Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Karyopharm Therapeutics Inc. | d845399d8k.htm |
| EX-10.1 - EX-10.1 - Karyopharm Therapeutics Inc. | d845399dex101.htm |
| EX-99.1 - EX-99.1 - Karyopharm Therapeutics Inc. | d845399dex991.htm |
Exhibit 99.2
Karyopharm Therapeutics Inc.
Overview
We are a clinical-stage pharmaceutical company focused on the discovery, development and subsequent commercialization of novel, first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases. Our scientific expertise is focused on the understanding of the regulation of intracellular transport between the nucleus and the cytoplasm. We have discovered and are developing wholly-owned, novel, small molecule Selective Inhibitor of Nuclear Export, or SINE™, compounds that inhibit the nuclear export protein XPO1. These SINE compounds represent a new class of drug candidates with a novel mechanism of action that have the potential to treat a variety of diseases in areas of unmet medical need. Our initial focus is on seeking the regulatory approval and commercialization of our lead drug candidate, selinexor (KPT-330), as a single orally administered agent in cancer indications with significant unmet clinical need. We then plan to seek additional approvals for the use of selinexor in combination therapy to expand the patient population that is eligible for selinexor as well as to move selinexor further towards front-line cancer therapy. To date, we have initiated three registration-directed trials in hematological cancers with selinexor, and anticipate initiating a fourth registration-directed trial in the first half of 2015. We intend to establish a commercial infrastructure to prepare for a potential launch of selinexor for hematologic indications in North America and Western Europe as early as the middle of 2017 assuming successful results of ongoing clinical trials and receipt of the necessary approvals from regulatory authorities to begin marketing selinexor in these geographies.
Selinexor is being evaluated in multiple open-label Phase 1 and Phase 2 clinical trials in patients with heavily pretreated relapsed and/or refractory hematological and solid tumor malignancies. To date, we have administered selinexor to over 550 patients across Phase 1 and Phase 2 clinical trials in hematologic and solid tumor indications, including over 450 patients that have been dosed at above 35 mg/m2, which is roughly equivalent to Phase 2 or Phase 3 recommended dosing. Evidence of anti-cancer activity has been observed in significant numbers of patients and selinexor has been sufficiently well-tolerated to allow many of these patients to remain on therapy for prolonged periods, including several patients who have remained on study for over 12 months, with the longest patient on study for over 24 months. During 2014, we initiated three registration-directed clinical trials of selinexor in three different hematological malignancy indications, older patients with relapsed or refractory acute myeloid leukemia, or AML, Richter’s transformation (also called Richter’s syndrome), or Richter’s, following chemotherapy and in diffuse large B-cell lymphoma, or DLBCL, in third-line or later therapy. We plan to initiate a single-arm trial in multiple myeloma, or MM, in the first half of 2015 that we intend to be registration-directed and are evaluating other potential registration-directed trials in hematological and solid tumor indications. These registration-directed trials are designed to serve as the basis for an application seeking regulatory approval for selinexor in particular indications. To our knowledge, no other XPO1 inhibitors are in clinical development at the present time.
Transient XPO1 Inhibition by SINE Compounds
One of the ways in which the cell regulates the function of a particular protein is by controlling the protein’s location within the cell, as a specific function may only occur within a particular location in the cell. In healthy cells, nuclear transport, both into and out of the nucleus, is a normal and regular occurrence that is tightly regulated and requires specific carrier proteins to be present. XPO1 mediates the export of approximately 220 different mammalian cargo proteins, including the vast majority of tumor suppressor proteins, as well as the transport of oncogenic mRNAs which are translated to proteins at high levels in the cytoplasm. Moreover, XPO1 appears to be the only nuclear exporter for most of these tumor suppressor proteins. Cancer cells have increased levels of XPO1, causing the increased export of these tumor suppressor proteins from the nucleus. Since the tumor suppressor proteins need to be located in the nucleus to promote programmed cell death, or apoptosis, XPO1 overexpression in cancer cells counteracts the natural apoptotic process that protects the body from cancer. Due to XPO1 inhibition by our SINE compounds, the export of tumor suppressor proteins is prevented, thereby leading to their accumulation in the nucleus. This subsequently reinitiates and amplifies their natural apoptotic function in cancer cells with minimal effects on normal cells. Further, SINE compounds reduce the translation of oncogenes by inhibiting the XPO1-mediated transport of oncogenes to the cytoplasm. The figure below depicts the process by which our SINE compounds inhibit the XPO1 nuclear export of tumor suppressor proteins.
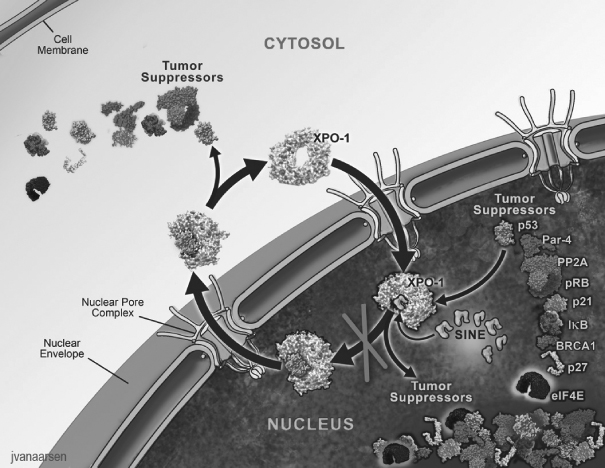
We believe that the XPO1-inhibiting SINE compounds that we have discovered and developed to date, including selinexor, have the potential to provide a novel targeted therapy that enables tumor suppressor proteins
to remain in the nucleus and promote apoptosis of potentially any type of cancer cell. Moreover, our SINE compounds spare normal cells, which, unlike cancer cells, do not have significant damage to their genetic material, and we believe this selectivity for cancer cells minimizes side effects. We believe that the oral administration of selinexor and the lack of cumulative or major organ toxicities observed to date in patients treated with selinexor in clinical trials create the potential for its broad use across many cancer types, including both hematological and solid tumor malignancies. We believe that no currently approved cancer treatments or current clinical-stage cancer drug candidates are selectively targeting the restoration and increase in the levels of multiple tumor suppressor proteins in the nucleus. No other XPO1 inhibitors are reported to be in clinical development based on clinicaltrials.gov listings. We own all intellectual property rights related to the SINE compounds that we are developing and our patent application claiming selinexor as a composition of matter was allowed by the U.S. Patent and Trademark Office in late 2014.
Summary of Clinical Progress
We are currently conducting multiple open-label clinical trials of selinexor, including three registration-directed Phase 2 or Phase 2b clinical trials in hematological indications. We refer to these trials as registration-directed because they are designed to serve as the basis for an application seeking regulatory approval of selinexor. In June 2014, we initiated a registration-directed clinical trial for selinexor in older patients with relapsed or refractory AML. In November 2014, we initiated a registration-directed clinical trial in Richter’s following chemotherapy. In December 2014, we initiated a registration-directed clinical trial in DLBCL in third-line or later therapy. We plan to initiate a clinical trial in MM during the first half of 2015 that we intend to be registration-directed and are evaluating other potential registration-directed trials in hematological and solid tumor indications. We plan to seek regulatory approvals of selinexor in North America and Western Europe in each such indication with respect to which we receive positive results and positive regulatory feedback. We may seek such approvals in other geographies as well.
We have also initiated Phase 2 studies of selinexor in several solid tumor indications, including prostate cancer, gynecologic malignancies (ovarian, cervical and uterine cancers), glioblastoma and squamous head, neck or lung cancers. We expect initial data from these studies during 2015. Approximately 17 investigator-sponsored trials, or ISTs, are currently ongoing and a number of additional combination studies, primarily ISTs, are expected to begin during 2015.
During 2014, we reported data from three Phase 1 clinical trials, the first in patients with various advanced hematological malignancies, the second in patients with various advanced or metastatic solid tumor malignancies and the third, a food effect study, in patients who have metastatic, locally advanced or locally recurrent soft tissue or bone sarcomas. In these trials, we have observed evidence of anti-cancer activity of selinexor across a spectrum of patients with advanced cancers who had received multiple previous treatments and, despite these treatments, had disease that was progressing at the time of enrollment in our clinical trials. Our hematological malignancy trial consists of seven arms, in which selinexor is administered as a monotherapy in Arms 1-5 and in combination in Arms 6 and 7. Arm 1 includes patients with certain chronic B-cell malignancies, including non-Hodgkin’s lymphoma, or NHL, chronic lymphocytic leukemia, or CLL, MM and Waldenström’s Macroglobulinemia, or WM; Arm 2 includes patients with AML; Arm 3 includes patients with T-cell lymphomas; Arm 4 includes patients with chronic myeloid leukemia, or CML; Arm 5 includes patients with acute lymphocytic leukemia, or ALL; Arm 6 includes patients on combination therapy who have MM and are taking 20 mg of dexamethasone (so called “low-dose dex”) in combination with each twice weekly dose of selinexor and Arm 7 includes patients on combination therapy who have DLBCL and are taking standard doses of rituximab in combination with twice weekly doses of selinexor.
Of the patients evaluated in our hematological malignancy trial, we have observed complete responses or remissions, partial responses or remissions, minimal responses or stable disease in a number of these patients, all as determined in accordance with commonly accepted evaluation criteria for the specific indication. Complete
or partial responses or stable disease were observed in 73% of evaluable patients with relapsed and/or refractory NHL through December 1, 2014. In patients with relapsed or refractory AML as of May 13, 2014, we have observed complete remissions, partial remissions, morphologic leukemia-free state or stable disease in 49% of patients, some for longer than three months, and as of December 1, 2014, there had been no material changes or adverse trends observed in the evaluable AML patient responses. Among nine evaluable patients with MM treated with selinexor in combination with low-dose (20 mg) dexamethasone dosed twice weekly, complete, partial or minor responses were observed in 89% of patients as of December 1, 2014. Of the patients in the solid tumor malignancy trial evaluated as of May 13, 2014, we have observed partial responses or stable disease in 49%, all as determined in accordance with Response Evaluation Criteria In Solid Tumors, or RECIST, and as of December 1, 2014, there had been no material changes or adverse trends observed in the evaluable solid tumor patient responses.
We are developing another SINE compound, verdinexor (KPT-335), which is closely related to selinexor, as an oral treatment of pet dogs with lymphomas. The approval of drugs to treat animals is based on a New Animal Drug Application, or NADA, reviewed by the FDA’s Center for Veterinary Medicine, or CVM. The use of verdinexor to treat canine lymphoma has been designated a “minor use” in accordance with the Minor Use Minor Species, or MUMS, Act, which makes verdinexor eligible for conditional approval similar to accelerated approvals used for submissions of human therapeutics. Two of the four major technical sections of the NADA for verdinexor, Effectiveness and Safety, are complete and may support conditional approval by the CVM and the applicability of the Environmental Impact section has been waived. Thus, CVM has indicated that three of the four major technical sections of the NADA support conditional approval. We may seek a commercial relationship with a marketing collaborator to complete the final major technical section of the NADA, the Chemicals/Manufacturing/Controls section, which covers the commercial-scale manufacturing, or CMC, of verdinexor. We believe that the acceptance of the Effectiveness and Safety sections of the NADA for verdinexor for the treatment of dogs with lymphoma helps confirm the activity and tolerability of our novel SINE compounds for the treatment of cancers.
In addition to cancer, we believe that our SINE compounds have the potential to provide therapeutic benefit in a number of additional indications, including autoimmune and inflammatory diseases, wound healing, HIV and influenza. We have discovered and are developing a pipeline of SINE compounds that have shown evidence of activity in preclinical models of inflammation, wound healing and viral infection. In the first quarter of 2015, we plan to initiate a Phase 1b clinical trial of topical selinexor in patients with diabetic foot ulcers and we expect to initiate Phase 1 clinical trials in healthy human volunteers for verdinexor and KPT-350, an oral SINE compound that we are developing for the treatment of autoimmune and inflammatory diseases, by the middle of 2015. In addition, we hope to initiate one or more Phase 1 clinical trials in heavily pretreated oncology patients for our oral PAK4 inhibitor during the second half of 2015. We may seek to enter into development, marketing and commercialization collaboration arrangements for selinexor in geographies outside North America and Western Europe and for our other SINE compounds in non-oncology indications globally.
The table below summarizes the current stages of development of our key human drug candidates and indications that we expect to initially focus on for each candidate. In addition, approximately 17 ISTs for selinexor as single-agent or in combination are currently ongoing. We expect a growing number of ISTs to be initiated for selinexor in a variety of cancer indications over the next year consisting of single agent or combination studies in both hematological and solid tumor malignancies.
Karyopharm’s Broad Therapeutic Pipeline
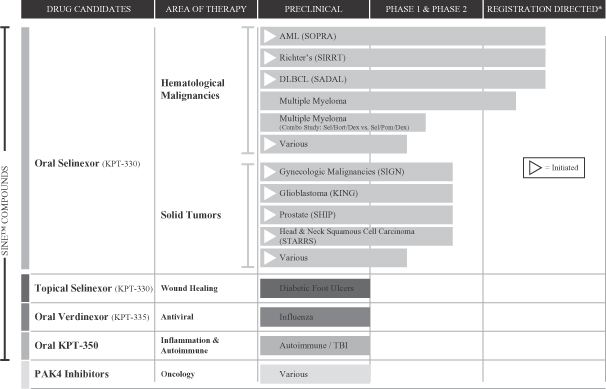
| * | Designed to serve as the basis for an application seeking regulatory approval for selinexor for the specified indication. |
Since our founding by Dr. Sharon Shacham in December 2008, our goal has been to establish a leading, independent oncology business. We are led by Dr. Shacham, our President and Chief Scientific Officer, and Dr. Michael Kauffman, our Chief Executive Officer. Dr. Kauffman played a leadership role in the development and approval of Velcade® at Millennium Pharmaceuticals and of Kyprolis® while serving as Chief Medical Officer at Proteolix and then Onyx Pharmaceuticals. Dr. Shacham has played a leadership role in the discovery and development of many novel drug candidates, which have been or are being tested in human clinical trials, prior to her founding of Karyopharm and while at Karyopharm.
Indications
Cancer is a leading cause of death worldwide, with approximately 580,000 people in the United States and 7.6 million people in the world projected to die of cancer in 2014 according to the American Cancer Society. The American Cancer Society projected that approximately 1.7 million new cancer cases would be diagnosed in the United States in 2014. The International Agency for Research on Cancer projects that, by 2030, 20 million to 26 million people will be diagnosed with cancer, and 13 million to 17 million will die of cancer, each year worldwide.
During 2014, we presented updated data from our ongoing Phase 1 clinical trials of selinexor in patients with various advanced hematological malignancies and in patients with various advanced or metastatic solid tumor malignancies. All patients entering these trials have progressive disease upon enrollment that is relapsed after and/or refractory to all available classes of anti-cancer agents, typically after multiple approved therapies and often after one or more experimental therapies. Anti-cancer activity has been observed with tumor reductions and durable disease control across many hematologic and solid tumor malignancies. Several patients have remained on single-agent oral selinexor for over 12 months with the longest on therapy for over 24 months. Adverse events observed in our most recent patient data are generally mild, responsive to standard supportive care and consistent with those previously reported in patients in our Phase 1 clinical trials.
Response data presented herein are interim unaudited data based on reports by physicians at the clinical trial sites. Responses in the hematological trial are measured using commonly accepted evaluation criteria for the specific indication. Responses in the solid tumor trial are measured using RECIST. Responses include:
| • | sCR—stringent complete response; |
| • | CR—complete response; |
| • | CR(i/p)—complete response without hematological recovery; |
| • | PR—partial response; |
| • | MLFS—morphological leukemia free state; |
| • | MR—minor response; |
| • | SD—stable disease; |
| • | PD—progressive disease; |
| • | WC—withdrew consent; or |
| • | NE—non-evaluable, meaning the patient’s response could not be evaluated due to a number of potential factors, including when a patient withdraws consent or fails to comply with the therapeutic protocol for the trial. |
Disease control rate, or DCR, refers to the percentage of responses that are SD or better (MR or better in the case of MM) and overall response rate, or ORR, refers to the percentage of responses that are PR or better.
Advanced Hematological Malignancies
During December 2014, we reported data from patients with NHL, including DLBCL, Richter’s, AML and MM, as part of our ongoing Phase 1 clinical trial of selinexor in patients with various advanced hematological malignancies. The primary objectives of the Phase 1 trial are to determine the safety, tolerability and recommended Phase 2 and Phase 3 dose of orally administered selinexor. Patients were dosed 3-80 mg/m2 (equivalent to approximately 5-130 mg) of selinexor orally over a four-week cycle, with lower doses initially given ten times per cycle and higher doses given twice weekly. Responses were evaluated every one to two cycles.
Non-Hodgkin’s Lymphoma
NHL is a cancer that starts in cells called lymphocytes, which are part of the body’s immune system. Lymphocytes are found in the lymph nodes and other lymphoid tissues, such as the spleen and bone marrow. The
World Health Organization estimated that 386,000 new cases of NHL would be diagnosed worldwide in 2012 and the American Cancer Society predicted that about 70,800 patients would be diagnosed with NHL in the United States in 2014. According to the Leukemia and Lymphoma Society, NHL rates, including DLBCL, have steadily increased 3 to 4% each year in the United States from 1973 to the mid-1990s.
We have initiated two registration-directed trials of selinexor in NHL indications, one in DLBCL and the other in Richter’s.
Diffuse Large B-cell Lymphoma
DLBCL is the most common of the aggressive NHLs. We estimate that approximately 25,000 patients are diagnosed with DLBCL in the United States each year.
The fundamental treatment of DLBCL has changed little in the past two decades, with no new or targeted agents approved for this indication. Initial therapy with multiagent, or three to four, cytotoxic drugs in combination with the monoclonal antibody rituximab (Rituxan®) leads to responses in greater than 75% of patients. In patients who are less than 65 years old, and who have good organ function, high dose chemotherapy with stem cell transplantation can lead to cures in approximately 50% of these patients. Older patients relapsing after initial chemotherapy, and those relapsing after stem cell transplantation, have a very poor prognosis, and the expected survival of such patients is less than one year. Newer targeted agents such as the BTK inhibitor ibrutinib (Imbruvica®) and the immunomodulatory drug lenalidomide (Revlimid®) have shown activity in the immunoblastic (activated B-cell) type of DLBCL in clinical trials, but responses are generally short-lived. Responses are much lower in the germinal center, or GCB, type of DLBCL. Therefore, we believe that novel, well-tolerated drugs are needed for the treatment of relapsed DLBCL, particularly because ibrutinib and lenalidomide have not been approved by the FDA for the treatment of DLBCL.
The first patient in our registration-directed trial in DLBCL, a Phase 2b clinical trial known as the SADAL, or Selinexor Against Diffuse Aggressive Lymphoma, study (NCT 02227251), was dosed in December 2014. This is a two-arm open-label trial in patients that are relapsed and/or refractory to two lines of chemotherapy. The primary endpoint of this trial is ORR to 100 mg with low dose dexamethasone compared to 60 mg with low dose dexamethasone, with both arms compared to a minimally effective lower threshold level of ORR at 20%. Patients will be randomized evenly between the two arms. In one arm, patients will receive a 60 mg dose of selinexor twice weekly, and in the other, patients will receive a 100 mg dose of selinexor twice weekly. The selinexor dose may be increased to 120 mg for patients in the 100 mg arm and to 80 mg for patients in the 60 mg arm on a case-by-case basis for those patients showing stable disease or partial response and who are tolerating treatment well. All patients will receive a minimum of 4-8 mg of dexamethasone with each dose of selinexor during the first cycle as supportive care, and dexamethasone will be optional in successive cycles. The SADAL study is expected to enroll approximately 200 patients and to take approximately two years from initiation to complete.
Richter’s Transformation
Richter’s describes the transformation from CLL to a type of NHL that is similar to DLBCL. The American Cancer Society estimates that 15,720 patients will be diagnosed with CLL in the United States in 2014. Approximately 5% to 10% of patients with CLL will experience Richter’s, which is characterized by a distinct worsening of symptoms. Although there are no specific therapies approved to treat Richter’s, multi-agent chemoimmunotherapy is typically used as a first line treatment.
The first patient in our registration-directed trial in Richter’s, a Phase 2 clinical trial known as the Selinexor In Relapsed/Refractory Richter’s Transformation, or SIRRT, study (NCT 02138786), was dosed in November 2014. This is a single-arm open-label trial in patients with CLL who experienced Richter’s and
subsequently relapsed after chemotherapy. The primary endpoints of this trial are ORR and duration of response, or DOR. The trial’s initial stage of enrollment is expected to be 21 patients, and following at least 2 ORR responders, an additional 30 patients will be enrolled in a second stage. Patients will be treated with selinexor given at a dose of 60 mg, administered orally two times per week in weeks 1-3 of each 4-week cycle, until disease progression or intolerability. The dose will be increased to 80 mg at cycle 3 day 1 unless clinically contraindicated. The SIRRT study is expected to take approximately two years from initiation to complete.
Phase 1 Clinical Trial Data in Non-Hodgkin’s Lymphoma
In our Phase 1 trial, as of December 1, 2014, 71 heavily pretreated patients with relapsed and/or refractory NHL, including DLBCL and Richter’s, and median prior treatment regimens of three were enrolled in this arm of this clinical trial. Of this group, 52 patients were evaluable and the DCR was 73% across all doses of selinexor and the ORR was 37%. Responses were observed across all subtypes of NHL, independent of genetic abnormalities, with durable cancer control observed across several patients who remained on study for longer than nine months, with the longest for 24 months. The median DOR in patients was approximately seven months as of December 1, 2014. Responses in these 52 evaluable patients are shown below.
Best Responses in NHL Patients as of December 1, 2014
| Type |
N | DCR (%) | ORR (%) | CR (%) | PR (%) | SD (%) | PD (%) | |||||||
| DLBCL |
32 | 21 (66%) | 11 (34%) | 4 (13%) | 7 (22%) | 10 (31%) | 11 (34%) | |||||||
| Richter’s |
4 | 4 (100%) | 2 (50%) | — | 2 (50%) | 2 (50%) | — | |||||||
| Other NHL |
16 | 13 (81%) | 6 (38%) | 1 (6%) | 5 (31%) | 7 (44%) | 3 (19%) | |||||||
| Total |
52 | 38 (73%) | 19 (37%) | 5 (10%) | 14 (27%) | 19 (37%) | 14 (27%) |
Among the 32 patients with heavily pretreated DLBCL who were evaluable as of December 1, 2014, ORR and DCR were nearly identical across all subtypes of DLBCL. There are two major subtypes of DLBCL, namely GCB and Activated B-Cell, or ABC, also called non-GCB. Many targeted therapies such as ibrutinib or lenalidomide show activity primarily against the ABC subtype, but relapsed/refractory GCB remains difficult to treat. However, as detailed in the table below and consistent with the broadly applicable mechanism of action of selinexor, selinexor shows activity across both major subtypes.
Best Responses in Diffuse Large B-Cell Patients as of December 1, 2014
| Type |
N | DCR (%) | ORR (%) | CR (%) | PR (%) | SD (%) | PD (%) | |||||||
| GCB |
11 | 9 (82%) | 4 (36%) | 1 (9%) | 3 (27%) | 5 (45%) | 2 (18%) | |||||||
| non-GCB |
5 | 4 (80%) | 2 (40%) | 1 (20%) | 1 (20%) | 2 (40%) | 1 (20%) | |||||||
| Unknown |
16 | 8 (50%) | 5 (31%) | 2 (13%) | 3 (19%) | 3 (19%) | 8 (50%) | |||||||
| Total |
32 | 21 (66%) | 11 (34%) | 4 (13%) | 7 (22%) | 10 (31%) | 11 (34%) |
In addition, a minority of DLBCL patients have “double-hit” disease because these tumors over-express the two oncogenes MYC and BCL2 (or BCL6). Double-hit DLBCL is particularly difficult to treat due in part to its resistance to multi-agent immunochemotherapy and many targeted agents. Of four patients with double-hit DLBCL as of December 1, 2014, there was one patient with a CR (on study 440 days and remained on study as of December 1, 2014), one patient with a PR (on study 214 days), one patient with SD (45% lymph node reduction on study 104 days) and one patient with PD (on study 57 days). We believe, together, these data and the consistent data across DLBCL subtypes indicate that selinexor has the potential to treat a broad range of subtypes of DLBCL, largely independent of the cell of origin or oncogenic drivers.
Among all NHL patients, Grade 3/4 adverse events occurring in more than three patients out of 67 included thrombocytopenia (39%), neutropenia (24%), hyponatremia (10%), fatigue (12%) and dehydration (6%). The most common Grade 1/2 adverse events were nausea (64%), anorexia (52%), fatigue (46%), vomiting
(36%) and diarrhea (23%). There has been one case of dose limiting toxicities among NHL patients as of December 1, 2014, grade 4 thrombocytopenia. The maximum tolerated dose was not reached, but based on biological activity and data from additional studies, the recommended Phase 2 or Phase 3 trial dose of oral selinexor for NHL is 60-100 mg twice weekly.
Multiple Myeloma
MM is a hematological malignancy characterized by the accumulation of monoclonal plasma cells in the bone marrow, the presence of monoclonal immunoglobulin, or M protein, in the serum or urine, bone disease, kidney disease and immunodeficiency. It is more common in elderly patients, with a median age at diagnosis of 65-70 years. In the United States, the American Cancer Society estimated that there would be approximately 24,050 new cases of MM in 2014. Approximately 114,000 cases are expected worldwide each year.
The treatment of MM has improved in the last 20 years due to the use of high-dose chemotherapy and autologous stem cell transplantation, and the subsequent introduction of the immunomodulatory agents thalidomide (Thalomid®) and lenalidomide (Revlimid®) and the proteasome inhibitor bortezomib (Velcade®). The median overall survival of MM patients, meaning the length of time an MM patient survives with the disease, has increased significantly in patients younger than 50 years old, with those patients experiencing a 10-year survival rate of around 40%, meaning that 40% of those patients are still alive after 10 years. However, despite the increased effectiveness of the first-line agents, the majority of patients will eventually relapse and become drug-resistant. Although a wide variety of new agents are being used in relapsed and/or refractory patients, including new proteasome inhibitors (carfilzomib (Kyprolis®), ixazomib, oprozomib and marizomib), immunomodulatory drugs (pomalidomide (Pomalyst®), monoclonal antibodies (elotuzumab and daratumumab), a signal transduction modulator (perifosine) and histone deacetylase inhibitors (vorinostat (Zolinza®) and panobinostat), we believe that there remains a need for therapies in these relapsed and/or refractory patients that can improve the overall survival rate.
Subject to ongoing discussions with regulatory authorities and key opinion leaders regarding trial design, we intend to initiate a Phase 2b clinical trial for selinexor in patients with MM during the first half of 2015. We currently intend for this trial to be able to serve as the basis for an application seeking regulatory approval. We expect to treat patients with “quadruple refractory” MM, meaning MM that has been treated with and is refractory to bortezomib, carfilzomib, lenalidomide and pomalidomide. We expect that patients will also have to have received prior alkylating agents (such as melphalan and/or cyclophosphamide) and glucocorticoids (such as dexamethasone) and some patients will also have received anti-CD38 monoclonal antibodies. We expect that the primary endpoint will be ORR and ORR compared to a minimally effective lower threshold of ORR (15%) and that the trial will have several secondary endpoints, including ORR in patients whose disease is relapsed/refractory to an anti-CD38 monoclonal antibody and DOR. Once initiated, this trial is expected to take approximately two and a half years to complete.
A second combination study evaluating selinexor in MM patients is ongoing. This Phase 1/2 investigator sponsored clinical trial is being conducted at the University of Chicago to evaluate tolerability and efficacy of the combination of selinexor with carfilzomib and low-dose dexamethasone. As of December 1, 2014, the best responses in the first three treated patients, all of whom have MM that is refractory to carfilzomib and dexamethasone, were one very good partial response, or VGPR, and two PRs, with good tolerability in each case. The primary objectives of the study are to determine the maximum tolerated dose and recommended Phase 2 and Phase 3 doses for selinexor in these combination therapies in the dose-escalation phase and to assess preliminary efficacy through ORR, clinical benefit rate, or CBR, and DOR in the expension phase. The dose escalation phase in this clinical study is ongoing.
In addition to the ongoing investigator sponsored trial of selinexor in combination with carfilzomib and dexamethasone, we intend to initiate a third combination trial, in this case a company sponsored Phase 1b/2 clinical trial for selinexor in combination with approved therapies to treat MM, during the first quarter of 2015. This two arm trial will evaluate selinexor in combination with dexamethasone and pomalidomide, or the SdP Arm, and selinexor in combination with dexamethasone and bortezomib, or the SdB Arm. The SdP Arm will accrue patients who received at least two prior therapies, including lenalidomide, a proteasome inhibitor, and glucocorticoids, with progression during or within 60 days of completion of last therapy (i.e., patients whose disease is refractory to their most recent therapy). The SdB Arm will accrue patients whose disease is relapsing after at least one line of therapy, and whose disease is not refractory to bortezomib in their most recent line of therapy. The primary objectives of the study are to determine the maximum tolerated dose and recommended Phase 2 and Phase 3 doses for selinexor in these combination therapies and to assess preliminary efficacy through ORR, CBR and DOR. We expect to determine the recommended Phase 2 and Phase 3 trial doses for selinexor in these combinations by the end of 2015 and the trial is expected to be complete by the end of 2016.
As part of our Phase 1 clinical trial of selinexor in patients with advanced hematological malignancies, patients with MM were treated with either single-agent selinexor or selinexor in combination with low-dose (20 mg) dexamethasone, all dosed twice weekly. As of December 1, 2014, nine evaluable patients were treated with 45 mg/m2 of oral selinexor and 20 mg of dexamethasone, each dosed twice weekly. This dose of selinexor, equivalent to approximately 80 mg, was determined to be the recommended Phase 2 and Phase 3 dose for this combination therapy as higher doses were not well tolerated and this dose of dexamethasone is the standard low dose dexamethasone (40 mg weekly or 20 mg twice weekly) used with other anti-myeloma drugs including lenalidomide or pomalidomide (and now commonly used with bortezomib and carfilzomib). The patients enrolled in this study had received a median of 7.5 prior lines of therapy, each line typically consisting of two to four separate anti-myeloma agents. All had received prior therapy with at least one proteasome inhibitor, such as carfilzomib or bortezomib, and at least one immunomodulatory agent, such as pomalidomide, steroids (typically two or more times) while nine of the ten patients also underwent high dose alkylating agent therapy (typically melphalan) with autologous stem cell transplantation. As of December 1, 2014, the best responses among the nine evaluable patients were one stringent complete response (sCR) (11%), five PRs (56%), two MRs (22%) and one PD (11%); one additional patient left the trial after two weeks due to severe infection which was not related to study drug and was therefore not evaluable for response. The clinical benefit response rate (sCR+PR+MR) was 89% and the ORR was 67%. Two of the nine responding patients remain on study as of December 1, 2014. Responses in these nine patients are described below.
Patients with Relapsed/Refractory MM Treated with Twice Weekly
Oral Selinexor 45 mg/m2 + Dexamethasone 20 mg
| MM Type |
Number of Prior therapies |
Maximal Change |
Response | Study Days | ||||
| IgG- k |
7 | -71% | PR | 301+ | ||||
| FLC- k |
3 | -53% | PR | 52 | ||||
| FLC- k |
5 | -99% | sCR | 280 | ||||
| IgG- k |
9 | -84% | PR | 170 | ||||
| IgG- k |
5 | 41% | PD | 31 | ||||
| IgA- k |
10 | -55% | PR | 121 | ||||
| IgG- k |
9 | -41% | MR | 114 | ||||
| IgG- l |
16 | -48% | MR | 79 | ||||
| IgA- k |
6 | -82% | PR | 201+ |
| + | patients remain on study as of December 1, 2014 |
Adverse events in patients receiving single-agent selinexor were generally low-grade, consistent with events observed in patients with other hematological malignancies and responsive to standard supportive care. Compared with selinexor given alone, fewer adverse events in patients receiving selinexor in combination with dexamethasone were reported, particularly levels of nausea, vomiting and weight loss consistent with dexamethasone’s reduction in selinexor’s main side effects of nausea, anorexia and fatigue.
In addition to patients with MM achieving durable responses and disease control on selinexor single-agent therapy, selinexor with low dose dexamethasone showed activity with rapid M-protein reductions and good tolerability, even in patients with disease refractory to pomalidomide and/or carfilzomib. We believe that selinexor may have synergistic potential with other therapies.
Acute Myeloid Leukemia
AML in elderly populations remains a vexing clinical problem. AML is a cancer that starts in the bone marrow and in most cases quickly moves into the blood. The incidence of AML dramatically increases after the age of 55. The American Cancer Society estimates that approximately 18,860 new cases of AML, most of which will be in adults, will be diagnosed in the United States annually with approximately 7,330 deaths. About 40% of AML patients are young enough and fit enough to undergo bone marrow transplantation for their AML, and about 50% of these patients can be cured of their disease. Those that are not cured, and patients who are elderly or unfit for transplant, have a very poor prognosis. The median survival for elderly patients with AML is less than a year and worsens continuously with advancing age to as low as one month for those who are older than 85 years of age.
Over the past two decades, many compounds have been evaluated in elderly patients with AML, but due to significant toxicities and/or lack of efficacy, none has been approved to date.
In June 2014, we dosed the first patient in our registration-directed Phase 2 study of selinexor in patients 60 years of age or older with relapsed or refractory AML who are ineligible for standard intensive chemotherapy and/or transplantation. In this trial, known as the SOPRA, or Selinexor in Older Patient with Relapsed/Refractory AML, study (NCT 02088541), we are evaluating approximately 150 patients with AML which has relapsed after, or was refractory to, first line therapy. Patients are randomized in a 2:1 fashion to selinexor provided orally twice weekly in a dose of 55 mg/m2 plus BSC versus one of three physician choices. Physician choices include (i) best supportive care, or BSC, alone, (ii) BSC plus either azacytidine (Vidaza®), decitabine (Dacogen®), or (iii) BSC plus low dose cytosine arabinoside (LD-AraC). Overall survival is the primary endpoint. The SOPRA study was designed based on data from the ongoing Phase 1 study of selinexor in patients with advanced hematologic malignancies, including AML. The SOPRA study is expected to take approximately two years from initiation to complete.
Sixty-five heavily pretreated patients with progressive, relapsed and/or refractory AML, most with three or more prior treatment regimens, were enrolled in our Phase 1 clinical trial in hematological malignancies as of May 13, 2014. These patients were dosed 16.8-70 mg/m2 of selinexor in a four-week cycle, with lower doses initially given ten times per cycle and higher doses given twice weekly. Of these 65 patients, two patients had tumors pending evaluation, and among the 63 other patients, the complete response rate with or without full hematologic recovery was 11%, the ORR was 16% and the DCR was 49%; 16 (25%) of the 63 patients with AML were non-evaluable but were included in the AML response rate calculation. Responses were observed across multiple genetic subtypes of AML. Higher doses of selinexor were associated with greater reductions in bone marrow blast counts, which were also observed across different AML subtypes. Responses in the 63 evaluable patients are shown below. As of December 1, 2014, there had been no material changes or adverse trends observed in the evaluable AML patient responses.
Best Responses in AML Patients as of May 13, 2014
| N |
DCR (%) |
ORR (%) |
CR (%) |
CR(i/p) (%) |
PR (%) |
MLFS (%) |
SD (%) |
PD (%) |
NE (%) | |||||||||
| 31 | 10 | 5 | 2 | 1 | 2 | 21 | 16 | 16 | ||||||||||
| 63 |
(49%) | (16%) | (8%) | (3%) | (2%) | (3%) | (33%) | (25%) | (25%) |
Grade 3/4 adverse events occurring in more than three patients included fatigue (18%), thrombocytopenia (15%), neutropenia (11%), and nausea (8%). The most common Grade 1/2 adverse events were diarrhea (82%), anorexia (78%), nausea (74%), and fatigue (65%). There have been no dose limiting toxicities in AML patients as of May 13, 2014 and the maximum tolerated dose is ³ 70mg/m2 twice weekly. The intended Phase 2 or Phase 3 trial dose of oral selinexor for AML is 60-80 mg twice weekly.
Advanced or Metastatic Solid Tumor Malignancies
The International Agency for Research on Cancer estimates that approximately 12.1 million adults were diagnosed with solid tumor malignancies in 2012. Based on preclinical data and our Phase 1 clinical data in heavily pretreated patients with progressive tumors, we initiated four Phase 2 trials in solid tumor indications: gynecologic malignancies (SIGN study), glioblastoma (KING study), prostate adenocarcinoma (SHIP study) and head and neck squamous cell carcinoma (STARRS study). We expect to report preliminary data from some of these studies at various scientific conferences throughout 2015.
During 2014, we reported data from our ongoing Phase 1 clinical trial of selinexor in patients with advanced or metastatic solid tumor malignancies. All patients entered the study with advanced or metastatic solid tumor cancers relapsed or refractory after multiple previous treatments and objectively progressing on study entry. The primary objectives of the Phase 1 dose escalation trial are to determine the safety, tolerability and recommended Phase 2 dose of orally administered selinexor. These patients were dosed 3-85 mg/m2 (equivalent to approximately 5-145 mg) of oral selinexor over a four-week cycle, with lower doses initially given ten times per cycle and higher doses given twice weekly. Response evaluation was done every two cycles in accordance with RECIST.
As of May 13, 2014, 129 patients were enrolled in this Phase 1 clinical trial. Enrolled patients had received a median of 3 prior therapies. Of these patients, 106 were evaluable and 23 patients were non-evaluable or pending evaluation as of May 13, 2014. Of these evaluable patients, the DCR was 49%. PRs were observed in four patients, one each with colorectal cancer (KRAS mutant), melanoma (BRAF wild type), ovarian adenocarcinoma and cervical cancer. SD was noted in 47 patients, with 17 patients (16%) experiencing SD for six months or longer. Eight of nine evaluable patients with hormone and chemotherapy refractory prostate cancer achieved stable disease and have remained on study for between 70 and 317 days. Among 14 evaluable patients with head and neck cancer, nine achieved stable disease with eight on study for 75 to over 401 days. Responses in 106 evaluable patients are shown below. As of December 1, 2014, there had been no material changes or adversetrends observed in the evaluable solid tumor patient responses.
Best Responses in Solid Tumor Patients as of May 13, 2014
| Cancer Type |
N | DCR (%) |
PR (%) | SD (%) | PD (%) | |||||
| Colorectal |
39 | 14 (36%) | 1 (3%) | 13 (33%) | 25 (64%) | |||||
| Head & Neck |
14 | 9 (64%) | — | 9 (64%) | 5 (36%) | |||||
| Prostate |
8 | 7 (88%) | — | 7 (88%) | 1 (12%) | |||||
| Cervical |
5 | 4 (80%) | 1 (20%) | 3 (60%) | 1 (20%) | |||||
| Ovarian |
5 | 3 (60%) | 1 (20%) | 2 (40%) | 2 (40%) | |||||
| GBM |
5 | — | — | — | 5 (100%) | |||||
| Melanoma |
3 | 2 (67%) | 1 (33%) | 1 (33%) | 1 (33%) | |||||
| Sarcoma |
8 | 7 (88%) | — | 7 (88%) | 1 (12%) | |||||
| Other |
19 | 6 (32%) | — | 6 (32%) | 13 (68%) | |||||
|
|
|
|
|
| ||||||
| Total |
106 | 52 (49%) | 4 (4%) | 48 (45%) | 54 (51%) | |||||
|
|
|
|
|
|
In addition to the response and disease stabilization summarized above across a variety of different cancer types, biopsies of tumors prior to and 3-5 weeks after initiation of selinexor treatment are available from some of the patients. These biopsies have confirmed the nuclear localization of tumor suppressor proteins and induction of cancer cell death (i.e., apoptosis) following selinexor treatment across multiple different cancer types. Given the broadly applicable, unique and confirmed mechanism of action of selinexor, we believe that selinexor has the potential to contribute to the treatment of a large variety of cancers. Consistent with this potential, in preclinical models, oral selinexor has shown broad single agent and synergistic or additive activity in combination with many different anti-cancer agents. Therefore, we are initiating a variety of company- and investigator- sponsored combination studies across multiple cancer types. We anticipate that these trials could inform the use of selinexor in earlier stages of cancer therapy across a variety of indications.
Side effects were generally low grade and typically gastrointestinal in nature, or fatigue. These common side effects decreased over time, in part due to prophylactic use of standard supportive care. Two dose-limiting toxicities (both reversible) were observed in solid tumor patients receiving 85 mg/m2 dosed twice weekly. Grade 3/4 adverse events occurring in six or more patients in the first cycle included hyponatremia (8%), fatigue (7%), and thrombocytopenia (7%). The most common Grade 1/2 adverse events in the first cycle were nausea (62%), fatigue (52%), anorexia (48%) and vomiting (35%). One was Grade 3 asymptomatic hyponatremia and the other was acute cerebellar syndrome that reversed over several weeks with markedly improving ataxia and dysarthria. No other similar central nervous system toxicities were observed in any other patients receiving selinexor to date. Major organ dysfunction or clinically significant cumulative toxicities have not been observed. The intended Phase 2 or Phase 3 trial dose of oral selinexor in solid tumors is 60-80 mg twice weekly.
Combination Therapy
Approximately 14.1 million adults worldwide were diagnosed with cancer in 2012, according to the International Agency for Research on Cancer. Based on its mechanism of action and supported by preclinical and clinical data, we believe that selinexor has the potential to be additive or synergistic with approved and experimental therapies in treating many of these patients. As a result, we believe that selinexor has the potential to serve as backbone therapy across multiple hematological and solid tumor malignancies as part of a variety of combination therapies. In addition to our company-sponsored combination studies in MM, we are evaluating several combination therapies across various indications, largely through ISTs. These include selinexor in combination with:
| • | paclitaxel and arboplatin in advanced ovarian or endometrial malignancies; |
| • | irinotecan in adenocarcinoma of stomach and distal esophagus; |
| • | low dose ara-C (LDAC) in AML; |
| • | decitabine in AML; |
| • | Ara-C and idarubicin in AML; |
| • | carfilzomib and dexamethasone in MM; |
| • | fludarabine and cytarabine in pediatric leukemia or myelodysplastic syndrome; |
| • | pegylated liposomal doxorubicin in MM; and |
| • | bortezomib and dexamethasone for induction and consolidation in MM. |
Data from these studies may be presented by our collaborators at scientific conferences or through other publications at various times. We expect such data will inform our Phase 2 and Phase 3 dosing for selinexor in these combinations and allow us to evaluate the combinations with the greatest potential for durable responses and increased survival.
Our Strategy
As a clinical-stage pharmaceutical company focused on the discovery and development of orally available, novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases, the critical components of our business strategy are to:
| • | Develop and seek regulatory approval of selinexor, our lead drug candidate, in North America and Western Europe. |
| • | Maximize the commercial value of selinexor in key geographies for hematological indications. |
| • | Maintain our competitive advantage and scientific expertise in the field of nuclear transport. |
| • | Develop novel drug candidates by leveraging our proprietary drug discovery and optimization platform and our understanding of nuclear transport. |
| • | Collaborate with key opinion leaders to conduct ISTs of selinexor. |
| • | Maximize the value of our other SINE compounds in non-oncology indications and veterinary oncology through collaborations. |
Company Information
We were incorporated under the laws of the State of Delaware in December 2008. Our executive offices are located at 85 Wells Avenue, 2nd Floor, Newton, Massachusetts 02459, and our telephone number is (617) 658-0600.
