Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Horizon Therapeutics Public Ltd Co | d804018d8k.htm |
| EX-99.1 - EX-99.1 - Horizon Therapeutics Public Ltd Co | d804018dex991.htm |
 Horizon Pharma
plc October 13, 2014
Friedreich's Ataxia Analyst Day Presentation
Exhibit 99.2
Non-Confidential Information – Horizon Pharma plc
|
 Forward-Looking Statements
2
Non-Confidential Information – Horizon Pharma
plc This presentation contains
forward-looking statements, including, but not limited to, statements related to the consummated business
combination transaction between Horizon Pharma, Inc. and Vidara Therapeutics
International plc and the benefits thereof, the strategy, plans, objectives,
expectations (financial or otherwise) and intentions, future financial results and growth potential of the combined company –
Horizon Pharma plc, development programs and management structure, and other statements that
are not historical facts. This presentation also includes forward-looking statements regarding results of the Phase
2 clinical trial of ACTIMMUNE and management expectations and plans regarding further
study of ACTIMMUNE in Friedreich’s ataxia (FA) , related discussions with the FDA, the potential for
ACTIMMUNE as a treatment for FA patients, and potential sales opportunity for
ACTIMMUNE. The statements made by representatives of the Friedreich’s
Ataxia Research Alliance and the Collaborative Clinical Research Network in Friedreich’s Ataxia represent their independent
conclusions and recommendations.
These forward-looking statements are based on Horizon Pharma plc’s current
expectations and inherently involve significant risks and uncertainties.
Actual results and the timing of events could differ materially from those anticipated in such forward looking statements as a
result of these risks and uncertainties, which include, without limitation, risks related to
future opportunities and plans for Horizon Pharma plc, including uncertainty of the
expected financial performance and results; disruption from the transaction, making it more difficult to conduct
business as usual or maintain relationships with customers, employees or suppliers; and the
possibility that if Horizon Pharma plc does not achieve the perceived benefits of the
transaction as rapidly or to the extent anticipated by financial analysts or investors, the market price of its
shares could decline, as well as other risks related to Horizon Pharma plc’s business,
including whether results of subsequent studies will be consistent with results of the
Phase 2 clinical trial of ACTIMMUNE; risks regarding timing and outcome of regulatory review and approval of
product candidates; its dependence on sales of its existing products and its ability to
increase sales of its existing products; Horizon Pharma plc’s ability to
successfully execute its commercial strategy and achieve projected financial results in 2014 and 2015; the fact that past financial or
operating results are not a guarantee of future results; competition, including potential
generic competition; the ability of Horizon Pharma plc to protect its intellectual
property and defend its patents; regulatory obligations and oversight; and those risks detailed from time-to-time under
the caption “Risk Factors” and elsewhere in Horizon Pharma plc’s SEC
filings and reports, including in its Annual Report on Form 10-K for the year ended
December 31, 2013. Horizon Pharma plc undertakes no duty or obligation to update any forward-looking statements contained in
this presentation as a result of new information, future events or changes in its
expectations. For full prescribing information, please see product websites;
www.DUEXIS.com
www.ACTIMMUNE.com
www.RAYOSrx.com
www.VIMOVO.com
,
;
;
. |
 Non-Confidential Information – Horizon Pharma
plc Forward-Looking Statements
– Friedreich’s Ataxia
Slides regarding the Friedreich’s ataxia population and market are from a report prepared
by L.E.K. Consulting LLC (“L.E.K.”) and are being provided for informational
purposes only. Recipients of such report and the information contained
therein are not permitted to rely on the report, and their access to the report is not
a substitute for the investigations and due diligence that any such recipient would
ordinarily undertake. L.E.K. is not and shall not be responsible for decisions made by any such recipient
of
the report. L.E.K. owes no obligations or duties to recipients
of this report in connection with the report, whether in contract,
tort (including negligence), breach of statutory duty or otherwise.
During the course of L.E.K.’s analysis, certain assumptions and forecasts may have been
developed. These assumptions are inherently subject to uncertainties, and
actual results may differ from those projected. While L.E.K. believes that the
assumptions and forecasts are reasonably based, L.E.K. does not provide any assurance that
these assumptions are correct, or that any forecasts will reflect actual
results. Therefore, no representation is made or intended to be made, nor should be
inferred, with respect to the likely occurrence of any particular future set of
facts. L.E.K.’s analysis is based on information available at the time
it prepared its analysis and on certain assumptions, including, but not limited to, assumptions regarding
future events, developments and uncertainties and contains “forward-looking
statements” (statements that may include, without
limitation, statements about projected revenues, earnings, market opportunities, strategies,
competition, expected activities and expenditures, and at times may be identified by
the use of words such as “may”, “could”, “should”,
“would”, “project”, “believe”, “anticipate”,
“expect”, “plan”, “estimate”, “forecast”, “potential”, “intend”, “continue”
and variations of these words or
comparable words). L.E.K. is not able to predict future events, developments and
uncertainties. Consequently, any of the forward-looking statements contained in
this report may prove to be incorrect or incomplete, and actual results could differ
materially from those projected or estimated in this report. L.E.K. undertakes no
obligation to update any forward-looking statements for revisions or changes after
the date of this report and L.E.K. makes no representation or warranty that any of the
projections or estimates in this report will be realized. Nothing contained herein is,
or should be relied upon as, a promise or representation as to the future.
3 |
 Non-Confidential Information – Horizon Pharma
plc Note Regarding Use of Non-GAAP
Financial Measures 4
Horizon provides certain financial measures such as adjusted non-GAAP net income (loss),
adjusted non-GAAP net income (loss) per share, non- GAAP gross profit margins
and non-GAAP cash from operations that include adjustments to GAAP figures. These adjustments to GAAP exclude
acquisition transaction related expenses as well as non-cash items such as stock
compensation, depreciation and amortization, accretion, non- cash interest expense,
and other non-cash adjustments such as the increase or decrease in the fair value of the embedded derivative associated
with the Company’s convertible senior notes. Certain other special items or
substantive events may also be included in the non-GAAP adjustments periodically
when their magnitude is significant within the periods incurred. EBITDA, or earnings before interest, taxes,
depreciation and amortization, and adjusted EBITDA are also used and provided by Horizon as
non-GAAP financial measures. Horizon believes that these non-GAAP
financial measures, when considered together with the GAAP figures, can enhance an overall understanding of Horizon's
financial performance. The non-GAAP financial measures are included with the intent
of providing investors with a more complete understanding of the Company’s
operational results, trends and expectations. In addition, these non-GAAP financial measures are among the
indicators Horizon’s management uses for planning and forecasting purposes and measuring
the Company's performance. These non-GAAP financial measures should be
considered in addition to, and not as a substitute for, or superior to, financial measures calculated in accordance
with GAAP. The non-GAAP financial measures used by the Company may be calculated
differently from, and therefore may not be comparable to, non-GAAP financial
measures used by other companies. The Company has not provided a reconciliation of full year 2014 or 2015 adjusted
EBITDA outlook to a net income (loss) outlook because certain items that are a component of
net income (loss) but not part of adjusted EBITDA, such as the gain (loss) on
derivative revaluation associated with the convertible senior notes, stock compensation, acquisition related expenses
and certain purchase accounting items such as intangibles and step-up inventory, cannot be
reasonably projected, either due to the significant impact of changes in Horizon’s
stock price on derivative revaluation and stock compensation, or the variability associated with acquisition
related expenses and purchase accounting items due to timing and other factors.
|
 Friedreich’s
Ataxia Analyst Day Agenda 5
Non-Confidential Information – Horizon Pharma
plc Overview & Vision for
ACTIMMUNE ®
Timothy P. Walbert
Chairman, President and Chief Executive Officer
FA Overview & ACTIMMUNE Phase 2
Clinical Trial Results
Jennifer Farmer, MS
Certified Genetic Counselor
Executive Director, FARA and Coordinator,
Collaborative Clinical Research Network in FA
FARA’s Mission and Strategy
Ronald J. Bartek
Co-Founder & President of FARA
ACTIMMUNE Clinical & Regulatory Plan
Jeffrey W. Sherman, M.D., FACP
Executive
Vice President, Research and
Development,
Chief Medical Officer
FA Market Overview
Brian Andersen
Group Vice President and General Manager,
Orphan Business Unit
Business Development Strategy
Robert F. Carey
Executive Vice President, Chief Business Officer
Q&A
All |
 6
HZNP Overview and Vision for ACTIMMUNE®
Timothy P. Walbert
Chairman, President and Chief Executive Officer
Non-Confidential Information – Horizon Pharma
plc |
 Non-Confidential Information – Horizon Pharma
plc (1)
On a non-GAAP basis
(2)
RAYOS is known as LODOTRA outside the United States
•
Profitable
(1)
specialty biopharmaceutical
company with accelerating growth
•
Integrated commercial model with track
record of execution
•
Four products targeting unmet
therapeutic needs in orphan diseases,
primary care and specialty segments
•
Experienced, proven leadership team
•
Tax efficient corporate platform
facilitating an aggressive business
development strategy via
product/company acquisitions
Horizon Pharma plc Overview
7
(2) |
 Non-Confidential Information – Horizon Pharma
plc Four U.S. Products in Three Market
Segments 8
•
7 clinical science associates
–
Academic medical centers
–
Infectious disease and
immunology
Orphan Diseases
Primary Care
•
250 sales reps
–
Primary care
–
Orthopedic surgeons
Specialty
•
42 sales reps
–
Rheumatology |
 Non-Confidential Information – Horizon Pharma
plc 2012
2013
2014E
(1)
2Q 2013
2Q 2014
2015E
(1)
~495% Year-over-Year
Net Sales Growth
2Q13 vs. 2Q14
Accelerating Growth in Net Sales and EBITDA
9
$18.8
$74.0
$270 -
$280
$380 -
$405
$11.1
$66.1
$(73.3)
$(33.5)
$80 -
$90
$(12.5)
$23.8
$(150.0)
$(50.0)
$50.0
$150.0
$250.0
$350.0
$450.0
Net Sales
Adjusted EBITDA
$150 -
$170
(1)
Guidance provided on September 19, 2014 which included estimated ACTIMMUNE results and
excluded transaction related expenses. By this presentation, Horizon is not confirming
or updating September 19, 2014 financial guidance. |
 Non-Confidential Information – Horizon Pharma
plc •
Adjusted 2Q 2014 non-GAAP net income was $20.7 million, or $0.28 non-GAAP
basic earnings per share and $0.21 non-GAAP diluted earnings per share
(1)
RAYOS is known as LODOTRA outside the United States
Consistently Improving Quarterly Growth
10
$9
$11
$24
$30
$52
$66
$0
$10
$20
$30
$40
$50
$60
$70
1Q 2013
2Q 2013
3Q 2013
4Q 2013
1Q 2014
2Q 2014
LODOTRA
(1)
RAYOS
(1)
DUEXIS
VIMOVO |
 Non-Confidential Information – Horizon Pharma
plc 11
ACTIMMUNE
–
Making
a
Difference
in
Patients’
Lives
See full prescribing information at www.ACTIMMUNE.com |
 ACTIMMUNE
- $500M Product Opportunity*
12
ACTIMMUNE in CGD/SMO
+
•
ACTIMMUNE (interferon gamma-1b)
•
Indications
–
Reduction of the frequency and
severity of serious infections
associated with Chronic
Granulomatous Disease (CGD)
–
Delaying time to disease
progression in patients with severe,
malignant osteopetrosis (SMO)
•
270 CGD/SMO patients on drug
ACTIMMUNE in FA
•
Friedreich's ataxia is a debilitating,
life-shortening, ultra-orphan
disorder
•
Prevalence of 1 in 50,000 people in
U.S. or ~3,700 U.S. patients
•
No FDA approved treatment for
patients
•
Strong efficacy signal in Phase 2
study
•
Plan to partner with FARA and
rapidly move into Phase 3
registration program
* Subject to approval in Friedreich’s ataxia
+
See full prescribing information at www.ACTIMMUNE.com
Non-Confidential Information – Horizon Pharma
plc |
  Non-Confidential Information – Horizon Pharma
plc •
$73.6 million annual sales and $39.1 million operating income run-rate based on 2Q
2014 performance
•
Q2 2014 ACTIMMUNE sales of $18.4M, an increase of $4.9 million from 2Q 2013
ACTIMMUNE Financial Overview –
Q2 2014
13
($ in millions)
2014
2013
2014
2013
Product Sales, net
18.4
$
13.5
$
35.7
$
25.9
$
Cost of product sales
0.8
1.7
1.7
3.2
Gross Profit
17.6
11.8
34.1
22.7
Gross Margin
96%
87%
95%
88%
Operating Expenses
7.8
6.1
17.1
12.6
Income from Operations
9.8
5.7
17.0
10.1
Operating Margin
53%
42%
47%
39%
Other Expenses
0.2
0.6
0.5
1.6
Income Taxes
0.4
(0.8)
0.7
(1.5)
Net Income
9.2
$
5.9
$
15.7
$
10.1
$
Net Margin
50%
44%
44%
39%
(Unaudited)
(Unaudited)
Three Months Ended June 30,
Six Months Ended June 30, |
 Non-Confidential Information – Horizon Pharma
plc Drive market penetration in
CGD (currently < 30%)
Meet with FDA and finalize Phase 3
program in Friedreich’s ataxia
Identify further potential
indications with proven science
Rapidly understand opportunity in
Cutaneous T-cell lymphoma and
Eczema Herpeticum
ACTIMMUNE Strategy
Drive CGD Penetration and Rapidly Expand Market Opportunity
14 |
 Non-Confidential Information – Horizon Pharma
plc 15
FA & ACTIMMUNE Phase 2 Clinical Trial Results
Jennifer Farmer, MS, Certified Genetic Counselor
Executive Director, FARA
Coordinator, Collaborative Clinical Research
Network in FA |
 Friedreich
Ataxia (FA) •
A rare, genetic condition that is a progressive
degenerative disease of children, adolescents and
adults
•
Affects 1 in 50,000
•
Estimated 4-5,000 individuals in US and 15,000
worldwide
•
Nicolaus Friedreich first described in 1860’s based on
6 patients from 2 families
•
Typically thought of as a neurodegenerative disease
as this is the most visible and common symptom;
however FA is a multi-system disease
•
Genotypic and phenotypic heterogeneity
16
–
Childhood onset is associated with more rapid progression.
•
Onset/symptoms appear between ages of 5-15 years
•
Late teens –
early twenties most individuals are wheelchair-bound
and require assistance with ADLs
–
Late onset (diagnosed after age 20) is associated with slower progression
and overall more mild phenotype |
 FA –
Clinical Features
Clinical features: Neuro
•
Loss of large sensory neurons -
proprioception
–
Loss of balance and coordination
–
Loss of reflexes
•
Loss of spinocerebellar tracts
–
Loss of balance and coordination
•
Loss of motor tracts to a lesser degree
•
Loss of dentate nucleus of the cerebellum
–
Dysarthria (slurred speech), modest eye movement
abnormalities
•
Loss of a few other specific sites.
–
Vision, hearing loss
•
Sparing of cerebellar cortex, cerebral cortex
–
Normal cognition
•
Overall loss of relatively few neurons, modest number of
axonal tracts----MRI scans are normal or almost normal in
early disease
17 |
 FA –
Not just Neuro but Multi-system condition
•
Heart: Cardiomyopathy and arrhythmia
–
Hypertrophic cardiomyopathy can be
progressive and severe and early
morbidity and mortality
–
Clinically significant arrhythmia
•
Endo: Diabetes –10-20%; >65% Insulin
resistance
•
ENT
–
Swallowing difficulty in later stage
patients
–
Hearing loss –
10-15% of patients
•
Ophthal
–
Optic atrophy –
present in 10% of pts
–
Fixation abnormalities common
(square wave jerks, ocular flutter)
•
Skeletal: Scoliosis
-Corrective surgery required in up to
50% of patients
-Pes cavus
•
Fatigue: Nearly all patients
experience significant fatigue that
impacts quality of life.
•
Cognition remains normal
18 |
 Friedreich’s Ataxia: Management
•
No treatment to stop progression of disease
•
Most individuals take vitamins and antioxidants
•
Annual Echocardiogram and EKG
–
Treat cardiac symptoms as they appear
–
No clinical treatment guidelines
•
Annual glucose
•
Annual scoliosis screening (especially kids ages
7-18)
–
Spinal fusion –
as needed
•
Annual vision and hearing screening
•
Annual PT/OT/Speech
19 |
 Diagnosis
- FXN
gene identified in 1996
Exon 1
2
3
4
5
4
GAA
FXN Gene
5
Transcription
Translation
Frataxin: highly conserved;
functions in mitochondria
20 |
 Diagnosis
-
FXN gene identified in 1996
Exon 1
2
3
4
GAA
5
Exon 1
2
3
4
GAA
5
GAA repeats
3-33 –
normal
GAA repeats
66-1700
-
abnormal
Frataxin
21 |
 Diagnosis
-
FXN gene identified in 1996
Exon 1
2
3
4
GAA
5
Exon 1
2
3
4
GAA
5
Mat
Pat
Low frataxin
22 |
 Frataxin
Correlates with GAA repeat length Deutsch et al., Mol Genet Metab. 2010
Measured frataxin levels inversely correlates with GAA repeat
length, suggesting that the assay is useful for assessing severity of
disease in buccal cells.
23 |
 p
<<< 0.0001 p <<< 0.0001
p <<< 0.0001
p <<< 0.0001
p <<< 0.0001
p <<< 0.0001
Two Encouraging Learnings: 1) Individuals with FA
make some Frataxin; 2) One does not need 100%
24 |
 Low frataxin =
mitochondrial dysfunction and energy deprivation
Frataxin: highly conserved; functions in
mitochondria
25 |
 Why Interferon
gamma for FA? 26 |
 Why Interferon
gamma for FA? 27 |
 Why Interferon
gamma for FA? •
FRDA mice improve both locomotor activity and
motor coordination, when treated with IFN
, and
that this is paralleled by both frataxin upregulation
and neuronal preservation in DRG.
28 |
 Pilot Study of
IFN in Children with FA
•
Investigator-initiated study; IND waiver
–
Goals
•
Safety and tolerability of IFN
•
Effect on frataxin protein levels
•
Effect on neurologic function
•
Design: Small, open-label
pilot study
•
12 Individuals with genetic confirmation of FA, ages 5-17
•
Dose-escalation
(10mcg/m2,
25mcg/m2,
50mcg/m2
–
3xwk)
every
two
weeks
based
on
tolerability
•
Overall treatment phase was 12 weeks
•
Reevaluation 1 month post treatment
29 |
 Study Outcome
Measures 30
•
Primary outcome measure
–
Effect
of
IFN-
on
frataxin
mRNA
and
protein
levels
in
whole
blood
(primary)
and buccal cells after 12 weeks of treatment.
•
Secondary outcome measures
•
Pharmacokinetic:
in
vivo
and
in
vitro
levels
of
IFN-
,
whole
blood
•
Neurologic:
Friedreich’s
Ataxia
Rating
Scale
(FARS),
Timed
25
Foot
Walk
Test (T25FW), 9-Hole Peg Test (9HPT), Low Contrast Sloan Letter Acuity
(LCSLA), Listening in Spatialized Noise –
Sentences (LiSN-S)
•
Overall
clinical
measures:
Global
Impression
of
Clinical
Severity,
Modified
Fatigue Impact Scale, Activities of Daily Living, Pediatrics Quality of Life
scales
•
Safety
and
Tolerability:
Clinical
safety
lab
testing,
electrocardiogram,
assessment of adverse events, flu-like symptoms (FLS), injection-site
reactions (ISR) |
 Demographics
31
8-17 years old, mean age of 12
Mean GAA repeat length 835
Mean age of onset was 6
12 of 12 subjects screened met criteria
Two subjects did not follow dose escalation protocol
due to adverse events
Two subjects withdrawn from the study for inability
to complete study procedures |
 No
drug-related serious adverse events (SAE) occurred
11 of 12 subjects experienced at least 1 AE
Low grade, not dose related
Largely known side-effects of IFN-
Two subjects were unable to continue with dose-
escalation of IFN-
due to moderate to severe flu-like
symptoms
Safety and tolerability
32 |
 Frataxin
Measurements A rapid, noninvasive
immunoassay
for
frataxin: utility in assessment of Friedreich
ataxia,
Deutsch
EC
1
,
Santani
AB,
Perlman
SL,
Farmer
JM,
Stolle
CA,
Marusich
MF,
Lynch
DR.
Mol
Genet
Metab.
2010
Oct-Nov;101(2-3):238-45.
Usefulness of
frataxin
immunoassays for the diagnosis of Friedreich ataxia,
Deutsch
EC
1
,
Oglesbee
D
,
Greeley
NR
,
Lynch
DR,
J
Neurol
Neurosurg
Psychiatry.
2014 Sep;85(9):994-1002.
Validation in whole blood and buccal cells
Exploratory –
RBCs, platelets and WBCs
–
While
there
was
statistically
significant
change
it
was
not
at
a
level
or
magnitude
that
could
be
clinically meaningful
–
Unaffected tissue
33
2
3 |
 34
|
 Friedreich
Ataxia Rating Scale (FARS)
35
Clinical neurological exam and ADL based scale developed by
S. Subramony
•
Good inter-rater reliability; correlates with duration, disability and ADL
•
Bulbar –
speech, facial strength, cough
•
Upper Extremity –
finger to nose, dysmetria, finger taps
•
Lower Extremity –
heel to shin –
slide and tap
•
Peripheral Nerves –
reflexes, proprioception, sensation
•
Upright Stability –
sitting, standing, walking
•
FARS, Functional Disability Rating Scale –
0-6
•
Clinician grading
•
FARS, Activities of Daily Living 0-36
•
Patient response
•
FARS Neuro, 5 separate subscores (0-125) |
 36
|
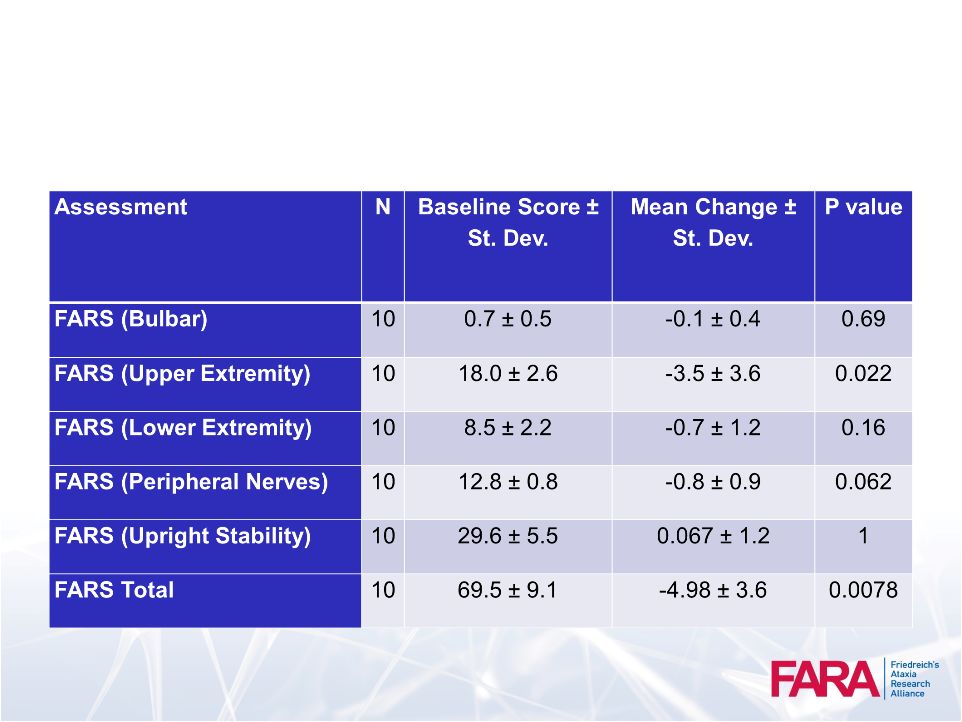 FARS:
Clinical Outcome Measure 37 |
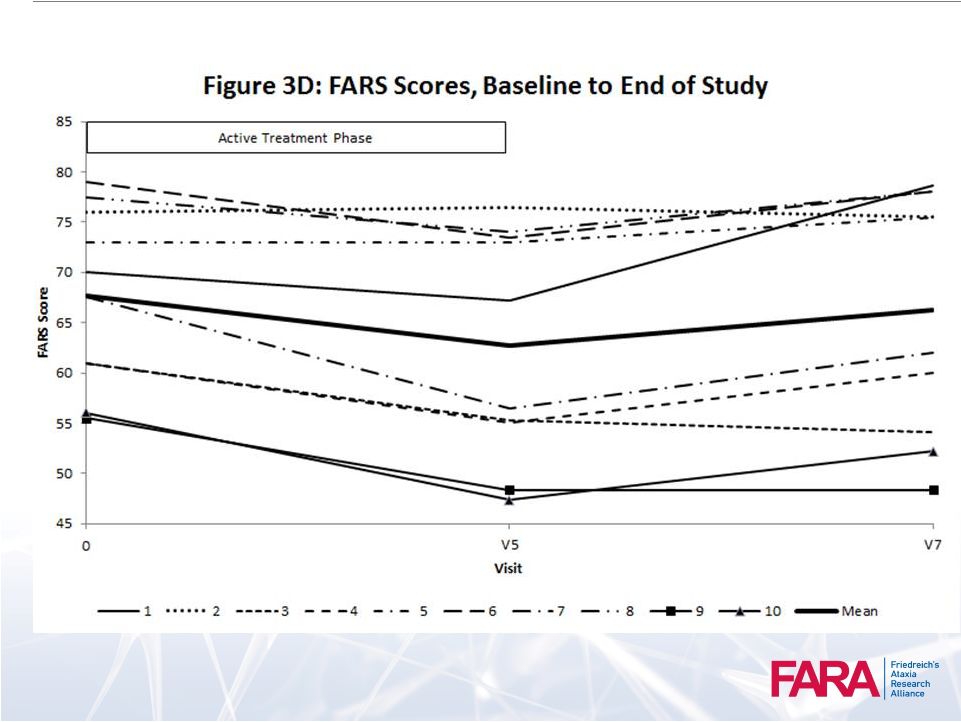 38
|
 Performance
Measures 39 |
 Conclusions
IFN-
was safe and well-tolerated
No overt significant increase in frataxin levels
–
Presence of increased levels through treatment
–
Sampling limited to unaffected tissue
–
Timing
of
sampling
-
short
half
life
of
gamma
interferon
–
Limited to FDA approved dose for other diseases
Clinically significant neurologic improvement
magnitude
40 |
 Limitations
•
The small size of the study
•
Absence of a placebo group
•
One site
41 |
 Future
Directions •
Double-blind, placebo-controlled trial
•
Larger number of subjects
•
Frataxin measurements more consistently timed
with dosing
•
Frataxin measurement in affected tissue
–
Muscle
•
Explore higher-dose
42 |
 43
Non-Confidential Information – Horizon Pharma
plc Ronald J. Bartek
Co-Founder & President of FARA
FARA’s Mission and Strategy
|
 FARA Mission
& Strategy Mission
is to marshal and focus the resources and relationships
needed to cure FA
Guiding Principle
-
Collaboration and Partnership
•
As a rare disease we can not afford to seek progress in isolation
Strategy
•
Growing the scientific community
•
Diverse and Deep Pursuit of Treatments
•
Focus on Infrastructure needs
•
Public-Private Partnership
•
Advocacy
44 |
 FARA Research
and Clinical Programs Grant
Program
Scientific
Conference
Program
Patient Registry &
Education
Program
Collaborative
Clinical
Research
Network
Advocacy
•24/7/365
•Peer-reviewed
•Basic,
Translational
and Clinical
Research
•
> $25 Million
in research
funding to
scientists in
both public and
private
organizations.
•Facilitating
rapid and open
sharing research
•Promoting
collaboration
•Nurturing young
investigators
•Patient
Recruitment
•2200 Registrants
•Athena Diagnostics
sends letter to
referring physicians
alerting them to the
FARA Patient
Registry when an
individual is
diagnosed.
•10 Network Sites
Worldwide
•>750 Individuals
seen on an annual
•Infrastructure for
clinical research,
including natural
history, outcome
measures,
biorepository,
biomarker studies
and trials.
•Trained physicians
and coordinators
•Promote
public-
private
partnership.
•Government
(NIH &FDA)
•Pharmaceutica
l Industry
•Other
advocacy
organizations
45 |
 FARA Patient
Registry •
The
FARA
Patient
Registry
is
the
only
worldwide
registry
of
FA
patients
•
Web-based, patient entered demographic and basic clinical
data
•
Proven successful for clinical research and trial recruitment.
–
7 clinical trials; 9 clinical research studies
–
Recruiting subjects in hours and days not weeks and months
•
Providing good evidence for geographic distribution and
population data
46 |
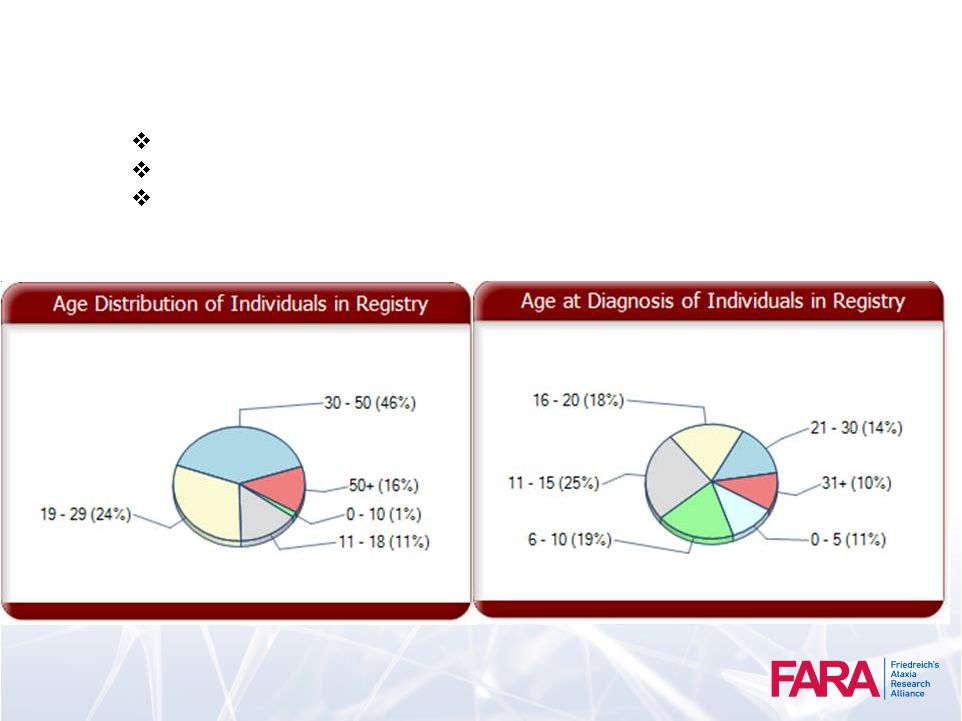 FARA Patient
Registry Over 2000 Patients Active Enrollment
From 65 Countries
Top Five Countries –
United States (64%), Canada, United Kingdom,
Australia, Spain
47 |
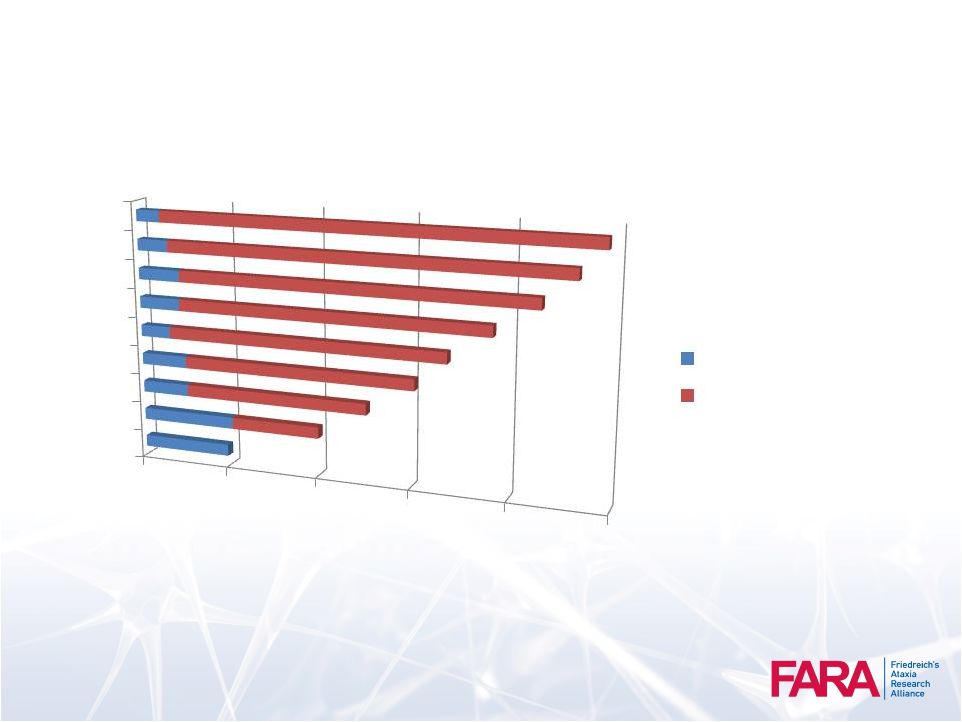 FARA Patient
Registry Average 200 new registrants per year
48
0
500
1000
1500
2000
2500
2006
2008
2010
2012
2014
>2400 individuals with FA Enrolled
New Enrollment /YR
Existing |
 Collaborative
Clinical Research Network in FA (CCRN in FA)
•
Established 10 years ago -
10 sites in U.S., Australia and
South America
•
Goals
•
Provide
quantitative
serial
clinical
data
on
patients
–
natural
history
•
Design
improved
clinical
measures
for
use
in
trials
•
Build
a
clinical
database
in
parallel
with
a
biorepository
for
use
in
translational research including modifier gene studies and biomarker studies
•
Create
a
mechanism
for
sharing
data
and
resources
•
Facilitate
the
implementation
and
conduct
of
clinical
trials
•
Define
best
practices
for
FA
and
provide
the
highest
level
of
clinical
care
for
patients
49 |
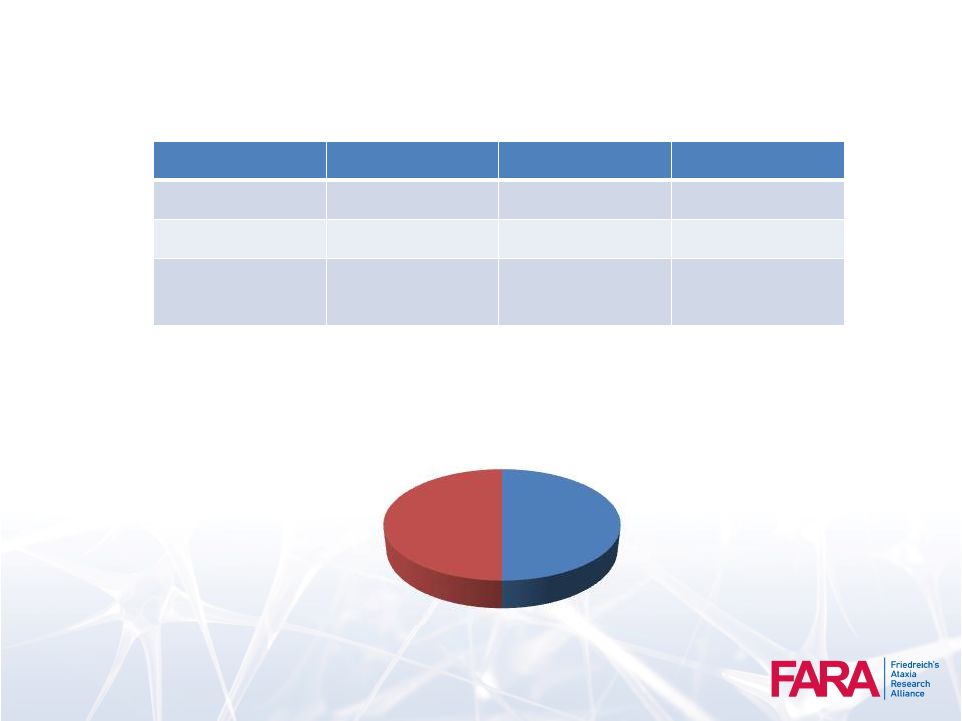 CCRN in FA
Cohort Assessment
N
Mean
SD
Age of Onset
746
13.7
10.03
Age at Baseline
746
26.2
15.17
Shorter GAA
repeat
746
632.7
241.14
50
Gender
Male
50%
Female
50% |
 Advocacy
•
NIH –
Member NINDS Council, Steering Committee NINDS non-profit
forum, Rare Disease Patient Registry Advisors
–
1
NIH RAID grant for non-cancer condition
•
Partnership –
Industry, Academia, Advocacy
•
Raising awareness and knowledge at FDA
–
2013 & 2014 –
Invited speakers at FDA training
–
FA community members on FDA Advisory panels
•
Institute of Medicine
–
Accelerating Drug Development for Rare Diseases
–
Independent Review of the RAC
51
st |
 Researchers
&
Physicians
Government
NIH, FDA
Pharma & Biotech
Patients and
Families
FARA
FARA
52 |
 53
Clinical and Regulatory Plan
Clinical and Regulatory Plan
Jeffrey W. Sherman, M.D., FACP
Executive Vice President, Research and
Development, Chief Medical Officer
Non-Confidential Information – Horizon Pharma
plc |
 Clinical/Regulatory Considerations for FA
Clinical/Regulatory Considerations for FA
•
FA is a debilitating, life-shortening, ultra orphan disorder
(prevalence of 1 in 50,000 people in U.S.)
•
No treatment for patients only monitored for symptoms
•
Increase in initiatives and incentives from FDA to expedite
treatments for conditions such as FA
–
Rare and orphan disease programs
–
Expedited programs for serious conditions
–
Best Pharmaceuticals for Children Act (BPCA)
•
ACTIMMUNE already FDA approved for two debilitating,
orphan diseases
•
Supplement approval pathway typically more straightforward
than new entity approval
54
Non-Confidential Information – Horizon Pharma
plc |
 Non-Confidential Information – Horizon Pharma
plc Potential to Address an Unmet Medical
Need Potential to Address an Unmet Medical Need
Before Treatment
55
Source: Patient Blog
http://fafysio.wordpress.com/2014/08/21/amazing-ilva/#comments |
 Potential to
Address an Unmet Medical Need Potential to Address an Unmet Medical Need
On Treatment
(200mcg 3x/week
[2x CGD Dose])
after 6 months
56
Non-Confidential Information – Horizon Pharma
plc Source: Patient Blog http://fafysio.wordpress.com/2014/08/21/amazing-ilva/#comments |
 57
Anticipated ACTIMMUNE FA Clinical/Regulatory Timeline
Anticipated ACTIMMUNE FA Clinical/Regulatory Timeline
October
2014
November
2014
October
2014
Q1
2015
Received Orphan
Drug Designation
for ACTIMMUNE
to treat FA
Pre-IND meeting
with FDA
Planning ongoing in
collaboration with
CCRN in FA and FARA
for FDA Pre-IND
meeting and next-step
development activities
leading to clinical
study and
supplemental BLA
Phase 3 clinical trial
start
pending
FDA
Pre-IND meeting
feedback and IND
submission
IND Submission:
Also planning a
submission at the
same time to FDA
expedited programs
for serious
conditions (e.g., Fast
Track Designation)
pending Pre-IND
meeting feedback
Q2
2015
Non-Confidential Information – Horizon Pharma
plc |
 Additional
Potential ACTIMMUNE Indications Additional Potential ACTIMMUNE Indications
58
•
Autosomal Dominant Osteopetrosis (ADO)
–
1 in 250,000 in the U.S.
(1)
•
Severe Atopic Dermatitis (AD) -
3.4 million
(2)
–
Methicillin Resistant Staphylococcus aureus
(MRSA)
infection -
colonization up to 11%
(3)
–
Eczema Herpeticum (EH)
-
< 3%; ~102,000
(4)
•
Cutaneous T-Cell Lymphoma (CTCL)
–
6 in 1 million in the U.S.
(5)
Non-Confidential Information – Horizon Pharma
plc (1)
Shaw, Tatyana E, Gabriel P Currie, Caroline W Koudelka, and Eric L Simpson. Eczema Prevalence
in the United States: Data from the 2003 National Survey of Children’s Health. The
Journal of Investigative Dermatology 131 (1): 67–73. doi:10.1038/jid.2010.251. (2) Hanifin, Jon M, Michael L Reed, and Eczema Prevalence and Impact
Working Group. 2007. “A Population-Based Survey of Eczema Prevalence in the United
States.” Dermatitis: Contact, Atopic, Occupational, Drug 18 (2): 82–91.
(3)
Huang, Jennifer T., Melissa Abrams, Brook Tlougan, Alfred Rademaker, Amy S. Paller.
Treatment of Staphylococcus aureus Colonization in Atopic Dermatitis Decreases Disease
Severity. Pediatrics Vol. 123 No 5 May1, 2009 pp. e808-e814 (dol:10,152/peds/2008-2217).
(4)
Leung, Donald Y M. 2013. “Why is Eczema Herpeticum Unexpectedly Rare? “
Antiviral Research 98 (2): 153-57 . Doi:10.1016/j.anitivral.2013.02.010. (5) Cutaneous T-Cell Lymphoma Facts. Leukemia & Lymphoma
Society. June 2014 .
|
 59
ACTIMMUNE Market Overview
ACTIMMUNE Market Overview
Brian Andersen
Group Vice President and
General Manager, Orphan Business Unit
Non-Confidential Information – Horizon Pharma
plc |
 60
ACTIMMUNE Impacts Patients’
ACTIMMUNE Impacts Patients’
Lives
Lives
Non-Confidential Information – Horizon Pharma
plc |
 Chronic
Granulomatous Disease (CGD) Overview •
CGD was identified in the 1950’s
•
Survival has improved over time and
most patients are able to manage their
disease and can live well into adulthood
•
CGD is a disease of the immune system
and described as a primary
immunodeficiency disorder
•
CGD is the most common disorder of
phagocytes making up 6% of primary
immune deficiencies
•
~1 in every 200,000 babies are born
with CGD. There are estimated to be
~1,600 CGD patients in the U.S.
61
Non-Confidential Information – Horizon Pharma
plc |
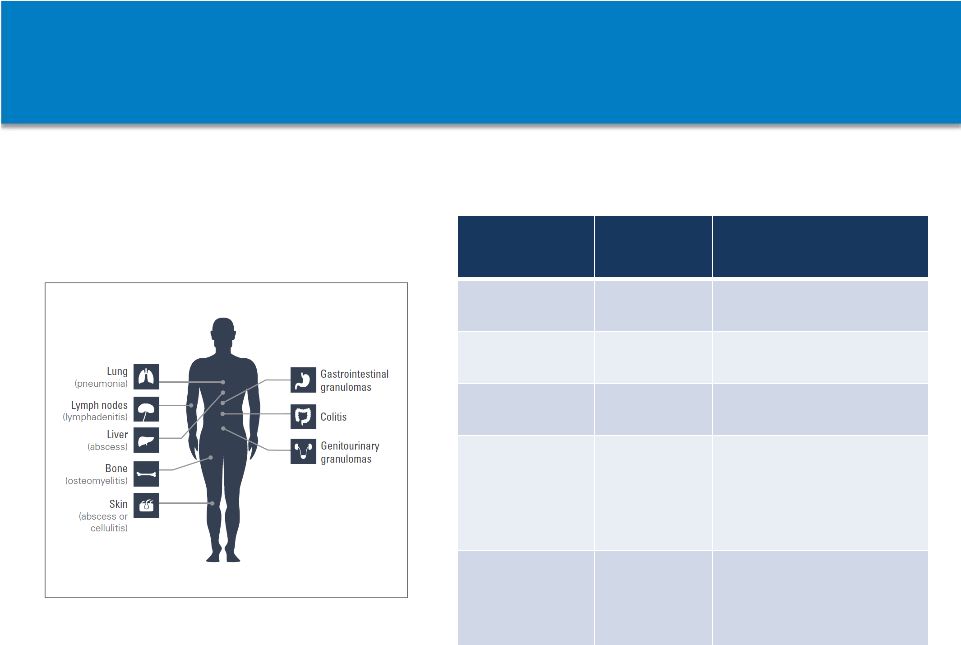 Non-Confidential
Information
–
Horizon
Pharma
plc
62
Common Presentation Indicating CGD
Common Presentation Indicating CGD
In the U.S., the majority of CGD-related
infections are caused by:
* This is not a complete list of pathogens. Infections may also be caused by other species of
bacteria, fungi, and yeast not listed here. Pathogen*
Type of
Infection
Common Presentation
Aspergillus
species
Fungal
Pneumonia, lymphadenitis,
osteomyelitis, brain abscess
Burkholderia
species
Bacterial
Pneumonia, sepsis
Nocardia
species
Bacterial
Pneumonia, osteomyelitis,
brain abscess
Serratia
marcescens
Bacterial
More common:
Osteomyelitis, soft tissue
infections
Less common: Pneumonia,
sepsis
Staphylococcus
aureus
Bacterial
Soft tissue infections,
lymphadenitis, liver abscess,
osteomyelitis, pneumonia,
sepsis
Most frequent sites of infection and
common inflammatory complications
resulting from CGD |
 van den Berg JM, et al. (2009) Chronic Granulomatous Disease: The European
Experience. PLoS ONE 4(4): e5234. Typically Diagnosed Before the Age of Five
•
CGD may become apparent any time from infancy to late adulthood
•
>75% are diagnosed before the age of five years
•
CGD is under-diagnosed and can be misdiagnosed
•
Diagnostic testing is readily available
63
Non-Confidential Information – Horizon Pharma
plc |
 64
ACTIMMUNE Clinical Benefit in CGD
ACTIMMUNE Clinical Benefit in CGD
Fewer Days of Hospitalization
Reduction in Relative Risk of Serious Infections
Non-Confidential Information – Horizon Pharma
plc * Serious infection was defined as
an infection requiring hospitalization and parenteral antibiotics **The
beneficial effect of ACTIMMUNE was demonstrated throughout a 12 month study •
67% reduction in relative risk of serious
infection* in patients receiving ACTIMMUNE
(p=0.0006)
•
64% reduction in total number of serious
infections* (p<0.0001)
•
Placebo patients required 3x as many inpatient
hospitalization days for treatment of clinical
events compared to patients receiving
ACTIMMUNE (p=0.02)
•
77% of patients receiving ACTIMMUNE were
free of serious infections vs. 30% receiving
placebo. ** (P= 0.0006).
The mean duration of
therapy for these patients was 8.9 months
•
A long-term observational follow-up study of
patients who received adjunctive prophylactic
therapy with ACTIMMUNE demonstrated an
incidence of 0.30 serious infections* per
patient-year |
 Severe Malignant
Osteopetrosis (SMO) Overview •
SMO is a one form of osteopetrosis and is sometimes referred to as
marble bone disease or malignant infantile osteopetrosis (MIOP)
•
Often misdiagnosed as hematologic cancer
•
~1 in every 250,000 babies are born with SMO
•
There are estimated to be ~200 SMO patients in the U.S.
65
Non-Confidential Information – Horizon Pharma
plc |
 Non-Confidential
Information
–
Horizon
Pharma
plc
66
•
Most severe form and diagnosis usually in first
months of life
•
Clinical Presentation
–
Failure to thrive
–
Impaired vision or blindness
–
Hearing loss
–
Fractures, frontal bossing, macrocephaly,
hydrocephalus, hypocalcaemia
–
Infections
–
Absence of the bone marrow cavity results in
severe anemia and thrombocytopenia
•
If left untreated, generally lethal < 10 years old
due to severe anaemia, bleeding or infections
associated with bone marrow failure
Severe Malignant Osteopetrosis
(SMO)
Severe Malignant Osteopetrosis
(SMO)
Clinical Presentation
Clinical Presentation
Sobacchi et al. Nature Rev Endocrinology. 2013 (9) 522-36 |
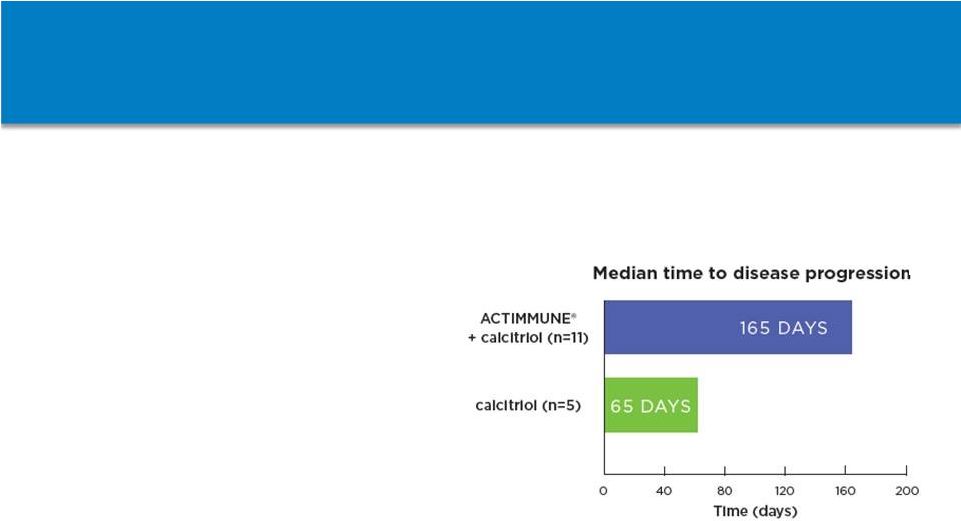 67
ACTIMMUNE Clinical Benefit in SMO
ACTIMMUNE Clinical Benefit in SMO
•
Median time to disease
progression was significantly
delayed in the ACTIMMUNE
plus calcitriol arm (165 days)
vs. calcitriol alone (65 days)
•
When combined with data
from a second study, 19 of 24
patients on ACTIMMUNE
therapy (+/-
calcitriol) for at
least 6 months had reduced
trabecular bone volume
compared to baseline
Delays the time to disease
progression
(1)
in patients with SMO
(1)
Disease progression is defined by death, significant reduction in hemoglobin or platelet
counts, a serious bacterial infection requiring antibiotics, a 50 dB decrease in
hearing, or progressive optic atrophy. Non-Confidential Information – Horizon
Pharma plc (1)
|
 Non-Confidential
Information
–
Horizon
Pharma
plc
Weekly CGD/SMO SP Vials Dispensed up 73%
Weekly CGD/SMO SP Vials Dispensed up 73%
Likely impacted by lack of patient
support programs and promotion
+73%
Jun-12: Vidara acquires
ACTIMMUNE
®
Sep-12:
COMPASS
HUB launched
Apr-13: Launched
patient ed. brochure
& HCP detailer
Jan-13: New
brand launched
with COMPASS
brochure
Jan-14: Launched new
ACTIMMUNE.com
website
Note: 8-week moving average CGD/SMO vials dispensed per SP data. Excludes OptumRx,
CignaTel Drug, Aetna SP and FEP. ~200+
new CGD patients have initiated ACTIMMUNE therapy since July 2012
68 |
 Non-Confidential
Information
–
Horizon
Pharma
plc
Our Commercial Effort is Focused Solely on CGD
Our Commercial Effort is Focused Solely on CGD
and SMO Patients
and SMO Patients
69
CGD / SMO
Other ICD-9
CGD / SMO
Other ICD-9
% of ACTIMMUNE®
Specialty Pharmacy Vials Dispensed
0%
5%
10%
15%
20%
25%
30%
35%
40%
45%
50%
55%
60%
65%
70%
Note: 8-week moving average vials dispensed per specialty pharmacy data.
Excludes OptumRx, CignaTel Drug, Aetna SP and FEP. |
 Non-Confidential
Information
–
Horizon
Pharma
plc
ACTIMMUNE CGD/SMO Patient Increase of 74%
ACTIMMUNE CGD/SMO Patient Increase of 74%
Note: Active Patient defined as a patient that has shipped in the last 120 days. Grossed up by
10% to account for restricted data from OptumRx, CignaTel, Aetna SP and FEP
patients. 70
0
25
50
75
100
125
150
175
200
225
250
275
300
Active CGD/SMO Specialty Pharmacy Patients
155 CGD/SMO Pts
270 CGD/SMO Pts |
 Non-Confidential Information – Horizon Pharma
plc 71
ACTIMMUNE COMPASS Program
ACTIMMUNE COMPASS Program
COMPASS
Advocacy & Support Services)
*SP= Specialty Pharmacy, **SD= Specialty Distributor,
†
For FDA approved indications
•
Benefits Investigation
•
Prior Authorization Support
†
•
Appeals Support
†
•
Co-Pay Assistance (Private Insurance)
•
Patient Assistance Program (Uninsured)
•
Foundation Referral (Public Insurance)
•
StarterRX (Private insurance)
•
BridgeRX (Private insurance)
•
Sharps Container Program/Syringe Disposal
–
Improved Patient Safety
–
Environmentally-friendly
•
Clinical Support Program
–
Improve persistency
–
Improve compliance
SP*
SP*
SD**
SD**
Patient Support
Patient Support
(Comprehensive Personalized Patient Prescription |
 FA Population
Overview: ~3,700 in the U.S. U.S.
Population
X
%
Caucasian*
X
Diagnosed FA
prevalence in
Caucasians
=
Diagnosed FA
prevalence in
Caucasians
~320M
X
78%
X
~1.5/100,000
=
~3,700
72
Total estimated diagnosed FA prevalence is ~3,700 in the U.S.
Methodology
Non-Confidential Information – Horizon Pharma
plc |
 85% of Diagnosed
Patients are Early Onset Patients 73
Source: FARA, L.E.K. interviews and analysis
•
The vast majority (~85%) of
diagnosed patients are early onset
patients (i.e., exhibit symptoms
before age 25) and are typically
diagnosed by age 12
Age of FA onset and current age
among diagnosed prevalent FA
patients (2014)
Adult
(
25 years)
Percent of Patients)
Adolescent
(12-17
years)
Non-Confidential Information – Horizon Pharma
plc 0
10
20
30
40
50
60
70
80
90
100
Late onset
(>25 years)
Early onset
Age of onset
Current age of patient
(N=8)
(
25 years)
Pre-adolescent
(12-17
years)
) |
 Non-Confidential
Information
–
Horizon
Pharma
plc
Neurological Examinations and Genetic Testing
Lead to Specialized Care Centers
74
•
Patients are typically referred by
their primary provider to
neurologists (including pediatric
neurologists) for evaluation and
diagnosis of FA
•
FA is typically suspected after a
neurological exam, though
genetic testing of frataxin is
needed for definitive diagnosis
•
KOLs estimate two thirds of FA
patients will eventually receive
care at a center that specializes in
muscular dystrophies, FA or
general ataxias
Source: L.E.K. interviews and analysis
FA patient journey
Pediatrician / PCP
Neurological (including
pediatric neurologist)
FA or ataxia
center
neurologist
MDA clinic
neurologist
Pediatric
Cardiologist
Clinical
geneticist
Specialized
care centers
33% of patients
are managed by
general
neurologists only
Return to
primary
neurologist
100%
20-40%
60-80%
33%
Return for
Regular
care
33%
FA or ataxia centers
are the primary care
center for ~50% of FA
patients
DIRECTIONAL
based on small N |
 Significant
Growth Opportunities for ACTIMMUNE •
Significant expansion opportunity in CGD & SMO
–
CGD & SMO still under-diagnosed or misdiagnosed
–
Increasing focus on supporting patient and advocacy groups to
help the CGD and SMO communities
•
We will continue to enhance the COMPASS program to serve
patients
–
Scalable for new indications
–
Easily adapted for new orphan products
75
Non-Confidential Information – Horizon Pharma
plc |
 76
Horizon Pharma plc Business Development Strategy
Robert F. Carey
Executive Vice President,
Chief Business Officer
Non-Confidential Information – Horizon Pharma
plc |
 77
Business Development Strategy
April 2010
Nitec Pharma
acquisition
November 2013
Acquired from
AstraZeneca
September 2014
Vidara Therapeutics
acquisition
Established track record of deal execution
Non-Confidential Information – Horizon Pharma
plc |
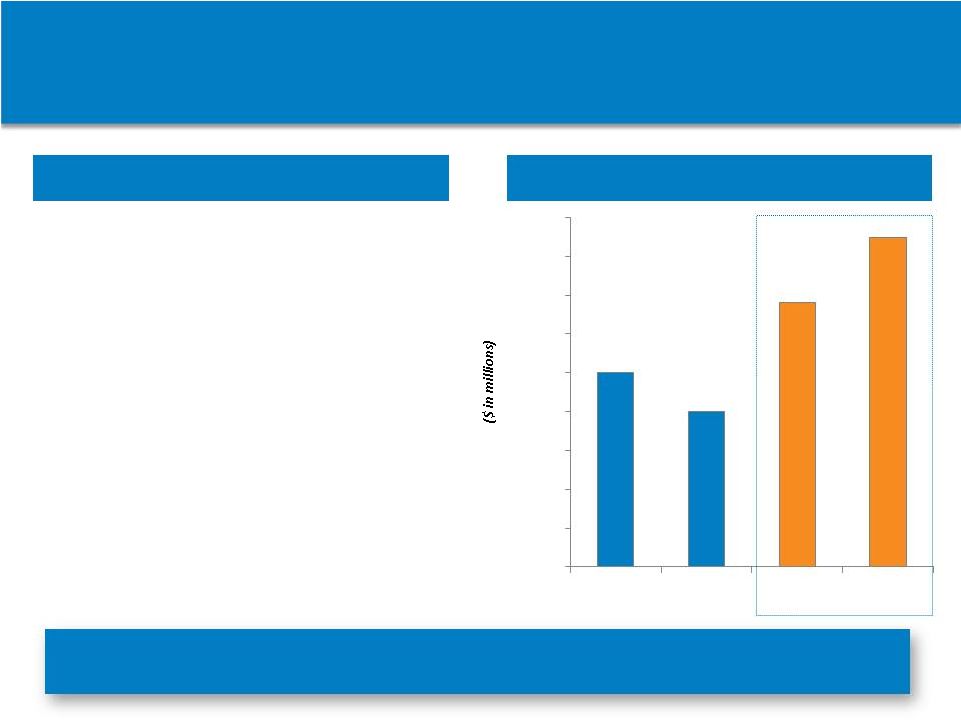 (1)
AstraZeneca Annual Reports
(1)
(1)
Rapidly Successful VIMOVO Acquisition
Perfect example of value arbitrage we intend to capture
through our BD strategy
Net Sales
Product Highlights
•
Acquired November 18, 2013
from AstraZeneca
•
$35 million one-time payment
•
Leverages existing commercial
infrastructure
•
Rapid growth in VIMOVO
prescriptions and revenues
•
Maximizing value through price
and lower patient co-pay
•
$76.4 million in 1H 2014
revenues
78
Non-Confidential Information – Horizon Pharma
plc $25.0
$20.0
$34.0
$42.4
$-
$5.0
$10.0
$15.0
$20.0
$25.0
$30.0
$35.0
$40.0
$45.0
2012
2013
1Q:14
2Q:14 |
 Plan to Execute
Aggressive Acquisition Strategy •
Executing on our aggressive business development strategy
via product/company acquisitions to maximize shareholder
value creation
79
Potential for
attractive financial
returns –
rapid
accretion, long-life
assets
Non-Confidential Information – Horizon Pharma
plc Near
term focus
on U.S. marketed
products/
companies to
leverage core
strengths
Pursuing
products with
differentiated
clinical benefits
Differentiated
and/or under
appreciated
assets
regardless of
therapeutic area
Targeting
products with
potential annual
revenues of $20
million to $200
million
-
- |
 80
Q&A
Non-Confidential Information – Horizon Pharma
plc |
 Non-Confidential
Information
–
Horizon
Pharma
plc
81
Appendix
Appendix |
 Non-Confidential
Information
–
Horizon
Pharma
plc
2011
2012
2013
2014
2013
2014
2013
Adjusted Non-GAAP Net Income (Loss):
GAAP Net Loss
(113,265)
$
(87,794)
$
(149,005)
$
(27,769)
$
(18,441)
$
(234,019)
$
(40,612)
$
Non-GAAP Adjustments:
Change in estimate of VIMOVO royalties
-
-
-
13,033
-
13,033
-
Loss on derivative revaluation
-
-
69,300
10,965
-
214,995
-
Vidara acquisition costs
-
-
-
10,125
-
14,174
-
Intangible impairment charge
69,621
-
-
-
-
-
-
Amortization and accretion:
Intangible amortization expense (net of tax effect)
3,012
3,782
6,790
4,683
1,311
9,363
2,635
Amortization of debt discount and deferred financing costs
2,708
2,740
17,245
2,333
919
4,666
1,829
Accretion of royalty liability
-
-
-
2,953
-
2,953
-
Amortization of deferred revenue
(166)
(217)
(930)
(161)
(147)
(322)
(215)
Stock-based compensation
2,530
4,661
5,014
4,160
1,021
6,087
2,100
Depreciation expense
446
806
1,174
404
299
780
558
Total of non-GAAP adjustments
78,151
11,772
98,593
48,495
3,403
265,729
6,907
Adjusted Non-GAAP Net Income (Loss)
(35,114)
$
(76,022)
$
(50,412)
$
20,726
$
(15,038)
$
31,710
$
(33,705)
$
Weighted average shares - basic
9,014,968
38,871,422
63,657,924
73,384,801
62,872,173
70,164,267
62,339,285
Weighted average shares - diluted
9,014,968
38,871,422
63,657,924
98,073,812
62,872,173
93,119,769
62,339,285
Adjusted Non-GAAP net income (loss) per common share-basic
0.28
$
0.28
$
0.28
$
0.28
$
(0.24)
$
0.45
$
(0.54)
$
Adjusted Non-GAAP net income (loss) per common share-diluted
0.21
$
0.21
$
0.21
$
0.21
$
(0.24)
$
0.34
$
(0.54)
$
(Unaudited)
(Unaudited)
Three Months Ended June 30,
Six Months Ended June 30,
Fiscal Year Ended December 31,
GAAP to Non-GAAP Reconciliation
GAAP to Non-GAAP Reconciliation
82 |
 Non-Confidential
Information
–
Horizon
Pharma
plc
GAAP to Non-GAAP Reconciliation (continued)
GAAP to Non-GAAP Reconciliation (continued)
83
2011
2012
2013
2014
2013
2014
2013
EBITDA and Adjusted EBITDA:
GAAP Net Loss
(113,265)
$
(87,794)
$
(149,005)
$
(27,769)
$
(18,441)
$
(234,019)
$
(40,612)
$
Depreciation
446
806
1,174
404
299
780
558
Amortization and accretion:
Intangible amortization expense
3,753
4,732
8,136
5,029
1,634
10,056
3,297
Accretion of royalty liability
-
-
-
2,953
-
2,953
-
Amortization of deferred revenue
(166)
(217)
(930)
(161)
(147)
(322)
(215)
Interest expense, net (including amortization of
debt discount and deferred financing costs)
6,284
14,525
39,178
4,207
3,442
8,414
7,045
Expense (benefit) for income taxes
(14,683)
(5,171)
(1,121)
880
(351)
(225)
(1,232)
EBITDA
(117,631)
$
(73,119)
$
(102,568)
$
(14,457)
$
(13,564)
$
(212,363)
$
(31,159)
$
Non-GAAP adjustments:
Change in estimate of VIMOVO reyalties
-
-
-
13,033
-
13,033
-
Intangible impairment charge
69,621
-
-
-
-
-
-
Loss on derivative revaluation
-
-
69,300
10,965
-
214,995
-
Vidara acquisition costs
-
-
1,252
10,125
-
14,174
-
Stock-based compensation
2,530
4,661
5,014
4,160
1,021
6,087
2,100
Total of Non-GAAP adjustments
72,151
$
4,661
$
75,566
$
38,283
$
1,021
$
248,289
$
2,100
$
Adjusted EBITDA
(45,480)
$
(68,458)
$
(27,002)
$
23,826
$
(12,543)
$
35,926
$
(29,059)
$
(Unaudited)
(Unaudited)
Three Months Ended June 30,
Six Months Ended June 30,
Fiscal Year Ended December 31, |
 Horizon Pharma
plc October 13, 2014
Friedreich's Ataxia Analyst Day Presentation
Non-Confidential Information – Horizon Pharma plc
|
