Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Recro Pharma, Inc. | d816641d8k.htm |
| EX-99.1 - EX-99.1 - Recro Pharma, Inc. | d816641dex991.htm |
  Relieving
Pain….Improving Lives Exhibit 99.2 |
 Special Note
Regarding Forward-Looking Statements
2
This presentation includes forward-looking statements within the meaning of
Section 27A of the Securities Act of 1933 and Section 21E of the Securities
Exchange Act of 1934. These statements, among other things, relate to
our business strategy, goals and expectations concerning our product
candidates, future operations, prospects, plans and objectives of
management. The words "anticipate", "believe", "could",
"estimate", "expect", "intend", "may",
"plan", "predict", "project", "will" and similar terms
and phrases are used to identify forward-looking statements in this
presentation. Our operations involve risks and uncertainties, many of
which are outside our control, and any one of which, or a combination of
which, could materially affect our results of operations and whether the
forward-looking statements ultimately prove to be correct. These
forward-looking statements should be considered together with the risks and
uncertainties that may affect our business and future results included in our
filings with the Securities and Exchange Commission at www.sec.gov.
These forward- looking statements are based on information currently
available to us, and we assume no obligation to update any
forward-looking statements except as required by applicable law.
|
 Company
Highlights •
Dex-IN –
intranasal, non-opioid in Phase II for acute
pain following surgery -
significant market opp’y
•
Multiple clinical studies demonstrate analgesic
effect, fast onset of action and well tolerated
•
Phase
II
post
op
pain
trial
ongoing
–
Day
1
dosing
•
Multiple clinical and regulatory milestones over next
few years
•
Experienced team with significant development,
regulatory and commercial experience
3 |
 Experienced
Management and Board 4
•
Gerri Henwood –
President and CEO
Founded Auxilium Pharmaceuticals (AUXL,
NASDAQ) and IBAH (former NASDAQ Co. –
acquired 1998); GSK
•
Chuck Garner –
CFO, CBO and Treasurer
Over 14 years of life sciences investment
banking experience –
Deutsche Bank, Burrill &
Co., Inverness Advisors; PwC
•
Randy Mack –
SVP, Development
Over 20 years of clinical development
experience –
Adolor, Auxilium, Abbott Labs
and Harris Labs
Board of Directors
Wayne B. Weisman –
Chairman
SCP VitaLife Partners
Winston J. Churchill
SCP VitaLife Partners
Gerri Henwood –
CEO
William L. Ashton
Harrison Consulting Group; frmly Amgen
Abraham Ludomirski, M.D.
SCP VitaLife Partners
Alfred Altomari
CEO, Agile Therapeutics
Michael Berelowitz
Former SVP, Specialty Care Business
Unit, Pfizer |
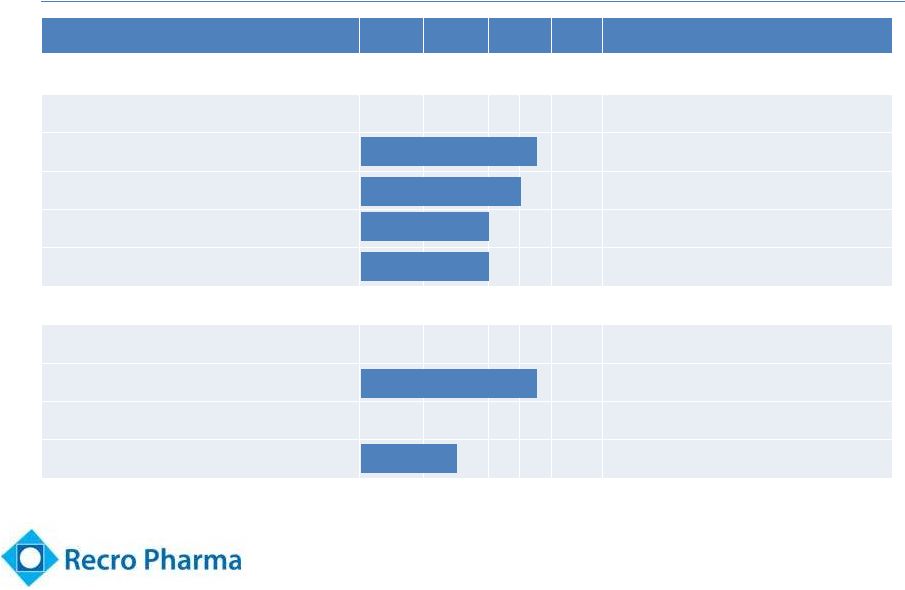 Clinical Stage
Pipeline Product
PC
I
II
III
Rights
Dexmedetomidine (“Dex”)
WW, exc. Europe, Turkey, CIS*
Dex-
IN (intranasal)
Acute post-operative pain
Cancer breakthrough pain
Dex-SL (sublingual)
Transdermal
Fadolmidine (“Fado”)
WW, exc. Europe, Turkey, CIS*
Intrathecal
Post-operative pain
Topical
Neuropathic pain
5
* CIS currently includes Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan,
Kyrgyzstan, Moldova, Russia, Tajikistan, Turkmenistan, Ukraine, and
Uzebekistan. |
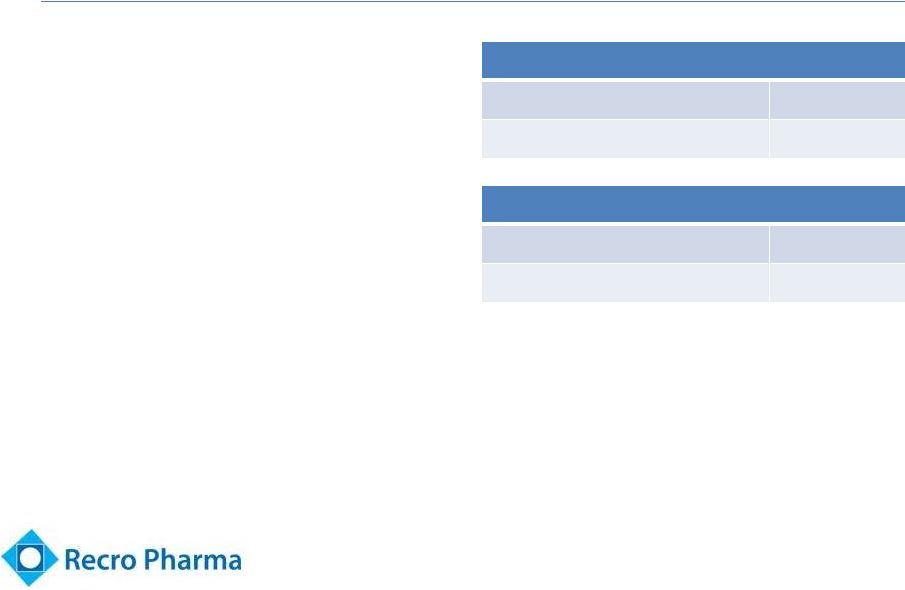 Post Op Pain
Market Underserved 6
•
$5.9 billion market
(1)
•
Predominantly opioid
use
•
Significant side effects /
issues associated with
opioids
•
Dearth of non-opioid
drugs in development
Inpatient procedures
Total procedures (2009)
47.9M
Addressable
>25M
Ambulatory procedures
Total procedures (2006)
53.3M
Addressable
>25M
Note: Addressable includes procedures expected to
utilize pain medication.
Source: National Center for Health Statistics and
management estimates.
(1) GBI Research, 2010 sales. |
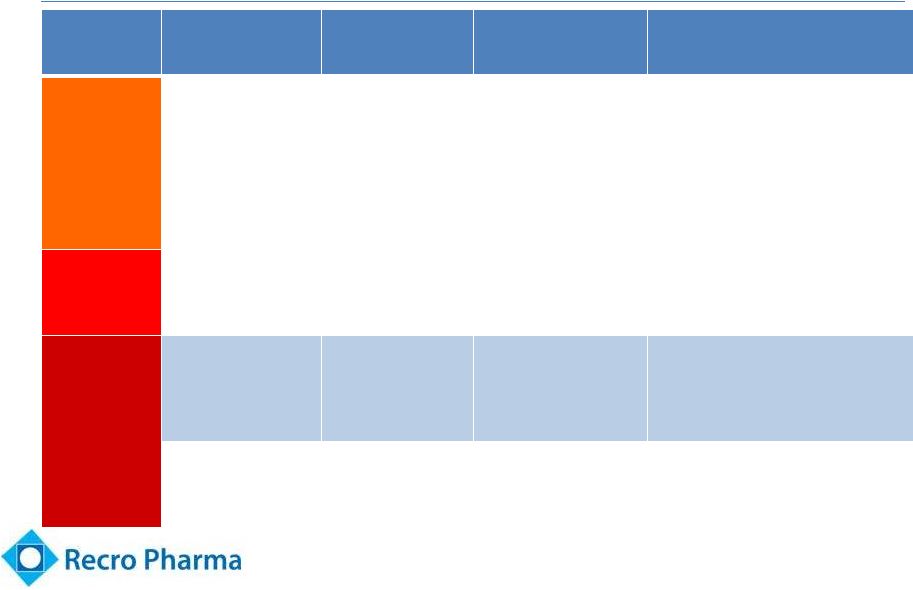 Limited Pain
Relief Options for Patients 7
Pain
Severity
Class
Compounds
Advantages
Disadvantages
Mild
Acetaminophen
Antipyretic properties;
Oral; no opioid AEs
Only effective for mild pain
NSAIDs
Ketorolac,
ibuprofen, aspirin
Mild to moderate
analgesia; oral; no
opioid AEs
Bleeding risk; GI and renal
complications
Moderate
Sodium channel
blockers
Bupivacaine,
lidocaine
Use directly at pain
site; mostly peri-
operative
Limited duration of action; some are
concerned about local tissue impact
Moderate to
Severe
Alpha 2 agonists
Dexmedetomidine
(Recro Pharma)
Good pain relief;
anxiolytic properties;
no respiratory
depression, impaired GI
or addictive properties
In development –
potential for first in
class to be approved for post-
operative pain
Opioids
Morphine,
hydrocodone,
oxycodone, fentanyl
Good pain relief
Respiratory depression, impaired GI
motility after even one dose;
frequent nausea and vomiting;
abuse/addiction potential
Note: Pain severity based upon market research / physician feedback
|
  Dexmedetomidine
(“Dex”) |
 Dex Has
Demonstrated Analgesia & Safety •
Alpha 2 agonist (non-opioid)
–
Injectable form (Precedex) marketed by Hospira in US as sedative
–
Multiple studies demonstrating analgesia of alpha 2 agonists
•
Intranasal formulation in clinical development for acute pain
–
In-licensed non-IV rights from Orion
–
Worldwide rights except Europe, Turkey, and CIS
•
Multiple studies demonstrate Dex pain relief and safe profile
–
Including our completed placebo controlled trials
•
Expect strong IP position
–
Pending IP coverage could run through 2030
•
Expect to file 505(b)(2) NDA shortly after completion of Ph III
9 |
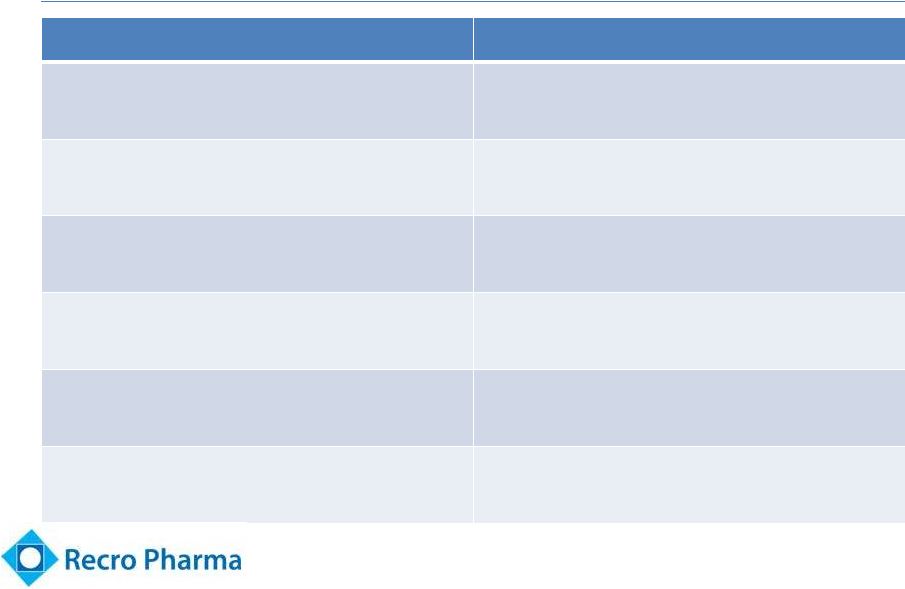 Dex Efficacy
and Safety in Multiple Studies 10
Beneficial effects
Source
Approved sedative and safe profile
NDA filing / pivotal trials -
Abbott/Hospira, Orion
Morphine sparing
NDA studies plus Literature
Analgesia by IV route
Chan, 2010; Grosu, 2010; Lin, 2009, Arain,
2010
Demonstration of pain relief (VAS)
Placebo controlled trials; L. Webster, MD
(Utah) CLBP study (Recro sponsored)
Positive PK/PD plasma levels
demonstrating analgesic potential
Clinical trials run by Recro
Relieves morphine “Max”
(‘hyperalgesia’)
University of Minnesota; M. Belgrade, MD |
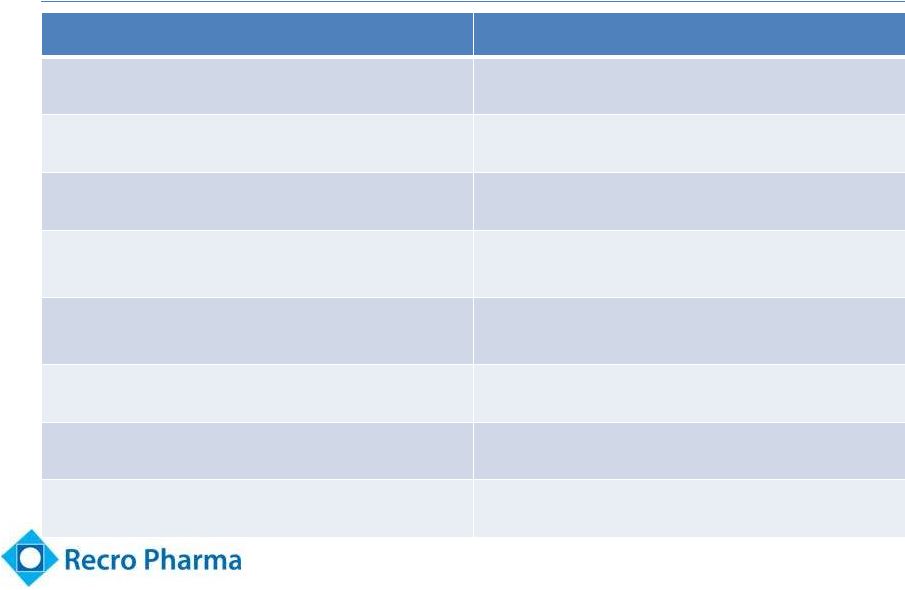 Significant
Advantages Over Opioids 11
Dex
Fast-acting Opioids
Non-opioid (Not controlled substance)
Opioid -
DEA scheduled product
No habituation effects
Addictive
Does not cause respiratory depression
Respiratory depression
Not associated with constipation,
nausea, or vomiting
Unwanted side-effects of constipation,
nausea and vomiting
Enhances morphine effectiveness
without morphine dose increase
Additive effect requires higher dose
More cognitively intact
Frequently “Foggy”/ may be confused
Anxiolytic properties
Not anxiolytic
Effective Analgesic
Effective Analgesic |
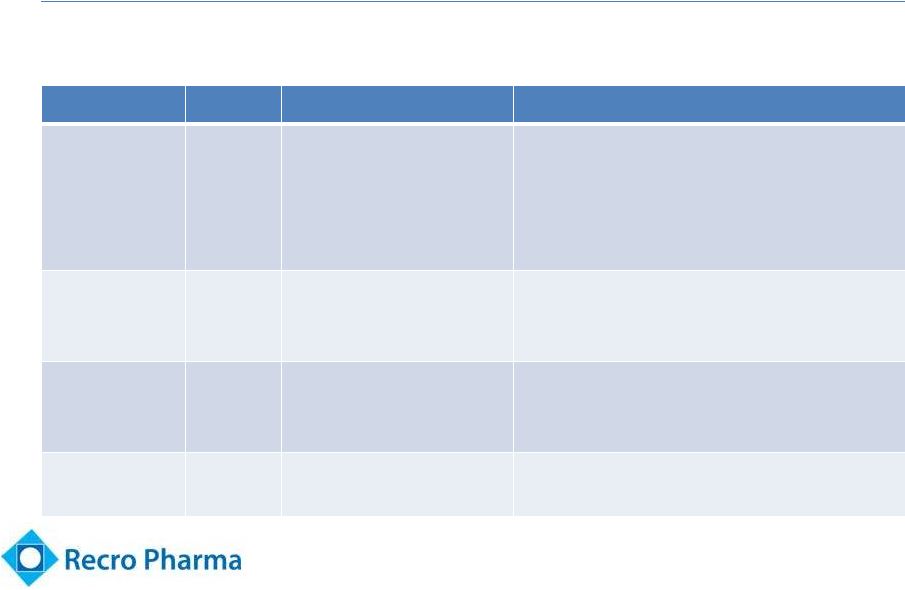 Dex Has Been
Well Studied by Recro 12
•
Evaluated proprietary formulations of Dex in 9 trials
Trial
Form
Design
Outcome
REC-13-012
Dex-IN
Acute pain following
bunionectomy surgery
(n=85 evaluable)
Within a subset of patients (n=42), with
baseline pain intensity of 6 or below,
there was a trend towards analgesia in
50 mcg and reduced opioid use versus
placebo
REC-11-010
Dex-IN
Chronic lower back
pain POC study (n=24)
Statistically significant pain relief within
30 minutes demonstrated in placebo
controlled trial –
single use device
REC-09-003
Dex-SL
Chronic lower back
pain POC study (n=21)
Statistically
significant reduction in pain
intensity demonstrated in placebo
controlled trial
REC-11-008
Dex-IN
Multi-dose PK study
(n=12)
Safety & tolerability of IN dosage form |
 Dex-IN
Study REC-13-012 (US placebo controlled trial)
13
•
Phase II bunionectomy study in approx. 150-200 pts
•
Three dosing groups –
50 mcg, 35 mcg and placebo
•
Preplanned interim analysis
–
Randomized, placebo controlled study
•
Primary endpoint –
summed pain intensity difference (SPID) over 48 hours
•
Rescue therapy allowed
•
Post Op Day 0 dosing
–
Scheduled after half of patients enrolled
–
Allowed for possible sample size adjustment
–
85 pts evaluable in interim analysis –
approx. 28 pts per group |
 Results of
Phase II Interim Analysis •
Analgesia and opioid reduction seen in subset of
patients
–
Patients with baseline pain score equal to 6 or lower
–
Approximately half of patients enrolled
•
However, trial was not expected to reach statistical
significance with current design
–
Post Op Day 0 dosing
•
Revised trial design –
Post Op Day 1 dosing
–
Stable / declining pain trajectory
14 |
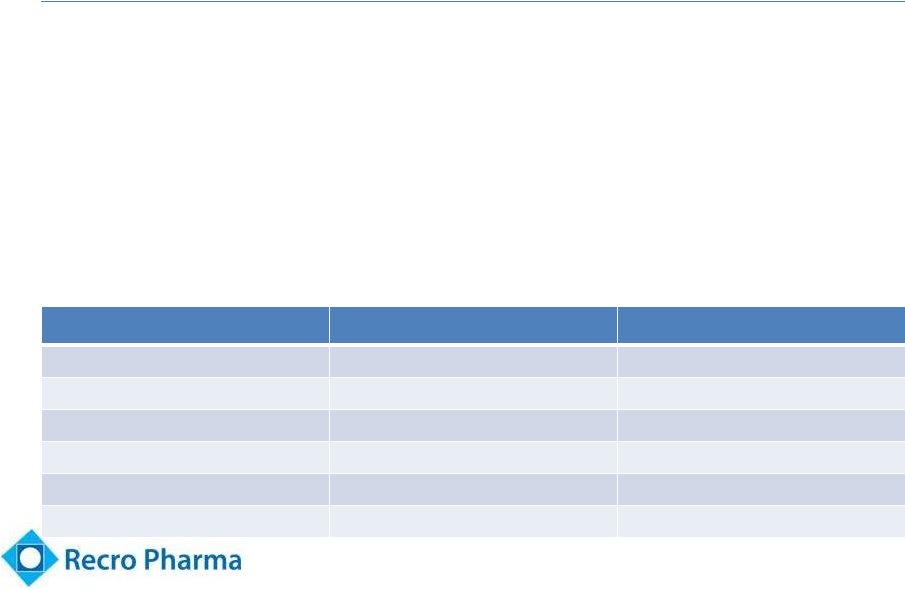 Summary of Key
Safety Data – No SAEs
Event
Dex-IN 50 mcg arm
Placebo arm
Drowsiness
17 (53%)
17 (53%)
Nausea
8 (25%)
14 (44%)
Vomiting
2 (6%)
6 (19%)
Dizziness
3 (9%)
5 (16%)
Nasal Irritation
2 (6%)
3 (9%)
Epistaxis
2 (6%)
3 (9%)
15
•
4 patients discontinued due to symptomatic hypotension (3 in
50 mcg arm, 1 in 35 mcg), 1 due to fever (35 mcg) and 1 due to
nausea and vomiting (placebo)
•
Adverse event of asymptomatic “BP decrease”
in 10 Dex-IN
patients (6 in 50 mcg arm)
•
1 patient in 50 mcg arm and 2 patients in placebo arm had heart
rate below 50 bpm and notable change from baseline |
 Select Opioid
Clinical Trials Side Effects Source: Stegmann et. al. (2008). The efficacy and
tolerability of multiple-dose tapentadol immediate release for the relief of acute
pain following orthopedic (bunionectomy) surgery. Current Medical Research and
Opinion Placebo
Tapentadol IR
50mg
Tapentadol IR
100mg
Oxycodone IR
10mg
Event
n = 67
n = 67
n = 68
n = 67
Nausea
17.9%
46.3%
66.2%
71.6%
Dizziness
14.9%
32.8%
64.7%
56.7%
Somnolence
7.5%
28.4%
36.8%
26.9%
Vomiting
1.5%
16.4%
35.3%
38.8%
Headache
10.4%
17.9%
22.1%
20.9%
Pruritus generalized
0.0%
7.5%
13.2%
10.4%
Hyperhidrosis
1.5%
6.0%
8.8%
10.4%
Constipation
1.5%
6.0%
7.4%
17.9%
Pruritus
3.0%
7.5%
7.4%
11.9%
Feeling Hot
4.5%
7.5%
2.9%
10.4%
16 |
 Dex-IN
Next Steps – REC-14-013
(US placebo controlled trial)
•
Phase II bunionectomy study in approx. 200 –
250pts
•
Post Op Day 1 dosing (previous trial Post Op Day 0)
•
Interim analysis for sample size adjustment planned
•
Top line results expected mid 2015
17
–
Randomized, placebo controlled study
–
Primary endpoint –
SPID over 48 hours
–
Rescue therapy allowed
–
Pain trajectory stable / declining
–
approximately half of the evaluable patients enrolled |
 Dex-IN
Study REC-11-010 (US placebo controlled POC trial)
•
24 chronic lower back pain (CLBP) patients
–
Chronic opioid users & non-opioid users
•
PBO controlled, cross-over to evaluate:
–
Analgesia –
Standard VAS for Pain Intensity and Pain Relief at multiple
timepoints
–
Safety –
Adverse Events, Vital Signs, Sedation
•
Single doses in a 3-way cross-over
–
PBO
–
Dex-IN 25 µg
–
Dex-IN 50 µg
•
Pain intensity measurements focused on 1 hour with
patients monitored for up to 24 hours
18 |
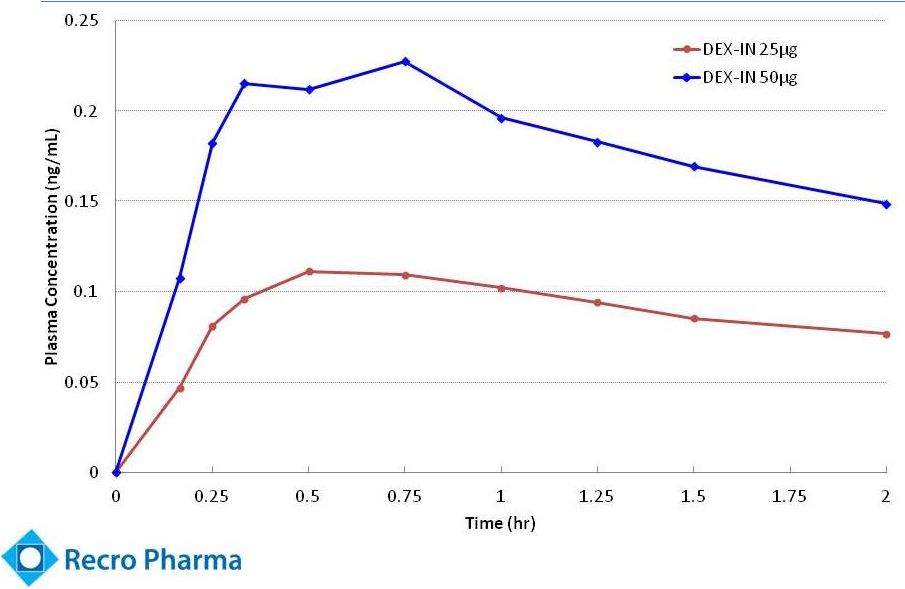 Fast Onset Of
and Prolonged Action (Clinical trial REC-11-010 –
Dex-IN pharmacokinetics)
Note: Administered with single unit device
19 |
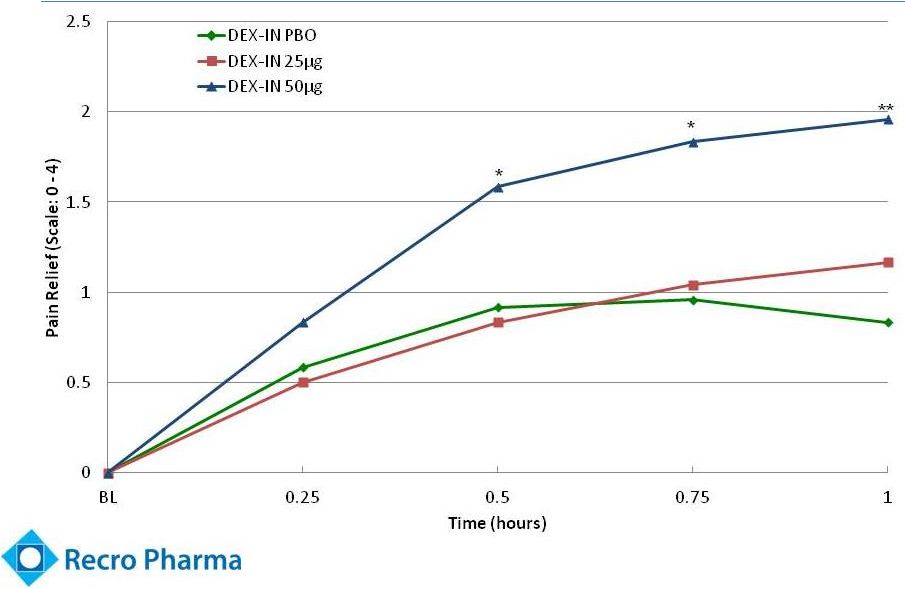 Statistically
Significant Pain Relief (Dex-IN –
REC-11-010)
Scale: 0 = No Relief, 4 = Complete Relief
* p < 0.05
** p < 0.01
20 |
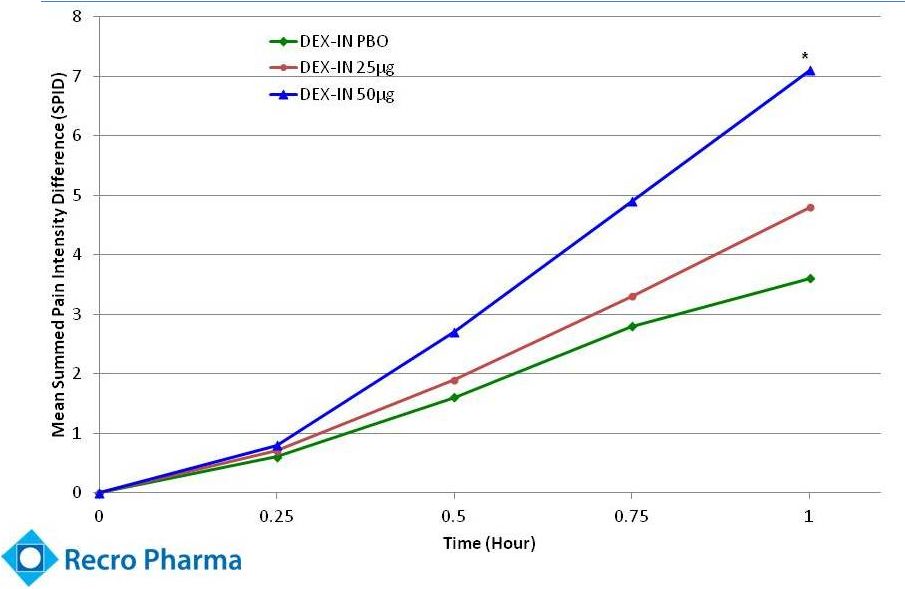 Significant
Pain Relief Over Time (Dex-IN –
REC-11-010 –
Summed Pain Intensity Difference)
* p < 0.05
21 |
 Dex-IN
Well Tolerated (Clinical trial REC-11-010 -
Adverse events†)
Placebo
(n=24)
DEX-IN 25 µg
(n=24)
DEX-IN 50 µg
(n=24)
Dry Mouth
-
2
2
Nausea
1
3
5
Vomiting
-
1
2
Feeling Abnormal
-
2
3
BP Decrease
-
-
2
Dizziness
4
5
10
Headache
1
4
4
Paraesthesia
-
-
2
Sinus Headache
-
2
1
Somnolence
-
6
18
Nasal Congestion
-
-
2
Nasal Discomfort
-
1
3
Hypotension
-
4
7
†Reported by more than one subject
22 |
 Dex-IN
Repeat Dosing Well Tolerated (Clinical trial REC-11-008)
•
7 consecutive doses of 35 mcg Dex-IN every 6 hours
•
Evaluated heart rate, blood pressure and BP upon
standing every 5 minutes for two hours after dosing
–
Transient effect after initial dosing
•
None of the above effects categorized by
investigators as AEs
23 |
 Well Tolerated
Profile – Repeated Dosing
(Study REC-11-008 –
35 mcg Dex-IN formulation)
Period 1
n = 12
Period 2
n = 10
Term
D1
D2
D1
D2
D3
D4
D5
D6
D7
Total
7am
1pm
7am
1pm
7pm
1am
7am
1pm
7pm
Back Pain
-
-
-
-
1
-
-
-
1
1
Muscle Spasms
-
-
-
-
-
-
-
-
-
1
Dizziness
-
1
2
-
-
-
-
-
-
3
Headache
-
-
-
1
-
-
-
-
-
1
Anxiety
-
-
1
-
-
-
-
-
-
1
Nasal Discomfort
-
3
-
5
-
-
-
-
-
6
Nasal Dryness
-
1
-
2
-
-
-
-
-
3
Rhinalgia
-
-
-
-
1
-
-
-
-
1
Rinorrhea
-
1
-
-
-
-
-
-
-
1
Number of Subjects
24 |
  Fadolmidine
(“Fado”) |
 Fado Effective
in Phase II for Pain Relief •
Alpha 2 agonist
–
more potent at the alpha 2c receptor than Dex
–
>20 fold less potent at the alpha 1b receptor than clonidine
•
Fado has demonstrated analgesia in multiple animal models
•
Positive Phase II analgesia study in bunionectomy patients
–
Intrathecal route of administration
•
Formulation work underway for topical prototype
–
Potential in regional neuropathies
•
WW rights to all human uses except Europe, Turkey and CIS
•
NCE patent w/ expected extension to 2021 / pursuing add’l IP
26 |
  Corporate
Overview |
 Intellectual
Property •
Dex applications for methods for treating/preventing
pain through intranasal, sublingual and transdermal
formulations without sedation
•
Dex composition of oral transmucosal (SL)
formulation and dispensing devices
•
Fado IP in-licensed from Orion
–
Composition of matter
–
Method of administration for analgesia
–
Treatment and prevention of hypotension and shock
•
Regulatory exclusivity
–
505(b)(2) –
3 years (Dex-IN, Dex-SL)
–
505(b)(1) –
NCE, 5 years (Fado)
28 |
 Company
Highlights •
Dex-IN –
intranasal, non-opioid in Phase II for acute
pain following surgery -
significant market opp’y
•
Multiple clinical studies demonstrate analgesic
effect, fast onset of action and well tolerated
•
Phase II post op pain trial ongoing –
Day 1 dosing
•
Multiple clinical and regulatory milestones over next
few years
•
Experienced team with significant development,
regulatory and commercial experience
29 |
