Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 19, 2014
Bone Biologics, Corp.
(Exact name of registrant as specified in its charter)
| Delaware | 000-53078 | 42-1743430 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 175 May Street, Suite 400, Edison, NJ | 08837 | |
| (Address of principal executive offices) | (Zip Code) |
(732) 661-2224
(Registrant’s telephone number, including area code)
AFH Acquisition X, Inc.
269 S. Beverly Drive, Suite 1600
Beverly Hills, CA 90212
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
[ ] Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
[ ] Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
[ ] Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
[ ] Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Current Report contains forward-looking statements. These statements are based on the Company’s (as hereinafter defined) current beliefs, expectations and assumptions about future events, conditions and results and on information currently available to them. All statements, other than statements of historical fact, included herein regarding the Company’s strategy, future operations, financial position, future revenues, projected costs, plans, prospects and objectives are forward-looking statements. Words such as “expect,” “may,” “anticipate,” “intend,” “would,” “plan,” “believe,” “estimate,” “should,” and similar words and expressions are intended to identify forward-looking statements, but are not the exclusive means of identifying forward-looking statements. Forward-looking statements in the Current Report include express or implied statements concerning the Company’s future revenues, expenditures, capital or other funding requirements, the adequacy of the Company’s current cash and working capital to fund present and planned operations and financing needs, expansion of and demand for product offerings, and the growth of the Company’s business and operations through acquisitions or otherwise, as well as future economic and other conditions both generally and in the Company’s specific geographic and product markets. These statements are based on currently available operating, financial and competitive information and are subject to various risks, uncertainties and assumptions that could cause actual results to differ materially from those anticipated or implied in the forward-looking statements due to a number of factors including, but not limited to, those set forth below in the section entitled “Risk Factors and Special Considerations” in this Current Report. Given those risks, uncertainties and other factors, many of which are beyond the Company’s control, you should not place undue reliance on these forward-looking statements.
Before purchasing the Shares, you should carefully read and consider the risks described under the section entitled “Risk Factors and Special Considerations.” You should be prepared to accept any and all of the risks associated with purchasing the securities, including a loss of all of your investment.
The forward-looking statements relate only to events as of the date on which the statements are made. Neither the Company nor Bone (as hereinafter defined) undertakes any obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, even if experience or future changes make it clear that any projected results or events expressed or implied therein will not be realized. You are advised, however, to consult any further disclosures the Company makes in future public filings, statements and press releases.
| i |
On September 19, 2014, AFH Acquisition X, Inc. (the “Company”) and its wholly-owned subsidiary, Bone Biologics Acquisition Corp., a Delaware corporation (“Merger Sub”), entered into an Agreement and Plan of Merger, dated September 19, 2014 (the “Merger Agreement”), by and among (i) the Company, (ii) Bone Biologics, Inc. (“Bone” or “Bone Biologics”), and (iii) Merger Sub. Pursuant to the terms of the Merger Agreement, Bone merged with Merger Sub on September 19, 2014, with Bone as the surviving entity, in exchange for the issuance of 19,897,587 shares of the Company’s common stock, par value $0.001 per share (“Common Stock”) (including 2,151,926 shares of Common Stock issuable upon the exercise of outstanding warrants and 5,648,658 shares issuable upon the conversion of debt) to the stockholders of Bone as set forth in the Merger Agreement (the “Merger”). After the Merger, the Company ceased to be a shell company, as defined in the rules of the SEC, and the Company officially changed its name to “Bone Biologics, Corp.” The 5,000,000 outstanding shares of Common Stock of the Company prior to the Merger were consolidated into 3,853,600 shares of Common Stock and the remaining shares were cancelled.
As used in this Current Report, the terms the “we,” “us,” and “our” refer to the Company, after giving effect to the Merger, unless otherwise stated or the context clearly indicates otherwise. The term “AFH Acquisition X” refers to the Company, as it was named “AFH Acquisition X, Inc.” before giving effect to the Merger.
This Current Report contains summaries of the material terms of various agreements executed in connection with the transactions described herein. The summaries of these agreements are subject to, and are qualified in their entirety by, reference to these agreements, all of which are incorporated herein by reference.
This Current Report is being filed in connection with a series of transactions consummated by the Company and certain related events and actions taken by the Company.
This Current Report responds to the following items on Form 8-K:
| Item 1.01 | Entry into a Material Definitive Agreement |
| Item 2.01 | Completion of Acquisition or Disposition of Assets |
| Item 3.02 | Unregistered Sales of Equity Securities |
| Item 5.01 | Changes in Control of Registrant |
| Item 5.02 | Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers; Compensatory Arrangements of Certain Officers |
| Item 5.03 | Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year |
| Item 5.06 | Change in Shell Company Status |
| Item 9.01 | Financial Statements and Exhibits |
| ii |
TABLE OF CONTENTS
| iii |
Item 1.01. Entry into a Material Definitive Agreement.
The Merger Agreement
On September 19, 2014, we entered into the Merger Agreement, and on September 19, 2014 we completed the Merger. For a description of the Merger and the material agreements entered into in connection with the Merger, please see the disclosures set forth in Item 2.01 to this Current Report, which disclosures are incorporated into this item by reference.
Indemnification Agreements
Effective September 19, 2014, our Board of Directors approved a form of indemnification agreement (the “Indemnification Agreement”) to be entered into between us and our current directors and certain executive officers. The Indemnification Agreement requires that we, under the circumstances and to the extent provided for therein, indemnify such persons to the fullest extent permitted by applicable law against certain expenses and other amounts incurred by any such person as a result of such person being made a party to certain actions, suits and proceedings by reason of the fact that such person is or was a director, officer, employee or agent of the Company, any entity that was a predecessor corporation of the Company or any of the Company’s affiliates. The rights of each person who is a party to an Indemnification Agreement are in addition to any other rights such person may have under applicable law, our Articles of Incorporation, our Bylaws, any other agreement, a vote of our stockholders, a resolution adopted by our Board of Directors or otherwise. Immediately following the closing of the Merger, we entered into indemnification agreements in the form of the Indemnification Agreement with each of our newly appointed executive officers and directors. The foregoing is only a brief description of the Indemnification Agreement, does not purport to be a complete description of the rights and obligations of the parties thereunder and is qualified in its entirety by reference to the form of Indemnification Agreement filed as Exhibit 10.17 to this Current Report on Form 8-K and incorporated herein by reference.
Effective as of September 19, 2014, our Board of Directors also approved a form of indemnification agreement (the “Former D&O Indemnification Agreement”) to be entered into between us, Don Hankey and Amir Heshmatpour. The Former D&O Indemnification Agreement requires that for a period of four (4) years from and after September 19, 2014, we will indemnify (including advancement of expenses) and hold harmless persons who were officers and directors of the Company (i) by reason of being an officer or director of the Company prior to the Merger, including through all transactions relating to the Merger, or (ii) is related to acts in connection with the Merger taken by the Former D&O Indemnified Persons, provided however, that the foregoing indemnity shall be excess of all any insurance coverage available to the Former D&O Indemnified Parties for any such loss. The accuracy of the affidavits executed by Mr. Hankey (the “Hankey Affidavit”) and Mr. Heshmatpour (the “Heshmatpour Affidavit”) in connection with the Former D&O Indemnification is a condition precedent to the foregoing indemnity (including advancement of expenses). The Company has no insurance coverage that would cover any claim asserted against the Company by any Former D&O Indemnified Person pursuant to this Former D&O Indemnification Agreement. This description is qualified in its entirety by the Former D&O Indemnification Agreement filed as Exhibit 10.18 to this Current Report on Form 8-K and incorporated herein by reference.
Item 2.01. Completion of Acquisition or Disposition of Assets.
THE MERGER AND RELATED TRANSACTIONS
The Merger
On September 19, 2014, the Company and its wholly-owned subsidiary, Merger Sub, entered into the Merger Agreement, dated September 19, 2014, by and among the Company, Merger Sub, and Bone Biologics. Pursuant to the Merger Agreement, Merger Sub merged with and into Bone Biologics, with Bone Biologics remaining as the surviving corporation in the Merger. Upon the consummation of the Merger, the separate existence of Merger Sub ceased, on September 22, 2014 the Company officially changed its name to “Bone Biologics, Corp.” to more accurately reflect the nature of its business, and Bone Biologics became a wholly-owned subsidiary of the Company. A copy of the Merger Agreement is attached as Exhibit 2.1 to this Current Report and is incorporated herein by reference.
| 1 |
In connection with the Merger, the 5,000,000 outstanding shares of Common Stock of the Company prior to the Merger were consolidated into 3,853,600 shares of Common Stock and the remaining shares were cancelled.
Additionally, at the Effective Time (as defined below), all of the issued and outstanding shares of Bone Biologics Inc.’s $0.0001 par value common stock (“Bone Biologics Common Stock”) converted into a combined total of 19,897,587 shares of the Company’s Common Stock (including 2,151,926 shares issuable upon the exercise of outstanding warrants and 5,648,658 shares issuable upon the conversion of debt) (the “Company Merger Consideration”). In exchange, Bone Biologics agreed to pay AFH Holding & Advisory, LLC (“AFH Advisory”) the principal sum of $590,000. On July 3, 2014, Bone Biologics paid AFH Advisory $250,000 of such amount and on July 31, 2014, Bone Biologics issued that certain Promissory Note, dated July 31, 2014 (the “Note”), pursuant to which the Bone Biologics promised to pay AFH Advisory the principal sum of $340,000. MTF has granted AFH Advisory a standby letter of credit in the amount of $340,000 for the remaining amount due under the Note. On September 19, 2014, the Note was assigned to the Company.
In addition, MTF converted all amounts due, $1,533,356, pursuant to a convertible promissory note dated January 18, 2008 in the original face amount of $1,107,000 entered into by and between the Bone Biologics and MTF prior to consummation of the Merger to shares of Series B Preferred Stock of Bone Biologics at $ 1.00 per share, then to shares of Common Stock of Bone Biologics at a 1:1 basis. MTF agreed to execute any additional agreements reasonably necessary to give effect to that provision. Prior to the Merger, MTF converted all of the outstanding shares of Series A preferred stock and Series B preferred stock of Bone Biologics that MTF held into shares of Bone Biologics Common Stock.
Prior to consummation of the Merger, the 2013 Bridge Note holders converted outstanding principal and accrued interest of $455,974 into Common Stock at a conversion price of $1.00 per share. The $50,000 that Bone Biologics borrowed from AFH Advisory pursuant to the sale and issuance of Notes and Warrants to AFH Advisory was contingent upon liquidation of the securities transferred to the Company by AFH Advisory, as describe in that certain Letter Agreement, dated September 26, 2013, by and between Amir F. Heshmatpour, an individual residing in the State of California, and the Company.
Each share of capital stock of Merger Sub that was issued and outstanding immediately prior to the effective time of the Merger (the “Effective Time”), by virtue of the Merger and without further action on the part of Bone Biologics, Merger Sub, or the Company, was converted into and became 100 shares of Bone Biologics Common Stock, which shares were the sole issued and outstanding shares of Bone Biologics Common Stock immediately after the Effective Time. After the Merger, each certificate evidencing ownership of shares of Merger Sub common stock evidenced ownership of such shares of Bone Biologics Common Stock.
At the Effective Time, there were no shares of Bone Biologics Common Stock that were owned by Bone Biologics as treasury stock or reserved for issuance by Bone Biologics.
Also at the Effective Time, all of the issued and outstanding options and warrants to purchase shares of Bone Biologics Common Stock converted, respectively, into options (the “New Options”) and warrants (the “New Warrants”) to purchase shares of Common Stock. The New Options and the New Warrants were re-issued on a 1 for 1 basis.
The Merger Agreement contains customary representations, warranties, and covenants of the Company, Bone Biologics, and Merger Sub for a reverse triangular merger (and accompanying transactions). Breaches of representations and warranties are secured by customary indemnification provisions.
The Merger will be treated as a “reverse merger,” and although the Company is the legal acquirer, Bone Biologics is deemed to be the acquirer for accounting purposes in the reverse merger. Consequently, the assets and liabilities and the historical operations that will be reflected in the financial statements prior to the Merger will be those of Bone Biologics, and the consolidated financial statement after completion of the Merger will include the assets and liabilities of Bone Biologics, historical operations of Bone Biologics and operations of Bone Biologics from the Closing Date of the Merger and in all future filings with the Securities and Exchange Commission (the “SEC”).
| 2 |
Following the Effective Time, our Board of Directors will be as identified in this Current Report under the heading “Directors and Executive Officers.”
Bridge Financing
Bone Biologics borrowed $400,000 pursuant to the sale and issuance of convertible promissory notes (the “Bridge Notes,” or singularly each a “Bridge Note”) and warrants to purchase Common Stock (as defined below) of the Company, as the successor to Bone Biologics. Prior to consummation of the Merger, the 2013 Bridge Note holders converted outstanding principal and accrued interest of $455,974 into Common Stock at a conversion price of $1.00 per share. MTF has purchased $100,000 of the Notes and Warrants. Orthofix Holdings Inc. (“Orthofix”) has purchased $250,000 of the Notes and Warrants. AFH Advisory purchased $50,000 of the Bridge Notes and Bridge Warrants which was contingent upon liquidation of the securities transferred to the Company by AFH Advisory, as described in that certain Letter Agreement, dated September 26, 2013, by and between Amir F. Heshmatpour and the Company. The Bridge Warrants and the amendment thereto are filed as Exhibits 4.1, 4.2, 4.3 and 4.4, respectively, to this Current Report and are incorporated herein by reference. The issuance of the Bridge Notes and Bridge Warrants are collectively referred to in this Current Report as the “Bridge Financing.”
Bone entered into a Security Agreement, dated March 17, 2009, wherein it pledged certain of its assets as collateral to the Bridge Note holders. Additionally, Bone and MTF entered into a Subordination Agreement, dated April 2013, wherein the parties agreed that the security interest granted to MTF pursuant to the March 17, 2009 Security Agreement between the parties wherein MTF agreed to subordinate any rights to any payment and any security interest it may have in Bone’s assets to the holders of the Bridge Notes. In addition, Benjamin Wu, Kang Ting, and Chia Soo executed a Pledge and Guarantee Agreement, dated April 18, 2013, in favor of the Bridge Note purchasers wherein Benjamin Wu, Kang Ting, and Chia Soo pledged their shares of Bone Biologics Common Stock to secure the full and punctual payment of Bone’s obligations to the Bridge Note holders and unconditionally and irrevocably guaranteed such payment. Upon consummation of the Merger, the notes converted into notes of the Company.
On September 26, 2013, Bone and AFH Advisory entered into a letter agreement wherein Bone agreed to accept shares of common stock of Targeted Medical Pharma, Inc. (the “TMP Shares”) with a market value of $50,000 on the date of transfer as consideration for the aforementioned purchase of the Bridge Note and the Bridge Warrant for AFH Advisory. Bone agreed to use its commercially reasonable best efforts to sell the TMP Shares on the Over-the-Counter Market within 90 days of such transfer. Because the gross proceeds from the sale of the TMP Shares during such 90-day period (the “Initial Gross Proceeds”) were less than $50,000 (the “Deficiency Amount”), AFH Advisory agreed to provide the Company with a cash infusion equal to the Deficiency Amount following the Merger.
In addition to and subsequent to the Bridge Notes and Bridge Warrants discussed in the preceding paragraph, Orthofix, on July 1, 2014, also (A) purchased $500,000 worth of Bone Biologics Common Stock (the “Subsequent Orthofix Shares”); (B) was issued two convertible promissory notes (the “Subsequent Orthofix Convertible Promissory Notes”), each in the principal amount of $250,000 and exercisable for $333,333 worth of Bone Biologics Common Stock; and (C) was issued two warrants (the “Subsequent Orthofix Warrants”), each exercisable for 166,667 shares of Bone Biologics Common Stock at an exercise price per share of $1.50. Upon subscribing for the Subsequent Orthofix Shares, the Subsequent Orthofix Convertible Promissory Notes converted by its terms into a combined total of $666,666 worth of shares of Bone Biologics Common Stock in accordance with the terms of the Subsequent Orthofix Convertible Promissory Notes. The Subsequent Orthofix Warrants converted into warrants of the Company with substantially identical terms upon consummation of the Merger. Amounts received by Bone Biologics in connection with the Subsequent Orthofix Convertible Promissory Notes and the Subsequent Orthofix Shares will be aggregated towards the $5 million amount to be raised in the Private Placement for purposes of determining when various parties will be paid their fees in connection with the Merger and the Private Placement.
| 3 |
Subsequent Closings
Upon filing this Current Report, the Company will commence a private placement of securities, whether debt or equity, of up to a maximum of $10.0 million that will include an over-allotment option of 15% at AFH Advisory’s discretion or at the discretion of any investment bank engaged for such offering (the “Private Placement”). Private Placement proceeds in excess of $1.0 million will be used upon receipt for: (i) payment of the expenses payable to AFH Advisory for providing a publicly-reporting company for the Merger and capped at $250,000 to reduce the Note and (ii) $750,000 to the working capital of the Company to be utilized for general corporate purposes. All proceeds received between $1.0 million and $1.5 million in the Private Placement shall be used to further retire the Note by reimbursement of an additional $250,000 of the Note and to provide an additional $250,000 for general corporate purposes of the Company, split evenly on a dollar for dollar basis up to $500,000. The proceeds received over $1.5 million will initially be paid on a pro rata basis, with 50% of such proceeds being directed to the Company for working capital purposes and 50% being used to pay off other fees incurred in connection with the Merger and the Private Placement, including the final $90,000 owed under the Note. Once all such fees in connection with the Merger and the Private Placement have been paid by the Company, the Company will use any additional proceeds received in the Private Placement to first pay all outstanding principal, interest and penalties then outstanding with respect to the MTF Short Term 2014 Loan (as such term is defined herein) and second for working capital purposes. The Private Placement will be structured such that any debt issued may either be convertible into Common Stock at a fixed conversion price or repayable from the proceeds of the Initial Public Offering (as defined below), if not due sooner pursuant to the terms thereof. The Company and MTF each agree that none of the proceeds from the Private Placement will be used for the repayment of the Bridge Financing or any outstanding long-term debt of the Company. The shares offered in the Private Placement will be offered pursuant to exemptions provided by Section 4(a)(2) and/or Section 4(6) of the Securities Act of 1933 (the “Securities Act”) and Rule 506 of Regulation D as promulgated by the SEC. No general solicitation will be made by us or any person acting on our behalf in the Private Placement. The $1 million investment received from Orthofix on July 1, 2014 will be counted towards the amounts received in the Private Placement for purposes of determining when the Private Placement will be deemed closed and for purposes of determining when and how proceeds from the Private Placement will be allocated.
After the Private Placement, the Company intends to procure an investment bank to handle a private investment in public entity offering in an amount between $8.0 million and $10.0 million at a valuation of not less than the post-money valuation of the Company at the closing of the Private Placement through the sale of securities of the Company (the “PIPE”). The PIPE will include a 15% over allotment option at AFH Advisory’s discretion or at the discretion of any investment bank engaged for such offering. Commencement of the PIPE offering is contingent upon (i) the appointment and continued support of an investment bank, and (ii) the filing of Financial Industry Regulatory Authority, Inc. (“FINRA”) Form 211.
Following to the PIPE offering, and at such time as it is deemed appropriate by AFH Advisory and the Company, the Company intends to procure an investment bank to act as underwriter for an initial public offering in an amount up to $40 million (the “Initial Public Offering”) at a valuation not less than the post-money valuation of the Company at the closing of the PIPE offering. The Initial Public Offering shall include a 15% over allotment option at AFH Advisory’s discretion or at the discretion of any investment bank engaged for such offering. Both parties recognize that the Initial Public Offering is contingent upon the appointment and continued support of an investment bank.
The Private Placement, the PIPE, and the IPO are collectively referred to in this Current Report as the “Subsequent Closings.” The Merger, the Subsequent Closings, the Bridge Financing and the related transactions are collectively referred to in this Current Report as the “Transactions.”
Registration Rights
All of the securities issued in connection with the Transactions, except the IPO, are “restricted securities,” and as such are subject to all applicable restrictions specified by federal and state securities laws.
Effective as of September 19, 2014, the Company entered into a Registration Rights Agreement with MTF, AFH Advisory and Hanky Investment Company, L.P. (“HIC”), each of which have certain demand registration rights and unlimited piggyback registration rights for the Company’s shares under the Registration Rights Agreement and subject to an agreed lock up period. Pursuant to the Registration Rights Agreement, within thirty (30) days hereof, the Company will seek registration under the Securities Act of all or part of the registrable shares of MTF, AFH Advisory and HIC. Within five (5) days hereof, the Company will provide written notice of such request to all other holders of registrable securities and will include in such registration all registrable shares with respect to which the Company has received written requests for inclusion within twenty-five (25) days after delivery of the Company’s notice. The Company has agreed to pay all registration expenses relating to up to two long-form registrations or short-form registrations for each of MTF, AFH Advisory and HIC.
| 4 |
Whenever the Company proposes to register any of its securities under the Securities Act (other than pursuant to a demand registration under the Registration Rights Agreement) and the registration form to be used may be used for the registration of any registrable shares, the Company will give prompt written notice to all holders of the registrable shares of its intention to effect such a registration and will include in such registration all registrable shares (in accordance with the priorities set forth in the Registration Rights Agreement) with respect to which the Company has received written requests for inclusion within fifteen (15) days after the delivery of the Company’s notice. Pursuant to Registration Rights Agreement, holders of registrable shares and the Company agree not to effect any public sale or distribution of equity securities of the Company, or any securities convertible into or exchangeable or exercisable for such securities, during the six (6) months following, the effective date of the Merger Agreement.
Restriction on Resale
None of the securities offered in the Transactions, other than the IPO, will be registered under the Securities Act and the certificates representing our securities will contain a legend restricting the distribution, resale, transfer, pledge, hypothecation or other disposition of the securities unless and until such securities are registered under the Securities Act or an opinion of counsel reasonably satisfactory to us is received that registration is not required under the Securities Act.
Current Ownership
The following table sets forth information with respect to the post-merger beneficial ownership of the Company’s common stock as of September 19, 2014, by each person or group of affiliated persons known to the Company to beneficially own 5% or more of its common stock, each director, each named executive officer, and all of its directors and named executive officers as a group.
| Name of Beneficial Owner or Identity of Group | Title of Class | Shares(1) | Percentage | |||||||
| 5% or greater stockholders: | ||||||||||
| The Musculoskeletal Transplant Foundation, Inc. 125 May Street Edison, NJ 08837 | Common Stock | 11,932,807 | 49.3 | % | ||||||
| AFH Holding & Advisory, LLC 10830 Massachusetts Ave., Penthouse Los Angeles, CA 90024 | Common Stock | 2,609,602 | 10.8 | % | ||||||
| Amir Heshmatpour 269 Beverly Drive, Ste. 1600 Beverly Hills, CA 90212 | Common Stock | 3,609,602 | (2) | 14.9 | % | |||||
| Dr. Kang Ting 115 North Doheny Drive Beverly Hills, CA 90211 | Common Stock | 2,000,000 | 8.3 | % | ||||||
| Orthofix Holdings Inc. 3451 Plana Parkway Lewisville, TX 75056 | Common Stock | 1,909,908 | 7.9 | % | ||||||
| 5 |
| Executive Officers and Directors: | ||||||||||
| Michael Schuler 175 May Street Edison, NJ 08837 | Common Stock | - | - | |||||||
| William J. Treat 175 May Street, Suite 400 Edison, NJ 08837 | Common Stock | 198,202 | 0.8 | % | ||||||
| Catherine Doll 175 May Street, Suite 400 Edison, NJ 08837 | Common Stock | 12,625 | 0.1 | % | ||||||
| Bruce Stroever 175 May Street, Suite 400 Edison, NJ 08837 | - | - | ||||||||
| Dr. Chia Soo 175 May Street, Suite 400 Edison, NJ 08837 | Common Stock | 1,119,318 | 4.6 | % | ||||||
| William Coffin 175 May Street, Suite 400 Edison, NJ 08837 | - | - | ||||||||
| John Booth 175 May Street, Suite 400 Edison, NJ 08837 | - | - | ||||||||
| Jimmy Delshad 175 May Street, Suite 400 Edison, NJ 08837 | - | - | ||||||||
| Steve Warnecke 1026 Anaconda Drive Castle Rock, CO 80108 | - | - | ||||||||
| Total Officers and Directors as a Group | Common Stock | 1,330,145 | 5.5 | % | ||||||
| Reserve for Future Issuance: | ||||||||||
| Option Plan | 2,444,696 | |||||||||
(1) The number of shares of Common Stock issued and outstanding that was used to calculate the percentage ownership of each listed person includes the shares of Common Stock underlying convertible debt, stock options and warrants that are exercisable 60 days after the close of the Merger.
(2) These shares of Common Stock are owned by AFH Holding & Advisory, LLC (“AFH Holding”), H&H (Hong Kong) Holdings Co., Limited (“H&H”) and Mr. Heshmatpour’s spouse and children. Mr. Heshmatpour is sole member of AFH Holding and has sole voting and investment control over the 2,609,602 shares of Common Stock owned of record by AFH Holding. Mr. Heshmatpour is the sole member of H&H and has sole voting and investment control over the 200,000 shares of Common Stock owned of record by H&H. Mr. Heshmatpour has voting and investment control over the 800,000 shares of Common Stock owned by his spouse and children. Accordingly, he may be deemed a beneficial owner with respect to these 3,609,602 shares of Common Stock.
| 6 |
Accounting Treatment; Change of Control
The Merger will be treated as a “reverse merger,” and although the Company is the legal acquirer, Bone Biologics is deemed to be the acquirer for accounting purposes in the reverse merger. Consequently, the assets and liabilities and the historical operations that will be reflected in the financial statements prior to the Merger will be those of Bone Biologics, and the consolidated financial statement after completion of the Merger will include the assets and liabilities of Bone Biologics, historical operations of Bone Biologics and operations of Bone Biologics from the Closing Date of the Merger.
Pursuant the Merger Agreement AFH Advisory and MTF have the right to appoint one individual with non-voting “observer status” to receive all board of director communications and attend all meetings of the board of directors for a period of 2 years from the Effective Time, including attending meetings of the board of directors related to engaging professional service providers and the right to review any credentials provided by such service providers.
Except as described in the previous paragraphs, no arrangements or understandings exist among present or former controlling stockholders with respect to the election of members of our Board of Directors and, to our knowledge, no other arrangements exist that might result in a change of control of the Company. Further, as a result of the issuance of the shares of Common Stock pursuant to the Merger, a change in control of the Company occurred as of the date of consummation of the Merger.
| 7 |
Immediately following the Merger, the business of Bone Biologics became our business. Bone Biologics was founded by University of California professors in collaboration with an Osaka University professor and a University of Southern California surgeon in 2004 as a privately-held company with proprietary, patented technology that has been clinically proven in non-human primate models to facilitate bone growth. Bone’s platform technology has application in delivering improved outcomes in the surgical specialties of spinal, orthopedic, general orthopedic, plastic reconstruction, neurosurgery, interventional radiology, and sports medicine. Lead product development and clinical studies are targeted on spinal fusion surgery, a growing market space. The following chart provides a segment overview of bone replacement products in the U.S.:
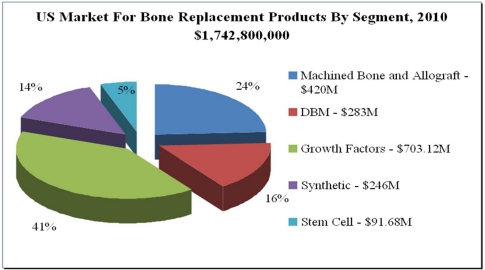
(See O’Reilly, Sharon. Beyond INFUSE: Spine Community Searches For Answers. INVIVO. November 2011, Vol. 29, No. 10.)
Products
Bone Biologics has developed stand-alone platform technologies through exhaustive lab and small animal research over the past seven years to generate the current applications across broad fields of use. The platform technologies are UCB-1TM, a proprietary skeletal specific growth factor in use with DBX™, a proprietary demineralized bone matrix, and UCS-1TM, a biomaterial that potentates and regulates growth factor in use with DBX, a proprietary demineralized bone matrix, activity in bone regeneration. Together, with DBX, or in isolation, Nell-1TM and UCS-1TM allow regulation over skeletal tissue formation and stem cell differentiation.
Bone Biologics is currently focused on bone regeneration in spinal fusion using its recombinant human protein, known as Nell-1. Nell-1 is an osteoinductive orthobiologic: a recombinant protein that provides control over bone regeneration. This patent protected technology has been exclusively licensed to Bone Biologics from the University of California, Los Angeles (“UCLA”). Leveraging the resources of investors and strategic partners, Bone Biologics has successfully surpassed two critical milestones:
| ● | Demonstrating a successful small laboratory scale pilot run for the recombinant manufacturing of the human Nell-1 protein in Chinese Hamster Ovary cells, which is a commercially established mammalian cell line for other recombinant proteins, which is well-defined and accepted by international regulatory agencies; and | |
| ● | Validation of protein dosing and efficacy in established large animal models and Rhesus Monkey primate models. |
| 8 |
Bone Biologics is targeting spinal fusion as the first clinical indication and is currently in the pre-investigational device exemption (“IDE”) phase. The lead product, purified Nell-1 recombinant protein, is expected to be dried onto ß-tricalcium phosphate (“TCP”) bone void filler to produce a medical device, known as Nell-1/TCP (“Nell/TCP Fusion Device”). This device will be mixed with 510(k) cleared DBX® Demineralized Bone Putty recommended for use in conjunction with a cleared intervertebral body fusion device. The Nell-1/TCP Fusion Device is intended for use in lumbar spinal fusion and may have a variety of other applications such as cervical spinal fusion.
While the product is initially targeted at the spine fusion market, Bone believes Nell-1’s unique set of characteristics, target specific mechanism of action, efficacy, safety, and affordability, position the product well for application in a variety of procedures, including:
Spine Implants. This is the largest market for bone substitute product, representing approximately 80% of the total 2009 U.S. market. While use of the patient’s own bone, also referred to as autograft, to enhance fusion of vertebral segments remains the gold standard for this type of treatment, complications associated with use of autograft bone including pain, increased surgical time and infection limit its use.
Non-Union Trauma Cases. While the majority of fractures heal without the need for osteosynthetic products, bone substitutes are used in complicated breaks where the bone does not mend naturally. Nell-1 is expected to perform as well as high-priced growth factors in this market.
Hip & Knee Revisions. The use of bone substitutes in reconstruction surgery is generally limited to revision cases where the products are used to account for the significant bone loss that accompanies these cases. The treatment of osteoporotic patients also represents a substantial opportunity for Nell-1 use in hip and knee reconstruction.
Implant Coating. The use of Nell-1 as a direct coating on hip and knee implants could have a very significant impact on the market. A Nell-1 coating may prolong the life of primary implants and allow for differentiation in a commodity market.
UCLA’s initial research was funded with approximately $18 million in resources from UCLA and government grants. After licensing the exclusive worldwide intellectual property rights from UCLA, development was funded with additional grant funding and $6.5 million in strategic investment from MTF. Bone anticipates that it will require an additional $2 million for preclinical studies, $4 million for completion of the filing of an IDE application completion, and $4 million for initiation of human studies. An estimated $50-60 million will be required to achieve product launch.
Nell-1’s powerful specific bone and cartilage forming properties derive from the ability of Nell-1 to only target cells that exhibit an activated “master switch” to develop into bone or cartilage. Nell-1 is a function specific recombinant human protein that has been proven in lab bench models to recapitulate normal human growth and development to provide control over bone and cartilage regeneration through research and development work at UCLA and written in publications.
The search for a target specific molecule to promote normal bone formation started in 1994. Nell-1 was isolated in 1996, and the first Nell-1 patent on bone regeneration was filed in 1999. Subsequent patents and continuations in part describing Nell-1 manufacturing, delivery, and cartilage regeneration were filed in 2002, 2003, 2006, 2007, 2008, and 2009 to further strengthen the patent portfolio.
The Nell-1/TCP Fusion Device will be comprised of a single dose vial of NELL-1 recombinant protein freeze dried onto TCP. A vial of Nell-1/TCP will be sold in a convenience kit with a diluent and a syringe of 510(k) cleared demineralized bone (“DBX® Putty”), produced by MTF. An elegant delivery device will allow the surgeon to mix the reconstituted Nell 1/TCP with the appropriate quantity of DBX® Putty just prior to implantation.
Research & Publications
Bone’s leading scientists have been published in notable scientific journals and publications in its field. These publications have served to highlight the work and achievements of its members.
| 9 |
Proposed Clinical Application
The Nell-1/TCP Fusion Device will be indicated for spinal fusion procedures in skeletally mature patients with degenerative disc disease (“DDD”) at one level from L4-S1. These DDD patients may also have up to Grade I spondylolisthesis at the involved level. The Nell-1/TCP Fusion Device is to be implanted via an anterior open or an anterior laparoscopic approach in conjunction with a cleared intervertebral body fusion device. Patients receiving the device should have had at least six months of non-operative treatment prior to treatment with the device. A cervical indication is currently under consideration. This indication for use would fill a current clinical gap, created by potentially dangerous inflammatory response caused by Infuse Bone Graft, the subject of a Public Health Notification from the United States Food and Drug Administration (the “FDA”) on July 1, 2008 about life threatening complications associated with rhBMP in cervical spine fusion. Bone would not expect to see the same adverse events with Nell-1/TCP as have been observed with BMP and OP-1. Bone has performed a rat femoral onlay model to compare proinflammatory response of BMP2 (Infuse Bone Graft) and Nell-1 within Helistate collagen sponges. While Nell-1 induced normal healing, BMP2 (Infuse Bone Graft) induced significant amounts of swelling and histological evidence of intense inflammatory response.
Description of the DBX® Putty to be used with Nell-1/TCP
The DBX® Demineralized Bone Putty provided in the convenience kit with Nell-1/TCP is a Class II device. The common name is “Bone Void Filler Containing Human Demineralized Bone Matrix.” The product is regulated under 21 C.F.R. §888.3045 Resorbable calcium salt bone void filler device, Product Codes MQV, GXP, and MBP. MTF is the manufacturer of the DBX® Putty. This product was cleared by the FDA under 510(k) number K053218 for spine indication in December 2006.
DBX® Putty is a matrix composed of processed human cortical bone. Demineralized bone granules are mixed with sodium hyaluronate to form the DBX® Putty. Every lot of final DBX® Putty product is tested in an athymic mouse model or in an alkaline phosphatase assay, which has been shown to have a positive correlation with the athymic mouse model, to ensure the osteoinductive potential of the final product.
The Bone Biologics instructions for use will recommend use of the Nell-1/TCP Fusion Device with a lumbar (or cervical) indication. The surgeon can therefore choose to use the intervertebral fusion device that he or she is most experienced with and in their judgment is the best option for successful treatment. Bone has three precedent products, which are also osteoinductive, with intended uses similar or the same as Nell-1, that have been cleared by the FDA as medical devices. These are as follow:
| ● | Infuse Bone Graft/LT-Cage Lumbar Tapered Fusion Device (PMA)-rhBMP-2 dissolved in water and applied to a collagen (bovine type I) sponge and placed in a cage; | |
| ● | GEM 21S (PMA)-rhPDGF-BB and -tricalcium phosphate (growth factor enhanced matrix); and | |
| ● | DBX® (510k)-human cortical bone (ground and demineralized) and mixed with sodium hyaluronate to form a putty. |
Based upon extensive discussions with regulatory experts and a specific communication from the FDA in response to a submission of our plan under the Exclusive License between UCLA and Bone Biologics we believe the Nell-1 TCP Fusion Device will be regulated as a Class III medical device and will therefore require submission and approval of a pre-market approval, (“PMA”). The FDA response to the submission of our plan is, “We have determined that the product is a combination product, that will be regulated under Device authorities, with CDRH (Center for Devices and Radiological Health) as the lead center.”
Bone’s Business Strategy
Bone’s business strategy has been to develop its target specific platform technology to meet a current established market with improvement in patient outcomes and reduction in costs to the healthcare delivery system. This narrowing of its focus from the research to the development stage is to allow for the approval for use of our target specific protein exhibiting efficacy and safety by matching or exceeding current market approved products. Identifying the best future strategic partners to facilitate the development through pre IDE, clinical, and ultimate commercialization is critical as Bone funds the pre-IDE work and continues achieving milestones. Bone believes that the licensing of the distribution of the Nell-1 product in the fields of use focused upon will generate sufficient funding to provide for the ongoing development of the Platform Technology across other surgical and therapeutic fields.
| 10 |
Material Agreements
UCLA Exclusive License Agreement
On March 15, 2006, Bone entered into an exclusive license agreement (the “Regents’ License”) with the Regents of the University of California (the “Regents”). The Regents’ License provides Bone with an exclusive license to several of the Regents’ patents covering, among other things, enhanced Nell-1 bone mineralization. The grant of the Regents’ License is subject to any license obligations to the U.S. government, and the term of the license lasts until the last-to-expire Regent patent licensed under the agreement expires. Under the Regents’ License, Bone is permitted to make, have made, use, sell, offer for sale and import any products covered by the Regents’ licensed patents in a certain field of use. By a subsequent Seventh Amendment entered into on August 7, 2012, the parties modified the applicable field of use that Bone is permitted to use the Regents’ patents in, which generally comprises musculoskeletal repair and regeneration, plus some related methods of manufacture. Bone has agreed to pay an annual maintenance fee to the Regents of $10,000 as well as to pay certain royalties to the Regents under the Regents’ License at the rate of 3% of net sales of licensed products. Bone must pay the royalties to the Regents on a quarterly basis, and Bone also must pay a minimum annual royalty of $25,000 to the Regents once earned royalties commence. If Bone is required to pay any third party any royalties as a result of Bone making use of the Regents’ patents, then Bone may reduce the royalty owed to the Regents by 0.333% for every percentage point paid to a third party. If Bone grants sublicensing rights to a third party to use the Regent’s patent, then Bone shall pay to the Regents 8% to 10% of the sublicensing income Bone receives from such sublicense.
By a subsequent Eighth Amendment entered into on October 22, 2013, the parties agreed that Bone is obligated to pay a milestone fee of 2% of the amount raised from this Private Placement. Additionally, if the Private Placement does not close or is less than $2.5 million, then a fee of $100,000 will be due and paid to the Regents by June 1, 2014. Furthermore, the Agreement was modified in that Bone shall pay the Regents $25,000 for dosing of Phase 1 clinical trial and $50,000 for dosing of Phase 3 clinical trial.
Bone is obligated to diligently proceed with developing and commercializing licensed products under the Regents’ patents set forth in the Regents’ License. The Regents have the right to either terminate the license or reduce the license to a non-exclusive license if Bone does not meet certain diligence milestone deadlines set forth in the Regents’ License.
Under a Fourth Amendment to the Regents’ License, entered into on August 19, 2009, Bone must reimburse or pre-pay the Regents for patent prosecution and maintenance costs incurred during the term of the Regents’ License. Bone has the right to bring infringement actions against third party infringers of the Regents’ License, the Regents may join voluntarily, at its own expense, or, at Bone’s expenses, be joined involuntarily to the action. Bone is required to indemnify the Regents against any third party claims arising out of Bone’s exercise of the rights under the Regents’ License or any sublicense.
Milestone Side Letter Agreement
Pursuant to a letter agreement, dated September 7, 2014, by and among AFH Advisory, Bone Biologics, and MTF (the “Milestone Side Letter Agreement”), Bone Biologics has agreed to use its commercially reasonable efforts to achieve the following milestones (the “Milestone Targets”) by the specified times following the closing of the Private Placement:
| (i) | Complete media screening studies of cell line within two (2) to three (3) months: | |
| (ii) | Initiate manufacturing of master cell bank within three (3) to four (4) months; | |
| (iii) | Initiate formulation studies for the cGMP manufacturing process once sufficient Nell-1 material is available within approximately eight (8) to ten (10) months; |
| 11 |
| (iv) | Initiate a pre-clinical bioreactor production run for toxicology material within nine (9) to twelve (12) months; | |
| (v) | Initiate pre-clinical toxicology studies to include carcinogenicity and reproductive within approximately eleven (11) to thirteen (13) months; | |
| (vi) | Finalize refinement of the manufacturing process within approximately twelve (12) to fourteen (14) months; | |
| (vii) | Initiate cGMP bioreactor run within twelve (12) to fourteen (14) months or after completion of (v), and | |
| (viii) | Request an IDE meeting to review the clinical safety plan within eighteen (18) to twenty (20) months; |
AFH Advisory and MTF will each receive restricted shares pursuant to the Milestone Targets equal to and not to exceed 2.5% of the fully diluted shares of the Company at the time of the completion of all Milestone Targets.
Placement Agent Agreement
On December 12, 2013, the Company and Bone Biologics entered into an engagement letter, which engagement letter was amended on September 22, 2014, with Forefront Capital Markets, LLC (“Forefront”) a registered FINRA broker-dealer, to act as placement agent for the Private Placement and the PIPE. Forefront shall be entitled to receive (i) a cash fee of 8% of the gross proceeds of the Private Placement, (ii) a warrant to purchase shares of the Company’s common stock (the “Agent Warrant”) equal to 8.0% of the Company’s common stock underlying the securities issued in the Private Placement, (iii) a cash fee of 3% of the gross proceeds received by the Company from any financing of non-convertible debt securities, and (iv) a warrant to purchase shares of the Company’s common stock (the “Advisory Warrant”) equal to 2.0% of the Company’s post-merger and financing fully diluted shares outstanding upon the closing of $2.5 million of investors on which Forefront is eligible to receive compensation. Forefront shall only be entitled to receive a management fee of 4% and a 4% Agent Warrant on the gross proceeds received from the sale of securities to investors introduced to the Company by AFH Advisory, Bone Biologics or their respective officers and directors at closing. The Agent Warrant will be issued at each closing and shall provide, among other things, that the Agent Warrant shall: (i) be exercisable at the price of the securities (or the exercise price of the securities) issued to the investors in the offering, (ii) expire five (5) years from the date of issuance, (iii) include customary registration rights, including the registration rights provided to the Investors, (iv) contain provisions for cashless exercise and (v) include such other terms that are normal and customary for warrants of this type. Forefront will serve as the Company’s exclusive placement agent in connection with the Private Placement through December 31, 2014, which exclusive period may be extended to 12 months at the discretion of the Company.
MTF Credit Agreement & Promissory Note
Bone and MTF entered into a loan agreement in 2008 and a credit agreement in 2009 (collectively, the “MTF Credit Agreements”), and accompanying promissory and convertible promissory notes in January 2008, November 2008, March 2009 and August 2009 to fund the development of Bone. On March 31, 2014, Bone and MTF entered into the Tenth Amendment to the MTF Credit Agreements and accompanying promisssory notes wherein MTF and Bone agreed that the aggregate principal amount of all advances would remain the same, but the maturity date of the notes would be extended to March 31, 2015. As of September 19, 2014, $5,192,684 in principal and interest was outstanding under the MTF Credit Agreements and $117,302 in principal and interst was outstanding under the 2013 Bridge Notes. On September 19, 2014, $1,533,356 of the amounts due under the MTF Credit Agreements were converted to shares of the Company. The remaining amounts due under the MTF Credit Agreements were cancelled and, as described in this Currert Report 8K under Recent Sales of Unregistered Securities, the New MTF Convertible Note (as defined herein) was issued by the Company.
| 12 |
In 2013, Bone and MTF also entered into a bridge note in the principal amount of $100,000. On June 6, 2014, the maturity date of the 2013 Bridge Note was extended to October 14, 2014.
MTF Short Term 2014 Loan
On September 15, 2014, Bone and MTF entered into a loan agreement and accompanying promissory note (the “MTF Short Term 2014 Loan”) to fund the continued operations of Bone prior to the Merger. Pursuant to the MTF Short Term 2014 Loan, MTF has agreed to advance an initial $250,000 to Bone and, at Bone’s request and subject to the terms and conditions of the MTF Short Term 2014 Loan, to advance up to an additional $250,000 to Bone. The MTF Short Term 2014 Loan has an interest rate of eight and one-half percent (8.5%) accruing annually. The MTF Short Term 2014 Loan matures on the earlier to occur of (i) the date on which at least $1 million is loaned to or invested in the Company and (ii) December 31, 2014. In further consideration of the MTF 2014 Loan, Bone granted to MTF 625,000 warrants at a strike price of $1.62. The MTF 2014 Loan was assigned to the Company on September 19, 2014.
Competition
Our most significant competitor is Infuse™ Bone Graft or BMP-2 (bone morphogenic protein 2) from Medtronics. BMP-2, despite dominant market position, is suffering from bad press related to negative off label cervical fusion outcomes due to inflammatory response. Bone believes that BMP2 also suffers from disadvantageous margins due to an unfavorable revenue sharing agreement with Wyeth. We believe that our product will not suffer from these same negative factors as to date, our products have not had inflammatory response issues and we are not burdened by an unfavorable revenue sharing agreement. A second potential competitor was OP-1 or BMP-7 from Stryker and sold to Olympus, which has had significant regulatory setbacks long delaying time to market beyond humanitarian use.
Customers
The customers for the product being developed by Bone are the acute care hospitals performing spinal fusion and long bone non-union fracture repair and regeneration. This universe of customers has been identified by Medtronic, with their bone growth product Infuse Bone Graft which is a bone morphogenic protein, and has grown over the past 10 years to a greater than $800 million market share dollar volume. FDA approval pathways, reimbursement pathways, and procedure acceptance by surgeons has been established by the Medtronic product. This does not provide any assurance that the Company will be approved by the FDA on the same pathway, reimbursed by payors comparably, and accepted by hospitals and surgeons as an alternative to Medtronic or any of the less efficacious modalities of therapy. Medrontic has experienced difficulties in this market from FDA questions relative to off label use, payors on reimbursement rates, and hospitals on procedural cost which create an environment that could be unfavorable to the Company achieving current forecasts for approval, commercialization, and revenue.
Intellectual Property
Bone Biologics has an intellectual property portfolio that includes exclusive, worldwide licenses from UCLA which Bone believes constitutes a formidable barrier to entry.
| 13 |
Additional patent applications are currently in preparation. The intellectual property is unique and comprehensively covers Nell-1 manufacture, Nell-1 compositions and Nell-1 use in wide ranging clinical and diagnostic applications. Bone Biologics protects its proprietary technology through all mechanisms including U.S. and foreign patent filings, trade secret protections, and collaboration agreements with domestic and international corporations, universities and research institutions. Bone is the exclusive licensee for the following twelve (12) UCLA issued patents:
| U.S Patent No. | Summary | Date Issued | ||
| 7052856 | NELL-1 Enhanced Bone Mineralization | 5/20/2006 | ||
| 7544486 | NELL-1 Peptide Expression Systems | 6/9/2009 | ||
| 7687462 | Composition for promoting Cartilage | 3/30/2010 | ||
| 7691607 | Expression system of NELL peptide | 4/6/2010 | ||
| 7776361 | NELL-1 Enhanced Bone Mineralization | 8/17/2010 | ||
| 7807787 | NELL-1 Peptide | 10/5/2010 | ||
| 7833968 | Pharmaceutical compositions for treating or preventing bone conditions | 11/16/2010 | ||
| 7844066 | Nell-1 Enhanced Bone Minerilization | 2/8/2011 | ||
| 8044026 | Composition for promoting cartilage | 10/25/2011 | ||
| 8048646 | NELL-1 peptide expression systems | 11/1/2011 | ||
| 8053412 | NELL-1 Peptides | 11/8/2011 | ||
| 8207120 | Nell-1 Enhanced Bone Mineralization | 6/26/2012 |
Government Regulation
The manufacturing and marketing of any product which Bone may formulate with its technologies as well as its related research and development activities are subject to regulation for safety, efficacy and quality by governmental authorities in the U.S. and other countries. Bone anticipates that these regulations will apply separately to each biotechnology product. Bone believes that complying with these regulations will involve a considerable level of time, expense and uncertainty.
In the U.S., drugs are subject to rigorous federal regulation and, to a lesser extent, state regulation. The Federal Food, Drug and Cosmetic Act, as amended, and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the testing, manufacture, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of Bone’s products. Drug development and approval within this regulatory framework is difficult to predict, requires a number of years and involves the expenditure of substantial resources. Moreover, ongoing legislation by U.S. Congress and rule making by the FDA presents an ever-changing landscape where Bone could be required to undertake additional activities before any governmental approval is granted allowing us to market Bone’s products. The steps required before a pharmaceutical agent may be marketed in the U.S. include:
| ● | Laboratory and non-clinical tests for safety and small scale manufacturing of the agent; | |
| ● | The submission to the FDA of an IDE which must become effective before human clinical trials can commence; | |
| ● | Clinical trials to characterize the efficacy and safety of the product in the intended patient population; | |
| ● | The submission of a New Drug Application (“NDA”) or PMA to the FDA; and | |
| ● | FDA approval of the NDA or PMA prior to any commercial sale or shipment of the product. |
In addition to obtaining FDA approval for each product, each manufacturing establishment must be registered with, and approved by, the FDA. Moreover, manufacturing establishments are subject to biennial inspections by the FDA and must comply with the FDA’s Good Manufacturing Practices for products, drugs and devices.
| 14 |
Non-clinical Trials
Non-clinical testing includes laboratory evaluation of chemistry and formulation as well as tissue culture and animal studies to assess the safety and potential efficacy of the product. Non-clinical safety tests must be conducted by laboratories that comply with FDA regulations regarding good laboratory practices. Non-clinical testing is inherently risky and the results can be unpredictable or difficult to interpret. The results of non-clinical testing are submitted to the FDA as part of an IDE and are reviewed by the FDA prior to the commencement of clinical trials. Unless the FDA objects to an IDE, clinical studies may begin 30 days after the IDE is submitted. Bone has relied and intends to continue to rely on third-party contractors to perform non-clinical trials.
Clinical Trials
Clinical trials involve the administration of the investigational product to healthy volunteers or to patients under the supervision of a qualified investigator. Clinical trials must be conducted in accordance with good clinical practices under protocols that detail the objectives of the study, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Each protocol must be submitted to the FDA prior to its conduct. Further, each clinical study must be conducted under the auspices of an independent institutional review board. The institutional review board will consider, among other things, ethical factors, the safety of human subjects and the possible liability of the institution. The drug product used in clinical trials must be manufactured according to the FDA’s Good Manufacturing Practices.
Clinical trials under IDE regulations are typically conducted in two sequential trials. In the Pilot trial, the initial introduction of the product into healthy human subjects, the drug is tested for safety (adverse side effects), absorption, metabolism, bio-distribution, excretion, food and drug interactions, abuse as well as limited measures of pharmacologic effect and proof of principle that involves studies in a limited patient population in order to:
| ● | assess the potential efficacy of the product for specific, targeted indications; | |
| ● | demonstrate efficacy in a limited patient population; | |
| ● | identify the range of doses likely to be effective for the indication; and | |
| ● | identify possible adverse events and safety risks. |
When there is evidence that the product may be effective and has an acceptable safety profile in Pilot evaluations, Pivotal trials are undertaken to establish and confirm the clinical efficacy and establish the safety profile of the product within a larger population at geographically dispersed clinical study sites. Pivotal trials frequently involve randomized controlled trials and, whenever possible, studies are conducted in a manner so that neither the patient nor the investigator knows what treatment is being administered. Bone, or the FDA, may suspend clinical trials at any time if it is believed that the individuals participating in such trials are being exposed to unacceptable health risks. Bone intends to rely upon third-party contractors to advise and assist us in the preparation of Bone’s IDEs and the conduct of clinical trials that will be conducted under the IDEs.
Premarket Approval and FDA Approval Process
The results of the manufacturing process, development work, non-clinical studies and clinical studies are submitted to the FDA in the form of a PMA prior to marketing and selling the product. The testing and approval process is likely to require substantial time and effort. In addition to the results of non-clinical and clinical testing, the PMA applicant must submit detailed information about chemistry, manufacturing and controls that will describe how the product is made and tested through the manufacturing process.
The PMA review process involves FDA investigation into the details of the manufacturing process, as well as the design and analysis of each of the non-clinical and clinical studies. This review includes inspection of the manufacturing facility, the data recording process for the clinical studies, the record keeping at a sample of clinical trial sites and a thorough review of the data collected and analyzed for each non-clinical and clinical study. Through this investigation, the FDA reaches a decision about the risk-benefit profile of a product candidate. If the benefit is worth the risk, the FDA begins negotiating with the company about the content of an acceptable package insert and associated Risk Evaluation and Mitigation Strategies (“REMS”), if required.
| 15 |
The approval process is affected by a number of factors, including the severity of the disease, the availability of alternative treatments and the risks and benefits demonstrated in clinical trials. Consequently, there is a risk that approval may not be granted on a timely basis, if at all. The FDA may deny a PMA if applicable regulatory criteria are not satisfied, require additional testing or information or require post-marketing testing (Phase 4) and surveillance to monitor the safety of a company’s product if it does not believe the PMA contains adequate evidence of the safety and efficacy of the product. Moreover, if regulatory approval of a product is granted, such approval may entail limitations on the indicated uses for which it may be marketed. Finally, product approvals may be withdrawn if compliance with regulatory standards is not maintained or health problems are identified that would alter the risk-benefit analysis for the product. Post-approval studies may be conducted to explore the use of the product for new indications or populations such as pediatrics.
Among the conditions for PMA approval is the requirement that any prospective manufacturer’s quality control and manufacturing procedures conform to the FDA’s Good Manufacturing Practices and the specifications approved in the PMA. In complying with standards set forth in these regulations, manufacturers must continue to expend time, money and effort in the area of product and quality control to ensure full technical compliance. Manufacturing establishments, both foreign and domestic, also are subject to inspections by or under the authority of the FDA and by other federal, state or local agencies. Additionally, in the event of non-compliance, FDA may issue warning letters and/or seek criminal and civil penalties, enjoin manufacture, seize product or revoke approval.
International Approval
Whether or not FDA approval has been obtained, approval of a product by regulatory authorities in foreign countries must be obtained prior to the commencement of commercial sales of the drug in such countries. The requirements governing the conduct of clinical trials and drug approvals vary widely from country to country, and the time required for approval may be longer or shorter than that required for FDA approval. Although there are some procedures for unified filings for certain European countries, in general, each country at this time has its own procedures and requirements.
Other Regulation
In addition to regulations enforced by the FDA, Bone is also subject to U.S. regulation under the Controlled Substances Act, the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential future federal, state, local or similar foreign regulations. Bone’s research and development may involve the controlled use of hazardous materials, chemicals and radioactive compounds. Although Bone believes that its safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of any accident, Bone could be held liable for any damages that result and any such liability could exceed Bone’s resources.
Employees
As of the date hereof, Bone has one part-time employee working for Bone as Bone’s President and Chief Technology Officer (“CTO”).
Strategic Partners
Musculoskeletal Transplant Foundation
Bone Biologics has formed a formal strategic alliance with MTF on the collaborative development of osteoinductive products that incorporate MTF’s current product line of natural bone graft substitutes with Nell-1™. MTF is the exclusive allograft supplier for the BIOBONE-X™. MTF has become one of the major investors of Bone Biologics. MTF is the world’s largest allograft bone supplier. It is also the country’s largest full service tissue organization dedicated to providing quality tissue through a commitment to excellence in education, research, recovery and care for recipients, donors and their families. A not-for-profit organization, MTF is a consortium of academic medical institutions and organ and tissue recovery organizations across the country. Bone anticipates that MTF, with its proven ISO 9001 manufacturing and packaging of FDA approved osteogenic carriers, will significantly accelerate the clinical development cycle of Nell-1TM related products.
| 16 |
Katayama Chemical Industries Co., Ltd
Katayama Chemical Industries Co., Ltd (“KCI”), based in Osaka, Japan, was founded in 1918. KCI focuses on the production of OEM laboratory products for many distributors such as Amersham Biosciences, Millipore, and Sigma-Aldrich Japan, the exclusive Japanese distributor for laboratory products manufactured by KCI. Under a strategic partnership with Bone Biologics, KCI is seeking to develop clinical diagnostic reagents related to bone metabolism and regeneration. KCI produced the Nell-1 protein in an insect cell line that was utilized in development work and for proof of concept validation in rodent models and large animal (sheep) spinal fusion trials.
Available Information
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). We are not required to deliver an annual report to our security holders, but will provide one voluntarily if a written request is sent to us at our principal executive office at 175 May Street, Suite 400, Edison, New Jersey. Reports filed with the SEC pursuant to the Exchange Act, including our annual and quarterly reports, and other reports we file, can be inspected and copied on official business days during the hours of 10 a.m. to 3 p.m. prevailing eastern time at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. Investors may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. Investors can request copies of these documents upon payment of a duplicating fee by writing to the SEC. The reports we file with the SEC are also available on the SEC’s website (http://www.sec.gov).
| 17 |
RISK FACTORS AND SPECIAL CONSIDERATIONS
This Report contains forward-looking statements.
Information provided in this Current Report may contain forward-looking statements which reflect management’s current view with respect to future events, the viability or efficacy of our products and our future performance. Such forward-looking statements may include projections with respect to market size and acceptance, revenues and earnings, marketing and sales strategies and business operations, as well as efficacy of our products.
We operate in a highly competitive and highly regulated business environment. Our business can be expected to be affected by government regulation, economic, political and social conditions, business’ response to new and existing products and services, technological developments and the ability to obtain and maintain patent and/or other intellectual property protection for our products and intellectual property. Our actual results could differ materially from management’s expectations because of changes both within and outside of our control. Due to such uncertainties and the risk factors set forth in this Current Report, prospective investors are cautioned not to place undue reliance upon such forward-looking statements.
Risks Related to Our Business
Our ability to grow and compete in the future will be adversely affected if adequate capital is not available to us or not available on terms favorable to us.
The ability of our business to grow and compete depends on the availability of adequate capital. Bone currently has no cash flow. We cannot assure you that we will be able to obtain equity or debt financing on acceptable terms or at all to implement our growth strategy. As a result, we cannot assure you that adequate capital will be available to finance our current growth plans, take advantage of business opportunities or respond to competitive pressures, any of which could harm our business.
Our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern.
Our recurring operating losses raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firms included an explanatory paragraph in their reports on our financial statements as and for the years ended December 31, 2013 and December 31, 2012 with respect to this uncertainty. The perception of our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could result in the loss of confidence by investors, suppliers and employees.
We have incurred losses for calendar year 2013 and we expect our operating expenses to increase in the foreseeable future, which may make it more difficult for us to achieve and maintain profitability.
We have no significant operating history and have never been profitable. From our inception on March 9, 2004 through June 30, 2014, we have generated a net loss of approximately $8.4 million. We have negative cash flow from operations, working capital deficiencies and have not established the commercial viability of our products. These conditions raise doubts as to the Company’s ability to continue as a going concern. The Company’s December 31, 2013 audited financial statements contained a notation by our auditors regarding the Company’s ability to continue as a going concern. Although we intend to raise additional capital or financing, we will continue to incur significant expenses for development activities for our lead product Nell-1. In addition, as a public company, we will incur additional accounting, legal and other expenses that we did not incur as a private company. These expenditures will make it harder for us to achieve profitability. As a result, we can provide no assurance as to whether or if we will ever be profitability. If we are not able to achieve and maintain profitability, the value of our company and our common stock could decline significantly.
There may be conflicts of interest between our management and our non-management stockholders and other affiliates.
Conflicts of interest create the risk that management may have an incentive to act adversely to the interests of the Company. A conflict of interest may arise between our management’s personal pecuniary interest and its fiduciary duty to our stockholders.
| 18 |
We face a number of risks associated with the completed Reverse Merger, including our incurrence of substantial debt which could adversely affect our financial condition.
On September 19, 2014, we completed the Merger. Completing the Merger increased our expenses, and we incurred substantial debt in completing the business combination, which could adversely affect our financial condition. In connection with the Merger, we incurred debt that includes, but is not limited to, a promissory note issued by the Company to AFH Advisory pursuant to which the Company agrees to pay AFH Advisory the amount of $340,000 out of the proceeds of the Private Placement for the remainder of the $590,000 owed to AFH Advisory in connection with the Merger. MTF has granted AFH Advisory a standby letter of credit in the amount of $340,000 for the remaining amount due under the Note. Principal and interest under the Note is due October 24, 2014.
Incurring a substantial amount of debt may require us to use a significant portion of any cash flow to pay principal and interest on the debt, which will reduce the amount available to fund working capital, capital expenditures, and other general purposes. Our indebtedness may negatively impact our ability to operate our business and limit our ability to borrow additional funds by increasing our borrowing costs, and impact the terms, conditions, and restrictions contained in possible future debt agreements, including the addition of more restrictive covenants; impact our flexibility in planning for and reacting to changes in our business as covenants and restrictions contained in possible future debt arrangements may require that we meet certain financial tests and place restrictions on the incurrence of additional indebtedness and place us at a disadvantage compared to similar companies in our industry that have less debt.
The business combination was completed through a “reverse merger.” As a result, we may not be able to attract the attention of major brokerage firms.
Securities analysts of major brokerage firms may not provide coverage of our Company since there is no incentive to brokerage firms to recommend the purchase of our Common Stock. No assurance can be given that brokerage firms will want to conduct any secondary offerings on behalf of our post-merger company.
We operate in a highly competitive environment.
The biotechnology industry is characterized by rapidly evolving technology and intense competition. Our competitors include major multi-national biotechnology companies developing both generic and proprietary therapies to treat serious diseases. Many of these companies are well-established and possess technical, human, research and development, financial and sales and marketing resources significantly greater than ours. In addition, many of our potential competitors have formed strategic collaborations, partnerships and other types of joint ventures with larger, well established industry competitors that afford these companies potential research and development and commercialization advantages in the therapeutic areas we are currently pursuing.
Academic research centers, governmental agencies and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those being developed by us. In addition, many of these competitors may be able to obtain patent protection, obtain FDA and other regulatory approvals, and begin commercial sales of their products before us.
Our limited operating history makes it difficult to evaluate our current business and future prospects.
We have a limited operating history, and there is a risk that we will be unable to continue as a going concern. We have minimal assets and no significant financial resources. Our limited operating history makes it difficult to evaluate our current business model and future prospects. Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development. Potential investors should carefully consider the risks and uncertainties that a new company with no operating history will face. In particular, potential investors should consider that there is a significant risk that we will not be able to:
| ● | implement or execute our current business plan, which may or may not be sound; | |
| ● | maintain our anticipated management and advisory team; and | |
| ● | raise sufficient funds in the capital markets to effectuate our business plan. |
| 19 |
If we cannot execute any one of the foregoing or similar matters relating to our business, the business may fail, in which case you would lose the entire amount of your investment in the Company.
Our future success is dependent, in part, on the performance and continued service of our officers and directors.
We are presently dependent to a great extent upon the experience, abilities and continued services of William Jay Treat, our President and Chief Technology Officer. The loss of services of Mr. Treat could have a material adverse effect on our business, financial condition or results of operation.
We rely upon consulting agreements with third parties to provide the services of our interim chief executive officer and interim chief financial officer.
We have entered into consulting agreements with MTF and The Gilson Group, LLC (the “Gilson Group”) to provide us with the services of Michael Schuler as our interim chief executive officer and Catherine Doll as our interim chief financial officer. If we are unable to continue to obtain the services of Mr. Schuler or Ms. Doll, from MTF and Gilson Group respectively, the loss of their services could have a material adverse effect on our business, financial condition and our operational results.
Acceptance of our formulations or products in the marketplace is uncertain and failure to achieve market acceptance will prevent or delay our ability to generate revenues.
Our future financial performance will depend, at least in part, upon the introduction and customer acceptance of our products. Even if approved for marketing by the necessary regulatory authorities, our formulations or products may not achieve market acceptance. The degree of market acceptance will depend upon a number of factors, including:
| ● | receipt of regulatory clearance of marketing claims for the uses that we are developing; | |
| ● | establishment and demonstration of the advantages, safety and efficacy of our formulations, products and technologies; | |
| ● | pricing and reimbursement policies of government and third-party payers such as insurance companies, health maintenance organizations and other health plan administrators; | |
| ● | Our ability to attract corporate partners, including pharmaceutical companies, to assist in commercializing our proposed products; and | |
| ● | Our ability to market our products. |
Physicians, patients, payers or the medical community in general may be unwilling to accept, utilize or recommend any of our proposed formulations or products. If we are unable to obtain regulatory approval, commercialize and market our proposed formulations or products when planned, we may not achieve any market acceptance or generate revenue.
| 20 |
Our long term capital requirements are subject to numerous risks.
We believe we will need an additional $8 million to $10 million to complete all regulatory requirements and complete the Pilot clinical trials in human patients which is required prior marketing or selling our products. We anticipate we will need to raise substantial additional funds for the Pivotal clinical trial prior to marketing our first product. Our long term capital requirements are expected to depend on many factors, including, among others:
| ● | the number of potential formulations, products and technologies in development; | |
| ● | continued progress and cost of our research and development programs; | |
| ● | progress with pre-clinical studies and clinical trials; | |
| ● | time and costs involved in obtaining regulatory (including FDA) clearance; | |
| ● | costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims; | |
| ● | costs of developing sales, marketing and distribution channels and our ability to sell our formulations or products; | |
| ● | costs involved in establishing manufacturing capabilities for commercial quantities of our products; | |
| ● | competing technological and market developments; | |
| ● | market acceptance of our drug formulations or products; | |
| ● | costs for recruiting and retaining employees and consultants; | |
| ● | costs for training physicians; and | |
| ● | legal, accounting and other professional costs. |
We may consume available resources more rapidly than currently anticipated, resulting in the need for additional funding. We may seek to raise any necessary additional funds through equity or debt financings, collaborative arrangements with corporate partners or other sources, which may be dilutive to existing stockholders or otherwise have a material effect on our current or future business prospects. If adequate funds are not available, we may be required to significantly reduce or refocus our development and commercialization efforts with regard to our delivery technologies and our proposed formulations and products.
Competitors could develop and/or gain FDA approval of our products for a different indication.
We cannot provide any assurances that any other company won’t obtain FDA approval for similar products that might adversely affect our ability to develop and market these products in the U.S. We are aware that other companies have intellectual property protection and have conducted clinical trials. Many of these companies may have more resources than us. We cannot provide any assurances that our products will be FDA-approved prior to our competitors.
The FDA does not regulate the practice of medicine and, as a result, cannot direct physicians to select certain products for their patients. Consequently, we might be limited in our ability to prevent off-label use of a competitor’s product to treat the diseases we intend to commercialize, even if we have issued method of use patents for that indication. If we are not able to obtain and enforce our patents, a competitor could develop and commercialize similar products for the same indications that we are pursuing. We cannot provide any assurances that a competitor will not obtain FDA approval for a product that contains the same active ingredients as our products.
| 21 |
We rely on method patents and patent applications and various regulatory exclusivities to protect some of our product candidates, and our ability to compete may be limited or eliminated if we are not able to protect our products.
The patent positions of biotechnology companies are uncertain and involve complex legal and factual questions. We may incur significant expenses in protecting our intellectual property and defending or assessing claims with respect to intellectual property owned by others. Any patent or other infringement litigation by or against us could cause us to incur significant expense and divert the attention of our management.
Others may file patent applications or obtain patents on similar technologies or compounds that compete with our products. We cannot predict how broad the claims in any such patents or applications will be and whether they will be allowed. Once claims have been issued, we cannot predict how they will be construed or enforced. We may infringe upon intellectual property rights of others without being aware of it. If another party claims we are infringing their technology, we could have to defend an expensive and time consuming lawsuit, pay a large sum if we are found to be infringing, or be prohibited from selling or licensing our products unless we obtain a license or redesign our product, which may not be possible.
We also rely on trade secrets and proprietary know-how to develop and maintain our competitive position. Some of our current or former employees, consultants, scientific advisors, current or prospective corporate collaborators, may unintentionally or willfully disclose our confidential information to competitors or use our proprietary technology for their own benefit. Furthermore, enforcing a claim alleging the infringement of our trade secrets would be expensive and difficult to prove, making the outcome uncertain. Our competitors may also independently develop similar knowledge, methods, and know-how or gain access to our proprietary information through some other means.
We may fail to retain or recruit necessary personnel, and we may be unable to secure the services of consultants.
As of the date of this Current Report, we have one part-time employee. We also engaged regulatory consultants to advise us on our dealings with the FDA and other foreign regulatory authorities and have been and will be required to retain additional consultants and employees. Our future performance will depend in part on our ability to successfully integrate newly hired officers into our management team and our ability to develop an effective working relationship among senior management.
Certain of our directors, officers, scientific advisors, and consultants serve as officers, directors, scientific advisors, or consultants of other biopharmaceutical or biotechnology companies or institutes that might be developing competitive products. Other than corporate opportunities, none of our directors are obligated under any agreement or understanding with us to make any additional products or technologies available to us. Similarly, we can give no assurances, and we do not expect and stockholders should not expect, that any biomedical or pharmaceutical product or technology identified by any of our directors or affiliates in the future would be made available to us other than corporate opportunities. We can give no assurances that any such other companies will not have interests that are in conflict with its interests.
Losing key personnel or failing to recruit necessary additional personnel would impede our ability to attain our development objectives. There is intense competition for qualified personnel in the drug-development field, and we may not be able to attract and retain the qualified personnel we need to develop our business.
We rely on independent organizations, advisors and consultants to perform certain services for us, including handling substantially all aspects of regulatory approval, clinical management, manufacturing, marketing, and sales. We expect that this will continue to be the case. Such services may not always be available to us on a timely basis.
We rely on third parties to supply our raw materials, and if certain manufacturing-related services do not timely supply these products and services, it may delay or impair our ability to develop, manufacture and market our products.
We rely on suppliers for raw materials and other third parties for certain manufacturing-related services to produce material that meets appropriate content, quality and stability standards and to use in clinical trials of its products. To succeed, clinical trials require adequate supplies of drug substance and drug product, which may be difficult or uneconomical to procure or manufacture. We and our suppliers and vendors may not be able to (i) produce our drug substance or drug product to appropriate standards for use in clinical studies, (ii) perform under any definitive manufacturing, supply or service agreements or (iii) remain in business for a sufficient time to successfully produce and market our product candidates. If we do not maintain important manufacturing and service relationships, we may fail to find a replacement supplier or required vendor or develop our own manufacturing capabilities which could delay or impair our ability to obtain regulatory approval for our products and substantially increase our costs or deplete profit margins, if any. If we do find replacement providers, we may not be able to enter into agreements with suppliers on favorable terms and conditions, or there could be a substantial delay before a new third party could be qualified and registered with the FDA and foreign regulatory authorities as a provider.
| 22 |
Clinical trials are very expensive, time-consuming, and difficult to implement.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The clinical trial process is also time-consuming. We estimate that clinical trials of our product candidates would take at least several years to complete. Furthermore, failure can occur at any stage of the trials, and we could encounter problems that cause us to abandon or repeat clinical trials. Commencement and completion of clinical trials may be delayed by several factors, including:
| ● | obtaining an IDE approval with the FDA to commence clinical trials; | |
| ● | identification of, and acceptable arrangements with, one or more clinical sites; | |
| ● | obtaining Institutional Review Board (“IRB”) approval to commence clinical trials; | |
| ● | unforeseen safety issues; | |
| ● | determination of dosing; | |
| ● | lack of effectiveness during clinical trials; | |
| ● | slower than expected rates of patient recruitment; | |
| ● | inability to monitor patients adequately during or after treatment; | |
| ● | inability or unwillingness of medical investigators to follow clinical protocols; and | |
| ● | unwillingness of the FDA or IRBs to permit the clinical trials to be initiated. |
In addition, we, IRBs or the FDA may suspend clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if IRBs or the FDA finds deficiencies in our submissions or the conduct of our trials.
The results of our clinical trials may not support our product candidate claims and the results of preclinical studies and completed clinical trials are not necessarily predictive of future results.
To date, long-term safety and efficacy have not yet been demonstrated in clinical trials for any of our diagnostic product candidates. Favorable results in early studies or trials, if any, may not be repeated in later studies or trials. Even if our clinical trials are initiated and completed as planned, it cannot be certain that the results will support our product candidate claims. Success in preclinical testing and Phase II clinical trials does not ensure that later Phase II or Phase III clinical trials will be successful. We cannot be sure that the results of later clinical trials would replicate the results of prior clinical trials and preclinical testing. In particular, the limited results we have obtained for our tests may not predict results from studies in larger numbers of subjects drawn from more diverse populations over a longer period of time. Clinical trials may fail to demonstrate that our product candidates are safe for humans and effective for indicated uses. Any such failure could cause us to abandon a product candidate and might delay development of other product candidates. Preclinical and clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals or commercialization. Any delay in, or termination of, our clinical trials would delay us in obtaining FDA approval for the affected product candidate and, ultimately, our ability to commercialize that product candidate.
| 23 |
We depend on third parties, including researchers, who are not under our control.
We depend upon independent investigators and scientific collaborators, such as universities and medical institutions or private physician scientists, to conduct our preclinical and clinical trials under agreements. These collaborators are not our employees, and they cannot control the amount or timing of resources that they devote to their programs or the timing of their procurement of clinical-trial data or their compliance with applicable regulatory guidelines. Should any of these scientific inventors/advisors become disabled or die unexpectedly, or should they fail to comply with applicable regulatory guidelines, we may be forced to scale back or terminate development of that program. They may not assign as great a priority to our programs or pursue them as diligently as we would if it were undertaking those programs itself. Failing to devote sufficient time and resources to our drug-development programs, or substandard performance and failure to comply with regulatory guidelines, could result in delay of any FDA applications and our commercialization of the drug candidate involved.
These collaborators may also have relationships with other commercial entities, some of which may compete with us. Our collaborators assisting our competitors at our expense could harm our competitive position. We have been and continue to be highly dependent on our strategic partner, MTF, for technical support and administrative support. We are also dependent on the support of the founding scientists who are UCLA employees for current scientific work in transitioning development work through and to contract vendors.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights, as well as costs associated with lawsuits.
If any other person files patent applications, or is issued patents, claiming technology also claimed by us, we may be required to participate in interference proceedings in the U.S. Patent and Trademark Office to determine priority of invention. We or our licensors may also need to participate in interference proceedings involving issued patents and pending applications of another entity.
The intellectual property environment in our industry is particularly complex, constantly evolving and highly fragmented. Other companies and institutions have issued patents and have filed or will file patent applications that may issue into patents that cover or attempt to cover products, processes or technologies similar to us. We have not conducted freedom-to-use patent searches on all aspects of our product candidates or potential product candidates and may be unaware of relevant patents and patent applications of third parties. In addition, the freedom-to-use patent searches that have been conducted may not have identified all relevant issued patents or pending patents. We cannot provide assurance that our proposed products in this area will not ultimately be held to infringe one or more valid claims owned by third parties which may exist or come to exist in the future or that in such case we will be able to obtain a license from such parties on acceptable terms.
We cannot guarantee that our technologies will not conflict with the rights of others. In some foreign jurisdictions, we could become involved in opposition proceedings, either by opposing the validity of another’s foreign patent or by persons opposing the validity of our foreign patents.
We may also face frivolous litigation or lawsuits from various competitors or from litigious securities attorneys. The cost of any litigation or other proceeding relating to these areas, even if deemed frivolous or resolved in our favor, could be substantial and could distract management from its business. Uncertainties resulting from initiation and continuation of any litigation could have a material adverse effect on our ability to continue our operations.
If we infringe the rights of others, we could be prevented from selling products or forced to pay damages.
If our products, methods, processes, and other technologies are found to infringe the proprietary rights of other parties, we could be required to pay damages, or may be required to cease using the technology or to license rights from the prevailing party. Any prevailing party may be unwilling to offer us a license on commercially acceptable terms.
| 24 |
Our product candidates are at an early stage of development and may not be successfully developed or commercialized.
Our products are in the early stage of development and will require substantial further capital expenditures, development, testing, and regulatory clearances prior to commercialization. The development and regulatory approval process takes several years, and it is not likely that our products, technologies or processes, even if successfully developed and approved by the FDA, would be commercially available for five or more years. Of the large number of drugs in development, only a small percentage successfully completes the FDA regulatory approval process and is commercialized. Accordingly, even if we are able to obtain the requisite financing to fund our development programs, we cannot assure you that our product candidates will be successfully developed or commercialized. Our failure to develop, manufacture or receive regulatory approval for or successfully commercialize any of our product candidates, could result in the failure of our business and a loss of all of your investment in our company.
Any product candidates advanced into clinical development are subject to extensive regulation, which can be costly and time consuming, cause unanticipated delays or prevent the receipt of the required approvals to commercialize such product candidates.
The clinical development, manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing and distribution of our product candidates are subject to extensive regulation by the FDA in the U.S. and by comparable health authorities in foreign markets. In the U.S., we may not be permitted to market our product candidates until we receive approval of our PMA from the FDA. The process of obtaining PMA approval is expensive, often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. In addition to the significant clinical testing requirements, our ability to obtain marketing approval for these products depends on obtaining the final results of required non-clinical testing, including characterization of the manufactured components of our product candidates and validation of our manufacturing processes. The FDA may determine that our product manufacturing processes, testing procedures or facilities are insufficient to justify approval. Approval policies or regulations may change and the FDA has substantial discretion in the approval process, including the ability to delay, limit or deny approval of a product candidate for many reasons. Despite the time and expense invested in clinical development of product candidates, regulatory approval is never guaranteed.
The FDA or another regulatory agency can delay, limit or deny approval of a product candidate for many reasons, including, but not limited to:
| ● | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of clinical trials; | |
| ● | We may be unable to demonstrate to the satisfaction of the FDA that a product candidate is safe and effective for any indication; | |
| ● | the FDA may not accept clinical data from trials which are conducted by individual investigators or in countries where the standard of care is potentially different from the U.S.; | |
| ● | the results of clinical trials may not meet the level of statistical significance required by the FDA for approval; | |
| ● | We may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; | |
| ● | the FDA may disagree with our interpretation of data from preclinical studies or clinical trials; | |
| ● | the FDA may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we or our collaborators contract for clinical and commercial supplies; or | |
| ● | the approval policies or regulations of the FDA may significantly change in a manner rendering our clinical data insufficient for approval. |
With respect to foreign markets, approval procedures vary among countries and, in addition to the aforementioned risks, can involve additional product testing, administrative review periods and agreements with pricing authorities. In addition, recent events raising questions about the safety of certain marketed pharmaceuticals may result in increased cautiousness by the FDA and comparable foreign regulatory authorities in reviewing new pharmaceuticals based on safety, efficacy or other regulatory considerations and may result in significant delays in obtaining regulatory approvals. Any delay in obtaining, or inability to obtain, applicable regulatory approvals could prevent us from commercializing our product candidates.
| 25 |
Any product candidate we advance into clinical trials may cause unacceptable adverse events or have other properties that may delay or prevent their regulatory approval or commercialization or limit their commercial potential.
Unacceptable adverse events caused by any of our product candidates that we advance into clinical trials could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications and markets. This, in turn, could prevent us from commercializing the affected product candidate and generating revenues from its sale.
We have not yet completed testing of any of our product candidates for the treatment of the indications for which we intend to seek product approval in humans, and we currently do not know the extent of adverse events, if any, that will be observed in patients who receive any of our product candidates. If any of our product candidates cause unacceptable adverse events in clinical trials, we may not be able to obtain regulatory approval or commercialize such product or, if such product candidate is approved for marketing, future adverse events could cause us to withdraw such product from the market.
Delays in the commencement of clinical trials could result in increased costs and delay our ability to pursue regulatory approval.
The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
| ● | obtaining regulatory clearance to commence a clinical trial; | |
| ● | identifying, recruiting and training suitable clinical investigators; | |
| ● | reaching agreement on acceptable terms with prospective clinical research organizations, and trial sites, the terms of which can be subject to extensive negotiation, may be subject to modification from time to time and may vary significantly among different clinical research organizations and trial sites; | |
| ● | obtaining sufficient quantities of a product candidate for use in clinical trials; | |
| ● | obtaining an IRB or ethics committee approval to conduct a clinical trial at a prospective site; | |
| ● | identifying, recruiting and enrolling patients to participate in a clinical trial; and | |
| ● | retaining patients who have initiated a clinical trial but may withdraw due to adverse events from the therapy, insufficient efficacy, fatigue with the clinical trial process or personal issues. |
Any delays in the commencement of clinical trials will delay our ability to pursue regulatory approval for our product candidates. In addition, many of the factors that cause, or lead to, a delay in the commencement of clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate.
Suspensions or delays in the completion of clinical testing could result in increased costs to us and delay or prevent our ability to complete development of that product or generate product revenues.
Once a clinical trial has begun, patient recruitment and enrollment may be slower than we anticipate. Clinical trials may also be delayed as a result of ambiguous or negative interim results or difficulties in obtaining sufficient quantities of product manufactured in accordance with regulatory requirements. Further, a clinical trial may be modified, suspended or terminated by us, an IRB, an ethics committee or a data safety monitoring committee overseeing the clinical trial, any clinical trial site with respect to that site, or the FDA or other regulatory authorities due to a number of factors, including:
| ● | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; | |
| ● | inspection of the clinical trial operations or clinical trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold; | |
| ● | stopping rules contained in the protocol; | |
| ● | unforeseen safety issues or any determination that the clinical trial presents unacceptable health risks; and/or | |
| ● | lack of adequate funding to continue the clinical trial. |
| 26 |
Any changes in the current regulatory requirements and guidance also may occur, and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit clinical trial protocols to IRBs for re-examination, which may impact the costs, timing and the likelihood of a successful completion of a clinical trial. If we experience delays in the completion of, or if we must suspend or terminate, any clinical trial of any product candidate, our ability to obtain regulatory approval for that product candidate will be delayed and the commercial prospects, if any, for the product candidate may suffer as a result. In addition, many of these factors may also ultimately lead to the denial of regulatory approval of a product candidate.
Privacy Provisions of HIPAA
HIPAA, among other things, protects the privacy and security of individually identifiable health information by limiting its use and disclosure. HIPAA directly regulates “covered entities” (healthcare providers, insurers and clearinghouses) and indirectly regulates “business associates” with respect to the privacy of patients’ medical information. All entities that receive and process protected health information are required to adopt certain procedures to safeguard the security of that information. It is uncertain whether we would be deemed to be a covered entity under HIPAA, and it is unlikely that we, based on our current business model, would be a business associate. Nevertheless, we may be contractually required to physically safeguard the integrity and security of any patient information that we receive, store, create or transmit. If we fail to adhere to our contractual commitments, then certain of our contract counterparties may be subject to civil monetary penalties and this could adversely affect our ability to market our product. If we are deemed to be a vendor, under the Health Information Technology for Economic and Clinical Health Act, enacted as part of the American Recovery and Reinvestment Act of 2009, then we will be obligated to adopt various security measures. We may also be subject to state and foreign privacy laws under which breaches could lead to substantial fines and liability.
We may be subject to claims that our consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of their other clients or former employers to us.
As is common in the biotechnology industry, we engage the services of consultants to assist in the development of our product candidates. Many of these consultants were previously employed at, or may have previously been or are currently providing consulting services to, other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may become subject to claims that we or our consultants have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of our former employers or their former or current customers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications for which there may be a greater likelihood of success.
Because we have limited financial and managerial resources, we are focused on one research program. As a result, we may forego or delay pursuit of opportunities with other product candidates or, for other indications for which there may be a greater likelihood of success or may prove to have greater commercial potential. Notwithstanding our investment to date and anticipated future expenditures, we may never successfully develop, any marketed treatments using these products. Research programs to identify new product candidates or pursue alternative indications for current product candidates require substantial technical, financial and administrative support.
| 27 |
We may incur substantial product liability or indemnification claims relating to the clinical testing of our product candidates.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials, and claims could be brought against us if use or misuse of one of our product candidates causes, or merely appears to have caused, personal injury or death. While we have and intend to maintain product liability insurance relating to our clinical trials, our coverage may not be sufficient to cover claims that may be made, and we may be unable to maintain such insurance. Any claims, regardless of their merit, could severely harm our financial condition, strain our management and other resources or destroy the prospects for commercialization of the product which is the subject of any such claim. We are unable to predict if we will be able to obtain or maintain product liability insurance for any products that may be approved for marketing. Additionally, it is expected that we will need to enter into various agreements where we indemnify third parties for certain claims relating to the testing of our product candidates. These indemnification obligations may require us to pay significant sums of money for claims that are covered by these indemnifications.
Our research and development activities could be affected or delayed as a result of possible restrictions on animal testing.
Certain laws and regulations require us to test our product candidates on animals before initiating clinical trials involving humans. Animal testing activities have been the subject of controversy and adverse publicity. Animal rights groups and other organizations and individuals have attempted to stop animal testing activities by pressing for legislation and regulation in these areas and by disrupting these activities through protests and other means. To the extent the activities of these groups are successful, our research and development activities may be interrupted, delayed or become more expensive.
We use biological materials and may use hazardous materials, and any claims relating to improper handling, storage or disposal of these materials could be time consuming or costly.
We may use hazardous materials, including chemicals and biological agents and compounds, that could be dangerous to human health and safety or the environment. Our operations also produce hazardous waste products. Federal, state and local laws and regulations govern the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair our product development efforts. In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes. We do not carry specific biological or hazardous waste insurance coverage, and our property and casualty and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or penalized with fines in an amount exceeding our resources, and our clinical trials or regulatory approvals could be suspended.
| 28 |
Risks Related to Ownership of Our Common Stock
There is no public trading market for our Common Stock, and you may not be able to resell your Common Stock.
There is no established public trading market for our securities. Our shares are not and have not been quoted on any exchange or quotation system. We cannot assure you that an application for listing will be approved or that a regular trading market will develop or that if developed, will be sustained. In the absence of a trading market, an investor may be unable to liquidate its investment, which will result in the loss of your investment.
We have no plans to pay dividends.
To date, we have paid no cash dividends on our Common Stock. For the foreseeable future, earnings generated from our operations will be retained for use in our business and not to pay dividends.
The application of the SEC’s “penny stock” rules to our Common Stock could limit trading activity in the market, and our stockholders may find it more difficult to sell their stock.
It is expected that our Common Stock will be trading at less than $5.00 per share and will therefore be subject to the SEC’s penny stock rules. Penny stocks generally are equity securities with a price of less than $5.00. Penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. The broker-dealer must also make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security that becomes subject to the penny stock rules. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our securities, which could severely limit their market price and liquidity of our securities. These requirements may restrict the ability of broker-dealers to sell our Common Stock and may affect your ability to resell our Common Stock.
If we are unable to establish appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations, result in the restatement of our financial statements, harm our operating results, subject us to regulatory scrutiny and sanction, cause investors to lose confidence in our reported financial information and have a negative effect on the market price for shares of our Common Stock.
Effective internal controls are necessary for us to provide reliable financial reports and to effectively prevent fraud. We maintain a system of internal control over financial reporting, which is defined as a process designed by, or under the supervision of, our principal executive officer and principal financial officer, or persons performing similar functions, and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
As a public company, we have significant additional requirements for enhanced financial reporting and internal controls. We are required to document and test our internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, which requires annual management assessments of the effectiveness of our internal controls over financial reporting and a report by our independent registered public accounting firm addressing these assessments. The process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company.
We cannot assure you that we will, in the future, identify areas requiring improvement in our internal control over financial reporting. We cannot assure you that the measures we will take to remediate any areas in need of improvement will be successful or that we will implement and maintain adequate controls over our financial processes and reporting in the future as we continue our growth. If we are unable to establish appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations, result in the restatement of our financial statements, harm our operating results, subject us to regulatory scrutiny and sanction, cause investors to lose confidence in our reported financial information and have a negative effect on the market price for shares of our Common Stock.
| 29 |
If the SEC does not declare a registration statement effective, you may not be able to sell shares in the amounts or at the times you might otherwise wish to.
The Company will enter into the Registration Rights Agreement at the closing of the Merger. Pursuant to the Registration Rights Agreement, MTF and AFH may, at any time, request registration under the Securities Act of all or part of their registrable shares. Within thirty (30) days of such request, the Company will provide written notice of such request to all other holders of registrable securities and will include in such registration all registrable shares with respect to which the Company has received written requests for inclusion within thirty (30) days after delivery of the Company’s notice. Although the Company believes that it and its advisors will be able to take all steps necessary to permit the SEC to declare the registration statement effective, it is possible that the SEC may, by application of policies or procedures that vary from past policies and procedures, delay the effectiveness of the registration statement or make it impractical for the Company to respond to the SEC in a manner that permits it to declare the registration statement effective. If the registration statement is not declared effective, you will need to rely on exemptions from the registration requirements of the Securities Act, such as Rule 144. Such exemptions typically limit the amount of shares that can be sold, require that shares be sold in certain types of transactions and require certain holding periods.
The market price of our Common Stock may be volatile.
The market price of our Common Stock may be highly volatile. Some of the factors that may materially affect the market price of our Common Stock are beyond our control, such as changes in financial estimates by industry and securities analysts, conditions or trends in the industry in which we operate or sales of our Common Stock. These factors may materially adversely affect the market price of our Common Stock, regardless of our performance. In addition, public stock markets have experienced extreme price and trading volume volatility. This volatility has significantly affected the market prices of securities of many companies for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our Common Stock.
Because our directors and executive officers are among our largest stockholders, they can exert significant control over our business and affairs and have actual or potential interests that may depart from those of investors in the Subsequent Closings.
The holdings of our directors and executive officers may increase in the future upon vesting or other maturation of exercise rights under any of the options or warrants they may hold or in the future be granted or if they otherwise acquire additional shares of Common Stock. The interests of such persons may differ from the interests of our other stockholders, including purchasers of shares of Common Stock in the Subsequent Closings. As a result, in addition to their board seats and offices, such persons will have significant influence over and control all corporate actions requiring stockholder approval, irrespective of how the Company’s other stockholders, including purchasers in the Subsequent Closings, may vote, including the following actions:
| ● | to elect or defeat the election of our directors; | |
| ● | to amend or prevent amendment of our Amended and Restated Certificate of Incorporation or By-laws; | |
| ● | to effect or prevent a merger, sale of assets or other corporate transaction; and | |
| ● | to control the outcome of any other matter submitted to our stockholders for vote. |
This concentration of ownership by itself may have the effect of impeding a merger, consolidation, takeover or other business consolidation, or discouraging a potential acquirer from making a tender offer for the Common Stock which in turn could reduce our stock price or prevent our stockholders from realizing a premium over our stock price.
| 30 |
We cannot assure you that the Common Stock will be listed on NASDAQ or any other securities exchange.
We intend to seek quotation on the Over-the-Counter Bulletin and possible listing of the Common Stock on NASDAQ. However, we cannot assure you that we will be able to meet the initial listing standards of either of those or any other stock exchange, or that we will be able to maintain a listing of the Common Stock on either of those or any other stock exchange. This would also make it more difficult for us to raise additional capital. There are no assurances that an active market for our shares will develop even if we are listed.
There is currently no trading market for our Common Stock, and liquidity of shares of our Common Stock is limited.
Shares of our Common Stock are not registered under the securities laws of any state or other jurisdiction, and accordingly there is no public trading market for the Common Stock. Further, no public trading market is expected to develop in the foreseeable future unless and until the Company files and obtains effectiveness of a registration statement under the Securities Act. Therefore, outstanding shares of Common Stock cannot be offered, sold, pledged or otherwise transferred unless subsequently registered pursuant to, or exempt from registration under, the Securities Act and any other applicable federal or state securities laws or regulations.
Compliance with the criteria for securing exemptions under federal securities laws and the securities laws of the various states is extremely complex, especially in respect of those exemptions affording flexibility and the elimination of trading restrictions in respect of securities received in exempt transactions and subsequently disposed of without registration under the Securities Act or state securities laws.
We may issue more shares in a future financing which will result in substantial dilution.
Our Amended and Restated Certificate of Incorporation authorizes the issuance of a maximum of 100,000,000 shares of Common Stock and a maximum of 20,000,000 shares of Preferred Stock. Any future merger or acquisition effected by us would result in the issuance of additional securities without stockholder approval and the substantial dilution in the percentage of our Common Stock held by our then existing stockholders. Moreover, the Common Stock issued in any such merger or acquisition transaction may be valued on an arbitrary or non-arm’s-length basis by our management, resulting in an additional reduction in the percentage of Common Stock held by our then existing stockholders. Additionally, we expect to seek additional financing in order to provide working capital to the operating business. Our Board of Directors has the power to issue any or all of such authorized but unissued shares without stockholder approval. To the extent that additional shares of Common Stock or Preferred Stock are issued in connection with and following a business combination or otherwise, dilution to the interests of our stockholders will occur and the rights of the holders of Common Stock might be materially and adversely affected.
Our Board of Directors is authorized to issue Preferred Stock without obtaining shareholder approval.
Our Amended and Restated Certificate of Incorporation authorizes the issuance of up to 20,000,000 shares of Preferred Stock with designations, rights and preferences determined from time to time by the Board of Directors. Accordingly, our Board of Directors is empowered, without stockholder approval, to issue Preferred Stock with dividend, liquidation, conversion, voting, or other rights which could adversely affect the voting power or other rights of the holders of the Common Stock. In the event of issuance, the Preferred Stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of the Company. Although we have no present intention to issue any shares of Preferred Stock, there can be no assurance that the Company will not do so in the future.
| 31 |
Risks Related to the Private Placement
Your ability to sell your shares is not assured because there are restrictions on their transfer.
The shares sold in the Private Placement are being sold as a private placement of securities. There is, at present, no trading market for such shares. The shares may not be sold or transferred unless registered under the Securities Act, and applicable state securities laws, or unless an opinion of counsel satisfactory to the Company is obtained to the effect that an exemption from registration is available and such transfer is approved by the Company. Each investor will be required to represent that it is acquiring the shares for its individual investment and not with a view to resale within the meaning of the Act. THERE CAN BE NO ASSURANCE THAT THE COMPANY WILL EVER FILE A REGISTRATION STATEMENT TO REGISTER SUCH SECURITIES, THAT SUCH REGISTRATION STATEMENT WILL BECOME EFFECTIVE, OR THAT ONCE EFFECTIVE, SUCH EFFECTIVENESS WILL BE MAINTAINED OR THAT A PUBLIC TRADING MARKET CAN EVER BE ESTABLISHED OR MAINTAINED.
Our management has discretion as to the use of proceeds from the Private Placement.
The net proceeds, if any, from the Private Placement will be used as described under Subsequent Closings in this Current Report. We may spend or invest these proceeds in a way with which our stockholders disagree, and we reserve the right to use the funds obtained from the Private Placement for other purposes not presently contemplated which we deem to be in our and our stockholders’ best interests in order to address changed circumstances and opportunities. Failure by our management to effectively use these funds could harm our business and financial condition. Investors will be entrusting their funds to our management, upon whose judgment and discretion the investors must depend, with only limited information concerning management’s specific intentions.
We may include purchases from affiliated investors in determining whether we have sold the shares.
Purchases made by affiliates of the Company will be used in determining whether the maximum offering amount has been sold. Investors in the Private Placement, therefore, should not assume that investments in the Company came from a large number of unaffiliated investors.
There can be no assurance that the results and events contemplated by forward-looking statements will, in fact, transpire.
There are statements in this Current Report that are not historical facts. These “forward-looking statements” can be identified by the use of terminology such as “believe,” “hope,” “may,” “anticipate,” “should,” “intend,” “plan,” “will,” “expect,” “estimate,” “project,” “positioned,” “strategy” and similar expressions. You should be aware that these forward-looking statements are subject to risks and uncertainties that are beyond our control. Actual results could differ significantly from these forward-looking statements. In light of these risks and uncertainties, there can be no assurance that the results and events contemplated by the forward-looking statements contained in this Registration Statement will in fact transpire. You are cautioned to not place undue reliance on these forward-looking statements, which speak only as of their dates. We do not undertake any obligation to update or revise any forward-looking statements.
The price of the shares and other terms of the Private Placement have been arbitrarily determined.
If you purchase shares of Common Stock in the Private Placement, you will pay a price that was not established in a competitive market. Rather, you will pay a price that was arbitrarily determined by the Company. The Private Placement price for the shares may bear no relationship to our assets, book value, historical results of operations or any other established criterion of value and may not be indicative of the fair value of the shares.
IN ADDITION TO THE ABOVE RISKS, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY OUR MANAGEMENT. IN REVIEWING THIS MEMORANDUM, POTENTIAL INVESTORS SHOULD KEEP IN MIND THAT THERE MAY BE OTHER POSSIBLE RISKS THAT COULD BE IMPORTANT.
| 32 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of the results of operations and financial condition for the six months ended June 30, 2014 and the year ended December 31, 2013 and 2012 of Bone Biologics should be read in conjunction with our financial statements and the notes to those financial statements that follow the signature page to this Current Report. The financial statements should not be relied on for an understanding of the current financial status of the Company.
Overview
Bone Biologics was incorporated in California on March 9, 2004. Bone Biologics is a privately-held biotechnology company that is currently focused on bone regeneration in spinal fusion using the recombinant human protein, known as Nell-1. The Nell-1 protein is an osteoinductive recombinant protein that provides target specific control over bone regeneration. The protein, as part of the UCB-1 technology platform has been licensed exclusively for worldwide applications to Bone through a technology transfer from UCLA. UCLA and Bone recently received guidance from the FDA that Nell-1 will be classified as a combination product with a device lead.
We are a development stage entity. The production and marketing of our products and ongoing research and development activities will be subject to extensive regulation by numerous governmental authorities in the United States. Prior to marketing in the United States, any combination product developed by us must undergo rigorous preclinical (animal) and clinical (human) testing and an extensive regulatory approval process implemented by the FDA under the Food, Drug and Cosmetic Act. There can be no assurance that we will not encounter problems in clinical trials that will cause us or the FDA to delay or suspend the clinical trial.
Our success will depend in part on our ability to obtain patents and product license rights, maintain trade secrets, and operate without infringing on the proprietary rights of others, both in the United States and other countries. There can be no assurance that patents issued to or licensed by us will not be challenged, invalidated, or circumvented, or that the rights granted thereunder will provide proprietary protection or competitive advantages to us.
Pursuant to the Milestone Side Letter Agreement, Bone agreed to use its commercially reasonable efforts to achieve the following Milestone Targets by the specified times following the closing of the Private Placement:
| (i) | Complete media screening studies of cell line within two (2) to three (3) months; | |
| (ii) | Initiate manufacturing of master cell bank within three (3) to four (4) months; | |
| (iii) | Initiate formulation studies for the cGMP manufacturing process once sufficient Nell-1 material is available within approximately eight (8) to ten (10) months; | |
| (iv) | Initiate a pre-clinical bioreactor production run for toxicology material within nine (9) to twelve (12) months following the closing of the Private Placement; | |
| (v) | Initiate pre-clinical toxicology studies to include carcinogenicity and reproductive within approximately eleven (11) to thirteen (13) months; | |
| (vi) | Finalize refinement of the manufacturing process within approximately twelve (12) to fourteen (14) months; | |
| (vii) | Initiate cGMP bioreactor run within twelve (12) to fourteen (14) months or after completion of (v), and | |
| (viii) | Request an IDE meeting to review the clinical safety plan within eighteen (18) to twenty (20) months; |
| 33 |
Results of Operations
Since its inception, Bone devoted substantially all of its efforts and funding to the development of the Nell-1 protein and raising capital. We have not generated revenues from our planned operations.
Year ended December 31, 2013 compared to Year ended December 31, 2012
| Year
Ended December 31, 2013 | Year
Ended December 31, 2012 | |||||||
| Operating expenses | ||||||||
| Research and development | $ | 188,236 | $ | 255,575 | ||||
| General and administrative | 483,749 | 180,089 | ||||||
| Total operating expenses | 671,984 | 435,664 | ||||||
| Loss from operations | (671,984 | ) | (435,664 | ) | ||||
| Interest expense, net | (409,419 | ) | (279,101 | ) | ||||
| Loss before provision for income taxes | (1,081,403 | ) | (714,765 | ) | ||||
| Provision for income taxes | 800 | 800 | ||||||
| Net loss | $ | (1,082,203 | ) | $ | (715,565 | ) | ||
Research and Development
Bone’s research and development expenses decreased from $255,575 during the year ended December 31, 2012 to $188,236 during the year ended December 31, 2013. The $67,339 or 26.3% decrease was related to patent costs and development activities for Bone’s lead product Nell-1. We will continue to incur significant expenses for development activities for Nell-1.
General and Administrative
Bone’s general and administrative expenses increased from $180,089 during the year ended December 31, 2012 to $483,749 during the year ended December 31, 2013. The $303,660 or 168.6% increase was primarily driven by increased expenses due to professional services and transaction costs related to the Bridge Financing and pursuing the merger related transactions.
Interest Expense
Bone’s net interest expense increased from $279,101 for the year ended December 31, 2012 to $409,419 for the year ended December 31, 2013. The increase in expenses of $130,318 or 46.7% was due to interest expense on our related party promissory notes and our bridge financing.
Net Loss
Bone’s net loss increased from $715,565 for the year ended December 31, 2012 to $1,082,203 for the year ended December 31, 2013, as a result of the increase in general and administrative expenses and interest expense.
| 34 |
Six Months ended June 30, 2014 compared to the Six Months ended June 30, 2013
| Six Months Ended June 30, 2014 | Six Months Ended June 30, 2013 | |||||||
| (unaudited) | (unaudited) | |||||||
| Operating expenses | ||||||||
| Research and development | $ | 183,111 | $ | 96,213 | ||||
| General and administrative | 307,948 | 215,937 | ||||||
| Total operating expenses | 491,059 | 312,150 | ||||||
| Loss from operations | (491,059 | ) | (312,150 | ) | ||||
| Other expense | (9,623 | ) | 0 | |||||
| Interest expense, net | (250,533 | ) | (172,524 | ) | ||||
| Total other income/expense | (260,156 | ) | (172,524 | ) | ||||
| Loss before provision for income taxes | (751,215 | ) | (484,674 | ) | ||||
| Provision for income taxes | 0 | 800 | ||||||
| Net loss | $ | (751,215 | ) | $ | (485,474 | ) | ||
Research and Development
Bone’s research and development expenses increased from $96,213 during the six months ended June 30, 2014 to $183,111 during the six months ended June 30, 2013. The $86,898 or 90.3% increase was driven by development activities for Bone’s lead product Nell-1. We will continue to incur significant expenses for development activities for Nell-1.
General and Administrative
Bone’s general and administrative expenses increased from $215,937 during the six months ended June 30, 2013 to $ 307,948 during the six months ended June 30, 2014. The $ 92,011 or 42.6% increase was primarily driven by professional fees surrounding additional financing and merger related transactions.
Other Expense
Bone’s other expense increased from $0 for the six months ended June 30, 2013 to $9,623 for the six months ended June 30, 2014. The increase in expense was due to the realized loss on sale of marketable securities received in lieu of cash on our 2013 Bridge Note with AFH.
Interest Expense
Bone’s net interest expense increased from $172,524 for the six months ended June 30, 2013 to $250,533 for the six months ended June 30, 2014. The $78,009 or 45.2% increase in interest expense was due to interest expense on related party notes due to MTF and the 2013 Bridge Financing.
Net Loss
Bone’s net loss increased from $485,474 for the six months ended June 30, 2013 to $751,215 for the six months ended June 30, 2014 as a result of the increases in research and development expenses, general and administrative expenses and interest expense.
Liquidity and Capital Resources
Bone has no significant operating history and, from March 9, 2004 (inception) to June 30, 2014, has generated a net loss of approximately $8.4 million. The financial statements for the six months ended June 30, 2014 and 2013 and the years ended December 31, 2013 and 2012 were prepared assuming we will continue as a going concern.
As of June 30, 2014 and December 31, 2013 and 2012, Bone had cash of $163, $1,538 and $2,370, respectively. Management intends to raise additional debt and/or equity financing to fund future operations and to provide additional working capital. However, there is no assurance that such financing will be consummated or obtained in sufficient amounts necessary to meet our needs. We can provide no assurance that we can continue to satisfy our cash requirements for the next twelve months.
| 35 |
In May, 2014, the Company entered into a convertible promissory note with MTF (the “2014 Note”) for $250,000 with interest at 7% per annum compounded annually and a maturity date of June 15, 2015. In the event of a financing of not less than $1 million, the 2014 Note automatically converts into Equity Securities, as defined in the 2014 Note, at a 25% discount to the price paid per share in such financing. In connection with the 2014 Note, the Company issued a warrant to purchase 166,667 shares of the Company’s common stock at an exercise price of $1.50 per share and 4 year term. The warrants had a fair value of $78,417, calculated using the Black-Scholes option pricing model with a volatility of 109%, a risk free rate of 0.39%. The Company incurred placement agent fees of $10,000 or 4% of the funds raised in connection with the financing and is obligated to issue a warrant for the purchase of 13,333 shares of common stock, which represents 4% of the common shares underlying the 2014 Note, with an exercise price of $1.00, a 5 year term and fair value of $8,181, calculated using the Black-Scholes model with a volatility of 109% and a risk free rate of 0.39%. The 2014 Note and related warrants were assigned to Orthofix in July 2014 and included in the Subsequent Orthofix Financing discussed below.
On July 1, 2014, Orthofix (A) purchased $500,000 worth of Bone Biologics Common Stock; (B) was issued two convertible promissory notes, each in the principal amount of $250,000 and exercisable for $333,333 worth of Bone Biologics Common Stock; and (C) was issued two warrants, each exercisable for 166,667 shares of Bone Biologics Common Stock at an exercise price per share of $1.50. Upon subscribing for the Subsequent Orthofix Shares, the Subsequent Orthofix Convertible Promissory Notes converted by their terms into a combined total of $666,666 worth of shares of Bone Biologics’ Common Stock in accordance with the terms of the Subsequent Orthofix Convertible Promissory Notes. The Subsequent Orthofix Warrants converted into warrants of the Company with substantially identical terms upon consummation of the Merger.
At the closing of the Subsequent Orthofix Shares and Notes, AFH Advisory was entitled to receive warrants to purchase up to 500,000 shares of Common Stock of the Company at the per share price of the shares offered or $1.00 per share, with a 5 year term and a cashless exercise provision (the “Extra Warrants”). AFH Advisory has normal and customary piggyback registration rights with respect to the shares of Common Stock issuable upon exercise of the Extra Warrants.
Forefront or its designees will receive an Agent Warrant equal to 8% of the Common Stock underlying the securities issued in the Private Placement (4% if investors are introduced by Bone Biologics, AFH Holdings & Advisory, LLC or their respective officers and directors). Such Agent Warrant will be issued at the closing of the Private Placement and shall provide, among other things, that the Agent Warrant shall: (i) be exercisable at the price of the securities (or the exercise price of the securities) issued to the investors in the offering, (ii) expire five (5) years from the date of issuance, (iii) include customary registration rights, including the registration rights provided to the Investors, (iv) contain provisions for cashless exercise and (v) include such other terms that are normal and customary for warrants of this type. In addition, Forefront or its designees will receive and Advisory Warrant equal to 2.0% of the Company’s post-merger and financing fully diluted shares outstanding upon the closing of $2.5 million of investors on which Forefront is eligible to receive compensation. Forefront was issued a warrant to purchase 46,667 shares of Common Stock at $1.00 per share upon completion of the Orthofix Subsequent Financing. Forefront will receive a cash fee equal to 8% of gross proceeds received and payable upon each closing (4% if investors are introduced to the Company by either Bone Biologics, AFH Holdings and Advisory, LLC, or their respective officers and directors, or an aggregate of $40,000 on the Orthofix Subsequent Financing, including $10,000 incurred in connection with the MTF 2014 Note assigned to Orthofix).
On September 15, 2014, Bone and MTF entered into the MTF Short Term 2014 Loan pursuant to which MTF has agreed to advance an initial $250,000 to Bone and, at Bone’s request and subject to the terms and conditions of the MTF Short Term 2014 Loan, to advance up to an additional $250,000 to Bone. The MTF Short Term 2014 Loan has an interest rate of eight and one-half percent (8.5%) accruing annually. The MTF Short Term 2014 Loan matures on the earlier to occur of (i) the date on which at least $1 million is loaned to or invested in the Company and (ii) December 31, 2014. In further consideration of the MTF 2014 Loan, Bone granted to MTF 625,000 warrants at a strike price of $1.62. The MTF 2014 Loan was assigned to the Company on September 19, 2014.
| 36 |
Cash Flows
The following is a summary of Bone’s cash flows provided by operating, investing and financing activities for the six months ended June 30, 2014 and 2013, and the years ended December 31, 2013 and 2012:
| Six
Months Ended June 30, 2014 | Six
Months Ended June 30, 2013 | Year
Ended December 31, 2013 | Year
Ended December 31, 2012 | |||||||||||||
| Net Cash Used In Operating Activities | $ | (288,752 | ) | $ | (339,011 | ) | $ | (525,365 | ) | $ | (447,486 | ) | ||||
| Net Cash Provided by Investing Activities | 37,377 | 0 | 0 | 0 | ||||||||||||
| Net Cash Provided by Financing Activities | 250,000 | 374,533 | 524,533 | 448,609 | ||||||||||||
| Net Increase (Decrease) in Cash and Cash Equivalents | $ | (1,375 | ) | $ | 35,522 | $ | (832 | ) | $ | 1,123 | ||||||
Operating activities
In the six months ended June 30, 2014 and 2013, cash used in operating activities was $288,752 and $339,011 respectively, which was driven by Bone’s net losses of $751,215 and $485,474, respectively. Cash expenditures in both the 2014 and 2013 periods increased primarily due to patent costs and professional fees as a result of Bone’s financing activities which included the Bridge Financing and matters related to financing and the merger transactions.
During the six months ended June 30, 2014, cash used in operating activities was partially offset by non-cash increases in accrued interest expense of $162,493, debt discount amortization of $91,111, advances due to related party of $89,374, other accrued expenses of $108,095 and a loss of $9,623 on the sale of marketable securities received in lieu of cash on our 2013 Bridge Note with AFH. During the six months ended June 30, 2013, cash used in operating activities included an increase in prepaid expenses and other current assets of $17,708 and a decrease in accrued expenses of $7,892, and were partially offset by non-cash increases in accrued interest expense of $161,616 and debt discount amortization of $10,447.
In the years ended December 31, 2013 and 2012, cash used in operating activities was $525,365 and $447,486, respectively, which was driven by Bone’s net losses of $1,082,203 and $715,565, respectively. Cash expenditures in 2013 and 2012 increased primarily due to patent costs and professional fees as a result of Bone’s financing activities which included the Bridge Financing and matters related to the merger transactions in 2013.
In the year ended December 31, 2013, cash used in operating activities increased due to an increase in prepaid expenses and other current assets of $10,767, and was offset by non-cash increases in accrued interest expense of $340,268 related to our Bridge Notes and notes due to MTF and an amortization of debt discount of $67,104, and increases in accrued expenses of $114,215 and accounts payable of $41,300. In the year ended December 31, 2012, cash used in operating activities increased due to a reduction in accounts payable of $29,657, which was offset by increases in accrued interest expense of $279,104 and accrued expenses of $18,632.
Investing activities
In the six months ended June 30, 2014, cash provided by investing activities of $37,377 resulted from the sale of marketable securities which were received in lieu of cash for our Bridge Note with AFH. There were no investing activities in the years ended December 31, 2013 or 2012.
Financing activities
In the six months ended June 30, 2014, cash provided by financing activities was $250,000 and resulted from the proceeds received from the issuance of the 2014 Note. In the six months ended June 30, 2013, cash provided by financing activities was $374,533 and resulted from the proceeds received from the issuance of the 2013 Bridge Notes and 2014 notes to MTF.
In the year ended December 31, 2013 and 2012, cash provided by financing activities of $524,533 and $448,609, respectively, was due to proceeds received from the issuance of promissory notes and convertible notes payable.
| 37 |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Critical Accounting Policies
Our financial statements are presented in accordance with accounting principles generally accepted in the U.S., or GAAP. The preparation of financial statements in conformity with GAAP requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of expenses during the reporting period. Significant estimates include warrants and income tax valuation allowances. Actual results could differ from those estimates.
Patents and Licenses
In March 2006, Bone entered into the Regents’ License with the Regents for the worldwide application of the Nell-1 protein through a technology transfer. Patent expenses include costs to acquire the license of Nell-1, which was de minimus, and costs to file patent applications related to Nell-1.
Bone Biologics expenses the costs incurred to file patent applications, all costs related to abandoned patent applications and maintenance costs, and these costs are included in research and development expenses. Costs associated with licenses acquired to be able to use products from third parties prior to receipt of regulatory approval to market the related products are also expensed. Our licensed technologies may have alternative future uses in that they are enabling (or platform) technologies that can be the basis for multiple products that would each target a specific indication. Costs of acquisition of licenses are expensed.
Research and Development Costs
Research and development costs include, but are not limited to, payroll and other personnel expenses, consultants, expenses incurred under agreements with contract research and manufacturing organizations and animal clinical investigative sites and the cost to manufacture clinical trial materials. Costs related to research, design and development of products are charged to research and development expense as incurred.
Income Taxes
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due and deferred taxes resulting from timing differences in the recording of transactions for tax purposes and financial reporting purposes.
Deferred tax assets and liabilities represent the future tax return consequences of those differences, which will either be taxable or deductible when the assets and liabilities are received or settled. Valuation allowances are established when necessary to reduce deferred tax assets to amounts expected to be realized.
The accounting provisions related to uncertain income tax positions require us to determine whether any tax position in all open years meets a more likely than not threshold of being sustained upon examination by the applicable taxing authority. Bone did not have any changes to its liability for uncertain tax positions for the years ended December 31, 2013 and 2012.
Our policy is to recognize interest and/or penalties related to income tax matters in income tax expense. No such amounts were accrued by Bone as of June 30, 2014 and December 31, 2013 and 2012.
New Accounting Standard
In June 2014, the FASB issued ASU 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements. ASU 2014-10 eliminates the distinction of a development stage entity and certain related disclosure requirements, including the elimination of inception-to-date information on the statements of operations, cash flows and stockholders’ equity. The amendments in ASU 2014-10 will be effective prospectively for annual reporting periods beginning after December 15, 2014, and interim periods within those annual periods, however early adoption is permitted. We adopted ASU 2014-10 during the quarter ended June 30, 2014, thereby are no longer presenting or disclosing any information required by Topic 915.
| 38 |
Our primary office, including administrative and laboratory space, is located at 175 May Street, Suite 400, Edison, NJ 08837. We also lease office space located at 100 Rancho Rd., Suite 7, Thousand Oaks, CA 91362.
| 39 |
SECURITY OWNERSHIP OF CERTAIN STOCKHOLDERS AND MANAGEMENT
Pre-Merger Beneficial Ownership
AFH Acquisition X, Inc.
The following table sets forth certain pre-merger information with respect to the beneficial ownership AFH Acquisition X, Inc. as of September 18, 2014, regarding (i) each person known by the Company to be the beneficial owner of more than 5% of the outstanding shares of Common Stock, (ii) each director, nominee and executive officer of the Company, and (iii) all officers and directors as a group.
| Name and Address of Beneficial Owner | Title of Class | Shares | Percentage of Class | |||||||||
| AFH Holding & Advisory, LLC | Common Stock | 3,175,000 | 63.50 | % | ||||||||
10830 Massachusetts Ave., Penthouse Los Angeles, CA 90024 | ||||||||||||
| Amir Heshmatpour | Common Stock | 4,175,000 | (1) | 83.50 | % | |||||||
269 Beverly Drive, Ste. 1600 Beverly Hills, CA 90212 | ||||||||||||
| Don Hankey | Common Stock | 450,000 | (2)(3) | 9 | % | |||||||
4751 Wilshire Blvd., Suite 110 Los Angeles, CA 90010 | ||||||||||||
Hankey Investment Company, L.P.
| Common Stock | 450,000 | 9 | % | ||||||||
| 4751 Wilshire Blvd., Suite 110 Los Angeles, CA 90010 | ||||||||||||
| All Officers and Directors as a group | Common Stock | 450,000 | 9 | % | ||||||||
| (1) | These shares of Common Stock are owned by AFH Holding, H&H and Mr. Heshmatpour’s spouse and children. Mr. Heshmatpour is sole member of AFH Holding and has sole voting and investment control over the 3,175,000 shares of Common Stock owned of record by AFH Holding. Mr. Heshmatpour is the sole member of H&H and has sole voting and investment control over the 200,000 shares of Common Stock owned of record by H&H. Mr. Heshmatpour has voting and investment control over the 800,000 shares of Common Stock owned by his spouse and children. Accordingly, he may be deemed a beneficial owner with respect to these 4,175,000 shares of Common Stock. | |
| (2) | Executive Officer and/or Director | |
| (3) | These shares of Common Stock are owned by Hankey Investment Company, L.P. (“HIC”). Mr. Hankey has voting and investment control over the shares of Common Stock owned of record by HIC. Accordingly, he may be deemed a beneficial owner with respect to the 450,000 shares. |
| 40 |
Bone Biologics
The following table sets forth certain pre-merger information with respect to the beneficial ownership of Bone Biologics as of September 18, 2014, regarding (i) each person known by Bone Biologics to be the beneficial owner of more than 5% of the outstanding shares of Common Stock, (ii) each director, nominee and executive officer of Bone Biologics, and (iii) all officers and directors as a group.
| Name and Address of Beneficial Owner | Title of Class | Amount
and Nature of | Percentage
of Class | |||||||||
| 5% or greater stockholders | ||||||||||||
| The Musculoskeletal Transplant Foundation, Inc. 125 May Street Edison, NJ 08837 | Common Stock | 11,307,807 | 56.8 | % | ||||||||
Dr. Kang Ting(1) 115 North Doheny Drive Beverly Hills, CA 90211 | Common Stock | 2,000,000 | 10.1 | % | ||||||||
| Orthofix Holdings Inc. 3451 Plana Parkway Lewisville, TX 75056 | Common Stock | 1,909,908 | 9.6 | % | ||||||||
Dr. Chia Soo(1) 115 North Doheny Drive Beverly Hills, CA 90211 | Common Stock | 1,119,318 | 5.6 | % | ||||||||
Dr. Benjamin Wu(1) 2740 Lorain Road San Marino, CA 91108 | Common Stock | 1,000,000 | 5.0 | % | ||||||||
Michael Schuler(1) 175 May Street, Suite 300 Edison, NJ 08837 | Common Stock | |||||||||||
Bruce Stroever(1) 175 May Street, Suite 300 Edison, NJ 08837 | Common Stock | |||||||||||
Mark Spilker(1) 175 May Street Edison, NJ 08837 | Common Stock | |||||||||||
| All Officers and Directors as a group | Common Stock | 4,131,943 | 20.8 | % | ||||||||
| (1) | Executive Officer and/or Director | |
| (2) | The number of shares of Common Stock issued and outstanding that was used to calculate the percentage ownership of each listed person includes the shares of Common Stock underlying convertible debt and warrants that are exercisable 60 days after the close of the Merger. |
| 41 |
Post-Merger Beneficial Ownership
Company
The following table sets forth information with respect to the post-merger beneficial ownership of the Company’s common stock as of September 19, 2014, by each person or group of affiliated persons known to the Company to beneficially own 5% or more of its common stock, each director, each named executive officer, and all of its directors and named executive officers as a group.
| Name of Beneficial Owner or Identity of Group | Title of Class | Shares(1) | Percentage | ||||||||
| 5% or greater stockholders: | |||||||||||
| The
Musculoskeletal Transplant Foundation, Inc. 125 May Street Edison, NJ 08837 | Common Stock | 11,932,807 | 49.3 | % | |||||||
| AFH Holding
& Advisory, LLC 10830 Massachusetts Ave., Penthouse Los Angeles, CA 900024 | Common Stock | 2,609,602 | 10.8 | % | |||||||
| Amir Heshmatpour
269 Beverly Drive, Ste. 1600 Beverly Hills, CA 90212 | Common Stock | 3,609,602 | (2) | 14.9 | % | ||||||
| Dr. Kang Ting
115 North Doheny Drive Beverly Hills, CA 90211 | Common Stock | 2,000,000 | 8.3 | % | |||||||
| Orthofix Holdings
Inc. 3451 Plana Parkway Lewisville, TX 75056 | Common Stock | 1,909,908 | 7.9 | % | |||||||
| Executive Officers and Directors: | |||||||||||
| Michael Schuler
175 May Street Edison, NJ 08837 | - | - | |||||||||
| William J.
Treat 175 May Street, Suite 400 Edison, NJ 08837 | Common Stock | 198,202 | 0.8 | % | |||||||
| Catherine
Doll 175 May Street, Suite 400 Edison, NJ 08837 | Common Stock | 12,625 | 0.1 | % | |||||||
| Bruce Stroever
175 May Street, Suite 400 Edison, NJ 08837 | - | - | |||||||||
| Dr. Chia Soo
175 May Street, Suite 400 Edison, NJ 08837 | Common Stock | 1,119,318 | 4.6 | % | |||||||
| William Coffin
175 May Street, Suite 400 Edison, NJ 08837 | - | - | |||||||||
| John Booth
175 May Street, Suite 400 Edison, NJ 08837 | - | - | |||||||||
| Jimmy Delshad
175 May Street, Suite 400 Edison, NJ 08837 | - | - | |||||||||
| Steve Warnecke
1026 Anaconda Drive Castle Rock, CO 80108 | - | - | |||||||||
| Total Officers and Directors as a Group | Common Stock | 1,330,145 | 5.5 | % | |||||||
| Reserve for Future Issuance: | |||||||||||
| Option Plan | 2,444,696 | ||||||||||
| (1) | The number of shares of Common Stock issued and outstanding that was used to calculate the percentage ownership of each listed person includes the shares of Common Stock underlying convertible debt, stock options and warrants that are exercisable 60 days after the close of the Merger. | |
| (2) | These shares of Common Stock are owned by AFH Holding, H&H and Mr. Heshmatpour’s spouse and children. Mr. Heshmatpour is sole member of AFH Holding and has sole voting and investment control over the 2,609,602 shares of Common Stock owned of record by AFH Holding. Mr. Heshmatpour is the sole member of H&H and has sole voting and investment control over the 200,000 shares of Common Stock owned of record by H&H. Mr. Heshmatpour has voting and investment control over the 800,000 shares of Common Stock owned by his spouse and children. Accordingly, he may be deemed a beneficial owner with respect to these 3,609,602 shares of Common Stock. |
| 42 |
DIRECTORS AND EXECUTIVE OFFICERS
The Company’s officers and directors are elected annually for a one year term or until their respective successors are duly elected and qualified or until their earlier resignation or removal. The following table sets forth certain information regarding the Company’s directors and executive officers:
| Name | Age | Position | Term Length | |||
| Michael Schuler | 64 | Interim Chief Executive Officer | 1 years | |||
| William J. Treat | 59 | President and Chief Technology Officer | 1 year | |||
| Catherine Doll | 53 | Interim Chief Financial Officer | 1 year | |||
| Bruce Stroever | 64 | Chairman of the Board of Directors | 1 year | |||
| Dr. Chia Soo | 46 | Director | 1 year | |||
| William Coffin | 69 | Director | 1 year | |||
| John Booth | 59 | Director | 1 year | |||
| Jimmy Delshad | 74 | Director | 1 year | |||
| Steve Warnecke | 57 | Director | 1 year |
Michael Schuler: Interim Chief Executive Officer
Pursuant to a consulting agreement by and between the Company and MTF, MTF has agreed to provide the services of Mr. Schuler to serve as the Company’s interim Chief Executive Officer. Mr. Schuler has served as Vice-President, New Business Development of MTF since 2002. In addition to his work at MTF, since 2000 Mr. Schuler is the founder and partner in Tri-Medics, LLC, an early stage company developing and marketing innovative medical devices. From 1995 to 2001, Mr. Schuler served as an independent strategic planner and new business development consultant with Olympus USA and SurgiNex, Inc. From 1993 to 1995, he was President of the Surgical Products Division of Imagyn Medical Technologies, a NASDAQ listed company. From 1992 to 1996, he served as Executive Vice President, Marketing and Sales, then President and CEO of Advanced Surgical, Inc., an innovator in surgical instruments for advanced procedures and a NASDAQ listed company. Prior to his roles at Advanced Surgical, Inc. Mr. Schuler spent 18 years with Johnson & Johnson, where he progressively held positions of greater responsibility, including a position in new business development which was responsible for the assessment and development of the Palmaz-Schatz Stent technology that was utilized in cardiology and radiology. As Director and then Vice-President of Marketing of Johnson & Johnson Interventional Systems from 1987 to 1992, he was involved in the launch of the Intraluminal Stent that grew to over $1 billion in annual sales. Mr. Schuler was the only Johnson & Johnson employee to receive both the Phillip B. Hoffman award for outstanding achievement in R&D and the Johnson & Johnson Entrepreneurial Award for creating over $50 million in new business. He is the holder of 13 US patents and numerous international patents. Mr. Schuler was a part-time professor at the Rutgers University Graduate School from 1978-1993. He has also served as a member of Bone’s Board of Directors since April 7, 2006. He earned a Bachelors of Science in Industrial Engineering in 1971, a Masters in Business Administration in 1973, and a Masters of Science in Engineering in 1974, all from Rutgers University. Given Mr. Schuler’s extensive background in medical device development and marketing, as well as his previous roles with Bone Biologics, the Company believes he is well qualified to fill the role of interim Chief Executive Officer.
William Jay Treat, Ph.D.: President and Chief Technology Officer
Dr. Treat has served as the President and Chief Technology Officer of Bone Biologics since November 2012. Since 2005 to present, he has run his own consulting business specializing in providing senior management leadership to clients in developing their products from concept to commercial launch. During this period he was one of the founders and remains active in America Stem Cell (now Targazyme) overseeing their manufacturing and clinical supplies. He also currently serves as Advisor for Systems Operations at PBS BioTech. From 2001 to 2005 he served first as Vice President of Business Development and later as Chief Operations Officer for Avid Bioservices, a contract manufacturing organization specializing in the production of monoclonal antibodies and other recombinant non-antibody proteins. As Chief Operating Officer of Avid Bioservices he presided over a successful PAI and commercial license of a biologic for Halozyme. Prior to joining Avid Bioservices, from 1999 to 2001 he was Vice President of Research and Development at Irvine Scientific where his team developed products for human assisted reproductive medicine and for cell culture medium formulations used to produce medically important biological molecules. From 1990 to 1999 he was with BioWhittaker (acquired by Cambrex), where he held positions of progressively greater responsibility in manufacturing, technical support, new business acquisitions and marketing while they were a privately held company and later as a public company. Dr. Treat started his career at Lipogen, where he quickly moved to Vice President of Manufacturing and managed the technology transfer to BioWhittaker. During his career, Dr. Treat has also served as Chief Operating Officer for BioTork, CSO for Algenol, Vice President of Manufacturing for Attenuion, CTO for Geneve Bio and Head of Commercial Manufacturing for DianaPlantSciences. Dr. Treat has served on the Scientific Advisory Boards for the Chemical Engineering Department at Texas A&M University from 2000 to 2013 and on the Emergent Biosolutions and XOMA External Advisory Boards in support of NAIAD/DOD bioterrorism programs. He earned a Bachelors of Science in Microbiology in 1979, a Masters of Science in Microbiology in 1982 and a Ph.D. in Agricultural and Biochemical Engineering in 1988 all from Texas A&M University. Given Dr. Treat’s extensive background with biologics and his previous roles with Bone Biologics, the Company believe Dr. Treat is well qualified to serve as its President and Chief Technology Officer.
| 43 |
Catherine Doll: Interim Chief Financial Officer
Pursuant to a consulting agreement by and between the Company and Gilson Group, Gilson Group has agreed to provide the services of Ms. Doll to serve as the Company’s interim Chief Financial Officer. Ms. Doll, a certified public accountant, brings more than 25 years of international corporate finance and accounting experience in both publicly and privately held companies to the Company. From 2004 through the present Ms. Doll has been a consultant at Gilson Group where she has assisted clients with the development of audit strategies and management assessments, as well as acting as an audit liaison for clients in preparation for or support of compliance audit under the Sarbanes-Oxley Act. She has also served as project lead and quality control for SOX implementation and monitoring for public companies. From 1999 through 2004 Ms. Doll was and associate at Resources Global Professionals where she provided management, financial and accounting services to clients. From 1996 through 1999, Ms. Doll served as the controller of National Directory Company. From 1993 through 1996 she served as the advisor to the secretary of finance for the Federated States of Micronesia. From 1987 through 1993 she served as the controller of Triquest Development Company. From 1983 through 1987 she served as a senior accountant at Deloitte & Touche, LLP. Ms. Doll received her Bachelor of Arts Degree in Economics with an emphasis in Accounting at the University of California Santa Barbara. Given Ms. Doll’s extensive accounting background and here work with a number of private and public companies, the Company believe Ms. Doll is well qualified to serve as its Interim Chief Financial Officer.
Bruce Stroever: Chairman of the Board of Directors
Mr. Stroever has forty years of product development and general management experience in the medical device and orthobiologics fields. Mr. Stroever joined MTF in late 1988 as General Manager and is currently the President and Chief Executive Officer of MTF. He has served MTF’s President since his appointment in 1992 and as Chief Executive Officer since 1996. Under Mr. Stroever’s leadership, MTF has grown to be the largest tissue bank in the world providing over 450,000 grafts per year. From 1971 to 1988, Mr. Stroever held several positions with Ethicon, Inc., a Johnson & Johnson, Inc. subsidiary. Mr. Stroever currently serves on the advisory boards for the Department of Bioengineering at UCLA and the New Jersey Organ and Tissue Sharing Network. He was elected to the Board of Governors of the American Association of Tissue Banks for a three year term in 1999 and subsequently in 2012. Mr. Stroever has served as the Chairman of Bone’s Board of Directors since 2012. Mr. Stroever received his B.E. in Mechanical/Chemical Engineering from Stevens Institute of Technology in 1972 and a Masters of Science in Bioengineering from Columbia University in 1977. Given Mr. Stroever’s forty years of experience in the medical device and orthobiologics fields, as well as, the roles he has held at Bone, the Company believes he is well qualified to serve as the Chairman of the Board of Directors.
Dr. Chia Soo: Director
Since 2009, Dr. Soo has served as a Professor in the Orthopedic Hospital Department of Orthopedics of UCLA. Dr. Soo and her colleagues have been studying the osteogenic potential of the Nell-1 protein for the last 13 years. In addition to currently being the PI of an NIH and a DOD translational grant to study the osteoinductive properties of Nell-1, Dr. Soo has served as PI for two NIH SBIR grants, as Co-PI on an RO1 grant, and managed two (2) University of California Discovery grants. Since 2011, Dr. Soo has served as a consultant to MTF, the world’s largest tissue bank for allograft product development. She also has consulted extensively on FDA issues related to allograft products, cGMP protein production, and FDA regulatory submissions. She is technically experienced with both large and small translational animal models of skeletal disease. Dr. Soo is certified by the American Board of Plastic Surgery and a Fellow of the American College of Surgeons. She has also served as a member of Bone’s Board of Directors since 2004. Given Dr. Soo’s background with grants, her studies of the Nell-1 protein and position on Bone’s Board of Directors, the Company thinks she is well qualified to serve as a Director of the Company.
William Coffin: Director
Founder and CEO of CCG, a national investor relations agency which during his leadership conducted business through offices in New York, Los Angeles, Beijing, Mr. Coffin was an investor relations counselor for over 25 years until his retirement in 2012. In this role, Mr. Coffin represented numerous publicly held and private companies, assisted in over 100 initial public offerings, counseled and participated in over 50 mergers and acquisitions, and worked with virtually every major investment banking firm in the country. Since 2004, Mr. Coffin has served as Chairman of the Board of the California Council on Economic Education, a nonprofit, nonpartisan organization that works towards implementing and increasing economic and financial literacy among California primary and secondary school students. Mr. Coffin is also an adjunct professor in the MBA program at Mount St. Mary’s college, a private liberal arts college in Los Angeles, where he teaches modern theories of corporate governance and corporate communications. Mr. Coffin received a B.A. in journalism from California State University, Los Angeles.
John Booth: Director
Mr. Booth has been CEO of Spineology Inc. since 2004 and has been a board member since its inception in 1998. Spineology is involved in the development and commercialization of minimally invasive spinal implants and access systems. Mr. Booth held various executive level positions at Phillips Plastics Corporation, most recently serving as CEO from June of 2001 to December 2002. Before serving as CEO of Phillips, he was CEO of Microvena Corporation, a cardiovascular device subsidiary of Phillips, from 1999 to 2001 and CEO of Phillips Origen Group Division from 1998 to 1999. Prior to Phillips, Mr. Booth was President and CEO of INCSTAR Corporation, a publicly held medical technology company involved in in-vitro diagnostics. He has held various positions in both financial and general management in the medical technology industry since 1981. Mr. Booth has also serve on the boards of directors of INCSTAR Corporation from 1994 to 1997, Microvena Corporation from 1998 to 2001, Phillips Plastics Corporation from 2000 to 2002, Imricor Medical Systems Inc. from 2007 to 2014, Spineology Inc. from 1998 to the present. Mr. Booth received a B.S. degree in accounting from Villanova University and an MBA from Seton Hall University.
| 44 |
Jimmy Delshad: Director
Mr. Delshad brings more than ten years of elected public service to the Company. From 2003 through 2011, Mr. Delshad served as Mayor and Councilmember of the City of Beverly Hills, California. In this role, Mr. Delshad was responsible for, among other things: the formulation of city policies and ordinances; the establishment and monitoring of a budget of approximately $500 million; and the management of more than 1000 city employees. Additionally, since his retirement as Mayor in 2011, Mr. Delshad has served as a Goodwill Ambassador for the City of Beverly Hills. Since 2012, Mr. Delshad has held the position of Chairman of Delshad Capital, which guides companies in technology, security, crowd-funding and marketing. During 2011 through 2012, Mr. Delshad was Vice Chairman at Pacific Capital Group where he evaluated and managed various projects, such as Smart City initiatives, fuel technology and software products. From 1978 through 2002, Mr. Delshad was founder and Chief Executive Officer of American International Business, Inc., a manufacturer of computer storage technologies with offices in Germany, London and Brussels. Mr. Delshad served on the board of directors of Evryx Corp from 2008 through 2010 and Dream Team Gaming from 2007 through 2009. Mr. Delshad has also served on the Boards of Directors of the Iranian American Jewish Federation from 2002 through the present, the World Affair Council from 2011 through 2012, Sheba Medical Center from 2003 through 2008, Maple Counseling Center from 2001 through 2003, Mount Sinai Mortuaries from 2001 through the present and Nessah Synagogue from 2001 through 2004. Mr. Delshad received his B.S. in Computer Science from California State University and completed additional post-graduate coursework at the University of Southern California.
Steve Warnecke: Director
Mr. Warnecke brings to the Company over thirty years of management experience in biotech, pharma, medical device, healthcare, software/telecom, travel/airline, construction/real estate and manufacturing/distribution. Since 2010 Mr. Warnecke has served as member of the board of directors of Evolutionary Genomics, Inc., unique biotechnology company that is changing how relevant gene/target discovery and validation is performed during the post-genomics era. From 2003 through 2008 and from 2011 through the present, Mr. Warnecke has held several positions with Children’s Hospital Colorado Foundation, including vice president, chief financial officer and chief operating officer. He currently leads all of their accounting, finance, gift processing and IT functions, and participates in Board/Executive Committee/Audit Committee/Investment Committee meetings. From 2012 through the present Mr. Warnecke has served as chairman of the board of directors of VETDC, Inc., a veterinary product development company that adapts innovative, underutilized human biomedical technologies for use in companion animals. Since 2013, Mr. Warnecke has served as a member of the board of directors of the University of Iowa Cardiovascular Research Center. Since 2004 he has also served as chairman of Children’s Partners Foundation. MR. Warnecke has also served as member of the board of directors of the Cystic Fibrosis Foundation since 2002. From 2003 through 2011, Mr. Warnecke has held various positions, including lead independent director, chairman of the audit committee, financial expert and member of the nominating and governance committee of Evolving Systems, Inc. In 2011, he also served as a member of the board of directors, chairman of the audit committee and financial expert for Emmaus Life Sciences. Mr. Warnecke also served as the chief financial officer of Targeted Medical Pharma, Inc. in 2011. From 2008 to 2010, Mr. Warnecke served as the chief financial officer and a member of the board of directors of Bacterin International, Inc. From 2005 through 2008 he served as a member of the board of directors and served as a member of the compensation committee of Boppy Company. From 2001 to 2002, he served as senior vice president of strategic planning at First Data/Western Union (NYSE-FDC). From 1999 to 2001 he served as the chief financial officer of Frontier Airlines (NASDAQ-FRNT). From 1982 to 1999 he served as the chief financial officer of Helm Corporation and affiliates. Mr. Warnecke earned a BBA with honors from the University of Iowa in 1979. Given Mr. Warnecke’s broad base of experience in serving as an officer and director of a variety of companies and his experience in serving on audit committees and compensation committees, the Company believes he is well suited to serve as a member of the Company’s Board of Directors.
Family Relationships
Drs. Ting and Soo are husband and wife.
Board of Directors and Corporate Governance
Our Board of Directors currently consists of six (6) members. On the Closing of the Merger, Don Hankey, the sole member of the Board of Directors of the Company, resigned, and simultaneously therewith, a new Board of Directors was appointed. Our Board consists of Bruce Stroever and Dr. Chia Soo, who were former directors of Bone Biologics, and William Coffin, John Booth, Jimmy Delshad and Steve Warnecke, who were appointed at the Closing of the Merger.
| 45 |
Board Independence and Committees
We are not currently listed on any national securities exchange or in an inter-dealer quotation system that has a requirement that the Board of Directors be independent. However, in evaluating the independence of our members and the composition of the committees of our Board of Directors, our Board utilizes the definition of “independence” as that term is defined by applicable listing standards of the Nasdaq Stock Market and SEC rules, including the rules relating to the independence standards of an audit committee and the non-employee director definition of Rule 16b-3 promulgated under the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”).
Our Board of Directors expects to continue to evaluate its independence standards and whether and to what extent the composition of the Board and its committees meets those standards. We ultimately intend to appoint such persons to our Board and committees of our Board as are expected to be required to meet the corporate governance requirements imposed by a national securities exchange. Therefore, we intend that a majority of our directors will be independent directors of which at least one director will qualify as an “audit committee financial expert,” within the meaning of Item 407(d)(5) of Regulation S-K, as promulgated by the SEC.
Additionally, our Board of Directors is expected to appoint an audit committee, governance committee and compensation committee and to adopt charters relative to each such committee.
Code of Ethics
We have not adopted a formal code of ethics within the meaning of Item 406 of Regulation S-K promulgated under the Securities Act, that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and that that establishes, among other things, procedures for handling actual or apparent conflicts of interest. Our Board of Directors intends to adopt such a formal code of ethics when it deems appropriate based on the size of our operations and personnel.
Indemnification Agreements
Our Board has approved a form of indemnification agreement for our directors and executive officers (“Indemnification Agreement”). Following Board approval, we entered into Indemnification Agreements with each of our current directors and executive officers.
The Indemnification Agreement provides for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The Indemnification Agreement also provides for the advancement of expenses in connection with a proceeding prior to a final, non-appealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to be entitled to indemnification by us. The Indemnification Agreement sets forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that will apply to any dispute between us and an indemnitee arising under the Indemnification Agreement.
The foregoing description is qualified in its entirety by reference to the form of Indemnification Agreement attached to this Report as Exhibit 10.17.
Effective as of September 19, 2014, our Board of Directors also approved the Former D&O Indemnification Agreement to be entered into between us, Don Hankey and Amir Heshmatpour. The Former D&O Indemnification Agreement requires that for a period of four (4) years from and after September 19, 2014, we will indemnify (including advancement of expenses) and hold harmless persons who were officers and directors of the Company (i) by reason of being an officer or director of the Company prior to the Merger, including through all transactions relating to the Merger, or (ii) is related to acts in connection with the Merger taken by the Former D&O Indemnified Persons, provided however, that the foregoing indemnity shall be excess of all any insurance coverage available to the Former D&O Indemnified Parties for any such loss. The accuracy of the Hankey Affidavit and Heshmatpour Affidavit in connection with the Former D&O Indemnification is a condition precedent to the foregoing indemnity (including advancement of expenses). The Company has no insurance coverage that would cover any claim asserted against the Company by any Former D&O Indemnified Person pursuant to this Former D&O Indemnification Agreement.
This description is qualified in its entirety by the Former D&O Indemnification Agreement filed as Exhibit 10.18 to this Current Report on Form 8-K and incorporated herein by reference.
Scientific Advisory Board
Mr. Gertzman has served as a member of Bone’s Scientific Advisory Board since 2005. He is the former Executive Vice President for Research and Development from MTF since 1996 and is currently a consultant to MTF in patent prosecution. He has been engaged in industrial product development of surgical implants for forty years. From 1964 to 1993, he was employed by Ethicon, Inc., a Johnson & Johnson Company as Director of Product Engineering Johnson & Johnson USA. From 1993 to 1996, he was employed by Xomed Medical Products and served in several positions of responsibility in research and product development, after his appointment as Vice President, Research and Development in 1993. Mr. Gertzman was appointed Vice President, Research and Development for MTF in 1996 and is currently employed in the development of new tissue forms and related processes. He holds over twenty-five (25) U.S. patents, with many more pending, both in the U.S. and internationally. He completed a Bachelors of Science at CCNY in 1960 and a Masters of Science degree in Chemistry from Boston University in 1963.
| 46 |
Dr. Shun’ichi Kuroda has served as a member of Bone’s Scientific Advisory Board since 2005. He taught as a professor at the Department of Bio-agricultural Sciences of Nagoya University since 2009 and has served as the Chairman of the Department since 2012. Dr. Kuroda has expertise is in recombinant protein engineering and manufacturing.
Dr. Jeffrey C. Wang has served as a member of Bone’s Scientific Advisory Board since 2005. Dr. Wang has been Chief of the Orthopaedic Spine Surgery Service since 1997, Fellowship Director of the UCLA Orthopaedic Spine Surgery Fellowship, and is Currently Professor of Orthopaedic Surgery and Neurosurgery. He is also the Vice Chair of Clinical Operations for the UCLA Department of Orthopaedic Surgery. He is Co-Director of the UCLA Spine Center. Dr. Wang’s research areas include the use of osteoinductive and osteoconductive materials for spinal fusion as well as novel gene therapy and minimally invasive techniques for spinal surgery. He obtained his undergraduate degree from Stanford University and his medical degree from the University of Pittsburgh. He then completed his Orthopaedic Surgery training at UCLA and his Spine Fellowship at Case Western Reserve University.
Dr. Xinli Zhang has served as a member of Bone’s Scientific Advisory Board since 2005. Since 2009, he has served as an Associate Professor at the UCLA School of Dentistry. Prior to joining UCLA, Dr. Zhang was Associate Professor in the Third Military Medical University in China from 1994 to 2000. Dr. Zhang combines his specialized training as a pathologist with a PhD in molecular biology. Dr. Zhang brings over twenty years of experience in medical and dental research in both China and the U.S. Dr. Zhang is an expert in developmental molecular biology and pathology of various bone and cartilaginous tissue related conditions.
Dr. Mark Spilker joined MTF in early 2009 and is currently managing the R&D, Project Management, Clinical Studies, Quality Assurance and Regulatory departments. Mark has been leading the continued development and expansion of MTF’s human allograft tissue portfolio. Under his leadership, the MTF team has developed new tissue forms including allograft bone designs for extremity surgery, allograft scaffolds for cartilage regeneration and a living allogeneic stem cell graft for orthopedic surgery. Over his career, Mark has held positions in Marketing, R&D and Program Management. Prior to MTF, Mark was formerly Vice President, R&D and Program Management for Integra NeuroSciences where he was responsible for development programs ranging from collagen-based scaffolds for bone and soft tissue regeneration to medical electronics and implantable fluid-handling shunts and valves. Prior to joining Integra he conducted his post-graduate studies at Massachusetts Institute of Technology, making significant contributions in the field of biomaterials technology and tissue engineering in neurosurgery. He received a B.S. degree in Mechanical Engineering from the University of Utah and an M.S. and Ph.D. in Mechanical Engineering from the Massachusetts Institute of Technology.
| 47 |
None of the directors, named executive officers or members acting in similar capacities of the Company received any compensation as that term is defined in Item 402(a)(2) of Regulation S-K for the years ending October 31, 2012 and 2013.
For the fiscal years ending December 31, 2012 and 2013, other than the amounts paid to William Jay Treat set forth below, none of the directors, named executive officers or members acting in similar capacities of Bone Biologics received any compensation as that term is defined in Item 402(a)(2) of Regulation S-K for the fiscal years ending December 31, 2012 and 2013.
| Name
and Principal Position | Year | Salary | Bonus
($) | Stock
Awards ($) | Option
Awards ($) | Non-Equity
Incentive Plan Compensation ($) | Deferred
Compensation ($) | All
Other Compensation ($) | Total
Compensation ($) | |||||||||||||||||||||||||||
| William Jay Treat | 2012 | $ | 15,000 | $ | 15,000 | |||||||||||||||||||||||||||||||
| President, Chief Technology Officer | 2013 | $ | 60,000 | $ | 60,000 | |||||||||||||||||||||||||||||||
Changes in Executive Compensation
The Company engaged a professional executive compensation expert that recommended to the Company that they institute a stock option plan. Accordingly, immediately following the Merger, our Company’s Board of Directors approved a compensation program for our named executive officers. Consistent with the size and nature of our Company, our executive compensation program is simple, consisting of a base salary, an annual performance-based cash award and an annual long-term equity award under our 2014 Stock Option Plan.
| ● | Base Salary: The Company’s base salaries are designed as a means to provide a fixed level of compensation in order to attract and retain talent. The base salaries of our named executive officers depend on their job responsibilities, the market rate of compensation paid by companies in our industry for similar positions, our financial position and the strength of our business. | |
| ● | Performance-Based Cash Awards: As part of the Company’s executive compensation program, the board intends to establish an annual performance-based cash award program for our executive officers and other key employees based upon individual performance and the Company’s performance. The award program will also be designed to reinforce the Company’s goals and then current strategic initiatives. The annual performance-based cash awards will be based on the achievement of Company and individual performance metrics established at the beginning of each fiscal year by the compensation committee and our Board of Directors. Following the end of each fiscal year, the compensation committee will be responsible for determining the bonus amount payable to the executive officer based on the achievement of the Company’s performance and the individual performance metrics established for such executive. | |
| ● | Long-Term Equity Awards: Our Board of Directors believes that equity ownership by our executive officers and key employees encourages them to create long-term value and aligns their interest with those of our stockholders. We intend to grant annual equity awards to our executive officers under our 2014 Stock Option Plan. Our Board of Directors adopted and approved the following 2014 Stock Option Plan and intends to submit it for approval by our stockholders. | |
| ● | 2014 Stock Option Plan: 2,642,898 shares of our common stock have been initially authorized and reserved for issuance under our 2014 Stock Plan as option awards. This reserve may be increased by the Board on January 1, 2015 and each subsequent anniversary through January 1, 2024 by up to the number of shares of stock equal to 5% of the number of shares of stock issued and outstanding on the immediately preceding December 31. Appropriate adjustments will be made in the number of authorized shares and other numerical limits in our 2014 Stock Option Plan and in outstanding awards to prevent dilution or enlargement of participants’ rights in the event of a stock split or other change in our capital structure. Shares subject to awards granted under our 2014 Stock Option Plan which expire, are repurchased or are cancelled or forfeited will again become available for issuance under our 2014 Stock Option Plan. The shares available will not be reduced by awards settled in cash. Shares withheld to satisfy tax withholding obligations will not again become available for grant. The gross number of shares issued upon the exercise of stock appreciation rights or options exercised by means of a net exercise or by tender of previously owned shares will be deducted from the shares available under our 2014 Stock Option Plan. | |
| ● | Awards may be granted under our 2014 Stock Option Plan to our employees, including officers, director or consultants, and our present or future affiliated entities. While we may grant incentive stock options only to employees, we may grant non-statutory stock options, stock appreciation rights, restricted stock purchase rights or bonuses, restricted stock units, performance shares, performance units and cash-based awards or other stock based awards to any eligible participant. | |
| ● | The 2014 Stock Option Plan will be administered by our compensation committee. Subject to the provisions of our 2014 Stock Option Plan, the compensation committee determines, in its discretion, the persons to whom, and the times at which, awards are granted, as well as the size, terms and conditions of each award. All awards are evidenced by a written agreement between us and the holder of the award. The compensation committee has the authority to construe and interpret the terms of our 2014 Stock Option Plan and awards granted under our 2014 Stock Option Plan. |
| 48 |
Our Board of Directors approved the following compensation for our named executives officers:
Michael Schuler, Interim Chief Executive Officer:
Base Salary: The compensation provided to MTF for Mr. Schuler’s monthly services will be $15,000, pro-rated based upon the actual amount of time Mr. Schuler provides services to the Company.
Warrants: For the services provide by Mr. Schuler, MTF shall receive warrants with a 10 year term to purchase 50,000 shares of the Company’s common stock at an exercise price of $1.00 per share upon completion of Consultant’s first year of service as the Company’s Chief Executive Officer. MTF shall also receive $50,000 worth of common stock upon completion of each year of service Mr. Schuler provides as the Company’s Chief Executive Officer. Such issuances of common stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The common stock will be valued at the average of the trading price for shares of common stock over the 10 day period prior to the issuance.
The board of directors believes that the compensation paid to MTF for Mr. Schuler’s services is in line with the Company’s goal of rapidly increasing its profitability and helps to ensure that MTF’s and Mr. Schuler’s interests are aligned with those of the Company’s stockholders.
Catherine Doll, Interim Chief Financial Officer:
Base Salary: The compensation provided to Gilson Group for the services provided by Ms. Doll shall be $10,000 per month for the first 66 hours of services provided by Ms. Doll each month. Any services provided in excess of 66 hours in a given month will be paid at a rate of $150/hour.
Warrants: For the services provided by Ms. Doll, Gilson Group shall receive 1 warrant for Company common stock for each dollar that is paid by Company for services provided by Ms. Doll. The first issuance of such warrants will be made at the completion of the initial 90 day term of the agreement. Any additional warrants to be provided for dollars paid for services rendered after the initial 90 day term of the agreement will be paid at the end of each 30 day period thereafter.
The board of directors believes that the compensation provided to Gilson Group for the services provided by Ms. Doll is in line with the Company’s goal of rapidly increasing its profitability and helps to ensure that Gilson Group’s and Ms. Doll’s interests are aligned with those of the Company’s stockholders.
William Jay Treat, President and Chief Technology Officer:
Base Salary: Mr. Treat’s base salary will be $300,000.
Bonus: During each calendar year beginning in 2014, Mr. Treat shall be eligible to earn an annual target bonus of thirty-five percent (35%) of his base salary as in-effect for the applicable calendar year, subject to the achievement of personal and corporate objectives or milestones to be established by the board of directors, or any compensation committee thereof, (after considering any input or recommendations from Mr. Treat) within sixty (60) days following the beginning of each calendar year during Mr. Treat’s employment. In order to earn the annual bonus under this provision, the applicable objectives must be achieved and Mr. Treat must be employed by Company at the time the annual bonus is distributed by Company. The annual bonus, if any, shall be paid on or before March 15th of the calendar year following the year in which it is considered earned. The actual annual bonus paid may be more or less than thirty-five percent (35%) of Mr. Treat’s base salary.
| 49 |
Stock Options: Subject to the approval of the Board of Directors, Mr. Treat will be granted an option to purchase 2.5% of the Company’s fully diluted shares of common stock outstanding as of the date of closing of the Merger. The option will be granted under Company’s stock plan and related stock option documents. The Option is intended to be an “incentive stock option” (within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended ) to the greatest extent permitted under the code. The option will have an exercise price per share equal to the fair market value of one share of Company’s common stock on the date of grant, as determined by the board of directors. As a condition of receipt of the option, Mr. Treat will be required to sign Company’s standard form of stock option agreement and the option will be subject to the terms and conditions of the plan, the option agreement and his employment agreement. The option will vest over a two-year period from the effective date subject to Mr. Treat’s continued Service (as defined in the plan), with 33.33% of the shares subject to the option becoming vested and exercisable on the date that Mr. Treat’s employment agreement is executed, 33.33% of the shares subject to the option becoming vested and exercisable on the date that is twelve (12) months after the effective date, and 33.34% of the shares subject to the option vesting and becoming exercisable on the date that is twenty four (24) months after the effective date; provided, however, that all unvested shares subject to the option (and any additional equity awards hereafter issued by Company to Mr. Treat pursuant to the plan) shall fully vest and be exercisable if Mr. Treat’s service ceases as a result of a “qualifying termination” occurring on or within twelve (12) months after a “change in control.”
The board of directors believes that Mr. Treat’s compensation is in line with the Company’s goal of rapidly increasing its profitability and helps to ensure that Mr. Treat’s interests are aligned with those of the Company’s stockholders. The board of directors also believes that the compensation awarded to Mr. Treat provides appropriate incentives and provides for rewards appropriate for the level of difficulty in achieving the applicable metrics.
Potential Payments upon Termination of Change in Control
There were no payments or benefits due to any of the Company’s named executive officers upon termination of their employment or a change in control of the Company as of the end of its fiscal year ending October 31, 2013.
There were no payments or benefits due to any of Bone Biologic’s named executive officers upon termination of their employment or a change in control of Bone Biologics at the end of its fiscal year ending December 31, 2013.
Changes to Potential Payments upon Termination of Change in Control
William Jay Treat: Pursuant to the employment agreement signed with Mr. Treat immediately following the Merger, if the Company terminates Mr. Treat’s employment for other than Cause, or if Mr. Treat terminates his employment due to a breach of his employment agreement by the Company, or if Mr. Treat terminates his employment agreement for Good Reason, Mr. Treat will receive the standard entitlements and shall be entitled to receive reimbursement of any business expenses, to the extent not previously reimbursed, in accordance with his employment agreement. In addition, Mr. Treat will receive (a) a severance payment in an amount which is equivalent to the greater of the remaining number of months left in the initial term of his employment or twelve (12) months of his base salary then in effect on the date of termination, payable in equal installments (but no less frequently than once per calendar month) for a duration equal to the greater of the remaining number of months left in the Initial Term or twelve (12) months, in accordance with Company’s regular payroll cycle, beginning on the first payroll date following the date on which the general release referenced below has become effective and (b) payment (or reimbursement) of monthly premiums for Mr. Treat and Mr. Treat’s dependents’ group health care coverage continuation pursuant to the Consolidated Omnibus Budget Reconciliation Act of 1986, for the severance period, provided Mr. Treat elects to continue and remains eligible for such benefits and does not become eligible for health coverage through another employer during the severance period. Mr. Treat will only receive the severance package and other severance benefits and payments described if Mr. Treat: (i) complies with all surviving provisions of his employment agreement; and (ii) executes a separation agreement and release of claims agreement and such release has become effective in accordance with its terms prior to the 60th day following the termination date. Except for any terms and conditions of Mr. Treat’s employment agreement that by their terms survive termination of Mr. Treat’s employment, all other Company obligations to Mr. Treat pursuant to Mr. Treat’s employment agreement will become automatically terminated and completely extinguished.
For purposes of Mr. Treat’s payments upon termination, “cause” and “good reason” shall have the following meanings:
“Cause” means (a) acts or omissions constituting gross negligence, recklessness or willful misconduct on the part of Mr. Treat with respect to Mr. Treat’s obligations or otherwise relating to the business of Company; (b) any acts or conduct by Mr. Treat that are materially adverse to Company’s interests; (c) Mr. Treat’s material breach of this Agreement; (d) Mr. Treat’s material breach of Company’s employee proprietary information and inventions agreement; (e) Mr. Treat’s conviction or entry of a plea of nolo contendere for fraud, misappropriation or embezzlement, or any felony or crime of moral turpitude or that otherwise materially negatively impacts Mr. Treat’s ability to effectively perform Mr. Treat’s duties hereunder; (f) Mr. Treat’s willful neglect of duties as determined in the good faith discretion of the board of directors (provided that poor performance and/or subpar results by themselves do not constitute “cause”); or (g) the winding down of Company’s business and/or dissolution or liquidation of Company (other than in connection with a change in control). In the event of termination of Mr. Treat’s employment based on clauses (a), (b) or (f) above, Mr. Treat will have fifteen (15) days following receipt of notice from Company to cure the issue, if curable.
| 50 |
“Good Reason” means that any one or more of the following events have occurred without Mr. Treat’s express prior written consent: (i) a material adverse change in Mr. Treat’s authority, duties and/or responsibilities such that Mr. Treat’s authority, duties and/or responsibilities are no longer commensurate with Mr. Treat being Company’s President or most senior technology officer; (ii) the relocation of the primary workplace to a location that increases Mr. Treat’s daily commute by more than thirty (30) miles from its location specified in his employment agreement; (iii) any material breach by Company of any material term of Mr. Treat’s employment agreement; or (iv) any material reduction by Company (or its successor) of (A) Mr. Treat’s base salary or (B) Mr. Treat’s target bonus, unless any such reduction is made as part of, and is generally consistent with, a general reduction of senior executive base salaries or target bonuses, respectively, in which case such a reduction shall not constitute Good Reason. In order to resign his employment for Good Reason, Mr. Treat must within 60 days of Mr. Treat’s awareness of the applicable Good Reason event(s) provide Company with written notice informing Company about Mr. Treat’s intention to resign his employment for Good Reason unless such event(s) is cured or remedied by Company. Company will have 30 days after its receipt of such notice to cure or remedy the good reason event(s). If Company does not timely cure or remedy the good reason event(s), then Mr. Treat can resign Mr. Treat’s employment for good reason at any time within 30 days following the expiration of the 30 day cure/remedy period.
Employment and Consulting Agreements for Executives
The Company has entered into employment agreements with the following executives: William Jay Treat, MTF (for the services of Michael Schuler) and Gilson Group (for the services of Catherine Doll).
MTF (for the services of Michael Schuler): Immediately following the Merger, the Company entered into a consulting agreement with MTF, which has agreed to provide the services of Mr. Schuler to the Company as a contractor. Pursuant to the agreement, Mr. Schuler will serve as the Company’s Interim Chief Executive Officer for a period of 6 months. The agreement shall automatically renew for successive three (3) month periods unless either party provides written notice to the other party at least 10 days in advance of the renewal term of its decision not to renew the term. The agreement is intended to be temporary in nature, and will cease once the Company retains a permanent Chief Executive Officer. There are no payments due to MTF or Mr. Schuler with respect to any change in control of the Company or termination of the consulting agreement. Please see our discussion of Changes in Executive Compensation for further information regarding the compensation to be paid to MTF for Mr. Schuler’s services pursuant to the consulting agreement with the Company.
Gilson Group (for the services of Catherine Doll): Immediately following the Merger, the Company entered into an consulting agreement with Gilson Group, which has agreed to provide the services of Ms. Doll to the Company as a contractor. Pursuant to the agreement, Ms. Doll will serve as the Company’s Interim Chief Financial Officer for a period of 90 days. The agreement shall automatically renew for successive one (1) month periods unless either party provides written notice to the other party at least 10 days in advance of the renewal term of its decision not to renew the term. The agreement is intended to be temporary in nature, and will cease once the Company retains a permanent Chief Executive Officer. There are no payments due to Gilson Group or Ms. Doll with respect to any change in control of the Company or termination of the consulting agreement. Please see our discussion of Changes in Executive Compensation for further information regarding Ms. Doll’s compensation pursuant to his employment agreement with the Company.
William J. Treat: Immediately following the Merger, the Company entered into an employment agreement with Mr. Treat. Pursuant to the agreement, Mr. Treat will serve as the Company’s President and Chief Technology Officer for an initial term of two years. The agreement shall automatically renew for successive one (1) year periods unless either party provides written notice to the other party at least thirty (30) days in advance of the renewal term of its decision not to renew the agreement. Please see our discussion of Changes in Executive Compensation for further information regarding Mr. Treat’s compensation pursuant to his employment agreement with the Company. Please see our discussion of Potential Payments Upon Termination or Change in Control for information regarding any termination payments that may become due to Mr. Treat pursuant to his employment agreement with the Company.
Grants of Plan-Based Awards
The Company did not grant any equity awards to our named executive officers during the fiscal year ended October 31, 2013. Additionally, Bone Biologics did not grant any equity awards to its named executive officers during its fiscal year ended December 31, 2013.
Outstanding Equity Awards at Fiscal Year End
The Company had no outstanding equity awards owed to our named executive officers that were outstanding as of the end of our fiscal year on October 31, 2013. Additionally, Bone Biologics had no outstanding equity awards owed to its named executive officers that were outstanding as of the end of its fiscal year on December 31, 2013.
Director Compensation
No compensation was paid to any of the Company’s directors for the fiscal years ending October 31, 2012 and October 31, 2013.
No compensation was paid to any directors of Bone Biologic’s for the fiscal years ended December 31, 2012 and December 31, 2013.
| 51 |
Changes in Director Compensation
Immediately following the Merger, the Board of Directors of the Company entered into employment agreements with and agreed to the following compensation for each member of the Company’s Board of Directors.
Bruce Stroever: Pursuant to a consulting agreement by and between the Company and MTF, MTF has agreed to provide the services of Mr. Stroever to serve as the chairman of the Company’s board of directors. MTF has agreed to provide Mr. Stroever’s services as chairman of the board of directors for a one year term. For the services being provided, MTF shall receive an annual payment of $25,000, paid quarterly. In addition, MTF shall receive warrants equal to 50,000 shares of the Company’s $0.001 par value per share common stock at an exercise price of $1.00 per share with a term of 10 years, upon completion of the year of service by Mr. Stroever as chairman of the Company’s board of directors.
Dr. Chia Soo: Dr. Soo shall serve as a director of the Company for a one year term. Dr. Soo shall receive annual compensation of $25,000, paid quarterly, during her tenure as a board member. In addition, Dr. Soo shall receive options equal to 50,000 shares of the Company’s $0.001 par value per share common stock at an exercise price of $1.00 per share, with a term of 10 years, upon completion of the year of service as a member of the board of directors.
William Coffin: Mr. Coffin shall serve as a director of the Company and chairman of the corporate governance committee for a one year term. Mr. Coffin shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. Mr. Coffin shall also receive $5,000 as annual compensation for his service as the chairman of the corporate governance committee. In addition, Mr. Coffin shall receive options equal to 50,000 shares of the Company’s $0.001 par value per share common stock at an exercise price of $1.00 per share with a term of 10 years, upon completion of the year of service as a member of the board of directors.
John Booth: Mr. Booth shall serve as a director of the Company and chairman of the compensation committee for a one year term. Mr. Booth shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. Mr. Booth shall also receive $5,000 as annual compensation for his service as the chairman of the compensation committee. In addition, Mr. Booth shall receive options equal to 50,000 shares of the Company’s $0.001 par value per share common stock at an exercise price of $1.00 per share with a term of 10 years, upon completion of the year of service as a member of the board of directors.
Jimmy Delshad: Mr. Delshad shall serve as a director of the Company for a one year term. Mr. Delshad shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. In addition, Mr. Delshad shall receive options equal to 50,000 shares of the Company’s $0.001 par value per share common stock at an exercise price of $1.00 per share with a term of 10 years, upon completion of the year of service as a member of the board of directors.
Steve Warnecke: Mr. Warnecke shall serve as a director of the Company and chairman of the audit committee for a one year term. Mr. Warnecke shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. Mr. Warnecke shall also receive $5,000 as annual compensation for his service as the chairman of the audit committee. In addition, Mr. Warnecke shall receive options equal to 50,000 shares of the Company’s $0.001 par value per share common stock at an exercise price of $1.00 per share with a term of 10 years, upon completion of the year of service as a member of the board of directors.
| 52 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Except as disclosed below, none of the following persons has any direct or indirect material interest in any transaction to which we are a party since our incorporation or in any proposed transaction to which we are proposed to be a party:
| ● | Any of our directors or officers; | |
| ● | Any proposed nominee for election as our director; | |
| ● | Any person who beneficially owns, directly or indirectly, shares carrying more than 5% of the voting rights attached to our Common Stock; or | |
| ● | Any relative or spouse of any of the foregoing persons, or any relative of such spouse, who has the same house as such person or who is a director or officer of any parent or subsidiary of our Company. |
Review, Approval or Ratification of Transactions with Related Persons
Due to the small size of our Company, we do not at this time have a formal written policy regarding the review of related party transactions, and rely on our full Board of Directors to review, approve or ratify such transactions and identify and prevent conflicts of interest. Our Board of Directors reviews any such transaction in light of the particular affiliation and interest of any involved director, officer or other employee or stockholder and, if applicable, any such person’s affiliates or immediate family members. Management aims to present transactions to our Board of Directors for approval before they are entered into or, if that is not possible, for ratification after the transaction has occurred. If our Board of Directors finds that a conflict of interest exists, then it will determine the appropriate action or remedial action, if any. Our Board of Directors approves or ratifies a transaction if it determines that the transaction is consistent with our best interests and the best interest of our stockholders.
Director Independence
In connection with the closing of the Merger, AFH Advisory selected three of the members of our Board of Directors and the remaining three directors were selected by MTF; all six members were thereafter added to our Board of Directors in accordance with our Bylaws. Our Board of Directors thereafter undertook a review of the composition of our Board of Directors and the independence of each director. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, our Board of Directors has determined that William Coffin, John Booth, Jimmy Delshad, Dr. Chia Soo and Steve Warnecke (the “Independent Directors”) would qualify as “independent” as that term is defined by NASDAQ Listing Rule 5605(a)(2). Further, although we do not presently have separately standing audit or governance committees of our Board of Directors, our Board of Directors has determined that each of the independent directors would qualify as “independent” under NASDAQ Listing Rules applicable to such board committees. Bruce Strover would not qualify as “independent” under applicable NASDAQ Listing Rules applicable to the Board of Directors generally or to separately designated board committees because he is the Chief Executive Officer of MTF, a significant shareholder of the Company and an entity to whom the Company continues to owe obligations to pursuant to notes outstanding to MTF. In making such determinations, our Board of Directors considered the relationships that each of our nonemployee directors has with the Company and all other facts and circumstances deemed relevant in determining independence, including the beneficial ownership of our capital stock by each non-employee director.
Subject to some exceptions, NASDAQ Listing Rule 5605(a)(2) provides that a director will only qualify as an “independent director” if, in the opinion of our Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, and that a director cannot be an “independent director” if (a) the director is, or in the past three years has been, an employee of ours; (b) a member of the director’s immediate family is, or in the past three years has been, an executive officer of ours; (c) the director or a member of the director’s immediate family has received more than $120,000 per year in direct compensation from us within the preceding three years, other than for service as a director or benefits under a tax-qualified retirement plan or non-discretionary compensation (or, for a family member, as a non-executive employee); (d) the director or a member of the director’s immediate family is a current partner of our independent public accounting firm, or has worked for such firm in any capacity on our audit at any time during the past three years; (e) the director or a member of the director’s immediate family is, or in the past three years has been, employed as an executive officer of a company where one of our executive officers serves on the compensation committee; or (f) the director or a member of the director’s immediate family is an executive officer, partner or controlling shareholder of a company that makes payments to, or receives payments from, us in an amount which, in any twelve-month period during our past three fiscal years, exceeds the greater of 5% of the recipient’s consolidated gross revenues for that year or $200,000 (except for payments arising solely from investments in our securities or payments under non-discretionary charitable contribution matching programs). Additionally, in order to be considered an independent member of an audit committee under Rule 10A-3 of the Exchange Act, a member of an audit committee may not, other than in his or her capacity as a member of the audit committee, the Board of Directors, or any other committee of the Board of Directors, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the applicable company or any of its subsidiaries or otherwise be an affiliated person of the applicable company or any of its subsidiaries.
| 53 |
Authorized Capital Stock
Our authorized capital stock consists of 100,000,000 shares of Common Stock at a par value of $0.001 per share and 20,000,000 shares of preferred stock at a par value of $0.001 per shares (“Preferred Stock”). As of September 19, 2014, 17,939,933 shares of our Common Stock and no shares of our Preferred Stock were issued and outstanding.
Common Stock
All outstanding shares of Common Stock are of the same class and have equal rights and attributes. The holders of Common Stock are entitled to one vote per share on all matters submitted to a vote of stockholders of the Company. All stockholders are entitled to share equally in dividends, if any, as may be declared from time to time by the Board of Directors out of funds legally available. In the event of liquidation, the holders of Common Stock are entitled to share ratably in all assets remaining after payment of all liabilities. The stockholders do not have cumulative or preemptive rights.
Preferred Stock
Our Amended and Restated Certificate of Incorporation provides that we are authorized to issue up to 20,000,000 shares of Preferred Stock. Our Board of Directors has the authority, without further action by the stockholders, to issue from time to time the Preferred Stock in one or more series for such consideration and with such relative rights, privileges, preferences and restrictions that the Board of Directors may determine. The preferences, powers, rights and restrictions of different series of Preferred Stock may differ with respect to dividend rates, amounts payable on liquidation, voting rights, conversion rights, redemption provisions, sinking fund provisions and purchase funds and other matters. The issuance of Preferred Stock could adversely affect the voting power or other rights of the holders of Common Stock.
Registration Rights Agreement
Effective as of September 19, 2014, the Company entered into a Registration Rights Agreement with MTF, AFH Advisory and Hanky Investment Company, L.P. (“HIC”), each of which have certain demand registration rights and unlimited piggyback registration rights for the Company’s shares under the Registration Rights Agreement and subject to an agreed lock up period. Pursuant to the Registration Rights Agreement, within thirty (30) days hereof, the Company will seek registration under the Securities Act of all or part of the registrable shares of MTF, AFH Advisory and HIC. Within five (5) days hereof, the Company will provide written notice of such request to all other holders of registrable securities and will include in such registration all registrable shares with respect to which the Company has received written requests for inclusion within twenty-five (25) days after delivery of the Company’s notice. The Company has agreed to pay all registration expenses relating to up to two long-form registrations or short-form registrations for each of MTF, AFH Advisory and HIC.
Whenever the Company proposes to register any of its securities under the Securities Act (other than pursuant to a demand registration under the Registration Rights Agreement) and the registration form to be used may be used for the registration of any registrable shares, the Company will give prompt written notice to all holders of the registrable shares of its intention to effect such a registration and will include in such registration all registrable shares (in accordance with the priorities set forth in the Registration Rights Agreement) with respect to which the Company has received written requests for inclusion within fifteen (15) days after the delivery of the Company’s notice. Pursuant to Registration Rights Agreement, holders of registrable shares and the Company agree not to effect any public sale or distribution of equity securities of the Company, or any securities convertible into or exchangeable or exercisable for such securities, during the six (6) months following, the effective date of the Merger Agreement.
Bridge Warrants
In April 2013, the Company’s Board approved the Company to borrow up to an aggregate principal amount of $300,000 pursuant to the sale and issuance of convertible promissory notes and warrants to purchase common stock of the Company. Each Bridge Note accrues interest at a rate of 12% per year and is payable per quarter. A Bridge Warrant to purchase the Company’s common stock equal to 50% of the original principal amount at $1.00 per share was issued to each Bridge Financing participant. Principal and unpaid accrued interest may be converted into equity securities issued in the Private Placement at a price equal to the price paid by investors in the Private Placement.
On April 29, 2013, June 5, 2013 and October 2013, Bone borrowed $100,000 from MTF and $100,000 and $150,000 from Orthofix, respectively, under the Bridge Financing. In August 2013, AFH Advisory purchased $50,000 of the Bridge Financing
| 54 |
Orthofix Subsequent Financing
On July 1, 2014, (i) Orthofix purchased $500,000 worth of Bone Biologics Common Stock or the Subsequent Orthofix Shares; (ii) was issued the Subsequent Orthofix Convertible Promissory Notes in the principal amount of $500,000 and exercisable for $666,666 worth of Bone Biologics Common Stock at $0.75 per share; and (iii) was issued the Subsequent Orthofix Warrants which were exercisable for 333,334 shares of Bone Biologics Common Stock at an exercise price per share of $1.50 (the “Orthofix Subsequent Financing”). Upon subscribing for the Subsequent Orthofix Shares, the Subsequent Orthofix Convertible Promissory Notes converted into a combined total of $666,666 worth of shares of Bone Biologics Common Stock in accordance with the terms of the Subsequent Orthofix Convertible Promissory Notes. The Subsequent Orthofix Warrants converted into warrants of the Company with substantially identical terms upon consummation of the Merger.
Extra Warrants
At the closing of the Subsequent Orthofix Shares and Notes, AFH Advisory was entitled to receive the Extra Warrants. AFH Advisory has normal and customary piggyback registration rights with respect to the shares of Common Stock issuable upon exercise of the Extra Warrants.
Agent Warrants
Forefront or its designees will receive the Agent Warrant. Such Agent Warrant will be issued at the closing of the Private Placement and shall provide, among other things, that the Agent Warrant shall: (i) be exercisable at the price of the securities (or the exercise price of the securities) issued to the investors in the offering, (ii) expire five (5) years from the date of issuance, (iii) include customary registration rights, including the registration rights provided to the Investors, (iv) contain provisions for cashless exercise and (v) include such other terms that are normal and customary for warrants of this type. In addition, Forefront or its designees will receive and Advisory Warrant equal to 2.0% of the Company’s post-merger and financing fully diluted shares outstanding upon the closing of $2.5 million of investors on which Forefront is eligible to receive compensation. Forefront was issued a warrant to purchase 46,667 shares of Common Stock at $1.00 per share upon completion of the Orthofix Subsequent Financing.
MTF Short Term 2014 Loan
On September 15, 2014, Bone and MTF entered into a loan agreement and accompanying promissory note to fund the continued operations of Bone prior to the Merger. Pursuant to the MTF Short Term 2014 Loan, MTF has agreed to advance an initial $250,000 to Bone and, at Bone's request and subject to the terms and conditions of the MTF Short Term 2014 Loan, to advance up to an additional $250,000 to Bone. The MTF Short Term 2014 Loan has an interest rate of eight and one-half percent (8.5%) accruing annually. The MTF Short Term 2014 Loan matures on the earlier to occur of (i) the date on which at least $1 million is loaned to or invested in the Company and (ii) December 31, 2014. In further consideration of the MTF 2014 Loan, Bone granted to MTF 625,000 warrants at a strike price of $1.62. The MTF 2014 Loan was assigned to the Company on September 19, 2014.
Anti-Takeover Effects of Provisions of Delaware State Law
Anti-takeover provisions in our Amended and Restated Certificate of Incorporation and Delaware law could make an acquisition more difficult and could prevent attempts by our stockholders to remove or replace current management.
Anti-takeover provisions of Delaware law and in our Amended and Restated Certificate of Incorporation and our Bylaws may discourage, delay or prevent a change in control of our company, even if a change in control would be beneficial to our stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our Board of Directors. In particular, under our Amended and Restated Certificate of Incorporation our Board of Directors may issue up to 20,000,000 shares of Preferred Stock with rights and privileges that might be senior to our Common Stock, without the consent of the holders of our Common Stock. Moreover, without any further vote or action on the part of the stockholders, the Board of Directors would have the authority to determine the price, rights, preferences, privileges, and restrictions of the Preferred Stock. This preferred stock, if it is ever issued, may have preference over, and harm the rights of, the holders of Common Stock. Although the issuance of this Preferred stock would provide us with flexibility in connection with possible acquisitions and other corporate purposes, this issuance may make it more difficult for a third party to acquire a majority of our outstanding voting stock. Similarly, our authorized but unissued Common Stock is available for future issuance without stockholder approval.
| 55 |
LIMITATIONS ON TRANSFER OF SHARES
The securities offered in the Private Placement have not been registered with the SEC pursuant to the Securities Act; however, they will be deemed to be exempt from such registration pursuant to Regulation D, Rule 506 of the Securities Act. Even so, the securities are subject to a restriction on re-sale and will be marked as such on the face of the certificate. In addition, there are limits on the resale of the securities by virtue of their corporate issuance. Accordingly, an investment in the securities offered in the Private Placement should be considered highly illiquid.
Changes in Control
We are not aware of any agreement or a party to arrangements, including any pledge by any person of our securities, the operation of which may at a subsequent date result in a change of control.
| 56 |
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Prior to September 19, 2014, our Common Stock has not been available for trading on the over-the-counter market since the Common Stock had never been traded.
Trades in our Common Stock may be subject to Rule 15g-9 of the Exchange Act, which imposes requirements on broker/dealers who sell securities subject to the rule to persons other than established customers and accredited investors. For transactions covered by the rule, broker/dealers must make a special suitability determination for purchasers of the securities and receive the purchaser’s written agreement to the transaction before the sale.
The SEC also has rules that regulate broker/dealer practices in connection with transactions in “penny stocks.” Penny stocks generally are equity securities with a price of less than $5.00 (other than securities listed on certain national exchanges, provided that the current price and volume information with respect to transactions in that security is provided by the applicable exchange or system). The penny stock rules require a broker/dealer, before effecting a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document prepared by the SEC that provides information about penny stocks and the nature and level of risks in the penny stock market. The broker/dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker/dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker/dealer and salesperson compensation information, must be given to the customer orally or in writing before effecting the transaction, and must be given to the customer in writing before or with the customer’s confirmation. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for shares of our Common Stock. As a result of these rules, investors may find it difficult to sell their shares.
Holders
As of September 3, 2014, there are approximately 25 record holders of 17,939,933 shares of Common Stock. As of the date of this filing, 2,581,043 shares of Common Stock are issuable upon the exercise of outstanding warrants and options, 3,659,328 shares of Common Stock are issuable upon exercise of the conversion features associated with our convertible promissory notes and 2,444,696 shares are reserved for future issuance to management, directors and consultants. The shares issued in connection with the Transactions, including the Common Stock issued to the former Bone Biologics stockholders and investors in the Private Placement, are “restricted securities,” which may be sold or otherwise transferred only if such shares are first registered under the Securities Act or are exempt from the registration requirements. As discussed elsewhere in this Current Report, we have agreed to file a registration statement within 90 days of the final Effective Time, to register the shares of the Common Stock and shares of Common Stock issuable upon exercise of the shares of Common Stock issuable upon exercise of the New Bridge Warrants.
Dividend Policy
We have never declared or paid dividends. We do not intend to pay cash dividends on our Common Stock for the foreseeable future, but currently intend to retain any future earnings to fund the development and growth of our business. The payment of dividends if any, on our Common Stock will rest solely within the discretion of our Board of Directors and will depend, among other things, upon our earnings, capital requirements, financial condition, and other relevant factors.
| 57 |
In the normal course of our business, Bone may periodically become subject to various lawsuits. However, there are currently no legal actions pending against us or, to our knowledge, are any such proceedings contemplated.
On January 24, 2007, Bone entered into a Biopharmaceutic Services Agreement with Cytovance, Inc. to provide certain services including the production of NELL protein in mammallian cells. In January 2008, Bone terminated the agreement based upon Cytovance’s alleged breach of contract. On July 31, 2008, Cytovance commenced legal action in the District Court of Oklahoma County State of Oklahoma against Bone for breach of contract, but the action was subsequently dismissed as the parties had initially agreed contractually to resolve all disputes through mediation. Bone alleges that, as a result of Cytovance’s breach of contract, the company is owed more than $150,000 for damages. Bone has attempted to amicably resolve this matter with a settlement offer made on April 15, 2009 but the matter has remained unresolved without further communication.
| 58 |
RECENT SALES OF UNREGISTERED SECURITIES
Sales by Bone Biologics
From January 2008 through December 2010, Bone Biologics sold unsecured promissory and convertible promissory notes to MTF. The notes included a (i) convertible promissory note dated January 18, 2008 in the face amount of $1,107,000 (the “2008 January Convertible Note”), (ii) promissory note dated as of November 4, 2008 in the face amount of $250,000 (the “2008 November Promissory Note”), (iii) promissory note dated as of March 17, 2009 in the face amount of $400,000 (the “2009 March Promissory Note”), (iv) promissory note dated as of August 24, 2009 in the face amount of $16,420 (the “2009 August Promissory Note”) and (v) promissory note dated as of September 30, 2009 in the face amount of $445,400, which was subsequently amended to, among other things, increase the maximum face amount to $2,090,000 (the “2009 September Promissory Note” and collectively with the 2008 November Promissory Note, the 2009 March Promissory Note and the 2009 August Promissory Note, the “MTF 2008 and 2009 Promissory Notes”).
Interest accrued at various rates of Prime plus 1.5% to 8% and Libor plus 8% to 12%. Under the terms of certain of the notes, unpaid principal and accrued interest was convertible into shares of Bone Biologics Series B Preferred Stock. In connection with the Security Agreement issued with the March 2009 Promissory Note, Bone Biologics also issued MTF a warrant to purchase 118,383 shares of common stock at an exercise price of $0.44. In July 2013, all notes held by MTF were amended to extend the maturity date to March 31, 2014 and amended again on April 1, 2014 to extend the maturity date to March 31, 2015.
On September 19, 2014, the MTF 2008 and 2009 Promissory Notes and any related loan agreements, credit agreements, guarantee agreements or other agreements related to the MTF 2008 and 2009 Promissory Notes were cancelled and Bone Biologics issued MTF a convertible promissory note in the face amount of $3,659,328 (the “New MTF Convertible Note”). Pursuant to the terms of the New MTF Convertible Note, 50% of all principal and accrued and unpaid interest due under the New MTF Convertible Note will be converted into common stock of the Company upon the closing of the PIPE. The remainder of the New MTF Convertible Note, including all accrued and unpaid interest, will be converted upon consummation of the Initial Public Offering.
Upon consummation of the merger, the 2008 January Convertible Note will be converted into 1,533,356 shares of common stock of the Company. Upon consummation of the merger, MTF also converted all their outstanding Series A and B Preferred Stock, 5,829,438 shares, into common stock.
In April 2013 and June 2013, Bone Biologics sold convertible promissory notes of $100,000 to MTF and $100,000 to Orthofix, Inc. under the Bridge Financing, and in October 2013 Bone Biologics sold an additional promissory note of $150,000 to Orthofix, Inc. The convertible promissory notes were issued with a one year term and accrued interest at a rate of 12% per year and payable quarterly. A warrant to purchase shares of Bone Biologics’ common stock equal to 50% of the original principal amount divided by $1.00 was issued to the holders of the Bridge Notes. Principal and unpaid accrued interest may be converted into equity securities issued in Bone Biologics’ next equity financing in an aggregate amount of at least $2.5 million at a price equal to the price paid by investors in the next equity financing or in the event Bone consummates its first underwritten public offering. AFH Advisory purchased $50,000 of the Bridge Notes and Bridge Warrants which was contingent upon liquidation of the securities transferred to Bone Biologics by AFH Advisory, and subject to adjustment, as described in that certain Letter Agreement, dated September 26, 2013, by and between Amir F. Heshmatpour and Bone Biologics. In June 2014, the note held by MTF under the April Bridge Financing was amended to extend the maturity date to October 14, 2014. Upon consummation of the merger, the bridge notes converted into 455,974 shares of common stock.
In May, 2014, the Company entered into a convertible promissory note with MTF (the “2014 Note”) for $250,000 with interest at 7% per annum compounded annually and a maturity date of June 15, 2015. In the event of a financing of not less than $1 million, the 2014 Note automatically converts into Equity Securities, as defined in the 2014 Note, at a 25% discount to the price paid per share in such financing. In connection with the 2014 Note, the Company issued a warrant to purchase 166,667 shares of the Company’s common stock at an exercise price of $1.50 per share and 4 year term. The Company accrued placement agent fees of $10,000 or 4% of the funds raised in connection with the financing and is obligated to issue a warrant for the purchase of 13,333 shares of common stock, which represents 4% of the common shares underlying the 2014 Note. In July 2014, the 2014 Note and related warrants were assigned to Orthofix and included in the Subsequent Orthofix Financing discussed below.
On July 1, 2014, Orthofix (A) purchased $500,000 worth of Bone Biologics Common Stock; (B) was issued two convertible promissory notes, each in the principal amount of $250,000 and exercisable for $333,333 worth of Bone Biologics Common Stock; and (C) was issued two warrants, each exercisable for 166,667 shares of Bone Biologics Common Stock at an exercise price per share of $1.50. Upon subscribing for the Subsequent Orthofix Shares, the Subsequent Orthofix Convertible Promissory Notes converted by their terms into a combined total of $666,666 worth of shares of Bone Biologics’ Common Stock in accordance with the terms of the Subsequent Orthofix Convertible Promissory Notes. The Subsequent Orthofix Warrants converted into warrants of the Company with substantially identical terms upon consummation of the Merger.
| 59 |
At the closing of the Subsequent Orthofix Shares and Notes, AFH Advisory was entitled to receive the Extra Warrants. Forefront or its designees will receive the Agent Warrant. Such Agent Warrant will be issued at the closing of the Private Placement and shall provide, among other things, that the Agent Warrant shall: (i) be exercisable at the price of the securities (or the exercise price of the securities) issued to the investors in the offering, (ii) expire five (5) years from the date of issuance, (iii) include customary registration rights, including the registration rights provided to the Investors, (iv) contain provisions for cashless exercise and (v) include such other terms that are normal and customary for warrants of this type. In addition, Forefront or its designees will receive and Advisory Warrant equal to 2.0% of the Company’s post-merger and financing fully diluted shares outstanding upon the closing of $2.5 million of investors on which Forefront is eligible to receive compensation. Forefront was issued a warrant to purchase 46,667 shares of Common Stock at $1.00 per share upon completion of the Orthofix Subsequent Financing.
On July 11, 2014, Catherine Doll, Internim CFO, was granted warrants to purchase up to 12,625 shares of Common Stock of the Company at a strike price of $0.00 per share, with a 4 year term.
On September 15, 2014, Bone and MTF entered into the MTF Short Term 2014 Loan pursuant to which MTF has agreed to advance an initial $250,000 to Bone and, at Bone’s request and subject to the terms and conditions of the MTF Short Term 2014 Loan, to advance up to an additional $250,000 to Bone. The MTF Short Term 2014 Loan has an interest rate of eight and one-half percent (8.5%) accruing annually. The MTF Short Term 2014 Loan matures on the earlier to occur of (i) the date on which at least $1 million is loaned to or invested in the Company and (ii) December 31, 2014. In further consideration of the MTF 2014 Loan, Bone granted to MTF 625,000 warrants at a strike price of $1.62. The MTF 2014 Loan was assigned to the Company on September 19, 2014.
The transactions described above were exempt from registration under Section 4(2) of the Securities Act and Rule 506 of Regulation D thereunder.
| 60 |
INDEMNIFICATION OF OFFICERS AND DIRECTORS
Current Officers and Directors of the Company
Under Section 145 of the General Corporation Law of the State of Delaware, we may indemnify our directors and officers against liabilities they may incur in such capacities, including liabilities under the Securities Act. Our Amended and Restated Certificate of Incorporation provides that no director shall be personally liable to the us or our stockholders for monetary damages for any breach of fiduciary duty by such director as a director. This provision does not eliminate liability (i) for breach of the director’s duty of loyalty to us or us stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the General Corporation Law of the State of Delaware or (iv) for any transaction from which the director derived an improper personal benefit. If the General Corporation Law of the State of Delaware is amended to authorize the further elimination or limitation of the liability of directors, then the liability of our directors, in addition to the limitation on personal liability provided herein, shall be limited to the fullest extent permitted by the amended DGCL.
Our Amended and Restated By-laws provide that we shall indemnify and hold harmless, to the fullest extent permitted by applicable law as it presently exists or may hereafter be amended, any person who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such person, or a person for whom such person is the legal representative, is or was a director or officer of the corporation or, while a director or officer of the corporation, is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or of a partnership, joint venture, limited liability company, trust, enterprise or nonprofit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including attorneys’ fees) reasonably incurred by such indemnified person in such proceeding. We shall pay the expenses (including attorneys’ fees) incurred by an indemnified person in defending any proceeding in advance of its final disposition; provided, however, that, to the extent required by law, such payment of expenses in advance of the final disposition of the proceeding shall be made only upon receipt of an undertaking by the indemnified person to repay all amounts advanced if it should be ultimately determined that the indemnified person is not entitled to be indemnified.
Our Amended and Restated By-laws provide that we may indemnify and advance expenses to any person who was or is made or is threatened to be made or is otherwise involved in any proceeding by reason of the fact that such person, or a person for whom such person is the legal representative, is or was an employee or agent of the corporation or, while an employee or agent of the corporation, is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or of a partnership, joint venture, limited liability company, trust, enterprise or nonprofit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including attorney’s fees) reasonably incurred by such person in connection with such proceeding. We may pay the expenses (including attorney’s fees) incurred by persons who are non-director or non-officer employees or agents in defending any proceeding in advance of its final disposition on such terms and conditions as may be determined by the Board of Directors.
We have been advised that in the opinion of the SEC, insofar as indemnification for liabilities arising under the Securities Act may be permitted to its directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event a claim for indemnification against such liabilities (other than the our payment of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Our Board has approved an Indemnification Agreement for our directors and executive officers. Following Board approval, we entered into Indemnification Agreements with each of our current directors and executive officers. The Indemnification Agreement provides for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The Indemnification Agreement also provides for the advancement of expenses in connection with a proceeding prior to a final, non-appealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to be entitled to indemnification by us. The Indemnification Agreement sets forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that will apply to any dispute between us and an indemnitee arising under the Indemnification Agreement.
Former Officers and Directors of the Company
Effective as of September 19, 2014, our Board of Directors also approved the Former D&O Indemnification Agreement to be entered into between us, Don Hankey and Amir Heshmatpour. The Former D&O Indemnification Agreement requires that for a period of four (4) years from and after September 19, 2014, we will indemnify (including advancement of expenses) and hold harmless persons who were officers and directors of the Company (i) by reason of being an officer or director of the Company prior to the Merger, including through all transactions relating to the Merger, or (ii) is related to acts in connection with the Merger taken by the Former D&O Indemnified Persons, provided however, that the foregoing indemnity shall be excess of all any insurance coverage available to the Former D&O Indemnified Parties for any such loss. The accuracy of the Hankey Affidavit and Heshmatpour Affidavit in connection with the Former D&O Indemnification is a condition precedent to the foregoing indemnity (including advancement of expenses). The Company has no insurance coverage that would cover any claim asserted against the Company by any Former D&O Indemnified Person pursuant to this Former D&O Indemnification Agreement.
| 61 |
Reference is made to the disclosure set forth under Item 9.01 of this Current Report, which disclosure is incorporated herein by reference.
| 62 |
See Item 9.01(d) below, which is incorporated by reference herein.
| 63 |
Item 3.02. Unregistered Sales of Equity Securities.
The disclosure set forth in Item 2.01 to this Current Report is incorporated into this item by reference.
Item 5.01. Changes in Control of the Registrant.
As a result of the transfer of a majority of the Common Stock to the previous stockholders of Bone Biologics in connection with the Merger, we experienced a change in control, with the former stockholders of Bone Biologics acquiring control of us. The disclosure set forth in Item 2.01 to this Current Report is incorporated into this item by reference.
Item 5.02. Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
The disclosure set forth in Item 2.01 to this Current Report is incorporated into this item by reference.
Item 5.03. Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year.
On September 19, 2014, concurrent with the Merger, we adopted the fiscal year end of Bone Biologics, thereby changing our fiscal year end from October 31 to December 31.
Item 5.06. Change in Shell Company Status.
The disclosure set forth in Item 2.01 to this Current Report is incorporated into this item by reference. As a result of the completion of the Merger, we believe that we are no longer a shell company, as defined in Rule 405 of the Securities Act and Rule 12b-2 of the Exchange Act.
Item 9.01. Financial Statements and Exhibits.
| (a) | Financial Statements of business acquired |
In accordance with Item 9.01(a), Bone Biologics’ audited financial statements for the years ended December 31, 2013 and 2012 are included with this Current Report beginning on Page F-1.
| (b) | Pro forma financial information |
In accordance with Item 9.01(b), unaudited pro-forma combined financial statements are included with this Current Report beginning on Page F-19.
| 64 |
| Exhibit No. | Description | |
| 2.1 | Agreement and Plan of Merger, dated as of September 19, 2014, by and among AFH Acquisition X, Inc., Bone Biologics Acquisition Corp., and Bone Biologics, Inc.* | |
| 2.2 | Certificate of Merger as filed with the California Secretary of State effective September 19, 2019* | |
| 3.1(i) | Amended and Restated Articles of Incorporation, of Bone Biologics, Corp., as filed with the Delaware Secretary of State on July 28, 2014* | |
| 3.1(ii) | Amended and Restated Bylaws of Bone Biologics, Corp.* | |
| 4.1 | Bone Biologics, Corp. September 2013 Warrant issued to AFH* | |
| 4.2 | Bone Biologics, Corp. June 2013 Warrant issued to Orthofix* | |
| 4.3 | Bone Biologics, Corp. April 2013 Warrant issued to MTF* | |
| 4.4 | Amendment to Bone Biologics, Corp. April 2013 Warrant issued to MTF, June 2013 Warrant issued to Orthofix and September 2013 Warrant issued to AFH* | |
| 4.5 | Bone Biologics, Corp. Warrant issued to Marie Antonia Gray* | |
| 4.6 | Bone Biologics, Corp. March 2009 Warrant issued to MTF* | |
| 4.7 | Bone Biologics, Corp. Warrant issued to T.O. Medical Development Inc.* | |
| 4.8 | Bone Biologics, Corp. Warrant issued to Chia Soo* | |
| 4.9 | Bone Biologics, Corp. Warrant issued to Aragen Bioscience, Inc.* | |
| 4.10 | Bone Biologics, Corp. Warrant issued to Alquest, Inc.* | |
| 4.11 | Bone Biologics, Corp. October 2013 Warrant issued to Orthofix* | |
| 4.12 | Bone Biologics, Corp. June 2014 Warrant issued to MTF, as thereafter assigned to Orthofix* | |
| 4.13 | Bone Biologics, Corp. July 2014 Warrant issued to Orthofix* | |
| 4.14 | Bone Biologics, Corp. July 2014 Warrant issued to AFH* | |
| 4.15 | Bone Biologics, Corp. Warrant issued to Catherine Doll * | |
| 4.16 | Bone Biologics, Corp. Warrant issued to Forefront Capital Markets, LLC* | |
| 4.17 | Bone Biologics, Corp. September 2014 Warrant issued to MTF* | |
| 4.18 | Form of Registration Rights Agreement, by and between Bone Biologics, Corp., AFH, HIC and MTF* | |
| 4.19 | Bone Biologics, Corp. Convertible Note Purchase Agreement, dated September 19, 2014 by and Between Bone Biologics, Corp. and MTF* | |
| 4.20 | Bone Biologics, Corp. Convertible Promissory Note, dated September 19, 2014, issued to MTF* | |
| 4.21 | $340,000 Note issued in Favor of AFH Advisory* | |
| 4.22 | Loan Agreement dated September 15, 2014, by and Between MTF and Bone Biologics, Inc. and Assignment, Assumption and Consent Agreement* | |
| 4.23 | Note dated as of September 15, 2014, issued in Favor of MTF* |
| 65 |
| 4.24 | Letter of Credit granted by MTF to AFH Advisory* | |
| 4.25 | Return to Treasury Agreement of Bone Biologics, Corp. with AFH Advisory* | |
| 10.1 | Letter Agreement, dated September 7, 2014, by and among AFH Holding & Advisory, LLC, Bone Biologics, Inc. and Musculoskeletal Transplant Foundation, Inc.* | |
| 10.2 | Letter agreement, dated December 18, 2013, as amended on September 22, 2014, by and among Bone Biologics, Inc., AFH Acquisition X, Inc., and Forefront Capital Markets, LLC* | |
| 10.3 | Director Offer Letter, dated July 2, 2014, by and between Chia Soo and Bone Biologics, Corp. *+ | |
| 10.4 | Director Offer Letter, dated July 1, 2014, by and between Bruce Stroever and Bone Biologics, Corp. *+ | |
| 10.5 | Director Offer Letter, dated August 22, 2014, by and between John Booth and Bone Biologics, Corp. *+ | |
| 10.6 | Director Offer Letter, dated June 23, 2014, by and between Jimmy Delshad and Bone Biologics, Corp. *+ | |
| 10.7 | Director Offer Letter, dated, June 25, 2014, by and between Steve Warnecke and Bone Biologics, Corp. *+ | |
| 10.8 | Director Offer Letter, dated, June 25, 2014, by and between William Coffin and Bone Biologics, Corp. *+ | |
| 10.9 | Management Consulting Agreement, dated September 19, 2014, by and between the Musculoskeletal Transplant Foundation, Inc. and Bone Biologics, Corp. (Mike Schuler) *+ | |
| 10.10 | Management Consulting Agreement, dated September 19, 2014, by and between the Musculoskeletal Transplant Foundation, Inc. and Bone Biologics, Corp. (Bruce Stroever) *+ | |
| 10.11 | President and Chief Technology Officer Employment agreement, dated September 19, 2014, by and between Bone Biologics, Corp. and William Jay Treat*+ | |
| 10.12 | Management Consulting Agreement, dated July 1, 2014, by and between The Gilson Group, LLC and Bone Biologics, Corp. *+ | |
| 10.13 | Consulting Agreement, dated September 19, 2014, by and between Bone Biologics, Corp. and T.O. Medical Development Inc. *+ | |
| 10.14 | Bone Biologics, Corp. 2014 Stock Plan*+ | |
| 10.15 | Form of Stock Option Award Agreement under the Bone Biologics, Corp. 2014 Equity Incentive Plan*+ | |
| 10.16 | Exclusive License Agreement, dated as of March 15, 2006, by and between Bone Biologics, Inc. and the Regents of the University of California* | |
| 10.17 | Form of Indemnification Agreement* | |
| 10.18 | Form of Former Officer and Director Indemnification Agreement* | |
| 21.1 | Subsidiaries* | |
| 101.INS | XBRL Instance Document* | |
| 101.SCH | XBRL Taxonomy Extension Schema Document* | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document* | |
| 101.DEF | XBRL Taxonomy Extension Definition Document* | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document* | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document* |
| * | Filed herewith. |
| + | Designates management contracts and compensation plans. |
| 66 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| BONE BIOLOGICS CORP. | ||
| Date: September 25, 2014 | By: | /s/ Michael Schuler |
| Name: | Michael Schuler | |
| Title: | Chief Executive Officer | |
| 67 |
BONE BIOLOGICS, INC.
TABLE OF CONTENTS
| F-1 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Bone Biologics, Inc.
We have audited the accompanying balance sheets of Bone Biologics, Inc. (the “Company”) as of December 31, 2013 and 2012, and the related statement of operations, changes in stockholders’ deficit and cash flows for the years then ended, and the period from March 9, 2004 (Inception) through December 31, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit includes consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Bone Biologics, Inc. as of December 31, 2013 and 2012, and the results of its operations and its cash flows for the years then ended, and the period from March 9, 2004 (Inception) through December 31, 2013, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company has recurring losses from operations and an accumulated deficit of $7,613,504 at December 31, 2013. As discussed in Note 1 to the financial statements, the Company has negative cash flows from operations, working capital deficiencies, has notes payable and related interest payable, and has not established commercial viability of its products. These conditions, among others, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding these matters, which are further described in Note 1, are to use its available borrowing capacity through its short-term credit facilities provided by related parties, raise additional debt or equity capital, and continue to progress towards commercial viability of its products. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ ANTON & CHIA, LLP | |
| Newport Beach, California | |
| June 6, 2014 |
| F-2 |
Financial Statements
As of and for the Years Ended December 31, 2013 and 2012 and for the
Period from March 9, 2004 (inception) through December 31, 2013
| F-3 |
Bone Biologics, Inc.
(A Development Stage Company)
| December 31, 2013 | December 31, 2012 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash | $ | 1,538 | $ | 2,370 | ||||
| Prepaid expenses | 10,767 | - | ||||||
| Deferred transaction costs | 75,000 | - | ||||||
| Total current assets | 87,305 | 2,370 | ||||||
| Total assets | $ | 87,305 | $ | 2,370 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 41,300 | $ | - | ||||
| Accrued expenses | 1,525,604 | 991,403 | ||||||
| Notes payable to related party, net of debt discount | 3,947,817 | 3,687,237 | ||||||
| Notes payable, net of debt discount | 180,690 | - | ||||||
| Total current liabilities | 5,695,411 | 4,678,640 | ||||||
| Total liabilities | 5,695,411 | 4,678,640 | ||||||
| Commitments and Contingencies | ||||||||
| Stockholders’ deficit | ||||||||
| Series A Preferred Stock, $0.0001 par value per share; 493,339 shares authorized; 493,339 shares issued and outstanding at December 31, 2013 and December 31, 2012 (liquidation preference $881,103 at December 31, 2013 and December 31, 2012, respectively) | 49 | 49 | ||||||
| Series B Preferred Stock, $0.0001 par value per share; 10,000,000 shares authorized; 5,336,099 shares issued and outstanding at December 31, 2013 and December 31, 2012 (liquidation preference $23,585,557 at December 31, 2013 and December 31, 2012, respectively) | 534 | 534 | ||||||
| Common stock, $0.0001 par value per share; 20,000,000 shares authorized; 5,098,661 shares issued and outstanding at December 31, 2013 and December 31, 2012 | 510 | 510 | ||||||
| Additional paid-in capital | 2,004,305 | 1,853,938 | ||||||
| Accumulated deficit | (7,613,504 | ) | (6,531,301 | ) | ||||
| Total stockholders’ deficit | (5,608,106 | ) | (4,676,270 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 87,305 | $ | 2,370 | ||||
See accompanying notes to financial statements.
| F-4 |
Bone Biologics, Inc.
(A Development Stage Company)
| Year
Ended December 31, 2013 | Year
Ended December 31, 2012 | Period
from March 9, 2004 (inception) to December 31, 2013 | ||||||||||
| Revenues | $ | - | $ | - | $ | - | ||||||
| Cost of revenues | - | - | - | |||||||||
| Gross profit | - | - | - | |||||||||
| Operating expenses | ||||||||||||
| Research and development | 188,236 | 255,575 | 5,089,482 | |||||||||
| General and administrative | 483,749 | 180,089 | 1,238,419 | |||||||||
| Total operating expenses | 671,985 | 435,664 | 6,327,901 | |||||||||
| Loss from operations | (671,985 | ) | (435,664 | ) | (6,327,901 | ) | ||||||
| Interest expense, net | (409,419 | ) | (279,101 | ) | (1,277,603 | ) | ||||||
| Loss before provision for income taxes | (1,081,404 | ) | (715,765 | ) | (7,605,504 | ) | ||||||
| Provision for income taxes | 800 | 800 | 8,000 | |||||||||
| Net loss | $ | (1,082,204 | ) | $ | (715,565 | ) | $ | (7,613,504 | ) | |||
See accompanying notes to financial statements.
| F-5 |
Bone Biologics, Inc.
(A Development Stage Company)
Statements of Stockholders’ Deficit
| Series A Preferred Stock | Series B Preferred Stock | Common Stock | Additional Paid-in | Accumulated Deficit During the Development | Total Stockholders’ Equity | |||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Stage | (Deficit) | ||||||||||||||||||||||||||||
| Balance at March 9, 2004 | - | $ | - | - | $ | - | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||||||||
| Capital contribution | - | - | - | - | - | - | 35,000 | - | 35,000 | |||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (32,327 | ) | (32,327 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2004 | - | - | - | - | - | - | 35,000 | (32,327 | ) | 2,673 | ||||||||||||||||||||||||||
| Capital contribution | - | - | - | - | - | - | (18,000 | ) | - | (18,000 | ) | |||||||||||||||||||||||||
| Issuance of common stock | - | - | - | - | 5,125,500 | 513 | 34,012 | - | 34,525 | |||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (50,762 | ) | (50,762 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2005 | - | - | - | - | 5,125,500 | 513 | 51,012 | (83,089 | ) | (31,564 | ) | |||||||||||||||||||||||||
| Issuance of common stock | - | - | - | - | 473,161 | 47 | (47 | ) | - | - | ||||||||||||||||||||||||||
| - | - | - | - | |||||||||||||||||||||||||||||||||
| Sale and issuance of Series A Preferred Stock in July 2006 at $1.786 per share for cash | 83,987 | 8 | - | - | - | - | 149,993 | - | 150,001 | |||||||||||||||||||||||||||
| Issuance of Series A Preferred Stock in conjunction with the conversion of notes payable in July 2006 | 409,352 | 41 | - | - | - | - | 731,062 | - | 731,103 | |||||||||||||||||||||||||||
| Issuance of warrants in November 2006 | - | - | - | - | - | - | 10,356 | - | 10,356 | |||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (476,474 | ) | (476,474 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2006 | 493,339 | 49 | - | - | 5,598,661 | 560 | 942,376 | (559,563 | ) | 383,422 | ||||||||||||||||||||||||||
| Sale and issuance of Series B Preferred Stock in July 2007 at $4.42 per share for cash | - | - | 147,846 | 15 | - | - | 653,467 | - | 653,482 | |||||||||||||||||||||||||||
| Repurchase common stock | - | - | - | - | (500,000 | ) | (50 | ) | (34,650 | ) | - | (34,700 | ) | |||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (1,218,678 | ) | (1,218,678 | ) | |||||||||||||||||||||||||
| F-6 |
Bone Biologics, Inc.
(A Development Stage Company)
Statements of Stockholders’ Deficit (Continued)
| Series A Preferred Stock | Series B Preferred Stock | Common Stock | Additional Paid-in | Deficit Accumulated During the Development | Total Stockholders’ Equity/ | |||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Stage | (Deficit) | ||||||||||||||||||||||||||||
| Balance at December 31, 2007 | 493,339 | 49 | 147,846 | 15 | 5,098,661 | 510 | 1,561,193 | (1,778,241 | ) | (216,474 | ) | |||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (1,001,573 | ) | (1,001,573 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2008 | 493,339 | 49 | 147,846 | 15 | 5,098,661 | 510 | 1,561,193 | (2,779,814 | ) | (1,218,047 | ) | |||||||||||||||||||||||||
| Warrant issuance in connection with March 2009 note | - | - | - | - | - | - | 47,970 | - | 47,970 | |||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (1,200,579 | ) | (1,200,579 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2009 | 493,339 | 49 | 147,846 | 15 | 5,098,661 | 510 | 1,609,163 | (3,980,393 | ) | (2,370,656 | ) | |||||||||||||||||||||||||
| Issuance of Series B Preferred Stock in conjunction with the conversion of September 2009 Convertible Note in February 2010 | - | - | 5,188,253 | 519 | - | - | 141,448 | - | 141,967 | |||||||||||||||||||||||||||
| Issuance of warrants in February 2010 | - | - | - | - | - | - | 103,327 | - | 103,327 | |||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (897,713 | ) | (897,713 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2010 | 493,339 | 49 | 5,336,099 | 534 | 5,098,661 | 510 | 1,853,938 | (4,878,106 | ) | (3,023,075 | ) | |||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (937,630 | ) | (937,630 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2011 | 493,339 | 49 | 5,336,099 | 534 | 5,098,661 | 510 | 1,853,938 | (5,815,736 | ) | (3,960,705 | ) | |||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (715,565 | ) | (715,565 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2012 | 493,339 | $ | 49 | 5,336,099 | $ | 534 | 5,098,661 | $ | 510 | $ | 1,853,938 | $ | (6,531,301 | ) | $ | (4,676,270 | ) | |||||||||||||||||||
| Net Loss | - | - | - | - | - | - | - | (1,082,203 | ) | (1,082,203 | ) | |||||||||||||||||||||||||
| Warrants issued in connection with Bridge Notes | - | - | - | - | - | - | 150,367 | - | 150,367 | |||||||||||||||||||||||||||
Balance at December 31, 2013 | 493,339 | $ | 49 | 5,336,099 | $ | 534 | 5,098,661 | $ | 510 | $ | 2,004,305 | $ | (7,613,504 | ) | $ | (5,608,106 | ) | |||||||||||||||||||
See accompanying notes to financial statements.
| F-7 |
Bone Biologics, Inc.
(A Development Stage Company)
| For
the Year Ended December 31, 2013 | For
the Year Ended December 31, 2012 | Period
from March 9, 2004 (inception) to December 31, 2013 | ||||||||||
| Operating Activities | ||||||||||||
| Net loss | $ | (1,082,203 | ) | $ | (715,565 | ) | $ | (7,613,504 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Accrued interest expense | 340,268 | 279,104 | 1,180,488 | |||||||||
| Debt discount | 67,104 | - | 67,104 | |||||||||
| Warrants issued in connection with deferred fees | - | - | 161,613 | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses and other current assets | (10,767 | ) | - | (10,767 | ) | |||||||
| Deferred transaction costs | 4,717 | - | 4,717 | |||||||||
| Accounts payable | 41,300 | (29,657 | ) | 41,300 | ||||||||
| Accrued expenses | 114,215 | 18,632 | 287,422 | |||||||||
| Net cash used in operating activities | (525,365 | ) | (447,486 | ) | (5,881,628 | ) | ||||||
| Financing Activities | ||||||||||||
| Capital contributions, net | - | - | 17,000 | |||||||||
| Proceeds from issuance of notes payable | 524,533 | 448,609 | 5,062,858 | |||||||||
| Proceeds from issuance of common stock | - | - | 34,525 | |||||||||
| Repurchase of common stock | - | - | (34,700 | ) | ||||||||
| Proceeds from sale of Series A preferred stock | - | - | 150,000 | |||||||||
| Proceeds from sale of Series B preferred stock | - | - | 653,482 | |||||||||
| Net cash provided by financing activities | 524,533 | 448,609 | 5,883,165 | |||||||||
| Net increase (decrease) in cash | (832 | ) | 1,123 | 1,538 | ||||||||
| Cash, beginning of period | 2,370 | 1,247 | - | |||||||||
| Cash, end of period | $ | 1,538 | $ | 2,370 | $ | 1,538 | ||||||
| Supplemental non-cash information | ||||||||||||
| Conversion of notes payable and Accrued interest to preferred stock | $ | - | $ | - | $ | 873,070 | ||||||
| Accrued transaction fees | $ | 129,717 | $ | - | $ | - | ||||||
| Interest paid | $ | 2,047 | $ | - | $ | 2,047 | ||||||
| Taxes paid | $ | 800 | $ | 800 | $ | 8,000 | ||||||
| Warrants issued in connection with notes payable | $ | 150,367 | $ | - | $ | - | ||||||
See accompanying notes to financial statements.
| F-8 |
Bone
Biologics, Inc.
(A Development Stage Company)
1. | The Company |
Bone Biologics, Inc. (“Bone” or the “Company”) was incorporated in California on March 9, 2004. Bone is a privately-held biotechnology company that is currently focused on bone regeneration in spinal fusion using the recombinant human protein, known as UCB-1 (or “Nell-1”). The UCB-1 protein is an osteoinductive recombinant protein that provides target specific control over bone regeneration. The protein has been licensed exclusively for worldwide applications to Bone Biologics through a technology transfer from the University of California, Los Angeles (“UCLA”). Bone Biologics recently received guidance from the United States Food and Drug Administration (“FDA”) that UCB-1 will be classified as a combination product with a device lead.
The Company is a development stage entity. The production and marketing of the Company’s products and its ongoing research and development activities will be subject to extensive regulation by numerous governmental authorities in the United States. Prior to marketing in the United States, any drug developed by the Company must undergo rigorous preclinical (animal) and clinical (human) testing and an extensive regulatory approval process implemented by the FDA under the Food, Drug and Cosmetic Act. The Company has limited experience in conducting and managing the preclinical and clinical testing necessary to obtain regulatory approval. There can be no assurance that the Company will not encounter problems in clinical trials that will cause the Company or the FDA to delay or suspend clinical trials.
The Company’s success will depend in part on its ability to obtain patents and product license rights, maintain trade secrets, and operate without infringing on the proprietary rights of others, both in the United States and other countries. There can be no assurance that patents issued to or licensed by the Company will not be challenged, invalidated, or circumvented, or that the rights granted thereunder will provide proprietary protection or competitive advantages to the Company.
In August 2012, the Company, along with its majority owner and debt holder, Musculoskeletal Transplant Foundation, Inc. (“MTF”) and AFH Holding & Advisory, LLC (“AFH”) entered into a Letter of Intent (“LOI”), as amended on August 19, 2013, to consummate a business combination through a share exchange, reverse merger, or other similar transactions resulting in the Company becoming a public entity (“The Transaction”) and the contemplated subsequent financings (see Note 4).
Going Concern and Liquidity
The Company has no significant operating history and, from March 9, 2004 (inception) to December 31, 2013, has generated an accumulated deficit of approximately $7.6 million. The Company will continue to incur significant expenses for development activities for their lead product Nell-1. The accompanying financial statements for the year ended December 31, 2013, have been prepared assuming the Company will continue as a going concern. In connection with the LOI, management intends to raise additional debt and/or equity financing to fund future operations and to provide additional working capital. However, there is no assurance that such financing will be consummated or obtained in sufficient amounts and on acceptable terms necessary to meet the Company’s needs.
The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the possible inability of the Company to continue as a going concern.
| 2. | Summary of Significant Accounting Policies |
Basis of Presentation
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”).
Use of Estimates
The preparation of the accompanying financial statements in conformity with GAAP requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of expenses during the reporting period. Significant estimates include warrants and income tax valuation allowances. Actual results could differ from those estimates.
| F-9 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
Research and Development Costs
Research and development costs include, but are not limited to, patents and license expenses, payroll and other personnel expenses, consultants, expenses incurred under agreements with contract research and manufacturing organizations and animal clinical investigative sites and the cost to manufacture clinical trial materials. Costs related to research, design and development of products are charged to research and development expense as incurred.
Patents and Licenses
In March 2006, the Company entered into an exclusive license agreement (“Exclusive License Agreement”) with UCLA for the worldwide application of the UCB-1 protein through a technology transfer. See Note 4 for commitments related to the Exclusive License Agreement. Patent expenses include costs to acquire the license of UCB-1, which were de minimus, and costs to file patent applications related to UCB-1.
Bone Biologics expenses the costs incurred to file patent applications, all costs related to abandoned patent applications and maintenance costs, and these costs are included in research and development expenses. Costs associated with licenses acquired to be able to use products from third parties prior to receipt of regulatory approval to market the related products are also expensed. The Company’s licensed technologies may have alternative future uses in that they are enabling (or platform) technologies that can be the basis for multiple products that would each target a specific indication. Costs of acquisition of licenses are expensed.
Concentration of Credit Risk and Other Risks and Uncertainties
Cash and cash equivalents are maintained at financial institutions and, at times, balances may exceed federally insured limits. The Company has never experienced any losses related to these balances. All of the non-interest bearing cash balances were fully insured at December 31, 2012 due to a temporary federal program in effect from December 31, 2010 through December 31, 2012. Under the program, there is no limit to the amount of insurance for eligible accounts. Beginning January 1, 2013, insurance coverage reverted to $250,000 per depositor at each financial institution, and the Company’s non-interest bearing cash balances may again exceed federally insured limits. There were no interest-bearing amounts on deposit in excess of federally insured limits at December 31, 2013 and December 31, 2012.
Income Taxes
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due and deferred taxes resulting from timing differences in recording of transactions for tax purposes and financial reporting purposes.
The deferred tax assets and liabilities represent the future tax return consequences of those differences, which will either be taxable or deductible when the assets and liabilities are received or settled. Valuation allowances are established when necessary to reduce deferred tax assets to amounts expected to be realized.
The accounting provisions related to uncertain income tax positions require the Company to determine whether any tax position in all open years meets a more likely than not threshold of being sustained upon examination by the applicable taxing authority. The Company did not have any changes to its liability for uncertain tax positions for the years ended December 31, 2013 and 2012.
The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense. No such amounts are accrued as of December 31, 2013 and 2012.
| F-10 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
| 3. | Accrued Expenses |
Accrued expenses consist of the following:
| December 31, 2013 | December 31, 2012 | |||||||
| Interest expense | $ | 1,158,465 | $ | 818,195 | ||||
| Professional services | 152,492 | 161,700 | ||||||
| Transaction costs | 137,585 | - | ||||||
| Patents | 75,383 | 10,031 | ||||||
| Payroll taxes | 1,679 | 1,477 | ||||||
| $ | 1,525,604 | $ | 991,403 | |||||
| 4. | Commitments and Contingencies |
Letter of Intent
In August of 2012, the Company, along with its majority owner and debt holder, MTF, entered into a Letter of Intent (“LOI”) with AFH to consummate a business combination through a share exchange, reverse merger, or other similar transactions resulting in the Company becoming a public entity (“The Transaction”). In August, 2013, the LOI was amended and restated, and on May 7, 2014, the LOI was again amended and restated. The Amended and Restated Letter of Intent dated May 7, 2014 (the “Amended LOI”) contemplates and defines the following events:
Consummation of Bridge Financings (“Closing I”)
In April 2013 and September 2013, the Company’s Board approved the Company to borrow up to an aggregate principal amount of $300,000 (April Bridge Financing) and $250,000 (September Bridge Financing) pursuant to the sale and issuance of convertible promissory notes and warrants to purchase common stock of the Company (collectively, the “Bridge Financings”). The note accrues interest at a rate of 12% per year and payable per quarter. A warrant to purchase the Company’s common stock equal to 50% of the original principal amount at $1.00 per share will be issued to each Bridge Financing participant. Principal and unpaid accrued interest may be converted into equity securities issued in the Company’s next equity financing in an aggregate amount of at least $2.5 million at a price equal to the price paid by investors in the next equity financing. On April 29, 2013 and on June 5, 2013, the Company borrowed $100,000 from MTF and $100,000 from Orthofix, Inc., respectively, under the April Bridge Financing. In August 2013, in conjunction with the Amended LOI, AFH agreed to purchase $50,000 of the April Bridge Financing prior to Closing II. In October 2013, the Company borrowed an additional $150,000 from Orthofix under the September Bridge Financing.
Consummation of Business Combination (“Closing II”)
Under the amended LOI, it is contemplated that the Company and its equity holders will consummate a share exchange, reverse merger, or other business combination, with a Delaware corporation publicly reporting pursuant to United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), or a private Delaware corporation (“Acquisition Co.”), either directly or indirectly through an affiliate. If the post-business combination entity is not already a corporation publicly reporting pursuant to the Exchange Act, AFH will assist the post business combination entity with the filing of an appropriate registration statement resulting in the Company becoming a public company (“PubCo”).
Consummation of the Private Placement (“Closing III”)
Subsequent to Closing II, AFH will use its best efforts to assist PubCo in procuring one or more investors for a private financing, whether debt or equity, of a minimum of $2.5 million up to a maximum of $5.0 million. Such transaction is to include an over-allotment option of 15% at AFH’s discretion (the “Private Placement”).
Consummation of the PIPE Transaction (“Closing IV”)
Subsequent to Closing III, AFH Advisory will use its best efforts to assist PubCo in procuring an investment bank (the “Bank”) to facilitate a private investment in public equity transaction in an amount between $8.0 million and $10.0 million through the sale of securities of PubCo (the “PIPE”). Such transaction will include a 15% over allotment at AFH and/or the Bank’s discretion. Such transaction is contingent upon the appointment of a Bank and filing appropriate forms with the Financial Industry Regulatory Authority, Inc. (“FINRA”).
| F-11 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
Consummation of Initial Public Offering (“Closing V”)
Subsequent to Closing IV, AFH will assist PubCo in procuring a Bank to act as underwriter for an initial public offering in an amount of up to $40.0 million (the “Initial Public Offering”). The Initial Public Offering shall include a 15% over allotment option at AFH and/or the Bank’s discretion. Such a transaction is contingent upon the appointment of the Bank.
At or prior to consummation of the Business Combination, MTF agrees to convert all of its outstanding shares of Series A preferred stock and Series B preferred stock of the Company into share of Common Stock.
In addition, MTF agrees to convert 30%, 35%, and 35% of all outstanding convertible promissory notes and promissory notes converting as amended (principal and accrued interest) at each of the consummation of Closing II, Closing IV, and Closing V, respectively.
Upon (i) the consummation of the Business Combination, (ii) after giving effect to the issuance of any securities by Acquisition Co. in connection with the Business Combination (the “Business Combination Shares”), (iii) the completion of the Private Placement and (iv) after giving effect to the PIPE, the existing stockholders of Acquisition Co., and its owners, relatives, assignees and affiliates (collectively, the “AFH Group”), will own an aggregate of ten percent of the issued and outstanding common shares (the “Advisor Shares”) of PubCo.
At the consummation of Closing III, AFH Group shall be entitled to receive warrants to purchase up to 500,000 share of common stock of PubCo at the per share price of the shares offered in the Private Placement with a 5 year term and a cashless exercise provision (the “Extra Warrants”).
In addition to the Advisor Shares and Extra Warrants, AFH Group shall be entitled to receive warrants to purchase shares of common stock of PubCo (“Advisor Warrants”) in the amount necessary to cause AFH Group, when combined with the Advisor Shares, to have ownership equal to 10% of the fully diluted outstanding Common Stock, options and warrants at Closing III.
AFH will also be entitled to a reimbursement of $590,000 in connection with the Business Combination, which shall be payable directly from the net proceeds of the Private Placement (Closing III) to AFH at closing. Each party will bear all of its own costs and expenses in connection with each Closing.
In conjunction with the Amended LOI, the Company has agreed to covenants for the period of time between signing of the Amended LOI and the consummation of the Business Combination (Closing II) or upon termination of the agreement. Such covenants include restrictions and limitations on additional indebtedness, liquidation, selling of equity securities, amending organizational document and certain other normal and customary covenants. The Amended LOI will expire on August 31, 2014 if Closing II has not occurred.
License Commitment
In connection with the Exclusive License Agreement, the Company is required to pay a royalty fee beginning in the first year of commercial sale of the licensed product equal to 3% of net sales on a quarterly basis with an annual minimum royalty of $25,000 for the life of the patent rights. In addition to the royalty fees, the Company is also required to pay UCLA a $10,000 annual maintenance fee, $50,000 upon FDA marketing approval, and $25,000 upon first commercial sale.
On October 22, 2013, the Exclusive License Agreement was amended. The following additional fees will be due to UCLA i) 2% of the amount raised in the Private Placement. If the Private Placement does not close or is less than $2.5 million then a fee of $100,000 will be due and payable by June 1, 2014, ii) $25,000 due upon dosing of Phase 1 clinical trial and iii) $50,000 due upon closing of Phase 3 clinical trial.
Contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company’s management does not believe that any such matters, individually or in the aggregate, will have a material adverse effect on the Company’s business, financial condition, results of operations or cash flows.
| F-12 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations. However, the Company may record charges in the future as a result of these indemnification obligations.
In accordance with its amended and restated certificate of incorporation and amended and restated bylaws, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacity. There have been no claims to date and the Company has a director and officer insurance policy that enables it to recover a portion of any amounts paid for future potential claims.
| 5. | Notes Payable to Related Party |
As of December 31, 2013 and December 31, 2012, the Company had a total of $5,095,427 and $4,505,432, respectively, of notes outstanding (principal and interest) with MTF, a related party, which consist of the following:
| Note Type | Issue Date | Maturity Date | Interest Rate | December 31, 2013 | December 31, 2012 | |||||||||||||||
| Convertible Promissory Note | 1/18/08 | 3/31/14 | PRIME + 1 ½% | $ | 1,479,654 | $ | 1,415,475 | |||||||||||||
| Promissory Note | 11/4/08 | 3/31/14 | PRIME + 3% | 343,429 | 322,963 | |||||||||||||||
| Promissory Note | 3/17/09 | 3/31/14 | PRIME + 8% | 584,745 | 543,128 | |||||||||||||||
| Promissory Note | 8/24/09 | 3/31/14 | LIBOR + 8% | 23,193 | 21,110 | |||||||||||||||
| Tranched Promissory Note | 9/30/09 | 3/31/14 | LIBOR + 8% | 2,570,126 | 2,202,756 | |||||||||||||||
| Bridge Note, net of discount | 4/29/13 | 4/29/14 | 12% | 94,280 | - | |||||||||||||||
| $ | 5,095,427 | $ | 4,505,432 | |||||||||||||||||
Accrued interest on the notes payable to related party of $1,158,465 (2012 - $818,195) is recorded in accrued expenses at December 31, 2013 and 2012.
Convertible Promissory Notes
The convertible promissory notes are considered hybrid instruments, which consist of a debt host instrument together with a conversion feature, thus giving the holder of a convertible note an option to convert into an equity instrument providing the holder a residual interest in the Company. The holder of a convertible promissory note also has the option to present its convertible promissory note to the Company and demand payment under the terms of the note after the maturity date or upon the occurrence of certain events such as the failure of the Company to make a payment on the note when due, bankruptcy or certain other liquidation events. The Company concluded that the convertible promissory notes would be accounted for as a typical debt instrument with related interest expense recorded in the Company’s statements of operations. The company concluded that there is no beneficial conversion feature as of the date of issuance of the convertible notes. However, the note contains a contingent feature whereby the conversion rate may be lowered if a financing occurs at a lower rate than the note’s conversion rate. If the contingency is met and the conversion feature is determined to be “beneficial” in a future accounting period, an additional financing cost would be recorded for the beneficial conversion feature in the Company’s statements of operations at that time.
In April 2005, the Company issued a $100,000 convertible promissory note (the “2005 Convertible Note”) to MTF in accordance with the Convertible Note Purchase Agreement and Convertible Promissory Note dated April 6, 2005. In April 2006 the Company issued an additional $612,000 convertible promissory note (the “2006 Convertible Note”) to MTF in accordance with the Convertible Note Purchase Agreement and Convertible Promissory Note dated April 7, 2006.
The 2005 Convertible Note and the 2006 Convertible Note bore interest at a fixed rate of 6% per annum and prime plus one and one-half percent per annum, respectively, and matured on September 30, 2008 and September 30, 2009, respectively. In July 2006, the 2005 Note and 2006 Note, respectively, and accrued interest thereon for a total of $731,103, were converted into an aggregate of 409,352 shares of Series A preferred stock which was based on the conversion price of $1.786 per share (see Note 6). The conversion of the notes did not trigger a contingency and no additional financing charge was recognized.
| F-13 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
In January 2008, the Company issued a $1,107,000 convertible promissory note (“January 2008 Note”) to MTF in accordance with the Convertible Promissory Note dated January 18, 2008, as amended. The January 2008 Note bears interest at prime plus one and one-half percent per annum. MTF has the right to convert the entire outstanding balance (principal plus accrued interest) into shares of Series B Preferred Stock at the initial conversion price of $4.42 per share (“Initial Conversion Price”). Such Initial Conversion Price shall be subject to adjustments including but not limited to stock splits, issuance of securities and next equity financing.
The Company issued promissory notes to MTF in November 2008 of $250,000 (“November 2008 Note”), in March 2009 of $400,000 (“March 2009 Note) and in August 2009 of $16,400 (August 2009 Note”). The November 2008 and the March 2009 Note bear interest at prime plus three percent per annum. The August 2009 Note bears interest at LIBOR plus eight percent per annum.
In connection with the March 2009 Note, the Company entered into a Security Agreement (the “Security Agreement”) which grants MTF a security interest in all of the Company’s right, title and interest, whether presently existing or hereafter acquired, in, to all intellectual property and all other collateral. In connection with the Security Agreement, the Company issued a warrant to purchase 118,383 shares of common stock at an exercise price of $0.44 (See Note 6).
In September 2009, the Company issued a $139,047 promissory note (the “2009 Convertible Note”) to MTF in accordance with the Convertible Note Purchase Agreement and Convertible Promissory Note dated September 30, 2009. The 2009 Convertible Note bears interest at the rate of LIBOR plus 8% per annum and matured on October 30, 2009, but could have extended to November 30, 2009 or December 31, 2009. If the note is was not repaid by the maturity date, MFT was entitled to (i) convert the amount due on the 2009 Convertible Note into shares of Series B Preferred stock sufficient to increase MTF’s ownership in the Company to 51% of the fully-diluted capitalization, and (ii) receive the right to designate up to three additional members of the Company’s Board of Directors.
Since the 2009 Convertible Note was not repaid by the maturity date, on February 4, 2010, the 2009 Convertible Note was converted into 5,188,253 shares of Series B Preferred stock, which increased MTF’s ownership in the Company to 51% of the fully-diluted capitalization.
In September 2009, the Company entered into a tranched promissory note with MTF (“Tranched Note”), allowing the Company to initially borrow up to $445,000 in a series of one or more tranches. The Tranched Note was subsequently amended which, among other things, increased the maximum advance amount to $2,190,000.
In July 2013, all notes held by MTF were amended to extend the maturity date to March 31, 2014, and amended again on April 1, 2014 to extend the maturity date to March 31, 2015.
Bridge Note
In April 2013 and June 2013, the Company borrowed $100,000 from MTF and $100,000 from Orthofix, Inc. under the April Bridge Financing, and in October the Company borrowed an additional $150,000 from Orthofix, Inc. under the September Bridge Financing (See Note 5). The convertible promissory note accrues interest at a rate of 12% per year and payable per quarter. A warrant to purchase the Company’s common stock equal to 50% of the original principal amount divided by $1.00 was issued to the Bridge Financing participant. Principal and unpaid accrued interest may be converted into equity securities issued in the Company’s next equity financing in an aggregate amount of at least $2.5 million at a price equal to the price paid by investors in the next equity financing. As of December 31, 2013 the total outstanding balance under the Bridge Financings was $266,737 (net of debt discount of $83,263) of which $94,280 is included in Notes Payable to Related Party and $180,690 is reported as Notes Payable, net of discount.
| F-14 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
| 6. | Stockholders’ Equity |
Preferred Stock
The Company’s amended second amended and restated certificate of incorporation authorizes the Company to issue a total of 10,493,339 shares of preferred stock (Series A and B Combined). The Company has reserved sufficient shares of common stock for issuance upon conversion of the preferred stock. As of December 31, 2013, the Company has the following outstanding shares of preferred stock as shown in the table below.
| Series | Date Issued | Issue Price | Shares Outstanding | Carrying Amount | Liquidation Preference | Dividend Rate | ||||||||||||||||
| A | July 2007 | $ | 1.786 | 493,339 | $ | 881,103 | $ | 881,103 | $ | 0.09 | ||||||||||||
| B | August 2007 | 4.420 | 147,846 | 653,479 | 653,479 | 0.09 | ||||||||||||||||
| B | February 2010 | 0.027 | 5,188,253 | 141,967 | 22,932,078 | 0.09 | ||||||||||||||||
| 5,829,438 | $ | 1,676,549 | $ | 24,466,660 | ||||||||||||||||||
Dividends - The holders of Series A and B preferred stock are entitled to receive noncumulative dividends prior to and in preference to any declaration of payment of any dividends on the common stock of the Company, at the rate of $0.09 per share per annum. Such dividends are payable only when, and if declared by the Board of Directors. No dividends on preferred stock were declared by the Board from inception through December 31, 2013.
Liquidation Preference - In the event of liquidation, dissolution, or winding up of the Company, the holders of the Series A and B preferred stock are entitled to receive an amount per share equal to $1.786 and $4.42, respectively, for each outstanding share of Series A & B preferred stock (as adjusted for stock splits, stock dividends, combinations or other recapitalizations), plus all declared and unpaid dividends on such shares. Thereafter, if assets or surplus funds remained in the Company, the holders of common stock are entitled to receive all of the remaining assets of the Company.
Deemed Liquidation - Any merger or consolidation which would result in the Company’s stockholders immediately prior to such transaction not holding at least 50% of the voting power of the surviving, continuing or purchasing entity, or the sale or lease of all or substantially all of the assets of the Company, was deemed to be a liquidation, dissolution or winding up. Upon this event, holders of all shares of Series A and Series B preferred stock, as well as holders of the Company’s common stock would have receive their liquidation preference, including any declared and unpaid dividends as of the liquidation date. As in an ordinary liquidation, no class or series of the Company’s equity securities has a right to receive a particular form of consideration (e.g., cash or shares) upon a deemed liquidation event. Accordingly, because the holders of the Company’s preferred stock did not have a right to receive cash redemption of their shares, the Preferred stock are classified as permanent equity.
Conversion Rights - The holder of each share of Series A and B preferred stock have the option to convert each share into such number of fully paid and non-assessable shares of the Company’s common stock as is determined by dividing $1.786 (Series A Conversion Price) and $4.42 (Series B Conversion Price).
If the value of the adjusted number of shares of common stock into which the convertible preferred stock was convertible, based on the market price of the common stock on the date the convertible preferred stock was issued, was greater than the value of the number of shares of common stock into which the convertible preferred stock was convertible prior to such adjustment, based on the market price of the common stock on the date the convertible preferred stock was issued, the Company will recognize a beneficial conversion feature associated with the preferred stock. Because the beneficial conversion feature meets the requirements for equity classification (i.e., is not required to be accounted for as a liability), such future beneficial conversion feature charge will be recorded as a preferred stock dividend and the amount will be presented in a reconciliation of “net loss” to arrive at “net loss attributable to common shareholders” on the face of the Company’s statements of operations.
Each share of Series A and Series B preferred is subject to automatic conversion into common stock upon the earlier of (i) the Company’s sales of its common stock in a firm commitment underwritten public offering pursuant to a registration statement under the Securities Act of 1933, as amended or (ii) the date specified by written consent of holders of a majority of the then outstanding shares of Series A and Series B preferred stock, voting together as a single class on an as-converted basis.
Redemption - The preferred stock is not redeemable.
Voting Rights - The holders of preferred stock has the same voting rights as the holders of common stock. The holders of each share of convertible preferred stock are entitled to the number of votes equal to the number of shares of common stock into which such shares of convertible preferred stock could be converted.
| F-15 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
Common Stock
The
Company’s amendment to the second amended and restated certificate of incorporation authorizes the Company to issue a total
of 20,000,000 shares of common stock. As of
December 31, 2013, the Company had an aggregate of 5,098,661 shares of
common stock outstanding of which 4,000,000 shares of the outstanding common stock were issued to the founders of the Company
in exchange for technology know how and services.
Each share of common stock has the right to one vote. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to the prior rights of holders of all classes of stock outstanding having priority rights as to dividends. No dividends have been declared by the Board from inception through December 31, 2013.
In the event of a liquidation dissolution, or winding up of the Company, after distribution to the holders of the Series A and B convertible preferred stock, all remaining assets or surplus funds of the Company shall be distributed on a pro-rata basis among the holders of the outstanding common stock and convertible preferred stock assuming full conversion of the convertible preferred stock.
Common Stock Warrants
As of December 31, 2013, the Company had an aggregate of 609,300 outstanding unexercised common stock warrants as follows:
| Date Issued | Exercise Price | Number of Shares | ||||||
| 2006 | $ | 0.17 | 60,920 | |||||
| 2009 | $ | 0.44 | 118,383 | |||||
| 2010 | $ | 0.44 | 254,997 | |||||
| 2013 | $ | 1.00 | 175,000 | |||||
| Total Shares at December 31, 2013 | 609,300 | |||||||
In November 2006 and February 2010, the Company issued warrants to purchase 60,920 shares of common stock at an exercise price of $0.17 per share and 254,997 shares of common stock at an exercise price of $0.44 per share, respectively. The warrants were issued to one of the co-founders of the Company and to certain consultants who previously rendered services to the Company for which they agreed to defer payment for their services. The warrants expire in ten years from issuance date and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrants was estimated at an aggregate value of $113,683, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 105.6%, risk-free interest rate of between 3.62% and 4.62%, contractual term of 10 years and dividend yield of 0%. The warrants are classified as permanent equity. As of December 31, 2013 and December 31, 2012, the unpaid deferred payment balance was $90,199 and is included in accrued professional services (see Note 3).
In March 2009, the Company entered a Credit Agreement with MTF, a related party, for which the Company may borrow up to $400,000 (see Note 5). In connection with this transaction, the Company entered into a Warrant Agreement whereby it issued to MTF a warrant to purchase 118,383 shares of the Company’s common stock (“Note Warrant”) at an exercise price of $0.44 which allowed the Company to extend the maturity dates of the notes dated January 18, 2008 and November 4, 2008 to December 31, 2009. The fair value of the warrants was recorded as a debt issuance cost and was being amortized to interest expense over the term of the loan. The initial fair value of the Note Warrant at the grant date was estimated at an aggregate value of $47,970, using the Black-Scholes option pricing model. The warrant was classified as permanent equity at December 31, 2013.
In the connection with the Bridge Financings (see Note 4), warrants were issued to purchase 125,000 shares of the Company’s common stock at an exercise price of $1.00 per share. The warrants expire in seven years from issuance date and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrant was estimated at an aggregate value of $150,367 using the Black-Scholes option pricing model. The fair value on the warrants was recorded as a debt issuance cost and is being amortized to interest expense over the term of the note. For the year ended December 31, 2013, $67,104 of the debt issuance costs was amortized to interest expense.
| F-16 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
| 7. | Income Taxes |
The provision for income taxes consists of the following:
| Year Ended December 31, 2013 | Year Ended December 31, 2012 | Period from March 9, 2004 (inception) to December 31, 2013 | ||||||||||
| Current: | ||||||||||||
| Federal | $ | - | $ | - | $ | - | ||||||
| State | 800 | 800 | 8,000 | |||||||||
| Total current | 800 | 800 | 8,000 | |||||||||
| Deferred: | ||||||||||||
| Federal | - | - | - | |||||||||
| State | - | - | - | |||||||||
| Total deferred | - | - | - | |||||||||
| Provision for income taxes | $ | 800 | $ | 800 | $ | 8,000 | ||||||
The components of deferred tax assets and liabilities consist of the following:
| December 31, | 2013 | 2012 | ||||||
| Deferred tax assets | ||||||||
| Net operating losses | $ | 1,866,000 | $ | 1,610,000 | ||||
| Patents | 560,000 | 520,000 | ||||||
| Accrued expenses | 550,000 | 390,000 | ||||||
| R&D credits | 57,000 | 45,000 | ||||||
| Warrants | 45,000 | 45,000 | ||||||
| Total | 3,078,000 | 2,610,000 | ||||||
| Less: Valuation allowance | (3,078,000 | ) | (2,610,000 | ) | ||||
| $ | - | $ | - | |||||
The Company’s federal and state net operating loss carryfowards at December 31, 2013 were approximately $4,681,000 and $4,713,000, respectively, and will begin to expire in 2019 if not utilized.
The Company reviews its deferred tax assets for realization based upon historical taxable income, prudent and feasible tax planning strategies, the expected timing of the reversals of existing temporary differences and expected future taxable income. The Company has concluded that it is more likely than not that the deferred tax assets will not be realized. Accordingly, the Company has recorded a valuation allowance against the net deferred tax assets in the amount of $3,078,000 at December 31, 2013. The net change in the valuation allowance for the year ended December 31, 2013 was $468,000.
The effective tax rate differs from the statutory tax rate principally due to the change in valuation allowance, nondeductible permanent differences, credits, and state income taxes.
| F-17 |
Bone
Biologics, Inc.
(A Development Stage Company)
Notes to Financial Statements
| 8. | Related Party Transactions |
In September 2006, the Company entered into a consulting agreement with one of its stockholders whom previously served as chairman, president and CEO of the Company. The Company paid $120,000 for each year ended December 31, 2013 and 2012, in consulting fees to this related party.
In addition, one of the Company’s co-founders had previously provided research and development consulting services to the Company and earned an aggregate of $320,000 of fees from inception to January 2010. Of the $320,000, $52,500 has been deferred for payment until the Company’s next equity financing. As of December 31, 2013 and December 31, 2012, the $52,500 deferred payment was included in the accrued expenses.
During the year ended December 31, 2013 a related party, MTF, advanced $41,300 (2012 - $0) to the Company.
See Note 5 for related party notes payable to MTF.
| F-18 |
(formerly AFH Acquisition X, Inc.)
Unaudited pro forma consolidated financial statements as at June 30, 2014
| F-19 |
Bone Biologics, Corp.
(formerly AFH Acquisition X, Inc.)
PRO
FORMA CONSOLIDATED STATEMENT OF FINANCIAL POSITION AS AT June 30, 2014
(Unaudited)
| AFH Acquisition X, Inc. | Bone Biologics, Inc. | Subtotal | Pro forma adjustments | Notes | Bone Biologics, Corp. | |||||||||||||||||||
| April 30, 2014 | June 30, 2014 | June 30, 2014 | ||||||||||||||||||||||
| Assets | ||||||||||||||||||||||||
| Current assets | ||||||||||||||||||||||||
| Cash | 58 | 163 | 221 | 250,000 | f | 715,221 | ||||||||||||||||||
| 500,000 | e | |||||||||||||||||||||||
| (35,000 | ) | h | ||||||||||||||||||||||
| Prepaid expenses and other current assets | 9,000 | 9,000 | 9,000 | |||||||||||||||||||||
| Deferred transaction costs | 100,335 | 100,335 | 366,496 | h | 461,831 | |||||||||||||||||||
| (5,000 | ) | h | ||||||||||||||||||||||
| Deferred financing fees | 16,581 | 16,581 | 13,105 | h | 16,581 | |||||||||||||||||||
| (13,105 | ) | h | ||||||||||||||||||||||
| Total Current Assets | 58 | 126,079 | 126,137 | 1, 202,633 | ||||||||||||||||||||
| Total Assets | 58 | 126,079 | 126,137 | 1,202,633 | ||||||||||||||||||||
| Liabilities | ||||||||||||||||||||||||
| Current liabilities | ||||||||||||||||||||||||
| Accrued liabilities | 4,454 | 1,829,927 | 1,834,381 | 590,000 | h | 2,167,294 | ||||||||||||||||||
| 200,000 | h | |||||||||||||||||||||||
| (410,726 | ) | j | ||||||||||||||||||||||
| (44,683 | ) | i | ||||||||||||||||||||||
| (1,678 | ) | g | ||||||||||||||||||||||
| Advances to related party | 130,674 | 130,674 | 130,674 | |||||||||||||||||||||
| Due to parent | 39,173 | 39,173 | (39,173 | ) | i | - | ||||||||||||||||||
| Convertible notes payable | (111,893 | ) | f | - | ||||||||||||||||||||
| 250,000 | f | |||||||||||||||||||||||
| 111,893 | g | |||||||||||||||||||||||
| (250,000 | ) | g | ||||||||||||||||||||||
| Notes payable, net | 277,066 | 277,066 | (250,000 | ) | k | - | ||||||||||||||||||
| 15,879 | k | |||||||||||||||||||||||
| (50,000 | ) | k | ||||||||||||||||||||||
| 7,055 | k | |||||||||||||||||||||||
| Notes payable to related parties, net | 4,106,972 | 4,106,972 | (1,107,000 | ) | j | 2,754,770 | ||||||||||||||||||
| (100,000 | ) | k | ||||||||||||||||||||||
| 104,798 | k | |||||||||||||||||||||||
| (250,000 | ) | k | ||||||||||||||||||||||
| Total current liabilities | 43,627 | 6,344,639 | 6,388,266 | 5,052,738 | ||||||||||||||||||||
| 43,627 | 6,344,639 | 6,388,266 | 5,052,738 | |||||||||||||||||||||
| Stockholders’ deficit | ||||||||||||||||||||||||
| Series A convertible preferred stock | 49 | 49 | (49 | ) | l | - | ||||||||||||||||||
| Series B convertible preferred stock | 534 | 534 | (534 | ) | l | - | ||||||||||||||||||
| Common stock | 5,000 | 510 | 5,510 | 50 | e,g | 17,913 | ||||||||||||||||||
| 5 | k | |||||||||||||||||||||||
| (5,000 | ) | i | ||||||||||||||||||||||
| 152 | j | |||||||||||||||||||||||
| 583 | l | |||||||||||||||||||||||
| 114 | k | |||||||||||||||||||||||
| 276 | k | |||||||||||||||||||||||
| 16,157 | o | |||||||||||||||||||||||
| 67 | g | |||||||||||||||||||||||
| Additional paid-in capital | 20,000 | 2,145,066 | 2,165,066 | 499,950 | e | 5,894,641 | ||||||||||||||||||
| 111,893 | f | |||||||||||||||||||||||
| (20,000 | ) | i | ||||||||||||||||||||||
| (43,569 | ) | i | ||||||||||||||||||||||
| 1,517,574 | j | |||||||||||||||||||||||
| 114,205 | k | |||||||||||||||||||||||
| 275,417 | k | |||||||||||||||||||||||
| (13,557 | ) | h | ||||||||||||||||||||||
| 393,158 | h | |||||||||||||||||||||||
| 501,611 | g | |||||||||||||||||||||||
| 54,665 | k | |||||||||||||||||||||||
| (2,500 | ) | h | ||||||||||||||||||||||
| 26,200 | h | |||||||||||||||||||||||
| (15,720 | ) | h | ||||||||||||||||||||||
| (23,333 | ) | h | ||||||||||||||||||||||
| (16,157 | ) | o | ||||||||||||||||||||||
| 39,173 | i | |||||||||||||||||||||||
| 330,564 | n | |||||||||||||||||||||||
| Accumulated deficit | (68,569 | ) | (8,364,719 | ) | (8,433,288 | ) | 68,569 | i | (9,762,660 | ) | ||||||||||||||
| (590,000 | ) | h | ||||||||||||||||||||||
| (200,000 | ) | h | ||||||||||||||||||||||
| (7,055 | ) | k | ||||||||||||||||||||||
| (15,879 | ) | k | ||||||||||||||||||||||
| (10,480 | ) | h | ||||||||||||||||||||||
| (11,667 | ) | h | ||||||||||||||||||||||
| (13,105 | ) | h | ||||||||||||||||||||||
| (216,691 | ) | g | ||||||||||||||||||||||
| (2,500 | ) | h | ||||||||||||||||||||||
| (330,564 | ) | n | ||||||||||||||||||||||
| Total stockholders’ deficit | (43,569 | ) | (6,218,560 | ) | (6,262,129 | ) | (3,850,105 | ) | ||||||||||||||||
| Total liabilities and stockholders’ deficit | 58 | 126,079 | 126,137 | 1,202,633 | ||||||||||||||||||||
See accompanying notes to pro forma consolidated financial statements
| F-20 |
Bone
Biologics, Corp.
(formerly AFH Acquisition X, Inc.)
PRO
FORMA CONSOLIDATED STATEMENT OF OPERATIONS AND COMPREHENSIVE INCOME
FOR THE YEAR ENDED DECEMBER 31, 2013
(Unaudited)
| AFH Acquisition X, Inc. | Bone Biologics, Inc. | Subtotal | Pro forma adjustments | Notes | Bone Biologics, Corp. | |||||||||||||||||||
| Year Ended October 31, 2013 | Year Ended December 31, 2013 | Year Ended December 31, 2013 | ||||||||||||||||||||||
| Revenues: | - | - | - | - | ||||||||||||||||||||
| Cost of revenue: | - | - | - | - | ||||||||||||||||||||
| - | - | - | - | |||||||||||||||||||||
| Gross profit | - | - | - | - | ||||||||||||||||||||
| Operating expenses | ||||||||||||||||||||||||
| Research and development | 188,236 | 188,236 | 188,236 | |||||||||||||||||||||
| General and administrative | 4,806 | 483,749 | 488,555 | (50,000 | ) | q | 1,730,288 | |||||||||||||||||
| 1,291,733 | t | |||||||||||||||||||||||
| Total operating expenses | 4,806 | 671,985 | 676,791 | 1,918,524 | ||||||||||||||||||||
| Loss from operations | (4,806 | ) | (671,985 | ) | (676,791 | ) | (1,918,524 | ) | ||||||||||||||||
| Other income (expense) | ||||||||||||||||||||||||
| Other expense | - | - | - | - | ||||||||||||||||||||
| Interest expense, net | - | (409,419 | ) | (409,419 | ) | (150,371 | ) | r,s | (259,048 | ) | ||||||||||||||
| Total other income (expense) | - | - | - | - | ||||||||||||||||||||
| Loss before provision for income taxes | (4,806 | ) | (1,081,404 | ) | (1,086,210 | ) | (2,177,572 | ) | ||||||||||||||||
| Provision for income taxes | 400 | 800 | 1,200 | p | 1,200 | |||||||||||||||||||
| Net loss and comprehensive loss | (5,206 | ) | (1,082,204 | ) | (1.087,410 | ) | (2,178,772 | ) | ||||||||||||||||
See accompanying notes to pro forma consolidated financial statements
| F-21 |
Bone
Biologics, Corp.
(formerly AFH Acquisition X, Inc.)
PRO
FORMA STATEMENT OF OPERATIONS AND COMPREHENSIVE INCOME
FOR THE SIX MONTHS ENDED JUNE 30, 2014
(Unaudited)
| AFH Acquisition X, Inc. | Bone Biologics, Inc. | Subtotal | Pro forma adjustments | Notes | Bone Biologics, Corp. | |||||||||||||||||||
| Six
Months Ended April 30, 2014 | Six
Months Ended June 30, 2014 | Six
Months Ended June 30, 2014 | ||||||||||||||||||||||
| Revenues: | - | - | - | - | ||||||||||||||||||||
| Cost of revenue: | - | - | - | - | ||||||||||||||||||||
| Gross profit | - | - | - | - | ||||||||||||||||||||
| Operating expenses | ||||||||||||||||||||||||
| Research and development | 183,111 | 183,111 | 183,111 | |||||||||||||||||||||
| General and administrative | 3,364 | 307,948 | 311,312 | 164,217 | w | 475,529 | ||||||||||||||||||
| Total operating expenses | 3,364 | 491,059 | 494,423 | 658,640 | ||||||||||||||||||||
| Loss from operations | (3,364 | ) | (491,059 | ) | (494,423 | ) | (658,640 | ) | ||||||||||||||||
| Other income (expense) | ||||||||||||||||||||||||
| Other expense | (9,623 | ) | (9,623 | ) | (9,623 | ) | ||||||||||||||||||
| Interest expense, net | - | (250,533 | ) | (250,533 | ) | (153,561 | ) | u,v | (96,972 | ) | ||||||||||||||
| Total other income (expense) | - | (260,156 | ) | (260,156 | ) | (106,595 | ) | |||||||||||||||||
| Loss before provision for income taxes | (3,364 | ) | (751,215 | ) | (754,579 | ) | (765,235 | ) | ||||||||||||||||
| Provision for income taxes | 400 | - | 400 | p | 400 | |||||||||||||||||||
| Net loss and comprehensive loss | (3,764 | ) | (751,215 | ) | (754,979 | ) | (765,635 | ) | ||||||||||||||||
See accompanying notes to pro forma consolidated financial statements
| F-22 |
Bone
Biologics, Corp.
(formerly AFH Acquisition X, Inc.)
NOTES
TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
Six Months ended June 30, 2014
(Unaudited)
| 1. | Basis of presentation |
On September 19, AFH Acquisition X, Inc. (“AFH”) and its wholly-owned subsidiary, Merger Sub, entered into the Merger Agreement between AFH, Merger Sub, and Bone Biologics, Inc. Pursuant to the Merger Agreement, Merger Sub merged with and into Bone Biologics with Bone Biologics remaining as the surviving corporation in the Merger. Upon the consummation of the Merger, the separate existence of Merger Sub ceased, on September 22, 2014 AFH officially changed its name to “Bone Biologics, Corp.” to more accurately reflect the nature of its business, and Bone Biologics became a wholly-owned subsidiary of the Company.
As used in this Current Report, the terms “we,” “us,”, “our” and “the Company” refer to AFH, after giving effect to the Merger or Bone Biologics, Corp., unless otherwise stated or the context clearly indicates otherwise. The term “AFH” refers to the Company, as it was named “AFH Acquisition X, Inc.” before giving effect to the Merger.
These pro forma consolidated financial statements have been prepared from, and should be read in conjunction with the audited financial statements as at October 31, 2013 and 2012 and for each of the years in the two years ended October 31, 2013; and the unaudited financial statements as at April 30, 2014 and for the six month periods ended April 30, 2014 and 2013 of Acquisition X, Inc. In addition, these pro forma consolidated financial statements have been prepared from, and should be read in conjunction with the audited financial statements as at December 31, 2013 and 2012 and for the two years then ended; and the unaudited financial statements as at June 30, 2014 and for the six month periods ended June 30, 2014 and 2013 of Bone Biologics. These financial statements are included elsewhere in this Filing Statement.
The Merger will be treated as a recapitalization of the Company for financial accounting purposes and is being accounted for as a “reverse merger,” and although the Company is the legal acquirer, Bone Biologics, Inc. is deemed to be the acquirer for accounting purposes in the reverse merger. Consequently, the assets and liabilities and the historical operations that will be reflected in the financial statements prior to the Merger will be those of Bone, and the consolidated financial statements after completion of the Merger will include the assets and liabilities of Bone, historical operations of Bone and operations of Bone from the Closing Date of the Merger and in all future filings with the Securities and Exchange Commission (the “SEC”).
The pro forma consolidated statement of financial position gives effect to the transactions described in Note 6 below as if they had occurred on June 30, 2014. The pro forma consolidated statement of financial position and results of operations are not necessarily indicative of the results that would have actually occurred if the transactions had been consummated on this date, nor is it necessarily indicative of the future financial position of the Company.
| 2. | Summary of significant accounting policies |
The accounting policies adopted by the Company are those of Bone Biologics, Inc. since it is the accounting acquirer. The unaudited pro forma consolidated financial information has been prepared based on the historical financial information of Bone Biologics, Inc. and AFH Acquisition X, Inc. giving effect to the transaction between Bone Biologics with AFH Acquisition X and related adjustments described in these notes.
| 3. | Transaction |
The pro forma adjustments to the pro forma consolidated statement of financial position have been prepared to reflect the following transactions:
| (a) | In connection with the Merger, all of the issued and outstanding shares of Bone Biologics Inc.’s $0.0001 par value common stock (“Bone Biologics Common Stock”) converted into 19,237,857 shares of the Company’s Common Stock (including 1,526,926 shares issuable upon the exercise of outstanding warrants and 5,568,016 shares issuable upon the conversion of debt including accrued interest through 6/30/2014) (the “Company Merger Consideration”). In exchange, Bone Biologics agreed to pay AFH Holding & Advisory, LLC (“AFH Advisory”) the principal sum of $590,000. On July 3, 2014, Bone Biologics paid AFH Advisory $250,000 of such amount and on July 31, 2014, Bone Biologics issued that certain Promissory Note, dated July 31, 2014 (the “Note”), pursuant to which the Bone Biologics promised to pay AFH Advisory the principal sum of $340,000. MTF has granted AFH Advisory a standby letter of credit in the amount of $340,000 for the remaining amount due under the Note. On September 19, 2014, the Note was assigned to the Company. | |
| The 5,000,000 outstanding shares of Common Stock of AFH Acquisition X, Inc. prior to the Merger were consolidated into 3,853,600 shares of Bone Biologics, Corp. Common Stock and the remaining shares were cancelled. | ||
| (b) | In addition, upon consummation of the Merger, MTF agreed to convert 30% or $1,517,726 of the outstanding convertible promissory notes (including accrued interest through 6/30/2014) entered into between the Company and MTF (the “MTF Notes”) into Series B Preferred Stock of the Company at $ 1.00, then to Common Stock of the Company at 1:1 basis. | |
| Upon consummation of the Merger, the 2013 Bridge Note holders agreed to convert all outstanding principal and accrued interest (through 6/30/2014) of $444,683 at $1.00 per share into 444,683 shares of Common Stock on a 1:1 basis. | ||
| Upon consummation of the Merger, the holders of the Subsequent Orthofix Notes converted $500,000 into Common Stock at $0.75 per share for a total of 668,904 shares of Common Stock (which includes the $250,000 May 2014 MTF Note, and related accrued interest, assigned to Orthofix). |
| F-23 |
| (c) | Upon consummation of the Merger, MTF agreed to (i) convert its entire outstanding shares of Series A preferred stock and Series B preferred stock of the Company held into shares of Common Stock on a 1:1 basis. | |
| (d) | A reserve was established for the 2014 Equity Incentive Plan of 2,642,898 shares of Common Stock, of which 583,059 options to purchase shares of Common Stock were issued to management of Bone Biologics, Corp. The stock options vest as to one third immediately upon issuance and the remainder vest 50% at the end of each of the next 2 years. An aggregate of 350,000 stock options and 350,000 restricted shares were granted to the board members and CEO of Bone Biologics, Corp. The stock options vest upon completion of 1 year of service and the restricted stock vests at the end of each quarter over 1 year. The estimated fair value of the 583,059 stock options, 350,000 board and CEO options and 350,000 restricted shares was $476,866, $282,799 and $350,000, respectively, and will be recognized over respective vesting periods. The stock options were valued using the Black-Scholes Option Pricing Model with an exercise price of $1.00, estimated volatility of 109%, a risk-free rate of 1.62% and expected life of 5.5-5.75 years. | |
| In addition, warrants to purchase 699,671 shares of Bone Biologics, Corp. Common Stock were issued to a consultant outside of the 2014 Equity Incentive Plan. The warrants vest as to one third immediately upon issuance with the remainder vesting 50% at the end of each of the next 2 years and were valued at $510,437 using the Black-Scholes Option Pricing Model with an exercise price of $1.00, volatility of 109%, risk free rate of 0.88% and have a 4 year term. |
| 4. | Pro forma adjustments – Consolidated Statement of Financial Position |
| (e) | Impact of Subsequent Orthofix Financing | |
| Reflect the aggregate of 500,000 subscription receipts issued at $1.00 per subscription to Orthofix for gross proceeds of $500,000 and recorded in Common Stock and additional paid-in capital. | ||
| (f) | Reflect the issuance of $250,000 Subsequent Orthofix Convertible Notes, which are convertible into 333,333 shares at $0.75 per share, and the issuance of Subsequent Orthofix Warrants to purchase 166,667 shares of the Company’s common stock at $1.50 per share. The fair value of the Subsequent Orthofix Warrants was $111,893 and was calculated using the Black-Scholes option pricing model with a risk-free rate of 0.88%, volatility of 109% and a 4 year term. | |
| (g) | Reflect the conversion of the Subsequent Orthofix Bridge Notes of $500,000 plus accrued interest of $1,678 into 668,904 shares of Common Stock and expensing of related debt discount of $216,691. Included in these amounts are the debt discount of $104,798 and interest of $1,678 associated with the $250,000 May 2014 MTF Note which was assigned to Orthofix. | |
| (h) | Offering costs incurred in connection with the Private Placement; allocated to additional paid in capital as issuance costs and deferred financing fees for debt placement. Reflect costs incurred in connection with the Merger. |
| ● | Promissory note due AFH Advisory of $590,000, which reflects a shell fee of $500,000 and legal expenses of $90,000 and expensed as a cost of the merger. Bone Biologics paid $250,000 as of July 3, 2014. In July 2014, Bone issued a secured promissory note for $340,000 and MTF granted AFH Advisory a standby letter of credit in the amount of $340,000 for the remaining amount due under the Note. | |
| ● | At the closing of the Private Placement, AFH Advisory is entitled to receive warrants (“Extra Warrants”) to purchase up to 500,000 shares of Common Stock at the closing of the Private Placement at the price per share offered in the Private Placement or $1.00 per share with a 5 year term. The warrants have an estimated fair value of $393,159 calculated using the Black-Scholes Option Pricing Model with a risk-free rate of 1.62% and volatility of 109%. The fair value of the warrants was allocated and charged (i) $13,105 to deferred financing fees related to the Subsequent Orthofix Notes, (ii) $13,557 to Subsequent Orthofix Subscription and offset against the related proceeds and (iii) $366,496 charged to Deferred Transaction Costs for allocation to PPM and PIPE. Allocations are based on corresponding amounts of debt raised to date and total estimated equity associated with the Subsequent Orthofix Raise, PPM and PIPE financing. | |
| ● | Legal expenses incurred in connection with the Merger of $200,000. | |
| ● | Forefront as Placement Agent was issued a warrant (the “Agent Warrant”) to purchase 46,667 shares of Common Stock at $1.00 per share upon completion of the Orthofix Subsequent Financing. The Agent Warrant is equal to 4% of the Common Stock underlying the securities issued in the Orthofix Subsequent Financing. The warrant has a 5 year term and total estimated fair value of $34,381 calculated using the Black-Scholes Option Pricing Model with a risk-free rate of 1.56% and volatility of 109%. An additional 33,334 shares were recorded in connection with the financing for an estimated fair value of $26,200. | |
| ● | The Company agreed to pay Forefront a cash fee equal to 4% of gross proceeds received from the Orthofix Subsequent Financing or an additional $30,000 and an estimated $5,000 in expenses, for an aggregate of $35,000 (in addition to $10,000 accrued at June 30, 2014 in connection with the May 2014 MTF Note assigned to Orthofix). Both the fair value of the Agent Warrant and the cash fee were allocated to debt (and written-off as the Subsequent Orthofix Notes were converted to equity) and additional paid-in capital (which offset the proceeds received from the Subsequent Orthofix Subscription), based on the corresponding amounts of debt and equity raised in the Subsequent Orthofix Financing. | |
| ● | Previously deferred transaction costs of $5,000 were allocated to the Subsequent Orthofix Financing (which includes the assigned MTF 2014 Note) and charged 50% against the proceeds from the Orthofix Subsequent Financing within additional paid-in capital and 50% to interest expense (accumulated deficit) for Orthofix Subsequent Notes which were converted to equity. | |
| ● | AFH Advisory and MTF will each receive restricted stock awards contingent upon completion of milestone targets equal to 2.5% of the fully diluted shares of the Company at the time of completion of all such targets. Compensation expense associated with the restricted shares will be recognized when probable over the performance period of 2 years. There was no impact to the Pro forma financial statements. |
| F-24 |
| (i) | Reflect the exchange of AFH Acquisition X shares for shares of the Company. |
| ● | Record the transfer of the value of AFH Acquisition X common shares of $5,000 to additional paid-in capital. | |
| ● | Record the transfer of the value of AFH Acquisition X additional paid-in capital of $20,000 to Bone Biologics additional paid-in capital. | |
| ● | Record the elimination of AFH Acquisition X deficit of $68,569. | |
| ● | Net amount of ($43,569), which represents the fair value of net assets of AFH Acquisition X and charged to additional paid-in capital as a cost of the listing. | |
| ● | Record the reclass of amount due to parent of $39,173 to equity – additional paid-in capital. |
| (j) | Record the conversion of Bone Biologics MTF Convertible Notes related party debt and accrued interest into Common Stock on a 1 for 1 basis. | |
| Convertible debt of $1,517,726, including accrued interest of $410,726 through 6/30/2014, converts into 1,517,726 shares of Series B Preferred stock at $1.00 per share and subsequently into Common Stock on a 1 for 1 basis. | ||
| (k) | Record the conversion of Bone Biologics 2013 Bridge Notes and accrued interest into Common Stock on a 1 for 1 basis. | |
| Bridge Notes of $444,683 including interest of $44,683 converts into 444,683 shares of Common Stock at $1.00 per share. The remaining debt discount of $22,935 is charged to interest expense (accumulated deficit). | ||
| (l) | Record the conversion of Bone Biologics Series A and Series B Preferred into Common Stock on a 1 for 1 basis. | |
| At the time of the Reverse Merger, MTF agreed to convert its entire outstanding holdings of Bone Biologics Preferred Stock into shares of Common Stock, which consist of 493,339 shares of Series A Preferred and 5,336,099 shares of Series B Preferred for an aggregate of 5,829,438 shares. | ||
| Since the value of the adjusted number of shares of common stock into which the convertible preferred stock was convertible was not greater than the value of the number of shares of common stock into which the convertible preferred stock was convertible prior to such adjustment, based on the market price of the common stock on the date the convertible preferred stock was issued, the Company did not recognize a beneficial conversion feature associated with the preferred stock. | ||
| (m) | Record the exchange of Bone Biologics outstanding warrants for warrants of the Company. | |
| As a result of the transaction, Bone Biologics warrants to purchase 1,526,926 shares of common stock were exchanged for warrants of Bone Biologics, Corp on a 1:1 basis. There was no impact to the Pro forma financial statements at June 30, 2014. | ||
| (n) | Record stock-based compensation associated with the issuance of new management and consultant stock options, for an aggregate total of $330,564. | |
| Reflect stock-based compensation of $162,103 related to the issuance of 583,059 options to purchase shares of Common Stock issued to management of Bone Biologics, Corp., of which 198,202 vest immediately upon issuance. | ||
| Reflect stock-based compensation of $168,461 related to the issuance of warrants to purchase 699,671 shares of Bone Biologics, Corp. Common Stock issued to a consultant outside of the 2014 Equity Incentive Plan, of which 230,915 vest immediately upon issuance. | ||
| (o) | Adjust the PAR value of Bone Biologics Common Stock | |
| PAR value was adjusted from $0.0001 per share to $0.001 per share of Bone Biologics, Corp. Common stock for a total of 17,913,012 issued and outstanding shares, and a reclass of $16,157 from APIC to Common Stock for a total value of $17,913. | ||
| (p) | Tax Impact | |
| Although the Company will be subject to applicable US and California income tax and other tax obligations, we do not expect that an income tax will be payable by the Company in the foreseeable future. The US statutory corporate tax rate on the operating entity of the Company is currently 35%. However, Bone Biologics has significant net operating loss carryovers and deferred tax assets that are fully reserved, which brings Bone Biologic’s effective tax rate down to 0%. As a result, there is no income tax impact reflected in the Pro forma financial statements. |
| 5. | Pro forma adjustment –Statement of Operations for the year ended December 31, 2013 |
The pro forma adjustments to the pro forma statement of operations for the year ended December 31, 2013 have been prepared to reflect the following transactions:
| (q) | Reversal of one-time legal fees of $50,000 associated with the LOI transactions including the PPM and Merger Agreement. | |
| (r) | Reversal of $83,267 of interest expense, of which $64,178 is related to the 30% conversion of MTF Notes and $19,088 related to the 2013 Bridge Notes. | |
| (s) | Reversal of amortization of debt discount related to the 2013 Bridge Notes of $67,104. | |
| (t) | Estimated stock-based compensation expense of $1,291,733 related to the management and consultant stock option grants, board and CEO stock option grants and restricted stock awards. |
| F-25 |
| 6. | Pro forma adjustments –Statement of Operations for the six months ended June 30, 2014 |
The pro forma adjustments to the pro forma statement of operations for the three months ended March 31, 2014 have been prepared to reflect the following transactions:
| (u) | Reversal of $62,450 of interest expense, of which $34,927 is related to the 30% conversion of MTF Notes, $25,845 is related to the 2013 Bridge Notes and $1,678 is related to the May 2014 MTF Note. | |
| (v) | Reversal of amortization of debt discount of $91,111, of which $20,959 is related to the 2013 MTF Bridge Notes and 2014 MTF Note, $53,430 is related to the Orthofix 2013 Bridge Notes and $16,721 is related to the AFH 2013 Bridge Note. | |
| (w) | Estimated stock-based compensation expense of $164,185, which is related to the management and consultant stock option grants. |
| 7. | Share capital |
Common shares
The following table summarizes the changes in share capital that will occur pursuant to the aforementioned transactions as of June 30, 2014:
| Common shares of AFH Acquisition X, Inc. issued and outstanding as at June 30, 2014(1) | 5,000,000 | $ | (43,569 | ) | Note 6(a) | |||||||
| Consolidation of AFH Acquisition X, Inc. shares(1) | (1,146,400 | ) | Note 6(a) | |||||||||
| Shares issued in exchange for Bone Biologics shares to effect reverse merger (2)(3) | 14,059,412 | (3,806,536 | ) | Note 6(a)(b)(c) | ||||||||
| Common shares of Bone Biologics, Corp. issued and outstanding, at June 30, 2014 after giving effect to Pro-forma adjustments | 17,913,012 | $ | (3,850,105 | ) |
(1) Reflects consolidation of the 5,000,000 outstanding shares of Common Stock of AFH Acquisition X, Inc. prior to the Merger into 3,853,600 shares of Bone Biologics, Corp. Common Stock and the remaining shares were cancelled.
(2) Assumes the following:
| a) | Outstanding 5,098,661 shares of Common Stock of Bone Biologics converted on a 1:1 basis; | |
| b) | Conversion of 30% MTF convertible debt principal and accrued interest (through 6/30/2014) of $1,517,726 into Common Stock at a price of $1.00 per share or 1,517,726 shares; | |
| c) | Conversion of $444,683 principal and accrued interest (through 6/30/2014) of 2013 Bridge Notes into Common Stock at a price of $1.00 per share or 444,683shares; | |
| d) | Conversion of all outstanding Bone Biologics Series A and B Preferred shares into Common Stock on a 1:1 basis, or 5,829,438 shares; and | |
| e) | Subsequent Orthofix Financing of 1,168,904 shares of Common Stock which includes the conversion of $500,000 Convertible Notes and $1,678 accrued interest at $0.75 per share (inclusive of the shares from conversion of the May 2014 MTF Note and related accrued interest which was assigned to Orthofix). |
(3) Does not include:
| e) | Conversion of remaining principal and interest (through 6/30/2014) of MTF Notes of $3,605,607; | |
| f) | The exercise of outstanding warrants and options to purchase shares of the Company’s Common stock as follows: |
| i. | Warrants issued to consultants for the purchase of 1,146,596 shares of Common Stock at prices per share ranging from $0.00 to $1.00. | |
| ii. | Warrants issued to 2013 Bridge Note holders for the purchase of 200,000 shares of Common Stock; | |
| iii. | Orthofix Subsequent Warrants for the purchase of 333,334 shares of Common Stock at $1.00 per share (including the 166,667 assigned to Orthofix from the May 2014 MTF Note); | |
| iv. | Extra Warrants issued to AFH as advisor for the purchase of 500,000 shares of Common Stock at a price per share of $1.50. | |
| v. | Advisor Warrants issued to Forefront as placement agent on Orthofix Subsequent Financing of 46,667 shares at $1.00 per share. | |
| vi. | Stock options issued to Bone Biologics, Corp. management for the purchase of 583,059 shares of Common Stock at $1.00 per share. | |
| vii. | Issuance of equity awards to Board members and CEO for an aggregate of 350,000 options with an exercise price of $1.00 and 350,000 restricted stock grants. The stock options vest after one year and the restricted stock grants are issued quarterly over one year. | |
| viii. | Shares reserved for the 2014 Equity Incentive Plan of 2,642,898, of which 1,359,839 shares are available for future issuance (after adjustment for the grants in vi and vii above). | |
| ix. | Warrants to purchase 625,000 shares of Common Stock at a price per share of $1.62 issued pursuant to the 8.5% MTF Omnibus Note of $500,000 entered into September 2014 and maturing on December 31, 2014. | |
| x. | The issuance of performance-based restricted stock awards granted to AFH and MTF, contingent and issuable upon completion of all milestone targets at 2.5% of fully diluted shares calculated at completion. |
| F-26 |
| Bone Biologics, Inc. | |
| Condensed Financial Statements | |
| For the Three and Six Months Ended June 30, 2014 and 2013 (unaudited) |
| F-27 |
Bone Biologics, Inc.
(A Development Stage Company)
Condensed Financial Statements
For the Three and Six Months Ended June 30, 2014 and 2013 (unaudited)
| F-28 |
Bone Biologics, Inc.
Contents
| Condensed Financial Statements | |
| Condensed Balance Sheets | F-30 |
| Condensed Statements of Operations | F-31 |
| Condensed Statements of Cash Flows | F-32 |
| Condensed Notes to Financial Statements | F-33 – F-46 |
| F-29 |
Bone Biologics, Inc.
| June 30, 2014 | December 31, 2013 | |||||||
| (unaudited) | ||||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash | $ | 163 | $ | 1,538 | ||||
| Prepaid expenses | 9,000 | 10,767 | ||||||
| Deferred transaction costs | 100,335 | 75,000 | ||||||
| Deferred financing fees | 16,581 | - | ||||||
| Total current assets | 126,079 | 87,305 | ||||||
| Total assets | $ | 126,079 | $ | 87,305 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities | ||||||||
| Accrued expenses | $ | 1,829,927 | $ | 1,525,604 | ||||
| Advances due to related party | 130,674 | 41,300 | ||||||
| Notes payable to related party, net of debt discount | 4,106,972 | 3,947,817 | ||||||
| Notes payable, net of debt discount | 277,066 | 180,690 | ||||||
| Total current liabilities | 6,344,639 | 5,695,411 | ||||||
| Total liabilities | 6,344,639 | 5,695,411 | ||||||
| Commitments and Contingencies | ||||||||
| Stockholders’ deficit | ||||||||
| Series A Preferred Stock, $0.0001 par value per share; 493,339 shares authorized; 493,339 shares issued and outstanding at June 30, 2014 and December 31, 2013 (liquidation preference $881,103 at June 30, 2014 and December 31, 2013, respectively) | 49 | 49 | ||||||
| Series B Preferred Stock, $0.0001 par value per share; 10,000,000 shares authorized; 5,336,099 shares issued and outstanding at June 30, 2014 and December 31, 2013 (liquidation preference $23,585,557 at June 30, 2014 and December 31, 2013, respectively) | 534 | 534 | ||||||
| Common stock, $0.0001 par value per share; 20,000,000 shares authorized; 5,098,661 shares issued and outstanding at June 30, 2014 and December 31, 2013 | 510 | 510 | ||||||
| Additional paid-in capital | 2,145,066 | 2,004,305 | ||||||
| Accumulated deficit | (8,364,719 | ) | (7,613,504 | ) | ||||
| Total stockholders’ deficit | (6,218,560 | ) | (5,608,106 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 126,079 | $ | 87,305 | ||||
See accompanying notes to condensed financial statement.
| F-30 |
Bone Biologics, Inc.
Condensed Statements of Operations
| Three
Months Ended June 30, 2014 | Three
Months Ended June 30, 2013 | Six
Months Ended June 30, 2014 | Six
Months Ended June 30, 2013 | |||||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | |||||||||||||
| Revenues | $ | - | $ | - | $ | - | $ | - | ||||||||
| Cost of revenues | - | - | - | - | ||||||||||||
| Gross profit | - | - | - | - | ||||||||||||
| Operating expenses | ||||||||||||||||
| Research and development | 155,257 | 56,619 | 183,111 | 96,213 | ||||||||||||
| General and administrative | 183,036 | 154,756 | 307,948 | 215,937 | ||||||||||||
| Total operating expenses | 338,293 | 211,375 | 491,059 | 312,150 | ||||||||||||
| Loss from operations | (338,293 | ) | (211,375 | ) | (491,059 | ) | (312,150 | ) | ||||||||
| Other Income (expense) | ||||||||||||||||
| Other expense | (9,623 | ) | - | (9,623 | ) | - | ||||||||||
| Interest expense, net | (116,294 | ) | (94,277 | ) | (250,533 | ) | (172,524 | ) | ||||||||
| Total other income (expense) | (125,917 | ) | (94,277 | ) | (260,156 | ) | (172,524 | ) | ||||||||
| Loss before provision for income taxes | (464,210 | ) | (305,652 | ) | (751,215 | ) | (484,674 | ) | ||||||||
| Provision for income taxes | - | 800 | - | 800 | ||||||||||||
| Net loss | $ | (464,210 | ) | $ | (306,452 | ) | $ | (751,215 | ) | $ | (485,474 | ) | ||||
See accompanying notes to condensed financial statements.
| F-31 |
Bone Biologics, Inc.
Condensed Statements of Cash Flows
| For the Six Months Ended June 30, 2014 | For the Six Months Ended June 30, 2013 | |||||||
| (unaudited) | (unaudited) | |||||||
| Cash flows from operating activities | ||||||||
| Net loss | $ | (751,215 | ) | $ | (485,474 | ) | ||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | ||||||||
| Accrued interest expense | 162,493 | 161,616 | ||||||
| Debt discount amortization | 91,111 | 10,447 | ||||||
| Loss on sale of marketable securities | 9,623 | |||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | 1,767 | (17,708 | ) | |||||
| Advances due to related party | 89,374 | - | ||||||
| Accrued expenses | 108,095 | (7,892 | ) | |||||
| Net cash provided by (used in) operating activities | (288,752 | ) | (339,011 | ) | ||||
| Cash flows from investing activities | ||||||||
| Proceeds from sale of marketable securities | 37,377 | - | ||||||
| Net cash provided by investing activities | 37,377 | - | ||||||
| Cash flows from financing activities | ||||||||
| Proceeds from issuance of notes payable | 250,000 | 374,533 | ||||||
| Net cash provided by financing activities | 250,000 | 374,533 | ||||||
| Net increase (decrease) in cash | (1,375 | ) | 35,522 | |||||
| Cash, beginning of period | 1,538 | 2,370 | ||||||
| Cash, end of period | $ | 163 | $ | 37,892 | ||||
| Supplemental non-cash information | ||||||||
| Issuance of warrants in connection with Notes Payable, net of amortization included above | $ | 104,798 | $ | 75,292 | ||||
| Issuance of warrants in payment of financing fees | $ | 8,180 | $ | - | ||||
| Interest paid | $ | - | $ | - | ||||
| Taxes paid | $ | - | $ | 800 | ||||
See accompanying notes to condensed financial statements.
| F-32 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
| 1. | The Company |
Bone Biologics, Inc. (“Bone” or the “Company”) was incorporated in California on March 9, 2004. Bone is a privately-held biotechnology company that is currently focused on bone regeneration in spinal fusion using the recombinant human protein, known as UCB-1 (or “Nell-1”). The Nell-1 protein is an osteoinductive recombinant protein that provides target specific control over bone regeneration. The protein has been licensed exclusively for worldwide applications to Bone Biologics through a technology transfer from the University of California, Los Angeles (“UCLA”). Bone Biologics recently received guidance from the United States Food and Drug Administration (“FDA”) that Nell-1 will be classified as a combination product with a device lead.
The production and marketing of the Company’s products and its ongoing research and development activities will be subject to extensive regulation by numerous governmental authorities in the United States. Prior to marketing in the United States, any drug developed by the Company must undergo rigorous preclinical (animal) and clinical (human) testing and an extensive regulatory approval process implemented by the FDA under the Food, Drug and Cosmetic Act. The Company has limited experience in conducting and managing the preclinical and clinical testing necessary to obtain regulatory approval. There can be no assurance that the Company will not encounter problems in preclinical and clinical trials that will cause the Company or the FDA to delay or suspend clinical trials.
The Company’s success will depend in part on its ability to obtain patents and product license rights, maintain trade secrets, and operate without infringing on the proprietary rights of others, both in the United States and other countries. There can be no assurance that patents issued to or licensed by the Company will not be challenged, invalidated, or circumvented, or that the rights granted thereunder will provide proprietary protection or competitive advantages to the Company.
In August 2012, the Company, along with its majority owner and debt holder, Musculoskeletal Transplant Foundation, Inc. (“MTF”) and AFH Holding & Advisory, LLC (“AFH”) entered into a Letter of Intent (“LOI”), as amended on August 19, 2013 and subsequently on May 7, 2014, to consummate a business combination through a share exchange, reverse merger, or other similar transactions resulting in the Company becoming a public entity (“The Transaction”) and the contemplated subsequent financings (see Note 4).
Going Concern and Liquidity
The Company has no significant operating history and, from March 9, 2004 (inception) to June 30, 2014, has generated a net loss of approximately $8.4 million. The Company will continue to incur significant expenses for development activities for their lead product Nell-1. The accompanying condensed financial statements for the three and six months ended June 30, 2014, have been prepared assuming the Company will continue as a going concern. In connection with the LOI, management intends to raise additional debt and/or equity financing to fund future operations and to provide additional working capital. However, there is no assurance that such financing will be consummated or obtained in sufficient amounts necessary to meet the Company’s needs.
The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the possible inability of the Company to continue as a going concern.
| F-33 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
| 2. | Summary of Significant Accounting Policies |
The unaudited interim condensed financial statements have been prepared by us pursuant to the rules and regulations of the Securities and Exchange Commission. The information furnished herein reflects all adjustments (consisting of normal recurring accruals and adjustments) which are, in the opinion of management, necessary to fairly present the operating results for the respective periods. Certain information and footnote disclosures normally present in the annual financial statements prepared in accordance with accounting principles generally accepted in the United States of America have been omitted pursuant to such rules and regulations. These financial statements should be read in conjunction with the audited financial statements and notes for the year ended December 31, 2013. The results of the three and six-month periods ended June 30, 2014 are not necessarily indicative of the results to be expected for the full year ending December 31, 2014.
Basis of Presentation
The accompanying condensed financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”).
Use of Estimates
The preparation of the accompanying condensed financial statements in conformity with GAAP requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of expenses during the reporting period. Significant estimates include warrants and income tax valuation allowances. Actual results could differ from those estimates.
Research and Development Costs
Research and development costs include, but are not limited to, patents and license expenses, payroll and other personnel expenses, consultants, expenses incurred under agreements with contract research and manufacturing organizations and animal clinical investigative sites and the cost to manufacture clinical trial materials. Costs related to research, design and development of products are charged to research and development expense as incurred.
Patents and Licenses
In March 2006, the Company entered into an exclusive license agreement (“Exclusive License Agreement”), with UCLA for the worldwide application of the UCB-1 protein through a technology transfer. See Note 4 for commitments related to the Exclusive License Agreement. Patent expenses include costs to acquire the license of UCB-1, which was de minimus, and costs to file patent applications related to UCB-1.
Bone Biologics expenses the costs incurred to file patent applications, all costs related to abandoned patent applications and maintenance costs, and these costs are included in research and development expenses. Costs associated with licenses acquired to be able to use products from third parties prior to receipt of regulatory approval to market the related products are also expensed. The Company’s licensed technologies may have alternative future uses in that they are enabling (or platform) technologies that can be the basis for multiple products that would each target a specific indication. Costs of acquisition of licenses are expensed.
| F-34 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
Deferred Financing and Transaction Costs
Deferred financing costs represent costs incurred in connection with the issuance of the convertible notes payable and private equity financing. Deferred financing costs related to the issuance of debt are being amortized over the term of the financing instrument using the effective interest method, while deferred transaction costs from equity financings are netted against the gross proceeds received from the equity financings.
During the three and six month periods ended June 30, 2014, the Company capitalized deferred financing costs of $18,180 in connection with the 2014 Note that closed in May (See Note 5). During the three and six months ended June 30, 2014, the Company incurred $25,335 of offering costs in connection with the future financings discussed in Note 4. As of June 30, 2014, all offering costs were included in accounts payable and accrued expenses in the accompanying financial statements. There were no deferred financing or transaction costs during the three and six months ended June 30, 2013.
Concentration of Credit Risk and Other Risks and Uncertainties
Cash and cash equivalents are maintained at financial institutions and, at times, balances may exceed federally insured limits. The Company has never experienced any losses related to these balances. All of the non-interest bearing cash balances were fully insured at June 30, 2014. As of January 1, 2013, federal insurance coverage is $250,000 per depositor at each financial institution. The Company’s non-interest bearing cash balances may from time to time exceed federally insured limits. There were no interest-bearing amounts on deposit in excess of federally insured limits at June 30, 2014 and December 31, 2013.
Income Taxes
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due and deferred taxes resulting from timing differences in recording of transactions for tax purposes and financial reporting purposes.
The deferred tax assets and liabilities represent the future tax return consequences of those differences, which will either be taxable or deductible when the assets and liabilities are received or settled. Valuation allowances are established when necessary to reduce deferred tax assets to amounts expected to be realized.
The accounting provisions related to uncertain income tax positions require the Company to determine whether any tax position in all open years meets a more likely than not threshold of being sustained upon examination by the applicable taxing authority. The Company did not have any changes to its liability for uncertain tax positions as at June 30, 2014 and December 31, 2013.
The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense. No such amounts are accrued as of June 30, 2014 and December 31, 2013.
| F-35 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
New Accounting Standards
The Company has reviewed all recently issued, but not yet adopted, accounting standards in order to determine their effects, if any, on its results of operation, financial position or cash flows. Based on that review, the Company believes that none of these pronouncements will have a significant effect on its condensed consolidated financial statements.
In June 2014, the FASB issued ASU 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements. ASU 2014-10 eliminates the distinction of a development stage entity and certain related disclosure requirements, including the elimination of inception-to-date information on the statements of operations, cash flows and stockholders’ equity. The amendments in ASU 2014-10 will be effective prospectively for annual reporting periods beginning after December 15, 2014, and interim periods within those annual periods, however early adoption is permitted. The Company adopted ASU 2014-10 during the quarter ended June 30, 2014, thereby no longer presenting or disclosing any information required by Topic 915.
| 3. | Accrued Expenses |
Accrued expenses consist of the following:
| June 30, 2014 | December 31, 2013 | |||||||
| Interest expense | $ | 1,311,618 | $ | 1,158,465 | ||||
| Professional services | 239,153 | 114,849 | ||||||
| Patents | 110,245 | 85,412 | ||||||
| Deferred compensation | 90,199 | 90,199 | ||||||
| Transaction costs | 75,000 | 75,000 | ||||||
| Payroll liabilities | 3,712 | 1,679 | ||||||
| $ | 1,829,927 | $ | 1,525,604 | |||||
| 4. | Commitments and Contingencies |
Letter of Intent
In August of 2012, the Company, along with its majority owner and debt holder, MTF, entered into a Letter of Intent (“LOI”) with AFH to consummate a business combination through a share exchange, reverse merger, or other similar transactions resulting in the Company becoming a public entity (“The Transaction”). In August, 2013, the LOI was amended and restated, and on May 7, 2014, the LOI was again amended and restated. The Amended and Restated Letter of Intent dated May 7, 2014 (the “Amended LOI”) contemplates and defines the following events:
| F-36 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
Consummation of Bridge Financings (“Closing I”)
In April 2013 and September 2013, the Company’s Board approved the Company to borrow up to an aggregate principal amount of $300,000 (April Bridge Financing) and $250,000 (September Bridge Financing) pursuant to the sale and issuance of convertible promissory notes and warrants to purchase common stock of the Company (collectively, the “Bridge Financings”). The note accrues interest at a rate of 12% per year and payable per quarter. A warrant to purchase the Company’s common stock equal to 50% of the original principal amount at $1.00 per share will be issued to each Bridge Financing participant. Principal and unpaid accrued interest may be converted into equity securities issued in the Company’s next equity financing in an aggregate amount of at least $2.5 million at a price equal to the price paid by investors in the next equity financing. On April 29, 2013 and on June 5, 2013, the Company borrowed $100,000 from MTF and $100,000 from Orthofix, Inc., respectively, under the April Bridge Financing. In September 2013, AFH purchased $50,000 of the April Bridge Financing. In October 2013, the Company borrowed an additional $150,000 from Orthofix under the September Bridge Financing.
Consummation of Business Combination (“Closing II”)
Under the amended LOI, it is contemplated that the Company and its equity holders will consummate a share exchange, reverse merger, or other business combination, with a Delaware corporation publicly reporting pursuant to United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), or a private Delaware corporation (“Acquisition Co.”), either directly or indirectly through an affiliate. If the post-business combination entity is not already a corporation publicly reporting pursuant to the Exchange Act, AFH will assist the post business combination entity with the filing of an appropriate registration statement resulting in the Company becoming a public company (“PubCo”).
Consummation of the Private Placement (“Closing III”)
Subsequent to Closing II, AFH will use its best efforts to assist PubCo in procuring one or more investors for a private financing, whether debt or equity, of a minimum of $2.5 million up to a maximum of $5.0 million. Such transaction is to include an over-allotment option of 15% at AFH’s discretion (the “Private Placement”).
Consummation of the PIPE Transaction (“Closing IV”)
Subsequent to Closing III, AFH Advisory will use its best efforts to assist PubCo in procuring an investment bank (the “Bank”) to facilitate a private investment in public equity transaction in an amount between $8.0 million and $10.0 million through the sale of securities of PubCo (the “PIPE”). Such transaction will include a 15% over allotment at AFH and/or the Bank’s discretion. Such transaction is contingent upon the appointment of a Bank and filing appropriate forms with the Financial Industry Regulatory Authority, Inc. (“FINRA”).
Consummation of Initial Public Offering (“Closing V”)
Subsequent to Closing IV, AFH will assist PubCo in procuring a Bank to act as underwriter for an initial public offering in an amount of up to $40.0 million (the “Initial Public Offering”). The Initial Public Offering shall include a 15% over allotment option at AFH and/or the Bank’s discretion. Such a transaction is contingent upon the appointment of the Bank.
At or prior to consummation of the Business Combination, MTF agrees to convert all of its outstanding shares of Series A preferred stock and Series B preferred stock of the Company into share of Common Stock.
| F-37 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
In addition, MTF agrees to convert 30%, 35%, and 35% of all outstanding convertible promissory notes and promissory notes converting as amended (principal and accrued interest) at each of the consummation of Closing II, Closing IV, and Closing V, respectively.
Upon (i) the consummation of the Business Combination, (ii) after giving effect to the issuance of any securities by Acquisition Co. in connection with the Business Combination (the “Business Combination Shares”), (iii) the completion of the Private Placement and (iv) after giving effect to the PIPE, the existing stockholders of Acquisition Co., and its owners, relatives, assignees and affiliates (collectively, the “AFH Group”), will own an aggregate of ten percent of the issued and outstanding common shares (the “Advisor Shares”) of PubCo.
At the consummation of Closing III, AFH Group shall be entitled to receive warrants to purchase up to 500,000 share of common stock of PubCo at the per share price of the shares offered in the Private Placement with a 5 year term and a cashless exercise provision (the “Extra Warrants”).
In addition to the Advisor Shares and Extra Warrants, AFH Group shall be entitled to receive warrants to purchase shares of common stock of PubCo (“Advisor Warrants”) in the amount necessary to cause AFH Group, when combined with the Advisor Shares, to have ownership equal to 10% of the fully diluted outstanding Common Stock, options and warrants at Closing III.
AFH will also be entitled to a reimbursement of $590,000 in connection with the Business Combination, which shall be payable directly from the net proceeds of the Private Placement (Closing III) to AFH at closing. Each party will bear all of its own costs and expenses in connection with each Closing.
In conjunction with the Amended LOI, the Company has agreed to covenants for the period of time between signing of the Amended LOI and the consummation of the Business Combination (Closing II) or upon termination of the agreement. Such covenants include restrictions and limitations on additional indebtedness, liquidation, selling of equity securities, amending organizational document and certain other normal and customary covenants. The Amended LOI will expire on August 31, 2014 if Closing II has not occurred. On August 28, 2014 the Amended LOI expiration date was extended to September 30, 2014.
License Commitment
In connection with the Exclusive License Agreement, the Company is required to pay a royalty fee beginning in the first year of commercial sale of the licensed product equal to 3% of net sales on a quarterly basis with an annual minimum royalty of $25,000 for the life of the patent rights. In addition to the royalty fees, the Company is also required to pay UCLA a $10,000 annual maintenance fee, $50,000 upon FDA marketing approval, and $25,000 upon first commercial sale.
On October 22, 2013, the Exclusive License Agreement was amended. The following additional fees will be due to UCLA i) 2% of the amount raised in the Private Placement. If the Private Placement did not close or was less than $2.5 million then a fee of $100,000 was due and payable by June 1, 2014, ii) $25,000 due upon closing of Phase 1 clinical trial and iii) $50,000 due upon dosing of Phase 3 clinical trial. The Company paid the fee of $100,000 in June 2014.
Contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company’s management does not believe that any such matters, individually or in the aggregate, will have a material adverse effect on the
Company’s business, financial condition, results of operations or cash flows.
| F-38 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations. However, the Company may record charges in the future as a result of these indemnification obligations.
In accordance with its amended and restated certificate of incorporation and amended and restated bylaws, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacity. There have been no claims to date and the Company has a director and officer insurance policy that enables it to recover a portion of any amounts paid for future potential claims.
| F-39 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
| 5. | Notes Payable to Related Party |
As of June 30, 2014 and December 31, 2013, the Company had a total of $5,486,133 and $5,095,427, respectively, of notes outstanding (principal and interest) including unamortized discount, with MTF a related party, which consisted of the following:
| Note Type | Issue Date | Maturity Date(1) | Interest Rate | June 30, 2014 | December 31, 2013 | |||||||||||||||
| Convertible Promissory Note | 1/18/08 | 3/31/15 | PRIME + 1 ½% | $ | 1,517,726 | $ | 1,479,654 | |||||||||||||
| Promissory Note | 11/04/08 | 3/31/15 | PRIME + 3% | 352,615 | 343,429 | |||||||||||||||
| Promissory Note | 3/17/09 | 3/31/15 | PRIME + 8% | 612,346 | 584,745 | |||||||||||||||
| Promissory Note | 8/24/09 | 3/31/15 | LIBOR + 8% | 24,271 | 23,193 | |||||||||||||||
| Tranched Promissory Note | 9/30/09 | 3/31/15 | LIBOR + 8% | 2,616,376 | 2,570,126 | |||||||||||||||
| Bridge Note, net of discount | 4/29/13 | 10/14/14 | 12% | 114,320 | 94,280 | |||||||||||||||
| Convertible Promissory Note, net of discount | 5/27/14 | 6/30/15 | 7% | 146,880 | - | |||||||||||||||
| 5,384,533 | 5,095,427 | |||||||||||||||||||
| Less: Accrued interest expense | 1,277,561 | 1,147,610 | ||||||||||||||||||
| Notes payable to related party, net of debt discount | $ | 4,106,972 | $ | 3,947,817 | ||||||||||||||||
| (1) | As amended. |
Accrued interest on the notes payable to related party of $1,277,561 (2013 - $1,147,610) is recorded in accrued expenses at June 30, 2014 and December 31, 2013.
Convertible Promissory Notes
The convertible promissory notes are considered hybrid instruments, which consist of a debt host instrument together with a conversion feature, thus giving the holder of a convertible note an option to convert into an equity instrument providing the holder a residual interest in the Company. The holder of a convertible promissory note also has the option to present its convertible promissory note to the Company and demand payment under the terms of the note after the maturity date or upon the occurrence of certain events such as the failure of the Company to make a payment on the note when due, bankruptcy or certain other liquidation events. The Company concluded that the convertible promissory notes would be accounted for as a typical debt instrument with related interest expense recorded in the Company’s statements of operations. The company concluded that there is no beneficial conversion feature as of the date of issuance of the convertible notes. However, the note contains a contingent feature whereby the conversion rate may be lowered if a financing occurs at a lower rate than the note’s conversion rate. If the contingency is met and the conversion feature is determined to be “beneficial” in a future accounting period, an additional financing cost would be recorded for the beneficial conversion feature in the Company’s statements of operations at that time.
In April 2005, the Company issued a $100,000 convertible promissory note (the “2005 Convertible Note”) to MTF in accordance with the Convertible Note Purchase Agreement and Convertible Promissory Note dated April 6, 2005. In April 2006 the Company issued an additional $612,000 convertible promissory note (the “2006 Convertible Note”) to MTF in accordance with the Convertible Note Purchase Agreement and Convertible Promissory Note dated April 7, 2006.
| F-40 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
The 2005 Convertible Note and the 2006 Convertible Note bore interest at a fixed rate of 6% per annum and prime plus one and one-half percent per annum, respectively, and matured on September 30, 2008 and September 30, 2009, respectively. In July 2006, the 2005 Note and 2006 Note, respectively, and accrued interest thereon for a total of $731,103, were converted into an aggregate of 409,352 shares of Series A preferred stock which was based on the conversion price of $1.786 per share (see Note 6). The conversion of the notes did not trigger a contingency and no additional financing charge was recognized.
In January 2008, the Company issued a $1,107,000 convertible promissory note (“January 2008 Note”) to MTF in accordance with the Convertible Promissory Note dated January 18, 2008, as amended. The January 2008 Note bears interest at prime plus one and one-half percent per annum. MTF has the right to convert the entire outstanding balance (principal plus accrued interest) into shares of Series B Preferred Stock at the initial conversion price of $4.42 per share (“Initial Conversion Price”). Such Initial Conversion Price shall be subject to adjustments including but not limited to stock splits, issuance of securities and next equity financing.
The Company issued promissory notes to MTF in November 2008 of $250,000 (“November 2008 Note”), in March 2009 of $400,000 (“March 2009 Note) and in August 2009 of $16,400 (August 2009 Note”). The November 2008 and the March 2009 Note bear interest at prime plus three percent per annum. The August 2009 Note bears interest at LIBOR plus eight percent per annum.
In connection with the March 2009 Note, the Company entered into a Security Agreement (the “Security Agreement”) which grants MTF a security interest in all of the Company’s right, title and interest, whether presently existing or hereafter acquired, in, to all intellectual property and all other collateral. In connection with the Security Agreement, the Company issued a warrant to purchase 118,383 shares of common stock at an exercise price of $0.44 (See Note 6).
In September 2009, the Company issued a $139,047 promissory note (the “2009 Convertible Note”) to MTF in accordance with the Convertible Note Purchase Agreement and Convertible Promissory Note dated September 30, 2009. The 2009 Convertible Note bears interest at the rate of LIBOR plus 8% per annum and matured on October 30, 2009, but could have extended to November 30, 2009 or December 31, 2009. If the note was not repaid by the maturity date, MTF was entitled to (i) convert the amount due on the 2009 Convertible Note into shares of Series B Preferred stock sufficient to increase MTF’s ownership in the Company to 51% of the fully-diluted capitalization, and (ii) receive the right to designate up to three additional members of the Company’s Board of Directors.
Since the 2009 Convertible Note was not repaid by the maturity date, on February 4, 2010, the 2009 Convertible Note was converted into 5,188,253 shares of Series B Preferred stock, which increased MTF’s ownership in the Company to 51% of the fully-diluted capitalization.
In September 2009, the Company entered into a tranched promissory note with MTF (“Tranched Note”), allowing the Company to initially borrow up to $445,000 in a series of one or more tranches. The Tranched Note was subsequently amended which, among other things, increased the maximum advance amount to $2,090,000. The Company borrowed a total of $2,088,350 under the Tranched Note through 2013.
In July 2013, all notes held by MTF were amended to extend the maturity date to March 31, 2014 and amended again on April 1, 2014 to extend the maturity date to March 31, 2015.
| F-41 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
In May, 2014, the Company entered into a convertible promissory note with MTF (the “2014 Note”) for $250,000 with interest at 7% per annum compounded annually and a maturity date of June 15, 2015. In the event of a financing of not less than $1 million, the 2014 Note automatically converts into Equity Securities, as defined in the 2014 Note, at a 25% discount to the price paid per share in such financing. In connection with the 2014 Note, the Company issued a warrant to purchase 166,667 shares of the Company’s common stock at an exercise price of $1.50 per share and 4 year term (See Note 6). The warrants had a fair value of $111,804, calculated using the Black-Scholes option pricing model with a volatility of 109%, a risk free rate of 0.79%. The Company accrued placement agent fees of $10,000 or 4% of the funds raised in connection with the financing and is obligated to issue a warrant for the purchase of 13,333 shares of common stock, which represents 4% of the common shares underlying the 2014 Note, with an exercise price of $1.00, a 5 year term and fair value of $8,181, calculated using the Black-Scholes model with a volatility of 109% and a risk free rate of 0.39%.
In July 2014, the 2014 Note and related warrants were assigned to Orthofix (see Note 9).
Bridge Notes
In April 2013 and June 2013, the Company borrowed $100,000 from MTF and $100,000 from Orthofix, Inc. under the April Bridge Financing, and in September 2013 and October 2013 the Company borrowed $50,000 from AFH and an additional $150,000 from Orthofix, Inc. under the September Bridge Financing (See Note 5). The convertible promissory note accrues interest at a rate of 12% per year and payable per quarter. A warrant to purchase the Company’s common stock equal to 50% of the original principal amount divided by $1.00 was issued to the Bridge Financing participant. Principal and unpaid accrued interest may be converted into equity securities issued in the Company’s next equity financing in an aggregate amount of at least $2.5 million at a price equal to the price paid by investors in the next equity financing. As of June 30, 2014 the total outstanding balance under the Bridge Financings was $377,066 (net of debt discount of $22,934) of which $100,000 is included in Notes Payable to Related Party and $277,066 is reported as Notes Payable, net of discount.
In June 2014, the note held by MTF under the April Bridge Financing was amended to extend the maturity date to October 14, 2014.
| 6. | Stockholders’ Equity |
Preferred Stock
The Company’s amended second amended and restated certificate of incorporation authorizes the Company to issue a total of 10,493,339 shares of preferred stock. The Company has reserved sufficient shares of common stock for issuance upon conversion of the preferred stock. As of June 30, 2014, the Company has the following outstanding shares of preferred stock as shown in the table below.
| Series | Date Issued | Issue Price | Shares Outstanding | Carrying Amount | Liquidation Preference | Dividend Rate | ||||||||||||||||
| A | July 2007 | $ | 1.786 | 493,339 | $ | 881,103 | $ | 881,103 | $ | 0.09 | ||||||||||||
| B | August 2007 | 4.420 | 147,846 | 653,479 | 653,479 | 0.09 | ||||||||||||||||
| B | February 2010 | 0.027 | 5,188,253 | 141,967 | 22,932,078 | 0.09 | ||||||||||||||||
| 5,829,438 | $ | 1,676,549 | $ | 24,466,660 | ||||||||||||||||||
Dividends - The holders of Series A and B preferred stock are entitled to receive noncumulative dividends prior to and in preference to any declaration of payment of any dividends on the common stock of the Company, at the rate of $0.09 per share per annum. Such dividends are payable only when, and if declared by the Board of Directors. No dividends on preferred stock were declared by the Board from inception through June 30, 2014.
| F-42 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
Liquidation Preference - In the event of liquidation, dissolution, or winding up of the Company, the holders of the Series A and B preferred stock are entitled to receive an amount per share equal to $1.786 and $4.42, respectively, for each outstanding share of Series A & B preferred stock (as adjusted for stock splits, stock dividends, combinations or other recapitalizations), plus all declared and unpaid dividends on such shares. Thereafter, if assets or surplus funds remained in the Company, the holders of common stock are entitled to receive all of the remaining assets of the Company.
Deemed Liquidation - Any merger or consolidation which would result in the Company’s stockholders immediately prior to such transaction not holding at least 50% of the voting power of the surviving, continuing or purchasing entity, or the sale or lease of all or substantially all of the assets of the Company, was deemed to be a liquidation, dissolution or winding up. Upon this event, holders of all shares of Series A and Series B preferred stock, as well as holders of the Company’s common stock would have receive their liquidation preference, including any declared and unpaid dividends as of the liquidation date. As in an ordinary liquidation, no class or series of the Company’s equity securities has a right to receive a particular form of consideration (e.g., cash or shares) upon a deemed liquidation event. Accordingly, because the holders of the Company’s preferred stock did not have a right to receive cash redemption of their shares, the Preferred stock is classified as permanent equity.
Conversion Rights - The holder of each share of Series A and B preferred stock have the option to convert each share into such number of fully paid and non-assessable shares of the Company’s common stock as is determined by dividing $1.786 (Series A Conversion Price) and $4.42 (Series B Conversion Price).
If the value of the adjusted number of shares of common stock into which the convertible preferred stock was convertible, based on the market price of the common stock on the date the convertible preferred stock was issued, was greater than the value of the number of shares of common stock into which the convertible preferred stock was convertible prior to such adjustment, based on the market price of the common stock on the date the convertible preferred stock was issued, the Company will recognize a beneficial conversion feature associated with the preferred stock. Because the beneficial conversion feature meets the requirements for equity classification (i.e., is not required to be accounted for as a liability), such future beneficial conversion feature charge will be recorded as a preferred stock dividend and the amount will be presented in a reconciliation of “net loss” to arrive at “net loss attributable to common shareholders” on the face of the Company’s statements of operations.
Each share of Series A and Series B preferred is subject to automatic conversion into common stock upon the earlier of (i) the Company’s sales of its common stock in a firm commitment underwritten public offering pursuant to a registration statement under the Securities Act of 1933, as amended or (ii) the date specified by written consent of holders of a majority of the then outstanding shares of Series A and Series B preferred stock, voting together as a single class on an as-converted basis.
Redemption - The preferred stock is not redeemable.
Voting Rights - The holders of preferred stock has the same voting rights as the holders of common stock. The holders of each share of convertible preferred stock are entitled to the number of votes equal to the number of shares of common stock into which such shares of convertible preferred stock could be converted.
Common Stock
The Company’s amendment to the second amended and restated certificate of incorporation authorizes the Company to issue a total of 20,000,000 shares of common stock. As of June 30, 2014, the Company had an aggregate of 5,098,661 shares of common stock outstanding of which 4,000,000 shares of the outstanding common stock were issued to the founders of the Company in exchange for technology know how and services.
| F-43 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
Each share of common stock has the right to one vote. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to the prior rights of holders of all classes of stock outstanding having priority rights as to dividends. No dividends have been declared by the Board from inception through June 30, 2014.
In the event of a liquidation dissolution, or winding up of the Company, after distribution to the holders of the Series A and B convertible preferred stock, all remaining assets or surplus funds of the Company shall be distributed on a pro-rata basis among the holders of the outstanding common stock and convertible preferred stock assuming full conversion of the convertible preferred stock.
Common Stock Warrants
As of June 30, 2014, the Company had an aggregate of 800,967 outstanding unexercised common stock warrants as follows:
| Date Issued | Exercise Price | Number of Shares | ||||||
| 2006 | $ | 0.17 | 60,920 | |||||
| 2009 | $ | 0.44 | 118,383 | |||||
| 2010 | $ | 0.44 | 254,997 | |||||
| 2013 | $ | 1.00 | 200,000 | |||||
| 2014 | $ | 1.50 | 166,667 | |||||
| Total Shares at June 30, 2014 | 800,967 | |||||||
In November 2006 and February 2010, the Company issued warrants to purchase 60,920 shares of common stock at an exercise price of $0.17 per share and 254,997 shares of common stock at an exercise price of $0.44 per share, respectively. The warrants were issued to one of the co-founders of the Company and to certain consultants who previously rendered services to the Company for which they agreed to defer payment for their services. The warrants expire in ten years from issuance date and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrants was estimated at an aggregate value of $113,683, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 105.6%, risk-free interest rate of between 3.62% and 4.62%, contractual term of 10 years and dividend yield of 0%. The warrants are classified as permanent equity. As of June 30, 2014 and December 31, 2013, the unpaid deferred payment balance was $90,199 (see Note 3).
In March 2009, the Company entered a Credit Agreement with MTF, a related party, for which the Company may borrow up to $400,000 (see Note 5). In connection with this transaction, the Company entered into a Warrant Agreement whereby it issued to MTF a warrant to purchase 118,383 shares of the Company’s common stock (“Note Warrant”) at an exercise price of $0.44 which allowed the Company to extend the maturity dates of the notes dated January 18, 2008 and November 4, 2008 to December 31, 2009. The fair value of the warrants was recorded as a debt issuance cost and was being amortized to interest expense over the term of the loan. The initial fair value of the Note Warrant at the grant date was estimated at an aggregate value of $47,970, using the Black-Scholes option pricing model. The warrant was classified as permanent equity at June 30, 2014.
| F-44 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
In the connection with the Bridge Financings (see Note 4), warrants were issued to purchase 200,000 shares of the Company’s common stock at an exercise price of $1.00 per share. The warrants expire in seven years from issuance date and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrant was estimated at an aggregate value of $171,143 using the Black-Scholes option pricing model. The fair value on the warrants was recorded as a debt issuance cost and is being amortized to interest expense over the term of the note. For the six months ended June 30, 2014 and the year ended December 31, 2013, $83,625 and $67,104 of the debt issuance costs was amortized to interest expense, respectively.
In connection with the 2014 Note, the Company issued a warrant to purchase 166,667 shares of the Company’s common stock at an exercise price of $1.50 per share and 4 year term (See Note 5).
| 7. | Income Taxes |
The Company’s effective tax rate is 0% for income tax for the six months ended June 30, 2014 and the Company expects that its effective tax rate for the full year 2014 will be 0%. Based on the weight of available evidence, including cumulative losses since inception and expected future losses, the Company has determined that it is more likely than not that the deferred tax asset amount will not be realized and therefore a valuation allowance has been provided on net deferred tax assets.
The Company files tax returns for U.S. Federal and State of California. The Company is not currently subject to any income tax examinations. Since the Company’s inception, the Company had incurred losses from operations, which generally allows all tax years to remain open.
Uncertain Tax Positions
The Company recognizes the financial statement effects of a tax position when it becomes more likely than not, based upon the technical merits, that the position will be sustained upon examination.
The Company recognizes interest and/or penalties related to uncertain tax positions. To the extent accrued interest and penalties do not ultimately become payable, amounts accrued will be reduced and reflected in the period that such determination is made. The interest and penalties are recognized as other expense and not tax expense. The Company currently has no interest and penalties related to uncertain tax positions.
| 8. | Related Party Transactions |
In September 2006, the Company entered into a consulting agreement with one of its stockholders whom previously served as chairman, president and CEO of the Company. The Company paid $70,000 and $60,000, respectively, for the six months ended June 30, 2014 and 2013 in consulting fees to this related party.
In addition, one of the Company’s co-founders had previously provided research and development consulting services to the Company and earned an aggregate of $320,000 of fees from inception to January 2010. Of the $320,000, $52,500 has been deferred for payment until the Company’s next equity financing. As of June 30, 2014 and December 31, 2013, the $52,500 deferred payment was included in the accrued expenses.
See Note 5 for related party notes payable to MTF.
| F-45 |
Bone Biologics, Inc.
Notes to Condensed Financial Statements
| 9. | Subsequent Events |
Orthofix Subsequent Financing
On July 1, 2014, (i) Orthofix purchased $500,000 worth of Bone Biologics Common Stock or the Subsequent Orthofix Shares; (ii) was issued the Subsequent Orthofix Convertible Promissory Notes in the principal amount of $500,000 (which includes the assignment of the $250,000 2014 Note from MTF) and convertible into 666,666 worth of the Company’s Common Stock at $0.75 per share; and (iii) was issued the Subsequent Orthofix Warrants (including the assignment of warrants by MTF issued in connection with the 2014 Note) which were exercisable for 333,334 shares of Bone Biologics Common Stock at an exercise price per share of $1.50 (the “Orthofix Subsequent Financing”). Upon subscribing for the Subsequent Orthofix Shares, the Subsequent Orthofix Convertible Promissory Notes and accrued interest converted into a combined total of 668,904 shares of Bone Biologics Common Stock in accordance with the terms of the Subsequent Orthofix Convertible Promissory Notes. The Subsequent Orthofix Warrants converted into warrants of the Company with substantially identical terms upon consummation of the Merger.
At the closing of the Subsequent Orthofix Shares and Notes, AFH Advisory was entitled to receive warrants to purchase up to 500,000 shares of Common Stock of the Company at the per share price of the shares offered or $1.00 per share, with a 5 year term and a cashless exercise provision (the “Extra Warrants”). AFH Advisory has normal and customary piggyback registration rights with respect to the shares of Common Stock issuable upon exercise of the Extra Warrants.
Forefront or its designees will receive a warrant to purchase shares of Common Stock (the “Agent Warrant”) equal to 8% of the Common Stock underlying the securities issued in the Private Placement (4% if investors are introduced by Bone Biologics, AFH Holdings & Advisory, LLC or their respective officers and directors). Such Agent Warrant will be issued at the closing of the Private Placement and shall provide, among other things, that the Agent Warrant shall: (i) be exercisable at the price of the securities (or the exercise price of the securities) issued to the investors in the offering, (ii) expire five (5) years from the date of issuance, (iii) include customary registration rights, including the registration rights provided to the Investors, (iv) contain provisions for cashless exercise and (v) include such other terms that are normal and customary for warrants of this type. In addition, Forefront or its designees will receive and Advisory Warrant equal to 2.0% of the Company’s post-merger and financing fully diluted shares outstanding upon the closing of $2.5 million of investors on which Forefront is eligible to receive compensation. Forefront was issued warrants to purchase 46,667 shares of Common Stock at $1.00 per share upon completion of the Orthofix Subsequent Financing (which includes 13,333 warrants in connection with the 2014 Note with MTF).
| F-46 |
