Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Durata Therapeutics, Inc. | Financial_Report.xls |
| EX-32.2 - EX-32.2 - Durata Therapeutics, Inc. | d773629dex322.htm |
| EX-32.1 - EX-32.1 - Durata Therapeutics, Inc. | d773629dex321.htm |
| EX-10.3 - EX-10.3 - Durata Therapeutics, Inc. | d773629dex103.htm |
| EX-31.1 - EX-31.1 - Durata Therapeutics, Inc. | d773629dex311.htm |
| EX-31.2 - EX-31.2 - Durata Therapeutics, Inc. | d773629dex312.htm |
| EX-10.5 - EX-10.5 - Durata Therapeutics, Inc. | d773629dex105.htm |
| 10-Q - 10-Q - Durata Therapeutics, Inc. | d773629d10q.htm |
| EX-10.4 - EX-10.4 - Durata Therapeutics, Inc. | d773629dex104.htm |
Exhibit 10.2
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Double asterisks denote omissions.
SUPPLY AGREEMENT
This Supply Agreement (“Agreement”), effective as of July 29, 2014 (the “Effective Date”), is made by and between Durata Therapeutics International B.V., a company registered in the Netherlands with offices at Spaces Zuidas II, Barbara Strozzilaan 101, 1083 HN Amsterdam, the Netherlands (“Durata”) and A.C.R.A.F. S.p.A.—a company with only one shareholder under direction and coordination of Finaf S.p.A., a company duly organized and validly existing under the laws of Italy, with its principal place of business at Viale Amelia, 70—00181 Rome Italy (“Angelini”).
WHEREAS, simultaneously with the execution of this Agreement, Durata and Angelini executed the License Agreement pursuant to Section 7.1 of which Angelini undertakes Durata will be the exclusive supplier of the Product to Angelini in accordance with the terms and conditions set forth below.
NOW THEREFORE, in consideration of the above stated premises and of the mutual covenants and agreements set forth below, and intending to be legally bound by the provisions of this Agreement, the Parties hereby agree as follows:
ARTICLE I
DEFINITIONS
Capitalized terms not otherwise defined herein shall have the meanings set forth in the License Agreement, unless otherwise specified. When used in this Agreement, the capitalized terms listed in this ARTICLE I shall have the following meanings:
1.1 “Agreed Tolerance” has the meaning set out in Section 3.1 (c).
1.2 “Agreement” means this Supply Agreement, including any exhibits or schedules hereto, as such may be amended from time to time, in writing, by mutual agreement of the Parties.
1.3 “Authorized Manufacturer” means the manufacturer of the Products for each country of the Territory as appointed by Durata and listed and described in Exhibit C authorized by EMA as well as by the local health authorities, which shall include the Back Up Manufacturers when they are ultimately qualified by EMA and the local health authorities.
1.4 “Back Up Manufacturers” has the meaning set forth in Section 2.6.
1.5 “Business Day” means Monday, Tuesday, Wednesday, Thursday or Friday of any week other than such days which constitute an official Italian or Dutch public or United States federal government holiday.
1.6 “Catastrophic Supply Failure” has the meaning set forth in Section 3.7 (b).
1.7 “Certificate of Analysis” means a certificate provided by Durata to Angelini for a Product certifying that the Product Lot meets the relevant Specifications.
1.8 “Commercially Reasonable Efforts” means that degree of skill, effort, expertise, and resources that a global pharmaceutical company under similar circumstances, in the matters addressed herein would reasonably utilize and otherwise apply with respect to fulfilling a particular obligation.
1.1 “Compound” shall mean dalbavancin, as described with greater specificity in Exhibit A.
1.9 “Effective Date” has the meaning set forth in the introductory paragraph hereto.
1.10 “Facility(ies)” means the manufacturing facility(ies) used by Durata’s contract manufacturers to practice the Processes as described in the Exhibit B.
1.11 “Firm Forecast” has the meaning set forth in Section 3.1(c).
1.12 “Firm Order” has the meaning set forth in Section 3.1(b).
1.13 “INCOTERMS 2010” means the specifications of the obligations for delivering goods in international contracts, as issued by the International Chamber of Commerce.
1.14 “License Agreement” means that certain License Agreement by and between Durata and Angelini, dated as of , 2014.
1.15 “Master Batch Record” means the master batch record of Durata, which shall include, without limitation, instructions for manufacturing, packaging, and in-process testing and release testing for Product, as amended from time to time.
1.16 “Non-Conforming Product” means Product that fails to conform to the Product Specifications.
1.17 “Notices” has the meaning set forth in Section 10.9.
1.18 “Planned Order” has the meaning set forth in Section 3.1(d).
1.19 “Process” or “Processing” means the act of purification, preparation, filling, storing, testing, and any other pharmaceutical manufacturing procedures, or any part thereof, involved in manufacturing the Products.
1.20 “Product” means a pharmaceutical composition to be marketed by Angelini in the Territory containing the Compound in (i) finished goods form with finished packaging and labelling as approved by the EMA and/or each country in the Territory (“Finished Goods Form”) or (ii) if agreed by the Parties pursuant to Section 2.1(b), bulk form without packaging or labelling (“Bulk Form”) as described in the Exhibit D.
1.21 “Product Forecast” has the meaning set forth in Section 3.1(a).
1.22 “Product Lot” means one production lot of the Product based on starting inputs, as stated in the Master Batch Record.
1.23 “Product Price” has the meaning set forth in Section 5.1.
1.24 “Product Specifications” means the manufacturing and quality specifications, including, without limitation, unit descriptions, which the Product must meet in order to be released for distribution, as approved by the EMA, as set forth in the Registration Dossier as well as in the Quality Agreement, as amended from time to time.
1.25 “Purchase Order” has the meaning set forth in Section 3.4.
1.26 “Quality Agreement” means the agreement described in Section 2.4.
1.27 “Registration Dossier” shall mean with respect to the Product, the package of technical and clinical information and data concerning such Product (and relevant manufacturing site) that is prepared by Durata for use in seeking any relevant Regulatory Approval.
1.28 “Specifications” means, with respect to the Product, the relevant Master Batch Record and Product Specifications in effect from time to time during the Term as described, as of the Effective Date, in Exhibit E.
1.29 “Supply Failure” has the meaning set forth in Section 3.7.
1.30 “Term” has the meaning set forth in Section 6.1 and for the avoidance of any doubt, shall mean the Initial Termas well as the Extended Term as set forth in Section 6.2.
1.31 “Territory” shall mean Italy (including San Marino and the Vatican City), Albania, Andorra, Armenia, Austria, Azerbaijan, Belarus, Bosnia-Herzegovina, Bulgaria, Croatia, Czech Republic, Estonia, Georgia, Greece, Hungary, Kazakhstan, Kosovo, Kyrgzstan, Lithuania, Latvia, Macedonia, Moldova, Montenegro, Poland, Portugal, Romania, Russia, Serbia, Slovenia, Slovak Republic, Spain, Tajikistan, Turkey, Turkmenistan, Ukraine, and Uzbekistan.
ARTICLE II
GENERAL SCOPE OF SERVICES
2.1 Requirements.
(a) Except as set forth in Section 3.7(b), during the Term of this Agreement, Angelini shall purchase Product and Durata shall manufacture and supply all of Angelini’s requirements for Product.
(b) As at the Effective Date, Durata shall manufacture the Product using the Authorized Manufacturers and supply the Product to Angelini in Finished Goods Form released and ready for sales in the Territory. At any time during the Term of this Agreement, Angelini may notify Durata in writing that it wishes for Durata to manufacture and supply the Product in Bulk Form. The Parties, shall promptly following the receipt of any notice under this Section 2.1(b), discuss in good faith and agree the terms applicable to the supply by Durata to Angelini of Bulk Product.
2.2 Inspections.
(a) Durata agrees to allow, after consultation, Angelini or parties contracted by Angelini and reasonably acceptable by Durata to conduct inspections reasonably required in connection with the manufacture of the Products. Angelini shall have the right, upon no less than [**] Business Days’ prior written notice to Durata, to conduct, during normal business hours and on the same dates and at the same times as any audit conducted by Durata, a quality assurance audit and inspection of Durata’s records and the Third Party production facilities used in the manufacture of the Products. Angelini shall comply, and shall ensure that its auditors comply, with the terms of any confidentiality or non-disclosure agreement requested by any of Durata’s Third Party production facilities and shall treat any Information received during any such audit as Confidential Information in accordance with the terms of Article VIII of the License Agreement. Except as expressly provided in the remaining provisions of this Section 2.2, Angelini may audit and inspect each facility that is used for any Processing of the Products by or on behalf of Durata pursuant to this Agreement no more than [**] years. More frequent audits by Angelini will be permitted by Durata in case of critical non conformities of the Products supplied hereunder or, if based on less than satisfactory results for previous audits of Durata facilities, subject to [**] Business Days’ prior written notice to Durata, and subject to Durata’s agreement, such agreement not to be unreasonably withheld. The duration of such audits shall not exceed [**] days per facility and such audits shall be performed by no more than [**] per facility. Authorized Manufacturers shall hold all necessary manufacturing authorizations granted by relevant European Government Authorities for the activities indicated in such manufacturing authorizations. Any Authorized Manufacturers (located inside and outside the EU) shall hold and maintain local authorizations, as required by local Laws. Durata will supply an EU-cGMP certification for all of the Authorized Manufacturers and for the relevant pharmaceutical finished dosage form released by a European Health Authority. Durata states and warrants that the Product shall be manufactured only by Authorized Manufacturers described in Exhibit C, as such list may be modified by the mutual agreement of the Parties, not to be unreasonably withheld.
(b) Angelini agrees to allow, after consultation, Durata or parties contracted by Durata and reasonably acceptable by Angelini to conduct inspections reasonably required in connection with Angelini’s storage and distribution of the
Products. Durata shall have the right, upon no less than [**] Business Days’ prior written notice to Angelini, to conduct, during normal business hours and on the same dates and at the same times as any audit conducted by Angelini, a quality assurance audit and inspection of Angelini’s records and the storage and distribution facilities used in the storage and distribution of the Products (including any Third Party facilities used by Angelini). Durata shall comply, and shall ensure that its auditors comply, with the terms of any confidentiality or non-disclosure agreement requested by any of Angelini’s Third Party facilities and shall treat any Information received during any such audit as Confidential Information in accordance with the terms of Article VIII of the License Agreement. Except as expressly provided in the remaining provisions of this Section 2.2, Durata may audit and inspect each facility that is used for any storage and distribution of the Products by or on behalf of Angelini pursuant to this Agreement no more than [**] years. More frequent audits by Durata will be permitted by Angelini in case of critical non conformities in the storage and handling of the Products or, if based on less than satisfactory results for previous audits of Angelini facilities, subject to [**] Business Days’ prior written notice to Angelini, and subject to Angelini’s agreement, such agreement not to be unreasonably withheld. The duration of such audits shall not exceed [**] days per facility and such audits shall be performed by no more than [**] per facility.
2.3 Specifications.
(a) Durata shall, and shall ensure that its Authorized Manufacturers shall, Process the Products in accordance with all applicable Laws, manufacturing authorizations and the Specifications. Products provided by Durata pursuant to this Agreement shall conform to the Specifications at the time of delivery by Durata. Durata is responsible for an Annual Product Quality Review, on-going stability and the audit of the active pharmaceutical ingredients manufacturer (for GMP requirement) and to ensure that Authorized Manufacturers comply with all of the EU-GMP/gdp requirements.
(b) The residual shelf life of the Product at the delivery date shall not be less than [**]% of the total shelf life of the Product, based upon the assumption that the shelf life shall be [**] months (similar to the already granted by FDA in the United States of America).
(c) Except as otherwise agreed by the Parties pursuant to Section 2.1(b), Durata shall package the Products in Finished Goods Form released and ready to sale with labels and other packaging in accordance with all applicable Laws and the Specifications. Angelini shall develop and provide to Durata all artwork, labelling (including all special labelling, such as Bollino), product inserts and other packaging designs for the Product and shall ensure that all such artwork, labelling, product inserts and other packaging is in compliance with all Regulatory Approvals for the Product and all applicable Law in each respective country in the Territory and is reasonably acceptable to Durata. Additionally, Angelini shall supply to Durata sufficient quantities of any and all special packaging and labeling required for any country in the Territory, including, but not limited to, required pricing imprinting, Italian Bollini labels, etc. These items must be supplied to Durata with sufficient lead time in order to include on the Product in Finished Goods Form. After receipt of the Product, Angelini may not re-package or re-label the Product without the prior written approval of Durata, not to be unreasonably withheld.
(d) Durata shall retain samples of each batch of the Products supplied to Angelini pursuant to this Agreement as described in the Quality Agreement and manage any stability programs required by commercially reasonable regulatory requirements including storage, testing and reporting, as necessary at its cost.
(e) Durata may implement Process, Specification and all other changes as approved or required by applicable Law (including by the EMA and European Union). Angelini must comply with any such changes in the Territory as requested by Durata. Any Process or Specification change requested by Angelini for any country in the Territory which is not subject to the jurisdiction of the EMA shall be discussed and shall require Durata’s prior written agreement to implement. The costs and expenses for the implementation of any such changes will be borne by the party requesting the change; provided, notwithstanding Section 5.1, any cost incurred by Durata as a result of changes required by applicable Law, will be appropriately reflected in changes to the Price.
(f) Any documentation or materials requested by Angelini for use with the Products for any country in the Territory which is not subject to the jurisdiction of the EMA shall be supplied by Durata free of any costs if already available. If such documentation or materials are not already available, Angelini will bear any incremental costs of Durata in supplying such documentation or materials.
2.4 Quality Agreement. The Parties shall discuss and agree, where appropriate, on specific procedures and guidelines for batch release, quality control testing and quality assurance review, acceptance testing, and other roles and responsibilities related to the Compound and the Products, which procedures and guidelines shall be set forth in the Quality Agreement. Durata guarantees that Authorized Manufacturers:
a) will comply with EU Directive 2001/83 as amended, EU-GMP (for active pharmaceutical ingredient and finished product) and relevant ICH/EMA guidelines (e.g. risk assessment for excipients, elemental impurities, etc.) and (for all the countries outside the EU territory) with the local GMP; and
b) will be duly authorized as a batch releaser in accordance with the local Law of the Territory
Further, the Quality Agreement shall reflect the terms detailed in the Exhibit F.
2.5 Back up Manufacturer. Durata shall use its Commercially Reasonable Efforts to qualify with the EMA as well as to the other health authorities in the Territory an appropriate back up manufacturer for the Compound and an appropriate back up manufacturer for the Product (collectively referred to as the “Back up Manufacturer”) as soon as reasonably practicable after the Effective Date.
ARTICLE III
FORECASTS AND PURCHASE ORDERS
3.1 Monthly Forecast.
(a) Product Forecast. Within [**] days from the date that EMA Regulatory Approval is granted and thereafter on or before the last Business Day of each calendar month, Angelini shall submit to Durata an [**] month rolling forecast that sets forth the total quantity of Products that Angelini either has ordered, desires to order, or expects to order from Durata within the next [**] month period (“Product Forecast”). For the avoidance of doubt, the Product Forecast shall not include the calendar month during which the Product Forecast is provided. The first Product Forecast shall be delivered to Durata within [**] months from the date on which the EMA Regulatory Approvals are obtained by Durata as described in Section 4.1 (a) of the License Agreement.
(b) Firm Orders. The forecast quantities for the [**] calendar months only in a Product Forecast shall include the requested delivery dates and shall be binding upon the Parties (“Firm Orders”).
(c) Non-Binding Forecasts. The forecast quantities for the [**] up to the [**] month in a Product Forecast are non-binding orders (“Planned Orders”), but orders which Angelini reasonably believes are likely over time to become Firm Orders. Angelini may revise the quantity and timing of Planned Orders at each scheduled update, provided that any such revision to a Planned Order for a given Calendar Month (whether increase or reduction) may not in any circumstances exceed a maximum of [**] per cent ([**]%) (“Agreed Tolerance”) from the quantity first specified when such Calendar Month was the [**] month in the Product Forecast. Durata shall be obliged to satisfy the relevant requirements of such Firm Order.
3.2 Information for long-term Planning. By [**] in each Calendar Year, Angelini shall submit to Durata its non-binding information for long-term planning of its estimated requirements for the Product, split into resale and sampling quantities for the following [**] years or the remaining portion of this Agreement, whichever is shorter. This information is for informational and planning purposes only and will not amend any Product Forecast or Purchase Order then in effect.
3.3 Amending Forecasts.
(a) Non-binding Forecasts. Planned Orders are to be considered to be estimated forecasts for planning purposes only and shall not be construed as a firm commitment by Durata to Angelini. Planned Orders may be reasonably increased or reduced by Angelini from time to time.
(b) Firm Orders. Once a Planned Order has become a Firm Order, it cannot be reduced, delayed, or increased by Angelini without the written consent of Durata.
3.4 Purchase Orders. Angelini shall, together with its monthly Product Forecast, deliver to Durata a written purchase order (“Purchase Order”) in respect of each Firm Order for which Angelini has not previously submitted a Purchase Order. Angelini will accompany its monthly update of the Product Forecast with a new Purchase Order for each new Firm Order that was a Planned Order in the previous month’s Product Forecast. Each Purchase Order shall specify the Products ordered, the quantities of the Product ordered, and the requested time, manner and address of delivery.
3.5 Order Size. Each Purchase Order shall specify a quantity of Products that is consistent with a minimum batch size, on a country by country basis, of [**] vials of Product per country. Durata has the right not to fulfill any Purchase Order with respect to Products intended for a specific country if such Purchase Order does not conform to the minimum batch size for such country. If Durata does not deliver the requested quantity ordered by Angelini within a deviation of ± [**]% Durata shall discuss in good faith with Angelini its requirements for the requested quantity ordered and, unless Angelini determines that the requested quantity ordered (or any of it) is not required, Durata shall manufacture and deliver such remaining amounts (or such of them as may be required by Angelini) to Angelini within [**] Business Days.
3.6 Durata’s Fulfillment of Purchase Orders. Durata shall use Commercially Reasonable Efforts to satisfy Angelini’s Purchase Orders in accordance with their terms.
3.7 Supply Failure.
Durata shall be deemed to have failed to supply the Products (a “Supply Failure”), if: (i) Durata fails to deliver Products (through its Authorized Manufacturers) as well as through its Back Up Manufacturer) in accordance with this Agreement (and ordered by Angelini in accordance with this Agreement) and, (ii) as a result, Angelini is unable to meet customer orders for Products in a given country in the Territory after depleting all of the Safety Stock and all Back-up Stock (as defined below);
For the avoidance of any doubt:
1) provided, that Angelini has, at all times, maintainedat least [**] months’ inventory (for each country in the Territory) on hand as safety stock to protect based upon the average sales made by Angelini in such a period (such inventory, the “Back-up Stock”) and
2) the date on which a Supply Failure occurs is the date on which the circumstance described in the previous para (ii) occurs (“Supply Failure Date”);
(a) “). Further, any interruption of supply experienced by Angelini when Angelini has less than [**] months’ of Back-up Stock on-hand of the applicable Product shall not be deemed a “Supply Failure”, unless Angelini’s failure to have [**] months’ Back-up Stock is due to Durata’s failure to supply Product in accordance with this Agreement. In addition, for clarity, any shortage of Product due in whole or in part as a result of a Force Majeure event will not be considered a Supply Failure.
(b) Quantity Limitation. In the event of a Supply Failure, Durata shall be obliged to reallocate to Angelini, until such time as the Supply Failure has been cured, quantities of the Product that are within its inventory or reasonable control, on a pro rata basis, based on the prior year sales of the Product in the Territory compared to the prior year total sales of the Product in all other territories (“Available Products”). In the event that the Supply Failure has not been cured by Durata within a period of [**] months from the Supply Failure Date, this shall be considered a “Catastrophic Supply Failure”.
(c) In the event of a Catastrophic Supply Failure, in addition to the rights granted to Angelini under the Marketing License in the License Agreement Angelini shall, subject to Section 3.7 (d), have a non-exclusive license, effective as of the date of the Catastrophic Supply Failure, under the Durata Patent Rights, the Durata Trademarks and the Durata Know How to the extent necessary to make, have made, and import the Product, including the right to manufacture and/or to have manufactured the Product by a Permitted CMO (as defined in Section 3.7 (d) below).
(d) In order to exercise its right to manufacture the Product under Section 3.7 (c) and to receive any technology transfer under Section 3.7 (e) Angelini and any Permitted CMO shall enter into a confidentiality and non-use agreement with Durata protecting the Durata Know-How relevant to the manufacture of the Product (the “Non-Use Agreement”). A “Permitted CMO” is a Third Party manufacturing organization selected by Angelini that is reasonably acceptable to Durata. Angelini understands that in determining whether a Third Party manufacturing organization is acceptable, Durata may, acting reasonably, take into consideration, among other factors, Durata’s ability to enforce the Non-Use Agreement against such Third Party.
(e) Durata shall, within [**] days from the date of the Catastrophic Supply Failure, use Commercially Reasonable Efforts to provide a technology transfer reasonably sufficient to permit Angelini (or its Permitted CMO) to manufacture or have manufactured the Product.
(f) Durata shall cooperate and reasonably support Angelini’s efforts to file the appropriate filings or otherwise amend the applicable Regulatory Approvals in the Territory as well as the EMA Regulatory Approval for the Product to permit Angelini (or its Permitted CMO) to manufacture the Product pursuant to Section 3.7 (c) above.
(g) Notwithstanding Section 11.3, or any other provision, of the License Agreement, (i) Durata will have no obligation to defend, indemnify or hold harmless Angelini (nor bear any other obligation or liability) with respect to Product Liability Claims arising from the manufacture of Products made by Angelini (or its Permitted CMO) following a Catastrophic Supply Failure and (ii) Angelini will, pursuant to Section 11.3 of the License Agreement, indemnify Durata for any such Product Liability Claims arising from the manufacture of Products made by Angelini (or its Permitted CMO) following a Catastrophic Supply Failure.
(h) Except as stated above, all the other terms and conditions described in this Agreement and the License Agreement shall remain fully valid and effective.
3.9 Safety Stock. For each of the Products, during the Term, Durata undertakes to keep a rolling safety stock of Product exclusively dedicated to Angelini, in Bulk Form, equal or higher than the amount of Product ordered by Angelini in the previous [**] month period (the “Safety Stock”).
ARTICLE IV
DELIVERY; INVOICES
4.1 Delivery.
(a) All deliveries of the Product in Finished Goods Form shall be made by Durata ExWorks (INCOTERMS 2010) the Facilities. Title and risk of loss will shift to Angelini upon delivery to the common carrier at the Facilities for shipment to Angelini’s warehouse located at Ancona, Italy, Europe, or such other locations in Europe as may be designated by Angelini to Durata from time to time within [**] months from the date of receipt of the relevant order. Angelini will then be responsible for coordinating and executing all logistics and documentation required to move Product into those markets in the Territory.
(b) Notwithstanding the foregoing, Durata shall not be obligated to deliver the Products to more than [**] locations requested by Angelini.
4.2 Certificates of Analysis. Durata shall provide to Angelini, in preparation for each delivery of the Products, a Certificate of Analysis as specified in the Quality Agreement for each batch of Products delivered.
4.3 Certificate of Origin. Durata shall, for customs purposes, upon delivery of the Products, provide Angelini with a valid declaration of origin, in a form reasonably acceptable to Angelini, in respect of all Products supplied to Angelini under this Agreement, together with such other supporting documents relating to the origin of such Products as Angelini may reasonably require.
4.4 Packaging. Durata shall package and label the Products appropriate for delivery to end-users in accordance with the Specifications.
4.5 Method of Invoicing. All orders under this Agreement shall be invoiced at the time of shipment.
ARTICLE V
PRICE
5.1 Product Price. Subject to Sections 5.2 and 5.3, the price for each vial of Product (the “Product Price”) shall be [**] Euros (€[**]).
5.2 Price Changes. From the [**] anniversary of the Effective Date, Durata shall have the right to increase the Product Price by [**] per year. In the event a generic product receives regulatory approval in the Territory, is commercialized, and affects Angelini’s revenues for the Product, the parties will discuss in good faith the potential to reduce the Product Price.
5.3 Remittance of Payments. Payments due to Durata under Section 5.1 shall be payable by Angelini no later than [**] days after the invoice date. Angelini shall make payment by wire transfer of Euros to a bank account designated by Durata or by such other payment method as the Parties may agree upon from time to time.
5.4 Currency. All payments under this Agreement shall be made in Euro.
5.5 Interest on Late Payments. Angelini shall be liable to pay interest to Durata at the average one-month European Interbank Offered Rate (EURIBOR) for the Euro as reported from time to time in the Wall Street Journal plus [**] percent ([**]%), but in no event higher than the highest rate permissible under Law on all overdue amounts from the due date until the date the amount is paid in full.
5.6 Deductions from Payments.
(a) Angelini will make all payments to Durata under this Agreement without deduction or withholding for taxes except to the extent that any such deduction or withholding is required by applicable Law in effect at the time of payment. Any tax required to be withheld on amounts payable under this Agreement will promptly be paid by Angelini on behalf of Durata to the appropriate governmental authority, and Angelini will furnish Durata with proof of payment of such tax. Any such tax required to be withheld will be an expense of and borne by Durata.
(b) Angelini and Durata will cooperate with respect to all documentation required by any taxing authority or reasonably requested by Durata to secure a reduction in the rate of applicable withholding taxes.
5.7 Value Added Tax. All payments under this ARTICLE V shall be exclusive of value added tax. Angelini shall pay for its own account all value added taxes as required by applicable Law.
ARTICLE VI
TERM AND TERMINATION
6.1 Term. This Agreement shall be in effect from the Effective Date and shall continue in effect until the tenth (10th) anniversary of the launch of the first Product into the Territory, unless terminated pursuant to Section 6.2 (the “Initial Term”).
6.2 Extended Term. Parties agree that at the expiry of the Initial Term above described, Angelini shall have the right to extend the term of this Agreement up to ten (10) times, each for a period of one (1) year at the same terms and conditions described in this Agreement (each such one year period, an “Extended Term”), upon written notice provided to Durata no later than ninety (90) days prior to the expiration of the Initial Term or each subsequent Extended Term.
6.3 Termination by Durata for breach. Durata shall be entitled to terminate this Agreement in the event that Angelini commits a material breach of this Agreement and (i) the breach is incurable, or (ii) Angelini fails to cure such breach within [**] days of receiving a Notice of default from Durata (or such longer period as Durata may reasonably agree if said breach is incapable of cure within such [**] days), by giving a Notice of termination to Angelini within [**] days of first becoming aware of such breach (if such breach is incurable) or within [**] days of the end of such cure period.
6.4 Termination by Angelini for breach. Angelini shall be entitled to terminate this Agreement in the event that Durata commits a material breach of this Agreement and (i) the breach is incurable, or (ii) Durata fails to cure such breach within [**] days of receiving a Notice of default from Angelini (or such longer period as Angelini may reasonably agree if said breach is incapable of cure within such [**] days), by giving a Notice of termination to Durata within [**] days of first becoming aware of such breach (if such breach is incurable) or within [**] days of the end of such cure period. Durata acknowledges that a Catastrophic Supply Failure would constitute a material breach of this Agreement for the purposes of this Section. The Parties acknowledge that if Angelini terminates this Agreement as a result of a Catastrophic Supply Failure the License Agreement shall continue to the extent detailed in Section 14.4] of the License Agreement
6.5 Cross Termination. If either Party terminates the License Agreement for material breach in accordance with Section 14.2 of the License Agreement, such Party may by giving written notice to the other Party, terminate this Agreement,, immediately as of the date specified in the notice of termination.
6.6 Effect of Termination. Termination of this Agreement for any reason is without prejudice to the Parties’ accrued rights and shall not be construed to release either Party of any obligation matured prior to the effective date of such termination. Except where this Agreement is terminated by Angelini as a result of a Catastrophic Supply Failure (in which case Angelini shall have the rights of manufacture detailed in Section 3.7), Angelini shall have no right or license to manufacture Products upon or after expiration or termination of this Agreement.
6.7 Survival. Sections 5.3, 6.6, 6.7, ARTICLE VIII, and ARTICLE X shall survive the expiration or termination of this Agreement.
ARTICLE VII
REPRESENTATIONS AND WARRANTIES
7.1 Representations and Warranties. Durata and Angelini each represents and warrants to the other that:
(a) Duly Executed. This Agreement has been duly executed and delivered by such Party and constitutes a legal, valid and binding obligation of such Party, enforceable against such Party in accordance with its terms, except as such enforceability may be limited by applicable insolvency and other laws affecting creditors’ rights generally or by the availability of equitable remedies.
(b) Duly Authorized; No Conflicts. The execution, delivery and performance of this Agreement by such Party and all instruments and documents to be delivered by such Party hereunder and the performance of such Party’s obligations hereunder
| (i) | are within the corporate power of such Party; |
| (ii) | have been duly authorized by all necessary or proper corporate action; |
| (iii) | do not conflict with any provision of the charter documents of such Party; |
| (iv) | do not conflict with or violate any requirement of applicable Laws; |
| (v) | do not and will not conflict with, violate or breach or constitute a default or require any consent under, any contractual obligations of such Party, except such consent as shall have been obtained prior to the Effective Date; and |
| (vi) | do not and will not require any filing or registration with or the license, permit, consent, approval or authorization of or any notice to any Governmental Authority, except such as shall have been obtained prior to the Effective Date. |
(c) Duly Organized. It:
| (i) | is a company duly organized, validly existing and in good standing under the Laws of its jurisdiction of incorporation; |
| (ii) | is duly qualified as a corporation and in good standing under the Laws of each jurisdiction where its ownership or lease of property or the conduct of its business requires such qualification, where the failure to be so qualified would have a material adverse effect on its financial condition or its ability to perform its obligations hereunder; |
| (iii) | has the requisite corporate power and authority and the legal right to conduct its business as now conducted and hereafter contemplated to be conducted; |
| (iv) | has all necessary licenses, permits, consents, authorizations or approvals from or by, and has made all necessary notices to, all Governmental Authorities having jurisdiction, to the extent required for such ownership and operation; and |
| (v) | is in compliance with its organizational documents. |
(d) Warranty by Durata. Durata hereby represents and warrants to Angelini that the quality (purity, physical and chemical properties) of the Products supplied by it to Angelini shall be in accordance with their respective Specifications and the Quality Agreement. This warranty is exclusive and is in lieu of all other warranties, whether written or oral express, implied or statutory including any such warranties of merchantability, satisfactory quality or fitness for any purpose and, also in view of article 7:17 of the Dutch Civil Code, exhaustively reflects all of the characteristics Angelini may reasonably expect the Products to have. Further, the parties agree that this Agreement is not governed by the United Nations Convention on the International Sale of Goods, the application of which is hereby expressly excluded.
ARTICLE VIII
REMEDIES FOR NON-CONFORMING PRODUCT
8.1 Non-Conforming Product.
(a) Durata shall promptly notify Angelini of any Non-Conforming Product of which it becomes aware, which have been delivered to Angelini, specifying the release testing of the Product and the relevant Master Batch Record.
(b) Angelini shall notify Durata of any Non-Conforming Product within (i) [**] business days of Angelini’s receipt of such Non-Conforming Product in the event of a defect discovered by Angelini through the use of reasonable testing methods and procedures mutually agreed to by the Parties or (ii) during the full shelf life of the product within [**] Business Days of Angelini’s confirmation of the Non-Conforming status of the Product in the event of a defect (latent or otherwise) which was not discovered through the use of such testing methods and procedures. Durata shall have the right to examine and test any Product in Angelini’s possession that Angelini claims is Non-Conforming. The Parties shall cooperate to determine the point at which the Product became Non-Conforming.
8.2 Disagreement. In case Durata and Angelini cannot agree as to whether a certain quantity of the Product delivered complies with the Specifications, then the situation shall be referred to an independent analytical laboratory mutually acceptable to both Parties for resolution. The resolution shall be final and binding on the Parties. The costs associated with the resolution proceedings shall be borne by the losing Party.
8.3 Remedies for Non-Conformance. Angelini shall be entitled to the replacement of any Non-Conforming Products delivered to Angelini by Durata with corresponding Product meeting their respective Specifications to be delivered within the
lead time described in Section 4.1. Durata shall not be responsible for Non-Conforming Products if the Non-Conformance arose (i) after receipt of the Product by Angelini (including, without limitation, as a result of improper storage by Angelini of the Product) or (ii) as a result of any change in artwork, labelling, product inserts and other packaging designs for the Product requested by Angelini and/or required a Governmental Authority in the Territory. Durata shall bear the costs of freight and insurance for return of the Non-Conforming Products which must be replaced by Durata pursuant to this Section 8.3. Angelini shall, at Durata’s option and cost, either return to Durata at the Facility or destroy the Non-Conforming Products. Except as set forth in this Section 8.3 and Section 11.3 of the License Agreement, Durata shall have no liability for Non-Conforming Product, and Angelini’s sole remedy shall be as set forth in this Section 8.3 and Section 11.3 of the License Agreement.
ARTICLE IX
REGULATORY MATTERS
9.1 Government Inspections. In the event that Durata or any Affiliate or Third Party contractor of Durata is audited or inspected by the EMA (or any other Governmental Authority whose inspection might reasonably be expected to impact the EMA certification of the relevant facility) with respect to a facility used for Processing the Products pursuant to this Agreement, Durata shall promptly (but in any event, within [**]) notify Angelini of such audit or inspection as well as of any alleged violations or deficiencies. Durata shall use Commercially Reasonable Efforts to correct all such deficiencies in a timely manner and advise Angelini periodically of progress being made, as well as when all such deficiencies have been corrected.
ARTICLE X
GENERAL PROVISIONS
10.1 Confidentiality. From the Effective Date, all information proprietary or confidential to a Party that the other Party acquires through its participation in the negotiation and performance of this Agreement shall be considered Confidential Information subject to Article VIII of the License Agreement.
10.2 Relationship of the Parties. Each Party shall bear its own costs incurred in the performance of its obligations hereunder without charge or expense to the other except as expressly provided in this Agreement. For all purposes, and notwithstanding any other provision of this Agreement to the contrary, Angelini’s legal relationship under this Agreement to Durata shall be that of independent contractor. Nothing in this Agreement shall be construed to establish a partnership or joint venture, or a relationship of co-partners or joint venturers between the Parties.
10.3 Limitation of Liability. TO THE EXTENT PERMITTED BY LAW, NOTWITHSTANDING ANY OTHER PROVISION CONTAINED HEREIN, UNLESS RESULTING FROM A PARTY’S FRAUDULENT BEHAVIOR, IN NO EVENT SHALL DURATA, ON THE ONE HAND, OR ANGELINI, ON THE OTHER HAND, BE LIABLE TO THE OTHER OR ANY OF THE OTHER’S AFFILIATES FOR ANY
CONSEQUENTIAL, INCIDENTAL, INDIRECT, SPECIAL, PUNITIVE OR EXEMPLARY DAMAGES (INCLUDING, WITHOUT LIMITATION, LOST PROFITS, BUSINESS OR GOODWILL) SUFFERED OR INCURRED BY SUCH OTHER PARTY OR ITS AFFILIATES IN CONNECTION WITH A BREACH OR ALLEGED BREACH OF THIS AGREEMENT.
10.4 Force Majeure. The occurrence of an event that materially interferes with the ability of a Party to perform its obligations or duties hereunder which is not within the reasonable control of the Party affected, not due to such Party’s malfeasance, and which could not with the exercise of due diligence have been avoided (“Force Majeure”), including, but not limited to, an injunction, order or action by a Governmental Authority, fire, accident, labor difficulty, strike, riot, civil commotion, act of God, delay or errors by shipping companies or change in Law, shall not excuse such Party from the performance of its obligations or duties under this Agreement, but shall merely suspend such performance during the continuation of Force Majeure. The Party prevented from performing its obligations or duties because of Force Majeure shall promptly notify the other Party hereto (the “Other Party”) of the occurrence and particulars of such Force Majeure and shall provide the Other Party, from time to time, with its best estimate of the duration of such Force Majeure and with notice of the termination thereof. The Party so affected shall use reasonable efforts to avoid or remove such causes of nonperformance. Upon termination of Force Majeure, the performance of any suspended obligation or duty shall promptly recommence.
10.5 Choice of Law. All disputes, claims or controversies arising out of, relating to or in connection with this Agreement (“Disputes”), including any question regarding its formation, existence, validity, enforceability, performance, interpretation or termination, shall be governed by the laws of the Netherlands (without reference to its choice of law or conflicts of law rules).
10.6 Dispute Resolution. The Parties irrevocably agree that any dispute, controversy or claim arising out, of or in relation to this Agreement, including the validity, invalidity, breach or termination thereof, will be settled by arbitration in accordance with the Rules of the International Chamber of Commerce in force on the date when the notice of arbitration is submitted. The number of arbitrators will be three (3). The seat of arbitration will be London, England. The arbitral proceedings will be conducted in English.
10.7 Remedies Cumulative. The remedies set forth in this Agreement (including without limitation termination rights) are cumulative and shall not be construed to restrict or otherwise affect any other remedies that may be available under any other agreement or under Law.
10.8 Assignment. This Agreement may not be assigned by either Party without the prior consent of the other Party; provided, however, that each Party shall have the right to assign its rights and obligations under this Agreement to any Third Party successor to all or substantially all of (i) its entire business or (ii) its pharmaceutical business. It is further understood and agreed that each Party shall assign, or otherwise
cause to be performed, its obligations under this Agreement (including, without limitation, obligations of confidentiality, processing and payment) to or by, as the case may be, one or more of its Affiliates to the extent necessary or appropriate in order to ensure that such obligations are fulfilled in accordance with the terms and intent of this Agreement, provided, however, that in the event a Party causes any of its obligations to be performed by an Affiliate, such Party shall guarantee such performance. This Agreement shall be binding upon, and subject to the terms of the foregoing sentence, inure to the benefit of the Parties hereto, their successors, legal representatives and assigns.
10.9 Notices. All demands, notices, consents, approvals, reports, requests and other communications hereunder (“Notices”) must be in writing and will be deemed to have been duly given only if delivered personally, by facsimile transmission (against confirmed receipt), by certified or registered mail, return receipt requested, or by overnight delivery using a globally-recognized carrier, to the Parties at the following addresses or facsimile numbers:
| Durata: |
Spaces Zuidas II, | |
| Kantoor 4.03 | ||
| 1083 HN Amsterdam, | ||
| The Netherlands | ||
| Attn: General Manager | ||
| With copy to: |
Durata Therapeutics, Inc. | |
| 200 S. Wacker, Suite 2550 | ||
| Chicago, IL 60606 | ||
| Attn: General Counsel | ||
| Phone: 312-219-7000 | ||
| Email: bhecht@duratatx.com |
And, a copy which shall not constitute notice to:
| Morrison & Foerster LLP | ||
| 2000 Pennsylvania Avenue, N.W. | ||
| Washington, D.C. 20006 | ||
| Attention: Van Ellis | ||
| Phone: 202-887-8776 | ||
| Email: vellis@mofo.com | ||
| Angelini: |
Viale Amelia 70 | |
| 00181 – Rome | ||
| Italy | ||
| Attn: CEO Ing.Gianluigi Maria Frozzi | ||
| Phone: +39 06 70853283 | ||
| Email: legale@angelini.it | ||
| With copy to: |
Finaf S.p.a. | |
| Viale Amelia 70 | ||
| 00181 – Rome | ||
| Italy | ||
| Attn: Adelmo Di Michele | ||
| Phone: +39 06 70853269 | ||
| Email: a.dimichele@angelini.it |
or to such other address as the addressee shall have last furnished in writing in accord with this provision to the addressor. All Notices shall be deemed effective upon receipt by the addressee.
10.10 Invalid Provisions. If any provision of this Agreement is held to be illegal, invalid or unenforceable under any applicable present or future Law (the “Unenforceable Provision”), then such Unenforceable Provision will be fully severable, and the remaining provisions of this Agreement will remain in full force and effect and will not be affected by the Unenforceable Provision or by its severance herefrom.
10.11 Headings. The headings used in this Agreement have been inserted for convenience of reference only and do not define or limit the provisions hereof.
10.12 Waiver. Any term or condition of this Agreement may be waived at any time by the Party that is entitled to the benefit thereof, but no such waiver shall be effective unless set forth in a written instrument duly executed by or on behalf of the Party waiving such term or condition. No waiver by any Party of any term or condition of this Agreement, in any one or more instances, shall be deemed to be or construed as a waiver of the same or any other term or condition of this Agreement on any future occasion. The failure of either Party at any time or times to require performance of any provision hereof shall in no manner affect its rights at a later time to enforce the same.
10.13 Entire Agreement. This Agreement (including the exhibits and schedules hereto), the License Agreement and the Quality Agreement constitute the entire agreement between the Parties hereto with respect to the within subject matter and supersedes all previous agreements and understandings between the Parties, whether written or oral. In the event of any inconsistency between the terms of this Agreement and the License Agreement, the License Agreement terms, including its dispute resolution terms, shall prevail. This Agreement may be altered, amended or changed only by a writing making specific reference to this Agreement and signed by duly authorized representatives of Durata and Angelini.
10.14 No License. Nothing in this Agreement shall be deemed to constitute the grant of any license or other right in either Party to or in respect of any product, patent, trademark, Confidential Information, trade secret or other data or any other intellectual property of the other Party except as expressly set forth herein.
10.15 Third Party Beneficiaries. None of the provisions of this Agreement shall be for the benefit of or enforceable by any Third Party, including, without limitation, any creditor of either Party hereto. No such Third Party shall obtain any right under any provision of this Agreement or shall by reasons of any such provision make any Claim in respect of any debt, liability or obligation (or otherwise) against either Party hereto.
10.16 Counterparts. This Agreement may be executed in any two or more counterparts, each of which, when executed, shall be deemed to be an original and all of which together shall constitute one and the same document.
10.17 Compliance with Law. Notwithstanding any other provision of this Agreement, neither Party shall be required to undertake any activity or obligation under this Agreement which it has reason to believe may violate any Laws; provided, however, a Party which so believes shall promptly inform the other Party of such belief. Furthermore, Durata shall ensure that all Product supplied to Angelini hereunder shall be manufactured in compliance with the relevant requirements of Governmental Authorities, and applicable Laws, including, without limitation, all Laws applicable to the transportation, storage, use, handling and disposal of hazardous materials used to manufacture Product. Durata shall obtain and maintain, at its expense, for so long as Durata is supplying Product to Angelini hereunder, all facility licenses and government permits, including, without limitation, health, safety, and environmental permits, necessary for the conduct of the actions and procedures undertaken to manufacture and supply the Product for importation and sale to Angelini.
10.18 List of Exhibits
A Compound
B Facilities
C Authorized Manufacturers
D Products
E Specifications
F Quality Agreement (minimum clauses)
THE BALANCE OF THIS PAGE IS LEFT BLANK INTENTIONALLY
IN WITNESS WHEREOF, the Parties hereto have caused this Supply Agreement to be executed by their respective officers hereunto duly authorized as of the Effective Date.
| DURATA TH. INT. B.V.(NL) | ||||
| By: | /s/ Corey Fishman | |||
| Name: | Corey Fishman | |||
| Title: | Director | |||
| By: | /s/ Alexander Van Oyen | |||
| Name: | Alexander Van Oyen | |||
| Title: | Managing Director | |||
| ACRAF S.P.A. | ||||
| By: | /s/ Gianluigi Maria Frozzi | |||
| Name: | Gianluigi Maria Frozzi | |||
| Title: | Managing Director | |||
EXHIBIT A
Compound
Dalbavancin drug substance is a semi-synthetic antibiotic manufactured by chemical modification of homologues of the lipoglycopeptide antibiotic A-40,926 which is produced through a fermentation process.
Dalbavancin drug substance is a mixture of five closely related homologues that can be grouped into two structural families, designated dalbavancin A (subtypes A0 and A1) and dalbavancin B (subtypes B0, B1, and B2). The components that comprise dalbavancin drug substance all share the same core structure (Table A-1). These homologues differ from one another primarily in the length and/or branching of their respective fatty acid side chains on the N-acylaminoglucuronic acid moiety (designated as R1) and/or the presence of an additional methyl group (designated as R2) on the terminal amino group. Dalbavancin drug substance is a hydrochloride salt.
Dalbavancin drug substance is isolated as a hydrochloric acid salt. However, the active moiety is the free base form. The dalbavancin drug product strength (i.e. 500 mg per vial) is reported as the free base form. The free base structure is provided below.
Figure A-1 General Structure of Dalbavancin Drug Substance
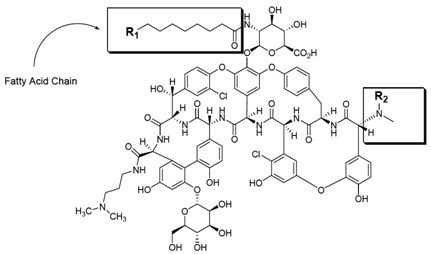
Table A-1 Substitution Patterns for Dalbavancin Homologues
| Homologue | R1 | Number of Carbons R1 | R2 | |||
| A0 |
CH(CH3)2 | 3 | H | |||
| A1 |
CH2CH2CH3 | 3 | H | |||
| B0 |
CH2CH(CH3)2 | 4 | H | |||
| B1 |
CH2CH2CH2CH3 | 4 | H | |||
|
B2 |
CH2CH(CH3)2 | 4 | CH3 |
The major dalbavancin homolog is dalbavancin B0.
Figure A-2 Structure of Dalbavancin B0
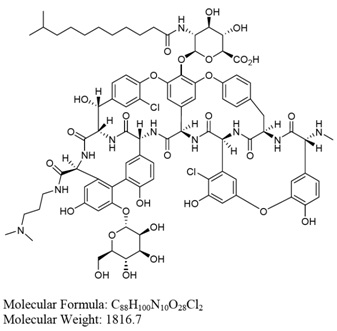
The B0 INN chemical name is: 5,31-dichloro-38-de(methoxycarbonyl)-7-demethyl-19-deoxy-56-O-[2-deoxy-2-[(10-methylundecanoyl)amino]-ß-D-glucopyranuronosyl]-38-[[3-(dimethylamino)propyl] carbamoyl]-42-O-a-D-mannopyranosyl-15-N-methyl(ristomycin A aglycone) hydrochloride.
EXHIBIT B
Facilities
Gnosis Bioresearch S.r.l – 75010 Pisticci Sala, Pisticci, Italy
Hospira Inc., 1776 Centennial Drive, McPherson, KS 67460, USA
EXHIBIT C
Authorized Manufacturers
Gnosis Bioresearch S.r.l – 75010 Pisticci Sala, Pisticci, Italy
Hospira Inc., 1776 Centennial Drive, McPherson, KS 67460, USA
EXHIBIT D
Products
The “Product” Dalbavancin for Injection, 500 mg, also known as << XYDALBA™>> , is supplied in single-use, clear glass vials as a sterile, lyophilized, preservative-free, white to off-white to pale yellow solid. Each vial contains dalbavancin HCl equivalent to 500 mg of anhydrous dalbavancin as the free base, plus lactose monohydrate and mannitol as excipients. Sodium hydroxide or hydrochloric acid may be added to adjust the pH at the time of manufacture. The powder is to be reconstituted and further diluted for IV infusion
The container closure system consists of a 48 mL glass vial with a rubber stopper and flip-off seal.
Table B-1 Quantitative Composition of Dalbavancin for Injection, 500 mg Vials
| Ingredient | Quantity per Viala | Function | ||
| Dalbavancin |
[**] mgb | Active Ingredient | ||
| Mannitol |
128.8 mg | Bulking Agent | ||
| Lactose Monohydrate |
128.8 mg | Bulking Agent | ||
| Water for Injectionc |
– | Solvent | ||
| Sodium hydroxided |
– | pH adjustment | ||
| Hydrochloric acidd |
– | pH adjustment | ||
| Total Weight |
[**] mg | |||
| a | Vials contain a [**]% overfill to ensure withdrawal of the label claim from each reconstituted vial. |
| b | Anhydrous dalbavancin free base. |
| c | Removed by lyophilization. |
| d | Added as a solution in water for pH adjustment |
A pack of Product is of one vial
EXHIBIT E
Specifications
Minimum batch size per country in the Territory is [**] vials of Product in Finished Goods Form.
EXHIBIT F
Quality Agreement (minimum clauses)
The Authorized Manufacturers of the pharmaceutical product are responsible for certifying that the API is manufactured under GMP (EUGMP Part II, ICH Q7 Guide and any other applicable territory GMP guidelines/requirements) including re-packaging, re-labeling or dividing-up of API.
All of the activities such as procuring, holding, supplying or exporting the API have to be performed in compliance with the European Commission Guidelines on the Principles of Good Distribution Practices for API for Medicinal Products for Human Use and any other applicable territory GDP guideline/requirements.
The Authorized Manufacturers of the pharmaceutical product shall verify compliance with applicable territory GMP and GDP by conducting audits at the manufacturing and distribution sites of the API manufacturer.
The Authorized Manufacturers of the pharmaceutical product shall verify such compliance themselves or, without prejudice to Directive 2011/62/EU, through an entity acting on their behalf under a contract.
A written confirmation that the Authorized Manufacturers of the pharmaceutical product have verified the compliance of the API manufacturer with applicable territory principles and guidelines of good manufacturing practice by conducting audits is mandatory.
The written confirmation shall contain a reference to the date of the audit and a declaration that the outcome of the audit confirms that the API manufacturing complies with the principles and guidelines of good manufacturing practice in the US and EU.
The Authorized Manufacturers of the pharmaceutical product shall have a written contract in place with manufacturers of API and shall audit such manufacturers, in the absence of critical quality incidence, at least [**], providing Angelini at least an abstract of the audit report.
The Authorized Manufacturers of the pharmaceutical product shall inform within [**] business days Angelini of any major GMP deviation relevant to these API.
The Authorized Manufacturers of the pharmaceutical product are responsible to certify that the API manufacturer has the supply chain traceability of starting materials, intermediates, reagents used for the manufacture of the API that should be established and documented.
This documentation should be made available for inspection at the request of the Angelini and competent authorities.
The holders of the manufacturing authorization of the pharmaceutical product shall inform within [**] business days Angelini of any major GMP deviation relevant to these GMP- starting materials.
The Authorized Manufacturers
The holders of the manufacturing authorization of the pharmaceutical product shall ensure that the excipients are suitable for use in medicinal products by ascertaining what the appropriate good manufacturing practice is.
This shall be ascertained on the basis of a formalised risk assessment in accordance with the applicable US/EU guidelines.
Such risk assessment shall take into account requirements under other appropriate quality systems as well as the source and intended use of the excipients and previous instances of quality defects.
The Authorized Manufacturers of the pharmaceutical product shall ensure that the appropriate good manufacturing practice so ascertained, is applied.
The Authorized Manufacturers of the pharmaceutical product shall verify the presence of metals listed in ICHQ3D, in EMEA/CHMP/SWP/4446/2000 and in USP <232> and <233> in the finished product supplied to Angelini.
This shall be ascertained on the basis of a formalised risk assessment in accordance with the ICHQ3D requirements.
Based on the risk assessment outcome the Authorized Manufacturers of the pharmaceutical product shall develop selective and quantitative analytical methods to verify that the content of each potential elemental impurity is within the limit calculated from the PDE defined by the ICH Q3D.
The need to add a specification for the elemental impurity control ([routine o by skip] has to be also evaluated and shared with Angelini.
The Authorized Manufacturers of the pharmaceutical product shall:
| • | inform the competent authority and the marketing authorisation holder immediately if Durata obtains information that medicinal products products which come under the scope of their manufacturing authorizations are, or are suspected of being, falsified irrespective of whether those medicinal products were distributed within the legal supply chain or by illegal means, including illegal sale by means of information society services; |
| • | verify that the manufacturers, importers or distributors from whom Durata obtains active substances are registered with the competent authority of the US or applicable EU Member State in which they are established, if applicable; and |
| • | verify the authenticity and quality of the active substances and the excipients. |
