Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a14-21561_18k.htm |
| EX-10.1 - EX-10.1 - AMAG PHARMACEUTICALS, INC. | a14-21561_1ex10d1.htm |
| EX-2.1 - EX-2.1 - AMAG PHARMACEUTICALS, INC. | a14-21561_1ex2d1.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a14-21561_1ex99d1.htm |
| EX-4.1 - EX-4.1 - AMAG PHARMACEUTICALS, INC. | a14-21561_1ex4d1.htm |
Exhibit 99.2
|
|
Transformative Acquisition of Lumara Health Maternal Health Business September 29, 2014 |
|
|
Forward-Looking Statements ` This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to, statements regarding: (1) our plans to build a multi-product specialty pharmaceutical company; (2) the transaction with Lumara Health and the addition of Makena to our product portfolio, including expected benefits, opportunities, synergies, financing, results and the impact on adjusted earnings per share (EPS), EBITDA and shareholder value, the impact on our financial profile, as well as the timing of the closing of the transaction and the issuance of stock consideration; (3) expectations that the combined company will have $350 million in U.S. product sales in 2015 and will be immediately accretive to adjusted EPS; (4) anticipated combined company adjusted EPS; (5) AMAG’s intentions to provide further guidance; (6) anticipated cost synergies of at least $20 million; (7) beliefs that Makena enjoys significant advantages over possible competition from generic alternatives; (8) expectations that Makena will retain long-term growth after exclusivity expires in February 2018; (9) expectations for Makena’s lifecycle management program; (10) anticipated benefits from rules and regulations, including the Drug Quality and Security Act, and product reimbursement trends; (11) the size of the market for Makena (potentially $1 billion-plus in revenue); (12) expectations for the Lumara Health maternal health commercial platform and our entry into a market important for the future growth of Feraheme® (ferumoxytol) injection; (13) our belief that this is a transformative transaction that propels us into a profitable, high-growth multi-product specialty pharmaceutical company positioned for continued revenue and bottom-line growth, further diversification and shareholder value creation; (14) expectations regarding Lumara Health’s impact on future product acquisitions; (15) beliefs about the commercial platform and the strategic fit of Lumara Health; (16) our plans to expand the market for Feraheme; (17) beliefs about Makena and its benefits, including that it is a unique product that serves an important medical need; (18) the impact of Makena on our product portfolio; (19) beliefs regarding the opportunity for Makena and the potential to leverage our in-office injectables commercial expertise; (20) expectations regarding the Makena commercial team, including its ability to grow the brand; (21) beliefs regarding the combined company’s scale, portfolio diversification, resources and commercial expertise, including the impact on AMAG’s long-term growth opportunities and patient service; (22) AMAG being the right partner to support the continued growth of Makena and its maternal health business; (23) plans for Lumara Health following the closing; (24) expectations regarding the financing for and the timeline for the Lumara Health transaction; (25) our tax attributes and expectations regarding the shareholder rights plan amendment; (26) our plans to expand our portfolio through the in-license or purchase of additional pharmaceutical products or companies; and (27) our goal of bringing to market therapies that provide clear benefits and improve patients’ lives are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Statements about AMAG’s or Lumara Health’s past financial results do not, and are not meant to, predict future results. AMAG can provide no assurance that such results and performance will continue. For the avoidance of doubt, any statements herein that relate to the acquisition of Lumara Health are forward-looking statements within the meaning of the PSLRA and other federal securities laws. Such risks and uncertainties include, among others: (1) the possibility that the closing conditions set forth in the definitive acquisition agreement, including those conditions related to antitrust clearance, will not be met and that the parties will be unable to consummate the proposed transaction, (2) the chance that, despite having a commitment in place, we will be unable to secure financing, or financing on satisfactory terms, in amounts sufficient to consummate the acquisition, (3) the possibility that if the acquisition is consummated, we may not realize the expected benefits, synergies and opportunities anticipated in connection with the transaction, including the anticipated costs synergies of $20 million, annualized sales of more than $180 million and continued revenue growth, annualized EBITDA of $100 million, the $1 billion market opportunity, the accelerated use of combined $500 million in net operating losses and that the transaction will be immediately accretive to adjusted EPS, (4) the challenges of integrating the Makena commercial team into AMAG, (5) the impact on sales of Makena from competitive, commercial payor, government (including federal and state Medicaid reimbursement policies), physician, patient or public responses with respect to product pricing, product access and sales and marketing initiatives, (6) the impact of patient compliance on units sales, (7) the uncertainty of achieving sales of Feraheme to OB/GYN specialists for the treatment of women who suffer from iron deficiency anemia (IDA), even assuming FDA approval for the broader indication, (8) we may face challenges in leveraging our in-office injectables commercial expertise, which could result in unforeseen expenses and disrupt business operations, (9) liabilities we assume from Lumara Health, including the class action litigation In Re K-V Pharmaceutical Company Securities Litigation, Case No. 4:11CV1816 AGF, may be higher than expected, (10) the possibility that sales of Makena will not meet expectations as a result of current and future competition from compounded products and/or future competition from generic alternatives upon expiration of exclusivity in February 2018, (11) the impact of reimbursement policies for Makena and the resulting coverage decisions and/or impact on pricing, (12) the number of preterm birth risk pregnancies for which Makena may be prescribed, its safety and side effects profile and acceptance of pricing, 1 |
|
|
Forward-Looking Statements (cont.) 2 (13) in connection with the Lumara Health acquisition, we will incur a substantial amount of indebtedness and will have to comply with restrictive and affirmative debt covenants, including a requirement that we reduce our leverage over time, (14) the possibility that we will need to raise additional capital from the sale of our common stock, which will cause significant dilution to our stockholders, in order to satisfy our contractual obligations, including our debt service, milestone payments that may become payable to Lumara Health’s stockholders /or in order to pursue business development activities, (15) upon consummation of the Lumara Health transaction, we will be highly leveraged and have limited cash and cash equivalent resources which may limit our ability to take advantage of attractive business development opportunities and execute on our strategic plan, (16) the possibility that our stockholders will not approve the amendment to our shareholder rights plan and that our tax benefits, including those acquired upon consummation of the Lumara Health acquisition, will not be available in the future, (17) the likelihood and timing of potential approval of Feraheme in the U.S. in the broader IDA indication in light of the complete response letter we received from the FDA informing us that our supplemental new drug application (sNDA) for the broader indication could not be approved in its present form and stating that we had not provided sufficient information to permit labeling of Feraheme for safe and effective use for the proposed broader indication, (18) the possibility that following FDA review of post-marketing safety data, including reports of serious anaphylaxis, cardiovascular events, and death, and/or in light of the label changes requested by the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) and confirmed by the Committee for Medicinal Products for Human Use (CHMP), the FDA (or other regulators) will request additional technical or scientific information, new studies or reanalysis of existing data, on-label warnings, post-marketing requirements/commitments or risk evaluation and mitigation strategies (REMS) in the current indication for Feraheme for IDA in adult patients with chronic kidney disease (CKD) and the additional costs and expenses that will or may be incurred in connection with such activities, (19) whether our proposed label changes will be acceptable to the FDA or other regulatory authorities and what impact such changes, or such additional changes as the FDA, CHMP or other regulators may require, will have on sales of Feraheme/ Rienso™ (Rienso is the trade name for ferumoxytol outside of the U.S. and Canada), (20) our and Takeda Pharmaceutical Limited’s ability to successfully compete in the IV iron replacement market both in the U.S. and outside the U.S., including the EU, as a result of limitations, restrictions or warnings in Feraheme’s/Rienso’s current or future label, including the changes recommended by PRAC and confirmed by CHMP that Rienso be administered to patients by infusion over at least 15-minutes (replacing injection) and that it be contraindicated for patients with any known history of drug allergy, (21) our ability to execute on our long-term strategic plan or to realize the expected results from our long-term strategic plan, (22) Takeda’s ability to obtain regulatory approval for Feraheme in Canada, and Rienso in the EU, in the broader IDA patient population, (23) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso and in turn affect sales, or our ability to market the product both in the U.S. and outside of the U.S., including the EU, (24) the relationship between Takeda and AMAG and the impact on commercialization efforts for Feraheme/Rienso in the EU and Canada, (25) the likelihood and timing of milestone payments, if any, in connection with AMAG’s licensing arrangement with Takeda, (26) the manufacture of Feraheme/Rienso or MuGard (or Makena if the acquisition is consummated), including any significant interruption in the supply of raw materials or finished product, (27) our patents and proprietary rights both in the U.S. and outside the U.S. (including those acquired in the acquisition), (28) the risk of an Abbreviated New Drug Application (ANDA) filing following the FDA’s draft bioequivalence recommendation for ferumoxytol published in December 2012, (29) the possibility that AMAG will disseminate future Dear Healthcare Provider letters in the U.S. (or, working Takeda, in Europe or other markets), (30) uncertainties regarding our ability to compete in the oral mucositis market in the U.S. and in the women’s maternal health market and (31) other risks identified in our filings with the U.S. Securities and Exchange Commission (SEC), including our Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and subsequent filings with the SEC. Any of the above risks and uncertainties could materially and adversely affect our results of operations, our profitability and our cash flows, which would, in turn, have a significant and adverse impact on our stock price. Use of the term “including” in the two paragraphs above shall mean in each case “including, but not limited to.” We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals and Feraheme are registered trademarks of AMAG Pharmaceuticals, Inc. MuGard is a registered trademark of Access Pharmaceuticals, Inc. Rienso is a trademark of Takeda Pharmaceutical Company Limited. Makena is a registered trademark of Lumara Health Inc. Lumara Health is a trademark of Lumara Health Inc. |
|
|
3 + |
|
|
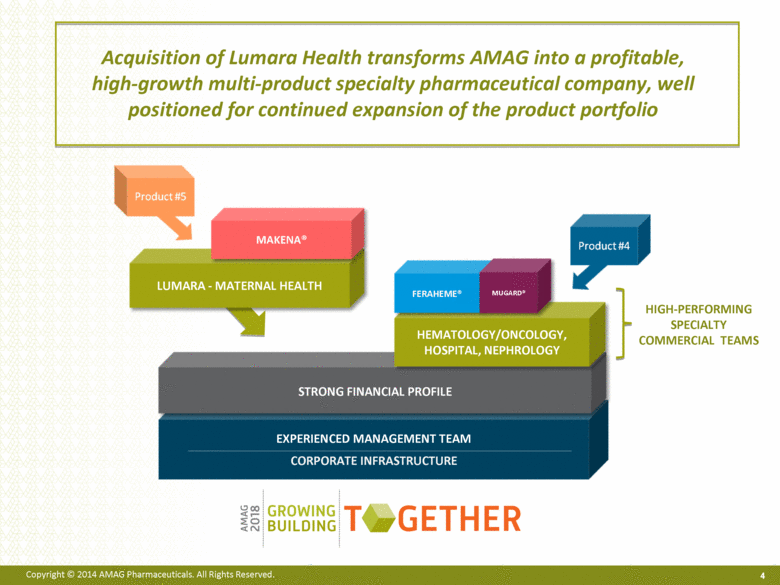
EXPERIENCED MANAGEMENT TEAM CORPORATE INFRASTRUCTURE Acquisition of Lumara Health transforms AMAG into a profitable, high-growth multi-product specialty pharmaceutical company, well positioned for continued expansion of the product portfolio 4 HEMATOLOGY/ONCOLOGY, HOSPITAL, NEPHROLOGY FERAHEME® MUGARD® HIGH-PERFORMING SPECIALTY COMMERCIAL TEAMS LUMARA - MATERNAL HEALTH MAKENA® |
|
|
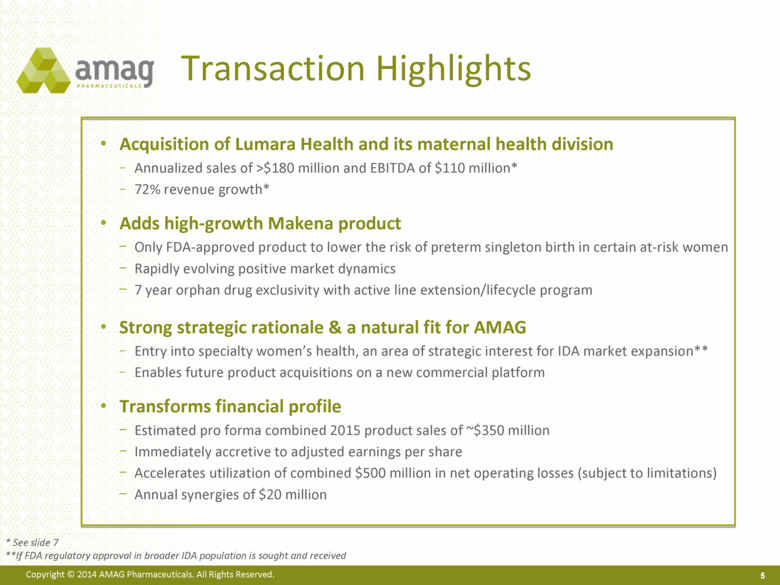
Transaction Highlights Acquisition of Lumara Health and its maternal health division Annualized sales of >$180 million and EBITDA of $110 million* 72% revenue growth* Adds high-growth Makena product Only FDA-approved product to lower the risk of preterm singleton birth in certain at-risk women Rapidly evolving positive market dynamics 7 year orphan drug exclusivity with active line extension/lifecycle program Strong strategic rationale & a natural fit for AMAG Entry into specialty women’s health, an area of strategic interest for IDA market expansion** Enables future product acquisitions on a new commercial platform Transforms financial profile Estimated pro forma combined 2015 product sales of ~$350 million Immediately accretive to adjusted earnings per share Accelerates utilization of combined $500 million in net operating losses (subject to limitations) Annual synergies of $20 million 5 * See slide 7 **If FDA regulatory approval in broader IDA population is sought and received |
|
|
6 Transaction Terms Performance-Based Financial Structure $675MM consideration (~3.75 times annualized sales) Up to $350MM of additional consideration based on Makena sales, including milestones of $300MM, $400MM and $500MM* Operational Structure Lumara Health will operate as separate business unit within AMAG Easy integration of Makena commercial team, including 75-person sales force Sources of Consideration $340MM committed term loan B financing, attractive terms $260MM in existing AMAG cash $75MM equity (subject to lock-up agreement) Timing Hart-Scott-Rodino (HSR) review Expect to complete transaction in 4Q14 * Based on consecutive 12 months net sales |
|
|
Lumara Maternal Health Business 7 Privately-held specialty pharmaceutical company focused on maternal health Flagship product, Makena Commercial team with deep experience with OB/GYN sub-specialty Lumara Health’s new commercial leadership is pursuing patient-centric business strategy Makena® is a progestin used to lower the risk of preterm birth in women who are pregnant with one baby and who have spontaneously delivered one preterm baby in the past Weekly intramuscular injections from 16 to 37 weeks of pregnancy, 18-22 total injections |
|
|
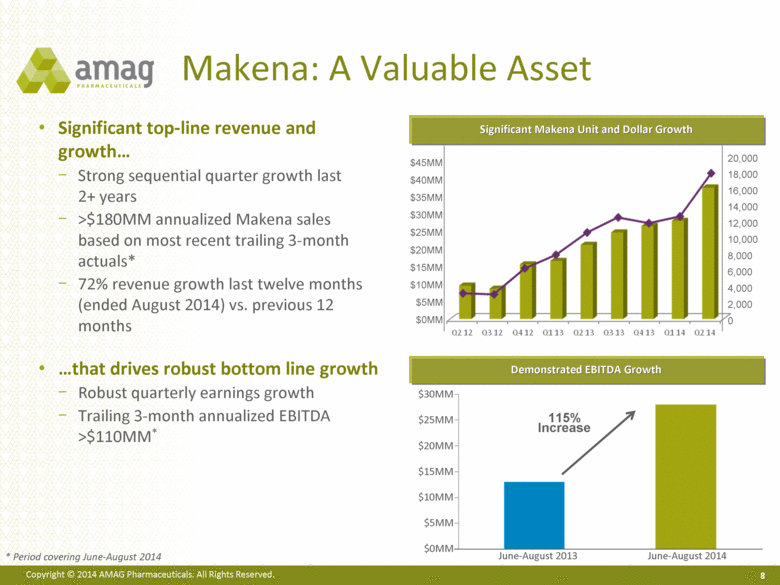
Makena: A Valuable Asset 8 Significant Makena Unit and Dollar Growth Demonstrated EBITDA Growth Significant top-line revenue and growth Strong sequential quarter growth last 2+ years >$180MM annualized Makena sales based on most recent trailing 3-month actuals* 72% revenue growth last twelve months (ended August 2014) vs. previous 12 months that drives robust bottom line growth Robust quarterly earnings growth Trailing 3-month annualized EBITDA >$110MM* June-August 2013 June-August 2014 $0MM $5MM $10MM $15MM $20MM $25MM $30MM 115% Increase $45MM $40MM $35MM $30MM $25MM $20MM $15MM $10MM $5MM $0MM 20,000 18,000 16,000 14,000 12,000 10,000 8,000 6,000 4,000 2,000 0 * Period covering June-August 2014 |
|
|
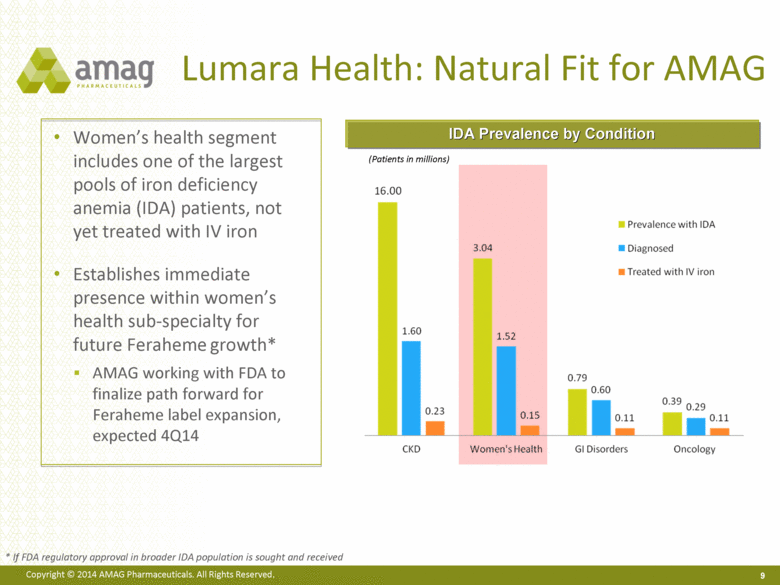
Lumara Health: Natural Fit for AMAG Women’s health segment includes one of the largest pools of iron deficiency anemia (IDA) patients, not yet treated with IV iron Establishes immediate presence within women’s health sub-specialty for future Feraheme growth* AMAG working with FDA to finalize path forward for Feraheme label expansion, expected 4Q14 (Patients in millions) IDA Prevalence by Condition * If FDA regulatory approval in broader IDA population is sought and received 9 |
|
|
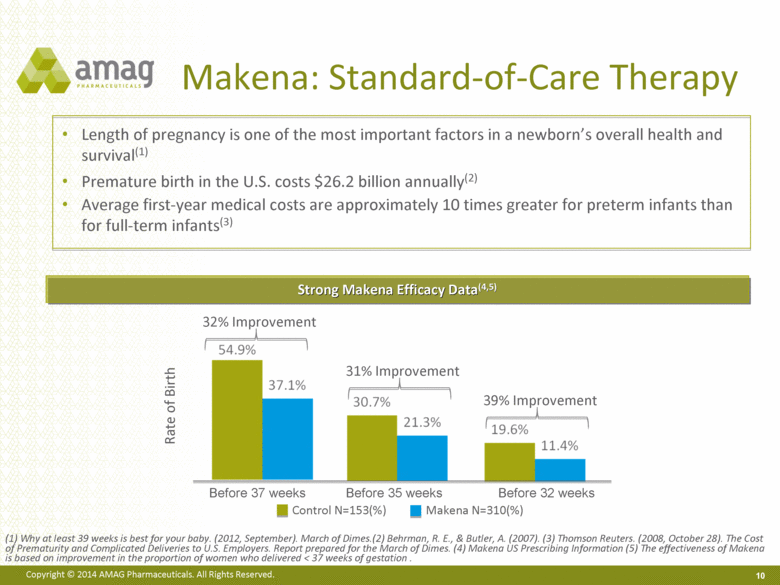
Makena: Standard-of-Care Therapy Strong Makena Efficacy Data(4,5) Rate of Birth 32% Improvement Control N=153(%) Makena N=310(%) Before 35 weeks Before 32 weeks Before 37 weeks 31% Improvement 39% Improvement Length of pregnancy is one of the most important factors in a newborn’s overall health and survival(1) Premature birth in the U.S. costs $26.2 billion annually(2) Average first-year medical costs are approximately 10 times greater for preterm infants than for full-term infants(3) (1) Why at least 39 weeks is best for your baby. (2012, September). March of Dimes.(2) Behrman, R. E., & Butler, A. (2007). (3) Thomson Reuters. (2008, October 28). The Cost of Prematurity and Complicated Deliveries to U.S. Employers. Report prepared for the March of Dimes. (4) Makena US Prescribing Information (5) The effectiveness of Makena is based on improvement in the proportion of women who delivered < 37 weeks of gestation . 10 |
|
|
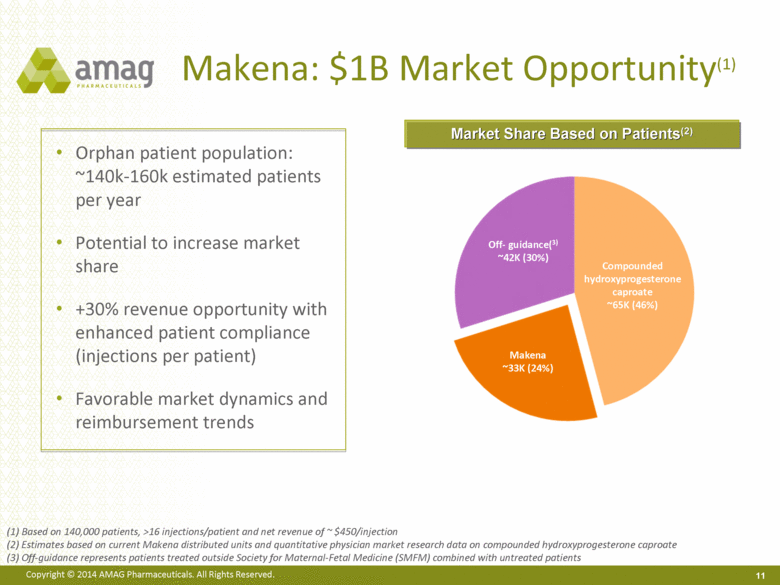
Orphan patient population: ~140k-160k estimated patients per year Potential to increase market share +30% revenue opportunity with enhanced patient compliance (injections per patient) Favorable market dynamics and reimbursement trends Market Share Based on Patients(2) Makena: $1B Market Opportunity(1) 11 Compounded hydroxyprogesterone caproate ~65K (46%) Off- guidance(3) ~42K (30%) Makena ~33K (24%) (1) Based on 140,000 patients, >16 injections/patient and net revenue of ~ $450/injection (2) Estimates based on current Makena distributed units and quantitative physician market research data on compounded hydroxyprogesterone caproate (3) Off-guidance represents patients treated outside Society for Maternal-Fetal Medicine (SMFM) combined with untreated patients 11 |
|
|
Favorable Market Dynamics Federal Drug Quality and Security Act (enacted 11/2013) Clarifies roles of traditional compounding and FDA-approved medicines Makes clear FDA's authority over drug compounding and requires agency to engage and coordinate with states 12 12 “If FDA identifies a registered outsourcing facility that compounds any drug products that are essentially copies of Makena as defined under section 503B, FDA intends to take action as it deems appropriate.“ – FDA Website, posted 7/13/2014 |
|
|
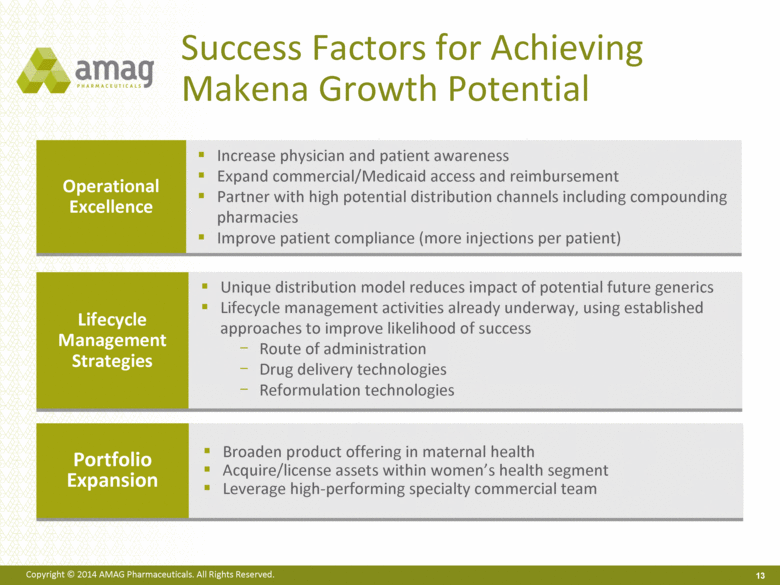
Success Factors for Achieving Makena Growth Potential 13 Operational Excellence Increase physician and patient awareness Expand commercial/Medicaid access and reimbursement Partner with high potential distribution channels including compounding pharmacies Improve patient compliance (more injections per patient) Lifecycle Management Strategies Unique distribution model reduces impact of potential future generics Lifecycle management activities already underway, using established approaches to improve likelihood of success Route of administration Drug delivery technologies Reformulation technologies Portfolio Expansion Broaden product offering in maternal health Acquire/license assets within women’s health segment Leverage high-performing specialty commercial team |
|
|
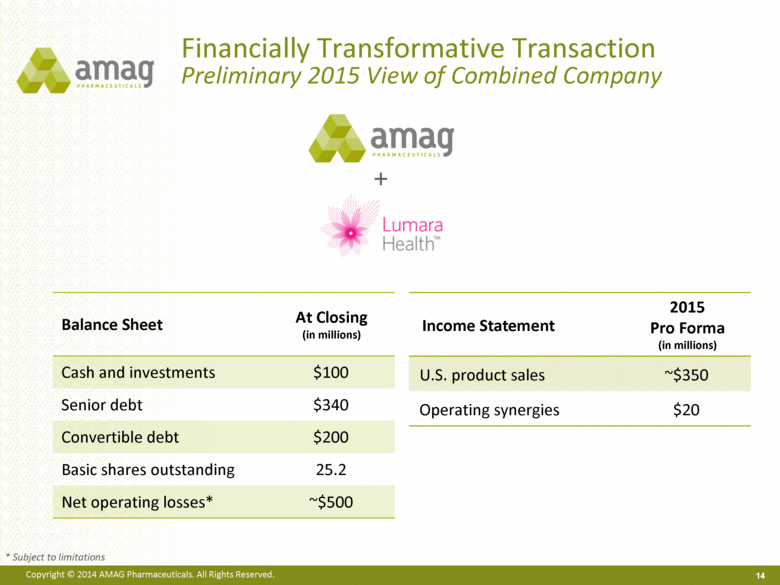
Financially Transformative Transaction Preliminary 2015 View of Combined Company 14 + Income Statement 2015 Pro Forma (in millions) U.S. product sales ~$350 Operating synergies $20 Balance Sheet At Closing (in millions) Cash and investments $100 Senior debt $340 Convertible debt $200 Basic shares outstanding 25.2 Net operating losses* ~$500 * Subject to limitations |
|
|
Transaction Creates Significant Shareholder Value Transforms AMAG into a profitable, high-growth, multi-product specialty pharmaceutical company Adds a proven product in a therapeutic area of strategic interest for Feraheme Provides a new commercial platform for portfolio expansion through business development Offers compelling financial benefits – adds significant revenues, profitability and synergies 15 |