Attached files
| file | filename |
|---|---|
| 8-K - BIOTIME INC 8-K 9-25-2014 - Lineage Cell Therapeutics, Inc. | form8k.htm |
Exhibit 99.1

Joseph Wagner, Ph.D. Chief Executive OfficerSeptember 25, 2014

OncoCyte Corporation * Our mission is to develop novel products for the diagnosis and treatment of cancer in order to improve both the quality and length of life of cancer patients Internally developed cancer gene discovery platformPlatform based on extensive microarray datasetMarker discovery principle based on similarity of gene expression in embryonic development and cancerScores of potential targets identified and IP filedMultiple product opportunities Goal: Develop and market low-cost molecular diagnostic tests for major cancers with rapid adoption by initial users followed by widespread use in large patient populations

OncoCyte Investment Opportunity * Multiple clinical studies reporting by year-end 2014, coupled with very large market potential of products may yield large, near-term valuation increases Initial clinical validation studies on three diagnostic products reporting by year-end 2014Novel molecular cancer diagnostics that address major unmet medical needsPotential for rapid initial adoption through KOL support and limitations of current diagnostic testsPotential for broad eventual adoption due to advantages over current screening diagnosticsFavorable reimbursement landscape with opportunity for value-based pricing of testsNear-term revenue potential (12-18 months)Potential for products to achieve “blockbuster” status (>$1B/year)

OncoCyte Presentation Highlights * Our mission is to develop novel products for the diagnosis and treatment of cancer in order to improve both the quality and length of life of cancer patients Technology OverviewBusiness StrategyProduct Development & Marketing PathProduct ProgramsBreast Cancer DiagnosticBladder Cancer DiagnosticLung Cancer DiagnosticReimbursement StrategyUpcoming Milestones

OncoCyte Technology * Many of the same genes that drive embryonic development drive tumor formation and growth A substantial proportion of the mammalian genome is exclusively activated during embryonic developmentThese embryo-exclusive genes regulate:Cell proliferationCell signalingOrgan formationAngiogenesisMany of these genes are accessed/activated by cells after oncogenic mutation & transformationMany of these genes have not been previously associated with cancer Developing Tissue & Tumors Adult Tissues

OncoCyte’s Product Development Path * Translating proprietary cancer marker platform into near-term revenue opportunities in oncology markets Cancer Marker Discovery (2011-):Assembled >700 sample microarray dataset Bioinformatics generated >3000 candidate markersProtection of Intellectual Property (2011-):Aggressive filings protecting all markers and all usesCancer Marker Validation (2012-):Verify gene expression using alternate methodologiesVerify protein expression in tumor tissueProof of Concept in Patient Samples (2013-):Verify gene/protein expression in retrospective clinical sample banksBuild candidate multiplex panels for large scale testingLarge Prospective Clinical Studies (2014-):Initiate larger studies in target patient populationsTest performance of multiplex panels using platform-neutral proprietary test kits

OncoCyte Publications * Validation of cancer marker biology through peer-review process Many more to come…

Example 1: COL10A1 * COL10A1 is normally only expressed during embryonic development and regulates long bone mineralization Tumors Tumor Cell Lines Normal Cells Normal Tissues Data from Illumina microarray shows high levels of COL10A1 expression in a majority of tumor samples tested with little expression in normal tissuesCOL10A1 represents a novel “pan-cancer” biomarker

Example 2: FSIP1 * FSIP1 is a protein normally only expressed in the tail of mature male sperm cells Data from Illumina microarray shows high levels of FSIP1 expression in a majority of breast cancer samples tested with no expression elsewhereFSIP1 represents a novel breast cancer-specific biomarker * Normal Cells Normal Tissues Tumors Tumor Cell Lines Breast Tumors

Multiplex Breast Cancer Biomarker Panel * Normal Donors Breast Cancer Patients Preliminary data compilation shows discrimination between breast cancer patient serum and normal donor serum. Individual biomarker expression in patient samples validates microarray dataCombining individual markers demonstrates high performance of multiplex approach necessary for user adoption

OncoCyte’s Business Formula * Designed to translate proprietary cancer marker platform into near-term revenue opportunities “Listen to Your Technology”“Listen to Your Customers”Create Scalable Business ModelEarly Revenues Fund Larger Trials
“Listen to Your Technology” * Technology platform defines product opportunities in subsectors of cancer diagnostics Potential products based on dataset structure:Screening diagnosticsRecurrence diagnosticsPotential products based on markers:Breast cancerColorectal/GI cancersBladder/urothelial cancersThyroid cancerLung cancerPotential products with low cost and ease of use:Blood-based testsUrine-based tests

“Listen to Your Customer” * Physician adoption is the key barrier to entry for cancer screening diagnostics User Will Adopt Tests That:Key Opinion Leaders supportResolve diagnostic dilemmasJustify the need for proceduresEliminate unnecessary proceduresHave reasonable cost/reimbursementUser Will Not Adopt Tests That:Create diagnostic ambiguityReplace proceduresCreate unnecessary costs Technology alone does not drive user adoption

OncoCyte Products: Business Strategy * How does OncoCyte identify and prioritize its products? Strategically develop diagnostic products that will be rapidly adopted and have short- and long-term revenue potentialFocus on developing products that:Fit with proprietary technologyAre simple to perform and interpretAre relatively low cost and easy to useAppeal to an immediate user baseHave long-term large market potentialAre based on protectable/enforceable IP

Product Development & Marketing Strategy * Create a scalable business model beginning with an immediate market with early adopters, successively expanding into larger opportunities $15M Initial Initial Use & Market:Early adopters with immediate needsCan be addressed rapidly with relatively low costExpanded Use & Market:Larger markets and user basesRequires additional clinical validationFinal Target Use & Market:Addresses very large markets and tens of thousands of usersRequires partnerships to meet ultimate revenue goals Expansion Total Market

Key Driver: Limitations of Current Tests * Initial users/use will be to clarify diagnostic dilemmas due to uncertainties in imaging and other traditional tests Current Tests With Significant Limitations Include:Mammography for breast cancerUrine cytology for bladder cancer recurrence surveillanceLow-dose CT for lung cancer screening Definite Negative Definite Positive Indeterminate Test Score

Current Product Programs * Simple, low cost cancer screens that address current unmet user needs in large patient markets ONC-BR-001:Blood-based breast cancer screenMeasures sera proteins via ELISAONC-BL-002:Urine-based bladder cancer screenMeasures RNA levels via PCRONC-LN-003:Blood-based lung cancer screenMeasures sera proteins and/or RNA

* Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-LN-003: Lung Cancer Product

* Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-LN-003: Lung Cancer Product
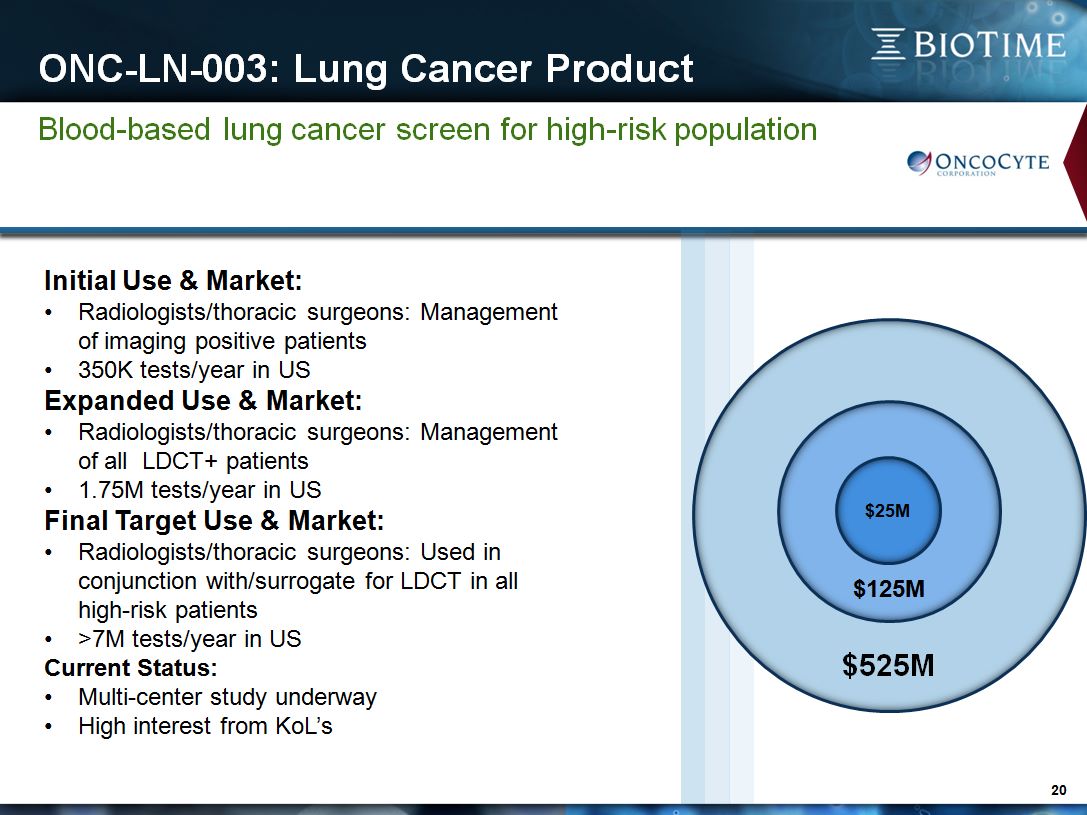
ONC-LN-003: Lung Cancer Product * Blood-based lung cancer screen for high-risk population Initial Use & Market:Radiologists/thoracic surgeons: Management of imaging positive patients350K tests/year in USExpanded Use & Market:Radiologists/thoracic surgeons: Management of all LDCT+ patients1.75M tests/year in USFinal Target Use & Market:Radiologists/thoracic surgeons: Used in conjunction with/surrogate for LDCT in all high-risk patients>7M tests/year in USCurrent Status:Multi-center study underwayHigh interest from KoL’s $125M $525M $25M

ONC-LN-003: Lung Cancer Clinical Study * Ongoing study in target population initiated by collaborators at the Wistar Institute Subjects:N = 600All high-risk, includes cancer-free, benign nodules, confirmed cancer patientsEndpoints:Primary: Correlation with diagnosisSecondary: Correlation with tumor origin, stage, gradeSites:6 total including NYU, Temple, Penn, ChristianaTiming:Study completed enrollment May 2014Sample analysis completed Sept 2014Initial data publication late 2014Study data presentations at ATS & ASCO 2015

* Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-BL-002: Bladder Cancer Product

* Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-BL-002: Bladder Cancer Product

ONC-BL-002: Bladder Cancer Diagnostic * Urine-based multiplex PCR recurrence diagnostic test $15M $15M Initial Use & Market:Pathologist: Resolution of indeterminate cytology during recurrence surveillance500K tests/year in USExpanded Use & Market:Oncologist: All recurrence surveillance1.5M test/year in USFinal Target Use & Market:Urologist: Management of hematuria>5M tests/year in USCurrent Status:Ongoing patient studies in US, ChinaAdditional sites to be selected $150M $500M

ONC-BL-002: Bladder Cancer Clinical Studies * Multisite clinical studies in setting of surveillance cytology testing or cystoscopy Subjects:N = 100/1200At time of surveillance cytology/cystoscopyEndpoints:Correlation of markers to cytology/cystoscopy resultsSites:Cytopathology: Johns HopkinsCystoscopy: Four large urology clinics in Texas, Ohio, Indiana and South CarolinaTiming:Cytopathology: Complete late 2014Cystoscopy: July 2015Data presentation at AUA & ASCO in May, 2015

* Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-BR-001: Breast Cancer Product

* ONC-BR-001: Breast Cancer Product Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier

ONC-BR-001: Breast Cancer Product * Blood-based screening diagnostic measures sera protein biomarkers using ELISA $15M $25M Initial Use & Market:Radiologist: Management of BIRADS 3-4 patients350K tests/year in USExpanded Use & Market:Radiologist: In conjunction with all diagnostic mammographyOncologist: Recurrence surveillance3M tests/year in USFinal Target Use & Market:PCP/Radiologists: In conjunction with or surrogate for screening mammography>30M tests/year in USCurrent Status:Clinical study underwayIn discussions with additional sites $250M $2.5B

ONC-BR-001: Breast Cancer Clinical Study * Prospective, multisite study in diagnostic mammography patients Subjects:N = 600At time of diagnostic mammographyEndpoints:Primary: Correlation with BIRADS scoreSecondary: Correlation of biomarker with pathologySites:Up to 6 totalCurrently enrolling: Ron Korn, SMIL, Scottsdale, AZAbcodia providing additional samplesTiming:First subject enrolled early Jan 2014Complete study by end of 2014Data presentation at ACR & ASCO in May, 2015

Adoption Strategy * Superior product at lower cost to drive rapid adoption in initial markets, followed by widespread adoption KOL endorsementsDrive adoption within KOLs’ own practices as well as among other physiciansCan lead to medical society recommendationsStrong Clinical Trial DataSupports positive health economics and patient outcomesCost effectiveness of testsDrives user willingness to acquire additional dataAs test volume increases, adoption point becomes earlier as test accuracy gains credibility

Reimbursement Strategy * Favorable reimbursement potential for multiplex molecular tests despite recent CMS changes Anticipated Reimbursement for Our Tests:Each test would receive new 815XX codeApril 2014 Medicare patch exempts molecular tests from pricing cutsPotential for value-based pricing is realOncoCyte would set price for first 3 quarters after launch, price negotiation with CMS regional contractors (i.e. Gap Filling) effective fourth quarterLow-cost test technology allows strong gross margins even at low reimbursement rates

Upcoming Development Milestones * Positive clinical trial results will drive commercialization efforts with first test launch in 2015 Q4 2014: Completion of breast, bladder and lung clinical trial enrollment and data analysisPublication of lung trial results; submission of clinical study data for presentation at major oncology meetingsQ1 2015: Initiation of CLIA lab testing certificationExpansion of executive management teamQ2 2015: Presentation of breast, bladder and lung clinical trial at major oncology meetingsPublication of breast and bladder clinical trial data in peer-reviewed journalsQ3 2015: Assay validation completed for lead testCalifornia CLIA certification obtainedQ4 2015: Commercial launch of first diagnostic test to be announced at major oncology meeting

OncoCyte: Investment Highlights * Beginning with an immediate market with early adopters, expanding into large opportunities Broad platform of cancer markersLow-cost tests using standard methodsPotential for rapid initial adoption and broad eventual adoptionScalable business model:Significant revenue in initial marketsEarly revenues fund studies necessary for broader useVery large long-term potential revenue opportunity
