Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ImmunoCellular Therapeutics, Ltd. | d821936d8k.htm |
| EX-99.2 - EX-99.2 - ImmunoCellular Therapeutics, Ltd. | d821936dex992.htm |
| Exhibit 99.1
|
Exhibit 99.1
A randomized double blind placebo-controlled phase 2 trial of dendritic cell (DC) vaccine ICT-107 following standard treatment in newly diagnosed patients with GBM
Patrick Y. Wen, David Reardon, Surasak Phuphanich, Robert Aiken, Joseph Landolfi, William Curry, Jay-Jiguang Zhu, Michael Glantz, David Peereboom, James Markert, Renato Larocca, Donald O’Rourke, Karen Fink, Lyndon Kim, Michael Gruber, Glenn Lesser, Ed Pan, Santosh Kesari, John Yu
Society for Neuro-Oncology 19th Annual Scientific Meeting
November 14, 2014
Copyright 2014
|
|
Rationale for Immunotherapy in GBM
Immunoprivilege of the CNS is circumvented in diseased brain tissue such as in brain tumors and MS
Patients with GBM demonstrate impaired immune function in numbers and function of cytotoxic and helper T cells, with decreased antigen presentation function of dendritic cells
DC vaccination removes DCs from immunosuppressive milieu, increasing the yield and potency of these antigen presenting cells
T cells generated from DCs can target intracranial glioblastoma
Copyright 2014
| 2 |
|
|
|
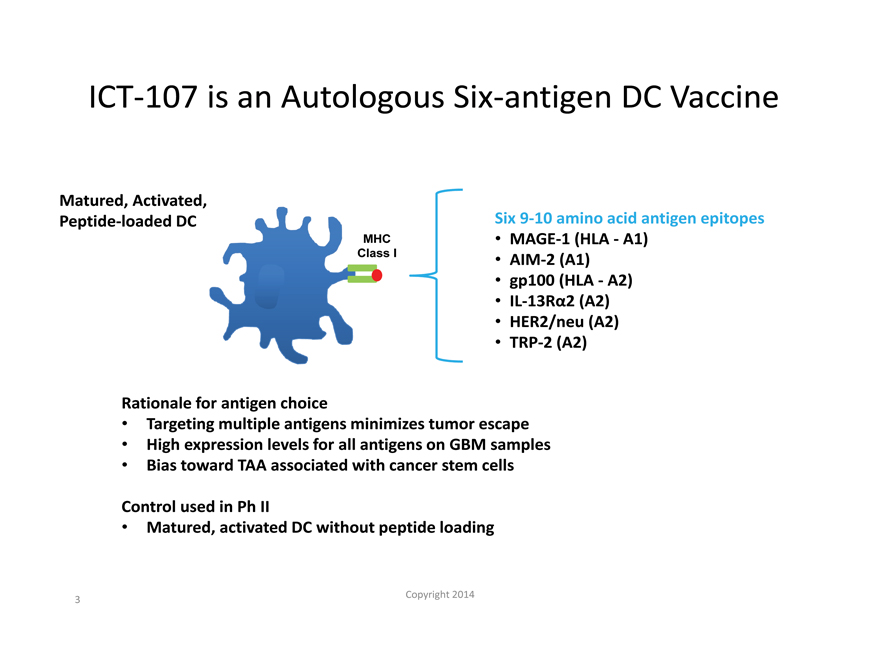
ICT-107 is an Autologous Six-antigen DC Vaccine
Matured, Activated,
Peptide-loaded DC
MHC
Class I
Six 9-10 amino acid antigen epitopes
MAGE-1 (HLA—A1)
AIM-2 (A1) gp100 (HLA—A2)
IL-13R 2 (A2)
HER2/neu (A2)
TRP-2 (A2)
Rationale for antigen choice
Targeting multiple antigens minimizes tumor escape
High expression levels for all antigens on GBM samples
Bias toward TAA associated with cancer stem cells
Control used in Ph II
Matured, activated DC without peptide loading
Copyright 2014
| 3 |
|
|
|
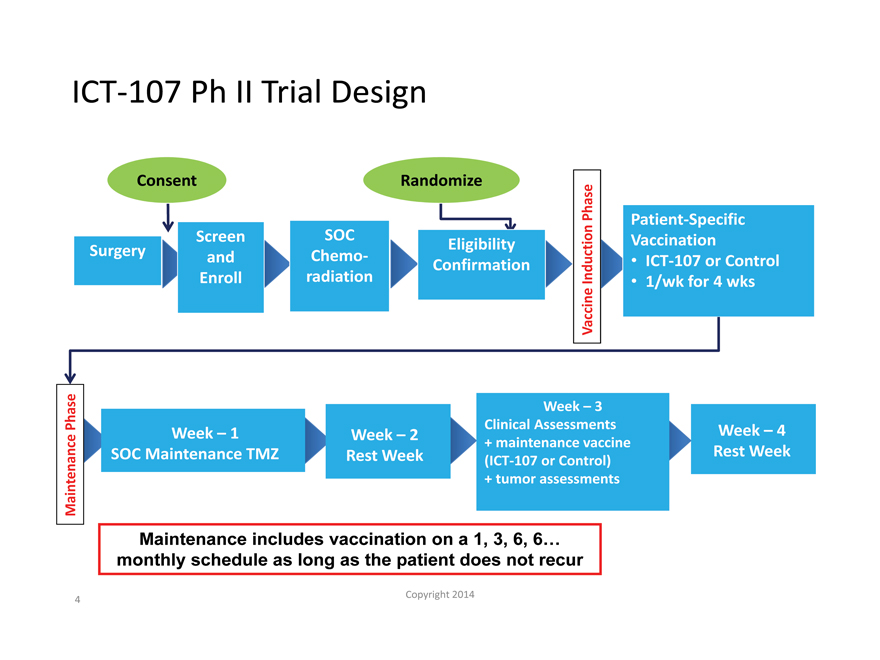
ICT-107 Ph II Trial Design
Consent Randomize
Phase Patient-Specific
Screen SOC Eligibility Vaccination
Surgery and Chemo- ICT-107 or Control
Confirmation Induction
Enroll radiation 1/wk for 4 wks
Vaccine
Week – 3
Phase Week – 1 Week – 2 Clinical Assessments Week – 4
+ maintenance vaccine
SOC Maintenance TMZ Rest Week (ICT-107 or Control) Rest Week
Maintenance + tumor assessments
Maintenance includes vaccination on a 1, 3, 6, 6… monthly schedule as long as the patient does not recur
Copyright 2014
| 4 |
|
|
|
Eligibility Criteria and Objectives
Key INCLUSION Criteria
– Complete surgical resection or minimum residual tumor <1 cm3 GBM
– Human leukocyte antigen (HLA) A1, HLA-A2, or HLA-A1/A2
– Karnofsky Performance Status (KPS) score of 70% Key EXCLUSION Criteria
– Recurrent disease assessed after surgery / before chemoradiation
– Radiosurgery and placement of Gliadel Primary Objective
– OS
Secondary Objectives
– PFS
– Safety and tolerability of ICT-107
– Describe the immune response to ICT-107
– Determine predictors of response Outcome Stratification
– MGMT methylation status: single largest factor for predicting survival
– Age category: 50 and >50
Copyright 2014
| 5 |
|
|
|
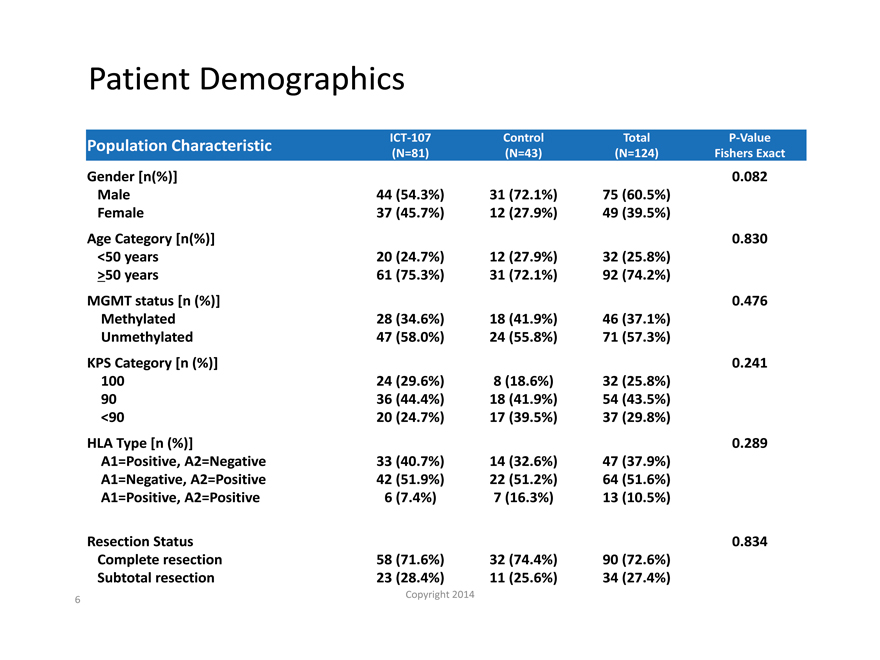
Patient Demographics
Population Characteristic ICT-107 Control Total P-Value
(N=81) (N=43) (N=124) Fishers Exact
Gender [n(%)] 0.082
Male 44 (54.3%) 31 (72.1%) 75 (60.5%)
Female 37 (45.7%) 12 (27.9%) 49 (39.5%)
Age Category [n(%)] 0.830
<50 years 20 (24.7%) 12 (27.9%) 32 (25.8%)
>50 years 61 (75.3%) 31 (72.1%) 92 (74.2%)
MGMT status [n (%)] 0.476
Methylated 28 (34.6%) 18 (41.9%) 46 (37.1%)
Unmethylated 47 (58.0%) 24 (55.8%) 71 (57.3%)
KPS Category [n (%)] 0.241
100 24 (29.6%) 8 (18.6%) 32 (25.8%)
90 36 (44.4%) 18 (41.9%) 54 (43.5%)
<90 20 (24.7%) 17 (39.5%) 37 (29.8%)
HLA Type [n (%)] 0.289
A1=Positive, A2=Negative 33 (40.7%) 14 (32.6%) 47 (37.9%)
A1=Negative, A2=Positive 42 (51.9%) 22 (51.2%) 64 (51.6%)
A1=Positive, A2=Positive 6 (7.4%) 7 (16.3%) 13 (10.5%)
Resection Status 0.834
Complete resection 58 (71.6%) 32 (74.4%) 90 (72.6%)
Subtotal resection 23 (28.4%) 11 (25.6%) 34 (27.4%)
Copyright 2014
| 6 |
|
|
|
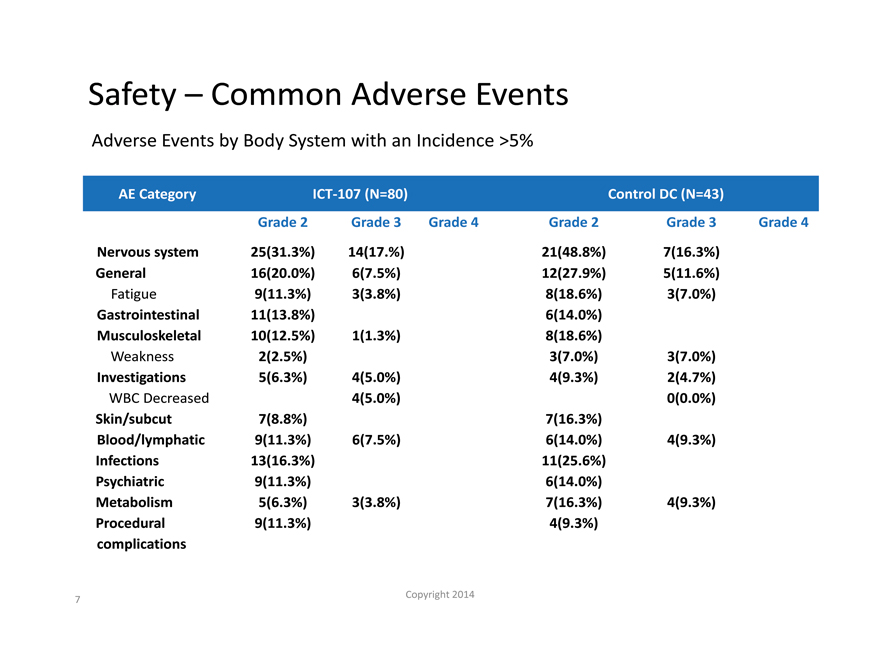
Safety – Common Adverse Events
Adverse Events by Body System with an Incidence >5%
AE Category ICT-107 (N=80) Control DC (N=43)
Grade 2 Grade 3 Grade 4 Grade 2 Grade 3 Grade 4
Nervous system 25(31.3%) 14(17.%) 21(48.8%) 7(16.3%)
General 16(20.0%) 6(7.5%) 12(27.9%) 5(11.6%)
Fatigue 9(11.3%) 3(3.8%) 8(18.6%) 3(7.0%)
Gastrointestinal 11(13.8%) 6(14.0%)
Musculoskeletal 10(12.5%) 1(1.3%) 8(18.6%)
Weakness 2(2.5%) 3(7.0%) 3(7.0%)
Investigations 5(6.3%) 4(5.0%) 4(9.3%) 2(4.7%)
WBC Decreased 4(5.0%) 0(0.0%)
Skin/subcut 7(8.8%) 7(16.3%)
Blood/lymphatic 9(11.3%) 6(7.5%) 6(14.0%) 4(9.3%)
Infections 13(16.3%) 11(25.6%)
Psychiatric 9(11.3%) 6(14.0%)
Metabolism 5(6.3%) 3(3.8%) 7(16.3%) 4(9.3%)
Procedural 9(11.3%) 4(9.3%)
complications
Copyright 2014
| 7 |
|
|
|
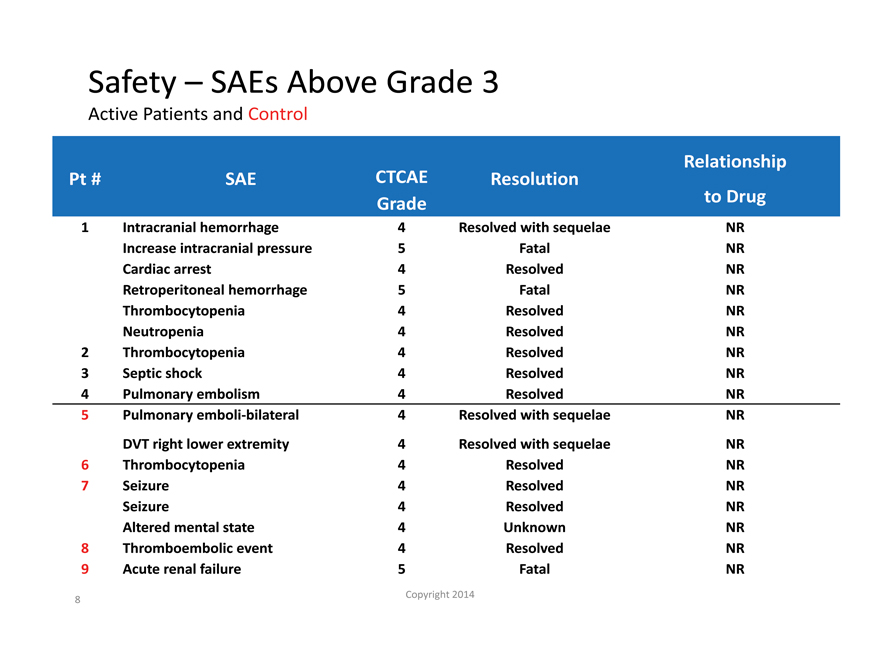
Safety – SAEs Above Grade 3
Active Patients and Control Patients
Relationship
Pt # SAE CTCAE Resolution
Grade to Drug
| 1 |
|
Intracranial hemorrhage 4 Resolved with sequelae NR |
Increase intracranial pressure 5 Fatal NR
Cardiac arrest 4 Resolved NR
Retroperitoneal hemorrhage 5 Fatal NR
Thrombocytopenia 4 Resolved NR
Neutropenia 4 Resolved NR
| 2 |
|
Thrombocytopenia 4 Resolved NR |
| 3 |
|
Septic shock 4 Resolved NR |
| 4 |
|
Pulmonary embolism 4 Resolved NR |
| 5 |
|
Pulmonary emboli-bilateral 4 Resolved with sequelae NR |
DVT right lower extremity 4 Resolved with sequelae NR
| 6 |
|
Thrombocytopenia 4 Resolved NR |
| 7 |
|
Seizure 4 Resolved NR |
Seizure 4 Resolved NR
Altered mental state 4 Unknown NR
| 8 |
|
Thromboembolic event 4 Resolved NR |
9 Acute renal failure 5 Fatal NR
Copyright 2014
| 8 |
|
|
|
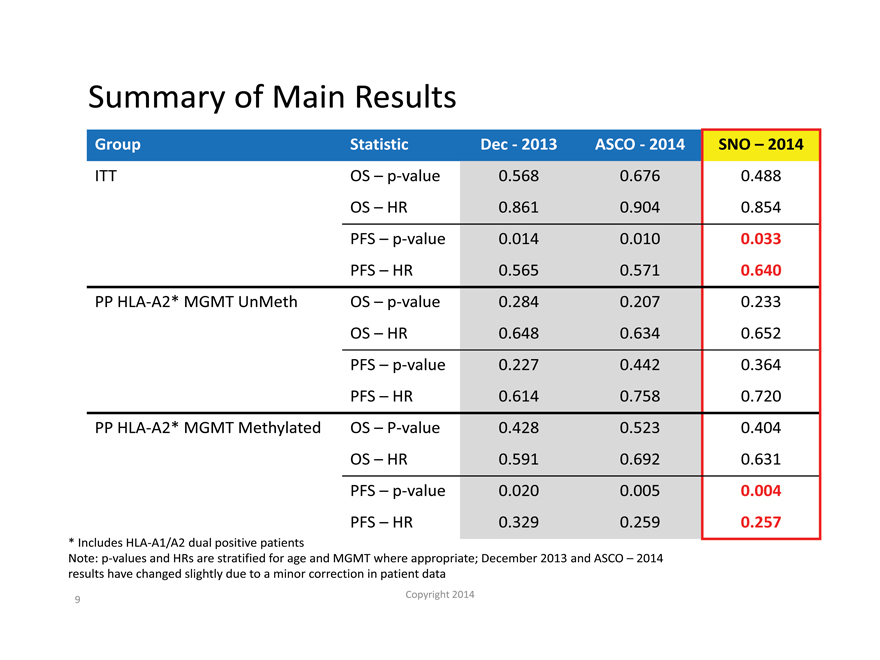
Summary of Main Results
Group Statistic Dec—2013 ASCO—2014 SNO – 2014
ITT OS – p-value 0.568 0.676 0.488
OS – HR 0.861 0.904 0.854
PFS – p-value 0.014 0.010 0.033
PFS – HR 0.565 0.571 0.640
PP HLA-A2* MGMT UnMeth OS – p-value 0.284 0.207 0.233
OS – HR 0.648 0.634 0.652
PFS – p-value 0.227 0.442 0.364
PFS – HR 0.614 0.758 0.720
PP HLA-A2* MGMT Methylated OS – P-value 0.428 0.523 0.404
OS – HR 0.591 0.692 0.631
PFS – p-value 0.020 0.005 0.004
PFS – HR 0.329 0.259 0.257
| * |
|
Includes HLA-A1/A2 dual positive patients |
Note: p-values and HRs are stratified for age and MGMT where appropriate; December 2013 and ASCO – 2014
results have changed slightly due to a minor correction in patient data
Copyright 2014
9
|
|
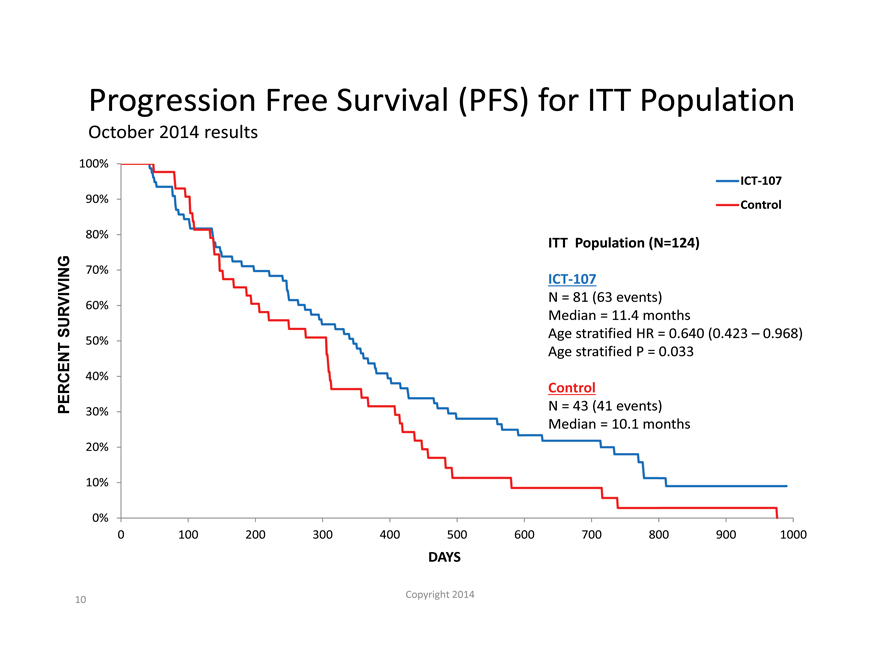
Progression Free Survival (PFS) for ITT Population
October 2014 results
100%
ICT-107
90% Control
80%
ITT Population (N=124)
70%
ICT-107
N = 81 (63 events)
60%
SURVIVING Median = 11.4 months
50% Age stratified HR = 0.640 (0.423 – 0.968)
Age stratified P = 0.033
40%
Control
PERCENT N = 43 (41 events)
30%
Median = 10.1 months
20%
10%
0%
0 100 200 300 400 500 600 700 800 900 1000
DAYS
Copyright 2014
10
|
|
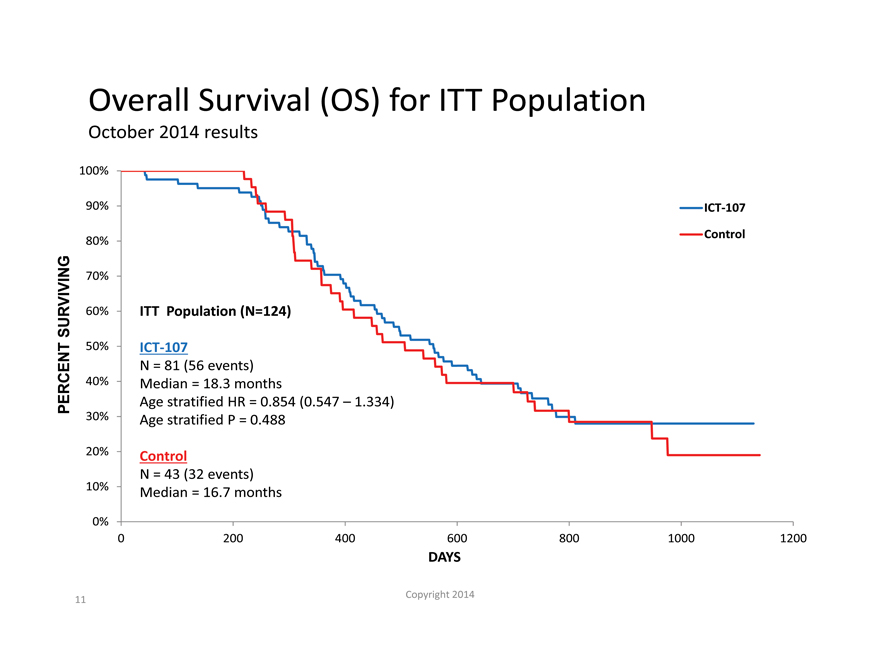
Overall Survival (OS) for ITT Population
October 2014 results
100%
90% ICT-107
80% Control
70%
SURVIVING 60% ITT Population (N=124)
50% ICT-107
N = 81 (56 events)
40% Median = 18.3 months
PERCENT Age stratified HR = 0.854 (0.547 – 1.334)
30% Age stratified P = 0.488
20% Control
N = 43 (32 events)
10% Median = 16.7 months
0%
0 200 400 600 800 1000 1200
DAYS
Copyright 2014
11
|
|
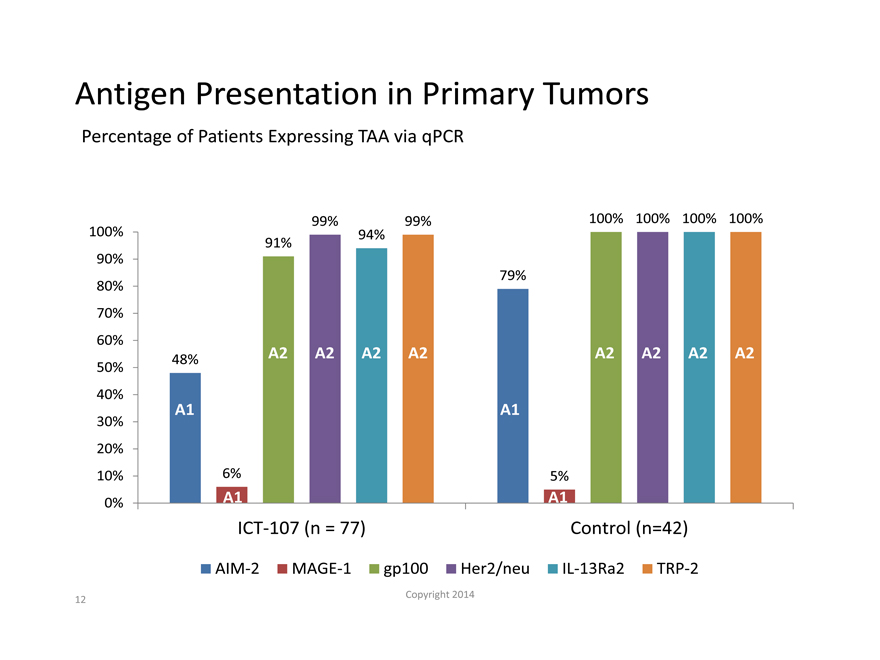
Antigen Presentation in Primary Tumors
Percentage of Patients Expressing TAA via qPCR
99% 99% 100% 100% 100% 100%
100% 94%
91%
90%
79%
80%
70%
60%
48% A2 A2 A2 A2 A2 A2 A2 A2
50%
40%
A1 A1
30%
20%
10% 6% 5%
0% A1 A1
ICT-107 (n = 77) Control (n=42)
AIM-2 MAGE-1 gp100 Her2/neu IL-13Ra2 TRP-2
Copyright 2014
12
|
|
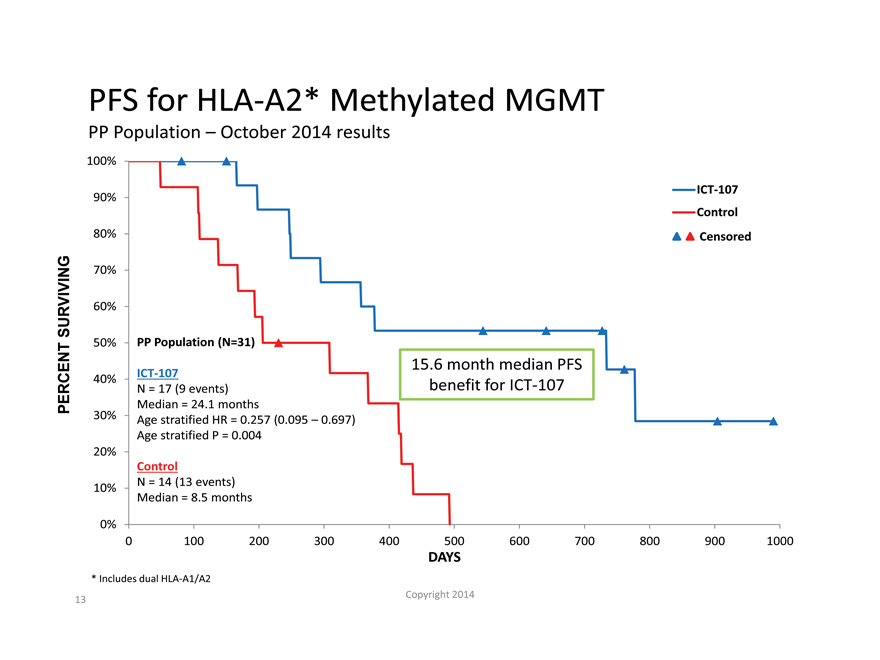
PFS for HLA-A2* Methylated MGMT
PP Population – October 2014 results
100%
ICT-107
90%
Control
80% Censored
70%
SURVIVING 60%
50% PP Population (N=31)
15.6 month median PFS
ICT-107
40% N = 17 (9 events) benefit for ICT-107
PERCENT Median = 24.1 months
30% Age stratified HR = 0.257 (0.095 – 0.697)
Age stratified P = 0.004
20%
Control
10% N = 14 (13 events)
Median = 8.5 months
0%
0 100 200 300 400 500 600 700 800 900 1000
DAYS
| * |
|
Includes dual HLA-A1/A2 |
Copyright 2014
13
|
|
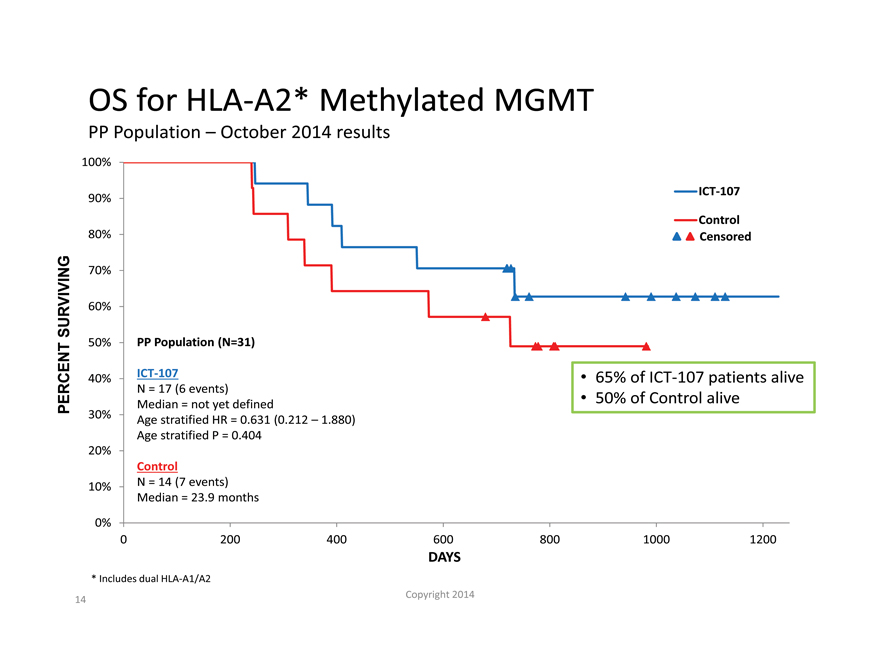
OS for HLA-A2* Methylated MGMT
PP Population – October 2014 results
100%
90% ICT-107
Control
80% Censored
70%
SURVIVING 60%
50% PP Population (N=31)
40% ICT-107 65% of ICT-107 patients alive
N = 17 (6 events)
PERCENT Median = not yet defined 50% of Control alive
30% Age stratified HR = 0.631 (0.212 – 1.880)
Age stratified P = 0.404
20%
Control
10% N = 14 (7 events)
Median = 23.9 months
0%
0 200 400 600 800 1000 1200
DAYS
| * |
|
Includes dual HLA-A1/A2 |
Copyright 2014
14
|
|
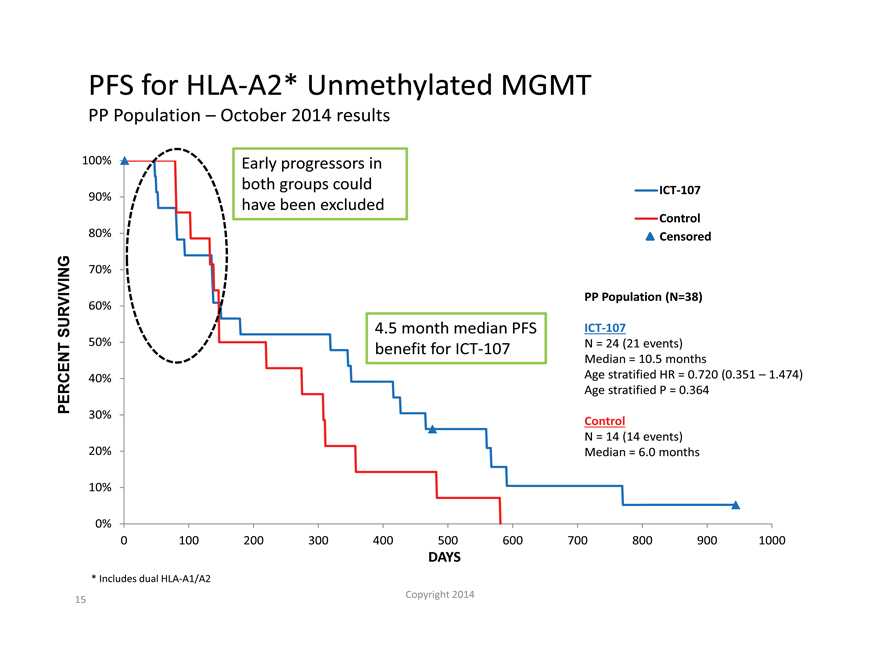
PFS for HLA-A2* Unmethylated MGMT
PP Population – October 2014 results
100% Early progressors in
both groups could ICT-107
90% have been excluded
Control
80% Censored
70%
PP Population (N=38)
60%
SURVIVING 4.5 month median PFS ICT-107
50% benefit for ICT-107 N = 24 (21 events)
Median = 10.5 months
40% Age stratified HR = 0.720 (0.351 – 1.474)
PERCENT Age stratified P = 0.364
30% Control
N = 14 (14 events)
20% Median = 6.0 months
10%
0%
0 100 200 300 400 500 600 700 800 900 1000
DAYS
| * |
|
Includes dual HLA-A1/A2 |
Copyright 2014
15
|
|
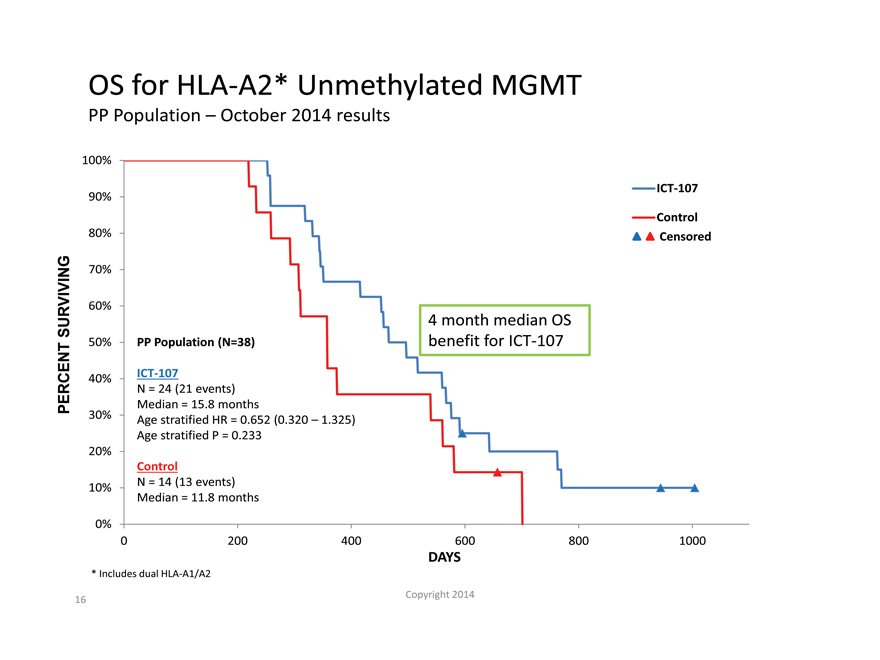
OS for HLA-A2* Unmethylated MGMT
PP Population – October 2014 results
100%
ICT-107
90%
Control
80% Censored
70%
60%
SURVIVING 4 month median OS
50% PP Population (N=38) benefit for ICT-107
40% ICT-107
N = 24 (21 events)
PERCENT Median = 15.8 months
30% Age stratified HR = 0.652 (0.320 – 1.325)
Age stratified P = 0.233
20%
Control
10% N = 14 (13 events)
Median = 11.8 months
0%
0 200 400 600 800 1000
DAYS
| * |
|
Includes dual HLA-A1/A2 |
Copyright 2014
16
|
|
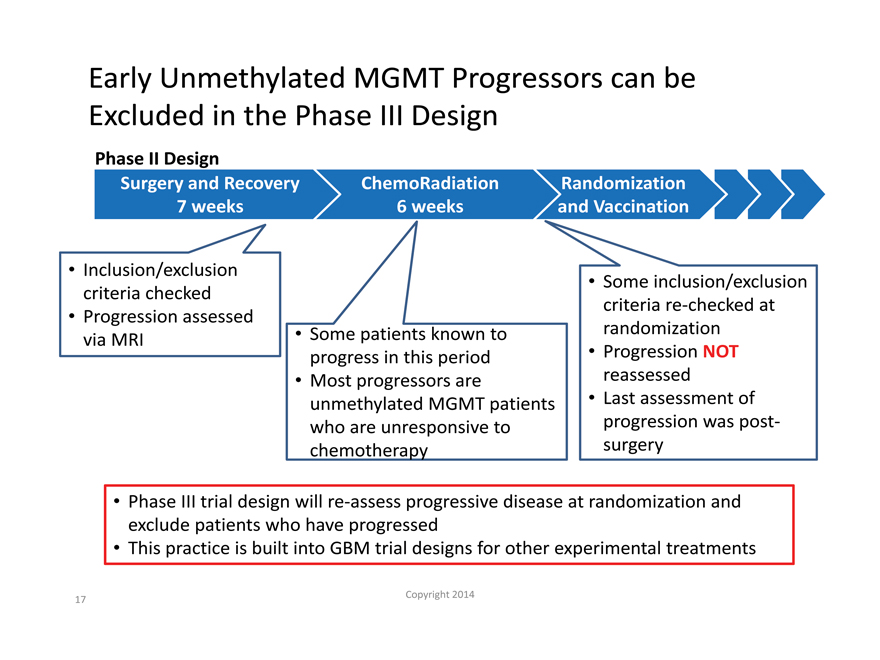
Early Unmethylated MGMT Progressors can be Excluded in the Phase III Design
Phase II Design
Surgery and Recovery ChemoRadiation Randomization
| 7 |
|
weeks 6 weeks and Vaccination |
Inclusion/exclusion criteria checked Progression assessed via MRI
Some patients known to progress in this period
Most progressors are unmethylated MGMT patients who are unresponsive to chemotherapy
Some inclusion/exclusion criteria re-checked at randomization
Progression NOT reassessed Last assessment of progression was post-surgery
Phase III trial design will re-assess progressive disease at randomization and exclude patients who have progressed This practice is built into GBM trial designs for other experimental treatments
Copyright 2014
17
|
|
Change in Treatment Effect when Early Progressors are Removed
PP Patients Who Progressed in 90 Days were Removed – 8 Total; 6 Active, 2 Control
Group / Endpoint Medians P-value† HR†
HLA-A2* Stayed the same at Changed from 0.364 Changed from 0.720
unmethylated PFS 4.5 months in favor to 0.149 to 0.556
of ICT-107
HLA-A2* Increased from 4.0 Changed from 0.233 Changed from 0.652
unmethylated OS months to 5.9 to 0.183 to 0.580
months in favor of
ICT-107
This analysis informs phase III design and statistics
| * |
|
Includes dual HLA-A1/A2 |
† Age stratified
Copyright 2014
18
|
|
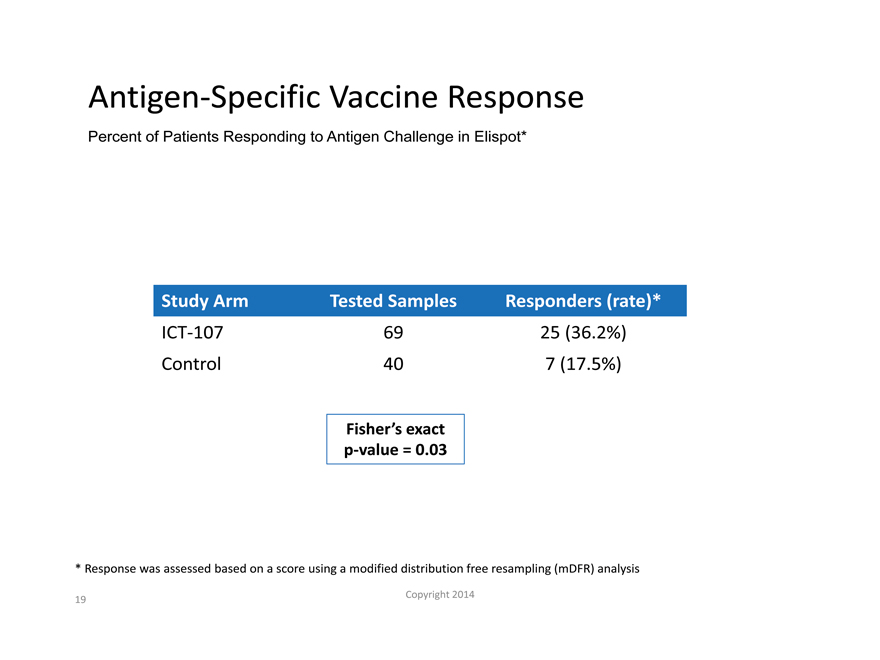
Antigen-Specific Vaccine Response
Percent of Patients Responding to Antigen Challenge in Elispot*
Study Arm Tested Samples Responders (rate)*
ICT-107 69 25 (36.2%)
Control 40 7 (17.5%)
Fisher’s exact
p-value = 0.03
| * |
|
Response was assessed based on a score using a modified distribution free resampling (mDFR) analysis |
Copyright 2014
19
|
|
Conclusions for the ICT-107 Phase II Trial
No significant difference in adverse events between ICT-107 and control The vaccine is biologically active in terms of producing an immune response PFS was statistically improved for the entire treated population
– No other immunotherapy has shown statistical benefit for a clinical outcome in newly diagnosed GBM in a controlled trial
ICT-107 activity is strongest in the predefined HLA-A2 subgroup
– Tumor antigen expression proportions were high for A2 antigens
– The MGMT methylated subgroup showed the largest treatment effect with statistically significant PFS and OS expected to trend toward significance as more events occur
– The MGMT unmethylated subgroup showed trends toward PFS and OS treatment benefit that were more pronounced when early progressors were removed
Results support advancement to phase III testing
– OS hazard ratios in both per-protocol HLA-A2 MGMT subgroups are better than 0.70
Copyright 2014
20
|
|
Acknowledgements
The Authors and ImmunoCellular Therapeutics Wish to Thank
Additional Investigators:
Andrew Sloan, Susan C. Pannullo, James Chandler, Jeffrey Raizer, David Schiff, Tina Mayer, Jay Grewel
Terri Armstrong for Analysis of the QOL Data
Trial Sites:
Johns Hopkins University, New York University, University of Texas at Houston, Northwestern University, Arizona Cancer Center, New Jersey Neuroscience Institute, UC San Diego, Moffitt Cancer Center, Penn State, University of Pennsylvania, University of Virginia, Wake Forest Cornell Presbyterian, Massachusetts General, Kentuckiana Cancer Institute, Cedars-Sinai Medical Center, University Hospital Case Medical Center, Rush University, Overlook Hospital, Baylor University, Cleveland Clinic, University of Alabama, Thomas Jefferson, Long Island Brain Center
Patients and Families
Copyright 2014
21