by
law. |
 3
Rhopressa
TM
Triple Action
All Products Fully
Owned by Aerie
Roclatan
TM
Quadruple Action
•
Fixed
combination
of
Rhopressa
TM
and
latanoprost
•
P2b achieved all clinical endpoints, P3 start mid-2015
•
Potentially most efficacious IOP-lowering therapy
•
Inhibits ROCK and NET, targets diseased tissue
•
Consistent
IOP
lowering,
lowers
Episcleral
Venous
Pressure
•
Expect P3 efficacy data mid-Q2 2015, NDA filing mid-2016
Large Market
Opportunity
•
$4.5B US/EU/JP Market with significant unmet needs -
growing to more than $8B
by 2023
•
Multiple MOAs, once daily, high efficacy and safety
•
Late-stage/potential blockbuster revenue opportunity
•
Full patent protection through at least 2030
•
Plan to commercialize products ourselves in North America
(and potentially Europe) while partnering in Japan
•
Grow
pipeline
via
R&D
efforts,
in-license
and/or
acquisition
Aerie –
Building a Major Ophthalmic
Pharmaceutical Company |
 4
Largest Rx market in ophthalmology: $4.5B US/EU/JP*
•
US
Glaucoma
patients:
2.7M
growing
to
4.3M
by
2030
1
•
Patients on more than one drug to control disease: 50%
No commonly prescribed drugs:
•
target the diseased tissue
•
relax the main fluid drain and lower EVP
•
show consistent efficacy across a broad range of pressures
*
IMS 2013
1
National Eye Institute
Glaucoma Market
Unmet Needs
Expanding Market |
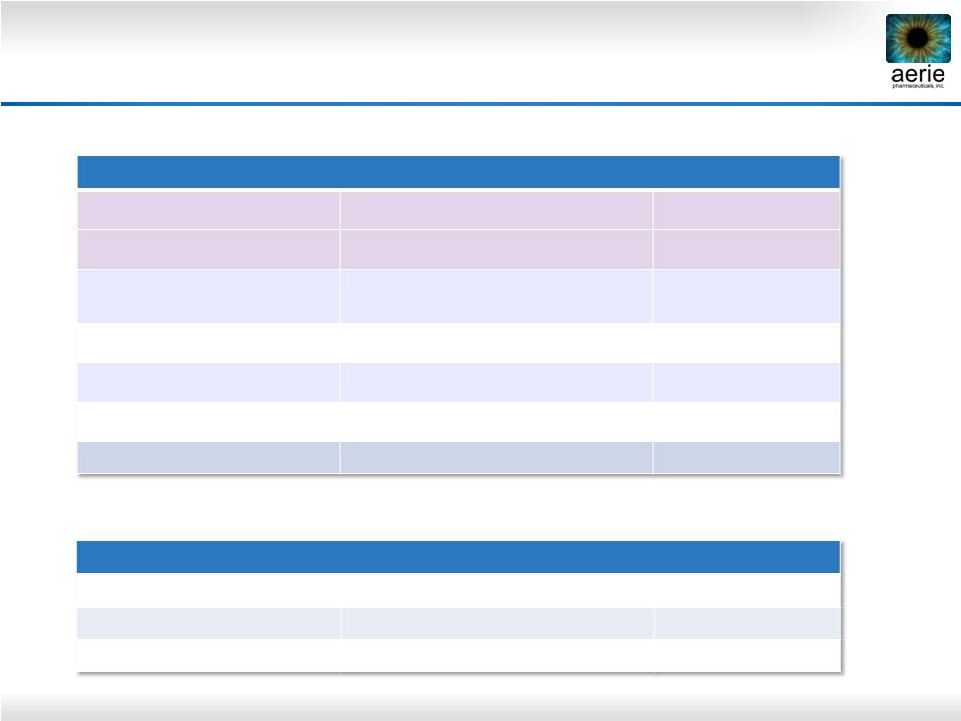
 5
FY 2013 U.S. Glaucoma Market = $2.0B; 31.5M TRx
Market Share in TRx
Current Product Dashboard
PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic
Anhydrase Inhibitor Once Daily
2-3 Times Daily
Bimatoprost
Travoprost
Latanoprost
BB
BB Fixed Combo
AA
CAI
15%
8%
10%
10%
10%
31%
14%
PGA Market
Non-PGA Market |
 6
New MOAs
Rhopressa
TM
(Aerie
AR-13324)
ROCK/NET inhibitor (qd)
Phase 3
Roclatan
TM
(Aerie PG324)
ROCK/NET inhibitor + PGA (qd)
Phase 3
K-115 (Kowa)
ROCK inhibitor (bid)
Approved in Japan*
Adjunctive Therapy
AMA0076 (Amakem)
ROCK inhibitor (bid)
Phase 2a
INO-8875 (Inotek)
Adenosine-A1 agonist (bid)
Phase 2
OPA-6566 (Acucela)
Adenosine-A2a agonist (bid)
Phase 1/2
SYL040012 (Sylentis)
RNAi beta blocker (qd)
Phase 2
New PGAs: not usable as add-on to current PGAs
Rhopressa
TM
and Roclatan
TM
: advanced triple and quadruple MOAs
New PGAs
BOL-303259 (B+L)
NO donating latanoprost (qd)
Phase 3
DE-117 (Santen)
EP2 agonist (qd)
Phase 2a
ONO-9054 (Ono)
FP/EP3 agonist (qd)
Phase 1
Glaucoma Competitors in Pipeline
* Approved in Japan 9/29/2014 |
 7
2023 U.S. Glaucoma Market
and Market Share Projections*
Projected Market Share in TRx
PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic
Anhydrase Inhibitor Data Source: IMS 2013 FY TRX. Internal projection
*Projected market shares compiled by an independent market research company
U.S. Glaucoma Market = $4.9B; 41.7 M TRx
TRx Growth 2013-2023 = 2.3%
Roclatan™
+ Rhopressa™
May Capture Over 40% Market Share
Based on 200 U.S. Physician Responses to Survey
PGA’s
BB
BB Fixed Combo
AA
CAI
Rhopressa
™
Roclatan™
25%
6%
9%
5%
5%
31%
18% |
 8
Managed Care Market Research Summary
* Respondents ranked products on a scale of 1-7
** After pricing was disclosed and discussed
Payers
were
impressed
by
Roclatan
TM
’s unprecedented lowering of IOP
Payers’
score
on
the
overall
utility
of
Rhopressa
TM
was
4.7*
(Market research firm states that scores above 4 are high)
Payers’
score
on
the
overall
utility
of
Roclatan
TM
was 5.2*
(Market research firm states that scores above 5 are rare)
100%
of
respondents
placed
Rhopressa
TM
and
Roclatan
TM
in
Tier 2 or Tier 3 of Commercial and Medicare Part D
formularies.**
There were no “Not Covered”
recommendations.
Interviews with 12 decision makers whose companies cover over 42
million Commercial, Medicare and Medicaid lives
Payers
were
receptive
to
Rhopressa
TM
’s new MOAs and efficacy at any baseline IOP |
 9
increase
Decreases Fluid
Inflow/Production
(Ciliary Processes)
Increases Fluid Outflow:
Secondary Drain
(Uveoscleral Pathway)
Increases Fluid Outflow:
Primary
Drain-
Lowers EVP -
(Episcleral Venous Pressure)
Rhopressa
™
Roclatan
™
AA, BB, CAI
PGAs
Aerie Products Cover the IOP-lowering Spectrum
Trabecular Meshwork (TM); |
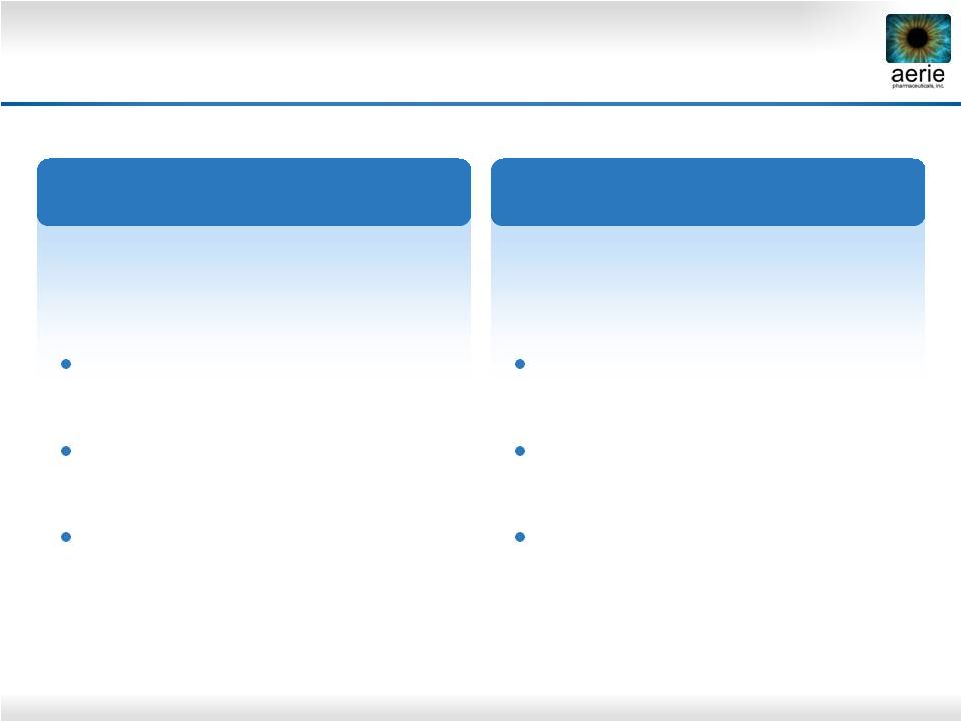 Aerie
Product Market Positioning* * Confirmed by Market Research
Triple-Action Rhopressa™
Triple-Action Rhopressa™
Quadruple-Action Roclatan™
Quadruple-Action Roclatan™
Also for PGA users as add-on
therapy
Also for PGA non-responders and
those with tolerability concerns
Also for patients with low-tension
glaucoma
For patients with IOPs above
26 mmHg
Also for patients at any IOP with
significant disease progression
10
Efficacy potentially greater than all
currently marketed drugs
Future product of choice for
patients requiring maximal IOP
lowering
Future drug of choice for the 80%
of patients with IOP of 26 mmHg
or less |
 11
Rhopressa
™
NET
RKI
NET
RKI
Trabecular
Meshwork
Triple-Action Rhopressa™
Episcleral
Veins
Schlemm’s
Canal
Ciliary Processes
Uveoscleral
Outflow
ROCK inhibition relaxes TM, increases outflow
NET inhibition reduces fluid production
ROCK inhibition lowers Episcleral Venous
Pressure (EVP)
IOP-Lowering Mechanisms
RKI
Cornea |
 12
Rhopressa™: Powerful Compound for Physiological
Lowering of IOP –
Phase 2b Results
Once-daily
PM
dosing
of
0.02%
Rhopressa™
is
highly
effective
IOP -5.7 and -6.2 mmHg on D28 and D14
Rhopressa™
efficacy results within ~1 mmHg of latanoprost
Favorable tolerability profile with no systemic side effects
Mean
Mean
Diurnal
Diurnal
IOP
IOP
–
–
Entry
Entry
IOP
IOP
22-36
22-36
mmHg
mmHg
(n=221)
(n=221)
25.6
19.5
20.0
25.5
18.4
18.7
13
18
23
28
Baseline
Day 14
Day 28
Rhopressa™
0.02% (n=71)
Latanoprost (n=76) |
 13
Rhopressa
™
Differentiated Efficacy Profile
Phase 2b baseline IOP entry requirements: 24,
22, 22 mmHg (8 AM, 10 AM, 4 PM)
Rhopressa
™
and latanoprost clinically
and statistically equivalent in patients
with moderately elevated IOPs of 22-26
mmHg
Latanoprost loses ~1 mmHg efficacy @
baselines of 22-26 mmHg
Rhopressa
™
maintains consistent
efficacy
Rhopressa
™; 1
st
product to treat the
diseased tissue (trabecular meshwork)
with a once-daily (QD) dose
Baseline: 22 –
36 mmHg (n=221)
Baseline: 22 –
26 mmHg (n=106)
-5.7
-6.8
-8
-6
-4
-2
0
0.02% Rhopressa™
Latanoprost
-5.8
-5.9
-8
-6
-4
-2
0
0.02% Rhopressa™
Latanoprost |
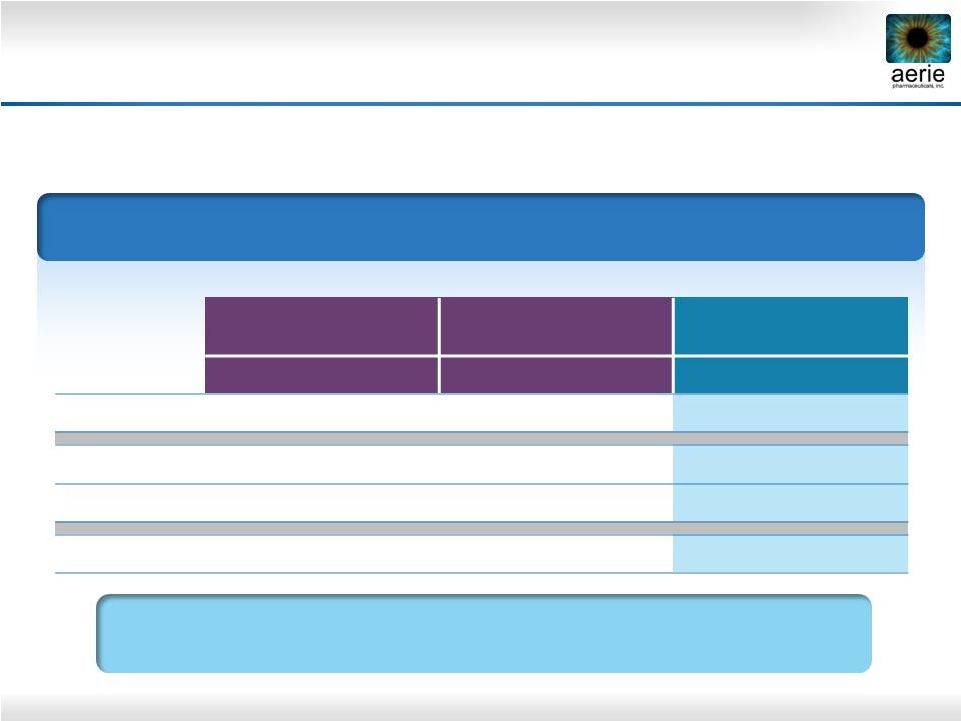 14
Sustained Effect of Rhopressa™
vs. Latanoprost
* 36 hours post dose
0.02% Rhopressa
™
Roclatan
™
Phase 2b
0.02% Rhopressa
™
Rhopressa
™
Phase 2b
0.005% latanoprost
Rhopressa
™
Phase 2b
(n = 78)
(n = 71)
(n = 76)
Baseline
26.6
27.2
26.8
Day 8
20.0
21.1
20.0
Day 29
20.3
21.2
19.2
Day 30*
21.0
22.2
22.4
8 AM Mean IOP (mmHg) by Treatment Group
Rhopressa
™
Duration is Superior to Latanoprost
36 Hours After Last Dose |
 15
Rhopressa™
EVP-Lowering Breakthrough
Phase 2b data provided first sign of EVP-lowering:
Phase 1 study in low baseline IOP subjects:
Preclinical in vivo study:
Note: Timolol and latanoprost reported to have no effect or to increase
EVP in animal models
Consistent Efficacy Across Baseline IOPs
Lowered Average IOP by Over 30%
From 16 Down to 11 mmHg
Lowered EVP by 35% |
 16
Baseline IOP*
~80% of U.S. Glaucoma Patients Have IOPs that
are
26 mmHg at Time of Diagnosis
The Baltimore Eye Survey
*
Sommer A, Tielsch JM, Katz J et al. Relationship between intraocular pressure and
primary open angle glaucoma among white and black Americans:
The
Baltimore
eye
survey.
Arch
Ophthalmol
1991;109:1090-1095
**
IWASE et al Tajimi study group. Japan Glaucoma Society. Ophthalmology, 2004 Sep, 111 (9): 1641-8.
21 mmHg
(Normal Tension Glaucoma)
>21
-
26
mmHg
>26 -
<35 mmHg
10,444 Individuals Were Screened for the Prevalence of Primary Open-Angle
Glaucoma (POAG) and the IOP at Time of Diagnosis
92%
of
Japanese
Patients
with
POAG,
IOPs
Were
21
mmHg**
20%
60%
20% |
 17
Latanoprost and Timolol Show Reduced Efficacy
at Lower Baseline IOPs
Pooled data from three latanoprost registration studies. Hedman and Alm;
European Journal Ophthalmology; 2000 Latanoprost and timolol
lose efficacy as baseline
IOPs decline
Timolol at least 1 mmHg less
effective than latanoprost
across all published baselines
Rhopressa™
equivalent/
non-inferior
to
latanoprost
at
baselines 22–26 mmHg
Timolol is the standard
comparator for glaucoma
Phase 3 trials
-16
-14
-12
-10
-8
-6
-4
-2
0
16
18
20
22
24
26
28
30
32
34
36
38
Untreated Diurnal IOP (mmHg)
Timolol (n=369)
Latanoprost (n=460) |
 18
Rhopressa™
Registration Trial Overview
Primary efficacy endpoint: IOP at nine time points through Day 90
Phase 3 entry IOP is >20 mmHg and <27 mmHg
Non-inferiority design vs. timolol
95% CI within 1.5 mmHg at all time points, within 1.0 mmHg at a majority
of time points
Combined trials to include approximately 1,300 total patients
100 patients with 12 months of safety data needed for NDA filing
Should meet efficacy requirements for EMA filing
300
patients
with
6
months
safety
data
needed
for
EMA
filing
and
100
with
12 months |
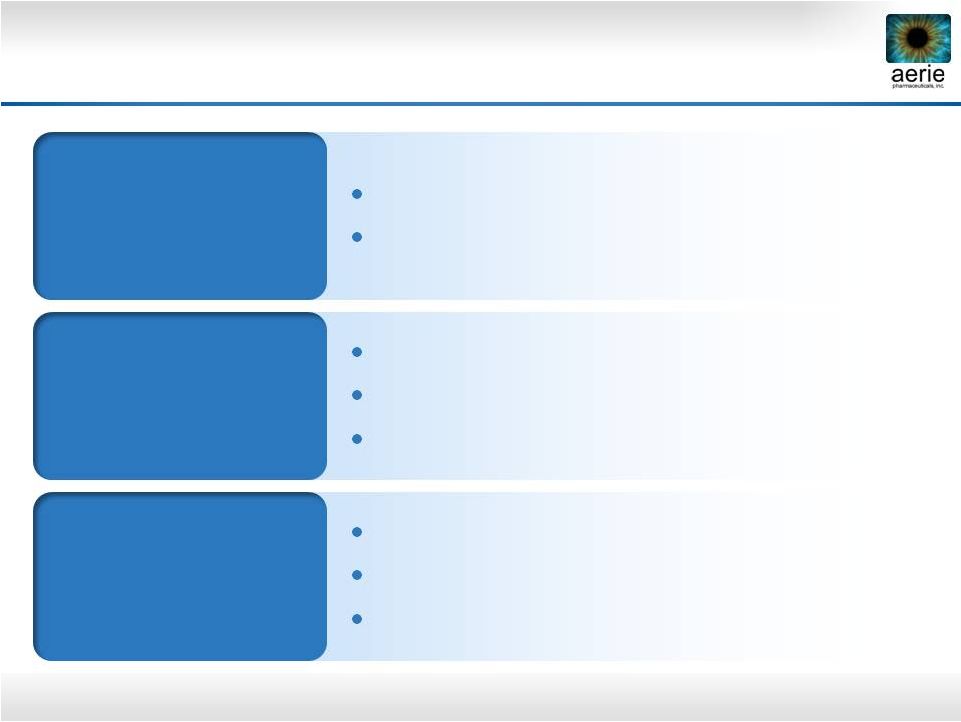 19
Rhopressa™
Registration Trial Design
*
PGAs have been shown to be less effective when dosed BID
“Rocket 1”
90-Day Efficacy
Registration Trial
Rhopressa™
0.02% QD
~200 patients
Timolol BID
~200 patients
“Rocket 2”
One Year Safety
(3 Mo. Interim
Efficacy)
Registration Trial
“Rocket 3”
One Year Safety
Registration Trial
Canada
Rhopressa™
0.02% QD
~230 patients
Rhopressa™
0.02% BID
*
~230 patients
Timolol BID
~230 patients
Rhopressa™
0.02% QD
~90 patients
Rhopressa™
0.02% BID
~90 patients
Timolol BID
~60 patients |
 20
Latanoprost
Rhopressa
™
Quadruple-Action Roclatan™
Fixed
Combination
of
Rhopressa™
with
Latanoprost
IOP-Lowering Mechanisms
ROCK inhibition relaxes TM, increases outflow
NET inhibition reduces fluid production
ROCK inhibition lowers EVP
PGA receptor activation
increases uveoscleral outflow
Ciliary Processes
Cornea
Uveoscleral
Outflow
NET
RKI
NET
Trabecular
Meshwork
Episcleral
Veins
Schlemm’s
Canal
RKI
PGA |
 21
Roclatan™
Phase 2b Clinical Trial Design
Phase 2b Protocol
Roclatan™
0.01%
vs.
Roclatan™
0.02%
vs.
Rhopressa™
0.02%
vs.
Latanoprost
All Dosed QD PM
~300 Patients
28 Days
Primary efficacy endpoint:
Mean diurnal IOP on Day 29
Two concentrations of
Roclatan™
vs. Rhopressa™
0.02% and latanoprost
Trial design follows FDA
requirement for fixed-dose
combination
Statistically significant difference
at measured time points
Higher combo efficacy vs.
components of at least 1–3
mmHg, as previously accepted
by FDA for product approval |
 22
Roclatan™
Phase 2b Clinical Trial Performance
Achieved primary efficacy measure
Superiority over each of the components on day 29
Achieved statistical superiority over the individual components at all
time points
More efficacious than latanoprost by 1.6 –
3.2 mmHg
More efficacious than Rhopressa™
by 1.7 –
3.4 mmHg
Main adverse event was hyperemia (eye redness):
Reported in 40 percent of patients
Mild for the large majority of patients
No systemic drug-related adverse events |
 23
Mean IOP at Each Time Point
Primary Efficacy Measure
0.02% Roclatan™
Achieved Statistical Superiority Over
Individual Components at All Time Points (p<0.001)
Roclatan™
Phase 2b, Intent to Treat
14
15
16
17
19
20
21
22
23
24
25
26
27
28
29
Pre
-
8AM
8AM
10AM
4PM
8AM
10AM
4PM
8AM
10AM
4PM
8AM
10AM
4PM
8AM
Study
Qual 1
Baseline
Day 8
Day 15
Day 29
Day 30
0.02% Rhopressa™
(n=78)
0.005% Latanoprost (n=73)
0.02% Roclatan™
(n=72)
18 |
 24
Roclatan™
Phase 2b, ITT
Mean IOP (mmHg)
*
Difference
between
0.02%
Roclatan™
and
latanoprost
or
Rhopressa™
Roclatan:
Produced lowest IOP
drop in any trial
Was superior to
latanoprost by 1.6–3.2
mmHg (p<0.001)
Was superior to
Rhopressa™
by 1.7–3.4
mmHg (p<0.001)
Impressive Rhopressa™
performance
0.02%
0.02%
Roclatan
Roclatan
™
™
(n = 72)
(n = 72)
0.005%
0.005%
latanoprost
latanoprost
(n = 73)
(n = 73)
0.02%
0.02%
Rhopressa
Rhopressa
™
™
(n = 78)
(n = 78)
Mean
Mean
Mean
Mean
Difference*
Difference*
Mean
Mean
Difference*
Difference*
Day 8
8 AM
17.0
19.6
-2.6
20.0
-3.1
10 AM
15.6
18.3
-2.7
18.0
-2.4
4 PM
15.6
18.6
-3.1
17.9
-2.3
Day 15
8 AM
16.5
19.6
-3.2
19.6
-3.1
10 AM
15.8
18.3
-2.4
18.7
-2.8
4 PM
15.7
18.3
-2.6
18.4
-2.7
Day 29
8 AM
16.9
19.2
-2.4
20.3
-3.4
10 AM
15.9
17.7
-1.8
18.6
-2.7
4 PM
16.8
18.4
-1.6
18.5
-1.7 |
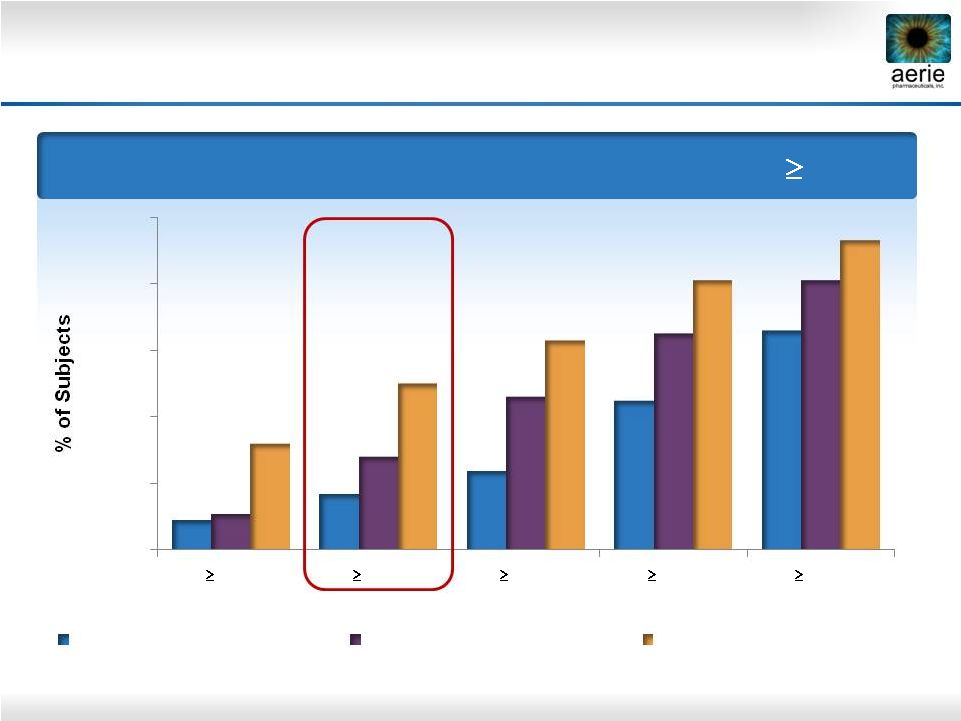 Day
29 – % of Patients with IOP Reductions of
20%
Roclatan™
Phase 2b Responder Analysis:
Goal is to Achieve Lowest IOP Possible
9%
17%
24%
45%
66%
11%
28%
46%
65%
81%
32%
50%
63%
81%
93%
0%
20%
40%
60%
80%
100%
40%
35%
30%
25%
20%
Reduction
0.02% Rhopressa™
(n=78)
0.005% Latanoprost (n=73)
0.02% Roclatan™
(n=72)
25 |
 26
Roclatan™
Phase 2b Responder Analysis:
Goal is to Achieve Lowest IOP Possible
Day 29 –
% of Subjects with IOP Reduced to <
18 mmHg
10%
21%
25%
40%
8%
18%
29%
47%
38%
46%
57%
69%
0%
20%
40%
60%
80%
100%
15 mmHg
16 mmHg
17 mmHg
18 mmHg
Reduction
0.02% Rhopressa™
(n=78)
0.005% Latanoprost (n=73)
0.02% Roclatan™
(n=72) |
 27
Diurnal
IOP in Subset Of High Responders 16 mmHg
Roclatan™
Phase 2b High Responders:
Consistent IOP Drop by Rhopressa™
and Roclatan™
-9.6
-7.2
-9.9
-9.4
-8.2
-10.6
-9.5
-9.5
-10.2
-12
-11
-10
-9
-8
-7
-6
-5
-4
-3
-2
-1
0
0.02% Rhopressa™
(n=16)
0.005% Latanoprost
(n=13)
0.02% Roclatan™
(n=31)
Day 8
Day 15
Day 29 |
 28
2014
2015
2016
2017
2018
Key Future Milestones
June
2014:
Roclatan™
Phase 2b clinical trial completed
Mid-2015:
Roclatan
™
Phase 3 initiated
Mid-2016:
Roclatan
™
Efficacy
results from Phase 3 expected
Mid-2017:
Roclatan
™
NDA filing expected
2H
2018:
Roclatan
™
Launch expected
3Q
2014:
Rhopressa
™
Phase 3 initiated
Mid-Q2
2015:
Rhopressa
™
Efficacy results from Phase 3
expected
Mid-2016:
Rhopressa
™
NDA filing expected
2H 2017: Rhopressa™
Launch expected |
 29
Financing the Future
Sufficient Capital to Fund:
$190+ Million Raised From IPO (10/13) and Follow-on Financing (9/14)
FDA
approval
and
launch
of
Rhopressa™
and
Roclatan™
Building of Aerie’s pipeline via internal discovery, licensing and M&A
|
 Building a Major
Ophthalmic
Pharmaceutical
Company |
