Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REPROS THERAPEUTICS INC. | v389711_8k.htm |
Exhibit 99.1

Androxal ® A Mechanistically Consistent Approach to the Treatment of Secondary Hypogonadism

Repros Disclaimer Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discu ssi on) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to var ious risks, uncertainties and other factors that could cause the Company’s actual results, performance or achiev
ements to differ m ate rially from those expressed or implied by such forward - looking statements. These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “believe,” “plan,” “seek,” “could,” “can,” “sh ould” or similar expressions. These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current conditions, expected future developments and oth er factors the Company believes are appropriate in these circumstances. Forward - looking statements include, but are not limited to, those relating to anticipated milestones for Androxal® and Proellex®, the conduct of planned clinical studies and the timing and na tur e of the results thereof, the markets for the Company’s products and the potential success of the Company in penetrating those markets an d that the Company’s need for and use of financial resources. Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factors that may cause actual events to be materially different fr om those expressed or implied by such forward - looking statements, including the ability to raise additional needed capital on a timely ba sis in order for it to continue to fund development of its Androxal® and Proellex® programs, the ability to have success in the clin ica l development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks as are identified in the Company's most recent Annual Report on Form 10 - K and the subsequent quarterly report on Form 10 - Q and in the prospectus supplement and the accompanying prospectus included in the registration statement mentioned below. These documents are available on request from Repros or at www.sec.gov. Repros disclaims any intention or obligation to update or revise any forw ard - looking statements, whether as a result of new information, future events or otherwise. In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry. We obtained t his information and these statistics from third - party sources, which we have supplemented where necessary with information from publ icly available sources and our own internal estimates. Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information. Whi le we believe that each of these studies and publications is reliable, we have not independently verified such data, and we make no an y representation as to the accuracy of such information. Similarly, we believe our internal research is reliable, but it has no t b een verified by any independent sources.

Hypogonadism Background Normal Hypothalamus - Pituitary - Testes (HPT) Axis A. Normal secretion of LH and FSH B. Normal stimulation of testes C. Results in normal testosterone levels and sperm production Seftel A. Male hypogonadism . Part II: etiology, pathophysiology , and diagnosis. Int J Impot Res. 2006;18(3):223 - 228. • The hypothalamus releases gonadotropin - releasing hormone (GnRH)
, which stimulates the release of luteinizing hormone (LH) and follicle - stimulating hormone (FSH) from the pituitary. These hormones are used to produce sperm and testosterone in the testes. • In a normally functioning hypothalamic - pituitary - testicular axis, rising testosterone levels create negative feedback to inhibit further production of GnRH by the hypothalamus .

Hypogonadism Background • Hypogonadism is a condition in which a male is unable to produce sufficient testosterone (T) . • A man is considered to be clinically hypogonadal if his morning testosterone is < 300 ng / dL (normal range 300 - 1040 ng / dL ). • In a recent study, hypogonadism was estimated to affect up to 13.8 million men in the United States . Another 17 million may be compromi
sed due to obesity • There are two types of hypogonadism , primary and secondary . Overview

Hypogonadism Background • The less frequent type is primary , which is typically a result of a genetic defect, trauma or viral infections such as mumps. • This type of disorder is easily diagnosed by assessing both testosterone and the pituitary hormone that drives testicular synthesis of testosterone, luteinizing hormone (LH) . – LH pulses during the day, and in men with primary testicular fa
ilure, these pulses yield much higher levels of the hormone (>20 mIU /mL) compared to the normal range of 4 - 15 mIU / mL. Primary Hypogonadism

Hypogonadism Background • The more common dysfunction is secondary hypogonadism (85 - 95% of hypogonadal men). • This dysfunction is characterized by low testosterone and low/low normal LH and is a result of the pituitary not responding to the low T state by secreting more LH to drive T synthesis. • Historically, many believed this disorder was primarily age - related but growing evidence po
ints to obesity playing a major role in this condition . – Obese men are estrogenized and estrogen provides negative feedback on the hypothalamic - pituitary axis suppressing the ability of the pituitary to sense the low T state and respond appropriately. Secondary Hypogonadism

Categorizing Gonadal Status by Testosterone (T) and LH in the European Male Ageing Study (EMAS) : A General Population Observational Cohort Study Thresholds for abnormal: <Total T 10.5 nmol /L (300 ng/ dL ) and >LH 9.4 U/L (Tajar et al J Clin Endocrinol Metab 2010 95:1810) 0 20 40 60 80 LH (U/L) 0 10 20 30 40 50 Total Testosterone (nmol/L) Secondary hypogonadism – Low T and normal or low LH Eug
onadal – Normal T and LH Primary hypogonadism – Low T and high LH Compensated ‘ hypogonadism ’ – Normal T but high LH (n = 3219)

Prevalence, Group Sizes and Hormonal Distribution in 4 Different Biochemical Diagnostic Categories In the European Male Ageing Study (EMAS) Thresholds for abnormal: Total T <10.5 nmol /L and >LH 9.4 U/L <10.5 nmol /L >9.4 U/L (Tajar et al 2010) Area of circles represents individual group sizes (n) Position of circles represents mean T and LH levels

Wu et al. JCEM 2008 Greater Effects of BMI ( cf to Age) on Reproductive Hormone Levels in EMAS - Indicating Secondary Hypogonadism in Obesity (n = 3210)

Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Two Separate Tracks of Hypogonadism 1. Secondary hypogonadism associated with obesity , independent of age and reversible 2. Primary hypogonadism - associated with aging ,
independent of obesity, largely irreversible • Distinct clinical features and outcomes between tracks/categories • Require different management strategies

Secondary Hypogonadism at Baseline Age (years) BMI ≥ 25 - <30 kg/m2 BMI ≥ 30 kg/m2 Smoking (current) Alcohol (frequent) Morbidity ( ≥ 1) 0 5 10 15 *** *** * Poor morning erection Sexual thoughts Erectile dysfunction Low vigorous activity Unable to bend Slow walking speed 0 2 4 6 * * Adjusted Relative Risk Ratio *p <0.05 **p<0.01 ***p<0.001 Adjusted Odds Ratio Risk factors Symptoms Tajar et al
. JCEM 2010

Primary Hypogonadism at Baseline Poor morning erection Sexual thoughts Erectile dysfunction Low vigorous activity Unable to bend Slow walking speed 0 2 4 6 Age (years) BMI ≥ 25 - <30 kg/m2 BMI ≥ 30 kg/m2 Smoking (current) Alcohol (frequent) Morbidity ( ≥ 1) 0 5 10 15 *** * ** *p <0.05 **p< 0.01 ***p<0.001 Adjusted Relative Risk Ratio Adjusted Odds Ratio Risk factors Symptoms

• A man’s weight affects his T level more than his age. • Obese men, at even a young age, experience obesity - related Low T. • Weight loss significantly improves T levels in the secondary hypogonadal male. • The secondary hypogonadal male has functional but under - stimulated testes due to obesity caused by the negative feedback of estrogen. 40 - 44 45 - 49 50 - 54 55 - 59 60 - 64 65 - 69 70 -
74 75 - 79 Age Hypogonadism Background Weight Effect on T Production in the Secondary Hypogonadal Male Late onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment, Ilpo Huhtaniemi, AJA (2014) 16, 192 - 202

The Average American Male at All Ages Was Overweight in 2000 Overweight BMI > 25 (6’ 190# male BMI=25.8) Obese BMI > 30 (6’ 230# male BMI =31.2) In 2010 there were ~90 million men in the US between the ages of 20 and 65 32% are obese

Hormone Changes (Rise/Fall) are Related to % Weight Loss/Gain (Data adjusted for age , centre, changes in smoking status, alcohol consumption co - morbidities & physical activity) n = 2395, *p<0.05, **p<0.01 % Weight loss Weight gain % 0 * ** * * * * * * ** ** ** ** * Camacho et al. European Journal of Endocrinolology 2013 Mean (SEM)

Age & BMI of Subjects in Early Repros Androxal Studies Study IND # of Subjects Mean Age Mean BMI ZN - 018 65,396 52 51.2 31.95 ZA - 003 65,396 194 52.6 32.4 ZA - 202 104,921 116 57.3 33.5 ZA - 203 65,396 108 50.7 32.0 ZA - 204 65,396 60 53.1 31.8

BMI Distribution in Study of Secondary Hypogonadism, Repros: ZA - 003 (% of Subjects) 0 10 20 30 40 50 60 70 80 90 100 Underweight <18.5 Normal 18.5-24.9 Overweight 25-29.9 Obese>30 Weight Classification of Subjects Enrolled in ZA - 003 (n=204) Screening Criteria: T<300 ng/dL
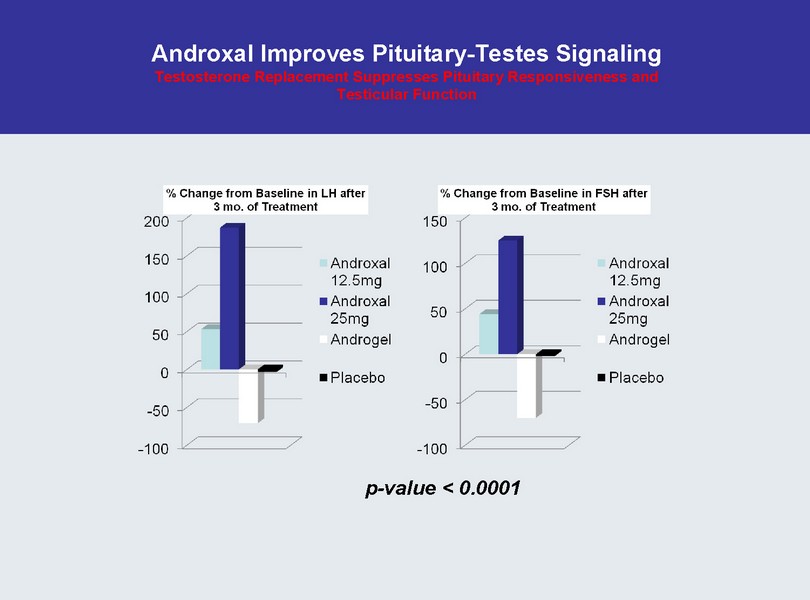
Androxal Improves Pituitary - Testes Signaling Testosterone Replacement Suppresses Pituitary Responsiveness and Testicular Function p - value < 0.0001

% of Subjects with FSH Below the Lower Limit of Normal and Below the Lower Limit of Detection (0.3 mIU/ml) after 3 months ZA - 003 “Completer” Analysis

Head to Head Comparison of Two Doses of Androxal to Testim and Placebo, Study ZA - 203 An approved T gel significantly suppresses LH and FSH in a clinically relevant manner and suppresses sperm concentration to near severe oligospermia (median sperm concentration 6.2 million/mL) after only three months of treatment 0 2 4 6 8 10 12 14 12.5 mg 25 mg PL Testim FSH Treatment Effect of Treatm
ent on Median FSH p versus Testim Before After p<0.00001 p=0.0004 0 20 40 60 80 100 12.5 mg 25 mg PL Testim [Sperm] in Millions/ml Treatment Effect of Treatment on Median Sperm Concentration p versus Testim Baseline EOS p=0.012 p=0.0021 p=0.0049 0 2 4 6 8 10 12 12.5 mg 25 mg PL Testim LH Treatment Effect of Treatment on Median LH p versus Testim Before After p=0.0004 0 50 100 150 200 250 300 350 400 450 500 12.5 mg 25 mg PL Testim TT in ng/dL Treatment Effect of Treatment on Median Serum TT p versus placebo Before After p<0.00001 p=0.0002 p<0.00001
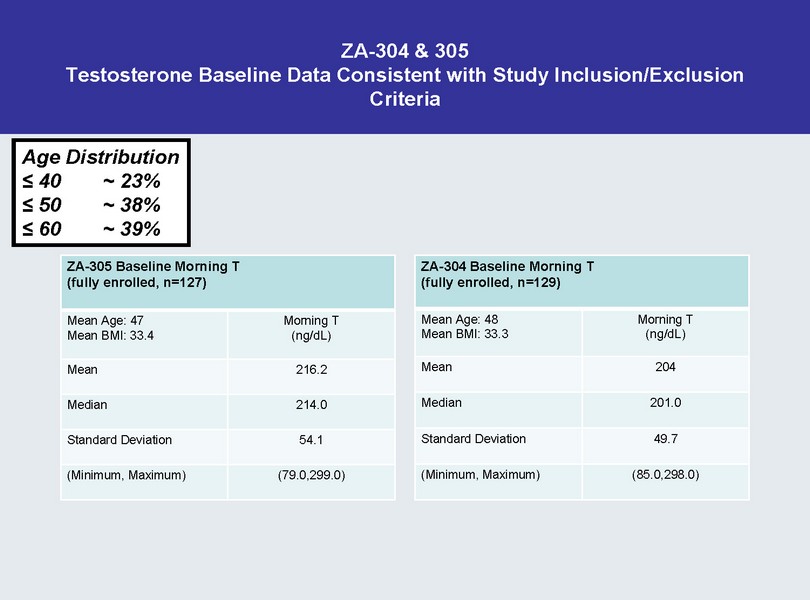
ZA - 304 & 305 Testosterone Baseline Data Consistent with Study Inclusion/Exclusion Criteria ZA - 305 Baseline Morning T (fully enrolled, n=127) Mean Age: 47 Mean BMI: 33.4 Morning T (ng/ dL ) Mean 216.2 Median 214.0 Standard Deviation 54.1 (Minimum, Maximum) (79.0,299.0) ZA - 304 Baseline Morning T (fully enrolled, n=129) Mean Age: 48 Mean BMI: 33.3 Morning T (ng/ dL ) Mean 204 Me
dian 201.0 Standard Deviation 49.7 (Minimum, Maximum) (85.0,298.0) Age Distribution ≤ 40 ~ 23% ≤ 50 ~ 38% ≤ 60 ~ 39%
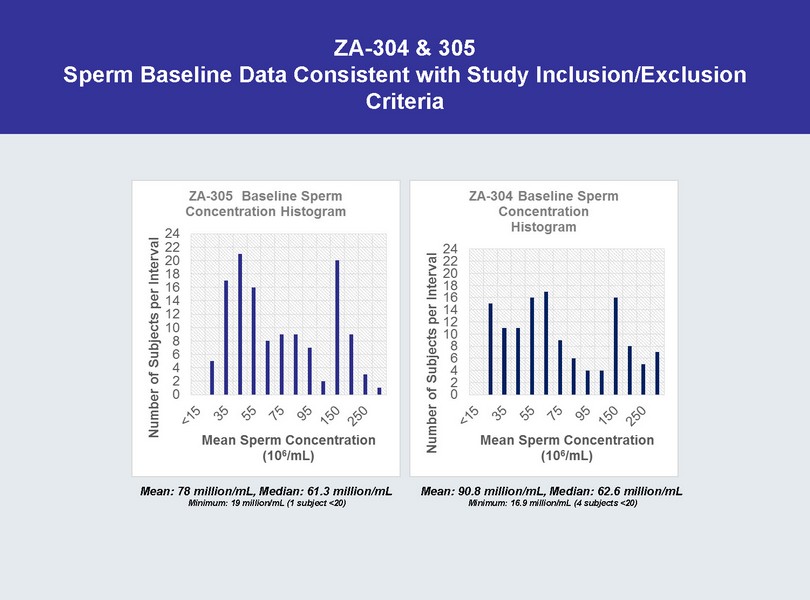
ZA - 304 & 305 Sperm Baseline Data Consistent with Study Inclusion/Exclusion Criteria Mean: 78 million/mL, Median: 61.3 million/mL Minimum: 19 million/mL (1 subject <20) Mean: 90.8 million/mL, Median: 62.6 million/mL Minimum: 16.9 million/mL (4 subjects <20)
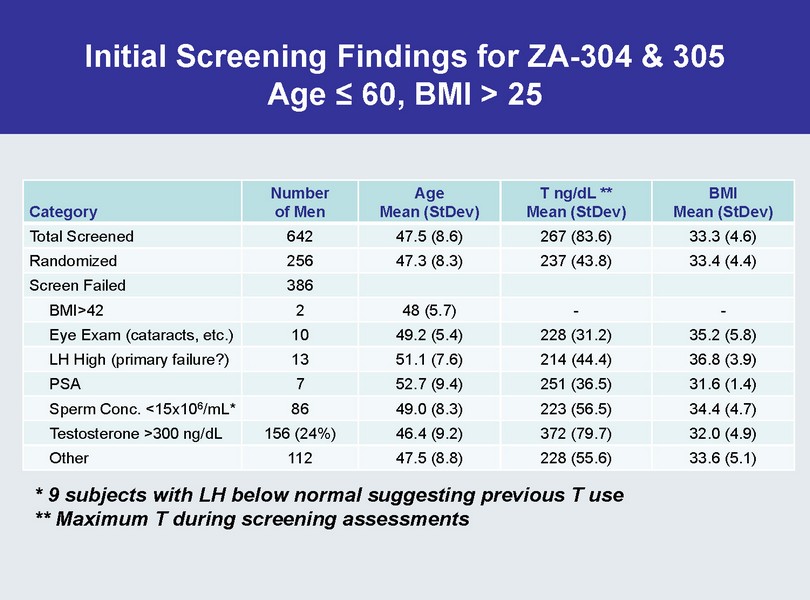
Initial Screening Findings for ZA - 304 & 305 Age ≤ 60, BMI > 25 Category Number of Men Age Mean ( StDev ) T ng/ dL ** Mean ( StDev ) BMI Mean ( StDev ) Total Screened 642 47.5 (8.6) 267 (83.6) 33.3 (4.6) Randomized 256 47.3 (8.3) 237 (43.8) 33.4 (4.4) Screen Failed 386 BMI>42 2 48 (5.7) - - Eye Exam (cataracts, etc.) 10 49.2 (5.4) 228 (31.2) 35.2 (5.8) LH High (primary failure?) 13 51.1 (7
.6) 214 (44.4) 36.8 (3.9) PSA 7 52.7 (9.4) 251 (36.5) 31.6 (1.4) Sperm Conc. <15x10 6 /mL* 86 49.0 (8.3) 223 (56.5) 34.4 (4.7) Testosterone >300 ng/ dL 156 (24%) 46.4 (9.2) 372 (79.7) 32.0 (4.9) Other 112 47.5 (8.8) 228 (55.6) 33.6 (5.1) * 9 subjects with LH below normal suggesting previous T use ** Maximum T during screening assessments
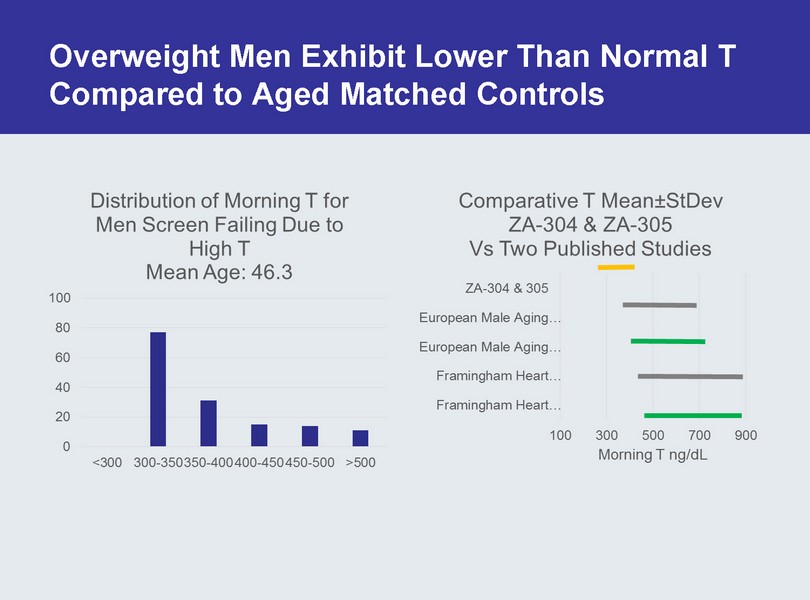
Overweight Men Exhibit Lower Than Normal T Compared to Aged Matched Controls
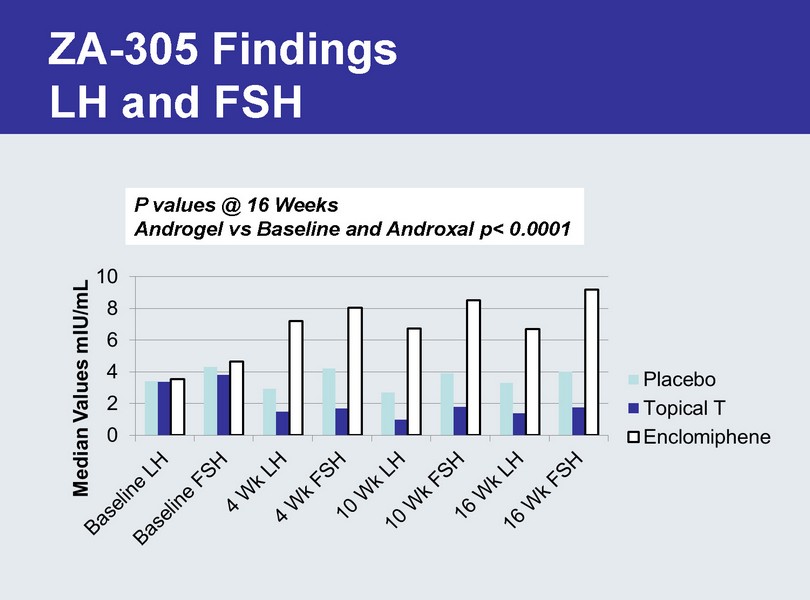
ZA - 305 Findings LH and FSH P values @ 16 Weeks Androgel vs Baseline and Androxal p< 0.0001
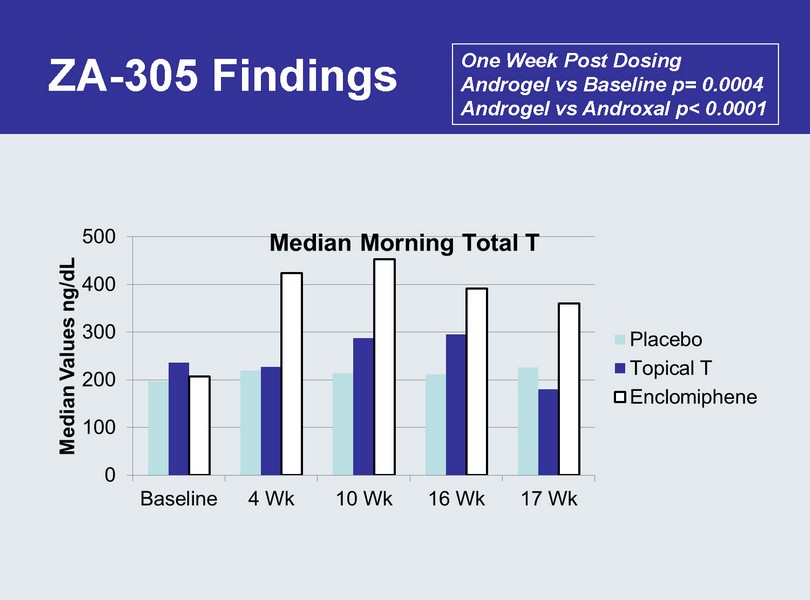
ZA - 305 Findings One Week Post Dosing Androgel vs Baseline p= 0.0004 Androgel vs Androxal p< 0.0001
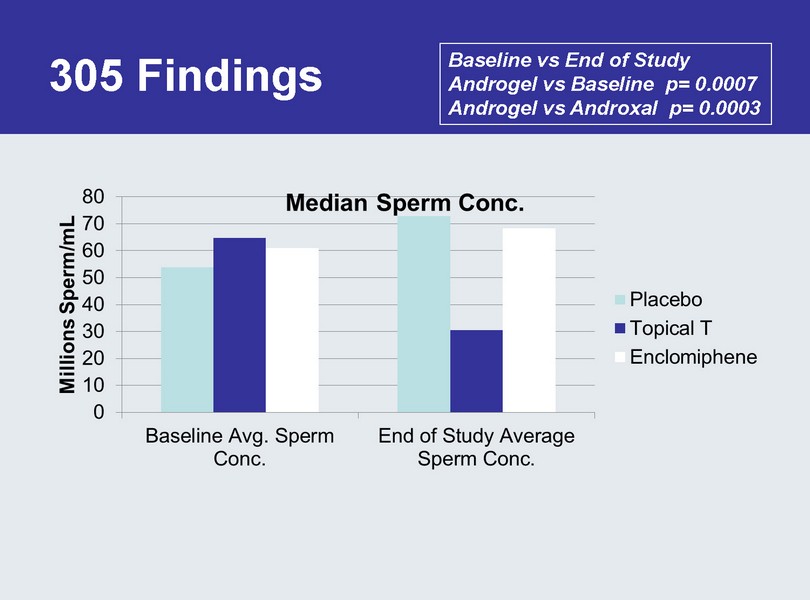
305 Findings Baseline vs End of Study Androgel vs Baseline p= 0.0007 Androgel vs Androxal p= 0.0003
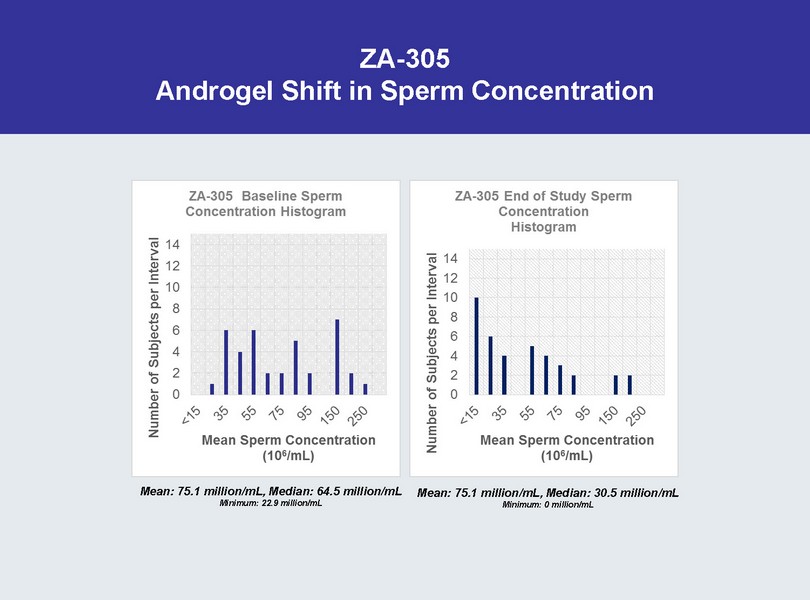
ZA - 305 Androgel Shift in Sperm Concentration Mean: 75.1 million/mL, Median: 64.5 million/mL Minimum: 22.9 million/mL Mean: 75.1 million/mL, Median: 30.5 million/mL Minimum: 0 million/mL

ZA - 305 Androxal Shift in Sperm Concentration Mean: 79 million/mL, Median: 60.9 million/mL Minimum: 19.6 million/mL Mean: 88.6 million/mL, Median: 68.2 million/mL Minimum : 9.4 million/mL
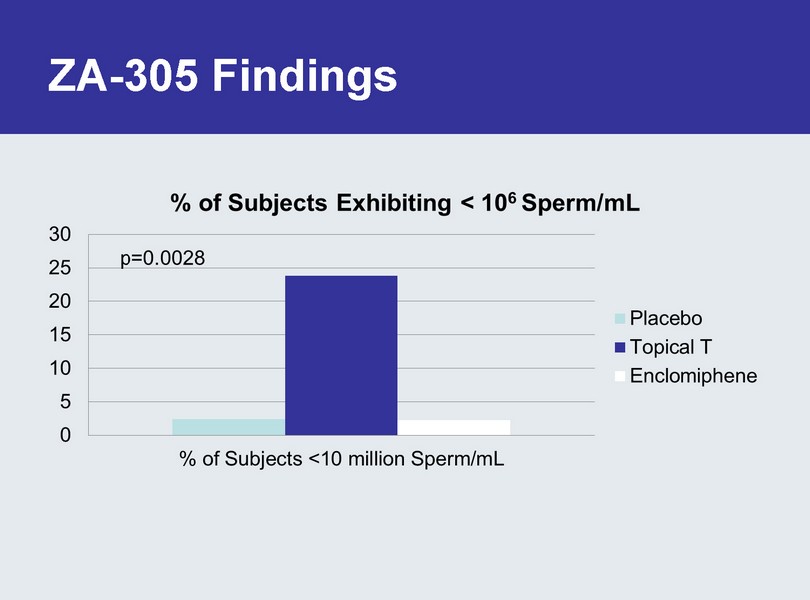
ZA - 305 Findings

Influence of Prior Testosterone ZA - 300, 6 month study

Androxal Safety • Generally well tolerated at all doses – > 1500 men exposed – > 900 men for 6 months – > 150 men for 12 months – > 700 patient years of exposure • No serious drug related CV events or dose dependent events • Adverse events consistent with increasing T – No exposure of T outside the normal range • Lower frequency of increased hematocrit

Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Clinical Implications • Secondary hypogonadism is closely associated with obesity and reversible suppression of hypothalamic/pituitary function • Diagnostic differentiation
of secondary from primary hypogonadism is essential – measure T and LH • First line standard of care for obese men with low T should be weight loss • Strategies to mitigate gonadotropin suppression thereby raising endogenous T and preserving fertility are preferable to exogenous T treatment in men with secondary hypogonadism

Upcoming Androxal Related Events • FDA AdCom Meeting 9/17/14 • Anticipated Release of 304 Data Imminent • NYC Analyst Day Morning of Halloween • Submit Androxal NDA Around Year - end ‘14

Financial Summary • Cash and equivalents (as of 8/31/14, unaudited) $55.5 M • Cash runway: Through 2016* – Anticipate Androxal approved and launched • Current shares outstanding: 24.0 M shares – Warrants Outstanding – Series A – 314,614 (purchased in unit deal @ $2.45); Series B – 429,704 @ $2.49 exercise price. *Excludes marketing and commercialization expenses for Androxal
