Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - HEAT BIOLOGICS, INC. | htbx_8k.htm |

Corporate Presentation November 2014

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer stem cells, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2013 and our quarterly report on Form 10-Q for the quarter ended March 31, 2014, June 30, 2014 and September 30, 2014 (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully our “Special Cautionary Notice Regarding Forward-Looking Statements” and the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. *

Heat Biologics (NASDAQ: HTBX): Company Snapshot Clinical-stage immunotherapy company developing fully allogeneic “off-the-shelf” cell-based cancer therapiesFounded in 2008; completed IPO in 2013Headquarters in Research Triangle Park area * *As of market close on Nov 14, 2014**Does not include $7.5 million milestone-based debt facility Shares Outstanding 6,452,341 Share Price* $4.44 Outstanding Shares 6.48M Market Capitalization* ~$28.7M Cash as of 9/30/14** $15.4M Enterprise Value* ~$13.3M

Investment Highlights Novel, “off-the-shelf” ImPACT therapy to combat a wide range of cancers39 patients dosed* in two phase 1 and two phase 2 trials with positive safety dataTwo product candidates in ongoing multicenter Phase 2 clinical trials:Non-Small Cell Lung Cancer (NSCLC): HS-110 (Viagenpumatucel-L)Bladder Cancer: HS-410 (Vesigenurtacel-L)Combination trial approach leverages ImPACT cost advantagesMultiple, fully-funded near-term catalystsProduct candidates target large markets with significant unmet medical needsDiverse strategic partnership and collaboration opportunities Broad, international intellectual property portfolioExperienced management team and world-renowned advisory boardsCash runway projected through the first quarter 2016 * *As of 11/14/14
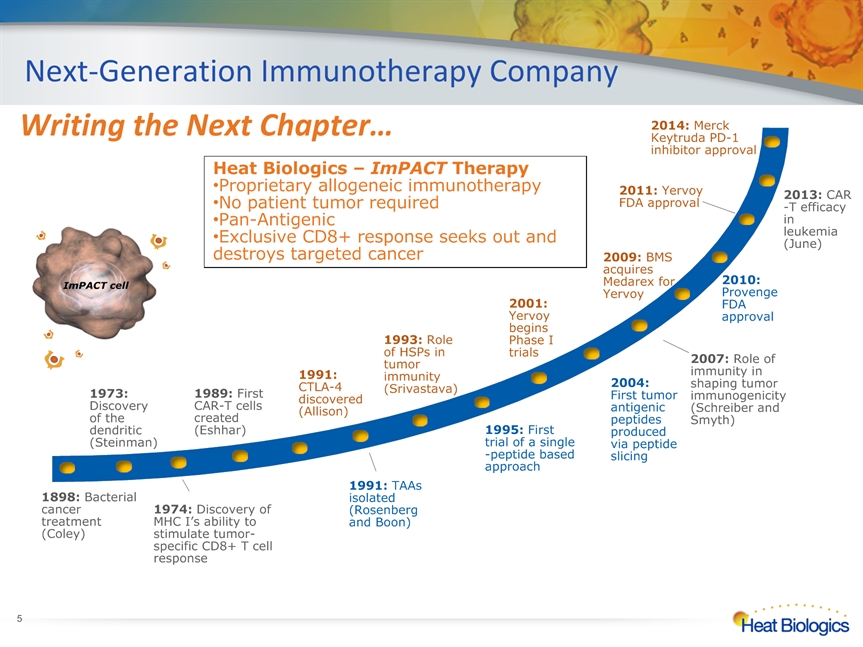
Next-Generation Immunotherapy Company * 1974: Discovery of MHC I’s ability to stimulate tumor-specific CD8+ T cell response Heat Biologics – ImPACT Therapy Proprietary allogeneic immunotherapy No patient tumor requiredPan-AntigenicExclusive CD8+ response seeks out and destroys targeted cancer ImPACT cell 2013: CAR-T efficacy in leukemia (June) Writing the Next Chapter… 2014: Merck Keytruda PD-1 inhibitor approval

Experienced Management and World-Class Advisors * Jeff WolfFounder & CEO Founded several biomedical companies including Avigen, TyRx Pharma (sold to Medtronic), EluSys Therapeutics, GenerationOne Matt CzajkowskiChief Financial Officer 15+ years CFO experience: Pozen, AAIPharma, NextRay; Investment Banking experience: Goldman Sachs & Co Anil Goyal, Ph.D.VP Business Development 20+ years biotech business development experience: Serenex (acquired by Pfizer), Millennium Pharmaceuticals, Genome Therapeutics, Qualiber, Ascletis Pharmaceuticals, Merck Taylor Schreiber, M.D., Ph.D.VP Research & Development Co-developer ImPACT and TNFRSF25 technologies; 10+ years laboratory experience; Author numerous immunology and immunotherapy abstracts and publications Melissa Price, Ph.D.VP Clinical & Regulatory Affairs 14+ years in oncology clinical development in biotech and CRO space: INC Research, Novaquest, Attenuon Scientific Advisory Board Clinical Advisory Board

ImPACT Pipeline ImPACT cancer immunotherapy unleashes immune system against a wide range of cancersTwo product candidates in ongoing multicenter phase 2 clinical trials with multiple fully-funded near-term catalystsNon-small cell lung cancer (NSCLC) - Phase 2Bladder Cancer - Phase 2 *

ImPACT® Modified Cell Vaccine Injection into Patient ImPACT Drug Development and Manufacturing ImPACT® Cell Killer T-Cell * Choose cancer of interest Proprietary screening assay to identify representative cancer cell lines Transform clonal cancer cells into ImPACT cells that secrete own antigens Mass produce ImPACT cells as drug to treat all patients Inject ImPACT cells into patients ImPACT cells secrete antigens to activate killer T-cells to destroy patient’s tumor ImPACT® cells secrete antigens to specifically activate patient’s killer T-Cells Killer T-Cells destroy patient’s tumor

Low COGS Allogeneic Therapy An “off-the shelf” therapy to generate a pan-antigen T-cell immune attack Approach Allogeneic, “off-the-shelf” treatment created from a master cell line No tumor cells, blood or anything else extracted from the patient Non-invasive Benefits Large-scale manufacturing process enables immediate treatment Frequent administration with no patient-specific processing Less expensive to produce and administer than autologous therapies with COGS < 5% of autologous approaches with fewer logistical hurdles Pan-antigen immune attackUnleashes an immune attack against a wide variety of known and unknown cancer antigensCytotoxic T-cell exclusive immune responsePatient HLA IrrelevantAntigen + adjuvant in a single complexAntigen + adjuvant presented simultaneouslyActivates robust and highly specific immune response against secreted cancer antigensContinuous secretion of gp96-antigen/adjuvant complexGenerates more robust and sustained antigen-specific immune response *

Cell-Based Immunotherapy Approaches Criteria/Benchmarks ImPACT® Therapy Other Allogeneic Autologous Cell-based Pan-antigen delivery (known and unknown) Some Some Targeted delivery of antigens to APC in vivo Exclusive cytotoxic CD8+ T cell response Some Dual antigen carrier/adjuvant(not general immune stimulus) Rapid and Efficient New Product Development Some Low Manufacturing COGS Some Patient HLA Irrelevant Some *

Heat’s Combination Approach * Heat’s StrategyPair with complementary cost-effective agents for enhanced payor adoption HS-110 NSCLC combined with cyclophosphamide (low cost) to reduce regulatory T-cell activity HS-410 Bladder combined with BCG (low-cost) for enhanced immune response Why think beyond Checkpoint blockade? Low response rates Immune-related adverse events High price limits viability of cost- effective combinations Neil Berinstein, M.D. Director, Translational Research, Ontario Institute for Cancer Research Llew Keltner, M.D., Ph.D.President and CEO Epistat 1Combination Cancer Immunotherapy, A Virtual Roundtable, Sep. 2014 “It is likely that the most successful cancer immunotherapy will be a combination of two or more therapies that are very effective in doing their part, rather than a single, do-it-all treatment.”1 “Many more than two constituents will possibly be needed to fully engage the immune system. In fact, some agents that have a negligible effect when administered alone may actually be quite active in the presence of others”1

Lung Cancer and HS-110 Little approved after 2nd-line setting in adenocarcinoma, and efficacy is minimalOpportunity to treat a non-immunogenic tumor likely to benefit from T-cell activationCombination immunotherapy approach with low-dose cyclophosphamide, an orally available, inexpensive, known modulator of tumor-induced immunosuppressionAllows treatment of patients who have received checkpoint inhibitors 225,000 new cases of lung cancer and 156,000 new deaths in US in 2014Annual mortality from lung cancer worldwide is 1.4 million, higher than that from colon, breast, and prostate carcinoma combined * “Without any chemotherapy, the average person will live about 4½ months.… Even with chemotherapy, the chance of being alive at one year is about 30-50%”— American Society for Clinical Oncology (ASCO) Guidelines

Comparable Efficacy of HS-110 and Checkpoint Blockade * Scott Gettinger, Yale Cancer Center, 2014 ASCO annual Meeting Nivolumab NSCLC 2nd Line Phase 1(BMS PD-1 blocking antibody) HS-110 NSCLC ≥ 2nd line Phase 1 1 year OS Rate 44% 2 year OS rate 19% Well-tolerated with no overt toxicity Single agent clinical activity in heavily pre-treated stage patients with advanced NSCLC7 of 15 treated patients exhibited stable disease after single course of therapy Median 1 year overall survival rate of patients in the study was 44% (95% CI: 21.6-65.1) comparing favorably to 42% rate in patients treated with Nivolumab in 2nd line

Immune Response Predictive of Survival Responders saw a threefold increase in median overall survival compared to non-responders in the trial, from 4.5 months to 16.9 months Non-Responders Responders >3x Extension of Median OS for Responders 6 12 18 24 30 36 0.25 0.50 1.00 0.75 Proportion Surviving Months From Treatment Start 0 (N=4) (N=11) Hazard Ratio: 0.02195% CI: 0.002 - 0.204 * Immune response observed in 73% (11 out of 15) of patients who completed their first course of therapyImmune response predictive of survival The immune responders exhibited a median survival of 16.9 months while the immune non-responders exhibited a median survival of 4.5 monthsLong-term survivalThree patients survive >3 yrs.One patient alive > 4 yrs.
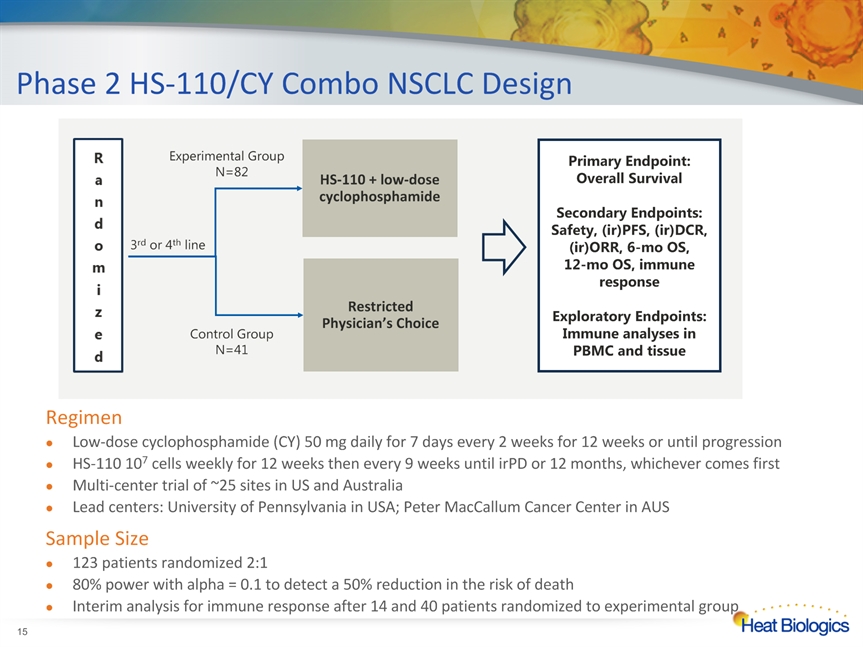
Phase 2 HS-110/CY Combo NSCLC Design * RegimenLow-dose cyclophosphamide (CY) 50 mg daily for 7 days every 2 weeks for 12 weeks or until progressionHS-110 107 cells weekly for 12 weeks then every 9 weeks until irPD or 12 months, whichever comes firstMulti-center trial of ~25 sites in US and AustraliaLead centers: University of Pennsylvania in USA; Peter MacCallum Cancer Center in AUSSample Size123 patients randomized 2:1 80% power with alpha = 0.1 to detect a 50% reduction in the risk of death Interim analysis for immune response after 14 and 40 patients randomized to experimental group 3rd or 4th line

HS-110 in Lung Cancer: Summary * Phase 1 clinical experienceEstimated median survival: 16.5 months (95% CI: 7.1-20.0). Three patients survived >3 yearsStrong immunologic responses Minimal toxicityPhase 2 in NSCLC initiated in Q1 2014Protocol developed in collaboration with world’s leading KOLs and lung expertsProgram specifically designed to leverage promising combination immunotherapy approaches HS-110 is a viable therapeutic intervention for advanced NSCLC patients and has potential to be highly attractive to potential development and commercialization partners

Bladder Cancer and HS-410 Currently-available treatments have high failure rate and are poorly toleratedAmong highest lifetime treatment cost per patient of any cancer due to a high recurrence rateOpportunity to treat patients with minimal residual disease and a tumor type sensitive to immunotherapy (BCG)No new approved drug for this patient population in >25 yearsLeverage potential synergy with BCG to promote trafficking of HS-410 activated cytotoxic T cells into the bladder * In 2012 Alone, There Were 73,000 New Cases of Bladder Cancer Reported and 15,000 Deaths* *American Cancer Society
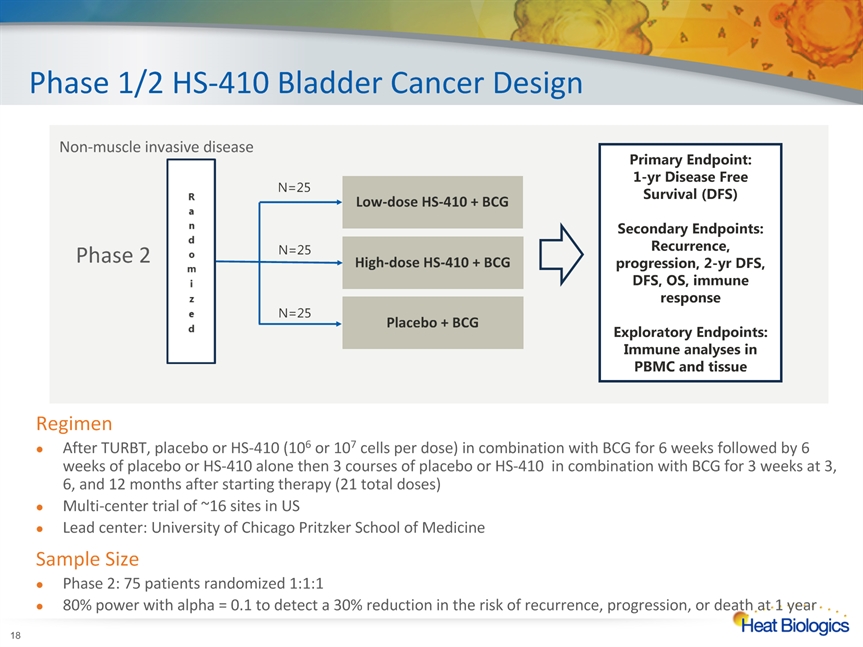
Phase 1/2 HS-410 Bladder Cancer Design * RegimenAfter TURBT, placebo or HS-410 (106 or 107 cells per dose) in combination with BCG for 6 weeks followed by 6 weeks of placebo or HS-410 alone then 3 courses of placebo or HS-410 in combination with BCG for 3 weeks at 3, 6, and 12 months after starting therapy (21 total doses)Multi-center trial of ~16 sites in USLead center: University of Chicago Pritzker School of MedicineSample SizePhase 2: 75 patients randomized 1:1:180% power with alpha = 0.1 to detect a 30% reduction in the risk of recurrence, progression, or death at 1 year Non-muscle invasive disease
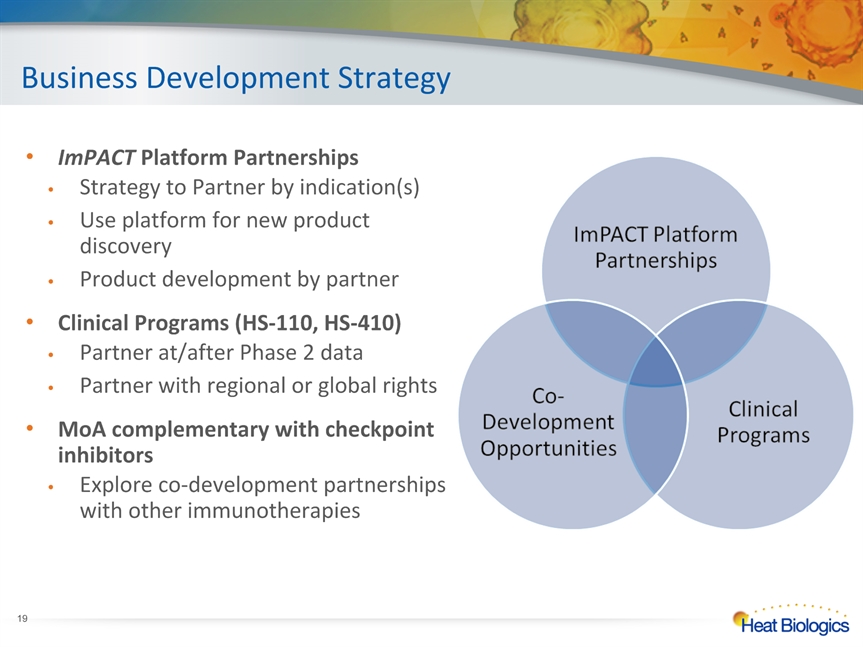
Business Development Strategy * ImPACT Platform PartnershipsStrategy to Partner by indication(s)Use platform for new product discoveryProduct development by partnerClinical Programs (HS-110, HS-410)Partner at/after Phase 2 dataPartner with regional or global rightsMoA complementary with checkpoint inhibitorsExplore co-development partnerships with other immunotherapies
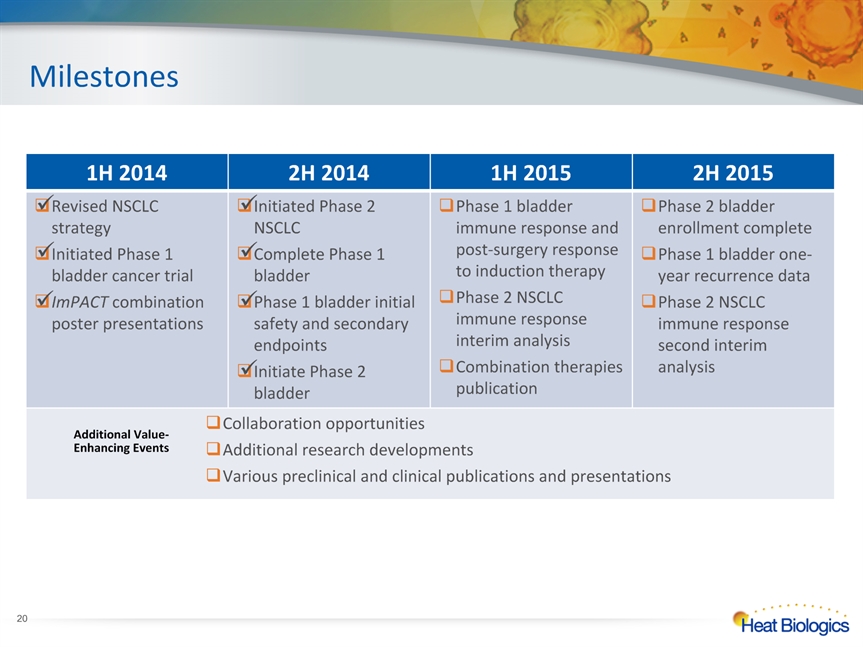
* 1H 2014 2H 2014 1H 2015 2H 2015 Revised NSCLC strategyInitiated Phase 1 bladder cancer trialImPACT combination poster presentations Initiated Phase 2 NSCLC Complete Phase 1 bladder Phase 1 bladder initial safety and secondary endpointsInitiate Phase 2 bladder Phase 1 bladder immune response and post-surgery response to induction therapy Phase 2 NSCLC immune response interim analysisCombination therapies publication Phase 2 bladder enrollment completePhase 1 bladder one-year recurrence dataPhase 2 NSCLC immune response second interim analysis Collaboration opportunitiesAdditional research developmentsVarious preclinical and clinical publications and presentations Collaboration opportunitiesAdditional research developmentsVarious preclinical and clinical publications and presentations Collaboration opportunitiesAdditional research developmentsVarious preclinical and clinical publications and presentations Collaboration opportunitiesAdditional research developmentsVarious preclinical and clinical publications and presentations Milestones Additional Value-Enhancing Events

SummaryClinical Stage Platform Technology Generating Promising Human Data Data to Date Demonstrate: Positive safety profile Powerful, disease-specific immune activation Immune activation corresponds with increased overall survival PromisingClinical Data Strong Clinical Pipeline Phase 2 NSCLC clinical trial and Phase 2 bladder cancer trial with additional other cancer products in preclinical development Multiple near-term enrollment and data readouts Potential for business development licensing activity Value Creating Milestones Transformational Technology Platform Unleash the immune system against a wide range of cancers Over a decade of published research and recent clinical data Combination strategy aligned with cutting-edge science Fully-allogeneic therapy with low COGS *

Living Drug Factories to Treat Cancer Thank youfor more information visit www.heatbio.comorContact Investor Relations: Michael Wood - investorrelations@heatbio.com
