Attached files
| file | filename |
|---|---|
| EX-10 - EX-10.19 - BIOCEPT INC | bioc-ex1019_2014093058.htm |
| EX-23 - EX-23.1 - BIOCEPT INC | bioc-ex231_2014093059.htm |
As filed with the Securities and Exchange Commission on January 9, 2015
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Biocept, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
8071 |
|
80-0943522 |
|
(State or other jurisdiction of incorporation or organization) |
|
(Primary Standard Industrial Classification Code Number) |
|
(I.R.S. Employer Identification Number) |
5810 Nancy Ridge Drive
San Diego, CA 92121
(858) 320-8200
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Michael W. Nall
Chief Executive Officer and President
Biocept, Inc.
5810 Nancy Ridge Drive
San Diego, CA 92121
(858) 320-8200
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
Frederick T. Muto Charles J. Bair Cooley LLP 4401 Eastgate Mall San Diego, CA 92121 (858) 550-6142
|
|
William G. Kachioff Senior Vice-President, Finance and Chief Financial Officer Biocept, Inc. 5810 Nancy Ridge Drive San Diego, CA 92121 (858) 320-8200 |
|
Ivan K. Blumenthal Merav Gershtenman Julia F. Gaffin Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. 666 Third Avenue New York, NY 10017 (212) 935-3000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 under the Securities Exchange Act of 1934. (Check one):
|
Large Accelerated Filer ¨ |
Accelerated Filer ¨ |
Non-accelerated Filer ¨ |
Smaller Reporting Company x |
CALCULATION OF REGISTRATION FEE
|
|
|
|
|
Title of Securities being Registered |
Proposed Maximum Aggregate Offering Price(1) |
Amount of Registration Fee |
|
Common Stock, $0.0001 par value per share |
$15,000,000 |
$1,743 |
|
(1) |
Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act. Includes the offering price of shares that the underwriters have the option to purchase to cover over-allotments, if any. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS
SUBJECT TO COMPLETION, DATED JANUARY 9, 2015
Shares
Common Stock

Biocept, Inc. is offering shares of its common stock. Our common stock is listed on The NASDAQ Capital Market under the symbol “BIOC.” On January 8, 2015, the last reported sale price of our common stock on The NASDAQ Capital Market was $3.04 per share.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012, and, as such, we have elected to take advantage of certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 17 of this prospectus for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
|
Per Share |
|
|
Total |
||
|
Public offering price |
|
$ |
|
|
|
$ |
|
|
Underwriting discounts and commissions(1) |
|
$ |
|
|
|
$ |
|
|
Offering proceeds to us, before expenses |
|
$ |
|
|
|
$ |
|
|
(1) |
We have agreed to reimburse the underwriters for certain expenses. See “Underwriting.” |
We have granted a 30-day option to the representative of the underwriters to purchase up to additional shares of common stock to cover over-allotments, if any.
The underwriters expect to deliver the shares to purchasers in this offering on or about , 2015.
Joint Book-Running Managers
|
Aegis Capital Corp |
|
Feltl and Company |
The date of this prospectus is , 2015.

4
TABLE OF CONTENTS
|
|
Page |
|
6 |
|
|
15 |
|
|
17 |
|
|
42 |
|
|
43 |
|
|
44 |
|
|
45 |
|
|
46 |
|
|
47 |
|
|
49 |
|
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
51 |
|
63 |
|
|
93 |
|
|
98 |
|
|
113 |
|
|
118 |
|
|
120 |
|
|
125 |
|
|
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF OUR COMMON STOCK |
127 |
|
130 |
|
|
137 |
|
|
137 |
|
|
137 |
|
|
138 |
|
|
139 |
Neither we nor the underwriters have authorized anyone to provide you with information that is different from that contained in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. When you make a decision about whether to invest in our common stock, you should not rely upon any information other than the information in this prospectus or in any free writing prospectus that we may authorize to be delivered or made available to you. Neither the delivery of this prospectus nor the sale of our common stock means that the information contained in this prospectus or any free writing prospectus is correct after the date of this prospectus or such free writing prospectus. This prospectus is not an offer to sell or the solicitation of an offer to buy the shares of common stock in any circumstances under which the offer or solicitation is unlawful.
__________________________
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations and market position, market opportunity and market share, is based on information from our own management estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. Our management estimates have not been verified by any independent source, and we have not independently verified any third-party information. In addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates. See “Special Note Regarding Forward-Looking Statements.”
__________________________
We use in this prospectus our BIOCEPT logo, for which a United States trademark application has been filed, our mark CEE, which is a registered United States trademark, and our marks OncoCEE-BR, OncoCEE-LU, CEE-Selector, CEE-Cap, CEE-Enhanced, CEE-Sure, OncoCEE-GA, OncoCEE-PR, OncoCEE-ME, OncoCEE-CR and OncoCEE, which in the United States are unregistered trademarks. This prospectus also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks and tradenames referred to in this prospectus appear (after the first usage) without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to these trademarks and tradenames.
5
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. You should read this entire prospectus carefully, especially the “Risk Factors” section of this prospectus and the financial statements and related notes appearing at the end of this prospectus before making an investment decision.
Unless the context provides otherwise, all references in this prospectus to “Biocept,” “we,” “us,” “our,” the “Company,” or similar terms, refer to Biocept, Inc. We reincorporated from California to Delaware in July 2013. Except where otherwise expressly stated, no distinction is made in this prospectus between historic activities and results of the California and Delaware corporations.
Our Company
We are a cancer diagnostics company that develops and commercializes proprietary circulating tumor cell, or CTC, and circulating tumor DNA, or ctDNA, tests utilizing a standard blood sample, or “liquid biopsy”. Our currently commercialized tests are OncoCEE-BRTM for breast cancer CTC enumeration and analysis, OncoCEE-GATM for gastric cancer CTC enumeration and analysis and OncoCEE-LUTM for non-small cell lung cancer, or NSCLC, CTC enumeration and analysis. OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our tests in development are designed to provide information to oncologists and other physicians to enable them to select appropriate treatment for their patients due to better, timelier and more-detailed data on the characteristics of tumors. Our marketed test and our tests in development for the enumeration and analysis of CTCs utilize our Cell Enrichment and Extraction, or CEE®, technology, and our tests in development for the detection and analysis of ctDNA utilize our CEE-Selector™ technology, each performed on a standard blood sample. CEE is an internally developed, microfluidics-based CTC capture and analysis platform, with enabling features that change how CTC testing can be used by clinicians by providing real-time biomarker monitoring with only a standard blood sample. The CEE-Selector technology enables mutation detection with enhanced sensitivity and specificity and is applicable to nucleic acid from CTCs or other sample types, such as blood plasma for ctDNA. From August 2011, when we launched OncoCEE-BR, to September 30, 2014, our revenues from OncoCEE-BR have totaled approximately $253,000. We launched OncoCEE-LU and OncoCEE-GA in late 2014 and have not yet recognized significant revenues from these tests. To achieve profitability, we would need to increase our revenue, from OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and any tests we introduce in the future, many-fold from historic levels. We are an emerging company.
At our corporate headquarters facility located in San Diego, California, we operate a clinical laboratory that is certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and accredited by the College of American Pathologists, or CAP. We also manufacture our CEE microfluidic channels, related equipment and certain reagents to perform our tests at this facility.
OncoCEE-BR is a breast cancer CTC test that is performed on a standard blood sample. It detects CTCs, which are typically very rare, and determines the patient’s human epidermal growth factor receptor 2, or HER2, status by fluorescence in situ hybridization, or FISH.
OncoCEE-GA is a gastric cancer CTC test that is performed on a standard blood sample. It detects CTCs, which are typically very rare, and determines the patient’s HER2 status by fluorescence in situ hybridization, or FISH.
We launched OncoCEE-LUTM, a test performed on a standard blood sample for NSCLC, in November of 2014. The OncoCEE-LU test’s biomarker analysis currently includes FISH testing for anaplastic lymphoma kinase, or ALK, c-ros oncogene 1, receptor tyrosine kinase, or ROS1, gene rearrangements and molecular analysis of the T790M mutation of the epidermal growth factor receptor or EGFR gene using our CEE-SelectorTM platform. We plan to add FISH testing for RET, MET, as well as mutation analysis for deletions 19 and l858R mutation in the ECFR gene, the K-ras gene and the B-raf gene in the future.
We plan to add additional biomarker analyses to OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests as their clinical relevance is demonstrated, for example, RET proto-oncogene gene fusions in NSCLC. In addition, we are developing a series of other CTC and ctDNA tests for different solid tumor types, including colorectal cancer, prostate cancer, gastric cancer and melanoma, each incorporating treatment-associated biomarker analyses specific to that cancer. We also have a research and development program focused on technology enhancements, novel platform development, and evaluating clinical applications for our cancer diagnostic tests in different cancer types and clinical settings. We plan to launch four new OncoCEETM cancer tests over the next two years.
6
We collaborate with physicians and researchers at The University of Texas MD Anderson Cancer Center, the Dana-Farber Cancer Institute, the University of California, San Diego and Columbia University and plan to expand our current collaborative relationships to include other key thought leaders for the types of cancer we are targeting with OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned CTC and ctDNA tests. Such relationships are designed to help us develop and validate the effectiveness and utility of our current tests and our planned tests in specific clinical settings and provide us access to patient samples and data.
Market Overview
Despite many advances in the treatment of cancer, cancer remains one of the greatest areas of unmet medical need. According to the World Cancer Report 2014, cancers figure among the leading causes of morbidity and mortality worldwide, with approximately 14 million new cases and 8.2 million cancer related deaths in 2012. It is also expected that the number of new cases will rise by approximately 70% over the next two decades. The incidence of, and deaths caused by, solid tumor cancers are staggering.
Cancer constitutes a heterogeneous class of diseases, characterized by uncontrollable cell growth, that result from a combination of both environmental and hereditary risk factors. Many different tissue types can become malignant, such as breast, lung, liver, and skin, and even within a particular tumor there is heterogeneity, with certain cancer cells in a patient bearing specific cellular or genetic biomarkers, while other cells in the tumor may not have these markers. It has only been in recent years that technology has progressed far enough to enable researchers to understand many cancers at a molecular level and attribute specific cancers to associated genetic changes.
Limitations of Traditional Cancer Diagnostic and Profiling Approaches
Cancer is difficult to diagnose and manage due to its heterogeneity at visual, genetic and clinical levels. Traditional methods of diagnosis for solid tumors, routinely used as the initial step in cancer detection, involve a tissue biopsy, followed by a pathologist examining a thin slice of potentially cancerous tissue under a microscope. The tissue sample must be used in combination with chemical staining techniques to enable analysis of the biopsy. Through visual inspection, the pathologist determines whether the biopsy contains normal or cancerous cells, with those cells that are deemed cancerous being graded on a level of aggressiveness. In recent years, molecular (or genetic) testing has become the standard of care and will also be performed in order to provide information about which drugs a patient is likely or unlikely to respond to. After the diagnosis, a clinical workup is performed according to established guidelines for the specific cancer type. From there, the physician determines the stage of progression of the cancer based on a series of clinical measures, such as size, grade, metastasis rates, symptoms and patient history, and decides on a treatment plan that may include surgery, watchful waiting, radiation, chemotherapy, or stem cell transplant.
Molecular analysis is dependent on the availability of a relevant tissue biopsy for the pathologist to analyze. Such a biopsy is often not available. A tumor may not be readily accessible for biopsy, a patient’s condition may be such that a biopsy is not advised, and for routine periodic patient monitoring to evaluate potential progression or recurrence, a biopsy is a fairly invasive procedure and not typically performed. As the length of time between when the original biopsy, diagnosis or surgery is conducted to the current evaluation of the patient increases, the likelihood that an original biopsy specimen is truly representative of the current disease condition declines, as does the usefulness of the original biopsy for making treatment decisions. This risk intensifies in situations where a drug therapy is being administered, because the drug can put selective pressure on the tumor cells to adapt and change. Similarly, the heterogeneity referred to above means that different parts or areas of the same tumor can have different molecular features or properties. In evaluating a biopsy specimen, the pathologist will take a few thin slices of the tumor for microscopic review rather than exhaustively analyzing the whole tumor mass. The pathologist can only report on the tumor sections analyzed, and if other parts of the tumor have different features, such as biomarkers corresponding to specific treatments, they can be missed. A more representative analysis of the entire tumor, as well as any metastases if they are present, could be very helpful.
CTCs, ctDNA and Cancer
Circulating tumor cells, or CTCs, are cancer cells that have detached from the tumor and invaded the patient’s blood or other bodily fluids. These cells are representative of the tumor and its metastases, and can function as their surrogates. Testing CTCs can complement pathologic information drawn from a biopsy or resected tissue sample, helping to ensure that the analysis is comprehensive and not biased by tumor heterogeneity and sampling issues. Testing CTCs can also provide critical data when a biopsy is not possible. Clinical studies have demonstrated that the presence and number of CTCs provides information on the likely course of certain types of cancer for the patient, or in other words they are considered “prognostic.” Since CTCs are understood to be representative of the tumor, they can also be used for biomarker analysis, for example, to help guide therapy selection. In this way they are “predictive” in that they offer insight into the likely responsiveness or resistance to particular therapies. After surgery and during any subsequent therapy or monitoring period, blood samples can periodically be drawn and analyzed to evaluate a therapy’s continuing effectiveness, as well as to detect other biomarkers, such as new genetic mutations that may arise as a result of selective pressure by a particular therapy or by chance. Physicians can use this information to determine which therapy is most likely to benefit
7
their patients at particular times through the course of their disease. Treatment decisions based on patient-specific information are the foundation of personalized medicine, and tests, or assays, that guide a physician in the selection of individualized therapy for a patient are termed “predictive assays.”
Nucleic acid that is released into blood by dying tumor cells is called ctDNA. Cell death occurs in all tissues, especially those that are rapidly dividing, and in cancer, where cell growth is not only rapid but also uncontrolled, parts of tumors often outgrow their blood supply, resulting in cell death. As a consequence, ctDNA is common in cancer patients, and like CTCs, scientists believe that it may be more representative of a patient’s tumor than a few thin sections from a tissue biopsy, thus reducing the heterogeneity problem. ctDNA is found in the plasma component of blood, and is readily accessible in a standard blood sample. Analyzing ctDNA for mutations that are used as biomarkers for therapy selection shows great promise. One of the strengths of utilizing this approach, in addition to not requiring a tissue biopsy, is that the process is not dependent on capturing rare tumor cells from blood to provide a sample for testing. The negative side of this approach is that the cellular context is lost, as the ctDNA is mixed with a much larger amount of circulating DNA from normal cells that are continuously dying and being replaced in the body, thus making analysis challenging. This requires a mutation detection methodology with enhanced sensitivity and specificity, to distinguish mutations in particular gene regions in cancer cells from the normal gene sequence which co-exist in blood as normal cells die and are replaced in the body. Our CEE-Selector technology provides the necessary sensitivity and specificity, creating an opportunity for ctDNA testing to complement CTC analysis or potentially to serve as stand-alone tests.
Use of CTC- and ctDNA-Derived Biomarker Data in Cancer Treatment
CTCs and ctDNA are derived from, and are understood to be representative of, a solid tumor and its metastases and can be analyzed as adjuncts to the tumor, especially when a recent tumor biopsy is not available. This is also referred to as a liquid biopsy. In theory, almost any analysis that can be performed on tumor tissue can also be performed on CTCs, while the number of currently available assays that can be performed on ctDNA is more limited. We have focused and will focus our analysis of CTCs and ctDNA on known biomarkers associated with specific therapies to support treatment decisions and therapy selection made by oncologists and other physicians. To analyze proteins and genetic aberrations and mutations which are detected in CTCs or ctDNA, we can use molecular diagnostic tests, such as PCR and gene sequencing. Specific examples include (i) the detection of the estrogen receptor protein in breast cancer, indicative of the likely responsiveness to hormonal therapies, (ii) the presence of an amplified HER2 gene in breast cancer, indicative of the likely responsiveness to HER2-targeted agents, and (iii) the presence of an EGFR activating mutation in Non-Small Cell Lung Cancer (NSCLC) , indicative of the likely responsiveness to EGFR-targeted agents. All of these biomarkers are currently tested on tumor tissue and can be tested on CTCs, while ctDNA only provides information on mutations. The resulting information is then used to guide patient care, specifically treatment selection.
To date, these types of molecular and genetic detection methods have been successfully utilized to provide predictive information for several cancers, including breast, colon, NSCLC, melanoma and others in the form of companion diagnostics, typically performed on tumor tissue. CTC and ctDNA tests analyze the same biomarkers in a more convenient, standard blood test format that permits periodic testing.
Our Business Strategy
We plan to provide oncologists and other physicians with a straightforward means to profile and characterize their patients’ tumors on a real-time basis by analyzing CTCs and ctDNA found in standard blood test draws. Biomarkers are currently detected and analyzed primarily in tissue biopsy specimens. We believe that our technology, which not only provides information on CTC enumeration (quantitation of CTCs) but also the assessment of treatment-associated biomarkers identified within the CTCs or in ctDNA, will provide information to physicians that improve patient treatment and management and will become a key component in the standard of care for personalized cancer treatment.
Our approach is to develop and commercialize CTC and ctDNA tests and services to enable us to offer to oncologists and other physicians standard blood sample based, real-time, testing solutions for a range of solid tumor types, starting with breast cancer and progressing to future launches of tests for NSCLC, gastric cancer, colorectal cancer, prostate cancer, melanoma and others, to improve patient treatment with better prognostic and predictive tools. To achieve this, we intend to:
|
· |
Develop and commercialize a portfolio of proprietary CTC and ctDNA tests and services, to enable physicians to develop personalized treatment plans. |
|
· |
Scale our internal sales and marketing capabilities. |
|
· |
Develop and expand our collaborations with leading university hospitals and research centers. |
|
· |
Enhance our efforts in reaching and educating community oncologists and other physicians about CTC and ctDNA tests and services. |
8
|
· |
Increase our efforts to provide biopharmaceutical companies and clinical research organizations with our current and planned CTC and ctDNA tests and services. |
|
· |
Conduct additional clinical studies of breast cancer, NSCLC and other CTC and ctDNA tests we plan to introduce. |
|
· |
Continue to enhance our current and planned CTC and ctDNA tests and reduce the costs associated with providing them through internal research and development and partnering with leading technology developers and reagent suppliers. |
Our Competitive Advantages
We believe that our competitive advantages are as follows:
OncoCEE-BR, OncoCEE-LU and OncoCEE-GA enable, and we anticipate our planned CTC and ctDNA tests will enable, detailed analysis of a patient’s cancer utilizing a standard blood sample, facilitating testing at any time, including when a biopsy is not available or inconclusive, offering real-time monitoring of the cancer and the response of the cancer to therapy, and allowing oncologists and other physicians to select timely modifications to treatment regimens. CTCs and ctDNA, because they are derived from the primary tumor or its metastases, function as surrogates for the tumor, with the advantage of being readily accessible in a standard blood sample, which is especially important in situations where a biopsy is not available or advised. The simplicity of obtaining a standard blood sample will permit repeat testing in a monitoring mode to detect recurrence or progression, and will offer information on treatment modifications based on a current assessment of the cancer’s properties.
OncoCEE-BR, OncoCEE-LU and OncoCEE-GA provide, and we anticipate our planned tests will provide, more information than competitors’ existing tests, including predictive information on biomarkers linked to specific therapies. We anticipate that such additional biomarker information will enable a physician to develop a personalized treatment plan. By including biomarker information in our analysis in addition to CTC enumeration, OncoCEE-BR, OncoCEE-LU and OncoCEE-GA and our planned tests are designed to provide a more complete profile of a patient’s disease than existing CTC tests can. The biomarker information assists physicians in selecting appropriate therapies for individual patients. Our ctDNA tests are expected to offer enhanced sensitivity and specificity based on the CEE-Selector technology, enabling earlier detection of therapy-associated mutation targets or resistance markers, again supporting treatment decisions.
OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned CTC tests are designed to capture and detect a broader range of CTCs than existing tests and to be applicable to, or quickly modifiable for, a wide range of cancer types. Our CEE-CapTM antibody capture cocktail is comprised of antibodies targeting not only EpCAM, the traditional epithelial CTC capture antigen utilized in Janssen Diagnostics, LLC’s CellSearch® system and in other platforms, but also other epithelial antigens and mesenchymal and cancer stem cell antigens, indicative of cells having undergone the epithelial-to-mesenchymal transition, or EMT. These cells may be more relevant for metastasis. Our detection modalities include cytokeratin staining, with a broader range of cytokeratin isotypes than existing CTC tests. We plan to introduce our CEE-EnhancedTM staining, which would enable detection of cells specifically captured with our antibody cocktail, including EMT cells lacking cytokeratin. We believe that through our planned CEE-Enhanced staining, more CTCs and different types of CTCs will be able to be identified and potentially at earlier stages of disease, resulting in fewer non-informative cases and more information for physicians.
OncoCEE-BR, OncoCEE-LU and OncoCEE-GA are and we anticipate our planned CTC and ctDNA tests will be, flexible and readily configurable to accommodate new biomarkers with clinical relevance as they are identified. In theory, our CEE platform permits almost any analysis that is currently performed on tumor tissue to be performed on CTCs. As new therapies are approved, we will be able to include them in our tests with minimal changes. This is attractive to pharmaceutical and biotechnology companies that are developing such therapies, or seeking ways to make their clinical trials more efficient, as this flexibility would enable them to focus on patients more likely to respond to a particular therapy and demonstrate a benefit from that therapy.
Collaborative relationships with physicians at The University of Texas MD Anderson Cancer Center. We work closely with a number of physicians at The University of Texas MD Anderson Cancer Center on various collaborative projects in different cancer types, including breast, NSCLC, prostate, colorectal, ovarian, bladder, renal and endometrial cancers. These projects provide us access to leading researchers, leading clinicians and key thought leaders, access to valuable patient samples and insight into clinical applications for tests. Some of these projects have resulted in publications in leading journals, such as Cancer Discovery and Cancer Medicine, which enhances our standing in the oncology community and supports our marketing efforts.
Our planned CEE-Selector mutation tests would not be platform dependent. These tests are being designed to be able to be performed on almost any molecular instrument, which will provide flexibility in laboratory operations. To the extent we elect to develop these tests as in vitro diagnostic kits, or IVDs, including pursuing CE marks for them to be marketed in Europe, the ability to rapidly deploy them on different approved instrument platforms already in many laboratories greatly simplifies their distribution and commercialization.
9
Our Tests and Services
We currently offer OncoCEE-BR for breast cancer, OncoCEE-GA for gastric cancer and OncoCEE-LU for NSCLC, and plan to continue to launch a series of tests for CTCs in different tumor types, including colorectal and prostate cancers and melanoma, incorporating analyses for different biomarkers, over the next two years. OncoCEE-BR, OncoCEE-GA and OncoCEE-LU are, and the planned future tests will be, based on the CEE technology platform. The CEE system isolates CTCs from blood samples of cancer patients for enumeration (or count) and genetic analysis. A sample is shipped to us in our specialized blood collection tube called the CEE-SureTM tube for recovery and analysis of CTCs. When performing the CTC assay, the sample is processed in our laboratory. The specimen of blood is separated into its parts (red blood cells, buffy coat and plasma). The buffy coat is incubated with the antibody solution and passed through a proprietary microfluidic channel containing 9,000 microscopic posts coated with reagents to capture antibody-labeled tumor cells. The captured cells are suitable for further testing of whole cells directly in the microfluidic channel or by releasing the cells from the microfluidic channel and performing CEE-Selector or similar techniques.
Clinicians acknowledge limitations of currently available CTC test systems such as CellSearch® that rely on capture solely by anti-EpCAM antibodies and detection by anti-cytokeratin antibodies. Capture and detection based only on these two antigens is unlikely to identify all CTCs, and clinically this may result in no CTCs being detected in cases in which they are present. For example, some tumor cells that have been released into the circulatory system have undergone an EMT. These mesenchymal cells are less differentiated than epithelial cells and more similar to stem cells. OncoCEE-BR, OncoCEE-LU and OncoCEE-GA enable, and we believe our planned assays will enable, the capture of significantly more CTCs than is accomplished through the use of traditional anti-EpCAM immuno-capture alone.
In addition to enhanced capture, we are also improving identification of CTCs. We have developed alternative methods of fluorescent cell staining that are uniquely possible within the CEE system to enhance detection of CTCs. This technology is called CEE-Enhanced. We believe that the combination of our assay with more sensitive fluorescent detection of CTCs through CEE-Enhanced staining will lead to major advances in the capture, enumeration and analysis of CTCs. CEE-Enhanced staining is expected to be included in our commercially available and planned tests by mid-2015.
Analysis of CTCs performed by us incorporates both standard and proprietary methods. Immunocytochemistry which looks at proteins, analogous to the immunohistochemistry, or IHC, performed on tissues, can be readily applied and performed in the microfluidic channel, dependent only on suitable biomarkers. Similarly, FISH, used to evaluate genetic abnormalities in cells, may be performed in our microfluidic channel using validated assays available from a number of vendors. For genetic mutation analysis, standard technologies can be applied. We have also developed proprietary CEE-Selector technology for mutation analysis in CTCs and ctDNA, with enhanced sensitivity and specificity.
CTCs are generally very rare and outnumbered many-fold by white blood cells. This complexity has been a challenge for standard technologies. CEE-Selector offers enhanced specificity and sensitivity (greater than 1 in 10,000 of mutated sequence to normal sequence in a complex genetic background) compared to other approaches, and potentially has broader application than just CTC analysis, including analysis of ctDNA in plasma, both in a CLIA lab setting and as an IVD.
OncoCEE-BR, OncoCEE-LU and OncoCEE-GA are Laboratory Developed Tests, and our planned CTC and ctDNA tests would be Laboratory Developed Tests. FDA clearance or approval is not currently required to offer these types of tests in our laboratory once they have been clinically and analytically validated. We have obtained licenses and approvals for our laboratory facility and for our LDTs from the appropriate regulatory authorities, such as the Centers for Medicare & Medicaid Services, or CMS, which oversees CLIA, and various state regulatory bodies such as California, Florida, Maryland, Pennsylvania and Rhode Island. Certain states, such as New York, in addition to those previously mentioned, require us to obtain state licensure in order for us to perform testing on specimens taken from patients or received from ordering physicians from those states. As part of this process, the State of New York requires validation of our tests. We are currently in the process of addressing the requirements for licensure in New York, and we expect to have soon re-obtained all required licenses and approvals from all other states requiring licensure for out-of-state laboratories.
We believe, based on research showing that approximately 54% of new cancers occur in persons age 65 and older and that almost all Americans age 65 and older are enrolled in Medicare, that a substantial portion of the patients for whom we would expect to perform cancer diagnostic tests will have Medicare as their primary medical insurance. Only in November 2013 did we first directly bill any payor for physician-ordered testing; until May 2013, our commercialization partner Clarient was responsible for all billing associated with our tests. We do not have data for Clarient’s billing and collection experience with respect to our test, because Clarient paid us a contracted amount per test performed regardless of their billing and collections. From May to December 2013, we performed an average of 1-3 physician-ordered tests per month, and from July to September 2014, we performed an average of 32 physician-ordered tests per month (in addition to the tests which we have been performing since January 2013 for a clinical utility study with investigators at the Dana-Farber Cancer Institute, with an average of 15-30 tests per month performed during the trial’s enrollment period through May 2014). Billing for these physician-ordered tests is now handled for us by a non-Clarient billing service provider.
10
Since May 2013, we invoiced, through this service provider, for 218 physician-ordered tests. Of these, 37 tests were billed to Medicare and the remainder were billed to other payors. As of December 2014, we have been paid by private payors for 66 of these tests, with an average price collected of $1,165 per test, while we have not yet had any response or adjudication from any payor as to the other bills submitted. Accordingly, we do not yet have any data regarding reimbursement history or collectability experience. To date, all of our revenue recognized has come from private payors. Medicare claims have not yet been processed due to a new application process due to a change in our tax identification number. We cannot assure you that, even if OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests are otherwise successful, reimbursement for the currently Medicare-covered portions of OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests would, without adequate Medicare reimbursement for the capture/enumeration portion, produce sufficient revenues to enable us to reach profitability and achieve our other commercial objectives.
Medicare has coverage policies that can be national or regional in scope. Coverage means that the test or assay is approved as a benefit for Medicare beneficiaries. If there is no coverage, neither the supplier nor any other party, such as a reference laboratory, may receive reimbursement from Medicare for the service. There is currently no national coverage policy regarding the CTC capture/enumeration portion of our testing. Because our laboratory is in California, the regional Medicare Administrative Contractor , or MAC, for California is the relevant MAC for all our testing. The previous MAC for California, Palmetto GBA, LLC, adopted a negative coverage policy for CTC capture/enumeration. The current MAC for California, Noridian Healthcare Solutions, LLC, is adopting the coverage policies from Palmetto GBA. Therefore the capture/enumeration portion of our OncoCEE testing is not currently covered and we will receive no payment from Medicare for this service unless and until the coverage policy is changed. On November 4, 2013, we submitted a comprehensive dossier explaining to Palmetto GBA and Noridian the benefits of the capture/enumeration testing in order to seek to persuade the MACs to allow coverage for this portion of our testing. Palmetto GBA responded on November 27, 2013, denying our request for Medicare coverage for the CTC capture/enumeration portion of our OncoCEE testing. We have not received any other indications to suggest that the negative coverage determination will be reversed. We intend to continue our efforts to obtain Medicare coverage for capture/enumeration.
FISH analysis is a covered benefit for Medicare beneficiaries and accordingly we expect that the FISH portion of OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests are and will be covered and that when and as we bill Medicare we will receive payment from Medicare under the Physician Fee Schedule for FISH analysis. Molecular testing for the mutations we currently plan to test for with CEE-Selector is also a covered benefit, so we believe that CEE-Selector testing would thereby be covered and that when and as we bill Medicare we would receive payment from Medicare under the Clinical Laboratory Fee Schedule, or CLFS, for CEE-Selector testing. As discussed above, we have not yet received from Medicare any response or adjudication regarding any of our late-2014 billings, including for the FISH portion of our testing.
We expect these analysis components to have a significantly greater billing value than the capture/enumeration components of our current and anticipated CTC tests, based on a comparison of what we believe CellSearch® capture/reimbursement rates currently are, versus existing reimbursement rates for analysis components such as FISH and immunocytochemistry analysis and molecular testing.
The processing of Medicare claims is subject to change at CMS’ discretion at any time. Cost containment initiatives may be a threat to Medicare reimbursement levels for the foreseeable future.
In addition, we are currently considered a “non-contracted provider” by the majority of third-party payors because we have not entered into a specific contract to provide cancer diagnostic tests to their insured patients at specified rates of reimbursement. If we were to become a contracted provider with additional payors in the future, the amount of overall reimbursement we receive would likely decrease because we could be reimbursed less money per test performed at a contracted rate than at a non-contracted rate, which could have a negative impact on our revenues. Further, we typically are unable to collect payments from patients beyond that which is paid by their insurance and will continue to experience lost revenue as a result.
Clinical Trial Services
Industry research has shown many promising drugs have produced disappointing results in clinical trials. For example, a study by Princess Margaret Hospital in Toronto estimated that over a five-year study period 85% of the new therapies for solid tumors which were tested in early clinical trials in the United States, Europe and Japan failed, and that of those that survive through to Phase III trials only half will actually be approved. Given such a high failure rate of oncology drugs in clinical development, combined with constrained budgets for biopharmaceutical companies, there is a significant need for drug developers to utilize molecular diagnostics to decrease these failure rates. For specific molecular-targeted therapeutics, the identification of appropriate biomarkers potentially may help to optimize clinical trial patient selection and success rates by helping clinicians identify patients that are most likely to benefit from a therapy based on their individual genetic profile.
11
Although through 2012 we had essentially no clinical trial testing services revenues, we believe clinical trial testing services can be an important part of our business in the future. We believe our testing and analysis can help increase the efficiency and economic viability of clinical trials for biopharmaceutical companies and clinical research organizations. Our clinical trial services could include developing customizable tests and techniques utilizing our proprietary CTC and ctDNA technologies to provide sensitive, real-time characterization of individual patient’s tumors using a standard blood sample. These tests may also be useful as, and ultimately developed into, companion diagnostics associated with a specific therapeutic. Additionally, through our services we would hope to gain further insights into disease progression and the latest drug development that we can incorporate into our tests and services.
In 2013 and 2014 we provided clinical trial testing services for the Dana-Farber Cancer Institute, and this project resulted in approximately 77% and 74% of our revenues for the year ended December 31, 2013 and the nine months ended September 30, 2014, respectively.
Risks That We Face
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. The risks are discussed more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. These risks include, but are not limited to, the following:
|
· |
we are an early-stage company with a history of substantial net losses. We have never been profitable and we have an accumulated deficit of approximately $134 million (as of September 30, 2014). Before 2008, we were pursuing a business plan relating to fetal genetic disorders and other fields, all of which were unrelated to cancer diagnostics. The portion of our accumulated deficit that relates to the period from inception through December 31, 2007 is approximately $66.5 million. |
|
· |
we expect to incur net losses in the future, and we may never achieve sustained profitability; |
|
· |
our business depends upon our ability to introduce additional tests and increase sales of our cancer diagnostic test; |
|
· |
our current cash resources are insufficient to fund our operations beyond March 2015 without this offering; |
|
· |
our business depends on executing on our sales and marketing strategy for our cancer diagnostic tests and gaining acceptance of our current tests and future tests in the market; |
|
· |
our business depends on our ability to continually develop new cancer diagnostic tests and enhance our current tests and future tests; |
|
· |
our business depends on being able to obtain coverage and adequate reimbursement from governmental and other third-party payors for tests and services; |
|
· |
our business depends on satisfying any applicable United States (including FDA) and international regulatory requirements with respect to tests and services; and many of these requirements are new and still evolving; |
|
· |
our business depends on our ability to effectively compete with other diagnostic tests, methods and services that now exist or may hereafter be developed; |
|
· |
we depend on our senior management and in August 2013 we hired a new chief executive officer; |
|
· |
we depend on our ability to attract and retain scientists, clinicians and sales personnel with extensive experience in oncology, who are in short supply; and |
|
· |
we need to obtain or maintain patents or other appropriate protection for the intellectual property utilized in our current and planned tests and services, and we must avoid infringement of third-party intellectual property. |
Company Information
We maintain our principal executive offices at 5810 Nancy Ridge Drive, San Diego, California 92121. Our telephone number is (858) 320-8200 and our website address is www.biocept.com. The information contained in, or that can be accessed through, our website is not incorporated into and is not part of this prospectus. We were incorporated in California on May 12, 1997 and reincorporated as a Delaware corporation on July 30, 2013.
12
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012. An “emerging growth company” may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
|
· |
being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations in this prospectus; |
|
· |
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
|
· |
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
|
· |
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We may take advantage of these provisions until December 31, 2019. However, if certain events occur prior to December 31, 2019, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.0 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company before such date.
We have elected to take advantage of certain of the reduced disclosure obligations and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than the information you might receive from other public reporting companies in which you hold equity interests.
13
The Offering
|
Common stock offered by us |
shares. |
|
Common stock outstanding after this offering |
shares. |
|
Over-allotment option |
We have granted the underwriters a 30-day option to purchase up to additional shares of our common stock from us at the public offering price less underwriting discounts and commissions. |
|
Use of proceeds |
Based on an assumed public offering price of $ per share (the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015), we estimate that the net proceeds from our sale of shares of our common stock in this offering will be approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We currently expect to use the net proceeds from this offering as follows: · approximately $ million to hire sales and marketing personnel and support increased sales and marketing activities; · approximately $ million to fund further research and development, clinical utility studies and future enhancements of our current tests and our planned tests and services; · approximately $ million to acquire equipment, implement automation and scale our capabilities to prepare for significant test volume; and · the balance for general corporate purposes and to fund ongoing operations and expansion of our business. |
|
|
For additional information please refer to the section entitled “Use of Proceeds” on page 43 of this prospectus. |
|
Risk Factors |
See the section entitled “Risk Factors” beginning on page 17 of this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
|
NASDAQ Capital Market symbol |
BIOC. |
___________________
The number of shares of our common stock to be outstanding after this offering is based on 4,449,603 shares of our common stock outstanding as of September 30, 2014 and excludes as of such date:
|
· |
875,042 shares of our common stock issuable upon the exercise of stock options, with a weighted average exercise price of $6.47 per share; |
|
· |
251,618 shares of our common stock issuable upon the settlement of outstanding restricted stock units; |
|
· |
610,774 shares of our common stock issuable upon the exercise of outstanding warrants, with a weighted average exercise price of $9.47 per share; |
|
· |
any shares of our common stock issuable upon exercise of the underwriters’ over-allotment option; and |
|
· |
other shares of our common stock reserved for future issuance under our 2013 and 2007 Equity Incentive Plans. |
Unless otherwise indicated, all information contained in this prospectus assumes no exercise by the underwriters of their over-allotment option to purchase up to an additional shares of our common stock.
14
The following tables set forth a summary of our historical financial data as of, and for the period ended on, the dates indicated. We have derived the statement of operations data for the years ended December 31, 2012 and 2013 and the balance sheet data as of December 31, 2013 from our audited financial statements appearing elsewhere in this prospectus. We have derived the statements of operations data for the nine months ended September 30, 2013 and 2014 and balance sheet data as of September 30, 2014 from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements included in this prospectus and, in the opinion of management, reflect all adjustments, consisting only of normal recurring adjustments, necessary to fairly state our financial position as of September 30, 2014 and results of operations for the nine months ended September 30, 2013 and 2014. You should read this data together with our financial statements and related notes appearing elsewhere in this prospectus and the sections in this prospectus entitled “Capitalization,” “Selected Historical Financial Data,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results for any prior period are not necessarily indicative of our future results.
|
|
|
Year ended December 31, |
|
|
For the nine months ended September 30, |
|
||||||||||
|
|
|
2012 |
|
|
2013 |
|
|
2013 |
|
|
2014 |
|
||||
|
|
|
|
|
|
|
|
|
|
|
(unaudited) |
|
|
(unaudited) |
|
||
|
|
|
(in thousands, except share and per share data) |
|
|||||||||||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
109 |
|
|
$ |
134 |
|
|
$ |
115 |
|
|
$ |
58 |
|
|
Cost of revenues |
|
|
1,202 |
|
|
|
2,330 |
|
|
|
1,760 |
|
|
|
1,556 |
|
|
Gross profit |
|
|
(1,093 |
) |
|
|
(2,196 |
) |
|
|
(1,645 |
) |
|
|
(1,498 |
) |
|
Research and development expenses |
|
|
6,562 |
|
|
|
3,086 |
|
|
|
2,376 |
|
|
|
3,428 |
|
|
General and administrative expenses |
|
|
2,063 |
|
|
|
2,513 |
|
|
|
1,736 |
|
|
|
3,971 |
|
|
Sales and marketing expenses |
|
|
785 |
|
|
|
149 |
|
|
|
129 |
|
|
|
1,246 |
|
|
Loss from operations |
|
|
(10,503 |
) |
|
|
(7,944 |
) |
|
|
(5,886 |
) |
|
|
(10,143 |
) |
|
Total other income/(expense) |
|
|
(1,756 |
) |
|
|
(1,288 |
) |
|
|
(874 |
) |
|
|
(1,841 |
) |
|
Loss Before Income Taxes |
|
$ |
(12,259 |
) |
|
$ |
(9,232 |
) |
|
$ |
(6,760 |
) |
|
$ |
(11,984 |
) |
|
Income tax expense |
|
|
(1 |
) |
|
|
(1 |
) |
|
|
(1 |
) |
|
|
(1 |
) |
|
Net loss & comprehensive loss |
|
$ |
(12,260 |
) |
|
$ |
(9,233 |
) |
|
$ |
(6,761 |
) |
|
$ |
(11,985 |
) |
|
Weighted-average shares outstanding used in computing net loss per common share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
160,393 |
|
|
|
181,762 |
|
|
|
182,199 |
|
|
|
3,845,540 |
|
|
Diluted |
|
|
160,393 |
|
|
|
181,762 |
|
|
|
182,199 |
|
|
|
3,845,540 |
|
|
Net loss per common share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(76.43 |
) |
|
$ |
(50.80 |
) |
|
$ |
(37.11 |
) |
|
$ |
(3.12 |
) |
|
Diluted |
|
$ |
(76.43 |
) |
|
$ |
(50.80 |
) |
|
$ |
(37.11 |
) |
|
$ |
(3.12 |
) |
15
|
|
|
As of December 31, 2013 |
|
|
As of September 30, 2014 |
|
||||||
|
|
|
Actual |
|
|
Actual |
|
|
Pro Forma(1) |
|
|||
|
|
|
|
|
|
|
(Unaudited) |
|
|
(Unaudited) |
|
||
|
Balance Sheet Data (in thousands): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
69 |
|
|
$ |
8,820 |
|
|
$ |
|
|
|
Total assets |
|
$ |
1,330 |
|
|
$ |
9,875 |
|
|
$ |
|
|
|
Notes payable, net of discount |
|
$ |
5,201 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Line of Credit |
|
$ |
1,981 |
|
|
$ |
— |
|
|
$ |
— |
|
|
April 2014 Credit Facility, net of discount |
|
$ |
— |
|
|
$ |
4,732 |
|
|
$ |
4,732 |
|
|
Total liabilities |
|
$ |
13,786 |
|
|
$ |
6,530 |
|
|
$ |
6,530 |
|
|
Convertible preferred stock |
|
$ |
7 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Total shareholders’ equity/(deficit) |
|
$ |
(12,456) |
|
|
$ |
3,345 |
|
|
$ |
|
|
|
(1) |
A $1.00 increase (decrease) in the assumed public offering price of $ per share, the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015, would increase (decrease) each of cash and cash equivalents, total assets and total stockholders’ equity/(deficit) by $ million, assuming the number of shares offered by us as stated on the cover page of this prospectus remain unchanged and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, a one million share increase (decrease) in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, total assets and total stockholders’ equity/(deficit) by $ million, assuming the public offering price of $ per share, the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. |
16
An investment in our common stock involves a high degree of risk. You should consider carefully the risks described below, together with all of the other information included in this prospectus, as well as in our other filings with the SEC, in evaluating our business. If any of the following risks actually occur, our business, financial condition, operating results and future prospects could be materially and adversely affected. In that case, the trading price of our common stock may decline and you might lose all or part of your investment. The risks described below are not the only ones we face. Additional risks that we currently do not know about or that we currently believe to be immaterial may also impair our business, financial condition, operating results and prospects. Certain statements below are forward-looking statements. For additional information, see the information included under the heading “Special Note Regarding Forward-Looking Statements.”
Risks Related to this Offering
If you purchase our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
Investors purchasing common stock in this offering will pay a price per share that substantially exceeds the pro forma book value (deficit) per share of our tangible assets after subtracting our liabilities. As a result, investors purchasing common stock in this offering will incur immediate dilution of $ per share, based on an assumed public offering price of $ per share (the last reported sale price of our common stock on The Nasdaq Capital Market on , 2015) and our net tangible book value (deficit) as of September 30, 2014. For information on how the foregoing amounts were calculated, see “Dilution.”
We have broad discretion in the use of the net proceeds we receive from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds we receive in this offering, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether our management are using the net proceeds appropriately. Because of the number and variability of factors that will determine our use of our net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business and cause the price of our common stock to decline. Pending their use, we may invest our net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders.
Risks Relating to Our Financial Condition and Capital Requirements
We are an early stage company with a history of net losses; we expect to incur net losses in the future, and we may never achieve sustained profitability.
We have historically incurred substantial net losses, including net losses of $12.3 million in 2012 and $9.2 million in 2013, as well as a net loss of $12.0 for the nine months ended September 30, 2014, and we have never been profitable. At September 30, 2014, our accumulated deficit was approximately $134.4 million. Before 2008, we were pursuing a business plan relating to fetal genetic disorders and other fields, all of which were unrelated to cancer diagnostics. The portion of our accumulated deficit that relates to the period from inception through December 31, 2007 is approximately $66.5 million.
We expect our losses to continue as a result of costs relating to our lab operations as well as increased sales and marketing costs and ongoing research and development expenses. These losses have had, and will continue to have, an adverse effect on our working capital, total assets and stockholders’ equity. Because of the numerous risks and uncertainties associated with our commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations and cash flows. Our chief executive officer Michael W. Nall, who joined us in August 2013, has not previously been the chief executive officer of a public or private company, and therefore his lack of experience may result in some of his time being spent acclimating to his new position and responsibilities. A lack of significant experience in being the chief executive officer of a public company could have an adverse effect on his ability to quickly respond to problems or effectively manage issues surrounding the operation of a public company.
We will need to raise additional capital.
We expect to need to raise additional capital to expand our business to meet our long-term business objectives. Additional financing may be from the sale of equity or convertible or other debt securities in a public or private offering, from a new credit facility or strategic partnership coupled with an investment in us or a combination of both. We may be unable to raise sufficient
17
additional financing on terms that are acceptable to us, if at all. Failure to raise additional capital in sufficient amounts would significantly impact our ability to expand our business. For further discussion of our liquidity requirements as they relate to our long-term plans, see the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources.”
Risks Relating to Our Business and Strategy
If we are unable to increase sales of our OncoCEE diagnostic tests or successfully develop and commercialize other tests, our revenues will be insufficient for us to achieve profitability.
We currently derive substantially all of our revenues from sales of cancer diagnostic tests. We recently began offering our OncoCEE cancer tests through our CLIA-certified, CAP accredited, and state-licensed laboratory. We are in varying stages of research and development for other cancer diagnostic tests that we may offer. If we are unable to increase sales of our OncoCEE-BR for breast cancer, OncoCEE-LU for NSCLC and OncoCEE-GA for gastric cancer diagnostic test or successfully develop and commercialize other cancer diagnostic tests, we will not produce sufficient revenues to become profitable.
If we are unable to execute our sales and marketing strategy for cancer diagnostic tests and are unable to gain acceptance in the market, we may be unable to generate sufficient revenue to sustain our business.
We are an early-stage company and have engaged in only limited sales and marketing activities for the OncoCEE-BR for breast cancer, OncoCEE-LU for NSCLC and OncoCEE-GA for gastric cancer diagnostic tests we offer through our CLIA-certified, CAP accredited, and state-licensed laboratory. To date, we have received very limited revenue.
Although we believe that our current tests and our planned diagnostic tests represent a promising commercial opportunity, our tests may never gain significant acceptance in the marketplace and therefore may never generate substantial revenue or profits for us. We will need to establish a market for our cancer diagnostic tests and build that market through physician education, awareness programs and the publication of clinical trial results. Gaining acceptance in medical communities requires, among other things, publication in leading peer-reviewed journals of results from studies using our current tests and/or our planned cancer tests. The process of publication in leading medical journals is subject to a peer review process and peer reviewers may not consider the results of our studies sufficiently novel or worthy of publication. Failure to have our studies published in peer-reviewed journals would limit the adoption of our current tests and our planned tests.
Our ability to successfully market the cancer diagnostic tests that we may develop will depend on numerous factors, including:
|
· |
conducting clinical utility studies of such tests in collaboration with key thought leaders to demonstrate their use and value in important medical decisions such as treatment selection; |
|
· |
whether our current or future partners, vigorously support our offerings; |
|
· |
the success of our sales force; |
|
· |
whether healthcare providers believe such diagnostic tests provide clinical utility; |
|
· |
whether the medical community accepts that such diagnostic tests are sufficiently sensitive and specific to be meaningful in patient care and treatment decisions; and |
|
· |
whether private health insurers, government health programs and other third-party payors will cover such cancer diagnostic tests and, if so, whether they will adequately reimburse us. |
Failure to achieve widespread market acceptance of our current tests and our planned cancer diagnostic tests would materially harm our business, financial condition and results of operations.
If we cannot develop tests to keep pace with rapid advances in technology, medicine and science, our operating results and competitive position could be harmed.
In recent years, there have been numerous advances in technologies relating to the diagnosis and treatment of cancer. Several new cancer drugs have been approved, and a number of new drugs in clinical development may increase patient survival time. There have also been advances in methods used to identify patients likely to benefit from these drugs based on analysis of biomarkers. We must continuously develop new cancer diagnostic tests and enhance any existing tests to keep pace with evolving standards of care. Our current tests and our planned tests could become obsolete unless we continually innovate and expand them to demonstrate benefit in the diagnosis, monitoring or prognosis of patients with cancer. New cancer therapies typically have only a few years of clinical data associated with them, which limits our ability to develop cancer diagnostic tests based on, for example, biomarker analysis related to
18
the appearance or development of resistance to those therapies. If we cannot adequately demonstrate the applicability of our current tests and our planned tests to new treatments, by incorporating important biomarker analysis, sales of our tests could decline, which would have a material adverse effect on our business, financial condition and results of operations.
If our current tests and our planned tests do not continue to perform as expected, our operating results, reputation and business will suffer.
Our success depends on the market’s confidence that we can continue to provide reliable, high-quality diagnostic results. We believe that our customers are likely to be particularly sensitive to test defects and errors. As a result, the failure of our current or planned tests to perform as expected would significantly impair our reputation and the public image of our cancer tests, and we may be subject to legal claims arising from any defects or errors.
If our sole laboratory facility becomes damaged or inoperable, or we are required to vacate the facility, our ability to sell and provide cancer diagnostic tests and pursue our research and development efforts may be jeopardized.
We currently derive our revenues from our OncoCEE-BR for breast cancer, OncoCEE-LU for NSCLC and OncoCEE-GA for gastric cancer diagnostic tests conducted in our CLIA-certified, CAP accredited, and state-licensed laboratory. We do not have any clinical reference laboratory facilities other than our facility in San Diego, California. Our facilities and equipment could be harmed or rendered inoperable by natural or man-made disasters, including fire, earthquake, flooding and power outages, which may render it difficult or impossible for us to perform our diagnostic tests for some period of time. The inability to perform our current tests and our planned tests or the backlog of tests that could develop if our facility is inoperable for even a short period of time may result in the loss of customers or harm to our reputation or relationships with scientific or clinical collaborators, and we may be unable to regain those customers or repair our reputation in the future. Furthermore, our facilities and the equipment we use to perform our research and development work could be costly and time-consuming to repair or replace.
The San Diego area has recently experienced serious fires and power outages, and is considered to lie in an area with earthquake risk.
Additionally, a key component of our research and development process involves using biological samples as the basis for our diagnostic test development. In some cases, these samples are difficult to obtain. If the parts of our laboratory facility where we store these biological samples were damaged or compromised, our ability to pursue our research and development projects, as well as our reputation, could be jeopardized. We carry insurance for damage to our property and the disruption of our business, but this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, if at all.
Further, if our CLIA-certified, CAP accredited, and state-licensed laboratory became inoperable we may not be able to license or transfer our technology to another facility with the necessary qualifications, including state licensure and CLIA certification, under the scope of which our current tests and our planned cancer diagnostic tests could be performed. Even if we find a facility with such qualifications to perform our tests, it may not be available to us on commercially reasonable terms.
If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenues or achieve and sustain profitability.
Our principal competition comes from mainstream diagnostic methods, used by pathologists and oncologists and other physicians for many years, which focus on tumor tissue analysis. It may be difficult to change the methods or behavior of oncologists and other physicians to incorporate our CTC and ctDNA testing, including molecular diagnostic testing, in their practices in conjunction with or instead of tissue biopsies and analysis. In addition, companies offering capital equipment and kits or reagents to local pathology laboratories represent another source of potential competition. These kits are used directly by the pathologist, which can facilitate adoption. We plan to focus our marketing and sales efforts on medical oncologists rather than pathologists.
We also face competition from companies that offer products or are conducting research to develop products for CTC or ctDNA testing in various cancers. In particular, Janssen Diagnostics, LLC markets its CellSearch® test and Atossa Genetics markets its ArgusCYTE® test, which are competitive to our tests for CTC enumeration and HER2 analysis. CTC and ctDNA testing is a new area of science and we cannot predict what tests others will develop that may compete with or provide results similar or superior to the results we are able to achieve with the tests we develop. In addition to Janssen Diagnostics and Atossa Genetics, our competitors also include public companies such as Alere (Adnagen) and Illumina as well as many private companies, including Apocell, EPIC Sciences, Clearbridge Biomedics, Cynvenio Biosystems, Fluxion Biosciences, Guardant Health, RareCells and Silicon Biosystems. Many of these groups, in addition to operating research and development laboratories, are establishing CLIA-certified testing laboratories while others are focused on selling equipment and reagents. Our sales and distribution agreements are non-exclusive and our partners could enter into agreements with competitors.
19
We expect that pharmaceutical and biopharmaceutical companies will increasingly focus attention and resources on the personalized cancer diagnostic sector as the potential and prevalence of molecularly targeted oncology therapies approved by the FDA along with companion diagnostics increases. For example, the FDA has recently approved two such agents—Xalkori® from Pfizer Inc. along with its companion anaplastic lymphoma kinase FISH test from Abbott Laboratories, Inc., Zelboraf® from Daiichi-Sankyo/Genentech/Roche along with its companion B-raf kinase V600 mutation test from Roche Molecular Systems, Inc. and Tafinlar® from GlaxoSmithKline along with its companion B-raf kinase V600 mutation test from bioMerieux. These recent FDA approvals are only the second, third and fourth instances of simultaneous approvals of a drug and companion diagnostic, the first being the 2010 approval of Genentech’s Herceptin® for HER2 positive breast cancer along with the HercepTest from partner Dako A/S. Our competitors may invent and commercialize technology platforms or tests that compete with ours.
There are a number of companies which are focused on the oncology diagnostic market, such as Biodesix, Caris, Clarient, Foundation Medicine, Neogenomics, Response Genetics, Agendia, Genomic Health, and Genoptix, who while not currently offering CTC or ctDNA tests are selling to the medical oncologists and pathologists and could develop or offer CTC or ctDNA tests. Large laboratory services companies, such as Sonic USA, Quest and LabCorp, provide more generalized cancer diagnostic testing.
Additionally, projects related to cancer diagnostics and particularly genomics have received increased government funding, both in the United States and internationally. As more information regarding cancer genomics becomes available to the public, we anticipate that more products aimed at identifying targeted treatment options will be developed and that these products may compete with ours. In addition, competitors may develop their own versions of our current or planned tests in countries where we did not apply for patents or where our patents have not issued and compete with us in those countries, including encouraging the use of their test by physicians or patients in other countries.
Some of our present and potential competitors have widespread brand recognition and substantially greater financial and technical resources and development, production and marketing capabilities than we do. Others may develop lower-priced, less complex tests that payors, pathologists and oncologists and other physicians could view as functionally equivalent to our current or planned tests, which could force us to lower the list price of our tests and impact our operating margins and our ability to achieve and maintain profitability. In addition, technological innovations that result in the creation of enhanced diagnostic tools that are more sensitive or specific than ours may enable other clinical laboratories, hospitals, physicians or medical providers to provide specialized diagnostic tests similar to ours in a more patient-friendly, efficient or cost-effective manner than is currently possible. If we cannot compete successfully against current or future competitors, we may be unable to increase or create market acceptance and sales of our current or planned tests, which could prevent us from increasing or sustaining our revenues or achieving or sustaining profitability.
We expect to continue to incur significant expenses to develop and market cancer diagnostic tests, which could make it difficult for us to achieve and sustain profitability.
In recent years, we have incurred significant costs in connection with the development of cancer diagnostic tests. For the year ended December 31, 2012, our research and development expenses were $6.6 million and our sales and marketing expenses were $0.8 million. For the year ended December 31, 2013, our research and development expenses were $3.1 million and our sales and marketing expenses were $0.1 million. For the nine months ended September 30, 2014, our research and development expenses were $3.4 million and our sales and marketing expenses were $1.2 million. We expect our expenses to continue to increase for the foreseeable future as we conduct studies of our current tests and our planned cancer diagnostic tests, establish a sales and marketing organization, drive adoption of and reimbursement for our diagnostic tests and develop new tests. As a result, we need to generate significant revenues in order to achieve sustained profitability.
If oncologists and other physicians decide not to order our OncoCEE cancer diagnostic tests or our future cancer diagnostic tests, we may be unable to generate sufficient revenue to sustain our business.
To generate demand for our current tests and our planned cancer diagnostic tests, we will need to educate oncologists, pathologists, and other health care professionals on the clinical utility, benefits and value of the tests we provide through published papers, presentations at scientific conferences, educational programs and one-on-one education sessions by members of our sales force. In addition, we need to assure oncologists and other physicians of our ability to obtain and maintain coverage and adequate from third-party payors. We need to hire additional commercial, scientific, technical and other personnel to support this process. Unless an adequate number of medical practitioners order our current tests and our planned tests, we will likely be unable to create demand in sufficient volume for us to achieve sustained profitability.
20
Clinical utility studies are important in demonstrating to both customers and payors a test’s clinical relevance and value. If we are unable to identify collaborators willing to work with us to conduct clinical utility studies, or the results of those studies do not demonstrate that a test provides clinically meaningful information and value, commercial adoption of such test may be slow, which would negatively impact our business.
Clinical utility studies show when and how to use a clinical test, and describe the particular clinical situations or settings in which it can be applied and the expected results. Clinical utility studies also show the impact of the test results on patient care and management. Clinical utility studies are typically performed with collaborating oncologists or other physicians at medical centers and hospitals, analogous to a clinical trial, and generally result in peer-reviewed publications. Sales and marketing representatives use these publications to demonstrate to customers how to use a clinical test, as well as why they should use it. These publications are also used with payors to obtain coverage for a test, helping to assure there is appropriate reimbursement.
Our OncoCEE-BR test is currently part of a clinical utility study led by investigators at the Dana-Farber Cancer Institute. We will need to conduct additional studies for this test, as well as other CTC and ctDNA tests we plan to introduce, to increase test adoption in the marketplace and obtain coverage and adequate reimbursement. Should we not be able to perform these studies, or should their results not provide clinically meaningful data and value for oncologists and other physicians, adoption of our tests could be impaired and we may not be able to obtain coverage and adequate reimbursement for them.
We are undergoing a management transition.
Until August 26, 2013, David F. Hale, our Chairman, served as our principal executive officer. On that date, Michael W. Nall began his employment with us as our Chief Executive Officer and President, with David F. Hale remaining employed as our Executive Chairman until February 10, 2014. Mr. Hale currently serves as non-Executive Chairman of our Board of Directors. We intend to recruit and hire other senior executives. Such a management transition subjects us to a number of risks, including risks pertaining to coordination of responsibilities and tasks, creation of new management systems and processes, differences in management style, effects on corporate culture, and the need for transfer of historical knowledge. In addition, Mr. Nall has not previously been the chief executive officer of a public or private company, and therefore his lack of experience may result in some of his time being spent acclimating to his new position and responsibilities. A lack of significant experience in being the chief executive officer of a public company could have an adverse effect on his ability to quickly respond to problems or effectively manage issues surrounding the operation of a public company.
The loss of key members of our executive management team could adversely affect our business.
Our success in implementing our business strategy depends largely on the skills, experience and performance of key members of our executive management team and others in key management positions, including Michael W. Nall, our Chief Executive Officer and President, Lyle J. Arnold, Ph.D., our Senior Vice-President of Research & Development and Chief Scientific Officer, and Veena M. Singh, M.D., our Senior Vice President and Senior Medical Director, William G. Kachioff, our Senior Vice-President of Finance and Chief Financial Officer and Raaj Trivedi, Vice President, Commercial Operations. The collective efforts of each of these persons and others working with them as a team are critical to us as we continue to develop our technologies, tests and research and development and sales programs. As a result of the difficulty in locating qualified new management, the loss or incapacity of existing members of our executive management team could adversely affect our operations. If we were to lose one or more of these key employees, we could experience difficulties in finding qualified successors, competing effectively, developing our technologies and implementing our business strategy. Our Chief Executive Officer and President, Chief Financial Officer, Chief Scientific Officer, Vice President, Commercial Operations and Senior Medical Director have employment agreements, however, the existence of an employment agreement does not guarantee retention of members of our executive management team and we may not be able to retain those individuals for the duration of or beyond the end of their respective terms. We do not maintain “key person” life insurance on any of our employees.
In addition, we rely on collaborators, consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our collaborators, consultants and advisors are generally employed by employers other than us and may have commitments under agreements with other entities that may limit their availability to us.
The loss of a key employee, the failure of a key employee to perform in his or her current position or our inability to attract and retain skilled employees could result in our inability to continue to grow our business or to implement our business strategy.
21
There is a scarcity of experienced professionals in our industry. If we are not able to retain and recruit personnel with the requisite technical skills, we may be unable to successfully execute our business strategy.
The specialized nature of our industry results in an inherent scarcity of experienced personnel in the field. Our future success depends upon our ability to attract and retain highly skilled personnel, including scientific, technical, commercial, business, regulatory and administrative personnel, necessary to support our anticipated growth, develop our business and perform certain contractual obligations. Given the scarcity of professionals with the scientific knowledge that we require and the competition for qualified personnel among life science businesses, we may not succeed in attracting or retaining the personnel we require to continue and grow our operations.
Our failure to continue to attract, hire and retain a sufficient number of qualified sales professionals would hamper our ability to increase demand for our cancer diagnostic test, to expand geographically and to successfully commercialize any other tests or products we may develop.
To succeed in selling our diagnostic tests and any other tests or products that we are able to develop, we must expand our sales force in the United States and/or internationally by recruiting additional sales representatives with extensive experience in oncology and established relationships with medical oncologists, surgeons, oncology nurses, pathologists and other hospital personnel. To achieve our marketing and sales goals, we will need to continue to build our sales and commercial infrastructure, with which to date we have had limited experience. Sales professionals with the necessary technical and business qualifications are in high demand, and there is a risk that we may be unable to attract, hire and retain the number of sales professionals with the right qualifications, scientific backgrounds and relationships with decision-makers at potential customers needed to achieve our sales goals. We expect to face competition from other companies in our industry, some of whom are much larger than us and who can pay greater compensation and benefits than we can, in seeking to attract and retain qualified sales and marketing employees. If we are unable to hire and retain qualified sales and marketing personnel, our business will suffer.
Our dependence on commercialization partners for sales of tests could limit our success in realizing revenue growth.
We intend to grow our business through the use of commercialization partners for the sales, marketing and commercialization of our current tests and our planned future tests, and to do so we must enter into agreements with these partners to sell, market or commercialize our tests. These agreements may contain exclusivity provisions and generally cannot be terminated without cause during the term of the agreement. We may need to attract additional partners to expand the markets in which we sell tests. These partners may not commit the necessary resources to market and sell our cancer diagnostics tests to the level of our expectations, and we may be unable to locate suitable alternatives should we terminate our agreement with such partners or if such partners terminate their agreement with us.
Any relationships we form with commercialization partners are subject to change over time. For example, over 75% of our revenue in 2012 was generated through our arrangement with Clarient, but Clarient is no longer marketing the OncoCEE-BR test as actively as before. In May 2013, we amended our commercialization agreement with Clarient such that Clarient is no longer the exclusive marketer of the OncoCEE-BR test. In 2013, only 11% of our revenue was generated through our arrangement with Clarient, and we expect that in the future the percentage of our revenue which is generated through our arrangement with Clarient will diminish further. If we cannot replace any diminution in revenues we receive through Clarient, our results will be weakened.
If current or future commercialization partners do not perform adequately, or we are unable to locate commercialization partners, we may not realize revenue growth.
We depend on third parties for the supply of blood samples and other biological materials that we use in our research and development efforts. If the costs of such samples and materials increase or our third party suppliers terminate their relationship with us, our business may be materially harmed.
We have relationships with suppliers and institutions that provide us with blood samples and other biological materials that we use in developing and validating our current tests and our planned future tests. If one or more suppliers terminate their relationship with us or are unable to meet our requirements for samples, we will need to identify other third parties to provide us with blood samples and biological materials, which could result in a delay in our research and development activities and negatively affect our business. In addition, as we grow, our research and academic institution collaborators may seek additional financial contributions from us, which may negatively affect our results of operations.
22
We currently rely on third-party suppliers for critical materials needed to perform our current tests and our planned future tests and any problems experienced by them could result in a delay or interruption of their supply to us.
We currently purchase raw materials for our microfluidic channels and testing reagents under purchase orders and do not have long-term contracts with most of the suppliers of these materials. If suppliers were to delay or stop producing our materials or reagents, or if the prices they charge us were to increase significantly, or if they elected not to sell to us, we would need to identify other suppliers. We could experience delays in manufacturing the microfluidic channels or performing tests while finding another acceptable supplier, which could impact our results of operations. The changes could also result in increased costs associated with qualifying the new materials or reagents and in increased operating costs. Further, any prolonged disruption in a supplier’s operations could have a significant negative impact on our ability to perform cancer diagnostic tests in a timely manner.
Some of the components used in our current or planned products are currently sole-source, and substitutes for these components might not be able to be obtained easily or may require substantial design or manufacturing modifications. Any significant problem experienced by one of our sole source suppliers may result in a delay or interruption in the supply of components to us until that supplier cures the problem or an alternative source of the component is located and qualified. Any delay or interruption would likely lead to a delay or interruption in our manufacturing operations. The inclusion of substitute components must meet our product specifications and could require us to qualify the new supplier with the appropriate government regulatory authorities.
If we were sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
The marketing, sale and use of our current tests and our planned future diagnostic tests could lead to the filing of product liability claims against us if someone alleges that our tests failed to perform as designed. We may also be subject to liability for errors in the test results we provide to physicians or for a misunderstanding of, or inappropriate reliance upon, the information we provide. A product liability or professional liability claim could result in substantial damages and be costly and time-consuming for us to defend.
Although we believe that our existing product and professional liability insurance is adequate, our insurance may not fully protect us from the financial impact of defending against product liability or professional liability claims. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could damage our reputation, result in the recall of tests, or cause current partners to terminate existing agreements and potential partners to seek other partners, any of which could impact our results of operations.
If we use biological and hazardous materials in a manner that causes injury, we could be liable for damages.
Our activities currently require the controlled use of potentially harmful biological materials and chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject to, on an ongoing basis, federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations may become significant and could have a material adverse effect on our financial condition, results of operations and cash flows. In the event of an accident or if we otherwise fail to comply with applicable regulations, we could lose our permits or approvals or be held liable for damages or penalized with fines.
We may acquire other businesses or form joint ventures or make investments in other companies or technologies that could harm our operating results, dilute our stockholders’ ownership, increase our debt or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of businesses and assets. We also may pursue strategic alliances and joint ventures that leverage our core technology and industry experience to expand our offerings or distribution. We have no experience with acquiring other companies and limited experience with forming strategic alliances and joint ventures. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could have a material adverse effect on our financial condition, results of operations and cash flows. Integration of an acquired company also may disrupt ongoing operations and require management resources that would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could have a material negative effect on our results of operations. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, technology license, strategic alliance or joint venture.
23
To finance any acquisitions or joint ventures, we may choose to issue shares of our common stock as consideration, which would dilute the ownership of our stockholders. If the price of our common stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
If we cannot support demand for our current tests and our planned future diagnostic tests, including successfully managing the evolution of our technology and manufacturing platforms, our business could suffer.
As our test volume grows, we will need to increase our testing capacity, implement automation, increase our scale and related processing, customer service, billing, collection and systems process improvements and expand our internal quality assurance program and technology to support testing on a larger scale. We will also need additional clinical laboratory scientists and other scientific and technical personnel to process these additional tests. Any increases in scale, related improvements and quality assurance may not be successfully implemented and appropriate personnel may not be available. As additional tests are commercialized, we may need to bring new equipment on line, implement new systems, technology, controls and procedures and hire personnel with different qualifications. Failure to implement necessary procedures or to hire the necessary personnel could result in a higher cost of processing or an inability to meet market demand. We cannot assure you that we will be able to perform tests on a timely basis at a level consistent with demand, that our efforts to scale our commercial operations will not negatively affect the quality of our test results or that we will respond successfully to the growing complexity of our testing operations. If we encounter difficulty meeting market demand or quality standards for our current tests and our planned future tests, our reputation could be harmed and our future prospects and business could suffer, which may have a material adverse effect on our financial condition, results of operations and cash flows.
We may encounter manufacturing problems or delays that could result in lost revenue.
We currently manufacture our proprietary microfluidic channels at our San Diego facility and intend to continue to do so. We believe we currently have adequate manufacturing capacity for our microfluidic channels. If demand for our current tests and our planned future tests increases significantly, we will need to either expand our manufacturing capabilities or outsource to other manufacturers. If we or third party manufacturers engaged by us fail to manufacture and deliver our microfluidic channels or certain reagents in a timely manner, our relationships with our customers could be seriously harmed. We cannot assure you that manufacturing or quality control problems will not arise as we attempt to increase the production of our microfluidic channels or reagents or that we can increase our manufacturing capabilities and maintain quality control in a timely manner or at commercially reasonable costs. If we cannot manufacture our microfluidic channels consistently on a timely basis because of these or other factors, it could have a significant negative impact on our ability to perform tests and generate revenues.
International expansion of our business would expose us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States.
Our business strategy contemplates possible international expansion, including partnering with academic and commercial testing laboratories, and introducing OncoCEE technology outside the United States as part of CE-marked IVD test kits and/or testing systems utilizing our CEE and/or CEE-Selector technologies. Doing business internationally involves a number of risks, including:
|
· |
multiple, conflicting and changing laws and regulations such as tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; |
|
· |
failure by us or our distributors to obtain regulatory approvals for the sale or use of our current tests and our planned future tests in various countries; |
|
· |
difficulties in managing foreign operations; |
|
· |
complexities associated with managing government payor systems, multiple payor-reimbursement regimes or self-pay systems; |
|
· |
logistics and regulations associated with shipping blood samples, including infrastructure conditions and transportation delays; |
|
· |
limits on our ability to penetrate international markets if our current tests and our planned future diagnostic tests cannot be processed by an appropriately qualified local laboratory; |
|
· |
financial risks, such as longer payment cycles, difficulty enforcing contracts and collecting accounts receivable and exposure to foreign currency exchange rate fluctuations; |
|
· |
reduced protection for intellectual property rights, or lack of them in certain jurisdictions, forcing more reliance on our trade secrets, if available; |
24
|
· |
natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; and |
|
· |
failure to comply with the Foreign Corrupt Practices Act, including its books and records provisions and its anti-bribery provisions, by maintaining accurate information and control over sales activities and distributors’ activities. |
|
· |
Any of these risks, if encountered, could significantly harm our future international expansion and operations and, consequently, have a material adverse effect on our financial condition, results of operations and cash flows. |
Declining general economic or business conditions may have a negative impact on our business.
Continuing concerns over United States health care reform legislation and energy costs, geopolitical issues, the availability and cost of credit and government stimulus programs in the United States and other countries have contributed to increased volatility and diminished expectations for the global economy. These factors, combined with low business and consumer confidence and high unemployment, precipitated an economic slowdown and recession. If the economic climate does not improve, or it deteriorates, our business, including our access to patient samples and the addressable market for diagnostic tests that we may successfully develop, as well as the financial condition of our suppliers and our third-party payors, could be adversely affected, resulting in a negative impact on our business, financial condition and results of operations.
Intrusions into our computer systems could result in compromise of confidential information.
Despite the implementation of security measures, our technology or systems that we interface with, including the Internet and related systems, may be vulnerable to physical break-ins, hackers, improper employee or contractor access, computer viruses, programming errors, or similar problems. Any of these might result in confidential medical, business or other information of other persons or of ourselves being revealed to unauthorized persons.
There are a number of state, federal and international laws protecting the privacy and security of health information and personal data. As part of the American Recovery and Reinvestment Act of 2009, or ARRA, Congress amended the privacy and security provisions of the Health Insurance Portability and Accountability Act, or HIPAA. HIPAA imposes limitations on the use and disclosure of an individual’s healthcare information by healthcare providers, healthcare clearinghouses, and health insurance plans, collectively referred to as covered entities, and also grants individuals rights with respect to their health information. HIPAA also imposes compliance obligations and corresponding penalties for non-compliance on individuals and entities that provide services to healthcare providers and other covered entities, collectively referred to as business associates. ARRA also made significant increases in the penalties for improper use or disclosure of an individual’s health information under HIPAA and extended enforcement authority to state attorneys general. As amended by ARRA and subsequently by the final omnibus rule adopted in 2013, or Final Omnibus Rule, HIPAA also imposes notification requirements on covered entities in the event that certain health information has been inappropriately accessed or disclosed: notification requirements to individuals, federal regulators, and in some cases, notification to local and national media. Notification is not required under HIPAA if the health information that is improperly used or disclosed is deemed secured in accordance with encryption or other standards developed by the U.S. Department of Health and Human Services, or HHS. Most states have laws requiring notification of affected individuals and/or state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms to ensure ongoing protection of personal information. Activities outside of the United States implicate local and national data protection standards, impose additional compliance requirements and generate additional risks of enforcement for non-compliance. We may be required to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws, to protect against security breaches and hackers or to alleviate problems caused by such breaches.
We depend on our information technology and telecommunications systems, and any failure of these systems could harm our business.
We depend on information technology and telecommunications systems for significant aspects of our operations. In addition, our third-party billing and collections provider depends upon telecommunications and data systems provided by outside vendors and information we provide on a regular basis. These information technology and telecommunications systems support a variety of functions, including test processing, sample tracking, quality control, customer service and support, billing and reimbursement, research and development activities and our general and administrative activities. Information technology and telecommunications systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our information technology and telecommunications systems, failures or significant downtime of our information technology or telecommunications systems or those used by our third-party service providers
25
could prevent us from processing tests, providing test results to oncologists, pathologists, billing payors, processing reimbursement appeals, handling patient or physician inquiries, conducting research and development activities and managing the administrative aspects of our business. Any disruption or loss of information technology or telecommunications systems on which critical aspects of our operations depend could have an adverse effect on our business.
Regulatory Risks Relating to Our Business
Healthcare policy changes, including recently enacted legislation reforming the U.S. health care system, may have a material adverse effect on our financial condition, results of operations and cash flows.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, enacted in March 2010, makes a number of substantial changes in the way health care is financed by both governmental and private insurers. Among other things, the ACA:
|
· |
Mandates a reduction in payments for clinical laboratory services paid under the Medicare Clinical Laboratory Fee Schedule, or CLFS, annual Consumer Price Index update of 1.75% for the years 2011 through 2015. In addition, a multifactor productivity adjustment is made to the fee schedule payment amount, which could further reduce payment rates. These changes in payments may apply to some or all of the tests we furnish to Medicare beneficiaries. |
|
· |
Establishes an Independent Payment Advisory Board to reduce the per capita rate of growth in Medicare spending if spending exceeds a target growth rate. The Independent Payment Advisory Board has broad discretion to propose policies, which may have a negative impact on payment rates for services, including clinical laboratory services, beginning in 2016, and for hospital services beginning in 2020. |
|
· |
Requires each medical device manufacturer to pay an excise tax equal to 2.3% of the price for which such manufacturer sells its medical devices that are listed with the FDA. We believe that at this time this tax does not apply to our current cancer diagnostic test or to our products that are in development; nevertheless, this could change in the future if either the FDA or the Internal Revenue Service, which regulates the payment of this excise tax, changes its position. |
Although some of these provisions may negatively impact payment rates for clinical laboratory tests, the ACA also extends coverage to over 30 million previously uninsured people, which may result in an increase in the demand for our current tests and our planned future cancer diagnostic tests. The mandatory purchase of insurance has been strenuously opposed by a number of state governors, resulting in lawsuits challenging the constitutionality of certain provisions of the ACA. In 2012, the Supreme Court upheld the constitutionality of the ACA, with the exception of certain provisions dealing with the expansion of Medicaid coverage under the law.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. The Protecting Access to Medicare Act of 2014, or PAMA, was signed to law, which, among other things, significantly alters the current payment methodology under the CLFS. Under the new law, starting January 1, 2016 and every three years thereafter (or annually in the case of advanced diagnostic lab tests), clinical laboratories must report laboratory test payment data for each Medicare-covered clinical diagnostic lab test that it furnishes during a time period to be defined by future regulations. The reported data must include the payment rate (reflecting all discounts, rebates, coupons and other price concessions) and the volume of each test that was paid by each private payor (including health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations). Beginning in 2017, the Medicare payment rate for each clinical diagnostic lab test will be equal to the weighted median amount for the test from the most recent data collection period. The payment rate will apply to laboratory tests furnished by a hospital laboratory if the test is separately paid under the hospital outpatient prospective payment system. Although the PAMA changes are generally viewed by industry as a favorable alternative to other proposals to update the CLFS payment methodology, it is too early to predict the impact on reimbursement for our products. Also under PAMA, the Centers for Medicare & Medicaid Services, or CMS, is required to adopt temporary billing codes to identify new tests and new advanced diagnostic laboratory tests that have been cleared or approved by the FDA. For an existing test that is cleared or approved by the FDA and for which Medicare payment is made as of April 1, 2014, CMS is required to assign a unique billing code if one has not already been assigned by the agency. In addition to assigning the code, CMS must publicly report payment for the tests no later than January 1, 2016. Also under PAMA, CMS is required to adopt temporary billing codes to identify new tests and new advanced diagnostic laboratory tests that have been cleared or approved by the FDA. We cannot determine at this time the full impact of PAMA on our business, financial condition and results of operations.
Additionally, the Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee did not achieve its targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers and suppliers of up to 2% per fiscal year, starting in 2013, and will remain in effect through 2024 unless additional congressional action is taken. The full impact on our business of the ACA and the
26
sequester law is uncertain. In addition, the Middle Class Tax Relief and Job Creation Act of 2012, or MCTRJCA, mandated an additional change in Medicare reimbursement for clinical laboratory tests.
Some of our laboratory test business is subject to the Medicare Physician Fee Schedule and, under the current statutory formula, the rates for these services are updated annually. For the past several years, the application of the statutory formula would have resulted in substantial payment reductions if Congress failed to intervene. In the past, Congress passed interim legislation to prevent the decreases. A recent legislative intervention was passed with PAMA, which provided for a 0.5% update from 2013 Medicare Physician Fee Schedule payment rates through 2014 and a 0% update from January 1 until April 1, 2015. If Congress fails to intervene to prevent the negative update factor in future years, the resulting decrease in payment may adversely affect our revenue and results of operations. If in future years Congress does not adopt interim legislation to block or offset, and/or CMS does not moderate, any substantial CMS-proposed reimbursement reductions, the resulting decrease in payments from Medicare could adversely impact our revenues and results of operations.
In November 2014, CMS issued the Physician Fee Schedule Final Rule to take effect January 1, 2015 the overall reduction was 2% but pricing for some codes including FISH pricing were reduced by approximately 53%.
We cannot predict whether future health care initiatives will be implemented at the federal or state level, or how any future legislation or regulation may affect us. The expansion of government’s role in the U.S. health care industry as a result of the ACA’s implementation, and changes to the reimbursement amounts paid by Medicare and other payors for our current tests and our planned future cancer diagnostic tests, may reduce our profits, if any, and have a materially adverse effect on our business, financial condition, results of operations and cash flows. Moreover, Congress has proposed on several occasions to impose a 20% coinsurance payment requirement on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. In the event that Congress were to ever enact such legislation, the cost of billing and collecting for our tests could often exceed the amount actually received from the patient.
Our commercial success could be compromised if hospitals or other clients do not pay our invoices or if third-party payors, including managed care organizations and Medicare, do not provide coverage and reimbursement, breach, rescind or modify their contracts or reimbursement policies or delay payments for our current tests and our planned future tests.
Oncologists and other physicians may not order our current tests and our planned future cancer diagnostic tests unless third-party payors, such as managed care organizations and government payors (e.g., Medicare and Medicaid), pay a substantial portion of the test price. Coverage and reimbursement by a third-party payor may depend on a number of factors, including a payor’s determination that tests using our technologies are:
|
· |
not experimental or investigational; |
|
· |
medically necessary; |
|
· |
appropriate for the specific patient; |
|
· |
cost-effective; |
|
· |
supported by peer-reviewed publications; and |
|
· |
included in clinical practice guidelines. |
Uncertainty surrounds third-party payor coverage and adequate reimbursement of any test incorporating new technology, including tests developed using our technologies. Technology assessments of new medical tests conducted by research centers and other entities may be disseminated to interested parties for informational purposes. Third-party payors and health care providers may use such technology assessments as grounds to deny coverage for a test or procedure. Technology assessments can include evaluation of clinical utility studies, which define how a test is used in a particular clinical setting or situation.
Because each payor generally determines for its own enrollees or insured patients whether to cover or otherwise establish a policy to reimburse our cancer diagnostic tests, seeking payor approvals is a time-consuming and costly process. We cannot be certain that coverage for our current tests and our planned future tests will be provided in the future by additional third-party payors or that existing agreements, policy decisions or reimbursement levels will remain in place or be fulfilled under existing terms and provisions. If we cannot obtain coverage and adequate reimbursement from private and governmental payors such as Medicare and Medicaid for our current tests, or new tests or test enhancements that we may develop in the future, our ability to generate revenues could be limited, which may have a material adverse effect on our financial condition, results of operations and cash flow. Further, we may experience delays and interruptions in the receipt of payments from third-party payors due to missing documentation and/or other issues, which could cause delay in collecting our revenue.
27
In addition, to the extent that our testing is ordered for Medicare inpatients and outpatients, only the hospital may receive payment from the Medicare program for the technical component of pathology services and any clinical laboratory services that we perform, unless the testing is ordered at least 14 days after discharge and certain other requirements are met. We therefore must look to the hospital for payment for these services under these circumstances. If hospitals refuse to pay for the services or fail to pay in a timely manner, our ability to generate revenues could be limited, which may have a material adverse effect on our financial condition, results of operations and cash flow.
We expect to depend on Medicare and a limited number of private payors for a significant portion of our revenues and if these or other payors stop providing reimbursement or decrease the amount of reimbursement for our current tests and our planned future tests, our revenues could decline.
We believe, based on research showing that approximately 54% of new cancers occur in persons age 65 and older and that almost all Americans age 65 and older are enrolled in Medicare, that a substantial portion of the patients for whom we would expect to perform cancer diagnostic tests will have Medicare as their primary medical insurance. Only in November 2013 did we first directly bill any payor for physician-ordered testing; until May 2013, our commercialization partner Clarient was responsible for all billing associated with our tests. We do not have data for Clarient’s billing and collection experience with respect to our tests, because Clarient paid us a contracted amount per test performed regardless of their billing and collections. From May to December 2013, we performed an average of 1-3 physician-ordered tests per month, and from July to September 2014, we performed an average of 32 physician-ordered tests per month (in addition to the tests which we have been performing since January 2013 for a clinical utility study with investigators at the Dana-Farber Cancer Institute, with an average of 15-30 tests per month performed during the trial’s enrollment period through May 2014). Billing for these physician-ordered tests is now handled for us by a non-Clarient billing service provider. Since May 2013, we invoiced, through this service provider, for 218 physician-ordered tests. Of these, 37 tests were billed to Medicare and the remainder were billed to other payors. As of December 2014, we have been paid by private payors for 66 of these tests, with an average price collected of $1,165 per test, while we have not yet had any response or adjudication from any payor as to the other bills submitted. Accordingly, we do not yet have any data regarding reimbursement history or collectability experience. To date, all of our revenue recognized has come from private payors. Medicare claims have not yet been processed due to a new application process due to a change in our tax identification number. We cannot assure you that, even if OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests are otherwise successful, reimbursement for the currently Medicare-covered portions of OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests would, without adequate Medicare reimbursement for the capture/enumeration portion, produce sufficient revenues to enable us to reach profitability and achieve our other commercial objectives.
Medicare and other third-party payors may change their coverage policies or cancel future contracts with us at any time, review and adjust the rate of reimbursement or stop paying for our tests altogether, which would reduce our total revenues. Payors have increased their efforts to control the cost, utilization and delivery of health care services. In the past, measures have been undertaken to reduce payment rates for and decrease utilization of the clinical laboratory testing generally. Because of the cost-trimming trends, third-party payors that currently cover and provide reimbursement for our current tests and our planned future cancer diagnostic tests may suspend, revoke or discontinue coverage at any time, or may reduce the reimbursement rates payable to us. Any such action could have a negative impact on our revenues, which may have a material adverse effect on our financial condition, results of operations and cash flows.
In addition, we are currently considered a “non-contracted provider” by the majority of third-party payors because we have not entered into a specific contract to provide cancer diagnostic tests to their insured patients at specified rates of reimbursement. If we were to become a contracted provider with additional payors in the future, the amount of overall reimbursement we receive would likely decrease because we could be reimbursed less money per test performed at a contracted rate than at a non-contracted rate, which could have a negative impact on our revenues. Further, we typically are unable to collect payments from patients beyond that which is paid by their insurance and will continue to experience lost revenue as a result.
Because of certain Medicare billing policies, we may not receive complete reimbursement for tests provided to Medicare patients. Medicare reimbursement revenues are an important component of our business model, and private payors sometimes look to Medicare determinations when making their own payment determinations; therefore, incomplete or inadequate reimbursement from Medicare would negatively affect our business.
Medicare has coverage policies that can be national or regional in scope. Coverage means that the test or assay is approved as a benefit for Medicare beneficiaries. If there is no coverage, neither the supplier nor any other party, such as a reference laboratory, may receive reimbursement from Medicare for the service. There is currently no national coverage policy regarding the CTC capture/enumeration portion of our testing. Because our laboratory is in California, the regional Medicare Administrative Contractor, or MAC, for California is the relevant MAC for all our testing. The previous MAC for California, Palmetto GBA, LLC, adopted a negative coverage policy for CTC capture/enumeration. The current MAC for California, Noridian Healthcare Solutions, LLC, is adopting the coverage policies from Palmetto GBA. Therefore the capture/enumeration portion of our OncoCEE testing is not
28
currently covered and we will receive no payment from Medicare for this service unless and until the coverage policy is changed. On November 4, 2013, we submitted a comprehensive dossier explaining to Palmetto GBA and Noridian the benefits of the capture/enumeration testing in order to seek to persuade the MACs to allow coverage for this portion of our testing. Palmetto GBA responded on November 27, 2013, denying our request for Medicare coverage for the CTC capture/enumeration portion of our OncoCEE testing. We have not received any other indications to suggest that the negative coverage determination will be reversed. We intend to continue our efforts to obtain Medicare coverage for capture/enumeration.
We cannot assure you that, even if OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests are otherwise successful, reimbursement for the currently Medicare-covered portions of OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests would, without Medicare reimbursement for the capture/enumeration portion, produce sufficient revenues to enable us to reach profitability and achieve our other commercial objectives.
The processing of Medicare claims is subject to change at CMS’ discretion at any time. Cost containment initiatives may be a threat to Medicare reimbursement levels (including for the covered components of OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests, including FISH analysis and molecular testing) for the foreseeable future.
Long payment cycles of Medicare, Medicaid and/or other third-party payors, or other payment delays, could hurt our cash flows and increase our need for working capital.
Medicare and Medicaid have complex billing and documentation requirements that we must satisfy in order to receive payment, and the programs can be expected to carefully audit and monitor our compliance with these requirements. We must also comply with numerous other laws applicable to billing and payment for healthcare services, including, for example, privacy laws. Failure to comply with these requirements may result in, among other things, non-payment, refunds, exclusion from government healthcare programs, and civil or criminal liabilities, any of which may have a material adverse effect on our revenues and earnings. In addition, failure by third-party payors to properly process our payment claims in a timely manner could delay our receipt of payment for our products and services, which may have a material adverse effect on our cash flows.
Complying with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
We are subject to CLIA, a federal law regulating clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. Our clinical laboratory must be certified under CLIA in order for us to perform testing on human specimens. CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality control, quality assurance and inspections. We have a current certificate of accreditation under CLIA to perform high complexity testing, and our laboratory is accredited by the College of American Pathologists, or CAP, one of six CLIA-approved accreditation organizations. To renew this certificate, we are subject to survey and inspection every two years. Moreover, CLIA inspectors may make periodic inspections of our clinical laboratory outside of the renewal process. The failure to comply with CLIA requirements can result in enforcement actions, including the revocation, suspension, or limitation of our CLIA certificate of accreditation, as well as a directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit and/or criminal penalties. We must maintain CLIA compliance and certification to be eligible to bill for tests provided to Medicare beneficiaries. If we were to be found out of compliance with CLIA program requirements and subjected to sanctions, our business and reputation could be harmed. Even if it were possible for us to bring our laboratory back into compliance, we could incur significant expenses and potentially lose revenue in doing so.
The failure to comply with CLIA requirements can result in enforcement actions, including the revocation, suspension, or limitation of our CLIA certificate of accreditation, as well as a directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit and/or criminal penalties. We must maintain CLIA compliance and certification to be eligible to bill for tests provided to Medicare beneficiaries. If we were to be found out of compliance with CLIA program requirements and subjected to sanctions, our business and reputation could be harmed. Even if it were possible for us to bring our laboratory back into compliance, we could incur significant expenses and potentially lose revenue in doing so.
In addition, our laboratory is located in California and is required by state law to have a California state license; as we expand our geographic focus, we may need to obtain laboratory licenses from additional states. California laws establish standards for operation of our clinical laboratory, including the training and skills required of personnel and quality control. In addition, we hold licenses from the states of Pennsylvania, Florida, Maryland and Rhode Island to test specimens from patients in those states or received from ordering physicians in those states. In addition, our clinical reference laboratory is required to be licensed on a product-specific basis by New York as an out of state laboratory and our products, as laboratory developed tests, must be approved by the New York State Department of Health before they are offered in New York. As part of this process, the State of New York requires validation of our tests. We currently do not have the necessary New York license, but we are in the process of addressing the
29
requirements for licensure in New York. Other states may have similar requirements or may adopt similar requirements in the future. Finally, we may be subject to regulation in foreign jurisdictions if we seek to expand international distribution of our tests outside the United States.
If we were to lose our CLIA certification or California laboratory license, whether as a result of a revocation, suspension or limitation, we would no longer be able to offer our tests, which would limit our revenues and harm our business. If we were to lose, or fail to obtain, a license in any other state where we are required to hold a license, we would not be able to test specimens from those states.
If the FDA were to begin requiring approval or clearance of our current tests and our planned future tests, we could incur substantial costs and time delays associated with meeting requirements for pre-market clearance or approval or we could experience decreased demand for, or reimbursement of, our tests.
Historically, the U.S. Food and Drug Administration, or FDA, has exercised enforcement discretion with respect to most LDTs and has not required laboratories that furnish LDTs to comply with the agency’s requirements for medical devices (e.g., establishment registration, device listing, quality systems regulations, premarket clearance or premarket approval, and post-market controls). In recent years, however, the FDA has stated it intends to end its policy of general enforcement discretion and regulate certain LDTs as medical devices. To this end, on October 3, 2014, the FDA issued two draft guidance documents, entitled “Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs)” and “FDA Notification and Medical Device Reporting for Laboratory Developed Tests (LDTs)”, respectively, that set forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs. The FDA has indicated that it does not intend to modify its policy of enforcement discretion until the draft guidance documents are finalized. It is unclear at this time when, or if, the draft guidance documents will be finalized, and even then, the new regulatory requirements are proposed to be phased-in consistent with the schedule set forth in the guidance (in as little as 12 months after the draft guidance is finalized for certain high-priority LDTs such as LDTs with the same intended use as a cleared or approved companion diagnostic). Nevertheless, the FDA may decide to regulate certain LDTs on a case-by-case basis at any time.
The container we provide for collection and transport of blood samples from a health care provider to our clinical laboratory may be a medical device subject to the FDA regulation but is currently exempt from pre-market review by the FDA. While we believe that we are currently in material compliance with applicable laws and regulations, we cannot assure you that the FDA or other regulatory agencies would agree with our determination, and a determination that we have violated these laws, or a public announcement that we are being investigated for possible violations of these laws, could adversely affect our business, prospects, results of operations or financial condition.
In addition, HHS requested that its Advisory Committee on Genetics, Health and Society make recommendations about the oversight of genetic testing. A final report was published in April 2008. If the report’s recommendations for increased oversight of genetic testing were to result in further regulatory burdens, they could negatively affect our business and delay the commercialization of tests in development.
The requirement of pre-market review could negatively affect our business until such review is completed and clearance to market or approval is obtained. The FDA could require that we stop selling our cancer diagnostic tests pending pre-market clearance or approval. If the FDA allows our tests to remain on the market but there is uncertainty about our tests, if they are labeled investigational by the FDA or if labeling claims the FDA allows us to make are very limited, orders from physicians or reimbursement may decline. The regulatory approval process may involve, among other things, successfully completing additional clinical trials and making a 510(k) submission, or filing a pre-market approval application with the FDA. If the FDA requires pre-market review, our tests may not be cleared or approved on a timely basis, if at all. We may also decide voluntarily to pursue FDA pre-market review of our tests if we determine that doing so would be appropriate.
Additionally, should future regulatory actions affect any of the reagents we obtain from suppliers and use in conducting our tests, our business could be adversely affected in the form of increased costs of testing or delays, limits or prohibitions on the purchase of reagents necessary to perform our testing.
If we were required to conduct additional clinical studies or trials before continuing to offer tests that we have developed or may develop as LDTs, those studies or trials could lead to delays or failure to obtain necessary regulatory approval, which could cause significant delays in commercializing any future products and harm our ability to achieve sustained profitability.
If the FDA decides to require that we obtain clearance or approvals to commercialize our current tests or our planned future cancer diagnostic tests, we may be required to conduct additional pre-market clinical testing before submitting a regulatory notification or application for commercial sales. In addition, as part of our long-term strategy we may plan to seek FDA clearance or approval so we can sell our tests outside our CLIA laboratory; however, we would need to conduct additional clinical validation activities on our tests before we can submit an application for FDA approval or clearance. Clinical trials must be conducted in
30
compliance with FDA regulations or the FDA may take enforcement action or reject the data. The data collected from these clinical trials may ultimately be used to support market clearance or approval for our tests. We believe it would likely take two years or more to conduct the clinical studies and trials necessary to obtain approval from the FDA to commercially launch our current tests and our planned future tests outside of our clinical laboratory. Even if our clinical trials are completed as planned, we cannot be certain that their results will support our test claims or that the FDA or foreign authorities will agree with our conclusions regarding our test results. Success in early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior clinical trials and studies. If we are required to conduct pre-market clinical trials, whether using prospectively acquired samples or archival samples, delays in the commencement or completion of clinical testing could significantly increase our test development costs and delay commercialization. Many of the factors that may cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or approval. The commencement of clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites and the eligibility criteria for the clinical trial. Moreover, the clinical trial process may fail to demonstrate that our current tests and our planned future tests are effective for the proposed indicated uses, which could cause us to abandon a test candidate and may delay development of other tests.
We may find it necessary to engage contract research organizations to perform data collection and analysis and other aspects of our clinical trials, which might increase the cost and complexity of our trials. We may also depend on clinical investigators, medical institutions and contract research organizations to perform the trials properly. If these parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, or if the quality, completeness or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or for other reasons, our clinical trials may have to be extended, delayed or terminated. Many of these factors would be beyond our control. We may not be able to enter into replacement arrangements without undue delays or considerable expenditures. If there are delays in testing or approvals as a result of the failure to perform by third parties, our research and development costs would increase, and we may not be able to obtain regulatory clearance or approval for our current tests and our planned future tests. In addition, we may not be able to establish or maintain relationships with these parties on favorable terms, if at all. Each of these outcomes would harm our ability to market our tests or to achieve sustained profitability.
We are subject to federal and state healthcare fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws.
We are subject to health care fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. These health care laws and regulations include, for example:
|
· |
the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from soliciting, receiving, offering or providing remuneration, directly or indirectly, overtly or covertly, in cash or in kind, in return for or to induce either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or services for which payment may be made under a federal health care program such as the Medicare and Medicaid programs; |
|
· |
the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits physicians from referring Medicare or Medicaid patients to providers of “designated health services” with whom the physician or a member of the physician’s immediate family has an ownership interest or compensation arrangement, unless a statutory or regulatory exception applies; |
|
· |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which established federal crimes for, among other things, knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services; |
|
· |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
|
· |
federal false claims and civil monetary penalties laws, which, prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, false or fraudulent claims for payment to the federal government; |
|
· |
The federal Physician Payment Sunshine Act requirements under the ACA, which require certain manufacturers of drugs, devices, biologics and medical supplies to report to the U.S. Department of Health and Human Services information related to payments and other transfers of value made to or at the request of covered recipients, such as physicians and teaching hospitals, and certain physician ownership and investment interests in such manufacturers; and |
|
· |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers. |
31
Further, the ACA, among other things, amends the intent requirement of the federal Anti-Kickback Statute and certain criminal health care fraud statutes. Where the intent requirement has been lowered, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the government may now assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Any action brought against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including, among others, administrative, civil and criminal penalties, damages and fines, and/or exclusion from participation in Medicare, Medicaid programs, including the California Medical Assistance Program (Medi-Cal—the California Medicaid program) or other state or federal health care programs. Additionally, we could be required to refund payments received by us, and we could be required to curtail or cease our operations. Any of the foregoing consequences could seriously harm our business and our financial results.
We may be required to comply with laws governing the transmission, security and privacy of health information that require significant compliance costs, and any failure to comply with these laws could result in material criminal and civil penalties.
Under the administrative simplification provisions of HIPAA, HHS has issued regulations which establish uniform standards governing the conduct of certain electronic health care transactions and protecting the privacy and security of Protected Health Information used or disclosed by health care providers and other covered entities.
The privacy regulations regulate the use and disclosure of Protected Health Information by covered entities engaging in certain electronic transactions or “standard transactions.” They also set forth certain rights that an individual has with respect to his or her Protected Health Information maintained by a covered entity, including the right to access or amend certain records containing Protected Health Information or to request restrictions on the use or disclosure of Protected Health Information. The HIPAA security regulations establish administrative, physical and technical standards for maintaining the confidentiality, integrity and availability of Protected Health Information in electronic form. These standards apply to covered entities and also to “business associates” or third parties providing services to covered entities involving the use or disclosure of Protected Health Information. The HIPAA privacy and security regulations establish a uniform federal “floor” and do not supersede state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing Protected Health Information. As a result, we may be required to comply with both HIPAA privacy regulations and varying state privacy and security laws.
Moreover, the Health Information Technology for Economic and Clinical Health Act, or HITECH, enacted as part of ARRA, among other things, established certain health information security breach notification requirements, which were later further modified by the Final Omnibus Rule. In the event of a breach of unsecured Protected Health Information, a covered entity must notify each individual whose Protected Health Information is breached, federal regulators and in some cases, must publicize the breach in local or national media. Breaches affecting 500 individuals or more may be publicized by federal regulators who publicly identify the breaching entity, the circumstances of the breach and the number of individuals affected.
These laws contain significant fines and other penalties for wrongful use or disclosure of Protected Health Information. Given the complexity of HIPAA and HITECH and their overlap with state privacy and security laws, and the fact that these laws are rapidly evolving and are subject to changing and potentially conflicting interpretation, our ability to comply with the HIPAA, HITECH and state privacy requirements is uncertain and the costs of compliance are significant. Adding to the complexity is that our operations are evolving and the requirements of these laws will apply differently depending on such things as whether or not we bill electronically for our services. The costs of complying with any changes to the HIPAA, HITECH and state privacy restrictions may have a negative impact on our operations. Noncompliance could subject us to criminal penalties, civil sanctions and significant monetary penalties as well as reputational damage.
Clinical research is heavily regulated and failure to comply with human subject protection regulations may disrupt our research program leading to significant expense, regulatory enforcement, private lawsuits and reputational damage.
Clinical research is subject to federal, state and, for studies conducted outside of the United States, international regulation. At the federal level, the FDA imposes regulations for the protection of human subjects and requirements such as initial and ongoing institutional review board review; informed consent requirements, adverse event reporting and other protections to minimize the risk and maximize the benefit to research participants. Many states impose human subject protection laws that mirror or in some cases exceed federal requirements. HIPAA also regulates the use and disclosure of Protected Health Information in connection with research activities. Research conducted overseas is subject to a variety of national protections such as mandatory ethics committee review, as well as laws regulating the use, disclosure and cross-border transfer of personal data. The costs of compliance with these laws may be significant and compliance with regulatory requirements may result in delay. Noncompliance may disrupt our research and result in data that is unacceptable to regulatory authorities, data lock or other sanctions that may significantly disrupt our operations.
32
Violation of a state’s prohibition on the corporate practice of medicine could result in a material adverse effect on our business.
A number of states, including California, do not allow business corporations to employ physicians to provide professional services. This prohibition against the “corporate practice of medicine” is aimed at preventing corporations such as us from exercising control over the medical judgments or decisions of physicians. The state licensure statutes and regulations and agency and court decisions that enumerate the specific corporate practice rules vary considerably from state to state and are enforced by both the courts and regulatory authorities, each with broad discretion. If regulatory authorities or other parties in any jurisdiction successfully assert that we are engaged in the unauthorized corporate practice of medicine, we could be required to restructure our contractual and other arrangements. In addition, violation of these laws may result in sanctions imposed against us and/or the professional through licensure proceedings, and we could be subject to civil and criminal penalties that could result in exclusion from state and federal health care programs.
Intellectual Property Risks Related to Our Business
If we are unable to obtain and maintain effective patent rights for our products or services, we may not be able to compete effectively in our markets.
We rely upon a combination of patents, trade secret protection, and confidentiality agreements to protect the intellectual property related to our technologies, products and services. Our success depends in large part on our ability to obtain and maintain patent and other intellectual property protection in the United States and in other countries with respect to our proprietary technology and products.
We have sought to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and products that are important to our business. This process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
The patent position of diagnostic companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unsolved. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our products or services in the United States or in other foreign countries. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our products and services, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our products and services, or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
We, independently or together with our licensors, have filed several patent applications covering various aspects of our products and services. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents owned by or licensed to us after patent issuance could deprive us of rights necessary for the successful commercialization of any products and services that we may offer. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product or service under patent protection could be reduced.
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We therefore cannot be certain that we or our licensors were the first to make the invention claimed in our owned and licensed patents or pending applications, or that we or our licensor were the first to file for patent protection of such inventions. Assuming the other requirements for patentability are met, in the United States prior to March 15, 2013, the first to make the claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act, or the Leahy-Smith Act, enacted on September 16, 2011, the United States has moved to a first to file system. The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. The effects of these changes are currently unclear as the USPTO must still implement various regulations, the courts have yet to address any of these provisions and the applicability of the act and new regulations on specific patents discussed herein have not been determined and would need to be reviewed. In general, the
33
Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
If we are unable to maintain effective proprietary rights for our products or services, we may not be able to compete effectively in our markets.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our products and services that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors.
Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors, and any third parties who have access to our proprietary know-how, information, or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There have been many lawsuits and other proceedings involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, and reexamination proceedings before the USPTO and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing products and services. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our products and services may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture, or methods for treatment related to the use or manufacture of our products and services. We have conducted freedom to operate analyses with respect to only certain of our products and services, and therefore we do not know whether there are any third-party patents that would impair our ability to commercialize these products and services. We also cannot guarantee that any of our analyses are complete and thorough, nor can we be sure that we have identified each and every patent and pending application in the United States and abroad that is relevant or necessary to the commercialization of our products and services. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our products or services may infringe.
In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover aspects of our products or services, the holders of any such patents may be able to block our ability to commercialize such products or services unless we obtained a license under the applicable patents, or until such patents expire or are finally determined to be invalid or unenforceable. Such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our products or services. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
34
We may not be successful in obtaining or maintaining necessary rights to our products or services through acquisitions and in-licenses.
We currently have rights to the intellectual property, through licenses from third parties and under patents that we own, to develop our products and services. Because our programs may require the use of proprietary rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, in-license, or use these proprietary rights. We may be unable to acquire or in-license any compositions, methods of use, processes, or other third-party intellectual property rights from third parties that we identify as necessary for our products or services. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources, and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment.
We sometimes collaborate with U.S. and foreign institutions to accelerate our research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such option, we may be unable to negotiate a license within the specified timeframe or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
If we are unable to successfully obtain rights to required third-party intellectual property rights or maintain the existing intellectual property rights we have, we may have to abandon development of that program and our business and financial condition could suffer.
Although we are not currently involved in any litigation, we may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. Although we are not currently involved in any litigation, if we or one of our licensing partners were to initiate legal proceedings against a third party to enforce a patent covering one of our products or services, the defendant could counterclaim that the patent covering our product or service is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Interference proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise sufficient capital to continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help commercialize our products or services.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We employ certain individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, and we are not currently subject to any claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties, we may in the future be subject to such claims. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or
35
personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
Although we are not currently experiencing any claims challenging the inventorship of our patents or ownership of our intellectual property, we may in the future be subject to claims that former employees, collaborators or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our products or services. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involves both technological and legal complexity. Therefore, obtaining and enforcing biotechnology patents is costly, time consuming, and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on products and services in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Our collaborators may assert ownership or commercial rights to inventions we develop from our use of the biological materials which they provide to us, or otherwise arising from the collaboration.
We collaborate with several institutions, physicians and researchers in scientific matters. We do not have written agreements with certain of such collaborators, or the written agreements we have do not cover intellectual property rights. Also, we rely on numerous third parties to provide us with blood samples and biological materials that we use to develop tests. If we cannot successfully negotiate sufficient ownership and commercial rights to any inventions that result from our use of a third party collaborator’s materials, or if disputes arise with respect to the intellectual property developed with the use of a collaborator’s samples, or data developed in a collaborator’s study, we may be limited in our ability to capitalize on the market potential of these inventions or developments.
36
Risks Relating to Our Common Stock
The price of our common stock may be volatile.
Before our recently completed initial public offering, there was no public market for our common stock. Market prices for securities of early-stage life sciences companies have historically been particularly volatile. The factors that may cause the market price of our common stock to fluctuate include, but are not limited to:
|
· |
progress, or lack of progress, in developing and commercializing our current tests and our planned future cancer diagnostic tests; |
|
· |
favorable or unfavorable decisions about our tests from government regulators, insurance companies or other third-party payors; |
|
· |
our ability to recruit and retain qualified research and development personnel; |
|
· |
changes in investors’ and securities analysts’ perception of the business risks and conditions of our business; |
|
· |
changes in our relationship with key collaborators; |
|
· |
changes in the market valuation or earnings of our competitors or companies viewed as similar to us; |
|
· |
changes in key personnel; |
|
· |
depth of the trading market in our common stock; |
|
· |
termination of the lock-up agreements or other restrictions on the ability of our existing stockholders to sell shares after our initial public offering; |
|
· |
changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
|
· |
the granting or exercise of employee stock options or other equity awards; |
|
· |
realization of any of the risks described under this section entitled “Risk Factors”; and |
|
· |
general market and economic conditions. |
In addition, the equity markets have experienced significant price and volume fluctuations that have affected the market prices for the securities of newly public companies for a number of reasons, including reasons that may be unrelated to our business or operating performance. These broad market fluctuations may result in a material decline in the market price of our common stock and you may not be able to sell your shares at prices you deem acceptable. In the past, following periods of volatility in the equity markets, securities class action lawsuits have been instituted against public companies. Such litigation, if instituted against us, could result in substantial cost and the diversion of management attention.
Our failure to meet the continued listing requirements of The NASDAQ Capital Market could result in a de-listing of our common stock.
If we fail to satisfy the continued listing requirements of The NASDAQ Capital Market, such as the corporate governance requirements or the minimum closing bid price requirement, NASDAQ may take steps to de-list our common stock. Such a de-listing would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a de-listing, we would take actions to restore our compliance with NASDAQ’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the NASDAQ minimum bid price requirement or prevent future non-compliance with NASDAQ’s listing requirements.
If our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not retain a listing on The NASDAQ Capital Market and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk
37
disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
Our quarterly operating results may fluctuate significantly.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
|
· |
the rate of adoption and/or continued use of our current tests and our planned future tests by healthcare practitioners; |
|
· |
variations in the level of expenses related to our development programs; |
|
· |
addition or reduction of resources for sales and marketing; |
|
· |
addition or termination of clinical utility studies; |
|
· |
any intellectual property infringement lawsuit in which we may become involved; |
|
· |
third party payor determinations affecting our tests; and |
|
· |
regulatory developments affecting our tests. |
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
Future sales of our common stock, or the perception that future sales may occur, may cause the market price of our common stock to decline, even if our business is doing well.
Sales of substantial amounts of our common stock, or the perception that these sales may occur, could materially and adversely affect the price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We had outstanding 4,449,603 shares of common stock as of December 31, 2014, 2,549,603 of which are restricted securities that may be sold only in accordance with the resale restrictions under Rule 144 of the Securities Act of 1933, as amended. In addition, as of December 31, 2014, we had outstanding options to purchase 906,194 shares of our common stock, 251,618 shares of common stock were issuable upon the settlement of outstanding restricted stock units and we had outstanding warrants to purchase 610,774 shares of our common stock. Shares issued upon the exercise of stock options or upon the settlement of outstanding restricted stock units generally will be eligible for sale in the public market, except that affiliates will continue to be subject to volume limitations and other requirements of Rule 144 under the Securities Act. The issuance or sale of such shares could depress the market price of our common stock.
In the future, we also may issue our securities if we need to raise additional capital. The number of new shares of our common stock issued in connection with raising additional capital could constitute a material portion of the then-outstanding shares of our common stock.
Our largest stockholder continues to have substantial influence over us and could delay or prevent a change in corporate control.
Claire K. T. Reiss beneficially owned approximately 44% of our common stock at December 31, 2014. Mrs. Reiss has significant influence over the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. Accordingly, this concentration of ownership might harm the market price of our common stock by:
|
· |
delaying, deferring or preventing a change in control; |
|
· |
impeding a merger, consolidation, takeover or other business combination involving us; or |
|
· |
discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
If we are unable to favorably assess the effectiveness of our internal control over financial reporting, investors may lose confidence in our financial reporting and our stock price could be materially adversely affected.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any
38
testing by us conducted in connection with Section 404(a) of the Sarbanes-Oxley Act, or the subsequent testing by our independent registered public accounting firm conducted in connection with Section 404(b) of the Sarbanes-Oxley Act after we no longer qualify as an “emerging growth company,” may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our consolidated financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
We are required to disclose changes made in our internal control procedures on a quarterly basis and our management is required to assess the effectiveness of these controls annually. However, for as long as we are an “emerging growth company” under the recently enacted JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404. An independent assessment of the effectiveness of our internal controls could detect problems that our management’s assessment might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an emerging growth company, as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements and exemptions from the requirements of holding nonbinding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company until December 31, 2019, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million as of any June 30 before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three year period before that time, we would cease to be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements. We cannot predict if investors find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, are subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. As a result, changes in rules of U.S. generally accepted accounting principles or their interpretation, the adoption of new guidance or the application of existing guidance to changes in our business could significantly affect our financial position and results of operations.
We have incurred and will continue to incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, the listing requirements of The NASDAQ Stock Market and other applicable securities rules and regulations. Compliance with these rules and regulations has increased and will continue to increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly, and increase demand on our systems and resources. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight may be required. As a result, management’s attention may be diverted from other business concerns, which could harm our business and operating results. Further, there are significant corporate governance and executive compensation related provisions in the Dodd-Frank Wall Street Reform and Consumer Protection Act, enacted in 2010, that require the SEC to adopt additional rules and regulations in these areas such as “say on pay” and proxy access. Recent legislation permits smaller “emerging growth companies” to implement many of these requirements over a longer period. We intend to continue taking advantage of this new legislation but cannot guarantee that we will not be required to implement these requirements sooner than budgeted or planned and thereby incur unexpected expenses. Stockholder activism, the current political environment and the current high level of
39
government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Anti-takeover provisions of our certificate of incorporation, our bylaws and Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board and management.
Certain provisions of our amended certificate of incorporation and amended and restated bylaws could discourage, delay or prevent a merger, acquisition or other change of control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove members of our board of directors. (For example, Delaware law provides that if a corporation has a classified board of directors, stockholders cannot remove any director during his or her term without cause.) These provisions also could limit the price that investors might be willing to pay in the future for our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions, among other things:
|
· |
classify our board of directors into three classes of equal (or roughly equal) size, with all directors serving for a three-year term and the directors of only one class being elected at each annual meeting of stockholders, so that the terms of the classes of directors are “staggered”; |
|
· |
allow the authorized number of directors to be changed only by resolution of our board of directors; |
|
· |
authorize our board of directors to issue, without stockholder approval, preferred stock, the rights of which will be determined at the discretion of the board of directors and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that our board of directors does not approve; |
|
· |
establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings; and |
|
· |
limit who may call a stockholders meeting. |
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
Because we do not expect to pay cash dividends for the foreseeable future, you must rely on appreciation of our common stock price for any return on your investment. Even if we change that policy, we may be restricted from paying dividends on our common stock.
We do not intend to pay cash dividends on shares of our common stock for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend upon results of operations, financial performance, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant. Accordingly, you will have to rely on capital appreciation, if any, to earn a return on your investment in our common stock. Investors seeking cash dividends in the foreseeable future should not purchase our common stock.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Our ability to utilize our federal net operating loss, carryforwards and federal tax credits may be limited under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code. The limitations apply if an “ownership change,” as defined by Section 382 of the Code, occurs. If we have experienced an “ownership change” at any time since our formation, we may already be subject to limitations on our ability to utilize our existing net operating losses and other tax attributes to offset taxable income. In
40
addition, future changes in our stock ownership (including in connection with this or future offerings, as well as other changes that may be outside of our control), may trigger an “ownership change” and, consequently, limitations under Sections 382 and 383 of the Code. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards and other tax attributes to offset United States federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. As of December 31, 2013, we had federal and state net operating loss carryforwards of approximately $111.7 million and $97.7 million, respectively, and federal and California research and development credits of $3.1 million and $3.0 million, respectively, which could be limited if we have experienced or do experience any “ownership changes.” We have not completed a study to assess whether an “ownership change” has occurred or whether there have been multiple “ownership changes” since our formation, due to the complexity and cost associated with such a study, and the fact that there may be additional ownership changes in the future.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because early-stage life sciences companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
41
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this prospectus are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of risks, uncertainties and assumptions described under the sections in this prospectus entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this prospectus. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. The forward-looking statements contained in this prospectus are excluded from the safe harbor protection provided by the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act.
This prospectus also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
42
We will receive net proceeds of approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, from the sale of the shares of common stock offered by us in this offering, based on an assumed public offering price of $ per share (the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each $1.00 increase (decrease) in the assumed public offering price of $ per share would increase (decrease) the net proceeds to us from this offering by approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, assuming the assumed public offering price of $ per share (the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015), the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently intend to use the net proceeds of the offering as follows:
|
· |
approximately $ million to hire sales and marketing personnel and support increased sales and marketing activities; |
|
· |
approximately $ million to fund further research and development, clinical utility studies and future enhancements of our current tests and our planned tests and services; |
|
· |
approximately $ million to acquire equipment, implement automation and scale our capabilities to prepare for significant test volume; and |
|
· |
the balance for general corporate purposes and to fund ongoing operations and expansion of our business. |
The expected use of net proceeds of this offering represents our current intentions based upon our present plan and business conditions. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. We will have broad discretion in the application of the net proceeds in the category of “for general corporate purposes and to fund ongoing operations and expansion of our business,” and investors will be relying on our judgment regarding the application of the proceeds of this offering. For example, if we identify opportunities that we believe are in the best interests of our stockholders, we may use a portion of the net proceeds from this offering to acquire, invest in or license complementary products, technologies or businesses although we have no current commitments, understandings or agreements to do so. The actual amounts and timing of our actual expenditures depend on numerous factors, including the success of our efforts to market OncoCEE-BR, OncoCEE-LU and OncoCEE-GA the timing and progress of our discovery, research and development activities for the tests in our pipeline, the success of our efforts to increase sales of our laboratory services, the success of our efforts to expand our international sales, changes in regulatory requirements for LDTs, and other unforeseen regulatory or compliance costs. The costs and timing of test discovery and development activities, particularly conducting clinical validation studies and obtaining regulatory clearance or approval, if required, are highly uncertain, subject to substantial risks and can often change. Depending on the outcome of these activities and other unforeseen events, our plans and priorities may change and we may apply the net proceeds of this offering in different proportions than we currently anticipate.
43
PRICE RANGE OF OUR COMMON STOCK
Our common stock has been listed on The NASDAQ Capital Market since February 5, 2014 under the symbol BIOC. Prior to that date, there was no public market for our common stock. Shares sold in our initial public offering on February 5, 2014 were priced at $10.00 per share.
On January 8, 2015, the closing price for our common stock as reported on The NASDAQ Capital Market was $3.04 per share. The following table sets forth the ranges of high and low sales prices per share of our common stock as reported on The NASDAQ Capital Market for the periods indicated. Such quotations represent inter-dealer prices without retail markup, markdown or commission and may not necessarily represent actual transactions.
|
Year Ended December 31, 2014 |
|
High |
|
|
Low |
|
|
First Quarter (beginning February 5, 2014) |
$ |
10.02 |
|
$ |
6.51 |
|
|
Second Quarter |
$ |
8.00 |
|
$ |
4.16 |
|
|
Third Quarter |
$ |
6.68 |
|
$ |
2.37 |
|
|
Fourth Quarter |
$ |
4.60 |
|
$ |
2.27 |
|
|
Year Ended December 31, 2015 |
|
|
|
|
|
|
|
First Quarter (through January 8, 2015) |
$ |
3.88 |
|
$ |
2.42 |
|
As of December 31, 2014, there were 206 stockholders of record of our common stock, which excludes stockholders whose shares were held in nominee or street name by brokers. The actual number of common stockholders is greater than the number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
44
We have never declared dividends on our equity securities, and currently do not plan to declare dividends on shares of our common stock in the foreseeable future. We expect to retain our future earnings, if any, for use in the operation and expansion of our business. Subject to the foregoing, the payment of cash dividends in the future, if any, will be at the discretion of our board of directors and will depend upon such factors as earnings levels, capital requirements, our overall financial condition and any other factors deemed relevant by our board of directors.
45
The following table sets forth our capitalization as of September 30, 2014:
|
· |
on an actual basis; and |
|
· |
on a pro forma as adjusted basis to reflect the sale by us of shares of our common stock in the offering at an assumed public offering price of $ per share (the last reported sale price of our common stock, as reported on The NASDAQ Capital Market on , 2015), after deducting the underwriting discounts and commissions and estimated offering costs payable by us. |
You should read this table together with the sections entitled “Use of Proceeds” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as our financial statements and the related notes, which appear elsewhere in this prospectus.
|
|
|
As of September 30, 2014 |
|
|||||
|
(dollars in thousands) |
|
(unaudited) |
|
|
(unaudited) |
|
||
|
Cash and cash equivalents |
|
$ |
8,820 |
|
|
$ |
|
|
|
Long term debt (inclusive of current portion) |
|
|
4,732 |
|
|
|
|
|
|
Common stock, par value $0.0001 per share, 40,000,000 authorized; 4,449,603 issued and outstanding at September 30, 2014, actual; 40,000,000 shares authorized, shares issued and outstanding, pro forma |
|
|
— |
|
|
|
|
|
|
Additional paid-in capital |
|
|
137,750 |
|
|
|
|
|
|
Accumulated deficit |
|
|
(134,405 |
) |
|
|
|
|
|
Total stockholders’ equity/(deficit) |
|
|
3,345 |
|
|
|
|
|
|
Total capitalization |
|
|
8,077 |
|
|
|
|
|
The number of shares of our common stock to be outstanding after this offering is based on 4,449,603 shares of our common stock outstanding as of September 30, 2014 and excludes as of such date:
|
· |
875,042 shares of our common stock issuable upon the exercise of stock options, with a weighted average exercise price of $6.47 per share; |
|
· |
251,618 shares of our common stock issuable upon the settlement of outstanding restricted stock units; |
|
· |
610,774 shares of our common stock issuable upon the exercise of outstanding warrants, with a weighted average exercise price of $9.47 per share; |
|
· |
any shares of our common stock issuable upon exercise of the underwriters’ over-allotment option; and |
|
· |
other shares of our common stock reserved for future issuance under our 2013 and 2007 Equity Incentive Plans. |
46
If you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the public offering price per share of our common stock in this offering and our pro forma net tangible book value per share immediately after this offering. We calculate net tangible book value per share by dividing our net tangible book value, which is tangible assets less total liabilities less debt discounts, by the number of outstanding shares of our common stock as of September 30, 2014. Our historical net tangible book value (deficit) as of September 30, 2014, was approximately $3.3 million, or $0.75 per share of our common stock. Net historical tangible book value (deficit) per share is our historical net tangible book value (deficit) divided by the number of shares of common stock outstanding as of September 30, 2014.
After giving effect to the sale of shares of our common stock offered by us at an assumed public offering price of $ per share (the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015), after deducting the underwriting discounts and commissions and estimated offering costs payable by us, our net tangible book value as of September 30, 2014, would have been approximately $ million, or $ per share of common stock. This represents an immediate increase in net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to investors purchasing shares of common stock in this offering at the assumed public offering price. The following table illustrates the per share dilution (unaudited):
|
Assumed public offering price per share |
|
|
|
|
$ |
|
|
|
Pro forma net tangible book value (deficit) per share as of September 30, 2014 |
$ |
|
|
|
|
|
|
|
Increase in pro forma net tangible book value (deficit) per share after this offering |
|
|
|
|
|
|
|
|
Pro forma net tangible book value (deficit) per share after this offering |
|
|
|
|
|
|
|
|
Dilution in pro forma net tangible book value (deficit) per share to new investors |
|
|
|
|
$ |
|
|
Each $1.00 increase (decrease) in the assumed public offering price of $ per share, the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015, would increase (decrease) our pro forma net tangible book value after this offering by approximately $ million, or approximately $ per share, and the dilution per share to new investors by approximately $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase of 1,000,000 shares in the number of shares offered by us would increase our pro forma net tangible book value after this offering by approximately $ million, or $ per share, and the dilution per share to new investors would be $ per share, assuming that the assumed public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, a decrease of 1,000,000 shares in the number of shares offered by us would decrease our pro forma net tangible book value after this offering by approximately $ million, or $ per share, and the dilution per share to new investors would be $ per share, assuming that the assumed public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will adjust based on the actual public offering price and other terms of this offering determined at pricing.
If the underwriters exercise in full their option to purchase up to additional shares of common stock at the assumed public offering price of $ per share, the as adjusted net tangible book value after this offering would be $ per share, representing an increase in net tangible book value of $ per share to existing stockholders and immediate dilution in net tangible book value of $ per share to investors purchasing our common stock in this offering at the assumed public offering price.
47
The number of shares of our common stock to be outstanding after this offering is based on 4,449,603 shares of our common stock outstanding as of September 30, 2014 and excludes as of such date:
|
· |
875,042 shares of our common stock issuable upon the exercise of stock options, with a weighted average exercise price of $6.47 per share; |
|
· |
251,618 shares of our common stock issuable upon the settlement of outstanding restricted stock units; |
|
· |
610,774 shares of our common stock issuable upon the exercise of outstanding warrants, with a weighted average exercise price of $9.47 per share; |
|
· |
any shares of our common stock issuable upon exercise of the underwriters’ over-allotment option; and |
|
· |
other shares of our common stock reserved for future issuance under our 2013 and 2007 Equity Incentive Plans. |
To the extent that the underwriters’ over-allotment option is exercised or any warrants or options are exercised, there will be further dilution to investors.
48
The following table summarizes our selected financial data for the periods and as of the dates indicated. Our selected statements of operations data for each of the years in the periods ended December 31, 2012 and 2013, and our selected balance sheet data as of December 31, 2013, have been derived from our audited financial statements and their related notes, which are included elsewhere in this prospectus. The unaudited selected statements of operations data for the nine months ended September 30, 2013 and 2014, and the unaudited balance sheet data as of September 30, 2014, are derived from our unaudited financial statements, which are included elsewhere in this prospectus. Our unaudited financial statements have been prepared on the same basis as the audited financial statements and, in the opinion of management, include all adjustments, consisting of normal recurring adjustments necessary for a fair presentation of our financial condition as of such dates and our results of operations for such periods. Our historical results are not necessarily indicative of the results to be expected for any future periods and our interim results are not necessarily indicative of the results to be expected for the full fiscal year. Our selected financial data should be read together with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and with our financial statements and their related notes, which are included elsewhere in this prospectus.
|
|
|
Year ended December 31, |
|
|
For the nine months ended September 30, |
|
|||||||||||
|
|
|
|
2012 |
|
|
|
2013 |
|
|
|
2013 |
|
|
|
2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(unaudited) |
|
|
|
(unaudited) |
|
|
|
|
|
(in thousands, except share and per share data) |
|
|
|||||||||||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
109 |
|
|
$ |
134 |
|
|
$ |
115 |
|
|
$ |
58 |
|
|
|
Cost of revenues |
|
|
1,202 |
|
|
|
2,330 |
|
|
|
1,760 |
|
|
|
1,556 |
|
|
|
Gross profit |
|
|
(1,093 |
) |
|
|
(2,196 |
) |
|
|
(1,645 |
) |
|
|
(1,498 |
) |
|
|
Research and development expenses |
|
|
6,562 |
|
|
|
3,086 |
|
|
|
2,376 |
|
|
|
3,428 |
|
|
|
General and administrative expenses |
|
|
2,063 |
|
|
|
2,513 |
|
|
|
1,736 |
|
|
|
3,971 |
|
|
|
Sales and marketing expenses |
|
|
785 |
|
|
|
149 |
|
|
|
129 |
|
|
|
1,246 |
|
|
|
Loss from operations |
|
|
(10,503 |
) |
|
|
(7,944 |
) |
|
|
(5,886 |
) |
|
|
(10,143 |
) |
|
|
Total other income/(expense) |
|
|
(1,756 |
) |
|
|
(1,288 |
) |
|
|
(874 |
) |
|
|
(1,841 |
) |
|
|
Loss Before Income Taxes |
|
$ |
(12,259 |
) |
|
$ |
(9,232 |
) |
|
$ |
(6,760 |
) |
|
$ |
(11,984 |
) |
|
|
Income tax expense |
|
|
(1 |
) |
|
|
(1 |
) |
|
|
(1 |
) |
|
|
(1 |
) |
|
|
Net loss & comprehensive loss |
|
$ |
(12,260 |
) |
|
$ |
(9,233 |
) |
|
$ |
(6,761 |
) |
|
$ |
(11,985 |
) |
|
|
Weighted average shares outstanding used in computing net loss per common share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
160,393 |
|
|
|
181,762 |
|
|
|
182,199 |
|
|
|
3,845,540 |
|
|
|
Diluted |
|
|
160,393 |
|
|
|
181,762 |
|
|
|
182,199 |
|
|
|
3,845,540 |
|
|
|
Net loss per common share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(76.43 |
) |
|
$ |
(50.80 |
) |
|
$ |
(37.11 |
) |
|
$ |
(3.12 |
) |
|
|
Diluted |
|
$ |
(76.43 |
) |
|
$ |
(50.80 |
) |
|
$ |
(37.11 |
) |
|
$ |
(3.12 |
) |
|
|
Weighted average shares outstanding used in computing pro forma net loss per share attributable to common shareholders, basic and diluted (unaudited) |
|
|
|
|
|
|
|
|
|
Pro forma net loss per common share |
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
|
|
|
$ |
|
|
|
Diluted |
|
$ |
|
|
|
$ |
|
|
49
|
|
|
As of December 31, 2013 |
|
|
As of September 30, 2014 |
|
||||||
|
|
|
|
Actual |
|
|
|
Actual |
|
|
|
Pro Forma(1) |
|
|
|
|
|
|
|
|
|
(Unaudited) |
|
|
|
(Unaudited) |
|
|
Balance Sheet Data (in thousands): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
69 |
|
|
$ |
8,820 |
|
|
$ |
|
|
|
Total assets |
|
$ |
1,330 |
|
|
$ |
9,875 |
|
|
$ |
|
|
|
Notes payable, net of discount |
|
$ |
5,201 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Line of credit |
|
$ |
1,981 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Credit facility, net of discount |
|
$ |
— |
|
|
$ |
4,732 |
|
|
$ |
4,732 |
|
|
Total liabilities |
|
$ |
13,786 |
|
|
$ |
6,530 |
|
|
$ |
6,530 |
|
|
Convertible preferred stock |
|
$ |
7 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Total shareholders’ equity/(deficit) |
|
$ |
(12,456 |
) |
|
$ |
3,345 |
|
|
$ |
|
|
|
(1) |
A $1.00 increase (decrease) in the assumed public offering price of $ per share, the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015, would increase (decrease) each of cash and cash equivalents, total assets and total stockholders’ equity/(deficit) by $ million, assuming the number of shares offered by us as stated on the cover page of this prospectus remain unchanged and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, a one million share increase (decrease) in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, total assets and total stockholders’ equity/(deficit) by $ million, assuming the public offering price of $ per share, the last reported sale price of our common stock on The NASDAQ Capital Market on , 2015, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. |
50
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read together with our financial statements and related notes included elsewhere in the prospectus. This discussion contains forward-looking statements based upon our current plans, estimates, beliefs and expectations that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under the sections entitled “Risk Factors,” “Special Note Regarding Forward-Looking Statements” and elsewhere in this prospectus.
We are an early-stage cancer diagnostics company that develops and commercializes proprietary circulating tumor cell, or CTC, and circulating tumor DNA, or ctDNA, tests utilizing a standard blood sample, or “liquid biopsy.” Our current CTC breast, lung and gastric cancer tests provide, and our planned future tests would provide, information to oncologists and other physicians that enable them to select appropriate personalized treatment for their patients based on better, timelier and more-detailed data on the characteristics of their patients’ tumors.
Our current breast, lung and gastric cancer tests and our planned future tests utilize our Cell Enrichment and Extraction (CEE) technology for the enumeration and analysis of CTCs, and our CEE-Selector technology for the detection and analysis of ctDNA from plasma, each performed on a standard blood sample. The CEE technology is an internally developed, microfluidics-based CTC capture and analysis platform, with enabling features that change how CTC testing can be used by clinicians by providing real-time biomarker monitoring with only a standard blood sample. The CEE-Selector technology enables mutation detection with enhanced sensitivity and specificity and is applicable to nucleic acid from CTCs or other sample types, such as blood plasma for ctDNA. We believe the CEE-Selector technology is an important part of our pipeline and will be a stand-alone test for molecular analysis of biomarkers.
At our corporate headquarters facility located in San Diego, California, we operate a clinical laboratory that is certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and accredited by the College of American Pathologists, or CAP. We manufacture our CEE microfluidic channels, related equipment and certain reagents to perform our current tests and our planned future tests at this facility. CLIA certification is required before any clinical laboratory, including ours, may perform testing on human specimens for the purpose of obtaining information for the diagnosis, prevention, or treatment of disease or the assessment of health. The tests we offer and intend to offer are classified as laboratory developed tests, or LDTs, under CLIA regulations.
We are in the process of commercializing our first test, OncoCEE-BR, for breast cancer, and recently launched our OncoCEE-LU test for non-small cell lung cancer, or NSCLC and our OncoCEE-GA test for gastric cancer in late 2014. These tests utilize our CEE technology platform and provide CTC enumeration as well as biomarker analysis from a standard blood sample. In the case of the OncoCEE-BR and OncoCEE-GA tests, biomarker analysis involves fluorescence in situ hybridization, or FISH, for the detection and quantitation of the human epidermal growth factor receptor 2, or HER2, gene copy number as well as immunocytochemical analysis of estrogen receptor (ER) protein, which is now launched. We plan to include immunocytochemical analysis of progesterone receptor proteins in the OncoCEE-BR test within the next year. A patient’s HER2 status provides the physician with information about the appropriateness of therapies such as Herceptin® or Tykerb®. Estrogen receptor and progesterone receptor (PR) status provides the physician with information about the appropriateness of endocrine therapies such as tamoxifen and aromatase inhibitors.
The OncoCEE-LU test’s biomarker analysis currently includes FISH testing for ALK and ROS1 gene rearrangements and molecular analysis of the T790M mutation of the epidermal growth factor receptor or EGFR gene using our CEE-SelectorTM platform. We plan to add FISH testing for RET, MET, as well as mutation analysis for deletions 19 and l858R mutation in the ECFR gene, the K-ras gene and the B-raf gene in the future. The L858R mutation of the EGFR gene and Exon 19 deletions as activators of EGFR kinase activity are linked to the drugs Tarceva®, Gilotrif® and Iressa®. The codon 12 and 13 mutations of the K-ras gene are found in patients whose tumors are unlikely to respond to the EGFR kinase inhibitors such as Erbitux® and Vectibix®, and the codon 600 mutations of the B-raf gene are linked to Zelboraf® and Tafinlar®, which are both approved for melanoma and are in clinical trials for lung cancer. Our OncoCEE-LU test is performed on a standard blood sample.
51
We plan to add other biomarker analyses on blood samples to our current tests and our planned future OncoCEE tests as their relevance is demonstrated in clinical trials, for example, RET proto-oncogene gene fusions in NSCLC, which may indicate a particular course of therapy, and NRAS for melanoma, which may predict therapy resistance. In addition, we are developing a series of other CTC and ctDNA tests for different solid tumor types, including colorectal cancer, prostate cancer, gastric cancer and melanoma, each incorporating treatment-associated biomarker analyses specific to that cancer, planned to be launched as noted in the table below.
|
Test Name/ Solid Tumor Type |
|
Biomarkers |
|
|
|
Status of Test or Project |
|
|
|
Targeted Quarter of Availability for Commercialization |
|
|
OncoCEE-BRTM / Breast Cancer |
|
Enumeration, HER2 by FISH, ER |
|
|
|
Currently available |
|
|
|
N/A |
|
|
|
|
PR |
|
|
|
Validation |
|
|
|
2015 Q2 |
|
|
OncoCEE-LUTM / Lung Cancer |
|
Enumeration, ALK by FISH |
|
|
|
Currently available |
|
|
|
N/A |
|
|
OncoCEE-LUTM / Lung Cancer |
|
EGFR T790M mutation by CEE-SelectorTM |
|
|
|
Currently available |
|
|
|
N/A |
|
|
|
|
ROS1 by FISH |
|
|
|
Validation |
|
|
|
2015 Q2 |
|
|
|
|
EGFR L858R and Del19, K-ras, B-raf, and ALK mutations by CEE-SelectorTM |
|
|
|
Development and Validation |
|
|
|
2015 Q1, Q2, Q3 |
|
|
OncoCEE-GATM / Gastric Cancer |
|
Enumeration, HER2 by FISH |
|
|
|
Currently available |
|
|
|
N/A |
|
|
OncoCEE-CRTM / Colorectal Cancer |
|
Enumeration, EGFR by FISH |
|
|
|
Validation |
|
|
|
2015 Q2 |
|
|
|
|
K-ras and B-raf by CEE-SelectorTM |
|
|
|
Development |
|
|
|
2015 Q2 |
|
|
OncoCEE-PRTM / Prostate Cancer |
|
Enumeration, PTEN deletion and AR by FISH |
|
|
|
Validation |
|
|
|
2015 Q3 |
|
|
OncoCEE-METM / Melanoma |
|
Enumeration, B-raf and N-ras mutations by CEE-SelectorTM |
|
|
|
Development |
|
|
|
2015 Q3 |
|
|
|
|
PDL-1 by ICC |
|
|
|
Development |
|
|
|
2015 Q3 |
|
|
OncoCEE-DTCTM/ Breast and Prostate Cancer |
|
DTC analysis in bone marrow; HER2 and AR/PTEN by FISH, respectively |
|
|
|
Currently available for Research and Pharma |
|
|
|
N/A |
|
|
CEE-SelectorTM/ Sequencing application for multiple cancer types |
|
K-ras, B-raf, EGFR and other mutations detected in plasma. |
|
|
|
Development |
|
|
|
2015 Q3 |
|
Our revenue generating efforts are focused in three areas:
|
· |
Providing clinical testing that physicians use in order to determine the best treatment plan for their patients; |
|
· |
Providing clinical trial, research and development services to biopharma companies developing cancer therapies; and |
|
· |
Licensing our proprietary testing and/or technologies to partners in the United States and abroad. |
52
We accessioned 96 commercial cases during the three months ended September 30, 2014 as compared to 10 commercial cases for the same period in 2013, an increase of 86 cases, or 860%. We accessioned 110 commercial cases during the nine months ended September 30, 2014 as compared to 42 cases for the same period in 2013, an increase of 68 cases, or 162%. Revenues from commercial cases are recognized as collected. The expected collection period for the commercial cases accessioned during the three months ended September 30, 2014 extends beyond the end of the reporting period.
Results of Operations
Three Months Ended September 30, 2013 and 2014
The following table sets forth certain information concerning our results of operations for the periods shown:
|
|
Three Months Ended September 30, |
|
|
|
Change |
|
|||||||||
|
|
2013 |
|
|
2014 |
|
|
$ |
|
|
% |
|
||||
|
(dollars in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
$ |
32 |
|
|
$ |
10 |
|
|
$ |
(22 |
) |
|
|
(69 |
%) |
|
Cost of revenues |
|
619 |
|
|
|
538 |
|
|
|
(81 |
) |
|
|
(13 |
%) |
|
Research and development expenses |
|
975 |
|
|
|
1,311 |
|
|
|
336 |
|
|
|
34 |
% |
|
General and administrative expenses |
|
807 |
|
|
|
1,061 |
|
|
|
254 |
|
|
|
31 |
% |
|
Sales and marketing expenses |
|
5 |
|
|
|
812 |
|
|
|
807 |
|
|
|
16,140 |
% |
|
Loss from operations |
|
(2,374 |
) |
|
|
(3,712 |
) |
|
|
(1,338 |
) |
|
|
56 |
% |
|
Interest expense, net |
|
(457 |
) |
|
|
(151 |
) |
|
|
306 |
|
|
|
(67 |
%) |
|
Change in fair value of warrant liability |
|
(8 |
) |
|
|
3 |
|
|
|
11 |
|
|
|
(138 |
%) |
|
Other income/(expense) |
|
(21 |
) |
|
|
— |
|
|
|
(21 |
) |
|
|
100 |
% |
|
Loss before income taxes |
|
(2,860 |
) |
|
|
(3,860 |
) |
|
|
(1,000 |
) |
|
|
35 |
% |
|
Income tax expense |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Net loss |
$ |
(2,860 |
) |
|
$ |
(3,860 |
) |
|
$ |
(1,000 |
) |
|
|
35 |
% |
Revenue
Revenues were approximately $10,000 for the three months ended September 30, 2014, compared with approximately $32,000 for the three months ended September 30, 2013, a decrease of $22,000, or 69%. The decrease was primarily related to lower Dana-Farber Cancer Institute sample volume as the trial’s enrollment approaches completion, with three development services tests performed during the three months ended September 30, 2014 as compared to 48 during the same period in 2013. The average price collected per commercial test increased from $1,011 for the three months ended September 30, 2013 to an average of $1,512 for the three months ended September 30, 2014, and the average price per development services test was $400 for the three months ended September 30, 2013 and 2014.
Cost of Revenues
Cost of revenues was approximately $538,000 for the three months ended September 30, 2014, compared with approximately $619,000 for the three months ended September 30, 2013, a decrease of $81,000, or 13%. The decrease was primarily due to a decrease in the proportion of lab volume that related to revenue-generating activities relative to the total number of samples processed for the three months ended September 30, 2014 as compared to the same period in 2013, partially offset by increases in personnel and allocated facilities costs.
Operating Expenses
Research and Development Expenses. Research and development expenses were approximately $1,311,000 for the three months ended September 30, 2014, compared with approximately $975,000 for the three months ended September 30, 2013, an increase of $336,000, or 34%. The increase was primarily due to an increase of $284,000 in validation samples and allocated costs related to the higher proportion of lab activities relating to product development, as well as increases of $229,000 in personnel costs primarily related to hiring additional development and lab personnel for new test validations and $73,000 in facilities, repairs and maintenance expenses for the three months ended September 30, 2014 as compared to the same period in 2013, partially offset by a decrease of $268,000 in stock-based compensation expense.
53
General and Administrative Expenses. General and administrative expenses were approximately $1,061,000 for the three months ended September 30, 2014, compared with approximately $807,000 for the three months ended September 30, 2013, an increase of $254,000, or 31%. The increase was primarily due to an increase of $151,000 in insurance, legal, accounting, and consulting expenses as a result of becoming a publicly traded company in February 2014, an increase of $115,000 in personnel costs, and an increase of $61,000 in legal fees associated with patents for the three months ended September 30, 2014 as compared to the same period in 2013, partially offset by a decrease of $101,000 in stock-based compensation expense.
Sales and Marketing Expenses. Sales and marketing expenses were approximately $812,000 for the three months ended September 30, 2014, compared with approximately $5,000 for the three months ended September 30, 2013, an increase of $807,000. The increase was primarily due to personnel-related expenses resulting from the deployment of our sales and marketing function. For the three months ended September 30, 2014, the sales and marketing function included an average of 10 employees. We had no sales and marketing function during the three month period ended September 30, 2013.
Interest Income and Expense
Interest expense was approximately $151,000 for the three months ended September 30, 2014, compared with approximately $457,000 for the three months ended September 30, 2013, a decrease of $306,000, or 67%. The decrease was due to a decrease of $400,000 in non-cash interest and discount amortization expense related to convertible notes payable that were converted into shares of common stock in conjunction with our initial public offering in February 2014, partially offset by an increase of $94,000 in cash interest expense primarily associated with the April 2014 Credit Facility.
Change in Fair Value of Warrant Liability
The non-cash gain resulting from the change in the fair value of warrant liability of approximately $3,000 for the three months ended September 30, 2014 compared with the non-cash loss of approximately $8,000 for the three months ended September 30, 2013 represents an increase in non-cash gain of $11,000, or 138%. The increase is due to a decline in the stock price underlying the warrants during the three months ended September 30, 2014, compared with an increase in the stock price underlying the warrants during the same period in 2013.
Income Taxes
Over the past several years we have generated operating losses in all jurisdictions in which we may be subject to income taxes. As a result, we have accumulated significant net operating losses and other deferred tax assets. Because of our history of losses and the uncertainty as to the realization of those deferred tax assets, a full valuation allowance has been recognized. We do not expect to report a provision for income taxes until we have a history of earnings, if ever, that would support the realization of our deferred tax assets.
We have not completed a study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since our formation, due to the complexity and cost associated with such a study, and the fact that there may be additional ownership changes in the future. We estimate that if such a change did occur, the federal and state net operating loss carryforwards and research and development credits that can be utilized in the future will be significantly limited.
54
Nine Months Ended September 30, 2013 and 2014
The following table sets forth certain information concerning our results of operations for the periods shown:
|
|
Nine Months Ended September 30, |
|
|
Change |
|
||||||||||
|
|
2013 |
|
|
2014 |
|
|
$ |
|
|
% |
|
||||
|
(dollars in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
$ |
115 |
|
|
$ |
58 |
|
|
$ |
(57 |
) |
|
|
(50 |
%) |
|
Cost of revenues |
|
1,760 |
|
|
|
1,556 |
|
|
|
(204 |
) |
|
|
(12 |
%) |
|
Research and development expenses |
|
2,376 |
|
|
|
3,428 |
|
|
|
1,052 |
|
|
|
44 |
% |
|
General and administrative expenses |
|
1,736 |
|
|
|
3,971 |
|
|
|
2,235 |
|
|
|
129 |
% |
|
Sales and marketing expenses |
|
129 |
|
|
|
1,246 |
|
|
|
1,117 |
|
|
|
866 |
% |
|
Loss from operations |
|
(5,886 |
) |
|
|
(10,143 |
) |
|
|
(4,257 |
) |
|
|
72 |
% |
|
Interest expense, net |
|
(1,435 |
) |
|
|
(1,640 |
) |
|
|
(205 |
) |
|
|
14 |
% |
|
Change in fair value of warrant liability |
|
593 |
|
|
|
(201 |
) |
|
|
(794 |
) |
|
|
(134 |
%) |
|
Other income/(expense) |
|
(32 |
) |
|
— |
|
|
|
(32 |
) |
|
|
100 |
% |
|
|
Loss before income taxes |
|
(6,760 |
) |
|
|
(11,984 |
) |
|
|
(5,224 |
) |
|
|
77 |
% |
|
Income tax expense |
|
(1 |
) |
|
|
(1 |
) |
|
— |
|
|
— |
|
||
|
Net loss |
$ |
(6,761 |
) |
|
$ |
(11,985 |
) |
|
$ |
(5,224 |
) |
|
|
77 |
% |
Revenue
Revenues were approximately $58,000 for the nine months ended September 30, 2014, compared with approximately $115,000 for the nine months ended September 30, 2013, a decrease of $57,000, or 50%. The decrease was primarily related to lower Dana-Farber Cancer Institute sample volume as the trial’s enrollment approaches completion, with 104 development services tests performed during the nine months ended September 30, 2014 as compared to 212 during the same period in 2013. The average price collected per commercial test increased from $636 for the nine months ended September 30, 2013 to an average of $1,261 for the nine months ended September 30, 2014, and the average price per development services test was $400 for the nine months ended September 30, 2013 and 2014.
Cost of Revenues
Cost of revenues was approximately $1,556,000 for the nine months ended September 30, 2014, compared with approximately $1,760,000 for the nine months ended September 30, 2013, a decrease of $204,000, or 12%. The decrease was primarily due to the decrease in the proportion of lab volume that related to revenue-generating activities relative to the total number of samples processed for the nine months ended September 30, 2014 as compared to the same period in 2013, partially offset by increases in personnel and allocated facilities costs.
Operating Expenses
Research and Development Expenses. Research and development expenses were approximately $3,428,000 for the nine months ended September 30, 2014, compared with approximately $2,376,000 for the nine months ended September 30, 2013, an increase of $1,052,000, or 44%. The increase was primarily due to an increase of $518,000 in personnel expense primarily related to hiring additional development and lab personnel for new test validations and partially related to non-recurring compensation triggered by our initial public offering, an increase of $417,000 in validation samples and allocated costs related to the higher proportion of lab activities relating to product development, and an increase of $258,000 in facilities, repairs and maintenance costs for the three months ended September 30, 2014 as compared to the same period in 2013, partially offset by a decrease of $147,000 in stock-based compensation expense.
General and Administrative Expenses. General and administrative expenses were approximately $3,971,000 for the nine months ended September 30, 2014, compared with approximately $1,736,000 for the nine months ended September 30, 2013, an increase of $2,235,000, or 129%. The increase was primarily due to an increase of $923,000 in stock-based compensation expense, an increase of $754,000 in insurance, legal, accounting, and consulting expenses as a result of becoming a publicly traded company in February 2014, an increase of $313,000 in personnel expense primarily related to non-recurring compensation triggered by our initial public offering, and increases of $99,000 in legal fees associated with patents and $85,000 in general corporate expenses primarily related to taxes for the nine months ended September 30, 2014 as compared to the same period in 2013.
Sales and Marketing Expenses. Sales and marketing expenses were approximately $1,246,000 for the nine months ended September 30, 2014, compared with approximately $129,000 for the nine months ended September 30, 2013, an increase of
55
$1,117,000. The increase was primarily due to an increase in personnel-related expenses resulting from an expansion in sales and marketing headcount from an average of one for the nine months ended September 30, 2013 to an average of six for the same period in 2014.
Interest Income and Expense
Interest expense was approximately $1,640,000 for the nine months ended September 30, 2014, compared with approximately $1,435,000 for the nine months ended September 30, 2013, an increase of $205,000, or 14%. The increase was due to an increase of $1,028,000 in non-cash amortization expense related to amortization and write-offs of discounts to convertible notes payable that were converted into shares of common stock in conjunction with our initial public offering in February 2014, as well as an increase of $187,000 in cash interest expense primarily associated with the April 2014 Credit Facility, partially offset by a decrease of $1,010,000 in non-cash interest expense related to the notes payable that were converted to common stock in February 2014.
Change in Fair Value of Warrant Liability
The non-cash loss resulting from the change in the fair value of warrant liability of approximately $201,000 for the nine months ended September 30, 2014 compared with the non-cash gain of approximately $593,000 for the nine months ended September 30, 2013 represents an increase in non-cash loss of $794,000, or 134%. The increase is due to a relative increase in the average price of the shares underlying warrants, as well as a greater number of average estimated warrants outstanding upon which the price of the shares underlying warrants is applied, during the nine months ended September 30, 2014 as compared to the nine months ended September 30, 2013.
Income Taxes
Over the past several years we have generated operating losses in all jurisdictions in which we may be subject to income taxes. As a result, we have accumulated significant net operating losses and other deferred tax assets. Because of our history of losses and the uncertainty as to the realization of those deferred tax assets, a full valuation allowance has been recognized. We do not expect to report a provision for income taxes until we have a history of earnings, if ever, that would support the realization of our deferred tax assets.
We have not completed a study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since our formation, due to the complexity and cost associated with such a study, and the fact that there may be additional ownership changes in the future. We estimate that if such a change did occur, the federal and state net operating loss carryforwards and research and development credits that can be utilized in the future will be significantly limited.
Years Ended December 31, 2012 and 2013
The following table sets forth certain information concerning our results of operations for the periods shown:
|
|
Year Ended December 31, |
|
|
Change |
|
||||||||||
|
|
2012 |
|
|
2013 |
|
|
$ |
|
|
% |
|
||||
|
(dollars in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
$ |
109 |
|
|
$ |
134 |
|
|
$ |
25 |
|
|
|
23 |
% |
|
Cost of revenues |
|
1,202 |
|
|
|
2,330 |
|
|
|
1,128 |
|
|
|
94 |
% |
|
Research and development expenses |
|
6,562 |
|
|
|
3,086 |
|
|
|
(3,476) |
|
|
|
(53 |
%) |
|
General and administrative expenses |
|
2,063 |
|
|
|
2,513 |
|
|
|
450 |
|
|
|
22 |
% |
|
Sales and marketing expenses |
|
785 |
|
|
|
149 |
|
|
|
(636) |
|
|
|
(81 |
%) |
|
Total Operating Loss |
|
(10,503 |
) |
|
|
(7,944 |
) |
|
|
2,559 |
|
|
|
72 |
% |
|
Interest income/(expense), net |
|
(2,187 |
) |
|
|
(2,070 |
) |
|
|
117 |
|
|
|
14 |
% |
|
Change in fair value of warrant liability |
|
454 |
|
|
|
782 |
|
|
|
328 |
|
|
|
(134 |
%) |
|
Other income/(expense) |
|
(23 |
) |
|
— |
|
|
|
23 |
|
|
|
(100 |
%) |
|
|
Income/(Loss) before income taxes |
|
(12,259 |
) |
|
|
(9,232 |
) |
|
|
3,027 |
|
|
|
(25 |
%) |
|
Income tax expense |
|
1 |
|
|
|
1 |
|
|
— |
|
|
— |
|
||
|
Net loss |
$ |
(12,260 |
) |
|
$ |
(9,233 |
) |
|
$ |
3,027 |
|
|
|
(25 |
%) |
Revenue
Revenues were approximately $134,000 for the year ended December 31, 2013, compared with approximately $109,000 for the year ended December 31, 2012, an increase of approximately $25,000, or 23%. The increase was primarily related to clinical trial
56
testing services for our development collaboration program with the Dana-Farber Cancer Institute, partially offset by a decrease in revenues from Clarient. The average price per commercial test decreased from $694 for the year ended December 31, 2012 to an average of $635 for the year ended December 31, 2013. The average price per clinical test was $400 for the year ended December 31, 2013.
Cost of Revenues
Cost of revenues was $2.3 million for the year ended December 31, 2013, compared with $1.2 million for the year ended December 31, 2012, an increase of $1.1 million, or 94%. The increase was primarily related to the volume of clinical tests performed, which increased from zero for the year ended December 31, 2012 to 258 for the year ended December 31, 2013. The volume of commercial tests performed decreased from 130 for the year ended December 31, 2012 to 23 for the year ended December 31, 2013. The net volume increase was due to clinical tests performed under our 2013 development collaboration program with the Dana-Farber Cancer Institute, partially offset by fewer tests performed under our arrangement with Clarient for the year ended December 31, 2013 as compared to 2012.
Operating Expenses
Research and Development Expenses. Research and development expenses were $3.1 million for the year ended December 31, 2013, compared with $6.6 million for the year ended December 31, 2012, a decrease of $3.5 million, or 53%.The decrease was primarily due to a $1.8 million decrease in personnel expenses relating to a reduction in research and development headcount from an average of 16 for the year ended December 31, 2012 to an average of 9 for the same period in 2013, and a $1.1 million decrease in research and development expenses due to the allocation of lab expenses to cost of revenues based on the number of samples processed.
General and Administrative Expenses. General and administrative expenses were $2.5 million for the year ended December 31, 2013, compared with $2.1 million for the year ended December 31, 2012, an increase of $0.4 million, or 22%. The increase was primarily due to an increase of $266,000 in legal fees, particularly fees pertaining to our patent portfolio, as well as an increase in stock-based compensation expense of $358,000, partially offset by a $310,000 decrease in personnel-related expenses resulting from a reduction in general and administrative headcount from an average of 8 for the year ended December 31, 2012 to an average of 6 for the same period in 2013.
Sales and Marketing Expenses. Sales and marketing expenses were $0.1 million for the year ended December 31, 2013, compared with $0.8 million for the year ended December 31, 2012, a decrease of $0.7 million, or 81%.The decrease was primarily due to a decrease in personnel-related expenses resulting from a reduction in sales and marketing headcount from an average of 2 for the year ended December 31, 2012 to an average of 1 for the same period in 2013.
Interest Income and Expense
Interest expense was $2.1 million for the year ended December 31, 2013, compared with $2.2 million for the year ended December 31, 2012, with the $0.1 million decrease primarily related to lower average debt balances, partially offset by increased debt discount amortization as a result of entering into new financing arrangements with associated discounts during 2013.
Change in Fair Value of Warrant Liability
The change in the fair value of warrant liability was $0.8 million for the year ended December 31, 2013 compared with $0.5 million for the year ended December 31, 2012, an increase of $0.3 million, or 72%. The increase is due to a larger decline in the price of the shares underlying warrants during the year ended December 31, 2013 as compared to the year ended December 31, 2012.
Income Taxes
Over the past several years we have generated operating losses in all jurisdictions in which we may be subject to income taxes. As a result, we have accumulated significant net operating losses and other deferred tax assets. Because of our history of losses and the uncertainty as to the realization of those deferred tax assets, a full valuation allowance has been recognized. We do not expect to report a provision for income taxes until we have a history of earnings, if ever, that would support the realization of our deferred tax assets.
We have not completed a study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since our formation, due to the complexity and cost associated with such a study, and the fact that there may be
57
additional ownership changes in the future. We estimate that if such a change did occur, the federal and state net operating loss carryforwards and research and development credits that can be utilized in the future will be significantly limited.
Liquidity and Capital Resources
Cash Flows
Our net cash flow from operating, investing and financing activities for the periods below were as follows:
|
|
Nine Months Ended |
|
|||||
|
|
September 30, |
|
|||||
|
|
2013 |
|
|
2014 |
|
||
|
(dollars in thousands) |
|
|
|
|
|
|
|
|
Cash provided by (used in): |
|
|
|
|
|
|
|
|
Operating activities |
$ |
(4,877 |
) |
|
$ |
(11,386 |
) |
|
Investing activities |
|
(1 |
) |
|
|
(202 |
) |
|
Financing activities |
|
4,996 |
|
|
|
20,338 |
|
|
Net increase (decrease) in cash and cash equivalents |
$ |
118 |
|
|
$ |
8,750 |
|
Cash Used in Operating Activities. Net cash used in operating activities was approximately $11,386,000 for the nine months ended September 30, 2014, compared to net cash used in operating activities of approximately $4,877,000 for the nine months ended September 30, 2013. In all periods the primary use of cash was to fund our net loss. Additionally, an increase of $2,926,000 in cash used to fund operating assets and liabilities, primarily related to the payment of deferred salaries, interest and taxes thereon as well as initial public offering costs, was partially offset by an increase of $1,641,000 in non-cash operating expenses during the nine months ended September 30, 2014 as compared to the same period in 2013.
Cash Used in Investing Activities. Net cash used in investing activities was approximately $202,000 for the nine months ended September 30, 2014, compared to net cash used in investing activities of approximately $1,000 for the nine months ended September 30, 2013. In all periods the primary use of cash was to acquire fixed assets.
Cash Provided by Financing Activities. Net cash provided by financing activities was approximately $20,338,000 for the nine months ended September 30, 2014, compared to net cash provided by financing activities of approximately $4,996,000 for the nine months ended September 30, 2013. Our primary source of financing in the nine months ended September 30, 2013 consisted of loans received from our major shareholder and members of our board of directors and their affiliates in exchange for convertible promissory notes and warrants, as well as proceeds from borrowings on our July 2013 line of credit with UBS Bank USA (our “Line of Credit”), and our primary sources of financing in the nine months ended September 30, 2014 consisted of proceeds from our initial public offering and borrowings on our credit facility and warrants.
Our net cash flow from operating, investing and financing activities for the periods below were as follows:
|
|
Year Ended |
|
|||||
|
|
December 31, |
|
|||||
|
|
2012 |
|
|
2013 |
|
||
|
(dollars in thousands) |
|
|
|
|
|
|
|
|
Cash provided by (used in): |
|
|
|
|
|
|
|
|
Operating activities |
$ |
(8,607 |
) |
|
$ |
(6,202 |
) |
|
Investing activities |
|
(8 |
) |
|
|
(1 |
) |
|
Financing activities |
|
8,365 |
|
|
|
6,087 |
|
|
Net increase (decrease) in cash and cash equivalents |
$ |
(250) |
|
|
$ |
(116) |
|
Cash Used in Operating Activities. Net cash used in operating activities was $6.2 million for the year ended December 31, 2013, compared to net cash used in operating activities of $8.6 million for the year ended December 31, 2012. In all periods the primary use of cash was to fund our net loss.
Cash Used in Investing Activities. Cash used in investing activities was $1,000 for the year ended December 31, 2013, compared to $8,000 for the year ended December 31, 2012. The cash used in investing activities in 2012 was primarily used to acquire laboratory equipment and software.
Cash Provided by Financing Activities. Net cash provided by financing activities was $6.1 million for the year ended December 31, 2013, compared to net cash provided by financing activities of $8.4 million for the year ended December 31, 2012. Our
58
primary source of financing in all periods consisted of loans received from our major shareholder and members of our board of directors and their affiliates, in exchange for convertible promissory notes and warrants.
Capital Resources and Expenditure Requirements
We expect to continue to incur substantial operating losses in the future. It may take several years to achieve positive operational cash flow or we may not ever achieve positive operational cash flow. We expect that we will use a portion of the net proceeds from our initial public offering and our revenues from operations to hire sales and marketing personnel, support increased sales and marketing activities, fund further research and development, clinical utility studies and future enhancements of our tests, acquire equipment, implement automation and scale our capabilities to prepare for significant test volume, for general corporate purposes and to fund ongoing operations and the expansion of our business, including the increased costs associated with being a public company. We may also use a portion of the net proceeds of our initial public offering to acquire or invest in businesses, technologies, services or products, although we do not have any current plans to do so.
As of September 30, 2014, our cash and cash equivalents totaled approximately $8,820,000. While we currently are in the commercialization stage of operations, we have not yet achieved profitability and anticipate that we will continue to incur net losses for the foreseeable future. On February 10, 2014, we received net cash proceeds of approximately $17,390,000 as a result of the closing of our initial public offering, after deducting approximately $1,610,000 of underwriting discounts and additional underwriting costs incurred.
On April 30, 2014, we received net cash proceeds of approximately $4,927,000 pursuant to the execution of a term loan agreement with Oxford Finance LLC, or the April 2014 Credit Facility. A second term loan of up to a principal amount of $5 million will be funded at our request prior to December 31, 2015, subject to our achieving product and services revenues of at least $9 million for the trailing six months, with such six-month period ending no later than November 30, 2015. Upon the entry into the April 2014 Credit Facility, we were required to pay the lenders a facility fee of $50,000 in conjunction with the funding of the first term loan. Another $50,000 facility fee will be due and payable to the lenders on the funding date of the second term loan (if such date occurs). The April 2014 Credit Facility is secured by substantially all of our personal property other than our intellectual property. Each term loan under the April 2014 Credit Facility bears interest at an annual rate equal to the greater of (i) 7.95% or (ii) the sum of (a) the three-month U.S. LIBOR rate reported in the Wall Street Journal three business days prior to the funding date of the applicable term loan, plus (b) 7.71%, such rate to be fixed at the time of borrowing. The first term loan bears interest at an annual rate of 7.95%. We are required to make interest-only payments on the first term loan through February 1, 2016 if the funding date of the second term loan occurs before June 30, 2015, or through August 1, 2015 otherwise. If we request and the lenders fund the second term loan, we are required to make interest-only payments on the second term loan through February 1, 2016 if the funding date of the second term loan occurs before June 30, 2015, or through the seventh month following the funding date of the second term loan otherwise. All outstanding term loans under the April 2014 Credit Facility will begin amortizing at the end of the applicable interest-only period, with monthly payments of principal and interest being made by us to the lenders in consecutive monthly installments following such interest-only period. The first term loan under the April 2014 Credit Facility matures on July 1, 2018, and the second term loan matures on the first day of the 29th month following the end of the applicable interest-only period. Upon repayment of each term loan, we are also required to make a final payment to the lenders equal to 5.50% of the original principal amount of such term loan funded. At our option, we may prepay the outstanding principal balance of the term loans in whole but not in part, subject to a prepayment fee of 3% of any amount prepaid if the prepayment occurs on or prior to April 30, 2015, 2% of the amount prepaid if the prepayment occurs after April 30, 2015 but on or prior to April 30, 2016, and 1% of any amount prepaid after April 30, 2016. The April 2014 Credit Facility includes affirmative and negative covenants applicable to us and any subsidiaries we create in the future. The affirmative covenants include, among others, covenants requiring us to maintain our legal existence and governmental approvals, deliver certain financial reports and maintain insurance coverage. The negative covenants include, among others, restrictions on our transferring collateral, incurring additional indebtedness, engaging in mergers or acquisitions, paying dividends or making other distributions, making investments, creating liens, selling assets, and suffering a change in control, in each case subject to certain exceptions. The April 2014 Credit Facility also includes events of default, the occurrence and continuation of which provide Oxford Finance LLC, as collateral agent, with the right to exercise remedies against us and the collateral securing the term loans under the April 2014 Credit Facility, including foreclosure against our properties securing the April 2014 Credit Facility, including our cash. These events of default include, among other things, our failure to pay any amounts due under the April 2014 Credit Facility, a breach of covenants under the April 2014 Credit Facility, our insolvency, a material adverse change, the occurrence of any default under certain other indebtedness in an amount greater than $250,000, and a final judgment against us in an amount greater than $250,000.
We expect that we will need additional financing in the future to execute on our current or future business strategies beyond March 2015. Until we can generate significant cash from operations, we expect to continue to fund operations with the proceeds of offerings of our equity and debt securities. We can provide no assurances that any sources of a sufficient amount of financing will be available to us on favorable terms, if at all. In addition to test revenues, such financing may be derived from one or more of the following types of transactions: debt, equity, product development, technology licensing or collaboration. If we are unable to raise a sufficient amount of financing in a timely manner, we would likely need to scale back our general and administrative activities and
59
certain of our research and development activities. Our forecast pertaining to our current financial resources and the costs to support our general and administrative and research and development activities are forward-looking statements and involve risks and uncertainties. Actual results could vary materially and negatively as a result of a number of factors, including:
|
· |
our ability to secure financing and the amount thereof; |
|
· |
the costs of operating and enhancing our laboratory facilities; |
|
· |
the costs of developing our anticipated internal sales and marketing capabilities; |
|
· |
the scope, progress and results of our research and development programs, including clinical utility studies; |
|
· |
the scope, progress, results, costs, timing and outcomes of the clinical utility studies for our cancer diagnostic tests; |
|
· |
our ability to manage the costs for manufacturing our microfluidic channels; |
|
· |
the costs of maintaining, expanding and protecting our intellectual property portfolio, including potential litigation costs and liabilities; |
|
· |
our ability to obtain adequate reimbursement from governmental and other third-party payors for our tests and services; |
|
· |
the costs of additional general and administrative personnel, including accounting and finance, legal and human resources, as a result of becoming a public company; |
|
· |
our ability to collect revenues; and |
|
· |
other risks discussed in our other filings with the SEC. |
We may raise additional capital to fund our current operations and to fund expansion of our business to meet our long-term business objectives through public or private equity offerings, debt financings, borrowings or strategic partnerships coupled with an investment in our company or a combination thereof. If we raise additional funds through the issuance of convertible debt securities, or other debt securities, these securities could be secured and could have rights senior to those of our common stock. In addition, any new debt incurred by us could impose covenants that restrict our operations. The issuance of any new equity securities will also dilute the interest of our current stockholders. Given the risks associated with our business, including our unprofitable operating history and our ability or inability to develop additional tests, additional capital may not be available when needed on acceptable terms, or at all. If adequate funds are not available, we will need to curb our expansion plans or limit our research and development activities, which would have a material adverse impact on our business prospects and results of operations.
Off-Balance Sheet Arrangements
We have not engaged in any off-balance sheet arrangements as defined in Item 303(a)(4) of Regulation S-K.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates based on historical experience and make various assumptions, which management believes to be reasonable under the circumstances, which form the basis for judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
The notes to our audited and unaudited financial statements, which are included elsewhere in this prospectus, contain a summary of our significant accounting policies. We consider the following accounting policies critical to the understanding of the results of our operations:
|
· |
Revenue recognition; |
|
· |
Accounts receivable and bad debts; |
|
· |
Stock-based compensation; |
|
· |
Common stock valuation; and |
|
· |
Warrant liability. |
60
Revenue Recognition
We recognize revenue in accordance with ASC 605, Revenue Recognition, and ASC 954-605, Health Care Entities, Revenue Recognition which requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred and title and the risks and rewards of ownership have been transferred to the client or services have been rendered; (3) the price is fixed or determinable; and (4) collectability is reasonably assured. For contract partners, revenue is recorded based upon the contractually agreed upon fee schedule. When assessing collectability, we consider whether we have sufficient payment history to reliably estimate a payor’s individual payment patterns. For new tests where there is limited evidence of payment history at the time the tests are completed, we recognize revenue equal to the amount of cash received until such time as reimbursement experience can be established.
Accounts Receivable and Bad Debts
We carry accounts receivable at original invoice amounts, less an estimate for doubtful receivables, based on a review of all outstanding amounts on a periodic basis. The estimate for doubtful receivables is determined from an analysis of the accounts receivable on a quarterly basis, and is recorded as bad debt expense. Since we only recognize revenue to the extent we expect to collect such amounts, bad debt expense related to receivables from patient service revenue is recorded in general and administrative expense in the statements of operations. Accounts receivable are written off when deemed uncollectible. Recoveries of accounts receivable previously written off are recorded when received.
Stock-Based Compensation Expense
We account for stock-based compensation under the provisions of ASC Topic 718, Compensation—Stock Compensation, which requires the measurement and recognition of compensation expense for all stock-based awards made to employees and directors based on estimated fair values on the grant date. We estimate the fair value of stock option awards on the date of grant using the Black-Scholes option pricing model, or Black-Scholes valuation model. The fair value of restricted stock unit awards is determined by the price of the Company’s common stock on the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods using the straight-line method. We estimate forfeitures at the time of grant and revise our estimates in subsequent periods if actual forfeitures differ from those estimates.
We account for stock-based compensation awards to non-employees in accordance with ASC Topic 505-50, Equity-Based Payments to Non-Employees. Under ASC 505-50, we determine the fair value of the warrants or stock-based compensation awards granted as either the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. All issuances of equity instruments issued to non-employees as consideration for goods or services received by us are accounted for based on the fair value of the equity instruments issued. These awards are recorded in expense and additional paid-in capital in stockholders’ equity over the applicable service periods based on the fair value of the options at the end of each period.
Calculating the fair value of stock-based awards requires the input of highly subjective assumptions into the Black-Scholes valuation model. Stock-based compensation expense is calculated using our best estimate, which involves inherent uncertainties, and the application of our management’s judgment. Significant estimates include the fair value of our common stock at the date of grant for awards granted prior to our initial public offering, the expected life of the stock option, stock price volatility, risk-free interest rate and forfeiture rate.
Common Stock Valuation
In the absence of a public trading market, our board of directors determined a reasonable estimate of the then-current fair value of our common stock for purposes of granting stock-based compensation based on input from management and valuation reports prepared by an independent third-party valuation specialist. We determined the fair value of our common stock utilizing methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants Practice Aid, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation,” which we refer to as the AICPA Practice Aid. In addition, we exercised judgment in evaluating and assessing the foregoing based on several factors including:
|
· |
the nature and history of our business; |
|
· |
our historical operating and financial results; |
|
· |
the market value of companies that are engaged in a similar business to ours; |
|
· |
the lack of marketability of our common stock; |
|
· |
the price at which shares of our equity instruments have been sold; |
61
|
· |
the overall inherent risks associated with our business at the time stock option grants or warrants were approved; and |
|
· |
the overall equity market conditions and general economic trends. |
Warrant Liability
Warrants for shares that are contingently redeemable and for which the exercise price is not fixed are classified as liabilities on the accompanying balance sheets and carried at their estimated fair value, determined through use of a probability-weighted Black-Scholes valuation model. At the end of each reporting period, any changes in fair value are recorded as a component of total other income/(expense). As of the closing of the Company’s initial public offering on February 10, 2014, the exercise price underlying the majority of the Company’s warrants was fixed and the fair value of those warrants was reclassified to shareholders’ deficit, while a preferred stock warrant to purchase an equivalent of 1,587 shares of common stock remains liability-classified at September 30, 2014.
62
Company Overview
We are a cancer diagnostics company that develops and commercializes proprietary circulating tumor cell, or CTC, and circulating tumor DNA, or ctDNA, tests utilizing a standard blood sample, or “liquid biopsy.” These tests provide information to oncologists and other physicians that enable them to select the most appropriate treatment for their patients based on better, timelier and more-detailed data on the characteristics of tumors. Our current OncoCEE-BR for breast cancer test, OncoCEE-LU for NSCLC test and OncoCEE-GA for gastric cancer test and our planned tests utilize our Cell Enrichment and Extraction (CEE) technology for the enumeration and analysis of CTCs, and our CEE-Selector technology for the detection and analysis of ctDNA, each performed on a standard blood sample. The CEE technology is an internally developed, microfluidics-based CTC capture and analysis platform, with enabling features that change how CTC testing can be used by clinicians by providing real-time biomarker monitoring with a standard blood sample. The CEE-Selector technology enables mutation detection with enhanced sensitivity and specificity and is applicable to nucleic acid from CTCs or other sample types, such as blood plasma for ctDNA. We believe CEE-Selector technology is an important part of certain of our pipeline CTC tests, and believe it could also be a stand-alone test for molecular analysis of biomarkers.
At our corporate headquarters facility located in San Diego, California, we operate a clinical laboratory that is certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and accredited by the College of American Pathologists, or CAP, and manufacture our CEE microfluidic channels, related equipment and certain reagents to perform our current tests and our planned future tests at this facility. CLIA certification is required before any clinical laboratory, including ours, may perform testing on human specimens for the purpose of obtaining information for the diagnosis, prevention, or treatment of disease, or impairment of, or the assessment of health. The OncoCEE-BR, OncoCEE-LU, and OncoCEE-GA tests and the tests we plan to offer are classified as laboratory developed tests, or LDTs, under CLIA regulations.
OncoCEE-BR is a breast cancer CTC test and OncoCEE-GA is a gastric cancer CTC test that are performed on a standard blood sample. They detect CTCs, which are typically very rare compared to normal blood cells, and determine the patient’s human epidermal growth factor receptor 2, or HER2, status by fluorescence in situ hybridization, or FISH. In addition, OncoCEE-BR is used to detect the presence of ER, which is the biomarker that indicates the likely responsiveness of a patient’s tumor to hormonal therapies.
We believe that the OncoCEE-BR and OncoCEE-GA tests offers advantages over other available CTC tests, with improved sensitivity and enumeration results as well as diagnostic biomarker analyses. Competitive CTC tests rely on the expression of the epithelial cell adhesion molecule, or EpCAM, and cytokeratins for CTC capture, detection and enumeration. This approach may exclude CTCs that have undergone intrinsic modifications of their phenotype, such as the epithelial-to-mesenchymal transition, or EMT, thought to be critical for metastasis. EMT may represent a possible explanation for many patients who, despite an aggressive disease, are found to be negative for the presence of CTCs by current technologies. OncoCEE™ captures and detects EpCAM and cytokeratin negative CTCs, which are more mesenchymal-like. Additionally, the OncoCEE platform enables evaluation of treatment-associated biomarkers, like HER2 status, which qualifies patients as candidates for HER2-targeted therapeutics, and ER status, which qualifies patients as candidates for hormone therapies. We plan to include immunocytochemical analysis of progesterone receptor proteins, as well as mutation analysis as appropriate, into the OncoCEE-BR test within the next year.
We launched OncoCEE-LU, a test performed on a standard blood sample for non-small cell lung cancer, or NSCLC, in November of 2014. The biomarkers to be analyzed in the OncoCEE-LU test includes ALK and ROS1 gene fusions by FISH, and the epidermal growth factor receptor, or EGFR, gene. We expect to add FISH testing for RET, MET, as well as mutation analysis for the EGFR gene, the K-ras gene and the B-raf gene during 2015.
Our OncoCEE-LU test is run against a standard blood sample.
We plan to add other biomarker analyses to our OncoCEE tests as their relevance is demonstrated in clinical trials, for example, RET proto-oncogene gene fusions in NSCLC, which may indicate a particular course of therapy. In addition, we are developing a series of other CTC and ctDNA tests for different solid tumor types, including colorectal cancer, prostate cancer, gastric cancer and melanoma, each incorporating treatment-associated biomarker analyses specific to that cancer, planned to be launched over the next two years.
63
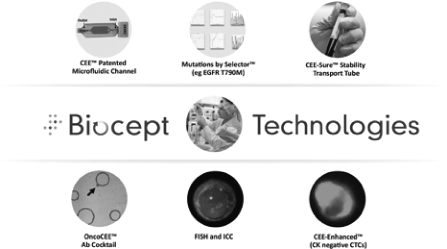
Biomarkers are molecular or cellular features of a cancer cell that indicate an abnormality. This abnormality, typically a genetic mutation or aberration, detected at either the gene, protein or metabolite level, may in fact be responsible for the transformation of the cell from a normal cell to a cancer cell. We have focused our efforts on biomarkers associated with specific targeted cancer therapeutics, or resistance to those therapeutics. Examples include an amplified HER2 gene, which is associated with HER2-targeted therapeutics like Herceptin®, Perjeta®, Kadcyla® and Tykerb® for the treatment of breast cancer, or a mutated B-raf gene, which is associated with the drugs Zelboraf® (Daiichi-Sankyo/Genentech/Roche) and Tafinlar® (GlaxoSmithKline) for the treatment of melanoma. This is important because the presence or level of these biomarkers indicates to a physician that the associated therapy is appropriate for the patient, or instead that the patient has, or has developed, resistance to that therapy.
Biomarkers have traditionally been detected in tumor tissue after biopsy or re-section, with the analysis performed by a pathologist. We are able to perform these same analyses on CTCs or ctDNA on a standard blood sample using our CEE and CEE-Selector technology in our CLIA laboratory, meaning that the biomarkers detected in a patient’s tumor can now be monitored on a real-time basis without the need for a tissue biopsy. Because of the difficulty or inability to obtain periodic tissue biopsies, especially at the time of recurrence, this offers the physician a new source and level of information than was previously available.
We also have a research and development program focused on technology enhancements and novel platform development and are evaluating clinical applications for cancer diagnostic tests in different cancer types and clinical settings. We offer our current and planned unique cancer diagnostic tests through our CLIA laboratory to physicians for patient care applications as well as to pharmaceutical and biopharmaceutical companies and academic centers using CTC or ctDNA testing, with biomarker analysis including genetic analysis, in their clinical trials and research efforts. CTC tests, particularly those that offer analysis of CTCs for treatment-associated biomarkers, are becoming powerful tools in the practice of personalized medicine. They enable physicians to utilize a standard blood sample as a “liquid biopsy” to assess the status of their patient’s cancer at a cellular and molecular level on an ongoing basis, and to select therapies that have the highest likelihood of benefiting their patients.
Historically, our average price received per OncoCEE-BR test performed for commercial customers has been approximately $2,412. This was heavily influenced by the fact that historically a high percentage of our sales were through our marketing partner, Clarient. We amended our arrangement with Clarient as of May 2013, and we do not expect a significant percentage of our future sales to come through Clarient. Our OncoCEE-LU and OncoCEE-GA tests were launched in late 2014 and we have not yet recognized significant revenues from these tests. Our future average price for commercial customers could increase from our historical figure, based on recognition of the medical value of our products, publication of clinical utility study results, possible improvement of the product, introduction of additional tests, increased demand generated by our future sales and marketing efforts, and similar commercial factors. Factors that could cause pricing for commercial customers to decrease include any perceived lack of clinical utility for CTC or ctDNA testing, or increased competition from other reference labs or IVD manufacturers. Third-party governmental and private payors have reimbursement policies and fee schedules which determine the amounts, if any, we would receive for performing tests for their covered patients. Such governmental and private third-party payors frequently make determinations about how much (if anything) they are willing to pay for tests such as ours, or for components of such tests; these determinations are important to our business and can have adverse or positive effects on the price we receive for our testing. For example, private payors often look to Medicare policies and rates when setting their reimbursement rates.
In addition, our reimbursement rates can vary based on whether we are considered by private third-party payors to be an “in-network” provider, a participating provider, a covered provider or an “out-of-network” provider. These definitions can vary from insurance company to insurance company, but we are generally considered an “out-of-network” or non-participating provider by the
64
vast majority of private third-party payors. It is not unusual for a company that offers highly specialized or unique testing to be an “out-of-network” provider. An “in-network” provider usually has a contracted arrangement with the insurance company or benefits provider. This contract governs, among other things, service-level agreements and reimbursement rates. In certain instances an insurance company may negotiate an “in-network” rate for our testing rather than pay the typical “out-of-network” rate. An “in-network” provider usually has rates that are lower per test than those that are “out-of-network”, and that rate can vary from a single digit percentage deduction discount to upwards of 25% to 30% lower than an “out-of-network” provider. The discount rate varies based on the insurance company, the testing type and often times the specifics of the patient’s insurance plan. In some plans, there is no benefit paid for out-of-network claims and our ability to collect from the patient may be hindered by the financial resources of the patient or by state laws that prohibit billing of patients for denied out-of-network claims.
We cannot predict whether, or under what circumstances, payors will reimburse for all components of our tests. Payment amounts can also vary across individual policies. Full or partial denial of coverage by payors, or reimbursement at inadequate levels, would have a material adverse impact on our business and on market acceptance of our tests.
To date, we have engaged in only limited sales and marketing activities. Such activities have primarily related to our OncoCEE-BR test. We have a sales and marketing team to market and sell OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA and our planned future cancer diagnostic tests directly to oncologists and other physicians. We have an initial group of 8 sales representatives, and, based on success and test volume, plan to grow this number to 15-20 within two years.
We collaborate with physicians and researchers at The University of Texas MD Anderson Cancer Center, the Dana-Farber Cancer Institute, the University of California, San Diego and Columbia University and plan to expand our collaborative relationships to include other key thought leaders at other institutions for the cancer types we target with OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA and our planned future CTC and ctDNA tests. Such relationships help us develop and validate the effectiveness and utility of OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA and our planned future tests in specific clinical settings and provide us access to patient samples and data. We completed a study, recently published in Cancer Medicine, utilizing our OncoCEE-BR test, and a version of this test adapted for use with bone marrow samples, with a group at The University of Texas MD Anderson Cancer Center comprised of breast cancer surgeons, pathologists and basic researchers. In this study, we demonstrated the ability to identify HER2 positive CTCs and disseminated tumor cells, or DTCs, seen in bone marrow in patients that had been previously classified as HER2 negative by analysis of their tumor tissue. A HER2 positive result in a patient with breast cancer provides an indication to the physician that there is likely to be a survival benefit from treatment with Herceptin®, which has been demonstrated in a number of large clinical studies.
We are currently involved in a clinical study following up on this finding in CTCs, employing OncoCEE-BR tests for patient selection and monitoring. This study, led by investigators at the Dana-Farber Cancer Institute, has completed enrolling patients. In the screening phase of this study, we tested in our CLIA-certified, CAP accredited, and state-licensed laboratory blood samples from HER2 negative patients based on standard tumor tissue analysis, to identify those patients that have HER2 positive CTCs. These patients were then being randomized to chemotherapy plus/minus Herceptin®, and followed for a period of time, with additional CTC tests, including biomarker analysis for HER2 using FISH, performed at subsequent time points. In December 2014 we announced preliminary findings that were presented at the San Antonio Breast Conference that 22 percent of 311 patients, who were previously HER2 negative according to a solid tumor biopsy, were found, upon disease progression, to be HER2 positive by CTC analysis, making them potential candidates for anti-HER2 therapy as the cancer evolves. Moreover, our multi-antibody CTC capture method identified a substantial subset of patients who would not likely be detected with commonly used CTC capture technologies. This added 10 percent (included in the 22 percent) to the number of women who were candidates for this highly specific targeted therapy.
We plan to grow our business by directly offering oncologists and other physicians our liquid biopsy CTC and ctDNA tests. Based on our product development data, as well as discussions with our collaborators, we believe that our planned tests should provide important information and clinical value to physicians. In particular, CTC and ctDNA tests should deliver important, actionable information not provided by other tests. For example, the market leading clinical CTC test is the United States Food and Drug Administration, or FDA, approved CellSearch® test (Janssen Diagnostics), which provides CTC enumeration, but is not FDA approved to perform biomarker analysis. We believe our ability to rapidly translate research insights about the utility of cytogenetic, immunocytochemical and molecular biomarkers to provide information to oncologists and other physicians for treatment decisions in the clinical setting will improve patient treatment and management, and that these tests will become a key component in the standard of care for personalized cancer treatment.
According to the National Cancer Institute, there will be approximately 230,000 new cases of breast cancer and approximately 220,000 new cases of lung cancer diagnosed in the United States in 2014, with over 3 million patients who have had a diagnosis of these cancers and either are living with these diseases and are undergoing treatment or are being monitored. For example, in breast cancer, many women have been deemed cancer-free, but continue to undergo periodic monitoring to assure there has been no disease recurrence. Our OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA tests and our other planned tests only require a readily accessible standard blood sample and thus may be used to help manage these patients, including supporting the selection of appropriate treatment, at multiple time points during the course of their disease. Because our tests require only a standard blood sample, they can be
65
particularly useful when no, old or inadequate amounts of, biopsy or surgical material is available, as is often the case in lung cancer, even at the time of initial evaluation. For example, up to 25% of patients with lung cancer are not surgically treated for various reasons, including patient status (consensus statement from the American College of Chest Physicians and the Society of Thoracic Surgeons; Chest, Dec. 2012). This is also the case with breast and lung cancers once surgical resection of the tumor has taken place and treatment has been initiated. Patients with breast and lung cancer must often undergo surgical resection of their primary tumor as part of their treatment. Therefore, at the time of progression or recurrence there may be no ability to obtain a tissue biopsy. Additionally, many studies have shown that most tumors mutate during treatment and as the disease progresses, so information from the initial tumor tissue may not be relevant. Again, a significant benefit of our technology is that it allows physicians to assess the current status of the tumors on a real-time basis utilizing a standard blood sample or liquid biopsy.
We currently offer and conduct our breast and lung cancer diagnostic tests and offer our clinical trial services at our CLIA-certified, CAP-accredited and state- licensed laboratory. Our current tests and our planned near-term cancer diagnostic tests and clinical trial services include:
|
· |
CTC and ctDNA Testing. Our current tests and our other planned cancer diagnostic tests are based on our CEE and CEE-Selector technologies and are currently intended to be performed only in our clinical laboratory. After completing testing, we or our partner provide our customers with an easy to understand report that describes the results of the analyses performed, designed to help oncologists and other physicians make better decisions about the treatment of their patients. |
|
· |
Clinical Trial Services. We plan to utilize our clinical laboratory and translational research capabilities to provide clinical trial and research services to pharmaceutical and biopharmaceutical companies and clinical research organizations to improve the efficiency and economic viability of their clinical trials. Our clinical trials and translational research services could leverage our knowledge of CTCs and ctDNA and our ability to develop and implement new cytogenetic, immunocytochemical and molecular diagnostic tests. Our current tests can, and our other planned cancer diagnostic tests and biomarker tests are anticipated to be able to, help optimize clinical trial patient selection, and as a result potentially improve the likelihood of success of the clinical trial. With positive results in a clinical trial, our tests would more easily then move into standard clinical practice, helping physicians select the most appropriate therapy for their patients. |
We intend to commercialize cancer diagnostic tests in the United States as LDTs performed in our CLIA-certified, CAP-accredited, and state-licensed laboratory. We plan to evaluate potential opportunities for the commercialization of our products in other countries. We are currently exploring the possibility of introducing OncoCEE technology outside the United States as part of CE-marked IVD test kits and/or testing systems utilizing our CEE and/or CEE-Selector technologies.
Our sales strategy is to engage oncologists and other physicians in the United States at private and group practices, hospitals and cancer centers. In addition, we market our clinical trial and research services to pharmaceutical and biopharmaceutical companies and clinical research organizations.
66
Market Overview
Cancer Market Overview
Despite many advances in the treatment of cancer, it remains one of the greatest areas of unmet medical need. According to the World Cancer Report 2014, cancers figure among the leading causes of morbidity and mortality worldwide, with approximately 14 million new cases and 8.2 million cancer related deaths in 2012. It is also expected that the number of new cases will rise by approximately 70% over the next two decades. The incidence of, and deaths caused by, the major cancers are staggering. The following data published by the National Cancer Institute shows estimated new cases and deaths for 2014, and prevalence in 2010, in the United States for the major solid cancers types:
|
Cancer Type |
|
Est. Incidence (New Cases/Year-2014) |
|
|
Est. Mortality (Deaths/Year-2014) |
|
|
Est. Prevalence (Diagnosed and Alive as of 2010)** |
|||
|
Bladder |
|
|
74,690 |
|
|
|
15,580 |
|
|
|
563,640 |
|
Breast* |
|
|
232,670 |
|
|
|
40,000 |
|
|
|
2,843,629 |
|
Cervical |
|
|
12,340 |
|
|
|
4,030 |
|
|
|
249,496 |
|
Colorectal* |
|
|
136,830 |
|
|
|
50,310 |
|
|
|
1,154,481 |
|
Endometrial |
|
|
52,630 |
|
|
|
8,590 |
|
|
|
600,346 |
|
Gastric* |
|
|
22,220 |
|
|
|
10,990 |
|
|
|
72,269 |
|
Kidney |
|
|
63,920 |
|
|
|
13,860 |
|
|
|
341,505 |
|
Lung* |
|
|
224,210 |
|
|
|
159,260 |
|
|
|
399,431 |
|
Melanoma* |
|
|
76,100 |
|
|
|
9,710 |
|
|
|
921,780 |
|
Ovarian |
|
|
22,240 |
|
|
|
14,030 |
|
|
|
186,138 |
|
Pancreatic |
|
|
46,420 |
|
|
|
39,590 |
|
|
|
41,609 |
|
Prostate* |
|
|
233,000 |
|
|
|
29,480 |
|
|
|
2,617,682 |
|
Thyroid |
|
|
62,980 |
|
|
|
1,890 |
|
|
|
534,973 |
|
* |
Areas where we currently have tests or active development programs. |
|
** |
Includes active disease and disease-free. |
In addition to the human toll, the financial cost of cancer is overwhelming. An independent study published in 2010 and conducted jointly by the American Cancer Society and LIVESTRONG ranked cancer as the most economically devastating cause of death in the world - estimated to be as high as $895 billion globally. According to an article in the Journal of the National Cancer Institute, the direct cost of cancer deaths in the United States in 2000 was over $115 billion, and if lost wages and caregiver costs were added, the total costs increased to over $230 billion.
Cancer is a Heterogeneous Disease
Cancer constitutes a heterogeneous class of diseases, characterized by uncontrolled cell growth that results from a combination of both environmental and hereditary risk factors. Many different tissue types can become malignant, such as breast, lung, liver, and skin, and even within a particular tumor there is heterogeneity, with certain cancer cells in a patient bearing specific cellular or genetic biomarkers which others lack. It has only been in recent years that technology has progressed far enough to enable researchers to understand many cancers at a cellular and molecular level, attribute specific cancers to associated genetic changes and determine the extent to which these changes are seen in a patient’s tumor.
Cancer cells contain genetic alterations compared to normal human cells. Common genetic abnormalities correlated to cancer include gains or losses of genetic material on specific chromosomal regions, or loci, or changes in specific genes, or mutations, which ultimately result in detrimental cellular changes followed by cancerous or pre-cancerous conditions. For example, multiple gains or losses of or on various chromosomes, and the rearrangement of genetic material among chromosomes, or chromosomal translocations, have been observed in different cancer types, such as HER2 in breast cancer and ALK rearrangements in NSCLC. In addition, mutations within gene sequences, or single nucleotide variations, can give rise to aberrant proteins that do not perform their functions correctly, leading to uncontrolled cell growth. Such genetic alterations can be a result of multiple factors, including genetic predisposition, environmental or lifestyle factors or viral infections. Importantly, these genetic changes can be used as biomarkers to help guide appropriate treatment. Detecting these biomarkers, particularly those representing drug targets, or those indicative of responsiveness or resistance of a tumor’s cells to specific therapies, helps clinicians to select drugs, design treatment regimens and optimize patient care and management. Tests that provide such predictive information have the potential to dramatically improve treatment outcomes for patients suffering from cancer.
67
Limitations of Traditional Cancer Diagnostic and Profiling Approaches
Cancer is difficult to diagnose and manage due to its heterogeneity at morphologic, genetic and clinical levels. Traditional methods of diagnosis for solid tumors, routinely used as the initial step in cancer detection, involve a tissue biopsy followed by a pathologist examining a thin slice of potentially cancerous tissue under a microscope. A recently obtained tissue sample is used in combination with chemical staining techniques to enable analysis of the biopsy. After staining, the pathologist determines through visual inspection whether the biopsy contains normal or cancerous cells, with those that are deemed cancerous being graded on a level of aggressiveness. Often an analysis of biomarkers relevant to that tumor type is also performed on the tissue, ranging from immunohistochemistry to FISH, to mutation analysis by various means such as microarrays and sequencing. After the diagnosis, a clinical workup is performed according to established guidelines for the specific cancer type. From there, the physician determines the stage of progression of the cancer based on a series of clinical measures, such as size, grade, metastasis rates, symptoms and patient history, and decides on a treatment plan that may include surgery, watchful waiting, radiation, chemotherapy, or stem cell transplant.
This type of analysis is dependent on the availability of a recently obtained tissue biopsy for the pathologist to analyze. Such a biopsy is often not available. A tumor may not be readily accessible for biopsy, a patient’s condition may be such that a biopsy is not advised, and for routine periodic patient monitoring to evaluate potential progression or recurrence, a biopsy is a fairly invasive procedure and not typically performed. As the length of time between when the original biopsy, diagnosis or surgery is conducted to the current evaluation of the patient increases, the likelihood that an original biopsy specimen is truly representative of the current disease condition declines, as does the usefulness of the original biopsy for making treatment decisions. This risk intensifies in situations where a drug therapy is being administered, because the drug can put selective pressure on the tumor cells to adapt and change.
Similarly, the heterogeneity referred to above means that different parts or areas of the same tumor can have different molecular features or properties. In evaluating a biopsy specimen, the pathologist will take a few thin slices of the tumor for microscopic review rather than exhaustively analyzing the whole tumor mass. The pathologist can only report on the tumor sections analyzed and if other parts of the tumor have different features, such as biomarkers corresponding to specific treatments, they can be missed. A more representative analysis of the entire tumor, as well as any metastases if they are present, is very helpful.
CTCs, ctDNA and Cancer
Circulating tumor cells, or CTCs, are cancer cells that have detached from the tumor matrix and invaded the patient’s blood or other bodily fluids. These cells are representative of the tumor and its metastases, and can function as their surrogates. Testing CTCs can complement pathologic information drawn from a biopsy or resected tissue sample, helping to insure that the analysis is comprehensive and not biased by tumor heterogeneity and sampling issues. They can also provide critical data when a biopsy is not possible. Clinical studies have demonstrated that the presence and number of CTCs provides information on the likely course of certain types of disease for the cancer patient, or in other words they are considered “prognostic.” Since CTCs are representative of the tumor, they can also be used for biomarker analysis, such as helping to guide therapy selection. Such analyses are “predictive” in that they offer insight into the likely responsiveness or resistance to particular therapies. After surgery and during any subsequent therapy or monitoring period, blood samples can periodically be drawn in a standard manner and analyzed to evaluate a therapy’s continuing effectiveness, as well as to detect other biomarkers such as new genetic mutations that may arise as a result of selection pressure by a particular therapy or by chance. Physicians can use this information to determine which therapy is most likely to benefit their patients at particular times through the course of their disease. Treatment decisions based on patient-specific information are the foundation of personalized medicine, and tests, or assays, that guide a physician in the selection of individualized therapy for a patient are termed “predictive assays.”
ctDNA is nucleic acid that is released into blood by dying tumor cells. Cell death occurs in all tissues, especially those that are rapidly dividing, and in cancer, where cell growth is not only rapid but also uncontrolled. Parts of tumors often outgrow their blood supply, resulting in cell death. Tumor cells dying as a result of therapy also release nucleic acid into blood. As a consequence, ctDNA is common in cancer patients and scientists believe that like CTCs, it may be more representative of a patient’s tumor than a few thin sections from a tissue biopsy, thus reducing the heterogeneity problem. ctDNA is found in the plasma component of blood and is readily accessible in a standard blood sample. Analyzing ctDNA for mutations that are used as biomarkers for therapy selection shows great promise. One of the strengths of this approach, in addition to not requiring a tissue biopsy, is that it is not dependent on capturing rare tumor cells from blood to provide a sample for testing. The difficulty with this approach is that the cellular context is lost since the ctDNA is mixed with a much larger amount of circulating DNA from normal cells that are continuously dying and being replaced in the body, thus making analysis challenging. This requires a mutation detection methodology with enhanced sensitivity and specificity, to distinguish mutations in particular gene regions in cancer cells from the normal gene sequence present in those same genes in normal cells which co-exist in blood as normal cells die and are replaced in the body. Our CEE-Selector technology provides this necessary sensitivity and specificity and creates an opportunity for ctDNA analysis to complement CTC analysis, or potentially to serve as the platform for stand-alone tests.
68
Given the incidence of cancer in the United States, with an estimated 925,000 new cases in 2014 for the major solid tumors targeted by our planned test products, the markets for our current and planned cancer diagnostic tests are very large. Furthermore, these market opportunities are even greater due to the benefits of CTC and ctDNA testing, including not only the ability to offer physicians a simple way to augment an initial tumor biopsy analysis but also to provide a means for relatively frequent monitoring of the tumor’s molecular status, utilizing a standard blood sample as a “liquid biopsy.” The latter application enables the physician to determine if or how a tumor is changing over time or is responding to therapy and what the next treatment should be. For example, in the United States, the incidence of new cases of breast cancer alone is estimated to be over 230,000 in 2014, and the prevalence of this disease is over 2.8 million (the number of women with a history of breast cancer in the United States, including women being treated and women who have finished treatment), with an estimated 330,000 lumpectomies performed annually in the United States. Of these lumpectomies, 20% need to be repeated because on pathological examination it is shown the procedure did not result in “clean margins,” thus suggesting not all the tumor was removed, according to a Johns Hopkins report. If a CTC test were performed at the time of initial diagnosis, at the time of surgery, or in lieu of, or as an adjunct to, a PET/CT scan (as a CTC test has the potential to identify a single tumor cell in a blood sample, while a scan requires a tumor mass of millions of cells to be detectable), to monitor disease progression or test for recurrence, thousands of tests, in breast cancer alone, could be performed per year with still relatively low market penetration.
Use of CTC- and ctDNA-Derived Biomarker Data in Cancer Treatment
CTCs and ctDNA are derived from, and are understood to be representative of, a solid tumor and its metastases and can be analyzed as adjuncts to or in place of the tumor, especially when a recent tumor biopsy is not available. This is also referred to as a liquid biopsy. In theory, almost any analysis that can be performed on tumor tissue can also be performed on CTCs, while ctDNA, because it is only nucleic acid, is more limited. We have focused our analysis of CTCs and ctDNA on known biomarkers associated with specific therapies to support treatment decisions and therapy selection made by physicians. The biomarkers we analyze and internal to analyze consist of proteins or protein modifications that can be identified by immunocytochemical means, cytogenetic or chromosomal aberrations, which are detected by FISH, and gene mutations which are detected in CTCs or ctDNA by molecular diagnostic tests, including CEE-Selector techniques and gene sequencing. Specific examples include (i) for immunocytochemistry, the detection of the estrogen receptor protein in breast cancer, indicative of the likely responsiveness to hormonal therapies like tamoxifen, often sold under the trade name Nolvadex®, (ii) for FISH, the presence of an amplified HER2 gene in breast cancer, indicative of the likely responsiveness to HER2-targeted agents like trastuzumab, often sold under the trade name Herceptin®, and (iii) for mutation detection, the presence of an EGFR activating mutation in NSCLC like L858R, indicative of the likely responsiveness to EGFR-targeted agents like Tarceva®. All of these biomarkers are currently tested on tumor tissue and can be tested on CTCs, and in the latter case on ctDNA. The resulting information could then be used to guide patient care, and specifically treatment selection.
To date these types of molecular and genetic detection methods have been successfully utilized to provide predictive information for several cancers, including breast, colon, NSCLC, melanoma and others in the form of companion diagnostics, typically performed on tumor tissue. CTC and ctDNA tests, which analyze the same biomarkers but in a more convenient standard blood sample test that also permits periodic monitoring, may be used in the same way.
Our Business Strategy
We plan to provide oncologists and other physicians with a straightforward means to profile and characterize their patients’ tumors on a real-time basis by analyzing CTCs and ctDNA found in standard blood draws. Biomarkers are currently detected and analyzed primarily in tissue biopsy specimens. We believe that our technology, which not only provides information on CTC enumeration but also the assessment of treatment-associated biomarkers identified within the CTCs or in ctDNA, will provide information to physicians that improves patient treatment and management and will become a key component in the standard of care for personalized cancer treatment.
Our approach is to develop and commercialize CTC and ctDNA tests and services to enable us to offer to oncologists standard blood sample based, real-time, testing solutions for a range of solid tumor types, starting with breast cancer and progressing to future launches of tests for NSCLC, gastric cancer, colorectal cancer, prostate cancer, melanoma and others, to improve patient treatment with better prognostic and predictive tools. To achieve this, we intend to:
|
· |
Develop and commercialize a portfolio of proprietary CTC and ctDNA tests and services, to enable physicians to develop personalized treatment plans. We intend to continue the development of additional prognostic and predictive tests and services to provide information that is essential to personalized cancer treatment. By including predictive information on biomarkers linked to specific therapies in our analysis in addition to CTC enumeration, our tests are designed to provide a more complete profile of a patient’s disease than existing CTC tests. The biomarker information will assist physicians in selecting appropriate therapies for individual patients. Our ctDNA tests are expected to offer enhanced sensitivity and specificity based on the CEE-Selector technology, enabling earlier detection of therapy-associated mutation targets or resistance markers, again supporting treatment decisions. We have launched our CTC tests, OncoCEE-BR for breast |
69
|
cancer, OncoCEE-GA for gastric cancer and OncoCEE-LU for NSCLC, performed in our CLIA-accredited testing facility. We are also developing a number of other CTC and ctDNA tests, including OncoCEE-CR for colorectal cancer, OncoCEE-PRTM for prostate cancer and OncoCEE-METM for melanoma. We plan to perform the necessary validation studies to allow us to commercialize these tests through our clinical laboratory. |
|
· |
Scale our internal sales and marketing capabilities. We are actively seeking additional partners to increase our market reach. Our specialized sales force with experience in cancer diagnostic testing focuses on key identified territories in order to provide geographic coverage throughout the United States. We have 8 sales representatives, and depending on test volume, expect to increase this group to 15-20 within two years and potentially 40-50 within five years. This team will educate physicians directly on the benefits of our tests and the clinical data supporting them, as well as provide support to and serve as technical specialists for our partners. |
|
· |
Develop and expand our collaborations with leading university hospitals and research centers. We collaborate with key thought leaders, physicians and clinical researchers, including those at The University of Texas MD Anderson Cancer Center, the Dana-Farber Cancer Institute, the University of California, San Diego, and Columbia University. Our collaborations enable us to test new technologies, validate the effectiveness and utility of our planned tests in a clinical setting and provide us access to clinically well-characterized and highly annotated patient data. These samples and data accelerate our validation process and facilitate the testing and refinement of our planned new tests. |
|
· |
Enhance our efforts in reaching and educating oncologists and other physicians about CTC and ctDNA tests. According to the State of Cancer Care in America 2014 Report, published in the Journal of Clinical Oncology in March of 2014 there were approximately 13,000 medical oncologists in the United States or 15,500 if gynecologic and pediatric oncologists are included. With the support of our key thought leader collaborators, we intend to focus on oncologists and other physicians who treat cancer patients by targeting our sales and marketing efforts on this important customer segment. We believe this will expand and optimize the oncology testing services and personalization of cancer treatment provided by oncologists and other physicians so that they can better serve their cancer patients. |
|
· |
Increase our efforts to provide biopharmaceutical companies and clinical research organizations with our current and planned CTC and ctDNA tests and services. Oncology drugs have the potential to be among the most personalized of therapeutics, yet oncology drugs have one of the worst approval rates, at 11% for leading indications and 2% for secondary indications of cancer drug compounds from first administration in humans to approval (2004-2011, Biotechnology Industry Organization). In an effort to improve the outcome of clinical trials for oncology drugs, and more rapidly advance targeted therapeutics, pharmaceutical and biopharmaceutical companies are increasingly looking to companies that have cancer diagnostic tests that specifically address their needs, including the ability to characterize and monitor a patient’s tumor over time using CTC and ctDNA tests to analyze biomarkers of interest. There are over 5,000 active trials in the United States in breast, lung, colorectal, prostate and gastric cancers and melanoma according to clinicaltrials.gov. We expect to increase our sales and marketing focus in this business as well as seek additional collaborations and partnerships with pharmaceutical and biopharmaceutical companies. |
|
· |
Conduct additional clinical studies of breast cancer, NSCLC and other CTC and ctDNA tests we plan to introduce. Clinical utility and validation studies for our planned ctDNA tests may rely on archived plasma or blood samples from clinical trials in which patient outcomes are already available, in a retrospective-prospective design that significantly shortens the length of such studies. |
|
· |
Continue to enhance our current and planned CTC and ctDNA tests and reduce the costs associated with providing them through internal research and development and partnering with leading technology developers and reagent suppliers. We intend to work closely with select key technology developers and suppliers to further automate the optical interpretation of our current tests and our planned additional CTC tests, including enumeration, immunocytochemical biomarker staining and FISH. We also intend to reduce the costs associated with key material components of these tests, including FISH probes. We have identified a technology group that, based on initial studies, can provide an automation system that will significantly reduce the hands-on time of our cytotechnicians for microfluidic channel analysis while increasing the uniformity, and potentially the sensitivity and quality, of the data we generate. This system is also expected to provide the ability to evaluate multiple fluorescent signals of different wavelengths simultaneously for multiplexed analysis, again enhancing efficiency. Similarly, we have identified suppliers that can provide FISH probes at reduced cost and with a broader choice of available fluors, enabling more extensive multiplexing of tests. |
Our Competitive Advantages
We believe that the competitive advantages of our tests, including our tests which are still under development, would include the following. In general, because OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA and our planned tests share our CEE platform, their competitive advantages would be the same.
70
OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA enable, and we anticipate our planned CTC and ctDNA tests will enable, detailed analysis of a patient’s cancer utilizing a standard blood sample, facilitating testing at any time, including when a biopsy is not available or inconclusive, offering real-time monitoring of the cancer and the response of the cancer to therapy, and allowing oncologists and other physicians to select timely modifications to treatment regimens. Because CTCs and ctDNA are derived from the primary tumor or its metastases, they function as surrogates for the tumor, with the advantage of being readily accessible in a standard blood sample. This is especially important in situations where a biopsy is not available or advised. The simplicity of obtaining a standard blood sample permits repeat testing in a monitoring mode to detect recurrence or progression and to offer information on treatment modifications based on a current assessment of the cancer’s properties.
OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA provide, and we anticipate our planned tests will provide, more information than competitors’ existing tests, including predictive information on biomarkers linked to specific therapies. We anticipate that such additional biomarker information will enable a physician to develop a personalized treatment plan. By including biomarker information in our analysis, in addition to CTC enumeration, our current tests and our planned tests are designed to provide a more complete profile of a patient’s disease than existing CTC tests. We intend for our tests to contain actionable information to assist physicians in selecting appropriate therapies for individual patients. Our ctDNA tests are expected to offer enhanced sensitivity and specificity based on the CEE-Selector technology, enabling earlier detection of therapy-associated mutation targets or resistance markers, again supporting treatment decisions.
OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA and our planned CTC tests are designed to capture and detect a broader range of CTCs than existing tests and to be applicable to, or quickly modifiable for, a wide range of cancer types. Our CEE-Cap antibody capture cocktail includes antibodies targeting not only EpCAM, the traditional epithelial CTC capture antigen utilized in the CellSearch® system and in other platforms, but also other epithelial antigens as well as mesenchymal and cancer stem cell antigens, indicative of cells having undergone the epithelial-to-mesenchymal transition. These cells may be more relevant for metastasis. Our detection methods include cytokeratin staining with a broader range of cytokeratin isotypes than existing CTC tests, and we plan to introduce our CEE-Enhanced staining which would enable detection of cells specifically captured with our antibody cocktail, including EMT cells lacking cytokeratin. We believe that through our planned CEE-Enhanced staining, more CTCs and different types of CTCs will be able to be identified and potentially at earlier stages of disease, resulting in fewer non-informative cases and more information for physicians.
OncoCEE-BR, Onco-CEE-LU, OncoCEE-GA are, and we anticipate our planned CTC and ctDNA tests will be, flexible and readily configurable to accommodate new biomarkers with clinical relevance as they are identified. In theory, our CEE platform permits essentially any analysis that is currently performed on tumor tissue to be performed on CTCs, including immunocytochemical staining, FISH and molecular analysis. As new therapies are approved, and to the extent that they are targeted therapies for which knowledge of a particular gene amplification event, mutation or presence, absence or modification, such as phosphorylation, of a protein are indicative of likely response or resistance to that therapy, we will be able to include them in our tests with minimal changes. This is attractive to pharmaceutical and biotechnology companies that are developing such therapies, or seeking ways to make their clinical trials more efficient, as this flexibility would enable them to focus on patients more likely to respond to a particular therapy and demonstrate a benefit from that therapy.
Collaborative relationships with physicians at The University of Texas MD Anderson Cancer Center. We have worked closely with a number of physicians at The University of Texas MD Anderson Cancer Center on various collaborative projects in different cancer types including breast, NSCLC, prostate, colorectal, ovarian, bladder, renal and endometrial. These projects provide us access to leading researchers, clinicians and key thought leaders, access to valuable patient samples and insight into clinical applications for our tests. Some of these projects have resulted in publications in leading journals, such as Cancer Discovery and Cancer Medicine, which enhances our standing in the oncology community and supports our marketing efforts.
Our planned CEE-Selector mutation tests would not be platform dependent. These tests are being designed to be able to be performed on almost any molecular instrument, which will provide flexibility in laboratory operations. To the extent we elect to develop these tests as IVDs, including pursuing CE marks for them to be marketed outside the United States, the ability to rapidly deploy them on different approved instrument platforms already in many laboratories should greatly simplify their distribution and commercialization.
71
Our Tests and Services
We have launched OncoCEE-BR for breast cancer, OncoCEE-GA for gastric cancer and OncoCEE-LU for NSCLC and plan to continue to launch a series of tests for CTCs in different tumor types, including colorectal and prostate cancers and melanoma, incorporating analyses for different biomarkers, over the next two years. OncoCEE-BR, OncoCEE-GA and OncoCEE-LU are and the planned tests will be based on the CEE technology platform. The CEE system isolates CTCs from blood samples of cancer patients for enumeration (or count) and genetic analysis. A sample is shipped to us in our specialized blood collection tube, called the CEE-Sure tube, for recovery and analysis of CTCs. When performing the CTC assay, the sample is processed in our laboratory. The specimen of blood is separated into its parts (red blood cells, buffy coat and plasma). The buffy coat is incubated with the antibody solution and passed through a proprietary microfluidic channel containing 9,000 microscopic posts coated with reagents to capture antibody-labeled tumor cells. The captured cells are suitable for further testing of whole cells directly in the microfluidic channel or by releasing the cells from the microfluidic channel and performing CEE-Selector or similar techniques.
Clinicians acknowledge limitations of currently available CTC test systems such as CellSearch® that rely on capture solely by anti-EpCAM antibodies and detection by anti-cytokeratin antibodies. Capture and detection based only on these two antigens is unlikely to identify all CTCs, and clinically this may result in no CTCs being detected in cases in which they are present. For example, some tumor cells that have been released into the circulatory system have undergone an EMT. These mesenchymal cells are less differentiated than epithelial cells and more similar to stem cells. OncoCEE-BR, Onco-CEE-LU and OncoCEE-GA enable, and we believe our planned assays will enable, the capture of significantly more CTCs than is accomplished through the use of traditional anti-EpCAM immuno-capture alone.
In addition to enhanced capture, our technology also improves the detection of CTCs. As with EpCAM, tumor cells that have undergone EMT can down-regulate the synthesis of cytokeratin, leading to an underestimate or even an apparent absence of CTCs since their positive identification has traditionally relied on anti-cytokeratin staining. We have developed alternative methods of fluorescent cell staining that are uniquely possible within the CEE system to enhance or enable detection of CTCs with low or no cytokeratin signal. This technology is called CEE-Enhanced. We believe that the combination of specific cocktails of tumor-associated capture antibodies and more sensitive fluorescent detection of CTCs through CEE-Enhanced methodology will lead to major advances in the capture, enumeration and analysis of CTCs. CEE-Enhanced methodology is expected to be included in our commercially available tests by mid-2015.
Analysis of CTCs performed by us incorporates both standard and proprietary methods. Immunocytochemistry which looks at proteins, analogous to the immunohistochemistry performed on tissues, can be readily applied and performed in the microfluidic channel, dependent only on suitable biomarkers. Similarly, FISH, used to evaluate cytogenetic abnormalities in cells, may be performed in our microfluidic channel using validated assays available from a number of vendors. For genetic mutation analysis, standard technologies can be applied. We have also developed proprietary CEE-Selector technology for mutation analysis in CTCs and ctDNA, with enhanced sensitivity and specificity.
CTCs are generally very rare and outnumbered many-fold by white blood cells. This complexity has been a challenge for standard technologies. We believe our CEE-Selector technology will offer enhanced specificity and sensitivity (greater than 1-in-10,000 of mutated sequence to normal sequence in a complex genetic background) compared to other approaches, and that it will potentially have broader application than just CTC analysis, including analysis of ctDNA in plasma, both in a CLIA-certified laboratory setting and as an IVD.
OncoCEE-BR, OncoCEE-GA and OncoCEE-LU are, and our planned tests would be, Laboratory Developed Tests. FDA clearance or approval is not currently required to offer these types of tests in our laboratory once they have been clinically and analytically validated. We seek licenses and approvals for our laboratory facility and for LDTs from the appropriate regulatory authorities, such as the Centers for Medicare & Medicaid Services, which oversees CLIA, and various state regulatory bodies. Certain states, such as New York, require us to obtain state licensure in order for us to perform testing on specimens taken from patients or received from ordering physicians from those states. In addition, our clinical reference laboratory is required to be licensed on a product-specific basis by New York as an out of state laboratory and our products, as laboratory developed tests, must be approved by the New York State Department of Health before they are offered in New York. As part of this process, the State of New York requires validation of our tests. We are currently in the process of addressing the requirements for licensure in New York, and we expect to have soon re-obtained all required licenses and approvals from all other states requiring licensure of out-of-state laboratories. (We were required to re-license in these other states as a result of our July 2013 reincorporation to Delaware.)
The following outline indicates our current and planned tests and indicates the stage the product is in and the targeted date of commercialization. As discussed in “Description of the Business—Test Development Process” below, prospective assays initially begin in research (stage 1) and progress through to development (stage 2), validation (stage 3) and finally availability for commercialization (stage 4). The OncoCEE-BR, OncoCEE-LU, OncoCEE-GA tests have completed all stages as to CTC and certain FISH test capabilities. Our remaining identified proposed tests have completed the research stage and are at the stages shown in the
72
table below with their respective estimated timetables for completing stage 4. As with all scientific endeavors, such timetables are only estimates; unanticipated problems might result in delays. We consider these timetables to be fairly aggressive, given the likelihood of our experiencing such unanticipated problems and associated delays.
In the development stage, there is still work to be done to finalize sensitivity and specificity of the assay. This work will vary as the assay is tested and fine-tuned in order to prepare it for validation and eventual commercial offering. In the validation stage, the assay has been fully developed and we are now able to run (or are in the process of running) a specific number of samples, both positive and negative, in order to validate that the assay results are reproducible. A validated assay is considered to have completed the availability for commercialization stage when the necessary training has been given and any necessary governmental licenses and approvals have been obtained so that we can start selling the assay through our commercial sales channel and provide patient results.
Our proposed tests have certain commonalities. For example, in each proposed test, biomarkers will be examined by one or both of FISH or CEE-Selector. Given the development, validation and commercialization of our first CTC/FISH test (OncoCEE-BR), all subsequent FISH- and Immunofluorescence-based assays have effectively been developed for the planned biomarker. Progression of these planned assays through stage 3 is largely dependent on the timing of our obtaining suitable validation specimens, although various scientific and other factors can also affect the pace of a particular proposed test’s progress through the validation stage. CTC-based OncoCEE-CR and OncoCEE-PR tests are targeted to be commercial in 2015 given our estimate of the timing to acquire appropriate positive and negative validation samples.
For ctDNA based assays, CEE-Selector will be used to detect each relevant mutation. CEE-Selector development was completed in 2014. Biomarker mutations (such as B-raf and K-ras) are often commonly seen in different tumor types, thus, once a particular mutation assay is developed for CEE-Selector, it can be applied to any tumor type. The OncoCEE-LU ctDNA test was our first CEE-Selector test to undergo validation. Given the nature of a molecular based test such as CEE-Selector, specimens can be batched and tested simultaneously, thereby reducing the validation time. All remaining currently proposed ctDNA tests will follow and are currently targeted to be commercial in 2015.
73
In “Use of Proceeds” above, we disclose that we currently intend to use approximately $ million of the net proceeds of this offering to fund further research and development and related activities. This includes all of the expenditures which we believe are needed to complete all four stages of development for the planned tests described below. Primarily these expenditures will be for existing and additional scientific personnel in the time periods reflected in the table below, and secondarily for obtaining a sufficient number of suitable validation specimens.
|
Test Name/ Solid Tumor Type |
Biomarkers |
Status of Test or Project |
Targeted Quarter of Availability for Commercialization |
|
OncoCEE-BRTM / Breast Cancer |
Enumeration, HER2 by FISH, ER |
Currently available |
N/A |
|
|
PR |
Validation |
2015 Q2 |
|
OncoCEE-LUTM / Lung Cancer |
Enumeration, ALK by FISH |
Currently available |
N/A |
|
OncoCEE-LUTM / Lung Cancer |
EGFR T790M mutation by CEE-SelectorTM |
Currently available |
N/A |
|
|
ROS1 by FISH |
Validation |
2015 Q2 |
|
|
EGFR L858R and Del19, K-ras, B-raf, and ALK mutations by CEE-SelectorTM |
Development and Validation |
2015 Q1, Q2, Q3 |
|
OncoCEE-GATM / Gastric Cancer |
Enumeration, HER2 by FISH |
Currently available |
N/A |
|
OncoCEE-CRTM / Colorectal Cancer |
Enumeration, EGFR by FISH |
Validation |
2015 Q2 |
|
|
K-ras and B-raf by CEE-SelectorTM |
Development |
2015 Q2 |
|
OncoCEE-PRTM / Prostate Cancer |
Enumeration, PTEN deletion and AR by FISH |
Validation |
2015 Q3 |
|
OncoCEE-METM / Melanoma |
Enumeration, B-raf and N-ras mutations by CEE-SelectorTM |
Development |
2015 Q3 |
|
|
PDL-1 by ICC |
Development |
2015 Q3 |
|
OncoCEE-DTCTM/ Breast and Prostate Cancer |
DTC analysis in bone marrow; HER2 and AR/PTEN by FISH, respectively |
Currently available for Research and Pharma |
N/A |
|
CEE-SelectorTM/ Sequencing application for multiple cancer types |
K-ras, B-raf, EGFR and other mutations detected in plasma. |
Development |
2015 Q3 |
Our Marketed OncoCEE CTC Tests
Our OncoCEE-BR breast cancer test was the first CTC test we developed and we are currently offering it to physicians through our CLIA laboratory. It is based on a standard blood sample and can be used at the time of diagnosis and for monitoring, including at the time of progression or recurrence. This allows the physician to characterize the tumor to help define treatment options, either augmenting tissue analysis or replacing it when a tumor biopsy is not available. The test currently includes CTC enumeration and determination of HER2 status by FISH and immunocytochemistry analysis of CTCs for estrogen receptor (ER) on the captured CTCs. HER2 status is used by physicians to determine suitability of a patient for treatment with HER2-targeted therapeutics. ER status provides information on suitability of breast cancer patients for endocrine or hormonal therapies. We plan to add immunocytochemistry analysis of CTCs for progesterone receptor to our OncoCEE-BR test, which will also provide information on suitability of breast cancer patients for endocrine or hormonal therapies.
74
OncoCEE-LU
Up to 25% of lung cancer patients, especially those diagnosed at Stage IIIB or Stage IV, do not have sufficient tissue for molecular profiling for various reasons, including tumor accessibility and status of the patient. In these cases, CTC and ctDNA tests are alternatives for obtaining more detailed information about the molecular status of the tumor that helps the physician select appropriate therapy. The OncoCEE-LU test’s biomarker analysis currently includes FISH testing for anaplastic lymphoma kinase, or ALK, c-ros oncogene 1, receptor tyrosine kinase, or ROS1, gene rearrangements and molecular analysis of the T790M mutation of the epidermal growth factor receptor or EGFR gene using our CEE-SelectorTM platform. We plan to add FISH testing for RET, MET, as well as mutation analysis for deletions 19 and l858R mutation in the ECFR gene, the K-ras gene and the B-raf gene in the future.
The L858R mutation of the EGFR gene and Exon 19 deletions are activators of EGFR kinase activity. The codon 12 and 13 mutations of the K-ras gene are linked to non-responsiveness to the EGFR kinase inhibitors, and the codon 600 mutations of the B-raf gene are linked to melanoma therapies in clinical trials for lung cancer. Our OncoCEE-LU test would be performed on a standard blood sample.
OncoCEE-GA
Our OncoCEE-GA test for gastric cancer is based on the identification of HER2 as a biomarker for this disease. We employ our CTC HER2 FISH test, which we previously developed for breast cancer, for the analysis of gastric cancer CTCs. Current clinical practice relies on a biopsy for tumor tissue analysis to detect elevated HER2, in the same manner as is done for breast cancer. Our tests circumvent this need for tissue, as well as providing straightforward monitoring of HER2 status from a standard blood sample, on a real-time basis during treatment.
Other OncoCEE CTC Tests in Development
We are now following a similar development path for additional OncoCEE CTC tests for other cancer types with a focus on large population solid tumor types, or cancers for which there are approved therapies that rely on biomarker tests we have previously developed. Examples of these tests include, OncoCEE-CRTM for colorectal cancer, OncoCEE-PR for prostate cancer, and OncoCEE-ME for melanoma, each described below.
OncoCEE-CR
Our current plan for our OncoCEE-CR test for colorectal cancer is to offer mutation testing analogous to that performed in lung cancer, namely detection of key mutations in the K-ras and B-raf genes, along with CTC enumeration. Testing on the K-ras gene would focus on codons 12 and 13 mutations. Testing on the B-raf gene would focus on V600 mutations. Our OncoCEE-CR test would be run on a standard blood sample.
This testing is important because certain targeted therapies for colorectal cancer, including the monoclonal antibodies targeting EGFR are ineffective in patients who have a K-ras mutation, which is found in up to 40% of cases according to the National Comprehensive Cancer Network. While for each of codons 12 and 13 in K-ras, up to 15-20 mutations have been reported, there are reports in the scientific literature that patients with one particular mutation, G13D, do respond well and that there may be variability in response to different chemotherapies based on the specific K-ras mutation, suggesting that detailed information on mutation status is clinically relevant.
OncoCEE-PR
Our OncoCEE-PR test for prostate cancer would be based on the analysis of CTCs found in a standard blood sample for key biomarkers: the androgen receptor, and phosphatase and tensin homolog (PTEN). The test would also include CTC enumeration, and our CEE-Cap antibody capture cocktail would be modified from that used for breast and lung cancer to include prostate specific membrane antigen.
The androgen receptor normally binds the hormones testosterone and dihydrotestosterone, and is the target for several drug molecules, including those acting directly as antagonists for the receptor and those acting indirectly through inhibition of androgen synthesis.
Phosphatase and tensin homolog, an enzyme that functions as a tumor suppressor, if mutated, deleted or otherwise functionally disrupted, removes a brake from cell replication and allows uncontrolled growth, which is seen in many cancers. If phosphatase and tensin homolog is mutated, deleted or disrupted, chemotherapy or polytherapy is usually recommended.
75
OncoCEE-ME
Our OncoCEE-ME melanoma test, performed on a standard blood sample, would provide information on the presence or absence and specific nature of the V600 mutation in the B-raf gene, which indicates whether the B-raf inhibitors are candidate therapies for the patient. CTC enumeration would also be a component of our test.
Disseminated Tumor Cell (DTC) Assays Performed on Bone Marrow
We have shown that our CEE-Sure blood collection tubes and CEE microfluidic channels work well with bone marrow samples, and we have further demonstrated the ability to perform FISH on disseminated tumor cells, or DTCs, from bone marrow that are isolated in this way. While bone marrow biopsies are not performed routinely in the United States, they are utilized in Europe, especially in prostate cancer. In addition, we were involved in a study at The University of Texas MD Anderson Cancer Center in which bone marrow was isolated from early stage operable breast cancer patients at the time of surgery. In this later study, published in Cancer Medicine (2013, 2(2) 226-233), we found a significant percentage of patients classified as HER2 negative by their primary tumor had HER2 positive DTCs, and hence could be considered for Herceptin® therapy. DTCs provide an interesting adjunct to CTC analysis that is well suited for our technology platform, and we plan to work with collaborators and key thought leaders to determine how best to introduce a series of tests based on a bone marrow sample type.
ctDNA Tests
We plan to introduce ctDNA tests for mutation analysis performed on blood plasma isolated from a standard blood sample using the CEE-Selector technology, based on increasing interest from physicians in this type of analysis. We plan to launch the first tests, for K-ras, B-raf and EGFR mutations, in conjunction with, or as a complement to, our OncoCEE-LU test. Tests for other mutations will be added as they are developed. These tests would be similar to those performed on CTCs but would instead focus on ctDNA in plasma. These tests would lack the cellular context provided by CTCs but would not require CTC isolation and would be simpler to perform. In addition, one of the benefits of this technology is its ability to detect and identify mutations in blood plasma. This indicates the importance of the enhanced sensitivity of the CEE-Selector technology and the ability of ctDNA tests to complement CTC tests.
Laboratory Testing
From our CLIA-certified laboratory in San Diego, California, we plan to provide test results from our current and planned CTC and ctDNA tests to oncologists and other physicians in community hospitals, cancer centers, group practices and offices. At the federal level, clinical laboratories, such as ours, must be certified under CLIA in order for us to perform testing on human specimens. Our laboratory is also accredited by CAP, which is one of six accreditation organizations approved by CMS under CLIA. Our clinical laboratory is located in California and we hold the requisite license from the California Department of Public Health to operate our laboratory. In addition, we hold licenses issued by the states of Florida, Maryland, Pennsylvania and Rhode Island to test specimens from patients in those states or received from ordering physicians from those states. In addition, our clinical reference laboratory is required to be licensed on a product-specific basis by New York as an out of state laboratory and our products, as laboratory developed tests, must be approved by the New York State Department of Health before they are offered in New York. As part of this process, the State of New York requires validation of our tests. We are currently in the process of addressing the requirements for licensure in New York, and we expect to have soon re-obtained all required licenses and approvals in all other states requiring licensure of out-of-state laboratories. (We were required to re-license in these other states as a result of our July 2013 reincorporation to Delaware.)
Clinical Trial Services
Industry research has shown many promising drugs have produced disappointing results in clinical trials. For example, a study by Princess Margaret Hospital in Toronto estimated that over a five-year study period 85% of the new therapies for solid tumors which were tested in early clinical trials in the United States, Europe and Japan failed, and that of those that survive through to Phase III trials only half will actually be approved. Given such a high failure rate of oncology drugs in clinical development, combined with constrained budgets for pharmaceutical and biopharmaceutical companies, there is a significant need for drug developers to utilize molecular diagnostics to help decrease these failure rates. For specific molecular-targeted therapeutics, the identification of appropriate biomarkers may help to optimize clinical trial patient selection and success rates by helping clinicians identify patients that are most likely to benefit from a therapy based on their individual genetic profile.
76
In addition to testing for physicians and their patients, we plan to offer clinical trials testing services to help increase the efficiency and economic viability of clinical trials for pharmaceutical and biopharmaceutical companies and clinical research organizations. Our clinical trial services will be aimed at developing customizable tests and techniques utilizing CTC and ctDNA technologies to provide sensitive, real-time characterization of individual patient’s tumors using a standard blood sample. These tests may be useful as, and ultimately developed into, companion diagnostics associated with a specific therapeutic. Additionally, through our services we may gain further insights into biomarkers for disease progression and drug resistance, as well as those associated with current drug development efforts, which we can incorporate into tests.
Test Development Process
Our OncoCEE-BR, OncoCEE-GA and OncoCEE-LU tests were, and our planned additional CTC and ctDNA tests are being, developed and validated in conjunction with leading academic and clinical research centers to ensure that the needs of the clinical community are being met with the latest research on key biomarkers that affect patient care. We utilize a research and validation process to help ensure that we are providing diagnostic, prognostic and predictive information that is clinically relevant and accurate. The time-frame for this process from design through development and market launch is dependent upon, among other things, the biomarkers in question having been discovered and validated before we incorporate them in a test, the specific clinical claims we plan to pursue, and the availability of high quality samples for validation. Our development protocol calls for us to monitor and review the process in four stages as detailed below:
|
· |
Stage 1, Research. We review known, validated biomarkers, preferably linked to a specific therapeutic or other high value treatment decision, and discuss with clinical collaborators and key thought leaders to characterize the opportunity, the specific clinical setting and the product profile of the candidate test. |
|
· |
Stage 2, Test Development. We design the test, which typically has two parts: efficient capture of CTCs and/or ctDNA from the targeted cancer type and development of the biomarker assays that will be included. For example, the first part may involve modification of the antibody capture cocktail and the second could include development of specific CEE-Selector mutation tests or testing of FISH probes. The test will be used on normal control specimens and clinical samples to assure performance and the process includes defining the performance characteristics of the test as well as developing standard protocols for our CLIA-certified, CAP accredited, and state-licensed laboratory, where the test will ultimately be performed. This assessment includes such features as reproducibility, accuracy, sensitivity, and specificity. |
|
· |
Stage 3, Clinical Validation. When the assay is performing as desired it is validated on clinical samples, typically in comparison to the existing gold standard for that biomarker, which is usually tumor tissue analysis. Depending on the tumor type and specimen requirement, samples are collected from patients through collaborators, or in the case of ctDNA tests, from sample banks, where clinical information on the patients, including outcomes, is already available. |
|
· |
Stage 4, Availability for Commercialization. As clinical validation is completed and before launch, we take several steps to prepare a test for marketing as a LDT. We create standard operating procedures and quality assurance and quality control measures to ensure repeatability and high standards of quality. We train both our commercial and laboratory staff on the interpretation and use of the data. Licenses and approvals for our laboratory to perform or use LDTs have been obtained from the appropriate regulatory authorities, such as CMS, which oversees CLIA, and different state regulatory bodies. |
Our CTC/FISH-based OncoCEE-BR, OncoCEE-GA and OncoCEE-LU tests, which have already launched, are considered to have completed this test development process. All other planned tests which are mentioned in this prospectus are all considered to currently be in Stage 2 or Stage 3 of this test development process.
We will be required to seek FDA clearance or approval to expand the commercial use of tests to other laboratories and testing sites in the United States. We will also need to complete additional activities to submit each of these tests for regulatory clearance or approval before commercialization in each of the international markets where we would plan to introduce them.
If the FDA finalizes its current draft guidance on a risk-based framework for regulation of LDTs, our process would also need to allow for obtaining FDA review, clearance or approval, as applicable, which would add delay, expense and risk to our current test development process.
Research and Development
We incurred research and development expenses of $3.1 million, which represents 2,299% of our net revenue, for the year ended December 31, 2013 and $3.4 million, which represents 5,931% of our net revenue, for the nine months ended September 30, 2014. Research and development expenses represented 54% of our total operating expenses for the year ended December 31, 2013 and
77
40% of our total operating expenses for the nine months ended September 30, 2014. Major components of the research and development expenses were direct personnel costs, laboratory equipment and consumables and overhead expenses.
Technology Development
In addition to developing new CTC and ctDNA tests for different cancers to be offered through our CLIA testing laboratory, and adapting additional predictive biomarkers to these tests as their importance is demonstrated by the scientific and clinical research communities, we continue to focus on improving the base technologies underlying our tests and processes. We are exploring various ways to improve CTC capture efficiency and detection, as well as approaches to sub-categorize CTCs into different populations that may have clinical relevance. For example, by determining which antigens individual CTCs expressed that enabled their capture, we could differentiate, and enumerate, various CTC phenotypes, for example, epithelial versus mesenchymal. We are also working to simplify the test process, and in general to provide a broader range of useful data on a patient’s cancer to assist the physician in determining an appropriate treatment. Some of these projects and initiatives include:
|
· |
Improve Ability to Capture CTCs |
|
· |
Continued modification and optimization of our CEE microfluidic channel as a way to further enhance CTC capture efficiency. Capture efficiency directly impacts sensitivity, informative rate, and the ability to perform accurate and reliable biomarker analyses on the CTCs, all of which increase the value of our offering. We are utilizing some of our early research experience to improve CTC capture rates and reduce background contamination from normal white blood cells. |
|
· |
Automation of Our Test Process |
|
· |
Development of automation throughout the test process, but particularly at the visual evaluation steps, which include enumeration, any immunocytochemistry for biomarkers beyond those used to identify CTCs, for example protein biomarkers, and FISH analysis, is a way to drive efficiencies, reduce costs, speed up turnaround time, and generate more reliable, uniform, and in some cases more sensitive data. We have identified an automation solution for the visual analysis, which is being validated in our CLIA laboratory. We have also adapted a semi-automated system for the separation, processing and washing steps before running a sample on the microfluidic channel, which is now being used in the research laboratory and similarly needs to be transferred and validated in the CLIA laboratory. These measures will reduce costs and time as well as allow for higher-throughput as sample volumes increase. |
|
· |
Development of Second Generation Platform for CTC Testing |
|
· |
Evaluating and developing techniques for CTC capture that take advantage of our CEE-Cap antibody capture cocktail and CEE-Enhanced staining technology to modify our current CTC process to a simpler, essentially IVD, format. In addition to reducing internal costs, such an advance would offer the opportunity for us to offer a product format that enable us to access the worldwide CTC testing market. The distribution of such kits could create a new business opportunity for us. |
|
· |
Utilization of CEE-Selector Technology for Highly Multiplexed Mutation Testing |
|
· |
The CEE-Selector technology should enable us to multiplex mutation testing such that larger panels of genes can be analyzed in a single step and interfaced with genetic sequencing. This should position us for the analysis at the molecular level of whole signaling pathways or enzyme cascades. We plan to take advantage of the sensitivity and specificity of the CEE-Selector technology and leverage interest in the clinical research community for detecting any actionable biomarker in a particular tumor, as opposed to only those that are known to occur at relatively higher frequencies in that type of tumor. Such multiplexed mutation tests, relying on our CEE-Selector technology, could provide a more global evaluation of a tumor through analysis of either CTCs or ctDNA. This would offer a broader range of potential treatment options as well as enable the monitoring of the effectiveness of those treatments over time. |
|
· |
Development of Single Cell CTC Isolation Techniques for Molecular Analysis |
|
· |
Tumor heterogeneity is a well-recognized problem for tissue analysis and is in part addressed by focusing on CTCs, which may provide a more universal sampling of a tumor. One result of this can be a diverse population of CTCs in a sample, with different phenotypes and genotypes represented. We are working with a collaborator on techniques for subsequent sorting of our highly enriched CTC samples released from our CEE microfluidic channels into pools of CTCs with similar phenotypes, and ultimately to single CTCs, for molecular analysis. |
78
Translational/Clinical Research
In the course of our research and validation studies, we have processed several hundred cancer patient samples and normal control samples for CTC enumeration and analysis. Our initial focus has been on breast cancer, where validation studies for the OncoCEE-BR test, including enumeration of CTCs compared to the CellSearch® system, and HER2 FISH performed on CTCs and compared with HER2 analysis performed on tumor tissue from the same patients, involved over 120 patient samples. The results of our validation studies, and the demonstration of a reliable and reproducible method for CTC capture and analysis using the OncoCEE platform were published in a paper entitled “Novel Platform for the Detection of Cytokeratin Positive (CK+) and Cytokeratin Negative (CK+) CTCs” appearing in the December 2011 issue of Cancer Discovery and a paper entitled “Efficient capture of circulating tumor cells with a novel immunocytochemical microfluidic device” appearing in the September 2011 issue of BioMicrofluidics.
Additional studies were conducted in breast and other tumor types, including lung, prostate and colorectal cancers, utilizing patient samples for comparison to the CellSearch® system. In head-to-head studies, the CEE system detected cytokeratin positive CTCs in comparable numbers of breast cancer patients, and in considerably more patients in the other cancer types (Cancer Discovery, December 2011). Moreover, the results clearly demonstrated that our use of the CEE-Cap capture antibody cocktail enabled recovery of more CTCs as compared to using only anti-EpCAM antibodies. This data served as a clinical validation study for CTC enumeration. When CEE-Enhanced staining is applied to detect cytokeratin-negative CTCs, we expect to see far more CTCs based on preliminary studies reported in a paper entitled “Detection of EpCAM-Negative and Cytokeratin-Negative CTCs in Peripheral Blood” appearing in the 2011 issue of the Journal of Oncology.
The CEE system has the added advantage of post-capture immunocytochemical, cytogenetic and molecular genomic analyses of the CTCs. The CEE system captured cells can be analyzed directly within the microfluidic channel, thereby removing the need to re-deposit cells on a slide, which could result in cell loss or damage. Furthermore, given the transparency of the microfluidic channel, it can be immediately analyzed on a microscope. Together these two important features allow for a very efficient process that is well suited for a LDT performed in a CLIA laboratory. The post-capture analyses, which focus on the evaluation of biomarkers, are particularly important and valuable to physicians and patients, as they focus on actionable information related to therapy selection. We have performed a number of clinical research studies in collaboration with The University of Texas MD Anderson Cancer Center investigators involving various tumor types, including breast, ovarian, endometrial, lung, colorectal, bladder and prostate cancers.
In a collaboration with physicians and researchers at The University of Texas MD Anderson Cancer Center, we evaluated matched samples of tumor tissue, blood for CTCs and bone marrow for DTCs in recently diagnosed breast cancer patients for evidence of HER2 amplification, which would indicate eligibility for HER2-targeted therapies like Herceptin®, a potentially life-saving treatment. These results were also presented at both the 2011 and 2012 annual meetings of the American Society of Clinical Oncology. In a study published in Cancer Medicine (2013, 2(2) 226-233) and involving 96 patients, HER2 positive CTCs and/or DTCs were identified in 18.8% of cases in which the primary tumor was HER2 negative. In the same cohort of patients, only 12.5% were HER2 positive in their primary tumor. In other words, beyond the 12 (of the 96) which traditional tumor tissue analysis had indicated could benefit from Herceptin-based therapy, the OncoCEE-BR test detected 18 (of the 96) patients who (despite the fact they were identified as being HER2 negative by primary-tumor testing) could benefit from Herceptin-based therapy. Patients classified as HER2 negative based on tumor tissue and found to have HER2 positive CTCs and/or DTCs will continue to be followed by our collaborators at The University of Texas MD Anderson Cancer Center to assess their overall and progression-free survival. Tumor heterogeneity is one likely cause of the discordance for HER2 status between tumor tissue and our test performed on blood and bone marrow samples. Tumor heterogeneity indicates an important clinical application for the OncoCEE-BR test, confirmation and crosschecking of the tissue analysis performed by the pathologist at the time of biopsy or surgery, especially if HER2 negative, with a CTC analysis derived from a standard blood sample.
Clinical utility studies, which demonstrate the specific clinical setting in which a particular CTC or ctDNA test is used, and how to use the information generated for medical, specifically treatment-related, decision making is a key part of our strategy and research and development plan. Data resulting from such studies is critical not only in the sales and marketing process, but also for reimbursement, as many payors now ask for peer-reviewed publications describing such studies and results before agreeing to coverage of a specific test. The study with Dana-Farber Cancer Institute is the first example of a clinical utility study for one of our tests and we plan to conduct additional studies in breast cancer and similar studies in NSCLC and other cancers for which we develop tests, including sponsoring such studies ourselves with some of the proceeds from this offering.
Sales and Marketing
Our sales organization currently consists of an initial group of 8 sales representatives placed in strategic locations around the country that have high concentrations of cancer patients, and we may, depending on test volume, potentially grow this number to 15-20 sales representatives within two years, and to 40-50 within five years. We have defined the initial sales territories and have hired sales professionals with an average of 10 years of successful experience in clinical oncology sales or oncology diagnostic testing sales
79
from leading biopharmaceutical, pharmaceutical or specialty reference laboratory companies. We plan on growing this specialized, oncology-focused sales force and supporting it with clinical specialists who bring significant technical knowledge in the use of CTC and ctDNA tests. We have also invested in sales headcount focusing on biopharma clinical trial opportunities.
Finally, we have invested in a managed care sales and marketing expert in order to pursue favorable payment and coverage for our testing. The key value proposition for these customers will be focused on cost savings by offering our tests as alternatives to expensive surgeries when tumor biopsy tissue is not available.
Our sales and marketing efforts are and will be based on a five-part marketing strategy:
|
· |
Work with oncologists, other physicians and group practices at community hospitals and cancer centers to educate them on the advantages and opportunities that CTC and ctDNA tests provide for better information, allowing them to select the most appropriate therapy for their patients, and how and when these tests are most effectively used; |
|
· |
Build relationships with key thought leaders in oncology, specifically in the cancers for which we are offering or plan to offer tests, to educate and support community oncologists; |
|
· |
Collaborate with leading research universities and institutions that enable the validation of our new tests, as well as the generation of clinical utility data; |
|
· |
Partner with pharmaceutical companies for clinical trial work focusing on CTC and ctDNA testing and analysis; and |
|
· |
Add value for the payor community by avoiding costly surgeries by providing the option of a simple blood test. |
We also take advantage of customary marketing channels commonly used by the diagnostic and pharmaceutical industries, such as medical meetings, broad-based publication of our scientific and clinical data, and the Internet. In addition, we provide easy-to-access information to our customers through our website and a data portal for physicians who wish to access test results electronically. Our customers value easily accessible information in order to quickly review their patients’ information and begin developing a treatment protocol.
Outside the United States
Outside the United States, where a central laboratory business model is less developed, we will evaluate opportunities with our existing and other partners for the conversion and/or development of our current and planned CTC and ctDNA tests to test systems or IVDs, and related strategies to develop and serve such regional oncology markets. We also plan to sell our clinical trial services to biopharmaceutical companies and research organizations outside the United States.
We plan to cooperate with partners on accessing markets internationally. We plan for this to be accomplished either through partnerships with local groups and distributors or the development of IVDs and/or test systems, including instrumentation.
Competition
As a cancer diagnostics company focused on current and planned tests for CTCs and ctDNA from standard blood samples, we rely extensively on our ability to combine novel technology and biomarker information with high-quality, state-of-the art clinical laboratory testing. We believe that we compete principally on the basis of:
|
· |
our ability to utilize standard blood samples, enabling testing of patients frequently through the course of their disease without a biopsy, thereby reducing cost and trauma, saving time, and providing real-time information on the current status of the tumor; |
|
· |
our ability to include biomarker information in our analysis, in addition to CTC enumeration, thereby providing a more complete profile of a patient’s disease than existing CTC tests can. This is actionable information that can assist physicians in selecting more personalized treatment plans for individual patients; |
|
· |
our current and planned CTC tests’ ability to capture and detect a broader range of CTC phenotypes than existing tests, and potentially at earlier stages of disease, resulting in fewer non-informative cases and more information for physicians. For example, our antibody capture cocktail targets not only EpCAM but also other epithelial antigens as well as mesenchymal and cancer stem cell antigens, indicative of cells having undergone the epithelial-to-mesenchymal transition. These cells may be more relevant for metastasis; |
|
· |
our ability to rapidly integrate new biomarkers, either validated in academic laboratories or of interest to pharmaceutical and biopharmaceutical companies in the context of their new therapies, into our current and planned tests, facilitating the expansion of actionable information for oncologists and other physicians; |
80
|
· |
our research and clinical collaborations with key academic and clinical study groups, which enhance our research and development resources and, by enhancing our standing in the oncology community, support our marketing efforts; and |
|
· |
our planned ctDNA tests based on the CEE-Selector technology are expected to offer enhanced sensitivity and specificity in detecting mutation targets or resistance markers, again supporting treatment decisions. |
We believe that we compete favorably with respect to these factors, although we cannot assure you that we will be able to continue to do so in the future or that new products or tests that perform better than our current and planned tests and services will not be introduced. We believe that our continued success depends on our ability to:
|
· |
expand and enhance our current and planned OncoCEE tests to provide clinically meaningful information in additional cancers; |
|
· |
work with clinicians to design and implement clinical studies that demonstrate the clinical utility of our products; |
|
· |
continue to innovate and maintain scientifically advanced technology; |
|
· |
successfully market and sell tests; |
|
· |
continue to comply with regulatory guidelines and obtain appropriate regulatory approvals in the United States and abroad as applicable; |
|
· |
continue to validate our pipeline of tests; |
|
· |
conduct or collaborate with clinical utility studies to demonstrate the application and medical value of our tests; |
|
· |
seek to obtain positive coverage and reimbursement decisions from Medicare and private third-party payors; |
|
· |
continue to enter into sales and marketing partnerships; |
|
· |
maintain existing and enter into new research and clinical collaborations with key academic and clinical study groups; |
|
· |
continue to attract and retain skilled scientific and clinical personnel; |
|
· |
continue to participate in and gain clinical trial work through biopharma partnerships; |
|
· |
receive payment for the testing we provide for patients; |
|
· |
obtain patents or other protection for our technologies, tests and services; and |
|
· |
obtain and maintain our clinical reference laboratory accreditations and licenses. |
Our principal competition comes from mainstream diagnostic methods, used by pathologists and oncologists and other physicians for many years, which focus on tumor tissue analysis. It may be difficult to change the methods or behavior of oncologists and other physicians to incorporate our CTC and ctDNA testing, including molecular diagnostic testing, into their practices in conjunction with or instead of tissue biopsies and analysis. In addition, companies offering capital equipment and kits or reagents to local pathology laboratories represent another source of potential competition. These kits are used directly by the pathologist, which can facilitate adoption. We plan to focus our marketing and sales efforts on medical oncologists rather than on pathologists.
We also face competition from companies that offer products or are conducting research to develop products for CTC or ctDNA testing in various cancers. In particular, Janssen Diagnostics, LLC markets its CellSearch® test and Atossa Genetics markets its ArgusCYTE® test, which are competitive to our OncoCEE-BR test for CTC enumeration, and HER2 analysis, respectively. However, the ArgusCYTE® test measures HER2 mRNA, which is not typically used for HER2 analysis, while we employ FISH for this analysis. FISH is generally considered to be the gold standard. CTC and ctDNA testing is a new area of science and we cannot predict what tests others will develop that may compete with or provide results similar or superior to the results we are able to achieve with the tests we develop. In addition to Janssen Diagnostics and Atossa Genetics, our competitors include public companies such as Alere (Adnagen) and Illumina as well as many private companies, including Apocell, EPIC Sciences, Clearbridge Biomedics, Cynvenio Biosystems, Fluxion Biosciences, RareCells, ScreenCell and Silicon Biosystems. Many of these groups, in addition to operating research and development laboratories, are establishing CLIA-certified testing laboratories while others are focused on selling equipment and reagents.
We expect that pharmaceutical and biopharmaceutical companies will increasingly focus attention and resources on the personalized cancer diagnostic sector as the potential and prevalence increases of molecularly targeted oncology therapies approved by the FDA along with companion diagnostics. For example, the FDA has recently approved three such agents—Xalkori® from Pfizer Inc. along with its companion anaplastic lymphoma kinase FISH test from Abbott Laboratories, Inc., Zelboraf® from Daiichi-Sankyo/Genentech/Roche along with its companion B-raf kinase V600 mutation test from Roche Molecular Systems, Inc. and
81
Tafinlar® from GlaxoSmithKline along with its companion B-raf kinase V600 mutation test from bioMerieux. These recent FDA approvals are only the second, third and fourth instances of simultaneous approvals of a drug and companion diagnostic. The first approval was the 2010 approval of Genentech’s Herceptin® for HER2 positive breast cancer along with the HercepTest from partner Dako A/S. Our competitors may invent and commercialize technology platforms or tests that compete with ours.
There are a number of companies which are focused on the oncology diagnostic market, such as Biodesix, Caris, Clarient, Foundation Medicine, Response Genetics, Neogenomics, Agendia, Genomic Health, and Genoptix, and which, while not currently offering CTC or ctDNA tests which are truly competitive with ours, are selling to the medical oncologists and pathologists. Large laboratory services companies, such as Sonic USA, Quest and LabCorp, provide more generalized cancer diagnostic testing.
Additionally, projects related to cancer diagnostics and genomics have received increased government funding, both in the United States and internationally. As more information regarding cancer genomics becomes available to the public, we anticipate that more products aimed at identifying targeted treatment options will be developed and that these products may compete with ours. In addition, competitors may develop their own versions of our current and planned tests in countries where we did not apply for patents or where our patents have not issued and compete with us in those countries, including encouraging the use of their test by physicians or patients in other countries.
Third-Party Suppliers and Manufacturers
Some of the components used in our current or planned products are currently sole-source, and substitutes for these components might not be able to be obtained easily or may require substantial design or manufacturing modifications. Any significant problem experienced by one of our sole source suppliers (particularly K.R. Anderson, Inc., which supplies a custom-packaged silicone compound used in our manufacturing) may result in a delay or interruption in the supply of components to us until that supplier cures the problem or an alternative source of the component is located and qualified. Any delay or interruption would likely lead to a delay or interruption in our manufacturing operations. The inclusion of substitute components must meet our product specifications and could require us to qualify the new supplier with the appropriate government regulatory authorities.
Patents and Technology
The proprietary nature of, and protection for, our products, services, processes, and know-how are important to our business. Our success depends in part on our ability to protect the proprietary nature of our products, services, technology, and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. We seek patent protection in the United States and internationally for our products, services and other technology. Our policy is to patent or in-license the technology, inventions and improvements that we consider important to the development of our business.
We also rely on trade secrets, know-how, and continuing innovation to develop and maintain our competitive position. We cannot be certain that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents granted to us in the future will be commercially useful in protecting our technology.
Our success depends on an intellectual property portfolio that supports our future revenue streams and erects barriers to our competitors. We are maintaining and building our patent portfolio through filing new patent applications, prosecuting existing applications, and licensing and acquiring new patents and patent applications.
Despite these measures, any of our intellectual property and proprietary rights could be challenged, invalidated, circumvented, infringed or misappropriated, or such intellectual property and proprietary rights may not be sufficient to permit us to take advantage of current market trends or otherwise to provide competitive advantages. For more information, please see “Intellectual Property Risks Related to Our Business.”
As of December 31, 2014, we owned 8 issued U.S. patents, 5 pending U.S. patent applications and corresponding patents and patent applications internationally. In addition, as of December 31, 2014, we co-owned 2 pending U.S. patent applications as well as corresponding foreign patents and applications. The patent portfolios for our leading programs as of December 31, 2014 are summarized below.
CEE Microfluidic Channels. We have 2 issued U.S. patents that are related to our current business, and a number of additional U.S. and foreign patent applications, which cover our microfluidic channel technology.
82
CEE-Sure Blood Collection Tubes. We have a U.S. patent application in prosecution for our CEE-Sure blood collection tubes, which contain reagents designed to prevent clumping of blood cells and CTCs that could clog the microfluidic channels and disrupt our assays.
CEE-Cap Antibody Capture Cocktail. We have 2 pending U.S. patent applications as well as their corresponding foreign patent applications directed to our antibody capture cocktail technology, which includes using antibodies to a number of tumor-associated antigens from cancer cells of both epithelial and mesenchymal phenotype, as well as cancer stem cells.
CEE-Enhanced Staining. We have 1 U.S. pending application as well as its corresponding foreign patent applications directed to this technology.
CEE-Selector Mutation Detection Technology. We co-own 2 pending U.S. patent applications with Aegea Biotechnologies, Inc., or Aegea. Under our agreement with Aegea, we have certain exclusive rights for oncology clinical testing and diagnostics as well as limited exclusive rights for oncology basic and clinical research. Aegea is responsible for the prosecution of 1 U.S. application and their corresponding foreign applications while we are responsible for the prosecution of the rest of U.S. applications and their corresponding foreign applications. Lyle J. Arnold, Ph.D., our Senior Vice-President of Research & Development and Chief Scientific Officer, is the controlling person of Aegea.
In addition to patents, we hold various U.S. registered trademarks, including a federal registration for the “CEE” mark, as well as several foreign registered trademarks and U.S. trademark applications for certain of our current and planned tests.
Operations and Production Facilities
Our research and development laboratories, our CLIA-certified, CAP accredited, and state-licensed diagnostic testing laboratory and our manufacturing facility are located in our San Diego, California headquarters. The laboratories employ commercial state-of-the-art equipment as well as custom-made components specific to our CTC process that are generated in a small in-house engineering shop. The manufacturing facility used for the production of our CEE microfluidic channels is a Class 10,000 suite in which polydimethylsiloxane is formed into the base of our proprietary microfluidic channels in a molding process. A glass cover slip suitable for optical analysis is added to seal the channels and make them watertight by making them reactive using plasma techniques. The inside of the microfluidic channels is subsequently chemically derivatized to enable the attachment of binding elements that strongly bind to antibody-tagged or coated CTCs. Because the microfluidic channels have micrometer dimensions, and we are seeking individual cells in a blood sample to interact with the surface of the microfluidic channel, dust particles and other microscopic debris that could clog the channel needs to be avoided.
The process of performing our tests is straightforward. When a health care professional takes a standard blood sample from a patient for CTC or ctDNA testing, he or she will place the blood sample in our CEE-Sure blood collection tubes, complete a requisition form, and package the specimen in our shipping kit for direct shipment to us. Once we receive the specimen at our laboratory and we enter all pertinent information about the specimen into our clinical laboratory information system, our laboratory technologists prepare the specimen for processing and analysis. Laboratory technologists, including clinical laboratory technologists and clinical laboratory scientists then conduct the analysis, including enumeration of CTCs and biomarker analysis such as FISH. The data, including images and the processed cells, are sent to our in-house or contracted pathologists or a commercialization partner’s pathologists who are experienced in the analysis and evaluation requested by the referring oncologist or pathologist.
After analysis, our in-house or contracted pathologists or a commercialization partner’s pathologists use laboratory information systems to prepare a comprehensive report, which may include selected relevant images associated with the specimen. Our Internet reporting portal allows a referring oncologist or pathologist to access his or her patient’s test results in real time in a secure manner that we believe to be compliant with HIPAA and other applicable standards. The reports are generated in industry standard .pdf formats which allows for high definition color images to be reproduced clearly. We send the results to the ordering physician and bill the payor through an arrangement we have with Xifin, Inc.
Quality Management Program
We are committed to providing reliable and accurate diagnostic testing to our customers. Accurate specimen identification, timely communication of test results, and prompt correction of errors, is critical. We monitor and improve our performance through a variety of methods, including performance improvement indicators, internal proficiency testing and external quality audits conducted by CAP. All quality concerns and incidents are subject to review and analysis, and our procedures are designed to ensure that we are providing the best services possible to our patients and customers. Protection of patient results from misuse and improper access is imperative and electronic and paper results are guarded via password-protection and identification cards.
83
We have established a Quality Management Program for our laboratory designed to help ensure accurate and timely test results, a consistent high quality of our testing services. The Quality Management Program documents the quality assurance and performance improvement plans and policies, the laboratory quality assurance and quality control procedures that are necessary to ensure that we offer the highest quality of diagnostic testing services. This program is designed to satisfy all the requirements necessary for local and state licensures and accreditation for clinical diagnostic laboratories by CAP. We follow the policies and procedures for patient and employee safety, hazardous waste disposal and fire codes stated in the general laboratory procedure manual. We believe that all pertinent regulations of CLIA, the Occupational Safety and Health Administration, the Environmental Protection Agency and the FDA are satisfied by following the established guidelines and procedures of our Quality Management Program.
In addition to the compulsory proficiency programs and external inspections required by CMS and other regulatory agencies, we have developed a variety of internal systems and procedures to emphasize, monitor and continuously improve the quality of our operations. We maintain internal quality controls by routinely processing specimens with known diagnoses in parallel with patient specimens. We also have an internally administered proficiency program for specimen testing.
The CAP accreditation program involves unannounced on-site inspections of our laboratories. CAP is an independent, non-governmental organization of board-certified pathologists that accredits laboratories nationwide on a voluntary basis and that has been recognized by CMS as an accreditation organization to inspect laboratories to determine adherence to the CLIA standards.
Third-Party Payor Reimbursement
Revenues from our clinical laboratory testing are derived from several different sources. Depending on the billing arrangement, the instruction of the ordering physician and applicable law, parties that reimburse us for our services include:
|
· |
third-party payors that provide coverage to the patient, such as an insurance company, a managed care organization or a governmental payor program; |
|
· |
physicians or other authorized parties, such as hospitals or independent laboratories, that order the testing service or otherwise refer the services to us; |
|
· |
patients in cases where the patient has no insurance, has insurance that partially covers the testing, or owes a co-payment, co-insurance or deductible amount; |
|
· |
collaboration partners ; or |
|
· |
biopharmaceutical companies, universities or researchers for clinical trial work. |
We are reimbursed for two categories of testing, anatomic pathology, which includes cell staining and the enumeration component of CTC tests, FISH, immunocytochemistry and immunofluorescence, and molecular pathology, which includes mutation analysis. Reimbursement under the Medicare program for the diagnostic services that we offer is based on either the Medicare Physician Fee Schedule or the Medicare Clinical Laboratory Fee Schedule, each of which is subject to geographic adjustments and is updated annually. Medical services provided to Medicare beneficiaries that require a degree of physician supervision, judgment or other physician involvement, such as pathology services, are generally reimbursed under the Medicare Physician Fee Schedule, whereas clinical diagnostic laboratory tests are generally reimbursed under the Medicare Clinical Laboratory Fee Schedule. Some of the services that we provide are genetic and molecular testing, which are reimbursed as clinical diagnostic laboratory tests.
Regardless of the applicable fee schedule, Medicare payment amounts are established for each CPT code. In addition, under the Clinical Laboratory Fee Schedule, Medicare also sets a cap on the amount that it will pay for any individual test. This cap, usually referred to as the National Limitation Amount, is set at a percentage of the median of all the contractor fee schedule amounts for each billing code.
Medicare also has policies that may limit when we can bill directly for our services and when we must instead bill another provider, such as a hospital. When the testing that we perform is done on a specimen that was collected while the patient was in the hospital, as either an inpatient or outpatient, we may be required to bill the hospital for clinical laboratory services and for the technical component of pathology services. Which party is to be billed depends primarily on whether the service was ordered at least 14 days after the patient’s discharge from the hospital. Complying with these requirements is complex and time-consuming and may affect our ability to collect for our services. In addition, hospitals may refuse to pay our invoices or may demand pricing that negatively affects our profit margin.
Medicare requires a beneficiary to pay a 20% co-insurance amount for services billed under the Physician Fee Schedule. Medicare covers the remaining 80%. There is currently no patient co-payment or co-insurance amount applicable to testing billed under the Clinical Laboratory Fee Schedule. Patients often have supplemental insurance policies that cover the co-insurance amount for physician services.
84
Medicare has coverage policies that can be national or regional in scope. Coverage means that the test or assay is approved as a benefit for Medicare beneficiaries. If there is no coverage, neither the supplier nor any other party, such as a reference laboratory, may receive reimbursement from Medicare for the service. There is currently no national coverage policy regarding the CTC capture/enumeration portion of our testing. Because our laboratory is in California, the regional Medicare Administrative Contractor, or MAC, for California is the relevant MAC for all our testing. The previous MAC for California, Palmetto GBA, LLC, adopted a negative coverage policy for CTC capture/enumeration. The current MAC for California, Noridian Healthcare Solutions, LLC, is adopting the coverage policies from Palmetto GBA. Therefore the capture/enumeration portion of our OncoCEE testing is not currently covered and we will receive no payment from Medicare for this service unless and until the coverage policy is changed. On November 4, 2013, we submitted a comprehensive dossier explaining to Palmetto GBA and Noridian the benefits of the capture/enumeration testing in order to seek to persuade the MACs to allow coverage for this portion of our testing. Palmetto GBA responded on November 27, 2013, denying our request for Medicare coverage for the CTC capture/enumeration portion of our OncoCEE testing. We have not received any other indications to suggest that the negative coverage determination will be reversed. We intend to continue our efforts to obtain Medicare coverage for capture/enumeration.
Reimbursement rates paid by private third-party payors can vary based on whether we are considered to be an “in-network” provider, a participating provider, a covered provider, an “out-of-network” provider or a non-participating provider. These definitions can vary among payors, but we are generally considered an “out-of-network” or non-participating provider by the vast majority of private third-party payors. An in-network provider usually has a contract with the payor or benefits provider. This contract governs, among other things, service-level agreements and reimbursement rates. In certain instances an insurance company may negotiate an in-network rate for our testing. An in-network provider may have rates that are lower per test than those that are out-of-network, and that rate can vary widely. The rate varies based on the payor, the testing type and often the specifics of the patient’s insurance plan. If a laboratory agrees to contract as an in-network provider, it generally expects to receive quicker payment and access to additional covered patients.
Billing and Billing Codes for Third-Party Payor Reimbursement
CPT codes are the main data code set used by physicians, hospitals, laboratories and other health care professionals to report separately-payable clinical laboratory and pathology services for reimbursement purposes. The CPT coding system is maintained and updated on an annual basis by the American Medical Association. We believe there are existing codes that describe nearly all of the other steps in our testing process. We currently use a combination of different codes to bill for our testing and analysis. Many of the CPT codes used to bill for molecular pathology tests such as those used for our OncoCEE-LU test were significantly revised by the CPT Code Editorial Panel effective January 1, 2013. These new codes replace the more general “stacking” codes that were previously used to bill for these services with more test-specific codes. In the Physician Fee Schedule Rule issued in November 2012, CMS stated that it had determined it would pay for the new codes as clinical laboratory tests under the Medicare Clinical Laboratory Fee Schedule. CMS has also started a process to “gapfill” the new codes. In other words, it will ask each of the MACs to determine a reasonable price for each of the new codes.
Changes in coding and reimbursement methods could have an adverse impact on our revenues going forward. However, we are currently working with our billing consultants to determine what will be required by the new coding changes. The elimination of the “stacking” codes will require us to either use the new more specific codes where applicable effective January 2013, or to use other “Not Otherwise Classified” codes when billing. The implementation of these new codes will vary from payor to payor, and it is too early to assess the impact, if any, that the migration to the new codes may have on our results of operations. The introduction of the new codes by CMS, in combination with the other actions it is considering with regard to pricing, could result in a reduction in the payments that we receive for our current tests and our planned future tests and make it more difficult to obtain coverage from Medicare or other payors. There can be no guarantees that Medicare and other payors will establish positive or adequate coverage policies or reimbursement rates.
We are moving forward with plans to obtain reimbursement coverage for the capture/enumeration components of OncoCEE-BR, OncoCEE-GA, OncoCEE-GA and our planned CTC tests. For other components and types of testing provided or anticipated to be provided by us, specific CPT codes were provided by the American Medical Association in January 2013 or we are able to utilize existing CPT codes from the Medicare Physician Fee Schedule. For these established CPT codes (for example, the codes for FISH and immunocytochemistry, or ICC), positive coverage determinations have been adopted as part of national Medicare policy or under applicable Local Coverage Determinations. Specific codes for our tests, however, do not assure an adequate coverage policy or reimbursement rate. Please see the section entitled “Legislative and Regulatory Changes Impacting Clinical Laboratory Tests” for further discussion of certain legislative and regulatory changes to these billing codes and the anticipated impact on our business.
85
Coverage and Reimbursement for our Current Tests and our Planned Future Tests
Because of our previous relationship with Clarient, under which Clarient had responsibility for billing and reimbursement until mid-2013, we do not have established coverage and reimbursement policies set with all third-party payors. Our Medicare Administrative Contractor has issued a negative coverage determination for the capture/enumeration component of all CTC tests. We have received reimbursement for the capture/enumeration component of our tests from some private payors, including major private third-party payors, based on submission of standard CPT codes. FISH, ICC and Molecular Testing CPT codes are the subject of positive coverage national or local Medicare determinations. We believe these codes can be used to bill for the analysis components of our current and anticipated CTC tests.
We expect these analysis components to have a significantly greater reimbursement value than the capture/enumeration components of our current and anticipated CTC tests, based on a comparison of what we believe CellSearch® capture/enumeration reimbursement rates currently are, versus existing reimbursement rates for analysis components such as FISH and ICC analysis and molecular testing.
We believe, based on research showing that approximately 54% of new cancers occur in persons age 65 and older and that almost all Americans age 65 and older are enrolled in Medicare, that a substantial portion of the patients for whom we would expect to perform cancer diagnostic tests will have Medicare as their primary medical insurance. Only in November 2013 did we first directly bill any payor for physician-ordered testing; until May 2013, our commercialization partner Clarient was responsible for all billing associated with our tests. We do not have data for Clarient’s billing and collection experience with respect to our tests, because Clarient paid us a contracted amount per test performed regardless of their billing and collections. From May to December 2013, we performed an average of 1-3 physician-ordered tests per month, and from July to September 2014, we performed an average of 32 physician-ordered tests per month (in addition to the tests which we have been performing since January 2013 for a clinical utility study with investigators at the Dana-Farber Cancer Institute, with an average of 15-30 tests per month performed during the trial’s enrollment period through May 2014). Billing for these physician-ordered tests is now handled for us by a non-Clarient billing service provider. Since May 2013, we invoiced, through this service provider, for 218 physician-ordered tests. Of these, 37 tests were billed to Medicare and the remainder were billed to other payors. As of December 2014, we have been paid by private payors for 66 of these tests, with an average price collected of $1,165 per test, while we have not yet had any response or adjudication from any payor as to the other bills submitted. Accordingly, we do not yet have any data regarding reimbursement history or collectability experience. To date, all of our revenue recognized has come from private payors. Medicare claims have not yet been processed due to a new application process due to a change in our tax identification number. We cannot assure you that, even if OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests are otherwise successful, reimbursement for the currently Medicare-covered portions of OncoCEE-BR, OncoCEE-LU, OncoCEE-GA and our planned tests would, without Medicare reimbursement for the capture/enumeration portion, produce sufficient revenues to enable us to reach profitability and achieve our other commercial objectives.
Where there is a private or governmental third-party payor coverage policy in place, we bill the payor and the patient in accordance with the established policy. Where there is no coverage policy in place, we pursue reimbursement on a case-by-case basis. Our efforts in obtaining reimbursement based on individual claims, including pursuing appeals or reconsiderations of claims denials, could take a substantial amount of time, and bills may not be paid for many months, if at all. Furthermore, if a third-party payor denies coverage after final appeal, payment may not be received at all. We are working to decrease risks of nonpayment by implementing a revenue cycle management system.
We cannot predict whether, or under what circumstances, payors will reimburse for all components of our tests. Payment amounts can also vary across individual policies. Full or partial denial of coverage by payors, or reimbursement at inadequate levels, would have a material adverse impact on our business and on market acceptance of our tests.
Legislative and Regulatory Changes Impacting Clinical Laboratory Tests
From time to time, Congress has revised the Medicare statute and the formulas it establishes for both the Medicare Clinical Laboratory Fee Schedule and the Medicare Physician Fee Schedule. The payment amounts under the Medicare fee schedules are important because they not only determine our reimbursement under Medicare, but those payment amounts are also often used as a basis for payment amounts set by other governmental and private third -party payors. For example, state Medicaid programs are prohibited from paying more than the Medicare fee schedule limit for clinical laboratory services furnished to Medicaid recipients.
Under the statutory formula for Medicare Clinical Laboratory Fee Schedule amounts, increases are made annually based on the Consumer Price Index for All Urban Consumers as of June 30 for the previous twelve-month period. From 2004-2008, Congress eliminated the Consumer Price Index for All Urban Consumers update in the Medicare Prescription Drug, Improvement and Modernization Act of 2003. In addition, for years 2009 through 2013, the Medicare Improvements for Patients and Providers Act of 2008 mandated an approximately 0.5% cut to the Consumer Price Index for All Urban Consumers update. Accordingly, the update for
86
2009 was reduced to 4.5% and negative 1.9% for 2010. The ACA has, among other things, imposed additional cuts to the Medicare reimbursement for clinical laboratories. The ACA replaced the 0.5% cut enacted by the Medicare Improvements for Patients and Providers Act with a “productivity adjustment” that will reduce the Consumer Price Index update in payments for clinical laboratory tests. In 2011, the productivity adjustment was -1.2%. In addition, the ACA includes a separate 1.75% reduction in the CPI update for clinical laboratories for the years 2011 through 2015. The MCTRJCA, enacted in 2012, mandated an additional change in reimbursement for clinical laboratory service programs. This legislation requires CMS to reduce the Medicare Clinical Laboratory Fee Schedule by 2% in 2013, which in turn will serve as a base for 2014 and subsequent years. CMS has projected that because of the changes required by ACA and MCTRJCA, payment for clinical laboratory services will go down by approximately 3% by 2013.
With respect to our diagnostic services for which we expect to be reimbursed under the Medicare Physician Fee Schedule, because of the statutory formula the rates would have decreased for the past several years if Congress failed to intervene. In the past, when the application of the statutory formula results in lower payment, Congress has passed interim legislation to prevent the reductions. In November 2013, CMS issued its 2014 Physician Fee Schedule Final Rule, or the 2014 Final Rule. In the 2014 Final Rule, CMS called for a reduction of approximately 23.7% in the 2014 conversion factor that is used to calculate physician reimbursement. If in future years Congress does not adopt interim legislation to block or offset, and/or CMS does not moderate, any substantial CMS-proposed reimbursement reductions, the resulting decrease in payments from Medicare could adversely impact our revenues and results of operations. In addition, for 2012, CMS requested that the American Medical Association’s Relative Value Scale Update Committee reexamine the relative values of certain codes, including FISH codes. The Relative Value Scale Update Committee is an expert panel that provides relative value recommendations to CMS for use in annual updates to the Medicare Physician Fee Schedule. These relative values are used by CMS to determine payments, and CMS seeks to assess whether such codes are misvalued and an adjustment is necessary. In July 2013 CMS published the proposed Physician Fee Schedule for 2014. As part of that proposed rule, CMS sought to decrease payment for approximately 200 CPT codes, including those for certain anatomic and molecular pathology services, to make payments to independent laboratories and hospital outpatient departments consistent. The proposed rates were generally lower than the current rates paid to independent laboratories and physicians for the same services. For example, CMS proposed to decrease the reimbursement rate for the technical component of FISH analysis by 47%. In fact, the 2014 Final Rule as adopted left FISH reimbursement rates for independent laboratories and physicians essentially unchanged from 2013 reimbursement levels.
In addition, the 2014 Final Rule included both increases and decreases in certain relative value units and geographic adjustment factors used to determine reimbursement for a number of codes used in our current tests and our planned future tests. These codes describe services that we must perform in connection with our tests and we bill for these codes in connection with the services that we provide.
Under the Protecting Access to Medicare Act of 2014, or PAMA, which was signed to law in April 2014, there will be major changes to the payment formula under the Medicare Clinical Laboratory Fee Schedule, or CLFS. Beginning January 1, 2016, clinical laboratories must report laboratory test payment data for each Medicare-covered clinical diagnostic lab test that it furnishes during a time period to be defined by future regulations. The reported data must include the payment rate (reflecting all discounts, rebates, coupons and other price concessions) and the volume of each test that was paid by each private payor (including health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations). Beginning in 2017, the Medicare payment rate for each clinical diagnostic lab test will be equal to the weighted median amount for the test from the most recent data collection period. The payment rate will apply to laboratory tests furnished by a hospital laboratory if the test is separately paid under the hospital outpatient prospective payment system. It is too early to predict the impact of this federal legislation on reimbursement for our products.
Further, with respect to the Medicare program, Congress has proposed on several occasions to impose a 20% coinsurance charge on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. Because of the relatively low reimbursement for many clinical laboratory tests, in the event that Congress were to ever enact such legislation, the cost of billing and collecting for these services would often exceed the amount actually received from the patient and effectively increase our costs of billing and collecting.
Some of our Medicare claims may be subject to policies issued by Palmetto GBA and Noridian Healthcare Solutions, our former and current Medicare Administrative Contractor for California, respectively. Palmetto GBA, acting on behalf of many MACs, recently issued a Local Coverage Decision that affects coverage, coding and billing of many molecular diagnostic tests. Under this Local Coverage Determination, Palmetto GBA will not cover any molecular diagnostic tests, such as the capture/enumeration component of our current tests and our planned future tests, unless the test is expressly included in a National Coverage Determination issued by CMS or a Local Coverage Determination or coverage article issued by Palmetto GBA. Currently, laboratories may submit coverage determination requests to Palmetto GBA for consideration and apply for a unique billing code for each test (which is a separate process from the coverage determination). In the event that a non-coverage determination is issued, the laboratory must wait six months following the determination to submit a new request. In addition, effective January 1, 2013, Palmetto GBA implemented its new Molecular Diagnostic Services Program, under which, among other things, laboratories must use the newly-assigned billing codes
87
specific to the test (as implemented by the American Medical Association), in order to receive the indicated reimbursement amounts. Reimbursement amounts under these new single molecular diagnostics billing codes were in some cases lower, and in some cases higher, than amounts allowed by Medicare before January 1, 2013, but most were significantly lower. Palmetto GBA currently has a negative coverage determination for the capture/enumeration component of CTC tests such as our current and anticipated CTC tests, but there is no such negative coverage determination for the analysis component of such CTC tests. Denial (or continuation of denial) of coverage for the capture/enumeration component of our current and anticipated CTC tests by Palmetto GBA or its successor MAC, Noridian Healthcare Solutions, or reimbursement at inadequate levels, would have a material adverse impact on our business and on market acceptance of our current tests and our planned future tests. Noridian Healthcare Solutions intends to follow, for CTC tests, the positive or negative coverage determinations which from time to time Palmetto GBA makes. On November 27, 2013, Palmetto GBA denied our request for coverage for the enumeration/detection portion of our OncoCEE testing. We intend to continue our efforts to obtain Medicare coverage for capture/enumeration.
Additionally, the Centers for Disease Control and Prevention, or CDC, CMS and the Office of Civil Rights, or OCR, issued a final rule in February 2014 to amend both the HIPAA and CLIA regulations. The final rule amended the HIPAA privacy rule to remove the CLIA laboratory exceptions, and as a result, HIPAA-covered laboratories are now required to provide individuals, upon request, with access to their completed test reports. Similarly, the final rule amended CLIA to state that CLIA laboratories and CLIA-exempt laboratories may provide copies of the patient’s completed rest reports that, using the laboratory’s authentication process, can be identified as belonging to that patient. Compliance was required to begin no later than October 6, 2014.
Governmental Regulations
Clinical Laboratory Improvement Amendments of 1988 and State Regulation
As a provider of laboratory testing on human specimens for the purpose of diagnosis, prevention, or treatment, we are required to hold certain federal, state and local licenses, certifications and permits to conduct our business. In 1988, Congress enacted CLIA, which established quality standards for all laboratories providing testing to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test was performed. Our laboratory holds a CLIA certificate of accreditation. As to state laws, we are required to meet certain laboratory licensing and other requirements. Our laboratory holds the required licenses from the applicable state agencies in which we operate. For more information on state licensing requirements, see the sections entitled see the section entitled “Description of the Business—Governmental Regulations—California State Laboratory Licensing” and “Description of the Business—Governmental Regulations—Other States’ Laboratory Licensing.”
Under CLIA, a laboratory is defined as any facility which performs laboratory testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the impairment of, or assessment of health of human beings. CLIA also requires that we hold a certificate applicable to the complexity of the categories of testing we perform and that we comply with certain standards. CLIA further regulates virtually all clinical laboratories by requiring they comply with various operational, personnel, facilities administration, quality and proficiency testing requirements intended to ensure that their clinical laboratory testing services are accurate, reliable and timely. CLIA certification is also a prerequisite to be eligible to be reimbursed for services provided to state and federal health care program beneficiaries. CLIA is user-fee funded. Therefore, all costs of administering the program must be covered by the regulated facilities, including certification and survey costs.
We are subject to survey and inspection every two years to assess compliance with program standards, and may be subject to additional unannounced inspections. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing less complex tests. In addition, a laboratory like ours that is certified as “high complexity” under CLIA may obtain analyte specific reagents, which are used to develop LDTs.
In addition to CLIA requirements, we must comply with the standards set by CAP, which accredits our laboratory. Under CMS requirements, accreditation by CAP is sufficient to satisfy the requirements of CLIA. Therefore, because we are accredited by CAP, we are deemed to also comply with CLIA. CLIA also provides that a state may adopt laboratory regulations that are more stringent than those under federal law, and certain states have implemented their own more stringent laboratory regulatory schemes.
Federal, State and Foreign Fraud and Abuse Laws
A variety of federal and state laws prohibit fraud and abuse. These laws are interpreted broadly and enforced aggressively by various state and federal agencies, including CMS, the Department of Justice, the Office of Inspector General for HHS, and various state agencies. In addition, the Medicare and Medicaid programs increasingly use a variety of contractors to review claims data and to identify improper payments as well as fraud and abuse. These contractors include Recovery Audit Contractors, Medicaid Integrity Contractors and Zone Program Integrity Contractors. In addition, CMS conducts Comprehensive Error Rate Testing audits, the purpose of which is to detect improper Medicare payments. Any overpayments identified must be repaid unless a favorable decision is
88
obtained on appeal. In some cases, these overpayments can be used as the basis for an extrapolation, by which the error rate is applied to a larger universe of claims, and which can result in even higher repayments.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, receiving, or providing remuneration, directly or indirectly, to induce or in return for either the referral of an individual, or the furnishing, recommending, or arranging for the purchase, lease or order of any health care item or service reimbursable, in whole or in part, under a federal health care program. The definition of “remuneration” has been broadly interpreted to include anything of value, including gifts, discounts, credit arrangements, payments of cash, ownership interests and providing anything at less than its fair market value. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements within the health care industry, the Office of Inspector General for HHS has issued a series of regulatory “safe harbors.” These safe harbor regulations set forth certain requirements that, if met, will assure immunity from prosecution under the federal Anti-Kickback Statute. Although full compliance with these provisions ensures against prosecution under the federal Anti-Kickback Statute, the failure of a transaction or arrangement to fit within a specific safe harbor does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the federal Anti-Kickback Statute will be pursued. For further discussion of the impact of federal and state health care fraud and abuse laws and regulations on our business, see the section entitled “Risk Factors—Regulatory Risks Relating to Our Business.” We are subject to federal and state health care fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws.
In addition, HIPAA also created new federal crimes, including health care fraud and false statements relating to health care matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including private third-party payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from federal health care programs, such as the Medicare and Medicaid programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from federal health care programs.
Finally, another development affecting the health care industry is the increased enforcement of the federal False Claims Act and, in particular, actions brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government and permit such individuals to share in any amounts paid by the entity to the government in fines or settlement. In addition, various states have enacted false claim laws analogous to the federal False Claims Act, and some of these state laws apply where a claim is submitted to any third-party payor. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties ranging from $5,500 to $11,000 for each false claim.
Additionally, the civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
Also, many states have laws similar to those listed above that may be broader in scope and may apply regardless of payor.
Additionally, in Europe various countries have adopted anti-bribery laws providing for severe consequences, in the form of criminal penalties and/or significant fines for individuals and/or companies committing a bribery offence. Violations of these anti-bribery laws, or allegations of such violations, could have a negative impact on our business, results of operations and reputation. For instance, in the United Kingdom, under the Bribery Act 2010, a bribery occurs when a person offers, gives or promises to give a financial or other advantage to induce or reward another individual to improperly perform certain functions or activities, including any function of a public nature. Bribery of foreign public officials also falls within the scope of the Bribery Act 2010. Under the new regime, an individual found in violation of the Bribery Act 2010 faces imprisonment of up to 10 years. In addition, the individual can be subject to an unlimited fine, as can commercial organizations for failure to prevent bribery.
Physician Referral Prohibitions
Under a federal law directed at “self-referral,” commonly known as the Stark Law, there are prohibitions, with certain exceptions, on Medicare and Medicaid payments for laboratory tests referred by physicians who personally, or through a family member, have a “financial relationship”—including an investment or ownership interest or a compensation arrangement—with the clinical laboratory performing the tests. Several Stark Law exceptions are relevant to arrangements involving clinical laboratories, including: (1) fair market value compensation for the provision of items or services; (2) payments by physicians to a laboratory for clinical laboratory services; (3) certain space and equipment rental arrangements that satisfy certain requirements, and (4) personal services arrangements that satisfy certain requirements. The laboratory cannot submit claims to the Medicare Part B program for
89
services furnished in violation of the Stark Law, and Medicaid reimbursements may be at risk as well. Penalties for violating the Stark Law include the return of funds received for all prohibited referrals, fines, civil monetary penalties and possible exclusion from the federal health care programs. Many states have comparable laws that are not limited to Medicare and Medicaid referrals.
Corporate Practice of Medicine
A number of states, including California, do not allow business corporations to employ physicians to provide professional services. This prohibition against the “corporate practice of medicine” is aimed at preventing corporations such as us from exercising control over the medical judgments or decisions of physicians. The state licensure statutes and regulations and agency and court decisions that enumerate the specific corporate practice rules vary considerably from state to state and are enforced by both the courts and regulatory authorities, each with broad discretion. If regulatory authorities or other parties in any jurisdiction successfully assert that we are engaged in the unauthorized corporate practice of medicine, we could be required to restructure our contractual and other arrangements. In addition, violation of these laws may result in sanctions imposed against us and/or the professional through licensure proceedings, and we could be subject to civil and criminal penalties that could result in exclusion from state and federal health care programs.
Direct Billing Laws and Other State Law Restrictions on Billing for Laboratory Services
Laws and regulations in certain states prohibit laboratories from billing physicians or other purchasers for testing that they order. Some of those laws and regulations apply only to anatomic pathology services while others extend to other types of testing. Some states may allow laboratories to bill physicians directly but may prohibit the physician (and, in some cases, other purchasers) from charging more than the purchase price for the services (or may allow only for the recovery of acquisition costs) or may require disclosure of certain information on the invoice. In some cases, and if not prohibited by law or regulation, we may bill physicians, hospitals and other laboratories directly for the services that they order. An increase in the number of states that impose similar restrictions could adversely affect us by encouraging physicians to perform laboratory services in-house or by causing physicians to refer services to other laboratories that are not subject to the same restrictions.
Physician Licensing
A number of the states where specimens originate require that the physician interpreting those specimens be licensed by that particular state. Physicians who fail to comply with these licensure requirements could face fines or other penalties for practicing medicine without a license and we could be required to pay those fines on behalf of our pathologists or subject to liability under the federal False Claims Act and similar state laws if we bill for services furnished by unlicensed pathologists. We do not believe that the services our pathologist performs constitute the practice of medicine in any state that requires out-of-state physician licensure. We believe that our pathologist thus is not required to obtain licensure in any state where he does not reside.
In addition, many states also prohibit the splitting or sharing of fees between physicians and non-physician entities. We do not believe that our contractual arrangements with physicians, physicians group practices or hospitals will subject us to claims under such regulations. However, changes in the laws may necessitate modifications in our relationships with our clients.
California State Laboratory Licensing
Our laboratory is licensed and in good standing under the State of California Department of Public Health standards. Our current licenses permit us to receive specimens obtained in California.
California state laws and regulations also establish standards for the day-to-day operations of clinical laboratories, including physical facility requirements and equipment, quality control and proficiency testing requirements. If we are found to be out of compliance with California statutory or regulatory standards, we may be subject to suspension, restriction or revocation of our laboratory license or assessed civil money penalties. The operator of a noncompliant laboratory may also be found guilty of a misdemeanor under California law. A finding of noncompliance, therefore, may result in harm to our business.
Other States’ Laboratory Licensing
Several states require the licensure of out-of-state laboratories that accept specimens from those states. We hold licenses from the states of Florida, Maryland, Pennsylvania and Rhode Island to test specimens from patients in those states or received from ordering physicians in those states. We are currently in the process of addressing the requirements for licensure in New York
90
From time to time, other states may require out of state laboratories to obtain licensure in order to accept specimens from such states. If we identify any other state with such requirements or if we are contacted by any other state advising us of such requirements, we intend to follow instructions from the state regulators as to how we should comply with such requirements.
U.S. Food and Drug Administration
We provide our tests as laboratory-developed tests, or LDTs. The Centers for Medicare and Medicaid Services, or CMS, and certain state agencies regulate the performance of LDTs (as authorized by the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and state law, respectively).
Historically, the FDA has exercised enforcement discretion with respect to most LDTs and has not required laboratories that furnish LDTs to comply with the agency’s requirements for medical devices (e.g., establishment registration, device listing, quality systems regulations, premarket clearance or premarket approval, and post-market controls). In recent years, however, the FDA has stated it intends to end its policy of general enforcement discretion and regulate certain LDTs as medical devices. To this end, on October 3, 2014, the FDA issued two draft guidance documents, entitled “Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs)” and “FDA Notification and Medical Device Reporting for Laboratory Developed Tests (LDTs)”, respectively, that set forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs. The FDA has indicated that it does not intend to modify its policy of enforcement discretion until the draft guidance documents are finalized. It is unclear at this time when, or if, the draft guidance documents will be finalized, and even then, the new regulatory requirements are proposed to be phased-in consistent with the schedule set forth in the guidance (in as little as 12 months after the draft guidance is finalized for certain high-priority LDTs). Nevertheless, the FDA may decide to regulate certain LDTs on a case-by-case basis at any time. LDTs with the same intended use as a cleared or approved companion diagnostic are defined in FDA’s draft guidance as “high-risk LDTs (Class III medical devices)” for which premarket review would be first to occur.
Failure to comply with applicable FDA regulatory requirements may trigger a range of enforcement actions by the FDA including warning letters, civil monetary penalties, injunctions, criminal prosecution, recall or seizure, operating restrictions, partial suspension or total shutdown of production, and denial of or challenges to applications for clearance or approval, as well as significant adverse publicity.
Other Regulatory Requirements
Our laboratory is subject to federal, state and local regulations relating to the handling and disposal of regulated medical waste, hazardous waste and biohazardous waste, including chemical, biological agents and compounds, blood and bone marrow samples and other human tissue. Typically, we use outside vendors who are contractually obligated to comply with applicable laws and regulations to dispose of such waste. These vendors are licensed or otherwise qualified to handle and dispose of such waste.
The Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for health care employers, including requirements to develop and implement programs to protect workers from exposure to blood-borne pathogens by preventing or minimizing any exposure through needle stick or similar penetrating injuries.
Segment and Geographical Information
We operate in one reportable business segment and historically have derived revenues only from the United States.
Employees
As of December 31, 2014, we had a total of 41 full-time employees and one part time employee, four of whom hold doctorate degrees and six of whom are engaged in full-time research and development activities. We plan to expand production, sales and marketing and our research and development programs, and we plan to hire additional staff as these initiatives are implemented. None of our employees is represented by a labor union.
Properties
We have a lease for approximately 48,000 square feet of space in San Diego, California for use as a clinical reference laboratory and corporate headquarters, including manufacturing and research laboratories. The average rent for the remaining lease period is approximately $106,500 per month. This lease expires in 2020.
91
In September 2013, we entered into an amendment of the lease, extending the term for 21 months so that it now ends on July 31, 2020 and providing for five months of free base rent (August 2013—December 2013). In return, we agreed, among other things, to forfeit our security deposit and to issue common stock warrants to the landlord exercisable for 50,260 shares, at a price of $10.00 per share. The warrants will expire on February 4, 2019.
Immediately following the execution of such amendment, we paid all amounts due under our lease. As of December 31, 2013 and September 30, 2014, we owed no rent in arrears.
In September 2012, in connection with an amendment of the lease, which included a rent deferral through November 30, 2012, we issued to our landlord warrants which, in connection with the closing of our initial public offering, became exercisable for 1,587 shares of our common stock at an exercise price of $25.20 per share.
Legal Proceedings
In the normal course of business, we may be involved in legal proceedings or threatened legal proceedings. We are not party to any legal proceedings or aware of any threatened legal proceedings which are expected to have a material adverse effect on our financial condition, results of operations or liquidity.
92
Executive Officers and Directors
The following table sets forth the name, age and position of each of our directors and executive officers.
|
Name |
|
Age |
|
Position |
|
Served as an Officer or Director Since |
|
David F. Hale |
|
65 |
|
Chairman of the Board of Directors |
|
2011 |
|
Marsha A. Chandler, Ph. D.(3) |
|
69 |
|
Director |
|
2013 |
|
Bruce E. Gerhardt, CPA(1) |
|
63 |
|
Director |
|
2010 |
|
Bruce A. Huebner(2) |
|
64 |
|
Director |
|
2013 |
|
Michael W. Nall |
|
52 |
|
Director, Chief Executive Officer and President |
|
2013 |
|
Edward Neff(1) |
|
64 |
|
Director |
|
2006 |
|
Ivor Royston, M.D.(2)(3) |
|
69 |
|
Director |
|
2010 |
|
M. Faye Wilson, CPA, MBA(1)(2)(3) |
|
77 |
|
Lead Director |
|
2009 |
|
Lyle J. Arnold, Ph. D. |
|
68 |
|
Senior Vice-President of Research & Development, Chief Scientific Officer |
|
2011 |
|
William G. Kachioff |
|
49 |
|
Senior Vice-President of Finance and Chief Financial Officer |
|
2011 |
|
Veena Singh, M.D. |
|
40 |
|
Senior Vice President and Senior Medical Director |
|
2014 |
|
Raaj Trivedi |
|
42 |
|
Vice-President, Commercial Operations |
|
2014 |
|
(1) |
Audit Committee |
|
(2) |
Compensation Committee |
|
(3) |
Nominating and Corporate Governance Committee |
Our board of directors is classified into three classes of two or three directors each, with all directors serving for a three-year term and the directors of only one class being elected at each annual meeting of stockholders, so that the terms of the classes of directors are “staggered.” The directors in Class I are Mr. Gerhardt and Mr. Neff. The next election of Class I directors by stockholders will be at our 2017 annual meeting of stockholders, with the elected candidates to then serve until our 2020 annual meeting of stockholders. The directors in Class II are Dr. Chandler, Mr. Huebner and Dr. Royston. The next election of Class II directors by stockholders will be at our 2015 annual meeting of stockholders, with the elected candidates to then serve until our 2018 annual meeting of stockholders. The directors in Class III are Mr. Hale, Mr. Nall and Ms. Wilson. The next election of Class III directors by stockholders will be at our 2016 annual meeting of stockholders, with the elected candidates to then serve until our 2019 annual meeting of stockholders.
Our executive officers are elected by, and serve at the discretion of, our board of directors. There are no family relationships among any of our directors and executive officers, except that Edward Neff is an uncle of Michael W. Nall. The business experience for the past five years (and, in some instances, for prior years) of each of our executive officers and directors is as follows:
93
David F. Hale
Mr. Hale was appointed as our Executive Chairman in March 2011, and currently serves as Chairman. He is the Chairman and CEO of Hale BioPharma Ventures LLC, a private company focused on the formation and development of biotechnology, specialty pharma, diagnostic and medical device companies. He has also been the Chairman of Santarus, Inc., a specialty biopharmaceutical company, since 2004 and a member of Santarus’ board since 2000. He also serves as Chairman of Conatus Pharmaceuticals, Inc. He was previously President and CEO of CancerVax Corporation from October 1999 through its merger in May 2006 with Micromet, Inc., a biotechnology company focused on the development of novel biological products for the treatment of cancer, when he became Chairman of the combined companies. He is a co-founder and served as Chairman of Somaxon Pharmaceuticals, Inc. before its acquisition by Pernix Therapeutics Holdings, Inc., and as Chairman of SkinMedica, Inc., before its acquisition by Allergan, Inc. He also serves as Chairman of Neurelis, Inc., Coloresciences, Inc., CRISI Medical Systems, Inc. and other private companies. Mr. Hale is a serial entrepreneur who has been involved in the founding and/or development of a number of life sciences technology companies. In 1982, after joining Hybritech, Inc., the first monoclonal antibody company, he served as COO, President and then Chief Executive Officer, until Hybritech was acquired by Eli Lilly and Co. in 1986. From 1987 until 1997 he was Chairman, President and CEO of Gensia, Inc., which merged with SICOR to become Gensia Sicor, Inc., which was later acquired by Teva Pharmaceuticals. He was a co-founder and Chairman of Viagene, Inc. from 1987 to 1995, when Viagene was acquired by Chiron, Inc. He was President and CEO of Women First HealthCare, Inc. from late 1997 to June 2000, before joining CancerVax in October 1999. Before joining Hybritech, Mr. Hale was Vice President and General Manager of BBL Microbiology Systems, a diagnostics division of Becton, Dickinson & Co. and from 1971 to 1980, held various marketing and sales management positions with Ortho Pharmaceutical Corporation, a division of Johnson & Johnson, Inc.
We selected Mr. Hale to serve on and lead our board of directors due to his public and private company board experience as well as his extensive experience with and knowledge of health care issues and the operational activities of life sciences companies.
Marsha A. Chandler
Dr. Chandler has been the Executive Vice President/Chief Operating Officer of the Salk Institute for Biological Studies since 2007. She manages approximately 1,000 scientific and administrative personnel and oversees all institutional fiscal, administrative and fund-raising activities. From 1997 to 2007 she served as Senior Vice Chancellor for Academic Affairs at the University of California, San Diego, where she was the chief academic officer responsible for the policies and decisions relating to all academic programs and faculty appointments and performance. She served as Acting Chancellor from 2003-04 and holds an appointment as Professor of Political Science in the Graduate School of International Relations and Pacific Studies at UCSD.
Dr. Chandler is a Fellow of the Royal Society of Canada, the highest academic honor bestowed in that country. She received her Ph.D. from The University of North Carolina at Chapel Hill.
We selected Dr. Chandler to serve on our board of directors due to her experience in organizational management and her stature in the life sciences community. Dr. Chandler also serves as chair of our nominating and corporate governance committee.
Bruce E. Gerhardt
Mr. Gerhardt has been self-employed, practicing as a Certified Public Accountant, since 1986. He is also a tax and business advisor providing tax compliance for small businesses and upper income individuals. He earned his Bachelor of Arts Degree from the University of Southern California in 1973 and is a member of the American Institute of Certified Public Accountants.
We selected Mr. Gerhardt to serve on our board of directors due to his experience and expertise in financial accounting and auditing. Mr. Gerhardt also serves as a member of our audit committee.
Bruce A. Huebner
Mr. Huebner is currently and has been since 2004 a managing director of LynxCom Partners LLC, a healthcare consulting firm with a focus on cancer diagnostics and personalized medicine. From March 2013 through December 2013, he was Chairman of Vermillion, Inc., a publicly held molecular diagnostics company. He served as Interim Chief Executive Officer and President of Vermillion from November 2012 to March 2013. From October 2009 to June 2010, Mr. Huebner served as President and Chief Executive Officer of TrovaGene, Inc., a developer of molecular diagnostics products. From 2005 to 2008, Mr. Huebner served as President of Osmetech Molecular Diagnostics, obtaining FDA clearance for four molecular diagnostic microarray products and introducing them to the marketplace. From 2002 to 2004, Mr. Huebner was President and Chief Operating Officer of Nanogen, Inc., a publicly held nanotechnology/microarray company. From 1996 to 2002, Mr. Huebner was Executive Vice President and Chief Operating Officer of Gen-Probe Incorporated, a leader in the development of nucleic acid tests.
94
Mr. Huebner received his Bachelor of Science degree in Chemistry from the University of Wisconsin-La Crosse and completed a graduate school senior executive program at Columbia University.
We selected Mr. Huebner to serve on our board of directors due to his strong background in cancer diagnostics sales, marketing, operations and reimbursement. Mr. Huebner also serves as a member of our compensation committee.
Michael W. Nall
Mr. Nall has over 25 years of healthcare sales and marketing experience, most recently serving at Clarient Diagnostic Services, Inc. in positions of increasing responsibility from 2002 through August 2013, with his last position being General Manager, North American Sales and Marketing. While at Clarient, Mr. Nall was also responsible for leading the team assimilating Clarient into GE Healthcare after Clarient was acquired in 2010.
From 1988 until joining Clarient, Mr. Nall served in the diagnostic and medical device industries in various commercial leadership roles for companies including Impath, American Cyanamid, Maquet Surgical, Strato Medical, Horizon Medical Products and Columbia Vital Systems.
Mr. Nall received a Bachelor of Science degree in Business Administration from Central Missouri State University (now known as the University of Central Missouri).
We selected Mr. Nall to serve on our board of directors due to his experience in the cancer diagnostics business, his expertise in the commercialization of products and services such as ours, his background in reimbursement and operations and his status as our chief executive officer and president.
Mr. Nall is a nephew of our director Edward Neff.
Edward Neff
Since 1990, Mr. Neff has been the Chief Executive Officer of Systems, Machines, Automation Components Corporation (also known as SMAC), a manufacturer of moving coil electric actuators.
Mr. Neff has received over 25 United States patents relating to robotics and precise automation. He is a graduate of the University of Michigan.
We selected Mr. Neff to serve on our board of directors due to his experience and expertise in business management and in automated systems. Mr. Neff also serves as a member of our audit committee.
Mr. Neff is an uncle of our Chief Executive Officer, President and director Michael W. Nall.
Ivor Royston, M.D.
Dr. Royston co-founded Forward Ventures and has served as its Managing Partner since 2000. From 1990 to 2000, he served as founding President and CEO of The Sidney Kimmel Cancer Center and from 1978 to 1990, he was a member of the oncology faculty of the University of California, San Diego. In addition to being a co-founder of Hybritech, Inc., in 1986 he co-founded IDEC Corporation, which later merged with Biogen to form BiogenIdec. Dr. Royston has been instrumental in the formation, financing and development of numerous biotechnology companies, including Applied Molecular Evolution (acquired by Eli Lilly), Corixa (acquired by GlaxoSmithKline), Dynavax, LigoCyte (acquired by Takeda), Morphotek (acquired by Eisai), Sequana Therapeutics (acquired by Celera), TargeGen (acquired by Sanofi-Aventis), and Triangle Pharmaceuticals (acquired by Gilead). He is currently a director of MMRGlobal, Inc., a publicly-traded health records management company. Dr. Royston received his B.A. and M.D. degrees from Johns Hopkins University and completed post-doctoral training in internal medicine and medical oncology at Stanford University. In 1997, President Clinton appointed Dr. Royston to a six-year term on the National Cancer Advisory Board.
We selected Dr. Royston to serve on our board of directors due to his extensive experience with emerging life sciences companies. Dr. Royston also serves as chair of our compensation committee and as a member of our nominating and governance committee.
95
M. Faye Wilson
Ms. Wilson has been a principal of Wilson Boyles & Co., LLC, a business management and strategic planning consulting firm, since 2003. Ms. Wilson is also a member of the board of directors of BioMed Realty Trust, Inc., a real estate investment trust. She served on the board of directors of Farmers Insurance Group of Companies from 1992 through 1998 and the board of directors of The Home Depot, Inc. from 1991 through 2001. Ms. Wilson was also a senior officer of Home Depot from 1998 through 2002. From 1992 until 1998, Ms. Wilson served in several senior management roles at Bank of America Corporation including Chairman of Security Pacific Financial Services and Executive Vice President and Chief Credit Officer for Bank of America’s National Consumer Banking Group. She earned her Master’s Degrees in International Relations and Business Administration from the University of Southern California and an undergraduate degree from Duke University.
We selected Ms. Wilson to serve on our board of directors due to her extensive experience as a director of public companies, her financial acumen and experience, and her expertise in business strategy. Ms. Wilson also serves as chair of our audit committee, as a member of our compensation committee and as a member of our nominating and governance committee.
Lyle J. Arnold, Ph. D.
Dr. Arnold joined us as Senior Vice President and Chief Scientific Officer in 2011. Before then, he consulted for us from May 2010 to April 2011. He is a biotechnology executive, entrepreneur, and developer of innovative technologies covering therapeutics, molecular diagnostics, and genomics. Dr. Arnold also serves as President of Aegea Biotechnologies, Inc., which he founded in 2010 to acquire, develop, and commercialize next generation nucleic acid technologies. Previously he was Vice President, Research at Gen-Probe Incorporated from September 2003 to October 2009. During the time between departing from Gen-Probe and joining us, Dr. Arnold worked as a consultant for various entities through Lyle Arnold Consulting LLC, and started Aegea Biotechnologies in February 2010. He has also held senior scientific and management positions at Molecular Biosystems (co-founder), Genta, Synteni, Incyte Genomics, and Oasis Biosciences (co-founder), where he was President and Chief Scientific Officer from October 2001 to September 2003. In addition, Dr. Arnold was a faculty member of the UCSD School of Medicine and a member of the UCSD Cancer Center. Dr. Arnold is an inventor or co-inventor on 39 issued U.S. patents and more than 140 issued and pending patents worldwide. He is the principal inventor of the chemiluminescent Hybridization Protection Assay (HPA) and associated technologies, core to Gen-Probe assays that have generated more than $5 billion in product revenue. In addition, he has authored more than 50 scientific publications. Dr. Arnold serves on the board of directors of Asuragen, a rapidly emerging biotechnology company in Austin, Texas, as well as on the board of Aegea.
He received a B.S. in Chemistry from the University of California at Los Angeles and a Ph.D. in Chemistry/Biochemistry from the University of California at San Diego.
William G. Kachioff
Mr. Kachioff, who joined us as Senior Vice President and Chief Financial Officer in August 2011, is experienced in corporate finance, investor relations, corporate governance and manufacturing accounting and systems. He has over twenty years of experience in the life science industry, having most recently served as Vice President and Chief Financial Officer at Althea Technologies, Inc., a pharmaceutical contract manufacturer, from 2009 to 2011. From 2007 to 2009 he was a CFO Partner with Tatum LLC, a national Executive Services firm, where he served a variety of life science industry clients in senior financial management roles. From 2002 to 2005, Mr. Kachioff was Chief Financial Officer at MicroIslet, a publicly traded biotechnology company developing cell transplant therapies for insulin dependent diabetes. From 1999 to 2001, he was Director of Finance at Cutera where he helped prepare the company for the commercial launch of its first product and its initial public offering. Mr. Kachioff has also served in a variety of financial management roles at Coulter Pharmaceutical, Vivus and Abbott Laboratories. He began his professional career as an auditor with Deloitte LLP.
Mr. Kachioff has a B.S. in Management from the University at Buffalo, State University of New York with concentrations in Accounting and Information Systems. He is a member of the American Institute of Certified Public Accountants and the Association of Bioscience Financial Officers.
Veena Singh, M.D.
Dr. Singh joined us as Senior Vice President and Senior Medical Director in December 2014. Prior to joining Biocept, she was the Medical Director at bioTheranostics, Inc. since July 2009. Dr. Singh brings experience in oncology molecular diagnostics, assay development and validation with expertise in CLIA regulations and is board certified in Anatomic and Clinical Pathology as well as in Molecular Pathology. Dr. Singh completed her Anatomic and Clinical pathology residency at the University of California, San Diego and her Molecular Pathology fellowship at Cedars-Sinai Medical Center in Los Angeles. Dr. Singh obtained her medical degree from the University of Transkei, South Africa.
96
Raaj Trivedi
Mr. Trivedi, who joined us as Vice President, Commercial Operations in March 2014, has more than 15 years of leadership experience in the biotechnology and diagnostic industry. He joined us from Life Technologies where from 2013 to 2014 he was involved in leading a number of business development and commercial initiatives geared towards taking their next generation sequencing, or NGS, applications and positioning them into the research, translation medicine and clinical markets. From 2005 to 2013, Mr. Trivedi worked at Clarient, a GE Healthcare Company, where he led both marketing and business development departments. From 2002 to 2005, Mr. Trivedi led commercial efforts and strategy for leukemia and lymphoma diagnostic services at US Labs, now part of LabCorp. He began his career at Ernst & Young LLP in 1998. Mr. Trivedi received a master’s degree in Biotechnology from the University of Maryland and earned his bachelor’s degree from the University of California, Irvine.
Director Independence
Our common stock is listed on The NASDAQ Capital Market under the symbol BIOC. Under the rules of The NASDAQ Stock Market, independent directors must comprise a majority of a listed company’s board of directors within 12 months after the completion of an initial public offering. In addition, the rules of The NASDAQ Stock Market require that, (i) on the date of the completion of this offering, at least one member of our audit, compensation and nominating and corporate governance committees be independent, (ii) within 90 days after the date of the completion of our initial public offering, a majority of the members of such committees be independent and (iii) within one year after the date of the completion of our initial public offering, all the members of such committees be independent. Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. Under the rules of The NASDAQ Stock Market, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
In order to be considered to be independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: (1) accept, directly or indirectly, any consulting, advisory or other compensatory fee from the listed company or any of its subsidiaries or (2) be an affiliated person of the listed company or any of its subsidiaries.
Our board of directors undertook a review of its composition, the composition of its committees and the independence of each director. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, our board of directors has determined that Dr. Chandler, Mr. Gerhardt, Mr. Huebner, Mr. Neff, Dr. Royston and Ms. Wilson, or six of our eight directors, do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the rules of The NASDAQ Stock Market.
Our board of directors also determined that (i) Messrs. Gerhardt and Neff and Ms. Wilson, who compose our audit committee, (ii) Mr. Huebner, Dr. Royston and Ms. Wilson, who compose our compensation committee, and (iii) Dr. Chandler, Dr. Royston and Ms. Wilson, who compose our nominating and corporate governance committee, each satisfy the independence standards for those committees established by the applicable rules and regulations of the SEC and The NASDAQ Stock Market. In making this determination, our board of directors considered the relationships that each non-employee director has with us and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director. We intend to comply with all size and independence requirements for committees within the applicable time periods.
97
Summary Compensation Table
The following table shows the compensation awarded to or earned in our last two fiscal years by our principal executive officer and our two most highly compensated executive officers other than our principal executive officer who were serving as executive officers as of December 31, 2014 and an individual who would have been among our two most highly compensated executive officers other than our principal executive officer if he had been serving as an executive officer of the company as of December 31, 2014. The persons listed in the following table are referred to herein as the “named executive officers.”
|
Name and Principal Position |
|
Year |
|
|
|
Salary ($)(1) |
|
|
|
Stock Awards ($)(2) |
|
|
|
Option Awards ($)(2) |
|
|
|
Other Compensation ($)(3) |
|
|
|
Total |
|
|
Michael W. Nall |
|
2014 |
|
|
|
418,782(4 |
) |
|
|
238,054 |
|
|
|
318,935 |
|
|
|
130,878(5 |
) |
|
$ |
1,106,649 |
|
|
President and Chief Executive Officer |
|
2013 |
|
|
|
66,091(4 |
) |
|
|
80,000 |
|
|
|
452,558 |
|
|
|
3,574(5 |
) |
|
$ |
602,223 |
|
|
William G. Kachioff |
|
2014 |
|
|
|
255,362(6 |
) |
|
|
— |
|
|
|
182,029 |
|
|
|
50,000(7 |
) |
|
$ |
487,391 |
|
|
SVP Finance, Chief Financial Officer |
|
2013 |
|
|
|
227,230 (6 |
) |
|
|
60,000 |
|
|
|
82,884 |
|
|
|
— |
|
|
$ |
370,114 |
|
|
Raaj Trivedi |
|
2014 |
|
|
|
177,014(8 |
) |
|
|
— |
|
|
|
256,587 |
|
|
|
34,800(9 |
) |
|
$ |
468,401 |
|
|
VP, Commercial Operations |
|
2013 |
|
|
|
— (8 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
David F. Hale |
|
2014 |
|
|
|
111,289(11 |
) |
|
|
— (12 |
) |
|
|
829,423 |
|
|
|
— |
|
|
$ |
940,712 |
|
|
Former Executive Chairman(10) |
|
2013 |
|
|
|
319,281(11 |
) |
|
|
80,000 |
|
|
|
108,785 |
|
|
|
— |
|
|
$ |
508,066 |
|
|
(1) |
The “Salary ($)” column includes both salary earned and salary amounts earned but deferred, or deferred salary, under each named executive officer’s amended and restated Salary Reduction and Contingent Payment Agreement, 8% annual interest (compounded monthly) on such deferred salary amounts, and the net increase/(decrease) in each named executive officer’s accrued vacation balance, or accrued vacation, in each year ended December 31. For information regarding the amended and restated Salary Reduction and Contingent Payment Agreement arrangements, see “Executive Compensation—Narrative Disclosure to Summary Compensation Table—Salary Deferrals.” |
|
(2) |
The amounts in the “Option Awards ($)” and “Stock Awards ($)” columns reflect the grant date fair values of stock option and RSU awards, respectively, granted during the year. These amounts are determined in accordance with the provisions of FASB ASC Topic 718, rather than an amount paid to or realized by the executive officer. For a description of these stock option and RSU awards, see “Narrative Disclosure to Summary Compensation Table” within this “Executive Compensation” section. |
|
(3) |
The “Other Compensation ($)” column includes amounts earned by each named executive officer but not otherwise included in amounts within the “Salary ($),” “Stock Awards ($)” or “Option Awards ($)” columns. |
|
(4) |
Mr. Nall commenced employment on August 26, 2013. 2014 salary amount includes a retroactive salary increase of $69,231 upon the closing of our initial public offering on February 10, 2014 and accrued vacation of $14,360. 2013 salary amount includes accrued vacation of $3,014. |
|
(5) |
2014 other compensation amount includes a bonus of $100,000, and $26,176 commuting expenses reimbursement benefit we provided to Mr. Nall plus $4,702 of income taxes we paid for Mr. Nall in respect of such benefit. 2013 other compensation amount includes a $2,383 commuting expenses reimbursement benefit we provided to Mr. Nall plus $1,191 of income taxes we paid for Mr. Nall in respect of such benefit. |
|
(6) |
2014 salary amount includes interest on deferred salary of $977. 2013 salary amount includes interest on deferred salary of $8,106 and accrued vacation of $4,124. |
|
(7) |
2014 other compensation amount includes a bonus of $50,000. |
|
(8) |
Mr. Trivedi commenced employment on March 24, 2014. 2014 salary amount includes accrued vacation of $1,341. |
|
(9) |
2014 other compensation amount includes a bonus of $30,000 and car allowances of $4,800 to Mr. Trivedi in such year ended December 31. |
|
(10) |
Mr. Hale was appointed as our Executive Chairman in March 2011. As of and in connection with the closing of our initial public offering on February 10, 2014, Mr. Hale now serves as non-executive Chairman. Amounts in the table above include 2014 salary, option awards, and restricted stock award amounts granted to Mr. Hale prior to February 10, 2014 as an employee, as well as fees earned or paid in cash, option awards, and restricted stock awards earned as a non-employee director since February 10, 2014. |
|
(11) |
2014 salary amount includes deferred salary of $8,081, interest on deferred salary of $5,669, and compensation earned as a non-employee director for services performed of $88,889. 2013 salary amount includes deferred salary of $255,182, interest on deferred salary of $36,417, and accrued vacation of $(4,636). |
|
(12) |
2014 stock awards amount excludes the estimated grant date fair value of RSU awards of $295,061 that vested upon the closing of our initial public offering on February 10, 2014, but were granted in 2011. |
98
Narrative Disclosure to Summary Compensation Table
Michael W. Nall
We entered into an employment agreement effective as of August 26, 2013 with Michael W. Nall, or the CEO Employment Agreement, in connection with his appointment as our Chief Executive Officer and President. The CEO Employment Agreement provides Mr. Nall the following: (i) a base salary of $200,000 per year, provided that the salary will increase retroactively to $350,000 per year upon completion of an initial public offering or an equity or debt financing of at least $5,000,000; (ii) a target bonus of $100,000 per year; (iii) a special one-time bonus of $100,000 in January 2014 if an initial public offering or an equity or debt financing of at least $5,000,000 has been completed by then; (iv) upon completion of an initial public offering or an equity or debt financing of at least $5,000,000, a housing allowance of $2,000 per month; (v) stock options under our 2013 Equity Incentive Plan to purchase a number of shares of common stock equal to at least 4% of our fully diluted stock outstanding as of August 26, 2013, vesting in equal monthly installments over four years beginning August 15, 2013 with a term of 10 years; and (vi) performance-based restricted stock units, or RSUs, under our 2013 Equity Incentive Plan for a number of shares of common stock equal to 1% of our common stock following completion of an initial public offering or an equity or debt financing of at least $5,000,000, subject to the establishment of goals and objectives to be agreed with and approved by our board of directors. The closing of our initial public offering on February 10, 2014 qualified as such a receipt of aggregate proceeds of $5,000,000 or more from an equity or debt financing. The CEO Employment Agreement calls for the vesting of such stock options to fully accelerate upon a change in control, and in the event Mr. Nall’s continuous service is terminated by us or our stockholders without cause or Mr. Nall resigns with good reason, for him to receive one year of additional vesting of such stock options.
The CEO Employment Agreement provides that in the event of termination of Mr. Nall’s employment by us without cause or his resignation for good reason, the vesting of any of his outstanding unvested stock options and RSUs which would have vested over the following 12 months will accelerate (unless the applicable stock option or RSU agreement provides for more favorable acceleration terms). Also, in the event of a change of control, the vesting of 50% of any of Mr. Nall’s outstanding unvested stock options and RSUs will accelerate on the date of the change of control and the remaining unvested stock options and RSUs will vest on the earliest of (i) the date of the termination of his employment by us without cause, (ii) the date of his resignation for good reason, or (iii) the first anniversary of the change of control (unless the applicable stock option or RSU agreement provides for more favorable acceleration terms). (For example, the foregoing would not apply to the initial stock options grant, which would fully accelerate upon a change in control.)
The CEO Employment Agreement provides that if Mr. Nall has a separation from service as a result of his discharge by us without cause or his resignation with good reason then, provided that he gives us an effective waiver and release of claims, he will be entitled to 12 months’ salary and up to 12 months of COBRA premiums (or substantially equivalent health insurance coverage). However, the CEO Employment Agreement further provides that Mr. Nall will have no entitlement to any severance benefits before our completion of an initial public offering or an equity or debt financing of at least $5,000,000. The closing of our initial public offering on February 10, 2014 qualified as such a receipt of aggregate proceeds of $5,000,000 or more from an equity or debt financing.
On August 8, 2013, and effective on his August 26, 2013 employment start date, an RSU award for 14,285 shares of common stock with a grant date fair value of $73,996 was granted to Mr. Nall under our 2013 Equity Incentive Plan. The share amount for the RSU award was determined by dividing the award value by $5.18, which was the estimated fair value of our common stock on the date of grant. Subsequent to the date of grant, and for the purposes of recording share-based compensation expense in accordance with FASB ASC Topic 718, the grant date fair value of this award was estimated to be $80,000. The RSU award vests 50% upon date of grant and the remaining 50% in equal monthly installments over 24 months beginning August 1, 2013.
On July 31, 2013, option awards exercisable into 100,000 shares of common stock with an aggregate grant date fair value of $452,558 were, effective upon his August 26, 2013 employment start date, granted to Mr. Nall under our 2013 Equity Incentive Plan. The exercise price of these awards of $5.18 per share is equal to the estimated fair value of our common stock on the date of grant. The share amounts for the option awards were determined by dividing the award values by $4.53, which is the fair value of the option exercisable into our common stock on the date of grant, estimated using a Black-Scholes pricing model. Subsequent to the date of grant, and for the purposes of recording share-based compensation expense in accordance with FASB ASC Topic 718, the grant date fair value of common stock was estimated to be $5.60 per share. The assumptions used in the Black-Scholes pricing model include a volatility of 105.0%, a risk free interest rate of 1.69%, a dividend yield of 0.00%, and an expected term of 6.02 years. The option awards vest in equal monthly installments over 48 months beginning August 1, 2013 with a term of 10 years.
99
On June 12, 2014, option awards exercisable into 75,000 shares of common stock with an aggregate grant date fair value of $318,935 were granted to Mr. Nall under our 2013 Equity Incentive Plan. The exercise price of these awards of $5.35 per share is equal to the closing price of our common stock on the date of grant. The share amounts for the option awards were determined by dividing the award values by $4.25, which is the fair value of the option exercisable into our common stock on the date of grant, estimated using a Black-Scholes pricing model. The assumptions used in the Black-Scholes pricing model include a volatility of 100.0%, a risk free interest rate of 1.94%, a dividend yield of 0.00%, and an expected term of 6.08 years. The option awards vest in equal monthly installments over 48 months beginning June 12, 2014 with a term of 10 years.
On June 12, 2014 a performance RSU award for 44,496 shares of common stock with a grant date fair value of $238,054 was granted to Mr. Nall under our 2013 Equity Incentive Plan. The share amount for the performance RSU award was determined by dividing the award value by $5.35, which was the closing price of our common stock on the date of grant. Vesting of the performance RSU award may occur based on our achievement of specified objectives as determined by our Board of Directors or Compensation Committee, as follows:
|
|
Percentage of |
|
|
|
|
Overall RSU |
|
|
|
|
Grant Subject to |
|
|
|
|
Vesting |
|
|
|
Target |
|
|
|
|
Minimum revenue in 2015 |
|
25 |
% |
|
Maximum EBITDA loss in 2015 |
|
15 |
% |
|
Attainment of financial plan for fiscal 2015 |
|
20 |
% |
|
Minimum value of strategic agreements by December 31, 2015 |
|
20 |
% |
|
Implementation of four new diagnostic test panels by December 31, 2015 |
|
20 |
% |
|
Total |
|
100 |
% |
William G. Kachioff
We entered into an employment agreement as of August 1, 2011 with William G. Kachioff, or the CFO Employment Agreement, in connection with his appointment as our Senior Vice-President and Chief Financial Officer. The CFO Employment Agreement provides Mr. Kachioff the following: (i) a base salary of $215,000 per year, provided that the salary will increase to $240,000 per year upon our receipt of aggregate proceeds of $15,000,000 or more from the sales of equity securities, excluding the conversion of outstanding indebtedness; (ii) a one-time bonus of $30,000 upon our receipt of aggregate proceeds of $15,000,000 or more from the sales of equity securities, excluding the conversion of outstanding indebtedness; (iii) stock options under our 2007 Equity Incentive Plan to purchase 5,952 shares of common stock with an exercise price of $4.62 and a term of 10 years, with 25% of all shares vesting on the one year anniversary of the grant and the remainder vesting in equal monthly installments over the following three year period; and (iv) an additional option to purchase 1,190 shares of common stock to be issued upon our receipt of aggregate proceeds of $15,000,000 or more from the sales of equity securities, excluding the conversion of outstanding indebtedness.
On August 8, 2013, an RSU award for 10,714 shares of common stock with a grant date fair value of $55,499 was granted to Mr. Kachioff under our 2013 Equity Incentive Plan. The share amount for the RSU award was determined by dividing the award value by $5.18, which was the estimated fair value of our common stock on the date of grant. Subsequent to the date of grant, and for the purposes of recording share-based compensation expense in accordance with FASB ASC Topic 718, the grant date fair value of this award was estimated to be $60,000. The RSU award vests 50% upon date of grant and the remaining 50% in equal monthly installments over 24 months beginning August 1, 2013.
On July 31, 2013, an option award exercisable into 19,047 shares of common stock with a grant date fair value of $82,884 was granted to Mr. Kachioff under our 2013 Equity Incentive Plan. The exercise price of this award of $5.18 per share is equal to the estimated fair value of our common stock on the date of grant. The share amount for the option award was determined by dividing the award value by $4.35, which is the fair value of the option exercisable into our common stock on the date of grant, estimated using a Black-Scholes pricing model. Subsequent to the date of grant, and for the purposes of recording share-based compensation expense in accordance with FASB ASC Topic 718, the grant date fair value of common stock was estimated to be $5.60 per share. The assumptions used in the Black-Scholes pricing model include a volatility of 105.0%, a risk free interest rate of 1.38%, a dividend yield of 0.00%, and an expected term of 5.26 years. The option award vests 50% upon date of grant and the remaining 50% in equal monthly installments over 24 months beginning August 1, 2013, with a term of 10 years.
100
The closing of our initial public offering on February 10, 2014 qualified as a receipt of aggregate proceeds of $15,000,000 or more from the sales of equity securities under the terms of the CFO Employment Agreement, and the one-time bonus of $30,000 was subsequently increased to $50,000 and paid to Mr. Kachioff, as approved by the compensation committee of our board of directors. Additionally, Mr. Kachioff’s annual base salary was subsequently increased to $260,000, as approved by the compensation committee of our board of directors. On February 21, 2014, in accordance with the terms of the CFO Employment Agreement, and in connection with the closing of our initial public offering on February 10, 2014, Mr. Kachioff received an additional option award to purchase 1,190 shares of common stock under our 2013 Equity Incentive Plan with an exercise price of $9.11 per share, which is the closing price of our common stock on the NASDAQ on the date of grant. The fair value of the option exercisable into our common stock on the date of grant of $8,589 was estimated using a Black-Scholes pricing model. The assumptions used in the Black-Scholes pricing model include a volatility of 100.0%, a risk free interest rate of 1.88%, a dividend yield of 0.00%, and an expected term of 6.02 years. The option award vests in equal monthly installments over 48 months from the date of grant with a term of 10 years.
On May 16, 2014, option awards exercisable into 50,000 shares of common stock with an aggregate grant date fair value of $173,440 were granted to Mr. Kachioff under our 2013 Equity Incentive Plan. The exercise price of these awards of $4.38 per share is equal to the closing price of our common stock on the date of grant. The share amounts for the option awards were determined by dividing the award values by $3.47, which is the fair value of the option exercisable into our common stock on the date of grant, estimated using a Black-Scholes pricing model. The assumptions used in the Black-Scholes pricing model include a volatility of 100.0%, a risk free interest rate of 1.83%, a dividend yield of 0.00%, and an expected term of 6.02 years. The option awards vest in equal monthly installments over 48 months beginning May 16, 2014 with a term of 10 years.
Raaj Trivedi
We entered into an employment agreement dated March 1, 2014 with Raaj Trivedi, or the VP Commercial Operations Employment Agreement, in connection with his appointment as our Vice President, Commercial Operations. The VP Commercial Operations Employment Agreement provides Mr. Trivedi the following: (i) a base salary of $225,000 per year; (ii) a target bonus of 20-40% of base salary per year, and (iii) stock options under our 2013 Equity Incentive Plan to purchase 43,000 shares of common stock with an exercise price of $7.50 and a term of 10 years, with 25% of all shares vesting on the one year anniversary of the grant and the remainder vesting in equal monthly installments over the following three year period.
On March 24, 2014, option awards exercisable into 43,000 shares of common stock with an aggregate grant date fair value of $256,587 were granted to Mr. Trivedi in accordance with the terms of the VP Commercial Operations Employment Agreement under our 2013 Equity Incentive Plan. The exercise price of these awards of $7.50 per share is equal to the closing price of our common stock on the date of grant. The share amounts for the option awards were determined by dividing the award values by $5.97, which is the fair value of the option exercisable into our common stock on the date of grant, estimated using a Black-Scholes pricing model. The assumptions used in the Black-Scholes pricing model include a volatility of 100.0%, a risk free interest rate of 2.06%, a dividend yield of 0.00%, and an expected term of 6.08 years. The option awards vest over a four year period with 25% of all shares vesting on the one year anniversary of the grant and the remainder vesting in equal monthly installments over the following three years beginning March 24, 2014, with a term of 10 years.
David F. Hale
As of March 10, 2011, we entered into an employment agreement, effective retroactive to January 1, 2011 with David F. Hale, or the Executive Chairman Agreement, in connection with his appointment as our Executive Chairman of the Board of Directors. The Executive Chairman Agreement was effective through December 31, 2013. The Executive Chairman Agreement provided Mr. Hale the following: (i) a monthly fee of $25,000 per month for each month before our board of directors appoints a chief executive officer and for each of the three months following the appointment of the new chief executive officer, with a reduction to $12,500 per month commencing with the fourth month following the appointment of the new chief executive officer (i.e., commencing with December 2013), subject to normal employee payroll deductions and withholdings; and (ii) stock options under our 2007 Equity Incentive Plan to purchase 10,204 shares of common stock with an exercise price of $4.62, vesting in equal monthly installments over a four year period with a 10 year term, with full vesting upon a change of control or initial public offering. In addition, vesting would accelerate upon his termination by us or our shareholders without cause, as defined in the 2007 Equity Incentive Plan, provided that he gives us an effective waiver and release of claims. Also, upon an equity financing such as an initial public offering, Mr. Hale is entitled to receive an additional stock option, on the same terms and conditions except for exercise price, to purchase a number of shares of common stock equal to the excess of (i) 1% of our fully-diluted equity capitalization as of immediately after the financing over (ii) the number of shares subject to the first stock option.
The Executive Chairman Agreement also entitled Mr. Hale to RSUs. Mr. Hale received a time-based RSU award for 428,597 shares of our preferred stock (equivalent to 10,204 shares of common stock), to fully vest and settle upon a change in control or initial public offering during the period of his continuous service. Mr. Hale would receive a prorated portion of such shares if the change in control or initial public offering occurred within 10 years after January 1, 2011 but after the involuntary termination of his continuous
101
service. The proration would be based upon the number of months he provided continuous service to us divided by 48; but the RSUs would be deemed vested in full upon his involuntary termination without cause, provided that he gives us an effective waiver and release of claims. In connection with the closing of our initial public offering on February 10, 2014, 10,204 shares of common stock vested as settlement of the time-based RSUs and became issuable subsequent to the expiration of the 180 day lock-up period.
The Executive Chairman Agreement also entitled Mr. Hale to a performance-based RSU award, which is divided into three equal tranches, each representing shares of our preferred stock equal to 0.5% of our fully-diluted equity capitalization, and each to fully vest (subject to satisfaction of milestones) and settle upon a change in control or initial public offering occurring within 10 years after January 1, 2011. The tranches were associated with achievement of a specified commercial milestone, a specified funding milestone, and specified leadership milestones. The Executive Chairman Agreement provided that if a change in control or initial public offering occurred during the time of his continuous service but before the performance requirements were achieved, he would be entitled to receive 0.5% of our fully-diluted equity capitalization as of immediately before such event for each of the three tranches. In connection with the closing of our initial public offering on February 10, 2014, 53,662 shares of common stock vested as settlement of the performance-based RSUs and became issuable subsequent to the expiration of the 180 day lock-up period, and was determined by the amount equal to 1.5% of our fully-diluted equity capitalization as of immediately before the closing of our initial public offering.
On August 8, 2013, an RSU award for 14,285 shares of common stock with a grant date fair value of $73,996 was granted to Mr. Hale under our 2013 Equity Incentive Plan. The share amount for the RSU award was determined by dividing the award value by $5.18, which was the estimated fair value of our common stock on the date of grant. Subsequent to the date of grant, and for the purposes of recording share-based compensation expense in accordance with FASB ASC Topic 718, the grant date fair value of this award was estimated to be $80,000. The RSU award vests 50% upon date of grant and the remaining 50% in equal monthly installments over 24 months beginning August 1, 2013.
On July 31, 2013, an option award exercisable into 25,000 shares of common stock with a grant date fair value of $108,785 was granted to Mr. Hale under our 2013 Equity Incentive Plan. The exercise price of this award of $5.18 per share is equal to the estimated fair value of our common stock on the date of grant. The share amount for the option award was determined by dividing the award value by $4.35, which is the fair value of the option exercisable into our common stock on the date of grant, estimated using a Black-Scholes pricing model. Subsequent to the date of grant, and for the purposes of recording share-based compensation expense in accordance with FASB ASC Topic 718, the grant date fair value of common stock was estimated to be $5.60 per share. The assumptions used in the Black-Scholes pricing model include a volatility of 105.0%, a risk free interest rate of 1.38%, a dividend yield of 0.00%, and an expected term of 5.26 years. The option award vests 50% upon date of grant and the remaining 50% in equal monthly installments over 24 months beginning August 1, 2013, with a term of 10 years.
At the request of our board of directors, Mr. Hale continued to serve as our Executive Chairman of the Board of Directors from January 1, 2014 until February 10, 2014, at a salary of $12,500 per month. As of and in connection with the closing of our initial public offering on February 10, 2014, Mr. Hale now serves as our non-executive Chairman of the Board of Directors. On February 21, 2014, in accordance with the terms of the Executive Chairman Agreement, and in connection with the closing of our initial public offering on February 10, 2014, Mr. Hale received an additional option award to purchase 53,108 shares of common stock under our 2013 Equity Incentive Plan with an exercise price of $9.11 per share, which is the closing price of our common stock on the NASDAQ on the date of grant. The number of stock options awarded was determined as the excess of (i) 1% of our fully-diluted equity capitalization as of immediately after the initial public offering over (ii) the number of shares subject to the first stock option previously granted to Mr. Hale. The fair value of the option exercisable into our common stock on the date of grant of $337,601 was estimated using a Black-Scholes pricing model. The assumptions used in the Black-Scholes pricing model include a volatility of 90.0%, a risk free interest rate of 1.56%, a dividend yield of 0.00%, and an expected term of 5.00 years. The option award vested fully on the date of grant with a term of 10 years.
On February 13, 2014, option awards exercisable into 70,000 shares of common stock with an aggregate grant date fair value of $491,822 were granted to Mr. Hale under our 2013 Equity Incentive Plan as a component of the automatic annual equity compensation for services performed as a non-employee member of our board of directors. The exercise price of these awards of $8.88 per share is equal to the closing price of our common stock on the date of grant. The share amounts for the option awards were determined by dividing the award values by $7.03, which is the fair value of the option exercisable into our common stock on the date of grant, estimated using a Black-Scholes pricing model. The assumptions used in the Black-Scholes pricing model include a volatility of 100.0%, a risk free interest rate of 1.84%, a dividend yield of 0.00%, and an expected term of 6.00 years. The option awards vest in equal annual installments over three years beginning February 13, 2014 with a term of 10 years.
Annual Incentive Plan
On May 19, 2014, the compensation committee of our board of directors approved an annual incentive plan, or the Annual Incentive Plan, to provide our employees, including our executive officers, with an incentive for such employees to perform to the best
102
of their abilities, to further the our growth, development and financial success, and to enable us to attract and retain highly qualified employees. Each executive officer is eligible for an award based upon the achievement of certain corporate performance goals and objectives approved by the compensation committee and, with respect to our executive officers other than our chief executive officer, individual performance. In 2014, no compensation was paid pursuant to the Annual Incentive Plan.
Salary Deferrals
Pursuant to written agreements with David F. Hale and one other employee, we deferred payment of portions of such individuals’ salaries in 2013 and 2014. In exchange we agreed to pay 8% per annum interest (compounded monthly) on the deferred amounts and to award them each, based on their election, either 357 common stock options or 357 RSU awards. On February 13, 2014, the compensation committee of our board of directors approved the payment of an aggregate $1,009,552 in deferred salary obligations, including contractual interest, to current and former named executive officers pursuant to previously existing agreements, which was fully disbursed by April 2014 using the net proceeds from our initial public offering. An additional $344,883 in deferred salary obligations and interest thereon was paid to former employees other than named executive officers. All deferred salaries and interest thereon have been paid by December 31, 2014.
Outstanding Equity Awards
The following table sets forth certain information, on an award-by-award basis, concerning unexercised options to purchase common stock and common stock that has not yet vested for each named executive officer and outstanding as of December 31, 2014.
|
|
|
|
|
Option Awards |
|
Restricted Stock Units |
||||||||
|
Name |
|
Grant Date |
|
Number of Securities Underlying Unexercised Options (#) Exercisable |
|
Number of Securities Underlying Unexercised Options (#) Unexercisable(1) |
|
Option Exercise Price ($) |
|
Option Expiration Date |
|
Number of Unvested Securities Underlying (#)(2) |
|
Market Value of Units that are Unvested ($)(3) |
|
Michael W. Nall |
|
7/31/2013 |
|
82,915 |
|
— |
|
5.18 |
|
7/31/2023 |
|
— |
|
— |
|
|
|
7/31/2013 |
|
17,085 |
|
— |
|
5.18 |
|
7/31/2023 |
|
— |
|
— |
|
|
|
6/12/2014 |
|
9,375 |
|
2,555 |
|
5.35 |
|
6/12/2024 |
|
— |
|
— |
|
|
|
6/12/2014 |
|
— |
|
63,070 |
|
5.35 |
|
6/12/2024 |
|
— |
|
— |
|
|
|
8/8/2013 |
|
— |
|
— |
|
— |
|
— |
|
2,381 |
|
5,905 |
|
|
|
6/12/2014 |
|
— |
|
— |
|
— |
|
— |
|
44,496 |
|
110,350 |
|
William G. Kachioff |
|
8/9/2011 |
|
2,480 |
|
496 |
|
4.62 |
|
8/9/2021 |
|
— |
|
— |
|
|
|
8/9/2011 |
|
2,480 |
|
496 |
|
4.62 |
|
8/9/2021 |
|
— |
|
— |
|
|
|
7/31/2013 |
|
16,269 |
|
2,778 |
|
5.18 |
|
7/31/2023 |
|
— |
|
— |
|
|
|
2/21/2014 |
|
1,190 |
|
— |
|
9.11 |
|
2/21/2024 |
|
— |
|
— |
|
|
|
5/16/2014 |
|
7,291 |
|
42,709 |
|
4.38 |
|
5/16/2024 |
|
— |
|
— |
|
|
|
8/8/2013 |
|
— |
|
— |
|
— |
|
— |
|
1,786 |
|
4,429 |
|
Raaj Trivedi |
|
3/24/2014 |
|
— |
|
37,521 |
|
7.50 |
|
3/24/2024 |
|
— |
|
— |
|
|
|
3/24/2014 |
|
— |
|
5,479 |
|
7.50 |
|
3/24/2024 |
|
— |
|
— |
|
David F. Hale |
|
7/31/2013 |
|
21,354 |
|
3,646 |
|
5.18 |
|
7/31/2023 |
|
— |
|
— |
|
|
|
2/13/2014 |
|
— |
|
70,000 |
|
8.88 |
|
2/13/2024 |
|
— |
|
— |
|
|
|
2/21/2014 |
|
53,108 |
|
— |
|
9.11 |
|
2/21/2024 |
|
— |
|
— |
|
|
|
8/8/2013 |
|
— |
|
— |
|
— |
|
— |
|
2,381 |
|
5,905 |
|
(1) |
The scheduled vesting dates, after December 31, 2014, of these options were as follows: |
Mr. Nall: For the first option award granted on July 31, 2013 in the table above, 33,333 shares are vested and exercisable, while 49,582 shares are unvested but exercisable as of December 31, 2014. 2,083 of the unvested option awards granted will vest each month from January 2015, subject to continuing service, until 100% of the option shares are vested. For the second option award granted on July 31, 2013 in the table above, no shares are vested and 17,085 shares are unvested but exercisable as of December 31, 2014. 410 of the unvested option awards granted will vest in December 2016, and 2,083 will vest each month from January 2017 until 100% of the option shares are vested, subject to continuing service. For the first option award granted on June 12, 2014 in the table above, 1,562 of the unvested option awards granted will vest each month from January 2015, subject to
103
continuing service, until 100% of the option shares are vested. For the second option award granted on June 12, 2014 in the table above, 569 of the unvested option awards granted will vest in February 2015, and 1,562 will vest each month from March 2015 until 100% of the option shares are vested, subject to continuing service.
Mr. Kachioff: For each of the two unvested option awards granted on August 9, 2011 in the table above, 62 of the unvested option shares will vest each month from January 2015, subject to continuing service, until 100% of the option shares are vested. 396 of the unvested option awards granted on July 31, 2013 in the table above will vest each month from January 2015, subject to continuing service, until 100% of the option shares are vested. 1,041 of the unvested option awards granted on May 16, 2014 in the table above will vest each month from January 2015, subject to continuing service, until 100% of the option shares are vested.
Mr. Trivedi: For the first option award granted on March 24, 2014 in the table above, 10,750 of the unvested option awards will vest in March 2015, and 895 of the unvested option awards granted will vest each month from April 2015, subject to continuing service, until 100% of the option shares are vested. For the second option award granted on March 24, 2014 in the table above, 79 of the unvested option awards granted will vest in September 2017, and 895 will vest each month from October 2017 until 100% of the option shares are vested, subject to continuing service.
Mr. Hale: 520 of the unvested option awards granted on July 31, 2013 in the table above will vest each month from January 2015, subject to continuing service, until 100% of the option shares are vested. 23,333 of the unvested option awards granted on February 13, 2014 in the table above will vest in each February 2015 and 2016, and 23,334 will vest in February 2017, subject to continuing service.
|
(2) |
The scheduled vesting dates, after December 31, 2014, of these unvested restricted shares are as follows: |
Mr. Nall: 297 of the unvested RSUs granted on August 8, 2013 in the table above will vest each month from January 2015, subject to continuing service, until 100% of the restricted shares are vested. For the 44,496 unvested performance RSU’s granted on June 12, 2014 in the table above, 11,125 will vest upon achievement of a minimum revenue target for 2015, 6,674 will vest upon achievement of a maximum EBITDA loss for 2015, 8,899 will vest upon achievement of a financial plan for 2015, 8,899 will vest upon achievement of a minimum value of strategic agreements for 2015, and 8,899 will vest upon achievement of implementing four new diagnostic test panels for 2015. The table above excludes 11,904 vested RSUs held by Mr. Nall that have not settled with a market value of $29,522 based on the closing price of our common stock of $2.48 on December 31, 2014.
Mr. Kachioff: 223 of the unvested RSUs granted on August 8, 2013 in the table above will vest each month from January 2015, subject to continuing service, until 100% of the restricted shares are vested. The table above excludes 8,928 vested RSUs held by Mr. Kachioff that have not settled with a market value of $22,141 based on the closing price of our common stock of $2.48 on December 31, 2014.
Mr. Hale: 297 of the unvested RSUs granted on August 8, 2013 in the table above will vest each month from January 2015, subject to continuing service, until 100% of the restricted shares are vested. The table above excludes 75,770 vested RSUs held by Mr. Hale that have not settled with a market value of $187,910 based on the closing price of our common stock of $2.48 on December 31, 2014.
|
(3) |
The market value is equal to the product of approximately $2.48, which is the closing price of our common stock on December 31, 2014, and the number of unvested RSUs. |
Potential Payments upon Termination or Change-In-Control
Our employment agreement with Mr. Nall provides that in the event of termination of his employment by us without cause or his resignation for good reason, the vesting of any of his outstanding unvested stock options and RSUs which would have vested over the following 12 months will accelerate (unless the applicable stock option or RSU agreement provides for more favorable acceleration terms). Also, in the event of a change of control, the vesting of 50% of any of Mr. Nall’s outstanding unvested stock options and RSUs will accelerate on the date of the change of control and the remaining unvested stock options and RSUs will vest on the earliest of (i) the date of the termination of his employment by us without cause, (ii) the date of his resignation for good reason, or (iii) the first anniversary of the change of control (unless the applicable stock option or RSU agreement provides for more favorable acceleration terms). (For example, the foregoing would not apply to Mr. Nall’s initial stock options grant, which would fully accelerate upon a change in control.) Our employment agreement with Mr. Nall further provides that if he has a separation from service as a result of his discharge by us without cause or his resignation with good reason then, provided that he gives us an effective waiver and release of claims, he will be entitled to 12 months’ salary and up to 12 months of COBRA premiums (or substantially equivalent health insurance coverage).
Our employment agreement with Mr. Kachioff provides that if his continuous service is terminated without cause or he resigns with good reason then, provided that he gives us an effective waiver and release of claims, he will be entitled to six months’ salary plus up to six months of COBRA premiums. However, if he is terminated without cause or he resigns with good reason within three
104
months before or 12 months after a change in control, then, provided that he gives us an effective waiver and release of claims, he will be entitled to 12 months’ salary plus up to 12 months of COBRA premiums. Additionally, all of his then-outstanding stock options will fully vest.
Our employment agreement with Mr. Hale provided that his stock option for 10,204 shares of common stock will fully vest in the event of a change in control (or upon the completion of our initial public offering). Because Mr. Hale early-exercised the stock option in November 2011, the same vesting and acceleration provisions now apply to the lapsing of our right to repurchase the exercised shares. The Executive Chairman Agreement also provided that Mr. Hale’s time-based RSU award for 428,597 shares of our preferred stock (equivalent to 10,204 shares of common stock) will fully vest and settle upon a change in control (or upon the completion of our initial public offering) during the period of his continuous service; he would receive a prorated portion of such shares if the change in control or initial public offering occurred within 10 years after January 1, 2011 but after the involuntary termination of his continuous service. The proration would be based upon the number of months he provided continuous service to us divided by 48; but the RSUs would be deemed vested in full upon his termination without cause, provided that he gives us an effective waiver and release of claims. The Executive Chairman Agreement also entitled Mr. Hale to a performance-based RSU award, which is divided into three equal tranches, each representing shares of our preferred stock equal to 0.5% of our fully-diluted equity capitalization, and each to settle upon a change in control (or upon the completion of our initial public offering) occurring within 10 years after January 1, 2011. The tranches were associated with achievement of a specified commercial milestone, a specified funding milestone, and specified leadership milestones. The Executive Chairman Agreement provides that if a change in control (or initial public offering) occurs during the time of his continuous service but before the performance requirements are achieved, he will be entitled to receive 0.5% of our fully-diluted equity capitalization as of immediately before such event for each of the three tranches. Because Mr. Hale’s time-based and performance-based RSUs under the Executive Chairman Agreement both vested upon the closing of our initial public offering on February 10, 2014, Mr. Hale would receive no additional payments thereunder if a change in control occurs after the closing of our initial public offering.
The common stock RSUs granted to five of our non-employee directors under the 2007 Equity Incentive Plan provide for acceleration of vesting in the event of a change in control or the director’s involuntary removal from the board of directors by our shareholders without cause.
A total of 13,095 stock options granted to five of our non-employee directors under the 2007 Equity Incentive Plan were amended in February 2012 to provide for acceleration of vesting in the event of the director’s involuntary removal from the board of directors by our shareholders without cause, provided that the director gives us an effective waiver and release of claims.
In October 2010, 390,000 preferred stock RSUs (equivalent to 9,285 shares of common stock) were granted to Dr. Royston, which vest only upon a change in control or the effectiveness of an underwriting agreement for an initial public offering within 10 years. Since Dr. Royston was still serving on the board at the closing of our initial public offering in February 2014, 9,285 shares of common stock vested then as settlement of the RSU’s and became issuable subsequent to the expiration of the 180 day IPO lock up period.
The vesting of all stock options and RSUs awarded under our 2013 Equity Incentive Plan will accelerate fully in the event that the optionee’s continuous service is terminated without cause, or the optionee resigns for good reason, within 10 days before or 12 months after a change in control. In addition, the vesting of all stock options and RSUs awarded in August 2013 to Mr. Hale, Mr. Nall, and Mr. Kachioff under our 2013 Equity Incentive Plan will, if the optionee’s continuous service persists through the first anniversary of a change in control, accelerate fully upon such first anniversary.
Director Compensation
In December 2010, our board of directors approved a resolution that each year on January 1, each non-employee director (with the exception of Mr. Neff) will be automatically granted an annual RSU award under the 2007 Equity Incentive Plan covering a number of shares of common stock equal to 0.25% of our fully diluted outstanding capital stock as of the December 31 immediately preceding the applicable grant date of the RSUs.
105
The following table reflects all compensation awarded to, earned by or paid to the non-employee directors during 2014:
|
Name |
|
Fees Earned or Paid in Cash ($)(1) |
|
|
Option Awards ($)(2) |
|
|
Restricted Stock Awards ($)(2) |
|
|
Total ($) |
|
|||
|
Marsha A. Chandler |
|
13,356 |
|
|
|
161,599 |
|
|
|
— |
|
|
$ |
174,955 |
|
|
Bruce E. Gerhardt |
|
13,356 |
|
|
|
161,599 |
|
|
|
— |
|
|
$ |
174,955 |
|
|
David F. Hale(3) |
|
88,889 |
|
|
|
491,822 |
|
|
|
— |
|
|
$ |
580,711 |
|
|
Bruce A. Huebner |
|
13,356 |
|
|
|
154,573 |
|
|
|
— |
|
|
$ |
167,929 |
|
|
Edward Neff |
|
13,356 |
|
|
|
161,599 |
|
|
|
— |
|
|
$ |
174,955 |
|
|
Ivor Royston, M.D. |
|
13,356 |
|
|
|
186,190 |
|
|
|
— |
|
|
$ |
199,546 |
|
|
M. Faye Wilson |
|
17,808 |
|
|
|
358,328 |
|
|
|
— |
|
|
$ |
376,136 |
|
|
(1) |
Cash compensation paid to non-employee directors for services performed during 2014 is effective as of the closing of our initial public offering on February 10, 2014 and pro-rated accordingly. |
|
(2) |
The amounts in the “Option Awards ($)” and “Restricted Stock Awards ($)” columns reflect the grant date fair values of stock option and RSU awards, respectively, granted during the year. These amounts are determined in accordance with the provisions of FASB ASC Topic 718, rather than an amount paid to or realized by the director. For a description of these stock option and RSU awards, see the first and second paragraphs of this “Director Compensation” section. |
|
(3) |
Mr. Hale was appointed as our Executive Chairman in March 2011. As of and in connection with the closing of our initial public offering on February 10, 2014, Mr. Hale now serves as non-executive Chairman. Amounts in the table above exclude fees earned or paid in cash, option awards, and restricted stock awards granted to Mr. Hale prior to February 10, 2014 as an employee. |
In 2013 our board of directors adopted a resolution that, beginning at the closing of our initial public offering, the previous non-employee directors automatic grant program will be terminated and, instead, non-employee members of our board of directors will be eligible to automatically receive annual cash and equity compensation, as follows:
|
· |
Annual Retainer. For service as a director: an annual cash retainer of $15,000. |
|
· |
Board Chair. For service as Board Chair: an annual cash retainer of $85,000 (in addition to an annual cash retainer of $15,000 as a director), plus an annual grant of an option to purchase 50,000 shares of common stock. |
|
· |
Lead Independent Director. For service as Lead Independent Director: an annual cash retainer of $20,000 (inclusive of the annual cash retainer of $15,000 as a director), plus an annual grant of an option to purchase 20,000 shares of common stock. |
|
· |
Audit Committee. |
|
· |
For service as Chair of the audit committee: an annual grant of an option to purchase 7,500 shares of common stock. |
|
· |
For service as member of the audit committee other than as its Chair: an annual grant of an option to purchase 3,000 shares of common stock. |
|
· |
Compensation Committee. |
|
· |
For service as Chair of the compensation committee: an annual grant of an option to purchase 5,000 shares of common stock. |
|
· |
For service as member of the compensation committee other than as its Chair: an annual grant of an option to purchase 2,000 shares of common stock. |
|
· |
Nominating and Corporate Governance Committee. |
|
· |
For service as Chair of the nominating and corporate governance committee: an annual grant of an option to purchase 3,000 shares of common stock. |
|
· |
For service as member of the nominating and corporate governance committee other than as its Chair: an annual grant of an option to purchase 1,500 shares of common stock. |
106
|
· |
Initial Post-IPO Equity Award. For each non-employee director serving at the time of the closing of our initial public offering: an annual grant of an option to purchase 20,000 shares of common stock. |
|
· |
Initial Awards. For each non-employee director who is initially elected or appointed to the board: an annual grant of an option to purchase 20,000 shares of common stock. |
|
· |
Subsequent Awards. |
|
· |
For each non-employee director who (i) has been serving on the board for at least six months as of the date of any annual meeting of our stockholders and (ii) will continue to serve as a non-employee director immediately following such meeting: an option to purchase 15,000 shares of common stock. |
|
· |
For each non-employee director who (i) has been serving as Chair of the board for at least six months as of the date of any annual meeting of our stockholders and (ii) will continue to serve as Chair of the board immediately following such meeting: an additional option to purchase 50,000 shares of common stock. |
The annual cash retainers will be earned and paid on a calendar quarterly basis, subject to proration in the case of service during only a portion of a calendar quarter.
The per share exercise price of each option granted under this program will equal the fair market value of a share of common stock on the date the option is granted. Each such stock option will vest and become exercisable in substantially equal installments on each of the first three anniversaries of the date of grant, subject to continuing in service on the board through each such vesting date; provided, that each such subsequent award will vest and/or become exercisable on the first anniversary of the date of grant, subject to continuing in service on the board through such vesting date; and provided further, that all stock options under the program will vest in full upon the occurrence of a change in control.
The term of each such stock option will be 10 years from the date the option is granted. Upon a non-employee director’s cessation of service on the board for any reason, his or her stock options granted under this program would, to the extent vested on the date of cessation of service, remain exercisable for 12 months following the cessation of his or her service on the board (or such longer period as the board may determine in its discretion on or after the date of such stock options).
Equity Compensation Plan Information
We have two equity incentive plans: the 2007 Equity Incentive Plan, and the 2013 Equity Incentive Plan. Each plan is described separately below, followed by a description of certain federal income tax consequences with respect to plans of these types.
2007 Equity Incentive Plan
The following is a summary of the material terms of our 2007 Equity Incentive Plan, as amended to date. This description is not complete. For more information, we refer you to the full text of the 2007 Equity Incentive Plan, which is filed as an exhibit to the registration statement of which this prospectus forms a part.
The purposes of the 2007 Equity Incentive Plan are: (i) to secure and retain the services of eligible employees, board members, consultants and other advisors to serve our company and its affiliates, (ii) to provide incentives for such persons to exert maximum efforts for the success of our company and its affiliates and (iii) to provide a means by which they can benefit from increases in the value of our common stock.
The 2007 Equity Incentive Plan authorizes the grant of the following types of awards: (i) nonstatutory stock options, or NSOs; (ii) incentive stock options, or ISOs; (iii) restricted stock awards; (iv) restricted stock unit awards, or RSUs; (v) stock appreciation rights, or SARs; (vi) performance stock awards; and (vii) other stock awards. Awards may be granted to employees, directors, consultants and other service providers of our company and its affiliates. However, ISOs may not be granted to non-employees.
We have authorized a total of 178,571 shares of common stock for issuance pursuant to all awards granted under the 2007 Equity Incentive Plan. The number of shares issued or reserved pursuant to the 2007 Equity Incentive Plan (or pursuant to outstanding awards) is subject to adjustment as a result of mergers, consolidations, reorganizations, stock splits, reverse stock splits, stock dividends and other changes in our common stock. Shares subject to awards that have been terminated, expired unexercised, forfeited, settled in cash or cancelled in accordance with the cancellation and regrant procedures under the 2007 Equity Incentive Plan will again become available for issuance under the 2007 Equity Incentive Plan. Shares of common stock used to pay the exercise price of awards will also again become available for issuance under the 2007 Equity Incentive Plan.
107
However, shares in the following categories may not again be made available for issuance as awards under the 2007 Equity Incentive Plan: (i) shares of common stock not issued or delivered as a result of the net settlement of outstanding awards, (ii) shares of common stock used to pay the exercise price of NSOs or ISOs, and (iii) shares of common stock used to pay withholding taxes related to awards.
As of December 31, 2014, 102,805 shares had been issued under the 2007 Equity Incentive Plan, 92,570 shares underlay outstanding awards, and 86,001 other shares remained available to be subjected to further awards.
Administration. Our board of directors administers the 2007 Equity Incentive Plan, subject to the board’s authority to delegate some or all of such administration to the Compensation Committee.
Performance Criteria. Vesting of any awards granted under the 2007 Equity Incentive Plan may be made subject to the satisfaction of one or more performance goals established by the board of directors, in addition to or instead of time-vesting. The performance goals may vary from participant to participant, group to group, and period to period. Performance goals may be weighted for different factors and measures.
Transferability. Unless otherwise determined by the board of directors, awards granted under the 2007 Equity Incentive Plan are generally not transferable other than by will or by the laws of descent and distribution.
Corporate Transaction. In the event we are acquired in a corporate transaction, as defined in the 2007 Equity Incentive Plan, unless otherwise provided in a written agreement between us and the holder of an outstanding 2007 Equity Incentive Plan award, the award will be assumed by the successor company or a similar award will be substituted by the successor company. If the successor company does not agree to assume or substitute the award, the vesting of the award will accelerate and the award will become exercisable in full.
Effectiveness of the 2007 Equity Incentive Plan; Amendment and Termination. The 2007 Equity Incentive Plan became effective on March 6, 2007. The 2007 Equity Incentive Plan will remain available for the grant of awards until the day before the tenth anniversary of the effective date. The board may amend, alter or discontinue the 2007 Equity Incentive Plan in any respect at any time, subject to certain exceptions, but no amendment may adversely affect the rights of a participant under any awards previously granted, without his or her consent, except that stockholder approval will be needed if required by applicable law.
The 2007 Equity Incentive Plan permits us to reprice any stock option granted under the plan without the approval of our stockholders.
2013 Equity Incentive Plan
The following is a summary of the material terms of our 2013 Equity Incentive Plan. This description is not complete. For more information, we refer you to the full text of the 2013 Equity Incentive Plan, which is filed as an exhibit to the registration statement of which this prospectus forms a part.
The purposes of the 2013 Equity Incentive Plan are: (i) to enable us to attract and retain the types of qualified employees, officers, directors, consultants and other service providers who will contribute to our long range success; (ii) to align the interests of employees, officers, directors, consultants and other service providers with those of our stockholders; and (iii) to promote the success of our business.
The 2013 Equity Incentive Plan authorizes the grant of the following types of awards: NSOs, ISOs, SARs, restricted stock, RSUs, and performance compensation awards. Awards may be granted to employees, officers, non-employee board members, consultants and other service providers of our company and its affiliates. However, ISOs may be granted only to employees, including officers.
We have authorized a total of 403,571 shares of common stock for issuance pursuant to all awards granted under the 2013 Equity Incentive Plan, subject to an increase of 800,000 shares upon the completion of our initial public offering and subject to additional increases every January 1 beginning January 1, 2015 equal to the lesser of (i) 5% of our outstanding common stock on such January 1 and (ii) a number of shares determined by our board in its discretion for use on such particular January 1. The number of shares issued or reserved pursuant to the 2013 Equity Incentive Plan, or pursuant to outstanding awards, is subject to adjustment as a result of mergers, consolidations, reorganizations, stock splits, reverse stock splits, stock dividends and other changes in our common stock. Shares subject to awards that have been cancelled, expired unexercised, or forfeited do not count as shares issued under the 2013 Equity Incentive Plan, and therefore will again to that extent become available for issuance under the 2013 Equity Incentive Plan. However, shares in the following categories may not again be made available for issuance as awards under the 2013 Equity Incentive
108
Plan: (i) shares of common stock not issued or delivered as a result of the net settlement of outstanding NSOs or ISOs, (ii) shares of common stock used to pay the exercise price of NSOs or ISOs, (iii) shares of common stock used to pay withholding taxes related to awards, or (iv) shares of common stock corresponding to the value of stock-designated SARs which are settled in cash.
In no event will any participant be granted under the 2013 Equity Incentive Plan in any one calendar year (i) NSOs, ISOs or SARs pursuant to which, in the case of NSOs or ISOs, the aggregate number of shares of common stock that may be acquired thereunder, or, in the case of SARs, the aggregate number of shares of common stock covered thereby, exceeds 357,142 shares, or (ii) any other types of awards covering in the aggregate over 35,714 shares of common stock. Also, the maximum number of shares of common stock subject to performance stock awards, other than NSOs, ISOs and SARs, payable to any one participant under the 2013 Equity Incentive Plan in any one performance period is 71,428 shares of common stock or, in the event such performance stock award is paid in cash, the equivalent cash value thereof on the first or last day of the performance period to which such award relates, as determined by the Compensation Committee. The maximum amount that can be paid in any calendar year to any participant pursuant to a performance cash bonus award under the 2013 Equity Incentive Plan is $1,000,000. In addition, the maximum number of shares of common stock that may be issued during the life of the 2013 Equity Incentive Plan under ISOs is 392,857 shares. If an award is settled in cash, the number of shares of common stock on which the award is based will count toward the applicable individual share limit.
As of December 31, 2014, 1,027,846 shares had been issued under the 2013 Equity Incentive Plan and underlay outstanding awards, and 175,725 other shares remained available to be subjected to further awards.
Administration. The 2013 Equity Incentive Plan is administered by our Compensation Committee. The Compensation Committee has the discretion to determine the individuals to whom awards may be granted under the 2013 Equity Incentive Plan, the number of shares of our common stock subject to each award, the type of award, the manner in which such awards will vest and the other conditions applicable to awards. The Compensation Committee is authorized to interpret the 2013 Equity Incentive Plan, to establish, amend and rescind any rules and regulations relating to the 2013 Equity Incentive Plan and to make any other determinations that it deems necessary or desirable for the administration of the 2013 Equity Incentive Plan. All decisions, determinations and interpretations by the Compensation Committee, and any rules and regulations under the 2013 Equity Incentive Plan and the terms and conditions of or operation of any award, are final and binding on all participants. Notwithstanding the foregoing, the board of directors also has authority to take action expressly or implicitly in the capacity of the administrator of the 2013 Equity Incentive Plan, and the board also may delegate, to the extent allowed under Delaware law, its authority to one or more of our officers with respect to awards that do not involve covered employees within the meaning of Internal Revenue Code Section 162(m) or “insiders” within the meaning of Section 16 of the Exchange Act.
Stock Options. The Compensation Committee will determine the exercise price and other terms for each option and whether the options will be NSOs or ISOs. The exercise price per share of each option will not be less than 100% of the fair market value of our common stock on the date of grant (or 110% of fair market value in the case of an ISO granted to a 10% stockholder), which, unless otherwise determined by the Committee, will be deemed to be the closing price of a share of our common stock on its principal exchange on the grant date. ISOs may be granted only to employees and are subject to certain other restrictions. To the extent an option intended to be an ISO does not qualify as an ISO, it will be treated as an NSO. A participant may exercise an option by written notice and payment of the exercise price in cash, or in the discretion of the Compensation Committee, in the form of an irrevocable commitment by a broker to pay over the net proceeds from a sale of the shares issuable under an option, the delivery of previously owned shares and/or withholding of shares deliverable upon exercise, net-exercise, or any combination of these methods, or in any other form of legal consideration that may be acceptable to the Compensation Committee. The maximum term of any option granted under the 2013 Equity Incentive Plan is 10 years from the grant date (or five years in the case of an ISO granted to a 10% stockholder). The 2013 Equity Incentive Plan does not permit us to reprice any stock option granted under the plan without the approval of our stockholders. The 2013 Equity Incentive Plan authorizes us to, but does not require us to, withhold from participants shares of common stock having a fair market value equal to our withholding obligation with respect to exercised NSOs.
Stock Appreciation Rights. The Compensation Committee may grant SARs independent of or in connection with an option. The Compensation Committee will determine the other terms applicable to SARs. The exercise price per share of each SAR will not be less than 100% of the fair market value of our common stock on the grant date, which, unless otherwise determined by the Committee, will be deemed to be the closing price of a share of our common stock on its principal exchange on the grant date. The price will be subject to adjustment for recapitalization or other changes in our common stock. The maximum term of any SAR granted under the 2013 Equity Incentive Plan will be 10 years from the grant date. Generally, each SAR will entitle a participant upon exercise to an amount equal to:
|
· |
the excess of the fair market value on the exercise date of one share of our common stock over the exercise price, multiplied by |
|
· |
the number of shares of common stock covered by the SAR. |
109
Payment may be made in shares of our common stock, in cash or partly in common stock and partly in cash, all as determined by the Compensation Committee.
Restricted Stock and Restricted Stock Units. The Compensation Committee will have the authority to award restricted common stock and/or RSUs under the 2013 Equity Incentive Plan. Restricted stock awards consist of shares of stock that are transferred to a participant subject to service and/or other restrictions that may result in forfeiture if specified conditions are not satisfied. Unless the Compensation Committee determines otherwise at the time the restricted stock award is granted, holders of restricted stock will have the right to vote the shares. RSUs confer the right to receive shares of our common stock, cash or a combination of shares and cash, at a future date upon or following the attainment of service and/or other conditions specified by the Compensation Committee. The Compensation Committee will determine the restrictions and conditions applicable to each award of restricted stock or RSUs, which may include performance-based conditions. The 2013 Equity Incentive Plan authorizes us to, but does not require us to, withhold from participants shares of common stock having a fair market value equal to our withholding obligation with respect to restricted stock and/or settled RSUs.
Performance Compensation Awards. The Compensation Committee may award performance stock awards under the 2013 Equity Incentive Plan. Performance stock awards are awards, denominated in shares of our common stock, cash or a combination thereof, which are earned during a specified performance period subject to the attainment of performance criteria, as established by the Compensation Committee. The Compensation Committee will determine the restrictions and conditions applicable to each stock award.
Performance Criteria. Vesting of awards granted under the 2013 Equity Incentive Plan may be subject to a requirement of continuous service and/or the satisfaction of one or more performance goals established by the Compensation Committee. The performance goals may vary from participant to participant, group to group, and period to period. Performance goals may be weighted for different factors and measures.
Transferability . Unless otherwise determined by the Compensation Committee, awards granted under the 2013 Equity Incentive Plan will generally not be transferable other than by will or by the laws of descent and distribution.
Change in Control. Unless otherwise provided in an award agreement, in the event of a participant’s termination of continuous service without cause or for good reason, but excluding termination as a result of resignation in the absence of good reason, during the 10 day period before a change in control or during the 12 month period following a change in control, all options and SARs will become immediately exercisable with respect to 100% of the shares subject to such options or SARs, and/or the restricted period will expire immediately with respect to 100% of the shares of restricted stock or RSUs as of the date of the participant’s termination of continuous service.
With respect to performance compensation awards, in the event of a change in control, all incomplete performance periods in respect of such award in effect on the date the change in control occurs will end on the date of such change and the Compensation Committee will (i) determine the extent to which performance goals with respect to each such performance period have been met based upon such audited or unaudited financial information then available as it deems relevant and (ii) cause to be paid to the applicable participant partial or full awards with respect to performance goals for each such performance period based upon the Compensation Committee’s determination of the degree of attainment of performance goals or, if not determinable, assuming that the applicable “target” levels of performance have been attained, or on such other basis determined by the Compensation Committee.
In addition, in the event of an anticipated change in control, the Compensation Committee may in its discretion and upon at least 10 days’ advance notice to the affected persons, cancel upon or immediately before the change in control any outstanding awards and pay to the holders thereof, in cash or stock, or any combination thereof, the value of such awards based upon the value per share of common stock received or to be received or deemed received by our other stockholders in the event. In the case of any option or SAR with an exercise price that equals or exceeds the price paid for a share of common stock in connection with the change in control, the Compensation Committee may cancel the option or SAR without the payment of consideration therefor.
Effectiveness of the 2013 Equity Incentive Plan; Amendment and Termination. The 2013 Equity Incentive Plan was adopted and approved by our board of directors on July 31, 2013 and approved by our stockholders on August 6, 2013. The 2013 Equity Incentive Plan will remain available for the grant of awards until the tenth anniversary of the effective date. The board may amend, alter or discontinue the 2013 Equity Incentive Plan in any respect at any time, but no amendment may impair the rights of a participant under any awards previously granted, without his or her consent, except that stockholder approval will be needed for any amendment that would increase the maximum number of shares available for awards (other than the increase that occurs every January 1), reduce the exercise price of outstanding options or SARs, or if otherwise required by applicable law or stock market requirements.
110
Federal Income Tax Consequences
Following is a summary of the federal income tax consequences of option and other awards under the 2007 Equity Incentive Plan and 2013 Equity Incentive Plan. Optionees and recipients of other rights and awards granted under the 2007 Equity Incentive Plan or the 2013 Equity Incentive Plan are advised to consult their personal tax advisors before exercising an option, stock appreciation right or award or disposing of any stock received pursuant to the exercise of an option, stock appreciation right or award. In addition, the following summary is based upon an analysis of the Code (the Internal Revenue Code of 1986, as amended and as currently in effect), existing laws, judicial decisions, administrative rulings, regulations and proposed regulations, all of which are subject to change and does not address state, local or other tax laws.
Treatment of Options. The Code treats ISOs and NSOs differently. However, as to both types of options, no income will be recognized to the optionee at the time of the grant of the options under the 2007 Equity Incentive Plan or the 2013 Equity Incentive Plan.
Generally, upon exercise of an NSO, including an option intended to be an ISO but which has not continued to so qualify at the time of exercise, an optionee will recognize ordinary income tax on the excess of the fair market value of the stock on the exercise date over the option price. In general, if an optionee, in exercising an NSO, tenders shares of our common stock in partial or full payment of the option price, no gain or loss will be recognized on the tender. However, if the tendered shares were previously acquired upon the exercise of an ISO and the tender is within two years after the date of grant or within one year after the date of exercise of the ISO, the tender will be a disqualifying disposition of the shares acquired upon exercise of the ISO.
For ISOs, there is no taxable income to an optionee at the time of exercise. However, the excess of the fair market value of the stock on the date of exercise over the exercise price will be taken into account in determining whether the alternative minimum tax will apply for the year of exercise. If the shares acquired upon exercise are held until at least two years from the date of grant and more than one year from the date of exercise, any gain or loss upon the sale of such shares, if held as capital assets, will be long-term capital gain or loss, measured by the difference between the sales price of the stock and the exercise price. Under current federal income tax law, a long-term capital gain will be taxed at a rate which is less than the maximum rate of tax on ordinary income. If the two-year and one-year holding period requirements are not met, an optionee will recognize ordinary income in the year of disposition in an amount equal to the lesser of (i) the fair market value of the stock on the date of exercise minus the exercise price or (ii) the amount realized on disposition minus the exercise price. The remainder of the gain will be treated as long-term capital gain, depending upon whether the stock has been held for more than a year. If an optionee makes such a disposition, he or she will be obligated to notify us.
In general, if an optionee, in exercising an ISO, tenders shares of our common stock in partial or full payment of the option price, no gain or loss will be recognized on the tender. However, if the tendered shares were previously acquired upon the exercise of another ISO and the tender is within two years after the date of grant or within one year after the date of exercise of the other option, the tender will be a disqualifying disposition of the shares acquired upon exercise of the other option.
As noted above, the exercise of an ISO could subject an optionee to the alternative minimum tax. The application of the alternative minimum tax to any particular optionee depends upon the particular facts and circumstances which exist with respect to the optionee in the year of exercise. However, as a general rule, the amount by which the fair market value of the common stock on the date of exercise of an option exceeds the exercise price of the option will constitute an item of “adjustment” for purposes of determining the alternative minimum taxable income on which the alternative tax may be imposed. As such, this item will enter into the tax base on which the alternative minimum tax is computed and may therefore cause the alternative minimum tax to become applicable in any given year.
Treatment of Stock Appreciation Rights. Generally, the recipient of a stock appreciation right will not recognize any income upon grant of the stock appreciation right. Upon exercise of a stock appreciation right, the holder will recognize ordinary income equal to the fair market value of our common stock at that time.
Treatment of Restricted Stock Awards. Generally, absent an election to be taxed currently under Section 83(b) of the Code, or a Section 83(b) Election, there will be no federal income tax consequences to the recipient upon the grant of a restricted stock award. At the expiration of the restriction period and the satisfaction of any other restrictions applicable to the restricted shares, the recipient will recognize ordinary income equal to the fair market value of our common stock at that time. If a Section 83(b) Election is made within 30 days after the date the restricted stock award is granted, the recipient will recognize an amount of ordinary income at the time of the receipt of the restricted shares equal to the fair market value, determined without regard to applicable restrictions, of the shares of our common stock at such time. If a Section 83(b) Election is made, no additional income will be recognized by the recipient upon the lapse of restrictions on the shares, and before the sale of such shares, but, if the shares are subsequently forfeited, the recipient may not deduct the income that was recognized pursuant to the Section 83(b) Election at the time of the receipt of the shares.
111
The recipient of an unrestricted stock award will recognize ordinary income equal to the fair market value of our common stock that is the subject of the award when the award is made.
The recipient of an RSU will recognize ordinary income as and when the units vest. The amount of the income will be equal to the fair market value of the shares of our common stock issued at that time. The recipient of an RSU will not be permitted to make a Section 83(b) Election with respect to such award.
Treatment of Performance Share Awards. The federal income tax consequences of performance share awards, performance unit awards, other cash-based awards and other stock-based awards will depend on the terms and conditions of those awards.
Tax Withholding. We have the right to deduct or withhold, or require a participant to remit to us, the amount required to satisfy minimum statutory withholding requirements of federal, state and local tax laws and regulations, domestic or foreign, with respect to any taxable event arising as a result of the 2007 Equity Incentive Plan or the 2013 Equity Incentive Plan.
Inapplicability of Code Sections and ERISA. Sections 401(a) and 401(k) of the Code and the provisions of the Employee Retirement Income Security Act of 1974 are not applicable to the 2007 Equity Incentive Plan or the 2013 Equity Incentive Plan.
112
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation arrangements for named executive officers and directors, we describe below each transaction and series of similar transactions, since January 1, 2012, to which we were a party or will be a party, in which the amount exceeds $120,000 (or, if less, 1% of the average of our total assets amount at December 31, 2013 and December 31, 2014) and in which any related person had or will have a direct or indirect material interest.
Compensation arrangements for our named executive officers and directors are described in the section entitled “Executive Compensation.”
Claire K. T. Reiss
From time to time, Claire K. T. Reiss, who is our controlling stockholder and at all times described in this section was also a director of Biocept, individually and through entities affiliated with her has loaned us operating funds through various convertible and non-convertible debt instruments. These entities consist of Reisung Enterprises, Inc., of which Mrs. Reiss is the owner and president, and family trusts of which Mrs. Reiss is the trustee. Mrs. Reiss resigned from the board of directors on August 14, 2013.
In February 2011, we executed a note and warrant purchase agreement with Mrs. Reiss’s trusts. In exchange for a series of loans, we issued secured convertible promissory notes and warrants to purchase shares of our preferred stock to the trusts. The aggregate borrowing amount allowable under the February 2011 note and warrant purchase agreement was initially $5.0 million and was subsequently raised to $6.0 million, then $12.0 million and then $15.0 million, and the funding period was extended first to February 2012 and then to December 2012. The notes bore interest at 8%, payable at maturity. Under this note and warrant purchase agreement, we issued notes payable of $1.25 million and $10.0 million to Mrs. Reiss’ family trusts and Reisung Enterprises, Inc. during 2012 and 2011, respectively. The notes matured during 2012, and all principal of these notes was unpaid at December 31, 2012. In June 2013, Mrs. Reiss’ family trusts and Reisung Enterprises, Inc. converted the entire principal amount of $11.25 million and accrued interest of $1.7 million due on these notes into 24,002,689 shares of Series A preferred stock. The family trusts and Reisung Enterprises, Inc. retained the 4,166,667 preferred stock warrants they received under the 2011 note and warrant purchase agreement. Such warrants terminated unexercised upon the closing of our initial public offering.
In January 2012, we executed a note and warrant purchase agreement with several shareholders, including Mrs. Reiss’ family trusts. The aggregate borrowing amount allowable under the January 2012 note and warrant purchase agreement was initially $3.35 million and was subsequently raised to $8.35 million, and the funding period was extended to December 2012. The notes bore interest at 10%, payable at maturity. Under this note and warrant purchase agreement, we issued notes payable to Mrs. Reiss’ family trusts and Reisung Enterprises, Inc. for an aggregate principal amount of $5.8 million during 2012. The notes matured during 2012, and all principal and accrued interest on these notes was unpaid at December 31, 2012. In June 2013, Mrs. Reiss’ family trusts and Reisung Enterprises, Inc. converted the entire principal amount of $5.8 million and accrued interest of $627,000 due on these notes into 11,921,156 shares of Series A preferred stock. The family trusts and Reisung Enterprises, Inc. retained the 2,151,852 preferred stock warrants they received under the 2012 note and warrant purchase agreement; such warrants terminated unexercised upon the closing of our initial public offering. The number of warrants exercisable under this series of warrant agreements was determined by dividing the warrant coverage amount of 20% by the exercise price. The exercise price of the warrants was $0.54.
As of June 2013, we executed a note and warrant purchase agreement with several shareholders, including a family trust affiliated with Mrs. Reiss and Reisung Enterprises, Inc., to reflect certain prior and possible future borrowings under a series of notes, totaling up to $7.0 million. We had borrowed $0.72 million under this arrangement from Mrs. Reiss’ family trust before December 31, 2012 and we borrowed another $1.8 million under it from her family trust and Reisung Enterprises, Inc. in 2013. The maturity date of each note was May 31, 2014 and may be extended for two successive six month periods. Each note bears interest at 8.0% per annum, payable at maturity. The principal amount of and accrued interest on each note automatically convert into common stock upon the closing of an underwritten initial public offering resulting in at least $8.0 million of gross proceeds to us, at a conversion price equal to the price per share of our common stock sold in our initial public offering. The number of shares underlying the associated common stock warrants is determined by dividing the warrant coverage amount, which is 50% of the loan principal, by the exercise price, which was set at the price per share of our common stock sold in our initial public offering. As of December 31, 2013, the aggregate amount of principal and accrued interest outstanding for amounts we borrowed from Mrs. Reiss and entities affiliated with her under this arrangement was $2,682,328. In connection with the closing of our initial public offering on February 10, 2014, the aggregate amount outstanding related to this arrangement of $2,704,839 converted at $10.00 per share into a total of 270,484 shares of common stock, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 125,250 shares of common stock. The warrants became exercisable for a five year period beginning on the closing of our initial public offering.
113
In July 2013, we and one of Mrs. Reiss’ family trusts amended a $1.4 million promissory note which we had issued to the trust in 2008 to provide that the entire principal amount of and accrued interest on such note would automatically convert, upon the closing of an initial public offering, into shares of our common stock at a price per share equal to the offering price per share to the public in such offering. As of December 31, 2013, the aggregate amount of principal and accrued interest outstanding on such note was $1,628,871. In connection with the closing of our initial public offering on February 10, 2014, the $1,633,982 aggregate amount outstanding related to this arrangement converted at $10.00 per share into a total of 163,399 shares of common stock.
As compensation for guaranteeing our Line of Credit, which had an initial credit availability of $1.5 million with two other guarantors and now approximately $2.6 million with five other guarantors, a family trust affiliated with Mrs. Reiss received common stock warrants from us. The number of shares underlying the common stock warrants is determined by dividing the warrant coverage amount, which is 50% of the fair market value of the collateral provided by the family trust to secure the trust’s guaranty obligations to UBS Bank USA, by the exercise price, which was set at the price per share of our common stock sold in our initial public offering. At the closing of our initial public offering on February 10, 2014, the fair market value of the collateral provided by the family trust under this arrangement was $1,176,042, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 58,802 shares of common stock. The warrants became exercisable for a two year period beginning on the closing of our initial public offering.
Edward Neff
Edward Neff, a member of our board of directors, is the chief executive officer and owner of Systems, Machines, Automation Components Corporation (SMAC), a company which has loaned us operating funds under convertible debt arrangements and provided financing for certain fixed asset purchases.
Under the note and warrant purchase agreement executed in February 2011, we borrowed $125,000 and $425,000 from SMAC in 2011 and 2012, respectively. See details of the February 2011 note and warrant purchase agreement in the description of transactions with Claire K. T. Reiss, above. The principal and accrued interest on these notes was unpaid at December 31, 2012. In June 2013, SMAC converted the principal of $550,000 and accrued interest of $53,000 due on these notes into 1,116,498 shares of Series A preferred stock. SMAC retained 203,698 preferred stock warrants it received under the 2011 note and warrant purchase agreement. Such warrants terminated unexercised upon the closing of our initial public offering.
During 2011, we entered into two financing arrangements with SMAC, for the purchase of lab equipment from SMAC totaling $256,000, of which $138,000 and $60,000 was outstanding as of December 31, 2011 and 2012, respectively. The stated interest rate on each financing agreement was 0.0%. Under the first financing arrangement, the maximum amount which could be borrowed was $147,000, the largest amount of principal outstanding during the period from January 1, 2012 to date was $72,000, the principal amount outstanding on September 30, 2013 was $22,000, the amount of principal paid during the period from January 1, 2012 to date was $50,000, and the amount of imputed interest (calculated using a 8.00% per annum imputed interest rate) during the period from January 1, 2012 to date was $7,000. Under the second financing arrangement, the maximum amount which could be borrowed was $109,000, the largest amount of principal outstanding during the period from January 1, 2012 to date was $66,000, the principal amount outstanding on September 30, 2013 was $39,000, the amount of principal paid during the period from January 1, 2012 to date was $27,000, and the amount of imputed interest (calculated using a 8.00% per annum imputed interest rate) during the period from January 1, 2012 to date was $5,000.
As of June 2013, we executed a note and warrant purchase agreement with several shareholders, including SMAC, to reflect certain prior and possible future borrowings under a series of notes, totaling up to $7.0 million. See details of the June 2013 note and warrant purchase agreement in the description of transactions with Claire K. T. Reiss, above. We borrowed $25,000 from SMAC under this arrangement in 2012 and an additional $925,000 in 2013. As of December 31, 2013, the aggregate amount of principal and accrued interest outstanding for amounts we borrowed from SMAC under this arrangement was $997,393. In connection with the closing of our initial public offering on February 10, 2014, the aggregate amount outstanding related to this arrangement of $1,081,401 converted at $10.00 per share into a total of 108,140 shares of common stock, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 51,249 shares of common stock. The warrants became exercisable for a five year period beginning on the closing of our initial public offering.
As compensation for guaranteeing our Line of Credit, which had an initial credit availability of $1.5 million with two other guarantors and now approximately $2.6 million with four other guarantors, SMAC received common stock warrants from us. The number of shares underlying the common stock warrants is determined by dividing the warrant coverage amount, which is 50% of the fair market value of the collateral provided by SMAC to secure its guaranty obligations to UBS Bank USA, by the exercise price, which was set at the price per share of our common stock sold in our initial public offering. At the closing of our initial public offering on February 10, 2014, the fair market value of the collateral provided by SMAC under this arrangement was $576,000, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 28,800 shares of common stock. The warrants became exercisable for a two year period beginning on the closing of our initial public offering.
114
David F. Hale
Under the note and warrant purchase agreement executed in February 2011, we issued a note payable of $50,000 during 2011 to Hale BioPharma Ventures LLC, which is controlled by our Executive Chairman David F. Hale. Under the note and warrant purchase agreement executed in January 2012, we issued notes payable of $100,000 to Hale BioPharma Ventures LLC. See details of the February 2011 and January 2012 note and warrant purchase agreements in the description of transactions with Claire K. T. Reiss, above. The principal and interest on these notes was unpaid at December 31, 2012. In June 2013, Hale BioPharma Ventures LLC converted the entire $150,000 principal balance of and accrued interest of $18,000 due on these notes into 310,392 shares of our Series A preferred stock. Hale BioPharma Ventures LLC retained 55,555 preferred stock warrants it received under the 2011 and 2012 note and warrant purchase agreements. Such warrants terminated unexercised upon the closing of our initial public offering.
As of June 2013, we executed a note and warrant purchase agreement with several shareholders, including Hale BioPharma Ventures LLC, to reflect certain prior and possible future borrowings under a series of notes, totaling up to $7.0 million. See details of the June 2013 note and warrant purchase agreement in the description of transactions with Claire K. T. Reiss, above. We borrowed $443,500 under this arrangement from Hale BioPharma Ventures LLC in 2013. As of December 31, 2013, the aggregate amount of principal and accrued interest outstanding for amounts we borrowed from Hale BioPharma Ventures LLC under this arrangement was $467,822. In connection with the closing of our initial public offering on February 10, 2014, the aggregate amount outstanding related to this arrangement of $471,807 converted at $10.00 per share into a total of 47,180 shares of common stock, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 22,175 shares of common stock. The warrants became exercisable for a five year period beginning on the closing of our initial public offering.
As compensation for guaranteeing our Line of Credit, which had an initial credit availability of $1.5 million with two other guarantors and now approximately $2.6 million with four other guarantors, Hale BioPharma Ventures LLC received common stock warrants from us. The number of shares underlying the common stock warrants is determined by dividing the warrant coverage amount, which is 50% of the fair market value of the collateral provided by Hale BioPharma Ventures LLC to secure its guaranty obligations to UBS Bank USA, by the exercise price, which was set at the price per share of our common stock sold in our initial public offering. At the closing of our initial public offering on February 10, 2014, the fair market value of the collateral provided by Hale BioPharma Ventures LLC under this arrangement was $726,034, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 36,301 shares of common stock. The warrants became exercisable for a two year period beginning on the closing of our initial public offering.
M. Faye Wilson
Under the note and warrant purchase agreement executed in February 2011, we issued notes payable of $75,200 during 2011 to our director M. Faye Wilson and Wilson Boyles & Co., LLC, which is controlled by Ms. Wilson. Under the note and warrant purchase agreement executed in January 2012, we issued a note payable of $20,000 to Ms. Wilson. See details of the February 2011 note and warrant purchase agreement in the description of transactions with Claire K. T. Reiss, above. The principal and interest on these notes was unpaid at December 31, 2012. In June 2013, Ms. Wilson and Wilson Boyles & Co., LLC converted the entire $95,200 principal balance of and accrued interest of $10,000 due on these notes into 194,859 shares of our Series A preferred stock. Ms. Wilson retained 30,536 preferred stock warrants she received under the 2011 and 2012 note and warrant purchase agreements and Wilson Boyles & Co., LLC retained 4,722 preferred stock warrants it received under the 2011 and 2012 note and warrant purchase agreements. Such warrants terminated unexercised upon the closing of our initial public offering.
As of June 2013, we executed a note and warrant purchase agreement with several shareholders, including Ms. Wilson, to reflect certain prior and possible future borrowings under a series of notes, totaling up to $7.0 million. See details of the June 2013 note and warrant purchase agreement in the description of transactions with Claire K. T. Reiss, above. We borrowed $25,000 under this arrangement from Ms. Wilson in 2013. As of December 31, 2013, the aggregate amount of principal and accrued interest outstanding for amounts we borrowed from Ms. Wilson under this arrangement was $26,271. In connection with the closing of our initial public offering on February 10, 2014, the aggregate amount outstanding related to this arrangement of $26,496 converted at $10.00 per share into a total of 2,649 shares of common stock, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 1,250 shares of common stock. The warrants became exercisable for a five year period beginning on the closing of our initial public offering.
Bruce E. Gerhardt
Under the note and warrant purchase agreement executed in February 2011, we issued a note payable of $25,000 during 2011 to our director Bruce E. Gerhardt. Under the note and warrant purchase agreement executed in January 2012, we issued notes payable of $30,000 to Mr. Gerhardt. See details of the February 2011 note and warrant purchase agreement in the description of transactions with Claire K. T. Reiss, above. The principal and interest on these notes was unpaid at December 31, 2012. In June 2013, Mr. Gerhardt converted the entire $55,000 principal balance of and accrued interest of $7,000 due on these notes into 115,084 shares of our Series A
115
preferred stock. Mr. Gerhardt retained 20,370 preferred stock warrants he received under the 2011 and 2012 note and warrant purchase agreements. Such warrants terminated unexercised upon the closing of our initial public offering.
As of June 2013, we executed a note and warrant purchase agreement with several shareholders, including Mr. Gerhardt, to reflect certain prior and possible future borrowings under a series of notes, totaling up to $7.0 million. See details of the June 2013 note and warrant purchase agreement in the description of transactions with Claire K. T. Reiss, above. We borrowed $10,000 under this arrangement from Mr. Gerhardt in 2013. As of December 31, 2013, the aggregate amount of principal and accrued interest outstanding for amounts we borrowed from Mr. Gerhardt under this arrangement was $10,458. In connection with the closing of our initial public offering on February 10, 2014, the aggregate amount outstanding related to this arrangement of $10,548 converted at $10.00 per share into a total of 1,054 shares of common stock, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 500 shares of common stock. The warrants became exercisable for a five year period beginning on the closing of our initial public offering.
Subsequent to December 31, 2013, as compensation for guaranteeing our Line of Credit, which had an initial credit availability of $1.5 million with a total of three guarantors and now approximately $2.6 million with four other guarantors, Mr. Gerhardt received common stock warrants from us. The number of shares underlying the common stock warrants is determined by dividing the warrant coverage amount, which is 50% of the fair market value of the collateral provided by Mr. Gerhardt to secure his guaranty obligations to UBS Bank USA, by the exercise price, which was set at the price per share of our common stock sold in our initial public offering. At the closing of our initial public offering on February 10, 2014, the fair market value of the collateral provided by Mr. Gerhardt under this arrangement was $50,000, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 2,500 shares of common stock. The warrants became exercisable for a two year period beginning on the closing of our initial public offering.
Ivor Royston, M.D.
Under the note and warrant purchase agreement executed in February 2011, we issued a note payable of $100,000 during 2011 to the individual retirement account of our director Ivor Royston, M.D. See details of the February 2011 note and warrant purchase agreement in the description of transactions with Claire K. T. Reiss, above. The principal and interest on this note was unpaid at December 31, 2012. In June 2013, Dr. Royston’s IRA converted the entire $100,000 principal balance of and accrued interest of $10,000 due on this note into 204,059 shares of our Series A preferred stock. Dr. Royston’s IRA retained 37,037 preferred stock warrants it received under the 2011 note and warrant purchase agreement. Such warrants terminated unexercised upon the closing of our initial public offering.
Subsequent to December 31, 2013, as compensation for guaranteeing our Line of Credit, which had an initial credit availability of $1.5 million with a total of three guarantors and now approximately $2.6 million with four other guarantors, Dr. Royston received common stock warrants from us. The number of shares underlying the common stock warrants is determined by dividing the warrant coverage amount, which is 50% of the fair market value of the collateral provided by Dr. Royston to secure his guaranty obligations to UBS Bank USA, by the exercise price, which was set at the price per share of our common stock sold in our initial public offering. At the closing of our initial public offering on February 10, 2014, the fair market value of the collateral provided by Dr. Royston under this arrangement was $50,000, and the exercise price of the associated warrants was fixed at $10.00 per share for an aggregate of 2,500 shares of common stock. The warrants became exercisable for a two year period beginning on the closing of our initial public offering.
Lyle J. Arnold
Lyle J. Arnold, Ph.D., our Senior Vice-President of Research and Development and Chief Scientific Officer, is the controlling person of Aegea Biotechnologies, Inc. On June 2, 2012, we entered into an Assignment and Exclusive Cross-License Agreement with Aegea in regard to the CEE-Selector technology. Under the Agreement, each party has an undivided joint ownership interest in all of the patents and other intellectual property rights for such technology. We obtained an exclusive, worldwide, royalty-free, fully-paid, irrevocable, sublicensable license for all applications in the fields of oncology clinical testing and oncology diagnostics (including both laboratory developed tests and IVD tests as applied to the oncology field) and oncology basic and clinical research that is performed internally by us, as a service offered by us, or in a bona fide collaboration between us and one or more third parties (where the sample types tested are tissue, whole blood, bone marrow, cerebrospinal fluid or derivatives of any of such sample types); provided that any such collaboration must not be solely or primarily directed to providing research reagents or research technologies to such collaborator, and must not involve the sale or resale of patented research reagents or the licensing of technologies for patented research applications by such collaborator to third parties. Under the Agreement’s license, we are free of any obligation to obtain further consent from Aegea or to account to Aegea. Aegea obtained an exclusive, worldwide, royalty-free, fully-paid, irrevocable sublicensable license for all applications in all other fields, without any obligation to obtain further consent from us or to account to us. We were given responsibility for prosecuting some of the relevant patent applications, and Aegea was given responsibility for prosecuting others, but the two parties will share all patent prosecution and maintenance costs equally.
116
Goodman Co. Ltd.
In June 2013, Goodman Co. Ltd., a beneficial owner of more than 5% of our common stock, converted the entire principal amount of $1,935,000 and accrued interest of approximately $105,000 due on a secured promissory note held by it into 3,777,324 shares of Series A preferred stock. In connection with this conversion, we issued to Goodman Co. Ltd. a warrant to purchase 23,809 shares of common stock at an exercise price equal to the price per share of our common stock sold in our initial public offering. The warrants became exercisable for a two year period beginning on the closing of our initial public offering.
Indemnification Agreements
We have entered into indemnification agreements with each of our current directors and executive officers. These agreements will require us to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to us and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. We also intend to enter into indemnification agreements with our future directors and executive officers. In addition, our predecessor company Biocept, Inc., a California corporation, entered into indemnification agreements with certain of our current directors and executive officers and certain prior directors and executive officers. These agreements will require us to indemnify these individuals to the fullest extent permitted under California law against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified.
Policies and Procedures for Related Party Transactions
We adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock, any members of the immediate family of any of the foregoing persons and any firms, corporations or other entities in which any of the foregoing persons is employed or is a partner or principal or in a similar position or in which such person has a 5% or greater beneficial ownership interest, collectively, related parties, are not permitted to enter into a transaction with us without the prior consent of our board of directors acting through the audit committee. Any request for us to enter into a transaction with a related party in which the amount involved exceeds $120,000, and in which such related party would have a direct or indirect interest, must first be presented to our audit committee for review, consideration and approval. In approving or rejecting any such proposal, our audit committee is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances, the extent of the benefits to us, the availability of other sources of comparable products or services and the extent of the related person’s interest in the transaction.
Equity Awards
We have granted stock options to our executive officers and directors. For additional information, see “Executive Compensation—Outstanding Equity Awards.”
117
The following table sets forth the beneficial ownership of our common stock as of January 1, 2015 by:
|
· |
each person, or group of affiliated persons, whom we know to beneficially own more than 5% of our common stock; |
|
· |
each of our named executive officers; |
|
· |
each of our directors; and |
|
· |
all of our executive officers and directors as a group. |
The percentage ownership information under the column entitled “Before Offering” is based on 4,449,603 shares of common stock outstanding as of January 1, 2015. The percentage ownership information under the column entitled “After Offering” is based on the sale of shares of common stock in this offering.
We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable on or before March 2, 2015, which is 60 days after January 1, 2015. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Except as otherwise noted below, the address for persons listed in the table is c/o Biocept, Inc., 5810 Nancy Ridge Drive, San Diego, California 92121.
|
|
|
Number of Shares Beneficially Owned |
|
|
|
Percentage of Shares |
|
|||||
|
Name of Beneficial Owner |
|
|
|
|
Before Offering |
|
|
|
After Offering |
|
||
|
5% Stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
Claire K. T. Reiss(1) |
|
|
2,041,807 |
|
|
|
44.1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Named Executive Officers, Executive Officers and Directors: |
|
|
|
|
|
|
|
|
|
|
|
|
|
David F. Hale(2) |
|
|
298,452 |
|
|
|
6.4 |
% |
|
|
|
|
|
Marsha A. Chandler(3) |
|
|
22,312 |
|
|
|
* |
% |
|
|
|
|
|
Bruce E. Gerhardt(4) |
|
|
38,193 |
|
|
|
* |
% |
|
|
|
|
|
Bruce A. Huebner(5) |
|
|
14,401 |
|
|
|
* |
% |
|
|
|
|
|
Michael W. Nall(6) |
|
|
125,000 |
|
|
|
2.7 |
% |
|
|
|
|
|
Edward Neff(7) |
|
|
248,014 |
|
|
|
5.5 |
% |
|
|
|
|
|
Ivor Royston, M.D.(8) |
|
|
47,753 |
|
|
|
1.1 |
% |
|
|
|
|
|
M. Faye Wilson(9) |
|
|
54,942 |
|
|
|
1.2 |
% |
|
|
|
|
|
William G. Kachioff(10) |
|
|
42,335 |
|
|
|
* |
% |
|
|
|
|
|
Raaj Trivedi |
|
|
— |
|
|
|
* |
% |
|
|
|
|
|
All Executive Officers and Directors as a Group (12 persons) |
|
|
933,203 |
|
|
|
18.1 |
% |
|
|
|
|
|
* |
denotes less than 1%. |
|
(1) |
The number of shares currently beneficially owned includes outstanding shares held by various family trusts and Reisung Enterprises, Inc., a private corporation controlled by Mrs. Reiss. The calculation of the percentage of shares beneficially owned also includes 184,052 shares for which common stock warrants held by various family trusts and Reisung Enterprises, Inc., a corporation controlled by Mrs. Reiss, are exercisable at a price of $10.00 per share, the price of our common stock sold in our initial public offering. The address of Mrs. Reiss is 9675 La Jolla Farms Road, La Jolla, California 92037. |
|
(2) |
Includes 98,836 shares of common stock underlying stock options and 76,366 shares of common stock underlying restricted stock awards. Includes shares held by Hale BioPharma Ventures LLC, which is controlled by Mr. Hale, and shares held by the Hale Family Trust, which is controlled by Mr. Hale as co-trustee. The calculation of the percentage of shares beneficially owned also includes 58,476 shares for which common stock warrants held by Hale BioPharma Ventures LLC are exercisable at a price of $10.00 per share, the price of our common stock sold in our initial public offering. |
|
(3) |
Includes 14,734 shares of common stock underlying stock options. The number of shares currently beneficially owned also includes outstanding shares held by a family trust affiliated with Dr. Chandler. The calculation of the percentage of shares |
118
|
beneficially owned includes 2,500 shares for which common stock warrants held by Dr. Chandler are exercisable at a price of $10.00 per share, the price of our common stock sold in our initial public offering. |
|
(4) |
Includes 10,285 shares of common stock underlying stock options and 19,658 shares of common stock underlying restricted stock awards. The calculation of the percentage of shares beneficially owned also includes 3,000 shares for which common stock warrants held by Mr. Gerhardt are exercisable at a price of $10.00 per share, the price of our common stock sold in our initial public offering. |
|
(5) |
Includes 14,401 shares of common stock underlying stock options. |
|
(6) |
Includes 112,500 shares which Mr. Nall has the right to acquire from us within 60 days of January 1, 2015 pursuant to the exercise of stock options, 62,502 of which will be unvested but exercisable as of March 2, 2015. Includes 12,500 shares of common stock underlying restricted stock awards. |
|
(7) |
Includes 7,666 shares of common stock underlying stock options and 8,735 shares of common stock underlying restricted stock awards. The number of shares currently beneficially owned includes outstanding shares held by Systems, Machines, Automation Components Corporation, which is controlled by Mr. Neff. The calculation of the percentage of shares beneficially owned after our initial public offering also includes 80,049 shares for which common stock warrants held by Systems, Machines, Automation Components Corporation are exercisable at a price of $10.00 per share, the price of our common stock sold in our initial public offering. |
|
(8) |
Includes 11,452 shares of common stock underlying stock options and 28,943 shares of common stock underlying restricted stock awards. Includes shares owned by Dr. Royston’s individual retirement account. The calculation of the percentage of shares beneficially owned also includes 2,500 shares for which common stock warrants held by Dr. Royston are exercisable at a price of $10.00 per share, the price of our common stock sold in our initial public offering. |
|
(9) |
Includes 19,619 shares of common stock underlying stock options and 25,208 shares of common stock underlying restricted stock awards. Includes shares held by Ms. Wilson’s individual retirement account as well as Wilson Boyles & Co., LLC, a company controlled by Ms. Wilson. The calculation of the percentage of shares beneficially owned also includes 1,250 shares for which common stock warrants held by Ms. Wilson are exercisable at a price of $10.00 per share, the price of our common stock sold in our initial public offering. |
|
(10) |
Includes 32,960 shares of common stock underlying stock options and 9,375 shares of common stock underlying restricted stock awards. |
119
General
Our amended certificate of incorporation authorizes us to issue up to 40,000,000 shares of common stock, par value $0.0001 per share, and 5,000,000 shares of preferred stock, par value $0.0001 per share.
As of December 31, 2014, there were 4,449,603 shares of common stock outstanding, held of record by 206 stockholders.
The following description of our capital stock is not complete and is subject to and qualified in its entirety by our amended certificate of incorporation and amended and restated bylaws, which are filed as exhibits to the registration statement of which this prospectus is a part, and by the relevant provisions of the Delaware General Corporation Law.
Common Stock
The holders of our common stock are entitled to the following rights:
Voting Rights
Holders of our common stock are entitled to one vote per share in the election of directors and on all other matters on which stockholders are entitled or permitted to vote. Holders of our common stock are not entitled to cumulative voting rights.
Dividend Rights
Subject to the terms of any then outstanding series of preferred stock, the holders of our common stock are entitled to dividends in the amounts and at times as may be declared by the board of directors out of funds legally available therefor.
Liquidation Rights
Upon liquidation or dissolution, holders of our common stock are entitled to share ratably in all net assets available for distribution to stockholders after we have paid, or provided for payment of, all of our debts and liabilities, and after payment of any liquidation preferences to holders of any then outstanding shares of preferred stock.
Other Matters
Holders of our common stock have no redemption, conversion or preemptive rights. There are no sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to the rights of the holders of shares of any series of preferred stock that we may issue in the future.
Preferred Stock
Our board of directors has the authority to issue preferred stock in one or more classes or series and to fix the designations, powers, preferences and rights, and the qualifications, limitations or restrictions thereof, including dividend rights, conversion right, voting rights, terms of redemption, liquidation preferences and the number of shares constituting any class or series, without further vote or action by the stockholders. Although we have no present plans to issue any shares of preferred stock, the issuance of shares of preferred stock, or the issuance of rights to purchase such shares, could decrease the amount of earnings and assets available for distribution to the holders of common stock, could adversely affect the rights and powers, including voting rights, of the common stock, and could have the effect of delaying, deterring or preventing a change of control of us or an unsolicited acquisition proposal.
Stock Options
As of December 31, 2014, we had outstanding options to purchase an aggregate of 906,194 shares of our common stock with exercise prices ranging from $4.38 to $9.11 per share, with an approximate weighted average exercise price of $6.29 per share.
120
Warrants
We have outstanding warrants to purchase shares of our common stock as follows:
|
· |
Warrants to purchase 23,809 shares of our common stock at an exercise price of $10.00 per share, issued to Goodman Co. Ltd. in connection with the June 28, 2013 conversion of its secured promissory note into shares of our Series A preferred stock. The warrants became exercisable for a two-year period beginning at the closing of our initial public offering. |
|
· |
Warrants exercisable for 258,249 shares of our common stock at an exercise price of $10.00 per share, issued to investors pursuant to our June 2013 note and warrant purchase agreement. The warrants became exercisable for a five-year period beginning at the closing of our initial public offering. |
|
· |
Warrants exercisable for 128,903 shares of our common stock at an exercise price of $10.00 per share, issued to guarantors of our Line of Credit. The warrants became exercisable for a two-year period beginning at the closing of our initial public offering. |
|
· |
Warrants exercisable for 50,260 shares of our common stock at an exercise price of $10.00 per share, issued to our landlord in connection with our September 2013 lease amendment which was effective as of August 1, 2013. The warrants became exercisable for a five-year period beginning at the closing of our initial public offering. |
|
· |
Warrants exercisable for 1,587 shares of our common stock at an exercise price of $25.20 per share, issued to our landlord in connection with our September 2012 lease amendment. These warrants are exercisable through September 2019. |
|
· |
Warrant exercisable for 95,000 shares of our common stock at an exercise price of $12.50 per share, issued to the representative of the underwriters in connection with our initial public offering. This warrant is exercisable through February 2019. |
|
· |
Warrant exercisable for 52,966 shares of our common stock at an exercise price of $4.72 per share, issued to Oxford Finance LLC in connection with a loan and security agreement dated April 30, 2014. This warrant is exercisable through April 2024. |
Registration Rights
Under our amended and restated investor rights agreement dated October 31, 2011, two trusts affiliated with our major stockholder Claire K. T. Reiss and our landlord, have the right to include their shares in any registration statement we file. If we register any securities for public sale, these stockholders with registration rights will have the right to include their shares in the registration statement, provided that the underwriters of any such underwritten offering will have the right to limit the number of shares to be included in the registration statement, except this offering in which the holders have waived any and all rights to have their shares included. We will pay all expenses, including for the fees and costs of one counsel to the stockholders exercising their registration rights (not to exceed $25,000) relating to all piggyback registrations.
The registration rights described above will terminate, as to a given stockholder, upon the earlier of (i) at any time when such holder can sell all of such holder’s shares pursuant to Rule 144 promulgated under the Securities Act during any three-month period and (ii) the date three years following the closing of our initial public offering.
Under the terms of the warrant issued to the representative of the underwriters in connection with our initial public offering, the representative has demand and piggyback registration rights. The holder(s) of at least 51% of the registrable securities, as defined in the warrant, have the right, subject to specified exceptions, to make one demand that we file a registration statement to register all or a portion of their shares. We are not required to comply with the demand if we have filed a registration statement with respect to which the holder is entitled to piggyback registration rights as described below and either (i) the holder has elected to participate in the offering covered by such registration statement or (ii) if such registration statement relates to an underwritten primary offering, until the offering covered by such registration has been withdrawn or until 30 days after such offering is consummated. These demand registration rights expire on February 4, 2019, and a demand pursuant to such rights must be made prior to February 4, 2018.
In addition, the representative has the right to include its shares in any registration statement we file. If we register any securities for public sale, the representative will have the right to include its shares in the registration statement, provided that the underwriters of any such underwritten offering will have the right to limit the number of shares to be included in the registration statement, except this offering in which the representative has waived any and all rights to have its shares included. These piggyback registration rights expire on February 4, 2021.
We will pay all expenses, other than any underwriting commissions and the expenses of any legal counsel of the representative, relating to the exercise of the registration rights pursuant to the warrant.
121
Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
The provisions of Delaware law, our certificate of incorporation and our bylaws described below may have the effect of delaying, deferring or discouraging another party from acquiring control of us.
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
|
· |
before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
|
· |
upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
|
· |
on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines business combination to include the following:
|
· |
any merger or consolidation involving the corporation and the interested stockholder; |
|
· |
any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
|
· |
subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
|
· |
any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
|
· |
the receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years before the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Certificate of Incorporation and Bylaws
Our certificate of incorporation and/or bylaws provide that:
|
· |
our board of directors is classified into three classes of equal (or roughly equal) size, with all directors serving for a three-year term and the directors of only one class being elected at each annual meeting of stockholders, so that the terms of the classes of directors are “staggered”; |
|
· |
the authorized number of directors can be changed only by resolution of our board of directors; |
|
· |
our bylaws may be amended or repealed by our board of directors or our stockholders; |
|
· |
no action can be taken by stockholders except at an annual or special meeting of the stockholders called in accordance with our bylaws, and stockholders may not act by written consent, unless the stockholders amend the certificate of incorporation to provide otherwise; |
|
· |
stockholders may not call special meetings of the stockholders or fill vacancies on the board; |
|
· |
our board of directors will be authorized to issue, without stockholder approval, preferred stock, the rights of which will be determined at the discretion of the board of directors and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that our board of directors does not approve; |
122
|
· |
our stockholders do not have cumulative voting rights, and therefore our stockholders holding a majority of the shares of common stock outstanding will be able to elect all of our directors; and |
|
· |
our stockholders must comply with advance notice provisions to bring business before or nominate directors for election at a stockholder meeting. |
Potential Effects of Authorized but Unissued Stock
We have shares of common stock and preferred stock available for future issuance without stockholder approval. We may utilize these additional shares for a variety of corporate purposes, including future public offerings to raise additional capital, to facilitate corporate acquisitions or payment as a dividend on the capital stock.
The existence of unissued and unreserved common stock and preferred stock may enable our board of directors to issue shares to persons friendly to current management or to issue preferred stock with terms that could render more difficult or discourage a third-party attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise, thereby protecting the continuity of our management. In addition, the board of directors has the discretion to determine designations, rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences of each series of preferred stock, all to the fullest extent permissible under the Delaware General Corporation Law and subject to any limitations set forth in our certificate of incorporation. The purpose of authorizing the board of directors to issue preferred stock and to determine the rights and preferences applicable to such preferred stock is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible financings, acquisitions and other corporate purposes, could have the effect of making it more difficult for a third-party to acquire, or could discourage a third-party from acquiring, a majority of our outstanding voting stock.
Limitations of Director Liability and Indemnification of Directors, Officers and Employees
Our certificate of incorporation limits the liability of directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
|
· |
breach of their duty of loyalty to us or our stockholders; |
|
· |
act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
|
· |
unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
|
· |
transaction from which the directors derived an improper personal benefit. |
These limitations of liability do not apply to liabilities arising under the federal or state securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission.
Our bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by law, and may indemnify employees and other agents. Our bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding.
We have obtained a policy of directors’ and officers’ liability insurance.
We enter into separate indemnification agreements with our directors and officers. These agreements, among other things, require us to indemnify our directors and officers for any and all expenses (including reasonable attorneys’ fees, retainers, court costs, transcript costs, fees of experts, witness fees, travel expenses, duplicating costs, printing and binding costs, telephone charges, postage, delivery service fees) judgments, fines and amounts paid in settlement actually and reasonably incurred by such directors or officers or on his or her behalf in connection with any action or proceeding arising out of their services as one of our directors or officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request provided that such person follows the procedures for determining entitlement to indemnification and advancement of expenses set forth in the indemnification agreement. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability and indemnification provisions in our certificate of incorporation and bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might provide a benefit to us and our
123
stockholders. Our results of operations and financial condition may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
At present, there is no pending litigation or proceeding involving any of our directors or officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
Transfer Agent
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company. Its address is 17 Battery Place, 8th Floor, New York, New York 10004 and its telephone number is (212) 509-4000.
Listing
Our common stock is listed on The NASDAQ Capital Market under the symbol “BIOC.”
124
SHARES ELIGIBLE FOR FUTURE SALE
Prior to our initial public offering in February 2014, there was no public market for our common stock and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options and warrants, or the anticipation of these sales, could adversely affect prevailing market prices from time to time and could impair our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as of January 1, 2015, upon the completion of this offering we will have shares of common stock outstanding, assuming (1) no exercise of the underwriters’ option to purchase additional shares of common stock and (2) no exercise of outstanding options or warrants. Of those shares, all of the shares sold in this offering and all 1,900,000 shares sold in our initial public offering will be freely tradable, except that any shares held by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, or Rule 144, may only be sold in compliance with the limitations described below.
Rule 144
In general, under Rule 144, any person who is not our affiliate and has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction, subject to the availability of current public information about us. In addition, under Rule 144, any person who is not an affiliate of ours and has held their shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares without regard to whether current public information about us is available. A person who is our affiliate or who was our affiliate at any time during the preceding three months, and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of shares within any three-month period that does not exceed the greater of:
|
· |
1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; or |
|
· |
the average weekly trading volume of our common stock on The NASDAQ Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements, and to the availability of current public information about us.
Rule 701
In general, under Rule 701 of the Securities Act, any of our stockholders who purchased shares from us in connection with a qualified compensatory stock plan or other written agreement before we became subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act is eligible to resell those shares in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirements of Rule 144, and a non-affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirements of Rule 144 and without regard to the volume of such sales or the availability of public information about the issuer.
As of December 31, 2014, options to purchase a total of 906,194 shares of common stock were outstanding, of which 256,471 were vested. In addition, there were 610,774 shares of our common stock that underlie outstanding warrants. Of the total number of shares of our common stock issuable under these options, substantially all are subject to contractual lock-up agreements with the underwriters described below, and will become eligible for sale at the expiration of those agreements unless held by an affiliate of ours.
Lock-Up Agreements
We, along with our directors and executive officers, have agreed with the underwriters that for a period of 90 days after the date of this prospectus, except with the prior written consent of Aegis Capital Corp. and subject to specified exceptions, we or they will not offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock.
125
Equity Plans
Shares of our common stock issued under our 2007 Equity Incentive Plan and our 2013 Equity Incentive Plan are available for sale in the open market, subject to Rule 144 volume limitations and the lock-up agreements described above, if applicable.
Registration Rights
We and two trusts affiliated with our major stockholder Claire K. T. Reiss are parties to an amended and restated investor rights agreement dated October 31, 2011. Under the agreement, the trusts are entitled to piggyback registration rights with respect to 1,347,394 shares of common stock. The piggyback registration rights expire in February 2017. In addition, our landlord has the right to partake in such piggyback registration rights with respect to the 1,587 shares of common stock issuable upon exercise of the warrant held by the landlord. Registration of these shares under the Securities Act would result in these shares becoming (subject to the expiration of or release from the terms of any applicable lock-up agreement) fully tradable without restriction under the Securities Act immediately upon the effectiveness of the resale registration statement.
The representative of the underwriters in connection with our initial public offering were issued a warrant for the purchase of 95,000 shares of our common stock. Under the warrant, the representative is entitled to demand and piggyback registration rights with respect to such shares of our common stock. The demand registration rights expire on February 4, 2019 and the piggyback registration rights expire on February 4, 2021. Registration of these shares under the Securities Act would result in these shares becoming (subject to the expiration of or release from the terms of any applicable lock-up agreement) fully tradable without restriction under the Securities Act immediately upon the effectiveness of the resale registration statement.
126
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO
NON-U.S. HOLDERS OF OUR COMMON STOCK
The following summary describes the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our common stock acquired in this offering by Non-U.S. Holders (as defined below). This discussion does not address all aspects of U.S. federal income tax law that may be relevant to Non-U.S. Holders in light of their particular circumstances, does not consider the potential application of the alternative minimum or Medicare contribution tax, does not deal with foreign, state, local, estate or gift tax consequences. Special rules different from those described below may apply to certain Non-U.S. Holders that are subject to special treatment under the Code, such as financial institutions, insurance companies, tax-exempt organizations, foreign governments, international organizations, broker-dealers and traders in securities, U.S. expatriates, controlled foreign corporations, passive foreign investment companies, corporations that accumulate earnings to avoid U.S. federal income tax, persons that hold our common stock as part of a straddle, hedge, conversion transaction, synthetic security or integrated investment or other risk reduction strategy, partnerships and other pass-through entities, and investors in such pass-through entities or an entity that is treated as a disregarded entity for U.S. federal income tax purposes (regardless of its place of organization or formation). Such Non-U.S. Holders are urged to consult their own tax advisors to determine the U.S. federal, state, local and other tax consequences that may be relevant to them. Furthermore, the discussion below is based upon the provisions of the Code, and Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively, so as to result in U.S. federal income tax consequences different from those discussed below. We have not requested a ruling from the U.S. Internal Revenue Service, or IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions. This discussion assumes that the Non-U.S. Holder holds our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment).
The following discussion is for general information only and is not tax advice. Persons considering the purchase of our common stock pursuant to this offering should consult their own tax advisors concerning the U.S. federal income tax consequences of acquiring, owning and disposing of our common stock in light of their particular situations as well as any consequences arising under other U.S. federal tax laws or the laws of any other taxing jurisdiction, including any state, local or foreign tax consequences.
For the purposes of this discussion, a “Non-U.S. Holder” is, for U.S. federal income tax purposes, a beneficial owner of common stock that has not been excluded from this discussion and is not a U.S. Holder. A “U.S. Holder” means a beneficial owner of our common stock that is for U.S. federal income tax purposes (a) an individual who is a citizen or resident of the United States, (b) a corporation or other entity treated as a corporation created or organized in or under the laws of the United States, any state thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
Distributions
Subject to the discussion below, distributions, if any, made on our common stock to a Non-U.S. Holder of our common stock to the extent made out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles) generally will constitute dividends for U.S. tax purposes and will be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide us with a properly executed IRS Form W-8BEN, W-8BEN-E or other appropriate form, certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. In the case of a Non-U.S. Holder that is an entity, Treasury regulations and the relevant tax treaty provide rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends will be treated as paid to the entity or to those holding an interest in that entity. If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. federal withholding tax under an income tax treaty, you should consult with your own tax advisor to determine if you are able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
We generally are not required to withhold tax on dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment that such holder maintains in the United States) if a properly executed IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to such agent). In general, such effectively connected dividends will be subject to U.S. federal income tax, on a net income basis at the regular graduated rates, unless a specific treaty exemption applies. A Non- U.S. Holder that is a corporation for U.S. federal income tax purposes that receives effectively connected dividends may also be subject to an additional “branch profits tax,” which is imposed,
127
under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected earnings and profits, subject to certain adjustments.
To the extent distributions on our common stock, if any, exceed our current and accumulated earnings and profits, they will constitute a non-taxable return of capital and will first reduce your adjusted basis in our common stock, but not below zero, and then will be treated as gain and taxed in the same manner as gain realized from a sale or other disposition of common stock as described in the next section.
Gain on Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding and foreign accounts, a Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock unless (a) the gain is effectively connected with a trade or business of such holder in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment that such holder maintains in the United States), (b) the Non-U.S. Holder is a nonresident alien individual and is present in the United States for 183 or more days in the taxable year of the disposition and certain other conditions are met, or (c) we are or have been a “United States real property holding corporation” within the meaning of Code Section 897(c)(2) at any time within the shorter of the five-year period preceding such disposition or such holder’s holding period. In general, we would be a United States real property holding corporation if interests in U.S. real estate comprised (by fair market value) at least half of our total worldwide interests in real property plus our business assets. We believe that we are not, and do not anticipate becoming, a United States real property holding corporation. Even if we are treated as a United States real property holding corporation, such treatment will not cause gain realized by a Non-U.S. Holder on a disposition of our common stock to be subject to U.S. federal income tax so long as (1) the Non-U.S. Holder owned, directly, indirectly and constructively, no more than 5% of our common stock at all times within the shorter of (i) the five-year period preceding the disposition or (ii) the holder’s holding period and (2) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will qualify or continue to qualify as regularly traded on an established securities market.
If you are a Non-U.S. Holder described in (a) above, you will be required to pay tax on the net gain derived from the sale at regular graduated U.S. federal income tax rates, unless a specific treaty exemption applies, and corporate Non-U.S. Holders described in (a) above may be subject to the additional branch profits tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. If you are an individual Non-U.S. Holder described in (b) above, you will be required to pay a flat 30% tax on the gain derived from the sale, which gain may be offset by U.S. source capital losses (even though you are not considered a resident of the United States).
Information Reporting Requirements and Backup Withholding
Generally, we or certain financial middlemen must report information to the IRS with respect to any dividends we pay on our common stock including the amount of any such dividends, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder to whom any such dividends are paid. Pursuant to tax treaties or certain other agreements, the IRS may make its reports available to tax authorities in the recipient’s country of residence.
Dividends paid by us (or our paying agents) to a Non-U.S. Holder may also be subject to U.S. backup withholding. U.S. backup withholding generally will not apply to a Non-U.S. Holder who provides a properly executed appropriate IRS Form W-8 or otherwise establishes an exemption. The current backup withholding rate is 28%.
Under current U.S. federal income tax law, U.S. information reporting and backup withholding requirements generally will apply to the proceeds from a disposition of our common stock effected by or through a U.S. office of any broker, U.S. or foreign, except that information reporting and such requirements may be avoided if the holder provides a properly executed appropriate IRS Form W-8 or otherwise meets documentary evidence requirements for establishing Non-U.S. Holder status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding requirements will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is considered effected outside the United States through a non-U.S. office of a non-U.S. broker. Information reporting and backup withholding requirements may, however, apply to a payment of disposition proceeds if the broker has actual knowledge, or reason to know, that the holder is, in fact, a U.S. person. For information reporting purposes, certain brokers with substantial U.S. ownership or operations will generally be treated in a manner similar to U.S. brokers.
If backup withholding is applied to you, you should consult with your own tax advisor to determine if you are able to obtain a credit with respect to such backup withholding.
128
Foreign Accounts
A U.S. federal withholding tax of 30% may apply to dividends paid, and the gross proceeds from a disposition of our common stock paid after December 31, 2016, to a foreign financial institution (as specifically defined for this purpose), including when the foreign financial institution holds our common stock on behalf of a non-U.S. Holder, unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners). This U.S. federal withholding tax of 30% will also apply to dividends paid, and the gross proceeds from a disposition of our common stock paid after December 31, 2016, to a non-financial foreign entity (as specifically defined for this purpose) unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect U.S. owners or provides information regarding direct and indirect U.S. owners of the entity. Under certain circumstances, a Non-U.S. Holder might be eligible for refunds or credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Holders are encouraged to consult with their own tax advisors regarding the possible implications of this withholding tax on their investment in our common stock.
THE PRECEDING DISCUSSION OF U.S. FEDERAL INCOME TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY. IT IS NOT TAX ADVICE. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAW, AS WELL AS ANY STATE, LOCAL, NON-U.S. OR U.S. FEDERAL NON-INCOME TAX LAWS.
129
Aegis Capital Corp. is acting as the representative of the underwriters. Subject to the terms and conditions set forth in an underwriting agreement dated the date of this prospectus among us and the representative of the underwriters named below, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase from us, the number of shares of common stock listed next to its name in the following table.
|
Underwriters |
|
Number of Shares |
|
Aegis Capital Corp. |
|
|
|
Feltl and Company, Inc. |
|
|
|
Total |
|
|
The underwriters are committed to purchase all the shares of common stock offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of nondefaulting underwriters may be increased or the offering may be terminated. The underwriters are not obligated to purchase the shares of common stock covered by the underwriters’ over-allotment option described below. The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Discounts and Commissions
The underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering of the shares, the public offering price and other selling terms may be changed by the representative.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise of the over-allotment option we granted to the representative of the underwriters.
|
|
|
Per |
|
|
Total Without Over- |
|
|
Total With Over- |
|
|||
|
Public offering price |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Underwriting discounts and commissions |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-accountable expense allowance |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds, before expenses, to us |
|
|
|
|
|
|
|
|
|
|
|
|
We have agreed to pay a non-accountable expense allowance to the representative of the underwriters equal to 1% of the gross proceeds received in the offering; provided, however, that an allowance shall not be paid in connection with the over-allotment option if the over-allotment option is exercised.
We have also agreed to pay the representative’s expenses relating to the offering, including (a) all actual filing fees incurred in connection with the review of this offering by the Financial Industry Regulatory Authority, or FINRA, and all fees and expenses relating to the listing of our shares of common stock on the NASDAQ Capital Market; (b) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $2,000 per individual, and not to exceed $15,000 in the aggregate; (c) all actual fees, expenses and disbursements relating to the registration or qualification of securities offered under state securities laws, or “blue sky” laws, or under the securities laws of foreign jurisdictions designated by the representative; (d) all actual fees, expenses and disbursements relating to the registration, qualification or exemption of our shares of common stock under the securities laws of such foreign jurisdictions as the representative may reasonably designate; (e) the costs of all mailing and printing of the underwriting documents as the representative may reasonably deem necessary; (f) the costs associated with bound volumes of the public offering materials as well as commemorative mementos and Lucite tombstones, in an amount not to exceed $1,000; (g) the fees and expenses of the representative’s legal counsel not to exceed $50,000, (h) $21,775 for the underwriters’ use of Ipreo’s book-building, prospectus tracking and compliance software for this offering; and (i) up to $20,000 of the representative’s actual accountable road show expenses for the offering.
The total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions, are approximately $ and are payable by us.
130
Over-Allotment Option
We have granted to the underwriters an option to purchase up to additional shares of common stock at the public offering price, less underwriting discounts and commissions. The underwriters may exercise this option for 45 days from the date of this prospectus solely to cover sales of shares of common stock by underwriters in excess of the total number of shares set forth in the table above. If any of these additional shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered. We will pay the expenses associated with the exercise of the over-allotment option.
Lock-Up Agreements
We, and our officers and directors have entered into lock-up agreements with the underwriters. Under these agreements, we and these other individuals have agreed, subject to specified exceptions, not to sell or transfer any common stock or securities convertible into, or exchangeable or exercisable for, common stock, during a period ending 90 days after the date of this prospectus, without first obtaining the written consent of representative of the underwriters.
Specifically, we and these other individuals have agreed not to:
|
· |
offer, pledge, sell or contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock; |
|
· |
enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock, whether any such transaction described above is to be settled by delivery of common stock or other securities, in cash or otherwise; |
|
· |
make any demand for or exercise any right with respect to the registration of any shares of our common stock or any securities convertible into or exchangeable or exercisable for shares of our common stock; or |
|
· |
publicly announce an intention to do any of the foregoing. |
The restrictions described above do not apply to:
|
· |
the sale of shares of common stock to the underwriters pursuant to the underwriting agreement; |
|
· |
the issuance by us of shares of common stock upon the exercise of an option or the conversion of a security outstanding on the date of this prospectus of which the underwriters have been advised in writing or that is described in this prospectus; |
|
· |
the grant by us of stock options or other stock-based awards, or the issuance of shares of common stock upon exercise thereof, to eligible participants pursuant to employee benefit or equity incentive plans described in this prospectus, provided that, before the grant of any such stock options or other stock-based awards that vest within the restricted period, each recipient of such grant shall sign and deliver a lock-up agreement agreeing to be subject to the restrictions on transfer described above; |
|
· |
the establishment of a Rule 10b5-1 trading plan under the Exchange Act by a security holder for the sale of shares of common stock, provided that such plan does not provide for the transfer of common stock during the restricted period; |
|
· |
transactions relating to shares of common stock acquired in open market transactions after the completion of this offering, provided that no filing under Section 16(a) of the Exchange Act is required or is voluntarily made in connection with subsequent dispositions of common stock acquired in such open market transactions during the 90 day lock-up period; |
|
· |
transfers to us of shares of common stock or other securities convertible into or exercisable or exchangeable for common stock upon a vesting event of our securities or the exercise of options issued under our equity incentive plans in full or partial payment of taxes or tax withholding obligations required to be paid or satisfied upon such vesting or exercise, provided that no filing under Section 16(a) of the Exchange Act, reporting a reduction in beneficial ownership of shares of common stock, is required or is voluntarily made during the 90 day lock-up period; |
|
· |
transfers by security holders of shares of common stock or other securities as a bona fide gift or by will or intestacy; |
|
· |
transfers by distribution by security holders of shares of common stock or other securities to partners, members, or shareholders of the security holder; or |
|
· |
transfers by security holders of shares of common stock or other securities to any trust for the direct or indirect benefit of the security holder or the immediate family of the security holder; |
131
provided that in the case of each of the preceding three types of transactions, the transfer does not involve a disposition for value and each transferee or distributee signs and delivers a lock-up agreement agreeing to be subject to the restrictions on transfer described above.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
NASDAQ Capital Market Listing
Our common stock is listed on The NASDAQ Capital Market under the symbol “BIOC.”
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of our common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of common stock in the offering. The underwriters may close out any covered short position by either exercising the over-allotment option or purchasing shares of common stock in the open market. In determining the source of shares of common stock to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. “Naked” short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares of common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market before the completion of the offering.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As result, the price of our common stock may be higher than the price that might otherwise exist in the open market.
The underwriters have advised us that, pursuant to Regulation M under the Exchange Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of our common stock, including the imposition of penalty bids. This means that if the representative of the underwriters purchases common stock in the open market in stabilizing transactions or to cover short sales, the representative can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
The underwriters make no representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares of common stock to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to underwriters and selling group members that may make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Notice to Non-U.S. Investors
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive, each of which we refer to as a relevant member state, with effect from and including the date on which the Prospectus Directive is implemented in
132
that relevant member state, or the relevant implementation date, an offer of securities described in this prospectus may not be made to the public in that relevant member state other than:
|
· |
to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
|
· |
to any legal entity that has two or more of (i) an average of at least 250 employees during the last financial year; (ii) a total balance sheet of more than €43,000,000 and (iii) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
|
· |
to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of representative for any such offer; or |
|
· |
in any other circumstances that do not require the publication of a prospectus pursuant to Article 3 of the Prospectus Directive; |
provided that no such offer of securities shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares of common stock in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe for the shares, as the same may be varied in that member state by any measure implementing the Prospectus Directive in that member state and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each relevant member state.
Other Relationships
From time to time, certain of the underwriters and their affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions. However, except as disclosed in this prospectus, we have no present arrangements with any of the underwriters for any further services. Aegis Capital Corp. and Feltl and Company, Inc. acted as the joint bookrunning managers for our initial public offering completed on February 10, 2014.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer for the offeree under this prospectus.
133
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area—Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
(a) to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €€ 43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €€ 50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements);
(c) to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive.
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority, or the ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in
134
Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
|
· |
qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
|
· |
in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
|
· |
made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
|
· |
in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act
135
(1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority.
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA.
This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49 (2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
136
The validity of the shares of common stock being offered by this prospectus will be passed upon for us by Cooley LLP, San Diego, California. The underwriters are being represented by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., New York, New York.
Mayer Hoffman McCann P.C., our independent registered public accounting firm, has audited our balance sheets as of December 31, 2012 and 2013, and the related statements of operations and comprehensive loss, changes in shareholders’ deficit and cash flows for each of the two years in the period ended December 31, 2013, as set forth in their report. We have included our financial statements in this prospectus and in this registration statement in reliance on the report of Mayer Hoffman McCann P.C. given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street NE, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. You may also request a copy of these filings, at no cost, by writing us at 5810 Nancy Ridge Drive, San Diego, California 92121 or telephoning us at (858) 320-8200.
We are subject to the information and periodic reporting requirements of the Exchange Act, and we file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information are available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at http://www.biocept.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this prospectus.
137
GLOSSARY OF SCIENTIFIC AND HEALTHCARE-RELATED ACRONYMS
|
ACA |
Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act |
|
ALK |
Anaplastic lymphoma kinase |
|
CAP |
College of American Pathologists; the leading organization of board-certified pathologists, serving patients, pathologists, and the public by fostering and advocating excellence in the practice of pathology and laboratory medicine worldwide |
|
CE |
Conformité Européenne; a conformity mark which is placed on all products including medical devices marketed in the European Economic Area |
|
CLIA |
Clinical Laboratory Improvement Amendments of 1988; federal regulatory standards that apply to all clinical laboratory testing performed on human samples in the United States |
|
CMS |
Centers for Medicare & Medicaid Services; a U.S. federal agency that administers Medicare, Medicaid and the Children’s Health Insurance Program |
|
CPT |
Current Procedure Terminology |
|
CTC |
Circulating tumor cell |
|
ctDNA |
Circulating tumor DNA |
|
DTC |
Disseminated tumor cell |
|
EGFR |
Epidermal growth factor receptor |
|
EMT |
Epithelial-to-mesenchymal transition |
|
EpCAM |
Epithelial cell adhesion molecule |
|
FDA |
United States Food and Drug Administration |
|
FISH |
Fluorescence in situ Hybridization; a molecular cytogenetic technique that is used to detect chromosomal aberrations that include deletions, amplifications and translocations; DNA FISH probes are fluorescently labeled segments of DNA that are complementary to specific sequences on a chromosome |
|
HER2 |
Human epidermal growth factor receptor 2 |
|
HHS |
United States Department of Health and Human Services |
|
HIPAA |
Health Insurance Portability and Accountability Act of 1996 |
|
HITECH |
Health Information Technology for Economic and Clinical Health Act |
|
IHC |
Immunohistochemistry |
|
IVD |
In vitro diagnostic |
|
LDTs |
Laboratory Developed Tests; assays developed in the laboratory for diagnostic or prognostic purposes |
|
MAC |
Medicare Administrative Contractor |
|
MCTRJCA |
Middle Class Tax Relief and Job Creation Act of 2012 |
|
NSCLC |
Non-small cell lung cancer |
|
PCR |
Polymerase chain reaction |
|
ROS1 |
c-ros oncogene 1, receptor tyrosine kinase |
138
BIOCEPT, INC.
139
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Biocept, Inc.
We have audited the accompanying balance sheets of Biocept, Inc. as of December 31, 2013 and 2012, and the related statements of operations and comprehensive loss, shareholders’ deficit and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Biocept, Inc. as of December 31, 2013 and 2012, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
/s/ Mayer Hoffman McCann P.C.
San Diego, California
March 28, 2014
140
Biocept, Inc.
|
|
|
December 31, |
|
|
December 31, |
|
|
Pro Forma |
|
|||
|
|
|
2012 |
|
|
2013 |
|
|
2013 |
|
|||
|
|
|
|
|
|
|
|
|
(unaudited) |
|
|||
|
Current assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash & cash equivalents |
|
$ |
185,256 |
|
|
$ |
69,178 |
|
|
$ |
69,178 |
|
|
Accounts receivable |
|
|
18,885 |
|
|
|
9,200 |
|
|
|
9,200 |
|
|
Inventories, net |
|
|
61,283 |
|
|
|
92,823 |
|
|
|
92,823 |
|
|
Prepaid expenses and other current assets |
|
|
310,442 |
|
|
|
799,131 |
|
|
|
260,813 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets |
|
|
575,866 |
|
|
|
970,332 |
|
|
|
432,014 |
|
|
Fixed assets, net |
|
|
624,730 |
|
|
|
358,887 |
|
|
|
358,887 |
|
|
Other non-current assets |
|
|
269,083 |
|
|
|
500 |
|
|
|
500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
1,469,679 |
|
|
$ |
1,329,719 |
|
|
$ |
791,401 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,387,677 |
|
|
$ |
1,540,618 |
|
|
$ |
1,540,618 |
|
|
Accrued liabilities |
|
|
3,346,806 |
|
|
|
2,242,058 |
|
|
|
1,745,899 |
|
|
Line of credit |
|
|
— |
|
|
|
1,981,000 |
|
|
|
1,665,757 |
|
|
Notes payable |
|
|
21,631,427 |
|
|
|
5,200,599 |
|
|
|
— |
|
|
Warrant liability |
|
|
981,747 |
|
|
|
2,140,532 |
|
|
|
— |
|
|
Supplier financings |
|
|
251,146 |
|
|
|
218,925 |
|
|
|
218,925 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
27,598,803 |
|
|
|
13,323,732 |
|
|
|
5,171,199 |
|
|
Notes payable, net of current portion |
|
|
745,000 |
|
|
|
— |
|
|
|
— |
|
|
Deferred rent |
|
|
510,771 |
|
|
|
462,001 |
|
|
|
462,001 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities |
|
|
28,854,574 |
|
|
|
13,785,733 |
|
|
|
5,633,200 |
|
|
|
|
|
|
|||||||||
|
Commitments and contingencies (see Note 16) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Shareholders’ deficit: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Series A convertible preferred stock, $0.0001 par value, 36,460,000 authorized; 27,175,213 issued and outstanding at December 31, 2012; 100,000,000 authorized; 69,421,047 issued and outstanding at December 31, 2013; liquidation preference of $16,305,127 at December 31, 2012 and $41,652,628 at December 31, 2013 (see Note 9); 5,000,000 shares authorized, no shares issued and outstanding on a pro forma basis at December 31, 2013 (see Unaudited Pro Forma Information paragraphs in Note 3). |
|
|
2,718 |
|
|
|
6,942 |
|
|
|
— |
|
|
Common stock, $0.0001 par value, 14,600,000 authorized; 160,393 issued and outstanding at December 31, 2012; 53,000,000 authorized; 185,550 issued and outstanding at December 31, 2013 (see Note 9); 40,000,000 authorized; 2,600,162 issued and outstanding on a pro forma basis at December 31, 2013 (see Unaudited Pro Forma Information paragraphs in Note 3). |
|
|
16 |
|
|
|
19 |
|
|
|
260 |
|
|
Additional paid-in capital |
|
|
85,800,164 |
|
|
|
109,958,001 |
|
|
|
118,453,075 |
|
|
Accumulated deficit |
|
|
(113,187,793 |
) |
|
|
(122,420,976 |
) |
|
|
(123,295,134 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total shareholders’ deficit |
|
|
(27,384,895 |
) |
|
|
(12,456,014 |
) |
|
|
(4,841,799 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and shareholders’ deficit |
|
$ |
1,469,679 |
|
|
$ |
1,329,719 |
|
|
$ |
791,401 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these financial statements
141
Biocept, Inc.
Statements of Operations and Comprehensive Loss
|
|
|
For the year ended December 31, |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Revenues |
|
$ |
109,289 |
|
|
$ |
134,245 |
|
|
Cost of revenues |
|
|
1,201,694 |
|
|
|
2,329,900 |
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit/(loss) |
|
|
(1,092,405 |
) |
|
|
(2,195,655 |
) |
|
Operating expenses |
|
|
|
|
|
|
|
|
|
Research and development expenses |
|
|
6,562,152 |
|
|
|
3,086,737 |
|
|
General and administrative expenses |
|
|
2,063,199 |
|
|
|
2,513,136 |
|
|
Sales and marketing expenses |
|
|
785,319 |
|
|
|
148,903 |
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
(10,503,075 |
) |
|
|
(7,944,431 |
) |
|
Other income/(expense) |
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
(2,187,499 |
) |
|
|
(2,070,064 |
) |
|
Change in fair value of warrant liability |
|
|
454,389 |
|
|
|
782,112 |
|
|
Other income/(expense) |
|
|
(22,541 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
Total other income/(expense) |
|
|
(1,755,651 |
) |
|
|
(1,287,952 |
) |
|
|
|
|
|
|
|
|
|
|
|
Loss before income taxes |
|
|
(12,258,726 |
) |
|
|
(9,232,383 |
) |
|
Income tax expense |
|
|
800 |
|
|
|
800 |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss & comprehensive loss |
|
$ |
(12,259,526 |
) |
|
$ |
(9,233,183 |
) |
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares outstanding used in computing net loss per share attributable to common shareholders: |
|
|
|
|
|
|
|
|
|
Basic |
|
|
160,393 |
|
|
|
181,762 |
|
|
|
|
|
|
|
|
|
|
|
|
Diluted |
|
|
160,393 |
|
|
|
181,762 |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share: |
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(76.43 |
) |
|
$ |
(50.80 |
) |
|
|
|
|
|
|
|
|
|
|
|
Diluted |
|
$ |
(76.43 |
) |
|
$ |
(50.80 |
) |
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these financial statements
142
Biocept, Inc.
Statements of Shareholders’ Deficit
|
|
|
Series A Preferred Stock |
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Total |
|
|||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
|
|||||||||||||
|
Balance at December 31, 2011 |
|
|
27,175,213 |
|
|
$ |
2,718 |
|
|
|
160,393 |
|
|
$ |
16 |
|
|
$ |
85,548,030 |
|
|
$ |
(100,928,267 |
) |
|
$ |
(15,377,503 |
) |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
252,134 |
|
|
|
— |
|
|
|
252,134 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(12,259,526 |
) |
|
|
(12,259,526 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2012 |
|
|
27,175,213 |
|
|
|
2,718 |
|
|
|
160,393 |
|
|
|
16 |
|
|
|
85,800,164 |
|
|
|
(113,187,793 |
) |
|
|
(27,384,895 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
952,521 |
|
|
|
— |
|
|
|
952,521 |
|
|
Stock issuance for RSU |
|
|
— |
|
|
|
— |
|
|
|
21,846 |
|
|
|
2 |
|
|
|
(2 |
) |
|
|
— |
|
|
|
— |
|
|
Exercise of stock options |
|
|
— |
|
|
|
— |
|
|
|
4,021 |
|
|
|
1 |
|
|
|
20,104 |
|
|
|
— |
|
|
|
20,105 |
|
|
Repurchase of common shares |
|
|
— |
|
|
|
— |
|
|
|
(710 |
) |
|
|
— |
|
|
|
(4,111 |
) |
|
|
— |
|
|
|
(4,111 |
) |
|
Shares issued for conversion of notes payable and accrued interest of $20.2 million and $2.6 million, respectively |
|
|
42,245,834 |
|
|
|
4,224 |
|
|
|
— |
|
|
|
— |
|
|
|
22,808,180 |
|
|
|
— |
|
|
|
22,812,404 |
|
|
Reclassification of warrant liability derivative due to triggering event |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
381,145 |
|
|
|
— |
|
|
|
381,145 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(9,233,183 |
) |
|
|
(9,233,183 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2013 |
|
|
69,421,047 |
|
|
$ |
6,942 |
|
|
|
185,550 |
|
|
$ |
19 |
|
|
$ |
109,958,001 |
|
|
$ |
(122,420,976 |
) |
|
$ |
(12,456,014 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these financial statements
143
Biocept, Inc.
|
|
|
For the year ended December 31, |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Cash Flows From Operating Activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(12,259,526 |
) |
|
$ |
(9,233,183 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
365,568 |
|
|
|
266,554 |
|
|
Inventory reserve |
|
|
56,004 |
|
|
|
(70,004 |
) |
|
Stock-based compensation |
|
|
252,134 |
|
|
|
952,521 |
|
|
Non-cash interest expense related to convertible debt and other financing activities |
|
|
2,159,234 |
|
|
|
2,066,287 |
|
|
Change in fair value of warrant liabilities |
|
|
(454,389 |
) |
|
|
(782,112 |
) |
|
Increase/(decrease) in cash resulting from changes in: |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(13,634 |
) |
|
|
9,685 |
|
|
Inventory |
|
|
(117,287 |
) |
|
|
38,464 |
|
|
Prepaid expenses and other current assets |
|
|
77,654 |
|
|
|
(37,691 |
) |
|
Other non-current assets |
|
|
— |
|
|
|
268,583 |
|
|
Accounts payable |
|
|
354,553 |
|
|
|
(175,280 |
) |
|
Accrued liabilities |
|
|
730,836 |
|
|
|
233,852 |
|
|
Deferred rent |
|
|
241,837 |
|
|
|
259,961 |
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities |
|
|
(8,607,016 |
) |
|
|
(6,202,363 |
) |
|
Cash Flows From Investing Activities |
|
|
|
|
|
|
|
|
|
Purchases of fixed assets |
|
|
(8,046 |
) |
|
|
(711 |
) |
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities |
|
|
(8,046 |
) |
|
|
(711 |
) |
|
Cash Flows From Financing Activities |
|
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options |
|
|
— |
|
|
|
20,105 |
|
|
Payments for repurchase of shares |
|
|
— |
|
|
|
(4,111 |
) |
|
Payments on supplier and other third party financings |
|
|
(164,974 |
) |
|
|
(154,998 |
) |
|
Proceeds from borrowings on line of credit |
|
|
— |
|
|
|
1,981,000 |
|
|
Proceeds from issuance of notes payable |
|
|
5,960,000 |
|
|
|
— |
|
|
Proceeds from issuance of convertible notes and warrants |
|
|
2,570,000 |
|
|
|
4,245,000 |
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities |
|
|
8,365,026 |
|
|
|
6,086,996 |
|
|
|
|
|
|
|
|
|
|
|
|
Net decrease in Cash and Cash Equivalents |
|
|
(250,036 |
) |
|
|
(116,078 |
) |
|
Cash and Cash Equivalents at Beginning of Period |
|
|
435,292 |
|
|
|
185,256 |
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents at End of Period |
|
$ |
185,256 |
|
|
$ |
69,178 |
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Disclosures of Cash Flow Information: |
|
|
|
|
|
|
|
|
|
Cash paid during the period for: |
|
|
|
|
|
|
|
|
|
Interest |
|
$ |
28,276 |
|
|
$ |
3,777 |
|
|
|
|
|
|
|
|
|
|
|
|
Taxes |
|
$ |
800 |
|
|
$ |
800 |
|
|
|
|
|
|
|
|
|
|
|
144
Non-cash Investing and Financing Activities:
For the years ended December 31, 2012 and 2013, the Company financed insurance premiums of $128,929 and $122,777, respectively, through third party financings. Such financings occur on an annual basis during the three months ended December 31 of each year.
During the year ended December 31, 2013, 21,846 shares of common stock, with a par value of $0.0001, were issued for restricted stock units.
During the year ended December 31, 2013, convertible notes with a principal balance of $20,231,000 and accrued interest of approximately $2,581,000 were converted into 42,245,834 shares of preferred stock with a par value of $0.0001. In conjunction with this conversion, $236,799 of derivative warrant liabilities were reclassified to additional paid-in capital, as the underlying exercise prices on the warrants were determined by the debt conversion. In addition, during the year ended December 31, 2013, an additional $144,346 of derivative warrant liabilities were reclassified to additional paid-in capital when their underlying exercise price was fixed.
During the year ended December 31, 2013, the Company issued to its landlord a warrant to purchase common shares with a warrant coverage amount of $502,605 and an exercise price equal to the price per share of the Company’s common stock sold in an initial public offering under the Securities Act (“IPO”). The fair value of the warrant as calculated under the Company’s probability weighted Black-Scholes valuation model was approximately $309,000 at issuance in September 2013 (see Note 7), and is recorded on the balance sheet as a component of deferred rent and warrant liability.
During the year ended December 31, 2013, the Company incurred $538,318 in costs directly associated with its anticipated IPO, which are reflected on the balance sheet as a component of prepaid expenses and other current assets at December 31, 2013. A liability of $328,221 for associated unpaid invoices is recorded as a component of accounts payable at December 31, 2013.
The accompanying notes are an integral part of these financial statements
145
BIOCEPT, INC.
1. The Company and Business Activities
Biocept, Inc. (“the Company”) was founded in California in May 1997 and is a commercial-stage cancer diagnostics company developing and commercializing proprietary circulating tumor cell (CTC) and circulating tumor DNA (ctDNA) tests utilizing a standard blood sample to improve the treatment that oncologists provide to their patients by providing better, more detailed information on the characteristics of their tumor.
The Company operates a clinical laboratory that is CLIA-certified (under the Clinical Laboratory Improvement Amendment of 1988) and CAP-accredited (by the College of American Pathologists), and manufactures CEE microfluidic channels, related equipment and certain reagents to perform the Company’s diagnostic tests in a facility located in San Diego, California. CLIA certification and accreditation are required before any clinical laboratory may perform testing on human specimens for the purpose of obtaining information for the diagnosis, prevention, treatment of disease, or assessment of health. The tests the Company offers are classified as laboratory developed tests (LDTs), under the CLIA regulations.
In July 2013, the Company effected a reincorporation to Delaware by merging itself with and into Biocept, Inc., a Delaware corporation, which had been formed to be and was a wholly-owned subsidiary of the Company since July 23, 2013.
2. Liquidity
At December 31, 2012 and 2013, the Company had accumulated deficits of approximately $113,188,000 and $122,421,000, respectively. For the years ended December 31, 2012 and 2013, the Company incurred net losses of approximately $12,260,000 and $9,233,000, respectively. In addition, as of December 31, 2013, the Company had notes payable and amounts outstanding under a line of credit due within one year, gross of applicable debt discounts, totaling approximately $8,371,000. The Company borrowed a total of $8,530,000, and $6,226,000 during the years ended December 31, 2012 and 2013, respectively, under note agreements with certain shareholders and a line of credit. While the Company is currently in the commercialization stage of operations, the Company has not yet achieved profitability and anticipates that it will continue to incur net losses in the foreseeable future.
Historically, the Company’s principal sources of cash have included revenues from clinical laboratory testing through contracted partners, proceeds from the issuance of common and preferred stock and proceeds from the issuance of debt. The Company’s principal uses of cash have included cash used in operations, payments relating to purchases of property and equipment and repayments of borrowings. The Company expects that the principal uses of cash in the future will be for hiring of sales and marketing personnel and increased sales and marketing activities, funding of research and development, capital expenditures, and general working capital requirements. The Company expects that, as revenues grow, sales and marketing and research and development expenses will continue to grow, albeit at a slower rate and, as a result, the Company will need to generate significant net revenues to achieve and sustain income from operations.
On February 10, 2014, the Company received net proceeds of approximately $16,673,000 as a result of the closing of its IPO, net of underwriting discounts and additional costs incurred (see Note 18). Management believes that its cash resources should be sufficient to support currently forecasted operations through at least the next twelve months. However, the Company operates in a market that makes its prospects difficult to evaluate, and the likelihood that the Company will need additional debt or equity financing in the future to execute on its current or future business strategies beyond the next twelve months is probable. Management also believes that, if necessary, it can implement plans in the short term to conserve existing cash should additional financing activities be delayed. Capital outlays and operating expenditures may occur over the next twelve months as the Company expands its infrastructure, commercialization, and research and development activities.
3. Summary of Significant Accounting Policies
Basis of Presentation
The financial statements and accompanying notes are prepared in accordance with accounting principles generally accepted in the United States of America.
Certain prior period amounts have been reclassified to conform to the current period presentation. Such reclassifications did not affect the Company’s Balance Sheets, Results of Operations or Cash Flows for the years ended December 31, 2012 and 2013.
146
Use of Estimates
The preparation of financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, management evaluates these estimates and judgments, including those related to inventories, long-lived assets, convertible debt, derivative liabilities, income taxes, and stock-based compensation. The Company bases its estimates on various assumptions that it believes are reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
Unaudited Pro Forma Information
The unaudited pro forma balance sheet information as of December 31, 2013 gives effect to (i) the automatic conversion of all outstanding shares of the Company’s Series A preferred stock into 1,652,851 shares of common stock, (ii) the conversion of convertible promissory notes and accrued interest of approximately $6,886,000 (as of December 31, 2013) into an aggregate of 688,610 shares of the Company’s common stock in connection with the closing of the Company’s IPO, (iii) the write-off of $874,158 to interest expense for the unamortized debt discount on notes payable, (iv) the reclassification to line of credit of $315,243 for the unamortized debt discount previously classified against notes payable, (v) the issuance of an estimated 73,151 shares of common stock upon such IPO pursuant to the settlement of certain restricted stock units in accordance with their terms, (vi) the termination of certain warrants upon the closing of the Company’s IPO in accordance with their terms and (vii) the reclassification to shareholders’ deficit of the fair value of certain warrants the exercise price and/or exercisability period length of which will be fixed upon the closing of the Company’s IPO in accordance with their terms, assuming for all such items an IPO price of $10.00 per share.
The unaudited pro forma balance sheet information as of December 31, 2013 assumes that the completion of the Company’s IPO had occurred as of December 31, 2013, and excludes shares of common stock issued in the IPO and any related net proceeds. In October 2013 the Board of Directors approved an amendment of the Company’s certificate of incorporation, to be filed in connection with the Company’s IPO, which would decrease the number of common shares authorized to 40,000,000 and decrease the number of preferred shares authorized to 5,000,000.
Reverse Stock Split and Change in Par Value of Common Stock and Preferred Stock
In November 2011, the Company effected a 1:3 reverse stock split of the Company’s common shares. In addition, in July 2013, in conjunction with its reincorporation in the state of Delaware, the Company initiated par values for preferred and common shares equal to $0.0001. On November 1, 2013, the Company effected a 1:14 reverse stock split for all common shares. All references to share and per share amounts in the financial statements and accompanying notes to the financial statements have been retroactively restated to reflect the 1:14 reverse stock split and the change in par value.
Revenue Recognition
Revenue is recognized in accordance with the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 605, Revenue Recognition, and ASC 954-605 Health Care Entities, Revenue Recognition which requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred and title and the risks and rewards of ownership have been transferred to the client or services have been rendered; (3) the price is fixed or determinable; and (4) collectability is reasonably assured. For contract partners, revenue is recorded based upon the contractually agreed upon fee schedule. When assessing collectability, the Company considers whether there is sufficient payment history to reliably estimate a payor’s individual payment patterns. For new tests where there is limited evidence of payment history at the time the tests are completed, the Company recognizes revenue equal to the amount of cash received until such time as reimbursement experience can be established.
The Company’s main source of revenue for the years ended December 31, 2012 and 2013 is through contracted partners. This revenue is derived from clinical laboratory testing performed in the Company’s laboratories under agreements with such partners. As there is a contractually agreed upon price, and collectability from the partners is reasonably assured, revenues for these tests are earned at the time the test is completed and the results are delivered to the partners or a third party.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents. The Company places its cash and cash equivalents with reputable financial institutions that are insured by the Federal Deposit Insurance Corporation (FDIC). At times, deposits held may exceed the amount of insurance provided by the FDIC. The Company has not experienced any losses in its cash and cash equivalents and believes they are not exposed to any significant credit risk.
147
Fair Value Measurement
The Company uses a three-tier fair value hierarchy to prioritize the inputs used in the Company’s fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions. The Company believes the carrying amount of cash and cash equivalents, accounts receivable, accounts payable and accrued expenses approximate their estimated fair values due to the short-term maturities of these financial instruments.
As of December 31, 2012 and 2013, the Company classified the fair value measurements of the Company’s warrant liability derivative as Level 3. See Note 7 for further details about the inputs and assumptions used to determine the fair value of the warrant liability at each balance sheet date.
The values attributed to such warrants as of December 31, 2012 and 2013 were as follows:
|
|
|
Fair Value Measurements Using |
|
|||||||||
|
|
|
Quoted Prices |
|
|
Significant |
|
|
Significant |
|
|||
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant Liability at December 31, 2012 |
|
|
— |
|
|
|
— |
|
|
$ |
981,747 |
|
|
Warrant Liability at December 31, 2013 |
|
|
— |
|
|
|
— |
|
|
$ |
2,140,532 |
|
The following table includes a summary of changes in the fair value of the warrants for the years ended December 31, 2012 and 2013:
|
|
|
Fair Value Measurements |
|
|
|
Balance at December 31, 2011 |
|
|
923,325 |
|
|
Warrant liability incurred in 2012 |
|
|
512,811 |
|
|
Change in fair value in 2012 |
|
|
(454,389 |
) |
|
|
|
|
|
|
|
Balance at December 31, 2012 |
|
|
981,747 |
|
|
Warrant liability incurred in 2013 |
|
|
2,322,042 |
|
|
Warrant liability reclassified to additional paid-in capital in 2013 |
|
|
(381,145 |
) |
|
Change in fair value in 2013 |
|
|
(782,112 |
) |
|
|
|
|
|
|
|
Balance at December 31, 2013 |
|
$ |
2,140,532 |
|
|
|
|
|
|
|
Concentration of Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of temporary cash investments. The Company has not experienced losses in such accounts. Management believes that the Company is not exposed to any significant credit risk with respect to its cash and cash equivalents.
In 2012, the Company launched commercial operations in partnership with a commercial partner, Clarient Diagnostic Services, Inc. (“Clarient”), a GE Healthcare Company. For the years ended December 31, 2012 and 2013, 79% and 10%, respectively, of the revenue earned was billed through this relationship. In addition, at December 31, 2012, 100% of the receivables were due from Clarient. In 2013, the Company entered into a research support agreement with a not-for-profit tax-exempt organization, Dana Farber Partners Cancer Care, Inc. (“Dana Farber”). For the year ended December 31, 2013, 77% of the revenue earned was billed through this relationship. In addition, 100% of the receivables were due from Dana Farber at December 31, 2013. For the year ended December 31, 2013, three customers made up 78%, 11% and 10% of total revenues.
All of the Company’s sales for all periods presented were generated in the United States of America.
Certain components used in the Company’s current or planned products are available from only one supplier, and substitutes for these components cannot be obtained easily or would require substantial design or manufacturing modifications or identification and qualification of alternative sources.
148
Accounts Receivable
Accounts receivable are carried at original invoice amounts, less an estimate for doubtful receivables, based on a review of all outstanding amounts on a periodic basis. The estimate for doubtful receivables is determined from an analysis of the accounts receivable on a quarterly basis, and is recorded as bad debt expense. As the Company only recognizes revenue to the extent collection is expected and reasonably assured, bad debt expense related to receivables from patient service revenue is recorded in general and administrative expense in the statement of operations and comprehensive loss. Accounts receivable are written off when deemed uncollectible. Recoveries of accounts receivable previously written off are recorded when received. As of December 31, 2012 and 2013, management determined that all of the amounts recorded as accounts receivable were collectible, and no allowance for doubtful accounts was needed.
Inventories
Inventories are valued at the lower of cost or market value. Cost is determined by the average cost method. The Company records adjustments to its inventory for estimated obsolescence or diminution in market value equal to the difference between the cost of the inventory and the estimated market value. At the point of loss recognition, a new cost basis for that inventory is established, and subsequent changes in facts and circumstances do not result in the restoration or increase in that newly established cost basis.
Fixed Assets
Fixed assets consist of machinery and equipment, furniture and fixtures, computer equipment and software, leasehold improvements, capital leased equipment and construction in process. Fixed assets are stated at cost less accumulated depreciation and amortization. Additions, improvements, and major renewals are capitalized. Maintenance, repairs, and minor renewals are expensed as incurred. Depreciation is determined using the straight-line method over the estimated useful lives of the assets, which range from three to five years. Leasehold improvements are amortized over the life of the lease or the asset, whichever is shorter. Depreciation expense for the years ended December 31, 2012 and 2013 was approximately $366,000 and $267,000, respectively.
Upon sale, retirement or disposal of fixed assets, the accounts are relieved of the cost and the related accumulated depreciation or amortization with any gain or loss recorded to the statement of operations.
Fixed assets are reviewed for impairment whenever changes in circumstances indicate that the carrying amount of an asset may not be recoverable. These computations utilize judgments and assumptions inherent in the estimates of future cash flows to determine recoverability of these assets. If the assumptions about these assets were to change as a result of events or circumstances, the Company may be required to record an impairment loss.
Warrant Liability
Warrants for shares that are contingently redeemable and for which the exercise price is not fixed are classified as liabilities on the accompanying balance sheets and carried at their estimated fair value, determined through use of a Black-Scholes valuation model. As of and for the years ended December 31, 2012 and 2013, the Company evaluated and concluded that the fair value obtained from the Black-Scholes method of valuing the warrant liability does not materially differ from the valuation of such warrants using the Monte Carlo or binomial lattice simulation models, and therefore the use of the Black-Scholes valuation model was considered a reasonable method to value the warrants. At the end of each reporting period, any changes in fair value are recorded as a component of other income (expense). The Company will continue to adjust the carrying value of the warrants until the completion of its IPO on February 10, 2014, at which time the exercise price was fixed and the fair value of those warrants was reclassified to shareholders’ deficit.
Stock-based Compensation
The Company accounts for stock-based compensation under the provisions of FASB ASC Topic 718, Compensation—Stock Compensation, which requires the measurement and recognition of compensation expense for all stock-based awards made to employees and directors based on estimated fair values on the grant date. The Company estimates the fair value of stock-based awards on the date of grant using the Black-Scholes option pricing model (“Black-Scholes valuation model”). The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods using the straight-line method. The Company estimates forfeitures at the time of grant and revises these estimates in subsequent periods if actual forfeitures differ from those estimates. See additional information in Note 10.
The Company accounts for stock-based compensation awards to non-employees in accordance with FASB ASC Topic 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). Under ASC 505-50, the Company determines the fair value of the warrants or stock-based compensation awards granted as either the fair value of the consideration received or the fair value of the equity
149
instruments issued, whichever is more reliably measurable. All issuances of equity instruments issued to non-employees as consideration for goods or services received by the Company are accounted for based on the fair value of the equity instruments issued. These awards are recorded in expense and additional paid-in capital in shareholders’ equity over the applicable service periods based on the fair value of the options at the end of each period.
Calculating the fair value of stock-based awards requires the input of highly subjective assumptions into the Black-Scholes valuation model. Stock-based compensation expense is calculated using the Company’s best estimates, which involves inherent uncertainties, and the application of management’s judgment. Significant estimates include the fair value of the Company’s common stock at the date of grant, the expected life of the stock option, stock price volatility, risk-free interest rate and forfeiture rates.
Research and Development
Research and development costs are expensed as incurred. The amounts expensed in the years ended December 31, 2012 and 2013 were approximately $6,562,000 and $3,087,000, respectively, which includes salaries of research and development personnel.
Income Taxes
The Company provides for income taxes utilizing the liability method. Under the liability method, current income tax expense or benefit is the amount of income taxes expected to be payable or refundable for the current year. A deferred income tax asset or liability is computed for the expected future impact of differences between the financial reporting and tax bases of assets and liabilities and for the expected future tax benefit to be derived from tax credits. Tax rate changes are reflected in the computation of the income tax provision during the period such changes are enacted.
Deferred tax assets are reduced by a valuation allowance when, in management’s opinion, it is more likely than not that some portion or all of the deferred tax assets will not be realized. The Company considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. The Company’s valuation allowance is based on available evidence, including its current year operating loss, evaluation of positive and negative evidence with respect to certain specific deferred tax assets including evaluation sources of future taxable income to support the realization of the deferred tax assets. The Company has established a full valuation allowance on the deferred tax assets as of December 31, 2012 and 2013, and therefore has not recognized any income tax benefit or expense in the periods presented.
ASC 740, Income Taxes (“ASC 740”), clarifies the accounting for uncertainty in income taxes recognized in the financial statements. ASC 740 provides that a tax benefit from uncertain tax positions may be recognized when it is more-likely-than- not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits of the position. Income tax positions must meet a more-likely-than-not recognition threshold to be recognized. ASC 740 also provides guidance on measurement, derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
The Company recognizes interest and/or penalties related to income tax matters in income tax expense. There is no accrual for interest or penalties for income taxes on the balance sheets at December 31, 2012 and 2013, and the Company has not recognized interest and/or penalties in the statements of operations for the years ended December 31, 2012 and 2013.
Recent Accounting Pronouncements
In July 2013, the FASB issued authoritative guidance which requires netting unrecognized tax benefits against deferred tax assets for a loss or other carryforward that would apply in settlement of uncertain tax positions. This guidance will be effective for annual reporting periods beginning after December 15, 2013. The Company does not believe that adoption of this guidance will have a material impact on the Company’s financial statements or disclosures.
150
4. Balance Sheet Details
The following provides certain balance sheet details:
|
|
|
December 31, |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Fixed Assets |
|
|
|
|
|
|
|
|
|
Machinery and equipment |
|
$ |
2,761,560 |
|
|
$ |
2,761,560 |
|
|
Furniture and office equipment |
|
|
209,844 |
|
|
|
209,844 |
|
|
Computer equipment and software |
|
|
681,508 |
|
|
|
681,508 |
|
|
Leasehold improvements |
|
|
373,653 |
|
|
|
373,653 |
|
|
Capital lease equipment |
|
|
677,000 |
|
|
|
677,000 |
|
|
Construction in process |
|
|
11,588 |
|
|
|
12,299 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,715,153 |
|
|
|
4,715,864 |
|
|
Accumulated depreciation and amortization |
|
|
4,090,423 |
|
|
|
4,356,977 |
|
|
|
|
|
|
|
|
|
|
|
|
Total fixed assets, net |
|
$ |
624,730 |
|
|
$ |
358,887 |
|
|
|
|
|
|
|
|
|
|
|
|
Accrued Liabilities |
|
|
|
|
|
|
|
|
|
Accrued interest |
|
$ |
1,963,007 |
|
|
$ |
524,885 |
|
|
Accrued payroll |
|
|
185,150 |
|
|
|
125,299 |
|
|
Deferred wages |
|
|
972,405 |
|
|
|
1,377,987 |
|
|
Accrued vacation |
|
|
224,187 |
|
|
|
213,601 |
|
|
Other |
|
|
2,057 |
|
|
|
286 |
|
|
|
|
|
|
|
|
|
|
|
|
Total accrued liabilities |
|
$ |
3,346,806 |
|
|
$ |
2,242,058 |
|
|
|
|
|
|
|
|
|
|
|
During the year ended December 31, 2013, the Company incurred $538,318 in costs directly associated with its anticipated IPO, which are reflected on the balance sheet as a component of prepaid expenses and other current assets at December 31, 2013. A liability of $328,221 for associated unpaid invoices is recorded as a component of accounts payable at December 31, 2013.
As of December 31, 2012 other non-current assets of $269,000 consisted solely of deposits for the San Diego building, which is leased under a non-cancelable operating lease. During the year ended December 31, 2013, the Company amended its lease agreement and forfeited the balance.
5. Line of Credit
In July 2013, the Company entered into a revolving line of credit with UBS Bank USA in the initial amount of $1.5 million. Interest accrues daily on the outstanding balance and is paid monthly at a variable rate which, as of December 31, 2013, was 2.75% over the 30 day LIBOR rate or a nominal annual interest rate of 2.92%. As of December 31, 2013, the amount outstanding under this revolving line of credit is approximately $2.0 million. Three of the Company’s related parties guaranteed the loan and pledged financial assets to the bank to secure their guaranties, as approved by the Company’s board of directors. In return, the Company issued common stock warrants to the guarantors. The number of shares subject to the common stock warrants will be determined by dividing the warrant coverage amount, which is 50% of the fair market value of the collateral provided by the respective guarantors to secure their respective guaranty obligations to the bank, by the exercise price, which will be set at the price per share of the Company’s common stock sold in its IPO. See Note 7 for further discussion of the warrant liabilities. The Company has entered into an agreement with the guarantors that provides for reimbursement of any amounts paid by them on their guaranties. This reimbursement obligation is secured by a security interest in the Company’s assets.
151
6. Notes Payable
The following is a summary of the Company’s short-term and long-term debt obligations:
|
|
|
December 31, |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Note payable to shareholder; principal and interest payable in quarterly installments until maturity on April 2015, bearing interest at a per annum fixed rate of 3.25%. As of June 28, 2013, the note payable was converted into preferred shares. (“Goodman Note”) (See Note 7) |
|
$ |
1,935,000 |
|
|
$ |
— |
|
|
Secured convertible note to a major shareholder. (“2008 Convertible Note”) (See Note 7) |
|
|
1,400,000 |
|
|
|
1,400,000 |
|
|
Secured convertible notes. Total includes convertible notes due to a major shareholder of $11,250,000 at December 31, 2012. As of June 28, 2013, the notes payable were converted into preferred shares. (“2011 Convertible Bridge Notes”) (See Note 7) |
|
|
12,336,427 |
|
|
|
— |
|
|
Notes payable to shareholders issued in 2012. Includes notes of $5,810,000 to a major shareholder at December 31, 2012. As of June 28, 2013, the notes payable were converted into preferred shares. (“2012 Revolver Notes”) (See Note 7) |
|
|
5,960,000 |
|
|
|
— |
|
|
Unsecured convertible notes, issued under a note and warrant purchase agreement dated as of June 28, 2013, net of discounts related to warrants aggregating $0 and $874,158 at December 31, 2012 and 2013, respectively. Includes notes of $720,000 and $2,505,000 to a major shareholder at December 31, 2012 and 2013, respectively. (“2013 Convertible Bridge Notes”) (See Note 7) |
|
|
745,000 |
|
|
|
4,115,842 |
|
|
Other debt discount (See Notes 5 and 7) |
|
|
— |
|
|
|
(315,243 |
) |
|
|
|
|
|
|
|
|
|
|
|
Total notes payable |
|
|
22,376,427 |
|
|
|
5,200,599 |
|
|
Less current portion |
|
|
21,631,427 |
|
|
|
5,200,599 |
|
|
|
|
|
|
|
|
|
|
|
|
Long-term portion |
|
$ |
745,000 |
|
|
$ |
— |
|
Except for the non-current balance of the 2013 Convertible Bridge Notes, all outstanding notes payable and convertible notes payable were classified as current as of December 31, 2012, as the Company was unable to make principal and interest payments on these notes during the year ended December 31, 2012, or prior to the conversion of certain of the notes as of June 28, 2013. None of the lenders had sought any remedy for this default as of December 31, 2012 or prior to the conversion of the notes as of June 28, 2013.
On June 28, 2013, approximately $20,231,000 of outstanding notes payable and $2,581,000 of accrued interest were converted into 42,245,834 preferred shares, in accordance with the provisions of the debt conversion agreements of that date. As of December 31, 2013, all remaining principal payments for outstanding notes payable and convertible notes are due within one year.
Total interest expense incurred for all notes, convertible notes, and the line of credit, including amortization of debt discounts, for the years ended December 31, 2012 and 2013 was approximately $2,125,000 and $1,964,000, respectively, of which approximately $1,957,000 and $516,000 was recorded as accrued interest as of December 31, 2012 and 2013, respectively.
7. Convertible Notes and Warrants
Outstanding Warrants—Preferred Shares
Goodman Note
During April 2005, the Company entered into an unsecured loan agreement for $15,000,000. The note required interest payments and principal settlement upon maturity at the earliest of (a) April 20, 2010, (b) the Company being acquired, or (c) the Company having a change in control, other than through the sale of preferred shares.
152
During January 2009, the Company entered into an amendment and restatement of the unsecured amended loan, whereby the parties agreed that the principal amount would be reduced to $3,000,000. The amended and restated unsecured note bears interest at a variable rate per annum based on prime plus 25 basis points. 25% of the accrued interest was due and payable quarterly in arrears on the last business day of each three-month quarter beginning February 1, 2009. The remaining 75% of the accrued interest was not to be compounded by becoming part of the principal, and was due and payable in a lump-sum payment on the maturity date. The principal and any interest amounts that remain outstanding was set to mature at the earlier of (a) April 20, 2010, or (b) the date immediately prior to the Company’s closing of an acquisition or asset transfer as defined by the Company’s amended and restated articles of incorporation.
In conjunction with the 2009 amendment, the Company issued a warrant to purchase preferred shares issued in the first equity financing to occur subsequent to the execution of the note, and in which the Company receives at least $2,000,000 in gross aggregate proceeds. The exercise price of the warrant is equal to the per share price of preferred shares sold in that equity financing, and the number of shares that may be exercised is equal to 10% of the principal amount of the convertible loan divided by the exercise price. Early termination of the warrant can occur upon an IPO, or if the Company is acquired. The holder of the warrant is to be given 20 days advance notice of such an event, and the warrant will terminate if not exercised before the date of the event.
A qualifying equity financing occurred during February 2009, which set the warrant exercise price at $0.60 per share.
During May 2010, the Company entered into a second amendment and restatement of the Goodman Note in order to extend the maturity date and amend the timing of payments to be made to the lender and to secure the Company’s obligations under the note. The secured amended and restated note bears interest at a per annum fixed rate of 3.25% and is due and payable quarterly in arrears on the last business day of each three-month quarter beginning May 1, 2010. On the effective date of the second amendment, the Company paid the lender $750,000 which was applied to the principal balance of $3,000,000. Beginning May 1, 2010, principal payments are due and payable quarterly in advance. For principal payments due and payable during the period of May 1, 2010 through January 31, 2011, the quarterly principal payment was equal to $45,000; for principal payments due and payable during the period of February 1, 2012 through January 31, 2014, the quarterly principal payment is equal to $90,000; and for principal payments due and payable during the period of February 1, 2014 through the maturity date, the quarterly principal payment is equal to $150,000. In addition to the $750,000 principal paid on the effective date of the amendment, the Company paid principal payments of $135,000 and $180,000 during the years ended December 31, 2010 and 2011, respectively. No principal payments were made during the years ended December 31, 2012 or 2013.
As of June 28, 2013 the holder of the Goodman Note agreed to convert the total principal balance owed under the Goodman Note of $1,935,000 and accrued interest of approximately $105,000 into 3,777,324 preferred shares at a conversion price of $0.54 per share. Although the conversion price of the debt was greater than the value of the preferred shares at the time of conversion, the Company did not record a gain on the conversion under the troubled debt restructuring accounting guidance since the transaction occurred between related parties, and thus, was treated as a capital transaction.
In July 2013, in connection with this conversion, the Company issued to such beneficial owner a warrant to purchase 23,809 shares of common stock at an exercise price which will be set at the price per share of the Company’s common stock sold in the Company’s IPO. The warrants will be exercisable for a two-year period beginning with the closing of the Company’s IPO. In accordance with guidance applicable to accounting for derivative financial instruments that are accounted for as liabilities, the warrants are initially recorded at their fair value and are then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. For the warrants for common shares issued under the Goodman Note agreement, the Company used a probability weighted Black-Scholes valuation model. The fair value of the Goodman Note warrants was approximately $62,000 and is included in warrant liabilities at December 31, 2013.
2008 Convertible Note
In December 2008, the Company issued a convertible note in the principal amount of $1,400,000 which is secured by all assets of the Company to an affiliate of a major shareholder. The 2008 Convertible Note bears interest at a variable rate based on prime per annum payable at maturity, and matures at the earliest occurrence of, (a) the passing of 48 months from inception of the note, (b) the closing date of an acquisition or asset transfer as defined by the note, or (c) the closing date of the issuance and sale of shares of common stock of the Company in the Company’s IPO.
Upon the closing of a sale by the Company of its preferred shares in which the Company receives an aggregate of at least $20,000,000 in cumulative gross proceeds, including conversion of the convertible loan amount before the maturity date, the unpaid principal and accrued interest shall automatically be converted into the number of preferred shares, of the series sold by the Company in such sale, equal to the unpaid principal and accrued interest divided by the per share purchase price of the preferred shares in such sale. The 2008 Convertible Note may also be converted before the maturity date at the option of the holder at the closing of an equity financing involving the sale of the Company’s preferred shares in which the Company receives an aggregate of at least $2,000,000 in cumulative
153
gross proceeds, with a conversion price equal to the per share price included in that equity financing. In July 2013, the Company amended the 2008 Convertible Note to provide that all principal and accrued interest on the note would automatically convert into common stock upon the closing of an IPO at the price per share at which common stock is sold in such IPO.
Issued with the 2008 Convertible Note was a warrant to purchase preferred shares issued in the first equity financing to occur subsequent to the execution of the 2008 Convertible Note, and in which the Company receives at least $2,000,000 in gross aggregate proceeds. The exercise price of the warrant is equal to the per share price of preferred shares sold in that equity financing, and the number of shares that may be exercised is equal to 10% of the principal amount of the convertible loan divided by the exercise price. Early termination of the warrant can occur upon an IPO or if the Company is acquired. The holder of the warrant is to be given 20 days advance notice of such an event, and the warrant will terminate if not exercised before the date of the event.
A qualifying equity financing occurred during February 2009, which set the 2008 Convertible Note conversion price and the warrant exercise price at $0.60 per share. The 2008 Convertible Note remains outstanding at December 31, 2012 and 2013.
2011 Convertible Bridge Notes
In February 2011, the Company executed a note and warrant purchase agreement with a major shareholder’s affiliates. In exchange for a series of loans in an aggregate amount equal to $5,000,000 over a period through September 1, 2011, the Company issued secured convertible promissory notes and warrants to purchase preferred shares. The aggregate amount was subsequently raised to $6,000,000 and then $15,000,000 during the year and the funding period was first extended to February 2012 and then to December 2012. Other investors, including related parties, also became party to this arrangement and purchased 2011 Convertible Bridge Notes and warrants.
All unpaid principal and interest outstanding was initially payable on December 31, 2011. During 2012, the maturity date was extended to December 31, 2012. The 2011 Convertible Bridge Notes are secured by virtually all of the assets of the Company. The 2011 Convertible Bridge Notes bear interest at 8%, payable at maturity. The number of preferred shares for which the warrants are exercisable is determined by dividing the warrant coverage amount, which is 20% of the principal amount of the notes issued under the agreement, by the exercise price.
Upon the closing of the sale by the Company of its preferred stock in which the Company receives an aggregate of at least $20,000,000 in cumulative gross proceeds, including conversion of the 2011 Convertible Bridge Notes, before the maturity date, the unpaid principal and accrued interest shall automatically be converted into the number of preferred shares, of the series sold by the Company in such sale, equal to the unpaid principal and accrued interest divided by the per share purchase price of the preferred shares in such sale. At any time before the maturity date the investor may elect to convert all or any amount of the unpaid principal and accrued interest into the Company’s Series A preferred shares at $0.54 per share. Early termination of the warrants can occur upon an IPO or if the Company is acquired. The holders of the warrants are to be given 20 days advance notice of such an event, and the warrants will terminate if not exercised before the date of the event.
In accordance with guidance applicable to accounting for derivative financial instruments that are accounted for as liabilities, the warrants are initially recorded at their fair value and are then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. For stock-based derivative financial instruments issued under the note and warrant purchase agreement dated February 2011, the Company used the Black-Scholes valuation model. The Company recorded approximately $1,400,000 related to the fair value of the warrants at the date of issuance, as a discount to the carrying value of the 2011 Convertible Bridge Notes, accreted as interest expense over the life of the debt. The Company valued the warrants at the date of each issuance using the Black-Scholes valuation model with the following underlying assumptions: contractual term of 5 years, an underlying preferred share price between $0.25 and $0.54, an exercise price of $0.54, an average risk-free interest rate between 0.70% and 2.26%, a dividend yield of 0%, and volatilities between 100.0% and 105.0%. Approximately $302,000 related to accretion of the discount was recognized as interest expense during the year ended December 31, 2012. The discount was fully accreted as of December 31, 2012.
As of December 31, 2012, the Company had issued the 2011 Convertible Bridge Notes with an aggregate principal amount of approximately $12,336,000. No further note or warrant issuances were made under this agreement during the year ended December 31, 2013. As of December 31, 2012, the Company was in default for payment on the 2011 Convertible Bridge Notes, and no principal payments were made in 2013 prior to their conversion. As of June 28, 2013 the investors under these notes elected to convert the total principal balance owed under the 2011 Convertible Bridge Notes of approximately $12,336,000 and accrued interest of approximately $1,832,000 into 26,237,611 preferred shares at a conversion price of $0.54 per share. Upon the conversion, the exercise price of the related warrants was set at $0.54 per share, and the $236,799 fair value of the warrants was reclassified into additional paid-in capital as of June 28, 2013. Although the conversion price of the debt was greater than the value of the preferred shares at the time of conversion, the Company did not record a gain on the conversion under the troubled debt restructuring accounting guidance since the transaction occurred between related parties, and thus, was treated as a capital transaction.
154
2012 Revolver Notes
On January 13, 2012, the Company executed a note and warrant purchase agreement with several shareholders, including a major shareholder, calling for (in addition to the issuance of certain related warrants) the issuance of a series of notes to be issued between January 13, 2012 and April 5, 2012 totaling up to $1,750,000, with an original maturity date in April 2012. The 2012 Revolver Notes were amended on April 5, 2012 to extend the maturity date to May 31, 2012 or July 31, 2012, depending on certain milestones, and to allow the Company to issue up to $5,000,000 in notes payable under this agreement, as needed. The 2012 Revolver Notes were amended again on November 8, 2012 to increase the amount of notes payable the Company can issue to $8,000,000, and to provide that all notes issued under this agreement shall have the same maturity date of either November 30, 2012 or December 31, 2012, depending on certain milestones. The 2012 Revolver Notes bear interest at 10%, payable at maturity.
Beginning on the closing of the sale by the Company of its preferred shares in which the Company receives an aggregate of at least $20,000,000 in cumulative gross proceeds, the warrants are exercisable for preferred shares of the series sold by the Company in such sale, at an exercise price equal to the purchase price per share of the preferred shares sold by the Company in such sale. The number of preferred shares for which the warrants are exercisable is determined by dividing the warrant coverage amount, which is 20% of the principal amount of the notes issued under the agreement on the issuance date of such 2012 Revolver Notes, by the exercise price. At any time prior to the maturity date, the investor may elect to convert all or any amount of the unpaid principal and accrued interest into the Company’s Series A preferred stock at $0.54 per share, or if a qualified financing has occurred, at the purchase price per share of the preferred shares sold by the Company in such qualified financing. Early termination of the warrant can occur upon an IPO, or if the Company is acquired. The holders of the warrants are to be given 20 days advance notice of such an event, and the warrants will terminate if not exercised before the date of the event.
In accordance with guidance applicable to accounting for derivative financial instruments that are accounted for as liabilities, the warrants are initially recorded at their fair value and are then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. For the 2012 Revolver Notes and warrants issued under the note and warrant purchase agreement dated January 13, 2012, the Company used the Black-Scholes valuation model. The Company recorded approximately $396,000 related to the fair value of the warrants issued, as a discount to the carrying value of the debt, accreted as interest expense over the life of the debt. The Company valued the warrants at the date of each issuance using the Black-Scholes valuation model with the following underlying assumptions: contractual term of 5 years, an underlying preferred share price between $0.24 and $0.30, an exercise price of $0.54, an average risk-free interest rate between 0.62% and 1.02%, a dividend yield of 0%, and volatility of 105.0%. Approximately $396,000 related to accretion of the discount was recognized as interest expense during the year ended December 31, 2012. The discount was fully accreted as of December 31, 2012.
As of December 31, 2012, the Company had issued $5,960,000 in notes payable under the 2012 Revolver Notes agreement. The Company was in default for payment of these notes as of December 31, 2012, and no principal payments were made in 2013 prior to conversion. As of June 28, 2013 the investors under the 2012 Revolver Notes elected to convert the total principal balance of approximately $5,960,000 owed under the 2012 Revolver Notes and accrued interest of approximately $645,000 into 12,230,899 preferred shares at a conversion price of $0.54 per share, pursuant to note conversion agreements of that date. Although the conversion price of the debt was greater than the value of the preferred shares at the time of conversion, the Company did not record a gain on the conversion under the troubled debt restructuring accounting guidance since the transaction occurred between related parties, and thus, was treated as a capital transaction. On September 13, 2013, the exercise price of the warrants was fixed at $0.54 per share, and the fair value of the warrant liability of approximately $144,000 on that date was reclassified to additional paid-in capital.
Other
On September 10, 2012, the Company issued a warrant to its landlord in exchange for a rent deferral through November 30, 2012. The number of Series A preferred shares exercisable under the warrant agreement is determined by dividing the warrant coverage amount of $40,000 by the exercise price. The exercise price of the warrants is $0.60, or, upon the closing of the sale by the Company of its preferred stock in which the Company receives an aggregate of at least $15,000,000 in cumulative gross proceeds, the warrant’s exercise price will be the price per share for which the Company sells its preferred shares in such sale. The term of the warrant is seven years. Early termination of the warrant can occur if the Company is acquired. The holder of the warrant is to be given 20 days advance notice of such an event, and the warrant will terminate if not exercised before the date of the event. The fair value of the preferred warrant due to the landlord at December 31, 2012 and 2013 is not material to the financial statements.
As of December 31, 2012, warrants to purchase preferred stock are reflected as a liability on the balance sheet, which is adjusted to estimated fair value at the end of each reporting period over the term of the warrants. These warrants were reclassified to additional paid-in capital during the year ended December 31, 2013. The fair value of the warrant liability for warrants to purchase preferred stock as of December 31, 2012 of approximately $982,000 was estimated using the Black-Scholes valuation model with the following assumptions: contractual term between 3.08 and 4.92 years, an underlying preferred share price of $0.25, an exercise price of $0.54, an average risk-free interest rate between 0.35% and 0.70%, a dividend yield of 0%, and volatility of 105.0%.
155
Outstanding Warrants—Common Shares
2013 Convertible Bridge Notes
The Company executed a convertible note and warrant purchase agreement as of June 28, 2013 with several shareholders, including a major shareholder, relating to the Company’s borrowing as needed of, and issuance of a series of unsecured convertible notes for, up to $7,000,000. The Company had borrowed $745,000 and $4,990,000 as of December 31, 2012 and 2013, respectively, against the 2013 Convertible Bridge Notes, including $720,000 and $2,505,000, respectively, from a major shareholder. The maturity date of the 2013 Convertible Bridge Notes is May 31, 2014 and may be extended at the option of the respective note holders for two successive six month periods. The 2013 Convertible Bridge Notes bear interest at 8.0% per annum, payable at maturity.
The 2013 Convertible Bridge Notes automatically convert into the Company’s common stock upon the closing of an IPO of at least $8,000,000 in cumulative gross proceeds, at a price equal to the price per share of the Company’s common stock sold in the IPO. The number of common shares for which the warrants are exercisable is determined by dividing the warrant coverage amount, which is 50% of the principal amount of the notes issued under the agreement, by the exercise price, which is the price per share of the Company’s common stock sold in the IPO. The warrants will be exercisable for a five-year period beginning with the closing of the Company’s IPO. Early termination of the warrants can occur upon any capital reorganization, any reclassification of the capital stock, or an asset transfer or acquisition of the Company. The holders of the warrants are to be given 20 days advance notice of such an event, and the warrants will terminate if not exercised prior to the date of the event.
In accordance with guidance applicable to accounting for derivative financial instruments that are accounted for as liabilities, the warrants are initially recorded at their fair value and are then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. For the warrants for common shares issued under the 2013 Convertible Bridge Notes agreement, the Company used a probability weighted Black-Scholes valuation model. The Company recorded approximately $1,559,000 related to the fair value of the warrants issued, as a discount to the carrying value of the debt, accreted to interest expense using the effective interest method from the date of issuance over the life of the debt. These warrants to purchase common stock were valued as of their date of issuance, using the following assumptions: exercise price of between $1.48 and $14.28 per share, contractual term of 5 years, a risk-free interest rate between 1.38% and 1.73%, a dividend yield of 0%, and volatility between 100.0%—105.0%. The value of the warrants using the probability weighted Black-Scholes valuation model accounted for a probability between 75% and 80%, while a fair value of $0 was weighted between 20% and 25%. The fair value of the warrants is recorded as a liability of approximately $1,399,000 at December 31, 2013. Approximately $685,000 related to accretion of the discount was recognized as interest expense during the year ended December 31, 2013, with approximately $874,000 remaining unamortized and reflected as a discount to the debt.
Line of Credit
Three of the Company’s related parties guaranteed the Company’s Line of Credit (see Note 5) and pledged financial assets to the bank to secure their guaranties, as approved by the Company’s board of directors. In return, the Company issued common stock warrants to the guarantors. The fair market value of the collateral provided by the respective guarantors at December 31, 2013 was $2,178,000. The number of shares subject to the common stock warrants will be determined by dividing the warrant coverage amount, which is 50% of the fair market value of the collateral provided by the respective guarantors to secure their respective guaranty obligations to the bank, by the exercise price, which will be set at the price per share of the Company’s common stock sold in its IPO. The warrants will be exercisable for a two-year period beginning with the closing of the Company’s IPO.
In accordance with guidance applicable to accounting for derivative financial instruments that are accounted for as liabilities, the warrants are initially recorded at their fair value and are then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. For the warrants for common shares issued in connection with the Company’s Line of Credit, the Company used a probability weighted Black-Scholes valuation model. The Company recorded approximately $454,000 related to the fair value of the warrants issued, as a discount to the carrying value of the debt, accreted to interest expense on a straight line basis from the date of issuance over the life of the debt. These warrants to purchase common stock were valued as of their date of issuance, using the following assumptions: exercise price between $1.48 and $14.28 per share, contractual term of 2 years, a risk-free interest rate between 0.38% and 1.38%, a dividend yield of 0%, and volatility between 90.0% and 105.0%. The value of the warrants using the probability weighted Black-Scholes valuation model accounted for a probability of 75%, while a fair value of $0 was weighted 25%. The fair value of the warrants is recorded as a liability of approximately $390,000 at December 31, 2013. Approximately $139,000 related to accretion of the discount was recognized as interest expense during the year ended December 31, 2013, with approximately $315,000 remaining unamortized and reflected as a discount to outstanding debt.
Other
On September 10, 2013, the Company, as part of a lease amendment for its non-cancellable operating lease for its office, laboratory, and warehouse space at its San Diego, California facility, issued a warrant to its landlord. The warrant coverage amount was
156
$502,605. The number of shares subject to the common stock warrants will be determined by dividing the warrant coverage amount by the exercise price, which will be set at the price per share of the Company’s common stock sold in its IPO. The warrants will be exercisable for a five-year period beginning with the closing of the Company’s IPO.
In accordance with guidance applicable to accounting for derivative financial instruments that are accounted for as liabilities, the warrant is initially recorded at fair value and then is re-valued at each reporting date, with changes in the fair value reported in the statements of operations. For the warrant for common shares issued to the landlord, the Company used a probability weighted Black-Scholes valuation model. The Company recorded approximately $309,000 related to the fair value of the warrant issued at issuance in September 2013, as a reduction in deferred rent liability, accreted to rent expense on a straight line basis from the date of issuance over the term of the amended lease. The warrant was valued as of the date of issuance, using the following assumptions: exercise price of between $3.08 and $14.28 per share, contractual term of 5 years, a risk-free interest rate of 1.38%, a dividend yield of 0%, and volatility of 105.0%. The value of the warrant using the probability weighted Black-Scholes valuation model accounted for a probability of 75%, while a fair value of $0 was weighted 25%. The fair value of the warrant is recorded as a liability of approximately $282,000 at December 31, 2013.
As of December 31, 2013, warrants to purchase common stock are reflected as a liability on the balance sheet, which is adjusted to estimated fair value at the end of each reporting period over the term of the warrants. The aggregate fair value of the warrant liability for warrants to purchase common stock as of December 31, 2013 of approximately $2,132,000 was estimated using a probability weighted Black-Scholes valuation model with the following assumptions for both the five-year and two-year common stock warrant terms separately:
|
|
|
Five-year term |
|
|
Two-year term |
|
||
|
Stock price |
|
$ |
1.48 – 7.69 |
|
|
$ |
1.48 – 7.69 |
|
|
Exercise price |
|
$ |
1.48 – 7.69 |
|
|
$ |
1.48 – 7.69 |
|
|
Expected dividend yield |
|
|
0 |
% |
|
|
0 |
% |
|
Discount rate-bond equivalent yield |
|
|
1.73 |
% |
|
|
0.38 |
% |
|
Expected life (in years) |
|
|
5.0 |
|
|
|
2.0 |
|
|
Expected volatility |
|
|
100.0 |
% |
|
|
90.0 |
% |
At December 31, 2013 the values of both the five-year and two-year common stock warrants using the probability weighted Black-Scholes valuation models accounted for a probability of 75%, while a fair value of $0 was weighted 25%.
Change in estimated fair value of warrant liability
The change in the estimated fair value of the total liability outstanding for all outstanding warrants of approximately $454,000 and $782,000 was recognized as a non-cash gain and included in total other income/(expense) in the Company’s statements of operations and comprehensive loss for the years ended December 31, 2012 and 2013, respectively.
8. Supplier Financing
In 2011, the Company purchased certain laboratory equipment under financing agreements with a supplier, a business owned by a member of the Company’s board of directors, totaling approximately $256,000. Financing was granted for the purchase of the equipment at a stated interest rate of 0.0%. The Company has utilized its average interest rate for 2012 and 2013 of 8.0% to amortize the payments and record interest expense of approximately $17,000 and $5,000 for the years ended December 31, 2012 and 2013, respectively, utilizing the effective interest expense method. The remaining balance owed under these financing agreements was approximately $60,000 and $66,000 as of December 31, 2012 and 2013, respectively. The remaining balance owed under these financing agreements was due in 2013 (see Note 18).
In 2011, the Company purchased laboratory software under a financing agreement with a supplier for approximately $177,000. This software financing agreement bears an interest rate of 7.4% per annum. The balance owed under these financing agreements was approximately $62,000 at both December 31, 2012 and 2013.
In 2012 and 2013, the Company obtained third-party financing for certain business insurance premiums. The financing bears an interest rate of 5.95% per annum, and all financing is due within one year. The balances due under these annual financing arrangements were approximately $129,000 and $91,000 as of December 31, 2012 and 2013, respectively.
157
9. Shareholders’ Deficit
(a) Common Stock
In November of 2011, the Company amended and restated its articles of incorporation to decrease the number of authorized shares of common stock from 44,260,000 to 14,600,000. The authorized number shares of common stock at December 31, 2011 and 2012 was 14,600,000. In conjunction with the November 2011 amendment, the Company declared a 1:3 reverse stock split for all common shares. On November 1, 2013, the Company effected a 1:14 reverse stock split for all common shares. All references to share and per share amounts in the financial statements and accompanying notes to the financial statements have been retroactively restated to reflect the 1:14 reverse stock split.
On July 22, 2013, the Company amended its articles of incorporation to increase the number of authorized shares of common stock from 14,600,000 to 53,000,000. The authorized number of shares of common stock at December 31, 2013 was 53,000,000. In addition, on July 30, 2013, the Company assigned a par value to its common shares of $0.0001 in conjunction with its reincorporation in Delaware. The new par value per common share has been retroactively reflected in the financial statements for all periods presented.
(b) Preferred Stock
In November of 2011, the Company amended and restated its articles of incorporation so that each share of the issued and outstanding Series AA preferred stock and each share of the issued and outstanding Series BB preferred stock of the Company was converted into one share of Series A preferred stock. As of December 31, 2011 and 2012, all 36,460,000 authorized shares of preferred stock are designated as Series A preferred stock. On July 22, 2013, the Company amended its articles of incorporation to increase the number of authorized preferred shares from 14,600,000 to 100,000,000. In addition, on July 30, 2013, the Company assigned a par value to its preferred shares of $0.0001 in conjunction with its reincorporation in Delaware. The new par value per preferred share has been retroactively reflected in the financial statements for all periods presented.
Holders of the Company’s preferred shares are entitled to receive, when and as declared by the board of directors and in preference to common shareholders, non-cumulative cash dividends at the rate of 8% per annum of the applicable original issue price on each outstanding preferred share. The original issue price of each share of Series A preferred stock was $0.60. No dividends were declared during 2011 or 2012 or in the first nine months of 2013. Dividends cannot be granted for common shareholders while shares of preferred stock remain outstanding.
The holders of preferred shares have the right to one vote for each common share into which the preferred shares are convertible. Upon the liquidation, dissolution, or winding up of the Company, either voluntary or involuntary, the preferred shareholders will be paid out an amount equal to the original issue price plus all declared and unpaid dividends. If, upon any liquidation, distribution, or winding up of the Company, and the assets of the Company are insufficient to make payment in full to all holders of preferred shares of the liquidation preference, then such assets shall be distributed among the holders of preferred shares ratably in proportion to the full amounts to which they would be entitled.
The convertible preferred shares may be converted into common shares at any time at the option of the holder utilizing the then effective Series A preferred conversion price. All preferred shares shall be automatically converted into common shares utilizing the then effective Series A preferred conversion price upon a) the election of the holders of a majority of the outstanding shares of Series A preferred stock, or b) the closing of a firmly underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933 covering the sale of the Company’s common stock if gross proceeds are at least $20,000,000 and the per share price is at least $25.20.
The effective conversion price is equal to the original issue price divided by $25.20 as may be adjusted for dilutive issuances of common shares, common share rights or options, common share splits and combinations, dividends, and distributions. The effective conversion rate is not adjusted for issuances of common share options, warrants or rights to employees, directors, or non-employee service providers.
During the year ended December 31, 2013, 42,245,834 shares of Series A preferred stock were issued for the conversion of approximately $20,231,000 of debt and $2,581,000 of accrued interest, primarily to related parties (see Notes 6 and 7).
10. Accounting for Stock-Based Compensation Expense
2007 Equity Incentive Plan
The 2007 Equity Incentive Plan (“2007 Plan”) authorizes the grant of the following types of awards: (i) nonstatutory stock options, or NSOs, (ii) incentive stock options, or ISOs, (iii) restricted stock awards, (iv) restricted stock unit awards, or RSUs, (v) stock
158
appreciation rights, or SARs, (vi) performance awards, and (vii) other stock awards. Awards may be granted to employees, officers, non-employee board members, consultants, and other service providers of the Company. However, ISOs may not be granted to non-employees. Prior to November 2011, the Company was authorized to issue 7,500,000 options under the 2007 Plan. In conjunction with the 1:3 reverse common stock split in November 2011, the number of shares authorized under the 2007 Plan decreased to 2,500,000 shares and further reduced to 178,571 shares as a result of the 1:14 reverse split in November 2013. As of December 31, 2012 and 2013, shares available for grant under the 2007 Plan were 50,127 and 77,061, respectively.
2013 Equity Incentive Plan
In July 2013, the Company adopted a new stock-based compensation plan entitled the 2013 Equity Incentive Plan (“2013 Plan”). The 2013 Plan authorizes the grant of the following types of awards: (i) nonstatutory stock options, (ii) ISOs, (iii) restricted stock awards, (iv) restricted stock unit awards, (v) stock appreciation rights, and (vi) performance compensation awards. Awards may be granted to employees, officers, non-employee board members, consultants, and other service providers of the Company. However, ISOs may not be granted to non-employees. The Company has authorized a total of 403,571 shares of common stock for issuance pursuant to all awards granted under the 2013 Equity Incentive Plan, subject to an increase of 800,000 shares upon the completion of an IPO, and subject to additional increases every January 1 equal to the lesser of (i) 5% of the Company’s outstanding common stock on such January 1, or (ii) a number of shares determined by the Company’s board of directors in its discretion for use on such particular January 1. As of December 31, 2013, 401,640 stock options and RSUs have been granted under the 2013 Plan, and 1,931 shares are available for grant under the 2013 Plan. On February 10, 2014, in connection with the closing of the Company’s IPO, the number of shares of common stock covered by the 2013 Plan increased by 800,000. Additionally, 335,798 options were granted under the 2013 Plan in February and March 2014 (see Note 18).
Options granted under either plan vest over a maximum period of four years and expire ten years from the date of grant. Options generally vest either (i) over four years, 25% on the one year anniversary of the date of grant and monthly thereafter for the remaining three years; or (ii) over four years, monthly vesting beginning month-one after the grant and monthly thereafter. Certain options have been granted which vest 50% on the grant date and monthly thereafter for the remaining two years.
The fair value of stock options is determined on the date of grant using the Black-Scholes valuation model. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight-line method. The determination of the fair value of stock options is affected by the Company’s stock price, as well as assumptions regarding a number of complex and subjective variables. The volatility assumption is based on a combination of the historical volatility of the Company’s common stock and the volatilities of similar companies over a period of time equal to the expected term of the stock options. The volatilities of similar companies are used in conjunction with the Company’s historical volatility because of the lack of sufficient relevant history for the Company’s common stock equal to the expected term. The expected term of employee stock options represents the weighted-average period the stock options are expected to remain outstanding. The expected term assumption is estimated based primarily on the options’ vesting terms and remaining contractual life and employees’ expected exercise and post-vesting employment termination behavior. The risk-free interest rate assumption is based upon observed interest rates on the grant date appropriate for the term of the employee stock options. The dividend yield assumption is based on the expectation of no future dividend payouts by the Company.
The assumptions used in the Black-Scholes pricing model for options granted during the years ended December 31, 2012 and 2013 are as follows:
|
|
|
For the years ended December 31, |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Volatility |
|
|
96.8 |
% |
|
|
105.0 |
% |
|
Risk-free interest rate |
|
|
0.79% – 1.15 |
% |
|
|
1.38 – 1.69 |
% |
|
Dividend yield |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
Expected term (years) |
|
|
6.08 |
|
|
|
5.26 – 6.02 |
|
|
Expected forfeiture rate |
|
|
0.00 |
% |
|
|
0.00 – 5.00 |
% |
Using the assumptions described above, the weighted-average estimated fair value of options granted in 2012 and 2013 were approximately $1.82 and $4.43, respectively.
159
A summary of stock option activity for 2012 and 2013 is as follows:
|
|
|
Number of |
|
|
Weighted |
|
|
Average |
|
|||
|
Outstanding at December 31, 2011 |
|
|
78,987 |
|
|
$ |
4.90 |
|
|
|
8.3 |
|
|
Granted |
|
|
330 |
|
|
|
4.62 |
|
|
|
|
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Cancelled/forfeited/expired |
|
|
(15,799 |
) |
|
|
4.92 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2012 |
|
|
63,518 |
|
|
$ |
4.97 |
|
|
|
6.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
300,438 |
|
|
|
5.18 |
|
|
|
|
|
|
Exercised |
|
|
(4,021 |
) |
|
|
5.00 |
|
|
|
|
|
|
Cancelled/forfeited/expired |
|
|
(26,829 |
) |
|
|
5.20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2013 |
|
|
333,106 |
|
|
$ |
5.14 |
|
|
|
9.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and unvested expected to vest, December 31, 2013 |
|
|
331,540 |
|
|
$ |
5.14 |
|
|
|
9.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The intrinsic value of options exercised during the year ended December 31, 2013 was $3,450. The intrinsic value of options outstanding and vested and unvested expected to vest at December 31, 2013 was $8,204 and $8,192, respectively.
The Company received $20,105 in proceeds from stock options exercised during the year ended December 31, 2013. The tax benefit related to stock options exercised during the year ended December 31, 2013 was not significant.
Further information about the options outstanding and exercisable at December 31, 2012 and 2013 is as follows:
|
Options Outstanding and Exercisable at December 31, 2012 |
|
|||||||||||
|
Weighted Average Exercise Price |
|
Total Shares |
|
|
Weighted |
|
|
Total Shares |
|
|||
|
$4.62 |
|
|
33,031 |
|
|
|
7.2 |
|
|
|
16,173 |
|
|
$5.04 |
|
|
30,408 |
|
|
|
5.1 |
|
|
|
28,244 |
|
|
$125.58 |
|
|
79 |
|
|
|
1.1 |
|
|
|
79 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
63,518 |
|
|
|
|
|
|
|
44,496 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Outstanding and Exercisable at December 31, 2013 |
|
|||||||||||
|
Weighted |
|
Total Shares |
|
|
Weighted |
|
|
Total Shares |
|
|||
|
$4.62 |
|
|
20,208 |
|
|
|
7.3 |
|
|
|
13,731 |
|
|
$5.04 |
|
|
12,460 |
|
|
|
5.5 |
|
|
|
12,455 |
|
|
$5.18 |
|
|
300,438 |
|
|
|
9.6 |
|
|
|
110,825 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
333,106 |
|
|
|
|
|
|
|
137,011 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The intrinsic value of options exercisable at December 31, 2013 was $5,575.
Restricted Stock Units (“RSUs”)
In November 2010, the Company issued to a member of the board of directors a restricted stock unit award for 390,000 shares of Series BB preferred stock. In November 2011, these RSUs were modified to be redeemable for Series A preferred stock under the same terms and conditions of the original grant. The shares will not vest unless a change in control, as defined, or IPO occurs within 10 years of the vesting commencement date of October 2010. There will be no expense to record for these awards unless and until it
160
becomes probable that the award will vest. As of December 31, 2012 and 2013, it was not probable that these awards will vest and therefore, no expense was recorded during the years ended December 31, 2012 or 2013.
In March 2011, the Company awarded a restricted stock unit award to a member of the board of directors for 428,597 shares of Series BB preferred stock. Also in March 2011, the Company awarded an additional performance-based restricted stock unit award for an estimated 574,108 shares of Series BB preferred stock to the same member. In November 2011, these RSUs were modified to be redeemable for Series A preferred stock under the same terms and conditions of the original grant. The number of shares in the restricted stock units is based on certain milestones to be achieved. None of the shares under either award vest unless a change in control or IPO occurs within 10 years after January 1, 2011. There will be no expense to record for these awards until it becomes probable that an award will vest. As of December 31, 2012 and 2013, it was not probable that these awards will vest and therefore, no expense was recorded during the years ended December 31, 2012 or 2013.
The board of directors approved a resolution in December 2010, that each January 1 each person (other than two identified individuals) who is serving as a non-employee director on such January 1 shall be automatically granted an annual restricted stock unit award covering a number of common shares equal to 0.25% of the fully diluted outstanding common stock of the Company as of the December 31 immediately preceding such January 1. These restricted stock unit awards will be granted automatically on each January 1 and will vest in equal monthly installments over 12 months from the date of the grant. Additionally, in January 2012, each person (other than two identified individuals) who is serving as a non-employee director is to be granted a “true up grant” in addition to the annual grant covering a number of common shares equal to 0.25% of the fully diluted outstanding common shares of the Company as of the immediately preceding December 31. These grants will vest 100% on the date of the grant. In January 2012, five restricted stock unit awards for a total of 20,930 common shares were granted in accordance with this resolution. In addition, on January 1, 2012, an additional five restricted stock unit awards were granted to non-employee directors for a total of 20,930 common shares, vesting immediately upon grant. Although vested, shares are only delivered on the earlier of (i) the date that is 10 years from the grant date, (ii) the date of a change in control, (iii) the date of termination of the holder from the Company, (iv) the date of death or disability, or (v) the date of an unforeseeable emergency as described in Internal Revenue Code section 409A.
The RSU awards due to be granted on January 1, 2013 were not granted during the year ended December 31, 2013. In lieu of this issuance, RSU awards for 8,735 shares of common stock each were granted to three directors and an RSU award for 14,285 shares of common stock was granted to another director, on July 31, 2013. All RSUs issued in July 2013 vest in equal monthly installments over five months beginning August 1, 2013. The shares underlying the 2013 awards will be distributed no later than August 20, 2014.
In August 2013, 60,712 RSU awards were granted to certain executive employees. These awards vest 50% on the date of grant, with the remaining 50% vesting in equal monthly installments over twenty-four months beginning August 31, 2013.
Stock-based Compensation Expense
The following table presents the effects of stock-based compensation related to equity awards to employees and nonemployees on the statement of operations during the periods presented:
|
|
|
For the years ended |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Stock Options |
|
|
|
|
||||
|
Research and development expenses |
|
$ |
32,210 |
|
|
$ |
298,618 |
|
|
General and administrative expenses |
|
|
22,530 |
|
|
|
221,726 |
|
|
Sales and marketing expenses |
|
|
3,994 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
Total expenses related to stock options |
|
|
58,734 |
|
|
|
520,344 |
|
|
|
|
|
||||||
|
RSUs |
|
|
|
|
|
|
|
|
|
Research and development expenses |
|
|
— |
|
|
|
72,500 |
|
|
General and administrative expenses |
|
|
193,400 |
|
|
|
359,677 |
|
|
|
|
|
|
|
|
|
|
|
|
Total stock-based compensation |
|
$ |
252,134 |
|
|
$ |
952,521 |
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2013, total unrecognized share-based compensation expense related to nonvested stock option and RSU awards, adjusted for estimated forfeitures, was approximately $861,000 and $135,000, respectively, and is expected to be recognized over a weighted-average period of approximately 2.8 years and 1.6 years, respectively.
161
11. Net Loss per Common Share
Basic and diluted net loss per common share is determined by dividing net loss applicable to common shareholders by the weighted-average common shares outstanding during the period. Because there is a net loss attributable to common shareholders for the years ended December 31, 2012 and 2013, the outstanding shares of Series A preferred stock, RSUs, convertible debt, warrants, and common stock options have been excluded from the calculation of diluted loss per common share because their effect would be anti-dilutive. Therefore, the weighted-average shares used to calculate both basic and diluted loss per share are the same.
In November 2013, the Company effected a 1:14 reverse stock split of all common shares outstanding. The calculation of weighted-average shares outstanding has been adjusted for this reverse split as if it had occurred on January 1, 2012.
The following potentially dilutive securities have been excluded from the computations of diluted weighted-average shares outstanding for the periods presented, as they would be anti-dilutive:
|
|
|
For the years ended December 31, |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Series A preferred (number of common stock equivalents) |
|
|
647,007 |
|
|
|
1,652,851 |
|
|
Preferred warrants outstanding (number of common stock equivalents) |
|
|
192,262 |
|
|
|
192,262 |
|
|
Notes payable convertible into preferred shares (number of common stock equivalents) |
|
|
599,466 |
|
|
|
— |
|
|
Preferred share RSUs (number of common stock equivalents) |
|
|
33,158 |
|
|
|
89,647 |
|
|
Common warrants outstanding |
|
|
— |
|
|
|
836,890 |
|
|
Notes payable convertible into common shares |
|
|
665,178 |
|
|
|
1,110,649 |
|
|
Common share RSUs |
|
|
54,615 |
|
|
|
133,971 |
|
|
Common options outstanding |
|
|
63,518 |
|
|
|
333,106 |
|
|
|
|
|
|
|
|
|
|
|
|
Total anti-dilutive common share equivalents |
|
|
2,255,204 |
|
|
|
4,349,376 |
|
|
|
|
|
|
|
|
|
|
|
12. 401(k) Plan
The Company sponsors a 401(k) savings plan for all eligible employees. The Company may make discretionary matching contributions to the plan to be allocated to employee accounts based upon employee deferrals and compensation. To date, the Company has not made any matching contributions into the savings plan.
13. Income Taxes
For the year ended December 31, 2012 and 2013, the provision for income taxes was calculated as follows: The following table provides a reconciliation between income taxes computed at the federal statutory rate and the Company’s provision for income taxes:
|
|
|
For the years ended December 31, |
|
||||||||
|
|
|
2012 |
|
|
2013 |
|
|||||
|
Current: |
|
|
|
|
|
|
|
|
|||
|
Federal |
|
$ |
— |
|
|
$ |
— |
|
|||
|
State |
|
|
800 |
|
|
|
800 |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
Total |
|
|
800 |
|
|
|
800 |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
Deferred |
|
|
|
|
|
|
|
|
|||
|
Federal |
|
|
— |
|
|
|
— |
|
|||
|
State |
|
|
— |
|
|
|
— |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
Total |
|
|
— |
|
|
|
— |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
Provision for income tax |
|
$ |
800 |
|
|
$ |
800 |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
162
The following table provides a reconciliation between income taxes computed at the federal statutory rate and the Company’s provision for income taxes:
|
|
|
For the years ended December 31, |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Income tax at statutory rate |
|
$ |
(4,167,967 |
) |
|
$ |
(3,139,368 |
) |
|
State liability |
|
|
(602,296 |
) |
|
|
(321,058 |
) |
|
Permanent items |
|
|
3,164 |
|
|
|
6,932 |
|
|
Stock Compensation |
|
|
19,298 |
|
|
|
171,003 |
|
|
Nondeductible Interest |
|
|
521,531 |
|
|
|
395,089 |
|
|
Expiration of net operating losses |
|
|
146,175 |
|
|
|
188,316 |
|
|
Other |
|
|
80 |
|
|
|
(6,723 |
) |
|
Research and development credit |
|
|
(215,502 |
) |
|
|
(103,500 |
) |
|
Valuation allowance |
|
|
4,296,317 |
|
|
|
2,810,109 |
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income tax |
|
$ |
800 |
|
|
$ |
800 |
|
|
|
|
|
|
|
|
|
|
|
Deferred income taxes are provided for temporary differences in recognizing certain income and expense items for financial and tax reporting purposes. The deferred tax assets consisted primarily of the income tax benefits from net operating loss carryforwards, deferred rent, and research and development credits. Valuation allowances have been recorded to fully offset deferred tax assets at December 31, 2012 and 2013, as it is more likely than not that the assets will not be utilized.
At December 31, 2013, the Company has federal net operating loss carryforwards of approximately $111,673,000 expiring beginning in 2020 and California net operating loss carryforwards of approximately $97,656,000 expiring beginning in 2014. Additionally, at December 31, 2013, the Company has research and development credits of approximately $3,132,000 and $3,004,000 for federal and California purposes, respectively. The federal research and development tax credits will begin to expire in 2018. The California research and development tax credits do not expire.
For the years ended December 31, 2012 and 2013, the Company has evaluated the various tax positions reflected in their income tax returns for both federal and state jurisdictions, and all open tax years in these jurisdictions, to determine if the Company has any uncertain tax positions on the historical tax returns. The Company recognizes the impact of an uncertain tax position on an income tax return at the largest amount that the relevant taxing authority is more-likely-than not to sustain upon audit. The Company does not recognize uncertain income tax positions if they have less than 50 percent likelihood of being sustained. Based on this assessment, the Company believes there are no tax positions for which a liability for unrecognized tax benefits should be recorded as of December 31, 2012 or 2013. The Company is subject to taxation in the United States and California. The Company’s federal filings prior to 2010 and the Company’s California filings prior to 2009 are no longer subject to examination. The Company’s policy is to recognize interest and penalties related to income tax matters in income tax expense. Due to the existence of the valuation allowance, future changes in unrecognized tax benefits will not impact the Company’s effective tax rate. The Company is currently not under examination by any taxing authorities and does not believe its unrecognized tax benefits will significantly change in the next twelve months.
The tax effects of carryforwards that give rise to deferred tax assets consist of the following:
|
|
|
For the years ended December 31, |
|
|||||
|
|
|
2012 |
|
|
2013 |
|
||
|
Net operating loss carryforward |
|
$ |
41,100,511 |
|
|
$ |
43,666,636 |
|
|
Research and development credits |
|
|
4,898,055 |
|
|
|
5,114,652 |
|
|
Accruals and other |
|
|
688,089 |
|
|
|
742,045 |
|
|
Deferred rent |
|
|
203,463 |
|
|
|
176,893 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
46,890,118 |
|
|
|
49,700,226 |
|
|
Less valuation allowance |
|
|
(46,890,118 |
) |
|
|
(49,700,226 |
) |
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
The Company has not completed a study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since its formation, due to the complexity and cost associated with such a study, and the fact that there may be additional ownership changes in the future. Based on preliminary assessments, the Company believes that ownership changes occurred in 2010 and 2013. The Company estimates that if such a change did occur, the federal and state net operating loss carryforwards and research and development credits that can be utilized in the future will be significantly limited.
163
On September 13, 2013, the U.S. Treasury Department released final income tax regulations on the deduction and capitalization of expenditures related to tangible property. These final regulations apply to tax years beginning on or after January 1, 2014, and may be adopted in earlier years. The Company does not intend to early adopt the tax treatment of expenditures to improve tangible property and the capitalization of inherently facilitative costs to acquire tangible property as of January 1, 2013. The tangible property regulations will require the Company to make additional tax accounting method changes as of January 1, 2014; however, management does not anticipate the impact of these changes to be material to the Company’s consolidated financial position, its results of operations and its footnote disclosures.
14. Collaborative Agreements
On August 17, 2011, the Company entered into a three year exclusive collaboration agreement with Clarient Diagnostic Services, Inc. to collaborate to promote and maximize the commercialization of the Company’s or jointly developed diagnostic tests (together, the “Diagnostic Tests”) in the United States. Clarient is responsible for marketing, providing customer service, and for third party billing on all Diagnostic Tests performed under the agreement, and for performing the professional component of the Diagnostic Tests. The Company is responsible for promoting sales of the Diagnostic Tests in the United States, as well as performing all technical components of all Diagnostic Tests sold by either party.
Under this agreement, the Company invoices Clarient for the performance of each of the Diagnostic Tests at a contractually agreed-upon rate. Clarient is responsible for billing the patient, provider and/or payer for each completed test, and bears all collection risk related to such billings. Sales of Diagnostic Tests under this agreement did not commence until 2012. The total amount of revenue the Company earned under this agreement was approximately $86,000 and $14,000 for the years ended December 31, 2012 and 2013, respectively.
The agreement was replaced as of May 2013 to remove exclusivity provisions and to modify the performance obligations of the parties. As a result of the replacement agreement, the Company will be responsible for billing third party payors for tests performed under the Clarient agreement. Revenue derived from the Clarient arrangement after the replacement date is recognized as collected, provided all other revenue recognition criteria are met.
In January 2013, the Company entered into a research support agreement with Dana Farber, a not-for-profit tax-exempt organization. The Company is responsible for performing all technical components of the diagnostic tests as ordered by Dana Farber and recognizes revenue as collected, provided all other revenue recognition criteria are met. The total amount of revenue the Company earned under this agreement was approximately $104,000 for the year ended December 31, 2013.
15. Related Party Transactions
During 2005, the Company executed the Goodman Note in favor of an investor which became a beneficial owner of more than 5% of the Company’s common stock. As of December 31, 2012, the Company had $1,935,000 outstanding on this note. In June 2013, the investor converted the entire principal amount of $1,935,000 and accrued interest of approximately $105,000 due on the Goodman Note into 3,777,324 shares of Series A preferred stock.
During 2008, the Company executed the 2008 Convertible Note with an affiliate of a major shareholder who is a member of the board of directors in the amount of $1,400,000. A warrant to purchase preferred shares was issued along with the convertible promissory note (see Notes 6 and 7). In July 2013, the Company amended the 2008 Convertible Note with a principal balance of $1,400,000, held by a related party, to provide that all principal of and accrued interest on the note would automatically convert into common stock upon the closing of an IPO at the price per share at which common stock is sold in such IPO.
As of December 31, 2012 and 2013, the Company had $17,780,000 and $3,905,000, respectively, of notes payable outstanding to affiliates of a major shareholder who is a member of the board of directors under several note and warrant purchase agreements (see Notes 6 and 7). As of June 28, 2013, $17,060,000 of principal and $2,339,000 of interest due on a portion of these notes was converted into shares of 35,923,845 Series A preferred stock.
As of December 31, 2012 and 2013, the Company had approximately $1,000,000 and $1,479,000, respectively, of notes payable outstanding with other board members under several different note and warrant purchase agreements (see Notes 6 and 7). As of June 28, 2013, approximately $975,000 of principal and $101,000 of interest due on a portion of these notes were converted into 1,993,591 preferred shares.
In September and December 2013, the Company issued common stock warrants to three shareholders in conjunction with their guarantees on the Company’s borrowings under the Company’s line of credit (see Notes 5 and 7).
164
During 2011, the Company entered into two supplier financing arrangements with a business owned by a member of the board of directors totaling $256,000, of which $60,000 and $66,000 is outstanding as of December 31, 2012 and 2013, respectively (see Note 18).
A member of the Company’s management is the controlling person of Aegea Biotechnologies, Inc. (“Aegea”). On September 2, 2012, the Company entered into an Assignment and Exclusive Cross-License Agreement with Aegea Biotechnologies, Inc. The total amount of invoices received by the Company from Aegea during the year ended December 31, 2013 was approximately $2,000, which are unpaid and recorded in accounts payable at December 31, 2013.
The Company believes that these transactions were on terms at least as favorable to the Company as could have been obtained from unrelated third parties.
16. Commitments and Contingencies
Operating Leases
The Company leases office, laboratory, and warehouse space at its San Diego, California facility under a non-cancelable operating lease. The initial lease was for an eight-year term expiring in 2012. In November 2011, the Company extended the lease term through October 31, 2018 and expanded the original premises by 9,849 square feet. Under the amended lease, the landlord delivered the expanded premises in May 2013. The Company records rent expense on a straight-line basis over the life of the lease and records the excess of expense over the amounts paid as deferred rent.
For the years ended December 31, 2012 and 2013, rent expense was approximately $937,000 and $1,143,000, respectively. As of December 31, 2012 the Company owed rent in arrears of approximately $185,000. As of December 31, 2013, the Company owed no rent in arrears. This amount is included in accounts payable on the balance sheet.
In September 2013, the Company amended its non-cancellable operating lease for its office, laboratory, and warehouse space at its San Diego, California facility. The amendment extends the maturity date of the lease through July 31, 2020. As part of this amendment, the landlord waived the lease payments due from August 1, 2013 through December 31, 2013 of approximately $503,000, and the Company forfeited its long-term deposit of approximately $269,000. In conjunction with this amendment, the Company granted to the landlord a warrant to purchase common shares with a warrant coverage amount of $502,605 and an exercise price equal to the price per share of the Company’s common stock sold in the Company’s IPO (see Note 7).
The future minimum lease payments under the amended lease agreement as December 31, 2013 are as follows:
|
|
|
|
|
|
|
2014 |
|
$ |
1,233,846 |
|
|
2015 |
|
|
1,270,861 |
|
|
2016 |
|
|
1,308,987 |
|
|
2017 |
|
|
1,348,257 |
|
|
2018 |
|
|
1,388,705 |
|
|
Thereafter |
|
|
2,285,501 |
|
|
|
|
|
|
|
|
Total |
|
$ |
8,836,157 |
|
|
|
|
|
|
|
Employment Agreements
Under the terms of certain employment agreements with executive officers, the Company would incur additional cash compensation expense of $150,000 immediately, and $225,000 annually, upon the closing of an IPO or the Company’s receipt of aggregate proceeds of $15,000,000 or more from the sales of equity securities. All payments required under these agreements as a result of the closing of the Company’s IPO on February 10, 2014 have been subsequently made in February and March 2014.
Legal Proceedings
In the normal course of business, the Company may be involved in legal proceedings or threatened legal proceedings. The Company is not party to any legal proceedings or aware of any threatened legal proceedings which are expected to have a material adverse effect on its financial condition, results of operations or liquidity.
The Company’s former Vice President of Operations filed an administrative proceeding against the Company with the California Labor Commissioner in April 2013, seeking damages for alleged unpaid wages and penalties. A hearing was held on August 19, 2013
165
which resulted in a finding against the Company for approximately $65,000, of which $40,000 was paid during the year ended December 31, 2013 and $25,000 was accrued as of December 31, 2013 (see Note 18).
17. Selected Quarterly Financial Data (Unaudited)
The following is selected quarterly financial data as of and for the periods ending:
|
|
|
First Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
|
Fourth Quarter |
|
||||
|
December 31, 2012 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance sheet data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash & cash equivalents |
|
$ |
82,486 |
|
|
$ |
361,062 |
|
|
$ |
72,483 |
|
|
$ |
185,256 |
|
|
Total assets |
|
|
1,544,311 |
|
|
|
1,870,220 |
|
|
|
1,265,874 |
|
|
|
1,469,679 |
|
|
Total non-current liabilities |
|
|
2,034,960 |
|
|
|
1,918,018 |
|
|
|
1,816,319 |
|
|
|
1,255,771 |
|
|
Total shareholders’ deficit |
|
|
(18,973,870 |
) |
|
|
(21,646,012 |
) |
|
|
(24,477,238 |
) |
|
|
(27,384,895 |
) |
|
Statement of operations and comprehensive loss data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
10,373 |
|
|
$ |
54,020 |
|
|
$ |
23,949 |
|
|
$ |
20,947 |
|
|
Gross profit/(loss) |
|
|
(145,056 |
) |
|
|
(255,677 |
) |
|
|
(267,449 |
) |
|
|
(424,223 |
) |
|
Research and development expenses |
|
|
2,253,303 |
|
|
|
1,544,206 |
|
|
|
1,506,935 |
|
|
|
1,257,708 |
|
|
General and administrative expenses |
|
|
623,018 |
|
|
|
542,116 |
|
|
|
447,586 |
|
|
|
450,479 |
|
|
Sales and marketing expenses |
|
|
212,447 |
|
|
|
189,360 |
|
|
|
201,739 |
|
|
|
181,773 |
|
|
Loss from operations |
|
|
(3,233,824 |
) |
|
|
(2,531,359 |
) |
|
|
(2,423,709 |
) |
|
|
(2,314,183 |
) |
|
Net loss |
|
$ |
(3,717,239 |
) |
|
$ |
(2,725,279 |
) |
|
$ |
(2,869,883 |
) |
|
$ |
(2,947,125 |
) |
|
Net loss per common share:1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(23.18 |
) |
|
$ |
(16.99 |
) |
|
$ |
(17.89 |
) |
|
$ |
(18.37 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted |
|
$ |
(23.18 |
) |
|
$ |
(16.99 |
) |
|
$ |
(17.89 |
) |
|
$ |
(18.37 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares outstanding used in computing net loss per share attributable to common shareholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
160,393 |
|
|
|
160,393 |
|
|
|
160,393 |
|
|
|
160,393 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted |
|
|
160,393 |
|
|
|
160,393 |
|
|
|
160,393 |
|
|
|
160,393 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
Basic and diluted net loss per common share are computed independently for each of the components and quarters presented. Therefore, the sum of quarterly basic and diluted per share information may not equal annual basic and diluted net loss per common share. |
166
|
|
|
First Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
|
Fourth Quarter |
|
||||
|
December 31, 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance sheet data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash & cash equivalents |
|
$ |
17,964 |
|
|
$ |
4,483 |
|
|
$ |
302,908 |
|
|
$ |
69,178 |
|
|
Total assets |
|
|
1,095,023 |
|
|
|
991,576 |
|
|
|
1,083,089 |
|
|
|
1,329,719 |
|
|
Total non-current liabilities |
|
|
1,252,921 |
|
|
|
508,527 |
|
|
|
167,291 |
|
|
|
462,001 |
|
|
Total shareholders’ deficit |
|
|
(29,300,361 |
) |
|
|
(8,215,261 |
) |
|
|
(10,272,840 |
) |
|
|
(12,456,014 |
) |
|
Statement of operations and comprehensive loss data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
35,154 |
|
|
$ |
48,369 |
|
|
$ |
31,922 |
|
|
$ |
18,800 |
|
|
Gross profit/(loss) |
|
|
(512,097 |
) |
|
|
(544,868 |
) |
|
|
(587,158 |
) |
|
|
(551,532 |
) |
|
Research and development expenses |
|
|
710,206 |
|
|
|
690,582 |
|
|
|
975,104 |
|
|
|
710,845 |
|
|
General and administrative expenses |
|
|
451,157 |
|
|
|
478,163 |
|
|
|
806,872 |
|
|
|
776,944 |
|
|
Sales and marketing expenses |
|
|
96,404 |
|
|
|
27,932 |
|
|
|
5,342 |
|
|
|
19,225 |
|
|
Loss from operations |
|
|
(1,769,864 |
) |
|
|
(1,741,545 |
) |
|
|
(2,374,476 |
) |
|
|
(2,058,546 |
) |
|
Net loss |
|
$ |
(1,925,974 |
) |
|
$ |
(1,975,009 |
) |
|
$ |
(2,860,191 |
) |
|
$ |
(2,472,009 |
) |
|
Net loss per common share:1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(10.67 |
) |
|
$ |
(10.83 |
) |
|
$ |
(15.72 |
) |
|
$ |
(13.57 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted |
|
$ |
(10.67 |
) |
|
$ |
(10.83 |
) |
|
$ |
(15.72 |
) |
|
$ |
(13.57 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares outstanding used in computing net loss per share attributable to common shareholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
180,540 |
|
|
|
182,304 |
|
|
|
181,954 |
|
|
|
182,203 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted |
|
|
180,540 |
|
|
|
182,304 |
|
|
|
181,954 |
|
|
|
182,203 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
Basic and diluted net loss per common share are computed independently for each of the components and quarters presented. Therefore, the sum of quarterly basic and diluted per share information may not equal annual basic and diluted net loss per common share. |
18. Subsequent Events
The Company has evaluated all events or transactions that occurred after the balance sheet dates of December 31, 2013 and 2012, through March 28, 2014.
Subsequent to December 31, 2013, the Company has borrowed approximately $175,000 under the 2013 Convertible Bridge Notes.
Subsequent to December 31, 2013, the Company repaid in full the remaining amounts outstanding of approximately $70,000 due for laboratory equipment under financing agreements with a supplier, which is a business owned by a member of the Company’s board of directors.
Subsequent to December 31, 2013, the maximum amount of the Company’s line of credit discussed in Note 5 above was increased to approximately $2.6 million and common stock warrants were issued to four shareholders in conjunction with their guarantees on the Company’s additional borrowings under the line of credit. On February 10, 2014, the current outstanding balance under the line of credit of $2,346,000 plus accrued interest of $27,043 was paid in full.
Pursuant to an underwriting agreement dated February 4, 2014 between the Company and Aegis Capital Corp. (“Aegis”), as representative of the several underwriters named therein, an IPO of 1,900,000 shares of common stock at $10.00 per share was effected on February 5, 2014. The closing of the sale of these shares to the underwriters occurred on February 10, 2014. The Company received, after deducting underwriting discounts and additional costs incurred, approximately $16.7 million from the sale of these 1,900,000 shares. The underwriters had the option, through March 21, 2014, to purchase up to 285,000 shares of common stock at $9.30 per share to cover overallotments, which was not exercised. In addition, designees of Aegis were issued warrants to buy (in the aggregate) up to 95,000 shares of common stock at $12.50 per share.
167
On February 4, 2014, as contemplated by the registration statement covering the IPO, 69,421,047 shares of outstanding Series A Preferred Stock were converted into 1,652,851 shares of common stock and the Company’s certificate of incorporation was amended to provide for an authorized capitalization of 40,000,000 shares of common stock and 5,000,000 shares of preferred stock.
In connection with the closing of the Company’s IPO on February 10, 2014, (i) the $1,400,000 principal amount and $233,982 of accrued interest related to the 2008 Convertible Note were converted at $10.00 per share into a total of 163,399 shares of common stock, (ii) the $5,165,000 principal amount and $313,017 of accrued interest related to the 2013 Convertible Bridge Notes were converted at $10.00 per share into a total of 547,794 shares of common stock, (iii) the exercise price of the warrants associated with the 2013 Bridge Notes was fixed at $10.00 per share for an aggregate of 258,249 shares of common stock, (iv) the exercise price of the warrants associated with the $2,578,104 of collateral provided to secure the Company’s Line of Credit was fixed at $10.00 per share for an aggregate of 128,903 shares of common stock, (v) 73,151 shares of common stock vested as settlement of certain restricted stock and became issuable subsequent to the expiration of the 180 day lock-up period, (vi) the Company’s Executive Chairman ceased to be an employee and continues to serve as (non-executive) Chairman, (vii) the number of shares of common stock covered by the 2013 Equity Incentive Plan increased by 800,000, (viii) the preferred warrants previously outstanding were canceled due to early termination clauses associated with the IPO, and (ix) the exercise prices of common warrants previously outstanding were fixed, whereby the carrying amount of the associated liability was adjusted to fair value and then reclassified to shareholders’ deficit.
On February 13, 2014, the Compensation Committee of the Company’s Board of Directors approved the payment of $1,009,552 in deferred salary obligations, including contractual interest, to current and former named executive officers pursuant to previously existing agreements. An additional $172,089 in deferred salary obligations and interest thereon was paid to former employees other than named executive officers. Also on February 13, 2014, the Company’s Board of Directors approved annual cash retainers to non-employee directors, and granted 238,500 stock options under the 2013 Equity Incentive Plan to non-employee directors. Subsequently in February and March 2014, the Company’s Board of Directors approved grants of 97,298 stock options to employees and a non-employee director of the company.
On February 25, 2014, the aforementioned administrative proceeding filed with the California Labor Commissioner by the Company’s former Vice President of Operations was settled in full following payment of the remaining $25,000 due (see Note 16).
168
Biocept, Inc.
|
|
December 31, |
|
|
September 30, |
|
||
|
|
2013 |
|
|
2014 |
|
||
|
|
|
|
|
|
(unaudited) |
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
$ |
69,178 |
|
|
$ |
8,819,872 |
|
|
Accounts receivable |
|
9,200 |
|
|
|
12,445 |
|
|
Inventories, net |
|
92,823 |
|
|
|
148,640 |
|
|
Prepaid expenses and other current assets |
|
799,131 |
|
|
|
340,469 |
|
|
Total current assets |
|
970,332 |
|
|
|
9,321,426 |
|
|
Fixed assets, net |
|
358,887 |
|
|
|
528,248 |
|
|
Other non-current assets, net |
|
500 |
|
|
|
25,365 |
|
|
Total assets |
$ |
1,329,719 |
|
|
$ |
9,875,039 |
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
$ |
1,540,618 |
|
|
$ |
552,994 |
|
|
Accrued liabilities |
|
2,242,058 |
|
|
|
580,602 |
|
|
Line of credit |
|
1,981,000 |
|
|
|
— |
|
|
Notes payable, net |
|
5,200,599 |
|
|
|
— |
|
|
Warrant liability |
|
2,140,532 |
|
|
|
1,128 |
|
|
Supplier financings |
|
218,925 |
|
|
|
— |
|
|
Current portion of equipment financing |
— |
|
|
|
55,800 |
|
|
|
Total current liabilities |
|
13,323,732 |
|
|
|
1,190,524 |
|
|
Non-current portion of equipment financing, net |
— |
|
|
|
78,933 |
|
|
|
Credit facility, net |
— |
|
|
|
4,731,541 |
|
|
|
Non-current interest payable |
— |
|
|
|
33,905 |
|
|
|
Deferred rent |
|
462,001 |
|
|
|
495,239 |
|
|
Total liabilities |
|
13,785,733 |
|
|
|
6,530,142 |
|
|
Commitments and contingencies (see Note 9) |
|
|
|
|
|
|
|
|
Shareholders’ equity/(deficit): |
|
|
|
|
|
|
|
|
Series A convertible preferred stock, $0.0001 par value, 100,000,000 authorized; 69,421,047 issued and outstanding at December 31, 2013; 5,000,000 shares authorized; no shares issued and outstanding at September 30, 2014; liquidation preference of $41,652,628 at December 31, 2013 (see Note 2). |
|
6,942 |
|
|
— |
|
|
|
Common stock, $0.0001 par value, 53,000,000 authorized; 185,550 issued and outstanding at December 31, 2013; 40,000,000 authorized; 4,449,603 issued and outstanding at September 30, 2014 (see Note 2). |
|
19 |
|
|
|
445 |
|
|
Additional paid-in capital |
|
109,958,001 |
|
|
|
137,749,933 |
|
|
Accumulated deficit |
|
(122,420,976 |
) |
|
|
(134,405,481 |
) |
|
Total shareholders’ equity/(deficit) |
|
(12,456,014 |
) |
|
|
3,344,897 |
|
|
Total liabilities and shareholders’ equity/(deficit) |
$ |
1,329,719 |
|
|
$ |
9,875,039 |
|
The accompanying notes are an integral part of these unaudited condensed financial statements
169
Biocept, Inc.
Condensed Statements of Operations and Comprehensive Loss
(Unaudited)
|
|
For the three months ended September 30, |
|
|
For the nine months ended September 30, |
|
||||||||||
|
|
2013 |
|
|
2014 |
|
|
2013 |
|
|
2014 |
|
||||
|
Revenues |
$ |
31,922 |
|
|
$ |
10,274 |
|
|
$ |
115,445 |
|
|
$ |
57,794 |
|
|
Cost of revenues |
|
619,080 |
|
|
|
538,181 |
|
|
|
1,759,568 |
|
|
|
1,555,861 |
|
|
Gross loss |
|
(587,158 |
) |
|
|
(527,907 |
) |
|
|
(1,644,123 |
) |
|
|
(1,498,067 |
) |
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses |
|
975,104 |
|
|
|
1,310,905 |
|
|
|
2,375,892 |
|
|
|
3,427,513 |
|
|
General and administrative expenses |
|
806,872 |
|
|
|
1,060,812 |
|
|
|
1,736,192 |
|
|
|
3,970,579 |
|
|
Sales and marketing expenses |
|
5,342 |
|
|
|
812,005 |
|
|
|
129,678 |
|
|
|
1,246,507 |
|
|
Loss from operations |
|
(2,374,476 |
) |
|
|
(3,711,629 |
) |
|
|
(5,885,885 |
) |
|
|
(10,142,666 |
) |
|
Other income/(expense) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
(457,250 |
) |
|
|
(151,491 |
) |
|
|
(1,435,087 |
) |
|
|
(1,640,045 |
) |
|
Change in fair value of warrant liability |
|
(7,647 |
) |
|
|
3,326 |
|
|
|
593,365 |
|
|
|
(200,994 |
) |
|
Other income/(expense) |
|
(20,818 |
) |
|
— |
|
|
|
(32,767 |
) |
|
— |
|
||
|
Total other income/(expense) |
|
(485,715 |
) |
|
|
(148,165 |
) |
|
|
(874,489 |
) |
|
|
(1,841,039 |
) |
|
Loss before income taxes |
|
(2,860,191 |
) |
|
|
(3,859,794 |
) |
|
|
(6,760,374 |
) |
|
|
(11,983,705 |
) |
|
Income tax expense |
|
— |
|
|
|
— |
|
|
|
(800 |
) |
|
|
(800 |
) |
|
Net loss & comprehensive loss |
$ |
(2,860,191 |
) |
|
$ |
(3,859,794 |
) |
|
$ |
(6,761,174 |
) |
|
$ |
(11,984,505 |
) |
|
Weighted-average shares outstanding used in computing net loss per share attributable to common shareholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
181,954 |
|
|
|
4,449,603 |
|
|
|
182,199 |
|
|
|
3,845,540 |
|
|
Diluted |
|
181,954 |
|
|
|
4,449,603 |
|
|
|
182,199 |
|
|
|
3,845,540 |
|
|
Net loss per common share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
$ |
(15.72 |
) |
|
$ |
(0.87 |
) |
|
$ |
(37.11 |
) |
|
$ |
(3.12 |
) |
|
Diluted |
$ |
(15.72 |
) |
|
$ |
(0.87 |
) |
|
$ |
(37.11 |
) |
|
$ |
(3.12 |
) |
The accompanying notes are an integral part of these unaudited condensed financial statements
170
Biocept, Inc.
Condensed Statements of Cash Flows
(Unaudited)
|
|
For the nine months ended September 30, |
|
|||||
|
|
2013 |
|
|
2014 |
|
||
|
Cash Flows From Operating Activities |
|
|
|
|
|
|
|
|
Net loss |
$ |
(6,761,174 |
) |
|
$ |
(11,984,505 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
202,641 |
|
|
|
177,516 |
|
|
Inventory reserve |
|
68,496 |
|
|
|
(9,616 |
) |
|
Stock-based compensation |
|
683,396 |
|
|
|
1,506,586 |
|
|
Non-cash interest expense related to convertible debt, credit facility and other financing activities |
|
1,302,136 |
|
|
|
1,428,324 |
|
|
Change in fair value of warrant liability |
|
(593,365 |
) |
|
|
200,994 |
|
|
Increase/(decrease) in cash resulting from changes in: |
|
|
|
|
|
|
|
|
Accounts receivable |
|
(41,599 |
) |
|
|
(3,245 |
) |
|
Inventory |
|
(97,342 |
) |
|
|
(46,201 |
) |
|
Prepaid expenses and other current assets |
|
(25,255 |
) |
|
|
(528,988 |
) |
|
Other non-current assets |
|
269,083 |
|
|
|
(28,894 |
) |
|
Accounts payable |
|
(120,781 |
) |
|
|
(992,399 |
) |
|
Accrued liabilities |
|
271,470 |
|
|
|
(1,172,611 |
) |
|
Non-current interest payable |
— |
|
|
|
33,905 |
|
|
|
Deferred rent |
|
(34,749 |
) |
|
|
33,238 |
|
|
Net cash used in operating activities |
|
(4,877,043 |
) |
|
|
(11,385,896 |
) |
|
Cash Flows From Investing Activities |
|
|
|
|
|
|
|
|
Purchases of fixed assets |
|
(711 |
) |
|
|
(201,835 |
) |
|
Net cash used in investing activities |
|
(711 |
) |
|
|
(201,835 |
) |
|
Cash Flows From Financing Activities |
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options |
|
395 |
|
|
|
— |
|
|
Payments for repurchase of shares |
|
(4,111 |
) |
|
|
— |
|
|
Principal payments on equipment financing |
— |
|
|
|
(9,300 |
) |
|
|
Net proceeds from issuance of common stock |
— |
|
|
|
17,390,240 |
|
|
|
Payments on supplier and other third party financings |
|
(61,874 |
) |
|
|
(163,411 |
) |
|
Payments on line of credit |
— |
|
|
|
(2,346,000 |
) |
|
|
Proceeds from borrowings on line of credit |
|
1,490,996 |
|
|
|
365,000 |
|
|
Proceeds from issuance of convertible notes and warrants |
|
3,570,000 |
|
|
|
175,000 |
|
|
Net proceeds from borrowings on credit facility and warrants |
— |
|
|
|
4,926,896 |
|
|
|
Net cash provided by financing activities |
|
4,995,406 |
|
|
|
20,338,425 |
|
|
Net increase/(decrease) in Cash and Cash Equivalents |
|
117,652 |
|
|
|
8,750,694 |
|
|
Cash and Cash Equivalents at Beginning of Period |
|
185,256 |
|
|
|
69,178 |
|
|
Cash and Cash Equivalents at End of Period |
$ |
302,908 |
|
|
$ |
8,819,872 |
|
|
Supplemental Disclosures of Cash Flow Information: |
|
|
|
|
|
|
|
|
Cash paid during the period for: |
|
|
|
|
|
|
|
|
Interest |
$ |
- |
|
|
$ |
298,381 |
|
|
Taxes |
$ |
800 |
|
|
$ |
800 |
|
171
Non-cash Investing and Financing Activities:
During the nine months ended September 30, 2013, 21,846 shares of common stock, with a par value of $0.0001, were issued for restricted stock units.
During the nine months ended September 30, 2013, convertible notes with a principal balance of $20,231,000 and accrued interest of approximately $2,581,000 were converted into 42,245,834 shares of preferred stock with a par value of $0.0001. In conjunction with this conversion, $236,799 of derivative warrant liabilities were reclassified to additional paid-in capital, as the underlying exercise prices on the warrants were determined by the debt conversion. Also during the nine months ended September 30, 2013, an additional $144,346 of derivative warrant liabilities were reclassified to additional paid-in capital when their underlying exercise price was fixed.
During the nine months ended September 30, 2013, the Company issued to its landlord a warrant to purchase common shares with a warrant coverage amount of $502,605 and an exercise price equal to the price per share of the Company’s common stock sold in the Company’s initial public offering (“IPO”) (see Note 2). The fair value of the warrant as calculated under the Company’s probability weighted Black-Scholes valuation model was approximately $309,000 at September 30, 2013, which was recorded on the condensed balance sheet as a component of deferred rent and warrant liability.
During the nine months ended September 30, 2014, the Company cancelled its private company directors and officers liability insurance policy. The previously financed premium balance of $44,559 was cancelled and a partial refund of $10,955 was received.
During the nine months ended September 30, 2014, common stock warrants with an estimated aggregate grant date fair value of $135,222 were issued in conjunction with guarantees on the Company’s additional borrowings under its Line of Credit and additional borrowings made under its 2013 Convertible Bridge Notes, and were recorded as a discount to outstanding debt at the date of issuance.
An IPO of the Company’s common stock was effected on February 5, 2014, the closing of which occurred on February 10, 2014 (see Note 2). On February 4, 2014, as contemplated by the registration statement covering the IPO, 69,421,047 shares of outstanding Series A Preferred Stock were automatically converted into 1,652,851 shares of common stock. In connection with the closing of the IPO on February 10, 2014, (i) the underwriters of the IPO were granted a 45 day option from the closing date of the IPO to purchase up to 285,000 shares of common stock at $9.30 per share to cover overallotments with a grant date fair value of $202,143 (see Note 4), which was not exercised and is recorded as an offset to additional paid-in capital within common stock issuance costs at September 30, 2014, (ii) certain designees of the representative of the underwriters were issued warrants to buy (in the aggregate) up to 95,000 shares of common stock at $12.50 per share with a term of five years and a grant date fair value of $544,116 (see Note 4), and is recorded as an offset to additional paid-in capital within common stock issuance costs at September 30, 2014, (iii) underwriter IPO costs and discounts of $279,760 and $1,330,000, respectively, were netted against the proceeds from the IPO and are reflected as an offset to additional paid-in capital, (iv) the $1,400,000 principal amount and $233,982 of accrued interest related to the 2008 Convertible Note were converted at $10.00 per share into a total of 163,399 shares of common stock, (v) the $5,165,000 principal amount and $313,017 of accrued interest related to the 2013 Convertible Bridge Notes were converted at $10.00 per share into a total of 547,794 shares of common stock, (vi) derivative warrant liabilities of $2,475,620 associated with an aggregate of 387,152 common stock warrants related to the 2013 Convertible Bridge Notes and Line of Credit were reclassified to additional paid-in capital when their underlying exercise price was fixed at $10.00 per share, and (vii) additional costs associated with the IPO of $932,136 were reclassified from prepaid expenses and other current assets to additional paid-in capital.
During the nine months ended September 30, 2014, a common stock warrant with an estimated grant date fair value of $233,107 was issued in conjunction with borrowings made under the Company’s 2014 Credit Facility, and was recorded as a discount to outstanding debt at the date of issuance (see Note 6).
Fixed assets purchased totaling $4,775 during the nine months ended September 30, 2014 remain unpaid as of September 30, 2014, and are excluded from cash purchases in the Company’s unaudited condensed statement of cash flows.
A fixed asset purchased for $140,267 during the nine months ended September 30, 2014 is recorded as an equipment financing obligation and is excluded from cash purchases in the Company’s unaudited condensed statement of cash flows.
The accompanying notes are an integral part of these unaudited condensed financial statements
172
BIOCEPT, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
(Unaudited)
1. Basis of Presentation
Basis of Presentation
The financial statements and accompanying notes are prepared in accordance with accounting principles generally accepted in the United States of America.
The unaudited condensed financial statements included in this prospectus have been prepared in accordance with the U.S. Securities and Exchange Commission (“SEC”) instructions for Quarterly Reports on Form 10-Q. Accordingly, the condensed financial statements are unaudited and do not contain all the information required by U.S. Generally Accepted Accounting Principles (“GAAP”) to be included in a full set of financial statements. The unaudited condensed balance sheet at December 31, 2013 has been derived from the audited financial statements at that date but does not include all of the information and footnotes required by GAAP for a complete set of financial statements. The audited financial statements for the year ended December 31, 2013, filed with the SEC with our Annual Report on Form 10-K on March 28, 2014, and included earlier in this prospectus, include a summary of our significant accounting policies and should be read in conjunction with these unaudited condensed financial statements. In the opinion of management, all material adjustments necessary to present fairly the results of operations for such periods have been included in this prospectus. All such adjustments are of a normal recurring nature. The results of operations for interim periods are not necessarily indicative of the results of operations for the entire year.
The Company and Business Activities
Biocept, Inc. (“the Company”) was founded in California in May 1997 and is a commercial-stage cancer diagnostics company developing and commercializing proprietary circulating tumor cell (CTC) and circulating tumor DNA (ctDNA) tests utilizing a standard blood sample to improve the treatment that oncologists provide to their patients by providing better, more detailed information on the characteristics of their tumor.
The Company operates a clinical laboratory that is CLIA-certified (under the Clinical Laboratory Improvement Amendment of 1988) and CAP-accredited (by the College of American Pathologists), and manufactures CEE microfluidic channels, related equipment and certain reagents to perform the Company’s diagnostic tests in a facility located in San Diego, California. CLIA certification and accreditation are required before any clinical laboratory may perform testing on human specimens for the purpose of obtaining information for the diagnosis, prevention, treatment of disease, or assessment of health. The tests the Company offers are classified as laboratory developed tests (LDTs), under the CLIA regulations.
In July 2013, the Company effected a reincorporation to Delaware by merging itself with and into Biocept, Inc., a Delaware corporation, which had been formed to be and was a wholly-owned subsidiary of the Company since July 23, 2013.
Recent Accounting Pronouncements
In July 2013, the Financial Accounting Standards Board (“FASB”) issued authoritative guidance that requires netting unrecognized tax benefits against deferred tax assets for a loss or other carryforward that would apply in settlement of uncertain tax positions. This guidance is effective for annual reporting periods beginning after December 15, 2013, and was effective for the Company’s fiscal year beginning January 1, 2014. The adoption of this guidance did not have a material impact on the Company’s financial statements or disclosures.
In May 2014, the FASB issued authoritative guidance that requires entities to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services. This guidance is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. Early adoption is not permitted. The Company is currently in the process of evaluating the impact of the adoption of this guidance on its financial statements and disclosures.
In June 2014, the FASB issued authoritative guidance requiring share-based payments with a performance target which affects vesting and that could be achieved after the requisite service period be treated as a performance condition. This guidance is effective for fiscal years, and interim periods within those years, beginning after December 15, 2015. The Company does not expect adoption of this guidance to have a material impact on its financial statements or disclosures.
173
In August 2014, the FASB issued authoritative guidance requiring management to evaluate whether there are conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. Certain additional financial statement disclosures are required if such conditions or events are identified. This guidance is effective for the annual reporting period ending after December 15, 2016, and for annual periods and interim periods thereafter. Early adoption is permitted. The Company is currently in the process of evaluating the impact of the adoption of this guidance on its financial statements and disclosures.
2. Initial Public Offering
Pursuant to an underwriting agreement dated February 4, 2014 between the Company and Aegis Capital Corp. (“Aegis”), as representative of the several underwriters named therein, an IPO of 1,900,000 shares of common stock at $10.00 per share was effected on February 5, 2014. The closing of the sale of these shares to the underwriters occurred on February 10, 2014. The Company received, after deducting underwriting discounts and additional costs paid to the underwriters, approximately $17,390,000 of net cash proceeds from the sale of these 1,900,000 shares. The total increase in capital as a result of the sale of these shares was approximately $16,458,000 after deducting $932,136 of additional non-underwriter costs incurred that are netted against these proceeds under applicable accounting guidance. Additionally, the underwriters were granted a 45 day option from the closing date of the IPO to purchase up to 285,000 shares of common stock at $9.30 per share to cover overallotments with a grant date fair value of $202,143 (see Note 4), which was not exercised. In addition, designees of Aegis were issued warrants to buy (in the aggregate) up to 95,000 shares of common stock at $12.50 per share with a term of five years and a grant date fair value of $544,116 (see Note 4).
On February 4, 2014, as contemplated by the registration statement covering the IPO, 69,421,047 shares of outstanding Series A Preferred Stock were converted into 1,652,851 shares of common stock and the Company’s certificate of incorporation was amended to provide for an authorized capitalization of 40,000,000 shares of common stock and 5,000,000 shares of preferred stock.
In connection with the closing of the Company’s IPO on February 10, 2014, (i) the $1,400,000 principal amount and $233,982 of accrued interest related to the 2008 Convertible Note were converted at $10.00 per share into a total of 163,399 shares of common stock, (ii) the $5,165,000 principal amount and $313,017 of accrued interest related to the 2013 Convertible Bridge Notes were converted at $10.00 per share into a total of 547,794 shares of common stock, (iii) the exercise price of the warrants associated with the 2013 Bridge Notes was fixed at $10.00 per share for an aggregate 258,249 shares of common stock, (iv) the exercise price of the warrants associated with the $2,578,104 of collateral provided to secure the Company’s Line of Credit was fixed at $10.00 per share for an aggregate 128,903 shares of common stock, (v) 73,151 shares of common stock vested as settlement of certain restricted stock units and became issuable subsequent to the expiration of the 180 day lock-up period, (vi) the Company’s Executive Chairman ceased to be an employee and continues to serve as non-executive Chairman, (vii) the number of shares of common stock covered by the 2013 Equity Incentive Plan increased by 800,000, (viii) all but 1,587 of the preferred warrants previously outstanding were canceled due to early termination clauses associated with the IPO, (ix) derivative warrant liabilities of $2,475,620 associated with the aggregate of 387,152 common stock warrants related to the Company’s 2013 Convertible Bridge Notes and Line of Credit were reclassified to additional paid-in capital when their underlying exercise price was fixed, (x) unamortized discounts of $996,024 related to the warrants associated with the 2013 Convertible Bridge Notes and Line of Credit were reclassified to interest expense, and (xi) offering costs associated with the IPO of $932,136 were reclassified from prepaid expenses and other current assets to additional paid-in capital, while additional underwriter IPO costs and discounts of $279,760 and $1,330,000, respectively, were netted against the proceeds from the IPO and are reflected as an offset to additional paid-in capital.
Subsequent to December 31, 2013, the maximum amount of the Company’s Line of Credit was increased to approximately $2.6 million and common stock warrants were issued to four shareholders in conjunction with their guarantees on the Company’s additional borrowings under the line of credit. On February 10, 2014, the current outstanding balance under the line of credit of $2,346,000 plus accrued interest of $27,043 was paid in full using the net proceeds from the IPO.
On February 13, 2014, the Compensation Committee of the Company’s Board of Directors approved the payment of an aggregate $1,009,552 in deferred salary obligations, including contractual interest, to current and former named executive officers pursuant to previously existing agreements, which was fully disbursed by April 2014 using the net proceeds from the IPO. An additional $344,883 in deferred salary obligations and interest thereon was paid to former employees other than named executive officers. Also on February 13, 2014, in connection with the closing of the IPO and pursuant to a Board resolution for a director compensation policy adopted in 2013, the Company’s Board of Directors approved annual cash retainers to non-employee directors, and granted 238,500 stock options under the 2013 Equity Incentive Plan to non-employee directors. These option awards vest in equal annual installments over 3 years from the date of grant with a 10 year term, subject to continuing service requirements (see Note 7). Subsequently in February 2014, the Company’s Board of Directors approved grants of 54,298 stock options as a result of the closing of the IPO pursuant to the terms of underlying employment agreements. Included in the stock options granted pursuant to the terms of underlying employment agreements are 53,108 option awards granted to the Company’s non-executive Chairman, which vested fully on the date of grant (see Note 7).
174
Under the terms of certain employment agreements with executive officers, the Company incurred additional cash compensation expense of $150,000 immediately, and $225,000 annually, upon the closing of its IPO. All payments required under these agreements as a result of the closing of the Company’s IPO on February 10, 2014 have been subsequently made in February and March 2014, using the net proceeds from the IPO.
During the nine months ended September 30, 2014, the Company repaid in full the remaining amounts outstanding of approximately $70,000 due for laboratory equipment under financing agreements with a supplier, which is a business owned by a member of the Company’s board of directors, using the net proceeds from the IPO.
3. Liquidity & Going Concern Uncertainty
These unaudited condensed financial statements have been prepared and presented on a basis assuming the Company will continue as a going concern. The factors below raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might be necessary from the outcome of this uncertainty.
At December 31, 2013 and September 30, 2014, the Company had accumulated deficits of approximately $122,421,000 and $134,405,000, respectively. For the three and nine months ended September 30, 2014, the Company incurred net losses of approximately $3,860,000 and $11,985,000, respectively. While the Company is currently in the commercialization stage of operations, the Company has not yet achieved profitability and anticipates that it will continue to incur net losses in the foreseeable future.
Historically, the Company’s principal sources of cash have included proceeds from the issuance of common and preferred stock, proceeds from the issuance of debt, and revenues from clinical laboratory testing through contracted partners. The Company’s principal uses of cash have included cash used in operations, payments relating to purchases of property and equipment and repayments of borrowings. The Company expects that the principal uses of cash in the future will be for continuing operations, hiring of sales and marketing personnel and increased sales and marketing activities, funding of research and development, capital expenditures, and general working capital requirements. The Company expects that, as revenues grow, sales and marketing and research and development expenses will continue to grow, albeit at a slower rate and, as a result, the Company will need to generate significant net revenues to achieve and sustain income from operations.
As of September 30, 2014, cash and cash equivalents totaled approximately $8,820,000. On February 10, 2014, the Company received cash proceeds of approximately $17,390,000 as a result of the closing of its IPO, net of underwriting discounts and additional underwriting costs incurred (see Note 2). On April 30, 2014, the Company received net cash proceeds of approximately $4,927,000 pursuant to the execution of a term loan agreement with Oxford Finance LLC (see Note 6). Management expects that the Company will need additional financing in the future to execute on its current or future business strategies beyond the next six months. Until the Company can generate significant cash from operations, the Company expects to continue to fund its operations with the proceeds of offerings of the Company’s equity and debt securities. Management can provide no assurances that any sources of a sufficient amount of financing will be available to the Company on favorable terms, if at all. In addition to test revenues, such financing may be derived from one or more of the following types of transactions: debt, equity, product development, technology licensing or collaboration.
4. Fair Value Measurement
The Company uses a three-tier fair value hierarchy to prioritize the inputs used in the Company’s fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions. The Company believes the carrying amount of cash and cash equivalents, accounts receivable, accounts payable and accrued expenses approximate their estimated fair values due to the short-term maturities of these financial instruments.
Warrant Liability Derivatives
The Company classified the fair value measurements of the Company’s warrant liability derivatives as Level 3 in all periods presented. The Company adjusted the carrying value of the warrants classified as liabilities until the completion of its IPO on February 10, 2014, at which time the exercise price was fixed at $10.00 per share and the fair value of the warrants was reclassified to shareholders’ deficit, except for a warrant for 1,587 preferred shares that remains outstanding at September 30, 2014 (see Note 2).
175
The aggregate fair value of the Company’s warrant liability at the closing of the IPO on February 10, 2014 was estimated using a Black-Scholes valuation model with the following assumptions for the five-year term and two-year term common stock warrants, respectively:
|
|
Five-year term |
|
|
Two-year term |
|
||
|
Stock price |
$ |
8.91 |
|
|
$ |
8.91 |
|
|
Exercise price |
$ |
10.00 |
|
|
$ |
10.00 |
|
|
Expected dividend yield |
|
0.00 |
% |
|
|
0.00 |
% |
|
Discount rate-bond equivalent yield |
|
1.48 |
% |
|
|
0.32 |
% |
|
Expected life (in years) |
|
5.00 |
|
|
|
2.00 |
|
|
Expected volatility |
|
90.0 |
% |
|
|
90.0 |
% |
The fair value attributed to such warrants as of December 31, 2013 and September 30, 2014 is as follows:
|
|
Fair Value Measurements Using |
|
|||||||||
|
|
Quoted Prices |
|
|
Significant |
|
|
|
|
|
||
|
|
in Active |
|
|
Other |
|
|
Significant |
|
|||
|
|
Markets for |
|
|
Observable |
|
|
Unobservable |
|
|||
|
|
Identical Assets |
|
|
Inputs |
|
|
Inputs |
|
|||
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
|||
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
Warrant Liability at December 31, 2013 |
|
— |
|
|
|
— |
|
|
|
2,140,532 |
|
|
Warrant Liability at September 30, 2014 |
|
— |
|
|
|
— |
|
|
|
1,128 |
|
The following table includes a summary of changes in the fair value of the warrants for the nine months ended September 30, 2014:
|
|
Fair Value Measurements |
|
|
|
|
at Reporting Date Using |
|
|
|
|
Significant Unobservable |
|
|
|
|
Inputs (Level 3) |
|
|
|
Balance at December 31, 2013 |
$ |
2,140,532 |
|
|
Warrant liability incurred |
|
135,222 |
|
|
Change in fair value included in expense |
|
200,994 |
|
|
Warrant liability reclassified to additional paid-in capital |
|
(2,475,620 |
) |
|
Balance at September 30, 2014 |
$ |
1,128 |
|
Other Fair Value Measurements
In connection with the closing of the Company’s IPO on February 10, 2014, the IPO’s underwriters were granted a 45 day option to purchase up to 285,000 shares of common stock to cover overallotments with a grant date fair value of $202,143, which was not exercised. Additionally, certain designees of the representative of the underwriters were issued warrants to buy (in the aggregate) up to 95,000 shares of common stock with a grant date fair value of $544,116. The fair values of these stock option and common stock warrants were estimated using probability weighted Black-Scholes valuation models with the following assumptions:
|
|
Options |
|
|
Warrants |
|
||
|
Stock price |
$ |
8.91 |
|
|
$ |
8.91 |
|
|
Exercise price |
$ |
9.30 |
|
|
$ |
12.50 |
|
|
Expected dividend yield |
|
0.00 |
% |
|
|
0.00 |
% |
|
Discount rate-bond equivalent yield |
|
0.07 |
% |
|
|
1.46 |
% |
|
Expected life (in years) |
0.12 |
|
|
|
5.00 |
|
|
|
Expected volatility |
|
70.0 |
% |
|
|
90.0 |
% |
The estimated grant date fair values of these non-cash equity classified instruments were recorded as an offset to additional paid-in capital within common stock issuance costs.
176
In connection with the closing of the Company’s Credit Facility on April 30, 2014, the lender was granted a warrant to purchase 52,966 shares of common stock with a 10 year term and an estimated grant date fair value of $233,107 (see Note 6). The fair value of this warrant was estimated using a Black-Scholes valuation model with the following assumptions:
|
Stock price |
$ |
4.74 |
|
|
Exercise price |
$ |
4.72 |
|
|
Expected dividend yield |
|
0.00 |
% |
|
Discount rate-bond equivalent yield |
|
2.67 |
% |
|
Expected life (in years) |
|
10.00 |
|
|
Expected volatility |
|
110.0 |
% |
The estimated grant date fair value of this non-cash equity classified instrument was recorded as a discount to outstanding debt and is amortized to interest expense utilizing the effective interest method over the underlying term of the loan.
The estimated fair value of the Company’s Credit Facility at September 30, 2014 approximated carrying value, which was determined using a discounted cash flow analysis. The analysis considered interest rates of instruments with similar maturity dates, which involved the use of significant unobservable Level 3 inputs (see Note 6).
5. Balance Sheet Details
The following provides certain balance sheet details:
|
|
December 31, |
|
|
September 30, |
|
||
|
|
2013 |
|
|
2014 |
|
||
|
Fixed Assets |
|
|
|||||
|
Machinery and equipment |
$ |
2,761,560 |
|
|
$ |
2,773,875 |
|
|
Furniture and office equipment |
|
209,844 |
|
|
|
209,844 |
|
|
Computer equipment and software |
|
681,508 |
|
|
|
681,508 |
|
|
Leasehold improvements |
|
373,653 |
|
|
|
506,328 |
|
|
Financed equipment |
|
677,000 |
|
|
|
878,447 |
|
|
Construction in process |
|
12,299 |
|
|
|
12,739 |
|
|
|
|
4,715,864 |
|
|
|
5,062,741 |
|
|
Less accumulated depreciation and amortization |
|
4,356,977 |
|
|
|
4,534,493 |
|
|
Total fixed assets, net |
$ |
358,887 |
|
|
$ |
528,248 |
|
|
Accrued Liabilities |
|
|
|
|
|
|
|
|
Accrued interest |
$ |
524,885 |
|
|
$ |
33,125 |
|
|
Accrued payroll |
|
125,299 |
|
|
|
148,664 |
|
|
Deferred wages |
|
1,377,987 |
|
|
|
— |
|
|
Accrued vacation |
|
213,601 |
|
|
|
251,108 |
|
|
Accrued bonuses |
|
— |
|
|
|
122,100 |
|
|
Other |
|
286 |
|
|
|
25,605 |
|
|
Total accrued liabilities |
$ |
2,242,058 |
|
|
$ |
580,602 |
|
As of December 31, 2013, the Company incurred $538,318 in costs directly associated with its IPO, which are reflected on the unaudited condensed balance sheet as a component of prepaid expenses and other current assets. As of September 30, 2014, a balance of $1,211,896 of such costs, in addition to underwriting discounts of $1,330,000 and an aggregate $746,259 of associated stock option and restricted stock awards, are offset against additional paid-in capital as a result of the closing of the Company’s IPO on February 10, 2014 (see Note 2).
6. Credit Facility
Effective as of April 30, 2014, the Company entered into a loan and security agreement (the “Credit Facility”) in an aggregate principal amount of up to $10.0 million with Oxford Finance LLC (“Oxford”) for working capital and general business purposes. The first term loan under the Credit Facility was funded on April 30, 2014 in a principal amount of $5.0 million. A second term loan of up to a principal amount of $5.0 million will be funded at the Company’s request prior to December 31, 2015, subject to the achievement of product and services revenues of at least $9.0 million for the trailing six month period by November 30, 2015. In connection with the first term loan under the Credit Facility, a facility fee of $50,000 was charged and an additional $50,000 facility fee will be due
177
upon execution of the second term loan under the Credit Facility. The Credit Facility is secured by substantially all of the Company’s assets other than its intellectual property. Each term loan under the credit facility bears interest at an annual rate equal to the greater of (i) 7.95% or (ii) the sum of (a) the three-month U.S. LIBOR rate reported in the Wall Street Journal three business days prior to the funding date of the applicable term loan, plus (b) 7.71%. The Company is required to make interest-only payments on the first term loan through February 1, 2016 if the funding date of the second term loan occurs before June 30, 2015, or through August 1, 2015 otherwise. If executed, interest-only payments are required to be made on the second term loan through February 1, 2016 if the funding date of the second term loan occurs before June 30, 2015, or through the seventh month following the funding date of the second term loan otherwise. The first term loan under the credit facility matures on July 1, 2018, and the second term loan matures on the first day of the 29th month following the end of the applicable interest-only period. Upon repayment of each term loan, the Company is also required to make a final payment equal to 5.50% of the original principal amount(s) funded. At the Company’s option, the outstanding principal balance of the term loans may be repaid in whole but not in part, subject to a prepayment fee of 3% of any amount prepaid if the prepayment occurs on or prior to April 30, 2015, 2% of the amount prepaid if the prepayment occurs after April 30, 2015 but on or prior to April 30, 2016, and 1% of any amount prepaid after April 30, 2016. Additionally, a warrant to purchase up to 52,966 shares of the Company’s common stock at an exercise price of $4.72 per share with a term of 10 years was issued to Oxford on April 30, 2014 (see Note 4). Additional warrants for shares of the Company’s common stock will be issued upon execution of the second term loan under the Credit Facility in an amount equal to 5.0% of the funded amount divided by the exercise price, which will be equal to the lower of (i) the closing price per share of the Company’s common stock on the NASDAQ on the date prior to the funding date of the second term loan or (ii) the ten-day average closing price per share prior to the funding date of the second term loan.
Issuance costs of $73,104 associated with the first term loan under the Credit Facility were deducted from the gross proceeds by the lender and were recorded as a discount to outstanding debt as of the closing date, resulting in net proceeds of $4,926,896. Other issuance costs of $28,932 directly related to the Credit Facility but not associated with the lender were recorded as a component of other non-current assets in the unaudited condensed balance sheet. The estimated fair value of the warrant issued of $233,107 was recorded as a discount to outstanding debt as of the closing date. The discounts and other issuance costs are amortized to interest expense utilizing the effective interest method over the underlying term of the loan. The total amount of interest expense recorded during the three and nine months ended September 30, 2014 related to the Credit Facility was $144,717 and $240,850, respectively. The Credit Facility bears an effective annual interest rate of 10.81% at both April 30, 2014 and September 30, 2014.
7. Stock-based Compensation
Stock Options
A summary of stock option activity for option awards granted under the Company’s 2007 Equity Incentive Plan and 2013 Equity Incentive Plan for the nine months ended September 30, 2014 is as follows:
|
|
|
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
|
Weighted |
|
|
Remaining |
|
||
|
|
Number of |
|
|
Average Exercise |
|
|
Contractual |
|
|||
|
|
Shares |
|
|
Price Per Share |
|
|
Term in Years |
|
|||
|
Vested and unvested expected to vest, December 31, 2013 |
|
331,540 |
|
|
$ |
5.14 |
|
|
9.3 |
|
|
|
Outstanding at December 31, 2013 |
|
333,106 |
|
|
$ |
5.14 |
|
|
9.3 |
|
|
|
Granted |
|
594,798 |
|
|
$ |
7.06 |
|
|
|
|
|
|
Exercised |
— |
|
|
— |
|
|
|
|
|
||
|
Cancelled/forfeited/expired |
|
(52,862 |
) |
|
$ |
4.66 |
|
|
|
|
|
|
Outstanding at September 30, 2014 |
|
875,042 |
|
|
$ |
6.47 |
|
|
|
9.2 |
|
|
Vested and unvested expected to vest, September 30, 2014 |
|
871,124 |
|
|
$ |
6.48 |
|
|
|
9.2 |
|
The intrinsic values of options outstanding and options vested and unvested expected to vest at September 30, 2014 were zero.
178
The fair values of option awards granted during the nine months ended September 30, 2014 were estimated using a Black-Scholes pricing model with the following assumptions:
|
Stock and exercise prices |
$4.38 - $9.11 |
|
Expected dividend yield |
0.00% |
|
Discount rate-bond equivalent yield |
1.56% – 2.06% |
|
Expected life (in years) |
5.00 – 6.08 |
|
Expected volatility |
90.0% – 100.0% |
|
Expected forfeiture rate |
0.00% – 5.00% |
Using the assumptions described above, with stock and exercise prices being equal on date of grant, the weighted-average estimated fair value of options granted in the nine months ended September 30, 2014 was $5.51 per share.
Further information about the options outstanding and exercisable at September 30, 2014 is as follows:
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
Average |
|
|
|
|
|
||
|
Average |
|
|
Total Shares |
|
|
Contractual |
|
|
Total Shares |
|
||||
|
Exercise Price |
|
|
Outstanding |
|
|
Life (in years) |
|
|
Exercisable |
|
||||
|
$ |
4.38 |
|
|
|
86,458 |
|
|
|
9.6 |
|
|
|
8,540 |
|
|
$ |
4.62 |
|
|
|
19,928 |
|
|
|
6.5 |
|
|
|
17,473 |
|
|
$ |
5.03 |
|
|
|
21,500 |
|
|
|
9.8 |
|
|
— |
|
|
|
$ |
5.04 |
|
|
|
8,233 |
|
|
|
4.8 |
|
|
|
8,233 |
|
|
$ |
5.18 |
|
|
|
285,625 |
|
|
|
8.8 |
|
|
|
159,406 |
|
|
$ |
5.35 |
|
|
|
117,500 |
|
|
|
9.7 |
|
|
|
4,686 |
|
|
$ |
7.50 |
|
|
|
43,000 |
|
|
|
9.5 |
|
|
— |
|
|
|
$ |
8.88 |
|
|
|
238,500 |
|
|
|
9.4 |
|
|
— |
|
|
|
$ |
9.11 |
|
|
|
54,298 |
|
|
|
9.4 |
|
|
|
54,298 |
|
|
|
|
|
|
|
875,042 |
|
|
|
|
|
|
|
252,636 |
|
The intrinsic value of options exercisable at September 30, 2014 was zero.
Performance Stock Units
On June 12, 2014, the Company’s Board of Directors approved the issuance of 44,496 Restricted Stock Units (“RSUs”) to its Chief Executive Officer pursuant to its 2013 Equity Incentive Plan. Vesting of the RSU’s may occur based on the Company’s achievement of specified objectives as determined by the Company’s Board of Directors or Compensation Committee, as follows:
|
|
Percentage of |
|
|
|
|
Overall RSU |
|
|
|
|
Grant Subject to |
|
|
|
|
Vesting |
|
|
|
Target |
|
|
|
|
Minimum revenue in 2015 |
|
25 |
% |
|
Maximum EBITDA loss in 2015 |
|
15 |
% |
|
Attainment of financial plan for fiscal 2015 |
|
20 |
% |
|
Minimum value of strategic agreements by December 31, 2015 |
|
20 |
% |
|
Implementation of four new diagnostic test panels by December 31, 2015 |
|
20 |
% |
|
Total |
|
100 |
% |
The amount of compensation expense recognized is based on management’s estimate of the most likely outcome.
179
Stock-based Compensation Expense
The following table presents the effects of stock-based compensation related to equity awards to employees and nonemployees on the unaudited condensed statement of operations and comprehensive loss during the periods presented:
|
|
For the three months ended |
|
|
For the nine months ended |
|
||||||||||
|
|
September 30, |
|
|
September 30, |
|
||||||||||
|
|
2013 |
|
|
2014 |
|
|
2013 |
|
|
2014 |
|
||||
|
Stock Options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses |
$ |
246,313 |
|
|
$ |
35,569 |
|
|
$ |
253,828 |
|
|
$ |
149,626 |
|
|
General and administrative expenses |
|
141,693 |
|
|
|
236,769 |
|
|
|
155,197 |
|
|
|
908,490 |
|
|
Sales and marketing expenses |
— |
|
|
|
27,834 |
|
|
— |
|
|
|
46,762 |
|
||
|
Total expenses related to stock options |
|
388,006 |
|
|
|
300,172 |
|
|
|
409,025 |
|
|
|
1,104,878 |
|
|
RSUs |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses |
— |
|
|
|
7,500 |
|
|
— |
|
|
|
22,500 |
|
||
|
General and administrative expenses |
— |
|
|
|
13,750 |
|
|
|
274,371 |
|
|
|
379,208 |
|
|
|
Total stock-based compensation |
$ |
388,006 |
|
|
$ |
321,422 |
|
|
$ |
683,396 |
|
|
$ |
1,506,586 |
|
As of September 30, 2014, total unrecognized stock-based compensation expense related to unvested stock option and RSU awards, adjusted for estimated forfeitures, was approximately $2,916,000 and $71,000, respectively, and is expected to be recognized over a weighted-average period of 2.7 years and 0.8 years, respectively.
8. Net Loss per Common Share
Basic and diluted net loss per common share is determined by dividing net loss applicable to common shareholders by the weighted-average common shares outstanding during the period. Because there is a net loss attributable to common shareholders for the three and nine months ended September 30, 2013 and 2014, the outstanding shares of Series A preferred stock, RSUs, convertible debt, warrants, and common stock options have been excluded from the calculation of diluted loss per common share because their effect would be anti-dilutive. Therefore, the weighted-average shares used to calculate both basic and diluted loss per share are the same.
In November 2013, the Company effected a 1:14 reverse stock split of all common shares outstanding. The calculation of weighted-average shares outstanding has been adjusted for this reverse split as if it had occurred on January 1, 2013.
The following potentially dilutive securities have been excluded from the computations of diluted weighted-average shares outstanding for the periods presented, as they would be anti-dilutive:
|
|
For the three and nine months ended |
|
|||||
|
|
September 30, |
|
|||||
|
|
2013 |
|
|
2014 |
|
||
|
Series A preferred (number of common stock equivalents) |
|
1,652,851 |
|
|
— |
|
|
|
Preferred warrants outstanding (number of common stock equivalents) |
|
192,262 |
|
|
|
1,587 |
|
|
Notes payable convertible into preferred shares (number of common stock equivalents) |
|
232,558 |
|
|
— |
|
|
|
Preferred share RSUs (number of common stock equivalents) |
|
68,546 |
|
|
|
73,151 |
|
|
Common warrants outstanding |
|
630,110 |
|
|
|
609,187 |
|
|
Notes payable convertible into common shares |
|
741,857 |
|
|
— |
|
|
|
Common share RSUs |
|
133,971 |
|
|
|
178,467 |
|
|
Common options outstanding |
|
344,565 |
|
|
|
875,042 |
|
|
Total anti-dilutive common share equivalents |
|
3,996,720 |
|
|
|
1,737,434 |
|
180
9. Commitments and Contingencies
In the normal course of business, the Company may be involved in legal proceedings or threatened legal proceedings. The Company is not party to any legal proceedings or aware of any threatened legal proceedings which are expected to have a material adverse effect on its financial condition, results of operations or liquidity.
The Company’s former Vice President of Operations filed an administrative proceeding against the Company with the California Labor Commissioner in April 2013, seeking damages for alleged unpaid wages and penalties. A hearing was held on August 19, 2013 which resulted in a finding against the Company for approximately $65,000, of which $40,000 was paid during the year ended December 31, 2013 and $25,000 was accrued as of December 31, 2013. On February 25, 2014, the aforementioned administrative proceeding filed with the California Labor Commissioner by the Company’s former Vice President of Operations was settled in full following payment of the remaining $25,000 due.
181
Shares
Common Stock

_____________________
PROSPECTUS
_____________________
Joint Book-Running Managers
|
Aegis Capital Corp |
Feltl and Company |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
|
Item 13. |
Other Expenses of Issuance and Distribution |
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable by Biocept, Inc. (the “Registrant”) in connection with the sale and distribution of the securities being registered. All amounts are estimated except the SEC registration fee, the FINRA filing fee and the NASDAQ listing fee.
|
Item |
|
Amount |
|
|
|
SEC registration fee |
|
$ |
1,743 |
|
|
FINRA filing fee |
|
|
2,750 |
|
|
Legal fees and expenses |
|
|
150,000 |
|
|
Accounting fees and expenses |
|
|
50,000 |
|
|
Printing and engraving expenses |
|
|
25,000 |
|
|
Transfer agent and registrar fees and expenses |
|
|
2,000 |
|
|
Miscellaneous fees and expenses |
|
|
5,000 |
|
|
|
|
|
|
|
|
Total |
|
$ |
236,493 |
|
|
|
|
|
|
|
|
Item 14. |
Indemnification of Directors and Officers |
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act.
The Registrant’s amended certificate of incorporation provides for indemnification of its directors and executive officers to the maximum extent permitted by the Delaware General Corporation Law, and the Registrant’s amended and restated bylaws provide for indemnification of its directors and executive officers to the maximum extent permitted by the Delaware General Corporation Law.
In addition, the Registrant has entered into indemnification agreements with each of its current directors and executive officers. These agreements will require the Registrant to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to the Registrant and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. The Registrant also intends to enter into indemnification agreements with its future directors and executive officers.
The Registrant plans to enter into an underwriting agreement which provides that the underwriters are obligated, under some circumstances, to indemnify the Registrant’s directors, officers and controlling persons against specified liabilities, including liabilities under the Securities Act.
|
Item 15. |
Recent Sales of Unregistered Securities. |
Since January 1, 2011, the Registrant made sales of the unregistered securities discussed below. The offers, sales and issuances of the securities described below were exempt from registration under the Securities Act by virtue of Section 4(a)(2) of the Securities Act and/or, in the case of compensatory issuances, Securities Act Rule 701, and/or, in the case of conversions, Section 3(a)(9) of the Securities Act. No commissions were paid.
Note and Warrant Financings
In 30 closings from February 2011 to November 2012, the Registrant sold secured convertible promissory notes with an aggregate principal amount of $12,336,247, together with warrants that subsequently became exercisable for 108,786 shares of its common stock at an exercise price of $10.00 per share, to 11 accredited investors, for aggregate gross proceeds of $12,336,247.
In 21 closings from January 2012 to December 2012, the Registrant sold promissory notes with an aggregate principal amount of $5,960,000, together with warrants that subsequently became exercisable for 52,557 shares of the Registrant’s common stock to five accredited investors at an exercise price of $10.00 per share, for aggregate gross proceeds of $5,960,000. These promissory notes were converted into shares of the Registrant’s common stock upon the closing of its initial public offering.
II-1
In 56 closings from December 2012 through January 2014, the Registrant sold promissory notes with an aggregate principal amount of $5,165,000, together with warrants that subsequently became exercisable for 258,249 shares of the Registrant’s common stock at an exercise price of $10.00 per share, to 14 accredited investors, for aggregate gross proceeds of $5,165,000.
Compensatory Issuances
In 2011 the Company issued 36,260 common stock options (at a $4.62 weighted average exercise price per share) and 23,874 preferred stock restricted stock units (number of common stock equivalents) to service providers.
In 2012 the Registrant issued 330 common stock options (at a $4.62 weighted average exercise price per share) and 32,769 common stock restricted stock units to service providers.
In 2013, the Registrant issued 300,438 common stock options (at a $5.18 weighted average exercise price per share) and 101,202 common stock restricted stock units to service providers.
In 2014, the Registrant issued 647,298 common stock options (at a $6.71 weighted average exercise price per share) and 44,496 common stock restricted stock units to service providers. Upon the completion of the Registrant’s initial public offering in February 2014, a total of 73,151 preferred stock restricted stock units (number of common stock equivalents) vested in accordance with underlying agreements, including the 23,874 preferred stock restricted stock units (number of common stock equivalents) issued in 2011.
Inducement Warrants
In September 2012, the Registrant issued 66,666 Series A preferred stock warrants, at an exercise price of $0.60 per share, to its landlord in exchange for certain real estate lease accommodations. The Series A preferred stock warrants are exercisable for 1,587 shares of the Registrant’s common stock at an exercise price of $25.20 per share.
In June 2013, the Registrant issued 23,809 common stock warrants, at an exercise price to be determined in accordance with contract, to a lender (a 5% beneficial holder) in connection with a note conversion. Upon the completion of the Registrant’s initial public offering in February 2014, the exercise price of these common stock warrants was fixed at $10.00 per share.
In July 2013 through January 2014, the Registrant issued common stock warrants which became exercisable upon the completion of the Registrant’s initial public offering in February 2014 for an aggregate 128,903 shares of the Registrant’s common stock, at an exercise price of $10.00 per share, to five guarantors in connection with their guaranties of its Line of Credit.
In September 2013, the Registrant issued an indeterminate number of common stock warrants, at an exercise price to be determined in accordance with contract, to its landlord in connection with a lease amendment. Upon the completion of the Registrant’s initial public offering in February 2014, the exercise price of the common stock warrants issued to its landlord was fixed at $10.00 per share for an aggregate 50,260 shares of the Registrant’s common stock.
Recapitalization
In November 2011, the Registrant effected a recapitalization in which all outstanding shares of, and warrants to purchase and restricted stock units to obtain, the Registrant’s outstanding Series AA preferred stock and Series BB preferred stock were converted into the same number of shares of, warrants to purchase and restricted stock units to obtain, Series A preferred stock. At the same time, the Registrant effected the 1-for-3 reverse split of its common stock.
Conversions and Exercises
In November 2011, the Registrant’s Executive Chairman exercised 10,204 common stock options, paying an aggregate exercise price of $47,183.
In October 2011, a major stockholder converted 2,064,520 shares of Series AA preferred stock into 49,155 shares of common stock.
In March and April 2013, two employees exercised stock options for 85 shares of common stock, paying an aggregate exercise price of $395.
II-2
In June 2013, the holders of promissory notes with an aggregate principal balance of approximately $20,231,000 and accrued but unpaid interest of approximately $2,581,000 voluntarily converted such principal and interest into 42,245,834 shares of the Registrant’s Series A preferred stock. Such shares of Series A preferred stock were subsequently converted into 1,652,851 shares of the Registrant’s common stock upon completion of the Registrant’s initial public offering in February 2014.
In the fourth quarter of 2013, four employees exercised 3,936 common stock options, paying an aggregate exercise price of $19,710.
In February 2014:
|
· |
The $1,400,000 principal amount of and the $233,982 accrued interest on the Registrant’s 2008 convertible note held by a trust affiliated with the Registrant’s majority stockholder, Claire K. T. Reiss, were converted at $10.00 per share into a total of 163,399 shares of common stock. |
|
· |
The $5,165,000 principal amount of and the $313,017 accrued interest on our “2013” convertible notes held by various persons, including several insiders, were converted at $10.00 per share into a total of 547,801 shares of common stock. The following persons received the following numbers of such shares: |
|
· |
Affiliates of Claire K. T. Reiss, majority stockholder—270,484 |
|
· |
Affiliate of David F. Hale, Chairman—47,181 |
|
· |
Affiliate of Edward Neff, Director—108,140 |
|
· |
Marsha Chandler, Director—5,078 |
|
· |
M. Faye Wilson, Director—2,650 |
|
· |
Bruce E. Gerhardt, Director—1,055 |
|
· |
Warrants for an aggregate 95,000 shares of the Registrant’s common stock were issued to the underwriters of the Registrant’s initial public offering and are exercisable at a price of $12.50 per share. |
|
· |
Common stock options for an aggregate 285,000 shares of the Registrant’s common stock were issued to the underwriters of the Registrant’s initial public offering and were exercisable through March 21, 2014 at an exercise price of $9.30 per share to cover overallotments, which was not exercised. |
Debt financing warrant
In April 2014, the Registrant issued Oxford Finance LLC a warrant to purchase up to 52,966 shares of the Registrant’s common stock, at an exercise price of $4.72 per share. The warrant was issued in connection with the April 2014 Credit Facility.
|
Item 16. |
Exhibits and Financial Statement Schedules. |
(a)Exhibits
See the Exhibit Index attached to this Registration Statement, which is incorporated by reference herein.
(b) Financial Statement Schedules
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
|
Item 17. |
Undertakings |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to the Registrant’s directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by one of its directors, officers or controlling persons in the successful defense of any action, suit, or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such
II-3
indemnification by the Registrant is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes to provide to the underwriters, at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
The undersigned Registrant hereby undertakes that:
|
(i) |
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and |
|
(ii) |
For purposes of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in San Diego, California, on the 9th day of January, 2015.
|
|
|
|
|
BIOCEPT, INC. |
||
|
|
|
|
|
By: |
|
/s/ Michael W. Nall |
|
|
|
Michael W. Nall |
|
|
|
Chief Executive Officer and President |
POWER OF ATTORNEY
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints David F. Hale, Michael W. Nall and William G. Kachioff, and each and either of them, his or her true and lawful agent, proxy and attorney-in-fact, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments (including post-effective amendments) to this registration statement together with all schedules and exhibits thereto and any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act, together with all schedules and exhibits thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith, (iii) act on and file any supplement to any prospectus included in this registration statement or any such amendment or any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act and (iv) take any and all actions which may be necessary or appropriate to be done, as fully for all intents and purposes as he or she might or could do in person, hereby approving, ratifying and confirming all that such agent, proxy and attorney-in-fact or any of his substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
||
|
/s/ Michael W. Nall |
|
Chief Executive Officer, President and Director (Principal Executive Officer) |
|
January 9, 2015 |
|
Michael W. Nall |
|
|
||
|
|
|
|
||
|
/s/ William G. Kachioff |
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|
January 9, 2015 |
|
William G. Kachioff |
|
|
||
|
|
|
|
||
|
/s/ David F. Hale |
|
Chairman and Director |
|
January 9, 2015 |
|
David F. Hale |
|
|
||
|
|
|
|
||
|
/s/ Marsha A. Chandler |
|
Director |
|
January 9, 2015 |
|
Marsha A. Chandler |
|
|
|
|
|
|
|
|
||
|
/s/ Bruce E. Gerhardt |
|
Director |
|
January 9, 2015 |
|
Bruce E. Gerhardt |
|
|
|
|
|
|
|
|
||
|
/s/ Bruce A. Huebner |
|
Director |
|
January 9, 2015 |
|
Bruce A. Huebner |
|
|
|
|
|
|
|
|
||
|
/s/ Edward Neff |
|
Director |
|
January 9, 2015 |
|
Edward Neff |
|
|
|
|
|
|
|
|
||
|
/s/ Ivor Royston |
|
Director |
|
January 9, 2015 |
|
Ivor Royston |
|
|
|
|
|
|
|
|
||
|
/s/ M. Faye Wilson |
|
Director |
|
January 9, 2015 |
|
M. Faye Wilson |
|
|
|
|
II-5
EXHIBIT INDEX
|
Exhibit No. |
|
Description of Exhibit |
|
1.1† |
|
Form of Underwriting Agreement. |
|
3.1 |
|
Certificate of Amendment of Certificate of Incorporation (incorporated by reference to Exhibit 3.1.4 of the Registrant’s Current Report on Form 8-K, filed with the SEC on February 14, 2014). |
|
3.2 |
|
Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2.1 of the Registrant’s Current Report on Form S-1, filed with the SEC on September 23, 2013). |
|
4.1 |
|
Reference is made to Exhibits 3.1 and 3.2. |
|
4.2 |
|
Specimen Common Stock certificate of Biocept, Inc. (incorporated by reference to Exhibit 4.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on November 5, 2013). |
|
4.3 |
|
Form of Representative’s Warrant, dated February 10, 2014 (incorporated by reference to Exhibit 4.2 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on November 20, 2013). |
|
4.4 |
|
Form of Warrant issued to the lenders under the Loan and Security Agreement, dated as of April 30, 2014, by and among Biocept, Inc., Oxford Finance LLC, as collateral agent, and the lenders party thereto from time to time, including Oxford Finance LLC (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed with the SEC on May 6, 2014). |
|
5.1† |
|
Opinion of Cooley LLP. |
|
10.1+ |
|
2007 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.2+ |
|
Form of Stock Option Grant Notice and Option Agreement under 2007 Equity Incentive Plan (incorporated by reference to Exhibit 10.1.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.3+ |
|
Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under 2007 Equity Incentive Plan (incorporated by reference to Exhibit 10.1.2 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.4+ |
|
2013 Equity Incentive Plan (incorporated by reference to Exhibit 10.2 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on November 20, 2013) |
|
10.5+ |
|
Form of Notice of Stock Option Grant under 2013 Equity Incentive Plan (incorporated by reference to Exhibit 10.2.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.6+ |
|
Form of Stock Option Agreement under 2013 Equity Incentive Plan (incorporated by reference to Exhibit 10.2.2 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.7+ |
|
Form of Restricted Stock Unit Agreement under 2013 Equity Incentive Plan (incorporated by reference to Exhibit 10.2.3 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.8+ |
|
Form of Restricted Stock Unit Agreement under 2013 Equity Incentive Plan (for senior officers: as used August 8, 2013) (incorporated by reference to Exhibit 10.2.4 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.9+ |
|
Form of Restricted Stock Unit Agreement under 2013 Equity Incentive Plan (for non-employee directors: as used August 8, 2013) (incorporated by reference to Exhibit 10.2.5 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.10+ |
|
Restricted Stock Unit Grant Notice / Agreement with David F. Hale, dated as of March 10, 2011 (“Performance-Based”) (incorporated by reference to Exhibit 99.3 of the Registrant’s Registration Statement on Form S-8 (File No. 333-194930), filed with the SEC on March 31, 2014). |
|
10.11+ |
|
Restricted Stock Unit Grant Notice / Agreement with David F. Hale, dated as of March 10, 2011 (“Time-Based”) (incorporated by reference to Exhibit 99.4 of the Registrant’s Registration Statement on Form S-8 (File No. 333-194930), filed with the SEC on March 31, 2014). |
|
10.12+ |
|
Restricted Stock Unit Grant Notice / Agreement with Ivor Royston, dated as of November 8, 2010, as amended on February 15, 2012 (incorporated by reference to Exhibit 10.23 to the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the Commission on September 23, 2013). |
|
Exhibit No. |
|
Description of Exhibit |
|
10.13+ |
|
2014 Annual Incentive Plan (incorporated by reference to Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q, filed with the SEC on August 8, 2014). |
|
10.14+ |
|
Form of Indemnification Agreement between the Registrant and its officers and directors (incorporated by reference to Exhibit 10.3 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.15+ |
|
Form of Indemnity Agreement between Biocept, Inc., a California corporation, and its officers and directors (incorporated by reference to Exhibit 10.4 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.16+ |
|
Employment Agreement, between the Registrant and Michael W. Nall, effective as of August 26, 2013 (incorporated by reference to Exhibit 10.6 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.17+ |
|
Employment Agreement, between the Registrant and Lyle J. Arnold, dated April 30, 2011(incorporated by reference to Exhibit 10.7 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.18+ |
|
Employment Agreement, between the Registrant and William G. Kachioff, dated August 1, 2011(incorporated by reference to Exhibit 10.8 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.19+ |
|
Employment Agreement, between the Registrant and Raaj Trivedi, dated March 1, 2014. |
|
10.20 |
|
Lease, between the Registrant and Nexus Equity VIII LLC, dated March 31, 2004 (incorporated by reference to Exhibit 10.11 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on November 5, 2013). |
|
10.21 |
|
First Amendment to Lease, between the Registrant and ARE-SD Region No. 18, LLC, dated November 1, 2011(incorporated by reference to Exhibit 10.11.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.22 |
|
Second Amendment to Lease, between the Registrant and ARE-SD Region No. 18, LLC, dated September 10, 2012 (incorporated by reference to Exhibit 10.11.2 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.23 |
|
Third Amendment to Lease, between the Registrant and ARE-SD Region No. 18, LLC, dated as of January 31, 2013, and effective as of January 1, 2013 (incorporated by reference to Exhibit 10.11.4 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.24 |
|
Fourth Amendment to Lease, between the Registrant and ARE-SD Region No. 18, LLC, dated as of September 10, 2013, and effective as of August 1, 2013 (incorporated by reference to Exhibit 10.11.5 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.25 |
|
Warrant to Purchase Preferred Stock, dated September 10, 2012, issued by the Registrant in favor of ARE-SD Region No. 18, LLC (incorporated by reference to Exhibit 10.11.3 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.26 |
|
Warrant to Purchase Common Stock, dated September 10, 2013, issued by the Registrant in favor of ARE-SD Region No. 18, LLC (incorporated by reference to Exhibit 10.11.6 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.27 |
|
Amended and Restated Investor Rights Agreement, dated as of October 31, 2011, among the Registrant and certain investors named therein (incorporated by reference to Exhibit 10.12 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.28* |
|
Collaboration Agreement dated as of November 2, 2012 between the Registrant and Life Technologies Corporation (incorporated by reference to Exhibit 10.13 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on January 30, 2014. |
|
10.29 |
|
Collaboration Agreement dated as of August 17, 2011 between the Registrant and Clarient Diagnostic Services, Inc. (incorporated by reference to Exhibit 10.14 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on January 8, 2014). |
|
Exhibit No. |
|
Description of Exhibit |
|
10.30 |
|
Warrant to Purchase Preferred Stock dated as of January 21, 2009, issued by the Registrant in favor of Goodman Co. Ltd. (incorporated by reference to Exhibit 10.17.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.31 |
|
Warrant to Purchase Common Stock dated as of July 31, 2013, issued by the Registrant in favor of Goodman Co. Ltd. (incorporated by reference to Exhibit 10.17.3 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.32 |
|
Form of Warrant to Purchase Preferred Stock, issued by the Registrant in favor of various investors under the Note and Warrant Purchase Agreement dated as of January 13, 2012 (incorporated by reference to Exhibit 10.19.3 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.33 |
|
Form of Amendment of Warrant to Purchase Preferred Stock, dated as of September 13, 2013 (incorporated by reference to Exhibit 10.19.4 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.34 |
|
Form of Warrant to Purchase Common Stock, issued by the Registrant in favor of various investors under the Note and Warrant Purchase Agreement dated as of June 28, 2013 (incorporated by reference to Exhibit 10.20.2 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.35 |
|
Form of Warrant to Purchase Common Stock, issued by the Registrant in favor of various guarantors under the Reimbursement Agreement dated as of July 11, 2013 (incorporated by reference to Exhibit 10.21.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.36 |
|
Assignment and Exclusive Cross-License Agreement between the Registrant and Aegea Biotechnologies, Inc. dated June 2, 2012 (incorporated by reference to Exhibit 10.22 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on January 30, 2014). |
|
10.37* |
|
Master Laboratory Research Support and Services Agreement dated as of July 9, 2012 between the Registrant and Dana Farber Partners Cancer Care, Inc. (incorporated by reference to Exhibit 10.15 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on November 5, 2013). |
|
10.38 |
|
Laboratory Services Agreement dated July 29, 2013, effective as of May 1, 2013, between the Registrant and Clarient Diagnostic Services, Inc. (incorporated by reference to Exhibit 10.14.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
10.39 |
|
Loan and Security Agreement by and among Biocept, Inc., Oxford Finance LLC, as collateral agent, and the lenders party thereto from time to time, including Oxford Finance LLC, dated as of April 30, 2014 (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K, filed with the SEC on May 6, 2014). |
|
10.40+ |
|
Employment Agreement, between us and David F. Hale, dated March 10, 2011 (incorporated by reference to Exhibit 10.5 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013). |
|
21.1 |
|
List of Subsidiaries (incorporated by reference to Exhibit 21.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013) . |
|
23.1 |
|
Consent of Mayer Hoffman McCann P.C. |
|
23.2† |
|
Consent of Cooley LLP (included in Exhibit 5.1). |
|
24.1 |
|
Power of Attorney (included on the signature page hereto). |
|
101.INS† |
|
XBRL Instance Document |
|
101.SCH† |
|
XBRL Taxonomy Extension Schema Document |
|
101.CAL† |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
101.DEF† |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
101.LAB† |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
101.PRE† |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
+ |
Indicates management contract or compensatory plan. |
|
* |
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to Rule 406 under the Securities Act of 1933. |
|
† |
To be filed by amendment. |
