Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ACCELERON PHARMA INC | xlrn-2015x1x12form8xk.htm |
| EX-99.1 - EXHIBIT 99.1 - ACCELERON PHARMA INC | xlrn-2015x1x12exhibit991.htm |

Page 0 Corporate Overview January 2015

Page 1 Acceleron Forward-Looking Statements This presentation contains forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. The words ‘‘anticipate’’, ‘‘believe’’, ‘‘estimate’’, ‘‘expect’’, ‘‘forecast’’, ‘‘goal’’, ‘‘intend’’, ‘‘may’’, ‘‘plan’’, ‘‘predict’’, ‘‘project’’, ‘‘target’’, ‘‘potential’’, ‘‘will’’, ‘‘would’’, ‘‘could’’, ‘‘should’’, ‘‘continue’’, ‘‘contemplate’’, or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward- looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. Risks and uncertainties are identified under the heading “Risk Factors” included in the Company’s Annual Report on Form 10-K which was filed with the Securities and Exchange Commission (SEC) on March 17, 2014, and other filings that the Company may make with the SEC in the future. The forward-looking statements contained in this presentation reflect the Company’s current views with respect to future events, and the Company does not undertake and specifically disclaims any obligation to update any forward-looking statements.

Page 2 The Mission of Acceleron Pharma Acceleron will be a leader in the discovery, development and commercialization of therapeutics based on the body’s ability to regulate the growth and repair of its cells and tissues. By leveraging this powerful biology, Acceleron seeks to provide medicines that can have profound effects on human health

Page 3 Acceleron is a leader in discovering and developing protein therapeutics that regulate cellular growth and repair Tissue Growth and Repair is Central to Human Disease and Health • Disease is often caused by either • Failure of cells or tissues to properly grow, differentiate and maintain the proper number/strength/function (e.g. anemia, muscular dystrophies) • Defective or uncontrolled cell growth (e.g. cancer) • Acceleron is unlocking the body’s ability to regulate the growth and repair of various cells and tissues including red blood cells, muscle, bone and the vasculature

Page 4 Red Blood Cells Acceleron – Leader in Cell and Tissue Growth and Repair Red Blood Cells Bone Vasculature Increases number of red blood cells Luspatercept Sotatercept Dalantercept Increases bone mass and strength, and RBC number Inhibits blood vessel growth Muscle ACE-083 Increases muscle mass, strength and function

Page 5 Acceleron -- One of the Industry’s Most Robust Pipelines of Internally Discovered Protein Therapeutics Creating Near-term and Sustainable Value Product Indication Development Stage Commercial Rights Luspatercept β-Thalassemia Phase 3 to start in 2015 • Co-development • North American co-promote • Celgene funds 100% • Royalties starting in low 20% range and escalating to mid 20% Luspatercept / Sotatercept Myelodysplastic Syndromes Phase 3 to start in 2015 Sotatercept Chronic Kidney Disease Phase 2b ongoing Luspatercept / Sotatercept Myelofibrosis Phase 2 ongoing (Investigator Sponsored Trial) Luspatercept / Sotatercept Multiple Myeloma Phase 2 ongoing (Investigator Sponsored Trial) Luspatercept / Sotatercept Diamond-Blackfan Anemia Phase 2 ongoing (Investigator Sponsored Trial) Dalantercept Renal Cell Carcinoma Phase 2 ongoing Hepatocellular Carcinoma Phase 1b ongoing ACE-083 Muscle Wasting Phase 1 ongoing Phase 2 to start in 2015 Undisclosed Muscle Preclinical Undisclosed Bone Preclinical Undisclosed Skin Preclinical Undisclosed Fibrosis Preclinical Entire pipeline of protein therapeutics internally discovered, designed, manufactured and developed

Page 6 Highly Productive 2014 Sets Stage for 2015 Value Creation Demonstrated phase 3-enabling data in MDS Demonstrated phase 3-enabling data in beta-thalassemia Demonstrated proof-of-concept on bone density and vascular calcification in ESRD patients Advanced exploratory phase 2 studies in myelofibrosis and multiple myeloma Demonstrated safety and preliminary activity of combination of VEGF and ALK1 inhibition in advanced renal cell cancer Advanced muscle program into human clinical trials Continued research productivity in muscle, bone, fibrosis and other undisclosed research programs

Page 7 Hematology Franchise Luspatercept and Sotatercept

Page 8 Myelodysplastic Syndromes (MDS)

Page 9 MDS Opportunity: Develop Better Treatment for Lower Risk Patients In the U.S., recombinant erythropoietin sales in MDS are approximately $600 million EPO MDS (125,000 patients in US/EU-G5)) Low Risk (70,000 patients US/EU-G5) High Risk (55K patients) RBC transfusions (± iron chelation) Revlimid® Vidaza® Dacogen® MDS are a group of hematologic disorders characterized by cytopenias (anemia) and ineffective hematopoiesis. Ineffective erythropoiesis contributes to anemia and severity of disease in MDS Epidemiology Current Treatment Therapeutic Goal • Increase hemoglobin levels • Achieve transfusion independence

Page 10 MDS Program Highlights • Luspatercept data in Myelodysplastic Syndromes selected for "Best of ASH" by the American Society of Hematology • Preliminary Phase 2 results demonstrated – Increases in hemoglobin levels – Decreases in transfusion burden with patients becoming transfusion independent – Biomarkers identified that align with mechanism of action of luspatercept and may be valuable as part of upcoming Phase 3 program • 2015 program goals – Complete ongoing dose-titration expansion cohort – Hold end of Phase 2 meetings with health authorities – Initiate Phase 3 clinical trial in MDS

Page 11 MDS Development Plan: Initiate Phase 3 in 2015 Q1 Q2 Q3 Q4 Confirm dose-titration in expansion cohort (n=30) Health Authority meetings re: Phase 3 Initiate Phase 3 trials 2015 Q3 Q4 2014 Completed dose escalation cohorts (n=27)

Page 12 MDS Phase 3 Development Opportunities EPO Low Risk MDS (70,000 patients US/EU-G5) Revlimid® Vidaza® Dacogen® 2nd Line Opportunity: ESA experienced patients • Endpoint: Incidence of RBC Transfusions • Randomized vs. best supportive care 1st Line Opportunity: ESA naïve patients • Endpoint: Incidence of RBC Transfusions • Randomized vs. ESA

Page 13 β-thalassemia

Page 14 Beta-Thalassemia Opportunity: Develop Treatment for Both Non-Transfusion and Transfusion Dependent Patients Beta-Thalassemia (40,000 patients in US/EU) Non-Transfusion Dependent (20,000 patients US/EU) Transfusion Dependent (20,000 patients US/EU) RBC transfusions (iron chelation used to manage iron overload) Epidemiology Current Treatment Therapeutic Goal • Increase hemoglobin levels • Reduce transfusion burden • Reduce iron overload • Improve disease complications No effective therapy (iron chelation used to manage iron overload)
Page 15 Beta-Thalassemia Program Highlights • Preliminary Phase 2 results demonstrated – Activity in both non-transfusion and transfusion dependent patients that met/exceeded endpoints expected to be used in phase 3 trials • Increases in hemoglobin levels • Decreases in transfusion burden • Reductions in iron overload • Improvements in disease complications such as leg ulcers • 2015 program goals – Complete ongoing dose-titration expansion cohort – Hold end of Phase 2 meetings with health authorities – Initiate Phase 3 clinical trial of luspatercept in beta-thalassemia
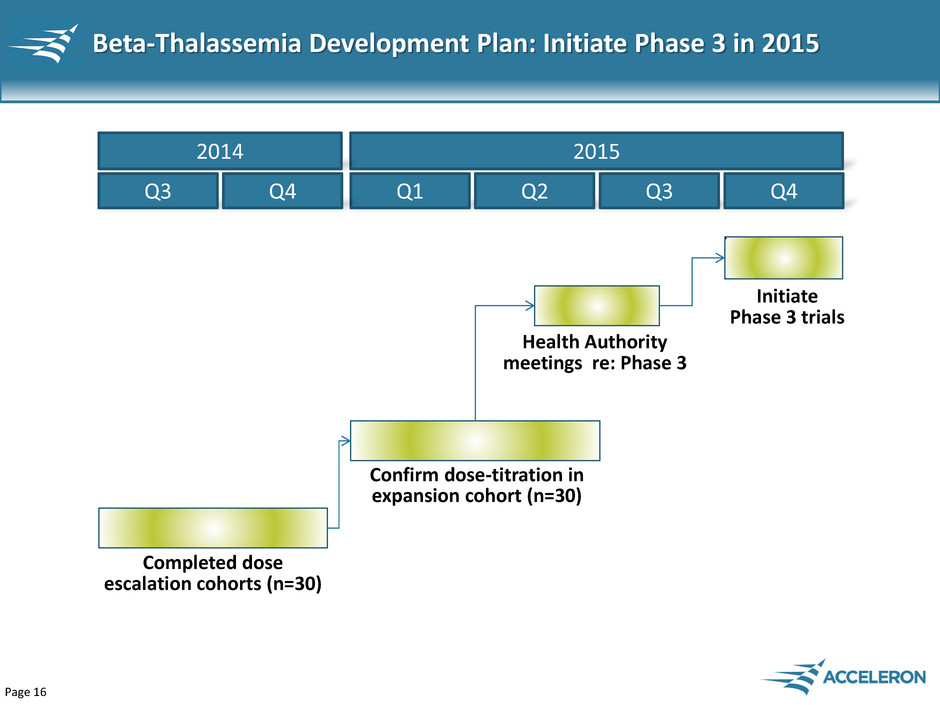
Page 16 Beta-Thalassemia Development Plan: Initiate Phase 3 in 2015 Q1 Q2 Q3 Q4 Confirm dose-titration in expansion cohort (n=30) Health Authority meetings re: Phase 3 Initiate Phase 3 trials 2015 Q3 Q4 2014 Completed dose escalation cohorts (n=30)

Page 17 Beta-Thalassemia Phase 3 Program Beta-Thalassemia (40K patients in US/EU) Non-Transfusion Dependent (20,000 patients US/EU) Transfusion Dependent (20,000 patients US/EU) Phase 3 – NTD Patients • Endpoints • Primary: hemoglobin increase ≥ 1.5 g/dL • Secondary: decreases in iron overload • Randomized, placebo- controlled Patient Populations Phase 3 – TD Patients • Endpoint • Primary: 25%-40% decrease in transfusion burden • Randomized, placebo- controlled

Page 18 Sotatercept Opportunity in Chronic Kidney Disease
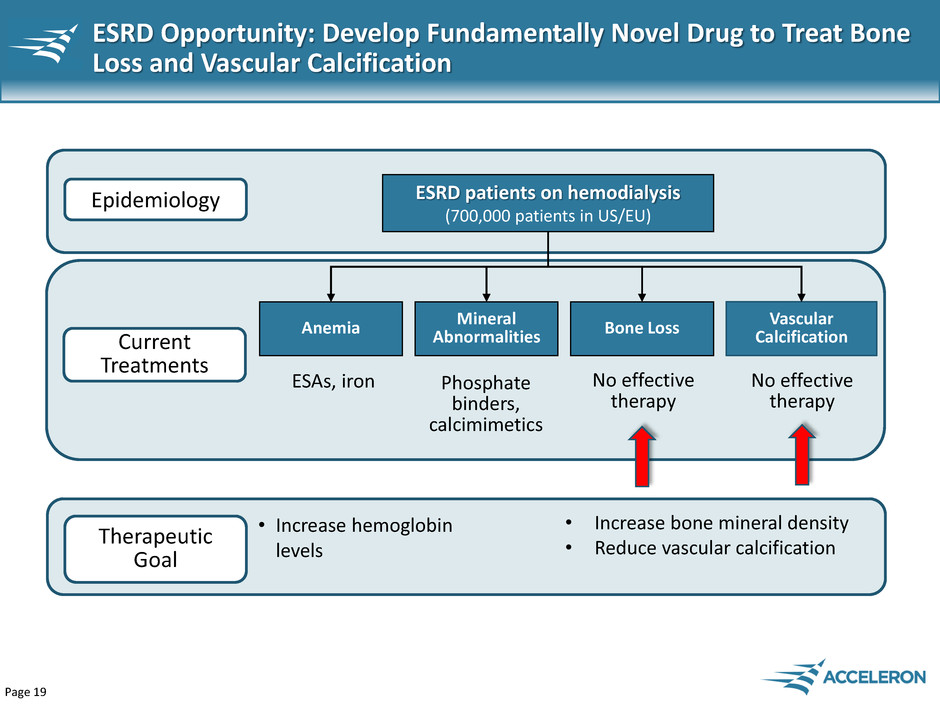
Page 19 ESRD Opportunity: Develop Fundamentally Novel Drug to Treat Bone Loss and Vascular Calcification ESRD patients on hemodialysis (700,000 patients in US/EU) Vascular Calcification Bone Loss Epidemiology Current Treatments Therapeutic Goal • Increase bone mineral density • Reduce vascular calcification Anemia Mineral Abnormalities ESAs, iron Phosphate binders, calcimimetics No effective therapy No effective therapy • Increase hemoglobin levels

Page 20 ESRD Program Highlights • Preliminary sotatercept Phase 2 results demonstrated – Increases in cortical bone mineral density – Reductions in vascular calcification – Increases in hemoglobin levels • 2015 program goals – Complete ongoing Phase 2a study – Compete dose finding stage of ongoing Phase 2b study – Initiate randomized, controlled stage of Phase 2b study
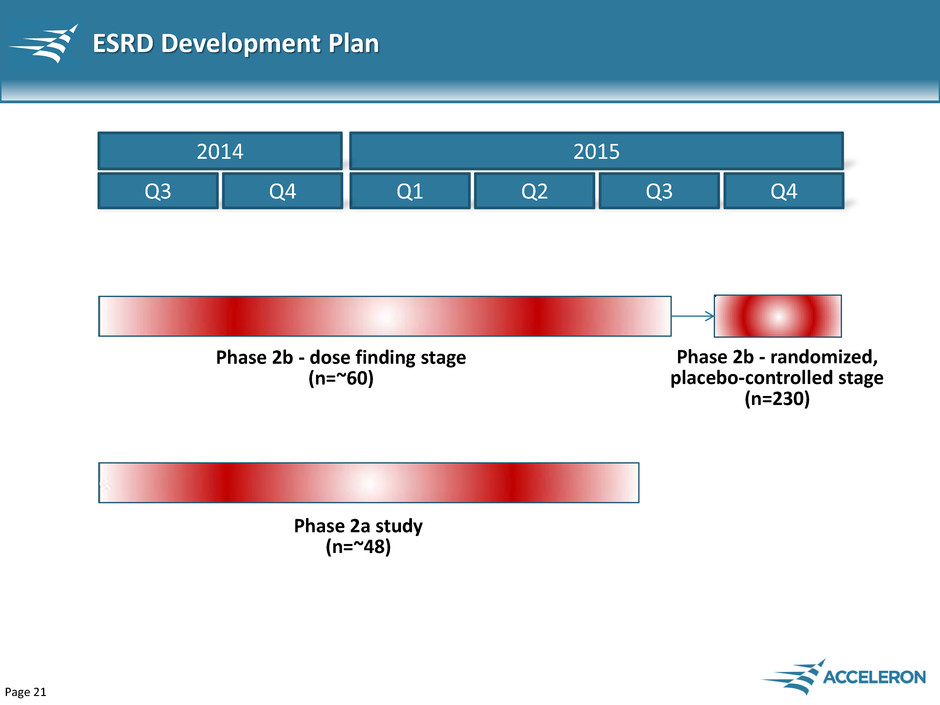
Page 21 ESRD Development Plan Q1 Q2 Q3 Q4 Phase 2b - dose finding stage (n=~60) 2015 Q3 Q4 2014 Phase 2a study (n=~48) Phase 2b - randomized, placebo-controlled stage (n=230)

Page 22 Muscle Franchise ACE-083
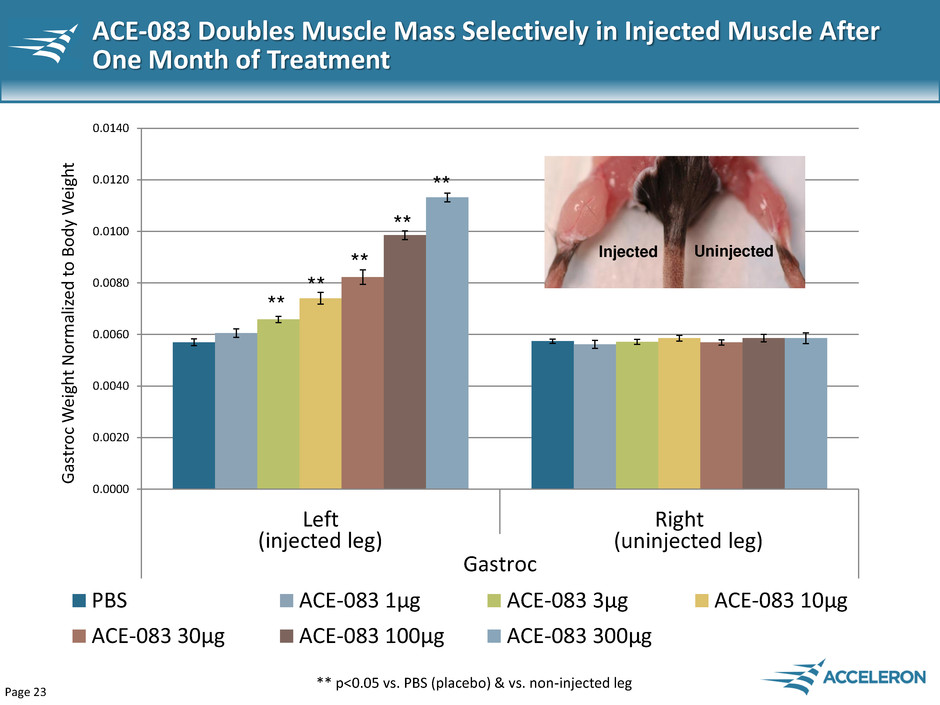
Page 23 ACE-083 Doubles Muscle Mass Selectively in Injected Muscle After One Month of Treatment 0.0000 0.0020 0.0040 0.0060 0.0080 0.0100 0.0120 0.0140 Left Right Gastroc Ga st roc W eig h t N o rm ali ze d t o B o d y W eig h t PBS ACE-083 1μg ACE-083 3μg ACE-083 10μg ACE-083 30μg ACE-083 100μg ACE-083 300μg ** p<0.05 vs. PBS (placebo) & vs. non-injected leg ** ** ** ** ** Injected Uninjected (injected leg) (uninjected leg)
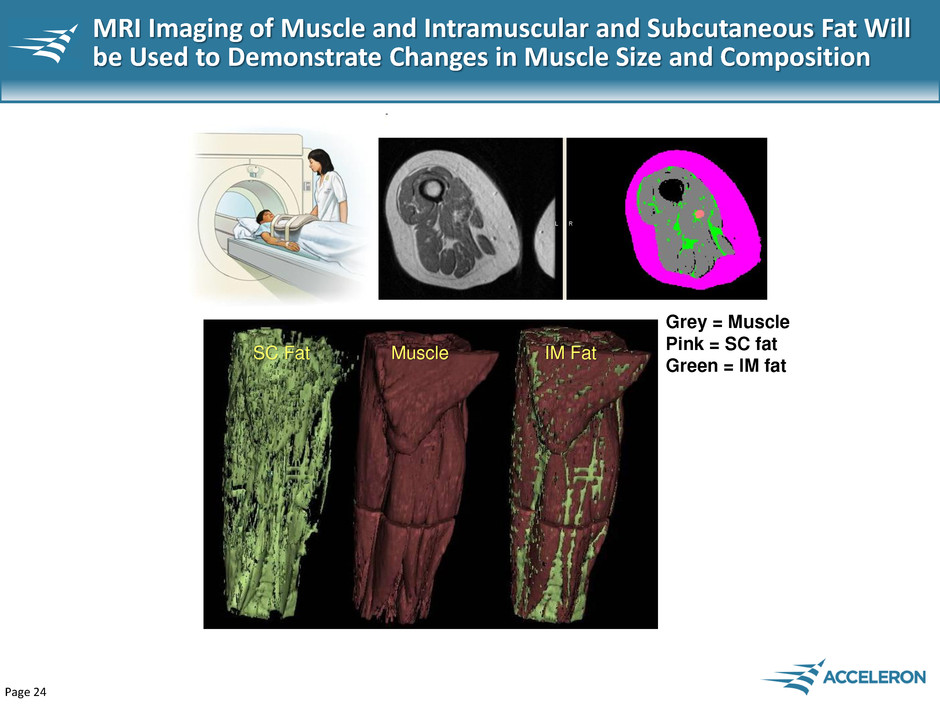
Page 24 MRI Imaging of Muscle and Intramuscular and Subcutaneous Fat Will be Used to Demonstrate Changes in Muscle Size and Composition SC Fat Muscle IM Fat Grey = Muscle Pink = SC fat Green = IM fat

Page 25 Affected Muscle Groups in Muscle Wasting Diseases Potential Clinical Indications For ACE-083 Facioscapulohumeral Muscular Dystrophy (16,000 patients – US) Inclusion Body Myositis (11,000 patients – US) Muscular Dystrophy Association USA

Page 26 ACE-083 Development Plan: Initiate Phase 2 Studies in 2015 Q1 Q2 Q3 Q4 2015 Q3 Q4 2014 Phase 1 study (n=40) Phase 2 study

Page 27 Dalantercept Novel anti-angiogenic agent based on the Activin Receptor-Like Kinase 1 (ALK1)

Page 28 Dalantercept Opportunity in RCC and HCC Renal Cell Carcinoma (65,000 new patients/year in US) Chemo, TACE Epidemiology Current Treatments VEGFR TKIs mTOR inhibitors Hepatocellular Carcinoma (31,000 new patients/year in US) • More than 70% of all RCC patients currently receive an anti-VEGF drug • Worldwide sales of anti-VEGFs in RCC were $2.6 billion in 2013 • More than 40% of all HCC patients currently receive sorafenib • Worldwide sales of anti-VEGFs (sorafenib) in HCC were $766 million in 2013 Immuno- Oncology VEGFR TKIs

Page 29 Dalantercept + anti-VEGF Development Plan Q1 Q2 Q3 Q4 Randomized, placebo-controlled stage (n=130) RCC (+ axitinib) HCC (+ sorafenib) HCC(+ sorafenib) 2015 Q3 Q4 2014 RCC (+ axitinib) Completed dose escalation cohorts (n=29) Dose escalation cohorts Dose expansion cohort

Page 30 Financial Highlights • Cash and cash equivalents at the end of September 2014 were $189 million • Cash and cash equivalents expected to provide the Company funding for three years into the second half of 2017
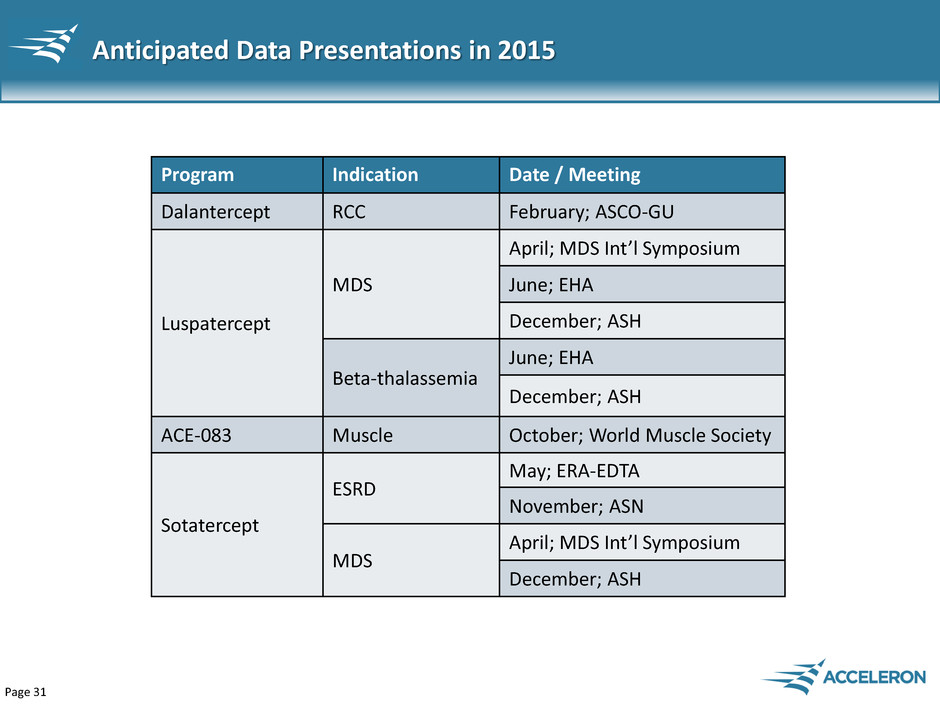
Page 31 Anticipated Data Presentations in 2015 Program Indication Date / Meeting Dalantercept RCC February; ASCO-GU Luspatercept MDS April; MDS Int’l Symposium June; EHA December; ASH Beta-thalassemia June; EHA December; ASH ACE-083 Muscle October; World Muscle Society Sotatercept ESRD May; ERA-EDTA November; ASN MDS April; MDS Int’l Symposium December; ASH
Page 32 Poised for Significant Value Creation in 2015 Complete phase 2 studies in MDS and beta-thalassemia Finalize phase 3 development plans with health authorities for MDS and beta-thalassemia Initiate phase 3 clinical trials in MDS Initiate phase 3 clinical trials in beta-thalassemia Initiate randomized, controlled stage of phase 2b study in ESRD patients Announce top line data from Phase 2 trial of dalantercept with axitinib in renal cell carcinoma Demonstrate safety of combination of dalantercept and sorafenib in hepatocellular carcinoma Initiate phase 2 trials with novel muscle agent ACE-083 Conduct IND-enabling work to advance at least one new protein therapeutic to the clinic in 2016

Page 33 • Two Phase 3 studies • Six Phase 2 studies • One Phase 1 study • 4 protein therapeutics in clinical trials • Numerous preclinical programs • Approvals in up to 5 indications • Multiple Phase 3 studies • 8 protein therapeutics in clinical trials • Sales and marketing organization in U.S. • Cashflow positive Acceleron’s 2020 Vision 2015 2020 Our highly productive discovery and development platform is creating one of the most impressive pipelines in the industry with the potential to transform Acceleron into a leading profitable, multi-billion dollar biopharmaceutical company

Page 34 Additional Information

Page 35 Myelodysplastic Syndromes (MDS) Presented at ASH 2014

Page 36 Luspatercept PACE-MDS Study Design Cohort 7 1.75 mg/kg (N=3) Cohort 5 1.0 mg/kg (N=3) Cohort 6 1.33 mg/kg (N=6) Cohort 1 0.125 mg/kg (N=3) Cohort 2 0.25 mg/kg (N=3) Cohort 3 0.5 mg/kg (N=3) Cohort 4 0.75 mg/kg (N=6) 3 Months Treatment Expansion Cohort Individually titrated dose (N=30) Data available Active Data from patients who completed treatment are presented; includes 2 patients in Cohort 7 Patients completing base study can enroll into a 12-month extension study

Page 37 Baseline Characteristics All Patients N = 26 Age, yr, median (range) 71 (27-88) Sex, males (%) 13 (50%) Prior ESA treatment, n (%) 14 (54%) Prior lenalidomide treatment, n (%) 5 (19%) Low Transfusion Burden (LTB) N = 7 (27%) Hemoglobin, g/dL, median (range) 9.1 (8.3-9.7) Units RBC/8 weeks, median (range) 0 (0-2) High Transfusion Burden (HTB) N = 19 (73%) Units RBC/8 weeks, median (range) 6 (4-13) Data as of 03 Oct 2014

Page 38 Pharmacokinetic and Safety Summary Pharmacokinetics Dose-dependent increase in Cmax and area under curve (AUC) Mean half-life (t½) is approximately 14 days, supporting Q3W dosing Safety No drug-related serious adverse events (AEs) One possibly related grade 3 AE of blast cell count increase Majority of adverse events were grade 1 or 2 Preferred Term n (%) 0.125 mg/kg (N=3) 0.25 mg/kg (N=3) 0.50 mg/kg (N=3) 0.75 mg/kg (N=6) 1.0 mg/kg (N=3) 1.33 mg/kg (N=6) 1.75 mg/kg (N=2) Overall (N=26) Diarrhea 0 1 1 1 0 1 0 4 (15%) Muscle Spasms 0 0 2 0 1 0 1 4 (15%) Bone Pain 0 0 1 0 2 0 0 3 (12%) Fatigue 0 0 0 0 0 3 0 3 (12%) Myalgia 0 1 1 0 1 0 0 3 (12%) Nasopharyngitis 0 1 0 2 0 0 0 3 (12%) Adverse Events in ≥ 10% of Patients (Regardless of Causality): Data as of 03 Oct 2014

Page 39 Patient Subgroup 0.125-0.5 mg/kg (N=9) n (%) 0.75-1.75 mg/kg (N=17) n (%) LTB patients (N=7) 0/2 (0%) 2/5 (40%) HTB patients (N=19) 2/7 (29%) 5/12 (42%) All patients (N=26) 2/9 (22%) 7/17 (41%) HI-E (IWG): LTB: Hemoglobin increase ≥1.5 g/dL for ≥8 weeks HTB: Reduction of ≥4 units RBCs transfused over 8 weeks HI-E, hematologic improvement-erythroid IWG, International Working Group LTB, low transfusion burden; HTB, high transfusion burden Data as of 03 Oct 2014 Luspatercept Increased Hemoglobin Levels and Reduced Transfusions in Lower Risk MDS Patients Luspatercept MDS Phase 2 Clinical Trial

Page 40 Data as of 03 Oct 2014 LTB, low transfusion burden M ean ( SD ) M ax. C h an ge in H emogl o b in ( g/ d L) 0.8 1.0 2.2 3.5 0.0 1.0 2.0 3.0 4.0 0.125 (n=1) 0.25 (n=1) 0.75 (n=3) 1.75 (n=2) Dose Group (mg/kg) Luspatercept Produced Dose Dependent Increases in LTB Patients Luspatercept MDS Phase 2 Clinical Trial Maximum Hemoglobin Increase
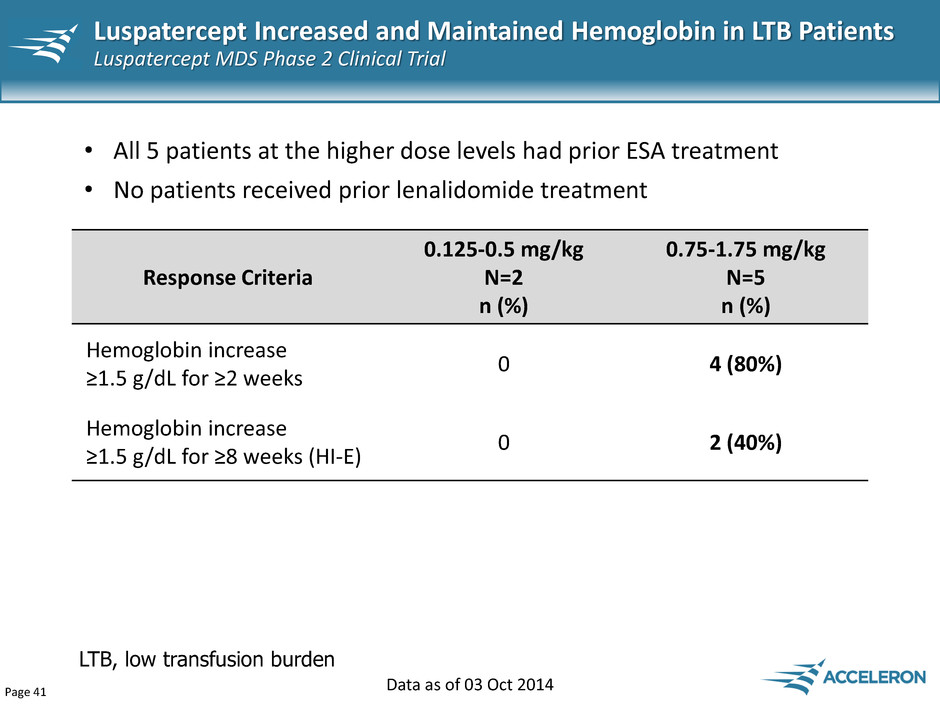
Page 41 Response Criteria 0.125-0.5 mg/kg N=2 n (%) 0.75-1.75 mg/kg N=5 n (%) Hemoglobin increase ≥1.5 g/dL for ≥2 weeks 0 4 (80%) Hemoglobin increase ≥1.5 g/dL for ≥8 weeks (HI-E) 0 2 (40%) • All 5 patients at the higher dose levels had prior ESA treatment • No patients received prior lenalidomide treatment Data as of 03 Oct 2014 LTB, low transfusion burden Luspatercept Increased and Maintained Hemoglobin in LTB Patients Luspatercept MDS Phase 2 Clinical Trial

Page 42 Hemoglobin ( g /d L) Weeks -- 0.56 mg/kg (dose reduced by 25%) Data as of 03 Oct 2014 LTB, low transfusion burden 2 0 1 2 3 4 5 6 7 8 6 7 8 9 10 11 12 13 -8 -6 -3 BL 3 6 9 12 16 20 24 Units Transfused Hemoglobin Follow-up Period Dose held for Hb >12 g/dL -- 0.75 mg/kg (starting dose) 78 year old male RCMD-RS EPO non-responder Luspatercept Activity in a LTB Patient Luspatercept MDS Phase 2 Clinical Trial
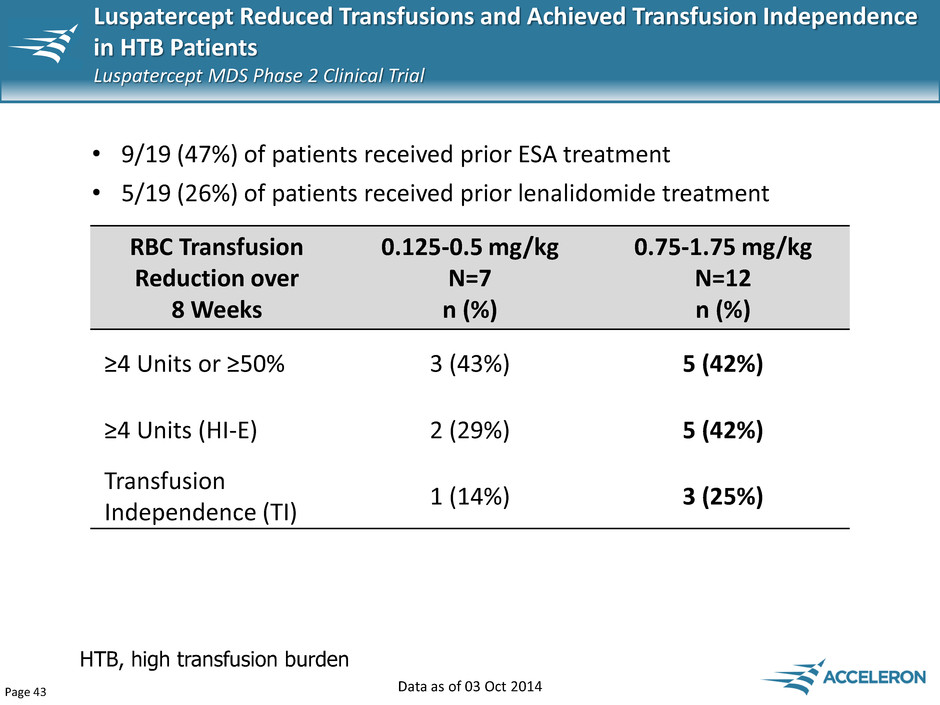
Page 43 HTB, high transfusion burden • 9/19 (47%) of patients received prior ESA treatment • 5/19 (26%) of patients received prior lenalidomide treatment RBC Transfusion Reduction over 8 Weeks 0.125-0.5 mg/kg N=7 n (%) 0.75-1.75 mg/kg N=12 n (%) ≥4 Units or ≥50% 3 (43%) 5 (42%) ≥4 Units (HI-E) 2 (29%) 5 (42%) Transfusion Independence (TI) 1 (14%) 3 (25%) Data as of 03 Oct 2014 Luspatercept Reduced Transfusions and Achieved Transfusion Independence in HTB Patients Luspatercept MDS Phase 2 Clinical Trial

Page 44 Weeks Data as of 03 Oct 2014 HTB, high transfusion burden Hemoglobin ( g /d L) Follow-up Period 2 2 0 2 4 6 8 10 12 5 6 7 8 9 10 11 -3 BL 3 6 9 12 16 20 24 Units Transfused Hemoglobin 0.75 mg/kg (starting dose) 71 year old female RCMD-RS Failed LEN, EPO Luspatercept Activity in a HTB Patient Luspatercept MDS Phase 2 Clinical Trial
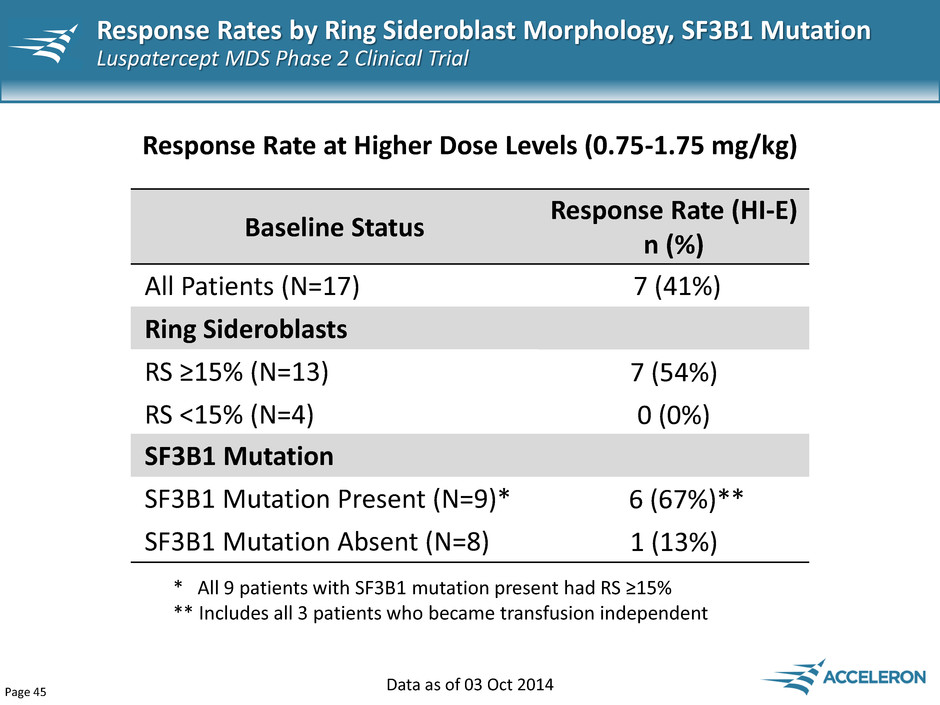
Page 45 Data as of 03 Oct 2014 Baseline Status Response Rate (HI-E) n (%) All Patients (N=17) 7 (41%) Ring Sideroblasts RS ≥15% (N=13) 7 (54%) RS <15% (N=4) 0 (0%) Response Rate at Higher Dose Levels (0.75-1.75 mg/kg) * All 9 patients with SF3B1 mutation present had RS ≥15% ** Includes all 3 patients who became transfusion independent SF3B1 Mutation SF3B1 Mutation Present (N=9)* 6 (67%)** SF3B1 Mutation Absent (N=8) 1 (13%) Response Rates by Ring Sideroblast Morphology, SF3B1 Mutation Luspatercept MDS Phase 2 Clinical Trial

Page 46 β-thalassemia Presented at ASH 2014

Page 47 Bone deformity, fractures and bone pain Iron overload, liver and heart failure Enlarged spleen, blood clots and ulcerations Chronic anemia β-thalassemia: Rare Hematologic Disease with Substantial Unmet Medical Need Beta-Thalassemia is red blood cell (RBC) disease Substantial unmet medical need • Transfusions are the only way to treat anemia but exacerbate iron overload • Iron chelators required to manage worsening iron overload from transfusions (Exjade $40K-$60K/year, $893M in WW sales1), but inadequate in removing excess iron (1) Evaluate Pharma
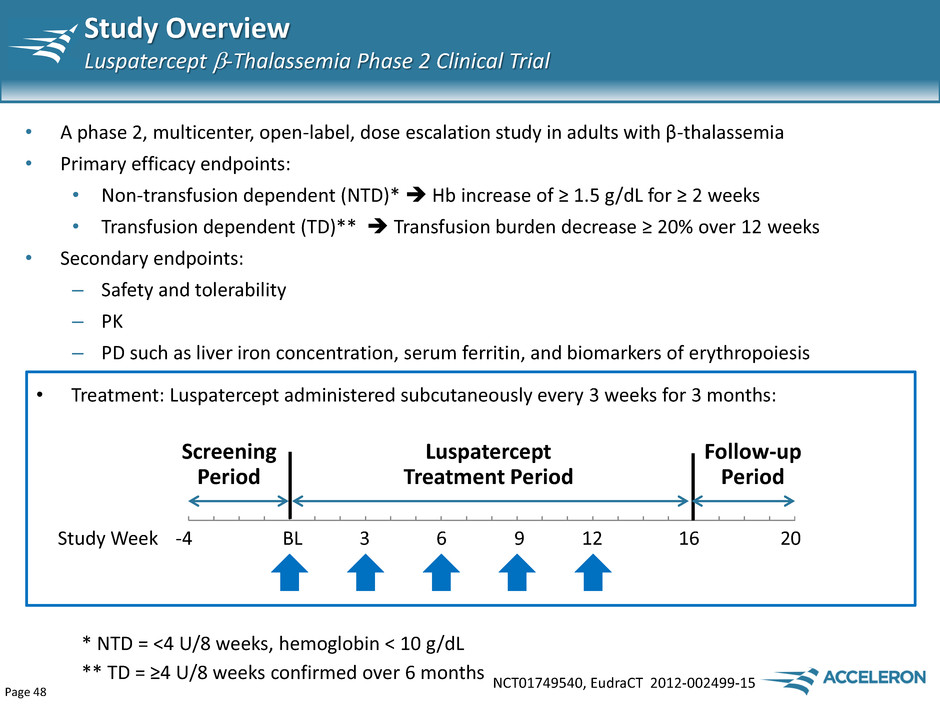
Page 48 Study Overview Luspatercept -Thalassemia Phase 2 Clinical Trial NCT01749540, EudraCT 2012-002499-15 • A phase 2, multicenter, open-label, dose escalation study in adults with β-thalassemia • Primary efficacy endpoints: • Non-transfusion dependent (NTD)* Hb increase of ≥ 1.5 g/dL for ≥ 2 weeks • Transfusion dependent (TD)** Transfusion burden decrease ≥ 20% over 12 weeks • Secondary endpoints: – Safety and tolerability – PK – PD such as liver iron concentration, serum ferritin, and biomarkers of erythropoiesis Luspatercept Treatment Period Screening Period Follow-up Period Study Week -4 BL 3 6 9 12 16 20 • Treatment: Luspatercept administered subcutaneously every 3 weeks for 3 months: * NTD = <4 U/8 weeks, hemoglobin < 10 g/dL ** TD = ≥4 U/8 weeks confirmed over 6 months
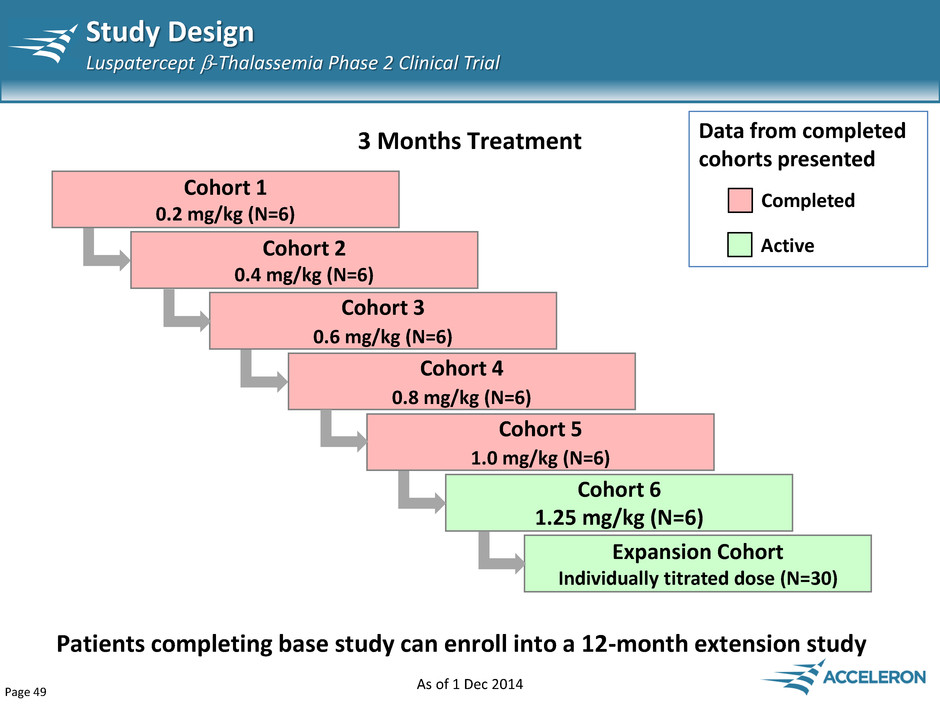
Page 49 Study Design Luspatercept -Thalassemia Phase 2 Clinical Trial Cohort 5 1.0 mg/kg (N=6) Cohort 1 0.2 mg/kg (N=6) Cohort 2 0.4 mg/kg (N=6) Cohort 3 0.6 mg/kg (N=6) Cohort 4 0.8 mg/kg (N=6) 3 Months Treatment Completed Data from completed cohorts presented Cohort 6 1.25 mg/kg (N=6) Expansion Cohort Individually titrated dose (N=30) Patients completing base study can enroll into a 12-month extension study Active As of 1 Dec 2014

Page 50 Baseline Characteristics Luspatercept -Thalassemia Phase 2 Clinical Trial All Patients N=30 Age, yr, median (range) 34.5 (20-57) Sex, male (%) 16 (53%) Splenectomy (%) 25 (83%) Non-Transfusion Dependent (NTD) N= 23 (77%) Hemoglobin, g/dL, mean ± SD 8.3 ± 0.9 Transfusion Dependent (TD) N=7 (23%) RBC Units/12 weeks, mean ± SD 7.3 ± 1.0 Data as of 10 Oct 2014
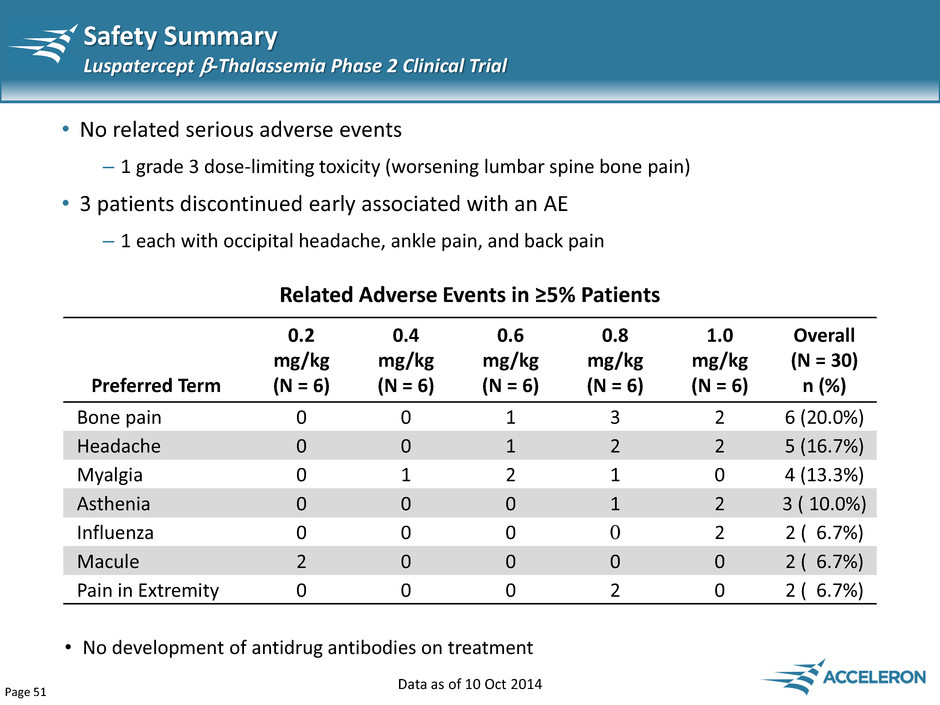
Page 51 Safety Summary Luspatercept -Thalassemia Phase 2 Clinical Trial • No related serious adverse events – 1 grade 3 dose-limiting toxicity (worsening lumbar spine bone pain) • 3 patients discontinued early associated with an AE – 1 each with occipital headache, ankle pain, and back pain Data as of 10 Oct 2014 Preferred Term 0.2 mg/kg (N = 6) 0.4 mg/kg (N = 6) 0.6 mg/kg (N = 6) 0.8 mg/kg (N = 6) 1.0 mg/kg (N = 6) Overall (N = 30) n (%) Bone pain 0 0 1 3 2 6 (20.0%) Headache 0 0 1 2 2 5 (16.7%) Myalgia 0 1 2 1 0 4 (13.3%) Asthenia 0 0 0 1 2 3 ( 10.0%) Influenza 0 0 0 0 2 2 ( 6.7%) Macule 2 0 0 0 0 2 ( 6.7%) Pain in Extremity 0 0 0 2 0 2 ( 6.7%) Related Adverse Events in ≥5% Patients • No development of antidrug antibodies on treatment
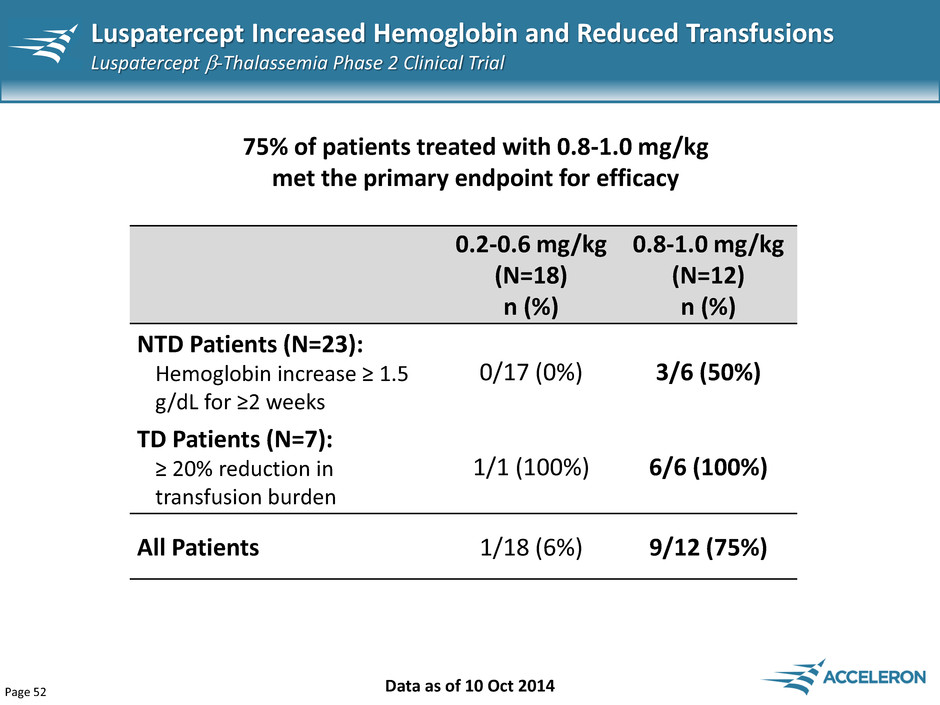
Page 52 Luspatercept Increased Hemoglobin and Reduced Transfusions Luspatercept -Thalassemia Phase 2 Clinical Trial Data as of 10 Oct 2014 75% of patients treated with 0.8-1.0 mg/kg met the primary endpoint for efficacy 0.2-0.6 mg/kg (N=18) n (%) 0.8-1.0 mg/kg (N=12) n (%) NTD Patients (N=23): Hemoglobin increase ≥ 1.5 g/dL for ≥2 weeks 0/17 (0%) 3/6 (50%) TD Patients (N=7): ≥ 20% reduction in transfusion burden 1/1 (100%) 6/6 (100%) All Patients 1/18 (6%) 9/12 (75%)

Page 53 Reduced Transfusion Burden in TD Patients Luspatercept -Thalassemia Phase 2 Clinical Trial -100 -80 -60 -40 -20 0 -79% -67% -70% -75% -63% -67% -100% • 7/7 (100%) patients had >60% reduction in transfusion burden over 12 weeks • Includes 2 patients with β0β0 genotype (79%, 75% reduction) TD, Transfusion dependent Data as of 10 Oct 2014 % Cha n ge in Tr an sfusi o n Bu rd e n 0.6 mg/kg 0.8 mg/kg 1.0 mg/kg - - - Protocol-defined threshold *Based on 8 weeks data *

Page 54 NTD, Non-transfusion dependent Maximum Hemoglobin Increase in NTD Patients Luspatercept -Thalassemia Phase 2 Clinical Trial Data as of 10 Oct 2014

Page 55 NTD Responder Hemoglobin Luspatercept -Thalassemia Phase 2 Clinical Trial Data as of 10 Oct 2014 NTD, Non-transfusion dependent He m og lo bi n (g /d L) Weeks 0 1 2 3 4 5 6 7 8 6 7 8 9 10 11 -3 BL 3 6 9 12 16 20 Hemoglobin 0.8 mg/kg 24 year old male Splenectomized Follow-up Period

Page 56 Liver Iron Concentration (LIC by MRI) in NTD Patients Luspatercept -Thalassemia Phase 2 Clinical Trial Data as of 10 Oct 2014 Baseline LIC ≥ 5 mg/g dry weight (dw) (n=12) • 8/12 patients had a decrease of ≥1 mg/g dw -5 -4 -3 -2 -1 0 1 2 3 On iron chelator No iron chelator C h an ge f ro m Bas e lin e a t 16 w e e ks in L IC ( m g/ g d w ) Dose (mg/kg) 0.2 0.4 0.6 1.0 NTD, Non-transfusion dependent
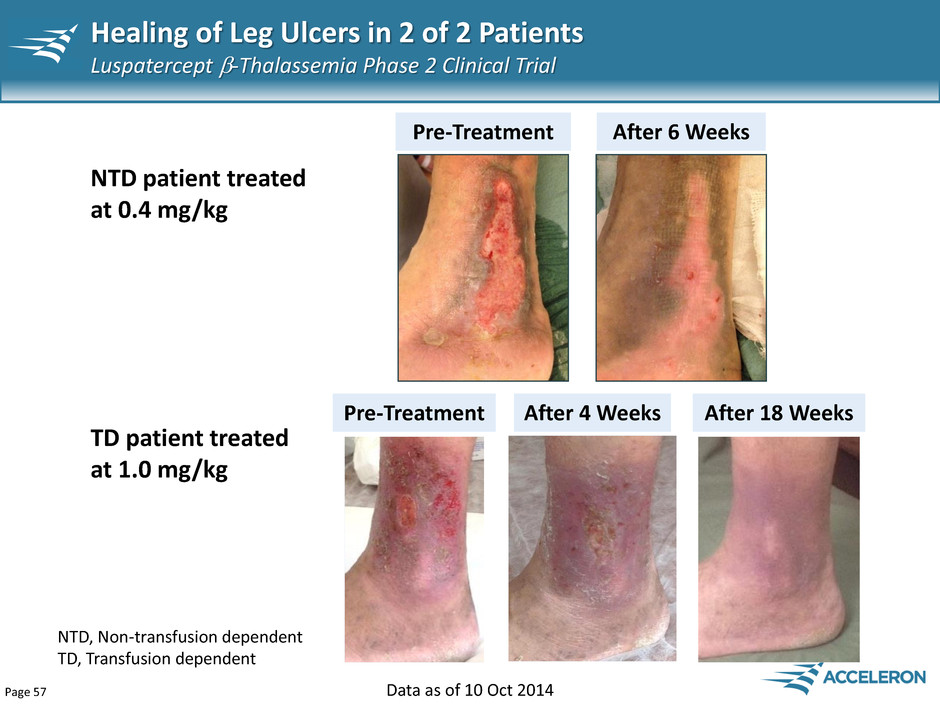
Page 57 Healing of Leg Ulcers in 2 of 2 Patients Luspatercept -Thalassemia Phase 2 Clinical Trial Pre-Treatment After 6 Weeks Pre-Treatment After 4 Weeks After 18 Weeks TD patient treated at 1.0 mg/kg NTD patient treated at 0.4 mg/kg NTD, Non-transfusion dependent TD, Transfusion dependent Data as of 10 Oct 2014

Page 58 Sotatercept Opportunity in Chronic Kidney Disease
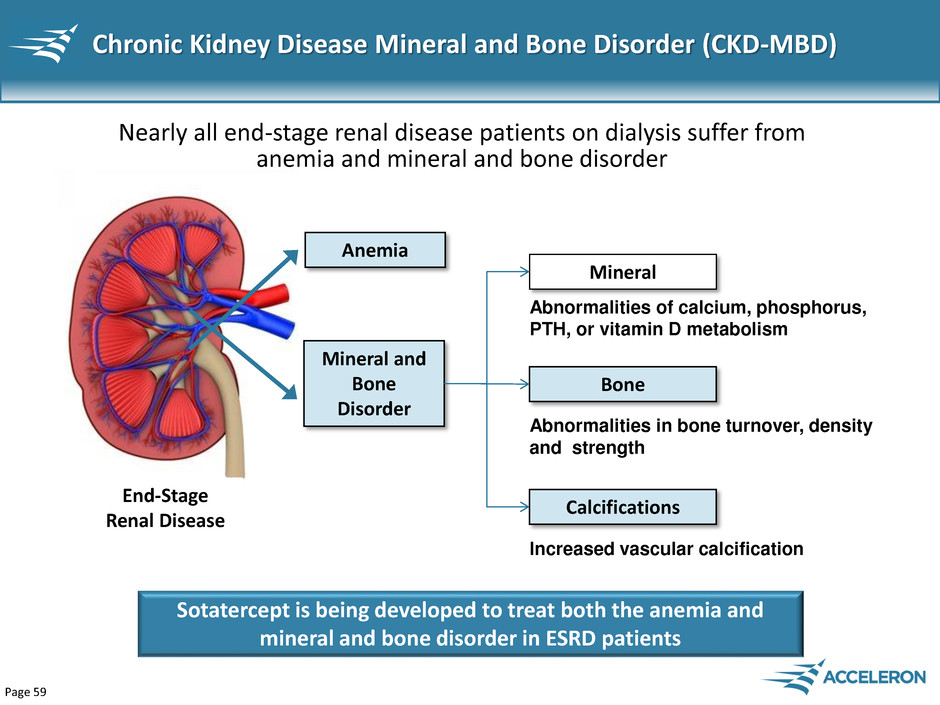
Page 59 Chronic Kidney Disease Mineral and Bone Disorder (CKD-MBD) End-Stage Renal Disease Anemia Nearly all end-stage renal disease patients on dialysis suffer from anemia and mineral and bone disorder Mineral and Bone Disorder Abnormalities of calcium, phosphorus, PTH, or vitamin D metabolism Abnormalities in bone turnover, density and strength Increased vascular calcification Mineral Bone Calcifications Sotatercept is being developed to treat both the anemia and mineral and bone disorder in ESRD patients
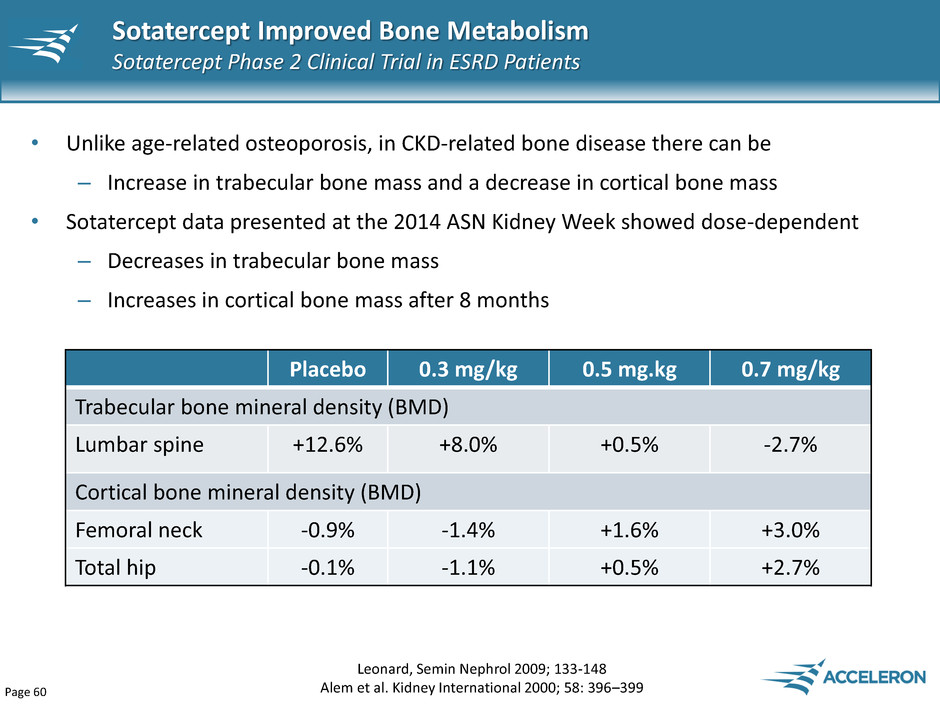
Page 60 • Unlike age-related osteoporosis, in CKD-related bone disease there can be – Increase in trabecular bone mass and a decrease in cortical bone mass • Sotatercept data presented at the 2014 ASN Kidney Week showed dose-dependent – Decreases in trabecular bone mass – Increases in cortical bone mass after 8 months Sotatercept Improved Bone Metabolism Sotatercept Phase 2 Clinical Trial in ESRD Patients Leonard, Semin Nephrol 2009; 133-148 Alem et al. Kidney International 2000; 58: 396–399 Placebo 0.3 mg/kg 0.5 mg.kg 0.7 mg/kg Trabecular bone mineral density (BMD) Lumbar spine +12.6% +8.0% +0.5% -2.7% Cortical bone mineral density (BMD) Femoral neck -0.9% -1.4% +1.6% +3.0% Total hip -0.1% -1.1% +0.5% +2.7%

Page 61 • Two types of vascular calcification - intimal (atherosclerotic) and medial • Both associated with osteoblastic transition of vascular smooth muscle cells • Medial calcification more common in ESRD patients • Vascular calcification is the strongest measure in cardiovascular risk prediction • Up to 80% of hemodialysis patients have evidence of coronary artery calcification (CAC) • Dialysis patients have 2-5 fold more arterial calcification than age- matched individuals with coronary artery disease Eddington et. al., Journal of Renal Care, 2009 Matsushita et al. JASN online 8/21/14 Chertow and Moe. Clin J Am Soc Neph 2006;1:697-703 Vascular Calcification in ESRD Patients

Page 62 Sotatercept Substantially Slowed Vascular Calcification Sotatercept Phase 2 Clinical Trial in ESRD Patients 58.4% 24.9% 17.3% 3.4% 0 10 20 30 40 50 60 70 Placebo 0.3 mg/kg 0.5 mg/kg 0.7 mg/kg Change in Agatston Score Over 8 Months Agatston score is a standard measure of vasculature calcification

Page 63 Dalantercept (ACE-041) Novel anti-angiogenic agent based on the Activin Receptor-Like Kinase 1 (ALK1)
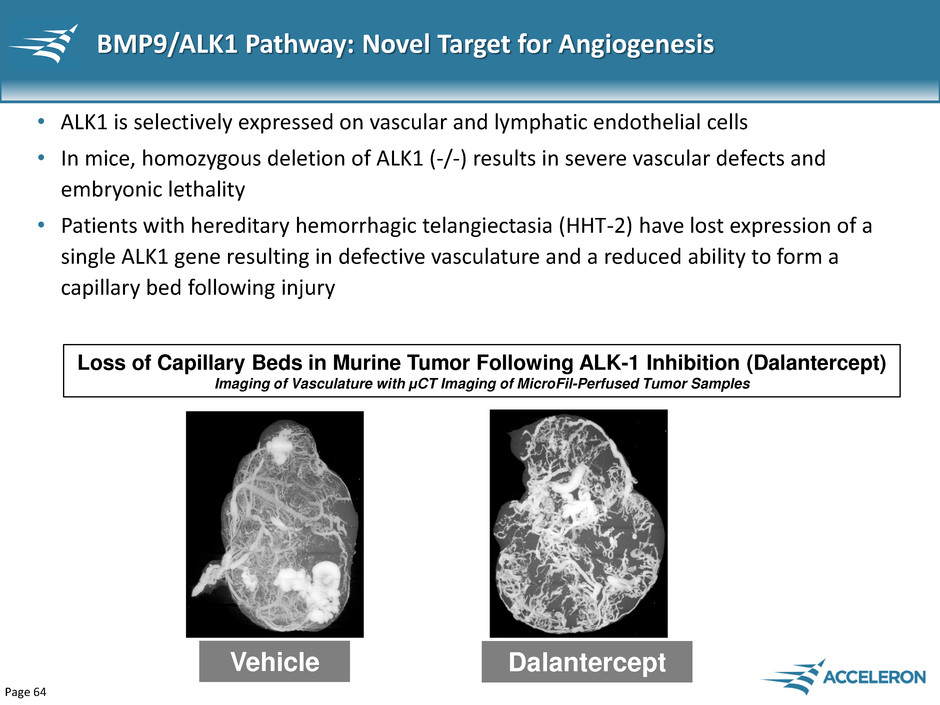
Page 64 BMP9/ALK1 Pathway: Novel Target for Angiogenesis • ALK1 is selectively expressed on vascular and lymphatic endothelial cells • In mice, homozygous deletion of ALK1 (-/-) results in severe vascular defects and embryonic lethality • Patients with hereditary hemorrhagic telangiectasia (HHT-2) have lost expression of a single ALK1 gene resulting in defective vasculature and a reduced ability to form a capillary bed following injury Vehicle Dalantercept Loss of Capillary Beds in Murine Tumor Following ALK-1 Inhibition (Dalantercept) Imaging of Vasculature with μCT Imaging of MicroFil-Perfused Tumor Samples

Page 65 Dalantercept Combination Development Strategy: Inhibit Two Key Steps in Angiogenesis to Improve Clinical Outcomes Concept: Inhibit sequential steps in pathway to augment angiogenic blockade Proliferation VEGF Receptor Maturation ALK1 Receptor Mature blood vessel supporting tumor growth

Page 66 Positive Results from Part 1 RCC Ph2 Study dalantercept + axitinib (presented at ASCO 2014) • Dalantercept combination therapy was well-tolerated and no dose-limiting toxicities were observed in the dose escalation cohorts • Encouraging preliminary efficacy of dalantercept: – Combination achieved greater than historical activity of axitinib alone • The objective response rate of axitinib alone in RCC was 11.3% in second line patients previously treated with sunitinib (source: FDA Medical Review documents) • The objective response rate of axitinib plus dalantercept was 25% overall in a group that included not only 2nd line patients but also 3rd and 4th line patients Randomized, placebo-controlled stage (part 2) of the study is underway

Page 67 IntrinsIQ (Diag Drug LOT Yearly Kidney Cancer April 2014), Evaluate Pharma By combining with established practice of using anti-VEGF therapies, dalantercept can quickly access a large segment of both the RCC and HCC markets Dalantercept Vision: Improve and Extend Response Rates • Worldwide sales of anti-VEGFs in RCC were $2.6 billion in 2013 • More than 70% of all RCC patients currently receive an anti-VEGF drug Renal Cell Carcinoma (RCC) Hepatocellular Carcinoma (HCC) • Worldwide sales of anti-VEGFs (sorafenib) in HCC were $766 million in 2013 • More than 40% of all HCC patients currently receive sorafenib
