Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ampio Pharmaceuticals, Inc. | d792397d8k.htm |
| EX-99.2 - EX-99.2 - Ampio Pharmaceuticals, Inc. | d792397dex992.htm |
 Shareholder Presentation
September 2014
Exhibit 99.1 |
 2
Agenda
Topic
Speaker
Formal Business
Greg Gould
Scientific Address
David Bar-Or, MD
Product Overview
Holli Loose, MsC
Clinical Review
Vaughan Clift, MD
Doctor’s Thoughts
John Ervin, MD
Doctor’s Thoughts
John Schwappach, MD
Luoxis Overview
Josh Disbrow, MBA
Company Updates
Michael Macaluso
Q&A
Michael Macaluso |
 3
Formal Business
2014 Annual Meeting of Shareholders
Formal Business |
 4
2014 Annual Meeting of Shareholders
Corporate Presentation
Formal Business |
 5
Safe Harbor Statement
This presentation includes forward-looking statements within the meaning of Section 27A of the
Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, or
the Exchange Act. All statements other than statements of historical facts contained in this
presentation, including statements regarding our anticipated future clinical and regulatory
events, future financial position, business strategy and plans and objectives of management for
future operations, are forward-looking statements. Forward looking statements are generally written
in the future tense and/or are preceded by words such as “may,” “will,”
“should,” “forecast,” “could,” “expect,”
“suggest,” “believe,” “estimate,” “continue,”
“anticipate,” “intend,” “plan,” or similar words, or the negatives of such
terms or other variations on such terms or comparable terminology. Such forward-looking statements
include, without limitation, statements regarding the potential future commercialization of our
product candidates, the anticipated start dates, durations and completion dates, as well as the
potential future results, of our ongoing and future clinical trials, the anticipated designs of
our future clinical trials, anticipated future regulatory submissions and events, our
anticipated future cash position and future events under our current and potential future
collaborations. These forward-looking statements are subject to a number of risks,
uncertainties and assumptions, including without limitation to the risks described in
“Risk Factors” in Part I, Item 1A of Ampio Pharmaceuticals, Inc. Annual Report on
Form 10-K and in the other reports and documents we file with the Securities and Exchange
Commission from time to time. These risks are not exhaustive. Other sections of Ampio
Pharmaceuticals, Inc. Annual Report on Form 10-K and such other filed reports and documents
include additional factors that could adversely impact our business and financial performance.
Moreover, we operate in a very competitive and rapidly changing environment. New risk factors
emerge from time to time and it is not possible for our management to predict all risk factors, nor can we
assess the impact of all factors on our business or the extent to which any factor, or combination of
factors, may cause actual results to differ materially from those contained in any
forward-looking statements. You should not rely upon forward-looking statements as
predictions of future events. We cannot assure you that the events and circumstances reflected
in the forward-looking statements will be achieved or occur and actual results could differ
materially from those projected in the forward looking statements. We assume no obligation to update
or supplement forward-looking statements.
|
 Scientific Address |
 7
Lead Products
Solid science comprising over 30 years of research
Ampion™
50 issued patents worldwide
118 pending patents worldwide
Optina™
83 issued patents worldwide
81 pending patents worldwide
Safe & Effective
Patent Portfolio:
Only orally available treatment for DME
Patent Portfolio: |
 8
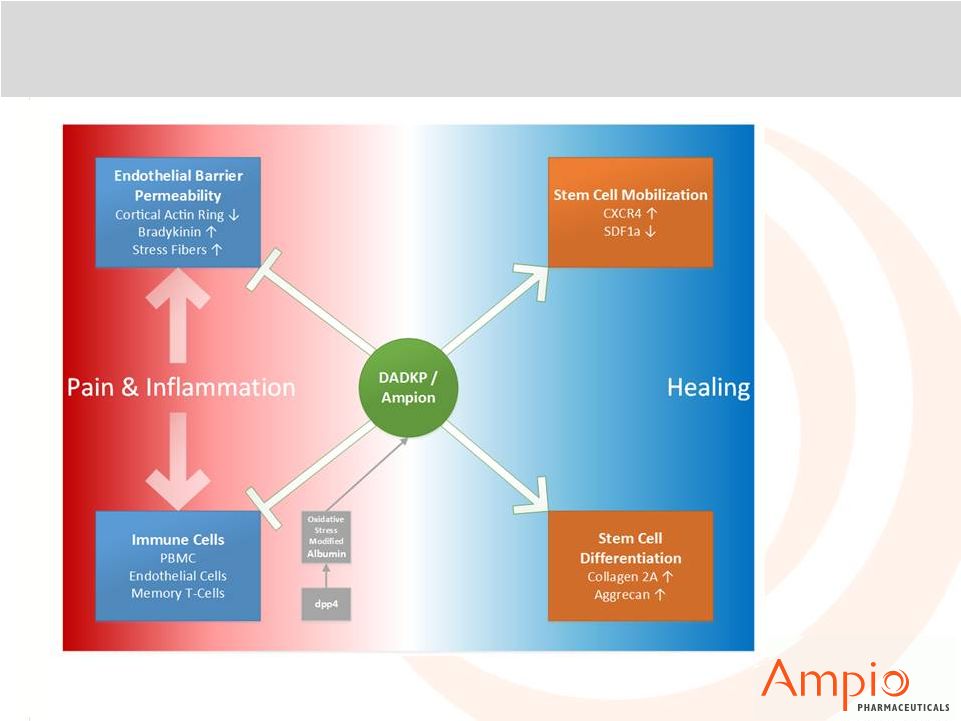
Ampion™: Overview of Anti-inflammatory and
Healing Effects |
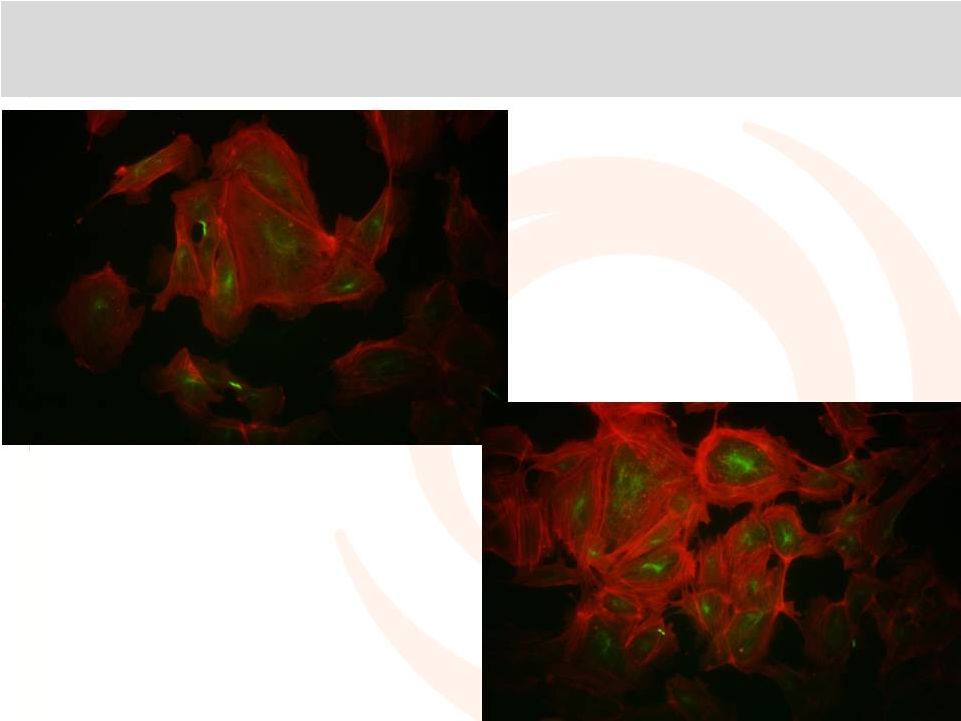 Ampion™: Cortical Actin Ring Formation in
Endothelial Cells
Saline
Ampion™ |
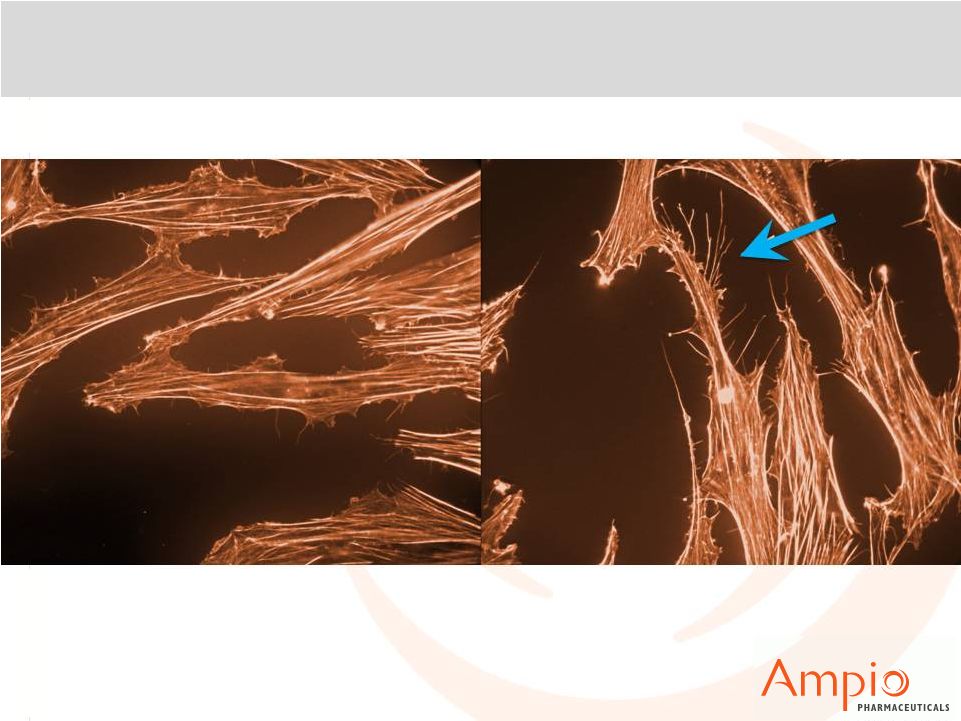 10
Ampion™: Filopodia Formation in Stem Cells
Saline
Ampion™ |
 11
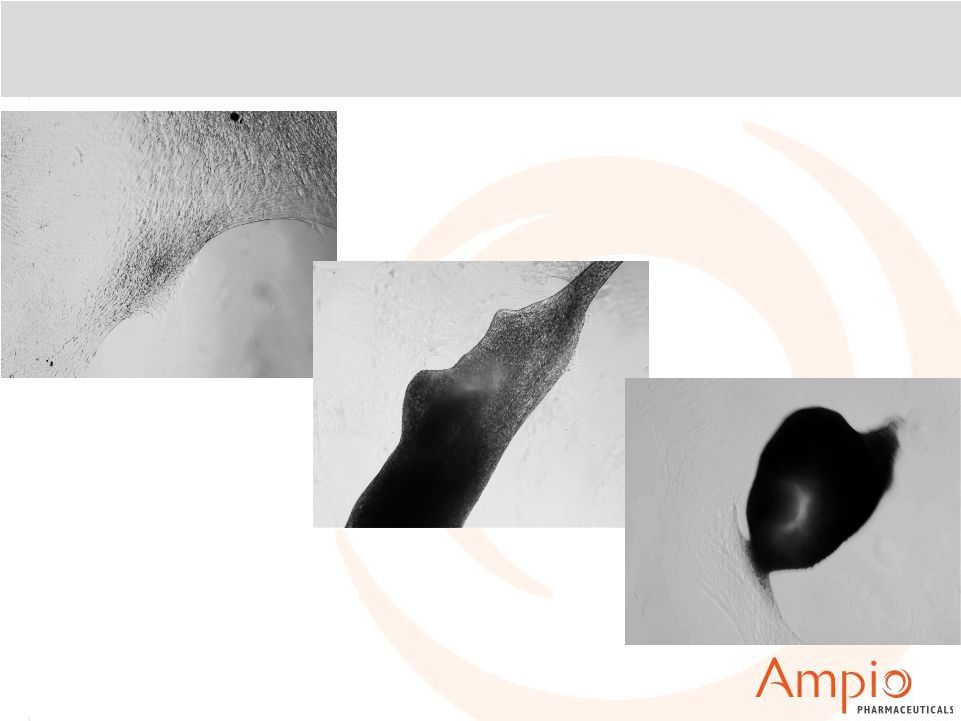
Ampion™: Micromass Condensation (Cartilage
Formation)
Saline
DADKP
Ampion™ |
 December 2013
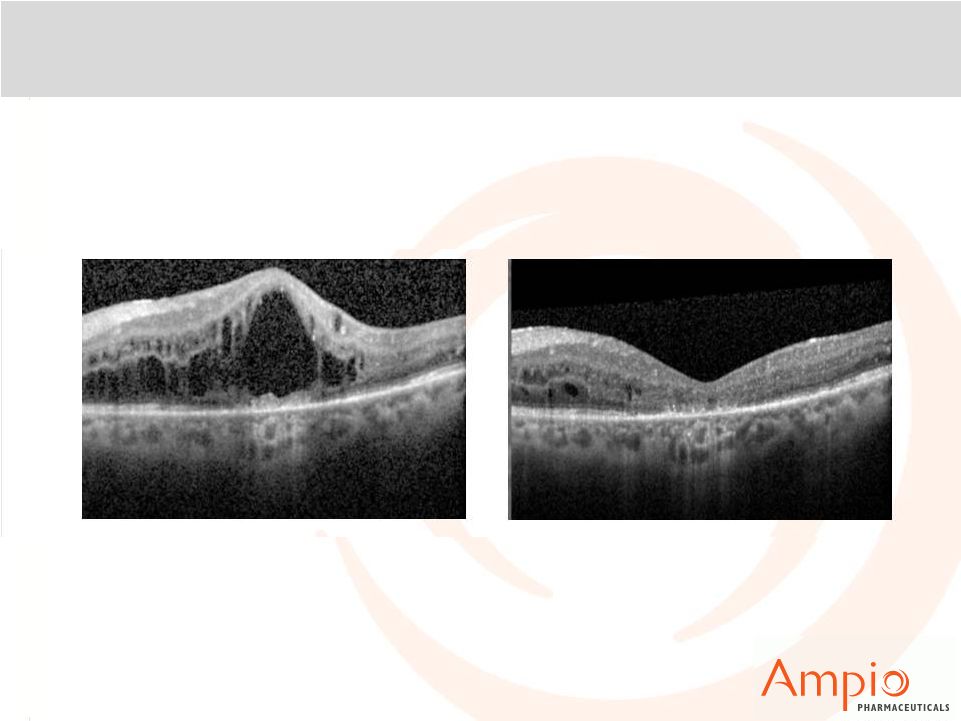
Optina™: OCT in Open-label Extension in Patient A
Beginning of OLE
+3 Months |
 13
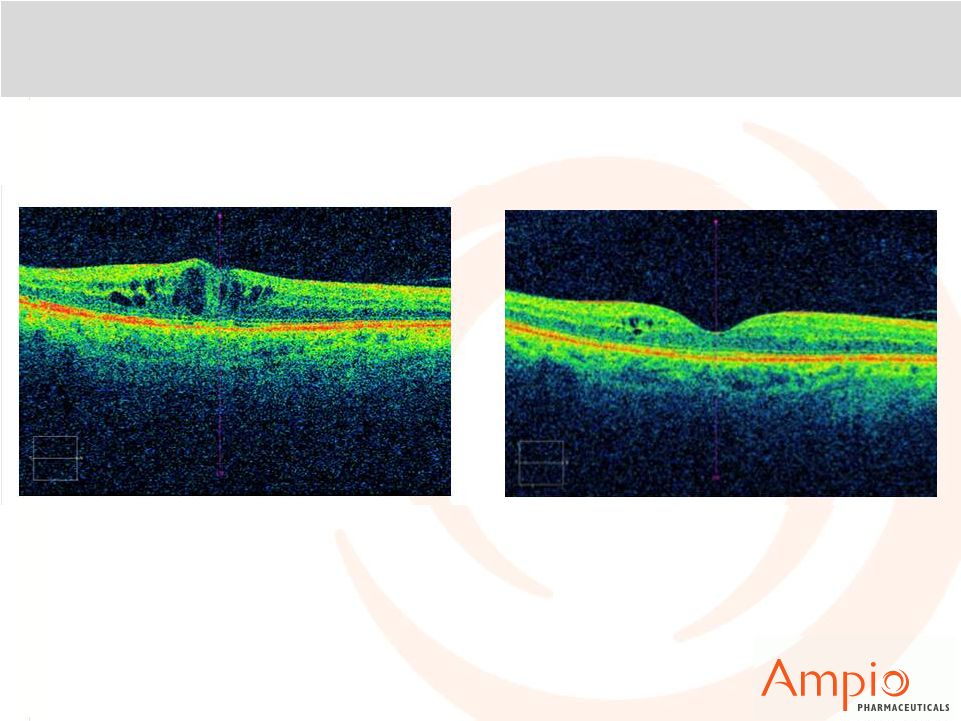
Optina™: Patient B
Kenalog intravitreal 8/22/06
PRP laser 8/28/04, 3/24/05, 6/20/07
Focal laser 8/20/12, 12/10/12
Avastin 10/1/12, 11/26/12, 4/14/13 |
 14
Optina™: VA and OCT in Patient B
OCT=437u
BCVA OD 20/50
ETDRS (62)
(37days no drug)
OCT=246u
BCVA OD 20/50
ETDRS (67) |
 Product Overview |
 16
What is a BLA?
BLA FILING
A Biologics License Application (BLA) is a request for permission to
introduce a biologic product into interstate commerce (21 CFR 601.2).
Requirements for a BLA include:
o
Applicant information
o
Product/Manufacturing information
o
Pre-clinical studies
o
Clinical studies
o
Labeling |
 17
•
Facility will have an annual
production capacity of ~10
million vials of Ampion
•
Source material, human
serum albumin (HSA),
required to meet this capacity
has already been secured
through a long-term, non-
exclusive agreement
•
cGMP compliant
–
US and European regulatory
compliant
Note:
Pictures are of rendering of new facility, not of actual facility.
Product/Manufacturing
Facility tour after the meeting today
Facility tour after the meeting today |
 18
Product/Manufacturing
FDA has issued a written response accepting the technical
transfer, analytical, scale-up and manufacturing plan for
production of Ampion™
in the new facility.
Technical transfer from contract manufacturing to commercial
facility in Englewood, CO is underway
Analysis developed by Ampio, and outsourced, will be brought
back in-house for commercial scale manufacture
Engineering runs to support scale-up to commercial
manufacture are underway
Registration batches for BLA have been scheduled to begin after
all validation activity is complete |
 19
Clinical Summary: Ampion ™
“Two adequate and well-controlled trials with
reproducible evidence of efficacy are required to
support approval of a novel product for…the
management of the pain of OA…[T]here is
one primary endpoint,
either a pain score such
as the NRS or the WOMAC pain subscale” -
FDA |
 FIRST PIVITOL
TRIAL: SPRING Study FIRST PIVITOL TRIAL: SPRING Study
20
Clinical Summary: Ampion™
‘Adequate’
(n=329 across 9 sites)
‘Well controlled’
(normal saline)
Trial was ‘well conducted’
The study met its primary endpoint
(change in WOMAC score at 12
weeks).
A randomized, placebo-controlled, double-blind study to evaluate the efficacy
and safety of two doses of intra-articular injection of Ampion™
in adults with
pain due to osteoarthritis of the knee.
“Upon review of the dataset
“Upon review of the dataset
received on November 5,
received on November 5,
2013, FDA concludes that
2013, FDA concludes that
study AP-003-A can be
study AP-003-A can be
considered as one of two
considered as one of two
“pivotal trials”
“pivotal trials”
required in
required in
support of a BLA.”
support of a BLA.”
-
-
FDA
FDA |
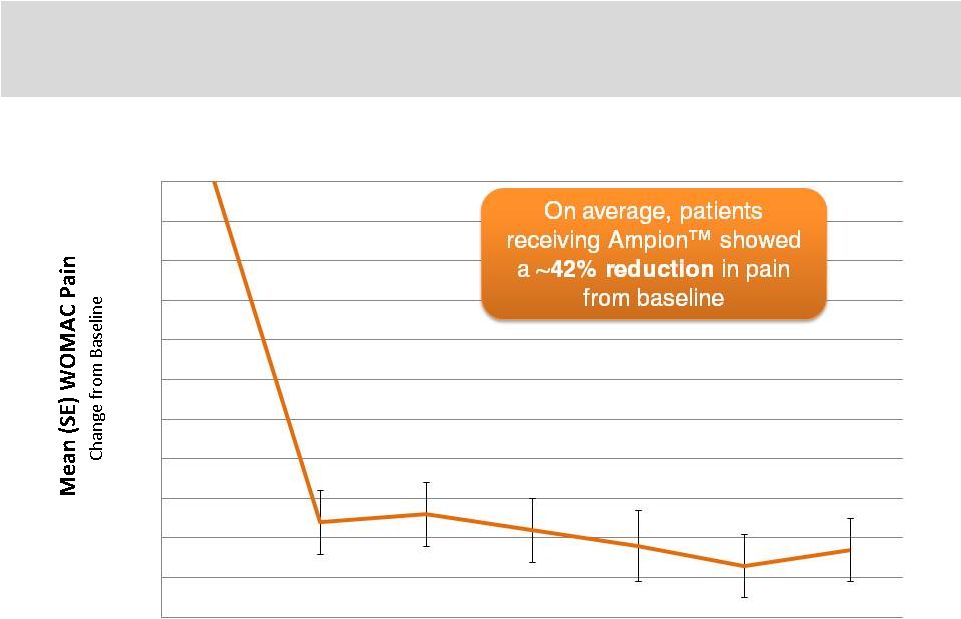 21
Clinical Summary: Ampion™
-1.1
-1
-0.9
-0.8
-0.7
-0.6
-0.5
-0.4
-0.3
-0.2
-0.1
0
0
2
4
6
8
10
12
Study Week
Effects of 4mL Ampion™
treatment on WOMAC pain over time |
 Due to the
strong results at Week 12, the study was amended and an Due to the strong results at Week
12, the study was amended and an optional follow up visit at Week 20 was added
optional follow up visit at Week 20 was added
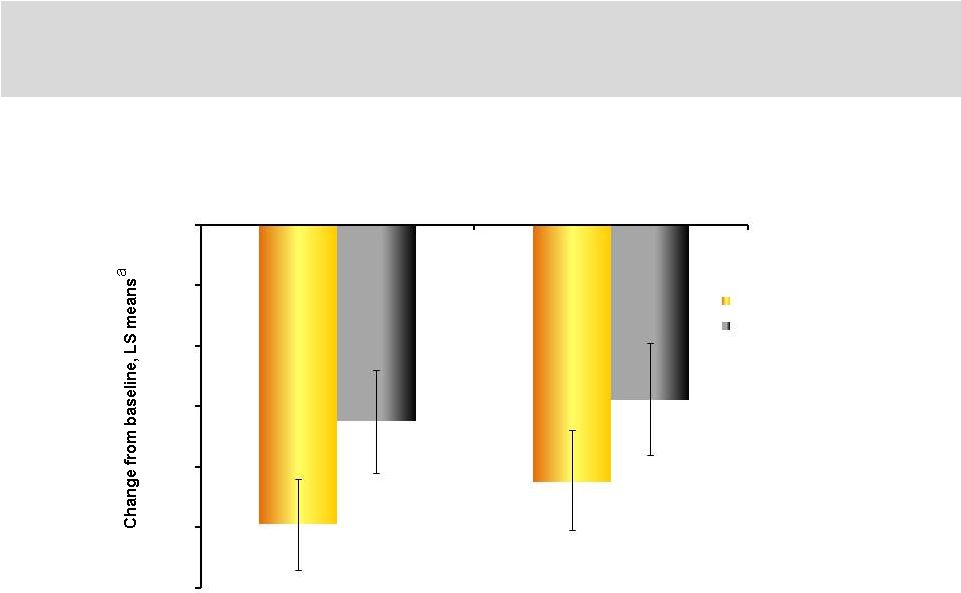
22
ANCOVA, analysis of covariance; K-L, Kellgren-Lawrence; LS, least squares; WOMAC,
Western Ontario and McMaster Universities
Osteoarthritis.
a
Repeated measures ANCOVA over 20 weeks, adjusted for baseline values.
P=0.005
P=0.04
SPRING Study Significant Pain Relief Over 20 Weeks
in K-L Grades 3 and 4
-0.99
-0.85
-0.65
-0.58
-1.2
-1
-0.8
-0.6
-0.4
-0.2
0
WOMAC A pain
WOMAC C function
LMWF-5A (n=36)
Control (n=28) |
 23
Clinical Summary: Ampion™
“FDA recommends that Ampio conduct an adequately
“FDA recommends that Ampio conduct an adequately
powered study to confirm the effect size of AP-003-A and
powered study to confirm the effect size of AP-003-A and
design the study to obtain information on the duration of
design the study to obtain information on the duration of
action and repeat dosing.”
action and repeat dosing.”
-
-
FDA
FDA
Adequately powered: 90%
Duration of action: 20 Weeks
Repeat dosing: Post-Approval |
 24
Clinical Summary: Ampion™
A prospective phase I/II study to evaluate the safety and exploratory efficacy of
three intra- articular
injection
of
Ampion™
(4
mL)
administered
two
weeks
a
part
in
adults
with pain due
to osteoarthritis of the knee.
Single center
Phase 1: Open label (N=7)
Phase II: Randomized (N=30*)
Primary endpoint: 20 Weeks
Study duration: 52 Weeks
Exploration of additional clinical benefits
High resolution MRI analysis
Knee aspirations to assess synovial fluid
Serum biomarkers
Activity and exercise logs
Investigating Healing
Investigating Healing
*study is being updated to N=40
|
 25
Clinical Summary: Ampion™
A randomized, placebo-controlled, double-blind study to evaluate the safety
and efficacy of three intra-articular injections of
Ampion (4 mL) administered two weeks apart in adults with
pain due to osteoarthritis of the knee. ‘Adequate’: N = 320 across
14 sites ‘Well controlled’: Normal saline
Primary endpoint: Change in WOMAC score at 20 weeks
Duration of action: Study extends over 24 weeks
Repeat dosing: 3 injections (Baseline, Week 2, Week 4)
SECOND PIVOTAL TRIAL: Stride Study
SECOND PIVOTAL TRIAL: Stride Study
TM |
 26
Clinical Summary: Ampion™
“FDA recommends that Ampio conduct an adequately
“FDA recommends that Ampio conduct an adequately
powered study
powered study
to confirm the effect size of AP-003-A
to confirm the effect size of AP-003-A
and design the study to obtain information on the
and design the study to obtain information on the
duration of action
duration of action
and repeat dosing.”
and repeat dosing.”
-
-
FDA
FDA
Adequately powered: 90%
Duration of action: 20 Weeks
Repeat dosing: Post-Approval
Adequately powered: 90%
Duration of action: 24 Weeks
Repeat dosing: 3 Doses
*Underline emphasis added to quotation for clarification and discussion
|
 27
R&D: Ampion™
Investigations into additional uses for
Ampion™
Crohn’s
disease
–
investigation
into
multiple
uses
and
indications
for
Ampion™
o
Oral formulation that can be made in our facility in Englewood, CO
o
Proof of Concept study was accepted by FDA |
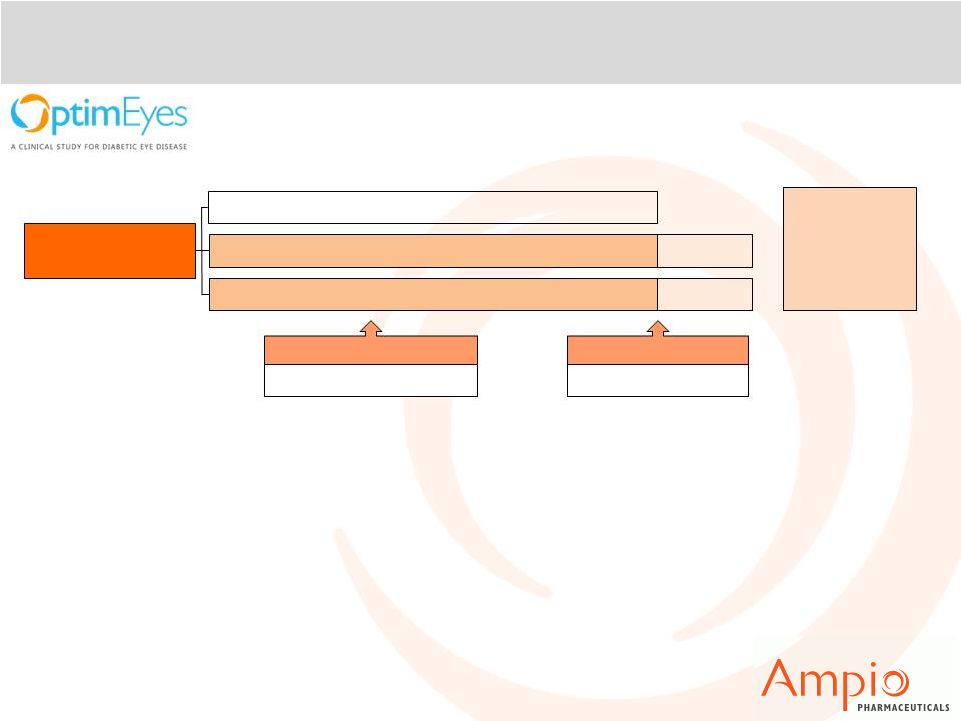 28
355 patients
randomized
0.5 mg per BMI
4 week interim analysis
Announced 4Q 2013
1.0 mg per BMI
Placebo
Washout
Washout
12 week analysis
Expected Q4 2014
12 Week
Open Label
Extension
•
~50% of patients are treatment naïve; ~50% have received anti-VEGF in
past •
Interim Analysis by independent committee identified optimal dosage
demonstrating potentially beneficial anatomic and clinical effect
•
Enrollment complete. Closed enrollment Q3 2014 with top line results expected Q4
2014
•
Optina 505(b)(2) designation enables efficient path toward NDA filing
Product Portfolio: Optina™
A randomized, placebo-controlled, double-masked study to evaluate the
efficacy and safety of two doses of oral
Optina™ in adult patients with diabetic macular
edema. |
 Regulatory Review |
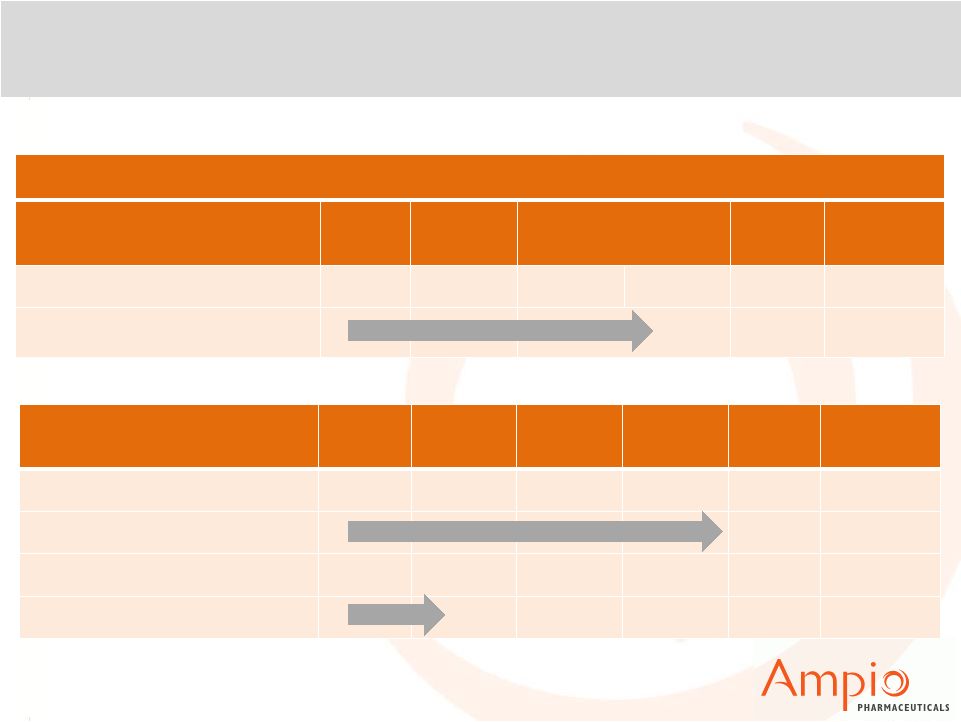 Ampio’s Pipeline Overview
Ampion™
PC
Phase 1
Phase 2
Phase 3
BLA
Filed
Approval
Degenerative Joint Diseases
Osteoarthritis of the Knee (OAK)
T-Cell Mediated Disease
Inflammatory Bowel Disease
Core Development Assets
Optina™
PC
Phase 1
Phase 2/Phase 3
NDA
Filed
Approval
Diabetic Angiopathies
Diabetic Macular Edema (DME)
30 |
 31
Product Portfolio: Optina™
A 505(b)(2) application is one for which one or more of the investigations relied
upon by the applicant for approval "were not conducted by or for the
applicant and for which the applicant has not obtained a right of reference
or use from the person by or for whom the investigations were conducted"
(21 U.S.C.355(b)(2)). 505(b)(2) Regulatory Pathway
Relatively LOW RISK
because of existing safety and efficacy information
LOWER COST
due to the smaller scope and number of potential studies
INCREASED SPEED
due to fewer studies
RAPID REGULATORY PATH
RAPID REGULATORY PATH |
 32
Optina™
Sub-Populations
•
22 centers, N=355, randomized 1:1:1, double-masked
•
Total Enrolled: 359 subjects; 432 study eyes
•
~50% of patients were refractory to anti-VEGEF intra-ocular therapy
•
Proportion of patients with renal impairment
•
Primary efficacy endpoint: Change in BCVA letters read from Baseline to
12 weeks
•
Safety examined as incidence and severity of adverse events and
glycemic, liver, renal, lipid, and hormone assays.
|
 33
Ampion™
addresses a clear medical need in the
treatment of Osteoarthritis of the Knee.
•
60% of OAK patients suffer from moderate or severe
disease.² •
Approximately 27 million OAK patients in the US are
symptomatic.³ •
Latest AAOS clinical practice guidelines recommend against the use of IA steroids
and HA. Ampion™
NSAIDs, COX-2 Inhibitors, IA Steroids, HA
Surgery
Arthroscopic,
osteotomy, total or
partial knee
arthroplasty, cartilage
grafting¹
Severe
Large osteophytes,
narrowing of joint space,
severe sclerosis and
deformity of bone ends¹
Moderate
Multiple osteophytes
and possible narrowing
of joint space¹
Mild
Osteophyte
development and
possible narrowing of
the joint space¹
1.
Hillary J. Braun and Garry E. Gold. Diagnosis of osteoarthritis: Imaging. Elsevier November
2011. 2. www.aaos.org “Arthritis of the knee” Accessed October 6, 2013.
2.
Relationship between patient-reported disease severity in osteoarthritis and
self-reported pain, function and work productivity. Sadosky et al. Arthritis Research & Therapy 2012.
3.
Osteoarthritis Epidemiology Report. DataMonitor, Inc. 2013.
4.
Treatment of Osteoarthritis of the Knee, Evidence-Based Guideline 2nd Edition. American
Academy of Orthopaedic Surgeons. May 18, 2013.
4 |
 34
Clinical Summary: Hurdles
A randomized, placebo-controlled, double-blind study to evaluate
the efficacy and safety of a single 4 mL intra-articular injection of
Ampion™
in adults with pain due to osteoarthritis of the knee.
Per FDA Good
Clinical Practice
5.13.3:
The investigational
product(s) should be
packaged to prevent
contamination and
unacceptable
deterioration during
transport and
storage.
“…Distribution Department will prepare each
shipment using pre-qualified insulated
shipping containers and gel packs…Shippers
are
pre-qualified
to
maintain
15°–
25°C
for 48 hours…” |
 Clinical Summary: Solutions
A randomized, placebo-controlled, double-blind study to evaluate the
safety and efficacy of three intra-articular injections of
Ampion™ (4 mL)
administered two weeks apart in adults with pain due to osteoarthritis of
the knee. |
 36
Ampion™
Clinical Development Progress &
Expected Milestones
•
Q3 2013: Positive results reported from pivotal SPRING Study
showing:
–
42% reduction in pain from baseline (12 week endpoint)
–
Pronounced effect in KL 3 and 4 patients with no serious drug-related
adverse events across entire treatment group
•
Q3
2014:
Initiation
of
operations
at
Ampion™
manufacturing
facility
•
Q3 2014: Pivotal STEP Study completed
–
Logistical issue identified related to shipping conditions of study drug
•
Q3 2014: Pivotal multiple injection (MI) study initiated
–
86% improvement in pain from baseline (6 week time point)
•
Q1/Q2
2015:
Expected
MI
results
and
filing
of
Ampion™
BLA
Q3
2013
Q3
2014
Q1/
Q2
2015 |
 Doctor’s Thoughts |
 38
62 yr old female; received Ampion™
in the SPRING study:
“I talked with a friend from work and he mentioned Dr. Ervin and
“I talked with a friend from work and he mentioned Dr. Ervin and
the clinical trials that he runs at his office. I enrolled in the Ampion
the clinical trials that he runs at his office. I enrolled in the Ampion
trial and although the actual injection really hurt, I now feel like a
trial and although the actual injection really hurt, I now feel like a
new person. I can walk up the stairs and am able to do so many
new person. I can walk up the stairs and am able to do so many
things I was previously unable to do. I can’t remember the last time
things I was previously unable to do. I can’t remember the last time
I have had to take pain medication for my knee. I am thrilled. I
I have had to take pain medication for my knee. I am thrilled. I
want to walk over to my orthopedic doctor and show him how well
want to walk over to my orthopedic doctor and show him how well
I am doing now.
I am doing now.
Just today, I found that I was on the real drug (Ampion™). It was
Just today, I found that I was on the real drug (Ampion™). It was
something I had long suspected since I had been having such
something I had long suspected since I had been having such
improvement in function. For some time, I have wished I could have
improvement in function. For some time, I have wished I could have
an injection in my other knee as well.”
an injection in my other knee as well.”
–
–
Patient 04-050
Patient 04-050
Doctor’s Thoughts and Patient Testimonials |
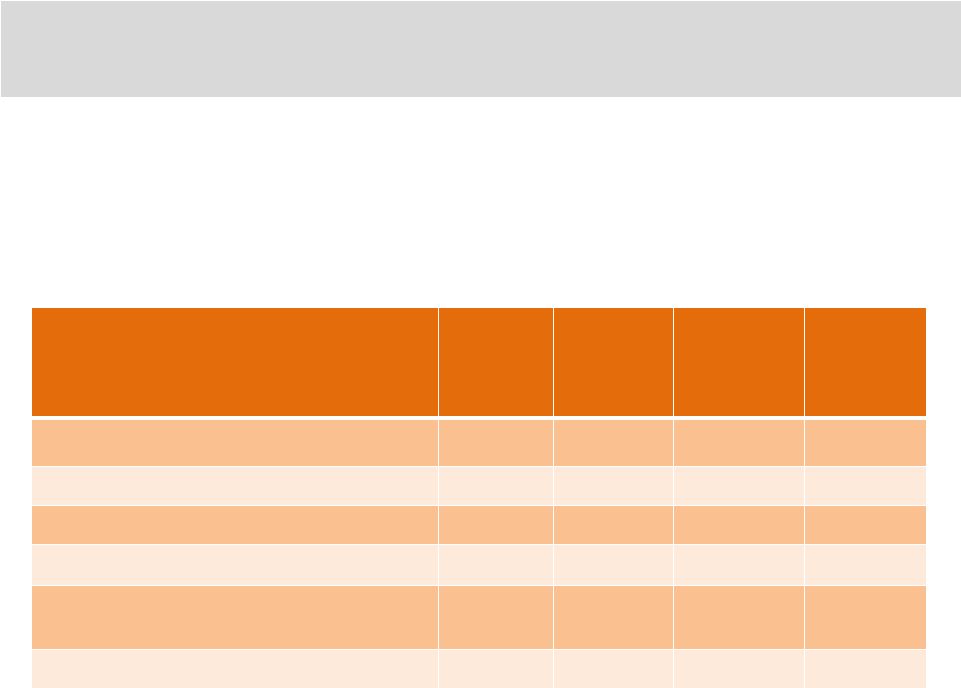 •
Ampion™
is the low molecular weight fraction of FDA approved /
regulated Human Serum Albumin (HSA)
•
HSA has its own approved safety record
•
In the AP-003-A trial there were no drug-related serious adverse
events Placebo
4 mL
Ampion™
4 mL
Placebo
4 mL +
10 mL
Ampion™
4 mL +
10 mL
(N=83)
(N=83)
(N=164)
(N=165)
Any Related AE
13 (16%)
9 (11%)
21 (13%)
17 (10%)
Any Related Serious AE
0 (0%)
0 (0%)
0 (0%)
0 (0%)
Any AE Resulting in Treatment Change
0 (0%)
0 (0%)
0 (0%)
0 (0%)
Any AE Resulting in Study
Discontinuation
0 (0%)
0 (0%)
0 (0%)
0 (0%)
Any Serious AE Resulting in Death
0 (0%)
0 (0%)
0 (0%)
0 (0%)
39
Inherent, Established Safety of Ampion™ |
 40
Doctor’s Thoughts
”The effects of Ampion™
”The effects of Ampion™
are truly remarkable. I have never
are truly remarkable. I have never
seen an injectable drug into the knee with this degree of pain
seen an injectable drug into the knee with this degree of pain
relief and improvement of function. This may change the way
relief and improvement of function. This may change the way
we treat patients with osteoarthritis of the knee”.
we treat patients with osteoarthritis of the knee”.
-
-
Dr. John Schwappach, orthopedic surgeon and the Principal
Dr. John Schwappach, orthopedic surgeon and the Principal
Investigator in the Ampion™
Investigator in the Ampion™
multiple injections trial
multiple injections trial |
 Luoxis Diagnostics,
subsidiary of
Ampio Pharmaceuticals |
 42
Oxidation-reduction potential: A ‘vital sign’
assessing the
body’s response to oxidative stress
•
Oxidative stress is the body’s response to physiologic stress
brought on by a depletion of natural antioxidants in response
to:
–
Illness
–
Injury
–
Inflammation
•
While well recognized, no good methods exist to measure
oxidative stress.
•
Oxidation-reduction potential is a measure of homeostasis
detecting the balance between antioxidants and pro-oxidants
in the body.
–
A complete picture of oxidative stress |
 43
Luoxis Diagnostics has significantly advanced development
of the oxidation-reduction potential (ORP) device platform.
•
Luoxis has a fully-developed IVD asset.
–
Clinical validation studies completed in traumatic brain injury (TBI),
multi-trauma, hip fracture, and stroke
–
ISO 13485:2003 certification awarded January 2014
–
Market development underway through research partnerships at
major research centers throughout the world
•
Luoxis is moving rapidly toward commercialization with
market development underway
–
CE Mark obtained in Q1 2014
–
Distribution partnering discussions underway in Europe
–
Distribution partnership discussions underway in Japan
–
First pharma research agreement signed in Q2 2014 |
 44
Corporate milestones since Jan 2014
Jan-2014: ISO 13485:2003 medical device certification
Apr-2014: CE Marking/Health Canada approval
Apr-2014: First clinical development project with Big Pharma
May-2014: First instrument placed with pharma company
June-2014: First instrument placed in Europe with major academic research
center
Jul-2014: Collaboration with first independent U.S. academic institution
Sept-2014: Presented TBI clinical study data at AAST (American Association for
the Surgery of Trauma)
Q4 2014-Q1 2015: Anticipate first distribution agreements signed in Europe and
Japan for introduction into research market and development of the clinical
market
Q4 2014: Expect additional pharma research agreements initiated
|
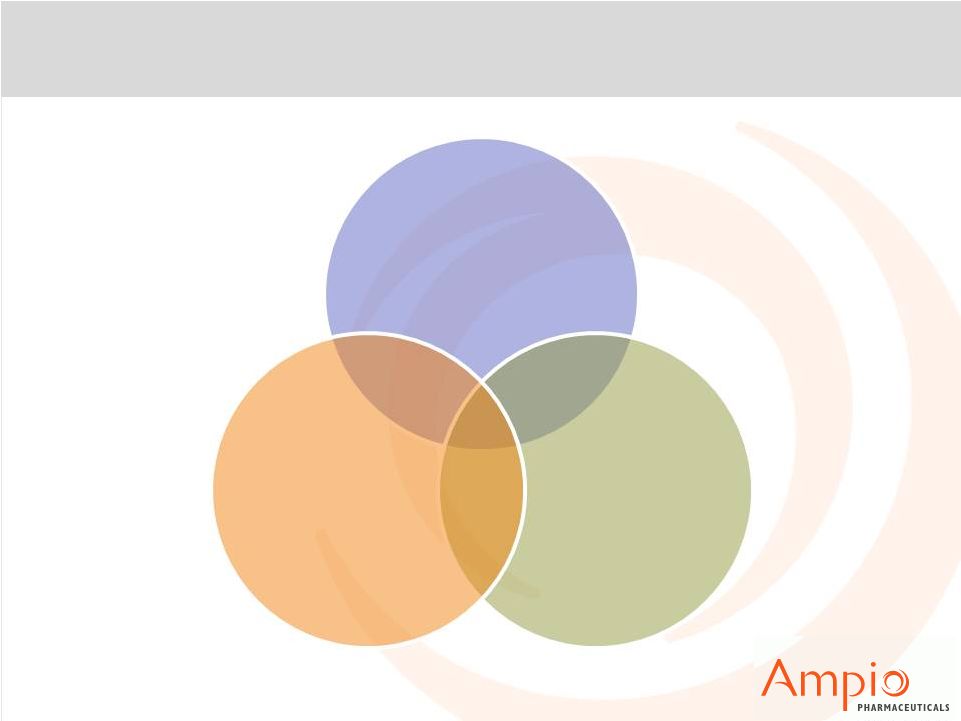 45
3-Pronged Approach for Broad Acceptance
Nearly 50 studies
underway or in
planning stages
Two large Phase
2b studies
underway utilizing
ORP, working with
5 pharma
companies
CE Mark enables
global market
clearances;
FDA studies
underway
Continue to Drive
Clinical Validation
Pursue Global
Regulatory
Approvals
Expand
Pharma
Relationships |
 46
Luoxis Diagnostics has significantly advanced development
of the oxidation-reduction potential (ORP) device platform.
•
Luoxis has a fully-developed IVD asset.
–
Clinical validation studies completed in traumatic brain injury and
multi-trauma
–
ISO 13485:2003 certification awarded January 2014
–
Research partnerships in place with major research centers
throughout the world
•
Luoxis is moving rapidly toward commercialization.
–
CE Mark in Q1 2014
–
Health Canada clearance in Q2 2014
–
Launching into research market for clinical market development
–
FDA submission expected in 2014 as 510k de novo
–
Confidential commercial partnering discussions actively underway
|
 Company Updates and
Q&A |
 Headquarters:
373 Inverness Parkway, Suite 200
Englewood, CO 80112
www.ampiopharma.com |
