Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - Lipocine Inc. | v395316_ex99-2.htm |
| 8-K - FORM 8-K - Lipocine Inc. | v395316_8-k.htm |

Corporate Presentation December 2014 Exhibit 99.1

2 This presentation contains and our discussions during this conference may include forward - looking statements about Lipocine Inc. (the “Company”). These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward - looking statements relate to the Company’s product candidates, clinical and regula tory processes and objectives, potential benefits of the Company’s product candidates, intellectual property and related matters, all of which involve known and unknown risks and uncertainties. Actual results may differ materially from the forward - looking statemen ts discussed in this presentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials, the potential advantages of the Company’s product candidates and the Company’s capital needs. Among other things, the projected commencement and completion of the Company’s clinical trials may be affected by difficulties or delays. In addition, the Com pan y’s results may be affected by its ability to manage its financial resources, difficulties or delays in developing manufacturing pro cesses for its product candidates, preclinical and toxicology testing and regulatory developments. Delays in clinical programs, whe the r caused by competitive developments, adverse events, patient enrollment rates, regulatory issues or other factors, could adver sel y affect the Company’s financial position and prospects. Prior clinical trial program designs and results are not necessarily pre dictive of future clinical trial designs or results. If the Company’s product candidates do not meet safety or efficacy endpoints in cl inical evaluations, they will not receive regulatory approval and the Company will not be able to market them. The Company may not b e able to enter into any strategic partnership agreements. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corporate priorities, the timing and outcomes of cli nical trials, competitive developments and the impact on expenditures and available capital from licensing and strategic collaborat ion opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to sig nif icantly delay, scale back or discontinue one or more of its drug development or discovery research programs. The Company is at an ea rly stage of development and may not ever have any products that generate significant revenue. The forward - looking statements contained in this presentation are further qualified by the detailed discussion of risks and uncertainties set forth in the d ocu ments filed by the Company with the Securities and Exchange Commission, all of which can be obtained on the Company’s website at www.lipocine.com or on the SEC website at www.sec.gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations. Forward Looking Statements

3 Lipocine Corporate Highlights Focused on innovative products for men’s and women’s health • “Transformative“ oral testosterone franchise • Oral alternative for the prevention of pre - term birth Technology platform capable of “multiple hits” Lead asset in Phase 3 • Positive top - line efficacy results in Phase 3 clinical study • Safety extension of Phase 3 clinical study ongoing Several near term value drivers with pipeline products
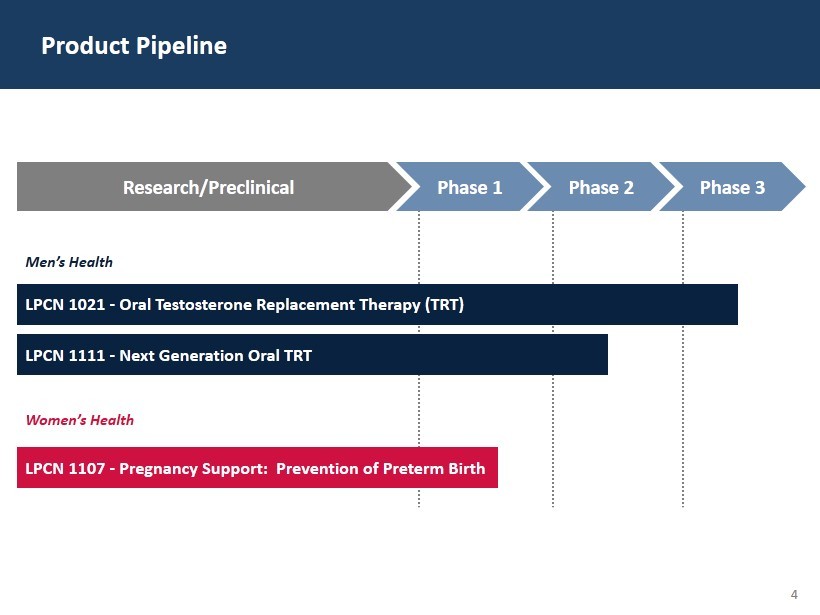
4 Product Pipeline LPCN 1021 - Oral Testosterone Replacement Therapy (TRT) Men’s Health LPCN 1111 - Next Generation Oral TRT Women’s Health LPCN 1107 - Pregnancy Support: Prevention of Preterm Birth Research/Preclinical Phase 1 Phase 2 Phase 3
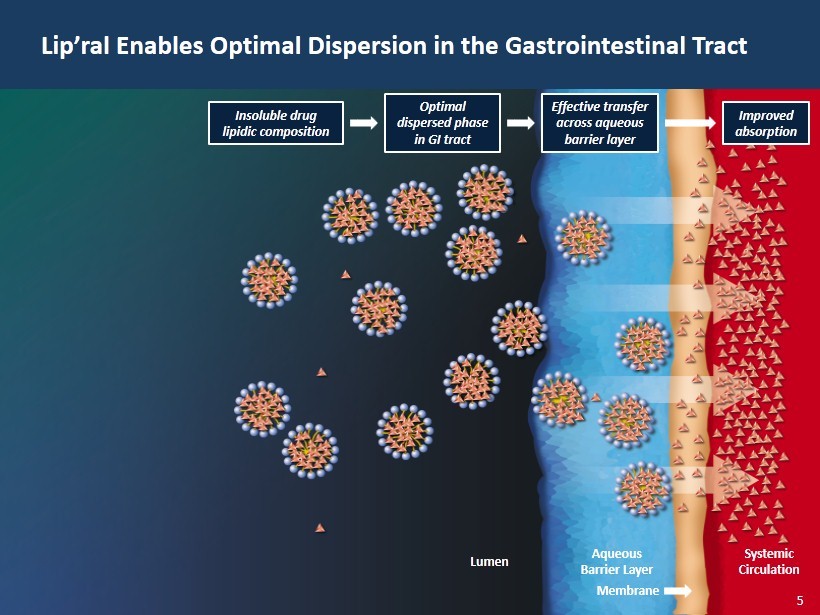
5 Lip’ral Enables Optimal Dispersion in the Gastrointestinal Tract Lumen Aqueous Barrier Layer Membrane Systemic Circulation Improved absorption Effective transfer across aqueous barrier layer Optimal dispersed phase in GI tract Insoluble drug lipidic composition 5
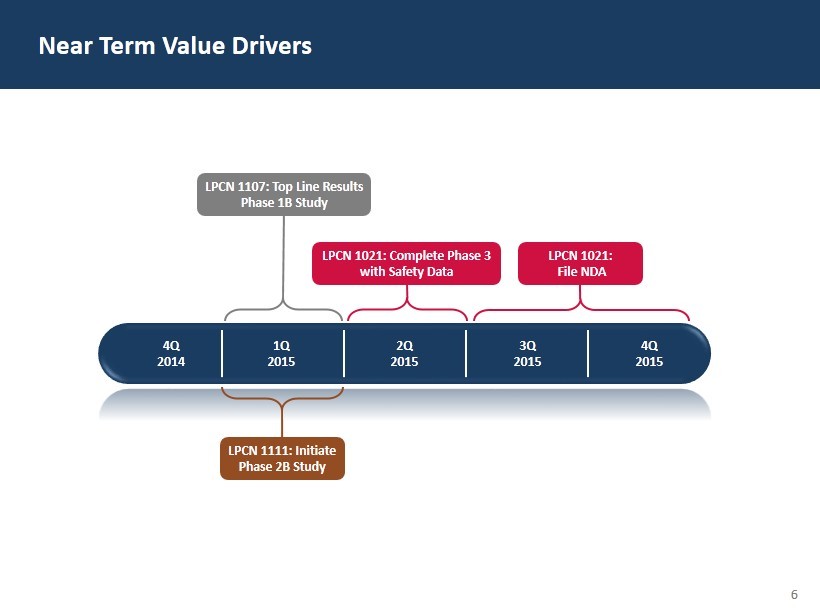
6 Near Term Value Drivers 4Q 2014 1 Q 2015 2 Q 2015 3 Q 2015 4Q 2015 LPCN 1021: Complete Phase 3 with Safety Data LPCN 1021: File NDA LPCN 1111: Initiate Phase 2B Study LPCN 1107: Top Line Results Phase 1B Study
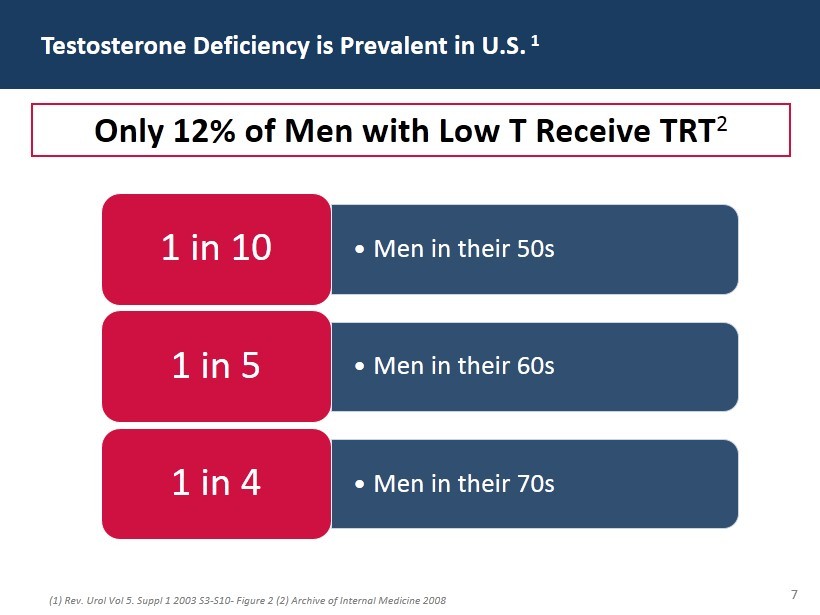
7 Testosterone Deficiency is Prevalent in U.S. 1 (1) Rev. Urol Vol 5. Suppl 1 2003 S3 - S10 - Figure 2 (2) Archive of Internal Medicine 2008 • Men in their 50s 1 in 10 • Men in their 60s 1 in 5 • Men in their 70s 1 in 4 Only 12% of Men with Low T Receive TRT 2

8 US market > $2.3B in 2013 1 International markets for TRT growing Current TRT “class label” under review by FDA Topicals dominate the market (~89 % of $ sales) • Inadvertent transference issue, black box warning • Poor compliance: 86 % discontinue at 12 months 1 Existing testosterone treatments are inadequate Testosterone Replacement Therapy Market 1. IMS data

9 TRT Advisory Committee Meeting Topics for Discussion • Appropriate population for testosterone replacement therapy • Potential for adverse cardiovascular outcomes associated with TRT Recommendations • Voted in favor of revising the current label for testosterone therapy • Recommended that companies be required to conduct a study to assess the potential cardiovascular risk only for certain indications

10 EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) review • Did not find consistent evidence that T use increases the risk of heart problems • Benefits of testosterone continue to outweigh its risks • Recommended that testosterone use should only occur after the lack of testosterone has been confirmed by signs and symptoms as well as laboratory tests • Suggested that the lack of testosterone itself could increase the risk of heart problems Recommended label changes by PRAC on 10/10/2014 • TRT should be prescribed only after confirming signs and symptoms as well as laboratory tests • Warnings against use in men suffering from severe heart, liver or kidney problems • Limited data on safety and effectiveness in patients over 65 years of age as well as the fact that testosterone levels decrease with age and that age - specific testosterone reference values do not exist will be highlighted in the product information Potential US Label Revision: Recent EU Recommendation
11 Novel product primarily directed at lymphatic delivery of Testosterone Undecanoate (TU) Enhanced profile over other forms of testosterone (T) • Methyl T is known to have liver safety issues • Natural T has short half life Maintains effective T blood levels when dosed BID Target label — consistent with TRT class label for other approved products LPCN 1021: Oral Treatment Option for TRT

12 Current Regulatory Paradigm 1 Meet primary end points • Responder analysis relating to average serum concentration 0 to 24 hours ( C avg ) and lower bound Confidence Interval (CI) Acceptable safety profile • Responder analysis relating to peak testosterone levels that is consistent with approved products • Long term safety data (at least 100 subjects, 1 year) 2 LPCN 1021 Target Success Criteria : (1) September 18, 2014 Joint Meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee Meeting, (2) ICH E1 guidance document “ The extent of population exposure to assess clinical safety for drugs intended for long - term treatment of non - life - threatening conditions”
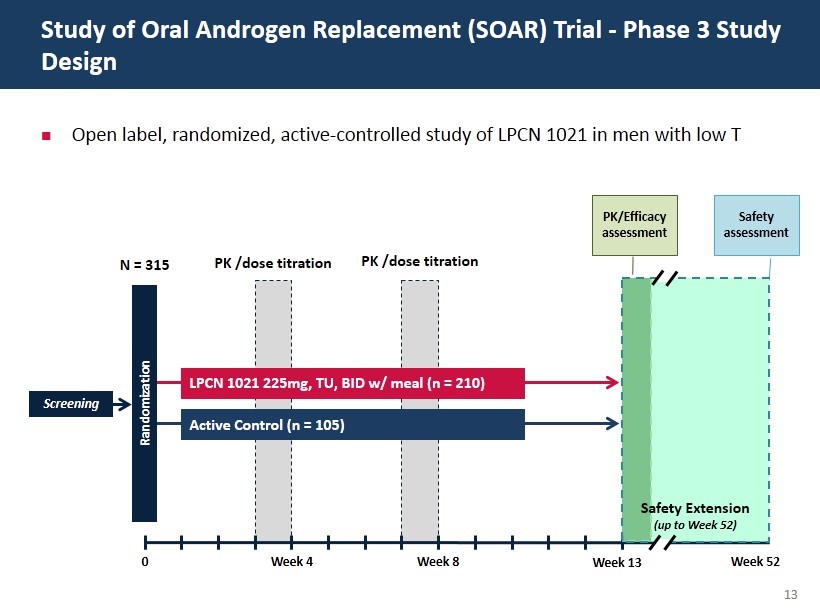
13 Open label, randomized, active - controlled study of LPCN 1021 in men with low T Study of Oral Androgen Replacement (SOAR) Trial - Phase 3 Study Design Screening N = 315 0 Week 4 Week 8 Randomization LPCN 1021 225mg, TU, BID w/ meal (n = 210) Active Control (n = 105) PK /dose titration PK /dose titration PK/Efficacy assessment Safety assessment Week 13 Week 52 Safety Extension (up to Week 52)
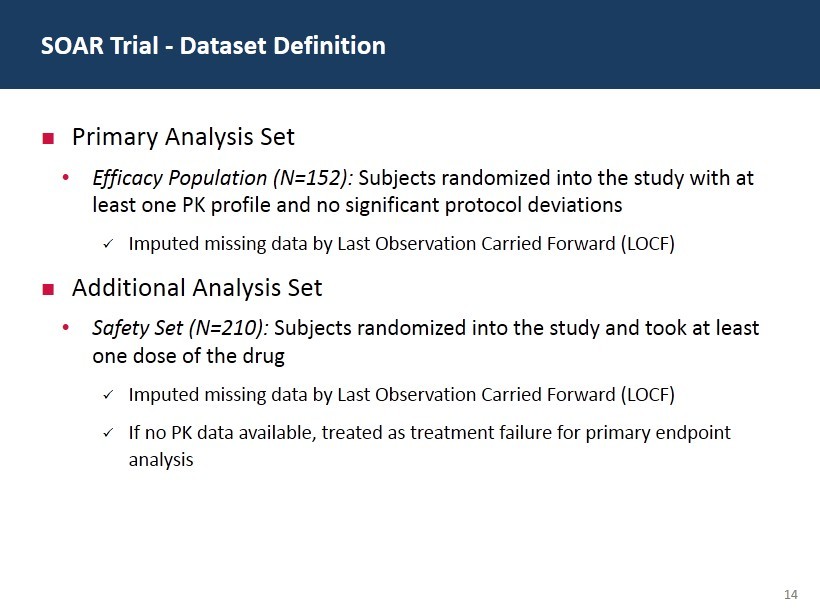
14 Primary Analysis Set • Efficacy Population (N=152): Subjects randomized into the study with at least one PK profile and no significant protocol deviations x Imputed missing data by Last Observation Carried Forward (LOCF) Additional Analysis Set • Safety Set (N=210): Subjects randomized into the study and took at least one dose of the drug x Imputed missing data by Last Observation Carried Forward (LOCF) x If no PK data available, treated as treatment failure for primary endpoint analysis SOAR Trial - Dataset Definition

15 SOAR Trial - Primary Endpoint: Responder Analysis and C avg Measure Efficacy Population Safety Set Number of subjects 152 210 % subjects with C avg w ithin normal range 88.2% 80.0% 95 % CI lower bound 81.9% 73.8% Parameter Mean (%CV) C avg ( ng / dL ) 447 (37%) Primary endpoint target ≥ 75% subjects should achieve C avg within normal range (300 ng/dL to 1140 ng/dL) and lower bound of 95% CI ≥ 65% x LPCN 1021 met both the primary endpoint targets in both population sets x Less than 12% of the subjects’ C avg were outside the normal range
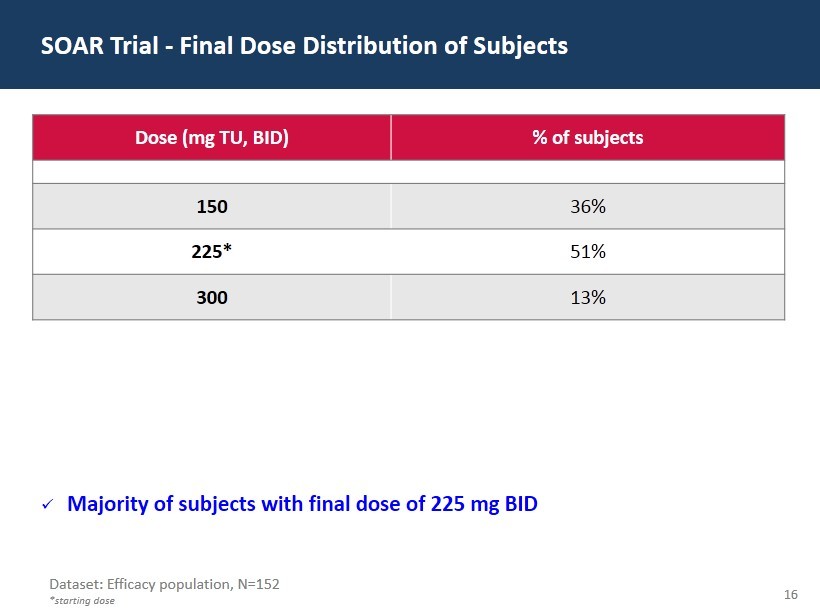
16 Dose (mg TU, BID) % of subjects 150 36% 225* 51% 300 13% SOAR Trial - Final Dose Distribution of Subjects Dataset: Efficacy population, N=152 *starting dose x Majority of subjects with final dose of 225 mg BID
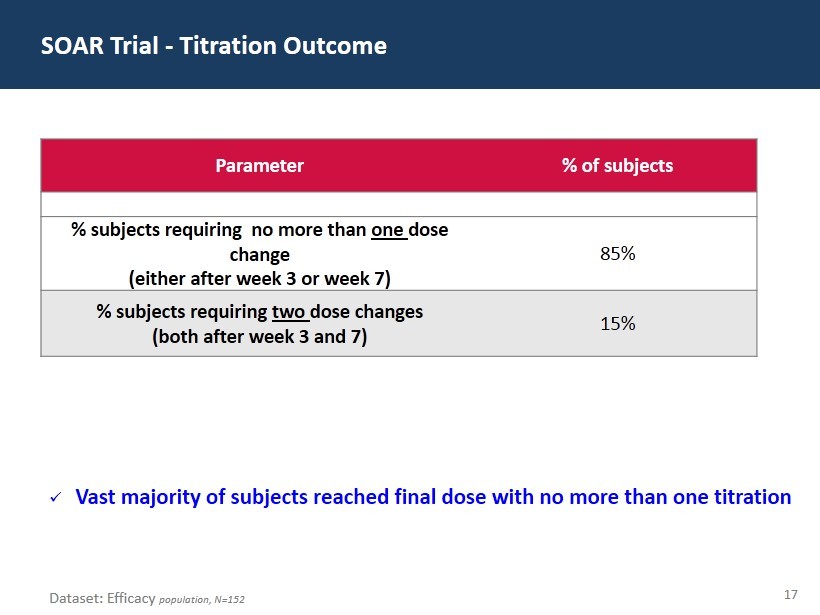
17 SOAR Trial - Titration Outcome Dataset: Efficacy population, N=152 Parameter % of subjects % subjects requiring no more than one dose change (either after week 3 or week 7) 85% % subjects requiring two dose changes (both after week 3 and 7) 15% x Vast majority of subjects reached final dose with no more than one titration
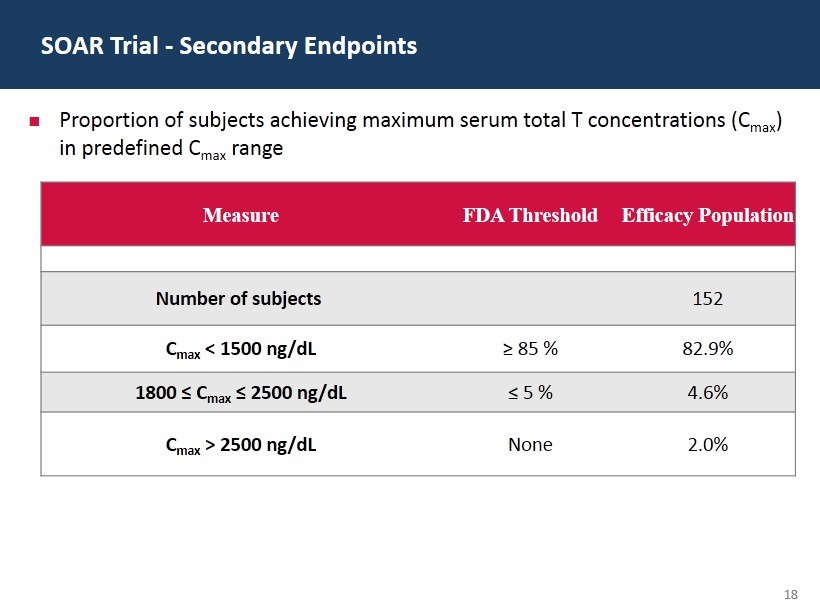
18 SOAR Trial - Secondary Endpoints Measure FDA Threshold Efficacy Population Number of subjects 152 C max < 1500 ng/ dL ≥ 85 % 82.9% 1800 ≤ C max ≤ 2500 ng/ dL ≤ 5 % 4.6% C max > 2500 ng/ dL None 2.0% Proportion of subjects achieving maximum serum total T concentrations (C max ) in predefined C max range

19 Excessive T repletion outliers were observed in three subjects These observed excessive T repletion outliers were transient, sporadic, isolated, and not clinically meaningful • Lack of dose or dosing time dependency • None of these subjects reported any adverse events SOAR Trial - LPCN 1021 Excessive T Repletion Outliers ( C max >2500 ng / dL ) Characteristics Dataset: Efficacy population , N=152

20 Safety study is on - going 3% of the subjects reported a serious adverse event • There were no drug related Serious AEs 46% of the subjects experienced at least one adverse event • Approximately one third of these events were drug related • All these adverse events were either mild or moderate (none were severe) Hematocrit and PSA increases were noted and consistent with other TRT products • 1 subject discontinued due to increase in PSA • 1 subject discontinued due to increase in hematocrit SOAR Trial - Adverse Events (AE) Summary in LPCN 1021 Arm

21 Unequivocally met primary efficacy end point Patient friendly dosing regimen • No more than one titration required in 85% of the subjects • Starting dose was the right dose for majority of the subjects Variability consistent with or better than approved products Consistent intra/inter day performance Proportion of subjects with maximum serum concentrations generally consistent with approved products To date LPCN 1021 treatment was well tolerated with no drug related serious adverse events SOAR Trial – LPCN 1021 Top Line Phase 3 Results Summary

22 Survey of 28 leading endocrinologists and urologists about oral testosterone compliance 1 • In your opinion, will oral testosterone improve patient compliance compared to existing options? Oral T is expected to increase per patient revenue through better retention rates Oral Expected to Improve Compliance and Patient Retention 94% 0% 6% Yes No Not Sure (1) Lipocine Survey 2014
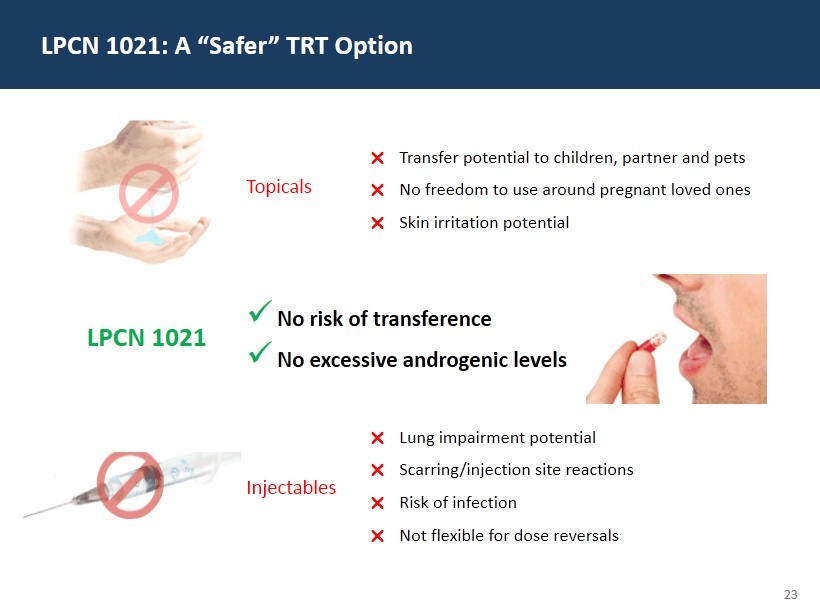
23 LPCN 1021: A “Safer” TRT Option Transfer potential to children, partner and pets No freedom to use around pregnant loved ones Skin irritation potential Lung impairment potential Scarring/injection site reactions Risk of infection Not flexible for dose reversals x No risk of transference x No excessive androgenic levels Topicals Injectables LPCN 1021
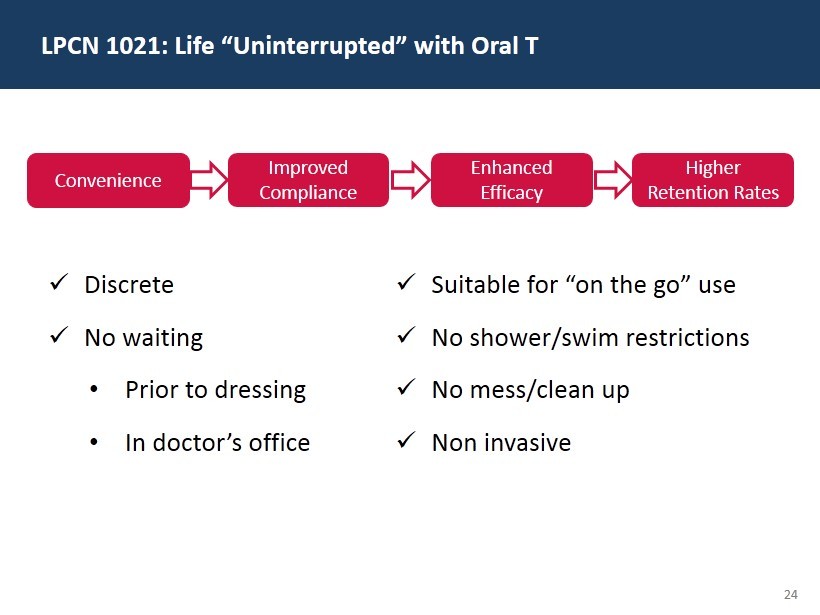
24 LPCN 1021: Life “Uninterrupted” with Oral T x Discrete x No waiting • Prior to dressing • In doctor’s office x Suitable for “on the go” use x No shower/swim restrictions x No mess/clean up x Non invasive Improved Compliance Convenience Enhanced Efficacy Higher Retention Rates
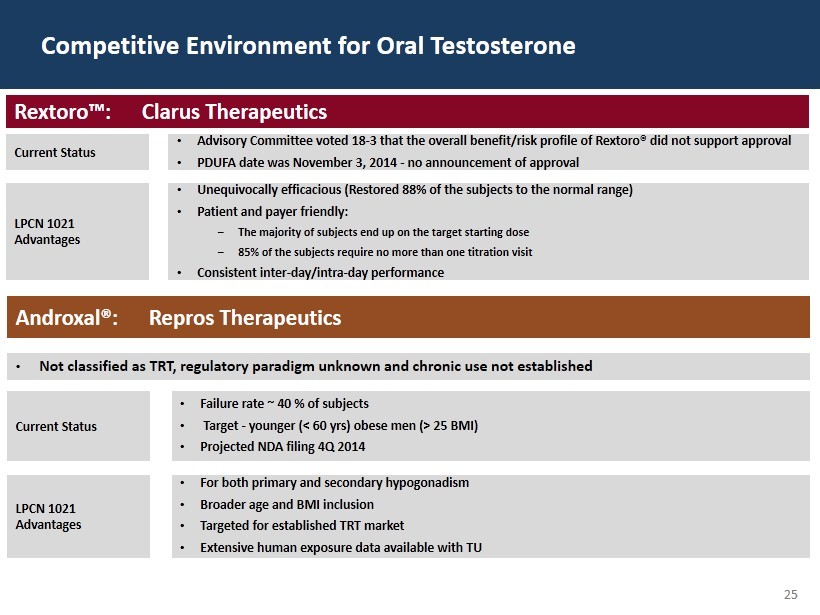
25 Competitive Environment for Oral Testosterone Rextoro™: Clarus Therapeutics Current Status • Advisory Committee voted 18 - 3 that the overall benefit/risk profile of Rextoro® did not support approval • PDUFA date was November 3, 2014 - no announcement of approval LPCN 1021 Advantages • Unequivocally efficacious (Restored 88% of the subjects to the normal range) • Patient and payer friendly: ‒ The majority of subjects end up on the target starting dose ‒ 85% of the subjects require no more than one titration visit • Consistent inter - day/intra - day performance Androxal®: Repros Therapeutics • Not classified as TRT, regulatory paradigm unknown and chronic use not established Current Status • Failure rate ~ 40 % of subjects • Target - younger (< 60 yrs) obese men (> 25 BMI) • Projected NDA filing 4Q 2014 LPCN 1021 Advantages • For both primary and secondary hypogonadism • Broader age and BMI inclusion • Targeted for established TRT market • Extensive human exposure data available with TU

26 Novel prodrug of testosterone for oral delivery through proprietary Lip’ral technology Maintains effective T blood levels when dosed once daily Status • Positive Phase 2a study results in hypogonadal men – Single daily oral dose provides T levels in the eugonadal range – No subject exceeded peak T levels of 1500 ng / dL • Initiate Phase 2b study - 1Q 2015 LPCN 1111: Next Generation Oral Testosterone
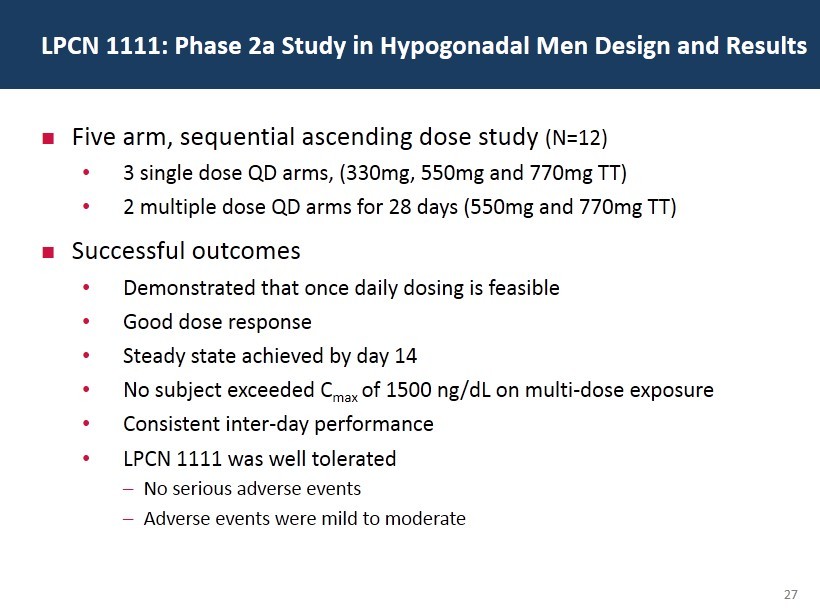
27 Five arm, sequential ascending dose study (N=12) • 3 single dose QD arms, (330mg, 550mg and 770mg TT) • 2 multiple dose QD arms for 28 days (550mg and 770mg TT) Successful outcomes • Demonstrated that once daily dosing is feasible • Good dose response • Steady state achieved by day 14 • No subject exceeded C max of 1500 ng / dL on multi - dose exposure • Consistent inter - day performance • LPCN 1111 was well tolerated – No serious adverse events – Adverse events were mild to moderate LPCN 1111: Phase 2a Study in Hypogonadal Men Design and Results
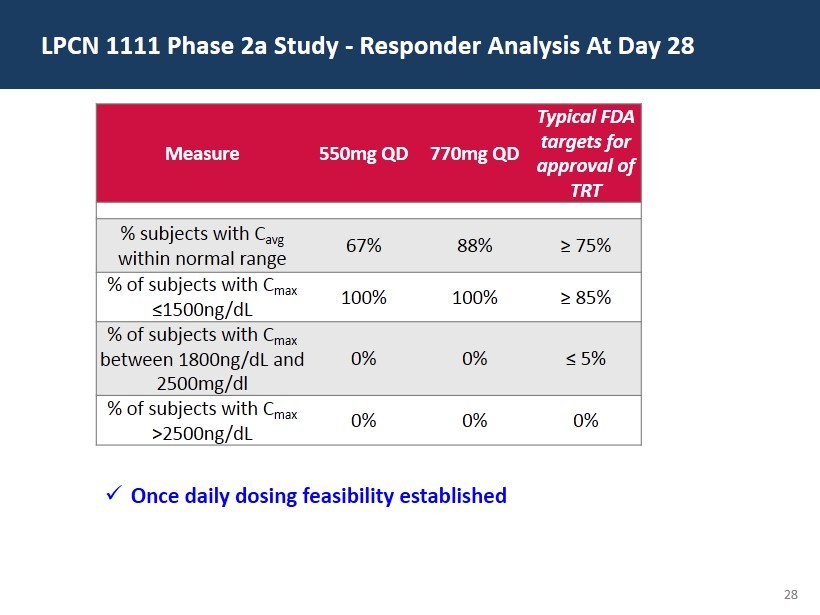
28 LPCN 1111 Phase 2a Study - Responder Analysis At Day 28 Measure 550mg QD 770mg QD Typical FDA targets for approval of TRT % subjects with C avg w ithin normal range 67% 88% ≥ 75% % of subjects with C max ≤ 1500ng/ dL 100% 100% ≥ 85% % of subjects with C max between 1800ng/ dL and 2500mg/dl 0% 0% ≤ 5% % of subjects with C max >2500ng/ dL 0% 0% 0% x Once daily dosing feasibility established

29 Preterm Birth (PTB) Represents a Significant Unmet Medical Need One Preterm Infant per Minute in the US 1 □ 11.7% of all US pregnancies 2 result in PTB - a leading cause of mortality and morbidity 3 □ ~10x more first year medical costs are for PTB infants than for full term infants 4 □ ≥ $26 billion economic impact: 4 $1 billion market opportunity 5 1 . Pediatric Research (2006) 60, 775 – 776; 2 . CDC (2010); 3 . J. Maternal - Fetal and Neonatal Medicine, Dec. 2006, 19(12), 773 – 782; 4 . Institute of Medicine of the National Academies. Jul.2006; 5 . AMAG Pharmaceuticals presentation 09/29/2014

30 Potential to be the first oral standard - of care therapy • Same API as in the only approved injectable product • Elimination of 18 - 22 injections • Potential for orphan drug designation Status • Successful Phase 1a POC study • Phase 1b pharmacokinetic study in pregnant women ongoing – Top line data expected 1Q 2015 LPCN 1107 : Oral Candidate for Prevention of Preterm B irth

31 Randomized cross - over design(N=10) • Single dose, 400 mg LPCN 1107 • Two doses, 400 mg LPCN 1107 administered 12 hours apart • Single dose of 250 mg IM HPC Successful outcomes • Significant absorption with good dose response upon oral dosing of LPCN 1107 • LPCN 1107 oral steady state 400mg BID exposure is about 55% of weekly 250mg IM product – Higher oral dose likely to match IM exposure • LPCN 1107 was well tolerated Abstract accepted for presentation at Society of Maternal Fetal Medicine meeting in February 2015 LPCN 1107: Phase 1a Study in Healthy Non - Pregnant Females Design and Results
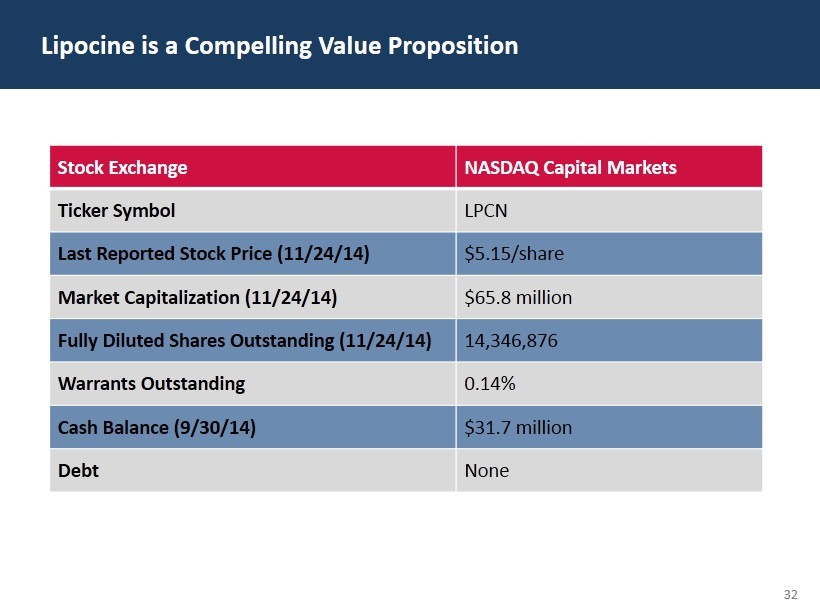
32 Lipocine is a Compelling Value Proposition Stock Exchange NASDAQ Capital Markets Ticker Symbol LPCN Last Reported Stock Price (11/24/14) $5.15/share Market Capitalization (11/24/14) $65.8 million Fully Diluted Shares Outstanding (11/24/14) 14,346,876 Warrants Outstanding 0.14% Cash Balance (9/30/14) $31.7 million Debt None
33 Lipocine Corporate Highlights Focused on innovative products for men’s and women’s health • “Transformative“ oral testosterone franchise • Oral alternative for the prevention of pre - term birth Technology platform capable of “multiple hits” Lead asset in Phase 3 • Positive top - line efficacy results in Phase 3 clinical study • Safety extension of Phase 3 clinical study ongoing Several near term value drivers with pipeline products
